User login
New AHA/ASA guideline on secondary stroke prevention
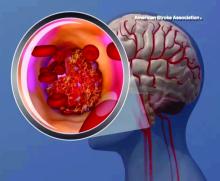
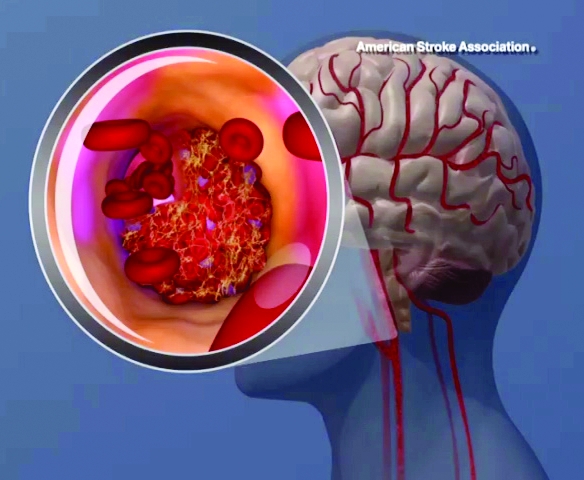
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
When possible, diagnostic tests to determine the cause of a first stroke or transient ischemic attack (TIA) should be completed within 48 hours after symptom onset, the American Heart Association/American Stroke Association said in an updated clinical practice guideline.
“It is critically important to understand the best ways to prevent another stroke once someone has had a stroke or a TIA,” Dawn O. Kleindorfer, MD, chair of the guideline writing group, said in a news release.
“If we can pinpoint the cause of the first stroke or TIA, we can tailor strategies to prevent a second stroke,” said Dr. Kleindorfer, professor and chair, department of neurology, University of Michigan, Ann Arbor.
The updated guideline was published online May 24, 2021, in Stroke.
“The secondary prevention of stroke guideline is one of the ASA’s ‘flagship’ guidelines, last updated in 2014,” Dr. Kleindorfer said.
The update includes “a number of changes to the writing and formatting of this guideline to make it easier for professionals to understand and locate information more quickly, ultimately greatly improving patient care and preventing more strokes in our patients,” she noted.
Let pathogenic subtype guide prevention
For patients who have survived a stroke or TIA, management of vascular risk factors, particularly hypertension, diabetes, cholesterol/triglyceride levels, and smoking cessation, are key secondary prevention tactics, the guideline said.
Limiting salt intake and/or following a heart-healthy Mediterranean diet is also advised, as is engaging in at least moderate-intensity aerobic activity for at least 10 minutes four times a week or vigorous-intensity aerobic activity for at least 20 minutes twice a week.
“Approximately 80% of strokes can be prevented by controlling blood pressure, eating a healthy diet, engaging in regular physical activity, not smoking and maintaining a healthy weight,” Amytis Towfighi, MD, vice chair of the guideline writing group and director of neurologic services, Los Angeles County Department of Health Services, noted in the release.
For health care professionals, the guideline said specific recommendations for secondary prevention often depend on the ischemic stroke/TIA subtype. “Therefore, new in this guideline is a section describing recommendations for the diagnostic workup after ischemic stroke, to define ischemic stroke pathogenesis (when possible), and to identify targets for treatment to reduce the risk of recurrent ischemic stroke. Recommendations are now segregated by pathogenetic subtype,” the guideline stated.
Among the recommendations:
- Use multidisciplinary care teams to personalize care for patients and employ shared decision-making with the patient to develop care plans that incorporate a patient’s wishes, goals, and concerns.
- Screen for and initiate anticoagulant drug therapy to reduce recurrent events.
- Prescribe antithrombotic therapy, including antiplatelets or anticoagulants, in the absence of contraindications. The guideline noted that the combination of antiplatelets and anticoagulation is typically not recommended for preventing second strokes and that dual antiplatelet therapy (DAPT) – taking along with a second medication to prevent blood clotting – is recommended in the short term and only for specific patients: those with early arriving minor stroke and high-risk TIA or severe symptomatic stenosis.
- Consider or carotid artery stenting for select patients with narrowing of carotid arteries.
- Aggressive medical management of risk factors and short-term DAPT are preferred for patients with severe intracranial stenosis thought to be the cause of first stroke or TIA.
- In some patients, it’s reasonable to consider percutaneous closure of .
The guideline is accompanied by a systematic review and meta-analysis regarding the benefits and risks of dual antiplatelet versus single antiplatelet therapy for secondary stroke prevention. The authors conclude that DAPT may be appropriate for select patients.
“Additional research is needed to determine: the optimal timing of starting treatment relative to the clinical event; the optimal duration of DAPT to maximize the risk-benefit ratio; whether additional populations excluded from POINT and CHANCE [two of the trials examined], such as those with major stroke, may also benefit from early DAPT; and whether certain genetic profiles eliminate the benefit of early DAPT,” concluded the reviewers, led by Devin Brown, MD, University of Michigan.
The guideline was prepared on behalf of and approved by the AHA Stroke Council’s Scientific Statements Oversight Committee on Clinical Practice Guidelines. The writing group included representatives from the AHA/ASA and the American Academy of Neurology. The guideline has been endorsed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons and the Society of Vascular and Interventional Neurology. It has also been affirmed by the AAN as an educational tool for neurologists.
The research had no commercial funding.
A version of this article first appeared on Medscape.com.
Rivaroxaban cut recurrent limb events in VOYAGER-PAD
After patients with peripheral artery disease undergo lower-extremity revascularization, they are at high risk for major adverse limb events, and new findings from a prespecified analysis of data from the VOYAGER-PAD trial show that treatment with the direct-acting oral anticoagulant rivaroxaban along with aspirin significantly cut the rate of total major adverse limb events in these patients.
These findings confirm the drop in first major adverse limb events linked to rivaroxaban treatment that was VOYAGER-PAD’s primary result, reported just over a year ago.
The new total-event analysis also provides important insight into the huge magnitude of total major adverse limb events that patients with PAD can develop following lower-extremity revascularization (LER).
The 6,564 patients who all received aspirin and were randomized to either rivaroxaban (Xarelto) or placebo had 4,714 total events during a median follow-up of 2.5 years following their revascularization procedure. This included 1,092 first primary events (a composite of acute limb ischemia, major amputation for vascular causes, MI, ischemic stroke, or cardiovascular death), 522 primary events that occurred as second or subsequent events among patients after a first primary event (a nearly 50% increase from first events only), and 3,100 additional vascular events that did not fit into the primary-event category, most often a peripheral revascularization procedure, Rupert M. Bauersachs, MD, said at the annual scientific sessions of the American College of Cardiology.
“We were all astonished by this high event rate,” Dr. Bauersachs said during his report.
The total-event analysis that he reported showed that treatment with rivaroxaban resulted in a significant 14% relative reduction, compared with placebo in the incidence of total primary events, which closely tracks the significant 15% relative reduction in first primary events reported from the VOYAGER-PAD trial in 2020. Treatment with rivaroxaban also significantly linked with a 14% cut in total vascular events, compared with placebo, including the many events not included in the primary endpoint, said Dr. Bauersachs, who until his retirement in May 2021 was director of the Clinic for Vascular Medicine at the Darmstadt (Germany) Clinic. Concurrently with the report, the results appeared online.
“If one focuses only on first events, you miss the totality of disease burden. There is even greater benefit by reducing total events,” Dr. Bauersachs said during a press briefing. Adding rivaroxaban prevented roughly 2.6 first primary events for every 100 patients treated, but it also prevented 4.4 total primary events and 12.5 total vascular events for every 100 treated patients.
An ‘incredibly high’ event rate
“I don’t think any of us imagined the level of morbidity in this population. The event rate is incredibly high,” commented Joshua A. Beckman, MD, professor and director of vascular medicine at Vanderbilt University Medical Center, Nashville, Tenn.
Because treatment with rivaroxaban showed clear efficacy for also preventing subsequent events it should not be considered to have failed in patients who have a vascular event while on rivaroxaban treatment, he added as designated discussant for the report. Treatment with rivaroxaban “should be continued indefinitely,” he concluded.
“It’s quite astonishing to see the magnitude of [total] events in these patients,” commented Sahil A. Parikh, MD, a cardiologist and director of endovascular services at Columbia University Medical Center in New York. “We’ve always known that these are high-risk patients, but exactly how high their risk is was not well understood until these data came to light.”
Dr. Parikh also noted that, despite the clear evidence reported from VOYAGER-PAD more than a year ago proving the efficacy and safety of adding rivaroxaban to aspirin for long-term treatment of patients with PAD following LER, this regimen has not yet become standard U.S. practice.
Rivaroxaban use falls short of the expected level
“This paradigm shift has not seen the level of adoption that we would expect based on the data,” he said. “There have been numerous editorials and discussions of this at every major medical meeting” during the past year, but those expert opinions have not translated into changed practice. “Perhaps the pandemic has muted enthusiasm for adoption of a new therapeutic paradigm,” suggested Dr. Parikh, and “on top of that guidelines have yet to be updated,” although he noted that updated guidelines from the ACC and American Heart Association for PAD that include the types of patients enrolled in VOYAGER-PAD are now under review and should be released by the first half of 2022.
“I think the additional data [reported by Dr. Bauersachs] will encourage us to use rivaroxaban in patients with claudication,” Dr. Parikh said. “Perhaps we should use rivaroxaban and aspirin in a broader swath of patients, but it will take time to convince some constituencies.”
VOYAGER-PAD randomized patients with PAD who underwent successful LER within 10 days prior to enrollment at 542 sites in 34 countries during 2015-2018. In addition to every patient receiving 100 mg aspirin daily and either 2.5 mg rivaroxaban twice daily or placebo once daily, patients who received an intra-arterial device such as a stent could also receive the antiplatelet agent clopidogrel for a planned maximum of 30 days after revascularization at the discretion of their physician, and the trial protocol allowed for extending clopidogrel treatment to as many as 60 days.
In addition to the efficacy outcomes, the safety results showed that adding rivaroxaban to aspirin appeared to increase bleeding episodes, but at rates that generally did not reach significance and that were dwarfed by the efficacy benefit. The study’s primary safety outcome was the incidence of Thrombolysis in Myocardial Infarction (TIMI) major bleeding episodes, which occurred in 2.65% of patients who received rivaroxaban and in 1.87% on those on placebo, a 43% relative increase that fell short of significance (P = .07). The analyses overall indicated that 10,000 similar patients treated for 1 year with rivaroxaban would have 181 fewer primary events, compared with placebo-treated patients at the cost of also having 29 additional TIMI major bleeding events compared with patient on placebo.
Adding clopidogrel adds little except bleeding
Further analysis showed that just over half of enrolled patients also received clopidogrel for a median of 29 days following their LER procedure. This added agent produced no significant added benefit during 3-year follow-up, but did boost bleeding risk, especially in patients who received clopidogrel for more than 30 days. This led the study investigators to suggest that, while rivaroxaban plus aspirin is indicated for long-term treatment, addition of clopidogrel on top of this should be limited to 30 days or fewer to minimize bleeding risk.
“I’m sure there is a bleeding hazard associated with rivaroxaban plus aspirin, but this is attenuated by using dual therapy and not using triple therapy” by also adding clopidogrel, noted Dr. Parikh.
The new VOYAGER-PAD results also showed that the ongoing risk faced by patients with PAD following LER applies globally to their peripheral arteries. Of the 3,034 total peripheral revascularizations performed in the cohort during follow-up, 64% occurred in the index limb and 36% in the contralateral limb. Another striking finding was that the need for ipsilateral repeat revascularization was more common after an index endovascular procedure, 2,329 repeat revascularizations in 4,379 of these patients (53%), compared with 2,185 patients who had surgical revascularization for their index procedure and subsequently 705 of these patients (32%) needed repeat revascularization.
But rivaroxaban treatment appeared to provide little benefit for the much less frequent incidence of first and subsequent events in the coronary and cerebral circulation. During follow-up, the rates of major adverse cardiovascular events – cardiovascular death, nonfatal MI, and nonfatal stroke – were virtually identical in the rivaroxaban and placebo groups.
“This study makes it clear that we are learning about differences in presentation between the vascular beds, and the benefits of specific treatments in each vascular bed,” Dr. Beckman said.
VOYAGER-PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). Dr. Bauersachs has received personal fees from Bayer, as well as from Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer, and has received grant support from Aspen Pharma. Dr. Beckman been a consultant to and received honoraria from Janssen, as well as from Amgen, JanOne, Novartis, and Sanofi, and he has served on a data and safety monitoring board for Bayer. Dr. Parikh has been a consultant to and received honoraria from Janssen, as well as from Abbott, Boston Scientific, Cordis, Medtronic, Penumbra, Philips, and Terumo, he has been a speaker on behalf of Inari, and he has received grant support from Abbott, Shockwave Medical, Surmodics, and TriReme Medical.
After patients with peripheral artery disease undergo lower-extremity revascularization, they are at high risk for major adverse limb events, and new findings from a prespecified analysis of data from the VOYAGER-PAD trial show that treatment with the direct-acting oral anticoagulant rivaroxaban along with aspirin significantly cut the rate of total major adverse limb events in these patients.
These findings confirm the drop in first major adverse limb events linked to rivaroxaban treatment that was VOYAGER-PAD’s primary result, reported just over a year ago.
The new total-event analysis also provides important insight into the huge magnitude of total major adverse limb events that patients with PAD can develop following lower-extremity revascularization (LER).
The 6,564 patients who all received aspirin and were randomized to either rivaroxaban (Xarelto) or placebo had 4,714 total events during a median follow-up of 2.5 years following their revascularization procedure. This included 1,092 first primary events (a composite of acute limb ischemia, major amputation for vascular causes, MI, ischemic stroke, or cardiovascular death), 522 primary events that occurred as second or subsequent events among patients after a first primary event (a nearly 50% increase from first events only), and 3,100 additional vascular events that did not fit into the primary-event category, most often a peripheral revascularization procedure, Rupert M. Bauersachs, MD, said at the annual scientific sessions of the American College of Cardiology.
“We were all astonished by this high event rate,” Dr. Bauersachs said during his report.
The total-event analysis that he reported showed that treatment with rivaroxaban resulted in a significant 14% relative reduction, compared with placebo in the incidence of total primary events, which closely tracks the significant 15% relative reduction in first primary events reported from the VOYAGER-PAD trial in 2020. Treatment with rivaroxaban also significantly linked with a 14% cut in total vascular events, compared with placebo, including the many events not included in the primary endpoint, said Dr. Bauersachs, who until his retirement in May 2021 was director of the Clinic for Vascular Medicine at the Darmstadt (Germany) Clinic. Concurrently with the report, the results appeared online.
“If one focuses only on first events, you miss the totality of disease burden. There is even greater benefit by reducing total events,” Dr. Bauersachs said during a press briefing. Adding rivaroxaban prevented roughly 2.6 first primary events for every 100 patients treated, but it also prevented 4.4 total primary events and 12.5 total vascular events for every 100 treated patients.
An ‘incredibly high’ event rate
“I don’t think any of us imagined the level of morbidity in this population. The event rate is incredibly high,” commented Joshua A. Beckman, MD, professor and director of vascular medicine at Vanderbilt University Medical Center, Nashville, Tenn.
Because treatment with rivaroxaban showed clear efficacy for also preventing subsequent events it should not be considered to have failed in patients who have a vascular event while on rivaroxaban treatment, he added as designated discussant for the report. Treatment with rivaroxaban “should be continued indefinitely,” he concluded.
“It’s quite astonishing to see the magnitude of [total] events in these patients,” commented Sahil A. Parikh, MD, a cardiologist and director of endovascular services at Columbia University Medical Center in New York. “We’ve always known that these are high-risk patients, but exactly how high their risk is was not well understood until these data came to light.”
Dr. Parikh also noted that, despite the clear evidence reported from VOYAGER-PAD more than a year ago proving the efficacy and safety of adding rivaroxaban to aspirin for long-term treatment of patients with PAD following LER, this regimen has not yet become standard U.S. practice.
Rivaroxaban use falls short of the expected level
“This paradigm shift has not seen the level of adoption that we would expect based on the data,” he said. “There have been numerous editorials and discussions of this at every major medical meeting” during the past year, but those expert opinions have not translated into changed practice. “Perhaps the pandemic has muted enthusiasm for adoption of a new therapeutic paradigm,” suggested Dr. Parikh, and “on top of that guidelines have yet to be updated,” although he noted that updated guidelines from the ACC and American Heart Association for PAD that include the types of patients enrolled in VOYAGER-PAD are now under review and should be released by the first half of 2022.
“I think the additional data [reported by Dr. Bauersachs] will encourage us to use rivaroxaban in patients with claudication,” Dr. Parikh said. “Perhaps we should use rivaroxaban and aspirin in a broader swath of patients, but it will take time to convince some constituencies.”
VOYAGER-PAD randomized patients with PAD who underwent successful LER within 10 days prior to enrollment at 542 sites in 34 countries during 2015-2018. In addition to every patient receiving 100 mg aspirin daily and either 2.5 mg rivaroxaban twice daily or placebo once daily, patients who received an intra-arterial device such as a stent could also receive the antiplatelet agent clopidogrel for a planned maximum of 30 days after revascularization at the discretion of their physician, and the trial protocol allowed for extending clopidogrel treatment to as many as 60 days.
In addition to the efficacy outcomes, the safety results showed that adding rivaroxaban to aspirin appeared to increase bleeding episodes, but at rates that generally did not reach significance and that were dwarfed by the efficacy benefit. The study’s primary safety outcome was the incidence of Thrombolysis in Myocardial Infarction (TIMI) major bleeding episodes, which occurred in 2.65% of patients who received rivaroxaban and in 1.87% on those on placebo, a 43% relative increase that fell short of significance (P = .07). The analyses overall indicated that 10,000 similar patients treated for 1 year with rivaroxaban would have 181 fewer primary events, compared with placebo-treated patients at the cost of also having 29 additional TIMI major bleeding events compared with patient on placebo.
Adding clopidogrel adds little except bleeding
Further analysis showed that just over half of enrolled patients also received clopidogrel for a median of 29 days following their LER procedure. This added agent produced no significant added benefit during 3-year follow-up, but did boost bleeding risk, especially in patients who received clopidogrel for more than 30 days. This led the study investigators to suggest that, while rivaroxaban plus aspirin is indicated for long-term treatment, addition of clopidogrel on top of this should be limited to 30 days or fewer to minimize bleeding risk.
“I’m sure there is a bleeding hazard associated with rivaroxaban plus aspirin, but this is attenuated by using dual therapy and not using triple therapy” by also adding clopidogrel, noted Dr. Parikh.
The new VOYAGER-PAD results also showed that the ongoing risk faced by patients with PAD following LER applies globally to their peripheral arteries. Of the 3,034 total peripheral revascularizations performed in the cohort during follow-up, 64% occurred in the index limb and 36% in the contralateral limb. Another striking finding was that the need for ipsilateral repeat revascularization was more common after an index endovascular procedure, 2,329 repeat revascularizations in 4,379 of these patients (53%), compared with 2,185 patients who had surgical revascularization for their index procedure and subsequently 705 of these patients (32%) needed repeat revascularization.
But rivaroxaban treatment appeared to provide little benefit for the much less frequent incidence of first and subsequent events in the coronary and cerebral circulation. During follow-up, the rates of major adverse cardiovascular events – cardiovascular death, nonfatal MI, and nonfatal stroke – were virtually identical in the rivaroxaban and placebo groups.
“This study makes it clear that we are learning about differences in presentation between the vascular beds, and the benefits of specific treatments in each vascular bed,” Dr. Beckman said.
VOYAGER-PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). Dr. Bauersachs has received personal fees from Bayer, as well as from Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer, and has received grant support from Aspen Pharma. Dr. Beckman been a consultant to and received honoraria from Janssen, as well as from Amgen, JanOne, Novartis, and Sanofi, and he has served on a data and safety monitoring board for Bayer. Dr. Parikh has been a consultant to and received honoraria from Janssen, as well as from Abbott, Boston Scientific, Cordis, Medtronic, Penumbra, Philips, and Terumo, he has been a speaker on behalf of Inari, and he has received grant support from Abbott, Shockwave Medical, Surmodics, and TriReme Medical.
After patients with peripheral artery disease undergo lower-extremity revascularization, they are at high risk for major adverse limb events, and new findings from a prespecified analysis of data from the VOYAGER-PAD trial show that treatment with the direct-acting oral anticoagulant rivaroxaban along with aspirin significantly cut the rate of total major adverse limb events in these patients.
These findings confirm the drop in first major adverse limb events linked to rivaroxaban treatment that was VOYAGER-PAD’s primary result, reported just over a year ago.
The new total-event analysis also provides important insight into the huge magnitude of total major adverse limb events that patients with PAD can develop following lower-extremity revascularization (LER).
The 6,564 patients who all received aspirin and were randomized to either rivaroxaban (Xarelto) or placebo had 4,714 total events during a median follow-up of 2.5 years following their revascularization procedure. This included 1,092 first primary events (a composite of acute limb ischemia, major amputation for vascular causes, MI, ischemic stroke, or cardiovascular death), 522 primary events that occurred as second or subsequent events among patients after a first primary event (a nearly 50% increase from first events only), and 3,100 additional vascular events that did not fit into the primary-event category, most often a peripheral revascularization procedure, Rupert M. Bauersachs, MD, said at the annual scientific sessions of the American College of Cardiology.
“We were all astonished by this high event rate,” Dr. Bauersachs said during his report.
The total-event analysis that he reported showed that treatment with rivaroxaban resulted in a significant 14% relative reduction, compared with placebo in the incidence of total primary events, which closely tracks the significant 15% relative reduction in first primary events reported from the VOYAGER-PAD trial in 2020. Treatment with rivaroxaban also significantly linked with a 14% cut in total vascular events, compared with placebo, including the many events not included in the primary endpoint, said Dr. Bauersachs, who until his retirement in May 2021 was director of the Clinic for Vascular Medicine at the Darmstadt (Germany) Clinic. Concurrently with the report, the results appeared online.
“If one focuses only on first events, you miss the totality of disease burden. There is even greater benefit by reducing total events,” Dr. Bauersachs said during a press briefing. Adding rivaroxaban prevented roughly 2.6 first primary events for every 100 patients treated, but it also prevented 4.4 total primary events and 12.5 total vascular events for every 100 treated patients.
An ‘incredibly high’ event rate
“I don’t think any of us imagined the level of morbidity in this population. The event rate is incredibly high,” commented Joshua A. Beckman, MD, professor and director of vascular medicine at Vanderbilt University Medical Center, Nashville, Tenn.
Because treatment with rivaroxaban showed clear efficacy for also preventing subsequent events it should not be considered to have failed in patients who have a vascular event while on rivaroxaban treatment, he added as designated discussant for the report. Treatment with rivaroxaban “should be continued indefinitely,” he concluded.
“It’s quite astonishing to see the magnitude of [total] events in these patients,” commented Sahil A. Parikh, MD, a cardiologist and director of endovascular services at Columbia University Medical Center in New York. “We’ve always known that these are high-risk patients, but exactly how high their risk is was not well understood until these data came to light.”
Dr. Parikh also noted that, despite the clear evidence reported from VOYAGER-PAD more than a year ago proving the efficacy and safety of adding rivaroxaban to aspirin for long-term treatment of patients with PAD following LER, this regimen has not yet become standard U.S. practice.
Rivaroxaban use falls short of the expected level
“This paradigm shift has not seen the level of adoption that we would expect based on the data,” he said. “There have been numerous editorials and discussions of this at every major medical meeting” during the past year, but those expert opinions have not translated into changed practice. “Perhaps the pandemic has muted enthusiasm for adoption of a new therapeutic paradigm,” suggested Dr. Parikh, and “on top of that guidelines have yet to be updated,” although he noted that updated guidelines from the ACC and American Heart Association for PAD that include the types of patients enrolled in VOYAGER-PAD are now under review and should be released by the first half of 2022.
“I think the additional data [reported by Dr. Bauersachs] will encourage us to use rivaroxaban in patients with claudication,” Dr. Parikh said. “Perhaps we should use rivaroxaban and aspirin in a broader swath of patients, but it will take time to convince some constituencies.”
VOYAGER-PAD randomized patients with PAD who underwent successful LER within 10 days prior to enrollment at 542 sites in 34 countries during 2015-2018. In addition to every patient receiving 100 mg aspirin daily and either 2.5 mg rivaroxaban twice daily or placebo once daily, patients who received an intra-arterial device such as a stent could also receive the antiplatelet agent clopidogrel for a planned maximum of 30 days after revascularization at the discretion of their physician, and the trial protocol allowed for extending clopidogrel treatment to as many as 60 days.
In addition to the efficacy outcomes, the safety results showed that adding rivaroxaban to aspirin appeared to increase bleeding episodes, but at rates that generally did not reach significance and that were dwarfed by the efficacy benefit. The study’s primary safety outcome was the incidence of Thrombolysis in Myocardial Infarction (TIMI) major bleeding episodes, which occurred in 2.65% of patients who received rivaroxaban and in 1.87% on those on placebo, a 43% relative increase that fell short of significance (P = .07). The analyses overall indicated that 10,000 similar patients treated for 1 year with rivaroxaban would have 181 fewer primary events, compared with placebo-treated patients at the cost of also having 29 additional TIMI major bleeding events compared with patient on placebo.
Adding clopidogrel adds little except bleeding
Further analysis showed that just over half of enrolled patients also received clopidogrel for a median of 29 days following their LER procedure. This added agent produced no significant added benefit during 3-year follow-up, but did boost bleeding risk, especially in patients who received clopidogrel for more than 30 days. This led the study investigators to suggest that, while rivaroxaban plus aspirin is indicated for long-term treatment, addition of clopidogrel on top of this should be limited to 30 days or fewer to minimize bleeding risk.
“I’m sure there is a bleeding hazard associated with rivaroxaban plus aspirin, but this is attenuated by using dual therapy and not using triple therapy” by also adding clopidogrel, noted Dr. Parikh.
The new VOYAGER-PAD results also showed that the ongoing risk faced by patients with PAD following LER applies globally to their peripheral arteries. Of the 3,034 total peripheral revascularizations performed in the cohort during follow-up, 64% occurred in the index limb and 36% in the contralateral limb. Another striking finding was that the need for ipsilateral repeat revascularization was more common after an index endovascular procedure, 2,329 repeat revascularizations in 4,379 of these patients (53%), compared with 2,185 patients who had surgical revascularization for their index procedure and subsequently 705 of these patients (32%) needed repeat revascularization.
But rivaroxaban treatment appeared to provide little benefit for the much less frequent incidence of first and subsequent events in the coronary and cerebral circulation. During follow-up, the rates of major adverse cardiovascular events – cardiovascular death, nonfatal MI, and nonfatal stroke – were virtually identical in the rivaroxaban and placebo groups.
“This study makes it clear that we are learning about differences in presentation between the vascular beds, and the benefits of specific treatments in each vascular bed,” Dr. Beckman said.
VOYAGER-PAD was sponsored by Bayer and Janssen, the companies that market rivaroxaban (Xarelto). Dr. Bauersachs has received personal fees from Bayer, as well as from Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer, and has received grant support from Aspen Pharma. Dr. Beckman been a consultant to and received honoraria from Janssen, as well as from Amgen, JanOne, Novartis, and Sanofi, and he has served on a data and safety monitoring board for Bayer. Dr. Parikh has been a consultant to and received honoraria from Janssen, as well as from Abbott, Boston Scientific, Cordis, Medtronic, Penumbra, Philips, and Terumo, he has been a speaker on behalf of Inari, and he has received grant support from Abbott, Shockwave Medical, Surmodics, and TriReme Medical.
FROM ACC 2021
Early aspirin withdrawal after PCI: More benefit for women?
A new analysis from the TWILIGHT study has shown that, in the high-risk population undergoing percutaneous coronary intervention (PCI) enrolled in the study, the benefits of early aspirin withdrawal and continuation on ticagrelor monotherapy were similar in women and men.
But there were some interesting observations in the analysis suggesting possible additional benefits of this strategy for women.
“These data support the use of ticagrelor monotherapy in women and men, and importantly show that the absolute risk reduction of bleeding was higher in women, as their bleeding rates were higher,” senior author Roxana Mehran, MD, the Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“These data also support the need for prospective dual antiplatelet therapy deescalation studies in women,” Dr. Mehran added.
The main results of the TWILIGHT study showed that after a short period of dual antiplatelet therapy, a strategy of ticagrelor monotherapy, compared with continued dual therapy led to reduced bleeding without an increase in ischemic events among patients at high risk for bleeding or ischemic events after PCI.
The new gender-based analysis was presented by Birgit Vogel, MD, on May 15 at the annual scientific sessions of the American College of Cardiology. It was also published online in JAMA Cardiology to coincide with the ACC presentation.
Dr. Vogel, also from Wiener Cardiovascular Institute, explained that the current analysis was undertaken to investigate whether the TWILIGHT results varied in relation to sex, given that women are believed to have an increased risk for bleeding after PCI, compared with men.
“The current analysis showed that, while women did have a higher bleeding risk, compared to men, this was no longer significant after adjustment for baseline characteristics; and ischemic events were similar between the two sexes,” she reported.
“Results showed that withdrawing aspirin while continuing ticagrelor after 3 months of dual antiplatelet therapy was associated with a reduction in bleeding and preserved ischemic benefits in both women and men,” she added.
The TWILIGHT trial randomized 7,119 patients at high risk of ischemic or bleeding events who had undergone successful PCI with at least one drug-eluting stent and had completed 3 months of dual antiplatelet therapy to aspirin or placebo for an additional 12 months plus open-label ticagrelor.
The main results showed that the primary endpoint of Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding at 1 year was almost halved with ticagrelor monotherapy, occurring in 4% of these patients, compared with 7.1% of the ticagrelor/aspirin group (hazard ratio, 0.56). Ischemic events were similar in the two groups.
The current analysis focused on whether these effects varied in relation to sex.
Dr. Vogel noted that women made up 23.9% of the study population, were older than the men, and were more likely to have diabetes, chronic kidney disease, anemia and hypertension, while the men were more likely to be current smokers. Men had a higher incidence of coronary heart disease history, while women were more likely to have an ACS indication for PCI.
Unadjusted results showed a higher rate of BARC 2, 3, or 5 bleeding at 1 year in women (6.8%) versus men (5.2%), giving an HR of 1.32 (95% CI, 1.06-1.64).
But after adjustment for baseline characteristics, this became nonsignificant (HR, 1.20; 95% CI, 0.95-1.52).
Dr. Vogel pointed out that the most severe type of bleeding (BARC 3 and 5) was not attenuated as much by adjustment for baseline characteristics, with the HR reducing from 1.57 to 1.49.
The ischemic endpoint of death/stroke or MI was similar in men (4.0%) and women (3.5%), and this did not change after adjustment for baseline characteristics.
In terms of the two treatment groups, BARC 2, 3, or 5 bleeding was reduced to a similar extent with ticagrelor monotherapy in both men and women. This endpoint decreased from 8.6% in women on dual-antiplatelet therapy to 5.0% in women on ticagrelor alone (adjusted HR, 0.62) and from 6.6% to 3.7% in men (aHR, 0.57). But she noted that the absolute risk reduction in bleeding was greater in women (3.6%) versus men (2.9%).
“If we have a relative risk reduction in bleeding with early withdrawal of aspirin that is similar between the sexes but an overall higher risk of bleeding in women, that results in a greater absolute risk reduction,” Dr. Vogel commented.
The primary ischemic endpoint of death/MI/stroke was not increased in the ticagrelor group vs the dual antiplatelet group in either men (aHR, 1.06) or women (aHR, 1.04).
Greater reduction in mortality in women?
However, Dr. Vogel reported that there was a suggestion of a greater reduction in all-cause mortality with ticagrelor monotherapy in women versus men. “We found a significant interaction for treatment effect and sex for all-cause mortality, a prespecified endpoint, which was significantly lower in women treated with ticagrelor monotherapy, compared to dual antiplatelet therapy, but this was not the case in men.”
However, this observation was based on few events and should not be considered definitive, she added.
Dr. Vogel noted that the analysis had the limitations of the study not being powered to show differences in men versus women, and the results are only applicable to the population studied who were at high risk of bleeding post PCI.
Commenting on the study at the ACC session, Jacqueline Tamis-Holland, MD, associate professor of medicine at the Icahn School of Medicine at Mount Sinai, described the presentation as “very interesting.”
“We know that women notoriously have higher bleeding risk than men, but this particular study did not show a difference in bleeding risk after adjusting for other confounding variables,” she said.
“In fact, one would think that the relative benefit of a treatment designed to decrease bleeding would be more favorable to women, but this analysis didn’t show that,” she added.
Dr. Vogel replied that the HR of the most serious type of bleeding (BARC 3 and 5) in women versus men was only reduced minimally after adjustment for baseline characteristics, “which still makes us think that there are additional factors that might be important and contribute to an increased risk for bleeding and especially more serous types of bleeding in women.”
She pointed out that, while there was a similar risk reduction in bleeding in women and men, there was a potential mortality benefit in women. “The question is whether this mortality benefit is due to reduced bleeding that might be greater in women than men, and the reality is we don’t have a lot of data on that.”
Dr. Vogel added: “We know about the relationship between bleeding and mortality very well but the impact of sex on this is really not well investigated. It would be worth investigating this further to come up with bleeding reduction strategies for women because this is a really important issue.”
This work was supported by an investigator-initiated grant from AstraZeneca. Dr. Mehran reported grants and personal fees (paid to the institution) from Abbott, Abiomed, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb, Chiesi, Concept Medical Research, Medtronic, Novartis and DSI Research; grants from Applied Therapeutics, AstraZeneca, Cerecor, CSL Behring, OrbusNeich, and Zoll; personal fees from Boston Scientific, California Institute for Regenerative Medicine, Cine-Med Research, Janssen Scientific Affairs, ACC, and WebMD; personal fees paid to the institution from CardiaWave, Duke University, and Idorsia Pharmaceuticals; serving as a consultant or committee or advisory board member for Society for Cardiovascular Angiography and Interventions, the American Medical Association, and Regeneron Pharmaceuticals; and owning stock in ControlRad, Elixir Medical, and STEL outside the submitted work. Dr. Vogel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis from the TWILIGHT study has shown that, in the high-risk population undergoing percutaneous coronary intervention (PCI) enrolled in the study, the benefits of early aspirin withdrawal and continuation on ticagrelor monotherapy were similar in women and men.
But there were some interesting observations in the analysis suggesting possible additional benefits of this strategy for women.
“These data support the use of ticagrelor monotherapy in women and men, and importantly show that the absolute risk reduction of bleeding was higher in women, as their bleeding rates were higher,” senior author Roxana Mehran, MD, the Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“These data also support the need for prospective dual antiplatelet therapy deescalation studies in women,” Dr. Mehran added.
The main results of the TWILIGHT study showed that after a short period of dual antiplatelet therapy, a strategy of ticagrelor monotherapy, compared with continued dual therapy led to reduced bleeding without an increase in ischemic events among patients at high risk for bleeding or ischemic events after PCI.
The new gender-based analysis was presented by Birgit Vogel, MD, on May 15 at the annual scientific sessions of the American College of Cardiology. It was also published online in JAMA Cardiology to coincide with the ACC presentation.
Dr. Vogel, also from Wiener Cardiovascular Institute, explained that the current analysis was undertaken to investigate whether the TWILIGHT results varied in relation to sex, given that women are believed to have an increased risk for bleeding after PCI, compared with men.
“The current analysis showed that, while women did have a higher bleeding risk, compared to men, this was no longer significant after adjustment for baseline characteristics; and ischemic events were similar between the two sexes,” she reported.
“Results showed that withdrawing aspirin while continuing ticagrelor after 3 months of dual antiplatelet therapy was associated with a reduction in bleeding and preserved ischemic benefits in both women and men,” she added.
The TWILIGHT trial randomized 7,119 patients at high risk of ischemic or bleeding events who had undergone successful PCI with at least one drug-eluting stent and had completed 3 months of dual antiplatelet therapy to aspirin or placebo for an additional 12 months plus open-label ticagrelor.
The main results showed that the primary endpoint of Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding at 1 year was almost halved with ticagrelor monotherapy, occurring in 4% of these patients, compared with 7.1% of the ticagrelor/aspirin group (hazard ratio, 0.56). Ischemic events were similar in the two groups.
The current analysis focused on whether these effects varied in relation to sex.
Dr. Vogel noted that women made up 23.9% of the study population, were older than the men, and were more likely to have diabetes, chronic kidney disease, anemia and hypertension, while the men were more likely to be current smokers. Men had a higher incidence of coronary heart disease history, while women were more likely to have an ACS indication for PCI.
Unadjusted results showed a higher rate of BARC 2, 3, or 5 bleeding at 1 year in women (6.8%) versus men (5.2%), giving an HR of 1.32 (95% CI, 1.06-1.64).
But after adjustment for baseline characteristics, this became nonsignificant (HR, 1.20; 95% CI, 0.95-1.52).
Dr. Vogel pointed out that the most severe type of bleeding (BARC 3 and 5) was not attenuated as much by adjustment for baseline characteristics, with the HR reducing from 1.57 to 1.49.
The ischemic endpoint of death/stroke or MI was similar in men (4.0%) and women (3.5%), and this did not change after adjustment for baseline characteristics.
In terms of the two treatment groups, BARC 2, 3, or 5 bleeding was reduced to a similar extent with ticagrelor monotherapy in both men and women. This endpoint decreased from 8.6% in women on dual-antiplatelet therapy to 5.0% in women on ticagrelor alone (adjusted HR, 0.62) and from 6.6% to 3.7% in men (aHR, 0.57). But she noted that the absolute risk reduction in bleeding was greater in women (3.6%) versus men (2.9%).
“If we have a relative risk reduction in bleeding with early withdrawal of aspirin that is similar between the sexes but an overall higher risk of bleeding in women, that results in a greater absolute risk reduction,” Dr. Vogel commented.
The primary ischemic endpoint of death/MI/stroke was not increased in the ticagrelor group vs the dual antiplatelet group in either men (aHR, 1.06) or women (aHR, 1.04).
Greater reduction in mortality in women?
However, Dr. Vogel reported that there was a suggestion of a greater reduction in all-cause mortality with ticagrelor monotherapy in women versus men. “We found a significant interaction for treatment effect and sex for all-cause mortality, a prespecified endpoint, which was significantly lower in women treated with ticagrelor monotherapy, compared to dual antiplatelet therapy, but this was not the case in men.”
However, this observation was based on few events and should not be considered definitive, she added.
Dr. Vogel noted that the analysis had the limitations of the study not being powered to show differences in men versus women, and the results are only applicable to the population studied who were at high risk of bleeding post PCI.
Commenting on the study at the ACC session, Jacqueline Tamis-Holland, MD, associate professor of medicine at the Icahn School of Medicine at Mount Sinai, described the presentation as “very interesting.”
“We know that women notoriously have higher bleeding risk than men, but this particular study did not show a difference in bleeding risk after adjusting for other confounding variables,” she said.
“In fact, one would think that the relative benefit of a treatment designed to decrease bleeding would be more favorable to women, but this analysis didn’t show that,” she added.
Dr. Vogel replied that the HR of the most serious type of bleeding (BARC 3 and 5) in women versus men was only reduced minimally after adjustment for baseline characteristics, “which still makes us think that there are additional factors that might be important and contribute to an increased risk for bleeding and especially more serous types of bleeding in women.”
She pointed out that, while there was a similar risk reduction in bleeding in women and men, there was a potential mortality benefit in women. “The question is whether this mortality benefit is due to reduced bleeding that might be greater in women than men, and the reality is we don’t have a lot of data on that.”
Dr. Vogel added: “We know about the relationship between bleeding and mortality very well but the impact of sex on this is really not well investigated. It would be worth investigating this further to come up with bleeding reduction strategies for women because this is a really important issue.”
This work was supported by an investigator-initiated grant from AstraZeneca. Dr. Mehran reported grants and personal fees (paid to the institution) from Abbott, Abiomed, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb, Chiesi, Concept Medical Research, Medtronic, Novartis and DSI Research; grants from Applied Therapeutics, AstraZeneca, Cerecor, CSL Behring, OrbusNeich, and Zoll; personal fees from Boston Scientific, California Institute for Regenerative Medicine, Cine-Med Research, Janssen Scientific Affairs, ACC, and WebMD; personal fees paid to the institution from CardiaWave, Duke University, and Idorsia Pharmaceuticals; serving as a consultant or committee or advisory board member for Society for Cardiovascular Angiography and Interventions, the American Medical Association, and Regeneron Pharmaceuticals; and owning stock in ControlRad, Elixir Medical, and STEL outside the submitted work. Dr. Vogel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis from the TWILIGHT study has shown that, in the high-risk population undergoing percutaneous coronary intervention (PCI) enrolled in the study, the benefits of early aspirin withdrawal and continuation on ticagrelor monotherapy were similar in women and men.
But there were some interesting observations in the analysis suggesting possible additional benefits of this strategy for women.
“These data support the use of ticagrelor monotherapy in women and men, and importantly show that the absolute risk reduction of bleeding was higher in women, as their bleeding rates were higher,” senior author Roxana Mehran, MD, the Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, said in an interview.
“These data also support the need for prospective dual antiplatelet therapy deescalation studies in women,” Dr. Mehran added.
The main results of the TWILIGHT study showed that after a short period of dual antiplatelet therapy, a strategy of ticagrelor monotherapy, compared with continued dual therapy led to reduced bleeding without an increase in ischemic events among patients at high risk for bleeding or ischemic events after PCI.
The new gender-based analysis was presented by Birgit Vogel, MD, on May 15 at the annual scientific sessions of the American College of Cardiology. It was also published online in JAMA Cardiology to coincide with the ACC presentation.
Dr. Vogel, also from Wiener Cardiovascular Institute, explained that the current analysis was undertaken to investigate whether the TWILIGHT results varied in relation to sex, given that women are believed to have an increased risk for bleeding after PCI, compared with men.
“The current analysis showed that, while women did have a higher bleeding risk, compared to men, this was no longer significant after adjustment for baseline characteristics; and ischemic events were similar between the two sexes,” she reported.
“Results showed that withdrawing aspirin while continuing ticagrelor after 3 months of dual antiplatelet therapy was associated with a reduction in bleeding and preserved ischemic benefits in both women and men,” she added.
The TWILIGHT trial randomized 7,119 patients at high risk of ischemic or bleeding events who had undergone successful PCI with at least one drug-eluting stent and had completed 3 months of dual antiplatelet therapy to aspirin or placebo for an additional 12 months plus open-label ticagrelor.
The main results showed that the primary endpoint of Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding at 1 year was almost halved with ticagrelor monotherapy, occurring in 4% of these patients, compared with 7.1% of the ticagrelor/aspirin group (hazard ratio, 0.56). Ischemic events were similar in the two groups.
The current analysis focused on whether these effects varied in relation to sex.
Dr. Vogel noted that women made up 23.9% of the study population, were older than the men, and were more likely to have diabetes, chronic kidney disease, anemia and hypertension, while the men were more likely to be current smokers. Men had a higher incidence of coronary heart disease history, while women were more likely to have an ACS indication for PCI.
Unadjusted results showed a higher rate of BARC 2, 3, or 5 bleeding at 1 year in women (6.8%) versus men (5.2%), giving an HR of 1.32 (95% CI, 1.06-1.64).
But after adjustment for baseline characteristics, this became nonsignificant (HR, 1.20; 95% CI, 0.95-1.52).
Dr. Vogel pointed out that the most severe type of bleeding (BARC 3 and 5) was not attenuated as much by adjustment for baseline characteristics, with the HR reducing from 1.57 to 1.49.
The ischemic endpoint of death/stroke or MI was similar in men (4.0%) and women (3.5%), and this did not change after adjustment for baseline characteristics.
In terms of the two treatment groups, BARC 2, 3, or 5 bleeding was reduced to a similar extent with ticagrelor monotherapy in both men and women. This endpoint decreased from 8.6% in women on dual-antiplatelet therapy to 5.0% in women on ticagrelor alone (adjusted HR, 0.62) and from 6.6% to 3.7% in men (aHR, 0.57). But she noted that the absolute risk reduction in bleeding was greater in women (3.6%) versus men (2.9%).
“If we have a relative risk reduction in bleeding with early withdrawal of aspirin that is similar between the sexes but an overall higher risk of bleeding in women, that results in a greater absolute risk reduction,” Dr. Vogel commented.
The primary ischemic endpoint of death/MI/stroke was not increased in the ticagrelor group vs the dual antiplatelet group in either men (aHR, 1.06) or women (aHR, 1.04).
Greater reduction in mortality in women?
However, Dr. Vogel reported that there was a suggestion of a greater reduction in all-cause mortality with ticagrelor monotherapy in women versus men. “We found a significant interaction for treatment effect and sex for all-cause mortality, a prespecified endpoint, which was significantly lower in women treated with ticagrelor monotherapy, compared to dual antiplatelet therapy, but this was not the case in men.”
However, this observation was based on few events and should not be considered definitive, she added.
Dr. Vogel noted that the analysis had the limitations of the study not being powered to show differences in men versus women, and the results are only applicable to the population studied who were at high risk of bleeding post PCI.
Commenting on the study at the ACC session, Jacqueline Tamis-Holland, MD, associate professor of medicine at the Icahn School of Medicine at Mount Sinai, described the presentation as “very interesting.”
“We know that women notoriously have higher bleeding risk than men, but this particular study did not show a difference in bleeding risk after adjusting for other confounding variables,” she said.
“In fact, one would think that the relative benefit of a treatment designed to decrease bleeding would be more favorable to women, but this analysis didn’t show that,” she added.
Dr. Vogel replied that the HR of the most serious type of bleeding (BARC 3 and 5) in women versus men was only reduced minimally after adjustment for baseline characteristics, “which still makes us think that there are additional factors that might be important and contribute to an increased risk for bleeding and especially more serous types of bleeding in women.”
She pointed out that, while there was a similar risk reduction in bleeding in women and men, there was a potential mortality benefit in women. “The question is whether this mortality benefit is due to reduced bleeding that might be greater in women than men, and the reality is we don’t have a lot of data on that.”
Dr. Vogel added: “We know about the relationship between bleeding and mortality very well but the impact of sex on this is really not well investigated. It would be worth investigating this further to come up with bleeding reduction strategies for women because this is a really important issue.”
This work was supported by an investigator-initiated grant from AstraZeneca. Dr. Mehran reported grants and personal fees (paid to the institution) from Abbott, Abiomed, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb, Chiesi, Concept Medical Research, Medtronic, Novartis and DSI Research; grants from Applied Therapeutics, AstraZeneca, Cerecor, CSL Behring, OrbusNeich, and Zoll; personal fees from Boston Scientific, California Institute for Regenerative Medicine, Cine-Med Research, Janssen Scientific Affairs, ACC, and WebMD; personal fees paid to the institution from CardiaWave, Duke University, and Idorsia Pharmaceuticals; serving as a consultant or committee or advisory board member for Society for Cardiovascular Angiography and Interventions, the American Medical Association, and Regeneron Pharmaceuticals; and owning stock in ControlRad, Elixir Medical, and STEL outside the submitted work. Dr. Vogel disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves new treatment option for rare anemia
A rare, life-threatening anemia now has a new treatment option. The Food and Drug Administration announced the approval of pegcetacoplan (Empaveli) injection to treat adults with paroxysmal nocturnal hemoglobinuria (PNH). Pegcetacoplan is the first PNH treatment that binds to complement protein C3, according to the FDA announcement. Complement protein C3 is a key component of host immunity and defense.
Special concern
Because of the risk of severe side effects, the drug is available only through a restricted program under a risk evaluation and mitigation strategy (REMS). Serious infections can occur in patients taking pegcetacoplan that can become life-threatening or fatal if not treated early. According to the FDA, REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication, and only a few drugs require a REMS.
The most common other side effects are injection site reactions, diarrhea, abdominal pain, and fatigue.
Pegcetacoplan was approved based upon a study of 80 patients with PNH and anemia who had been taking eculizumab, a previously approved treatment. During 16 weeks of treatment, patients in the pegcetacoplan group had an average increase in their hemoglobin of 2.4 g/dL, while patients in the eculizumab group had an average decrease in their hemoglobin of 1.5 g/dL.
About the disease
PNH is caused by gene mutations that affect red blood cells, causing them to be defective and susceptible to destruction by a patient’s own immune system. Red blood cells in people with these mutations are defective and can be destroyed by the immune system, causing anemia.
Other symptoms include blood clots and destruction of bone marrow. The disease affects 1-1.5 people per million, with diagnosis typically occurring around ages 35-40, and a median survival of only 10 years after diagnosis, according to the FDA.
A rare, life-threatening anemia now has a new treatment option. The Food and Drug Administration announced the approval of pegcetacoplan (Empaveli) injection to treat adults with paroxysmal nocturnal hemoglobinuria (PNH). Pegcetacoplan is the first PNH treatment that binds to complement protein C3, according to the FDA announcement. Complement protein C3 is a key component of host immunity and defense.
Special concern
Because of the risk of severe side effects, the drug is available only through a restricted program under a risk evaluation and mitigation strategy (REMS). Serious infections can occur in patients taking pegcetacoplan that can become life-threatening or fatal if not treated early. According to the FDA, REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication, and only a few drugs require a REMS.
The most common other side effects are injection site reactions, diarrhea, abdominal pain, and fatigue.
Pegcetacoplan was approved based upon a study of 80 patients with PNH and anemia who had been taking eculizumab, a previously approved treatment. During 16 weeks of treatment, patients in the pegcetacoplan group had an average increase in their hemoglobin of 2.4 g/dL, while patients in the eculizumab group had an average decrease in their hemoglobin of 1.5 g/dL.
About the disease
PNH is caused by gene mutations that affect red blood cells, causing them to be defective and susceptible to destruction by a patient’s own immune system. Red blood cells in people with these mutations are defective and can be destroyed by the immune system, causing anemia.
Other symptoms include blood clots and destruction of bone marrow. The disease affects 1-1.5 people per million, with diagnosis typically occurring around ages 35-40, and a median survival of only 10 years after diagnosis, according to the FDA.
A rare, life-threatening anemia now has a new treatment option. The Food and Drug Administration announced the approval of pegcetacoplan (Empaveli) injection to treat adults with paroxysmal nocturnal hemoglobinuria (PNH). Pegcetacoplan is the first PNH treatment that binds to complement protein C3, according to the FDA announcement. Complement protein C3 is a key component of host immunity and defense.
Special concern
Because of the risk of severe side effects, the drug is available only through a restricted program under a risk evaluation and mitigation strategy (REMS). Serious infections can occur in patients taking pegcetacoplan that can become life-threatening or fatal if not treated early. According to the FDA, REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication, and only a few drugs require a REMS.
The most common other side effects are injection site reactions, diarrhea, abdominal pain, and fatigue.
Pegcetacoplan was approved based upon a study of 80 patients with PNH and anemia who had been taking eculizumab, a previously approved treatment. During 16 weeks of treatment, patients in the pegcetacoplan group had an average increase in their hemoglobin of 2.4 g/dL, while patients in the eculizumab group had an average decrease in their hemoglobin of 1.5 g/dL.
About the disease
PNH is caused by gene mutations that affect red blood cells, causing them to be defective and susceptible to destruction by a patient’s own immune system. Red blood cells in people with these mutations are defective and can be destroyed by the immune system, causing anemia.
Other symptoms include blood clots and destruction of bone marrow. The disease affects 1-1.5 people per million, with diagnosis typically occurring around ages 35-40, and a median survival of only 10 years after diagnosis, according to the FDA.
LAAOS III: Surgical LAA closure cuts AFib stroke risk by one third
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2021
ADAPTABLE: Low-dose aspirin as good as high-dose in CHD?
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM ACC 2021
Update in Hospital Medicine relays important findings
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
FROM SHM CONVERGE 2021
High teen BMI linked to stroke risk in young adulthood
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
High and even high-normal body mass index (BMI) were linked to increased ischemic stroke risk, regardless of whether or not individuals had diabetes.
Overweight and obese adolescent groups in the study had a roughly two- to threefold increased risk of ischemic stroke, which was apparent even before age 30 years in the study that was based on records of Israeli adolescents evaluated prior to mandatory military service.
These findings highlight the importance of treating and preventing high BMI among adolescence, study coauthor Gilad Twig, MD, MPH, PhD, said in a press release.
“Adults who survive stroke earlier in life face poor functional outcomes, which can lead to unemployment, depression and anxiety,” said Dr. Twig, associate professor in the department of military medicine in The Hebrew University in Jerusalem.
The costs of stroke prevention and care, already high, are expected to become even higher as the adolescent obesity prevalence goes up, fueling further increases in stroke rate, Dr. Twig added.
This is believed to be the first study showing that stroke risk is associated with higher BMI values in both men and women, not just men, Dr. Twig and coauthors said in their article, published May 13, 2021 in the journal Stroke. Previous studies assessing the stroke-BMI relationship in adolescents were based on records of Swedish men evaluated during military conscription at age 18.
In the present study, Dr. Twig and coauthors assessed the linkage between adolescent BMI and first stroke event in 1.9 million male and female adolescents in Israel who were evaluated 1 year prior to mandatory military service, between the years of 1985 and 2013.
They cross-referenced that information with stroke events in a national registry to which all hospitals in Israel are required to report.
The adolescents were about 17 years of age on average at the time of evaluation, 58% were male, and 84% were born in Israel. The mean age at the beginning of follow-up for stroke was about 31 years.
Over the follow-up period, investigators identified 1,088 first stroke events, including 921 ischemic and 167 hemorrhagic strokes.
A gradual increase in stroke rate was seen across BMI categories for ischemic strokes, but not so much for hemorrhagic strokes, investigators found.
Hazard ratios for first ischemic stroke event were 1.4 (95% confidence interval, 1.2-1.6) for the high-normal BMI group, 2.0 (95% CI, 1.6-2.4) for the overweight group, and 3.5 (95% CI, 2.8-4.5) for the obese group after adjusting for age and sex at beginning of follow-up, investigators reported.
When the adjusted results were stratified by presence or absence of diabetes, estimates were similar to what was seen in the overall risk model, they added.
Among those young adults who developed ischemic stroke, 43% smoked, 29% had high blood pressure, 17% had diabetes, and 32% had abnormal lipids at the time of diagnosis, the reported data showed.
The clinical and public health implications of these findings could be substantial, since strokes are associated with worse medical and socioeconomic outcomes in younger as compared with older individuals, according to Dr. Twig and coauthors.
Younger individuals with stroke have a higher risk of recurrent stroke, heart attack, long-term care, or death, they said. Moreover, about half of young-adult stroke survivors have poor functional outcomes, and their risk of unemployment and depression/anxiety is higher than in young individuals without stroke.
One limitation of the study is that follow-up BMI data were not available for all participants. As a result, the contribution of obesity to stroke risk over time could not be assessed, and the independent risk of BMI during adolescence could not be determined. In addition, the authors said the study underrepresents orthodox and ultraorthodox Jewish women, as they are not obligated to serve in the Israeli military.
The study authors had no disclosures related to the study, which was supported by a medical corps Israel Defense Forces research grant.
FROM STROKE
Trends in the management of pulmonary embolism
One of the newest trends in pulmonary embolism management is treatment of cancer associated venous thromboembolism (VTE) which encompasses deep vein thrombosis (DVT) and PE. Following the clinical management of cancer-associated venous thromboembolism in the hospital, direct oral anticoagulant therapy at discharge is your starting point, except in cases of intact luminal cancers, Scott Kaatz, DO, MSc, FACP, SFHM, said during SHM Converge, the annual conference of the Society of Hospital Medicine.
Dr. Kaatz, of the division of hospital medicine at Henry Ford Hospital, Detroit, based his remarks on emerging recommendations from leading medical societies on the topic, as well as a one-page algorithm from the Anticoagulation Forum that can be accessed at https://acforum-excellence.org/Resource-Center/resource_files/1638-2020-11-30-121425.pdf.
For the short-term treatment of VTE (3-6 months) for patients with active cancer, the American Society of Hematology guideline panel suggests direct oral anticoagulants, such as apixaban, edoxaban, or rivaroxaban, over low-molecular-weight heparin (LMWH) – a conditional recommendation based on low certainty in the evidence of effects.
Dr. Kaatz also discussed the latest recommendations regarding length of VTE treatment. After completion of primary treatment for patients with DVT and/or PE provoked by a chronic risk factor such as a surgery, pregnancy, or having a leg in a cast, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “On the other hand, patients with DVT and/or PE provoked by a transient factor typically do not require antithrombotic therapy after completion of primary treatment,” said Dr. Kaatz, who is also a clinical professor of medicine at Wayne State University, Detroit.
After completion of primary treatment for patients with unprovoked DVT and/or PE, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “The recommendation does not apply to patients who have a high risk for bleeding complications,” he noted.
Transient or reversible risk factors should be also considered in length of VTE treatment. For example, according to guidelines from the European Society of Cardiology, the estimated risk for long-term VTE recurrence is high (defined as greater than 8% per year) for patients with active cancer, for patients with one or more previous episodes of VTE in the absence of a major transient or reversible factor, and for those with antiphospholipid antibody syndrome.
Dr. Kaatz also highlighted recommendations for the acute treatment of intermediate risk, or submassive PE. The ESC guidelines state that if anticoagulation is initiated parenterally, LMWH or fondaparinux is recommended over unfractionated heparin (UFH) for most patients. “The reason for that is, one drug-use evaluation study found that, after 24 hours using UFH, only about 24% of patients had reached their therapeutic goal,” Dr. Kaatz said. Guidelines for intermediate risk patients from ASH recommend anticoagulation as your starting point, while thrombolysis is reasonable to consider for submassive PE and low risk for bleeding in selected younger patients or for patients at high risk for decompensation because of concomitant cardiopulmonary disease. “The bleeding rates get much higher in patients over age 65,” he said.
Another resource Dr. Kaatz mentioned is the Pulmonary Embolism Response Team (PERT) Consortium, which was developed after initial efforts of a multidisciplinary team of physicians at Massachusetts General Hospital. The first PERT sought to coordinate and expedite the treatment of pulmonary embolus with a team of physicians from a variety of specialties. In 2019 the PERT Consortium published guidelines on the diagnosis, treatment, and follow-up of acute PE. “It includes detailed algorithms that are a little different from the ASH and ESC guidelines,” Dr. Kaatz said.
Dr. Kaatz disclosed that he is a consultant for Janssen, Pfizer, Portola/Alexion, Bristol-Myers Squibb, Novartis, and CSL Behring. He has also received research funding from Janssen, Bristol-Myers Squibb, and Osmosis. He also holds board positions with the AC Forum and the National Blood Clot Alliance Medical and Scientific Advisory Board.
One of the newest trends in pulmonary embolism management is treatment of cancer associated venous thromboembolism (VTE) which encompasses deep vein thrombosis (DVT) and PE. Following the clinical management of cancer-associated venous thromboembolism in the hospital, direct oral anticoagulant therapy at discharge is your starting point, except in cases of intact luminal cancers, Scott Kaatz, DO, MSc, FACP, SFHM, said during SHM Converge, the annual conference of the Society of Hospital Medicine.
Dr. Kaatz, of the division of hospital medicine at Henry Ford Hospital, Detroit, based his remarks on emerging recommendations from leading medical societies on the topic, as well as a one-page algorithm from the Anticoagulation Forum that can be accessed at https://acforum-excellence.org/Resource-Center/resource_files/1638-2020-11-30-121425.pdf.
For the short-term treatment of VTE (3-6 months) for patients with active cancer, the American Society of Hematology guideline panel suggests direct oral anticoagulants, such as apixaban, edoxaban, or rivaroxaban, over low-molecular-weight heparin (LMWH) – a conditional recommendation based on low certainty in the evidence of effects.
Dr. Kaatz also discussed the latest recommendations regarding length of VTE treatment. After completion of primary treatment for patients with DVT and/or PE provoked by a chronic risk factor such as a surgery, pregnancy, or having a leg in a cast, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “On the other hand, patients with DVT and/or PE provoked by a transient factor typically do not require antithrombotic therapy after completion of primary treatment,” said Dr. Kaatz, who is also a clinical professor of medicine at Wayne State University, Detroit.
After completion of primary treatment for patients with unprovoked DVT and/or PE, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “The recommendation does not apply to patients who have a high risk for bleeding complications,” he noted.
Transient or reversible risk factors should be also considered in length of VTE treatment. For example, according to guidelines from the European Society of Cardiology, the estimated risk for long-term VTE recurrence is high (defined as greater than 8% per year) for patients with active cancer, for patients with one or more previous episodes of VTE in the absence of a major transient or reversible factor, and for those with antiphospholipid antibody syndrome.
Dr. Kaatz also highlighted recommendations for the acute treatment of intermediate risk, or submassive PE. The ESC guidelines state that if anticoagulation is initiated parenterally, LMWH or fondaparinux is recommended over unfractionated heparin (UFH) for most patients. “The reason for that is, one drug-use evaluation study found that, after 24 hours using UFH, only about 24% of patients had reached their therapeutic goal,” Dr. Kaatz said. Guidelines for intermediate risk patients from ASH recommend anticoagulation as your starting point, while thrombolysis is reasonable to consider for submassive PE and low risk for bleeding in selected younger patients or for patients at high risk for decompensation because of concomitant cardiopulmonary disease. “The bleeding rates get much higher in patients over age 65,” he said.
Another resource Dr. Kaatz mentioned is the Pulmonary Embolism Response Team (PERT) Consortium, which was developed after initial efforts of a multidisciplinary team of physicians at Massachusetts General Hospital. The first PERT sought to coordinate and expedite the treatment of pulmonary embolus with a team of physicians from a variety of specialties. In 2019 the PERT Consortium published guidelines on the diagnosis, treatment, and follow-up of acute PE. “It includes detailed algorithms that are a little different from the ASH and ESC guidelines,” Dr. Kaatz said.
Dr. Kaatz disclosed that he is a consultant for Janssen, Pfizer, Portola/Alexion, Bristol-Myers Squibb, Novartis, and CSL Behring. He has also received research funding from Janssen, Bristol-Myers Squibb, and Osmosis. He also holds board positions with the AC Forum and the National Blood Clot Alliance Medical and Scientific Advisory Board.
One of the newest trends in pulmonary embolism management is treatment of cancer associated venous thromboembolism (VTE) which encompasses deep vein thrombosis (DVT) and PE. Following the clinical management of cancer-associated venous thromboembolism in the hospital, direct oral anticoagulant therapy at discharge is your starting point, except in cases of intact luminal cancers, Scott Kaatz, DO, MSc, FACP, SFHM, said during SHM Converge, the annual conference of the Society of Hospital Medicine.
Dr. Kaatz, of the division of hospital medicine at Henry Ford Hospital, Detroit, based his remarks on emerging recommendations from leading medical societies on the topic, as well as a one-page algorithm from the Anticoagulation Forum that can be accessed at https://acforum-excellence.org/Resource-Center/resource_files/1638-2020-11-30-121425.pdf.
For the short-term treatment of VTE (3-6 months) for patients with active cancer, the American Society of Hematology guideline panel suggests direct oral anticoagulants, such as apixaban, edoxaban, or rivaroxaban, over low-molecular-weight heparin (LMWH) – a conditional recommendation based on low certainty in the evidence of effects.
Dr. Kaatz also discussed the latest recommendations regarding length of VTE treatment. After completion of primary treatment for patients with DVT and/or PE provoked by a chronic risk factor such as a surgery, pregnancy, or having a leg in a cast, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “On the other hand, patients with DVT and/or PE provoked by a transient factor typically do not require antithrombotic therapy after completion of primary treatment,” said Dr. Kaatz, who is also a clinical professor of medicine at Wayne State University, Detroit.
After completion of primary treatment for patients with unprovoked DVT and/or PE, the ASH guideline panel suggests indefinite antithrombotic therapy over stopping anticoagulation. “The recommendation does not apply to patients who have a high risk for bleeding complications,” he noted.
Transient or reversible risk factors should be also considered in length of VTE treatment. For example, according to guidelines from the European Society of Cardiology, the estimated risk for long-term VTE recurrence is high (defined as greater than 8% per year) for patients with active cancer, for patients with one or more previous episodes of VTE in the absence of a major transient or reversible factor, and for those with antiphospholipid antibody syndrome.
Dr. Kaatz also highlighted recommendations for the acute treatment of intermediate risk, or submassive PE. The ESC guidelines state that if anticoagulation is initiated parenterally, LMWH or fondaparinux is recommended over unfractionated heparin (UFH) for most patients. “The reason for that is, one drug-use evaluation study found that, after 24 hours using UFH, only about 24% of patients had reached their therapeutic goal,” Dr. Kaatz said. Guidelines for intermediate risk patients from ASH recommend anticoagulation as your starting point, while thrombolysis is reasonable to consider for submassive PE and low risk for bleeding in selected younger patients or for patients at high risk for decompensation because of concomitant cardiopulmonary disease. “The bleeding rates get much higher in patients over age 65,” he said.
Another resource Dr. Kaatz mentioned is the Pulmonary Embolism Response Team (PERT) Consortium, which was developed after initial efforts of a multidisciplinary team of physicians at Massachusetts General Hospital. The first PERT sought to coordinate and expedite the treatment of pulmonary embolus with a team of physicians from a variety of specialties. In 2019 the PERT Consortium published guidelines on the diagnosis, treatment, and follow-up of acute PE. “It includes detailed algorithms that are a little different from the ASH and ESC guidelines,” Dr. Kaatz said.
Dr. Kaatz disclosed that he is a consultant for Janssen, Pfizer, Portola/Alexion, Bristol-Myers Squibb, Novartis, and CSL Behring. He has also received research funding from Janssen, Bristol-Myers Squibb, and Osmosis. He also holds board positions with the AC Forum and the National Blood Clot Alliance Medical and Scientific Advisory Board.
FROM SHM CONVERGE 2021
Ruling out PE in patients with low C-PTP and D dimer of less than 1,000 ng/mL
Background: A pulmonary embolism can be considered ruled out if patients have a low C-PTP for PE and a D-dimer level of less than 500 ng/mL. However, this occurs in approximately 30% of outpatients only. By increasing the D-dimer threshold used to define a negative test to 1,000 ng/mL in patients with a low C-PTP, we might be able to rule out a larger segment of patients and avoid chest imaging.
Study design: Prospective study.
Setting: University-based clinical centers in Canada.
Synopsis: This study enrolled 2,017 patients presenting with symptoms of PE. The Wells’ criteria was used to categorize the patient’s C-PTP as low (0-4.0), moderate (4.5-6.0), or high (6.5 or more). Patients with a low or moderate C-PTP had a D dimer drawn. Those with a low C-PTP and D dimer of less than 1,000 ng/mL or moderate C-PTP and a D dimer of less than 500 ng/mL underwent no further testing. Outcomes were assessed at 90 days. Of the 1,325 patients with a low C-PTP or moderate C-PTP and a negative D-dimer test (less than 1,000 or 500 ng/mL, respectively), none had venous thromboembolism during follow-up (95% confidence interval, 0.00-0.29). This strategy resulted in the use of chest imaging in only 34.3% of patients versus 51.9% using the prior criteria of a D-dimer level of less than 500 ng/mL (difference, –17.6 percentage points; 95% CI, −19.2 to −15.9). One limitation of the study is that almost all patients enrolled were outpatients (only one inpatient).
Bottom line: A combination of a low C-PTP and a D-dimer level of less than 1,000 ng/mL identified a group of patients at low risk for pulmonary embolism during follow-up.
Citation: Kearon C et al. Diagnosis of pulmonary embolism with D-dimer adjusted to clinical probability. N Engl J Med 2019 Nov 28;381:2125-34.
Dr. Santa is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: A pulmonary embolism can be considered ruled out if patients have a low C-PTP for PE and a D-dimer level of less than 500 ng/mL. However, this occurs in approximately 30% of outpatients only. By increasing the D-dimer threshold used to define a negative test to 1,000 ng/mL in patients with a low C-PTP, we might be able to rule out a larger segment of patients and avoid chest imaging.
Study design: Prospective study.
Setting: University-based clinical centers in Canada.
Synopsis: This study enrolled 2,017 patients presenting with symptoms of PE. The Wells’ criteria was used to categorize the patient’s C-PTP as low (0-4.0), moderate (4.5-6.0), or high (6.5 or more). Patients with a low or moderate C-PTP had a D dimer drawn. Those with a low C-PTP and D dimer of less than 1,000 ng/mL or moderate C-PTP and a D dimer of less than 500 ng/mL underwent no further testing. Outcomes were assessed at 90 days. Of the 1,325 patients with a low C-PTP or moderate C-PTP and a negative D-dimer test (less than 1,000 or 500 ng/mL, respectively), none had venous thromboembolism during follow-up (95% confidence interval, 0.00-0.29). This strategy resulted in the use of chest imaging in only 34.3% of patients versus 51.9% using the prior criteria of a D-dimer level of less than 500 ng/mL (difference, –17.6 percentage points; 95% CI, −19.2 to −15.9). One limitation of the study is that almost all patients enrolled were outpatients (only one inpatient).
Bottom line: A combination of a low C-PTP and a D-dimer level of less than 1,000 ng/mL identified a group of patients at low risk for pulmonary embolism during follow-up.
Citation: Kearon C et al. Diagnosis of pulmonary embolism with D-dimer adjusted to clinical probability. N Engl J Med 2019 Nov 28;381:2125-34.
Dr. Santa is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.
Background: A pulmonary embolism can be considered ruled out if patients have a low C-PTP for PE and a D-dimer level of less than 500 ng/mL. However, this occurs in approximately 30% of outpatients only. By increasing the D-dimer threshold used to define a negative test to 1,000 ng/mL in patients with a low C-PTP, we might be able to rule out a larger segment of patients and avoid chest imaging.
Study design: Prospective study.
Setting: University-based clinical centers in Canada.
Synopsis: This study enrolled 2,017 patients presenting with symptoms of PE. The Wells’ criteria was used to categorize the patient’s C-PTP as low (0-4.0), moderate (4.5-6.0), or high (6.5 or more). Patients with a low or moderate C-PTP had a D dimer drawn. Those with a low C-PTP and D dimer of less than 1,000 ng/mL or moderate C-PTP and a D dimer of less than 500 ng/mL underwent no further testing. Outcomes were assessed at 90 days. Of the 1,325 patients with a low C-PTP or moderate C-PTP and a negative D-dimer test (less than 1,000 or 500 ng/mL, respectively), none had venous thromboembolism during follow-up (95% confidence interval, 0.00-0.29). This strategy resulted in the use of chest imaging in only 34.3% of patients versus 51.9% using the prior criteria of a D-dimer level of less than 500 ng/mL (difference, –17.6 percentage points; 95% CI, −19.2 to −15.9). One limitation of the study is that almost all patients enrolled were outpatients (only one inpatient).
Bottom line: A combination of a low C-PTP and a D-dimer level of less than 1,000 ng/mL identified a group of patients at low risk for pulmonary embolism during follow-up.
Citation: Kearon C et al. Diagnosis of pulmonary embolism with D-dimer adjusted to clinical probability. N Engl J Med 2019 Nov 28;381:2125-34.
Dr. Santa is assistant professor in the division of hospital medicine, Loyola University Medical Center, Maywood, Ill.