User login
Lusutrombopag found safe, effective for severe thrombocytopenia in patients with hepatocellular carcinoma
For patients with severe thrombocytopenia and chronic liver diseases, including hepatocellular carcinoma, treatment with lusutrombopag prior to invasive procedures significantly decreased the need for platelet transfusions without increasing the need for rescue treatment for bleeding or the rate of thromboembolic events.
In a post hoc analysis of data from 270 patients in two manufacturer-sponsored, multicenter, randomized, double-blind, placebo-controlled, phase 3 trials, significantly more lusutrombopag recipients met the primary efficacy endpoint, including patients with hepatocellular carcinoma (68.0% vs. 8.9% in the placebo group; P < .0001) and those without it (77.0% vs. 21.6%; P < .0001). Rates of treatment-emergent adverse events were similar between the lusutrombopag and placebo groups, and patients with hepatocellular carcinoma were not at increased risk for thrombosis, Naim Alkhouri, MD, of Texas Liver Institute in San Antonio, and associates wrote in Clinical Gastroenterology and Hepatology.
Platelet transfusion is the treatment mainstay for patients with thrombocytopenia related to cirrhosis who are undergoing invasive procedures, but its effects are short-lived, and at least one in five transfusions fails. Thrombopoietin agonists such as lusutrombopag are efficacious and approved in this setting, but they can be prothrombotic, particularly in patients with hepatocellular carcinoma, who already are at heightened risk for portal vein thrombosis.
Dr. Alkhouri and associates performed an integrated analysis of the PLUS 1 trial (Japan, October 2013–May 2014) and the L-PLUS 2 (global, June 2015–April 2017). Participants were adults with Child-Pugh Class A or B chronic liver disease and baseline platelet counts under 50 x 109 per L who were scheduled for invasive procedures. Of the 270 patients, 95 had hepatocellular carcinoma. Patients were randomly assigned on a one-to-one basis to receive either lusutrombopag (3 mg) or placebo daily for up to 7 days before procedures. The primary endpoint was the percentage of patients in the per-protocol population who did not need a platelet transfusion before the invasive procedure or rescue therapy within 7 days afterward.
The treatment and placebo arms were similar except that patients with hepatocellular carcinoma were about 10 years older on average. In patients with hepatocellular carcinoma, 60.5% more lusutrombopag recipients than placebo recipients met the primary endpoint, and rates of bleeding-related adverse events were 9.1% and 15.7%, respectively. In patients with other chronic liver diseases, 52.6% more lusutrombopag recipients met the primary endpoint. Rates of bleeding-related adverse events were 5% and 10.6%.
“Approximately 88% of patients with hepatocellular carcinoma underwent a liver-related procedure, compared with approximately 10% of patients without hepatocellular carcinoma,” the investigators wrote. “This is significant because ablations or transcatheter arterial chemoembolizations can be associated with serious bleeding complications. It is clinically important that, given the greater number of liver-related procedures, the incidence of bleeding-related adverse events was lower in patients treated with lusutrombopag than placebo.”
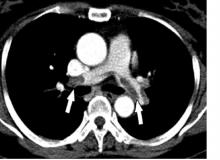
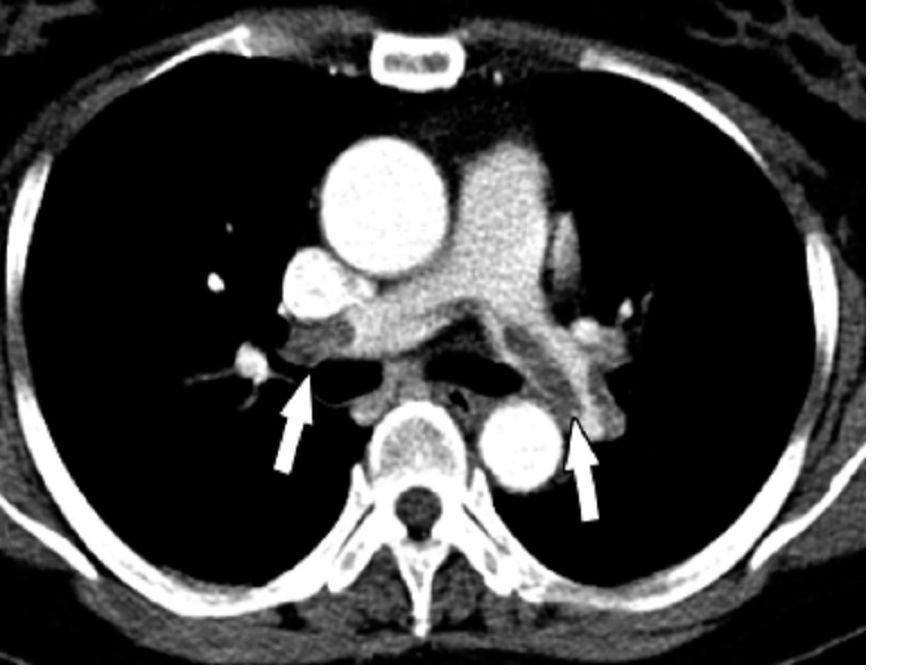
Imaging after the procedures confirmed low rates of thromboses in both groups and subgroups. Four patients developed portal vein thromboses, including two lusutrombopag recipients (one of whom had hepatocellular carcinoma) and two placebo recipients without hepatocellular carcinoma.
These trials excluded patients undergoing major surgical procedures and those with decompensated cirrhosis; portal vein thrombosis; hematopoietic tumors; aplastic anemia; myelodysplastic syndrome; myelofibrosis; liver transplantation; splenectomy; and thrombocytopenia that was congenital, autoimmune, or drug induced. “A limitation of this study was the high rate of protocol violations related to platelet transfusions,” the researchers noted. “A number of patients [42 in all] were excluded from the per-protocol population owing to receipt of unnecessary platelet transfusions, or because they did not receive a needed platelet transfusion.”Shionogi makes lusutrombopag and sponsored the study. Dr. Alkhouri reported an advisory relationship with Shionogi and Dova Pharma. Two coinvestigators reported being employed by Shionogi. Three coinvestigators also disclosed ties to Shionogi and to several other pharmaceutical companies.
SOURCE: Alkhouri N et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.032.
Thrombocytopenia is of clinical concern in patients with cirrhosis, as it complicates routine patient care and results in delayed or canceled procedures due to concern for risk of bleeding. In the last few years, availability of thrombopoietin (TPO) receptor agonists have facilitated the performance of elective invasive procedures in cirrhotic patients with severe thrombocytopenia.
In this integrated analysis of data from two phase 3 studies, Alkhouri et al. demonstrated the efficacy of a novel TPO receptor agonist, lusutrombopag, in reducing bleeding events and need for platelet transfusion in cirrhotic patients undergoing invasive procedures. The risk for thrombosis-related adverse events was not increased in lusutrombopag recipients with or without HCC. Previous studies with another TPO, eltrombopag, resulted in high rate of symptomatic portal vein thrombosis. Avatrombopag, a recently approved TPO receptor agonist reported few thrombotic symptomatic events but no prospective imaging for evaluation of thrombotic events was included in the protocol. A unique strength of this study was inclusion of prospective imaging for evaluation of portal vein thrombosis. Strategic scheduling is required with use of TPO agonists. Lusutrombopag can be given orally in convenient daily doses and provides a 7-10-day procedural window for scheduling and performing elective invasive procedures. However, because of several days of lag period for platelet production, these agents cannot be used for emergent cases.
Gagan K. Sood, MD, AGAF, FAASLD, is an associate professor of medicine and surgery, division of gastroenterology and hepatology and division of abdominal transplantation, Baylor College of Medicine, Houston. He has no conflicts of interest.
Thrombocytopenia is of clinical concern in patients with cirrhosis, as it complicates routine patient care and results in delayed or canceled procedures due to concern for risk of bleeding. In the last few years, availability of thrombopoietin (TPO) receptor agonists have facilitated the performance of elective invasive procedures in cirrhotic patients with severe thrombocytopenia.
In this integrated analysis of data from two phase 3 studies, Alkhouri et al. demonstrated the efficacy of a novel TPO receptor agonist, lusutrombopag, in reducing bleeding events and need for platelet transfusion in cirrhotic patients undergoing invasive procedures. The risk for thrombosis-related adverse events was not increased in lusutrombopag recipients with or without HCC. Previous studies with another TPO, eltrombopag, resulted in high rate of symptomatic portal vein thrombosis. Avatrombopag, a recently approved TPO receptor agonist reported few thrombotic symptomatic events but no prospective imaging for evaluation of thrombotic events was included in the protocol. A unique strength of this study was inclusion of prospective imaging for evaluation of portal vein thrombosis. Strategic scheduling is required with use of TPO agonists. Lusutrombopag can be given orally in convenient daily doses and provides a 7-10-day procedural window for scheduling and performing elective invasive procedures. However, because of several days of lag period for platelet production, these agents cannot be used for emergent cases.
Gagan K. Sood, MD, AGAF, FAASLD, is an associate professor of medicine and surgery, division of gastroenterology and hepatology and division of abdominal transplantation, Baylor College of Medicine, Houston. He has no conflicts of interest.
Thrombocytopenia is of clinical concern in patients with cirrhosis, as it complicates routine patient care and results in delayed or canceled procedures due to concern for risk of bleeding. In the last few years, availability of thrombopoietin (TPO) receptor agonists have facilitated the performance of elective invasive procedures in cirrhotic patients with severe thrombocytopenia.
In this integrated analysis of data from two phase 3 studies, Alkhouri et al. demonstrated the efficacy of a novel TPO receptor agonist, lusutrombopag, in reducing bleeding events and need for platelet transfusion in cirrhotic patients undergoing invasive procedures. The risk for thrombosis-related adverse events was not increased in lusutrombopag recipients with or without HCC. Previous studies with another TPO, eltrombopag, resulted in high rate of symptomatic portal vein thrombosis. Avatrombopag, a recently approved TPO receptor agonist reported few thrombotic symptomatic events but no prospective imaging for evaluation of thrombotic events was included in the protocol. A unique strength of this study was inclusion of prospective imaging for evaluation of portal vein thrombosis. Strategic scheduling is required with use of TPO agonists. Lusutrombopag can be given orally in convenient daily doses and provides a 7-10-day procedural window for scheduling and performing elective invasive procedures. However, because of several days of lag period for platelet production, these agents cannot be used for emergent cases.
Gagan K. Sood, MD, AGAF, FAASLD, is an associate professor of medicine and surgery, division of gastroenterology and hepatology and division of abdominal transplantation, Baylor College of Medicine, Houston. He has no conflicts of interest.
For patients with severe thrombocytopenia and chronic liver diseases, including hepatocellular carcinoma, treatment with lusutrombopag prior to invasive procedures significantly decreased the need for platelet transfusions without increasing the need for rescue treatment for bleeding or the rate of thromboembolic events.
In a post hoc analysis of data from 270 patients in two manufacturer-sponsored, multicenter, randomized, double-blind, placebo-controlled, phase 3 trials, significantly more lusutrombopag recipients met the primary efficacy endpoint, including patients with hepatocellular carcinoma (68.0% vs. 8.9% in the placebo group; P < .0001) and those without it (77.0% vs. 21.6%; P < .0001). Rates of treatment-emergent adverse events were similar between the lusutrombopag and placebo groups, and patients with hepatocellular carcinoma were not at increased risk for thrombosis, Naim Alkhouri, MD, of Texas Liver Institute in San Antonio, and associates wrote in Clinical Gastroenterology and Hepatology.
Platelet transfusion is the treatment mainstay for patients with thrombocytopenia related to cirrhosis who are undergoing invasive procedures, but its effects are short-lived, and at least one in five transfusions fails. Thrombopoietin agonists such as lusutrombopag are efficacious and approved in this setting, but they can be prothrombotic, particularly in patients with hepatocellular carcinoma, who already are at heightened risk for portal vein thrombosis.
Dr. Alkhouri and associates performed an integrated analysis of the PLUS 1 trial (Japan, October 2013–May 2014) and the L-PLUS 2 (global, June 2015–April 2017). Participants were adults with Child-Pugh Class A or B chronic liver disease and baseline platelet counts under 50 x 109 per L who were scheduled for invasive procedures. Of the 270 patients, 95 had hepatocellular carcinoma. Patients were randomly assigned on a one-to-one basis to receive either lusutrombopag (3 mg) or placebo daily for up to 7 days before procedures. The primary endpoint was the percentage of patients in the per-protocol population who did not need a platelet transfusion before the invasive procedure or rescue therapy within 7 days afterward.
The treatment and placebo arms were similar except that patients with hepatocellular carcinoma were about 10 years older on average. In patients with hepatocellular carcinoma, 60.5% more lusutrombopag recipients than placebo recipients met the primary endpoint, and rates of bleeding-related adverse events were 9.1% and 15.7%, respectively. In patients with other chronic liver diseases, 52.6% more lusutrombopag recipients met the primary endpoint. Rates of bleeding-related adverse events were 5% and 10.6%.
“Approximately 88% of patients with hepatocellular carcinoma underwent a liver-related procedure, compared with approximately 10% of patients without hepatocellular carcinoma,” the investigators wrote. “This is significant because ablations or transcatheter arterial chemoembolizations can be associated with serious bleeding complications. It is clinically important that, given the greater number of liver-related procedures, the incidence of bleeding-related adverse events was lower in patients treated with lusutrombopag than placebo.”
Imaging after the procedures confirmed low rates of thromboses in both groups and subgroups. Four patients developed portal vein thromboses, including two lusutrombopag recipients (one of whom had hepatocellular carcinoma) and two placebo recipients without hepatocellular carcinoma.
These trials excluded patients undergoing major surgical procedures and those with decompensated cirrhosis; portal vein thrombosis; hematopoietic tumors; aplastic anemia; myelodysplastic syndrome; myelofibrosis; liver transplantation; splenectomy; and thrombocytopenia that was congenital, autoimmune, or drug induced. “A limitation of this study was the high rate of protocol violations related to platelet transfusions,” the researchers noted. “A number of patients [42 in all] were excluded from the per-protocol population owing to receipt of unnecessary platelet transfusions, or because they did not receive a needed platelet transfusion.”Shionogi makes lusutrombopag and sponsored the study. Dr. Alkhouri reported an advisory relationship with Shionogi and Dova Pharma. Two coinvestigators reported being employed by Shionogi. Three coinvestigators also disclosed ties to Shionogi and to several other pharmaceutical companies.
SOURCE: Alkhouri N et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.032.
For patients with severe thrombocytopenia and chronic liver diseases, including hepatocellular carcinoma, treatment with lusutrombopag prior to invasive procedures significantly decreased the need for platelet transfusions without increasing the need for rescue treatment for bleeding or the rate of thromboembolic events.
In a post hoc analysis of data from 270 patients in two manufacturer-sponsored, multicenter, randomized, double-blind, placebo-controlled, phase 3 trials, significantly more lusutrombopag recipients met the primary efficacy endpoint, including patients with hepatocellular carcinoma (68.0% vs. 8.9% in the placebo group; P < .0001) and those without it (77.0% vs. 21.6%; P < .0001). Rates of treatment-emergent adverse events were similar between the lusutrombopag and placebo groups, and patients with hepatocellular carcinoma were not at increased risk for thrombosis, Naim Alkhouri, MD, of Texas Liver Institute in San Antonio, and associates wrote in Clinical Gastroenterology and Hepatology.
Platelet transfusion is the treatment mainstay for patients with thrombocytopenia related to cirrhosis who are undergoing invasive procedures, but its effects are short-lived, and at least one in five transfusions fails. Thrombopoietin agonists such as lusutrombopag are efficacious and approved in this setting, but they can be prothrombotic, particularly in patients with hepatocellular carcinoma, who already are at heightened risk for portal vein thrombosis.
Dr. Alkhouri and associates performed an integrated analysis of the PLUS 1 trial (Japan, October 2013–May 2014) and the L-PLUS 2 (global, June 2015–April 2017). Participants were adults with Child-Pugh Class A or B chronic liver disease and baseline platelet counts under 50 x 109 per L who were scheduled for invasive procedures. Of the 270 patients, 95 had hepatocellular carcinoma. Patients were randomly assigned on a one-to-one basis to receive either lusutrombopag (3 mg) or placebo daily for up to 7 days before procedures. The primary endpoint was the percentage of patients in the per-protocol population who did not need a platelet transfusion before the invasive procedure or rescue therapy within 7 days afterward.
The treatment and placebo arms were similar except that patients with hepatocellular carcinoma were about 10 years older on average. In patients with hepatocellular carcinoma, 60.5% more lusutrombopag recipients than placebo recipients met the primary endpoint, and rates of bleeding-related adverse events were 9.1% and 15.7%, respectively. In patients with other chronic liver diseases, 52.6% more lusutrombopag recipients met the primary endpoint. Rates of bleeding-related adverse events were 5% and 10.6%.
“Approximately 88% of patients with hepatocellular carcinoma underwent a liver-related procedure, compared with approximately 10% of patients without hepatocellular carcinoma,” the investigators wrote. “This is significant because ablations or transcatheter arterial chemoembolizations can be associated with serious bleeding complications. It is clinically important that, given the greater number of liver-related procedures, the incidence of bleeding-related adverse events was lower in patients treated with lusutrombopag than placebo.”
Imaging after the procedures confirmed low rates of thromboses in both groups and subgroups. Four patients developed portal vein thromboses, including two lusutrombopag recipients (one of whom had hepatocellular carcinoma) and two placebo recipients without hepatocellular carcinoma.
These trials excluded patients undergoing major surgical procedures and those with decompensated cirrhosis; portal vein thrombosis; hematopoietic tumors; aplastic anemia; myelodysplastic syndrome; myelofibrosis; liver transplantation; splenectomy; and thrombocytopenia that was congenital, autoimmune, or drug induced. “A limitation of this study was the high rate of protocol violations related to platelet transfusions,” the researchers noted. “A number of patients [42 in all] were excluded from the per-protocol population owing to receipt of unnecessary platelet transfusions, or because they did not receive a needed platelet transfusion.”Shionogi makes lusutrombopag and sponsored the study. Dr. Alkhouri reported an advisory relationship with Shionogi and Dova Pharma. Two coinvestigators reported being employed by Shionogi. Three coinvestigators also disclosed ties to Shionogi and to several other pharmaceutical companies.
SOURCE: Alkhouri N et al. Clin Gastroenterol Hepatol. 2020 Mar 20. doi: 10.1016/j.cgh.2020.03.032.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
IMPACT-AFib: Single mailing fails to budge oral anticoagulant uptake for AFib
A single educational mailing sent by several U.S. health plans to their patients with atrial fibrillation who were candidates for oral anticoagulation, but had not yet started a regimen, failed to boost them over their prescription hurdle and facilitate starting an antithrombotic regimen.
By 1 year following the intervention, a mere 10% of patients in both the intervention and a control arm of the randomized trial had begun treatment, with no signal of incremental uptake because of the mailing, Sean D. Pokorney, MD, said at the virtual annual congress of the European Society of Cardiology. Included in the mailing was an educational letter citing the patient’s atrial fibrillation (AFib) diagnosis, a statement regarding their suitability for oral anticoagulation, some information about the treatment, and a suggestion that recipients discuss this with their personal physician.
Dr. Pokorney acknowledged that the single mailing to patients may not have been adequate to capture patients’ attention and trigger an action, and that repeated messaging via multiple platforms and in coordination with interventions aimed at their health care providers may be what’s needed.
“It will take repeated interventions and engagements. We will need different methods to move the needle,” said Dr. Pokorney, a cardiac electrophysiologist at Duke University in Durham, N.C. The goal is to “empower patients to talk with their health care providers, and to become agents of change” in their care, he explained, but the single, mailed prod wasn’t enough.
An earlier study run by Dr. Pokorney and several of his colleagues used a broader panel of interventions aimed at both patients and clinicians to encourage increased prescribing of oral anticoagulants in five middle income countries, and documented successfully increasing the uptake rate by threefold compared with control patients (Lancet. 2017 Oct 14;390[10104]:1737-46). The current study tested the efficacy of a “much lower-impact intervention,” he admitted.
“The data are “sobering and eye-opening,” said Kalyanam Shivkumar, MD, a cardiac electrophysiologist and professor of medicine at the University of California, Los Angeles. “We’re stuck with this big challenge,” the gap between “what medicine can do and what it actually does” when evidence-based interventions fail to gain traction in everyday practice, he said in an interview.
The numbers collected during the new study highlighted the treatment gap. The IMPACT-AFib study randomized 23,546 patients with AFib and a CHA2DS2-VASc score of at least 2, denoting a stroke risk that warrants oral anticoagulation, to the intervention group, and 23,787 patients to the control arm. The patient selection process began with nearly 200,000 patients who met these criteria, but the researchers excluded 67% because they were already on an oral anticoagulant regimen, an uptake level that roughly matched the 50%-60% level usually seen among U.S. patients, Dr. Pokorney noted. That number coupled with the incremental uptake rate of only 10% of the enrolled patients during the trial, despite their uniform suitability for treatment, underscored how low uptake rates tend to remain stuck over time.
Enrolled patients averaged 78 years of age, with nearly two-thirds at least 75 years old, and with an average CHA2DS2-VASc score of 4.5.
The trial featured a novel design as the first clinical trial to take advantage of the Sentinel program for phase 4 data collection and study devised by the Food and Drug Administration, said Dr. Pokorney. The Sentinel program relies on data partners to provide information; for the IMPACT-AFib study, data came from five large U.S. health systems: Aetna, HealthCore, Humana, Harvard Pilgrim Healthcare, and Optum. Each of these systems sent the mailing to their targeted member patients.
In addition to sending just a single, mailed intervention, the study may have also been limited by the mailing’s content. The educational text, presented by Dr. Pokorney during his talk, focused largely on the potential risks of oral anticoagulation, the limited availability of antidote agents, potential drug and food interactions, and a brief entry about the risk for stroke associated with AFib along with a chart that a patient could use to hand calculate their CHA2DS2-VASc score. What the mailing lacked was discussion of the benefits of oral anticoagulation, noted study discussant Christophe LeClercq, MD, a cardiac electrophysiologist and professor of cardiology at the University of Rennes, France.
IMPACT-AFib received no commercial funding, and Dr. Pokorney and Dr. Shivkumar had no disclosures. Dr. Leclercq has received honoraria from Abbott, Biotronik, Boston Scientific, Livanova, and Medtronic.
A single educational mailing sent by several U.S. health plans to their patients with atrial fibrillation who were candidates for oral anticoagulation, but had not yet started a regimen, failed to boost them over their prescription hurdle and facilitate starting an antithrombotic regimen.
By 1 year following the intervention, a mere 10% of patients in both the intervention and a control arm of the randomized trial had begun treatment, with no signal of incremental uptake because of the mailing, Sean D. Pokorney, MD, said at the virtual annual congress of the European Society of Cardiology. Included in the mailing was an educational letter citing the patient’s atrial fibrillation (AFib) diagnosis, a statement regarding their suitability for oral anticoagulation, some information about the treatment, and a suggestion that recipients discuss this with their personal physician.
Dr. Pokorney acknowledged that the single mailing to patients may not have been adequate to capture patients’ attention and trigger an action, and that repeated messaging via multiple platforms and in coordination with interventions aimed at their health care providers may be what’s needed.
“It will take repeated interventions and engagements. We will need different methods to move the needle,” said Dr. Pokorney, a cardiac electrophysiologist at Duke University in Durham, N.C. The goal is to “empower patients to talk with their health care providers, and to become agents of change” in their care, he explained, but the single, mailed prod wasn’t enough.
An earlier study run by Dr. Pokorney and several of his colleagues used a broader panel of interventions aimed at both patients and clinicians to encourage increased prescribing of oral anticoagulants in five middle income countries, and documented successfully increasing the uptake rate by threefold compared with control patients (Lancet. 2017 Oct 14;390[10104]:1737-46). The current study tested the efficacy of a “much lower-impact intervention,” he admitted.
“The data are “sobering and eye-opening,” said Kalyanam Shivkumar, MD, a cardiac electrophysiologist and professor of medicine at the University of California, Los Angeles. “We’re stuck with this big challenge,” the gap between “what medicine can do and what it actually does” when evidence-based interventions fail to gain traction in everyday practice, he said in an interview.
The numbers collected during the new study highlighted the treatment gap. The IMPACT-AFib study randomized 23,546 patients with AFib and a CHA2DS2-VASc score of at least 2, denoting a stroke risk that warrants oral anticoagulation, to the intervention group, and 23,787 patients to the control arm. The patient selection process began with nearly 200,000 patients who met these criteria, but the researchers excluded 67% because they were already on an oral anticoagulant regimen, an uptake level that roughly matched the 50%-60% level usually seen among U.S. patients, Dr. Pokorney noted. That number coupled with the incremental uptake rate of only 10% of the enrolled patients during the trial, despite their uniform suitability for treatment, underscored how low uptake rates tend to remain stuck over time.
Enrolled patients averaged 78 years of age, with nearly two-thirds at least 75 years old, and with an average CHA2DS2-VASc score of 4.5.
The trial featured a novel design as the first clinical trial to take advantage of the Sentinel program for phase 4 data collection and study devised by the Food and Drug Administration, said Dr. Pokorney. The Sentinel program relies on data partners to provide information; for the IMPACT-AFib study, data came from five large U.S. health systems: Aetna, HealthCore, Humana, Harvard Pilgrim Healthcare, and Optum. Each of these systems sent the mailing to their targeted member patients.
In addition to sending just a single, mailed intervention, the study may have also been limited by the mailing’s content. The educational text, presented by Dr. Pokorney during his talk, focused largely on the potential risks of oral anticoagulation, the limited availability of antidote agents, potential drug and food interactions, and a brief entry about the risk for stroke associated with AFib along with a chart that a patient could use to hand calculate their CHA2DS2-VASc score. What the mailing lacked was discussion of the benefits of oral anticoagulation, noted study discussant Christophe LeClercq, MD, a cardiac electrophysiologist and professor of cardiology at the University of Rennes, France.
IMPACT-AFib received no commercial funding, and Dr. Pokorney and Dr. Shivkumar had no disclosures. Dr. Leclercq has received honoraria from Abbott, Biotronik, Boston Scientific, Livanova, and Medtronic.
A single educational mailing sent by several U.S. health plans to their patients with atrial fibrillation who were candidates for oral anticoagulation, but had not yet started a regimen, failed to boost them over their prescription hurdle and facilitate starting an antithrombotic regimen.
By 1 year following the intervention, a mere 10% of patients in both the intervention and a control arm of the randomized trial had begun treatment, with no signal of incremental uptake because of the mailing, Sean D. Pokorney, MD, said at the virtual annual congress of the European Society of Cardiology. Included in the mailing was an educational letter citing the patient’s atrial fibrillation (AFib) diagnosis, a statement regarding their suitability for oral anticoagulation, some information about the treatment, and a suggestion that recipients discuss this with their personal physician.
Dr. Pokorney acknowledged that the single mailing to patients may not have been adequate to capture patients’ attention and trigger an action, and that repeated messaging via multiple platforms and in coordination with interventions aimed at their health care providers may be what’s needed.
“It will take repeated interventions and engagements. We will need different methods to move the needle,” said Dr. Pokorney, a cardiac electrophysiologist at Duke University in Durham, N.C. The goal is to “empower patients to talk with their health care providers, and to become agents of change” in their care, he explained, but the single, mailed prod wasn’t enough.
An earlier study run by Dr. Pokorney and several of his colleagues used a broader panel of interventions aimed at both patients and clinicians to encourage increased prescribing of oral anticoagulants in five middle income countries, and documented successfully increasing the uptake rate by threefold compared with control patients (Lancet. 2017 Oct 14;390[10104]:1737-46). The current study tested the efficacy of a “much lower-impact intervention,” he admitted.
“The data are “sobering and eye-opening,” said Kalyanam Shivkumar, MD, a cardiac electrophysiologist and professor of medicine at the University of California, Los Angeles. “We’re stuck with this big challenge,” the gap between “what medicine can do and what it actually does” when evidence-based interventions fail to gain traction in everyday practice, he said in an interview.
The numbers collected during the new study highlighted the treatment gap. The IMPACT-AFib study randomized 23,546 patients with AFib and a CHA2DS2-VASc score of at least 2, denoting a stroke risk that warrants oral anticoagulation, to the intervention group, and 23,787 patients to the control arm. The patient selection process began with nearly 200,000 patients who met these criteria, but the researchers excluded 67% because they were already on an oral anticoagulant regimen, an uptake level that roughly matched the 50%-60% level usually seen among U.S. patients, Dr. Pokorney noted. That number coupled with the incremental uptake rate of only 10% of the enrolled patients during the trial, despite their uniform suitability for treatment, underscored how low uptake rates tend to remain stuck over time.
Enrolled patients averaged 78 years of age, with nearly two-thirds at least 75 years old, and with an average CHA2DS2-VASc score of 4.5.
The trial featured a novel design as the first clinical trial to take advantage of the Sentinel program for phase 4 data collection and study devised by the Food and Drug Administration, said Dr. Pokorney. The Sentinel program relies on data partners to provide information; for the IMPACT-AFib study, data came from five large U.S. health systems: Aetna, HealthCore, Humana, Harvard Pilgrim Healthcare, and Optum. Each of these systems sent the mailing to their targeted member patients.
In addition to sending just a single, mailed intervention, the study may have also been limited by the mailing’s content. The educational text, presented by Dr. Pokorney during his talk, focused largely on the potential risks of oral anticoagulation, the limited availability of antidote agents, potential drug and food interactions, and a brief entry about the risk for stroke associated with AFib along with a chart that a patient could use to hand calculate their CHA2DS2-VASc score. What the mailing lacked was discussion of the benefits of oral anticoagulation, noted study discussant Christophe LeClercq, MD, a cardiac electrophysiologist and professor of cardiology at the University of Rennes, France.
IMPACT-AFib received no commercial funding, and Dr. Pokorney and Dr. Shivkumar had no disclosures. Dr. Leclercq has received honoraria from Abbott, Biotronik, Boston Scientific, Livanova, and Medtronic.
FROM ESC CONGRESS 2020
HOME-PE trial clarifies which pulmonary embolism patients to treat at home
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
The pragmatic Hestia criteria proved as safe as the more structured, points-based simplified Pulmonary Embolism Severity Index (sPESI) score for selection of patients with acute pulmonary embolism for outpatient care in the large, randomized HOME-PE trial presented at the virtual annual congress of the European Society of Cardiology.
“These results support outpatient management of acute pulmonary embolism patients using either the Hestia method or the sPESI score with the option for the physician-in-charge to override the decision. In hospitals organized for outpatient management, both triaging strategies enable more than a third of pulmonary embolism patients to be managed at home with a low rate of complications,” Pierre-Marie Roy, MD, said in presenting the HOME-PE findings.
The study clarifies a transatlantic controversy regarding how best to triage patients with acute pulmonary embolism (PE) for outpatient care. The answer? It’s basically a tie between the points-based sPESI score recommended in the current ESC guidelines (Eur Respir J. 2019 Oct 9;54[3]:1901647) and the Hestia method endorsed in the American College of Chest Physician guidelines (Chest. 2016 Feb;149[2]:315-52).
The sPESI is a validated tool that grants 1 point each for age over 80 years, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%. A patient needs a score of zero to be eligible for outpatient management. In contrast, the Hestia method relies upon 11 simple bedside criteria rather than a points system, explained Dr. Roy of University Hospital of Angers, France (J Thromb Haemost. 2011 Aug;9[8]:1500-7).
HOME-PE was a randomized, open-label, noninferiority trial conducted at 26 hospitals in France, Belgium, Switzerland, and the Netherlands. The study included 1,974 patients presenting to the emergency department with non–high-risk acute PE as defined by hemodynamic stability. About 39% of patients in the Hestia group were eligible for outpatient care on the basis of ‘no’ answers regarding all 11 criteria, while 48% of patients had an sPESI score of 0 and were thus initially considered appropriate for outpatient management.
However, the investigators recognized that no scoring system for acute PE is perfect, and that the judgment of a physician with extensive experience in managing this life-threatening condition counts for a lot. So they stipulated that a patient’s physician-in-charge could overrule a decision for early discharge. This happened 29% of the time in patients with a sPESI score of 0, as compared with a 3% overrule rate with the Hestia rule. The physician-in-charge also moved small numbers of patients who were Hestia or sPESI positive into the outpatient care group. As a result, a similar proportion of patients in both groups were discharged home within 24 hours for outpatient treatment: 38% of the total Hestia group and 37% in the sPESI arm.
Major adverse event rates were reassuringly low in both groups managed on an outpatient basis. The composite of recurrent venous thromboembolism, bleeding, or death within 30 days occurred in 1.3% of Hestia outpatients and 1.1% of sPESI outpatients. Among patients managed in the hospital, these rates were 5.6% in the Hestia group and 4.7% in the sPESI group.
Discussant Stavros V. Konstantinides, MD, who chaired the ESC guideline committee, asked rhetorically, “who’s happy with the HOME-PE trial? I think everybody.”
“The Hestia criteria integrate the feasibility of family support of the individual patient. This is a good thing. And eligibility based on the Hestia criteria, unlike sPESI, does not require age younger than 80 years or no cancer, and it appears from the HOME-PE study that this is okay,” observed Dr. Konstantinides of the Center for Thrombosis and Hemostasis at the University of Mainz (Germany).
In an interview, Hadley Wilson, MD, called the HOME-PE trial “transformative” and predicted it will change clinical practice. He was particularly impressed with the high quality of the trial, noting that 87% of participants managed as outpatients received a direct oral anticoagulant.
The Hestia rule is simpler and more user-friendly. And greater use of this triaging strategy might have advantages in terms of economics and health care utilization by potentially encouraging movement of decision-making regarding outpatient management of acute PE out of the hospital wards and into emergency departments, said Dr. Wilson, executive vice chair of the Sanger Heart and Vascular Institute and a cardiologist at the University of North Carolina at Chapel Hill.
Dr. Roy reported receiving research grants to conduct HOME-PE from the French Ministry of Health, the study sponsor. In addition, he is on scientific advisory boards and/or speakers’ panels for Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Aspen, Daiichi Sankyo, and Sanofi Aventis.
REPORTING FROM ESC CONGRESS 2020
ESC’s revised NSTE-ACS guidelines embrace hsT, personalized anti-ischemia treatments
The first revision since 2015 to the European Society of Cardiology’s guidelines for diagnosing and managing non ST-elevation acute coronary syndrome placed increased reliance on high-sensitivity cardiac troponin testing for diagnosis, and embraced coronary CT to rule out lower-risk patients.
It also highlighted the need for personalized antiplatelet regimens, systems of care, and quality improvement.
The society released the new guidelines on August 29 (Eur Heart J. 2020 Aug 29;doi: 10.1093/eurheartj/ehaa575), and then devoted a session to them the next day at the virtual annual congress of the European Society of Cardiology to highlight some of the key updates, starting with the further emphasis placed on high-sensitivity cardiac troponin (hs-cTn), a reliance that contrasts with what remains inconsistent use of this metric in U.S. practice.
An hs-cTn test is the “preferred” diagnostic test and a “key” testing element, said Marco Roffi, MD, professor and director of interventional cardiology at University Hospital, Geneva, and a member of the guideline committee. He also stressed an update to the time frame of initial hs-cTn testing, which now involves a baseline reading and then a second measure after 2 hours to discern how the marker level is evolving with time. The guidelines advise against measuring any other biomarker of myocardial injury.
U.S. lags in measuring high-sensitivity cardiac troponin
U.S. medical systems and centers “are not uniform in adopting hs-cTn,” noted Richard J. Kovacs, MD, professor of cardiology at the Indiana University School of Medicine in Indianapolis. “The new European guidelines should spur U.S. institutions to at least take a close look at the advantages of hs-cTn. There is a strong case that it leads to more efficient emergency care and allows for quicker decisions and triage,” added Dr. Kovacs in an interview.
The new guideline’s emphasis on hs-cTn should hasten broader uptake in U.S. practice, agreed Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and a member of the guideline-writing panel
Another plus of the guidelines is its endorsement of an “organized approach to risk assessment” early on in these patients, said Dr. Kovacs, who is also the immediate past-president of the American College of Cardiology (ACC). An ACC committee is developing a new set of recommendations for managing patients with cardiac chest pain and is on track for release in 2021. It would represent the first update to U.S. guidelines for non ST-elevation ACS patients since 2014.
The new ESC guidelines give coronary CT angiography a class Ia rating as an alternative to invasive coronary angiography for assessing patients with a low or intermediate risk of having coronary disease, a “tremendous upgrade,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medical Heart Institute in Phoenix. While he welcomes this support for using coronary CT angiography in this setting, he acknowledged that the method remains limited in availability as it requires highly trained technicians to obtain good images and experienced clinicians to interpret the results.
Personalizing antiplatelet and antithrombotic treatments
Notable revisions to medical treatments to minimize ischemia included an admonition not to use routine pretreatment with a P2Y12 receptor inhibitor (such as clopidogrel) before testing determines coronary anatomy.
Not using one of these antiplatelet drugs upfront on all patients “is a tremendous change,” Dr. Pershad said in an interview. Many patients currently get these drugs while awaiting an angiogram, but a more selected and deferred antiplatelet approach would be better when angiography shows that some patients need coronary bypass surgery, he noted. Recent study results have shown no added benefit from pretreatment, and its use can be especially problematic for patients who are slated for a planned invasive strategy, said Dr. Bhatt.
Dr. Pershad, Dr. Bhatt, and Dr. Kovacs all praised the overall emphasis on a personalized approach to treating patients with antiplatelet and antithrombotic drugs, with endorsement of a flexible approach to treatment intensity and duration. The guideline acknowledges the need to take into account a patient’s bleeding risk and comorbidities, and specifically endorsed the Academic Research Consortium’s formula for identifying and stratifying high bleeding risk (Eur Heart J. 2019 Aug 14;40[31]:2632-53).
The new guidelines also provide guidance on how to apply recent study results that addressed balancing efficacy and safety when pairing an antiplatelet drug with a direct-acting oral anticoagulant (DOAC) for patients who potentially need both drug classes, such as patients with atrial fibrillation and a recent ACS event. “It’s tremendous to get clarity on this issue; there’s been a lot of uncertainty,” said Dr. Pershad. The guidelines call for a week of triple therapy with a DOAC, aspirin, and a second antiplatelet drug, followed by 12 months on a DOAC plus a single antiplatelet drug, and then the DOAC alone as the “default” strategy for most patients, but also presents alternative options for patients with high risk for either bleeding or ischemia.
The new guidelines also give much-needed direction on how to apply an invasive strategy, with an emphasis on immediate intervention for within 2 hours for very-high-risk patients, and early intervention within 24 hours for high-risk patients. Adhering to this timetable can mean increasing catheter laboratory availability on an urgent basis over weekends, Dr. Bhatt noted.
Improving quality of care
A novel section in the new guidelines was devoted to nine quality measures that can help health systems and medical centers monitor their adherence to the guideline recommendations, track their performance relative to peer institutions, and follow changes in performance that result from quality improvement steps. It’s something of a “futuristic” step for a guideline to take, with a goal of persuading administrators to implement tracking of these measures and improve patient outcomes, noted Dr. Bhatt.
“It’s very important to see that this is not just a set of guidelines but also a tool to improve quality of care,” commented Dr. Kovacs. The key to success in this effort will be to follow registered patients, set benchmarks that systems can aspire to achieve, and use this to improve the quality of care.
Until now, optimizing care for patients with NSTE-ACS has been “challenging,” he said. “The focus must be on moving toward systems of care” that can provide consistent patient evaluation and care, and do it quickly, said Dr. Kovacs.
Dr. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, and Medtronic. Dr. Kovacs was formerly an employee of Eli Lilly. Dr. Bhatt has been a consultant to and has received research funding from several companies. Dr. Pershad had no disclosures.
The first revision since 2015 to the European Society of Cardiology’s guidelines for diagnosing and managing non ST-elevation acute coronary syndrome placed increased reliance on high-sensitivity cardiac troponin testing for diagnosis, and embraced coronary CT to rule out lower-risk patients.
It also highlighted the need for personalized antiplatelet regimens, systems of care, and quality improvement.
The society released the new guidelines on August 29 (Eur Heart J. 2020 Aug 29;doi: 10.1093/eurheartj/ehaa575), and then devoted a session to them the next day at the virtual annual congress of the European Society of Cardiology to highlight some of the key updates, starting with the further emphasis placed on high-sensitivity cardiac troponin (hs-cTn), a reliance that contrasts with what remains inconsistent use of this metric in U.S. practice.
An hs-cTn test is the “preferred” diagnostic test and a “key” testing element, said Marco Roffi, MD, professor and director of interventional cardiology at University Hospital, Geneva, and a member of the guideline committee. He also stressed an update to the time frame of initial hs-cTn testing, which now involves a baseline reading and then a second measure after 2 hours to discern how the marker level is evolving with time. The guidelines advise against measuring any other biomarker of myocardial injury.
U.S. lags in measuring high-sensitivity cardiac troponin
U.S. medical systems and centers “are not uniform in adopting hs-cTn,” noted Richard J. Kovacs, MD, professor of cardiology at the Indiana University School of Medicine in Indianapolis. “The new European guidelines should spur U.S. institutions to at least take a close look at the advantages of hs-cTn. There is a strong case that it leads to more efficient emergency care and allows for quicker decisions and triage,” added Dr. Kovacs in an interview.
The new guideline’s emphasis on hs-cTn should hasten broader uptake in U.S. practice, agreed Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and a member of the guideline-writing panel
Another plus of the guidelines is its endorsement of an “organized approach to risk assessment” early on in these patients, said Dr. Kovacs, who is also the immediate past-president of the American College of Cardiology (ACC). An ACC committee is developing a new set of recommendations for managing patients with cardiac chest pain and is on track for release in 2021. It would represent the first update to U.S. guidelines for non ST-elevation ACS patients since 2014.
The new ESC guidelines give coronary CT angiography a class Ia rating as an alternative to invasive coronary angiography for assessing patients with a low or intermediate risk of having coronary disease, a “tremendous upgrade,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medical Heart Institute in Phoenix. While he welcomes this support for using coronary CT angiography in this setting, he acknowledged that the method remains limited in availability as it requires highly trained technicians to obtain good images and experienced clinicians to interpret the results.
Personalizing antiplatelet and antithrombotic treatments
Notable revisions to medical treatments to minimize ischemia included an admonition not to use routine pretreatment with a P2Y12 receptor inhibitor (such as clopidogrel) before testing determines coronary anatomy.
Not using one of these antiplatelet drugs upfront on all patients “is a tremendous change,” Dr. Pershad said in an interview. Many patients currently get these drugs while awaiting an angiogram, but a more selected and deferred antiplatelet approach would be better when angiography shows that some patients need coronary bypass surgery, he noted. Recent study results have shown no added benefit from pretreatment, and its use can be especially problematic for patients who are slated for a planned invasive strategy, said Dr. Bhatt.
Dr. Pershad, Dr. Bhatt, and Dr. Kovacs all praised the overall emphasis on a personalized approach to treating patients with antiplatelet and antithrombotic drugs, with endorsement of a flexible approach to treatment intensity and duration. The guideline acknowledges the need to take into account a patient’s bleeding risk and comorbidities, and specifically endorsed the Academic Research Consortium’s formula for identifying and stratifying high bleeding risk (Eur Heart J. 2019 Aug 14;40[31]:2632-53).
The new guidelines also provide guidance on how to apply recent study results that addressed balancing efficacy and safety when pairing an antiplatelet drug with a direct-acting oral anticoagulant (DOAC) for patients who potentially need both drug classes, such as patients with atrial fibrillation and a recent ACS event. “It’s tremendous to get clarity on this issue; there’s been a lot of uncertainty,” said Dr. Pershad. The guidelines call for a week of triple therapy with a DOAC, aspirin, and a second antiplatelet drug, followed by 12 months on a DOAC plus a single antiplatelet drug, and then the DOAC alone as the “default” strategy for most patients, but also presents alternative options for patients with high risk for either bleeding or ischemia.
The new guidelines also give much-needed direction on how to apply an invasive strategy, with an emphasis on immediate intervention for within 2 hours for very-high-risk patients, and early intervention within 24 hours for high-risk patients. Adhering to this timetable can mean increasing catheter laboratory availability on an urgent basis over weekends, Dr. Bhatt noted.
Improving quality of care
A novel section in the new guidelines was devoted to nine quality measures that can help health systems and medical centers monitor their adherence to the guideline recommendations, track their performance relative to peer institutions, and follow changes in performance that result from quality improvement steps. It’s something of a “futuristic” step for a guideline to take, with a goal of persuading administrators to implement tracking of these measures and improve patient outcomes, noted Dr. Bhatt.
“It’s very important to see that this is not just a set of guidelines but also a tool to improve quality of care,” commented Dr. Kovacs. The key to success in this effort will be to follow registered patients, set benchmarks that systems can aspire to achieve, and use this to improve the quality of care.
Until now, optimizing care for patients with NSTE-ACS has been “challenging,” he said. “The focus must be on moving toward systems of care” that can provide consistent patient evaluation and care, and do it quickly, said Dr. Kovacs.
Dr. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, and Medtronic. Dr. Kovacs was formerly an employee of Eli Lilly. Dr. Bhatt has been a consultant to and has received research funding from several companies. Dr. Pershad had no disclosures.
The first revision since 2015 to the European Society of Cardiology’s guidelines for diagnosing and managing non ST-elevation acute coronary syndrome placed increased reliance on high-sensitivity cardiac troponin testing for diagnosis, and embraced coronary CT to rule out lower-risk patients.
It also highlighted the need for personalized antiplatelet regimens, systems of care, and quality improvement.
The society released the new guidelines on August 29 (Eur Heart J. 2020 Aug 29;doi: 10.1093/eurheartj/ehaa575), and then devoted a session to them the next day at the virtual annual congress of the European Society of Cardiology to highlight some of the key updates, starting with the further emphasis placed on high-sensitivity cardiac troponin (hs-cTn), a reliance that contrasts with what remains inconsistent use of this metric in U.S. practice.
An hs-cTn test is the “preferred” diagnostic test and a “key” testing element, said Marco Roffi, MD, professor and director of interventional cardiology at University Hospital, Geneva, and a member of the guideline committee. He also stressed an update to the time frame of initial hs-cTn testing, which now involves a baseline reading and then a second measure after 2 hours to discern how the marker level is evolving with time. The guidelines advise against measuring any other biomarker of myocardial injury.
U.S. lags in measuring high-sensitivity cardiac troponin
U.S. medical systems and centers “are not uniform in adopting hs-cTn,” noted Richard J. Kovacs, MD, professor of cardiology at the Indiana University School of Medicine in Indianapolis. “The new European guidelines should spur U.S. institutions to at least take a close look at the advantages of hs-cTn. There is a strong case that it leads to more efficient emergency care and allows for quicker decisions and triage,” added Dr. Kovacs in an interview.
The new guideline’s emphasis on hs-cTn should hasten broader uptake in U.S. practice, agreed Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and a member of the guideline-writing panel
Another plus of the guidelines is its endorsement of an “organized approach to risk assessment” early on in these patients, said Dr. Kovacs, who is also the immediate past-president of the American College of Cardiology (ACC). An ACC committee is developing a new set of recommendations for managing patients with cardiac chest pain and is on track for release in 2021. It would represent the first update to U.S. guidelines for non ST-elevation ACS patients since 2014.
The new ESC guidelines give coronary CT angiography a class Ia rating as an alternative to invasive coronary angiography for assessing patients with a low or intermediate risk of having coronary disease, a “tremendous upgrade,” commented Ashish Pershad, MD, an interventional cardiologist at Banner-University Medical Heart Institute in Phoenix. While he welcomes this support for using coronary CT angiography in this setting, he acknowledged that the method remains limited in availability as it requires highly trained technicians to obtain good images and experienced clinicians to interpret the results.
Personalizing antiplatelet and antithrombotic treatments
Notable revisions to medical treatments to minimize ischemia included an admonition not to use routine pretreatment with a P2Y12 receptor inhibitor (such as clopidogrel) before testing determines coronary anatomy.
Not using one of these antiplatelet drugs upfront on all patients “is a tremendous change,” Dr. Pershad said in an interview. Many patients currently get these drugs while awaiting an angiogram, but a more selected and deferred antiplatelet approach would be better when angiography shows that some patients need coronary bypass surgery, he noted. Recent study results have shown no added benefit from pretreatment, and its use can be especially problematic for patients who are slated for a planned invasive strategy, said Dr. Bhatt.
Dr. Pershad, Dr. Bhatt, and Dr. Kovacs all praised the overall emphasis on a personalized approach to treating patients with antiplatelet and antithrombotic drugs, with endorsement of a flexible approach to treatment intensity and duration. The guideline acknowledges the need to take into account a patient’s bleeding risk and comorbidities, and specifically endorsed the Academic Research Consortium’s formula for identifying and stratifying high bleeding risk (Eur Heart J. 2019 Aug 14;40[31]:2632-53).
The new guidelines also provide guidance on how to apply recent study results that addressed balancing efficacy and safety when pairing an antiplatelet drug with a direct-acting oral anticoagulant (DOAC) for patients who potentially need both drug classes, such as patients with atrial fibrillation and a recent ACS event. “It’s tremendous to get clarity on this issue; there’s been a lot of uncertainty,” said Dr. Pershad. The guidelines call for a week of triple therapy with a DOAC, aspirin, and a second antiplatelet drug, followed by 12 months on a DOAC plus a single antiplatelet drug, and then the DOAC alone as the “default” strategy for most patients, but also presents alternative options for patients with high risk for either bleeding or ischemia.
The new guidelines also give much-needed direction on how to apply an invasive strategy, with an emphasis on immediate intervention for within 2 hours for very-high-risk patients, and early intervention within 24 hours for high-risk patients. Adhering to this timetable can mean increasing catheter laboratory availability on an urgent basis over weekends, Dr. Bhatt noted.
Improving quality of care
A novel section in the new guidelines was devoted to nine quality measures that can help health systems and medical centers monitor their adherence to the guideline recommendations, track their performance relative to peer institutions, and follow changes in performance that result from quality improvement steps. It’s something of a “futuristic” step for a guideline to take, with a goal of persuading administrators to implement tracking of these measures and improve patient outcomes, noted Dr. Bhatt.
“It’s very important to see that this is not just a set of guidelines but also a tool to improve quality of care,” commented Dr. Kovacs. The key to success in this effort will be to follow registered patients, set benchmarks that systems can aspire to achieve, and use this to improve the quality of care.
Until now, optimizing care for patients with NSTE-ACS has been “challenging,” he said. “The focus must be on moving toward systems of care” that can provide consistent patient evaluation and care, and do it quickly, said Dr. Kovacs.
Dr. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, and Medtronic. Dr. Kovacs was formerly an employee of Eli Lilly. Dr. Bhatt has been a consultant to and has received research funding from several companies. Dr. Pershad had no disclosures.
FROM ESC CONGRESS 2020
Aspirin alone preferred antithrombotic strategy after TAVI
Aspirin alone after transcatheter aortic valve implantation (TAVI) significantly reduced bleeding, compared with aspirin plus clopidogrel, without increasing thromboembolic events, in the latest results from the POPular TAVI study.
“Physicians can easily and safely reduce rate of bleeding by omitting clopidogrel after TAVI,” lead author, Jorn Brouwer, MD, St Antonius Hospital, Nieuwegein, the Netherlands, said.
“Aspirin alone should be used in patients undergoing TAVI who are not on oral anticoagulants and have not recently undergone coronary stenting,” he concluded.
Senior author, Jurriën ten Berg, MD, PhD, also from St Antonius Hospital, said in an interview: “I think we can say for TAVI patients, when it comes to antithrombotic therapy, less is definitely more.”
“This is a major change to clinical practice, with current guidelines recommending 3-6 months of dual antiplatelet therapy after a TAVI procedure,” he added. “We expected that these guidelines will change after our results.”
These latest results from POPular TAVI were presented at the virtual European Society of Cardiology Congress 2020 and simultaneously published online in The New England Journal of Medicine.
The trial was conducted in two cohorts of patients undergoing TAVI. The results from cohort B – in patients who were already taking an anticoagulant for another indication – were reported earlier this year and showed no benefit of adding clopidogrel and an increase in bleeding. Now the current results in cohort A – patients undergoing TAVI who do not have an established indication for long-term anticoagulation – show similar results, with aspirin alone preferred over aspirin plus clopidogrel.
Dr. ten Berg explained that the recommendation for dual antiplatelet therapy (DAPT) was adopted mainly because this has been shown to be beneficial in patients undergoing percutaneous coronary intervention (PCI) with stenting; it was thought the same benefits would be seen in TAVI, which also uses a stent-based delivery system.
“However, TAVI patients are a different population – they are generally much older than PCI patients, with an average age of 80 plus, and they have many more comorbidities, so they are much higher bleeding risk,” Dr. ten Berg explained. “In addition, the catheters used for TAVI are larger than those used for PCI, forcing the femoral route to be employed, and both of these factors increases bleeding risk.”
“We saw that, in the trial, patients on dual antiplatelet therapy had a much greater rate of major bleeding and the addition of clopidogrel did not reduce the risk of major thrombotic events,” such as stroke, myocardial infarction (MI), or cardiovascular (CV) death.
Given that the TAVI procedure is associated with an increase in stroke in the immediate few days after the procedure, it would seem logical that increased antiplatelet therapy would be beneficial in reducing this, Dr. ten Berg noted.
“But this is not what we are seeing,” he said. “The stroke incidence was similar in the two groups in POPular TAVI. This suggests that the strokes may not be platelet mediated. They might be caused by another mechanism, such as dislodgement of calcium from the valve or tissue from the aorta.”
For the current part of the study, 690 patients who were undergoing TAVI and did not have an indication for long-term anticoagulation were randomly assigned to receive aspirin alone or aspirin plus clopidogrel for 3 months.
The two primary outcomes were all bleeding (including minor, major, and life-threatening or disabling bleeding) and non–procedure-related bleeding over a period of 12 months. Most bleeding at the TAVI puncture site was counted as not procedure related.
Results showed that a bleeding event occurred in 15.1% of patients receiving aspirin alone and 26.6% of those receiving aspirin plus clopidogrel (risk ratio, 0.57; P = .001). Non–procedure-related bleeding occurred 15.1% of patients receiving aspirin alone vs 24.9% of those receiving aspirin plus clopidogrel (risk ratio, 0.61; P = .005). Major, life-threatening, or disabling bleeding occurred in 5.1% of the aspirin-alone group versus 10.8% of those in the aspirin plus clopidogrel group.
Two secondary outcomes included thromboembolic events. The secondary composite one endpoint of death from cardiovascular causes, non–procedure-related bleeding, stroke, or MI at 1 year occurred in 23.0% of those receiving aspirin alone and in 31.1% of those receiving aspirin plus clopidogrel (difference, −8.2 percentage points; P for noninferiority < .001; risk ratio, 0.74; P for superiority = .04).
The secondary composite two endpoint of death from cardiovascular causes, ischemic stroke, or MI at 1 year occurred in 9.7% of the aspirin-alone group versus 9.9% of the dual-antiplatelet group (difference, −0.2 percentage points; P for noninferiority = .004; risk ratio, 0.98; P for superiority = .93).
Dr. ten Berg pointed out that the trial was not strictly powered to look at thrombotic events, but he added: “There was no hint of an increase in the aspirin-alone group and there was quite a high event rate, so we should have seen something if it was there.”
The group has also performed a meta-analysis of these results, with some previous smaller studies also comparing aspirin and DAPT in TAVI which again showed no reduction in thrombotic events with dual-antiplatelet therapy.
Dr. ten Berg noted that the trial included all-comer TAVI patients. “The overall risk was quite a low [STS score, 2.5]. This is a reflection of the typical TAVI patient we are seeing but I would say our results apply to patients of all risk.”
Simplifies and clarifies
Discussant of the trial at the ESC Hotline session, Anna Sonia Petronio, MD, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy, said, “This was an excellent and essential study that simplifies and clarifies aspects of TAVI treatment and needs to change the guidelines.”
“These results will have a large impact on clinical practice in this elderly population,” she said. But she added that more data are needed for younger patients and more complicated cases, such as valve-in-valve and bicuspid valves.
Commenting on the results, Robert Bonow, MD, Northwestern University, Chicago, said, “The optimal antithrombotic management of patients undergoing TAVI who do not otherwise have an indication for anticoagulation [such as atrial fibrillation] has been uncertain and debatable. Aspirin plus clopidogrel for 3-6 months has been the standard, based on the experience with coronary stents.”
“Thus, the current results of cohort A of the POPular TAVI trial showing significant reduction in bleeding events with aspirin alone compared to DAPT for 3 months, with no difference in ischemic events, are important observations,” he said. “It is noteworthy that most of the bleeding events occurred in the first 30 days.
“This is a relatively small randomized trial, so whether these results will be practice changing will depend on confirmation by additional studies, but it is reassuring to know that patients at higher risk for bleeding would appear to do well with low-dose aspirin alone after TAVI,” Dr. Bonow added.
“These results complete the circle in terms of antithrombotic therapy after TAVI,” commented Michael Reardon, MD, Houston Methodist DeBakey Heart & Vascular Institute, Texas.
“I would add two caveats: First is that most of the difference in the primary endpoint occurs in the first month and levels out between the groups after that,” Dr. Reardon said. “Second is that this does not address the issue of leaflet thickening and immobility.”
Ashish Pershad, MD, Banner – University Medicine Heart Institute, Phoenix, added: “This trial answers a very important question and shows dual-antiplatelet therapy is hazardous in TAVI patients. Clopidogrel is not needed.”
Dr. Pershad says he still wonders about patients who receive very small valves who may have a higher risk for valve-induced thrombosis. “While there were some of these patients in the trial, the numbers were small, so we need more data on this group,” he commented.
“But for bread-and-butter TAVI, aspirin alone is the best choice, and the previous results showed, for patients already taking oral anticoagulation, no additional antithrombotic therapy is required,” Dr. Pershad concluded. “This is a big deal and will change the way we treat patients.”
The POPular trial was supported by the Netherlands Organization for Health Research and Development. Brouwer reports no disclosures.
A version of this article originally appeared on Medscape.com.
Aspirin alone after transcatheter aortic valve implantation (TAVI) significantly reduced bleeding, compared with aspirin plus clopidogrel, without increasing thromboembolic events, in the latest results from the POPular TAVI study.
“Physicians can easily and safely reduce rate of bleeding by omitting clopidogrel after TAVI,” lead author, Jorn Brouwer, MD, St Antonius Hospital, Nieuwegein, the Netherlands, said.
“Aspirin alone should be used in patients undergoing TAVI who are not on oral anticoagulants and have not recently undergone coronary stenting,” he concluded.
Senior author, Jurriën ten Berg, MD, PhD, also from St Antonius Hospital, said in an interview: “I think we can say for TAVI patients, when it comes to antithrombotic therapy, less is definitely more.”
“This is a major change to clinical practice, with current guidelines recommending 3-6 months of dual antiplatelet therapy after a TAVI procedure,” he added. “We expected that these guidelines will change after our results.”
These latest results from POPular TAVI were presented at the virtual European Society of Cardiology Congress 2020 and simultaneously published online in The New England Journal of Medicine.
The trial was conducted in two cohorts of patients undergoing TAVI. The results from cohort B – in patients who were already taking an anticoagulant for another indication – were reported earlier this year and showed no benefit of adding clopidogrel and an increase in bleeding. Now the current results in cohort A – patients undergoing TAVI who do not have an established indication for long-term anticoagulation – show similar results, with aspirin alone preferred over aspirin plus clopidogrel.
Dr. ten Berg explained that the recommendation for dual antiplatelet therapy (DAPT) was adopted mainly because this has been shown to be beneficial in patients undergoing percutaneous coronary intervention (PCI) with stenting; it was thought the same benefits would be seen in TAVI, which also uses a stent-based delivery system.
“However, TAVI patients are a different population – they are generally much older than PCI patients, with an average age of 80 plus, and they have many more comorbidities, so they are much higher bleeding risk,” Dr. ten Berg explained. “In addition, the catheters used for TAVI are larger than those used for PCI, forcing the femoral route to be employed, and both of these factors increases bleeding risk.”
“We saw that, in the trial, patients on dual antiplatelet therapy had a much greater rate of major bleeding and the addition of clopidogrel did not reduce the risk of major thrombotic events,” such as stroke, myocardial infarction (MI), or cardiovascular (CV) death.
Given that the TAVI procedure is associated with an increase in stroke in the immediate few days after the procedure, it would seem logical that increased antiplatelet therapy would be beneficial in reducing this, Dr. ten Berg noted.
“But this is not what we are seeing,” he said. “The stroke incidence was similar in the two groups in POPular TAVI. This suggests that the strokes may not be platelet mediated. They might be caused by another mechanism, such as dislodgement of calcium from the valve or tissue from the aorta.”
For the current part of the study, 690 patients who were undergoing TAVI and did not have an indication for long-term anticoagulation were randomly assigned to receive aspirin alone or aspirin plus clopidogrel for 3 months.
The two primary outcomes were all bleeding (including minor, major, and life-threatening or disabling bleeding) and non–procedure-related bleeding over a period of 12 months. Most bleeding at the TAVI puncture site was counted as not procedure related.
Results showed that a bleeding event occurred in 15.1% of patients receiving aspirin alone and 26.6% of those receiving aspirin plus clopidogrel (risk ratio, 0.57; P = .001). Non–procedure-related bleeding occurred 15.1% of patients receiving aspirin alone vs 24.9% of those receiving aspirin plus clopidogrel (risk ratio, 0.61; P = .005). Major, life-threatening, or disabling bleeding occurred in 5.1% of the aspirin-alone group versus 10.8% of those in the aspirin plus clopidogrel group.
Two secondary outcomes included thromboembolic events. The secondary composite one endpoint of death from cardiovascular causes, non–procedure-related bleeding, stroke, or MI at 1 year occurred in 23.0% of those receiving aspirin alone and in 31.1% of those receiving aspirin plus clopidogrel (difference, −8.2 percentage points; P for noninferiority < .001; risk ratio, 0.74; P for superiority = .04).
The secondary composite two endpoint of death from cardiovascular causes, ischemic stroke, or MI at 1 year occurred in 9.7% of the aspirin-alone group versus 9.9% of the dual-antiplatelet group (difference, −0.2 percentage points; P for noninferiority = .004; risk ratio, 0.98; P for superiority = .93).
Dr. ten Berg pointed out that the trial was not strictly powered to look at thrombotic events, but he added: “There was no hint of an increase in the aspirin-alone group and there was quite a high event rate, so we should have seen something if it was there.”
The group has also performed a meta-analysis of these results, with some previous smaller studies also comparing aspirin and DAPT in TAVI which again showed no reduction in thrombotic events with dual-antiplatelet therapy.
Dr. ten Berg noted that the trial included all-comer TAVI patients. “The overall risk was quite a low [STS score, 2.5]. This is a reflection of the typical TAVI patient we are seeing but I would say our results apply to patients of all risk.”
Simplifies and clarifies
Discussant of the trial at the ESC Hotline session, Anna Sonia Petronio, MD, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy, said, “This was an excellent and essential study that simplifies and clarifies aspects of TAVI treatment and needs to change the guidelines.”
“These results will have a large impact on clinical practice in this elderly population,” she said. But she added that more data are needed for younger patients and more complicated cases, such as valve-in-valve and bicuspid valves.
Commenting on the results, Robert Bonow, MD, Northwestern University, Chicago, said, “The optimal antithrombotic management of patients undergoing TAVI who do not otherwise have an indication for anticoagulation [such as atrial fibrillation] has been uncertain and debatable. Aspirin plus clopidogrel for 3-6 months has been the standard, based on the experience with coronary stents.”
“Thus, the current results of cohort A of the POPular TAVI trial showing significant reduction in bleeding events with aspirin alone compared to DAPT for 3 months, with no difference in ischemic events, are important observations,” he said. “It is noteworthy that most of the bleeding events occurred in the first 30 days.
“This is a relatively small randomized trial, so whether these results will be practice changing will depend on confirmation by additional studies, but it is reassuring to know that patients at higher risk for bleeding would appear to do well with low-dose aspirin alone after TAVI,” Dr. Bonow added.
“These results complete the circle in terms of antithrombotic therapy after TAVI,” commented Michael Reardon, MD, Houston Methodist DeBakey Heart & Vascular Institute, Texas.
“I would add two caveats: First is that most of the difference in the primary endpoint occurs in the first month and levels out between the groups after that,” Dr. Reardon said. “Second is that this does not address the issue of leaflet thickening and immobility.”
Ashish Pershad, MD, Banner – University Medicine Heart Institute, Phoenix, added: “This trial answers a very important question and shows dual-antiplatelet therapy is hazardous in TAVI patients. Clopidogrel is not needed.”
Dr. Pershad says he still wonders about patients who receive very small valves who may have a higher risk for valve-induced thrombosis. “While there were some of these patients in the trial, the numbers were small, so we need more data on this group,” he commented.
“But for bread-and-butter TAVI, aspirin alone is the best choice, and the previous results showed, for patients already taking oral anticoagulation, no additional antithrombotic therapy is required,” Dr. Pershad concluded. “This is a big deal and will change the way we treat patients.”
The POPular trial was supported by the Netherlands Organization for Health Research and Development. Brouwer reports no disclosures.
A version of this article originally appeared on Medscape.com.
Aspirin alone after transcatheter aortic valve implantation (TAVI) significantly reduced bleeding, compared with aspirin plus clopidogrel, without increasing thromboembolic events, in the latest results from the POPular TAVI study.
“Physicians can easily and safely reduce rate of bleeding by omitting clopidogrel after TAVI,” lead author, Jorn Brouwer, MD, St Antonius Hospital, Nieuwegein, the Netherlands, said.
“Aspirin alone should be used in patients undergoing TAVI who are not on oral anticoagulants and have not recently undergone coronary stenting,” he concluded.
Senior author, Jurriën ten Berg, MD, PhD, also from St Antonius Hospital, said in an interview: “I think we can say for TAVI patients, when it comes to antithrombotic therapy, less is definitely more.”
“This is a major change to clinical practice, with current guidelines recommending 3-6 months of dual antiplatelet therapy after a TAVI procedure,” he added. “We expected that these guidelines will change after our results.”
These latest results from POPular TAVI were presented at the virtual European Society of Cardiology Congress 2020 and simultaneously published online in The New England Journal of Medicine.
The trial was conducted in two cohorts of patients undergoing TAVI. The results from cohort B – in patients who were already taking an anticoagulant for another indication – were reported earlier this year and showed no benefit of adding clopidogrel and an increase in bleeding. Now the current results in cohort A – patients undergoing TAVI who do not have an established indication for long-term anticoagulation – show similar results, with aspirin alone preferred over aspirin plus clopidogrel.
Dr. ten Berg explained that the recommendation for dual antiplatelet therapy (DAPT) was adopted mainly because this has been shown to be beneficial in patients undergoing percutaneous coronary intervention (PCI) with stenting; it was thought the same benefits would be seen in TAVI, which also uses a stent-based delivery system.
“However, TAVI patients are a different population – they are generally much older than PCI patients, with an average age of 80 plus, and they have many more comorbidities, so they are much higher bleeding risk,” Dr. ten Berg explained. “In addition, the catheters used for TAVI are larger than those used for PCI, forcing the femoral route to be employed, and both of these factors increases bleeding risk.”
“We saw that, in the trial, patients on dual antiplatelet therapy had a much greater rate of major bleeding and the addition of clopidogrel did not reduce the risk of major thrombotic events,” such as stroke, myocardial infarction (MI), or cardiovascular (CV) death.
Given that the TAVI procedure is associated with an increase in stroke in the immediate few days after the procedure, it would seem logical that increased antiplatelet therapy would be beneficial in reducing this, Dr. ten Berg noted.
“But this is not what we are seeing,” he said. “The stroke incidence was similar in the two groups in POPular TAVI. This suggests that the strokes may not be platelet mediated. They might be caused by another mechanism, such as dislodgement of calcium from the valve or tissue from the aorta.”
For the current part of the study, 690 patients who were undergoing TAVI and did not have an indication for long-term anticoagulation were randomly assigned to receive aspirin alone or aspirin plus clopidogrel for 3 months.
The two primary outcomes were all bleeding (including minor, major, and life-threatening or disabling bleeding) and non–procedure-related bleeding over a period of 12 months. Most bleeding at the TAVI puncture site was counted as not procedure related.
Results showed that a bleeding event occurred in 15.1% of patients receiving aspirin alone and 26.6% of those receiving aspirin plus clopidogrel (risk ratio, 0.57; P = .001). Non–procedure-related bleeding occurred 15.1% of patients receiving aspirin alone vs 24.9% of those receiving aspirin plus clopidogrel (risk ratio, 0.61; P = .005). Major, life-threatening, or disabling bleeding occurred in 5.1% of the aspirin-alone group versus 10.8% of those in the aspirin plus clopidogrel group.
Two secondary outcomes included thromboembolic events. The secondary composite one endpoint of death from cardiovascular causes, non–procedure-related bleeding, stroke, or MI at 1 year occurred in 23.0% of those receiving aspirin alone and in 31.1% of those receiving aspirin plus clopidogrel (difference, −8.2 percentage points; P for noninferiority < .001; risk ratio, 0.74; P for superiority = .04).
The secondary composite two endpoint of death from cardiovascular causes, ischemic stroke, or MI at 1 year occurred in 9.7% of the aspirin-alone group versus 9.9% of the dual-antiplatelet group (difference, −0.2 percentage points; P for noninferiority = .004; risk ratio, 0.98; P for superiority = .93).
Dr. ten Berg pointed out that the trial was not strictly powered to look at thrombotic events, but he added: “There was no hint of an increase in the aspirin-alone group and there was quite a high event rate, so we should have seen something if it was there.”
The group has also performed a meta-analysis of these results, with some previous smaller studies also comparing aspirin and DAPT in TAVI which again showed no reduction in thrombotic events with dual-antiplatelet therapy.
Dr. ten Berg noted that the trial included all-comer TAVI patients. “The overall risk was quite a low [STS score, 2.5]. This is a reflection of the typical TAVI patient we are seeing but I would say our results apply to patients of all risk.”
Simplifies and clarifies
Discussant of the trial at the ESC Hotline session, Anna Sonia Petronio, MD, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy, said, “This was an excellent and essential study that simplifies and clarifies aspects of TAVI treatment and needs to change the guidelines.”
“These results will have a large impact on clinical practice in this elderly population,” she said. But she added that more data are needed for younger patients and more complicated cases, such as valve-in-valve and bicuspid valves.
Commenting on the results, Robert Bonow, MD, Northwestern University, Chicago, said, “The optimal antithrombotic management of patients undergoing TAVI who do not otherwise have an indication for anticoagulation [such as atrial fibrillation] has been uncertain and debatable. Aspirin plus clopidogrel for 3-6 months has been the standard, based on the experience with coronary stents.”
“Thus, the current results of cohort A of the POPular TAVI trial showing significant reduction in bleeding events with aspirin alone compared to DAPT for 3 months, with no difference in ischemic events, are important observations,” he said. “It is noteworthy that most of the bleeding events occurred in the first 30 days.
“This is a relatively small randomized trial, so whether these results will be practice changing will depend on confirmation by additional studies, but it is reassuring to know that patients at higher risk for bleeding would appear to do well with low-dose aspirin alone after TAVI,” Dr. Bonow added.
“These results complete the circle in terms of antithrombotic therapy after TAVI,” commented Michael Reardon, MD, Houston Methodist DeBakey Heart & Vascular Institute, Texas.
“I would add two caveats: First is that most of the difference in the primary endpoint occurs in the first month and levels out between the groups after that,” Dr. Reardon said. “Second is that this does not address the issue of leaflet thickening and immobility.”
Ashish Pershad, MD, Banner – University Medicine Heart Institute, Phoenix, added: “This trial answers a very important question and shows dual-antiplatelet therapy is hazardous in TAVI patients. Clopidogrel is not needed.”
Dr. Pershad says he still wonders about patients who receive very small valves who may have a higher risk for valve-induced thrombosis. “While there were some of these patients in the trial, the numbers were small, so we need more data on this group,” he commented.
“But for bread-and-butter TAVI, aspirin alone is the best choice, and the previous results showed, for patients already taking oral anticoagulation, no additional antithrombotic therapy is required,” Dr. Pershad concluded. “This is a big deal and will change the way we treat patients.”
The POPular trial was supported by the Netherlands Organization for Health Research and Development. Brouwer reports no disclosures.
A version of this article originally appeared on Medscape.com.
Aspirin may accelerate cancer progression in older adults
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
Aspirin may accelerate the progression of advanced cancers and lead to an earlier death as a result, new data from the ASPREE study suggest.
The results showed that patients 65 years and older who started taking daily low-dose aspirin had a 19% higher chance of being diagnosed with metastatic cancer, a 22% higher chance of being diagnosed with a stage 4 tumor, and a 31% increased risk of death from stage 4 cancer, when compared with patients who took a placebo.
John J. McNeil, MBBS, PhD, of Monash University in Melbourne, Australia, and colleagues detailed these findings in the Journal of the National Cancer Institute.
“If confirmed, the clinical implications of these findings could be important for the use of aspirin in an older population,” the authors wrote.
When results of the ASPREE study were first reported in 2018, they “raised important concerns,” Ernest Hawk, MD, and Karen Colbert Maresso wrote in an editorial related to the current publication.
“Unlike ARRIVE, ASCEND, and nearly all prior primary prevention CVD [cardiovascular disease] trials of aspirin, ASPREE surprisingly demonstrated increased all-cause mortality in the aspirin group, which appeared to be driven largely by an increase in cancer-related deaths,” wrote the editorialists, who are both from the University of Texas MD Anderson Cancer Center in Houston.
Even though the ASPREE investigators have now taken a deeper dive into their data, the findings “neither explain nor alleviate the concerns raised by the initial ASPREE report,” the editorialists noted.
ASPREE design and results
ASPREE is a multicenter, double-blind trial of 19,114 older adults living in Australia (n = 16,703) or the United States (n = 2,411). Most patients were 70 years or older at baseline. However, the U.S. group also included patients 65 years and older who were racial/ethnic minorities (n = 564).
Patients were randomized to receive 100 mg of enteric-coated aspirin daily (n = 9,525) or matching placebo (n = 9,589) from March 2010 through December 2014.
At inclusion, all participants were free from cardiovascular disease, dementia, or physical disability. A previous history of cancer was not used to exclude participants, and 19.1% of patients had cancer at randomization. Most patients (89%) had not used aspirin regularly before entering the trial.
At a median follow-up of 4.7 years, there were 981 incident cancer events in the aspirin-treated group and 952 in the placebo-treated group, with an overall incident cancer rate of 10.1%.
Of the 1,933 patients with newly diagnosed cancer, 65.7% had a localized cancer, 18.8% had a new metastatic cancer, 5.8% had metastatic disease from an existing cancer, and 9.7% had a new hematologic or lymphatic cancer.
A quarter of cancer patients (n = 495) died as a result of their malignancy, with 52 dying from a cancer they already had at randomization.
Aspirin was not associated with the risk of first incident cancer diagnosis or incident localized cancer diagnosis. The hazard ratios were 1.04 for all incident cancers (95% confidence interval, 0.95-1.14) and 0.99 for incident localized cancers (95% CI, 0.89-1.11).
However, aspirin was associated with an increased risk of metastatic cancer and cancer presenting at stage 4. The HR for metastatic cancer was 1.19 (95% CI, 1.00-1.43), and the HR for newly diagnosed stage 4 cancer was 1.22 (95% CI, 1.02-1.45).
Furthermore, “an increased progression to death was observed amongst those randomized to aspirin, regardless of whether the initial cancer presentation had been localized or metastatic,” the investigators wrote.
The HRs for death were 1.35 for all cancers (95% CI, 1.13-1.61), 1.47 for localized cancers (95% CI, 1.07-2.02), and 1.30 for metastatic cancers (95% CI, 1.03-1.63).
“Deaths were particularly high among those on aspirin who were diagnosed with advanced solid cancers,” study author Andrew Chan, MD, of Massachusetts General Hospital in Boston, said in a press statement.
Indeed, HRs for death in patients with solid tumors presenting at stage 3 and 4 were a respective 2.11 (95% CI, 1.03-4.33) and 1.31 (95% CI, 1.04-1.64). This suggests a possible adverse effect of aspirin on the growth of cancers once they have already developed in older adults, Dr. Chan said.
Where does that leave aspirin for cancer prevention?
“Although these results suggest that we should be cautious about starting aspirin therapy in otherwise healthy older adults, this does not mean that individuals who are already taking aspirin – particularly if they began taking it at a younger age – should stop their aspirin regimen,” Dr. Chan said.
There are decades of data supporting the use of daily aspirin to prevent multiple cancer types, particularly colorectal cancer, in individuals under the age of 70 years. In a recent meta-analysis, for example, regular aspirin use was linked to a 27% reduced risk for colorectal cancer, a 33% reduced risk for squamous cell esophageal cancer, a 39% decreased risk for adenocarcinoma of the esophagus and gastric cardia, a 36% decreased risk for stomach cancer, a 38% decreased risk for hepatobiliary tract cancer, and a 22% decreased risk for pancreatic cancer.
While these figures are mostly based on observational and case-control studies, it “reaffirms the fact that, overall, when you look at all of the ages, that there is still a benefit of aspirin for cancer,” John Cuzick, PhD, of Queen Mary University of London (England), said in an interview.
In fact, the meta-analysis goes as far as suggesting that perhaps the dose of aspirin being used is too low, with the authors noting that there was a 35% risk reduction in colorectal cancer with a dose of 325 mg daily. That’s a new finding, Dr. Cuzick said.
He noted that the ASPREE study largely consists of patients 70 years of age or older, and the authors “draw some conclusions which we can’t ignore about potential safety.”
One of the safety concerns is the increased risk for gastrointestinal bleeding, which is why Dr. Cuzick and colleagues previously recommended caution in the use of aspirin to prevent cancer in elderly patients. The group published a study in 2015 that suggested a benefit of taking aspirin daily for 5-10 years in patients aged 50-65 years, but the risk/benefit ratio was unclear for patients 70 years and older.
The ASPREE data now add to those uncertainties and suggest “there may be some side effects that we do not understand,” Dr. Cuzick said.
“I’m still optimistic that aspirin is going to be important for cancer prevention, but probably focusing on ages 50-70,” he added. “[The ASPREE data] reinforce the caution that we have to take in terms of trying to understand what the side effects are and what’s going on at these older ages.”
Dr. Cuzick is currently leading the AsCaP Project, an international effort to better understand why aspirin might work in preventing some cancer types but not others. AsCaP is supported by Cancer Research UK and also includes Dr. Chan among the researchers attempting to find out which patients may benefit the most from aspirin and which may be at greater risk of adverse effects.
The ASPREE trial was funded by grants from the National Institute on Aging, the National Cancer Institute, the National Health and Medical Research Council of Australia, Monash University, and the Victorian Cancer Agency. Several ASPREE investigators disclosed financial relationships with Bayer Pharma. The editorialists had no conflicts of interest. Dr. Cuzick has been an advisory board member for Bayer in the past.
SOURCE: McNeil J et al. J Natl Cancer Inst. 2020 Aug 11. doi: 10.1093/jnci/djaa114.
FROM JOURNAL OF THE NATIONAL CANCER INSTITUTE
Continued extension of time for thrombolysis in stroke
Background: Current guidelines for ischemic stroke recommend the time to thrombolysis be within 4.5 hours after onset of stroke. Guidelines are based on noncontrasted CT, but CT perfusion and perfusion-diffusion MRI may show salvageable brain tissue beyond the 4.5 hours. Studies have shown better outcomes in patients who were chosen for reperfusion based on tissue viability rather than time from onset of stroke. This has resulted in a disparity between the time windows used for thrombolysis.
Study design: Multicenter, randomized, placebo-controlled trial.
Setting: Hospitalized patients with acute ischemic stroke from 16 centers in Australia, 10 centers in Taiwan, 1 center in New Zealand, and 1 center in Finland.
Synopsis: 225 patients (aged 18 years and older) with acute ischemic stroke with hypoperfused but salvageable areas of brain detected on CT perfusion imaging or perfusion-diffusion MRI were randomly assigned to receive IV alteplase or placebo between 4.5 and 9 hours after onset of stroke or on awakening with stroke. Primary outcome measured on modified Rankin scale was 0 (no neurologic deficit) or 1. Before the trial was fully enrolled, it was terminated because of a loss of equipoise based on positive results from a previous trial. Of the patients enrolled, the primary outcome occurred in 35.4% of the alteplase group and 29.5% in the placebo group (adjusted risk ratio, 1.44). Symptomatic intracerebral hemorrhage was experienced in 6.2% of the patients in the alteplase group and 0.9% of patients in the placebo group (adjusted risk ratio, 7.22).
Not all centers may have access to perfusion imaging, so the study’s findings may not be applicable to multiple sites.
Bottom line: Diffusion-perfusion imaging may be useful in determining salvageable brain tissue in acute ischemic stroke that may benefit from thrombolysis after the standard 4.5-hour window, but further studies need to be conducted before guidelines are changed.
Citation: Ma H et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795-803.
Dr. Rogers is a hospitalist at Ochsner Health System, New Orleans.
Background: Current guidelines for ischemic stroke recommend the time to thrombolysis be within 4.5 hours after onset of stroke. Guidelines are based on noncontrasted CT, but CT perfusion and perfusion-diffusion MRI may show salvageable brain tissue beyond the 4.5 hours. Studies have shown better outcomes in patients who were chosen for reperfusion based on tissue viability rather than time from onset of stroke. This has resulted in a disparity between the time windows used for thrombolysis.
Study design: Multicenter, randomized, placebo-controlled trial.
Setting: Hospitalized patients with acute ischemic stroke from 16 centers in Australia, 10 centers in Taiwan, 1 center in New Zealand, and 1 center in Finland.
Synopsis: 225 patients (aged 18 years and older) with acute ischemic stroke with hypoperfused but salvageable areas of brain detected on CT perfusion imaging or perfusion-diffusion MRI were randomly assigned to receive IV alteplase or placebo between 4.5 and 9 hours after onset of stroke or on awakening with stroke. Primary outcome measured on modified Rankin scale was 0 (no neurologic deficit) or 1. Before the trial was fully enrolled, it was terminated because of a loss of equipoise based on positive results from a previous trial. Of the patients enrolled, the primary outcome occurred in 35.4% of the alteplase group and 29.5% in the placebo group (adjusted risk ratio, 1.44). Symptomatic intracerebral hemorrhage was experienced in 6.2% of the patients in the alteplase group and 0.9% of patients in the placebo group (adjusted risk ratio, 7.22).
Not all centers may have access to perfusion imaging, so the study’s findings may not be applicable to multiple sites.
Bottom line: Diffusion-perfusion imaging may be useful in determining salvageable brain tissue in acute ischemic stroke that may benefit from thrombolysis after the standard 4.5-hour window, but further studies need to be conducted before guidelines are changed.
Citation: Ma H et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795-803.
Dr. Rogers is a hospitalist at Ochsner Health System, New Orleans.
Background: Current guidelines for ischemic stroke recommend the time to thrombolysis be within 4.5 hours after onset of stroke. Guidelines are based on noncontrasted CT, but CT perfusion and perfusion-diffusion MRI may show salvageable brain tissue beyond the 4.5 hours. Studies have shown better outcomes in patients who were chosen for reperfusion based on tissue viability rather than time from onset of stroke. This has resulted in a disparity between the time windows used for thrombolysis.
Study design: Multicenter, randomized, placebo-controlled trial.
Setting: Hospitalized patients with acute ischemic stroke from 16 centers in Australia, 10 centers in Taiwan, 1 center in New Zealand, and 1 center in Finland.
Synopsis: 225 patients (aged 18 years and older) with acute ischemic stroke with hypoperfused but salvageable areas of brain detected on CT perfusion imaging or perfusion-diffusion MRI were randomly assigned to receive IV alteplase or placebo between 4.5 and 9 hours after onset of stroke or on awakening with stroke. Primary outcome measured on modified Rankin scale was 0 (no neurologic deficit) or 1. Before the trial was fully enrolled, it was terminated because of a loss of equipoise based on positive results from a previous trial. Of the patients enrolled, the primary outcome occurred in 35.4% of the alteplase group and 29.5% in the placebo group (adjusted risk ratio, 1.44). Symptomatic intracerebral hemorrhage was experienced in 6.2% of the patients in the alteplase group and 0.9% of patients in the placebo group (adjusted risk ratio, 7.22).
Not all centers may have access to perfusion imaging, so the study’s findings may not be applicable to multiple sites.
Bottom line: Diffusion-perfusion imaging may be useful in determining salvageable brain tissue in acute ischemic stroke that may benefit from thrombolysis after the standard 4.5-hour window, but further studies need to be conducted before guidelines are changed.
Citation: Ma H et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380(19):1795-803.
Dr. Rogers is a hospitalist at Ochsner Health System, New Orleans.
HM20 Virtual Product Theater: Aug. 12
Aug. 12, 2020. 12:00 p.m. to 1:00 p.m. ET
Clinical Insights in VTE: Treatment and Risk Reduction Through Prophylaxis
Speaker: Michael S. Oleksyk, MD, FACP, CPE, CMPE
Clinical assistant professor, Florida State University, Pensacola
Hospitalist & palliative care physician, Baptist Hospital, Pensacola.
Program description:
This lecture will discuss venous thromboembolism prophylaxis, as well as treatment options for patients with deep vein thrombosis and/or pulmonary embolism, and how these treatment options may reduce the risk of recurrent thrombotic events.
Sponsored by Janssen Pharmaceuticals Inc.
Aug. 12, 2020. 12:00 p.m. to 1:00 p.m. ET
Clinical Insights in VTE: Treatment and Risk Reduction Through Prophylaxis
Speaker: Michael S. Oleksyk, MD, FACP, CPE, CMPE
Clinical assistant professor, Florida State University, Pensacola
Hospitalist & palliative care physician, Baptist Hospital, Pensacola.
Program description:
This lecture will discuss venous thromboembolism prophylaxis, as well as treatment options for patients with deep vein thrombosis and/or pulmonary embolism, and how these treatment options may reduce the risk of recurrent thrombotic events.
Sponsored by Janssen Pharmaceuticals Inc.
Aug. 12, 2020. 12:00 p.m. to 1:00 p.m. ET
Clinical Insights in VTE: Treatment and Risk Reduction Through Prophylaxis
Speaker: Michael S. Oleksyk, MD, FACP, CPE, CMPE
Clinical assistant professor, Florida State University, Pensacola
Hospitalist & palliative care physician, Baptist Hospital, Pensacola.
Program description:
This lecture will discuss venous thromboembolism prophylaxis, as well as treatment options for patients with deep vein thrombosis and/or pulmonary embolism, and how these treatment options may reduce the risk of recurrent thrombotic events.
Sponsored by Janssen Pharmaceuticals Inc.
Patent foramen ovale linked with increased risk of ischemic stroke in PE
Background: Studies have demonstrated the increased risk for ischemic stroke in patients diagnosed with acute PE, and data support the mechanism of paradoxical embolism via PFO. However, the frequency of this phenomenon is unknown and the strength of the association between PFO and ischemic stroke in patients with PE is unclear.
Study design: Prospective cohort study.
Setting: Four French hospitals.
Synopsis: 315 patients aged 18 years and older presenting with acute symptomatic PE were evaluated at the time of diagnosis for PFO with contrast transthoracic echocardiography and for ischemic stroke with cerebral magnetic resonance imaging. The overall frequency of ischemic stroke at the time of PE diagnosis was high (7.6%), and was nearly four times higher in the PFO group than the non-PFO group (21.4% vs. 5.5%; difference in proportions, 15.9 percentage points; 95% confidence interval, 4.7-30.7).
This study adds to the growing body of data which supports the association of ischemic stroke with PFO and PE. Given the moderate indication for indefinite anticoagulation in patients at high risk for recurrent PE and stroke, there may be a role for screening for PFO in patients with acute PE so that they can be appropriately risk stratified.
Bottom line: The presence of ischemic stroke in patients with acute pulmonary embolism is high, and there is a strong association with PFO.
Citation: Le Moigne E et al. Patent Foramen Ovale and Ischemic Stroke in Patients With Pulmonary Embolism: A Prospective Cohort Study. Ann Intern Med. 2019;170:756-63.
Dr. McIntyre is a hospitalist at Ochsner Health System, New Orleans.
Background: Studies have demonstrated the increased risk for ischemic stroke in patients diagnosed with acute PE, and data support the mechanism of paradoxical embolism via PFO. However, the frequency of this phenomenon is unknown and the strength of the association between PFO and ischemic stroke in patients with PE is unclear.
Study design: Prospective cohort study.
Setting: Four French hospitals.
Synopsis: 315 patients aged 18 years and older presenting with acute symptomatic PE were evaluated at the time of diagnosis for PFO with contrast transthoracic echocardiography and for ischemic stroke with cerebral magnetic resonance imaging. The overall frequency of ischemic stroke at the time of PE diagnosis was high (7.6%), and was nearly four times higher in the PFO group than the non-PFO group (21.4% vs. 5.5%; difference in proportions, 15.9 percentage points; 95% confidence interval, 4.7-30.7).
This study adds to the growing body of data which supports the association of ischemic stroke with PFO and PE. Given the moderate indication for indefinite anticoagulation in patients at high risk for recurrent PE and stroke, there may be a role for screening for PFO in patients with acute PE so that they can be appropriately risk stratified.
Bottom line: The presence of ischemic stroke in patients with acute pulmonary embolism is high, and there is a strong association with PFO.
Citation: Le Moigne E et al. Patent Foramen Ovale and Ischemic Stroke in Patients With Pulmonary Embolism: A Prospective Cohort Study. Ann Intern Med. 2019;170:756-63.
Dr. McIntyre is a hospitalist at Ochsner Health System, New Orleans.
Background: Studies have demonstrated the increased risk for ischemic stroke in patients diagnosed with acute PE, and data support the mechanism of paradoxical embolism via PFO. However, the frequency of this phenomenon is unknown and the strength of the association between PFO and ischemic stroke in patients with PE is unclear.
Study design: Prospective cohort study.
Setting: Four French hospitals.
Synopsis: 315 patients aged 18 years and older presenting with acute symptomatic PE were evaluated at the time of diagnosis for PFO with contrast transthoracic echocardiography and for ischemic stroke with cerebral magnetic resonance imaging. The overall frequency of ischemic stroke at the time of PE diagnosis was high (7.6%), and was nearly four times higher in the PFO group than the non-PFO group (21.4% vs. 5.5%; difference in proportions, 15.9 percentage points; 95% confidence interval, 4.7-30.7).
This study adds to the growing body of data which supports the association of ischemic stroke with PFO and PE. Given the moderate indication for indefinite anticoagulation in patients at high risk for recurrent PE and stroke, there may be a role for screening for PFO in patients with acute PE so that they can be appropriately risk stratified.
Bottom line: The presence of ischemic stroke in patients with acute pulmonary embolism is high, and there is a strong association with PFO.
Citation: Le Moigne E et al. Patent Foramen Ovale and Ischemic Stroke in Patients With Pulmonary Embolism: A Prospective Cohort Study. Ann Intern Med. 2019;170:756-63.
Dr. McIntyre is a hospitalist at Ochsner Health System, New Orleans.
No rise in major hemorrhagic events with antiplatelet therapy after ICH
Background: Antiplatelet agents reduce the risk of major vascular events in patient with established vaso-occlusive disease, but they may increase the risk of ICH. Patients with prior ICH are at risk for both vaso-occlusive and hemorrhagic events. Clarification of the relative risk and benefit of antiplatelet agent use in this clinical scenario would serve to guide therapy.
Study design: Prospective, open-label, randomized parallel group trial.
Setting: 122 hospitals located in the United Kingdom.
Synopsis: The study included 537 adult patients with imaging-confirmed, nontraumatic intracerebral hemorrhage who were previously prescribed antithrombotic medications were randomized in 1:1 fashion to either start or avoid antiplatelet therapy. Participants were followed up on an annual basis with postal questionnaires both to the participants and their primary care providers. No significant difference was identified in rates of recurrent ICH (adjusted hazard ratio, 0.51; 95% confidence interval, 0.25-1.03), major hemorrhagic events (aHR, 0.71; 95% CI, 0.39-1.30), or major occlusive vascular events (aHR, 1.02; 95% CI, 0.65-1.60) between groups.
Hospitalists should be aware that these data suggest that the risk assessment for resumption of antiplatelet agents should not be affected by a history of nontraumatic intracerebral hemorrhage when weighed against the benefit of these medications in patients with occlusive vascular disease.
Bottom line: Resumption of antiplatelet agents following intracerebral hemorrhage showed no evidence of increased risk of recurrent intracerebral hemorrhage or major hemorrhagic events.
Citation: RESTART Collaboration. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): A randomized, open-label trial. Lancet. 2019. doi: 10.1016/S0140-6736(19)30840-2.
Dr. Deitelzweig is system department chair of hospital medicine at Ochsner Health System, New Orleans.
Background: Antiplatelet agents reduce the risk of major vascular events in patient with established vaso-occlusive disease, but they may increase the risk of ICH. Patients with prior ICH are at risk for both vaso-occlusive and hemorrhagic events. Clarification of the relative risk and benefit of antiplatelet agent use in this clinical scenario would serve to guide therapy.
Study design: Prospective, open-label, randomized parallel group trial.
Setting: 122 hospitals located in the United Kingdom.
Synopsis: The study included 537 adult patients with imaging-confirmed, nontraumatic intracerebral hemorrhage who were previously prescribed antithrombotic medications were randomized in 1:1 fashion to either start or avoid antiplatelet therapy. Participants were followed up on an annual basis with postal questionnaires both to the participants and their primary care providers. No significant difference was identified in rates of recurrent ICH (adjusted hazard ratio, 0.51; 95% confidence interval, 0.25-1.03), major hemorrhagic events (aHR, 0.71; 95% CI, 0.39-1.30), or major occlusive vascular events (aHR, 1.02; 95% CI, 0.65-1.60) between groups.
Hospitalists should be aware that these data suggest that the risk assessment for resumption of antiplatelet agents should not be affected by a history of nontraumatic intracerebral hemorrhage when weighed against the benefit of these medications in patients with occlusive vascular disease.
Bottom line: Resumption of antiplatelet agents following intracerebral hemorrhage showed no evidence of increased risk of recurrent intracerebral hemorrhage or major hemorrhagic events.
Citation: RESTART Collaboration. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): A randomized, open-label trial. Lancet. 2019. doi: 10.1016/S0140-6736(19)30840-2.
Dr. Deitelzweig is system department chair of hospital medicine at Ochsner Health System, New Orleans.
Background: Antiplatelet agents reduce the risk of major vascular events in patient with established vaso-occlusive disease, but they may increase the risk of ICH. Patients with prior ICH are at risk for both vaso-occlusive and hemorrhagic events. Clarification of the relative risk and benefit of antiplatelet agent use in this clinical scenario would serve to guide therapy.
Study design: Prospective, open-label, randomized parallel group trial.
Setting: 122 hospitals located in the United Kingdom.
Synopsis: The study included 537 adult patients with imaging-confirmed, nontraumatic intracerebral hemorrhage who were previously prescribed antithrombotic medications were randomized in 1:1 fashion to either start or avoid antiplatelet therapy. Participants were followed up on an annual basis with postal questionnaires both to the participants and their primary care providers. No significant difference was identified in rates of recurrent ICH (adjusted hazard ratio, 0.51; 95% confidence interval, 0.25-1.03), major hemorrhagic events (aHR, 0.71; 95% CI, 0.39-1.30), or major occlusive vascular events (aHR, 1.02; 95% CI, 0.65-1.60) between groups.
Hospitalists should be aware that these data suggest that the risk assessment for resumption of antiplatelet agents should not be affected by a history of nontraumatic intracerebral hemorrhage when weighed against the benefit of these medications in patients with occlusive vascular disease.
Bottom line: Resumption of antiplatelet agents following intracerebral hemorrhage showed no evidence of increased risk of recurrent intracerebral hemorrhage or major hemorrhagic events.
Citation: RESTART Collaboration. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): A randomized, open-label trial. Lancet. 2019. doi: 10.1016/S0140-6736(19)30840-2.
Dr. Deitelzweig is system department chair of hospital medicine at Ochsner Health System, New Orleans.