User login
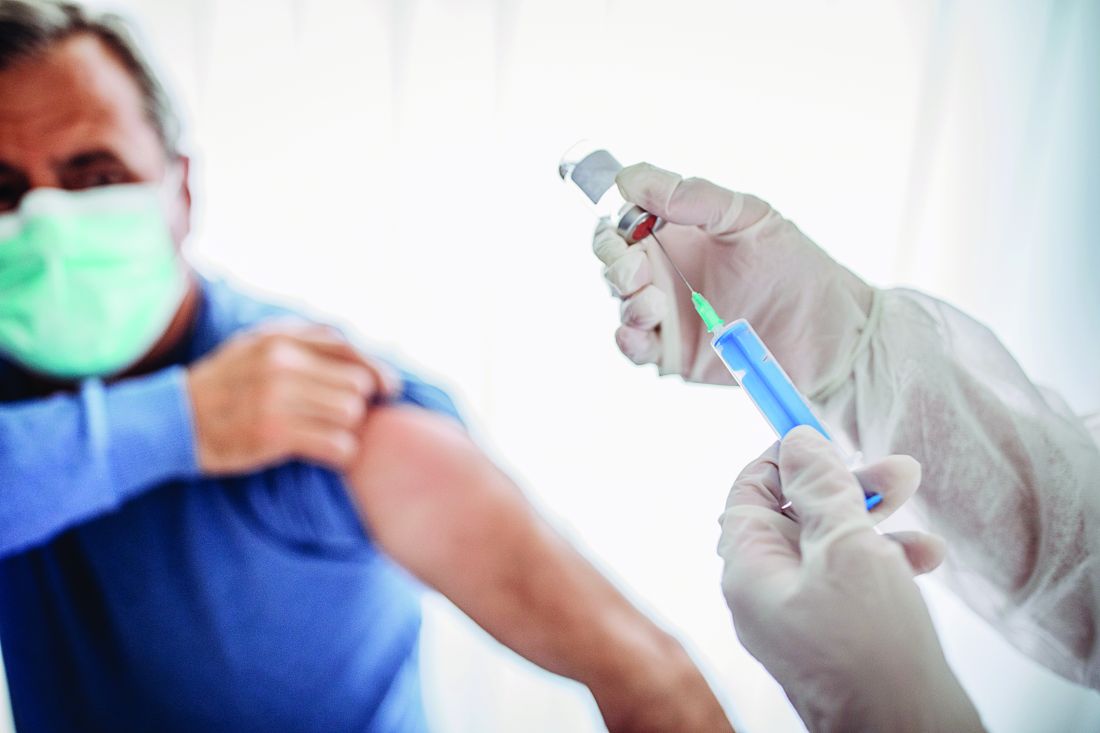
Babies better protected from COVID if mother vaccinated during pregnancy: study
In a first of its kind study, researchers found women who received two mRNA COVID vaccine doses during pregnancy were 61% less likely to have a baby hospitalized for COVID-19 during the first 6 months of life.
In addition, two doses of the Pfizer/BioNTech or Moderna COVID vaccine later in a pregnancy were linked to an even higher level of protection, 80%, compared with 32% when given before 20 weeks’ gestation.
This finding suggests a greater transfer of maternal antibodies closer to birth, but more research is needed, cautioned senior study author Manish Patel, MD, during a Tuesday media telebriefing held by the Centers for Disease Control and Prevention.
Unanswered questions include how the babies got infected or if there is any protection afforded to babies for women vaccinated before pregnancy.
“We cannot be sure about the source of the infection,” said Dr. Patel, a medical epidemiologist with the CDC COVID-19 Emergency Response Team.
Dana Meaney-Delman, MD, MPH, agreed, but added that “perinatal transmission of the virus is very rare” with SARS-CoV-2. She is a practicing obstetrician and gynecologist and chief of the CDC Infant Outcomes Monitoring Research and Prevention Branch.
The study numbers were too small to show if a booster shot during pregnancy or breastfeeding could provide even greater protection for babies, Dr. Patel said.
The early release study was published online Feb. 15 in the CDC’s Morbidity and Mortality Weekly Report (MMWR).
Many previous studies looking at COVID-19 immunization during pregnancy focused on maternal health and “have clearly shown that receiving an mRNA COVID-19 vaccine during pregnancy reduces the risk for severe illness,” Dr. Meaney-Delman said.
Some dual protection suggested
Now there is evidence for a potential benefit to babies as well when a pregnant woman gets vaccinated. The study “provides real-world evidence that getting COVID-19 vaccination during pregnancy might help protect infants less than 6 months [of age],” Dr. Meaney-Delman said.
“These findings continue to emphasize the importance of COVID-19 vaccination during pregnancy to protect people who are pregnant and also to protect their babies,” she said.
Dr. Patel and colleagues studied 379 infants younger than 6 months hospitalized between July 1, 2021 and Jan. 17 of this year. Delta and then the Omicron variant predominated during this time.
The infants were admitted to one of 20 children’s hospitals in 17 states. The researchers compared 176 infants admitted with a positive COVID-19 PCR test to another 203 infants with a negative PCR test who served as controls.
Half as many mothers of infants admitted with COVID-19 were vaccinated during pregnancy, 16%, versus 32% of mothers of the control infants.
Vaccination with two doses of mRNA vaccine during pregnancy was 61% effective (95% confidence interval, 31%-78%) at preventing hospitalization among these infants. Because the study was epidemiological, the lower risk was an association, not a cause-and-effect finding, Dr. Patel said.
Babies admitted to the hospital positive for COVID-19 were more likely to be non-Hispanic Black, 18%, versus 9% of control group babies; and more likely to be Hispanic, 34% versus 28%, respectively.
A total 24% of infants with COVID-19 were admitted to the ICU, including the baby of an unvaccinated mother who required extracorporeal membrane oxygenation (ECMO). Another baby of an unvaccinated mother was the only infant death during the study.
Maternal vaccination trends
A reporter pointed out that COVID-19 vaccination rates tend to be low among pregnant women. “So there is some exciting news,” Dr. Meaney-Delman said, referring to a steady increase in the percentages of pregnant women in the U.S. choosing to get vaccinated, according to the CDC Data Tracker website.
“The numbers are encouraging, [but] they’re not quite where we need them to be, and they do differ by race and ethnicity,” she added.
A version of this article first appeared on Medscape.com.
In a first of its kind study, researchers found women who received two mRNA COVID vaccine doses during pregnancy were 61% less likely to have a baby hospitalized for COVID-19 during the first 6 months of life.
In addition, two doses of the Pfizer/BioNTech or Moderna COVID vaccine later in a pregnancy were linked to an even higher level of protection, 80%, compared with 32% when given before 20 weeks’ gestation.
This finding suggests a greater transfer of maternal antibodies closer to birth, but more research is needed, cautioned senior study author Manish Patel, MD, during a Tuesday media telebriefing held by the Centers for Disease Control and Prevention.
Unanswered questions include how the babies got infected or if there is any protection afforded to babies for women vaccinated before pregnancy.
“We cannot be sure about the source of the infection,” said Dr. Patel, a medical epidemiologist with the CDC COVID-19 Emergency Response Team.
Dana Meaney-Delman, MD, MPH, agreed, but added that “perinatal transmission of the virus is very rare” with SARS-CoV-2. She is a practicing obstetrician and gynecologist and chief of the CDC Infant Outcomes Monitoring Research and Prevention Branch.
The study numbers were too small to show if a booster shot during pregnancy or breastfeeding could provide even greater protection for babies, Dr. Patel said.
The early release study was published online Feb. 15 in the CDC’s Morbidity and Mortality Weekly Report (MMWR).
Many previous studies looking at COVID-19 immunization during pregnancy focused on maternal health and “have clearly shown that receiving an mRNA COVID-19 vaccine during pregnancy reduces the risk for severe illness,” Dr. Meaney-Delman said.
Some dual protection suggested
Now there is evidence for a potential benefit to babies as well when a pregnant woman gets vaccinated. The study “provides real-world evidence that getting COVID-19 vaccination during pregnancy might help protect infants less than 6 months [of age],” Dr. Meaney-Delman said.
“These findings continue to emphasize the importance of COVID-19 vaccination during pregnancy to protect people who are pregnant and also to protect their babies,” she said.
Dr. Patel and colleagues studied 379 infants younger than 6 months hospitalized between July 1, 2021 and Jan. 17 of this year. Delta and then the Omicron variant predominated during this time.
The infants were admitted to one of 20 children’s hospitals in 17 states. The researchers compared 176 infants admitted with a positive COVID-19 PCR test to another 203 infants with a negative PCR test who served as controls.
Half as many mothers of infants admitted with COVID-19 were vaccinated during pregnancy, 16%, versus 32% of mothers of the control infants.
Vaccination with two doses of mRNA vaccine during pregnancy was 61% effective (95% confidence interval, 31%-78%) at preventing hospitalization among these infants. Because the study was epidemiological, the lower risk was an association, not a cause-and-effect finding, Dr. Patel said.
Babies admitted to the hospital positive for COVID-19 were more likely to be non-Hispanic Black, 18%, versus 9% of control group babies; and more likely to be Hispanic, 34% versus 28%, respectively.
A total 24% of infants with COVID-19 were admitted to the ICU, including the baby of an unvaccinated mother who required extracorporeal membrane oxygenation (ECMO). Another baby of an unvaccinated mother was the only infant death during the study.
Maternal vaccination trends
A reporter pointed out that COVID-19 vaccination rates tend to be low among pregnant women. “So there is some exciting news,” Dr. Meaney-Delman said, referring to a steady increase in the percentages of pregnant women in the U.S. choosing to get vaccinated, according to the CDC Data Tracker website.
“The numbers are encouraging, [but] they’re not quite where we need them to be, and they do differ by race and ethnicity,” she added.
A version of this article first appeared on Medscape.com.
In a first of its kind study, researchers found women who received two mRNA COVID vaccine doses during pregnancy were 61% less likely to have a baby hospitalized for COVID-19 during the first 6 months of life.
In addition, two doses of the Pfizer/BioNTech or Moderna COVID vaccine later in a pregnancy were linked to an even higher level of protection, 80%, compared with 32% when given before 20 weeks’ gestation.
This finding suggests a greater transfer of maternal antibodies closer to birth, but more research is needed, cautioned senior study author Manish Patel, MD, during a Tuesday media telebriefing held by the Centers for Disease Control and Prevention.
Unanswered questions include how the babies got infected or if there is any protection afforded to babies for women vaccinated before pregnancy.
“We cannot be sure about the source of the infection,” said Dr. Patel, a medical epidemiologist with the CDC COVID-19 Emergency Response Team.
Dana Meaney-Delman, MD, MPH, agreed, but added that “perinatal transmission of the virus is very rare” with SARS-CoV-2. She is a practicing obstetrician and gynecologist and chief of the CDC Infant Outcomes Monitoring Research and Prevention Branch.
The study numbers were too small to show if a booster shot during pregnancy or breastfeeding could provide even greater protection for babies, Dr. Patel said.
The early release study was published online Feb. 15 in the CDC’s Morbidity and Mortality Weekly Report (MMWR).
Many previous studies looking at COVID-19 immunization during pregnancy focused on maternal health and “have clearly shown that receiving an mRNA COVID-19 vaccine during pregnancy reduces the risk for severe illness,” Dr. Meaney-Delman said.
Some dual protection suggested
Now there is evidence for a potential benefit to babies as well when a pregnant woman gets vaccinated. The study “provides real-world evidence that getting COVID-19 vaccination during pregnancy might help protect infants less than 6 months [of age],” Dr. Meaney-Delman said.
“These findings continue to emphasize the importance of COVID-19 vaccination during pregnancy to protect people who are pregnant and also to protect their babies,” she said.
Dr. Patel and colleagues studied 379 infants younger than 6 months hospitalized between July 1, 2021 and Jan. 17 of this year. Delta and then the Omicron variant predominated during this time.
The infants were admitted to one of 20 children’s hospitals in 17 states. The researchers compared 176 infants admitted with a positive COVID-19 PCR test to another 203 infants with a negative PCR test who served as controls.
Half as many mothers of infants admitted with COVID-19 were vaccinated during pregnancy, 16%, versus 32% of mothers of the control infants.
Vaccination with two doses of mRNA vaccine during pregnancy was 61% effective (95% confidence interval, 31%-78%) at preventing hospitalization among these infants. Because the study was epidemiological, the lower risk was an association, not a cause-and-effect finding, Dr. Patel said.
Babies admitted to the hospital positive for COVID-19 were more likely to be non-Hispanic Black, 18%, versus 9% of control group babies; and more likely to be Hispanic, 34% versus 28%, respectively.
A total 24% of infants with COVID-19 were admitted to the ICU, including the baby of an unvaccinated mother who required extracorporeal membrane oxygenation (ECMO). Another baby of an unvaccinated mother was the only infant death during the study.
Maternal vaccination trends
A reporter pointed out that COVID-19 vaccination rates tend to be low among pregnant women. “So there is some exciting news,” Dr. Meaney-Delman said, referring to a steady increase in the percentages of pregnant women in the U.S. choosing to get vaccinated, according to the CDC Data Tracker website.
“The numbers are encouraging, [but] they’re not quite where we need them to be, and they do differ by race and ethnicity,” she added.
A version of this article first appeared on Medscape.com.
COVID vaccines open rifts between parents, children
The picture of rebellious teenagers sneaking “shots” has widened beyond breaking into Mom and Dad’s liquor cabinet. For some teens now, it means getting a COVID-19 vaccination without their parents’ consent – and, unlike the cabinet raids for the booze, they have adults willing to endorse the practice.
Since the U.S. Food and Drug Administration first granted emergency use authorization to Pfizer’s COVID-19 vaccine for teenagers in mid-2021, health officials have had to deal with a small subset of vaccine hesitancy where minors want the shot over the objections of their reluctant parents. The split has buoyed groups that were formed initially to convince teenagers to get vaccinated against other diseases.
When 14-year-old Arin Parsa of San Jose, California founded Teens for Vaccines in 2019 after a measles outbreak among unvaccinated children, “hardly anyone was interested,” he said. “Many teens were into climate change and other causes. Then, when the pandemic hit, so many were suddenly aware.”
Heavy toll on teens
Mr. Parsa’s parents fully supported Teens for Vaccines, he said, but he quickly found out how “politicized” COVID shots had become.
“We find people who are sad, angry, and frustrated at this stage of the pandemic,” he told this news organization. “The anti-vax lobby is riding the coat-tails of other movements. It has a very severe effect on their mental health. They can’t go out with their friends and socialize.”
In the pandemic’s initial stages, children were less likely to fall sick with COVID, but the Omicron variant led to a dramatic increase in illnesses among young people. The American Academy of Pediatrics has found that 3.5 million of the 11.4 million pediatric cases of the virus in the United States were reported in January 2022 alone. Meanwhile, vaccination rates for children aged 12-17, which were only 34% in June 2021 and lagged through the fall, are now at about 61% thanks to a sharp uptick during the Omicron surge, according to polling by the Kaiser Family Foundation.
No statistics are available on how many minors have received a COVID vaccine against their parents’ wishes.
“It’s not like there’s a big movement,” said Arthur Caplan, PhD, who heads the Division of Medical Ethics at the NYU Grossman School of Medicine. He said he noticed a divide around the HPV and hepatitis B vaccines. “They were tied up with sexual behavior,” he said, but “there were also some kids whose parents were really antivaxxers.”
Mr. Parsa said his and similar teen-oriented groups, such as VaxTeen, seek to educate their teen cohort, convince family members of the vaccines’ benefits, and to connect them with resources to get a shot. They also strive to change laws to make it easier for teenagers to receive the vaccine.
Consent laws vary from state to state (and within states), and proposed changes are afoot – some to loosen the laws and some to tighten them. Currently a 14-year-old in Alabama may get a COVID shot without parental permission, according to VaxTeen. In California, minors may receive the HPV shot without parental consent but not a COVID vaccine, although groups like Teens for Vaccines are pushing to change that. A bill now before the state legislature, the Teens Choose Vaccines Act (Senate Bill 866), would allow adolescents aged 12 and older to be able receive any FDA-approved vaccine – including COVID vaccines – without parental consent.
A second bill in California, the Keep Schools Open and Safe Act, would add the COVID-19 vaccines to the required list of immunizations needed to attend school in the state as well as eliminate the “personal belief” exemption against immunization.
California Sen. Richard Pan, MD (D-6th District), cowrote both bills with fellow Democrat Sen. Scott Wiener (D-11th District) and teen advocates from Teens for Vaccines and Generation Up, who helped draft the language in consultation with the lawmakers.
“As a pediatrician, I have seen all manner of situations where the requirement for a signed form has prevented teens from being able to get a vaccine that otherwise they and their guardians approved of them getting,” Dr. Pan told this news organization. “As a father, I don’t want to see my kids or any teen that wishes to protect themselves from deadly diseases unable to do so, particularly as we continue to fight off the dangers of the COVID-19 pandemic. I always encourage parents or teens that have questions about vaccines to speak directly with their pediatrician.”
Lawmakers in Philadelphia passed a provision last year to allow anyone age 11 or over to get the COVID vaccine without parental permission, keeping it in line with other vaccinations like hepatitis or HPV. “People from surrounding counties have come into the city, but it hasn’t been a huge rush,” says James Garrow, MPH, a spokesman for the city’s Department of Health.
Strive for collaboration, but listen to the children
Experts say the best solution is to for a doctor to meet with minors and their reluctant parents to get them on board for a COVID shot.
“Physicians are still the trusted messengers,” said Emma Olivera, MD, a pediatrician in suburban Chicago who advises groups that combat COVID misinformation.
Dr. Olivera said she often finds that internet-savvy teenagers have access to more information than older people, including their parents.
Thanks to COVID policies, office meetings are “difficult to do,” NYU’s Dr. Caplan added. In such a meeting, Dr. Caplan said he would try to convince the parents that the shots are needed for their children to stay in school or play sports. In the end, he said minors should get the shot but would also notify the parents before that happens: “My duty is to them.”
If parents take opposite stances, the pro-vaccine side is likely to prevail, even in California, said Patrick Baghdaserians, JD, a family law attorney in Pasadena. Mr. Baghdaserians said he is now representing a father who wants his teenager to get vaccinated but the mother doesn’t. “The court will fall on our side,” he predicted.
A version of this article first appeared on Medscape.com.
The picture of rebellious teenagers sneaking “shots” has widened beyond breaking into Mom and Dad’s liquor cabinet. For some teens now, it means getting a COVID-19 vaccination without their parents’ consent – and, unlike the cabinet raids for the booze, they have adults willing to endorse the practice.
Since the U.S. Food and Drug Administration first granted emergency use authorization to Pfizer’s COVID-19 vaccine for teenagers in mid-2021, health officials have had to deal with a small subset of vaccine hesitancy where minors want the shot over the objections of their reluctant parents. The split has buoyed groups that were formed initially to convince teenagers to get vaccinated against other diseases.
When 14-year-old Arin Parsa of San Jose, California founded Teens for Vaccines in 2019 after a measles outbreak among unvaccinated children, “hardly anyone was interested,” he said. “Many teens were into climate change and other causes. Then, when the pandemic hit, so many were suddenly aware.”
Heavy toll on teens
Mr. Parsa’s parents fully supported Teens for Vaccines, he said, but he quickly found out how “politicized” COVID shots had become.
“We find people who are sad, angry, and frustrated at this stage of the pandemic,” he told this news organization. “The anti-vax lobby is riding the coat-tails of other movements. It has a very severe effect on their mental health. They can’t go out with their friends and socialize.”
In the pandemic’s initial stages, children were less likely to fall sick with COVID, but the Omicron variant led to a dramatic increase in illnesses among young people. The American Academy of Pediatrics has found that 3.5 million of the 11.4 million pediatric cases of the virus in the United States were reported in January 2022 alone. Meanwhile, vaccination rates for children aged 12-17, which were only 34% in June 2021 and lagged through the fall, are now at about 61% thanks to a sharp uptick during the Omicron surge, according to polling by the Kaiser Family Foundation.
No statistics are available on how many minors have received a COVID vaccine against their parents’ wishes.
“It’s not like there’s a big movement,” said Arthur Caplan, PhD, who heads the Division of Medical Ethics at the NYU Grossman School of Medicine. He said he noticed a divide around the HPV and hepatitis B vaccines. “They were tied up with sexual behavior,” he said, but “there were also some kids whose parents were really antivaxxers.”
Mr. Parsa said his and similar teen-oriented groups, such as VaxTeen, seek to educate their teen cohort, convince family members of the vaccines’ benefits, and to connect them with resources to get a shot. They also strive to change laws to make it easier for teenagers to receive the vaccine.
Consent laws vary from state to state (and within states), and proposed changes are afoot – some to loosen the laws and some to tighten them. Currently a 14-year-old in Alabama may get a COVID shot without parental permission, according to VaxTeen. In California, minors may receive the HPV shot without parental consent but not a COVID vaccine, although groups like Teens for Vaccines are pushing to change that. A bill now before the state legislature, the Teens Choose Vaccines Act (Senate Bill 866), would allow adolescents aged 12 and older to be able receive any FDA-approved vaccine – including COVID vaccines – without parental consent.
A second bill in California, the Keep Schools Open and Safe Act, would add the COVID-19 vaccines to the required list of immunizations needed to attend school in the state as well as eliminate the “personal belief” exemption against immunization.
California Sen. Richard Pan, MD (D-6th District), cowrote both bills with fellow Democrat Sen. Scott Wiener (D-11th District) and teen advocates from Teens for Vaccines and Generation Up, who helped draft the language in consultation with the lawmakers.
“As a pediatrician, I have seen all manner of situations where the requirement for a signed form has prevented teens from being able to get a vaccine that otherwise they and their guardians approved of them getting,” Dr. Pan told this news organization. “As a father, I don’t want to see my kids or any teen that wishes to protect themselves from deadly diseases unable to do so, particularly as we continue to fight off the dangers of the COVID-19 pandemic. I always encourage parents or teens that have questions about vaccines to speak directly with their pediatrician.”
Lawmakers in Philadelphia passed a provision last year to allow anyone age 11 or over to get the COVID vaccine without parental permission, keeping it in line with other vaccinations like hepatitis or HPV. “People from surrounding counties have come into the city, but it hasn’t been a huge rush,” says James Garrow, MPH, a spokesman for the city’s Department of Health.
Strive for collaboration, but listen to the children
Experts say the best solution is to for a doctor to meet with minors and their reluctant parents to get them on board for a COVID shot.
“Physicians are still the trusted messengers,” said Emma Olivera, MD, a pediatrician in suburban Chicago who advises groups that combat COVID misinformation.
Dr. Olivera said she often finds that internet-savvy teenagers have access to more information than older people, including their parents.
Thanks to COVID policies, office meetings are “difficult to do,” NYU’s Dr. Caplan added. In such a meeting, Dr. Caplan said he would try to convince the parents that the shots are needed for their children to stay in school or play sports. In the end, he said minors should get the shot but would also notify the parents before that happens: “My duty is to them.”
If parents take opposite stances, the pro-vaccine side is likely to prevail, even in California, said Patrick Baghdaserians, JD, a family law attorney in Pasadena. Mr. Baghdaserians said he is now representing a father who wants his teenager to get vaccinated but the mother doesn’t. “The court will fall on our side,” he predicted.
A version of this article first appeared on Medscape.com.
The picture of rebellious teenagers sneaking “shots” has widened beyond breaking into Mom and Dad’s liquor cabinet. For some teens now, it means getting a COVID-19 vaccination without their parents’ consent – and, unlike the cabinet raids for the booze, they have adults willing to endorse the practice.
Since the U.S. Food and Drug Administration first granted emergency use authorization to Pfizer’s COVID-19 vaccine for teenagers in mid-2021, health officials have had to deal with a small subset of vaccine hesitancy where minors want the shot over the objections of their reluctant parents. The split has buoyed groups that were formed initially to convince teenagers to get vaccinated against other diseases.
When 14-year-old Arin Parsa of San Jose, California founded Teens for Vaccines in 2019 after a measles outbreak among unvaccinated children, “hardly anyone was interested,” he said. “Many teens were into climate change and other causes. Then, when the pandemic hit, so many were suddenly aware.”
Heavy toll on teens
Mr. Parsa’s parents fully supported Teens for Vaccines, he said, but he quickly found out how “politicized” COVID shots had become.
“We find people who are sad, angry, and frustrated at this stage of the pandemic,” he told this news organization. “The anti-vax lobby is riding the coat-tails of other movements. It has a very severe effect on their mental health. They can’t go out with their friends and socialize.”
In the pandemic’s initial stages, children were less likely to fall sick with COVID, but the Omicron variant led to a dramatic increase in illnesses among young people. The American Academy of Pediatrics has found that 3.5 million of the 11.4 million pediatric cases of the virus in the United States were reported in January 2022 alone. Meanwhile, vaccination rates for children aged 12-17, which were only 34% in June 2021 and lagged through the fall, are now at about 61% thanks to a sharp uptick during the Omicron surge, according to polling by the Kaiser Family Foundation.
No statistics are available on how many minors have received a COVID vaccine against their parents’ wishes.
“It’s not like there’s a big movement,” said Arthur Caplan, PhD, who heads the Division of Medical Ethics at the NYU Grossman School of Medicine. He said he noticed a divide around the HPV and hepatitis B vaccines. “They were tied up with sexual behavior,” he said, but “there were also some kids whose parents were really antivaxxers.”
Mr. Parsa said his and similar teen-oriented groups, such as VaxTeen, seek to educate their teen cohort, convince family members of the vaccines’ benefits, and to connect them with resources to get a shot. They also strive to change laws to make it easier for teenagers to receive the vaccine.
Consent laws vary from state to state (and within states), and proposed changes are afoot – some to loosen the laws and some to tighten them. Currently a 14-year-old in Alabama may get a COVID shot without parental permission, according to VaxTeen. In California, minors may receive the HPV shot without parental consent but not a COVID vaccine, although groups like Teens for Vaccines are pushing to change that. A bill now before the state legislature, the Teens Choose Vaccines Act (Senate Bill 866), would allow adolescents aged 12 and older to be able receive any FDA-approved vaccine – including COVID vaccines – without parental consent.
A second bill in California, the Keep Schools Open and Safe Act, would add the COVID-19 vaccines to the required list of immunizations needed to attend school in the state as well as eliminate the “personal belief” exemption against immunization.
California Sen. Richard Pan, MD (D-6th District), cowrote both bills with fellow Democrat Sen. Scott Wiener (D-11th District) and teen advocates from Teens for Vaccines and Generation Up, who helped draft the language in consultation with the lawmakers.
“As a pediatrician, I have seen all manner of situations where the requirement for a signed form has prevented teens from being able to get a vaccine that otherwise they and their guardians approved of them getting,” Dr. Pan told this news organization. “As a father, I don’t want to see my kids or any teen that wishes to protect themselves from deadly diseases unable to do so, particularly as we continue to fight off the dangers of the COVID-19 pandemic. I always encourage parents or teens that have questions about vaccines to speak directly with their pediatrician.”
Lawmakers in Philadelphia passed a provision last year to allow anyone age 11 or over to get the COVID vaccine without parental permission, keeping it in line with other vaccinations like hepatitis or HPV. “People from surrounding counties have come into the city, but it hasn’t been a huge rush,” says James Garrow, MPH, a spokesman for the city’s Department of Health.
Strive for collaboration, but listen to the children
Experts say the best solution is to for a doctor to meet with minors and their reluctant parents to get them on board for a COVID shot.
“Physicians are still the trusted messengers,” said Emma Olivera, MD, a pediatrician in suburban Chicago who advises groups that combat COVID misinformation.
Dr. Olivera said she often finds that internet-savvy teenagers have access to more information than older people, including their parents.
Thanks to COVID policies, office meetings are “difficult to do,” NYU’s Dr. Caplan added. In such a meeting, Dr. Caplan said he would try to convince the parents that the shots are needed for their children to stay in school or play sports. In the end, he said minors should get the shot but would also notify the parents before that happens: “My duty is to them.”
If parents take opposite stances, the pro-vaccine side is likely to prevail, even in California, said Patrick Baghdaserians, JD, a family law attorney in Pasadena. Mr. Baghdaserians said he is now representing a father who wants his teenager to get vaccinated but the mother doesn’t. “The court will fall on our side,” he predicted.
A version of this article first appeared on Medscape.com.
FDA delays action on Pfizer vaccine for kids under 5
for younger children until data on the effects of three doses is available.
Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the plan for a meeting the week of Feb. 14 of the FDA’s Vaccines and Related Biological Products Advisory Committee was to “understand if two doses would provide sufficient protection to move forward.”
Pfizer has asked the FDA to authorize the use of its mRNA vaccine for children under the age of 5. But, Dr. Marks said, “in looking through the data we realized now … that at this time it makes sense for us to wait until we have the data of the evaluation of a third dose before taking action.”
“If we feel something doesn’t meet (our) standard, we can’t go forward,” he said. “Rather than an issue of having anyone question the process, I hope this reassures people that the process has a standard.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, predicted in January that the Pfizer vaccine for younger kids could be available this month. But, he also predicted three doses would be required.
Pfizer announced in mid-December that it planned to submit data to the FDA during the first half of 2022 if the three-dose study was successful. At that time, Pfizer said it didn’t identify any safety concerns with the 3-microgram dose for children ages 6 months to 4 years, which is much lower than the 30-microgram dose given to adults.
A version of this article first appeared on WebMD.com.
for younger children until data on the effects of three doses is available.
Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the plan for a meeting the week of Feb. 14 of the FDA’s Vaccines and Related Biological Products Advisory Committee was to “understand if two doses would provide sufficient protection to move forward.”
Pfizer has asked the FDA to authorize the use of its mRNA vaccine for children under the age of 5. But, Dr. Marks said, “in looking through the data we realized now … that at this time it makes sense for us to wait until we have the data of the evaluation of a third dose before taking action.”
“If we feel something doesn’t meet (our) standard, we can’t go forward,” he said. “Rather than an issue of having anyone question the process, I hope this reassures people that the process has a standard.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, predicted in January that the Pfizer vaccine for younger kids could be available this month. But, he also predicted three doses would be required.
Pfizer announced in mid-December that it planned to submit data to the FDA during the first half of 2022 if the three-dose study was successful. At that time, Pfizer said it didn’t identify any safety concerns with the 3-microgram dose for children ages 6 months to 4 years, which is much lower than the 30-microgram dose given to adults.
A version of this article first appeared on WebMD.com.
for younger children until data on the effects of three doses is available.
Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the plan for a meeting the week of Feb. 14 of the FDA’s Vaccines and Related Biological Products Advisory Committee was to “understand if two doses would provide sufficient protection to move forward.”
Pfizer has asked the FDA to authorize the use of its mRNA vaccine for children under the age of 5. But, Dr. Marks said, “in looking through the data we realized now … that at this time it makes sense for us to wait until we have the data of the evaluation of a third dose before taking action.”
“If we feel something doesn’t meet (our) standard, we can’t go forward,” he said. “Rather than an issue of having anyone question the process, I hope this reassures people that the process has a standard.”
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, predicted in January that the Pfizer vaccine for younger kids could be available this month. But, he also predicted three doses would be required.
Pfizer announced in mid-December that it planned to submit data to the FDA during the first half of 2022 if the three-dose study was successful. At that time, Pfizer said it didn’t identify any safety concerns with the 3-microgram dose for children ages 6 months to 4 years, which is much lower than the 30-microgram dose given to adults.
A version of this article first appeared on WebMD.com.
Universal hepatitis B screening, vaccination deemed cost effective for pregnant women
Screening for hepatitis B antibodies and vaccinating pregnant women without immunity appears to be a cost-effective health measure, according to a recent analysis published in Obstetrics & Gynecology.
Malavika Prabhu, MD, of the division of maternal-fetal medicine and department of obstetrics and gynecology at Weill Cornell Medicine in New York, said in an interview that the impetus to conduct the study came from the idea that hepatitis B is a concern throughout a woman’s life, but not necessarily during pregnancy. While vaccination is not routine during pregnancy, guidelines from the American College of Obstetricians and Gynecologists state that at-risk women should be screened and vaccinated for hepatitis B during pregnancy.
“What we thought made more sense just from thinking about other principles of prenatal care was that it would make sense for us to screen, see who’s susceptible, counsel them on the risk of hepatitis B, and then vaccinate them in the course of the pregnancy,” Dr. Prabhu said.
After writing a commentary arguing in favor of universal screening and vaccination, she and her colleagues noted it was still unclear whether that approach was cost effective, she said. “Health care costs in this country are so expensive at baseline that, as we continue to add more things to health care, we have to make sure that it’s value added.”
Dr. Prabhu and her colleagues evaluated a theoretical cohort of 3.6 million pregnant women in the United States and created a decision-analytic model to determine how universal hepatitis B surface antibody screening and vaccination for hepatitis B affected factors such as cost, cost-effectiveness, and outcomes. They included hepatitis B virus cases as well as long-term problems associated with hepatitis B infection such as hepatocellular carcinoma, decompensated cirrhosis, liver transplant, and death. Assumptions of the model were that 84% of the women would undergo the screening, 61% would receive the vaccine, and 90% would seroconvert after the vaccine series, and were based on probabilities from other studies.
The cost-effectiveness ratio was calculated as the total cost and quality-adjusted life-years (QALYs) relative to the lifetime of the woman after the index pregnancy, with $50,000 per QALY set as the willingness-to-pay threshold. The researchers also performed an additional analysis and simulations to estimate which variables had the most effect, and an additional model was created to estimate the effect of universal screening and vaccination if at-risk patients were removed.
Dr. Prabhu and colleagues found the universal screening and vaccination program was cost effective, with 1,702 fewer cases of hepatitis B, 11 fewer deaths, 7 fewer decompensated cirrhosis cases, and 4 fewer liver transplants in their model. The incremental cost-effectiveness ratio was $1,890 per QALY, and the total increased lifetime cohort cost was $13,841,889. The researchers said the model held up in scenarios where there was a high level of hepatitis B immunity, and when at-risk women were removed from the model.
“While it does increase some costs to the health care system to screen everyone and vaccinate those susceptible; overall, it would cost more to not do that because we’re avoiding all of those long-term devastating health outcomes by vaccinating in pregnancy,” Dr. Prabhu said in an interview.
Hepatitis B screening and vaccination for all pregnant women?
Is universal hepatitis B screening and vaccination for pregnant women an upcoming change in prenatal care? In a related editorial, Martina L. Badell, MD, of the division of maternal-fetal medicine and department of gynecology and obstetrics at Emory University School of Medicine in Atlanta, emphasized the hepatitis B vaccine’s safety and effectiveness during pregnancy based on prior studies and compared a universal hepatitis B screening and vaccination program for pregnant women to how clinicians screen universally for rubella as standard of care in this group.
“Owing to the success of rubella vaccination campaigns, today there are fewer than 10 cases of rubella in the United States annually, and, since 2012, all of these cases have been in persons infected when living in or traveling to other countries,” she wrote. “Approximately 850,000 people are living with hepatitis B infection in the United States, and approximately 21,900 acute hepatitis B infections occurred in 2015. Despite the very different prevalence in these infections, we currently screen pregnant and nonpregnant patients for rubella immunity but not hepatitis B.”
If real-world studies bear out that a hepatitis B universal screening and vaccination program is cost effective, guidelines on who should be screened and vaccinated might need to be reconsidered, Dr. Prabhu said. Although following women for decades to see whether hepatitis B screening and vaccination is cost effective is impractical, “a lot of medicine has been predicated on risk-based strategies and risk stratifying, and there is a lot of value to approaching patients like that,” she explained.
How an ob.gyn. determines whether a patient is high risk and qualifies for hepatitis B vaccination under current guidelines is made more complicated by the large amount of information covered in a prenatal visit. There is a “laundry list” of risk factors to consider, and “patients are just meeting you for the first time, and so they may not feel comfortable completely sharing what their risk factors may or may not be,” Dr. Prabhu said. In addition, they may not know the risk factors of their partners.
Under guidelines where all pregnant women are screened and vaccinated for hepatitis B regardless of risk, “it doesn’t harm a woman to check one extra blood test when she’s already having this bevy of blood tests at the first prenatal visit,” she said.
Clinicians may be more aware of the need to add hepatitis B screening to prenatal care given that routine hepatitis C screening for pregnant women was recently released by ACOG as a practice advisory. “I think hepatitis is a little bit more on the forefront of the obstetrician or prenatal care provider’s mind as a result of that recent shift,” she said.
“A lot of women only really access care and access consistent care during their pregnancy, either due to insurance reasons or work reasons. People do things for their developing fetus that they might not do for themselves,” Dr. Prabhu said. “It’s a unique opportunity to have the time to build a relationship, build some trust in the health care system and also educate women about their health and what they can do to keep themselves in good health.
“It’s more than just about the next 9 months and keeping you and your baby safe, so I think there’s a real opportunity for us to think about the public health and the long-term health of a woman.”
One author reported receiving funding from UpToDate; the other authors reported no relevant financial disclosures. Dr. Badell reported no relevant financial disclosures.
Screening for hepatitis B antibodies and vaccinating pregnant women without immunity appears to be a cost-effective health measure, according to a recent analysis published in Obstetrics & Gynecology.
Malavika Prabhu, MD, of the division of maternal-fetal medicine and department of obstetrics and gynecology at Weill Cornell Medicine in New York, said in an interview that the impetus to conduct the study came from the idea that hepatitis B is a concern throughout a woman’s life, but not necessarily during pregnancy. While vaccination is not routine during pregnancy, guidelines from the American College of Obstetricians and Gynecologists state that at-risk women should be screened and vaccinated for hepatitis B during pregnancy.
“What we thought made more sense just from thinking about other principles of prenatal care was that it would make sense for us to screen, see who’s susceptible, counsel them on the risk of hepatitis B, and then vaccinate them in the course of the pregnancy,” Dr. Prabhu said.
After writing a commentary arguing in favor of universal screening and vaccination, she and her colleagues noted it was still unclear whether that approach was cost effective, she said. “Health care costs in this country are so expensive at baseline that, as we continue to add more things to health care, we have to make sure that it’s value added.”
Dr. Prabhu and her colleagues evaluated a theoretical cohort of 3.6 million pregnant women in the United States and created a decision-analytic model to determine how universal hepatitis B surface antibody screening and vaccination for hepatitis B affected factors such as cost, cost-effectiveness, and outcomes. They included hepatitis B virus cases as well as long-term problems associated with hepatitis B infection such as hepatocellular carcinoma, decompensated cirrhosis, liver transplant, and death. Assumptions of the model were that 84% of the women would undergo the screening, 61% would receive the vaccine, and 90% would seroconvert after the vaccine series, and were based on probabilities from other studies.
The cost-effectiveness ratio was calculated as the total cost and quality-adjusted life-years (QALYs) relative to the lifetime of the woman after the index pregnancy, with $50,000 per QALY set as the willingness-to-pay threshold. The researchers also performed an additional analysis and simulations to estimate which variables had the most effect, and an additional model was created to estimate the effect of universal screening and vaccination if at-risk patients were removed.
Dr. Prabhu and colleagues found the universal screening and vaccination program was cost effective, with 1,702 fewer cases of hepatitis B, 11 fewer deaths, 7 fewer decompensated cirrhosis cases, and 4 fewer liver transplants in their model. The incremental cost-effectiveness ratio was $1,890 per QALY, and the total increased lifetime cohort cost was $13,841,889. The researchers said the model held up in scenarios where there was a high level of hepatitis B immunity, and when at-risk women were removed from the model.
“While it does increase some costs to the health care system to screen everyone and vaccinate those susceptible; overall, it would cost more to not do that because we’re avoiding all of those long-term devastating health outcomes by vaccinating in pregnancy,” Dr. Prabhu said in an interview.
Hepatitis B screening and vaccination for all pregnant women?
Is universal hepatitis B screening and vaccination for pregnant women an upcoming change in prenatal care? In a related editorial, Martina L. Badell, MD, of the division of maternal-fetal medicine and department of gynecology and obstetrics at Emory University School of Medicine in Atlanta, emphasized the hepatitis B vaccine’s safety and effectiveness during pregnancy based on prior studies and compared a universal hepatitis B screening and vaccination program for pregnant women to how clinicians screen universally for rubella as standard of care in this group.
“Owing to the success of rubella vaccination campaigns, today there are fewer than 10 cases of rubella in the United States annually, and, since 2012, all of these cases have been in persons infected when living in or traveling to other countries,” she wrote. “Approximately 850,000 people are living with hepatitis B infection in the United States, and approximately 21,900 acute hepatitis B infections occurred in 2015. Despite the very different prevalence in these infections, we currently screen pregnant and nonpregnant patients for rubella immunity but not hepatitis B.”
If real-world studies bear out that a hepatitis B universal screening and vaccination program is cost effective, guidelines on who should be screened and vaccinated might need to be reconsidered, Dr. Prabhu said. Although following women for decades to see whether hepatitis B screening and vaccination is cost effective is impractical, “a lot of medicine has been predicated on risk-based strategies and risk stratifying, and there is a lot of value to approaching patients like that,” she explained.
How an ob.gyn. determines whether a patient is high risk and qualifies for hepatitis B vaccination under current guidelines is made more complicated by the large amount of information covered in a prenatal visit. There is a “laundry list” of risk factors to consider, and “patients are just meeting you for the first time, and so they may not feel comfortable completely sharing what their risk factors may or may not be,” Dr. Prabhu said. In addition, they may not know the risk factors of their partners.
Under guidelines where all pregnant women are screened and vaccinated for hepatitis B regardless of risk, “it doesn’t harm a woman to check one extra blood test when she’s already having this bevy of blood tests at the first prenatal visit,” she said.
Clinicians may be more aware of the need to add hepatitis B screening to prenatal care given that routine hepatitis C screening for pregnant women was recently released by ACOG as a practice advisory. “I think hepatitis is a little bit more on the forefront of the obstetrician or prenatal care provider’s mind as a result of that recent shift,” she said.
“A lot of women only really access care and access consistent care during their pregnancy, either due to insurance reasons or work reasons. People do things for their developing fetus that they might not do for themselves,” Dr. Prabhu said. “It’s a unique opportunity to have the time to build a relationship, build some trust in the health care system and also educate women about their health and what they can do to keep themselves in good health.
“It’s more than just about the next 9 months and keeping you and your baby safe, so I think there’s a real opportunity for us to think about the public health and the long-term health of a woman.”
One author reported receiving funding from UpToDate; the other authors reported no relevant financial disclosures. Dr. Badell reported no relevant financial disclosures.
Screening for hepatitis B antibodies and vaccinating pregnant women without immunity appears to be a cost-effective health measure, according to a recent analysis published in Obstetrics & Gynecology.
Malavika Prabhu, MD, of the division of maternal-fetal medicine and department of obstetrics and gynecology at Weill Cornell Medicine in New York, said in an interview that the impetus to conduct the study came from the idea that hepatitis B is a concern throughout a woman’s life, but not necessarily during pregnancy. While vaccination is not routine during pregnancy, guidelines from the American College of Obstetricians and Gynecologists state that at-risk women should be screened and vaccinated for hepatitis B during pregnancy.
“What we thought made more sense just from thinking about other principles of prenatal care was that it would make sense for us to screen, see who’s susceptible, counsel them on the risk of hepatitis B, and then vaccinate them in the course of the pregnancy,” Dr. Prabhu said.
After writing a commentary arguing in favor of universal screening and vaccination, she and her colleagues noted it was still unclear whether that approach was cost effective, she said. “Health care costs in this country are so expensive at baseline that, as we continue to add more things to health care, we have to make sure that it’s value added.”
Dr. Prabhu and her colleagues evaluated a theoretical cohort of 3.6 million pregnant women in the United States and created a decision-analytic model to determine how universal hepatitis B surface antibody screening and vaccination for hepatitis B affected factors such as cost, cost-effectiveness, and outcomes. They included hepatitis B virus cases as well as long-term problems associated with hepatitis B infection such as hepatocellular carcinoma, decompensated cirrhosis, liver transplant, and death. Assumptions of the model were that 84% of the women would undergo the screening, 61% would receive the vaccine, and 90% would seroconvert after the vaccine series, and were based on probabilities from other studies.
The cost-effectiveness ratio was calculated as the total cost and quality-adjusted life-years (QALYs) relative to the lifetime of the woman after the index pregnancy, with $50,000 per QALY set as the willingness-to-pay threshold. The researchers also performed an additional analysis and simulations to estimate which variables had the most effect, and an additional model was created to estimate the effect of universal screening and vaccination if at-risk patients were removed.
Dr. Prabhu and colleagues found the universal screening and vaccination program was cost effective, with 1,702 fewer cases of hepatitis B, 11 fewer deaths, 7 fewer decompensated cirrhosis cases, and 4 fewer liver transplants in their model. The incremental cost-effectiveness ratio was $1,890 per QALY, and the total increased lifetime cohort cost was $13,841,889. The researchers said the model held up in scenarios where there was a high level of hepatitis B immunity, and when at-risk women were removed from the model.
“While it does increase some costs to the health care system to screen everyone and vaccinate those susceptible; overall, it would cost more to not do that because we’re avoiding all of those long-term devastating health outcomes by vaccinating in pregnancy,” Dr. Prabhu said in an interview.
Hepatitis B screening and vaccination for all pregnant women?
Is universal hepatitis B screening and vaccination for pregnant women an upcoming change in prenatal care? In a related editorial, Martina L. Badell, MD, of the division of maternal-fetal medicine and department of gynecology and obstetrics at Emory University School of Medicine in Atlanta, emphasized the hepatitis B vaccine’s safety and effectiveness during pregnancy based on prior studies and compared a universal hepatitis B screening and vaccination program for pregnant women to how clinicians screen universally for rubella as standard of care in this group.
“Owing to the success of rubella vaccination campaigns, today there are fewer than 10 cases of rubella in the United States annually, and, since 2012, all of these cases have been in persons infected when living in or traveling to other countries,” she wrote. “Approximately 850,000 people are living with hepatitis B infection in the United States, and approximately 21,900 acute hepatitis B infections occurred in 2015. Despite the very different prevalence in these infections, we currently screen pregnant and nonpregnant patients for rubella immunity but not hepatitis B.”
If real-world studies bear out that a hepatitis B universal screening and vaccination program is cost effective, guidelines on who should be screened and vaccinated might need to be reconsidered, Dr. Prabhu said. Although following women for decades to see whether hepatitis B screening and vaccination is cost effective is impractical, “a lot of medicine has been predicated on risk-based strategies and risk stratifying, and there is a lot of value to approaching patients like that,” she explained.
How an ob.gyn. determines whether a patient is high risk and qualifies for hepatitis B vaccination under current guidelines is made more complicated by the large amount of information covered in a prenatal visit. There is a “laundry list” of risk factors to consider, and “patients are just meeting you for the first time, and so they may not feel comfortable completely sharing what their risk factors may or may not be,” Dr. Prabhu said. In addition, they may not know the risk factors of their partners.
Under guidelines where all pregnant women are screened and vaccinated for hepatitis B regardless of risk, “it doesn’t harm a woman to check one extra blood test when she’s already having this bevy of blood tests at the first prenatal visit,” she said.
Clinicians may be more aware of the need to add hepatitis B screening to prenatal care given that routine hepatitis C screening for pregnant women was recently released by ACOG as a practice advisory. “I think hepatitis is a little bit more on the forefront of the obstetrician or prenatal care provider’s mind as a result of that recent shift,” she said.
“A lot of women only really access care and access consistent care during their pregnancy, either due to insurance reasons or work reasons. People do things for their developing fetus that they might not do for themselves,” Dr. Prabhu said. “It’s a unique opportunity to have the time to build a relationship, build some trust in the health care system and also educate women about their health and what they can do to keep themselves in good health.
“It’s more than just about the next 9 months and keeping you and your baby safe, so I think there’s a real opportunity for us to think about the public health and the long-term health of a woman.”
One author reported receiving funding from UpToDate; the other authors reported no relevant financial disclosures. Dr. Badell reported no relevant financial disclosures.
FROM OBSTETRICS & GYNECOLOGY
Updated guidance for COVID vaccination in rheumatology patients arrives amid continued hesitancy
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
FROM ARTHRITIS & RHEUMATOLOGY
Childhood trauma may influence vaccine hesitancy
, data published Feb. 1 suggest.
The findings by Mark A. Bellis, DSc, College of Human Sciences, Bangor (Wales) University, and colleagues were published online in BMJ Open.
The results are especially significant, the authors say, because of the prevalence of adverse childhood experiences (ACEs) globally, with proportions of people having multiple traumas in some countries at 10% or more of the population.
The authors wrote that hesitancy or refusal to get the vaccine increased with the number of traumas reported.
For example, hesitancy was three times higher among people who had experienced four or more types of childhood trauma than among those who did not report any traumatic events.
Dr. Bellis told this news organization that though their work suggests that higher levels of ACEs are linked with higher vaccine hesitancy, it is by no means the only reason people choose not to get vaccinated.
However, he said, the association they found may have key messages for clinicians.
“For clinicians, simply being trauma informed can help,” Dr. Bellis said. “Understanding how such childhood adversity can affect people may help them when discussing vaccines, and in understanding resistance to what is a complex medical issue and one that requires considerable trust. What can appear routine to a clinician may be a difficult leap of faith especially for those who have poorer experiences of trusting even within family settings.”
More trauma, less trust
The authors used responses to a nationally representative telephone survey of adults in Wales taken between December 2020 and March 2021, when COVID-19 restrictions were in force. Out of 6,763 people contacted, 2,285 met all criteria and answered all the questions and were included in the final analysis.
The survey asked about nine types of ACEs before the age of 18, including: parental separation; physical, verbal, and sexual abuse; exposure to domestic violence; and living with a household member who has mental illness, misuses alcohol and/or drugs, or who was incarcerated.
It also included personal details and long-term health information.
About half of the respondents said they hadn’t experienced any childhood trauma. Of those who did, one in five said they had experienced one type, 17% reported two to three types, and 10% reported four or more.
According to the authors, prevalence of ACEs reported was consistent with other comparable population surveys, including those conducted face to face.
They also investigated measures of trust and preference for different health regulations.
People with more ACEs were more likely to have low trust in National Health Service COVID-19 information.
“Other sociodemographics and a history of either chronic disease or COVID-19 infection were not significantly associated with low trust,” the authors pointed out.
People reporting higher ACEs also were more likely to report that they felt they were unfairly restricted by the government. People with four or more ACEs were twice as likely than were those with no ACEs to say they felt unfairly restricted and wanted rules such as mandatory masking to stop.
People with four or more types of trauma were almost twice as likely to ignore the restrictions as were those who hadn’t experienced any – 38% versus 21% – to ignore the restrictions, even after the researchers accounted for associations with sociodemographic factors and previous COVID-19 infection or a history of long-term conditions.
“Clinicians can be a powerful voice to counter more alarmist or even conspiratorial messages that might otherwise resonate with those who find trust difficult,” Dr. Bellis said.
He said that the effect of childhood adversity needs to be considered at all levels in health systems. Overarching public health strategists should include ways to earn trust to counter resistance in some of the most vulnerable communities where ACEs can be higher.
It will also be important in the short-term to “provide reassurance, build community champions, and understand the low base from which trust needs to be built,” he said.
Loss of control
“Past traumatic experiences can predispose someone to avoid things that remind them of that trauma. This avoidance protects them from re-experiencing the negative symptoms and behaviors that come with it. Whether this results into hesitancy of something that would benefit their health is not well known,” Consuelo Cagande, MD, senior associate program director and fellowship adviser in the department of child and adolescent psychiatry and behavioral sciences, Children’s Hospital of Philadelphia, told this news organization.
She pointed out a limitation the authors mention that is common when using ACEs as a measure linking to future negative behaviors – that people self-report them and may misremember or misreport them.
Another limitation is the potential for self-selection bias, as participation level was 36.4%, though the authors noted that is not unusual for unsolicited telephone surveys.
Dr. Cagande said that fearing loss of control may be another factor at play in having to follow restrictions, such as quarantining and masking, social distancing, or mandated vaccinations.
She said it’s important to understand a person’s reason for hesitancy to vaccines and work with the person with the help of the community, to help them trust and feel safe.
Young adults of particular concern
The 18- to 29-year-old age group is of particular concern, Dr. Bellis said.
The researchers estimated the likely rates of vaccine hesitancy according to childhood trauma and age, and the numbers ranged from around 3.5% among those aged 70 and older with no experience of childhood adversity to 38% among 18- to 29-year-olds who had experienced four or more types of childhood trauma.
“Childhood adversity can be an especially raw issue in this group,” he explained. “Some have already been obliged to sacrifice substantial proportions of their teenage lives and some will have suffered greater exposure to adverse childhood experiences as a result of being isolated during the pandemic, sometimes in difficult home environments. Our results suggest that this age group and especially those with high levels of ACEs are some of the most likely to be vaccine hesitant.”
This work was supported by Public Health Wales. The study authors and Dr. Cagande reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, data published Feb. 1 suggest.
The findings by Mark A. Bellis, DSc, College of Human Sciences, Bangor (Wales) University, and colleagues were published online in BMJ Open.
The results are especially significant, the authors say, because of the prevalence of adverse childhood experiences (ACEs) globally, with proportions of people having multiple traumas in some countries at 10% or more of the population.
The authors wrote that hesitancy or refusal to get the vaccine increased with the number of traumas reported.
For example, hesitancy was three times higher among people who had experienced four or more types of childhood trauma than among those who did not report any traumatic events.
Dr. Bellis told this news organization that though their work suggests that higher levels of ACEs are linked with higher vaccine hesitancy, it is by no means the only reason people choose not to get vaccinated.
However, he said, the association they found may have key messages for clinicians.
“For clinicians, simply being trauma informed can help,” Dr. Bellis said. “Understanding how such childhood adversity can affect people may help them when discussing vaccines, and in understanding resistance to what is a complex medical issue and one that requires considerable trust. What can appear routine to a clinician may be a difficult leap of faith especially for those who have poorer experiences of trusting even within family settings.”
More trauma, less trust
The authors used responses to a nationally representative telephone survey of adults in Wales taken between December 2020 and March 2021, when COVID-19 restrictions were in force. Out of 6,763 people contacted, 2,285 met all criteria and answered all the questions and were included in the final analysis.
The survey asked about nine types of ACEs before the age of 18, including: parental separation; physical, verbal, and sexual abuse; exposure to domestic violence; and living with a household member who has mental illness, misuses alcohol and/or drugs, or who was incarcerated.
It also included personal details and long-term health information.
About half of the respondents said they hadn’t experienced any childhood trauma. Of those who did, one in five said they had experienced one type, 17% reported two to three types, and 10% reported four or more.
According to the authors, prevalence of ACEs reported was consistent with other comparable population surveys, including those conducted face to face.
They also investigated measures of trust and preference for different health regulations.
People with more ACEs were more likely to have low trust in National Health Service COVID-19 information.
“Other sociodemographics and a history of either chronic disease or COVID-19 infection were not significantly associated with low trust,” the authors pointed out.
People reporting higher ACEs also were more likely to report that they felt they were unfairly restricted by the government. People with four or more ACEs were twice as likely than were those with no ACEs to say they felt unfairly restricted and wanted rules such as mandatory masking to stop.
People with four or more types of trauma were almost twice as likely to ignore the restrictions as were those who hadn’t experienced any – 38% versus 21% – to ignore the restrictions, even after the researchers accounted for associations with sociodemographic factors and previous COVID-19 infection or a history of long-term conditions.
“Clinicians can be a powerful voice to counter more alarmist or even conspiratorial messages that might otherwise resonate with those who find trust difficult,” Dr. Bellis said.
He said that the effect of childhood adversity needs to be considered at all levels in health systems. Overarching public health strategists should include ways to earn trust to counter resistance in some of the most vulnerable communities where ACEs can be higher.
It will also be important in the short-term to “provide reassurance, build community champions, and understand the low base from which trust needs to be built,” he said.
Loss of control
“Past traumatic experiences can predispose someone to avoid things that remind them of that trauma. This avoidance protects them from re-experiencing the negative symptoms and behaviors that come with it. Whether this results into hesitancy of something that would benefit their health is not well known,” Consuelo Cagande, MD, senior associate program director and fellowship adviser in the department of child and adolescent psychiatry and behavioral sciences, Children’s Hospital of Philadelphia, told this news organization.
She pointed out a limitation the authors mention that is common when using ACEs as a measure linking to future negative behaviors – that people self-report them and may misremember or misreport them.
Another limitation is the potential for self-selection bias, as participation level was 36.4%, though the authors noted that is not unusual for unsolicited telephone surveys.
Dr. Cagande said that fearing loss of control may be another factor at play in having to follow restrictions, such as quarantining and masking, social distancing, or mandated vaccinations.
She said it’s important to understand a person’s reason for hesitancy to vaccines and work with the person with the help of the community, to help them trust and feel safe.
Young adults of particular concern
The 18- to 29-year-old age group is of particular concern, Dr. Bellis said.
The researchers estimated the likely rates of vaccine hesitancy according to childhood trauma and age, and the numbers ranged from around 3.5% among those aged 70 and older with no experience of childhood adversity to 38% among 18- to 29-year-olds who had experienced four or more types of childhood trauma.
“Childhood adversity can be an especially raw issue in this group,” he explained. “Some have already been obliged to sacrifice substantial proportions of their teenage lives and some will have suffered greater exposure to adverse childhood experiences as a result of being isolated during the pandemic, sometimes in difficult home environments. Our results suggest that this age group and especially those with high levels of ACEs are some of the most likely to be vaccine hesitant.”
This work was supported by Public Health Wales. The study authors and Dr. Cagande reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, data published Feb. 1 suggest.
The findings by Mark A. Bellis, DSc, College of Human Sciences, Bangor (Wales) University, and colleagues were published online in BMJ Open.
The results are especially significant, the authors say, because of the prevalence of adverse childhood experiences (ACEs) globally, with proportions of people having multiple traumas in some countries at 10% or more of the population.
The authors wrote that hesitancy or refusal to get the vaccine increased with the number of traumas reported.
For example, hesitancy was three times higher among people who had experienced four or more types of childhood trauma than among those who did not report any traumatic events.
Dr. Bellis told this news organization that though their work suggests that higher levels of ACEs are linked with higher vaccine hesitancy, it is by no means the only reason people choose not to get vaccinated.
However, he said, the association they found may have key messages for clinicians.
“For clinicians, simply being trauma informed can help,” Dr. Bellis said. “Understanding how such childhood adversity can affect people may help them when discussing vaccines, and in understanding resistance to what is a complex medical issue and one that requires considerable trust. What can appear routine to a clinician may be a difficult leap of faith especially for those who have poorer experiences of trusting even within family settings.”
More trauma, less trust
The authors used responses to a nationally representative telephone survey of adults in Wales taken between December 2020 and March 2021, when COVID-19 restrictions were in force. Out of 6,763 people contacted, 2,285 met all criteria and answered all the questions and were included in the final analysis.
The survey asked about nine types of ACEs before the age of 18, including: parental separation; physical, verbal, and sexual abuse; exposure to domestic violence; and living with a household member who has mental illness, misuses alcohol and/or drugs, or who was incarcerated.
It also included personal details and long-term health information.
About half of the respondents said they hadn’t experienced any childhood trauma. Of those who did, one in five said they had experienced one type, 17% reported two to three types, and 10% reported four or more.
According to the authors, prevalence of ACEs reported was consistent with other comparable population surveys, including those conducted face to face.
They also investigated measures of trust and preference for different health regulations.
People with more ACEs were more likely to have low trust in National Health Service COVID-19 information.
“Other sociodemographics and a history of either chronic disease or COVID-19 infection were not significantly associated with low trust,” the authors pointed out.
People reporting higher ACEs also were more likely to report that they felt they were unfairly restricted by the government. People with four or more ACEs were twice as likely than were those with no ACEs to say they felt unfairly restricted and wanted rules such as mandatory masking to stop.
People with four or more types of trauma were almost twice as likely to ignore the restrictions as were those who hadn’t experienced any – 38% versus 21% – to ignore the restrictions, even after the researchers accounted for associations with sociodemographic factors and previous COVID-19 infection or a history of long-term conditions.
“Clinicians can be a powerful voice to counter more alarmist or even conspiratorial messages that might otherwise resonate with those who find trust difficult,” Dr. Bellis said.
He said that the effect of childhood adversity needs to be considered at all levels in health systems. Overarching public health strategists should include ways to earn trust to counter resistance in some of the most vulnerable communities where ACEs can be higher.
It will also be important in the short-term to “provide reassurance, build community champions, and understand the low base from which trust needs to be built,” he said.
Loss of control
“Past traumatic experiences can predispose someone to avoid things that remind them of that trauma. This avoidance protects them from re-experiencing the negative symptoms and behaviors that come with it. Whether this results into hesitancy of something that would benefit their health is not well known,” Consuelo Cagande, MD, senior associate program director and fellowship adviser in the department of child and adolescent psychiatry and behavioral sciences, Children’s Hospital of Philadelphia, told this news organization.
She pointed out a limitation the authors mention that is common when using ACEs as a measure linking to future negative behaviors – that people self-report them and may misremember or misreport them.
Another limitation is the potential for self-selection bias, as participation level was 36.4%, though the authors noted that is not unusual for unsolicited telephone surveys.
Dr. Cagande said that fearing loss of control may be another factor at play in having to follow restrictions, such as quarantining and masking, social distancing, or mandated vaccinations.
She said it’s important to understand a person’s reason for hesitancy to vaccines and work with the person with the help of the community, to help them trust and feel safe.
Young adults of particular concern
The 18- to 29-year-old age group is of particular concern, Dr. Bellis said.
The researchers estimated the likely rates of vaccine hesitancy according to childhood trauma and age, and the numbers ranged from around 3.5% among those aged 70 and older with no experience of childhood adversity to 38% among 18- to 29-year-olds who had experienced four or more types of childhood trauma.
“Childhood adversity can be an especially raw issue in this group,” he explained. “Some have already been obliged to sacrifice substantial proportions of their teenage lives and some will have suffered greater exposure to adverse childhood experiences as a result of being isolated during the pandemic, sometimes in difficult home environments. Our results suggest that this age group and especially those with high levels of ACEs are some of the most likely to be vaccine hesitant.”
This work was supported by Public Health Wales. The study authors and Dr. Cagande reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
CDC issues new pneumococcal vaccine recommendations for adults
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
Moderna launches clinical trials for HIV vaccine
Human clinical trials have started for an experimental HIV vaccine that uses the same kind of mRNA technology found in Moderna’s successful COVID-19 vaccine, the drug company has announced.
The first vaccinations were given Jan. 27 at George Washington University School of Medicine and Health Sciences, Washington, the company said in a news release. Phase I trials will also be run at the Hope Clinic of Emory Vaccine Center, Atlanta, the Fred Hutchinson Cancer Research Center, Seattle, and the University of Texas Health Science Center, San Antonio.
The vaccine is designed to prompt white blood cells to turn into antibodies that can neutralize HIV, ABC News reported. A booster shot to work with the HIV vaccine is also being studied.
For 4 decades, the human immunodeficiency virus has managed to dodge the immune system’s attempts to destroy it. Scientists have not been able to develop a vaccine, though they have made advancements in treatments, such as long-acting injectables for pre- and post-exposure prevention and treatment. HIV can lead to AIDS, which can be fatal.
The release said 56 healthy HIV-negative adults are taking part in the clinical trial, with 48 getting one or two doses of the mRNA vaccine and 32 also getting the booster. Eight people will just get the booster. All of them will be monitored for up to 6 months after receiving a final dose.
The immunogens – antigens that elicit an immune response – that are being tested were developed by the International AIDS Vaccine Initiative (IAVI) and Scripps Research. They will be delivered using the same messenger RNA (mRNA) technology in Moderna’s successful COVID-19 vaccine, the news release said.
About 1.2 million people in the United States had HIV at the end of 2019, according to the CDC, with more than 36,000 people being diagnosed in 2019.
The World Health Organization says 37.7 million people in the world had HIV in 2020.
“We are tremendously excited to be advancing this new direction in HIV vaccine design with Moderna’s mRNA platform,” Mark Feinberg, MD, president and CEO of IAVI, said in the news release. “The search for an HIV vaccine has been long and challenging, and having new tools in terms of immunogens and platforms could be the key to making rapid progress toward an urgently needed, effective HIV vaccine.”
A version of this article first appeared on WebMD.com.
Human clinical trials have started for an experimental HIV vaccine that uses the same kind of mRNA technology found in Moderna’s successful COVID-19 vaccine, the drug company has announced.
The first vaccinations were given Jan. 27 at George Washington University School of Medicine and Health Sciences, Washington, the company said in a news release. Phase I trials will also be run at the Hope Clinic of Emory Vaccine Center, Atlanta, the Fred Hutchinson Cancer Research Center, Seattle, and the University of Texas Health Science Center, San Antonio.
The vaccine is designed to prompt white blood cells to turn into antibodies that can neutralize HIV, ABC News reported. A booster shot to work with the HIV vaccine is also being studied.
For 4 decades, the human immunodeficiency virus has managed to dodge the immune system’s attempts to destroy it. Scientists have not been able to develop a vaccine, though they have made advancements in treatments, such as long-acting injectables for pre- and post-exposure prevention and treatment. HIV can lead to AIDS, which can be fatal.
The release said 56 healthy HIV-negative adults are taking part in the clinical trial, with 48 getting one or two doses of the mRNA vaccine and 32 also getting the booster. Eight people will just get the booster. All of them will be monitored for up to 6 months after receiving a final dose.
The immunogens – antigens that elicit an immune response – that are being tested were developed by the International AIDS Vaccine Initiative (IAVI) and Scripps Research. They will be delivered using the same messenger RNA (mRNA) technology in Moderna’s successful COVID-19 vaccine, the news release said.
About 1.2 million people in the United States had HIV at the end of 2019, according to the CDC, with more than 36,000 people being diagnosed in 2019.
The World Health Organization says 37.7 million people in the world had HIV in 2020.
“We are tremendously excited to be advancing this new direction in HIV vaccine design with Moderna’s mRNA platform,” Mark Feinberg, MD, president and CEO of IAVI, said in the news release. “The search for an HIV vaccine has been long and challenging, and having new tools in terms of immunogens and platforms could be the key to making rapid progress toward an urgently needed, effective HIV vaccine.”
A version of this article first appeared on WebMD.com.
Human clinical trials have started for an experimental HIV vaccine that uses the same kind of mRNA technology found in Moderna’s successful COVID-19 vaccine, the drug company has announced.
The first vaccinations were given Jan. 27 at George Washington University School of Medicine and Health Sciences, Washington, the company said in a news release. Phase I trials will also be run at the Hope Clinic of Emory Vaccine Center, Atlanta, the Fred Hutchinson Cancer Research Center, Seattle, and the University of Texas Health Science Center, San Antonio.
The vaccine is designed to prompt white blood cells to turn into antibodies that can neutralize HIV, ABC News reported. A booster shot to work with the HIV vaccine is also being studied.
For 4 decades, the human immunodeficiency virus has managed to dodge the immune system’s attempts to destroy it. Scientists have not been able to develop a vaccine, though they have made advancements in treatments, such as long-acting injectables for pre- and post-exposure prevention and treatment. HIV can lead to AIDS, which can be fatal.
The release said 56 healthy HIV-negative adults are taking part in the clinical trial, with 48 getting one or two doses of the mRNA vaccine and 32 also getting the booster. Eight people will just get the booster. All of them will be monitored for up to 6 months after receiving a final dose.
The immunogens – antigens that elicit an immune response – that are being tested were developed by the International AIDS Vaccine Initiative (IAVI) and Scripps Research. They will be delivered using the same messenger RNA (mRNA) technology in Moderna’s successful COVID-19 vaccine, the news release said.
About 1.2 million people in the United States had HIV at the end of 2019, according to the CDC, with more than 36,000 people being diagnosed in 2019.
The World Health Organization says 37.7 million people in the world had HIV in 2020.
“We are tremendously excited to be advancing this new direction in HIV vaccine design with Moderna’s mRNA platform,” Mark Feinberg, MD, president and CEO of IAVI, said in the news release. “The search for an HIV vaccine has been long and challenging, and having new tools in terms of immunogens and platforms could be the key to making rapid progress toward an urgently needed, effective HIV vaccine.”
A version of this article first appeared on WebMD.com.
Immunocompromised patients should receive fourth COVID shot: CDC
The Centers for Disease Control and Prevention contacted pharmacies on Jan. 26 to reinforce the message that people with moderate to severe immune suppression should receive a fourth COVID-19 vaccine, according to Kaiser Health News.
The conference call came a day after the news outlet reported that immunocompromised people were being turned away by pharmacies. White House officials also emphasized on Jan. 26 that immunocompromised people should receive an additional shot.
During the call, the CDC “reiterated the recommendations, running through case examples,” Mitchel Rothholz, RPh, MBA, chief of governance and state affiliates for the American Pharmacists Association, told KHN.
While on the call, Mr. Rothholz asked for a “prepared document” with the CDC’s recommendations “so we can clearly and consistently communicate the message.” The CDC officials on the call said they would create a document but “don’t know how long that will take,” Mr. Rothholz told KHN.
The CDC recommends an additional shot -– or a fourth shot – for those who have weak immune systems, which makes them more at risk for severe COVID-19 and death. About 7 million American adults are considered immunocompromised, KHN reported, which includes people who have certain medical conditions that impair their immune response or who take immune-suppressing drugs because of organ transplants, cancer, or autoimmune diseases.
The CDC first recommended fourth shots for immunocompromised people in October. This month, the CDC shortened the time for booster shots from 6 months to 5 months, and some immunocompromised people who are due for another shot have begun to seek them. The agency has been educating pharmacists and other health providers since then, a CDC spokesperson told KHN.
While patients don’t need to provide proof that they are immunocompromised, according to the CDC, some have been turned away, KHN reported.
To improve communication with the public, large pharmacies could issue news releases and update their websites “explicitly stating that they are offering fourth doses” to immunocompromised people, Ameet Kini, MD, a professor of pathology and laboratory medicine at Loyola University Medical Center in Chicago, told KHN.
Pharmacies should also update their patient portals and provide “clear guidance for their pharmacists,” he said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention contacted pharmacies on Jan. 26 to reinforce the message that people with moderate to severe immune suppression should receive a fourth COVID-19 vaccine, according to Kaiser Health News.
The conference call came a day after the news outlet reported that immunocompromised people were being turned away by pharmacies. White House officials also emphasized on Jan. 26 that immunocompromised people should receive an additional shot.
During the call, the CDC “reiterated the recommendations, running through case examples,” Mitchel Rothholz, RPh, MBA, chief of governance and state affiliates for the American Pharmacists Association, told KHN.
While on the call, Mr. Rothholz asked for a “prepared document” with the CDC’s recommendations “so we can clearly and consistently communicate the message.” The CDC officials on the call said they would create a document but “don’t know how long that will take,” Mr. Rothholz told KHN.
The CDC recommends an additional shot -– or a fourth shot – for those who have weak immune systems, which makes them more at risk for severe COVID-19 and death. About 7 million American adults are considered immunocompromised, KHN reported, which includes people who have certain medical conditions that impair their immune response or who take immune-suppressing drugs because of organ transplants, cancer, or autoimmune diseases.
The CDC first recommended fourth shots for immunocompromised people in October. This month, the CDC shortened the time for booster shots from 6 months to 5 months, and some immunocompromised people who are due for another shot have begun to seek them. The agency has been educating pharmacists and other health providers since then, a CDC spokesperson told KHN.
While patients don’t need to provide proof that they are immunocompromised, according to the CDC, some have been turned away, KHN reported.
To improve communication with the public, large pharmacies could issue news releases and update their websites “explicitly stating that they are offering fourth doses” to immunocompromised people, Ameet Kini, MD, a professor of pathology and laboratory medicine at Loyola University Medical Center in Chicago, told KHN.
Pharmacies should also update their patient portals and provide “clear guidance for their pharmacists,” he said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention contacted pharmacies on Jan. 26 to reinforce the message that people with moderate to severe immune suppression should receive a fourth COVID-19 vaccine, according to Kaiser Health News.
The conference call came a day after the news outlet reported that immunocompromised people were being turned away by pharmacies. White House officials also emphasized on Jan. 26 that immunocompromised people should receive an additional shot.
During the call, the CDC “reiterated the recommendations, running through case examples,” Mitchel Rothholz, RPh, MBA, chief of governance and state affiliates for the American Pharmacists Association, told KHN.
While on the call, Mr. Rothholz asked for a “prepared document” with the CDC’s recommendations “so we can clearly and consistently communicate the message.” The CDC officials on the call said they would create a document but “don’t know how long that will take,” Mr. Rothholz told KHN.
The CDC recommends an additional shot -– or a fourth shot – for those who have weak immune systems, which makes them more at risk for severe COVID-19 and death. About 7 million American adults are considered immunocompromised, KHN reported, which includes people who have certain medical conditions that impair their immune response or who take immune-suppressing drugs because of organ transplants, cancer, or autoimmune diseases.
The CDC first recommended fourth shots for immunocompromised people in October. This month, the CDC shortened the time for booster shots from 6 months to 5 months, and some immunocompromised people who are due for another shot have begun to seek them. The agency has been educating pharmacists and other health providers since then, a CDC spokesperson told KHN.
While patients don’t need to provide proof that they are immunocompromised, according to the CDC, some have been turned away, KHN reported.
To improve communication with the public, large pharmacies could issue news releases and update their websites “explicitly stating that they are offering fourth doses” to immunocompromised people, Ameet Kini, MD, a professor of pathology and laboratory medicine at Loyola University Medical Center in Chicago, told KHN.
Pharmacies should also update their patient portals and provide “clear guidance for their pharmacists,” he said.
A version of this article first appeared on WebMD.com.
Rituximab and COVID-19 vaccines: Studies begin to answer key questions
Rituximab has presented something of a conundrum for patients taking the monoclonal antibody during the COVID-19 pandemic.
Used to manage a variety of autoimmune diseases and cancers, rituximab acts against CD20 proteins expressed on the surface of B cells, causing B-cell depletion. However, it is this B-cell depletion that may put these patients at greater risk of COVID-19 development, progression to more severe disease, and in-hospital mortality. Evidence for this appears to be mixed, with studies showing both that patients using rituximab to manage various diseases are and are not at increased risk for SARS-CoV-2 infection, COVID-19 progression, and mortality.
As COVID-19 vaccine rollouts take place across the world, more questions have been raised about the relationship between B-cell depletion from anti-CD20 therapies and COVID-19 vaccines. Do rituximab and other anti-CD20 therapies affect a patient’s response to COVID-19 vaccines? If this is the case, does the timing of anti-CD20 treatment matter to maximize B-cell levels and improve the vaccine’s effectiveness? And how do COVID-19 vaccine booster doses factor into the equation?
Humoral and cell-mediated responses following COVID-19 vaccination
First, the bad news: The vaccine is unquestionably safe to administer in patients taking rituximab, but one thing that has been well established is that antibody response to COVID-19 vaccination in these individuals does is reduced. This isn’t entirely unprecedented, as previous studies have shown a weakened immune response to pneumococcal polysaccharide and keyhole limpet hemocyanin vaccines among patients taking rituximab.
“Compromised immunogenicity to the SARS-CoV-2 vaccines has been demonstrated in rituximab-treated patients, which is of particular concern given the observation that B-cell–depleting therapies may be associated with worse COVID outcomes,” Robert F. Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York, said in an interview.
For example, in a recent study from the Medical University of Vienna, 29 (39%) of 74 patients receiving rituximab (43% as monotherapy, 57% with conventional-synthetic disease-modifying antirheumatic drugs) who were vaccinated with either the Comirnaty (Pfizer-BioNTech) or Spikevax (Moderna) COVID-19 vaccine achieved seroconversion, compared with 100% of patients in a healthy control group, and all but 1 patient without detectable CD19+ peripheral B cells did not develop anti–SARS-CoV-2 receptor-binding domain antibodies.
“There is an increasing number of studies in this field, and they confirm that patients treated with rituximab and other anti-CD20 agents have severely reduced serological responses to COVID-19 vaccines,” Ingrid Jyssum, MD, of the division of rheumatology and research at Diakonhjemmet Hospital in Oslo, said in an interview.
One silver lining is that patients treated with anti-CD20 therapies appear to have a cell-mediated response following vaccination even if they don’t develop SARS-CoV-2 antibodies. “Studies that also investigate T-cell responses are starting to emerge, and so far, they show that, even if the patients do not have antibodies, they may have T-cell responses,” Dr. Jyssum said.
One study of 24 patients with autoimmune diseases taking rituximab that evaluated humoral and T-cell responses following vaccination with the Comirnaty vaccine found that none had a humoral response to the vaccine, but the T-cell response from that group did not significantly differ from 35 patients receiving other immunosuppressants and 26 patients in a healthy control group. In another study of rituximab- or ocrelizumab-treated patients who received mRNA-based COVID-19 vaccines, 69.4% developed SARS-CoV-2–specific antibodies, compared with a control group, but 96.2% of patients taking ocrelizumab and 81.8% of patients taking rituximab mounted a spike-specific CD8+ T-cell response, compared with 66.7% in the control group, and there were comparable rates (85%-90%) of spike-specific CD4+ T cells in all groups. In the study from the Medical University of Vienna, T-cell response was detected in rituximab-treated patients who both did and did not mount an antibody response.
The clinical relevance of how a blunted humoral immune response but a respectable T-cell response to COVID-19 vaccines affects patients treated with anti-CD20 therapies isn’t currently known, Dr. Jyssum said.
While these data are reassuring, they’re also incomplete, Dr. Spiera noted. “The ultimate outcome of relevance to assess vaccine efficacy is protection from COVID and from severe outcomes of COVID infection (i.e., hospitalization, mechanical ventilation, death). That data will require assessment of very large numbers of rituximab-treated vaccinated patients to be compared with rituximab-treated unvaccinated patients, and is unlikely to be forthcoming in the very near future.
“In the meantime, however, achieving serologic positivity, meaning having evidence of serologic as well as cellular immunity following vaccination, is a desired outcome, and likely implies more robust immunity.”
Does treatment timing impact COVID-19 vaccine response?
Given enough time, B-cell reconstitution will occur in patients taking rituximab. With that in mind, is it beneficial to wait a certain amount of time after a patient has stopped rituximab therapy or time since their last dose before giving them a COVID-19 vaccine? In their guidance on COVID-19 vaccines for patients with rheumatic and musculoskeletal diseases, the American College of Rheumatology said there is moderate evidence to consider “optimal timing of dosing and vaccination with the rheumatology provider before proceeding.”
“Guidelines and preliminary studies of serologic response to COVID vaccine in rituximab-treated patients have suggested that longer time from last rituximab exposure is associated with a greater likelihood of a serologic response,” Dr. Spiera said.
In a brief report published in Arthritis & Rheumatology, Dr. Spiera and colleagues performed a retrospective chart review of 56 patients with varying levels of last exposure to rituximab who received a COVID-19 vaccine. Their results showed that, when patients were vaccinated 6-12 months after the last rituximab dose, 55% were seronegative, and when this was more than 12 months, only 13% were seronegative, compared with seronegativity in 86% who were vaccinated less than 6 months after their last rituximab dose.
The RituxiVac trial, conducted by researchers in Switzerland, also examined vaccine responses of 96 rituximab-treated patients who received Comirnaty or Spikevax; results recently published in The Lancet Rheumatology showed findings similar to other studies, with reduced humoral and cell-mediated responses. In the RituxiVac trial, the median time to last anti-CD20 treatment was 1.07 years.
“The typical interval between rituximab doses [for treatment of rheumatoid arthritis, as well as for remission maintenance in antineutrophil cytoplasmic antibody–associated vasculitis] is typically 6 months, and this has become widely used as the interval from last rituximab to time of COVID vaccination, with a recommendation to wait 4 weeks (if possible) from time of vaccination until the next rituximab administration,” Dr. Spiera explained. However, this window seems to vary depending on the study.
Recent research published in Arthritis & Rheumatology indicates B-cell levels could be a relevant indicator for humoral and cell-mediated response in patients with rheumatic diseases treated with rituximab, with a level of 10 B cells/mcL (0.4% of lymphocytes) identified as one potential marker for likely seroconversion following COVID-19 vaccination.
“In some smaller case series, it has been further recognized that rituximab-treated patients who were beginning to reconstitute peripheral B cells were most likely to respond serologically. Our present study confirmed those findings, demonstrating that the presence of detectable B cells was strongly associated with vaccine responsiveness, and affords complementary information to time from last [rituximab dose] in informing the likelihood of a vaccine response,” Dr. Spiera said.
However, the literature is limited in this area, and an exact cutoff for B-cell counts in these patients isn’t currently known, Dr. Jyssum said. A better metric is time away from anti-CD20 therapies, with CD19 cell count being highly correlated with last infusion.
Dr. Spiera agreed that there is no consistent B-cell percentage that works as a cutoff. “In our study, we looked at it as a binary variable, although we did find that a higher percentage of B cells in the peripheral lymphocyte population was associated with a higher likelihood of seroconversion. We did not, however, identify a ‘threshold’ for vaccine serologic responsiveness.”
Should clinicians measure antibodies?
The Food and Drug Administration and the Centers for Disease Control and Prevention have recommended that health care providers and the public not use COVID-19 antibody tests as a way to gauge immunity after exposure to SARS-CoV-2 and after receiving a COVID-19 vaccination. The ACR’s guidance on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases strongly recommends against ordering antibody tests for patients with autoimmune inflammatory rheumatic diseases as a way to measure immunity.
“Generally, such measurements are not recommended as the clinical correlate of various antibody levels are not known,” Dr. Jyssum said. “With regular infusions of rituximab or other anti-CD20 agents, one cannot expect that these patients will develop significant levels of antibodies.”
However, she said there might be situations where it’s useful to know whether a patient has developed antibodies at all. “Assessing the significance of specific antibody levels is difficult, and the subject of scientific studies. Patients lacking a humoral vaccine response are left to rely on their T-cell responses and on infectious control measures to prevent disease.”
Dr. Spiera said he disagreed with guidelines recommending against checking antibody levels after vaccination, “particularly in patients treated with immunosuppressive medications that might be expected to blunt their serologic response to the vaccines.
“Although we cannot be sure what level of measurable antibodies offer what level of protection, most clinicians would agree that patients who demonstrate no detectable antibodies (which is a common finding in rituximab-treated patients) should be considered at higher risk,” he said. “Indeed, recommendations regarding booster vaccine administration in general was initially based on the observation of declining antibody levels with longer time from vaccination.”
Do COVID-19 vaccine boosters help patients on anti-CD20 therapy?
As of January 2022, the FDA and CDC have recommended a third primary series shot of COVID-19 vaccines for some moderately to severely immunocompromised patients as young as 5 years old (for Comirnaty vaccine) or a booster shot of either Comirnaty or Spikevax for everyone aged 12 years and older, including immunocompromised people, while the ACR goes into more detail and recommends clinicians time a patient’s booster shot with temporary treatment interruption.
In The Lancet Rheumatology, Dr. Jyssum and colleagues recently published results from the prospective Nor-vaC study examining the humoral and cell-mediated immune responses of 87 patients with RA being treated with rituximab who received the Comirnaty, Spikevax, or Vaxzevria (AstraZeneca) COVID-19 vaccines; of these, 49 patients received a booster dose at a median of 70 days after completing their primary series. The results showed 19 patients (28.1%) had a serologic response after their primary series, while 8 of 49 patients (16.3%) who received their booster dose had a serologic response.
All patients who received a third dose in the study had a T-cell response, Dr. Jyssum said. “This is reassuring for patients and clinicians. T cells have been found to be important in countering COVID-19 disease, but whether we can rely on the T-cell response alone in the absence of antibodies to protect patients from infection or from serious COVID disease is still not determined,” she said.
When asked if she would recommend COVID-19 vaccine booster doses for patients on rituximab, Dr. Jyssum replied: “Absolutely.”
Another study, recently published in Annals of the Rheumatic Diseases, examined heterologous and homologous booster doses for 60 patients receiving rituximab without seroconversion after their COVID-19 vaccine primary series. The results showed no significant difference in new seroconversion at 4 weeks based on whether the patient received a vector or mRNA vaccine (22% vs. 32%), but all patients who received a booster dose with a vector vaccine had specific T-cell responses, compared with 81% of patients who received an mRNA vaccine booster. There was a new humoral and/or cellular response in 9 of 11 patients (82%), and most patients with peripheral B cells (12 of 18 patients; 67%) achieved seroconversion.
“Our data show that a cellular and/or humoral immune response can be achieved on a third COVID-19 vaccination in most of the patients who initially developed neither a humoral nor a cellular immune response,” the researchers concluded. “The efficacy data together with the safety data seen in our trial provide a favorable risk/benefit ratio and support the implementation of a third vaccination for nonseroconverted high-risk autoimmune disease patients treated with B-cell–depleting agents.”
Dr. Spiera said booster doses are an important part of the equation, and “it is important to consider factors that would be associated with a greater likelihood of achieving a serologic response, particularly in those patients who did not demonstrate a serologic response to the initial vaccines series.
“Preliminary data shows that the beginnings of B-cell reconstitution is also associated with a positive serologic response following a booster of the COVID-19 vaccine,” he said.
The authors of the cited studies reported numerous relevant financial disclosures. Dr. Spiera and Dr. Jyssum reported no relevant financial disclosures.
Rituximab has presented something of a conundrum for patients taking the monoclonal antibody during the COVID-19 pandemic.
Used to manage a variety of autoimmune diseases and cancers, rituximab acts against CD20 proteins expressed on the surface of B cells, causing B-cell depletion. However, it is this B-cell depletion that may put these patients at greater risk of COVID-19 development, progression to more severe disease, and in-hospital mortality. Evidence for this appears to be mixed, with studies showing both that patients using rituximab to manage various diseases are and are not at increased risk for SARS-CoV-2 infection, COVID-19 progression, and mortality.
As COVID-19 vaccine rollouts take place across the world, more questions have been raised about the relationship between B-cell depletion from anti-CD20 therapies and COVID-19 vaccines. Do rituximab and other anti-CD20 therapies affect a patient’s response to COVID-19 vaccines? If this is the case, does the timing of anti-CD20 treatment matter to maximize B-cell levels and improve the vaccine’s effectiveness? And how do COVID-19 vaccine booster doses factor into the equation?
Humoral and cell-mediated responses following COVID-19 vaccination
First, the bad news: The vaccine is unquestionably safe to administer in patients taking rituximab, but one thing that has been well established is that antibody response to COVID-19 vaccination in these individuals does is reduced. This isn’t entirely unprecedented, as previous studies have shown a weakened immune response to pneumococcal polysaccharide and keyhole limpet hemocyanin vaccines among patients taking rituximab.
“Compromised immunogenicity to the SARS-CoV-2 vaccines has been demonstrated in rituximab-treated patients, which is of particular concern given the observation that B-cell–depleting therapies may be associated with worse COVID outcomes,” Robert F. Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York, said in an interview.
For example, in a recent study from the Medical University of Vienna, 29 (39%) of 74 patients receiving rituximab (43% as monotherapy, 57% with conventional-synthetic disease-modifying antirheumatic drugs) who were vaccinated with either the Comirnaty (Pfizer-BioNTech) or Spikevax (Moderna) COVID-19 vaccine achieved seroconversion, compared with 100% of patients in a healthy control group, and all but 1 patient without detectable CD19+ peripheral B cells did not develop anti–SARS-CoV-2 receptor-binding domain antibodies.
“There is an increasing number of studies in this field, and they confirm that patients treated with rituximab and other anti-CD20 agents have severely reduced serological responses to COVID-19 vaccines,” Ingrid Jyssum, MD, of the division of rheumatology and research at Diakonhjemmet Hospital in Oslo, said in an interview.
One silver lining is that patients treated with anti-CD20 therapies appear to have a cell-mediated response following vaccination even if they don’t develop SARS-CoV-2 antibodies. “Studies that also investigate T-cell responses are starting to emerge, and so far, they show that, even if the patients do not have antibodies, they may have T-cell responses,” Dr. Jyssum said.
One study of 24 patients with autoimmune diseases taking rituximab that evaluated humoral and T-cell responses following vaccination with the Comirnaty vaccine found that none had a humoral response to the vaccine, but the T-cell response from that group did not significantly differ from 35 patients receiving other immunosuppressants and 26 patients in a healthy control group. In another study of rituximab- or ocrelizumab-treated patients who received mRNA-based COVID-19 vaccines, 69.4% developed SARS-CoV-2–specific antibodies, compared with a control group, but 96.2% of patients taking ocrelizumab and 81.8% of patients taking rituximab mounted a spike-specific CD8+ T-cell response, compared with 66.7% in the control group, and there were comparable rates (85%-90%) of spike-specific CD4+ T cells in all groups. In the study from the Medical University of Vienna, T-cell response was detected in rituximab-treated patients who both did and did not mount an antibody response.
The clinical relevance of how a blunted humoral immune response but a respectable T-cell response to COVID-19 vaccines affects patients treated with anti-CD20 therapies isn’t currently known, Dr. Jyssum said.
While these data are reassuring, they’re also incomplete, Dr. Spiera noted. “The ultimate outcome of relevance to assess vaccine efficacy is protection from COVID and from severe outcomes of COVID infection (i.e., hospitalization, mechanical ventilation, death). That data will require assessment of very large numbers of rituximab-treated vaccinated patients to be compared with rituximab-treated unvaccinated patients, and is unlikely to be forthcoming in the very near future.
“In the meantime, however, achieving serologic positivity, meaning having evidence of serologic as well as cellular immunity following vaccination, is a desired outcome, and likely implies more robust immunity.”
Does treatment timing impact COVID-19 vaccine response?
Given enough time, B-cell reconstitution will occur in patients taking rituximab. With that in mind, is it beneficial to wait a certain amount of time after a patient has stopped rituximab therapy or time since their last dose before giving them a COVID-19 vaccine? In their guidance on COVID-19 vaccines for patients with rheumatic and musculoskeletal diseases, the American College of Rheumatology said there is moderate evidence to consider “optimal timing of dosing and vaccination with the rheumatology provider before proceeding.”
“Guidelines and preliminary studies of serologic response to COVID vaccine in rituximab-treated patients have suggested that longer time from last rituximab exposure is associated with a greater likelihood of a serologic response,” Dr. Spiera said.
In a brief report published in Arthritis & Rheumatology, Dr. Spiera and colleagues performed a retrospective chart review of 56 patients with varying levels of last exposure to rituximab who received a COVID-19 vaccine. Their results showed that, when patients were vaccinated 6-12 months after the last rituximab dose, 55% were seronegative, and when this was more than 12 months, only 13% were seronegative, compared with seronegativity in 86% who were vaccinated less than 6 months after their last rituximab dose.
The RituxiVac trial, conducted by researchers in Switzerland, also examined vaccine responses of 96 rituximab-treated patients who received Comirnaty or Spikevax; results recently published in The Lancet Rheumatology showed findings similar to other studies, with reduced humoral and cell-mediated responses. In the RituxiVac trial, the median time to last anti-CD20 treatment was 1.07 years.
“The typical interval between rituximab doses [for treatment of rheumatoid arthritis, as well as for remission maintenance in antineutrophil cytoplasmic antibody–associated vasculitis] is typically 6 months, and this has become widely used as the interval from last rituximab to time of COVID vaccination, with a recommendation to wait 4 weeks (if possible) from time of vaccination until the next rituximab administration,” Dr. Spiera explained. However, this window seems to vary depending on the study.
Recent research published in Arthritis & Rheumatology indicates B-cell levels could be a relevant indicator for humoral and cell-mediated response in patients with rheumatic diseases treated with rituximab, with a level of 10 B cells/mcL (0.4% of lymphocytes) identified as one potential marker for likely seroconversion following COVID-19 vaccination.
“In some smaller case series, it has been further recognized that rituximab-treated patients who were beginning to reconstitute peripheral B cells were most likely to respond serologically. Our present study confirmed those findings, demonstrating that the presence of detectable B cells was strongly associated with vaccine responsiveness, and affords complementary information to time from last [rituximab dose] in informing the likelihood of a vaccine response,” Dr. Spiera said.
However, the literature is limited in this area, and an exact cutoff for B-cell counts in these patients isn’t currently known, Dr. Jyssum said. A better metric is time away from anti-CD20 therapies, with CD19 cell count being highly correlated with last infusion.
Dr. Spiera agreed that there is no consistent B-cell percentage that works as a cutoff. “In our study, we looked at it as a binary variable, although we did find that a higher percentage of B cells in the peripheral lymphocyte population was associated with a higher likelihood of seroconversion. We did not, however, identify a ‘threshold’ for vaccine serologic responsiveness.”
Should clinicians measure antibodies?
The Food and Drug Administration and the Centers for Disease Control and Prevention have recommended that health care providers and the public not use COVID-19 antibody tests as a way to gauge immunity after exposure to SARS-CoV-2 and after receiving a COVID-19 vaccination. The ACR’s guidance on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases strongly recommends against ordering antibody tests for patients with autoimmune inflammatory rheumatic diseases as a way to measure immunity.
“Generally, such measurements are not recommended as the clinical correlate of various antibody levels are not known,” Dr. Jyssum said. “With regular infusions of rituximab or other anti-CD20 agents, one cannot expect that these patients will develop significant levels of antibodies.”
However, she said there might be situations where it’s useful to know whether a patient has developed antibodies at all. “Assessing the significance of specific antibody levels is difficult, and the subject of scientific studies. Patients lacking a humoral vaccine response are left to rely on their T-cell responses and on infectious control measures to prevent disease.”
Dr. Spiera said he disagreed with guidelines recommending against checking antibody levels after vaccination, “particularly in patients treated with immunosuppressive medications that might be expected to blunt their serologic response to the vaccines.
“Although we cannot be sure what level of measurable antibodies offer what level of protection, most clinicians would agree that patients who demonstrate no detectable antibodies (which is a common finding in rituximab-treated patients) should be considered at higher risk,” he said. “Indeed, recommendations regarding booster vaccine administration in general was initially based on the observation of declining antibody levels with longer time from vaccination.”
Do COVID-19 vaccine boosters help patients on anti-CD20 therapy?
As of January 2022, the FDA and CDC have recommended a third primary series shot of COVID-19 vaccines for some moderately to severely immunocompromised patients as young as 5 years old (for Comirnaty vaccine) or a booster shot of either Comirnaty or Spikevax for everyone aged 12 years and older, including immunocompromised people, while the ACR goes into more detail and recommends clinicians time a patient’s booster shot with temporary treatment interruption.
In The Lancet Rheumatology, Dr. Jyssum and colleagues recently published results from the prospective Nor-vaC study examining the humoral and cell-mediated immune responses of 87 patients with RA being treated with rituximab who received the Comirnaty, Spikevax, or Vaxzevria (AstraZeneca) COVID-19 vaccines; of these, 49 patients received a booster dose at a median of 70 days after completing their primary series. The results showed 19 patients (28.1%) had a serologic response after their primary series, while 8 of 49 patients (16.3%) who received their booster dose had a serologic response.
All patients who received a third dose in the study had a T-cell response, Dr. Jyssum said. “This is reassuring for patients and clinicians. T cells have been found to be important in countering COVID-19 disease, but whether we can rely on the T-cell response alone in the absence of antibodies to protect patients from infection or from serious COVID disease is still not determined,” she said.
When asked if she would recommend COVID-19 vaccine booster doses for patients on rituximab, Dr. Jyssum replied: “Absolutely.”
Another study, recently published in Annals of the Rheumatic Diseases, examined heterologous and homologous booster doses for 60 patients receiving rituximab without seroconversion after their COVID-19 vaccine primary series. The results showed no significant difference in new seroconversion at 4 weeks based on whether the patient received a vector or mRNA vaccine (22% vs. 32%), but all patients who received a booster dose with a vector vaccine had specific T-cell responses, compared with 81% of patients who received an mRNA vaccine booster. There was a new humoral and/or cellular response in 9 of 11 patients (82%), and most patients with peripheral B cells (12 of 18 patients; 67%) achieved seroconversion.
“Our data show that a cellular and/or humoral immune response can be achieved on a third COVID-19 vaccination in most of the patients who initially developed neither a humoral nor a cellular immune response,” the researchers concluded. “The efficacy data together with the safety data seen in our trial provide a favorable risk/benefit ratio and support the implementation of a third vaccination for nonseroconverted high-risk autoimmune disease patients treated with B-cell–depleting agents.”
Dr. Spiera said booster doses are an important part of the equation, and “it is important to consider factors that would be associated with a greater likelihood of achieving a serologic response, particularly in those patients who did not demonstrate a serologic response to the initial vaccines series.
“Preliminary data shows that the beginnings of B-cell reconstitution is also associated with a positive serologic response following a booster of the COVID-19 vaccine,” he said.
The authors of the cited studies reported numerous relevant financial disclosures. Dr. Spiera and Dr. Jyssum reported no relevant financial disclosures.
Rituximab has presented something of a conundrum for patients taking the monoclonal antibody during the COVID-19 pandemic.
Used to manage a variety of autoimmune diseases and cancers, rituximab acts against CD20 proteins expressed on the surface of B cells, causing B-cell depletion. However, it is this B-cell depletion that may put these patients at greater risk of COVID-19 development, progression to more severe disease, and in-hospital mortality. Evidence for this appears to be mixed, with studies showing both that patients using rituximab to manage various diseases are and are not at increased risk for SARS-CoV-2 infection, COVID-19 progression, and mortality.
As COVID-19 vaccine rollouts take place across the world, more questions have been raised about the relationship between B-cell depletion from anti-CD20 therapies and COVID-19 vaccines. Do rituximab and other anti-CD20 therapies affect a patient’s response to COVID-19 vaccines? If this is the case, does the timing of anti-CD20 treatment matter to maximize B-cell levels and improve the vaccine’s effectiveness? And how do COVID-19 vaccine booster doses factor into the equation?
Humoral and cell-mediated responses following COVID-19 vaccination
First, the bad news: The vaccine is unquestionably safe to administer in patients taking rituximab, but one thing that has been well established is that antibody response to COVID-19 vaccination in these individuals does is reduced. This isn’t entirely unprecedented, as previous studies have shown a weakened immune response to pneumococcal polysaccharide and keyhole limpet hemocyanin vaccines among patients taking rituximab.
“Compromised immunogenicity to the SARS-CoV-2 vaccines has been demonstrated in rituximab-treated patients, which is of particular concern given the observation that B-cell–depleting therapies may be associated with worse COVID outcomes,” Robert F. Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York, said in an interview.
For example, in a recent study from the Medical University of Vienna, 29 (39%) of 74 patients receiving rituximab (43% as monotherapy, 57% with conventional-synthetic disease-modifying antirheumatic drugs) who were vaccinated with either the Comirnaty (Pfizer-BioNTech) or Spikevax (Moderna) COVID-19 vaccine achieved seroconversion, compared with 100% of patients in a healthy control group, and all but 1 patient without detectable CD19+ peripheral B cells did not develop anti–SARS-CoV-2 receptor-binding domain antibodies.
“There is an increasing number of studies in this field, and they confirm that patients treated with rituximab and other anti-CD20 agents have severely reduced serological responses to COVID-19 vaccines,” Ingrid Jyssum, MD, of the division of rheumatology and research at Diakonhjemmet Hospital in Oslo, said in an interview.
One silver lining is that patients treated with anti-CD20 therapies appear to have a cell-mediated response following vaccination even if they don’t develop SARS-CoV-2 antibodies. “Studies that also investigate T-cell responses are starting to emerge, and so far, they show that, even if the patients do not have antibodies, they may have T-cell responses,” Dr. Jyssum said.
One study of 24 patients with autoimmune diseases taking rituximab that evaluated humoral and T-cell responses following vaccination with the Comirnaty vaccine found that none had a humoral response to the vaccine, but the T-cell response from that group did not significantly differ from 35 patients receiving other immunosuppressants and 26 patients in a healthy control group. In another study of rituximab- or ocrelizumab-treated patients who received mRNA-based COVID-19 vaccines, 69.4% developed SARS-CoV-2–specific antibodies, compared with a control group, but 96.2% of patients taking ocrelizumab and 81.8% of patients taking rituximab mounted a spike-specific CD8+ T-cell response, compared with 66.7% in the control group, and there were comparable rates (85%-90%) of spike-specific CD4+ T cells in all groups. In the study from the Medical University of Vienna, T-cell response was detected in rituximab-treated patients who both did and did not mount an antibody response.
The clinical relevance of how a blunted humoral immune response but a respectable T-cell response to COVID-19 vaccines affects patients treated with anti-CD20 therapies isn’t currently known, Dr. Jyssum said.
While these data are reassuring, they’re also incomplete, Dr. Spiera noted. “The ultimate outcome of relevance to assess vaccine efficacy is protection from COVID and from severe outcomes of COVID infection (i.e., hospitalization, mechanical ventilation, death). That data will require assessment of very large numbers of rituximab-treated vaccinated patients to be compared with rituximab-treated unvaccinated patients, and is unlikely to be forthcoming in the very near future.
“In the meantime, however, achieving serologic positivity, meaning having evidence of serologic as well as cellular immunity following vaccination, is a desired outcome, and likely implies more robust immunity.”
Does treatment timing impact COVID-19 vaccine response?
Given enough time, B-cell reconstitution will occur in patients taking rituximab. With that in mind, is it beneficial to wait a certain amount of time after a patient has stopped rituximab therapy or time since their last dose before giving them a COVID-19 vaccine? In their guidance on COVID-19 vaccines for patients with rheumatic and musculoskeletal diseases, the American College of Rheumatology said there is moderate evidence to consider “optimal timing of dosing and vaccination with the rheumatology provider before proceeding.”
“Guidelines and preliminary studies of serologic response to COVID vaccine in rituximab-treated patients have suggested that longer time from last rituximab exposure is associated with a greater likelihood of a serologic response,” Dr. Spiera said.
In a brief report published in Arthritis & Rheumatology, Dr. Spiera and colleagues performed a retrospective chart review of 56 patients with varying levels of last exposure to rituximab who received a COVID-19 vaccine. Their results showed that, when patients were vaccinated 6-12 months after the last rituximab dose, 55% were seronegative, and when this was more than 12 months, only 13% were seronegative, compared with seronegativity in 86% who were vaccinated less than 6 months after their last rituximab dose.
The RituxiVac trial, conducted by researchers in Switzerland, also examined vaccine responses of 96 rituximab-treated patients who received Comirnaty or Spikevax; results recently published in The Lancet Rheumatology showed findings similar to other studies, with reduced humoral and cell-mediated responses. In the RituxiVac trial, the median time to last anti-CD20 treatment was 1.07 years.
“The typical interval between rituximab doses [for treatment of rheumatoid arthritis, as well as for remission maintenance in antineutrophil cytoplasmic antibody–associated vasculitis] is typically 6 months, and this has become widely used as the interval from last rituximab to time of COVID vaccination, with a recommendation to wait 4 weeks (if possible) from time of vaccination until the next rituximab administration,” Dr. Spiera explained. However, this window seems to vary depending on the study.
Recent research published in Arthritis & Rheumatology indicates B-cell levels could be a relevant indicator for humoral and cell-mediated response in patients with rheumatic diseases treated with rituximab, with a level of 10 B cells/mcL (0.4% of lymphocytes) identified as one potential marker for likely seroconversion following COVID-19 vaccination.
“In some smaller case series, it has been further recognized that rituximab-treated patients who were beginning to reconstitute peripheral B cells were most likely to respond serologically. Our present study confirmed those findings, demonstrating that the presence of detectable B cells was strongly associated with vaccine responsiveness, and affords complementary information to time from last [rituximab dose] in informing the likelihood of a vaccine response,” Dr. Spiera said.
However, the literature is limited in this area, and an exact cutoff for B-cell counts in these patients isn’t currently known, Dr. Jyssum said. A better metric is time away from anti-CD20 therapies, with CD19 cell count being highly correlated with last infusion.
Dr. Spiera agreed that there is no consistent B-cell percentage that works as a cutoff. “In our study, we looked at it as a binary variable, although we did find that a higher percentage of B cells in the peripheral lymphocyte population was associated with a higher likelihood of seroconversion. We did not, however, identify a ‘threshold’ for vaccine serologic responsiveness.”
Should clinicians measure antibodies?
The Food and Drug Administration and the Centers for Disease Control and Prevention have recommended that health care providers and the public not use COVID-19 antibody tests as a way to gauge immunity after exposure to SARS-CoV-2 and after receiving a COVID-19 vaccination. The ACR’s guidance on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases strongly recommends against ordering antibody tests for patients with autoimmune inflammatory rheumatic diseases as a way to measure immunity.
“Generally, such measurements are not recommended as the clinical correlate of various antibody levels are not known,” Dr. Jyssum said. “With regular infusions of rituximab or other anti-CD20 agents, one cannot expect that these patients will develop significant levels of antibodies.”
However, she said there might be situations where it’s useful to know whether a patient has developed antibodies at all. “Assessing the significance of specific antibody levels is difficult, and the subject of scientific studies. Patients lacking a humoral vaccine response are left to rely on their T-cell responses and on infectious control measures to prevent disease.”
Dr. Spiera said he disagreed with guidelines recommending against checking antibody levels after vaccination, “particularly in patients treated with immunosuppressive medications that might be expected to blunt their serologic response to the vaccines.
“Although we cannot be sure what level of measurable antibodies offer what level of protection, most clinicians would agree that patients who demonstrate no detectable antibodies (which is a common finding in rituximab-treated patients) should be considered at higher risk,” he said. “Indeed, recommendations regarding booster vaccine administration in general was initially based on the observation of declining antibody levels with longer time from vaccination.”
Do COVID-19 vaccine boosters help patients on anti-CD20 therapy?
As of January 2022, the FDA and CDC have recommended a third primary series shot of COVID-19 vaccines for some moderately to severely immunocompromised patients as young as 5 years old (for Comirnaty vaccine) or a booster shot of either Comirnaty or Spikevax for everyone aged 12 years and older, including immunocompromised people, while the ACR goes into more detail and recommends clinicians time a patient’s booster shot with temporary treatment interruption.
In The Lancet Rheumatology, Dr. Jyssum and colleagues recently published results from the prospective Nor-vaC study examining the humoral and cell-mediated immune responses of 87 patients with RA being treated with rituximab who received the Comirnaty, Spikevax, or Vaxzevria (AstraZeneca) COVID-19 vaccines; of these, 49 patients received a booster dose at a median of 70 days after completing their primary series. The results showed 19 patients (28.1%) had a serologic response after their primary series, while 8 of 49 patients (16.3%) who received their booster dose had a serologic response.
All patients who received a third dose in the study had a T-cell response, Dr. Jyssum said. “This is reassuring for patients and clinicians. T cells have been found to be important in countering COVID-19 disease, but whether we can rely on the T-cell response alone in the absence of antibodies to protect patients from infection or from serious COVID disease is still not determined,” she said.
When asked if she would recommend COVID-19 vaccine booster doses for patients on rituximab, Dr. Jyssum replied: “Absolutely.”
Another study, recently published in Annals of the Rheumatic Diseases, examined heterologous and homologous booster doses for 60 patients receiving rituximab without seroconversion after their COVID-19 vaccine primary series. The results showed no significant difference in new seroconversion at 4 weeks based on whether the patient received a vector or mRNA vaccine (22% vs. 32%), but all patients who received a booster dose with a vector vaccine had specific T-cell responses, compared with 81% of patients who received an mRNA vaccine booster. There was a new humoral and/or cellular response in 9 of 11 patients (82%), and most patients with peripheral B cells (12 of 18 patients; 67%) achieved seroconversion.
“Our data show that a cellular and/or humoral immune response can be achieved on a third COVID-19 vaccination in most of the patients who initially developed neither a humoral nor a cellular immune response,” the researchers concluded. “The efficacy data together with the safety data seen in our trial provide a favorable risk/benefit ratio and support the implementation of a third vaccination for nonseroconverted high-risk autoimmune disease patients treated with B-cell–depleting agents.”
Dr. Spiera said booster doses are an important part of the equation, and “it is important to consider factors that would be associated with a greater likelihood of achieving a serologic response, particularly in those patients who did not demonstrate a serologic response to the initial vaccines series.
“Preliminary data shows that the beginnings of B-cell reconstitution is also associated with a positive serologic response following a booster of the COVID-19 vaccine,” he said.
The authors of the cited studies reported numerous relevant financial disclosures. Dr. Spiera and Dr. Jyssum reported no relevant financial disclosures.