User login
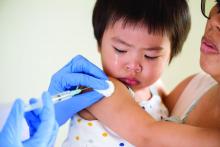
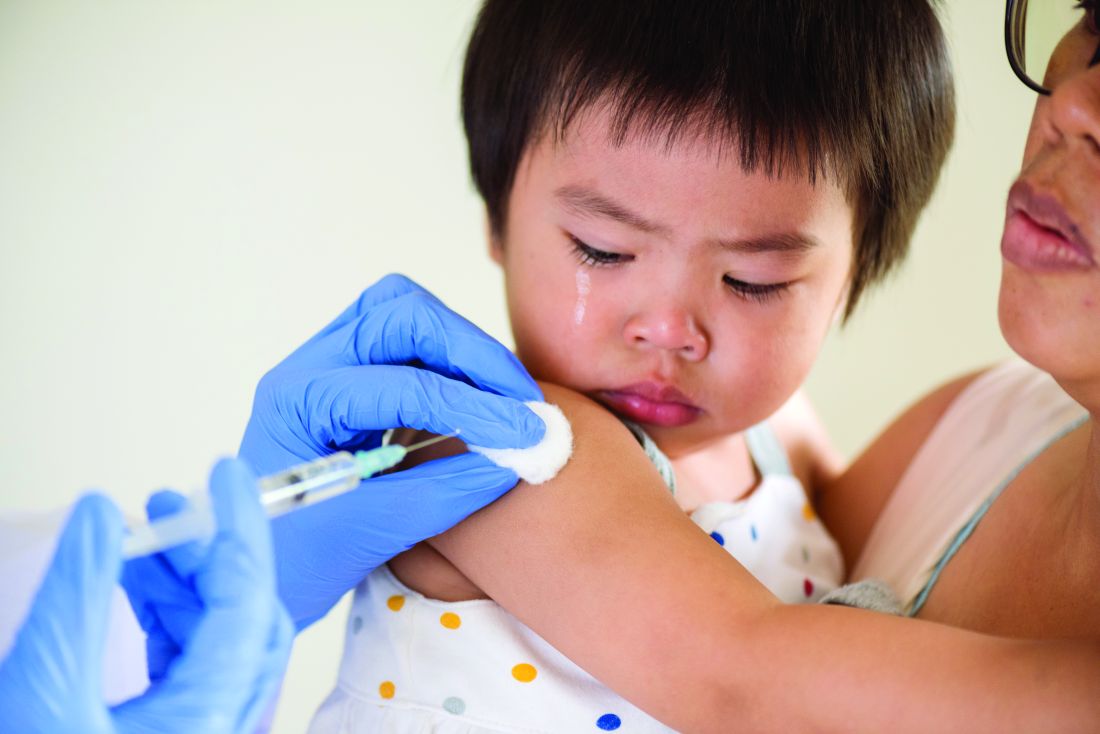
Standing orders for vaccines may improve pediatric vaccination rates
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
The biggest barrier to using standing orders for childhood immunizations is concern that patients will receive the wrong vaccine, according to a survey of pediatricians published in Pediatrics.
The other top reasons pediatricians give for not using standing orders for vaccines are concerns that parents may want to talk to the doctor about the vaccine before their child gets it, and a belief that the doctor should be the one who personally recommends a vaccine for their patient.
But with severe drops in vaccination rates resulting from the COVID-19 pandemic, standing orders may be a valuable tool for ensuring children get their vaccines on time, suggested lead author, Jessica Cataldi, MD, of the University of Colorado and Children’s Hospital Colorado in Aurora.
“As we work to bring more families back to their pediatrician’s office for well-child checks, standing orders are one process that can streamline the visit by saving providers time and increasing vaccine delivery,” she said in an interview. “We will also need use standing orders to support different ways to get children their immunizations during times of social distancing. This could take the form of drive-through immunization clinics or telehealth well-child checks that are paired with a quick immunization-only visit.”
The American Academy of Pediatrics issued guidance April 14 that emphasizes the need to prioritize immunization of children through 2-years-old.
Paul A. Offit, MD, director of the Vaccine Education Center and an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, agreed that it’s essential children do not fall behind on the recommended schedule during the pandemic.
“It’s important not to have greater collateral damage from this COVID-19 pandemic by putting children at increased risk from other infections that are circulating, like measles and pertussis,” he said, noting that nearly 1,300 measles cases and more than 15,000 pertussis cases occurred in the United States in 2019.
It’s important “not to delay those primary vaccines because it’s hard to catch up,” he said in an interview
Although “standing orders” may go by other names in non–inpatient settings, the researchers defined them in their survey as “a written or verbal policy that persons other than a medical provider, such as a nurse or medical assistant, may consent and vaccinate a person without speaking with the physician or advanced care provider first.” Further, the “vaccine may be given before or after a physician encounter or in the absence of a physician encounter altogether.”
Research strongly suggests that standing orders for childhood vaccines are cost-effective and increase immunization rates, the authors noted. The Centers for Disease Control and Prevention, its Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the federal National Vaccine Advisory Committee all recommend using standing orders to improve vaccination access and rates.
The authors sought to understand how many pediatricians use standing orders and what reasons stop them from doing so. During June-September 2017, they sent out 471 online and mail surveys to a nationally representative sample of AAP members who spent at least half their time in primary care.
The 372 pediatricians who completed the survey made up a response rate of 79%, with no differences in response based on age, sex, years in practice, practice setting, region or rural/urban location.
More than half the respondents (59%) used standing orders for childhood immunizations. Just over a third of respondents (36%) said they use standing orders for all routinely recommended vaccines, and 23% use them for some vaccines.
Among those who did not use standing orders, 68% cited the concern that patients would get the incorrect vaccine by mistake as a barrier to using them. That came as a surprise to Dr Offit, who would expect standing orders to reduce the likelihood of error.
“The standing order should make things a little more foolproof so that you’re less likely to make a mistake,” Dr Offit said.
No studies have shown that vaccine errors occur more often in clinics that use standing orders for immunizations, but the question merits continued monitoring, Dr Cataldi said.
“It is important for any clinic that is new to the use of standing orders to provide adequate education to providers and other staff about when and how to use standing orders, and to always leave room for staff to bring vaccination questions to the provider,” Dr Cataldi told this newspaper
Nearly as many physicians (62%) believed that families would want to speak to the doctor about a vaccine before getting it, and 57% of respondents who didn’t use standing orders believed they should be the one who recommends a vaccine to their patient’s parents.
All three of these reasons also ranked highest as barriers in responses from all respondents, including those who use standing orders. But those who didn’t use them were significantly more likely to cite these reasons (P less than .0001).
Since the survey occurred in 2017, however, it’s possible the pandemic and the rapid increase in telehealth as a result may influence perceptions moving forward.
“With provider concerns that standing orders remove physicians from the vaccination conversation, it may be that those conversations become less crucial as some families may start to value and accept immunizations more as a result of this pandemic,” Dr Cataldi said. “Or for families with vaccine questions, telehealth might support those conversations with a provider well.”
After adjusting for potential confounders, the only practice or physician factor significantly associated with not using standing orders for vaccines was physicians’ having a higher “physician responsibility score.” Doctors with these higher scores also were marginally more likely to make independent decisions about vaccines than counterparts working at practices where system-level decisions occur.
“Perhaps physicians who feel more personal responsibility about their role in vaccination are more likely to choose practice settings where they have more independent decision-making ability,” the authors wrote. “Alternatively, knowing the level of decision-making about vaccines in the practice may influence the amount of personal responsibility that pediatricians feel about their role in vaccine delivery.”
Again, attitudes may have shifted since the coronavirus pandemic began. The biggest risk to children in terms of immunizations is not getting them, Dr Offit said.
“The parents are scared, and the doctors are scared,” he said. “They feel that going to a doctor’s office is going to a concentrated area where they’re more likely to pick up this virus.”
He’s expressed uncertainty about whether standing orders could play a role in alleviating that anxiety. But Dr Cataldi suggests it’s possible.
“I think standing orders will be important to increasing vaccination rates during a pandemic as they can be used to support delivery of vaccines through public health departments and through vaccine-only nurse visits,” she said.
The research was funded by the Centers for Disease Control and Prevention. The authors had no relevant financial disclosures.
SOURCE: Cataldi J et al. Pediatrics. 2020 Apr;e20191855.
FROM PEDIATRICS
Do prophylactic antipyretics reduce vaccination-associated symptoms in children?
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
EVIDENCE-BASED ANSWER:
Yes for acetaminophen, not so much for ibuprofen. Prophylactic acetaminophen reduces the odds of febrile reactions in the first 48 hours after vaccination by 40% to 65% and pain of all grades by 36% to 43%. In contrast, prophylactic ibuprofen reduces pain of all grades by 34% only after primary vaccination and doesn’t alter pain after boosters. Nor does it alter early febrile reactions (strength of recommendation [SOR]: B, meta-analysis of randomized clinical trials [RCTs] with moderate-to-high risk of bias).
Prophylactic administration of acetaminophen or ibuprofen is associated with a reduction in antibody response to the primary vaccine series and to influenza vaccine, but antibody responses still achieve seroprotective levels (SOR: C, bench research).
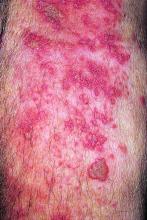
Live zoster vaccine confers limited protection during tofacitinib therapy
The live attenuated zoster vaccine (Zostavax) does not provide adequate long-term protection in patients with rheumatoid arthritis (RA) starting tofacitinib, suggests the ORAL Sequel extension study.
The incidence of herpes zoster in patients with RA taking tofacitinib (Xeljanz), an oral Janus kinase inhibitor, is about double the rate seen with biologic disease-modifying antirheumatic drugs, noted the investigators, who were led by Kevin L. Winthrop, MD, professor of infectious diseases, ophthalmology, public health, and preventive medicine at Oregon Health & Science University, Portland. The American College of Rheumatology’s guideline for the treatment of RA recommends herpes zoster vaccination before patients aged 50 years or older initiate any of these agents.
The investigators studied 100 patients with RA from an index randomized, placebo-controlled trial of tofacitinib who started the long-term extension study 14 weeks after receiving the live attenuated zoster vaccine. All were given open-label tofacitinib, at 5 or 10 mg two times per day, along with background RA therapy as needed.
With a follow-up of 27 months, five patients (5%) developed herpes zoster, including two treated with the 5-mg dose and three treated with the 10-mg dose, according to results reported in Annals of the Rheumatic Diseases. Cases occurred between 218 and 741 days after vaccination.
Four of the patients had herpes zoster involving a single dermatome, while one had involvement of five dermatomes. All episodes were mild or moderate, and resolved with antiviral therapy.
Humoral and cell-mediated immunity to the varicella zoster virus were assessed with immunoglobulin G titer and an interferon-gamma enzyme-linked immunosorbent spot assay, respectively. Results showed that three of the patients developing herpes zoster had undetectable cell-mediated immunity to the virus at baseline and week 6 after vaccination. The other two patients had an adequate humoral and cell-mediated immune response to the vaccine, as assessed from changes from baseline, but had below-average immunoglobulin G titer at baseline and week 6.
“These results suggest that [live attenuated zoster vaccine] may not provide adequate long-term protection, as previously demonstrated in healthy individuals aged ≥60 years 3 years post-vaccination, in which [herpes zoster] risk was reduced by 51%,” Dr. Winthrop and colleagues wrote.
“While it is possible that [live attenuated zoster vaccine] booster vaccinations may improve vaccine efficacy, to date there is a lack of data on the use and timing of booster vaccinations, and no recommendations on the use of [live attenuated zoster vaccine] booster vaccinations currently exist,” they concluded. “This highlights the importance of evaluating the newly approved subunit non-live vaccine (Shingrix) in patients with RA receiving tofacitinib.”
The study was sponsored by Pfizer. Dr. Winthrop disclosed consulting for AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, and UCB and receiving grant/research support from Bristol-Myers Squibb. Two coauthors disclosed financial relationships with Pfizer and other pharmaceutical companies, and the other seven coauthors were employees and shareholders of Pfizer.
SOURCE: Winthrop KL et al. Ann Rheum Dis. 2020 Mar 11. doi: 10.1136/annrheumdis-2019-216566.
The live attenuated zoster vaccine (Zostavax) does not provide adequate long-term protection in patients with rheumatoid arthritis (RA) starting tofacitinib, suggests the ORAL Sequel extension study.
The incidence of herpes zoster in patients with RA taking tofacitinib (Xeljanz), an oral Janus kinase inhibitor, is about double the rate seen with biologic disease-modifying antirheumatic drugs, noted the investigators, who were led by Kevin L. Winthrop, MD, professor of infectious diseases, ophthalmology, public health, and preventive medicine at Oregon Health & Science University, Portland. The American College of Rheumatology’s guideline for the treatment of RA recommends herpes zoster vaccination before patients aged 50 years or older initiate any of these agents.
The investigators studied 100 patients with RA from an index randomized, placebo-controlled trial of tofacitinib who started the long-term extension study 14 weeks after receiving the live attenuated zoster vaccine. All were given open-label tofacitinib, at 5 or 10 mg two times per day, along with background RA therapy as needed.
With a follow-up of 27 months, five patients (5%) developed herpes zoster, including two treated with the 5-mg dose and three treated with the 10-mg dose, according to results reported in Annals of the Rheumatic Diseases. Cases occurred between 218 and 741 days after vaccination.
Four of the patients had herpes zoster involving a single dermatome, while one had involvement of five dermatomes. All episodes were mild or moderate, and resolved with antiviral therapy.
Humoral and cell-mediated immunity to the varicella zoster virus were assessed with immunoglobulin G titer and an interferon-gamma enzyme-linked immunosorbent spot assay, respectively. Results showed that three of the patients developing herpes zoster had undetectable cell-mediated immunity to the virus at baseline and week 6 after vaccination. The other two patients had an adequate humoral and cell-mediated immune response to the vaccine, as assessed from changes from baseline, but had below-average immunoglobulin G titer at baseline and week 6.
“These results suggest that [live attenuated zoster vaccine] may not provide adequate long-term protection, as previously demonstrated in healthy individuals aged ≥60 years 3 years post-vaccination, in which [herpes zoster] risk was reduced by 51%,” Dr. Winthrop and colleagues wrote.
“While it is possible that [live attenuated zoster vaccine] booster vaccinations may improve vaccine efficacy, to date there is a lack of data on the use and timing of booster vaccinations, and no recommendations on the use of [live attenuated zoster vaccine] booster vaccinations currently exist,” they concluded. “This highlights the importance of evaluating the newly approved subunit non-live vaccine (Shingrix) in patients with RA receiving tofacitinib.”
The study was sponsored by Pfizer. Dr. Winthrop disclosed consulting for AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, and UCB and receiving grant/research support from Bristol-Myers Squibb. Two coauthors disclosed financial relationships with Pfizer and other pharmaceutical companies, and the other seven coauthors were employees and shareholders of Pfizer.
SOURCE: Winthrop KL et al. Ann Rheum Dis. 2020 Mar 11. doi: 10.1136/annrheumdis-2019-216566.
The live attenuated zoster vaccine (Zostavax) does not provide adequate long-term protection in patients with rheumatoid arthritis (RA) starting tofacitinib, suggests the ORAL Sequel extension study.
The incidence of herpes zoster in patients with RA taking tofacitinib (Xeljanz), an oral Janus kinase inhibitor, is about double the rate seen with biologic disease-modifying antirheumatic drugs, noted the investigators, who were led by Kevin L. Winthrop, MD, professor of infectious diseases, ophthalmology, public health, and preventive medicine at Oregon Health & Science University, Portland. The American College of Rheumatology’s guideline for the treatment of RA recommends herpes zoster vaccination before patients aged 50 years or older initiate any of these agents.
The investigators studied 100 patients with RA from an index randomized, placebo-controlled trial of tofacitinib who started the long-term extension study 14 weeks after receiving the live attenuated zoster vaccine. All were given open-label tofacitinib, at 5 or 10 mg two times per day, along with background RA therapy as needed.
With a follow-up of 27 months, five patients (5%) developed herpes zoster, including two treated with the 5-mg dose and three treated with the 10-mg dose, according to results reported in Annals of the Rheumatic Diseases. Cases occurred between 218 and 741 days after vaccination.
Four of the patients had herpes zoster involving a single dermatome, while one had involvement of five dermatomes. All episodes were mild or moderate, and resolved with antiviral therapy.
Humoral and cell-mediated immunity to the varicella zoster virus were assessed with immunoglobulin G titer and an interferon-gamma enzyme-linked immunosorbent spot assay, respectively. Results showed that three of the patients developing herpes zoster had undetectable cell-mediated immunity to the virus at baseline and week 6 after vaccination. The other two patients had an adequate humoral and cell-mediated immune response to the vaccine, as assessed from changes from baseline, but had below-average immunoglobulin G titer at baseline and week 6.
“These results suggest that [live attenuated zoster vaccine] may not provide adequate long-term protection, as previously demonstrated in healthy individuals aged ≥60 years 3 years post-vaccination, in which [herpes zoster] risk was reduced by 51%,” Dr. Winthrop and colleagues wrote.
“While it is possible that [live attenuated zoster vaccine] booster vaccinations may improve vaccine efficacy, to date there is a lack of data on the use and timing of booster vaccinations, and no recommendations on the use of [live attenuated zoster vaccine] booster vaccinations currently exist,” they concluded. “This highlights the importance of evaluating the newly approved subunit non-live vaccine (Shingrix) in patients with RA receiving tofacitinib.”
The study was sponsored by Pfizer. Dr. Winthrop disclosed consulting for AbbVie, Bristol-Myers Squibb, Eli Lilly, Galapagos, Gilead, Pfizer, and UCB and receiving grant/research support from Bristol-Myers Squibb. Two coauthors disclosed financial relationships with Pfizer and other pharmaceutical companies, and the other seven coauthors were employees and shareholders of Pfizer.
SOURCE: Winthrop KL et al. Ann Rheum Dis. 2020 Mar 11. doi: 10.1136/annrheumdis-2019-216566.
FROM ANNALS OF THE RHEUMATIC DISEASES
Preventable diseases could gain a foothold because of COVID-19
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
There is a highly infectious virus spreading around the world and it is targeting the most vulnerable among us. It is among the most contagious of human diseases, spreading through the air unseen. No, it isn’t the novel coronavirus, COVID-19. It’s measles.
Remember measles? Outbreaks in recent years have brought the disease, which once was declared eliminated in the United States, back into the news and public awareness, but measles never has really gone away. Every year there are millions of cases worldwide – in 2018 alone there were nearly 10 million estimated cases and 142,300 deaths, according to the World Health Organization. The good news is that measles vaccination is highly effective, at about 97% after the recommended two doses. According to the Centers for Disease Control and Prevention, “because of vaccination, more than 21 million lives have been saved and measles deaths have been reduced by 80% since 2000.” This is a tremendous public health success and a cause for celebration. But our work is not done. The recent increases in vaccine hesitancy and refusal in many countries has contributed to the resurgence of measles worldwide.
Influenza still is in full swing with the CDC reporting high activity in 1 states for the week ending April 4th. Seasonal influenza, according to currently available data, has a lower fatality rate than COVID-19, but that doesn’t mean it is harmless. Thus far in the 2019-2020 flu season, there have been at least 24,000 deaths because of influenza in the United States alone, 166 of which were among pediatric patients.*
Like many pediatricians, I have seen firsthand the impact of vaccine-preventable illnesses like influenza, pertussis, and varicella. I have personally cared for an infant with pertussis who had to be intubated and on a ventilator for nearly a week. I have told the family of a child with cancer that they would have to be admitted to the hospital yet again for intravenous antiviral medication because that little rash turned out to be varicella. I have performed CPR on a previously healthy teenager with the flu whose heart was failing despite maximum ventilator support. All these illnesses might have been prevented had these patients or those around them been appropriately vaccinated.
Right now, the United States and governments around the world are taking unprecedented public health measures to prevent the spread of COVID-19, directing the public to stay home, avoid unnecessary contact with other people, practice good hand-washing and infection-control techniques. In order to promote social distancing, many primary care clinics are canceling nonurgent appointments or converting them to virtual visits, including some visits for routine vaccinations for older children, teens, and adults. This is a responsible choice to keep potentially asymptomatic people from spreading COVID-19, but once restrictions begin to lift, we all will need to act to help our patients catch up on these missing vaccinations.
This pandemic has made it more apparent than ever that we all rely upon each other to stay healthy. While this pandemic has disrupted nearly every aspect of daily life, we can’t let it disrupt one of the great successes in health care today: the prevention of serious illnesses. As soon as it is safe to do so, we must help and encourage patients to catch up on missing vaccinations. It’s rare that preventative public health measures and vaccine developments are in the nightly news, so we should use this increased public awareness to ensure patients are well educated and protected from every disease. As part of this, we must continue our efforts to share accurate information on the safety and efficacy of routine vaccination. And when there is a vaccine for COVID-19? Let’s make sure everyone gets that too.
Dr. Leighton is a pediatrician in the ED at Children’s National Hospital and currently is completing her MPH in health policy at George Washington University, both in Washington. She had no relevant financial disclosures.*
* This article was updated 4/10/2020.
Inactivated flu vaccine succeeds among autoimmune rheumatic disease patients
Use of the inactivated influenza vaccine by adults with autoimmune rheumatic diseases significantly reduced their risk of influenza-like illness, hospitalization for pneumonia and chronic obstructive pulmonary disease, and death from pneumonia, according to findings from an observational study of more than 30,000 patients in the U.K. Clinical Practice Research Datalink.
Although the inactivated vaccine has been recommended for patients with autoimmune rheumatic diseases (AIRDs), including rheumatoid arthritis and spondyloarthritis, the vaccine’s impact on patient outcomes including pneumonia, hospitalization, and death has not been well studied, wrote Georgina Nakafero, PhD, of the University of Nottingham, England, and colleagues.
In a study published in Rheumatology, the researchers identified 30,788 adults with AIRDs from the longitudinal Clinical Practice Research Datalink database in the United Kingdom. Of these, 66% were women, 76% had rheumatoid arthritis, and 61% had been prescribed methotrexate. The study included a total of 125,034 flu cycles between 2006 and 2009 and between 2010 and 2015.
Overall, vaccination with the inactivated influenza vaccine (IIV) reduced the risk of primary care consultation for influenza-like illness (adjusted odds ratio, 0.70), hospitalization for pneumonia (aOR, 0.61), exacerbation of chronic obstructive pulmonary disease (aOR, 0.67), and death caused by pneumonia (aOR, 0.48) in the study population. In a propensity score–adjusted analysis, only protection from influenza-like illness lost statistical significance.
In addition, vaccination was associated with a reduction in all-cause mortality among AIRDs patients, but restricting the outcomes to the active influenza periods may have confounded this result, the researchers said.
The study findings were limited by several factors including observational design, the use of a single vaccine efficacy estimate for each outcome, potential missed vaccination cycles, and potential confounding by indication and healthy user bias that could inflate the vaccine effectiveness, the researchers noted. However, the results were strengthened by the large sample size, including a range of AIRDs, and the use of both diagnostic and prescription codes, they said.
“The findings of this study, together with the results of our previous study demonstrating the safety of IIV in people with AIRDs, provides evidence to promote seasonal flu vaccination in this population,” they concluded. They still emphasized that randomized, controlled trials are needed for an assessment of vaccine efficacy.
The study was supported by Versus Arthritis and the National Institute of Health Research. Lead author Dr. Nakafero had no financial conflicts to disclose. Several coauthors disclosed relationships with companies, including AstraZeneca, Roche, and Pfizer.
SOURCE: Nakafero G et al. Rheumatology. 2020 Mar 11. doi: 10.1093/rheumatology/keaa078.
Use of the inactivated influenza vaccine by adults with autoimmune rheumatic diseases significantly reduced their risk of influenza-like illness, hospitalization for pneumonia and chronic obstructive pulmonary disease, and death from pneumonia, according to findings from an observational study of more than 30,000 patients in the U.K. Clinical Practice Research Datalink.
Although the inactivated vaccine has been recommended for patients with autoimmune rheumatic diseases (AIRDs), including rheumatoid arthritis and spondyloarthritis, the vaccine’s impact on patient outcomes including pneumonia, hospitalization, and death has not been well studied, wrote Georgina Nakafero, PhD, of the University of Nottingham, England, and colleagues.
In a study published in Rheumatology, the researchers identified 30,788 adults with AIRDs from the longitudinal Clinical Practice Research Datalink database in the United Kingdom. Of these, 66% were women, 76% had rheumatoid arthritis, and 61% had been prescribed methotrexate. The study included a total of 125,034 flu cycles between 2006 and 2009 and between 2010 and 2015.
Overall, vaccination with the inactivated influenza vaccine (IIV) reduced the risk of primary care consultation for influenza-like illness (adjusted odds ratio, 0.70), hospitalization for pneumonia (aOR, 0.61), exacerbation of chronic obstructive pulmonary disease (aOR, 0.67), and death caused by pneumonia (aOR, 0.48) in the study population. In a propensity score–adjusted analysis, only protection from influenza-like illness lost statistical significance.
In addition, vaccination was associated with a reduction in all-cause mortality among AIRDs patients, but restricting the outcomes to the active influenza periods may have confounded this result, the researchers said.
The study findings were limited by several factors including observational design, the use of a single vaccine efficacy estimate for each outcome, potential missed vaccination cycles, and potential confounding by indication and healthy user bias that could inflate the vaccine effectiveness, the researchers noted. However, the results were strengthened by the large sample size, including a range of AIRDs, and the use of both diagnostic and prescription codes, they said.
“The findings of this study, together with the results of our previous study demonstrating the safety of IIV in people with AIRDs, provides evidence to promote seasonal flu vaccination in this population,” they concluded. They still emphasized that randomized, controlled trials are needed for an assessment of vaccine efficacy.
The study was supported by Versus Arthritis and the National Institute of Health Research. Lead author Dr. Nakafero had no financial conflicts to disclose. Several coauthors disclosed relationships with companies, including AstraZeneca, Roche, and Pfizer.
SOURCE: Nakafero G et al. Rheumatology. 2020 Mar 11. doi: 10.1093/rheumatology/keaa078.
Use of the inactivated influenza vaccine by adults with autoimmune rheumatic diseases significantly reduced their risk of influenza-like illness, hospitalization for pneumonia and chronic obstructive pulmonary disease, and death from pneumonia, according to findings from an observational study of more than 30,000 patients in the U.K. Clinical Practice Research Datalink.
Although the inactivated vaccine has been recommended for patients with autoimmune rheumatic diseases (AIRDs), including rheumatoid arthritis and spondyloarthritis, the vaccine’s impact on patient outcomes including pneumonia, hospitalization, and death has not been well studied, wrote Georgina Nakafero, PhD, of the University of Nottingham, England, and colleagues.
In a study published in Rheumatology, the researchers identified 30,788 adults with AIRDs from the longitudinal Clinical Practice Research Datalink database in the United Kingdom. Of these, 66% were women, 76% had rheumatoid arthritis, and 61% had been prescribed methotrexate. The study included a total of 125,034 flu cycles between 2006 and 2009 and between 2010 and 2015.
Overall, vaccination with the inactivated influenza vaccine (IIV) reduced the risk of primary care consultation for influenza-like illness (adjusted odds ratio, 0.70), hospitalization for pneumonia (aOR, 0.61), exacerbation of chronic obstructive pulmonary disease (aOR, 0.67), and death caused by pneumonia (aOR, 0.48) in the study population. In a propensity score–adjusted analysis, only protection from influenza-like illness lost statistical significance.
In addition, vaccination was associated with a reduction in all-cause mortality among AIRDs patients, but restricting the outcomes to the active influenza periods may have confounded this result, the researchers said.
The study findings were limited by several factors including observational design, the use of a single vaccine efficacy estimate for each outcome, potential missed vaccination cycles, and potential confounding by indication and healthy user bias that could inflate the vaccine effectiveness, the researchers noted. However, the results were strengthened by the large sample size, including a range of AIRDs, and the use of both diagnostic and prescription codes, they said.
“The findings of this study, together with the results of our previous study demonstrating the safety of IIV in people with AIRDs, provides evidence to promote seasonal flu vaccination in this population,” they concluded. They still emphasized that randomized, controlled trials are needed for an assessment of vaccine efficacy.
The study was supported by Versus Arthritis and the National Institute of Health Research. Lead author Dr. Nakafero had no financial conflicts to disclose. Several coauthors disclosed relationships with companies, including AstraZeneca, Roche, and Pfizer.
SOURCE: Nakafero G et al. Rheumatology. 2020 Mar 11. doi: 10.1093/rheumatology/keaa078.
FROM RHEUMATOLOGY
Key clinical point: Adults with autoimmune rheumatic diseases who received the inactivated flu vaccine had lower rates of flu-like illness, hospitalization, and death than did those not vaccinated.
Major finding: Vaccination significantly reduced the risk of flu-like illness, hospitalization for pneumonia or COPD exacerbation, and death from pneumonia by 30%, 39%, 33%, and 52%, respectively.
Study details: The data come from 30,788 adults with AIRD and included 125,034 influenza cycles.
Disclosures: The study was supported by Versus Arthritis and the National Institute of Health Research. Lead author Dr. Nakafero had no financial conflicts to disclose. Several coauthors disclosed relationships with companies, including AstraZeneca, Roche, and Pfizer.
Source: Nakafero G et al. Rheumatology. 2020 Mar 11. doi: 10.1093/rheumatology/keaa078.
HPV vaccine-chemo combo prolongs cervical cancer survival
Longer survival was observed in women who had a stronger immune response to an investigational human papillomavirus (HPV) vaccine while treated with standard chemotherapy for advanced, metastatic, or recurrent cervical cancer.
The results, from a phase 1/2 study, showed that women with a vaccine-induced immune response higher than the median had a median overall survival of 16.8 months, compared with a median overall survival of 11.2 months for women with an immune response lower than the median (hazard ratio, 0.491; P = .012).
Cornelis “Kees” Melief, MD, chief scientific officer of ISA Pharmaceuticals in Leiden, the Netherlands, and colleagues reported these findings in Science Translational Medicine.
The researchers previously evaluated the HPV16 vaccine, ISA101, in combination with carboplatin and paclitaxel in a pilot study. Results showed that carboplatin and paclitaxel reduced abnormally high numbers of immunosuppressive myeloid cells, which allowed for “much stronger” ISA101-induced tumor immunity.
To investigate further, the researchers tested the chemotherapy-ISA101 combination in a phase 1/2 study (NCT02128126) of 79 women with advanced, metastatic, or recurrent HPV16-positive cervical cancer.
The patients received the vaccine 2 weeks after starting the second, third, and fourth cycles of chemotherapy. They received various doses of the vaccine (20, 40, 100, or 300 mcg) with or without pegylated type 1 interferon (1 mcg/kg body weight).
“ISA101 was generally safe and well tolerated in that its safety profile was not different from chemotherapy alone,” Dr. Melief and colleagues wrote.
Chemotherapy-associated adverse events occurred in 98.9% of patients, with more than 80% of patients reporting adverse events possibly related to the vaccine or interferon-alpha. However, less than 16% of patients withdrew from the study because of an adverse event.
Of the 72 patients evaluable for efficacy, 43% experienced tumor regression, and 43% had stable disease. The researchers observed regression of the target lesion in 29 of 59 patients with a measurable target lesion.
The team noted that, since all patients received chemotherapy, it is “difficult to interpret short-term clinical outcomes as being due to chemotherapy alone or to the combination,” although they noted that the use of interferon-alpha did not seem to provide any additional benefit.
“Eleven of 14 patients still alive at the end of the study displayed a strong vaccine-induced response and included 9 patients with FIGO stage IVa/IVb cancer who had a mean OS [overall survival] of 3 years,” the researchers noted.
Considering that patients with higher vaccine-induced immune responses lived longer, the researchers concluded that “chemoimmunotherapy can be exploited to the benefit of patients with advanced cancer based on a defined mode of action.”
This trial was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
SOURCE: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
Longer survival was observed in women who had a stronger immune response to an investigational human papillomavirus (HPV) vaccine while treated with standard chemotherapy for advanced, metastatic, or recurrent cervical cancer.
The results, from a phase 1/2 study, showed that women with a vaccine-induced immune response higher than the median had a median overall survival of 16.8 months, compared with a median overall survival of 11.2 months for women with an immune response lower than the median (hazard ratio, 0.491; P = .012).
Cornelis “Kees” Melief, MD, chief scientific officer of ISA Pharmaceuticals in Leiden, the Netherlands, and colleagues reported these findings in Science Translational Medicine.
The researchers previously evaluated the HPV16 vaccine, ISA101, in combination with carboplatin and paclitaxel in a pilot study. Results showed that carboplatin and paclitaxel reduced abnormally high numbers of immunosuppressive myeloid cells, which allowed for “much stronger” ISA101-induced tumor immunity.
To investigate further, the researchers tested the chemotherapy-ISA101 combination in a phase 1/2 study (NCT02128126) of 79 women with advanced, metastatic, or recurrent HPV16-positive cervical cancer.
The patients received the vaccine 2 weeks after starting the second, third, and fourth cycles of chemotherapy. They received various doses of the vaccine (20, 40, 100, or 300 mcg) with or without pegylated type 1 interferon (1 mcg/kg body weight).
“ISA101 was generally safe and well tolerated in that its safety profile was not different from chemotherapy alone,” Dr. Melief and colleagues wrote.
Chemotherapy-associated adverse events occurred in 98.9% of patients, with more than 80% of patients reporting adverse events possibly related to the vaccine or interferon-alpha. However, less than 16% of patients withdrew from the study because of an adverse event.
Of the 72 patients evaluable for efficacy, 43% experienced tumor regression, and 43% had stable disease. The researchers observed regression of the target lesion in 29 of 59 patients with a measurable target lesion.
The team noted that, since all patients received chemotherapy, it is “difficult to interpret short-term clinical outcomes as being due to chemotherapy alone or to the combination,” although they noted that the use of interferon-alpha did not seem to provide any additional benefit.
“Eleven of 14 patients still alive at the end of the study displayed a strong vaccine-induced response and included 9 patients with FIGO stage IVa/IVb cancer who had a mean OS [overall survival] of 3 years,” the researchers noted.
Considering that patients with higher vaccine-induced immune responses lived longer, the researchers concluded that “chemoimmunotherapy can be exploited to the benefit of patients with advanced cancer based on a defined mode of action.”
This trial was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
SOURCE: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
Longer survival was observed in women who had a stronger immune response to an investigational human papillomavirus (HPV) vaccine while treated with standard chemotherapy for advanced, metastatic, or recurrent cervical cancer.
The results, from a phase 1/2 study, showed that women with a vaccine-induced immune response higher than the median had a median overall survival of 16.8 months, compared with a median overall survival of 11.2 months for women with an immune response lower than the median (hazard ratio, 0.491; P = .012).
Cornelis “Kees” Melief, MD, chief scientific officer of ISA Pharmaceuticals in Leiden, the Netherlands, and colleagues reported these findings in Science Translational Medicine.
The researchers previously evaluated the HPV16 vaccine, ISA101, in combination with carboplatin and paclitaxel in a pilot study. Results showed that carboplatin and paclitaxel reduced abnormally high numbers of immunosuppressive myeloid cells, which allowed for “much stronger” ISA101-induced tumor immunity.
To investigate further, the researchers tested the chemotherapy-ISA101 combination in a phase 1/2 study (NCT02128126) of 79 women with advanced, metastatic, or recurrent HPV16-positive cervical cancer.
The patients received the vaccine 2 weeks after starting the second, third, and fourth cycles of chemotherapy. They received various doses of the vaccine (20, 40, 100, or 300 mcg) with or without pegylated type 1 interferon (1 mcg/kg body weight).
“ISA101 was generally safe and well tolerated in that its safety profile was not different from chemotherapy alone,” Dr. Melief and colleagues wrote.
Chemotherapy-associated adverse events occurred in 98.9% of patients, with more than 80% of patients reporting adverse events possibly related to the vaccine or interferon-alpha. However, less than 16% of patients withdrew from the study because of an adverse event.
Of the 72 patients evaluable for efficacy, 43% experienced tumor regression, and 43% had stable disease. The researchers observed regression of the target lesion in 29 of 59 patients with a measurable target lesion.
The team noted that, since all patients received chemotherapy, it is “difficult to interpret short-term clinical outcomes as being due to chemotherapy alone or to the combination,” although they noted that the use of interferon-alpha did not seem to provide any additional benefit.
“Eleven of 14 patients still alive at the end of the study displayed a strong vaccine-induced response and included 9 patients with FIGO stage IVa/IVb cancer who had a mean OS [overall survival] of 3 years,” the researchers noted.
Considering that patients with higher vaccine-induced immune responses lived longer, the researchers concluded that “chemoimmunotherapy can be exploited to the benefit of patients with advanced cancer based on a defined mode of action.”
This trial was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
SOURCE: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: There may be an overall survival benefit of combining human papillomavirus vaccination with standard-of-care chemotherapy for cervical cancer.
Major finding: The median overall survival was 16.8 months for patients with immune responses to the vaccine that were higher than the median and 11.2 months for patients with immune responses lower than the median (hazard ratio, 0.491; P = .012).
Study details: A phase 1/2 study of 77 women with HPV16-positive advanced, metastatic, or recurrent cervical cancer.
Disclosures: The study was funded by ISA Pharmaceuticals and a Dutch Cancer Society grant. Investigators disclosed relationships with ISA Pharmaceuticals and other companies.
Source: Melief CJM et al. Sci Transl Med. 2020;12:eaaz8235.
Upcoming vaccine may offset surge in polio subtypes
Although wild poliovirus type 3 has not been detected globally for 7 years, the number of wild type 1 cases increased from 33 in 2018 to 173 in 2019. In response, a modified oral vaccine is being developed, according to Stephen Cochi, MD, of the Centers for Disease Control and Prevention’s Center for Global Health.
Several factors, including a Taliban ban on house-to-house vaccination in Afghanistan and a delay of large-scale vaccinations in Pakistan contributed to the surge in polio infections, Dr. Cochi said in a presentation at the February meeting of the CDC’s Advisory Committee on Immunization Practices (ACIP).
In addition, circulating vaccine-derived polioviruses (cVDPV) outbreaks have occurred in multiple countries including sub-Saharan Africa, China, Pakistan, and the Philippines. These outbreaks threaten the success of the bivalent oral polio vaccine introduced in April 2016 in 155 countries, Dr. Cochi said.
Outbreaks tend to occur just outside targeted areas for campaigns, caused by decreasing population immunity, he said.
The novel OPV2 (nOPV2) is a genetic modification of the existing OPV2 vaccine designed to improve genetic stability, Dr. Cochi explained. The modifications would “decrease the risk of seeding new cVDPVs and the risk of vaccine-associated paralytic poliomyelitis (VAPP),” he said.
The Emergency Use Listing (EUL) was developed by the World Health Organization in response to the Ebola virus outbreak in 2014-2016 and is the fastest way to obtain regulatory review and approval of drug products, said Dr. Cochi.
A pilot plant has been established in Indonesia, and upon EUL approval, 4-8 million doses of the nOPV2 should be available for use in the second quarter of 2020, he concluded.
Dr. Cochi had no relevant financial conflicts to disclose.
Although wild poliovirus type 3 has not been detected globally for 7 years, the number of wild type 1 cases increased from 33 in 2018 to 173 in 2019. In response, a modified oral vaccine is being developed, according to Stephen Cochi, MD, of the Centers for Disease Control and Prevention’s Center for Global Health.
Several factors, including a Taliban ban on house-to-house vaccination in Afghanistan and a delay of large-scale vaccinations in Pakistan contributed to the surge in polio infections, Dr. Cochi said in a presentation at the February meeting of the CDC’s Advisory Committee on Immunization Practices (ACIP).
In addition, circulating vaccine-derived polioviruses (cVDPV) outbreaks have occurred in multiple countries including sub-Saharan Africa, China, Pakistan, and the Philippines. These outbreaks threaten the success of the bivalent oral polio vaccine introduced in April 2016 in 155 countries, Dr. Cochi said.
Outbreaks tend to occur just outside targeted areas for campaigns, caused by decreasing population immunity, he said.
The novel OPV2 (nOPV2) is a genetic modification of the existing OPV2 vaccine designed to improve genetic stability, Dr. Cochi explained. The modifications would “decrease the risk of seeding new cVDPVs and the risk of vaccine-associated paralytic poliomyelitis (VAPP),” he said.
The Emergency Use Listing (EUL) was developed by the World Health Organization in response to the Ebola virus outbreak in 2014-2016 and is the fastest way to obtain regulatory review and approval of drug products, said Dr. Cochi.
A pilot plant has been established in Indonesia, and upon EUL approval, 4-8 million doses of the nOPV2 should be available for use in the second quarter of 2020, he concluded.
Dr. Cochi had no relevant financial conflicts to disclose.
Although wild poliovirus type 3 has not been detected globally for 7 years, the number of wild type 1 cases increased from 33 in 2018 to 173 in 2019. In response, a modified oral vaccine is being developed, according to Stephen Cochi, MD, of the Centers for Disease Control and Prevention’s Center for Global Health.
Several factors, including a Taliban ban on house-to-house vaccination in Afghanistan and a delay of large-scale vaccinations in Pakistan contributed to the surge in polio infections, Dr. Cochi said in a presentation at the February meeting of the CDC’s Advisory Committee on Immunization Practices (ACIP).
In addition, circulating vaccine-derived polioviruses (cVDPV) outbreaks have occurred in multiple countries including sub-Saharan Africa, China, Pakistan, and the Philippines. These outbreaks threaten the success of the bivalent oral polio vaccine introduced in April 2016 in 155 countries, Dr. Cochi said.
Outbreaks tend to occur just outside targeted areas for campaigns, caused by decreasing population immunity, he said.
The novel OPV2 (nOPV2) is a genetic modification of the existing OPV2 vaccine designed to improve genetic stability, Dr. Cochi explained. The modifications would “decrease the risk of seeding new cVDPVs and the risk of vaccine-associated paralytic poliomyelitis (VAPP),” he said.
The Emergency Use Listing (EUL) was developed by the World Health Organization in response to the Ebola virus outbreak in 2014-2016 and is the fastest way to obtain regulatory review and approval of drug products, said Dr. Cochi.
A pilot plant has been established in Indonesia, and upon EUL approval, 4-8 million doses of the nOPV2 should be available for use in the second quarter of 2020, he concluded.
Dr. Cochi had no relevant financial conflicts to disclose.
FROM AN ACIP MEETING
ACIP vaccination update
Every year the Advisory Committee on Immunization Practices (ACIP) updates the recommended immunization schedules for children/adolescents and adults on the Web site of the Centers for Disease Control and Prevention (www.cdc.gov/vaccines/schedules/hcp/index.html). The schedules for 2020 reflect additions and changes adopted by ACIP in 2019 and are discussed in this Practice Alert.
Hepatitis A: New directives on homelessness, HIV, and vaccine catch-up
Hepatitis A (HepA) vaccination is recommended for children ages 12 to 23 months, and for those at increased risk for hepatitis A virus (HAV) infection or for complications from HAV infection (TABLE 1).1-3 Routine vaccination is either 2 doses of HepA given 6 months apart or a 3-dose schedule of combined hepatitis A and B vaccine (Twinrix). Vaccines licensed in the United States for the prevention of HAV infection are listed in TABLE 2.1
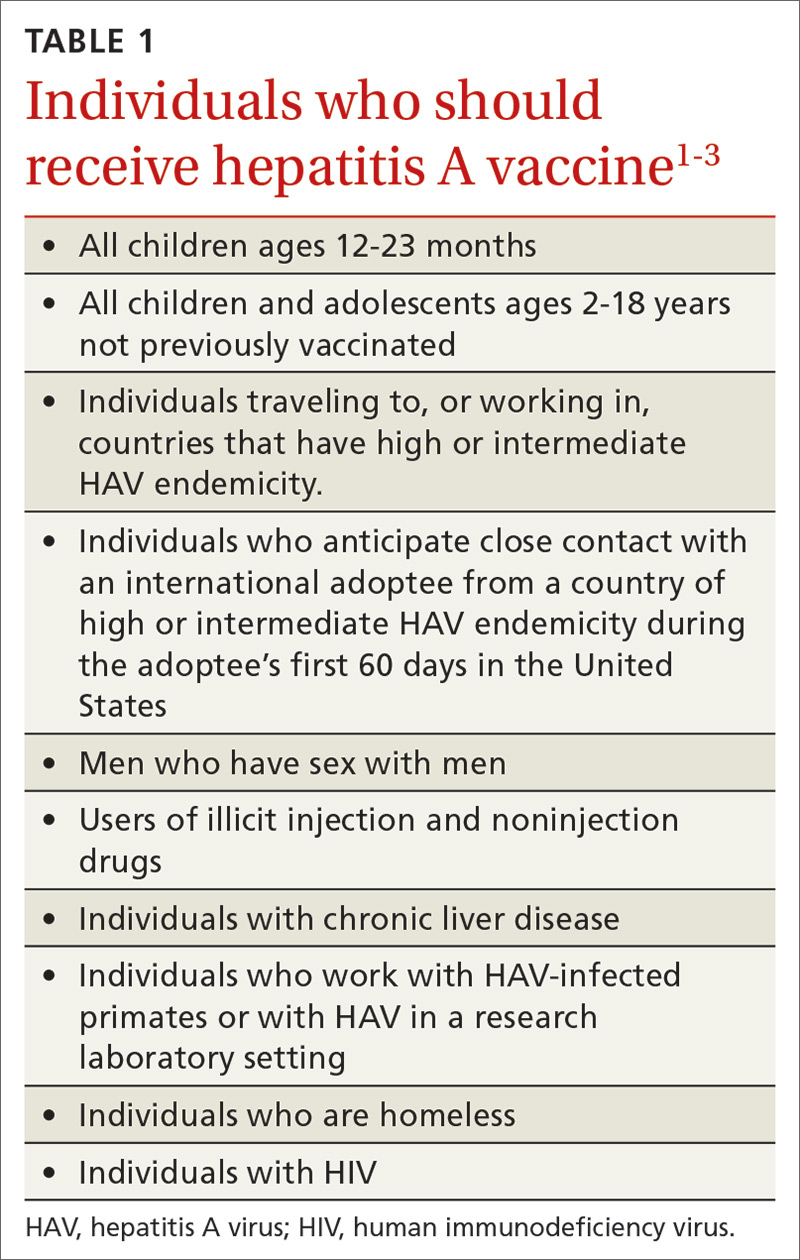
ACIP recently added homeless individuals to the list of those who should receive HepA vaccine.4 This step was taken in response to numerous outbreaks among those who are homeless or who use illicit drugs. These outbreaks have increased rates of HAV infection overall as well as rates of hospitalization (71%) and death (3%) among those infected.5 Concern about a homeless individual’s ability to complete a 2- or 3-dose series should not preclude initiating HepA vaccination; even 1 dose achieves protective immunity in 94% to 100% of those who have intact immune systems.2
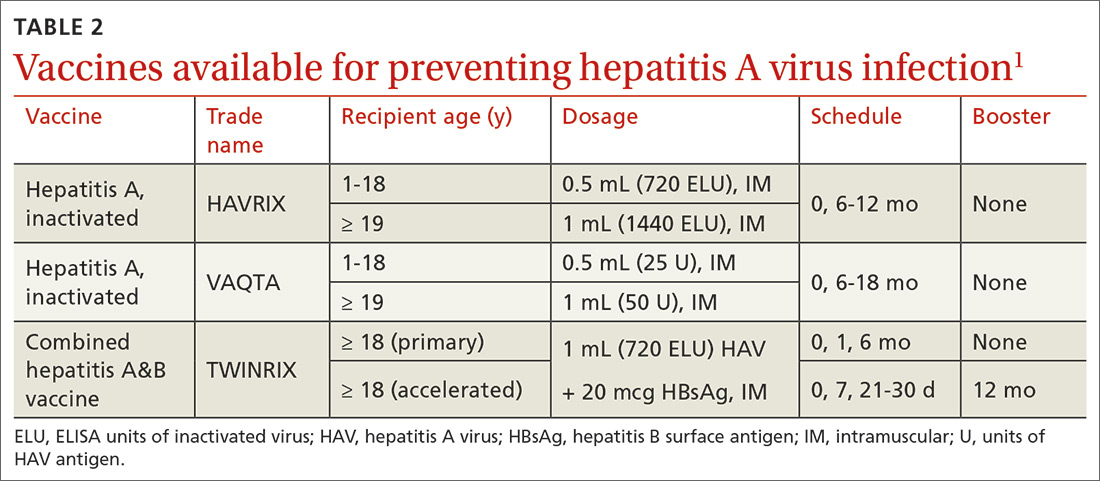
At its June 2019 meeting, ACIP made 2 other additions to its recommendations regarding HepA vaccination.1 First, those infected with the human immunodeficiency virus (HIV) are now among the individuals who should receive HepA vaccine. Those who are HIV-positive and ≥ 1 year old were recommended for HepA vaccination because they often have one of the other risks for HAV infection and have higher rates of complications and prolonged infections if they contract HAV.1 Second, catch-up HepA vaccination is indicated for children and adolescents ages 2 through 18 years who have not been previously vaccinated.1Also at the June 2019 meeting, the safety of HepA vaccination during pregnancy was confirmed. ACIP recommends HepA vaccine for any pregnant woman not previously vaccinated who is at risk for HAV infection or for a severe outcome from HAV infection.1
Japanese encephalitis: Vaccination can be accelerated
Japanese encephalitis (JE) is a serious mosquito-borne vaccine-preventable infection endemic to most of Asia and parts of the western Pacific. Most travelers to countries with endemic JE are at low risk of infection. But risk increases with prolonged visits to these areas and particularly during the JE virus transmission season (summer/fall in temperate areas; year-round in tropical climates). Risk is also heightened by traveling to, or living in, rural Asian areas, by participating in extensive outdoor activities, and by staying in accommodations without air-conditioning, screens, or bed nets.6
The only JE vaccine licensed in the United States is JE-VC (Ixiaro), manufactured by Valneva Austria GmbH. It is approved for use in children ≥ 2 months and adults. It requires a 2-dose series with 28 days between doses, and a booster after 1 year. ACIP recently approved an accelerated schedule for adults ages 18 to 65 years that allows the second dose to be administered as early as 7 days after the first. A full description of the epidemiology of JE and ACIP recommendations regarding JE-VC were published in July 2019.6
Meningococcal B vaccine booster doses recommended
Meningococcal B (MenB) vaccine is recommended for individuals ≥ 10 years old who are at increased risk of meningococcal infection, including those with complement deficiency, complement inhibitor use, or asplenia; microbiologists; and individuals exposed during an outbreak.7 It is also recommended for those ages 16 to 23 years who desire vaccination after individual clinical decision making.8
Continue to: Two MenB vaccines...
Two MenB vaccines are available in the United States: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). Either MenB vaccine can be used; however, they are not interchangeable and the same product must be used for all doses an individual receives. MenB-FHbp is licensed as a 3-dose series given at 0, 1-2, and 6 months, or as a 2-dose series given at 0 and 6 months. ACIP recommends the 3-dose schedule for individuals at increased risk for meningococcal disease or for use during community outbreaks of serogroup B meningococcal disease.9 For healthy adolescents who are not at increased risk for meningococcal disease, ACIP recommends using the 2-dose schedule of MenB-FHbp.9 MenB-4C is licensed as a 2-dose series, with doses administered at least 1 month apart.
At the June 2019 meeting, ACIP voted to recommend a MenB booster dose for those who are still at increased risk 1 year following completion of a MenB primary series, followed by booster doses every 2 to 3 years thereafter for as long as increased risk remains. This recommendation was made because of a rapid waning of immunity following the primary series and subsequent booster doses. A booster dose was not recommended for those who choose to be vaccinated after clinical decision making unless they are exposed during an outbreak and it has been at least a year since they received the primary series. An interval of 6 months for the booster can be considered, depending on the outbreak situation.10
A new DTaP product, and substituting Tdap for Td is approved
Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) is recommended for children as a 3-dose primary series (2, 4, 6 months) followed by 2 booster doses (at 15-18 months and at 4-6 years). These 3 antigens are available as DTaP products solely or as part of vaccines that combine other antigens with DTaP (TABLE 3).11,12 In addition, as a joint venture between Merck and Sanofi Pasteur, a new pediatric hexavalent vaccine containing DTaP5, polio, Haemophilus influenzae type b, and hepatitis B antigens is now available to be given at ages 2, 4, and 6 months.12
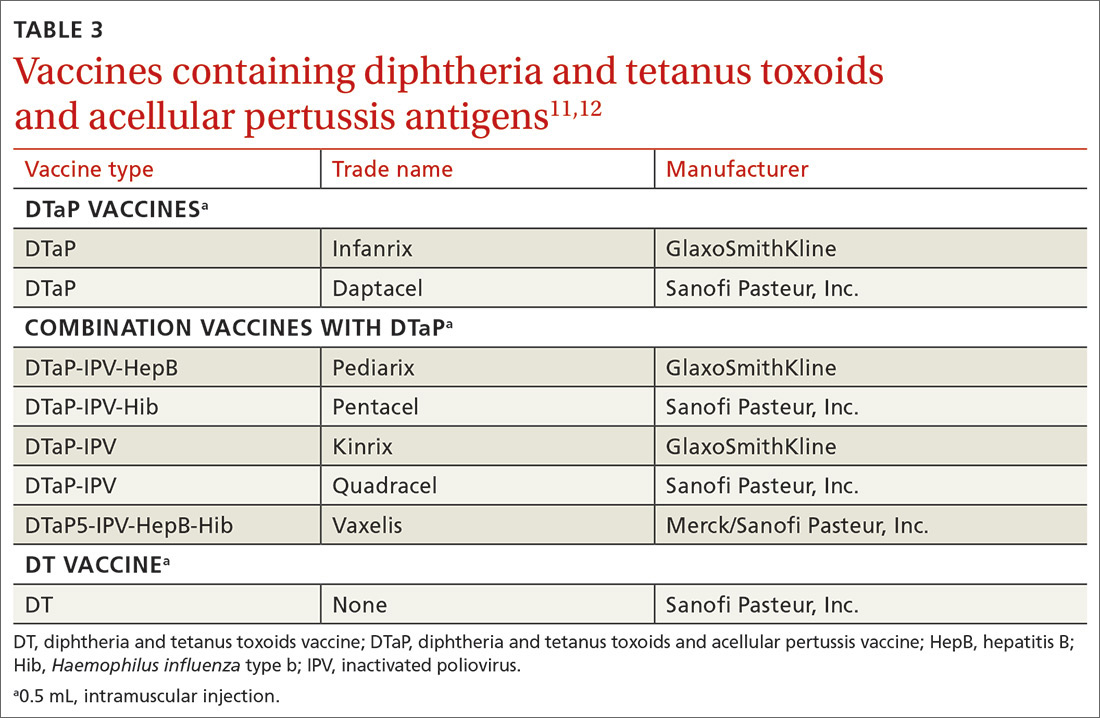
Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine is recommended for adolescents ages 11 to 12 years.11 It is also recommended once for adults who have not previously received it. The exception to the single Tdap dose for adults is during pregnancy; it is recommended as a single dose during each pregnancy regardless of the previous number of Tdap doses received.11
Td is recommended every 10 years after Tdap given at ages 11 to 12, for protection against tetanus and diphtheria. Tdap can be substituted for one of these decennial Td boosters. Tdap can also be substituted for Td for tetanus prophylaxis after a patient sustains a wound.11 The recommended single dose of Tdap for adolescents/adults also can be administered as part of a catch-up 3-dose Td series in previously unvaccinated adolescents and adults.
Continue to: It has become common...
It has become common practice throughout the country to substitute Tdap for Td when Td is indicated, even if Tdap has been received previously. ACIP looked at the safety of repeated doses of Tdap and found no safety concerns. For practicality, ACIP voted to recommend either Td or Tdap for these situations: the decennial booster, when tetanus prophylaxis is indicated in wound management, and when catch-up is needed in previously unvaccinated or inadequately vaccinated individuals who are 7 years of age and older. The resulting increase in the number of Tdap doses is not expected to have a major impact on the incidence of pertussis.13
Additional recommendations
Recommendations for preventing influenza in the 2019-2020 season are discussed in a previous Practice Alert.14
In 2019, ACIP also changed a previous recommendation on the routine use of 13-valent pneumococcal conjugate vaccine (PCV13) in adults ≥ 65 years. The new recommendation, covered in another Practice Alert, states that PCV13 should be used in immunocompetent adults ≥ 65 years only after individual clinical decision making.15
ACIP also changed its recommendations pertaining to human papillomavirus (HPV) vaccine. Catch-up vaccination is now recommended for all individuals through age 26 years. Previously catch up was recommended only for women and for men who have sex with men. And, even though use of HPV vaccine has been approved by the US Food and Drug Administration for adults ages 27 to 45 years, ACIP did not recommend its routine use in this age group but instead recommended it only after individual clinical decision making.16,17
1. Nelson N. Hepatitis A vaccine. Presentation to the ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Hepatitis-2-Nelson-508.pdf. Accessed February 24, 2020.
2. Fiore AE, Wasley A, Bell BP; Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(No. RR-7):1-23.
3. CDC. Prevention of hepatitis A through active or passive immunization. MMWR Wkly. 2006;55:1-23.
4. Doshani M, Weng M, Moore KL, et al. Recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for persons experiencing homelessness. MMWR Morb Mortal Wkly Rep. 2019;68:153-156.
5. Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208-1210.
6. Hills SL, Walter EB, Atmar RL, et al. Japanese encephalitis vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2019;68:1-33.
7. CDC. Meningococcal vaccination: what everyone should know. www.cdc.gov/vaccines/vpd/mening/public/index.html. Accessed February 24, 2020.
8. MacNeil JR, Rubin L, Folaranmi T, et al. Use of seroproup B meningococcal vaccine in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015; 64:1171-1176.
9. Patton M, Stephens D, Moore K, et al. Updated recommendations for use of MenB-FHbp seropgroup B meningococcal vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:509-513.
10. Mbaeyi S. Serogroup B Meningococcal vaccine booster doses. Presentation to ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Meningococcal-2-Mbaeyi-508.pdf. Accessed February 24, 2020.
11. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(No. RR-2):1-44.
12. Lee A. Immunogenicity and safety of DTaP5-IPV-HepB-Hib (Vaxelis™), a pediatric hexavalent combination vaccine. Presentation to the Advisory Committee on Immunization Practices; February 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/Combo-vaccine-2-Lee-508.pdf. Accessed February 24, 2020.
13. Havers F. Tdap and Td: summary of work group considerations and proposed policy options. Presentation to ACIP; October 23, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-10/Pertussis-03-Havers-508.pdf. Accessed February 24, 2020.
14. Campos-Outcalt D. Influenza update. J Fam Pract. 2019;68:456-458.
15. Campos-Outcalt D. Pneumococcal conjugate vaccine update. J Fam Pract. 2019;68:564-566.
16. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68:698–702.
17. Campos-Outcalt D. ACIP issues 2 new recs on HPV vaccine [audio]. J Fam Pract. September 2019. www.mdedge.com/familymedicine/article/205784/vaccines/acip-issues-2-new-recs-hpv-vaccination. Accessed February 24, 2020.
Every year the Advisory Committee on Immunization Practices (ACIP) updates the recommended immunization schedules for children/adolescents and adults on the Web site of the Centers for Disease Control and Prevention (www.cdc.gov/vaccines/schedules/hcp/index.html). The schedules for 2020 reflect additions and changes adopted by ACIP in 2019 and are discussed in this Practice Alert.
Hepatitis A: New directives on homelessness, HIV, and vaccine catch-up
Hepatitis A (HepA) vaccination is recommended for children ages 12 to 23 months, and for those at increased risk for hepatitis A virus (HAV) infection or for complications from HAV infection (TABLE 1).1-3 Routine vaccination is either 2 doses of HepA given 6 months apart or a 3-dose schedule of combined hepatitis A and B vaccine (Twinrix). Vaccines licensed in the United States for the prevention of HAV infection are listed in TABLE 2.1

ACIP recently added homeless individuals to the list of those who should receive HepA vaccine.4 This step was taken in response to numerous outbreaks among those who are homeless or who use illicit drugs. These outbreaks have increased rates of HAV infection overall as well as rates of hospitalization (71%) and death (3%) among those infected.5 Concern about a homeless individual’s ability to complete a 2- or 3-dose series should not preclude initiating HepA vaccination; even 1 dose achieves protective immunity in 94% to 100% of those who have intact immune systems.2

At its June 2019 meeting, ACIP made 2 other additions to its recommendations regarding HepA vaccination.1 First, those infected with the human immunodeficiency virus (HIV) are now among the individuals who should receive HepA vaccine. Those who are HIV-positive and ≥ 1 year old were recommended for HepA vaccination because they often have one of the other risks for HAV infection and have higher rates of complications and prolonged infections if they contract HAV.1 Second, catch-up HepA vaccination is indicated for children and adolescents ages 2 through 18 years who have not been previously vaccinated.1Also at the June 2019 meeting, the safety of HepA vaccination during pregnancy was confirmed. ACIP recommends HepA vaccine for any pregnant woman not previously vaccinated who is at risk for HAV infection or for a severe outcome from HAV infection.1
Japanese encephalitis: Vaccination can be accelerated
Japanese encephalitis (JE) is a serious mosquito-borne vaccine-preventable infection endemic to most of Asia and parts of the western Pacific. Most travelers to countries with endemic JE are at low risk of infection. But risk increases with prolonged visits to these areas and particularly during the JE virus transmission season (summer/fall in temperate areas; year-round in tropical climates). Risk is also heightened by traveling to, or living in, rural Asian areas, by participating in extensive outdoor activities, and by staying in accommodations without air-conditioning, screens, or bed nets.6
The only JE vaccine licensed in the United States is JE-VC (Ixiaro), manufactured by Valneva Austria GmbH. It is approved for use in children ≥ 2 months and adults. It requires a 2-dose series with 28 days between doses, and a booster after 1 year. ACIP recently approved an accelerated schedule for adults ages 18 to 65 years that allows the second dose to be administered as early as 7 days after the first. A full description of the epidemiology of JE and ACIP recommendations regarding JE-VC were published in July 2019.6
Meningococcal B vaccine booster doses recommended
Meningococcal B (MenB) vaccine is recommended for individuals ≥ 10 years old who are at increased risk of meningococcal infection, including those with complement deficiency, complement inhibitor use, or asplenia; microbiologists; and individuals exposed during an outbreak.7 It is also recommended for those ages 16 to 23 years who desire vaccination after individual clinical decision making.8
Continue to: Two MenB vaccines...
Two MenB vaccines are available in the United States: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). Either MenB vaccine can be used; however, they are not interchangeable and the same product must be used for all doses an individual receives. MenB-FHbp is licensed as a 3-dose series given at 0, 1-2, and 6 months, or as a 2-dose series given at 0 and 6 months. ACIP recommends the 3-dose schedule for individuals at increased risk for meningococcal disease or for use during community outbreaks of serogroup B meningococcal disease.9 For healthy adolescents who are not at increased risk for meningococcal disease, ACIP recommends using the 2-dose schedule of MenB-FHbp.9 MenB-4C is licensed as a 2-dose series, with doses administered at least 1 month apart.
At the June 2019 meeting, ACIP voted to recommend a MenB booster dose for those who are still at increased risk 1 year following completion of a MenB primary series, followed by booster doses every 2 to 3 years thereafter for as long as increased risk remains. This recommendation was made because of a rapid waning of immunity following the primary series and subsequent booster doses. A booster dose was not recommended for those who choose to be vaccinated after clinical decision making unless they are exposed during an outbreak and it has been at least a year since they received the primary series. An interval of 6 months for the booster can be considered, depending on the outbreak situation.10
A new DTaP product, and substituting Tdap for Td is approved
Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) is recommended for children as a 3-dose primary series (2, 4, 6 months) followed by 2 booster doses (at 15-18 months and at 4-6 years). These 3 antigens are available as DTaP products solely or as part of vaccines that combine other antigens with DTaP (TABLE 3).11,12 In addition, as a joint venture between Merck and Sanofi Pasteur, a new pediatric hexavalent vaccine containing DTaP5, polio, Haemophilus influenzae type b, and hepatitis B antigens is now available to be given at ages 2, 4, and 6 months.12

Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine is recommended for adolescents ages 11 to 12 years.11 It is also recommended once for adults who have not previously received it. The exception to the single Tdap dose for adults is during pregnancy; it is recommended as a single dose during each pregnancy regardless of the previous number of Tdap doses received.11
Td is recommended every 10 years after Tdap given at ages 11 to 12, for protection against tetanus and diphtheria. Tdap can be substituted for one of these decennial Td boosters. Tdap can also be substituted for Td for tetanus prophylaxis after a patient sustains a wound.11 The recommended single dose of Tdap for adolescents/adults also can be administered as part of a catch-up 3-dose Td series in previously unvaccinated adolescents and adults.
Continue to: It has become common...
It has become common practice throughout the country to substitute Tdap for Td when Td is indicated, even if Tdap has been received previously. ACIP looked at the safety of repeated doses of Tdap and found no safety concerns. For practicality, ACIP voted to recommend either Td or Tdap for these situations: the decennial booster, when tetanus prophylaxis is indicated in wound management, and when catch-up is needed in previously unvaccinated or inadequately vaccinated individuals who are 7 years of age and older. The resulting increase in the number of Tdap doses is not expected to have a major impact on the incidence of pertussis.13
Additional recommendations
Recommendations for preventing influenza in the 2019-2020 season are discussed in a previous Practice Alert.14
In 2019, ACIP also changed a previous recommendation on the routine use of 13-valent pneumococcal conjugate vaccine (PCV13) in adults ≥ 65 years. The new recommendation, covered in another Practice Alert, states that PCV13 should be used in immunocompetent adults ≥ 65 years only after individual clinical decision making.15
ACIP also changed its recommendations pertaining to human papillomavirus (HPV) vaccine. Catch-up vaccination is now recommended for all individuals through age 26 years. Previously catch up was recommended only for women and for men who have sex with men. And, even though use of HPV vaccine has been approved by the US Food and Drug Administration for adults ages 27 to 45 years, ACIP did not recommend its routine use in this age group but instead recommended it only after individual clinical decision making.16,17
Every year the Advisory Committee on Immunization Practices (ACIP) updates the recommended immunization schedules for children/adolescents and adults on the Web site of the Centers for Disease Control and Prevention (www.cdc.gov/vaccines/schedules/hcp/index.html). The schedules for 2020 reflect additions and changes adopted by ACIP in 2019 and are discussed in this Practice Alert.
Hepatitis A: New directives on homelessness, HIV, and vaccine catch-up
Hepatitis A (HepA) vaccination is recommended for children ages 12 to 23 months, and for those at increased risk for hepatitis A virus (HAV) infection or for complications from HAV infection (TABLE 1).1-3 Routine vaccination is either 2 doses of HepA given 6 months apart or a 3-dose schedule of combined hepatitis A and B vaccine (Twinrix). Vaccines licensed in the United States for the prevention of HAV infection are listed in TABLE 2.1

ACIP recently added homeless individuals to the list of those who should receive HepA vaccine.4 This step was taken in response to numerous outbreaks among those who are homeless or who use illicit drugs. These outbreaks have increased rates of HAV infection overall as well as rates of hospitalization (71%) and death (3%) among those infected.5 Concern about a homeless individual’s ability to complete a 2- or 3-dose series should not preclude initiating HepA vaccination; even 1 dose achieves protective immunity in 94% to 100% of those who have intact immune systems.2

At its June 2019 meeting, ACIP made 2 other additions to its recommendations regarding HepA vaccination.1 First, those infected with the human immunodeficiency virus (HIV) are now among the individuals who should receive HepA vaccine. Those who are HIV-positive and ≥ 1 year old were recommended for HepA vaccination because they often have one of the other risks for HAV infection and have higher rates of complications and prolonged infections if they contract HAV.1 Second, catch-up HepA vaccination is indicated for children and adolescents ages 2 through 18 years who have not been previously vaccinated.1Also at the June 2019 meeting, the safety of HepA vaccination during pregnancy was confirmed. ACIP recommends HepA vaccine for any pregnant woman not previously vaccinated who is at risk for HAV infection or for a severe outcome from HAV infection.1
Japanese encephalitis: Vaccination can be accelerated
Japanese encephalitis (JE) is a serious mosquito-borne vaccine-preventable infection endemic to most of Asia and parts of the western Pacific. Most travelers to countries with endemic JE are at low risk of infection. But risk increases with prolonged visits to these areas and particularly during the JE virus transmission season (summer/fall in temperate areas; year-round in tropical climates). Risk is also heightened by traveling to, or living in, rural Asian areas, by participating in extensive outdoor activities, and by staying in accommodations without air-conditioning, screens, or bed nets.6
The only JE vaccine licensed in the United States is JE-VC (Ixiaro), manufactured by Valneva Austria GmbH. It is approved for use in children ≥ 2 months and adults. It requires a 2-dose series with 28 days between doses, and a booster after 1 year. ACIP recently approved an accelerated schedule for adults ages 18 to 65 years that allows the second dose to be administered as early as 7 days after the first. A full description of the epidemiology of JE and ACIP recommendations regarding JE-VC were published in July 2019.6
Meningococcal B vaccine booster doses recommended
Meningococcal B (MenB) vaccine is recommended for individuals ≥ 10 years old who are at increased risk of meningococcal infection, including those with complement deficiency, complement inhibitor use, or asplenia; microbiologists; and individuals exposed during an outbreak.7 It is also recommended for those ages 16 to 23 years who desire vaccination after individual clinical decision making.8
Continue to: Two MenB vaccines...
Two MenB vaccines are available in the United States: MenB-FHbp (Trumenba, Wyeth Pharmaceuticals, Inc.) and MenB-4C (Bexsero, GlaxoSmithKline). Either MenB vaccine can be used; however, they are not interchangeable and the same product must be used for all doses an individual receives. MenB-FHbp is licensed as a 3-dose series given at 0, 1-2, and 6 months, or as a 2-dose series given at 0 and 6 months. ACIP recommends the 3-dose schedule for individuals at increased risk for meningococcal disease or for use during community outbreaks of serogroup B meningococcal disease.9 For healthy adolescents who are not at increased risk for meningococcal disease, ACIP recommends using the 2-dose schedule of MenB-FHbp.9 MenB-4C is licensed as a 2-dose series, with doses administered at least 1 month apart.
At the June 2019 meeting, ACIP voted to recommend a MenB booster dose for those who are still at increased risk 1 year following completion of a MenB primary series, followed by booster doses every 2 to 3 years thereafter for as long as increased risk remains. This recommendation was made because of a rapid waning of immunity following the primary series and subsequent booster doses. A booster dose was not recommended for those who choose to be vaccinated after clinical decision making unless they are exposed during an outbreak and it has been at least a year since they received the primary series. An interval of 6 months for the booster can be considered, depending on the outbreak situation.10
A new DTaP product, and substituting Tdap for Td is approved
Diphtheria and tetanus toxoids and acellular pertussis vaccine (DTaP) is recommended for children as a 3-dose primary series (2, 4, 6 months) followed by 2 booster doses (at 15-18 months and at 4-6 years). These 3 antigens are available as DTaP products solely or as part of vaccines that combine other antigens with DTaP (TABLE 3).11,12 In addition, as a joint venture between Merck and Sanofi Pasteur, a new pediatric hexavalent vaccine containing DTaP5, polio, Haemophilus influenzae type b, and hepatitis B antigens is now available to be given at ages 2, 4, and 6 months.12

Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine is recommended for adolescents ages 11 to 12 years.11 It is also recommended once for adults who have not previously received it. The exception to the single Tdap dose for adults is during pregnancy; it is recommended as a single dose during each pregnancy regardless of the previous number of Tdap doses received.11
Td is recommended every 10 years after Tdap given at ages 11 to 12, for protection against tetanus and diphtheria. Tdap can be substituted for one of these decennial Td boosters. Tdap can also be substituted for Td for tetanus prophylaxis after a patient sustains a wound.11 The recommended single dose of Tdap for adolescents/adults also can be administered as part of a catch-up 3-dose Td series in previously unvaccinated adolescents and adults.
Continue to: It has become common...
It has become common practice throughout the country to substitute Tdap for Td when Td is indicated, even if Tdap has been received previously. ACIP looked at the safety of repeated doses of Tdap and found no safety concerns. For practicality, ACIP voted to recommend either Td or Tdap for these situations: the decennial booster, when tetanus prophylaxis is indicated in wound management, and when catch-up is needed in previously unvaccinated or inadequately vaccinated individuals who are 7 years of age and older. The resulting increase in the number of Tdap doses is not expected to have a major impact on the incidence of pertussis.13
Additional recommendations
Recommendations for preventing influenza in the 2019-2020 season are discussed in a previous Practice Alert.14
In 2019, ACIP also changed a previous recommendation on the routine use of 13-valent pneumococcal conjugate vaccine (PCV13) in adults ≥ 65 years. The new recommendation, covered in another Practice Alert, states that PCV13 should be used in immunocompetent adults ≥ 65 years only after individual clinical decision making.15
ACIP also changed its recommendations pertaining to human papillomavirus (HPV) vaccine. Catch-up vaccination is now recommended for all individuals through age 26 years. Previously catch up was recommended only for women and for men who have sex with men. And, even though use of HPV vaccine has been approved by the US Food and Drug Administration for adults ages 27 to 45 years, ACIP did not recommend its routine use in this age group but instead recommended it only after individual clinical decision making.16,17
1. Nelson N. Hepatitis A vaccine. Presentation to the ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Hepatitis-2-Nelson-508.pdf. Accessed February 24, 2020.
2. Fiore AE, Wasley A, Bell BP; Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(No. RR-7):1-23.
3. CDC. Prevention of hepatitis A through active or passive immunization. MMWR Wkly. 2006;55:1-23.
4. Doshani M, Weng M, Moore KL, et al. Recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for persons experiencing homelessness. MMWR Morb Mortal Wkly Rep. 2019;68:153-156.
5. Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208-1210.
6. Hills SL, Walter EB, Atmar RL, et al. Japanese encephalitis vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2019;68:1-33.
7. CDC. Meningococcal vaccination: what everyone should know. www.cdc.gov/vaccines/vpd/mening/public/index.html. Accessed February 24, 2020.
8. MacNeil JR, Rubin L, Folaranmi T, et al. Use of seroproup B meningococcal vaccine in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015; 64:1171-1176.
9. Patton M, Stephens D, Moore K, et al. Updated recommendations for use of MenB-FHbp seropgroup B meningococcal vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:509-513.
10. Mbaeyi S. Serogroup B Meningococcal vaccine booster doses. Presentation to ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Meningococcal-2-Mbaeyi-508.pdf. Accessed February 24, 2020.
11. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(No. RR-2):1-44.
12. Lee A. Immunogenicity and safety of DTaP5-IPV-HepB-Hib (Vaxelis™), a pediatric hexavalent combination vaccine. Presentation to the Advisory Committee on Immunization Practices; February 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/Combo-vaccine-2-Lee-508.pdf. Accessed February 24, 2020.
13. Havers F. Tdap and Td: summary of work group considerations and proposed policy options. Presentation to ACIP; October 23, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-10/Pertussis-03-Havers-508.pdf. Accessed February 24, 2020.
14. Campos-Outcalt D. Influenza update. J Fam Pract. 2019;68:456-458.
15. Campos-Outcalt D. Pneumococcal conjugate vaccine update. J Fam Pract. 2019;68:564-566.
16. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68:698–702.
17. Campos-Outcalt D. ACIP issues 2 new recs on HPV vaccine [audio]. J Fam Pract. September 2019. www.mdedge.com/familymedicine/article/205784/vaccines/acip-issues-2-new-recs-hpv-vaccination. Accessed February 24, 2020.
1. Nelson N. Hepatitis A vaccine. Presentation to the ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Hepatitis-2-Nelson-508.pdf. Accessed February 24, 2020.
2. Fiore AE, Wasley A, Bell BP; Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(No. RR-7):1-23.
3. CDC. Prevention of hepatitis A through active or passive immunization. MMWR Wkly. 2006;55:1-23.
4. Doshani M, Weng M, Moore KL, et al. Recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for persons experiencing homelessness. MMWR Morb Mortal Wkly Rep. 2019;68:153-156.
5. Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208-1210.
6. Hills SL, Walter EB, Atmar RL, et al. Japanese encephalitis vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2019;68:1-33.
7. CDC. Meningococcal vaccination: what everyone should know. www.cdc.gov/vaccines/vpd/mening/public/index.html. Accessed February 24, 2020.
8. MacNeil JR, Rubin L, Folaranmi T, et al. Use of seroproup B meningococcal vaccine in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015; 64:1171-1176.
9. Patton M, Stephens D, Moore K, et al. Updated recommendations for use of MenB-FHbp seropgroup B meningococcal vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:509-513.
10. Mbaeyi S. Serogroup B Meningococcal vaccine booster doses. Presentation to ACIP; June 27, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-06/Meningococcal-2-Mbaeyi-508.pdf. Accessed February 24, 2020.
11. Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(No. RR-2):1-44.
12. Lee A. Immunogenicity and safety of DTaP5-IPV-HepB-Hib (Vaxelis™), a pediatric hexavalent combination vaccine. Presentation to the Advisory Committee on Immunization Practices; February 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/Combo-vaccine-2-Lee-508.pdf. Accessed February 24, 2020.
13. Havers F. Tdap and Td: summary of work group considerations and proposed policy options. Presentation to ACIP; October 23, 2019. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-10/Pertussis-03-Havers-508.pdf. Accessed February 24, 2020.
14. Campos-Outcalt D. Influenza update. J Fam Pract. 2019;68:456-458.
15. Campos-Outcalt D. Pneumococcal conjugate vaccine update. J Fam Pract. 2019;68:564-566.
16. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019; 68:698–702.
17. Campos-Outcalt D. ACIP issues 2 new recs on HPV vaccine [audio]. J Fam Pract. September 2019. www.mdedge.com/familymedicine/article/205784/vaccines/acip-issues-2-new-recs-hpv-vaccination. Accessed February 24, 2020.
Dengue vaccine deemed acceptable by most doctors, fewer parents
Adults are interested in a dengue vaccine for themselves and their children, and physicians recognize that dengue is a public health problem, according to data from parents and physicians in Puerto Rico. Most doctors, but fewer parents, found the idea of protecting children with Dengue vaccine acceptable.
Lack of detailed information about the vaccine is the greatest barrier to parents’ consent to vaccination, noted Ines Esquilin, MD, of the University of Puerto Rico, San Juan, in a presentation at the February meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The ACIP dengue vaccines work group reviewed data from 102 physicians in Puerto Rico, 82% of which were pediatricians, regarding potential dengue vaccination. Overall, 98% said they considered dengue a significant public health problem in Puerto Rico, and 73% said they would recommend the dengue vaccine to patients if a laboratory test with acceptable specificity were available. Among the physicians who said they would not recommend the vaccine, the most common reason (71%) was concern about the risks of vaccinating individuals with false-positive tests.
The availability of a test that can be performed in the medical office and avoid repeat visits is a major factor in the feasibility of dengue vaccination, Dr. Esquilin said.
The ACIP dengue vaccines work group also sought public opinion on the acceptability of a generic dengue vaccine through focus group sessions with parents of children aged 9-16 years in Puerto Rico, said Dr. Esquilin.
Approximately one-third of the parents said they were willing to vaccinate their children, one-third were unwilling, and one-third were unsure. The most commonly identified barriers to vaccination included lack of information or inconsistent information about the vaccine, high cost/lack of insurance coverage, time-consuming lab test to confirm infection, side effects, potential for false-positive lab results, and low vaccine effectiveness.
Motivating factors for vaccination included correct information about the vaccine, desire to prevent infection, lab-confirmed positive test, support from public health organizations, the presence of a dengue epidemic, and educational forums.
Based in part on these findings, the ACIP dengue vaccines work group noted that the need for an acceptably specific screening lab test is the greatest concern in their consideration of recommendations, and the work group expects to review a CDC assessment of laboratory tests for prevaccination screening at a future meeting.
Dr. Esquilin had no financial conflicts to disclose.
SOURCE: Esquilin E. 2020. February meeting of the CDC Advisory Committee on Immunization Practices (ACIP) presentation.
Adults are interested in a dengue vaccine for themselves and their children, and physicians recognize that dengue is a public health problem, according to data from parents and physicians in Puerto Rico. Most doctors, but fewer parents, found the idea of protecting children with Dengue vaccine acceptable.
Lack of detailed information about the vaccine is the greatest barrier to parents’ consent to vaccination, noted Ines Esquilin, MD, of the University of Puerto Rico, San Juan, in a presentation at the February meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The ACIP dengue vaccines work group reviewed data from 102 physicians in Puerto Rico, 82% of which were pediatricians, regarding potential dengue vaccination. Overall, 98% said they considered dengue a significant public health problem in Puerto Rico, and 73% said they would recommend the dengue vaccine to patients if a laboratory test with acceptable specificity were available. Among the physicians who said they would not recommend the vaccine, the most common reason (71%) was concern about the risks of vaccinating individuals with false-positive tests.
The availability of a test that can be performed in the medical office and avoid repeat visits is a major factor in the feasibility of dengue vaccination, Dr. Esquilin said.
The ACIP dengue vaccines work group also sought public opinion on the acceptability of a generic dengue vaccine through focus group sessions with parents of children aged 9-16 years in Puerto Rico, said Dr. Esquilin.
Approximately one-third of the parents said they were willing to vaccinate their children, one-third were unwilling, and one-third were unsure. The most commonly identified barriers to vaccination included lack of information or inconsistent information about the vaccine, high cost/lack of insurance coverage, time-consuming lab test to confirm infection, side effects, potential for false-positive lab results, and low vaccine effectiveness.
Motivating factors for vaccination included correct information about the vaccine, desire to prevent infection, lab-confirmed positive test, support from public health organizations, the presence of a dengue epidemic, and educational forums.
Based in part on these findings, the ACIP dengue vaccines work group noted that the need for an acceptably specific screening lab test is the greatest concern in their consideration of recommendations, and the work group expects to review a CDC assessment of laboratory tests for prevaccination screening at a future meeting.
Dr. Esquilin had no financial conflicts to disclose.
SOURCE: Esquilin E. 2020. February meeting of the CDC Advisory Committee on Immunization Practices (ACIP) presentation.
Adults are interested in a dengue vaccine for themselves and their children, and physicians recognize that dengue is a public health problem, according to data from parents and physicians in Puerto Rico. Most doctors, but fewer parents, found the idea of protecting children with Dengue vaccine acceptable.
Lack of detailed information about the vaccine is the greatest barrier to parents’ consent to vaccination, noted Ines Esquilin, MD, of the University of Puerto Rico, San Juan, in a presentation at the February meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP).
The ACIP dengue vaccines work group reviewed data from 102 physicians in Puerto Rico, 82% of which were pediatricians, regarding potential dengue vaccination. Overall, 98% said they considered dengue a significant public health problem in Puerto Rico, and 73% said they would recommend the dengue vaccine to patients if a laboratory test with acceptable specificity were available. Among the physicians who said they would not recommend the vaccine, the most common reason (71%) was concern about the risks of vaccinating individuals with false-positive tests.
The availability of a test that can be performed in the medical office and avoid repeat visits is a major factor in the feasibility of dengue vaccination, Dr. Esquilin said.
The ACIP dengue vaccines work group also sought public opinion on the acceptability of a generic dengue vaccine through focus group sessions with parents of children aged 9-16 years in Puerto Rico, said Dr. Esquilin.
Approximately one-third of the parents said they were willing to vaccinate their children, one-third were unwilling, and one-third were unsure. The most commonly identified barriers to vaccination included lack of information or inconsistent information about the vaccine, high cost/lack of insurance coverage, time-consuming lab test to confirm infection, side effects, potential for false-positive lab results, and low vaccine effectiveness.
Motivating factors for vaccination included correct information about the vaccine, desire to prevent infection, lab-confirmed positive test, support from public health organizations, the presence of a dengue epidemic, and educational forums.
Based in part on these findings, the ACIP dengue vaccines work group noted that the need for an acceptably specific screening lab test is the greatest concern in their consideration of recommendations, and the work group expects to review a CDC assessment of laboratory tests for prevaccination screening at a future meeting.
Dr. Esquilin had no financial conflicts to disclose.
SOURCE: Esquilin E. 2020. February meeting of the CDC Advisory Committee on Immunization Practices (ACIP) presentation.
FROM AN ACIP MEETING
Antibiotic resistance rises among pneumococcus strains in kids
Antibiotic resistance in strains of Streptococcus pneumoniae has been rising since 2013 because of changing susceptibility profiles, based on data from 1,201 isolates collected from 448 children in primary care settings.
“New strains expressing capsular serotypes not included in the 13-valent pneumococcal conjugate vaccine are emerging to cause disease, and strains that acquire antibiotic resistance are increasing in frequency due to their survival of the fittest advantage,” wrote Ravinder Kaur, PhD, of Rochester (N.Y.) General Hospital Research Institute, and colleagues.
Similar Darwinian principles occurred after the introduction of PCV-7, the study authors added.
In a prospective cohort study published in Clinical Infectious Diseases, the researchers reviewed 1,201 isolates collected from the nasopharynx during healthy periods, and from the nasopharynx and middle ear fluid (MEF) during episodes of acute otitis media, in children aged 6-36 months who were seen in primary care settings.
The isolates were collected during 2006-2016 to reflect the pre- and post-PCV13 era. Children received PCV-7 from 2006 until April 2010, and received PCV-13 after April 2010.
Overall, the number of acute otitis media (AOM) cases caused by S. pneumoniae was not significantly different between the PCV-7 and PCV-13 eras, nor was the frequency of pneumococci identified in the nasopharynx during healthy visits and visits at the start of an AOM infection.
The researchers examined susceptibility using minimum inhibitory concentrations (MIC). During healthy visits, the MIC50 of isolated pneumococci was low (no greater than 0.06 mcg/mL) for all four beta-lactam drugs tested. And it didn’t change significantly over the study years.
In contrast, among the nasopharyngeal and MEF isolates during AOM, the MIC50 to penicillin, amoxicillin, ceftriaxone, and meropenem during 2013-2016 rose significantly, the investigators said.
A change in antibiotic susceptibility within a subtype also contributed to the development of PCV-13 resistance.
The study authors identified three serotypes that affected the changes in susceptibility in their study population. Serotypes 35B and 35F increased their beta-lactam resistance during 2013-2016, and serotype 11A had a higher MIC to quinolones and became more prevalent during 2013-2016. Those three serotypes accounted for most of the change in antibiotic susceptibility, the researchers said.
In addition, “the frequency of strains resistant to penicillin and amoxicillin decreased with the introduction of PCV-13, but rebounded to levels similar to those before PCV-13 introduction by 2015-2016,” the investigators noted.
The study findings were limited by several factors, including the homogeneous study population and potential lack of generalizability to other settings. In addition, the researchers did not study antibiotic consumption or antibiotic treatment failure, and they could not account for potential AOM cases that may have been treated in settings other than primary care.
However, the investigators said the results support the need for additional studies and attention to the development of the next generation of PCVs, the PCV-15 and PCV-20. Both include serotypes 22F and 33F, but neither includes 35B or 35F. The PCV-20 also includes 11A and 15B.
The study was supported in part by the National Institutes of Health and Sanofi Pasteur. Some isolates collected during the 2010-2013 time period were part of a study supported by Pfizer. The researchers had no relevant financial conflicts to disclose.
SOURCE: Kaur R et al. Clin Inf Dis. 2020 Feb 18. doi: 10.1093/cid/ciaa157.
Dr. Kaur and colleagues report their analysis of pneumococcal resistance among nasopharyngeal and middle ear isolates (90% nasopharyngeal and 10% middle ear) collected between 2008 and 2016. They demonstrate the dominant role that nonvaccine serotypes play in carriage and acute otitis media (AOM) in children, and by extension potentially the entire spectrum of pneumococcal disease in the 13-valent pneumococcal conjugate vaccine (PCV13) era. Nonsusceptibility to beta-lactams was reported for one-third of isolates with the increase in the most recent reported years (2013-2016).
What are the implications for treatment of pneumococcal infections? For AOM, amoxicillin minimum inhibitory concentrations (MIC) were all less than 4 mcg/mL, which is the pharmacodynamic breakpoint for high-dose (90 mg/kg per day) AOM regimens; these data support continued use of high-dose amoxicillin for children with AOM that requires antimicrobial treatment. Resistance to macrolides (erythromycin and likely azithromycin) occurred in approximately one-third of isolates; however, in contrast to beta-lactams (amoxicillin), higher macrolide doses do not overcome resistance. Thus macrolide use for AOM appears limited to those with beta-lactam allergy and no better alternative drug, i.e., expect failure in one-third of AOM patients if macrolides are used. For ceftriaxone, no 2013-2016 isolate had a MIC over 0.5 mcg/mL, implying that ceftriaxone remains appropriate first-line therapy for serious pneumococcal disease and effective for pneumococcal AOM when oral drugs have failed or are not an option because of repeated emesis. Interestingly, trimethoprim/sulfamethoxazole (T/S) had lower resistance rates against the nonvaccine “bad boy” serogroup 35 (8%-15%), compared with cephalosporins (32%-57%). Perhaps we are back to the future and T/S will again have a role against pneumococcal AOM. Of note, no isolate was resistant to levofloxacin or linezolid. Linezolid or macrolide use alone must be considered with the caveat that nontypeable Haemophilus influenzae now likely surpasses pneumococcus as an AOM pathogen, and neither drug class is active against nontypeable H. influenzae.
What are the implications for prevention? This is one of many studies in the post-PCV era reporting serotype replacement with nonvaccine serotypes. But most prior studies reported reduced overall disease burden; in other words, the absolute number of pneumococcal infections was reduced, but residual AOM nonvaccine types dominated as the etiology. The current study, however, suggests that the overall number of AOM episodes may not be less because increases in AOM caused by nonvaccine serotypes may be offsetting declines in AOM caused by vaccine serotypes. This concept contrasts to multiple large epidemiologic studies demonstrating a decline in overall incidence of AOM office visits/episodes and several Israeli studies reporting a decline in pneumococcal AOM in children who warrant tympanocentesis. These new data are food for thought, but antibiotic resistance can vary regionally, so confirmation based on data from other regions seems warranted.
Next-generation vaccines will need to consider which serotypes are prevalent in pneumococcal disease, including AOM, as we continue into the PCV13 era. However, serotypes causing invasive pneumococcal disease and pneumonia would be higher priorities than AOM. Indeed, several candidate PCV vaccines are currently in clinical trials adding up to seven serotypes, including most of the newly emerging invasive disease serotypes. One downside to the newer PCVs is lack of serogroup 35, a prominent culprit in AOM resistance in the current report.
Stephen I. Pelton, MD, is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Christopher J. Harrison, MD, is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Dr. Pelton has received honorarium from Merck Vaccines, Pfizer, and Sanofi for participation in advisory board meeting on pneumococcal vaccine and/or membership on the Data and Safety Monitoring Board. Boston Medical Center has received investigator-initiated research grants from Merck Vaccines and Pfizer.
Children’s Mercy Hospital – Kansas City Boston Medical Center has received funding from GlaxoSmithKline, Merck, and Pfizer for research vaccine studies, and from Pfizer and Merck for investigator-initiated research grants for in vitro pneumococcal investigations on which Dr. Harrison is an investigator.
Dr. Kaur and colleagues report their analysis of pneumococcal resistance among nasopharyngeal and middle ear isolates (90% nasopharyngeal and 10% middle ear) collected between 2008 and 2016. They demonstrate the dominant role that nonvaccine serotypes play in carriage and acute otitis media (AOM) in children, and by extension potentially the entire spectrum of pneumococcal disease in the 13-valent pneumococcal conjugate vaccine (PCV13) era. Nonsusceptibility to beta-lactams was reported for one-third of isolates with the increase in the most recent reported years (2013-2016).
What are the implications for treatment of pneumococcal infections? For AOM, amoxicillin minimum inhibitory concentrations (MIC) were all less than 4 mcg/mL, which is the pharmacodynamic breakpoint for high-dose (90 mg/kg per day) AOM regimens; these data support continued use of high-dose amoxicillin for children with AOM that requires antimicrobial treatment. Resistance to macrolides (erythromycin and likely azithromycin) occurred in approximately one-third of isolates; however, in contrast to beta-lactams (amoxicillin), higher macrolide doses do not overcome resistance. Thus macrolide use for AOM appears limited to those with beta-lactam allergy and no better alternative drug, i.e., expect failure in one-third of AOM patients if macrolides are used. For ceftriaxone, no 2013-2016 isolate had a MIC over 0.5 mcg/mL, implying that ceftriaxone remains appropriate first-line therapy for serious pneumococcal disease and effective for pneumococcal AOM when oral drugs have failed or are not an option because of repeated emesis. Interestingly, trimethoprim/sulfamethoxazole (T/S) had lower resistance rates against the nonvaccine “bad boy” serogroup 35 (8%-15%), compared with cephalosporins (32%-57%). Perhaps we are back to the future and T/S will again have a role against pneumococcal AOM. Of note, no isolate was resistant to levofloxacin or linezolid. Linezolid or macrolide use alone must be considered with the caveat that nontypeable Haemophilus influenzae now likely surpasses pneumococcus as an AOM pathogen, and neither drug class is active against nontypeable H. influenzae.
What are the implications for prevention? This is one of many studies in the post-PCV era reporting serotype replacement with nonvaccine serotypes. But most prior studies reported reduced overall disease burden; in other words, the absolute number of pneumococcal infections was reduced, but residual AOM nonvaccine types dominated as the etiology. The current study, however, suggests that the overall number of AOM episodes may not be less because increases in AOM caused by nonvaccine serotypes may be offsetting declines in AOM caused by vaccine serotypes. This concept contrasts to multiple large epidemiologic studies demonstrating a decline in overall incidence of AOM office visits/episodes and several Israeli studies reporting a decline in pneumococcal AOM in children who warrant tympanocentesis. These new data are food for thought, but antibiotic resistance can vary regionally, so confirmation based on data from other regions seems warranted.
Next-generation vaccines will need to consider which serotypes are prevalent in pneumococcal disease, including AOM, as we continue into the PCV13 era. However, serotypes causing invasive pneumococcal disease and pneumonia would be higher priorities than AOM. Indeed, several candidate PCV vaccines are currently in clinical trials adding up to seven serotypes, including most of the newly emerging invasive disease serotypes. One downside to the newer PCVs is lack of serogroup 35, a prominent culprit in AOM resistance in the current report.
Stephen I. Pelton, MD, is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Christopher J. Harrison, MD, is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Dr. Pelton has received honorarium from Merck Vaccines, Pfizer, and Sanofi for participation in advisory board meeting on pneumococcal vaccine and/or membership on the Data and Safety Monitoring Board. Boston Medical Center has received investigator-initiated research grants from Merck Vaccines and Pfizer.
Children’s Mercy Hospital – Kansas City Boston Medical Center has received funding from GlaxoSmithKline, Merck, and Pfizer for research vaccine studies, and from Pfizer and Merck for investigator-initiated research grants for in vitro pneumococcal investigations on which Dr. Harrison is an investigator.
Dr. Kaur and colleagues report their analysis of pneumococcal resistance among nasopharyngeal and middle ear isolates (90% nasopharyngeal and 10% middle ear) collected between 2008 and 2016. They demonstrate the dominant role that nonvaccine serotypes play in carriage and acute otitis media (AOM) in children, and by extension potentially the entire spectrum of pneumococcal disease in the 13-valent pneumococcal conjugate vaccine (PCV13) era. Nonsusceptibility to beta-lactams was reported for one-third of isolates with the increase in the most recent reported years (2013-2016).
What are the implications for treatment of pneumococcal infections? For AOM, amoxicillin minimum inhibitory concentrations (MIC) were all less than 4 mcg/mL, which is the pharmacodynamic breakpoint for high-dose (90 mg/kg per day) AOM regimens; these data support continued use of high-dose amoxicillin for children with AOM that requires antimicrobial treatment. Resistance to macrolides (erythromycin and likely azithromycin) occurred in approximately one-third of isolates; however, in contrast to beta-lactams (amoxicillin), higher macrolide doses do not overcome resistance. Thus macrolide use for AOM appears limited to those with beta-lactam allergy and no better alternative drug, i.e., expect failure in one-third of AOM patients if macrolides are used. For ceftriaxone, no 2013-2016 isolate had a MIC over 0.5 mcg/mL, implying that ceftriaxone remains appropriate first-line therapy for serious pneumococcal disease and effective for pneumococcal AOM when oral drugs have failed or are not an option because of repeated emesis. Interestingly, trimethoprim/sulfamethoxazole (T/S) had lower resistance rates against the nonvaccine “bad boy” serogroup 35 (8%-15%), compared with cephalosporins (32%-57%). Perhaps we are back to the future and T/S will again have a role against pneumococcal AOM. Of note, no isolate was resistant to levofloxacin or linezolid. Linezolid or macrolide use alone must be considered with the caveat that nontypeable Haemophilus influenzae now likely surpasses pneumococcus as an AOM pathogen, and neither drug class is active against nontypeable H. influenzae.
What are the implications for prevention? This is one of many studies in the post-PCV era reporting serotype replacement with nonvaccine serotypes. But most prior studies reported reduced overall disease burden; in other words, the absolute number of pneumococcal infections was reduced, but residual AOM nonvaccine types dominated as the etiology. The current study, however, suggests that the overall number of AOM episodes may not be less because increases in AOM caused by nonvaccine serotypes may be offsetting declines in AOM caused by vaccine serotypes. This concept contrasts to multiple large epidemiologic studies demonstrating a decline in overall incidence of AOM office visits/episodes and several Israeli studies reporting a decline in pneumococcal AOM in children who warrant tympanocentesis. These new data are food for thought, but antibiotic resistance can vary regionally, so confirmation based on data from other regions seems warranted.
Next-generation vaccines will need to consider which serotypes are prevalent in pneumococcal disease, including AOM, as we continue into the PCV13 era. However, serotypes causing invasive pneumococcal disease and pneumonia would be higher priorities than AOM. Indeed, several candidate PCV vaccines are currently in clinical trials adding up to seven serotypes, including most of the newly emerging invasive disease serotypes. One downside to the newer PCVs is lack of serogroup 35, a prominent culprit in AOM resistance in the current report.
Stephen I. Pelton, MD, is professor of pediatrics and epidemiology at Boston University and senior attending physician at Boston Medical Center. Christopher J. Harrison, MD, is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Dr. Pelton has received honorarium from Merck Vaccines, Pfizer, and Sanofi for participation in advisory board meeting on pneumococcal vaccine and/or membership on the Data and Safety Monitoring Board. Boston Medical Center has received investigator-initiated research grants from Merck Vaccines and Pfizer.
Children’s Mercy Hospital – Kansas City Boston Medical Center has received funding from GlaxoSmithKline, Merck, and Pfizer for research vaccine studies, and from Pfizer and Merck for investigator-initiated research grants for in vitro pneumococcal investigations on which Dr. Harrison is an investigator.
Antibiotic resistance in strains of Streptococcus pneumoniae has been rising since 2013 because of changing susceptibility profiles, based on data from 1,201 isolates collected from 448 children in primary care settings.
“New strains expressing capsular serotypes not included in the 13-valent pneumococcal conjugate vaccine are emerging to cause disease, and strains that acquire antibiotic resistance are increasing in frequency due to their survival of the fittest advantage,” wrote Ravinder Kaur, PhD, of Rochester (N.Y.) General Hospital Research Institute, and colleagues.
Similar Darwinian principles occurred after the introduction of PCV-7, the study authors added.
In a prospective cohort study published in Clinical Infectious Diseases, the researchers reviewed 1,201 isolates collected from the nasopharynx during healthy periods, and from the nasopharynx and middle ear fluid (MEF) during episodes of acute otitis media, in children aged 6-36 months who were seen in primary care settings.
The isolates were collected during 2006-2016 to reflect the pre- and post-PCV13 era. Children received PCV-7 from 2006 until April 2010, and received PCV-13 after April 2010.
Overall, the number of acute otitis media (AOM) cases caused by S. pneumoniae was not significantly different between the PCV-7 and PCV-13 eras, nor was the frequency of pneumococci identified in the nasopharynx during healthy visits and visits at the start of an AOM infection.
The researchers examined susceptibility using minimum inhibitory concentrations (MIC). During healthy visits, the MIC50 of isolated pneumococci was low (no greater than 0.06 mcg/mL) for all four beta-lactam drugs tested. And it didn’t change significantly over the study years.
In contrast, among the nasopharyngeal and MEF isolates during AOM, the MIC50 to penicillin, amoxicillin, ceftriaxone, and meropenem during 2013-2016 rose significantly, the investigators said.
A change in antibiotic susceptibility within a subtype also contributed to the development of PCV-13 resistance.
The study authors identified three serotypes that affected the changes in susceptibility in their study population. Serotypes 35B and 35F increased their beta-lactam resistance during 2013-2016, and serotype 11A had a higher MIC to quinolones and became more prevalent during 2013-2016. Those three serotypes accounted for most of the change in antibiotic susceptibility, the researchers said.
In addition, “the frequency of strains resistant to penicillin and amoxicillin decreased with the introduction of PCV-13, but rebounded to levels similar to those before PCV-13 introduction by 2015-2016,” the investigators noted.
The study findings were limited by several factors, including the homogeneous study population and potential lack of generalizability to other settings. In addition, the researchers did not study antibiotic consumption or antibiotic treatment failure, and they could not account for potential AOM cases that may have been treated in settings other than primary care.
However, the investigators said the results support the need for additional studies and attention to the development of the next generation of PCVs, the PCV-15 and PCV-20. Both include serotypes 22F and 33F, but neither includes 35B or 35F. The PCV-20 also includes 11A and 15B.
The study was supported in part by the National Institutes of Health and Sanofi Pasteur. Some isolates collected during the 2010-2013 time period were part of a study supported by Pfizer. The researchers had no relevant financial conflicts to disclose.
SOURCE: Kaur R et al. Clin Inf Dis. 2020 Feb 18. doi: 10.1093/cid/ciaa157.
Antibiotic resistance in strains of Streptococcus pneumoniae has been rising since 2013 because of changing susceptibility profiles, based on data from 1,201 isolates collected from 448 children in primary care settings.
“New strains expressing capsular serotypes not included in the 13-valent pneumococcal conjugate vaccine are emerging to cause disease, and strains that acquire antibiotic resistance are increasing in frequency due to their survival of the fittest advantage,” wrote Ravinder Kaur, PhD, of Rochester (N.Y.) General Hospital Research Institute, and colleagues.
Similar Darwinian principles occurred after the introduction of PCV-7, the study authors added.
In a prospective cohort study published in Clinical Infectious Diseases, the researchers reviewed 1,201 isolates collected from the nasopharynx during healthy periods, and from the nasopharynx and middle ear fluid (MEF) during episodes of acute otitis media, in children aged 6-36 months who were seen in primary care settings.
The isolates were collected during 2006-2016 to reflect the pre- and post-PCV13 era. Children received PCV-7 from 2006 until April 2010, and received PCV-13 after April 2010.
Overall, the number of acute otitis media (AOM) cases caused by S. pneumoniae was not significantly different between the PCV-7 and PCV-13 eras, nor was the frequency of pneumococci identified in the nasopharynx during healthy visits and visits at the start of an AOM infection.
The researchers examined susceptibility using minimum inhibitory concentrations (MIC). During healthy visits, the MIC50 of isolated pneumococci was low (no greater than 0.06 mcg/mL) for all four beta-lactam drugs tested. And it didn’t change significantly over the study years.
In contrast, among the nasopharyngeal and MEF isolates during AOM, the MIC50 to penicillin, amoxicillin, ceftriaxone, and meropenem during 2013-2016 rose significantly, the investigators said.
A change in antibiotic susceptibility within a subtype also contributed to the development of PCV-13 resistance.
The study authors identified three serotypes that affected the changes in susceptibility in their study population. Serotypes 35B and 35F increased their beta-lactam resistance during 2013-2016, and serotype 11A had a higher MIC to quinolones and became more prevalent during 2013-2016. Those three serotypes accounted for most of the change in antibiotic susceptibility, the researchers said.
In addition, “the frequency of strains resistant to penicillin and amoxicillin decreased with the introduction of PCV-13, but rebounded to levels similar to those before PCV-13 introduction by 2015-2016,” the investigators noted.
The study findings were limited by several factors, including the homogeneous study population and potential lack of generalizability to other settings. In addition, the researchers did not study antibiotic consumption or antibiotic treatment failure, and they could not account for potential AOM cases that may have been treated in settings other than primary care.
However, the investigators said the results support the need for additional studies and attention to the development of the next generation of PCVs, the PCV-15 and PCV-20. Both include serotypes 22F and 33F, but neither includes 35B or 35F. The PCV-20 also includes 11A and 15B.
The study was supported in part by the National Institutes of Health and Sanofi Pasteur. Some isolates collected during the 2010-2013 time period were part of a study supported by Pfizer. The researchers had no relevant financial conflicts to disclose.
SOURCE: Kaur R et al. Clin Inf Dis. 2020 Feb 18. doi: 10.1093/cid/ciaa157.
FROM CLINICAL INFECTIOUS DISEASES