User login
Woman with transplanted uterus gives birth to boy
It’s the first time that a baby has been born to a woman with a transplanted uterus outside of a clinical trial. Officials from University of Alabama–Birmingham Hospital, where the 2-year process took place, said in a statement on July 24 that the birth sets its uterus transplant program on track to perhaps become covered under insurance plans.
The process of uterus transplant, in vitro fertilization, and pregnancy involves 50 medical providers and is open to women who have uterine factor infertility (UFI). The condition may affect up to 5% of reproductive-age women worldwide. Women with UFI cannot carry a pregnancy to term because they were either born without a uterus, had it removed via hysterectomy, or have a uterus that does not function properly.
The woman, whom the hospital identified as Mallory, moved with her family to the Birmingham area to enter the transplant program, which is one of four programs operating in the United States. Mallory learned when she was 17 years old that she was born without a uterus because of Mayer-Rokitansky-Küster-Hauser syndrome. Her first child, a daughter, was born after her sister carried the pregnancy as a surrogate.
Mallory received her uterus from a deceased donor. Her son was born in May.
“As with other types of organ transplants, the woman must take immunosuppressive medications to prevent the body from rejecting the transplanted uterus,” the transplant program’s website states. “After the baby is born and if the woman does not want more children, the transplanted uterus is removed with a hysterectomy procedure, and the woman no longer needs to take antirejection medications.”
“There are all different ways to grow your family if you have uterine factor infertility, but this [uterus transplantation] is what I feel like I knew that I was supposed to do,” Mallory said in a statement. “I mean, just hearing the cry at first was just, you know, mind blowing.”
A version of this article first appeared on WebMD.com.
It’s the first time that a baby has been born to a woman with a transplanted uterus outside of a clinical trial. Officials from University of Alabama–Birmingham Hospital, where the 2-year process took place, said in a statement on July 24 that the birth sets its uterus transplant program on track to perhaps become covered under insurance plans.
The process of uterus transplant, in vitro fertilization, and pregnancy involves 50 medical providers and is open to women who have uterine factor infertility (UFI). The condition may affect up to 5% of reproductive-age women worldwide. Women with UFI cannot carry a pregnancy to term because they were either born without a uterus, had it removed via hysterectomy, or have a uterus that does not function properly.
The woman, whom the hospital identified as Mallory, moved with her family to the Birmingham area to enter the transplant program, which is one of four programs operating in the United States. Mallory learned when she was 17 years old that she was born without a uterus because of Mayer-Rokitansky-Küster-Hauser syndrome. Her first child, a daughter, was born after her sister carried the pregnancy as a surrogate.
Mallory received her uterus from a deceased donor. Her son was born in May.
“As with other types of organ transplants, the woman must take immunosuppressive medications to prevent the body from rejecting the transplanted uterus,” the transplant program’s website states. “After the baby is born and if the woman does not want more children, the transplanted uterus is removed with a hysterectomy procedure, and the woman no longer needs to take antirejection medications.”
“There are all different ways to grow your family if you have uterine factor infertility, but this [uterus transplantation] is what I feel like I knew that I was supposed to do,” Mallory said in a statement. “I mean, just hearing the cry at first was just, you know, mind blowing.”
A version of this article first appeared on WebMD.com.
It’s the first time that a baby has been born to a woman with a transplanted uterus outside of a clinical trial. Officials from University of Alabama–Birmingham Hospital, where the 2-year process took place, said in a statement on July 24 that the birth sets its uterus transplant program on track to perhaps become covered under insurance plans.
The process of uterus transplant, in vitro fertilization, and pregnancy involves 50 medical providers and is open to women who have uterine factor infertility (UFI). The condition may affect up to 5% of reproductive-age women worldwide. Women with UFI cannot carry a pregnancy to term because they were either born without a uterus, had it removed via hysterectomy, or have a uterus that does not function properly.
The woman, whom the hospital identified as Mallory, moved with her family to the Birmingham area to enter the transplant program, which is one of four programs operating in the United States. Mallory learned when she was 17 years old that she was born without a uterus because of Mayer-Rokitansky-Küster-Hauser syndrome. Her first child, a daughter, was born after her sister carried the pregnancy as a surrogate.
Mallory received her uterus from a deceased donor. Her son was born in May.
“As with other types of organ transplants, the woman must take immunosuppressive medications to prevent the body from rejecting the transplanted uterus,” the transplant program’s website states. “After the baby is born and if the woman does not want more children, the transplanted uterus is removed with a hysterectomy procedure, and the woman no longer needs to take antirejection medications.”
“There are all different ways to grow your family if you have uterine factor infertility, but this [uterus transplantation] is what I feel like I knew that I was supposed to do,” Mallory said in a statement. “I mean, just hearing the cry at first was just, you know, mind blowing.”
A version of this article first appeared on WebMD.com.
Pregnancy risks elevated in women with chronic pancreatitis
TOPLINE:
METHODOLOGY:
- A retrospective analysis of hospital discharge records from the National Inpatient Sample database between 2009 and 2019 was conducted.
- The sample included 3,094 pregnancies with chronic pancreatitis and roughly 40.8 million pregnancies without this condition.
- The study focused on primary maternal outcomes and primary perinatal outcomes in pregnancies affected by chronic pancreatitis after accounting for relevant covariates.
TAKEAWAY:
- Chronic pancreatitis pregnancies had elevated rates of gestational diabetes (adjusted odds ratio, 1.63), gestational hypertensive complications (aOR, 2.48), preterm labor (aOR, 3.10), and small size for gestational age (aOR, 2.40).
- Women with chronic pancreatitis and a history of renal failure were more prone to gestational hypertensive complications (aOR, 20.09).
- Women with alcohol-induced chronic pancreatitis had a 17-fold higher risk for fetal death (aOR, 17.15).
- Pregnancies with chronic pancreatitis were associated with longer hospital stays and higher hospital costs.
IN PRACTICE:
“Our study provides novel insights into the impact of chronic pancreatitis on maternal and fetal health. The implications of our findings are critical for health care professionals, particularly those involved in preconception counseling. Pregnant women with chronic pancreatitis should be under the care of a multidisciplinary team of health care providers,” the authors advise.
SOURCE:
The study was led by Chengu Niu, MD, with Rochester General Hospital, Rochester, N.Y. It was published online July 18 in Digestive and Liver Disease. The study had no specific funding.
LIMITATIONS:
The authors note potential inaccuracies because of coding in the National Inpatient Sample database, a lack of detailed information regarding medication use, and a lack of follow-up clinical information. The findings are specific to the United States and may not be applicable to other countries.
DISCLOSURES:
The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A retrospective analysis of hospital discharge records from the National Inpatient Sample database between 2009 and 2019 was conducted.
- The sample included 3,094 pregnancies with chronic pancreatitis and roughly 40.8 million pregnancies without this condition.
- The study focused on primary maternal outcomes and primary perinatal outcomes in pregnancies affected by chronic pancreatitis after accounting for relevant covariates.
TAKEAWAY:
- Chronic pancreatitis pregnancies had elevated rates of gestational diabetes (adjusted odds ratio, 1.63), gestational hypertensive complications (aOR, 2.48), preterm labor (aOR, 3.10), and small size for gestational age (aOR, 2.40).
- Women with chronic pancreatitis and a history of renal failure were more prone to gestational hypertensive complications (aOR, 20.09).
- Women with alcohol-induced chronic pancreatitis had a 17-fold higher risk for fetal death (aOR, 17.15).
- Pregnancies with chronic pancreatitis were associated with longer hospital stays and higher hospital costs.
IN PRACTICE:
“Our study provides novel insights into the impact of chronic pancreatitis on maternal and fetal health. The implications of our findings are critical for health care professionals, particularly those involved in preconception counseling. Pregnant women with chronic pancreatitis should be under the care of a multidisciplinary team of health care providers,” the authors advise.
SOURCE:
The study was led by Chengu Niu, MD, with Rochester General Hospital, Rochester, N.Y. It was published online July 18 in Digestive and Liver Disease. The study had no specific funding.
LIMITATIONS:
The authors note potential inaccuracies because of coding in the National Inpatient Sample database, a lack of detailed information regarding medication use, and a lack of follow-up clinical information. The findings are specific to the United States and may not be applicable to other countries.
DISCLOSURES:
The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A retrospective analysis of hospital discharge records from the National Inpatient Sample database between 2009 and 2019 was conducted.
- The sample included 3,094 pregnancies with chronic pancreatitis and roughly 40.8 million pregnancies without this condition.
- The study focused on primary maternal outcomes and primary perinatal outcomes in pregnancies affected by chronic pancreatitis after accounting for relevant covariates.
TAKEAWAY:
- Chronic pancreatitis pregnancies had elevated rates of gestational diabetes (adjusted odds ratio, 1.63), gestational hypertensive complications (aOR, 2.48), preterm labor (aOR, 3.10), and small size for gestational age (aOR, 2.40).
- Women with chronic pancreatitis and a history of renal failure were more prone to gestational hypertensive complications (aOR, 20.09).
- Women with alcohol-induced chronic pancreatitis had a 17-fold higher risk for fetal death (aOR, 17.15).
- Pregnancies with chronic pancreatitis were associated with longer hospital stays and higher hospital costs.
IN PRACTICE:
“Our study provides novel insights into the impact of chronic pancreatitis on maternal and fetal health. The implications of our findings are critical for health care professionals, particularly those involved in preconception counseling. Pregnant women with chronic pancreatitis should be under the care of a multidisciplinary team of health care providers,” the authors advise.
SOURCE:
The study was led by Chengu Niu, MD, with Rochester General Hospital, Rochester, N.Y. It was published online July 18 in Digestive and Liver Disease. The study had no specific funding.
LIMITATIONS:
The authors note potential inaccuracies because of coding in the National Inpatient Sample database, a lack of detailed information regarding medication use, and a lack of follow-up clinical information. The findings are specific to the United States and may not be applicable to other countries.
DISCLOSURES:
The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
Cancer Patients: Who’s at Risk for Venous Thromboembolism?
Patients with cancer are at a high risk of venous thromboembolism (VTE)—in fact, it’s one of the leading causes of death in patients who receive systemic therapy for cancer. But as cancer treatment has evolved, have the incidence and risk of VTE changed too?
Researchers from Veterans Affairs Boston Healthcare System in Massachusetts conducted a study with 434,203 veterans to evaluate the pattern of VTE incidence over 16 years, focusing on the types of cancer, treatment, race and ethnicity, and other factors related to cancer-associated thrombosis (CAT).
In contrast with other large population studies, this study found the overall incidence of CAT remained largely stable over time. At 12 months, the incidence was 4.5%, with yearly trends ranging between 4.2% and 4.7%. “As expected,” the researchers say, the subset of patients receiving systemic therapy had a higher incidence of VTE at 12 months (7.7%) than did the overall cohort. The pattern was “particularly pronounced” in gynecologic, testicular, and kidney cancers, where the incidence of VTE was 2 to 3 times higher in the treated cohort compared with the overall cohort.
Cancer type and diagnosis were the most statistically and clinically significant associations with CAT, with up to a 6-fold difference between cancer subtypes. The patients at the highest risk of VTE were those with pancreatic cancer and acute lymphoblastic leukemia.
Most studies have focused only on patients with solid tumors, but these researchers observed novel patterns among patients with hematologic neoplasms. Specifically, a higher incidence of VTE among patients with aggressive vs indolent leukemias and lymphomas. This trend, the researchers say, may be associated in part with catheter-related events.
Furthermore, the type of system treatment was associated with the risk of VTE, the researchers say, although to a lesser extent. Chemotherapy- and immunotherapy-based regimens had the highest risk of VTE, relative to no treatment. Targeted and endocrine therapy also carried a higher risk compared with no treatment but to a lesser degree.
The researchers found significant heterogeneity by race and ethnicity across cancer types. Non-Hispanic Black patients had about 20% higher risk of VTE compared with non-Hispanic White patients. Asian and Pacific Islander patients had about 20% lower risk compared with non-Hispanic White patients.
Male sex was also associated with VTE. However, “interestingly,” the researchers note, neighborhood-level socioeconomic factors and patients’ comorbidities were not associated with CAT but were associated with mortality.
Their results suggest that patient- and treatment-specific factors play a critical role in assessing the risk of CAT, and “ongoing efforts to identify these patterns are of utmost importance for risk stratification and prognostic assessment.”
Patients with cancer are at a high risk of venous thromboembolism (VTE)—in fact, it’s one of the leading causes of death in patients who receive systemic therapy for cancer. But as cancer treatment has evolved, have the incidence and risk of VTE changed too?
Researchers from Veterans Affairs Boston Healthcare System in Massachusetts conducted a study with 434,203 veterans to evaluate the pattern of VTE incidence over 16 years, focusing on the types of cancer, treatment, race and ethnicity, and other factors related to cancer-associated thrombosis (CAT).
In contrast with other large population studies, this study found the overall incidence of CAT remained largely stable over time. At 12 months, the incidence was 4.5%, with yearly trends ranging between 4.2% and 4.7%. “As expected,” the researchers say, the subset of patients receiving systemic therapy had a higher incidence of VTE at 12 months (7.7%) than did the overall cohort. The pattern was “particularly pronounced” in gynecologic, testicular, and kidney cancers, where the incidence of VTE was 2 to 3 times higher in the treated cohort compared with the overall cohort.
Cancer type and diagnosis were the most statistically and clinically significant associations with CAT, with up to a 6-fold difference between cancer subtypes. The patients at the highest risk of VTE were those with pancreatic cancer and acute lymphoblastic leukemia.
Most studies have focused only on patients with solid tumors, but these researchers observed novel patterns among patients with hematologic neoplasms. Specifically, a higher incidence of VTE among patients with aggressive vs indolent leukemias and lymphomas. This trend, the researchers say, may be associated in part with catheter-related events.
Furthermore, the type of system treatment was associated with the risk of VTE, the researchers say, although to a lesser extent. Chemotherapy- and immunotherapy-based regimens had the highest risk of VTE, relative to no treatment. Targeted and endocrine therapy also carried a higher risk compared with no treatment but to a lesser degree.
The researchers found significant heterogeneity by race and ethnicity across cancer types. Non-Hispanic Black patients had about 20% higher risk of VTE compared with non-Hispanic White patients. Asian and Pacific Islander patients had about 20% lower risk compared with non-Hispanic White patients.
Male sex was also associated with VTE. However, “interestingly,” the researchers note, neighborhood-level socioeconomic factors and patients’ comorbidities were not associated with CAT but were associated with mortality.
Their results suggest that patient- and treatment-specific factors play a critical role in assessing the risk of CAT, and “ongoing efforts to identify these patterns are of utmost importance for risk stratification and prognostic assessment.”
Patients with cancer are at a high risk of venous thromboembolism (VTE)—in fact, it’s one of the leading causes of death in patients who receive systemic therapy for cancer. But as cancer treatment has evolved, have the incidence and risk of VTE changed too?
Researchers from Veterans Affairs Boston Healthcare System in Massachusetts conducted a study with 434,203 veterans to evaluate the pattern of VTE incidence over 16 years, focusing on the types of cancer, treatment, race and ethnicity, and other factors related to cancer-associated thrombosis (CAT).
In contrast with other large population studies, this study found the overall incidence of CAT remained largely stable over time. At 12 months, the incidence was 4.5%, with yearly trends ranging between 4.2% and 4.7%. “As expected,” the researchers say, the subset of patients receiving systemic therapy had a higher incidence of VTE at 12 months (7.7%) than did the overall cohort. The pattern was “particularly pronounced” in gynecologic, testicular, and kidney cancers, where the incidence of VTE was 2 to 3 times higher in the treated cohort compared with the overall cohort.
Cancer type and diagnosis were the most statistically and clinically significant associations with CAT, with up to a 6-fold difference between cancer subtypes. The patients at the highest risk of VTE were those with pancreatic cancer and acute lymphoblastic leukemia.
Most studies have focused only on patients with solid tumors, but these researchers observed novel patterns among patients with hematologic neoplasms. Specifically, a higher incidence of VTE among patients with aggressive vs indolent leukemias and lymphomas. This trend, the researchers say, may be associated in part with catheter-related events.
Furthermore, the type of system treatment was associated with the risk of VTE, the researchers say, although to a lesser extent. Chemotherapy- and immunotherapy-based regimens had the highest risk of VTE, relative to no treatment. Targeted and endocrine therapy also carried a higher risk compared with no treatment but to a lesser degree.
The researchers found significant heterogeneity by race and ethnicity across cancer types. Non-Hispanic Black patients had about 20% higher risk of VTE compared with non-Hispanic White patients. Asian and Pacific Islander patients had about 20% lower risk compared with non-Hispanic White patients.
Male sex was also associated with VTE. However, “interestingly,” the researchers note, neighborhood-level socioeconomic factors and patients’ comorbidities were not associated with CAT but were associated with mortality.
Their results suggest that patient- and treatment-specific factors play a critical role in assessing the risk of CAT, and “ongoing efforts to identify these patterns are of utmost importance for risk stratification and prognostic assessment.”
Regional Meeting Focuses on Women’s Cancer Survivorship
As the number of female veterans continues to grow, the US Department of Veterans Affairs (VA) is adjusting by focusing more on breast/gynecological cancer and referring fewer cases to outside clinicians.
The VA’s effort reflects the reality that female veterans from the wars in Afghanistan and Iraq are approaching the ages—50s, 60s, and 70s—when cancer diagnoses become more common, said Sarah Colonna, MD, national medical director of breast oncology for VA's Breast and Gynecologic Oncology System of Excellence and an oncologist at the Huntsman Cancer Institute and Wahlen VA Medical Center in Salt Lake City, Utah. “This is preparation for the change that we know is coming.”
In response, the Association of VA Hematology/Oncology (AVAHO) is devoting a regional meeting in Tampa, Florida (July 29, 2023) to improving survivorship for patients with women’s cancers. “This meeting is designed to educate both cancer experts and primary care providers on the care of women who have already gone through breast and gynecological cancer treatment,” Colonna explained.
Adherence Challenges
Colonna will speak in a session about the importance of adherence to endocrine therapy. “When we prescribe endocrine therapy for breast cancer, we usually ask women to stay on it for 5 to 10 years, and sometimes that’s hard for them,” she said. “I’ll talk about tips and tricks to help women stay on endocrine therapy for the long haul because we know that is linked to better survival.”
Between two-thirds and three-quarters of women with breast cancer are advised to stay on endocrine drugs, she said, but the medications can be difficult to tolerate due to adverse effects such as hot flashes and sleep disturbances.
In addition, patients are often anxious about the medications. “Women are very leery of anything that changes or makes their hormones different,” Colonna noted. “They feel like it’s messing with something that is natural for them.”
Colonna urges colleagues to focus on their “soft skills,” the ability to absorb and validate the worries of patients. Instead of dismissing them, she said, focus on messages that acknowledge concerns but are also firm: “That’s real, that sucks. But we’ve got to do it.”
It’s also helpful to guide patients away from thinking that taking a pill every day means they’re sick. “I try to flip that paradigm: ‘You’re taking this pill every day because you have power over this thing that happened to you.’”
Education is also key, she said, so that patients “understand very clearly why this medication is important for them: It increases the chance of surviving breast cancer or it increases the chances that the cancer will never come back in your arm or in your breast. Then, whether they make a decision to take it or not, at least they’re making the choice with knowledge.”
As for adverse effects, Colonna said medications such as antidepressants and painkillers can relieve hot flashes, which can disturb sleep.
Identifying the best strategy to address adverse effects “requires keeping in frequent contact with the patient during the first 6 months of endocrine therapy, which are really critical,” she said. “Once they’ve been on it for a year, they can see the light at the end of the tunnel and hang in there even if they have adverse effects.”
Some guidelines suggest that no doctor visits are needed until the 6-month mark, but Colonna prefers to check in at the 4- to 6-week mark, even if it’s just via a phone call. Otherwise, “often they’ll stop taking the pill, and then you won’t know about it until you see them at 6 six months.” At that point, she said, a critical period for treatment has passed.
The Role of Nurse Navigators
In another session at the Tampa regional meeting, AVAHO president-elect Cindy Bowman, MSN, RN, OCN, will moderate a session about the role of nurse navigators in VA cancer care. She is the coordinator of the Cancer Care Navigation Program at the C. W. Bill Young VA Medical Center in Bay Pines, Florida.
“Veterans become survivors the day they’re diagnosed with cancer,” she said. Within the VA, cancer-care navigator teams developed over the past decade aim to help patients find their way forward through survivorship, she said, and nurses are crucial to the effort.
As Sharp and Scheid reported in a 2018 Journal Oncology Navigation Survivorship article, “research demonstrates that navigation can improve access to the cancer care system by addressing barriers, as well as facilitating quality care. The benefits of patient navigation for improving cancer patient outcomes is considerable.” McKenney and colleagues found that “patient navigation has been demonstrated to increase access to screening, shorten time to diagnostic resolution, and improve cancer outcomes, particularly in health disparity populations, such as women of color, rural populations, and poor women.”
According to Bowman, “it has become standard practice to have nurse navigators be there each step of the way from a high suspicion of cancer to diagnosis and through the clinical workup into active treatment and survivorship.” Within the VA, she said, “the focus right now is to look at standardizing care that all VAs will be able to offer holistic, comprehensive cancer-care navigation teams.”
At the regional meeting, Bowman’s session will include updates from nurse navigators about helping patients through breast/gynecological cancer, abnormal mammograms, and survivorship.
Nurse navigators are typically the second medical professionals who talk to cancer patients after their physicians, Bowman said. The unique knowledge of oncology nurse navigators gives them invaluable insight into treatment plans and cancer drug regimens, she said.
“They’re able to sit down and discuss the actual cancer drug regimen with patients—what each of those drugs do, how they’re administered, the short-term and long-term side effects,” she said. “They have the knowledge about all aspects of cancer care that can really only come from somebody who’s specialty trained.”
Other sessions at the AVAHO regional meeting will highlight breast cancer and lymphedema, breast cancer and bone health; diet, exercise and cancer; sexual health for breast/gynecological cancer survivors; and imaging surveillance after diagnosis.
As the number of female veterans continues to grow, the US Department of Veterans Affairs (VA) is adjusting by focusing more on breast/gynecological cancer and referring fewer cases to outside clinicians.
The VA’s effort reflects the reality that female veterans from the wars in Afghanistan and Iraq are approaching the ages—50s, 60s, and 70s—when cancer diagnoses become more common, said Sarah Colonna, MD, national medical director of breast oncology for VA's Breast and Gynecologic Oncology System of Excellence and an oncologist at the Huntsman Cancer Institute and Wahlen VA Medical Center in Salt Lake City, Utah. “This is preparation for the change that we know is coming.”
In response, the Association of VA Hematology/Oncology (AVAHO) is devoting a regional meeting in Tampa, Florida (July 29, 2023) to improving survivorship for patients with women’s cancers. “This meeting is designed to educate both cancer experts and primary care providers on the care of women who have already gone through breast and gynecological cancer treatment,” Colonna explained.
Adherence Challenges
Colonna will speak in a session about the importance of adherence to endocrine therapy. “When we prescribe endocrine therapy for breast cancer, we usually ask women to stay on it for 5 to 10 years, and sometimes that’s hard for them,” she said. “I’ll talk about tips and tricks to help women stay on endocrine therapy for the long haul because we know that is linked to better survival.”
Between two-thirds and three-quarters of women with breast cancer are advised to stay on endocrine drugs, she said, but the medications can be difficult to tolerate due to adverse effects such as hot flashes and sleep disturbances.
In addition, patients are often anxious about the medications. “Women are very leery of anything that changes or makes their hormones different,” Colonna noted. “They feel like it’s messing with something that is natural for them.”
Colonna urges colleagues to focus on their “soft skills,” the ability to absorb and validate the worries of patients. Instead of dismissing them, she said, focus on messages that acknowledge concerns but are also firm: “That’s real, that sucks. But we’ve got to do it.”
It’s also helpful to guide patients away from thinking that taking a pill every day means they’re sick. “I try to flip that paradigm: ‘You’re taking this pill every day because you have power over this thing that happened to you.’”
Education is also key, she said, so that patients “understand very clearly why this medication is important for them: It increases the chance of surviving breast cancer or it increases the chances that the cancer will never come back in your arm or in your breast. Then, whether they make a decision to take it or not, at least they’re making the choice with knowledge.”
As for adverse effects, Colonna said medications such as antidepressants and painkillers can relieve hot flashes, which can disturb sleep.
Identifying the best strategy to address adverse effects “requires keeping in frequent contact with the patient during the first 6 months of endocrine therapy, which are really critical,” she said. “Once they’ve been on it for a year, they can see the light at the end of the tunnel and hang in there even if they have adverse effects.”
Some guidelines suggest that no doctor visits are needed until the 6-month mark, but Colonna prefers to check in at the 4- to 6-week mark, even if it’s just via a phone call. Otherwise, “often they’ll stop taking the pill, and then you won’t know about it until you see them at 6 six months.” At that point, she said, a critical period for treatment has passed.
The Role of Nurse Navigators
In another session at the Tampa regional meeting, AVAHO president-elect Cindy Bowman, MSN, RN, OCN, will moderate a session about the role of nurse navigators in VA cancer care. She is the coordinator of the Cancer Care Navigation Program at the C. W. Bill Young VA Medical Center in Bay Pines, Florida.
“Veterans become survivors the day they’re diagnosed with cancer,” she said. Within the VA, cancer-care navigator teams developed over the past decade aim to help patients find their way forward through survivorship, she said, and nurses are crucial to the effort.
As Sharp and Scheid reported in a 2018 Journal Oncology Navigation Survivorship article, “research demonstrates that navigation can improve access to the cancer care system by addressing barriers, as well as facilitating quality care. The benefits of patient navigation for improving cancer patient outcomes is considerable.” McKenney and colleagues found that “patient navigation has been demonstrated to increase access to screening, shorten time to diagnostic resolution, and improve cancer outcomes, particularly in health disparity populations, such as women of color, rural populations, and poor women.”
According to Bowman, “it has become standard practice to have nurse navigators be there each step of the way from a high suspicion of cancer to diagnosis and through the clinical workup into active treatment and survivorship.” Within the VA, she said, “the focus right now is to look at standardizing care that all VAs will be able to offer holistic, comprehensive cancer-care navigation teams.”
At the regional meeting, Bowman’s session will include updates from nurse navigators about helping patients through breast/gynecological cancer, abnormal mammograms, and survivorship.
Nurse navigators are typically the second medical professionals who talk to cancer patients after their physicians, Bowman said. The unique knowledge of oncology nurse navigators gives them invaluable insight into treatment plans and cancer drug regimens, she said.
“They’re able to sit down and discuss the actual cancer drug regimen with patients—what each of those drugs do, how they’re administered, the short-term and long-term side effects,” she said. “They have the knowledge about all aspects of cancer care that can really only come from somebody who’s specialty trained.”
Other sessions at the AVAHO regional meeting will highlight breast cancer and lymphedema, breast cancer and bone health; diet, exercise and cancer; sexual health for breast/gynecological cancer survivors; and imaging surveillance after diagnosis.
As the number of female veterans continues to grow, the US Department of Veterans Affairs (VA) is adjusting by focusing more on breast/gynecological cancer and referring fewer cases to outside clinicians.
The VA’s effort reflects the reality that female veterans from the wars in Afghanistan and Iraq are approaching the ages—50s, 60s, and 70s—when cancer diagnoses become more common, said Sarah Colonna, MD, national medical director of breast oncology for VA's Breast and Gynecologic Oncology System of Excellence and an oncologist at the Huntsman Cancer Institute and Wahlen VA Medical Center in Salt Lake City, Utah. “This is preparation for the change that we know is coming.”
In response, the Association of VA Hematology/Oncology (AVAHO) is devoting a regional meeting in Tampa, Florida (July 29, 2023) to improving survivorship for patients with women’s cancers. “This meeting is designed to educate both cancer experts and primary care providers on the care of women who have already gone through breast and gynecological cancer treatment,” Colonna explained.
Adherence Challenges
Colonna will speak in a session about the importance of adherence to endocrine therapy. “When we prescribe endocrine therapy for breast cancer, we usually ask women to stay on it for 5 to 10 years, and sometimes that’s hard for them,” she said. “I’ll talk about tips and tricks to help women stay on endocrine therapy for the long haul because we know that is linked to better survival.”
Between two-thirds and three-quarters of women with breast cancer are advised to stay on endocrine drugs, she said, but the medications can be difficult to tolerate due to adverse effects such as hot flashes and sleep disturbances.
In addition, patients are often anxious about the medications. “Women are very leery of anything that changes or makes their hormones different,” Colonna noted. “They feel like it’s messing with something that is natural for them.”
Colonna urges colleagues to focus on their “soft skills,” the ability to absorb and validate the worries of patients. Instead of dismissing them, she said, focus on messages that acknowledge concerns but are also firm: “That’s real, that sucks. But we’ve got to do it.”
It’s also helpful to guide patients away from thinking that taking a pill every day means they’re sick. “I try to flip that paradigm: ‘You’re taking this pill every day because you have power over this thing that happened to you.’”
Education is also key, she said, so that patients “understand very clearly why this medication is important for them: It increases the chance of surviving breast cancer or it increases the chances that the cancer will never come back in your arm or in your breast. Then, whether they make a decision to take it or not, at least they’re making the choice with knowledge.”
As for adverse effects, Colonna said medications such as antidepressants and painkillers can relieve hot flashes, which can disturb sleep.
Identifying the best strategy to address adverse effects “requires keeping in frequent contact with the patient during the first 6 months of endocrine therapy, which are really critical,” she said. “Once they’ve been on it for a year, they can see the light at the end of the tunnel and hang in there even if they have adverse effects.”
Some guidelines suggest that no doctor visits are needed until the 6-month mark, but Colonna prefers to check in at the 4- to 6-week mark, even if it’s just via a phone call. Otherwise, “often they’ll stop taking the pill, and then you won’t know about it until you see them at 6 six months.” At that point, she said, a critical period for treatment has passed.
The Role of Nurse Navigators
In another session at the Tampa regional meeting, AVAHO president-elect Cindy Bowman, MSN, RN, OCN, will moderate a session about the role of nurse navigators in VA cancer care. She is the coordinator of the Cancer Care Navigation Program at the C. W. Bill Young VA Medical Center in Bay Pines, Florida.
“Veterans become survivors the day they’re diagnosed with cancer,” she said. Within the VA, cancer-care navigator teams developed over the past decade aim to help patients find their way forward through survivorship, she said, and nurses are crucial to the effort.
As Sharp and Scheid reported in a 2018 Journal Oncology Navigation Survivorship article, “research demonstrates that navigation can improve access to the cancer care system by addressing barriers, as well as facilitating quality care. The benefits of patient navigation for improving cancer patient outcomes is considerable.” McKenney and colleagues found that “patient navigation has been demonstrated to increase access to screening, shorten time to diagnostic resolution, and improve cancer outcomes, particularly in health disparity populations, such as women of color, rural populations, and poor women.”
According to Bowman, “it has become standard practice to have nurse navigators be there each step of the way from a high suspicion of cancer to diagnosis and through the clinical workup into active treatment and survivorship.” Within the VA, she said, “the focus right now is to look at standardizing care that all VAs will be able to offer holistic, comprehensive cancer-care navigation teams.”
At the regional meeting, Bowman’s session will include updates from nurse navigators about helping patients through breast/gynecological cancer, abnormal mammograms, and survivorship.
Nurse navigators are typically the second medical professionals who talk to cancer patients after their physicians, Bowman said. The unique knowledge of oncology nurse navigators gives them invaluable insight into treatment plans and cancer drug regimens, she said.
“They’re able to sit down and discuss the actual cancer drug regimen with patients—what each of those drugs do, how they’re administered, the short-term and long-term side effects,” she said. “They have the knowledge about all aspects of cancer care that can really only come from somebody who’s specialty trained.”
Other sessions at the AVAHO regional meeting will highlight breast cancer and lymphedema, breast cancer and bone health; diet, exercise and cancer; sexual health for breast/gynecological cancer survivors; and imaging surveillance after diagnosis.
Number of cervical cancer screenings linked to higher preterm birth risk
For each additional recommended screening before childbirth, there was a direct increase in absolute PTD risk of 0.073 (95% confidence interval, 0.026-0.120), according to a study led by Rebecca A. Bromley-Dulfano, MS, an MD candidate at Stanford (Calif.) University and a PhD candidate in health policy at Harvard University, Cambridge, Mass.
There was no significant change in very preterm delivery (VPTD) risk, but mothers with hypertension or diabetes were at higher PTD risk.
Women in this younger age group are more prone to PTD. According to the study’s estimate, an additional 73 PTDs per 100,000 women could be expected for every 1 additional recommended screening before childbirth. For the year 2018, that translated to an estimated 1,348 PTDs that could have been averted, with reduced screening requirements (3% relative reduction).
“If you screen someone for cervical cancer and find a cervical lesion, the possible next steps can include a biopsy and an excisional procedure to remove the lesion,” Ms. Bromley-Dulfano explained, “and these procedures which remove a small (mostly diseased) part of the cervix have been shown to slightly increase the risk of PTD. Particularly in young individuals with a cervix who are known to have high rates of lesion regression and who have more potential childbearing years ahead of them, it is important to weigh the oncological benefits with the adverse birth outcome risks.”
Young women are more likely to have false-positive results on Papanicolaou tests and lesion regression within 2 years but may undergo unnecessary treatment, the authors noted.
Cervical excision procedures have previously been associated in clinical trials with an increase in PTB risk.
In their 2017 decision model in a fictive cohort, for example, Kamphuis and colleagues found the most intensive screening program was associated with an increase in maternal life years of 9%, a decrease in cervical cancer incidence of 67%, and a decrease in cervical cancer deaths of 75%. But those gains came at the cost of 250% more preterm births, compared with the least intensive program.
“These results can be used in future simulation models integrating oncological trade-offs to help ascertain optimal screening strategies,” the researchers wrote.
While the optimal screening strategy must trade off the oncologic benefits of cancer detection against the neonatal harms of overtreatment, the ideal age of cervical cancer screening onset and frequency remain uncertain, the authors noted. Recent American Cancer Society guidelines recommending less frequent screening for some diverge from those of other societies.
“The first and foremost priority is for gynecologists to continue to have individualized conversations with patients about all of the benefits and risks of procedures that patients undergo and to understand the benefits and risks influencing screening guidelines,” Ms. Bromley-Dulfano said.
Cross-sectional study
The study used data from the Centers for Disease Control and Prevention’s National Center for Health Statistics to analyze associations between cervical cancer screening guidelines and birth outcomes women who had a singleton nulliparous birth from 19916 to 2018. Gestational age and maternal characteristics were drawn from birth certificates.
The mean age of the 11,333,151 multiracial cohort of women was 20.9 years, and 6.8% had hypertension or diabetes. The mean number of guideline-recommended screenings by time of childbirth was 2.4. Overall, PTD and very PTD occurred in 1,140,490 individuals (10.1%) and 333,040 (2.9%) of births, respectively.
Those with hypertension or diabetes had a somewhat higher PTD risk: 0.26% (95% CI, 0.11-0.4) versus 0.06% (95% CI, 0.01-0.10; Wald test, P < .001).
Offering an outsider’s perspective on the analysis, ob.gyn. Fidel A. Valea, MD, director of gynecologic oncology at the Northwell Health Cancer Institute in New Hyde Park, N.Y., urged caution in drawing conclusions from large population analyses such as this.
“This study had over 11 million data points. Often these large numbers will show statistical differences that are not clinically significant,” he said in an interview. He noted that while small studies have shown a possible impact of frequent Pap tests on cervical function, “this is not 100% proven. Research from Texas showed that screening made a difference only in cases of dysplasia.”
Dr. Valea also noted that screening guidelines have already changed over the lengthy time span of the study and do reflect the concerns of the study authors.
“We know that the HPV virus is cleared more readily by young women than older women and so we have made adjustments and test them less frequently and we test them less early.” He added that conservative options are recommended even in the case of dysplasia.
In defense of the Pap smear test, he added: “It has virtually wiped out cervical cancer in the U.S., bringing it from No. 1 to No. 13.” While broadening HPV vaccination programs may impact guidelines in the future, “vaccination is still in its infancy. We have to wait until women have lived long to enough to see an impact.”
As to why this age group is more vulnerable to PTD, Dr. Valea said, “It’s likely multifactorial, with lifestyle and other factors involved.” Although based on U.S. data, the authors said their results may be useful for other public health entities, particularly in countries where cervical cancer is considerably more prevalent.
This work received no specific funding. The authors and Dr. Valea disclosed no competing interests.
For each additional recommended screening before childbirth, there was a direct increase in absolute PTD risk of 0.073 (95% confidence interval, 0.026-0.120), according to a study led by Rebecca A. Bromley-Dulfano, MS, an MD candidate at Stanford (Calif.) University and a PhD candidate in health policy at Harvard University, Cambridge, Mass.
There was no significant change in very preterm delivery (VPTD) risk, but mothers with hypertension or diabetes were at higher PTD risk.
Women in this younger age group are more prone to PTD. According to the study’s estimate, an additional 73 PTDs per 100,000 women could be expected for every 1 additional recommended screening before childbirth. For the year 2018, that translated to an estimated 1,348 PTDs that could have been averted, with reduced screening requirements (3% relative reduction).
“If you screen someone for cervical cancer and find a cervical lesion, the possible next steps can include a biopsy and an excisional procedure to remove the lesion,” Ms. Bromley-Dulfano explained, “and these procedures which remove a small (mostly diseased) part of the cervix have been shown to slightly increase the risk of PTD. Particularly in young individuals with a cervix who are known to have high rates of lesion regression and who have more potential childbearing years ahead of them, it is important to weigh the oncological benefits with the adverse birth outcome risks.”
Young women are more likely to have false-positive results on Papanicolaou tests and lesion regression within 2 years but may undergo unnecessary treatment, the authors noted.
Cervical excision procedures have previously been associated in clinical trials with an increase in PTB risk.
In their 2017 decision model in a fictive cohort, for example, Kamphuis and colleagues found the most intensive screening program was associated with an increase in maternal life years of 9%, a decrease in cervical cancer incidence of 67%, and a decrease in cervical cancer deaths of 75%. But those gains came at the cost of 250% more preterm births, compared with the least intensive program.
“These results can be used in future simulation models integrating oncological trade-offs to help ascertain optimal screening strategies,” the researchers wrote.
While the optimal screening strategy must trade off the oncologic benefits of cancer detection against the neonatal harms of overtreatment, the ideal age of cervical cancer screening onset and frequency remain uncertain, the authors noted. Recent American Cancer Society guidelines recommending less frequent screening for some diverge from those of other societies.
“The first and foremost priority is for gynecologists to continue to have individualized conversations with patients about all of the benefits and risks of procedures that patients undergo and to understand the benefits and risks influencing screening guidelines,” Ms. Bromley-Dulfano said.
Cross-sectional study
The study used data from the Centers for Disease Control and Prevention’s National Center for Health Statistics to analyze associations between cervical cancer screening guidelines and birth outcomes women who had a singleton nulliparous birth from 19916 to 2018. Gestational age and maternal characteristics were drawn from birth certificates.
The mean age of the 11,333,151 multiracial cohort of women was 20.9 years, and 6.8% had hypertension or diabetes. The mean number of guideline-recommended screenings by time of childbirth was 2.4. Overall, PTD and very PTD occurred in 1,140,490 individuals (10.1%) and 333,040 (2.9%) of births, respectively.
Those with hypertension or diabetes had a somewhat higher PTD risk: 0.26% (95% CI, 0.11-0.4) versus 0.06% (95% CI, 0.01-0.10; Wald test, P < .001).
Offering an outsider’s perspective on the analysis, ob.gyn. Fidel A. Valea, MD, director of gynecologic oncology at the Northwell Health Cancer Institute in New Hyde Park, N.Y., urged caution in drawing conclusions from large population analyses such as this.
“This study had over 11 million data points. Often these large numbers will show statistical differences that are not clinically significant,” he said in an interview. He noted that while small studies have shown a possible impact of frequent Pap tests on cervical function, “this is not 100% proven. Research from Texas showed that screening made a difference only in cases of dysplasia.”
Dr. Valea also noted that screening guidelines have already changed over the lengthy time span of the study and do reflect the concerns of the study authors.
“We know that the HPV virus is cleared more readily by young women than older women and so we have made adjustments and test them less frequently and we test them less early.” He added that conservative options are recommended even in the case of dysplasia.
In defense of the Pap smear test, he added: “It has virtually wiped out cervical cancer in the U.S., bringing it from No. 1 to No. 13.” While broadening HPV vaccination programs may impact guidelines in the future, “vaccination is still in its infancy. We have to wait until women have lived long to enough to see an impact.”
As to why this age group is more vulnerable to PTD, Dr. Valea said, “It’s likely multifactorial, with lifestyle and other factors involved.” Although based on U.S. data, the authors said their results may be useful for other public health entities, particularly in countries where cervical cancer is considerably more prevalent.
This work received no specific funding. The authors and Dr. Valea disclosed no competing interests.
For each additional recommended screening before childbirth, there was a direct increase in absolute PTD risk of 0.073 (95% confidence interval, 0.026-0.120), according to a study led by Rebecca A. Bromley-Dulfano, MS, an MD candidate at Stanford (Calif.) University and a PhD candidate in health policy at Harvard University, Cambridge, Mass.
There was no significant change in very preterm delivery (VPTD) risk, but mothers with hypertension or diabetes were at higher PTD risk.
Women in this younger age group are more prone to PTD. According to the study’s estimate, an additional 73 PTDs per 100,000 women could be expected for every 1 additional recommended screening before childbirth. For the year 2018, that translated to an estimated 1,348 PTDs that could have been averted, with reduced screening requirements (3% relative reduction).
“If you screen someone for cervical cancer and find a cervical lesion, the possible next steps can include a biopsy and an excisional procedure to remove the lesion,” Ms. Bromley-Dulfano explained, “and these procedures which remove a small (mostly diseased) part of the cervix have been shown to slightly increase the risk of PTD. Particularly in young individuals with a cervix who are known to have high rates of lesion regression and who have more potential childbearing years ahead of them, it is important to weigh the oncological benefits with the adverse birth outcome risks.”
Young women are more likely to have false-positive results on Papanicolaou tests and lesion regression within 2 years but may undergo unnecessary treatment, the authors noted.
Cervical excision procedures have previously been associated in clinical trials with an increase in PTB risk.
In their 2017 decision model in a fictive cohort, for example, Kamphuis and colleagues found the most intensive screening program was associated with an increase in maternal life years of 9%, a decrease in cervical cancer incidence of 67%, and a decrease in cervical cancer deaths of 75%. But those gains came at the cost of 250% more preterm births, compared with the least intensive program.
“These results can be used in future simulation models integrating oncological trade-offs to help ascertain optimal screening strategies,” the researchers wrote.
While the optimal screening strategy must trade off the oncologic benefits of cancer detection against the neonatal harms of overtreatment, the ideal age of cervical cancer screening onset and frequency remain uncertain, the authors noted. Recent American Cancer Society guidelines recommending less frequent screening for some diverge from those of other societies.
“The first and foremost priority is for gynecologists to continue to have individualized conversations with patients about all of the benefits and risks of procedures that patients undergo and to understand the benefits and risks influencing screening guidelines,” Ms. Bromley-Dulfano said.
Cross-sectional study
The study used data from the Centers for Disease Control and Prevention’s National Center for Health Statistics to analyze associations between cervical cancer screening guidelines and birth outcomes women who had a singleton nulliparous birth from 19916 to 2018. Gestational age and maternal characteristics were drawn from birth certificates.
The mean age of the 11,333,151 multiracial cohort of women was 20.9 years, and 6.8% had hypertension or diabetes. The mean number of guideline-recommended screenings by time of childbirth was 2.4. Overall, PTD and very PTD occurred in 1,140,490 individuals (10.1%) and 333,040 (2.9%) of births, respectively.
Those with hypertension or diabetes had a somewhat higher PTD risk: 0.26% (95% CI, 0.11-0.4) versus 0.06% (95% CI, 0.01-0.10; Wald test, P < .001).
Offering an outsider’s perspective on the analysis, ob.gyn. Fidel A. Valea, MD, director of gynecologic oncology at the Northwell Health Cancer Institute in New Hyde Park, N.Y., urged caution in drawing conclusions from large population analyses such as this.
“This study had over 11 million data points. Often these large numbers will show statistical differences that are not clinically significant,” he said in an interview. He noted that while small studies have shown a possible impact of frequent Pap tests on cervical function, “this is not 100% proven. Research from Texas showed that screening made a difference only in cases of dysplasia.”
Dr. Valea also noted that screening guidelines have already changed over the lengthy time span of the study and do reflect the concerns of the study authors.
“We know that the HPV virus is cleared more readily by young women than older women and so we have made adjustments and test them less frequently and we test them less early.” He added that conservative options are recommended even in the case of dysplasia.
In defense of the Pap smear test, he added: “It has virtually wiped out cervical cancer in the U.S., bringing it from No. 1 to No. 13.” While broadening HPV vaccination programs may impact guidelines in the future, “vaccination is still in its infancy. We have to wait until women have lived long to enough to see an impact.”
As to why this age group is more vulnerable to PTD, Dr. Valea said, “It’s likely multifactorial, with lifestyle and other factors involved.” Although based on U.S. data, the authors said their results may be useful for other public health entities, particularly in countries where cervical cancer is considerably more prevalent.
This work received no specific funding. The authors and Dr. Valea disclosed no competing interests.
FROM JAMA HEALTH FORUM
An STI upsurge requires a nimble approach to care
Except for a drop in the number of sexually transmitted infections (STIs) early in the COVID-19 pandemic (March and April 2020), the incidence of STIs has been rising throughout this century.1 In 2018, 1 in 5 people in the United States had an STI; 26 million new cases were reported that year, resulting in direct costs of $16 billion—85% of which was for the care of HIV infection.2 Also that year, infection with Chlamydia trachomatis (chlamydia), Trichomonas vaginalis (trichomoniasis), herpesvirus type 2 (genital herpes), and/or human papillomavirus (condylomata acuminata) constituted 97.6% of all prevalent and 93.1% of all incident STIs.3 Almost half (45.5%) of new cases of STIs occur in people between the ages of 15 and 24 years.3
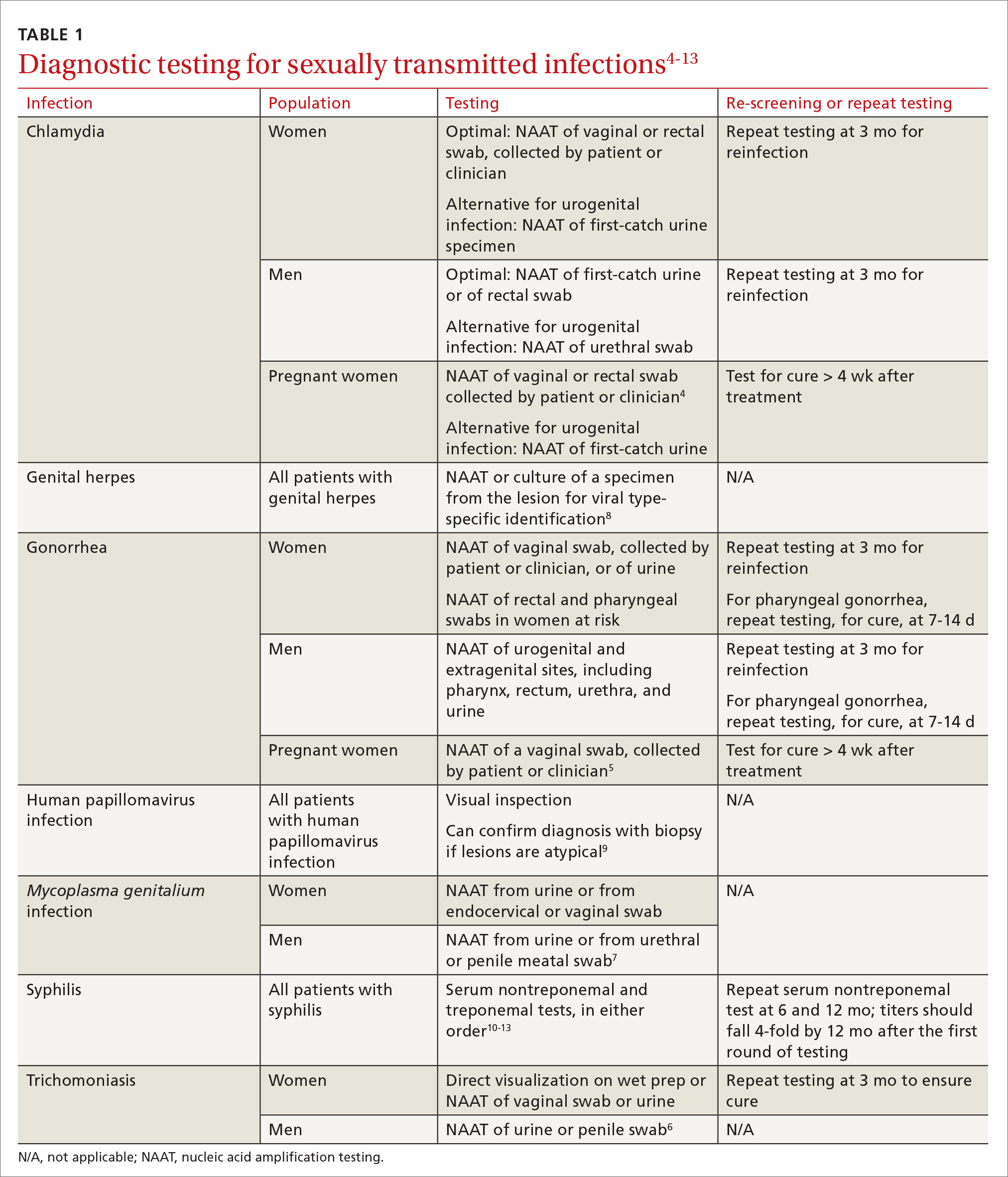
Three factors—changing social patterns, including the increase of social networking; the ability of antiviral therapy to decrease the spread of HIV, leading to a reduction in condom use; and increasing antibiotic resistance—have converged to force changes in screening and treatment recommendations. In this article, we summarize updated guidance for primary care clinicians from several sources—including the Centers for Disease Control and Prevention (CDC), the US Preventive Services Task Force (USPSTF), and the American Society for Colposcopy and Cervical Pathology (ASCCP)—on diagnosing STIs (TABLE 14-13) and providing guideline-based treatment (Table 214). Because of the breadth and complexity of HIV disease, it is not addressed here.
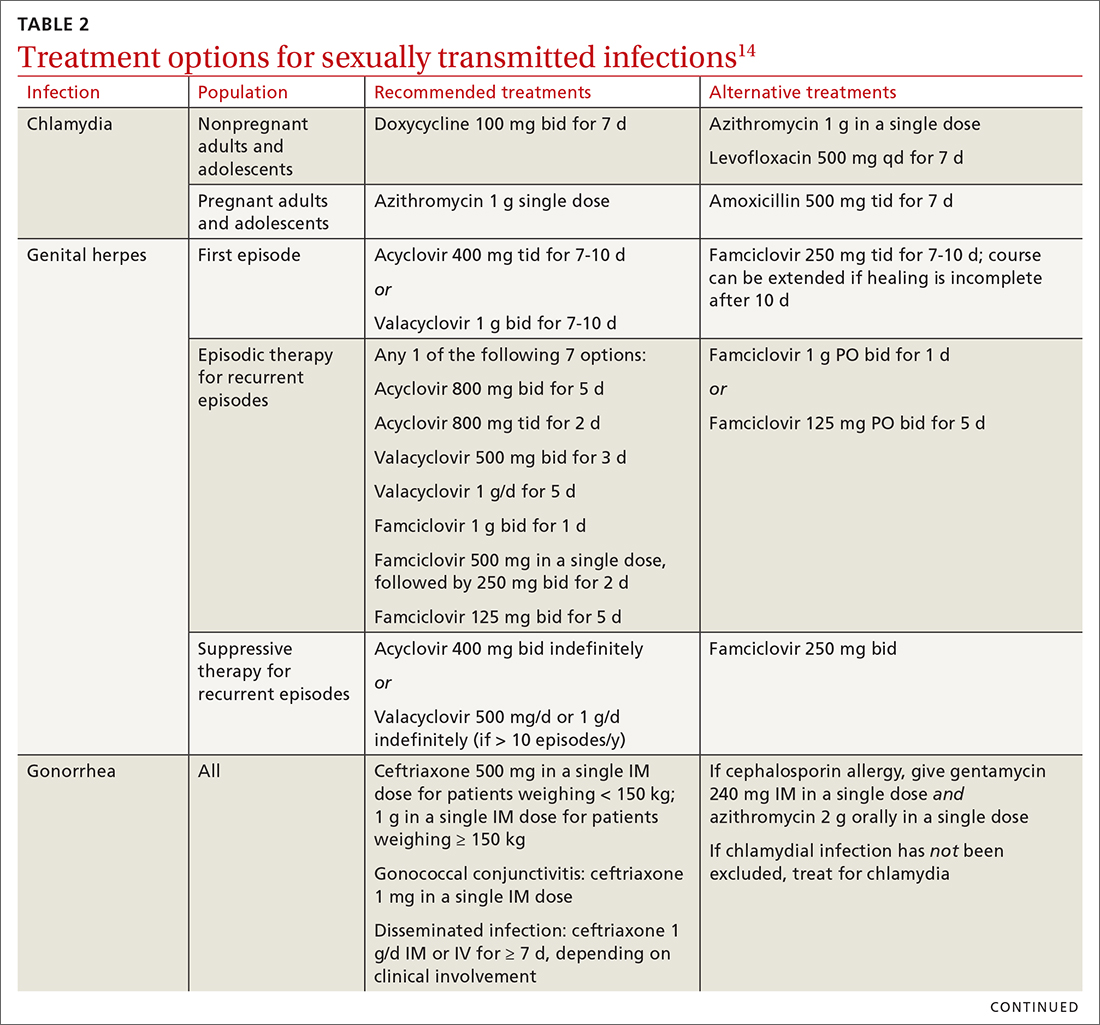
Chlamydia
Infection with Chlamydia trachomatis—the most commonly reported bacterial STI in the United States—primarily causes cervicitis in women and proctitis in men, and can cause urethritis and pharyngitis in men and women. Prevalence is highest in sexually active people younger than 24 years.15
Because most infected people are asymptomatic and show no signs of illness on physical exam, screening is recommended for all sexually active women younger than 25 years and all men who have sex with men (MSM).4 No studies have established proper screening intervals; a reasonable approach, therefore, is to repeat screening for patients who have a sexual history that confers a new or persistent risk for infection since their last negative result.
Depending on the location of the infection, symptoms of chlamydia can include vaginal or penile irritation or discharge, dysuria, pelvic or rectal pain, and sore throat. Breakthrough bleeding in a patient who is taking an oral contraceptive should raise suspicion for chlamydia.
Untreated chlamydia can lead to pelvic inflammatory disease (PID), tubo-ovarian abscess, tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. Infection can be transmitted vertically (mother to baby) antenatally, which can cause ophthalmia neonatorum and pneumonia in these newborns.
Diagnosis. The diagnosis of chlamydia is made using nucleic acid amplification testing (NAAT). Specimens can be collected by the clinician or the patient (self collected) using a vaginal, rectal, or oropharyngeal swab, or a combination of these, and can be obtained from urine or liquid-based cytology material.16
Continue to: Treatment
Treatment. Recommendations for treating chlamydia were updated by the CDC in its 2021 treatment guidelines (Table 214). Doxycycline 100 mg bid for 7 days is the preferred regimen; alternative regiments are (1) azithromycin 1 g in a single dose and (2) levofloxacin 500 mg daily for 7 days.4 A meta-analysis17 and a Cochrane review18 showed that the rate of treatment failure was higher among men when they were treated with azithromycin instead of doxycycline; furthermore, a randomized controlled trial demonstrated that doxycycline is more effective than azithromycin (cure rate, 100%, compared to 74%) at treating rectal chlamydia in MSM.19
Azithromycin is efficacious for urogenital infection in women; however, there is concern that the 33% to 83% of women who have concomitant rectal infection (despite reporting no receptive anorectal sexual activity) would be insufficiently treated. Outside pregnancy, the CDC does not recommend a test of cure but does recommend follow-up testing for reinfection in 3 months. Patients should abstain from sexual activity until 7 days after all sexual partners have been treated.
Expedited partner therapy (EPT) is the practice of treating sexual partners of patients with known chlamydia (and patients with gonococcal infection). Unless prohibited by law in your state, offer EPT to patients with chlamydia if they cannot ensure that their sexual partners from the past 60 days will seek timely treatment.a
Evidence to support EPT comes from 3 US clinical trials, whose subjects comprised heterosexual men and women with chlamydia or gonorrhea.21-23 The role of EPT for MSM is unclear; data are limited. Shared decision-making is recommended to determine whether EPT should be provided, to ensure that co-infection with other bacterial STIs (eg, syphilis) or HIV is not missed.24-26
a Visit www.cdc.gov/std/ept to read updated information about laws and regulations regarding EPT in your state.20
Gonorrhea
Gonorrhea is the second most-reported bacterial communicable disease.5 Infection with Neisseria gonorrhoeae causes urethral discharge in men, leading them to seek treatment; infected women, however, are often asymptomatic. Infected men and women might not recognize symptoms until they have transmitted the disease. Women have a slower natural clearance of gonococcal infection, which might explain their higher prevalence.27 Delayed recognition of symptoms can result in complications, including PID.5
Diagnosis. Specimens for NAAT can be obtained from urine, endocervical, vaginal, rectal, pharyngeal, and male urethral specimens. Reported sexual behaviors and exposures of women and transgender or gender-diverse people should be taken into consideration to determine whether rectal or pharyngeal testing, or both, should be performed.28 MSM should be screened annually at sites of contact, including the urethra, rectum, and pharynx.28 All patients with urogenital or rectal gonorrhea should be asked about oral sexual exposure; if reported, pharyngeal testing should be performed.5
NAAT of urine is at least as sensitive as testing of an endocervical specimen; the same specimen can be used to test for chlamydia and gonorrhea. Patient-collected specimens are a reasonable alternative to clinician-collected swab specimens.29
Continue to: Treatment
Treatment is complicated by the ability of gonorrhea to develop resistance. Intramuscular ceftriaxone 500 mg in a single dose cures 98% to 99% of infections in the United States; however, monitoring local resistance patterns in the community is an important component of treatment.28 (See Table 214 for an alternative regimen for cephalosporin-allergic patients and for treating gonococcal conjunctivitis and disseminated infection.)
In 2007, the CDC identified widespread quinolone-resistant gonococcal strains; therefore, fluoroquinolones no longer are recommended for treating gonorrhea.30 Cefixime has demonstrated only limited success in treating pharyngeal gonorrhea and does not attain a bactericidal level as high as ceftriaxone does; cefixime therefore is recommended only if ceftriaxone is unavailable.28 The national Gonococcal Isolate Surveillance Project is finding emerging evidence of the reduced susceptibility of N gonorrhoeae to azithromycin—making dual therapy for gonococcal infection no longer a recommendation.28
Patients should abstain from sex until 7 days after all sex partners have been treated for gonorrhea. As with chlamydia, the CDC does not recommend a test of cure for uncomplicated urogenital or rectal gonorrhea unless the patient is pregnant, but does recommend testing for reinfection 3 months after treatment.14 For patients with pharyngeal gonorrhea, a test of cure is recommended 7 to 14 days after initial treatment, due to challenges in treatment and because this site of infection is a potential source of antibiotic resistance.28
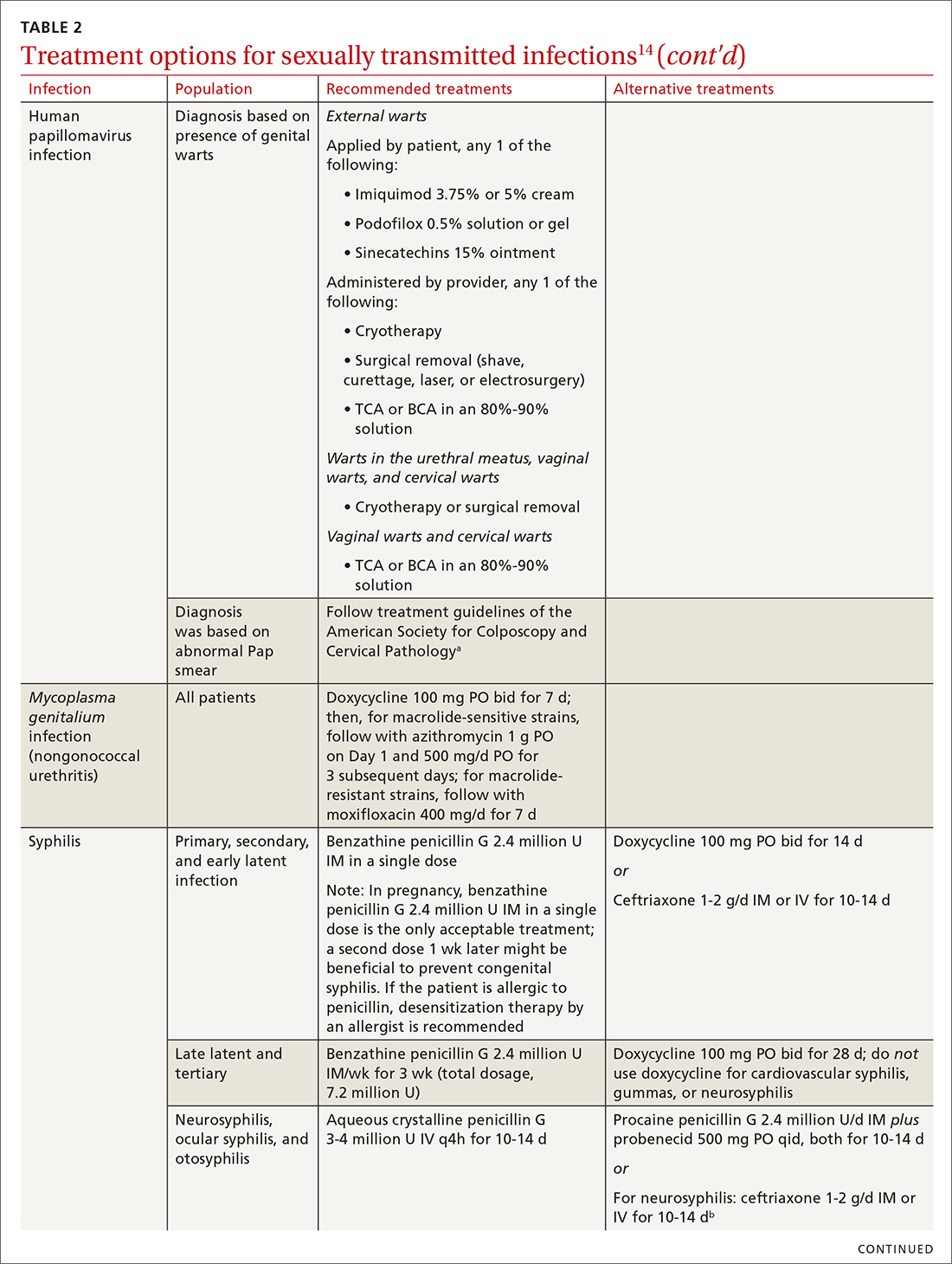
Trichomoniasis
T vaginalis, the most common nonviral STI worldwide,31 can manifest as a yellow-green vaginal discharge with or without vaginal discomfort, dysuria, epididymitis, and prostatitis; most cases, however, are asymptomatic. On examination, the cervix might be erythematous with punctate lesions (known as strawberry cervix).
Unlike most STIs, trichomoniasis is as common in women older than 24 years as it is in younger women. Infection is associated with a lower educational level, lower socioeconomic status, and having ≥ 2 sexual partners in the past year.32 Prevalence is approximately 10 times as high in Black women as it is in White women.
T vaginalis infection is associated with an increase in the risk for preterm birth, premature rupture of membranes, cervical cancer, and HIV infection. With a lack of high-quality clinical trials on the efficacy of screening, women with HIV are the only group for whom routine screening is recommended.6
Diagnosis. NAAT for trichomoniasis is now available in conjunction with gonorrhea and chlamydia testing of specimens on vaginal or urethral swabs and of urine specimens and liquid Pap smears.
Continue to: Treatment
Treatment. Because of greater efficacy, the treatment recommendation for women has changed from a single 2-g dose of oral metronidazole to 500 mg twice daily for 7 days. The 2-g single oral dose is still recommended for men7 (Table 214 lists alternative regimens).
Mycoplasma genitalium
Infection with M genitalium is common and often asymptomatic. The disease causes approximately 20% of all cases of nongonococcal and nonchlamydial urethritis in men and about 40% of persistent or recurrent infections. M genitalium is present in approximately 20% of women with cervicitis and has been associated with PID, preterm delivery, spontaneous abortion, and infertility.
There are limited and conflicting data regarding outcomes in infected patients other than those with persistent or recurrent infection; furthermore, resistance to azithromycin is increasing rapidly, resulting in an increase in treatment failures. Screening therefore is not recommended, and testing is recommended only in men with nongonococcal urethritis.33,34
Diagnosis. NAAT can be performed on urine or on a urethral, penile meatal, endocervical, or vaginal swab; men with recurrent urethritis or women with recurrent cervicitis should be tested. NAAT also can be considered in women with PID. Testing the specimen for the microorganism’s resistance to macrolide antibiotics is recommended (if such testing is available).
Treatment is initiated with doxycycline 100 mg twice daily for 7 days. If the organism is macrolide sensitive, follow with azithromycin 1 g orally on Day 1, then 500 mg/d for 3 more days. If the organism is macrolide resistant or testing is unavailable, follow doxycycline with oral moxifloxacin 400 mg/d for 7 days.33
Genital herpes (mostly herpesvirus type 2)
Genital herpes, characterized by painful, recurrent outbreaks of genital and anal lesions,35 is a lifelong infection that increases in prevalence with age.8 Because many infected people have disease that is undiagnosed or mild or have unrecognizable symptoms during viral shedding, most genital herpes infections are transmitted by people who are unaware that they are contagious.36 Herpesvirus type 2 (HSV-2) causes most cases of genital herpes, although an increasing percentage of cases are attributed to HSV type 1 (HSV-1) through receptive oral sex from a person who has an oral HSV-1 lesion.
Importantly, HSV-2–infected people are 2 to 3 times more likely to become infected with HIV than people who are not HSV-2 infected.37 This is because CD4+ T cells concentrate at the site of HSV lesions and express a higher level of cell-surface receptors that HIV uses to enter cells. HIV replicates 3 to 5 times more quickly in HSV-infected tissue.38
Continue to: HSV can become disseminated...
HSV can become disseminated, particularly in immunosuppressed people, and can manifest as encephalitis, hepatitis, and pneumonitis. Beyond its significant burden on health, HSV carries significant psychosocial consequences.9
Diagnosis. Clinical diagnosis can be challenging if classic lesions are absent at evaluation. If genital lesions are present, HSV can be identified by NAAT or culture of a specimen of those lesions. False-negative antibody results might be more frequent in early stages of infection; repeating antibody testing 12 weeks after presumed time of acquisition might therefore be indicated, based on clinical judgment. HSV-2 antibody positivity implies anogenital infection because almost all HSV-2 infections are sexually acquired.
HSV-1 antibody positivity alone is more difficult to interpret because this finding does not distinguish between oral and genital lesions, and most HSV-1 seropositivity is acquired during childhood.36 HSV polymerase chain reaction (PCR) testing of blood should not be performed to diagnose genital herpes infection, except in settings in which there is concern about disseminated infection.
Treatment. Management should address the acute episode and the chronic nature of genital herpes. Antivirals will not eradicate latent
- attenuate current infection
- prevent recurrence
- improve quality of life
- suppress the virus to prevent transmission to sexual partners.
All patients experiencing an initial episode of genital herpes should be treated, regardless of symptoms, due to the potential for prolonged or severe symptoms during recurrent episodes.9 Three drugs—acyclovir, valacyclovir, and famciclovir—are approved by the US Food and Drug Administration (FDA) to treat genital herpes and appear equally effective (TABLE 214).
Antiviral therapy for recurrent genital HSV infection can be administered either as suppressive therapy to reduce the frequency of recurrences or episodically to shorten the duration of lesions:
- Suppressive therapy reduces the frequency of recurrence by 70% to 80% among patients with frequent outbreaks. Long-term safety and efficacy are well established.
- Episodic therapy is most effective if started within 1 day after onset of lesions or during the prodrome.36
There is no specific recommendation for when to choose suppressive over episodic therapy; most patients prefer suppressive therapy because it improves quality of life. Use shared clinical decision-making to determine the best option for an individual patient.
Continue to: Human papillomavirus
Human papillomavirus
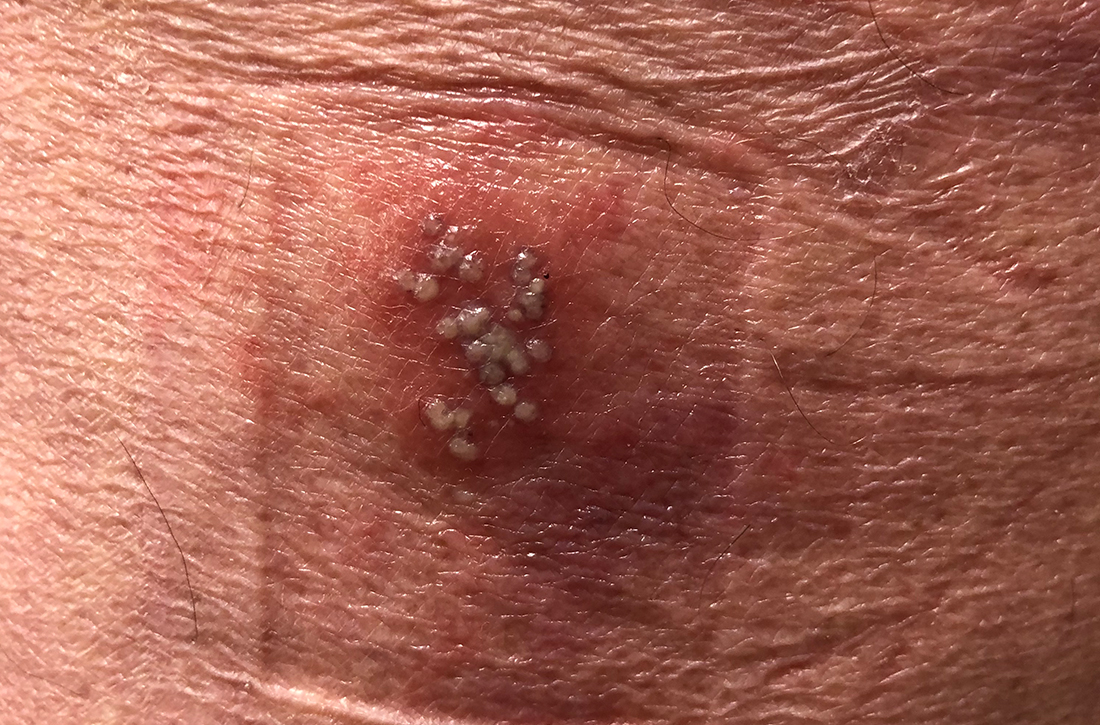
Condylomata acuminata (genital warts) are caused by human papillomavirus (HPV), most commonly types 6 and 11, which manifest as soft papules or plaques on the external genitalia, perineum, perianal skin, and groin. The warts are usually asymptomatic but can be painful or pruritic, depending on size and location.
Diagnosis is made by visual inspection and can be confirmed by biopsy if lesions are atypical. Lesions can resolve spontaneously, remain unchanged, or grow in size or number.
Treatment. The aim of treatment is relief of symptoms and removal of warts. Treatment does not eradicate HPV infection. Multiple treatments are available that can be applied by the patient as a cream, gel, or ointment or administered by the provider, including cryotherapy, surgical removal, and solutions. The decision on how to treat should be based on the number, size, and
HPV-associated cancers and precancers. This is a broad (and separate) topic. HPV types 16 and 18 cause most cases of cervical, penile, vulvar, vaginal, anal, and oropharyngeal cancer and precancer.39 The USPSTF, the American Cancer Society, and the American College of Obstetricians and Gynecologists all have recommendations for cervical cancer screening in the United States.40 Refer to guidelines of the ASCCP for recommendations on abnormal screening tests.41
Prevention of genital warts. The 9-valent HPV vaccine available in the United States is safe and effective and helps protect against viral types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Types 6 and 11 are the principal causes of genital warts. Types 16 and 18 cause 66% of cervical cancer. The vaccination series can be started at age 9 years and is recommended for everyone through age 26 years. Only 2 doses are needed if the first dose is given prior to age 15 years; given after that age, a 3-dose series is utilized. Refer to CDC vaccine guidelines42 for details on the exact timing of vaccination.
Vaccination for women ages 27 to 45 years is not universally recommended because most people have been exposed to HPV by that age. However, the vaccine can still be administered, depending on clinical circumstances and the risk for new infection.42
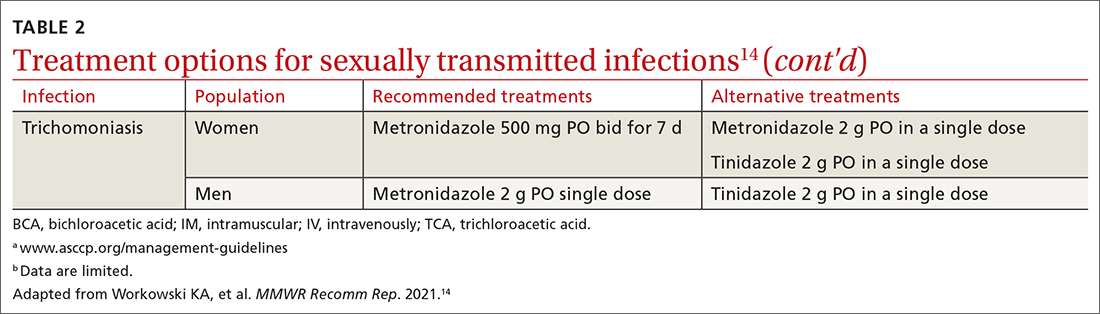
Syphilis
Caused by the spirochete Treponema pallidum, syphilis manifests across a spectrum—from congenital to tertiary. The inability of medical science to develop a method for culturing the spirochete has confounded diagnosis and treatment.
Continue to: Since reaching a historic...
Since reaching a historic nadir of incidence in 2000 (5979 cases in the United States), there has been an increasingly rapid rise in that number: to 130,000 in 2020. More than 50% of cases are in MSM; however, the number of cases in heterosexual women is rapidly increasing.43
Routine screening for syphilis should be performed in any person who is at risk: all pregnant women in the first trimester (and in the third trimester and at delivery if they are at risk or live in a community where prevalence is high) and annually in sexually active MSM or anyone with HIV infection.10
Diagnosis. Examination by dark-field microscopy, testing by PCR, and direct fluorescent antibody assay for T pallidum from lesion tissue or exudate provide definitive diagnosis for early and congenital syphilis, but are often unavailable.
Presumptive diagnosis requires 2 serologic tests:
- Nontreponemal tests (the VDRL and rapid plasma reagin tests) identify anticardiolipin antibodies released during syphilis infection, although results also can be elevated in autoimmune disease or after certain immunizations, including the COVID-19 vaccine.
- Treponemal tests (the fluorescent treponemal antibody absorbed assay, T pallidum particulate agglutination assay, enzyme immunoassay, and chemiluminescence immunoassay) are specific antibody tests.
Historically, reactive nontreponemal tests, which are less expensive and easier to perform, were followed by a treponemal test to confirm the presumptive diagnosis. This method continues to be reasonable when screening patients in a low-prevalence population.11 The reverse sequence screening algorithm (ie, begin with a treponemal test) is now frequently used. With this method, a positive treponemal test must be confirmed with a nontreponemal test. If the treponemal test is positive and the nontreponemal test is negative, another treponemal test must be positive to confirm the diagnosis. This algorithm is useful in high-risk populations because it provides earlier detection of recently acquired syphilis and enhanced detection of late latent syphilis.12,13,44 The CDC has not stated a diagnostic preference.
Once the diagnosis is made, a complete history (including a sexual history and a history of syphilis testing and treatment) and a physical exam are necessary to confirm stage of disease.45
Special circumstances. Neurosyphilis, ocular syphilis, and otosyphilis refer to the site of infection and can occur at any stage of disease. The nervous system usually is infected within hours of initial infection, but symptoms might take weeks or years to develop—or might never manifest. Any time a patient develops neurologic, ophthalmologic, or audiologic symptoms, careful neurologic and ophthalmologic evaluation should be performed and the patient should be tested for HIV.
Continue to: Lumbar puncture is warranted...
Lumbar puncture is warranted for evaluation of cerebrospinal fluid if neurologic symptoms are present but is not necessary for isolated ocular syphilis or otosyphilis without neurologic findings. Treatment should not be delayed for test results if ocular syphilis is suspected because permanent blindness can develop. Any patient at high risk for an STI who presents with neurologic or ophthalmologic symptoms should be tested for syphilis and HIV.45
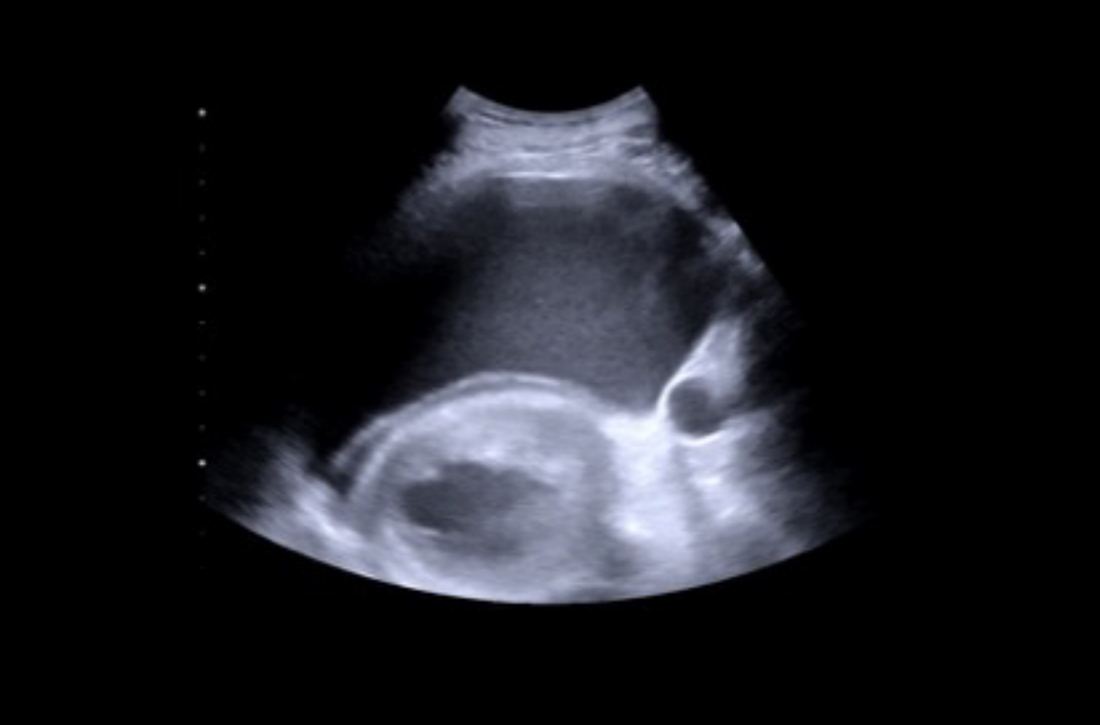
Pregnant women who have a diagnosis of syphilis should be treated with penicillin immediately because treatment ≥ 30 days prior to delivery is likely to prevent most cases of congenital syphilis. However, a course of penicillin might not prevent stillbirth or congenital syphilis in a gravely infected fetus, evidenced by fetal syphilis on a sonogram at the time of treatment. Additional doses of penicillin in pregnant women with early syphilis might be indicated if there is evidence of fetal syphilis on ultrasonography. All women who deliver a stillborn infant (≥ 20 weeks’ gestation) should be tested for syphilis at delivery.46
All patients in whom primary or secondary syphilis has been diagnosed should be tested for HIV at the time of diagnosis and treatment; if the result is negative, they should be offered preexposure prophylaxis (PrEP; discussed shortly). If the incidence of HIV in your community is high, repeat testing for HIV in 3 months. Clinical and serologic evaluation should be performed 6 and 12 months after treatment.47
Treatment. Penicillin remains the standard treatment for syphilis. Primary, secondary, and early tertiary stages (including in pregnancy) are treated with benzathine penicillin G 2.4 million units intramuscular (IM) in a single dose. For pregnant patients, repeating that dose in 1 week generally is recommended. Patients in the late latent (> 1 year) or tertiary stage receive the same dose of penicillin, which is then repeated weekly, for a total of 3 doses. Doxycycline and ceftriaxone are alternatives, except in pregnancy.
Warn patients of the Jarisch-Herxheimer reaction: fever, headache, and myalgias associated with initiation of treatment in the presence of the high bacterial load seen in early syphilis. Treatment is symptomatic, but the Jarisch-Herxheimer reaction can cause fetal distress in pregnancy.
Otosyphilis, ocular syphilis, and neurosyphilis require intravenous (IV) aqueous crystalline penicillin G 3 to 4 million U every 4 hours for 10 to 14 days.45 Alternatively, procaine penicillin G 2.4 million U/d IM can be given daily with oral probenecid 500 mg qid, both for 10 to 14 days (TABLE 214).
Screening andprevention of STIs
Screening recommendations
Follow USPSTF screening guidelines for STIs.10,48-54 Screen annually for:
- gonorrhea and chlamydia in women ages 15 to 24 years and in women older than 25 years if they are at increased risk
- gonorrhea, chlamydia, syphilis, and HIV in MSM, and hepatitis C if they are HIV positive
- trichomoniasis in women who are HIV positive.
Continue to: Consider the community in which...
Consider the community in which you practice when determining risk; you might want to consult local public health authorities for information about local epidemiology and guidance on determining which of your patients are at increased risk.
Preexposure prophylaxis
According to the CDC, all sexually active adults and adolescents should be informed about the availability of PrEP to prevent HIV infection. PrEP should be (1) available to anyone who requests it and (2) recommended for anyone who is sexually active and who practices sexual behaviors that place them at substantial risk for exposure to or acquisition of HIV, or both.
The recommended treatment protocol for men and women who have either an HIV-positive partner or inconsistent condom use or who have had a bacterial STI in the previous 6 months is oral emtricitabine 200 mg plus tenofovir disoproxil fumarate 300 mg/d (sold as Truvada-F/TDF). Men and transgender women (ie, assigned male at birth) with at-risk behaviors also can use emtricitabine plus tenofovir alafenamide 25 mg/d (sold as Descovy-F/TAF).
In addition, cabotegravir plus rilpirivine (sold as Cabenuva), IM every 2 months, was approved by the FDA for PrEP in 2021.
Creatinine clearance should be assessed at baseline and yearly (every 6 months for those older than 50 years) in patients taking PrEP. All patients must be tested for HIV at initiation of treatment and every 3 months thereafter (every 4 months for cabotegravir plus rilpirivine). Patients should be screened for bacterial STIs every 6 months (every 3 months for MSM and transgender women); screening for chlamydia should be done yearly. For patients being treated with emtricitabine plus tenofovir alafenamide, weight and a lipid profile (cholesterol and triglycerides) should be assessed annually.55
Postexposure prophylaxis
The sharp rise in the incidence of STIs in the past few years has brought renewed interest in postexposure prophylaxis (PEP) for STIs. Although PEP should be standard in cases of sexual assault, this protocol also can be considered in other instances of high-risk exposure.
CDC recommendations for PEP in cases of assault are56:
- ceftriaxone 500 mg IM in a single dose (1 g if weight is ≥ 150 kg) plus
- doxycycline 100 mg bid for 7 days plus
- metronidazole 2 g bid for 7 days (for vaginal exposure)
- pregnancy evaluation and emergency contraception
- hepatitis B risk evaluation and vaccination, with or without hepatitis B immune globulin
- HIV risk evaluation, based on CDC guidelines, and possible HIV prophylaxis (PrEP)
- HPV vaccination for patients ages 9 to 26 years if they are not already fully vaccinated.
CORRESPONDENCE
Belinda Vail, MD, 3901 Rainbow Boulevard, Mail Stop 4010, Kansas City, KS 66160; [email protected]
1. Pagaoa M, Grey J, Torrone E, et al. Trends in nationally notifiable sexually transmitted disease case reports during the US COVID-19 pandemic, January to December 2020. Sex Transm Dis. 2021;48:798-804. doi: 10.1097/OLQ.0000000000001506
2. Chesson HW, Spicknall IH; Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis. 2021;48:215-221. doi: 10.1097/OLQ.0000000000001380
3. Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48:208-214. doi: 10.1097/OLQ.0000000000001355
4. CDC. Sexually transmitted infections treatment guidelines, 2021: Chlamydial infections among adolescents and adults. US Department of Health and Human Services. July 21, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/chlamydia.htm
5. CDC. Sexually transmitted infections treatment guidelines, 2021: Gonococcal infections among adolescents and adults. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
6. Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;
7. CDC. Sexually transmitted infections treatment guidelines, 2021. Trichomoniasis. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. December 27, 2021. www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
8. Spicknall IH, Flagg EW, Torrone EA. Estimates of the prevalence and incidence of genital herpes, United States, 2018. Sex Transm Dis. 2021;48:260-265. doi: 10.1097/OLQ.0000000000001375
9. Mark H, Gilbert L, Nanda J. Psychosocial well-being and quality of life among women newly diagnosed with genital herpes. J Obstet Gynecol Neonatal Nurs.
10. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2321-2327. doi: 10.1001/jama.2016.5824
11. Ricco J, Westby A. Syphilis: far from ancient history. Am Fam Physician. 2020;102:91-98.
12. Goza M, Kulwicki B, Akers JM, et al. Syphilis screening: a review of the Syphilis Health Check rapid immunochromatographic test. J Pharm Technol. 2017;33:53-59. doi:10.1177/8755122517691308
13. Henao- AF, Johnson SC. Diagnostic tests for syphilis: new tests and new algorithms. Neurol Clin Pract. 2014;4:114-122. doi: 10.1212/01.CPJ.0000435752.17621.48
14. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
15. CDC. Sexually Transmitted Disease Surveillance 2021. National overview of STDs. US Department of Health and Human Services. April 2023. Accessed May 9, 2023. www.cdc.gov/std/statistics/2021/overview.htm#Chlamydia
16. CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63:1-19.
17. Kong FYS, Tabrizi SN, Law M, et al. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59:193-205. doi: 10.1093/cid/ciu220
18. Páez-Canro C, Alzate JP, González LM, et al. Antibiotics for treating urogenital Chlamydia trachomatis infection in men and non-pregnant women. Cochrane Database Syst Rev. 2019;1:CD010871. doi: 10.1002/14651858.CD010871.pub2
19. Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline versus azithromycin for the treatment of rectal chlamydia in men who have sex with men: a randomized controlled trial. Clin Infect Dis. 2021;73:824-831. doi: 10.1093/cid/ciab153
20. CDC. Sexually transmitted infections treatment guidelines, 2021: Expedited partner therapy. US Department of Health and Human Services. July 22, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/clinical-EPT.htm
21. Golden MR, Whittington WLH, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-685. doi: 10.1056/NEJMoa041681
22. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
23. Cameron ST, Glasier A, Scott G, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod. 2009;24:888-895. doi: 10.1093/humrep/den475
24. McNulty A, Teh MF, Freedman E. Patient delivered partner therapy for chlamydial infection—what would be missed? Sex Transm Dis. 2008;35:834-836. doi: 10.1097/OLQ.0b013e3181761993
25. Stekler J, Bachmann L, Brotman RM, et al. Concurrent sexually transmitted infections (STIs) in sex partners of patients with selected STIs: implications for patient-delivered partner therapy. Clin Infect Dis. 2005;40:787-793. doi: 10.1086/428043
26. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
27. Stupiansky NW, Van der Pol B, Williams JA, et al. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis. 2011;38:750-754. doi: 10.1097/OLQ.0b013e31820ff9a4
28. CDC. Sexually transmitted infections treatment guidelines, 2021: Screening recommendations and considerations referenced in treatment guidelines and original sources. US Department of Health and Human Services. June 6, 2022. Accessed May 9, 2023. www.cdc.gov/std/treatment-guidelines/screening-recommen dations.htm
29. Cantor A, Dana T, Griffen JC, et al. Screening for chlamydial and gonococcal infections: a systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 206. AHRQ Report No. 21-05275-EF-1. Agency for Healthcare Research and Quality. September 2021. www.ncbi.nlm.nih.gov/books/NBK574045
30. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
31. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019:97:548-562P. doi: 10.2471/BLT.18.228486
32. Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217. doi: 10.1093/cid/ciy079
33. CDC. Sexually transmitted infections treatment guidelines, 2021. Mycoplasma genitalium. US Department of Health and Human Services. July 22, 2021. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm
34. Manhart LE, Broad JM, Bolden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis. 2011;53(suppl 3):S129-S142. doi:10.1093/cid/cir702.
35. Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. McGraw-Hill; 2008:399-437.
36. CDC. Sexually transmitted infections treatment guidelines, 2021: Genital herpes. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/herpes.htm
37. Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1303-1316. doi: 10.1016/S1473-3099(17)30405-X
38. Rollenhagen C, Lathrop M, Macura SL, et al. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol. 2014;7:1165-1174. doi: 10.1038/mi.2014.3
39. Cogliano V, Baan R, Straif K, et al; WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol.
40. Simon MA, Tseng CW, Wong JB. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897
41. Perkins RB, Guido RS, Castle PE, et al; . 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
42. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
43. Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the United States: an assessment of contributing factors. Infect Dis (Auckl). 2019;12:1178633719883282. doi: 10.1177/1178633719883282
44. Boog GHP, Lopes JVZ, Mahler JV, et al. Diagnostic tools for neurosyphilis: a systematic review. BMC Infect Dis. 2021;21:568. doi: 10.1186/s12879-021-06264-8
45. CDC. Sexually transmitted infections treatment guidelines, 2021. Syphilis. US Department of Health and Human Services. April 20, 2023. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/syphilis.htm
46. Matthias JM, Rahman MM, Newman DR, et al. Effectiveness of prenatal screening and treatment to prevent congenital syphilis, Louisiana and Florida, 2013-2014. Sex Transm Dis. 2017;44:498-502. doi: 10.1097/OLQ.0000000000000638
47. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014;312:1905-1917. doi: 10.1001/jama.2014.13259
48. Davidson KW, Barry MJ, Mangione CM, et al; US Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:949-956. doi: 10.1001/jama.2021.14081
49. Krist AH, Davidson KW, Mangione CM, et al; US Preventive Services Task Force. Screening for hepatitis B virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2415-2422. doi: 10.1001/jama.2020.22980
50. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:970-975. doi: 10.1001/jama.2020.1123
51. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530. doi: 10.1001/jama.2016.16776
52. Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi: 10.1001/jama.2018.10897
53. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336. doi: 10.1001/jama.2019.6587
54. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36:502-509. doi: 10.1016/s0091-7435(02)00058-0
55. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. A clinical practice guideline. Centers for Disease Control and Prevention. Accessed April 24, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
56. CDC. Sexually transmitted infections treatment guidelines, 2021: Sexual assault and abuse and STIs—adolescents and adults, 2021. US Department of Health and Human Services. July 22, 2021. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/sexual-assault-adults.htm
Except for a drop in the number of sexually transmitted infections (STIs) early in the COVID-19 pandemic (March and April 2020), the incidence of STIs has been rising throughout this century.1 In 2018, 1 in 5 people in the United States had an STI; 26 million new cases were reported that year, resulting in direct costs of $16 billion—85% of which was for the care of HIV infection.2 Also that year, infection with Chlamydia trachomatis (chlamydia), Trichomonas vaginalis (trichomoniasis), herpesvirus type 2 (genital herpes), and/or human papillomavirus (condylomata acuminata) constituted 97.6% of all prevalent and 93.1% of all incident STIs.3 Almost half (45.5%) of new cases of STIs occur in people between the ages of 15 and 24 years.3

Three factors—changing social patterns, including the increase of social networking; the ability of antiviral therapy to decrease the spread of HIV, leading to a reduction in condom use; and increasing antibiotic resistance—have converged to force changes in screening and treatment recommendations. In this article, we summarize updated guidance for primary care clinicians from several sources—including the Centers for Disease Control and Prevention (CDC), the US Preventive Services Task Force (USPSTF), and the American Society for Colposcopy and Cervical Pathology (ASCCP)—on diagnosing STIs (TABLE 14-13) and providing guideline-based treatment (Table 214). Because of the breadth and complexity of HIV disease, it is not addressed here.



Chlamydia
Infection with Chlamydia trachomatis—the most commonly reported bacterial STI in the United States—primarily causes cervicitis in women and proctitis in men, and can cause urethritis and pharyngitis in men and women. Prevalence is highest in sexually active people younger than 24 years.15
Because most infected people are asymptomatic and show no signs of illness on physical exam, screening is recommended for all sexually active women younger than 25 years and all men who have sex with men (MSM).4 No studies have established proper screening intervals; a reasonable approach, therefore, is to repeat screening for patients who have a sexual history that confers a new or persistent risk for infection since their last negative result.
Depending on the location of the infection, symptoms of chlamydia can include vaginal or penile irritation or discharge, dysuria, pelvic or rectal pain, and sore throat. Breakthrough bleeding in a patient who is taking an oral contraceptive should raise suspicion for chlamydia.
Untreated chlamydia can lead to pelvic inflammatory disease (PID), tubo-ovarian abscess, tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. Infection can be transmitted vertically (mother to baby) antenatally, which can cause ophthalmia neonatorum and pneumonia in these newborns.
Diagnosis. The diagnosis of chlamydia is made using nucleic acid amplification testing (NAAT). Specimens can be collected by the clinician or the patient (self collected) using a vaginal, rectal, or oropharyngeal swab, or a combination of these, and can be obtained from urine or liquid-based cytology material.16
Continue to: Treatment
Treatment. Recommendations for treating chlamydia were updated by the CDC in its 2021 treatment guidelines (Table 214). Doxycycline 100 mg bid for 7 days is the preferred regimen; alternative regiments are (1) azithromycin 1 g in a single dose and (2) levofloxacin 500 mg daily for 7 days.4 A meta-analysis17 and a Cochrane review18 showed that the rate of treatment failure was higher among men when they were treated with azithromycin instead of doxycycline; furthermore, a randomized controlled trial demonstrated that doxycycline is more effective than azithromycin (cure rate, 100%, compared to 74%) at treating rectal chlamydia in MSM.19
Azithromycin is efficacious for urogenital infection in women; however, there is concern that the 33% to 83% of women who have concomitant rectal infection (despite reporting no receptive anorectal sexual activity) would be insufficiently treated. Outside pregnancy, the CDC does not recommend a test of cure but does recommend follow-up testing for reinfection in 3 months. Patients should abstain from sexual activity until 7 days after all sexual partners have been treated.
Expedited partner therapy (EPT) is the practice of treating sexual partners of patients with known chlamydia (and patients with gonococcal infection). Unless prohibited by law in your state, offer EPT to patients with chlamydia if they cannot ensure that their sexual partners from the past 60 days will seek timely treatment.a
Evidence to support EPT comes from 3 US clinical trials, whose subjects comprised heterosexual men and women with chlamydia or gonorrhea.21-23 The role of EPT for MSM is unclear; data are limited. Shared decision-making is recommended to determine whether EPT should be provided, to ensure that co-infection with other bacterial STIs (eg, syphilis) or HIV is not missed.24-26
a Visit www.cdc.gov/std/ept to read updated information about laws and regulations regarding EPT in your state.20
Gonorrhea
Gonorrhea is the second most-reported bacterial communicable disease.5 Infection with Neisseria gonorrhoeae causes urethral discharge in men, leading them to seek treatment; infected women, however, are often asymptomatic. Infected men and women might not recognize symptoms until they have transmitted the disease. Women have a slower natural clearance of gonococcal infection, which might explain their higher prevalence.27 Delayed recognition of symptoms can result in complications, including PID.5
Diagnosis. Specimens for NAAT can be obtained from urine, endocervical, vaginal, rectal, pharyngeal, and male urethral specimens. Reported sexual behaviors and exposures of women and transgender or gender-diverse people should be taken into consideration to determine whether rectal or pharyngeal testing, or both, should be performed.28 MSM should be screened annually at sites of contact, including the urethra, rectum, and pharynx.28 All patients with urogenital or rectal gonorrhea should be asked about oral sexual exposure; if reported, pharyngeal testing should be performed.5
NAAT of urine is at least as sensitive as testing of an endocervical specimen; the same specimen can be used to test for chlamydia and gonorrhea. Patient-collected specimens are a reasonable alternative to clinician-collected swab specimens.29
Continue to: Treatment
Treatment is complicated by the ability of gonorrhea to develop resistance. Intramuscular ceftriaxone 500 mg in a single dose cures 98% to 99% of infections in the United States; however, monitoring local resistance patterns in the community is an important component of treatment.28 (See Table 214 for an alternative regimen for cephalosporin-allergic patients and for treating gonococcal conjunctivitis and disseminated infection.)
In 2007, the CDC identified widespread quinolone-resistant gonococcal strains; therefore, fluoroquinolones no longer are recommended for treating gonorrhea.30 Cefixime has demonstrated only limited success in treating pharyngeal gonorrhea and does not attain a bactericidal level as high as ceftriaxone does; cefixime therefore is recommended only if ceftriaxone is unavailable.28 The national Gonococcal Isolate Surveillance Project is finding emerging evidence of the reduced susceptibility of N gonorrhoeae to azithromycin—making dual therapy for gonococcal infection no longer a recommendation.28
Patients should abstain from sex until 7 days after all sex partners have been treated for gonorrhea. As with chlamydia, the CDC does not recommend a test of cure for uncomplicated urogenital or rectal gonorrhea unless the patient is pregnant, but does recommend testing for reinfection 3 months after treatment.14 For patients with pharyngeal gonorrhea, a test of cure is recommended 7 to 14 days after initial treatment, due to challenges in treatment and because this site of infection is a potential source of antibiotic resistance.28
Trichomoniasis
T vaginalis, the most common nonviral STI worldwide,31 can manifest as a yellow-green vaginal discharge with or without vaginal discomfort, dysuria, epididymitis, and prostatitis; most cases, however, are asymptomatic. On examination, the cervix might be erythematous with punctate lesions (known as strawberry cervix).
Unlike most STIs, trichomoniasis is as common in women older than 24 years as it is in younger women. Infection is associated with a lower educational level, lower socioeconomic status, and having ≥ 2 sexual partners in the past year.32 Prevalence is approximately 10 times as high in Black women as it is in White women.
T vaginalis infection is associated with an increase in the risk for preterm birth, premature rupture of membranes, cervical cancer, and HIV infection. With a lack of high-quality clinical trials on the efficacy of screening, women with HIV are the only group for whom routine screening is recommended.6
Diagnosis. NAAT for trichomoniasis is now available in conjunction with gonorrhea and chlamydia testing of specimens on vaginal or urethral swabs and of urine specimens and liquid Pap smears.
Continue to: Treatment
Treatment. Because of greater efficacy, the treatment recommendation for women has changed from a single 2-g dose of oral metronidazole to 500 mg twice daily for 7 days. The 2-g single oral dose is still recommended for men7 (Table 214 lists alternative regimens).
Mycoplasma genitalium
Infection with M genitalium is common and often asymptomatic. The disease causes approximately 20% of all cases of nongonococcal and nonchlamydial urethritis in men and about 40% of persistent or recurrent infections. M genitalium is present in approximately 20% of women with cervicitis and has been associated with PID, preterm delivery, spontaneous abortion, and infertility.
There are limited and conflicting data regarding outcomes in infected patients other than those with persistent or recurrent infection; furthermore, resistance to azithromycin is increasing rapidly, resulting in an increase in treatment failures. Screening therefore is not recommended, and testing is recommended only in men with nongonococcal urethritis.33,34
Diagnosis. NAAT can be performed on urine or on a urethral, penile meatal, endocervical, or vaginal swab; men with recurrent urethritis or women with recurrent cervicitis should be tested. NAAT also can be considered in women with PID. Testing the specimen for the microorganism’s resistance to macrolide antibiotics is recommended (if such testing is available).
Treatment is initiated with doxycycline 100 mg twice daily for 7 days. If the organism is macrolide sensitive, follow with azithromycin 1 g orally on Day 1, then 500 mg/d for 3 more days. If the organism is macrolide resistant or testing is unavailable, follow doxycycline with oral moxifloxacin 400 mg/d for 7 days.33
Genital herpes (mostly herpesvirus type 2)
Genital herpes, characterized by painful, recurrent outbreaks of genital and anal lesions,35 is a lifelong infection that increases in prevalence with age.8 Because many infected people have disease that is undiagnosed or mild or have unrecognizable symptoms during viral shedding, most genital herpes infections are transmitted by people who are unaware that they are contagious.36 Herpesvirus type 2 (HSV-2) causes most cases of genital herpes, although an increasing percentage of cases are attributed to HSV type 1 (HSV-1) through receptive oral sex from a person who has an oral HSV-1 lesion.
Importantly, HSV-2–infected people are 2 to 3 times more likely to become infected with HIV than people who are not HSV-2 infected.37 This is because CD4+ T cells concentrate at the site of HSV lesions and express a higher level of cell-surface receptors that HIV uses to enter cells. HIV replicates 3 to 5 times more quickly in HSV-infected tissue.38
Continue to: HSV can become disseminated...
HSV can become disseminated, particularly in immunosuppressed people, and can manifest as encephalitis, hepatitis, and pneumonitis. Beyond its significant burden on health, HSV carries significant psychosocial consequences.9
Diagnosis. Clinical diagnosis can be challenging if classic lesions are absent at evaluation. If genital lesions are present, HSV can be identified by NAAT or culture of a specimen of those lesions. False-negative antibody results might be more frequent in early stages of infection; repeating antibody testing 12 weeks after presumed time of acquisition might therefore be indicated, based on clinical judgment. HSV-2 antibody positivity implies anogenital infection because almost all HSV-2 infections are sexually acquired.
HSV-1 antibody positivity alone is more difficult to interpret because this finding does not distinguish between oral and genital lesions, and most HSV-1 seropositivity is acquired during childhood.36 HSV polymerase chain reaction (PCR) testing of blood should not be performed to diagnose genital herpes infection, except in settings in which there is concern about disseminated infection.
Treatment. Management should address the acute episode and the chronic nature of genital herpes. Antivirals will not eradicate latent
- attenuate current infection
- prevent recurrence
- improve quality of life
- suppress the virus to prevent transmission to sexual partners.
All patients experiencing an initial episode of genital herpes should be treated, regardless of symptoms, due to the potential for prolonged or severe symptoms during recurrent episodes.9 Three drugs—acyclovir, valacyclovir, and famciclovir—are approved by the US Food and Drug Administration (FDA) to treat genital herpes and appear equally effective (TABLE 214).
Antiviral therapy for recurrent genital HSV infection can be administered either as suppressive therapy to reduce the frequency of recurrences or episodically to shorten the duration of lesions:
- Suppressive therapy reduces the frequency of recurrence by 70% to 80% among patients with frequent outbreaks. Long-term safety and efficacy are well established.
- Episodic therapy is most effective if started within 1 day after onset of lesions or during the prodrome.36
There is no specific recommendation for when to choose suppressive over episodic therapy; most patients prefer suppressive therapy because it improves quality of life. Use shared clinical decision-making to determine the best option for an individual patient.
Continue to: Human papillomavirus
Human papillomavirus
Condylomata acuminata (genital warts) are caused by human papillomavirus (HPV), most commonly types 6 and 11, which manifest as soft papules or plaques on the external genitalia, perineum, perianal skin, and groin. The warts are usually asymptomatic but can be painful or pruritic, depending on size and location.
Diagnosis is made by visual inspection and can be confirmed by biopsy if lesions are atypical. Lesions can resolve spontaneously, remain unchanged, or grow in size or number.
Treatment. The aim of treatment is relief of symptoms and removal of warts. Treatment does not eradicate HPV infection. Multiple treatments are available that can be applied by the patient as a cream, gel, or ointment or administered by the provider, including cryotherapy, surgical removal, and solutions. The decision on how to treat should be based on the number, size, and
HPV-associated cancers and precancers. This is a broad (and separate) topic. HPV types 16 and 18 cause most cases of cervical, penile, vulvar, vaginal, anal, and oropharyngeal cancer and precancer.39 The USPSTF, the American Cancer Society, and the American College of Obstetricians and Gynecologists all have recommendations for cervical cancer screening in the United States.40 Refer to guidelines of the ASCCP for recommendations on abnormal screening tests.41
Prevention of genital warts. The 9-valent HPV vaccine available in the United States is safe and effective and helps protect against viral types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Types 6 and 11 are the principal causes of genital warts. Types 16 and 18 cause 66% of cervical cancer. The vaccination series can be started at age 9 years and is recommended for everyone through age 26 years. Only 2 doses are needed if the first dose is given prior to age 15 years; given after that age, a 3-dose series is utilized. Refer to CDC vaccine guidelines42 for details on the exact timing of vaccination.
Vaccination for women ages 27 to 45 years is not universally recommended because most people have been exposed to HPV by that age. However, the vaccine can still be administered, depending on clinical circumstances and the risk for new infection.42
Syphilis
Caused by the spirochete Treponema pallidum, syphilis manifests across a spectrum—from congenital to tertiary. The inability of medical science to develop a method for culturing the spirochete has confounded diagnosis and treatment.
Continue to: Since reaching a historic...
Since reaching a historic nadir of incidence in 2000 (5979 cases in the United States), there has been an increasingly rapid rise in that number: to 130,000 in 2020. More than 50% of cases are in MSM; however, the number of cases in heterosexual women is rapidly increasing.43
Routine screening for syphilis should be performed in any person who is at risk: all pregnant women in the first trimester (and in the third trimester and at delivery if they are at risk or live in a community where prevalence is high) and annually in sexually active MSM or anyone with HIV infection.10
Diagnosis. Examination by dark-field microscopy, testing by PCR, and direct fluorescent antibody assay for T pallidum from lesion tissue or exudate provide definitive diagnosis for early and congenital syphilis, but are often unavailable.
Presumptive diagnosis requires 2 serologic tests:
- Nontreponemal tests (the VDRL and rapid plasma reagin tests) identify anticardiolipin antibodies released during syphilis infection, although results also can be elevated in autoimmune disease or after certain immunizations, including the COVID-19 vaccine.
- Treponemal tests (the fluorescent treponemal antibody absorbed assay, T pallidum particulate agglutination assay, enzyme immunoassay, and chemiluminescence immunoassay) are specific antibody tests.
Historically, reactive nontreponemal tests, which are less expensive and easier to perform, were followed by a treponemal test to confirm the presumptive diagnosis. This method continues to be reasonable when screening patients in a low-prevalence population.11 The reverse sequence screening algorithm (ie, begin with a treponemal test) is now frequently used. With this method, a positive treponemal test must be confirmed with a nontreponemal test. If the treponemal test is positive and the nontreponemal test is negative, another treponemal test must be positive to confirm the diagnosis. This algorithm is useful in high-risk populations because it provides earlier detection of recently acquired syphilis and enhanced detection of late latent syphilis.12,13,44 The CDC has not stated a diagnostic preference.
Once the diagnosis is made, a complete history (including a sexual history and a history of syphilis testing and treatment) and a physical exam are necessary to confirm stage of disease.45
Special circumstances. Neurosyphilis, ocular syphilis, and otosyphilis refer to the site of infection and can occur at any stage of disease. The nervous system usually is infected within hours of initial infection, but symptoms might take weeks or years to develop—or might never manifest. Any time a patient develops neurologic, ophthalmologic, or audiologic symptoms, careful neurologic and ophthalmologic evaluation should be performed and the patient should be tested for HIV.
Continue to: Lumbar puncture is warranted...
Lumbar puncture is warranted for evaluation of cerebrospinal fluid if neurologic symptoms are present but is not necessary for isolated ocular syphilis or otosyphilis without neurologic findings. Treatment should not be delayed for test results if ocular syphilis is suspected because permanent blindness can develop. Any patient at high risk for an STI who presents with neurologic or ophthalmologic symptoms should be tested for syphilis and HIV.45
Pregnant women who have a diagnosis of syphilis should be treated with penicillin immediately because treatment ≥ 30 days prior to delivery is likely to prevent most cases of congenital syphilis. However, a course of penicillin might not prevent stillbirth or congenital syphilis in a gravely infected fetus, evidenced by fetal syphilis on a sonogram at the time of treatment. Additional doses of penicillin in pregnant women with early syphilis might be indicated if there is evidence of fetal syphilis on ultrasonography. All women who deliver a stillborn infant (≥ 20 weeks’ gestation) should be tested for syphilis at delivery.46
All patients in whom primary or secondary syphilis has been diagnosed should be tested for HIV at the time of diagnosis and treatment; if the result is negative, they should be offered preexposure prophylaxis (PrEP; discussed shortly). If the incidence of HIV in your community is high, repeat testing for HIV in 3 months. Clinical and serologic evaluation should be performed 6 and 12 months after treatment.47
Treatment. Penicillin remains the standard treatment for syphilis. Primary, secondary, and early tertiary stages (including in pregnancy) are treated with benzathine penicillin G 2.4 million units intramuscular (IM) in a single dose. For pregnant patients, repeating that dose in 1 week generally is recommended. Patients in the late latent (> 1 year) or tertiary stage receive the same dose of penicillin, which is then repeated weekly, for a total of 3 doses. Doxycycline and ceftriaxone are alternatives, except in pregnancy.
Warn patients of the Jarisch-Herxheimer reaction: fever, headache, and myalgias associated with initiation of treatment in the presence of the high bacterial load seen in early syphilis. Treatment is symptomatic, but the Jarisch-Herxheimer reaction can cause fetal distress in pregnancy.
Otosyphilis, ocular syphilis, and neurosyphilis require intravenous (IV) aqueous crystalline penicillin G 3 to 4 million U every 4 hours for 10 to 14 days.45 Alternatively, procaine penicillin G 2.4 million U/d IM can be given daily with oral probenecid 500 mg qid, both for 10 to 14 days (TABLE 214).
Screening andprevention of STIs
Screening recommendations
Follow USPSTF screening guidelines for STIs.10,48-54 Screen annually for:
- gonorrhea and chlamydia in women ages 15 to 24 years and in women older than 25 years if they are at increased risk
- gonorrhea, chlamydia, syphilis, and HIV in MSM, and hepatitis C if they are HIV positive
- trichomoniasis in women who are HIV positive.
Continue to: Consider the community in which...
Consider the community in which you practice when determining risk; you might want to consult local public health authorities for information about local epidemiology and guidance on determining which of your patients are at increased risk.
Preexposure prophylaxis
According to the CDC, all sexually active adults and adolescents should be informed about the availability of PrEP to prevent HIV infection. PrEP should be (1) available to anyone who requests it and (2) recommended for anyone who is sexually active and who practices sexual behaviors that place them at substantial risk for exposure to or acquisition of HIV, or both.
The recommended treatment protocol for men and women who have either an HIV-positive partner or inconsistent condom use or who have had a bacterial STI in the previous 6 months is oral emtricitabine 200 mg plus tenofovir disoproxil fumarate 300 mg/d (sold as Truvada-F/TDF). Men and transgender women (ie, assigned male at birth) with at-risk behaviors also can use emtricitabine plus tenofovir alafenamide 25 mg/d (sold as Descovy-F/TAF).
In addition, cabotegravir plus rilpirivine (sold as Cabenuva), IM every 2 months, was approved by the FDA for PrEP in 2021.
Creatinine clearance should be assessed at baseline and yearly (every 6 months for those older than 50 years) in patients taking PrEP. All patients must be tested for HIV at initiation of treatment and every 3 months thereafter (every 4 months for cabotegravir plus rilpirivine). Patients should be screened for bacterial STIs every 6 months (every 3 months for MSM and transgender women); screening for chlamydia should be done yearly. For patients being treated with emtricitabine plus tenofovir alafenamide, weight and a lipid profile (cholesterol and triglycerides) should be assessed annually.55
Postexposure prophylaxis
The sharp rise in the incidence of STIs in the past few years has brought renewed interest in postexposure prophylaxis (PEP) for STIs. Although PEP should be standard in cases of sexual assault, this protocol also can be considered in other instances of high-risk exposure.
CDC recommendations for PEP in cases of assault are56:
- ceftriaxone 500 mg IM in a single dose (1 g if weight is ≥ 150 kg) plus
- doxycycline 100 mg bid for 7 days plus
- metronidazole 2 g bid for 7 days (for vaginal exposure)
- pregnancy evaluation and emergency contraception
- hepatitis B risk evaluation and vaccination, with or without hepatitis B immune globulin
- HIV risk evaluation, based on CDC guidelines, and possible HIV prophylaxis (PrEP)
- HPV vaccination for patients ages 9 to 26 years if they are not already fully vaccinated.
CORRESPONDENCE
Belinda Vail, MD, 3901 Rainbow Boulevard, Mail Stop 4010, Kansas City, KS 66160; [email protected]
Except for a drop in the number of sexually transmitted infections (STIs) early in the COVID-19 pandemic (March and April 2020), the incidence of STIs has been rising throughout this century.1 In 2018, 1 in 5 people in the United States had an STI; 26 million new cases were reported that year, resulting in direct costs of $16 billion—85% of which was for the care of HIV infection.2 Also that year, infection with Chlamydia trachomatis (chlamydia), Trichomonas vaginalis (trichomoniasis), herpesvirus type 2 (genital herpes), and/or human papillomavirus (condylomata acuminata) constituted 97.6% of all prevalent and 93.1% of all incident STIs.3 Almost half (45.5%) of new cases of STIs occur in people between the ages of 15 and 24 years.3

Three factors—changing social patterns, including the increase of social networking; the ability of antiviral therapy to decrease the spread of HIV, leading to a reduction in condom use; and increasing antibiotic resistance—have converged to force changes in screening and treatment recommendations. In this article, we summarize updated guidance for primary care clinicians from several sources—including the Centers for Disease Control and Prevention (CDC), the US Preventive Services Task Force (USPSTF), and the American Society for Colposcopy and Cervical Pathology (ASCCP)—on diagnosing STIs (TABLE 14-13) and providing guideline-based treatment (Table 214). Because of the breadth and complexity of HIV disease, it is not addressed here.



Chlamydia
Infection with Chlamydia trachomatis—the most commonly reported bacterial STI in the United States—primarily causes cervicitis in women and proctitis in men, and can cause urethritis and pharyngitis in men and women. Prevalence is highest in sexually active people younger than 24 years.15
Because most infected people are asymptomatic and show no signs of illness on physical exam, screening is recommended for all sexually active women younger than 25 years and all men who have sex with men (MSM).4 No studies have established proper screening intervals; a reasonable approach, therefore, is to repeat screening for patients who have a sexual history that confers a new or persistent risk for infection since their last negative result.
Depending on the location of the infection, symptoms of chlamydia can include vaginal or penile irritation or discharge, dysuria, pelvic or rectal pain, and sore throat. Breakthrough bleeding in a patient who is taking an oral contraceptive should raise suspicion for chlamydia.
Untreated chlamydia can lead to pelvic inflammatory disease (PID), tubo-ovarian abscess, tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. Infection can be transmitted vertically (mother to baby) antenatally, which can cause ophthalmia neonatorum and pneumonia in these newborns.
Diagnosis. The diagnosis of chlamydia is made using nucleic acid amplification testing (NAAT). Specimens can be collected by the clinician or the patient (self collected) using a vaginal, rectal, or oropharyngeal swab, or a combination of these, and can be obtained from urine or liquid-based cytology material.16
Continue to: Treatment
Treatment. Recommendations for treating chlamydia were updated by the CDC in its 2021 treatment guidelines (Table 214). Doxycycline 100 mg bid for 7 days is the preferred regimen; alternative regiments are (1) azithromycin 1 g in a single dose and (2) levofloxacin 500 mg daily for 7 days.4 A meta-analysis17 and a Cochrane review18 showed that the rate of treatment failure was higher among men when they were treated with azithromycin instead of doxycycline; furthermore, a randomized controlled trial demonstrated that doxycycline is more effective than azithromycin (cure rate, 100%, compared to 74%) at treating rectal chlamydia in MSM.19
Azithromycin is efficacious for urogenital infection in women; however, there is concern that the 33% to 83% of women who have concomitant rectal infection (despite reporting no receptive anorectal sexual activity) would be insufficiently treated. Outside pregnancy, the CDC does not recommend a test of cure but does recommend follow-up testing for reinfection in 3 months. Patients should abstain from sexual activity until 7 days after all sexual partners have been treated.
Expedited partner therapy (EPT) is the practice of treating sexual partners of patients with known chlamydia (and patients with gonococcal infection). Unless prohibited by law in your state, offer EPT to patients with chlamydia if they cannot ensure that their sexual partners from the past 60 days will seek timely treatment.a
Evidence to support EPT comes from 3 US clinical trials, whose subjects comprised heterosexual men and women with chlamydia or gonorrhea.21-23 The role of EPT for MSM is unclear; data are limited. Shared decision-making is recommended to determine whether EPT should be provided, to ensure that co-infection with other bacterial STIs (eg, syphilis) or HIV is not missed.24-26
a Visit www.cdc.gov/std/ept to read updated information about laws and regulations regarding EPT in your state.20
Gonorrhea
Gonorrhea is the second most-reported bacterial communicable disease.5 Infection with Neisseria gonorrhoeae causes urethral discharge in men, leading them to seek treatment; infected women, however, are often asymptomatic. Infected men and women might not recognize symptoms until they have transmitted the disease. Women have a slower natural clearance of gonococcal infection, which might explain their higher prevalence.27 Delayed recognition of symptoms can result in complications, including PID.5
Diagnosis. Specimens for NAAT can be obtained from urine, endocervical, vaginal, rectal, pharyngeal, and male urethral specimens. Reported sexual behaviors and exposures of women and transgender or gender-diverse people should be taken into consideration to determine whether rectal or pharyngeal testing, or both, should be performed.28 MSM should be screened annually at sites of contact, including the urethra, rectum, and pharynx.28 All patients with urogenital or rectal gonorrhea should be asked about oral sexual exposure; if reported, pharyngeal testing should be performed.5
NAAT of urine is at least as sensitive as testing of an endocervical specimen; the same specimen can be used to test for chlamydia and gonorrhea. Patient-collected specimens are a reasonable alternative to clinician-collected swab specimens.29
Continue to: Treatment
Treatment is complicated by the ability of gonorrhea to develop resistance. Intramuscular ceftriaxone 500 mg in a single dose cures 98% to 99% of infections in the United States; however, monitoring local resistance patterns in the community is an important component of treatment.28 (See Table 214 for an alternative regimen for cephalosporin-allergic patients and for treating gonococcal conjunctivitis and disseminated infection.)
In 2007, the CDC identified widespread quinolone-resistant gonococcal strains; therefore, fluoroquinolones no longer are recommended for treating gonorrhea.30 Cefixime has demonstrated only limited success in treating pharyngeal gonorrhea and does not attain a bactericidal level as high as ceftriaxone does; cefixime therefore is recommended only if ceftriaxone is unavailable.28 The national Gonococcal Isolate Surveillance Project is finding emerging evidence of the reduced susceptibility of N gonorrhoeae to azithromycin—making dual therapy for gonococcal infection no longer a recommendation.28
Patients should abstain from sex until 7 days after all sex partners have been treated for gonorrhea. As with chlamydia, the CDC does not recommend a test of cure for uncomplicated urogenital or rectal gonorrhea unless the patient is pregnant, but does recommend testing for reinfection 3 months after treatment.14 For patients with pharyngeal gonorrhea, a test of cure is recommended 7 to 14 days after initial treatment, due to challenges in treatment and because this site of infection is a potential source of antibiotic resistance.28
Trichomoniasis
T vaginalis, the most common nonviral STI worldwide,31 can manifest as a yellow-green vaginal discharge with or without vaginal discomfort, dysuria, epididymitis, and prostatitis; most cases, however, are asymptomatic. On examination, the cervix might be erythematous with punctate lesions (known as strawberry cervix).
Unlike most STIs, trichomoniasis is as common in women older than 24 years as it is in younger women. Infection is associated with a lower educational level, lower socioeconomic status, and having ≥ 2 sexual partners in the past year.32 Prevalence is approximately 10 times as high in Black women as it is in White women.
T vaginalis infection is associated with an increase in the risk for preterm birth, premature rupture of membranes, cervical cancer, and HIV infection. With a lack of high-quality clinical trials on the efficacy of screening, women with HIV are the only group for whom routine screening is recommended.6
Diagnosis. NAAT for trichomoniasis is now available in conjunction with gonorrhea and chlamydia testing of specimens on vaginal or urethral swabs and of urine specimens and liquid Pap smears.
Continue to: Treatment
Treatment. Because of greater efficacy, the treatment recommendation for women has changed from a single 2-g dose of oral metronidazole to 500 mg twice daily for 7 days. The 2-g single oral dose is still recommended for men7 (Table 214 lists alternative regimens).
Mycoplasma genitalium
Infection with M genitalium is common and often asymptomatic. The disease causes approximately 20% of all cases of nongonococcal and nonchlamydial urethritis in men and about 40% of persistent or recurrent infections. M genitalium is present in approximately 20% of women with cervicitis and has been associated with PID, preterm delivery, spontaneous abortion, and infertility.
There are limited and conflicting data regarding outcomes in infected patients other than those with persistent or recurrent infection; furthermore, resistance to azithromycin is increasing rapidly, resulting in an increase in treatment failures. Screening therefore is not recommended, and testing is recommended only in men with nongonococcal urethritis.33,34
Diagnosis. NAAT can be performed on urine or on a urethral, penile meatal, endocervical, or vaginal swab; men with recurrent urethritis or women with recurrent cervicitis should be tested. NAAT also can be considered in women with PID. Testing the specimen for the microorganism’s resistance to macrolide antibiotics is recommended (if such testing is available).
Treatment is initiated with doxycycline 100 mg twice daily for 7 days. If the organism is macrolide sensitive, follow with azithromycin 1 g orally on Day 1, then 500 mg/d for 3 more days. If the organism is macrolide resistant or testing is unavailable, follow doxycycline with oral moxifloxacin 400 mg/d for 7 days.33
Genital herpes (mostly herpesvirus type 2)
Genital herpes, characterized by painful, recurrent outbreaks of genital and anal lesions,35 is a lifelong infection that increases in prevalence with age.8 Because many infected people have disease that is undiagnosed or mild or have unrecognizable symptoms during viral shedding, most genital herpes infections are transmitted by people who are unaware that they are contagious.36 Herpesvirus type 2 (HSV-2) causes most cases of genital herpes, although an increasing percentage of cases are attributed to HSV type 1 (HSV-1) through receptive oral sex from a person who has an oral HSV-1 lesion.
Importantly, HSV-2–infected people are 2 to 3 times more likely to become infected with HIV than people who are not HSV-2 infected.37 This is because CD4+ T cells concentrate at the site of HSV lesions and express a higher level of cell-surface receptors that HIV uses to enter cells. HIV replicates 3 to 5 times more quickly in HSV-infected tissue.38
Continue to: HSV can become disseminated...
HSV can become disseminated, particularly in immunosuppressed people, and can manifest as encephalitis, hepatitis, and pneumonitis. Beyond its significant burden on health, HSV carries significant psychosocial consequences.9
Diagnosis. Clinical diagnosis can be challenging if classic lesions are absent at evaluation. If genital lesions are present, HSV can be identified by NAAT or culture of a specimen of those lesions. False-negative antibody results might be more frequent in early stages of infection; repeating antibody testing 12 weeks after presumed time of acquisition might therefore be indicated, based on clinical judgment. HSV-2 antibody positivity implies anogenital infection because almost all HSV-2 infections are sexually acquired.
HSV-1 antibody positivity alone is more difficult to interpret because this finding does not distinguish between oral and genital lesions, and most HSV-1 seropositivity is acquired during childhood.36 HSV polymerase chain reaction (PCR) testing of blood should not be performed to diagnose genital herpes infection, except in settings in which there is concern about disseminated infection.
Treatment. Management should address the acute episode and the chronic nature of genital herpes. Antivirals will not eradicate latent
- attenuate current infection
- prevent recurrence
- improve quality of life
- suppress the virus to prevent transmission to sexual partners.
All patients experiencing an initial episode of genital herpes should be treated, regardless of symptoms, due to the potential for prolonged or severe symptoms during recurrent episodes.9 Three drugs—acyclovir, valacyclovir, and famciclovir—are approved by the US Food and Drug Administration (FDA) to treat genital herpes and appear equally effective (TABLE 214).
Antiviral therapy for recurrent genital HSV infection can be administered either as suppressive therapy to reduce the frequency of recurrences or episodically to shorten the duration of lesions:
- Suppressive therapy reduces the frequency of recurrence by 70% to 80% among patients with frequent outbreaks. Long-term safety and efficacy are well established.
- Episodic therapy is most effective if started within 1 day after onset of lesions or during the prodrome.36
There is no specific recommendation for when to choose suppressive over episodic therapy; most patients prefer suppressive therapy because it improves quality of life. Use shared clinical decision-making to determine the best option for an individual patient.
Continue to: Human papillomavirus
Human papillomavirus
Condylomata acuminata (genital warts) are caused by human papillomavirus (HPV), most commonly types 6 and 11, which manifest as soft papules or plaques on the external genitalia, perineum, perianal skin, and groin. The warts are usually asymptomatic but can be painful or pruritic, depending on size and location.
Diagnosis is made by visual inspection and can be confirmed by biopsy if lesions are atypical. Lesions can resolve spontaneously, remain unchanged, or grow in size or number.
Treatment. The aim of treatment is relief of symptoms and removal of warts. Treatment does not eradicate HPV infection. Multiple treatments are available that can be applied by the patient as a cream, gel, or ointment or administered by the provider, including cryotherapy, surgical removal, and solutions. The decision on how to treat should be based on the number, size, and
HPV-associated cancers and precancers. This is a broad (and separate) topic. HPV types 16 and 18 cause most cases of cervical, penile, vulvar, vaginal, anal, and oropharyngeal cancer and precancer.39 The USPSTF, the American Cancer Society, and the American College of Obstetricians and Gynecologists all have recommendations for cervical cancer screening in the United States.40 Refer to guidelines of the ASCCP for recommendations on abnormal screening tests.41
Prevention of genital warts. The 9-valent HPV vaccine available in the United States is safe and effective and helps protect against viral types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Types 6 and 11 are the principal causes of genital warts. Types 16 and 18 cause 66% of cervical cancer. The vaccination series can be started at age 9 years and is recommended for everyone through age 26 years. Only 2 doses are needed if the first dose is given prior to age 15 years; given after that age, a 3-dose series is utilized. Refer to CDC vaccine guidelines42 for details on the exact timing of vaccination.
Vaccination for women ages 27 to 45 years is not universally recommended because most people have been exposed to HPV by that age. However, the vaccine can still be administered, depending on clinical circumstances and the risk for new infection.42
Syphilis
Caused by the spirochete Treponema pallidum, syphilis manifests across a spectrum—from congenital to tertiary. The inability of medical science to develop a method for culturing the spirochete has confounded diagnosis and treatment.
Continue to: Since reaching a historic...
Since reaching a historic nadir of incidence in 2000 (5979 cases in the United States), there has been an increasingly rapid rise in that number: to 130,000 in 2020. More than 50% of cases are in MSM; however, the number of cases in heterosexual women is rapidly increasing.43
Routine screening for syphilis should be performed in any person who is at risk: all pregnant women in the first trimester (and in the third trimester and at delivery if they are at risk or live in a community where prevalence is high) and annually in sexually active MSM or anyone with HIV infection.10
Diagnosis. Examination by dark-field microscopy, testing by PCR, and direct fluorescent antibody assay for T pallidum from lesion tissue or exudate provide definitive diagnosis for early and congenital syphilis, but are often unavailable.
Presumptive diagnosis requires 2 serologic tests:
- Nontreponemal tests (the VDRL and rapid plasma reagin tests) identify anticardiolipin antibodies released during syphilis infection, although results also can be elevated in autoimmune disease or after certain immunizations, including the COVID-19 vaccine.
- Treponemal tests (the fluorescent treponemal antibody absorbed assay, T pallidum particulate agglutination assay, enzyme immunoassay, and chemiluminescence immunoassay) are specific antibody tests.
Historically, reactive nontreponemal tests, which are less expensive and easier to perform, were followed by a treponemal test to confirm the presumptive diagnosis. This method continues to be reasonable when screening patients in a low-prevalence population.11 The reverse sequence screening algorithm (ie, begin with a treponemal test) is now frequently used. With this method, a positive treponemal test must be confirmed with a nontreponemal test. If the treponemal test is positive and the nontreponemal test is negative, another treponemal test must be positive to confirm the diagnosis. This algorithm is useful in high-risk populations because it provides earlier detection of recently acquired syphilis and enhanced detection of late latent syphilis.12,13,44 The CDC has not stated a diagnostic preference.
Once the diagnosis is made, a complete history (including a sexual history and a history of syphilis testing and treatment) and a physical exam are necessary to confirm stage of disease.45
Special circumstances. Neurosyphilis, ocular syphilis, and otosyphilis refer to the site of infection and can occur at any stage of disease. The nervous system usually is infected within hours of initial infection, but symptoms might take weeks or years to develop—or might never manifest. Any time a patient develops neurologic, ophthalmologic, or audiologic symptoms, careful neurologic and ophthalmologic evaluation should be performed and the patient should be tested for HIV.
Continue to: Lumbar puncture is warranted...
Lumbar puncture is warranted for evaluation of cerebrospinal fluid if neurologic symptoms are present but is not necessary for isolated ocular syphilis or otosyphilis without neurologic findings. Treatment should not be delayed for test results if ocular syphilis is suspected because permanent blindness can develop. Any patient at high risk for an STI who presents with neurologic or ophthalmologic symptoms should be tested for syphilis and HIV.45
Pregnant women who have a diagnosis of syphilis should be treated with penicillin immediately because treatment ≥ 30 days prior to delivery is likely to prevent most cases of congenital syphilis. However, a course of penicillin might not prevent stillbirth or congenital syphilis in a gravely infected fetus, evidenced by fetal syphilis on a sonogram at the time of treatment. Additional doses of penicillin in pregnant women with early syphilis might be indicated if there is evidence of fetal syphilis on ultrasonography. All women who deliver a stillborn infant (≥ 20 weeks’ gestation) should be tested for syphilis at delivery.46
All patients in whom primary or secondary syphilis has been diagnosed should be tested for HIV at the time of diagnosis and treatment; if the result is negative, they should be offered preexposure prophylaxis (PrEP; discussed shortly). If the incidence of HIV in your community is high, repeat testing for HIV in 3 months. Clinical and serologic evaluation should be performed 6 and 12 months after treatment.47
Treatment. Penicillin remains the standard treatment for syphilis. Primary, secondary, and early tertiary stages (including in pregnancy) are treated with benzathine penicillin G 2.4 million units intramuscular (IM) in a single dose. For pregnant patients, repeating that dose in 1 week generally is recommended. Patients in the late latent (> 1 year) or tertiary stage receive the same dose of penicillin, which is then repeated weekly, for a total of 3 doses. Doxycycline and ceftriaxone are alternatives, except in pregnancy.
Warn patients of the Jarisch-Herxheimer reaction: fever, headache, and myalgias associated with initiation of treatment in the presence of the high bacterial load seen in early syphilis. Treatment is symptomatic, but the Jarisch-Herxheimer reaction can cause fetal distress in pregnancy.
Otosyphilis, ocular syphilis, and neurosyphilis require intravenous (IV) aqueous crystalline penicillin G 3 to 4 million U every 4 hours for 10 to 14 days.45 Alternatively, procaine penicillin G 2.4 million U/d IM can be given daily with oral probenecid 500 mg qid, both for 10 to 14 days (TABLE 214).
Screening andprevention of STIs
Screening recommendations
Follow USPSTF screening guidelines for STIs.10,48-54 Screen annually for:
- gonorrhea and chlamydia in women ages 15 to 24 years and in women older than 25 years if they are at increased risk
- gonorrhea, chlamydia, syphilis, and HIV in MSM, and hepatitis C if they are HIV positive
- trichomoniasis in women who are HIV positive.
Continue to: Consider the community in which...
Consider the community in which you practice when determining risk; you might want to consult local public health authorities for information about local epidemiology and guidance on determining which of your patients are at increased risk.
Preexposure prophylaxis
According to the CDC, all sexually active adults and adolescents should be informed about the availability of PrEP to prevent HIV infection. PrEP should be (1) available to anyone who requests it and (2) recommended for anyone who is sexually active and who practices sexual behaviors that place them at substantial risk for exposure to or acquisition of HIV, or both.
The recommended treatment protocol for men and women who have either an HIV-positive partner or inconsistent condom use or who have had a bacterial STI in the previous 6 months is oral emtricitabine 200 mg plus tenofovir disoproxil fumarate 300 mg/d (sold as Truvada-F/TDF). Men and transgender women (ie, assigned male at birth) with at-risk behaviors also can use emtricitabine plus tenofovir alafenamide 25 mg/d (sold as Descovy-F/TAF).
In addition, cabotegravir plus rilpirivine (sold as Cabenuva), IM every 2 months, was approved by the FDA for PrEP in 2021.
Creatinine clearance should be assessed at baseline and yearly (every 6 months for those older than 50 years) in patients taking PrEP. All patients must be tested for HIV at initiation of treatment and every 3 months thereafter (every 4 months for cabotegravir plus rilpirivine). Patients should be screened for bacterial STIs every 6 months (every 3 months for MSM and transgender women); screening for chlamydia should be done yearly. For patients being treated with emtricitabine plus tenofovir alafenamide, weight and a lipid profile (cholesterol and triglycerides) should be assessed annually.55
Postexposure prophylaxis
The sharp rise in the incidence of STIs in the past few years has brought renewed interest in postexposure prophylaxis (PEP) for STIs. Although PEP should be standard in cases of sexual assault, this protocol also can be considered in other instances of high-risk exposure.
CDC recommendations for PEP in cases of assault are56:
- ceftriaxone 500 mg IM in a single dose (1 g if weight is ≥ 150 kg) plus
- doxycycline 100 mg bid for 7 days plus
- metronidazole 2 g bid for 7 days (for vaginal exposure)
- pregnancy evaluation and emergency contraception
- hepatitis B risk evaluation and vaccination, with or without hepatitis B immune globulin
- HIV risk evaluation, based on CDC guidelines, and possible HIV prophylaxis (PrEP)
- HPV vaccination for patients ages 9 to 26 years if they are not already fully vaccinated.
CORRESPONDENCE
Belinda Vail, MD, 3901 Rainbow Boulevard, Mail Stop 4010, Kansas City, KS 66160; [email protected]
1. Pagaoa M, Grey J, Torrone E, et al. Trends in nationally notifiable sexually transmitted disease case reports during the US COVID-19 pandemic, January to December 2020. Sex Transm Dis. 2021;48:798-804. doi: 10.1097/OLQ.0000000000001506
2. Chesson HW, Spicknall IH; Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis. 2021;48:215-221. doi: 10.1097/OLQ.0000000000001380
3. Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48:208-214. doi: 10.1097/OLQ.0000000000001355
4. CDC. Sexually transmitted infections treatment guidelines, 2021: Chlamydial infections among adolescents and adults. US Department of Health and Human Services. July 21, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/chlamydia.htm
5. CDC. Sexually transmitted infections treatment guidelines, 2021: Gonococcal infections among adolescents and adults. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
6. Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;
7. CDC. Sexually transmitted infections treatment guidelines, 2021. Trichomoniasis. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. December 27, 2021. www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
8. Spicknall IH, Flagg EW, Torrone EA. Estimates of the prevalence and incidence of genital herpes, United States, 2018. Sex Transm Dis. 2021;48:260-265. doi: 10.1097/OLQ.0000000000001375
9. Mark H, Gilbert L, Nanda J. Psychosocial well-being and quality of life among women newly diagnosed with genital herpes. J Obstet Gynecol Neonatal Nurs.
10. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2321-2327. doi: 10.1001/jama.2016.5824
11. Ricco J, Westby A. Syphilis: far from ancient history. Am Fam Physician. 2020;102:91-98.
12. Goza M, Kulwicki B, Akers JM, et al. Syphilis screening: a review of the Syphilis Health Check rapid immunochromatographic test. J Pharm Technol. 2017;33:53-59. doi:10.1177/8755122517691308
13. Henao- AF, Johnson SC. Diagnostic tests for syphilis: new tests and new algorithms. Neurol Clin Pract. 2014;4:114-122. doi: 10.1212/01.CPJ.0000435752.17621.48
14. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
15. CDC. Sexually Transmitted Disease Surveillance 2021. National overview of STDs. US Department of Health and Human Services. April 2023. Accessed May 9, 2023. www.cdc.gov/std/statistics/2021/overview.htm#Chlamydia
16. CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63:1-19.
17. Kong FYS, Tabrizi SN, Law M, et al. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59:193-205. doi: 10.1093/cid/ciu220
18. Páez-Canro C, Alzate JP, González LM, et al. Antibiotics for treating urogenital Chlamydia trachomatis infection in men and non-pregnant women. Cochrane Database Syst Rev. 2019;1:CD010871. doi: 10.1002/14651858.CD010871.pub2
19. Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline versus azithromycin for the treatment of rectal chlamydia in men who have sex with men: a randomized controlled trial. Clin Infect Dis. 2021;73:824-831. doi: 10.1093/cid/ciab153
20. CDC. Sexually transmitted infections treatment guidelines, 2021: Expedited partner therapy. US Department of Health and Human Services. July 22, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/clinical-EPT.htm
21. Golden MR, Whittington WLH, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-685. doi: 10.1056/NEJMoa041681
22. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
23. Cameron ST, Glasier A, Scott G, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod. 2009;24:888-895. doi: 10.1093/humrep/den475
24. McNulty A, Teh MF, Freedman E. Patient delivered partner therapy for chlamydial infection—what would be missed? Sex Transm Dis. 2008;35:834-836. doi: 10.1097/OLQ.0b013e3181761993
25. Stekler J, Bachmann L, Brotman RM, et al. Concurrent sexually transmitted infections (STIs) in sex partners of patients with selected STIs: implications for patient-delivered partner therapy. Clin Infect Dis. 2005;40:787-793. doi: 10.1086/428043
26. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
27. Stupiansky NW, Van der Pol B, Williams JA, et al. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis. 2011;38:750-754. doi: 10.1097/OLQ.0b013e31820ff9a4
28. CDC. Sexually transmitted infections treatment guidelines, 2021: Screening recommendations and considerations referenced in treatment guidelines and original sources. US Department of Health and Human Services. June 6, 2022. Accessed May 9, 2023. www.cdc.gov/std/treatment-guidelines/screening-recommen dations.htm
29. Cantor A, Dana T, Griffen JC, et al. Screening for chlamydial and gonococcal infections: a systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 206. AHRQ Report No. 21-05275-EF-1. Agency for Healthcare Research and Quality. September 2021. www.ncbi.nlm.nih.gov/books/NBK574045
30. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
31. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019:97:548-562P. doi: 10.2471/BLT.18.228486
32. Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217. doi: 10.1093/cid/ciy079
33. CDC. Sexually transmitted infections treatment guidelines, 2021. Mycoplasma genitalium. US Department of Health and Human Services. July 22, 2021. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm
34. Manhart LE, Broad JM, Bolden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis. 2011;53(suppl 3):S129-S142. doi:10.1093/cid/cir702.
35. Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. McGraw-Hill; 2008:399-437.
36. CDC. Sexually transmitted infections treatment guidelines, 2021: Genital herpes. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/herpes.htm
37. Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1303-1316. doi: 10.1016/S1473-3099(17)30405-X
38. Rollenhagen C, Lathrop M, Macura SL, et al. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol. 2014;7:1165-1174. doi: 10.1038/mi.2014.3
39. Cogliano V, Baan R, Straif K, et al; WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol.
40. Simon MA, Tseng CW, Wong JB. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897
41. Perkins RB, Guido RS, Castle PE, et al; . 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
42. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
43. Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the United States: an assessment of contributing factors. Infect Dis (Auckl). 2019;12:1178633719883282. doi: 10.1177/1178633719883282
44. Boog GHP, Lopes JVZ, Mahler JV, et al. Diagnostic tools for neurosyphilis: a systematic review. BMC Infect Dis. 2021;21:568. doi: 10.1186/s12879-021-06264-8
45. CDC. Sexually transmitted infections treatment guidelines, 2021. Syphilis. US Department of Health and Human Services. April 20, 2023. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/syphilis.htm
46. Matthias JM, Rahman MM, Newman DR, et al. Effectiveness of prenatal screening and treatment to prevent congenital syphilis, Louisiana and Florida, 2013-2014. Sex Transm Dis. 2017;44:498-502. doi: 10.1097/OLQ.0000000000000638
47. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014;312:1905-1917. doi: 10.1001/jama.2014.13259
48. Davidson KW, Barry MJ, Mangione CM, et al; US Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:949-956. doi: 10.1001/jama.2021.14081
49. Krist AH, Davidson KW, Mangione CM, et al; US Preventive Services Task Force. Screening for hepatitis B virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2415-2422. doi: 10.1001/jama.2020.22980
50. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:970-975. doi: 10.1001/jama.2020.1123
51. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530. doi: 10.1001/jama.2016.16776
52. Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi: 10.1001/jama.2018.10897
53. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336. doi: 10.1001/jama.2019.6587
54. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36:502-509. doi: 10.1016/s0091-7435(02)00058-0
55. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. A clinical practice guideline. Centers for Disease Control and Prevention. Accessed April 24, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
56. CDC. Sexually transmitted infections treatment guidelines, 2021: Sexual assault and abuse and STIs—adolescents and adults, 2021. US Department of Health and Human Services. July 22, 2021. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/sexual-assault-adults.htm
1. Pagaoa M, Grey J, Torrone E, et al. Trends in nationally notifiable sexually transmitted disease case reports during the US COVID-19 pandemic, January to December 2020. Sex Transm Dis. 2021;48:798-804. doi: 10.1097/OLQ.0000000000001506
2. Chesson HW, Spicknall IH; Bingham A, et al. The estimated direct lifetime medical costs of sexually transmitted infections acquired in the United States in 2018. Sex Transm Dis. 2021;48:215-221. doi: 10.1097/OLQ.0000000000001380
3. Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis. 2021;48:208-214. doi: 10.1097/OLQ.0000000000001355
4. CDC. Sexually transmitted infections treatment guidelines, 2021: Chlamydial infections among adolescents and adults. US Department of Health and Human Services. July 21, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/chlamydia.htm
5. CDC. Sexually transmitted infections treatment guidelines, 2021: Gonococcal infections among adolescents and adults. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm
6. Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;
7. CDC. Sexually transmitted infections treatment guidelines, 2021. Trichomoniasis. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. December 27, 2021. www.cdc.gov/std/treatment-guidelines/trichomoniasis.htm
8. Spicknall IH, Flagg EW, Torrone EA. Estimates of the prevalence and incidence of genital herpes, United States, 2018. Sex Transm Dis. 2021;48:260-265. doi: 10.1097/OLQ.0000000000001375
9. Mark H, Gilbert L, Nanda J. Psychosocial well-being and quality of life among women newly diagnosed with genital herpes. J Obstet Gynecol Neonatal Nurs.
10. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Screening for syphilis infection in nonpregnant adults and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2321-2327. doi: 10.1001/jama.2016.5824
11. Ricco J, Westby A. Syphilis: far from ancient history. Am Fam Physician. 2020;102:91-98.
12. Goza M, Kulwicki B, Akers JM, et al. Syphilis screening: a review of the Syphilis Health Check rapid immunochromatographic test. J Pharm Technol. 2017;33:53-59. doi:10.1177/8755122517691308
13. Henao- AF, Johnson SC. Diagnostic tests for syphilis: new tests and new algorithms. Neurol Clin Pract. 2014;4:114-122. doi: 10.1212/01.CPJ.0000435752.17621.48
14. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
15. CDC. Sexually Transmitted Disease Surveillance 2021. National overview of STDs. US Department of Health and Human Services. April 2023. Accessed May 9, 2023. www.cdc.gov/std/statistics/2021/overview.htm#Chlamydia
16. CDC. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep. 2014;63:1-19.
17. Kong FYS, Tabrizi SN, Law M, et al. Azithromycin versus doxycycline for the treatment of genital chlamydia infection: a meta-analysis of randomized controlled trials. Clin Infect Dis. 2014;59:193-205. doi: 10.1093/cid/ciu220
18. Páez-Canro C, Alzate JP, González LM, et al. Antibiotics for treating urogenital Chlamydia trachomatis infection in men and non-pregnant women. Cochrane Database Syst Rev. 2019;1:CD010871. doi: 10.1002/14651858.CD010871.pub2
19. Dombrowski JC, Wierzbicki MR, Newman LM, et al. Doxycycline versus azithromycin for the treatment of rectal chlamydia in men who have sex with men: a randomized controlled trial. Clin Infect Dis. 2021;73:824-831. doi: 10.1093/cid/ciab153
20. CDC. Sexually transmitted infections treatment guidelines, 2021: Expedited partner therapy. US Department of Health and Human Services. July 22, 2021. Accessed April 19, 2023. www.cdc.gov/std/treatment-guidelines/clinical-EPT.htm
21. Golden MR, Whittington WLH, Handsfield HH, et al. Effect of expedited treatment of sex partners on recurrent or persistent gonorrhea or chlamydial infection. N Engl J Med. 2005;352:676-685. doi: 10.1056/NEJMoa041681
22. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
23. Cameron ST, Glasier A, Scott G, et al. Novel interventions to reduce re-infection in women with chlamydia: a randomized controlled trial. Hum Reprod. 2009;24:888-895. doi: 10.1093/humrep/den475
24. McNulty A, Teh MF, Freedman E. Patient delivered partner therapy for chlamydial infection—what would be missed? Sex Transm Dis. 2008;35:834-836. doi: 10.1097/OLQ.0b013e3181761993
25. Stekler J, Bachmann L, Brotman RM, et al. Concurrent sexually transmitted infections (STIs) in sex partners of patients with selected STIs: implications for patient-delivered partner therapy. Clin Infect Dis. 2005;40:787-793. doi: 10.1086/428043
26. Schillinger JA, Kissinger P, Calvet H, et al. Patient-delivered partner treatment with azithromycin to prevent repeated Chlamydia trachomatis infection among women: a randomized, controlled trial. Sex Transm Dis. 2003;30:49-56. doi: 10.1097/00007435-200301000-00011
27. Stupiansky NW, Van der Pol B, Williams JA, et al. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis. 2011;38:750-754. doi: 10.1097/OLQ.0b013e31820ff9a4
28. CDC. Sexually transmitted infections treatment guidelines, 2021: Screening recommendations and considerations referenced in treatment guidelines and original sources. US Department of Health and Human Services. June 6, 2022. Accessed May 9, 2023. www.cdc.gov/std/treatment-guidelines/screening-recommen dations.htm
29. Cantor A, Dana T, Griffen JC, et al. Screening for chlamydial and gonococcal infections: a systematic review update for the US Preventive Services Task Force. Evidence Synthesis No. 206. AHRQ Report No. 21-05275-EF-1. Agency for Healthcare Research and Quality. September 2021. www.ncbi.nlm.nih.gov/books/NBK574045
30. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep. 2007;56:332-336.
31. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019:97:548-562P. doi: 10.2471/BLT.18.228486
32. Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217. doi: 10.1093/cid/ciy079
33. CDC. Sexually transmitted infections treatment guidelines, 2021. Mycoplasma genitalium. US Department of Health and Human Services. July 22, 2021. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm
34. Manhart LE, Broad JM, Bolden MR. Mycoplasma genitalium: should we treat and how? Clin Infect Dis. 2011;53(suppl 3):S129-S142. doi:10.1093/cid/cir702.
35. Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Stamm WE, et al, eds. Sexually Transmitted Diseases. 4th ed. McGraw-Hill; 2008:399-437.
36. CDC. Sexually transmitted infections treatment guidelines, 2021: Genital herpes. US Department of Health and Human Services. September 21, 2022. Accessed April 23, 2023. www.cdc.gov/std/treatment-guidelines/herpes.htm
37. Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1303-1316. doi: 10.1016/S1473-3099(17)30405-X
38. Rollenhagen C, Lathrop M, Macura SL, et al. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol. 2014;7:1165-1174. doi: 10.1038/mi.2014.3
39. Cogliano V, Baan R, Straif K, et al; WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol.
40. Simon MA, Tseng CW, Wong JB. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi:10.1001/jama.2018.10897
41. Perkins RB, Guido RS, Castle PE, et al; . 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131. doi: 10.1097/LGT.0000000000000525
42. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702. doi: 10.15585/mmwr.mm6832a3
43. Schmidt R, Carson PJ, Jansen RJ. Resurgence of syphilis in the United States: an assessment of contributing factors. Infect Dis (Auckl). 2019;12:1178633719883282. doi: 10.1177/1178633719883282
44. Boog GHP, Lopes JVZ, Mahler JV, et al. Diagnostic tools for neurosyphilis: a systematic review. BMC Infect Dis. 2021;21:568. doi: 10.1186/s12879-021-06264-8
45. CDC. Sexually transmitted infections treatment guidelines, 2021. Syphilis. US Department of Health and Human Services. April 20, 2023. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/syphilis.htm
46. Matthias JM, Rahman MM, Newman DR, et al. Effectiveness of prenatal screening and treatment to prevent congenital syphilis, Louisiana and Florida, 2013-2014. Sex Transm Dis. 2017;44:498-502. doi: 10.1097/OLQ.0000000000000638
47. Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014;312:1905-1917. doi: 10.1001/jama.2014.13259
48. Davidson KW, Barry MJ, Mangione CM, et al; US Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:949-956. doi: 10.1001/jama.2021.14081
49. Krist AH, Davidson KW, Mangione CM, et al; US Preventive Services Task Force. Screening for hepatitis B virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2415-2422. doi: 10.1001/jama.2020.22980
50. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:970-975. doi: 10.1001/jama.2020.1123
51. Bibbins-Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force. Serologic screening for genital herpes infection: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:2525-2530. doi: 10.1001/jama.2016.16776
52. Curry SJ, Krist AH, Owens DK, et al; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686. doi: 10.1001/jama.2018.10897
53. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336. doi: 10.1001/jama.2019.6587
54. Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36:502-509. doi: 10.1016/s0091-7435(02)00058-0
55. US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update. A clinical practice guideline. Centers for Disease Control and Prevention. Accessed April 24, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
56. CDC. Sexually transmitted infections treatment guidelines, 2021: Sexual assault and abuse and STIs—adolescents and adults, 2021. US Department of Health and Human Services. July 22, 2021. Accessed April 24, 2023. www.cdc.gov/std/treatment-guidelines/sexual-assault-adults.htm
PRACTICE RECOMMENDATIONS
› Focus efforts to prevent sexually transmitted infections (STIs) on patients ages 15 to 24 years—because half of new STIs in the United States occur in this age group. A
› Screen for other STIs, including HIV infection, if a person tests positive for a single STI. A
› Treat STIs by following updated (2021) guidelines developed by the Centers for Disease Control and Prevention. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
30-year-old woman • progressive dyspnea and peripheral edema • 35th week of gestation with a history of mild preeclampsia • Dx?
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.
An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.

An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
THE CASE
A 30-year-old woman sought care at her rural family physician’s office for progressive dyspnea and peripheral edema, which she had been experiencing for several weeks. She was G1P0 and in her 35th week of gestation.
Her medical history was remarkable for mild preeclampsia, which was being managed observantly by her obstetrician in consultation with a maternal-fetal medicine specialist. She had been evaluated by her local hospital’s labor and delivery department and her maternal-fetal medicine specialist earlier in the week and seen the previous day by her obstetrician for these signs and symptoms. They all reassured her and told her these symptoms were normal during pregnancy. No diagnostic studies were performed. However, she remained concerned and decided to see her family physician for another opinion.
Upon presentation to her family physician, the patient was afebrile. Her blood pressure was 135/98 mm Hg; heart rate, 96 beats/min; and respiration, 20 breaths/min and slightly labored. Edema of 2 to 3+ was noted in her lower extremities, hands, and face. Bibasilar breath sounds were diminished, and her abdomen was nontender.
The family physician suspected left ventricular systolic dysfunction. He worked in a small office that lacked access to a laboratory or radiographic studies. However, he did have an ultrasound machine available, and although he was not skilled in echocardiography to assess cardiac function, he was able to obtain a bedside lung ultrasound.
THE DIAGNOSIS
While no B-lines were seen on the lung ultrasound, bilateral plural effusions were noted (FIGURE). This finding, paired with the patient’s signs and symptoms, prompted the family physician to suspect a diagnosis of acute decompensated heart failure with presumptive peripartum cardiomyopathy. The patient was immediately driven to the hospital by her family physician for emergency admission with stat obstetric and cardiology consultations.

An in-hospital echocardiogram revealed severe global hypokinesia with a left ventricular ejection fraction of 25% to 30%, which confirmed the family physician’s suspicions. Laboratory studies were significant for elevated N-terminal pro-brain natriuretic peptide (43,449 pg/mL; normal, < 125 pg/mL), troponin (1.12 ng/mL; normal range, 0-0.10 ng/mL), and white blood cell count (27.6 x 103/µL). She also had evidence of acute renal injury, with blood urea nitrogen of 46 mg/dL (normal range, 7-18 mg/dL), creatinine of 2.0 mg/dL (normal range, 0.5-1.0 mg/dL), and potassium of 7.6 mmol/L (normal range, 3.5-5.1 mmol/L). Emergency delivery was induced by amniotomy, resulting in the birth of a baby girl weighing 5 lb 4 oz (Apgar scores 6, 8, and 9).
Following delivery, the patient was placed on a milrinone infusion and required dialysis. She was emergently transferred to a tertiary care hospital, where she was admitted to the cardiac intensive care unit by the cardiology/heart transplant service with nephrology and obstetric consultations. Hematology and infectious disease specialists were consulted to rule out HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome and sepsis, respectively. Her course of care remained complicated with further testing, including cardiac catheterization and biopsy, which was negative for additional pathology.
Continue to: One week after admission...
One week after admission, she was discharged home with a 24-hour wearable external cardiac defibrillator and a confirmed diagnosis of peripartum cardiomyopathy. Her medication regimen included digoxin (125 µg 3 times/wk), spironolactone (25 mg/d), carvedilol (3.125 mg twice daily), sacubitril/valsartan (24 mg/26 mg twice daily), furosemide (20 mg/d as needed for weight gain > 3-4 lb or leg swelling), magnesium oxide (400 mg twice daily), and ferrous sulfate (325 mg/d).
DISCUSSION
Peripartum cardiomyopathy is a rare, life-threatening, idiopathic cardiomyopathy that is responsible for one-half to two-thirds of cardiovascular disease–related maternal deaths in the United States.1,2 It manifests in late pregnancy or early in the postpartum period and is characterized by left ventricular systolic dysfunction with resultant heart failure and an ejection fraction of less than 45%.1,2
Recognized as early as the 1800s by Virchow,2,3 the incidence of peripartum cardiomyopathy in the United States ranges from 1 in 1000 to 4000 live births and is increasing worldwide.1,2 While the cause of peripartum cardiomyopathy remains unknown, risk factors include advanced maternal age, African descent, hypertension, preeclampsia, and multiple gestation pregnancy.1,2
Early diagnosis of peripartum cardiomyopathy is imperative for survival of both mother and baby.4 This may be difficult because the signs and symptoms of heart failure—such as dyspnea, edema, orthopnea, cough, and chest and abdominal pain—overlap with those of a typical pregnancy, resulting in it often being missed on evaluation.1,2
Dx with echocardiography; in a pinch, consider lung ultrasound
Usually a diagnosis of peripartum cardiomyopathy is established with echocardiography.1,2 Thus, this case is of significant importance because it illustrates the successful use of lung ultrasound—a simple and easy test—by a rural family doctor to identify this potentially fatal, elusive condition with no additional studies.
Continue to: Use of lung ultrasound...
Use of lung ultrasound in the detection of acute decompensated heart failure is accepted in the medical literature.5-7 Given clinical correlation, a positive scan is defined by the presence of at least 3 B-lines on a longitudinal plane between 2 ribs or, as seen in our case, by the presence of pleural effusion.5-8 Lung ultrasound is readily available worldwide, is completely safe in pregnancy, and is considered one of the easiest studies to perform.7-10
At the patient’s 9-month follow-up visit, she had made a full clinical recovery. Her ejection fraction was 59.8%, and she had stopped all medications. The patient and her child did not experience any continued complications.
THE TAKEAWAY
Family physicians should be aware of peripartum cardiomyopathy—one of the most elusive and life-threatening diseases of pregnancy. When managing a pregnant patient, it is imperative to follow up on complaints such as dyspnea, peripheral edema, and chest and/or abdominal pain. While these symptoms are not unusual during pregnancy, they should always prompt a more thorough evaluation. If peripartum cardiomyopathy is suspected, lung ultrasound is a valuable diagnostic tool for family physicians. Further research is needed before the findings of this case report can be universally applied in the routine prenatal care of women at risk for peripartum cardiomyopathy.
The authors thank their daughter, Nickel Cielo Abarbanell, for her help in the preparation of this manuscript.
CORRESPONDENCE
Neal Robert Abarbanell, MD, First Choice Healthcare, 1867 20th Avenue, Vero Beach, FL 32960; neal.abarbanell@ gmail.com
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
1. Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287
2. Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133:1397-1409. doi: 10.1161/CIRCULATIONAHA.115.020491
3. Porak C. De L’influence reciproque de la grossesse et del maladies du Coceur [thesis]. Medical Faculty of Paris, France: 1880.
4. Lewey J, Levine LD, Elovitz MA, et al. Importance of early diagnosis in peripartum cardiomyopathy. Hypertension. 2020;75:91-97. doi: 10.1161/HYPERTENSIONAHA.119.13291
5. Volpicelli G, Caramello V, Cardinale L, et al. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585-591. doi: 10.1016/j.ajem.2007.09.014
6. Muniz RT, Mesquita ET, Souza CV Jr, et al. Pulmonary ultrasound in patients with heart failure-systematic review. Arq Bras Cardiol. 2018;110:577-584. doi: 10.5935/abc.20180097
7. Russell FM, Rutz M, Pang PS. Focused ultrasound in the emergency department for patients with acute heart failure. Card Fail Rev. 2015;1:83-86. doi: 10.15420/cfr.2015.1.2.83
8. Gustafsson M, Alehagen U, Johansson P. Imaging congestion with a pocket ultrasound device: prognostic implications in patients with chronic heart failure. J Card Fail. 2015;21:548-554. doi: 10.1016/j.cardfail.2015.02.004
9. Ntusi NA, Samuels P, Moosa S, et al. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95-103. doi: 10.5830/CVJA-2016-022
10. Kimberly HH, Murray A, Mennicke M, et al. Focused maternal ultrasound by midwives in rural Zambia. Ultrasound Med Biol. 2010;36:1267-1272. doi: 10.1016/j.ultrasmedbio.2010.05.017
► Progressive dyspnea and peripheral edema
► 35th week of gestation with a history of mild preeclampsia
Clinical index predicts common postpartum mental health disorders
Developed by Canadian researchers, the easily implementable PMH CAREPLAN index “creates a framework for clinically actionable risk stratification that could assist patients and providers in determining an individual’s level of risk for common postpartum mental health disorders and direct them to appropriate intervention,” wrote a group led by Simone N. Vigod, MD, MSc, head of the department of psychiatry at Women’s College Hospital, Toronto, in the British Journal of Psychiatry.
After giving birth, women are especially vulnerable to major depression, anxiety, PTSD, and obsessive-compulsive disorder, which have a general postpartum prevalence of 7%-20%.
Common PMH disorders are to be distinguished from the more rare but severe PMH disorders such as postpartum psychosis and bipolar disorder, the researchers stressed.
“We know there are interventions that can prevent these disorders, but these seem to work best in people who are at high risk for developing the illnesses, “ Dr. Vigod said. “So, we wanted to be able to determine the level of risk that a person might actually experience them.”
In an ideal world, she continued, physicians might be able to say to a patient: “You have a 50% chance of developing postpartum depression and anxiety, so it may be worth investing your time and resources in a course of preventive psychotherapy.” Or: “You have a 90% chance of developing these disorders, so it might be worth going back on your medications even though you are breastfeeding.” Or: “You have only a 1% chance of developing them, so probably it’s not worthwhile to go back on your medication prophylactically.”
A need for a new assessment tool, akin to the Framingham Risk Score for 10-year cardiovascular events and the FRAX scoring system for 10-year fracture risk, was evident since previous indices based largely on patient self-reporting have had moderate predictive capacity, and have not been adopted in clinical practice, Dr. Vigod and associates noted.
Split-cohort design
Using population-based health administrative data and hospital birth records from Ontario during 2012-2015, Dr. Vigod’s group created and internally validated a predictive model for common PMH disorders in a cohort of 152,362 mothers. They then converted it to a risk index after validation in an additional cohort of 75,772 mothers. The women had delivered live infants during 2012-2014.
A common PMH disorder occurred in 13,608 mothers, while 214,526 were unaffected.
Independently associated PMH variables were many: prenatal care provider, mental health diagnosis history and medications during pregnancy, psychiatric hospital admissions or ED visits, conception type and complications, and apprehension of newborn by child services. Other factors were region of maternal origin, extremes of gestational age at birth, primary maternal language, lactation intention, maternal age, and number of prenatal visits.
Based on a broad span of scores from 0 to 39, 1-year common PMH disorder risk ranged from 1.5% to 40.5%, with an overall 1-year prevalence of 6%, consistent with previous studies. That included 11,262 (5%) mothers with an anxiety or related disorder, 3,392 (1.5%) with a depressive episode, and 1,046 (0.5%) with both. The best trade-off of sensitivity/specificity for risk appeared to be at a screening threshold score of 17 or above.
Risk drivers
PMH-affected mothers were slightly younger than unaffected women (mean age, 29.9 years vs. 30.6 years), more likely to be primiparous (45.2% vs. 42%), and less likely to be recent immigrants (16.7% vs. 27.2%).
They were also more likely to have previously experienced postpartum depression (4.4% vs. 1.4%), any depression (15.3% vs. 4.4%), and any anxiety disorder (13.8% vs. 4.3%).
As to lifestyle, smoking was more common in women with PMH (15.0% vs. 10.2%), as were the use of nonprescribed substances (3% vs. 1.4%) and intimate partner violence in pregnancy (2.7% vs. 1.5%).
In addition, the affected group experienced more pregnancy complications than their unaffected peers (16% vs. 13.9%), preterm birth (8.2% vs. 6.8%), and Apgar scores below 7 at 1 or 5 minutes (10.5% vs. 7.6%).
Low income did not appear to have an impact since just over 20% in either group fell into the lowest neighborhood income quintile.
Commenting on the index but not involved in developing it, LaTasha D. Nelson, MD, an associate professor or medicine and a maternal-fetal medicine specialist at Northwestern Medicine in Chicago, doubted the Canadian model would work as well in the more fragmented U.S. health care system, compared with Canada’s universal model with its large provincial health databases.
She also found the large number of variables and broad score range potentially problematic, especially if the risk threshold is set at less than half the maximum score at 17, at which some low-risk mothers might get screening and perhaps intervention. “Are we going to use up the resources we have for those who might not need help, or are we going to treat someone who really needs it?” she asked.
Another concern is the postpartum timing of assessment. At Dr. Nelson’s center, mothers are checked for mental health at two points during pregnancy and those with higher scores are triaged for further care.
Dr. Nelson was also puzzled by the score-lowering impact of prenatal care given by a nurse practitioner and “other” provider : –5 and –2, respectively, versus +3 for a midwife and +1 for a family doctor. “This may capture more relaxed, easy-going multiparous mothers who felt comfortable turning to an NP,” she said.
It may indeed reflect that the risk level of a person who sees those providers is overall lower, Dr. Vigod agreed. “This is one reason why we would want to see replication of these results in other jurisdictions and by other ways of diagnosis before putting it out into clinical practice.”
As to the score-lowering effect of not speaking English as the primary tongue, Dr. Nelson wondered, “is that because we’re taking better care of mothers who speak the main language and missing those who speak other languages? Are they not getting the same level of interrogation?”
It may be that individuals in these groups were less likely to access mental health care, Dr. Vigod agreed, or it might reflect the so-called healthy immigrant effect or culturally different levels of postpartum support. “It might mean that there are more people who benefit from community-level protective factors in these groups. We know that social support is an important protective factor.”
Despite her reservations about the index, Dr. Nelson said that increasing attention to the pre- and postnatal mental health of mothers is an important part of maternal care. “This is an issue that needs to be recognized.”
The next step, Dr. Vigod said, is to determine whether the index holds up in other populations. “Then, we would want to test it out to see if recommending interventions based on a certain level of risk improves outcomes. At what percentage risk would starting an antidepressant medication result in a reduced risk for postpartum depression or anxiety – 90%, 80%, 70%, or less?”
The study received funding from the Canadian Institutes of Health Research. Data were analyzed by ICES, an independent nonprofit research organization that holds population-based data. Dr. Vigod reported royalties from UpToDate for materials related to depression and pregnancy. Dr. Nelson disclosed no relevant competing interests.
Developed by Canadian researchers, the easily implementable PMH CAREPLAN index “creates a framework for clinically actionable risk stratification that could assist patients and providers in determining an individual’s level of risk for common postpartum mental health disorders and direct them to appropriate intervention,” wrote a group led by Simone N. Vigod, MD, MSc, head of the department of psychiatry at Women’s College Hospital, Toronto, in the British Journal of Psychiatry.
After giving birth, women are especially vulnerable to major depression, anxiety, PTSD, and obsessive-compulsive disorder, which have a general postpartum prevalence of 7%-20%.
Common PMH disorders are to be distinguished from the more rare but severe PMH disorders such as postpartum psychosis and bipolar disorder, the researchers stressed.
“We know there are interventions that can prevent these disorders, but these seem to work best in people who are at high risk for developing the illnesses, “ Dr. Vigod said. “So, we wanted to be able to determine the level of risk that a person might actually experience them.”
In an ideal world, she continued, physicians might be able to say to a patient: “You have a 50% chance of developing postpartum depression and anxiety, so it may be worth investing your time and resources in a course of preventive psychotherapy.” Or: “You have a 90% chance of developing these disorders, so it might be worth going back on your medications even though you are breastfeeding.” Or: “You have only a 1% chance of developing them, so probably it’s not worthwhile to go back on your medication prophylactically.”
A need for a new assessment tool, akin to the Framingham Risk Score for 10-year cardiovascular events and the FRAX scoring system for 10-year fracture risk, was evident since previous indices based largely on patient self-reporting have had moderate predictive capacity, and have not been adopted in clinical practice, Dr. Vigod and associates noted.
Split-cohort design
Using population-based health administrative data and hospital birth records from Ontario during 2012-2015, Dr. Vigod’s group created and internally validated a predictive model for common PMH disorders in a cohort of 152,362 mothers. They then converted it to a risk index after validation in an additional cohort of 75,772 mothers. The women had delivered live infants during 2012-2014.
A common PMH disorder occurred in 13,608 mothers, while 214,526 were unaffected.
Independently associated PMH variables were many: prenatal care provider, mental health diagnosis history and medications during pregnancy, psychiatric hospital admissions or ED visits, conception type and complications, and apprehension of newborn by child services. Other factors were region of maternal origin, extremes of gestational age at birth, primary maternal language, lactation intention, maternal age, and number of prenatal visits.
Based on a broad span of scores from 0 to 39, 1-year common PMH disorder risk ranged from 1.5% to 40.5%, with an overall 1-year prevalence of 6%, consistent with previous studies. That included 11,262 (5%) mothers with an anxiety or related disorder, 3,392 (1.5%) with a depressive episode, and 1,046 (0.5%) with both. The best trade-off of sensitivity/specificity for risk appeared to be at a screening threshold score of 17 or above.
Risk drivers
PMH-affected mothers were slightly younger than unaffected women (mean age, 29.9 years vs. 30.6 years), more likely to be primiparous (45.2% vs. 42%), and less likely to be recent immigrants (16.7% vs. 27.2%).
They were also more likely to have previously experienced postpartum depression (4.4% vs. 1.4%), any depression (15.3% vs. 4.4%), and any anxiety disorder (13.8% vs. 4.3%).
As to lifestyle, smoking was more common in women with PMH (15.0% vs. 10.2%), as were the use of nonprescribed substances (3% vs. 1.4%) and intimate partner violence in pregnancy (2.7% vs. 1.5%).
In addition, the affected group experienced more pregnancy complications than their unaffected peers (16% vs. 13.9%), preterm birth (8.2% vs. 6.8%), and Apgar scores below 7 at 1 or 5 minutes (10.5% vs. 7.6%).
Low income did not appear to have an impact since just over 20% in either group fell into the lowest neighborhood income quintile.
Commenting on the index but not involved in developing it, LaTasha D. Nelson, MD, an associate professor or medicine and a maternal-fetal medicine specialist at Northwestern Medicine in Chicago, doubted the Canadian model would work as well in the more fragmented U.S. health care system, compared with Canada’s universal model with its large provincial health databases.
She also found the large number of variables and broad score range potentially problematic, especially if the risk threshold is set at less than half the maximum score at 17, at which some low-risk mothers might get screening and perhaps intervention. “Are we going to use up the resources we have for those who might not need help, or are we going to treat someone who really needs it?” she asked.
Another concern is the postpartum timing of assessment. At Dr. Nelson’s center, mothers are checked for mental health at two points during pregnancy and those with higher scores are triaged for further care.
Dr. Nelson was also puzzled by the score-lowering impact of prenatal care given by a nurse practitioner and “other” provider : –5 and –2, respectively, versus +3 for a midwife and +1 for a family doctor. “This may capture more relaxed, easy-going multiparous mothers who felt comfortable turning to an NP,” she said.
It may indeed reflect that the risk level of a person who sees those providers is overall lower, Dr. Vigod agreed. “This is one reason why we would want to see replication of these results in other jurisdictions and by other ways of diagnosis before putting it out into clinical practice.”
As to the score-lowering effect of not speaking English as the primary tongue, Dr. Nelson wondered, “is that because we’re taking better care of mothers who speak the main language and missing those who speak other languages? Are they not getting the same level of interrogation?”
It may be that individuals in these groups were less likely to access mental health care, Dr. Vigod agreed, or it might reflect the so-called healthy immigrant effect or culturally different levels of postpartum support. “It might mean that there are more people who benefit from community-level protective factors in these groups. We know that social support is an important protective factor.”
Despite her reservations about the index, Dr. Nelson said that increasing attention to the pre- and postnatal mental health of mothers is an important part of maternal care. “This is an issue that needs to be recognized.”
The next step, Dr. Vigod said, is to determine whether the index holds up in other populations. “Then, we would want to test it out to see if recommending interventions based on a certain level of risk improves outcomes. At what percentage risk would starting an antidepressant medication result in a reduced risk for postpartum depression or anxiety – 90%, 80%, 70%, or less?”
The study received funding from the Canadian Institutes of Health Research. Data were analyzed by ICES, an independent nonprofit research organization that holds population-based data. Dr. Vigod reported royalties from UpToDate for materials related to depression and pregnancy. Dr. Nelson disclosed no relevant competing interests.
Developed by Canadian researchers, the easily implementable PMH CAREPLAN index “creates a framework for clinically actionable risk stratification that could assist patients and providers in determining an individual’s level of risk for common postpartum mental health disorders and direct them to appropriate intervention,” wrote a group led by Simone N. Vigod, MD, MSc, head of the department of psychiatry at Women’s College Hospital, Toronto, in the British Journal of Psychiatry.
After giving birth, women are especially vulnerable to major depression, anxiety, PTSD, and obsessive-compulsive disorder, which have a general postpartum prevalence of 7%-20%.
Common PMH disorders are to be distinguished from the more rare but severe PMH disorders such as postpartum psychosis and bipolar disorder, the researchers stressed.
“We know there are interventions that can prevent these disorders, but these seem to work best in people who are at high risk for developing the illnesses, “ Dr. Vigod said. “So, we wanted to be able to determine the level of risk that a person might actually experience them.”
In an ideal world, she continued, physicians might be able to say to a patient: “You have a 50% chance of developing postpartum depression and anxiety, so it may be worth investing your time and resources in a course of preventive psychotherapy.” Or: “You have a 90% chance of developing these disorders, so it might be worth going back on your medications even though you are breastfeeding.” Or: “You have only a 1% chance of developing them, so probably it’s not worthwhile to go back on your medication prophylactically.”
A need for a new assessment tool, akin to the Framingham Risk Score for 10-year cardiovascular events and the FRAX scoring system for 10-year fracture risk, was evident since previous indices based largely on patient self-reporting have had moderate predictive capacity, and have not been adopted in clinical practice, Dr. Vigod and associates noted.
Split-cohort design
Using population-based health administrative data and hospital birth records from Ontario during 2012-2015, Dr. Vigod’s group created and internally validated a predictive model for common PMH disorders in a cohort of 152,362 mothers. They then converted it to a risk index after validation in an additional cohort of 75,772 mothers. The women had delivered live infants during 2012-2014.
A common PMH disorder occurred in 13,608 mothers, while 214,526 were unaffected.
Independently associated PMH variables were many: prenatal care provider, mental health diagnosis history and medications during pregnancy, psychiatric hospital admissions or ED visits, conception type and complications, and apprehension of newborn by child services. Other factors were region of maternal origin, extremes of gestational age at birth, primary maternal language, lactation intention, maternal age, and number of prenatal visits.
Based on a broad span of scores from 0 to 39, 1-year common PMH disorder risk ranged from 1.5% to 40.5%, with an overall 1-year prevalence of 6%, consistent with previous studies. That included 11,262 (5%) mothers with an anxiety or related disorder, 3,392 (1.5%) with a depressive episode, and 1,046 (0.5%) with both. The best trade-off of sensitivity/specificity for risk appeared to be at a screening threshold score of 17 or above.
Risk drivers
PMH-affected mothers were slightly younger than unaffected women (mean age, 29.9 years vs. 30.6 years), more likely to be primiparous (45.2% vs. 42%), and less likely to be recent immigrants (16.7% vs. 27.2%).
They were also more likely to have previously experienced postpartum depression (4.4% vs. 1.4%), any depression (15.3% vs. 4.4%), and any anxiety disorder (13.8% vs. 4.3%).
As to lifestyle, smoking was more common in women with PMH (15.0% vs. 10.2%), as were the use of nonprescribed substances (3% vs. 1.4%) and intimate partner violence in pregnancy (2.7% vs. 1.5%).
In addition, the affected group experienced more pregnancy complications than their unaffected peers (16% vs. 13.9%), preterm birth (8.2% vs. 6.8%), and Apgar scores below 7 at 1 or 5 minutes (10.5% vs. 7.6%).
Low income did not appear to have an impact since just over 20% in either group fell into the lowest neighborhood income quintile.
Commenting on the index but not involved in developing it, LaTasha D. Nelson, MD, an associate professor or medicine and a maternal-fetal medicine specialist at Northwestern Medicine in Chicago, doubted the Canadian model would work as well in the more fragmented U.S. health care system, compared with Canada’s universal model with its large provincial health databases.
She also found the large number of variables and broad score range potentially problematic, especially if the risk threshold is set at less than half the maximum score at 17, at which some low-risk mothers might get screening and perhaps intervention. “Are we going to use up the resources we have for those who might not need help, or are we going to treat someone who really needs it?” she asked.
Another concern is the postpartum timing of assessment. At Dr. Nelson’s center, mothers are checked for mental health at two points during pregnancy and those with higher scores are triaged for further care.
Dr. Nelson was also puzzled by the score-lowering impact of prenatal care given by a nurse practitioner and “other” provider : –5 and –2, respectively, versus +3 for a midwife and +1 for a family doctor. “This may capture more relaxed, easy-going multiparous mothers who felt comfortable turning to an NP,” she said.
It may indeed reflect that the risk level of a person who sees those providers is overall lower, Dr. Vigod agreed. “This is one reason why we would want to see replication of these results in other jurisdictions and by other ways of diagnosis before putting it out into clinical practice.”
As to the score-lowering effect of not speaking English as the primary tongue, Dr. Nelson wondered, “is that because we’re taking better care of mothers who speak the main language and missing those who speak other languages? Are they not getting the same level of interrogation?”
It may be that individuals in these groups were less likely to access mental health care, Dr. Vigod agreed, or it might reflect the so-called healthy immigrant effect or culturally different levels of postpartum support. “It might mean that there are more people who benefit from community-level protective factors in these groups. We know that social support is an important protective factor.”
Despite her reservations about the index, Dr. Nelson said that increasing attention to the pre- and postnatal mental health of mothers is an important part of maternal care. “This is an issue that needs to be recognized.”
The next step, Dr. Vigod said, is to determine whether the index holds up in other populations. “Then, we would want to test it out to see if recommending interventions based on a certain level of risk improves outcomes. At what percentage risk would starting an antidepressant medication result in a reduced risk for postpartum depression or anxiety – 90%, 80%, 70%, or less?”
The study received funding from the Canadian Institutes of Health Research. Data were analyzed by ICES, an independent nonprofit research organization that holds population-based data. Dr. Vigod reported royalties from UpToDate for materials related to depression and pregnancy. Dr. Nelson disclosed no relevant competing interests.
FROM THE BRITISH JOURNAL OF PSYCHIATRY
U.S. mammogram update sparks concern, reignites debates
, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screening mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
“The study’s central finding that some women who have an abnormal result on a mammogram may not get appropriate follow-up because of cost is worrisome,” said Dr. Fendrick and Ilana B. Richman, MD, MHS, in an accompanying commentary to the JAMA analysis. “On an individual level, high out-of-pocket costs may directly contribute to worse health outcomes or require individuals to use scarce financial resources that may otherwise be used for critical items such as food or rent.”
For patients to fully benefit from early detection, the USPSTF would also need to make clear that follow-up diagnostic mammograms are covered, Dr. Fendrick said.
The ongoing debates
Concerns over the costs of potential follow-up tests are not the only issues experts have highlighted since USPSTF released its updated draft guidance on screening mammography.
The task force’s proposed update has also reignited questions and uncertainties surrounding when to screen, how often, and what types are best.
When it comes to frequency, the major organizations that provide screening guidance don’t see eye to eye. The USPSTF recommends breast cancer screening every other year, while the American College of Radiology recommends screening every year because that approach leads to saves “the most lives.”
At this time, the American College of Obstetricians and Gynecologists guidance currently teeters in the middle, suggesting either annual or biennial screening and highlighting the pros and cons of either approach. According to ACOG, “annual screening intervals appear to result in the least number of breast cancer deaths, particularly in younger women, but at the cost of additional callbacks and biopsies.”
When to begin screening represents another point of contention. While some experts, such as ACOG, agree with the task force’s decision to lower the screening start age to 40, others point to the need for greater nuance on setting the appropriate screening age. The main issue: the task force’s draft sets a uniform age to begin screening, but the risk for breast cancer and breast cancer mortality is not uniform across different racial and ethnic groups.
A recent study published in JAMA Network Open found that, among women aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years) followed by White women (15 deaths per 100,000 person-years). Based on a recommended screening age of 50, the authors suggested that Black women should start screening at age 42, whereas White women could start at 51.
“These findings suggest that health policy makers and clinicians could consider an alternative, race and ethnicity–adapted approach in which Black female patients start screening earlier,” writes Tianhui Chen, PhD, of China’s Zhejiang Cancer Hospital and coauthor of the study.
Weighing in on the guidance, the nonprofit National Center for Health Research urged the task force to consider suggesting different screening schedules based on race and ethnicity data. That would mean the recommendation to start at age 40 should only apply to Black women and other groups with higher-than-average risk for breast cancer at a younger age.
“Women are capable of understanding why the age to start mammography screening may be different for women with different risk factors,” the National Center for Health Research wrote in a comment to USPSTF, provided to this news organization by request. “What is confusing is when some physician groups recommend annual mammograms for all women starting at age 40, even though the data do not support that recommendation.”
While the ACR agreed with the task force’s recommendation to lower the screening age, the organization suggested starting risk assessments based on racial variations in breast cancer incidence and death even earlier. Specifically, the ACR recommended that high-risk groups, such as Black women, get risk assessments by age 25 to determine whether mammography before age 40 is needed.
Screening options for women with dense breasts may be some of the most challenging to weigh. Having dense breasts increases an individual’s risk for breast cancer, and mammography alone is not as effective at identifying breast cancer among these women. However, the evidence on the benefits vs. harms of additional screening beyond mammography remains mixed.
As a result, the task force decided to maintain its “I” grade on additional screening beyond mammography for these women – a grade that indicates insufficient evidence to determine the benefits and harms for a service.
The task force largely based its decision on the findings of two key reports. One report from the Cancer Intervention and Surveillance Modeling Network, which modeled potential outcomes of different screening strategies, indicated that extra screening might reduce breast cancer mortality in those with dense breasts, but at a cost of more false-positive reports.
The second report, a review from the Kaiser Permanente Evidence-based Practice Center, reaffirmed the benefits of routine mammography for reducing deaths from breast cancer, but found no solid evidence that different strategies – including supplemental screening in women with denser breasts – lowered breast cancer mortality or the risk of progression to advanced cancer. Further studies may show which approaches work best to reduce breast cancer deaths, the report said.
In this instance, ACOG agreed with USPSTF: “Based on the lack of data, ACOG does not recommend routine use of alternative or adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.”
Women with dense breasts should still be encouraged to receive regular screening mammography, even if the results they get may not be as accurate as those for women with less dense breasts, said Diana L. Miglioretti, PhD, of the University of California, Davis, who worked on a report for the USPSTF guidelines.
What’s next?
Despite ongoing debate and uncertainties surrounding some breast screening guidance, support for ending copay requirements for follow-up tests after a positive mammogram finding is widespread.
According to Dr. Fendrick, the USPSTF should expand coverage of follow-up testing after a positive mammogram to ensure people receive routine screening and any necessary diagnostic tests, as it did with colon cancer.
Before 2021, patients could face high costs for a colonoscopy following a positive stool-based Cologuard test. But in 2021, the USPSTF said that positive results on stool-based tests would require follow-up with colonoscopy, defining this follow-up as part of the screening benefit. In 2022, Medicare followed by setting a policy that ended the copay for these follow-up colonoscopies.
For breast screening, there are efforts underway in Congress to end copays for breast screening. In May, Rep. Rosa DeLauro (D-Conn.) introduced a bill, the Find It Early Act, that would require both private and government insurers to cover the out-of-pocket costs for many women receiving screening with ultrasound and MRI.
When the USPSTF finalizes its breast screening guidelines, the recommendations will be woven into discussions between primary care physicians and patients about breast cancer screening.
As guidelines and evidence evolve, “we’re learning to adjust” and communicate these changes to patients, said Tochi Iroku-Malize, MD, president of the American Academy of Family Physicians.
However, gaps in the guidance will leave some open-ended questions about optimal screening practices and how much screening may cost.
Given that, Dr. Iroku-Malize takes many factors into account when discussing screening options with her patients. Based on the new information and the patient’s information, she said she will tell her patients, “We’re going to adjust our guidance as to what you need.”
A version of this article first appeared on Medscape.com.
, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screening mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
“The study’s central finding that some women who have an abnormal result on a mammogram may not get appropriate follow-up because of cost is worrisome,” said Dr. Fendrick and Ilana B. Richman, MD, MHS, in an accompanying commentary to the JAMA analysis. “On an individual level, high out-of-pocket costs may directly contribute to worse health outcomes or require individuals to use scarce financial resources that may otherwise be used for critical items such as food or rent.”
For patients to fully benefit from early detection, the USPSTF would also need to make clear that follow-up diagnostic mammograms are covered, Dr. Fendrick said.
The ongoing debates
Concerns over the costs of potential follow-up tests are not the only issues experts have highlighted since USPSTF released its updated draft guidance on screening mammography.
The task force’s proposed update has also reignited questions and uncertainties surrounding when to screen, how often, and what types are best.
When it comes to frequency, the major organizations that provide screening guidance don’t see eye to eye. The USPSTF recommends breast cancer screening every other year, while the American College of Radiology recommends screening every year because that approach leads to saves “the most lives.”
At this time, the American College of Obstetricians and Gynecologists guidance currently teeters in the middle, suggesting either annual or biennial screening and highlighting the pros and cons of either approach. According to ACOG, “annual screening intervals appear to result in the least number of breast cancer deaths, particularly in younger women, but at the cost of additional callbacks and biopsies.”
When to begin screening represents another point of contention. While some experts, such as ACOG, agree with the task force’s decision to lower the screening start age to 40, others point to the need for greater nuance on setting the appropriate screening age. The main issue: the task force’s draft sets a uniform age to begin screening, but the risk for breast cancer and breast cancer mortality is not uniform across different racial and ethnic groups.
A recent study published in JAMA Network Open found that, among women aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years) followed by White women (15 deaths per 100,000 person-years). Based on a recommended screening age of 50, the authors suggested that Black women should start screening at age 42, whereas White women could start at 51.
“These findings suggest that health policy makers and clinicians could consider an alternative, race and ethnicity–adapted approach in which Black female patients start screening earlier,” writes Tianhui Chen, PhD, of China’s Zhejiang Cancer Hospital and coauthor of the study.
Weighing in on the guidance, the nonprofit National Center for Health Research urged the task force to consider suggesting different screening schedules based on race and ethnicity data. That would mean the recommendation to start at age 40 should only apply to Black women and other groups with higher-than-average risk for breast cancer at a younger age.
“Women are capable of understanding why the age to start mammography screening may be different for women with different risk factors,” the National Center for Health Research wrote in a comment to USPSTF, provided to this news organization by request. “What is confusing is when some physician groups recommend annual mammograms for all women starting at age 40, even though the data do not support that recommendation.”
While the ACR agreed with the task force’s recommendation to lower the screening age, the organization suggested starting risk assessments based on racial variations in breast cancer incidence and death even earlier. Specifically, the ACR recommended that high-risk groups, such as Black women, get risk assessments by age 25 to determine whether mammography before age 40 is needed.
Screening options for women with dense breasts may be some of the most challenging to weigh. Having dense breasts increases an individual’s risk for breast cancer, and mammography alone is not as effective at identifying breast cancer among these women. However, the evidence on the benefits vs. harms of additional screening beyond mammography remains mixed.
As a result, the task force decided to maintain its “I” grade on additional screening beyond mammography for these women – a grade that indicates insufficient evidence to determine the benefits and harms for a service.
The task force largely based its decision on the findings of two key reports. One report from the Cancer Intervention and Surveillance Modeling Network, which modeled potential outcomes of different screening strategies, indicated that extra screening might reduce breast cancer mortality in those with dense breasts, but at a cost of more false-positive reports.
The second report, a review from the Kaiser Permanente Evidence-based Practice Center, reaffirmed the benefits of routine mammography for reducing deaths from breast cancer, but found no solid evidence that different strategies – including supplemental screening in women with denser breasts – lowered breast cancer mortality or the risk of progression to advanced cancer. Further studies may show which approaches work best to reduce breast cancer deaths, the report said.
In this instance, ACOG agreed with USPSTF: “Based on the lack of data, ACOG does not recommend routine use of alternative or adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.”
Women with dense breasts should still be encouraged to receive regular screening mammography, even if the results they get may not be as accurate as those for women with less dense breasts, said Diana L. Miglioretti, PhD, of the University of California, Davis, who worked on a report for the USPSTF guidelines.
What’s next?
Despite ongoing debate and uncertainties surrounding some breast screening guidance, support for ending copay requirements for follow-up tests after a positive mammogram finding is widespread.
According to Dr. Fendrick, the USPSTF should expand coverage of follow-up testing after a positive mammogram to ensure people receive routine screening and any necessary diagnostic tests, as it did with colon cancer.
Before 2021, patients could face high costs for a colonoscopy following a positive stool-based Cologuard test. But in 2021, the USPSTF said that positive results on stool-based tests would require follow-up with colonoscopy, defining this follow-up as part of the screening benefit. In 2022, Medicare followed by setting a policy that ended the copay for these follow-up colonoscopies.
For breast screening, there are efforts underway in Congress to end copays for breast screening. In May, Rep. Rosa DeLauro (D-Conn.) introduced a bill, the Find It Early Act, that would require both private and government insurers to cover the out-of-pocket costs for many women receiving screening with ultrasound and MRI.
When the USPSTF finalizes its breast screening guidelines, the recommendations will be woven into discussions between primary care physicians and patients about breast cancer screening.
As guidelines and evidence evolve, “we’re learning to adjust” and communicate these changes to patients, said Tochi Iroku-Malize, MD, president of the American Academy of Family Physicians.
However, gaps in the guidance will leave some open-ended questions about optimal screening practices and how much screening may cost.
Given that, Dr. Iroku-Malize takes many factors into account when discussing screening options with her patients. Based on the new information and the patient’s information, she said she will tell her patients, “We’re going to adjust our guidance as to what you need.”
A version of this article first appeared on Medscape.com.
, while also renewing debates about the timing of these tests and the screening approaches used.
The U.S. Preventive Services Task Force is currently finalizing an update to its recommendations on breast cancer screening. In May, the task force released a proposed update that dropped the initial age for routine mammogram screening from 50 to 40.
The task force intends to give a “B” rating to this recommendation, which covers screening every other year up to age 74 for women deemed average risk for breast cancer.
The task force’s rating carries clout, A. Mark Fendrick, MD, director of the Value-Based Insurance Design at the University of Michigan, Ann Arbor, said in an interview.
For one, the Affordable Care Act requires that private insurers cover services that get top A or B marks from USPSTF without charging copays.
However, Dr. Fendrick noted, such coverage does not necessarily apply to follow-up testing when a routine mammogram comes back with a positive finding. The expense of follow-up testing may deter some women from seeking follow-up diagnostic imaging or biopsies after an abnormal result on a screening mammogram.
A recent analysis in JAMA Network Open found that women facing higher anticipated out-of-pocket costs for breast cancer diagnostic tests, based on their health insurance plan, were less likely to get that follow-up screening. For instance, the use of breast MRI decreased by nearly 24% between patients undergoing subsequent diagnostic testing in plans with the lowest out-of-pocket costs vs. those with the highest.
“The study’s central finding that some women who have an abnormal result on a mammogram may not get appropriate follow-up because of cost is worrisome,” said Dr. Fendrick and Ilana B. Richman, MD, MHS, in an accompanying commentary to the JAMA analysis. “On an individual level, high out-of-pocket costs may directly contribute to worse health outcomes or require individuals to use scarce financial resources that may otherwise be used for critical items such as food or rent.”
For patients to fully benefit from early detection, the USPSTF would also need to make clear that follow-up diagnostic mammograms are covered, Dr. Fendrick said.
The ongoing debates
Concerns over the costs of potential follow-up tests are not the only issues experts have highlighted since USPSTF released its updated draft guidance on screening mammography.
The task force’s proposed update has also reignited questions and uncertainties surrounding when to screen, how often, and what types are best.
When it comes to frequency, the major organizations that provide screening guidance don’t see eye to eye. The USPSTF recommends breast cancer screening every other year, while the American College of Radiology recommends screening every year because that approach leads to saves “the most lives.”
At this time, the American College of Obstetricians and Gynecologists guidance currently teeters in the middle, suggesting either annual or biennial screening and highlighting the pros and cons of either approach. According to ACOG, “annual screening intervals appear to result in the least number of breast cancer deaths, particularly in younger women, but at the cost of additional callbacks and biopsies.”
When to begin screening represents another point of contention. While some experts, such as ACOG, agree with the task force’s decision to lower the screening start age to 40, others point to the need for greater nuance on setting the appropriate screening age. The main issue: the task force’s draft sets a uniform age to begin screening, but the risk for breast cancer and breast cancer mortality is not uniform across different racial and ethnic groups.
A recent study published in JAMA Network Open found that, among women aged 40-49, breast cancer mortality was highest among Black women (27 deaths per 100,000 person-years) followed by White women (15 deaths per 100,000 person-years). Based on a recommended screening age of 50, the authors suggested that Black women should start screening at age 42, whereas White women could start at 51.
“These findings suggest that health policy makers and clinicians could consider an alternative, race and ethnicity–adapted approach in which Black female patients start screening earlier,” writes Tianhui Chen, PhD, of China’s Zhejiang Cancer Hospital and coauthor of the study.
Weighing in on the guidance, the nonprofit National Center for Health Research urged the task force to consider suggesting different screening schedules based on race and ethnicity data. That would mean the recommendation to start at age 40 should only apply to Black women and other groups with higher-than-average risk for breast cancer at a younger age.
“Women are capable of understanding why the age to start mammography screening may be different for women with different risk factors,” the National Center for Health Research wrote in a comment to USPSTF, provided to this news organization by request. “What is confusing is when some physician groups recommend annual mammograms for all women starting at age 40, even though the data do not support that recommendation.”
While the ACR agreed with the task force’s recommendation to lower the screening age, the organization suggested starting risk assessments based on racial variations in breast cancer incidence and death even earlier. Specifically, the ACR recommended that high-risk groups, such as Black women, get risk assessments by age 25 to determine whether mammography before age 40 is needed.
Screening options for women with dense breasts may be some of the most challenging to weigh. Having dense breasts increases an individual’s risk for breast cancer, and mammography alone is not as effective at identifying breast cancer among these women. However, the evidence on the benefits vs. harms of additional screening beyond mammography remains mixed.
As a result, the task force decided to maintain its “I” grade on additional screening beyond mammography for these women – a grade that indicates insufficient evidence to determine the benefits and harms for a service.
The task force largely based its decision on the findings of two key reports. One report from the Cancer Intervention and Surveillance Modeling Network, which modeled potential outcomes of different screening strategies, indicated that extra screening might reduce breast cancer mortality in those with dense breasts, but at a cost of more false-positive reports.
The second report, a review from the Kaiser Permanente Evidence-based Practice Center, reaffirmed the benefits of routine mammography for reducing deaths from breast cancer, but found no solid evidence that different strategies – including supplemental screening in women with denser breasts – lowered breast cancer mortality or the risk of progression to advanced cancer. Further studies may show which approaches work best to reduce breast cancer deaths, the report said.
In this instance, ACOG agreed with USPSTF: “Based on the lack of data, ACOG does not recommend routine use of alternative or adjunctive tests to screening mammography in women with dense breasts who are asymptomatic and have no additional risk factors.”
Women with dense breasts should still be encouraged to receive regular screening mammography, even if the results they get may not be as accurate as those for women with less dense breasts, said Diana L. Miglioretti, PhD, of the University of California, Davis, who worked on a report for the USPSTF guidelines.
What’s next?
Despite ongoing debate and uncertainties surrounding some breast screening guidance, support for ending copay requirements for follow-up tests after a positive mammogram finding is widespread.
According to Dr. Fendrick, the USPSTF should expand coverage of follow-up testing after a positive mammogram to ensure people receive routine screening and any necessary diagnostic tests, as it did with colon cancer.
Before 2021, patients could face high costs for a colonoscopy following a positive stool-based Cologuard test. But in 2021, the USPSTF said that positive results on stool-based tests would require follow-up with colonoscopy, defining this follow-up as part of the screening benefit. In 2022, Medicare followed by setting a policy that ended the copay for these follow-up colonoscopies.
For breast screening, there are efforts underway in Congress to end copays for breast screening. In May, Rep. Rosa DeLauro (D-Conn.) introduced a bill, the Find It Early Act, that would require both private and government insurers to cover the out-of-pocket costs for many women receiving screening with ultrasound and MRI.
When the USPSTF finalizes its breast screening guidelines, the recommendations will be woven into discussions between primary care physicians and patients about breast cancer screening.
As guidelines and evidence evolve, “we’re learning to adjust” and communicate these changes to patients, said Tochi Iroku-Malize, MD, president of the American Academy of Family Physicians.
However, gaps in the guidance will leave some open-ended questions about optimal screening practices and how much screening may cost.
Given that, Dr. Iroku-Malize takes many factors into account when discussing screening options with her patients. Based on the new information and the patient’s information, she said she will tell her patients, “We’re going to adjust our guidance as to what you need.”
A version of this article first appeared on Medscape.com.
Progesterone might benefit women in perimenopause
In a randomized, placebo-controlled trial of about 180 women with vasomotor symptoms (VMS), women who received progesterone perceived a significantly greater decrease in night sweats (P = .023) and improved sleep quality (P = .005), compared with controls. VMS score did not differ significantly by treatment group, however.
“Women who have menstruated within the last year, who are waking twice or more times a week with night sweats and bothered by sleep disturbances would benefit from taking oral micronized progesterone 300 mg at bedtime,” principal investigator Jerilynn C. Prior, MD, professor of endocrinology at the University of British Columbia in Vancouver, British Columbia, Canada, said in an interview.
The study was published online in Scientific Reports.
A neglected group?
The best management for symptoms in perimenopause is an often-neglected topic of research, said Dr. Prior. Yet perimenopause is often associated with significant symptoms for women, including heavy menstrual bleeding, sore breasts, mood swings, night sweats, and insomnia – all when many women are at the peak of their careers.
Dr. Prior herself had a difficult perimenopause. “I began having cyclic night sweats, clustered around flow, when I was still having regular menstrual cycles, plus breast tenderness and sleep problems,” she said. “I knew from my research and my own experience that my estrogen levels were very high. Higher estrogen levels are not suppressible by exogenous estrogen, so it made no sense to me to ask my family doctor for a prescription for estrogen – or hormone replacement therapy, as it was then called. However, medroxyprogesterone acetate had been reported to be effective for menopausal hot flushes. I tried it, and it helped my night sweats and hot flushes but not my sleep. When oral micronized progesterone became available, I switched to that.”
In the current study, which was performed at the UBC Centre for Menstrual Cycle and Ovulation Research, the investigators studied 189 community-dwelling women from across Canada who were aged 35-58 years, had menstruated in the past year, and were bothered by daytime flushes or night sweats at least twice per week.
Participants were randomly assigned to receive either 300 mg of oral micronized progesterone or placebo at bedtime for 3 months. They recorded VMS number and intensity while awake and asleep each day. Some women participated remotely by web conference, telephone, or email. The experimental medicine was delivered to these participants by courier. The primary outcome was VMS score during the 3rd month.
Most (87%) participants were White, and about 57% had a college degree. The population’s average body mass index was 26.7, and 66.7% of participants were in late perimenopause.
The mean baseline VMS score among the women was 12.2. The average frequency of VMS per 24-hour day was 4.9. Average VMS intensity was 2.3 on a scale of 0-4. VMS scores decreased over time in both treatment groups.
At month 3, the VMS score was 5.5 in the progesterone group and 7.1 in the placebo group. The difference between groups was not statistically significant.
Compared with controls, however, women in the progesterone group perceived a significantly greater decrease in night sweats and improved sleep quality. Progesterone also was associated with significantly decreased perception of physical and emotional interference with their daily activities, compared with placebo (P = .017). Moreover, progesterone did not increase depression.
There were no serious adverse events.
“I hope that when women who look young and are still menstruating in their late 30s to early 50s go to the doctor and ask for help with night sweats and sleep problems, they will be told about this trial and offered progesterone therapy. I also hope they won’t be told, ‘You are too young,’ or ‘You are not in menopause,’ with the inference that the issue is all in their minds,” said Dr. Prior.
Useful dosing information
Mitchell S. Kramer, MD, chair of obstetrics and gynecology at Huntington (N.Y.) Hospital Northwell Health, said in a comment that “progesterone has been used for quite a while. I’ve been treating menopausal and perimenopausal hormonal disturbances and VMS for many years, and progesterone has been a real staple of treatment for these symptoms, especially in perimenopausal patients who are not good candidates for estrogen or who won’t accept treatment with estrogen. It’s actually nice to see a study that addresses this issue in a randomized controlled fashion and that confirms the efficacy of progesterone.”
The most helpful aspect of the study is the dosing information, Dr. Kramer added. “They recommend a 300-mg dose of oral micronized progesterone, which is much higher than I normally use. I may start to prescribe the higher dose and perhaps get a better or more complete response. There were no adverse events reported in this study, so the higher dose was enlightening to me,” he said.
Perimenopause is a time that is challenging to manage, said Michelle Jacobson, MD, of the department of obstetrics and gynecology at the University of Toronto, and obstetrician-gynecologist at Women’s College and Mount Sinai Hospitals in Toronto.
“There are so many nuances to the management. Women are suffering oftentimes from classic menopausal symptoms. There are fluctuating levels of estrogen, sometimes high. Sometimes there are complications of bleeding. There is the potential need for contraception because they are still menstruating,” she said in an interview.
“It’s important to specifically study this group of women with their own unique needs. Dr. Prior is a longtime proponent of using progesterone therapy, and kudos to her for doing this study in perimenopausal women, which is a group that is probably underrepresented in the menopause management literature,” she said.
Dr. Prior and Dr. Kramer reported no relevant financial relationships. Dr. Jacobson reported financial relationships with Astellas, AbbVie, Bayer, BioSyent, Duchesnay, Eisai, Lupin, Organon, Pfizer, and Searchlight.
A version of this article first appeared on Medscape.com.
In a randomized, placebo-controlled trial of about 180 women with vasomotor symptoms (VMS), women who received progesterone perceived a significantly greater decrease in night sweats (P = .023) and improved sleep quality (P = .005), compared with controls. VMS score did not differ significantly by treatment group, however.
“Women who have menstruated within the last year, who are waking twice or more times a week with night sweats and bothered by sleep disturbances would benefit from taking oral micronized progesterone 300 mg at bedtime,” principal investigator Jerilynn C. Prior, MD, professor of endocrinology at the University of British Columbia in Vancouver, British Columbia, Canada, said in an interview.
The study was published online in Scientific Reports.
A neglected group?
The best management for symptoms in perimenopause is an often-neglected topic of research, said Dr. Prior. Yet perimenopause is often associated with significant symptoms for women, including heavy menstrual bleeding, sore breasts, mood swings, night sweats, and insomnia – all when many women are at the peak of their careers.
Dr. Prior herself had a difficult perimenopause. “I began having cyclic night sweats, clustered around flow, when I was still having regular menstrual cycles, plus breast tenderness and sleep problems,” she said. “I knew from my research and my own experience that my estrogen levels were very high. Higher estrogen levels are not suppressible by exogenous estrogen, so it made no sense to me to ask my family doctor for a prescription for estrogen – or hormone replacement therapy, as it was then called. However, medroxyprogesterone acetate had been reported to be effective for menopausal hot flushes. I tried it, and it helped my night sweats and hot flushes but not my sleep. When oral micronized progesterone became available, I switched to that.”
In the current study, which was performed at the UBC Centre for Menstrual Cycle and Ovulation Research, the investigators studied 189 community-dwelling women from across Canada who were aged 35-58 years, had menstruated in the past year, and were bothered by daytime flushes or night sweats at least twice per week.
Participants were randomly assigned to receive either 300 mg of oral micronized progesterone or placebo at bedtime for 3 months. They recorded VMS number and intensity while awake and asleep each day. Some women participated remotely by web conference, telephone, or email. The experimental medicine was delivered to these participants by courier. The primary outcome was VMS score during the 3rd month.
Most (87%) participants were White, and about 57% had a college degree. The population’s average body mass index was 26.7, and 66.7% of participants were in late perimenopause.
The mean baseline VMS score among the women was 12.2. The average frequency of VMS per 24-hour day was 4.9. Average VMS intensity was 2.3 on a scale of 0-4. VMS scores decreased over time in both treatment groups.
At month 3, the VMS score was 5.5 in the progesterone group and 7.1 in the placebo group. The difference between groups was not statistically significant.
Compared with controls, however, women in the progesterone group perceived a significantly greater decrease in night sweats and improved sleep quality. Progesterone also was associated with significantly decreased perception of physical and emotional interference with their daily activities, compared with placebo (P = .017). Moreover, progesterone did not increase depression.
There were no serious adverse events.
“I hope that when women who look young and are still menstruating in their late 30s to early 50s go to the doctor and ask for help with night sweats and sleep problems, they will be told about this trial and offered progesterone therapy. I also hope they won’t be told, ‘You are too young,’ or ‘You are not in menopause,’ with the inference that the issue is all in their minds,” said Dr. Prior.
Useful dosing information
Mitchell S. Kramer, MD, chair of obstetrics and gynecology at Huntington (N.Y.) Hospital Northwell Health, said in a comment that “progesterone has been used for quite a while. I’ve been treating menopausal and perimenopausal hormonal disturbances and VMS for many years, and progesterone has been a real staple of treatment for these symptoms, especially in perimenopausal patients who are not good candidates for estrogen or who won’t accept treatment with estrogen. It’s actually nice to see a study that addresses this issue in a randomized controlled fashion and that confirms the efficacy of progesterone.”
The most helpful aspect of the study is the dosing information, Dr. Kramer added. “They recommend a 300-mg dose of oral micronized progesterone, which is much higher than I normally use. I may start to prescribe the higher dose and perhaps get a better or more complete response. There were no adverse events reported in this study, so the higher dose was enlightening to me,” he said.
Perimenopause is a time that is challenging to manage, said Michelle Jacobson, MD, of the department of obstetrics and gynecology at the University of Toronto, and obstetrician-gynecologist at Women’s College and Mount Sinai Hospitals in Toronto.
“There are so many nuances to the management. Women are suffering oftentimes from classic menopausal symptoms. There are fluctuating levels of estrogen, sometimes high. Sometimes there are complications of bleeding. There is the potential need for contraception because they are still menstruating,” she said in an interview.
“It’s important to specifically study this group of women with their own unique needs. Dr. Prior is a longtime proponent of using progesterone therapy, and kudos to her for doing this study in perimenopausal women, which is a group that is probably underrepresented in the menopause management literature,” she said.
Dr. Prior and Dr. Kramer reported no relevant financial relationships. Dr. Jacobson reported financial relationships with Astellas, AbbVie, Bayer, BioSyent, Duchesnay, Eisai, Lupin, Organon, Pfizer, and Searchlight.
A version of this article first appeared on Medscape.com.
In a randomized, placebo-controlled trial of about 180 women with vasomotor symptoms (VMS), women who received progesterone perceived a significantly greater decrease in night sweats (P = .023) and improved sleep quality (P = .005), compared with controls. VMS score did not differ significantly by treatment group, however.
“Women who have menstruated within the last year, who are waking twice or more times a week with night sweats and bothered by sleep disturbances would benefit from taking oral micronized progesterone 300 mg at bedtime,” principal investigator Jerilynn C. Prior, MD, professor of endocrinology at the University of British Columbia in Vancouver, British Columbia, Canada, said in an interview.
The study was published online in Scientific Reports.
A neglected group?
The best management for symptoms in perimenopause is an often-neglected topic of research, said Dr. Prior. Yet perimenopause is often associated with significant symptoms for women, including heavy menstrual bleeding, sore breasts, mood swings, night sweats, and insomnia – all when many women are at the peak of their careers.
Dr. Prior herself had a difficult perimenopause. “I began having cyclic night sweats, clustered around flow, when I was still having regular menstrual cycles, plus breast tenderness and sleep problems,” she said. “I knew from my research and my own experience that my estrogen levels were very high. Higher estrogen levels are not suppressible by exogenous estrogen, so it made no sense to me to ask my family doctor for a prescription for estrogen – or hormone replacement therapy, as it was then called. However, medroxyprogesterone acetate had been reported to be effective for menopausal hot flushes. I tried it, and it helped my night sweats and hot flushes but not my sleep. When oral micronized progesterone became available, I switched to that.”
In the current study, which was performed at the UBC Centre for Menstrual Cycle and Ovulation Research, the investigators studied 189 community-dwelling women from across Canada who were aged 35-58 years, had menstruated in the past year, and were bothered by daytime flushes or night sweats at least twice per week.
Participants were randomly assigned to receive either 300 mg of oral micronized progesterone or placebo at bedtime for 3 months. They recorded VMS number and intensity while awake and asleep each day. Some women participated remotely by web conference, telephone, or email. The experimental medicine was delivered to these participants by courier. The primary outcome was VMS score during the 3rd month.
Most (87%) participants were White, and about 57% had a college degree. The population’s average body mass index was 26.7, and 66.7% of participants were in late perimenopause.
The mean baseline VMS score among the women was 12.2. The average frequency of VMS per 24-hour day was 4.9. Average VMS intensity was 2.3 on a scale of 0-4. VMS scores decreased over time in both treatment groups.
At month 3, the VMS score was 5.5 in the progesterone group and 7.1 in the placebo group. The difference between groups was not statistically significant.
Compared with controls, however, women in the progesterone group perceived a significantly greater decrease in night sweats and improved sleep quality. Progesterone also was associated with significantly decreased perception of physical and emotional interference with their daily activities, compared with placebo (P = .017). Moreover, progesterone did not increase depression.
There were no serious adverse events.
“I hope that when women who look young and are still menstruating in their late 30s to early 50s go to the doctor and ask for help with night sweats and sleep problems, they will be told about this trial and offered progesterone therapy. I also hope they won’t be told, ‘You are too young,’ or ‘You are not in menopause,’ with the inference that the issue is all in their minds,” said Dr. Prior.
Useful dosing information
Mitchell S. Kramer, MD, chair of obstetrics and gynecology at Huntington (N.Y.) Hospital Northwell Health, said in a comment that “progesterone has been used for quite a while. I’ve been treating menopausal and perimenopausal hormonal disturbances and VMS for many years, and progesterone has been a real staple of treatment for these symptoms, especially in perimenopausal patients who are not good candidates for estrogen or who won’t accept treatment with estrogen. It’s actually nice to see a study that addresses this issue in a randomized controlled fashion and that confirms the efficacy of progesterone.”
The most helpful aspect of the study is the dosing information, Dr. Kramer added. “They recommend a 300-mg dose of oral micronized progesterone, which is much higher than I normally use. I may start to prescribe the higher dose and perhaps get a better or more complete response. There were no adverse events reported in this study, so the higher dose was enlightening to me,” he said.
Perimenopause is a time that is challenging to manage, said Michelle Jacobson, MD, of the department of obstetrics and gynecology at the University of Toronto, and obstetrician-gynecologist at Women’s College and Mount Sinai Hospitals in Toronto.
“There are so many nuances to the management. Women are suffering oftentimes from classic menopausal symptoms. There are fluctuating levels of estrogen, sometimes high. Sometimes there are complications of bleeding. There is the potential need for contraception because they are still menstruating,” she said in an interview.
“It’s important to specifically study this group of women with their own unique needs. Dr. Prior is a longtime proponent of using progesterone therapy, and kudos to her for doing this study in perimenopausal women, which is a group that is probably underrepresented in the menopause management literature,” she said.
Dr. Prior and Dr. Kramer reported no relevant financial relationships. Dr. Jacobson reported financial relationships with Astellas, AbbVie, Bayer, BioSyent, Duchesnay, Eisai, Lupin, Organon, Pfizer, and Searchlight.
A version of this article first appeared on Medscape.com.
FROM SCIENTIFIC REPORTS