User login
Pregnant, postpartum women with disabilities at higher risk for violence
Pregnant or postpartum women with disabilities are at relatively high risk of experiencing violence, often from the people closest to them, new research suggests.
The researchers set out to measure risk of interpersonal violence, which the World Health Organization defines as “the intentional use of physical force or power against an individual by an intimate partner, family member, or other community member.”
Hilary K. Brown, PhD, with the department of health & society, University of Toronto, led the study published online in Obstetrics and Gynecology.
Large, population-based dataset
The population study included people 15-49 years old with births in Ontario from 2004 to 2019. They included 147,414 people with physical disabilities; 47,459 people with intellectual disabilities; 2,557 with developmental disabilities; and 9,598 with multiple disabilities.
The control group was 1,594,441 million people without disabilities.
The outcome measured was “any emergency department visit, hospital admission, or death related to physical, sexual, or psychological violence between fertilization and 365 days post partum.”
Researchers found that the adjusted relative risk of interpersonal violence for those with disabilities, compared with those with no disabilities was 1.40 (95% confidence interval, 1.31-1.50) in those with physical disabilities; 2.39 (95% CI, 1.98-2.88) in those with intellectual or developmental disabilities; and 1.96 (95% CI, 1.66-2.30) in those with multiple disabilities.
History of violence means higher risk
Those with a history of interpersonal violence and a disability were at particularly high risk for perinatal violence.
The authors note that pregnancy is a high-risk period for interpersonal violence for all women, particularly by an intimate partner.
“More than 30% of intimate partner violence begins during pregnancy, and preexisting violence tends to escalate perinatally,” they write.
The authors cite previous research that found women with disabilities experience higher rates of abuse overall and by an intimate partner – two to four times rates reported by those without disabilities.
Opportunities for provider intervention
Since the period surrounding pregnancy is a time of increased contact with medical providers and resources, there may be opportunities for identifying abuse and providing interventions.
Those might include better screening, access to violence-related information and services, and education of health care professionals to support people with disabilities. For example, “Tools used for violence screening perinatally do not include items about forms of violence that are unique to individuals with disabilities, such as refusal to assist with activities of daily living.”
The authors add: “[G]iven that the strongest risk factor for interpersonal violence in the perinatal period, particularly in those with disabilities, was a prepregnancy history of interpersonal violence, our findings suggest that more could be done before pregnancy to offer screening and support at the index encounter.”
Violence can lead to adverse outcomes
Implications are important as the violence can result in barriers to care and adverse perinatal outcomes.
Jeanne L. Alhusen, PhD, CRNP, RN, University of Virginia Medical Center professor of nursing and associate dean for research, was not part of this research but wrote a paper earlier this year on the subject and had similar conclusions.
She said before this study by Brown et al., “our understanding of the risk of violence by disability type throughout the perinatal period, on a population-based level, was quite limited.”
With the size of this dataset, she said, this paper provides critical information for health care providers. It extends physicians’ ability to examine risk of violence by disability type as well as these patients’ risk of experiencing different types of violence.
She pointed out that the Pregnancy Risk Assessment Monitoring System (PRAMS) recently incorporated a disability supplement that allows better understanding of pregnancy risks in people with disabilities.
“It will be critical that U.S. states continue to incorporate the disability questions into their PRAMS administration [because] without that information, persons with disabilities will continue to experience unconscionable inequities,” she said.
Barriers to equitable care
Dr. Alhusen added that people with disabilities experience significant barriers in accessing equitable care – both at the provider and the system level.
She said it is critical that we recognize and address the sexual and reproductive health needs of all persons with disability. “This includes screening every person for violence and [ensuring] the tools we utilize are accessible and include items specific to disability-related abuse. In our qualitative studies, we have heard from pregnant persons that they were never screened or that they were screened with their abusive partner sitting next to them.”
Screening questions to ask
The American College of Obstetricians and Gynecologists provides examples of screening questions that are specific to people with disabilities such as asking if a partner has ever prevented the individual from using an assistive device (for example, a wheelchair, cane, or respirator) or refused to help with an important personal need, such as taking medication or getting out of bed.
“For many reasons, people with disabilities are less likely to disclose violence, and health care professionals are less likely to ask them about it,” said coauthor of the current study, Yona Lunsky, PhD, clinician-scientist, Centre for Addiction and Mental Health, Toronto, in a statement. Based on the findings, she said, she hopes clinicians will see the need to develop disability-informed screening tools to capture abuse and identify the appropriate resources for this population before, during, and after pregnancy.
Coauthor Dr. Natasha Saunders receives an honorarium from the BMJ Group (Archives of Diseases in Childhood). Coauthor Dr. Simone N. Vigod receives royalties from UpToDate for authorship of materials related to depression and pregnancy. The other authors did not report any potential conflicts of interest. Dr. Alhusen reported no relevant financial relationships.
Pregnant or postpartum women with disabilities are at relatively high risk of experiencing violence, often from the people closest to them, new research suggests.
The researchers set out to measure risk of interpersonal violence, which the World Health Organization defines as “the intentional use of physical force or power against an individual by an intimate partner, family member, or other community member.”
Hilary K. Brown, PhD, with the department of health & society, University of Toronto, led the study published online in Obstetrics and Gynecology.
Large, population-based dataset
The population study included people 15-49 years old with births in Ontario from 2004 to 2019. They included 147,414 people with physical disabilities; 47,459 people with intellectual disabilities; 2,557 with developmental disabilities; and 9,598 with multiple disabilities.
The control group was 1,594,441 million people without disabilities.
The outcome measured was “any emergency department visit, hospital admission, or death related to physical, sexual, or psychological violence between fertilization and 365 days post partum.”
Researchers found that the adjusted relative risk of interpersonal violence for those with disabilities, compared with those with no disabilities was 1.40 (95% confidence interval, 1.31-1.50) in those with physical disabilities; 2.39 (95% CI, 1.98-2.88) in those with intellectual or developmental disabilities; and 1.96 (95% CI, 1.66-2.30) in those with multiple disabilities.
History of violence means higher risk
Those with a history of interpersonal violence and a disability were at particularly high risk for perinatal violence.
The authors note that pregnancy is a high-risk period for interpersonal violence for all women, particularly by an intimate partner.
“More than 30% of intimate partner violence begins during pregnancy, and preexisting violence tends to escalate perinatally,” they write.
The authors cite previous research that found women with disabilities experience higher rates of abuse overall and by an intimate partner – two to four times rates reported by those without disabilities.
Opportunities for provider intervention
Since the period surrounding pregnancy is a time of increased contact with medical providers and resources, there may be opportunities for identifying abuse and providing interventions.
Those might include better screening, access to violence-related information and services, and education of health care professionals to support people with disabilities. For example, “Tools used for violence screening perinatally do not include items about forms of violence that are unique to individuals with disabilities, such as refusal to assist with activities of daily living.”
The authors add: “[G]iven that the strongest risk factor for interpersonal violence in the perinatal period, particularly in those with disabilities, was a prepregnancy history of interpersonal violence, our findings suggest that more could be done before pregnancy to offer screening and support at the index encounter.”
Violence can lead to adverse outcomes
Implications are important as the violence can result in barriers to care and adverse perinatal outcomes.
Jeanne L. Alhusen, PhD, CRNP, RN, University of Virginia Medical Center professor of nursing and associate dean for research, was not part of this research but wrote a paper earlier this year on the subject and had similar conclusions.
She said before this study by Brown et al., “our understanding of the risk of violence by disability type throughout the perinatal period, on a population-based level, was quite limited.”
With the size of this dataset, she said, this paper provides critical information for health care providers. It extends physicians’ ability to examine risk of violence by disability type as well as these patients’ risk of experiencing different types of violence.
She pointed out that the Pregnancy Risk Assessment Monitoring System (PRAMS) recently incorporated a disability supplement that allows better understanding of pregnancy risks in people with disabilities.
“It will be critical that U.S. states continue to incorporate the disability questions into their PRAMS administration [because] without that information, persons with disabilities will continue to experience unconscionable inequities,” she said.
Barriers to equitable care
Dr. Alhusen added that people with disabilities experience significant barriers in accessing equitable care – both at the provider and the system level.
She said it is critical that we recognize and address the sexual and reproductive health needs of all persons with disability. “This includes screening every person for violence and [ensuring] the tools we utilize are accessible and include items specific to disability-related abuse. In our qualitative studies, we have heard from pregnant persons that they were never screened or that they were screened with their abusive partner sitting next to them.”
Screening questions to ask
The American College of Obstetricians and Gynecologists provides examples of screening questions that are specific to people with disabilities such as asking if a partner has ever prevented the individual from using an assistive device (for example, a wheelchair, cane, or respirator) or refused to help with an important personal need, such as taking medication or getting out of bed.
“For many reasons, people with disabilities are less likely to disclose violence, and health care professionals are less likely to ask them about it,” said coauthor of the current study, Yona Lunsky, PhD, clinician-scientist, Centre for Addiction and Mental Health, Toronto, in a statement. Based on the findings, she said, she hopes clinicians will see the need to develop disability-informed screening tools to capture abuse and identify the appropriate resources for this population before, during, and after pregnancy.
Coauthor Dr. Natasha Saunders receives an honorarium from the BMJ Group (Archives of Diseases in Childhood). Coauthor Dr. Simone N. Vigod receives royalties from UpToDate for authorship of materials related to depression and pregnancy. The other authors did not report any potential conflicts of interest. Dr. Alhusen reported no relevant financial relationships.
Pregnant or postpartum women with disabilities are at relatively high risk of experiencing violence, often from the people closest to them, new research suggests.
The researchers set out to measure risk of interpersonal violence, which the World Health Organization defines as “the intentional use of physical force or power against an individual by an intimate partner, family member, or other community member.”
Hilary K. Brown, PhD, with the department of health & society, University of Toronto, led the study published online in Obstetrics and Gynecology.
Large, population-based dataset
The population study included people 15-49 years old with births in Ontario from 2004 to 2019. They included 147,414 people with physical disabilities; 47,459 people with intellectual disabilities; 2,557 with developmental disabilities; and 9,598 with multiple disabilities.
The control group was 1,594,441 million people without disabilities.
The outcome measured was “any emergency department visit, hospital admission, or death related to physical, sexual, or psychological violence between fertilization and 365 days post partum.”
Researchers found that the adjusted relative risk of interpersonal violence for those with disabilities, compared with those with no disabilities was 1.40 (95% confidence interval, 1.31-1.50) in those with physical disabilities; 2.39 (95% CI, 1.98-2.88) in those with intellectual or developmental disabilities; and 1.96 (95% CI, 1.66-2.30) in those with multiple disabilities.
History of violence means higher risk
Those with a history of interpersonal violence and a disability were at particularly high risk for perinatal violence.
The authors note that pregnancy is a high-risk period for interpersonal violence for all women, particularly by an intimate partner.
“More than 30% of intimate partner violence begins during pregnancy, and preexisting violence tends to escalate perinatally,” they write.
The authors cite previous research that found women with disabilities experience higher rates of abuse overall and by an intimate partner – two to four times rates reported by those without disabilities.
Opportunities for provider intervention
Since the period surrounding pregnancy is a time of increased contact with medical providers and resources, there may be opportunities for identifying abuse and providing interventions.
Those might include better screening, access to violence-related information and services, and education of health care professionals to support people with disabilities. For example, “Tools used for violence screening perinatally do not include items about forms of violence that are unique to individuals with disabilities, such as refusal to assist with activities of daily living.”
The authors add: “[G]iven that the strongest risk factor for interpersonal violence in the perinatal period, particularly in those with disabilities, was a prepregnancy history of interpersonal violence, our findings suggest that more could be done before pregnancy to offer screening and support at the index encounter.”
Violence can lead to adverse outcomes
Implications are important as the violence can result in barriers to care and adverse perinatal outcomes.
Jeanne L. Alhusen, PhD, CRNP, RN, University of Virginia Medical Center professor of nursing and associate dean for research, was not part of this research but wrote a paper earlier this year on the subject and had similar conclusions.
She said before this study by Brown et al., “our understanding of the risk of violence by disability type throughout the perinatal period, on a population-based level, was quite limited.”
With the size of this dataset, she said, this paper provides critical information for health care providers. It extends physicians’ ability to examine risk of violence by disability type as well as these patients’ risk of experiencing different types of violence.
She pointed out that the Pregnancy Risk Assessment Monitoring System (PRAMS) recently incorporated a disability supplement that allows better understanding of pregnancy risks in people with disabilities.
“It will be critical that U.S. states continue to incorporate the disability questions into their PRAMS administration [because] without that information, persons with disabilities will continue to experience unconscionable inequities,” she said.
Barriers to equitable care
Dr. Alhusen added that people with disabilities experience significant barriers in accessing equitable care – both at the provider and the system level.
She said it is critical that we recognize and address the sexual and reproductive health needs of all persons with disability. “This includes screening every person for violence and [ensuring] the tools we utilize are accessible and include items specific to disability-related abuse. In our qualitative studies, we have heard from pregnant persons that they were never screened or that they were screened with their abusive partner sitting next to them.”
Screening questions to ask
The American College of Obstetricians and Gynecologists provides examples of screening questions that are specific to people with disabilities such as asking if a partner has ever prevented the individual from using an assistive device (for example, a wheelchair, cane, or respirator) or refused to help with an important personal need, such as taking medication or getting out of bed.
“For many reasons, people with disabilities are less likely to disclose violence, and health care professionals are less likely to ask them about it,” said coauthor of the current study, Yona Lunsky, PhD, clinician-scientist, Centre for Addiction and Mental Health, Toronto, in a statement. Based on the findings, she said, she hopes clinicians will see the need to develop disability-informed screening tools to capture abuse and identify the appropriate resources for this population before, during, and after pregnancy.
Coauthor Dr. Natasha Saunders receives an honorarium from the BMJ Group (Archives of Diseases in Childhood). Coauthor Dr. Simone N. Vigod receives royalties from UpToDate for authorship of materials related to depression and pregnancy. The other authors did not report any potential conflicts of interest. Dr. Alhusen reported no relevant financial relationships.
FROM OBSTETRICS AND GYNECOLOGY
How do patients with chronic urticaria fare during pregnancy?
In addition, the rates of preterm births and medical problems of newborns in patients with CU are similar to those of the normal population and not linked to treatment used during pregnancy.
Those are the key findings from an analysis of new data from PREG-CU, an international, multicenter study of the Urticaria Centers of Reference and Excellence (UCARE) network. Results from the first PREG-CU analysis published in 2021 found that CU improved in about half of patients with CU during pregnancy. “However, two in five patients reported acute exacerbations of CU especially at the beginning and end of pregnancy,” investigators led by Emek Kocatürk, MD, of the department of dermatology and UCARE at Koç University School of Medicine, Istanbul, wrote in the new study, recently published in the Journal of the European Academy of Dermatology and Venereology.
“In addition, 1 in 10 pregnant CU patients required urticaria emergency care and 1 of 6 had angioedema during pregnancy,” they said. Risk factors for worsening CU during pregnancy, they added, were “mild disease and no angioedema before pregnancy, not taking treatment before pregnancy, chronic inducible urticaria, CU worsening during a previous pregnancy, stress as a driver of exacerbations, and treatment during pregnancy.”
Analysis involved 288 pregnant women
To optimize treatment of CU during pregnancy and to better understand how treatment affects pregnancy outcomes, the researchers analyzed 288 pregnancies in 288 women with CU from 13 countries and 21 centers worldwide. Their mean age at pregnancy was 32.1 years, and their mean duration of CU was 84.9 months. Prior to pregnancy, 35.7% of patients rated the severity of their CU symptoms as mild, 34.2% rated it as moderate, and 29.7% rated it as severe.
The researchers found that during pregnancy, 60% of patients used urticaria medication, including standard-dose second-generation H1-antihistamines (35.1%), first-generation H1-antihistamines (7.6%), high-dose second-generation H1-antihistamines (5.6%), and omalizumab (5.6%). The preterm birth rate was 10.2%, which was similar between patients who did and did not receive treatment during pregnancy (11.6% vs. 8.7%, respectively; P = .59).
On multivariate logistic regression, two predictors for preterm birth emerged: giving birth to twins (a 13.3-fold increased risk; P = .016) and emergency referrals for CU (a 4.3-fold increased risk; P =.016). The cesarean delivery rate was 51.3%, and more than 90% of newborns were healthy at birth. There was no link between any patient or disease characteristics or treatments and medical problems at birth.
In other findings, 78.8% of women with CU breastfed their babies. Of the 58 patients who did not breastfeed, 20.7% indicated severe urticaria/angioedema and/or taking medications as the main reason for not breastfeeding.
“Most CU patients use treatment during pregnancy and such treatments, especially second generation H1 antihistamines, seem to be safe during pregnancy regardless of the trimester,” the researchers concluded. “Outcomes of pregnancy in patients with CU were similar compared to the general population and not linked to treatment used during pregnancy. Notably, emergency referral for CU was an independent risk factor for preterm birth,” and the high cesarean delivery rate was “probably linked to comorbidities associated with the disease,” they added. “Overall, these findings suggest that patients should continue their treatments using an individualized dose to provide optimal symptom control.”
International guidelines
The authors noted that international guidelines for the management of urticaria published in 2022 suggest that modern second-generation H1-antihistamines should be used for pregnant patients, preferably loratadine with a possible extrapolation to desloratadine, cetirizine, or levocetirizine.
“Similarly, in this population, we found that cetirizine and loratadine were the most commonly used antihistamines, followed by levocetirizine and fexofenadine,” Dr. Kocatürk and colleagues wrote.
“Guidelines also suggest that the use of first-generation H1-antihistamines should be avoided given their sedative effects; but if these are to be given, it would be wise to know that use of first-generation H1-antihistamines immediately before parturition could cause respiratory depression and other adverse effects in the neonate,” they added, noting that chlorpheniramine and diphenhydramine are the first-generation H1-antihistamines with the greatest evidence of safety in pregnancy.
They acknowledged certain limitations of the analysis, including its retrospective design and the fact that there were no data on low birth weight, small for gestational age, or miscarriage rates. In addition, disease activity or severity during pregnancy and after birth were not monitored.
Asked to comment on these results, Raj Chovatiya, MD, PhD, who directs the center for eczema and itch in the department of dermatology at Northwestern University, Chicago, noted that despite a higher prevalence of CU among females compared with males, very little is known about how the condition is managed during pregnancy. “This retrospective study shows that most patients continue to utilize CU treatment during pregnancy (primarily second-generation antihistamines), with similar birth outcomes as the general population,” he said. “Interestingly, cesarean rates were higher among mothers with CU, and emergency CU referral was a risk factor for preterm birth. While additional prospective studies are needed, these results suggest that CU patients should be carefully managed, particularly during pregnancy, when treatment should be optimized.”
Dr. Kocatürk reported having received personal fees from Novartis, Ibrahim Etem-Menarini, and Sanofi, outside the submitted work. Many coauthors reported having numerous financial disclosures. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
In addition, the rates of preterm births and medical problems of newborns in patients with CU are similar to those of the normal population and not linked to treatment used during pregnancy.
Those are the key findings from an analysis of new data from PREG-CU, an international, multicenter study of the Urticaria Centers of Reference and Excellence (UCARE) network. Results from the first PREG-CU analysis published in 2021 found that CU improved in about half of patients with CU during pregnancy. “However, two in five patients reported acute exacerbations of CU especially at the beginning and end of pregnancy,” investigators led by Emek Kocatürk, MD, of the department of dermatology and UCARE at Koç University School of Medicine, Istanbul, wrote in the new study, recently published in the Journal of the European Academy of Dermatology and Venereology.
“In addition, 1 in 10 pregnant CU patients required urticaria emergency care and 1 of 6 had angioedema during pregnancy,” they said. Risk factors for worsening CU during pregnancy, they added, were “mild disease and no angioedema before pregnancy, not taking treatment before pregnancy, chronic inducible urticaria, CU worsening during a previous pregnancy, stress as a driver of exacerbations, and treatment during pregnancy.”
Analysis involved 288 pregnant women
To optimize treatment of CU during pregnancy and to better understand how treatment affects pregnancy outcomes, the researchers analyzed 288 pregnancies in 288 women with CU from 13 countries and 21 centers worldwide. Their mean age at pregnancy was 32.1 years, and their mean duration of CU was 84.9 months. Prior to pregnancy, 35.7% of patients rated the severity of their CU symptoms as mild, 34.2% rated it as moderate, and 29.7% rated it as severe.
The researchers found that during pregnancy, 60% of patients used urticaria medication, including standard-dose second-generation H1-antihistamines (35.1%), first-generation H1-antihistamines (7.6%), high-dose second-generation H1-antihistamines (5.6%), and omalizumab (5.6%). The preterm birth rate was 10.2%, which was similar between patients who did and did not receive treatment during pregnancy (11.6% vs. 8.7%, respectively; P = .59).
On multivariate logistic regression, two predictors for preterm birth emerged: giving birth to twins (a 13.3-fold increased risk; P = .016) and emergency referrals for CU (a 4.3-fold increased risk; P =.016). The cesarean delivery rate was 51.3%, and more than 90% of newborns were healthy at birth. There was no link between any patient or disease characteristics or treatments and medical problems at birth.
In other findings, 78.8% of women with CU breastfed their babies. Of the 58 patients who did not breastfeed, 20.7% indicated severe urticaria/angioedema and/or taking medications as the main reason for not breastfeeding.
“Most CU patients use treatment during pregnancy and such treatments, especially second generation H1 antihistamines, seem to be safe during pregnancy regardless of the trimester,” the researchers concluded. “Outcomes of pregnancy in patients with CU were similar compared to the general population and not linked to treatment used during pregnancy. Notably, emergency referral for CU was an independent risk factor for preterm birth,” and the high cesarean delivery rate was “probably linked to comorbidities associated with the disease,” they added. “Overall, these findings suggest that patients should continue their treatments using an individualized dose to provide optimal symptom control.”
International guidelines
The authors noted that international guidelines for the management of urticaria published in 2022 suggest that modern second-generation H1-antihistamines should be used for pregnant patients, preferably loratadine with a possible extrapolation to desloratadine, cetirizine, or levocetirizine.
“Similarly, in this population, we found that cetirizine and loratadine were the most commonly used antihistamines, followed by levocetirizine and fexofenadine,” Dr. Kocatürk and colleagues wrote.
“Guidelines also suggest that the use of first-generation H1-antihistamines should be avoided given their sedative effects; but if these are to be given, it would be wise to know that use of first-generation H1-antihistamines immediately before parturition could cause respiratory depression and other adverse effects in the neonate,” they added, noting that chlorpheniramine and diphenhydramine are the first-generation H1-antihistamines with the greatest evidence of safety in pregnancy.
They acknowledged certain limitations of the analysis, including its retrospective design and the fact that there were no data on low birth weight, small for gestational age, or miscarriage rates. In addition, disease activity or severity during pregnancy and after birth were not monitored.
Asked to comment on these results, Raj Chovatiya, MD, PhD, who directs the center for eczema and itch in the department of dermatology at Northwestern University, Chicago, noted that despite a higher prevalence of CU among females compared with males, very little is known about how the condition is managed during pregnancy. “This retrospective study shows that most patients continue to utilize CU treatment during pregnancy (primarily second-generation antihistamines), with similar birth outcomes as the general population,” he said. “Interestingly, cesarean rates were higher among mothers with CU, and emergency CU referral was a risk factor for preterm birth. While additional prospective studies are needed, these results suggest that CU patients should be carefully managed, particularly during pregnancy, when treatment should be optimized.”
Dr. Kocatürk reported having received personal fees from Novartis, Ibrahim Etem-Menarini, and Sanofi, outside the submitted work. Many coauthors reported having numerous financial disclosures. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
In addition, the rates of preterm births and medical problems of newborns in patients with CU are similar to those of the normal population and not linked to treatment used during pregnancy.
Those are the key findings from an analysis of new data from PREG-CU, an international, multicenter study of the Urticaria Centers of Reference and Excellence (UCARE) network. Results from the first PREG-CU analysis published in 2021 found that CU improved in about half of patients with CU during pregnancy. “However, two in five patients reported acute exacerbations of CU especially at the beginning and end of pregnancy,” investigators led by Emek Kocatürk, MD, of the department of dermatology and UCARE at Koç University School of Medicine, Istanbul, wrote in the new study, recently published in the Journal of the European Academy of Dermatology and Venereology.
“In addition, 1 in 10 pregnant CU patients required urticaria emergency care and 1 of 6 had angioedema during pregnancy,” they said. Risk factors for worsening CU during pregnancy, they added, were “mild disease and no angioedema before pregnancy, not taking treatment before pregnancy, chronic inducible urticaria, CU worsening during a previous pregnancy, stress as a driver of exacerbations, and treatment during pregnancy.”
Analysis involved 288 pregnant women
To optimize treatment of CU during pregnancy and to better understand how treatment affects pregnancy outcomes, the researchers analyzed 288 pregnancies in 288 women with CU from 13 countries and 21 centers worldwide. Their mean age at pregnancy was 32.1 years, and their mean duration of CU was 84.9 months. Prior to pregnancy, 35.7% of patients rated the severity of their CU symptoms as mild, 34.2% rated it as moderate, and 29.7% rated it as severe.
The researchers found that during pregnancy, 60% of patients used urticaria medication, including standard-dose second-generation H1-antihistamines (35.1%), first-generation H1-antihistamines (7.6%), high-dose second-generation H1-antihistamines (5.6%), and omalizumab (5.6%). The preterm birth rate was 10.2%, which was similar between patients who did and did not receive treatment during pregnancy (11.6% vs. 8.7%, respectively; P = .59).
On multivariate logistic regression, two predictors for preterm birth emerged: giving birth to twins (a 13.3-fold increased risk; P = .016) and emergency referrals for CU (a 4.3-fold increased risk; P =.016). The cesarean delivery rate was 51.3%, and more than 90% of newborns were healthy at birth. There was no link between any patient or disease characteristics or treatments and medical problems at birth.
In other findings, 78.8% of women with CU breastfed their babies. Of the 58 patients who did not breastfeed, 20.7% indicated severe urticaria/angioedema and/or taking medications as the main reason for not breastfeeding.
“Most CU patients use treatment during pregnancy and such treatments, especially second generation H1 antihistamines, seem to be safe during pregnancy regardless of the trimester,” the researchers concluded. “Outcomes of pregnancy in patients with CU were similar compared to the general population and not linked to treatment used during pregnancy. Notably, emergency referral for CU was an independent risk factor for preterm birth,” and the high cesarean delivery rate was “probably linked to comorbidities associated with the disease,” they added. “Overall, these findings suggest that patients should continue their treatments using an individualized dose to provide optimal symptom control.”
International guidelines
The authors noted that international guidelines for the management of urticaria published in 2022 suggest that modern second-generation H1-antihistamines should be used for pregnant patients, preferably loratadine with a possible extrapolation to desloratadine, cetirizine, or levocetirizine.
“Similarly, in this population, we found that cetirizine and loratadine were the most commonly used antihistamines, followed by levocetirizine and fexofenadine,” Dr. Kocatürk and colleagues wrote.
“Guidelines also suggest that the use of first-generation H1-antihistamines should be avoided given their sedative effects; but if these are to be given, it would be wise to know that use of first-generation H1-antihistamines immediately before parturition could cause respiratory depression and other adverse effects in the neonate,” they added, noting that chlorpheniramine and diphenhydramine are the first-generation H1-antihistamines with the greatest evidence of safety in pregnancy.
They acknowledged certain limitations of the analysis, including its retrospective design and the fact that there were no data on low birth weight, small for gestational age, or miscarriage rates. In addition, disease activity or severity during pregnancy and after birth were not monitored.
Asked to comment on these results, Raj Chovatiya, MD, PhD, who directs the center for eczema and itch in the department of dermatology at Northwestern University, Chicago, noted that despite a higher prevalence of CU among females compared with males, very little is known about how the condition is managed during pregnancy. “This retrospective study shows that most patients continue to utilize CU treatment during pregnancy (primarily second-generation antihistamines), with similar birth outcomes as the general population,” he said. “Interestingly, cesarean rates were higher among mothers with CU, and emergency CU referral was a risk factor for preterm birth. While additional prospective studies are needed, these results suggest that CU patients should be carefully managed, particularly during pregnancy, when treatment should be optimized.”
Dr. Kocatürk reported having received personal fees from Novartis, Ibrahim Etem-Menarini, and Sanofi, outside the submitted work. Many coauthors reported having numerous financial disclosures. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arcutis, Arena, Incyte, Pfizer, Regeneron, and Sanofi Genzyme.
FROM JEADV
Mother-to-child transmission of SARS-CoV-2 may be underestimated
ANAHEIM, CALIF. – The rate of mother-to-child transmission of SARS-CoV-2 infection is likely higher than the current estimate of 2%-8%, suggests a recent study using cord blood serology to determine incidence. The study was presented at the American Academy of Pediatrics National Conference.
“Cord blood screening is a potential tool to identify SARS-CoV-2 infected and/or exposed neonates who should then be followed for long-term consequences of mother-to-child transmission,” Amy Yeh, MD, an assistant professor of clinical pediatrics at the University of Southern California, Los Angeles, told attendees at the meeting.
Dr. Yeh and her colleagues collected cord blood from more than 500 mothers at LAC+USC Medical Center from October 2021 to April 2022 and tested them for IgG antibodies against three SARS-CoV-2 antigens: nucleoprotein (N), receptor-binding domain (RBD), and spike protein (S1). Results with an IgG mean fluorescence intensity (MFI) above 700 were considered positive for IgG antibodies. A positive result for N as well as RBD or S1 indicated a natural infection while a positive result for only RBD or S1 indicated a vaccine response or past infection.
The researchers also tested a subset of the IgG positive samples for IgM and IgA antibodies against N, S1, and RBD, with an IgM MFI greater than 24 and an IgA MFI greater than 102 used as the thresholds for positive results.
Among 384 cord blood samples analyzed, 85.4% were positive for IgG against RBD, indicating that the mother had SARS-CoV-2 immunity from either a past infection or vaccination. Of these anti-RBD positive samples, 60.7% were anti-N IgG negative, suggesting that N had waned since vaccination or the past infection.
Since the other 39.3% that were anti-N IgG positive suggest a past maternal infection, the researchers assessed these 129 samples for IgM and IgA antibodies against RBD. They found that 16 of them had high levels of anti-RBD IgA and/or IgM antibodies, pointing to a rate of mother-to-child-transmission of up to 12.4%.
Sallie Permar, MD, PhD, a professor and the chair of pediatrics at Weill Cornell Medicine in New York, who was not involved in the research, said most studies of placental transmission have focused on virologic testing, such as PCR. “Serologic tests for congenital infections are inherently challenged by the transfer of maternal IgG across the placenta and therefore must rely on non-IgG isotype response detection, which have inherently been more susceptible to false-positive results than IgG-based tests,” Dr. Permar said.
Also, “it is unclear if virologic testing was performed in the infants, which, if positive in the same infants for which cord blood IgM/IgA responses were identified, could further validate positive serologic findings,” added Dr. Permar, who is also pediatrician-in-chief at New York-Presbyterian Komansky Children’s Hospital.
Given these limitations, Dr. Permar reiterated that diagnostics for congenital SARS-CoV-2 continue to evolve, even if congenital SARS-CoV-2 infection currently appears rare. Dr. Permar said she agreed with Dr. Yeh that following those who do develop this infection is important.
“There have been initial reports of neurodevelopmental and other outcomes from long-term follow-up cohorts of infants exposed to SARS-CoV-2 infection in utero with variable results and it should continue to be pursued using cohorts both enrolled early in the pandemic and those enrolled more recently after population-level immunity to SARS-CoV-2 was achieved,” said Dr. Permar.
Dr. Permar serves as a consultant to Moderna, Pfizer, Merck, Dynavax, and Hoopika on their CMV vaccine programs and has led sponsored research programs with Moderna and Merck. Information on study funding and on disclosures for Dr. Yeh was unavailable.
ANAHEIM, CALIF. – The rate of mother-to-child transmission of SARS-CoV-2 infection is likely higher than the current estimate of 2%-8%, suggests a recent study using cord blood serology to determine incidence. The study was presented at the American Academy of Pediatrics National Conference.
“Cord blood screening is a potential tool to identify SARS-CoV-2 infected and/or exposed neonates who should then be followed for long-term consequences of mother-to-child transmission,” Amy Yeh, MD, an assistant professor of clinical pediatrics at the University of Southern California, Los Angeles, told attendees at the meeting.
Dr. Yeh and her colleagues collected cord blood from more than 500 mothers at LAC+USC Medical Center from October 2021 to April 2022 and tested them for IgG antibodies against three SARS-CoV-2 antigens: nucleoprotein (N), receptor-binding domain (RBD), and spike protein (S1). Results with an IgG mean fluorescence intensity (MFI) above 700 were considered positive for IgG antibodies. A positive result for N as well as RBD or S1 indicated a natural infection while a positive result for only RBD or S1 indicated a vaccine response or past infection.
The researchers also tested a subset of the IgG positive samples for IgM and IgA antibodies against N, S1, and RBD, with an IgM MFI greater than 24 and an IgA MFI greater than 102 used as the thresholds for positive results.
Among 384 cord blood samples analyzed, 85.4% were positive for IgG against RBD, indicating that the mother had SARS-CoV-2 immunity from either a past infection or vaccination. Of these anti-RBD positive samples, 60.7% were anti-N IgG negative, suggesting that N had waned since vaccination or the past infection.
Since the other 39.3% that were anti-N IgG positive suggest a past maternal infection, the researchers assessed these 129 samples for IgM and IgA antibodies against RBD. They found that 16 of them had high levels of anti-RBD IgA and/or IgM antibodies, pointing to a rate of mother-to-child-transmission of up to 12.4%.
Sallie Permar, MD, PhD, a professor and the chair of pediatrics at Weill Cornell Medicine in New York, who was not involved in the research, said most studies of placental transmission have focused on virologic testing, such as PCR. “Serologic tests for congenital infections are inherently challenged by the transfer of maternal IgG across the placenta and therefore must rely on non-IgG isotype response detection, which have inherently been more susceptible to false-positive results than IgG-based tests,” Dr. Permar said.
Also, “it is unclear if virologic testing was performed in the infants, which, if positive in the same infants for which cord blood IgM/IgA responses were identified, could further validate positive serologic findings,” added Dr. Permar, who is also pediatrician-in-chief at New York-Presbyterian Komansky Children’s Hospital.
Given these limitations, Dr. Permar reiterated that diagnostics for congenital SARS-CoV-2 continue to evolve, even if congenital SARS-CoV-2 infection currently appears rare. Dr. Permar said she agreed with Dr. Yeh that following those who do develop this infection is important.
“There have been initial reports of neurodevelopmental and other outcomes from long-term follow-up cohorts of infants exposed to SARS-CoV-2 infection in utero with variable results and it should continue to be pursued using cohorts both enrolled early in the pandemic and those enrolled more recently after population-level immunity to SARS-CoV-2 was achieved,” said Dr. Permar.
Dr. Permar serves as a consultant to Moderna, Pfizer, Merck, Dynavax, and Hoopika on their CMV vaccine programs and has led sponsored research programs with Moderna and Merck. Information on study funding and on disclosures for Dr. Yeh was unavailable.
ANAHEIM, CALIF. – The rate of mother-to-child transmission of SARS-CoV-2 infection is likely higher than the current estimate of 2%-8%, suggests a recent study using cord blood serology to determine incidence. The study was presented at the American Academy of Pediatrics National Conference.
“Cord blood screening is a potential tool to identify SARS-CoV-2 infected and/or exposed neonates who should then be followed for long-term consequences of mother-to-child transmission,” Amy Yeh, MD, an assistant professor of clinical pediatrics at the University of Southern California, Los Angeles, told attendees at the meeting.
Dr. Yeh and her colleagues collected cord blood from more than 500 mothers at LAC+USC Medical Center from October 2021 to April 2022 and tested them for IgG antibodies against three SARS-CoV-2 antigens: nucleoprotein (N), receptor-binding domain (RBD), and spike protein (S1). Results with an IgG mean fluorescence intensity (MFI) above 700 were considered positive for IgG antibodies. A positive result for N as well as RBD or S1 indicated a natural infection while a positive result for only RBD or S1 indicated a vaccine response or past infection.
The researchers also tested a subset of the IgG positive samples for IgM and IgA antibodies against N, S1, and RBD, with an IgM MFI greater than 24 and an IgA MFI greater than 102 used as the thresholds for positive results.
Among 384 cord blood samples analyzed, 85.4% were positive for IgG against RBD, indicating that the mother had SARS-CoV-2 immunity from either a past infection or vaccination. Of these anti-RBD positive samples, 60.7% were anti-N IgG negative, suggesting that N had waned since vaccination or the past infection.
Since the other 39.3% that were anti-N IgG positive suggest a past maternal infection, the researchers assessed these 129 samples for IgM and IgA antibodies against RBD. They found that 16 of them had high levels of anti-RBD IgA and/or IgM antibodies, pointing to a rate of mother-to-child-transmission of up to 12.4%.
Sallie Permar, MD, PhD, a professor and the chair of pediatrics at Weill Cornell Medicine in New York, who was not involved in the research, said most studies of placental transmission have focused on virologic testing, such as PCR. “Serologic tests for congenital infections are inherently challenged by the transfer of maternal IgG across the placenta and therefore must rely on non-IgG isotype response detection, which have inherently been more susceptible to false-positive results than IgG-based tests,” Dr. Permar said.
Also, “it is unclear if virologic testing was performed in the infants, which, if positive in the same infants for which cord blood IgM/IgA responses were identified, could further validate positive serologic findings,” added Dr. Permar, who is also pediatrician-in-chief at New York-Presbyterian Komansky Children’s Hospital.
Given these limitations, Dr. Permar reiterated that diagnostics for congenital SARS-CoV-2 continue to evolve, even if congenital SARS-CoV-2 infection currently appears rare. Dr. Permar said she agreed with Dr. Yeh that following those who do develop this infection is important.
“There have been initial reports of neurodevelopmental and other outcomes from long-term follow-up cohorts of infants exposed to SARS-CoV-2 infection in utero with variable results and it should continue to be pursued using cohorts both enrolled early in the pandemic and those enrolled more recently after population-level immunity to SARS-CoV-2 was achieved,” said Dr. Permar.
Dr. Permar serves as a consultant to Moderna, Pfizer, Merck, Dynavax, and Hoopika on their CMV vaccine programs and has led sponsored research programs with Moderna and Merck. Information on study funding and on disclosures for Dr. Yeh was unavailable.
AT AAP 2022
FDA: Newborns protected by whooping cough vaccine
The Food and Drug Administration has approved a whooping cough vaccine that protects newborns under 2 months of age.
The vaccine, manufactured by GlaxoSmithKline, was previously approved among pregnant people for their own protection.
“Infants younger than 2 months of age are too young to be protected by the childhood pertussis vaccine series,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a press release. “This is the first vaccine approved specifically for use during pregnancy to prevent a disease in young infants whose mothers are vaccinated during pregnancy.”
Pertussis is a highly contagious respiratory tract infection caused by the bacterium Bordetella pertussis. Most cases that result in hospitalizations and death are among infants within 2 months of birth.
The FDA said its decision was based on data from observational studies, which included 108 cases of pertussis in infants younger than 2 months old. According to data evaluated by the agency, the vaccine was 78% effective in preventing whooping cough.
Boostrix is administered as a single 0.5-mL dose.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a whooping cough vaccine that protects newborns under 2 months of age.
The vaccine, manufactured by GlaxoSmithKline, was previously approved among pregnant people for their own protection.
“Infants younger than 2 months of age are too young to be protected by the childhood pertussis vaccine series,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a press release. “This is the first vaccine approved specifically for use during pregnancy to prevent a disease in young infants whose mothers are vaccinated during pregnancy.”
Pertussis is a highly contagious respiratory tract infection caused by the bacterium Bordetella pertussis. Most cases that result in hospitalizations and death are among infants within 2 months of birth.
The FDA said its decision was based on data from observational studies, which included 108 cases of pertussis in infants younger than 2 months old. According to data evaluated by the agency, the vaccine was 78% effective in preventing whooping cough.
Boostrix is administered as a single 0.5-mL dose.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a whooping cough vaccine that protects newborns under 2 months of age.
The vaccine, manufactured by GlaxoSmithKline, was previously approved among pregnant people for their own protection.
“Infants younger than 2 months of age are too young to be protected by the childhood pertussis vaccine series,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a press release. “This is the first vaccine approved specifically for use during pregnancy to prevent a disease in young infants whose mothers are vaccinated during pregnancy.”
Pertussis is a highly contagious respiratory tract infection caused by the bacterium Bordetella pertussis. Most cases that result in hospitalizations and death are among infants within 2 months of birth.
The FDA said its decision was based on data from observational studies, which included 108 cases of pertussis in infants younger than 2 months old. According to data evaluated by the agency, the vaccine was 78% effective in preventing whooping cough.
Boostrix is administered as a single 0.5-mL dose.
A version of this article first appeared on Medscape.com.
HPV infection in pregnancy higher among women living with HIV
Pregnant women living with HIV were more likely to be infected with human papillomavirus (HPV) than were pregnant women without HIV, a recent systematic review and meta-analysis reports.
“High prevalence of HPV was documented in pregnant WLWH [women living with HIV], exceeding the prevalence among pregnant women without HIV,” Elisabeth McClymont, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in the Journal of Acquired Immune Deficiency Syndrome.
Their results contribute to two major global public health goals: eliminating cervical cancer and improving the health outcomes of newborn babies.
“Our findings of a high prevalence of HPV infection during pregnancy in WLWH, particularly of highly oncogenic HPV types, emphasize the need for HPV screening and vaccination in WLWH,” they added. “WLWH are a key population for both HPV and adverse pregnancy outcome prevention.”
Emerging evidence suggests that being infected with HPV during pregnancy may be linked with adverse pregnancy outcomes. Although women living with HIV have higher rates of HPV infection and adverse pregnancy outcomes, no prior reviews have reported on HPV infection during pregnancy in women living with HIV, the authors explained.
A study of studies
Dr. McClymont and colleagues searched the standard medical research databases through Jan. 18, 2022, for pooled and type-specific HPV prevalence and associated pregnancy outcomes among pregnant women living with HIV, including available within-study comparators of women without HIV.
They performed subgroup analyses according to polymerase chain reaction primers used to detect HPV type and according to region (Africa, Asia and Europe, the Americas).
Their analysis of 10 studies describing HPV prevalence in 1,594 pregnant women living with HIV found:
- The pooled HPV prevalence in pregnant women living with HIV was 75.5% (95% confidence interval, 50.2%-90.4%) but ranged from 23% to 98% between individual studies.
- Among the five studies that also analyzed HPV prevalence in pregnant women without HIV, the pooled prevalence was 48.1% (95% CI, 27.1%-69.8%).
- Pregnant women living with HIV had 54% higher odds of being HPV positive than did pregnant women without HIV.
- HPV-16 was the most common HPV type detected in pregnant women living with HIV, followed by HPV-52; other common types included HPV-18 and HPV-58.
- One study provided data on pregnancy outcomes in women living with HIV but did not correlate pregnancy outcomes with HPV status.
Experts urge HPV, cervical cancer screening for women living with HIV
“HPV is a common virus that can lead to cervical dysplasia and cervical cancer,” cautioned Clara Paik, MD, professor and clinic medical director of obstetrics and gynecology at UC Davis Health, Sacramento.
“HPV can also be associated with adverse pregnancy outcomes, including preterm birth and premature membrane rupture,” she said in an interview. “It is important to know the prevalence of HPV infection in pregnant women living with HIV in order to assess if this specific population is at higher risk for adverse pregnancy outcomes.”
Dr. Paik, who was not involved in the study, would like these results to lead to better HPV screening in pregnant women living with HIV.
“The study’s strengths include the large number of women studied when all the research studies were pooled,” she said. “A weakness is that, if individual studies had limitations, a systematic review based on weaker studies may not necessarily yield results that are conclusive.”
Linda Eckert, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, said that the study highlights the importance of including cervical cancer screening in antepartum care, especially in areas of high HIV prevalence.
“Women living with HIV have a sixfold increased rate of developing cervical cancer compared to women without HIV,” she added, citing a 2020 analysis in The Lancet Global Health that estimated global cervical cancer risk among women living with HIV.
“This [new] study allows us to definitively say that pregnant women living with HIV have higher rates of HPV than do pregnant women without HIV,” noted Dr. Eckert, who was not involved in either study. “And HPV type 16 – the HPV type most associated with developing cervical cancer – was the most common high-risk HPV type found in these patients.”
HPV vaccination recommended
The World Health Organization’s call to eliminate cervical cancer has generated interest and funding for cervical cancer screening of women with HIV, Dr. Eckert said. “WHO recommends that women living with HIV who are 25 years of age and above be screened for cervical cancer annually.”
The authors urged that women living with HIV not only be screened for HPV but that they also be vaccinated against HPV.
“We know that HPV vaccination is unprecedented in its ability to prevent HPV infections when it is received prior to acquiring HPV infection,” Dr. Eckert said, “but currently data showing that HPV vaccination would treat HPV16 in pregnant women already infected with HPV16 are lacking.
“This study points to the need for a trial to investigate HPV vaccination in pregnant women living with HIV who have the high-risk HPV types,” she suggested.
Dr. Eckert contributed to the American College of Obstetricians and Gynecologists’ 2020 Human Papillomavirus Vaccination Committee Opinion. One study coauthor reported financial relationships with Merck. Dr. McClymont, the other coauthors, as well as Dr. Paik and Dr. Eckert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women living with HIV were more likely to be infected with human papillomavirus (HPV) than were pregnant women without HIV, a recent systematic review and meta-analysis reports.
“High prevalence of HPV was documented in pregnant WLWH [women living with HIV], exceeding the prevalence among pregnant women without HIV,” Elisabeth McClymont, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in the Journal of Acquired Immune Deficiency Syndrome.
Their results contribute to two major global public health goals: eliminating cervical cancer and improving the health outcomes of newborn babies.
“Our findings of a high prevalence of HPV infection during pregnancy in WLWH, particularly of highly oncogenic HPV types, emphasize the need for HPV screening and vaccination in WLWH,” they added. “WLWH are a key population for both HPV and adverse pregnancy outcome prevention.”
Emerging evidence suggests that being infected with HPV during pregnancy may be linked with adverse pregnancy outcomes. Although women living with HIV have higher rates of HPV infection and adverse pregnancy outcomes, no prior reviews have reported on HPV infection during pregnancy in women living with HIV, the authors explained.
A study of studies
Dr. McClymont and colleagues searched the standard medical research databases through Jan. 18, 2022, for pooled and type-specific HPV prevalence and associated pregnancy outcomes among pregnant women living with HIV, including available within-study comparators of women without HIV.
They performed subgroup analyses according to polymerase chain reaction primers used to detect HPV type and according to region (Africa, Asia and Europe, the Americas).
Their analysis of 10 studies describing HPV prevalence in 1,594 pregnant women living with HIV found:
- The pooled HPV prevalence in pregnant women living with HIV was 75.5% (95% confidence interval, 50.2%-90.4%) but ranged from 23% to 98% between individual studies.
- Among the five studies that also analyzed HPV prevalence in pregnant women without HIV, the pooled prevalence was 48.1% (95% CI, 27.1%-69.8%).
- Pregnant women living with HIV had 54% higher odds of being HPV positive than did pregnant women without HIV.
- HPV-16 was the most common HPV type detected in pregnant women living with HIV, followed by HPV-52; other common types included HPV-18 and HPV-58.
- One study provided data on pregnancy outcomes in women living with HIV but did not correlate pregnancy outcomes with HPV status.
Experts urge HPV, cervical cancer screening for women living with HIV
“HPV is a common virus that can lead to cervical dysplasia and cervical cancer,” cautioned Clara Paik, MD, professor and clinic medical director of obstetrics and gynecology at UC Davis Health, Sacramento.
“HPV can also be associated with adverse pregnancy outcomes, including preterm birth and premature membrane rupture,” she said in an interview. “It is important to know the prevalence of HPV infection in pregnant women living with HIV in order to assess if this specific population is at higher risk for adverse pregnancy outcomes.”
Dr. Paik, who was not involved in the study, would like these results to lead to better HPV screening in pregnant women living with HIV.
“The study’s strengths include the large number of women studied when all the research studies were pooled,” she said. “A weakness is that, if individual studies had limitations, a systematic review based on weaker studies may not necessarily yield results that are conclusive.”
Linda Eckert, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, said that the study highlights the importance of including cervical cancer screening in antepartum care, especially in areas of high HIV prevalence.
“Women living with HIV have a sixfold increased rate of developing cervical cancer compared to women without HIV,” she added, citing a 2020 analysis in The Lancet Global Health that estimated global cervical cancer risk among women living with HIV.
“This [new] study allows us to definitively say that pregnant women living with HIV have higher rates of HPV than do pregnant women without HIV,” noted Dr. Eckert, who was not involved in either study. “And HPV type 16 – the HPV type most associated with developing cervical cancer – was the most common high-risk HPV type found in these patients.”
HPV vaccination recommended
The World Health Organization’s call to eliminate cervical cancer has generated interest and funding for cervical cancer screening of women with HIV, Dr. Eckert said. “WHO recommends that women living with HIV who are 25 years of age and above be screened for cervical cancer annually.”
The authors urged that women living with HIV not only be screened for HPV but that they also be vaccinated against HPV.
“We know that HPV vaccination is unprecedented in its ability to prevent HPV infections when it is received prior to acquiring HPV infection,” Dr. Eckert said, “but currently data showing that HPV vaccination would treat HPV16 in pregnant women already infected with HPV16 are lacking.
“This study points to the need for a trial to investigate HPV vaccination in pregnant women living with HIV who have the high-risk HPV types,” she suggested.
Dr. Eckert contributed to the American College of Obstetricians and Gynecologists’ 2020 Human Papillomavirus Vaccination Committee Opinion. One study coauthor reported financial relationships with Merck. Dr. McClymont, the other coauthors, as well as Dr. Paik and Dr. Eckert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women living with HIV were more likely to be infected with human papillomavirus (HPV) than were pregnant women without HIV, a recent systematic review and meta-analysis reports.
“High prevalence of HPV was documented in pregnant WLWH [women living with HIV], exceeding the prevalence among pregnant women without HIV,” Elisabeth McClymont, PhD, of the University of British Columbia, Vancouver, and colleagues wrote in the Journal of Acquired Immune Deficiency Syndrome.
Their results contribute to two major global public health goals: eliminating cervical cancer and improving the health outcomes of newborn babies.
“Our findings of a high prevalence of HPV infection during pregnancy in WLWH, particularly of highly oncogenic HPV types, emphasize the need for HPV screening and vaccination in WLWH,” they added. “WLWH are a key population for both HPV and adverse pregnancy outcome prevention.”
Emerging evidence suggests that being infected with HPV during pregnancy may be linked with adverse pregnancy outcomes. Although women living with HIV have higher rates of HPV infection and adverse pregnancy outcomes, no prior reviews have reported on HPV infection during pregnancy in women living with HIV, the authors explained.
A study of studies
Dr. McClymont and colleagues searched the standard medical research databases through Jan. 18, 2022, for pooled and type-specific HPV prevalence and associated pregnancy outcomes among pregnant women living with HIV, including available within-study comparators of women without HIV.
They performed subgroup analyses according to polymerase chain reaction primers used to detect HPV type and according to region (Africa, Asia and Europe, the Americas).
Their analysis of 10 studies describing HPV prevalence in 1,594 pregnant women living with HIV found:
- The pooled HPV prevalence in pregnant women living with HIV was 75.5% (95% confidence interval, 50.2%-90.4%) but ranged from 23% to 98% between individual studies.
- Among the five studies that also analyzed HPV prevalence in pregnant women without HIV, the pooled prevalence was 48.1% (95% CI, 27.1%-69.8%).
- Pregnant women living with HIV had 54% higher odds of being HPV positive than did pregnant women without HIV.
- HPV-16 was the most common HPV type detected in pregnant women living with HIV, followed by HPV-52; other common types included HPV-18 and HPV-58.
- One study provided data on pregnancy outcomes in women living with HIV but did not correlate pregnancy outcomes with HPV status.
Experts urge HPV, cervical cancer screening for women living with HIV
“HPV is a common virus that can lead to cervical dysplasia and cervical cancer,” cautioned Clara Paik, MD, professor and clinic medical director of obstetrics and gynecology at UC Davis Health, Sacramento.
“HPV can also be associated with adverse pregnancy outcomes, including preterm birth and premature membrane rupture,” she said in an interview. “It is important to know the prevalence of HPV infection in pregnant women living with HIV in order to assess if this specific population is at higher risk for adverse pregnancy outcomes.”
Dr. Paik, who was not involved in the study, would like these results to lead to better HPV screening in pregnant women living with HIV.
“The study’s strengths include the large number of women studied when all the research studies were pooled,” she said. “A weakness is that, if individual studies had limitations, a systematic review based on weaker studies may not necessarily yield results that are conclusive.”
Linda Eckert, MD, professor of obstetrics and gynecology at the University of Washington, Seattle, said that the study highlights the importance of including cervical cancer screening in antepartum care, especially in areas of high HIV prevalence.
“Women living with HIV have a sixfold increased rate of developing cervical cancer compared to women without HIV,” she added, citing a 2020 analysis in The Lancet Global Health that estimated global cervical cancer risk among women living with HIV.
“This [new] study allows us to definitively say that pregnant women living with HIV have higher rates of HPV than do pregnant women without HIV,” noted Dr. Eckert, who was not involved in either study. “And HPV type 16 – the HPV type most associated with developing cervical cancer – was the most common high-risk HPV type found in these patients.”
HPV vaccination recommended
The World Health Organization’s call to eliminate cervical cancer has generated interest and funding for cervical cancer screening of women with HIV, Dr. Eckert said. “WHO recommends that women living with HIV who are 25 years of age and above be screened for cervical cancer annually.”
The authors urged that women living with HIV not only be screened for HPV but that they also be vaccinated against HPV.
“We know that HPV vaccination is unprecedented in its ability to prevent HPV infections when it is received prior to acquiring HPV infection,” Dr. Eckert said, “but currently data showing that HPV vaccination would treat HPV16 in pregnant women already infected with HPV16 are lacking.
“This study points to the need for a trial to investigate HPV vaccination in pregnant women living with HIV who have the high-risk HPV types,” she suggested.
Dr. Eckert contributed to the American College of Obstetricians and Gynecologists’ 2020 Human Papillomavirus Vaccination Committee Opinion. One study coauthor reported financial relationships with Merck. Dr. McClymont, the other coauthors, as well as Dr. Paik and Dr. Eckert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ACQUIRED IMMUNE DEFICIENCY SYNDROME.
Nifedipine during labor controls BP in severe preeclampsia
Women with preeclampsia with severe features benefit from treatment with oral nifedipine during labor and delivery, results of a randomized controlled trial suggest.
The study showed that intrapartum administration of extended-release oral nifedipine was safe and reduced the need for acute intravenous or immediate-release oral hypertensive therapy. There was a trend toward fewer cesarean deliveries and less need for neonatal intensive care.
The results suggest that providers “consider initiating long-acting nifedipine every 24 hours for individuals with preeclampsia with severe features who are undergoing induction of labor,” Erin M. Cleary, MD, with the Ohio State University, Columbus, told this news organization.
“There is no need to wait until patients require one or more doses of acute [antihypertensive] therapy before starting long-acting nifedipine, as long as they otherwise meet criteria for preeclampsia with severe features,” Dr. Cleary said.
The study was published online in Hypertension.
Clear benefits for mom and baby
Preeclampsia complicates up to 8% of pregnancies and often leads to significant maternal and perinatal morbidity.
“We know that bringing down very high blood pressure to a safer range will help prevent maternal and fetal complications. However, besides rapid-acting, intravenous medicines for severe hypertension during pregnancy, optimal management for hypertension during the labor and delivery process has not been studied,” Dr. Cleary explains in a news release.
In a randomized, triple-blind, placebo-controlled study, the researchers assessed whether treatment with long-acting nifedipine could prevent severe hypertension in women with a singleton or twin gestation and preeclampsia with severe features, as defined according to American College of Obstetrics and Gynecology criteria.
During induction of labor between 22 and 41 weeks’ gestation, 55 women were assigned to 30-mg oral extended-release nifedipine, and 55 received matching placebo, administered every 24 hours until delivery.
The primary outcome was receipt of one or more doses of acute hypertension therapy for blood pressure of at least 160/110 mm Hg that was sustained for 10 minutes or longer.
The primary outcome occurred in significantly fewer women in the nifedipine group than in the placebo group (34% vs. 55%; relative risk, 0.62; 95% CI, 0.39-0.97; number needed to treat, 4.7).
Fewer women in the nifedipine group than in the placebo group required cesarean delivery, although this difference did not meet statistical significance (21% vs. 35%; RR, 0.60; 95% CI, 0.31-1.15).
There was no between-group difference in the rate of hypotensive episodes, including symptomatic hypotension requiring phenylephrine for pressure support following neuraxial anesthesia (9.4% vs. 8.2%; RR, 1.15; 95% CI, 0.33-4.06).
After delivery, there was no difference in the rate of persistently severe blood pressure that required acute therapy and maintenance therapy at time of discharge home.
Birth weight and rates of births of neonates who were small for gestational age were similar in the two groups. There was a trend for decreased rates of neonatal intensive care unit admission among infants born to mothers who received nifedipine (29% vs. 47%; RR, 0.62; 95% CI, 0.37-1.02).
The neonatal composite outcome was also similar between the nifedipine group and the placebo group (36% vs. 41%; RR, 0.83; 95% CI, 0.51-1.37). The composite outcome included Apgar score of less than 7 at 5 minutes, hyperbilirubinemia requiring phototherapy, hypoglycemia requiring intravenous therapy, or supplemental oxygen therapy beyond the first 24 hours of life.
“Our findings support the growing trend in more active management of hypertension in pregnancy with daily maintenance medications,” Dr. Cleary and colleagues note in their article.
“Even in the absence of preeclampsia, emerging research suggests pregnant individuals may benefit from initiating and titrating antihypertensive therapy at goals similar to the nonobstetric population,” they add.
Potentially practice changing
Reached for comment, Vesna Garovic, MD, PhD, with Mayo Clinic, Rochester, Minn., said that this is an “important initial paper to start a very important conversation about blood pressure treatment goals in preeclampsia.”
Dr. Garovic noted that for chronic hypertension in pregnancy, the blood pressure treatment goal is now less than or equal to 140/90 mm Hg.
“However, this does not apply to preeclampsia, where quite high blood pressures, such 160/110 mm Hg or higher, are still allowed before treatment is considered,” Dr. Garovic said.
“This study shows that as soon as you reach that level, treatment with oral nifedipine should be initiated and that timely initiation of oral nifedipine may optimize blood pressure control and decrease the need for intravenous therapy subsequently, and that has good effects on the mother without adversely affecting the baby,” Dr. Garovic said.
“This is potentially practice changing,” Dr. Garovic added. “But the elephant in the room is the question of why we are waiting for blood pressure to reach such dangerous levels before initiating treatment, and whether initiating treatment at a blood pressure of 140/90 or higher may prevent blood pressure reaching these high levels and women developing complications that are the consequence of severe hypertension.”
The study was funded by the Ohio State University’s Department of Obstetrics and Gynecology. Dr. Cleary and Dr. Garovic have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with preeclampsia with severe features benefit from treatment with oral nifedipine during labor and delivery, results of a randomized controlled trial suggest.
The study showed that intrapartum administration of extended-release oral nifedipine was safe and reduced the need for acute intravenous or immediate-release oral hypertensive therapy. There was a trend toward fewer cesarean deliveries and less need for neonatal intensive care.
The results suggest that providers “consider initiating long-acting nifedipine every 24 hours for individuals with preeclampsia with severe features who are undergoing induction of labor,” Erin M. Cleary, MD, with the Ohio State University, Columbus, told this news organization.
“There is no need to wait until patients require one or more doses of acute [antihypertensive] therapy before starting long-acting nifedipine, as long as they otherwise meet criteria for preeclampsia with severe features,” Dr. Cleary said.
The study was published online in Hypertension.
Clear benefits for mom and baby
Preeclampsia complicates up to 8% of pregnancies and often leads to significant maternal and perinatal morbidity.
“We know that bringing down very high blood pressure to a safer range will help prevent maternal and fetal complications. However, besides rapid-acting, intravenous medicines for severe hypertension during pregnancy, optimal management for hypertension during the labor and delivery process has not been studied,” Dr. Cleary explains in a news release.
In a randomized, triple-blind, placebo-controlled study, the researchers assessed whether treatment with long-acting nifedipine could prevent severe hypertension in women with a singleton or twin gestation and preeclampsia with severe features, as defined according to American College of Obstetrics and Gynecology criteria.
During induction of labor between 22 and 41 weeks’ gestation, 55 women were assigned to 30-mg oral extended-release nifedipine, and 55 received matching placebo, administered every 24 hours until delivery.
The primary outcome was receipt of one or more doses of acute hypertension therapy for blood pressure of at least 160/110 mm Hg that was sustained for 10 minutes or longer.
The primary outcome occurred in significantly fewer women in the nifedipine group than in the placebo group (34% vs. 55%; relative risk, 0.62; 95% CI, 0.39-0.97; number needed to treat, 4.7).
Fewer women in the nifedipine group than in the placebo group required cesarean delivery, although this difference did not meet statistical significance (21% vs. 35%; RR, 0.60; 95% CI, 0.31-1.15).
There was no between-group difference in the rate of hypotensive episodes, including symptomatic hypotension requiring phenylephrine for pressure support following neuraxial anesthesia (9.4% vs. 8.2%; RR, 1.15; 95% CI, 0.33-4.06).
After delivery, there was no difference in the rate of persistently severe blood pressure that required acute therapy and maintenance therapy at time of discharge home.
Birth weight and rates of births of neonates who were small for gestational age were similar in the two groups. There was a trend for decreased rates of neonatal intensive care unit admission among infants born to mothers who received nifedipine (29% vs. 47%; RR, 0.62; 95% CI, 0.37-1.02).
The neonatal composite outcome was also similar between the nifedipine group and the placebo group (36% vs. 41%; RR, 0.83; 95% CI, 0.51-1.37). The composite outcome included Apgar score of less than 7 at 5 minutes, hyperbilirubinemia requiring phototherapy, hypoglycemia requiring intravenous therapy, or supplemental oxygen therapy beyond the first 24 hours of life.
“Our findings support the growing trend in more active management of hypertension in pregnancy with daily maintenance medications,” Dr. Cleary and colleagues note in their article.
“Even in the absence of preeclampsia, emerging research suggests pregnant individuals may benefit from initiating and titrating antihypertensive therapy at goals similar to the nonobstetric population,” they add.
Potentially practice changing
Reached for comment, Vesna Garovic, MD, PhD, with Mayo Clinic, Rochester, Minn., said that this is an “important initial paper to start a very important conversation about blood pressure treatment goals in preeclampsia.”
Dr. Garovic noted that for chronic hypertension in pregnancy, the blood pressure treatment goal is now less than or equal to 140/90 mm Hg.
“However, this does not apply to preeclampsia, where quite high blood pressures, such 160/110 mm Hg or higher, are still allowed before treatment is considered,” Dr. Garovic said.
“This study shows that as soon as you reach that level, treatment with oral nifedipine should be initiated and that timely initiation of oral nifedipine may optimize blood pressure control and decrease the need for intravenous therapy subsequently, and that has good effects on the mother without adversely affecting the baby,” Dr. Garovic said.
“This is potentially practice changing,” Dr. Garovic added. “But the elephant in the room is the question of why we are waiting for blood pressure to reach such dangerous levels before initiating treatment, and whether initiating treatment at a blood pressure of 140/90 or higher may prevent blood pressure reaching these high levels and women developing complications that are the consequence of severe hypertension.”
The study was funded by the Ohio State University’s Department of Obstetrics and Gynecology. Dr. Cleary and Dr. Garovic have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women with preeclampsia with severe features benefit from treatment with oral nifedipine during labor and delivery, results of a randomized controlled trial suggest.
The study showed that intrapartum administration of extended-release oral nifedipine was safe and reduced the need for acute intravenous or immediate-release oral hypertensive therapy. There was a trend toward fewer cesarean deliveries and less need for neonatal intensive care.
The results suggest that providers “consider initiating long-acting nifedipine every 24 hours for individuals with preeclampsia with severe features who are undergoing induction of labor,” Erin M. Cleary, MD, with the Ohio State University, Columbus, told this news organization.
“There is no need to wait until patients require one or more doses of acute [antihypertensive] therapy before starting long-acting nifedipine, as long as they otherwise meet criteria for preeclampsia with severe features,” Dr. Cleary said.
The study was published online in Hypertension.
Clear benefits for mom and baby
Preeclampsia complicates up to 8% of pregnancies and often leads to significant maternal and perinatal morbidity.
“We know that bringing down very high blood pressure to a safer range will help prevent maternal and fetal complications. However, besides rapid-acting, intravenous medicines for severe hypertension during pregnancy, optimal management for hypertension during the labor and delivery process has not been studied,” Dr. Cleary explains in a news release.
In a randomized, triple-blind, placebo-controlled study, the researchers assessed whether treatment with long-acting nifedipine could prevent severe hypertension in women with a singleton or twin gestation and preeclampsia with severe features, as defined according to American College of Obstetrics and Gynecology criteria.
During induction of labor between 22 and 41 weeks’ gestation, 55 women were assigned to 30-mg oral extended-release nifedipine, and 55 received matching placebo, administered every 24 hours until delivery.
The primary outcome was receipt of one or more doses of acute hypertension therapy for blood pressure of at least 160/110 mm Hg that was sustained for 10 minutes or longer.
The primary outcome occurred in significantly fewer women in the nifedipine group than in the placebo group (34% vs. 55%; relative risk, 0.62; 95% CI, 0.39-0.97; number needed to treat, 4.7).
Fewer women in the nifedipine group than in the placebo group required cesarean delivery, although this difference did not meet statistical significance (21% vs. 35%; RR, 0.60; 95% CI, 0.31-1.15).
There was no between-group difference in the rate of hypotensive episodes, including symptomatic hypotension requiring phenylephrine for pressure support following neuraxial anesthesia (9.4% vs. 8.2%; RR, 1.15; 95% CI, 0.33-4.06).
After delivery, there was no difference in the rate of persistently severe blood pressure that required acute therapy and maintenance therapy at time of discharge home.
Birth weight and rates of births of neonates who were small for gestational age were similar in the two groups. There was a trend for decreased rates of neonatal intensive care unit admission among infants born to mothers who received nifedipine (29% vs. 47%; RR, 0.62; 95% CI, 0.37-1.02).
The neonatal composite outcome was also similar between the nifedipine group and the placebo group (36% vs. 41%; RR, 0.83; 95% CI, 0.51-1.37). The composite outcome included Apgar score of less than 7 at 5 minutes, hyperbilirubinemia requiring phototherapy, hypoglycemia requiring intravenous therapy, or supplemental oxygen therapy beyond the first 24 hours of life.
“Our findings support the growing trend in more active management of hypertension in pregnancy with daily maintenance medications,” Dr. Cleary and colleagues note in their article.
“Even in the absence of preeclampsia, emerging research suggests pregnant individuals may benefit from initiating and titrating antihypertensive therapy at goals similar to the nonobstetric population,” they add.
Potentially practice changing
Reached for comment, Vesna Garovic, MD, PhD, with Mayo Clinic, Rochester, Minn., said that this is an “important initial paper to start a very important conversation about blood pressure treatment goals in preeclampsia.”
Dr. Garovic noted that for chronic hypertension in pregnancy, the blood pressure treatment goal is now less than or equal to 140/90 mm Hg.
“However, this does not apply to preeclampsia, where quite high blood pressures, such 160/110 mm Hg or higher, are still allowed before treatment is considered,” Dr. Garovic said.
“This study shows that as soon as you reach that level, treatment with oral nifedipine should be initiated and that timely initiation of oral nifedipine may optimize blood pressure control and decrease the need for intravenous therapy subsequently, and that has good effects on the mother without adversely affecting the baby,” Dr. Garovic said.
“This is potentially practice changing,” Dr. Garovic added. “But the elephant in the room is the question of why we are waiting for blood pressure to reach such dangerous levels before initiating treatment, and whether initiating treatment at a blood pressure of 140/90 or higher may prevent blood pressure reaching these high levels and women developing complications that are the consequence of severe hypertension.”
The study was funded by the Ohio State University’s Department of Obstetrics and Gynecology. Dr. Cleary and Dr. Garovic have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION
What role does the uterine microbiome play in fertility?
Until the second half of the 20th century, it was believed that the uterine cavity was sterile. Since then, technological advances have provided insight into the nature of the microbiome throughout the female reproductive tract. The role of these microorganisms on the fertility of women of reproductive age has been the subject of research. Is there an “optimal microbiome” for fertility? Can changing the microbiome of the uterine cavity affect fertility? There is still no definitive scientific response to these questions.
Several studies describe the healthy state of the uterine microbiota in women of reproductive age, with most of these studies reporting dominance of Lactobacillus species. However, by contrast, some studies did not observe Lactobacillus predominance inside the uterine cavity in cases of healthy uterine microbiomes. The presence of other microorganisms, such as Gardnerella vaginalis, was associated with reduced success in patients attempting in vitro fertilization (IVF) treatment, such as, for example, embryo implantation failure and miscarriage.
It is also possible that a physiologic endometrial microbiome could be considered healthy despite a minor presence of pathogenic bacteria. Importantly, responses from the host also modulate many aspects of human conception. These shifts correlate with parameters such as age, hormonal changes, ethnicity, sexual activity, and intrauterine devices.
Carlos Simón, MD, PhD, is a gynecologist and obstetrician and professor at the University of Valencia in Valencia, Spain; Harvard University, Cambridge, Mass.; and Baylor College of Medicine, Houston. He was in São Paulo at the time of the XXVI Brazilian Congress of Assisted Reproduction and agreed to be interviewed by Medscape Portuguese Edition. Dr. Simón, who is Spanish and is an international reference in uterine microbiome studies, created an endometrial receptivity analysis (ERA).
“What we know is that the human uterus has its own microbiome. Thanks to next-generation sequencing (NGS), we can detect microbial DNA. We’re talking about a microbiome that, if changed, affects [embryo] implantation. We have identified that Lactobacilli are the good [microorganisms], but if there are Streptococci, Gardnerella, or other bacteria, the implantation [of the embryo] is affected.”
In 2018, Dr. Simón’s team published a pilot study assessing the microbiome of 30 patients during fertilization treatment. It was observed that, when there is a change in the microbiome, the implantation rate drops to half and the miscarriage rate doubles.
Following this study, also in 2018, the team published a multicenter, prospective, observational study. A 16S ribosomal RNA (16S rRNA) gene sequencing technique was used to analyze endometrial fluid and biopsy samples before embryo transfer in a cohort of 342 infertile patients asymptomatic for infection. Participants underwent fertilization procedures in 13 centers on three continents.
A dysbiotic endometrial microbiota profile composed of Atopobium, Bifidobacterium, Chryseobacterium, Gardnerella, Haemophilus, Klebsiella, Neisseria, Staphylococcus, and Streptococcus was associated with unsuccessful outcomes. In contrast, Lactobacillus was consistently enriched in patients with live birth outcomes. The authors concluded that endometrial microbiota composition before embryo transfer is a useful biomarker to predict reproductive outcome.
“You see a microbial signature in patients who become pregnant, another in those who do not become pregnant, and yet another in those who miscarry,” Dr. Simón summarized. “By knowing this signature, the microbiome can be analyzed and treated so that it is stabilized before the embryo is transferred.”
What should be done?
Endometrial microbiome profiles do not use microbial cultures. They are obtained by NGS of the endometrial sample. This is because the 16S rRNA gene, which can be found in bacteria, presents hypervariable regions that serve as markers to identify the bacteria present.
If a microbiome is found to be somewhat unhealthy, it is theoretically possible to change its composition, increasing the chances of successful assisted reproduction. The administration of antibiotics and vaginal probiotics are two treatment approaches.
According to Dr. Simón, treatment is specific to the bacterium (metronidazole, and, if that fails, rifampicin for Gardnerella, amoxicillin and clavulanic acid for Streptococci). Once the pathogenic bacterium has been treated, the probiotics can be administered. “If all is well, we can then go ahead with the procedure,” he explained.
Dr. Simón pointed out that, with respect to treatment, knowledge is still limited and primarily based on case reports. “You look for issues in the microbiome when the patient experiences reproductive failure and there are no other causes,” he emphasized. “Microbiology plays a role in reproduction, affecting the human uterus. It’s good to know about it to improve reproductive outcomes. When there are repeated [embryo] implantation failures, we suggest an endometrial biopsy to identify the implantation window and determine whether the uterine microbiome is healthy or not. And if there are any abnormalities in the microbiome, they can be treated.”
There are still many open questions, such as how long the “good microbiome” lasts after antibiotic therapy. “We suggest checking the microbiome after [antibiotic] treatment and before implanting the embryo,” said Dr. Simón.
Although there is no consensus on how the endometrial microbiota relate to reproductive outcomes, the analysis and change in microbiome are already being offered in clinical practice as a way to increase the chances of conception. Márcia Riboldi, PhD, a genetics specialist serving as Country Manager for Igenomix Brasil and Argentina, the company that offers the analyses, provides an idea of the market for such analyses in Brazil. “We perform approximately 500 analyses per month,” she said, adding that most patients have a history of [embryo] implantation failure or miscarriage.
Matheus Roque, MD, PhD, an IVF specialist, shared two IVF case reports from the Mater Prime Human Reproduction Clinic in the southern region of the city of São Paulo. He emphasized that the decision to perform a microbiome analysis was made only after repeated implantation failure.
“With the outcomes the doctors started to see, the paradigm started to shift,” said Dr. Riboldi. “Why wait for the patient to have [an embryo] transfer failure? Let’s study the endometrium, check the ideal moment for the transfer, see whether it’s receptive or not, if there’s any disease and if there are Lactobacilli,” she proposed. “We need medical training and awareness, and we need to use them appropriately. We have the tests. Doctors need to learn about them and know when and how to use them.” The microbiome analysis costs approximately BRL 2,000, plus expenses for the medical procedure.
Is it too early?
Caio Parente Barbosa, MD, PhD, is an obstetrician/gynecologist specializing in human reproduction, as well as the director general and founder of the Fertile Idea Institute for Reproductive Health. He shared a few of his experiences in an interview with this news organization. “I would say it is still too early to confirm that [the microbiome analysis] produces effective outcomes.”
Dr. Barbosa, who is also provost of graduate studies, research, and innovation of the ABC School of Medicine, Santo André, Brazil, emphasized there is still little global experience with these analyses. “There are doubts worldwide regarding whether these analyses produce effective outcomes. Scientific studies are entirely controversial.”
He stated that some professionals recommend the microbiome analysis for “patients who don’t know what else to do,” but also recognized that there is already a demand for patients who don’t fit this category, who research the analyses on social networks and YouTube. “But it is the smallest of demands. Patients are not as worried about this yet.”
Dr. Barbosa recognized that the idea of an increasingly tailored treatment plan is inevitable. He believes that the study and treatment of the microbiome will become more critical in the future, but he thinks it still “does not offer any value.”
Dr. Barbosa emphasized that the financial side of things must also be considered. “If we add all these tests when investigating a patient’s issues, the treatment becomes ridiculously expensive.” He pointed out that health care professionals need to be careful to perform minimal testing. “We have already added some tests, such as the karyotype test, to the minimal testing for all patients.”
Dr. Simón responded to this criticism, stating: “The cost of repeating cycles is always greater than that of being thorough and knowing what’s going on. Nothing is certain, but if my daughter or wife needed it, I would like to have as much information as possible to make this decision.”
Dr. Barbosa and Dr. Simón reported no relevant financial relationships. Dr. Riboldi is Country Manager for Igenomix Brasil and Argentina, the company that offers the analyses.
This article was translated from the Medscape Portuguese edition and appeared on Medscape.com.
Until the second half of the 20th century, it was believed that the uterine cavity was sterile. Since then, technological advances have provided insight into the nature of the microbiome throughout the female reproductive tract. The role of these microorganisms on the fertility of women of reproductive age has been the subject of research. Is there an “optimal microbiome” for fertility? Can changing the microbiome of the uterine cavity affect fertility? There is still no definitive scientific response to these questions.
Several studies describe the healthy state of the uterine microbiota in women of reproductive age, with most of these studies reporting dominance of Lactobacillus species. However, by contrast, some studies did not observe Lactobacillus predominance inside the uterine cavity in cases of healthy uterine microbiomes. The presence of other microorganisms, such as Gardnerella vaginalis, was associated with reduced success in patients attempting in vitro fertilization (IVF) treatment, such as, for example, embryo implantation failure and miscarriage.
It is also possible that a physiologic endometrial microbiome could be considered healthy despite a minor presence of pathogenic bacteria. Importantly, responses from the host also modulate many aspects of human conception. These shifts correlate with parameters such as age, hormonal changes, ethnicity, sexual activity, and intrauterine devices.
Carlos Simón, MD, PhD, is a gynecologist and obstetrician and professor at the University of Valencia in Valencia, Spain; Harvard University, Cambridge, Mass.; and Baylor College of Medicine, Houston. He was in São Paulo at the time of the XXVI Brazilian Congress of Assisted Reproduction and agreed to be interviewed by Medscape Portuguese Edition. Dr. Simón, who is Spanish and is an international reference in uterine microbiome studies, created an endometrial receptivity analysis (ERA).
“What we know is that the human uterus has its own microbiome. Thanks to next-generation sequencing (NGS), we can detect microbial DNA. We’re talking about a microbiome that, if changed, affects [embryo] implantation. We have identified that Lactobacilli are the good [microorganisms], but if there are Streptococci, Gardnerella, or other bacteria, the implantation [of the embryo] is affected.”
In 2018, Dr. Simón’s team published a pilot study assessing the microbiome of 30 patients during fertilization treatment. It was observed that, when there is a change in the microbiome, the implantation rate drops to half and the miscarriage rate doubles.
Following this study, also in 2018, the team published a multicenter, prospective, observational study. A 16S ribosomal RNA (16S rRNA) gene sequencing technique was used to analyze endometrial fluid and biopsy samples before embryo transfer in a cohort of 342 infertile patients asymptomatic for infection. Participants underwent fertilization procedures in 13 centers on three continents.
A dysbiotic endometrial microbiota profile composed of Atopobium, Bifidobacterium, Chryseobacterium, Gardnerella, Haemophilus, Klebsiella, Neisseria, Staphylococcus, and Streptococcus was associated with unsuccessful outcomes. In contrast, Lactobacillus was consistently enriched in patients with live birth outcomes. The authors concluded that endometrial microbiota composition before embryo transfer is a useful biomarker to predict reproductive outcome.
“You see a microbial signature in patients who become pregnant, another in those who do not become pregnant, and yet another in those who miscarry,” Dr. Simón summarized. “By knowing this signature, the microbiome can be analyzed and treated so that it is stabilized before the embryo is transferred.”
What should be done?
Endometrial microbiome profiles do not use microbial cultures. They are obtained by NGS of the endometrial sample. This is because the 16S rRNA gene, which can be found in bacteria, presents hypervariable regions that serve as markers to identify the bacteria present.
If a microbiome is found to be somewhat unhealthy, it is theoretically possible to change its composition, increasing the chances of successful assisted reproduction. The administration of antibiotics and vaginal probiotics are two treatment approaches.
According to Dr. Simón, treatment is specific to the bacterium (metronidazole, and, if that fails, rifampicin for Gardnerella, amoxicillin and clavulanic acid for Streptococci). Once the pathogenic bacterium has been treated, the probiotics can be administered. “If all is well, we can then go ahead with the procedure,” he explained.
Dr. Simón pointed out that, with respect to treatment, knowledge is still limited and primarily based on case reports. “You look for issues in the microbiome when the patient experiences reproductive failure and there are no other causes,” he emphasized. “Microbiology plays a role in reproduction, affecting the human uterus. It’s good to know about it to improve reproductive outcomes. When there are repeated [embryo] implantation failures, we suggest an endometrial biopsy to identify the implantation window and determine whether the uterine microbiome is healthy or not. And if there are any abnormalities in the microbiome, they can be treated.”
There are still many open questions, such as how long the “good microbiome” lasts after antibiotic therapy. “We suggest checking the microbiome after [antibiotic] treatment and before implanting the embryo,” said Dr. Simón.
Although there is no consensus on how the endometrial microbiota relate to reproductive outcomes, the analysis and change in microbiome are already being offered in clinical practice as a way to increase the chances of conception. Márcia Riboldi, PhD, a genetics specialist serving as Country Manager for Igenomix Brasil and Argentina, the company that offers the analyses, provides an idea of the market for such analyses in Brazil. “We perform approximately 500 analyses per month,” she said, adding that most patients have a history of [embryo] implantation failure or miscarriage.
Matheus Roque, MD, PhD, an IVF specialist, shared two IVF case reports from the Mater Prime Human Reproduction Clinic in the southern region of the city of São Paulo. He emphasized that the decision to perform a microbiome analysis was made only after repeated implantation failure.
“With the outcomes the doctors started to see, the paradigm started to shift,” said Dr. Riboldi. “Why wait for the patient to have [an embryo] transfer failure? Let’s study the endometrium, check the ideal moment for the transfer, see whether it’s receptive or not, if there’s any disease and if there are Lactobacilli,” she proposed. “We need medical training and awareness, and we need to use them appropriately. We have the tests. Doctors need to learn about them and know when and how to use them.” The microbiome analysis costs approximately BRL 2,000, plus expenses for the medical procedure.
Is it too early?
Caio Parente Barbosa, MD, PhD, is an obstetrician/gynecologist specializing in human reproduction, as well as the director general and founder of the Fertile Idea Institute for Reproductive Health. He shared a few of his experiences in an interview with this news organization. “I would say it is still too early to confirm that [the microbiome analysis] produces effective outcomes.”
Dr. Barbosa, who is also provost of graduate studies, research, and innovation of the ABC School of Medicine, Santo André, Brazil, emphasized there is still little global experience with these analyses. “There are doubts worldwide regarding whether these analyses produce effective outcomes. Scientific studies are entirely controversial.”
He stated that some professionals recommend the microbiome analysis for “patients who don’t know what else to do,” but also recognized that there is already a demand for patients who don’t fit this category, who research the analyses on social networks and YouTube. “But it is the smallest of demands. Patients are not as worried about this yet.”
Dr. Barbosa recognized that the idea of an increasingly tailored treatment plan is inevitable. He believes that the study and treatment of the microbiome will become more critical in the future, but he thinks it still “does not offer any value.”
Dr. Barbosa emphasized that the financial side of things must also be considered. “If we add all these tests when investigating a patient’s issues, the treatment becomes ridiculously expensive.” He pointed out that health care professionals need to be careful to perform minimal testing. “We have already added some tests, such as the karyotype test, to the minimal testing for all patients.”
Dr. Simón responded to this criticism, stating: “The cost of repeating cycles is always greater than that of being thorough and knowing what’s going on. Nothing is certain, but if my daughter or wife needed it, I would like to have as much information as possible to make this decision.”
Dr. Barbosa and Dr. Simón reported no relevant financial relationships. Dr. Riboldi is Country Manager for Igenomix Brasil and Argentina, the company that offers the analyses.
This article was translated from the Medscape Portuguese edition and appeared on Medscape.com.
Until the second half of the 20th century, it was believed that the uterine cavity was sterile. Since then, technological advances have provided insight into the nature of the microbiome throughout the female reproductive tract. The role of these microorganisms on the fertility of women of reproductive age has been the subject of research. Is there an “optimal microbiome” for fertility? Can changing the microbiome of the uterine cavity affect fertility? There is still no definitive scientific response to these questions.
Several studies describe the healthy state of the uterine microbiota in women of reproductive age, with most of these studies reporting dominance of Lactobacillus species. However, by contrast, some studies did not observe Lactobacillus predominance inside the uterine cavity in cases of healthy uterine microbiomes. The presence of other microorganisms, such as Gardnerella vaginalis, was associated with reduced success in patients attempting in vitro fertilization (IVF) treatment, such as, for example, embryo implantation failure and miscarriage.
It is also possible that a physiologic endometrial microbiome could be considered healthy despite a minor presence of pathogenic bacteria. Importantly, responses from the host also modulate many aspects of human conception. These shifts correlate with parameters such as age, hormonal changes, ethnicity, sexual activity, and intrauterine devices.
Carlos Simón, MD, PhD, is a gynecologist and obstetrician and professor at the University of Valencia in Valencia, Spain; Harvard University, Cambridge, Mass.; and Baylor College of Medicine, Houston. He was in São Paulo at the time of the XXVI Brazilian Congress of Assisted Reproduction and agreed to be interviewed by Medscape Portuguese Edition. Dr. Simón, who is Spanish and is an international reference in uterine microbiome studies, created an endometrial receptivity analysis (ERA).
“What we know is that the human uterus has its own microbiome. Thanks to next-generation sequencing (NGS), we can detect microbial DNA. We’re talking about a microbiome that, if changed, affects [embryo] implantation. We have identified that Lactobacilli are the good [microorganisms], but if there are Streptococci, Gardnerella, or other bacteria, the implantation [of the embryo] is affected.”
In 2018, Dr. Simón’s team published a pilot study assessing the microbiome of 30 patients during fertilization treatment. It was observed that, when there is a change in the microbiome, the implantation rate drops to half and the miscarriage rate doubles.
Following this study, also in 2018, the team published a multicenter, prospective, observational study. A 16S ribosomal RNA (16S rRNA) gene sequencing technique was used to analyze endometrial fluid and biopsy samples before embryo transfer in a cohort of 342 infertile patients asymptomatic for infection. Participants underwent fertilization procedures in 13 centers on three continents.
A dysbiotic endometrial microbiota profile composed of Atopobium, Bifidobacterium, Chryseobacterium, Gardnerella, Haemophilus, Klebsiella, Neisseria, Staphylococcus, and Streptococcus was associated with unsuccessful outcomes. In contrast, Lactobacillus was consistently enriched in patients with live birth outcomes. The authors concluded that endometrial microbiota composition before embryo transfer is a useful biomarker to predict reproductive outcome.
“You see a microbial signature in patients who become pregnant, another in those who do not become pregnant, and yet another in those who miscarry,” Dr. Simón summarized. “By knowing this signature, the microbiome can be analyzed and treated so that it is stabilized before the embryo is transferred.”
What should be done?
Endometrial microbiome profiles do not use microbial cultures. They are obtained by NGS of the endometrial sample. This is because the 16S rRNA gene, which can be found in bacteria, presents hypervariable regions that serve as markers to identify the bacteria present.
If a microbiome is found to be somewhat unhealthy, it is theoretically possible to change its composition, increasing the chances of successful assisted reproduction. The administration of antibiotics and vaginal probiotics are two treatment approaches.
According to Dr. Simón, treatment is specific to the bacterium (metronidazole, and, if that fails, rifampicin for Gardnerella, amoxicillin and clavulanic acid for Streptococci). Once the pathogenic bacterium has been treated, the probiotics can be administered. “If all is well, we can then go ahead with the procedure,” he explained.
Dr. Simón pointed out that, with respect to treatment, knowledge is still limited and primarily based on case reports. “You look for issues in the microbiome when the patient experiences reproductive failure and there are no other causes,” he emphasized. “Microbiology plays a role in reproduction, affecting the human uterus. It’s good to know about it to improve reproductive outcomes. When there are repeated [embryo] implantation failures, we suggest an endometrial biopsy to identify the implantation window and determine whether the uterine microbiome is healthy or not. And if there are any abnormalities in the microbiome, they can be treated.”
There are still many open questions, such as how long the “good microbiome” lasts after antibiotic therapy. “We suggest checking the microbiome after [antibiotic] treatment and before implanting the embryo,” said Dr. Simón.
Although there is no consensus on how the endometrial microbiota relate to reproductive outcomes, the analysis and change in microbiome are already being offered in clinical practice as a way to increase the chances of conception. Márcia Riboldi, PhD, a genetics specialist serving as Country Manager for Igenomix Brasil and Argentina, the company that offers the analyses, provides an idea of the market for such analyses in Brazil. “We perform approximately 500 analyses per month,” she said, adding that most patients have a history of [embryo] implantation failure or miscarriage.
Matheus Roque, MD, PhD, an IVF specialist, shared two IVF case reports from the Mater Prime Human Reproduction Clinic in the southern region of the city of São Paulo. He emphasized that the decision to perform a microbiome analysis was made only after repeated implantation failure.
“With the outcomes the doctors started to see, the paradigm started to shift,” said Dr. Riboldi. “Why wait for the patient to have [an embryo] transfer failure? Let’s study the endometrium, check the ideal moment for the transfer, see whether it’s receptive or not, if there’s any disease and if there are Lactobacilli,” she proposed. “We need medical training and awareness, and we need to use them appropriately. We have the tests. Doctors need to learn about them and know when and how to use them.” The microbiome analysis costs approximately BRL 2,000, plus expenses for the medical procedure.
Is it too early?
Caio Parente Barbosa, MD, PhD, is an obstetrician/gynecologist specializing in human reproduction, as well as the director general and founder of the Fertile Idea Institute for Reproductive Health. He shared a few of his experiences in an interview with this news organization. “I would say it is still too early to confirm that [the microbiome analysis] produces effective outcomes.”
Dr. Barbosa, who is also provost of graduate studies, research, and innovation of the ABC School of Medicine, Santo André, Brazil, emphasized there is still little global experience with these analyses. “There are doubts worldwide regarding whether these analyses produce effective outcomes. Scientific studies are entirely controversial.”
He stated that some professionals recommend the microbiome analysis for “patients who don’t know what else to do,” but also recognized that there is already a demand for patients who don’t fit this category, who research the analyses on social networks and YouTube. “But it is the smallest of demands. Patients are not as worried about this yet.”
Dr. Barbosa recognized that the idea of an increasingly tailored treatment plan is inevitable. He believes that the study and treatment of the microbiome will become more critical in the future, but he thinks it still “does not offer any value.”
Dr. Barbosa emphasized that the financial side of things must also be considered. “If we add all these tests when investigating a patient’s issues, the treatment becomes ridiculously expensive.” He pointed out that health care professionals need to be careful to perform minimal testing. “We have already added some tests, such as the karyotype test, to the minimal testing for all patients.”
Dr. Simón responded to this criticism, stating: “The cost of repeating cycles is always greater than that of being thorough and knowing what’s going on. Nothing is certain, but if my daughter or wife needed it, I would like to have as much information as possible to make this decision.”
Dr. Barbosa and Dr. Simón reported no relevant financial relationships. Dr. Riboldi is Country Manager for Igenomix Brasil and Argentina, the company that offers the analyses.
This article was translated from the Medscape Portuguese edition and appeared on Medscape.com.
Lasker awardee pioneered prenatal DNA testing
For Yuk Ming Dennis Lo, BM BCh, DPhil, a 1996 paper showing the detection of tumor DNA in blood plasma would prove a turning point.
Since the 1960s, clinicians had been searching for a way to glimpse into a fetus’ genetic makeup without disturbing the pregnancy – a fascination Dr. Lo, at the time a graduate student in the United Kingdom, shared.
But the article triggered a thought. If cancer cells could release their DNA into blood plasma, then maybe fetuses could, too. “I had the strange thought that the cancer growing in the patients is a little bit like the placenta that has implanted into the uterus,” he told The New York Times.
The answer was yes. In 1997, having returned home to Hong Kong, Dr. Lo published a seminal article showing that cell-free fetal DNA could be detected in maternal blood.
He went on to devise methods to detect markers for Down syndrome, creating a noninvasive test that is more than 99% accurate for ruling out the disorder, along with screenings for trisomy 18 (Edwards syndrome), trisomy 13 (Patau syndrome), and other chromosome abnormalities.
With the commercial launch of noninvasive prenatal testing (NIPT) in 2011, health care centers around the world quickly embraced the technology as a safe alternative to more invasive methods, such as amniocentesis, for identifying fetal abnormalities. NIPT is now available in over 60 countries and is widely used by clinicians, according to the Lasker Foundation, which granted him the 2022 Lasker DeBakey Clinical Medical Research Award, along with a $250,000 prize.
“I am pleased that since its launch, noninvasive prenatal testing has become a standard of care,” Dr. Lo, chair of the department of chemical pathology at The Chinese University of Hong Kong, said in a video on the Lasker website. “It has also stimulated a global interest in the diagnostic applications of plasma DNA, especially in the area of cancer liquid biopsies and transplantation monitoring. I look forward to seeing these and other yet to be developed applications improving health care worldwide.”
Dr. Lo’s work has inspired clinical advances and applications, including Rh factor assessments, innovations in cancer technology, transplantation, and beyond, according to Lasker.
Iris Krishna, MD, MPH, director of Perinatal Quality in the Emory Perinatal Center at Emory University School of Medicine, Atlanta, said Dr. Lo’s work has also provided opportunities to screen for other genetic disorders, such as microdeletion syndromes and single gene disorders
“As we continue to learn about the possibilities of this technology, it is imperative for the clinician to be knowledgeable of the benefits and limitations of cell-free DNA screening to be able to counsel their patients appropriately,” Dr. Krishna said.
A COVID clearinghouse
Lauren Gardner, PhD, professor in the department of civil and systems engineering at Johns Hopkins University, Baltimore, received the Lasker Bloomberg Public Service Award for her work on the Johns Hopkins’ COVID-19 dashboard, a critical tool for the dissemination of public health data in real time.
According to the Los Angeles Times, Ensheng Dong, Dr. Gardner’s graduate student, approached her about tracking cases of the emerging infection in his home country of China. Mr. Dong mined Chinese websites for early cases of COVID-19 and created online maps using the information. At Dr. Gardner’s suggestion, he expanded the database to include global data.
At the time, according to Lasker, no other institution was providing this information. The World Health Organization created summaries of daily COVID-19 counts, but the data were not as accessible. Dr. Gardner said timely and obtainable information was crucial to craft nimble and rational strategies for combating the pandemic.
“Given the amount of misinformation in circulation and the highly politicized nature of the COVID-19 public health crisis, our work enabled individuals to access the information they needed to make informed decisions to protect themselves, which was especially critical in those locations with delayed or nonexistent policies in place,” Dr. Gardner said in a statement.
Dr. Gardner said she was excited to pursue additional data-centric projects. “I am optimistic that in the future, timely public health information will become increasingly available, especially in times of crisis,” she said. “Moving forward, I am excited to build on our learnings from COVID-19 and transfer that knowledge to address other problems facing societies.”
New knowledge of cells, immunology, and disease
The 2022 Albert Lasker Basic Research Award honored three scientists who helped identify a family of proteins that connect cells and assist the immune system in attaching to its targets. The proteins, called integrins, are needed for cells to interact with each other to build complex structures in the body. They are also key to the process T cells undergo to recognize and attack cancer cells.
Awardees Richard O. Hynes, MA, PhD, distinguished professor of cancer research at Massachusetts Institute of Technology; Erkki Ruoslahti, MD, PhD, distinguished professor emeritus at Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California; and Timothy A. Springer, PhD, professor of biological chemistry and molecular biology at Boston Children’s Hospital and Harvard Medical School, independently identified a cell-surface–associated protein that helps cells attach to the extracellular matrix.
“Many of the mysteries of how integrins work are only being discovered today,” Dr. Springer said in his acceptance remarks online.
The discoveries related to integrins have led to several clinical advances, including the development of drugs like the eyedrops lifitegrast, the biologic agent vedolizumab (made using integrins Springer discovered), and tirofiban, a medication used to hamper clotting in cardiovascular diseases.
A version of this article first appeared on Medscape.com.
For Yuk Ming Dennis Lo, BM BCh, DPhil, a 1996 paper showing the detection of tumor DNA in blood plasma would prove a turning point.
Since the 1960s, clinicians had been searching for a way to glimpse into a fetus’ genetic makeup without disturbing the pregnancy – a fascination Dr. Lo, at the time a graduate student in the United Kingdom, shared.
But the article triggered a thought. If cancer cells could release their DNA into blood plasma, then maybe fetuses could, too. “I had the strange thought that the cancer growing in the patients is a little bit like the placenta that has implanted into the uterus,” he told The New York Times.
The answer was yes. In 1997, having returned home to Hong Kong, Dr. Lo published a seminal article showing that cell-free fetal DNA could be detected in maternal blood.
He went on to devise methods to detect markers for Down syndrome, creating a noninvasive test that is more than 99% accurate for ruling out the disorder, along with screenings for trisomy 18 (Edwards syndrome), trisomy 13 (Patau syndrome), and other chromosome abnormalities.
With the commercial launch of noninvasive prenatal testing (NIPT) in 2011, health care centers around the world quickly embraced the technology as a safe alternative to more invasive methods, such as amniocentesis, for identifying fetal abnormalities. NIPT is now available in over 60 countries and is widely used by clinicians, according to the Lasker Foundation, which granted him the 2022 Lasker DeBakey Clinical Medical Research Award, along with a $250,000 prize.
“I am pleased that since its launch, noninvasive prenatal testing has become a standard of care,” Dr. Lo, chair of the department of chemical pathology at The Chinese University of Hong Kong, said in a video on the Lasker website. “It has also stimulated a global interest in the diagnostic applications of plasma DNA, especially in the area of cancer liquid biopsies and transplantation monitoring. I look forward to seeing these and other yet to be developed applications improving health care worldwide.”
Dr. Lo’s work has inspired clinical advances and applications, including Rh factor assessments, innovations in cancer technology, transplantation, and beyond, according to Lasker.
Iris Krishna, MD, MPH, director of Perinatal Quality in the Emory Perinatal Center at Emory University School of Medicine, Atlanta, said Dr. Lo’s work has also provided opportunities to screen for other genetic disorders, such as microdeletion syndromes and single gene disorders
“As we continue to learn about the possibilities of this technology, it is imperative for the clinician to be knowledgeable of the benefits and limitations of cell-free DNA screening to be able to counsel their patients appropriately,” Dr. Krishna said.
A COVID clearinghouse
Lauren Gardner, PhD, professor in the department of civil and systems engineering at Johns Hopkins University, Baltimore, received the Lasker Bloomberg Public Service Award for her work on the Johns Hopkins’ COVID-19 dashboard, a critical tool for the dissemination of public health data in real time.
According to the Los Angeles Times, Ensheng Dong, Dr. Gardner’s graduate student, approached her about tracking cases of the emerging infection in his home country of China. Mr. Dong mined Chinese websites for early cases of COVID-19 and created online maps using the information. At Dr. Gardner’s suggestion, he expanded the database to include global data.
At the time, according to Lasker, no other institution was providing this information. The World Health Organization created summaries of daily COVID-19 counts, but the data were not as accessible. Dr. Gardner said timely and obtainable information was crucial to craft nimble and rational strategies for combating the pandemic.
“Given the amount of misinformation in circulation and the highly politicized nature of the COVID-19 public health crisis, our work enabled individuals to access the information they needed to make informed decisions to protect themselves, which was especially critical in those locations with delayed or nonexistent policies in place,” Dr. Gardner said in a statement.
Dr. Gardner said she was excited to pursue additional data-centric projects. “I am optimistic that in the future, timely public health information will become increasingly available, especially in times of crisis,” she said. “Moving forward, I am excited to build on our learnings from COVID-19 and transfer that knowledge to address other problems facing societies.”
New knowledge of cells, immunology, and disease
The 2022 Albert Lasker Basic Research Award honored three scientists who helped identify a family of proteins that connect cells and assist the immune system in attaching to its targets. The proteins, called integrins, are needed for cells to interact with each other to build complex structures in the body. They are also key to the process T cells undergo to recognize and attack cancer cells.
Awardees Richard O. Hynes, MA, PhD, distinguished professor of cancer research at Massachusetts Institute of Technology; Erkki Ruoslahti, MD, PhD, distinguished professor emeritus at Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California; and Timothy A. Springer, PhD, professor of biological chemistry and molecular biology at Boston Children’s Hospital and Harvard Medical School, independently identified a cell-surface–associated protein that helps cells attach to the extracellular matrix.
“Many of the mysteries of how integrins work are only being discovered today,” Dr. Springer said in his acceptance remarks online.
The discoveries related to integrins have led to several clinical advances, including the development of drugs like the eyedrops lifitegrast, the biologic agent vedolizumab (made using integrins Springer discovered), and tirofiban, a medication used to hamper clotting in cardiovascular diseases.
A version of this article first appeared on Medscape.com.
For Yuk Ming Dennis Lo, BM BCh, DPhil, a 1996 paper showing the detection of tumor DNA in blood plasma would prove a turning point.
Since the 1960s, clinicians had been searching for a way to glimpse into a fetus’ genetic makeup without disturbing the pregnancy – a fascination Dr. Lo, at the time a graduate student in the United Kingdom, shared.
But the article triggered a thought. If cancer cells could release their DNA into blood plasma, then maybe fetuses could, too. “I had the strange thought that the cancer growing in the patients is a little bit like the placenta that has implanted into the uterus,” he told The New York Times.
The answer was yes. In 1997, having returned home to Hong Kong, Dr. Lo published a seminal article showing that cell-free fetal DNA could be detected in maternal blood.
He went on to devise methods to detect markers for Down syndrome, creating a noninvasive test that is more than 99% accurate for ruling out the disorder, along with screenings for trisomy 18 (Edwards syndrome), trisomy 13 (Patau syndrome), and other chromosome abnormalities.
With the commercial launch of noninvasive prenatal testing (NIPT) in 2011, health care centers around the world quickly embraced the technology as a safe alternative to more invasive methods, such as amniocentesis, for identifying fetal abnormalities. NIPT is now available in over 60 countries and is widely used by clinicians, according to the Lasker Foundation, which granted him the 2022 Lasker DeBakey Clinical Medical Research Award, along with a $250,000 prize.
“I am pleased that since its launch, noninvasive prenatal testing has become a standard of care,” Dr. Lo, chair of the department of chemical pathology at The Chinese University of Hong Kong, said in a video on the Lasker website. “It has also stimulated a global interest in the diagnostic applications of plasma DNA, especially in the area of cancer liquid biopsies and transplantation monitoring. I look forward to seeing these and other yet to be developed applications improving health care worldwide.”
Dr. Lo’s work has inspired clinical advances and applications, including Rh factor assessments, innovations in cancer technology, transplantation, and beyond, according to Lasker.
Iris Krishna, MD, MPH, director of Perinatal Quality in the Emory Perinatal Center at Emory University School of Medicine, Atlanta, said Dr. Lo’s work has also provided opportunities to screen for other genetic disorders, such as microdeletion syndromes and single gene disorders
“As we continue to learn about the possibilities of this technology, it is imperative for the clinician to be knowledgeable of the benefits and limitations of cell-free DNA screening to be able to counsel their patients appropriately,” Dr. Krishna said.
A COVID clearinghouse
Lauren Gardner, PhD, professor in the department of civil and systems engineering at Johns Hopkins University, Baltimore, received the Lasker Bloomberg Public Service Award for her work on the Johns Hopkins’ COVID-19 dashboard, a critical tool for the dissemination of public health data in real time.
According to the Los Angeles Times, Ensheng Dong, Dr. Gardner’s graduate student, approached her about tracking cases of the emerging infection in his home country of China. Mr. Dong mined Chinese websites for early cases of COVID-19 and created online maps using the information. At Dr. Gardner’s suggestion, he expanded the database to include global data.
At the time, according to Lasker, no other institution was providing this information. The World Health Organization created summaries of daily COVID-19 counts, but the data were not as accessible. Dr. Gardner said timely and obtainable information was crucial to craft nimble and rational strategies for combating the pandemic.
“Given the amount of misinformation in circulation and the highly politicized nature of the COVID-19 public health crisis, our work enabled individuals to access the information they needed to make informed decisions to protect themselves, which was especially critical in those locations with delayed or nonexistent policies in place,” Dr. Gardner said in a statement.
Dr. Gardner said she was excited to pursue additional data-centric projects. “I am optimistic that in the future, timely public health information will become increasingly available, especially in times of crisis,” she said. “Moving forward, I am excited to build on our learnings from COVID-19 and transfer that knowledge to address other problems facing societies.”
New knowledge of cells, immunology, and disease
The 2022 Albert Lasker Basic Research Award honored three scientists who helped identify a family of proteins that connect cells and assist the immune system in attaching to its targets. The proteins, called integrins, are needed for cells to interact with each other to build complex structures in the body. They are also key to the process T cells undergo to recognize and attack cancer cells.
Awardees Richard O. Hynes, MA, PhD, distinguished professor of cancer research at Massachusetts Institute of Technology; Erkki Ruoslahti, MD, PhD, distinguished professor emeritus at Sanford Burnham Prebys Medical Discovery Institute, La Jolla, California; and Timothy A. Springer, PhD, professor of biological chemistry and molecular biology at Boston Children’s Hospital and Harvard Medical School, independently identified a cell-surface–associated protein that helps cells attach to the extracellular matrix.
“Many of the mysteries of how integrins work are only being discovered today,” Dr. Springer said in his acceptance remarks online.
The discoveries related to integrins have led to several clinical advances, including the development of drugs like the eyedrops lifitegrast, the biologic agent vedolizumab (made using integrins Springer discovered), and tirofiban, a medication used to hamper clotting in cardiovascular diseases.
A version of this article first appeared on Medscape.com.
Mouse embryo experiment could teach us about miscarriages
Miscarriages are a devastating, if natural, occurrence. Nearly 1 million pregnant people in the United States experience a miscarriage every year, according to the National Advocates for Pregnant Women. New research could lend insight into the causes of some types of early pregnancy loss and maybe one day help prevent miscarriages.
In the bioengineering breakthrough, scientists created a mouse embryo in a lab without using sperm or eggs. The experimental embryo, called a model, was grown out of stem cells and developed further than any earlier experiments, with a beating heart and the foundation of a brain within a yolk sac, according to the researchers.
The experiment, while conducted with mouse stem cells, could help explain why some human pregnancies fail. Miscarriages occur in up to 15% of pregnancies confirmed by doctors, according to some studies, and also for many pregnant people before they even knew of the pregnancy. This experiment gives researchers a glimpse of a critical developmental stage for the first time.
“We are building mouse embryo models, but they have exactly the same principle as real human embryos,” says lead researcher Magdalena Zernicka-Goetz, PhD, professor in mammalian development and stem cell biology at Cambridge (England) University. “That’s why they tell us about real pregnancy.”
With the new mouse models, the researchers can study implantation, the stage when embryos embed themselves in the mother’s body – a stage that’s often difficult for embryos to survive. The same process happens in mouse embryos, which develop very similarly to human embryos at this early stage of life.
Six years ago, researchers from the University of Cambridge and the California Institute of Technology set out to create models that would allow them to study fetal development in three-dimensional form but without the need for human embryos.
“We are trying to understand the major principles of time and space that have to be fulfilled” to form a successful pregnancy, Dr. Zernicka-Goetz explains. “If those principles are not fulfilled, the pregnancies are terminated, even before women know they’re pregnant.”
There are limits on using human embryos for research, and previous experiments have tended to replicate only one aspect of development. That led to two-dimensional experiments: flat cells on the bottom of a petri dish that lack the structural organization of real tissue.
The new models are three-dimensional with beating hearts and the yolk sacs in which embryos feed and grow. The models even progressed to forming the beginning of a brain – a research first.
The scientists used the foundational cellular “building blocks” called stem cells and managed to get the cells to communicate along a timeline that mimicked natural development, simulating those developmental stages, says Dr. Zernicka-Goetz. Those “building blocks” are actually three types of stem cells: pluripotent stem cells that build body tissue, and two other types of stem cells that build the placenta and the amniotic sac.
Completing the experiment required the right quantity of each stem cell type. The researchers also needed to understand how those cells exchange information before they can begin to grow. The researchers were able to “decipher the code” of how the cells talk to each other, Dr. Zernicka-Goetz says.
Initially, the three types of stem cells combine, almost like a soup, but when the timing is right, they have to recognize each other and sort themselves. Next, each stem cell type must start building a different structure necessary for fetal development. Dr. Zernicka-Goetz thinks of this construction as the architecture of human tissue.
With the new technique, researchers can continue investigating the implantation stage and beyond. And they did – tweaking the experiment to create a genetically flawed embryo on purpose.
Dr. Zernicka-Goetz and her team eliminated a certain gene known to regulate how cells establish their own identities. Doing so resulted in the same brain development flaws as in human embryos, providing “a proof of concept” that the experimental models can be used to study other genetic mysteries, she says.
Scientists are still in the dark about what some genes do, as well as the point when they become critical to brain development.
“Many genes have very early roles in specifying, for example, the position of the head and also how our brain will function,” Dr. Zernicka-Goetz says. “We can now use this model system to assess the function of those genes.”
A version of this article first appeared on WebMD.com.
Miscarriages are a devastating, if natural, occurrence. Nearly 1 million pregnant people in the United States experience a miscarriage every year, according to the National Advocates for Pregnant Women. New research could lend insight into the causes of some types of early pregnancy loss and maybe one day help prevent miscarriages.
In the bioengineering breakthrough, scientists created a mouse embryo in a lab without using sperm or eggs. The experimental embryo, called a model, was grown out of stem cells and developed further than any earlier experiments, with a beating heart and the foundation of a brain within a yolk sac, according to the researchers.
The experiment, while conducted with mouse stem cells, could help explain why some human pregnancies fail. Miscarriages occur in up to 15% of pregnancies confirmed by doctors, according to some studies, and also for many pregnant people before they even knew of the pregnancy. This experiment gives researchers a glimpse of a critical developmental stage for the first time.
“We are building mouse embryo models, but they have exactly the same principle as real human embryos,” says lead researcher Magdalena Zernicka-Goetz, PhD, professor in mammalian development and stem cell biology at Cambridge (England) University. “That’s why they tell us about real pregnancy.”
With the new mouse models, the researchers can study implantation, the stage when embryos embed themselves in the mother’s body – a stage that’s often difficult for embryos to survive. The same process happens in mouse embryos, which develop very similarly to human embryos at this early stage of life.
Six years ago, researchers from the University of Cambridge and the California Institute of Technology set out to create models that would allow them to study fetal development in three-dimensional form but without the need for human embryos.
“We are trying to understand the major principles of time and space that have to be fulfilled” to form a successful pregnancy, Dr. Zernicka-Goetz explains. “If those principles are not fulfilled, the pregnancies are terminated, even before women know they’re pregnant.”
There are limits on using human embryos for research, and previous experiments have tended to replicate only one aspect of development. That led to two-dimensional experiments: flat cells on the bottom of a petri dish that lack the structural organization of real tissue.
The new models are three-dimensional with beating hearts and the yolk sacs in which embryos feed and grow. The models even progressed to forming the beginning of a brain – a research first.
The scientists used the foundational cellular “building blocks” called stem cells and managed to get the cells to communicate along a timeline that mimicked natural development, simulating those developmental stages, says Dr. Zernicka-Goetz. Those “building blocks” are actually three types of stem cells: pluripotent stem cells that build body tissue, and two other types of stem cells that build the placenta and the amniotic sac.
Completing the experiment required the right quantity of each stem cell type. The researchers also needed to understand how those cells exchange information before they can begin to grow. The researchers were able to “decipher the code” of how the cells talk to each other, Dr. Zernicka-Goetz says.
Initially, the three types of stem cells combine, almost like a soup, but when the timing is right, they have to recognize each other and sort themselves. Next, each stem cell type must start building a different structure necessary for fetal development. Dr. Zernicka-Goetz thinks of this construction as the architecture of human tissue.
With the new technique, researchers can continue investigating the implantation stage and beyond. And they did – tweaking the experiment to create a genetically flawed embryo on purpose.
Dr. Zernicka-Goetz and her team eliminated a certain gene known to regulate how cells establish their own identities. Doing so resulted in the same brain development flaws as in human embryos, providing “a proof of concept” that the experimental models can be used to study other genetic mysteries, she says.
Scientists are still in the dark about what some genes do, as well as the point when they become critical to brain development.
“Many genes have very early roles in specifying, for example, the position of the head and also how our brain will function,” Dr. Zernicka-Goetz says. “We can now use this model system to assess the function of those genes.”
A version of this article first appeared on WebMD.com.
Miscarriages are a devastating, if natural, occurrence. Nearly 1 million pregnant people in the United States experience a miscarriage every year, according to the National Advocates for Pregnant Women. New research could lend insight into the causes of some types of early pregnancy loss and maybe one day help prevent miscarriages.
In the bioengineering breakthrough, scientists created a mouse embryo in a lab without using sperm or eggs. The experimental embryo, called a model, was grown out of stem cells and developed further than any earlier experiments, with a beating heart and the foundation of a brain within a yolk sac, according to the researchers.
The experiment, while conducted with mouse stem cells, could help explain why some human pregnancies fail. Miscarriages occur in up to 15% of pregnancies confirmed by doctors, according to some studies, and also for many pregnant people before they even knew of the pregnancy. This experiment gives researchers a glimpse of a critical developmental stage for the first time.
“We are building mouse embryo models, but they have exactly the same principle as real human embryos,” says lead researcher Magdalena Zernicka-Goetz, PhD, professor in mammalian development and stem cell biology at Cambridge (England) University. “That’s why they tell us about real pregnancy.”
With the new mouse models, the researchers can study implantation, the stage when embryos embed themselves in the mother’s body – a stage that’s often difficult for embryos to survive. The same process happens in mouse embryos, which develop very similarly to human embryos at this early stage of life.
Six years ago, researchers from the University of Cambridge and the California Institute of Technology set out to create models that would allow them to study fetal development in three-dimensional form but without the need for human embryos.
“We are trying to understand the major principles of time and space that have to be fulfilled” to form a successful pregnancy, Dr. Zernicka-Goetz explains. “If those principles are not fulfilled, the pregnancies are terminated, even before women know they’re pregnant.”
There are limits on using human embryos for research, and previous experiments have tended to replicate only one aspect of development. That led to two-dimensional experiments: flat cells on the bottom of a petri dish that lack the structural organization of real tissue.
The new models are three-dimensional with beating hearts and the yolk sacs in which embryos feed and grow. The models even progressed to forming the beginning of a brain – a research first.
The scientists used the foundational cellular “building blocks” called stem cells and managed to get the cells to communicate along a timeline that mimicked natural development, simulating those developmental stages, says Dr. Zernicka-Goetz. Those “building blocks” are actually three types of stem cells: pluripotent stem cells that build body tissue, and two other types of stem cells that build the placenta and the amniotic sac.
Completing the experiment required the right quantity of each stem cell type. The researchers also needed to understand how those cells exchange information before they can begin to grow. The researchers were able to “decipher the code” of how the cells talk to each other, Dr. Zernicka-Goetz says.
Initially, the three types of stem cells combine, almost like a soup, but when the timing is right, they have to recognize each other and sort themselves. Next, each stem cell type must start building a different structure necessary for fetal development. Dr. Zernicka-Goetz thinks of this construction as the architecture of human tissue.
With the new technique, researchers can continue investigating the implantation stage and beyond. And they did – tweaking the experiment to create a genetically flawed embryo on purpose.
Dr. Zernicka-Goetz and her team eliminated a certain gene known to regulate how cells establish their own identities. Doing so resulted in the same brain development flaws as in human embryos, providing “a proof of concept” that the experimental models can be used to study other genetic mysteries, she says.
Scientists are still in the dark about what some genes do, as well as the point when they become critical to brain development.
“Many genes have very early roles in specifying, for example, the position of the head and also how our brain will function,” Dr. Zernicka-Goetz says. “We can now use this model system to assess the function of those genes.”
A version of this article first appeared on WebMD.com.
The role of repeat uterine curettage in postmolar gestational trophoblastic neoplasia
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.
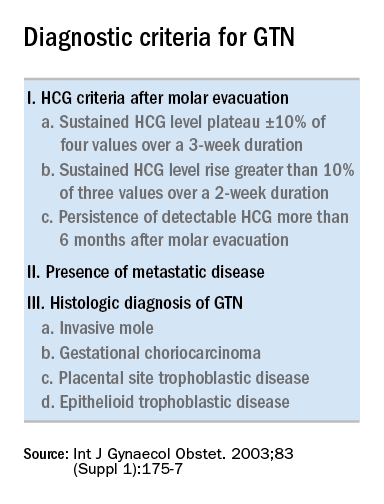
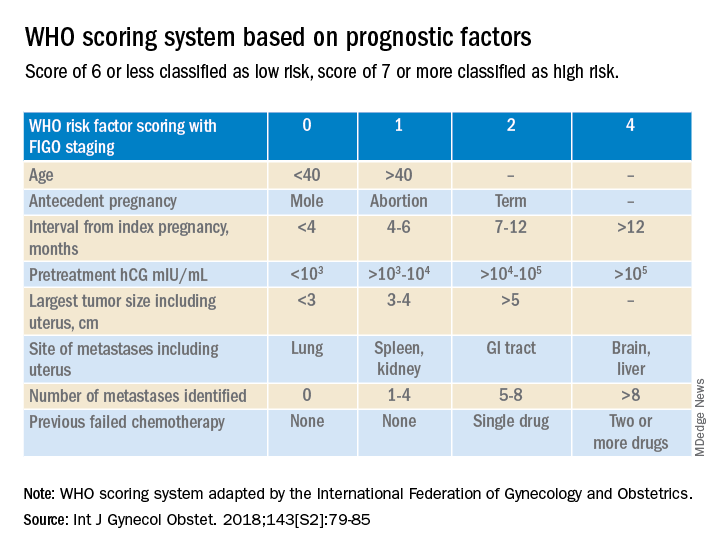
The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.


The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.


The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.




