User login
Exercise may counteract genetics for gestational diabetes
Women giving birth for the first time have significantly higher odds of developing gestational diabetes if they have a high polygenic risk score (PRS) and low physical activity, new data suggest.
Researchers, led by Kymberleigh A. Pagel, PhD, with the department of computer science, Indiana University, Bloomington, concluded that physical activity early in pregnancy is associated with reduced risk of gestational diabetes and may help women who are at high risk because of genetic predisposition, age, family history of diabetes, and body mass index.
The researchers included 3,533 women in the analysis (average age, 28.6 years) which was a subcohort of a larger study. They found that physical activity’s association with lower gestational diabetes risk “was particularly significant in individuals who were genetically predisposed to diabetes through PRS or family history,” the authors wrote.
Women with high PRS and low level of physical activity had three times the odds of developing gestational diabetes (odds ratio, 3.4; 95% confidence interval, 2.3-5.3).
Those with high PRS and moderate to high activity levels in early pregnancy (metabolic equivalents of task [METs] of at least 450) had gestational diabetes risk similar to that of the general population, according to the researchers.
The findings were published in JAMA Network Open.
Maisa Feghali, MD, a maternal-fetal specialist at the University of Pittsburgh Medical Center, who was not part of the study, said in an interview she found the link of physical activity and compensation for high predisposition to gestational diabetes most interesting.
“That’s interesting because a lot of studies that have looked at prevention of gestational diabetes either through limited weight gain or through some form of counseling on physical activity have not really shown any benefit,” she noted. “It might just be it’s not just one size fits all and it may be that physical activity is mostly beneficial in those with a high predisposition.”
Research in this area is particularly important as 7% of pregnancies in the United States each year are affected by gestational diabetes and the risk for developing type 2 diabetes “has doubled in the past decade among patients with GD [gestational diabetes],” the authors wrote.
Researchers looked at risks for gestational diabetes in high-risk subgroups, including women who had a body mass index of more than 25 kg/m2 or were at least 35 years old. In that group, women who were either in the in the top 25th percentile for PRS or had low physical activity (METs less than 450) had from 25% to 75% greater risk of developing gestational diabetes.
The findings are consistent with previous research and suggest exercise interventions may be important in improving pregnancy outcomes, the authors wrote.
Christina Han, MD, division director for maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, pointed out several limitations of the study, however.
One of the biggest limitations, she said, was that “they excluded two-thirds of the original study. Essentially, they took only Caucasian [White] patients, which is about one-third of the study.” Additionally, the cohort was made up of people who had never had babies.
“Lots of our gestational diabetes patients are not first-time moms, so this makes the generalizability of the study very limited,” Dr. Han said.
She added that none of the sites where the study was conducted were in the South or Northwest, which also adds questions about generalizability.
Dr. Feghali and Dr. Han reported no relevant financial relationships.
Women giving birth for the first time have significantly higher odds of developing gestational diabetes if they have a high polygenic risk score (PRS) and low physical activity, new data suggest.
Researchers, led by Kymberleigh A. Pagel, PhD, with the department of computer science, Indiana University, Bloomington, concluded that physical activity early in pregnancy is associated with reduced risk of gestational diabetes and may help women who are at high risk because of genetic predisposition, age, family history of diabetes, and body mass index.
The researchers included 3,533 women in the analysis (average age, 28.6 years) which was a subcohort of a larger study. They found that physical activity’s association with lower gestational diabetes risk “was particularly significant in individuals who were genetically predisposed to diabetes through PRS or family history,” the authors wrote.
Women with high PRS and low level of physical activity had three times the odds of developing gestational diabetes (odds ratio, 3.4; 95% confidence interval, 2.3-5.3).
Those with high PRS and moderate to high activity levels in early pregnancy (metabolic equivalents of task [METs] of at least 450) had gestational diabetes risk similar to that of the general population, according to the researchers.
The findings were published in JAMA Network Open.
Maisa Feghali, MD, a maternal-fetal specialist at the University of Pittsburgh Medical Center, who was not part of the study, said in an interview she found the link of physical activity and compensation for high predisposition to gestational diabetes most interesting.
“That’s interesting because a lot of studies that have looked at prevention of gestational diabetes either through limited weight gain or through some form of counseling on physical activity have not really shown any benefit,” she noted. “It might just be it’s not just one size fits all and it may be that physical activity is mostly beneficial in those with a high predisposition.”
Research in this area is particularly important as 7% of pregnancies in the United States each year are affected by gestational diabetes and the risk for developing type 2 diabetes “has doubled in the past decade among patients with GD [gestational diabetes],” the authors wrote.
Researchers looked at risks for gestational diabetes in high-risk subgroups, including women who had a body mass index of more than 25 kg/m2 or were at least 35 years old. In that group, women who were either in the in the top 25th percentile for PRS or had low physical activity (METs less than 450) had from 25% to 75% greater risk of developing gestational diabetes.
The findings are consistent with previous research and suggest exercise interventions may be important in improving pregnancy outcomes, the authors wrote.
Christina Han, MD, division director for maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, pointed out several limitations of the study, however.
One of the biggest limitations, she said, was that “they excluded two-thirds of the original study. Essentially, they took only Caucasian [White] patients, which is about one-third of the study.” Additionally, the cohort was made up of people who had never had babies.
“Lots of our gestational diabetes patients are not first-time moms, so this makes the generalizability of the study very limited,” Dr. Han said.
She added that none of the sites where the study was conducted were in the South or Northwest, which also adds questions about generalizability.
Dr. Feghali and Dr. Han reported no relevant financial relationships.
Women giving birth for the first time have significantly higher odds of developing gestational diabetes if they have a high polygenic risk score (PRS) and low physical activity, new data suggest.
Researchers, led by Kymberleigh A. Pagel, PhD, with the department of computer science, Indiana University, Bloomington, concluded that physical activity early in pregnancy is associated with reduced risk of gestational diabetes and may help women who are at high risk because of genetic predisposition, age, family history of diabetes, and body mass index.
The researchers included 3,533 women in the analysis (average age, 28.6 years) which was a subcohort of a larger study. They found that physical activity’s association with lower gestational diabetes risk “was particularly significant in individuals who were genetically predisposed to diabetes through PRS or family history,” the authors wrote.
Women with high PRS and low level of physical activity had three times the odds of developing gestational diabetes (odds ratio, 3.4; 95% confidence interval, 2.3-5.3).
Those with high PRS and moderate to high activity levels in early pregnancy (metabolic equivalents of task [METs] of at least 450) had gestational diabetes risk similar to that of the general population, according to the researchers.
The findings were published in JAMA Network Open.
Maisa Feghali, MD, a maternal-fetal specialist at the University of Pittsburgh Medical Center, who was not part of the study, said in an interview she found the link of physical activity and compensation for high predisposition to gestational diabetes most interesting.
“That’s interesting because a lot of studies that have looked at prevention of gestational diabetes either through limited weight gain or through some form of counseling on physical activity have not really shown any benefit,” she noted. “It might just be it’s not just one size fits all and it may be that physical activity is mostly beneficial in those with a high predisposition.”
Research in this area is particularly important as 7% of pregnancies in the United States each year are affected by gestational diabetes and the risk for developing type 2 diabetes “has doubled in the past decade among patients with GD [gestational diabetes],” the authors wrote.
Researchers looked at risks for gestational diabetes in high-risk subgroups, including women who had a body mass index of more than 25 kg/m2 or were at least 35 years old. In that group, women who were either in the in the top 25th percentile for PRS or had low physical activity (METs less than 450) had from 25% to 75% greater risk of developing gestational diabetes.
The findings are consistent with previous research and suggest exercise interventions may be important in improving pregnancy outcomes, the authors wrote.
Christina Han, MD, division director for maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, pointed out several limitations of the study, however.
One of the biggest limitations, she said, was that “they excluded two-thirds of the original study. Essentially, they took only Caucasian [White] patients, which is about one-third of the study.” Additionally, the cohort was made up of people who had never had babies.
“Lots of our gestational diabetes patients are not first-time moms, so this makes the generalizability of the study very limited,” Dr. Han said.
She added that none of the sites where the study was conducted were in the South or Northwest, which also adds questions about generalizability.
Dr. Feghali and Dr. Han reported no relevant financial relationships.
FROM JAMA NETWORK OPEN
Hormonal therapy a safe, long term option for older women with recalcitrant acne
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
PORTLAND, ORE. – During her dermatology residency training at the University of California, Irvine, Medical Center, Jenny Murase, MD, remembers hearing a colleague say that her most angry patients of the day were adult women with recalcitrant acne who present to the clinic with questions like, “My skin has been clear my whole life! What’s going on?”
Such . In fact, 82% fail multiple courses of systemic antibiotics and 32% relapse after using isotretinoin, Dr. Murase, director of medical dermatology consultative services and patch testing at the Palo Alto Foundation Medical Group, said at the annual meeting of the Pacific Dermatologic Association.
In her clinical experience, hormonal therapy is a safe long-term option for recalcitrant acne in postmenarcheal females over the age of 14. “Although oral antibiotics are going to be superior to hormonal therapy in the first month or two, when you get to about six months, they have equivalent efficacy,” she said.
Telltale signs of acne associated with androgen excess include the development of nodulocystic papules along the jawline and small comedones over the forehead. Female patients with acne may request that labs be ordered to check their hormone levels, but that often is not necessary, according to Dr. Murase, who is also associate clinical professor of dermatology at the University of California, San Francisco. “There aren’t strict guidelines to indicate when you should perform hormonal testing, but warning signs that warrant further evaluation include hirsutism, androgenetic alopecia, virilization, infertility, oligomenorrhea or amenorrhea, and sudden onset of severe acne. The most common situation that warrants hormonal testing is polycystic ovary syndrome (PCOS).”
When there is a strong suspicion for hyperandrogenism, essential labs include free and total testosterone. Free testosterone is commonly elevated in patients with PCOS and total testosterone levels over 200 ng/dL is suggestive of an ovarian tumor. Other essential labs include 17-hyydroxyprogesterone (values greater than 200 ng/dL indicate congenital adrenal hyperplasia), and dehydroepiandrosterone sulfate (DHEA-S); levels over 8,000 mcg/dL indicate an adrenal tumor, while levels in the 4,000-8,000 mcg/dL range indicate congenital adrenal hyperplasia.
Helpful lab tests to consider include the ratio of luteinizing hormone to follicle-stimulating hormone; a 3:1 ratio or greater is suggestive for PCOS. “Ordering a prolactin level can also help, especially if patients are describing issues with headaches, which could indicate a pituitary tumor,” Dr. Murase added. Measuring sex hormone binding globulin (SHBG) levels can also be helpful. “If a patient has been on oral contraceptives for a long time, it increases their SHBG,” which, in older women, she said, “is inversely related to the development of type 2 diabetes.”
All labs for hyperandrogenism should be performed early in the morning on day 3 of the patient’s menstrual cycle. “If patients are on some kind of hormonal therapy, they need to be off of it for at least 6 weeks in order for you get a relevant test,” she said. Other relevant labs to consider include fasting glucose and lipids, cortisol, and thyroid-stimulating hormone.
Oral contraceptives
Estrogen contained in oral contraceptives (OCs) provides the most benefit to acne patients. “It reduces sebum production, decreases free testosterone and DHEA-S by stimulating SHBG synthesis in the liver, inhibits 5-alpha-reductase, which decreases peripheral testosterone conversion, and it decreases the production of ovarian and adrenal androgens,” Dr. Murase explained. “On average, you can get about 40%-70% reduction of lesion count, which is pretty good.”
Progestins with low androgenetic activity are the most helpful for acne, including norgestimate, desogestrel, and drospirenone. FDA-approved OC options include Ortho Tri-Cyclen, EstroStep, Yaz, and Beyaz. None has data showing superior efficacy.
No Pap smear or pelvic exam is required when prescribing OCs, but the risk of clotting should be discussed with patients. According to Dr. Murase, the risk of deep vein thrombosis (DVT) at baseline is about 1 per 10,000 woman-years, while the risk of DVT after 1 year on an OC is 3.4 per 10,000 years.
“This is a very mild increased risk that we’re talking about, but it is relevant in smokers, in those with hypertension, and in those who are diabetic,” she said. As for the risk of cancer associated with the use of OCs, a large collaborative study found a relative risk of 1.24 for developing breast cancer (not dose or duration related), but a risk reduction for endometrial, colorectal, and ovarian cancer.
The most common side effects associated with OCs are unscheduled bleeding, nausea, breast tenderness, and possible weight gain. Concomitant antibiotics can be used, with the exception of CYP3A4 inducers, such as rifampin. “That’s the main antibiotic we have to worry about that could affect the efficacy of the birth control pill,” she said. “It accounts for about three-quarters of pregnancies on antibiotics.”
Tetracyclines do not appear to increase the rate of birth defects with incidental first-trimester exposure, and data are reassuring but “tetracycline should be stopped within the first trimester as soon as the patient discovers she is pregnant,” Dr. Murase said.
Contraindications for OCs include being pregnant or breastfeeding; history of stroke, venous thromboembolism, or MI; history of smoking and being over age 35; uncontrolled hypertension; migraines with focal symptoms/aura; current or past breast cancer; hypercholesterolemia; diabetes with end-organ damage or having diabetes over age 35; liver issues such as a tumor, viral hepatitis, or cirrhosis; and a history of major surgery with prolonged immobilization.
Spironolactone
Another treatment option is spironolactone, a potassium-sparing diuretic that blocks aldosterone at a dose of 25 mg/day. At doses of 50-100 mg/day, it blocks androgen. “It can be used in combination with an oral contraceptive, with the rates of efficacy reported to range between 33% and 85%,” Dr. Murase said.
Spironolactone can also reduce hirsutism, improve androgenetic alopecia, and lower blood pressure by about 5 mm Hg systolic and 2.5 mm Hg diastolic. Dr. Murase usually checks blood pressure in patients, and “only if they’re really low I’ll talk about the potential for postural hypotension and the fact that you can get a little bit dizzy when going from a position of lying down to standing up.” Potassium levels should be checked at baseline and 4 weeks in patients older than age 46, in those with cardiac and/or renal disease, or in those on concomitant drospirenone or a third-generation progestin.
Spironolactone is classified as a pregnancy category D drug that could compromise the genital development of a male fetus. “So the onus is on us as providers to have the conversation with our patient,” she said. “If you’re putting a patient on spironolactone and they are of child-bearing age, you need to make sure that you’ve had the conversation with them about the fact that they should not get pregnant while on the medicine.”
Spironolactone also has a boxed warning citing the development of benign tumors in animal studies. That warning is based on studies in rats at doses of 10-150 mg/kg per day, “which is an extremely high dose and would never be given in humans,” said Dr. Murase, who has coauthored CME content regarding the safety of dermatologic medications in pregnancy and lactation.
In humans, there has been no evidence of the development of benign tumors associated with spironolactone therapy, and “there has been a decreased risk of prostate cancer and no association with its use and the development of breast, ovarian, bladder, kidney, gastric, or esophageal cancer,” she said.
Dr. Murase noted that during pregnancy, first-line oral antibiotics include amoxicillin for acne rosacea and cefadroxil for acne vulgaris. Macrolides are a second-line choice because of an increase in atrial/ventricular septal defects and pyloric stenosis that have been reported with first-trimester exposure.
“Erythromycin is the preferred choice over azithromycin and clarithromycin because it has the most data, [but] erythromycin estolate has been associated with increased AST levels in the second trimester,” she said. “It occurs in about 10% of cases and is reversible. Erythromycin base and erythromycin ethylsuccinate do not have this risk, and those are preferable.”
Dr. Murase disclosed that she has been a paid speaker of unbranded medical content for Regeneron and UCB. She is also a member of the advisory board for Leo Pharma, Eli Lilly, UCB, and Genzyme/Sanofi.
AT PDA 2022
New ovulatory disorder classifications from FIGO replace 50-year-old system
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
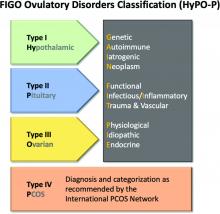
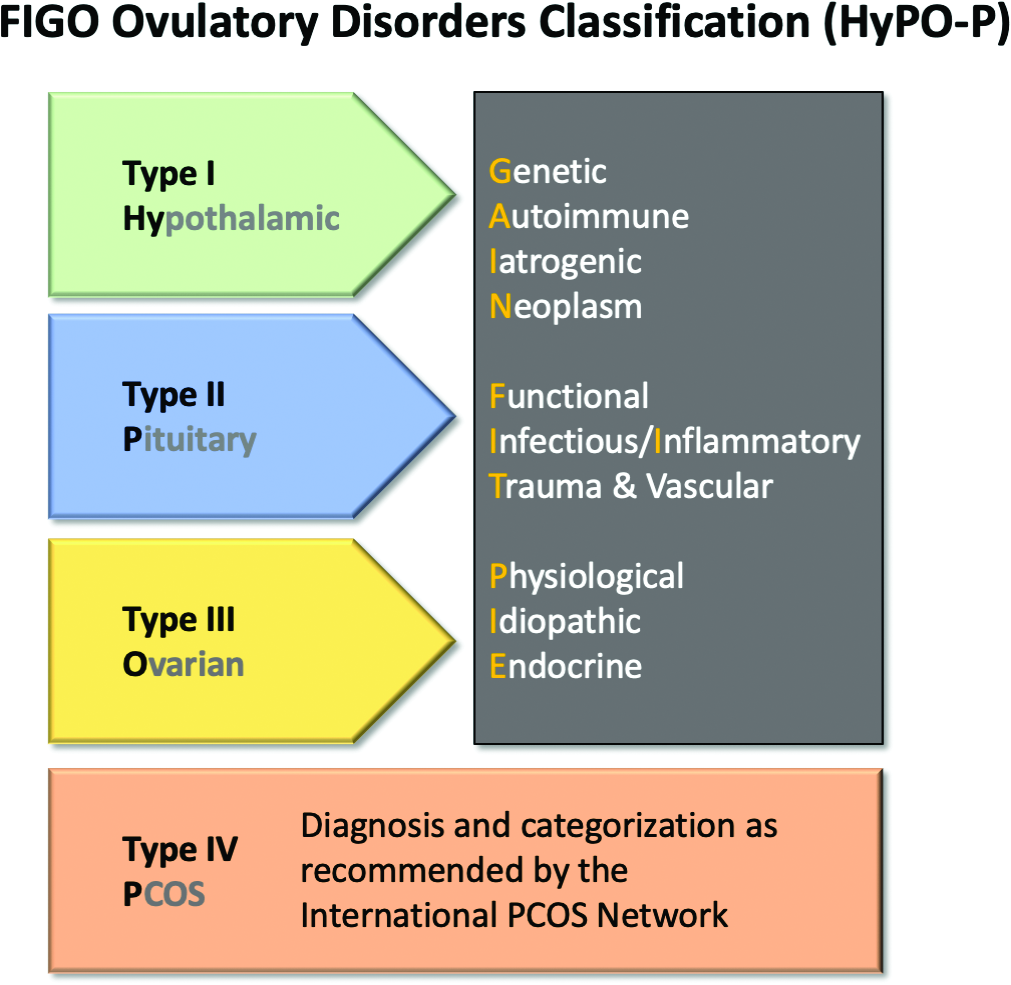
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
The first major revision in the systematic description of ovulatory disorders in nearly 50 years has been proposed by a consensus of experts organized by the International Federation of Gynecology and Obstetrics.
“The FIGO HyPO-P system for the classification of ovulatory disorders is submitted for consideration as a worldwide standard,” according to the writing committee, who published their methodology and their proposed applications in the International Journal of Gynecology and Obstetrics.
The classification system was created to replace the much-modified World Health Organization system first described in 1973. Since that time, many modifications have been proposed to accommodate advances in imaging and new information about underlying pathologies, but there has been no subsequent authoritative reference with these modifications or any other newer organizing system.
The new consensus was developed under the aegis of FIGO, but the development group consisted of representatives from national organizations and the major subspecialty societies. Recognized experts in ovulatory disorders and representatives from lay advocacy organizations also participated.
The HyPO-P system is based largely on anatomy. The acronym refers to ovulatory disorders related to the hypothalamus (type I), the pituitary (type II), and the ovary (type III).
Polycystic ovary syndrome (PCOS), one of the most common ovulatory disorders, was given a separate category (type IV) because of its complexity as well as the fact that PCOS is a heterogeneous systemic disorder with manifestations not limited to an impact on ovarian function.
As the first level of classification, three of the four primary categories (I-III) focus attention on the dominant anatomic source of the change in ovulatory function. The original WHO classification system identified as many as seven major groups, but they were based primarily on assays for gonadotropins and estradiol.
The new system “provides a different structure for determining the diagnosis. Blood tests are not a necessary first step,” explained Malcolm G. Munro, MD, clinical professor, department of obstetrics and gynecology, University of California, Los Angeles. Dr. Munro was the first author of the publication.
The classification system “is not as focused on the specific steps for investigation of ovulatory dysfunction as much as it explains how to structure an investigation of the girl or woman with an ovulatory disorder and then how to characterize the underlying cause,” Dr. Munro said in an interview. “It is designed to allow everyone, whether clinicians, researchers, or patients, to speak the same language.”
New system employs four categories
The four primary categories provide just the first level of classification. The next step is encapsulated in the GAIN-FIT-PIE acronym, which frames the presumed or documented categories of etiologies for the primary categories. GAIN stands for genetic, autoimmune, iatrogenic, or neoplasm etiologies. FIT stands for functional, infectious/inflammatory, or trauma and vascular etiologies. PIE stands for physiological, idiopathic, and endocrine etiologies.
By this methodology, a patient with irregular menses, galactorrhea, and elevated prolactin and an MRI showing a pituitary tumor would be identified a type 2-N, signifying pituitary (type 2) involvement with a neoplasm (N).
A third level of classification permits specific diagnostic entities to be named, allowing the patient in the example above to receive a diagnosis of a prolactin-secreting adenoma.
Not all etiologies can be identified with current diagnostic studies, even assuming clinicians have access to the resources, such as advanced imaging, that will increase diagnostic yield. As a result, the authors acknowledged that the classification system will be “aspirational” in at least some patients, but the structure of this system is expected to lead to greater precision in understanding the causes and defining features of ovulatory disorders, which, in turn, might facilitate new research initiatives.
In the published report, diagnostic protocols based on symptoms were described as being “beyond the spectrum” of this initial description. Rather, Dr. Munro explained that the most important contribution of this new classification system are standardization and communication. The system will be amenable for educating trainees and patients, for communicating between clinicians, and as a framework for research where investigators focus on more homogeneous populations of patients.
“There are many causes of ovulatory disorders that are not related to ovarian function. This is one message. Another is that ovulatory disorders are not binary. They occur on a spectrum. These range from transient instances of delayed or failed ovulation to chronic anovulation,” he said.
The new system is “ a welcome update,” according to Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, both in Orlando.
Dr. Trolice pointed to the clinical value of placing PCOS in a separate category. He noted that it affects 8%-13% of women, making it the most common single cause of ovulatory dysfunction.
“Another area that required clarification from prior WHO classifications was hyperprolactinemia, which is now placed in the type II category,” Dr. Trolice said in an interview.
Better terminology can help address a complex set of disorders with multiple causes and variable manifestations.
“In the evaluation of ovulation dysfunction, it is important to remember that regular menstrual intervals do not ensure ovulation,” Dr. Trolice pointed out. Even though a serum progesterone level of higher than 3 ng/mL is one of the simplest laboratory markers for ovulation, this level, he noted, “can vary through the luteal phase and even throughout the day.”
The proposed classification system, while providing a framework for describing ovulatory disorders, is designed to be adaptable, permitting advances in the understanding of the causes of ovulatory dysfunction, in the diagnosis of the causes, and in the treatments to be incorporated.
“No system should be considered permanent,” according to Dr. Munro and his coauthors. “Review and careful modification and revision should be carried out regularly.”
Dr. Munro reports financial relationships with AbbVie, American Regent, Daiichi Sankyo, Hologic, Myovant, and Pharmacosmos. Dr. Trolice reports no potential conflicts of interest.
FROM INTERNATIONAL JOURNAL OF GYNECOLOGY AND OBSTETRICS
Increased risk of dyspareunia following cesarean section
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.
There is no evidence to support postulated associations between mode of delivery and subsequent maternal sexual enjoyment or frequency of intercourse, according to a new study from the University of Bristol (England). However, cesarean section was shown to be associated with a 74% increased risk of dyspareunia, and this was not necessarily due to abdominal scarring, the researchers said.
A team from the University of Bristol and the Karolinska Institutet in Sweden used data from participants in the Avon Longitudinal Study of Parents and Children, a prospective longitudinal birth cohort study also dubbed “Children of the 90s” and involving more than 14,000 women in the United Kingdom who were pregnant in 1991 and 1992. The study has been following the health and development of the parents, their children, and now their grandchildren in detail ever since.
The new study, published in BJOG, aimed to assess whether cesarean section maintains sexual well-being compared with vaginal delivery, as has been suggested to occur because of the reduced risk of genital damage – less chance of tearing – and the maintenance of vaginal tone. There is some evidence that cesarean section is associated with an increased risk of sexual problems such as dyspareunia, but few studies have looked at the postbirth period long term.
Mode of delivery was abstracted from routine obstetric records and recorded as one of either spontaneous vaginal delivery (SVD), cesarean section, assisted breech, breech extraction, forceps, or vacuum extraction. Women whose records showed “other” as mode of delivery or whose notes contained conflicting modes of delivery were excluded.
Self-reported questionnaires asking about general health and lifestyle and including questions relating to sexual enjoyment and frequency were collected at 33 months and at 5, 12, and 18 years postpartum. Women were asked if they enjoyed sexual intercourse, with possible responses of:
- Yes, very much.
- Yes, somewhat.
- No, not a lot.
- No, not at all.
- No sex at the moment.
Possible sexual frequency responses were:
- Not at all.
- Less than once a month.
- 1-3 times a month.
- About once a week.
- 2-4 times a week.
- 5 or more times a week.
First study to look at sexual frequency
The team noted that theirs is the first study investigating the association of mode of delivery with sexual frequency. “Although it may be less important for well-being than sexual enjoyment or sex-related pain, it is an important measure to observe alongside other sexual outcomes,” they said.
Separately, sex-related pain, in the vagina during sex or elsewhere after sex, was assessed once, at 11 years post partum.
The data showed that women who had a cesarean section (11% of the sample) tended to be older than those who had vaginal delivery, with a higher mean body mass index (24.2 versus 22.8 kg/m2), and were more likely to be nulliparous at the time of the index pregnancy (54% versus 44%).
There was no significant difference between cesarean section and vaginal delivery in terms of responses for sexual enjoyment or frequency at any time after childbirth, the authors said. Nor, in adjusted models, was there evidence of associations between the type of vaginal delivery and sexual enjoyment or frequency outcomes.
Pain during sex increased more than a decade after cesarean
However, while the majority of respondents reported no intercourse-related pain, those who delivered via cesarean were more likely than those who gave birth vaginally to report sex-related pain at 11 years post partum. This was specifically an elevated incidence of pain in the vagina during sex, with an odds ratio of 1.74 (95% confidence interval, 1.46-2.08) in the adjusted model. This finding was consistent for emergency and elective cesarean section separately – both types were associated with increased dyspareunia, compared with vaginal delivery.
The dataset did not include measures of individual prenatal sex-related pain and, therefore, “it is unknown from this study whether Caesarean section causes sex-related pain, as suggested by the findings, or whether prenatal sex-related pain predicts both Caesarean section and postnatal sex-related pain,” the researchers said.
“Longitudinal data on sex-related pain need to be collected both before and after parturition,” they recommend, to clarify the direction of a possible effect between cesarean section and dyspareunia.
Cesarean does not protect against sexual dysfunction
Meanwhile, “For women considering a planned Caesarean section in an uncomplicated pregnancy, evidence suggesting that Caesarean section may not protect against sexual dysfunction may help inform their decision-making in the antenatal period.”
Lead author Flo Martin, a PhD student in epidemiology at the University of Bristol, said: “Rates of Caesarean section have been rising over the last 20 years due to many contributing factors and, importantly, it has been suggested that Caesarean section maintains sexual wellbeing compared with vaginal delivery. It is crucial that a whole range of maternal and foetal outcomes following Caesarean section are investigated, including sexual wellbeing, to appropriately inform decision-making both pre- and postnatally.
“This research provides expectant mothers, as well as women who have given birth, with really important information and demonstrates that there was no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via Caesarean section and those who delivered vaginally. It also suggests that Caesarean section may not help protect against sexual dysfunction, as previously thought, where sex-related pain was higher among women who gave birth via Caesarean section more than 10 years postpartum.”
Asked to comment on the research, Dr. Leila Frodsham, consultant gynecologist and spokesperson for the Royal College of Obstetricians and Gynaecologists, told this news organization: “Sexual pain disorders affect 7.5% of women of all ages, but there are peaks: during the start of sexual activity, if subfertility is an issue, after childbirth, and in the peri/menopause. It can be up to three times more prevalent at these peak times.
“Many women with sexual pain are worried when they consider starting a family and request a Caesarean birth to reduce risk of worsening their pain. However, this study has demonstrated that a Caesarean birth is associated with increased sexual pain longer term, which is very useful for helping women to plan their births.
“While more research about postpartum sexual wellbeing is needed, the findings of this study are reassuring to those who are pregnant as it found no difference in the enjoyment or frequency of sex in the years after a vaginal or a Caesarean birth.
“Most women in the U.K. recover well whether they have a vaginal or a Caesarean birth. Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences.”
A version of this article first appeared on Medscape UK.
State of the science in PCOS: Emerging neuroendocrine involvement driving research
Polycystic ovary syndrome (PCOS) affects an estimated 8%-13% of women, and yet “it has been quite a black box for many years,” as Margo Hudson, MD, an assistant professor of endocrinology, diabetes, and hypertension at Harvard Medical School, Boston, puts it. That black box encompasses not only uncertainty about the etiology and pathophysiology of the condition but even what constitutes a diagnosis.
Even the international guidelines on PCOS management endorsed by the American Society for Reproductive Medicine – a document developed over 15 months with the input of 37 medical organizations covering 71 countries – notes that PCOS diagnosis is “controversial and assessment and management are inconsistent.” The result, the guidelines note, is that “the needs of women with PCOS are not being adequately met.”
One of the earliest diagnostic criteria, defined in 1990 by the National Institutes of Health, required only hyperandrogenism and irregular menstruation. Then the 2003 Rotterdam Criteria added presence of polycystic ovaries on ultrasound as a third criterion. Then the Androgen Excess Society determined that PCOS required presence of hyperandrogenism with either polycystic ovaries or oligo/amenorrhea anovulation. Yet the Endocrine Society notes that excess androgen levels are seen in 60%-80% of those with PCOS, suggesting it’s not an essential requirement for diagnosis, leaving most to diagnose it in people who have two of the three key criteria. The only real agreement on diagnosis is the need to eliminate other potential diagnoses first, making PCOS always a diagnosis of exclusion.
Further, though PCOS is known as the leading cause of infertility in women, it is more than a reproductive condition, with metabolic and psychological features as well. Then there is the range of comorbidities, none of which occur in all patients with PCOS but all of which occur in a majority and which are themselves interrelated. Insulin resistance is a common feature, occurring in 50%-70% of people with PCOS. Accordingly, metabolic syndrome occurs in at least a third of people with PCOS and type 2 diabetes prevalence is higher in those with PCOS as well.
Obesity occurs in an estimated 80% of women with PCOS in the United States, though it affects only about 50% of women with PCOS outside the United States, and those with PCOS have an increased risk of hypertension. Mood disorders, particularly anxiety and depression but also, to a lesser extent, bipolar disorder and obsessive-compulsive disorder, are more likely in people with PCOS. And given that these comorbidities are all cardiovascular risk factors, it’s unsurprising that recent studies are finding those with PCOS to be at greater risk for cardiometabolic disease and major cardiovascular events.
“The reality is that PCOS is a heterogenous entity. It’s not one thing – it’s a syndrome,” Lubna Pal, MBBS, a professor of ob.gyn. and director of the PCOS Program at Yale University, New Haven, Conn., said in an interview. A whole host of factors are likely playing a role in the causes of PCOS, and those factors interact differently within different people. “We’re looking at things like lipid metabolism, fetal origins, the gut microbiome, genetics, epigenetics, and then dietary and environmental factors,” Nichole Tyson, MD, division chief of pediatric and adolescent gynecology and a clinical associate professor at Stanford (Calif.) Medicine Children’s Health, said in an interview. And most studies have identified associations that may or may not be causal. Take, for example, endocrine disruptors. BPA levels have been shown to be higher in women with PCOS than women without, but that correlation may or may not be related to the etiology of the condition.
The hypothalamic-pituitary-gonadal axis
In trying to understand the pathophysiology of the condition, much of the latest research has zeroed in on potential mechanisms in the hypothalamic-pituitary-gonadal axis. “A consistent feature of PCOS is disordered gonadotropin secretion with elevated mean LH [luteinizing hormone], low or low normal FSH [follicle-stimulating hormone], and a persistently rapid frequency of GnRH [gonadotropin-releasing hormone] pulse secretion,” wrote authors of a scientific statement on aspects of PCOS.
“I think the balance is heading more to central neurologic control of the reproductive system and that disturbances there impact the GnRH cells in the hypothalamus, which then go on to give us the findings that we can measure peripherally with the LH-FSH ratio,” Dr. Hudson said in an interview.
The increased LH levels are thought to be a major driver of increased androgen levels. Current thinking suggests that the primary driver of increased LH is GnRH pulsatility, supported not only by human studies but by animal models as well. This leads to the question of what drives GnRH dysregulation. One hypothesis posits that GABA neurons play a role here, given findings that GABA levels in cerebrospinal fluid were higher in women with PCOS than those with normal ovulation.
But the culprit garnering the most attention is kisspeptin, a protein encoded by the KISS1 gene that stimulates GnRH neurons and has been linked to regulation of LH and FSH secretion. Kisspeptin, along with neurokinin B and dynorphin, is part of the triumvirate that comprises KNDy neurons, also recently implicated in menopausal vasomotor symptoms. Multiple systematic reviewsand meta-analyses have found a correlation between higher kisspeptin levels in the blood and higher circulating LH levels, regardless of body mass index. While kisspeptin is expressed in several tissues, including liver, pancreas, gonad, and adipose, it’s neural kisspeptin signaling that appears most likely to play a role in activating GnRH hormones and disrupting normal function of the hypothalamic-pituitary-gonadal axis.
But as noted, in at least one systematic review of kisspeptin and PCOS, “findings from animal studies suggest that kisspeptin levels are not increased in all subtypes of PCOS.” And another review found “altered” levels of kisspeptin levels in non-PCOS patients who had obesity, potentially raising questions about any associations between kisspeptin and obesity or insulin resistance.
Remaining chicken-and-egg questions
A hallmark of PCOS has long been, and continues to be, the string of chicken-or-egg questions that plague understanding of it. One of these is how depression and anxiety fit into the etiology of PCOS. Exploring the role of specific neurons that may overstimulate GnRH pulsatility may hold clues to a common underlying mechanism for the involvement of depression and anxiety in patients with PCOS, Dr. Hudson speculated. While previous assumptions often attributed depression and anxiety in PCOS to the symptoms – such as thin scalp hair and increased facial hair, excess weight, acne, and irregular periods – Dr. Hudson pointed out that women can address many of these symptoms with laser hair removal, weight loss, acne treatment, and similar interventions, yet they still have a lot of underlying mental health issues.
It’s also unclear whether metabolic factors so common with PCOS, particularly insulin resistance and obesity, are a result of the condition or are contributors to it. Is insulin resistance contributing to dysregulation in the neurons that interferes with normal functioning of the hypothalamic-pituitary-adrenal axis? Is abnormal functioning along this axis contributing to insulin resistance? Or neither? Or both? Or does it depend? The authors of one paper wrote that “insulin may play both direct and indirect roles in the pathogenesis of androgen excess in PCOS,” since insulin can “stimulate ovarian androgen production” and “enhance ovarian growth and follicular cyst formation in rats.”
Dr. Pal noted that “obesity itself can evolve into a PCOS-like picture,” raising questions about whether obesity or insulin resistance might be part of the causal pathway to PCOS, or whether either can trigger its development in those genetically predisposed.
“Obesity does appear to exacerbate many aspects of the PCOS phenotype, particularly those risk factors related to metabolic syndrome,” wrote the authors of a scientific statement on aspects of PCOS, but they add that “it is currently debated whether obesity per se can cause PCOS.” While massive weight loss in those with PCOS and obesity has improved multiple reproductive and metabolic issues, it hasn’t resolved all of them, they write.
Dr. Hudson said she expects there’s “some degree of appetite dysregulation and metabolic dysregulation” that contributes, but then there are other women who don’t have much of an appetite or overeat and still struggle with their weight. Evidence has also found insulin resistance in women of normal weight with PCOS. “There may be some kind of metabolic dysregulation that they have at some level, and others are clearly bothered by overeating,” Dr. Hudson said.
Similarly, it’s not clear whether the recent discovery of increased cardiovascular risks in people with PCOS is a result of the comorbidities so common with PCOS, such as obesity, or whether an underlying mechanism links the cardiovascular risk and the dysregulation of hormones. Dr. Pal would argue that, again, it’s probably both, depending on the patient.
Then there is the key feature of hyperandrogenemia. “An outstanding debate is whether the elevated androgens in PCOS women are merely a downstream endocrine response to hyperactive GnRH and LH secretion driving the ovary, or do the elevated androgens themselves act in the brain (or pituitary) during development and/or adulthood to sculpt and maintain the hypersecretion of GnRH and LH?” wrote Eulalia A. Coutinho, PhD, and Alexander S. Kauffman, PhD, in a 2019 review of the brain’s role in PCOS.
These problems may be bidirectional or part of various feedback loops. Sleep apnea is more common in people with PCOS, Dr. Tyson noted, but sleep apnea is also linked to cardiovascular, metabolic, and depression risks, and depression can play a role in obesity, which increases the risk of obstructive sleep apnea. “So you’re in this vicious cycle,” Dr. Tyson said. That’s why she also believes it’s important to change the dialogue and perspective on PCOS, to reduce the stigma attached to it, and work with patients to empower them in treating its symptoms and reducing their risk of comorbidities.
Recent and upcoming changes in treatment
Current treatment of PCOS already changes according to the symptoms posing the greatest problems at each stage of a person’s life, Dr. Hudson said. Younger women tend to be more bothered about the cosmetic effects of PCOS, including hair growth patterns and acne, but as they grow out of adolescence and into their 20s and 30s, infertility becomes a bigger concern for many. Then, as they start approaching menopause, metabolic and cardiovascular issues take the lead, with more of a focus on lipids, diabetes risk, and heart health.
In some ways, management of PCOS hasn’t changed much in the past several decades, except in an increased awareness of the metabolic and cardiovascular risks, which has led to more frequent screening to catch potential conditions earlier in life. What has changed, however, is improvements in the treatments used for symptoms, such as expanded bariatric surgery options and GLP-1 agonists for treating obesity. Other examples include better options for menstrual management, such as new progesterone IUDs, and optimized fertility treatments, Dr. Tyson said.
“I think with more of these large-scale studies about the pathophysiology of PCOS and how it may look in different people and the different outcomes, we may be able to tailor our treatments even further,” Dr. Tyson said. She emphasized the importance of identifying the condition early, particularly in adolescents, even if it’s identifying young people at risk for the condition rather than actually having it yet.
Early identification “gives us this chance to do a lot of preventative care and motivate older teens to have a great lifestyle, work on their diet and exercise, and manage cardiovascular” risk factors, Dr. Tyson said.
“What we do know and recognize is that there’s so many spokes to this PCOS wheel that there really should be a multidisciplinary approach to care,” Dr. Tyson said. “When I think about who would be the real doctors for patients with PCOS, these would be gynecologists, endocrinologists, dermatologists, nutritionists, psychologists, sleep specialists, and primary care at a minimum.”
Dr. Pal worries that the label of PCOS leaves it in the laps of ob.gyns. whereas, “if it was called something else, everybody would be involved in being vigilant and managing those patients.” She frequently reiterated that the label of PCOS is less important than ensuring clinicians treat the symptoms that most bother the patient.
And even if kisspeptin does play a causal role in PCOS for some patients, it’s only a subset of individuals with PCOS who would benefit from therapies developed to target it. Given the complexity of the syndrome and its many manifestations, a “galaxy of pathways” are involved in different potential subtypes of the condition. “You can’t treat PCOS as one entity,” Dr. Pal said.
Still, Dr. Hudson is optimistic that the research into potential neuroendocrine contributions to PCOS will yield therapies that go beyond just managing symptoms.
“There aren’t a lot of treatments available yet, but there may be some on the horizon,” Dr. Hudson said. “We’re still in this very primitive stage in terms of therapeutics, where we’re only addressing specific symptoms, and we haven’t been able to really address the underlying cause because we haven’t understood it as well and because we don’t have therapies that can target it,” Dr. Hudson said. “But once there are therapies developed that will target some of these central mechanisms, I think it will change completely the approach to treating PCOS for patients.”
This story was updated on Sept. 6, 2022.
Polycystic ovary syndrome (PCOS) affects an estimated 8%-13% of women, and yet “it has been quite a black box for many years,” as Margo Hudson, MD, an assistant professor of endocrinology, diabetes, and hypertension at Harvard Medical School, Boston, puts it. That black box encompasses not only uncertainty about the etiology and pathophysiology of the condition but even what constitutes a diagnosis.
Even the international guidelines on PCOS management endorsed by the American Society for Reproductive Medicine – a document developed over 15 months with the input of 37 medical organizations covering 71 countries – notes that PCOS diagnosis is “controversial and assessment and management are inconsistent.” The result, the guidelines note, is that “the needs of women with PCOS are not being adequately met.”
One of the earliest diagnostic criteria, defined in 1990 by the National Institutes of Health, required only hyperandrogenism and irregular menstruation. Then the 2003 Rotterdam Criteria added presence of polycystic ovaries on ultrasound as a third criterion. Then the Androgen Excess Society determined that PCOS required presence of hyperandrogenism with either polycystic ovaries or oligo/amenorrhea anovulation. Yet the Endocrine Society notes that excess androgen levels are seen in 60%-80% of those with PCOS, suggesting it’s not an essential requirement for diagnosis, leaving most to diagnose it in people who have two of the three key criteria. The only real agreement on diagnosis is the need to eliminate other potential diagnoses first, making PCOS always a diagnosis of exclusion.
Further, though PCOS is known as the leading cause of infertility in women, it is more than a reproductive condition, with metabolic and psychological features as well. Then there is the range of comorbidities, none of which occur in all patients with PCOS but all of which occur in a majority and which are themselves interrelated. Insulin resistance is a common feature, occurring in 50%-70% of people with PCOS. Accordingly, metabolic syndrome occurs in at least a third of people with PCOS and type 2 diabetes prevalence is higher in those with PCOS as well.
Obesity occurs in an estimated 80% of women with PCOS in the United States, though it affects only about 50% of women with PCOS outside the United States, and those with PCOS have an increased risk of hypertension. Mood disorders, particularly anxiety and depression but also, to a lesser extent, bipolar disorder and obsessive-compulsive disorder, are more likely in people with PCOS. And given that these comorbidities are all cardiovascular risk factors, it’s unsurprising that recent studies are finding those with PCOS to be at greater risk for cardiometabolic disease and major cardiovascular events.
“The reality is that PCOS is a heterogenous entity. It’s not one thing – it’s a syndrome,” Lubna Pal, MBBS, a professor of ob.gyn. and director of the PCOS Program at Yale University, New Haven, Conn., said in an interview. A whole host of factors are likely playing a role in the causes of PCOS, and those factors interact differently within different people. “We’re looking at things like lipid metabolism, fetal origins, the gut microbiome, genetics, epigenetics, and then dietary and environmental factors,” Nichole Tyson, MD, division chief of pediatric and adolescent gynecology and a clinical associate professor at Stanford (Calif.) Medicine Children’s Health, said in an interview. And most studies have identified associations that may or may not be causal. Take, for example, endocrine disruptors. BPA levels have been shown to be higher in women with PCOS than women without, but that correlation may or may not be related to the etiology of the condition.
The hypothalamic-pituitary-gonadal axis
In trying to understand the pathophysiology of the condition, much of the latest research has zeroed in on potential mechanisms in the hypothalamic-pituitary-gonadal axis. “A consistent feature of PCOS is disordered gonadotropin secretion with elevated mean LH [luteinizing hormone], low or low normal FSH [follicle-stimulating hormone], and a persistently rapid frequency of GnRH [gonadotropin-releasing hormone] pulse secretion,” wrote authors of a scientific statement on aspects of PCOS.
“I think the balance is heading more to central neurologic control of the reproductive system and that disturbances there impact the GnRH cells in the hypothalamus, which then go on to give us the findings that we can measure peripherally with the LH-FSH ratio,” Dr. Hudson said in an interview.
The increased LH levels are thought to be a major driver of increased androgen levels. Current thinking suggests that the primary driver of increased LH is GnRH pulsatility, supported not only by human studies but by animal models as well. This leads to the question of what drives GnRH dysregulation. One hypothesis posits that GABA neurons play a role here, given findings that GABA levels in cerebrospinal fluid were higher in women with PCOS than those with normal ovulation.
But the culprit garnering the most attention is kisspeptin, a protein encoded by the KISS1 gene that stimulates GnRH neurons and has been linked to regulation of LH and FSH secretion. Kisspeptin, along with neurokinin B and dynorphin, is part of the triumvirate that comprises KNDy neurons, also recently implicated in menopausal vasomotor symptoms. Multiple systematic reviewsand meta-analyses have found a correlation between higher kisspeptin levels in the blood and higher circulating LH levels, regardless of body mass index. While kisspeptin is expressed in several tissues, including liver, pancreas, gonad, and adipose, it’s neural kisspeptin signaling that appears most likely to play a role in activating GnRH hormones and disrupting normal function of the hypothalamic-pituitary-gonadal axis.
But as noted, in at least one systematic review of kisspeptin and PCOS, “findings from animal studies suggest that kisspeptin levels are not increased in all subtypes of PCOS.” And another review found “altered” levels of kisspeptin levels in non-PCOS patients who had obesity, potentially raising questions about any associations between kisspeptin and obesity or insulin resistance.
Remaining chicken-and-egg questions
A hallmark of PCOS has long been, and continues to be, the string of chicken-or-egg questions that plague understanding of it. One of these is how depression and anxiety fit into the etiology of PCOS. Exploring the role of specific neurons that may overstimulate GnRH pulsatility may hold clues to a common underlying mechanism for the involvement of depression and anxiety in patients with PCOS, Dr. Hudson speculated. While previous assumptions often attributed depression and anxiety in PCOS to the symptoms – such as thin scalp hair and increased facial hair, excess weight, acne, and irregular periods – Dr. Hudson pointed out that women can address many of these symptoms with laser hair removal, weight loss, acne treatment, and similar interventions, yet they still have a lot of underlying mental health issues.
It’s also unclear whether metabolic factors so common with PCOS, particularly insulin resistance and obesity, are a result of the condition or are contributors to it. Is insulin resistance contributing to dysregulation in the neurons that interferes with normal functioning of the hypothalamic-pituitary-adrenal axis? Is abnormal functioning along this axis contributing to insulin resistance? Or neither? Or both? Or does it depend? The authors of one paper wrote that “insulin may play both direct and indirect roles in the pathogenesis of androgen excess in PCOS,” since insulin can “stimulate ovarian androgen production” and “enhance ovarian growth and follicular cyst formation in rats.”
Dr. Pal noted that “obesity itself can evolve into a PCOS-like picture,” raising questions about whether obesity or insulin resistance might be part of the causal pathway to PCOS, or whether either can trigger its development in those genetically predisposed.
“Obesity does appear to exacerbate many aspects of the PCOS phenotype, particularly those risk factors related to metabolic syndrome,” wrote the authors of a scientific statement on aspects of PCOS, but they add that “it is currently debated whether obesity per se can cause PCOS.” While massive weight loss in those with PCOS and obesity has improved multiple reproductive and metabolic issues, it hasn’t resolved all of them, they write.
Dr. Hudson said she expects there’s “some degree of appetite dysregulation and metabolic dysregulation” that contributes, but then there are other women who don’t have much of an appetite or overeat and still struggle with their weight. Evidence has also found insulin resistance in women of normal weight with PCOS. “There may be some kind of metabolic dysregulation that they have at some level, and others are clearly bothered by overeating,” Dr. Hudson said.
Similarly, it’s not clear whether the recent discovery of increased cardiovascular risks in people with PCOS is a result of the comorbidities so common with PCOS, such as obesity, or whether an underlying mechanism links the cardiovascular risk and the dysregulation of hormones. Dr. Pal would argue that, again, it’s probably both, depending on the patient.
Then there is the key feature of hyperandrogenemia. “An outstanding debate is whether the elevated androgens in PCOS women are merely a downstream endocrine response to hyperactive GnRH and LH secretion driving the ovary, or do the elevated androgens themselves act in the brain (or pituitary) during development and/or adulthood to sculpt and maintain the hypersecretion of GnRH and LH?” wrote Eulalia A. Coutinho, PhD, and Alexander S. Kauffman, PhD, in a 2019 review of the brain’s role in PCOS.
These problems may be bidirectional or part of various feedback loops. Sleep apnea is more common in people with PCOS, Dr. Tyson noted, but sleep apnea is also linked to cardiovascular, metabolic, and depression risks, and depression can play a role in obesity, which increases the risk of obstructive sleep apnea. “So you’re in this vicious cycle,” Dr. Tyson said. That’s why she also believes it’s important to change the dialogue and perspective on PCOS, to reduce the stigma attached to it, and work with patients to empower them in treating its symptoms and reducing their risk of comorbidities.
Recent and upcoming changes in treatment
Current treatment of PCOS already changes according to the symptoms posing the greatest problems at each stage of a person’s life, Dr. Hudson said. Younger women tend to be more bothered about the cosmetic effects of PCOS, including hair growth patterns and acne, but as they grow out of adolescence and into their 20s and 30s, infertility becomes a bigger concern for many. Then, as they start approaching menopause, metabolic and cardiovascular issues take the lead, with more of a focus on lipids, diabetes risk, and heart health.
In some ways, management of PCOS hasn’t changed much in the past several decades, except in an increased awareness of the metabolic and cardiovascular risks, which has led to more frequent screening to catch potential conditions earlier in life. What has changed, however, is improvements in the treatments used for symptoms, such as expanded bariatric surgery options and GLP-1 agonists for treating obesity. Other examples include better options for menstrual management, such as new progesterone IUDs, and optimized fertility treatments, Dr. Tyson said.
“I think with more of these large-scale studies about the pathophysiology of PCOS and how it may look in different people and the different outcomes, we may be able to tailor our treatments even further,” Dr. Tyson said. She emphasized the importance of identifying the condition early, particularly in adolescents, even if it’s identifying young people at risk for the condition rather than actually having it yet.
Early identification “gives us this chance to do a lot of preventative care and motivate older teens to have a great lifestyle, work on their diet and exercise, and manage cardiovascular” risk factors, Dr. Tyson said.
“What we do know and recognize is that there’s so many spokes to this PCOS wheel that there really should be a multidisciplinary approach to care,” Dr. Tyson said. “When I think about who would be the real doctors for patients with PCOS, these would be gynecologists, endocrinologists, dermatologists, nutritionists, psychologists, sleep specialists, and primary care at a minimum.”
Dr. Pal worries that the label of PCOS leaves it in the laps of ob.gyns. whereas, “if it was called something else, everybody would be involved in being vigilant and managing those patients.” She frequently reiterated that the label of PCOS is less important than ensuring clinicians treat the symptoms that most bother the patient.
And even if kisspeptin does play a causal role in PCOS for some patients, it’s only a subset of individuals with PCOS who would benefit from therapies developed to target it. Given the complexity of the syndrome and its many manifestations, a “galaxy of pathways” are involved in different potential subtypes of the condition. “You can’t treat PCOS as one entity,” Dr. Pal said.
Still, Dr. Hudson is optimistic that the research into potential neuroendocrine contributions to PCOS will yield therapies that go beyond just managing symptoms.
“There aren’t a lot of treatments available yet, but there may be some on the horizon,” Dr. Hudson said. “We’re still in this very primitive stage in terms of therapeutics, where we’re only addressing specific symptoms, and we haven’t been able to really address the underlying cause because we haven’t understood it as well and because we don’t have therapies that can target it,” Dr. Hudson said. “But once there are therapies developed that will target some of these central mechanisms, I think it will change completely the approach to treating PCOS for patients.”
This story was updated on Sept. 6, 2022.
Polycystic ovary syndrome (PCOS) affects an estimated 8%-13% of women, and yet “it has been quite a black box for many years,” as Margo Hudson, MD, an assistant professor of endocrinology, diabetes, and hypertension at Harvard Medical School, Boston, puts it. That black box encompasses not only uncertainty about the etiology and pathophysiology of the condition but even what constitutes a diagnosis.
Even the international guidelines on PCOS management endorsed by the American Society for Reproductive Medicine – a document developed over 15 months with the input of 37 medical organizations covering 71 countries – notes that PCOS diagnosis is “controversial and assessment and management are inconsistent.” The result, the guidelines note, is that “the needs of women with PCOS are not being adequately met.”
One of the earliest diagnostic criteria, defined in 1990 by the National Institutes of Health, required only hyperandrogenism and irregular menstruation. Then the 2003 Rotterdam Criteria added presence of polycystic ovaries on ultrasound as a third criterion. Then the Androgen Excess Society determined that PCOS required presence of hyperandrogenism with either polycystic ovaries or oligo/amenorrhea anovulation. Yet the Endocrine Society notes that excess androgen levels are seen in 60%-80% of those with PCOS, suggesting it’s not an essential requirement for diagnosis, leaving most to diagnose it in people who have two of the three key criteria. The only real agreement on diagnosis is the need to eliminate other potential diagnoses first, making PCOS always a diagnosis of exclusion.
Further, though PCOS is known as the leading cause of infertility in women, it is more than a reproductive condition, with metabolic and psychological features as well. Then there is the range of comorbidities, none of which occur in all patients with PCOS but all of which occur in a majority and which are themselves interrelated. Insulin resistance is a common feature, occurring in 50%-70% of people with PCOS. Accordingly, metabolic syndrome occurs in at least a third of people with PCOS and type 2 diabetes prevalence is higher in those with PCOS as well.
Obesity occurs in an estimated 80% of women with PCOS in the United States, though it affects only about 50% of women with PCOS outside the United States, and those with PCOS have an increased risk of hypertension. Mood disorders, particularly anxiety and depression but also, to a lesser extent, bipolar disorder and obsessive-compulsive disorder, are more likely in people with PCOS. And given that these comorbidities are all cardiovascular risk factors, it’s unsurprising that recent studies are finding those with PCOS to be at greater risk for cardiometabolic disease and major cardiovascular events.
“The reality is that PCOS is a heterogenous entity. It’s not one thing – it’s a syndrome,” Lubna Pal, MBBS, a professor of ob.gyn. and director of the PCOS Program at Yale University, New Haven, Conn., said in an interview. A whole host of factors are likely playing a role in the causes of PCOS, and those factors interact differently within different people. “We’re looking at things like lipid metabolism, fetal origins, the gut microbiome, genetics, epigenetics, and then dietary and environmental factors,” Nichole Tyson, MD, division chief of pediatric and adolescent gynecology and a clinical associate professor at Stanford (Calif.) Medicine Children’s Health, said in an interview. And most studies have identified associations that may or may not be causal. Take, for example, endocrine disruptors. BPA levels have been shown to be higher in women with PCOS than women without, but that correlation may or may not be related to the etiology of the condition.
The hypothalamic-pituitary-gonadal axis
In trying to understand the pathophysiology of the condition, much of the latest research has zeroed in on potential mechanisms in the hypothalamic-pituitary-gonadal axis. “A consistent feature of PCOS is disordered gonadotropin secretion with elevated mean LH [luteinizing hormone], low or low normal FSH [follicle-stimulating hormone], and a persistently rapid frequency of GnRH [gonadotropin-releasing hormone] pulse secretion,” wrote authors of a scientific statement on aspects of PCOS.
“I think the balance is heading more to central neurologic control of the reproductive system and that disturbances there impact the GnRH cells in the hypothalamus, which then go on to give us the findings that we can measure peripherally with the LH-FSH ratio,” Dr. Hudson said in an interview.
The increased LH levels are thought to be a major driver of increased androgen levels. Current thinking suggests that the primary driver of increased LH is GnRH pulsatility, supported not only by human studies but by animal models as well. This leads to the question of what drives GnRH dysregulation. One hypothesis posits that GABA neurons play a role here, given findings that GABA levels in cerebrospinal fluid were higher in women with PCOS than those with normal ovulation.
But the culprit garnering the most attention is kisspeptin, a protein encoded by the KISS1 gene that stimulates GnRH neurons and has been linked to regulation of LH and FSH secretion. Kisspeptin, along with neurokinin B and dynorphin, is part of the triumvirate that comprises KNDy neurons, also recently implicated in menopausal vasomotor symptoms. Multiple systematic reviewsand meta-analyses have found a correlation between higher kisspeptin levels in the blood and higher circulating LH levels, regardless of body mass index. While kisspeptin is expressed in several tissues, including liver, pancreas, gonad, and adipose, it’s neural kisspeptin signaling that appears most likely to play a role in activating GnRH hormones and disrupting normal function of the hypothalamic-pituitary-gonadal axis.
But as noted, in at least one systematic review of kisspeptin and PCOS, “findings from animal studies suggest that kisspeptin levels are not increased in all subtypes of PCOS.” And another review found “altered” levels of kisspeptin levels in non-PCOS patients who had obesity, potentially raising questions about any associations between kisspeptin and obesity or insulin resistance.
Remaining chicken-and-egg questions
A hallmark of PCOS has long been, and continues to be, the string of chicken-or-egg questions that plague understanding of it. One of these is how depression and anxiety fit into the etiology of PCOS. Exploring the role of specific neurons that may overstimulate GnRH pulsatility may hold clues to a common underlying mechanism for the involvement of depression and anxiety in patients with PCOS, Dr. Hudson speculated. While previous assumptions often attributed depression and anxiety in PCOS to the symptoms – such as thin scalp hair and increased facial hair, excess weight, acne, and irregular periods – Dr. Hudson pointed out that women can address many of these symptoms with laser hair removal, weight loss, acne treatment, and similar interventions, yet they still have a lot of underlying mental health issues.
It’s also unclear whether metabolic factors so common with PCOS, particularly insulin resistance and obesity, are a result of the condition or are contributors to it. Is insulin resistance contributing to dysregulation in the neurons that interferes with normal functioning of the hypothalamic-pituitary-adrenal axis? Is abnormal functioning along this axis contributing to insulin resistance? Or neither? Or both? Or does it depend? The authors of one paper wrote that “insulin may play both direct and indirect roles in the pathogenesis of androgen excess in PCOS,” since insulin can “stimulate ovarian androgen production” and “enhance ovarian growth and follicular cyst formation in rats.”
Dr. Pal noted that “obesity itself can evolve into a PCOS-like picture,” raising questions about whether obesity or insulin resistance might be part of the causal pathway to PCOS, or whether either can trigger its development in those genetically predisposed.
“Obesity does appear to exacerbate many aspects of the PCOS phenotype, particularly those risk factors related to metabolic syndrome,” wrote the authors of a scientific statement on aspects of PCOS, but they add that “it is currently debated whether obesity per se can cause PCOS.” While massive weight loss in those with PCOS and obesity has improved multiple reproductive and metabolic issues, it hasn’t resolved all of them, they write.
Dr. Hudson said she expects there’s “some degree of appetite dysregulation and metabolic dysregulation” that contributes, but then there are other women who don’t have much of an appetite or overeat and still struggle with their weight. Evidence has also found insulin resistance in women of normal weight with PCOS. “There may be some kind of metabolic dysregulation that they have at some level, and others are clearly bothered by overeating,” Dr. Hudson said.
Similarly, it’s not clear whether the recent discovery of increased cardiovascular risks in people with PCOS is a result of the comorbidities so common with PCOS, such as obesity, or whether an underlying mechanism links the cardiovascular risk and the dysregulation of hormones. Dr. Pal would argue that, again, it’s probably both, depending on the patient.
Then there is the key feature of hyperandrogenemia. “An outstanding debate is whether the elevated androgens in PCOS women are merely a downstream endocrine response to hyperactive GnRH and LH secretion driving the ovary, or do the elevated androgens themselves act in the brain (or pituitary) during development and/or adulthood to sculpt and maintain the hypersecretion of GnRH and LH?” wrote Eulalia A. Coutinho, PhD, and Alexander S. Kauffman, PhD, in a 2019 review of the brain’s role in PCOS.
These problems may be bidirectional or part of various feedback loops. Sleep apnea is more common in people with PCOS, Dr. Tyson noted, but sleep apnea is also linked to cardiovascular, metabolic, and depression risks, and depression can play a role in obesity, which increases the risk of obstructive sleep apnea. “So you’re in this vicious cycle,” Dr. Tyson said. That’s why she also believes it’s important to change the dialogue and perspective on PCOS, to reduce the stigma attached to it, and work with patients to empower them in treating its symptoms and reducing their risk of comorbidities.
Recent and upcoming changes in treatment
Current treatment of PCOS already changes according to the symptoms posing the greatest problems at each stage of a person’s life, Dr. Hudson said. Younger women tend to be more bothered about the cosmetic effects of PCOS, including hair growth patterns and acne, but as they grow out of adolescence and into their 20s and 30s, infertility becomes a bigger concern for many. Then, as they start approaching menopause, metabolic and cardiovascular issues take the lead, with more of a focus on lipids, diabetes risk, and heart health.
In some ways, management of PCOS hasn’t changed much in the past several decades, except in an increased awareness of the metabolic and cardiovascular risks, which has led to more frequent screening to catch potential conditions earlier in life. What has changed, however, is improvements in the treatments used for symptoms, such as expanded bariatric surgery options and GLP-1 agonists for treating obesity. Other examples include better options for menstrual management, such as new progesterone IUDs, and optimized fertility treatments, Dr. Tyson said.
“I think with more of these large-scale studies about the pathophysiology of PCOS and how it may look in different people and the different outcomes, we may be able to tailor our treatments even further,” Dr. Tyson said. She emphasized the importance of identifying the condition early, particularly in adolescents, even if it’s identifying young people at risk for the condition rather than actually having it yet.
Early identification “gives us this chance to do a lot of preventative care and motivate older teens to have a great lifestyle, work on their diet and exercise, and manage cardiovascular” risk factors, Dr. Tyson said.
“What we do know and recognize is that there’s so many spokes to this PCOS wheel that there really should be a multidisciplinary approach to care,” Dr. Tyson said. “When I think about who would be the real doctors for patients with PCOS, these would be gynecologists, endocrinologists, dermatologists, nutritionists, psychologists, sleep specialists, and primary care at a minimum.”
Dr. Pal worries that the label of PCOS leaves it in the laps of ob.gyns. whereas, “if it was called something else, everybody would be involved in being vigilant and managing those patients.” She frequently reiterated that the label of PCOS is less important than ensuring clinicians treat the symptoms that most bother the patient.
And even if kisspeptin does play a causal role in PCOS for some patients, it’s only a subset of individuals with PCOS who would benefit from therapies developed to target it. Given the complexity of the syndrome and its many manifestations, a “galaxy of pathways” are involved in different potential subtypes of the condition. “You can’t treat PCOS as one entity,” Dr. Pal said.
Still, Dr. Hudson is optimistic that the research into potential neuroendocrine contributions to PCOS will yield therapies that go beyond just managing symptoms.
“There aren’t a lot of treatments available yet, but there may be some on the horizon,” Dr. Hudson said. “We’re still in this very primitive stage in terms of therapeutics, where we’re only addressing specific symptoms, and we haven’t been able to really address the underlying cause because we haven’t understood it as well and because we don’t have therapies that can target it,” Dr. Hudson said. “But once there are therapies developed that will target some of these central mechanisms, I think it will change completely the approach to treating PCOS for patients.”
This story was updated on Sept. 6, 2022.
Early menopause linked with increased risk of heart problems
SEOUL, South Korea – Menopause before age 40 is associated with elevated risk of heart failure and atrial fibrillation, according to a study published in European Heart Journal, from the European Society of Cardiology (ESC). The study of more than 1.4 million women revealed that the younger the age at menopause, the higher the risk of heart failure and atrial fibrillation.
“Women with premature menopause should be aware that they may be more likely to develop heart failure or atrial fibrillation than their peers,” said study author Ga Eun Nam, MD, PhD, of Korea University College of Medicine, Seoul. “This may be good motivation to improve lifestyle habits known to be linked with heart disease, such as quitting smoking and exercising.”
Cardiovascular disease typically occurs up to 10 years later in women than men. Premenopausal women are thought to benefit from estrogen’s protective effect on the cardiovascular system. The cessation of menstruation and subsequent decline of estrogen levels may make women more vulnerable to cardiovascular disease.
A national population
Premature menopause affects 1% of women younger than 40 years, the ESC press release stated. Prior studies have found a link between premature (before age 40 years) and early (before age 45 years) menopause and cardiovascular disease overall, but the evidence for heart failure or atrial fibrillation alone is limited. This study examined the associations between premature menopause, age at menopause, and incident heart failure and atrial fibrillation. Data were obtained from the Korean National Health Insurance System (NHIS), which provides health screening at least every 2 years and includes 97% of the population.
The study included 1,401,175 postmenopausal women aged 30 years and older who completed the NHIS health checkup in 2009. Participants were monitored until the end of 2018 for new-onset heart failure and atrial fibrillation. Information was collected on demographics, health behaviors, and reproductive factors, including age at menopause and use of hormone replacement therapy (HRT). Age at menopause was split into four categories: younger than 40 years, 40-44 years, 45-49 years, and 50 years or older. Premature menopause was defined as having the final menstrual period before age 40 years.
Some 28,111 (2%) participants had a history of premature menopause. For these women, the average age at menopause was 36.7 years. The average age at study enrollment for women with and for those without a history of premature menopause was 60 and 61.5 years, respectively. During an average follow-up of 9.1 years, 42,699 (3.0%) developed heart failure, and 44,834 (3.2%) developed atrial fibrillation.
The researchers analyzed the association between history of premature menopause and incident heart failure and atrial fibrillation after adjusting for age, smoking, alcohol use, physical activity, income, body mass index, hypertension, type 2 diabetes, dyslipidemia, chronic kidney disease, coronary heart disease, HRT, and age at menarche. Women who experienced premature menopause had a 33% higher risk for heart failure and 9% higher risk for atrial fibrillation, compared with those who did not.
Reproductive history
The researchers then analyzed the associations between age at menopause and incidence of heart failure and atrial fibrillation after adjusting for the same factors as in the previous analyses. The risk for incident heart failure increased as the age at menopause decreased. Compared with women aged 50 years and older at menopause, those aged 45-49 years, 40-44 years, and younger than 40 years at menopause had 11%, 23%, and 39% greater risk for incident heart failure, respectively. Similarly, the risk for incident atrial fibrillation increased as the age at menopause decreased; the risk was 4%, 10%, and 11% higher for those aged 45-49 years, 40-44 years, and younger than 40 years at menopause, respectively, compared with women aged 50 years and older at menopause.
The authors said that several factors may explain the associations between menopausal age, heart failure, and atrial fibrillation, such as the drop in estrogen levels and changes in body fat distribution.
Dr. Nam concluded, “The misconception that heart disease primarily affects men has meant that sex-specific risk factors have been largely ignored. Evidence is growing that undergoing menopause before the age of 40 years may increase the likelihood of heart disease later in life. Our study indicates that reproductive history should be routinely considered in addition to traditional risk factors such as smoking when evaluating the future likelihood of heart failure and atrial fibrillation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.
SEOUL, South Korea – Menopause before age 40 is associated with elevated risk of heart failure and atrial fibrillation, according to a study published in European Heart Journal, from the European Society of Cardiology (ESC). The study of more than 1.4 million women revealed that the younger the age at menopause, the higher the risk of heart failure and atrial fibrillation.
“Women with premature menopause should be aware that they may be more likely to develop heart failure or atrial fibrillation than their peers,” said study author Ga Eun Nam, MD, PhD, of Korea University College of Medicine, Seoul. “This may be good motivation to improve lifestyle habits known to be linked with heart disease, such as quitting smoking and exercising.”
Cardiovascular disease typically occurs up to 10 years later in women than men. Premenopausal women are thought to benefit from estrogen’s protective effect on the cardiovascular system. The cessation of menstruation and subsequent decline of estrogen levels may make women more vulnerable to cardiovascular disease.
A national population
Premature menopause affects 1% of women younger than 40 years, the ESC press release stated. Prior studies have found a link between premature (before age 40 years) and early (before age 45 years) menopause and cardiovascular disease overall, but the evidence for heart failure or atrial fibrillation alone is limited. This study examined the associations between premature menopause, age at menopause, and incident heart failure and atrial fibrillation. Data were obtained from the Korean National Health Insurance System (NHIS), which provides health screening at least every 2 years and includes 97% of the population.
The study included 1,401,175 postmenopausal women aged 30 years and older who completed the NHIS health checkup in 2009. Participants were monitored until the end of 2018 for new-onset heart failure and atrial fibrillation. Information was collected on demographics, health behaviors, and reproductive factors, including age at menopause and use of hormone replacement therapy (HRT). Age at menopause was split into four categories: younger than 40 years, 40-44 years, 45-49 years, and 50 years or older. Premature menopause was defined as having the final menstrual period before age 40 years.
Some 28,111 (2%) participants had a history of premature menopause. For these women, the average age at menopause was 36.7 years. The average age at study enrollment for women with and for those without a history of premature menopause was 60 and 61.5 years, respectively. During an average follow-up of 9.1 years, 42,699 (3.0%) developed heart failure, and 44,834 (3.2%) developed atrial fibrillation.
The researchers analyzed the association between history of premature menopause and incident heart failure and atrial fibrillation after adjusting for age, smoking, alcohol use, physical activity, income, body mass index, hypertension, type 2 diabetes, dyslipidemia, chronic kidney disease, coronary heart disease, HRT, and age at menarche. Women who experienced premature menopause had a 33% higher risk for heart failure and 9% higher risk for atrial fibrillation, compared with those who did not.
Reproductive history
The researchers then analyzed the associations between age at menopause and incidence of heart failure and atrial fibrillation after adjusting for the same factors as in the previous analyses. The risk for incident heart failure increased as the age at menopause decreased. Compared with women aged 50 years and older at menopause, those aged 45-49 years, 40-44 years, and younger than 40 years at menopause had 11%, 23%, and 39% greater risk for incident heart failure, respectively. Similarly, the risk for incident atrial fibrillation increased as the age at menopause decreased; the risk was 4%, 10%, and 11% higher for those aged 45-49 years, 40-44 years, and younger than 40 years at menopause, respectively, compared with women aged 50 years and older at menopause.
The authors said that several factors may explain the associations between menopausal age, heart failure, and atrial fibrillation, such as the drop in estrogen levels and changes in body fat distribution.
Dr. Nam concluded, “The misconception that heart disease primarily affects men has meant that sex-specific risk factors have been largely ignored. Evidence is growing that undergoing menopause before the age of 40 years may increase the likelihood of heart disease later in life. Our study indicates that reproductive history should be routinely considered in addition to traditional risk factors such as smoking when evaluating the future likelihood of heart failure and atrial fibrillation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.
SEOUL, South Korea – Menopause before age 40 is associated with elevated risk of heart failure and atrial fibrillation, according to a study published in European Heart Journal, from the European Society of Cardiology (ESC). The study of more than 1.4 million women revealed that the younger the age at menopause, the higher the risk of heart failure and atrial fibrillation.
“Women with premature menopause should be aware that they may be more likely to develop heart failure or atrial fibrillation than their peers,” said study author Ga Eun Nam, MD, PhD, of Korea University College of Medicine, Seoul. “This may be good motivation to improve lifestyle habits known to be linked with heart disease, such as quitting smoking and exercising.”
Cardiovascular disease typically occurs up to 10 years later in women than men. Premenopausal women are thought to benefit from estrogen’s protective effect on the cardiovascular system. The cessation of menstruation and subsequent decline of estrogen levels may make women more vulnerable to cardiovascular disease.
A national population
Premature menopause affects 1% of women younger than 40 years, the ESC press release stated. Prior studies have found a link between premature (before age 40 years) and early (before age 45 years) menopause and cardiovascular disease overall, but the evidence for heart failure or atrial fibrillation alone is limited. This study examined the associations between premature menopause, age at menopause, and incident heart failure and atrial fibrillation. Data were obtained from the Korean National Health Insurance System (NHIS), which provides health screening at least every 2 years and includes 97% of the population.
The study included 1,401,175 postmenopausal women aged 30 years and older who completed the NHIS health checkup in 2009. Participants were monitored until the end of 2018 for new-onset heart failure and atrial fibrillation. Information was collected on demographics, health behaviors, and reproductive factors, including age at menopause and use of hormone replacement therapy (HRT). Age at menopause was split into four categories: younger than 40 years, 40-44 years, 45-49 years, and 50 years or older. Premature menopause was defined as having the final menstrual period before age 40 years.
Some 28,111 (2%) participants had a history of premature menopause. For these women, the average age at menopause was 36.7 years. The average age at study enrollment for women with and for those without a history of premature menopause was 60 and 61.5 years, respectively. During an average follow-up of 9.1 years, 42,699 (3.0%) developed heart failure, and 44,834 (3.2%) developed atrial fibrillation.
The researchers analyzed the association between history of premature menopause and incident heart failure and atrial fibrillation after adjusting for age, smoking, alcohol use, physical activity, income, body mass index, hypertension, type 2 diabetes, dyslipidemia, chronic kidney disease, coronary heart disease, HRT, and age at menarche. Women who experienced premature menopause had a 33% higher risk for heart failure and 9% higher risk for atrial fibrillation, compared with those who did not.
Reproductive history
The researchers then analyzed the associations between age at menopause and incidence of heart failure and atrial fibrillation after adjusting for the same factors as in the previous analyses. The risk for incident heart failure increased as the age at menopause decreased. Compared with women aged 50 years and older at menopause, those aged 45-49 years, 40-44 years, and younger than 40 years at menopause had 11%, 23%, and 39% greater risk for incident heart failure, respectively. Similarly, the risk for incident atrial fibrillation increased as the age at menopause decreased; the risk was 4%, 10%, and 11% higher for those aged 45-49 years, 40-44 years, and younger than 40 years at menopause, respectively, compared with women aged 50 years and older at menopause.
The authors said that several factors may explain the associations between menopausal age, heart failure, and atrial fibrillation, such as the drop in estrogen levels and changes in body fat distribution.
Dr. Nam concluded, “The misconception that heart disease primarily affects men has meant that sex-specific risk factors have been largely ignored. Evidence is growing that undergoing menopause before the age of 40 years may increase the likelihood of heart disease later in life. Our study indicates that reproductive history should be routinely considered in addition to traditional risk factors such as smoking when evaluating the future likelihood of heart failure and atrial fibrillation.”
A version of this article appeared on Medscape.com. This article was translated from the Medscape French edition.
Monkeypox in children and women remains rare, CDC data show
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Use of antidotes in pregnancy and lactation
The human pregnancy data reported for these 16 agents are very limited as only 8 of the drugs have this data. However, the 8 reports indicated that the use of these drugs was highly important for the mother and did not cause embryo/fetal harm.
- Acetylcysteine
The need for this antidote in a pregnant or lactating woman is most likely a rare requirement. However, the need for this agent does occur in women who have taken a potentially hepatic toxic dose of acetaminophen (e.g., Tylenol).
- Black widow spider antivenin
Only three reports of the use of this agent in a pregnant woman have been located. In each case, the symptoms from the spider bite did not respond to other therapies but did within 1 hour to the antivenin. There was no fetal harm in these cases.
- Deferasirox
This agent is an oral iron-chelating agent used for the treatment of chronic iron overload. Five case reports have described its use without causing any fetal harm.
- Deferoxamine
This agent has been used in more than 65 pregnancies for acute iron overdose or for transfusion-dependent thalassemia. No reports have observed adverse human developmental effects.
- Digoxin immune FAB (ovine)
Several reports have described the use of this agent in pregnancy. No fetal harm has been observed, but none of the reports involved exposure during organogenesis. However, in cases of digoxin overdose, the maternal benefits of therapy should take priority over the embryo/fetus.
- Dimercaprol
Although the limited animal data suggest low risk, there are no reports of the use of this drug in human organogenesis. The absence of data prevents an assessment of the embryo-fetal risk, but the maternal benefit and indirect embryo-fetal benefit appears to outweigh that risk.
- Edetate calcium disodium
This agent is used to treat acute or chronic lead poisoning. It is compatible in pregnancy because the maternal and possibly the embryo-fetal benefit appears to outweigh any unknown direct or indirect risks.
- Flumazenil
The use of this drug in the third trimester has been reported in two cases. Because the drug is indicated to reverse the effects of benzodiazepines on the central nervous system, the maternal benefit should far outweigh the unknown embryo-fetal risk.
- Glucagon
The embryo-fetal risks appear to be very low. Apparently, the drug does not cross the placenta.
- Glucarpidase
This drug is indicated for the treatment of methotrexate toxicity. There are no reports describing the use of this drug in pregnancy or during breastfeeding.
- Idarucizumab
This agent is a humanized monoclonal antibody fragment that is indicated for the reversal of the anticoagulant effects of dabigatran. No reports describing its use in human or animal pregnancy have been located. However, the maternal benefit appears to be high and probably outweighs the unknown risk to the embryo/fetus.
- Lanthanum carbonate
There are no human pregnancy or lactation data. It is used to reduce blood levels of phosphate in people with kidney disease.
- Pralidoxime
This agent relieves the paralysis of the muscles of respiration caused by an organophosphate pesticide or related compound. The human pregnancy experience is limited to two cases, one at 36 weeks and the other at 16 weeks, both of which delivered normal infants.
- Sapropterin
Four reports have described the use of sapropterin to lower blood phenylalanine levels in 31 pregnancies. There were no embryo-fetal adverse effects attributable to the drug.
- Sevelamer
Sevelamer is used to control high blood levels of phosphorus in people with chronic kidney disease who are on dialysis. There are no human pregnancy or breastfeeding data.
- Succimer
This drug is a heavy metal–chelating agent that is indicated for the treatment of lead poisoning in pediatric patients. The drug was teratogenic in rats and mice. Two reports described the use of the drug in two pregnant women for lead poisoning. It has also been used as an antidote for the treatment of arsenic, mercury, and cadmium poisoning in adults, but there have been no reports of this use in pregnant patients.
Mr. Briggs, now retired, was a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
The human pregnancy data reported for these 16 agents are very limited as only 8 of the drugs have this data. However, the 8 reports indicated that the use of these drugs was highly important for the mother and did not cause embryo/fetal harm.
- Acetylcysteine
The need for this antidote in a pregnant or lactating woman is most likely a rare requirement. However, the need for this agent does occur in women who have taken a potentially hepatic toxic dose of acetaminophen (e.g., Tylenol).
- Black widow spider antivenin
Only three reports of the use of this agent in a pregnant woman have been located. In each case, the symptoms from the spider bite did not respond to other therapies but did within 1 hour to the antivenin. There was no fetal harm in these cases.
- Deferasirox
This agent is an oral iron-chelating agent used for the treatment of chronic iron overload. Five case reports have described its use without causing any fetal harm.
- Deferoxamine
This agent has been used in more than 65 pregnancies for acute iron overdose or for transfusion-dependent thalassemia. No reports have observed adverse human developmental effects.
- Digoxin immune FAB (ovine)
Several reports have described the use of this agent in pregnancy. No fetal harm has been observed, but none of the reports involved exposure during organogenesis. However, in cases of digoxin overdose, the maternal benefits of therapy should take priority over the embryo/fetus.
- Dimercaprol
Although the limited animal data suggest low risk, there are no reports of the use of this drug in human organogenesis. The absence of data prevents an assessment of the embryo-fetal risk, but the maternal benefit and indirect embryo-fetal benefit appears to outweigh that risk.
- Edetate calcium disodium
This agent is used to treat acute or chronic lead poisoning. It is compatible in pregnancy because the maternal and possibly the embryo-fetal benefit appears to outweigh any unknown direct or indirect risks.
- Flumazenil
The use of this drug in the third trimester has been reported in two cases. Because the drug is indicated to reverse the effects of benzodiazepines on the central nervous system, the maternal benefit should far outweigh the unknown embryo-fetal risk.
- Glucagon
The embryo-fetal risks appear to be very low. Apparently, the drug does not cross the placenta.
- Glucarpidase
This drug is indicated for the treatment of methotrexate toxicity. There are no reports describing the use of this drug in pregnancy or during breastfeeding.
- Idarucizumab
This agent is a humanized monoclonal antibody fragment that is indicated for the reversal of the anticoagulant effects of dabigatran. No reports describing its use in human or animal pregnancy have been located. However, the maternal benefit appears to be high and probably outweighs the unknown risk to the embryo/fetus.
- Lanthanum carbonate
There are no human pregnancy or lactation data. It is used to reduce blood levels of phosphate in people with kidney disease.
- Pralidoxime
This agent relieves the paralysis of the muscles of respiration caused by an organophosphate pesticide or related compound. The human pregnancy experience is limited to two cases, one at 36 weeks and the other at 16 weeks, both of which delivered normal infants.
- Sapropterin
Four reports have described the use of sapropterin to lower blood phenylalanine levels in 31 pregnancies. There were no embryo-fetal adverse effects attributable to the drug.
- Sevelamer
Sevelamer is used to control high blood levels of phosphorus in people with chronic kidney disease who are on dialysis. There are no human pregnancy or breastfeeding data.
- Succimer
This drug is a heavy metal–chelating agent that is indicated for the treatment of lead poisoning in pediatric patients. The drug was teratogenic in rats and mice. Two reports described the use of the drug in two pregnant women for lead poisoning. It has also been used as an antidote for the treatment of arsenic, mercury, and cadmium poisoning in adults, but there have been no reports of this use in pregnant patients.
Mr. Briggs, now retired, was a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
The human pregnancy data reported for these 16 agents are very limited as only 8 of the drugs have this data. However, the 8 reports indicated that the use of these drugs was highly important for the mother and did not cause embryo/fetal harm.
- Acetylcysteine
The need for this antidote in a pregnant or lactating woman is most likely a rare requirement. However, the need for this agent does occur in women who have taken a potentially hepatic toxic dose of acetaminophen (e.g., Tylenol).
- Black widow spider antivenin
Only three reports of the use of this agent in a pregnant woman have been located. In each case, the symptoms from the spider bite did not respond to other therapies but did within 1 hour to the antivenin. There was no fetal harm in these cases.
- Deferasirox
This agent is an oral iron-chelating agent used for the treatment of chronic iron overload. Five case reports have described its use without causing any fetal harm.
- Deferoxamine
This agent has been used in more than 65 pregnancies for acute iron overdose or for transfusion-dependent thalassemia. No reports have observed adverse human developmental effects.
- Digoxin immune FAB (ovine)
Several reports have described the use of this agent in pregnancy. No fetal harm has been observed, but none of the reports involved exposure during organogenesis. However, in cases of digoxin overdose, the maternal benefits of therapy should take priority over the embryo/fetus.
- Dimercaprol
Although the limited animal data suggest low risk, there are no reports of the use of this drug in human organogenesis. The absence of data prevents an assessment of the embryo-fetal risk, but the maternal benefit and indirect embryo-fetal benefit appears to outweigh that risk.
- Edetate calcium disodium
This agent is used to treat acute or chronic lead poisoning. It is compatible in pregnancy because the maternal and possibly the embryo-fetal benefit appears to outweigh any unknown direct or indirect risks.
- Flumazenil
The use of this drug in the third trimester has been reported in two cases. Because the drug is indicated to reverse the effects of benzodiazepines on the central nervous system, the maternal benefit should far outweigh the unknown embryo-fetal risk.
- Glucagon
The embryo-fetal risks appear to be very low. Apparently, the drug does not cross the placenta.
- Glucarpidase
This drug is indicated for the treatment of methotrexate toxicity. There are no reports describing the use of this drug in pregnancy or during breastfeeding.
- Idarucizumab
This agent is a humanized monoclonal antibody fragment that is indicated for the reversal of the anticoagulant effects of dabigatran. No reports describing its use in human or animal pregnancy have been located. However, the maternal benefit appears to be high and probably outweighs the unknown risk to the embryo/fetus.
- Lanthanum carbonate
There are no human pregnancy or lactation data. It is used to reduce blood levels of phosphate in people with kidney disease.
- Pralidoxime
This agent relieves the paralysis of the muscles of respiration caused by an organophosphate pesticide or related compound. The human pregnancy experience is limited to two cases, one at 36 weeks and the other at 16 weeks, both of which delivered normal infants.
- Sapropterin
Four reports have described the use of sapropterin to lower blood phenylalanine levels in 31 pregnancies. There were no embryo-fetal adverse effects attributable to the drug.
- Sevelamer
Sevelamer is used to control high blood levels of phosphorus in people with chronic kidney disease who are on dialysis. There are no human pregnancy or breastfeeding data.
- Succimer
This drug is a heavy metal–chelating agent that is indicated for the treatment of lead poisoning in pediatric patients. The drug was teratogenic in rats and mice. Two reports described the use of the drug in two pregnant women for lead poisoning. It has also been used as an antidote for the treatment of arsenic, mercury, and cadmium poisoning in adults, but there have been no reports of this use in pregnant patients.
Mr. Briggs, now retired, was a clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at [email protected].
After cancer, abortion experience highlights post-Roe reality
The drive from Texas to the clinic in Albuquerque, N.M., took 10 hours. It was mid-April of this year. There wasn’t much to see along the mostly barren stretch, and there wasn’t much for Kailee DeSpain to do aside from think about where she was going and why.
Her husband was driving. She sensed his nervous glances toward the passenger seat where she sat struggling to quiet her thoughts.
No, she wasn’t having any pain, she told him. No, she wasn’t feeling like she did the last time or the two times before that.
This pregnancy was different. It was the first in which she feared for her own life. Her fetus – Finley – had triploidy, a rare chromosomal abnormality. Because of the condition, which affects 1%-3% of pregnancies, his heart, brain, and kidneys were not developing properly.
At 19 weeks, Finley was already struggling to draw breath from lungs squeezed inside an overcrowded chest cavity. Ms. DeSpain wanted nothing more than to carry Finley to term, hold him, meet him even for a moment before saying goodbye.
But his condition meant he would likely suffocate in utero well before that. And Ms. DeSpain knew that carrying him longer would likely raise her risk of bleeding and of her blood pressure increasing to dangerous highs.
“This could kill you,” her husband told her. “Do you realize you could die bringing a baby into this world who is not going to live? I don’t want to lose you.’”
Unlike her other pregnancies, the timing of this one and the decision she faced to end it put her health in even greater danger.
Imminent danger
On Sept. 1, 2021, a bill went into effect in Texas that banned abortions from as early as 6 weeks’ gestation. Texas Senate Bill 8 (SB8) became one of the most restrictive abortion laws in the country. It prohibited abortions whenever a fetal heartbeat, defined by lawmakers, could be detected on an ultrasound, often before many women knew they were pregnant.
The Texas abortion law was hardly the last word on the topic. Ms. DeSpain didn’t know it on her drive to New Mexico in April, but the U.S. Supreme Court was weeks away from overturning the landmark Roe v. Wade decision.
On June 24, the Supreme Court delivered its 6-3 ruling overturning Roe v. Wade, the 1973 case that granted women the right to abortion.
This decision set in motion “trigger laws” in some states – laws that essentially fully banned abortions. Those states included Ms. DeSpain’s home state of Texas, where abortion is now a felony except when the life of the mother is in peril.
However, legal definitions of what qualifies as “life-threatening” remain murky.
The law is unclear, says Lisa Harris, MD, PhD, professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor. “What does the risk of death have to be, and how imminent must it be?” she asked in a recent editorial in the New England Journal of Medicine. Is 25% enough? 50%? Or does a woman have to be moments from dying?
“This whole thing makes me so angry,” says Shikha Jain, MD, a medical oncologist at University of Illinois Health, Chicago. “A patient may not be experiencing an emergency right now, but if we don’t take care of the situation, it may become an emergency in 2 hours or 2 days.”
Even before the Roe v. Wade decision, pregnancy had been a high-stakes endeavor for many women. In 2019, more than 750 women died from pregnancy-related events in the United States. In 2020, that number rose to 850. Each year dozens more suffer pregnancy-related events that require lifesaving interventions.
Now, in a post-Roe world, the number of maternal deaths will likely climb as more abortion bans take effect and fewer women have access to lifesaving care, experts say. A 2021 study that compared 2017 maternal mortality rates in states with different levels of abortion restrictions found that the rate of maternal mortality was almost two times higher in states that restricted abortion access compared with those that protected it – 28.5 per 100,000 women vs. 15.7.
Some women living in states with abortion bans won’t have the resources to cross state lines for care.
“This is just going to widen the health care disparities that are already so prevalent in this country,” Dr. Jain says.
Navigating a crossroads
Ms. DeSpain’s medical history reads like a checklist of pregnancy-related perils: chronic high blood pressure, persistent clotting problems, and a high risk of hemorrhage. She was also diagnosed with cervical cancer in 2020, which left her body more fragile.
Cardiovascular conditions, including hypertension and hemorrhage, are the leading causes of maternal mortality, responsible for more than one-third of pregnancy-related deaths. Preeclampsia, characterized by high blood pressure, accounts for more than 7% of maternal deaths in the United States. Although less common, genetic disorders, such as spinal muscular atrophy and triploidy, or cancer during pregnancy can put a mother and fetus at risk.
Cancer – which affects about 1 in 1,000 pregnant women and results in termination in as many as 28% of cases – brings sharp focus to the new dangers and complex decision-making patients and their doctors face as abortion bans take hold.
Before the Supreme Court decision, a pregnant woman with cancer was already facing great uncertainty. The decision to treat cancer during pregnancy involves “weighing the risk of exposing the fetus to medication vs. the risk to the mother’s untreated illness if you don’t expose the fetus to medication,” Elyce Cardonick, MD, an obstetrician at Cooper University Health Care, Camden, N.J., who specializes in high-risk pregnancies, told the National Cancer Institute.
Oncologists generally agree that it’s safe for pregnant women to receive chemotherapy during the second and third trimesters. But for women with aggressive cancers that are diagnosed in the first trimester, chemotherapy is dangerous. For women who need immunotherapy, the risks of treatment remain unclear.
In these cases, Alice S. Mims, MD, must broach the possibility of terminating the pregnancy.
“Cancer is a very urgent condition,” says Dr. Mims, a hematology specialist at the Ohio State University Comprehensive Cancer Center, Columbus, who sees patients who are pregnant. “These women may have other children at home, and they want to do their best to fight the disease so they can be around for their family long term.”
Now the changing legal landscape on abortion will put hundreds more pregnant women with cancer in danger. In a recent viewpoint article published in JAMA Oncology, Jordyn Silverstein and Katherine Van Loon, MD, MPH, estimate that during the next year, up to 420 pregnant women living in states with restricted abortion access will face threats to their cancer care and potentially their life.
“The repercussions of overturning Roe v. Wade – and the failure of the Supreme Court to provide any guidance on exceptions related to the life and health of the mother – are potentially catastrophic for a subset of women who face a life-threating diagnosis of [pregnancy-associated cancer],” they write.
The choice Ms. DeSpain faced after her cervical cancer diagnosis was different. She was not pregnant at the time, but she was at a crossroads.
Although it was caught early, the cancer was aggressive. Her oncologist recommended that she undergo a hysterectomy – the surgery that would give her the best chance for a cancer-free future. It would also mean she could no longer become pregnant.
With a less invasive procedure, on the other hand, she could still carry a child, but she would face a much greater chance that the cancer would come back.
At 27, Ms. DeSpain was not ready to close the pregnancy door. She opted for a surgery in which part of her cervix was removed, allowing her to try for another baby.
But she faced a ticking clock in the event her cancer returned.
If you want to have a baby, “try soon,” her doctor warned.
A dead end
After her cancer surgery and a third miscarriage, Ms. DeSpain and her husband were surprised and excited when in late 2021 she again became pregnant.
The first trimester seemed blissfully uneventful. As the weeks passed, Finley’s heart started to beat.
But the 16-week ultrasound signaled a turning point. The sonographer was too quiet.
“This is really bad, isn’t it?” Ms. DeSpain asked her sonographer.
The doctors told her he wouldn’t survive. Finley had no heart chambers. His heart couldn’t pump blood properly. He was missing one kidney, and his brain was split in the back. With almost no amniotic fluid, her doctor said he would likely die in utero, crushed to death without support from the protective liquid.
She fought for him anyway. She sought specialty care, followed bed rest orders, and traveled 3 hours to Houston to enroll in a clinical trial.
But every road was a dead end.
Ultimately, testing revealed Finley had triploidy, and all lines led to one point.
“There were too many things wrong, too much wrong for them to fix,” says Ms. DeSpain, recalling the news from her doctor in Houston. “I was in shock. My husband was just sitting with his hands flat on the table, staring at nothing, shaking a little bit.”
However, Finley still had a heartbeat, making an abortion after 6 weeks a felony in Texas. Even a compassionate induction was now out of the question unless her death was imminent.
Ms. DeSpain called the abortion clinic in Albuquerque and made an appointment. She would have to wait 2 weeks because of an influx of pregnant patients coming from Texas.
She welcomed the wait … just in case she changed her mind.
“At that point I wanted to carry him as far as I could,” she says.
For those 2 weeks, Ms. DeSpain remained on bed rest. She cried all day every day. She worried that Finley was experiencing pain.
Through this process, her doctor’s support helped keep her grounded.
“She cried with us in her office and said, ‘I wish that you didn’t have to go, but I think you’re doing the right thing, doing what keeps you safest,’ “ Ms. DeSpain recalls.
Ms. DeSpain declined to share the name of her doctor out of fear that even expressing compassion for a patient’s safety could put the physician in legal jeopardy and provoke harassment.
That fear is warranted. Some doctors will be forced to choose between doing what is legal – even though the law is vague – and doing what is right for patients, says law professor Jamie Abrams, who was recently diagnosed with breast cancer.
To live in a world where there’s talk of criminalizing doctors for taking care of their patients, where there’s “this national movement to position some women to be shunned and exiled for seeking care that’s right for them, their health, and might save their life is staggering and beyond comprehension,” says Ms. Abrams, professor of law at the American University Washington College of Law.
Ms. Abrams, who was diagnosed with hormone receptor–positive invasive breast cancer the same day she read the leaked Supreme Court draft on the decision to end of Roe v. Wade, said that “overnight, I became a person who would need an abortion if I became pregnant, because my treatment would compromise a healthy birth or delay necessary cancer care.” Ms. Abrams was also told she could no longer use hormonal contraception.
Dr. Harris’s advice to clinicians is to try to do what they feel is best for patients, including referring them to centers that have legal resources and protections regarding abortions.
Dr. Mims agrees and recommends that doctors reach out to those with more resources and legal backing for support. “I would advise doctors in [states with restrictive laws] to familiarize themselves with available resources and organizations taking action to deal with questionable cases,” Dr. Mims says.
‘Baby killers work here’
Following her 10-hour drive to Albuquerque, Ms. DeSpain encountered lines of protesters at the clinic. They were holding signs that said, “Abortion is murder,” and “Baby killers work here.”
“Please don’t kill your baby – we have resources for you,” a woman screeched through a megaphone as Ms. DeSpain, nearly 20 weeks’ pregnant, stepped out of the car to enter the clinic.
“I remember turning around, looking at her and making eye contact, and yelling back, ‘My baby has triploidy – he is dying! He is going to suffocate if I carry him full term. You don’t know what you’re talking about!’ “
A nurse held her hand during the procedure.
“He said, ‘You’re doing great, you’re okay,’ “ she recalls. She knew there was a chance that Finley’s face would be crushed by contractions during labor because of the lack of amniotic fluid, but she hoped not. Ms. DeSpain longed for a photo.
There was no photo to take home the next day, but Ms. DeSpain did receive Finley’s footprints, and his heartbeat – as captured by the specialty team in Houston – lives on in a stuffed giraffe.
His ashes arrived a few weeks later.
By then, the Supreme Court draft had been leaked. Ms. DeSpain knew her predicament in Texas would soon affect women across the United States and make any future pregnancy attempt for her even more risky.
The weeks and months that followed were a blur of grief, anger, and medical testing.
But she received some good news. A second triploidy pregnancy was extremely unlikely.
Several weeks later, Ms. DeSpain got more good news.
“I had a follow-up cancer appointment, and everything was completely clear,” she says.
She remains hopeful that she will be able to give birth, but her doctor cautioned that it’s no longer safe to become pregnant in Texas.
“I need you to understand that if you get pregnant and you have complications, we can’t intervene unless the baby doesn’t have a heartbeat, even if it would save your life,” Ms. DeSpain recalls her doctor saying.
If Texas remains a dangerous place to be pregnant, Ms. DeSpain and her husband will have to move.
For now, Ms. DeSpain wants people to know her story and to continue to fight for her right to govern her body.
In a public post to Facebook, she laid bare her pregnancy journey.
“No one should have to share a story like mine to justify abortion,” she wrote. “My choice is not yours to judge, and my rights are not yours to gleefully take away.”
Ms. Abrams, Ms. DeSpain, Dr. Harris, Dr. Jain, and Dr. Mims have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The drive from Texas to the clinic in Albuquerque, N.M., took 10 hours. It was mid-April of this year. There wasn’t much to see along the mostly barren stretch, and there wasn’t much for Kailee DeSpain to do aside from think about where she was going and why.
Her husband was driving. She sensed his nervous glances toward the passenger seat where she sat struggling to quiet her thoughts.
No, she wasn’t having any pain, she told him. No, she wasn’t feeling like she did the last time or the two times before that.
This pregnancy was different. It was the first in which she feared for her own life. Her fetus – Finley – had triploidy, a rare chromosomal abnormality. Because of the condition, which affects 1%-3% of pregnancies, his heart, brain, and kidneys were not developing properly.
At 19 weeks, Finley was already struggling to draw breath from lungs squeezed inside an overcrowded chest cavity. Ms. DeSpain wanted nothing more than to carry Finley to term, hold him, meet him even for a moment before saying goodbye.
But his condition meant he would likely suffocate in utero well before that. And Ms. DeSpain knew that carrying him longer would likely raise her risk of bleeding and of her blood pressure increasing to dangerous highs.
“This could kill you,” her husband told her. “Do you realize you could die bringing a baby into this world who is not going to live? I don’t want to lose you.’”
Unlike her other pregnancies, the timing of this one and the decision she faced to end it put her health in even greater danger.
Imminent danger
On Sept. 1, 2021, a bill went into effect in Texas that banned abortions from as early as 6 weeks’ gestation. Texas Senate Bill 8 (SB8) became one of the most restrictive abortion laws in the country. It prohibited abortions whenever a fetal heartbeat, defined by lawmakers, could be detected on an ultrasound, often before many women knew they were pregnant.
The Texas abortion law was hardly the last word on the topic. Ms. DeSpain didn’t know it on her drive to New Mexico in April, but the U.S. Supreme Court was weeks away from overturning the landmark Roe v. Wade decision.
On June 24, the Supreme Court delivered its 6-3 ruling overturning Roe v. Wade, the 1973 case that granted women the right to abortion.
This decision set in motion “trigger laws” in some states – laws that essentially fully banned abortions. Those states included Ms. DeSpain’s home state of Texas, where abortion is now a felony except when the life of the mother is in peril.
However, legal definitions of what qualifies as “life-threatening” remain murky.
The law is unclear, says Lisa Harris, MD, PhD, professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor. “What does the risk of death have to be, and how imminent must it be?” she asked in a recent editorial in the New England Journal of Medicine. Is 25% enough? 50%? Or does a woman have to be moments from dying?
“This whole thing makes me so angry,” says Shikha Jain, MD, a medical oncologist at University of Illinois Health, Chicago. “A patient may not be experiencing an emergency right now, but if we don’t take care of the situation, it may become an emergency in 2 hours or 2 days.”
Even before the Roe v. Wade decision, pregnancy had been a high-stakes endeavor for many women. In 2019, more than 750 women died from pregnancy-related events in the United States. In 2020, that number rose to 850. Each year dozens more suffer pregnancy-related events that require lifesaving interventions.
Now, in a post-Roe world, the number of maternal deaths will likely climb as more abortion bans take effect and fewer women have access to lifesaving care, experts say. A 2021 study that compared 2017 maternal mortality rates in states with different levels of abortion restrictions found that the rate of maternal mortality was almost two times higher in states that restricted abortion access compared with those that protected it – 28.5 per 100,000 women vs. 15.7.
Some women living in states with abortion bans won’t have the resources to cross state lines for care.
“This is just going to widen the health care disparities that are already so prevalent in this country,” Dr. Jain says.
Navigating a crossroads
Ms. DeSpain’s medical history reads like a checklist of pregnancy-related perils: chronic high blood pressure, persistent clotting problems, and a high risk of hemorrhage. She was also diagnosed with cervical cancer in 2020, which left her body more fragile.
Cardiovascular conditions, including hypertension and hemorrhage, are the leading causes of maternal mortality, responsible for more than one-third of pregnancy-related deaths. Preeclampsia, characterized by high blood pressure, accounts for more than 7% of maternal deaths in the United States. Although less common, genetic disorders, such as spinal muscular atrophy and triploidy, or cancer during pregnancy can put a mother and fetus at risk.
Cancer – which affects about 1 in 1,000 pregnant women and results in termination in as many as 28% of cases – brings sharp focus to the new dangers and complex decision-making patients and their doctors face as abortion bans take hold.
Before the Supreme Court decision, a pregnant woman with cancer was already facing great uncertainty. The decision to treat cancer during pregnancy involves “weighing the risk of exposing the fetus to medication vs. the risk to the mother’s untreated illness if you don’t expose the fetus to medication,” Elyce Cardonick, MD, an obstetrician at Cooper University Health Care, Camden, N.J., who specializes in high-risk pregnancies, told the National Cancer Institute.
Oncologists generally agree that it’s safe for pregnant women to receive chemotherapy during the second and third trimesters. But for women with aggressive cancers that are diagnosed in the first trimester, chemotherapy is dangerous. For women who need immunotherapy, the risks of treatment remain unclear.
In these cases, Alice S. Mims, MD, must broach the possibility of terminating the pregnancy.
“Cancer is a very urgent condition,” says Dr. Mims, a hematology specialist at the Ohio State University Comprehensive Cancer Center, Columbus, who sees patients who are pregnant. “These women may have other children at home, and they want to do their best to fight the disease so they can be around for their family long term.”
Now the changing legal landscape on abortion will put hundreds more pregnant women with cancer in danger. In a recent viewpoint article published in JAMA Oncology, Jordyn Silverstein and Katherine Van Loon, MD, MPH, estimate that during the next year, up to 420 pregnant women living in states with restricted abortion access will face threats to their cancer care and potentially their life.
“The repercussions of overturning Roe v. Wade – and the failure of the Supreme Court to provide any guidance on exceptions related to the life and health of the mother – are potentially catastrophic for a subset of women who face a life-threating diagnosis of [pregnancy-associated cancer],” they write.
The choice Ms. DeSpain faced after her cervical cancer diagnosis was different. She was not pregnant at the time, but she was at a crossroads.
Although it was caught early, the cancer was aggressive. Her oncologist recommended that she undergo a hysterectomy – the surgery that would give her the best chance for a cancer-free future. It would also mean she could no longer become pregnant.
With a less invasive procedure, on the other hand, she could still carry a child, but she would face a much greater chance that the cancer would come back.
At 27, Ms. DeSpain was not ready to close the pregnancy door. She opted for a surgery in which part of her cervix was removed, allowing her to try for another baby.
But she faced a ticking clock in the event her cancer returned.
If you want to have a baby, “try soon,” her doctor warned.
A dead end
After her cancer surgery and a third miscarriage, Ms. DeSpain and her husband were surprised and excited when in late 2021 she again became pregnant.
The first trimester seemed blissfully uneventful. As the weeks passed, Finley’s heart started to beat.
But the 16-week ultrasound signaled a turning point. The sonographer was too quiet.
“This is really bad, isn’t it?” Ms. DeSpain asked her sonographer.
The doctors told her he wouldn’t survive. Finley had no heart chambers. His heart couldn’t pump blood properly. He was missing one kidney, and his brain was split in the back. With almost no amniotic fluid, her doctor said he would likely die in utero, crushed to death without support from the protective liquid.
She fought for him anyway. She sought specialty care, followed bed rest orders, and traveled 3 hours to Houston to enroll in a clinical trial.
But every road was a dead end.
Ultimately, testing revealed Finley had triploidy, and all lines led to one point.
“There were too many things wrong, too much wrong for them to fix,” says Ms. DeSpain, recalling the news from her doctor in Houston. “I was in shock. My husband was just sitting with his hands flat on the table, staring at nothing, shaking a little bit.”
However, Finley still had a heartbeat, making an abortion after 6 weeks a felony in Texas. Even a compassionate induction was now out of the question unless her death was imminent.
Ms. DeSpain called the abortion clinic in Albuquerque and made an appointment. She would have to wait 2 weeks because of an influx of pregnant patients coming from Texas.
She welcomed the wait … just in case she changed her mind.
“At that point I wanted to carry him as far as I could,” she says.
For those 2 weeks, Ms. DeSpain remained on bed rest. She cried all day every day. She worried that Finley was experiencing pain.
Through this process, her doctor’s support helped keep her grounded.
“She cried with us in her office and said, ‘I wish that you didn’t have to go, but I think you’re doing the right thing, doing what keeps you safest,’ “ Ms. DeSpain recalls.
Ms. DeSpain declined to share the name of her doctor out of fear that even expressing compassion for a patient’s safety could put the physician in legal jeopardy and provoke harassment.
That fear is warranted. Some doctors will be forced to choose between doing what is legal – even though the law is vague – and doing what is right for patients, says law professor Jamie Abrams, who was recently diagnosed with breast cancer.
To live in a world where there’s talk of criminalizing doctors for taking care of their patients, where there’s “this national movement to position some women to be shunned and exiled for seeking care that’s right for them, their health, and might save their life is staggering and beyond comprehension,” says Ms. Abrams, professor of law at the American University Washington College of Law.
Ms. Abrams, who was diagnosed with hormone receptor–positive invasive breast cancer the same day she read the leaked Supreme Court draft on the decision to end of Roe v. Wade, said that “overnight, I became a person who would need an abortion if I became pregnant, because my treatment would compromise a healthy birth or delay necessary cancer care.” Ms. Abrams was also told she could no longer use hormonal contraception.
Dr. Harris’s advice to clinicians is to try to do what they feel is best for patients, including referring them to centers that have legal resources and protections regarding abortions.
Dr. Mims agrees and recommends that doctors reach out to those with more resources and legal backing for support. “I would advise doctors in [states with restrictive laws] to familiarize themselves with available resources and organizations taking action to deal with questionable cases,” Dr. Mims says.
‘Baby killers work here’
Following her 10-hour drive to Albuquerque, Ms. DeSpain encountered lines of protesters at the clinic. They were holding signs that said, “Abortion is murder,” and “Baby killers work here.”
“Please don’t kill your baby – we have resources for you,” a woman screeched through a megaphone as Ms. DeSpain, nearly 20 weeks’ pregnant, stepped out of the car to enter the clinic.
“I remember turning around, looking at her and making eye contact, and yelling back, ‘My baby has triploidy – he is dying! He is going to suffocate if I carry him full term. You don’t know what you’re talking about!’ “
A nurse held her hand during the procedure.
“He said, ‘You’re doing great, you’re okay,’ “ she recalls. She knew there was a chance that Finley’s face would be crushed by contractions during labor because of the lack of amniotic fluid, but she hoped not. Ms. DeSpain longed for a photo.
There was no photo to take home the next day, but Ms. DeSpain did receive Finley’s footprints, and his heartbeat – as captured by the specialty team in Houston – lives on in a stuffed giraffe.
His ashes arrived a few weeks later.
By then, the Supreme Court draft had been leaked. Ms. DeSpain knew her predicament in Texas would soon affect women across the United States and make any future pregnancy attempt for her even more risky.
The weeks and months that followed were a blur of grief, anger, and medical testing.
But she received some good news. A second triploidy pregnancy was extremely unlikely.
Several weeks later, Ms. DeSpain got more good news.
“I had a follow-up cancer appointment, and everything was completely clear,” she says.
She remains hopeful that she will be able to give birth, but her doctor cautioned that it’s no longer safe to become pregnant in Texas.
“I need you to understand that if you get pregnant and you have complications, we can’t intervene unless the baby doesn’t have a heartbeat, even if it would save your life,” Ms. DeSpain recalls her doctor saying.
If Texas remains a dangerous place to be pregnant, Ms. DeSpain and her husband will have to move.
For now, Ms. DeSpain wants people to know her story and to continue to fight for her right to govern her body.
In a public post to Facebook, she laid bare her pregnancy journey.
“No one should have to share a story like mine to justify abortion,” she wrote. “My choice is not yours to judge, and my rights are not yours to gleefully take away.”
Ms. Abrams, Ms. DeSpain, Dr. Harris, Dr. Jain, and Dr. Mims have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The drive from Texas to the clinic in Albuquerque, N.M., took 10 hours. It was mid-April of this year. There wasn’t much to see along the mostly barren stretch, and there wasn’t much for Kailee DeSpain to do aside from think about where she was going and why.
Her husband was driving. She sensed his nervous glances toward the passenger seat where she sat struggling to quiet her thoughts.
No, she wasn’t having any pain, she told him. No, she wasn’t feeling like she did the last time or the two times before that.
This pregnancy was different. It was the first in which she feared for her own life. Her fetus – Finley – had triploidy, a rare chromosomal abnormality. Because of the condition, which affects 1%-3% of pregnancies, his heart, brain, and kidneys were not developing properly.
At 19 weeks, Finley was already struggling to draw breath from lungs squeezed inside an overcrowded chest cavity. Ms. DeSpain wanted nothing more than to carry Finley to term, hold him, meet him even for a moment before saying goodbye.
But his condition meant he would likely suffocate in utero well before that. And Ms. DeSpain knew that carrying him longer would likely raise her risk of bleeding and of her blood pressure increasing to dangerous highs.
“This could kill you,” her husband told her. “Do you realize you could die bringing a baby into this world who is not going to live? I don’t want to lose you.’”
Unlike her other pregnancies, the timing of this one and the decision she faced to end it put her health in even greater danger.
Imminent danger
On Sept. 1, 2021, a bill went into effect in Texas that banned abortions from as early as 6 weeks’ gestation. Texas Senate Bill 8 (SB8) became one of the most restrictive abortion laws in the country. It prohibited abortions whenever a fetal heartbeat, defined by lawmakers, could be detected on an ultrasound, often before many women knew they were pregnant.
The Texas abortion law was hardly the last word on the topic. Ms. DeSpain didn’t know it on her drive to New Mexico in April, but the U.S. Supreme Court was weeks away from overturning the landmark Roe v. Wade decision.
On June 24, the Supreme Court delivered its 6-3 ruling overturning Roe v. Wade, the 1973 case that granted women the right to abortion.
This decision set in motion “trigger laws” in some states – laws that essentially fully banned abortions. Those states included Ms. DeSpain’s home state of Texas, where abortion is now a felony except when the life of the mother is in peril.
However, legal definitions of what qualifies as “life-threatening” remain murky.
The law is unclear, says Lisa Harris, MD, PhD, professor in the department of obstetrics and gynecology at the University of Michigan, Ann Arbor. “What does the risk of death have to be, and how imminent must it be?” she asked in a recent editorial in the New England Journal of Medicine. Is 25% enough? 50%? Or does a woman have to be moments from dying?
“This whole thing makes me so angry,” says Shikha Jain, MD, a medical oncologist at University of Illinois Health, Chicago. “A patient may not be experiencing an emergency right now, but if we don’t take care of the situation, it may become an emergency in 2 hours or 2 days.”
Even before the Roe v. Wade decision, pregnancy had been a high-stakes endeavor for many women. In 2019, more than 750 women died from pregnancy-related events in the United States. In 2020, that number rose to 850. Each year dozens more suffer pregnancy-related events that require lifesaving interventions.
Now, in a post-Roe world, the number of maternal deaths will likely climb as more abortion bans take effect and fewer women have access to lifesaving care, experts say. A 2021 study that compared 2017 maternal mortality rates in states with different levels of abortion restrictions found that the rate of maternal mortality was almost two times higher in states that restricted abortion access compared with those that protected it – 28.5 per 100,000 women vs. 15.7.
Some women living in states with abortion bans won’t have the resources to cross state lines for care.
“This is just going to widen the health care disparities that are already so prevalent in this country,” Dr. Jain says.
Navigating a crossroads
Ms. DeSpain’s medical history reads like a checklist of pregnancy-related perils: chronic high blood pressure, persistent clotting problems, and a high risk of hemorrhage. She was also diagnosed with cervical cancer in 2020, which left her body more fragile.
Cardiovascular conditions, including hypertension and hemorrhage, are the leading causes of maternal mortality, responsible for more than one-third of pregnancy-related deaths. Preeclampsia, characterized by high blood pressure, accounts for more than 7% of maternal deaths in the United States. Although less common, genetic disorders, such as spinal muscular atrophy and triploidy, or cancer during pregnancy can put a mother and fetus at risk.
Cancer – which affects about 1 in 1,000 pregnant women and results in termination in as many as 28% of cases – brings sharp focus to the new dangers and complex decision-making patients and their doctors face as abortion bans take hold.
Before the Supreme Court decision, a pregnant woman with cancer was already facing great uncertainty. The decision to treat cancer during pregnancy involves “weighing the risk of exposing the fetus to medication vs. the risk to the mother’s untreated illness if you don’t expose the fetus to medication,” Elyce Cardonick, MD, an obstetrician at Cooper University Health Care, Camden, N.J., who specializes in high-risk pregnancies, told the National Cancer Institute.
Oncologists generally agree that it’s safe for pregnant women to receive chemotherapy during the second and third trimesters. But for women with aggressive cancers that are diagnosed in the first trimester, chemotherapy is dangerous. For women who need immunotherapy, the risks of treatment remain unclear.
In these cases, Alice S. Mims, MD, must broach the possibility of terminating the pregnancy.
“Cancer is a very urgent condition,” says Dr. Mims, a hematology specialist at the Ohio State University Comprehensive Cancer Center, Columbus, who sees patients who are pregnant. “These women may have other children at home, and they want to do their best to fight the disease so they can be around for their family long term.”
Now the changing legal landscape on abortion will put hundreds more pregnant women with cancer in danger. In a recent viewpoint article published in JAMA Oncology, Jordyn Silverstein and Katherine Van Loon, MD, MPH, estimate that during the next year, up to 420 pregnant women living in states with restricted abortion access will face threats to their cancer care and potentially their life.
“The repercussions of overturning Roe v. Wade – and the failure of the Supreme Court to provide any guidance on exceptions related to the life and health of the mother – are potentially catastrophic for a subset of women who face a life-threating diagnosis of [pregnancy-associated cancer],” they write.
The choice Ms. DeSpain faced after her cervical cancer diagnosis was different. She was not pregnant at the time, but she was at a crossroads.
Although it was caught early, the cancer was aggressive. Her oncologist recommended that she undergo a hysterectomy – the surgery that would give her the best chance for a cancer-free future. It would also mean she could no longer become pregnant.
With a less invasive procedure, on the other hand, she could still carry a child, but she would face a much greater chance that the cancer would come back.
At 27, Ms. DeSpain was not ready to close the pregnancy door. She opted for a surgery in which part of her cervix was removed, allowing her to try for another baby.
But she faced a ticking clock in the event her cancer returned.
If you want to have a baby, “try soon,” her doctor warned.
A dead end
After her cancer surgery and a third miscarriage, Ms. DeSpain and her husband were surprised and excited when in late 2021 she again became pregnant.
The first trimester seemed blissfully uneventful. As the weeks passed, Finley’s heart started to beat.
But the 16-week ultrasound signaled a turning point. The sonographer was too quiet.
“This is really bad, isn’t it?” Ms. DeSpain asked her sonographer.
The doctors told her he wouldn’t survive. Finley had no heart chambers. His heart couldn’t pump blood properly. He was missing one kidney, and his brain was split in the back. With almost no amniotic fluid, her doctor said he would likely die in utero, crushed to death without support from the protective liquid.
She fought for him anyway. She sought specialty care, followed bed rest orders, and traveled 3 hours to Houston to enroll in a clinical trial.
But every road was a dead end.
Ultimately, testing revealed Finley had triploidy, and all lines led to one point.
“There were too many things wrong, too much wrong for them to fix,” says Ms. DeSpain, recalling the news from her doctor in Houston. “I was in shock. My husband was just sitting with his hands flat on the table, staring at nothing, shaking a little bit.”
However, Finley still had a heartbeat, making an abortion after 6 weeks a felony in Texas. Even a compassionate induction was now out of the question unless her death was imminent.
Ms. DeSpain called the abortion clinic in Albuquerque and made an appointment. She would have to wait 2 weeks because of an influx of pregnant patients coming from Texas.
She welcomed the wait … just in case she changed her mind.
“At that point I wanted to carry him as far as I could,” she says.
For those 2 weeks, Ms. DeSpain remained on bed rest. She cried all day every day. She worried that Finley was experiencing pain.
Through this process, her doctor’s support helped keep her grounded.
“She cried with us in her office and said, ‘I wish that you didn’t have to go, but I think you’re doing the right thing, doing what keeps you safest,’ “ Ms. DeSpain recalls.
Ms. DeSpain declined to share the name of her doctor out of fear that even expressing compassion for a patient’s safety could put the physician in legal jeopardy and provoke harassment.
That fear is warranted. Some doctors will be forced to choose between doing what is legal – even though the law is vague – and doing what is right for patients, says law professor Jamie Abrams, who was recently diagnosed with breast cancer.
To live in a world where there’s talk of criminalizing doctors for taking care of their patients, where there’s “this national movement to position some women to be shunned and exiled for seeking care that’s right for them, their health, and might save their life is staggering and beyond comprehension,” says Ms. Abrams, professor of law at the American University Washington College of Law.
Ms. Abrams, who was diagnosed with hormone receptor–positive invasive breast cancer the same day she read the leaked Supreme Court draft on the decision to end of Roe v. Wade, said that “overnight, I became a person who would need an abortion if I became pregnant, because my treatment would compromise a healthy birth or delay necessary cancer care.” Ms. Abrams was also told she could no longer use hormonal contraception.
Dr. Harris’s advice to clinicians is to try to do what they feel is best for patients, including referring them to centers that have legal resources and protections regarding abortions.
Dr. Mims agrees and recommends that doctors reach out to those with more resources and legal backing for support. “I would advise doctors in [states with restrictive laws] to familiarize themselves with available resources and organizations taking action to deal with questionable cases,” Dr. Mims says.
‘Baby killers work here’
Following her 10-hour drive to Albuquerque, Ms. DeSpain encountered lines of protesters at the clinic. They were holding signs that said, “Abortion is murder,” and “Baby killers work here.”
“Please don’t kill your baby – we have resources for you,” a woman screeched through a megaphone as Ms. DeSpain, nearly 20 weeks’ pregnant, stepped out of the car to enter the clinic.
“I remember turning around, looking at her and making eye contact, and yelling back, ‘My baby has triploidy – he is dying! He is going to suffocate if I carry him full term. You don’t know what you’re talking about!’ “
A nurse held her hand during the procedure.
“He said, ‘You’re doing great, you’re okay,’ “ she recalls. She knew there was a chance that Finley’s face would be crushed by contractions during labor because of the lack of amniotic fluid, but she hoped not. Ms. DeSpain longed for a photo.
There was no photo to take home the next day, but Ms. DeSpain did receive Finley’s footprints, and his heartbeat – as captured by the specialty team in Houston – lives on in a stuffed giraffe.
His ashes arrived a few weeks later.
By then, the Supreme Court draft had been leaked. Ms. DeSpain knew her predicament in Texas would soon affect women across the United States and make any future pregnancy attempt for her even more risky.
The weeks and months that followed were a blur of grief, anger, and medical testing.
But she received some good news. A second triploidy pregnancy was extremely unlikely.
Several weeks later, Ms. DeSpain got more good news.
“I had a follow-up cancer appointment, and everything was completely clear,” she says.
She remains hopeful that she will be able to give birth, but her doctor cautioned that it’s no longer safe to become pregnant in Texas.
“I need you to understand that if you get pregnant and you have complications, we can’t intervene unless the baby doesn’t have a heartbeat, even if it would save your life,” Ms. DeSpain recalls her doctor saying.
If Texas remains a dangerous place to be pregnant, Ms. DeSpain and her husband will have to move.
For now, Ms. DeSpain wants people to know her story and to continue to fight for her right to govern her body.
In a public post to Facebook, she laid bare her pregnancy journey.
“No one should have to share a story like mine to justify abortion,” she wrote. “My choice is not yours to judge, and my rights are not yours to gleefully take away.”
Ms. Abrams, Ms. DeSpain, Dr. Harris, Dr. Jain, and Dr. Mims have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Indiana’s new abortion ban may drive some young ob.gyns. to leave a state where they’re needed
On a Monday morning, a group of obstetrics and gynecology residents, dressed in blue scrubs and white coats, gathered in an auditorium at Indiana University, Indianapolis. After the usual updates and announcements, Nicole Scott, MD, the residency program director, addressed the elephant in the room. “Any more abortion care questions?” she asked the trainees.
After a few moments of silence, one resident asked: “How’s Dr. Bernard doing?”
“Bernard is actually in really good spirits – I mean, relatively,” Dr. Scott answered. “She has 24/7 security, has her own lawyer.”
They were talking about Caitlin Bernard, MD, an Indiana ob.gyn. who provides abortions and trains residents at the university hospital. Dr. Bernard was recently caught in a political whirlwind after she spoke about an abortion she provided to a 10-year-old rape victim from Ohio. Dr. Bernard was the target of false accusations made on national television by pundits and political leaders, including Indiana’s attorney general.
The doctors interviewed for this article said that they are not speaking on behalf of their school of medicine but rather about their personal experiences during a tumultuous moment that they worry will affect the way they care for their patients.
The vitriol directed at Dr. Bernard hit home for this group of residents. She has mentored most of them for years. Many of the young doctors were certain they wanted to practice in Indiana after their training. But lately, some have been ambivalent about that prospect.
Beatrice Soderholm, DO, a fourth-year ob.gyn. resident, said watching what Dr. Bernard went through was “scary.” “I think that was part of the point for those who were putting her through that,” Dr. Soderholm said. They were trying “to scare other people out of doing the work that she does.”
In early August, Gov. Eric Holcomb, a Republican, signed a near-total abortion ban into law, making Indiana the first state to adopt new restrictions on abortion access since the Supreme Court struck down Roe v. Wade in June. When the ban takes effect Sept. 15, medical providers who violate the law risk losing their licenses or serving up to six years in prison.
These days, Dr. Scott, the residency program director, uses some meeting time with residents to fill them in on political updates and available mental health services. She also reminds them that legal counsel is on call round the clock to help if they’re ever unsure about the care they should provide a patient.
“Our residents are devastated,” Dr. Scott said, holding back tears. “They signed up to provide comprehensive health care to women, and they are being told that they can’t do that.”
She expects this will “deeply impact” how Indiana hospitals recruit and retain medical professionals.
A 2018 report from the March of Dimes found that 27% of Indiana counties are considered maternity care deserts, with no or limited access to maternal care. The state has one of the nation’s highest maternal mortality rates.
Dr. Scott said new laws restricting abortion will only worsen those statistics.
Dr. Scott shared results from a recent survey of nearly 1,400 residents and fellows across all specialties at IU, nearly 80% of the trainees said they were less likely to stay and practice in Indiana after the abortion ban.
Wendy Tian, MD, a third-year resident, said she is worried about her safety. Dr. Tian grew up and went to medical school in Chicago and chose to do her residency in Indiana because the program has a strong family-planning focus. She was open to practicing in Indiana when she completed her training.
But that’s changed.
“I, for sure, don’t know if I would be able to stay in Indiana post graduation with what’s going on,” Dr. Tian said.
Still, she feels guilty for “giving up” on Indiana’s most vulnerable patients.
Even before Roe fell, Dr. Tian said, the climate in Indiana could be hostile and frustrating for ob.gyns. Indiana, like other states with abortion restrictions, allows nearly all health care providers to opt out of providing care to patients having an abortion.
“We encounter other people who we work with on a daily basis who are opposed to what we do,” Dr. Tian said, adding that she and her colleagues have had to cancel scheduled procedures because the nurses on call were not comfortable assisting during an abortion.
Dr. Scott said the ob.gyn. program at the IU has provided residents with comprehensive training, including on abortion care and family planning. Since miscarriages are managed the same way as first-trimester abortions, she said, the training gives residents lots of hands-on experience. “What termination procedures allow you to do is that kind of repetition and that understanding of the female anatomy and how to manage complications that may happen with miscarriages.”
The ban on abortions dramatically reduces the hands-on opportunities for ob.gyn. residents, and that’s a huge concern, she said.
The program is exploring ways to offer training. One option is to send residents to learn in states without abortion restrictions, but Dr. Scott said that would be a logistical nightmare. “This is not as simple as just showing up to an office and saying: ‘Can I observe?’ This includes getting a medical license for out-of-state trainees. This includes funding for travel and lodging,” Dr. Scott said. “It adds a lot to what we already do to educate future ob.gyns.”
Four in 10 of all ob.gyn. residents in the United States are in states where abortion is banned or likely to be banned, so there could be a surge of residents looking to go out of state to make up for lost training opportunities. The Accreditation Council for Graduate Medical Education, the body that accredits residency programs, proposed modifications to the graduation requirements for ob.gyn. residents to account for the changing landscape.
For some of the Indiana ob.gyn. residents – including Veronica Santana, MD, a first-year resident – these political hurdles are a challenge they’re more than willing to take on. Dr. Santana is Latina, grew up in Seattle, and has been involved in community organizing since she was a teenager. One reason she chose obstetrics and gynecology was because of how the field intersects with social justice. “It’s political. It always has been, and it continues to be,” she said, “And, obviously, especially now.”
After Roe was overturned, Dr. Santana, alongside other residents and mentors, took to the streets of Indianapolis to participate in rallies in support of abortion rights.
Indiana could be the perfect battleground for Dr. Santana’s advocacy and social activism. But lately, she said, she is “very unsure” whether staying in Indiana to practice after residency makes sense, since she wants to provide the entire range of ob.gyn. services.
Dr. Soderholm, who grew up in Minnesota, has felt a strong connection to patients at the county hospital in Indianapolis. She had been certain she wanted to practice in Indiana. But her family in Minnesota – where abortion remains largely protected – has recently questioned why she would stay in a state with such a hostile climate for ob.gyns. “There’s been a lot of hesitation,” she said. But the patients make leaving difficult. “Sorry,” she said, starting to cry.
It’s for those patients that Dr. Soderholm decided she’ll likely stay. Other young doctors may make a different decision.
This story is part of a partnership that includes Side Effects Public Media, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On a Monday morning, a group of obstetrics and gynecology residents, dressed in blue scrubs and white coats, gathered in an auditorium at Indiana University, Indianapolis. After the usual updates and announcements, Nicole Scott, MD, the residency program director, addressed the elephant in the room. “Any more abortion care questions?” she asked the trainees.
After a few moments of silence, one resident asked: “How’s Dr. Bernard doing?”
“Bernard is actually in really good spirits – I mean, relatively,” Dr. Scott answered. “She has 24/7 security, has her own lawyer.”
They were talking about Caitlin Bernard, MD, an Indiana ob.gyn. who provides abortions and trains residents at the university hospital. Dr. Bernard was recently caught in a political whirlwind after she spoke about an abortion she provided to a 10-year-old rape victim from Ohio. Dr. Bernard was the target of false accusations made on national television by pundits and political leaders, including Indiana’s attorney general.
The doctors interviewed for this article said that they are not speaking on behalf of their school of medicine but rather about their personal experiences during a tumultuous moment that they worry will affect the way they care for their patients.
The vitriol directed at Dr. Bernard hit home for this group of residents. She has mentored most of them for years. Many of the young doctors were certain they wanted to practice in Indiana after their training. But lately, some have been ambivalent about that prospect.
Beatrice Soderholm, DO, a fourth-year ob.gyn. resident, said watching what Dr. Bernard went through was “scary.” “I think that was part of the point for those who were putting her through that,” Dr. Soderholm said. They were trying “to scare other people out of doing the work that she does.”
In early August, Gov. Eric Holcomb, a Republican, signed a near-total abortion ban into law, making Indiana the first state to adopt new restrictions on abortion access since the Supreme Court struck down Roe v. Wade in June. When the ban takes effect Sept. 15, medical providers who violate the law risk losing their licenses or serving up to six years in prison.
These days, Dr. Scott, the residency program director, uses some meeting time with residents to fill them in on political updates and available mental health services. She also reminds them that legal counsel is on call round the clock to help if they’re ever unsure about the care they should provide a patient.
“Our residents are devastated,” Dr. Scott said, holding back tears. “They signed up to provide comprehensive health care to women, and they are being told that they can’t do that.”
She expects this will “deeply impact” how Indiana hospitals recruit and retain medical professionals.
A 2018 report from the March of Dimes found that 27% of Indiana counties are considered maternity care deserts, with no or limited access to maternal care. The state has one of the nation’s highest maternal mortality rates.
Dr. Scott said new laws restricting abortion will only worsen those statistics.
Dr. Scott shared results from a recent survey of nearly 1,400 residents and fellows across all specialties at IU, nearly 80% of the trainees said they were less likely to stay and practice in Indiana after the abortion ban.
Wendy Tian, MD, a third-year resident, said she is worried about her safety. Dr. Tian grew up and went to medical school in Chicago and chose to do her residency in Indiana because the program has a strong family-planning focus. She was open to practicing in Indiana when she completed her training.
But that’s changed.
“I, for sure, don’t know if I would be able to stay in Indiana post graduation with what’s going on,” Dr. Tian said.
Still, she feels guilty for “giving up” on Indiana’s most vulnerable patients.
Even before Roe fell, Dr. Tian said, the climate in Indiana could be hostile and frustrating for ob.gyns. Indiana, like other states with abortion restrictions, allows nearly all health care providers to opt out of providing care to patients having an abortion.
“We encounter other people who we work with on a daily basis who are opposed to what we do,” Dr. Tian said, adding that she and her colleagues have had to cancel scheduled procedures because the nurses on call were not comfortable assisting during an abortion.
Dr. Scott said the ob.gyn. program at the IU has provided residents with comprehensive training, including on abortion care and family planning. Since miscarriages are managed the same way as first-trimester abortions, she said, the training gives residents lots of hands-on experience. “What termination procedures allow you to do is that kind of repetition and that understanding of the female anatomy and how to manage complications that may happen with miscarriages.”
The ban on abortions dramatically reduces the hands-on opportunities for ob.gyn. residents, and that’s a huge concern, she said.
The program is exploring ways to offer training. One option is to send residents to learn in states without abortion restrictions, but Dr. Scott said that would be a logistical nightmare. “This is not as simple as just showing up to an office and saying: ‘Can I observe?’ This includes getting a medical license for out-of-state trainees. This includes funding for travel and lodging,” Dr. Scott said. “It adds a lot to what we already do to educate future ob.gyns.”
Four in 10 of all ob.gyn. residents in the United States are in states where abortion is banned or likely to be banned, so there could be a surge of residents looking to go out of state to make up for lost training opportunities. The Accreditation Council for Graduate Medical Education, the body that accredits residency programs, proposed modifications to the graduation requirements for ob.gyn. residents to account for the changing landscape.
For some of the Indiana ob.gyn. residents – including Veronica Santana, MD, a first-year resident – these political hurdles are a challenge they’re more than willing to take on. Dr. Santana is Latina, grew up in Seattle, and has been involved in community organizing since she was a teenager. One reason she chose obstetrics and gynecology was because of how the field intersects with social justice. “It’s political. It always has been, and it continues to be,” she said, “And, obviously, especially now.”
After Roe was overturned, Dr. Santana, alongside other residents and mentors, took to the streets of Indianapolis to participate in rallies in support of abortion rights.
Indiana could be the perfect battleground for Dr. Santana’s advocacy and social activism. But lately, she said, she is “very unsure” whether staying in Indiana to practice after residency makes sense, since she wants to provide the entire range of ob.gyn. services.
Dr. Soderholm, who grew up in Minnesota, has felt a strong connection to patients at the county hospital in Indianapolis. She had been certain she wanted to practice in Indiana. But her family in Minnesota – where abortion remains largely protected – has recently questioned why she would stay in a state with such a hostile climate for ob.gyns. “There’s been a lot of hesitation,” she said. But the patients make leaving difficult. “Sorry,” she said, starting to cry.
It’s for those patients that Dr. Soderholm decided she’ll likely stay. Other young doctors may make a different decision.
This story is part of a partnership that includes Side Effects Public Media, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On a Monday morning, a group of obstetrics and gynecology residents, dressed in blue scrubs and white coats, gathered in an auditorium at Indiana University, Indianapolis. After the usual updates and announcements, Nicole Scott, MD, the residency program director, addressed the elephant in the room. “Any more abortion care questions?” she asked the trainees.
After a few moments of silence, one resident asked: “How’s Dr. Bernard doing?”
“Bernard is actually in really good spirits – I mean, relatively,” Dr. Scott answered. “She has 24/7 security, has her own lawyer.”
They were talking about Caitlin Bernard, MD, an Indiana ob.gyn. who provides abortions and trains residents at the university hospital. Dr. Bernard was recently caught in a political whirlwind after she spoke about an abortion she provided to a 10-year-old rape victim from Ohio. Dr. Bernard was the target of false accusations made on national television by pundits and political leaders, including Indiana’s attorney general.
The doctors interviewed for this article said that they are not speaking on behalf of their school of medicine but rather about their personal experiences during a tumultuous moment that they worry will affect the way they care for their patients.
The vitriol directed at Dr. Bernard hit home for this group of residents. She has mentored most of them for years. Many of the young doctors were certain they wanted to practice in Indiana after their training. But lately, some have been ambivalent about that prospect.
Beatrice Soderholm, DO, a fourth-year ob.gyn. resident, said watching what Dr. Bernard went through was “scary.” “I think that was part of the point for those who were putting her through that,” Dr. Soderholm said. They were trying “to scare other people out of doing the work that she does.”
In early August, Gov. Eric Holcomb, a Republican, signed a near-total abortion ban into law, making Indiana the first state to adopt new restrictions on abortion access since the Supreme Court struck down Roe v. Wade in June. When the ban takes effect Sept. 15, medical providers who violate the law risk losing their licenses or serving up to six years in prison.
These days, Dr. Scott, the residency program director, uses some meeting time with residents to fill them in on political updates and available mental health services. She also reminds them that legal counsel is on call round the clock to help if they’re ever unsure about the care they should provide a patient.
“Our residents are devastated,” Dr. Scott said, holding back tears. “They signed up to provide comprehensive health care to women, and they are being told that they can’t do that.”
She expects this will “deeply impact” how Indiana hospitals recruit and retain medical professionals.
A 2018 report from the March of Dimes found that 27% of Indiana counties are considered maternity care deserts, with no or limited access to maternal care. The state has one of the nation’s highest maternal mortality rates.
Dr. Scott said new laws restricting abortion will only worsen those statistics.
Dr. Scott shared results from a recent survey of nearly 1,400 residents and fellows across all specialties at IU, nearly 80% of the trainees said they were less likely to stay and practice in Indiana after the abortion ban.
Wendy Tian, MD, a third-year resident, said she is worried about her safety. Dr. Tian grew up and went to medical school in Chicago and chose to do her residency in Indiana because the program has a strong family-planning focus. She was open to practicing in Indiana when she completed her training.
But that’s changed.
“I, for sure, don’t know if I would be able to stay in Indiana post graduation with what’s going on,” Dr. Tian said.
Still, she feels guilty for “giving up” on Indiana’s most vulnerable patients.
Even before Roe fell, Dr. Tian said, the climate in Indiana could be hostile and frustrating for ob.gyns. Indiana, like other states with abortion restrictions, allows nearly all health care providers to opt out of providing care to patients having an abortion.
“We encounter other people who we work with on a daily basis who are opposed to what we do,” Dr. Tian said, adding that she and her colleagues have had to cancel scheduled procedures because the nurses on call were not comfortable assisting during an abortion.
Dr. Scott said the ob.gyn. program at the IU has provided residents with comprehensive training, including on abortion care and family planning. Since miscarriages are managed the same way as first-trimester abortions, she said, the training gives residents lots of hands-on experience. “What termination procedures allow you to do is that kind of repetition and that understanding of the female anatomy and how to manage complications that may happen with miscarriages.”
The ban on abortions dramatically reduces the hands-on opportunities for ob.gyn. residents, and that’s a huge concern, she said.
The program is exploring ways to offer training. One option is to send residents to learn in states without abortion restrictions, but Dr. Scott said that would be a logistical nightmare. “This is not as simple as just showing up to an office and saying: ‘Can I observe?’ This includes getting a medical license for out-of-state trainees. This includes funding for travel and lodging,” Dr. Scott said. “It adds a lot to what we already do to educate future ob.gyns.”
Four in 10 of all ob.gyn. residents in the United States are in states where abortion is banned or likely to be banned, so there could be a surge of residents looking to go out of state to make up for lost training opportunities. The Accreditation Council for Graduate Medical Education, the body that accredits residency programs, proposed modifications to the graduation requirements for ob.gyn. residents to account for the changing landscape.
For some of the Indiana ob.gyn. residents – including Veronica Santana, MD, a first-year resident – these political hurdles are a challenge they’re more than willing to take on. Dr. Santana is Latina, grew up in Seattle, and has been involved in community organizing since she was a teenager. One reason she chose obstetrics and gynecology was because of how the field intersects with social justice. “It’s political. It always has been, and it continues to be,” she said, “And, obviously, especially now.”
After Roe was overturned, Dr. Santana, alongside other residents and mentors, took to the streets of Indianapolis to participate in rallies in support of abortion rights.
Indiana could be the perfect battleground for Dr. Santana’s advocacy and social activism. But lately, she said, she is “very unsure” whether staying in Indiana to practice after residency makes sense, since she wants to provide the entire range of ob.gyn. services.
Dr. Soderholm, who grew up in Minnesota, has felt a strong connection to patients at the county hospital in Indianapolis. She had been certain she wanted to practice in Indiana. But her family in Minnesota – where abortion remains largely protected – has recently questioned why she would stay in a state with such a hostile climate for ob.gyns. “There’s been a lot of hesitation,” she said. But the patients make leaving difficult. “Sorry,” she said, starting to cry.
It’s for those patients that Dr. Soderholm decided she’ll likely stay. Other young doctors may make a different decision.
This story is part of a partnership that includes Side Effects Public Media, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.