User login
Official Newspaper of the American College of Surgeons
Elevated glucose after colorectal surgery spells trouble
INDIANAPOLIS – Even a single brief episode of postoperative hyperglycemia after colorectal resection in nondiabetic patients was independently associated with increased morbidity and mortality in a large consecutive patient series.
The risks of a variety of complications, both infectious and noninfectious, in nondiabetic patients with postoperative hyperglycemia were similar in magnitude to those seen in diabetic patients experiencing postoperative hyperglycemia.
"A take-home point from our study would be that since it’s known that in diabetic patients it’s absolutely paramount to control hyperglycemia, perhaps nondiabetic patients undergoing major operations – especially colorectal surgery – need to be carefully monitored and have their glucose managed," Dr. P. Ravi Kiran said at the annual meeting of the American Surgical Association.
He and his coinvestigators evaluated the significance of hyperglycemia occurring within 48 hours postoperatively in 2,628 consecutive patients undergoing elective colorectal resection at the Cleveland Clinic in a recent 2-year period.
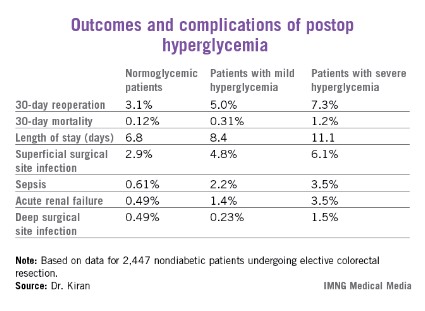
A total of 2,447 of these patients were nondiabetic. They collectively had 16,404 randomly obtained postoperative blood glucose measurements. One-third of them remained normoglycemic, with all their blood glucose values remaining at 125 mg/dL or less. Another 52.7% had one or more episodes of mild hyperglycemia as defined by a blood glucose value of 126-200 mg/dL. And 14% of nondiabetic subjects experienced postoperative severe hyperglycemia, with a level in excess of 200 mg/dL.
Those rates were similar to those of the known-diabetic patients, 35% of whom remained normoglycemic postoperatively, while 54% became mildly hyperglycemic and 11% severely hyperglycemic.
Postoperative hyperglycemia in nondiabetic patients was associated with greater intraoperative estimated blood loss. The transfusion rate was 4.8% in normoglycemic patients, 10.3% in mildly hyperglycemic ones, and 18.1% in those with severe hyperglycemia. The length of surgery averaged 137 minutes in normoglycemic patients, 166 minutes in patients with postoperative mild hyperglycemia, and 181 minutes in patients with severe hyperglycemia.
Any episode of postoperative severe hyperglycemia in the nondiabetic patients was associated with significantly higher rates of both superficial and deep surgical site infections, greater length of stay, and higher 30-day mortality, compared with normoglycemic patients (see chart). Mild hyperglycemia wasn’t associated with as many complications; however, it was linked to a significantly increased rate of sepsis and a greater length of stay.
Moreover, in a multivariate analysis mild hyperglycemia was independently associated with a 2.1-fold increased risk of reoperation within 30 days, while severe hyperglycemia carried a 3.8-fold increased risk, compared with normoglycemia, continued Dr. Kiran of the Cleveland Clinic.
The investigators identified two major independent risk factors for sepsis in nondiabetic patients: an American Society of Anesthesiologists Physical Status class of 3 or more (odds ratio, 4.2), and postoperative hyperglycemia, which was associated with roughly an 8-fold risk regardless of whether the hyperglycemia was mild or severe.
Studies from the cardiovascular and trauma surgery literature suggest postoperative uncontrolled blood glucose may lead to adverse outcomes. Dr. Kiran and his coworkers performed this study because the impact of elevated blood glucose after elective major abdominal surgery had not been well defined, although colorectal surgery entails bacterial contamination, so background rates of surgical site and other infectious complications already tend to run relatively high.
Dr. Kiran said he believes postoperative hyperglycemia is probably a surrogate marker, perhaps for a distressed physiologic state or for looming complications. The next major question he and his coinvestigators want to tackle is this: Do prompt recognition and management of postoperative hyperglycemia in nondiabetic colorectal resection patients improve outcomes?
A cautionary note was sounded by discussant Dr. Hiram C. Polk Jr.,who emphasized that management strategies involving tight glucose control entail the risk of potentially disastrous postsurgical hypoglycemia.
"As many of you know, there are more than half a dozen patients in the midwestern U.S. who’ve been rendered badly hurt with hypoglycemia and cerebral damage. They’re working their way through the legal system at this point. It’s a fine balance: Sometimes perfection is the enemy of good in this situation," warned Dr. Polk, professor and chairman of the surgery department at the University of Louisville (Ky.).
He noted as an aside that just as Dr. Kiran found that even a single episode of postoperative hyperglycemia has adverse consequences, he and his Louisville coworkers have found the same is true for hypothermia.
"A single brief episode of hypothermia seems to throw the wheels off the track. It disrupts something in host defenses and makes everything very difficult," Dr. Polk observed.
The study was sponsored by the Cleveland Clinic Foundation. Dr. Kiran reported no financial conflicts.
INDIANAPOLIS – Even a single brief episode of postoperative hyperglycemia after colorectal resection in nondiabetic patients was independently associated with increased morbidity and mortality in a large consecutive patient series.
The risks of a variety of complications, both infectious and noninfectious, in nondiabetic patients with postoperative hyperglycemia were similar in magnitude to those seen in diabetic patients experiencing postoperative hyperglycemia.
"A take-home point from our study would be that since it’s known that in diabetic patients it’s absolutely paramount to control hyperglycemia, perhaps nondiabetic patients undergoing major operations – especially colorectal surgery – need to be carefully monitored and have their glucose managed," Dr. P. Ravi Kiran said at the annual meeting of the American Surgical Association.

He and his coinvestigators evaluated the significance of hyperglycemia occurring within 48 hours postoperatively in 2,628 consecutive patients undergoing elective colorectal resection at the Cleveland Clinic in a recent 2-year period.
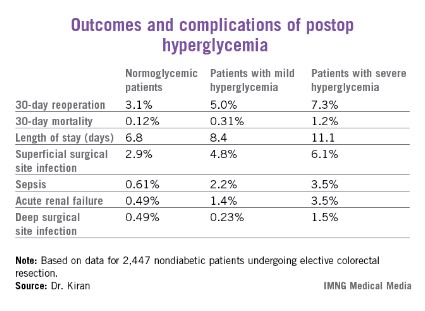
A total of 2,447 of these patients were nondiabetic. They collectively had 16,404 randomly obtained postoperative blood glucose measurements. One-third of them remained normoglycemic, with all their blood glucose values remaining at 125 mg/dL or less. Another 52.7% had one or more episodes of mild hyperglycemia as defined by a blood glucose value of 126-200 mg/dL. And 14% of nondiabetic subjects experienced postoperative severe hyperglycemia, with a level in excess of 200 mg/dL.
Those rates were similar to those of the known-diabetic patients, 35% of whom remained normoglycemic postoperatively, while 54% became mildly hyperglycemic and 11% severely hyperglycemic.
Postoperative hyperglycemia in nondiabetic patients was associated with greater intraoperative estimated blood loss. The transfusion rate was 4.8% in normoglycemic patients, 10.3% in mildly hyperglycemic ones, and 18.1% in those with severe hyperglycemia. The length of surgery averaged 137 minutes in normoglycemic patients, 166 minutes in patients with postoperative mild hyperglycemia, and 181 minutes in patients with severe hyperglycemia.
Any episode of postoperative severe hyperglycemia in the nondiabetic patients was associated with significantly higher rates of both superficial and deep surgical site infections, greater length of stay, and higher 30-day mortality, compared with normoglycemic patients (see chart). Mild hyperglycemia wasn’t associated with as many complications; however, it was linked to a significantly increased rate of sepsis and a greater length of stay.
Moreover, in a multivariate analysis mild hyperglycemia was independently associated with a 2.1-fold increased risk of reoperation within 30 days, while severe hyperglycemia carried a 3.8-fold increased risk, compared with normoglycemia, continued Dr. Kiran of the Cleveland Clinic.
The investigators identified two major independent risk factors for sepsis in nondiabetic patients: an American Society of Anesthesiologists Physical Status class of 3 or more (odds ratio, 4.2), and postoperative hyperglycemia, which was associated with roughly an 8-fold risk regardless of whether the hyperglycemia was mild or severe.
Studies from the cardiovascular and trauma surgery literature suggest postoperative uncontrolled blood glucose may lead to adverse outcomes. Dr. Kiran and his coworkers performed this study because the impact of elevated blood glucose after elective major abdominal surgery had not been well defined, although colorectal surgery entails bacterial contamination, so background rates of surgical site and other infectious complications already tend to run relatively high.
Dr. Kiran said he believes postoperative hyperglycemia is probably a surrogate marker, perhaps for a distressed physiologic state or for looming complications. The next major question he and his coinvestigators want to tackle is this: Do prompt recognition and management of postoperative hyperglycemia in nondiabetic colorectal resection patients improve outcomes?
A cautionary note was sounded by discussant Dr. Hiram C. Polk Jr.,who emphasized that management strategies involving tight glucose control entail the risk of potentially disastrous postsurgical hypoglycemia.
"As many of you know, there are more than half a dozen patients in the midwestern U.S. who’ve been rendered badly hurt with hypoglycemia and cerebral damage. They’re working their way through the legal system at this point. It’s a fine balance: Sometimes perfection is the enemy of good in this situation," warned Dr. Polk, professor and chairman of the surgery department at the University of Louisville (Ky.).
He noted as an aside that just as Dr. Kiran found that even a single episode of postoperative hyperglycemia has adverse consequences, he and his Louisville coworkers have found the same is true for hypothermia.
"A single brief episode of hypothermia seems to throw the wheels off the track. It disrupts something in host defenses and makes everything very difficult," Dr. Polk observed.
The study was sponsored by the Cleveland Clinic Foundation. Dr. Kiran reported no financial conflicts.
INDIANAPOLIS – Even a single brief episode of postoperative hyperglycemia after colorectal resection in nondiabetic patients was independently associated with increased morbidity and mortality in a large consecutive patient series.
The risks of a variety of complications, both infectious and noninfectious, in nondiabetic patients with postoperative hyperglycemia were similar in magnitude to those seen in diabetic patients experiencing postoperative hyperglycemia.
"A take-home point from our study would be that since it’s known that in diabetic patients it’s absolutely paramount to control hyperglycemia, perhaps nondiabetic patients undergoing major operations – especially colorectal surgery – need to be carefully monitored and have their glucose managed," Dr. P. Ravi Kiran said at the annual meeting of the American Surgical Association.

He and his coinvestigators evaluated the significance of hyperglycemia occurring within 48 hours postoperatively in 2,628 consecutive patients undergoing elective colorectal resection at the Cleveland Clinic in a recent 2-year period.
A total of 2,447 of these patients were nondiabetic. They collectively had 16,404 randomly obtained postoperative blood glucose measurements. One-third of them remained normoglycemic, with all their blood glucose values remaining at 125 mg/dL or less. Another 52.7% had one or more episodes of mild hyperglycemia as defined by a blood glucose value of 126-200 mg/dL. And 14% of nondiabetic subjects experienced postoperative severe hyperglycemia, with a level in excess of 200 mg/dL.
Those rates were similar to those of the known-diabetic patients, 35% of whom remained normoglycemic postoperatively, while 54% became mildly hyperglycemic and 11% severely hyperglycemic.
Postoperative hyperglycemia in nondiabetic patients was associated with greater intraoperative estimated blood loss. The transfusion rate was 4.8% in normoglycemic patients, 10.3% in mildly hyperglycemic ones, and 18.1% in those with severe hyperglycemia. The length of surgery averaged 137 minutes in normoglycemic patients, 166 minutes in patients with postoperative mild hyperglycemia, and 181 minutes in patients with severe hyperglycemia.
Any episode of postoperative severe hyperglycemia in the nondiabetic patients was associated with significantly higher rates of both superficial and deep surgical site infections, greater length of stay, and higher 30-day mortality, compared with normoglycemic patients (see chart). Mild hyperglycemia wasn’t associated with as many complications; however, it was linked to a significantly increased rate of sepsis and a greater length of stay.
Moreover, in a multivariate analysis mild hyperglycemia was independently associated with a 2.1-fold increased risk of reoperation within 30 days, while severe hyperglycemia carried a 3.8-fold increased risk, compared with normoglycemia, continued Dr. Kiran of the Cleveland Clinic.
The investigators identified two major independent risk factors for sepsis in nondiabetic patients: an American Society of Anesthesiologists Physical Status class of 3 or more (odds ratio, 4.2), and postoperative hyperglycemia, which was associated with roughly an 8-fold risk regardless of whether the hyperglycemia was mild or severe.
Studies from the cardiovascular and trauma surgery literature suggest postoperative uncontrolled blood glucose may lead to adverse outcomes. Dr. Kiran and his coworkers performed this study because the impact of elevated blood glucose after elective major abdominal surgery had not been well defined, although colorectal surgery entails bacterial contamination, so background rates of surgical site and other infectious complications already tend to run relatively high.
Dr. Kiran said he believes postoperative hyperglycemia is probably a surrogate marker, perhaps for a distressed physiologic state or for looming complications. The next major question he and his coinvestigators want to tackle is this: Do prompt recognition and management of postoperative hyperglycemia in nondiabetic colorectal resection patients improve outcomes?
A cautionary note was sounded by discussant Dr. Hiram C. Polk Jr.,who emphasized that management strategies involving tight glucose control entail the risk of potentially disastrous postsurgical hypoglycemia.
"As many of you know, there are more than half a dozen patients in the midwestern U.S. who’ve been rendered badly hurt with hypoglycemia and cerebral damage. They’re working their way through the legal system at this point. It’s a fine balance: Sometimes perfection is the enemy of good in this situation," warned Dr. Polk, professor and chairman of the surgery department at the University of Louisville (Ky.).
He noted as an aside that just as Dr. Kiran found that even a single episode of postoperative hyperglycemia has adverse consequences, he and his Louisville coworkers have found the same is true for hypothermia.
"A single brief episode of hypothermia seems to throw the wheels off the track. It disrupts something in host defenses and makes everything very difficult," Dr. Polk observed.
The study was sponsored by the Cleveland Clinic Foundation. Dr. Kiran reported no financial conflicts.
AT THE ASA ANNUAL MEETING
Major finding: Mild hyperglycemia occurring within 48 hours after major colorectal surgery in nondiabetic patients was independently associated with a doubled risk of reoperation within 30 days and a 7.9-fold increase in sepsis, compared with patients who remained normoglycemic. Severe hyperglycemia – a blood glucose measurement in excess of 200 mg/dL – was associated with even worse outcomes.
Data source: A single-center study of more than 16,000 postoperative blood glucose measurements in 2,628 consecutive patients who underwent colorectal resection.
Disclosures: The study was sponsored by the Cleveland Clinic Foundation. Dr. Kiran reported no financial conflicts.
The American Urogynecologic Society fights back against transvaginal mesh bans
The American Urogynecologic Society is pushing back against attempts to ban transvaginal mesh surgery in the treatment of pelvic floor disorders.
In a recent position statement, officials at the American Urogynecologic Society (AUGS) voiced their strong opposition to restrictions on the use of transvaginal mesh surgery when performed by qualified, credentialed surgeons. The policy statement comes after a state medical organization, a health care system, and a medical malpractice company have considered or adopted bans on the use of transvaginal mesh for pelvic organ prolapse or stress urinary incontinence.
"We really feel that the best decision making is left between the patient and her physician without outside interference," Dr. Anthony G. Visco, AUGS president and division chief of urogynecology at Duke University, Durham, N.C. said in an interview.
Rather than an across-the-board ban, AUGS is urging hospitals to do a better job in assessing the competence of the surgeons performing these procedures.
"Instead of a ban on mesh, I think hospitals and health systems should adopt strict credentialing guidelines so that qualified surgeons are performing these procedures," Dr. Visco said.
Some hospitals have already adopted guidelines issued by AUGS that outline how to credential and audit surgeons who perform transvaginal placement of surgical mesh for pelvic organ prolapse and sacrocolpopexy for pelvic organ prolapse, he said. AUGS also recommends that hospitals form a group of pelvic floor reconstructive experts who can review cases and complications in both mesh and nonmesh prolapse repair. Additionally AUGS has released an informed consent toolkit that it recommends to hospitals to help standardize the process of reviewing risks and benefits with patients.
The use of transvaginal mesh to treat pelvic organ prolapse and stress urinary continence has been controversial in recent years as reports of adverse events from the devices have mounted. In July 2011, the Food and Drug Administration issued a safety notice warning that serious complications associated with surgical mesh for the transvaginal repair of pelvic organ prolapse are "not rare." The surgery may expose patients to greater risks without the benefit of being more effective than traditional nonmesh repairs, the FDA wrote.
The agency advised physicians to obtain specialized training for each mesh placement technique and to be vigilant about potential adverse events including mesh erosion and infection. In the same 2011 notice, the FDA said it was still evaluating the impact of transvaginal mesh in the treatment of stress urinary incontinence.
While the FDA has not acted to remove these devices from the market, it has ordered manufacturers of urogynecologic surgical mesh devices to conduct postmarket surveillance studies to look at the safety and effectiveness of surgical mesh used for transvaginal repair of pelvic organ prolapse and mini-sling devices used to treat stress urinary incontinence.
In December 2011, AUGS and the American College of Obstetricians and Gynecologists issued their own statement recommending that pelvic organ prolapse vaginal mesh repair be reserved for high-risk cases such as women with recurrent prolapse or who have medical comorbidities that prevent more invasive endoscopic procedures (Obstet. Gynecol. 2011;118:1459-64).
The safety warnings from the FDA don’t warrant a ban on the procedure, Dr. Visco said. But enacting such a ban could have a number of negative consequences from limiting women’s medical options or limiting the ability of researchers to gather and analyze safety data, he said.
In certain cases, the use of transvaginal mesh for pelvic organ prolapse could be the patient’s best surgical option, according to AUGS. For example, patients with recurrent prolapse after a nonmesh, native tissue repair would be good candidates. Also, the use of transvaginal mesh might be preferred in patients with significant intra-abdominal adhesions because an abdominal approach would pose greater surgical risks.
Dr. Visco said he’s also concerned that news of bans on the procedure, combined with all the advertising about transvaginal mesh lawsuits, could lead some women to leave pelvic floor disorders untreated.
"I think patients are afraid," he said. "We’re worried that they’re so afraid that they may not even seek care."
Bans on transvaginal mesh surgeries could also put a stop to critical research on the products, according to AUGS. For instance, the Pelvic Floor Disorders Network, a clinical trials network funded by the National Institutes of Health, is preparing to launch a randomized trial of transvaginal mesh vs. nonmesh, native tissue repair for uterine prolapse.
And AUGS is building a national registry for pelvic floor disorders with the FDA, the NIH, and ACOG. The registry, which will include data from at least four major device manufacturers, is slated to launch later in 2013. Once the registry is up and running, surgeons will be able to track their outcomes and compare themselves to others.
"Our concern is that we don’t prematurely make a decision about these procedures before there’s a full assessment out there," Dr. Visco said.
On Twitter @MaryEllenNY
The American Urogynecologic Society is pushing back against attempts to ban transvaginal mesh surgery in the treatment of pelvic floor disorders.
In a recent position statement, officials at the American Urogynecologic Society (AUGS) voiced their strong opposition to restrictions on the use of transvaginal mesh surgery when performed by qualified, credentialed surgeons. The policy statement comes after a state medical organization, a health care system, and a medical malpractice company have considered or adopted bans on the use of transvaginal mesh for pelvic organ prolapse or stress urinary incontinence.
"We really feel that the best decision making is left between the patient and her physician without outside interference," Dr. Anthony G. Visco, AUGS president and division chief of urogynecology at Duke University, Durham, N.C. said in an interview.
Rather than an across-the-board ban, AUGS is urging hospitals to do a better job in assessing the competence of the surgeons performing these procedures.
"Instead of a ban on mesh, I think hospitals and health systems should adopt strict credentialing guidelines so that qualified surgeons are performing these procedures," Dr. Visco said.
Some hospitals have already adopted guidelines issued by AUGS that outline how to credential and audit surgeons who perform transvaginal placement of surgical mesh for pelvic organ prolapse and sacrocolpopexy for pelvic organ prolapse, he said. AUGS also recommends that hospitals form a group of pelvic floor reconstructive experts who can review cases and complications in both mesh and nonmesh prolapse repair. Additionally AUGS has released an informed consent toolkit that it recommends to hospitals to help standardize the process of reviewing risks and benefits with patients.
The use of transvaginal mesh to treat pelvic organ prolapse and stress urinary continence has been controversial in recent years as reports of adverse events from the devices have mounted. In July 2011, the Food and Drug Administration issued a safety notice warning that serious complications associated with surgical mesh for the transvaginal repair of pelvic organ prolapse are "not rare." The surgery may expose patients to greater risks without the benefit of being more effective than traditional nonmesh repairs, the FDA wrote.
The agency advised physicians to obtain specialized training for each mesh placement technique and to be vigilant about potential adverse events including mesh erosion and infection. In the same 2011 notice, the FDA said it was still evaluating the impact of transvaginal mesh in the treatment of stress urinary incontinence.
While the FDA has not acted to remove these devices from the market, it has ordered manufacturers of urogynecologic surgical mesh devices to conduct postmarket surveillance studies to look at the safety and effectiveness of surgical mesh used for transvaginal repair of pelvic organ prolapse and mini-sling devices used to treat stress urinary incontinence.
In December 2011, AUGS and the American College of Obstetricians and Gynecologists issued their own statement recommending that pelvic organ prolapse vaginal mesh repair be reserved for high-risk cases such as women with recurrent prolapse or who have medical comorbidities that prevent more invasive endoscopic procedures (Obstet. Gynecol. 2011;118:1459-64).
The safety warnings from the FDA don’t warrant a ban on the procedure, Dr. Visco said. But enacting such a ban could have a number of negative consequences from limiting women’s medical options or limiting the ability of researchers to gather and analyze safety data, he said.
In certain cases, the use of transvaginal mesh for pelvic organ prolapse could be the patient’s best surgical option, according to AUGS. For example, patients with recurrent prolapse after a nonmesh, native tissue repair would be good candidates. Also, the use of transvaginal mesh might be preferred in patients with significant intra-abdominal adhesions because an abdominal approach would pose greater surgical risks.
Dr. Visco said he’s also concerned that news of bans on the procedure, combined with all the advertising about transvaginal mesh lawsuits, could lead some women to leave pelvic floor disorders untreated.
"I think patients are afraid," he said. "We’re worried that they’re so afraid that they may not even seek care."
Bans on transvaginal mesh surgeries could also put a stop to critical research on the products, according to AUGS. For instance, the Pelvic Floor Disorders Network, a clinical trials network funded by the National Institutes of Health, is preparing to launch a randomized trial of transvaginal mesh vs. nonmesh, native tissue repair for uterine prolapse.
And AUGS is building a national registry for pelvic floor disorders with the FDA, the NIH, and ACOG. The registry, which will include data from at least four major device manufacturers, is slated to launch later in 2013. Once the registry is up and running, surgeons will be able to track their outcomes and compare themselves to others.
"Our concern is that we don’t prematurely make a decision about these procedures before there’s a full assessment out there," Dr. Visco said.
On Twitter @MaryEllenNY
The American Urogynecologic Society is pushing back against attempts to ban transvaginal mesh surgery in the treatment of pelvic floor disorders.
In a recent position statement, officials at the American Urogynecologic Society (AUGS) voiced their strong opposition to restrictions on the use of transvaginal mesh surgery when performed by qualified, credentialed surgeons. The policy statement comes after a state medical organization, a health care system, and a medical malpractice company have considered or adopted bans on the use of transvaginal mesh for pelvic organ prolapse or stress urinary incontinence.
"We really feel that the best decision making is left between the patient and her physician without outside interference," Dr. Anthony G. Visco, AUGS president and division chief of urogynecology at Duke University, Durham, N.C. said in an interview.
Rather than an across-the-board ban, AUGS is urging hospitals to do a better job in assessing the competence of the surgeons performing these procedures.
"Instead of a ban on mesh, I think hospitals and health systems should adopt strict credentialing guidelines so that qualified surgeons are performing these procedures," Dr. Visco said.
Some hospitals have already adopted guidelines issued by AUGS that outline how to credential and audit surgeons who perform transvaginal placement of surgical mesh for pelvic organ prolapse and sacrocolpopexy for pelvic organ prolapse, he said. AUGS also recommends that hospitals form a group of pelvic floor reconstructive experts who can review cases and complications in both mesh and nonmesh prolapse repair. Additionally AUGS has released an informed consent toolkit that it recommends to hospitals to help standardize the process of reviewing risks and benefits with patients.
The use of transvaginal mesh to treat pelvic organ prolapse and stress urinary continence has been controversial in recent years as reports of adverse events from the devices have mounted. In July 2011, the Food and Drug Administration issued a safety notice warning that serious complications associated with surgical mesh for the transvaginal repair of pelvic organ prolapse are "not rare." The surgery may expose patients to greater risks without the benefit of being more effective than traditional nonmesh repairs, the FDA wrote.
The agency advised physicians to obtain specialized training for each mesh placement technique and to be vigilant about potential adverse events including mesh erosion and infection. In the same 2011 notice, the FDA said it was still evaluating the impact of transvaginal mesh in the treatment of stress urinary incontinence.
While the FDA has not acted to remove these devices from the market, it has ordered manufacturers of urogynecologic surgical mesh devices to conduct postmarket surveillance studies to look at the safety and effectiveness of surgical mesh used for transvaginal repair of pelvic organ prolapse and mini-sling devices used to treat stress urinary incontinence.
In December 2011, AUGS and the American College of Obstetricians and Gynecologists issued their own statement recommending that pelvic organ prolapse vaginal mesh repair be reserved for high-risk cases such as women with recurrent prolapse or who have medical comorbidities that prevent more invasive endoscopic procedures (Obstet. Gynecol. 2011;118:1459-64).
The safety warnings from the FDA don’t warrant a ban on the procedure, Dr. Visco said. But enacting such a ban could have a number of negative consequences from limiting women’s medical options or limiting the ability of researchers to gather and analyze safety data, he said.
In certain cases, the use of transvaginal mesh for pelvic organ prolapse could be the patient’s best surgical option, according to AUGS. For example, patients with recurrent prolapse after a nonmesh, native tissue repair would be good candidates. Also, the use of transvaginal mesh might be preferred in patients with significant intra-abdominal adhesions because an abdominal approach would pose greater surgical risks.
Dr. Visco said he’s also concerned that news of bans on the procedure, combined with all the advertising about transvaginal mesh lawsuits, could lead some women to leave pelvic floor disorders untreated.
"I think patients are afraid," he said. "We’re worried that they’re so afraid that they may not even seek care."
Bans on transvaginal mesh surgeries could also put a stop to critical research on the products, according to AUGS. For instance, the Pelvic Floor Disorders Network, a clinical trials network funded by the National Institutes of Health, is preparing to launch a randomized trial of transvaginal mesh vs. nonmesh, native tissue repair for uterine prolapse.
And AUGS is building a national registry for pelvic floor disorders with the FDA, the NIH, and ACOG. The registry, which will include data from at least four major device manufacturers, is slated to launch later in 2013. Once the registry is up and running, surgeons will be able to track their outcomes and compare themselves to others.
"Our concern is that we don’t prematurely make a decision about these procedures before there’s a full assessment out there," Dr. Visco said.
On Twitter @MaryEllenNY
Perioperative beta-blockers cut 30-day mortality, cardiac morbidity
For patients at elevated cardiac risk who are undergoing major noncardiac surgery, early perioperative use of beta-blockers is associated with significantly lower 30-day all-cause mortality and cardiac morbidity, according to a report in the April 24 issue of JAMA.
In a retrospective cohort study, this association was strongest in the patients at highest cardiac risk – those with two or more factors on the six-item Cardiac Risk Index, said Dr. Martin J. London of the department of anesthesia and perioperative care, U.S. Department of Veterans Affairs Medical Center, San Francisco, and his associates.
Beta-blocker use in this setting remains controversial, and the use of perioperative beta-blockers has been declining, because of safety concerns. To examine whether high-risk patients are helped or harmed by the treatment, Dr. London and his colleagues analyzed data from the VA Surgical Quality Improvement Program database, a VA pharmacy database, and a VA administrative database.
They assessed the records of 136,745 patients who had vascular, general, neurologic, orthopedic, thoracic, urologic, or otolaryngologic surgery at 104 VA medical centers in 2005-2010.
A total of 40.3% of these subjects had received beta-blockers on the day of or the day after surgery, and 33.2% were given outpatient prescriptions for beta-blockers within 7 days of surgery.
There was a modest but significant decline in beta-blocker use during the 5-year study period, from 43.5% in the first year to 36.2% in the last. A similar national trend has been reported in previous studies. "This may be related to the findings of the POISE trial of increased stroke and death in treated patients, leading to more conservative guideline recommendations within this period," the investigators said.
They performed 1:1 propensity matching and identified 37,805 matched pairs of exposed and nonexposed patients for the primary outcome analysis. The primary outcome measure – all-cause mortality at 30 days – was significantly lower (relative risk 0.73) among patients who used beta-blockers than among those who did not, with a number needed to treat of 241 (JAMA 2013;309:1704-13).
The study subjects were categorized according to their scores on the revised Cardiac Risk Index, which includes six variables: high-risk surgery, cerebrovascular disease, ischemic heart disease, heart failure, diabetes, and renal insufficiency. Patients who had two or more of these CRI risk factors showed the greatest mortality benefit from perioperative beta-blocker therapy, with an relative risk of 0.63.
When the data were broken down by type of surgery, mortality remained significantly lower in beta-blocker users, compared with nonusers for every category except vascular surgery. In previous studies, beta-blockers have shown equivocal benefit in this same subgroup of surgery patients, Dr. London and his associates noted.
The secondary outcome measure of the study was a composite of Q-wave myocardial infarction or nonfatal cardiac arrest at 30 days. These may be rare complications but they are highly predictive of subsequent mortality, the researchers said.
Again, patients who took beta-blockers showed significantly less cardiac morbidity than those who did not, with a relative risk of 0.67 and a number needed to treat of 339.
The study results remained robust in a sensitivity analysis that categorized patients according to whether they had been taking beta-blockers before hospitalization, as well as in a sensitivity analysis that categorized patients as either acute or chronic users of beta-blockers.
This study confirmed the previous finding that withdrawal of beta-blockers within 30 days of surgery is associated with increased mortality. In this study, the risk of death was approximately doubled if beta-blockers were withdrawn perioperatively.
In a post hoc analysis, "we were unable to demonstrate significant associations of perioperative beta-blockade with the risk of postoperative stroke." There was no significant difference in stroke rates between patients who received beta-blockers and those who did not, Dr. London and his colleagues said.
This study was supported by a grant from the Anesthesia Patient Safety Foundation. Dr. London reported no financial conflicts of interest. An associate reported ties to Roche, Resverlogie, Anthera, and Sanofi.
For patients at elevated cardiac risk who are undergoing major noncardiac surgery, early perioperative use of beta-blockers is associated with significantly lower 30-day all-cause mortality and cardiac morbidity, according to a report in the April 24 issue of JAMA.
In a retrospective cohort study, this association was strongest in the patients at highest cardiac risk – those with two or more factors on the six-item Cardiac Risk Index, said Dr. Martin J. London of the department of anesthesia and perioperative care, U.S. Department of Veterans Affairs Medical Center, San Francisco, and his associates.
Beta-blocker use in this setting remains controversial, and the use of perioperative beta-blockers has been declining, because of safety concerns. To examine whether high-risk patients are helped or harmed by the treatment, Dr. London and his colleagues analyzed data from the VA Surgical Quality Improvement Program database, a VA pharmacy database, and a VA administrative database.
They assessed the records of 136,745 patients who had vascular, general, neurologic, orthopedic, thoracic, urologic, or otolaryngologic surgery at 104 VA medical centers in 2005-2010.
A total of 40.3% of these subjects had received beta-blockers on the day of or the day after surgery, and 33.2% were given outpatient prescriptions for beta-blockers within 7 days of surgery.
There was a modest but significant decline in beta-blocker use during the 5-year study period, from 43.5% in the first year to 36.2% in the last. A similar national trend has been reported in previous studies. "This may be related to the findings of the POISE trial of increased stroke and death in treated patients, leading to more conservative guideline recommendations within this period," the investigators said.
They performed 1:1 propensity matching and identified 37,805 matched pairs of exposed and nonexposed patients for the primary outcome analysis. The primary outcome measure – all-cause mortality at 30 days – was significantly lower (relative risk 0.73) among patients who used beta-blockers than among those who did not, with a number needed to treat of 241 (JAMA 2013;309:1704-13).
The study subjects were categorized according to their scores on the revised Cardiac Risk Index, which includes six variables: high-risk surgery, cerebrovascular disease, ischemic heart disease, heart failure, diabetes, and renal insufficiency. Patients who had two or more of these CRI risk factors showed the greatest mortality benefit from perioperative beta-blocker therapy, with an relative risk of 0.63.
When the data were broken down by type of surgery, mortality remained significantly lower in beta-blocker users, compared with nonusers for every category except vascular surgery. In previous studies, beta-blockers have shown equivocal benefit in this same subgroup of surgery patients, Dr. London and his associates noted.
The secondary outcome measure of the study was a composite of Q-wave myocardial infarction or nonfatal cardiac arrest at 30 days. These may be rare complications but they are highly predictive of subsequent mortality, the researchers said.
Again, patients who took beta-blockers showed significantly less cardiac morbidity than those who did not, with a relative risk of 0.67 and a number needed to treat of 339.
The study results remained robust in a sensitivity analysis that categorized patients according to whether they had been taking beta-blockers before hospitalization, as well as in a sensitivity analysis that categorized patients as either acute or chronic users of beta-blockers.
This study confirmed the previous finding that withdrawal of beta-blockers within 30 days of surgery is associated with increased mortality. In this study, the risk of death was approximately doubled if beta-blockers were withdrawn perioperatively.
In a post hoc analysis, "we were unable to demonstrate significant associations of perioperative beta-blockade with the risk of postoperative stroke." There was no significant difference in stroke rates between patients who received beta-blockers and those who did not, Dr. London and his colleagues said.
This study was supported by a grant from the Anesthesia Patient Safety Foundation. Dr. London reported no financial conflicts of interest. An associate reported ties to Roche, Resverlogie, Anthera, and Sanofi.
For patients at elevated cardiac risk who are undergoing major noncardiac surgery, early perioperative use of beta-blockers is associated with significantly lower 30-day all-cause mortality and cardiac morbidity, according to a report in the April 24 issue of JAMA.
In a retrospective cohort study, this association was strongest in the patients at highest cardiac risk – those with two or more factors on the six-item Cardiac Risk Index, said Dr. Martin J. London of the department of anesthesia and perioperative care, U.S. Department of Veterans Affairs Medical Center, San Francisco, and his associates.
Beta-blocker use in this setting remains controversial, and the use of perioperative beta-blockers has been declining, because of safety concerns. To examine whether high-risk patients are helped or harmed by the treatment, Dr. London and his colleagues analyzed data from the VA Surgical Quality Improvement Program database, a VA pharmacy database, and a VA administrative database.
They assessed the records of 136,745 patients who had vascular, general, neurologic, orthopedic, thoracic, urologic, or otolaryngologic surgery at 104 VA medical centers in 2005-2010.
A total of 40.3% of these subjects had received beta-blockers on the day of or the day after surgery, and 33.2% were given outpatient prescriptions for beta-blockers within 7 days of surgery.
There was a modest but significant decline in beta-blocker use during the 5-year study period, from 43.5% in the first year to 36.2% in the last. A similar national trend has been reported in previous studies. "This may be related to the findings of the POISE trial of increased stroke and death in treated patients, leading to more conservative guideline recommendations within this period," the investigators said.
They performed 1:1 propensity matching and identified 37,805 matched pairs of exposed and nonexposed patients for the primary outcome analysis. The primary outcome measure – all-cause mortality at 30 days – was significantly lower (relative risk 0.73) among patients who used beta-blockers than among those who did not, with a number needed to treat of 241 (JAMA 2013;309:1704-13).
The study subjects were categorized according to their scores on the revised Cardiac Risk Index, which includes six variables: high-risk surgery, cerebrovascular disease, ischemic heart disease, heart failure, diabetes, and renal insufficiency. Patients who had two or more of these CRI risk factors showed the greatest mortality benefit from perioperative beta-blocker therapy, with an relative risk of 0.63.
When the data were broken down by type of surgery, mortality remained significantly lower in beta-blocker users, compared with nonusers for every category except vascular surgery. In previous studies, beta-blockers have shown equivocal benefit in this same subgroup of surgery patients, Dr. London and his associates noted.
The secondary outcome measure of the study was a composite of Q-wave myocardial infarction or nonfatal cardiac arrest at 30 days. These may be rare complications but they are highly predictive of subsequent mortality, the researchers said.
Again, patients who took beta-blockers showed significantly less cardiac morbidity than those who did not, with a relative risk of 0.67 and a number needed to treat of 339.
The study results remained robust in a sensitivity analysis that categorized patients according to whether they had been taking beta-blockers before hospitalization, as well as in a sensitivity analysis that categorized patients as either acute or chronic users of beta-blockers.
This study confirmed the previous finding that withdrawal of beta-blockers within 30 days of surgery is associated with increased mortality. In this study, the risk of death was approximately doubled if beta-blockers were withdrawn perioperatively.
In a post hoc analysis, "we were unable to demonstrate significant associations of perioperative beta-blockade with the risk of postoperative stroke." There was no significant difference in stroke rates between patients who received beta-blockers and those who did not, Dr. London and his colleagues said.
This study was supported by a grant from the Anesthesia Patient Safety Foundation. Dr. London reported no financial conflicts of interest. An associate reported ties to Roche, Resverlogie, Anthera, and Sanofi.
FROM JAMA
Major finding: All-cause mortality at 30 days was significantly lower in high-risk patients who received perioperative beta-blockers than in those who did not, with an RR of 0.73.
Data source: A retrospective cohort study involving 136,745 VA patients undergoing noncardiac surgery during a 5-year period.
Disclosures: This study was supported by a grant from the Anesthesia Patient Safety Foundation. Dr. London reported no financial conflicts of interest. An associate reported ties to Roche, Resverlogie, Anthera, and Sanofi.
More than 50 injured by bombs still in Boston hospitals
Fifty-one runners and spectators remained hospitalized April 23 with injuries from the Boston Marathon bombings, according to the Boston Public Health Commission.
With a total of 264 patients treated at Boston area hospitals, "the team approach [was] vital," said Dr. Jeffrey Kalish, director of endovascular surgery at Boston Medical Center. "These types of injuries require multiple teams talking together and working together. It taught us really how to work together across multiple disciplines in a really acute time frame."
At BMC, "the initial approach was gathering every single surgeon available and figuring out who could do which specific operations," Dr. Kalish said.
"On the first day, the goal was to get the patients in and out of the operating room and stop the bleeding," he said. The next day [Tuesday], "we got together as a big group and talked about every patient and decided who was going to take over their care."
Teams met twice a day and while the surgeons focused on the patients, the hospital leadership made sure that adequate staff and infrastructure were available.
"This [experience] really taught me the importance of practicing for the unthinkable and practicing for the unknown, so when these things happen, it’s not as much of a surprise," said Dr. Kalish. "Talking about command structure and incident reporting and how to deploy the resources to the best of hospital’s knowledge is a very worthwhile thing."
Five patients at BMC have had above- or below-the-knee amputations and have averaged at least two surgical procedures each. One patient has had five.
For those with amputations, wound healing and pain control are now the most important factors, according to Dr. Simona Manasian, a rehabilitation medicine physician at BMC. Once the wounds are healed, the focus turns to shaping the stumps for prostheses.
Patients have begun early rehabilitation and physical therapy to strengthen the lower limbs and their upper bodies for mobility and support.
Patients also have been receiving psychological support, Dr. Manasian said. She added that they have been visited by several veterans who were similarly injured in Iraq or Afghanistan and who are farther along in adapting to amputations and prostheses. "That was very helpful."
Dr. Kalish added, "We’ve seen a full range of emotions over the past week. But now that we’re a week into this, everyone’s emotionally doing much better. [The patients are] optimistic, and they know that life will be a little different, but life is not over."
On Twitter @NaseemSMiller
Fifty-one runners and spectators remained hospitalized April 23 with injuries from the Boston Marathon bombings, according to the Boston Public Health Commission.
With a total of 264 patients treated at Boston area hospitals, "the team approach [was] vital," said Dr. Jeffrey Kalish, director of endovascular surgery at Boston Medical Center. "These types of injuries require multiple teams talking together and working together. It taught us really how to work together across multiple disciplines in a really acute time frame."
At BMC, "the initial approach was gathering every single surgeon available and figuring out who could do which specific operations," Dr. Kalish said.
"On the first day, the goal was to get the patients in and out of the operating room and stop the bleeding," he said. The next day [Tuesday], "we got together as a big group and talked about every patient and decided who was going to take over their care."
Teams met twice a day and while the surgeons focused on the patients, the hospital leadership made sure that adequate staff and infrastructure were available.
"This [experience] really taught me the importance of practicing for the unthinkable and practicing for the unknown, so when these things happen, it’s not as much of a surprise," said Dr. Kalish. "Talking about command structure and incident reporting and how to deploy the resources to the best of hospital’s knowledge is a very worthwhile thing."
Five patients at BMC have had above- or below-the-knee amputations and have averaged at least two surgical procedures each. One patient has had five.
For those with amputations, wound healing and pain control are now the most important factors, according to Dr. Simona Manasian, a rehabilitation medicine physician at BMC. Once the wounds are healed, the focus turns to shaping the stumps for prostheses.
Patients have begun early rehabilitation and physical therapy to strengthen the lower limbs and their upper bodies for mobility and support.
Patients also have been receiving psychological support, Dr. Manasian said. She added that they have been visited by several veterans who were similarly injured in Iraq or Afghanistan and who are farther along in adapting to amputations and prostheses. "That was very helpful."
Dr. Kalish added, "We’ve seen a full range of emotions over the past week. But now that we’re a week into this, everyone’s emotionally doing much better. [The patients are] optimistic, and they know that life will be a little different, but life is not over."
On Twitter @NaseemSMiller
Fifty-one runners and spectators remained hospitalized April 23 with injuries from the Boston Marathon bombings, according to the Boston Public Health Commission.
With a total of 264 patients treated at Boston area hospitals, "the team approach [was] vital," said Dr. Jeffrey Kalish, director of endovascular surgery at Boston Medical Center. "These types of injuries require multiple teams talking together and working together. It taught us really how to work together across multiple disciplines in a really acute time frame."
At BMC, "the initial approach was gathering every single surgeon available and figuring out who could do which specific operations," Dr. Kalish said.
"On the first day, the goal was to get the patients in and out of the operating room and stop the bleeding," he said. The next day [Tuesday], "we got together as a big group and talked about every patient and decided who was going to take over their care."
Teams met twice a day and while the surgeons focused on the patients, the hospital leadership made sure that adequate staff and infrastructure were available.
"This [experience] really taught me the importance of practicing for the unthinkable and practicing for the unknown, so when these things happen, it’s not as much of a surprise," said Dr. Kalish. "Talking about command structure and incident reporting and how to deploy the resources to the best of hospital’s knowledge is a very worthwhile thing."
Five patients at BMC have had above- or below-the-knee amputations and have averaged at least two surgical procedures each. One patient has had five.
For those with amputations, wound healing and pain control are now the most important factors, according to Dr. Simona Manasian, a rehabilitation medicine physician at BMC. Once the wounds are healed, the focus turns to shaping the stumps for prostheses.
Patients have begun early rehabilitation and physical therapy to strengthen the lower limbs and their upper bodies for mobility and support.
Patients also have been receiving psychological support, Dr. Manasian said. She added that they have been visited by several veterans who were similarly injured in Iraq or Afghanistan and who are farther along in adapting to amputations and prostheses. "That was very helpful."
Dr. Kalish added, "We’ve seen a full range of emotions over the past week. But now that we’re a week into this, everyone’s emotionally doing much better. [The patients are] optimistic, and they know that life will be a little different, but life is not over."
On Twitter @NaseemSMiller
Reflux after surgery increases risk of esophageal cancer
Patients who experience recurrent reflux despite surgical treatment are three times more likely to develop esophageal adenocarcinoma than are those who have a successful surgery.
The findings of a national database study suggest that careful observation may be key to prevention of cancer when antireflux surgery doesn’t deliver, reported Dr. Hedvig E. Lofdahl and colleagues. The study was published in the April issue of Annals of Surgery.
"From a clinical point of view, this study suggests that it might be valuable to carefully evaluate the result of the antireflux surgery, and consider the patients with recurrent GERD, particularly those with Barrett’s esophagus, for endoscopic surveillance," wrote Dr. Lofdahl of Karolinska Institutet, Stockholm, and his coauthors (Ann. Surg. 2013;257:579-82).
The case-control study drew its data from the Swedish Cancer Register. It comprised 295 patients who underwent antireflux surgery from 1996 to 2006. Fifty-five of the patients developed an adenocarcinoma of the esophagus sometime during the 7-year follow-up period.
Most of the patients in the study were male (87%). Smoking status did not differ significantly between the cases and controls (47% vs.42%, respectively). Recurrent reflux was significantly more common among the cases than among the controls (35% vs. 18%).
The multivariate analysis controlled for body mass index, smoking, and the type of antireflux surgery. In the final adjusted model, recurrent reflux conferred a threefold increase in the risk of a later esophageal adenocarcinoma. A BMI of more than 25 kg/m2 also increased the risk, but not significantly (odds ratio [OR] 1.6; confidence interval [CI]: 0.8-3.5). There were not enough patients with a BMI of greater than 30 kg/m2 to further tease out the effect of weight.
Having ever smoked tobacco also increased the risk of esophageal cancer, but again, the increase was not statistically significant (OR 1.4; CI: 0.7-2.8).
Compared with a partial fundoplication, a total 360-degree fundoplication was associated with a lower risk of cancer, but the difference was not statistically significant (OR 0.6; CI: 0.3-1.3).
"This finding might at least partly explain the lack of cancer-preventive effect of antireflux surgery," the investigators wrote.
The study was supported by the Swedish Research Council, the Swedish Cancer Society, and the Stockholm Cancer Society. None of the authors had financial disclosures.
Patients who experience recurrent reflux despite surgical treatment are three times more likely to develop esophageal adenocarcinoma than are those who have a successful surgery.
The findings of a national database study suggest that careful observation may be key to prevention of cancer when antireflux surgery doesn’t deliver, reported Dr. Hedvig E. Lofdahl and colleagues. The study was published in the April issue of Annals of Surgery.
"From a clinical point of view, this study suggests that it might be valuable to carefully evaluate the result of the antireflux surgery, and consider the patients with recurrent GERD, particularly those with Barrett’s esophagus, for endoscopic surveillance," wrote Dr. Lofdahl of Karolinska Institutet, Stockholm, and his coauthors (Ann. Surg. 2013;257:579-82).
The case-control study drew its data from the Swedish Cancer Register. It comprised 295 patients who underwent antireflux surgery from 1996 to 2006. Fifty-five of the patients developed an adenocarcinoma of the esophagus sometime during the 7-year follow-up period.
Most of the patients in the study were male (87%). Smoking status did not differ significantly between the cases and controls (47% vs.42%, respectively). Recurrent reflux was significantly more common among the cases than among the controls (35% vs. 18%).
The multivariate analysis controlled for body mass index, smoking, and the type of antireflux surgery. In the final adjusted model, recurrent reflux conferred a threefold increase in the risk of a later esophageal adenocarcinoma. A BMI of more than 25 kg/m2 also increased the risk, but not significantly (odds ratio [OR] 1.6; confidence interval [CI]: 0.8-3.5). There were not enough patients with a BMI of greater than 30 kg/m2 to further tease out the effect of weight.
Having ever smoked tobacco also increased the risk of esophageal cancer, but again, the increase was not statistically significant (OR 1.4; CI: 0.7-2.8).
Compared with a partial fundoplication, a total 360-degree fundoplication was associated with a lower risk of cancer, but the difference was not statistically significant (OR 0.6; CI: 0.3-1.3).
"This finding might at least partly explain the lack of cancer-preventive effect of antireflux surgery," the investigators wrote.
The study was supported by the Swedish Research Council, the Swedish Cancer Society, and the Stockholm Cancer Society. None of the authors had financial disclosures.
Patients who experience recurrent reflux despite surgical treatment are three times more likely to develop esophageal adenocarcinoma than are those who have a successful surgery.
The findings of a national database study suggest that careful observation may be key to prevention of cancer when antireflux surgery doesn’t deliver, reported Dr. Hedvig E. Lofdahl and colleagues. The study was published in the April issue of Annals of Surgery.
"From a clinical point of view, this study suggests that it might be valuable to carefully evaluate the result of the antireflux surgery, and consider the patients with recurrent GERD, particularly those with Barrett’s esophagus, for endoscopic surveillance," wrote Dr. Lofdahl of Karolinska Institutet, Stockholm, and his coauthors (Ann. Surg. 2013;257:579-82).
The case-control study drew its data from the Swedish Cancer Register. It comprised 295 patients who underwent antireflux surgery from 1996 to 2006. Fifty-five of the patients developed an adenocarcinoma of the esophagus sometime during the 7-year follow-up period.
Most of the patients in the study were male (87%). Smoking status did not differ significantly between the cases and controls (47% vs.42%, respectively). Recurrent reflux was significantly more common among the cases than among the controls (35% vs. 18%).
The multivariate analysis controlled for body mass index, smoking, and the type of antireflux surgery. In the final adjusted model, recurrent reflux conferred a threefold increase in the risk of a later esophageal adenocarcinoma. A BMI of more than 25 kg/m2 also increased the risk, but not significantly (odds ratio [OR] 1.6; confidence interval [CI]: 0.8-3.5). There were not enough patients with a BMI of greater than 30 kg/m2 to further tease out the effect of weight.
Having ever smoked tobacco also increased the risk of esophageal cancer, but again, the increase was not statistically significant (OR 1.4; CI: 0.7-2.8).
Compared with a partial fundoplication, a total 360-degree fundoplication was associated with a lower risk of cancer, but the difference was not statistically significant (OR 0.6; CI: 0.3-1.3).
"This finding might at least partly explain the lack of cancer-preventive effect of antireflux surgery," the investigators wrote.
The study was supported by the Swedish Research Council, the Swedish Cancer Society, and the Stockholm Cancer Society. None of the authors had financial disclosures.
FROM ANNALS OF SURGERY
Major finding: Patients with recurrent reflux after surgical treatment are three times more likely to develop an esophageal adenocarcinoma than are those whose symptoms resolve after surgery,
Data source: The case-control study comprised 295 patients included in a national cancer registry.
Disclosures: The study was supported by the Swedish Research Council, the Swedish Cancer Society, and the Stockholm Cancer Society. None of the authors had financial disclosures.
Study IDs risks for bladder test failure following sling procedure
CHARLESTON, S.C. – Most women who undergo an isolated midurethral sling procedure pass active bladder testing on the first attempt, but the likelihood of being discharged with an indwelling catheter increases as bladder capacity increases, maximum flow rate decreases, or detrusor overactivity occurs, findings from a retrospective cross-sectional study have shown.
Of 112 patients who underwent an isolated midurethral sling procedure at a single site, 90 (80.4%) passed active bladder testing (ABT) on the first attempt prior to discharge home, and 22 (19.6%) failed, Dr. Meadow M. Good reported at the annual meeting of the Society of Gynecologic Surgeons.
Bladder testing involved filling the bladder with sterile fluid to 300 mL or to patient discomfort, whichever came first. Passing the test required that two-thirds of the instilled volume was voided.
Average bladder capacity among those who failed ABT was 415 cc, compared with 381 cc in those who passed; average maximum flow rate in those who failed ABT was 15.6 cc/second, compared with 21.6 cc/second in those who passed; and detrusor overactivity was observed in 41% of those who failed ABT, compared with 20% of those who passed, said Dr. Good of the University of Texas Southwestern Medical Center, Dallas.
Furthermore, the capacity-to-infused volume ratio was 1:5 for those who failed ABT and 1:3 for those who passed. Most (81.8%) of those who failed had bladder capacity greater than the infused amount, but no significant difference in the capacity-to-infused volume ratios was seen in the group who passed ABT.
On multivariate analysis, every 50-cc increase in bladder capacity significantly increased the odds of failing ABT (odds ratio, 1.25); every unit increase in the maximum flow rate significantly decreased the odds of ABT failure (OR, 0.91); and the presence of detrusor overactivity was associated with a fivefold increase in the odds of failure (OR, 5.0).
Age, race, body mass index, maximum urethral closure pressure, and maximum detrusor pressure during pressure-flow studies were not found to be associated with ABT outcomes.
Patients included in this study were all those who underwent an isolated midurethral sling procedure at the medical center between January 2011 and August 2012, excluding those who had intraoperative complications requiring discharge with a Foley catheter.
The findings are important because the rates of postoperative urinary retention following midurethral sling procedures are highly variable, and the identification of factors associated with ABT failure could help improve outcomes.
"Patients with postoperative retention are generally discharged home with an indwelling catheter or intermittent self-catheterization; catheter-associated bacteria lead to increased urinary tract infections and health care costs, and to decreased quality of life," Dr. Good said, adding that reducing the rates of postoperative catheter use should be a priority.
"While further research is needed, filling patients to cystometric capacity may improve the success of postoperative bladder testing," she said.
Dr. Good reported having no relevant financial disclosures.
CHARLESTON, S.C. – Most women who undergo an isolated midurethral sling procedure pass active bladder testing on the first attempt, but the likelihood of being discharged with an indwelling catheter increases as bladder capacity increases, maximum flow rate decreases, or detrusor overactivity occurs, findings from a retrospective cross-sectional study have shown.
Of 112 patients who underwent an isolated midurethral sling procedure at a single site, 90 (80.4%) passed active bladder testing (ABT) on the first attempt prior to discharge home, and 22 (19.6%) failed, Dr. Meadow M. Good reported at the annual meeting of the Society of Gynecologic Surgeons.
Bladder testing involved filling the bladder with sterile fluid to 300 mL or to patient discomfort, whichever came first. Passing the test required that two-thirds of the instilled volume was voided.
Average bladder capacity among those who failed ABT was 415 cc, compared with 381 cc in those who passed; average maximum flow rate in those who failed ABT was 15.6 cc/second, compared with 21.6 cc/second in those who passed; and detrusor overactivity was observed in 41% of those who failed ABT, compared with 20% of those who passed, said Dr. Good of the University of Texas Southwestern Medical Center, Dallas.
Furthermore, the capacity-to-infused volume ratio was 1:5 for those who failed ABT and 1:3 for those who passed. Most (81.8%) of those who failed had bladder capacity greater than the infused amount, but no significant difference in the capacity-to-infused volume ratios was seen in the group who passed ABT.
On multivariate analysis, every 50-cc increase in bladder capacity significantly increased the odds of failing ABT (odds ratio, 1.25); every unit increase in the maximum flow rate significantly decreased the odds of ABT failure (OR, 0.91); and the presence of detrusor overactivity was associated with a fivefold increase in the odds of failure (OR, 5.0).
Age, race, body mass index, maximum urethral closure pressure, and maximum detrusor pressure during pressure-flow studies were not found to be associated with ABT outcomes.
Patients included in this study were all those who underwent an isolated midurethral sling procedure at the medical center between January 2011 and August 2012, excluding those who had intraoperative complications requiring discharge with a Foley catheter.
The findings are important because the rates of postoperative urinary retention following midurethral sling procedures are highly variable, and the identification of factors associated with ABT failure could help improve outcomes.
"Patients with postoperative retention are generally discharged home with an indwelling catheter or intermittent self-catheterization; catheter-associated bacteria lead to increased urinary tract infections and health care costs, and to decreased quality of life," Dr. Good said, adding that reducing the rates of postoperative catheter use should be a priority.
"While further research is needed, filling patients to cystometric capacity may improve the success of postoperative bladder testing," she said.
Dr. Good reported having no relevant financial disclosures.
CHARLESTON, S.C. – Most women who undergo an isolated midurethral sling procedure pass active bladder testing on the first attempt, but the likelihood of being discharged with an indwelling catheter increases as bladder capacity increases, maximum flow rate decreases, or detrusor overactivity occurs, findings from a retrospective cross-sectional study have shown.
Of 112 patients who underwent an isolated midurethral sling procedure at a single site, 90 (80.4%) passed active bladder testing (ABT) on the first attempt prior to discharge home, and 22 (19.6%) failed, Dr. Meadow M. Good reported at the annual meeting of the Society of Gynecologic Surgeons.
Bladder testing involved filling the bladder with sterile fluid to 300 mL or to patient discomfort, whichever came first. Passing the test required that two-thirds of the instilled volume was voided.
Average bladder capacity among those who failed ABT was 415 cc, compared with 381 cc in those who passed; average maximum flow rate in those who failed ABT was 15.6 cc/second, compared with 21.6 cc/second in those who passed; and detrusor overactivity was observed in 41% of those who failed ABT, compared with 20% of those who passed, said Dr. Good of the University of Texas Southwestern Medical Center, Dallas.
Furthermore, the capacity-to-infused volume ratio was 1:5 for those who failed ABT and 1:3 for those who passed. Most (81.8%) of those who failed had bladder capacity greater than the infused amount, but no significant difference in the capacity-to-infused volume ratios was seen in the group who passed ABT.
On multivariate analysis, every 50-cc increase in bladder capacity significantly increased the odds of failing ABT (odds ratio, 1.25); every unit increase in the maximum flow rate significantly decreased the odds of ABT failure (OR, 0.91); and the presence of detrusor overactivity was associated with a fivefold increase in the odds of failure (OR, 5.0).
Age, race, body mass index, maximum urethral closure pressure, and maximum detrusor pressure during pressure-flow studies were not found to be associated with ABT outcomes.
Patients included in this study were all those who underwent an isolated midurethral sling procedure at the medical center between January 2011 and August 2012, excluding those who had intraoperative complications requiring discharge with a Foley catheter.
The findings are important because the rates of postoperative urinary retention following midurethral sling procedures are highly variable, and the identification of factors associated with ABT failure could help improve outcomes.
"Patients with postoperative retention are generally discharged home with an indwelling catheter or intermittent self-catheterization; catheter-associated bacteria lead to increased urinary tract infections and health care costs, and to decreased quality of life," Dr. Good said, adding that reducing the rates of postoperative catheter use should be a priority.
"While further research is needed, filling patients to cystometric capacity may improve the success of postoperative bladder testing," she said.
Dr. Good reported having no relevant financial disclosures.
AT THE SGS ANNUAL MEETING
Major finding: Every 50-cc increase in bladder capacity significantly increased the odds of failing active bladder testing (OR, 1.25); every unit increase in the maximum flow rate significantly decreased the odds of ABT failure (OR, 0.91); and the presence of detrusor overactivity was associated with a fivefold increase in the odds of failure (OR, 5.0).
Data source: A single-site, retrospective cross-sectional study involving 112 patients.
Disclosures: Dr. Good reported having no relevant financial disclosures.
Antibiotics after damage control laparotomy up infection risk
LAS VEGAS – Trauma patients should not get antibiotics after damage control or primarily closed laparotomies because this treatment may increase the risk of postsurgical intra-abdominal infections, according to a study from Virginia Commonwealth University, Richmond, a Level 1 trauma center.
The abdomen is often left open for a while after a damage control laparotomy (DCL), especially when patients are coagulopathic, acidotic, or at risk for an abdominal compartment syndrome. In those cases, "people just automatically assume ‘Open abdomen: Throw on the antibiotics.’ What we are showing here is don’t throw on the antibiotics," said lead investigator Dr. Stephanie Goldberg of the trauma, critical care, and emergency surgery faculty at VCU. The worry is probably the same for primarily closed (PC) laparotomies, when the fascia is closed but skin is sometimes left open.
The findings are important because although – and as the team found – preoperative antibiotics are known to reduce the risk of postsurgical abdominal infections, there’s not much evidence in either direction for their use after trauma laparotomies, so "no one knows what to do." Some surgeons opt for antibiotics, others don’t, Dr. Goldberg said.
To help figure out the right approach, her team analyzed perioperative antibiotic use and infection rates in 28 DCL patients whose abdomens were left open, and 93 PC patients. The PC group had a mean injury severity score of 18; 35.5% (33) had bowel injuries. The DCL group was in worse shape, with a mean severity score of 31.4 and bowel injuries in 53.6% (15).
Everyone should have been dosed with an antibiotic before surgery; 94.6% (88) PC patients, but only 69.2% (19) DCL patients, actually were. "It’s likely," in the DCL cases especially, "that patients were so sick and there was so much chaos in the operating room that giving pre-op antibiotics got missed," Dr. Goldberg said.
Postop antibiotic use differed significantly between the groups; 50.5% (47) of PC patients got no antibiotics, 21.5% (20) got a day’s worth, and 28% (26) were treated for more than a day. In the DCL group, 21.4% (6) got no antibiotics, 25.0% (7) a 1-day course, and 53.6% (15) more than a 1-day course.
As expected, preop antibiotics protected against intra-abdominal infections (odds ratio, 0.20; 95% confidence interval 0.05-0.91; P = .037). Postoperative antibiotics, however, substantially increased the risk (OR, 6.7; 95% CI 1.33 – 33.8; P= .044).
The longer patients were on antibiotics, the greater that risk became. Among the 6 DCL patients who received no postsurgical antibiotics, 16.7% (1) developed an intra-abdominal infection. Among the 7 treated for a day, 28.6% (2) developed an intra-abdominal infection; 40% (6) did so among the 15 treated for more than a day. The trend was similar for PC patients, although the overall infection rates were lower.
Antimicrobial resistance could be to blame. As normal flora were wiped out, maybe the field was cleared for "bugs to cause problems that otherwise would not have," explained senior investigator Dr. Thèrese Duane of the department of surgery at VCU. Surgeons there tend to favor Zosyn or Cefoxitin.
The project was just the first step toward building a robust evidence base about antibiotic use after trauma laparotomies. Next on the team’s agenda is a multicenter, prospective trial.
"We need more numbers," Dr. Duane said.
Dr. Goldberg has no relevant disclosures. Dr. Duane speaks for Pfizer on behalf of its antibiotic, linezolid.
Antibiotics after damage-control laparotomy up the infection risk. This study by Dr. Goldberg and her colleagues highlights areas for process improvement with antibiotic prophylaxis to prevent organ space surgical site infection (SSI) in trauma patients undergoing damage control or primarily closed laparotomies. Preoperative antibiotic prophylaxis has been consistently demonstrated across meta-analyses of randomized trials to be effective in preventing SSIs regardless of the type of surgery; this study supports similar benefits in trauma laparotomy patients. Furthermore, given that only 69% of DCL patients received preoperative antibiotics, there was significant room for improvement.
Antibiotic stewardship has also been at the forefront of recent efforts to improve perioperative care. Level I evidence exists for not continuing postoperative antibiotics beyond 24 hours in patients undergoing trauma laparotomies for penetrating injuries. Although less well studied, there is no evidence to suggest that antibiotic prophylaxis practices after laparotomy for blunt injuries or for damage control should differ. Further studies are necessary to determine optimal methods for delivering appropriate preoperative antibiotic prophylaxis and to identify additional perioperative strategies to reduce superficial, deep, and organ space SSIs in these high-risk patients.
Dr. Lillian S. Kao is in the department of surgery at the University of Texas Health Science Center at Houston. Dr. Kao has no conflict of interest disclosures.
Antibiotics after damage-control laparotomy up the infection risk. This study by Dr. Goldberg and her colleagues highlights areas for process improvement with antibiotic prophylaxis to prevent organ space surgical site infection (SSI) in trauma patients undergoing damage control or primarily closed laparotomies. Preoperative antibiotic prophylaxis has been consistently demonstrated across meta-analyses of randomized trials to be effective in preventing SSIs regardless of the type of surgery; this study supports similar benefits in trauma laparotomy patients. Furthermore, given that only 69% of DCL patients received preoperative antibiotics, there was significant room for improvement.
Antibiotic stewardship has also been at the forefront of recent efforts to improve perioperative care. Level I evidence exists for not continuing postoperative antibiotics beyond 24 hours in patients undergoing trauma laparotomies for penetrating injuries. Although less well studied, there is no evidence to suggest that antibiotic prophylaxis practices after laparotomy for blunt injuries or for damage control should differ. Further studies are necessary to determine optimal methods for delivering appropriate preoperative antibiotic prophylaxis and to identify additional perioperative strategies to reduce superficial, deep, and organ space SSIs in these high-risk patients.
Dr. Lillian S. Kao is in the department of surgery at the University of Texas Health Science Center at Houston. Dr. Kao has no conflict of interest disclosures.
Antibiotics after damage-control laparotomy up the infection risk. This study by Dr. Goldberg and her colleagues highlights areas for process improvement with antibiotic prophylaxis to prevent organ space surgical site infection (SSI) in trauma patients undergoing damage control or primarily closed laparotomies. Preoperative antibiotic prophylaxis has been consistently demonstrated across meta-analyses of randomized trials to be effective in preventing SSIs regardless of the type of surgery; this study supports similar benefits in trauma laparotomy patients. Furthermore, given that only 69% of DCL patients received preoperative antibiotics, there was significant room for improvement.
Antibiotic stewardship has also been at the forefront of recent efforts to improve perioperative care. Level I evidence exists for not continuing postoperative antibiotics beyond 24 hours in patients undergoing trauma laparotomies for penetrating injuries. Although less well studied, there is no evidence to suggest that antibiotic prophylaxis practices after laparotomy for blunt injuries or for damage control should differ. Further studies are necessary to determine optimal methods for delivering appropriate preoperative antibiotic prophylaxis and to identify additional perioperative strategies to reduce superficial, deep, and organ space SSIs in these high-risk patients.
Dr. Lillian S. Kao is in the department of surgery at the University of Texas Health Science Center at Houston. Dr. Kao has no conflict of interest disclosures.
LAS VEGAS – Trauma patients should not get antibiotics after damage control or primarily closed laparotomies because this treatment may increase the risk of postsurgical intra-abdominal infections, according to a study from Virginia Commonwealth University, Richmond, a Level 1 trauma center.
The abdomen is often left open for a while after a damage control laparotomy (DCL), especially when patients are coagulopathic, acidotic, or at risk for an abdominal compartment syndrome. In those cases, "people just automatically assume ‘Open abdomen: Throw on the antibiotics.’ What we are showing here is don’t throw on the antibiotics," said lead investigator Dr. Stephanie Goldberg of the trauma, critical care, and emergency surgery faculty at VCU. The worry is probably the same for primarily closed (PC) laparotomies, when the fascia is closed but skin is sometimes left open.
The findings are important because although – and as the team found – preoperative antibiotics are known to reduce the risk of postsurgical abdominal infections, there’s not much evidence in either direction for their use after trauma laparotomies, so "no one knows what to do." Some surgeons opt for antibiotics, others don’t, Dr. Goldberg said.
To help figure out the right approach, her team analyzed perioperative antibiotic use and infection rates in 28 DCL patients whose abdomens were left open, and 93 PC patients. The PC group had a mean injury severity score of 18; 35.5% (33) had bowel injuries. The DCL group was in worse shape, with a mean severity score of 31.4 and bowel injuries in 53.6% (15).
Everyone should have been dosed with an antibiotic before surgery; 94.6% (88) PC patients, but only 69.2% (19) DCL patients, actually were. "It’s likely," in the DCL cases especially, "that patients were so sick and there was so much chaos in the operating room that giving pre-op antibiotics got missed," Dr. Goldberg said.
Postop antibiotic use differed significantly between the groups; 50.5% (47) of PC patients got no antibiotics, 21.5% (20) got a day’s worth, and 28% (26) were treated for more than a day. In the DCL group, 21.4% (6) got no antibiotics, 25.0% (7) a 1-day course, and 53.6% (15) more than a 1-day course.
As expected, preop antibiotics protected against intra-abdominal infections (odds ratio, 0.20; 95% confidence interval 0.05-0.91; P = .037). Postoperative antibiotics, however, substantially increased the risk (OR, 6.7; 95% CI 1.33 – 33.8; P= .044).
The longer patients were on antibiotics, the greater that risk became. Among the 6 DCL patients who received no postsurgical antibiotics, 16.7% (1) developed an intra-abdominal infection. Among the 7 treated for a day, 28.6% (2) developed an intra-abdominal infection; 40% (6) did so among the 15 treated for more than a day. The trend was similar for PC patients, although the overall infection rates were lower.
Antimicrobial resistance could be to blame. As normal flora were wiped out, maybe the field was cleared for "bugs to cause problems that otherwise would not have," explained senior investigator Dr. Thèrese Duane of the department of surgery at VCU. Surgeons there tend to favor Zosyn or Cefoxitin.
The project was just the first step toward building a robust evidence base about antibiotic use after trauma laparotomies. Next on the team’s agenda is a multicenter, prospective trial.
"We need more numbers," Dr. Duane said.
Dr. Goldberg has no relevant disclosures. Dr. Duane speaks for Pfizer on behalf of its antibiotic, linezolid.
LAS VEGAS – Trauma patients should not get antibiotics after damage control or primarily closed laparotomies because this treatment may increase the risk of postsurgical intra-abdominal infections, according to a study from Virginia Commonwealth University, Richmond, a Level 1 trauma center.
The abdomen is often left open for a while after a damage control laparotomy (DCL), especially when patients are coagulopathic, acidotic, or at risk for an abdominal compartment syndrome. In those cases, "people just automatically assume ‘Open abdomen: Throw on the antibiotics.’ What we are showing here is don’t throw on the antibiotics," said lead investigator Dr. Stephanie Goldberg of the trauma, critical care, and emergency surgery faculty at VCU. The worry is probably the same for primarily closed (PC) laparotomies, when the fascia is closed but skin is sometimes left open.
The findings are important because although – and as the team found – preoperative antibiotics are known to reduce the risk of postsurgical abdominal infections, there’s not much evidence in either direction for their use after trauma laparotomies, so "no one knows what to do." Some surgeons opt for antibiotics, others don’t, Dr. Goldberg said.
To help figure out the right approach, her team analyzed perioperative antibiotic use and infection rates in 28 DCL patients whose abdomens were left open, and 93 PC patients. The PC group had a mean injury severity score of 18; 35.5% (33) had bowel injuries. The DCL group was in worse shape, with a mean severity score of 31.4 and bowel injuries in 53.6% (15).
Everyone should have been dosed with an antibiotic before surgery; 94.6% (88) PC patients, but only 69.2% (19) DCL patients, actually were. "It’s likely," in the DCL cases especially, "that patients were so sick and there was so much chaos in the operating room that giving pre-op antibiotics got missed," Dr. Goldberg said.
Postop antibiotic use differed significantly between the groups; 50.5% (47) of PC patients got no antibiotics, 21.5% (20) got a day’s worth, and 28% (26) were treated for more than a day. In the DCL group, 21.4% (6) got no antibiotics, 25.0% (7) a 1-day course, and 53.6% (15) more than a 1-day course.
As expected, preop antibiotics protected against intra-abdominal infections (odds ratio, 0.20; 95% confidence interval 0.05-0.91; P = .037). Postoperative antibiotics, however, substantially increased the risk (OR, 6.7; 95% CI 1.33 – 33.8; P= .044).
The longer patients were on antibiotics, the greater that risk became. Among the 6 DCL patients who received no postsurgical antibiotics, 16.7% (1) developed an intra-abdominal infection. Among the 7 treated for a day, 28.6% (2) developed an intra-abdominal infection; 40% (6) did so among the 15 treated for more than a day. The trend was similar for PC patients, although the overall infection rates were lower.
Antimicrobial resistance could be to blame. As normal flora were wiped out, maybe the field was cleared for "bugs to cause problems that otherwise would not have," explained senior investigator Dr. Thèrese Duane of the department of surgery at VCU. Surgeons there tend to favor Zosyn or Cefoxitin.
The project was just the first step toward building a robust evidence base about antibiotic use after trauma laparotomies. Next on the team’s agenda is a multicenter, prospective trial.
"We need more numbers," Dr. Duane said.
Dr. Goldberg has no relevant disclosures. Dr. Duane speaks for Pfizer on behalf of its antibiotic, linezolid.
AT THE ANNUAL MEETING OF THE SURGICAL INFECTION SOCIETY
Major finding: Patients who were given antibiotics after trauma laparotomies are six times more likely to develop an intra-abdominal infection than were those who are not (OR, 6.7; 95% CI 1.33-33.8; P = .044).
Data Source: Retrospective review of 121 trauma laparotomies
Disclosures: The lead investigator has no relevant disclosures. The senior investigator speaks for Pfizer on behalf of its antibiotic, linezolid.
Survey: Women Prefer Uterine-Preserving Treatment for Prolapse
CHARLESTON, S.C. – Women with pelvic organ prolapse symptoms are more likely to favor treatment that allows for uterine preservation than treatment involving hysterectomy, according to findings from a Fellow’s Pelvic Research Network study.
Of 213 women who participated in the multicenter cross-sectional study, 36% said they would prefer uterine preservation and 20% said they would prefer hysterectomy in a scenario in which outcomes were equal, Dr. Nicole. Korbly reported at the annual meeting of the Society of Gynecologic Surgeons.
The remaining 44% had no strong preference, said Dr. Korbly of the Women and Infants’ Hospital of Rhode Island and Brown University, both in Providence, R.I.
"In a scenario in which uterine preservation was superior, the proportion of women preferring uterine preservation increased from 36% to 44%; 11% preferred hysterectomy," she said.
In a scenario in which hysterectomy was superior, the proportion preferring hysterectomy increased from 20% to 36%. Of note, in this scenario, 21% of women still preferred uterine preservation, despite its inferior efficacy, she said.
Responses in some cases differed by geographic region. For example, in the scenarios in which outcomes would be equal and in which uterine preservation would be superior, those living in the West and Northeast regions of the United States were more likely than those in the South and Midwest regions to prefer uterine preservation. The responses did not differ significantly by region in the scenario in which hysterectomy was superior.
On logistic regression analysis, those living in the South had a lower odds of preferring uterine preservation compared with those living in the Northeast (odds ratio, 0.2), Dr. Korbly noted.
Also, women with at least some college education and those who said they considered the uterus important for a sense of self had increased odds of preferring uterine preservation (OR, 3.37 and 26.3, respectively). Age, race, and ethnicity, however, were not significant predictors.
Women included in the study were patients with a mean age of 59 years who presented with prolapse symptoms at one of nine participating centers between May 2011 and October 2012. They completed a 35-item questionnaire designed to assess uterine preservation and hysterectomy preference, as well as attitudes about the potential benefits and risks of uterine preservation and knowledge about pelvic organ prolapse.
The findings are timely, given a recent increased interest in prolapse treatment options that allow for uterine preservation, Dr. Korbly said, explaining that because long-term efficacy of these treatment methods remains unclear, patient preference remains a particularly important consideration when determining the best treatment approach.
Few studies have looked at patient preference regarding uterine preservation, she said at the meeting, jointly sponsored by the American College of Surgeons.
"Well-designed clinical trials comparing uterine-preserving versus hysterectomy-based techniques for prolapse are needed to improve the decision-making process and to balance patients’ values and preferences with evidence-based treatment outcomes data," she concluded.
Dr. Korbly reported having no relevant financial disclosures.
CHARLESTON, S.C. – Women with pelvic organ prolapse symptoms are more likely to favor treatment that allows for uterine preservation than treatment involving hysterectomy, according to findings from a Fellow’s Pelvic Research Network study.
Of 213 women who participated in the multicenter cross-sectional study, 36% said they would prefer uterine preservation and 20% said they would prefer hysterectomy in a scenario in which outcomes were equal, Dr. Nicole. Korbly reported at the annual meeting of the Society of Gynecologic Surgeons.
The remaining 44% had no strong preference, said Dr. Korbly of the Women and Infants’ Hospital of Rhode Island and Brown University, both in Providence, R.I.
"In a scenario in which uterine preservation was superior, the proportion of women preferring uterine preservation increased from 36% to 44%; 11% preferred hysterectomy," she said.
In a scenario in which hysterectomy was superior, the proportion preferring hysterectomy increased from 20% to 36%. Of note, in this scenario, 21% of women still preferred uterine preservation, despite its inferior efficacy, she said.
Responses in some cases differed by geographic region. For example, in the scenarios in which outcomes would be equal and in which uterine preservation would be superior, those living in the West and Northeast regions of the United States were more likely than those in the South and Midwest regions to prefer uterine preservation. The responses did not differ significantly by region in the scenario in which hysterectomy was superior.
On logistic regression analysis, those living in the South had a lower odds of preferring uterine preservation compared with those living in the Northeast (odds ratio, 0.2), Dr. Korbly noted.
Also, women with at least some college education and those who said they considered the uterus important for a sense of self had increased odds of preferring uterine preservation (OR, 3.37 and 26.3, respectively). Age, race, and ethnicity, however, were not significant predictors.
Women included in the study were patients with a mean age of 59 years who presented with prolapse symptoms at one of nine participating centers between May 2011 and October 2012. They completed a 35-item questionnaire designed to assess uterine preservation and hysterectomy preference, as well as attitudes about the potential benefits and risks of uterine preservation and knowledge about pelvic organ prolapse.
The findings are timely, given a recent increased interest in prolapse treatment options that allow for uterine preservation, Dr. Korbly said, explaining that because long-term efficacy of these treatment methods remains unclear, patient preference remains a particularly important consideration when determining the best treatment approach.
Few studies have looked at patient preference regarding uterine preservation, she said at the meeting, jointly sponsored by the American College of Surgeons.
"Well-designed clinical trials comparing uterine-preserving versus hysterectomy-based techniques for prolapse are needed to improve the decision-making process and to balance patients’ values and preferences with evidence-based treatment outcomes data," she concluded.
Dr. Korbly reported having no relevant financial disclosures.
CHARLESTON, S.C. – Women with pelvic organ prolapse symptoms are more likely to favor treatment that allows for uterine preservation than treatment involving hysterectomy, according to findings from a Fellow’s Pelvic Research Network study.
Of 213 women who participated in the multicenter cross-sectional study, 36% said they would prefer uterine preservation and 20% said they would prefer hysterectomy in a scenario in which outcomes were equal, Dr. Nicole. Korbly reported at the annual meeting of the Society of Gynecologic Surgeons.
The remaining 44% had no strong preference, said Dr. Korbly of the Women and Infants’ Hospital of Rhode Island and Brown University, both in Providence, R.I.
"In a scenario in which uterine preservation was superior, the proportion of women preferring uterine preservation increased from 36% to 44%; 11% preferred hysterectomy," she said.
In a scenario in which hysterectomy was superior, the proportion preferring hysterectomy increased from 20% to 36%. Of note, in this scenario, 21% of women still preferred uterine preservation, despite its inferior efficacy, she said.
Responses in some cases differed by geographic region. For example, in the scenarios in which outcomes would be equal and in which uterine preservation would be superior, those living in the West and Northeast regions of the United States were more likely than those in the South and Midwest regions to prefer uterine preservation. The responses did not differ significantly by region in the scenario in which hysterectomy was superior.
On logistic regression analysis, those living in the South had a lower odds of preferring uterine preservation compared with those living in the Northeast (odds ratio, 0.2), Dr. Korbly noted.
Also, women with at least some college education and those who said they considered the uterus important for a sense of self had increased odds of preferring uterine preservation (OR, 3.37 and 26.3, respectively). Age, race, and ethnicity, however, were not significant predictors.
Women included in the study were patients with a mean age of 59 years who presented with prolapse symptoms at one of nine participating centers between May 2011 and October 2012. They completed a 35-item questionnaire designed to assess uterine preservation and hysterectomy preference, as well as attitudes about the potential benefits and risks of uterine preservation and knowledge about pelvic organ prolapse.
The findings are timely, given a recent increased interest in prolapse treatment options that allow for uterine preservation, Dr. Korbly said, explaining that because long-term efficacy of these treatment methods remains unclear, patient preference remains a particularly important consideration when determining the best treatment approach.
Few studies have looked at patient preference regarding uterine preservation, she said at the meeting, jointly sponsored by the American College of Surgeons.
"Well-designed clinical trials comparing uterine-preserving versus hysterectomy-based techniques for prolapse are needed to improve the decision-making process and to balance patients’ values and preferences with evidence-based treatment outcomes data," she concluded.
Dr. Korbly reported having no relevant financial disclosures.
AT THE SGS ANNUAL MEETING
Major finding: 36% vs. 20% of women preferred uterine preservation over hysterectomy given equal outcomes.
Data source: A multicenter cross-sectional study of 213 women with pelvic organ prolapse symptoms.
Disclosures: Dr. Korbly reported having no relevant financial disclosures.
Navigating the new insurance exchanges
Starting Oct. 1, individuals and small businesses will have the chance to shop for and purchase private insurance through the new health insurance exchanges, but they are likely to have plenty of questions about the products and the process for enrollment. With that in mind, the Affordable Care Act (ACA) also mandates that the exchanges award grants to "navigators" and other personnel to provide impartial information to consumers about health plans, eligibility, and their potential to qualify for financial subsidies.
The Health and Human Services department recently issued regulations outlining the roles, training, and certifications required of navigators. Shelly Ten Napel, an expert on state health care coverage issues, explained what these navigators will do and how physicians may be affected.
Question: Who can be a navigator under the HHS regulations?
Ms. Ten Napel: It may be helpful to think in these terms: "navigator entities" are organizations that have existing relationships with the target populations of the health insurance exchange. Individual navigators are people who work for navigator entities and receive in-depth training on health coverage options under the ACA. Each health insurance exchange can set its own target population, but most will emphasize outreach to those who qualify for the tax credits (people with household incomes between 138% and 400% of the federal poverty level), the uninsured, and populations that have traditionally been underserved or hard to reach.
Federal regulations require that navigators include at least one community-based organization or consumer-focused nonprofit as well as at least one of the following: trade, industry, and professional associations; commercial fishing industry organizations; ranching and farming organizations; chambers of commerce; unions; resource partners of the Small Business Administration; licensed agents and brokers; and other public or private entities or individuals that meet the requirements of this section, such as Indian tribes, tribal organizations, and state or local human service agencies.
Navigators and "in-person assisters" cannot have a financial or nonfinancial conflict of interest. Some states may allow health care providers to serve as navigators, while others may perceive providers as having a conflict of interest because they receive payments from health plans.
Question: Has HHS included enough safeguards to ensure that the navigators don’t have financial conflicts of interest?
Ms. Ten Napel: Yes, there are strong requirements to prevent navigators from having both financial and nonfinancial conflicts of interest. Specifically, navigators cannot receive financial or nonfinancial consideration from any health insurer. States must monitor the enrollment patterns of navigators and develop civil and criminal penalties for those who fail to comply with the conflict-of-interest standards.
Question: How will the navigators help consumers?
Ms. Ten Napel: They will conduct education and outreach to help the public learn about the new health care coverage options under the ACA. Navigators will help consumers determine whether they are eligible for Medicaid or tax credits. They will walk consumers through the decision tools on the health insurance exchange websites to help individuals understand plan choices and enroll in plans. There is an emphasis on providing services in a culturally and linguistically appropriate manner.
Question: Does anything like this exist in the current insurance marketplace?
Ms. Ten Napel: There are individuals in the current market who perform similar functions. For example, Medicaid enrollment brokers and eligibility workers perform some similar functions, though they focus on the Medicaid program. Brokers or producers help people find and enroll in private health coverage. Navigators are unique in that they will be trained to help people understand the range of options across public programs and the private market in the health insurance exchanges. In addition, they will have an outreach and education function. They will be selected based on their unique ability to reach uninsured and underserved populations.
Question: Will the navigators work only with consumers or will they be in contact with physician offices?
Ms. Ten Napel: Navigators will work primarily with consumers, though providers may feel the impact of navigators in a few ways. First, new functionality in health insurance exchange websites will enable individuals to determine which plans offer in-network coverage for their providers. Thus, navigators can help patients who want to remain with their current physician select the right plan. Second, navigators will have a role in helping people understand some of the key features of health insurance, such as copayments and deductibles. The hope is that consumers will not only gain coverage but develop a better understanding of how to use that coverage. Third – and this is the exciting part of the program – if the navigators and other outreach efforts are successful, more people will gain coverage, enabling them to seek needed health care.
Starting in October, states and federal government will be embarking on an all-hands-on-deck effort to educate consumers about their new health care coverage options and encourage them to become insured. Providers absolutely can be helpful in that effort. Providers who want to offer information to their patients should reach out to their professional associations or state health insurance exchanges to learn more about how they can help.
Shelly Ten Napel is a senior manager at AcademyHealth, a health services research organization in Washington, D.C., where she works on state health care coverage initiatives.
Starting Oct. 1, individuals and small businesses will have the chance to shop for and purchase private insurance through the new health insurance exchanges, but they are likely to have plenty of questions about the products and the process for enrollment. With that in mind, the Affordable Care Act (ACA) also mandates that the exchanges award grants to "navigators" and other personnel to provide impartial information to consumers about health plans, eligibility, and their potential to qualify for financial subsidies.
The Health and Human Services department recently issued regulations outlining the roles, training, and certifications required of navigators. Shelly Ten Napel, an expert on state health care coverage issues, explained what these navigators will do and how physicians may be affected.
Question: Who can be a navigator under the HHS regulations?
Ms. Ten Napel: It may be helpful to think in these terms: "navigator entities" are organizations that have existing relationships with the target populations of the health insurance exchange. Individual navigators are people who work for navigator entities and receive in-depth training on health coverage options under the ACA. Each health insurance exchange can set its own target population, but most will emphasize outreach to those who qualify for the tax credits (people with household incomes between 138% and 400% of the federal poverty level), the uninsured, and populations that have traditionally been underserved or hard to reach.
Federal regulations require that navigators include at least one community-based organization or consumer-focused nonprofit as well as at least one of the following: trade, industry, and professional associations; commercial fishing industry organizations; ranching and farming organizations; chambers of commerce; unions; resource partners of the Small Business Administration; licensed agents and brokers; and other public or private entities or individuals that meet the requirements of this section, such as Indian tribes, tribal organizations, and state or local human service agencies.
Navigators and "in-person assisters" cannot have a financial or nonfinancial conflict of interest. Some states may allow health care providers to serve as navigators, while others may perceive providers as having a conflict of interest because they receive payments from health plans.
Question: Has HHS included enough safeguards to ensure that the navigators don’t have financial conflicts of interest?
Ms. Ten Napel: Yes, there are strong requirements to prevent navigators from having both financial and nonfinancial conflicts of interest. Specifically, navigators cannot receive financial or nonfinancial consideration from any health insurer. States must monitor the enrollment patterns of navigators and develop civil and criminal penalties for those who fail to comply with the conflict-of-interest standards.
Question: How will the navigators help consumers?
Ms. Ten Napel: They will conduct education and outreach to help the public learn about the new health care coverage options under the ACA. Navigators will help consumers determine whether they are eligible for Medicaid or tax credits. They will walk consumers through the decision tools on the health insurance exchange websites to help individuals understand plan choices and enroll in plans. There is an emphasis on providing services in a culturally and linguistically appropriate manner.
Question: Does anything like this exist in the current insurance marketplace?
Ms. Ten Napel: There are individuals in the current market who perform similar functions. For example, Medicaid enrollment brokers and eligibility workers perform some similar functions, though they focus on the Medicaid program. Brokers or producers help people find and enroll in private health coverage. Navigators are unique in that they will be trained to help people understand the range of options across public programs and the private market in the health insurance exchanges. In addition, they will have an outreach and education function. They will be selected based on their unique ability to reach uninsured and underserved populations.
Question: Will the navigators work only with consumers or will they be in contact with physician offices?
Ms. Ten Napel: Navigators will work primarily with consumers, though providers may feel the impact of navigators in a few ways. First, new functionality in health insurance exchange websites will enable individuals to determine which plans offer in-network coverage for their providers. Thus, navigators can help patients who want to remain with their current physician select the right plan. Second, navigators will have a role in helping people understand some of the key features of health insurance, such as copayments and deductibles. The hope is that consumers will not only gain coverage but develop a better understanding of how to use that coverage. Third – and this is the exciting part of the program – if the navigators and other outreach efforts are successful, more people will gain coverage, enabling them to seek needed health care.
Starting in October, states and federal government will be embarking on an all-hands-on-deck effort to educate consumers about their new health care coverage options and encourage them to become insured. Providers absolutely can be helpful in that effort. Providers who want to offer information to their patients should reach out to their professional associations or state health insurance exchanges to learn more about how they can help.
Shelly Ten Napel is a senior manager at AcademyHealth, a health services research organization in Washington, D.C., where she works on state health care coverage initiatives.
Starting Oct. 1, individuals and small businesses will have the chance to shop for and purchase private insurance through the new health insurance exchanges, but they are likely to have plenty of questions about the products and the process for enrollment. With that in mind, the Affordable Care Act (ACA) also mandates that the exchanges award grants to "navigators" and other personnel to provide impartial information to consumers about health plans, eligibility, and their potential to qualify for financial subsidies.
The Health and Human Services department recently issued regulations outlining the roles, training, and certifications required of navigators. Shelly Ten Napel, an expert on state health care coverage issues, explained what these navigators will do and how physicians may be affected.
Question: Who can be a navigator under the HHS regulations?
Ms. Ten Napel: It may be helpful to think in these terms: "navigator entities" are organizations that have existing relationships with the target populations of the health insurance exchange. Individual navigators are people who work for navigator entities and receive in-depth training on health coverage options under the ACA. Each health insurance exchange can set its own target population, but most will emphasize outreach to those who qualify for the tax credits (people with household incomes between 138% and 400% of the federal poverty level), the uninsured, and populations that have traditionally been underserved or hard to reach.
Federal regulations require that navigators include at least one community-based organization or consumer-focused nonprofit as well as at least one of the following: trade, industry, and professional associations; commercial fishing industry organizations; ranching and farming organizations; chambers of commerce; unions; resource partners of the Small Business Administration; licensed agents and brokers; and other public or private entities or individuals that meet the requirements of this section, such as Indian tribes, tribal organizations, and state or local human service agencies.
Navigators and "in-person assisters" cannot have a financial or nonfinancial conflict of interest. Some states may allow health care providers to serve as navigators, while others may perceive providers as having a conflict of interest because they receive payments from health plans.
Question: Has HHS included enough safeguards to ensure that the navigators don’t have financial conflicts of interest?
Ms. Ten Napel: Yes, there are strong requirements to prevent navigators from having both financial and nonfinancial conflicts of interest. Specifically, navigators cannot receive financial or nonfinancial consideration from any health insurer. States must monitor the enrollment patterns of navigators and develop civil and criminal penalties for those who fail to comply with the conflict-of-interest standards.
Question: How will the navigators help consumers?
Ms. Ten Napel: They will conduct education and outreach to help the public learn about the new health care coverage options under the ACA. Navigators will help consumers determine whether they are eligible for Medicaid or tax credits. They will walk consumers through the decision tools on the health insurance exchange websites to help individuals understand plan choices and enroll in plans. There is an emphasis on providing services in a culturally and linguistically appropriate manner.
Question: Does anything like this exist in the current insurance marketplace?
Ms. Ten Napel: There are individuals in the current market who perform similar functions. For example, Medicaid enrollment brokers and eligibility workers perform some similar functions, though they focus on the Medicaid program. Brokers or producers help people find and enroll in private health coverage. Navigators are unique in that they will be trained to help people understand the range of options across public programs and the private market in the health insurance exchanges. In addition, they will have an outreach and education function. They will be selected based on their unique ability to reach uninsured and underserved populations.
Question: Will the navigators work only with consumers or will they be in contact with physician offices?
Ms. Ten Napel: Navigators will work primarily with consumers, though providers may feel the impact of navigators in a few ways. First, new functionality in health insurance exchange websites will enable individuals to determine which plans offer in-network coverage for their providers. Thus, navigators can help patients who want to remain with their current physician select the right plan. Second, navigators will have a role in helping people understand some of the key features of health insurance, such as copayments and deductibles. The hope is that consumers will not only gain coverage but develop a better understanding of how to use that coverage. Third – and this is the exciting part of the program – if the navigators and other outreach efforts are successful, more people will gain coverage, enabling them to seek needed health care.
Starting in October, states and federal government will be embarking on an all-hands-on-deck effort to educate consumers about their new health care coverage options and encourage them to become insured. Providers absolutely can be helpful in that effort. Providers who want to offer information to their patients should reach out to their professional associations or state health insurance exchanges to learn more about how they can help.
Shelly Ten Napel is a senior manager at AcademyHealth, a health services research organization in Washington, D.C., where she works on state health care coverage initiatives.
Supreme Court ponders patenting of human genes
Can human genes be patented? The Supreme Court is set to decide just that later this summer in a decision that could have a deep and lasting effect on medical research.
In Association for Molecular Pathology, et al. v. Myriad Genetics, the high court is considering the validity of patents held by Utah-based Myriad Genetics for the BRCA1 and BRCA2 genes, which are associated with an increased risk of breast and ovarian cancer.
Scientists at Myriad Genetics uncovered the link between the BRCA1 and BRCA2 genes and the increased risk for cancer and patented the discovery in the mid-1990s. Since then, they have held the exclusive rights to the diagnostic testing for the mutations.
The company, however, has been criticized for setting the price of its BRACAnalysis, which tests for mutations on both genes, too high. Also, critics say that, since no one else can run the test, it’s impossible to get a second opinion.
So in 2009, the Association for Molecular Pathology, the American Civil Liberties Union, and several women’s health groups filed suit against Myriad in an effort to invalidate the company’s patents. In the most recent court ruling, a federal appeals court sided with Myriad and upheld the patents.
The Supreme Court recently heard oral arguments in the case and is expected to release its decision at the end of June.
Opponents of gene patenting, including the American Medical Association, contend that Myriad doesn’t have the right to patent human genes because these are products of nature and have not been altered. The AMA, which filed an amicus brief in the case, is also opposed to the patents on ethical grounds.
"Medical innovations that provide insight into natural human biology must remain freely accessible and widely disseminated, not hidden behind a vast thicket of exclusive rights," Dr. Jeremy Lazarus, AMA president, said in a statement. "Blocking this information interferes with diagnosis and treatment of patients and inhibits new medical discoveries."
Myriad argues that its patents aren’t for the genes themselves but for the synthetic molecules based on the genes, which are created in the lab. These molecules are different from what is found in nature or the human body, according to Myriad.
On a practical level, invalidating the patents would "chill" future biomedical research, according to the Biotechnology Industry Organization (BIO). In an amicus brief in support of Myriad, the organization warned that the chilling effect would apply to nondiagnostic uses for isolated human DNA, the untapped potential of isolated nonhuman DNA, the use of RNA molecules such as microRNA, and other isolated molecules such as therapeutic proteins and antibiotics. BIO said that future advances in vaccination also could be threatened if the Myriad patent case failed. For example, without the hope of patent protection, companies might not continue to work toward making vaccines that use small pieces of DNA to trigger antibody production. This area of research, which is in the early stages, holds the potential to produce vaccines for diseases such as HIV and cancer, according to BIO.
But bioethicist Arthur Caplan, Ph.D., said that the idea that research would be stifled if patents on genes were overturned is "sheer nonsense."
"Most of the money to understand human genes came from the taxpayer, through the form of the genome mapping project," said Dr. Caplan, who is the director of the division of medical ethics at the New York University Langone Medical Center. "It wasn’t funded by private companies."
No matter what the court decides, there isn’t likely to be a major practical impact, Dr. Caplan said. Since Myriad’s 20-year patent exclusivity is about to expire, diagnostic testing on BRCA1 and BRCA2 will soon open up. So in terms of a fight about cost and access, the case is a little late, he said.
"I don’t think the case is momentous if you’re someone thinking about getting a breast cancer test because the situation is going to likely change pretty soon no matter what the court says," Dr. Caplan said.
Dr. James P. Evans, professor of genetics and medicine at the University of North Carolina at Chapel Hill, agreed.
"This is a classic example of the science having outstripped the legislative and judicial arenas," Dr. Evans said.
For starters, genome sequencing has become so routine that there is patent infringement going on "right and left," he said, which is likely to continue regardless of what the Supreme Court rules in the case. "I think the horse is out of the barn," he said.
As for research, Dr. Evans said that the removal of patents is likely to open up this area. Most of the progress in genetics so far has come from researchers who have not been seeking patents. And patents would still be available for other advances, such as the creation of new gene-sequencing platforms, he said.
On Twitter @MaryEllenNY
Opponents of gene patenting, American Medical Association,
Can human genes be patented? The Supreme Court is set to decide just that later this summer in a decision that could have a deep and lasting effect on medical research.
In Association for Molecular Pathology, et al. v. Myriad Genetics, the high court is considering the validity of patents held by Utah-based Myriad Genetics for the BRCA1 and BRCA2 genes, which are associated with an increased risk of breast and ovarian cancer.
Scientists at Myriad Genetics uncovered the link between the BRCA1 and BRCA2 genes and the increased risk for cancer and patented the discovery in the mid-1990s. Since then, they have held the exclusive rights to the diagnostic testing for the mutations.
The company, however, has been criticized for setting the price of its BRACAnalysis, which tests for mutations on both genes, too high. Also, critics say that, since no one else can run the test, it’s impossible to get a second opinion.
So in 2009, the Association for Molecular Pathology, the American Civil Liberties Union, and several women’s health groups filed suit against Myriad in an effort to invalidate the company’s patents. In the most recent court ruling, a federal appeals court sided with Myriad and upheld the patents.
The Supreme Court recently heard oral arguments in the case and is expected to release its decision at the end of June.
Opponents of gene patenting, including the American Medical Association, contend that Myriad doesn’t have the right to patent human genes because these are products of nature and have not been altered. The AMA, which filed an amicus brief in the case, is also opposed to the patents on ethical grounds.
"Medical innovations that provide insight into natural human biology must remain freely accessible and widely disseminated, not hidden behind a vast thicket of exclusive rights," Dr. Jeremy Lazarus, AMA president, said in a statement. "Blocking this information interferes with diagnosis and treatment of patients and inhibits new medical discoveries."
Myriad argues that its patents aren’t for the genes themselves but for the synthetic molecules based on the genes, which are created in the lab. These molecules are different from what is found in nature or the human body, according to Myriad.
On a practical level, invalidating the patents would "chill" future biomedical research, according to the Biotechnology Industry Organization (BIO). In an amicus brief in support of Myriad, the organization warned that the chilling effect would apply to nondiagnostic uses for isolated human DNA, the untapped potential of isolated nonhuman DNA, the use of RNA molecules such as microRNA, and other isolated molecules such as therapeutic proteins and antibiotics. BIO said that future advances in vaccination also could be threatened if the Myriad patent case failed. For example, without the hope of patent protection, companies might not continue to work toward making vaccines that use small pieces of DNA to trigger antibody production. This area of research, which is in the early stages, holds the potential to produce vaccines for diseases such as HIV and cancer, according to BIO.
But bioethicist Arthur Caplan, Ph.D., said that the idea that research would be stifled if patents on genes were overturned is "sheer nonsense."
"Most of the money to understand human genes came from the taxpayer, through the form of the genome mapping project," said Dr. Caplan, who is the director of the division of medical ethics at the New York University Langone Medical Center. "It wasn’t funded by private companies."
No matter what the court decides, there isn’t likely to be a major practical impact, Dr. Caplan said. Since Myriad’s 20-year patent exclusivity is about to expire, diagnostic testing on BRCA1 and BRCA2 will soon open up. So in terms of a fight about cost and access, the case is a little late, he said.
"I don’t think the case is momentous if you’re someone thinking about getting a breast cancer test because the situation is going to likely change pretty soon no matter what the court says," Dr. Caplan said.
Dr. James P. Evans, professor of genetics and medicine at the University of North Carolina at Chapel Hill, agreed.
"This is a classic example of the science having outstripped the legislative and judicial arenas," Dr. Evans said.
For starters, genome sequencing has become so routine that there is patent infringement going on "right and left," he said, which is likely to continue regardless of what the Supreme Court rules in the case. "I think the horse is out of the barn," he said.
As for research, Dr. Evans said that the removal of patents is likely to open up this area. Most of the progress in genetics so far has come from researchers who have not been seeking patents. And patents would still be available for other advances, such as the creation of new gene-sequencing platforms, he said.
On Twitter @MaryEllenNY
Can human genes be patented? The Supreme Court is set to decide just that later this summer in a decision that could have a deep and lasting effect on medical research.
In Association for Molecular Pathology, et al. v. Myriad Genetics, the high court is considering the validity of patents held by Utah-based Myriad Genetics for the BRCA1 and BRCA2 genes, which are associated with an increased risk of breast and ovarian cancer.
Scientists at Myriad Genetics uncovered the link between the BRCA1 and BRCA2 genes and the increased risk for cancer and patented the discovery in the mid-1990s. Since then, they have held the exclusive rights to the diagnostic testing for the mutations.
The company, however, has been criticized for setting the price of its BRACAnalysis, which tests for mutations on both genes, too high. Also, critics say that, since no one else can run the test, it’s impossible to get a second opinion.
So in 2009, the Association for Molecular Pathology, the American Civil Liberties Union, and several women’s health groups filed suit against Myriad in an effort to invalidate the company’s patents. In the most recent court ruling, a federal appeals court sided with Myriad and upheld the patents.
The Supreme Court recently heard oral arguments in the case and is expected to release its decision at the end of June.
Opponents of gene patenting, including the American Medical Association, contend that Myriad doesn’t have the right to patent human genes because these are products of nature and have not been altered. The AMA, which filed an amicus brief in the case, is also opposed to the patents on ethical grounds.
"Medical innovations that provide insight into natural human biology must remain freely accessible and widely disseminated, not hidden behind a vast thicket of exclusive rights," Dr. Jeremy Lazarus, AMA president, said in a statement. "Blocking this information interferes with diagnosis and treatment of patients and inhibits new medical discoveries."
Myriad argues that its patents aren’t for the genes themselves but for the synthetic molecules based on the genes, which are created in the lab. These molecules are different from what is found in nature or the human body, according to Myriad.
On a practical level, invalidating the patents would "chill" future biomedical research, according to the Biotechnology Industry Organization (BIO). In an amicus brief in support of Myriad, the organization warned that the chilling effect would apply to nondiagnostic uses for isolated human DNA, the untapped potential of isolated nonhuman DNA, the use of RNA molecules such as microRNA, and other isolated molecules such as therapeutic proteins and antibiotics. BIO said that future advances in vaccination also could be threatened if the Myriad patent case failed. For example, without the hope of patent protection, companies might not continue to work toward making vaccines that use small pieces of DNA to trigger antibody production. This area of research, which is in the early stages, holds the potential to produce vaccines for diseases such as HIV and cancer, according to BIO.
But bioethicist Arthur Caplan, Ph.D., said that the idea that research would be stifled if patents on genes were overturned is "sheer nonsense."
"Most of the money to understand human genes came from the taxpayer, through the form of the genome mapping project," said Dr. Caplan, who is the director of the division of medical ethics at the New York University Langone Medical Center. "It wasn’t funded by private companies."
No matter what the court decides, there isn’t likely to be a major practical impact, Dr. Caplan said. Since Myriad’s 20-year patent exclusivity is about to expire, diagnostic testing on BRCA1 and BRCA2 will soon open up. So in terms of a fight about cost and access, the case is a little late, he said.
"I don’t think the case is momentous if you’re someone thinking about getting a breast cancer test because the situation is going to likely change pretty soon no matter what the court says," Dr. Caplan said.
Dr. James P. Evans, professor of genetics and medicine at the University of North Carolina at Chapel Hill, agreed.
"This is a classic example of the science having outstripped the legislative and judicial arenas," Dr. Evans said.
For starters, genome sequencing has become so routine that there is patent infringement going on "right and left," he said, which is likely to continue regardless of what the Supreme Court rules in the case. "I think the horse is out of the barn," he said.
As for research, Dr. Evans said that the removal of patents is likely to open up this area. Most of the progress in genetics so far has come from researchers who have not been seeking patents. And patents would still be available for other advances, such as the creation of new gene-sequencing platforms, he said.
On Twitter @MaryEllenNY
Opponents of gene patenting, American Medical Association,
Opponents of gene patenting, American Medical Association,