User login
Asthma Across a Woman’s Lifespan
1. Chowdhury NU et al. Eur Respir Rev. 2021;30(162):210067. doi:10.1183/16000617.0067-2021
2. Perikleous EP et al. J Pers Med. 2022;12(6):999. doi:10.3390/jpm12060999
3. Khaleva E et al. Clin Transl Allergy. 2020;10:40. doi:10.1186/s13601-020-00340-z
4. Robijn AL et al. Curr Opin Pulm Med. 2019;25(1):11-17. doi:10.1097/MCP.0000000000000538
5. Bravo-Solarte DC et al. Allergy Asthma Proc. 2023;44(1):24-34. doi:10.2500/aap.2023.44.220077
6. Wang G et al. J Matern Fetal Neonatal Med. 2014;27(9):934-942. doi:10.3109/14767058.2013.847080
7. Hough KP et al. Front Med (Lausanne). 2020;7:191. doi:10.3389/fmed.2020.00191
8. Triebner K et al. Am J Respir Crit Care Med. 2017;195(8):1058-1065. doi:10.1164/rccm.201605-0968OC
9. Bacharier LB, Jackson DJ. J Allergy Clin Immunol. 2023;151(3):581-589. doi:10.1016/j.jaci.2023.01.002
10. An amazing journey: how young lungs develop. American Lung Association. Published May 11, 2018. Accessed June 28, 2023. https://www.lung.org/blog/how-young-lungs-develop
11. Strunk RC et al. J Allergy Clin Immunol. 2006;118(5):1040-1047. doi:10.1016/j.jaci.2006.07.053
12. Kaplan A, Price D. J Asthma Allergy. 2020;13:39-49. doi:10.2147/JAA.S233268
1. Chowdhury NU et al. Eur Respir Rev. 2021;30(162):210067. doi:10.1183/16000617.0067-2021
2. Perikleous EP et al. J Pers Med. 2022;12(6):999. doi:10.3390/jpm12060999
3. Khaleva E et al. Clin Transl Allergy. 2020;10:40. doi:10.1186/s13601-020-00340-z
4. Robijn AL et al. Curr Opin Pulm Med. 2019;25(1):11-17. doi:10.1097/MCP.0000000000000538
5. Bravo-Solarte DC et al. Allergy Asthma Proc. 2023;44(1):24-34. doi:10.2500/aap.2023.44.220077
6. Wang G et al. J Matern Fetal Neonatal Med. 2014;27(9):934-942. doi:10.3109/14767058.2013.847080
7. Hough KP et al. Front Med (Lausanne). 2020;7:191. doi:10.3389/fmed.2020.00191
8. Triebner K et al. Am J Respir Crit Care Med. 2017;195(8):1058-1065. doi:10.1164/rccm.201605-0968OC
9. Bacharier LB, Jackson DJ. J Allergy Clin Immunol. 2023;151(3):581-589. doi:10.1016/j.jaci.2023.01.002
10. An amazing journey: how young lungs develop. American Lung Association. Published May 11, 2018. Accessed June 28, 2023. https://www.lung.org/blog/how-young-lungs-develop
11. Strunk RC et al. J Allergy Clin Immunol. 2006;118(5):1040-1047. doi:10.1016/j.jaci.2006.07.053
12. Kaplan A, Price D. J Asthma Allergy. 2020;13:39-49. doi:10.2147/JAA.S233268
1. Chowdhury NU et al. Eur Respir Rev. 2021;30(162):210067. doi:10.1183/16000617.0067-2021
2. Perikleous EP et al. J Pers Med. 2022;12(6):999. doi:10.3390/jpm12060999
3. Khaleva E et al. Clin Transl Allergy. 2020;10:40. doi:10.1186/s13601-020-00340-z
4. Robijn AL et al. Curr Opin Pulm Med. 2019;25(1):11-17. doi:10.1097/MCP.0000000000000538
5. Bravo-Solarte DC et al. Allergy Asthma Proc. 2023;44(1):24-34. doi:10.2500/aap.2023.44.220077
6. Wang G et al. J Matern Fetal Neonatal Med. 2014;27(9):934-942. doi:10.3109/14767058.2013.847080
7. Hough KP et al. Front Med (Lausanne). 2020;7:191. doi:10.3389/fmed.2020.00191
8. Triebner K et al. Am J Respir Crit Care Med. 2017;195(8):1058-1065. doi:10.1164/rccm.201605-0968OC
9. Bacharier LB, Jackson DJ. J Allergy Clin Immunol. 2023;151(3):581-589. doi:10.1016/j.jaci.2023.01.002
10. An amazing journey: how young lungs develop. American Lung Association. Published May 11, 2018. Accessed June 28, 2023. https://www.lung.org/blog/how-young-lungs-develop
11. Strunk RC et al. J Allergy Clin Immunol. 2006;118(5):1040-1047. doi:10.1016/j.jaci.2006.07.053
12. Kaplan A, Price D. J Asthma Allergy. 2020;13:39-49. doi:10.2147/JAA.S233268
Pulmonology Data Trends 2023 (Slideshow)
CHEST Physician presents the 2023 edition of Pulmonology Data Trends (click to read). This special issue provides updates on hot topics in pulmonology through original infographics and visual storytelling.
In this issue:
Long-Awaited RSV Vaccines Now Available for Older Adults and Pediatric Patients
Burton L. Lesnick, MD, FCCP
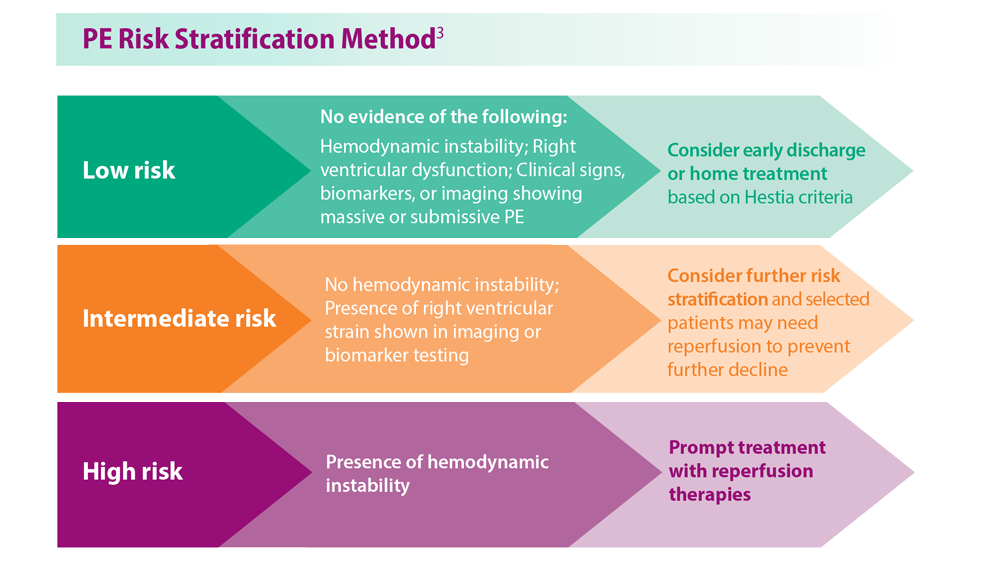
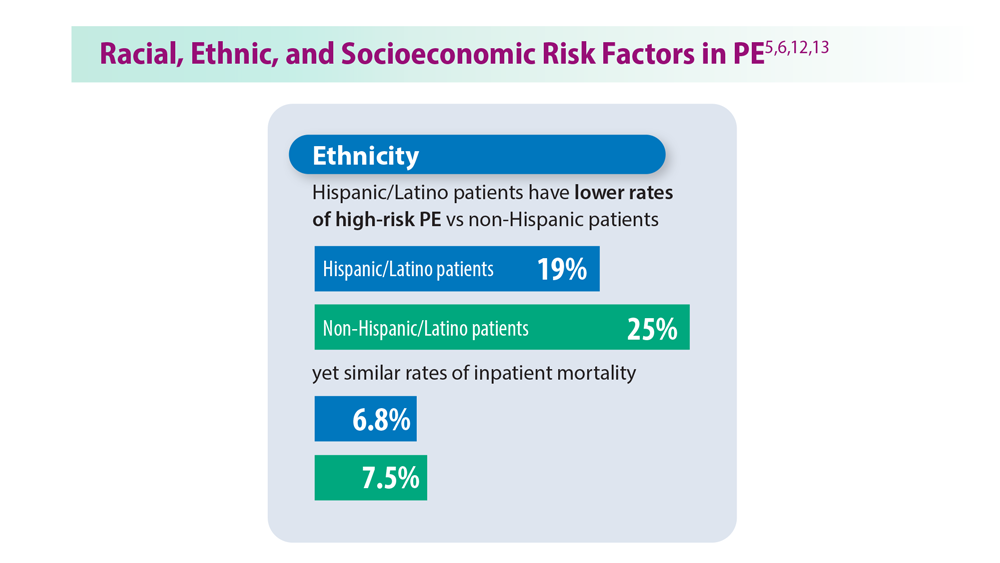
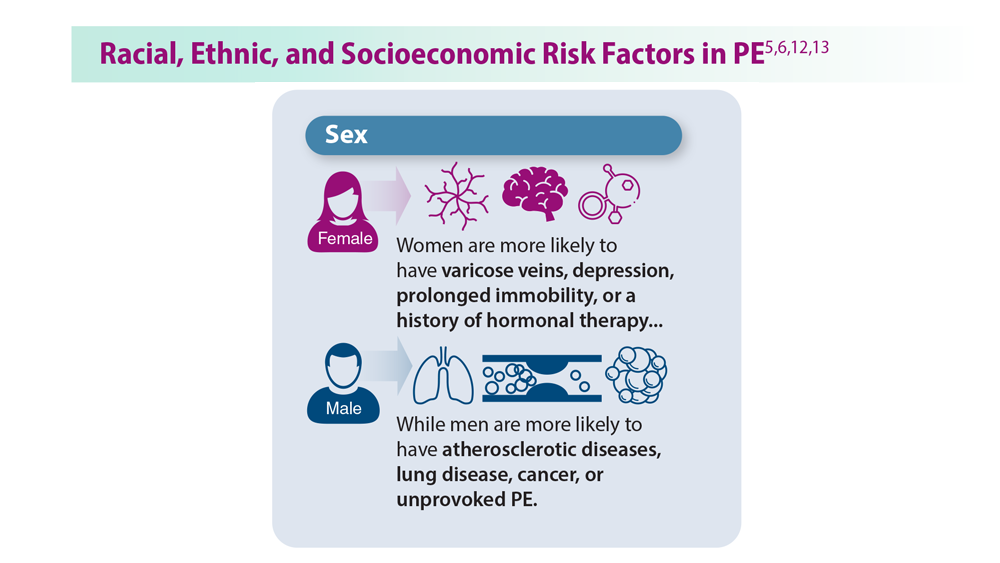
Decreasing Pulmonary Embolism-Related Mortality
Parth Rali, MD
Addressing Physician Burnout in Pulmonology and Critical Care
Kelly Vranas, MD, MCR
Updated Guidelines for COPD Management: 2023 GOLD Strategy Report
Muhammad Adrish, MD, MBA, FCCP, FCCM
Progressive Pulmonary Fibrosis: Understanding Its Many Forms
Tejaswini Kulkarni, MD, MPH, FCCP
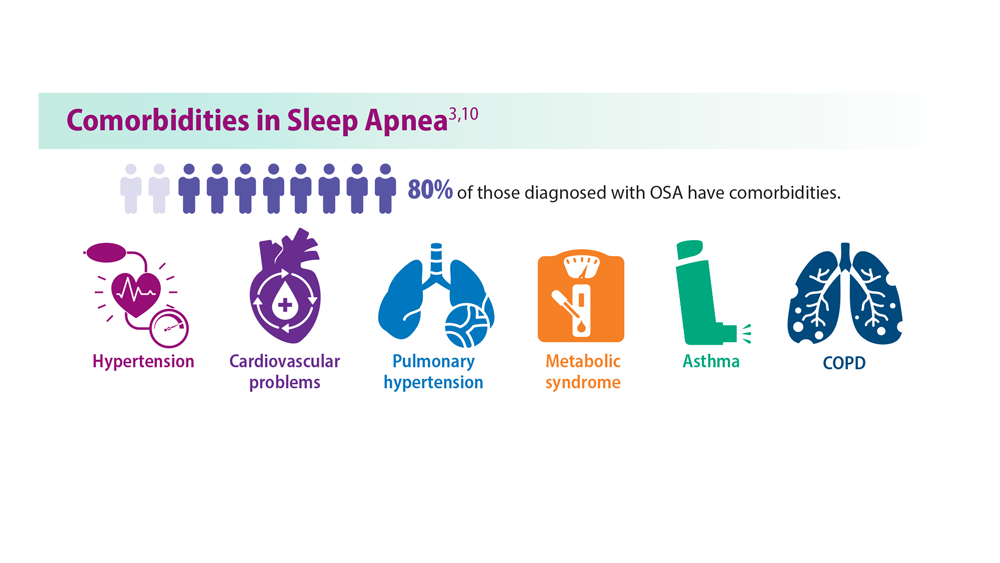
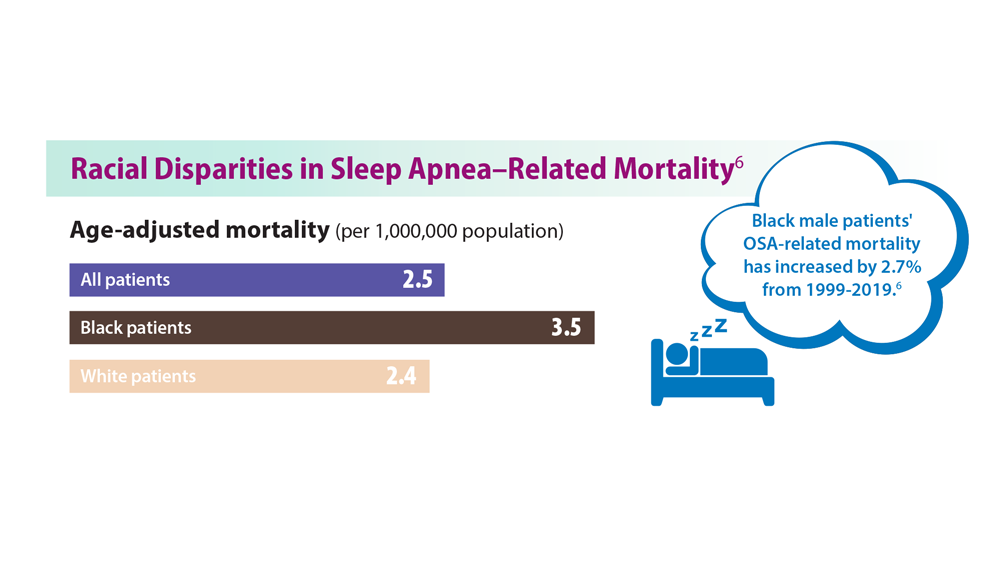
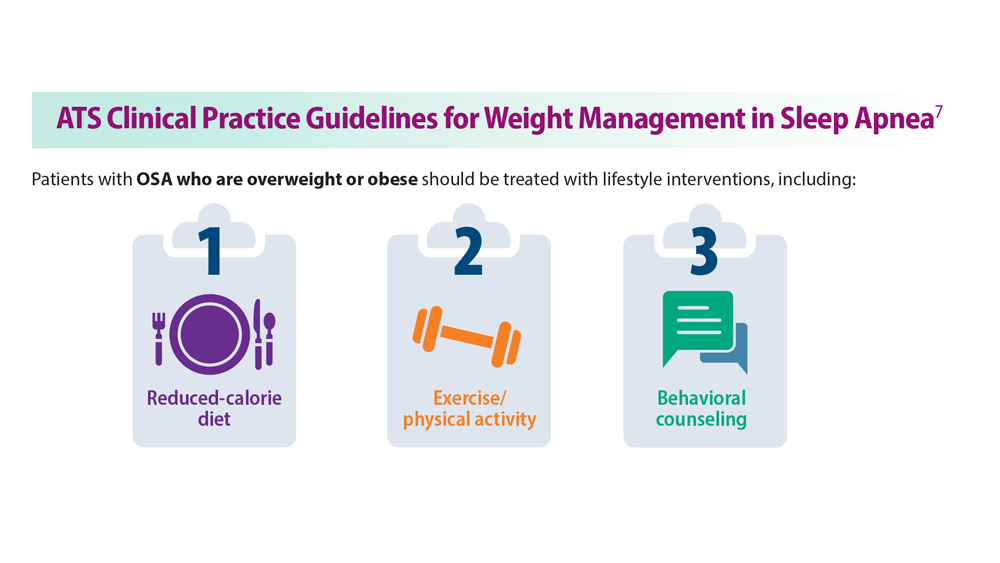
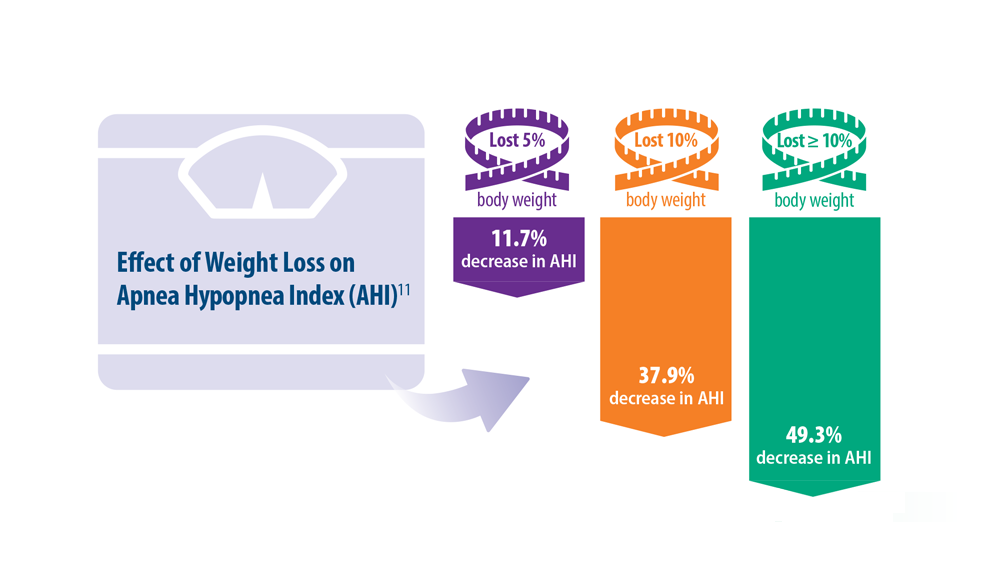
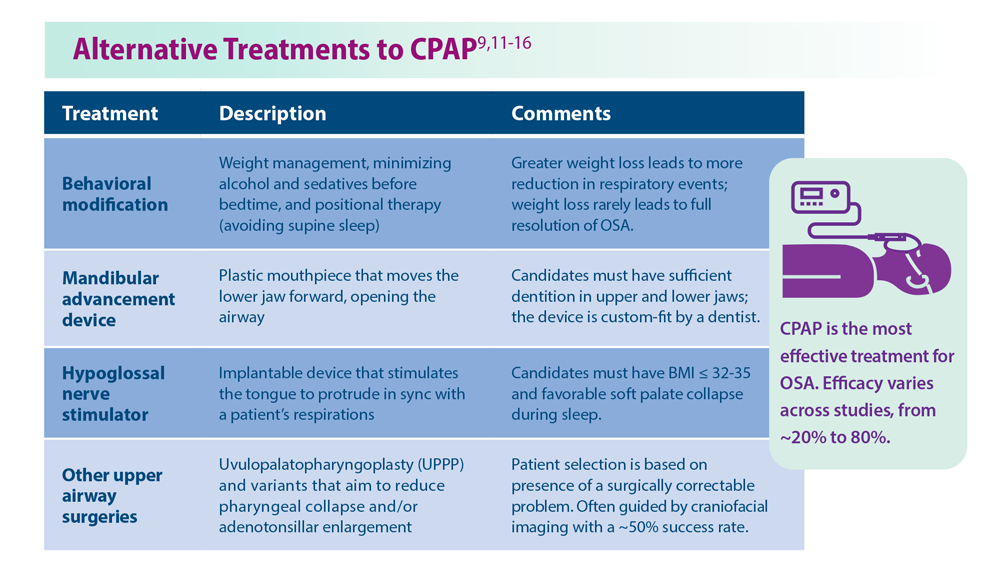
Sleep Apnea: Comorbidities, Racial Disparities, Weight Guidelines, and Alternatives to CPAP
Lauren Tobias, MD, FCCP
Lung Cancer Screening: A Need for Adjunctive Testing
Eric S. Edell, MD, FCCP
Asthma Across a Woman’s Lifespan
Navitha Ramesh, MD, FCCP
Tuberculosis Management: Returning to Pre-Pandemic Priorities
Patricio Escalante, MD, MSc, FCCP, and Paige K. Marty, MD
Long COVID: Advocating for Patients and Implementing Effective Techniques
Kyle B. Enfield, MD, MS, FSHEA, FCCM
CHEST Physician presents the 2023 edition of Pulmonology Data Trends (click to read). This special issue provides updates on hot topics in pulmonology through original infographics and visual storytelling.
In this issue:
Long-Awaited RSV Vaccines Now Available for Older Adults and Pediatric Patients
Burton L. Lesnick, MD, FCCP
Decreasing Pulmonary Embolism-Related Mortality
Parth Rali, MD
Addressing Physician Burnout in Pulmonology and Critical Care
Kelly Vranas, MD, MCR
Updated Guidelines for COPD Management: 2023 GOLD Strategy Report
Muhammad Adrish, MD, MBA, FCCP, FCCM
Progressive Pulmonary Fibrosis: Understanding Its Many Forms
Tejaswini Kulkarni, MD, MPH, FCCP
Sleep Apnea: Comorbidities, Racial Disparities, Weight Guidelines, and Alternatives to CPAP
Lauren Tobias, MD, FCCP
Lung Cancer Screening: A Need for Adjunctive Testing
Eric S. Edell, MD, FCCP
Asthma Across a Woman’s Lifespan
Navitha Ramesh, MD, FCCP
Tuberculosis Management: Returning to Pre-Pandemic Priorities
Patricio Escalante, MD, MSc, FCCP, and Paige K. Marty, MD
Long COVID: Advocating for Patients and Implementing Effective Techniques
Kyle B. Enfield, MD, MS, FSHEA, FCCM
CHEST Physician presents the 2023 edition of Pulmonology Data Trends (click to read). This special issue provides updates on hot topics in pulmonology through original infographics and visual storytelling.
In this issue:
Long-Awaited RSV Vaccines Now Available for Older Adults and Pediatric Patients
Burton L. Lesnick, MD, FCCP
Decreasing Pulmonary Embolism-Related Mortality
Parth Rali, MD
Addressing Physician Burnout in Pulmonology and Critical Care
Kelly Vranas, MD, MCR
Updated Guidelines for COPD Management: 2023 GOLD Strategy Report
Muhammad Adrish, MD, MBA, FCCP, FCCM
Progressive Pulmonary Fibrosis: Understanding Its Many Forms
Tejaswini Kulkarni, MD, MPH, FCCP
Sleep Apnea: Comorbidities, Racial Disparities, Weight Guidelines, and Alternatives to CPAP
Lauren Tobias, MD, FCCP
Lung Cancer Screening: A Need for Adjunctive Testing
Eric S. Edell, MD, FCCP
Asthma Across a Woman’s Lifespan
Navitha Ramesh, MD, FCCP
Tuberculosis Management: Returning to Pre-Pandemic Priorities
Patricio Escalante, MD, MSc, FCCP, and Paige K. Marty, MD
Long COVID: Advocating for Patients and Implementing Effective Techniques
Kyle B. Enfield, MD, MS, FSHEA, FCCM
Sleep Apnea: Comorbidities, Racial Disparities, Weight Guidelines, and Alternatives to CPAP
1. Gottlieb DJ, Punjabi NM. JAMA. 2020;323(14):1389-1400. doi:10.1001/jama.2020.3514
2. Slowik JM et al. Obstructive Sleep Apnea. In: StatPearls. Treasure Island (FL): StatPearls Publishing; December 11, 2022.
3. Bonsignore MR et al. Multidiscip Respir Med. 2019;14:8. doi:10.1186/s40248-019-0172-9
4. Schwartz SW et al. Sleep Breath. 2016;20(3):947-955. doi:10.1007/s11325-016-1316-1
5. Grandner MA et al. Sleep Med. 2016;18:7-18. doi:10.1016/j.sleep.2015.01.020
6. Lee YC et al. Sleep Med. 2022;90:204-213. doi:10.1016/j.sleep.2021.11.014
7. Hudgel DW et al. Am J Respir Crit Care Med. 2018;198(6):e70-e87. doi:10.1164/rccm.201807-1326ST
8. Lloyd R et al. J Clin Sleep Med. 2022;18(11):2673-2680. doi:10.5664/jcsm.10244
9. Nokes B et al. Expert Rev Respir Med. 2022;16(8):917-929. doi:10.1080/17476348.2022.2112669
10. Pinto JA et al. Int Arch Otorhinolaryngol. 2016;20(2):145-150.doi:10.1055/s-0036-1579546
11. Georgoulis M et al. J Clin Sleep Med. 2022;18(5):1251-1261. doi:10.5664/jcsm.9834
12. Askland K et al. Cochrane Database Syst Rev. 2020;4(4):CD007736. doi:10.1002/14651858.CD007736.pub3
13. Jugé L et al. Sleep. 2022;45(6):zsac044. doi:10.1093/sleep/zsac044
14. Strollo PJ Jr et al. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
15. Fattal D et al. J Clin Sleep Med. 2022;18(12):2723-2729. doi:10.5664/jcsm.10190
16. He M et al. Otolaryngol Head Neck Surg. 2019;161(3):401-411. doi:10.1177/0194599819840356
1. Gottlieb DJ, Punjabi NM. JAMA. 2020;323(14):1389-1400. doi:10.1001/jama.2020.3514
2. Slowik JM et al. Obstructive Sleep Apnea. In: StatPearls. Treasure Island (FL): StatPearls Publishing; December 11, 2022.
3. Bonsignore MR et al. Multidiscip Respir Med. 2019;14:8. doi:10.1186/s40248-019-0172-9
4. Schwartz SW et al. Sleep Breath. 2016;20(3):947-955. doi:10.1007/s11325-016-1316-1
5. Grandner MA et al. Sleep Med. 2016;18:7-18. doi:10.1016/j.sleep.2015.01.020
6. Lee YC et al. Sleep Med. 2022;90:204-213. doi:10.1016/j.sleep.2021.11.014
7. Hudgel DW et al. Am J Respir Crit Care Med. 2018;198(6):e70-e87. doi:10.1164/rccm.201807-1326ST
8. Lloyd R et al. J Clin Sleep Med. 2022;18(11):2673-2680. doi:10.5664/jcsm.10244
9. Nokes B et al. Expert Rev Respir Med. 2022;16(8):917-929. doi:10.1080/17476348.2022.2112669
10. Pinto JA et al. Int Arch Otorhinolaryngol. 2016;20(2):145-150.doi:10.1055/s-0036-1579546
11. Georgoulis M et al. J Clin Sleep Med. 2022;18(5):1251-1261. doi:10.5664/jcsm.9834
12. Askland K et al. Cochrane Database Syst Rev. 2020;4(4):CD007736. doi:10.1002/14651858.CD007736.pub3
13. Jugé L et al. Sleep. 2022;45(6):zsac044. doi:10.1093/sleep/zsac044
14. Strollo PJ Jr et al. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
15. Fattal D et al. J Clin Sleep Med. 2022;18(12):2723-2729. doi:10.5664/jcsm.10190
16. He M et al. Otolaryngol Head Neck Surg. 2019;161(3):401-411. doi:10.1177/0194599819840356
1. Gottlieb DJ, Punjabi NM. JAMA. 2020;323(14):1389-1400. doi:10.1001/jama.2020.3514
2. Slowik JM et al. Obstructive Sleep Apnea. In: StatPearls. Treasure Island (FL): StatPearls Publishing; December 11, 2022.
3. Bonsignore MR et al. Multidiscip Respir Med. 2019;14:8. doi:10.1186/s40248-019-0172-9
4. Schwartz SW et al. Sleep Breath. 2016;20(3):947-955. doi:10.1007/s11325-016-1316-1
5. Grandner MA et al. Sleep Med. 2016;18:7-18. doi:10.1016/j.sleep.2015.01.020
6. Lee YC et al. Sleep Med. 2022;90:204-213. doi:10.1016/j.sleep.2021.11.014
7. Hudgel DW et al. Am J Respir Crit Care Med. 2018;198(6):e70-e87. doi:10.1164/rccm.201807-1326ST
8. Lloyd R et al. J Clin Sleep Med. 2022;18(11):2673-2680. doi:10.5664/jcsm.10244
9. Nokes B et al. Expert Rev Respir Med. 2022;16(8):917-929. doi:10.1080/17476348.2022.2112669
10. Pinto JA et al. Int Arch Otorhinolaryngol. 2016;20(2):145-150.doi:10.1055/s-0036-1579546
11. Georgoulis M et al. J Clin Sleep Med. 2022;18(5):1251-1261. doi:10.5664/jcsm.9834
12. Askland K et al. Cochrane Database Syst Rev. 2020;4(4):CD007736. doi:10.1002/14651858.CD007736.pub3
13. Jugé L et al. Sleep. 2022;45(6):zsac044. doi:10.1093/sleep/zsac044
14. Strollo PJ Jr et al. N Engl J Med. 2014;370(2):139-149. doi:10.1056/NEJMoa1308659
15. Fattal D et al. J Clin Sleep Med. 2022;18(12):2723-2729. doi:10.5664/jcsm.10190
16. He M et al. Otolaryngol Head Neck Surg. 2019;161(3):401-411. doi:10.1177/0194599819840356
New insight into genetic link between schizophrenia and CVD
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
There is an extensive genetic overlap between schizophrenia and smoking, but there are also schizophrenia genes that may protect against obesity, illustrating the bidirectional effects of shared loci across cardiovascular disease (CVD) risk factors, results of new research suggest.
METHODOLOGY:
- Researchers obtained what they call an “unprecedentedly large” set of GWAS samples, including schizophrenia (53,386 patients and 77,258 controls) and various CVD risk factors.
- They used analytic approaches to identify genetic links between schizophrenia and CVD risk factors, including bivariate causal mixture model (MiXeR), which estimates the number of shared genetic variants between pairs of phenotypes, and conditional and conjunctional false discovery rate (condFDR and conjFDR), to identify specific genetic loci; these approaches can identify genetic overlap regardless of the effect directions.
TAKEAWAY:
- Using MiXeR, the study showed that several genetic variants underlying schizophrenia also influence CVD phenotypes, particularly risk factors of smoking and BMI.
- A total of 825 distinct loci were jointly associated with schizophrenia and CVD phenotypes at conjFDR < .05.
- Most of the loci shared with smoking were in line with positive genetic correlations; the authors noted individuals with schizophrenia are more nicotine dependent than the general population, and they experience greater reinforcing effects of nicotine and worse withdrawal symptoms during abstinence than the general population.
- The overlapping loci with BMI had effect directions consistent with negative genetic correlations, suggesting people with schizophrenia are genetically predisposed to lower BMI; this is in line with evidence of low BMI being a risk factor for schizophrenia, although obesity is more common in people with schizophrenia.
- There was a pattern of mixed effect directions among loci jointly associated with schizophrenia and lipids, blood pressure, type 2 diabetes, waist-to-hip ratio, and coronary artery disease, which may reflect variation in genetic susceptibility to CVD across subgroups of schizophrenia.
IN PRACTICE:
The new results “shed light” on biological pathways associated with comorbidity between CVD and schizophrenia, said the authors, adding future work could provide insights into mechanisms underlying the comorbidity and could facilitate development of antipsychotics with lower metabolic side effects, which could help prevent comorbid CVD, “thereby helping to mitigate a major clinical and health care problem.”
SOURCE:
The study was led by Linn Rødevand, PhD, Norwegian Center for Mental Disorders Research, Division of Mental Health and Addiction, Institute of Clinical Medicine, Oslo University Hospital, University of Oslo, and colleagues. It was published online in the American Journal of Psychiatry.
LIMITATIONS:
Methods used in the study are limited by uncertainties in translating genetic loci to causal variants, which restricts the biological interpretation of the shared genetic variants. Among other methodological limitations are that discrepancies between the linkage disequilibrium structure of the samples used for the GWAS and that of the reference panel may have biased estimates underlying MiXeR.
DISCLOSURES:
The study received support from the Research Council of Norway, Norwegian Health Association, South-East Norway Regional Health Authority, and the European Union. Dr. Rødevand reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
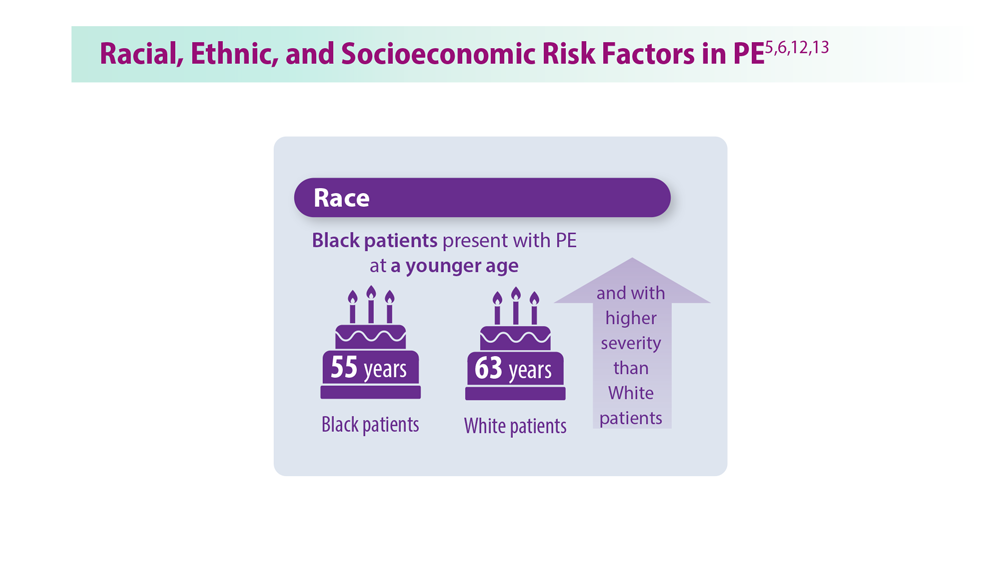
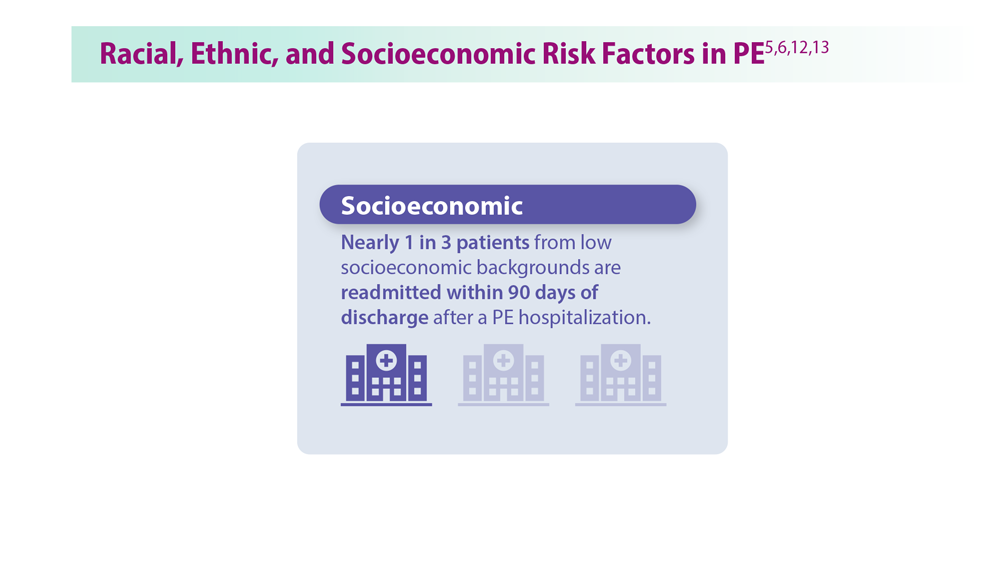
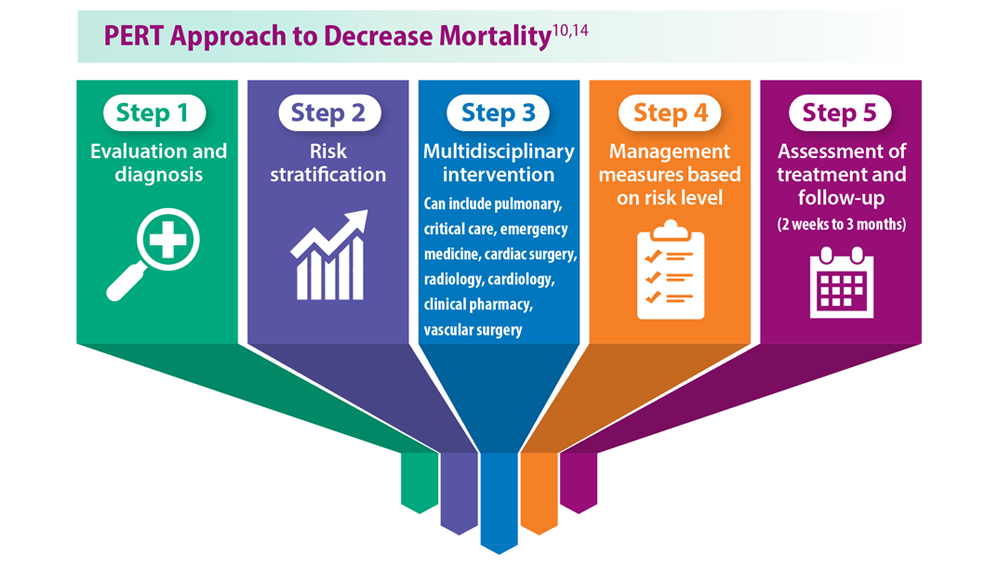
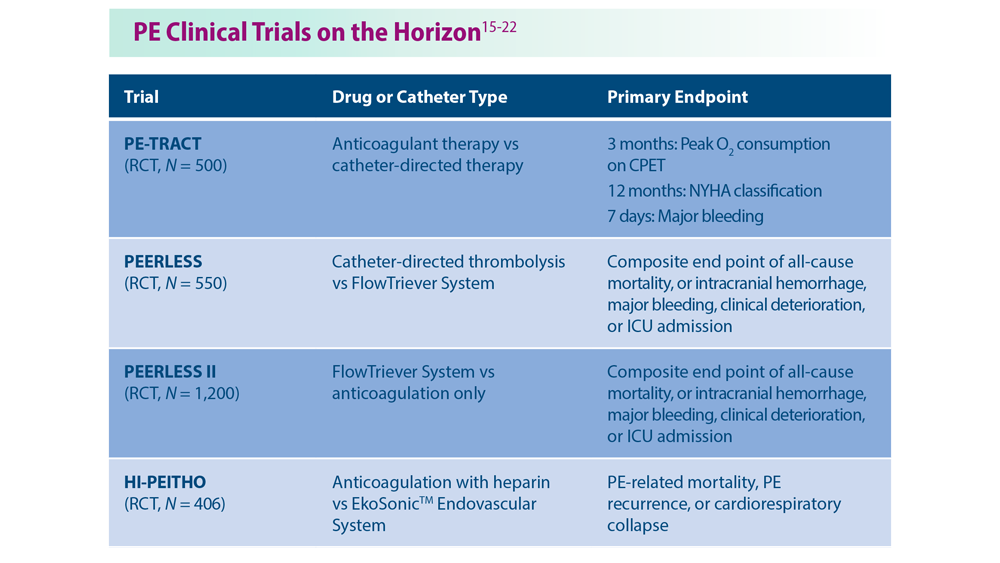
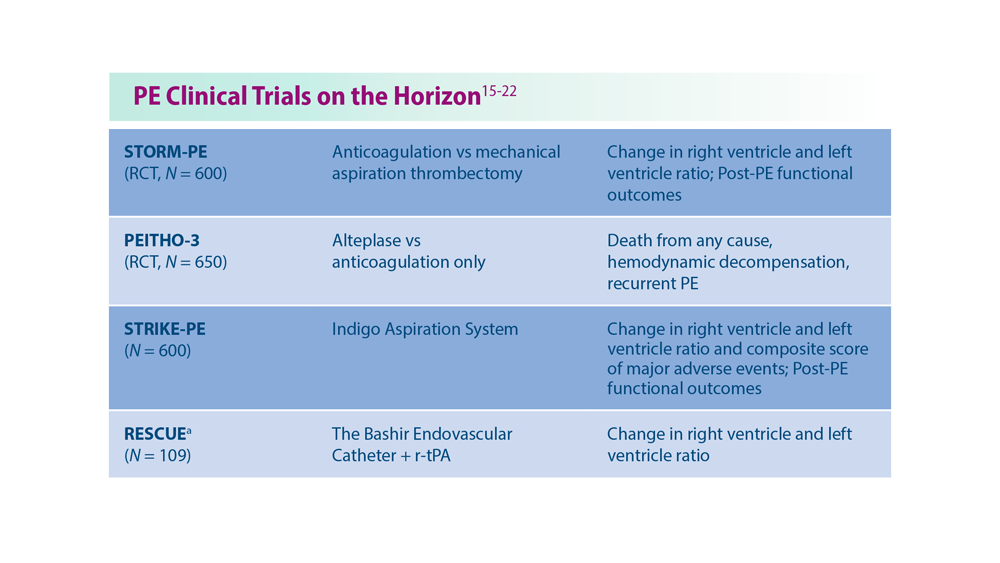
Decreasing Pulmonary Embolism-Related Mortality
- Centers for Disease Control and Prevention. Data and statistics on venous thromboembolism. Last reviewed June 28, 2023. Accessed July 18, 2023. https://www.cdc.gov/ncbddd/dvt/data.html
- Becattini C et al. Chest. 2016;149(1):192-200. doi:10.1378/chest.15-0808
- Triantafyllou GA et al. Semin Respir Crit Care Med. 2021;42(2):183-198.doi:10.1055/s-0041-1722898
- Ng ACC et al. Respiration. 2013;85(5):408-416. doi:10.1159/000342024
- Phillips AR et al. J Am Heart Assoc. 2021;10(17):e021818. doi:10.1161/JAHA.121.021818
- Wadhera RK et al. J Am Heart Assoc. 2021;10(13):e021117. doi:10.1161/JAHA.121.021117
- Bashir R et al. JACC Cardiovasc Interv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
- Patel NJ et al. Int J Cardiol. 2019;287:116-117. doi:10.1016/j.ijcard.2019.04.029
- Li X et al. Ann Transl Med. 2021;9(10):838. doi:10.21037/atm-21-975
- Rivera-Lebron BN et al. Chest. 2021;159(1):347-355. doi:10.1016/j.chest.2020.07.065
- Noto JG, Rali P. Pulm Circ. 2022;12(1):e12021. doi:10.1002/pul2.12021
- Snyder DJ et al. Vasc Med. 2023;28(3):222-232. doi:10.1177/1358863X231157441
- Bikdeli B et al. Semin Thromb Hemost. 2023. doi:10.1055/s-0043-1764231
- Fleitas Sosa D et al. Eur Respir Rev. 2022;31(165):220023. doi:10.1183/16000617.0023-2022
- Pulmonary embolism - thrombus removal with catheter-directed therapy (PE-TRACT). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05591118
- The PEERLESS study (PEERLESS). ClinicalTrials.gov. Updated Jun 23, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05111613
- Inari Medical, Inc. Inari Medical announces Peerless II, a randomized controlled trial evaluating clinical outcomes of the FlowTriever® system vs. anticoagulation in pulmonary embolism patients [press release]. Published May 22,2023. Accessed July 18, 2023. https://ir.inarimedical.com/news-releases/news-release-details/inari-medical-announces-peerless-ii-randomized-controlled-trial
- Ultrasound-facilitated, catheter-directed, thrombolysis in intermediate-high risk pulmonary embolism (HI-PEITHO). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04790370
- Comparison of two pulmonary embolism treatments. ClinicalTrials.gov. Updated May 31, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05684796
- Pulmonary Embolism International THrOmbolysis Study-3 (PEITHO-3).ClinicalTrials.gov. Updated June 8, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04430569
- Study of the long-term safety and outcomes of treating pulmonary embolism with the Indigo Aspiration System. ClinicalTrials.gov. Updated May 11, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04798261
- Bashir R et al. J Am Coll Cardiol Intv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
- Centers for Disease Control and Prevention. Data and statistics on venous thromboembolism. Last reviewed June 28, 2023. Accessed July 18, 2023. https://www.cdc.gov/ncbddd/dvt/data.html
- Becattini C et al. Chest. 2016;149(1):192-200. doi:10.1378/chest.15-0808
- Triantafyllou GA et al. Semin Respir Crit Care Med. 2021;42(2):183-198.doi:10.1055/s-0041-1722898
- Ng ACC et al. Respiration. 2013;85(5):408-416. doi:10.1159/000342024
- Phillips AR et al. J Am Heart Assoc. 2021;10(17):e021818. doi:10.1161/JAHA.121.021818
- Wadhera RK et al. J Am Heart Assoc. 2021;10(13):e021117. doi:10.1161/JAHA.121.021117
- Bashir R et al. JACC Cardiovasc Interv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
- Patel NJ et al. Int J Cardiol. 2019;287:116-117. doi:10.1016/j.ijcard.2019.04.029
- Li X et al. Ann Transl Med. 2021;9(10):838. doi:10.21037/atm-21-975
- Rivera-Lebron BN et al. Chest. 2021;159(1):347-355. doi:10.1016/j.chest.2020.07.065
- Noto JG, Rali P. Pulm Circ. 2022;12(1):e12021. doi:10.1002/pul2.12021
- Snyder DJ et al. Vasc Med. 2023;28(3):222-232. doi:10.1177/1358863X231157441
- Bikdeli B et al. Semin Thromb Hemost. 2023. doi:10.1055/s-0043-1764231
- Fleitas Sosa D et al. Eur Respir Rev. 2022;31(165):220023. doi:10.1183/16000617.0023-2022
- Pulmonary embolism - thrombus removal with catheter-directed therapy (PE-TRACT). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05591118
- The PEERLESS study (PEERLESS). ClinicalTrials.gov. Updated Jun 23, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05111613
- Inari Medical, Inc. Inari Medical announces Peerless II, a randomized controlled trial evaluating clinical outcomes of the FlowTriever® system vs. anticoagulation in pulmonary embolism patients [press release]. Published May 22,2023. Accessed July 18, 2023. https://ir.inarimedical.com/news-releases/news-release-details/inari-medical-announces-peerless-ii-randomized-controlled-trial
- Ultrasound-facilitated, catheter-directed, thrombolysis in intermediate-high risk pulmonary embolism (HI-PEITHO). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04790370
- Comparison of two pulmonary embolism treatments. ClinicalTrials.gov. Updated May 31, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05684796
- Pulmonary Embolism International THrOmbolysis Study-3 (PEITHO-3).ClinicalTrials.gov. Updated June 8, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04430569
- Study of the long-term safety and outcomes of treating pulmonary embolism with the Indigo Aspiration System. ClinicalTrials.gov. Updated May 11, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04798261
- Bashir R et al. J Am Coll Cardiol Intv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
- Centers for Disease Control and Prevention. Data and statistics on venous thromboembolism. Last reviewed June 28, 2023. Accessed July 18, 2023. https://www.cdc.gov/ncbddd/dvt/data.html
- Becattini C et al. Chest. 2016;149(1):192-200. doi:10.1378/chest.15-0808
- Triantafyllou GA et al. Semin Respir Crit Care Med. 2021;42(2):183-198.doi:10.1055/s-0041-1722898
- Ng ACC et al. Respiration. 2013;85(5):408-416. doi:10.1159/000342024
- Phillips AR et al. J Am Heart Assoc. 2021;10(17):e021818. doi:10.1161/JAHA.121.021818
- Wadhera RK et al. J Am Heart Assoc. 2021;10(13):e021117. doi:10.1161/JAHA.121.021117
- Bashir R et al. JACC Cardiovasc Interv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
- Patel NJ et al. Int J Cardiol. 2019;287:116-117. doi:10.1016/j.ijcard.2019.04.029
- Li X et al. Ann Transl Med. 2021;9(10):838. doi:10.21037/atm-21-975
- Rivera-Lebron BN et al. Chest. 2021;159(1):347-355. doi:10.1016/j.chest.2020.07.065
- Noto JG, Rali P. Pulm Circ. 2022;12(1):e12021. doi:10.1002/pul2.12021
- Snyder DJ et al. Vasc Med. 2023;28(3):222-232. doi:10.1177/1358863X231157441
- Bikdeli B et al. Semin Thromb Hemost. 2023. doi:10.1055/s-0043-1764231
- Fleitas Sosa D et al. Eur Respir Rev. 2022;31(165):220023. doi:10.1183/16000617.0023-2022
- Pulmonary embolism - thrombus removal with catheter-directed therapy (PE-TRACT). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05591118
- The PEERLESS study (PEERLESS). ClinicalTrials.gov. Updated Jun 23, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05111613
- Inari Medical, Inc. Inari Medical announces Peerless II, a randomized controlled trial evaluating clinical outcomes of the FlowTriever® system vs. anticoagulation in pulmonary embolism patients [press release]. Published May 22,2023. Accessed July 18, 2023. https://ir.inarimedical.com/news-releases/news-release-details/inari-medical-announces-peerless-ii-randomized-controlled-trial
- Ultrasound-facilitated, catheter-directed, thrombolysis in intermediate-high risk pulmonary embolism (HI-PEITHO). ClinicalTrials.gov. Updated July 17, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04790370
- Comparison of two pulmonary embolism treatments. ClinicalTrials.gov. Updated May 31, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05684796
- Pulmonary Embolism International THrOmbolysis Study-3 (PEITHO-3).ClinicalTrials.gov. Updated June 8, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04430569
- Study of the long-term safety and outcomes of treating pulmonary embolism with the Indigo Aspiration System. ClinicalTrials.gov. Updated May 11, 2023. Accessed July 18, 2023. https://clinicaltrials.gov/ct2/show/NCT04798261
- Bashir R et al. J Am Coll Cardiol Intv. 2022;15(23):2427-2436. doi:10.1016/j.jcin.2022.09.011
Updated guidance from USPSTF on PrEP for HIV prevention
The US Preventive Services Task Force (USPSTF) recently released their final recommendation update on the use of antiretroviral therapy to prevent HIV infection in adolescents and adults who are at increased risk.1 The Task Force last addressed this topic in 2019; since then, 2 additional antiretroviral regimens have been approved for preexposure prophylaxis (PrEP). The update also includes revised wording on who should consider receiving PrEP.
HIV remains a significant public health problem in the United States. The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million people in the United States are living with HIV, and approximately 30,000 new infections occur each year.2 Men who have sex with men account for 68% of new infections, and there are marked racial disparities in both incidence and prevalence of infection, with Black/African Americans accounting for 42% of new infections.2
PrEP decreases the risk for HIV by about 50% overall, with higher rates of protection correlated to higher adherence (close to 100% protection with daily adherence to oral regimens).3 The 3 approved regimens for PrEP are outlined in TABLE 13.
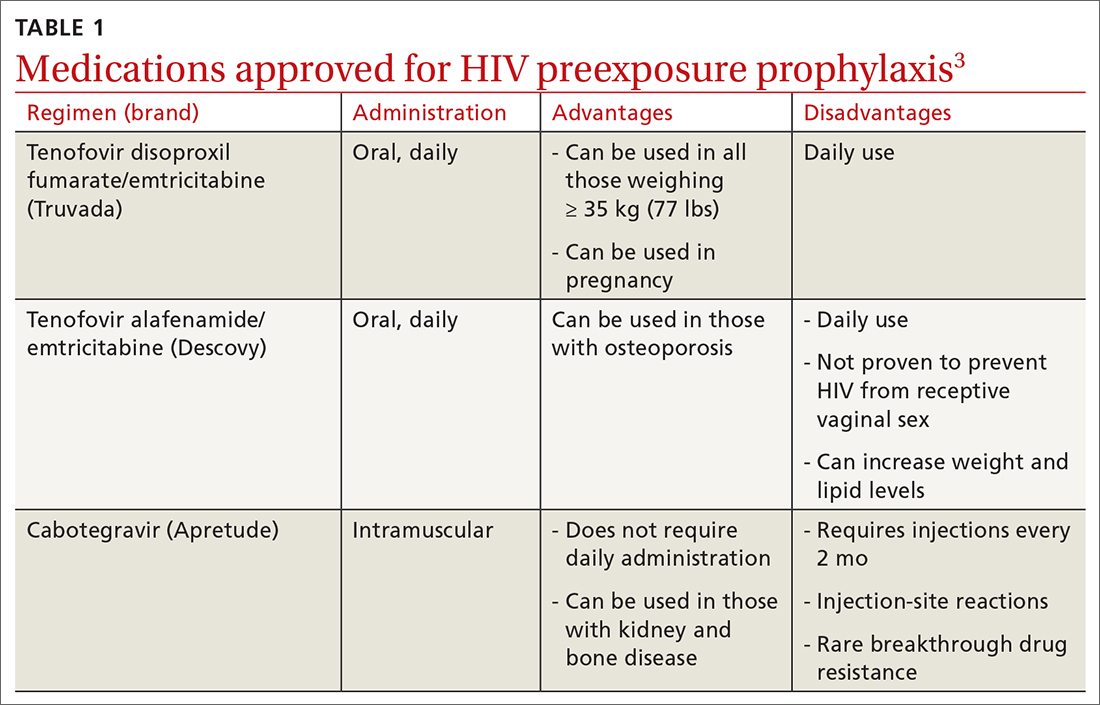
Who’s at increased risk? The USPSTF did not find any risk assessment tools with proven accuracy in identifying those at increased risk for HIV infection but did document risk factors and behaviors that can be used to predict risk. They encourage discussion about HIV prevention with all adults and adolescents who are sexually active or who inject drugs.
Those people for whom the Task Force recommends considering PrEP are listed in TABLE 21. However, the USPSTF recommends providing PrEP to anyone who requests it, as they may not want to disclose their risk factors.

What to keep in mind. Family physicians are encouraged to read the full USPSTF report and refer to CDC guidelines on prescribing PrEP, which provide details on each regimen and the routine laboratory testing that should be performed.4 The most important clinical considerations described in the USPSTF report are:
- Before starting PrEP, document a negative HIV antigen/antibody test result and continue to test for HIV every 3 months. PrEP regimens should not be used to treat HIV.
- Document a negative HIV RNA assay if the patient has taken oral PrEP in the past 3 months or injectable PrEP in the past 12 months.
- At PrEP initiation, consider ordering other recommended tests, such as those for kidney function, chronic hepatitis B infection (if using tenofovir disoproxil fumarate/emtricitabine), lipid levels (if using tenofovir alafenamide/emtricitabine), and other sexually transmitted infection (STIs).
- Encourage the use of condoms, as PrEP does not protect from other STIs.
- Follow up regularly, and at each patient visit stress the need for medication adherence to achieve maximum protection.
1. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
2. CDC. HIV surveillance report: diagnoses of HIV infection in the United States and dependent areas, 2020. Published May 2022. Accessed September 29, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
3. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final evidence review. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-review/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
4. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed September 28, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
The US Preventive Services Task Force (USPSTF) recently released their final recommendation update on the use of antiretroviral therapy to prevent HIV infection in adolescents and adults who are at increased risk.1 The Task Force last addressed this topic in 2019; since then, 2 additional antiretroviral regimens have been approved for preexposure prophylaxis (PrEP). The update also includes revised wording on who should consider receiving PrEP.
HIV remains a significant public health problem in the United States. The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million people in the United States are living with HIV, and approximately 30,000 new infections occur each year.2 Men who have sex with men account for 68% of new infections, and there are marked racial disparities in both incidence and prevalence of infection, with Black/African Americans accounting for 42% of new infections.2
PrEP decreases the risk for HIV by about 50% overall, with higher rates of protection correlated to higher adherence (close to 100% protection with daily adherence to oral regimens).3 The 3 approved regimens for PrEP are outlined in TABLE 13.

Who’s at increased risk? The USPSTF did not find any risk assessment tools with proven accuracy in identifying those at increased risk for HIV infection but did document risk factors and behaviors that can be used to predict risk. They encourage discussion about HIV prevention with all adults and adolescents who are sexually active or who inject drugs.
Those people for whom the Task Force recommends considering PrEP are listed in TABLE 21. However, the USPSTF recommends providing PrEP to anyone who requests it, as they may not want to disclose their risk factors.

What to keep in mind. Family physicians are encouraged to read the full USPSTF report and refer to CDC guidelines on prescribing PrEP, which provide details on each regimen and the routine laboratory testing that should be performed.4 The most important clinical considerations described in the USPSTF report are:
- Before starting PrEP, document a negative HIV antigen/antibody test result and continue to test for HIV every 3 months. PrEP regimens should not be used to treat HIV.
- Document a negative HIV RNA assay if the patient has taken oral PrEP in the past 3 months or injectable PrEP in the past 12 months.
- At PrEP initiation, consider ordering other recommended tests, such as those for kidney function, chronic hepatitis B infection (if using tenofovir disoproxil fumarate/emtricitabine), lipid levels (if using tenofovir alafenamide/emtricitabine), and other sexually transmitted infection (STIs).
- Encourage the use of condoms, as PrEP does not protect from other STIs.
- Follow up regularly, and at each patient visit stress the need for medication adherence to achieve maximum protection.
The US Preventive Services Task Force (USPSTF) recently released their final recommendation update on the use of antiretroviral therapy to prevent HIV infection in adolescents and adults who are at increased risk.1 The Task Force last addressed this topic in 2019; since then, 2 additional antiretroviral regimens have been approved for preexposure prophylaxis (PrEP). The update also includes revised wording on who should consider receiving PrEP.
HIV remains a significant public health problem in the United States. The Centers for Disease Control and Prevention (CDC) estimates that 1.2 million people in the United States are living with HIV, and approximately 30,000 new infections occur each year.2 Men who have sex with men account for 68% of new infections, and there are marked racial disparities in both incidence and prevalence of infection, with Black/African Americans accounting for 42% of new infections.2
PrEP decreases the risk for HIV by about 50% overall, with higher rates of protection correlated to higher adherence (close to 100% protection with daily adherence to oral regimens).3 The 3 approved regimens for PrEP are outlined in TABLE 13.

Who’s at increased risk? The USPSTF did not find any risk assessment tools with proven accuracy in identifying those at increased risk for HIV infection but did document risk factors and behaviors that can be used to predict risk. They encourage discussion about HIV prevention with all adults and adolescents who are sexually active or who inject drugs.
Those people for whom the Task Force recommends considering PrEP are listed in TABLE 21. However, the USPSTF recommends providing PrEP to anyone who requests it, as they may not want to disclose their risk factors.

What to keep in mind. Family physicians are encouraged to read the full USPSTF report and refer to CDC guidelines on prescribing PrEP, which provide details on each regimen and the routine laboratory testing that should be performed.4 The most important clinical considerations described in the USPSTF report are:
- Before starting PrEP, document a negative HIV antigen/antibody test result and continue to test for HIV every 3 months. PrEP regimens should not be used to treat HIV.
- Document a negative HIV RNA assay if the patient has taken oral PrEP in the past 3 months or injectable PrEP in the past 12 months.
- At PrEP initiation, consider ordering other recommended tests, such as those for kidney function, chronic hepatitis B infection (if using tenofovir disoproxil fumarate/emtricitabine), lipid levels (if using tenofovir alafenamide/emtricitabine), and other sexually transmitted infection (STIs).
- Encourage the use of condoms, as PrEP does not protect from other STIs.
- Follow up regularly, and at each patient visit stress the need for medication adherence to achieve maximum protection.
1. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
2. CDC. HIV surveillance report: diagnoses of HIV infection in the United States and dependent areas, 2020. Published May 2022. Accessed September 29, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
3. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final evidence review. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-review/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
4. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed September 28, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
1. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final recommendation statement. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
2. CDC. HIV surveillance report: diagnoses of HIV infection in the United States and dependent areas, 2020. Published May 2022. Accessed September 29, 2023. www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2020-updated-vol-33.pdf
3. USPSTF. Prevention of acquisition of HIV: preexposure prophylaxis. Final evidence review. Published August 22, 2023. Accessed September 28, 2023. https://uspreventiveservicestaskforce.org/uspstf/document/final-evidence-review/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis
4. CDC. Preexposure prophylaxis for the prevention of HIV infection in the United States—2021 update: a clinical practice guideline. Accessed September 28, 2023. www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
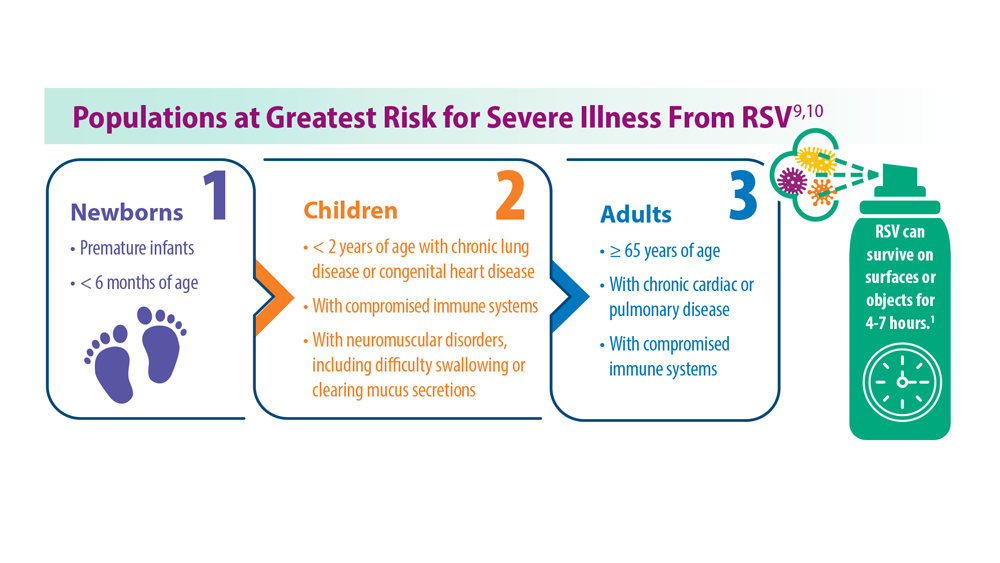
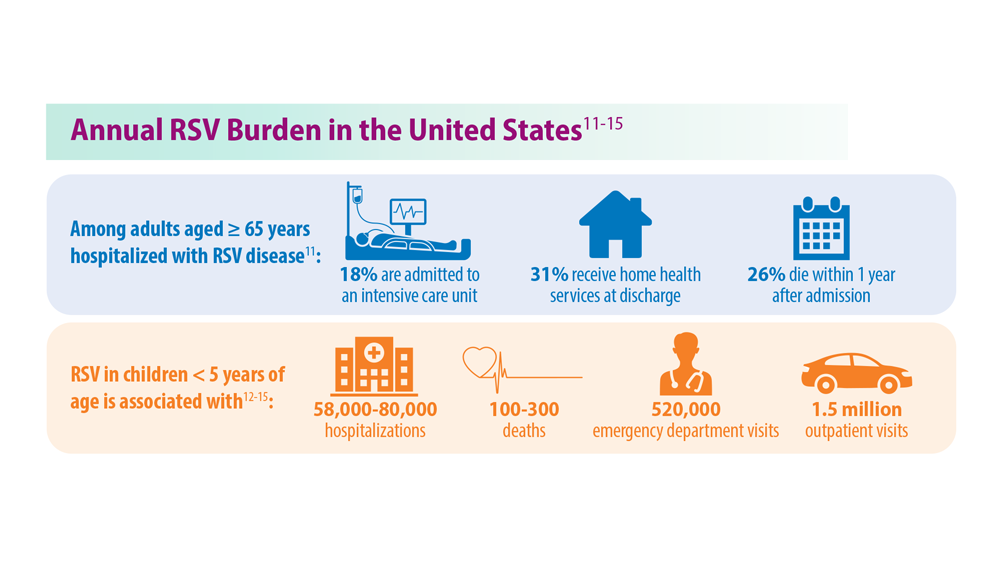
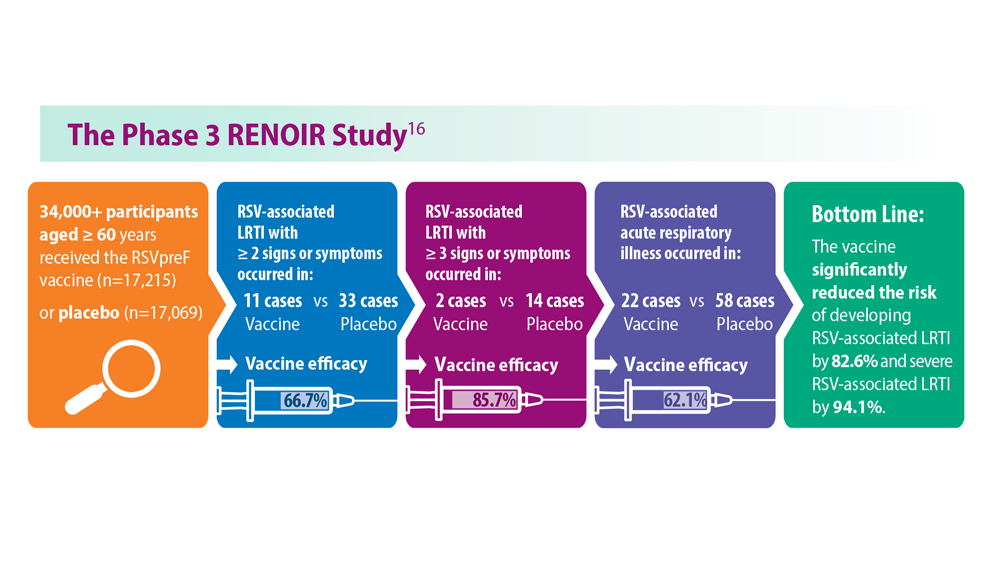
Long-Awaited RSV Vaccines Now Available for Older Adults and Pediatric Patients
- Jha A et al. Respiratory syncytial virus. In: Hui DS, Rossi GA, Johnston SL, eds. Respiratory Syncytial Virus. SARS, MERS and Other Viral Lung Infections. European Respiratory Society; 2016:chap 5. Accessed May 17, 2023.
- Ginsburg SA, Srikantiah P. Lancet Glob Health. 2021;9(12):e1644-e6145. doi:10.1016/S2214-109X(21)00455-1
- US Food and Drug Administration. FDA approves first respiratory syncytial virus (RSV) vaccine [press release]. Published May 3, 2023. Accessed May 17, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine
- US Food and Drug Administration. FDA Approves New Drug to Prevent RSV in Babies and Toddlers [press release]. Published July 17, 2023. Accessed August 11, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers
- US Food and Drug Administration. FDA Approves First Vaccine for Pregnant Individuals to Prevent RSV in Infants. Published August 21, 2023. Accessed August 22, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants
- Madhi SA et al. N Engl J Med. 2020;383(5):426-439. doi:10.1056/ NEJMoa1908380
- Centers for Disease Control. Advisory Committee on Immunization Practices (ACIP) Meeting recommendations, August 2023. https://www.cdc.gov/vaccines/acip/recommendations.html
- Hammit LL et al. N Engl J Med. 2022;386(9):837-846. doi:10.1056/ NEJMoa2110275
- Centers for Disease Control and Prevention. RSV in infants and young children. Updated October 28, 2022. Accessed May 30, 2023. https://www.cdc.gov/rsv/ high-risk/infants-young-children.html
- Centers for Disease Control and Prevention. RSV in older adults and adults with chronic medical conditions. Updated October 28, 2022. Accessed May 30, 2023. https://www.cdc.gov/rsv/high-risk/older-adults.html
- Widmer K et al. J Infect Dis. 2012;206(1):56-62. doi:10.1093/infdis/jis309
- Hall CB et al. N Engl J Med. 2009;360(6):588-598. doi:10.1056/NEJMoa0804877
- McLaughlin JM et al. Open Forum Infect Dis. 2022;9(7):ofac300. doi:10.1093/ofid/ofac300
- Thompson et al. JAMA. 2003;289(2):179-186. doi:10.1001/jama.289.2.179
- Hansen CL et al. JAMA Netw Open. 2022;5(2):e220527. doi:10.1001/jamanetworkopen.2022.0527
- Walsh EE et al; RENOIR Clinical Trial Group. N Engl J Med. 2023;388(16):1465-1477. doi:10.1056/NEJMoa2213836
- Martin JA et al. Natl Vital Stat Rep. 2019;68(13):1-47. PMID:32501202
- Townsi N et al. Eur Clin Respir J. 2018;5(1):1487214. doi:10.1080/20018525.20 18.1487214
- Malek A et al. Am J Reprod Immunol. 1994;32(1):8-14. doi:10.1111/j.1600-0897.1994.tb00873.x
- Kampmann B et al; MATISSE Study Group. N Engl J Med. 2023;388(16):1451- 1464. doi:10.1056/NEJMoa2216480
- Synagis (palivizumab) injection prescribing information. Published June 2023. Accessed August 2023. https://www.synagis.com/synagis.pdf
- Jha A et al. Respiratory syncytial virus. In: Hui DS, Rossi GA, Johnston SL, eds. Respiratory Syncytial Virus. SARS, MERS and Other Viral Lung Infections. European Respiratory Society; 2016:chap 5. Accessed May 17, 2023.
- Ginsburg SA, Srikantiah P. Lancet Glob Health. 2021;9(12):e1644-e6145. doi:10.1016/S2214-109X(21)00455-1
- US Food and Drug Administration. FDA approves first respiratory syncytial virus (RSV) vaccine [press release]. Published May 3, 2023. Accessed May 17, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine
- US Food and Drug Administration. FDA Approves New Drug to Prevent RSV in Babies and Toddlers [press release]. Published July 17, 2023. Accessed August 11, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers
- US Food and Drug Administration. FDA Approves First Vaccine for Pregnant Individuals to Prevent RSV in Infants. Published August 21, 2023. Accessed August 22, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants
- Madhi SA et al. N Engl J Med. 2020;383(5):426-439. doi:10.1056/ NEJMoa1908380
- Centers for Disease Control. Advisory Committee on Immunization Practices (ACIP) Meeting recommendations, August 2023. https://www.cdc.gov/vaccines/acip/recommendations.html
- Hammit LL et al. N Engl J Med. 2022;386(9):837-846. doi:10.1056/ NEJMoa2110275
- Centers for Disease Control and Prevention. RSV in infants and young children. Updated October 28, 2022. Accessed May 30, 2023. https://www.cdc.gov/rsv/ high-risk/infants-young-children.html
- Centers for Disease Control and Prevention. RSV in older adults and adults with chronic medical conditions. Updated October 28, 2022. Accessed May 30, 2023. https://www.cdc.gov/rsv/high-risk/older-adults.html
- Widmer K et al. J Infect Dis. 2012;206(1):56-62. doi:10.1093/infdis/jis309
- Hall CB et al. N Engl J Med. 2009;360(6):588-598. doi:10.1056/NEJMoa0804877
- McLaughlin JM et al. Open Forum Infect Dis. 2022;9(7):ofac300. doi:10.1093/ofid/ofac300
- Thompson et al. JAMA. 2003;289(2):179-186. doi:10.1001/jama.289.2.179
- Hansen CL et al. JAMA Netw Open. 2022;5(2):e220527. doi:10.1001/jamanetworkopen.2022.0527
- Walsh EE et al; RENOIR Clinical Trial Group. N Engl J Med. 2023;388(16):1465-1477. doi:10.1056/NEJMoa2213836
- Martin JA et al. Natl Vital Stat Rep. 2019;68(13):1-47. PMID:32501202
- Townsi N et al. Eur Clin Respir J. 2018;5(1):1487214. doi:10.1080/20018525.20 18.1487214
- Malek A et al. Am J Reprod Immunol. 1994;32(1):8-14. doi:10.1111/j.1600-0897.1994.tb00873.x
- Kampmann B et al; MATISSE Study Group. N Engl J Med. 2023;388(16):1451- 1464. doi:10.1056/NEJMoa2216480
- Synagis (palivizumab) injection prescribing information. Published June 2023. Accessed August 2023. https://www.synagis.com/synagis.pdf
- Jha A et al. Respiratory syncytial virus. In: Hui DS, Rossi GA, Johnston SL, eds. Respiratory Syncytial Virus. SARS, MERS and Other Viral Lung Infections. European Respiratory Society; 2016:chap 5. Accessed May 17, 2023.
- Ginsburg SA, Srikantiah P. Lancet Glob Health. 2021;9(12):e1644-e6145. doi:10.1016/S2214-109X(21)00455-1
- US Food and Drug Administration. FDA approves first respiratory syncytial virus (RSV) vaccine [press release]. Published May 3, 2023. Accessed May 17, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine
- US Food and Drug Administration. FDA Approves New Drug to Prevent RSV in Babies and Toddlers [press release]. Published July 17, 2023. Accessed August 11, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers
- US Food and Drug Administration. FDA Approves First Vaccine for Pregnant Individuals to Prevent RSV in Infants. Published August 21, 2023. Accessed August 22, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants
- Madhi SA et al. N Engl J Med. 2020;383(5):426-439. doi:10.1056/ NEJMoa1908380
- Centers for Disease Control. Advisory Committee on Immunization Practices (ACIP) Meeting recommendations, August 2023. https://www.cdc.gov/vaccines/acip/recommendations.html
- Hammit LL et al. N Engl J Med. 2022;386(9):837-846. doi:10.1056/ NEJMoa2110275
- Centers for Disease Control and Prevention. RSV in infants and young children. Updated October 28, 2022. Accessed May 30, 2023. https://www.cdc.gov/rsv/ high-risk/infants-young-children.html
- Centers for Disease Control and Prevention. RSV in older adults and adults with chronic medical conditions. Updated October 28, 2022. Accessed May 30, 2023. https://www.cdc.gov/rsv/high-risk/older-adults.html
- Widmer K et al. J Infect Dis. 2012;206(1):56-62. doi:10.1093/infdis/jis309
- Hall CB et al. N Engl J Med. 2009;360(6):588-598. doi:10.1056/NEJMoa0804877
- McLaughlin JM et al. Open Forum Infect Dis. 2022;9(7):ofac300. doi:10.1093/ofid/ofac300
- Thompson et al. JAMA. 2003;289(2):179-186. doi:10.1001/jama.289.2.179
- Hansen CL et al. JAMA Netw Open. 2022;5(2):e220527. doi:10.1001/jamanetworkopen.2022.0527
- Walsh EE et al; RENOIR Clinical Trial Group. N Engl J Med. 2023;388(16):1465-1477. doi:10.1056/NEJMoa2213836
- Martin JA et al. Natl Vital Stat Rep. 2019;68(13):1-47. PMID:32501202
- Townsi N et al. Eur Clin Respir J. 2018;5(1):1487214. doi:10.1080/20018525.20 18.1487214
- Malek A et al. Am J Reprod Immunol. 1994;32(1):8-14. doi:10.1111/j.1600-0897.1994.tb00873.x
- Kampmann B et al; MATISSE Study Group. N Engl J Med. 2023;388(16):1451- 1464. doi:10.1056/NEJMoa2216480
- Synagis (palivizumab) injection prescribing information. Published June 2023. Accessed August 2023. https://www.synagis.com/synagis.pdf
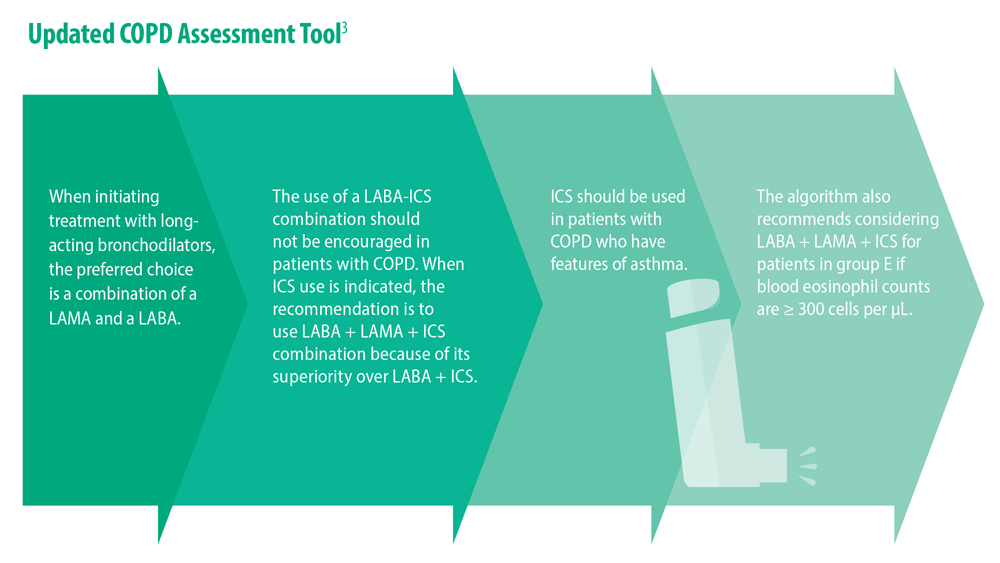
Updated Guidelines for COPD Management: 2023 GOLD Strategy Report
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). Published 2023. Accessed June 6, 2023. https://goldcopd.org/2023-gold-report-2/
- Celli B et al. Am J Respir Crit Care Med. 2022;206(11):1317. doi:10.1164/rccm.202204-0671PP
- Han M et al. Lancet Respir Med. 2013;1(1):43-50. doi:10.1016/S2213-2600(12)70044-9
- Klijn SL et al. NPJ Prim Care Respir Med. 2017;27(1):24. doi:10.1038/s41533-017-0022-1
- Chan AH et al. J Allergy Clin Immunol Pract. 2015;3(3):335-349.e1-e5. doi:10.1016/j.jaip.2015.01.024
- Brusselle G et al. Int J Chron Obstruct Pulmon Dis. 2015;10:2207-2217. doi:10.2147/COPD.S91694
- Salvi SS, Barnes PJ. Lancet. 2009;374(9691):733-743. doi:10.1016/S0140-6736(09)61303-9
- Trupin L et al. Eur Respir J. 2003;22(3):462-469. doi:10.1183/09031936.03.00094203
- Celli BR et al. Am J Respir Crit Care Med. 2021;204(11):1251-1258. doi:10.1164/rccm.202108-1819PP
- Barnes PJ, Celli BR. Eur Respir J. 2009;33(5):1165-1185. doi:10.1183/09031936.00128008
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). Published 2023. Accessed June 6, 2023. https://goldcopd.org/2023-gold-report-2/
- Celli B et al. Am J Respir Crit Care Med. 2022;206(11):1317. doi:10.1164/rccm.202204-0671PP
- Han M et al. Lancet Respir Med. 2013;1(1):43-50. doi:10.1016/S2213-2600(12)70044-9
- Klijn SL et al. NPJ Prim Care Respir Med. 2017;27(1):24. doi:10.1038/s41533-017-0022-1
- Chan AH et al. J Allergy Clin Immunol Pract. 2015;3(3):335-349.e1-e5. doi:10.1016/j.jaip.2015.01.024
- Brusselle G et al. Int J Chron Obstruct Pulmon Dis. 2015;10:2207-2217. doi:10.2147/COPD.S91694
- Salvi SS, Barnes PJ. Lancet. 2009;374(9691):733-743. doi:10.1016/S0140-6736(09)61303-9
- Trupin L et al. Eur Respir J. 2003;22(3):462-469. doi:10.1183/09031936.03.00094203
- Celli BR et al. Am J Respir Crit Care Med. 2021;204(11):1251-1258. doi:10.1164/rccm.202108-1819PP
- Barnes PJ, Celli BR. Eur Respir J. 2009;33(5):1165-1185. doi:10.1183/09031936.00128008
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 Report). Published 2023. Accessed June 6, 2023. https://goldcopd.org/2023-gold-report-2/
- Celli B et al. Am J Respir Crit Care Med. 2022;206(11):1317. doi:10.1164/rccm.202204-0671PP
- Han M et al. Lancet Respir Med. 2013;1(1):43-50. doi:10.1016/S2213-2600(12)70044-9
- Klijn SL et al. NPJ Prim Care Respir Med. 2017;27(1):24. doi:10.1038/s41533-017-0022-1
- Chan AH et al. J Allergy Clin Immunol Pract. 2015;3(3):335-349.e1-e5. doi:10.1016/j.jaip.2015.01.024
- Brusselle G et al. Int J Chron Obstruct Pulmon Dis. 2015;10:2207-2217. doi:10.2147/COPD.S91694
- Salvi SS, Barnes PJ. Lancet. 2009;374(9691):733-743. doi:10.1016/S0140-6736(09)61303-9
- Trupin L et al. Eur Respir J. 2003;22(3):462-469. doi:10.1183/09031936.03.00094203
- Celli BR et al. Am J Respir Crit Care Med. 2021;204(11):1251-1258. doi:10.1164/rccm.202108-1819PP
- Barnes PJ, Celli BR. Eur Respir J. 2009;33(5):1165-1185. doi:10.1183/09031936.00128008
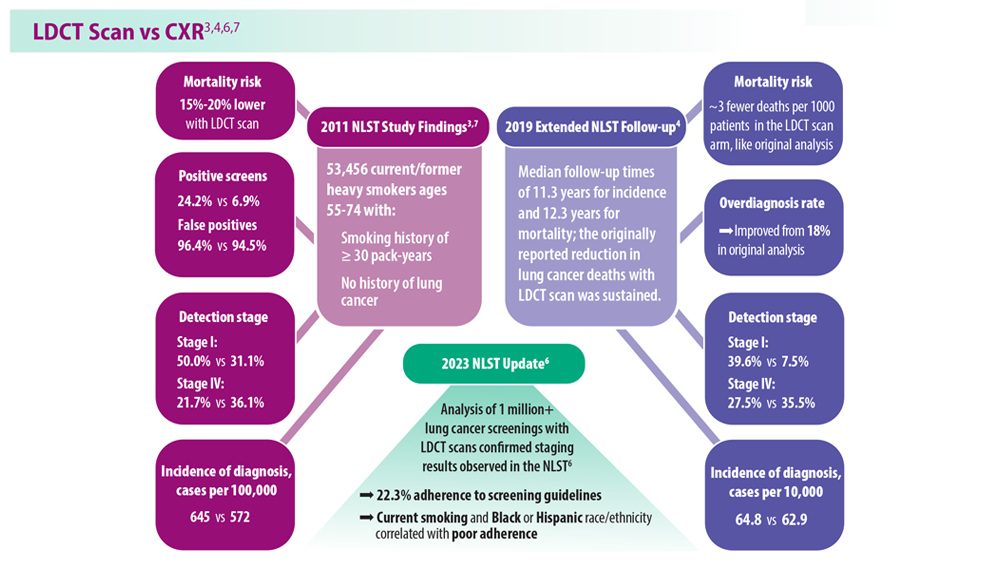
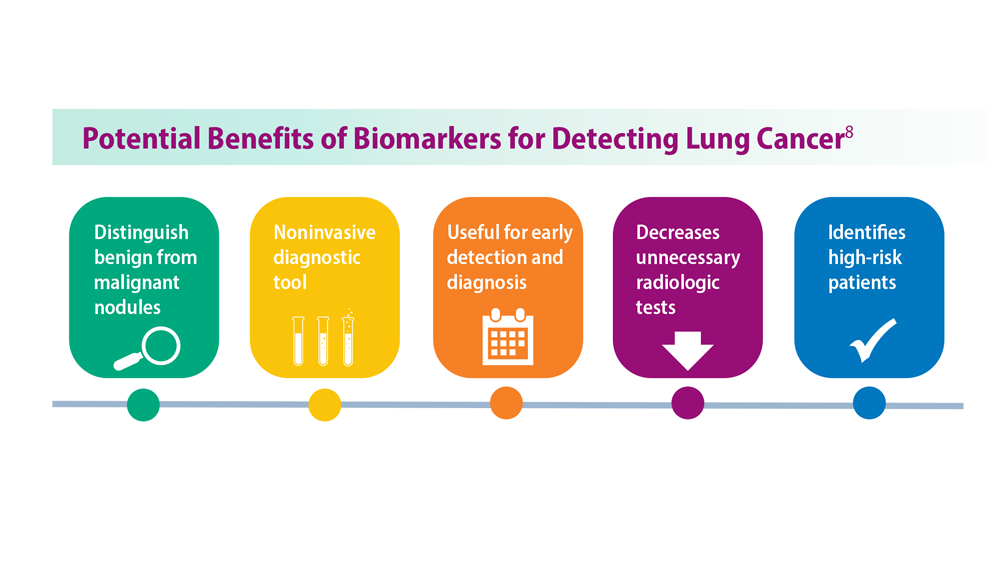
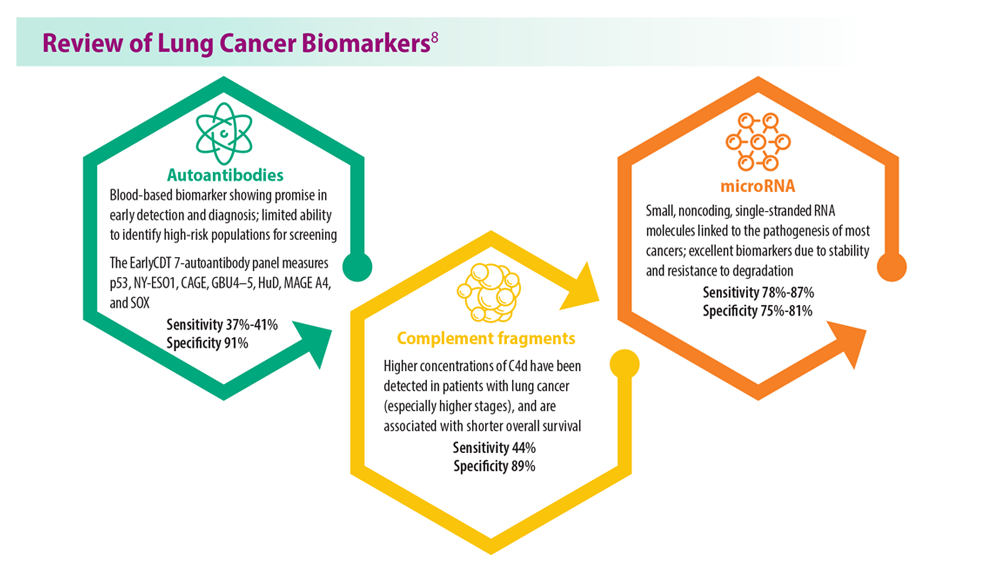
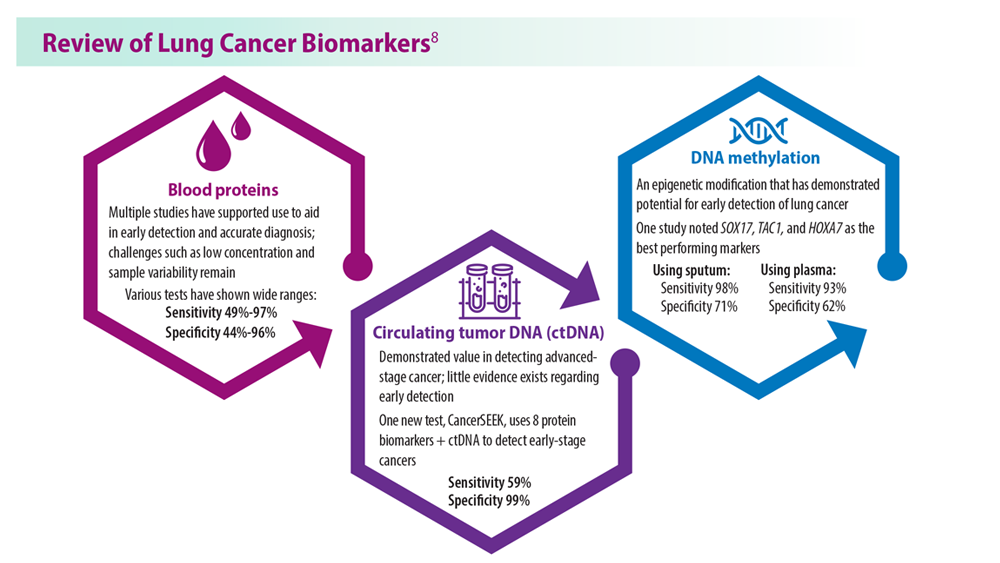
Lung Cancer Screening: A Need for Adjunctive Testing
- Naidch DP et al. Radiology. 1990;175(3):729-731. doi:10.1148/radiology.175.3.2343122
- Kaneko M et al. Radiology. 1996;201(3):798-802. doi:10.1148/radiology.201.3.8939234
- National Lung Screening Trial Research Team. Radiology. 2011;258(1):243-253. doi:10.1148/radiol.10091808
- National Lung Screening Trial Research Team. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
- Mazzone PJ et al. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
- Tanner NT et al. Chest. 2023;S0012-3692(23)00175-7. doi:10.1016/j.chest.2023.02.003
- National Lung Screening Trial Research Team. N Engl J Med. 2011;365(5):395- 409. doi:10.1056/NEJMoa1102873
- Marmor HN et al. Curr Chall Thorac Surg. 2023;5:5. doi:10.21037/ccts-20-171
- Naidch DP et al. Radiology. 1990;175(3):729-731. doi:10.1148/radiology.175.3.2343122
- Kaneko M et al. Radiology. 1996;201(3):798-802. doi:10.1148/radiology.201.3.8939234
- National Lung Screening Trial Research Team. Radiology. 2011;258(1):243-253. doi:10.1148/radiol.10091808
- National Lung Screening Trial Research Team. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
- Mazzone PJ et al. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
- Tanner NT et al. Chest. 2023;S0012-3692(23)00175-7. doi:10.1016/j.chest.2023.02.003
- National Lung Screening Trial Research Team. N Engl J Med. 2011;365(5):395- 409. doi:10.1056/NEJMoa1102873
- Marmor HN et al. Curr Chall Thorac Surg. 2023;5:5. doi:10.21037/ccts-20-171
- Naidch DP et al. Radiology. 1990;175(3):729-731. doi:10.1148/radiology.175.3.2343122
- Kaneko M et al. Radiology. 1996;201(3):798-802. doi:10.1148/radiology.201.3.8939234
- National Lung Screening Trial Research Team. Radiology. 2011;258(1):243-253. doi:10.1148/radiol.10091808
- National Lung Screening Trial Research Team. J Thorac Oncol. 2019;14(10):1732-1742. doi:10.1016/j.jtho.2019.05.044
- Mazzone PJ et al. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
- Tanner NT et al. Chest. 2023;S0012-3692(23)00175-7. doi:10.1016/j.chest.2023.02.003
- National Lung Screening Trial Research Team. N Engl J Med. 2011;365(5):395- 409. doi:10.1056/NEJMoa1102873
- Marmor HN et al. Curr Chall Thorac Surg. 2023;5:5. doi:10.21037/ccts-20-171
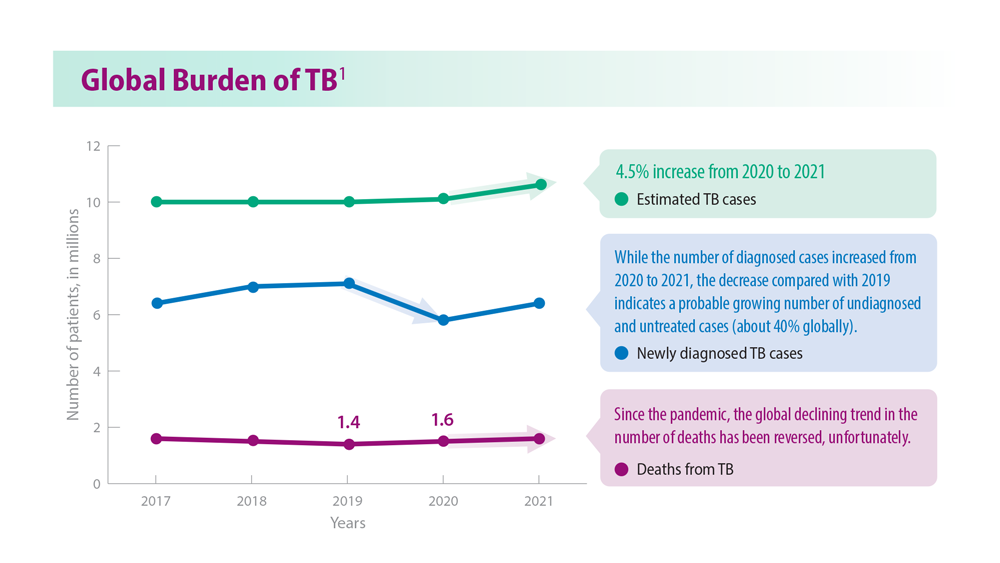
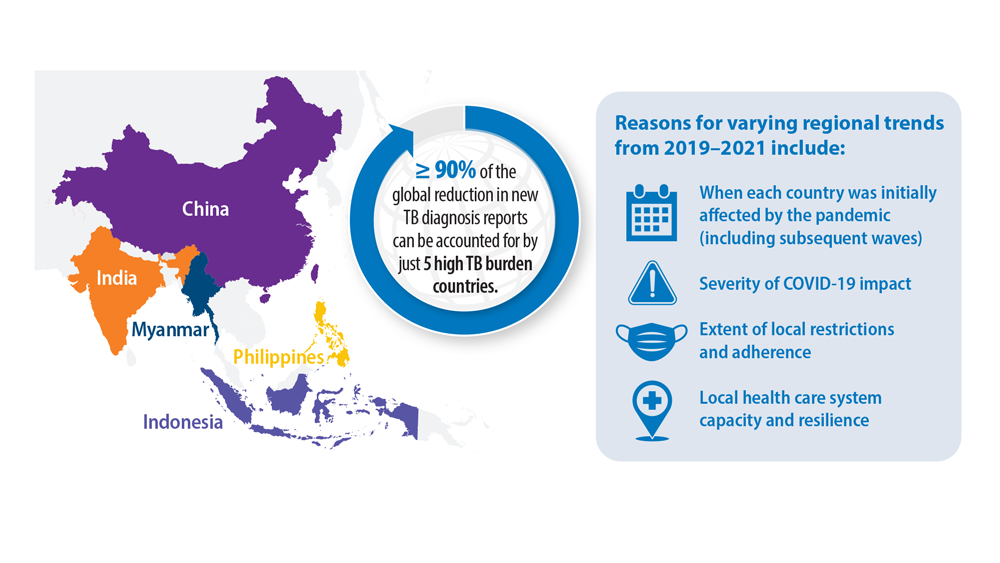
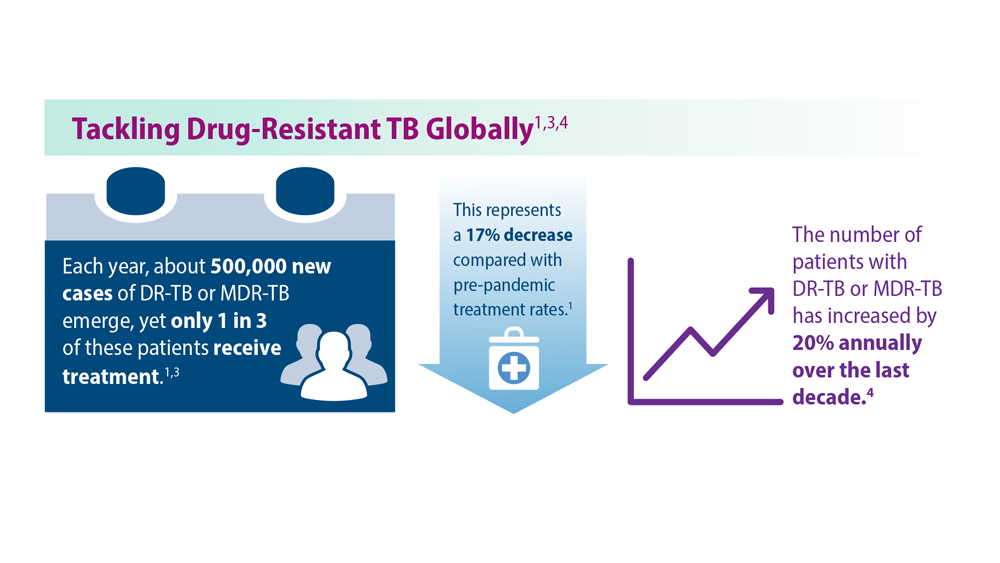
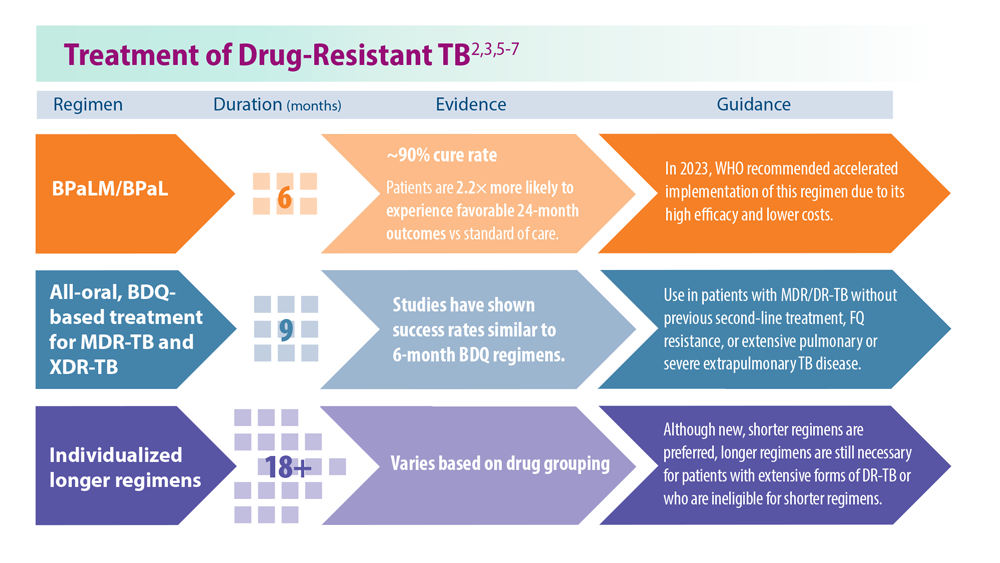
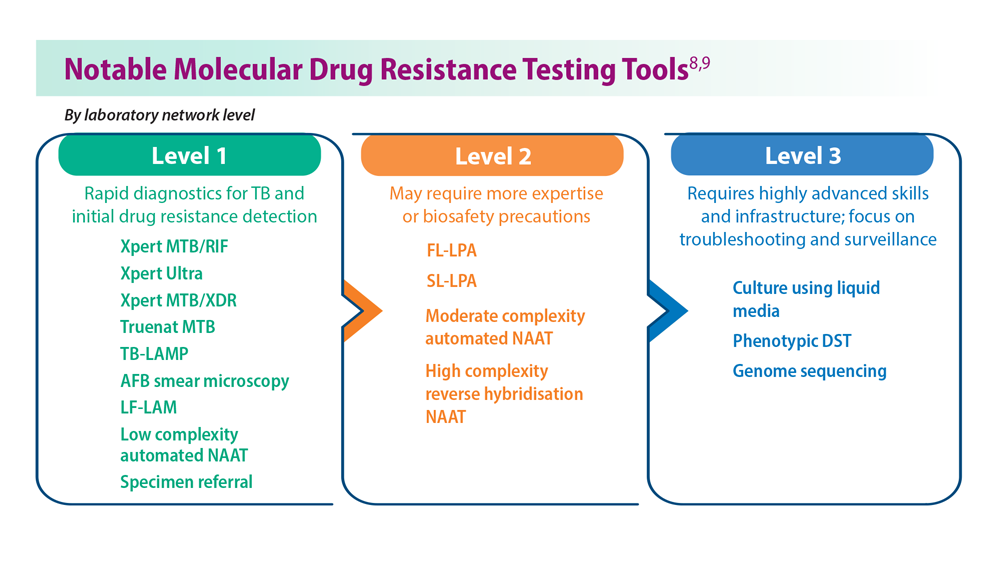
Tuberculosis Management: Returning to Pre-Pandemic Priorities
- Global tuberculosis report 2022. World Health Organization. Published October 27, 2022. Accessed June 26, 2023. https://www.who.int/publications/i/item/9789240061729
- WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment, 2022 update. World Health Organization. Published December 15, 2022. Accessed June 26, 2023. https://www.who.int/publications/i/item/9789240063129
- Migliori GB, Tiberi S. Int J Tuberc Lung Dis. 2022 ;26(7):590-591. doi:10.5588/ijtld.22.0263.
- Lange C et al. Am J Respir Crit Care Med. 2022;205(10):1142-1144. doi:10.1164/rccm.202202-0393ED
- Esmail A et al. Am J Respir Crit Care Med. 2022;205(10):1214-1227. doi:10.1164/rccm.202107-1779OC
- WHO BPaLM Accelerator Platform: to support the call to action for implementation of the shorter and more effective treatment for all people suffering from drug-resistant TB. World Health Organization. Published May 9, 2023. Accessed June 26, 2023. https://www.who.int/news-room/events/detail/2023/05/09/default-calendar/who-bpalm-accelerator-platform–to-support-the-call-to-action-for-implementation-of-the-shorter-and-moreeffective-
- Trevisi L et al. Am J Respir Crit Care Med. 2023;207(11):1525-1532. doi:10.1164/rccm.202211-2125OC
- Domínguez J et al; TBnet and RESIST-TB networks. Lancet Infect Dis. 2023;23(4):e122-e137. doi:10.1016/S1473-3099(22)00875-1
- WHO operational handbook on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection, 2021 update. World Health Organization. Published July 7, 2021. Accessed June 26, 2023. https://www.who.int/publications/i/item/9789240030589treatment-for-all-people-suffering-from-drug-resistant-tb
- Global tuberculosis report 2022. World Health Organization. Published October 27, 2022. Accessed June 26, 2023. https://www.who.int/publications/i/item/9789240061729
- WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment, 2022 update. World Health Organization. Published December 15, 2022. Accessed June 26, 2023. https://www.who.int/publications/i/item/9789240063129
- Migliori GB, Tiberi S. Int J Tuberc Lung Dis. 2022 ;26(7):590-591. doi:10.5588/ijtld.22.0263.
- Lange C et al. Am J Respir Crit Care Med. 2022;205(10):1142-1144. doi:10.1164/rccm.202202-0393ED
- Esmail A et al. Am J Respir Crit Care Med. 2022;205(10):1214-1227. doi:10.1164/rccm.202107-1779OC
- WHO BPaLM Accelerator Platform: to support the call to action for implementation of the shorter and more effective treatment for all people suffering from drug-resistant TB. World Health Organization. Published May 9, 2023. Accessed June 26, 2023. https://www.who.int/news-room/events/detail/2023/05/09/default-calendar/who-bpalm-accelerator-platform–to-support-the-call-to-action-for-implementation-of-the-shorter-and-moreeffective-
- Trevisi L et al. Am J Respir Crit Care Med. 2023;207(11):1525-1532. doi:10.1164/rccm.202211-2125OC
- Domínguez J et al; TBnet and RESIST-TB networks. Lancet Infect Dis. 2023;23(4):e122-e137. doi:10.1016/S1473-3099(22)00875-1
- WHO operational handbook on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection, 2021 update. World Health Organization. Published July 7, 2021. Accessed June 26, 2023. https://www.who.int/publications/i/item/9789240030589treatment-for-all-people-suffering-from-drug-resistant-tb
- Global tuberculosis report 2022. World Health Organization. Published October 27, 2022. Accessed June 26, 2023. https://www.who.int/publications/i/item/9789240061729
- WHO consolidated guidelines on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment, 2022 update. World Health Organization. Published December 15, 2022. Accessed June 26, 2023. https://www.who.int/publications/i/item/9789240063129
- Migliori GB, Tiberi S. Int J Tuberc Lung Dis. 2022 ;26(7):590-591. doi:10.5588/ijtld.22.0263.
- Lange C et al. Am J Respir Crit Care Med. 2022;205(10):1142-1144. doi:10.1164/rccm.202202-0393ED
- Esmail A et al. Am J Respir Crit Care Med. 2022;205(10):1214-1227. doi:10.1164/rccm.202107-1779OC
- WHO BPaLM Accelerator Platform: to support the call to action for implementation of the shorter and more effective treatment for all people suffering from drug-resistant TB. World Health Organization. Published May 9, 2023. Accessed June 26, 2023. https://www.who.int/news-room/events/detail/2023/05/09/default-calendar/who-bpalm-accelerator-platform–to-support-the-call-to-action-for-implementation-of-the-shorter-and-moreeffective-
- Trevisi L et al. Am J Respir Crit Care Med. 2023;207(11):1525-1532. doi:10.1164/rccm.202211-2125OC
- Domínguez J et al; TBnet and RESIST-TB networks. Lancet Infect Dis. 2023;23(4):e122-e137. doi:10.1016/S1473-3099(22)00875-1
- WHO operational handbook on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection, 2021 update. World Health Organization. Published July 7, 2021. Accessed June 26, 2023. https://www.who.int/publications/i/item/9789240030589treatment-for-all-people-suffering-from-drug-resistant-tb