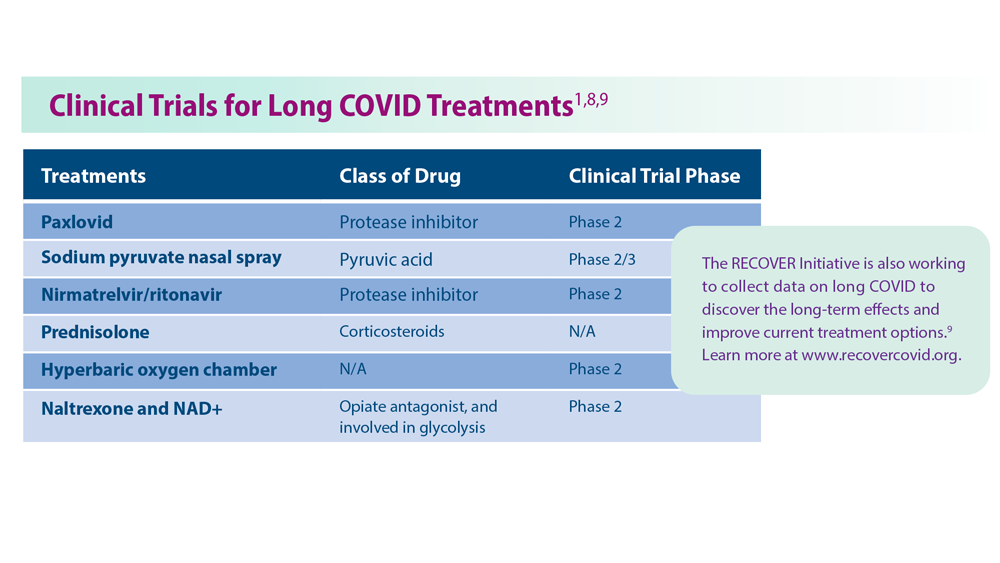
User login
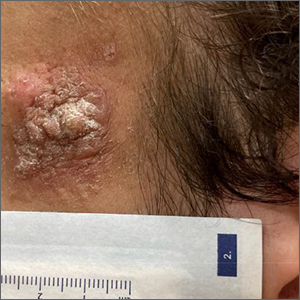
Enlarging lesion on temple
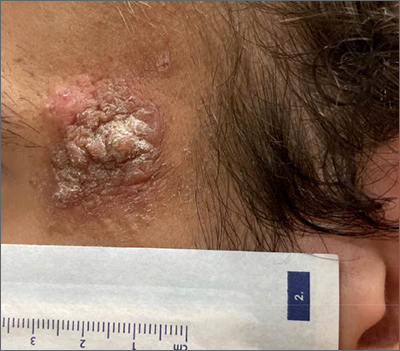
A shave biopsy revealed acanthosis, papillomatosis, hyperkeratosis, hypergranulosis, parakeratosis, and cytoplasmic viral-like inclusions without atypia, consistent with a diagnosis of a common wart. The biopsy ruled out other possible diagnoses, which included keratoacanthoma, seborrheic keratosis, and squamous cell carcinoma.
Cutaneous warts can manifest as common warts (verruca vulgaris), plantar warts (verruca plantaris), or plane warts (verruca plana). These benign skin lesions are caused by human papillomavirus and can manifest in areas of skin trauma; this is known as the Koebner phenomenon. Most warts can be diagnosed through clinical history and examination. Dermoscopy, if performed, may reveal thrombosed capillaries as dotted structures, but there is an increased risk of cross-contamination.1 That said, some dermatoscopes have disposable covers or can be cleaned with antiviral, antibacterial wipes. If the diagnosis is unclear or the exam is clinically suspicious, a biopsy may be required.
Cases with progressive enlargement and extensive involvement of the skin (as was seen here) are generally associated with certain predisposing conditions, such as atopic dermatitis and immunosuppression.2 Our patient screened negative for HIV infection, and further evaluation did not reveal any concerns for immunosuppression.
Treatment for a common wart depends on patient characteristics, preferences, cost, and possible adverse effects. Standard treatment options are topical salicylic acid and cryotherapy with liquid nitrogen. Depending on the location and type of the wart, multiple treatments may be required, and recurrences are common. Intralesional injection with bleomycin, 5‐fluorouracil, or cidofovir is often used for recurrent and refractory warts.
Patients unable to tolerate cryotherapy or local injections may benefit from thermotherapy by heating the wart with a pulsed dye laser.3 Observation is also a reasonable course of action for new warts, as they may spontaneously resolve within a year.
In this case, the patient opted for over-the-counter salicylic acid 17% to be applied nightly until resolution. Cryosurgery would be a next step for him if the lesion does not resolve after 3 months of treatment.
Image courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mun JH, Park SM, Ko HC, et al. Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion. Dermatol Pract Concept. 2013;3:33-34. doi: 10.5826/dpc.0304a07
2. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030-1048. doi: 10.1016/j.jaci.2012.07.049
3. Zhu P, Qi RQ, Yang Y, et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J Evid Based Med. 2022;15:284-301. doi: 10.1111/jebm.12494

A shave biopsy revealed acanthosis, papillomatosis, hyperkeratosis, hypergranulosis, parakeratosis, and cytoplasmic viral-like inclusions without atypia, consistent with a diagnosis of a common wart. The biopsy ruled out other possible diagnoses, which included keratoacanthoma, seborrheic keratosis, and squamous cell carcinoma.
Cutaneous warts can manifest as common warts (verruca vulgaris), plantar warts (verruca plantaris), or plane warts (verruca plana). These benign skin lesions are caused by human papillomavirus and can manifest in areas of skin trauma; this is known as the Koebner phenomenon. Most warts can be diagnosed through clinical history and examination. Dermoscopy, if performed, may reveal thrombosed capillaries as dotted structures, but there is an increased risk of cross-contamination.1 That said, some dermatoscopes have disposable covers or can be cleaned with antiviral, antibacterial wipes. If the diagnosis is unclear or the exam is clinically suspicious, a biopsy may be required.
Cases with progressive enlargement and extensive involvement of the skin (as was seen here) are generally associated with certain predisposing conditions, such as atopic dermatitis and immunosuppression.2 Our patient screened negative for HIV infection, and further evaluation did not reveal any concerns for immunosuppression.
Treatment for a common wart depends on patient characteristics, preferences, cost, and possible adverse effects. Standard treatment options are topical salicylic acid and cryotherapy with liquid nitrogen. Depending on the location and type of the wart, multiple treatments may be required, and recurrences are common. Intralesional injection with bleomycin, 5‐fluorouracil, or cidofovir is often used for recurrent and refractory warts.
Patients unable to tolerate cryotherapy or local injections may benefit from thermotherapy by heating the wart with a pulsed dye laser.3 Observation is also a reasonable course of action for new warts, as they may spontaneously resolve within a year.
In this case, the patient opted for over-the-counter salicylic acid 17% to be applied nightly until resolution. Cryosurgery would be a next step for him if the lesion does not resolve after 3 months of treatment.
Image courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

A shave biopsy revealed acanthosis, papillomatosis, hyperkeratosis, hypergranulosis, parakeratosis, and cytoplasmic viral-like inclusions without atypia, consistent with a diagnosis of a common wart. The biopsy ruled out other possible diagnoses, which included keratoacanthoma, seborrheic keratosis, and squamous cell carcinoma.
Cutaneous warts can manifest as common warts (verruca vulgaris), plantar warts (verruca plantaris), or plane warts (verruca plana). These benign skin lesions are caused by human papillomavirus and can manifest in areas of skin trauma; this is known as the Koebner phenomenon. Most warts can be diagnosed through clinical history and examination. Dermoscopy, if performed, may reveal thrombosed capillaries as dotted structures, but there is an increased risk of cross-contamination.1 That said, some dermatoscopes have disposable covers or can be cleaned with antiviral, antibacterial wipes. If the diagnosis is unclear or the exam is clinically suspicious, a biopsy may be required.
Cases with progressive enlargement and extensive involvement of the skin (as was seen here) are generally associated with certain predisposing conditions, such as atopic dermatitis and immunosuppression.2 Our patient screened negative for HIV infection, and further evaluation did not reveal any concerns for immunosuppression.
Treatment for a common wart depends on patient characteristics, preferences, cost, and possible adverse effects. Standard treatment options are topical salicylic acid and cryotherapy with liquid nitrogen. Depending on the location and type of the wart, multiple treatments may be required, and recurrences are common. Intralesional injection with bleomycin, 5‐fluorouracil, or cidofovir is often used for recurrent and refractory warts.
Patients unable to tolerate cryotherapy or local injections may benefit from thermotherapy by heating the wart with a pulsed dye laser.3 Observation is also a reasonable course of action for new warts, as they may spontaneously resolve within a year.
In this case, the patient opted for over-the-counter salicylic acid 17% to be applied nightly until resolution. Cryosurgery would be a next step for him if the lesion does not resolve after 3 months of treatment.
Image courtesy of Faryal Tahir, MD. Text courtesy of Faryal Tahir, MD, Assistant Professor, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mun JH, Park SM, Ko HC, et al. Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion. Dermatol Pract Concept. 2013;3:33-34. doi: 10.5826/dpc.0304a07
2. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030-1048. doi: 10.1016/j.jaci.2012.07.049
3. Zhu P, Qi RQ, Yang Y, et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J Evid Based Med. 2022;15:284-301. doi: 10.1111/jebm.12494
1. Mun JH, Park SM, Ko HC, et al. Prevention of possible cross-infection among patients by dermoscopy: a brief review of the literature and our suggestion. Dermatol Pract Concept. 2013;3:33-34. doi: 10.5826/dpc.0304a07
2. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030-1048. doi: 10.1016/j.jaci.2012.07.049
3. Zhu P, Qi RQ, Yang Y, et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J Evid Based Med. 2022;15:284-301. doi: 10.1111/jebm.12494
Commentary: "Migraine Plus" Symptoms, October 2023
This month we will discuss "migraine plus" conditions: menstrual migraine as well as migraine-associated symptoms, including allodynia, photophobia, and nausea.
Migraine is one of the most common and disabling conditions worldwide, and it is three times more likely to be found in women than men. This is even more so during reproductive years, where many women experience hormonally triggered migraine attacks. Although some women will experience migraine exclusively perimenstrually, most women who experience menstrual migraine attacks also will have migraine attacks that are not hormonally triggered. It is often challenging to find the correct acute treatment for specific kinds of migraine attacks, and many women will describe specific acute medications as more effective for their nonmenstrual or "regular" migraine attacks compared with their perimenstrual attacks. The study by MacGregor and colleagues investigated the use of ubrogepant and compared its effect between these two subtypes of attacks.
This trial was an extension of the initial phase 3 trial of ubrogepant, called ACHIEVE II. Initial investigators enrolled over 700 patients into an open-label extension, and the participants were randomly assigned 1:1:1 to their "usual care," 50 mg ubrogepant, or 100 mg ubrogepant. Participants were blinded to the dose of ubrogepant even though they knew that they were taking ubrogepant or their standard acute medication. The purpose of the "usual care" arm was not to collect efficacy results; rather, it was for safety, specifically to evaluate the long-term hepatic safety with ubrogepant.
Participants were allowed to treat up to eight migraine attacks per 4-week interval. The duration of the trial was 52 weeks, and a second dose of medication was allowed, identical to the initial dose. Women in this trial recorded their menstrual start date and whether they treated menstrually related attacks. An attack was considered menstrually related if the headache was within a 5-day window of the onset of menstruation. Of the 734 women enrolled in the intention-to-treat population, 354 reported at least one menstrual cycle start date with a headache day. Efficacy outcome measures included pain freedom at 2 hours post-dose, pain relief at 2 hours post-dose, absence of photophobia, phonophobia, and nausea at 2 hours post-dose, normal function at 2 hours post-dose, and use of rescue medication within 24 hours of the initial dose. All information was collected via an electronic diary.
There was no statistically significant difference between 2-hour pain freedom outcomes of menstrual and nonmenstrual migraine attacks, although there was a numerically higher mean percentage of menstrual attacks that was not statistically significant. This was noted for both doses of ubrogepant. This was also the case for 2-hour pain relief; the migraine-associated symptoms of photophobia, phonophobia, and nausea; for functional disability; and the use of a rescue medication. Among all outcomes it appears that both doses of ubrogepant are equally effective for both menstrual and nonmenstrual migraine attacks. On the basis of this evidence, clinicians may be able to consolidate different acute medications for different migraine subtypes and consider the use of this calcitonin gene-related peptide (CGRP) antagonist for all the patient's attacks.
Allodynia is a condition whereby a nonpainful stimulus is perceived as painful. In the context of migraine, this often will occur in the head and neck region and as a result of the chronification of migraine — headache frequency increasing to > 15 days per month. One significant risk factor for the development of chronic migraine is medication overuse, when an acute medication for migraine is used more often than its recommended use. Pijpers and colleagues sought to determine whether the presence of allodynia was predictive for the prognosis of chronic migraine complicated by medication overuse.
This study was a subset of the Chronification And Reversibility of Migraine (CHARM) study, a randomized, double-blind, placebo-controlled trial that aimed to investigate whether treatment with botulinum toxin A was of added value in addition to withdrawal therapy in chronic migraine patients with medication overuse headache. Diagnoses were made in consultation with headache experts and confirmed by a headache diary. Exclusion criteria were: (1) other primary headache or neurologic disorders; (2) other chronic pain disorders with medium to high pain intensity or requiring pain medication; (3) major psychiatric disorders other than depression; (4) major cognitive, behavioral, or oncologic disorders; (5) contraindications for treatment or inability to adhere to the study protocol; (6) (planned) pregnancy or breastfeeding; (7) use of ergots, opioids, or barbiturates; or (8) abuse of drugs in the past 12 months. Allodynia was determined by the Allodynia Symptom Checklist (ASC) .
The primary outcome was reversion from chronic to episodic migraine; secondary outcomes were > 50% reduction in monthly migraine days and reduction in number of monthly headache days. A total of 173 participants in the CHARM trial provided baseline allodynia data and were included in this current study. Participants with cutaneous allodynia were mainly women and did not differ significantly in age, number of monthly migraine or headache days, age of onset, use of acute or prophylactic treatment, or being treated with botulinum toxin.
The absence of cutaneous allodynia was predictive for good outcome after 12 weeks. For the primary endpoint, the odds for reversion from chronic migraine to episodic migraine were 2.5 times higher for participants without allodynia vs with allodynia. In all, 75.0% of participants without allodynia vs 57.4% of participants with allodynia reverted to episodic migraine. These helpful data will allow us to better predict accurately the disease process and better set expectations for our patients with chronic migraine.
In the earlier days of headache treatment, the focus for both acute and preventive medications was a decrease in the severity or frequency of pain. As time has progressed and our understanding of migraine has broadened, we now consider pain one of the many features of migraine, albeit usually the most prominent feature. The CGRP antagonist class of migraine medications has revolutionized how migraine is treated, both acutely and preventively; however, the initial studies all focused on pain-related outcomes. Alpuente and colleagues sought to better determine the effect of CGRP monoclonal antibody medications on other migraine-associated symptoms, specifically photophobia, photophobia, nausea, dizziness, and aura.
All injectable CGRP antibody medications were studied. Responses were recorded in an electronic diary. Patients were followed at 3 and 6 months and were excluded if their diary was < 80% complete; a total of 158 patients were included in this study. At 3 months, groups of patients were further divided between those who had > 50% decrease in monthly headache days and those that had < 50% reduction.
The > 50% group showed statistically significant reductions in the ratios of photophobia, phonophobia, and aura after 6 months of treatment, and, of note, these symptoms decreased at a higher rate than the reduction in headache days per month after 6 months. Rates of nausea and dizziness only reduced proportionally to the monthly headache days. For the < 50% group, there was a rebound of dizziness in between months 3 and 6, but all other outcomes decreased in proportion to the monthly headache days.
Our patients all experience symptoms other than headache pain as part of their migraine attacks. When we discuss the risks and benefits of a new treatment, we can now more accurately address many of the other associated symptoms and explain what our patients are likely to expect when starting a new medication. Similar studies have described these findings with the oral CGRP antagonists as well, and most acute migraine studies now use "most bothersome symptom" rather than pain severity as their primary outcome.
This month we will discuss "migraine plus" conditions: menstrual migraine as well as migraine-associated symptoms, including allodynia, photophobia, and nausea.
Migraine is one of the most common and disabling conditions worldwide, and it is three times more likely to be found in women than men. This is even more so during reproductive years, where many women experience hormonally triggered migraine attacks. Although some women will experience migraine exclusively perimenstrually, most women who experience menstrual migraine attacks also will have migraine attacks that are not hormonally triggered. It is often challenging to find the correct acute treatment for specific kinds of migraine attacks, and many women will describe specific acute medications as more effective for their nonmenstrual or "regular" migraine attacks compared with their perimenstrual attacks. The study by MacGregor and colleagues investigated the use of ubrogepant and compared its effect between these two subtypes of attacks.
This trial was an extension of the initial phase 3 trial of ubrogepant, called ACHIEVE II. Initial investigators enrolled over 700 patients into an open-label extension, and the participants were randomly assigned 1:1:1 to their "usual care," 50 mg ubrogepant, or 100 mg ubrogepant. Participants were blinded to the dose of ubrogepant even though they knew that they were taking ubrogepant or their standard acute medication. The purpose of the "usual care" arm was not to collect efficacy results; rather, it was for safety, specifically to evaluate the long-term hepatic safety with ubrogepant.
Participants were allowed to treat up to eight migraine attacks per 4-week interval. The duration of the trial was 52 weeks, and a second dose of medication was allowed, identical to the initial dose. Women in this trial recorded their menstrual start date and whether they treated menstrually related attacks. An attack was considered menstrually related if the headache was within a 5-day window of the onset of menstruation. Of the 734 women enrolled in the intention-to-treat population, 354 reported at least one menstrual cycle start date with a headache day. Efficacy outcome measures included pain freedom at 2 hours post-dose, pain relief at 2 hours post-dose, absence of photophobia, phonophobia, and nausea at 2 hours post-dose, normal function at 2 hours post-dose, and use of rescue medication within 24 hours of the initial dose. All information was collected via an electronic diary.
There was no statistically significant difference between 2-hour pain freedom outcomes of menstrual and nonmenstrual migraine attacks, although there was a numerically higher mean percentage of menstrual attacks that was not statistically significant. This was noted for both doses of ubrogepant. This was also the case for 2-hour pain relief; the migraine-associated symptoms of photophobia, phonophobia, and nausea; for functional disability; and the use of a rescue medication. Among all outcomes it appears that both doses of ubrogepant are equally effective for both menstrual and nonmenstrual migraine attacks. On the basis of this evidence, clinicians may be able to consolidate different acute medications for different migraine subtypes and consider the use of this calcitonin gene-related peptide (CGRP) antagonist for all the patient's attacks.
Allodynia is a condition whereby a nonpainful stimulus is perceived as painful. In the context of migraine, this often will occur in the head and neck region and as a result of the chronification of migraine — headache frequency increasing to > 15 days per month. One significant risk factor for the development of chronic migraine is medication overuse, when an acute medication for migraine is used more often than its recommended use. Pijpers and colleagues sought to determine whether the presence of allodynia was predictive for the prognosis of chronic migraine complicated by medication overuse.
This study was a subset of the Chronification And Reversibility of Migraine (CHARM) study, a randomized, double-blind, placebo-controlled trial that aimed to investigate whether treatment with botulinum toxin A was of added value in addition to withdrawal therapy in chronic migraine patients with medication overuse headache. Diagnoses were made in consultation with headache experts and confirmed by a headache diary. Exclusion criteria were: (1) other primary headache or neurologic disorders; (2) other chronic pain disorders with medium to high pain intensity or requiring pain medication; (3) major psychiatric disorders other than depression; (4) major cognitive, behavioral, or oncologic disorders; (5) contraindications for treatment or inability to adhere to the study protocol; (6) (planned) pregnancy or breastfeeding; (7) use of ergots, opioids, or barbiturates; or (8) abuse of drugs in the past 12 months. Allodynia was determined by the Allodynia Symptom Checklist (ASC) .
The primary outcome was reversion from chronic to episodic migraine; secondary outcomes were > 50% reduction in monthly migraine days and reduction in number of monthly headache days. A total of 173 participants in the CHARM trial provided baseline allodynia data and were included in this current study. Participants with cutaneous allodynia were mainly women and did not differ significantly in age, number of monthly migraine or headache days, age of onset, use of acute or prophylactic treatment, or being treated with botulinum toxin.
The absence of cutaneous allodynia was predictive for good outcome after 12 weeks. For the primary endpoint, the odds for reversion from chronic migraine to episodic migraine were 2.5 times higher for participants without allodynia vs with allodynia. In all, 75.0% of participants without allodynia vs 57.4% of participants with allodynia reverted to episodic migraine. These helpful data will allow us to better predict accurately the disease process and better set expectations for our patients with chronic migraine.
In the earlier days of headache treatment, the focus for both acute and preventive medications was a decrease in the severity or frequency of pain. As time has progressed and our understanding of migraine has broadened, we now consider pain one of the many features of migraine, albeit usually the most prominent feature. The CGRP antagonist class of migraine medications has revolutionized how migraine is treated, both acutely and preventively; however, the initial studies all focused on pain-related outcomes. Alpuente and colleagues sought to better determine the effect of CGRP monoclonal antibody medications on other migraine-associated symptoms, specifically photophobia, photophobia, nausea, dizziness, and aura.
All injectable CGRP antibody medications were studied. Responses were recorded in an electronic diary. Patients were followed at 3 and 6 months and were excluded if their diary was < 80% complete; a total of 158 patients were included in this study. At 3 months, groups of patients were further divided between those who had > 50% decrease in monthly headache days and those that had < 50% reduction.
The > 50% group showed statistically significant reductions in the ratios of photophobia, phonophobia, and aura after 6 months of treatment, and, of note, these symptoms decreased at a higher rate than the reduction in headache days per month after 6 months. Rates of nausea and dizziness only reduced proportionally to the monthly headache days. For the < 50% group, there was a rebound of dizziness in between months 3 and 6, but all other outcomes decreased in proportion to the monthly headache days.
Our patients all experience symptoms other than headache pain as part of their migraine attacks. When we discuss the risks and benefits of a new treatment, we can now more accurately address many of the other associated symptoms and explain what our patients are likely to expect when starting a new medication. Similar studies have described these findings with the oral CGRP antagonists as well, and most acute migraine studies now use "most bothersome symptom" rather than pain severity as their primary outcome.
This month we will discuss "migraine plus" conditions: menstrual migraine as well as migraine-associated symptoms, including allodynia, photophobia, and nausea.
Migraine is one of the most common and disabling conditions worldwide, and it is three times more likely to be found in women than men. This is even more so during reproductive years, where many women experience hormonally triggered migraine attacks. Although some women will experience migraine exclusively perimenstrually, most women who experience menstrual migraine attacks also will have migraine attacks that are not hormonally triggered. It is often challenging to find the correct acute treatment for specific kinds of migraine attacks, and many women will describe specific acute medications as more effective for their nonmenstrual or "regular" migraine attacks compared with their perimenstrual attacks. The study by MacGregor and colleagues investigated the use of ubrogepant and compared its effect between these two subtypes of attacks.
This trial was an extension of the initial phase 3 trial of ubrogepant, called ACHIEVE II. Initial investigators enrolled over 700 patients into an open-label extension, and the participants were randomly assigned 1:1:1 to their "usual care," 50 mg ubrogepant, or 100 mg ubrogepant. Participants were blinded to the dose of ubrogepant even though they knew that they were taking ubrogepant or their standard acute medication. The purpose of the "usual care" arm was not to collect efficacy results; rather, it was for safety, specifically to evaluate the long-term hepatic safety with ubrogepant.
Participants were allowed to treat up to eight migraine attacks per 4-week interval. The duration of the trial was 52 weeks, and a second dose of medication was allowed, identical to the initial dose. Women in this trial recorded their menstrual start date and whether they treated menstrually related attacks. An attack was considered menstrually related if the headache was within a 5-day window of the onset of menstruation. Of the 734 women enrolled in the intention-to-treat population, 354 reported at least one menstrual cycle start date with a headache day. Efficacy outcome measures included pain freedom at 2 hours post-dose, pain relief at 2 hours post-dose, absence of photophobia, phonophobia, and nausea at 2 hours post-dose, normal function at 2 hours post-dose, and use of rescue medication within 24 hours of the initial dose. All information was collected via an electronic diary.
There was no statistically significant difference between 2-hour pain freedom outcomes of menstrual and nonmenstrual migraine attacks, although there was a numerically higher mean percentage of menstrual attacks that was not statistically significant. This was noted for both doses of ubrogepant. This was also the case for 2-hour pain relief; the migraine-associated symptoms of photophobia, phonophobia, and nausea; for functional disability; and the use of a rescue medication. Among all outcomes it appears that both doses of ubrogepant are equally effective for both menstrual and nonmenstrual migraine attacks. On the basis of this evidence, clinicians may be able to consolidate different acute medications for different migraine subtypes and consider the use of this calcitonin gene-related peptide (CGRP) antagonist for all the patient's attacks.
Allodynia is a condition whereby a nonpainful stimulus is perceived as painful. In the context of migraine, this often will occur in the head and neck region and as a result of the chronification of migraine — headache frequency increasing to > 15 days per month. One significant risk factor for the development of chronic migraine is medication overuse, when an acute medication for migraine is used more often than its recommended use. Pijpers and colleagues sought to determine whether the presence of allodynia was predictive for the prognosis of chronic migraine complicated by medication overuse.
This study was a subset of the Chronification And Reversibility of Migraine (CHARM) study, a randomized, double-blind, placebo-controlled trial that aimed to investigate whether treatment with botulinum toxin A was of added value in addition to withdrawal therapy in chronic migraine patients with medication overuse headache. Diagnoses were made in consultation with headache experts and confirmed by a headache diary. Exclusion criteria were: (1) other primary headache or neurologic disorders; (2) other chronic pain disorders with medium to high pain intensity or requiring pain medication; (3) major psychiatric disorders other than depression; (4) major cognitive, behavioral, or oncologic disorders; (5) contraindications for treatment or inability to adhere to the study protocol; (6) (planned) pregnancy or breastfeeding; (7) use of ergots, opioids, or barbiturates; or (8) abuse of drugs in the past 12 months. Allodynia was determined by the Allodynia Symptom Checklist (ASC) .
The primary outcome was reversion from chronic to episodic migraine; secondary outcomes were > 50% reduction in monthly migraine days and reduction in number of monthly headache days. A total of 173 participants in the CHARM trial provided baseline allodynia data and were included in this current study. Participants with cutaneous allodynia were mainly women and did not differ significantly in age, number of monthly migraine or headache days, age of onset, use of acute or prophylactic treatment, or being treated with botulinum toxin.
The absence of cutaneous allodynia was predictive for good outcome after 12 weeks. For the primary endpoint, the odds for reversion from chronic migraine to episodic migraine were 2.5 times higher for participants without allodynia vs with allodynia. In all, 75.0% of participants without allodynia vs 57.4% of participants with allodynia reverted to episodic migraine. These helpful data will allow us to better predict accurately the disease process and better set expectations for our patients with chronic migraine.
In the earlier days of headache treatment, the focus for both acute and preventive medications was a decrease in the severity or frequency of pain. As time has progressed and our understanding of migraine has broadened, we now consider pain one of the many features of migraine, albeit usually the most prominent feature. The CGRP antagonist class of migraine medications has revolutionized how migraine is treated, both acutely and preventively; however, the initial studies all focused on pain-related outcomes. Alpuente and colleagues sought to better determine the effect of CGRP monoclonal antibody medications on other migraine-associated symptoms, specifically photophobia, photophobia, nausea, dizziness, and aura.
All injectable CGRP antibody medications were studied. Responses were recorded in an electronic diary. Patients were followed at 3 and 6 months and were excluded if their diary was < 80% complete; a total of 158 patients were included in this study. At 3 months, groups of patients were further divided between those who had > 50% decrease in monthly headache days and those that had < 50% reduction.
The > 50% group showed statistically significant reductions in the ratios of photophobia, phonophobia, and aura after 6 months of treatment, and, of note, these symptoms decreased at a higher rate than the reduction in headache days per month after 6 months. Rates of nausea and dizziness only reduced proportionally to the monthly headache days. For the < 50% group, there was a rebound of dizziness in between months 3 and 6, but all other outcomes decreased in proportion to the monthly headache days.
Our patients all experience symptoms other than headache pain as part of their migraine attacks. When we discuss the risks and benefits of a new treatment, we can now more accurately address many of the other associated symptoms and explain what our patients are likely to expect when starting a new medication. Similar studies have described these findings with the oral CGRP antagonists as well, and most acute migraine studies now use "most bothersome symptom" rather than pain severity as their primary outcome.
Difficult-to-Control Diabetes: Is Cortisol at Play?
Is Cortisol at Play?
Daniel Einhorn, MD; John Buse, MD, PhD; Ralph DeFronzo, MD; Juan Pablo Frias, MD, and Christopher Lucci, MD, share their insights and perspectives on the connection between difficult-to control T2DM and untreated hypercortisolism:
• Almost a quarter of patients with type 2 diabetes (T2DM) require 3 or more medications to manage their disease, and even then, many patients have difficulty getting their diabetes under control1
• Elevated cortisol activity can exacerbate the pathophysiology of T2DM and can counter the impact of traditional anti-diabetic medications, making diabetes control challenging2-6
• Studies emerging over the last two decades suggest that up to 10% of patients with T2DM may have hypercortisolism7-11
• Patients with treatment-resistant T2DM should therefore be evaluated for hypercortisolism
• Treating the underlying hypercortisolism is important in these patients because managing comorbidities (alone) has not significantly reduced morbidity and mortality12,13

Daniel Einhorn, MD
Meeting Moderator
Vice President
Endocrine Strategy
Corcept Therapeutics
Menlo Park, CA

John Buse, MD, PhD
University of North Carolina
School of Medicine
UNC Diabetes and Endocrinology Clinic
Chapel Hill, NC

Ralph DeFronzo, MD
University of Texas
Health Science Center
San Antonio, TX

Juan Pablo Frias, MD
Velocity Clinical Research
Los Angeles, CA

Christopher Lucci, MD
Diabetes and Cardiovascular of Rockport
Rockport, TX
Click HERE to read the supplement.
References
- Fang M, et al. N Engl J Med. 2021;384(23):2219-2228.
- Scaroni C, et al. Endocr Rev. 2017;38(3):189-219.
- Mazziotti G, et al. Trends Endocrinol Metab. 2011;22(12):499-506.
- Pivonello R, et al. Neuroendocrinology. 2010;92(suppl 1):77-81.
- Mason IC, et al. Diabetologia. 2020;63(3):462-472.
- Thau L, et al. StatPearls [Internet]. Updated August 29, 2022. Accessed February 3, 2023. https://www.ncbi.nlm.nih.gov/books/NBK538239/
- Chiodini I, et al. Eur J Endocrinol. 2005;153(6):837-844.
- Catargi B, et al. J Clin Endocrinol Metab. 2003;88(12):5808-5813.
- Costa DS, et al. J Diabetes Complications. 2016;30(6):1032-1038.
- Leon-Justel A, et al. J Clin Endocrinol Metab. 2016;101(10):3747-3754.
- Steffensen C, et al. Horm Metab Res. 2019;51(1):62-68.
- Petramala L, et al. Endocrine. 2020;70(1):150-163.
- Morelli V, et al. Front Endocrinol (Lausanne). 2022;13:898084.
©2023 Corcept Therapeutics Incorporated. All Rights Reserved. DSE-01055 SEP 2023
Daniel Einhorn, MD; John Buse, MD, PhD; Ralph DeFronzo, MD; Juan Pablo Frias, MD, and Christopher Lucci, MD, share their insights and perspectives on the connection between difficult-to control T2DM and untreated hypercortisolism:
• Almost a quarter of patients with type 2 diabetes (T2DM) require 3 or more medications to manage their disease, and even then, many patients have difficulty getting their diabetes under control1
• Elevated cortisol activity can exacerbate the pathophysiology of T2DM and can counter the impact of traditional anti-diabetic medications, making diabetes control challenging2-6
• Studies emerging over the last two decades suggest that up to 10% of patients with T2DM may have hypercortisolism7-11
• Patients with treatment-resistant T2DM should therefore be evaluated for hypercortisolism
• Treating the underlying hypercortisolism is important in these patients because managing comorbidities (alone) has not significantly reduced morbidity and mortality12,13

Daniel Einhorn, MD
Meeting Moderator
Vice President
Endocrine Strategy
Corcept Therapeutics
Menlo Park, CA

John Buse, MD, PhD
University of North Carolina
School of Medicine
UNC Diabetes and Endocrinology Clinic
Chapel Hill, NC

Ralph DeFronzo, MD
University of Texas
Health Science Center
San Antonio, TX

Juan Pablo Frias, MD
Velocity Clinical Research
Los Angeles, CA

Christopher Lucci, MD
Diabetes and Cardiovascular of Rockport
Rockport, TX
Click HERE to read the supplement.
References
- Fang M, et al. N Engl J Med. 2021;384(23):2219-2228.
- Scaroni C, et al. Endocr Rev. 2017;38(3):189-219.
- Mazziotti G, et al. Trends Endocrinol Metab. 2011;22(12):499-506.
- Pivonello R, et al. Neuroendocrinology. 2010;92(suppl 1):77-81.
- Mason IC, et al. Diabetologia. 2020;63(3):462-472.
- Thau L, et al. StatPearls [Internet]. Updated August 29, 2022. Accessed February 3, 2023. https://www.ncbi.nlm.nih.gov/books/NBK538239/
- Chiodini I, et al. Eur J Endocrinol. 2005;153(6):837-844.
- Catargi B, et al. J Clin Endocrinol Metab. 2003;88(12):5808-5813.
- Costa DS, et al. J Diabetes Complications. 2016;30(6):1032-1038.
- Leon-Justel A, et al. J Clin Endocrinol Metab. 2016;101(10):3747-3754.
- Steffensen C, et al. Horm Metab Res. 2019;51(1):62-68.
- Petramala L, et al. Endocrine. 2020;70(1):150-163.
- Morelli V, et al. Front Endocrinol (Lausanne). 2022;13:898084.
©2023 Corcept Therapeutics Incorporated. All Rights Reserved. DSE-01055 SEP 2023
Daniel Einhorn, MD; John Buse, MD, PhD; Ralph DeFronzo, MD; Juan Pablo Frias, MD, and Christopher Lucci, MD, share their insights and perspectives on the connection between difficult-to control T2DM and untreated hypercortisolism:
• Almost a quarter of patients with type 2 diabetes (T2DM) require 3 or more medications to manage their disease, and even then, many patients have difficulty getting their diabetes under control1
• Elevated cortisol activity can exacerbate the pathophysiology of T2DM and can counter the impact of traditional anti-diabetic medications, making diabetes control challenging2-6
• Studies emerging over the last two decades suggest that up to 10% of patients with T2DM may have hypercortisolism7-11
• Patients with treatment-resistant T2DM should therefore be evaluated for hypercortisolism
• Treating the underlying hypercortisolism is important in these patients because managing comorbidities (alone) has not significantly reduced morbidity and mortality12,13

Daniel Einhorn, MD
Meeting Moderator
Vice President
Endocrine Strategy
Corcept Therapeutics
Menlo Park, CA

John Buse, MD, PhD
University of North Carolina
School of Medicine
UNC Diabetes and Endocrinology Clinic
Chapel Hill, NC

Ralph DeFronzo, MD
University of Texas
Health Science Center
San Antonio, TX

Juan Pablo Frias, MD
Velocity Clinical Research
Los Angeles, CA

Christopher Lucci, MD
Diabetes and Cardiovascular of Rockport
Rockport, TX
Click HERE to read the supplement.
References
- Fang M, et al. N Engl J Med. 2021;384(23):2219-2228.
- Scaroni C, et al. Endocr Rev. 2017;38(3):189-219.
- Mazziotti G, et al. Trends Endocrinol Metab. 2011;22(12):499-506.
- Pivonello R, et al. Neuroendocrinology. 2010;92(suppl 1):77-81.
- Mason IC, et al. Diabetologia. 2020;63(3):462-472.
- Thau L, et al. StatPearls [Internet]. Updated August 29, 2022. Accessed February 3, 2023. https://www.ncbi.nlm.nih.gov/books/NBK538239/
- Chiodini I, et al. Eur J Endocrinol. 2005;153(6):837-844.
- Catargi B, et al. J Clin Endocrinol Metab. 2003;88(12):5808-5813.
- Costa DS, et al. J Diabetes Complications. 2016;30(6):1032-1038.
- Leon-Justel A, et al. J Clin Endocrinol Metab. 2016;101(10):3747-3754.
- Steffensen C, et al. Horm Metab Res. 2019;51(1):62-68.
- Petramala L, et al. Endocrine. 2020;70(1):150-163.
- Morelli V, et al. Front Endocrinol (Lausanne). 2022;13:898084.
©2023 Corcept Therapeutics Incorporated. All Rights Reserved. DSE-01055 SEP 2023
Is Cortisol at Play?
Is Cortisol at Play?
Syracuse Hemoglobinopathy Presenting With Tophaceous Gout: A Case Report
Hemoglobinopathies are inherited disorders of hemoglobin that alter oxygen binding capacity by affecting the production of a specific subset of globin chains or their structure.1 A lesser-known subtype, Syracuse hemoglobinopathy (SH), was first identified in 4 generations of a family in the 1970s.2 As with other disorders of hemoglobin structure, there is an inherent risk of increased cell breakdown and turnover. This case discusses the presentation of gout in a patient with a history of SH.
Case presentation
A 44-year-old man with known SH, tobacco use disorder, and shoulder osteoarthritis presented with pain and palpable nodular masses on bilateral elbows, metacarpophalangeal joints, and feet progressively over 5 years. Of note, he was initially misdiagnosed with polycythemia vera after an incidental finding of elevated hematocrit more than 10 years prior. His mother, maternal aunt, and maternal grandmother have all been treated for polycythemia vera.
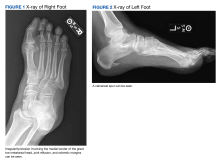
On examination, there were irregular palpable masses of varying sizes, erythema, and tenderness over the second metacarpophalangeal joint of the left hand, bilateral elbows, and bilateral metatarsophalangeal joints. Laboratory studies were remarkable for 19.8 g/dL hemoglobin (reference range, 12.0-16.0 g/dL); 63.4% hematocrit (reference range, 37.0%-47.0%); 219 × 103 µL platelets (reference range, 150-450 × 103 µL); 79.3 fL mean corpuscular volume (reference range, 81.0-99.0 fL); 14 mg/dL blood urea nitrogen (reference range, 8-27 mg/dL); 1.18 mg/dL creatinine (reference range, 0.60-1.60 mg/dL); 3 mmol/h erythrocyte sedimentation rate (reference range, 0-30 mmol/h); 88 IU/L alkaline phosphatase (reference range, 34-130 IU/L); and 11.3 mg/dL uric acid (reference range, 2.4-7.9 mg/dL). Hemoglobin electrophoresis studies showed a 49% hemoglobin A1 (reference range, 95%-98%); 3.0% hemoglobin A2 (reference range, 2%-3%); 3.1% hemoglobin F (reference range, < 0.6%); and 44.9% hemoglobin Syracuse (reference range, absent). It was negative for JAK2 V617F mutation. An X-ray of the bilateral feet showed irregularity/erosion involving the medial border of the great toe metatarsal head, joint effusions, and sclerotic margins (Figure 1). A prominent plantar calcaneal spur was present (Figure 2). Synovial fluid analysis detected the presence of negatively birefringent needle-shaped urate crystals.
Per the Clinical Gout Diagnosis tool, which has a sensitivity of 97%, this patient scored high given the findings of greater than one attack of acute arthritis, mono/oligoarthritic attacks, podagra, erythema, probable tophi, and hyperuricemia. This raised the likelihood of his presentation being an acute flare of tophaceous gout.3 He was treated with colchicine and prednisone for acute exacerbation. Once the exacerbation subsided, the colchicine was discontinued, and allopurinol was added. The uric acid goal was < 6 mg/dL and was consistently maintained. Over the subsequent months, he reported mild joint pain if he stopped taking allopurinol but did not report a recurrence in disease exacerbation.
Discussion
Hemoglobin Syracuse was first identified in the early 1970s after the discovery of similar familial hemoglobinopathies unique for their high oxygen affinity hemoglobin.1 High oxygen affinity hemoglobin functions by causing a leftward shift in the hemoglobin dissociation curve and therefore slower off-loading of oxygen into tissues.4 The hypoxic state at the tissue level created by the hemoglobin binding tightly to oxygen promotes the production of erythropoietin, increasing red blood cell and hemoglobin production.5 A study looking at uric acid levels in patients living at high altitudes (which can imitate the low-oxygen state seen in high affinity hemoglobinopathy) theorized that increased erythroblast turnover in the setting of polycythemia involves increased purine metabolism and consequently, uric acid as a breakdown product.6 Uric acid levels have also been used as a marker for hypoxia in studies regarding sleep apnea. Tissue hypoxia can increase adenosine triphosphate breakdown. One byproduct of this breakdown is hypoxanthine, which is further metabolized by xanthine oxidase, which, in turn, produces uric acid.7
The relationship between elevated uric acid and gout was first studied in the mid-nineteenth century after Alfred Barring Garrod identified urate deposits in the articular cartilage of patients with gout.1 These urate deposits garner a proinflammatory response with the activation of the complement cascade, resulting in the recruitment of neutrophils, macrophages, and lymphocytes. Recurrent gout flares eventually result in a chronic granulomatous inflammatory response to the deposited crystals resulting in the classic tophi.8 A study looking at patients with thalassemia showed that while elevated serum uric acid levels were common in these patients, only 6% developed gout. Significant risk factors were noted to be intact spleen and inefficient urinary excretion of urea due to chronic kidney disease.9
Current treatment of gout flares consistsof pain control in the acute phase and prevention in the long-term setting. The first-line treatment for acute gout attack is colchicine, prednisone, or nonsteroidal anti-inflammatory drugs. Clinicians can consider switching or combining these therapies if ineffective or in the event of severe exacerbation. Prophylactic therapy involves urate-lowering agents, such as allopurinol and febuxostat.10
Conclusions
This case illustrates how a rare disorder of high oxygen affinity hemoglobin, SH, can present itself with findings of elevated serum uric acid and tophaceous gout. Most patients with hyperuricemia never develop gout, but having a condition that increases their serum levels of uric acid can increase their chances.11 It is important for clinicians to consider this increased risk when a patient with hemoglobinopathy presents with joint pain.
1. Garrod AB. The Nature and Treatment of Gout and Rheumatic Gout. 2nd ed. Walton and Maberly; 1859.
2. Jensen M, Oski FA, Nathan DG, Bunn HF. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975;55(3):469-477. doi:10.1172/JCI107953
3. Vázquez-Mellado J, Hernández-Cuevas CB, Alvarez-Hernández E, et al. The diagnostic value of the proposal for clinical gout diagnosis (CGD). Clin Rheumatol. 2012;31(3):429-434. doi:10.1007/s10067-011-1873-4
4. Kaufman DP, Kandle PF, Murray IV, et al. Physiology, Oxyhemoglobin Dissociation Curve. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499818/
5. Yudin J, Verhovsek M. How we diagnose and manage altered oxygen affinity hemoglobin variants. Am J Hematol. 2019;94(5):597-603. doi:10.1002/ajh.25425
6. Jefferson JA, Escudero E, Hurtado ME, et al. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis. 2002;39(6):1135-1142. doi:10.1053/ajkd.2002.33380
7. Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PLoS One. 2013;8(6):e66891. Published 2013 Jun 24. doi:10.1371/journal.pone.0066891
8. Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. 2018;77(7):1048-1052. doi:10.1136/annrheumdis-2017-212288
9. Ballou SP, Khan MA, Kushner I, Harris JW. Secondary gout in hemoglobinopathies: report of two cases and review of the literature. Am J Hematol. 1977;2(4):397-402. doi:10.1002/ajh.2830020410
10. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. doi:10.1002/acr.21773
11. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. Published 2019 Sep 26. doi:10.1038/s41572-019-0115-y
Hemoglobinopathies are inherited disorders of hemoglobin that alter oxygen binding capacity by affecting the production of a specific subset of globin chains or their structure.1 A lesser-known subtype, Syracuse hemoglobinopathy (SH), was first identified in 4 generations of a family in the 1970s.2 As with other disorders of hemoglobin structure, there is an inherent risk of increased cell breakdown and turnover. This case discusses the presentation of gout in a patient with a history of SH.
Case presentation
A 44-year-old man with known SH, tobacco use disorder, and shoulder osteoarthritis presented with pain and palpable nodular masses on bilateral elbows, metacarpophalangeal joints, and feet progressively over 5 years. Of note, he was initially misdiagnosed with polycythemia vera after an incidental finding of elevated hematocrit more than 10 years prior. His mother, maternal aunt, and maternal grandmother have all been treated for polycythemia vera.
On examination, there were irregular palpable masses of varying sizes, erythema, and tenderness over the second metacarpophalangeal joint of the left hand, bilateral elbows, and bilateral metatarsophalangeal joints. Laboratory studies were remarkable for 19.8 g/dL hemoglobin (reference range, 12.0-16.0 g/dL); 63.4% hematocrit (reference range, 37.0%-47.0%); 219 × 103 µL platelets (reference range, 150-450 × 103 µL); 79.3 fL mean corpuscular volume (reference range, 81.0-99.0 fL); 14 mg/dL blood urea nitrogen (reference range, 8-27 mg/dL); 1.18 mg/dL creatinine (reference range, 0.60-1.60 mg/dL); 3 mmol/h erythrocyte sedimentation rate (reference range, 0-30 mmol/h); 88 IU/L alkaline phosphatase (reference range, 34-130 IU/L); and 11.3 mg/dL uric acid (reference range, 2.4-7.9 mg/dL). Hemoglobin electrophoresis studies showed a 49% hemoglobin A1 (reference range, 95%-98%); 3.0% hemoglobin A2 (reference range, 2%-3%); 3.1% hemoglobin F (reference range, < 0.6%); and 44.9% hemoglobin Syracuse (reference range, absent). It was negative for JAK2 V617F mutation. An X-ray of the bilateral feet showed irregularity/erosion involving the medial border of the great toe metatarsal head, joint effusions, and sclerotic margins (Figure 1). A prominent plantar calcaneal spur was present (Figure 2). Synovial fluid analysis detected the presence of negatively birefringent needle-shaped urate crystals.
Per the Clinical Gout Diagnosis tool, which has a sensitivity of 97%, this patient scored high given the findings of greater than one attack of acute arthritis, mono/oligoarthritic attacks, podagra, erythema, probable tophi, and hyperuricemia. This raised the likelihood of his presentation being an acute flare of tophaceous gout.3 He was treated with colchicine and prednisone for acute exacerbation. Once the exacerbation subsided, the colchicine was discontinued, and allopurinol was added. The uric acid goal was < 6 mg/dL and was consistently maintained. Over the subsequent months, he reported mild joint pain if he stopped taking allopurinol but did not report a recurrence in disease exacerbation.
Discussion
Hemoglobin Syracuse was first identified in the early 1970s after the discovery of similar familial hemoglobinopathies unique for their high oxygen affinity hemoglobin.1 High oxygen affinity hemoglobin functions by causing a leftward shift in the hemoglobin dissociation curve and therefore slower off-loading of oxygen into tissues.4 The hypoxic state at the tissue level created by the hemoglobin binding tightly to oxygen promotes the production of erythropoietin, increasing red blood cell and hemoglobin production.5 A study looking at uric acid levels in patients living at high altitudes (which can imitate the low-oxygen state seen in high affinity hemoglobinopathy) theorized that increased erythroblast turnover in the setting of polycythemia involves increased purine metabolism and consequently, uric acid as a breakdown product.6 Uric acid levels have also been used as a marker for hypoxia in studies regarding sleep apnea. Tissue hypoxia can increase adenosine triphosphate breakdown. One byproduct of this breakdown is hypoxanthine, which is further metabolized by xanthine oxidase, which, in turn, produces uric acid.7
The relationship between elevated uric acid and gout was first studied in the mid-nineteenth century after Alfred Barring Garrod identified urate deposits in the articular cartilage of patients with gout.1 These urate deposits garner a proinflammatory response with the activation of the complement cascade, resulting in the recruitment of neutrophils, macrophages, and lymphocytes. Recurrent gout flares eventually result in a chronic granulomatous inflammatory response to the deposited crystals resulting in the classic tophi.8 A study looking at patients with thalassemia showed that while elevated serum uric acid levels were common in these patients, only 6% developed gout. Significant risk factors were noted to be intact spleen and inefficient urinary excretion of urea due to chronic kidney disease.9
Current treatment of gout flares consistsof pain control in the acute phase and prevention in the long-term setting. The first-line treatment for acute gout attack is colchicine, prednisone, or nonsteroidal anti-inflammatory drugs. Clinicians can consider switching or combining these therapies if ineffective or in the event of severe exacerbation. Prophylactic therapy involves urate-lowering agents, such as allopurinol and febuxostat.10
Conclusions
This case illustrates how a rare disorder of high oxygen affinity hemoglobin, SH, can present itself with findings of elevated serum uric acid and tophaceous gout. Most patients with hyperuricemia never develop gout, but having a condition that increases their serum levels of uric acid can increase their chances.11 It is important for clinicians to consider this increased risk when a patient with hemoglobinopathy presents with joint pain.
Hemoglobinopathies are inherited disorders of hemoglobin that alter oxygen binding capacity by affecting the production of a specific subset of globin chains or their structure.1 A lesser-known subtype, Syracuse hemoglobinopathy (SH), was first identified in 4 generations of a family in the 1970s.2 As with other disorders of hemoglobin structure, there is an inherent risk of increased cell breakdown and turnover. This case discusses the presentation of gout in a patient with a history of SH.
Case presentation
A 44-year-old man with known SH, tobacco use disorder, and shoulder osteoarthritis presented with pain and palpable nodular masses on bilateral elbows, metacarpophalangeal joints, and feet progressively over 5 years. Of note, he was initially misdiagnosed with polycythemia vera after an incidental finding of elevated hematocrit more than 10 years prior. His mother, maternal aunt, and maternal grandmother have all been treated for polycythemia vera.
On examination, there were irregular palpable masses of varying sizes, erythema, and tenderness over the second metacarpophalangeal joint of the left hand, bilateral elbows, and bilateral metatarsophalangeal joints. Laboratory studies were remarkable for 19.8 g/dL hemoglobin (reference range, 12.0-16.0 g/dL); 63.4% hematocrit (reference range, 37.0%-47.0%); 219 × 103 µL platelets (reference range, 150-450 × 103 µL); 79.3 fL mean corpuscular volume (reference range, 81.0-99.0 fL); 14 mg/dL blood urea nitrogen (reference range, 8-27 mg/dL); 1.18 mg/dL creatinine (reference range, 0.60-1.60 mg/dL); 3 mmol/h erythrocyte sedimentation rate (reference range, 0-30 mmol/h); 88 IU/L alkaline phosphatase (reference range, 34-130 IU/L); and 11.3 mg/dL uric acid (reference range, 2.4-7.9 mg/dL). Hemoglobin electrophoresis studies showed a 49% hemoglobin A1 (reference range, 95%-98%); 3.0% hemoglobin A2 (reference range, 2%-3%); 3.1% hemoglobin F (reference range, < 0.6%); and 44.9% hemoglobin Syracuse (reference range, absent). It was negative for JAK2 V617F mutation. An X-ray of the bilateral feet showed irregularity/erosion involving the medial border of the great toe metatarsal head, joint effusions, and sclerotic margins (Figure 1). A prominent plantar calcaneal spur was present (Figure 2). Synovial fluid analysis detected the presence of negatively birefringent needle-shaped urate crystals.
Per the Clinical Gout Diagnosis tool, which has a sensitivity of 97%, this patient scored high given the findings of greater than one attack of acute arthritis, mono/oligoarthritic attacks, podagra, erythema, probable tophi, and hyperuricemia. This raised the likelihood of his presentation being an acute flare of tophaceous gout.3 He was treated with colchicine and prednisone for acute exacerbation. Once the exacerbation subsided, the colchicine was discontinued, and allopurinol was added. The uric acid goal was < 6 mg/dL and was consistently maintained. Over the subsequent months, he reported mild joint pain if he stopped taking allopurinol but did not report a recurrence in disease exacerbation.
Discussion
Hemoglobin Syracuse was first identified in the early 1970s after the discovery of similar familial hemoglobinopathies unique for their high oxygen affinity hemoglobin.1 High oxygen affinity hemoglobin functions by causing a leftward shift in the hemoglobin dissociation curve and therefore slower off-loading of oxygen into tissues.4 The hypoxic state at the tissue level created by the hemoglobin binding tightly to oxygen promotes the production of erythropoietin, increasing red blood cell and hemoglobin production.5 A study looking at uric acid levels in patients living at high altitudes (which can imitate the low-oxygen state seen in high affinity hemoglobinopathy) theorized that increased erythroblast turnover in the setting of polycythemia involves increased purine metabolism and consequently, uric acid as a breakdown product.6 Uric acid levels have also been used as a marker for hypoxia in studies regarding sleep apnea. Tissue hypoxia can increase adenosine triphosphate breakdown. One byproduct of this breakdown is hypoxanthine, which is further metabolized by xanthine oxidase, which, in turn, produces uric acid.7
The relationship between elevated uric acid and gout was first studied in the mid-nineteenth century after Alfred Barring Garrod identified urate deposits in the articular cartilage of patients with gout.1 These urate deposits garner a proinflammatory response with the activation of the complement cascade, resulting in the recruitment of neutrophils, macrophages, and lymphocytes. Recurrent gout flares eventually result in a chronic granulomatous inflammatory response to the deposited crystals resulting in the classic tophi.8 A study looking at patients with thalassemia showed that while elevated serum uric acid levels were common in these patients, only 6% developed gout. Significant risk factors were noted to be intact spleen and inefficient urinary excretion of urea due to chronic kidney disease.9
Current treatment of gout flares consistsof pain control in the acute phase and prevention in the long-term setting. The first-line treatment for acute gout attack is colchicine, prednisone, or nonsteroidal anti-inflammatory drugs. Clinicians can consider switching or combining these therapies if ineffective or in the event of severe exacerbation. Prophylactic therapy involves urate-lowering agents, such as allopurinol and febuxostat.10
Conclusions
This case illustrates how a rare disorder of high oxygen affinity hemoglobin, SH, can present itself with findings of elevated serum uric acid and tophaceous gout. Most patients with hyperuricemia never develop gout, but having a condition that increases their serum levels of uric acid can increase their chances.11 It is important for clinicians to consider this increased risk when a patient with hemoglobinopathy presents with joint pain.
1. Garrod AB. The Nature and Treatment of Gout and Rheumatic Gout. 2nd ed. Walton and Maberly; 1859.
2. Jensen M, Oski FA, Nathan DG, Bunn HF. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975;55(3):469-477. doi:10.1172/JCI107953
3. Vázquez-Mellado J, Hernández-Cuevas CB, Alvarez-Hernández E, et al. The diagnostic value of the proposal for clinical gout diagnosis (CGD). Clin Rheumatol. 2012;31(3):429-434. doi:10.1007/s10067-011-1873-4
4. Kaufman DP, Kandle PF, Murray IV, et al. Physiology, Oxyhemoglobin Dissociation Curve. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499818/
5. Yudin J, Verhovsek M. How we diagnose and manage altered oxygen affinity hemoglobin variants. Am J Hematol. 2019;94(5):597-603. doi:10.1002/ajh.25425
6. Jefferson JA, Escudero E, Hurtado ME, et al. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis. 2002;39(6):1135-1142. doi:10.1053/ajkd.2002.33380
7. Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PLoS One. 2013;8(6):e66891. Published 2013 Jun 24. doi:10.1371/journal.pone.0066891
8. Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. 2018;77(7):1048-1052. doi:10.1136/annrheumdis-2017-212288
9. Ballou SP, Khan MA, Kushner I, Harris JW. Secondary gout in hemoglobinopathies: report of two cases and review of the literature. Am J Hematol. 1977;2(4):397-402. doi:10.1002/ajh.2830020410
10. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. doi:10.1002/acr.21773
11. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. Published 2019 Sep 26. doi:10.1038/s41572-019-0115-y
1. Garrod AB. The Nature and Treatment of Gout and Rheumatic Gout. 2nd ed. Walton and Maberly; 1859.
2. Jensen M, Oski FA, Nathan DG, Bunn HF. Hemoglobin Syracuse (alpha2beta2-143(H21)His leads to Pro), a new high-affinity variant detected by special electrophoretic methods. Observations on the auto-oxidation of normal and variant hemoglobins. J Clin Invest. 1975;55(3):469-477. doi:10.1172/JCI107953
3. Vázquez-Mellado J, Hernández-Cuevas CB, Alvarez-Hernández E, et al. The diagnostic value of the proposal for clinical gout diagnosis (CGD). Clin Rheumatol. 2012;31(3):429-434. doi:10.1007/s10067-011-1873-4
4. Kaufman DP, Kandle PF, Murray IV, et al. Physiology, Oxyhemoglobin Dissociation Curve. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499818/
5. Yudin J, Verhovsek M. How we diagnose and manage altered oxygen affinity hemoglobin variants. Am J Hematol. 2019;94(5):597-603. doi:10.1002/ajh.25425
6. Jefferson JA, Escudero E, Hurtado ME, et al. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis. 2002;39(6):1135-1142. doi:10.1053/ajkd.2002.33380
7. Hirotsu C, Tufik S, Guindalini C, Mazzotti DR, Bittencourt LR, Andersen ML. Association between uric acid levels and obstructive sleep apnea syndrome in a large epidemiological sample. PLoS One. 2013;8(6):e66891. Published 2013 Jun 24. doi:10.1371/journal.pone.0066891
8. Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. 2018;77(7):1048-1052. doi:10.1136/annrheumdis-2017-212288
9. Ballou SP, Khan MA, Kushner I, Harris JW. Secondary gout in hemoglobinopathies: report of two cases and review of the literature. Am J Hematol. 1977;2(4):397-402. doi:10.1002/ajh.2830020410
10. Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64(10):1447-1461. doi:10.1002/acr.21773
11. Dalbeth N, Choi HK, Joosten LAB, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69. Published 2019 Sep 26. doi:10.1038/s41572-019-0115-y
Are Text Pages an Effective Nudge to Increase Attendance at Internal Medicine Morning Report Conferences? A Cluster Randomized Controlled Trial
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
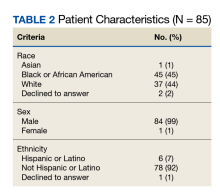
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
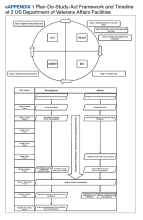
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
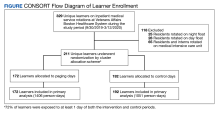
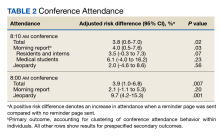
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
Regularly scheduled educational conferences, such as case-based morning reports, have been a standard part of internal medicine residencies for decades.1-4 In addition to better patient care from the knowledge gained at educational conferences, attendance by interns and residents (collectively called house staff) may be associated with higher in-service examination scores.5 Unfortunately, competing priorities, including patient care and trainee supervision, may contribute to an action-intention gap among house staff that reduces attendance.6-8 Low attendance at morning reports represents wasted effort and lost educational opportunities; therefore, strategies to increase attendance are needed. Of several methods studied, more resource-intensive interventions (eg, providing food) were the most successful.6,9-12
Using the behavioral economics framework of nudge strategies, we hypothesized that a less intensive intervention of a daily reminder text page would encourage medical students, interns, and residents (collectively called learners) to attend the morning report conference.8,13 However, given the high cognitive load created by frequent task switching, a reminder text page could disrupt workflow and patient care without promoting the intended behavior change.14-17 Because of this uncertainty, our objective was to determine whether a preconference text page increased learner attendance at morning report conferences.
Methods
This study was a single-center, multiple-crossover cluster randomized controlled trial conducted at the Veteran Affairs Boston Healthcare System (VABHS) in Massachusetts. Study participants included house staff rotating on daytime inpatient rotations from 4 residency programs and students from 2 medical schools. The setting was the morning report, an in-person, interactive, case-based conference held Monday through Thursday, from 8:00
Learners assigned to rotate on the inpatient medicine, cardiology, medicine consultation, and patient safety rotations were eligible to attend these conferences and for inclusion in the study. Learners rotating in the medical intensive care unit, on night float, or on day float (an admitting shift for which residents are not on-site until late afternoon) were excluded. Additional details of the study population are available in the supplement (eAppendix). The study period was originally planned for September 30, 2019, to March 31, 2020, but data collection was stopped on March 12, 2020, due to the COVID-19 pandemic and suspension of in-person conferences. We chose the study period, which determined our sample size, to exclude the first 3 months of the academic year (July-September) because during that time learners acclimate to the inpatient workflow. We also chose not to include the last 3 months of the academic year to provide time for data analysis and preparation of the manuscript within the academic year.
Intervention and Outcome Assessment
Each intervention and control period was 3 weeks long; the first period was randomly determined by coin flip and alternated thereafter. Additional details of randomization are available in the supplement (Appendix 1). During intervention periods, all house staff received a page at 7:55
A daily facesheet (a roster of house staff names and photos) was used to identify learners for conference attendance. This facesheet was already used for other purposes at VABHS. At 8:00
During control periods, no text page reminder of upcoming conferences was sent, but the attendance of total learners at 8:00
Statistical Analysis
The primary outcome was the proportion of eligible learners present at 8:10
To estimate the primary outcome, we modeled the risk difference adjusted for covariates using a generalized estimating equation accounting for the clustering of attendance behavior within individuals and controlling for date and team. Secondary outcomes were estimated similarly. To evaluate the robustness of the primary outcome, we performed a sensitivity analysis using a multilevel generalized linear model with clustering by individual learner and team. Additional details on our statistical analysis plan, including accessing our raw data and analysis code, are available in Appendices 2 and 3. Categorical variables were compared using the χ2 or Fisher exact test. Continuous variables were compared using the t test or Wilcoxon rank-sum tests. All P values were 2-sided, and a significance level of ≤ .05 was considered statistically significant. Analysis was performed in Stata v16.1. Our study was deemed exempt by the VABHS Institutional Review Board, and this article was prepared following the CONSORT reporting guidelines. The trial protocol has been registered with the International Standard Randomized Controlled Trial Number registry
Results
Over the study period, 329 unique learners rotated on inpatient medical services at the VABHS and 211 were eligible to attend 85 morning report conferences and 22 Jeopardy conferences (Figure). Outcomes data were available for 100% of eligible participants. Forty-seven (55%) of the morning report conferences occurred during the intervention period (Table 1).
Morning report attendance observed at 8:10
On-time attendance was lower than at 8:10
To estimate the impact of rotating on teams with lighter clinical workloads on the association between receipt of a reminder page and conference attendance, we repeated our primary analysis with a test of interaction between team assignment and the intervention, which was not significant (P = .90). To estimate the impact of morning workload on the association between receipt of a reminder page and conference attendance, we performed a subgroup analysis limited to learners rotating on teams eligible to receive overnight admissions and included the number of overnight admissions as a covariate in our regression model. A test of interaction between the intervention and the number of overnight admissions on conference attendance was not significant (P = .73).
In a subgroup analysis limited to learners on teams eligible to receive overnight admissions and controlling for the number of overnight admissions (a proxy for morning workload), no significant interaction between the intervention and admissions was observed. We also assessed for interaction between learner type and receipt of a reminder page on conference attendance and found no evidence of such an effect.
Discussion
Among a diverse population of learners from multiple academic institutions rotating at a single, large, urban VA medical center, a nudge strategy of sending a reminder text page before morning report conferences was associated with a 4.0% absolute increase in attendance measured 10 minutes after the conference started compared with not sending a reminder page. Overall, only one-quarter of learners attended the morning report at the start at 8:00
We designed our analysis to overcome several limitations of prior studies on the effect of reminder text pages on conference attendance. First, to account for differences in conference attendance behavior of individual learners, we used a generalized estimating equation model that allowed clustering of outcomes by individual. Second, we controlled for the date to account for secular trends in conference attendance over the academic year. Finally, we controlled for the team to account for the possibility that the conference attendance behavior of one learner on a team influences the behavior of other learners on the same team.
We also evaluated the effect of a reminder page on attendance at a weekly Jeopardy conference. Interestingly, reminder pages seemed to increase on-time Jeopardy attendance, although this effect was no longer statistically significant at 8:10
We also assessed the interaction between sending a reminder page and learner type and its effect on conference attendance and found no evidence to support such an effect. Because medical students do not receive reminder pages, their conference attendance behavior can be thought of as indicative of clustering within teams. Though there was no evidence of a significant interaction, given the small number of students, our study may be underpowered to find a benefit for this group.
The results of this study differ from Smith and colleagues, who found that reminder pages had no overall effect on conference attendance for fellows; however, no sample size justification was provided in that study, making it difficult to evaluate the likelihood of a false-negative finding.7 Our study differs in several ways: the timing of the reminder page (5 minutes vs 30 minutes prior to the conference), the method by which attendance was recorded (by an independent observer vs learner sign-in), and the time that attendance was recorded (2 prespecified times vs continuously). As far as we know, our study is the first to evaluate the nudge effect of reminder text pages on internal medicine resident attendance at conferences, with attendance taken by an observer.
Limitations
This study has some limitations. First, it was conducted at a single VA medical center. An additional limitation was our decision to classify learners who arrived after 8:10
Unfortunately, due to the COVID-19 pandemic and the suspension of in-person conferences, our study ended earlier than anticipated. This resulted in an imbalance of morning report conferences that occurred during each period: 55% during the intervention period, and 45% during the control period. However, because we accounted for the clustering of conference attendance behavior within individuals in our model, this imbalance is unlikely to introduce bias in our estimation of the effect of the intervention.
Another limitation relates to the evolving landscape of educational conferences in the postpandemic era.18 Whether our results can be generalized to increase virtual conference attendance is unknown. Finally, it is not clear whether a 4% absolute increase in conference attendance is educationally meaningful or justifies the effort of sending a reminder page.
Conclusions
In this cluster randomized controlled trial conducted at a single VA medical center, reminder pages sent 5 minutes before the start of morning report conferences resulted in a 4% increase in conference attendance. Our results suggest that reminder pages are one strategy that may result in a small increase in conference attendance, but whether this small increase is educationally significant will vary across training programs applying this strategy.
Acknowledgments
The authors are indebted to Kenneth J. Mukamal and Katharine A. Robb, who provided invaluable guidance in data analysis. Todd Reese assisted in data organization and presentation of data, and Mark Tuttle designed the facesheet. None of these individuals received compensation for their assistance.
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
1. Daniels VJ, Goldstein CE. Changing morning report: an educational intervention to address curricular needs. J Biomed Educ. 2014;2014:1-5. doi:10.1155/2014/830701
2. Parrino TA, Villanueva AG. The principles and practice of morning report. JAMA. 1986;256(6):730-733. doi:10.1001/jama.1986.03380060056025
3. Wenger NS, Shpiner RB. An analysis of morning report: implications for internal medicine education. Ann Intern Med. 1993;119(5):395-399. doi:10.7326/0003-4819-119-5-199309010-00008
4. Ways M, Kroenke K, Umali J, Buchwald D. Morning report. A survey of resident attitudes. Arch Intern Med. 1995;155(13):1433-1437. doi:10.1001/archinte.155.13.1433
5. McDonald FS, Zeger SL, Kolars JC. Associations of conference attendance with internal medicine in-training examination scores. Mayo Clin Proc. 2008;83(4):449-453. doi:10.4065/83.4.449
6. FitzGerald JD, Wenger NS. Didactic teaching conferences for IM residents: who attends, and is attendance related to medical certifying examination scores? Acad Med. 2003;78(1):84-89. doi:10.1097/00001888-200301000-00015
7. Smith J, Zaffiri L, Clary J, Davis T, Bosslet GT. The effect of paging reminders on fellowship conference attendance: a multi-program randomized crossover study. J Grad Med Educ. 2016;8(3):372-377. doi:10.4300/JGME-D-15-00487.1
8. Sheeran P, Webb TL. The intention-behavior gap. Soc Personal Psychol Compass. 2016;10(9):503-518. doi:10.1111/spc3.12265
9. McDonald RJ, Luetmer PH, Kallmes DF. If you starve them, will they still come? Do complementary food provisions affect faculty meeting attendance in academic radiology? J Am Coll Radiol. 2011;8(11):809-810. doi:10.1016/j.jacr.2011.06.003
10. Segovis CM, Mueller PS, Rethlefsen ML, et al. If you feed them, they will come: a prospective study of the effects of complimentary food on attendance and physician attitudes at medical grand rounds at an academic medical center. BMC Med Educ. 2007;7:22. Published 2007 Jul 12. doi:10.1186/1472-6920-7-22
11. Mueller PS, Litin SC, Sowden ML, Habermann TM, LaRusso NF. Strategies for improving attendance at medical grand rounds at an academic medical center. Mayo Clin Proc. 2003;78(5):549-553. doi:10.4065/78.5.549
12. Tarabichi S, DeLeon M, Krumrei N, Hanna J, Maloney Patel N. Competition as a means for improving academic scores and attendance at education conference. J Surg Educ. 2018;75(6):1437-1440. doi:10.1016/j.jsurg.2018.04.020
13. Thaler RH, Sunstein CR. Nudge: Improving Decisions About Health, Wealth, and Happiness. Rev. and Expanded Ed. Penguin Books; 2009.
14. Weijers RJ, de Koning BB, Paas F. Nudging in education: from theory towards guidelines for successful implementation. Eur J Psychol Educ. 2021;36:883-902. Published 2020 Aug 24. doi:10.1007/s10212-020-00495-0
15. Wieland ML, Loertscher LL, Nelson DR, Szostek JH, Ficalora RD. A strategy to reduce interruptions at hospital morning report. J Grad Med Educ. 2010;2(1):83-84. doi:10.4300/JGME-D-09-00084.1
16. Witherspoon L, Nham E, Abdi H, et al. Is it time to rethink how we page physicians? Understanding paging patterns in a tertiary care hospital. BMC Health Serv Res. 2019;19(1):992. Published 2019 Dec 23. doi:10.1186/s12913-019-4844-0
17. Fargen KM, O’Connor T, Raymond S, Sporrer JM, Friedman WA. An observational study of hospital paging practices and workflow interruption among on-call junior neurological surgery residents. J Grad Med Educ. 2012;4(4):467-471. doi:10.4300/JGME-D-11-00306.1
18. Chick RC, Clifton GT, Peace KM, et al. Using technology to maintain the education of residents during the COVID-19 pandemic. J Surg Educ. 2020;77(4):729-732. doi:10.1016/j.jsurg.2020.03.018
Nephrology–Palliative Care Collaboration to Promote Outpatient Hemodialysis Goals of Care Conversations
Estimates of chronic kidney disease (CKD) among veterans range between 34% and 47% higher than in the general population.1 As patients progress to end-stage kidney disease and begin chronic dialysis, they often experience further functional and cognitive decline and a high symptom burden, leading to poor quality of life.2 Clinicians should initiate goals of care conversations (GOCCs) to support high-risk patients on dialysis to ensure that the interventions they receive align with their goals and preferences, since many patients on dialysis prefer measures focused on pain relief and discomfort.3,4 While proactive GOCCs are supported among nephrology associations, few such conversations take place.5,6 In one study, more than half of patients on dialysis stated they had not discussed end-of-life preferences in the past 12 months.4 As a result, patients may not consider the larger implications of receiving dialysis indefinitely as a life-sustaining treatment (LST).
In May 2018, the US Department of Veterans Affairs (VA) National Center for Ethics in Health Care rolled out the Life-Sustaining Treatment Decisions Initiative to proactively engage patients with serious illnesses, such as those with end-stage kidney disease, in GOCCs to clarify their preferences for LSTs.7 After comprehensive training, a preliminary audit at the Edward Hines, Jr. VA Hospital (EHJVAH) in Hines, Illinois, revealed that only 27% of patients on dialysis had LST preferences documented in a standardized LST note.
Nephrologists cite multiple barriers to proactively addressing goals of care with patients with advanced CKD, including clinician discomfort, perceived lack of time, infrastructure, and training.8,9 Similarly, the absence of a multidisciplinary advance care planning approach—specifically bringing together palliative care (PC) clinicians with nephrologists—has been highlighted but not as well studied.10,11
In this quality improvement (QI) project, we aimed to establish a workflow to enhance collaboration between nephrology and PC and to increase the percentage of VA patients on outpatient hemodialysis who engaged in GOCCs, as documented by completion of an LST progress note in the VA’s electronic health record (EHR). We developed a collaboration among PC, nephrology, and social work to improve the rates of documented GOCCs and LST patients on dialysis.
Implementation
EHJVAH is a 1A facility with > 80 patients who receive outpatient hemodialysis on campus. At the time of this collaboration in the fall of 2019, the collaborative dialysis team comprised 2 social workers and a nephrologist. The PC team included a coordinator, 2 nurse practitioners, and 3 physicians. A QI nurse was involved in the initial data gathering for this project.
The PC and nephrology medical directors developed a workflow process that reflected organizational and clinical steps in planning, initiating, and completing GOCCs with patients on outpatient dialysis (Table 1). The proposed process engaged an interdisciplinary PC and nephrology group and was revised to incorporate staff suggestions.
A prospective review of 85 EHJVAH hemodialysis unit patient records was conducted between September 1, 2019, and September 30, 2020 (Table 2). We reviewed LST completion rates for all patients receiving dialysis within this timeframe. During the intervention period, the PC team approached 40 patients without LST notes to engage in GOCCs.
Discussion
Over the 13-month collaboration, LST note completion rates increased from 27% to 81%, with 69 of 85 patients having a documented LST progress note in the EHR. PC approached nearly half of all patients on dialysis. Most patients agreed to be seen by the PC team, with 72% of those approached agreeing to a PC consultation. Previous research has suggested that having a trusted dialysis staff member included in GOCCs contributes to high acceptance rates.12
PC is a relatively uncommon partnership for nephrologists, and PC and hospice services are underutilized in patients on dialysis both nationally and within the VA.13-15 Our outcomes could be replicated, as PC is required at all VA sites.
Conclusions
The innovation of an interdisciplinary nephrology–PC collaboration was an important step in increasing high-quality GOCCs and eliciting patient preferences for LSTs among patients on dialysis. PC integration for patients on dialysis is associated with improved symptom management, fewer aggressive health care measures, and a higher likelihood of dying in one’s preferred setting.16 While this partnership focused on patients already receiving dialysis, successful PC interventions are felt most keenly upstream, before dialysis initiation.
Acknowledgments
The authors acknowledge the contributions of their colleague, Mary McCabe, DNP, Quality Systems Improvement, Edward Hines, Jr. Veterans Affairs Hospital. The authors also acknowledge the clinical dedication of the dialysis social workers, Sarah Adam, LCSW, and Sarah Kraner, LCSW, without which this collaboration would not have been possible.
1. Patel N, Golzy M, Nainani N, et al. Prevalence of various comorbidities among veterans with chronic kidney disease and its comparison with other datasets. Ren Fail. 2016;38(2):204-208. doi:10.3109/0886022X.2015.1117924
2. Weisbord SD, Carmody SS, Bruns FJ, et al. Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant. 2003;18(7):1345-1352. doi:10.1093/ndt/gfg105
3. Wachterman MW, Marcantonio ER, Davis RB, et al. Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med. 2013;173(13):1206-1214. doi:10.1001/jamainternmed.2013.6036
4. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(2):195-204. doi:10.2215/CJN.05960809
5. Williams AW, Dwyer AC, Eddy AA, et al; American Society of Nephrology Quality, and Patient Safety Task Force. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664-1672. doi:10.2215/CJN.04970512
6. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed. Renal Physicians Association; 2010.
7. US Department of Veterans Affairs, National Center for Ethics in Health Care. Goals of care conversations training for nurses, social workers, psychologists, and chaplains. Updated October 9, 2018. Accessed August 31, 2023. https://www.ethics.va.gov/goalsofcaretraining/team.asp
8. Goff SL, Unruh ML, Klingensmith J, et al. Advance care planning with patients on hemodialysis: an implementation study. BMC Palliat Care. 2019;18(1):64. Published 2019 Jul 26. doi:10.1186/s12904-019-0437-2
9. O’Hare AM, Szarka J, McFarland LV, et al. Provider perspectives on advance care planning for patients with kidney disease: whose job is it anyway? Clin J Am Soc Nephrol. 2016;11(5):855-866. doi:10.2215/CJN.11351015
10. Koncicki HM, Schell JO. Communication skills and decision making for elderly patients with advanced kidney disease: a guide for nephrologists. Am J Kidney Dis. 2016;67(4):688-695. doi:10.1053/j.ajkd.2015.09.032
11. Holley JL, Davison SN. Advance care planning for patients with advanced CKD: a need to move forward. Clin J Am Soc Nephrol. 2015;10(3):344-346. doi:10.2215/CJN.00290115
12. Davison SN. Facilitating advance care planning for patients with end-stage renal disease: the patient perspective. Clin J Am Soc Nephrol. 2006;1(5):1023-1028. doi:10.2215/CJN.01050306
13. Murray AM, Arko C, Chen SC, Gilbertson DT, Moss AH. Use of hospice in the United States dialysis population. Clin J Am Soc Nephrol. 2006;1(6):1248-1255. doi:10.2215/CJN.00970306
14. Williams ME, Sandeep J, Catic A. Aging and ESRD demographics: consequences for the practice of dialysis. Semin Dial. 2012;25(6):617-622. doi:10.1111/sdi.12029
15. US Dept of Veterans Affairs. FY 2020 annual report. Palliative and hospice care.
16. Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614. doi:10.1093/ndt/gfq630
17. Fadem SZ, Fadem J. HD mortality predictor. Accessed August 31, 2023. http://touchcalc.com/calculators/sq
18. National Center for Ethics in Health Care. Setting health care goals: a guide for people with hearth problems. Updated June 2016. Accessed August 31, 2023. https://www.ethics.va.gov/docs/GoCC/lst_booklet_for_patients_setting_health_care_goals_final.pdf
Estimates of chronic kidney disease (CKD) among veterans range between 34% and 47% higher than in the general population.1 As patients progress to end-stage kidney disease and begin chronic dialysis, they often experience further functional and cognitive decline and a high symptom burden, leading to poor quality of life.2 Clinicians should initiate goals of care conversations (GOCCs) to support high-risk patients on dialysis to ensure that the interventions they receive align with their goals and preferences, since many patients on dialysis prefer measures focused on pain relief and discomfort.3,4 While proactive GOCCs are supported among nephrology associations, few such conversations take place.5,6 In one study, more than half of patients on dialysis stated they had not discussed end-of-life preferences in the past 12 months.4 As a result, patients may not consider the larger implications of receiving dialysis indefinitely as a life-sustaining treatment (LST).
In May 2018, the US Department of Veterans Affairs (VA) National Center for Ethics in Health Care rolled out the Life-Sustaining Treatment Decisions Initiative to proactively engage patients with serious illnesses, such as those with end-stage kidney disease, in GOCCs to clarify their preferences for LSTs.7 After comprehensive training, a preliminary audit at the Edward Hines, Jr. VA Hospital (EHJVAH) in Hines, Illinois, revealed that only 27% of patients on dialysis had LST preferences documented in a standardized LST note.
Nephrologists cite multiple barriers to proactively addressing goals of care with patients with advanced CKD, including clinician discomfort, perceived lack of time, infrastructure, and training.8,9 Similarly, the absence of a multidisciplinary advance care planning approach—specifically bringing together palliative care (PC) clinicians with nephrologists—has been highlighted but not as well studied.10,11
In this quality improvement (QI) project, we aimed to establish a workflow to enhance collaboration between nephrology and PC and to increase the percentage of VA patients on outpatient hemodialysis who engaged in GOCCs, as documented by completion of an LST progress note in the VA’s electronic health record (EHR). We developed a collaboration among PC, nephrology, and social work to improve the rates of documented GOCCs and LST patients on dialysis.
Implementation
EHJVAH is a 1A facility with > 80 patients who receive outpatient hemodialysis on campus. At the time of this collaboration in the fall of 2019, the collaborative dialysis team comprised 2 social workers and a nephrologist. The PC team included a coordinator, 2 nurse practitioners, and 3 physicians. A QI nurse was involved in the initial data gathering for this project.
The PC and nephrology medical directors developed a workflow process that reflected organizational and clinical steps in planning, initiating, and completing GOCCs with patients on outpatient dialysis (Table 1). The proposed process engaged an interdisciplinary PC and nephrology group and was revised to incorporate staff suggestions.
A prospective review of 85 EHJVAH hemodialysis unit patient records was conducted between September 1, 2019, and September 30, 2020 (Table 2). We reviewed LST completion rates for all patients receiving dialysis within this timeframe. During the intervention period, the PC team approached 40 patients without LST notes to engage in GOCCs.
Discussion
Over the 13-month collaboration, LST note completion rates increased from 27% to 81%, with 69 of 85 patients having a documented LST progress note in the EHR. PC approached nearly half of all patients on dialysis. Most patients agreed to be seen by the PC team, with 72% of those approached agreeing to a PC consultation. Previous research has suggested that having a trusted dialysis staff member included in GOCCs contributes to high acceptance rates.12
PC is a relatively uncommon partnership for nephrologists, and PC and hospice services are underutilized in patients on dialysis both nationally and within the VA.13-15 Our outcomes could be replicated, as PC is required at all VA sites.
Conclusions
The innovation of an interdisciplinary nephrology–PC collaboration was an important step in increasing high-quality GOCCs and eliciting patient preferences for LSTs among patients on dialysis. PC integration for patients on dialysis is associated with improved symptom management, fewer aggressive health care measures, and a higher likelihood of dying in one’s preferred setting.16 While this partnership focused on patients already receiving dialysis, successful PC interventions are felt most keenly upstream, before dialysis initiation.
Acknowledgments
The authors acknowledge the contributions of their colleague, Mary McCabe, DNP, Quality Systems Improvement, Edward Hines, Jr. Veterans Affairs Hospital. The authors also acknowledge the clinical dedication of the dialysis social workers, Sarah Adam, LCSW, and Sarah Kraner, LCSW, without which this collaboration would not have been possible.
Estimates of chronic kidney disease (CKD) among veterans range between 34% and 47% higher than in the general population.1 As patients progress to end-stage kidney disease and begin chronic dialysis, they often experience further functional and cognitive decline and a high symptom burden, leading to poor quality of life.2 Clinicians should initiate goals of care conversations (GOCCs) to support high-risk patients on dialysis to ensure that the interventions they receive align with their goals and preferences, since many patients on dialysis prefer measures focused on pain relief and discomfort.3,4 While proactive GOCCs are supported among nephrology associations, few such conversations take place.5,6 In one study, more than half of patients on dialysis stated they had not discussed end-of-life preferences in the past 12 months.4 As a result, patients may not consider the larger implications of receiving dialysis indefinitely as a life-sustaining treatment (LST).
In May 2018, the US Department of Veterans Affairs (VA) National Center for Ethics in Health Care rolled out the Life-Sustaining Treatment Decisions Initiative to proactively engage patients with serious illnesses, such as those with end-stage kidney disease, in GOCCs to clarify their preferences for LSTs.7 After comprehensive training, a preliminary audit at the Edward Hines, Jr. VA Hospital (EHJVAH) in Hines, Illinois, revealed that only 27% of patients on dialysis had LST preferences documented in a standardized LST note.
Nephrologists cite multiple barriers to proactively addressing goals of care with patients with advanced CKD, including clinician discomfort, perceived lack of time, infrastructure, and training.8,9 Similarly, the absence of a multidisciplinary advance care planning approach—specifically bringing together palliative care (PC) clinicians with nephrologists—has been highlighted but not as well studied.10,11
In this quality improvement (QI) project, we aimed to establish a workflow to enhance collaboration between nephrology and PC and to increase the percentage of VA patients on outpatient hemodialysis who engaged in GOCCs, as documented by completion of an LST progress note in the VA’s electronic health record (EHR). We developed a collaboration among PC, nephrology, and social work to improve the rates of documented GOCCs and LST patients on dialysis.
Implementation
EHJVAH is a 1A facility with > 80 patients who receive outpatient hemodialysis on campus. At the time of this collaboration in the fall of 2019, the collaborative dialysis team comprised 2 social workers and a nephrologist. The PC team included a coordinator, 2 nurse practitioners, and 3 physicians. A QI nurse was involved in the initial data gathering for this project.
The PC and nephrology medical directors developed a workflow process that reflected organizational and clinical steps in planning, initiating, and completing GOCCs with patients on outpatient dialysis (Table 1). The proposed process engaged an interdisciplinary PC and nephrology group and was revised to incorporate staff suggestions.
A prospective review of 85 EHJVAH hemodialysis unit patient records was conducted between September 1, 2019, and September 30, 2020 (Table 2). We reviewed LST completion rates for all patients receiving dialysis within this timeframe. During the intervention period, the PC team approached 40 patients without LST notes to engage in GOCCs.
Discussion
Over the 13-month collaboration, LST note completion rates increased from 27% to 81%, with 69 of 85 patients having a documented LST progress note in the EHR. PC approached nearly half of all patients on dialysis. Most patients agreed to be seen by the PC team, with 72% of those approached agreeing to a PC consultation. Previous research has suggested that having a trusted dialysis staff member included in GOCCs contributes to high acceptance rates.12
PC is a relatively uncommon partnership for nephrologists, and PC and hospice services are underutilized in patients on dialysis both nationally and within the VA.13-15 Our outcomes could be replicated, as PC is required at all VA sites.
Conclusions
The innovation of an interdisciplinary nephrology–PC collaboration was an important step in increasing high-quality GOCCs and eliciting patient preferences for LSTs among patients on dialysis. PC integration for patients on dialysis is associated with improved symptom management, fewer aggressive health care measures, and a higher likelihood of dying in one’s preferred setting.16 While this partnership focused on patients already receiving dialysis, successful PC interventions are felt most keenly upstream, before dialysis initiation.
Acknowledgments
The authors acknowledge the contributions of their colleague, Mary McCabe, DNP, Quality Systems Improvement, Edward Hines, Jr. Veterans Affairs Hospital. The authors also acknowledge the clinical dedication of the dialysis social workers, Sarah Adam, LCSW, and Sarah Kraner, LCSW, without which this collaboration would not have been possible.
1. Patel N, Golzy M, Nainani N, et al. Prevalence of various comorbidities among veterans with chronic kidney disease and its comparison with other datasets. Ren Fail. 2016;38(2):204-208. doi:10.3109/0886022X.2015.1117924
2. Weisbord SD, Carmody SS, Bruns FJ, et al. Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant. 2003;18(7):1345-1352. doi:10.1093/ndt/gfg105
3. Wachterman MW, Marcantonio ER, Davis RB, et al. Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med. 2013;173(13):1206-1214. doi:10.1001/jamainternmed.2013.6036
4. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(2):195-204. doi:10.2215/CJN.05960809
5. Williams AW, Dwyer AC, Eddy AA, et al; American Society of Nephrology Quality, and Patient Safety Task Force. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664-1672. doi:10.2215/CJN.04970512
6. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed. Renal Physicians Association; 2010.
7. US Department of Veterans Affairs, National Center for Ethics in Health Care. Goals of care conversations training for nurses, social workers, psychologists, and chaplains. Updated October 9, 2018. Accessed August 31, 2023. https://www.ethics.va.gov/goalsofcaretraining/team.asp
8. Goff SL, Unruh ML, Klingensmith J, et al. Advance care planning with patients on hemodialysis: an implementation study. BMC Palliat Care. 2019;18(1):64. Published 2019 Jul 26. doi:10.1186/s12904-019-0437-2
9. O’Hare AM, Szarka J, McFarland LV, et al. Provider perspectives on advance care planning for patients with kidney disease: whose job is it anyway? Clin J Am Soc Nephrol. 2016;11(5):855-866. doi:10.2215/CJN.11351015
10. Koncicki HM, Schell JO. Communication skills and decision making for elderly patients with advanced kidney disease: a guide for nephrologists. Am J Kidney Dis. 2016;67(4):688-695. doi:10.1053/j.ajkd.2015.09.032
11. Holley JL, Davison SN. Advance care planning for patients with advanced CKD: a need to move forward. Clin J Am Soc Nephrol. 2015;10(3):344-346. doi:10.2215/CJN.00290115
12. Davison SN. Facilitating advance care planning for patients with end-stage renal disease: the patient perspective. Clin J Am Soc Nephrol. 2006;1(5):1023-1028. doi:10.2215/CJN.01050306
13. Murray AM, Arko C, Chen SC, Gilbertson DT, Moss AH. Use of hospice in the United States dialysis population. Clin J Am Soc Nephrol. 2006;1(6):1248-1255. doi:10.2215/CJN.00970306
14. Williams ME, Sandeep J, Catic A. Aging and ESRD demographics: consequences for the practice of dialysis. Semin Dial. 2012;25(6):617-622. doi:10.1111/sdi.12029
15. US Dept of Veterans Affairs. FY 2020 annual report. Palliative and hospice care.
16. Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614. doi:10.1093/ndt/gfq630
17. Fadem SZ, Fadem J. HD mortality predictor. Accessed August 31, 2023. http://touchcalc.com/calculators/sq
18. National Center for Ethics in Health Care. Setting health care goals: a guide for people with hearth problems. Updated June 2016. Accessed August 31, 2023. https://www.ethics.va.gov/docs/GoCC/lst_booklet_for_patients_setting_health_care_goals_final.pdf
1. Patel N, Golzy M, Nainani N, et al. Prevalence of various comorbidities among veterans with chronic kidney disease and its comparison with other datasets. Ren Fail. 2016;38(2):204-208. doi:10.3109/0886022X.2015.1117924
2. Weisbord SD, Carmody SS, Bruns FJ, et al. Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant. 2003;18(7):1345-1352. doi:10.1093/ndt/gfg105
3. Wachterman MW, Marcantonio ER, Davis RB, et al. Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med. 2013;173(13):1206-1214. doi:10.1001/jamainternmed.2013.6036
4. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(2):195-204. doi:10.2215/CJN.05960809
5. Williams AW, Dwyer AC, Eddy AA, et al; American Society of Nephrology Quality, and Patient Safety Task Force. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664-1672. doi:10.2215/CJN.04970512
6. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed. Renal Physicians Association; 2010.
7. US Department of Veterans Affairs, National Center for Ethics in Health Care. Goals of care conversations training for nurses, social workers, psychologists, and chaplains. Updated October 9, 2018. Accessed August 31, 2023. https://www.ethics.va.gov/goalsofcaretraining/team.asp
8. Goff SL, Unruh ML, Klingensmith J, et al. Advance care planning with patients on hemodialysis: an implementation study. BMC Palliat Care. 2019;18(1):64. Published 2019 Jul 26. doi:10.1186/s12904-019-0437-2
9. O’Hare AM, Szarka J, McFarland LV, et al. Provider perspectives on advance care planning for patients with kidney disease: whose job is it anyway? Clin J Am Soc Nephrol. 2016;11(5):855-866. doi:10.2215/CJN.11351015
10. Koncicki HM, Schell JO. Communication skills and decision making for elderly patients with advanced kidney disease: a guide for nephrologists. Am J Kidney Dis. 2016;67(4):688-695. doi:10.1053/j.ajkd.2015.09.032
11. Holley JL, Davison SN. Advance care planning for patients with advanced CKD: a need to move forward. Clin J Am Soc Nephrol. 2015;10(3):344-346. doi:10.2215/CJN.00290115
12. Davison SN. Facilitating advance care planning for patients with end-stage renal disease: the patient perspective. Clin J Am Soc Nephrol. 2006;1(5):1023-1028. doi:10.2215/CJN.01050306
13. Murray AM, Arko C, Chen SC, Gilbertson DT, Moss AH. Use of hospice in the United States dialysis population. Clin J Am Soc Nephrol. 2006;1(6):1248-1255. doi:10.2215/CJN.00970306
14. Williams ME, Sandeep J, Catic A. Aging and ESRD demographics: consequences for the practice of dialysis. Semin Dial. 2012;25(6):617-622. doi:10.1111/sdi.12029
15. US Dept of Veterans Affairs. FY 2020 annual report. Palliative and hospice care.
16. Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614. doi:10.1093/ndt/gfq630
17. Fadem SZ, Fadem J. HD mortality predictor. Accessed August 31, 2023. http://touchcalc.com/calculators/sq
18. National Center for Ethics in Health Care. Setting health care goals: a guide for people with hearth problems. Updated June 2016. Accessed August 31, 2023. https://www.ethics.va.gov/docs/GoCC/lst_booklet_for_patients_setting_health_care_goals_final.pdf
Shifting Culture Toward Age-Friendly Care: Lessons From VHA Early Adopters
Nearly 50% of living US veterans are aged ≥ 65 years compared with 18.3% of the general population.1,2 The Veterans Health Administration (VHA), the largest integrated health care system in the US, has a vested interest in improving the quality and effectiveness of care for older veterans.3
Health care systems are often unprepared to care for the complex needs of older adults. There are roughly 7300 certified geriatricians practicing in the US, and about 250 new geriatricians are trained each year while the American Geriatrics Society expects > 12,000 geriatricians will be required by 2030.4,5 More geriatricians are needed to serve as the primary health care professionals (HCPs) for older adults.4,6 Health care systems like the VHA must find ways to increase geriatrics skills, knowledge, and practices among their entire health care workforce. A culture shift toward age-friendly care for older adults across care settings and inclusive of all HCPs may help meet this escalating workforce need.7
The Age-Friendly Health System (AFHS) is an initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement (IHI) in partnership with the American Hospital Association and the Catholic Health Association of the United States.8,9 AFHS uses a what matters, medication, mentation, and mobility (4Ms) framework to ensure reliable, evidence-based care for older adults (Table 1).10,11 In an AFHS, the 4Ms are integrated into every discipline and care setting for older adults.11 The 4Ms neither replace formal training in geriatrics nor create the level of expertise needed for geriatrics teachers, researchers, and program leaders. However, the systematic approach of AFHS to assess and act on each of the 4Ms offers one solution to expand geriatrics skills and knowledge beyond geriatric care settings in all disciplines by engaging each HCP to meet the needs of older adults.12 To act on what matters, HCPs need to align the care plan with what is important to the older adult.
Hospitals and health care systems are encouraged to begin implementing the 4Ms in ≥ 1 care setting.13 Care settings may get started on a do-it-yourself track or by joining an IHI Action Community, which provides a series of webinars to help adopt the 4Ms over 7 months.14 By creating a plan for how each M will be assessed, documented, and acted on, care settings may earn level 1 recognition from the IHI.14 As of July 2023, there are at least 3100 AFHS participants and > 1900 have achieved level 2 recognition, which requires 3 months of clinical data to demonstrate the impact of the 4Ms.13,14
The main cultural shift of the AFHS movement is to focus on what matters to older adults by prioritizing each older adult’s personal health goals and care preferences across all care settings.9,11 Medication addresses age-appropropriate prescribing, making dose adjustments, if needed, and avoiding/deprescribing high-risk medications that may interfere with what matters, mentation, or mobility. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults is often used as a guide and includes lists of medications that are potentially harmful for older adults.11 Mentation focuses on preventing, identifying, treating, and managing dementia, depression, and delirium across care settings. Mobility includes assisting or encouraging older adults to move safely every day to maintain functional ability and do what matters.15,16 Each of the 4Ms has the potential to improve health outcomes for older adults, reduce waste from low-quality services, and increase the use of cost-effective services.11,17
In March 2020, the VHA Office of Geriatrics and Extended Care (GEC) set the goal for the VHA to be recognized by the IHI as an AFHS.18,19 US Department of Veterans Affairs (VA) facilities that joined the AFHS movement in 2020 are considered early adopters. We describe early adopter AFHS implementation at Birmingham VA Health Care System (BVAHCS) hospital, geriatrics assessment clinic (GAC), and Home Based Primary Care (HBPC) and at the Atlanta VA Medical Center (AVAMC) HBPC.
Implementing 4Ms Care
The IHI identifies 6 steps in the Plan-Do-Study-Act cycle to reliably practice the 4Ms. eAppendix 1 provides a side-by-side comparison of the steps over a 9-month timeline independently taken by BVAHCS and AVAMC to achieve both levels of AFHS recognition.
Step 1: Understand the Current State
In March 2020 the BVAHCS enrolled in the IHI Action Community. Three BVAHCS care settings were identified for the Action Community: the inpatient hospital, GAC (an outpatient clinic), and HBPC. The AVAMC HBPC enrolled in the IHI Action Community in March 2021.
Before joining the AFHS movement, the BVAHCS implemented a hospital-wide delirium standard operating procedure (SOP) whereby every veteran admitted to the 313-bed hospital is screened for delirium risk, with positive screens linked to nursing-led interventions. Nursing leadership supported AFHS due to its recognized value and an exemplary process in place to assess mentation/delirium and background understanding for screening and acting on medication, mobility, and what matters most to the veteran. The BVAHCS GAC, which was led by a single geriatrician, integrated the 4Ms into all geriatrics assessment appointments.
For the BVAHCS HBPC, the 4Ms supported key performance measures, such as fall prevention, patient satisfaction, decreasing medication errors, and identification of cognition and mood disorders. For the AVAMC HBPC, joining the AFHS movement represented an opportunity to improve performance measures, interdisciplinary teamwork, and care coordination for patients. For both HBPC sites, the shift to virtual meeting modalities due to the COVID-19 pandemic enabled HBPC team members to garner support for AFHS and collectively develop a 4Ms plan.
Step 2: Describe 4Ms Care
In March 2020 as guided by the Action Community, BVAHCS created a plan for each of its 3 care settings that described assessment tools, frequency, documentation, and responsible team members. All BVAHCS care settings achieved level 1 recognition in April 2020. Of the approximately 300 veterans served by the AVAMC HBPC, 83% are aged > 65 years. They achieved level 1 recognition in August 2021.
Step 3: Design and Adapt Workflows
From April to August 2020, BVAHCS implemented its 4Ms plans. In the hospital, a 4Ms overview was provided with education on the delirium SOP at nursing meetings. Updates were requested to the electronic health record (EHR) templates for the GAC to streamline documentation. For the BVAHCS HBPC, 4Ms assessments were added to the EHR quarterly care plan template, which was updated by all team members (Table 2).
From April through June 2021, the AVAMC HBPC formed teams led by 4Ms champions: what matters was led by a nurse care manager, medication by a nurse practitioner and pharmacist, mentation by a social worker, and mobility by a physical therapist. The champions initially focused on a plan for each M, incorporating all 4Ms as a set for optimal effectiveness into their quarterly care plan meeting using what matters to drive the entire care plan.
Step 4: Provide Care
Each of the 4Ms was to be assessed, documented, and acted on for each veteran within a short period, such as a hospitalization or 1 or 2 outpatient visits. BVAHCS implemented 4Ms care in each care setting from August to October 2020. The AVAMC HBPC implemented 4Ms from July to September 2021.
Step 5: Study Performance
The IHI identifies 3 methods for measuring older adults who receive 4Ms care: real-time observation, chart review, or EHR report. For chart review, the IHI recommends using a random sample to calculate the number of patients who received 4Ms in 1 month, which provides evidence of progress toward reliable practice.
Both facilities used chart review with random sampling. Each setting estimated the number of veterans receiving 4Ms care by multiplying the percentage of sampled charts with documented 4Ms care by unique patient encounters (eAppendix 2).
From August through October 2020, BVAHCS sites reached an estimated 97% of older veterans with complete 4Ms care: hospital, 100%; GAC, 90%; and HBPC, 85%. AVAMC HBPC increased 4Ms care from 52% to 100% between July and September 2021. Both teams demonstrated the feasibility of reliably providing 4Ms care to > 85% of older veterans in these care settings and earned level 2 recognition. Through satisfaction surveys and informal feedback, notable positive changes were evident to veterans, their families, and the VA staff providing 4Ms age-friendly care.
Step 6: Improve and Sustain Care
Each site acknowledged barriers and facilitators for adopting the 4Ms. The COVID-19 pandemic was an ongoing barrier for both sites, with teams transitioning to virtual modalities for telehealth visits and team meetings, and higher staff turnover. However, the greater use of technology facilitated 4Ms adoption by allowing physically distant team members to collaborate.
One of the largest barriers was the lack of 4Ms documentation in the EHR, which could not be implemented in the BVAHCS inpatient hospital due to existing standardized nursing templates. Both sites recognized that 4Ms documentation in the EHR for all care settings would facilitate achieving level 2 recognition and tracking and reporting 4Ms care in the future.
Discussion
The AFHS 4Ms approach offers a method to impart geriatrics knowledge, skills, and practice throughout an entire health care system in a short time. The AFHS framework provides a structured pathway to the often daunting challenge of care for complex, multimorbid, and highly heterogeneous older adults. The 4Ms approach promotes the provision of evidence-based care that is reliable, efficient, patient centered, and avoids unwanted care: worthy goals not only for geriatrics but for all members of a high-reliability organization.
Through the implementation of the 4Ms framework, consistent use of AFHS practices, measurement, and feedback, the staff in each VA care setting reported here reached a level of reliability in which at least 85% of patients had all 4Ms addressed. Notably, adoption was strong and improvements in reliably addressing all 4Ms were observed in both geriatrics (HBPC and outpatient clinics) and nongeriatrics (inpatient medicine) settings. Although one might expect that high-functioning interdisciplinary teams in geriatrics-focused VA settings were routinely addressing all 4Ms for most of their patients, our experience was consistent with prior teams indicating that this is often not the case. Although many of these teams were addressing some of the 4Ms in their usual practice, the 4Ms framework facilitated addressing all 4Ms as a set with input from all team members. Most importantly, it fostered a culture of asking the older adult what matters most and documenting, sharing, and aligning this with the care plan. Within 6 months, all VA care settings achieved level 1 recognition, and within 9 months, all achieved level 2 recognition.
Lessons Learned
Key lessons learned include the importance of identifying, preparing, and supporting a champion to lead this effort; garnering facility and system leadership support at the outset; and integration with the EHR for reliable and efficient data capture, reporting, and feedback. Preparing and supporting champions was achieved through national and individual calls and peer support. Guidance was provided on garnering leadership support, including local needs assessment and data analysis, meeting with leadership to first understand their key challenges and priorities and provide information on the AFHS movement, requesting a follow-up meeting to discuss local needs and data, and exploring how an AFHS might help address one or more of their priorities.
In September 2022, an AFHS 4Ms note template was introduced into the EHR for all VA sites for data capture and reporting, to standardize and facilitate documentation across all age-friendly VA sites, and decrease the reporting burden for staff. This effort is critically important: The ability to document, track, and analyze 4Ms measures, provide feedback, and synergize efforts across systems is vital to design studies to determine whether the AFHS 4Ms approach to care achieves substantive improvements in patient care across settings.
Limitations
Limitations of this analysis include the small sample of care settings, which did not include a skilled nursing or long-term care facility, nor general primary care. Although the short timeframe assessed did not allow us to report on the anticipated clinical outcomes of 4Ms care, it does set up a foundation for evaluation of the 4Ms and EHR integration and dashboard development.
Conclusions
The VHA provides a comprehensive spectrum of geriatrics services and innovative models of care that often serve as exemplars to other health care systems. Implementing the AFHS framework to assess and act on the 4Ms provides a structure for confronting the HCP shortage with geriatrics expertise by infusing geriatrics knowledge, skills, and practices throughout all care settings and disciplines. Enhancing patient-centered care to older veterans through AFHS implementation exemplifies the VHA as a learning health care system.
Acknowledgments
We thank the Veterans Health Administration Office of Geriatrics and Extended Care and the clinical staff from the Atlanta Veterans Affairs Healthcare System and the Birmingham Veterans Affairs Health Care System for assisting us in this work.
1. US Census Bureau. Older Americans month: May 2023. Accessed September 11, 2023. https://www.census.gov/newsroom/stories/older-americans-month.html
2. Vespa J. Aging veterans: America’s veteran population in later life. July 2023. Accessed September 11, 2023. https://www.census.gov/content/dam/Census/library/publications/2023/acs/acs-54.pdf
3. O’Hanlon C, Huang C, Sloss E, et al. Comparing VA and non-VA quality of care: a systematic review. J Gen Intern Med. 2017;32(1):105-121. doi:10.1007/s11606-016-3775-2
4. Fulmer T, Reuben DB, Auerbach J, Fick DM, Galambos C, Johnson KS. Actualizing better health and health care for older adults: commentary describes six vital directions to improve the care and quality of life for all older Americans. Health Aff (Millwood). 2021;40(2):219-225. doi:10.1377/hlthaff.2020.01470
5. ChenMed. The physician shortage in geriatrics. March 18, 2022. Accessed September 6, 2023. https://www.chenmed.com/blog/physician-shortage-geriatrics
6. American Geriatrics Society. Projected future need for geriatricians. Updated May 2016. Accessed September 6, 2023. https://www.americangeriatrics.org/sites/default/files/inline-files/Projected-Future-Need-for-Geriatricians.pdf 7. Carmody J, Black K, Bonner A, Wolfe M, Fulmer T. Advancing gerontological nursing at the intersection of age-friendly communities, health systems, and public health. J Gerontol Nurs. 2021;47(3):13-17. doi:10.3928/00989134-20210125-01
8. Lesser S, Zakharkin S, Louie C, Escobedo MR, Whyte J, Fulmer T. Clinician knowledge and behaviors related to the 4Ms framework of Age‐Friendly Health Systems. J Am Geriatr Soc. 2022;70(3):789-800. doi:10.1111/jgs.17571
9. Edelman LS, Drost J, Moone RP, et al. Applying the Age-Friendly Health System framework to long term care settings. J Nutr Health Aging. 2021;25(2):141-145. doi:10.1007/s12603-020-1558-2
10. Emery-Tiburcio EE, Mack L, Zonsius MC, Carbonell E, Newman M. The 4Ms of an Age-Friendly Health System: an evidence-based framework to ensure older adults receive the highest quality care. Home Healthc Now. 2022;40(5):252-257. doi:10.1097/NHH.0000000000001113
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
12. Mate KS, Berman A, Laderman M, Kabcenell A, Fulmer T. Creating age-friendly health systems – a vision for better care of older adults. Healthc (Amst). 2018;6(1):4-6. doi:10.1016/j.hjdsi.2017.05.005
13. Institute for Healthcare Improvement. What is an Age-Friendly Health System? Accessed September 6, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/default.aspx
14. Institute for Healthcare Improvement. Health systems recognized by IHI. Updated September 2023. Accessed September 6, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/recognized-systems.aspx
15. Burke RE, Ashcraft LE, Manges K, et al. What matters when it comes to measuring Age‐Friendly Health System transformation. J Am Geriatr Soc. 2022;70(10):2775-2785. doi:10.1111/jgs.18002
16. Wang J, Shen JY, Conwell Y, et al. How “age-friendly” are deprescribing interventions? A scoping review of deprescribing trials. Health Serv Res. 202;58(suppl 1):123-138. doi:10.1111/1475-6773.14083
17. Pohnert AM, Schiltz NK, Pino L, et al. Achievement of age‐friendly health systems committed to care excellence designation in a convenient care health care system. Health Serv Res. 2023;58 (suppl 1):89-99. doi:10.1111/1475-6773.14071
18. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2022;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
19. Farrell TW, Volden TA, Butler JM, et al. Age‐friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. doi:10.1111/jgs.18070
Nearly 50% of living US veterans are aged ≥ 65 years compared with 18.3% of the general population.1,2 The Veterans Health Administration (VHA), the largest integrated health care system in the US, has a vested interest in improving the quality and effectiveness of care for older veterans.3
Health care systems are often unprepared to care for the complex needs of older adults. There are roughly 7300 certified geriatricians practicing in the US, and about 250 new geriatricians are trained each year while the American Geriatrics Society expects > 12,000 geriatricians will be required by 2030.4,5 More geriatricians are needed to serve as the primary health care professionals (HCPs) for older adults.4,6 Health care systems like the VHA must find ways to increase geriatrics skills, knowledge, and practices among their entire health care workforce. A culture shift toward age-friendly care for older adults across care settings and inclusive of all HCPs may help meet this escalating workforce need.7
The Age-Friendly Health System (AFHS) is an initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement (IHI) in partnership with the American Hospital Association and the Catholic Health Association of the United States.8,9 AFHS uses a what matters, medication, mentation, and mobility (4Ms) framework to ensure reliable, evidence-based care for older adults (Table 1).10,11 In an AFHS, the 4Ms are integrated into every discipline and care setting for older adults.11 The 4Ms neither replace formal training in geriatrics nor create the level of expertise needed for geriatrics teachers, researchers, and program leaders. However, the systematic approach of AFHS to assess and act on each of the 4Ms offers one solution to expand geriatrics skills and knowledge beyond geriatric care settings in all disciplines by engaging each HCP to meet the needs of older adults.12 To act on what matters, HCPs need to align the care plan with what is important to the older adult.
Hospitals and health care systems are encouraged to begin implementing the 4Ms in ≥ 1 care setting.13 Care settings may get started on a do-it-yourself track or by joining an IHI Action Community, which provides a series of webinars to help adopt the 4Ms over 7 months.14 By creating a plan for how each M will be assessed, documented, and acted on, care settings may earn level 1 recognition from the IHI.14 As of July 2023, there are at least 3100 AFHS participants and > 1900 have achieved level 2 recognition, which requires 3 months of clinical data to demonstrate the impact of the 4Ms.13,14
The main cultural shift of the AFHS movement is to focus on what matters to older adults by prioritizing each older adult’s personal health goals and care preferences across all care settings.9,11 Medication addresses age-appropropriate prescribing, making dose adjustments, if needed, and avoiding/deprescribing high-risk medications that may interfere with what matters, mentation, or mobility. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults is often used as a guide and includes lists of medications that are potentially harmful for older adults.11 Mentation focuses on preventing, identifying, treating, and managing dementia, depression, and delirium across care settings. Mobility includes assisting or encouraging older adults to move safely every day to maintain functional ability and do what matters.15,16 Each of the 4Ms has the potential to improve health outcomes for older adults, reduce waste from low-quality services, and increase the use of cost-effective services.11,17
In March 2020, the VHA Office of Geriatrics and Extended Care (GEC) set the goal for the VHA to be recognized by the IHI as an AFHS.18,19 US Department of Veterans Affairs (VA) facilities that joined the AFHS movement in 2020 are considered early adopters. We describe early adopter AFHS implementation at Birmingham VA Health Care System (BVAHCS) hospital, geriatrics assessment clinic (GAC), and Home Based Primary Care (HBPC) and at the Atlanta VA Medical Center (AVAMC) HBPC.
Implementing 4Ms Care
The IHI identifies 6 steps in the Plan-Do-Study-Act cycle to reliably practice the 4Ms. eAppendix 1 provides a side-by-side comparison of the steps over a 9-month timeline independently taken by BVAHCS and AVAMC to achieve both levels of AFHS recognition.
Step 1: Understand the Current State
In March 2020 the BVAHCS enrolled in the IHI Action Community. Three BVAHCS care settings were identified for the Action Community: the inpatient hospital, GAC (an outpatient clinic), and HBPC. The AVAMC HBPC enrolled in the IHI Action Community in March 2021.
Before joining the AFHS movement, the BVAHCS implemented a hospital-wide delirium standard operating procedure (SOP) whereby every veteran admitted to the 313-bed hospital is screened for delirium risk, with positive screens linked to nursing-led interventions. Nursing leadership supported AFHS due to its recognized value and an exemplary process in place to assess mentation/delirium and background understanding for screening and acting on medication, mobility, and what matters most to the veteran. The BVAHCS GAC, which was led by a single geriatrician, integrated the 4Ms into all geriatrics assessment appointments.
For the BVAHCS HBPC, the 4Ms supported key performance measures, such as fall prevention, patient satisfaction, decreasing medication errors, and identification of cognition and mood disorders. For the AVAMC HBPC, joining the AFHS movement represented an opportunity to improve performance measures, interdisciplinary teamwork, and care coordination for patients. For both HBPC sites, the shift to virtual meeting modalities due to the COVID-19 pandemic enabled HBPC team members to garner support for AFHS and collectively develop a 4Ms plan.
Step 2: Describe 4Ms Care
In March 2020 as guided by the Action Community, BVAHCS created a plan for each of its 3 care settings that described assessment tools, frequency, documentation, and responsible team members. All BVAHCS care settings achieved level 1 recognition in April 2020. Of the approximately 300 veterans served by the AVAMC HBPC, 83% are aged > 65 years. They achieved level 1 recognition in August 2021.
Step 3: Design and Adapt Workflows
From April to August 2020, BVAHCS implemented its 4Ms plans. In the hospital, a 4Ms overview was provided with education on the delirium SOP at nursing meetings. Updates were requested to the electronic health record (EHR) templates for the GAC to streamline documentation. For the BVAHCS HBPC, 4Ms assessments were added to the EHR quarterly care plan template, which was updated by all team members (Table 2).
From April through June 2021, the AVAMC HBPC formed teams led by 4Ms champions: what matters was led by a nurse care manager, medication by a nurse practitioner and pharmacist, mentation by a social worker, and mobility by a physical therapist. The champions initially focused on a plan for each M, incorporating all 4Ms as a set for optimal effectiveness into their quarterly care plan meeting using what matters to drive the entire care plan.
Step 4: Provide Care
Each of the 4Ms was to be assessed, documented, and acted on for each veteran within a short period, such as a hospitalization or 1 or 2 outpatient visits. BVAHCS implemented 4Ms care in each care setting from August to October 2020. The AVAMC HBPC implemented 4Ms from July to September 2021.
Step 5: Study Performance
The IHI identifies 3 methods for measuring older adults who receive 4Ms care: real-time observation, chart review, or EHR report. For chart review, the IHI recommends using a random sample to calculate the number of patients who received 4Ms in 1 month, which provides evidence of progress toward reliable practice.
Both facilities used chart review with random sampling. Each setting estimated the number of veterans receiving 4Ms care by multiplying the percentage of sampled charts with documented 4Ms care by unique patient encounters (eAppendix 2).
From August through October 2020, BVAHCS sites reached an estimated 97% of older veterans with complete 4Ms care: hospital, 100%; GAC, 90%; and HBPC, 85%. AVAMC HBPC increased 4Ms care from 52% to 100% between July and September 2021. Both teams demonstrated the feasibility of reliably providing 4Ms care to > 85% of older veterans in these care settings and earned level 2 recognition. Through satisfaction surveys and informal feedback, notable positive changes were evident to veterans, their families, and the VA staff providing 4Ms age-friendly care.
Step 6: Improve and Sustain Care
Each site acknowledged barriers and facilitators for adopting the 4Ms. The COVID-19 pandemic was an ongoing barrier for both sites, with teams transitioning to virtual modalities for telehealth visits and team meetings, and higher staff turnover. However, the greater use of technology facilitated 4Ms adoption by allowing physically distant team members to collaborate.
One of the largest barriers was the lack of 4Ms documentation in the EHR, which could not be implemented in the BVAHCS inpatient hospital due to existing standardized nursing templates. Both sites recognized that 4Ms documentation in the EHR for all care settings would facilitate achieving level 2 recognition and tracking and reporting 4Ms care in the future.
Discussion
The AFHS 4Ms approach offers a method to impart geriatrics knowledge, skills, and practice throughout an entire health care system in a short time. The AFHS framework provides a structured pathway to the often daunting challenge of care for complex, multimorbid, and highly heterogeneous older adults. The 4Ms approach promotes the provision of evidence-based care that is reliable, efficient, patient centered, and avoids unwanted care: worthy goals not only for geriatrics but for all members of a high-reliability organization.
Through the implementation of the 4Ms framework, consistent use of AFHS practices, measurement, and feedback, the staff in each VA care setting reported here reached a level of reliability in which at least 85% of patients had all 4Ms addressed. Notably, adoption was strong and improvements in reliably addressing all 4Ms were observed in both geriatrics (HBPC and outpatient clinics) and nongeriatrics (inpatient medicine) settings. Although one might expect that high-functioning interdisciplinary teams in geriatrics-focused VA settings were routinely addressing all 4Ms for most of their patients, our experience was consistent with prior teams indicating that this is often not the case. Although many of these teams were addressing some of the 4Ms in their usual practice, the 4Ms framework facilitated addressing all 4Ms as a set with input from all team members. Most importantly, it fostered a culture of asking the older adult what matters most and documenting, sharing, and aligning this with the care plan. Within 6 months, all VA care settings achieved level 1 recognition, and within 9 months, all achieved level 2 recognition.
Lessons Learned
Key lessons learned include the importance of identifying, preparing, and supporting a champion to lead this effort; garnering facility and system leadership support at the outset; and integration with the EHR for reliable and efficient data capture, reporting, and feedback. Preparing and supporting champions was achieved through national and individual calls and peer support. Guidance was provided on garnering leadership support, including local needs assessment and data analysis, meeting with leadership to first understand their key challenges and priorities and provide information on the AFHS movement, requesting a follow-up meeting to discuss local needs and data, and exploring how an AFHS might help address one or more of their priorities.
In September 2022, an AFHS 4Ms note template was introduced into the EHR for all VA sites for data capture and reporting, to standardize and facilitate documentation across all age-friendly VA sites, and decrease the reporting burden for staff. This effort is critically important: The ability to document, track, and analyze 4Ms measures, provide feedback, and synergize efforts across systems is vital to design studies to determine whether the AFHS 4Ms approach to care achieves substantive improvements in patient care across settings.
Limitations
Limitations of this analysis include the small sample of care settings, which did not include a skilled nursing or long-term care facility, nor general primary care. Although the short timeframe assessed did not allow us to report on the anticipated clinical outcomes of 4Ms care, it does set up a foundation for evaluation of the 4Ms and EHR integration and dashboard development.
Conclusions
The VHA provides a comprehensive spectrum of geriatrics services and innovative models of care that often serve as exemplars to other health care systems. Implementing the AFHS framework to assess and act on the 4Ms provides a structure for confronting the HCP shortage with geriatrics expertise by infusing geriatrics knowledge, skills, and practices throughout all care settings and disciplines. Enhancing patient-centered care to older veterans through AFHS implementation exemplifies the VHA as a learning health care system.
Acknowledgments
We thank the Veterans Health Administration Office of Geriatrics and Extended Care and the clinical staff from the Atlanta Veterans Affairs Healthcare System and the Birmingham Veterans Affairs Health Care System for assisting us in this work.
Nearly 50% of living US veterans are aged ≥ 65 years compared with 18.3% of the general population.1,2 The Veterans Health Administration (VHA), the largest integrated health care system in the US, has a vested interest in improving the quality and effectiveness of care for older veterans.3
Health care systems are often unprepared to care for the complex needs of older adults. There are roughly 7300 certified geriatricians practicing in the US, and about 250 new geriatricians are trained each year while the American Geriatrics Society expects > 12,000 geriatricians will be required by 2030.4,5 More geriatricians are needed to serve as the primary health care professionals (HCPs) for older adults.4,6 Health care systems like the VHA must find ways to increase geriatrics skills, knowledge, and practices among their entire health care workforce. A culture shift toward age-friendly care for older adults across care settings and inclusive of all HCPs may help meet this escalating workforce need.7
The Age-Friendly Health System (AFHS) is an initiative of the John A. Hartford Foundation and the Institute for Healthcare Improvement (IHI) in partnership with the American Hospital Association and the Catholic Health Association of the United States.8,9 AFHS uses a what matters, medication, mentation, and mobility (4Ms) framework to ensure reliable, evidence-based care for older adults (Table 1).10,11 In an AFHS, the 4Ms are integrated into every discipline and care setting for older adults.11 The 4Ms neither replace formal training in geriatrics nor create the level of expertise needed for geriatrics teachers, researchers, and program leaders. However, the systematic approach of AFHS to assess and act on each of the 4Ms offers one solution to expand geriatrics skills and knowledge beyond geriatric care settings in all disciplines by engaging each HCP to meet the needs of older adults.12 To act on what matters, HCPs need to align the care plan with what is important to the older adult.
Hospitals and health care systems are encouraged to begin implementing the 4Ms in ≥ 1 care setting.13 Care settings may get started on a do-it-yourself track or by joining an IHI Action Community, which provides a series of webinars to help adopt the 4Ms over 7 months.14 By creating a plan for how each M will be assessed, documented, and acted on, care settings may earn level 1 recognition from the IHI.14 As of July 2023, there are at least 3100 AFHS participants and > 1900 have achieved level 2 recognition, which requires 3 months of clinical data to demonstrate the impact of the 4Ms.13,14
The main cultural shift of the AFHS movement is to focus on what matters to older adults by prioritizing each older adult’s personal health goals and care preferences across all care settings.9,11 Medication addresses age-appropropriate prescribing, making dose adjustments, if needed, and avoiding/deprescribing high-risk medications that may interfere with what matters, mentation, or mobility. The Beers Criteria for Potentially Inappropriate Medication Use in Older Adults is often used as a guide and includes lists of medications that are potentially harmful for older adults.11 Mentation focuses on preventing, identifying, treating, and managing dementia, depression, and delirium across care settings. Mobility includes assisting or encouraging older adults to move safely every day to maintain functional ability and do what matters.15,16 Each of the 4Ms has the potential to improve health outcomes for older adults, reduce waste from low-quality services, and increase the use of cost-effective services.11,17
In March 2020, the VHA Office of Geriatrics and Extended Care (GEC) set the goal for the VHA to be recognized by the IHI as an AFHS.18,19 US Department of Veterans Affairs (VA) facilities that joined the AFHS movement in 2020 are considered early adopters. We describe early adopter AFHS implementation at Birmingham VA Health Care System (BVAHCS) hospital, geriatrics assessment clinic (GAC), and Home Based Primary Care (HBPC) and at the Atlanta VA Medical Center (AVAMC) HBPC.
Implementing 4Ms Care
The IHI identifies 6 steps in the Plan-Do-Study-Act cycle to reliably practice the 4Ms. eAppendix 1 provides a side-by-side comparison of the steps over a 9-month timeline independently taken by BVAHCS and AVAMC to achieve both levels of AFHS recognition.
Step 1: Understand the Current State
In March 2020 the BVAHCS enrolled in the IHI Action Community. Three BVAHCS care settings were identified for the Action Community: the inpatient hospital, GAC (an outpatient clinic), and HBPC. The AVAMC HBPC enrolled in the IHI Action Community in March 2021.
Before joining the AFHS movement, the BVAHCS implemented a hospital-wide delirium standard operating procedure (SOP) whereby every veteran admitted to the 313-bed hospital is screened for delirium risk, with positive screens linked to nursing-led interventions. Nursing leadership supported AFHS due to its recognized value and an exemplary process in place to assess mentation/delirium and background understanding for screening and acting on medication, mobility, and what matters most to the veteran. The BVAHCS GAC, which was led by a single geriatrician, integrated the 4Ms into all geriatrics assessment appointments.
For the BVAHCS HBPC, the 4Ms supported key performance measures, such as fall prevention, patient satisfaction, decreasing medication errors, and identification of cognition and mood disorders. For the AVAMC HBPC, joining the AFHS movement represented an opportunity to improve performance measures, interdisciplinary teamwork, and care coordination for patients. For both HBPC sites, the shift to virtual meeting modalities due to the COVID-19 pandemic enabled HBPC team members to garner support for AFHS and collectively develop a 4Ms plan.
Step 2: Describe 4Ms Care
In March 2020 as guided by the Action Community, BVAHCS created a plan for each of its 3 care settings that described assessment tools, frequency, documentation, and responsible team members. All BVAHCS care settings achieved level 1 recognition in April 2020. Of the approximately 300 veterans served by the AVAMC HBPC, 83% are aged > 65 years. They achieved level 1 recognition in August 2021.
Step 3: Design and Adapt Workflows
From April to August 2020, BVAHCS implemented its 4Ms plans. In the hospital, a 4Ms overview was provided with education on the delirium SOP at nursing meetings. Updates were requested to the electronic health record (EHR) templates for the GAC to streamline documentation. For the BVAHCS HBPC, 4Ms assessments were added to the EHR quarterly care plan template, which was updated by all team members (Table 2).
From April through June 2021, the AVAMC HBPC formed teams led by 4Ms champions: what matters was led by a nurse care manager, medication by a nurse practitioner and pharmacist, mentation by a social worker, and mobility by a physical therapist. The champions initially focused on a plan for each M, incorporating all 4Ms as a set for optimal effectiveness into their quarterly care plan meeting using what matters to drive the entire care plan.
Step 4: Provide Care
Each of the 4Ms was to be assessed, documented, and acted on for each veteran within a short period, such as a hospitalization or 1 or 2 outpatient visits. BVAHCS implemented 4Ms care in each care setting from August to October 2020. The AVAMC HBPC implemented 4Ms from July to September 2021.
Step 5: Study Performance
The IHI identifies 3 methods for measuring older adults who receive 4Ms care: real-time observation, chart review, or EHR report. For chart review, the IHI recommends using a random sample to calculate the number of patients who received 4Ms in 1 month, which provides evidence of progress toward reliable practice.
Both facilities used chart review with random sampling. Each setting estimated the number of veterans receiving 4Ms care by multiplying the percentage of sampled charts with documented 4Ms care by unique patient encounters (eAppendix 2).
From August through October 2020, BVAHCS sites reached an estimated 97% of older veterans with complete 4Ms care: hospital, 100%; GAC, 90%; and HBPC, 85%. AVAMC HBPC increased 4Ms care from 52% to 100% between July and September 2021. Both teams demonstrated the feasibility of reliably providing 4Ms care to > 85% of older veterans in these care settings and earned level 2 recognition. Through satisfaction surveys and informal feedback, notable positive changes were evident to veterans, their families, and the VA staff providing 4Ms age-friendly care.
Step 6: Improve and Sustain Care
Each site acknowledged barriers and facilitators for adopting the 4Ms. The COVID-19 pandemic was an ongoing barrier for both sites, with teams transitioning to virtual modalities for telehealth visits and team meetings, and higher staff turnover. However, the greater use of technology facilitated 4Ms adoption by allowing physically distant team members to collaborate.
One of the largest barriers was the lack of 4Ms documentation in the EHR, which could not be implemented in the BVAHCS inpatient hospital due to existing standardized nursing templates. Both sites recognized that 4Ms documentation in the EHR for all care settings would facilitate achieving level 2 recognition and tracking and reporting 4Ms care in the future.
Discussion
The AFHS 4Ms approach offers a method to impart geriatrics knowledge, skills, and practice throughout an entire health care system in a short time. The AFHS framework provides a structured pathway to the often daunting challenge of care for complex, multimorbid, and highly heterogeneous older adults. The 4Ms approach promotes the provision of evidence-based care that is reliable, efficient, patient centered, and avoids unwanted care: worthy goals not only for geriatrics but for all members of a high-reliability organization.
Through the implementation of the 4Ms framework, consistent use of AFHS practices, measurement, and feedback, the staff in each VA care setting reported here reached a level of reliability in which at least 85% of patients had all 4Ms addressed. Notably, adoption was strong and improvements in reliably addressing all 4Ms were observed in both geriatrics (HBPC and outpatient clinics) and nongeriatrics (inpatient medicine) settings. Although one might expect that high-functioning interdisciplinary teams in geriatrics-focused VA settings were routinely addressing all 4Ms for most of their patients, our experience was consistent with prior teams indicating that this is often not the case. Although many of these teams were addressing some of the 4Ms in their usual practice, the 4Ms framework facilitated addressing all 4Ms as a set with input from all team members. Most importantly, it fostered a culture of asking the older adult what matters most and documenting, sharing, and aligning this with the care plan. Within 6 months, all VA care settings achieved level 1 recognition, and within 9 months, all achieved level 2 recognition.
Lessons Learned
Key lessons learned include the importance of identifying, preparing, and supporting a champion to lead this effort; garnering facility and system leadership support at the outset; and integration with the EHR for reliable and efficient data capture, reporting, and feedback. Preparing and supporting champions was achieved through national and individual calls and peer support. Guidance was provided on garnering leadership support, including local needs assessment and data analysis, meeting with leadership to first understand their key challenges and priorities and provide information on the AFHS movement, requesting a follow-up meeting to discuss local needs and data, and exploring how an AFHS might help address one or more of their priorities.
In September 2022, an AFHS 4Ms note template was introduced into the EHR for all VA sites for data capture and reporting, to standardize and facilitate documentation across all age-friendly VA sites, and decrease the reporting burden for staff. This effort is critically important: The ability to document, track, and analyze 4Ms measures, provide feedback, and synergize efforts across systems is vital to design studies to determine whether the AFHS 4Ms approach to care achieves substantive improvements in patient care across settings.
Limitations
Limitations of this analysis include the small sample of care settings, which did not include a skilled nursing or long-term care facility, nor general primary care. Although the short timeframe assessed did not allow us to report on the anticipated clinical outcomes of 4Ms care, it does set up a foundation for evaluation of the 4Ms and EHR integration and dashboard development.
Conclusions
The VHA provides a comprehensive spectrum of geriatrics services and innovative models of care that often serve as exemplars to other health care systems. Implementing the AFHS framework to assess and act on the 4Ms provides a structure for confronting the HCP shortage with geriatrics expertise by infusing geriatrics knowledge, skills, and practices throughout all care settings and disciplines. Enhancing patient-centered care to older veterans through AFHS implementation exemplifies the VHA as a learning health care system.
Acknowledgments
We thank the Veterans Health Administration Office of Geriatrics and Extended Care and the clinical staff from the Atlanta Veterans Affairs Healthcare System and the Birmingham Veterans Affairs Health Care System for assisting us in this work.
1. US Census Bureau. Older Americans month: May 2023. Accessed September 11, 2023. https://www.census.gov/newsroom/stories/older-americans-month.html
2. Vespa J. Aging veterans: America’s veteran population in later life. July 2023. Accessed September 11, 2023. https://www.census.gov/content/dam/Census/library/publications/2023/acs/acs-54.pdf
3. O’Hanlon C, Huang C, Sloss E, et al. Comparing VA and non-VA quality of care: a systematic review. J Gen Intern Med. 2017;32(1):105-121. doi:10.1007/s11606-016-3775-2
4. Fulmer T, Reuben DB, Auerbach J, Fick DM, Galambos C, Johnson KS. Actualizing better health and health care for older adults: commentary describes six vital directions to improve the care and quality of life for all older Americans. Health Aff (Millwood). 2021;40(2):219-225. doi:10.1377/hlthaff.2020.01470
5. ChenMed. The physician shortage in geriatrics. March 18, 2022. Accessed September 6, 2023. https://www.chenmed.com/blog/physician-shortage-geriatrics
6. American Geriatrics Society. Projected future need for geriatricians. Updated May 2016. Accessed September 6, 2023. https://www.americangeriatrics.org/sites/default/files/inline-files/Projected-Future-Need-for-Geriatricians.pdf 7. Carmody J, Black K, Bonner A, Wolfe M, Fulmer T. Advancing gerontological nursing at the intersection of age-friendly communities, health systems, and public health. J Gerontol Nurs. 2021;47(3):13-17. doi:10.3928/00989134-20210125-01
8. Lesser S, Zakharkin S, Louie C, Escobedo MR, Whyte J, Fulmer T. Clinician knowledge and behaviors related to the 4Ms framework of Age‐Friendly Health Systems. J Am Geriatr Soc. 2022;70(3):789-800. doi:10.1111/jgs.17571
9. Edelman LS, Drost J, Moone RP, et al. Applying the Age-Friendly Health System framework to long term care settings. J Nutr Health Aging. 2021;25(2):141-145. doi:10.1007/s12603-020-1558-2
10. Emery-Tiburcio EE, Mack L, Zonsius MC, Carbonell E, Newman M. The 4Ms of an Age-Friendly Health System: an evidence-based framework to ensure older adults receive the highest quality care. Home Healthc Now. 2022;40(5):252-257. doi:10.1097/NHH.0000000000001113
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
12. Mate KS, Berman A, Laderman M, Kabcenell A, Fulmer T. Creating age-friendly health systems – a vision for better care of older adults. Healthc (Amst). 2018;6(1):4-6. doi:10.1016/j.hjdsi.2017.05.005
13. Institute for Healthcare Improvement. What is an Age-Friendly Health System? Accessed September 6, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/default.aspx
14. Institute for Healthcare Improvement. Health systems recognized by IHI. Updated September 2023. Accessed September 6, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/recognized-systems.aspx
15. Burke RE, Ashcraft LE, Manges K, et al. What matters when it comes to measuring Age‐Friendly Health System transformation. J Am Geriatr Soc. 2022;70(10):2775-2785. doi:10.1111/jgs.18002
16. Wang J, Shen JY, Conwell Y, et al. How “age-friendly” are deprescribing interventions? A scoping review of deprescribing trials. Health Serv Res. 202;58(suppl 1):123-138. doi:10.1111/1475-6773.14083
17. Pohnert AM, Schiltz NK, Pino L, et al. Achievement of age‐friendly health systems committed to care excellence designation in a convenient care health care system. Health Serv Res. 2023;58 (suppl 1):89-99. doi:10.1111/1475-6773.14071
18. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2022;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
19. Farrell TW, Volden TA, Butler JM, et al. Age‐friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. doi:10.1111/jgs.18070
1. US Census Bureau. Older Americans month: May 2023. Accessed September 11, 2023. https://www.census.gov/newsroom/stories/older-americans-month.html
2. Vespa J. Aging veterans: America’s veteran population in later life. July 2023. Accessed September 11, 2023. https://www.census.gov/content/dam/Census/library/publications/2023/acs/acs-54.pdf
3. O’Hanlon C, Huang C, Sloss E, et al. Comparing VA and non-VA quality of care: a systematic review. J Gen Intern Med. 2017;32(1):105-121. doi:10.1007/s11606-016-3775-2
4. Fulmer T, Reuben DB, Auerbach J, Fick DM, Galambos C, Johnson KS. Actualizing better health and health care for older adults: commentary describes six vital directions to improve the care and quality of life for all older Americans. Health Aff (Millwood). 2021;40(2):219-225. doi:10.1377/hlthaff.2020.01470
5. ChenMed. The physician shortage in geriatrics. March 18, 2022. Accessed September 6, 2023. https://www.chenmed.com/blog/physician-shortage-geriatrics
6. American Geriatrics Society. Projected future need for geriatricians. Updated May 2016. Accessed September 6, 2023. https://www.americangeriatrics.org/sites/default/files/inline-files/Projected-Future-Need-for-Geriatricians.pdf 7. Carmody J, Black K, Bonner A, Wolfe M, Fulmer T. Advancing gerontological nursing at the intersection of age-friendly communities, health systems, and public health. J Gerontol Nurs. 2021;47(3):13-17. doi:10.3928/00989134-20210125-01
8. Lesser S, Zakharkin S, Louie C, Escobedo MR, Whyte J, Fulmer T. Clinician knowledge and behaviors related to the 4Ms framework of Age‐Friendly Health Systems. J Am Geriatr Soc. 2022;70(3):789-800. doi:10.1111/jgs.17571
9. Edelman LS, Drost J, Moone RP, et al. Applying the Age-Friendly Health System framework to long term care settings. J Nutr Health Aging. 2021;25(2):141-145. doi:10.1007/s12603-020-1558-2
10. Emery-Tiburcio EE, Mack L, Zonsius MC, Carbonell E, Newman M. The 4Ms of an Age-Friendly Health System: an evidence-based framework to ensure older adults receive the highest quality care. Home Healthc Now. 2022;40(5):252-257. doi:10.1097/NHH.0000000000001113
11. Mate K, Fulmer T, Pelton L, et al. Evidence for the 4Ms: interactions and outcomes across the care continuum. J Aging Health. 2021;33(7-8):469-481. doi:10.1177/0898264321991658
12. Mate KS, Berman A, Laderman M, Kabcenell A, Fulmer T. Creating age-friendly health systems – a vision for better care of older adults. Healthc (Amst). 2018;6(1):4-6. doi:10.1016/j.hjdsi.2017.05.005
13. Institute for Healthcare Improvement. What is an Age-Friendly Health System? Accessed September 6, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/default.aspx
14. Institute for Healthcare Improvement. Health systems recognized by IHI. Updated September 2023. Accessed September 6, 2023. https://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/recognized-systems.aspx
15. Burke RE, Ashcraft LE, Manges K, et al. What matters when it comes to measuring Age‐Friendly Health System transformation. J Am Geriatr Soc. 2022;70(10):2775-2785. doi:10.1111/jgs.18002
16. Wang J, Shen JY, Conwell Y, et al. How “age-friendly” are deprescribing interventions? A scoping review of deprescribing trials. Health Serv Res. 202;58(suppl 1):123-138. doi:10.1111/1475-6773.14083
17. Pohnert AM, Schiltz NK, Pino L, et al. Achievement of age‐friendly health systems committed to care excellence designation in a convenient care health care system. Health Serv Res. 2023;58 (suppl 1):89-99. doi:10.1111/1475-6773.14071
18. Church K, Munro S, Shaughnessy M, Clancy C. Age-Friendly Health Systems: improving care for older adults in the Veterans Health Administration. Health Serv Res. 2022;58(suppl 1):5-8. doi:10.1111/1475-6773.14110
19. Farrell TW, Volden TA, Butler JM, et al. Age‐friendly care in the Veterans Health Administration: past, present, and future. J Am Geriatr Soc. doi:10.1111/jgs.18070
Community Nursing Home Program Oversight: Can the VA Meet Increased Demand for Community-Based Care?
The US Department of Veterans Affairs (VA) Community Nursing Home (CNH) program provides 24-hour skilled nursing care for eligible veterans in public or private community-based facilities that have established a contract to care for veterans. Veteran eligibility is based on service-connected status and level of disability, covering the cost of care for veterans who need long-term care because of their service-connected disability or for veterans with disabilities rated at ≥ 70%.1 Between 2014 and 2018, the average daily census of veterans in CNHs increased by 26% and the percentage of funds obligated to this program increased by 49%.2 The VA projects that the number of veterans receiving care in a CNH program will increase by 80% between 2017 and 2037, corresponding to a 149% increase in CNH expenditures.2
CNH program oversight teams are mandated at each VA medical center (VAMC) to monitor care coordination within the CNH program. These teams include nurses and social workers (SWs) who perform regular on-site assessments to monitor the clinical, functional, and psychosocial needs of veterans. These assessments include a review of the electronic health record (EHR) and face-to-face contact with veterans and CNH staff, regardless of the purchasing authority (hospice, long-term care, short-term rehabilitation, respite care).3 These teams represent key stakeholders impacted by CNH program expansion.
While the CNH program has focused primarily on the provision of long-term care, the VA is now expanding to include short-term rehabilitation through Veteran Care Agreements.4 These agreements are authorized under the MISSION Act, designed to improve care for veterans.5 Veteran Care Agreements are expected to be less burdensome to execute than traditional contracts and will permit the VA to partner with more CNHs, as noted in a Congressional Research Service report regarding long-term care services for veterans.6 However, increasing the number of CNHs increases demands on oversight teams, particularly if the coordinators are compelled to perform monthly on-site visits to facilities required under current guidelines.3
The objective of this study was to describe the experiences of VA and CNH staff involved in care coordination and the oversight of veterans receiving CNH care amid Veteran Care Agreement implementation and in anticipation of CNH program expansion. The results are intended to inform expansion efforts within the CNH program.
METHODS
This study was a component of a larger research project examining VA-purchased CNH care; recruitment methods are available in previous publications describing this work.7 Participants provided written or verbal consent before video and phone interviews, respectively. This study was approved by the Colorado Multiple Institutional Review Board (Protocol #18-1186).
Video and phone interviews were conducted by 3 team members from October 2018 to March 2020 with CNH staff and VA CNH program oversight team members. Participant recruitment was paused from May to October 2020 as a result of the COVID-19 pandemic and ambiguity about VA NH care purchasing policies following the passage of the VA MISSION Act.5 We used semistructured interview guides (eAppendix 1 for VA staff and eAppendix 2 for NH staff, available online at doi:10.12788/fp.0421). Recorded and transcribed interviews ranged from 15 to 90 minutes.
Two members of the research team analyzed transcripts using both deductive and inductive content analysis.8 The interview guide informed an a priori codebook, and in vivo codes were included as they emerged. We jointly coded 6 transcripts to reach a consensus on coding approaches and analyzed the remaining transcripts independently with frequent meetings to develop themes with a qualitative methodologist. All qualitative data were analyzed using ATLAS.ti software.
This was a retrospective observational study of veterans who received VA-paid care in CNHs during the 2019 fiscal year (10/1/2018-9/30/2019) using data from the enrollment, inpatient and outpatient encounters, and other care paid for by the VA in the VA Corporate Data Warehouse. We linked Centers for Medicare and Medicaid monthly Nursing Home Compare reports and the Brown University Long Term Care: Facts on Care in the US (LTC FoCUS) annual files to identify facility addresses.9
Descriptive analyses of quantitative data were conducted in parallel with the qualitative findings.8 Distance from the contracting VAMC to CNH was calculated using the greater-circle formula to find the linear distance between geographic coordinates. Quantitative and qualitative data were collected concurrently, analyzed independently, and integrated into the interpretation of results.10
RESULTS
We conducted 36 interviews with VA and NH staff who were affiliated with 6 VAMCs and 17 CNHs. Four themes emerged concerning CNH oversight: (1) benefits of VA CNH team engagement/visits; (2) burden of VA CNH oversight; (3) burden of oversight limited the ability to contract with additional NHs; and (4) factors that ease the burden and facilitate successful oversight.
Benefits of Engagement/Visits
VA SWs and nurses visit each veteran every 30 to 45 days to review their health records, meet with them, and check in with NH staff. In addition, VA SWs and nurses coordinate each veteran’s care by working as liaisons between the VA and the NH to help NH staff problem solve veteran-related issues through care conferences. VA SWs and nurses act as extra advocates for veterans to make sure their needs are met. “This program definitely helps ensure that veterans are receiving higher quality care because if we see that they aren’t, then we do something about it,” a VA NH coordinator reported in an interview.
NH staff noted benefits to monthly VA staff visits, including having an additional person coordinating care and built-in VA liaisons. “It’s nice to have that extra set of eyes, people that you can care plan with,” an NH administrator shared. “It’s definitely a true partnership, and we have open and honest conversations so we can really provide a good service for our veterans.”
Distance & High Veteran Census Burdens
VA participants described oversight components as burdensome. Specifically, several VA participants mentioned that the charting they completed in the facility during each visit proved time consuming and onerous, particularly for distant NHs. To accommodate veterans’ preferences to receive care in a facility close to their homes and families, VAMCs contract with NHs that are geographically spread out. “We’re just all spread out… staff have issues driving 2 and a half hours just to review charts all day,” a VA CNH coordinator explained. In 2019, the mean distance between VAMC and NH was 48 miles, with half located > 32 miles from the VAMC. One-quarter of NHs were > 70 miles and 44% were located > 50 miles from the VAMC (Figure 1).
Participants highlighted how regular oversight visits were particularly time consuming at CNHs with a large contracted population. VA nurses and SWs spend multiple days and up to a week conducting oversight visits at facilities with large numbers of veterans. Another VA nurse highlighted how charting requirements resulted in several days of documentation outside of the NH visit for facilities with many contracted veteran residents. Multiple VA participants noted that having many veterans at an NH exacerbated the oversight burdens. In 2019, 252 (28%) of VA CNHs had > 10 contracted veterans and 1 facility had 34 veterans (Figure 2). VA participants perceived having too many veterans concentrated at 1 facility as potentially challenging for CNHs due to the complex care needs of veterans and the added need for care coordination with the VA. One VA NH coordinator noted that while some facilities were “adept at being able to handle higher numbers” of veterans, others were “overwhelmed.” Too many veterans at an NH, an SW explained, might lead the “facility to fail because we are such a cumbersome system.”
Oversight & Staffing Burden
While several participants described wanting to contract with more NHs to avoid overwhelming existing CNHs and to increase choice for veterans, they expressed concerns about their ability to provide oversight at more facilities due to limited staffing and oversight requirements. Across VAMCs, the median number of VA CNHs varied substantially (Figure 3). One VA participant with about 35 CNHs explained that while adding more NHs could create “more opportunities and options” for veterans, it needs to be balanced with the required oversight responsibilities. One VA nurse insisted that more staff were needed to meet current and future oversight needs. “We’re all getting stretched pretty thin, and just so we don’t drop the ball on things… I would like to see a little more staff if we’re gonna have a lot more nursing homes.”
Participants had concerns related to the VA MISSION Act and the possibility of more VA-paid NHs for rehabilitation or short-term care. Participants underscored the necessity for additional staff to account for the increased oversight burden or a reduction in oversight requirements. One SW felt that increasing the number of CNHs would increase the required oversight and the need for collaboration with NH staff, which would limit her ability to establish close and trusting working relationships with NH staff. Participants also described the challenges of meeting their current oversight requirements, which limited extra visits for acute issues and care conferences. This was attributed to a lack of adequate staffing in the VA CNH program, given the time-intensive nature of VA oversight requirements.
Easing Burden & Facilitating Oversight
Participants noted how obtaining remote access to veterans’ EHRs allowed them to conduct chart reviews before oversight visits. This permitted more time for interaction with veterans and CNH staff as well as coordinating care. While providing access to the VA EHR would not change the chart review component of VA oversight, some participants felt it might improve care coordination between VA and NH staff during monthly visits.
Participants felt they were able to build strong working relationships with facilities with more veterans due to frequent communication and collaboration. VA participants also noted that CNHs with larger veteran censuses were more likely to respond to VA concerns about care to maintain the business relationship and contract. To optimize strong working relationships and decrease the challenges of having too many veterans at a facility, some VA participants suggested that CNH programs create a local policy to recommend the number of veterans placed in a CNH.
Discussion
Participants interviewed for this study echoed findings from previous work that identified the importance of developing trusted working relationships with CNHs to care for veterans.11,12 However, interorganizational care coordination, a shortage of health care professionals, and resource demands associated with caring for veterans reported in other community care settings were also noted in our findings.12,13
Building upon prior recommendations related to community care of veterans, our analysis identified key areas that could improve CNH program oversight efficiency, including: (1) improving the interoperability of EHRs to facilitate coordination of care and oversight; (2) addressing inefficiencies associated with traveling to geographically dispersed CNHs; and (3) “right-sizing” the number of veterans residing in each CNH.
The interoperability of EHRs has been cited by multiple studies of VA community care programs as critical to reducing inefficiencies and allowing more in-person time with veterans and staff in care coordination, especially at rural locations.11-15 The Veterans Health Information Exchange Program is designed to optimize data sharing as veterans are increasingly referred to non-VA care through the MISSION Act. This program is organized around patient engagement, clinician adoption, partner engagement, and technological capabilities.16
Unfortunately, significant barriers exist for the VA CNH program within each of these information exchange domains. For example, patient engagement requires veteran consent for consumer-initiated exchange of medical information, which is not practical due to the high prevalence of cognitive impairment in NHs. Similarly, VA consent requirements prohibit EHR download and sharing with non-VA facilities like CNHs, limiting use. eHealth Exchange partnerships allow organizations caring for veterans to connect with the VA via networks that provide a common trust agreement and technical compliance testing. Unfortunately, in 2017, only 257 NHs in which veterans received care nationally were eHealth Exchange partners, which indicates that while NHs could partner in this information exchange, very few did.16
Finally, while the exchange is possible, it is not practical; most CNHs lack the staff that would be required to support data transfer on their technology platform into the appropriate translational gateways. Although remote access to EHRs in CNHs improved during the pandemic, the Veterans Health Information Exchange Program is not designed to facilitate interoperability of VA and CNH records and remains a significant barrier for this and many other VA community care programs.
The dispersal of veterans across CNHs that are > 50 miles from the nearest VAMC represents an additional area to improve program efficiency and meet growing demands for rural care. While recent field guidance to CNH oversight teams reduces the frequency of visits by VA CNH teams, the burden of driving to each facility is not likely to decrease as CNHs increasingly offer rehabilitation as a part of Veteran Care Agreements.17 Visits performed by telehealth or by trained local VA staff may represent alternatives.15
Finally, interview participants described the ideal range of the number of veterans in each CNH necessary to optimize efficiencies. Veterans who rely more heavily upon VA care tend to have more medical and mental health comorbidities than average Medicare beneficiaries.18,19 This was reflected in participants’ recommendation to have enough veterans to benefit from economies of scale but to also identify a limit when efficiencies are lost. Given that most CNHs cared for 8 to 15 veterans, facilities seem to have identified how best to match the resources available with veterans’ care needs. Based on these observations, new care networks that will be established because of the MISSION Act may benefit from establishing evidence-based policies that support flexibility in oversight frequency and either allow for remote oversight or consolidate the number of CNHs to improve efficiencies in care provision and oversight.20
Limitations
Limitations include the unique relationship between VA and CNH staff overseeing the quality of care provided to veterans in CNHs, which is not replicated in other models of care. Data collection was interrupted following the passage of the MISSION Act in 2018 until guidance on changes to practice resulting from the law were clarified in 2020. Interviews were also interrupted at the onset of the COVID-19 pandemic.
Conclusions
The current quality of the CNH care oversight structure will require adaptation as demand for CNH care increases. While the VA CNH program is one of the longest-standing programs collaborating with non-VA community care partners, it is now only one of many following the MISSION Act. The success of this and other programs will depend on matching available CNH resources to the complex medical and psychological needs of veterans. At a time when strategies to ease the burden on NHs and VA CNH coordinators are desperately needed, Veterans Health Information Exchange capabilities need to improve. Evidence is needed to guide the scaling of the program to meet the needs of the rapidly expanding veteran population who are eligible for CNH care.
Acknowledgments
The authors acknowledge Amy Mochel of the Providence Veterans Affairs Medical Center for project management support of this project.
1. Miller EA, Gadbois E, Gidmark S, Intrator O. Purchasing nursing home care within the Veterans Health Administration: lessons for nursing home recruitment, contracting, and oversight. J Health Admin Educ. 2015;32(2):165-197.
2. GAO. VA health care. Veterans’ use of long-term care is increasing, and VA faces challenges in meeting the demand. February 19, 2020. Accessed September 19, 2023. https://www.gao.gov/assets/gao-20-284.pdf
3. VHA Handbook 1143.2, VHA community nursing home oversight procedures. US Department of Veterans Affairs, Veterans Health Administration. June 2004. https://www.vendorportal.ecms.va.gov/FBODocumentServer/DocumentServer.aspx?DocumentId=3740930&FileName=VA259-17-Q-0501-007.pdf
4. Community care: veteran care agreements. US Department of Veterans Affairs. 2022. Updated August 8, 2023. Accessed September 7, 2023. https://www.va.gov/COMMUNITYCARE/providers/Veterans_Care_Agreements.asp
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION Act and the future of veterans’ access to quality health care. JAMA. 2020;324(4):343-344. doi:10.1001/jama.2020.4505
6. Colello KJ, Panangala SV; Congressional Research Service. Long-term care services for veterans. February 14, 2017. Accessed September 7, 2023. https://crsreports.congress.gov/product/pdf/R/R44697
7. Magid KH, Galenbeck E, Haverhals LM, et al. Purchasing high-quality community nursing home care: a will to work with VHA diminished by contracting burdens. J Am Med Dir Assoc. 2022;23(11):1757-1764. doi:10.1016/j.jamda.2022.03.007
8. Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398-405. doi:10.1111/nhs.12048
9. Brown University. LTC Focus. Accessed September 18, 2023. https://ltcfocus.org/about
10. Zhang W, Creswell J. The use of “mixing” procedure of mixed methods in health services research. Med Care. 2013;51(8):e51-e57. doi:10.1097/MLR.0b013e31824642fd
11. Haverhals LM, Magid KH, Blanchard KN, Levy CR. Veterans Health Administration staff perceptions of overseeing care in community nursing homes during COVID-19. Gerontol Geriatr Med. 2022;8:23337214221080307. Published 2022 Feb 15. doi:10.1177/23337214221080307
12. Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
13. Schlosser J, Kollisch D, Johnson D, Perkins T, Olson A. VA-community dual care: veteran and clinician perspectives. J Community Health. 2020;45(4):795-802. doi:10.1007/s10900-020-00795-y
14. Nevedal AL, Wong EP, Urech TH, Peppiatt JL, Sorie MR, Vashi AA. Veterans’ experiences with accessing community emergency care. Mil Med. 2023;188(1-2):e58-e64. doi:10.1093/milmed/usab196
15. Levenson SA. Smart case review: a model for successful remote medical direction and enhanced nursing home quality improvement. J Am Med Dir Assoc. 2021;22(10):2212-2215.e6. doi:10.1016/j.jamda.2021.05.043
16. Donahue M, Bouhaddou O, Hsing N, et al. Veterans Health Information Exchange: successes and challenges of nationwide interoperability. AMIA Annu Symp Proc. 2018;2018:385-394. Published 2018 Dec 5.
17. US Department of Veterans Affairs. VHA Notice 2023-07. Community Nursing Home Program. September 5, 2023:1-4.
18. Helmer DA, Dwibedi N, Rowneki M, et al. Mental health conditions and hospitalizations for ambulatory care sensitive conditions among veterans with diabetes. Am Health Drug Benefits. 2020;13(2):61-71.
19. Rosen AK, Wagner TH, Pettey WBP, et al. Differences in risk scores of veterans receiving community care purchased by the Veterans Health Administration. Health Serv Res. 2018;53(suppl 3):5438-5454. doi:10.1111/1475-6773.13051
20. Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
The US Department of Veterans Affairs (VA) Community Nursing Home (CNH) program provides 24-hour skilled nursing care for eligible veterans in public or private community-based facilities that have established a contract to care for veterans. Veteran eligibility is based on service-connected status and level of disability, covering the cost of care for veterans who need long-term care because of their service-connected disability or for veterans with disabilities rated at ≥ 70%.1 Between 2014 and 2018, the average daily census of veterans in CNHs increased by 26% and the percentage of funds obligated to this program increased by 49%.2 The VA projects that the number of veterans receiving care in a CNH program will increase by 80% between 2017 and 2037, corresponding to a 149% increase in CNH expenditures.2
CNH program oversight teams are mandated at each VA medical center (VAMC) to monitor care coordination within the CNH program. These teams include nurses and social workers (SWs) who perform regular on-site assessments to monitor the clinical, functional, and psychosocial needs of veterans. These assessments include a review of the electronic health record (EHR) and face-to-face contact with veterans and CNH staff, regardless of the purchasing authority (hospice, long-term care, short-term rehabilitation, respite care).3 These teams represent key stakeholders impacted by CNH program expansion.
While the CNH program has focused primarily on the provision of long-term care, the VA is now expanding to include short-term rehabilitation through Veteran Care Agreements.4 These agreements are authorized under the MISSION Act, designed to improve care for veterans.5 Veteran Care Agreements are expected to be less burdensome to execute than traditional contracts and will permit the VA to partner with more CNHs, as noted in a Congressional Research Service report regarding long-term care services for veterans.6 However, increasing the number of CNHs increases demands on oversight teams, particularly if the coordinators are compelled to perform monthly on-site visits to facilities required under current guidelines.3
The objective of this study was to describe the experiences of VA and CNH staff involved in care coordination and the oversight of veterans receiving CNH care amid Veteran Care Agreement implementation and in anticipation of CNH program expansion. The results are intended to inform expansion efforts within the CNH program.
METHODS
This study was a component of a larger research project examining VA-purchased CNH care; recruitment methods are available in previous publications describing this work.7 Participants provided written or verbal consent before video and phone interviews, respectively. This study was approved by the Colorado Multiple Institutional Review Board (Protocol #18-1186).
Video and phone interviews were conducted by 3 team members from October 2018 to March 2020 with CNH staff and VA CNH program oversight team members. Participant recruitment was paused from May to October 2020 as a result of the COVID-19 pandemic and ambiguity about VA NH care purchasing policies following the passage of the VA MISSION Act.5 We used semistructured interview guides (eAppendix 1 for VA staff and eAppendix 2 for NH staff, available online at doi:10.12788/fp.0421). Recorded and transcribed interviews ranged from 15 to 90 minutes.
Two members of the research team analyzed transcripts using both deductive and inductive content analysis.8 The interview guide informed an a priori codebook, and in vivo codes were included as they emerged. We jointly coded 6 transcripts to reach a consensus on coding approaches and analyzed the remaining transcripts independently with frequent meetings to develop themes with a qualitative methodologist. All qualitative data were analyzed using ATLAS.ti software.
This was a retrospective observational study of veterans who received VA-paid care in CNHs during the 2019 fiscal year (10/1/2018-9/30/2019) using data from the enrollment, inpatient and outpatient encounters, and other care paid for by the VA in the VA Corporate Data Warehouse. We linked Centers for Medicare and Medicaid monthly Nursing Home Compare reports and the Brown University Long Term Care: Facts on Care in the US (LTC FoCUS) annual files to identify facility addresses.9
Descriptive analyses of quantitative data were conducted in parallel with the qualitative findings.8 Distance from the contracting VAMC to CNH was calculated using the greater-circle formula to find the linear distance between geographic coordinates. Quantitative and qualitative data were collected concurrently, analyzed independently, and integrated into the interpretation of results.10
RESULTS
We conducted 36 interviews with VA and NH staff who were affiliated with 6 VAMCs and 17 CNHs. Four themes emerged concerning CNH oversight: (1) benefits of VA CNH team engagement/visits; (2) burden of VA CNH oversight; (3) burden of oversight limited the ability to contract with additional NHs; and (4) factors that ease the burden and facilitate successful oversight.
Benefits of Engagement/Visits
VA SWs and nurses visit each veteran every 30 to 45 days to review their health records, meet with them, and check in with NH staff. In addition, VA SWs and nurses coordinate each veteran’s care by working as liaisons between the VA and the NH to help NH staff problem solve veteran-related issues through care conferences. VA SWs and nurses act as extra advocates for veterans to make sure their needs are met. “This program definitely helps ensure that veterans are receiving higher quality care because if we see that they aren’t, then we do something about it,” a VA NH coordinator reported in an interview.
NH staff noted benefits to monthly VA staff visits, including having an additional person coordinating care and built-in VA liaisons. “It’s nice to have that extra set of eyes, people that you can care plan with,” an NH administrator shared. “It’s definitely a true partnership, and we have open and honest conversations so we can really provide a good service for our veterans.”
Distance & High Veteran Census Burdens
VA participants described oversight components as burdensome. Specifically, several VA participants mentioned that the charting they completed in the facility during each visit proved time consuming and onerous, particularly for distant NHs. To accommodate veterans’ preferences to receive care in a facility close to their homes and families, VAMCs contract with NHs that are geographically spread out. “We’re just all spread out… staff have issues driving 2 and a half hours just to review charts all day,” a VA CNH coordinator explained. In 2019, the mean distance between VAMC and NH was 48 miles, with half located > 32 miles from the VAMC. One-quarter of NHs were > 70 miles and 44% were located > 50 miles from the VAMC (Figure 1).
Participants highlighted how regular oversight visits were particularly time consuming at CNHs with a large contracted population. VA nurses and SWs spend multiple days and up to a week conducting oversight visits at facilities with large numbers of veterans. Another VA nurse highlighted how charting requirements resulted in several days of documentation outside of the NH visit for facilities with many contracted veteran residents. Multiple VA participants noted that having many veterans at an NH exacerbated the oversight burdens. In 2019, 252 (28%) of VA CNHs had > 10 contracted veterans and 1 facility had 34 veterans (Figure 2). VA participants perceived having too many veterans concentrated at 1 facility as potentially challenging for CNHs due to the complex care needs of veterans and the added need for care coordination with the VA. One VA NH coordinator noted that while some facilities were “adept at being able to handle higher numbers” of veterans, others were “overwhelmed.” Too many veterans at an NH, an SW explained, might lead the “facility to fail because we are such a cumbersome system.”
Oversight & Staffing Burden
While several participants described wanting to contract with more NHs to avoid overwhelming existing CNHs and to increase choice for veterans, they expressed concerns about their ability to provide oversight at more facilities due to limited staffing and oversight requirements. Across VAMCs, the median number of VA CNHs varied substantially (Figure 3). One VA participant with about 35 CNHs explained that while adding more NHs could create “more opportunities and options” for veterans, it needs to be balanced with the required oversight responsibilities. One VA nurse insisted that more staff were needed to meet current and future oversight needs. “We’re all getting stretched pretty thin, and just so we don’t drop the ball on things… I would like to see a little more staff if we’re gonna have a lot more nursing homes.”
Participants had concerns related to the VA MISSION Act and the possibility of more VA-paid NHs for rehabilitation or short-term care. Participants underscored the necessity for additional staff to account for the increased oversight burden or a reduction in oversight requirements. One SW felt that increasing the number of CNHs would increase the required oversight and the need for collaboration with NH staff, which would limit her ability to establish close and trusting working relationships with NH staff. Participants also described the challenges of meeting their current oversight requirements, which limited extra visits for acute issues and care conferences. This was attributed to a lack of adequate staffing in the VA CNH program, given the time-intensive nature of VA oversight requirements.
Easing Burden & Facilitating Oversight
Participants noted how obtaining remote access to veterans’ EHRs allowed them to conduct chart reviews before oversight visits. This permitted more time for interaction with veterans and CNH staff as well as coordinating care. While providing access to the VA EHR would not change the chart review component of VA oversight, some participants felt it might improve care coordination between VA and NH staff during monthly visits.
Participants felt they were able to build strong working relationships with facilities with more veterans due to frequent communication and collaboration. VA participants also noted that CNHs with larger veteran censuses were more likely to respond to VA concerns about care to maintain the business relationship and contract. To optimize strong working relationships and decrease the challenges of having too many veterans at a facility, some VA participants suggested that CNH programs create a local policy to recommend the number of veterans placed in a CNH.
Discussion
Participants interviewed for this study echoed findings from previous work that identified the importance of developing trusted working relationships with CNHs to care for veterans.11,12 However, interorganizational care coordination, a shortage of health care professionals, and resource demands associated with caring for veterans reported in other community care settings were also noted in our findings.12,13
Building upon prior recommendations related to community care of veterans, our analysis identified key areas that could improve CNH program oversight efficiency, including: (1) improving the interoperability of EHRs to facilitate coordination of care and oversight; (2) addressing inefficiencies associated with traveling to geographically dispersed CNHs; and (3) “right-sizing” the number of veterans residing in each CNH.
The interoperability of EHRs has been cited by multiple studies of VA community care programs as critical to reducing inefficiencies and allowing more in-person time with veterans and staff in care coordination, especially at rural locations.11-15 The Veterans Health Information Exchange Program is designed to optimize data sharing as veterans are increasingly referred to non-VA care through the MISSION Act. This program is organized around patient engagement, clinician adoption, partner engagement, and technological capabilities.16
Unfortunately, significant barriers exist for the VA CNH program within each of these information exchange domains. For example, patient engagement requires veteran consent for consumer-initiated exchange of medical information, which is not practical due to the high prevalence of cognitive impairment in NHs. Similarly, VA consent requirements prohibit EHR download and sharing with non-VA facilities like CNHs, limiting use. eHealth Exchange partnerships allow organizations caring for veterans to connect with the VA via networks that provide a common trust agreement and technical compliance testing. Unfortunately, in 2017, only 257 NHs in which veterans received care nationally were eHealth Exchange partners, which indicates that while NHs could partner in this information exchange, very few did.16
Finally, while the exchange is possible, it is not practical; most CNHs lack the staff that would be required to support data transfer on their technology platform into the appropriate translational gateways. Although remote access to EHRs in CNHs improved during the pandemic, the Veterans Health Information Exchange Program is not designed to facilitate interoperability of VA and CNH records and remains a significant barrier for this and many other VA community care programs.
The dispersal of veterans across CNHs that are > 50 miles from the nearest VAMC represents an additional area to improve program efficiency and meet growing demands for rural care. While recent field guidance to CNH oversight teams reduces the frequency of visits by VA CNH teams, the burden of driving to each facility is not likely to decrease as CNHs increasingly offer rehabilitation as a part of Veteran Care Agreements.17 Visits performed by telehealth or by trained local VA staff may represent alternatives.15
Finally, interview participants described the ideal range of the number of veterans in each CNH necessary to optimize efficiencies. Veterans who rely more heavily upon VA care tend to have more medical and mental health comorbidities than average Medicare beneficiaries.18,19 This was reflected in participants’ recommendation to have enough veterans to benefit from economies of scale but to also identify a limit when efficiencies are lost. Given that most CNHs cared for 8 to 15 veterans, facilities seem to have identified how best to match the resources available with veterans’ care needs. Based on these observations, new care networks that will be established because of the MISSION Act may benefit from establishing evidence-based policies that support flexibility in oversight frequency and either allow for remote oversight or consolidate the number of CNHs to improve efficiencies in care provision and oversight.20
Limitations
Limitations include the unique relationship between VA and CNH staff overseeing the quality of care provided to veterans in CNHs, which is not replicated in other models of care. Data collection was interrupted following the passage of the MISSION Act in 2018 until guidance on changes to practice resulting from the law were clarified in 2020. Interviews were also interrupted at the onset of the COVID-19 pandemic.
Conclusions
The current quality of the CNH care oversight structure will require adaptation as demand for CNH care increases. While the VA CNH program is one of the longest-standing programs collaborating with non-VA community care partners, it is now only one of many following the MISSION Act. The success of this and other programs will depend on matching available CNH resources to the complex medical and psychological needs of veterans. At a time when strategies to ease the burden on NHs and VA CNH coordinators are desperately needed, Veterans Health Information Exchange capabilities need to improve. Evidence is needed to guide the scaling of the program to meet the needs of the rapidly expanding veteran population who are eligible for CNH care.
Acknowledgments
The authors acknowledge Amy Mochel of the Providence Veterans Affairs Medical Center for project management support of this project.
The US Department of Veterans Affairs (VA) Community Nursing Home (CNH) program provides 24-hour skilled nursing care for eligible veterans in public or private community-based facilities that have established a contract to care for veterans. Veteran eligibility is based on service-connected status and level of disability, covering the cost of care for veterans who need long-term care because of their service-connected disability or for veterans with disabilities rated at ≥ 70%.1 Between 2014 and 2018, the average daily census of veterans in CNHs increased by 26% and the percentage of funds obligated to this program increased by 49%.2 The VA projects that the number of veterans receiving care in a CNH program will increase by 80% between 2017 and 2037, corresponding to a 149% increase in CNH expenditures.2
CNH program oversight teams are mandated at each VA medical center (VAMC) to monitor care coordination within the CNH program. These teams include nurses and social workers (SWs) who perform regular on-site assessments to monitor the clinical, functional, and psychosocial needs of veterans. These assessments include a review of the electronic health record (EHR) and face-to-face contact with veterans and CNH staff, regardless of the purchasing authority (hospice, long-term care, short-term rehabilitation, respite care).3 These teams represent key stakeholders impacted by CNH program expansion.
While the CNH program has focused primarily on the provision of long-term care, the VA is now expanding to include short-term rehabilitation through Veteran Care Agreements.4 These agreements are authorized under the MISSION Act, designed to improve care for veterans.5 Veteran Care Agreements are expected to be less burdensome to execute than traditional contracts and will permit the VA to partner with more CNHs, as noted in a Congressional Research Service report regarding long-term care services for veterans.6 However, increasing the number of CNHs increases demands on oversight teams, particularly if the coordinators are compelled to perform monthly on-site visits to facilities required under current guidelines.3
The objective of this study was to describe the experiences of VA and CNH staff involved in care coordination and the oversight of veterans receiving CNH care amid Veteran Care Agreement implementation and in anticipation of CNH program expansion. The results are intended to inform expansion efforts within the CNH program.
METHODS
This study was a component of a larger research project examining VA-purchased CNH care; recruitment methods are available in previous publications describing this work.7 Participants provided written or verbal consent before video and phone interviews, respectively. This study was approved by the Colorado Multiple Institutional Review Board (Protocol #18-1186).
Video and phone interviews were conducted by 3 team members from October 2018 to March 2020 with CNH staff and VA CNH program oversight team members. Participant recruitment was paused from May to October 2020 as a result of the COVID-19 pandemic and ambiguity about VA NH care purchasing policies following the passage of the VA MISSION Act.5 We used semistructured interview guides (eAppendix 1 for VA staff and eAppendix 2 for NH staff, available online at doi:10.12788/fp.0421). Recorded and transcribed interviews ranged from 15 to 90 minutes.
Two members of the research team analyzed transcripts using both deductive and inductive content analysis.8 The interview guide informed an a priori codebook, and in vivo codes were included as they emerged. We jointly coded 6 transcripts to reach a consensus on coding approaches and analyzed the remaining transcripts independently with frequent meetings to develop themes with a qualitative methodologist. All qualitative data were analyzed using ATLAS.ti software.
This was a retrospective observational study of veterans who received VA-paid care in CNHs during the 2019 fiscal year (10/1/2018-9/30/2019) using data from the enrollment, inpatient and outpatient encounters, and other care paid for by the VA in the VA Corporate Data Warehouse. We linked Centers for Medicare and Medicaid monthly Nursing Home Compare reports and the Brown University Long Term Care: Facts on Care in the US (LTC FoCUS) annual files to identify facility addresses.9
Descriptive analyses of quantitative data were conducted in parallel with the qualitative findings.8 Distance from the contracting VAMC to CNH was calculated using the greater-circle formula to find the linear distance between geographic coordinates. Quantitative and qualitative data were collected concurrently, analyzed independently, and integrated into the interpretation of results.10
RESULTS
We conducted 36 interviews with VA and NH staff who were affiliated with 6 VAMCs and 17 CNHs. Four themes emerged concerning CNH oversight: (1) benefits of VA CNH team engagement/visits; (2) burden of VA CNH oversight; (3) burden of oversight limited the ability to contract with additional NHs; and (4) factors that ease the burden and facilitate successful oversight.
Benefits of Engagement/Visits
VA SWs and nurses visit each veteran every 30 to 45 days to review their health records, meet with them, and check in with NH staff. In addition, VA SWs and nurses coordinate each veteran’s care by working as liaisons between the VA and the NH to help NH staff problem solve veteran-related issues through care conferences. VA SWs and nurses act as extra advocates for veterans to make sure their needs are met. “This program definitely helps ensure that veterans are receiving higher quality care because if we see that they aren’t, then we do something about it,” a VA NH coordinator reported in an interview.
NH staff noted benefits to monthly VA staff visits, including having an additional person coordinating care and built-in VA liaisons. “It’s nice to have that extra set of eyes, people that you can care plan with,” an NH administrator shared. “It’s definitely a true partnership, and we have open and honest conversations so we can really provide a good service for our veterans.”
Distance & High Veteran Census Burdens
VA participants described oversight components as burdensome. Specifically, several VA participants mentioned that the charting they completed in the facility during each visit proved time consuming and onerous, particularly for distant NHs. To accommodate veterans’ preferences to receive care in a facility close to their homes and families, VAMCs contract with NHs that are geographically spread out. “We’re just all spread out… staff have issues driving 2 and a half hours just to review charts all day,” a VA CNH coordinator explained. In 2019, the mean distance between VAMC and NH was 48 miles, with half located > 32 miles from the VAMC. One-quarter of NHs were > 70 miles and 44% were located > 50 miles from the VAMC (Figure 1).
Participants highlighted how regular oversight visits were particularly time consuming at CNHs with a large contracted population. VA nurses and SWs spend multiple days and up to a week conducting oversight visits at facilities with large numbers of veterans. Another VA nurse highlighted how charting requirements resulted in several days of documentation outside of the NH visit for facilities with many contracted veteran residents. Multiple VA participants noted that having many veterans at an NH exacerbated the oversight burdens. In 2019, 252 (28%) of VA CNHs had > 10 contracted veterans and 1 facility had 34 veterans (Figure 2). VA participants perceived having too many veterans concentrated at 1 facility as potentially challenging for CNHs due to the complex care needs of veterans and the added need for care coordination with the VA. One VA NH coordinator noted that while some facilities were “adept at being able to handle higher numbers” of veterans, others were “overwhelmed.” Too many veterans at an NH, an SW explained, might lead the “facility to fail because we are such a cumbersome system.”
Oversight & Staffing Burden
While several participants described wanting to contract with more NHs to avoid overwhelming existing CNHs and to increase choice for veterans, they expressed concerns about their ability to provide oversight at more facilities due to limited staffing and oversight requirements. Across VAMCs, the median number of VA CNHs varied substantially (Figure 3). One VA participant with about 35 CNHs explained that while adding more NHs could create “more opportunities and options” for veterans, it needs to be balanced with the required oversight responsibilities. One VA nurse insisted that more staff were needed to meet current and future oversight needs. “We’re all getting stretched pretty thin, and just so we don’t drop the ball on things… I would like to see a little more staff if we’re gonna have a lot more nursing homes.”
Participants had concerns related to the VA MISSION Act and the possibility of more VA-paid NHs for rehabilitation or short-term care. Participants underscored the necessity for additional staff to account for the increased oversight burden or a reduction in oversight requirements. One SW felt that increasing the number of CNHs would increase the required oversight and the need for collaboration with NH staff, which would limit her ability to establish close and trusting working relationships with NH staff. Participants also described the challenges of meeting their current oversight requirements, which limited extra visits for acute issues and care conferences. This was attributed to a lack of adequate staffing in the VA CNH program, given the time-intensive nature of VA oversight requirements.
Easing Burden & Facilitating Oversight
Participants noted how obtaining remote access to veterans’ EHRs allowed them to conduct chart reviews before oversight visits. This permitted more time for interaction with veterans and CNH staff as well as coordinating care. While providing access to the VA EHR would not change the chart review component of VA oversight, some participants felt it might improve care coordination between VA and NH staff during monthly visits.
Participants felt they were able to build strong working relationships with facilities with more veterans due to frequent communication and collaboration. VA participants also noted that CNHs with larger veteran censuses were more likely to respond to VA concerns about care to maintain the business relationship and contract. To optimize strong working relationships and decrease the challenges of having too many veterans at a facility, some VA participants suggested that CNH programs create a local policy to recommend the number of veterans placed in a CNH.
Discussion
Participants interviewed for this study echoed findings from previous work that identified the importance of developing trusted working relationships with CNHs to care for veterans.11,12 However, interorganizational care coordination, a shortage of health care professionals, and resource demands associated with caring for veterans reported in other community care settings were also noted in our findings.12,13
Building upon prior recommendations related to community care of veterans, our analysis identified key areas that could improve CNH program oversight efficiency, including: (1) improving the interoperability of EHRs to facilitate coordination of care and oversight; (2) addressing inefficiencies associated with traveling to geographically dispersed CNHs; and (3) “right-sizing” the number of veterans residing in each CNH.
The interoperability of EHRs has been cited by multiple studies of VA community care programs as critical to reducing inefficiencies and allowing more in-person time with veterans and staff in care coordination, especially at rural locations.11-15 The Veterans Health Information Exchange Program is designed to optimize data sharing as veterans are increasingly referred to non-VA care through the MISSION Act. This program is organized around patient engagement, clinician adoption, partner engagement, and technological capabilities.16
Unfortunately, significant barriers exist for the VA CNH program within each of these information exchange domains. For example, patient engagement requires veteran consent for consumer-initiated exchange of medical information, which is not practical due to the high prevalence of cognitive impairment in NHs. Similarly, VA consent requirements prohibit EHR download and sharing with non-VA facilities like CNHs, limiting use. eHealth Exchange partnerships allow organizations caring for veterans to connect with the VA via networks that provide a common trust agreement and technical compliance testing. Unfortunately, in 2017, only 257 NHs in which veterans received care nationally were eHealth Exchange partners, which indicates that while NHs could partner in this information exchange, very few did.16
Finally, while the exchange is possible, it is not practical; most CNHs lack the staff that would be required to support data transfer on their technology platform into the appropriate translational gateways. Although remote access to EHRs in CNHs improved during the pandemic, the Veterans Health Information Exchange Program is not designed to facilitate interoperability of VA and CNH records and remains a significant barrier for this and many other VA community care programs.
The dispersal of veterans across CNHs that are > 50 miles from the nearest VAMC represents an additional area to improve program efficiency and meet growing demands for rural care. While recent field guidance to CNH oversight teams reduces the frequency of visits by VA CNH teams, the burden of driving to each facility is not likely to decrease as CNHs increasingly offer rehabilitation as a part of Veteran Care Agreements.17 Visits performed by telehealth or by trained local VA staff may represent alternatives.15
Finally, interview participants described the ideal range of the number of veterans in each CNH necessary to optimize efficiencies. Veterans who rely more heavily upon VA care tend to have more medical and mental health comorbidities than average Medicare beneficiaries.18,19 This was reflected in participants’ recommendation to have enough veterans to benefit from economies of scale but to also identify a limit when efficiencies are lost. Given that most CNHs cared for 8 to 15 veterans, facilities seem to have identified how best to match the resources available with veterans’ care needs. Based on these observations, new care networks that will be established because of the MISSION Act may benefit from establishing evidence-based policies that support flexibility in oversight frequency and either allow for remote oversight or consolidate the number of CNHs to improve efficiencies in care provision and oversight.20
Limitations
Limitations include the unique relationship between VA and CNH staff overseeing the quality of care provided to veterans in CNHs, which is not replicated in other models of care. Data collection was interrupted following the passage of the MISSION Act in 2018 until guidance on changes to practice resulting from the law were clarified in 2020. Interviews were also interrupted at the onset of the COVID-19 pandemic.
Conclusions
The current quality of the CNH care oversight structure will require adaptation as demand for CNH care increases. While the VA CNH program is one of the longest-standing programs collaborating with non-VA community care partners, it is now only one of many following the MISSION Act. The success of this and other programs will depend on matching available CNH resources to the complex medical and psychological needs of veterans. At a time when strategies to ease the burden on NHs and VA CNH coordinators are desperately needed, Veterans Health Information Exchange capabilities need to improve. Evidence is needed to guide the scaling of the program to meet the needs of the rapidly expanding veteran population who are eligible for CNH care.
Acknowledgments
The authors acknowledge Amy Mochel of the Providence Veterans Affairs Medical Center for project management support of this project.
1. Miller EA, Gadbois E, Gidmark S, Intrator O. Purchasing nursing home care within the Veterans Health Administration: lessons for nursing home recruitment, contracting, and oversight. J Health Admin Educ. 2015;32(2):165-197.
2. GAO. VA health care. Veterans’ use of long-term care is increasing, and VA faces challenges in meeting the demand. February 19, 2020. Accessed September 19, 2023. https://www.gao.gov/assets/gao-20-284.pdf
3. VHA Handbook 1143.2, VHA community nursing home oversight procedures. US Department of Veterans Affairs, Veterans Health Administration. June 2004. https://www.vendorportal.ecms.va.gov/FBODocumentServer/DocumentServer.aspx?DocumentId=3740930&FileName=VA259-17-Q-0501-007.pdf
4. Community care: veteran care agreements. US Department of Veterans Affairs. 2022. Updated August 8, 2023. Accessed September 7, 2023. https://www.va.gov/COMMUNITYCARE/providers/Veterans_Care_Agreements.asp
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION Act and the future of veterans’ access to quality health care. JAMA. 2020;324(4):343-344. doi:10.1001/jama.2020.4505
6. Colello KJ, Panangala SV; Congressional Research Service. Long-term care services for veterans. February 14, 2017. Accessed September 7, 2023. https://crsreports.congress.gov/product/pdf/R/R44697
7. Magid KH, Galenbeck E, Haverhals LM, et al. Purchasing high-quality community nursing home care: a will to work with VHA diminished by contracting burdens. J Am Med Dir Assoc. 2022;23(11):1757-1764. doi:10.1016/j.jamda.2022.03.007
8. Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398-405. doi:10.1111/nhs.12048
9. Brown University. LTC Focus. Accessed September 18, 2023. https://ltcfocus.org/about
10. Zhang W, Creswell J. The use of “mixing” procedure of mixed methods in health services research. Med Care. 2013;51(8):e51-e57. doi:10.1097/MLR.0b013e31824642fd
11. Haverhals LM, Magid KH, Blanchard KN, Levy CR. Veterans Health Administration staff perceptions of overseeing care in community nursing homes during COVID-19. Gerontol Geriatr Med. 2022;8:23337214221080307. Published 2022 Feb 15. doi:10.1177/23337214221080307
12. Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
13. Schlosser J, Kollisch D, Johnson D, Perkins T, Olson A. VA-community dual care: veteran and clinician perspectives. J Community Health. 2020;45(4):795-802. doi:10.1007/s10900-020-00795-y
14. Nevedal AL, Wong EP, Urech TH, Peppiatt JL, Sorie MR, Vashi AA. Veterans’ experiences with accessing community emergency care. Mil Med. 2023;188(1-2):e58-e64. doi:10.1093/milmed/usab196
15. Levenson SA. Smart case review: a model for successful remote medical direction and enhanced nursing home quality improvement. J Am Med Dir Assoc. 2021;22(10):2212-2215.e6. doi:10.1016/j.jamda.2021.05.043
16. Donahue M, Bouhaddou O, Hsing N, et al. Veterans Health Information Exchange: successes and challenges of nationwide interoperability. AMIA Annu Symp Proc. 2018;2018:385-394. Published 2018 Dec 5.
17. US Department of Veterans Affairs. VHA Notice 2023-07. Community Nursing Home Program. September 5, 2023:1-4.
18. Helmer DA, Dwibedi N, Rowneki M, et al. Mental health conditions and hospitalizations for ambulatory care sensitive conditions among veterans with diabetes. Am Health Drug Benefits. 2020;13(2):61-71.
19. Rosen AK, Wagner TH, Pettey WBP, et al. Differences in risk scores of veterans receiving community care purchased by the Veterans Health Administration. Health Serv Res. 2018;53(suppl 3):5438-5454. doi:10.1111/1475-6773.13051
20. Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
1. Miller EA, Gadbois E, Gidmark S, Intrator O. Purchasing nursing home care within the Veterans Health Administration: lessons for nursing home recruitment, contracting, and oversight. J Health Admin Educ. 2015;32(2):165-197.
2. GAO. VA health care. Veterans’ use of long-term care is increasing, and VA faces challenges in meeting the demand. February 19, 2020. Accessed September 19, 2023. https://www.gao.gov/assets/gao-20-284.pdf
3. VHA Handbook 1143.2, VHA community nursing home oversight procedures. US Department of Veterans Affairs, Veterans Health Administration. June 2004. https://www.vendorportal.ecms.va.gov/FBODocumentServer/DocumentServer.aspx?DocumentId=3740930&FileName=VA259-17-Q-0501-007.pdf
4. Community care: veteran care agreements. US Department of Veterans Affairs. 2022. Updated August 8, 2023. Accessed September 7, 2023. https://www.va.gov/COMMUNITYCARE/providers/Veterans_Care_Agreements.asp
5. Massarweh NN, Itani KMF, Morris MS. The VA MISSION Act and the future of veterans’ access to quality health care. JAMA. 2020;324(4):343-344. doi:10.1001/jama.2020.4505
6. Colello KJ, Panangala SV; Congressional Research Service. Long-term care services for veterans. February 14, 2017. Accessed September 7, 2023. https://crsreports.congress.gov/product/pdf/R/R44697
7. Magid KH, Galenbeck E, Haverhals LM, et al. Purchasing high-quality community nursing home care: a will to work with VHA diminished by contracting burdens. J Am Med Dir Assoc. 2022;23(11):1757-1764. doi:10.1016/j.jamda.2022.03.007
8. Vaismoradi M, Turunen H, Bondas T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs Health Sci. 2013;15(3):398-405. doi:10.1111/nhs.12048
9. Brown University. LTC Focus. Accessed September 18, 2023. https://ltcfocus.org/about
10. Zhang W, Creswell J. The use of “mixing” procedure of mixed methods in health services research. Med Care. 2013;51(8):e51-e57. doi:10.1097/MLR.0b013e31824642fd
11. Haverhals LM, Magid KH, Blanchard KN, Levy CR. Veterans Health Administration staff perceptions of overseeing care in community nursing homes during COVID-19. Gerontol Geriatr Med. 2022;8:23337214221080307. Published 2022 Feb 15. doi:10.1177/23337214221080307
12. Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
13. Schlosser J, Kollisch D, Johnson D, Perkins T, Olson A. VA-community dual care: veteran and clinician perspectives. J Community Health. 2020;45(4):795-802. doi:10.1007/s10900-020-00795-y
14. Nevedal AL, Wong EP, Urech TH, Peppiatt JL, Sorie MR, Vashi AA. Veterans’ experiences with accessing community emergency care. Mil Med. 2023;188(1-2):e58-e64. doi:10.1093/milmed/usab196
15. Levenson SA. Smart case review: a model for successful remote medical direction and enhanced nursing home quality improvement. J Am Med Dir Assoc. 2021;22(10):2212-2215.e6. doi:10.1016/j.jamda.2021.05.043
16. Donahue M, Bouhaddou O, Hsing N, et al. Veterans Health Information Exchange: successes and challenges of nationwide interoperability. AMIA Annu Symp Proc. 2018;2018:385-394. Published 2018 Dec 5.
17. US Department of Veterans Affairs. VHA Notice 2023-07. Community Nursing Home Program. September 5, 2023:1-4.
18. Helmer DA, Dwibedi N, Rowneki M, et al. Mental health conditions and hospitalizations for ambulatory care sensitive conditions among veterans with diabetes. Am Health Drug Benefits. 2020;13(2):61-71.
19. Rosen AK, Wagner TH, Pettey WBP, et al. Differences in risk scores of veterans receiving community care purchased by the Veterans Health Administration. Health Serv Res. 2018;53(suppl 3):5438-5454. doi:10.1111/1475-6773.13051
20. Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
Progressive Pulmonary Fibrosis: Understanding Its Many Forms
- Raghu G et al. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/rccm.202202-0399ST
- Cottin V et al. Front Med (Lausanne). 2022;9:799912. doi:10.3389/fmed.2022.799912
- Molina-Molina M et al. Expert Rev Respir Med. 2022;16(7):765-774. doi:10.1080/17476348.2022.2107508
- Cottin V. Am J Respir Crit Care Med. 2023;207(1):11-13. doi:10.1164/rccm.202208-1639ED
- Wijsenbeek M, Cottin V. N Engl J Med. 2020;383(10):958-968. doi:10.1056/NEJMra2005230
- Chiu YH et al. Front Med (Lausanne). 2023;10:1106560. doi:10.3389/fmed.2023.1106560
- Wong AW et al. BMC Pulm Med. 2022;22(1):148. doi:10.1186/s12890-022-01922-2
- Raghu G et al. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/rccm.202202-0399ST
- Cottin V et al. Front Med (Lausanne). 2022;9:799912. doi:10.3389/fmed.2022.799912
- Molina-Molina M et al. Expert Rev Respir Med. 2022;16(7):765-774. doi:10.1080/17476348.2022.2107508
- Cottin V. Am J Respir Crit Care Med. 2023;207(1):11-13. doi:10.1164/rccm.202208-1639ED
- Wijsenbeek M, Cottin V. N Engl J Med. 2020;383(10):958-968. doi:10.1056/NEJMra2005230
- Chiu YH et al. Front Med (Lausanne). 2023;10:1106560. doi:10.3389/fmed.2023.1106560
- Wong AW et al. BMC Pulm Med. 2022;22(1):148. doi:10.1186/s12890-022-01922-2
- Raghu G et al. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/rccm.202202-0399ST
- Cottin V et al. Front Med (Lausanne). 2022;9:799912. doi:10.3389/fmed.2022.799912
- Molina-Molina M et al. Expert Rev Respir Med. 2022;16(7):765-774. doi:10.1080/17476348.2022.2107508
- Cottin V. Am J Respir Crit Care Med. 2023;207(1):11-13. doi:10.1164/rccm.202208-1639ED
- Wijsenbeek M, Cottin V. N Engl J Med. 2020;383(10):958-968. doi:10.1056/NEJMra2005230
- Chiu YH et al. Front Med (Lausanne). 2023;10:1106560. doi:10.3389/fmed.2023.1106560
- Wong AW et al. BMC Pulm Med. 2022;22(1):148. doi:10.1186/s12890-022-01922-2
Long COVID: Advocating for Patients and Implementing Effective Techniques
1. Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
2. Davis HE et al. Nat Rev Microbiol. 2023;21(3):133-146. doi:10.1038/s41579-022-00846-2
3. Ahmed H et al. J Rehabil Med. 2020;52(5):jrm00063. doi:10.2340/16501977-2694
4. Resources. Long COVID Physio. Accessed May 31, 2023. https://longcovid.physio/resources
5. Long COVID: What do the latest data show? KFF. Published January 26, 2023. Accessed May 31, 2023. https://www.kff.org/policy-watch/long-covid-what-do-latest-data-show/
6. Castanares-Zapatero D et al. Ann Med. 2022;54(1):1473-1487. doi:10.1080/07853890.2022.2076901
7. Mehandru S, Merad M. Nat Immunol. 2022;23(2):194-202. doi:10.1038/s41590-021-01104-y
8. Dhooria S et al. Eur Respir J. 2022;59(2):2102930. doi:10.1183/13993003.02930-2021
9. Researching COVID to enhance recovery. RECOVER. Accessed May 31, 2023. https://recovercovid.org/
1. Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
2. Davis HE et al. Nat Rev Microbiol. 2023;21(3):133-146. doi:10.1038/s41579-022-00846-2
3. Ahmed H et al. J Rehabil Med. 2020;52(5):jrm00063. doi:10.2340/16501977-2694
4. Resources. Long COVID Physio. Accessed May 31, 2023. https://longcovid.physio/resources
5. Long COVID: What do the latest data show? KFF. Published January 26, 2023. Accessed May 31, 2023. https://www.kff.org/policy-watch/long-covid-what-do-latest-data-show/
6. Castanares-Zapatero D et al. Ann Med. 2022;54(1):1473-1487. doi:10.1080/07853890.2022.2076901
7. Mehandru S, Merad M. Nat Immunol. 2022;23(2):194-202. doi:10.1038/s41590-021-01104-y
8. Dhooria S et al. Eur Respir J. 2022;59(2):2102930. doi:10.1183/13993003.02930-2021
9. Researching COVID to enhance recovery. RECOVER. Accessed May 31, 2023. https://recovercovid.org/
1. Lutchmansingh DD et al. Semin Respir Crit Care Med. 2023;44(1):130-142. doi:10.1055/s-0042-1759568
2. Davis HE et al. Nat Rev Microbiol. 2023;21(3):133-146. doi:10.1038/s41579-022-00846-2
3. Ahmed H et al. J Rehabil Med. 2020;52(5):jrm00063. doi:10.2340/16501977-2694
4. Resources. Long COVID Physio. Accessed May 31, 2023. https://longcovid.physio/resources
5. Long COVID: What do the latest data show? KFF. Published January 26, 2023. Accessed May 31, 2023. https://www.kff.org/policy-watch/long-covid-what-do-latest-data-show/
6. Castanares-Zapatero D et al. Ann Med. 2022;54(1):1473-1487. doi:10.1080/07853890.2022.2076901
7. Mehandru S, Merad M. Nat Immunol. 2022;23(2):194-202. doi:10.1038/s41590-021-01104-y
8. Dhooria S et al. Eur Respir J. 2022;59(2):2102930. doi:10.1183/13993003.02930-2021
9. Researching COVID to enhance recovery. RECOVER. Accessed May 31, 2023. https://recovercovid.org/