User login
Implementation of an Interfacility Telehealth Cancer Genetics Clinic
BACKGROUND
Cancer risk assessment and genetic counseling are the processes to identify and counsel people at risk for familial or hereditary cancer syndromes. They serve to inform, educate and empower patients and family members to make informed decisions about testing, cancer screening, and prevention. Additionally, genetic testing can also provide therapeutic options and opportunities for research.
METHODS
Prior to this program initiative, there were no cancer genetics services available at the VA Pittsburgh Medical Center (VAPHS) and 100% of genetics consults were referred to the community. Each year over $100,000 was spent outside of VAPHS on genetic testing and counseling. Community care referral resulted in fragmented care, prolonged wait times of 3 to 5 months, communication issues, and added financial cost to the institution. Corporal Michael J. Crescenz VA Medical Center (CMCVAMC) had previously created a genetics consultation service staffed with an advanced practice nurse that increased access to genetics services and testing rates at the facility-level. VAPHS recently established an interfacility telegenetics clinic with CMCVAMC to provide virtual genetic counseling services to Veterans at VAPHS. Under this program, VAPHS providers place an interfacility consult for Veterans who need cancer genetics services. The consult is received and reviewed by the CMCVAMC team. VAPHS patients are then seen by CMCVAMC providers via VVC or CVT and provide recommendations regarding additional genetic testing and follow-up.
RESULTS
The telegenetics clinic opened in October 2022. The clinic initially focused on patients with metastatic prostate cancer but has since expanded to provide care for all patients for whom genetics testing and/ or counseling is recommended by NCCN guidelines. Since initiation, 29 consults have been placed and 26 have been completed or are in process (89.6%). In the year prior to creation of the clinic, only 31 of 67 (46%) of referred patients completed genetics evaluation.
CONCLUSIONS
Due to the success of the clinic, plans to expand services to the VISN-level and within VAPHS to include high risk breast cancer assessment are underway. Efforts to provide genetic counseling services via virtual care modalities have the potential to increase access to care and to improve outcomes for veterans with cancer.
BACKGROUND
Cancer risk assessment and genetic counseling are the processes to identify and counsel people at risk for familial or hereditary cancer syndromes. They serve to inform, educate and empower patients and family members to make informed decisions about testing, cancer screening, and prevention. Additionally, genetic testing can also provide therapeutic options and opportunities for research.
METHODS
Prior to this program initiative, there were no cancer genetics services available at the VA Pittsburgh Medical Center (VAPHS) and 100% of genetics consults were referred to the community. Each year over $100,000 was spent outside of VAPHS on genetic testing and counseling. Community care referral resulted in fragmented care, prolonged wait times of 3 to 5 months, communication issues, and added financial cost to the institution. Corporal Michael J. Crescenz VA Medical Center (CMCVAMC) had previously created a genetics consultation service staffed with an advanced practice nurse that increased access to genetics services and testing rates at the facility-level. VAPHS recently established an interfacility telegenetics clinic with CMCVAMC to provide virtual genetic counseling services to Veterans at VAPHS. Under this program, VAPHS providers place an interfacility consult for Veterans who need cancer genetics services. The consult is received and reviewed by the CMCVAMC team. VAPHS patients are then seen by CMCVAMC providers via VVC or CVT and provide recommendations regarding additional genetic testing and follow-up.
RESULTS
The telegenetics clinic opened in October 2022. The clinic initially focused on patients with metastatic prostate cancer but has since expanded to provide care for all patients for whom genetics testing and/ or counseling is recommended by NCCN guidelines. Since initiation, 29 consults have been placed and 26 have been completed or are in process (89.6%). In the year prior to creation of the clinic, only 31 of 67 (46%) of referred patients completed genetics evaluation.
CONCLUSIONS
Due to the success of the clinic, plans to expand services to the VISN-level and within VAPHS to include high risk breast cancer assessment are underway. Efforts to provide genetic counseling services via virtual care modalities have the potential to increase access to care and to improve outcomes for veterans with cancer.
BACKGROUND
Cancer risk assessment and genetic counseling are the processes to identify and counsel people at risk for familial or hereditary cancer syndromes. They serve to inform, educate and empower patients and family members to make informed decisions about testing, cancer screening, and prevention. Additionally, genetic testing can also provide therapeutic options and opportunities for research.
METHODS
Prior to this program initiative, there were no cancer genetics services available at the VA Pittsburgh Medical Center (VAPHS) and 100% of genetics consults were referred to the community. Each year over $100,000 was spent outside of VAPHS on genetic testing and counseling. Community care referral resulted in fragmented care, prolonged wait times of 3 to 5 months, communication issues, and added financial cost to the institution. Corporal Michael J. Crescenz VA Medical Center (CMCVAMC) had previously created a genetics consultation service staffed with an advanced practice nurse that increased access to genetics services and testing rates at the facility-level. VAPHS recently established an interfacility telegenetics clinic with CMCVAMC to provide virtual genetic counseling services to Veterans at VAPHS. Under this program, VAPHS providers place an interfacility consult for Veterans who need cancer genetics services. The consult is received and reviewed by the CMCVAMC team. VAPHS patients are then seen by CMCVAMC providers via VVC or CVT and provide recommendations regarding additional genetic testing and follow-up.
RESULTS
The telegenetics clinic opened in October 2022. The clinic initially focused on patients with metastatic prostate cancer but has since expanded to provide care for all patients for whom genetics testing and/ or counseling is recommended by NCCN guidelines. Since initiation, 29 consults have been placed and 26 have been completed or are in process (89.6%). In the year prior to creation of the clinic, only 31 of 67 (46%) of referred patients completed genetics evaluation.
CONCLUSIONS
Due to the success of the clinic, plans to expand services to the VISN-level and within VAPHS to include high risk breast cancer assessment are underway. Efforts to provide genetic counseling services via virtual care modalities have the potential to increase access to care and to improve outcomes for veterans with cancer.
Comparing Outcomes and Toxicities With Standard and Reduced Dose Melphalan in Autologous Stem Cell Transplant Patients With Multiple Myeloma
BACKGROUND
Multiple myeloma, an incurable plasma cell malignancy, has an average age of diagnosis over 65 years. For transplant-eligible patients, high-dose melphalan 200 mg/m2 (MEL200), followed by autologous stem cell rescue (ASCR) is the standard in consolidation therapy. Most clinical trials evaluating MEL200 with ASCR excluded patients over 65 due to concerns for toxicity and treatment-related mortality, leading to use of reduced dose melphalan 140 mg/m2 (MEL140) in clinical practice for older patients. As this dose has limited studies surrounding its reduction, the purpose of this study was to compare outcomes and toxicities of MEL140 in patients over the age of 65 to MEL200 in patients 65 and under.
METHODS
This single-center institutional review board approved retrospective study was conducted at VA Tennessee Valley Healthcare System. All multiple myeloma patients greater than 18 years of age who received melphalan with ASCR from January 1, 2018, to December 31, 2021, were included. Patients were divided into two arms: age < 65 treated with MEL200 and age >65 treated with MEL140. The primary endpoint was oneyear progression-free survival (PFS). The secondary endpoints were one-year overall survival (OS), treatment related mortality, time to neutrophil engraftment, and toxicities including febrile neutropenia, diarrhea, mucositis, infection, and intensive care unit transfers.
RESULTS
A total of 222 patients were included, 114 patients in the MEL200 arm and 108 patients in the MEL140 arm. The primary endpoint of one-year PFS had no significant difference, with 103 (90.4%) patients in the MEL200 group compared to 99 (91.7%) patients in the MEL140 group (p=0.732). Similarly, there was no statistically significant difference in the secondary endpoint of one-year OS with 112 (98.3%) patients in the MEL200 group compared to 106 (98.2%) in the MEL140 group (p=0.956). Toxicities were similar; however, grade 3 mucositis was higher in the MEL200 arm.
CONCLUSIONS
Our study found no difference in oneyear PFS or one-year OS when comparing MEL140 to MEL200 with minimal differences in regimen-related toxicities. Although not powered to detect statistical difference, results suggests that dose reduction with MEL140 in patients >65 years does not impact one-year PFS when compared to patients <65 receiving standard MEL200.
BACKGROUND
Multiple myeloma, an incurable plasma cell malignancy, has an average age of diagnosis over 65 years. For transplant-eligible patients, high-dose melphalan 200 mg/m2 (MEL200), followed by autologous stem cell rescue (ASCR) is the standard in consolidation therapy. Most clinical trials evaluating MEL200 with ASCR excluded patients over 65 due to concerns for toxicity and treatment-related mortality, leading to use of reduced dose melphalan 140 mg/m2 (MEL140) in clinical practice for older patients. As this dose has limited studies surrounding its reduction, the purpose of this study was to compare outcomes and toxicities of MEL140 in patients over the age of 65 to MEL200 in patients 65 and under.
METHODS
This single-center institutional review board approved retrospective study was conducted at VA Tennessee Valley Healthcare System. All multiple myeloma patients greater than 18 years of age who received melphalan with ASCR from January 1, 2018, to December 31, 2021, were included. Patients were divided into two arms: age < 65 treated with MEL200 and age >65 treated with MEL140. The primary endpoint was oneyear progression-free survival (PFS). The secondary endpoints were one-year overall survival (OS), treatment related mortality, time to neutrophil engraftment, and toxicities including febrile neutropenia, diarrhea, mucositis, infection, and intensive care unit transfers.
RESULTS
A total of 222 patients were included, 114 patients in the MEL200 arm and 108 patients in the MEL140 arm. The primary endpoint of one-year PFS had no significant difference, with 103 (90.4%) patients in the MEL200 group compared to 99 (91.7%) patients in the MEL140 group (p=0.732). Similarly, there was no statistically significant difference in the secondary endpoint of one-year OS with 112 (98.3%) patients in the MEL200 group compared to 106 (98.2%) in the MEL140 group (p=0.956). Toxicities were similar; however, grade 3 mucositis was higher in the MEL200 arm.
CONCLUSIONS
Our study found no difference in oneyear PFS or one-year OS when comparing MEL140 to MEL200 with minimal differences in regimen-related toxicities. Although not powered to detect statistical difference, results suggests that dose reduction with MEL140 in patients >65 years does not impact one-year PFS when compared to patients <65 receiving standard MEL200.
BACKGROUND
Multiple myeloma, an incurable plasma cell malignancy, has an average age of diagnosis over 65 years. For transplant-eligible patients, high-dose melphalan 200 mg/m2 (MEL200), followed by autologous stem cell rescue (ASCR) is the standard in consolidation therapy. Most clinical trials evaluating MEL200 with ASCR excluded patients over 65 due to concerns for toxicity and treatment-related mortality, leading to use of reduced dose melphalan 140 mg/m2 (MEL140) in clinical practice for older patients. As this dose has limited studies surrounding its reduction, the purpose of this study was to compare outcomes and toxicities of MEL140 in patients over the age of 65 to MEL200 in patients 65 and under.
METHODS
This single-center institutional review board approved retrospective study was conducted at VA Tennessee Valley Healthcare System. All multiple myeloma patients greater than 18 years of age who received melphalan with ASCR from January 1, 2018, to December 31, 2021, were included. Patients were divided into two arms: age < 65 treated with MEL200 and age >65 treated with MEL140. The primary endpoint was oneyear progression-free survival (PFS). The secondary endpoints were one-year overall survival (OS), treatment related mortality, time to neutrophil engraftment, and toxicities including febrile neutropenia, diarrhea, mucositis, infection, and intensive care unit transfers.
RESULTS
A total of 222 patients were included, 114 patients in the MEL200 arm and 108 patients in the MEL140 arm. The primary endpoint of one-year PFS had no significant difference, with 103 (90.4%) patients in the MEL200 group compared to 99 (91.7%) patients in the MEL140 group (p=0.732). Similarly, there was no statistically significant difference in the secondary endpoint of one-year OS with 112 (98.3%) patients in the MEL200 group compared to 106 (98.2%) in the MEL140 group (p=0.956). Toxicities were similar; however, grade 3 mucositis was higher in the MEL200 arm.
CONCLUSIONS
Our study found no difference in oneyear PFS or one-year OS when comparing MEL140 to MEL200 with minimal differences in regimen-related toxicities. Although not powered to detect statistical difference, results suggests that dose reduction with MEL140 in patients >65 years does not impact one-year PFS when compared to patients <65 receiving standard MEL200.
Quality Improvement Project of All Resected Lung Specimens for Pathologic Findings and Synoptic Surgical Reports for Accuracy in Staging: A Critical Review of 91 Specimens
BACKGROUND
In 2017, the Thoracic Tumor Board realized that there were patients whose lung resections had critical review of the slides and reports prior to presentation. Errors were found which resulted in a change of the pathology Tumor Nodal Metastases (pTNM) staging for the patient. The impacts were important for determining appropriate therapy. It was decided to systematically review all lung cancer resections for accuracy before determining definitive therapy recommendations.
METHODS
All lung resections for malignancy were examined prior and up to 2 days of completion for accuracy of tumor type, tumor size, tumor grade, lymph node metastases and pathologic stage (pTNM). Any errors found were given to the original pathologist for a change in the report before release or for a modified report to be issued.
RESULTS
From June 2017 to December 2020, there were 91 lung resections with 28 (30.77%) errors. Errors included: 16 incorrect pathologic staging, 5 missed tumors in lung and lymph nodes, 2 unexamined stapled surgical margins, 1 wrong site, 1 incorrect lymph node number and 2 missed tumor vascular invasion.
IMPLICATIONS
Quality improvement (QI) review of lung resections by a second pathologist is important and may clearly improve pathologic staging for lung cancer patients. It can be added to QI programs currently used in Surgical Pathology. It is important in directing appropriate postsurgical therapies.
BACKGROUND
In 2017, the Thoracic Tumor Board realized that there were patients whose lung resections had critical review of the slides and reports prior to presentation. Errors were found which resulted in a change of the pathology Tumor Nodal Metastases (pTNM) staging for the patient. The impacts were important for determining appropriate therapy. It was decided to systematically review all lung cancer resections for accuracy before determining definitive therapy recommendations.
METHODS
All lung resections for malignancy were examined prior and up to 2 days of completion for accuracy of tumor type, tumor size, tumor grade, lymph node metastases and pathologic stage (pTNM). Any errors found were given to the original pathologist for a change in the report before release or for a modified report to be issued.
RESULTS
From June 2017 to December 2020, there were 91 lung resections with 28 (30.77%) errors. Errors included: 16 incorrect pathologic staging, 5 missed tumors in lung and lymph nodes, 2 unexamined stapled surgical margins, 1 wrong site, 1 incorrect lymph node number and 2 missed tumor vascular invasion.
IMPLICATIONS
Quality improvement (QI) review of lung resections by a second pathologist is important and may clearly improve pathologic staging for lung cancer patients. It can be added to QI programs currently used in Surgical Pathology. It is important in directing appropriate postsurgical therapies.
BACKGROUND
In 2017, the Thoracic Tumor Board realized that there were patients whose lung resections had critical review of the slides and reports prior to presentation. Errors were found which resulted in a change of the pathology Tumor Nodal Metastases (pTNM) staging for the patient. The impacts were important for determining appropriate therapy. It was decided to systematically review all lung cancer resections for accuracy before determining definitive therapy recommendations.
METHODS
All lung resections for malignancy were examined prior and up to 2 days of completion for accuracy of tumor type, tumor size, tumor grade, lymph node metastases and pathologic stage (pTNM). Any errors found were given to the original pathologist for a change in the report before release or for a modified report to be issued.
RESULTS
From June 2017 to December 2020, there were 91 lung resections with 28 (30.77%) errors. Errors included: 16 incorrect pathologic staging, 5 missed tumors in lung and lymph nodes, 2 unexamined stapled surgical margins, 1 wrong site, 1 incorrect lymph node number and 2 missed tumor vascular invasion.
IMPLICATIONS
Quality improvement (QI) review of lung resections by a second pathologist is important and may clearly improve pathologic staging for lung cancer patients. It can be added to QI programs currently used in Surgical Pathology. It is important in directing appropriate postsurgical therapies.
Development of a National Precision Oncology Program (NPOP) Dashboard Suite and Data Mart For Monitoring Somatic Molecular Testing Use
BACKGROUND
As of May 2023, the Veterans Affairs (VA) National Precision Oncology Program (NPOP) has provided somatic molecular testing for nearly 36,000 Veterans with cancer. Automated tools to monitor test use (locally and nationally) have only been available for NGS testing in advanced stage lung and prostate cancer. To better track utilization of NPOP supported testing across all cancer indications, and to support strategies to promote wider adoption, we developed an automated data architecture to monitor program operations. Here, we describe the development of the NPOP data mart and summarize the core components of the NPOP Somatic Molecular Testing dashboards.
METHODS
SQL Server Integration Services was used to build the backend data mart, which required the ingestion of vendor-specific XML data and subsequent harmonization with data found in the VA’s Corporate Data Warehouse (CDW). The NPOP somatic testing dashboards, developed using Power BI, are securely hosted in the cloud, and accessible through SharePoint.
DATA ANALYSIS
The NPOP dashboard suite displays key measures using descriptive statistics, including counts, proportions, means, medians, and standard deviations. To support the visualization of comparisons we leveraged stacked and clustered bar charts, and violin plots.
RESULTS
The NPOP data mart refreshes nightly providing users with near real-time data. The NPOP somatic testing dashboards include an all vendor combined report and sub-reports organized by vendors: Foundation Medicine, Personalis, and Personal Genome Diagnostics and Tempus. All reports contain four views with the ability to toggle between tests ordered or completed. For current NPOP vendors, patient level data and turnaround time views were developed. Data are stratified by test category (i.e., NGS Solid, NGS Liquid, Heme, IHC) and can be viewed longitudinally (i.e., across time) and filtered by test date, VA facility, patient demographics, and cancer characteristics (diagnosis, stage). To date, over 50,000 tests have been ordered (90% through Foundation Medicine).
IMPLICATIONS
The NPOP data mart and operational dashboards synthesizes vast amounts of data into a visually consumable format that supports monitoring the uptake and variation of somatic molecular testing services being offered across the VA.
BACKGROUND
As of May 2023, the Veterans Affairs (VA) National Precision Oncology Program (NPOP) has provided somatic molecular testing for nearly 36,000 Veterans with cancer. Automated tools to monitor test use (locally and nationally) have only been available for NGS testing in advanced stage lung and prostate cancer. To better track utilization of NPOP supported testing across all cancer indications, and to support strategies to promote wider adoption, we developed an automated data architecture to monitor program operations. Here, we describe the development of the NPOP data mart and summarize the core components of the NPOP Somatic Molecular Testing dashboards.
METHODS
SQL Server Integration Services was used to build the backend data mart, which required the ingestion of vendor-specific XML data and subsequent harmonization with data found in the VA’s Corporate Data Warehouse (CDW). The NPOP somatic testing dashboards, developed using Power BI, are securely hosted in the cloud, and accessible through SharePoint.
DATA ANALYSIS
The NPOP dashboard suite displays key measures using descriptive statistics, including counts, proportions, means, medians, and standard deviations. To support the visualization of comparisons we leveraged stacked and clustered bar charts, and violin plots.
RESULTS
The NPOP data mart refreshes nightly providing users with near real-time data. The NPOP somatic testing dashboards include an all vendor combined report and sub-reports organized by vendors: Foundation Medicine, Personalis, and Personal Genome Diagnostics and Tempus. All reports contain four views with the ability to toggle between tests ordered or completed. For current NPOP vendors, patient level data and turnaround time views were developed. Data are stratified by test category (i.e., NGS Solid, NGS Liquid, Heme, IHC) and can be viewed longitudinally (i.e., across time) and filtered by test date, VA facility, patient demographics, and cancer characteristics (diagnosis, stage). To date, over 50,000 tests have been ordered (90% through Foundation Medicine).
IMPLICATIONS
The NPOP data mart and operational dashboards synthesizes vast amounts of data into a visually consumable format that supports monitoring the uptake and variation of somatic molecular testing services being offered across the VA.
BACKGROUND
As of May 2023, the Veterans Affairs (VA) National Precision Oncology Program (NPOP) has provided somatic molecular testing for nearly 36,000 Veterans with cancer. Automated tools to monitor test use (locally and nationally) have only been available for NGS testing in advanced stage lung and prostate cancer. To better track utilization of NPOP supported testing across all cancer indications, and to support strategies to promote wider adoption, we developed an automated data architecture to monitor program operations. Here, we describe the development of the NPOP data mart and summarize the core components of the NPOP Somatic Molecular Testing dashboards.
METHODS
SQL Server Integration Services was used to build the backend data mart, which required the ingestion of vendor-specific XML data and subsequent harmonization with data found in the VA’s Corporate Data Warehouse (CDW). The NPOP somatic testing dashboards, developed using Power BI, are securely hosted in the cloud, and accessible through SharePoint.
DATA ANALYSIS
The NPOP dashboard suite displays key measures using descriptive statistics, including counts, proportions, means, medians, and standard deviations. To support the visualization of comparisons we leveraged stacked and clustered bar charts, and violin plots.
RESULTS
The NPOP data mart refreshes nightly providing users with near real-time data. The NPOP somatic testing dashboards include an all vendor combined report and sub-reports organized by vendors: Foundation Medicine, Personalis, and Personal Genome Diagnostics and Tempus. All reports contain four views with the ability to toggle between tests ordered or completed. For current NPOP vendors, patient level data and turnaround time views were developed. Data are stratified by test category (i.e., NGS Solid, NGS Liquid, Heme, IHC) and can be viewed longitudinally (i.e., across time) and filtered by test date, VA facility, patient demographics, and cancer characteristics (diagnosis, stage). To date, over 50,000 tests have been ordered (90% through Foundation Medicine).
IMPLICATIONS
The NPOP data mart and operational dashboards synthesizes vast amounts of data into a visually consumable format that supports monitoring the uptake and variation of somatic molecular testing services being offered across the VA.
Reversal of Ptosis in Metastatic Prostatic Adenocarcinoma Presenting as Cavernous Sinus Syndrome
INTRODUCTION
Prostate cancer rarely metastasizes to the pituitary gland and the close relationship of these sellar masses to cavernous sinuses and major vessels makes management challenging. We describe a unique case of complete reversal of ptosis in metastatic prostate cancer presenting as cavernous sinus syndrome
CASE REPORT
A 76-year-old male presented with left diplopia, ptosis, and facial numbness. Examination showed left oculomotor palsy and numbness in the V1 and V2 distribution of trigeminal nerve. MRI revealed an 11 × 26 × 17 mm posterior sellar mass extending into the left cavernous sinus. Prolactin was slightly elevated, but rest of the pituitary hormones were normal. Resection of the sellar mass showed metastatic prostatic adenocarcinoma positive for NKX-3.1 and prostate-specific antigen (PSA), and Gleason score 4. PSA was elevated at 32 ng/ mL. Positron emission tomography (PET) showed lesions in the left prostatic lobe, pelvic lymph nodes, L5 spine, and right femoral head. FoundationOne testing found no actionable mutations. He was started on leuprorelin-docetaxel and received radiation for the brain and bony lesions. He is currently being maintained on leuprorelin-abiraterone and prednisone, which he is tolerating well.
DISCUSSION
Pituitary metastases (PMs) from prostate cancer are rare and are usually confined to the posterior pituitary. The close relationship of pituitary masses to the cavernous sinuses and internal carotid artery can lead to catastrophic neurovascular consequences. Imaging has limited sensitivity for differentiating non-invasive metastases from adenomas. Older age, new-onset diabetes insipidus, invasive masses, and rapidly growing lesions should raise suspicion for PMs. Intracranial prostatic metastases indicate poor prognosis with a reported median survival of 6-10 months. Timely diagnosis and management can prevent permanent neurologic damage as illustrated by our case in which ptosis and extraocular symptoms were completely resolved by surgery and radiation. Such unique cases underline the significance of suspecting metastatic disease in appropriate demographic groups and the crucial role of multidisciplinary care for oncologic patients.
CONCLUSIONS
A high index of suspicion for PMs in elderly people with new-onset sellar symptoms and early involvement of multidisciplinary teams can lead to prevention and even reversal of serious neurologic symptoms.
INTRODUCTION
Prostate cancer rarely metastasizes to the pituitary gland and the close relationship of these sellar masses to cavernous sinuses and major vessels makes management challenging. We describe a unique case of complete reversal of ptosis in metastatic prostate cancer presenting as cavernous sinus syndrome
CASE REPORT
A 76-year-old male presented with left diplopia, ptosis, and facial numbness. Examination showed left oculomotor palsy and numbness in the V1 and V2 distribution of trigeminal nerve. MRI revealed an 11 × 26 × 17 mm posterior sellar mass extending into the left cavernous sinus. Prolactin was slightly elevated, but rest of the pituitary hormones were normal. Resection of the sellar mass showed metastatic prostatic adenocarcinoma positive for NKX-3.1 and prostate-specific antigen (PSA), and Gleason score 4. PSA was elevated at 32 ng/ mL. Positron emission tomography (PET) showed lesions in the left prostatic lobe, pelvic lymph nodes, L5 spine, and right femoral head. FoundationOne testing found no actionable mutations. He was started on leuprorelin-docetaxel and received radiation for the brain and bony lesions. He is currently being maintained on leuprorelin-abiraterone and prednisone, which he is tolerating well.
DISCUSSION
Pituitary metastases (PMs) from prostate cancer are rare and are usually confined to the posterior pituitary. The close relationship of pituitary masses to the cavernous sinuses and internal carotid artery can lead to catastrophic neurovascular consequences. Imaging has limited sensitivity for differentiating non-invasive metastases from adenomas. Older age, new-onset diabetes insipidus, invasive masses, and rapidly growing lesions should raise suspicion for PMs. Intracranial prostatic metastases indicate poor prognosis with a reported median survival of 6-10 months. Timely diagnosis and management can prevent permanent neurologic damage as illustrated by our case in which ptosis and extraocular symptoms were completely resolved by surgery and radiation. Such unique cases underline the significance of suspecting metastatic disease in appropriate demographic groups and the crucial role of multidisciplinary care for oncologic patients.
CONCLUSIONS
A high index of suspicion for PMs in elderly people with new-onset sellar symptoms and early involvement of multidisciplinary teams can lead to prevention and even reversal of serious neurologic symptoms.
INTRODUCTION
Prostate cancer rarely metastasizes to the pituitary gland and the close relationship of these sellar masses to cavernous sinuses and major vessels makes management challenging. We describe a unique case of complete reversal of ptosis in metastatic prostate cancer presenting as cavernous sinus syndrome
CASE REPORT
A 76-year-old male presented with left diplopia, ptosis, and facial numbness. Examination showed left oculomotor palsy and numbness in the V1 and V2 distribution of trigeminal nerve. MRI revealed an 11 × 26 × 17 mm posterior sellar mass extending into the left cavernous sinus. Prolactin was slightly elevated, but rest of the pituitary hormones were normal. Resection of the sellar mass showed metastatic prostatic adenocarcinoma positive for NKX-3.1 and prostate-specific antigen (PSA), and Gleason score 4. PSA was elevated at 32 ng/ mL. Positron emission tomography (PET) showed lesions in the left prostatic lobe, pelvic lymph nodes, L5 spine, and right femoral head. FoundationOne testing found no actionable mutations. He was started on leuprorelin-docetaxel and received radiation for the brain and bony lesions. He is currently being maintained on leuprorelin-abiraterone and prednisone, which he is tolerating well.
DISCUSSION
Pituitary metastases (PMs) from prostate cancer are rare and are usually confined to the posterior pituitary. The close relationship of pituitary masses to the cavernous sinuses and internal carotid artery can lead to catastrophic neurovascular consequences. Imaging has limited sensitivity for differentiating non-invasive metastases from adenomas. Older age, new-onset diabetes insipidus, invasive masses, and rapidly growing lesions should raise suspicion for PMs. Intracranial prostatic metastases indicate poor prognosis with a reported median survival of 6-10 months. Timely diagnosis and management can prevent permanent neurologic damage as illustrated by our case in which ptosis and extraocular symptoms were completely resolved by surgery and radiation. Such unique cases underline the significance of suspecting metastatic disease in appropriate demographic groups and the crucial role of multidisciplinary care for oncologic patients.
CONCLUSIONS
A high index of suspicion for PMs in elderly people with new-onset sellar symptoms and early involvement of multidisciplinary teams can lead to prevention and even reversal of serious neurologic symptoms.
A Rare Case of Leptomeningeal Carcinomatosis From Gastroesophageal Adenocarcinoma Masquerading as Polyneuropathy
INTRODUCTION
Leptomeningeal metastasis (LM) is an extremely rare complication of gastroesophageal (GE) cancer. Diagnosis is challenging due to frequently nonspecific clinical presentations, limited sensitivity of diagnostic testing, and potential overlap with neurologic immune-related adverse events (irAE). We describe a case of metastatic gastroesophageal cancer on immunotherapy presenting with LM masquerading as polyneuropathy.
CASE REPORT
A 74-year-old male with HER2+ GE junction cancer with peritoneal metastases diagnosed 6 months ago, on maintenance trastuzumab/pembrolizumab and with no previous history of cranial or spinal disease, presented with worsening ataxia, headache, and diplopia for one month with multiple negative outpatient MRIs. Examination showed left abducens nerve palsy, dysmetria and absent deep tendon reflexes in upper and lower extremities. CT head was unremarkable, and MRI showed non-specific mild enhancement of the right optic nerve, symmetrical lumbosacral nerve roots and cauda equina concerning for paraneoplastic versus immunotherapy-related polyneuropathy. He was started on empiric high-dose corticosteroids. PET-CT was negative for FDG-avid lesions. Cerebrospinal fluid (CSF) analysis revealed moderate pleocytosis with many large atypical cells, elevated protein (118 mg/dL) and LDH (28 IU/L). Immunohistochemistry was positive for CDX2, CA 19-9, CK7, and pankeratin, consistent with metastatic adenocarcinoma, negative for HER2 in contrast to the original tumor. He subsequently developed hydrocephalus requiring a ventriculoperitoneal shunt. He received ten fractions of whole brain irradiation before electing to pursue hospice care.
DISCUSSION
LM is an extremely rare complication of GE cancer with an incidence of <0.2% and carries a poor prognosis. Differentiation between LM and irAE in patients on immunotherapy can be challenging. Diagnosis relies mostly on CSF cytology, and lumbar puncture should not be delayed in patients with new neurologic symptoms. Treatment options are intrathecal chemotherapy, radiation and steroids. A recent phase II trial has shown promise for intrathecal trastuzumab in patients with HER2+ cancers, but options for HER2 negative disease remain mostly palliative.
CONCLUSIONS
Our case highlights the need for suspecting this rare metastatic site, as early diagnosis and genetic characterization allow for exploring more treatment options including targeted therapies which may improve overall survival and quality of life.
INTRODUCTION
Leptomeningeal metastasis (LM) is an extremely rare complication of gastroesophageal (GE) cancer. Diagnosis is challenging due to frequently nonspecific clinical presentations, limited sensitivity of diagnostic testing, and potential overlap with neurologic immune-related adverse events (irAE). We describe a case of metastatic gastroesophageal cancer on immunotherapy presenting with LM masquerading as polyneuropathy.
CASE REPORT
A 74-year-old male with HER2+ GE junction cancer with peritoneal metastases diagnosed 6 months ago, on maintenance trastuzumab/pembrolizumab and with no previous history of cranial or spinal disease, presented with worsening ataxia, headache, and diplopia for one month with multiple negative outpatient MRIs. Examination showed left abducens nerve palsy, dysmetria and absent deep tendon reflexes in upper and lower extremities. CT head was unremarkable, and MRI showed non-specific mild enhancement of the right optic nerve, symmetrical lumbosacral nerve roots and cauda equina concerning for paraneoplastic versus immunotherapy-related polyneuropathy. He was started on empiric high-dose corticosteroids. PET-CT was negative for FDG-avid lesions. Cerebrospinal fluid (CSF) analysis revealed moderate pleocytosis with many large atypical cells, elevated protein (118 mg/dL) and LDH (28 IU/L). Immunohistochemistry was positive for CDX2, CA 19-9, CK7, and pankeratin, consistent with metastatic adenocarcinoma, negative for HER2 in contrast to the original tumor. He subsequently developed hydrocephalus requiring a ventriculoperitoneal shunt. He received ten fractions of whole brain irradiation before electing to pursue hospice care.
DISCUSSION
LM is an extremely rare complication of GE cancer with an incidence of <0.2% and carries a poor prognosis. Differentiation between LM and irAE in patients on immunotherapy can be challenging. Diagnosis relies mostly on CSF cytology, and lumbar puncture should not be delayed in patients with new neurologic symptoms. Treatment options are intrathecal chemotherapy, radiation and steroids. A recent phase II trial has shown promise for intrathecal trastuzumab in patients with HER2+ cancers, but options for HER2 negative disease remain mostly palliative.
CONCLUSIONS
Our case highlights the need for suspecting this rare metastatic site, as early diagnosis and genetic characterization allow for exploring more treatment options including targeted therapies which may improve overall survival and quality of life.
INTRODUCTION
Leptomeningeal metastasis (LM) is an extremely rare complication of gastroesophageal (GE) cancer. Diagnosis is challenging due to frequently nonspecific clinical presentations, limited sensitivity of diagnostic testing, and potential overlap with neurologic immune-related adverse events (irAE). We describe a case of metastatic gastroesophageal cancer on immunotherapy presenting with LM masquerading as polyneuropathy.
CASE REPORT
A 74-year-old male with HER2+ GE junction cancer with peritoneal metastases diagnosed 6 months ago, on maintenance trastuzumab/pembrolizumab and with no previous history of cranial or spinal disease, presented with worsening ataxia, headache, and diplopia for one month with multiple negative outpatient MRIs. Examination showed left abducens nerve palsy, dysmetria and absent deep tendon reflexes in upper and lower extremities. CT head was unremarkable, and MRI showed non-specific mild enhancement of the right optic nerve, symmetrical lumbosacral nerve roots and cauda equina concerning for paraneoplastic versus immunotherapy-related polyneuropathy. He was started on empiric high-dose corticosteroids. PET-CT was negative for FDG-avid lesions. Cerebrospinal fluid (CSF) analysis revealed moderate pleocytosis with many large atypical cells, elevated protein (118 mg/dL) and LDH (28 IU/L). Immunohistochemistry was positive for CDX2, CA 19-9, CK7, and pankeratin, consistent with metastatic adenocarcinoma, negative for HER2 in contrast to the original tumor. He subsequently developed hydrocephalus requiring a ventriculoperitoneal shunt. He received ten fractions of whole brain irradiation before electing to pursue hospice care.
DISCUSSION
LM is an extremely rare complication of GE cancer with an incidence of <0.2% and carries a poor prognosis. Differentiation between LM and irAE in patients on immunotherapy can be challenging. Diagnosis relies mostly on CSF cytology, and lumbar puncture should not be delayed in patients with new neurologic symptoms. Treatment options are intrathecal chemotherapy, radiation and steroids. A recent phase II trial has shown promise for intrathecal trastuzumab in patients with HER2+ cancers, but options for HER2 negative disease remain mostly palliative.
CONCLUSIONS
Our case highlights the need for suspecting this rare metastatic site, as early diagnosis and genetic characterization allow for exploring more treatment options including targeted therapies which may improve overall survival and quality of life.
A Multi-Disciplinary Approach to Increasing Germline Genetic Testing for Prostate Cancer
PURPOSE
This quality improvement project aims to enhance the rate of germline genetic testing for prostate cancer at the Stratton VA Medical Center, improving risk reduction strategies and therapeutic options for patients.
BACKGROUND
Prostate cancer is prevalent at the Stratton VA Medical Center, yet the rate of genetic evaluation for prostate cancer remains suboptimal. National guidelines recommend genetic counseling and testing in specific patient populations. To address this gap, an interdisciplinary working group conducted gap analysis and root cause analysis, identifying four significant barriers.
METHODS
The working group comprised medical oncologists, urologists, primary care physicians, genetics counselors, data experts, and a LEAN coach. Interventions included implementing a prostate cancer pathway to educate staff on genetic testing indications and integrating genetic testing screening into clinic visits. After the interventions were implemented in January 2022, patient charts were reviewed for all genetic referrals and new prostate cancer diagnoses from January to December 2022.
DATA ANALYSIS
Descriptive analysis was conducted on referral rates, evaluation visit completion rates, and genetic testing outcomes among prostate cancer patients.
RESULTS
During the study period, 59 prostate cancer patients were referred for genetic evaluation. Notably, this was a large increase from no genetic referrals for prostate cancer in the previous year. Among them, 43 completed the evaluation visit, and 34 underwent genetic testing. Noteworthy findings were observed in 5 patients, including 3 variants of unknown significance and 2 pathogenic germline variants: HOXB13 and BRCA2 mutations.
IMPLICATIONS
This project highlights the power of a collaborative, multidisciplinary approach to overcome barriers and enhance the quality of care for prostate cancer patients. The team’s use of gap analysis and root cause analysis successfully identified barriers and proposed solutions, leading to increased referrals and the identification of significant genetic findings. Continued efforts to improve access to germline genetic testing are crucial for enhanced patient care and improved outcomes.
PURPOSE
This quality improvement project aims to enhance the rate of germline genetic testing for prostate cancer at the Stratton VA Medical Center, improving risk reduction strategies and therapeutic options for patients.
BACKGROUND
Prostate cancer is prevalent at the Stratton VA Medical Center, yet the rate of genetic evaluation for prostate cancer remains suboptimal. National guidelines recommend genetic counseling and testing in specific patient populations. To address this gap, an interdisciplinary working group conducted gap analysis and root cause analysis, identifying four significant barriers.
METHODS
The working group comprised medical oncologists, urologists, primary care physicians, genetics counselors, data experts, and a LEAN coach. Interventions included implementing a prostate cancer pathway to educate staff on genetic testing indications and integrating genetic testing screening into clinic visits. After the interventions were implemented in January 2022, patient charts were reviewed for all genetic referrals and new prostate cancer diagnoses from January to December 2022.
DATA ANALYSIS
Descriptive analysis was conducted on referral rates, evaluation visit completion rates, and genetic testing outcomes among prostate cancer patients.
RESULTS
During the study period, 59 prostate cancer patients were referred for genetic evaluation. Notably, this was a large increase from no genetic referrals for prostate cancer in the previous year. Among them, 43 completed the evaluation visit, and 34 underwent genetic testing. Noteworthy findings were observed in 5 patients, including 3 variants of unknown significance and 2 pathogenic germline variants: HOXB13 and BRCA2 mutations.
IMPLICATIONS
This project highlights the power of a collaborative, multidisciplinary approach to overcome barriers and enhance the quality of care for prostate cancer patients. The team’s use of gap analysis and root cause analysis successfully identified barriers and proposed solutions, leading to increased referrals and the identification of significant genetic findings. Continued efforts to improve access to germline genetic testing are crucial for enhanced patient care and improved outcomes.
PURPOSE
This quality improvement project aims to enhance the rate of germline genetic testing for prostate cancer at the Stratton VA Medical Center, improving risk reduction strategies and therapeutic options for patients.
BACKGROUND
Prostate cancer is prevalent at the Stratton VA Medical Center, yet the rate of genetic evaluation for prostate cancer remains suboptimal. National guidelines recommend genetic counseling and testing in specific patient populations. To address this gap, an interdisciplinary working group conducted gap analysis and root cause analysis, identifying four significant barriers.
METHODS
The working group comprised medical oncologists, urologists, primary care physicians, genetics counselors, data experts, and a LEAN coach. Interventions included implementing a prostate cancer pathway to educate staff on genetic testing indications and integrating genetic testing screening into clinic visits. After the interventions were implemented in January 2022, patient charts were reviewed for all genetic referrals and new prostate cancer diagnoses from January to December 2022.
DATA ANALYSIS
Descriptive analysis was conducted on referral rates, evaluation visit completion rates, and genetic testing outcomes among prostate cancer patients.
RESULTS
During the study period, 59 prostate cancer patients were referred for genetic evaluation. Notably, this was a large increase from no genetic referrals for prostate cancer in the previous year. Among them, 43 completed the evaluation visit, and 34 underwent genetic testing. Noteworthy findings were observed in 5 patients, including 3 variants of unknown significance and 2 pathogenic germline variants: HOXB13 and BRCA2 mutations.
IMPLICATIONS
This project highlights the power of a collaborative, multidisciplinary approach to overcome barriers and enhance the quality of care for prostate cancer patients. The team’s use of gap analysis and root cause analysis successfully identified barriers and proposed solutions, leading to increased referrals and the identification of significant genetic findings. Continued efforts to improve access to germline genetic testing are crucial for enhanced patient care and improved outcomes.
Pacify the Prostate, Pop Goes the Pituitary
INTRODUCTION
Excluding skin cancer, prostate cancer is the most common malignancy affecting men in the United States, accounting for ~33% of VA cancer cases. Androgen deprivation therapy (ADT) is considered standard of care in treating advanced prostate cancer. Pituitary apoplexy is a rare and morbid adverse event associated with GnRH agonist treatment. We describe a patient with advanced prostate cancer who developed pituitary apoplexy shortly after leuprolide therapy.
CASE PRESENTATION
A 70-year-old African-American male was diagnosed with a T2aN1M1 stage IVB prostate cancer, Gleason 4+5, PSA 19.5. Four hours after his first leuprolide injection, he developed vomiting, diaphoresis, myalgia, and a severe frontal headache. Brain MRI revealed a 2.4 × 1.3 × 1.3cm pituitary mass, suspicious for an adenoma with hemorrhage. Labs noted low TSH, prolactin, LH, growth hormone, ACTH, cortisol, and testosterone, consistent with pituitary apoplexy. He was treated with steroids. Three weeks later, testosterone levels remained very low. He started abiraterone and prednisone without further leuprolide.
DISCUSSION
Prostate cancer is ubiquitous among VA patients, and ADT with GnRH agonist is vital in their care. These medications stimulate the pituitary to release LH and FSH resulting in a negative feedback loop, ultimately decreasing the levels of testosterone. Common side effects of GnRH agonists include hot flashes, diaphoresis, and sexual dysfunction. We present a patient who started leuprolide for prostate cancer. Symptoms including a severe headache led to an evaluation confirming pituitary apoplexy. Literature review reveals ~ 21 cases of pituitary apoplexy associated with GnRH agonist treatment for prostate cancer, and apoplexy can occur immediately to months later Undiagnosed pituitary adenomas are common among these patients. Treatment includes pituitary surgery or conservative management. Further prostate cancer treatment needs investigation, but we propose that GnRH modifying treatment can be withheld while testosterone levels remain low.
CONCLUSIONS
Prostate cancer is extremely common in the VA population, and treatment with leuprolide is standard. Pituitary apoplexy is a rare, but devastating complication of this treatment, and providers should be aware of the symptoms in order to intervene quickly. Further testosterone lowering treatment may be withheld if testosterone levels remain low.
INTRODUCTION
Excluding skin cancer, prostate cancer is the most common malignancy affecting men in the United States, accounting for ~33% of VA cancer cases. Androgen deprivation therapy (ADT) is considered standard of care in treating advanced prostate cancer. Pituitary apoplexy is a rare and morbid adverse event associated with GnRH agonist treatment. We describe a patient with advanced prostate cancer who developed pituitary apoplexy shortly after leuprolide therapy.
CASE PRESENTATION
A 70-year-old African-American male was diagnosed with a T2aN1M1 stage IVB prostate cancer, Gleason 4+5, PSA 19.5. Four hours after his first leuprolide injection, he developed vomiting, diaphoresis, myalgia, and a severe frontal headache. Brain MRI revealed a 2.4 × 1.3 × 1.3cm pituitary mass, suspicious for an adenoma with hemorrhage. Labs noted low TSH, prolactin, LH, growth hormone, ACTH, cortisol, and testosterone, consistent with pituitary apoplexy. He was treated with steroids. Three weeks later, testosterone levels remained very low. He started abiraterone and prednisone without further leuprolide.
DISCUSSION
Prostate cancer is ubiquitous among VA patients, and ADT with GnRH agonist is vital in their care. These medications stimulate the pituitary to release LH and FSH resulting in a negative feedback loop, ultimately decreasing the levels of testosterone. Common side effects of GnRH agonists include hot flashes, diaphoresis, and sexual dysfunction. We present a patient who started leuprolide for prostate cancer. Symptoms including a severe headache led to an evaluation confirming pituitary apoplexy. Literature review reveals ~ 21 cases of pituitary apoplexy associated with GnRH agonist treatment for prostate cancer, and apoplexy can occur immediately to months later Undiagnosed pituitary adenomas are common among these patients. Treatment includes pituitary surgery or conservative management. Further prostate cancer treatment needs investigation, but we propose that GnRH modifying treatment can be withheld while testosterone levels remain low.
CONCLUSIONS
Prostate cancer is extremely common in the VA population, and treatment with leuprolide is standard. Pituitary apoplexy is a rare, but devastating complication of this treatment, and providers should be aware of the symptoms in order to intervene quickly. Further testosterone lowering treatment may be withheld if testosterone levels remain low.
INTRODUCTION
Excluding skin cancer, prostate cancer is the most common malignancy affecting men in the United States, accounting for ~33% of VA cancer cases. Androgen deprivation therapy (ADT) is considered standard of care in treating advanced prostate cancer. Pituitary apoplexy is a rare and morbid adverse event associated with GnRH agonist treatment. We describe a patient with advanced prostate cancer who developed pituitary apoplexy shortly after leuprolide therapy.
CASE PRESENTATION
A 70-year-old African-American male was diagnosed with a T2aN1M1 stage IVB prostate cancer, Gleason 4+5, PSA 19.5. Four hours after his first leuprolide injection, he developed vomiting, diaphoresis, myalgia, and a severe frontal headache. Brain MRI revealed a 2.4 × 1.3 × 1.3cm pituitary mass, suspicious for an adenoma with hemorrhage. Labs noted low TSH, prolactin, LH, growth hormone, ACTH, cortisol, and testosterone, consistent with pituitary apoplexy. He was treated with steroids. Three weeks later, testosterone levels remained very low. He started abiraterone and prednisone without further leuprolide.
DISCUSSION
Prostate cancer is ubiquitous among VA patients, and ADT with GnRH agonist is vital in their care. These medications stimulate the pituitary to release LH and FSH resulting in a negative feedback loop, ultimately decreasing the levels of testosterone. Common side effects of GnRH agonists include hot flashes, diaphoresis, and sexual dysfunction. We present a patient who started leuprolide for prostate cancer. Symptoms including a severe headache led to an evaluation confirming pituitary apoplexy. Literature review reveals ~ 21 cases of pituitary apoplexy associated with GnRH agonist treatment for prostate cancer, and apoplexy can occur immediately to months later Undiagnosed pituitary adenomas are common among these patients. Treatment includes pituitary surgery or conservative management. Further prostate cancer treatment needs investigation, but we propose that GnRH modifying treatment can be withheld while testosterone levels remain low.
CONCLUSIONS
Prostate cancer is extremely common in the VA population, and treatment with leuprolide is standard. Pituitary apoplexy is a rare, but devastating complication of this treatment, and providers should be aware of the symptoms in order to intervene quickly. Further testosterone lowering treatment may be withheld if testosterone levels remain low.
Depression Workup
From Breakouts to Bargains: Strategies for Patient-Centered, Cost-effective Acne Care
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).
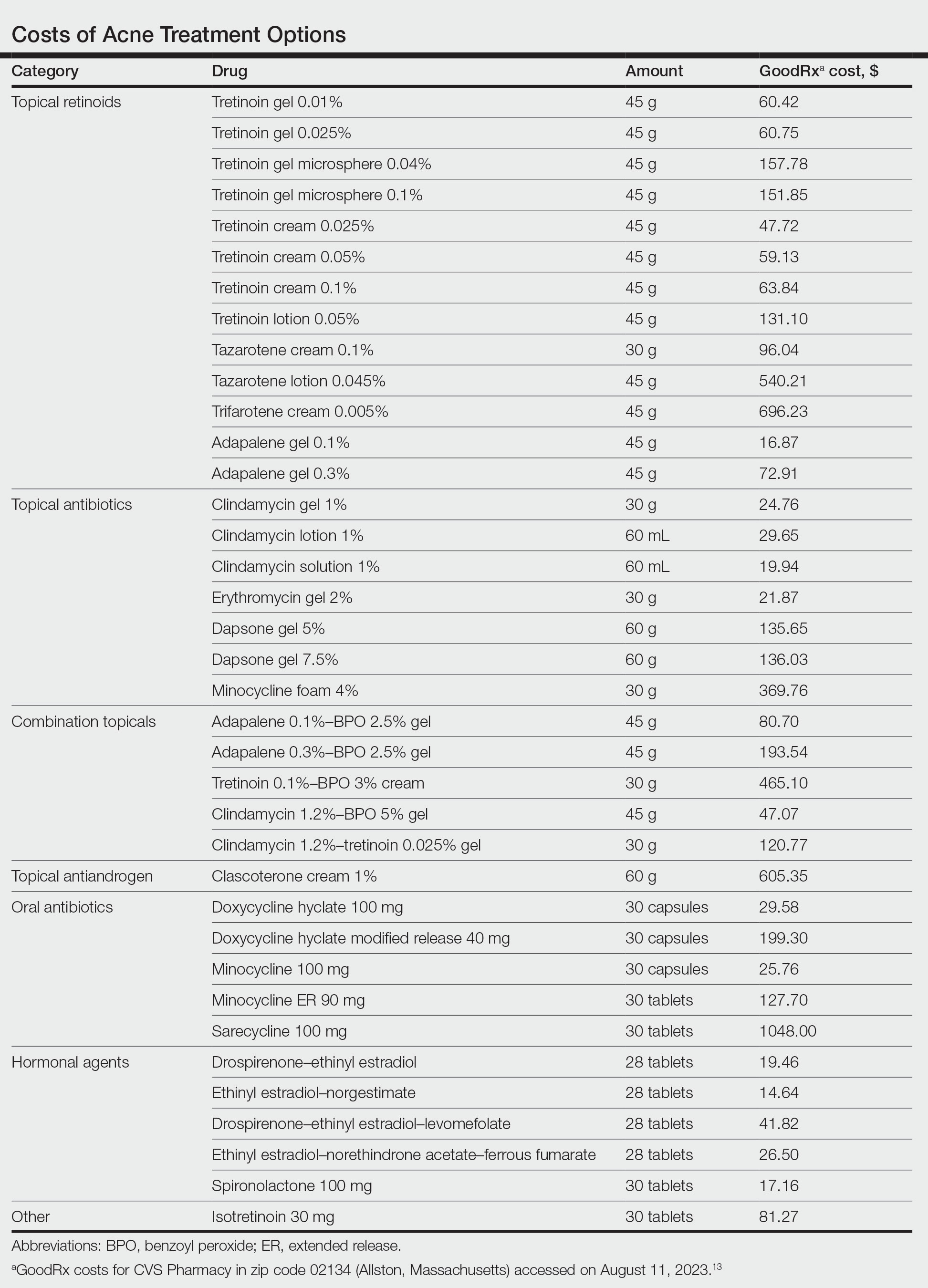
In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
Practice Points
- For mild to moderate acne, fixed-dose combination adapalene–benzoyl peroxide and clindamycin–benzoyl peroxide are highly cost-effective options for most patients.
- For moderate to severe acne, doxycycline or hormonal therapy (ie, combined oral contraceptives, spironolactone) are highly cost-effective options.
- Reduction of laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.