User login
Environmental and Lifestyle Triggers of Rosacea
Environmental and Lifestyle Triggers of Rosacea
Rosacea is a chronic inflammatory skin disease characterized by erythema, flushing, telangiectasias, papules, pustules, and rarely, phymatous changes that primarily manifest in a centrofacial distribution.1,2 Although establishing the true prevalence of rosacea may be challenging due to a wide spectrum of clinical manifestations, current studies estimate that it is between 5% to 6% of the global adult population and that rosacea most commonly is diagnosed in patients aged 30 and 60 years, though it occasionally can affect adolescents and children.3,4 Although the origin and pathophysiology of rosacea remain incompletely understood, the condition arises from a complex interplay of genetic, environmental, immune, microbial, and neurovascular factors; this interplay ultimately leads to excessive production of inflammatory and vasoactive peptides, chronic inflammation, and neurovascular hyperreactivity.1,5-7
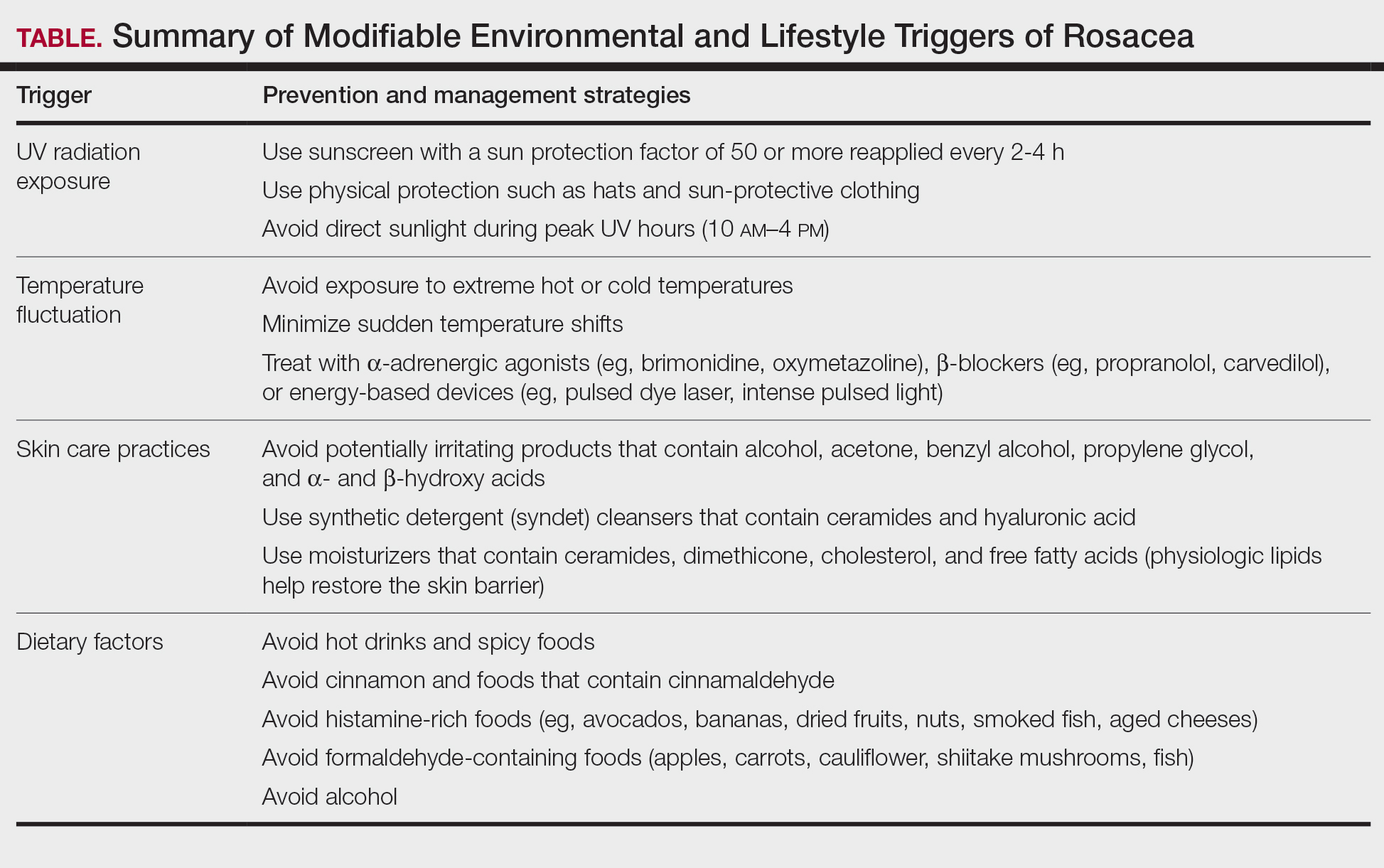
Identifying triggers can be valuable in managing rosacea, as avoidance of these exposures may lead to disease improvement. In this review, we highlight 4 major environmental triggers of rosacea—UV radiation exposure, temperature fluctuation, skin care practices, and diet—and their roles in its pathogenesis and management. A high-level summary of recommendations can be found in the Table.
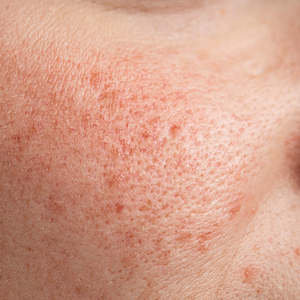
UV Radiation Exposure
Exposure to UV radiation is a known trigger of rosacea and may worsen symptoms through several mechanisms.8,9 It increases the production of inflammatory cytokines, which enhance the release of vascular endothelial growth factor, promoting angiogenesis and vasodilation.10 Exposure to UV radiation also contributes to tissue inflammation through the production of reactive oxygen species, further mediating inflammatory cascades and leading to immune dysregulation.11,12 Interestingly, though the mechanisms by which UV radiation may contribute to the pathophysiology of rosacea are well described, it remains unclear whether chronic UV exposure plays a major role in the pathogenesis or disease progression of rosacea.1 Studies have observed that increased exposure to sunlight seems to be correlated with increased severity of redness but not of papules and pustules.13,14
Despite some uncertainty regarding the relationship between rosacea and chronic UV exposure, sun protection is a prudent recommendation in this patient population, particularly given other risks of exposure to UV radiation, such as photoaging and skin cancer.9,15,16 Sun protection can be accomplished using broad-spectrum sunscreen (sun protection factor 50 or higher, reapplied every 2 to 4 hours) or by wearing physical protection (eg, hats, sun-protective clothing) along with avoidance of sun exposure during peak UV hours (ie, 10 am-
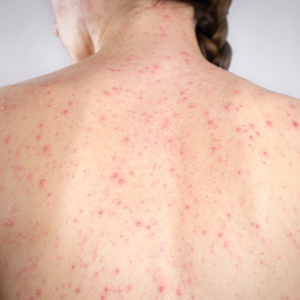
Temperature Fluctuation
Both heat and cold exposure have been suggested as triggers for rosacea, thought to be mediated through dysregulations in neurovascular and thermal pathways, resulting in increased flushing and erythema.6 Skin affected by rosacea exhibits a lower threshold for temperature and pain stimuli, resulting in heightened hypersensitivity compared to normal skin.18 Exposure to heat activates thermosensitive receptors found in neuronal and nonneuronal tissues, triggering the release of vasoactive neuropeptides.1 Among these, transient receptor potential (TRP) channels seem to play a crucial role in neurovascular reactivity and have been studied in the pathophysiology of rosacea.1,8 Overexpression or excessive stimulation of TRPs by various environmental triggers, such as heat or cold, leads to increased neuropeptide production, ultimately contributing to persistent erythema and vascular dysfunction, as well as a burning or stinging sensation.1,2 Moreover, rapid temperature changes, such as moving from freezing outdoor conditions into a heated environment, may also trigger flushing due to sudden vasodilation.2
Adopting behavioral strategies such as preventing overheating, minimizing sudden temperature shifts, and protecting the skin from cold can help reduce rosacea flare-ups, particularly flushing. For patients who do not achieve sufficient relief through lifestyle modifications alone, targeted pharmacologic treatments are available to help manage these symptoms. Topical α-adrenergic agonists (eg, brimonidine, oxymetazoline) are effective in reducing erythema and flushing by causing vasoconstriction.15,19 For persistent erythema and telangiectasias, pulsed dye laser and intense pulsed light therapies can be effective treatments, as they target hemoglobin in blood vessels, leading to their destruction and a subsequent reduction in erythema.20 Other medications such as topical metronidazole, azelaic acid, calcitonin-gene related peptide inhibitors, and systemic ß-blockers also can be used to treat flushing and redness.15,21
Skin Care Practices
Due to the increased tissue inflammation and potential skin barrier dysfunction, rosacea-affected skin is highly sensitive, and skin care practices or products that disrupt the already compromised skin barrier can contribute to flare-ups. General recommendations should include use of gentle cleansers and moisturizers to prevent dry skin and improve skin barrier function22 as well as avoidance of ingredients that are common irritants and inducers of allergic contact dermatitis (eg, fragrances).9
Cleansing the face should be limited to 1 to 2 times daily, as excessive cleansing and use of harsh formulations with exfoliative ingredients can lead to skin irritation and worsening of symptoms.9 Overcleansing can lead to alterations in cutaneous pH and strip the stratum corneum of healthy components such as lipids and natural moisturizing factors. Common ingredients in cleansers that should be avoided due to their irritant nature include alcohol, acetone, benzyl alcohol, propylene glycol, and α- and ß-hydroxy acids. Instead, syndet (synthetic detergent) cleansers that contain ceramides, hyaluronic acid, or other hydrating agents with a near-physiological pH can be helpful for dry and sensitive skin.23 Toners with high alcohol content and astringent-based products also should be avoided.
Optimal moisturizers for rosacea-affected skin should contain physiologic lipids that help replace a healthy skin barrier as well as relieve dryness and seal in moisture. Beneficial barrier-restoring ingredients include ceramides, dimethicone, cholesterol, and free fatty acids as well as humectants such as glycerin and hyaluronic acid.9,23,24 Applying moisturizer immediately after cleansing and prior to the application of any topical treatments also can help decrease irritation.
As mentioned previously, sun protection is a cornerstone in the management of rosacea and can help reduce redness and skin irritation. Using combination formulas, such as moisturizers with a sun protection factor of at least 50, can be effective.25 Additionally, products with antioxidant or anti-inflammatory ingredients such as niacinamide and allantoin can further support skin health. Lastly, formulations containing green pigments may also be beneficial, as they provide cosmetic camouflage to neutralize redness.26
Dietary Factors
Several dietary factors have been proposed as triggers for rosacea, but conclusive evidence remains limited.27 Foods and beverages that generate heat (eg, hot drinks, spicy foods) may exacerbate rosacea by causing vasodilation and stimulating TRP channels, resulting in flushing.18 While capsaicin, found in spicy foods, may lead to flushing through similar activation of TRP channels, current evidence has not proved a specific and consistent role in the pathogenesis of rosacea.18,27 Similarly, cinnamaldehyde, found in cinnamon and many commercial cinnamon-containing foods as well as various fruits and vegetables, activates thermosensitive receptors that may worsen rosacea symptoms.28 Other potential triggers include histamine-rich foods (eg, avocados, bananas, dried fruits, nuts, smoked fish, aged cheeses), which can lead to skin hypersensitivity and flushing, and formaldehyde-containing foods (eg, apples, carrots, cauliflower, shiitake mushrooms, fish), though the role these types of foods play in rosacea remains unclear.1,29-31
The relationship between caffeine and rosacea is complex. While caffeine commonly is found in coffee, tea, and soda, some studies have suggested that coffee consumption may reduce rosacea risk due to its vasoconstrictive and anti-inflammatory effects.28,32 In contrast, alcohol—particularly white wine and liquor—has been associated with increased rosacea risk due to its effect on vasodilation, inflammation, and oxidative stress.33 Despite anecdotal reports, the role of dairy products in rosacea remains unclear, with conflicting studies suggesting dairy consumption may exacerbate or protect against rosacea.27,28 Given the variability in dietary triggers, patients with rosacea may benefit from using a dietary journal to identify and avoid foods that exacerbate their symptoms, though more research is needed to establish clear recommendations.
Conclusion
Rosacea is a complex condition influenced by genetic, immune, microbial, and environmental factors. Triggers such as UV exposure, temperature fluctuations, alterations in the skin microbiome, and diet contribute to disease exacerbation through mechanisms like vasodilation, neurogenic inflammation, and immune dysregulation. These triggers often interact, compounding their effects and making symptom management more challenging and multifaceted.
Successful rosacea treatment relies on identifying and minimizing patient-specific triggers, as lifestyle modifications can reduce flare-ups and improve outcomes. When combined with interventional, oral, and topical therapies, these adjustments enhance treatment effectiveness and contribute to better long-term disease control. Clinicians should adopt a personalized holistic approach by educating patients on common triggers, recommending lifestyle changes, and integrating medical treatments as necessary. Future research should continue exploring the relationships between rosacea and environmental factors to develop more targeted and evidence-based recommendations.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289. doi:10.1111/bjd.16481
- Chamaillard M, Mortemousque B, Boralevi F, et al. Cutaneous and ocular signs of childhood rosacea. Arch Dermatol. 2008;144:167-171.
- Abram K, Silm H, Maaroos H, et al. Risk factors associated with rosacea. J Eur Acad Dermatol Venereol. 2010;24:565-571.
- Gerber PA, Buhren BA, Steinhoff M, et al. Rosacea: the cytokine and chemokine network. J Investig Dermatol Symp Proc. 2011;15:40-47.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:749-758.
- Morgado‐Carrasco D, Granger C, Trullas C, et al. Impact of ultraviolet radiation and exposome on rosacea: key role of photoprotection in optimizing treatment. J Cosmet Dermatol. 2021;20:3415-3421.
- Suhng E, Kim BH, Choi YW, et al. Increased expression of IL‐33 in rosacea skin and UVB‐irradiated and LL‐37‐treated HaCaT cells. Exp Dermatol. 2018;27:1023-1029.
- Tisma VS, Basta-Juzbasic A, Jaganjac M, et al. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol. 2009;60:270-276.
- Kulkarni NN, Takahashi T, Sanford JA, et al. Innate immune dysfunction in rosacea promotes photosensitivity and vascular adhesion molecule expression. J Invest Dermatol. 2020;140:645-655.E6.
- Bae YI, Yun SJ, Lee JB, et al. Clinical evaluation of 168 Korean patients with rosacea: the sun exposure correlates with the erythematotelangiectatic subtype. Ann Dermatol. 2009;21:243-249.
- McAleer, MA, Fitzpatrick P, Powell FC. Papulopustular rosacea: prevalence and relationship to photodamage. J Am Acad Dermatol. 2010;63:33-39.
- Van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:761-770.
- Nichols K, Desai N, Lebwohl MG. Effective sunscreen ingredients and cutaneous irritation in patients with rosacea. Cutis. 1998;61:344-346.
- Guzman-Sanchez DA, Ishiuji Y, Patel T, et al. Enhanced skin blood flow and sensitivity to noxious heat stimuli in papulopustular rosacea. J Am Acad Dermatol. 2007;57:800-805.
- Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- van Zuuren EJ, Fedorowicz Z, Tan J, et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol.2019;181:65-79.
- Wienholtz NKF, Christensen CE, Do TP, et al. Erenumab for treatment of persistent erythema and flushing in rosacea: a nonrandomized controlled trial. JAMA Dermatol.2024;160:612-619.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
- Baldwin H, Alexis AF, Andriessen A, et al. Evidence of barrier deficiency in rosacea and the importance of integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2021;20:384-392.
- Schlesinger TE, Powell CR. Efficacy and tolerability of low molecular weight hyaluronic acid sodium salt 0.2% cream in rosacea. J Drugs Dermatol. 2013;12:664-667.
- Williams JD, Maitra P, Atillasoy E, et al. SPF 100+ sunscreen is more protective against sunburn than SPF 50+ in actual use: results of a randomized, double-blind, split-face, natural sunlight exposure clinical trial. J Am Acad Dermatol. 2018;78:902-910.E2.
- Draelos ZD. Cosmeceuticals for rosacea. Clin Dermatol. 2017;35:213-217.
- Yuan X, Huang X, Wang B, et al. Relationship between rosacea and dietary factors: a multicenter retrospective case–control survey. J Dermatol. 2019;46:219-225.
- Alia E, Feng H. Rosacea pathogenesis, common triggers, and dietary role: the cause, the trigger, and the positive effects of different foods. Clin Dermatol. 2022;40:122-127.
- Branco ACCC, Yoshikawa FSY, Pietrobon AJ, et al. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm. 2018;2018:1-10.
- Darrigade A, Dendooven E, Aerts O. Contact allergy to fragrances and formaldehyde contributing to papulopustular rosacea. Contact Dermatitis. 2019;81:395-397.
- Linauskiene K, Isaksson M. Allergic contact dermatitis from formaldehyde mimicking impetigo and initiating rosacea. Contact Dermatitis. 2018;78:359-361.
- Al Reef T, Ghanem E. Caffeine: well-known as psychotropic substance, but little as immunomodulator. Immunobiology. 2018;223:818-825.
- Drago F, Ciccarese G, Herzum A, et al. Rosacea and alcohol intake. J Am Acad Dermatol. 2018;78:E25.
Rosacea is a chronic inflammatory skin disease characterized by erythema, flushing, telangiectasias, papules, pustules, and rarely, phymatous changes that primarily manifest in a centrofacial distribution.1,2 Although establishing the true prevalence of rosacea may be challenging due to a wide spectrum of clinical manifestations, current studies estimate that it is between 5% to 6% of the global adult population and that rosacea most commonly is diagnosed in patients aged 30 and 60 years, though it occasionally can affect adolescents and children.3,4 Although the origin and pathophysiology of rosacea remain incompletely understood, the condition arises from a complex interplay of genetic, environmental, immune, microbial, and neurovascular factors; this interplay ultimately leads to excessive production of inflammatory and vasoactive peptides, chronic inflammation, and neurovascular hyperreactivity.1,5-7
Identifying triggers can be valuable in managing rosacea, as avoidance of these exposures may lead to disease improvement. In this review, we highlight 4 major environmental triggers of rosacea—UV radiation exposure, temperature fluctuation, skin care practices, and diet—and their roles in its pathogenesis and management. A high-level summary of recommendations can be found in the Table.

UV Radiation Exposure
Exposure to UV radiation is a known trigger of rosacea and may worsen symptoms through several mechanisms.8,9 It increases the production of inflammatory cytokines, which enhance the release of vascular endothelial growth factor, promoting angiogenesis and vasodilation.10 Exposure to UV radiation also contributes to tissue inflammation through the production of reactive oxygen species, further mediating inflammatory cascades and leading to immune dysregulation.11,12 Interestingly, though the mechanisms by which UV radiation may contribute to the pathophysiology of rosacea are well described, it remains unclear whether chronic UV exposure plays a major role in the pathogenesis or disease progression of rosacea.1 Studies have observed that increased exposure to sunlight seems to be correlated with increased severity of redness but not of papules and pustules.13,14
Despite some uncertainty regarding the relationship between rosacea and chronic UV exposure, sun protection is a prudent recommendation in this patient population, particularly given other risks of exposure to UV radiation, such as photoaging and skin cancer.9,15,16 Sun protection can be accomplished using broad-spectrum sunscreen (sun protection factor 50 or higher, reapplied every 2 to 4 hours) or by wearing physical protection (eg, hats, sun-protective clothing) along with avoidance of sun exposure during peak UV hours (ie, 10 am-
Temperature Fluctuation
Both heat and cold exposure have been suggested as triggers for rosacea, thought to be mediated through dysregulations in neurovascular and thermal pathways, resulting in increased flushing and erythema.6 Skin affected by rosacea exhibits a lower threshold for temperature and pain stimuli, resulting in heightened hypersensitivity compared to normal skin.18 Exposure to heat activates thermosensitive receptors found in neuronal and nonneuronal tissues, triggering the release of vasoactive neuropeptides.1 Among these, transient receptor potential (TRP) channels seem to play a crucial role in neurovascular reactivity and have been studied in the pathophysiology of rosacea.1,8 Overexpression or excessive stimulation of TRPs by various environmental triggers, such as heat or cold, leads to increased neuropeptide production, ultimately contributing to persistent erythema and vascular dysfunction, as well as a burning or stinging sensation.1,2 Moreover, rapid temperature changes, such as moving from freezing outdoor conditions into a heated environment, may also trigger flushing due to sudden vasodilation.2
Adopting behavioral strategies such as preventing overheating, minimizing sudden temperature shifts, and protecting the skin from cold can help reduce rosacea flare-ups, particularly flushing. For patients who do not achieve sufficient relief through lifestyle modifications alone, targeted pharmacologic treatments are available to help manage these symptoms. Topical α-adrenergic agonists (eg, brimonidine, oxymetazoline) are effective in reducing erythema and flushing by causing vasoconstriction.15,19 For persistent erythema and telangiectasias, pulsed dye laser and intense pulsed light therapies can be effective treatments, as they target hemoglobin in blood vessels, leading to their destruction and a subsequent reduction in erythema.20 Other medications such as topical metronidazole, azelaic acid, calcitonin-gene related peptide inhibitors, and systemic ß-blockers also can be used to treat flushing and redness.15,21
Skin Care Practices
Due to the increased tissue inflammation and potential skin barrier dysfunction, rosacea-affected skin is highly sensitive, and skin care practices or products that disrupt the already compromised skin barrier can contribute to flare-ups. General recommendations should include use of gentle cleansers and moisturizers to prevent dry skin and improve skin barrier function22 as well as avoidance of ingredients that are common irritants and inducers of allergic contact dermatitis (eg, fragrances).9
Cleansing the face should be limited to 1 to 2 times daily, as excessive cleansing and use of harsh formulations with exfoliative ingredients can lead to skin irritation and worsening of symptoms.9 Overcleansing can lead to alterations in cutaneous pH and strip the stratum corneum of healthy components such as lipids and natural moisturizing factors. Common ingredients in cleansers that should be avoided due to their irritant nature include alcohol, acetone, benzyl alcohol, propylene glycol, and α- and ß-hydroxy acids. Instead, syndet (synthetic detergent) cleansers that contain ceramides, hyaluronic acid, or other hydrating agents with a near-physiological pH can be helpful for dry and sensitive skin.23 Toners with high alcohol content and astringent-based products also should be avoided.
Optimal moisturizers for rosacea-affected skin should contain physiologic lipids that help replace a healthy skin barrier as well as relieve dryness and seal in moisture. Beneficial barrier-restoring ingredients include ceramides, dimethicone, cholesterol, and free fatty acids as well as humectants such as glycerin and hyaluronic acid.9,23,24 Applying moisturizer immediately after cleansing and prior to the application of any topical treatments also can help decrease irritation.
As mentioned previously, sun protection is a cornerstone in the management of rosacea and can help reduce redness and skin irritation. Using combination formulas, such as moisturizers with a sun protection factor of at least 50, can be effective.25 Additionally, products with antioxidant or anti-inflammatory ingredients such as niacinamide and allantoin can further support skin health. Lastly, formulations containing green pigments may also be beneficial, as they provide cosmetic camouflage to neutralize redness.26
Dietary Factors
Several dietary factors have been proposed as triggers for rosacea, but conclusive evidence remains limited.27 Foods and beverages that generate heat (eg, hot drinks, spicy foods) may exacerbate rosacea by causing vasodilation and stimulating TRP channels, resulting in flushing.18 While capsaicin, found in spicy foods, may lead to flushing through similar activation of TRP channels, current evidence has not proved a specific and consistent role in the pathogenesis of rosacea.18,27 Similarly, cinnamaldehyde, found in cinnamon and many commercial cinnamon-containing foods as well as various fruits and vegetables, activates thermosensitive receptors that may worsen rosacea symptoms.28 Other potential triggers include histamine-rich foods (eg, avocados, bananas, dried fruits, nuts, smoked fish, aged cheeses), which can lead to skin hypersensitivity and flushing, and formaldehyde-containing foods (eg, apples, carrots, cauliflower, shiitake mushrooms, fish), though the role these types of foods play in rosacea remains unclear.1,29-31
The relationship between caffeine and rosacea is complex. While caffeine commonly is found in coffee, tea, and soda, some studies have suggested that coffee consumption may reduce rosacea risk due to its vasoconstrictive and anti-inflammatory effects.28,32 In contrast, alcohol—particularly white wine and liquor—has been associated with increased rosacea risk due to its effect on vasodilation, inflammation, and oxidative stress.33 Despite anecdotal reports, the role of dairy products in rosacea remains unclear, with conflicting studies suggesting dairy consumption may exacerbate or protect against rosacea.27,28 Given the variability in dietary triggers, patients with rosacea may benefit from using a dietary journal to identify and avoid foods that exacerbate their symptoms, though more research is needed to establish clear recommendations.
Conclusion
Rosacea is a complex condition influenced by genetic, immune, microbial, and environmental factors. Triggers such as UV exposure, temperature fluctuations, alterations in the skin microbiome, and diet contribute to disease exacerbation through mechanisms like vasodilation, neurogenic inflammation, and immune dysregulation. These triggers often interact, compounding their effects and making symptom management more challenging and multifaceted.
Successful rosacea treatment relies on identifying and minimizing patient-specific triggers, as lifestyle modifications can reduce flare-ups and improve outcomes. When combined with interventional, oral, and topical therapies, these adjustments enhance treatment effectiveness and contribute to better long-term disease control. Clinicians should adopt a personalized holistic approach by educating patients on common triggers, recommending lifestyle changes, and integrating medical treatments as necessary. Future research should continue exploring the relationships between rosacea and environmental factors to develop more targeted and evidence-based recommendations.
Rosacea is a chronic inflammatory skin disease characterized by erythema, flushing, telangiectasias, papules, pustules, and rarely, phymatous changes that primarily manifest in a centrofacial distribution.1,2 Although establishing the true prevalence of rosacea may be challenging due to a wide spectrum of clinical manifestations, current studies estimate that it is between 5% to 6% of the global adult population and that rosacea most commonly is diagnosed in patients aged 30 and 60 years, though it occasionally can affect adolescents and children.3,4 Although the origin and pathophysiology of rosacea remain incompletely understood, the condition arises from a complex interplay of genetic, environmental, immune, microbial, and neurovascular factors; this interplay ultimately leads to excessive production of inflammatory and vasoactive peptides, chronic inflammation, and neurovascular hyperreactivity.1,5-7
Identifying triggers can be valuable in managing rosacea, as avoidance of these exposures may lead to disease improvement. In this review, we highlight 4 major environmental triggers of rosacea—UV radiation exposure, temperature fluctuation, skin care practices, and diet—and their roles in its pathogenesis and management. A high-level summary of recommendations can be found in the Table.

UV Radiation Exposure
Exposure to UV radiation is a known trigger of rosacea and may worsen symptoms through several mechanisms.8,9 It increases the production of inflammatory cytokines, which enhance the release of vascular endothelial growth factor, promoting angiogenesis and vasodilation.10 Exposure to UV radiation also contributes to tissue inflammation through the production of reactive oxygen species, further mediating inflammatory cascades and leading to immune dysregulation.11,12 Interestingly, though the mechanisms by which UV radiation may contribute to the pathophysiology of rosacea are well described, it remains unclear whether chronic UV exposure plays a major role in the pathogenesis or disease progression of rosacea.1 Studies have observed that increased exposure to sunlight seems to be correlated with increased severity of redness but not of papules and pustules.13,14
Despite some uncertainty regarding the relationship between rosacea and chronic UV exposure, sun protection is a prudent recommendation in this patient population, particularly given other risks of exposure to UV radiation, such as photoaging and skin cancer.9,15,16 Sun protection can be accomplished using broad-spectrum sunscreen (sun protection factor 50 or higher, reapplied every 2 to 4 hours) or by wearing physical protection (eg, hats, sun-protective clothing) along with avoidance of sun exposure during peak UV hours (ie, 10 am-
Temperature Fluctuation
Both heat and cold exposure have been suggested as triggers for rosacea, thought to be mediated through dysregulations in neurovascular and thermal pathways, resulting in increased flushing and erythema.6 Skin affected by rosacea exhibits a lower threshold for temperature and pain stimuli, resulting in heightened hypersensitivity compared to normal skin.18 Exposure to heat activates thermosensitive receptors found in neuronal and nonneuronal tissues, triggering the release of vasoactive neuropeptides.1 Among these, transient receptor potential (TRP) channels seem to play a crucial role in neurovascular reactivity and have been studied in the pathophysiology of rosacea.1,8 Overexpression or excessive stimulation of TRPs by various environmental triggers, such as heat or cold, leads to increased neuropeptide production, ultimately contributing to persistent erythema and vascular dysfunction, as well as a burning or stinging sensation.1,2 Moreover, rapid temperature changes, such as moving from freezing outdoor conditions into a heated environment, may also trigger flushing due to sudden vasodilation.2
Adopting behavioral strategies such as preventing overheating, minimizing sudden temperature shifts, and protecting the skin from cold can help reduce rosacea flare-ups, particularly flushing. For patients who do not achieve sufficient relief through lifestyle modifications alone, targeted pharmacologic treatments are available to help manage these symptoms. Topical α-adrenergic agonists (eg, brimonidine, oxymetazoline) are effective in reducing erythema and flushing by causing vasoconstriction.15,19 For persistent erythema and telangiectasias, pulsed dye laser and intense pulsed light therapies can be effective treatments, as they target hemoglobin in blood vessels, leading to their destruction and a subsequent reduction in erythema.20 Other medications such as topical metronidazole, azelaic acid, calcitonin-gene related peptide inhibitors, and systemic ß-blockers also can be used to treat flushing and redness.15,21
Skin Care Practices
Due to the increased tissue inflammation and potential skin barrier dysfunction, rosacea-affected skin is highly sensitive, and skin care practices or products that disrupt the already compromised skin barrier can contribute to flare-ups. General recommendations should include use of gentle cleansers and moisturizers to prevent dry skin and improve skin barrier function22 as well as avoidance of ingredients that are common irritants and inducers of allergic contact dermatitis (eg, fragrances).9
Cleansing the face should be limited to 1 to 2 times daily, as excessive cleansing and use of harsh formulations with exfoliative ingredients can lead to skin irritation and worsening of symptoms.9 Overcleansing can lead to alterations in cutaneous pH and strip the stratum corneum of healthy components such as lipids and natural moisturizing factors. Common ingredients in cleansers that should be avoided due to their irritant nature include alcohol, acetone, benzyl alcohol, propylene glycol, and α- and ß-hydroxy acids. Instead, syndet (synthetic detergent) cleansers that contain ceramides, hyaluronic acid, or other hydrating agents with a near-physiological pH can be helpful for dry and sensitive skin.23 Toners with high alcohol content and astringent-based products also should be avoided.
Optimal moisturizers for rosacea-affected skin should contain physiologic lipids that help replace a healthy skin barrier as well as relieve dryness and seal in moisture. Beneficial barrier-restoring ingredients include ceramides, dimethicone, cholesterol, and free fatty acids as well as humectants such as glycerin and hyaluronic acid.9,23,24 Applying moisturizer immediately after cleansing and prior to the application of any topical treatments also can help decrease irritation.
As mentioned previously, sun protection is a cornerstone in the management of rosacea and can help reduce redness and skin irritation. Using combination formulas, such as moisturizers with a sun protection factor of at least 50, can be effective.25 Additionally, products with antioxidant or anti-inflammatory ingredients such as niacinamide and allantoin can further support skin health. Lastly, formulations containing green pigments may also be beneficial, as they provide cosmetic camouflage to neutralize redness.26
Dietary Factors
Several dietary factors have been proposed as triggers for rosacea, but conclusive evidence remains limited.27 Foods and beverages that generate heat (eg, hot drinks, spicy foods) may exacerbate rosacea by causing vasodilation and stimulating TRP channels, resulting in flushing.18 While capsaicin, found in spicy foods, may lead to flushing through similar activation of TRP channels, current evidence has not proved a specific and consistent role in the pathogenesis of rosacea.18,27 Similarly, cinnamaldehyde, found in cinnamon and many commercial cinnamon-containing foods as well as various fruits and vegetables, activates thermosensitive receptors that may worsen rosacea symptoms.28 Other potential triggers include histamine-rich foods (eg, avocados, bananas, dried fruits, nuts, smoked fish, aged cheeses), which can lead to skin hypersensitivity and flushing, and formaldehyde-containing foods (eg, apples, carrots, cauliflower, shiitake mushrooms, fish), though the role these types of foods play in rosacea remains unclear.1,29-31
The relationship between caffeine and rosacea is complex. While caffeine commonly is found in coffee, tea, and soda, some studies have suggested that coffee consumption may reduce rosacea risk due to its vasoconstrictive and anti-inflammatory effects.28,32 In contrast, alcohol—particularly white wine and liquor—has been associated with increased rosacea risk due to its effect on vasodilation, inflammation, and oxidative stress.33 Despite anecdotal reports, the role of dairy products in rosacea remains unclear, with conflicting studies suggesting dairy consumption may exacerbate or protect against rosacea.27,28 Given the variability in dietary triggers, patients with rosacea may benefit from using a dietary journal to identify and avoid foods that exacerbate their symptoms, though more research is needed to establish clear recommendations.
Conclusion
Rosacea is a complex condition influenced by genetic, immune, microbial, and environmental factors. Triggers such as UV exposure, temperature fluctuations, alterations in the skin microbiome, and diet contribute to disease exacerbation through mechanisms like vasodilation, neurogenic inflammation, and immune dysregulation. These triggers often interact, compounding their effects and making symptom management more challenging and multifaceted.
Successful rosacea treatment relies on identifying and minimizing patient-specific triggers, as lifestyle modifications can reduce flare-ups and improve outcomes. When combined with interventional, oral, and topical therapies, these adjustments enhance treatment effectiveness and contribute to better long-term disease control. Clinicians should adopt a personalized holistic approach by educating patients on common triggers, recommending lifestyle changes, and integrating medical treatments as necessary. Future research should continue exploring the relationships between rosacea and environmental factors to develop more targeted and evidence-based recommendations.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289. doi:10.1111/bjd.16481
- Chamaillard M, Mortemousque B, Boralevi F, et al. Cutaneous and ocular signs of childhood rosacea. Arch Dermatol. 2008;144:167-171.
- Abram K, Silm H, Maaroos H, et al. Risk factors associated with rosacea. J Eur Acad Dermatol Venereol. 2010;24:565-571.
- Gerber PA, Buhren BA, Steinhoff M, et al. Rosacea: the cytokine and chemokine network. J Investig Dermatol Symp Proc. 2011;15:40-47.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:749-758.
- Morgado‐Carrasco D, Granger C, Trullas C, et al. Impact of ultraviolet radiation and exposome on rosacea: key role of photoprotection in optimizing treatment. J Cosmet Dermatol. 2021;20:3415-3421.
- Suhng E, Kim BH, Choi YW, et al. Increased expression of IL‐33 in rosacea skin and UVB‐irradiated and LL‐37‐treated HaCaT cells. Exp Dermatol. 2018;27:1023-1029.
- Tisma VS, Basta-Juzbasic A, Jaganjac M, et al. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol. 2009;60:270-276.
- Kulkarni NN, Takahashi T, Sanford JA, et al. Innate immune dysfunction in rosacea promotes photosensitivity and vascular adhesion molecule expression. J Invest Dermatol. 2020;140:645-655.E6.
- Bae YI, Yun SJ, Lee JB, et al. Clinical evaluation of 168 Korean patients with rosacea: the sun exposure correlates with the erythematotelangiectatic subtype. Ann Dermatol. 2009;21:243-249.
- McAleer, MA, Fitzpatrick P, Powell FC. Papulopustular rosacea: prevalence and relationship to photodamage. J Am Acad Dermatol. 2010;63:33-39.
- Van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:761-770.
- Nichols K, Desai N, Lebwohl MG. Effective sunscreen ingredients and cutaneous irritation in patients with rosacea. Cutis. 1998;61:344-346.
- Guzman-Sanchez DA, Ishiuji Y, Patel T, et al. Enhanced skin blood flow and sensitivity to noxious heat stimuli in papulopustular rosacea. J Am Acad Dermatol. 2007;57:800-805.
- Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- van Zuuren EJ, Fedorowicz Z, Tan J, et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol.2019;181:65-79.
- Wienholtz NKF, Christensen CE, Do TP, et al. Erenumab for treatment of persistent erythema and flushing in rosacea: a nonrandomized controlled trial. JAMA Dermatol.2024;160:612-619.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
- Baldwin H, Alexis AF, Andriessen A, et al. Evidence of barrier deficiency in rosacea and the importance of integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2021;20:384-392.
- Schlesinger TE, Powell CR. Efficacy and tolerability of low molecular weight hyaluronic acid sodium salt 0.2% cream in rosacea. J Drugs Dermatol. 2013;12:664-667.
- Williams JD, Maitra P, Atillasoy E, et al. SPF 100+ sunscreen is more protective against sunburn than SPF 50+ in actual use: results of a randomized, double-blind, split-face, natural sunlight exposure clinical trial. J Am Acad Dermatol. 2018;78:902-910.E2.
- Draelos ZD. Cosmeceuticals for rosacea. Clin Dermatol. 2017;35:213-217.
- Yuan X, Huang X, Wang B, et al. Relationship between rosacea and dietary factors: a multicenter retrospective case–control survey. J Dermatol. 2019;46:219-225.
- Alia E, Feng H. Rosacea pathogenesis, common triggers, and dietary role: the cause, the trigger, and the positive effects of different foods. Clin Dermatol. 2022;40:122-127.
- Branco ACCC, Yoshikawa FSY, Pietrobon AJ, et al. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm. 2018;2018:1-10.
- Darrigade A, Dendooven E, Aerts O. Contact allergy to fragrances and formaldehyde contributing to papulopustular rosacea. Contact Dermatitis. 2019;81:395-397.
- Linauskiene K, Isaksson M. Allergic contact dermatitis from formaldehyde mimicking impetigo and initiating rosacea. Contact Dermatitis. 2018;78:359-361.
- Al Reef T, Ghanem E. Caffeine: well-known as psychotropic substance, but little as immunomodulator. Immunobiology. 2018;223:818-825.
- Drago F, Ciccarese G, Herzum A, et al. Rosacea and alcohol intake. J Am Acad Dermatol. 2018;78:E25.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289. doi:10.1111/bjd.16481
- Chamaillard M, Mortemousque B, Boralevi F, et al. Cutaneous and ocular signs of childhood rosacea. Arch Dermatol. 2008;144:167-171.
- Abram K, Silm H, Maaroos H, et al. Risk factors associated with rosacea. J Eur Acad Dermatol Venereol. 2010;24:565-571.
- Gerber PA, Buhren BA, Steinhoff M, et al. Rosacea: the cytokine and chemokine network. J Investig Dermatol Symp Proc. 2011;15:40-47.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:749-758.
- Morgado‐Carrasco D, Granger C, Trullas C, et al. Impact of ultraviolet radiation and exposome on rosacea: key role of photoprotection in optimizing treatment. J Cosmet Dermatol. 2021;20:3415-3421.
- Suhng E, Kim BH, Choi YW, et al. Increased expression of IL‐33 in rosacea skin and UVB‐irradiated and LL‐37‐treated HaCaT cells. Exp Dermatol. 2018;27:1023-1029.
- Tisma VS, Basta-Juzbasic A, Jaganjac M, et al. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol. 2009;60:270-276.
- Kulkarni NN, Takahashi T, Sanford JA, et al. Innate immune dysfunction in rosacea promotes photosensitivity and vascular adhesion molecule expression. J Invest Dermatol. 2020;140:645-655.E6.
- Bae YI, Yun SJ, Lee JB, et al. Clinical evaluation of 168 Korean patients with rosacea: the sun exposure correlates with the erythematotelangiectatic subtype. Ann Dermatol. 2009;21:243-249.
- McAleer, MA, Fitzpatrick P, Powell FC. Papulopustular rosacea: prevalence and relationship to photodamage. J Am Acad Dermatol. 2010;63:33-39.
- Van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:761-770.
- Nichols K, Desai N, Lebwohl MG. Effective sunscreen ingredients and cutaneous irritation in patients with rosacea. Cutis. 1998;61:344-346.
- Guzman-Sanchez DA, Ishiuji Y, Patel T, et al. Enhanced skin blood flow and sensitivity to noxious heat stimuli in papulopustular rosacea. J Am Acad Dermatol. 2007;57:800-805.
- Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- van Zuuren EJ, Fedorowicz Z, Tan J, et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol.2019;181:65-79.
- Wienholtz NKF, Christensen CE, Do TP, et al. Erenumab for treatment of persistent erythema and flushing in rosacea: a nonrandomized controlled trial. JAMA Dermatol.2024;160:612-619.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
- Baldwin H, Alexis AF, Andriessen A, et al. Evidence of barrier deficiency in rosacea and the importance of integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2021;20:384-392.
- Schlesinger TE, Powell CR. Efficacy and tolerability of low molecular weight hyaluronic acid sodium salt 0.2% cream in rosacea. J Drugs Dermatol. 2013;12:664-667.
- Williams JD, Maitra P, Atillasoy E, et al. SPF 100+ sunscreen is more protective against sunburn than SPF 50+ in actual use: results of a randomized, double-blind, split-face, natural sunlight exposure clinical trial. J Am Acad Dermatol. 2018;78:902-910.E2.
- Draelos ZD. Cosmeceuticals for rosacea. Clin Dermatol. 2017;35:213-217.
- Yuan X, Huang X, Wang B, et al. Relationship between rosacea and dietary factors: a multicenter retrospective case–control survey. J Dermatol. 2019;46:219-225.
- Alia E, Feng H. Rosacea pathogenesis, common triggers, and dietary role: the cause, the trigger, and the positive effects of different foods. Clin Dermatol. 2022;40:122-127.
- Branco ACCC, Yoshikawa FSY, Pietrobon AJ, et al. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm. 2018;2018:1-10.
- Darrigade A, Dendooven E, Aerts O. Contact allergy to fragrances and formaldehyde contributing to papulopustular rosacea. Contact Dermatitis. 2019;81:395-397.
- Linauskiene K, Isaksson M. Allergic contact dermatitis from formaldehyde mimicking impetigo and initiating rosacea. Contact Dermatitis. 2018;78:359-361.
- Al Reef T, Ghanem E. Caffeine: well-known as psychotropic substance, but little as immunomodulator. Immunobiology. 2018;223:818-825.
- Drago F, Ciccarese G, Herzum A, et al. Rosacea and alcohol intake. J Am Acad Dermatol. 2018;78:E25.
Environmental and Lifestyle Triggers of Rosacea
Environmental and Lifestyle Triggers of Rosacea
PRACTICE POINTS
- It is important to routinely assess and individualize rosacea management by encouraging use of symptom and trigger diaries to guide lifestyle modifications.
- Patients with rosacea should be encouraged to use mild, fragrance-free cleansers, barrier-supporting moisturizers, and daily broad-spectrum sunscreen and to avoid common irritants.
- Address flushing and erythema with behavioral and medical strategies; counsel patients on minimizing abrupt temperature shifts and consider topical Symbolα-adrenergic agonists, anti-inflammatory agents, or laser therapies when lifestyle measures alone are insufficient.
- Lifestyle recommendations (eg, optimal skin care practices, avoidance of dietary triggers) should be incorporated in treatment plans.
Spironolactone for Acne: Practical Strategies for Optimal Clinical Outcomes
Spironolactone for Acne: Practical Strategies for Optimal Clinical Outcomes
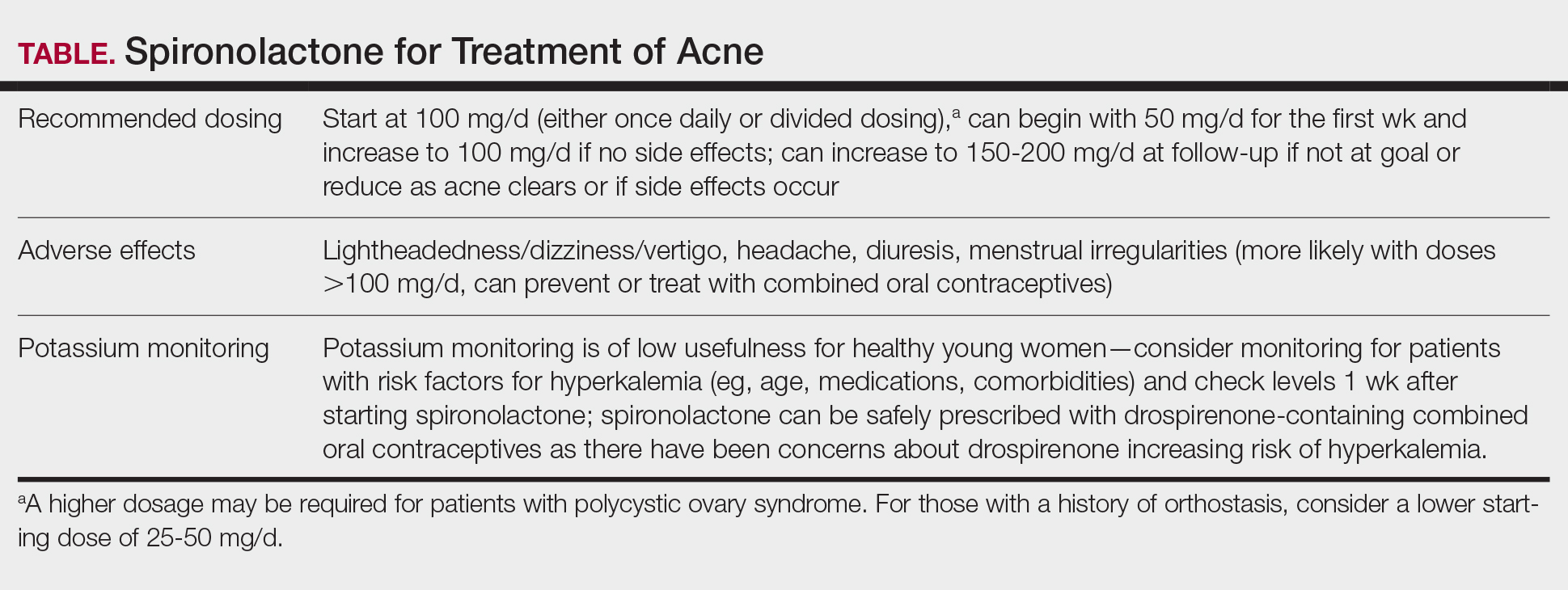
Spironolactone is increasingly used off label for acne treatment and is now being prescribed for women with acne at a frequency similar to oral antibiotics.1,2 In this article, we provide an overview of spironolactone use for acne treatment and discuss recent clinical trials and practical strategies for patient selection, dosing, adverse effect management, and monitoring (Table).
History and Mechanism of Action
Because sebaceous gland activity is an important component of acne pathogenesis and is regulated by androgens,3 there has long been interest in identifying treatment strategies that can target the role of hormones in activating the sebaceous gland. In the 1980s, it became apparent that spironolactone, originally developed as a potassium-sparing diuretic, also might possess antiandrogenic properties that could be useful in the treatment of acne.4 Spironolactone has been found to decrease testosterone production, inhibit testosterone and dihydrotestosterone binding to androgen receptors,5-8 and block 5α-reductase receptors of the sebaceous glands of skin.9
In 1984, Goodfellow et al10 conducted a trial in which 36 male and female patients with severe acne were randomized to placebo or spironolactone doses ranging from 50 to 200 mg/d. They found that spironolactone resulted in dose-dependent reductions of sebum production as well as improvement in patient- and clinician-reported assessments of acne. In 1986, another placebo-controlled crossover trial by Muhlemann et al11 provided further support for the effectiveness of spironolactone for acne. This trial randomized 21 women to placebo or spironolactone 200 mg/d and found that spironolactone was associated with statistically significant (P<.001) improvements in acne lesion counts.
Recent Observational Studies and Trials
Following these early trials, several large case series have been published describing the successful use of spironolactone for acne, including a 2020 retrospective case series from the Mayo Clinic describing 395 patients.12 The investigators found that almost 66% of patients had a complete response and almost 85% had a complete response or a partial response greater than 50%. They also found that the median time to initial response and maximal response were 3 and 5 months, respectively, and that efficacy was observed across acne subtypes, including for nodulocystic acne.12 In addition, a 2021 case series describing 403 patients treated with spironolactone found that approximately 80% had reduction or complete clearance of acne, with improvements observed for both facial and truncal acne. In this cohort, doses of 100 to 150 mg/d typically were the most successful.13 A case series of 80 adolescent females also highlighted the efficacy of spironolactone in younger populations.14
Adding to these observational data, the multicenter, phase 3, double-blind Spironolactone for Adult Female Acne (SAFA) trial included 410 women (mean age, 29.2 years) who were randomized to receive either placebo or intervention (spironolactone 50 mg/d until week 6 and 100 mg/d until week 24).15 At 24 weeks, greater improvement in quality of life and participant self-assessed improvement were observed in the spironolactone group. In addition, at 12 weeks, rates of success were higher in the spironolactone group using the Investigator Global Assessment score (adjusted odds ratio 5.18 [95% CI, 2.18- 12.28]). Those randomized to receive spironolactone also had lower rates of oral antibiotic use at 52 weeks than the placebo group did (5.8% vs 13.5%, respectively).
In the SAFA trial, spironolactone was well tolerated; the most common adverse effects relative to placebo were lightheadedness (19% for spironolactone vs 12% for placebo) and headache (20% for spironolactone vs 12% for placebo). Notably, more than 95% of patients were able to increase from 50 mg/d to 100 mg/d at week 6, with greater than 90% tolerating 100 mg/d. As observational data suggest that spironolactone takes 3 to 5 months to reach peak efficacy, these findings provide further support that starting at a dose of at least 100 mg/d is likely optimal for most patients.16
A Potential Alternative to Oral Antibiotics
Oral antibiotics such as tetracyclines have long played a central role in the treatment of acne and remain a first-line treatment option.17 In addition, many of these antibiotic courses exceed 6 months in duration.1 In fact, dermatologists prescribe more antibiotics per capita than any other specialty1,18-20; however, this can be associated with the development of antibiotic resistance,21,22 as well as other antibiotic-associated complications, including inflammatory bowel disease,23 pharyngitis,24 Clostridium difficile infections, and cancer.25-29
In addition to these concerns, many patients may prefer nonantibiotic alternatives to oral antibiotics, with more than 75% preferring a nonantibiotic option if available. For female patients with acne, antiandrogens such as spironolactone have been suggested as a potential alternative.30 A 10-year retrospective study of female patients with acne found that those who had ever received hormonal therapy (ie, spironolactone or a combined oral contraceptive) received fewer cumulative days of oral antibiotics than those who did not (226 days vs 302 days, respectively).31 In addition, while oral antibiotics were the most common initial therapy prescribed for patients, as they progressed through their treatment course, more patients ended up on hormonal therapy than oral antibiotics. This study suggests that hormonal therapy such as spironolactone could represent an alternative to the use of systemic antibiotics.31
Further supporting the role of spironolactone as an alternative to oral antibiotics, a 2018 analysis of claims data found that spironolactone may have similar effectiveness to oral antibiotics for the treatment of acne.32 After adjusting for age and topical retinoid and oral contraceptive use, this study found that there was no significant difference in the odds of being prescribed a different systemic treatment within 1 year (ie, treatment failure) among those starting spironolactone vs those starting oral tetracycline-class antibiotics as their initial therapy for acne.
A multicenter, randomized, double-blind trial (Female Acne Spironolactone vs doxyCycline Efficacy [FASCE]) also evaluated the comparative effectiveness of doxycycline 100 mg/d for 3 months followed by an oral placebo for 3 months vs spironolactone 150 mg/d for 6 months among 133 adult women with acne. This study found that spironolactone had statistically significantly greater rates of Investigator Global Assessment treatment success after 6 months (odds ratio 2.87 [95% CI, 1.38-5.99; P=.007]).33 Since spironolactone historically has been prescribed less often than oral antibiotics for women with acne, these findings support spironolactone as an underutilized treatment alternative. The ongoing Spironolactone versus Doxycycline for Acne: A Comparative Effectiveness, Noninferiority Evaluation trial—a 16-week, blinded trial comparing 100 mg/d doses of both drugs—should provide additional evidence regarding the relative role of spironolactone and oral antibiotics in the management of acne.34
Ultimately, the decision to use spironolactone or other treatments such as oral antibiotics should be based on shared decision making between clinician and patient. Spironolactone has a relatively slow onset of efficacy, and other options such as oral antibiotics might be preferred by those looking for more immediate results; however, as women with acne often have activity that persists into adulthood, spironolactone might be preferable as a long-term maintenance therapy to avoid complications of prolonged antibiotic use.35 Comorbidities also will influence the optimal choice of therapy (eg, spironolactone might be preferred in someone with inflammatory bowel disease, and oral antibiotics might be preferred in someone with orthostatic hypotension).
Patient Selection
Acne occurring along the lower face or jawline in adult women sometimes is referred to as hormonal acne, but this dogma is not particularly evidence based. An observational study of 374 patients found that almost 90% of adult women had acne involving multiple facial zones with a spectrum of facial acne severity similar to that in adolescents.36 Only a small subset of these patients (11.2%) had acne localized solely to the mandibular area. In addition, acne along the lower face is not predictive of hyperandrogenism (eg, polycystic ovary syndrome).37 Antiandrogen therapies such as spironolactone and clascoterone are effective in both men and women with acne10,38 and in adolescents and adults, suggesting that hormones play a fundamental role in all acne and that addressing this mechanism can be useful broadly. Therefore, hormonal therapies such as spironolactone should not be restricted to only adult women with acne along the lower face.
While spironolactone can be effective for acne treatment in any age group, it may be most effective for adult women with acne. In the SAFA trial, prespecified subgroup analyses showed a statistically significant (P=.005) interaction term for age (categorized as <25 years and ≥25 years), which suggested that spironolactone might be a more effective treatment for women 25 years and older.15 In addition, subgroup analyses in the aforementioned 2018 analysis of claims data found that spironolactone was more effective relative to oral antibiotics in adults vs adolescents.32 Despite these limitations, several case series have highlighted that spironolactone is effective among adolescent populations with acne. A case series of spironolactone use in 73 patients aged 19 years or younger found that 68% of patients demonstrated resolution or improvement in their acne after spironolactone treatment.39 Another case series among 80 adolescent females reported 80% of patients experiencing improvement of their acne.14
For those with more severe acne, spironolactone can be combined with other complementary treatment approaches such as topicals, oral antibiotics, or procedural modalities.40
Dosing
We recommend starting spironolactone at a dose of 100 mg/d (the patient can take 50 mg/d for 1 week, then increase to 100 mg/d if there are no adverse effects at the lower dose). In the 1984 trial by Goodfellow et al,10 participants were randomized to doses of 50 mg/d, 100 mg/d, 150 mg/d, and 200 mg/d. In this trial, efficacy assessed by objective and subjective outcomes did not plateau until doses of 100 mg/d to 150 mg/d. In addition, a case series of 403 patients found that the most successful dosage of spironolactone generally was 100 mg/d or higher.13 Most of the patients who were started at this dosage either stayed at this level or escalated, whereas patients who started at lower dosages (25-75 mg/d) frequently increased their dosage over time. The SAFA trial also highlighted that most patients can tolerate a spironolactone dose of 100 mg/d.15 For specific populations, such as patients with polycystic ovary syndrome, a higher dose (mean dosage of 143 mg/d) may be required for efficacy.41 Given the slow onset of efficacy, typically taking 3 to 5 months, and the low rate of adverse effects, we believe the optimal starting dose is 100 mg/s to 150 mg/d. If adverse effects occur or lesions clear, then the dosage may be reduced.
Adverse Effects
Spironolactone generally is well tolerated; in the SAFA and FASCE trials, fewer than 1% of participants discontinued due to adverse effects.15,33 Rates of discontinuation due to adverse effects typically have been less than 5% in case series of patients treated in routine clinical practice.12-14
Because spironolactone is a diuretic and antihypertensive, the most common adverse effects are related to these characteristics. In the SAFA trial, dizziness, lightheadedness, and vertigo were reported more commonly in the spironolactone group than in the placebo group (19% vs 12%, respectively). Similarly, headaches also were reported more frequently in the spironolactone group than in the placebo group (20% vs 12%, respectively).15 One case series found that, among the 267 patients on spironolactone whose blood pressure was monitored, the mean reduction in systolic blood pressure was 3.5 mm Hg and the mean reduction in diastolic blood pressure was 0.9 mm Hg.13 For those with baseline orthostasis or in those who experience adverse effects related to hypotension, reducing the dose often can be helpful. Of note, while doses of 100 mg/d to 150 mg/d often are the most effective, randomized trials have found that spironolactone still can be effective for acne at doses as low as 25 mg/d to 50 mg/d.10,38
Menstrual irregularities are another commonly cited adverse effect of spironolactone. While a systematic review found that 15% to 30% of patients treated with spironolactone experience menstrual irregularities, it has been difficult to evaluate whether this is due to the medication or other comorbidities, such as polycystic ovary syndrome.42 Notably, in the SAFA trial, rates of menstrual irregularities were equivalent between the spironolactone and placebo groups at a dose of 100 mg/d (32% vs 35%, respectively).15 In contrast, in the FASCE trial, menstrual irregularities were more commonly reported at a dose of 150 mg/d.33 These findings are consistent with observational data suggesting that menstrual irregularities are much more common at spironolactone doses greater than 100 mg/d.42 Additionally, some evidence supports that for some patients these menstrual irregularities may resolve within 2 to 3 months of continued treatment.43 It has been noted in several studies that menstrual irregularities are less likely to occur in patients who are using combined oral contraceptives; therefore, for patients who are amenable and have no contraindications, combined oral contraceptives can be considered to prevent or address menstrual irregularities.13,42,44
More generally, combined oral contraceptives can be an excellent combination with spironolactone, as they have complementary characteristics. Spironolactone primarily blocks the effects of androgens, while combined oral contraceptives predominantly block the production of androgens. Whereas spironolactone typically causes hypotension and menstrual irregularities, combined oral contraceptives cause hypertension and help to regulate the menstrual cycle.
Spironolactone carries an official US Food and Drug Administration warning regarding possible tumorigenicity that is based on animal studies that used up to 150 times the normal dose of spironolactone used in humans45; however, observational studies in humans have not identified such an association when spironolactone is used in normal clinical settings. A systematic review and metanalysis in 2022 reviewed data from a total population of more than 4 million individuals and found that there was no statistically significant association between spironolactone use and the risk for breast, ovarian, bladder, kidney, gastric, or esophageal cancers.46 Additional studies also found no association between spironolactone use and cancers.48 A more recent cohort study specifically among patients treated with spironolactone for acne also found no significant increased risk for breast cancer.49
Combined oral contraceptives are associated with an increased risk for venous thromboembolisms, and there have been concerns that this risk may be greater in combined oral contraceptives that contain drospirenone.50 Drospirenone is molecularly related to spironolactone, which has prompted the consideration of whether spironolactone use also conveys a risk for venous thromboembolism. Reassuringly, a retrospective study of claims data found that individuals on spironolactone were not more likely to develop a pulmonary embolism or a deep venous thrombosis than matched controls treated with tetracycline antibiotics, with a point estimate favoring decreased risk.51
Monitoring
Given that one of spironolactone’s mechanisms of action is aldosterone antagonism and thus the inhibition of potassium excretion, there have been concerns regarding risk for hyperkalemia. A retrospective study analyzing data from 2000 to 2014 found that, among 974 young women receiving spironolactone therapy, the rate of hyperkalemia was 0.72%, which is equivalent to the 0.76% baseline rate of hyperkalemia in the same population.52 Subsequent studies also have found that spironolactone does not appear to be associated with a meaningful risk for hyperkalemia among young healthy patients treated for acne.38,53 These studies suggest that routine potassium monitoring is of low usefulness for healthy young women taking spironolactone for acne. The 2024 American Academy of Dermatology guidelines on the management of acne also state that potassium monitoring is not needed in healthy patients but that potassium testing should be considered for those with risk factors for hyperkalemia (eg, older age, medical comorbidities, medications).40 Clinicians should still engage in shared decision making with patients to determine whether to check potassium. If potassium is to be monitored, it should be checked 1 to 2 weeks after spironolactone is started.45,54
Since drospirenone also has aldosterone antagonistic properties,55 there have been concerns about whether concomitant use of spironolactone and drospirenone-containing combined oral contraceptives might increase the risk for hyperkalemia.56 However, a retrospective cohort study analyzing data from more than 1 million women found that drospirenone is not any more likely than levonorgestrel to cause hyperkalemia and that there is no interaction between drospirenone and spironolactone for hyperkalemia.57 A subsequent prospective study of 27 women treated with combined oral contraceptives containing ethinyl estradiol/drospirenone and spironolactone also did not find any significant elevations in potassium.58 Data from these studies suggest that spironolactone can safely be co-administered with drospirenone-containing combined oral contraceptives.
Reproductive Risks
Despite its utility in treating acne, spironolactone should not be used during pregnancy, and appropriate pregnancy prevention is recommended. Spironolactone crosses the placenta, and some animal studies have shown feminization of male fetuses.59 While human data are limited to a few case reports that did not demonstrate an association of major malformations,60 it generally is recommended to avoid spironolactone during pregnancy. Small studies have found that spironolactone has minimal transfer to breastmilk and is not associated with adverse effects in breastfed infants.61-63 Accordingly, the World Health Organization considers spironolactone to be compatible with breastfeeding.64 Notably, spironolactone may be associated with lactation suppression65,66; therefore, it may be best if lactating patients ensure that their milk production is established prior to starting spironolactone and to increase their water intake to offset the diuretic effects.
Spironolactone also can result in gynecomastia in men and therefore typically is not prescribed for the treatment of acne in this population in oral form10; however, topical antiandrogens such as clascoterone can be used in both women and men with acne.67
Conclusion
Spironolactone is a well-tolerated and effective treatment for women with acne, both in adult and adolescent populations. It is a potentially underutilized alternative to oral antibiotics. Spironolactone also is affordable, fully covered without any requirements in almost 90% of states under Medicaid and with a monthly cost of only $4.00 when obtained through major retailers in the United States, making it an optimal long-term treatment option for many patients.52,68 We recommend a starting dose of 100 mg/d, which can be increased to 150 mg/d to 200 mg/d if needed for better acne control or decreased if adverse effects occur or acne clears. Potassium monitoring is of low usefulness in young healthy women, and studies have not identified an association between spironolactone use and increased risk for cancer.
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS. Temporal trends in the use of systemic medications for acne from 2017 to 2020. JAMA Dermatol. 2023;159:1135-1136. doi:10.1001 /jamadermatol.2023.2363
- Strauss JS, Pochi PE, Downing DT. Acne: perspectives. J Invest Dermatol. 1974;62:321-325. doi:10.1111/1523-1747.ep12724280
- Luderschmidt C, Bidlingmaier F, Plewig G. Inhibition of sebaceous gland activity by spironolactone in Syrian hamster. J Invest Dermatol. 1982;78:253-255. doi:10.1111/1523-1747.ep12506612
- Boisselle A, Dionne FT, Tremblay RR. Interaction of spironolactone with rat skin androgen receptor. Can J Biochem. 1979;57:1042-1046. doi:10.1139/o79-131
- Menard RH, Stripp B, Gillette JR. Spironolactone and testicular cytochrome P-450: decreased testosterone formation in several species and changes in hepatic drug metabolism. Endocrinology. 1974;94:1628-1636. doi:10.1210/endo-94-6-1628
- Rifka SM, Pita JC, Vigersky RA, et al. Interaction of digitalis and spironolactone with human sex steroid receptors. J Clin Endocrinol Metab. 1978;46:338-344. doi:10.1210/jcem-46-2-338
- Corvol P, Michaud A, Menard J, et al. Antiandrogenic effect of spirolactones: mechanism of action. Endocrinology. 1975;97:52-58. doi:10.1210/endo-97-1-52
- Akamatsu H, Zouboulis CC, Orfanos CE. Spironolactone directly inhibits proliferation of cultured human facial sebocytes and acts antagonistically to testosterone and 5 alpha-dihydrotestosterone in vitro. J Invest Dermatol. 1993;100:660-662. doi:10.1111/1523-1747 .ep12472325
- Goodfellow A, Alaghband-Zadeh J, Carter G, et al. Oral spironolactone improves acne vulgaris and reduces sebum excretion. Br J Dermatol. 1984;111:209-214. doi:10.1111/j.1365-2133.1984.tb04045.x
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232. doi:10.1111/j.1365-2133.1986.tb05722.x
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Santer M, Lawrence M, Renz S, et al. Effectiveness of spironolactone for women with acne vulgaris (SAFA) in England and Wales: pragmatic, multicentre, phase 3, double blind, randomised controlled trial. BMJ. 2023;381:E074349. doi:10.1136/bmj-2022-074349
- Shields A, Barbieri JS. Effectiveness of spironolactone for women with acne vulgaris (SAFA) trial: a critically appraised topic. Br J Dermatol. 2023;189:509-510. doi:10.1093/bjd/ljad270
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344. doi:10.1007/s40257-018-00417-3
- Knutsen-Larson S, Dawson AL, Dunnick CA, et al. Acne vulgaris: pathogenesis, treatment, and needs assessment. Dermatol Clin. 2012;30:99-106, viii-ix. doi:10.1016/j.det.2011.09.001
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States, 2021. Accessed May 21, 2025. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
- Adler BL, Kornmehl H, Armstrong AW. Antibiotic resistance in acne treatment. JAMA Dermatol. 2017;153:810-811. doi:10.1001 /jamadermatol.2017.1297
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33. doi:10.1016/S1473-3099(15)00527-7
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616. doi:10.1038/ajg.2010.303?
- Margolis DJ, Fanelli M, Kupperman E, et al. Association of pharyngitis with oral antibiotic use for the treatment of acne: a cross-sectional and prospective cohort study. Arch Dermatol. 2012;148:326-332. doi:10.1001 /archdermatol.2011.355
- Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531-534. doi:10.1056/NEJM197803092981003
- Carroll KC, Bartlett JG. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu Rev Microbiol. 2011;65:501-521. doi:10.1146/annurev-micro-090110-102824
- Velicer CM, Heckbert SR, Lampe JW, et al. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291:827-835. doi:10.1001/jama.291.7.827
- Song M, Nguyen LH, Emilsson L, et al. Antibiotic use associated with risk of colorectal polyps in a nationwide study. Clin Gastroenterol Hepatol. 2021;19:1426-1435.e6. doi:10.1016/j.cgh.2020.05.036
- Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67:672-678. doi:10.1136 /gutjnl-2016-313413
- Del Rosso JQ, Rosen T, Palceski D, et al. Patient awareness of antimicrobial resistance and antibiotic use in acne vulgaris. J Clin Aesthetic Dermatol. 2019;12:30-41.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455. doi:10.1007/s40257-018-0349-6
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) Study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Barbieri JS, Ellenberg S, Grice E, et al. Challenges in designing a randomized, double-blind noninferiority trial for treatment of acne: The SDACNE trial. Clin Trials. 2025;22:66-76. doi:10.1177/17407745241265094
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106. doi:10.1111/jdv.12757
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2016;152:391-398. doi:10.1001/jamadermatol.2015.4498
- Plante J, Robinson I, Elston D. The need for potassium monitoring in women on spironolactone for dermatologic conditions. J Am Acad Dermatol. 2022;87:1097-1099. doi:10.1016/j.jaad.2022.01.010
- Berman HS, Cheng CE, Hogeling M. Spironolactone in the treatment of adolescent acne: a retrospective review. J Am Acad Dermatol. 2021;85:269-271. doi:10.1016/j.jaad.2020.11.044
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006.e1-1006 .e30. doi:10.1016/j.jaad.2023.12.017
- Basu P. High-dose spironolactone for acne in patients with polycystic ovarian syndrome: a single-institution retrospective study. J Am Acad Dermatol. 2021;85:740-741.
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Yemisci A, Gorgulu A, Piskin S. Effects and side-effects of spironolactone therapy in women with acne. J Eur Acad Dermatol Venereol. 2005;19:163-166. doi:10.1111/j.1468-3083.2005.01072.x
- Patiyasikunt M, Chancheewa B, Asawanonda P, et al. Efficacy and tolerability of low-dose spironolactone and topical benzoyl peroxide in adult female acne: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2020;47:1411-1416. doi:10.1111/1346-8138.15559
- Aldactone (spironolactone) tablets. Prescribing information. Pfizer; 2008. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/012151s062lbl.pdf
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001/jamadermatol.2021.5866
- Mackenzie IS, Morant SV, Wei L, et al. Spironolactone use and risk of incident cancers: a retrospective, matched cohort study. Br J Clin Pharmacol. 2017;83:653-663. doi:10.1111/bcp.13152
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875. doi:10.1016/j.canep.2013.10.004
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007/s00403-024-02936-y
- Jick SS, Hernandez RK. Risk of nonfatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: casecontrol study using United States claims data. BMJ. 2011;342:d2151. doi:10.1136/bmj.d2151
- Shields A, Flood K, Barbieri JS. Spironolactone use for acne is not associated with an increased risk of venous thromboembolism: a matched, retrospective cohort study. J Am Acad Dermatol. 2023;88:1396-1397. doi:10.1016/j.jaad.2023.02.028
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Muhn P, Fuhrmann U, Fritzemeier KH, et al. Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Ann N Y Acad Sci. 1995;761:311-335. doi:10.1111/j.1749-6632.1995.tb31386.x
- Yaz (drospirenone/ethinyl estradiol) tablets. Prescribing information. Bayer HealthCare Pharmaceuticals; 2012. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021676s012lbl.pdf
- Bird ST, Pepe SR, Etminan M, et al. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol. 2011;11:23. doi:10.1186/1472-6904-11-23
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Liszewski W, Boull C. Lack of evidence for feminization of males exposed to spironolactone in utero: a systematic review. J Am Acad Dermatol. 2019;80:1147-1148. doi:10.1016/j.jaad.2018.10.023
- de Jong MFC, Riphagen IJ, Kootstra-Ros JE, et al. Potassium and magnesium in breast milk of a woman with gitelman syndrome. Kidney Int Rep. 2022;7:1720-1721. doi:10.1016/j.ekir.2022.05.006
- Reisman T, Goldstein Z. Case report: induced lactation in a transgender woman. Transgender Health. 2018;3:24-26. doi:10.1089 /trgh.2017.0044
- Phelps DL, Karim A. Spironolactone: relationship between concentrations of dethioacetylated metabolite in human serum and milk. J Pharm Sci. 1977;66:1203. doi:10.1002/jps.2600660841
- World Health Organization. Breastfeeding and maternal medication: recommendations for drugs in the eleventh WHO model list of essential drugs. February 25, 2002. Accessed May 21, 2025. https://www.who.int/publications/i/item/55732
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. Lactation. J Am Acad Dermatol. 2014;70:417.e1-10; quiz 427. doi:10.1016/j.jaad.2013.09.009
- Cominos DC, van der Walt A, van Rooyen AJ. Suppression of postpartum lactation with furosemide. S Afr Med J. 1976;50:251-252.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Ershadi S, Choe J, Barbieri JS. Medicaid formularies for acne treatments are difficult to access and reflect inconsistent coverage policies. J Am Acad Dermatol. 2024;90:1074-1076. doi:10.1016/j.jaad.2024.01.033
Spironolactone is increasingly used off label for acne treatment and is now being prescribed for women with acne at a frequency similar to oral antibiotics.1,2 In this article, we provide an overview of spironolactone use for acne treatment and discuss recent clinical trials and practical strategies for patient selection, dosing, adverse effect management, and monitoring (Table).

History and Mechanism of Action
Because sebaceous gland activity is an important component of acne pathogenesis and is regulated by androgens,3 there has long been interest in identifying treatment strategies that can target the role of hormones in activating the sebaceous gland. In the 1980s, it became apparent that spironolactone, originally developed as a potassium-sparing diuretic, also might possess antiandrogenic properties that could be useful in the treatment of acne.4 Spironolactone has been found to decrease testosterone production, inhibit testosterone and dihydrotestosterone binding to androgen receptors,5-8 and block 5α-reductase receptors of the sebaceous glands of skin.9
In 1984, Goodfellow et al10 conducted a trial in which 36 male and female patients with severe acne were randomized to placebo or spironolactone doses ranging from 50 to 200 mg/d. They found that spironolactone resulted in dose-dependent reductions of sebum production as well as improvement in patient- and clinician-reported assessments of acne. In 1986, another placebo-controlled crossover trial by Muhlemann et al11 provided further support for the effectiveness of spironolactone for acne. This trial randomized 21 women to placebo or spironolactone 200 mg/d and found that spironolactone was associated with statistically significant (P<.001) improvements in acne lesion counts.
Recent Observational Studies and Trials
Following these early trials, several large case series have been published describing the successful use of spironolactone for acne, including a 2020 retrospective case series from the Mayo Clinic describing 395 patients.12 The investigators found that almost 66% of patients had a complete response and almost 85% had a complete response or a partial response greater than 50%. They also found that the median time to initial response and maximal response were 3 and 5 months, respectively, and that efficacy was observed across acne subtypes, including for nodulocystic acne.12 In addition, a 2021 case series describing 403 patients treated with spironolactone found that approximately 80% had reduction or complete clearance of acne, with improvements observed for both facial and truncal acne. In this cohort, doses of 100 to 150 mg/d typically were the most successful.13 A case series of 80 adolescent females also highlighted the efficacy of spironolactone in younger populations.14
Adding to these observational data, the multicenter, phase 3, double-blind Spironolactone for Adult Female Acne (SAFA) trial included 410 women (mean age, 29.2 years) who were randomized to receive either placebo or intervention (spironolactone 50 mg/d until week 6 and 100 mg/d until week 24).15 At 24 weeks, greater improvement in quality of life and participant self-assessed improvement were observed in the spironolactone group. In addition, at 12 weeks, rates of success were higher in the spironolactone group using the Investigator Global Assessment score (adjusted odds ratio 5.18 [95% CI, 2.18- 12.28]). Those randomized to receive spironolactone also had lower rates of oral antibiotic use at 52 weeks than the placebo group did (5.8% vs 13.5%, respectively).
In the SAFA trial, spironolactone was well tolerated; the most common adverse effects relative to placebo were lightheadedness (19% for spironolactone vs 12% for placebo) and headache (20% for spironolactone vs 12% for placebo). Notably, more than 95% of patients were able to increase from 50 mg/d to 100 mg/d at week 6, with greater than 90% tolerating 100 mg/d. As observational data suggest that spironolactone takes 3 to 5 months to reach peak efficacy, these findings provide further support that starting at a dose of at least 100 mg/d is likely optimal for most patients.16
A Potential Alternative to Oral Antibiotics
Oral antibiotics such as tetracyclines have long played a central role in the treatment of acne and remain a first-line treatment option.17 In addition, many of these antibiotic courses exceed 6 months in duration.1 In fact, dermatologists prescribe more antibiotics per capita than any other specialty1,18-20; however, this can be associated with the development of antibiotic resistance,21,22 as well as other antibiotic-associated complications, including inflammatory bowel disease,23 pharyngitis,24 Clostridium difficile infections, and cancer.25-29
In addition to these concerns, many patients may prefer nonantibiotic alternatives to oral antibiotics, with more than 75% preferring a nonantibiotic option if available. For female patients with acne, antiandrogens such as spironolactone have been suggested as a potential alternative.30 A 10-year retrospective study of female patients with acne found that those who had ever received hormonal therapy (ie, spironolactone or a combined oral contraceptive) received fewer cumulative days of oral antibiotics than those who did not (226 days vs 302 days, respectively).31 In addition, while oral antibiotics were the most common initial therapy prescribed for patients, as they progressed through their treatment course, more patients ended up on hormonal therapy than oral antibiotics. This study suggests that hormonal therapy such as spironolactone could represent an alternative to the use of systemic antibiotics.31
Further supporting the role of spironolactone as an alternative to oral antibiotics, a 2018 analysis of claims data found that spironolactone may have similar effectiveness to oral antibiotics for the treatment of acne.32 After adjusting for age and topical retinoid and oral contraceptive use, this study found that there was no significant difference in the odds of being prescribed a different systemic treatment within 1 year (ie, treatment failure) among those starting spironolactone vs those starting oral tetracycline-class antibiotics as their initial therapy for acne.
A multicenter, randomized, double-blind trial (Female Acne Spironolactone vs doxyCycline Efficacy [FASCE]) also evaluated the comparative effectiveness of doxycycline 100 mg/d for 3 months followed by an oral placebo for 3 months vs spironolactone 150 mg/d for 6 months among 133 adult women with acne. This study found that spironolactone had statistically significantly greater rates of Investigator Global Assessment treatment success after 6 months (odds ratio 2.87 [95% CI, 1.38-5.99; P=.007]).33 Since spironolactone historically has been prescribed less often than oral antibiotics for women with acne, these findings support spironolactone as an underutilized treatment alternative. The ongoing Spironolactone versus Doxycycline for Acne: A Comparative Effectiveness, Noninferiority Evaluation trial—a 16-week, blinded trial comparing 100 mg/d doses of both drugs—should provide additional evidence regarding the relative role of spironolactone and oral antibiotics in the management of acne.34
Ultimately, the decision to use spironolactone or other treatments such as oral antibiotics should be based on shared decision making between clinician and patient. Spironolactone has a relatively slow onset of efficacy, and other options such as oral antibiotics might be preferred by those looking for more immediate results; however, as women with acne often have activity that persists into adulthood, spironolactone might be preferable as a long-term maintenance therapy to avoid complications of prolonged antibiotic use.35 Comorbidities also will influence the optimal choice of therapy (eg, spironolactone might be preferred in someone with inflammatory bowel disease, and oral antibiotics might be preferred in someone with orthostatic hypotension).
Patient Selection
Acne occurring along the lower face or jawline in adult women sometimes is referred to as hormonal acne, but this dogma is not particularly evidence based. An observational study of 374 patients found that almost 90% of adult women had acne involving multiple facial zones with a spectrum of facial acne severity similar to that in adolescents.36 Only a small subset of these patients (11.2%) had acne localized solely to the mandibular area. In addition, acne along the lower face is not predictive of hyperandrogenism (eg, polycystic ovary syndrome).37 Antiandrogen therapies such as spironolactone and clascoterone are effective in both men and women with acne10,38 and in adolescents and adults, suggesting that hormones play a fundamental role in all acne and that addressing this mechanism can be useful broadly. Therefore, hormonal therapies such as spironolactone should not be restricted to only adult women with acne along the lower face.
While spironolactone can be effective for acne treatment in any age group, it may be most effective for adult women with acne. In the SAFA trial, prespecified subgroup analyses showed a statistically significant (P=.005) interaction term for age (categorized as <25 years and ≥25 years), which suggested that spironolactone might be a more effective treatment for women 25 years and older.15 In addition, subgroup analyses in the aforementioned 2018 analysis of claims data found that spironolactone was more effective relative to oral antibiotics in adults vs adolescents.32 Despite these limitations, several case series have highlighted that spironolactone is effective among adolescent populations with acne. A case series of spironolactone use in 73 patients aged 19 years or younger found that 68% of patients demonstrated resolution or improvement in their acne after spironolactone treatment.39 Another case series among 80 adolescent females reported 80% of patients experiencing improvement of their acne.14
For those with more severe acne, spironolactone can be combined with other complementary treatment approaches such as topicals, oral antibiotics, or procedural modalities.40
Dosing
We recommend starting spironolactone at a dose of 100 mg/d (the patient can take 50 mg/d for 1 week, then increase to 100 mg/d if there are no adverse effects at the lower dose). In the 1984 trial by Goodfellow et al,10 participants were randomized to doses of 50 mg/d, 100 mg/d, 150 mg/d, and 200 mg/d. In this trial, efficacy assessed by objective and subjective outcomes did not plateau until doses of 100 mg/d to 150 mg/d. In addition, a case series of 403 patients found that the most successful dosage of spironolactone generally was 100 mg/d or higher.13 Most of the patients who were started at this dosage either stayed at this level or escalated, whereas patients who started at lower dosages (25-75 mg/d) frequently increased their dosage over time. The SAFA trial also highlighted that most patients can tolerate a spironolactone dose of 100 mg/d.15 For specific populations, such as patients with polycystic ovary syndrome, a higher dose (mean dosage of 143 mg/d) may be required for efficacy.41 Given the slow onset of efficacy, typically taking 3 to 5 months, and the low rate of adverse effects, we believe the optimal starting dose is 100 mg/s to 150 mg/d. If adverse effects occur or lesions clear, then the dosage may be reduced.
Adverse Effects
Spironolactone generally is well tolerated; in the SAFA and FASCE trials, fewer than 1% of participants discontinued due to adverse effects.15,33 Rates of discontinuation due to adverse effects typically have been less than 5% in case series of patients treated in routine clinical practice.12-14
Because spironolactone is a diuretic and antihypertensive, the most common adverse effects are related to these characteristics. In the SAFA trial, dizziness, lightheadedness, and vertigo were reported more commonly in the spironolactone group than in the placebo group (19% vs 12%, respectively). Similarly, headaches also were reported more frequently in the spironolactone group than in the placebo group (20% vs 12%, respectively).15 One case series found that, among the 267 patients on spironolactone whose blood pressure was monitored, the mean reduction in systolic blood pressure was 3.5 mm Hg and the mean reduction in diastolic blood pressure was 0.9 mm Hg.13 For those with baseline orthostasis or in those who experience adverse effects related to hypotension, reducing the dose often can be helpful. Of note, while doses of 100 mg/d to 150 mg/d often are the most effective, randomized trials have found that spironolactone still can be effective for acne at doses as low as 25 mg/d to 50 mg/d.10,38
Menstrual irregularities are another commonly cited adverse effect of spironolactone. While a systematic review found that 15% to 30% of patients treated with spironolactone experience menstrual irregularities, it has been difficult to evaluate whether this is due to the medication or other comorbidities, such as polycystic ovary syndrome.42 Notably, in the SAFA trial, rates of menstrual irregularities were equivalent between the spironolactone and placebo groups at a dose of 100 mg/d (32% vs 35%, respectively).15 In contrast, in the FASCE trial, menstrual irregularities were more commonly reported at a dose of 150 mg/d.33 These findings are consistent with observational data suggesting that menstrual irregularities are much more common at spironolactone doses greater than 100 mg/d.42 Additionally, some evidence supports that for some patients these menstrual irregularities may resolve within 2 to 3 months of continued treatment.43 It has been noted in several studies that menstrual irregularities are less likely to occur in patients who are using combined oral contraceptives; therefore, for patients who are amenable and have no contraindications, combined oral contraceptives can be considered to prevent or address menstrual irregularities.13,42,44
More generally, combined oral contraceptives can be an excellent combination with spironolactone, as they have complementary characteristics. Spironolactone primarily blocks the effects of androgens, while combined oral contraceptives predominantly block the production of androgens. Whereas spironolactone typically causes hypotension and menstrual irregularities, combined oral contraceptives cause hypertension and help to regulate the menstrual cycle.
Spironolactone carries an official US Food and Drug Administration warning regarding possible tumorigenicity that is based on animal studies that used up to 150 times the normal dose of spironolactone used in humans45; however, observational studies in humans have not identified such an association when spironolactone is used in normal clinical settings. A systematic review and metanalysis in 2022 reviewed data from a total population of more than 4 million individuals and found that there was no statistically significant association between spironolactone use and the risk for breast, ovarian, bladder, kidney, gastric, or esophageal cancers.46 Additional studies also found no association between spironolactone use and cancers.48 A more recent cohort study specifically among patients treated with spironolactone for acne also found no significant increased risk for breast cancer.49
Combined oral contraceptives are associated with an increased risk for venous thromboembolisms, and there have been concerns that this risk may be greater in combined oral contraceptives that contain drospirenone.50 Drospirenone is molecularly related to spironolactone, which has prompted the consideration of whether spironolactone use also conveys a risk for venous thromboembolism. Reassuringly, a retrospective study of claims data found that individuals on spironolactone were not more likely to develop a pulmonary embolism or a deep venous thrombosis than matched controls treated with tetracycline antibiotics, with a point estimate favoring decreased risk.51
Monitoring
Given that one of spironolactone’s mechanisms of action is aldosterone antagonism and thus the inhibition of potassium excretion, there have been concerns regarding risk for hyperkalemia. A retrospective study analyzing data from 2000 to 2014 found that, among 974 young women receiving spironolactone therapy, the rate of hyperkalemia was 0.72%, which is equivalent to the 0.76% baseline rate of hyperkalemia in the same population.52 Subsequent studies also have found that spironolactone does not appear to be associated with a meaningful risk for hyperkalemia among young healthy patients treated for acne.38,53 These studies suggest that routine potassium monitoring is of low usefulness for healthy young women taking spironolactone for acne. The 2024 American Academy of Dermatology guidelines on the management of acne also state that potassium monitoring is not needed in healthy patients but that potassium testing should be considered for those with risk factors for hyperkalemia (eg, older age, medical comorbidities, medications).40 Clinicians should still engage in shared decision making with patients to determine whether to check potassium. If potassium is to be monitored, it should be checked 1 to 2 weeks after spironolactone is started.45,54
Since drospirenone also has aldosterone antagonistic properties,55 there have been concerns about whether concomitant use of spironolactone and drospirenone-containing combined oral contraceptives might increase the risk for hyperkalemia.56 However, a retrospective cohort study analyzing data from more than 1 million women found that drospirenone is not any more likely than levonorgestrel to cause hyperkalemia and that there is no interaction between drospirenone and spironolactone for hyperkalemia.57 A subsequent prospective study of 27 women treated with combined oral contraceptives containing ethinyl estradiol/drospirenone and spironolactone also did not find any significant elevations in potassium.58 Data from these studies suggest that spironolactone can safely be co-administered with drospirenone-containing combined oral contraceptives.
Reproductive Risks
Despite its utility in treating acne, spironolactone should not be used during pregnancy, and appropriate pregnancy prevention is recommended. Spironolactone crosses the placenta, and some animal studies have shown feminization of male fetuses.59 While human data are limited to a few case reports that did not demonstrate an association of major malformations,60 it generally is recommended to avoid spironolactone during pregnancy. Small studies have found that spironolactone has minimal transfer to breastmilk and is not associated with adverse effects in breastfed infants.61-63 Accordingly, the World Health Organization considers spironolactone to be compatible with breastfeeding.64 Notably, spironolactone may be associated with lactation suppression65,66; therefore, it may be best if lactating patients ensure that their milk production is established prior to starting spironolactone and to increase their water intake to offset the diuretic effects.
Spironolactone also can result in gynecomastia in men and therefore typically is not prescribed for the treatment of acne in this population in oral form10; however, topical antiandrogens such as clascoterone can be used in both women and men with acne.67
Conclusion
Spironolactone is a well-tolerated and effective treatment for women with acne, both in adult and adolescent populations. It is a potentially underutilized alternative to oral antibiotics. Spironolactone also is affordable, fully covered without any requirements in almost 90% of states under Medicaid and with a monthly cost of only $4.00 when obtained through major retailers in the United States, making it an optimal long-term treatment option for many patients.52,68 We recommend a starting dose of 100 mg/d, which can be increased to 150 mg/d to 200 mg/d if needed for better acne control or decreased if adverse effects occur or acne clears. Potassium monitoring is of low usefulness in young healthy women, and studies have not identified an association between spironolactone use and increased risk for cancer.
Spironolactone is increasingly used off label for acne treatment and is now being prescribed for women with acne at a frequency similar to oral antibiotics.1,2 In this article, we provide an overview of spironolactone use for acne treatment and discuss recent clinical trials and practical strategies for patient selection, dosing, adverse effect management, and monitoring (Table).

History and Mechanism of Action
Because sebaceous gland activity is an important component of acne pathogenesis and is regulated by androgens,3 there has long been interest in identifying treatment strategies that can target the role of hormones in activating the sebaceous gland. In the 1980s, it became apparent that spironolactone, originally developed as a potassium-sparing diuretic, also might possess antiandrogenic properties that could be useful in the treatment of acne.4 Spironolactone has been found to decrease testosterone production, inhibit testosterone and dihydrotestosterone binding to androgen receptors,5-8 and block 5α-reductase receptors of the sebaceous glands of skin.9
In 1984, Goodfellow et al10 conducted a trial in which 36 male and female patients with severe acne were randomized to placebo or spironolactone doses ranging from 50 to 200 mg/d. They found that spironolactone resulted in dose-dependent reductions of sebum production as well as improvement in patient- and clinician-reported assessments of acne. In 1986, another placebo-controlled crossover trial by Muhlemann et al11 provided further support for the effectiveness of spironolactone for acne. This trial randomized 21 women to placebo or spironolactone 200 mg/d and found that spironolactone was associated with statistically significant (P<.001) improvements in acne lesion counts.
Recent Observational Studies and Trials
Following these early trials, several large case series have been published describing the successful use of spironolactone for acne, including a 2020 retrospective case series from the Mayo Clinic describing 395 patients.12 The investigators found that almost 66% of patients had a complete response and almost 85% had a complete response or a partial response greater than 50%. They also found that the median time to initial response and maximal response were 3 and 5 months, respectively, and that efficacy was observed across acne subtypes, including for nodulocystic acne.12 In addition, a 2021 case series describing 403 patients treated with spironolactone found that approximately 80% had reduction or complete clearance of acne, with improvements observed for both facial and truncal acne. In this cohort, doses of 100 to 150 mg/d typically were the most successful.13 A case series of 80 adolescent females also highlighted the efficacy of spironolactone in younger populations.14
Adding to these observational data, the multicenter, phase 3, double-blind Spironolactone for Adult Female Acne (SAFA) trial included 410 women (mean age, 29.2 years) who were randomized to receive either placebo or intervention (spironolactone 50 mg/d until week 6 and 100 mg/d until week 24).15 At 24 weeks, greater improvement in quality of life and participant self-assessed improvement were observed in the spironolactone group. In addition, at 12 weeks, rates of success were higher in the spironolactone group using the Investigator Global Assessment score (adjusted odds ratio 5.18 [95% CI, 2.18- 12.28]). Those randomized to receive spironolactone also had lower rates of oral antibiotic use at 52 weeks than the placebo group did (5.8% vs 13.5%, respectively).
In the SAFA trial, spironolactone was well tolerated; the most common adverse effects relative to placebo were lightheadedness (19% for spironolactone vs 12% for placebo) and headache (20% for spironolactone vs 12% for placebo). Notably, more than 95% of patients were able to increase from 50 mg/d to 100 mg/d at week 6, with greater than 90% tolerating 100 mg/d. As observational data suggest that spironolactone takes 3 to 5 months to reach peak efficacy, these findings provide further support that starting at a dose of at least 100 mg/d is likely optimal for most patients.16
A Potential Alternative to Oral Antibiotics
Oral antibiotics such as tetracyclines have long played a central role in the treatment of acne and remain a first-line treatment option.17 In addition, many of these antibiotic courses exceed 6 months in duration.1 In fact, dermatologists prescribe more antibiotics per capita than any other specialty1,18-20; however, this can be associated with the development of antibiotic resistance,21,22 as well as other antibiotic-associated complications, including inflammatory bowel disease,23 pharyngitis,24 Clostridium difficile infections, and cancer.25-29
In addition to these concerns, many patients may prefer nonantibiotic alternatives to oral antibiotics, with more than 75% preferring a nonantibiotic option if available. For female patients with acne, antiandrogens such as spironolactone have been suggested as a potential alternative.30 A 10-year retrospective study of female patients with acne found that those who had ever received hormonal therapy (ie, spironolactone or a combined oral contraceptive) received fewer cumulative days of oral antibiotics than those who did not (226 days vs 302 days, respectively).31 In addition, while oral antibiotics were the most common initial therapy prescribed for patients, as they progressed through their treatment course, more patients ended up on hormonal therapy than oral antibiotics. This study suggests that hormonal therapy such as spironolactone could represent an alternative to the use of systemic antibiotics.31
Further supporting the role of spironolactone as an alternative to oral antibiotics, a 2018 analysis of claims data found that spironolactone may have similar effectiveness to oral antibiotics for the treatment of acne.32 After adjusting for age and topical retinoid and oral contraceptive use, this study found that there was no significant difference in the odds of being prescribed a different systemic treatment within 1 year (ie, treatment failure) among those starting spironolactone vs those starting oral tetracycline-class antibiotics as their initial therapy for acne.
A multicenter, randomized, double-blind trial (Female Acne Spironolactone vs doxyCycline Efficacy [FASCE]) also evaluated the comparative effectiveness of doxycycline 100 mg/d for 3 months followed by an oral placebo for 3 months vs spironolactone 150 mg/d for 6 months among 133 adult women with acne. This study found that spironolactone had statistically significantly greater rates of Investigator Global Assessment treatment success after 6 months (odds ratio 2.87 [95% CI, 1.38-5.99; P=.007]).33 Since spironolactone historically has been prescribed less often than oral antibiotics for women with acne, these findings support spironolactone as an underutilized treatment alternative. The ongoing Spironolactone versus Doxycycline for Acne: A Comparative Effectiveness, Noninferiority Evaluation trial—a 16-week, blinded trial comparing 100 mg/d doses of both drugs—should provide additional evidence regarding the relative role of spironolactone and oral antibiotics in the management of acne.34
Ultimately, the decision to use spironolactone or other treatments such as oral antibiotics should be based on shared decision making between clinician and patient. Spironolactone has a relatively slow onset of efficacy, and other options such as oral antibiotics might be preferred by those looking for more immediate results; however, as women with acne often have activity that persists into adulthood, spironolactone might be preferable as a long-term maintenance therapy to avoid complications of prolonged antibiotic use.35 Comorbidities also will influence the optimal choice of therapy (eg, spironolactone might be preferred in someone with inflammatory bowel disease, and oral antibiotics might be preferred in someone with orthostatic hypotension).
Patient Selection
Acne occurring along the lower face or jawline in adult women sometimes is referred to as hormonal acne, but this dogma is not particularly evidence based. An observational study of 374 patients found that almost 90% of adult women had acne involving multiple facial zones with a spectrum of facial acne severity similar to that in adolescents.36 Only a small subset of these patients (11.2%) had acne localized solely to the mandibular area. In addition, acne along the lower face is not predictive of hyperandrogenism (eg, polycystic ovary syndrome).37 Antiandrogen therapies such as spironolactone and clascoterone are effective in both men and women with acne10,38 and in adolescents and adults, suggesting that hormones play a fundamental role in all acne and that addressing this mechanism can be useful broadly. Therefore, hormonal therapies such as spironolactone should not be restricted to only adult women with acne along the lower face.
While spironolactone can be effective for acne treatment in any age group, it may be most effective for adult women with acne. In the SAFA trial, prespecified subgroup analyses showed a statistically significant (P=.005) interaction term for age (categorized as <25 years and ≥25 years), which suggested that spironolactone might be a more effective treatment for women 25 years and older.15 In addition, subgroup analyses in the aforementioned 2018 analysis of claims data found that spironolactone was more effective relative to oral antibiotics in adults vs adolescents.32 Despite these limitations, several case series have highlighted that spironolactone is effective among adolescent populations with acne. A case series of spironolactone use in 73 patients aged 19 years or younger found that 68% of patients demonstrated resolution or improvement in their acne after spironolactone treatment.39 Another case series among 80 adolescent females reported 80% of patients experiencing improvement of their acne.14
For those with more severe acne, spironolactone can be combined with other complementary treatment approaches such as topicals, oral antibiotics, or procedural modalities.40
Dosing
We recommend starting spironolactone at a dose of 100 mg/d (the patient can take 50 mg/d for 1 week, then increase to 100 mg/d if there are no adverse effects at the lower dose). In the 1984 trial by Goodfellow et al,10 participants were randomized to doses of 50 mg/d, 100 mg/d, 150 mg/d, and 200 mg/d. In this trial, efficacy assessed by objective and subjective outcomes did not plateau until doses of 100 mg/d to 150 mg/d. In addition, a case series of 403 patients found that the most successful dosage of spironolactone generally was 100 mg/d or higher.13 Most of the patients who were started at this dosage either stayed at this level or escalated, whereas patients who started at lower dosages (25-75 mg/d) frequently increased their dosage over time. The SAFA trial also highlighted that most patients can tolerate a spironolactone dose of 100 mg/d.15 For specific populations, such as patients with polycystic ovary syndrome, a higher dose (mean dosage of 143 mg/d) may be required for efficacy.41 Given the slow onset of efficacy, typically taking 3 to 5 months, and the low rate of adverse effects, we believe the optimal starting dose is 100 mg/s to 150 mg/d. If adverse effects occur or lesions clear, then the dosage may be reduced.
Adverse Effects
Spironolactone generally is well tolerated; in the SAFA and FASCE trials, fewer than 1% of participants discontinued due to adverse effects.15,33 Rates of discontinuation due to adverse effects typically have been less than 5% in case series of patients treated in routine clinical practice.12-14
Because spironolactone is a diuretic and antihypertensive, the most common adverse effects are related to these characteristics. In the SAFA trial, dizziness, lightheadedness, and vertigo were reported more commonly in the spironolactone group than in the placebo group (19% vs 12%, respectively). Similarly, headaches also were reported more frequently in the spironolactone group than in the placebo group (20% vs 12%, respectively).15 One case series found that, among the 267 patients on spironolactone whose blood pressure was monitored, the mean reduction in systolic blood pressure was 3.5 mm Hg and the mean reduction in diastolic blood pressure was 0.9 mm Hg.13 For those with baseline orthostasis or in those who experience adverse effects related to hypotension, reducing the dose often can be helpful. Of note, while doses of 100 mg/d to 150 mg/d often are the most effective, randomized trials have found that spironolactone still can be effective for acne at doses as low as 25 mg/d to 50 mg/d.10,38
Menstrual irregularities are another commonly cited adverse effect of spironolactone. While a systematic review found that 15% to 30% of patients treated with spironolactone experience menstrual irregularities, it has been difficult to evaluate whether this is due to the medication or other comorbidities, such as polycystic ovary syndrome.42 Notably, in the SAFA trial, rates of menstrual irregularities were equivalent between the spironolactone and placebo groups at a dose of 100 mg/d (32% vs 35%, respectively).15 In contrast, in the FASCE trial, menstrual irregularities were more commonly reported at a dose of 150 mg/d.33 These findings are consistent with observational data suggesting that menstrual irregularities are much more common at spironolactone doses greater than 100 mg/d.42 Additionally, some evidence supports that for some patients these menstrual irregularities may resolve within 2 to 3 months of continued treatment.43 It has been noted in several studies that menstrual irregularities are less likely to occur in patients who are using combined oral contraceptives; therefore, for patients who are amenable and have no contraindications, combined oral contraceptives can be considered to prevent or address menstrual irregularities.13,42,44
More generally, combined oral contraceptives can be an excellent combination with spironolactone, as they have complementary characteristics. Spironolactone primarily blocks the effects of androgens, while combined oral contraceptives predominantly block the production of androgens. Whereas spironolactone typically causes hypotension and menstrual irregularities, combined oral contraceptives cause hypertension and help to regulate the menstrual cycle.
Spironolactone carries an official US Food and Drug Administration warning regarding possible tumorigenicity that is based on animal studies that used up to 150 times the normal dose of spironolactone used in humans45; however, observational studies in humans have not identified such an association when spironolactone is used in normal clinical settings. A systematic review and metanalysis in 2022 reviewed data from a total population of more than 4 million individuals and found that there was no statistically significant association between spironolactone use and the risk for breast, ovarian, bladder, kidney, gastric, or esophageal cancers.46 Additional studies also found no association between spironolactone use and cancers.48 A more recent cohort study specifically among patients treated with spironolactone for acne also found no significant increased risk for breast cancer.49
Combined oral contraceptives are associated with an increased risk for venous thromboembolisms, and there have been concerns that this risk may be greater in combined oral contraceptives that contain drospirenone.50 Drospirenone is molecularly related to spironolactone, which has prompted the consideration of whether spironolactone use also conveys a risk for venous thromboembolism. Reassuringly, a retrospective study of claims data found that individuals on spironolactone were not more likely to develop a pulmonary embolism or a deep venous thrombosis than matched controls treated with tetracycline antibiotics, with a point estimate favoring decreased risk.51
Monitoring
Given that one of spironolactone’s mechanisms of action is aldosterone antagonism and thus the inhibition of potassium excretion, there have been concerns regarding risk for hyperkalemia. A retrospective study analyzing data from 2000 to 2014 found that, among 974 young women receiving spironolactone therapy, the rate of hyperkalemia was 0.72%, which is equivalent to the 0.76% baseline rate of hyperkalemia in the same population.52 Subsequent studies also have found that spironolactone does not appear to be associated with a meaningful risk for hyperkalemia among young healthy patients treated for acne.38,53 These studies suggest that routine potassium monitoring is of low usefulness for healthy young women taking spironolactone for acne. The 2024 American Academy of Dermatology guidelines on the management of acne also state that potassium monitoring is not needed in healthy patients but that potassium testing should be considered for those with risk factors for hyperkalemia (eg, older age, medical comorbidities, medications).40 Clinicians should still engage in shared decision making with patients to determine whether to check potassium. If potassium is to be monitored, it should be checked 1 to 2 weeks after spironolactone is started.45,54
Since drospirenone also has aldosterone antagonistic properties,55 there have been concerns about whether concomitant use of spironolactone and drospirenone-containing combined oral contraceptives might increase the risk for hyperkalemia.56 However, a retrospective cohort study analyzing data from more than 1 million women found that drospirenone is not any more likely than levonorgestrel to cause hyperkalemia and that there is no interaction between drospirenone and spironolactone for hyperkalemia.57 A subsequent prospective study of 27 women treated with combined oral contraceptives containing ethinyl estradiol/drospirenone and spironolactone also did not find any significant elevations in potassium.58 Data from these studies suggest that spironolactone can safely be co-administered with drospirenone-containing combined oral contraceptives.
Reproductive Risks
Despite its utility in treating acne, spironolactone should not be used during pregnancy, and appropriate pregnancy prevention is recommended. Spironolactone crosses the placenta, and some animal studies have shown feminization of male fetuses.59 While human data are limited to a few case reports that did not demonstrate an association of major malformations,60 it generally is recommended to avoid spironolactone during pregnancy. Small studies have found that spironolactone has minimal transfer to breastmilk and is not associated with adverse effects in breastfed infants.61-63 Accordingly, the World Health Organization considers spironolactone to be compatible with breastfeeding.64 Notably, spironolactone may be associated with lactation suppression65,66; therefore, it may be best if lactating patients ensure that their milk production is established prior to starting spironolactone and to increase their water intake to offset the diuretic effects.
Spironolactone also can result in gynecomastia in men and therefore typically is not prescribed for the treatment of acne in this population in oral form10; however, topical antiandrogens such as clascoterone can be used in both women and men with acne.67
Conclusion
Spironolactone is a well-tolerated and effective treatment for women with acne, both in adult and adolescent populations. It is a potentially underutilized alternative to oral antibiotics. Spironolactone also is affordable, fully covered without any requirements in almost 90% of states under Medicaid and with a monthly cost of only $4.00 when obtained through major retailers in the United States, making it an optimal long-term treatment option for many patients.52,68 We recommend a starting dose of 100 mg/d, which can be increased to 150 mg/d to 200 mg/d if needed for better acne control or decreased if adverse effects occur or acne clears. Potassium monitoring is of low usefulness in young healthy women, and studies have not identified an association between spironolactone use and increased risk for cancer.
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS. Temporal trends in the use of systemic medications for acne from 2017 to 2020. JAMA Dermatol. 2023;159:1135-1136. doi:10.1001 /jamadermatol.2023.2363
- Strauss JS, Pochi PE, Downing DT. Acne: perspectives. J Invest Dermatol. 1974;62:321-325. doi:10.1111/1523-1747.ep12724280
- Luderschmidt C, Bidlingmaier F, Plewig G. Inhibition of sebaceous gland activity by spironolactone in Syrian hamster. J Invest Dermatol. 1982;78:253-255. doi:10.1111/1523-1747.ep12506612
- Boisselle A, Dionne FT, Tremblay RR. Interaction of spironolactone with rat skin androgen receptor. Can J Biochem. 1979;57:1042-1046. doi:10.1139/o79-131
- Menard RH, Stripp B, Gillette JR. Spironolactone and testicular cytochrome P-450: decreased testosterone formation in several species and changes in hepatic drug metabolism. Endocrinology. 1974;94:1628-1636. doi:10.1210/endo-94-6-1628
- Rifka SM, Pita JC, Vigersky RA, et al. Interaction of digitalis and spironolactone with human sex steroid receptors. J Clin Endocrinol Metab. 1978;46:338-344. doi:10.1210/jcem-46-2-338
- Corvol P, Michaud A, Menard J, et al. Antiandrogenic effect of spirolactones: mechanism of action. Endocrinology. 1975;97:52-58. doi:10.1210/endo-97-1-52
- Akamatsu H, Zouboulis CC, Orfanos CE. Spironolactone directly inhibits proliferation of cultured human facial sebocytes and acts antagonistically to testosterone and 5 alpha-dihydrotestosterone in vitro. J Invest Dermatol. 1993;100:660-662. doi:10.1111/1523-1747 .ep12472325
- Goodfellow A, Alaghband-Zadeh J, Carter G, et al. Oral spironolactone improves acne vulgaris and reduces sebum excretion. Br J Dermatol. 1984;111:209-214. doi:10.1111/j.1365-2133.1984.tb04045.x
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232. doi:10.1111/j.1365-2133.1986.tb05722.x
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Santer M, Lawrence M, Renz S, et al. Effectiveness of spironolactone for women with acne vulgaris (SAFA) in England and Wales: pragmatic, multicentre, phase 3, double blind, randomised controlled trial. BMJ. 2023;381:E074349. doi:10.1136/bmj-2022-074349
- Shields A, Barbieri JS. Effectiveness of spironolactone for women with acne vulgaris (SAFA) trial: a critically appraised topic. Br J Dermatol. 2023;189:509-510. doi:10.1093/bjd/ljad270
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344. doi:10.1007/s40257-018-00417-3
- Knutsen-Larson S, Dawson AL, Dunnick CA, et al. Acne vulgaris: pathogenesis, treatment, and needs assessment. Dermatol Clin. 2012;30:99-106, viii-ix. doi:10.1016/j.det.2011.09.001
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States, 2021. Accessed May 21, 2025. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
- Adler BL, Kornmehl H, Armstrong AW. Antibiotic resistance in acne treatment. JAMA Dermatol. 2017;153:810-811. doi:10.1001 /jamadermatol.2017.1297
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33. doi:10.1016/S1473-3099(15)00527-7
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616. doi:10.1038/ajg.2010.303?
- Margolis DJ, Fanelli M, Kupperman E, et al. Association of pharyngitis with oral antibiotic use for the treatment of acne: a cross-sectional and prospective cohort study. Arch Dermatol. 2012;148:326-332. doi:10.1001 /archdermatol.2011.355
- Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531-534. doi:10.1056/NEJM197803092981003
- Carroll KC, Bartlett JG. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu Rev Microbiol. 2011;65:501-521. doi:10.1146/annurev-micro-090110-102824
- Velicer CM, Heckbert SR, Lampe JW, et al. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291:827-835. doi:10.1001/jama.291.7.827
- Song M, Nguyen LH, Emilsson L, et al. Antibiotic use associated with risk of colorectal polyps in a nationwide study. Clin Gastroenterol Hepatol. 2021;19:1426-1435.e6. doi:10.1016/j.cgh.2020.05.036
- Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67:672-678. doi:10.1136 /gutjnl-2016-313413
- Del Rosso JQ, Rosen T, Palceski D, et al. Patient awareness of antimicrobial resistance and antibiotic use in acne vulgaris. J Clin Aesthetic Dermatol. 2019;12:30-41.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455. doi:10.1007/s40257-018-0349-6
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) Study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Barbieri JS, Ellenberg S, Grice E, et al. Challenges in designing a randomized, double-blind noninferiority trial for treatment of acne: The SDACNE trial. Clin Trials. 2025;22:66-76. doi:10.1177/17407745241265094
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106. doi:10.1111/jdv.12757
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2016;152:391-398. doi:10.1001/jamadermatol.2015.4498
- Plante J, Robinson I, Elston D. The need for potassium monitoring in women on spironolactone for dermatologic conditions. J Am Acad Dermatol. 2022;87:1097-1099. doi:10.1016/j.jaad.2022.01.010
- Berman HS, Cheng CE, Hogeling M. Spironolactone in the treatment of adolescent acne: a retrospective review. J Am Acad Dermatol. 2021;85:269-271. doi:10.1016/j.jaad.2020.11.044
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006.e1-1006 .e30. doi:10.1016/j.jaad.2023.12.017
- Basu P. High-dose spironolactone for acne in patients with polycystic ovarian syndrome: a single-institution retrospective study. J Am Acad Dermatol. 2021;85:740-741.
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Yemisci A, Gorgulu A, Piskin S. Effects and side-effects of spironolactone therapy in women with acne. J Eur Acad Dermatol Venereol. 2005;19:163-166. doi:10.1111/j.1468-3083.2005.01072.x
- Patiyasikunt M, Chancheewa B, Asawanonda P, et al. Efficacy and tolerability of low-dose spironolactone and topical benzoyl peroxide in adult female acne: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2020;47:1411-1416. doi:10.1111/1346-8138.15559
- Aldactone (spironolactone) tablets. Prescribing information. Pfizer; 2008. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/012151s062lbl.pdf
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001/jamadermatol.2021.5866
- Mackenzie IS, Morant SV, Wei L, et al. Spironolactone use and risk of incident cancers: a retrospective, matched cohort study. Br J Clin Pharmacol. 2017;83:653-663. doi:10.1111/bcp.13152
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875. doi:10.1016/j.canep.2013.10.004
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007/s00403-024-02936-y
- Jick SS, Hernandez RK. Risk of nonfatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: casecontrol study using United States claims data. BMJ. 2011;342:d2151. doi:10.1136/bmj.d2151
- Shields A, Flood K, Barbieri JS. Spironolactone use for acne is not associated with an increased risk of venous thromboembolism: a matched, retrospective cohort study. J Am Acad Dermatol. 2023;88:1396-1397. doi:10.1016/j.jaad.2023.02.028
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Muhn P, Fuhrmann U, Fritzemeier KH, et al. Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Ann N Y Acad Sci. 1995;761:311-335. doi:10.1111/j.1749-6632.1995.tb31386.x
- Yaz (drospirenone/ethinyl estradiol) tablets. Prescribing information. Bayer HealthCare Pharmaceuticals; 2012. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021676s012lbl.pdf
- Bird ST, Pepe SR, Etminan M, et al. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol. 2011;11:23. doi:10.1186/1472-6904-11-23
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Liszewski W, Boull C. Lack of evidence for feminization of males exposed to spironolactone in utero: a systematic review. J Am Acad Dermatol. 2019;80:1147-1148. doi:10.1016/j.jaad.2018.10.023
- de Jong MFC, Riphagen IJ, Kootstra-Ros JE, et al. Potassium and magnesium in breast milk of a woman with gitelman syndrome. Kidney Int Rep. 2022;7:1720-1721. doi:10.1016/j.ekir.2022.05.006
- Reisman T, Goldstein Z. Case report: induced lactation in a transgender woman. Transgender Health. 2018;3:24-26. doi:10.1089 /trgh.2017.0044
- Phelps DL, Karim A. Spironolactone: relationship between concentrations of dethioacetylated metabolite in human serum and milk. J Pharm Sci. 1977;66:1203. doi:10.1002/jps.2600660841
- World Health Organization. Breastfeeding and maternal medication: recommendations for drugs in the eleventh WHO model list of essential drugs. February 25, 2002. Accessed May 21, 2025. https://www.who.int/publications/i/item/55732
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. Lactation. J Am Acad Dermatol. 2014;70:417.e1-10; quiz 427. doi:10.1016/j.jaad.2013.09.009
- Cominos DC, van der Walt A, van Rooyen AJ. Suppression of postpartum lactation with furosemide. S Afr Med J. 1976;50:251-252.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Ershadi S, Choe J, Barbieri JS. Medicaid formularies for acne treatments are difficult to access and reflect inconsistent coverage policies. J Am Acad Dermatol. 2024;90:1074-1076. doi:10.1016/j.jaad.2024.01.033
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS. Temporal trends in the use of systemic medications for acne from 2017 to 2020. JAMA Dermatol. 2023;159:1135-1136. doi:10.1001 /jamadermatol.2023.2363
- Strauss JS, Pochi PE, Downing DT. Acne: perspectives. J Invest Dermatol. 1974;62:321-325. doi:10.1111/1523-1747.ep12724280
- Luderschmidt C, Bidlingmaier F, Plewig G. Inhibition of sebaceous gland activity by spironolactone in Syrian hamster. J Invest Dermatol. 1982;78:253-255. doi:10.1111/1523-1747.ep12506612
- Boisselle A, Dionne FT, Tremblay RR. Interaction of spironolactone with rat skin androgen receptor. Can J Biochem. 1979;57:1042-1046. doi:10.1139/o79-131
- Menard RH, Stripp B, Gillette JR. Spironolactone and testicular cytochrome P-450: decreased testosterone formation in several species and changes in hepatic drug metabolism. Endocrinology. 1974;94:1628-1636. doi:10.1210/endo-94-6-1628
- Rifka SM, Pita JC, Vigersky RA, et al. Interaction of digitalis and spironolactone with human sex steroid receptors. J Clin Endocrinol Metab. 1978;46:338-344. doi:10.1210/jcem-46-2-338
- Corvol P, Michaud A, Menard J, et al. Antiandrogenic effect of spirolactones: mechanism of action. Endocrinology. 1975;97:52-58. doi:10.1210/endo-97-1-52
- Akamatsu H, Zouboulis CC, Orfanos CE. Spironolactone directly inhibits proliferation of cultured human facial sebocytes and acts antagonistically to testosterone and 5 alpha-dihydrotestosterone in vitro. J Invest Dermatol. 1993;100:660-662. doi:10.1111/1523-1747 .ep12472325
- Goodfellow A, Alaghband-Zadeh J, Carter G, et al. Oral spironolactone improves acne vulgaris and reduces sebum excretion. Br J Dermatol. 1984;111:209-214. doi:10.1111/j.1365-2133.1984.tb04045.x
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232. doi:10.1111/j.1365-2133.1986.tb05722.x
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Santer M, Lawrence M, Renz S, et al. Effectiveness of spironolactone for women with acne vulgaris (SAFA) in England and Wales: pragmatic, multicentre, phase 3, double blind, randomised controlled trial. BMJ. 2023;381:E074349. doi:10.1136/bmj-2022-074349
- Shields A, Barbieri JS. Effectiveness of spironolactone for women with acne vulgaris (SAFA) trial: a critically appraised topic. Br J Dermatol. 2023;189:509-510. doi:10.1093/bjd/ljad270
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344. doi:10.1007/s40257-018-00417-3
- Knutsen-Larson S, Dawson AL, Dunnick CA, et al. Acne vulgaris: pathogenesis, treatment, and needs assessment. Dermatol Clin. 2012;30:99-106, viii-ix. doi:10.1016/j.det.2011.09.001
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States, 2021. Accessed May 21, 2025. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
- Adler BL, Kornmehl H, Armstrong AW. Antibiotic resistance in acne treatment. JAMA Dermatol. 2017;153:810-811. doi:10.1001 /jamadermatol.2017.1297
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33. doi:10.1016/S1473-3099(15)00527-7
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616. doi:10.1038/ajg.2010.303?
- Margolis DJ, Fanelli M, Kupperman E, et al. Association of pharyngitis with oral antibiotic use for the treatment of acne: a cross-sectional and prospective cohort study. Arch Dermatol. 2012;148:326-332. doi:10.1001 /archdermatol.2011.355
- Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531-534. doi:10.1056/NEJM197803092981003
- Carroll KC, Bartlett JG. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu Rev Microbiol. 2011;65:501-521. doi:10.1146/annurev-micro-090110-102824
- Velicer CM, Heckbert SR, Lampe JW, et al. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291:827-835. doi:10.1001/jama.291.7.827
- Song M, Nguyen LH, Emilsson L, et al. Antibiotic use associated with risk of colorectal polyps in a nationwide study. Clin Gastroenterol Hepatol. 2021;19:1426-1435.e6. doi:10.1016/j.cgh.2020.05.036
- Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67:672-678. doi:10.1136 /gutjnl-2016-313413
- Del Rosso JQ, Rosen T, Palceski D, et al. Patient awareness of antimicrobial resistance and antibiotic use in acne vulgaris. J Clin Aesthetic Dermatol. 2019;12:30-41.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455. doi:10.1007/s40257-018-0349-6
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) Study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Barbieri JS, Ellenberg S, Grice E, et al. Challenges in designing a randomized, double-blind noninferiority trial for treatment of acne: The SDACNE trial. Clin Trials. 2025;22:66-76. doi:10.1177/17407745241265094
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106. doi:10.1111/jdv.12757
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2016;152:391-398. doi:10.1001/jamadermatol.2015.4498
- Plante J, Robinson I, Elston D. The need for potassium monitoring in women on spironolactone for dermatologic conditions. J Am Acad Dermatol. 2022;87:1097-1099. doi:10.1016/j.jaad.2022.01.010
- Berman HS, Cheng CE, Hogeling M. Spironolactone in the treatment of adolescent acne: a retrospective review. J Am Acad Dermatol. 2021;85:269-271. doi:10.1016/j.jaad.2020.11.044
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006.e1-1006 .e30. doi:10.1016/j.jaad.2023.12.017
- Basu P. High-dose spironolactone for acne in patients with polycystic ovarian syndrome: a single-institution retrospective study. J Am Acad Dermatol. 2021;85:740-741.
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Yemisci A, Gorgulu A, Piskin S. Effects and side-effects of spironolactone therapy in women with acne. J Eur Acad Dermatol Venereol. 2005;19:163-166. doi:10.1111/j.1468-3083.2005.01072.x
- Patiyasikunt M, Chancheewa B, Asawanonda P, et al. Efficacy and tolerability of low-dose spironolactone and topical benzoyl peroxide in adult female acne: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2020;47:1411-1416. doi:10.1111/1346-8138.15559
- Aldactone (spironolactone) tablets. Prescribing information. Pfizer; 2008. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/012151s062lbl.pdf
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001/jamadermatol.2021.5866
- Mackenzie IS, Morant SV, Wei L, et al. Spironolactone use and risk of incident cancers: a retrospective, matched cohort study. Br J Clin Pharmacol. 2017;83:653-663. doi:10.1111/bcp.13152
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875. doi:10.1016/j.canep.2013.10.004
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007/s00403-024-02936-y
- Jick SS, Hernandez RK. Risk of nonfatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: casecontrol study using United States claims data. BMJ. 2011;342:d2151. doi:10.1136/bmj.d2151
- Shields A, Flood K, Barbieri JS. Spironolactone use for acne is not associated with an increased risk of venous thromboembolism: a matched, retrospective cohort study. J Am Acad Dermatol. 2023;88:1396-1397. doi:10.1016/j.jaad.2023.02.028
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Muhn P, Fuhrmann U, Fritzemeier KH, et al. Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Ann N Y Acad Sci. 1995;761:311-335. doi:10.1111/j.1749-6632.1995.tb31386.x
- Yaz (drospirenone/ethinyl estradiol) tablets. Prescribing information. Bayer HealthCare Pharmaceuticals; 2012. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021676s012lbl.pdf
- Bird ST, Pepe SR, Etminan M, et al. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol. 2011;11:23. doi:10.1186/1472-6904-11-23
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Liszewski W, Boull C. Lack of evidence for feminization of males exposed to spironolactone in utero: a systematic review. J Am Acad Dermatol. 2019;80:1147-1148. doi:10.1016/j.jaad.2018.10.023
- de Jong MFC, Riphagen IJ, Kootstra-Ros JE, et al. Potassium and magnesium in breast milk of a woman with gitelman syndrome. Kidney Int Rep. 2022;7:1720-1721. doi:10.1016/j.ekir.2022.05.006
- Reisman T, Goldstein Z. Case report: induced lactation in a transgender woman. Transgender Health. 2018;3:24-26. doi:10.1089 /trgh.2017.0044
- Phelps DL, Karim A. Spironolactone: relationship between concentrations of dethioacetylated metabolite in human serum and milk. J Pharm Sci. 1977;66:1203. doi:10.1002/jps.2600660841
- World Health Organization. Breastfeeding and maternal medication: recommendations for drugs in the eleventh WHO model list of essential drugs. February 25, 2002. Accessed May 21, 2025. https://www.who.int/publications/i/item/55732
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. Lactation. J Am Acad Dermatol. 2014;70:417.e1-10; quiz 427. doi:10.1016/j.jaad.2013.09.009
- Cominos DC, van der Walt A, van Rooyen AJ. Suppression of postpartum lactation with furosemide. S Afr Med J. 1976;50:251-252.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Ershadi S, Choe J, Barbieri JS. Medicaid formularies for acne treatments are difficult to access and reflect inconsistent coverage policies. J Am Acad Dermatol. 2024;90:1074-1076. doi:10.1016/j.jaad.2024.01.033
Spironolactone for Acne: Practical Strategies for Optimal Clinical Outcomes
Spironolactone for Acne: Practical Strategies for Optimal Clinical Outcomes
PRACTICE POINTS
- Spironolactone is an effective systemic treatment for women with acne and likely is an underutilized alternative to oral antibiotics.
- We recommend a starting dose of 100 mg/d, which is well tolerated by most patients and has superior effectiveness to lower doses.
- Potassium monitoring is of low usefulness in young healthy women, and an association between spironolactone use and increased risk for cancer has not been identified.
From Breakouts to Bargains: Strategies for Patient-Centered, Cost-effective Acne Care
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
In the United States, acne affects 85% of adolescents and can persist into adulthood at a prevalence of 30% to 50% in adult women. 1,2 The pathogenesis of acne is multifactorial and involves hyperkeratinization of the follicle, bacterial colonization with Cutibacterium acnes , and increased androgen-induced sebum production, which together lead to inflammation. 3,4 A wide range of treatment guideline–recommended options are available, including benzoyl peroxide (BPO), topical retinoids, topical and oral antibiotics, antiandrogens, and isotretinoin. 5 However, these options vary widely in their clinical uses, effectiveness, and costs.
Why Cost-effective Acne Care Matters
Out-of-pocket spending by patients on acne treatments can be substantial, with surveys finding that acne patients often spend hundreds to thousands of dollars per year.6,7 In a poll conducted in 2019 by the Kaiser Family Foundation, 3 in 10 patients said they had not taken their medicine as prescribed because of costs.8 A mixed methods study by Ryskina et al9 found that 65% (17/26) of participants who reported primary nonadherence—intended to fill prescriptions but were unable to do so—cited cost or coverage-related barriers as the reason. With the continued rise of dermatologic drug prices and increased prevalence of high-deductible health plans, cost-effective treatment continues to grow in importance. Failure to consider cost-effective, patient-centered care may lead to increased financial toxicity, reduced adherence, and ultimately worse outcomes and patient satisfaction. We aim to review the cost-effectiveness of current prescription therapies for acne management and highlight the most cost-effective approaches to patients with mild to moderate acne as well as moderate to severe acne.
In this review, we will take a value-oriented framework.10 Value can be defined as the cost per outcome of interest. Therefore, a treatment does not necessarily need to be inexpensive to provide high value if it delivers outstanding clinical outcomes. In addition, we will focus on incremental cost-effectiveness relative to common alternatives (eg, a retinoid could deliver high value relative to a vehicle but still provide limited value compared to other available retinoids if it is more expensive but not more efficacious). When possible, we present data from cost-effectiveness studies.11,12 We also use recent available price data obtained from GoodRx on August 11, 2023, to guide this discussion.13 However, as comparative-effectiveness and cost-effectiveness studies rarely are performed for acne medications, much of this discussion will be based on expert opinion.
Treatment Categories
Topical Retinoids—There currently are 4 topical retinoids that are approved by the US Food and Drug Administration (FDA) for the treatment of acne: tretinoin, tazarotene, trifarotene, and adapalene. These drugs are vitamin A derivatives that bind retinoic acid receptors and function as comedolytic and anti-inflammatory agents.5 In general, generic tretinoin and adapalene products have the lowest cost (Table).

In network meta-analyses, tretinoin and adapalene often are highly ranked topical treatment options with respect to efficacy.14 Combined with their low cost, generic tretinoin and adapalene likely are excellent initial options for topical therapy from the standpoint of cost-effectiveness.15 Adapalene may be preferred in many situations because of its better photostability and compatibility with BPO.
Due to the importance of the vehicle in determining retinoid tolerability, efforts have been made to use encapsulation and polymeric emulsion technology to improve tolerability. Recently, polymeric lotion formulations of tretinoin and tazarotene have become available. In a phase 2 study, tazarotene lotion 0.045% was found to have equivalent efficacy and superior tolerability to tazarotene cream 0.1%.16 Although head-to-head data are not available, it is likely that tretinoin lotion may offer similar tolerability improvements.17 Although these formulations currently are more costly, this improved tolerability may be critical for some patients to be able to use topical retinoids, and the additional cost may be worthwhile. In addition, as these products lose market exclusivity, they may become more affordable and similarly priced to other topical retinoids. It is important to keep in mind that in clinical trials of tretinoin and adapalene, rates of dropout due to adverse events typically were 1% to 2%; therefore, because many patients can tolerate generic tretinoin and adapalene, at current prices the lotion formulations of retinoids may not be cost-effective relative to these generics.14
Trifarotene cream 0.005%, a fourth-generation topical retinoid that is highly sensitive for retinoic acid receptor γ, recently was FDA approved for the treatment of acne. Although trifarotene is efficacious for both facial and truncal acne, there is a lack of active comparator data compared to other topical retinoids.18 In a 2023 network meta-analysis, trifarotene was found to be both less efficacious and less tolerable compared to other topical retinoids.19 Thus, it is unclear if trifarotene offers any improved efficacy compared to other options, and it comes at a much higher cost (Table). In a tolerability study, trifarotene was found to be significantly more irritating than tazarotene lotion 0.045% and adapalene gel 0.3% (P<.05).20 Therefore, trifarotene cream 0.005% is unlikely to be a cost-effective option; in fact, it may be overall inferior to other topical retinoids, given its potentially lower tolerability.
Topical Antibiotics—There are 4 commonly prescribed topical antibiotics that are approved by the FDA for the treatment of acne: clindamycin, erythromycin, dapsone, and minocycline. The American Academy of Dermatology guidelines for the treatment of acne recommend concomitant use of BPO to prevent antibiotic resistance.5 Clindamycin is favored over erythromycin because of increasing antibiotic resistance to erythromycin.21 Inexpensive generic options in multiple vehicles (eg, solution, foam, gel) make clindamycin a highly cost-effective option when antibiotic therapy is desired as part of a topical regimen (Table).
The cost-effectiveness of dapsone gel and minocycline foam relative to clindamycin are less certain. Rates of resistance to minocycline are lower than clindamycin, and minocycline foam may be a reasonable alternative in patients who have not had success with other topical antibiotics, such as clindamycin.22 However, given the absence of comparative effectiveness data to suggest minocycline is more effective than clindamycin, it is difficult to justify the substantially higher cost for the typical patient. Although dapsone gel has been suggested as an option for adult women with acne, there are no data to support that it is any more effective than other topical antibiotics in this patient population.23 As generic dapsone prices decrease, it may become a reasonable alternative to clindamycin. In addition, the antineutrophil properties of dapsone may be useful in other acneform and inflammatory eruptions, such as scalp folliculitis and folliculitis decalvans.24
Combination Topicals—Current combination topical products include antibiotic and BPO, antibiotic and retinoid, and retinoid and BPO. Use of combination agents is recommended to reduce the risk for resistance and to enhance effectiveness. Combination products offer improved convenience, which is associated with better adherence and outcomes.25 Generic fixed-dose adapalene-BPO can be a highly cost-effective option that can sometimes be less expensive than the individual component products (Table). Similarly, fixed-dose clindamycin-BPO also is likely to be highly cost-effective. A network meta-analysis found fixed-dose adapalene-BPO to be the most efficacious topical treatment, though it also was found to be the most irritating—more so than fixed-dose clindamycin-BPO, which may have similar efficacy.14,26,27 Generic fixed-dose tretinoin-clindamycin offers improved convenience and adherence compared to the individual components, but it is more expensive, and its cost-effectiveness may be influenced by the importance of convenience for the patient.25 An encapsulated, fixed-dose tretinoin 0.1%–BPO 3% cream is FDA approved for acne, but the cost is high and there is a lack of comparative effectiveness data demonstrating advantages over generic fixed-dose adapalene-BPO products.
Topical Antiandrogen—Clascoterone was introduced in 2020 as the first FDA-approved topical medication to target the hormonal pathogenesis of acne, inhibiting the androgen receptors in the sebaceous gland.28 Because it is rapidly metabolized to cortexolone and does not have systemic antiandrogen effects, clascoterone can be used in both men and women with acne. In clinical trials, it had minimal side effects, including no evidence of irritability, which is an advantage over topical retinoids and BPO.29 In addition, a phase 2 study found that clascoterone may have similar to superior efficacy to tretinoin cream 0.05%.30 Although clascoterone has several strengths, including its efficacy, tolerability, and unique mechanism of action, its cost-effectiveness is limited due to its high cost (Table) and the need for twice-daily application, which reduces convenience. Clascoterone likely is best reserved for patients with a strong hormonal pathogenesis of their acne or difficulty tolerating other topicals, or as an additional therapy to complement other topicals.
Oral Antibiotics—Oral antibiotics are the most commonly prescribed systemic treatments for acne, particularly tetracyclines such as doxycycline, minocycline, and sarecycline.31-34 Doxycycline and minocycline are considered first-line oral antibiotic therapy in the United States and are inexpensive and easily accessible.5 Doxycycline generally is recommended over minocycline given lack of evidence of superior efficacy of minocycline and concerns about severe adverse cutaneous reactions and drug-induced lupus with minocycline.35
In recent years, there has been growing concern of the development of antibiotic resistance.5 Sarecycline is a narrow-spectrum tetracycline that was FDA approved for acne in 2018. In vitro studies demonstrate sarecycline maintains high efficacy against C acnes with less activity against other bacteria, particularly gram-negative enterobes.36 The selectivity of sarecycline may lessen alterations of the gut microbiome seen with other oral antibiotics and reduce gastrointestinal tract side effects. Although comparative effectiveness studies are lacking, sarecycline was efficacious in phase 3 trials with few side effects compared with placebo.37 However, at this time, given the absence of comparative effectiveness data and its high cost (Table), sarecycline likely is best reserved for patients with comorbidities (eg, gastrointestinal disease), those requiring long-term antibiotic therapy, or those with acne that has failed to respond to other oral antibiotics.
Hormonal Treatments—Hormonal treatments such as combined oral contraceptives (COCs) and spironolactone often are considered second-line options, though they may represent cost-effective and safe alternatives to oral antibiotics for women with moderate to severe acne.38-41 There currently are 4 COCs approved by the FDA for the treatment of moderate acne in postmenarcheal females: drospirenone-ethinyl estradiol (Yaz [Bayer HealthCare Pharmaceuticals, Inc]), ethinyl estradiol-norgestimate (Ortho Tri-Cyclen [Ortho-McNeil Pharmaceuticals, Inc]), drospirenone-ethinyl estradiol-levomefolate (Beyaz [Bayer HealthCare Pharmaceuticals, Inc]), and ethinyl estradiol-norethindrone acetate-ferrous fumarate (Estrostep Fe [Allergan USA, Inc]).5 Treatment with COCs has been shown to cause substantial reductions in lesion counts across all lesion types compared to placebo, and a meta-analysis of 24 randomized trials conducted by Arowojolu et al42 demonstrated no consistent differences in acne reduction among different COCs.43,44 Although oral antibiotics are associated with faster improvement than COCs, there is some evidence that they have similar efficacy at 6 months of therapy.45 Combined oral contraceptives are inexpensive and likely reflect a highly cost-effective option (Table).
Spironolactone is an aldosterone inhibitor and androgen receptor blocker that is used off label to treat acne. It is one of the least expensive systemic medications for acne (Table). Although randomized controlled trials are lacking, several large case series support the effectiveness of spironolactone for women with acne.38,46 In addition, observational data suggest spironolactone may have similar effectiveness to oral antibiotics.41 Spironolactone generally is well tolerated, with the most common adverse effects being menstrual irregularities, breast tenderness, and diuresis.47,48 Many of these adverse effects are dose dependent and less likely with the dosing used in acne care. Additionally, menstrual irregularities can be reduced by concomitant use of a COC.48
Although frequent potassium monitoring remains common among patients being treated with spironolactone, there is growing evidence to suggest that potassium monitoring is of low value in young healthy women with acne.49-51 Reducing this laboratory monitoring likely represents an opportunity to provide higher-value care to patients being treated with spironolactone. However, laboratory monitoring should be considered if risk factors for hyperkalemia are present (eg, older age, comorbidities, medications).51
Isotretinoin—Isotretinoin is the most efficacious treatment available for acne and has the unique property of being able to induce a remission of acne activity for many patients.5 Although it remains modestly expensive (Table), it may be less costly overall relative to other treatments that may need continued use over many years because it can induce a remission of acne activity. As with spironolactone, frequent laboratory monitoring remains common among patients being treated with isotretinoin. There is no evidence to support checking complete blood cell counts.52 Several observational studies and a Delphi consensus support reduced monitoring, such as checking lipids and alanine aminotransferase at baseline and peak dose in otherwise young healthy patients.53,54 A recent critically appraised topic published in the British Journal of Dermatology has proposed eliminating laboratory monitoring entirely.55 Reducing laboratory monitoring for patients being treated with isotretinoin has been estimated to potentially save $100 million to $200 million per year in the United States.52-54
Other Strategies to Reduce Patient Costs
Although choosing a cost-effective treatment approach is critical to preventing financial toxicity given poor coverage for acne care and the growth of high-deductible insurance plans, some patients may still experience high treatment costs.56 Because pharmacy costs often are inflated, potentially related to practices of pharmacy benefit managers, it often is possible to find better prices than the presented list price, either by using platforms such as GoodRx or through direct-to-patient mail-order pharmacies such as Cost Plus Drug.57 For branded medications, some patients may be eligible for patient-assistance programs, though they typically are not available for those with public insurance such as Medicare or Medicaid. Compounding pharmacies offer another approach to reduce cost and improve convenience for patients, but because the vehicle can influence the efficacy and tolerability of some topical medications, it is possible that these compounded formulations may not perform similarly to the original FDA-approved products.
Conclusion
For mild to moderate acne, multimodal topical therapy often is required. Fixed-dose combination adapalene-BPO and clindamycin-BPO are highly cost-effective options for most patients. Lotion formulations of topical retinoids may be useful in patients with difficulty tolerating other formulations. Clascoterone is a novel topical antiandrogen that is more expensive than other topical therapies but can complement other topical therapies and is well tolerated.
For moderate to severe acne, doxycycline or hormonal therapy (ie, COCs, spironolactone) are highly cost-effective options. Isotretinoin is recommended for severe or scarring acne. Reduced laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
- Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485. doi:10.1111/bjd.12149
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Webster GF. The pathophysiology of acne. Cutis. 2005;76(2 suppl):4-7.
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316-323. doi:10.1111/j.1610-0387.2007.06274.x
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33. doi:10.1016/j.jaad.2015.12.037
- Felmingham C, Kerr A, Veysey E. Costs incurred by patients with acne prior to dermatological consultation and their relation to patient income. Australas J Dermatol. 2020;61:384-386. doi:10.1111/ajd.13324
- Perche P, Singh R, Feldman S. Patient preferences for acne vulgaris treatment and barriers to care: a survey study. J Drugs Dermatol. 2022;21:1191-1195. doi:10.36849/JDD.6940
- KFF Health Tracking Poll—February 2019. Accessed August 9, 2023. https://files.kff.org/attachment/Topline-KFF-Health-Tracking-Poll-February-2019
- Ryskina KL, Goldberg E, Lott B, et al. The role of the physician in patient perceptions of barriers to primary adherence with acne medications. JAMA Dermatol. 2018;154:456-459. doi:10.1001/jamadermatol.2017.6144
- Porter ME. What is value in health care? N Engl J Med. 2010;363:2477-2481. doi:10.1056/NEJMp1011024
- Barbieri JS, Tan JKL, Adamson AS. Active comparator trial designs used to promote development of innovative new medications. Cutis. 2020;106:E4-E6. doi:10.12788/cutis.0067
- Miller J, Ly S, Mostaghimi A, et al. Use of active comparator trials for topical medications in dermatology. JAMA Dermatol. 2021;157:597-599. doi:10.1001/jamadermatol.2021.0356
- GoodRx. Accessed August 11, 2023. https://www.goodrx.com
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525. doi:10.1111/bjd.20080
- Mavranezouli I, Welton NJ, Daly CH, et al. Cost-effectiveness of topical pharmacological, oral pharmacological, physical and combined treatments for acne vulgaris. Clin Exp Dermatol. 2022;47:2176-2187. doi:10.1111/ced.15356
- Tanghetti E, Werschler W, Lain T, et al. Tazarotene 0.045% lotion for once-daily treatment of moderate-to-severe acne vulgaris: results from two phase 3 trials. J Drugs Dermatol. 2020;19:70-77. doi:10.36849/JDD.2020.3977
- Tyring SK, Kircik LH, Pariser DM, et al. Novel tretinoin 0.05% lotion for the once-daily treatment of moderate-to-severe acne vulgaris: assessment of efficacy and safety in patients aged 9 years and older. J Drugs Dermatol. 2018;17:1084-1091.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699. doi:10.1016/j.jaad.2019.02.044
- Huang CY, Chang IJ, Bolick N, et al. Comparative efficacy of pharmacological treatments for acne vulgaris: a network meta-analysis of 221 randomized controlled trials. Ann Fam Med. 2023;21:358-369. doi:10.1370/afm.2995
- Draelos ZD. Low irritation potential of tazarotene 0.045% lotion: head-to-head comparison to adapalene 0.3% gel and trifarotene 0.005% cream in two studies. J Dermatolog Treat. 2023;34:2166346. doi:10.1080/09546634.2023.2166346
- Dessinioti C, Katsambas A. Antibiotics and antimicrobial resistance in acne: epidemiological trends and clinical practice considerations. Yale J Biol Med. 2022;95:429-443.
- Gold LS, Dhawan S, Weiss J, et al. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: results of 2 randomized, double-blind, phase 3 studies. J Am Acad Dermatol. 2019;80:168-177. doi:10.1016/j.jaad.2018.08.020
- Wang X, Wang Z, Sun L, et al. Efficacy and safety of dapsone gel for acne: a systematic review and meta-analysis. Ann Palliat Med. 2022;11:611-620. doi:10.21037/apm-21-3935
- Melián-Olivera A, Burgos-Blasco P, Selda-Enríquez G, et al. Topical dapsone for folliculitis decalvans: a retrospective cohort study. J Am Acad Dermatol. 2022;87:150-151. doi:10.1016/j.jaad.2021.07.004
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103-108.
- Ting W. Randomized, observer-blind, split-face study to compare the irritation potential of 2 topical acne formulations over a 14-day treatment period. Cutis. 2012;90:91-96.
- Aschoff R, Möller S, Haase R, et al. Tolerability and efficacy ofclindamycin/tretinoin versus adapalene/benzoyl peroxide in the treatment of acne vulgaris. J Drugs Dermatol. 2021;20:295-301. doi:10.36849/JDD.2021.5641
- Rosette C, Agan FJ, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18:412-418.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165:177-183. doi:10.1111/j.1365-2133.2011.10332.x
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319. doi:10.1001/jamadermatol.2019.4818
- Guzman AK, Barbieri JS. Comparative analysis of prescribing patterns of tetracycline class antibiotics and spironolactone between advanced practice providers and physicians in the treatment of acne vulgaris. J Am Acad Dermatol. 2021;84:1119-1121. doi:10.1016/j.jaad.2020.06.044
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297. doi:10.1001/jamadermatol.2018.4944
- Garner SE, Eady A, Bennett C, et al. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;2012:CD002086. doi:10.1002/14651858.CD002086.pub2
- Zhanel G, Critchley I, Lin LY, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2018;63:e01297-18. doi:10.1128/AAC.01297-18
- Moore A, Green LJ, Bruce S, et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: results from two identically designed, phase 3, randomized, double-blind clinical trials. J Drugs Dermatol. 2018;17:987-996.
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, James WD, et al. Real-world drug usage survival of spironolactone versus oral antibiotics for the management of female patients with acne. J Am Acad Dermatol. 2019;81:848-851. doi:10.1016/j.jaad.2019.03.036
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549. doi:10.1016/j.jaad.2018.09.055
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012;7:CD004425. doi:10.1002/14651858.CD004425.pub6
- Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen. Obstet Gynecol. 2008;112:773-781. doi:10.1097/AOG.0b013e318187e1c5
- Lucky AW, Koltun W, Thiboutot D, et al. A combined oral contraceptive containing 3-mg drospirenone/20-microg ethinyl estradiol in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled study evaluating lesion counts and participant self-assessment. Cutis. 2008;82:143-150.
- Koo EB, Petersen TD, Kimball AB. Meta-analysis comparing efficacy of antibiotics versus oral contraceptives in acne vulgaris. J Am Acad Dermatol. 2014;71:450-459. doi:10.1016/j.jaad.2014.03.051
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502. doi:10.1067/mjd.2000.105557
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300. doi:10.1001/jamadermatol.2020.5468
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001/jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Barbieri JS, Shin DB, Wang S, et al. The clinical utility of laboratory monitoring during isotretinoin therapy for acne and changes to monitoring practices over time. J Am Acad Dermatol. 2020;82:72-79. doi:10.1016/j.jaad.2019.06.025
- Lee YH, Scharnitz TP, Muscat J, et al. Laboratory monitoring during isotretinoin therapy for acne: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:35-44. doi:10.1001/jamadermatol.2015.3091
- Xia E, Han J, Faletsky A, et al. Isotretinoin laboratory monitoring in acne treatment: a Delphi consensus study. JAMA Dermatol. 2022;158:942-948. doi:10.1001/jamadermatol.2022.2044
- Affleck A, Jackson D, Williams HC, et al. Is routine laboratory testing in healthy young patients taking isotretinoin necessary: a critically appraised topic. Br J Dermatol. 2022;187:857-865. doi:10.1111/bjd.21840
- Barbieri JS, LaChance A, Albrecht J. Double standards and inconsistencies in access to care-what constitutes a cosmetic treatment? JAMA Dermatol. 2023;159:245-246. doi:10.1001/jamadermatol.2022.6322
- Trish E, Van Nuys K, Popovian R. US consumers overpay for generic drugs. Schaeffer Center White Paper Series. May 31, 2022. doi:10.25549/m589-2268
Practice Points
- For mild to moderate acne, fixed-dose combination adapalene–benzoyl peroxide and clindamycin–benzoyl peroxide are highly cost-effective options for most patients.
- For moderate to severe acne, doxycycline or hormonal therapy (ie, combined oral contraceptives, spironolactone) are highly cost-effective options.
- Reduction of laboratory monitoring for spironolactone and isotretinoin is an opportunity to provide higher-value care.
Telemedicine and Home Pregnancy Testing for iPLEDGE: A Survey of Clinician Perspectives
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).

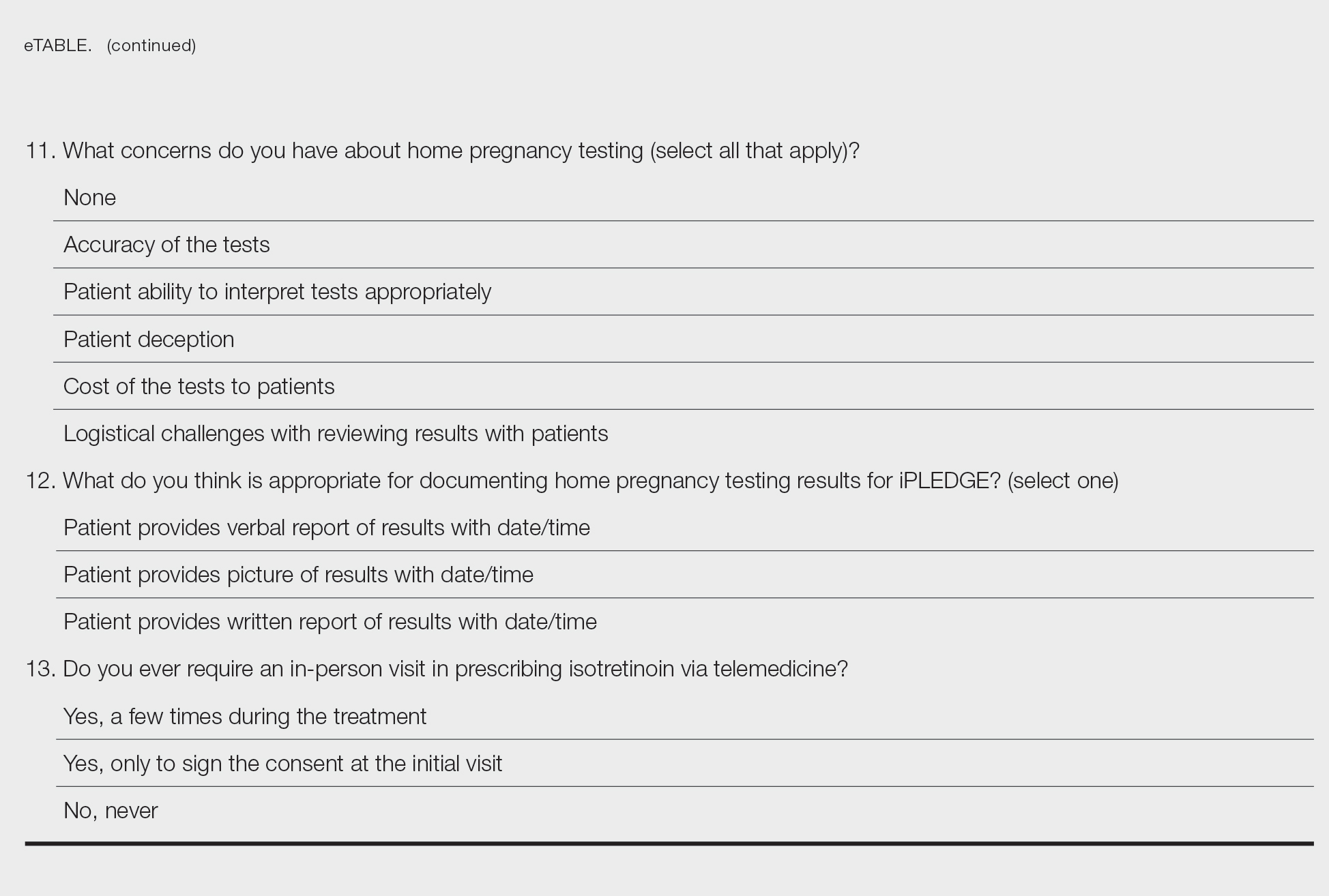
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
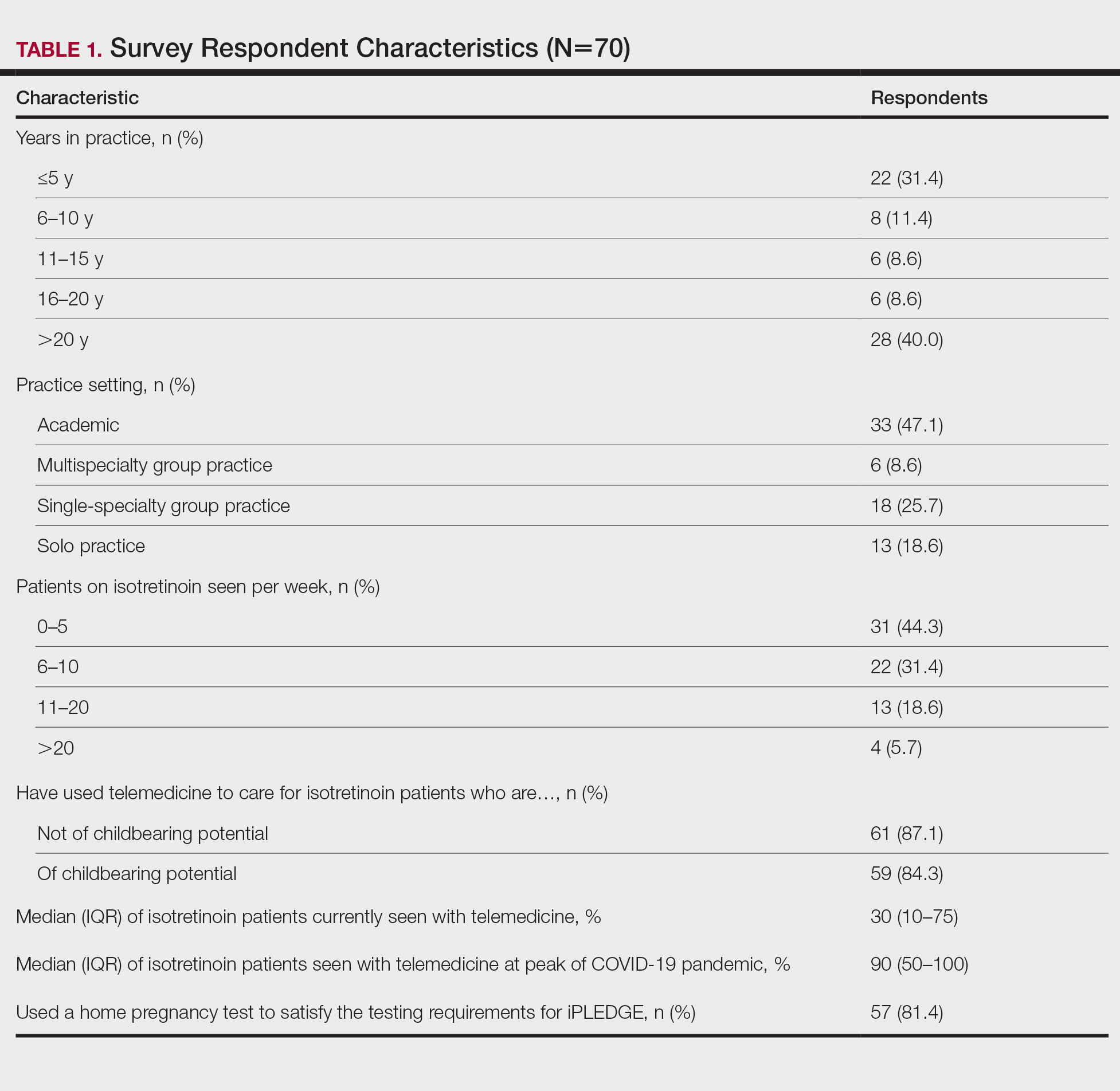
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
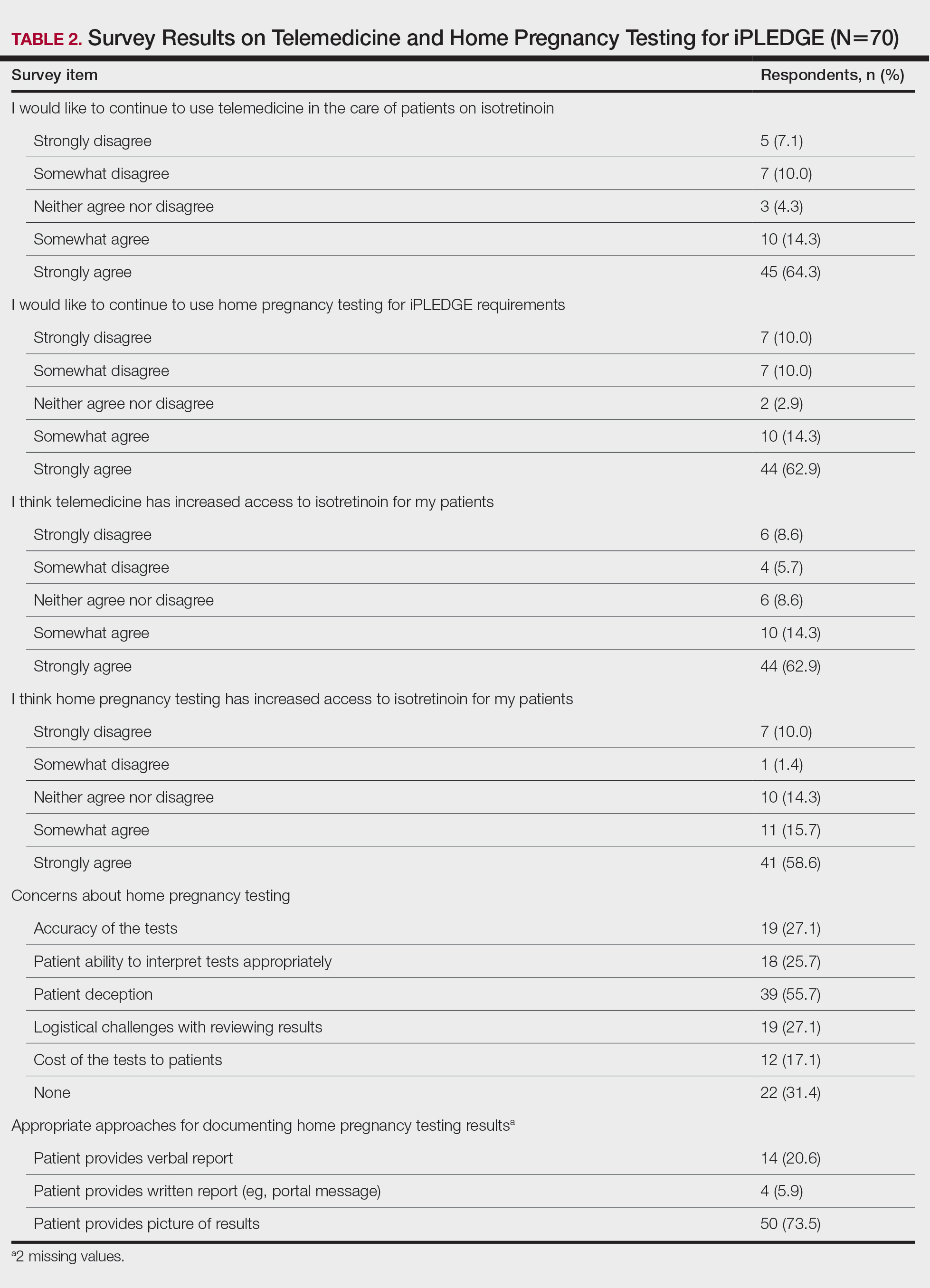
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
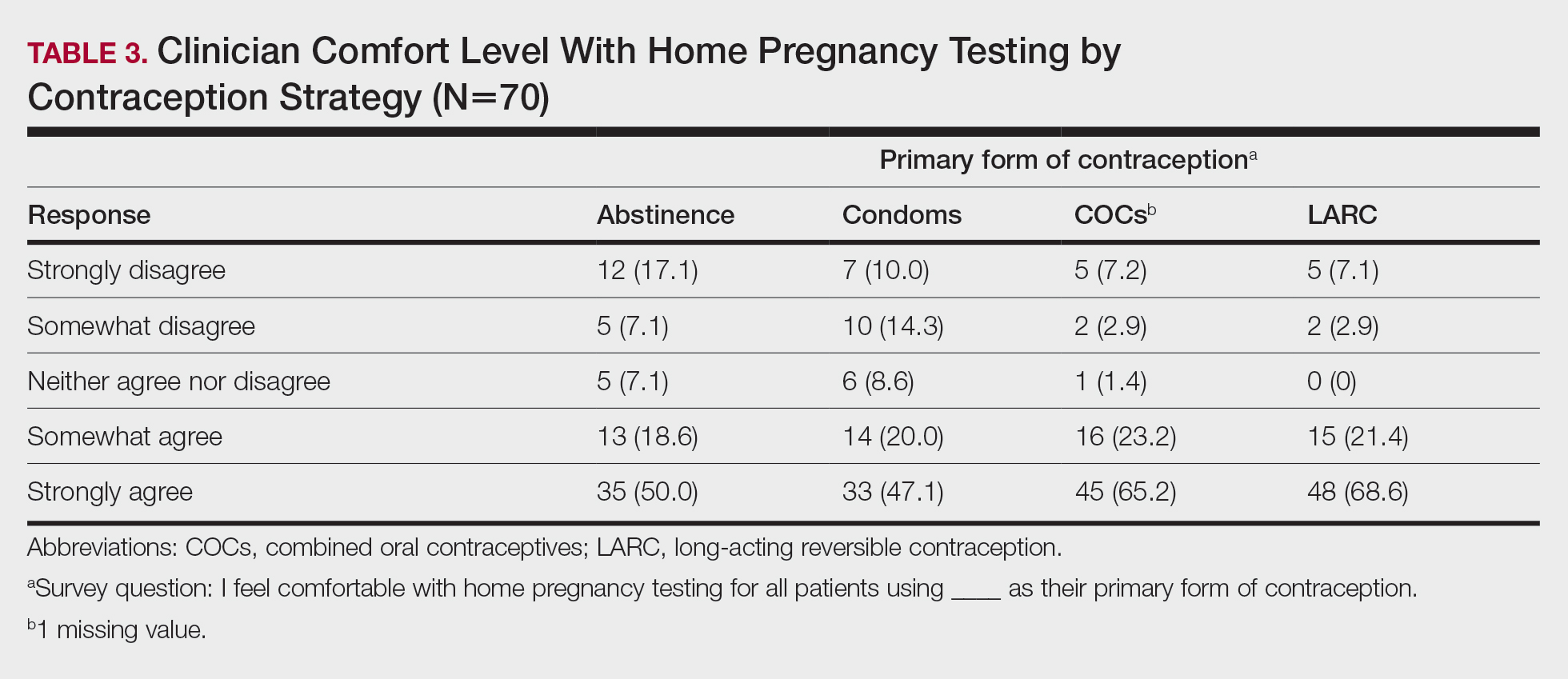
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).


Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).

The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).

In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2

Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).


Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).

The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).

In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2

Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
PRACTICE POINTS
- The majority of clinicians report that the use of telemedicine and home pregnancy testing for iPLEDGE has improved access to care and that they would like to continue these practices.
- Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care for patients treated with isotretinoin.
Comparison of Shave and Punch Biopsy Utilization Among Dermatology Practices
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.
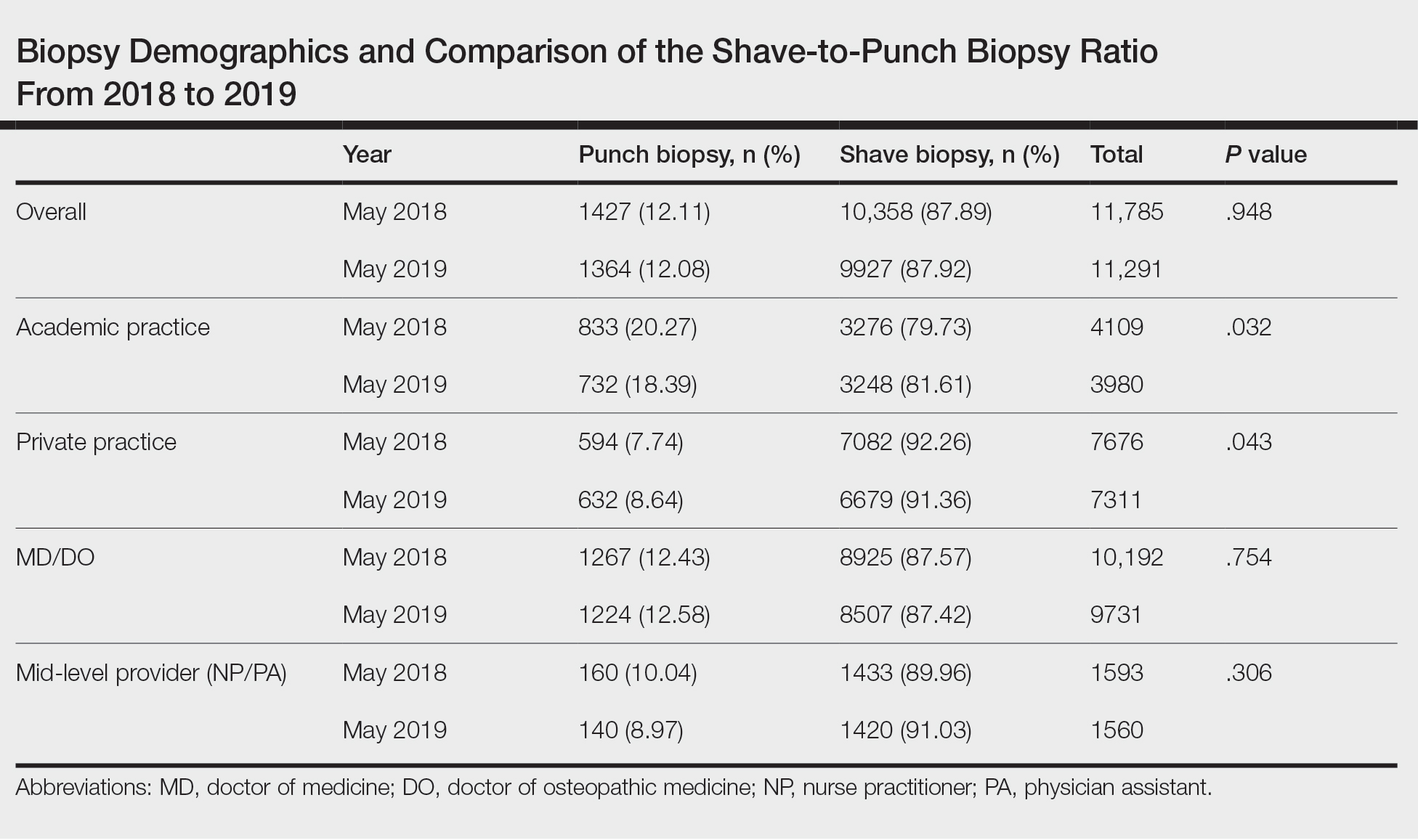
Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.

Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
In 2019, the 2 Current Procedural Terminology (CPT) codes for skin biopsies (11100 and 11101) were replaced with 6 new CPT codes that specify biopsy technique and associated procedural complexity. 1,2 Prior to the coding changes, all biopsies were reimbursed at the same payment level, but a punch biopsy (11104; national nonfacility Medicare payment, $133.29) is now reimbursed more than a shave biopsy (11102; national nonfacility Medicare payment, $106.42). 3 We sought to evaluate whether the decrease in reimbursement for shave biopsies and concurrent increase in reimbursement for punch biopsies led to a shift from shave to punch biopsy utilization.
Methods
We examined shave and punch biopsies submitted for pathologic examination at Brigham and Women’s Hospital, Massachusetts General Hospital, and Massachusetts General Physician’s Organization (all in Boston, Massachusetts), and Penn Medicine, University of Pennsylvania Health System (Philadelphia, Pennsylvania), in May 2018 vs May 2019 (four months after new codes were implemented). This study was approved by Partners HealthCare (Boston, Massachusetts) and the University of Pennsylvania institutional review boards.
We included shave and punch biopsies of skin performed by physician dermatologists and mid-level providers (ie, physician assistants, nurse practitioners) at dermatology practices. All biopsies performed by a technique other than shave or punch, unspecified biopsy type, consultation cases, nonskin biopsies (eg, mucosa), and biopsies performed at nondermatology practices were excluded. We also excluded biopsies by providers who were not present during both study periods to account for provider mix.
Statistical Analysis
To evaluate for changes in the ratio of shave to punch biopsy utilization over time, we performed χ2 tests. Because care practices may differ between private and academic settings as well as between physicians and mid-level providers, we performed subgroup analyses by practice setting and provider type.4
Results
We identified 11,785 biopsies (12.11% of which were punch) submitted for pathologic examination in May 2018 compared to 11,291 biopsies (12.08% of which were punch) in May 2019 (Table). The overall use of punch biopsies relative to shave biopsies did not change between the years. There was a relative decrease in punch biopsy use among academic practices (−1.88%; P=.032) and a relative increase in punch biopsy use among private practices (+0.90%; P=.043). Provider type was not associated with differing utilization of biopsy type.

Comment
Overall, there was not a considerable shift in utilization behavior from shave to punch biopsies after the introduction of new coding changes. However, our study does demonstrate a small yet significant increase in punch biopsy utilization among private practices, and a decrease among academic practices. Although the change in biopsy utilization behavior is small in magnitude, it may have a substantial impact when extrapolated to behavior across the entire United States.
We were unable to assess additional factors, such as clinical diagnosis, body site, and cosmetic concerns, that may impact biopsy type selection in this study. Although we included multiple study sites to improve generalizability, our findings may not be representative of all biopsies performed in the dermatology setting. The baseline difference in relative punch biopsy use in academic vs private practices may reflect differences in patient populations and chief concerns, but assuming these features are stable over a 1-year time period, our findings should remain valid. Future studies should focus on qualitative evaluations of physician decision-making and evaluate whether similar trends persist over time.
Conclusion
Skin biopsy utilization trends among differing practice and provider types should continue to be monitored to assess for longitudinal trends in utilization within the context of updated billing codes and associated reimbursements.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
- Grider D. 2019 CPT® coding for skin biopsies. ICD10 monitor website. September 17, 2018. Updated January 7, 2019. Accessed February 17, 2021. https://www.icd10monitor.com/2019-cpt-coding-for-skin-biopsies 2.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Search the physician fee schedule. Centers for Medicare & Medicaid Services website. Updated January 20, 2021. Accessed February 17, 2021. https://www.cms.gov/medicare/physician-fee-schedule/search
- Zhang M, Zippin J, Kaffenberger B. Trends and scope of dermatology procedures billed by advanced practice professionals from 2012 through 2015. JAMA Dermatol. 2018;154:1040-1044.
Practice Points
- Dermatologists should be aware that skin biopsy billing codes and reimbursements were changed in 2019 to reflect their level of complexity, which may impact how often each type of biopsy is used.
- Even small shifts in biopsy utilization behavior among dermatologists in the context of reimbursement changes can have a large impact on net reimbursements.
Active Comparator Trial Designs Used to Promote Development of Innovative New Medications
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Practice Points
- When evaluating a new treatment, it is important to consider not only whether it is effective but also whether it provides additional value compared to existing treatment options.
- Encouraging active comparator trials will provide clinicians and patients with important data to guide decision-making regarding the most appropriate treatment options.
Amylase Testing for Acute Pancreatitis
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 37‐year‐old man presents to the emergency department complaining of acute onset abdominal pain associated with nausea and vomiting. The pain is constant and achy in nature. It is located in the upper abdomen and radiates to the back. The patient reports binge alcohol consumption the day prior to the onset of his pain.
His physical examination is remarkable for fever, with a temperature of 100.6F and epigastric tenderness to palpation without rebound or guarding. He is not hypotensive, and there is no evidence of the Cullen sign or Grey‐Turner sign.
In this patient presenting with acute abdominal pain, is ordering amylase alone, lipase alone, or amylase and lipase together the most high‐value method to evaluate him for acute pancreatitis?
WHY YOU MIGHT THINK AMYLASE TESTING IS HELPFUL
Amylase was one of the earliest, easily measurable laboratory tests that provided a relatively high degree of sensitivity and specificity for identifying patients with acute pancreatitis among those presenting with acute abdominal pain.[1] Since the introduction of amylase, additional tests, including lipase, have been introduced into clinical practice, which offer superior sensitivity and specificity compared to amylase for the diagnosis of acute pancreatitis.[2] However, amylase testing is routinely ordered at many healthcare institutions, with co‐ordering of these tests occurring greater than 90% of the time in some cases.[3, 4] Amylase testing may remain in clinical practice for several reasons including: (1) greater experience with amylase given its earlier introduction into clinical practice, (2) the belief that co‐ordering amylase and lipase provides greater accuracy than either test alone, (3) the notion that pancreatic enzymes provide prognostic information or allow monitoring of clinical progress, and (4) coupling of amylase and lipase in electronic order sets or including the tests together as part of routine lab panels used for the evaluation of abdominal pain.
WHY AMYLASE TESTING OFFERS NO ADDITIONAL VALUE TO LIPASE TESTING
Is Amylase More Accurate Than Lipase in the Diagnosis of Acute Pancreatitis?
In a number of studies, lipase has generally been found to be both more sensitive and more specific than amylase for the diagnosis of acute pancreatitis.[5, 6, 7, 8, 9] A large study by Smith and colleagues, which included 8937 patients who were initially evaluated in the emergency department, found that lipase had a superior area under the receiver operating curve when compared to amylase (0.948 vs 0.906). At a diagnostic threshold of 208 U/L for lipase and 114 U/L for amylase, the authors found that lipase compared to amylase had a superior sensitivity (90.3% vs 78.7%), specificity (93.0% vs 92.6%), positive likelihood ratio (14.1 vs 10.6), and a similar negative likelihood ratio (0.1 vs 0.1).[5]
The observed superiority of lipase over amylase may be related to a number of underlying factors. For instance, amylase measurements often include ‐amylase from the salivary glands and various macroamylase molecules that may not be related to pancreatic injury, whereas lipase measurements are more specific to the pancreas itself.[5] As a result, amylase can be elevated in a number of conditions that are unrelated to acute pancreatitis including parotitis, macroamylasemia, and some cancers.[10] In addition, because lipase remains elevated longer than amylase, it may be more accurate in the setting of delayed presentations of acute pancreatitis.
Does Amylase Co‐Ordered With Lipase Increase Diagnostic Accuracy?
Multiple studies have explored whether amylase provides additional diagnostic information when co‐ordered with lipase. A study by Chase et al. found that amylase and lipase were closely correlated, making them likely redundant measures.[7] Viel and colleagues developed a logistic regression model exploring the value of various parameters in the diagnosis of acute pancreatitis. Although they found that lipase and amylase were both accurate in univariate analyses, a multivariate analysis found that the addition of amylase did not improve the model when compared to lipase alone.[9] In a more recent study by Treacy et al., the investigators explored the accuracy of amylase and lipase in the diagnosis of acute pancreatitis, either alone or in combination, at days 1, 2, and 3 following presentation. In addition to showing that lipase was more accurate than amylase, their results also demonstrated that amylase in addition to lipase did not provide additional diagnostic accuracy compared to lipase alone, as assessed by partial area under the receiver operating curve (0.125 vs 0.128 at day 1, 0.050 vs 0.054 at day 3).[6] Finally, although some early reports suggested that the lipase to amylase ratio could be helpful to distinguish alcoholic pancreatitis from nonalcoholic pancreatitis, later studies have not confirmed these results.[11, 12]
Does Either Amylase or Lipase Add Prognostic Information?
Although both amylase and lipase are useful in the diagnosis of pancreatitis, neither correlates well with severity of illness or clinical resolution of pancreatitis.[10] As a result, the level of elevation of pancreatic enzymes is not included in the major tools used to assess severity of illness, including Ranson's criteria,[13] APACHE II (Acute Physiology and Chronic Health Evaluation II), or the computed tomography (CT) severity index.[14] Newer scoring systems, including the Bedside Index of Severity in Acute Pancreatitis[15] and the Harmless Acute Pancreatitis Score[16] also do not include pancreatic enzymes in their algorithms.
What Do Guidelines and Thought Leaders Say About Using Amylase?
The 2006 Practice Guidelines in Acute Pancreatitis state that it is not necessary to order amylase and lipase together under normal circumstances, and also note that serum lipase is the preferred diagnostic study. In addition, these guidelines state that daily measurement of pancreatic enzymes after the initial diagnosis has limited value in assessing the clinical progress of the illness or ultimate prognosis.[10] The 2013 American College of Gastroenterology guidelines provide stronger support for ordering lipase alone, stating serum amylase alone cannot be used reliably and that serum lipase is preferred.[2]
International guidelines also support the use of lipase alone for the diagnosis of acute pancreatitis. The UK guidelines for the management of acute pancreatitis offer their strongest recommendation supporting the use of lipase alone rather than amylase, unless lipase testing is not available.[17] The Japanese guidelines state the lipase level is the best pancreatic enzyme parameter and additionally do not support the co‐ordering of amylase and lipase together.[18]
Finally, a group of experts in pathology and laboratory medicine at major academic medical centers have identified serum amylase as one of the top 10 antiquated tests within the clinical pathology laboratory.[19]
WHAT YOU SHOULD DO INSTEAD: ORDER LIPASE ALONE
In all cases where a patient presents with abdominal pain concerning for acute pancreatitis, we recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, we suggest that healthcare providers do not perform daily measurement of pancreatic enzymes.
When lipase testing is available, amylase testing provides no clinical value in assisting with the diagnosis or management of acute pancreatitis, but comes at a significant cost to patients. At an average charge of $35 per test, amylase testing represents at least $19 million in annual charges to Medicare alone, according to 2013 payment data.[20] Given that the average charge for a lipase test is $41, it is also difficult to argue that amylase testing is significantly less costly than lipase testing. In addition to the direct costs, amylase tests in these settings can result in diagnostic delays or misdiagnosis, imposing additional costs on patients and the health system. For instance, a patient who presents with symptoms of pancreatitis, a positive lipase, and a negative amylase could receive an unnecessary CT scan to further assess for acute pancreatitis, despite already meeting diagnostic criteria based on the symptoms and lipase test alone.
At institutions where amylase and lipase are listed together in common order sets, removing amylase from these order sets may be a simple, durable intervention to reduce amylase testing.[3] Educational interventions aimed at alleviating some of the cognitive factors associated with amylase and lipase co‐ordering described above should also be considered.
RECOMMENDATIONS
- In patients suspected of having acute pancreatitis, lipase should be ordered alone rather than ordering either amylase alone or amylase and lipase together.
- Pancreatic enzymes should not be repeated after making the diagnosis of acute pancreatitis, as this practice does not provide additional information that is of clinical utility.
CONCLUSION
In the evaluation of acute pancreatitis, the majority of the evidence suggests that amylase, when compared to lipase, has inferior sensitivity and specificity, adds no additional diagnostic information when co‐ordered, and does not provide additional prognostic information (Table 1). In this setting, many guidelines and thought leaders recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, daily monitoring of pancreatic enzymes is not recommended because it does not help assess clinical progress or severity of illness. As a result, we believe that amylase testing should no longer be ordered for patients with suspected acute pancreatitis. Ordering amylase for the evaluation of abdominal pain is a thing we do for no reason.
| Test Characteristic | Amylase | Lipase |
|---|---|---|
| Sensitivity3 | 78.7% | 90.3% |
| Specificity3 | 92.6% | 93.0% |
| Useful when presentation of pancreatitis is delayed | Sometimes | Almost always |
| Useful to assess severity | No | No |
| Useful to assess clinical resolution | No | No |
| Peak | 1272 hours | 24 hours |
| Return to normal | 35 days | 814 days |
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 37‐year‐old man presents to the emergency department complaining of acute onset abdominal pain associated with nausea and vomiting. The pain is constant and achy in nature. It is located in the upper abdomen and radiates to the back. The patient reports binge alcohol consumption the day prior to the onset of his pain.
His physical examination is remarkable for fever, with a temperature of 100.6F and epigastric tenderness to palpation without rebound or guarding. He is not hypotensive, and there is no evidence of the Cullen sign or Grey‐Turner sign.
In this patient presenting with acute abdominal pain, is ordering amylase alone, lipase alone, or amylase and lipase together the most high‐value method to evaluate him for acute pancreatitis?
WHY YOU MIGHT THINK AMYLASE TESTING IS HELPFUL
Amylase was one of the earliest, easily measurable laboratory tests that provided a relatively high degree of sensitivity and specificity for identifying patients with acute pancreatitis among those presenting with acute abdominal pain.[1] Since the introduction of amylase, additional tests, including lipase, have been introduced into clinical practice, which offer superior sensitivity and specificity compared to amylase for the diagnosis of acute pancreatitis.[2] However, amylase testing is routinely ordered at many healthcare institutions, with co‐ordering of these tests occurring greater than 90% of the time in some cases.[3, 4] Amylase testing may remain in clinical practice for several reasons including: (1) greater experience with amylase given its earlier introduction into clinical practice, (2) the belief that co‐ordering amylase and lipase provides greater accuracy than either test alone, (3) the notion that pancreatic enzymes provide prognostic information or allow monitoring of clinical progress, and (4) coupling of amylase and lipase in electronic order sets or including the tests together as part of routine lab panels used for the evaluation of abdominal pain.
WHY AMYLASE TESTING OFFERS NO ADDITIONAL VALUE TO LIPASE TESTING
Is Amylase More Accurate Than Lipase in the Diagnosis of Acute Pancreatitis?
In a number of studies, lipase has generally been found to be both more sensitive and more specific than amylase for the diagnosis of acute pancreatitis.[5, 6, 7, 8, 9] A large study by Smith and colleagues, which included 8937 patients who were initially evaluated in the emergency department, found that lipase had a superior area under the receiver operating curve when compared to amylase (0.948 vs 0.906). At a diagnostic threshold of 208 U/L for lipase and 114 U/L for amylase, the authors found that lipase compared to amylase had a superior sensitivity (90.3% vs 78.7%), specificity (93.0% vs 92.6%), positive likelihood ratio (14.1 vs 10.6), and a similar negative likelihood ratio (0.1 vs 0.1).[5]
The observed superiority of lipase over amylase may be related to a number of underlying factors. For instance, amylase measurements often include ‐amylase from the salivary glands and various macroamylase molecules that may not be related to pancreatic injury, whereas lipase measurements are more specific to the pancreas itself.[5] As a result, amylase can be elevated in a number of conditions that are unrelated to acute pancreatitis including parotitis, macroamylasemia, and some cancers.[10] In addition, because lipase remains elevated longer than amylase, it may be more accurate in the setting of delayed presentations of acute pancreatitis.
Does Amylase Co‐Ordered With Lipase Increase Diagnostic Accuracy?
Multiple studies have explored whether amylase provides additional diagnostic information when co‐ordered with lipase. A study by Chase et al. found that amylase and lipase were closely correlated, making them likely redundant measures.[7] Viel and colleagues developed a logistic regression model exploring the value of various parameters in the diagnosis of acute pancreatitis. Although they found that lipase and amylase were both accurate in univariate analyses, a multivariate analysis found that the addition of amylase did not improve the model when compared to lipase alone.[9] In a more recent study by Treacy et al., the investigators explored the accuracy of amylase and lipase in the diagnosis of acute pancreatitis, either alone or in combination, at days 1, 2, and 3 following presentation. In addition to showing that lipase was more accurate than amylase, their results also demonstrated that amylase in addition to lipase did not provide additional diagnostic accuracy compared to lipase alone, as assessed by partial area under the receiver operating curve (0.125 vs 0.128 at day 1, 0.050 vs 0.054 at day 3).[6] Finally, although some early reports suggested that the lipase to amylase ratio could be helpful to distinguish alcoholic pancreatitis from nonalcoholic pancreatitis, later studies have not confirmed these results.[11, 12]
Does Either Amylase or Lipase Add Prognostic Information?
Although both amylase and lipase are useful in the diagnosis of pancreatitis, neither correlates well with severity of illness or clinical resolution of pancreatitis.[10] As a result, the level of elevation of pancreatic enzymes is not included in the major tools used to assess severity of illness, including Ranson's criteria,[13] APACHE II (Acute Physiology and Chronic Health Evaluation II), or the computed tomography (CT) severity index.[14] Newer scoring systems, including the Bedside Index of Severity in Acute Pancreatitis[15] and the Harmless Acute Pancreatitis Score[16] also do not include pancreatic enzymes in their algorithms.
What Do Guidelines and Thought Leaders Say About Using Amylase?
The 2006 Practice Guidelines in Acute Pancreatitis state that it is not necessary to order amylase and lipase together under normal circumstances, and also note that serum lipase is the preferred diagnostic study. In addition, these guidelines state that daily measurement of pancreatic enzymes after the initial diagnosis has limited value in assessing the clinical progress of the illness or ultimate prognosis.[10] The 2013 American College of Gastroenterology guidelines provide stronger support for ordering lipase alone, stating serum amylase alone cannot be used reliably and that serum lipase is preferred.[2]
International guidelines also support the use of lipase alone for the diagnosis of acute pancreatitis. The UK guidelines for the management of acute pancreatitis offer their strongest recommendation supporting the use of lipase alone rather than amylase, unless lipase testing is not available.[17] The Japanese guidelines state the lipase level is the best pancreatic enzyme parameter and additionally do not support the co‐ordering of amylase and lipase together.[18]
Finally, a group of experts in pathology and laboratory medicine at major academic medical centers have identified serum amylase as one of the top 10 antiquated tests within the clinical pathology laboratory.[19]
WHAT YOU SHOULD DO INSTEAD: ORDER LIPASE ALONE
In all cases where a patient presents with abdominal pain concerning for acute pancreatitis, we recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, we suggest that healthcare providers do not perform daily measurement of pancreatic enzymes.
When lipase testing is available, amylase testing provides no clinical value in assisting with the diagnosis or management of acute pancreatitis, but comes at a significant cost to patients. At an average charge of $35 per test, amylase testing represents at least $19 million in annual charges to Medicare alone, according to 2013 payment data.[20] Given that the average charge for a lipase test is $41, it is also difficult to argue that amylase testing is significantly less costly than lipase testing. In addition to the direct costs, amylase tests in these settings can result in diagnostic delays or misdiagnosis, imposing additional costs on patients and the health system. For instance, a patient who presents with symptoms of pancreatitis, a positive lipase, and a negative amylase could receive an unnecessary CT scan to further assess for acute pancreatitis, despite already meeting diagnostic criteria based on the symptoms and lipase test alone.
At institutions where amylase and lipase are listed together in common order sets, removing amylase from these order sets may be a simple, durable intervention to reduce amylase testing.[3] Educational interventions aimed at alleviating some of the cognitive factors associated with amylase and lipase co‐ordering described above should also be considered.
RECOMMENDATIONS
- In patients suspected of having acute pancreatitis, lipase should be ordered alone rather than ordering either amylase alone or amylase and lipase together.
- Pancreatic enzymes should not be repeated after making the diagnosis of acute pancreatitis, as this practice does not provide additional information that is of clinical utility.
CONCLUSION
In the evaluation of acute pancreatitis, the majority of the evidence suggests that amylase, when compared to lipase, has inferior sensitivity and specificity, adds no additional diagnostic information when co‐ordered, and does not provide additional prognostic information (Table 1). In this setting, many guidelines and thought leaders recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, daily monitoring of pancreatic enzymes is not recommended because it does not help assess clinical progress or severity of illness. As a result, we believe that amylase testing should no longer be ordered for patients with suspected acute pancreatitis. Ordering amylase for the evaluation of abdominal pain is a thing we do for no reason.
| Test Characteristic | Amylase | Lipase |
|---|---|---|
| Sensitivity3 | 78.7% | 90.3% |
| Specificity3 | 92.6% | 93.0% |
| Useful when presentation of pancreatitis is delayed | Sometimes | Almost always |
| Useful to assess severity | No | No |
| Useful to assess clinical resolution | No | No |
| Peak | 1272 hours | 24 hours |
| Return to normal | 35 days | 814 days |
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 37‐year‐old man presents to the emergency department complaining of acute onset abdominal pain associated with nausea and vomiting. The pain is constant and achy in nature. It is located in the upper abdomen and radiates to the back. The patient reports binge alcohol consumption the day prior to the onset of his pain.
His physical examination is remarkable for fever, with a temperature of 100.6F and epigastric tenderness to palpation without rebound or guarding. He is not hypotensive, and there is no evidence of the Cullen sign or Grey‐Turner sign.
In this patient presenting with acute abdominal pain, is ordering amylase alone, lipase alone, or amylase and lipase together the most high‐value method to evaluate him for acute pancreatitis?
WHY YOU MIGHT THINK AMYLASE TESTING IS HELPFUL
Amylase was one of the earliest, easily measurable laboratory tests that provided a relatively high degree of sensitivity and specificity for identifying patients with acute pancreatitis among those presenting with acute abdominal pain.[1] Since the introduction of amylase, additional tests, including lipase, have been introduced into clinical practice, which offer superior sensitivity and specificity compared to amylase for the diagnosis of acute pancreatitis.[2] However, amylase testing is routinely ordered at many healthcare institutions, with co‐ordering of these tests occurring greater than 90% of the time in some cases.[3, 4] Amylase testing may remain in clinical practice for several reasons including: (1) greater experience with amylase given its earlier introduction into clinical practice, (2) the belief that co‐ordering amylase and lipase provides greater accuracy than either test alone, (3) the notion that pancreatic enzymes provide prognostic information or allow monitoring of clinical progress, and (4) coupling of amylase and lipase in electronic order sets or including the tests together as part of routine lab panels used for the evaluation of abdominal pain.
WHY AMYLASE TESTING OFFERS NO ADDITIONAL VALUE TO LIPASE TESTING
Is Amylase More Accurate Than Lipase in the Diagnosis of Acute Pancreatitis?
In a number of studies, lipase has generally been found to be both more sensitive and more specific than amylase for the diagnosis of acute pancreatitis.[5, 6, 7, 8, 9] A large study by Smith and colleagues, which included 8937 patients who were initially evaluated in the emergency department, found that lipase had a superior area under the receiver operating curve when compared to amylase (0.948 vs 0.906). At a diagnostic threshold of 208 U/L for lipase and 114 U/L for amylase, the authors found that lipase compared to amylase had a superior sensitivity (90.3% vs 78.7%), specificity (93.0% vs 92.6%), positive likelihood ratio (14.1 vs 10.6), and a similar negative likelihood ratio (0.1 vs 0.1).[5]
The observed superiority of lipase over amylase may be related to a number of underlying factors. For instance, amylase measurements often include ‐amylase from the salivary glands and various macroamylase molecules that may not be related to pancreatic injury, whereas lipase measurements are more specific to the pancreas itself.[5] As a result, amylase can be elevated in a number of conditions that are unrelated to acute pancreatitis including parotitis, macroamylasemia, and some cancers.[10] In addition, because lipase remains elevated longer than amylase, it may be more accurate in the setting of delayed presentations of acute pancreatitis.
Does Amylase Co‐Ordered With Lipase Increase Diagnostic Accuracy?
Multiple studies have explored whether amylase provides additional diagnostic information when co‐ordered with lipase. A study by Chase et al. found that amylase and lipase were closely correlated, making them likely redundant measures.[7] Viel and colleagues developed a logistic regression model exploring the value of various parameters in the diagnosis of acute pancreatitis. Although they found that lipase and amylase were both accurate in univariate analyses, a multivariate analysis found that the addition of amylase did not improve the model when compared to lipase alone.[9] In a more recent study by Treacy et al., the investigators explored the accuracy of amylase and lipase in the diagnosis of acute pancreatitis, either alone or in combination, at days 1, 2, and 3 following presentation. In addition to showing that lipase was more accurate than amylase, their results also demonstrated that amylase in addition to lipase did not provide additional diagnostic accuracy compared to lipase alone, as assessed by partial area under the receiver operating curve (0.125 vs 0.128 at day 1, 0.050 vs 0.054 at day 3).[6] Finally, although some early reports suggested that the lipase to amylase ratio could be helpful to distinguish alcoholic pancreatitis from nonalcoholic pancreatitis, later studies have not confirmed these results.[11, 12]
Does Either Amylase or Lipase Add Prognostic Information?
Although both amylase and lipase are useful in the diagnosis of pancreatitis, neither correlates well with severity of illness or clinical resolution of pancreatitis.[10] As a result, the level of elevation of pancreatic enzymes is not included in the major tools used to assess severity of illness, including Ranson's criteria,[13] APACHE II (Acute Physiology and Chronic Health Evaluation II), or the computed tomography (CT) severity index.[14] Newer scoring systems, including the Bedside Index of Severity in Acute Pancreatitis[15] and the Harmless Acute Pancreatitis Score[16] also do not include pancreatic enzymes in their algorithms.
What Do Guidelines and Thought Leaders Say About Using Amylase?
The 2006 Practice Guidelines in Acute Pancreatitis state that it is not necessary to order amylase and lipase together under normal circumstances, and also note that serum lipase is the preferred diagnostic study. In addition, these guidelines state that daily measurement of pancreatic enzymes after the initial diagnosis has limited value in assessing the clinical progress of the illness or ultimate prognosis.[10] The 2013 American College of Gastroenterology guidelines provide stronger support for ordering lipase alone, stating serum amylase alone cannot be used reliably and that serum lipase is preferred.[2]
International guidelines also support the use of lipase alone for the diagnosis of acute pancreatitis. The UK guidelines for the management of acute pancreatitis offer their strongest recommendation supporting the use of lipase alone rather than amylase, unless lipase testing is not available.[17] The Japanese guidelines state the lipase level is the best pancreatic enzyme parameter and additionally do not support the co‐ordering of amylase and lipase together.[18]
Finally, a group of experts in pathology and laboratory medicine at major academic medical centers have identified serum amylase as one of the top 10 antiquated tests within the clinical pathology laboratory.[19]
WHAT YOU SHOULD DO INSTEAD: ORDER LIPASE ALONE
In all cases where a patient presents with abdominal pain concerning for acute pancreatitis, we recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, we suggest that healthcare providers do not perform daily measurement of pancreatic enzymes.
When lipase testing is available, amylase testing provides no clinical value in assisting with the diagnosis or management of acute pancreatitis, but comes at a significant cost to patients. At an average charge of $35 per test, amylase testing represents at least $19 million in annual charges to Medicare alone, according to 2013 payment data.[20] Given that the average charge for a lipase test is $41, it is also difficult to argue that amylase testing is significantly less costly than lipase testing. In addition to the direct costs, amylase tests in these settings can result in diagnostic delays or misdiagnosis, imposing additional costs on patients and the health system. For instance, a patient who presents with symptoms of pancreatitis, a positive lipase, and a negative amylase could receive an unnecessary CT scan to further assess for acute pancreatitis, despite already meeting diagnostic criteria based on the symptoms and lipase test alone.
At institutions where amylase and lipase are listed together in common order sets, removing amylase from these order sets may be a simple, durable intervention to reduce amylase testing.[3] Educational interventions aimed at alleviating some of the cognitive factors associated with amylase and lipase co‐ordering described above should also be considered.
RECOMMENDATIONS
- In patients suspected of having acute pancreatitis, lipase should be ordered alone rather than ordering either amylase alone or amylase and lipase together.
- Pancreatic enzymes should not be repeated after making the diagnosis of acute pancreatitis, as this practice does not provide additional information that is of clinical utility.
CONCLUSION
In the evaluation of acute pancreatitis, the majority of the evidence suggests that amylase, when compared to lipase, has inferior sensitivity and specificity, adds no additional diagnostic information when co‐ordered, and does not provide additional prognostic information (Table 1). In this setting, many guidelines and thought leaders recommend ordering lipase alone rather than either amylase alone or co‐ordering amylase and lipase. In addition, daily monitoring of pancreatic enzymes is not recommended because it does not help assess clinical progress or severity of illness. As a result, we believe that amylase testing should no longer be ordered for patients with suspected acute pancreatitis. Ordering amylase for the evaluation of abdominal pain is a thing we do for no reason.
| Test Characteristic | Amylase | Lipase |
|---|---|---|
| Sensitivity3 | 78.7% | 90.3% |
| Specificity3 | 92.6% | 93.0% |
| Useful when presentation of pancreatitis is delayed | Sometimes | Almost always |
| Useful to assess severity | No | No |
| Useful to assess clinical resolution | No | No |
| Peak | 1272 hours | 24 hours |
| Return to normal | 35 days | 814 days |
Disclosure: Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
© 2016 Society of Hospital Medicine