User login
Impact of Stewardship Assistance Pilot Program for Veterans on Adherence and Persistence to Oral mCRPC Therapies
Background
Given the poor prognosis of patients with metastatic castration-resistant prostate cancer (mCRPC), interventions aimed at increasing adherence to oral treatments have the potential to improve patient outcomes. This study evaluates the impact of a patient stewardship assistance pilot program (stewardship program) on the adherence and persistence to oral treatments among patients with mCRPC at VA medical centers (VAMCs).
Methods
A non-randomized controlled study design and data from the VA Corporate Data Warehouse were used. The study included patients treated with an oral mCRPC therapy (i.e., abiraterone acetate or enzalutamide) between 08/2018 and 12/2019. Patients participating in the stewardship program formed the intervention arm and patients not participating the controls. Control patients were selected and matched 1:3 based on age, race and index year. The index date was the date of initiation of abiraterone acetate or enzalutamide. Outcomes included persistence (no gap >60 days of supply) and adherence (proportion of days covered [PDC] ≥80%) to oral mCRPC treatment post-index. Persistence and adherence were compared between the two arms using a Cox proportional hazard model and logistic regression model, respectively, adjusted for baseline characteristics.
Results
The study included 108 intervention patients (mean age: 74.6, 19.4% Black or African American, 44.4% from South, mean Quan-CCI: 6.7) and 324 control patients (mean age: 74.6, 19.4% Black or African American, 31.5% from South, mean Quan-CCI: 6.2). There was no statistically significant difference in persistence between the intervention and control arms (hazard ratio [95% confidence interval]: 0.84 [0.66-1.10], p-value: 0.211), with respective median times to discontinuation of 18 and 19 months. Over the first 12 months post-index, the proportion of adherent patients was not significantly different between the intervention arm and the control arm (50.6% vs. 50.9%; odds ratio [95% confidence interval]: 1.05 [0.80-1.38], p-value: 0.729).
Conclusions
In this racially diverse study of patients treated at VAMCs, high levels of persistence and adherence to oral mCRPC therapy were observed. The absence of any significant difference in adherence and persistence from the study intervention suggests that a stewardship assistance program aimed at improving adherence and persistence of patients with mCRPC may not be required at VAMCs.
Background
Given the poor prognosis of patients with metastatic castration-resistant prostate cancer (mCRPC), interventions aimed at increasing adherence to oral treatments have the potential to improve patient outcomes. This study evaluates the impact of a patient stewardship assistance pilot program (stewardship program) on the adherence and persistence to oral treatments among patients with mCRPC at VA medical centers (VAMCs).
Methods
A non-randomized controlled study design and data from the VA Corporate Data Warehouse were used. The study included patients treated with an oral mCRPC therapy (i.e., abiraterone acetate or enzalutamide) between 08/2018 and 12/2019. Patients participating in the stewardship program formed the intervention arm and patients not participating the controls. Control patients were selected and matched 1:3 based on age, race and index year. The index date was the date of initiation of abiraterone acetate or enzalutamide. Outcomes included persistence (no gap >60 days of supply) and adherence (proportion of days covered [PDC] ≥80%) to oral mCRPC treatment post-index. Persistence and adherence were compared between the two arms using a Cox proportional hazard model and logistic regression model, respectively, adjusted for baseline characteristics.
Results
The study included 108 intervention patients (mean age: 74.6, 19.4% Black or African American, 44.4% from South, mean Quan-CCI: 6.7) and 324 control patients (mean age: 74.6, 19.4% Black or African American, 31.5% from South, mean Quan-CCI: 6.2). There was no statistically significant difference in persistence between the intervention and control arms (hazard ratio [95% confidence interval]: 0.84 [0.66-1.10], p-value: 0.211), with respective median times to discontinuation of 18 and 19 months. Over the first 12 months post-index, the proportion of adherent patients was not significantly different between the intervention arm and the control arm (50.6% vs. 50.9%; odds ratio [95% confidence interval]: 1.05 [0.80-1.38], p-value: 0.729).
Conclusions
In this racially diverse study of patients treated at VAMCs, high levels of persistence and adherence to oral mCRPC therapy were observed. The absence of any significant difference in adherence and persistence from the study intervention suggests that a stewardship assistance program aimed at improving adherence and persistence of patients with mCRPC may not be required at VAMCs.
Background
Given the poor prognosis of patients with metastatic castration-resistant prostate cancer (mCRPC), interventions aimed at increasing adherence to oral treatments have the potential to improve patient outcomes. This study evaluates the impact of a patient stewardship assistance pilot program (stewardship program) on the adherence and persistence to oral treatments among patients with mCRPC at VA medical centers (VAMCs).
Methods
A non-randomized controlled study design and data from the VA Corporate Data Warehouse were used. The study included patients treated with an oral mCRPC therapy (i.e., abiraterone acetate or enzalutamide) between 08/2018 and 12/2019. Patients participating in the stewardship program formed the intervention arm and patients not participating the controls. Control patients were selected and matched 1:3 based on age, race and index year. The index date was the date of initiation of abiraterone acetate or enzalutamide. Outcomes included persistence (no gap >60 days of supply) and adherence (proportion of days covered [PDC] ≥80%) to oral mCRPC treatment post-index. Persistence and adherence were compared between the two arms using a Cox proportional hazard model and logistic regression model, respectively, adjusted for baseline characteristics.
Results
The study included 108 intervention patients (mean age: 74.6, 19.4% Black or African American, 44.4% from South, mean Quan-CCI: 6.7) and 324 control patients (mean age: 74.6, 19.4% Black or African American, 31.5% from South, mean Quan-CCI: 6.2). There was no statistically significant difference in persistence between the intervention and control arms (hazard ratio [95% confidence interval]: 0.84 [0.66-1.10], p-value: 0.211), with respective median times to discontinuation of 18 and 19 months. Over the first 12 months post-index, the proportion of adherent patients was not significantly different between the intervention arm and the control arm (50.6% vs. 50.9%; odds ratio [95% confidence interval]: 1.05 [0.80-1.38], p-value: 0.729).
Conclusions
In this racially diverse study of patients treated at VAMCs, high levels of persistence and adherence to oral mCRPC therapy were observed. The absence of any significant difference in adherence and persistence from the study intervention suggests that a stewardship assistance program aimed at improving adherence and persistence of patients with mCRPC may not be required at VAMCs.
Unexpected Findings: A Rare Case of Signet Ring Cell Adenocarcinoma in the Small Intestine
Introduction
Signet ring cell carcinoma (SRCC) of the small intestine is very rare. It is characterized by the presence of malignant cells that contain mucin that push nuclei to the periphery. It is more aggressive compared to other adenocarcinomas due to early metastasis and poorer prognosis.
Case Presentation
A 59-year-old male with a history of HIV/AIDS, presented with complaints of anorexia, vomiting and weight loss. Initial abdominal CT showed a retroperitoneal mass causing gastric outlet obstruction. The patient elected to go home after supportive treatment and follow up as an outpatient, however, he presented 10 days later with worsening symptoms. Evaluation with CT abdomen and pelvis showed enlarging soft tissue density in the retrocrural space extending into the retroperitoneum around the aorta, as well as a 1.5 cm intraluminal cystic lesion in the duodenum. Endoscopic ultrasound revealed lymphadenopathy of celiac and porta hepatis regions, along with duodenal stenosis, stent placement for decompression was not feasible and biopsies were inconclusive. The decision was made to proceed with laparotomy for decompression and additional biopsies from the retroperitoneal mass and omental lymph nodes, which confirmed poorly differentiated adenocarcinoma with signet ring cells. The presence of a mass in the duodenum strongly suggested adenocarcinoma of small intestine origin. As the patient’s symptoms worsened, imaging revealed progression with lung metastases. The patient continued to deteriorate rapidly requiring dialysis and gangrenous cholecystitis. Given his complex medical history, patient decided to transition to comfort care.
Discussion
SRCC can present with any GI symptoms. Most important step in diagnosing SRCC is biopsy. Current treatment options for small intestinal malignancies include wide resection that includes the mesentery and corresponding lymph nodes. The use of adjuvant chemotherapy has been described only in small retrospective studies. Due to its scarcity, there isn’t sufficient data for optimal treatment strategies compared to gastric SRCC.
Conclusions
This case report highlights the importance of how rare and aggressive signet ring cell adenocarcinoma of the small intestine. There are only a few cases documented in the literature, which is why we lack data on how to manage the disease.
Introduction
Signet ring cell carcinoma (SRCC) of the small intestine is very rare. It is characterized by the presence of malignant cells that contain mucin that push nuclei to the periphery. It is more aggressive compared to other adenocarcinomas due to early metastasis and poorer prognosis.
Case Presentation
A 59-year-old male with a history of HIV/AIDS, presented with complaints of anorexia, vomiting and weight loss. Initial abdominal CT showed a retroperitoneal mass causing gastric outlet obstruction. The patient elected to go home after supportive treatment and follow up as an outpatient, however, he presented 10 days later with worsening symptoms. Evaluation with CT abdomen and pelvis showed enlarging soft tissue density in the retrocrural space extending into the retroperitoneum around the aorta, as well as a 1.5 cm intraluminal cystic lesion in the duodenum. Endoscopic ultrasound revealed lymphadenopathy of celiac and porta hepatis regions, along with duodenal stenosis, stent placement for decompression was not feasible and biopsies were inconclusive. The decision was made to proceed with laparotomy for decompression and additional biopsies from the retroperitoneal mass and omental lymph nodes, which confirmed poorly differentiated adenocarcinoma with signet ring cells. The presence of a mass in the duodenum strongly suggested adenocarcinoma of small intestine origin. As the patient’s symptoms worsened, imaging revealed progression with lung metastases. The patient continued to deteriorate rapidly requiring dialysis and gangrenous cholecystitis. Given his complex medical history, patient decided to transition to comfort care.
Discussion
SRCC can present with any GI symptoms. Most important step in diagnosing SRCC is biopsy. Current treatment options for small intestinal malignancies include wide resection that includes the mesentery and corresponding lymph nodes. The use of adjuvant chemotherapy has been described only in small retrospective studies. Due to its scarcity, there isn’t sufficient data for optimal treatment strategies compared to gastric SRCC.
Conclusions
This case report highlights the importance of how rare and aggressive signet ring cell adenocarcinoma of the small intestine. There are only a few cases documented in the literature, which is why we lack data on how to manage the disease.
Introduction
Signet ring cell carcinoma (SRCC) of the small intestine is very rare. It is characterized by the presence of malignant cells that contain mucin that push nuclei to the periphery. It is more aggressive compared to other adenocarcinomas due to early metastasis and poorer prognosis.
Case Presentation
A 59-year-old male with a history of HIV/AIDS, presented with complaints of anorexia, vomiting and weight loss. Initial abdominal CT showed a retroperitoneal mass causing gastric outlet obstruction. The patient elected to go home after supportive treatment and follow up as an outpatient, however, he presented 10 days later with worsening symptoms. Evaluation with CT abdomen and pelvis showed enlarging soft tissue density in the retrocrural space extending into the retroperitoneum around the aorta, as well as a 1.5 cm intraluminal cystic lesion in the duodenum. Endoscopic ultrasound revealed lymphadenopathy of celiac and porta hepatis regions, along with duodenal stenosis, stent placement for decompression was not feasible and biopsies were inconclusive. The decision was made to proceed with laparotomy for decompression and additional biopsies from the retroperitoneal mass and omental lymph nodes, which confirmed poorly differentiated adenocarcinoma with signet ring cells. The presence of a mass in the duodenum strongly suggested adenocarcinoma of small intestine origin. As the patient’s symptoms worsened, imaging revealed progression with lung metastases. The patient continued to deteriorate rapidly requiring dialysis and gangrenous cholecystitis. Given his complex medical history, patient decided to transition to comfort care.
Discussion
SRCC can present with any GI symptoms. Most important step in diagnosing SRCC is biopsy. Current treatment options for small intestinal malignancies include wide resection that includes the mesentery and corresponding lymph nodes. The use of adjuvant chemotherapy has been described only in small retrospective studies. Due to its scarcity, there isn’t sufficient data for optimal treatment strategies compared to gastric SRCC.
Conclusions
This case report highlights the importance of how rare and aggressive signet ring cell adenocarcinoma of the small intestine. There are only a few cases documented in the literature, which is why we lack data on how to manage the disease.
Registered Dietitian Staffing and Nutrition Practices in High-Risk Cancer Patients Across the Veterans Health Administration
Background
Nutrition disorders, such as sarcopenia, malnutrition, and cachexia are prevalent in cancer patients and correlated with negative outcomes, increased costs, and reduced quality of life (QOL). Registered dietitians (RDs) effectively diagnose and treat nutrition disorders. RD staffing guidelines in outpatient cancer centers are non-specific and unvalidated. This study explored RD staffing ratios to determine trends which may indicate best practices.
Methods
Facility-level measures including full time equivalents (FTE), referral practices, RD participation interdisciplinary round participation, and nutrition referral practices were obtained from survey data of RDs working in oncology clinics and from cancer registries across VHA between 2016-2017. A proactive score was calculated based on interdisciplinary meeting attendances, use of validated screening tools, and standardized protocols for nutrition referrals. Chart review was conducted for 681 Veterans from 13 VHA cancer centers and 207 oncology providers (OPs) to determine weight change, malnutrition, oral nutrition supplement (ONS) use, time to RD referral, and survival. Logistic regression was used for statistical analysis.
Results
Mean and median RD FTE assigned to oncology clinics was 0.5. The total RD:OP ratio ranged from 1:4 to 1:850 with an average of 1 RD to 48.5 OP. An increase in RD:OP ratio from 0:1 to 1:1 was associated with a 16-fold increased odds of weight maintenance during cancer treatment (95% CI: 2.01, 127.53). A 10% increase in the RD:OP ratio increased probability of weight maintenance by 32%. Being seen by an RD was associated with 2.87 times odds of being diagnosed with malnutrition (95% CI: 1.62, 5.08). Each unit increase in a facility’s proactive score was associated with 38% increased odds of a patient being seen by an RD (95% CI: 1.08, 1.76), and 21% reduced odds of being prescribed an ONS (95% CI: 0.63, 0.98).
Conclusions
Few cancer centers employ dedicated fulltime RDs and nutrition practices vary across cancer centers. Improved RD:OP ratios may contribute to improved nutrition outcomes for this population. When RDs are active in interdisciplinary cancer teams, nutrition treatment improves. These efforts support patient complexity, facility funding, and QOL. These data may be used to support cancer care guidelines across VHA.
Background
Nutrition disorders, such as sarcopenia, malnutrition, and cachexia are prevalent in cancer patients and correlated with negative outcomes, increased costs, and reduced quality of life (QOL). Registered dietitians (RDs) effectively diagnose and treat nutrition disorders. RD staffing guidelines in outpatient cancer centers are non-specific and unvalidated. This study explored RD staffing ratios to determine trends which may indicate best practices.
Methods
Facility-level measures including full time equivalents (FTE), referral practices, RD participation interdisciplinary round participation, and nutrition referral practices were obtained from survey data of RDs working in oncology clinics and from cancer registries across VHA between 2016-2017. A proactive score was calculated based on interdisciplinary meeting attendances, use of validated screening tools, and standardized protocols for nutrition referrals. Chart review was conducted for 681 Veterans from 13 VHA cancer centers and 207 oncology providers (OPs) to determine weight change, malnutrition, oral nutrition supplement (ONS) use, time to RD referral, and survival. Logistic regression was used for statistical analysis.
Results
Mean and median RD FTE assigned to oncology clinics was 0.5. The total RD:OP ratio ranged from 1:4 to 1:850 with an average of 1 RD to 48.5 OP. An increase in RD:OP ratio from 0:1 to 1:1 was associated with a 16-fold increased odds of weight maintenance during cancer treatment (95% CI: 2.01, 127.53). A 10% increase in the RD:OP ratio increased probability of weight maintenance by 32%. Being seen by an RD was associated with 2.87 times odds of being diagnosed with malnutrition (95% CI: 1.62, 5.08). Each unit increase in a facility’s proactive score was associated with 38% increased odds of a patient being seen by an RD (95% CI: 1.08, 1.76), and 21% reduced odds of being prescribed an ONS (95% CI: 0.63, 0.98).
Conclusions
Few cancer centers employ dedicated fulltime RDs and nutrition practices vary across cancer centers. Improved RD:OP ratios may contribute to improved nutrition outcomes for this population. When RDs are active in interdisciplinary cancer teams, nutrition treatment improves. These efforts support patient complexity, facility funding, and QOL. These data may be used to support cancer care guidelines across VHA.
Background
Nutrition disorders, such as sarcopenia, malnutrition, and cachexia are prevalent in cancer patients and correlated with negative outcomes, increased costs, and reduced quality of life (QOL). Registered dietitians (RDs) effectively diagnose and treat nutrition disorders. RD staffing guidelines in outpatient cancer centers are non-specific and unvalidated. This study explored RD staffing ratios to determine trends which may indicate best practices.
Methods
Facility-level measures including full time equivalents (FTE), referral practices, RD participation interdisciplinary round participation, and nutrition referral practices were obtained from survey data of RDs working in oncology clinics and from cancer registries across VHA between 2016-2017. A proactive score was calculated based on interdisciplinary meeting attendances, use of validated screening tools, and standardized protocols for nutrition referrals. Chart review was conducted for 681 Veterans from 13 VHA cancer centers and 207 oncology providers (OPs) to determine weight change, malnutrition, oral nutrition supplement (ONS) use, time to RD referral, and survival. Logistic regression was used for statistical analysis.
Results
Mean and median RD FTE assigned to oncology clinics was 0.5. The total RD:OP ratio ranged from 1:4 to 1:850 with an average of 1 RD to 48.5 OP. An increase in RD:OP ratio from 0:1 to 1:1 was associated with a 16-fold increased odds of weight maintenance during cancer treatment (95% CI: 2.01, 127.53). A 10% increase in the RD:OP ratio increased probability of weight maintenance by 32%. Being seen by an RD was associated with 2.87 times odds of being diagnosed with malnutrition (95% CI: 1.62, 5.08). Each unit increase in a facility’s proactive score was associated with 38% increased odds of a patient being seen by an RD (95% CI: 1.08, 1.76), and 21% reduced odds of being prescribed an ONS (95% CI: 0.63, 0.98).
Conclusions
Few cancer centers employ dedicated fulltime RDs and nutrition practices vary across cancer centers. Improved RD:OP ratios may contribute to improved nutrition outcomes for this population. When RDs are active in interdisciplinary cancer teams, nutrition treatment improves. These efforts support patient complexity, facility funding, and QOL. These data may be used to support cancer care guidelines across VHA.
Telehealth Research and Innovation for Veterans With Cancer (THRIVE): Understanding Experiences of National TeleOncology Service Providers
Background
Currently within the Veterans Health Administration, nearly 38% of VA users reside in rural areas. Approximately 70% of rural areas do not have an oncologist, resulting in a high proportion of Veterans who lack access to specialized cancer services. The National TeleOncology Service (NTO) was designed to increase access to specialty and subspecialty cancer care for Veterans regardless of geographical location, and for those who may experience additional barriers to in-person care due to medical complexity or other social determinants of health. Purpose: THRIVE focuses on health equity for telehealth-delivered cancer care. We are specifically interested in the intersection of poverty, rurality, and race. As part of this inquiry, we examined provider experiences of the NTO to better understand the benefits, drawbacks, facilitators and barriers to implementing NTO care.
Methods
We conducted two focus groups with NTO providers. We developed guides using the Consolidated Framework for Implementation Research (CFIR 2.0) and utilized rapid qualitative analysis. We arrayed data in matrices based on CFIR 2.0-based guide for analysis.
Results
The focus groups included NTO physicians (n=4) and non-physicians (n=19). Providers agreed that NTO provides valuable cancer care to Veterans facing in-person access issues. The technology is easy to use for many patients, but those in rural areas experiencing poverty struggle most. NTO’s technical support resources reduce technical skill and equipment barriers and facilitate connection for both patients and providers. Providers enjoyed the team-based approach of NTO and believed it increases care quality through access to multiple providers and resources within the clinical encounter. The NTO’s work could be strengthened by standardizing technology to facilitate records transfer and enable sharing of documentation and education between NTO and patients. Implications: This study examined providers’ perceived acceptability, feasibility, barriers, and facilitators of NTO-delivered cancer care within VA, demonstrating that NTO service is well-liked and a valuable emerging resource of VA care.
Conclusions
In an era when CMMS shifts away from reimbursing telehealth, VA has committed to continue such care providing a variety of patient-centered approaches. NTO may serve as a model for expanding telehealth-delivered care for other serious and chronic diseases and conditions.
Background
Currently within the Veterans Health Administration, nearly 38% of VA users reside in rural areas. Approximately 70% of rural areas do not have an oncologist, resulting in a high proportion of Veterans who lack access to specialized cancer services. The National TeleOncology Service (NTO) was designed to increase access to specialty and subspecialty cancer care for Veterans regardless of geographical location, and for those who may experience additional barriers to in-person care due to medical complexity or other social determinants of health. Purpose: THRIVE focuses on health equity for telehealth-delivered cancer care. We are specifically interested in the intersection of poverty, rurality, and race. As part of this inquiry, we examined provider experiences of the NTO to better understand the benefits, drawbacks, facilitators and barriers to implementing NTO care.
Methods
We conducted two focus groups with NTO providers. We developed guides using the Consolidated Framework for Implementation Research (CFIR 2.0) and utilized rapid qualitative analysis. We arrayed data in matrices based on CFIR 2.0-based guide for analysis.
Results
The focus groups included NTO physicians (n=4) and non-physicians (n=19). Providers agreed that NTO provides valuable cancer care to Veterans facing in-person access issues. The technology is easy to use for many patients, but those in rural areas experiencing poverty struggle most. NTO’s technical support resources reduce technical skill and equipment barriers and facilitate connection for both patients and providers. Providers enjoyed the team-based approach of NTO and believed it increases care quality through access to multiple providers and resources within the clinical encounter. The NTO’s work could be strengthened by standardizing technology to facilitate records transfer and enable sharing of documentation and education between NTO and patients. Implications: This study examined providers’ perceived acceptability, feasibility, barriers, and facilitators of NTO-delivered cancer care within VA, demonstrating that NTO service is well-liked and a valuable emerging resource of VA care.
Conclusions
In an era when CMMS shifts away from reimbursing telehealth, VA has committed to continue such care providing a variety of patient-centered approaches. NTO may serve as a model for expanding telehealth-delivered care for other serious and chronic diseases and conditions.
Background
Currently within the Veterans Health Administration, nearly 38% of VA users reside in rural areas. Approximately 70% of rural areas do not have an oncologist, resulting in a high proportion of Veterans who lack access to specialized cancer services. The National TeleOncology Service (NTO) was designed to increase access to specialty and subspecialty cancer care for Veterans regardless of geographical location, and for those who may experience additional barriers to in-person care due to medical complexity or other social determinants of health. Purpose: THRIVE focuses on health equity for telehealth-delivered cancer care. We are specifically interested in the intersection of poverty, rurality, and race. As part of this inquiry, we examined provider experiences of the NTO to better understand the benefits, drawbacks, facilitators and barriers to implementing NTO care.
Methods
We conducted two focus groups with NTO providers. We developed guides using the Consolidated Framework for Implementation Research (CFIR 2.0) and utilized rapid qualitative analysis. We arrayed data in matrices based on CFIR 2.0-based guide for analysis.
Results
The focus groups included NTO physicians (n=4) and non-physicians (n=19). Providers agreed that NTO provides valuable cancer care to Veterans facing in-person access issues. The technology is easy to use for many patients, but those in rural areas experiencing poverty struggle most. NTO’s technical support resources reduce technical skill and equipment barriers and facilitate connection for both patients and providers. Providers enjoyed the team-based approach of NTO and believed it increases care quality through access to multiple providers and resources within the clinical encounter. The NTO’s work could be strengthened by standardizing technology to facilitate records transfer and enable sharing of documentation and education between NTO and patients. Implications: This study examined providers’ perceived acceptability, feasibility, barriers, and facilitators of NTO-delivered cancer care within VA, demonstrating that NTO service is well-liked and a valuable emerging resource of VA care.
Conclusions
In an era when CMMS shifts away from reimbursing telehealth, VA has committed to continue such care providing a variety of patient-centered approaches. NTO may serve as a model for expanding telehealth-delivered care for other serious and chronic diseases and conditions.
Blaschkolinear Lupus Erythematosus: Strategies for Early Detection and Management
To the Editor:
Chronic cutaneous lupus erythematosus (CCLE) is an inflammatory condition with myriad cutaneous manifestations. Most forms of CCLE have the potential to progress to systemic lupus erythematosus (SLE).1
Blaschkolinear lupus erythematosus (BLE) is an exceedingly rare subtype of cutaneous lupus erythematosus that usually manifests during childhood as linear plaques along the lines of Blaschko.2,3 Under normal conditions, Blaschko lines are not noticeable; they correspond to the direction of ectodermal cell migration during cutaneous embryogenesis.4,5 The embryonic cells travel ventrolaterally, forming a V-shaped pattern on the back, an S-shaped pattern on the trunk, and an hourglass-shaped pattern on the face with several perpendicular intersections near the mouth and nose.6 During their migration, the cells are susceptible to somatic mutations and clonal expansion, resulting in a monoclonal population of genetically heterogenous cells. This phenomenon is known as somatic mosaicism and may lead to an increased susceptibility to an array of congenital and inflammatory dermatoses, such as cutaneous lupus erythematosus.4 Blaschkolinear entities tend to manifest in a unilateral distribution following exposure to a certain environmental trigger, such as trauma, viral illness, or UV radiation, although a trigger is not always present.7 We report a case of BLE manifesting on the head and neck in an adult patient.
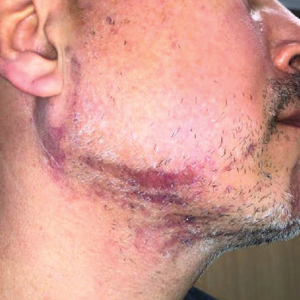
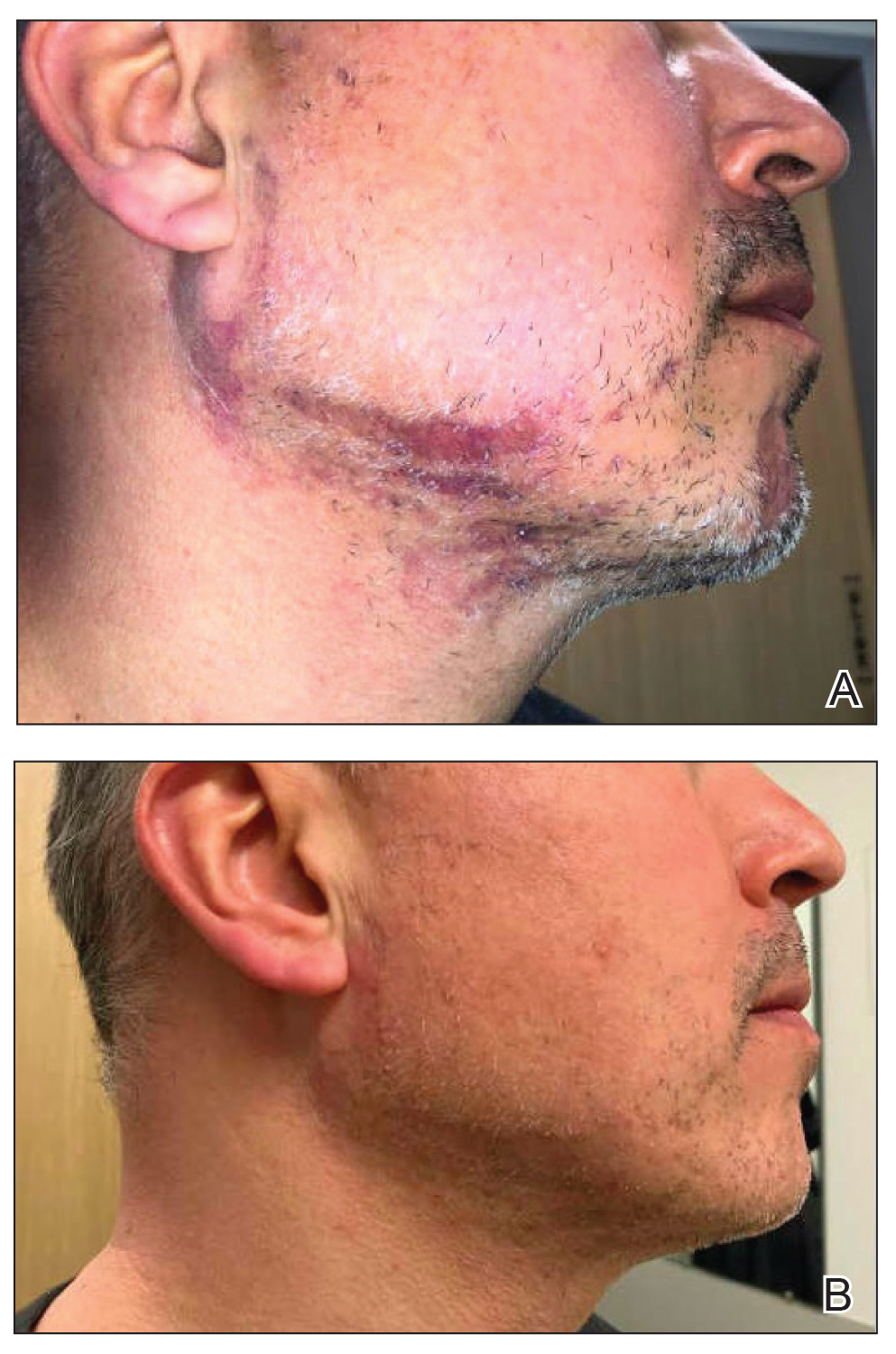
A 46-year-old man presented with a pruritic rash of 3 months’ duration on the right cheek that extended inferiorly to the right upper chest. He had a medical history of well-controlled psoriasis, and he denied any antecedent trauma, fevers, chills, arthralgia, or night sweats. There had been no improvement with mometasone ointment 0.1% applied daily for 2 months as prescribed by his primary care provider. Physical examination revealed indurated, red-brown, atrophic plaques in a blaschkolinear distribution around the nose, right upper jaw, right side of the neck, and right upper chest (Figure, A).
Histopathology of punch biopsies from the right jaw and right upper chest showed an atrophic epidermis with scattered dyskeratotic keratinocytes and vacuolar alteration of the basal cell layer. A superficial and deep perivascular and periadnexal lymphocytic infiltrate was observed in both biopsies. Staining with Verhoeff-van Gieson elastin and periodic acid–Schiff highlighted prominent basement membrane thickening and loss of elastic fibers in the superficial dermis. These findings favored a diagnosis of CCLE, and the clinical blaschkolinear distribution of the rash led to our specific diagnosis of BLE. Laboratory workup for SLE including a complete blood cell count; urine analysis; and testing for liver and kidney function, antinuclearantibodies, complement levels, and erythrocyte sedimentation rate revealed no abnormalities.
The patient started hydroxychloroquine 200 mg twice daily and methotrexate 25 mg weekly along with strict photoprotection measures, including wearing photoprotective clothing and avoiding sunlight during the most intense hours of the day.
Linear lichen planus is an important differential diagnosis to consider in patients with a blaschkolinear eruption.7 Although the clinical manifestations of BLE and linear lichen planus are similar, they differ histopathologically. One study found that only 33.3% of patients (6/18) who clinically presented with blaschkolinear eruptions were correctly diagnosed before histologic examination.7 Visualization of the adnexa as well as the superficial and deep vascular plexuses is paramount in distinguishing between linear lichen planus and BLE; linear lichen planus does not have perivascular and periadnexal infiltration, while BLE does. Thus, in our experience, a punch biopsy—rather than a shave biopsy—should be performed to access the deeper layers of the skin.
Because these 2 entities have noteworthy differences in their management, prognosis, and long-term follow-up, accurate diagnosis is critical. To start, BLE is treated with the use of photoprotection, whereas linear lichen planus is commonly treated with phototherapy. Given the potential for forms of CCLE to progress to SLE, serial monitoring is indicated in patients with BLE. As the risk for progression to SLE is highest in the first 3 years after diagnosis, a review of systems and laboratory testing should occur every 2 to 3 months in the first year after diagnosis (sooner if the disease presentation is more severe).9 Also, treatment with hydroxychloroquine likely delays transformation to SLE and is important in the early management of BLE.10 On the other hand, linear lichen planus tends to self-resolve without progression to systemic involvement, warranting limited follow-up.9
Blaschkolinear lupus erythematosus typically manifests in childhood, but it also can be seen in adults, such as in our patient. Adult-onset BLE is rare but may be underrecognized or underreported in the literature.11 However, dermatologists should consider it in the differential diagnosis for any patient with a blaschkolinear eruption, as establishing the correct diagnosis is key to ensuring prompt and effective treatment for this rare inflammatory condition.
- Grönhagen CM, Fored CM, Granath F, et al. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164:1335-1341. doi:10.1111/j.1365-2133.2011.10272.x
- Requena C, Torrelo A, de Prada I, et al. Linear childhood cutaneous lupus erythematosus following Blaschko lines. J Eur Acad Dermatol Venereol. 2002;16:618-620. doi:10.1046/j.1468-3083.2002.00588.x
- Lim D, Hatami A, Kokta V, et al. Linear cutaneous lupus erythematosus in children-report of two cases and review of the literature: a case report. SAGE Open Med Case Rep. 2020;8:2050313x20979206. doi:10.1177/2050313X20979206
- Jin H, Zhang G, Zhou Y, et al. Old lines tell new tales: Blaschko linear lupus erythematosus. Autoimmun Rev. 2016;15:291-306. doi:10.1016/j.autrev.2015.11.014
- Yu S, Yu H-S. A patient with subacute cutaneous lupus erythematosus along Blaschko lines: implications for the role of keratinocytes in lupus erythematosus. Dermatologica Sinica. 2016;34:144-147. doi:10.1016/j.dsi.2015.12.002
- Kouzak SS, Mendes MST, Costa IMC. Cutaneous mosaicisms: concepts, patterns and classifications. An Bras Dermatol. 2013;88:507-517. doi:10.1590/abd1806-4841.20132015
- Liu W, Vano-Galvan S, Liu J-W, et al. Pigmented linear discoid lupus erythematosus following the lines of Blaschko: a retrospective study of a Chinese series. Indian J Dermatol Venereol Leprol. 2020;86:359-365. doi:10.4103/ijdvl.IJDVL_341_19
- O’Brien JC, Chong BF. Not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Invest Dermatol Symp Proc. 2017;18:S69-S74. doi:10.1016/j.jisp.2016.09.001
- Curtiss P, Walker AM, Chong BF. A systematic review of the progression of cutaneous lupus to systemic lupus erythematosus. Front Immunol. 2022:13:866319. doi:10.3389/fimmu.2022.866319
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. doi:10.1016/j.berh.2013.07.008
- Milosavljevic K, Fibeger E, Virata AR. A case of linear cutaneous lupus erythematosus in a 55-year-old woman. Am J Case Rep. 2020;21:E921495. doi:10.12659/AJCR.921495
To the Editor:
Chronic cutaneous lupus erythematosus (CCLE) is an inflammatory condition with myriad cutaneous manifestations. Most forms of CCLE have the potential to progress to systemic lupus erythematosus (SLE).1
Blaschkolinear lupus erythematosus (BLE) is an exceedingly rare subtype of cutaneous lupus erythematosus that usually manifests during childhood as linear plaques along the lines of Blaschko.2,3 Under normal conditions, Blaschko lines are not noticeable; they correspond to the direction of ectodermal cell migration during cutaneous embryogenesis.4,5 The embryonic cells travel ventrolaterally, forming a V-shaped pattern on the back, an S-shaped pattern on the trunk, and an hourglass-shaped pattern on the face with several perpendicular intersections near the mouth and nose.6 During their migration, the cells are susceptible to somatic mutations and clonal expansion, resulting in a monoclonal population of genetically heterogenous cells. This phenomenon is known as somatic mosaicism and may lead to an increased susceptibility to an array of congenital and inflammatory dermatoses, such as cutaneous lupus erythematosus.4 Blaschkolinear entities tend to manifest in a unilateral distribution following exposure to a certain environmental trigger, such as trauma, viral illness, or UV radiation, although a trigger is not always present.7 We report a case of BLE manifesting on the head and neck in an adult patient.
A 46-year-old man presented with a pruritic rash of 3 months’ duration on the right cheek that extended inferiorly to the right upper chest. He had a medical history of well-controlled psoriasis, and he denied any antecedent trauma, fevers, chills, arthralgia, or night sweats. There had been no improvement with mometasone ointment 0.1% applied daily for 2 months as prescribed by his primary care provider. Physical examination revealed indurated, red-brown, atrophic plaques in a blaschkolinear distribution around the nose, right upper jaw, right side of the neck, and right upper chest (Figure, A).

Histopathology of punch biopsies from the right jaw and right upper chest showed an atrophic epidermis with scattered dyskeratotic keratinocytes and vacuolar alteration of the basal cell layer. A superficial and deep perivascular and periadnexal lymphocytic infiltrate was observed in both biopsies. Staining with Verhoeff-van Gieson elastin and periodic acid–Schiff highlighted prominent basement membrane thickening and loss of elastic fibers in the superficial dermis. These findings favored a diagnosis of CCLE, and the clinical blaschkolinear distribution of the rash led to our specific diagnosis of BLE. Laboratory workup for SLE including a complete blood cell count; urine analysis; and testing for liver and kidney function, antinuclearantibodies, complement levels, and erythrocyte sedimentation rate revealed no abnormalities.
The patient started hydroxychloroquine 200 mg twice daily and methotrexate 25 mg weekly along with strict photoprotection measures, including wearing photoprotective clothing and avoiding sunlight during the most intense hours of the day.
Linear lichen planus is an important differential diagnosis to consider in patients with a blaschkolinear eruption.7 Although the clinical manifestations of BLE and linear lichen planus are similar, they differ histopathologically. One study found that only 33.3% of patients (6/18) who clinically presented with blaschkolinear eruptions were correctly diagnosed before histologic examination.7 Visualization of the adnexa as well as the superficial and deep vascular plexuses is paramount in distinguishing between linear lichen planus and BLE; linear lichen planus does not have perivascular and periadnexal infiltration, while BLE does. Thus, in our experience, a punch biopsy—rather than a shave biopsy—should be performed to access the deeper layers of the skin.
Because these 2 entities have noteworthy differences in their management, prognosis, and long-term follow-up, accurate diagnosis is critical. To start, BLE is treated with the use of photoprotection, whereas linear lichen planus is commonly treated with phototherapy. Given the potential for forms of CCLE to progress to SLE, serial monitoring is indicated in patients with BLE. As the risk for progression to SLE is highest in the first 3 years after diagnosis, a review of systems and laboratory testing should occur every 2 to 3 months in the first year after diagnosis (sooner if the disease presentation is more severe).9 Also, treatment with hydroxychloroquine likely delays transformation to SLE and is important in the early management of BLE.10 On the other hand, linear lichen planus tends to self-resolve without progression to systemic involvement, warranting limited follow-up.9
Blaschkolinear lupus erythematosus typically manifests in childhood, but it also can be seen in adults, such as in our patient. Adult-onset BLE is rare but may be underrecognized or underreported in the literature.11 However, dermatologists should consider it in the differential diagnosis for any patient with a blaschkolinear eruption, as establishing the correct diagnosis is key to ensuring prompt and effective treatment for this rare inflammatory condition.
To the Editor:
Chronic cutaneous lupus erythematosus (CCLE) is an inflammatory condition with myriad cutaneous manifestations. Most forms of CCLE have the potential to progress to systemic lupus erythematosus (SLE).1
Blaschkolinear lupus erythematosus (BLE) is an exceedingly rare subtype of cutaneous lupus erythematosus that usually manifests during childhood as linear plaques along the lines of Blaschko.2,3 Under normal conditions, Blaschko lines are not noticeable; they correspond to the direction of ectodermal cell migration during cutaneous embryogenesis.4,5 The embryonic cells travel ventrolaterally, forming a V-shaped pattern on the back, an S-shaped pattern on the trunk, and an hourglass-shaped pattern on the face with several perpendicular intersections near the mouth and nose.6 During their migration, the cells are susceptible to somatic mutations and clonal expansion, resulting in a monoclonal population of genetically heterogenous cells. This phenomenon is known as somatic mosaicism and may lead to an increased susceptibility to an array of congenital and inflammatory dermatoses, such as cutaneous lupus erythematosus.4 Blaschkolinear entities tend to manifest in a unilateral distribution following exposure to a certain environmental trigger, such as trauma, viral illness, or UV radiation, although a trigger is not always present.7 We report a case of BLE manifesting on the head and neck in an adult patient.
A 46-year-old man presented with a pruritic rash of 3 months’ duration on the right cheek that extended inferiorly to the right upper chest. He had a medical history of well-controlled psoriasis, and he denied any antecedent trauma, fevers, chills, arthralgia, or night sweats. There had been no improvement with mometasone ointment 0.1% applied daily for 2 months as prescribed by his primary care provider. Physical examination revealed indurated, red-brown, atrophic plaques in a blaschkolinear distribution around the nose, right upper jaw, right side of the neck, and right upper chest (Figure, A).

Histopathology of punch biopsies from the right jaw and right upper chest showed an atrophic epidermis with scattered dyskeratotic keratinocytes and vacuolar alteration of the basal cell layer. A superficial and deep perivascular and periadnexal lymphocytic infiltrate was observed in both biopsies. Staining with Verhoeff-van Gieson elastin and periodic acid–Schiff highlighted prominent basement membrane thickening and loss of elastic fibers in the superficial dermis. These findings favored a diagnosis of CCLE, and the clinical blaschkolinear distribution of the rash led to our specific diagnosis of BLE. Laboratory workup for SLE including a complete blood cell count; urine analysis; and testing for liver and kidney function, antinuclearantibodies, complement levels, and erythrocyte sedimentation rate revealed no abnormalities.
The patient started hydroxychloroquine 200 mg twice daily and methotrexate 25 mg weekly along with strict photoprotection measures, including wearing photoprotective clothing and avoiding sunlight during the most intense hours of the day.
Linear lichen planus is an important differential diagnosis to consider in patients with a blaschkolinear eruption.7 Although the clinical manifestations of BLE and linear lichen planus are similar, they differ histopathologically. One study found that only 33.3% of patients (6/18) who clinically presented with blaschkolinear eruptions were correctly diagnosed before histologic examination.7 Visualization of the adnexa as well as the superficial and deep vascular plexuses is paramount in distinguishing between linear lichen planus and BLE; linear lichen planus does not have perivascular and periadnexal infiltration, while BLE does. Thus, in our experience, a punch biopsy—rather than a shave biopsy—should be performed to access the deeper layers of the skin.
Because these 2 entities have noteworthy differences in their management, prognosis, and long-term follow-up, accurate diagnosis is critical. To start, BLE is treated with the use of photoprotection, whereas linear lichen planus is commonly treated with phototherapy. Given the potential for forms of CCLE to progress to SLE, serial monitoring is indicated in patients with BLE. As the risk for progression to SLE is highest in the first 3 years after diagnosis, a review of systems and laboratory testing should occur every 2 to 3 months in the first year after diagnosis (sooner if the disease presentation is more severe).9 Also, treatment with hydroxychloroquine likely delays transformation to SLE and is important in the early management of BLE.10 On the other hand, linear lichen planus tends to self-resolve without progression to systemic involvement, warranting limited follow-up.9
Blaschkolinear lupus erythematosus typically manifests in childhood, but it also can be seen in adults, such as in our patient. Adult-onset BLE is rare but may be underrecognized or underreported in the literature.11 However, dermatologists should consider it in the differential diagnosis for any patient with a blaschkolinear eruption, as establishing the correct diagnosis is key to ensuring prompt and effective treatment for this rare inflammatory condition.
- Grönhagen CM, Fored CM, Granath F, et al. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164:1335-1341. doi:10.1111/j.1365-2133.2011.10272.x
- Requena C, Torrelo A, de Prada I, et al. Linear childhood cutaneous lupus erythematosus following Blaschko lines. J Eur Acad Dermatol Venereol. 2002;16:618-620. doi:10.1046/j.1468-3083.2002.00588.x
- Lim D, Hatami A, Kokta V, et al. Linear cutaneous lupus erythematosus in children-report of two cases and review of the literature: a case report. SAGE Open Med Case Rep. 2020;8:2050313x20979206. doi:10.1177/2050313X20979206
- Jin H, Zhang G, Zhou Y, et al. Old lines tell new tales: Blaschko linear lupus erythematosus. Autoimmun Rev. 2016;15:291-306. doi:10.1016/j.autrev.2015.11.014
- Yu S, Yu H-S. A patient with subacute cutaneous lupus erythematosus along Blaschko lines: implications for the role of keratinocytes in lupus erythematosus. Dermatologica Sinica. 2016;34:144-147. doi:10.1016/j.dsi.2015.12.002
- Kouzak SS, Mendes MST, Costa IMC. Cutaneous mosaicisms: concepts, patterns and classifications. An Bras Dermatol. 2013;88:507-517. doi:10.1590/abd1806-4841.20132015
- Liu W, Vano-Galvan S, Liu J-W, et al. Pigmented linear discoid lupus erythematosus following the lines of Blaschko: a retrospective study of a Chinese series. Indian J Dermatol Venereol Leprol. 2020;86:359-365. doi:10.4103/ijdvl.IJDVL_341_19
- O’Brien JC, Chong BF. Not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Invest Dermatol Symp Proc. 2017;18:S69-S74. doi:10.1016/j.jisp.2016.09.001
- Curtiss P, Walker AM, Chong BF. A systematic review of the progression of cutaneous lupus to systemic lupus erythematosus. Front Immunol. 2022:13:866319. doi:10.3389/fimmu.2022.866319
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. doi:10.1016/j.berh.2013.07.008
- Milosavljevic K, Fibeger E, Virata AR. A case of linear cutaneous lupus erythematosus in a 55-year-old woman. Am J Case Rep. 2020;21:E921495. doi:10.12659/AJCR.921495
- Grönhagen CM, Fored CM, Granath F, et al. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164:1335-1341. doi:10.1111/j.1365-2133.2011.10272.x
- Requena C, Torrelo A, de Prada I, et al. Linear childhood cutaneous lupus erythematosus following Blaschko lines. J Eur Acad Dermatol Venereol. 2002;16:618-620. doi:10.1046/j.1468-3083.2002.00588.x
- Lim D, Hatami A, Kokta V, et al. Linear cutaneous lupus erythematosus in children-report of two cases and review of the literature: a case report. SAGE Open Med Case Rep. 2020;8:2050313x20979206. doi:10.1177/2050313X20979206
- Jin H, Zhang G, Zhou Y, et al. Old lines tell new tales: Blaschko linear lupus erythematosus. Autoimmun Rev. 2016;15:291-306. doi:10.1016/j.autrev.2015.11.014
- Yu S, Yu H-S. A patient with subacute cutaneous lupus erythematosus along Blaschko lines: implications for the role of keratinocytes in lupus erythematosus. Dermatologica Sinica. 2016;34:144-147. doi:10.1016/j.dsi.2015.12.002
- Kouzak SS, Mendes MST, Costa IMC. Cutaneous mosaicisms: concepts, patterns and classifications. An Bras Dermatol. 2013;88:507-517. doi:10.1590/abd1806-4841.20132015
- Liu W, Vano-Galvan S, Liu J-W, et al. Pigmented linear discoid lupus erythematosus following the lines of Blaschko: a retrospective study of a Chinese series. Indian J Dermatol Venereol Leprol. 2020;86:359-365. doi:10.4103/ijdvl.IJDVL_341_19
- O’Brien JC, Chong BF. Not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Invest Dermatol Symp Proc. 2017;18:S69-S74. doi:10.1016/j.jisp.2016.09.001
- Curtiss P, Walker AM, Chong BF. A systematic review of the progression of cutaneous lupus to systemic lupus erythematosus. Front Immunol. 2022:13:866319. doi:10.3389/fimmu.2022.866319
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. doi:10.1016/j.berh.2013.07.008
- Milosavljevic K, Fibeger E, Virata AR. A case of linear cutaneous lupus erythematosus in a 55-year-old woman. Am J Case Rep. 2020;21:E921495. doi:10.12659/AJCR.921495
Practice Points
- Blaschkolinear lupus erythematosus (BLE), an exceedingly rare subtype of chronic cutaneous lupus erythematosus, usually presents during childhood as linear plaques along the lines of Blaschko.
- It is important to consider linear lichen planus in patients with a blaschkolinear eruption, as the clinical manifestations are similar but there are differences in histopathology, management, prognosis, and long-term follow-up.
- Serial monitoring is indicated in patients with BLE given the potential for progression to systemic lupus erythematosus, which may be delayed with early use of hydroxychloroquine.
Commentary: Targeted Therapies in PsA, September 2024
The question of whether effective targeted therapies for psoriasis reduce the incidence or "prevent" psoriatic arthritis (PsA) has increasingly become a topic of interest. Also of interest is whether there are differences between different drug classes for treating psoriasis and PsA. To evaluate whether there is a difference between patients treated with interleukin (IL)-23 vs IL-12/23 inhibitors, Tsai and colleagues conducted a retrospective cohort study that included the propensity score–matched data of patients with psoriasis from the TriNetX database who were treated with either IL-23 inhibitors (n = 2142) or IL-12/23 inhibitors (n = 2142). Patients treated with IL-23 inhibitors vs IL-12/23 inhibitors demonstrated no significant difference in the risk for PsA (hazard ratio 0.96; P = .812) and cumulative incidence of PsA (P = .812). Given the many drawbacks of administrative database-based retrospective studies, I would ideally like to see prospective studies conducted to evaluate the differential risk for PsA between targeted therapies for psoriasis. However, patients can be assured that the beneficial effect, if any, is likely to be similar between these two drug classes in regard to PsA prevention.
One important question when treating patients with PsA with biologic therapies is whether treatment with methotrexate needs to be continued. In a post hoc analysis of phase 3 trials (BE OPTIMAL, BE COMPLETE, and BE VITAL) that included patients with PsA who were biologic-naive (n = 852) or had an incomplete response to a tumor necrosis factor (TNF) inhibitor (n = 400), McInnes and colleagues evaluated the efficacy and safety of bimekizumab in patients with active PsA with or without concomitant methotrexate treatment at baseline. They demonstrated that through week 52, nearly half of the patients receiving bimekizumab with or without methotrexate achieved a ≥50% improvement in American College of Rheumatology response (biologic-naive ~55%; TNF inhibitor ~48-56%) and minimal disease activity (biologic-naive ~55%; TNF inhibitor ~47%). Thus, bimekizumab demonstrated similar sustained efficacy for 52 weeks, regardless of concomitant methotrexate use. Therefore, concomitant treatment with methotrexate may not be necessary when treating PsA patients with bimekizumab.
Nonpharmacologic interventions, such as diet and exercise, are likely to be of benefit to PsA patients, but studies on such therapies are lacking. In a cross-sectional study that enrolled 279 patients with PsA and 76 patients with psoriasis, Katsimbri and colleagues showed that patients reporting high vs low levels of exercise had significantly lower median values of Disease Activity Index for PsA and erythrocyte sedimentation rate, and fewer tender and swollen joints. Similarly, high vs low adherence to the Mediterranean diet was associated with a lower Psoriasis Area and Severity Index and body surface area affected by psoriasis. Thus, exercise and a Mediterranean diet may improve disease activity outcomes in PsA, and may be an important adjunct to immunomodulatory therapy. However, prospective interventional trials are required.
Finally, a study evaluated whether the initiation of targeted therapies, such as biologics, led to a decrease in the use of other arthritis-related treatments and healthcare use in PsA. Using data from the French health insurance database, Pina Vegas and colleagues evaluated the difference in the proportion of users of associated treatments, hospitalizations, and sick leaves between 6 months before and 3-9 months after treatment initiation. In a cohort of 9793 patients, they found that first-line targeted therapy significantly reduced the use of nonsteroidal anti-inflammatory drugs (NSAID; −15%), prednisone (−9%), methotrexate (−15%), and mood disorder treatments (−2%), and lowered the rate of hospitalizations (−12%) and sick leave (−4%; all P < 10-4). TNF inhibitors showed greater reductions in NSAID and prednisone use compared with IL-17 inhibitors, with similar outcomes for IL-12/23 inhibitors.
The question of whether effective targeted therapies for psoriasis reduce the incidence or "prevent" psoriatic arthritis (PsA) has increasingly become a topic of interest. Also of interest is whether there are differences between different drug classes for treating psoriasis and PsA. To evaluate whether there is a difference between patients treated with interleukin (IL)-23 vs IL-12/23 inhibitors, Tsai and colleagues conducted a retrospective cohort study that included the propensity score–matched data of patients with psoriasis from the TriNetX database who were treated with either IL-23 inhibitors (n = 2142) or IL-12/23 inhibitors (n = 2142). Patients treated with IL-23 inhibitors vs IL-12/23 inhibitors demonstrated no significant difference in the risk for PsA (hazard ratio 0.96; P = .812) and cumulative incidence of PsA (P = .812). Given the many drawbacks of administrative database-based retrospective studies, I would ideally like to see prospective studies conducted to evaluate the differential risk for PsA between targeted therapies for psoriasis. However, patients can be assured that the beneficial effect, if any, is likely to be similar between these two drug classes in regard to PsA prevention.
One important question when treating patients with PsA with biologic therapies is whether treatment with methotrexate needs to be continued. In a post hoc analysis of phase 3 trials (BE OPTIMAL, BE COMPLETE, and BE VITAL) that included patients with PsA who were biologic-naive (n = 852) or had an incomplete response to a tumor necrosis factor (TNF) inhibitor (n = 400), McInnes and colleagues evaluated the efficacy and safety of bimekizumab in patients with active PsA with or without concomitant methotrexate treatment at baseline. They demonstrated that through week 52, nearly half of the patients receiving bimekizumab with or without methotrexate achieved a ≥50% improvement in American College of Rheumatology response (biologic-naive ~55%; TNF inhibitor ~48-56%) and minimal disease activity (biologic-naive ~55%; TNF inhibitor ~47%). Thus, bimekizumab demonstrated similar sustained efficacy for 52 weeks, regardless of concomitant methotrexate use. Therefore, concomitant treatment with methotrexate may not be necessary when treating PsA patients with bimekizumab.
Nonpharmacologic interventions, such as diet and exercise, are likely to be of benefit to PsA patients, but studies on such therapies are lacking. In a cross-sectional study that enrolled 279 patients with PsA and 76 patients with psoriasis, Katsimbri and colleagues showed that patients reporting high vs low levels of exercise had significantly lower median values of Disease Activity Index for PsA and erythrocyte sedimentation rate, and fewer tender and swollen joints. Similarly, high vs low adherence to the Mediterranean diet was associated with a lower Psoriasis Area and Severity Index and body surface area affected by psoriasis. Thus, exercise and a Mediterranean diet may improve disease activity outcomes in PsA, and may be an important adjunct to immunomodulatory therapy. However, prospective interventional trials are required.
Finally, a study evaluated whether the initiation of targeted therapies, such as biologics, led to a decrease in the use of other arthritis-related treatments and healthcare use in PsA. Using data from the French health insurance database, Pina Vegas and colleagues evaluated the difference in the proportion of users of associated treatments, hospitalizations, and sick leaves between 6 months before and 3-9 months after treatment initiation. In a cohort of 9793 patients, they found that first-line targeted therapy significantly reduced the use of nonsteroidal anti-inflammatory drugs (NSAID; −15%), prednisone (−9%), methotrexate (−15%), and mood disorder treatments (−2%), and lowered the rate of hospitalizations (−12%) and sick leave (−4%; all P < 10-4). TNF inhibitors showed greater reductions in NSAID and prednisone use compared with IL-17 inhibitors, with similar outcomes for IL-12/23 inhibitors.
The question of whether effective targeted therapies for psoriasis reduce the incidence or "prevent" psoriatic arthritis (PsA) has increasingly become a topic of interest. Also of interest is whether there are differences between different drug classes for treating psoriasis and PsA. To evaluate whether there is a difference between patients treated with interleukin (IL)-23 vs IL-12/23 inhibitors, Tsai and colleagues conducted a retrospective cohort study that included the propensity score–matched data of patients with psoriasis from the TriNetX database who were treated with either IL-23 inhibitors (n = 2142) or IL-12/23 inhibitors (n = 2142). Patients treated with IL-23 inhibitors vs IL-12/23 inhibitors demonstrated no significant difference in the risk for PsA (hazard ratio 0.96; P = .812) and cumulative incidence of PsA (P = .812). Given the many drawbacks of administrative database-based retrospective studies, I would ideally like to see prospective studies conducted to evaluate the differential risk for PsA between targeted therapies for psoriasis. However, patients can be assured that the beneficial effect, if any, is likely to be similar between these two drug classes in regard to PsA prevention.
One important question when treating patients with PsA with biologic therapies is whether treatment with methotrexate needs to be continued. In a post hoc analysis of phase 3 trials (BE OPTIMAL, BE COMPLETE, and BE VITAL) that included patients with PsA who were biologic-naive (n = 852) or had an incomplete response to a tumor necrosis factor (TNF) inhibitor (n = 400), McInnes and colleagues evaluated the efficacy and safety of bimekizumab in patients with active PsA with or without concomitant methotrexate treatment at baseline. They demonstrated that through week 52, nearly half of the patients receiving bimekizumab with or without methotrexate achieved a ≥50% improvement in American College of Rheumatology response (biologic-naive ~55%; TNF inhibitor ~48-56%) and minimal disease activity (biologic-naive ~55%; TNF inhibitor ~47%). Thus, bimekizumab demonstrated similar sustained efficacy for 52 weeks, regardless of concomitant methotrexate use. Therefore, concomitant treatment with methotrexate may not be necessary when treating PsA patients with bimekizumab.
Nonpharmacologic interventions, such as diet and exercise, are likely to be of benefit to PsA patients, but studies on such therapies are lacking. In a cross-sectional study that enrolled 279 patients with PsA and 76 patients with psoriasis, Katsimbri and colleagues showed that patients reporting high vs low levels of exercise had significantly lower median values of Disease Activity Index for PsA and erythrocyte sedimentation rate, and fewer tender and swollen joints. Similarly, high vs low adherence to the Mediterranean diet was associated with a lower Psoriasis Area and Severity Index and body surface area affected by psoriasis. Thus, exercise and a Mediterranean diet may improve disease activity outcomes in PsA, and may be an important adjunct to immunomodulatory therapy. However, prospective interventional trials are required.
Finally, a study evaluated whether the initiation of targeted therapies, such as biologics, led to a decrease in the use of other arthritis-related treatments and healthcare use in PsA. Using data from the French health insurance database, Pina Vegas and colleagues evaluated the difference in the proportion of users of associated treatments, hospitalizations, and sick leaves between 6 months before and 3-9 months after treatment initiation. In a cohort of 9793 patients, they found that first-line targeted therapy significantly reduced the use of nonsteroidal anti-inflammatory drugs (NSAID; −15%), prednisone (−9%), methotrexate (−15%), and mood disorder treatments (−2%), and lowered the rate of hospitalizations (−12%) and sick leave (−4%; all P < 10-4). TNF inhibitors showed greater reductions in NSAID and prednisone use compared with IL-17 inhibitors, with similar outcomes for IL-12/23 inhibitors.
Necrotic Papules in a Pediatric Patient
The Diagnosis: Pityriasis Lichenoides et Varioliformis Acuta
Sectioned punch biopsies were performed on the patient’s right arm. Histopathology showed acanthosis and parakeratosis in the epidermis, with vacuolar degeneration and dyskeratosis in the basal layer. Dermal changes included extravasated red blood cells in the papillary dermis as well as perivascular lymphocytic infiltrates in both the papillary and reticular dermis (Figure). Direct immunofluorescence of a perilesional biopsy using anti–human IgG, IgM, IgA, C3, and fibrin conjugates showed no findings of immune deposition. Biopsy results were consistent with pityriasis lichenoides et varioliformis acuta (PLEVA), and the patient was treated with a 5-day course of oral azithromycin, triamcinolone ointment 0.1% twice daily, and phototherapy with narrowband UVB 3 times weekly. Rapid improvement was noted at 2-month follow-up.
Pityriasis lichenoides et varioliformis acuta is a form of pityriasis lichenoides, a group of inflammatory dermatoses that are characterized clinically by successive crops of morphologically diverse lesions. Epidemiologic studies have shown a slight male predominance. It primarily affects children and young adults, with peak ages of 8 and 32 years in pediatric and adult populations, respectively.1
The pathogenesis of PLEVA remains unclear. An abnormal immune response to Toxoplasma, Epstein-Barr virus, HIV, and other pathogens has been suggested based on serologic evidence of concurrent disease activity with the onset of lesions as well as cutaneous improvement in some patients after treatment of the infection.1 A T-cell lymphoproliferative etiology also has been considered based on histopathologic similarities between PLEVA and lymphomatoid papulosis (LyP) as well as findings of clonality in T-cell receptor gene rearrangement in many patients.1,2 Some clinicians consider LyP and PLEVA as separate entities on one disease spectrum.
Eruptions of PLEVA tend to favor the trunk and proximal extremities. Lesions may begin as macules measuring 2 to 3 mm in diameter that quickly evolve into papules with fine scale that remains attached centrally. Ulcerations with hemorrhagic crusts also may be noted as the lesions progress in stage. The rash may persist for weeks to years, and overlapping crops of macules and papules at varying stages of development may be seen in the same patient.1
Histopathologic findings of PLEVA include spongiosis, dyskeratosis, parakeratosis, and focal keratinocyte necrosis within the epidermis, as well as vacuolar degeneration of the basal layer. Lymphocyte and erythrocyte extravasation may extend into the epidermis. Dermal findings may include edema and wedge-shaped perivascular lymphocytic infiltrates extending into the reticular dermis.1

Important differential diagnoses to consider include LyP, mycosis fungoides (MF), pemphigus foliaceus, and varicella. Lymphomatoid papulosis is a benign CD30+ lymphoproliferative disorder that is characterized by an indolent course of recurrent, often self-resolving papules that occur most frequently on the trunk, arms, and legs of older patients. There are several histologic subtypes of LyP, but the most common (type A) may manifest with wedge-shaped perivascular lymphocytic infiltrates in the dermis, similar to PLEVA. T-cell receptor gene rearrangement studies characteristically reveal clonality in LyP, and clonality has been reported in PLEVA. However, LyP demonstrates a higher cytologic grade and lacks the characteristic parakeratotic scale and superficial dermal microhemorrhage of PLEVA.3
Mycosis fungoides is a malignant lymphoproliferative disorder that is characterized by an indolent clinical course of persistent patches, plaques, or tumors of various sizes that often manifest in non–sun-exposed areas of the skin. Early stages of MF are difficult to detect histologically, but biopsies may show atypical lymphocytes with hyperchromatic, irregularly contoured nuclei arranged along the basal layer of the epidermis. Epidermal aggregates of atypical lymphocytes (also known as Pautrier microabscesses) are considered highly specific for MF. T-cell receptor and immunopathologic studies also are important adjuncts in the diagnosis of MF.4
Pemphigus foliaceus is an autoimmune blistering disease caused by antibodies directed against desmoglein 1, which is found in the granular layer of the epidermis. It manifests with a subtle onset of scattered crusted lesions in the seborrheic areas, such as the scalp, face, chest, and upper back. Histopathologic findings of early blisters may include acantholysis and dyskeratosis in the stratum granulosum as well as vacuolization of the granular layer. The blisters may coalesce into superficial bullae containing fibrin and neutrophils. Immunofluorescence studies that demonstrate intraepidermal C3 and IgG deposition are key to the diagnosis of pemphigus.5
Varicella (also known as chickenpox) manifests with crops of vesicles on an erythematous base in a centripetal distribution favoring the trunk and proximal extremities. It often is preceded by prodromal fever, malaise, and myalgia. Histopathologic evaluation of varicella is uncommon but may reveal acantholysis, multinucleation, and nuclear margination of keratinocytes. Viral culture or nucleic acid amplification testing of lesions can be used to verify the diagnosis.6
Most cases of PLEVA resolve without intervention.7 Treatment is directed at speeding recovery, providing symptomatic relief, and limiting permanent sequelae. Topical steroids often are used to alleviate inflammation and pruritus. Systemic antibiotics such as doxycycline, minocycline, and erythromycin have been used for their anti-inflammatory properties. Phototherapy of various wavelengths, including broadband and narrowband UVB as well as psoralen plus UVA, have led to improvements in affected patients. Refractory disease may warrant consideration of therapy with methotrexate, acitretin, dapsone, or cyclosporine.7
There have been rare reports of PLEVA evolving into its potentially lethal variant, febrile ulceronecrotic Mucha-Habermann disease, which is differentiated by the presence of systemic manifestations, including high fever, sore throat, diarrhea, central nervous system symptoms, abdominal pain, interstitial pneumonitis, splenomegaly, arthritis, sepsis, megaloblastic anemia, or conjunctival ulcers. The orogenital mucosa may be affected. Cutaneous lesions may rapidly progress to large, generalized, coalescent ulcers with necrotic crusts and vasculitic features on biopsy.8 Malignant transformation of PLEVA into LyP or MF rarely may occur and warrants continued follow-up of unresolved lesions.9
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-572. doi:10.1016/j.jaad.2005.07.058
- Teklehaimanot F, Gade A, Rubenstein R. Pityriasis lichenoides et varioliformis acuta (PLEVA). In: StatPearls. StatPearls Publishing; 2023.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73. doi:10.1111/jdv.15931
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Lepe K, Yarrarapu SNS, Zito PM. Pemphigus foliaceus. In: StatPearls. StatPearls Publishing; 2023.
- Ayoade F, Kumar S. Varicella zoster (chickenpox). In: StatPearls. StatPearls Publishing; 2023.
- Bellinato F, Maurelli M, Gisondi P, et al. A systematic review of treatments for pityriasis lichenoides. J Eur Acad Dermatol Venereol. 2019;33:2039-2049. doi:10.1111/jdv.15813
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738. doi:10.1111/ijd.13195
- Thomson KF, Whittaker SJ, Russell-Jones R, et al. Childhood cutaneous T-cell lymphoma in association with pityriasis lichenoides chronica. Br J Dermatol. 1999;141:1136-1152. doi:10.1046/j.1365-2133.1999.03232.x
The Diagnosis: Pityriasis Lichenoides et Varioliformis Acuta
Sectioned punch biopsies were performed on the patient’s right arm. Histopathology showed acanthosis and parakeratosis in the epidermis, with vacuolar degeneration and dyskeratosis in the basal layer. Dermal changes included extravasated red blood cells in the papillary dermis as well as perivascular lymphocytic infiltrates in both the papillary and reticular dermis (Figure). Direct immunofluorescence of a perilesional biopsy using anti–human IgG, IgM, IgA, C3, and fibrin conjugates showed no findings of immune deposition. Biopsy results were consistent with pityriasis lichenoides et varioliformis acuta (PLEVA), and the patient was treated with a 5-day course of oral azithromycin, triamcinolone ointment 0.1% twice daily, and phototherapy with narrowband UVB 3 times weekly. Rapid improvement was noted at 2-month follow-up.
Pityriasis lichenoides et varioliformis acuta is a form of pityriasis lichenoides, a group of inflammatory dermatoses that are characterized clinically by successive crops of morphologically diverse lesions. Epidemiologic studies have shown a slight male predominance. It primarily affects children and young adults, with peak ages of 8 and 32 years in pediatric and adult populations, respectively.1
The pathogenesis of PLEVA remains unclear. An abnormal immune response to Toxoplasma, Epstein-Barr virus, HIV, and other pathogens has been suggested based on serologic evidence of concurrent disease activity with the onset of lesions as well as cutaneous improvement in some patients after treatment of the infection.1 A T-cell lymphoproliferative etiology also has been considered based on histopathologic similarities between PLEVA and lymphomatoid papulosis (LyP) as well as findings of clonality in T-cell receptor gene rearrangement in many patients.1,2 Some clinicians consider LyP and PLEVA as separate entities on one disease spectrum.
Eruptions of PLEVA tend to favor the trunk and proximal extremities. Lesions may begin as macules measuring 2 to 3 mm in diameter that quickly evolve into papules with fine scale that remains attached centrally. Ulcerations with hemorrhagic crusts also may be noted as the lesions progress in stage. The rash may persist for weeks to years, and overlapping crops of macules and papules at varying stages of development may be seen in the same patient.1
Histopathologic findings of PLEVA include spongiosis, dyskeratosis, parakeratosis, and focal keratinocyte necrosis within the epidermis, as well as vacuolar degeneration of the basal layer. Lymphocyte and erythrocyte extravasation may extend into the epidermis. Dermal findings may include edema and wedge-shaped perivascular lymphocytic infiltrates extending into the reticular dermis.1

Important differential diagnoses to consider include LyP, mycosis fungoides (MF), pemphigus foliaceus, and varicella. Lymphomatoid papulosis is a benign CD30+ lymphoproliferative disorder that is characterized by an indolent course of recurrent, often self-resolving papules that occur most frequently on the trunk, arms, and legs of older patients. There are several histologic subtypes of LyP, but the most common (type A) may manifest with wedge-shaped perivascular lymphocytic infiltrates in the dermis, similar to PLEVA. T-cell receptor gene rearrangement studies characteristically reveal clonality in LyP, and clonality has been reported in PLEVA. However, LyP demonstrates a higher cytologic grade and lacks the characteristic parakeratotic scale and superficial dermal microhemorrhage of PLEVA.3
Mycosis fungoides is a malignant lymphoproliferative disorder that is characterized by an indolent clinical course of persistent patches, plaques, or tumors of various sizes that often manifest in non–sun-exposed areas of the skin. Early stages of MF are difficult to detect histologically, but biopsies may show atypical lymphocytes with hyperchromatic, irregularly contoured nuclei arranged along the basal layer of the epidermis. Epidermal aggregates of atypical lymphocytes (also known as Pautrier microabscesses) are considered highly specific for MF. T-cell receptor and immunopathologic studies also are important adjuncts in the diagnosis of MF.4
Pemphigus foliaceus is an autoimmune blistering disease caused by antibodies directed against desmoglein 1, which is found in the granular layer of the epidermis. It manifests with a subtle onset of scattered crusted lesions in the seborrheic areas, such as the scalp, face, chest, and upper back. Histopathologic findings of early blisters may include acantholysis and dyskeratosis in the stratum granulosum as well as vacuolization of the granular layer. The blisters may coalesce into superficial bullae containing fibrin and neutrophils. Immunofluorescence studies that demonstrate intraepidermal C3 and IgG deposition are key to the diagnosis of pemphigus.5
Varicella (also known as chickenpox) manifests with crops of vesicles on an erythematous base in a centripetal distribution favoring the trunk and proximal extremities. It often is preceded by prodromal fever, malaise, and myalgia. Histopathologic evaluation of varicella is uncommon but may reveal acantholysis, multinucleation, and nuclear margination of keratinocytes. Viral culture or nucleic acid amplification testing of lesions can be used to verify the diagnosis.6
Most cases of PLEVA resolve without intervention.7 Treatment is directed at speeding recovery, providing symptomatic relief, and limiting permanent sequelae. Topical steroids often are used to alleviate inflammation and pruritus. Systemic antibiotics such as doxycycline, minocycline, and erythromycin have been used for their anti-inflammatory properties. Phototherapy of various wavelengths, including broadband and narrowband UVB as well as psoralen plus UVA, have led to improvements in affected patients. Refractory disease may warrant consideration of therapy with methotrexate, acitretin, dapsone, or cyclosporine.7
There have been rare reports of PLEVA evolving into its potentially lethal variant, febrile ulceronecrotic Mucha-Habermann disease, which is differentiated by the presence of systemic manifestations, including high fever, sore throat, diarrhea, central nervous system symptoms, abdominal pain, interstitial pneumonitis, splenomegaly, arthritis, sepsis, megaloblastic anemia, or conjunctival ulcers. The orogenital mucosa may be affected. Cutaneous lesions may rapidly progress to large, generalized, coalescent ulcers with necrotic crusts and vasculitic features on biopsy.8 Malignant transformation of PLEVA into LyP or MF rarely may occur and warrants continued follow-up of unresolved lesions.9
The Diagnosis: Pityriasis Lichenoides et Varioliformis Acuta
Sectioned punch biopsies were performed on the patient’s right arm. Histopathology showed acanthosis and parakeratosis in the epidermis, with vacuolar degeneration and dyskeratosis in the basal layer. Dermal changes included extravasated red blood cells in the papillary dermis as well as perivascular lymphocytic infiltrates in both the papillary and reticular dermis (Figure). Direct immunofluorescence of a perilesional biopsy using anti–human IgG, IgM, IgA, C3, and fibrin conjugates showed no findings of immune deposition. Biopsy results were consistent with pityriasis lichenoides et varioliformis acuta (PLEVA), and the patient was treated with a 5-day course of oral azithromycin, triamcinolone ointment 0.1% twice daily, and phototherapy with narrowband UVB 3 times weekly. Rapid improvement was noted at 2-month follow-up.
Pityriasis lichenoides et varioliformis acuta is a form of pityriasis lichenoides, a group of inflammatory dermatoses that are characterized clinically by successive crops of morphologically diverse lesions. Epidemiologic studies have shown a slight male predominance. It primarily affects children and young adults, with peak ages of 8 and 32 years in pediatric and adult populations, respectively.1
The pathogenesis of PLEVA remains unclear. An abnormal immune response to Toxoplasma, Epstein-Barr virus, HIV, and other pathogens has been suggested based on serologic evidence of concurrent disease activity with the onset of lesions as well as cutaneous improvement in some patients after treatment of the infection.1 A T-cell lymphoproliferative etiology also has been considered based on histopathologic similarities between PLEVA and lymphomatoid papulosis (LyP) as well as findings of clonality in T-cell receptor gene rearrangement in many patients.1,2 Some clinicians consider LyP and PLEVA as separate entities on one disease spectrum.
Eruptions of PLEVA tend to favor the trunk and proximal extremities. Lesions may begin as macules measuring 2 to 3 mm in diameter that quickly evolve into papules with fine scale that remains attached centrally. Ulcerations with hemorrhagic crusts also may be noted as the lesions progress in stage. The rash may persist for weeks to years, and overlapping crops of macules and papules at varying stages of development may be seen in the same patient.1
Histopathologic findings of PLEVA include spongiosis, dyskeratosis, parakeratosis, and focal keratinocyte necrosis within the epidermis, as well as vacuolar degeneration of the basal layer. Lymphocyte and erythrocyte extravasation may extend into the epidermis. Dermal findings may include edema and wedge-shaped perivascular lymphocytic infiltrates extending into the reticular dermis.1

Important differential diagnoses to consider include LyP, mycosis fungoides (MF), pemphigus foliaceus, and varicella. Lymphomatoid papulosis is a benign CD30+ lymphoproliferative disorder that is characterized by an indolent course of recurrent, often self-resolving papules that occur most frequently on the trunk, arms, and legs of older patients. There are several histologic subtypes of LyP, but the most common (type A) may manifest with wedge-shaped perivascular lymphocytic infiltrates in the dermis, similar to PLEVA. T-cell receptor gene rearrangement studies characteristically reveal clonality in LyP, and clonality has been reported in PLEVA. However, LyP demonstrates a higher cytologic grade and lacks the characteristic parakeratotic scale and superficial dermal microhemorrhage of PLEVA.3
Mycosis fungoides is a malignant lymphoproliferative disorder that is characterized by an indolent clinical course of persistent patches, plaques, or tumors of various sizes that often manifest in non–sun-exposed areas of the skin. Early stages of MF are difficult to detect histologically, but biopsies may show atypical lymphocytes with hyperchromatic, irregularly contoured nuclei arranged along the basal layer of the epidermis. Epidermal aggregates of atypical lymphocytes (also known as Pautrier microabscesses) are considered highly specific for MF. T-cell receptor and immunopathologic studies also are important adjuncts in the diagnosis of MF.4
Pemphigus foliaceus is an autoimmune blistering disease caused by antibodies directed against desmoglein 1, which is found in the granular layer of the epidermis. It manifests with a subtle onset of scattered crusted lesions in the seborrheic areas, such as the scalp, face, chest, and upper back. Histopathologic findings of early blisters may include acantholysis and dyskeratosis in the stratum granulosum as well as vacuolization of the granular layer. The blisters may coalesce into superficial bullae containing fibrin and neutrophils. Immunofluorescence studies that demonstrate intraepidermal C3 and IgG deposition are key to the diagnosis of pemphigus.5
Varicella (also known as chickenpox) manifests with crops of vesicles on an erythematous base in a centripetal distribution favoring the trunk and proximal extremities. It often is preceded by prodromal fever, malaise, and myalgia. Histopathologic evaluation of varicella is uncommon but may reveal acantholysis, multinucleation, and nuclear margination of keratinocytes. Viral culture or nucleic acid amplification testing of lesions can be used to verify the diagnosis.6
Most cases of PLEVA resolve without intervention.7 Treatment is directed at speeding recovery, providing symptomatic relief, and limiting permanent sequelae. Topical steroids often are used to alleviate inflammation and pruritus. Systemic antibiotics such as doxycycline, minocycline, and erythromycin have been used for their anti-inflammatory properties. Phototherapy of various wavelengths, including broadband and narrowband UVB as well as psoralen plus UVA, have led to improvements in affected patients. Refractory disease may warrant consideration of therapy with methotrexate, acitretin, dapsone, or cyclosporine.7
There have been rare reports of PLEVA evolving into its potentially lethal variant, febrile ulceronecrotic Mucha-Habermann disease, which is differentiated by the presence of systemic manifestations, including high fever, sore throat, diarrhea, central nervous system symptoms, abdominal pain, interstitial pneumonitis, splenomegaly, arthritis, sepsis, megaloblastic anemia, or conjunctival ulcers. The orogenital mucosa may be affected. Cutaneous lesions may rapidly progress to large, generalized, coalescent ulcers with necrotic crusts and vasculitic features on biopsy.8 Malignant transformation of PLEVA into LyP or MF rarely may occur and warrants continued follow-up of unresolved lesions.9
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-572. doi:10.1016/j.jaad.2005.07.058
- Teklehaimanot F, Gade A, Rubenstein R. Pityriasis lichenoides et varioliformis acuta (PLEVA). In: StatPearls. StatPearls Publishing; 2023.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73. doi:10.1111/jdv.15931
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Lepe K, Yarrarapu SNS, Zito PM. Pemphigus foliaceus. In: StatPearls. StatPearls Publishing; 2023.
- Ayoade F, Kumar S. Varicella zoster (chickenpox). In: StatPearls. StatPearls Publishing; 2023.
- Bellinato F, Maurelli M, Gisondi P, et al. A systematic review of treatments for pityriasis lichenoides. J Eur Acad Dermatol Venereol. 2019;33:2039-2049. doi:10.1111/jdv.15813
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738. doi:10.1111/ijd.13195
- Thomson KF, Whittaker SJ, Russell-Jones R, et al. Childhood cutaneous T-cell lymphoma in association with pityriasis lichenoides chronica. Br J Dermatol. 1999;141:1136-1152. doi:10.1046/j.1365-2133.1999.03232.x
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-572. doi:10.1016/j.jaad.2005.07.058
- Teklehaimanot F, Gade A, Rubenstein R. Pityriasis lichenoides et varioliformis acuta (PLEVA). In: StatPearls. StatPearls Publishing; 2023.
- Martinez-Cabriales SA, Walsh S, Sade S, et al. Lymphomatoid papulosis: an update and review. J Eur Acad Dermatol Venereol. 2020;34:59-73. doi:10.1111/jdv.15931
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Lepe K, Yarrarapu SNS, Zito PM. Pemphigus foliaceus. In: StatPearls. StatPearls Publishing; 2023.
- Ayoade F, Kumar S. Varicella zoster (chickenpox). In: StatPearls. StatPearls Publishing; 2023.
- Bellinato F, Maurelli M, Gisondi P, et al. A systematic review of treatments for pityriasis lichenoides. J Eur Acad Dermatol Venereol. 2019;33:2039-2049. doi:10.1111/jdv.15813
- Nofal A, Assaf M, Alakad R, et al. Febrile ulceronecrotic Mucha-Habermann disease: proposed diagnostic criteria and therapeutic evaluation. Int J Dermatol. 2016;55:729-738. doi:10.1111/ijd.13195
- Thomson KF, Whittaker SJ, Russell-Jones R, et al. Childhood cutaneous T-cell lymphoma in association with pityriasis lichenoides chronica. Br J Dermatol. 1999;141:1136-1152. doi:10.1046/j.1365-2133.1999.03232.x
A 7-year-old boy was referred to the dermatology clinic for evaluation of a diffuse pruritic rash of 3 months’ duration. The rash began as scant erythematous papules on the face, and crops of similar lesions later erupted on the trunk, arms, and legs. He was treated previously by a pediatrician for scabies with topical permethrin followed by 2 doses of oral ivermectin 200 μg/kg without improvement. Physical examination revealed innumerable erythematous macules and papules with centrally adherent scaling distributed on the trunk, arms, and legs, as well as scant necrotic papules with a hemorrhagic crust and a peripheral rim of scale.

Commentary: Migraine and Lifestyle Factors, September 2024
Migraine pathophysiology has been shown to be associated with vascular and inflammatory processes. Diet and lifestyle can have an effect on an individual's inflammatory process, and research regarding the steps between these factors and inflammation is vague and nonspecific. The Dietary Inflammation Score (DIS), which is calculated on the basis of a questionnaire, is used to score the inflammatory potential of an individual's diet. The Dietary and Lifestyle Inflammation Score (DLIS) includes the DIS questions, and also incorporates body mass index (BMI), physical activity, smoking, and alcohol consumption. A recent study, based on a secondary analysis of previous data, examined the correlation between migraine and DIS and DLIS among 285 women, 40% of whom had a chronic migraine diagnosis. Results published in Scientific Reports in July 2024 noted that participants with chronic migraine had a significantly higher DIS and DLIS than those who were not diagnosed with chronic migraine. It is important to note that migraine-associated inflammation can also result from genetic factors. A previous study, published in 2023 in Nature Genetics, described a correlation between genetic markers of inflammatory disorders, such as endometriosis, asthma, and migraine.1 These results, consistent with our current understanding of the genetic contribution to migraine risk, emphasize that lifestyle modifications alone are not usually adequate for complete management of migraines.
Patients who experience chronic migraine may be inclined to reduce their time spent exercising and engaging in physical activity, as these activities can exacerbate migraine symptoms. Additionally, after recovering from a migraine, patients often need to catch up on tasks and responsibilities, which can squeeze out time for physical activity and exercise (often considered luxuries that can be done during leisure time). Results of a small cross-sectional retrospective study published in Scientific Reports in 2024 suggested a correlation between daily walking steps and response to calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb). According to the study, which included 22 patients who were diagnosed with migraine and treated with CGRP mAb, patients who experienced an improvement of their migraine symptoms also increased their average daily steps by almost 1000 steps per day. The authors suggested that steps can be used as a marker of treatment response in migraine.
Screen time is often blamed as a cause for a number of different ailments, including obesity, anxiety, depression, insomnia, and migraine. An article published in June 2024 in European Journal of Pain described results of a meta-analysis examining the association between sedentary lifestyle and migraine. The authors noted that time spent watching television could be causally associated with an increased risk for migraine.2 Another study, with results published in July 2024 in The Journal of Headache and Pain, examined the relationship between migraine and leisure screen time. The researchers used data from 661,399 European individuals from 53 studies to look at genetically predicted leisure screen time, rather than actual leisure screen time. They reported that genetically predicted leisure screen time was associated with a 27.7% increase in migraine risk. While the results are consistent with what is already widely accepted about screen time and migraine, the inclusion of genetic predisposition to screen time is interesting in suggesting that some underlying drive could be contributing to increased screen time among patients who have migraine.
The results of these studies reemphasize the importance of the link between lifestyle factors and migraine but warn against oversimplifying the correlation. There is a bidirectional relationship between migraine and inflammation. We know that inflammation is mediated by diet as well as physical activity. During a migraine, patients may turn to foods that have a high inflammatory potential. Furthermore, migraine can influence a person's inclination to participate in physical activity, as the pain and discomfort can make it difficult engage in exercise. During a migraine, patients may prefer sedentary activities. Screen time can be appealing or relaxing while recovering from a migraine. Genetic predisposition is an interesting additional contributor to this link. Acknowledging genetic predisposition to inflammation or sedentary activity can be a step in helping patients recognize that it could be challenging to overcome these genetically inherent drives or conditions, while providing encouragement regarding the potential benefits of doing so.
Additional References
1. Rahmioglu N, Mortlock S, Ghiasi M, et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat Genet. 2023;55(3):423-436. Doi: 10.1038/s41588-023-01323-z Source
2. Li P, Li J, Zhu H, et al. Causal effects of sedentary behaviours on the risk of migraine: A univariable and multivariable Mendelian randomization study. Eur J Pain. 2024 (Jun 4). Doi: 10.1002/ejp.2296 Source
Migraine pathophysiology has been shown to be associated with vascular and inflammatory processes. Diet and lifestyle can have an effect on an individual's inflammatory process, and research regarding the steps between these factors and inflammation is vague and nonspecific. The Dietary Inflammation Score (DIS), which is calculated on the basis of a questionnaire, is used to score the inflammatory potential of an individual's diet. The Dietary and Lifestyle Inflammation Score (DLIS) includes the DIS questions, and also incorporates body mass index (BMI), physical activity, smoking, and alcohol consumption. A recent study, based on a secondary analysis of previous data, examined the correlation between migraine and DIS and DLIS among 285 women, 40% of whom had a chronic migraine diagnosis. Results published in Scientific Reports in July 2024 noted that participants with chronic migraine had a significantly higher DIS and DLIS than those who were not diagnosed with chronic migraine. It is important to note that migraine-associated inflammation can also result from genetic factors. A previous study, published in 2023 in Nature Genetics, described a correlation between genetic markers of inflammatory disorders, such as endometriosis, asthma, and migraine.1 These results, consistent with our current understanding of the genetic contribution to migraine risk, emphasize that lifestyle modifications alone are not usually adequate for complete management of migraines.
Patients who experience chronic migraine may be inclined to reduce their time spent exercising and engaging in physical activity, as these activities can exacerbate migraine symptoms. Additionally, after recovering from a migraine, patients often need to catch up on tasks and responsibilities, which can squeeze out time for physical activity and exercise (often considered luxuries that can be done during leisure time). Results of a small cross-sectional retrospective study published in Scientific Reports in 2024 suggested a correlation between daily walking steps and response to calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb). According to the study, which included 22 patients who were diagnosed with migraine and treated with CGRP mAb, patients who experienced an improvement of their migraine symptoms also increased their average daily steps by almost 1000 steps per day. The authors suggested that steps can be used as a marker of treatment response in migraine.
Screen time is often blamed as a cause for a number of different ailments, including obesity, anxiety, depression, insomnia, and migraine. An article published in June 2024 in European Journal of Pain described results of a meta-analysis examining the association between sedentary lifestyle and migraine. The authors noted that time spent watching television could be causally associated with an increased risk for migraine.2 Another study, with results published in July 2024 in The Journal of Headache and Pain, examined the relationship between migraine and leisure screen time. The researchers used data from 661,399 European individuals from 53 studies to look at genetically predicted leisure screen time, rather than actual leisure screen time. They reported that genetically predicted leisure screen time was associated with a 27.7% increase in migraine risk. While the results are consistent with what is already widely accepted about screen time and migraine, the inclusion of genetic predisposition to screen time is interesting in suggesting that some underlying drive could be contributing to increased screen time among patients who have migraine.
The results of these studies reemphasize the importance of the link between lifestyle factors and migraine but warn against oversimplifying the correlation. There is a bidirectional relationship between migraine and inflammation. We know that inflammation is mediated by diet as well as physical activity. During a migraine, patients may turn to foods that have a high inflammatory potential. Furthermore, migraine can influence a person's inclination to participate in physical activity, as the pain and discomfort can make it difficult engage in exercise. During a migraine, patients may prefer sedentary activities. Screen time can be appealing or relaxing while recovering from a migraine. Genetic predisposition is an interesting additional contributor to this link. Acknowledging genetic predisposition to inflammation or sedentary activity can be a step in helping patients recognize that it could be challenging to overcome these genetically inherent drives or conditions, while providing encouragement regarding the potential benefits of doing so.
Additional References
1. Rahmioglu N, Mortlock S, Ghiasi M, et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat Genet. 2023;55(3):423-436. Doi: 10.1038/s41588-023-01323-z Source
2. Li P, Li J, Zhu H, et al. Causal effects of sedentary behaviours on the risk of migraine: A univariable and multivariable Mendelian randomization study. Eur J Pain. 2024 (Jun 4). Doi: 10.1002/ejp.2296 Source
Migraine pathophysiology has been shown to be associated with vascular and inflammatory processes. Diet and lifestyle can have an effect on an individual's inflammatory process, and research regarding the steps between these factors and inflammation is vague and nonspecific. The Dietary Inflammation Score (DIS), which is calculated on the basis of a questionnaire, is used to score the inflammatory potential of an individual's diet. The Dietary and Lifestyle Inflammation Score (DLIS) includes the DIS questions, and also incorporates body mass index (BMI), physical activity, smoking, and alcohol consumption. A recent study, based on a secondary analysis of previous data, examined the correlation between migraine and DIS and DLIS among 285 women, 40% of whom had a chronic migraine diagnosis. Results published in Scientific Reports in July 2024 noted that participants with chronic migraine had a significantly higher DIS and DLIS than those who were not diagnosed with chronic migraine. It is important to note that migraine-associated inflammation can also result from genetic factors. A previous study, published in 2023 in Nature Genetics, described a correlation between genetic markers of inflammatory disorders, such as endometriosis, asthma, and migraine.1 These results, consistent with our current understanding of the genetic contribution to migraine risk, emphasize that lifestyle modifications alone are not usually adequate for complete management of migraines.
Patients who experience chronic migraine may be inclined to reduce their time spent exercising and engaging in physical activity, as these activities can exacerbate migraine symptoms. Additionally, after recovering from a migraine, patients often need to catch up on tasks and responsibilities, which can squeeze out time for physical activity and exercise (often considered luxuries that can be done during leisure time). Results of a small cross-sectional retrospective study published in Scientific Reports in 2024 suggested a correlation between daily walking steps and response to calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb). According to the study, which included 22 patients who were diagnosed with migraine and treated with CGRP mAb, patients who experienced an improvement of their migraine symptoms also increased their average daily steps by almost 1000 steps per day. The authors suggested that steps can be used as a marker of treatment response in migraine.
Screen time is often blamed as a cause for a number of different ailments, including obesity, anxiety, depression, insomnia, and migraine. An article published in June 2024 in European Journal of Pain described results of a meta-analysis examining the association between sedentary lifestyle and migraine. The authors noted that time spent watching television could be causally associated with an increased risk for migraine.2 Another study, with results published in July 2024 in The Journal of Headache and Pain, examined the relationship between migraine and leisure screen time. The researchers used data from 661,399 European individuals from 53 studies to look at genetically predicted leisure screen time, rather than actual leisure screen time. They reported that genetically predicted leisure screen time was associated with a 27.7% increase in migraine risk. While the results are consistent with what is already widely accepted about screen time and migraine, the inclusion of genetic predisposition to screen time is interesting in suggesting that some underlying drive could be contributing to increased screen time among patients who have migraine.
The results of these studies reemphasize the importance of the link between lifestyle factors and migraine but warn against oversimplifying the correlation. There is a bidirectional relationship between migraine and inflammation. We know that inflammation is mediated by diet as well as physical activity. During a migraine, patients may turn to foods that have a high inflammatory potential. Furthermore, migraine can influence a person's inclination to participate in physical activity, as the pain and discomfort can make it difficult engage in exercise. During a migraine, patients may prefer sedentary activities. Screen time can be appealing or relaxing while recovering from a migraine. Genetic predisposition is an interesting additional contributor to this link. Acknowledging genetic predisposition to inflammation or sedentary activity can be a step in helping patients recognize that it could be challenging to overcome these genetically inherent drives or conditions, while providing encouragement regarding the potential benefits of doing so.
Additional References
1. Rahmioglu N, Mortlock S, Ghiasi M, et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat Genet. 2023;55(3):423-436. Doi: 10.1038/s41588-023-01323-z Source
2. Li P, Li J, Zhu H, et al. Causal effects of sedentary behaviours on the risk of migraine: A univariable and multivariable Mendelian randomization study. Eur J Pain. 2024 (Jun 4). Doi: 10.1002/ejp.2296 Source
Recommended Use of Anticoagulant Reversal in Bleeding Events
The number of patients treated with anticoagulants has significantly increased over the past decade, largely owing to the introduction of direct oral anticoagulants (DOACs). Currently, more than 6 million people nationwide are taking anticoagulants; these include patients receiving care through the Veterans Health Administration.
However, the growing use of oral anticoagulants has been accompanied by a rise in anticoagulant-related bleeding incidents. Dr Geoffrey Barnes from the University of Michigan discusses strategies to assess and manage bleeding events, and he reviews the most current recommendations on the appropriate selection and use of anticoagulation reversal agents.
Dr Barnes also underscores the importance of monitoring for thromboembolic complications in patients treated for life-threatening bleeding to prevent post-bleed thromboembolic events.
--
Associate Professor, Frankel Cardiovascular Center, University of Michigan, Ann Arbor, Michigan
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Pfizer; Bristol-Myers Squibb; Janssen; Bayer; AstraZeneca; Sanofi; Anthos; Abbott Vascular; Boston Scientific
Received research grant from: Boston Scientific
The number of patients treated with anticoagulants has significantly increased over the past decade, largely owing to the introduction of direct oral anticoagulants (DOACs). Currently, more than 6 million people nationwide are taking anticoagulants; these include patients receiving care through the Veterans Health Administration.
However, the growing use of oral anticoagulants has been accompanied by a rise in anticoagulant-related bleeding incidents. Dr Geoffrey Barnes from the University of Michigan discusses strategies to assess and manage bleeding events, and he reviews the most current recommendations on the appropriate selection and use of anticoagulation reversal agents.
Dr Barnes also underscores the importance of monitoring for thromboembolic complications in patients treated for life-threatening bleeding to prevent post-bleed thromboembolic events.
--
Associate Professor, Frankel Cardiovascular Center, University of Michigan, Ann Arbor, Michigan
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Pfizer; Bristol-Myers Squibb; Janssen; Bayer; AstraZeneca; Sanofi; Anthos; Abbott Vascular; Boston Scientific
Received research grant from: Boston Scientific
The number of patients treated with anticoagulants has significantly increased over the past decade, largely owing to the introduction of direct oral anticoagulants (DOACs). Currently, more than 6 million people nationwide are taking anticoagulants; these include patients receiving care through the Veterans Health Administration.
However, the growing use of oral anticoagulants has been accompanied by a rise in anticoagulant-related bleeding incidents. Dr Geoffrey Barnes from the University of Michigan discusses strategies to assess and manage bleeding events, and he reviews the most current recommendations on the appropriate selection and use of anticoagulation reversal agents.
Dr Barnes also underscores the importance of monitoring for thromboembolic complications in patients treated for life-threatening bleeding to prevent post-bleed thromboembolic events.
--
Associate Professor, Frankel Cardiovascular Center, University of Michigan, Ann Arbor, Michigan
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Pfizer; Bristol-Myers Squibb; Janssen; Bayer; AstraZeneca; Sanofi; Anthos; Abbott Vascular; Boston Scientific
Received research grant from: Boston Scientific

Applications for the CUTIS 2025 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2025 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2025.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Alicia Sonners ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2025 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2025.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Alicia Sonners ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2025 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2025.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Alicia Sonners ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!