User login
My pet peeves about the current state of primary care
For this month’s column, I wanted to share some frustrations I have had about the current state of primary care. We all find those things that are going on in medicine that seem crazy and we just have to find a way to adapt to them. It is good to be able to share some of these thoughts with a community as distinguished as you readers. I know some of these are issues that you all struggle with and I wanted to give a voice to them. I wish I had answers to fix them.
Faxes from insurance companies
I find faxes from insurance companies immensely annoying. First, it takes time to go through lots of unwanted faxes but these faxes are extremely inaccurate. Today I received a fax telling me I might want to consider starting a statin in my 64-year-old HIV patient who has hypertension. He has been on a statin for 10 years.
Another fax warned me to not combine ACE inhibitors and angiotensin II receptor blockers (ARBs) in a patient who was switched from an ACE inhibitor in July to an ARB because of a cough. The fax that was sent to me has a documented end date for the ACE inhibitor before the start date of the ARB.
We only have so much time in the day and piles of faxes are not helpful.
Speaking of faxes: Why do physical therapy offices and nursing homes fax the same form every day? Physicians do not always work in clinic every single day and it increases the workload and burden when you have to sort through three copies of the same fax. I once worked in a world where these would be sent by mail, and mailed back a week later, which seemed to work just fine.
Misinformation
Our patients have many sources of health information. Much of the information they get comes from family, friends, social media posts, and Internet sites. The accuracy of the information is often questionable, and in some cases, they are victims of intentional misinformation.
It is frustrating and time consuming to counter the bogus, unsubstantiated information patients receive. It is especially difficult when patients have done their own research on proven therapies (such as statins) and do not want to use them because of the many websites they have looked at that make unscientific claims about the dangers of the proposed therapy. I share evidence-based websites with my patients for their research; my favorite is medlineplus.gov.
Access crisis
The availability of specialty care is extremely limited now. In my health care system, there is up to a 6-month wait for appointments in neurology, cardiology, and endocrinology. This puts the burden on the primary care professional to manage the patient’s health, even when the patient really needs specialty care. It also increases the calls we receive to interpret the echocardiograms, MRIs, or lab tests ordered by specialists who do not share the interpretation of the results with their patients.
What can be done to improve this situation? Automatic consults in the hospital should be limited. Every patient who has a transient ischemic attack with a negative workup does not need neurology follow-up. The same goes for patients who have chest pain but a negative cardiac workup in the hospital – they do not need follow-up by a cardiologist, nor do those who have stable, well-managed coronary disease. We have to find a way to keep our specialists seeing the patients whom they can help the most and available for consultation in a timely fashion.
Please share your pet peeves with me. I will try to give them voice in the future. Hang in there, you are the glue that keeps this flawed system together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
For this month’s column, I wanted to share some frustrations I have had about the current state of primary care. We all find those things that are going on in medicine that seem crazy and we just have to find a way to adapt to them. It is good to be able to share some of these thoughts with a community as distinguished as you readers. I know some of these are issues that you all struggle with and I wanted to give a voice to them. I wish I had answers to fix them.
Faxes from insurance companies
I find faxes from insurance companies immensely annoying. First, it takes time to go through lots of unwanted faxes but these faxes are extremely inaccurate. Today I received a fax telling me I might want to consider starting a statin in my 64-year-old HIV patient who has hypertension. He has been on a statin for 10 years.
Another fax warned me to not combine ACE inhibitors and angiotensin II receptor blockers (ARBs) in a patient who was switched from an ACE inhibitor in July to an ARB because of a cough. The fax that was sent to me has a documented end date for the ACE inhibitor before the start date of the ARB.
We only have so much time in the day and piles of faxes are not helpful.
Speaking of faxes: Why do physical therapy offices and nursing homes fax the same form every day? Physicians do not always work in clinic every single day and it increases the workload and burden when you have to sort through three copies of the same fax. I once worked in a world where these would be sent by mail, and mailed back a week later, which seemed to work just fine.
Misinformation
Our patients have many sources of health information. Much of the information they get comes from family, friends, social media posts, and Internet sites. The accuracy of the information is often questionable, and in some cases, they are victims of intentional misinformation.
It is frustrating and time consuming to counter the bogus, unsubstantiated information patients receive. It is especially difficult when patients have done their own research on proven therapies (such as statins) and do not want to use them because of the many websites they have looked at that make unscientific claims about the dangers of the proposed therapy. I share evidence-based websites with my patients for their research; my favorite is medlineplus.gov.
Access crisis
The availability of specialty care is extremely limited now. In my health care system, there is up to a 6-month wait for appointments in neurology, cardiology, and endocrinology. This puts the burden on the primary care professional to manage the patient’s health, even when the patient really needs specialty care. It also increases the calls we receive to interpret the echocardiograms, MRIs, or lab tests ordered by specialists who do not share the interpretation of the results with their patients.
What can be done to improve this situation? Automatic consults in the hospital should be limited. Every patient who has a transient ischemic attack with a negative workup does not need neurology follow-up. The same goes for patients who have chest pain but a negative cardiac workup in the hospital – they do not need follow-up by a cardiologist, nor do those who have stable, well-managed coronary disease. We have to find a way to keep our specialists seeing the patients whom they can help the most and available for consultation in a timely fashion.
Please share your pet peeves with me. I will try to give them voice in the future. Hang in there, you are the glue that keeps this flawed system together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
For this month’s column, I wanted to share some frustrations I have had about the current state of primary care. We all find those things that are going on in medicine that seem crazy and we just have to find a way to adapt to them. It is good to be able to share some of these thoughts with a community as distinguished as you readers. I know some of these are issues that you all struggle with and I wanted to give a voice to them. I wish I had answers to fix them.
Faxes from insurance companies
I find faxes from insurance companies immensely annoying. First, it takes time to go through lots of unwanted faxes but these faxes are extremely inaccurate. Today I received a fax telling me I might want to consider starting a statin in my 64-year-old HIV patient who has hypertension. He has been on a statin for 10 years.
Another fax warned me to not combine ACE inhibitors and angiotensin II receptor blockers (ARBs) in a patient who was switched from an ACE inhibitor in July to an ARB because of a cough. The fax that was sent to me has a documented end date for the ACE inhibitor before the start date of the ARB.
We only have so much time in the day and piles of faxes are not helpful.
Speaking of faxes: Why do physical therapy offices and nursing homes fax the same form every day? Physicians do not always work in clinic every single day and it increases the workload and burden when you have to sort through three copies of the same fax. I once worked in a world where these would be sent by mail, and mailed back a week later, which seemed to work just fine.
Misinformation
Our patients have many sources of health information. Much of the information they get comes from family, friends, social media posts, and Internet sites. The accuracy of the information is often questionable, and in some cases, they are victims of intentional misinformation.
It is frustrating and time consuming to counter the bogus, unsubstantiated information patients receive. It is especially difficult when patients have done their own research on proven therapies (such as statins) and do not want to use them because of the many websites they have looked at that make unscientific claims about the dangers of the proposed therapy. I share evidence-based websites with my patients for their research; my favorite is medlineplus.gov.
Access crisis
The availability of specialty care is extremely limited now. In my health care system, there is up to a 6-month wait for appointments in neurology, cardiology, and endocrinology. This puts the burden on the primary care professional to manage the patient’s health, even when the patient really needs specialty care. It also increases the calls we receive to interpret the echocardiograms, MRIs, or lab tests ordered by specialists who do not share the interpretation of the results with their patients.
What can be done to improve this situation? Automatic consults in the hospital should be limited. Every patient who has a transient ischemic attack with a negative workup does not need neurology follow-up. The same goes for patients who have chest pain but a negative cardiac workup in the hospital – they do not need follow-up by a cardiologist, nor do those who have stable, well-managed coronary disease. We have to find a way to keep our specialists seeing the patients whom they can help the most and available for consultation in a timely fashion.
Please share your pet peeves with me. I will try to give them voice in the future. Hang in there, you are the glue that keeps this flawed system together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A focus on women with diabetes and their offspring
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
In 2021, diabetes and related complications was the 8th leading cause of death in the United States.1 As of 2022, more than 11% of the U.S. population had diabetes and 38% of the adult U.S. population had prediabetes.2 Diabetes is the most expensive chronic condition in the United States, where $1 of every $4 in health care costs is spent on care.3
Where this is most concerning is diabetes in pregnancy. While childbirth rates in the United States have decreased since the 2007 high of 4.32 million births4 to 3.66 million in 2021,5 the incidence of diabetes in pregnancy – both pregestational and gestational – has increased. The rate of pregestational diabetes in 2021 was 10.9 per 1,000 births, a 27% increase from 2016 (8.6 per 1,000).6 The percentage of those giving birth who also were diagnosed with gestational diabetes mellitus (GDM) was 8.3% in 2021, up from 6.0% in 2016.7
Adverse outcomes for an infant born to a mother with diabetes include a higher risk of obesity and diabetes as adults, potentially leading to a forward-feeding cycle.
We and our colleagues established the Diabetes in Pregnancy Study Group of North America in 1997 because we had witnessed too frequently the devastating diabetes-induced pregnancy complications in our patients. The mission we set forth was to provide a forum for dialogue among maternal-fetal medicine subspecialists. The three main goals we set forth to support this mission were to provide a catalyst for research, contribute to the creation and refinement of medical policies, and influence professional practices in diabetes in pregnancy.8
In the last quarter century, DPSG-NA, through its annual and biennial meetings, has brought together several hundred practitioners that include physicians, nurses, statisticians, researchers, nutritionists, and allied health professionals, among others. As a group, it has improved the detection and management of diabetes in pregnant women and their offspring through knowledge sharing and influencing policies on GDM screening, diagnosis, management, and treatment. Our members have shown that preconceptional counseling for women with diabetes can significantly reduce congenital malformation and perinatal mortality compared with those women with pregestational diabetes who receive no counseling.9,10
We have addressed a wide variety of topics including the paucity of data in determining the timing of delivery for women with diabetes and the Institute of Medicine/National Academy of Medicine recommendations of gestational weight gain and risks of not adhering to them. We have learned about new scientific discoveries that reveal underlying mechanisms to diabetes-related birth defects and potential therapeutic targets; and we have discussed the health literacy requirements, ethics, and opportunities for lifestyle intervention.11-16
But we need to do more.
Two risk factors are at play: Women continue to choose to have babies at later ages and their pregnancies continue to be complicated by the rising incidence of obesity (see Figure 1 and Figure 2). 
The global obesity epidemic has become a significant concern for all aspects of health and particularly for diabetes in pregnancy. 
In 1990, 24.9% of women in the United States were obese; in 2010, 35.8%; and now more than 41%. Some experts project that by 2030 more than 80% of women in the United States will be overweight or obese.21
If we are to stop this cycle of diabetes begets more diabetes, now more than ever we need to come together and accelerate the research and education around the diabetes in pregnancy. Join us at this year’s DPSG-NA meeting Oct. 26-28 to take part in the knowledge sharing, discussions, and planning. More information can be found online at https://events.dpsg-na.com/home.
Dr. Miodovnik is adjunct professor of obstetrics, gynecology, and reproductive sciences at University of Maryland School of Medicine. Dr. Reece is professor of obstetrics, gynecology, and reproductive sciences and senior scientist at the Center for Birth Defects Research at University of Maryland School of Medicine.
References
1. Xu J et al. Mortality in the United States, 2021. NCHS Data Brief. 2022 Dec;(456):1-8. PMID: 36598387.
2. Centers for Disease Control and Prevention, diabetes data and statistics.
3. American Diabetes Association. The Cost of Diabetes.
4. Martin JA et al. Births: Final data for 2007. Natl Vital Stat Rep. 2010 Aug 9;58(24):1-85. PMID: 21254725.
5. Osterman MJK et al. Births: Final data for 2021. Natl Vital Stat Rep. 2023 Jan;72(1):1-53. PMID: 36723449.
6. Gregory ECW and Ely DM. Trends and characteristics in prepregnancy diabetes: United States, 2016-2021. Natl Vital Stat Rep. 2023 May;72(6):1-13. PMID: 37256333.
7. QuickStats: Percentage of mothers with gestational diabetes, by maternal age – National Vital Statistics System, United States, 2016 and 2021. MMWR Morb Mortal Wkly Rep. 2023 Jan 6;72(1):16. doi: 10.15585/mmwr.mm7201a4.
8. Langer O et al. The Diabetes in Pregnancy Study Group of North America – Introduction and summary statement. Prenat Neonat Med. 1998;3(6):514-6.
9. Willhoite MB et al. The impact of preconception counseling on pregnancy outcomes. The experience of the Maine Diabetes in Pregnancy Program. Diabetes Care. 1993 Feb;16(2):450-5. doi: 10.2337/diacare.16.2.450.
10. McElvy SS et al. A focused preconceptional and early pregnancy program in women with type 1 diabetes reduces perinatal mortality and malformation rates to general population levels. J Matern Fetal Med. 2000 Jan-Feb;9(1):14-20. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<14::AID-MFM5>3.0.CO;2-K.
11. Rosen JA et al. The history and contributions of the Diabetes in Pregnancy Study Group of North America (1997-2015). Am J Perinatol. 2016 Nov;33(13):1223-6. doi: 10.1055/s-0036-1585082.
12. Driggers RW and Baschat A. The 12th meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA): Introduction and overview. J Matern Fetal Neonatal Med. 2012 Jan;25(1):3-4. doi: 10.3109/14767058.2012.626917.
13. Langer O et al. The proceedings of the Diabetes in Pregnancy Study Group of North America 2009 conference. J Matern Fetal Neonatal Med. 2010 Mar;23(3):196-8. doi: 10.3109/14767050903550634.
14. Reece EA et al. A consensus report of the Diabetes in Pregnancy Study Group of North America Conference, Little Rock, Ark., May 2002. J Matern Fetal Neonatal Med. 2002 Dec;12(6):362-4. doi: 10.1080/jmf.12.6.362.364.
15. Reece EA and Maulik D. A consensus conference of the Diabetes in Pregnancy Study Group of North America. J Matern Fetal Neonatal Med. 2002 Dec;12(6):361. doi: 10.1080/jmf.12.6.361.361.
16. Gabbe SG. Summation of the second meeting of the Diabetes in Pregnancy Study Group of North America (DPSG-NA). J Matern Fetal Med. 2000 Jan-Feb;9(1):3-9.
17. Vital Statistics of the United States 1990: Volume I – Natality.
18. Martin JA et al. Births: final data for 2000. Natl Vital Stat Rep. 2002 Feb 12;50(5):1-101. PMID: 11876093.
19. Martin JA et al. Births: final data for 2010. Natl Vital Stat Rep. 2012 Aug 28;61(1):1-72. PMID: 24974589.
20. CDC Website. Normal weight, overweight, and obesity among adults aged 20 and over, by selected characteristics: United States.
21. Wang Y et al. Has the prevalence of overweight, obesity, and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020 Jun 1;49(3):810-23. doi: 10.1093/ije/dyz273.
Employed physicians: A survival guide
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The strike by health care workers at Kaiser Permanente may not involve physicians (yet). But as more doctors in the United States are finding themselves working as salaried employees, physicians can – and probably will – become a powerful force for change in a health care system that has shown itself to be increasingly hostile to employee concerns over issues involving patient care, wages and benefits, safety, and well-being.
Salaried employment has its challenges. Physician-employees may have less autonomy and voice in decision-making that affects patients. They may splinter into fragmented work groups; feel isolated; and have different imperatives based on who they are, what they want, and where they work. They may feel more removed from their patients and struggle to build strong relationships, with their employers in the way.
Yet important opportunities exist for doctors when embracing their employee side. Examples of these interests include adequate compensation, wellness, job security, patient and worker safety, health care quality, reasonable workloads and schedules, and fair treatment by employers, including the need to exhibit a strong collective voice in organizational decision-making.
Some believe that physician-employees must be unionized to maximize their rights and power as employees. Many expect physician unionization to take hold more fully over time. Medical residents, the doctors of tomorrow, are already considering unionization in greater numbers. Some are also doing it in the same employment setting alongside other health professionals, such as nurses.
Having studied doctors and their employment situations for years, I am convinced that whether through unionization or another approach, physicians must also change how they think about control; train and learn alongside other health care workers who share similar interests; and elevate at an early career stage their knowledge of the business side of health care.
Adopt a more pragmatic definition of autonomy
Doctors must embrace an updated definition of autonomy – one that matches their status as highly paid labor.
When I have spoken to physicians in my research about what autonomy means to them, many seem unable to reconceptualize it from a vague and absolute form of their profession’s strategic control over their economic fates and technical skills toward an individualized control that is situation-specific, one centered on winning the daily fights about workplace bread-and-butter issues such as those mentioned above.
But a more pragmatic definition of autonomy could get doctors focused on influencing important issues of the patient-care day and enhance their negotiating power with employers. It would allow physicians to break out of what often seems a paralysis of inaction – waiting for employers, insurers, or the government to reinstate the profession’s idealized version of control by handing it back the keys to the health care system through major regulatory, structural, and reimbursement-related changes. This fantasy is unlikely to become reality.
Physician-employees I’ve talked to over the years understand their everyday challenges. But when it comes to engaging in localized and sustained action to overcome them, they often perform less well, leading to feelings of helplessness and burnout. Valuing tactical control over their jobs and work setting will yield smaller but more impactful wins as employees intent on making their everyday work lives better.
Train alongside other health care professionals
Physicians must accept that how they are trained no longer prepares them for the employee world into which most are dropped. For instance, unless doctors are trained collaboratively alongside other health care professionals – such as nurses – they are less likely to identify closely with these colleagues once in practice. There is strength in numbers, so this mutual identification empowers both groups of employees. Yet, medical education remains largely the same: training young medical students in isolation for the first couple of years, then placing them into clerkships and residencies where true interprofessional care opportunities remain stunted and secondary to the “physician as captain of the team” mantra.
Unfortunately, the “hidden curriculum” of medicine helps convince medical students and residents early in their careers that they are the unquestioned leaders in patient care settings. This hierarchy encourages some doctors to keep their psychological distance from other members of the health care team and to resist sharing power, concerns, or insights with less skilled health care workers. This socialization harms the ability of physicians to act in a unified fashion alongside these other workers. Having physicians learn and train alongside other health professionals yields positive benefits for collective advocacy, including a shared sense of purpose, positive views on collaboration with others in the health setting, and greater development of bonds with nonphysician coworkers.
Integrate business with medical training in real time
Medical students and residents generally lack exposure to the everyday business realities of the U.S. health care system. This gap hinders their ability to understand the employee world and push for the types of changes and work conditions that benefit all health care workers. Formal business and management training should be a required part of every U.S. medical school and residency curriculum from day one. If you see it at all in medical schools now, it is mostly by accident, or given separate treatment in the form of standalone MBA or MPH degrees that rarely integrate organically and in real time with actual medical training. Not every doctor needs an MBA or MPH degree. However, all of them require a stronger contextual understanding of how the medicine they wish to practice is shaped by the economic and fiscal circumstances surrounding it – circumstances they do not control.
This is another reason why young doctors are unhappy and burned out. They cannot push for specific changes or properly critique the pros and cons of how their work is structured because they have not been made aware, in real time as they learn clinical practice, how their jobs are shaped by realities such as insurance coverage and reimbursement, the fragmentation of the care delivery system, their employer’s financial health , and the socioeconomic circumstances of their patients. They aren’t given the methods and tools related to process and quality improvement, budgeting, negotiation, risk management, leadership, and talent management that might help them navigate these undermining forces. They also get little advance exposure in their training to important workplace “soft” skills in such areas as how to work in teams, networking, communication and listening, empathy, and problem-solving – all necessary foci for bringing them closer to other health care workers and advocating alongside them effectively with health care employers.
Now is the time for physicians to embrace their identity as employees. Doing so is in their own best interest as professionals. It will help others in the health care workforce as well as patients. Moreover, it provides a needed counterbalance to the powerful corporate ethos now ascendant in U.S. health care.
Timothy Hoff, PhD, is a professor of management and healthcare systems at Northeastern University, Boston, and an associate fellow at the University of Oxford, England. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Why legal pot makes this physician sick
Last year, my husband and I took a 16-day road trip from Kentucky through Massachusetts to Maine. On our first morning in Boston, we exited the Park Street Station en route to Boston Common, but instead of being greeted by the aroma of molasses, we were hit full-on with a pungent, repulsive odor. “That’s skunk weed,” my husband chuckled as we stepped right into the middle of the Boston Freedom Rally, a celebration of all things cannabis.
As we boarded a hop-on-hop-off bus, we learned that this was the one week of the year that the city skips testing tour bus drivers for tetrahydrocannabinol (THC), “because we all test positive,” the driver quipped. As our open-air bus circled the Common, a crowd of pot enthusiasts displayed signs in support of relaxed regulation for public consumption.
The 34-year-old Boston Freedom Rally is a sign that U.S. culture has transformed forever. Mary Jane is no friend of emergency physicians nor of staff on hospital wards and offices.
Toking boomers and millennials
Researchers at the University of California, San Diego, looked at cannabis-related emergency department visits from all acute-care hospitals in the state from 2005 to 2019 and found an 1,808% increase in patients aged 65 or older (that is not a typo) who were there for complications from cannabis use.
The lead author said in an interview that, “older patients taking marijuana or related products may have dizziness and falls, heart palpitations, panic attacks, confusion, anxiety or worsening of underlying lung diseases, such as asthma or [chronic obstructive pulmonary disease].”
A recent study from Canada suggests that commercialization has been associated with an increase in related hospitalizations, including cannabis-induced psychosis.
According to a National Study of Drug Use and Health, marijuana use in young adults reached an all-time high (pun intended) in 2021. Nearly 10% of eighth graders and 20% of 10th graders reported using marijuana this past year.
The full downside of any drug, legal or illegal, is largely unknown until it infiltrates the mainstream market, but these are the typical cases we see:
Let’s start with the demotivated high school honors student who dropped out of college to work at the local cinema. He stumbled and broke his clavicle outside a bar at 2 AM, but he wasn’t sure if he passed out, so a cardiology consult was requested to “rule out” arrhythmia associated with syncope. He related that his plan to become a railway conductor had been upended because he knew he would be drug tested and just couldn’t give up pot. After a normal cardiac exam, ECG, labs, a Holter, and an echocardiogram were also requested and normal at a significant cost.
Cannabinoid hyperemesis syndrome
One of my Midwest colleagues related her encounter with two middle-aged pot users with ventricular tachycardia (VT). These episodes coincided with potassium levels less than 3.0 mEq/L in the setting of repetitive vomiting. The QTc interval didn’t normalize despite a corrected potassium level in one patient. They were both informed that they should never smoke pot because vomiting would predictably drop their K+ levels again and prolong their QTc intervals. Then began “the circular argument,” as my friend described it. The patient claims, “I smoke pot to relieve my nausea,” to which she explains that “in many folks, pot use induces nausea.” Of course, the classic reply is, “Not me.” Predictably one of these stoners soon returned with more VT, more puking, and more hypokalemia. “Consider yourself ‘allergic’ to pot smoke,” my friend advised, but “was met with no meaningful hint of understanding or hope for transformative change,” she told me.
I’ve seen cannabinoid hyperemesis syndrome several times in the past few years. It occurs in daily to weekly pot users. Very rarely, it can cause cerebral edema, but it is also associated with seizures and dehydration that can lead to hypovolemic shock and kidney failure.
Heart and brain harm
Then there are the young patients who for various reasons have developed heart failure. Unfortunately, some are repetitively tox screen positive with varying trifectas of methamphetamine (meth), cocaine, and THC; opiates, meth, and THC; alcohol, meth, and THC; or heroin, meth, and THC. THC, the ever present and essential third leg of the stool of stupor. These unfortunate patients often need heart failure medications that they can’t afford or won’t take because illicit drug use is expensive and dulls their ability to prioritize their health. Some desperately need a heart transplant, but the necessary negative drug screen is a pipe dream.
And it’s not just the heart that is affected. There are data linking cannabis use to a higher risk for both ischemic and hemorrhagic stroke. A retrospective study published in Stroke, of more than 1,000 people diagnosed with an aneurysmal subarachnoid hemorrhage, found that more than half of the 46 who tested positive for THC at admission developed delayed cerebral ischemia (DCI), which increases the risk for disability or early death. This was after adjusting for several patient characteristics as well as recent exposure to other illicit substances; cocaine, meth, and tobacco use were not associated with DCI.
Natural my ...
I’m certain my anti-cannabis stance will strike a nerve with those who love their recreational THC and push for its legal sale; after all, “It’s perfectly natural.” But I counter with the fact that tornadoes, earthquakes, cyanide, and appendicitis are all natural but certainly not optimal. And what we are seeing in the vascular specialties is completely unnatural. We are treating a different mix of complications than before pot was readily accessible across several states.
Our most effective action is to educate our patients. We should encourage those who don’t currently smoke cannabis to never start and those who do to quit. People who require marijuana for improved quality of life for terminal care or true (not supposed) disorders that mainstream medicine fails should be approached with empathy and caution.
A good rule of thumb is to never breathe anything you can see. Never put anything in your body that comes off the street: Drug dealers who sell cannabis cut with fentanyl will be ecstatic to take someone’s money then merely keep scrolling when their obituary comes up.
Let’s try to reverse the rise of vascular complications, orthopedic injuries, and vomiting across America. We can start by encouraging our patients to avoid “skunk weed” and get back to the sweet smells of nature in our cities and parks.
Some details have been changed to protect the patients’ identities, but the essence of their diagnoses has been preserved.
Dr. Walton-Shirley is a retired clinical cardiologist from Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Last year, my husband and I took a 16-day road trip from Kentucky through Massachusetts to Maine. On our first morning in Boston, we exited the Park Street Station en route to Boston Common, but instead of being greeted by the aroma of molasses, we were hit full-on with a pungent, repulsive odor. “That’s skunk weed,” my husband chuckled as we stepped right into the middle of the Boston Freedom Rally, a celebration of all things cannabis.
As we boarded a hop-on-hop-off bus, we learned that this was the one week of the year that the city skips testing tour bus drivers for tetrahydrocannabinol (THC), “because we all test positive,” the driver quipped. As our open-air bus circled the Common, a crowd of pot enthusiasts displayed signs in support of relaxed regulation for public consumption.
The 34-year-old Boston Freedom Rally is a sign that U.S. culture has transformed forever. Mary Jane is no friend of emergency physicians nor of staff on hospital wards and offices.
Toking boomers and millennials
Researchers at the University of California, San Diego, looked at cannabis-related emergency department visits from all acute-care hospitals in the state from 2005 to 2019 and found an 1,808% increase in patients aged 65 or older (that is not a typo) who were there for complications from cannabis use.
The lead author said in an interview that, “older patients taking marijuana or related products may have dizziness and falls, heart palpitations, panic attacks, confusion, anxiety or worsening of underlying lung diseases, such as asthma or [chronic obstructive pulmonary disease].”
A recent study from Canada suggests that commercialization has been associated with an increase in related hospitalizations, including cannabis-induced psychosis.
According to a National Study of Drug Use and Health, marijuana use in young adults reached an all-time high (pun intended) in 2021. Nearly 10% of eighth graders and 20% of 10th graders reported using marijuana this past year.
The full downside of any drug, legal or illegal, is largely unknown until it infiltrates the mainstream market, but these are the typical cases we see:
Let’s start with the demotivated high school honors student who dropped out of college to work at the local cinema. He stumbled and broke his clavicle outside a bar at 2 AM, but he wasn’t sure if he passed out, so a cardiology consult was requested to “rule out” arrhythmia associated with syncope. He related that his plan to become a railway conductor had been upended because he knew he would be drug tested and just couldn’t give up pot. After a normal cardiac exam, ECG, labs, a Holter, and an echocardiogram were also requested and normal at a significant cost.
Cannabinoid hyperemesis syndrome
One of my Midwest colleagues related her encounter with two middle-aged pot users with ventricular tachycardia (VT). These episodes coincided with potassium levels less than 3.0 mEq/L in the setting of repetitive vomiting. The QTc interval didn’t normalize despite a corrected potassium level in one patient. They were both informed that they should never smoke pot because vomiting would predictably drop their K+ levels again and prolong their QTc intervals. Then began “the circular argument,” as my friend described it. The patient claims, “I smoke pot to relieve my nausea,” to which she explains that “in many folks, pot use induces nausea.” Of course, the classic reply is, “Not me.” Predictably one of these stoners soon returned with more VT, more puking, and more hypokalemia. “Consider yourself ‘allergic’ to pot smoke,” my friend advised, but “was met with no meaningful hint of understanding or hope for transformative change,” she told me.
I’ve seen cannabinoid hyperemesis syndrome several times in the past few years. It occurs in daily to weekly pot users. Very rarely, it can cause cerebral edema, but it is also associated with seizures and dehydration that can lead to hypovolemic shock and kidney failure.
Heart and brain harm
Then there are the young patients who for various reasons have developed heart failure. Unfortunately, some are repetitively tox screen positive with varying trifectas of methamphetamine (meth), cocaine, and THC; opiates, meth, and THC; alcohol, meth, and THC; or heroin, meth, and THC. THC, the ever present and essential third leg of the stool of stupor. These unfortunate patients often need heart failure medications that they can’t afford or won’t take because illicit drug use is expensive and dulls their ability to prioritize their health. Some desperately need a heart transplant, but the necessary negative drug screen is a pipe dream.
And it’s not just the heart that is affected. There are data linking cannabis use to a higher risk for both ischemic and hemorrhagic stroke. A retrospective study published in Stroke, of more than 1,000 people diagnosed with an aneurysmal subarachnoid hemorrhage, found that more than half of the 46 who tested positive for THC at admission developed delayed cerebral ischemia (DCI), which increases the risk for disability or early death. This was after adjusting for several patient characteristics as well as recent exposure to other illicit substances; cocaine, meth, and tobacco use were not associated with DCI.
Natural my ...
I’m certain my anti-cannabis stance will strike a nerve with those who love their recreational THC and push for its legal sale; after all, “It’s perfectly natural.” But I counter with the fact that tornadoes, earthquakes, cyanide, and appendicitis are all natural but certainly not optimal. And what we are seeing in the vascular specialties is completely unnatural. We are treating a different mix of complications than before pot was readily accessible across several states.
Our most effective action is to educate our patients. We should encourage those who don’t currently smoke cannabis to never start and those who do to quit. People who require marijuana for improved quality of life for terminal care or true (not supposed) disorders that mainstream medicine fails should be approached with empathy and caution.
A good rule of thumb is to never breathe anything you can see. Never put anything in your body that comes off the street: Drug dealers who sell cannabis cut with fentanyl will be ecstatic to take someone’s money then merely keep scrolling when their obituary comes up.
Let’s try to reverse the rise of vascular complications, orthopedic injuries, and vomiting across America. We can start by encouraging our patients to avoid “skunk weed” and get back to the sweet smells of nature in our cities and parks.
Some details have been changed to protect the patients’ identities, but the essence of their diagnoses has been preserved.
Dr. Walton-Shirley is a retired clinical cardiologist from Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Last year, my husband and I took a 16-day road trip from Kentucky through Massachusetts to Maine. On our first morning in Boston, we exited the Park Street Station en route to Boston Common, but instead of being greeted by the aroma of molasses, we were hit full-on with a pungent, repulsive odor. “That’s skunk weed,” my husband chuckled as we stepped right into the middle of the Boston Freedom Rally, a celebration of all things cannabis.
As we boarded a hop-on-hop-off bus, we learned that this was the one week of the year that the city skips testing tour bus drivers for tetrahydrocannabinol (THC), “because we all test positive,” the driver quipped. As our open-air bus circled the Common, a crowd of pot enthusiasts displayed signs in support of relaxed regulation for public consumption.
The 34-year-old Boston Freedom Rally is a sign that U.S. culture has transformed forever. Mary Jane is no friend of emergency physicians nor of staff on hospital wards and offices.
Toking boomers and millennials
Researchers at the University of California, San Diego, looked at cannabis-related emergency department visits from all acute-care hospitals in the state from 2005 to 2019 and found an 1,808% increase in patients aged 65 or older (that is not a typo) who were there for complications from cannabis use.
The lead author said in an interview that, “older patients taking marijuana or related products may have dizziness and falls, heart palpitations, panic attacks, confusion, anxiety or worsening of underlying lung diseases, such as asthma or [chronic obstructive pulmonary disease].”
A recent study from Canada suggests that commercialization has been associated with an increase in related hospitalizations, including cannabis-induced psychosis.
According to a National Study of Drug Use and Health, marijuana use in young adults reached an all-time high (pun intended) in 2021. Nearly 10% of eighth graders and 20% of 10th graders reported using marijuana this past year.
The full downside of any drug, legal or illegal, is largely unknown until it infiltrates the mainstream market, but these are the typical cases we see:
Let’s start with the demotivated high school honors student who dropped out of college to work at the local cinema. He stumbled and broke his clavicle outside a bar at 2 AM, but he wasn’t sure if he passed out, so a cardiology consult was requested to “rule out” arrhythmia associated with syncope. He related that his plan to become a railway conductor had been upended because he knew he would be drug tested and just couldn’t give up pot. After a normal cardiac exam, ECG, labs, a Holter, and an echocardiogram were also requested and normal at a significant cost.
Cannabinoid hyperemesis syndrome
One of my Midwest colleagues related her encounter with two middle-aged pot users with ventricular tachycardia (VT). These episodes coincided with potassium levels less than 3.0 mEq/L in the setting of repetitive vomiting. The QTc interval didn’t normalize despite a corrected potassium level in one patient. They were both informed that they should never smoke pot because vomiting would predictably drop their K+ levels again and prolong their QTc intervals. Then began “the circular argument,” as my friend described it. The patient claims, “I smoke pot to relieve my nausea,” to which she explains that “in many folks, pot use induces nausea.” Of course, the classic reply is, “Not me.” Predictably one of these stoners soon returned with more VT, more puking, and more hypokalemia. “Consider yourself ‘allergic’ to pot smoke,” my friend advised, but “was met with no meaningful hint of understanding or hope for transformative change,” she told me.
I’ve seen cannabinoid hyperemesis syndrome several times in the past few years. It occurs in daily to weekly pot users. Very rarely, it can cause cerebral edema, but it is also associated with seizures and dehydration that can lead to hypovolemic shock and kidney failure.
Heart and brain harm
Then there are the young patients who for various reasons have developed heart failure. Unfortunately, some are repetitively tox screen positive with varying trifectas of methamphetamine (meth), cocaine, and THC; opiates, meth, and THC; alcohol, meth, and THC; or heroin, meth, and THC. THC, the ever present and essential third leg of the stool of stupor. These unfortunate patients often need heart failure medications that they can’t afford or won’t take because illicit drug use is expensive and dulls their ability to prioritize their health. Some desperately need a heart transplant, but the necessary negative drug screen is a pipe dream.
And it’s not just the heart that is affected. There are data linking cannabis use to a higher risk for both ischemic and hemorrhagic stroke. A retrospective study published in Stroke, of more than 1,000 people diagnosed with an aneurysmal subarachnoid hemorrhage, found that more than half of the 46 who tested positive for THC at admission developed delayed cerebral ischemia (DCI), which increases the risk for disability or early death. This was after adjusting for several patient characteristics as well as recent exposure to other illicit substances; cocaine, meth, and tobacco use were not associated with DCI.
Natural my ...
I’m certain my anti-cannabis stance will strike a nerve with those who love their recreational THC and push for its legal sale; after all, “It’s perfectly natural.” But I counter with the fact that tornadoes, earthquakes, cyanide, and appendicitis are all natural but certainly not optimal. And what we are seeing in the vascular specialties is completely unnatural. We are treating a different mix of complications than before pot was readily accessible across several states.
Our most effective action is to educate our patients. We should encourage those who don’t currently smoke cannabis to never start and those who do to quit. People who require marijuana for improved quality of life for terminal care or true (not supposed) disorders that mainstream medicine fails should be approached with empathy and caution.
A good rule of thumb is to never breathe anything you can see. Never put anything in your body that comes off the street: Drug dealers who sell cannabis cut with fentanyl will be ecstatic to take someone’s money then merely keep scrolling when their obituary comes up.
Let’s try to reverse the rise of vascular complications, orthopedic injuries, and vomiting across America. We can start by encouraging our patients to avoid “skunk weed” and get back to the sweet smells of nature in our cities and parks.
Some details have been changed to protect the patients’ identities, but the essence of their diagnoses has been preserved.
Dr. Walton-Shirley is a retired clinical cardiologist from Nashville, Tenn. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Addressing obesity bias in health care
Obesity is a major factor affecting the health of many Americans. It is estimated by the Centers for Disease Control and Prevention that 41% of adults and 19.7% of children in our country now meet the criteria for being obese. Obesity costs the United States approximately $147 billion annually in health care costs. While these numbers are staggering, they continue to rise.
The recent craze over medications such as Ozempic, Wegovy, and Mounjaro shows how eager people are to lose weight. Yet, many of them face bias, not just in their daily lives, but from health care professionals who should do better. No one should feel stigmatized when they come for medical help. This just drives away patients who need us and who may then suffer more severe consequences of obesity-related illnesses.
Earlier this year, the American Association of Clinical Endocrinology issued a consensus statement on the role stigma and weight bias play in the management of obesity. They proposed a staging system to address the severity of obesity and suggested stigma and bias should be assessed in all patients.
While we are good at diagnosing obesity, many of us fail at addressing it empathetically with patients. I’ve seen many patients cry about past encounters they’ve had in the health care system. We need to address the emotional effect that obesity has as well as the physical complications.
Obesity is a major contributor to many diseases such as diabetes and heart disease, but we are finding it also plays a role in other diseases such as certain cancers. Treating obesity is imperative to prevent these diseases as well as to promote better treatment outcomes. We’ve all seen the diabetic patient lose weight and have their blood glucose levels come under control.
Many patients have tried hard to lose weight yet health care providers talk to them as if they haven’t made any efforts. This is very frustrating for patients. Simply telling a patient to diet and lose weight is a setup for failure. We need to address their past efforts and see what has worked and what hasn’t. Redoing the same thing over and over again is not a recipe for success.
Additionally, the focus on “diet and exercise” fails to account for emotional factors that may be contributing to a person’s obesity. Some people eat when they are stressed or depressed. It can become a habit or even an addiction. If this contributor to obesity isn’t fixed, nothing will work.
However, no medication will work well without the basic building blocks of diet and exercise. Routinely prescribing weight-loss medications without discussing diet and exercise will not result in much weight loss. Some patients simply don’t know how to eat healthfully or what they should do for exercise. A little education can go a long way. Ancillary staff, such as nutritionists or diabetic counselors, can help and free up the doctor’s time. In small practices, we can’t afford to provide those services in house but we should learn where patients can go for these services.
The AACE guidelines do a great job staging obesity. The guidelines make it easier to measure progress and decide on treatment plans. With this system, it is no longer necessary to use terms such as “excess weight” or “morbid obesity.” Patients already know they are overweight. What they need to know are clear steps so that they can reach goals. These guidelines greatly assist with providing those steps.
Most of us can do better when treating patients with obesity, We are probably not even aware of the times we have been guilty of stigmatization or weight bias. When we start treating obesity as a serious medical problem rather than something that’s the fault of the patient, it becomes much easier. When we remind ourselves what can happen to our patients when we fail to treat their obesity, we can become more serious about trying to help them reverse this critical medical problem. Bring an end to throwing out a “lose weight” or “eat healthier” suggestion to our already stressed patients. In order to address the obesity crisis that is here, we need to look inside ourselves and ask how we are going to contribute to the solution.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant of medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
Obesity is a major factor affecting the health of many Americans. It is estimated by the Centers for Disease Control and Prevention that 41% of adults and 19.7% of children in our country now meet the criteria for being obese. Obesity costs the United States approximately $147 billion annually in health care costs. While these numbers are staggering, they continue to rise.
The recent craze over medications such as Ozempic, Wegovy, and Mounjaro shows how eager people are to lose weight. Yet, many of them face bias, not just in their daily lives, but from health care professionals who should do better. No one should feel stigmatized when they come for medical help. This just drives away patients who need us and who may then suffer more severe consequences of obesity-related illnesses.
Earlier this year, the American Association of Clinical Endocrinology issued a consensus statement on the role stigma and weight bias play in the management of obesity. They proposed a staging system to address the severity of obesity and suggested stigma and bias should be assessed in all patients.
While we are good at diagnosing obesity, many of us fail at addressing it empathetically with patients. I’ve seen many patients cry about past encounters they’ve had in the health care system. We need to address the emotional effect that obesity has as well as the physical complications.
Obesity is a major contributor to many diseases such as diabetes and heart disease, but we are finding it also plays a role in other diseases such as certain cancers. Treating obesity is imperative to prevent these diseases as well as to promote better treatment outcomes. We’ve all seen the diabetic patient lose weight and have their blood glucose levels come under control.
Many patients have tried hard to lose weight yet health care providers talk to them as if they haven’t made any efforts. This is very frustrating for patients. Simply telling a patient to diet and lose weight is a setup for failure. We need to address their past efforts and see what has worked and what hasn’t. Redoing the same thing over and over again is not a recipe for success.
Additionally, the focus on “diet and exercise” fails to account for emotional factors that may be contributing to a person’s obesity. Some people eat when they are stressed or depressed. It can become a habit or even an addiction. If this contributor to obesity isn’t fixed, nothing will work.
However, no medication will work well without the basic building blocks of diet and exercise. Routinely prescribing weight-loss medications without discussing diet and exercise will not result in much weight loss. Some patients simply don’t know how to eat healthfully or what they should do for exercise. A little education can go a long way. Ancillary staff, such as nutritionists or diabetic counselors, can help and free up the doctor’s time. In small practices, we can’t afford to provide those services in house but we should learn where patients can go for these services.
The AACE guidelines do a great job staging obesity. The guidelines make it easier to measure progress and decide on treatment plans. With this system, it is no longer necessary to use terms such as “excess weight” or “morbid obesity.” Patients already know they are overweight. What they need to know are clear steps so that they can reach goals. These guidelines greatly assist with providing those steps.
Most of us can do better when treating patients with obesity, We are probably not even aware of the times we have been guilty of stigmatization or weight bias. When we start treating obesity as a serious medical problem rather than something that’s the fault of the patient, it becomes much easier. When we remind ourselves what can happen to our patients when we fail to treat their obesity, we can become more serious about trying to help them reverse this critical medical problem. Bring an end to throwing out a “lose weight” or “eat healthier” suggestion to our already stressed patients. In order to address the obesity crisis that is here, we need to look inside ourselves and ask how we are going to contribute to the solution.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant of medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
Obesity is a major factor affecting the health of many Americans. It is estimated by the Centers for Disease Control and Prevention that 41% of adults and 19.7% of children in our country now meet the criteria for being obese. Obesity costs the United States approximately $147 billion annually in health care costs. While these numbers are staggering, they continue to rise.
The recent craze over medications such as Ozempic, Wegovy, and Mounjaro shows how eager people are to lose weight. Yet, many of them face bias, not just in their daily lives, but from health care professionals who should do better. No one should feel stigmatized when they come for medical help. This just drives away patients who need us and who may then suffer more severe consequences of obesity-related illnesses.
Earlier this year, the American Association of Clinical Endocrinology issued a consensus statement on the role stigma and weight bias play in the management of obesity. They proposed a staging system to address the severity of obesity and suggested stigma and bias should be assessed in all patients.
While we are good at diagnosing obesity, many of us fail at addressing it empathetically with patients. I’ve seen many patients cry about past encounters they’ve had in the health care system. We need to address the emotional effect that obesity has as well as the physical complications.
Obesity is a major contributor to many diseases such as diabetes and heart disease, but we are finding it also plays a role in other diseases such as certain cancers. Treating obesity is imperative to prevent these diseases as well as to promote better treatment outcomes. We’ve all seen the diabetic patient lose weight and have their blood glucose levels come under control.
Many patients have tried hard to lose weight yet health care providers talk to them as if they haven’t made any efforts. This is very frustrating for patients. Simply telling a patient to diet and lose weight is a setup for failure. We need to address their past efforts and see what has worked and what hasn’t. Redoing the same thing over and over again is not a recipe for success.
Additionally, the focus on “diet and exercise” fails to account for emotional factors that may be contributing to a person’s obesity. Some people eat when they are stressed or depressed. It can become a habit or even an addiction. If this contributor to obesity isn’t fixed, nothing will work.
However, no medication will work well without the basic building blocks of diet and exercise. Routinely prescribing weight-loss medications without discussing diet and exercise will not result in much weight loss. Some patients simply don’t know how to eat healthfully or what they should do for exercise. A little education can go a long way. Ancillary staff, such as nutritionists or diabetic counselors, can help and free up the doctor’s time. In small practices, we can’t afford to provide those services in house but we should learn where patients can go for these services.
The AACE guidelines do a great job staging obesity. The guidelines make it easier to measure progress and decide on treatment plans. With this system, it is no longer necessary to use terms such as “excess weight” or “morbid obesity.” Patients already know they are overweight. What they need to know are clear steps so that they can reach goals. These guidelines greatly assist with providing those steps.
Most of us can do better when treating patients with obesity, We are probably not even aware of the times we have been guilty of stigmatization or weight bias. When we start treating obesity as a serious medical problem rather than something that’s the fault of the patient, it becomes much easier. When we remind ourselves what can happen to our patients when we fail to treat their obesity, we can become more serious about trying to help them reverse this critical medical problem. Bring an end to throwing out a “lose weight” or “eat healthier” suggestion to our already stressed patients. In order to address the obesity crisis that is here, we need to look inside ourselves and ask how we are going to contribute to the solution.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant of medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
Artificial intelligence in the office: Part 2
In the year since generative artificial intelligence (AI) software first began to emerge for use, the staggering pace and breadth of development has condensed years of growth and change into months and weeks. Among the settings where these tools may find the greatest straight-line relevance is private medical practice.
. A multitude of generative AI products with potential medical applications are now available, with new ones appearing almost weekly. (As always, I have no financial interest in any product or service mentioned in this column.)
Last month, I discussed ChatGPT, the best-known AI algorithm, and some of its applications in clinical practice, such as generating website, video, and blog content. ChatGPT can also provide rapid and concise answers to general medical questions, like a search engine – but with more natural language processing and contextual understanding. Additionally, the algorithm can draft generic medical documents, including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts.
Another useful feature of ChatGPT is its ability to provide accurate and conversational language translations, thus serving as an interpreter during clinic visits in situations where a human translator is not available. It also has potential uses in clinical research by finding resources, formulating hypotheses, drafting study protocols, and collecting large amounts of data in short periods of time. Other possibilities include survey administration, clinical trial recruitment, and automatic medication monitoring.
GPT-4, the latest version of ChatGPT, is reported to have greater problem-solving abilities and an even broader knowledge base. Among its claimed skills are the ability to find the latest literature in a given area, write a discharge summary for a patient following an uncomplicated surgery, and an image analysis feature to identify objects in photos. GPT-4 has been praised as having “the potential to help drive medical innovation, from aiding with patient discharge notes, summarizing recent clinical trials, providing information on ethical guidelines, and much more.”
Bard, an AI “chat bot” introduced by Google earlier this year, intends to leverage Google’s enormous database to compete with ChatGPT in providing answers to medical questions. Bard also hopes to play a pivotal role in expanding telemedicine and remote care via Google’s secure connections and access to patient records and medical history, and “facilitate seamless communication through appointment scheduling, messaging, and sharing medical images,” according to PackT, a website for IT professionals. The company claims that Bard’s integration of AI and machine learning capabilities will serve to elevate health care efficiency and patient outcomes, PackT says, and “the platform’s AI system quickly and accurately analyzes patient records, identifies patterns and trends, and aids medical professionals in developing effective treatment plans.”
Doximity has introduced an AI engine called DocsGPT, an encrypted, HIPAA-compliant writing assistant that, the company says, can draft any form of professional correspondence, including prior authorization letters, insurance appeals, patient support letters, and patient education materials. The service is available at no charge to all U.S. physicians and medical students through their Doximity accounts.
Microsoft has introduced several AI products. BioGPT is a language model specifically designed for health care. Compared with GPT models that are trained on more general text data, BioGPT is purported to have a deeper understanding of the language used in biomedical research and can generate more accurate and relevant outputs for biomedical tasks, such as drug discovery, disease classification, and clinical decision support. Fabric is another health care–specific data and analytics platform the company described in an announcement in May. It can combine data from sources such as electronic health records, images, lab systems, medical devices, and claims systems so hospitals and offices can standardize it and access it in the same place. Microsoft said the new tools will help eliminate the “time-consuming” process of searching through these sources one by one. Microsoft will also offer a new generative AI chatbot called the Azure Health Bot, which can pull information from a health organization’s own internal data as well as reputable external sources such as the Food and Drug Administration and the National Institutes of Health.
Several other AI products are available for clinicians. Tana served as an administrative aid and a clinical helper during the height of the COVID-19 pandemic, answering frequently asked questions, facilitating appointment management, and gathering preliminary medical information prior to teleconsultations. Dougall GPT is another AI chatbot tailored for health care professionals. It provides clinicians with AI-tuned answers to their queries, augmented by links to relevant, up-to-date, authoritative resources. It also assists in drafting patient instructions, consultation summaries, speeches, and professional correspondence. Wang has created Clinical Camel, an open-source health care–focused chatbot that assembles medical data with a combination of user-shared conversations and synthetic conversations derived from curated clinical articles. The Chinese company Baidu has rolled out Ernie as a potential rival to ChatGPT. You get the idea.
Of course, the inherent drawbacks of AI, such as producing false or biased information, perpetuating harmful stereotypes, and presenting information that has since been proven inaccurate or out-of-date, must always be kept in mind. All AI algorithms have been criticized for giving wrong answers, as their datasets are generally culled from information published in 2021 or earlier. Several of them have been shown to fabricate information – a phenomenon labeled “artificial hallucinations” in one article. “The scientific community must be vigilant in verifying the accuracy and reliability of the information provided by AI tools,” wrote the authors of that paper. “Researchers should use AI as an aid rather than a replacement for critical thinking and fact-checking.”
In the year since generative artificial intelligence (AI) software first began to emerge for use, the staggering pace and breadth of development has condensed years of growth and change into months and weeks. Among the settings where these tools may find the greatest straight-line relevance is private medical practice.
. A multitude of generative AI products with potential medical applications are now available, with new ones appearing almost weekly. (As always, I have no financial interest in any product or service mentioned in this column.)
Last month, I discussed ChatGPT, the best-known AI algorithm, and some of its applications in clinical practice, such as generating website, video, and blog content. ChatGPT can also provide rapid and concise answers to general medical questions, like a search engine – but with more natural language processing and contextual understanding. Additionally, the algorithm can draft generic medical documents, including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts.
Another useful feature of ChatGPT is its ability to provide accurate and conversational language translations, thus serving as an interpreter during clinic visits in situations where a human translator is not available. It also has potential uses in clinical research by finding resources, formulating hypotheses, drafting study protocols, and collecting large amounts of data in short periods of time. Other possibilities include survey administration, clinical trial recruitment, and automatic medication monitoring.
GPT-4, the latest version of ChatGPT, is reported to have greater problem-solving abilities and an even broader knowledge base. Among its claimed skills are the ability to find the latest literature in a given area, write a discharge summary for a patient following an uncomplicated surgery, and an image analysis feature to identify objects in photos. GPT-4 has been praised as having “the potential to help drive medical innovation, from aiding with patient discharge notes, summarizing recent clinical trials, providing information on ethical guidelines, and much more.”
Bard, an AI “chat bot” introduced by Google earlier this year, intends to leverage Google’s enormous database to compete with ChatGPT in providing answers to medical questions. Bard also hopes to play a pivotal role in expanding telemedicine and remote care via Google’s secure connections and access to patient records and medical history, and “facilitate seamless communication through appointment scheduling, messaging, and sharing medical images,” according to PackT, a website for IT professionals. The company claims that Bard’s integration of AI and machine learning capabilities will serve to elevate health care efficiency and patient outcomes, PackT says, and “the platform’s AI system quickly and accurately analyzes patient records, identifies patterns and trends, and aids medical professionals in developing effective treatment plans.”
Doximity has introduced an AI engine called DocsGPT, an encrypted, HIPAA-compliant writing assistant that, the company says, can draft any form of professional correspondence, including prior authorization letters, insurance appeals, patient support letters, and patient education materials. The service is available at no charge to all U.S. physicians and medical students through their Doximity accounts.
Microsoft has introduced several AI products. BioGPT is a language model specifically designed for health care. Compared with GPT models that are trained on more general text data, BioGPT is purported to have a deeper understanding of the language used in biomedical research and can generate more accurate and relevant outputs for biomedical tasks, such as drug discovery, disease classification, and clinical decision support. Fabric is another health care–specific data and analytics platform the company described in an announcement in May. It can combine data from sources such as electronic health records, images, lab systems, medical devices, and claims systems so hospitals and offices can standardize it and access it in the same place. Microsoft said the new tools will help eliminate the “time-consuming” process of searching through these sources one by one. Microsoft will also offer a new generative AI chatbot called the Azure Health Bot, which can pull information from a health organization’s own internal data as well as reputable external sources such as the Food and Drug Administration and the National Institutes of Health.
Several other AI products are available for clinicians. Tana served as an administrative aid and a clinical helper during the height of the COVID-19 pandemic, answering frequently asked questions, facilitating appointment management, and gathering preliminary medical information prior to teleconsultations. Dougall GPT is another AI chatbot tailored for health care professionals. It provides clinicians with AI-tuned answers to their queries, augmented by links to relevant, up-to-date, authoritative resources. It also assists in drafting patient instructions, consultation summaries, speeches, and professional correspondence. Wang has created Clinical Camel, an open-source health care–focused chatbot that assembles medical data with a combination of user-shared conversations and synthetic conversations derived from curated clinical articles. The Chinese company Baidu has rolled out Ernie as a potential rival to ChatGPT. You get the idea.
Of course, the inherent drawbacks of AI, such as producing false or biased information, perpetuating harmful stereotypes, and presenting information that has since been proven inaccurate or out-of-date, must always be kept in mind. All AI algorithms have been criticized for giving wrong answers, as their datasets are generally culled from information published in 2021 or earlier. Several of them have been shown to fabricate information – a phenomenon labeled “artificial hallucinations” in one article. “The scientific community must be vigilant in verifying the accuracy and reliability of the information provided by AI tools,” wrote the authors of that paper. “Researchers should use AI as an aid rather than a replacement for critical thinking and fact-checking.”
In the year since generative artificial intelligence (AI) software first began to emerge for use, the staggering pace and breadth of development has condensed years of growth and change into months and weeks. Among the settings where these tools may find the greatest straight-line relevance is private medical practice.
. A multitude of generative AI products with potential medical applications are now available, with new ones appearing almost weekly. (As always, I have no financial interest in any product or service mentioned in this column.)
Last month, I discussed ChatGPT, the best-known AI algorithm, and some of its applications in clinical practice, such as generating website, video, and blog content. ChatGPT can also provide rapid and concise answers to general medical questions, like a search engine – but with more natural language processing and contextual understanding. Additionally, the algorithm can draft generic medical documents, including templates for after-visit summaries, postprocedure instructions, referrals, prior authorization appeal letters, and educational handouts.
Another useful feature of ChatGPT is its ability to provide accurate and conversational language translations, thus serving as an interpreter during clinic visits in situations where a human translator is not available. It also has potential uses in clinical research by finding resources, formulating hypotheses, drafting study protocols, and collecting large amounts of data in short periods of time. Other possibilities include survey administration, clinical trial recruitment, and automatic medication monitoring.
GPT-4, the latest version of ChatGPT, is reported to have greater problem-solving abilities and an even broader knowledge base. Among its claimed skills are the ability to find the latest literature in a given area, write a discharge summary for a patient following an uncomplicated surgery, and an image analysis feature to identify objects in photos. GPT-4 has been praised as having “the potential to help drive medical innovation, from aiding with patient discharge notes, summarizing recent clinical trials, providing information on ethical guidelines, and much more.”
Bard, an AI “chat bot” introduced by Google earlier this year, intends to leverage Google’s enormous database to compete with ChatGPT in providing answers to medical questions. Bard also hopes to play a pivotal role in expanding telemedicine and remote care via Google’s secure connections and access to patient records and medical history, and “facilitate seamless communication through appointment scheduling, messaging, and sharing medical images,” according to PackT, a website for IT professionals. The company claims that Bard’s integration of AI and machine learning capabilities will serve to elevate health care efficiency and patient outcomes, PackT says, and “the platform’s AI system quickly and accurately analyzes patient records, identifies patterns and trends, and aids medical professionals in developing effective treatment plans.”
Doximity has introduced an AI engine called DocsGPT, an encrypted, HIPAA-compliant writing assistant that, the company says, can draft any form of professional correspondence, including prior authorization letters, insurance appeals, patient support letters, and patient education materials. The service is available at no charge to all U.S. physicians and medical students through their Doximity accounts.
Microsoft has introduced several AI products. BioGPT is a language model specifically designed for health care. Compared with GPT models that are trained on more general text data, BioGPT is purported to have a deeper understanding of the language used in biomedical research and can generate more accurate and relevant outputs for biomedical tasks, such as drug discovery, disease classification, and clinical decision support. Fabric is another health care–specific data and analytics platform the company described in an announcement in May. It can combine data from sources such as electronic health records, images, lab systems, medical devices, and claims systems so hospitals and offices can standardize it and access it in the same place. Microsoft said the new tools will help eliminate the “time-consuming” process of searching through these sources one by one. Microsoft will also offer a new generative AI chatbot called the Azure Health Bot, which can pull information from a health organization’s own internal data as well as reputable external sources such as the Food and Drug Administration and the National Institutes of Health.
Several other AI products are available for clinicians. Tana served as an administrative aid and a clinical helper during the height of the COVID-19 pandemic, answering frequently asked questions, facilitating appointment management, and gathering preliminary medical information prior to teleconsultations. Dougall GPT is another AI chatbot tailored for health care professionals. It provides clinicians with AI-tuned answers to their queries, augmented by links to relevant, up-to-date, authoritative resources. It also assists in drafting patient instructions, consultation summaries, speeches, and professional correspondence. Wang has created Clinical Camel, an open-source health care–focused chatbot that assembles medical data with a combination of user-shared conversations and synthetic conversations derived from curated clinical articles. The Chinese company Baidu has rolled out Ernie as a potential rival to ChatGPT. You get the idea.
Of course, the inherent drawbacks of AI, such as producing false or biased information, perpetuating harmful stereotypes, and presenting information that has since been proven inaccurate or out-of-date, must always be kept in mind. All AI algorithms have been criticized for giving wrong answers, as their datasets are generally culled from information published in 2021 or earlier. Several of them have been shown to fabricate information – a phenomenon labeled “artificial hallucinations” in one article. “The scientific community must be vigilant in verifying the accuracy and reliability of the information provided by AI tools,” wrote the authors of that paper. “Researchers should use AI as an aid rather than a replacement for critical thinking and fact-checking.”
Neoadjuvant advantages: Treating locally advanced lung cancer
Many of you saw the press release from Merck announcing that their randomized trial comparing chemo with chemo plus pembrolizumab in the neoadjuvant setting led to improved event-free survival and also improved pathologic complete response rate.
This comes in addition to the data from the AstraZeneca trial with durvalumab saying they’ve already achieved their endpoint of higher pathologic complete response rate vs. chemotherapy alone and also the data with nivolumab from Bristol-Myers Squibb saying that nivolumab plus chemotherapy leads to a better event-free survival and a better pathologic complete response rate. That information has led to Food and Drug Administration approval for their regimen.
We’re running the table with these very positive data, and I think it’s just a sign that the approach is safe and effective.
A huge question has come up. I just came from a meeting of lung cancer experts asking what to do if you have a patient with a small tumor, for example, a 3-cm tumor. Do you recommend immediate surgery followed by adjuvant therapy, chemotherapy, and then a checkpoint inhibitor if appropriate? Or do you proceed with neoadjuvant therapy if appropriate? The truth is that it’s a very difficult decision.
We have overwhelming data that the neoadjuvant approach works for that patient. Please remember that this is a clinically staged patient. This is not the patient after their surgery, where I think we have a very clear path. We have adjuvant data and adjuvant trials for those patients.
For the patient who’s in your office with a small tumor or a small tumor and only hilar lymphadenopathy, the decision there isn’t data driven, but rather it is experience driven. The data that are out there right now suggest that neoadjuvant therapy is a better way to go. Why is that?
Well, I think that the first reason is that it is probably a better regimen. I think many of you saw the recent clinical trial by Patel and colleagues in the New England Journal of Medicine with melanoma. It was an interesting trial. They gave a checkpoint inhibitor for 18 doses after surgery for melanoma versus three doses of checkpoint inhibitor, surgery, and then 15 doses of the checkpoint inhibitor.
It was 18 doses versus 18 doses, with the only difference being the three doses before surgery. Lo and behold, the three doses before surgery led to a better event-free survival.
There are preclinical data in lung cancer demonstrating that the same thing is true. Tina Cascone published on that years ago. We could talk about why, but it appears that neoadjuvant is just better.
There are other advantages to it as well. I think a big one is that all the information shows that it’s better tolerated, so you’re more likely to give all the drug. You can see if the drug isn’t working, and you can stop the drug. Also, if the drug is causing a side effect, you can see whether it’s working or not and use that decision to stop. It’s different than when you’re giving a drug in the adjuvant setting where you don’t really know whether it is working or not.
I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group. You need to weigh the pros and cons I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group coming in. It’s already an FDA-approved regimen with nivolumab and chemotherapy, and I think we’re moving to making that our standard of care now.
The way to handle it today, though, is to convene your multidisciplinary panel about every patient other than those with the tiniest of lung cancers and put your heads together to see what the best treatment is for that patient.
Dr. Kris is professor of medicine, Weill Cornell Medicine, and the William and Joy Ruane Chair in Thoracic Oncology, Memorial Sloan Kettering Cancer Center, both in New York. He disclosed ties with Ariad Pharmaceuticals, AstraZeneca, Pfizer, PUMA, and Roche/Genentech.
A version of this article appeared on Medscape.com.
Many of you saw the press release from Merck announcing that their randomized trial comparing chemo with chemo plus pembrolizumab in the neoadjuvant setting led to improved event-free survival and also improved pathologic complete response rate.
This comes in addition to the data from the AstraZeneca trial with durvalumab saying they’ve already achieved their endpoint of higher pathologic complete response rate vs. chemotherapy alone and also the data with nivolumab from Bristol-Myers Squibb saying that nivolumab plus chemotherapy leads to a better event-free survival and a better pathologic complete response rate. That information has led to Food and Drug Administration approval for their regimen.
We’re running the table with these very positive data, and I think it’s just a sign that the approach is safe and effective.
A huge question has come up. I just came from a meeting of lung cancer experts asking what to do if you have a patient with a small tumor, for example, a 3-cm tumor. Do you recommend immediate surgery followed by adjuvant therapy, chemotherapy, and then a checkpoint inhibitor if appropriate? Or do you proceed with neoadjuvant therapy if appropriate? The truth is that it’s a very difficult decision.
We have overwhelming data that the neoadjuvant approach works for that patient. Please remember that this is a clinically staged patient. This is not the patient after their surgery, where I think we have a very clear path. We have adjuvant data and adjuvant trials for those patients.
For the patient who’s in your office with a small tumor or a small tumor and only hilar lymphadenopathy, the decision there isn’t data driven, but rather it is experience driven. The data that are out there right now suggest that neoadjuvant therapy is a better way to go. Why is that?
Well, I think that the first reason is that it is probably a better regimen. I think many of you saw the recent clinical trial by Patel and colleagues in the New England Journal of Medicine with melanoma. It was an interesting trial. They gave a checkpoint inhibitor for 18 doses after surgery for melanoma versus three doses of checkpoint inhibitor, surgery, and then 15 doses of the checkpoint inhibitor.
It was 18 doses versus 18 doses, with the only difference being the three doses before surgery. Lo and behold, the three doses before surgery led to a better event-free survival.
There are preclinical data in lung cancer demonstrating that the same thing is true. Tina Cascone published on that years ago. We could talk about why, but it appears that neoadjuvant is just better.
There are other advantages to it as well. I think a big one is that all the information shows that it’s better tolerated, so you’re more likely to give all the drug. You can see if the drug isn’t working, and you can stop the drug. Also, if the drug is causing a side effect, you can see whether it’s working or not and use that decision to stop. It’s different than when you’re giving a drug in the adjuvant setting where you don’t really know whether it is working or not.
I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group. You need to weigh the pros and cons I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group coming in. It’s already an FDA-approved regimen with nivolumab and chemotherapy, and I think we’re moving to making that our standard of care now.
The way to handle it today, though, is to convene your multidisciplinary panel about every patient other than those with the tiniest of lung cancers and put your heads together to see what the best treatment is for that patient.
Dr. Kris is professor of medicine, Weill Cornell Medicine, and the William and Joy Ruane Chair in Thoracic Oncology, Memorial Sloan Kettering Cancer Center, both in New York. He disclosed ties with Ariad Pharmaceuticals, AstraZeneca, Pfizer, PUMA, and Roche/Genentech.
A version of this article appeared on Medscape.com.
Many of you saw the press release from Merck announcing that their randomized trial comparing chemo with chemo plus pembrolizumab in the neoadjuvant setting led to improved event-free survival and also improved pathologic complete response rate.
This comes in addition to the data from the AstraZeneca trial with durvalumab saying they’ve already achieved their endpoint of higher pathologic complete response rate vs. chemotherapy alone and also the data with nivolumab from Bristol-Myers Squibb saying that nivolumab plus chemotherapy leads to a better event-free survival and a better pathologic complete response rate. That information has led to Food and Drug Administration approval for their regimen.
We’re running the table with these very positive data, and I think it’s just a sign that the approach is safe and effective.
A huge question has come up. I just came from a meeting of lung cancer experts asking what to do if you have a patient with a small tumor, for example, a 3-cm tumor. Do you recommend immediate surgery followed by adjuvant therapy, chemotherapy, and then a checkpoint inhibitor if appropriate? Or do you proceed with neoadjuvant therapy if appropriate? The truth is that it’s a very difficult decision.
We have overwhelming data that the neoadjuvant approach works for that patient. Please remember that this is a clinically staged patient. This is not the patient after their surgery, where I think we have a very clear path. We have adjuvant data and adjuvant trials for those patients.
For the patient who’s in your office with a small tumor or a small tumor and only hilar lymphadenopathy, the decision there isn’t data driven, but rather it is experience driven. The data that are out there right now suggest that neoadjuvant therapy is a better way to go. Why is that?
Well, I think that the first reason is that it is probably a better regimen. I think many of you saw the recent clinical trial by Patel and colleagues in the New England Journal of Medicine with melanoma. It was an interesting trial. They gave a checkpoint inhibitor for 18 doses after surgery for melanoma versus three doses of checkpoint inhibitor, surgery, and then 15 doses of the checkpoint inhibitor.
It was 18 doses versus 18 doses, with the only difference being the three doses before surgery. Lo and behold, the three doses before surgery led to a better event-free survival.
There are preclinical data in lung cancer demonstrating that the same thing is true. Tina Cascone published on that years ago. We could talk about why, but it appears that neoadjuvant is just better.
There are other advantages to it as well. I think a big one is that all the information shows that it’s better tolerated, so you’re more likely to give all the drug. You can see if the drug isn’t working, and you can stop the drug. Also, if the drug is causing a side effect, you can see whether it’s working or not and use that decision to stop. It’s different than when you’re giving a drug in the adjuvant setting where you don’t really know whether it is working or not.
I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group. You need to weigh the pros and cons I think that it’s time to change some of our standards. When patients appear with lung cancers other than tiny ones that might be detected through screening, you need to convene your multidisciplinary group coming in. It’s already an FDA-approved regimen with nivolumab and chemotherapy, and I think we’re moving to making that our standard of care now.
The way to handle it today, though, is to convene your multidisciplinary panel about every patient other than those with the tiniest of lung cancers and put your heads together to see what the best treatment is for that patient.
Dr. Kris is professor of medicine, Weill Cornell Medicine, and the William and Joy Ruane Chair in Thoracic Oncology, Memorial Sloan Kettering Cancer Center, both in New York. He disclosed ties with Ariad Pharmaceuticals, AstraZeneca, Pfizer, PUMA, and Roche/Genentech.
A version of this article appeared on Medscape.com.
New kids on the block for migraine treatment and prophylaxis
This transcript has been edited for clarity.
Dear colleagues, I’m Hans-Christoph Diener from the Faculty of Medicine at the University of Duisburg-Essen in Germany.
CGRP receptor agonists
Let me start with the treatment of acute migraine attacks. Until recently, we had analgesics, nonsteroidal anti-inflammatory drugs like ibuprofen, ergot alkaloids, and triptans. There are new developments, which are small molecules that are antagonists at the calcitonin gene-related peptide (CGRP) receptor. At the moment, we have three of them: rimegepant 75 mg, ubrogepant 50 mg or 100 mg, and zavegepant (a nasal spray) 10 mg.
These are all effective and superior to placebo. The 2-hour pain-free rate is somewhere between 25% and 30%. They have very few side effects; these include a little bit of nausea, somnolence, nasopharyngitis, and for zavegepant, the nasal spray, taste disturbance. In indirect comparisons, the so-called gepants are about as effective as ibuprofen and aspirin, and they seem to be less effective than sumatriptan 100 mg.
Unfortunately, until now, we have no direct comparison with triptans and we have no data demonstrating whether they are effective in people where triptans do not work. The major shortcoming is the cost in the United States. The cost per tablet or nasal spray is somewhere between $80 and $200. This means we definitely need more studies for these gepants.
Migraine prophylaxis
Let me move to the prophylaxis of migraine with drugs. Previously and still, we have all medications like beta-blockers, flunarizine, topiramate, valproic acid, amitriptyline, and candesartan, and for chronic migraine, onabotulinumtoxinA. We have now 5 years’ experience with the monoclonal antibodies against CGRP or the CGRP receptor like eptinezumab, erenumab, fremanezumab, and galcanezumab.
These are all equally effective. They reduce migraine-days between 3 and 7 per month. They are effective both in episodic and chronic migraine, and most importantly, they are effective in people with medication overuse headaches. The 50% responder rates are somewhere between 40% and 60%, and there are no significant differences between the four monoclonal antibodies.
The major advantage is a very good tolerability profile; very few patients terminate treatment because of adverse events. There has been, with one exception, no direct comparison of the monoclonal antibodies with traditional migraine preventive drugs or onabotulinumtoxinA. The only exception is a trial that compared topiramate and erenumab, showing that erenumab was definitely more effective and better tolerated.
At the moment, the recommendation is to use these monoclonal antibodies for 12 months in episodic migraine and 24 months in chronic migraine and then pause. It usually turns out that between 50% and 70% of these patients need to continue the treatment. If they are not working, there is a possibility to switch between the monoclonal antibodies, and the success rate after this is somewhere between 15% and 30%.
Gepants were also developed for the prevention of migraine. Here, we have rimegepant 75 mg every other day or atogepant 60 mg daily. They are effective, but in indirect comparisons, they are less effective than the monoclonal antibodies. At present, we have no comparative trials with monoclonal antibodies or the traditional migraine preventive drugs.
Potential patients are those who have needle phobia or patients who do not respond to monoclonal antibodies. Again, the biggest shortcoming is cost in the United States. The cost per year for migraine prevention or prophylaxis is between $12,000 and $20,000.
Finally, we also had very exciting news. There is a new therapeutic approach via PACAP. PACAP is pituitary adenylate cyclase-activating polypeptide, which has similar biological actions as CGRP but with additional actions. It could very well be that people who do not respond to a monoclonal antibody would respond to a monoclonal antibody against PACAP.
At the congress, the first randomized, placebo-controlled trial with a monoclonal antibody against PACAP was presented. This monoclonal antibody was effective in a population of people in whom prior preventive therapy had failed. A phase 3 study is planned, and most probably the PACAP monoclonal could work in people who do not respond to monoclonal antibodies against CGRP.
Dear colleagues, we have now many choices for the acute treatment of migraine and migraine prophylaxis. We have new kids on the block, and we have to learn more about how to use these drugs, their benefits, and their shortcomings.
He has disclosed the following relevant financial relationships:Received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Abbott; Addex Pharma; Alder; Allergan; Almirall; Amgen; Autonomic Technology; AstraZeneca; Bayer Vital; Berlin Chemie; Bristol-Myers Squibb; Boehringer Ingelheim; Chordate; CoAxia; Corimmun; Covidien; Coherex; CoLucid; Daiichi-Sankyo; D-Pharm; Electrocore; Fresenius; GlaxoSmithKline; Grunenthal; Janssen-Cilag; Labrys Biologics Lilly; La Roche; 3M Medica; MSD; Medtronic; Menarini; MindFrame; Minster; Neuroscore; Neurobiological Technologies; Novartis; Novo Nordisk; Johnson & Johnson; Knoll; Paion; Parke-Davis; Pierre Fabre; Pfizer; Schaper and Brummer; Sanofi-Aventis; Schering-Plough; Servier; Solvay; Syngis; St. Jude; Talecris; Thrombogenics; WebMD Global; Weber and Weber; Wyeth; and Yamanouchi.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I’m Hans-Christoph Diener from the Faculty of Medicine at the University of Duisburg-Essen in Germany.
CGRP receptor agonists
Let me start with the treatment of acute migraine attacks. Until recently, we had analgesics, nonsteroidal anti-inflammatory drugs like ibuprofen, ergot alkaloids, and triptans. There are new developments, which are small molecules that are antagonists at the calcitonin gene-related peptide (CGRP) receptor. At the moment, we have three of them: rimegepant 75 mg, ubrogepant 50 mg or 100 mg, and zavegepant (a nasal spray) 10 mg.
These are all effective and superior to placebo. The 2-hour pain-free rate is somewhere between 25% and 30%. They have very few side effects; these include a little bit of nausea, somnolence, nasopharyngitis, and for zavegepant, the nasal spray, taste disturbance. In indirect comparisons, the so-called gepants are about as effective as ibuprofen and aspirin, and they seem to be less effective than sumatriptan 100 mg.
Unfortunately, until now, we have no direct comparison with triptans and we have no data demonstrating whether they are effective in people where triptans do not work. The major shortcoming is the cost in the United States. The cost per tablet or nasal spray is somewhere between $80 and $200. This means we definitely need more studies for these gepants.
Migraine prophylaxis
Let me move to the prophylaxis of migraine with drugs. Previously and still, we have all medications like beta-blockers, flunarizine, topiramate, valproic acid, amitriptyline, and candesartan, and for chronic migraine, onabotulinumtoxinA. We have now 5 years’ experience with the monoclonal antibodies against CGRP or the CGRP receptor like eptinezumab, erenumab, fremanezumab, and galcanezumab.
These are all equally effective. They reduce migraine-days between 3 and 7 per month. They are effective both in episodic and chronic migraine, and most importantly, they are effective in people with medication overuse headaches. The 50% responder rates are somewhere between 40% and 60%, and there are no significant differences between the four monoclonal antibodies.
The major advantage is a very good tolerability profile; very few patients terminate treatment because of adverse events. There has been, with one exception, no direct comparison of the monoclonal antibodies with traditional migraine preventive drugs or onabotulinumtoxinA. The only exception is a trial that compared topiramate and erenumab, showing that erenumab was definitely more effective and better tolerated.
At the moment, the recommendation is to use these monoclonal antibodies for 12 months in episodic migraine and 24 months in chronic migraine and then pause. It usually turns out that between 50% and 70% of these patients need to continue the treatment. If they are not working, there is a possibility to switch between the monoclonal antibodies, and the success rate after this is somewhere between 15% and 30%.
Gepants were also developed for the prevention of migraine. Here, we have rimegepant 75 mg every other day or atogepant 60 mg daily. They are effective, but in indirect comparisons, they are less effective than the monoclonal antibodies. At present, we have no comparative trials with monoclonal antibodies or the traditional migraine preventive drugs.
Potential patients are those who have needle phobia or patients who do not respond to monoclonal antibodies. Again, the biggest shortcoming is cost in the United States. The cost per year for migraine prevention or prophylaxis is between $12,000 and $20,000.
Finally, we also had very exciting news. There is a new therapeutic approach via PACAP. PACAP is pituitary adenylate cyclase-activating polypeptide, which has similar biological actions as CGRP but with additional actions. It could very well be that people who do not respond to a monoclonal antibody would respond to a monoclonal antibody against PACAP.
At the congress, the first randomized, placebo-controlled trial with a monoclonal antibody against PACAP was presented. This monoclonal antibody was effective in a population of people in whom prior preventive therapy had failed. A phase 3 study is planned, and most probably the PACAP monoclonal could work in people who do not respond to monoclonal antibodies against CGRP.
Dear colleagues, we have now many choices for the acute treatment of migraine and migraine prophylaxis. We have new kids on the block, and we have to learn more about how to use these drugs, their benefits, and their shortcomings.
He has disclosed the following relevant financial relationships:Received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Abbott; Addex Pharma; Alder; Allergan; Almirall; Amgen; Autonomic Technology; AstraZeneca; Bayer Vital; Berlin Chemie; Bristol-Myers Squibb; Boehringer Ingelheim; Chordate; CoAxia; Corimmun; Covidien; Coherex; CoLucid; Daiichi-Sankyo; D-Pharm; Electrocore; Fresenius; GlaxoSmithKline; Grunenthal; Janssen-Cilag; Labrys Biologics Lilly; La Roche; 3M Medica; MSD; Medtronic; Menarini; MindFrame; Minster; Neuroscore; Neurobiological Technologies; Novartis; Novo Nordisk; Johnson & Johnson; Knoll; Paion; Parke-Davis; Pierre Fabre; Pfizer; Schaper and Brummer; Sanofi-Aventis; Schering-Plough; Servier; Solvay; Syngis; St. Jude; Talecris; Thrombogenics; WebMD Global; Weber and Weber; Wyeth; and Yamanouchi.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I’m Hans-Christoph Diener from the Faculty of Medicine at the University of Duisburg-Essen in Germany.
CGRP receptor agonists
Let me start with the treatment of acute migraine attacks. Until recently, we had analgesics, nonsteroidal anti-inflammatory drugs like ibuprofen, ergot alkaloids, and triptans. There are new developments, which are small molecules that are antagonists at the calcitonin gene-related peptide (CGRP) receptor. At the moment, we have three of them: rimegepant 75 mg, ubrogepant 50 mg or 100 mg, and zavegepant (a nasal spray) 10 mg.
These are all effective and superior to placebo. The 2-hour pain-free rate is somewhere between 25% and 30%. They have very few side effects; these include a little bit of nausea, somnolence, nasopharyngitis, and for zavegepant, the nasal spray, taste disturbance. In indirect comparisons, the so-called gepants are about as effective as ibuprofen and aspirin, and they seem to be less effective than sumatriptan 100 mg.
Unfortunately, until now, we have no direct comparison with triptans and we have no data demonstrating whether they are effective in people where triptans do not work. The major shortcoming is the cost in the United States. The cost per tablet or nasal spray is somewhere between $80 and $200. This means we definitely need more studies for these gepants.
Migraine prophylaxis
Let me move to the prophylaxis of migraine with drugs. Previously and still, we have all medications like beta-blockers, flunarizine, topiramate, valproic acid, amitriptyline, and candesartan, and for chronic migraine, onabotulinumtoxinA. We have now 5 years’ experience with the monoclonal antibodies against CGRP or the CGRP receptor like eptinezumab, erenumab, fremanezumab, and galcanezumab.
These are all equally effective. They reduce migraine-days between 3 and 7 per month. They are effective both in episodic and chronic migraine, and most importantly, they are effective in people with medication overuse headaches. The 50% responder rates are somewhere between 40% and 60%, and there are no significant differences between the four monoclonal antibodies.
The major advantage is a very good tolerability profile; very few patients terminate treatment because of adverse events. There has been, with one exception, no direct comparison of the monoclonal antibodies with traditional migraine preventive drugs or onabotulinumtoxinA. The only exception is a trial that compared topiramate and erenumab, showing that erenumab was definitely more effective and better tolerated.
At the moment, the recommendation is to use these monoclonal antibodies for 12 months in episodic migraine and 24 months in chronic migraine and then pause. It usually turns out that between 50% and 70% of these patients need to continue the treatment. If they are not working, there is a possibility to switch between the monoclonal antibodies, and the success rate after this is somewhere between 15% and 30%.
Gepants were also developed for the prevention of migraine. Here, we have rimegepant 75 mg every other day or atogepant 60 mg daily. They are effective, but in indirect comparisons, they are less effective than the monoclonal antibodies. At present, we have no comparative trials with monoclonal antibodies or the traditional migraine preventive drugs.
Potential patients are those who have needle phobia or patients who do not respond to monoclonal antibodies. Again, the biggest shortcoming is cost in the United States. The cost per year for migraine prevention or prophylaxis is between $12,000 and $20,000.
Finally, we also had very exciting news. There is a new therapeutic approach via PACAP. PACAP is pituitary adenylate cyclase-activating polypeptide, which has similar biological actions as CGRP but with additional actions. It could very well be that people who do not respond to a monoclonal antibody would respond to a monoclonal antibody against PACAP.
At the congress, the first randomized, placebo-controlled trial with a monoclonal antibody against PACAP was presented. This monoclonal antibody was effective in a population of people in whom prior preventive therapy had failed. A phase 3 study is planned, and most probably the PACAP monoclonal could work in people who do not respond to monoclonal antibodies against CGRP.
Dear colleagues, we have now many choices for the acute treatment of migraine and migraine prophylaxis. We have new kids on the block, and we have to learn more about how to use these drugs, their benefits, and their shortcomings.
He has disclosed the following relevant financial relationships:Received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Abbott; Addex Pharma; Alder; Allergan; Almirall; Amgen; Autonomic Technology; AstraZeneca; Bayer Vital; Berlin Chemie; Bristol-Myers Squibb; Boehringer Ingelheim; Chordate; CoAxia; Corimmun; Covidien; Coherex; CoLucid; Daiichi-Sankyo; D-Pharm; Electrocore; Fresenius; GlaxoSmithKline; Grunenthal; Janssen-Cilag; Labrys Biologics Lilly; La Roche; 3M Medica; MSD; Medtronic; Menarini; MindFrame; Minster; Neuroscore; Neurobiological Technologies; Novartis; Novo Nordisk; Johnson & Johnson; Knoll; Paion; Parke-Davis; Pierre Fabre; Pfizer; Schaper and Brummer; Sanofi-Aventis; Schering-Plough; Servier; Solvay; Syngis; St. Jude; Talecris; Thrombogenics; WebMD Global; Weber and Weber; Wyeth; and Yamanouchi.
Dr. Diener is professor, department of neurology, Stroke Center-Headache Center, University Duisburg-Essen (Germany).
A version of this article appeared on Medscape.com.
Where do you stand on the Middle East conflict?
“What do you think about the whole Israel thing?”
That question came at the end of an otherwise routine appointment.
Maybe she was just chatting. Maybe she wanted something deeper. I have no idea. I just said, “I don’t discuss those things with patients.”
My answer surprised her, but she didn’t push it. She paid her copay, scheduled a follow-up for 3 months, and left.
As I’ve written before, I try to avoid all news except the local weather. The sad reality is that most of it is bad and there’s nothing I can really do about it. It only upsets me, which isn’t good for my mental health and blood pressure, and if I can’t change it, what’s the point of knowing? It falls under the serenity prayer.
Of course, some news stories are too big not to hear something. I pass TVs in the doctors lounge or coffee house, hear others talking as I stand in line for the elevator, or see blurbs go by when checking the weather. It’s not entirely unavoidable.
I’m not trivializing the Middle East. But, to me, it’s not part of the doctor-patient relationship any more than my political views are. You run the risk of driving a wedge between you and the person you’re caring for. If you don’t like their opinion, you may find yourself less interested in them and their care. If they don’t like your opinion on news, they may start to question your ability as a doctor.
That’s not what we strive for, but it can be human nature. For better or worse we often reduce things to “us against them,” and learning someone is on the opposite side may, even subconsciously, alter how you treat them.
That’s not good, so to me it’s best not to know.
Some may think I’m being petty, or aloof, to be unwilling to discuss nonmedical issues of significance, but I don’t see it that way. Time is limited at the appointment and is best spent on medical care. Something unrelated to the visit that may alter my objective opinion of a patient – or theirs of me as a doctor – is best left out of it.
I’m here to be your doctor, and to do the best I can for you. I’m not here to be a debate partner. Whenever a patient asks me a question on politics or news I always think of the Monty Python skit “Argument Clinic.” That’s not why you’re here. There are plenty places to discuss such things. My office isn’t one of them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“What do you think about the whole Israel thing?”
That question came at the end of an otherwise routine appointment.
Maybe she was just chatting. Maybe she wanted something deeper. I have no idea. I just said, “I don’t discuss those things with patients.”
My answer surprised her, but she didn’t push it. She paid her copay, scheduled a follow-up for 3 months, and left.
As I’ve written before, I try to avoid all news except the local weather. The sad reality is that most of it is bad and there’s nothing I can really do about it. It only upsets me, which isn’t good for my mental health and blood pressure, and if I can’t change it, what’s the point of knowing? It falls under the serenity prayer.
Of course, some news stories are too big not to hear something. I pass TVs in the doctors lounge or coffee house, hear others talking as I stand in line for the elevator, or see blurbs go by when checking the weather. It’s not entirely unavoidable.
I’m not trivializing the Middle East. But, to me, it’s not part of the doctor-patient relationship any more than my political views are. You run the risk of driving a wedge between you and the person you’re caring for. If you don’t like their opinion, you may find yourself less interested in them and their care. If they don’t like your opinion on news, they may start to question your ability as a doctor.
That’s not what we strive for, but it can be human nature. For better or worse we often reduce things to “us against them,” and learning someone is on the opposite side may, even subconsciously, alter how you treat them.
That’s not good, so to me it’s best not to know.
Some may think I’m being petty, or aloof, to be unwilling to discuss nonmedical issues of significance, but I don’t see it that way. Time is limited at the appointment and is best spent on medical care. Something unrelated to the visit that may alter my objective opinion of a patient – or theirs of me as a doctor – is best left out of it.
I’m here to be your doctor, and to do the best I can for you. I’m not here to be a debate partner. Whenever a patient asks me a question on politics or news I always think of the Monty Python skit “Argument Clinic.” That’s not why you’re here. There are plenty places to discuss such things. My office isn’t one of them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“What do you think about the whole Israel thing?”
That question came at the end of an otherwise routine appointment.
Maybe she was just chatting. Maybe she wanted something deeper. I have no idea. I just said, “I don’t discuss those things with patients.”
My answer surprised her, but she didn’t push it. She paid her copay, scheduled a follow-up for 3 months, and left.
As I’ve written before, I try to avoid all news except the local weather. The sad reality is that most of it is bad and there’s nothing I can really do about it. It only upsets me, which isn’t good for my mental health and blood pressure, and if I can’t change it, what’s the point of knowing? It falls under the serenity prayer.
Of course, some news stories are too big not to hear something. I pass TVs in the doctors lounge or coffee house, hear others talking as I stand in line for the elevator, or see blurbs go by when checking the weather. It’s not entirely unavoidable.
I’m not trivializing the Middle East. But, to me, it’s not part of the doctor-patient relationship any more than my political views are. You run the risk of driving a wedge between you and the person you’re caring for. If you don’t like their opinion, you may find yourself less interested in them and their care. If they don’t like your opinion on news, they may start to question your ability as a doctor.
That’s not what we strive for, but it can be human nature. For better or worse we often reduce things to “us against them,” and learning someone is on the opposite side may, even subconsciously, alter how you treat them.
That’s not good, so to me it’s best not to know.
Some may think I’m being petty, or aloof, to be unwilling to discuss nonmedical issues of significance, but I don’t see it that way. Time is limited at the appointment and is best spent on medical care. Something unrelated to the visit that may alter my objective opinion of a patient – or theirs of me as a doctor – is best left out of it.
I’m here to be your doctor, and to do the best I can for you. I’m not here to be a debate partner. Whenever a patient asks me a question on politics or news I always think of the Monty Python skit “Argument Clinic.” That’s not why you’re here. There are plenty places to discuss such things. My office isn’t one of them.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
AI in medicine has a major Cassandra problem
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.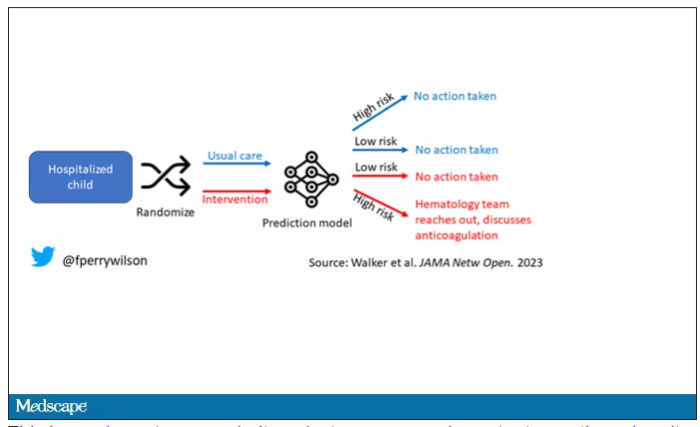
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.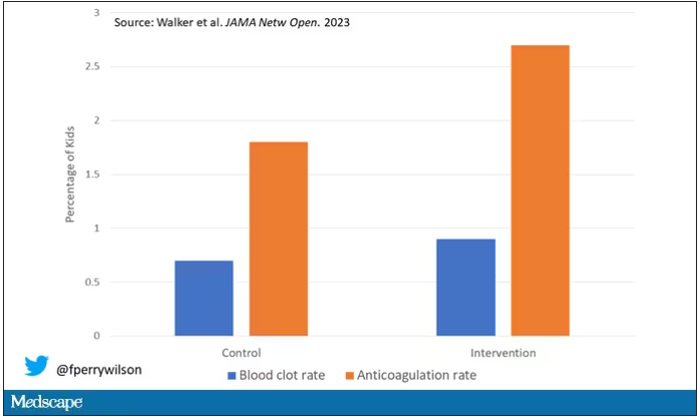
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.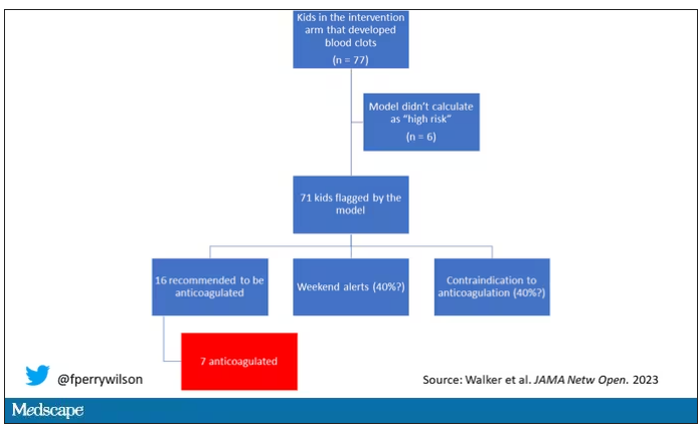
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.







