User login
April 2019 Highlights
DDNA19: The role of the microbiome in liver disease

Stephen Brant, MD, and Nikolaos Pyrsopoulos, MD, discuss the latest news and the role of the microbiome in liver diseases at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
Global Academy and this news organization are owned by the same company.

Stephen Brant, MD, and Nikolaos Pyrsopoulos, MD, discuss the latest news and the role of the microbiome in liver diseases at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
Global Academy and this news organization are owned by the same company.

Stephen Brant, MD, and Nikolaos Pyrsopoulos, MD, discuss the latest news and the role of the microbiome in liver diseases at Digestive Diseases: New Advances, jointly provided by Rutgers and Global Academy for Medical Education.
Global Academy and this news organization are owned by the same company.
REPORTING FROM DIGESTIVE DISEASES: NEW ADVANCES
HM19 Day One highlights: Plenary and sepsis updates (VIDEO)

Dr. Kranthi Sitammagari of Atrium Health in Monroe, N.C., and Dr. Marina Farah of Farah MD Consulting in Corvallis, Ore., offer their expert analysis of the plenary session and Updates on Sepsis session at HM19.

Dr. Kranthi Sitammagari of Atrium Health in Monroe, N.C., and Dr. Marina Farah of Farah MD Consulting in Corvallis, Ore., offer their expert analysis of the plenary session and Updates on Sepsis session at HM19.

Dr. Kranthi Sitammagari of Atrium Health in Monroe, N.C., and Dr. Marina Farah of Farah MD Consulting in Corvallis, Ore., offer their expert analysis of the plenary session and Updates on Sepsis session at HM19.
Novel chemo/PARP inhibitor strategy promising for advanced pancreatic cancer
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
ATLANTA – The current standard of care for patients with advanced pancreatic cancer is chemotherapy continued until patients experience disease progression, unacceptable toxicities, clinical decline, or death.
But a subset of patients with pancreatic cancer – approximately 5%-8% – have pathogenic mutations in homologous recombination genes such as BRCA1, BRCA2, or PALB2. The resulting homologous recombination deficiencies (HRD) make their cancers especially sensitive to platinum-based chemotherapy and, potentially, to poly (ADP-ribose) polymerase (PARP) inhibitors.
Now, investigators at the University of Pennsylvania, Philadelphia, are proposing to upend the conventional approach by treating patients with advanced pancreatic cancer and HRD with a novel strategy consisting of induction chemotherapy, followed by maintenance with the PARP inhibitor rucaparib (Rubraca).
In a video interview at the annual meeting of the American Society for Cancer Research, Kim A. Reiss Binder, MD, of the University of Pennsylvania, describes the rationale for treating this subset of patients with this novel strategy, outlines the promising progression-free and overall survival results in a clinical study, and discusses the potential for chemotherapy and PARP inhibitors in neoadjuvant or adjuvant settings for some patients with pancreatic cancer.
The study is sponsored by the Abramson Cancer Center and is funded by Clovis Oncology. Dr. Reiss Binder receives research funding from Clovis Oncology, Tesaro, Bristol-Myers Squibb, and Lilly Oncology.
REPORTING FROM AACR 2019
UNITY-NHL: Interim findings show activity, tolerability of umbralisib for R/R MZL
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
ATLANTA – The phosphoinositide 3-kinase (PI3K) delta inhibitor umbralisib is active and well tolerated as single-agent therapy in patients with relapsed or refractory marginal zone lymphoma, according to findings from the ongoing phase 2 UNITY-NHL study.
The best overall response rate (ORR) among 42 study participants with at least 9 months of follow-up was 52% by both independent review committee (IRC) and investigator assessment, and the complete response rate was 19%, Nathan H. Fowler, MD, reported at the annual meeting of the American Association for Cancer Research.
The ORR by IRC assessment for those with extranodal, nodal, and splenic disease was 57%, 42%, and 43%, respectively, and for those with prior chemo-immunotherapy and those refractory to their last line of therapy, the ORR was 53% and 38%, respectively, said Dr. Fowler of MD Anderson Cancer Center, Houston.
The clinical benefit rate was 88%, and the median duration of response and progression-free survival (PFS) have not been reached; 12-month PFS is estimated at 66%, he added.
Patients in the marginal zone lymphoma cohort of the multicohort trial, which is evaluating umbralisib both as monotherapy and as part of various combinations in patients with previously treated non-Hodgkin’s lymphoma, received single agent umbralisib at a dose of 800 mg daily until disease progression or unacceptable toxicity.
“Most patients who have responded continue on drug,” he said in a video interview.
Dr. Fowler also further discussed the findings to date with respect to response, adverse events, and future directions in the wake of the breakthrough therapy designation recently granted by the Food and Drug Administration for umbralisib based on these findings.
“Despite the fact that a lot of things work in the front line, when patients relapse, especially when they become refractory to rituximab, there are limited options available,” he said, noting that currently used treatments are associated with significant adverse event and short remission times. “We still need better options.”
REPORTING FROM AACR 2019
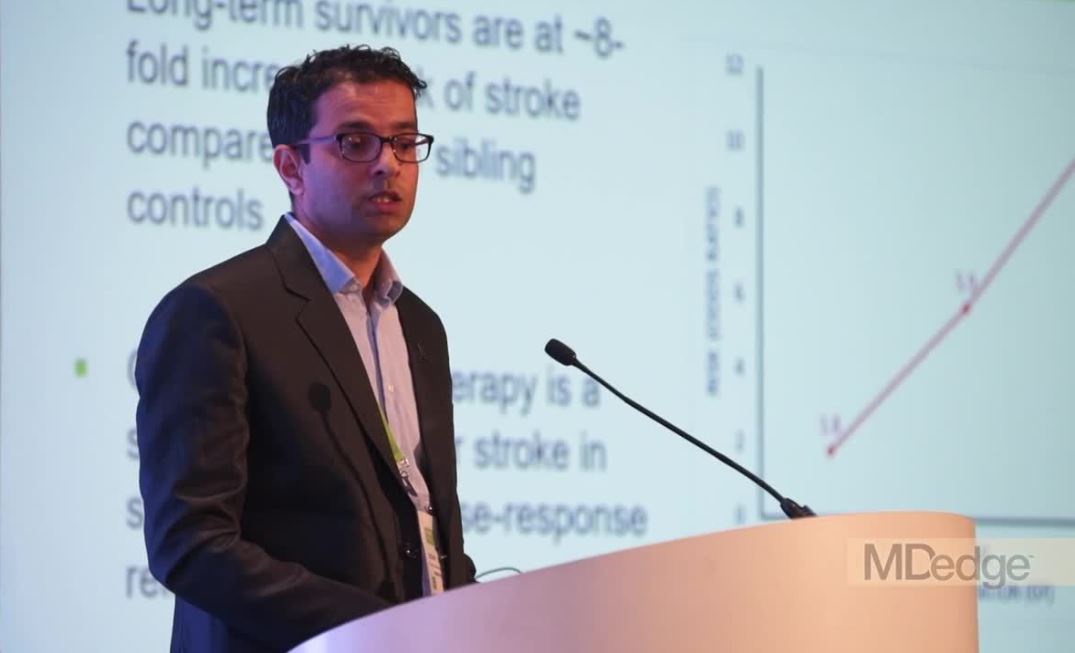
Genetic variant increases stroke risk in childhood cancer survivors
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
ATLANTA – Adult survivors of childhood cancers are at significantly greater risk than the general population for late-term complications related to therapy, including secondary cancers, cardiovascular disease, and cerebrovascular complications, including ischemic and hemorrhagic strokes.
In particular, childhood cancer survivors have an approximately eightfold higher risk for stroke, compared with their siblings, with a history of cranial irradiation being a strong, dose-dependent risk factor for stroke.
Researchers at St. Jude Children’s Research Hospital in Memphis, Tenn., are conducting a retrospective cohort study with prospective clinical follow-up and ongoing enrollment of childhood cancer survivors who are 5 or more years out of therapy.
The study includes publicly available, whole-genome sequencing data on 4,500 participants. Sifting through these data, Yadav Sapkota, PhD, a clinical research scientist at St. Jude, and his colleagues have identified a genetic variant strongly associated with stroke risk in survivors of European ancestry, and they have replicated the finding in survivors of African ancestry.
In a video interview at the annual meeting of the American Association for Cancer Research, Dr. Sapkota describes his group’s findings and potential research and clinical implications.
The study was sponsored by the National Cancer Institute and ALSAC, the fundraising and awareness organization of St. Jude. Dr. Sapkota declared no conflict of interest.
REPORTING FROM AACR 2019
Noninfected children of HIV-positive mothers have high rates of obesity
NEW ORLEANS – than are those with no such exposure, according to research that provides a compelling link between inflammatory activity in utero and subsequent risk of metabolic disorders.
Most supportive of that link was a near-linear inverse relationship between CD4 counts during the time of pregnancy and risk of both obesity and reactive respiratory disease more than a decade later, according to research presented by Lindsay Fourman, MD, an instructor in medicine at Massachusetts General Hospital, Boston, during the annual meeting of the Endocrine Society.
In this video interview, Dr. Fourman discusses the effort to understand the long-term health consequences of being exposed to HIV and antiretroviral therapies while in utero, a group known by the acronym HIV-exposed uninfected (HEU). With effective therapies now routinely preventing mother-to-child transmission, this population of children is growing quickly.
For this study, 50 HEU individuals were identified from a patient database. They were matched in a 3:1 ratio to a control group for a variety of demographic and socioeconomic variables. At a median age of 18 years, the HEU population was found to have a “strikingly” higher rate of obesity, compared with controls (42% vs. 25%, respectively; P = .04). The rate of reactive airway disease was similarly increased in the HEU group (40% vs. 24%; P = .04).
These data are important for considering health risks in an HEU population, but Dr. Fourman explained that it provides support for looking at metabolic risks from other in utero exposures linked to upregulated inflammation, such as gestational diabetes or obesity.
Dr Fourman and her colleagues reported no disclosures or financial conflicts of interest.
SOURCE: Fourman L et al. ENDO 2019, Session P10 (SAT-256).
NEW ORLEANS – than are those with no such exposure, according to research that provides a compelling link between inflammatory activity in utero and subsequent risk of metabolic disorders.
Most supportive of that link was a near-linear inverse relationship between CD4 counts during the time of pregnancy and risk of both obesity and reactive respiratory disease more than a decade later, according to research presented by Lindsay Fourman, MD, an instructor in medicine at Massachusetts General Hospital, Boston, during the annual meeting of the Endocrine Society.
In this video interview, Dr. Fourman discusses the effort to understand the long-term health consequences of being exposed to HIV and antiretroviral therapies while in utero, a group known by the acronym HIV-exposed uninfected (HEU). With effective therapies now routinely preventing mother-to-child transmission, this population of children is growing quickly.
For this study, 50 HEU individuals were identified from a patient database. They were matched in a 3:1 ratio to a control group for a variety of demographic and socioeconomic variables. At a median age of 18 years, the HEU population was found to have a “strikingly” higher rate of obesity, compared with controls (42% vs. 25%, respectively; P = .04). The rate of reactive airway disease was similarly increased in the HEU group (40% vs. 24%; P = .04).
These data are important for considering health risks in an HEU population, but Dr. Fourman explained that it provides support for looking at metabolic risks from other in utero exposures linked to upregulated inflammation, such as gestational diabetes or obesity.
Dr Fourman and her colleagues reported no disclosures or financial conflicts of interest.
SOURCE: Fourman L et al. ENDO 2019, Session P10 (SAT-256).
NEW ORLEANS – than are those with no such exposure, according to research that provides a compelling link between inflammatory activity in utero and subsequent risk of metabolic disorders.
Most supportive of that link was a near-linear inverse relationship between CD4 counts during the time of pregnancy and risk of both obesity and reactive respiratory disease more than a decade later, according to research presented by Lindsay Fourman, MD, an instructor in medicine at Massachusetts General Hospital, Boston, during the annual meeting of the Endocrine Society.
In this video interview, Dr. Fourman discusses the effort to understand the long-term health consequences of being exposed to HIV and antiretroviral therapies while in utero, a group known by the acronym HIV-exposed uninfected (HEU). With effective therapies now routinely preventing mother-to-child transmission, this population of children is growing quickly.
For this study, 50 HEU individuals were identified from a patient database. They were matched in a 3:1 ratio to a control group for a variety of demographic and socioeconomic variables. At a median age of 18 years, the HEU population was found to have a “strikingly” higher rate of obesity, compared with controls (42% vs. 25%, respectively; P = .04). The rate of reactive airway disease was similarly increased in the HEU group (40% vs. 24%; P = .04).
These data are important for considering health risks in an HEU population, but Dr. Fourman explained that it provides support for looking at metabolic risks from other in utero exposures linked to upregulated inflammation, such as gestational diabetes or obesity.
Dr Fourman and her colleagues reported no disclosures or financial conflicts of interest.
SOURCE: Fourman L et al. ENDO 2019, Session P10 (SAT-256).
REPORTING FROM ENDO 2019
Anti-EGFR TKI, MET inhibitor team up against drug-resistant NSCLC
ATLANTA – About 10%-25% of patients with epithelial growth factor receptor–(EGFR) mutant non–small cell lung cancer (NSCLC) have tumors with either MET amplification or another MET-based mechanism that leads to drug resistance.
In the TATTON trial, investigators are evaluating a combination of the EGFR-targeted tyrosine kinase inhibitor (TKI) osimertinib (Tagrisso) with savolitinib, an investigational MET inhibitor, for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents.
In a video interview at the annual meeting of the American Association for Cancer Research, Lecia Sequist, MD, from the Massachusetts General Hospital Cancer Center in Boston, discusses early results with the osimertinib/savolitinib combination in patients with disease progression after a first and/or second-generation EGFR TKI, or after a third-generation agent.
Dr. Sequist said results of TATTON suggest that it may be possible to overcome MET-driven drug-resistance mechanisms.
The TATTON trial is sponsored by AstraZeneca. Dr. Sequist reported serving as an advisory board member and receiving research support and honoraria from the company.
ATLANTA – About 10%-25% of patients with epithelial growth factor receptor–(EGFR) mutant non–small cell lung cancer (NSCLC) have tumors with either MET amplification or another MET-based mechanism that leads to drug resistance.
In the TATTON trial, investigators are evaluating a combination of the EGFR-targeted tyrosine kinase inhibitor (TKI) osimertinib (Tagrisso) with savolitinib, an investigational MET inhibitor, for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents.
In a video interview at the annual meeting of the American Association for Cancer Research, Lecia Sequist, MD, from the Massachusetts General Hospital Cancer Center in Boston, discusses early results with the osimertinib/savolitinib combination in patients with disease progression after a first and/or second-generation EGFR TKI, or after a third-generation agent.
Dr. Sequist said results of TATTON suggest that it may be possible to overcome MET-driven drug-resistance mechanisms.
The TATTON trial is sponsored by AstraZeneca. Dr. Sequist reported serving as an advisory board member and receiving research support and honoraria from the company.
ATLANTA – About 10%-25% of patients with epithelial growth factor receptor–(EGFR) mutant non–small cell lung cancer (NSCLC) have tumors with either MET amplification or another MET-based mechanism that leads to drug resistance.
In the TATTON trial, investigators are evaluating a combination of the EGFR-targeted tyrosine kinase inhibitor (TKI) osimertinib (Tagrisso) with savolitinib, an investigational MET inhibitor, for safety and activity against MET-driven NSCLC in patients with disease that has progressed on one or more prior EGFR-targeted agents.
In a video interview at the annual meeting of the American Association for Cancer Research, Lecia Sequist, MD, from the Massachusetts General Hospital Cancer Center in Boston, discusses early results with the osimertinib/savolitinib combination in patients with disease progression after a first and/or second-generation EGFR TKI, or after a third-generation agent.
Dr. Sequist said results of TATTON suggest that it may be possible to overcome MET-driven drug-resistance mechanisms.
The TATTON trial is sponsored by AstraZeneca. Dr. Sequist reported serving as an advisory board member and receiving research support and honoraria from the company.
REPORTING FROM AACR 2019
CAR T cells target HER2 expression in advanced sarcomas
ATLANTA – Sarcomas of bone and soft tissues are considered to be “antigenically cold” tumors, with few identifiable mutations that may be susceptible to targeted therapies.
Some sarcoma subtypes such as osteosarcoma and rhabomyosarcoma, however, frequently express the human epidermal growth factor receptor 2 on tumor surfaces. Although HER2 expression in these tumors is at too low a level for HER2-targeted therapies such as trastuzumab (Herceptin), HER2 appears to be an opportunistic target for chimeric antigen receptor (CAR) T-cell therapy, according to Shoba Navai, MD, from Baylor College of Medicine, Houston.
In a video interview at the 2019 annual meeting of the American Association for Cancer Research, Dr. Navai describes her team’s early experience using a HER2-targeted CAR-T cell construct and preinfusion lymphodepletion in patients with advanced sarcomas.
Development of the CAR-T cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai reported having no disclosures.
ATLANTA – Sarcomas of bone and soft tissues are considered to be “antigenically cold” tumors, with few identifiable mutations that may be susceptible to targeted therapies.
Some sarcoma subtypes such as osteosarcoma and rhabomyosarcoma, however, frequently express the human epidermal growth factor receptor 2 on tumor surfaces. Although HER2 expression in these tumors is at too low a level for HER2-targeted therapies such as trastuzumab (Herceptin), HER2 appears to be an opportunistic target for chimeric antigen receptor (CAR) T-cell therapy, according to Shoba Navai, MD, from Baylor College of Medicine, Houston.
In a video interview at the 2019 annual meeting of the American Association for Cancer Research, Dr. Navai describes her team’s early experience using a HER2-targeted CAR-T cell construct and preinfusion lymphodepletion in patients with advanced sarcomas.
Development of the CAR-T cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai reported having no disclosures.
ATLANTA – Sarcomas of bone and soft tissues are considered to be “antigenically cold” tumors, with few identifiable mutations that may be susceptible to targeted therapies.
Some sarcoma subtypes such as osteosarcoma and rhabomyosarcoma, however, frequently express the human epidermal growth factor receptor 2 on tumor surfaces. Although HER2 expression in these tumors is at too low a level for HER2-targeted therapies such as trastuzumab (Herceptin), HER2 appears to be an opportunistic target for chimeric antigen receptor (CAR) T-cell therapy, according to Shoba Navai, MD, from Baylor College of Medicine, Houston.
In a video interview at the 2019 annual meeting of the American Association for Cancer Research, Dr. Navai describes her team’s early experience using a HER2-targeted CAR-T cell construct and preinfusion lymphodepletion in patients with advanced sarcomas.
Development of the CAR-T cell construct is supported by the Cancer Prevention & Research Institute of Texas, Stand Up to Cancer, the St. Baldrick’s Foundation, Cookies for Kids’ Cancer, Alex’s Lemonade Stand, and a grant from the National Institutes of Health. Dr. Navai reported having no disclosures.
REPORTING FROM AACR 2019
HM19 Day One highlights: Pulmonary, critical care, and perioperative care updates (VIDEO)
Marina Farah, MD, MHA, and Kranthi Sitammagari, MD, editorial board members for The Hospitalist, discuss Day One highlights from HM19.

Marina Farah, MD, MHA, and Kranthi Sitammagari, MD, editorial board members for The Hospitalist, discuss Day One highlights from HM19.

Marina Farah, MD, MHA, and Kranthi Sitammagari, MD, editorial board members for The Hospitalist, discuss Day One highlights from HM19.