User login
Tanuja Chitnis: “It’s the right time” for precision medicine in MS
DALLAS – In an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, the conference’s cochair, Tanuja Chitnis, MD, explained why this is the right time to take a deep dive into what precision medicine means in MS, for patients and physicians alike.
“We chose the topic of precision medicine for this forum because it’s a really timely issue,” said Dr. Chitnis, noting that there are now over 16 approved treatments for MS, and an increasing recognition that “not every patient has the same disease course.”
“It’s the right time to think about individualized treatment, and not a one-size-fits-all approach,” she said, noting that clinicians and patients stand to benefit from guidance about treatment choices.
“In addition, we are aided by the number of biomarkers that are becoming available,” including quantitative MRI and serum biomarkers. “I think we – as a field – need to understand how to use these in clinical settings in order to guide treatment decisions,” said Dr. Chitnis, professor of neurology at Harvard Medical School, Boston.
Advances in data science are allowing the connection of disparate kinds of data for discovery and hypothesis testing and validation, said Dr. Chitnis, who serves as medical director for the large longitudinal CLIMB study. The study follows about 2,000 patients who have yearly neurologic examinations and brain MRI; serum biomarkers and self-report data are also acquired annually.
“Network science can help in the precision medicine approach to multiple sclerosis, because we have a very clear understanding that MS is a complex disease. It is not one gene; it is not one modality,” she said.
Dr. Chitnis reported that she has received research funding from multiple pharmaceutical companies.
DALLAS – In an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, the conference’s cochair, Tanuja Chitnis, MD, explained why this is the right time to take a deep dive into what precision medicine means in MS, for patients and physicians alike.
“We chose the topic of precision medicine for this forum because it’s a really timely issue,” said Dr. Chitnis, noting that there are now over 16 approved treatments for MS, and an increasing recognition that “not every patient has the same disease course.”
“It’s the right time to think about individualized treatment, and not a one-size-fits-all approach,” she said, noting that clinicians and patients stand to benefit from guidance about treatment choices.
“In addition, we are aided by the number of biomarkers that are becoming available,” including quantitative MRI and serum biomarkers. “I think we – as a field – need to understand how to use these in clinical settings in order to guide treatment decisions,” said Dr. Chitnis, professor of neurology at Harvard Medical School, Boston.
Advances in data science are allowing the connection of disparate kinds of data for discovery and hypothesis testing and validation, said Dr. Chitnis, who serves as medical director for the large longitudinal CLIMB study. The study follows about 2,000 patients who have yearly neurologic examinations and brain MRI; serum biomarkers and self-report data are also acquired annually.
“Network science can help in the precision medicine approach to multiple sclerosis, because we have a very clear understanding that MS is a complex disease. It is not one gene; it is not one modality,” she said.
Dr. Chitnis reported that she has received research funding from multiple pharmaceutical companies.
DALLAS – In an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis, the conference’s cochair, Tanuja Chitnis, MD, explained why this is the right time to take a deep dive into what precision medicine means in MS, for patients and physicians alike.
“We chose the topic of precision medicine for this forum because it’s a really timely issue,” said Dr. Chitnis, noting that there are now over 16 approved treatments for MS, and an increasing recognition that “not every patient has the same disease course.”
“It’s the right time to think about individualized treatment, and not a one-size-fits-all approach,” she said, noting that clinicians and patients stand to benefit from guidance about treatment choices.
“In addition, we are aided by the number of biomarkers that are becoming available,” including quantitative MRI and serum biomarkers. “I think we – as a field – need to understand how to use these in clinical settings in order to guide treatment decisions,” said Dr. Chitnis, professor of neurology at Harvard Medical School, Boston.
Advances in data science are allowing the connection of disparate kinds of data for discovery and hypothesis testing and validation, said Dr. Chitnis, who serves as medical director for the large longitudinal CLIMB study. The study follows about 2,000 patients who have yearly neurologic examinations and brain MRI; serum biomarkers and self-report data are also acquired annually.
“Network science can help in the precision medicine approach to multiple sclerosis, because we have a very clear understanding that MS is a complex disease. It is not one gene; it is not one modality,” she said.
Dr. Chitnis reported that she has received research funding from multiple pharmaceutical companies.
REPORTING FROM ACTRIMS FORUM 2019
Smartphone-based visual tests for MS patients show promise
DALLAS – A battery of smartphone-based tests has been developed to help detect visual pathway disturbances in MS patients and to follow them over time.
“One of the ideas is, can you design something that’s so easy to use and quick that it’s not a burden on the patient?” Randy H. Kardon, MD, PhD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “The other was to test a couple of different modalities. By that I mean we test visual acuity, contrast sensitivity, and critical flicker fusion, which is a way of measuring the speed of conduction of nerves in the visual system.”
Dr. Kardon, professor of neuro-ophthalmology at the University of Iowa, Iowa City, worked with colleagues from Aalborg University, Denmark, to study these tests and a novel measure known as the vanishing optotype on 117 patients with MS and 103 age-matched controls. They found that the tests “very nicely discriminated between normal eyes from patients that had MS,” said Dr. Kardon, director of the Iowa City VA Center for Prevention and Treatment of Visual Loss. “Furthermore, we could determine which eyes from the MS patients had previous optic neuritis and which eyes hadn’t. We’re now looking for partners to go forward with larger studies to validate it further and refine these tests even more.”
Dr. Kardon disclosed that he has received funding from the National Eye Institute, the Department of Defense, and from VA Rehabilitation Research and Development. He was also a member of the Novartis steering committee for the OCTiMS study and is a cofounder of MedFace and FaceX.
DALLAS – A battery of smartphone-based tests has been developed to help detect visual pathway disturbances in MS patients and to follow them over time.
“One of the ideas is, can you design something that’s so easy to use and quick that it’s not a burden on the patient?” Randy H. Kardon, MD, PhD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “The other was to test a couple of different modalities. By that I mean we test visual acuity, contrast sensitivity, and critical flicker fusion, which is a way of measuring the speed of conduction of nerves in the visual system.”
Dr. Kardon, professor of neuro-ophthalmology at the University of Iowa, Iowa City, worked with colleagues from Aalborg University, Denmark, to study these tests and a novel measure known as the vanishing optotype on 117 patients with MS and 103 age-matched controls. They found that the tests “very nicely discriminated between normal eyes from patients that had MS,” said Dr. Kardon, director of the Iowa City VA Center for Prevention and Treatment of Visual Loss. “Furthermore, we could determine which eyes from the MS patients had previous optic neuritis and which eyes hadn’t. We’re now looking for partners to go forward with larger studies to validate it further and refine these tests even more.”
Dr. Kardon disclosed that he has received funding from the National Eye Institute, the Department of Defense, and from VA Rehabilitation Research and Development. He was also a member of the Novartis steering committee for the OCTiMS study and is a cofounder of MedFace and FaceX.
DALLAS – A battery of smartphone-based tests has been developed to help detect visual pathway disturbances in MS patients and to follow them over time.
“One of the ideas is, can you design something that’s so easy to use and quick that it’s not a burden on the patient?” Randy H. Kardon, MD, PhD, said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “The other was to test a couple of different modalities. By that I mean we test visual acuity, contrast sensitivity, and critical flicker fusion, which is a way of measuring the speed of conduction of nerves in the visual system.”
Dr. Kardon, professor of neuro-ophthalmology at the University of Iowa, Iowa City, worked with colleagues from Aalborg University, Denmark, to study these tests and a novel measure known as the vanishing optotype on 117 patients with MS and 103 age-matched controls. They found that the tests “very nicely discriminated between normal eyes from patients that had MS,” said Dr. Kardon, director of the Iowa City VA Center for Prevention and Treatment of Visual Loss. “Furthermore, we could determine which eyes from the MS patients had previous optic neuritis and which eyes hadn’t. We’re now looking for partners to go forward with larger studies to validate it further and refine these tests even more.”
Dr. Kardon disclosed that he has received funding from the National Eye Institute, the Department of Defense, and from VA Rehabilitation Research and Development. He was also a member of the Novartis steering committee for the OCTiMS study and is a cofounder of MedFace and FaceX.
REPORTING FROM ACTRIMS FORUM 2019
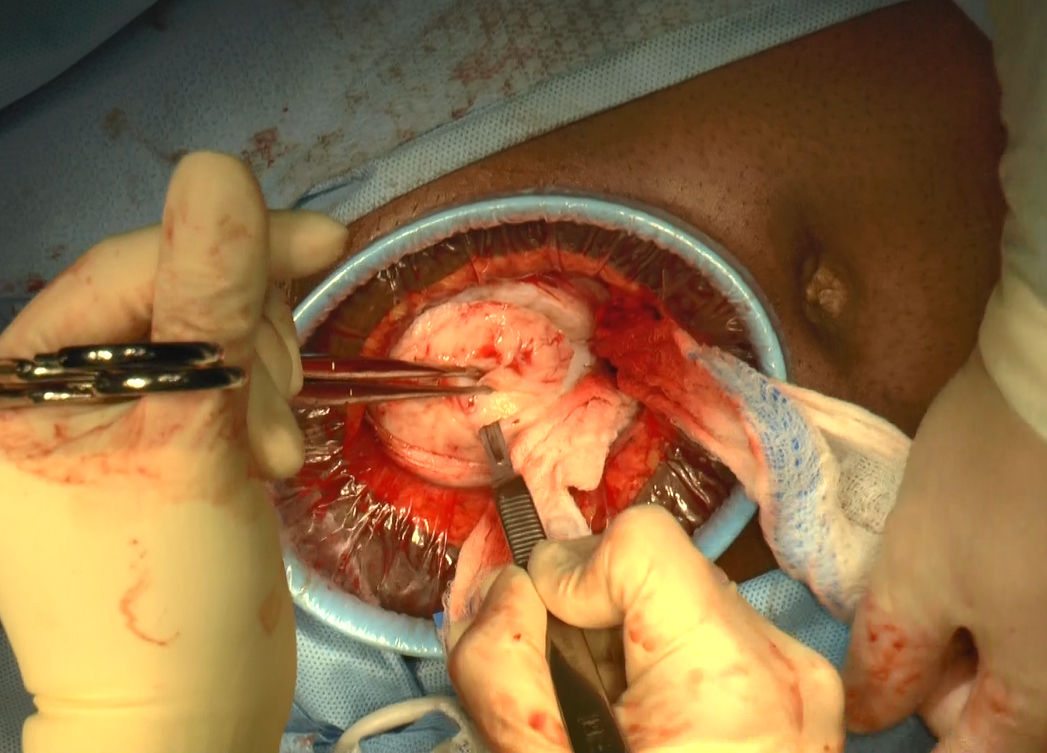
Minilaparotomy: Minimally invasive approach to abdominal myomectomy
A minilaparotomy is loosely defined as a laparotomy measuring between 4 cm and 6 cm. For the appropriate surgical candidate, a minilaparotomy is a useful alternative to laparotomy or laparoscopy, especially for large pathology.1 Benefits of minilaparotomy include improved pain management and postoperative recovery, as well as improved cosmetic outcome, with comparable blood loss and operative time.2,3

In this video, we illustrate the key surgical steps of a minilaparotomy for the removal of large fibroids. These steps include:
- strategic vertical skin incision
- use of a self-retaining retractor
- infiltrate myometrium with dilute vasopressin
- strategic hysterotomy
- use of tenaculum for upward traction
- 10# blade scalpels for the “lemon wedge” coring technique
- layered closure.
Minilaparotomy myomectomy can be an excellent minimally invasive alternative to a traditional “full laparotomy” for women with large fibroids.
We hope that you find this video beneficial to your clinical practice.
>> Arnold P. Advincula, MD
Laparoscopic bilateral salpingo-oophorectomy via minilaparotomy assistance for the massively enlarged adnexal mass
- Pelosi MA 2nd, Pelosi MA 3rd. Pelosi minilaparotomy hysterectomy: a non-endoscopic minimally invasive alternative to laparoscopy and laparotomy. Surg Technol Int. 2004;13:157-167.
- Fanafani F, Fagotti A, Longo R. Minilaparotomy in the management of benign gynecologic disease. Eur J Obstet Gynecol Reprod Biol. 2005;119:232-236.
- Glasser MH. Minilaparotomy: a minimally invasive alternative for major gynecologic abdominal surgery. Perm J. 2005;9:41-45.
A minilaparotomy is loosely defined as a laparotomy measuring between 4 cm and 6 cm. For the appropriate surgical candidate, a minilaparotomy is a useful alternative to laparotomy or laparoscopy, especially for large pathology.1 Benefits of minilaparotomy include improved pain management and postoperative recovery, as well as improved cosmetic outcome, with comparable blood loss and operative time.2,3

In this video, we illustrate the key surgical steps of a minilaparotomy for the removal of large fibroids. These steps include:
- strategic vertical skin incision
- use of a self-retaining retractor
- infiltrate myometrium with dilute vasopressin
- strategic hysterotomy
- use of tenaculum for upward traction
- 10# blade scalpels for the “lemon wedge” coring technique
- layered closure.
Minilaparotomy myomectomy can be an excellent minimally invasive alternative to a traditional “full laparotomy” for women with large fibroids.
We hope that you find this video beneficial to your clinical practice.
>> Arnold P. Advincula, MD
Laparoscopic bilateral salpingo-oophorectomy via minilaparotomy assistance for the massively enlarged adnexal mass
A minilaparotomy is loosely defined as a laparotomy measuring between 4 cm and 6 cm. For the appropriate surgical candidate, a minilaparotomy is a useful alternative to laparotomy or laparoscopy, especially for large pathology.1 Benefits of minilaparotomy include improved pain management and postoperative recovery, as well as improved cosmetic outcome, with comparable blood loss and operative time.2,3

In this video, we illustrate the key surgical steps of a minilaparotomy for the removal of large fibroids. These steps include:
- strategic vertical skin incision
- use of a self-retaining retractor
- infiltrate myometrium with dilute vasopressin
- strategic hysterotomy
- use of tenaculum for upward traction
- 10# blade scalpels for the “lemon wedge” coring technique
- layered closure.
Minilaparotomy myomectomy can be an excellent minimally invasive alternative to a traditional “full laparotomy” for women with large fibroids.
We hope that you find this video beneficial to your clinical practice.
>> Arnold P. Advincula, MD
Laparoscopic bilateral salpingo-oophorectomy via minilaparotomy assistance for the massively enlarged adnexal mass
- Pelosi MA 2nd, Pelosi MA 3rd. Pelosi minilaparotomy hysterectomy: a non-endoscopic minimally invasive alternative to laparoscopy and laparotomy. Surg Technol Int. 2004;13:157-167.
- Fanafani F, Fagotti A, Longo R. Minilaparotomy in the management of benign gynecologic disease. Eur J Obstet Gynecol Reprod Biol. 2005;119:232-236.
- Glasser MH. Minilaparotomy: a minimally invasive alternative for major gynecologic abdominal surgery. Perm J. 2005;9:41-45.
- Pelosi MA 2nd, Pelosi MA 3rd. Pelosi minilaparotomy hysterectomy: a non-endoscopic minimally invasive alternative to laparoscopy and laparotomy. Surg Technol Int. 2004;13:157-167.
- Fanafani F, Fagotti A, Longo R. Minilaparotomy in the management of benign gynecologic disease. Eur J Obstet Gynecol Reprod Biol. 2005;119:232-236.
- Glasser MH. Minilaparotomy: a minimally invasive alternative for major gynecologic abdominal surgery. Perm J. 2005;9:41-45.
TCA and punch excision are two options for icepick acne scars
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Dupilumab relieves severe sinusitis with polyposis
SAN FRANCISCO – Dupilumab, an anti-inflammatory drug already approved for use in the United States, met its efficacy endpoints for treating chronic rhinosinusitis with nasal polyps in a pivotal trial with 276 patients.
The results make it likely that dupilumab (Dupixent) will receive a new indication from the Food and Drug Administration, pending similar results in a second pivotal trial for nasal polyps that researchers will report soon. Dupilumab, which works by blocking a receptor for both interleukin 4 and interleukin 13 and thereby shutting down type 2 inflammation, is already approved in the United States for treating atopic dermatitis and asthma.
Type 2 inflammation drives polyp formation in patients with chronic rhinosinusitis that can produce severe nasal congestion, breathing difficulty, and substantially reduced quality of life.
In the new trial, the drug showed efficacy by significantly improving both the nasal congestion score reported by patients and the nasal polyp score measured by sinus endoscopy after 24 weeks on treatment, when compared with control patients on placebo, Joseph K. Han, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Patients enrolled in the study had chronic, severe sinusitis and nasal polyps that remained uncontrolled despite prior surgery, for 75% of enrolled patients, or treatment with systemic corticosteroids, used on about 90% of the patients within the prior 2 years.
During the 24 weeks of treatment, 23% of patients in the control arm had to restart systemic corticosteroid treatment or have surgery, compared with 7% of patients on dupilumab treatment, a statistically significant difference.
The new drug is a “game changer,” for these patients, Dr. Han said in a video interview.
In some patients, treatment produced complete polyp resolution. He and his colleagues in the otolaryngology field are now trying to decide exactly which patients with polyps secondary to sinusitis will be good candidates for dupilumab after it receives an expected indication for shrinking nasal polyps.
Roughly 4% of the adult population has chronic rhinosinusitis that generates polyps. How many of these patients are affected severely enough to warrant dupilumab treatment is not clear, but will likely include several hundreds of thousands of U.S. adults, said Dr. Han, professor of otolaryngology and chief of the division of allergy at Eastern Virginia Medical School in Norfolk.
The SINUS-24 (A Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps) trial enrolled patients at 76 sites in the United States and in several European countries. The study randomized 143 patients who received standard treatment plus a 300-mg dupilumab subcutaneous injection every 2 weeks, and 133 patients who received standard treatment plus placebo injections. Standard treatment included a nasal corticosteroid spray.
After 24 weeks of treatment, the endoscopically-measured nasal polyp score, which averaged about 6 at baseline on a scale of 0-8, fell by an average of 2.06 points, compared with controls, which was a statistically significant and clinically meaningful change, said Dr. Han.
The second primary endpoint, patient self-assessment of nasal congestion on a scale of 0-3, showed an average 0.89 improvement, compared with controls, which was also a statistically significant and meaningful change from the average baseline score of about 2.4.
Other efficacy measures also showed benefits from treatment, including a substantial improvement compared with controls in a quality-of-life measure. The safety profile was benign compared with placebo, and consistent with existing safety data for the drug.SINUS-24 was funded by Regeneron and Sanofi, the companies that market dupilumab. Dr. Han has been an adviser to Regeneron and Sanofi.
SOURCE: Han JK et al. AAAAI 2019, Abstract L4.
SAN FRANCISCO – Dupilumab, an anti-inflammatory drug already approved for use in the United States, met its efficacy endpoints for treating chronic rhinosinusitis with nasal polyps in a pivotal trial with 276 patients.
The results make it likely that dupilumab (Dupixent) will receive a new indication from the Food and Drug Administration, pending similar results in a second pivotal trial for nasal polyps that researchers will report soon. Dupilumab, which works by blocking a receptor for both interleukin 4 and interleukin 13 and thereby shutting down type 2 inflammation, is already approved in the United States for treating atopic dermatitis and asthma.
Type 2 inflammation drives polyp formation in patients with chronic rhinosinusitis that can produce severe nasal congestion, breathing difficulty, and substantially reduced quality of life.
In the new trial, the drug showed efficacy by significantly improving both the nasal congestion score reported by patients and the nasal polyp score measured by sinus endoscopy after 24 weeks on treatment, when compared with control patients on placebo, Joseph K. Han, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Patients enrolled in the study had chronic, severe sinusitis and nasal polyps that remained uncontrolled despite prior surgery, for 75% of enrolled patients, or treatment with systemic corticosteroids, used on about 90% of the patients within the prior 2 years.
During the 24 weeks of treatment, 23% of patients in the control arm had to restart systemic corticosteroid treatment or have surgery, compared with 7% of patients on dupilumab treatment, a statistically significant difference.
The new drug is a “game changer,” for these patients, Dr. Han said in a video interview.
In some patients, treatment produced complete polyp resolution. He and his colleagues in the otolaryngology field are now trying to decide exactly which patients with polyps secondary to sinusitis will be good candidates for dupilumab after it receives an expected indication for shrinking nasal polyps.
Roughly 4% of the adult population has chronic rhinosinusitis that generates polyps. How many of these patients are affected severely enough to warrant dupilumab treatment is not clear, but will likely include several hundreds of thousands of U.S. adults, said Dr. Han, professor of otolaryngology and chief of the division of allergy at Eastern Virginia Medical School in Norfolk.
The SINUS-24 (A Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps) trial enrolled patients at 76 sites in the United States and in several European countries. The study randomized 143 patients who received standard treatment plus a 300-mg dupilumab subcutaneous injection every 2 weeks, and 133 patients who received standard treatment plus placebo injections. Standard treatment included a nasal corticosteroid spray.
After 24 weeks of treatment, the endoscopically-measured nasal polyp score, which averaged about 6 at baseline on a scale of 0-8, fell by an average of 2.06 points, compared with controls, which was a statistically significant and clinically meaningful change, said Dr. Han.
The second primary endpoint, patient self-assessment of nasal congestion on a scale of 0-3, showed an average 0.89 improvement, compared with controls, which was also a statistically significant and meaningful change from the average baseline score of about 2.4.
Other efficacy measures also showed benefits from treatment, including a substantial improvement compared with controls in a quality-of-life measure. The safety profile was benign compared with placebo, and consistent with existing safety data for the drug.SINUS-24 was funded by Regeneron and Sanofi, the companies that market dupilumab. Dr. Han has been an adviser to Regeneron and Sanofi.
SOURCE: Han JK et al. AAAAI 2019, Abstract L4.
SAN FRANCISCO – Dupilumab, an anti-inflammatory drug already approved for use in the United States, met its efficacy endpoints for treating chronic rhinosinusitis with nasal polyps in a pivotal trial with 276 patients.
The results make it likely that dupilumab (Dupixent) will receive a new indication from the Food and Drug Administration, pending similar results in a second pivotal trial for nasal polyps that researchers will report soon. Dupilumab, which works by blocking a receptor for both interleukin 4 and interleukin 13 and thereby shutting down type 2 inflammation, is already approved in the United States for treating atopic dermatitis and asthma.
Type 2 inflammation drives polyp formation in patients with chronic rhinosinusitis that can produce severe nasal congestion, breathing difficulty, and substantially reduced quality of life.
In the new trial, the drug showed efficacy by significantly improving both the nasal congestion score reported by patients and the nasal polyp score measured by sinus endoscopy after 24 weeks on treatment, when compared with control patients on placebo, Joseph K. Han, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Patients enrolled in the study had chronic, severe sinusitis and nasal polyps that remained uncontrolled despite prior surgery, for 75% of enrolled patients, or treatment with systemic corticosteroids, used on about 90% of the patients within the prior 2 years.
During the 24 weeks of treatment, 23% of patients in the control arm had to restart systemic corticosteroid treatment or have surgery, compared with 7% of patients on dupilumab treatment, a statistically significant difference.
The new drug is a “game changer,” for these patients, Dr. Han said in a video interview.
In some patients, treatment produced complete polyp resolution. He and his colleagues in the otolaryngology field are now trying to decide exactly which patients with polyps secondary to sinusitis will be good candidates for dupilumab after it receives an expected indication for shrinking nasal polyps.
Roughly 4% of the adult population has chronic rhinosinusitis that generates polyps. How many of these patients are affected severely enough to warrant dupilumab treatment is not clear, but will likely include several hundreds of thousands of U.S. adults, said Dr. Han, professor of otolaryngology and chief of the division of allergy at Eastern Virginia Medical School in Norfolk.
The SINUS-24 (A Controlled Clinical Study of Dupilumab in Patients With Nasal Polyps) trial enrolled patients at 76 sites in the United States and in several European countries. The study randomized 143 patients who received standard treatment plus a 300-mg dupilumab subcutaneous injection every 2 weeks, and 133 patients who received standard treatment plus placebo injections. Standard treatment included a nasal corticosteroid spray.
After 24 weeks of treatment, the endoscopically-measured nasal polyp score, which averaged about 6 at baseline on a scale of 0-8, fell by an average of 2.06 points, compared with controls, which was a statistically significant and clinically meaningful change, said Dr. Han.
The second primary endpoint, patient self-assessment of nasal congestion on a scale of 0-3, showed an average 0.89 improvement, compared with controls, which was also a statistically significant and meaningful change from the average baseline score of about 2.4.
Other efficacy measures also showed benefits from treatment, including a substantial improvement compared with controls in a quality-of-life measure. The safety profile was benign compared with placebo, and consistent with existing safety data for the drug.SINUS-24 was funded by Regeneron and Sanofi, the companies that market dupilumab. Dr. Han has been an adviser to Regeneron and Sanofi.
SOURCE: Han JK et al. AAAAI 2019, Abstract L4.
REPORTING FROM AAAAI
Simple treatments can address bleeding in dermatologic surgery
WAIKOLOA, HAWAII – “Nothing can ruin your day more than a lot of bleeding,” said Mohs surgeon Daniel Siegel, MD, clinical professor of dermatology at the State University of New York Downstate Medical Center, New York.

“Part of planning for surgery is to prevent that sort of problem,” and fortunately, and some are very inexpensive. There’s even a “fancy form of potato starch” that can be left in the wound and sewn over, he said in an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
During the interview, Dr. Siegel described several options and how to use them. They are good products to have on hand in the clinic, just in case, he said.
SDEF/Global Academy for Medical Education and this news organization and are owned by the same parent company.
WAIKOLOA, HAWAII – “Nothing can ruin your day more than a lot of bleeding,” said Mohs surgeon Daniel Siegel, MD, clinical professor of dermatology at the State University of New York Downstate Medical Center, New York.

“Part of planning for surgery is to prevent that sort of problem,” and fortunately, and some are very inexpensive. There’s even a “fancy form of potato starch” that can be left in the wound and sewn over, he said in an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
During the interview, Dr. Siegel described several options and how to use them. They are good products to have on hand in the clinic, just in case, he said.
SDEF/Global Academy for Medical Education and this news organization and are owned by the same parent company.
WAIKOLOA, HAWAII – “Nothing can ruin your day more than a lot of bleeding,” said Mohs surgeon Daniel Siegel, MD, clinical professor of dermatology at the State University of New York Downstate Medical Center, New York.

“Part of planning for surgery is to prevent that sort of problem,” and fortunately, and some are very inexpensive. There’s even a “fancy form of potato starch” that can be left in the wound and sewn over, he said in an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
During the interview, Dr. Siegel described several options and how to use them. They are good products to have on hand in the clinic, just in case, he said.
SDEF/Global Academy for Medical Education and this news organization and are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Dupilumab conjunctivitis does not always require an ophthalmologist referral
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
New Topical Treatments for Psoriasis



Don’t miss early joint involvement in psoriasis
WAIKOLOA, HAWAII – advised Alan Menter, MD.
About a third of patients with psoriasis will go on to develop joint involvement, and about half of those will go on to develop permanent joint destruction “if left untreated,” he noted. But early joint involvement has to be caught first, and dermatologists aren’t doing a very good job at early detection, according to Dr. Menter, clinical professor of dermatology at the University of Texas, Dallas.
The consequences, including arthritis mutilans, can be devastating. “It’s vitally important for us to prevent any permanent joint disease by” picking it up early, he said. “Our job as dermatologists is to diagnose it early.”
It’s not hard to do, just a few extra questions and a few extra steps on the physical exam, which takes a minute or two during each visit with psoriasis patients, are needed, he said.
Dr. Menter reviewed questions to ask patients, and explained how to examine patients for joint involvement and alter treatment when it’s found, in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – advised Alan Menter, MD.
About a third of patients with psoriasis will go on to develop joint involvement, and about half of those will go on to develop permanent joint destruction “if left untreated,” he noted. But early joint involvement has to be caught first, and dermatologists aren’t doing a very good job at early detection, according to Dr. Menter, clinical professor of dermatology at the University of Texas, Dallas.
The consequences, including arthritis mutilans, can be devastating. “It’s vitally important for us to prevent any permanent joint disease by” picking it up early, he said. “Our job as dermatologists is to diagnose it early.”
It’s not hard to do, just a few extra questions and a few extra steps on the physical exam, which takes a minute or two during each visit with psoriasis patients, are needed, he said.
Dr. Menter reviewed questions to ask patients, and explained how to examine patients for joint involvement and alter treatment when it’s found, in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – advised Alan Menter, MD.
About a third of patients with psoriasis will go on to develop joint involvement, and about half of those will go on to develop permanent joint destruction “if left untreated,” he noted. But early joint involvement has to be caught first, and dermatologists aren’t doing a very good job at early detection, according to Dr. Menter, clinical professor of dermatology at the University of Texas, Dallas.
The consequences, including arthritis mutilans, can be devastating. “It’s vitally important for us to prevent any permanent joint disease by” picking it up early, he said. “Our job as dermatologists is to diagnose it early.”
It’s not hard to do, just a few extra questions and a few extra steps on the physical exam, which takes a minute or two during each visit with psoriasis patients, are needed, he said.
Dr. Menter reviewed questions to ask patients, and explained how to examine patients for joint involvement and alter treatment when it’s found, in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Onychomycosis that fails terbinafine probably isn’t T. rubrum
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR