User login
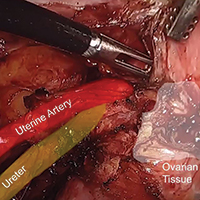
Prevention of ovarian remnant syndrome: Adnexal adhesiolysis from the pelvic sidewall

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
SAMHSA’s new general embarks on a new mission
Elinore F. McCance-Katz, MD, PhD, is by turns passionate and impatient, empathetic and brusquely no-nonsense. She is a person who knows what she wants to do and knows how to do it.
Accomplishing that on the idealogic battlefield of a presidential appointment is the focus of her considerable energy.
Dr. McCance-Katz carries the newly minted banner of assistant secretary for mental health and substance use. Her troops comprise more than 100 related federal agencies. Her charge is at once simple and mind-numbingly complex: Overhaul the nation’s mental health care system. for their illnesses before they can begin to heal.
It’s the precise opposite of the Substance Abuse and Mental Health Services Administration’s prior targeting, which focused heavily on providing healthy social support for patients with mental health and substance abuse disorders. To Dr. McCance-Katz’s way of thinking, dollars spent on such are largely wasted, because terribly ill people simply can’t function in healthy ways until they begin to get better. Why, she reasoned, should the government waste money on peer-support services while ignoring – or even openly rejecting – evidence-based psychiatric treatment paradigms that are proven to help heal?
It’s this philosophical dichotomy – combined with what she perceived as a palpable dismissal of psychiatric science – that drove her away from her first SAMHSA appointment as the agency’s first chief medical officer. She aired her frustration in an editorial, published 2 years ago in Psychiatric Times.
“There is a perceptible hostility toward psychiatric medicine: a resistance to addressing the treatment needs of those with serious mental illness and a questioning by some at SAMHSA as to whether mental disorders even exist – for example, is psychosis just a ‘different way of thinking for some experiencing stress?’ … Nowhere in SAMHSA’s strategic initiatives is psychiatric treatment of mental illness a priority. The occasional vague reference to treatment is no substitute for the urgent need for programs that address these issues.”
In the same letter, she outlined a new battle plan, one that includes funding for better outpatient treatments, more psychiatric hospital beds, clinician education and support, and money to beef up the dwindling supply of psychiatrists, advanced-practice psychiatric nurses, and psychologists.
In an exclusive video interview, Dr. McCance-Katz sat down with MDedge Psychiatry to discuss the path that brought her to this station and the long road ahead.
Elinore F. McCance-Katz, MD, PhD, is by turns passionate and impatient, empathetic and brusquely no-nonsense. She is a person who knows what she wants to do and knows how to do it.
Accomplishing that on the idealogic battlefield of a presidential appointment is the focus of her considerable energy.
Dr. McCance-Katz carries the newly minted banner of assistant secretary for mental health and substance use. Her troops comprise more than 100 related federal agencies. Her charge is at once simple and mind-numbingly complex: Overhaul the nation’s mental health care system. for their illnesses before they can begin to heal.
It’s the precise opposite of the Substance Abuse and Mental Health Services Administration’s prior targeting, which focused heavily on providing healthy social support for patients with mental health and substance abuse disorders. To Dr. McCance-Katz’s way of thinking, dollars spent on such are largely wasted, because terribly ill people simply can’t function in healthy ways until they begin to get better. Why, she reasoned, should the government waste money on peer-support services while ignoring – or even openly rejecting – evidence-based psychiatric treatment paradigms that are proven to help heal?
It’s this philosophical dichotomy – combined with what she perceived as a palpable dismissal of psychiatric science – that drove her away from her first SAMHSA appointment as the agency’s first chief medical officer. She aired her frustration in an editorial, published 2 years ago in Psychiatric Times.
“There is a perceptible hostility toward psychiatric medicine: a resistance to addressing the treatment needs of those with serious mental illness and a questioning by some at SAMHSA as to whether mental disorders even exist – for example, is psychosis just a ‘different way of thinking for some experiencing stress?’ … Nowhere in SAMHSA’s strategic initiatives is psychiatric treatment of mental illness a priority. The occasional vague reference to treatment is no substitute for the urgent need for programs that address these issues.”
In the same letter, she outlined a new battle plan, one that includes funding for better outpatient treatments, more psychiatric hospital beds, clinician education and support, and money to beef up the dwindling supply of psychiatrists, advanced-practice psychiatric nurses, and psychologists.
In an exclusive video interview, Dr. McCance-Katz sat down with MDedge Psychiatry to discuss the path that brought her to this station and the long road ahead.
Elinore F. McCance-Katz, MD, PhD, is by turns passionate and impatient, empathetic and brusquely no-nonsense. She is a person who knows what she wants to do and knows how to do it.
Accomplishing that on the idealogic battlefield of a presidential appointment is the focus of her considerable energy.
Dr. McCance-Katz carries the newly minted banner of assistant secretary for mental health and substance use. Her troops comprise more than 100 related federal agencies. Her charge is at once simple and mind-numbingly complex: Overhaul the nation’s mental health care system. for their illnesses before they can begin to heal.
It’s the precise opposite of the Substance Abuse and Mental Health Services Administration’s prior targeting, which focused heavily on providing healthy social support for patients with mental health and substance abuse disorders. To Dr. McCance-Katz’s way of thinking, dollars spent on such are largely wasted, because terribly ill people simply can’t function in healthy ways until they begin to get better. Why, she reasoned, should the government waste money on peer-support services while ignoring – or even openly rejecting – evidence-based psychiatric treatment paradigms that are proven to help heal?
It’s this philosophical dichotomy – combined with what she perceived as a palpable dismissal of psychiatric science – that drove her away from her first SAMHSA appointment as the agency’s first chief medical officer. She aired her frustration in an editorial, published 2 years ago in Psychiatric Times.
“There is a perceptible hostility toward psychiatric medicine: a resistance to addressing the treatment needs of those with serious mental illness and a questioning by some at SAMHSA as to whether mental disorders even exist – for example, is psychosis just a ‘different way of thinking for some experiencing stress?’ … Nowhere in SAMHSA’s strategic initiatives is psychiatric treatment of mental illness a priority. The occasional vague reference to treatment is no substitute for the urgent need for programs that address these issues.”
In the same letter, she outlined a new battle plan, one that includes funding for better outpatient treatments, more psychiatric hospital beds, clinician education and support, and money to beef up the dwindling supply of psychiatrists, advanced-practice psychiatric nurses, and psychologists.
In an exclusive video interview, Dr. McCance-Katz sat down with MDedge Psychiatry to discuss the path that brought her to this station and the long road ahead.
SUSTAIN-7: GLP-1 receptor agonists effective in elderly
BOSTON – Efficacy and safety of two glucagonlike peptide 1 (GLP-1) receptor agonists in type 2 diabetes mellitus were similar between older and younger adults, according to a post hoc analysis of the SUSTAIN 7 clinical trial data.
However, said the study’s first author, Vanita Aroda, MD, associate director for clinical diabetes research at Brigham and Women’s Hospital, Boston.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The SUSTAIN 7 trial compared low-dose semaglutide (0.5 mg) with low-dose dulaglutide (0.75 mg), and high-dose semaglutide (1.0 mg) with high-dose dulaglutide (1.5 mg) as add-on therapy to metformin for adults with type 2 diabetes. All medications were given as once-weekly subcutaneous injections.
Dr. Aroda and her collaborators performed a subgroup analysis of the SUSTAIN 7 data that compared 260 patients aged 65 years and older (mean, 69.3 years) with 939 patients younger than 65 years (mean, 51.9 years).
“What we found is that the efficacy results … were similar to what we saw in the general population. We did not lose the efficacy in the older adult population,” said Dr. Aroda in an interview at the annual meeting of the American Association for Clinical Endocrinology.
Weight loss was similar in older and younger patients, though there was “maybe a tiny bit more in the older adults,” said Dr. Aroda: Older participants had a 4.4-kg reduction in weight, compared with 4.9-kg reduction in the younger population, on low-dose semaglutide. For low-dose dulaglutide, losses were an average 2.6 kg in the elderly versus 2.2 kg in the younger participants.
The higher doses of each resulted in greater weight loss, up to a mean 6.7 kg in elderly participants on high-dose semaglutide, with the same marginally greater losses seen in older participants.
Both agents were efficacious in the older population, Dr. Aroda said, with slightly better efficacy than in younger patients. As a caveat, she noted that the older patients came into the study with slightly better glycemic control and slightly lower body weight.
An array of endpoints for the 40-week study included achieving hemoglobin A1c less than 7%, less than or equal to 6.5%, and less than 7% without weight gain or hypoglycemia. A higher proportion of elderly patients met these endpoints; for example, 83% of elderly patients on high-dose semaglutide reached the composite endpoint of HbA1c less than 7% with no weight gain or hypoglycemia, compared to 72.1% of younger participants.
The analysis also looked at safety data for SUSTAIN 7. “The next question is, are you seeing this efficacy in terms of glycemic change and weight loss, at any cost of hypoglycemia? And the answer to that was no,” said Dr. Aroda. There were very rare to zero hypoglycemic events in the various study arms, she said.
However, older adults taking the higher doses of both GLP-1 receptor agonists had a high incidence of nausea, vomiting, and other gastrointestinal disturbances. These adverse events were seen in 52.8% of older patients on high-dose semaglutide and 52.2% of those on high-dose dulaglutide. Rates for the younger study population at these doses were 42.5% for semaglutide and 46.6% for dulaglutide.
The clinical take-home message? Don’t be afraid to reach for a GLP-1 receptor agonist for an older patient who’s not reaching target on metformin. “You have good efficacy with both of the therapies … but you just need to watch out for tolerability at the higher doses,” said Dr. Aroda.
Dr. Aroda reported receiving research funding from AstraZeneca, Calibri, Eisai, Novo Nordisk, Sanofi, and Theracos. She was formerly affiliated with Medstar Health Research Institute, Hyattsville, Md.
SOURCE: Aroda V et al. AACE 2018. Abstract 245.
BOSTON – Efficacy and safety of two glucagonlike peptide 1 (GLP-1) receptor agonists in type 2 diabetes mellitus were similar between older and younger adults, according to a post hoc analysis of the SUSTAIN 7 clinical trial data.
However, said the study’s first author, Vanita Aroda, MD, associate director for clinical diabetes research at Brigham and Women’s Hospital, Boston.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The SUSTAIN 7 trial compared low-dose semaglutide (0.5 mg) with low-dose dulaglutide (0.75 mg), and high-dose semaglutide (1.0 mg) with high-dose dulaglutide (1.5 mg) as add-on therapy to metformin for adults with type 2 diabetes. All medications were given as once-weekly subcutaneous injections.
Dr. Aroda and her collaborators performed a subgroup analysis of the SUSTAIN 7 data that compared 260 patients aged 65 years and older (mean, 69.3 years) with 939 patients younger than 65 years (mean, 51.9 years).
“What we found is that the efficacy results … were similar to what we saw in the general population. We did not lose the efficacy in the older adult population,” said Dr. Aroda in an interview at the annual meeting of the American Association for Clinical Endocrinology.
Weight loss was similar in older and younger patients, though there was “maybe a tiny bit more in the older adults,” said Dr. Aroda: Older participants had a 4.4-kg reduction in weight, compared with 4.9-kg reduction in the younger population, on low-dose semaglutide. For low-dose dulaglutide, losses were an average 2.6 kg in the elderly versus 2.2 kg in the younger participants.
The higher doses of each resulted in greater weight loss, up to a mean 6.7 kg in elderly participants on high-dose semaglutide, with the same marginally greater losses seen in older participants.
Both agents were efficacious in the older population, Dr. Aroda said, with slightly better efficacy than in younger patients. As a caveat, she noted that the older patients came into the study with slightly better glycemic control and slightly lower body weight.
An array of endpoints for the 40-week study included achieving hemoglobin A1c less than 7%, less than or equal to 6.5%, and less than 7% without weight gain or hypoglycemia. A higher proportion of elderly patients met these endpoints; for example, 83% of elderly patients on high-dose semaglutide reached the composite endpoint of HbA1c less than 7% with no weight gain or hypoglycemia, compared to 72.1% of younger participants.
The analysis also looked at safety data for SUSTAIN 7. “The next question is, are you seeing this efficacy in terms of glycemic change and weight loss, at any cost of hypoglycemia? And the answer to that was no,” said Dr. Aroda. There were very rare to zero hypoglycemic events in the various study arms, she said.
However, older adults taking the higher doses of both GLP-1 receptor agonists had a high incidence of nausea, vomiting, and other gastrointestinal disturbances. These adverse events were seen in 52.8% of older patients on high-dose semaglutide and 52.2% of those on high-dose dulaglutide. Rates for the younger study population at these doses were 42.5% for semaglutide and 46.6% for dulaglutide.
The clinical take-home message? Don’t be afraid to reach for a GLP-1 receptor agonist for an older patient who’s not reaching target on metformin. “You have good efficacy with both of the therapies … but you just need to watch out for tolerability at the higher doses,” said Dr. Aroda.
Dr. Aroda reported receiving research funding from AstraZeneca, Calibri, Eisai, Novo Nordisk, Sanofi, and Theracos. She was formerly affiliated with Medstar Health Research Institute, Hyattsville, Md.
SOURCE: Aroda V et al. AACE 2018. Abstract 245.
BOSTON – Efficacy and safety of two glucagonlike peptide 1 (GLP-1) receptor agonists in type 2 diabetes mellitus were similar between older and younger adults, according to a post hoc analysis of the SUSTAIN 7 clinical trial data.
However, said the study’s first author, Vanita Aroda, MD, associate director for clinical diabetes research at Brigham and Women’s Hospital, Boston.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The SUSTAIN 7 trial compared low-dose semaglutide (0.5 mg) with low-dose dulaglutide (0.75 mg), and high-dose semaglutide (1.0 mg) with high-dose dulaglutide (1.5 mg) as add-on therapy to metformin for adults with type 2 diabetes. All medications were given as once-weekly subcutaneous injections.
Dr. Aroda and her collaborators performed a subgroup analysis of the SUSTAIN 7 data that compared 260 patients aged 65 years and older (mean, 69.3 years) with 939 patients younger than 65 years (mean, 51.9 years).
“What we found is that the efficacy results … were similar to what we saw in the general population. We did not lose the efficacy in the older adult population,” said Dr. Aroda in an interview at the annual meeting of the American Association for Clinical Endocrinology.
Weight loss was similar in older and younger patients, though there was “maybe a tiny bit more in the older adults,” said Dr. Aroda: Older participants had a 4.4-kg reduction in weight, compared with 4.9-kg reduction in the younger population, on low-dose semaglutide. For low-dose dulaglutide, losses were an average 2.6 kg in the elderly versus 2.2 kg in the younger participants.
The higher doses of each resulted in greater weight loss, up to a mean 6.7 kg in elderly participants on high-dose semaglutide, with the same marginally greater losses seen in older participants.
Both agents were efficacious in the older population, Dr. Aroda said, with slightly better efficacy than in younger patients. As a caveat, she noted that the older patients came into the study with slightly better glycemic control and slightly lower body weight.
An array of endpoints for the 40-week study included achieving hemoglobin A1c less than 7%, less than or equal to 6.5%, and less than 7% without weight gain or hypoglycemia. A higher proportion of elderly patients met these endpoints; for example, 83% of elderly patients on high-dose semaglutide reached the composite endpoint of HbA1c less than 7% with no weight gain or hypoglycemia, compared to 72.1% of younger participants.
The analysis also looked at safety data for SUSTAIN 7. “The next question is, are you seeing this efficacy in terms of glycemic change and weight loss, at any cost of hypoglycemia? And the answer to that was no,” said Dr. Aroda. There were very rare to zero hypoglycemic events in the various study arms, she said.
However, older adults taking the higher doses of both GLP-1 receptor agonists had a high incidence of nausea, vomiting, and other gastrointestinal disturbances. These adverse events were seen in 52.8% of older patients on high-dose semaglutide and 52.2% of those on high-dose dulaglutide. Rates for the younger study population at these doses were 42.5% for semaglutide and 46.6% for dulaglutide.
The clinical take-home message? Don’t be afraid to reach for a GLP-1 receptor agonist for an older patient who’s not reaching target on metformin. “You have good efficacy with both of the therapies … but you just need to watch out for tolerability at the higher doses,” said Dr. Aroda.
Dr. Aroda reported receiving research funding from AstraZeneca, Calibri, Eisai, Novo Nordisk, Sanofi, and Theracos. She was formerly affiliated with Medstar Health Research Institute, Hyattsville, Md.
SOURCE: Aroda V et al. AACE 2018. Abstract 245.
REPORTING FROM AACE 2018
Atopic dermatitis severity reduced by topical microbiome treatment
The Beginning Assessment of Cutaneous Treatment Efficacy of Roseomonas in Atopic Dermatitis trial; BACTERiAD I/II study, an open-label phase I/II trial, first looked at the therapeutic use of R. mucosa in 10 adults aged 18 years or older. Three sucrose mixtures with increasing doses of live R. mucosa bacteria were topically applied to two body areas – the antecubital fossae and a body surface of their choice – twice per week for 2 weeks per dose. At 6 weeks, the patients stopped using the mixtures and followed a 4-week washout phase.
Treatment was found to reduce mean antecubital SCORAD (SCORing Atopic Dermatitis) scores by 59.8%. Reduction in pruritus was even more pronounced, with a mean decrease of 78.5%. Treating the hands did not improve disease severity, even in patients whose symptoms improved in other body areas. One explanation may be the increased exposure of the hands to topical antimicrobials and environmental exposures, the researchers noted.
With the success in the adult cohort, the researchers enrolled five children aged 7-17 years in the study. These patients were treated twice weekly for 16 weeks. The pediatric patients experienced a mean decrease of 70.3% in their SCORAD scores. The mean decrease in pruritis was 78.8%.
All adults who responded continued to report improved symptoms after the washout period. The pediatric patients are now being evaluated in a washout period.
Four patients did not respond; three of them had a family history of AD persisting into adulthood. “The association between these complex medical histories and the lack of clinical response suggests that differences in heritable host and/or microbial factors may impact treatment responses,” wrote Ian A. Myles, MD, and his colleagues.
“Overall, our findings suggest the safety of topical R. mucosa therapy and justify continuation of our ongoing trial to assess safety and activity in a pediatric cohort of patients with AD. These studies will additionally assess changes in host serum markers, skin metabolomics, and the skin microbiota by culture and genomic methods.”
The researchers noted that expanding to the pediatric population will deepen understanding of topical microbiome transplantation and lay the foundation for placebo-controlled trials to assess efficacy.
This work was supported by the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases and the National Institutes of Health. The researchers had no disclosures.
SOURCE: Myles IA et al. JCI Insight. 2018 May 3. doi: 10.1172/jci.insight.120608.
The Beginning Assessment of Cutaneous Treatment Efficacy of Roseomonas in Atopic Dermatitis trial; BACTERiAD I/II study, an open-label phase I/II trial, first looked at the therapeutic use of R. mucosa in 10 adults aged 18 years or older. Three sucrose mixtures with increasing doses of live R. mucosa bacteria were topically applied to two body areas – the antecubital fossae and a body surface of their choice – twice per week for 2 weeks per dose. At 6 weeks, the patients stopped using the mixtures and followed a 4-week washout phase.
Treatment was found to reduce mean antecubital SCORAD (SCORing Atopic Dermatitis) scores by 59.8%. Reduction in pruritus was even more pronounced, with a mean decrease of 78.5%. Treating the hands did not improve disease severity, even in patients whose symptoms improved in other body areas. One explanation may be the increased exposure of the hands to topical antimicrobials and environmental exposures, the researchers noted.
With the success in the adult cohort, the researchers enrolled five children aged 7-17 years in the study. These patients were treated twice weekly for 16 weeks. The pediatric patients experienced a mean decrease of 70.3% in their SCORAD scores. The mean decrease in pruritis was 78.8%.
All adults who responded continued to report improved symptoms after the washout period. The pediatric patients are now being evaluated in a washout period.
Four patients did not respond; three of them had a family history of AD persisting into adulthood. “The association between these complex medical histories and the lack of clinical response suggests that differences in heritable host and/or microbial factors may impact treatment responses,” wrote Ian A. Myles, MD, and his colleagues.
“Overall, our findings suggest the safety of topical R. mucosa therapy and justify continuation of our ongoing trial to assess safety and activity in a pediatric cohort of patients with AD. These studies will additionally assess changes in host serum markers, skin metabolomics, and the skin microbiota by culture and genomic methods.”
The researchers noted that expanding to the pediatric population will deepen understanding of topical microbiome transplantation and lay the foundation for placebo-controlled trials to assess efficacy.
This work was supported by the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases and the National Institutes of Health. The researchers had no disclosures.
SOURCE: Myles IA et al. JCI Insight. 2018 May 3. doi: 10.1172/jci.insight.120608.
The Beginning Assessment of Cutaneous Treatment Efficacy of Roseomonas in Atopic Dermatitis trial; BACTERiAD I/II study, an open-label phase I/II trial, first looked at the therapeutic use of R. mucosa in 10 adults aged 18 years or older. Three sucrose mixtures with increasing doses of live R. mucosa bacteria were topically applied to two body areas – the antecubital fossae and a body surface of their choice – twice per week for 2 weeks per dose. At 6 weeks, the patients stopped using the mixtures and followed a 4-week washout phase.
Treatment was found to reduce mean antecubital SCORAD (SCORing Atopic Dermatitis) scores by 59.8%. Reduction in pruritus was even more pronounced, with a mean decrease of 78.5%. Treating the hands did not improve disease severity, even in patients whose symptoms improved in other body areas. One explanation may be the increased exposure of the hands to topical antimicrobials and environmental exposures, the researchers noted.
With the success in the adult cohort, the researchers enrolled five children aged 7-17 years in the study. These patients were treated twice weekly for 16 weeks. The pediatric patients experienced a mean decrease of 70.3% in their SCORAD scores. The mean decrease in pruritis was 78.8%.
All adults who responded continued to report improved symptoms after the washout period. The pediatric patients are now being evaluated in a washout period.
Four patients did not respond; three of them had a family history of AD persisting into adulthood. “The association between these complex medical histories and the lack of clinical response suggests that differences in heritable host and/or microbial factors may impact treatment responses,” wrote Ian A. Myles, MD, and his colleagues.
“Overall, our findings suggest the safety of topical R. mucosa therapy and justify continuation of our ongoing trial to assess safety and activity in a pediatric cohort of patients with AD. These studies will additionally assess changes in host serum markers, skin metabolomics, and the skin microbiota by culture and genomic methods.”
The researchers noted that expanding to the pediatric population will deepen understanding of topical microbiome transplantation and lay the foundation for placebo-controlled trials to assess efficacy.
This work was supported by the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases and the National Institutes of Health. The researchers had no disclosures.
SOURCE: Myles IA et al. JCI Insight. 2018 May 3. doi: 10.1172/jci.insight.120608.
FROM JCI INSIGHT
Key clinical point: Roseomonas mucosa reduces disease severity.
Major finding: There were reductions in SCORAD scores of 78.5% and 70.3% in the adult and pediatric cohorts, respectively.
Study details: Case study of 10 adult and 5 pediatric patients with atopic dermatitis.
Disclosures: No relevant financial disclosures were reported.
Source: Myles IA et al. JCI Insight. 2018 May 3. doi: 10.1172/jci.insight.120608.
VIDEO: Health advisers boost type 2 diabetes adherence
BOSTON – A company that partners patients and health advisers claims to help patients with type 2 diabetes to manage their symptoms and lower their HbA1c levels.
The Pack Health program connects each patient with a health adviser, who contacts them five times a week using a mix of phone calls, text messages, and emails. The goals include helping patients to find clinicians in their area, make appointments, and adhere to treatment, Dhiren Patel, PharmD, medical director at Pack Health, said at the annual meeting of the American Association of Clinical Endocrinologists.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients lost an average of 5 pounds, and annual eye and foot exams increased by 17% and 19%, respectively.
Similar remote health coach initiatives are being used to help other patients with chronic health conditions as part of the Pack Health services, which Dr. Patel discussed in a video interview.
SOURCE: Patel D et al. AACE 2018. Abstract 1209.
BOSTON – A company that partners patients and health advisers claims to help patients with type 2 diabetes to manage their symptoms and lower their HbA1c levels.
The Pack Health program connects each patient with a health adviser, who contacts them five times a week using a mix of phone calls, text messages, and emails. The goals include helping patients to find clinicians in their area, make appointments, and adhere to treatment, Dhiren Patel, PharmD, medical director at Pack Health, said at the annual meeting of the American Association of Clinical Endocrinologists.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients lost an average of 5 pounds, and annual eye and foot exams increased by 17% and 19%, respectively.
Similar remote health coach initiatives are being used to help other patients with chronic health conditions as part of the Pack Health services, which Dr. Patel discussed in a video interview.
SOURCE: Patel D et al. AACE 2018. Abstract 1209.
BOSTON – A company that partners patients and health advisers claims to help patients with type 2 diabetes to manage their symptoms and lower their HbA1c levels.
The Pack Health program connects each patient with a health adviser, who contacts them five times a week using a mix of phone calls, text messages, and emails. The goals include helping patients to find clinicians in their area, make appointments, and adhere to treatment, Dhiren Patel, PharmD, medical director at Pack Health, said at the annual meeting of the American Association of Clinical Endocrinologists.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Patients lost an average of 5 pounds, and annual eye and foot exams increased by 17% and 19%, respectively.
Similar remote health coach initiatives are being used to help other patients with chronic health conditions as part of the Pack Health services, which Dr. Patel discussed in a video interview.
SOURCE: Patel D et al. AACE 2018. Abstract 1209.
REPORTING FROM AACE 2018
VIDEO: Canagliflozin’s HbA1c effect muted over time by placebo group effects
BOSTON – In a large, long-term study of canagliflozin versus placebo, an excess of discontinuations in the placebo group contributed to a dampening in the magnitude of improvement in hemoglobin A1c.
That’s according to investigators who reported the findings at the annual meeting of the American Association of Clinical Endocrinologists.
A higher rate of starting new antihyperglycemic agents in the placebo arm also likely contributed to the decrease in the difference in HbA1c after 52 weeks in CANVAS (Canagliflozin Cardiovascular Assessment Study), according to investigator Carol Wysham, MD, of the University of Washington, Spokane.
As previously reported, the two randomized trials in the CANVAS program showed that canagliflozin reduced risk of cardiovascular events in patients with type 2 diabetes at elevated risk of cardiovascular disease.
While the CANVAS program was not designed to assess glucose lowering – and, in fact, allowed adjustment of background antihyperglycemic agents at any time – canagliflozin patients were more likely than placebo-treated patients to achieve HbA1c less than 7.0%.
However, the mean placebo-subtracted reduction in HbA1c peaked at –0.64% at week 26 but shrank to –0.24% by week 338, the end of the study.
“In this case, [the analysis] is helping to understand why we might have seen a deterioration in A1c control over a very long period of time,” Dr. Wysham explained.
The analysis showed that, after week 52 of the study, more patients discontinued placebo, compared with those taking canagliflozin. Over the entire CANVAS program, investigators said, the rate of discontinuation was 118.0 per 1,000 patient-years in the placebo group and 94.1 per 1,000 patient-years for canagliflozin.
In addition, while the concomitant use of antihyperglycemic agents was well balanced at baseline, the number of participants initiating new antihyperglycemic agents over the course of the study was 27% for the placebo-treated patients and 17.8% for the canagliflozin-treated patients, investigators found.
All together, the CANVAS program has provided the longest-term experience to date regarding glycemic control with canagliflozin, demonstrating greater reductions in HbA1c, versus placebo, over about 6.5 years, Dr. Wysham and her coinvestigators said in their poster presentation.
The new analysis provides better clarity on why the durability in HbA1c benefit seemed somewhat attenuated over time, according to Dr. Wysham.
“Most of us think that, if you start an SGLT2 inhibitor, especially starting it relatively early in diagnosis of diabetes, it gives you better durability than what you might see with other agents,” she said. “In fact, that’s been seen in many of the clinical trials, compared to sulphonylurea or DPP-4 inhibitor.”
Dr. Wysham reported disclosures related to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Insulet, Janssen Scientific Affairs, Novo Nordisk, and Sanofi Pasteur.
SOURCE: Wysham C et al. AACE 2018, Abstract 262.
BOSTON – In a large, long-term study of canagliflozin versus placebo, an excess of discontinuations in the placebo group contributed to a dampening in the magnitude of improvement in hemoglobin A1c.
That’s according to investigators who reported the findings at the annual meeting of the American Association of Clinical Endocrinologists.
A higher rate of starting new antihyperglycemic agents in the placebo arm also likely contributed to the decrease in the difference in HbA1c after 52 weeks in CANVAS (Canagliflozin Cardiovascular Assessment Study), according to investigator Carol Wysham, MD, of the University of Washington, Spokane.
As previously reported, the two randomized trials in the CANVAS program showed that canagliflozin reduced risk of cardiovascular events in patients with type 2 diabetes at elevated risk of cardiovascular disease.
While the CANVAS program was not designed to assess glucose lowering – and, in fact, allowed adjustment of background antihyperglycemic agents at any time – canagliflozin patients were more likely than placebo-treated patients to achieve HbA1c less than 7.0%.
However, the mean placebo-subtracted reduction in HbA1c peaked at –0.64% at week 26 but shrank to –0.24% by week 338, the end of the study.
“In this case, [the analysis] is helping to understand why we might have seen a deterioration in A1c control over a very long period of time,” Dr. Wysham explained.
The analysis showed that, after week 52 of the study, more patients discontinued placebo, compared with those taking canagliflozin. Over the entire CANVAS program, investigators said, the rate of discontinuation was 118.0 per 1,000 patient-years in the placebo group and 94.1 per 1,000 patient-years for canagliflozin.
In addition, while the concomitant use of antihyperglycemic agents was well balanced at baseline, the number of participants initiating new antihyperglycemic agents over the course of the study was 27% for the placebo-treated patients and 17.8% for the canagliflozin-treated patients, investigators found.
All together, the CANVAS program has provided the longest-term experience to date regarding glycemic control with canagliflozin, demonstrating greater reductions in HbA1c, versus placebo, over about 6.5 years, Dr. Wysham and her coinvestigators said in their poster presentation.
The new analysis provides better clarity on why the durability in HbA1c benefit seemed somewhat attenuated over time, according to Dr. Wysham.
“Most of us think that, if you start an SGLT2 inhibitor, especially starting it relatively early in diagnosis of diabetes, it gives you better durability than what you might see with other agents,” she said. “In fact, that’s been seen in many of the clinical trials, compared to sulphonylurea or DPP-4 inhibitor.”
Dr. Wysham reported disclosures related to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Insulet, Janssen Scientific Affairs, Novo Nordisk, and Sanofi Pasteur.
SOURCE: Wysham C et al. AACE 2018, Abstract 262.
BOSTON – In a large, long-term study of canagliflozin versus placebo, an excess of discontinuations in the placebo group contributed to a dampening in the magnitude of improvement in hemoglobin A1c.
That’s according to investigators who reported the findings at the annual meeting of the American Association of Clinical Endocrinologists.
A higher rate of starting new antihyperglycemic agents in the placebo arm also likely contributed to the decrease in the difference in HbA1c after 52 weeks in CANVAS (Canagliflozin Cardiovascular Assessment Study), according to investigator Carol Wysham, MD, of the University of Washington, Spokane.
As previously reported, the two randomized trials in the CANVAS program showed that canagliflozin reduced risk of cardiovascular events in patients with type 2 diabetes at elevated risk of cardiovascular disease.
While the CANVAS program was not designed to assess glucose lowering – and, in fact, allowed adjustment of background antihyperglycemic agents at any time – canagliflozin patients were more likely than placebo-treated patients to achieve HbA1c less than 7.0%.
However, the mean placebo-subtracted reduction in HbA1c peaked at –0.64% at week 26 but shrank to –0.24% by week 338, the end of the study.
“In this case, [the analysis] is helping to understand why we might have seen a deterioration in A1c control over a very long period of time,” Dr. Wysham explained.
The analysis showed that, after week 52 of the study, more patients discontinued placebo, compared with those taking canagliflozin. Over the entire CANVAS program, investigators said, the rate of discontinuation was 118.0 per 1,000 patient-years in the placebo group and 94.1 per 1,000 patient-years for canagliflozin.
In addition, while the concomitant use of antihyperglycemic agents was well balanced at baseline, the number of participants initiating new antihyperglycemic agents over the course of the study was 27% for the placebo-treated patients and 17.8% for the canagliflozin-treated patients, investigators found.
All together, the CANVAS program has provided the longest-term experience to date regarding glycemic control with canagliflozin, demonstrating greater reductions in HbA1c, versus placebo, over about 6.5 years, Dr. Wysham and her coinvestigators said in their poster presentation.
The new analysis provides better clarity on why the durability in HbA1c benefit seemed somewhat attenuated over time, according to Dr. Wysham.
“Most of us think that, if you start an SGLT2 inhibitor, especially starting it relatively early in diagnosis of diabetes, it gives you better durability than what you might see with other agents,” she said. “In fact, that’s been seen in many of the clinical trials, compared to sulphonylurea or DPP-4 inhibitor.”
Dr. Wysham reported disclosures related to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Insulet, Janssen Scientific Affairs, Novo Nordisk, and Sanofi Pasteur.
SOURCE: Wysham C et al. AACE 2018, Abstract 262.
REPORTING FROM AACE 2018
Key clinical point: and a higher rate of starting new antihyperglycemic agents in the placebo arm.
Major finding: After week 52 of the study, more patients discontinued placebo versus canagliflozin. The number of participants initiating new antihyperglycemic agents over the course of the study was 17.8% for the canagliflozin arm and 27% for placebo.
Study details: An analysis of the effects of canagliflozin on HbA1c and changes in use of antihyperglycemic agents in the CANVAS study.
Disclosures: Dr. Wysham reported disclosures related to AstraZeneca, Boehringer Ingelheim, Eli Lilly, Insulet, Janssen Scientific Affairs, Novo Nordisk, and Sanofi Pasteur.
Source: Wysham C et al. AACE 2018, Abstract 262.
VIDEO: Acromegaly study reveals gender-specific differences
BOSTON – , according to study results presented at the annual meeting of the American Association of Clinical Endocrinologists.
The study was based on data for 112 patients (54 male, 58 female) operated on by one neurosurgeon between 1994 and 2016. The mean age at surgery was 43.6 years in men and 48.7 in women (P = .04), according to Talin Handa, Emory University, Atlanta, who presented the retrospective analysis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Men had higher mean IGF-1 levels (874 ng/mL vs. 716 ng/mL for women; P less than .01), and were more likely to have hypopituitarism.
Adjuvant treatment for acromegaly was needed in 57% of men and 49% of women. Following adjuvant treatment, 72% of men maintained surgical remission or achieved normal IGF-1 levels, compared with 89% of women (P = .03). Mean follow-up was shorter in men, 3.6 years, versus 5.2 years for women (P = .02), the researchers reported.
Six-year event-free survival was higher in women (P less than .01), according to the researchers.
For more study findings, watch our video interview.
SOURCE: Handa T et al. AACE 2018. Abstract #824.
BOSTON – , according to study results presented at the annual meeting of the American Association of Clinical Endocrinologists.
The study was based on data for 112 patients (54 male, 58 female) operated on by one neurosurgeon between 1994 and 2016. The mean age at surgery was 43.6 years in men and 48.7 in women (P = .04), according to Talin Handa, Emory University, Atlanta, who presented the retrospective analysis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Men had higher mean IGF-1 levels (874 ng/mL vs. 716 ng/mL for women; P less than .01), and were more likely to have hypopituitarism.
Adjuvant treatment for acromegaly was needed in 57% of men and 49% of women. Following adjuvant treatment, 72% of men maintained surgical remission or achieved normal IGF-1 levels, compared with 89% of women (P = .03). Mean follow-up was shorter in men, 3.6 years, versus 5.2 years for women (P = .02), the researchers reported.
Six-year event-free survival was higher in women (P less than .01), according to the researchers.
For more study findings, watch our video interview.
SOURCE: Handa T et al. AACE 2018. Abstract #824.
BOSTON – , according to study results presented at the annual meeting of the American Association of Clinical Endocrinologists.
The study was based on data for 112 patients (54 male, 58 female) operated on by one neurosurgeon between 1994 and 2016. The mean age at surgery was 43.6 years in men and 48.7 in women (P = .04), according to Talin Handa, Emory University, Atlanta, who presented the retrospective analysis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Men had higher mean IGF-1 levels (874 ng/mL vs. 716 ng/mL for women; P less than .01), and were more likely to have hypopituitarism.
Adjuvant treatment for acromegaly was needed in 57% of men and 49% of women. Following adjuvant treatment, 72% of men maintained surgical remission or achieved normal IGF-1 levels, compared with 89% of women (P = .03). Mean follow-up was shorter in men, 3.6 years, versus 5.2 years for women (P = .02), the researchers reported.
Six-year event-free survival was higher in women (P less than .01), according to the researchers.
For more study findings, watch our video interview.
SOURCE: Handa T et al. AACE 2018. Abstract #824.
REPORTING FROM AACE 2018
Key clinical point: Men with acromegaly present at a younger age, have higher IGF-1 levels, and achieve lower biochemical control rates than do women with the disorder.
Major finding: Men were less likely than women to have surgical remissions or normal IGF-1 levels after adjuvant treatment (72% for men and 89% for women; P = .03).
Study details: A retrospective analysis of 112 patients (54 male, 58 female) operated on by one neurosurgeon during 1994-2016.
Disclosures: The presenter had no disclosures related to the presentation.
Source: Handa T et al. AACE 2018. Abstract #824.
VIDEO: Location of thyroid nodules may predict malignancy
BOSTON – according to the results of a retrospective, single-center study presented at the annual meeting of the American Association of Clinical Endocrinologists.
When nodules were located in the upper pole of the gland, the risk of malignancy was about 4 times higher than it was for nodules at other locations in the gland. Researchers confirmed the association using a multiple logistic regression model with adjustment for age, gender, body mass index, laterality, and number of nodules (odds ratio, 4.6; P = 0.03). The findings are believed to be the first to show an association between location of thyroid nodules on ultrasound and malignancy risk.
The results appear to elevate the value of ultrasound for predicting thyroid malignancy and should affect guidelines for ultrasound classification of thyroid nodules, researcher Fan Zhang, MD, PhD, a fellow at State University of New York, Brooklyn, said in a video interview. “In the future, I would recommend maybe we could consider including the location of thyroid nodules in the guidelines for better predictive value of malignancies,” she said.
Other ultrasound characteristics known to be associated with malignancy include findings of microcalcifications, increased vascularity, and nodules that are taller than they are wide, according to Dr. Zhang.
The retrospective review included data on 219 clinic patients with thyroid nodules who underwent fine-needle aspiration biopsy between July 2016 and June 2017. Nearly 80% of the nodules in the review were located in the lower pole of the gland, about 10% were in the upper pole, 7% were in the middle pole, and about 2% were found in the isthmus.
Fourteen nodules, or 7.4%, were found to be malignant, Dr. Zhang and her coauthors said in their presentation. Of those 14 malignancies, 7 were among the 149 nodules in the lower pole, 4 were among the 18 in the upper pole, and 3 were among the 21 in the middle pole.
The anatomy of the thyroid gland may be a factor in why upper pole nodules would be more likely to be associated with malignancy, according to Dr. Zhang. “The veins in the upper lobe are more tortuous compared to in the lower lobe,” she said, noting that slow venous drainage may increase the possibility of developing malignancy.
Dr. Zhang had no relevant disclosures to report.
SOURCE: Zhang F et al. AACE 2018, Abstract 1204.
BOSTON – according to the results of a retrospective, single-center study presented at the annual meeting of the American Association of Clinical Endocrinologists.
When nodules were located in the upper pole of the gland, the risk of malignancy was about 4 times higher than it was for nodules at other locations in the gland. Researchers confirmed the association using a multiple logistic regression model with adjustment for age, gender, body mass index, laterality, and number of nodules (odds ratio, 4.6; P = 0.03). The findings are believed to be the first to show an association between location of thyroid nodules on ultrasound and malignancy risk.
The results appear to elevate the value of ultrasound for predicting thyroid malignancy and should affect guidelines for ultrasound classification of thyroid nodules, researcher Fan Zhang, MD, PhD, a fellow at State University of New York, Brooklyn, said in a video interview. “In the future, I would recommend maybe we could consider including the location of thyroid nodules in the guidelines for better predictive value of malignancies,” she said.
Other ultrasound characteristics known to be associated with malignancy include findings of microcalcifications, increased vascularity, and nodules that are taller than they are wide, according to Dr. Zhang.
The retrospective review included data on 219 clinic patients with thyroid nodules who underwent fine-needle aspiration biopsy between July 2016 and June 2017. Nearly 80% of the nodules in the review were located in the lower pole of the gland, about 10% were in the upper pole, 7% were in the middle pole, and about 2% were found in the isthmus.
Fourteen nodules, or 7.4%, were found to be malignant, Dr. Zhang and her coauthors said in their presentation. Of those 14 malignancies, 7 were among the 149 nodules in the lower pole, 4 were among the 18 in the upper pole, and 3 were among the 21 in the middle pole.
The anatomy of the thyroid gland may be a factor in why upper pole nodules would be more likely to be associated with malignancy, according to Dr. Zhang. “The veins in the upper lobe are more tortuous compared to in the lower lobe,” she said, noting that slow venous drainage may increase the possibility of developing malignancy.
Dr. Zhang had no relevant disclosures to report.
SOURCE: Zhang F et al. AACE 2018, Abstract 1204.
BOSTON – according to the results of a retrospective, single-center study presented at the annual meeting of the American Association of Clinical Endocrinologists.
When nodules were located in the upper pole of the gland, the risk of malignancy was about 4 times higher than it was for nodules at other locations in the gland. Researchers confirmed the association using a multiple logistic regression model with adjustment for age, gender, body mass index, laterality, and number of nodules (odds ratio, 4.6; P = 0.03). The findings are believed to be the first to show an association between location of thyroid nodules on ultrasound and malignancy risk.
The results appear to elevate the value of ultrasound for predicting thyroid malignancy and should affect guidelines for ultrasound classification of thyroid nodules, researcher Fan Zhang, MD, PhD, a fellow at State University of New York, Brooklyn, said in a video interview. “In the future, I would recommend maybe we could consider including the location of thyroid nodules in the guidelines for better predictive value of malignancies,” she said.
Other ultrasound characteristics known to be associated with malignancy include findings of microcalcifications, increased vascularity, and nodules that are taller than they are wide, according to Dr. Zhang.
The retrospective review included data on 219 clinic patients with thyroid nodules who underwent fine-needle aspiration biopsy between July 2016 and June 2017. Nearly 80% of the nodules in the review were located in the lower pole of the gland, about 10% were in the upper pole, 7% were in the middle pole, and about 2% were found in the isthmus.
Fourteen nodules, or 7.4%, were found to be malignant, Dr. Zhang and her coauthors said in their presentation. Of those 14 malignancies, 7 were among the 149 nodules in the lower pole, 4 were among the 18 in the upper pole, and 3 were among the 21 in the middle pole.
The anatomy of the thyroid gland may be a factor in why upper pole nodules would be more likely to be associated with malignancy, according to Dr. Zhang. “The veins in the upper lobe are more tortuous compared to in the lower lobe,” she said, noting that slow venous drainage may increase the possibility of developing malignancy.
Dr. Zhang had no relevant disclosures to report.
SOURCE: Zhang F et al. AACE 2018, Abstract 1204.
REPORTING FROM AACE 2018
Key clinical point: Thyroid nodules located in the upper pole may be considered a risk factor for malignancy.
Major finding: Assessment by location showed that 28.6% of nodules found in the upper pole were malignant, compared with 4.9% in the lower pole, 18.2% in the middle pole, and 14.3% in the isthmus (odds ratio, 5.8; P = 0.01).
Study details: A retrospective review including data on 219 clinic patients with thyroid nodules who underwent fine-needle aspiration biopsy between July 2016 and June 2017.
Disclosures: Dr. Zhang had no relevant disclosures to report.
Source: Zhang F et al. AACE 2018, Abstract 1204.
VIDEO: Move beyond BMI to see obesity as a disease
BOSTON – , W. Timothy Garvey, MD, said.
“The term ‘obesity’ means so many things to different people,” Dr. Garvey explained in a video interview at the annual meeting of the American Association of Clinical Endocrinologists. “It doesn’t tell you what the impact is of excess adiposity on health.”
In fact, obesity meets the criteria needed to be defined as a disease, said Dr. Garvey, who coauthored a 2017 AACE position statement recommending a new diagnostic term for obesity: adiposity-based chronic disease, or ABCD.
“It’s not going to replace the general use of the term ‘obesity,’ of course; but for medical diagnosis, this term does tell you what we’re treating, and why we’re treating it,” noted Dr. Garvey, of the University of Alabama at Birmingham.
Instead of relying on BMI [body mass index], the ABCD model emphasizes a “complications-centric” approach that drives therapeutic decisions, which may include medication.
“A structured lifestyle intervention is the key to therapy, but if we add medications on to any lifestyle intervention, we’re going to get more bang for the buck,” Dr. Garvey explained.
“We’re going to get more weight loss and be able to keep it off for a longer period of time,” he added. “We want that in situations in particular where the patient really has complications. This could be diabetes, it could be prediabetes, it could be obstructive sleep apnea, symptomatic osteoarthritis in the knees, stress incontinence, hypertension – any one of a number of weight-related complications that are really impairing health.”
The five medications approved for chronic management of obesity all have been shown to be safe and effective in clinical trials. But they have different mechanisms of action, different side effect profiles, and different warnings and precautions, Dr. Garvey noted.
Understanding the pharmacology of all five drugs is important to help a specific patient achieve the best outcomes.
“There’s no drug that can be recommended, in a hierarchical sense, as being better than any others across the board in all patients,” Dr. Garvey explained. “We really need to individualize therapy based on their side effect profile and their types of complications that present with the patient.”
Endocrinologists can be particularly helpful in incorporating weight loss therapy into the overall therapeutic plan for refractory cases, he said, or in patients significantly burdened with metabolic complications, including dysglycemia, diabetes, hypertriglyceridemia, and nonalcoholic fatty liver disease.
Primary care physicians, advanced practice clinicians, dietitians, and others are needed on the team to engineer a successful lifestyle intervention for the obese patient. However, Dr. Garvey emphasized that the endocrinology subspecialty encompasses not only endocrinology and diabetes, but also metabolism.
“We need to take the lead here,” Dr. Garvey said. “Obesity is the most common metabolic disease on the planet.”
Dr. Garvey reported disclosures related to Janssen, Novo Nordisk, and Sanofi.
BOSTON – , W. Timothy Garvey, MD, said.
“The term ‘obesity’ means so many things to different people,” Dr. Garvey explained in a video interview at the annual meeting of the American Association of Clinical Endocrinologists. “It doesn’t tell you what the impact is of excess adiposity on health.”
In fact, obesity meets the criteria needed to be defined as a disease, said Dr. Garvey, who coauthored a 2017 AACE position statement recommending a new diagnostic term for obesity: adiposity-based chronic disease, or ABCD.
“It’s not going to replace the general use of the term ‘obesity,’ of course; but for medical diagnosis, this term does tell you what we’re treating, and why we’re treating it,” noted Dr. Garvey, of the University of Alabama at Birmingham.
Instead of relying on BMI [body mass index], the ABCD model emphasizes a “complications-centric” approach that drives therapeutic decisions, which may include medication.
“A structured lifestyle intervention is the key to therapy, but if we add medications on to any lifestyle intervention, we’re going to get more bang for the buck,” Dr. Garvey explained.
“We’re going to get more weight loss and be able to keep it off for a longer period of time,” he added. “We want that in situations in particular where the patient really has complications. This could be diabetes, it could be prediabetes, it could be obstructive sleep apnea, symptomatic osteoarthritis in the knees, stress incontinence, hypertension – any one of a number of weight-related complications that are really impairing health.”
The five medications approved for chronic management of obesity all have been shown to be safe and effective in clinical trials. But they have different mechanisms of action, different side effect profiles, and different warnings and precautions, Dr. Garvey noted.
Understanding the pharmacology of all five drugs is important to help a specific patient achieve the best outcomes.
“There’s no drug that can be recommended, in a hierarchical sense, as being better than any others across the board in all patients,” Dr. Garvey explained. “We really need to individualize therapy based on their side effect profile and their types of complications that present with the patient.”
Endocrinologists can be particularly helpful in incorporating weight loss therapy into the overall therapeutic plan for refractory cases, he said, or in patients significantly burdened with metabolic complications, including dysglycemia, diabetes, hypertriglyceridemia, and nonalcoholic fatty liver disease.
Primary care physicians, advanced practice clinicians, dietitians, and others are needed on the team to engineer a successful lifestyle intervention for the obese patient. However, Dr. Garvey emphasized that the endocrinology subspecialty encompasses not only endocrinology and diabetes, but also metabolism.
“We need to take the lead here,” Dr. Garvey said. “Obesity is the most common metabolic disease on the planet.”
Dr. Garvey reported disclosures related to Janssen, Novo Nordisk, and Sanofi.
BOSTON – , W. Timothy Garvey, MD, said.
“The term ‘obesity’ means so many things to different people,” Dr. Garvey explained in a video interview at the annual meeting of the American Association of Clinical Endocrinologists. “It doesn’t tell you what the impact is of excess adiposity on health.”
In fact, obesity meets the criteria needed to be defined as a disease, said Dr. Garvey, who coauthored a 2017 AACE position statement recommending a new diagnostic term for obesity: adiposity-based chronic disease, or ABCD.
“It’s not going to replace the general use of the term ‘obesity,’ of course; but for medical diagnosis, this term does tell you what we’re treating, and why we’re treating it,” noted Dr. Garvey, of the University of Alabama at Birmingham.
Instead of relying on BMI [body mass index], the ABCD model emphasizes a “complications-centric” approach that drives therapeutic decisions, which may include medication.
“A structured lifestyle intervention is the key to therapy, but if we add medications on to any lifestyle intervention, we’re going to get more bang for the buck,” Dr. Garvey explained.
“We’re going to get more weight loss and be able to keep it off for a longer period of time,” he added. “We want that in situations in particular where the patient really has complications. This could be diabetes, it could be prediabetes, it could be obstructive sleep apnea, symptomatic osteoarthritis in the knees, stress incontinence, hypertension – any one of a number of weight-related complications that are really impairing health.”
The five medications approved for chronic management of obesity all have been shown to be safe and effective in clinical trials. But they have different mechanisms of action, different side effect profiles, and different warnings and precautions, Dr. Garvey noted.
Understanding the pharmacology of all five drugs is important to help a specific patient achieve the best outcomes.
“There’s no drug that can be recommended, in a hierarchical sense, as being better than any others across the board in all patients,” Dr. Garvey explained. “We really need to individualize therapy based on their side effect profile and their types of complications that present with the patient.”
Endocrinologists can be particularly helpful in incorporating weight loss therapy into the overall therapeutic plan for refractory cases, he said, or in patients significantly burdened with metabolic complications, including dysglycemia, diabetes, hypertriglyceridemia, and nonalcoholic fatty liver disease.
Primary care physicians, advanced practice clinicians, dietitians, and others are needed on the team to engineer a successful lifestyle intervention for the obese patient. However, Dr. Garvey emphasized that the endocrinology subspecialty encompasses not only endocrinology and diabetes, but also metabolism.
“We need to take the lead here,” Dr. Garvey said. “Obesity is the most common metabolic disease on the planet.”
Dr. Garvey reported disclosures related to Janssen, Novo Nordisk, and Sanofi.
REPORTING FROM AACE 2018
VIDEO: Calming microglia might control fibromyalgia
SANDESTIN, FLA. – Activated microglia may be a root cause of fibromyalgia, and bringing them back to a resting state an effective path to symptom relief.
Jarred Younger, PhD, is particularly interested in dextronaltrexone, the right-handed isomer of the drug commonly employed in addiction medicine, for calming microglia in fibromyalgia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Unlike the commercially available levo-naltrexone, which binds at both the mu-opioid receptor and Toll-like receptor 4 (TLR4), dextronaltrexone blocks only TLR4. Blocking this receptor interferes with the cells’ ability to recruit peripheral immune cells, which may enter the brain, release cytokines, and induce a proinflammatory environment. By targeting only TLR4 and sparing opioid receptors, , Dr. Younger said in a video interview at the annual Congress of Clinical Rheumatology.
He already has investigated low-dose levo-naltrexone in a small positive crossover trial in 31 fibromyalgia patients. While taking the drug, patients reported significantly less pain and improved mood.
Dr. Younger also recently published a study suggesting that low-dose naltrexone actively improves peripheral proinflammatory cytokine levels.
The placebo-controlled crossover trial enrolled eight women with moderately severe fibromyalgia who took 4.5 mg naltrexone daily for 8 weeks. Compared with baseline, they had significantly reduced plasma levels of a variety of interleukin (IL) subtypes. Also reduced were interferon-alpha, transforming growth factor-alpha and -beta, TNF-alpha, and granulocyte-colony stimulating factor. Patients experienced a mean 15% reduction in fibromyalgia pain and an 18% reduction in overall symptoms.
But proving the drug’s method of action continues to be a challenge, he admitted. It’s not easy to observe microglial trafficking and cellular response to immune signaling in the brain.
Dr. Younger is preparing to launch an innovative PET study that should prove whether activated microglia are recruiting peripheral leukocytes into the brains of fibromyalgia patients. He intends to isolate T and B cells from blood, tag them with a PET radioligand, and reinject them into the subject.
“Since those cells are tagged, a few days later, we can scan the person and see if those cells made it into the brain,” Dr. Younger explained. “If we find T cells and B cells in the brain, that’s clear evidence that the peripheral immune system is attacking and infiltrating the brain, which would be very good in telling us what’s going on in fibromyalgia.”
Low-dose naltrexone is not approved for treating fibromyalgia, he noted. However, during the discussion period after Dr. Younger’s presentation, a number of physicians said they have been using the drug in fibromyalgia patients; some said it has been useful for patients with multiple sclerosis, as well.
Dr. Younger had no relevant financial disclosures.
SANDESTIN, FLA. – Activated microglia may be a root cause of fibromyalgia, and bringing them back to a resting state an effective path to symptom relief.
Jarred Younger, PhD, is particularly interested in dextronaltrexone, the right-handed isomer of the drug commonly employed in addiction medicine, for calming microglia in fibromyalgia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Unlike the commercially available levo-naltrexone, which binds at both the mu-opioid receptor and Toll-like receptor 4 (TLR4), dextronaltrexone blocks only TLR4. Blocking this receptor interferes with the cells’ ability to recruit peripheral immune cells, which may enter the brain, release cytokines, and induce a proinflammatory environment. By targeting only TLR4 and sparing opioid receptors, , Dr. Younger said in a video interview at the annual Congress of Clinical Rheumatology.
He already has investigated low-dose levo-naltrexone in a small positive crossover trial in 31 fibromyalgia patients. While taking the drug, patients reported significantly less pain and improved mood.
Dr. Younger also recently published a study suggesting that low-dose naltrexone actively improves peripheral proinflammatory cytokine levels.
The placebo-controlled crossover trial enrolled eight women with moderately severe fibromyalgia who took 4.5 mg naltrexone daily for 8 weeks. Compared with baseline, they had significantly reduced plasma levels of a variety of interleukin (IL) subtypes. Also reduced were interferon-alpha, transforming growth factor-alpha and -beta, TNF-alpha, and granulocyte-colony stimulating factor. Patients experienced a mean 15% reduction in fibromyalgia pain and an 18% reduction in overall symptoms.
But proving the drug’s method of action continues to be a challenge, he admitted. It’s not easy to observe microglial trafficking and cellular response to immune signaling in the brain.
Dr. Younger is preparing to launch an innovative PET study that should prove whether activated microglia are recruiting peripheral leukocytes into the brains of fibromyalgia patients. He intends to isolate T and B cells from blood, tag them with a PET radioligand, and reinject them into the subject.
“Since those cells are tagged, a few days later, we can scan the person and see if those cells made it into the brain,” Dr. Younger explained. “If we find T cells and B cells in the brain, that’s clear evidence that the peripheral immune system is attacking and infiltrating the brain, which would be very good in telling us what’s going on in fibromyalgia.”
Low-dose naltrexone is not approved for treating fibromyalgia, he noted. However, during the discussion period after Dr. Younger’s presentation, a number of physicians said they have been using the drug in fibromyalgia patients; some said it has been useful for patients with multiple sclerosis, as well.
Dr. Younger had no relevant financial disclosures.
SANDESTIN, FLA. – Activated microglia may be a root cause of fibromyalgia, and bringing them back to a resting state an effective path to symptom relief.
Jarred Younger, PhD, is particularly interested in dextronaltrexone, the right-handed isomer of the drug commonly employed in addiction medicine, for calming microglia in fibromyalgia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Unlike the commercially available levo-naltrexone, which binds at both the mu-opioid receptor and Toll-like receptor 4 (TLR4), dextronaltrexone blocks only TLR4. Blocking this receptor interferes with the cells’ ability to recruit peripheral immune cells, which may enter the brain, release cytokines, and induce a proinflammatory environment. By targeting only TLR4 and sparing opioid receptors, , Dr. Younger said in a video interview at the annual Congress of Clinical Rheumatology.
He already has investigated low-dose levo-naltrexone in a small positive crossover trial in 31 fibromyalgia patients. While taking the drug, patients reported significantly less pain and improved mood.
Dr. Younger also recently published a study suggesting that low-dose naltrexone actively improves peripheral proinflammatory cytokine levels.
The placebo-controlled crossover trial enrolled eight women with moderately severe fibromyalgia who took 4.5 mg naltrexone daily for 8 weeks. Compared with baseline, they had significantly reduced plasma levels of a variety of interleukin (IL) subtypes. Also reduced were interferon-alpha, transforming growth factor-alpha and -beta, TNF-alpha, and granulocyte-colony stimulating factor. Patients experienced a mean 15% reduction in fibromyalgia pain and an 18% reduction in overall symptoms.
But proving the drug’s method of action continues to be a challenge, he admitted. It’s not easy to observe microglial trafficking and cellular response to immune signaling in the brain.
Dr. Younger is preparing to launch an innovative PET study that should prove whether activated microglia are recruiting peripheral leukocytes into the brains of fibromyalgia patients. He intends to isolate T and B cells from blood, tag them with a PET radioligand, and reinject them into the subject.
“Since those cells are tagged, a few days later, we can scan the person and see if those cells made it into the brain,” Dr. Younger explained. “If we find T cells and B cells in the brain, that’s clear evidence that the peripheral immune system is attacking and infiltrating the brain, which would be very good in telling us what’s going on in fibromyalgia.”
Low-dose naltrexone is not approved for treating fibromyalgia, he noted. However, during the discussion period after Dr. Younger’s presentation, a number of physicians said they have been using the drug in fibromyalgia patients; some said it has been useful for patients with multiple sclerosis, as well.
Dr. Younger had no relevant financial disclosures.
REPORTING FROM CCR 2018