User login
Direct and Indirect Patient Costs of Dermatology Clinic Visits and Their Impact on Access to Care and Provider Preference
Access to outpatient specialty care is notably limited due to time and out-of-pocket costs to patients, leading to patient dissatisfaction and worsened clinical outcomes. Lost time and earnings pose considerable opportunity costs for patients, with the total opportunity cost for all physician visits per year estimated at $52 billion in 2010 in the United States.1
The field of dermatology exemplifies the access issues patients may face when seeking specialty care given the ongoing national shortage of dermatologists and notably long wait times exceeding 60 days in major cities.2-4 With the high demand and limited number of providers, patients may have longer wait times to see dermatologists in their communities or have to travel further to see dermatologists in distant locations who have available appointments; therefore, patients may be subject to higher associated time, travel, and monetary costs. According to the 2013 Medical Expenditure Panel Survey, dermatology visits in the United States cost an average of $221 per visit compared to $166 for primary care. Dermatology visits had the highest median cost per office visit ($124) and were more often associated with out-of-pocket expenses (60.7%) compared to other specialties.5 Despite these high costs, the number of dermatology visits is increasing each year, with more than 38 million dermatology visits in 2012.6
In light of these factors that limit patient access to dermatologists compared to other specialists, we performed an evaluation of the direct and indirect costs to patients visiting an outpatient dermatology clinic in Boston, Massachusetts, to better understand obstacles to receiving dermatologic care. The impact that time and money have on how patients prefer to receive their care also was evaluated. Conducting this study in Boston may best reflect patient barriers to obtaining dermatologic treatment, as nationwide surveys have found that Boston has the highest cumulative average wait times for physician appointments compared to other US metropolitan cities, with an average wait time of 72 days to see a dermatologist.4 New studies of patient costs associated with dermatology clinic visits are lacking, and existing economic analyses rarely include time costs. Understanding time burden and opportunity costs from the patient perspective may motivate patients and physicians to alter how they receive and provide health care, respectively, to minimize these expenses. Advances in health care technology such as telecommunication may facilitate these changes.
Methods
Study Design
This survey study took place from October 1, 2015, to March 4, 2016, at the department of dermatology outpatient clinic of Tufts Medical Center, an academic university hospital located in downtown Boston, Massachusetts, with no satellite clinics. Five general dermatologists, 2 dermatologic surgeons, and 9 dermatology residents comprised the dermatology department. The study protocol and questionnaire received exemption status from the Tufts University Health Science’s institutional review board.
All adult patients (aged ≥18 years) attending a scheduled dermatology clinic visit within the designated time frame were invited to complete a questionnaire available in English, Spanish, or Chinese. Patients completed the questionnaire on paper or electronically using handheld tablets. Data were then compiled into the REDCap (Research Electronic Data Capture) online database. The questionnaire surveyed patient age; gender; ethnicity; language spoken; highest level of education; employment status; reason for visit (ie, skin condition); duration, cost, and mode of transportation; duration of visit including wait time; companion accompaniment; profession; hourly wage; and number of work hours requested off to attend the visit. Lastly, patients were surveyed on whether they prefer to receive dermatologic care at Tufts, to receive in-person care elsewhere, to use teledermatology, or none of the above.
Statistical Analysis
Total time attributed to the visit was the sum of time for round-trip travel to and from the clinic, wait time, and face-to-face time with care providers. Out-of-pocket patient expenses included round-trip travel expenses, child care expenses, and direct payments such as deductibles and co-pays. Opportunity cost for employed patients was calculated as the patient’s average hourly wage multiplied by either the number of hours taken off from work or the number of hours the patient attributed to the visit, whichever value was higher at the individual level. For the purpose of calculating opportunity costs, travel time, wait time, and face-to-face time were imputed using average values for these variables when not reported. Patients could provide exact hourly wage and annual income or select the closest approximation from 10 wage ranges. For patients who selected a wage range, the midpoint of the range was used as the hourly wage. Total costs were the sum of reported out-of-pocket expenses and calculated opportunity costs. For unemployed patients and those who did not report employment status, hourly wage was assumed to be $0, resulting in opportunity costs of $0. Costs are tabulated for individual patients and analyzed in aggregate.
Differences in patient characteristics between those who preferred their current care provider versus those who preferred to seek care elsewhere or via teledermatology were compared using the χ2 and Student t test. A multivariate logistic regression was then performed to identify predictors of patient preference for their current provider. Potential predictors for regression model were time and cost variables as well as factors selected based on results from bivariate analysis. Data analysis was performed using statistical software.
Results
Demographics
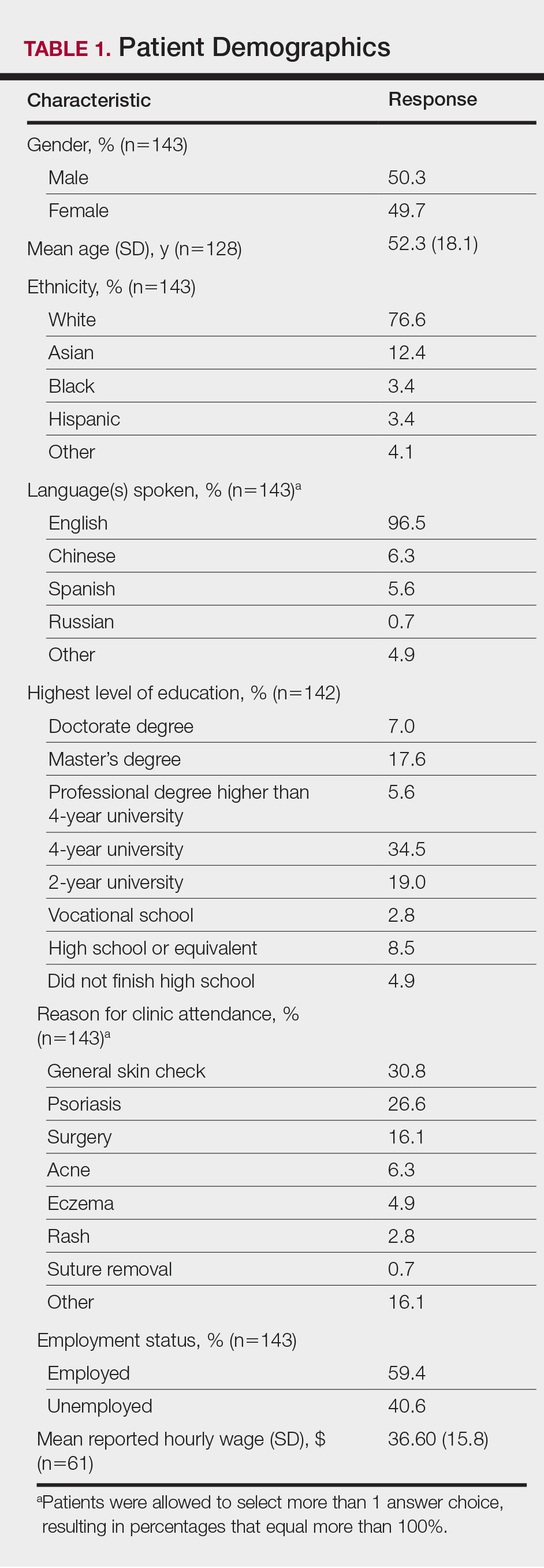
Demographic data for respondents are outlined in Table 1. Of 145 patients who completed the survey, the majority had already seen a dermatologist for their presenting condition (87.4%), and were English speaking (96.5%), white (76.6%), employed (59.4%), and male (50.3%), with a mean age (SD) of 52.3 (18.1) years and education level of 4-year university or higher (64.7%). The most common reasons for dermatology clinic attendance were general skin checks (30.8%) and psoriasis (26.6%). A smaller proportion of patients (16.1%) presented for surgical visits. Other less common conditions that brought patients into the clinic included acne (6.3%), eczema (4.9%), and skin rash (2.8%).
The mean (SD) reported hourly wage of employed patients was $36.60 (15.8). The most common reasons for unemployment were retirement (65.5% [38/58]), disability (10.3% [6/58]), and schooling (10.3% [6/58]).
Time Attributed to Attending Dermatology Clinic Visits
Time costs are reported in Table 2. Patients traveled to the clinic mainly by car (56.5% [78/138]) or train/subway (25.3% [35/138]). One in approximately 5 patients (21.3%) spent more than 1 hour traveling one-way to the clinic. Most patients waited less than 20 minutes to see their care providers. Face-to-face time with providers (ie, residents and attending physicians) ranged from less than 21 minutes to more than 1 hour, with a mean (SD) time of 36.8 (18.9) minutes.
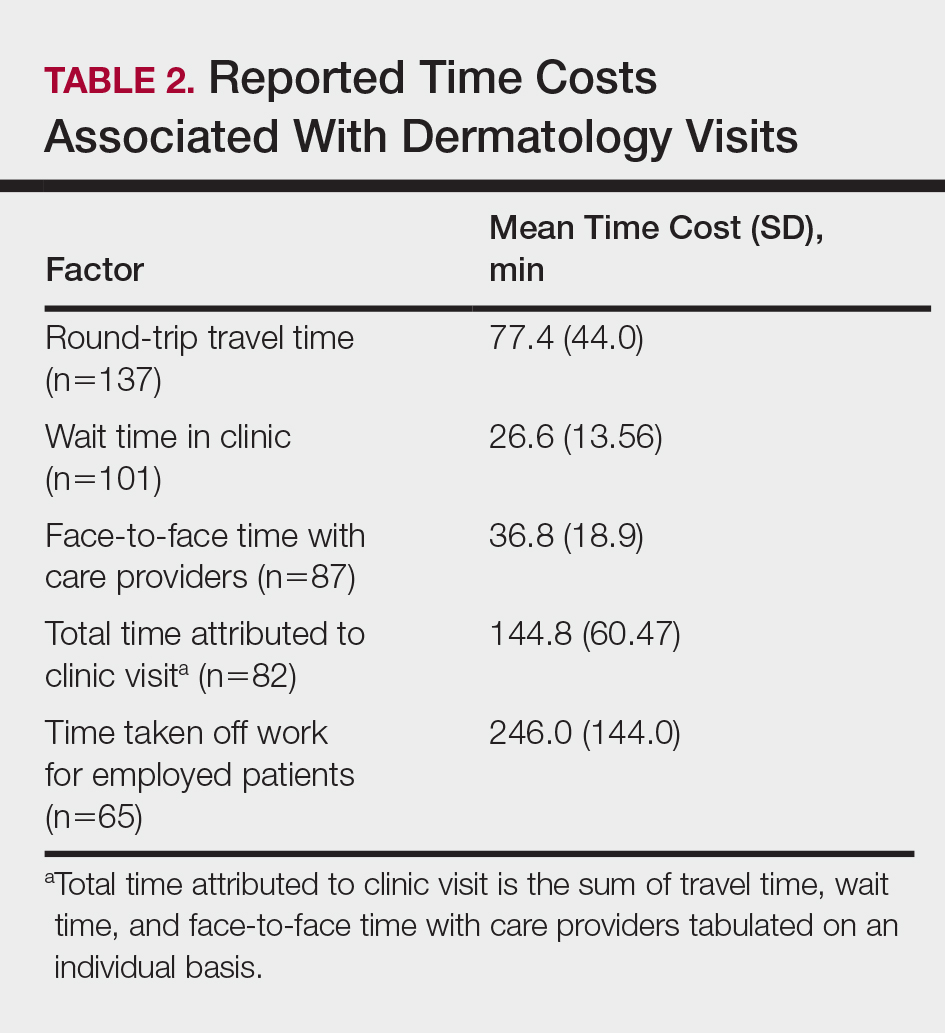
Of the employed respondents, 76.5% (65/85) took off time from work for the appointment. Patients took a mean (SD) of 4.1 (2.4) hours off from work, which was considered sick pay (35%), paid time off (36.6%), or unpaid time (28.3%). The total mean (SD) time dedicated to attending the clinic appointment averaged 144.8 (60.47) minutes. On average, the time spent traveling for the clinic visit was double the amount of time spent with the care provider (77.4 vs 36.8 minutes).
Monetary and Opportunity Costs
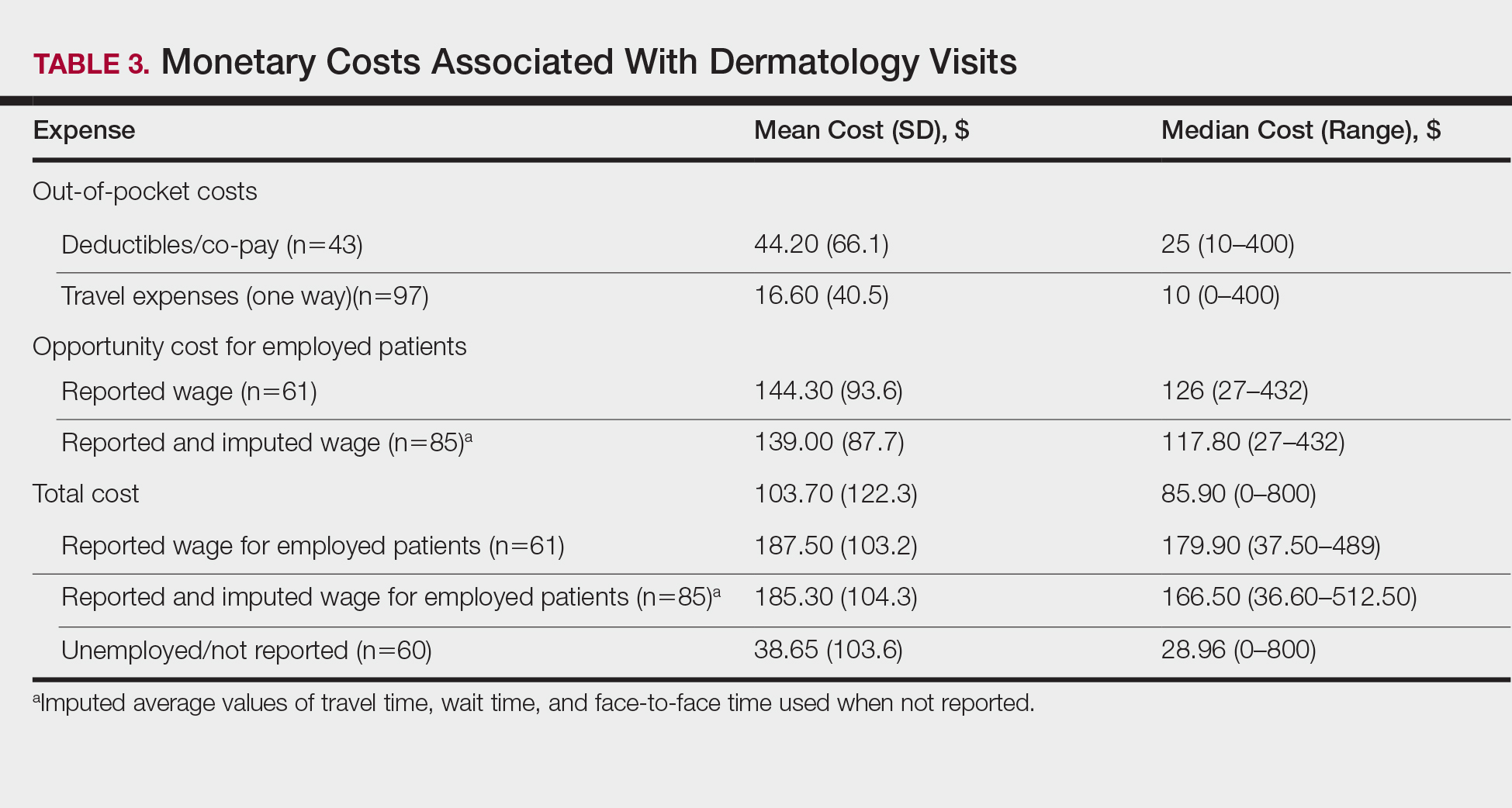
The mean (SD) monetary cost associated with clinic attendance for employed patients who reported their wages was $187.50 (103.2)(range, $37.50–$489), most of which was opportunity cost from loss of potential work income (mean [SD], $144.30 [93.6]; range, $27–$432)(Table 3). Similar total and opportunity costs were found for employed patients using the imputed average wage. The mean (SD) total cost per visit for unemployed patients or those who did not report employment status was $38.65 (103.6)(range, $0–$800), which was 4-times less than the cost per visit for employed patients. Mean (SD) and median one-way travel expenses were $16.60 (40.5) and $10, respectively. Mean (SD) and median reported costs for deductibles/co-pays were $44.20 (66.1) and $25, respectively. Only 2 patients reported child care costs, which were valued at $65 and $75.
Patient Provider Preference
The majority (59.3% [67/113]) of patients preferred their current care providers, whereas 33.6% (38/113) preferred providers closer to work, home, or in a different unspecified setting. Only 7.0% (8/113) of patients who answered this survey question would choose teledermatology over their current providers.
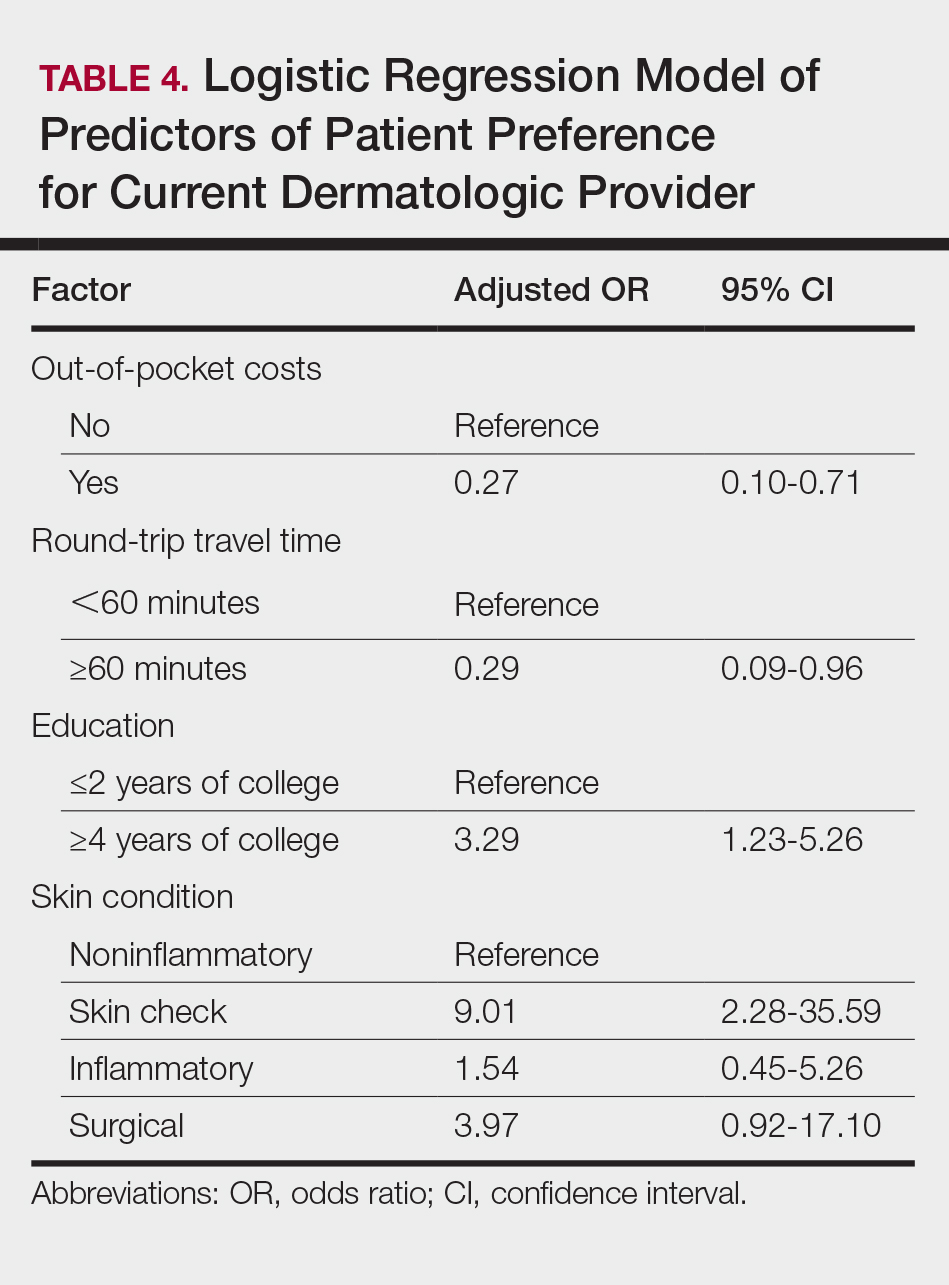
On multivariate logistic regression (Table 4), patients who had additional out-of-pocket costs were significantly less likely to prefer their current care provider compared to patients with no out-of-pocket costs (odds ratio [OR], 0.27; 95% confidence interval [CI], 0.10-0.71; P<.05). Opportunity costs were not a significant predictor of provider preference. For every minute the travel time increased, the likelihood of preference for the current care provider decreased by 2% (OR, 0.98; 95% CI, 0.95–0.99), and patients who traveled 60 minutes or more round-trip were 71% less likely to choose current provider care than those who traveled less than 60 minutes (OR, 0.29; 95% CI, 0.09-0.96; P<.05). Patients with higher education (≥4 years of college) were 3.29-times more likely to stay with their current care provider than those with lower education (≤2 years of college). Those presenting for skin checks also preferred the current provider more than those with noninflammatory skin conditions such as alopecia and warts (OR, 9.01; 95% CI, 2.28-35.59). Age and gender were not statistically significant predictors of patient provider preference.
Comment
Our study revealed that patients spend a substantial amount of time and money attending dermatology clinic appointments. Round-trip travel time exceeded 2 hours for 20% of patients and accounted for the majority of the total time attributed to the visit. Patients who were employed typically requested an average of 4 hours off from work, resulting in a mean (SD) opportunity cost of $144.30 (93.6) due to lost wages. Direct costs such as co-pays, deductibles, travel expenses, and child care accounted for a smaller proportion of total costs. The study assumed a wage of $0 for unemployed patients, thus underestimating the true costs of the visit for these patients whose time may otherwise have been spent on leisure, education, volunteerism, or other activities that contribute to individual and societal productivity. The total costs for unemployed patients reflected only direct costs, and thus were notably lower than those for employed patients.
Direct out-of-pocket costs and travel time negatively impacted provider preference. Patients with out-of-pocket costs were much less likely to stay with their current care provider (OR, 0.27; 95% CI, 0.10-0.71), preferring to seek care closer to home/work or teledermatology services. Similarly, for each minute that travel time increased, preference for current care provider decreased by 2%. Those who traveled 60 minutes or more were 71% less likely than those who traveled less than 60 minutes to stay with their current provider when given other options for care. Opportunity costs did not affect provider preference, even though they far exceeded direct costs for employed patients. Perhaps opportunity costs are not as immediately apparent to patients as out-of-pocket costs and travel time, and thus they do not factor as heavily in provider preference.
Despite high time and monetary costs, the majority of patients (60%) still preferred their current care provider, especially those with 4-year university degrees or higher education level (OR, 3.29; 95% CI, 1.23-5.26) and those presenting for skin checks (OR, 9.01; 95% CI, 2.28-35.59). Patients with higher levels of education likely have higher incomes and thus may not be as adversely affected by direct and/or indirect visit costs. Patients presenting for skin checks may value continuity and prefer providers with whom they already have an established therapeutic relationship. Future studies are needed to analyze the impact of these nonmonetary factors on provider preference.
Seeking Alternative Care
Tufts Medical Center does not have satellite dermatology clinics, making it the only option for patients who wish to receive care within the Tufts hospital network. However, patients do have the option of visiting non–Tufts-affiliated dermatology clinics outside of the city. To our knowledge, no formal studies have been performed comparing wait times for dermatology appointments in suburban versus urban Boston areas; however, it has been reported that rural practitioners have longer wait times than urban dermatologists, possibly due to the fact that physicians tend to aggregate in metropolitan areas.2 Thus, the potential for shorter wait times in the Boston metropolitan area may make it a more desirable location to receive care compared to more suburban or even rural areas of Massachusetts, but additional data are needed to substantiate this hypothesis. Additionally, health insurance restrictions, refractory or complex dermatologic conditions, and referring providers’ preference may affect patients’ decisions to seek care at a particular clinic. However, these factors do not alter our finding that those who travel long distances to our dermatology clinic are less likely to stay with their current provider if given the choice to seek care closer to home/work or utilize teledermatology services.
Prior studies have demonstrated patient preference and willingness to accept alternative modes of care delivery to reduce time and monetary costs associated with in-person medical visits.7,8 Dermatology patients at a clinic in Ontario, Canada, considered the time they spent attending the clinic to be even more burdensome than the monetary cost.7 Patients with nondermatologic chronic diseases and high out-of-pocket costs would prefer email rather than a clinic visit as the first method of contact with care providers.8 The explosive growth of direct-to-consumer (DTC) teledermatology services in the last 10 years speaks to patient demand for alternative care delivery that saves time and money. Although telemedicine has been implemented in various specialties, including ophthalmology and neurology, one of the most common applications is teledermatology. With DTC teledermatology, patients can take photographs or videos using personal smartphones and communicate directly with care providers using mobile or online applications. More recent review articles have identified 22 to 29 DTC mobile and web-based teledermatology services, with costs varying from $0 to $250.9-11 The median consultation fee of $59 for DTC teledermatology services is substantially less than total visit costs for employed patients in our study.9 Teledermatology has become an accessible and affordable modality of care, though perhaps not yet fully optimized for quality of care.
With increasingly higher co-pays and high-deductible insurance plans, time and monetary factors play increasingly important roles in patient preference for specialty care providers,12 as demonstrated by our study. Dermatologists can work with patients to reduce the costs of medical visits. Perhaps monitoring of chronic but stable conditions can be accomplished through telecommunication to reduce the number of follow-up visits. For instance, psoriasis patients enrolled in telemonitoring perceived savings of time and expenses through reduction of clinic visits, resulting in high patient satisfaction levels.13 Telephone calls and secure email messaging are other feasible alternatives shown to aid in clinical management and decrease the need for in-person care.8,14 Fewer unnecessary follow-up visits also means more availability for new patients and those with acute needs.
Barriers to obtaining care are not limited to dermatology and are pervasive across most medical specialties. Issues of patient time burden and out-of-pocket expenses are reflected in recent reports focused on quantifying these costs throughout ambulatory care visits and services such as colorectal, cervical, and breast cancer screenings.1,15-18 Similar to our findings, many of these studies also show high time and opportunity costs from the patient perspective. Expansion of telemedicine to reduce patient costs is becoming a viable option for many specialists, though low reimbursement rates restrict its widespread application.9,19 However, this obstacle is not impossible to surmount. One study found that offering teledermatology to Medicaid patients through their primary care providers significantly improved access, allowing for a 63.8% increase in the number of patients visiting a dermatologist (P<.01).20 Currently, a total of 48 state Medicaid programs now cover telemedicine, and a growing number of states are requiring private insurers to cover telehealth services.21 As more dermatologists adopt telemedicine practices, it may allow for better access as well as expanded insurance coverage.
Limitations
The results of our study are limited by the single-institution survey design. Patients were asked to complete the survey while still at the clinic visit to minimize recall bias. Because these patients actually attended their appointments, they might perceive the time and monetary costs associated with the visit to be less problematic than those who canceled their appointments or transferred care elsewhere; however, we were still able to detect a significant impact of time and monetary costs on provider preference in this cohort (P<.05). Larger studies in different geographic settings and other specialty clinics are needed to confirm our findings and to determine if nonmonetary factors such as specific diagnoses, length of time with a certain care provider, or patient socioeconomic status can modulate the impact of time and monetary costs on provider preference.
Conclusion
This study showed that patients expend a substantial amount of time and monetary costs to attend dermatology clinic visits. Data from the current and prior studies suggest that these costs affect patient provider preference for dermatologic care and may pose barriers to necessary medical care. Recognizing direct and indirect patient costs may drive critical changes in health care delivery, such as increased telecommunication utilization, the more cost-saving alternative. Telemedicine, when integrated appropriately, can help minimize expenses for patients while continuing to maintain a high level of care.
- Ray KN, Chari AV, Engberg J, et al. Opportunity costs of ambulatory medical care in the United States. Am J Manag Care. 2015;21:567-574.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Lipton S, Pletcher MJ. Short wait times for patients seeking cosmetic botulinum toxin appointments with dermatologists. J Am Acad Dermatol. 2007;57:985-989.
- Physician appointment wait times & Medicaid and Medicare acceptance rates. Merritt Hawkins website. https://www.merritthawkins.com/2014-survey/patientwaittime.aspx. Accessed February 15, 2017.
- Machlin SR, Adams SA. Expenses for office-based physician visits by specialty, 2013. Agency for Healthcare Research and Quality website. https://meps.ahrq.gov/data_files/publications/st484/stat484.pdf. Published November 2015. Accessed February 15, 2017.
- National ambulatory medical care survey: 2012 state and national summary tables. CDC website. www.cdc.gov/nchs/data/ahcd/namcs_summary/2012_namcs_web_tables.pdf. Accessed February 15, 2017.
- Vignjevic PM, Hux JE, Fisher BK, et al. Monetary and nonmonetary costs to patients attending an ambulatory dermatology clinic. J Cutan Med Surg. 1999;3:188-192.
- Reed M, Graetz I, Gordon N, Fung V. Patient-initiated e-mails to providers: associations with out-of-pocket visit costs, and impact on care-seeking and health. Am J Manag Care. 2015;21:E632-E639.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Kochmann M, Locatis C. Direct to consumer mobile teledermatology apps: an exploratory study. Telemed J E Health. 2016;22:689-693.
- Helms AD. High-deductible health plans can ruin finances. Kaiser Health News website. https://khn.org/news/high-deductible-health-plans-can-ruin-finances/. Published April 6, 2015. Accessed February 15, 2017.
- Fruhauf J, Schwantzer G, Ambros-Rudolph CM, et al. Pilot study on the acceptance of mobile teledermatology for the home monitoring of high-need patients with psoriasis. Australas J Dermatol. 2012;53:41-46.
- Eisenberg D, Hwa K, Wren SM. Telephone follow-up by a midlevel provider after laparoscopic inguinal hernia repair instead of face-to-face clinic visit. JSLS. 2015;19:e2014.00205.
- Yabroff KR, Guy GP Jr, Ekwueme DU, et al. Annual patient time costs associated with medical care among cancer survivors in the United States. Med Care. 2014;52:594-601.
- Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14-23.
- Jonas DE, Russell LB, Sandler RS, et al. Value of patient time invested in the colonoscopy screening process: time requirements for colonoscopy study. Med Decis Making. 2008;28:56-65.
- Shireman TI, Tsevat J, Goldie SJ. Time costs associated with cervical cancer screening. Int J Technol Assess Health Care. 2001;17:146-152.
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375:154-161.
- Uscher-Pines L, Malsberger R, Burgette L, et al. Effect of teledermatology on access to dermatology care among Medicaid enrollees. JAMA Dermatol. 2016;152:905-912.
- Thomas L, Capistrant G. State telemedicine gaps analysis: coverage & reimbursement. Telehealth website. http://www.mtelehealth.com/state-telemedicine-gaps-analysis-coverage-reimbursement/. Published January 19, 2016. Accessed February 15, 2017.
Access to outpatient specialty care is notably limited due to time and out-of-pocket costs to patients, leading to patient dissatisfaction and worsened clinical outcomes. Lost time and earnings pose considerable opportunity costs for patients, with the total opportunity cost for all physician visits per year estimated at $52 billion in 2010 in the United States.1
The field of dermatology exemplifies the access issues patients may face when seeking specialty care given the ongoing national shortage of dermatologists and notably long wait times exceeding 60 days in major cities.2-4 With the high demand and limited number of providers, patients may have longer wait times to see dermatologists in their communities or have to travel further to see dermatologists in distant locations who have available appointments; therefore, patients may be subject to higher associated time, travel, and monetary costs. According to the 2013 Medical Expenditure Panel Survey, dermatology visits in the United States cost an average of $221 per visit compared to $166 for primary care. Dermatology visits had the highest median cost per office visit ($124) and were more often associated with out-of-pocket expenses (60.7%) compared to other specialties.5 Despite these high costs, the number of dermatology visits is increasing each year, with more than 38 million dermatology visits in 2012.6
In light of these factors that limit patient access to dermatologists compared to other specialists, we performed an evaluation of the direct and indirect costs to patients visiting an outpatient dermatology clinic in Boston, Massachusetts, to better understand obstacles to receiving dermatologic care. The impact that time and money have on how patients prefer to receive their care also was evaluated. Conducting this study in Boston may best reflect patient barriers to obtaining dermatologic treatment, as nationwide surveys have found that Boston has the highest cumulative average wait times for physician appointments compared to other US metropolitan cities, with an average wait time of 72 days to see a dermatologist.4 New studies of patient costs associated with dermatology clinic visits are lacking, and existing economic analyses rarely include time costs. Understanding time burden and opportunity costs from the patient perspective may motivate patients and physicians to alter how they receive and provide health care, respectively, to minimize these expenses. Advances in health care technology such as telecommunication may facilitate these changes.
Methods
Study Design
This survey study took place from October 1, 2015, to March 4, 2016, at the department of dermatology outpatient clinic of Tufts Medical Center, an academic university hospital located in downtown Boston, Massachusetts, with no satellite clinics. Five general dermatologists, 2 dermatologic surgeons, and 9 dermatology residents comprised the dermatology department. The study protocol and questionnaire received exemption status from the Tufts University Health Science’s institutional review board.
All adult patients (aged ≥18 years) attending a scheduled dermatology clinic visit within the designated time frame were invited to complete a questionnaire available in English, Spanish, or Chinese. Patients completed the questionnaire on paper or electronically using handheld tablets. Data were then compiled into the REDCap (Research Electronic Data Capture) online database. The questionnaire surveyed patient age; gender; ethnicity; language spoken; highest level of education; employment status; reason for visit (ie, skin condition); duration, cost, and mode of transportation; duration of visit including wait time; companion accompaniment; profession; hourly wage; and number of work hours requested off to attend the visit. Lastly, patients were surveyed on whether they prefer to receive dermatologic care at Tufts, to receive in-person care elsewhere, to use teledermatology, or none of the above.
Statistical Analysis
Total time attributed to the visit was the sum of time for round-trip travel to and from the clinic, wait time, and face-to-face time with care providers. Out-of-pocket patient expenses included round-trip travel expenses, child care expenses, and direct payments such as deductibles and co-pays. Opportunity cost for employed patients was calculated as the patient’s average hourly wage multiplied by either the number of hours taken off from work or the number of hours the patient attributed to the visit, whichever value was higher at the individual level. For the purpose of calculating opportunity costs, travel time, wait time, and face-to-face time were imputed using average values for these variables when not reported. Patients could provide exact hourly wage and annual income or select the closest approximation from 10 wage ranges. For patients who selected a wage range, the midpoint of the range was used as the hourly wage. Total costs were the sum of reported out-of-pocket expenses and calculated opportunity costs. For unemployed patients and those who did not report employment status, hourly wage was assumed to be $0, resulting in opportunity costs of $0. Costs are tabulated for individual patients and analyzed in aggregate.
Differences in patient characteristics between those who preferred their current care provider versus those who preferred to seek care elsewhere or via teledermatology were compared using the χ2 and Student t test. A multivariate logistic regression was then performed to identify predictors of patient preference for their current provider. Potential predictors for regression model were time and cost variables as well as factors selected based on results from bivariate analysis. Data analysis was performed using statistical software.
Results
Demographics
Demographic data for respondents are outlined in Table 1. Of 145 patients who completed the survey, the majority had already seen a dermatologist for their presenting condition (87.4%), and were English speaking (96.5%), white (76.6%), employed (59.4%), and male (50.3%), with a mean age (SD) of 52.3 (18.1) years and education level of 4-year university or higher (64.7%). The most common reasons for dermatology clinic attendance were general skin checks (30.8%) and psoriasis (26.6%). A smaller proportion of patients (16.1%) presented for surgical visits. Other less common conditions that brought patients into the clinic included acne (6.3%), eczema (4.9%), and skin rash (2.8%).

The mean (SD) reported hourly wage of employed patients was $36.60 (15.8). The most common reasons for unemployment were retirement (65.5% [38/58]), disability (10.3% [6/58]), and schooling (10.3% [6/58]).
Time Attributed to Attending Dermatology Clinic Visits
Time costs are reported in Table 2. Patients traveled to the clinic mainly by car (56.5% [78/138]) or train/subway (25.3% [35/138]). One in approximately 5 patients (21.3%) spent more than 1 hour traveling one-way to the clinic. Most patients waited less than 20 minutes to see their care providers. Face-to-face time with providers (ie, residents and attending physicians) ranged from less than 21 minutes to more than 1 hour, with a mean (SD) time of 36.8 (18.9) minutes.

Of the employed respondents, 76.5% (65/85) took off time from work for the appointment. Patients took a mean (SD) of 4.1 (2.4) hours off from work, which was considered sick pay (35%), paid time off (36.6%), or unpaid time (28.3%). The total mean (SD) time dedicated to attending the clinic appointment averaged 144.8 (60.47) minutes. On average, the time spent traveling for the clinic visit was double the amount of time spent with the care provider (77.4 vs 36.8 minutes).
Monetary and Opportunity Costs
The mean (SD) monetary cost associated with clinic attendance for employed patients who reported their wages was $187.50 (103.2)(range, $37.50–$489), most of which was opportunity cost from loss of potential work income (mean [SD], $144.30 [93.6]; range, $27–$432)(Table 3). Similar total and opportunity costs were found for employed patients using the imputed average wage. The mean (SD) total cost per visit for unemployed patients or those who did not report employment status was $38.65 (103.6)(range, $0–$800), which was 4-times less than the cost per visit for employed patients. Mean (SD) and median one-way travel expenses were $16.60 (40.5) and $10, respectively. Mean (SD) and median reported costs for deductibles/co-pays were $44.20 (66.1) and $25, respectively. Only 2 patients reported child care costs, which were valued at $65 and $75.

Patient Provider Preference
The majority (59.3% [67/113]) of patients preferred their current care providers, whereas 33.6% (38/113) preferred providers closer to work, home, or in a different unspecified setting. Only 7.0% (8/113) of patients who answered this survey question would choose teledermatology over their current providers.
On multivariate logistic regression (Table 4), patients who had additional out-of-pocket costs were significantly less likely to prefer their current care provider compared to patients with no out-of-pocket costs (odds ratio [OR], 0.27; 95% confidence interval [CI], 0.10-0.71; P<.05). Opportunity costs were not a significant predictor of provider preference. For every minute the travel time increased, the likelihood of preference for the current care provider decreased by 2% (OR, 0.98; 95% CI, 0.95–0.99), and patients who traveled 60 minutes or more round-trip were 71% less likely to choose current provider care than those who traveled less than 60 minutes (OR, 0.29; 95% CI, 0.09-0.96; P<.05). Patients with higher education (≥4 years of college) were 3.29-times more likely to stay with their current care provider than those with lower education (≤2 years of college). Those presenting for skin checks also preferred the current provider more than those with noninflammatory skin conditions such as alopecia and warts (OR, 9.01; 95% CI, 2.28-35.59). Age and gender were not statistically significant predictors of patient provider preference.

Comment
Our study revealed that patients spend a substantial amount of time and money attending dermatology clinic appointments. Round-trip travel time exceeded 2 hours for 20% of patients and accounted for the majority of the total time attributed to the visit. Patients who were employed typically requested an average of 4 hours off from work, resulting in a mean (SD) opportunity cost of $144.30 (93.6) due to lost wages. Direct costs such as co-pays, deductibles, travel expenses, and child care accounted for a smaller proportion of total costs. The study assumed a wage of $0 for unemployed patients, thus underestimating the true costs of the visit for these patients whose time may otherwise have been spent on leisure, education, volunteerism, or other activities that contribute to individual and societal productivity. The total costs for unemployed patients reflected only direct costs, and thus were notably lower than those for employed patients.
Direct out-of-pocket costs and travel time negatively impacted provider preference. Patients with out-of-pocket costs were much less likely to stay with their current care provider (OR, 0.27; 95% CI, 0.10-0.71), preferring to seek care closer to home/work or teledermatology services. Similarly, for each minute that travel time increased, preference for current care provider decreased by 2%. Those who traveled 60 minutes or more were 71% less likely than those who traveled less than 60 minutes to stay with their current provider when given other options for care. Opportunity costs did not affect provider preference, even though they far exceeded direct costs for employed patients. Perhaps opportunity costs are not as immediately apparent to patients as out-of-pocket costs and travel time, and thus they do not factor as heavily in provider preference.
Despite high time and monetary costs, the majority of patients (60%) still preferred their current care provider, especially those with 4-year university degrees or higher education level (OR, 3.29; 95% CI, 1.23-5.26) and those presenting for skin checks (OR, 9.01; 95% CI, 2.28-35.59). Patients with higher levels of education likely have higher incomes and thus may not be as adversely affected by direct and/or indirect visit costs. Patients presenting for skin checks may value continuity and prefer providers with whom they already have an established therapeutic relationship. Future studies are needed to analyze the impact of these nonmonetary factors on provider preference.
Seeking Alternative Care
Tufts Medical Center does not have satellite dermatology clinics, making it the only option for patients who wish to receive care within the Tufts hospital network. However, patients do have the option of visiting non–Tufts-affiliated dermatology clinics outside of the city. To our knowledge, no formal studies have been performed comparing wait times for dermatology appointments in suburban versus urban Boston areas; however, it has been reported that rural practitioners have longer wait times than urban dermatologists, possibly due to the fact that physicians tend to aggregate in metropolitan areas.2 Thus, the potential for shorter wait times in the Boston metropolitan area may make it a more desirable location to receive care compared to more suburban or even rural areas of Massachusetts, but additional data are needed to substantiate this hypothesis. Additionally, health insurance restrictions, refractory or complex dermatologic conditions, and referring providers’ preference may affect patients’ decisions to seek care at a particular clinic. However, these factors do not alter our finding that those who travel long distances to our dermatology clinic are less likely to stay with their current provider if given the choice to seek care closer to home/work or utilize teledermatology services.
Prior studies have demonstrated patient preference and willingness to accept alternative modes of care delivery to reduce time and monetary costs associated with in-person medical visits.7,8 Dermatology patients at a clinic in Ontario, Canada, considered the time they spent attending the clinic to be even more burdensome than the monetary cost.7 Patients with nondermatologic chronic diseases and high out-of-pocket costs would prefer email rather than a clinic visit as the first method of contact with care providers.8 The explosive growth of direct-to-consumer (DTC) teledermatology services in the last 10 years speaks to patient demand for alternative care delivery that saves time and money. Although telemedicine has been implemented in various specialties, including ophthalmology and neurology, one of the most common applications is teledermatology. With DTC teledermatology, patients can take photographs or videos using personal smartphones and communicate directly with care providers using mobile or online applications. More recent review articles have identified 22 to 29 DTC mobile and web-based teledermatology services, with costs varying from $0 to $250.9-11 The median consultation fee of $59 for DTC teledermatology services is substantially less than total visit costs for employed patients in our study.9 Teledermatology has become an accessible and affordable modality of care, though perhaps not yet fully optimized for quality of care.
With increasingly higher co-pays and high-deductible insurance plans, time and monetary factors play increasingly important roles in patient preference for specialty care providers,12 as demonstrated by our study. Dermatologists can work with patients to reduce the costs of medical visits. Perhaps monitoring of chronic but stable conditions can be accomplished through telecommunication to reduce the number of follow-up visits. For instance, psoriasis patients enrolled in telemonitoring perceived savings of time and expenses through reduction of clinic visits, resulting in high patient satisfaction levels.13 Telephone calls and secure email messaging are other feasible alternatives shown to aid in clinical management and decrease the need for in-person care.8,14 Fewer unnecessary follow-up visits also means more availability for new patients and those with acute needs.
Barriers to obtaining care are not limited to dermatology and are pervasive across most medical specialties. Issues of patient time burden and out-of-pocket expenses are reflected in recent reports focused on quantifying these costs throughout ambulatory care visits and services such as colorectal, cervical, and breast cancer screenings.1,15-18 Similar to our findings, many of these studies also show high time and opportunity costs from the patient perspective. Expansion of telemedicine to reduce patient costs is becoming a viable option for many specialists, though low reimbursement rates restrict its widespread application.9,19 However, this obstacle is not impossible to surmount. One study found that offering teledermatology to Medicaid patients through their primary care providers significantly improved access, allowing for a 63.8% increase in the number of patients visiting a dermatologist (P<.01).20 Currently, a total of 48 state Medicaid programs now cover telemedicine, and a growing number of states are requiring private insurers to cover telehealth services.21 As more dermatologists adopt telemedicine practices, it may allow for better access as well as expanded insurance coverage.
Limitations
The results of our study are limited by the single-institution survey design. Patients were asked to complete the survey while still at the clinic visit to minimize recall bias. Because these patients actually attended their appointments, they might perceive the time and monetary costs associated with the visit to be less problematic than those who canceled their appointments or transferred care elsewhere; however, we were still able to detect a significant impact of time and monetary costs on provider preference in this cohort (P<.05). Larger studies in different geographic settings and other specialty clinics are needed to confirm our findings and to determine if nonmonetary factors such as specific diagnoses, length of time with a certain care provider, or patient socioeconomic status can modulate the impact of time and monetary costs on provider preference.
Conclusion
This study showed that patients expend a substantial amount of time and monetary costs to attend dermatology clinic visits. Data from the current and prior studies suggest that these costs affect patient provider preference for dermatologic care and may pose barriers to necessary medical care. Recognizing direct and indirect patient costs may drive critical changes in health care delivery, such as increased telecommunication utilization, the more cost-saving alternative. Telemedicine, when integrated appropriately, can help minimize expenses for patients while continuing to maintain a high level of care.
Access to outpatient specialty care is notably limited due to time and out-of-pocket costs to patients, leading to patient dissatisfaction and worsened clinical outcomes. Lost time and earnings pose considerable opportunity costs for patients, with the total opportunity cost for all physician visits per year estimated at $52 billion in 2010 in the United States.1
The field of dermatology exemplifies the access issues patients may face when seeking specialty care given the ongoing national shortage of dermatologists and notably long wait times exceeding 60 days in major cities.2-4 With the high demand and limited number of providers, patients may have longer wait times to see dermatologists in their communities or have to travel further to see dermatologists in distant locations who have available appointments; therefore, patients may be subject to higher associated time, travel, and monetary costs. According to the 2013 Medical Expenditure Panel Survey, dermatology visits in the United States cost an average of $221 per visit compared to $166 for primary care. Dermatology visits had the highest median cost per office visit ($124) and were more often associated with out-of-pocket expenses (60.7%) compared to other specialties.5 Despite these high costs, the number of dermatology visits is increasing each year, with more than 38 million dermatology visits in 2012.6
In light of these factors that limit patient access to dermatologists compared to other specialists, we performed an evaluation of the direct and indirect costs to patients visiting an outpatient dermatology clinic in Boston, Massachusetts, to better understand obstacles to receiving dermatologic care. The impact that time and money have on how patients prefer to receive their care also was evaluated. Conducting this study in Boston may best reflect patient barriers to obtaining dermatologic treatment, as nationwide surveys have found that Boston has the highest cumulative average wait times for physician appointments compared to other US metropolitan cities, with an average wait time of 72 days to see a dermatologist.4 New studies of patient costs associated with dermatology clinic visits are lacking, and existing economic analyses rarely include time costs. Understanding time burden and opportunity costs from the patient perspective may motivate patients and physicians to alter how they receive and provide health care, respectively, to minimize these expenses. Advances in health care technology such as telecommunication may facilitate these changes.
Methods
Study Design
This survey study took place from October 1, 2015, to March 4, 2016, at the department of dermatology outpatient clinic of Tufts Medical Center, an academic university hospital located in downtown Boston, Massachusetts, with no satellite clinics. Five general dermatologists, 2 dermatologic surgeons, and 9 dermatology residents comprised the dermatology department. The study protocol and questionnaire received exemption status from the Tufts University Health Science’s institutional review board.
All adult patients (aged ≥18 years) attending a scheduled dermatology clinic visit within the designated time frame were invited to complete a questionnaire available in English, Spanish, or Chinese. Patients completed the questionnaire on paper or electronically using handheld tablets. Data were then compiled into the REDCap (Research Electronic Data Capture) online database. The questionnaire surveyed patient age; gender; ethnicity; language spoken; highest level of education; employment status; reason for visit (ie, skin condition); duration, cost, and mode of transportation; duration of visit including wait time; companion accompaniment; profession; hourly wage; and number of work hours requested off to attend the visit. Lastly, patients were surveyed on whether they prefer to receive dermatologic care at Tufts, to receive in-person care elsewhere, to use teledermatology, or none of the above.
Statistical Analysis
Total time attributed to the visit was the sum of time for round-trip travel to and from the clinic, wait time, and face-to-face time with care providers. Out-of-pocket patient expenses included round-trip travel expenses, child care expenses, and direct payments such as deductibles and co-pays. Opportunity cost for employed patients was calculated as the patient’s average hourly wage multiplied by either the number of hours taken off from work or the number of hours the patient attributed to the visit, whichever value was higher at the individual level. For the purpose of calculating opportunity costs, travel time, wait time, and face-to-face time were imputed using average values for these variables when not reported. Patients could provide exact hourly wage and annual income or select the closest approximation from 10 wage ranges. For patients who selected a wage range, the midpoint of the range was used as the hourly wage. Total costs were the sum of reported out-of-pocket expenses and calculated opportunity costs. For unemployed patients and those who did not report employment status, hourly wage was assumed to be $0, resulting in opportunity costs of $0. Costs are tabulated for individual patients and analyzed in aggregate.
Differences in patient characteristics between those who preferred their current care provider versus those who preferred to seek care elsewhere or via teledermatology were compared using the χ2 and Student t test. A multivariate logistic regression was then performed to identify predictors of patient preference for their current provider. Potential predictors for regression model were time and cost variables as well as factors selected based on results from bivariate analysis. Data analysis was performed using statistical software.
Results
Demographics
Demographic data for respondents are outlined in Table 1. Of 145 patients who completed the survey, the majority had already seen a dermatologist for their presenting condition (87.4%), and were English speaking (96.5%), white (76.6%), employed (59.4%), and male (50.3%), with a mean age (SD) of 52.3 (18.1) years and education level of 4-year university or higher (64.7%). The most common reasons for dermatology clinic attendance were general skin checks (30.8%) and psoriasis (26.6%). A smaller proportion of patients (16.1%) presented for surgical visits. Other less common conditions that brought patients into the clinic included acne (6.3%), eczema (4.9%), and skin rash (2.8%).

The mean (SD) reported hourly wage of employed patients was $36.60 (15.8). The most common reasons for unemployment were retirement (65.5% [38/58]), disability (10.3% [6/58]), and schooling (10.3% [6/58]).
Time Attributed to Attending Dermatology Clinic Visits
Time costs are reported in Table 2. Patients traveled to the clinic mainly by car (56.5% [78/138]) or train/subway (25.3% [35/138]). One in approximately 5 patients (21.3%) spent more than 1 hour traveling one-way to the clinic. Most patients waited less than 20 minutes to see their care providers. Face-to-face time with providers (ie, residents and attending physicians) ranged from less than 21 minutes to more than 1 hour, with a mean (SD) time of 36.8 (18.9) minutes.

Of the employed respondents, 76.5% (65/85) took off time from work for the appointment. Patients took a mean (SD) of 4.1 (2.4) hours off from work, which was considered sick pay (35%), paid time off (36.6%), or unpaid time (28.3%). The total mean (SD) time dedicated to attending the clinic appointment averaged 144.8 (60.47) minutes. On average, the time spent traveling for the clinic visit was double the amount of time spent with the care provider (77.4 vs 36.8 minutes).
Monetary and Opportunity Costs
The mean (SD) monetary cost associated with clinic attendance for employed patients who reported their wages was $187.50 (103.2)(range, $37.50–$489), most of which was opportunity cost from loss of potential work income (mean [SD], $144.30 [93.6]; range, $27–$432)(Table 3). Similar total and opportunity costs were found for employed patients using the imputed average wage. The mean (SD) total cost per visit for unemployed patients or those who did not report employment status was $38.65 (103.6)(range, $0–$800), which was 4-times less than the cost per visit for employed patients. Mean (SD) and median one-way travel expenses were $16.60 (40.5) and $10, respectively. Mean (SD) and median reported costs for deductibles/co-pays were $44.20 (66.1) and $25, respectively. Only 2 patients reported child care costs, which were valued at $65 and $75.

Patient Provider Preference
The majority (59.3% [67/113]) of patients preferred their current care providers, whereas 33.6% (38/113) preferred providers closer to work, home, or in a different unspecified setting. Only 7.0% (8/113) of patients who answered this survey question would choose teledermatology over their current providers.
On multivariate logistic regression (Table 4), patients who had additional out-of-pocket costs were significantly less likely to prefer their current care provider compared to patients with no out-of-pocket costs (odds ratio [OR], 0.27; 95% confidence interval [CI], 0.10-0.71; P<.05). Opportunity costs were not a significant predictor of provider preference. For every minute the travel time increased, the likelihood of preference for the current care provider decreased by 2% (OR, 0.98; 95% CI, 0.95–0.99), and patients who traveled 60 minutes or more round-trip were 71% less likely to choose current provider care than those who traveled less than 60 minutes (OR, 0.29; 95% CI, 0.09-0.96; P<.05). Patients with higher education (≥4 years of college) were 3.29-times more likely to stay with their current care provider than those with lower education (≤2 years of college). Those presenting for skin checks also preferred the current provider more than those with noninflammatory skin conditions such as alopecia and warts (OR, 9.01; 95% CI, 2.28-35.59). Age and gender were not statistically significant predictors of patient provider preference.

Comment
Our study revealed that patients spend a substantial amount of time and money attending dermatology clinic appointments. Round-trip travel time exceeded 2 hours for 20% of patients and accounted for the majority of the total time attributed to the visit. Patients who were employed typically requested an average of 4 hours off from work, resulting in a mean (SD) opportunity cost of $144.30 (93.6) due to lost wages. Direct costs such as co-pays, deductibles, travel expenses, and child care accounted for a smaller proportion of total costs. The study assumed a wage of $0 for unemployed patients, thus underestimating the true costs of the visit for these patients whose time may otherwise have been spent on leisure, education, volunteerism, or other activities that contribute to individual and societal productivity. The total costs for unemployed patients reflected only direct costs, and thus were notably lower than those for employed patients.
Direct out-of-pocket costs and travel time negatively impacted provider preference. Patients with out-of-pocket costs were much less likely to stay with their current care provider (OR, 0.27; 95% CI, 0.10-0.71), preferring to seek care closer to home/work or teledermatology services. Similarly, for each minute that travel time increased, preference for current care provider decreased by 2%. Those who traveled 60 minutes or more were 71% less likely than those who traveled less than 60 minutes to stay with their current provider when given other options for care. Opportunity costs did not affect provider preference, even though they far exceeded direct costs for employed patients. Perhaps opportunity costs are not as immediately apparent to patients as out-of-pocket costs and travel time, and thus they do not factor as heavily in provider preference.
Despite high time and monetary costs, the majority of patients (60%) still preferred their current care provider, especially those with 4-year university degrees or higher education level (OR, 3.29; 95% CI, 1.23-5.26) and those presenting for skin checks (OR, 9.01; 95% CI, 2.28-35.59). Patients with higher levels of education likely have higher incomes and thus may not be as adversely affected by direct and/or indirect visit costs. Patients presenting for skin checks may value continuity and prefer providers with whom they already have an established therapeutic relationship. Future studies are needed to analyze the impact of these nonmonetary factors on provider preference.
Seeking Alternative Care
Tufts Medical Center does not have satellite dermatology clinics, making it the only option for patients who wish to receive care within the Tufts hospital network. However, patients do have the option of visiting non–Tufts-affiliated dermatology clinics outside of the city. To our knowledge, no formal studies have been performed comparing wait times for dermatology appointments in suburban versus urban Boston areas; however, it has been reported that rural practitioners have longer wait times than urban dermatologists, possibly due to the fact that physicians tend to aggregate in metropolitan areas.2 Thus, the potential for shorter wait times in the Boston metropolitan area may make it a more desirable location to receive care compared to more suburban or even rural areas of Massachusetts, but additional data are needed to substantiate this hypothesis. Additionally, health insurance restrictions, refractory or complex dermatologic conditions, and referring providers’ preference may affect patients’ decisions to seek care at a particular clinic. However, these factors do not alter our finding that those who travel long distances to our dermatology clinic are less likely to stay with their current provider if given the choice to seek care closer to home/work or utilize teledermatology services.
Prior studies have demonstrated patient preference and willingness to accept alternative modes of care delivery to reduce time and monetary costs associated with in-person medical visits.7,8 Dermatology patients at a clinic in Ontario, Canada, considered the time they spent attending the clinic to be even more burdensome than the monetary cost.7 Patients with nondermatologic chronic diseases and high out-of-pocket costs would prefer email rather than a clinic visit as the first method of contact with care providers.8 The explosive growth of direct-to-consumer (DTC) teledermatology services in the last 10 years speaks to patient demand for alternative care delivery that saves time and money. Although telemedicine has been implemented in various specialties, including ophthalmology and neurology, one of the most common applications is teledermatology. With DTC teledermatology, patients can take photographs or videos using personal smartphones and communicate directly with care providers using mobile or online applications. More recent review articles have identified 22 to 29 DTC mobile and web-based teledermatology services, with costs varying from $0 to $250.9-11 The median consultation fee of $59 for DTC teledermatology services is substantially less than total visit costs for employed patients in our study.9 Teledermatology has become an accessible and affordable modality of care, though perhaps not yet fully optimized for quality of care.
With increasingly higher co-pays and high-deductible insurance plans, time and monetary factors play increasingly important roles in patient preference for specialty care providers,12 as demonstrated by our study. Dermatologists can work with patients to reduce the costs of medical visits. Perhaps monitoring of chronic but stable conditions can be accomplished through telecommunication to reduce the number of follow-up visits. For instance, psoriasis patients enrolled in telemonitoring perceived savings of time and expenses through reduction of clinic visits, resulting in high patient satisfaction levels.13 Telephone calls and secure email messaging are other feasible alternatives shown to aid in clinical management and decrease the need for in-person care.8,14 Fewer unnecessary follow-up visits also means more availability for new patients and those with acute needs.
Barriers to obtaining care are not limited to dermatology and are pervasive across most medical specialties. Issues of patient time burden and out-of-pocket expenses are reflected in recent reports focused on quantifying these costs throughout ambulatory care visits and services such as colorectal, cervical, and breast cancer screenings.1,15-18 Similar to our findings, many of these studies also show high time and opportunity costs from the patient perspective. Expansion of telemedicine to reduce patient costs is becoming a viable option for many specialists, though low reimbursement rates restrict its widespread application.9,19 However, this obstacle is not impossible to surmount. One study found that offering teledermatology to Medicaid patients through their primary care providers significantly improved access, allowing for a 63.8% increase in the number of patients visiting a dermatologist (P<.01).20 Currently, a total of 48 state Medicaid programs now cover telemedicine, and a growing number of states are requiring private insurers to cover telehealth services.21 As more dermatologists adopt telemedicine practices, it may allow for better access as well as expanded insurance coverage.
Limitations
The results of our study are limited by the single-institution survey design. Patients were asked to complete the survey while still at the clinic visit to minimize recall bias. Because these patients actually attended their appointments, they might perceive the time and monetary costs associated with the visit to be less problematic than those who canceled their appointments or transferred care elsewhere; however, we were still able to detect a significant impact of time and monetary costs on provider preference in this cohort (P<.05). Larger studies in different geographic settings and other specialty clinics are needed to confirm our findings and to determine if nonmonetary factors such as specific diagnoses, length of time with a certain care provider, or patient socioeconomic status can modulate the impact of time and monetary costs on provider preference.
Conclusion
This study showed that patients expend a substantial amount of time and monetary costs to attend dermatology clinic visits. Data from the current and prior studies suggest that these costs affect patient provider preference for dermatologic care and may pose barriers to necessary medical care. Recognizing direct and indirect patient costs may drive critical changes in health care delivery, such as increased telecommunication utilization, the more cost-saving alternative. Telemedicine, when integrated appropriately, can help minimize expenses for patients while continuing to maintain a high level of care.
- Ray KN, Chari AV, Engberg J, et al. Opportunity costs of ambulatory medical care in the United States. Am J Manag Care. 2015;21:567-574.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Lipton S, Pletcher MJ. Short wait times for patients seeking cosmetic botulinum toxin appointments with dermatologists. J Am Acad Dermatol. 2007;57:985-989.
- Physician appointment wait times & Medicaid and Medicare acceptance rates. Merritt Hawkins website. https://www.merritthawkins.com/2014-survey/patientwaittime.aspx. Accessed February 15, 2017.
- Machlin SR, Adams SA. Expenses for office-based physician visits by specialty, 2013. Agency for Healthcare Research and Quality website. https://meps.ahrq.gov/data_files/publications/st484/stat484.pdf. Published November 2015. Accessed February 15, 2017.
- National ambulatory medical care survey: 2012 state and national summary tables. CDC website. www.cdc.gov/nchs/data/ahcd/namcs_summary/2012_namcs_web_tables.pdf. Accessed February 15, 2017.
- Vignjevic PM, Hux JE, Fisher BK, et al. Monetary and nonmonetary costs to patients attending an ambulatory dermatology clinic. J Cutan Med Surg. 1999;3:188-192.
- Reed M, Graetz I, Gordon N, Fung V. Patient-initiated e-mails to providers: associations with out-of-pocket visit costs, and impact on care-seeking and health. Am J Manag Care. 2015;21:E632-E639.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Kochmann M, Locatis C. Direct to consumer mobile teledermatology apps: an exploratory study. Telemed J E Health. 2016;22:689-693.
- Helms AD. High-deductible health plans can ruin finances. Kaiser Health News website. https://khn.org/news/high-deductible-health-plans-can-ruin-finances/. Published April 6, 2015. Accessed February 15, 2017.
- Fruhauf J, Schwantzer G, Ambros-Rudolph CM, et al. Pilot study on the acceptance of mobile teledermatology for the home monitoring of high-need patients with psoriasis. Australas J Dermatol. 2012;53:41-46.
- Eisenberg D, Hwa K, Wren SM. Telephone follow-up by a midlevel provider after laparoscopic inguinal hernia repair instead of face-to-face clinic visit. JSLS. 2015;19:e2014.00205.
- Yabroff KR, Guy GP Jr, Ekwueme DU, et al. Annual patient time costs associated with medical care among cancer survivors in the United States. Med Care. 2014;52:594-601.
- Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14-23.
- Jonas DE, Russell LB, Sandler RS, et al. Value of patient time invested in the colonoscopy screening process: time requirements for colonoscopy study. Med Decis Making. 2008;28:56-65.
- Shireman TI, Tsevat J, Goldie SJ. Time costs associated with cervical cancer screening. Int J Technol Assess Health Care. 2001;17:146-152.
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375:154-161.
- Uscher-Pines L, Malsberger R, Burgette L, et al. Effect of teledermatology on access to dermatology care among Medicaid enrollees. JAMA Dermatol. 2016;152:905-912.
- Thomas L, Capistrant G. State telemedicine gaps analysis: coverage & reimbursement. Telehealth website. http://www.mtelehealth.com/state-telemedicine-gaps-analysis-coverage-reimbursement/. Published January 19, 2016. Accessed February 15, 2017.
- Ray KN, Chari AV, Engberg J, et al. Opportunity costs of ambulatory medical care in the United States. Am J Manag Care. 2015;21:567-574.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Lipton S, Pletcher MJ. Short wait times for patients seeking cosmetic botulinum toxin appointments with dermatologists. J Am Acad Dermatol. 2007;57:985-989.
- Physician appointment wait times & Medicaid and Medicare acceptance rates. Merritt Hawkins website. https://www.merritthawkins.com/2014-survey/patientwaittime.aspx. Accessed February 15, 2017.
- Machlin SR, Adams SA. Expenses for office-based physician visits by specialty, 2013. Agency for Healthcare Research and Quality website. https://meps.ahrq.gov/data_files/publications/st484/stat484.pdf. Published November 2015. Accessed February 15, 2017.
- National ambulatory medical care survey: 2012 state and national summary tables. CDC website. www.cdc.gov/nchs/data/ahcd/namcs_summary/2012_namcs_web_tables.pdf. Accessed February 15, 2017.
- Vignjevic PM, Hux JE, Fisher BK, et al. Monetary and nonmonetary costs to patients attending an ambulatory dermatology clinic. J Cutan Med Surg. 1999;3:188-192.
- Reed M, Graetz I, Gordon N, Fung V. Patient-initiated e-mails to providers: associations with out-of-pocket visit costs, and impact on care-seeking and health. Am J Manag Care. 2015;21:E632-E639.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- Kochmann M, Locatis C. Direct to consumer mobile teledermatology apps: an exploratory study. Telemed J E Health. 2016;22:689-693.
- Helms AD. High-deductible health plans can ruin finances. Kaiser Health News website. https://khn.org/news/high-deductible-health-plans-can-ruin-finances/. Published April 6, 2015. Accessed February 15, 2017.
- Fruhauf J, Schwantzer G, Ambros-Rudolph CM, et al. Pilot study on the acceptance of mobile teledermatology for the home monitoring of high-need patients with psoriasis. Australas J Dermatol. 2012;53:41-46.
- Eisenberg D, Hwa K, Wren SM. Telephone follow-up by a midlevel provider after laparoscopic inguinal hernia repair instead of face-to-face clinic visit. JSLS. 2015;19:e2014.00205.
- Yabroff KR, Guy GP Jr, Ekwueme DU, et al. Annual patient time costs associated with medical care among cancer survivors in the United States. Med Care. 2014;52:594-601.
- Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14-23.
- Jonas DE, Russell LB, Sandler RS, et al. Value of patient time invested in the colonoscopy screening process: time requirements for colonoscopy study. Med Decis Making. 2008;28:56-65.
- Shireman TI, Tsevat J, Goldie SJ. Time costs associated with cervical cancer screening. Int J Technol Assess Health Care. 2001;17:146-152.
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375:154-161.
- Uscher-Pines L, Malsberger R, Burgette L, et al. Effect of teledermatology on access to dermatology care among Medicaid enrollees. JAMA Dermatol. 2016;152:905-912.
- Thomas L, Capistrant G. State telemedicine gaps analysis: coverage & reimbursement. Telehealth website. http://www.mtelehealth.com/state-telemedicine-gaps-analysis-coverage-reimbursement/. Published January 19, 2016. Accessed February 15, 2017.
Practice Points
- Physicians should be cognizant of the direct and indirect costs patients are subject to when attending dermatology clinic appointments and implement changes to reduce these costs.
- Telephone calls and secure email messaging are feasible alternatives shown to aid in clinical management and decrease the need for in-person care.
- Telecommunication may be used for the monitoring of chronic but stable conditions to reduce the number of follow-up visits.
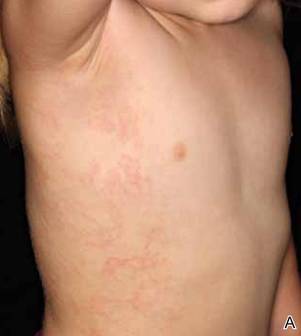
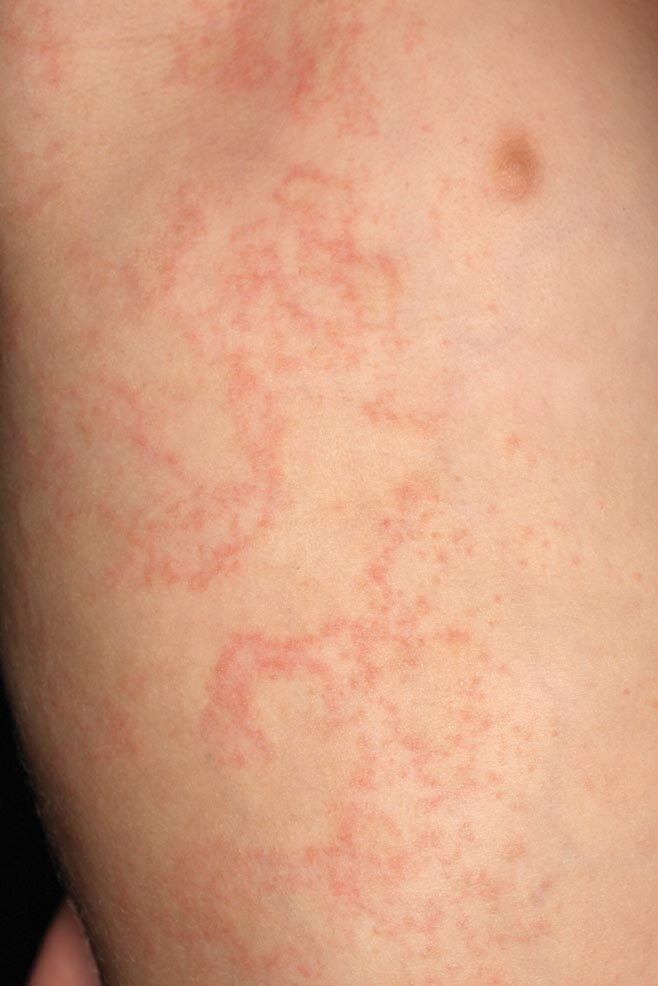
Erythematous Papules and Plaques on the Flank of a Child
The Diagnosis: Asymmetric Periflexural Exanthem of Childhood (Unilateral Laterothoracic Exanthem)
Asymmetric periflexural exanthem of childhood (APEC), also known as unilateral laterothoracic exanthem, is a self-limited eruptive dermatosis that occurs most frequently in infants and young children. The term unilateral laterothoracic exanthem was first coined by Bodemer and de Prost1 in 1992 due to its characteristic distribution. The eruption occurs in children aged 4 months to 10 years, with most cases presenting between 2 and 3 years of age.2 Isolated cases also have been reported in adults.3 It affects girls more often than boys (2:1), and the majority of reported cases have occurred in white individuals. The disease is seen throughout Europe and North America, and seasonal variation has been noted with most cases occurring in late winter and early spring.4,5
|
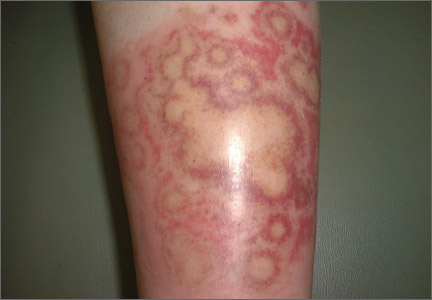
Clinically, APEC is characterized by its asymmetric localization and unilateral onset. In the majority of patients, the eruption presents as discrete erythematous papules that coalesce to form morbilliform plaques that may have reticular or annular configuration (Figure).4 The exanthem begins unilaterally near a flexural area, most commonly the axilla (75% of cases), and spreads centrifugally to the adjacent trunk and proximal extremity. There is no right or left dominance.4,6 There is eventual involvement of the contralateral side in 70% of cases, but a unilateral predominance is maintained throughout the disease course.4 Rarely, the eruption may involve the face, genitals, and palmoplantar surfaces. As in our case, up to three-quarters of affected children report symptoms of an upper respiratory tract or gastrointestinal prodrome, including mild fever, diarrhea, and rhinitis.4 Accompanying regional lymphadenopathy has been reported in the majority of cases, and mild to moderate pruritus is not uncommon. The syndrome is self-limited, with spontaneous resolution commonly occurring 3 to 6 weeks after onset. Although no treatment is required, systemic antihistamines and topical steroids have been used to alleviate pruritus in symptomatic patients. Our patient was treated with triamcinolone cream 0.1% twice daily as well as oral diphenhydramine 25 mg every 6 hours as needed for associated pruritus. The eruption spontaneously resolved over the following 4 weeks.
Although the cause of APEC remains unknown, an infectious etiology has been presumed. The seasonal pattern, lack of efficacy of broad-spectrum antibiotics, frequently reported prodromal symptoms, and reports of familial cases suggest a viral etiology.1 Additionally, the predilection to affect infants and young children as well as lack of recurrence in the same patient suggests that immunity may develop. Although no etiologic agent has been consistently detected, several reports have suggested a possible relationship to parvovirus B19.7,8 Parainfluenzavirus 2, parainfluenzavirus 3, and adenovirus also have been isolated but may represent incidental viral infection.2 An inoculation dermatosis from an arthropod bite also has been suggested, but this claim has not been substantiated.1
The diagnosis often can be made on clinical features alone, and histopathologic evaluation is not required. Histologic features are nonspecific and include a superficial perivascular infiltrate of lymphocytes, often involving the dermal eccrine ducts without involvement of the secretory coils.4,6 Mild lichenoid changes as well as spongiosis with exocytosis of lymphocytes into the acrosyringium also may be present.4 The clinical differential diagnosis of APEC includes allergic contact dermatitis, a nonspecific drug or viral eruption, atypical pityriasis rosea, miliaria, scabies, tinea corporis, and Gianotti-Crosti syndrome. Asymmetric periflexural exanthem of childhood lacks the peripheral scale present in tinea corporis or pityriasis rosea, but when an annular or reticular configuration predominates, a potassium hydroxide preparation of skin scrapings can exclude the presence of a dermatophyte. Similar to APEC, Gianotti-Crosti syndrome affects young children, is preceded by symptoms of a viral prodrome, and spontaneously resolves over several weeks. This condition is distinguished from APEC by the presence of papulovesicles located symmetrically on the face, buttocks, and extensor surface of the extremities, which largely spare the trunk.
Asymmetric periflexural exanthem of childhood is a unique morbilliform eruption of infants and young children characterized by a stereotypical distribution and self-limited course. The cause of this syndrome remains unclear, but most authors suggest a viral etiology. Recognition of this entity and an ability to distinguish it from other common pediatric dermatoses is required to provide reassurance to parents and avoid unnecessary diagnostic procedures and treatments.
1. Bodemer C, de Prost Y. Unilateral laterothoracic exanthem in children: a new disease? J Am Acad Dermatol. 1992;27(5, pt 1):693-696.
2. Nahm WK, Paiva C, Golomb C, et al. Asymmetric periflexural exanthema of childhood: a case involving a 4-month-old infant. Pediatr Dermatol. 2002;19:461-462.
3. Chan PK, To KF, Zawar V, et al. Asymmetric periflexural exanthema in an adult. Clin Exp Dermatol. 2004;29:320-321.
4. McCuaig CC, Russo P, Powell J, et al. Unilateral laterothoracic exanthem. a clinicopathologic study of forty-eight patients. J Am Acad Dermatol. 1996;34:979-984.
5. Taieb A, Megraud F, Legrain V, et al. Asymmetric periflexural exanthem of childhood. J Am Acad Dermatol. 1993;29:391-393.
6. Coustou D, Léauté-Labrèze C, Bioulac-Sage P, et al. Asymmetric periflexural exanthem of childhood. a clinical, pathologic, and epidemiologic prospective study. Arch Dermatol. 1999;135:799-803.
7. Guimerá-Martín-Neda F, Fagundo E, Rodríguez F, et al. Asymmetric periflexural exanthem of childhood: report of two cases with parvovirus B19. J Eur Acad Dermatol Venereol. 2006;20:461-462.
8. Pauluzzi P, Festini G, Gelmetti C. Asymmetric periflexural exanthem of childhood in an adult patient with parvovirus B19. J Eur Acad Dermatol Venereol. 2001;15:372-374.
The Diagnosis: Asymmetric Periflexural Exanthem of Childhood (Unilateral Laterothoracic Exanthem)
Asymmetric periflexural exanthem of childhood (APEC), also known as unilateral laterothoracic exanthem, is a self-limited eruptive dermatosis that occurs most frequently in infants and young children. The term unilateral laterothoracic exanthem was first coined by Bodemer and de Prost1 in 1992 due to its characteristic distribution. The eruption occurs in children aged 4 months to 10 years, with most cases presenting between 2 and 3 years of age.2 Isolated cases also have been reported in adults.3 It affects girls more often than boys (2:1), and the majority of reported cases have occurred in white individuals. The disease is seen throughout Europe and North America, and seasonal variation has been noted with most cases occurring in late winter and early spring.4,5
|
Clinically, APEC is characterized by its asymmetric localization and unilateral onset. In the majority of patients, the eruption presents as discrete erythematous papules that coalesce to form morbilliform plaques that may have reticular or annular configuration (Figure).4 The exanthem begins unilaterally near a flexural area, most commonly the axilla (75% of cases), and spreads centrifugally to the adjacent trunk and proximal extremity. There is no right or left dominance.4,6 There is eventual involvement of the contralateral side in 70% of cases, but a unilateral predominance is maintained throughout the disease course.4 Rarely, the eruption may involve the face, genitals, and palmoplantar surfaces. As in our case, up to three-quarters of affected children report symptoms of an upper respiratory tract or gastrointestinal prodrome, including mild fever, diarrhea, and rhinitis.4 Accompanying regional lymphadenopathy has been reported in the majority of cases, and mild to moderate pruritus is not uncommon. The syndrome is self-limited, with spontaneous resolution commonly occurring 3 to 6 weeks after onset. Although no treatment is required, systemic antihistamines and topical steroids have been used to alleviate pruritus in symptomatic patients. Our patient was treated with triamcinolone cream 0.1% twice daily as well as oral diphenhydramine 25 mg every 6 hours as needed for associated pruritus. The eruption spontaneously resolved over the following 4 weeks.
Although the cause of APEC remains unknown, an infectious etiology has been presumed. The seasonal pattern, lack of efficacy of broad-spectrum antibiotics, frequently reported prodromal symptoms, and reports of familial cases suggest a viral etiology.1 Additionally, the predilection to affect infants and young children as well as lack of recurrence in the same patient suggests that immunity may develop. Although no etiologic agent has been consistently detected, several reports have suggested a possible relationship to parvovirus B19.7,8 Parainfluenzavirus 2, parainfluenzavirus 3, and adenovirus also have been isolated but may represent incidental viral infection.2 An inoculation dermatosis from an arthropod bite also has been suggested, but this claim has not been substantiated.1
The diagnosis often can be made on clinical features alone, and histopathologic evaluation is not required. Histologic features are nonspecific and include a superficial perivascular infiltrate of lymphocytes, often involving the dermal eccrine ducts without involvement of the secretory coils.4,6 Mild lichenoid changes as well as spongiosis with exocytosis of lymphocytes into the acrosyringium also may be present.4 The clinical differential diagnosis of APEC includes allergic contact dermatitis, a nonspecific drug or viral eruption, atypical pityriasis rosea, miliaria, scabies, tinea corporis, and Gianotti-Crosti syndrome. Asymmetric periflexural exanthem of childhood lacks the peripheral scale present in tinea corporis or pityriasis rosea, but when an annular or reticular configuration predominates, a potassium hydroxide preparation of skin scrapings can exclude the presence of a dermatophyte. Similar to APEC, Gianotti-Crosti syndrome affects young children, is preceded by symptoms of a viral prodrome, and spontaneously resolves over several weeks. This condition is distinguished from APEC by the presence of papulovesicles located symmetrically on the face, buttocks, and extensor surface of the extremities, which largely spare the trunk.
Asymmetric periflexural exanthem of childhood is a unique morbilliform eruption of infants and young children characterized by a stereotypical distribution and self-limited course. The cause of this syndrome remains unclear, but most authors suggest a viral etiology. Recognition of this entity and an ability to distinguish it from other common pediatric dermatoses is required to provide reassurance to parents and avoid unnecessary diagnostic procedures and treatments.
The Diagnosis: Asymmetric Periflexural Exanthem of Childhood (Unilateral Laterothoracic Exanthem)
Asymmetric periflexural exanthem of childhood (APEC), also known as unilateral laterothoracic exanthem, is a self-limited eruptive dermatosis that occurs most frequently in infants and young children. The term unilateral laterothoracic exanthem was first coined by Bodemer and de Prost1 in 1992 due to its characteristic distribution. The eruption occurs in children aged 4 months to 10 years, with most cases presenting between 2 and 3 years of age.2 Isolated cases also have been reported in adults.3 It affects girls more often than boys (2:1), and the majority of reported cases have occurred in white individuals. The disease is seen throughout Europe and North America, and seasonal variation has been noted with most cases occurring in late winter and early spring.4,5
|
Clinically, APEC is characterized by its asymmetric localization and unilateral onset. In the majority of patients, the eruption presents as discrete erythematous papules that coalesce to form morbilliform plaques that may have reticular or annular configuration (Figure).4 The exanthem begins unilaterally near a flexural area, most commonly the axilla (75% of cases), and spreads centrifugally to the adjacent trunk and proximal extremity. There is no right or left dominance.4,6 There is eventual involvement of the contralateral side in 70% of cases, but a unilateral predominance is maintained throughout the disease course.4 Rarely, the eruption may involve the face, genitals, and palmoplantar surfaces. As in our case, up to three-quarters of affected children report symptoms of an upper respiratory tract or gastrointestinal prodrome, including mild fever, diarrhea, and rhinitis.4 Accompanying regional lymphadenopathy has been reported in the majority of cases, and mild to moderate pruritus is not uncommon. The syndrome is self-limited, with spontaneous resolution commonly occurring 3 to 6 weeks after onset. Although no treatment is required, systemic antihistamines and topical steroids have been used to alleviate pruritus in symptomatic patients. Our patient was treated with triamcinolone cream 0.1% twice daily as well as oral diphenhydramine 25 mg every 6 hours as needed for associated pruritus. The eruption spontaneously resolved over the following 4 weeks.
Although the cause of APEC remains unknown, an infectious etiology has been presumed. The seasonal pattern, lack of efficacy of broad-spectrum antibiotics, frequently reported prodromal symptoms, and reports of familial cases suggest a viral etiology.1 Additionally, the predilection to affect infants and young children as well as lack of recurrence in the same patient suggests that immunity may develop. Although no etiologic agent has been consistently detected, several reports have suggested a possible relationship to parvovirus B19.7,8 Parainfluenzavirus 2, parainfluenzavirus 3, and adenovirus also have been isolated but may represent incidental viral infection.2 An inoculation dermatosis from an arthropod bite also has been suggested, but this claim has not been substantiated.1
The diagnosis often can be made on clinical features alone, and histopathologic evaluation is not required. Histologic features are nonspecific and include a superficial perivascular infiltrate of lymphocytes, often involving the dermal eccrine ducts without involvement of the secretory coils.4,6 Mild lichenoid changes as well as spongiosis with exocytosis of lymphocytes into the acrosyringium also may be present.4 The clinical differential diagnosis of APEC includes allergic contact dermatitis, a nonspecific drug or viral eruption, atypical pityriasis rosea, miliaria, scabies, tinea corporis, and Gianotti-Crosti syndrome. Asymmetric periflexural exanthem of childhood lacks the peripheral scale present in tinea corporis or pityriasis rosea, but when an annular or reticular configuration predominates, a potassium hydroxide preparation of skin scrapings can exclude the presence of a dermatophyte. Similar to APEC, Gianotti-Crosti syndrome affects young children, is preceded by symptoms of a viral prodrome, and spontaneously resolves over several weeks. This condition is distinguished from APEC by the presence of papulovesicles located symmetrically on the face, buttocks, and extensor surface of the extremities, which largely spare the trunk.
Asymmetric periflexural exanthem of childhood is a unique morbilliform eruption of infants and young children characterized by a stereotypical distribution and self-limited course. The cause of this syndrome remains unclear, but most authors suggest a viral etiology. Recognition of this entity and an ability to distinguish it from other common pediatric dermatoses is required to provide reassurance to parents and avoid unnecessary diagnostic procedures and treatments.
1. Bodemer C, de Prost Y. Unilateral laterothoracic exanthem in children: a new disease? J Am Acad Dermatol. 1992;27(5, pt 1):693-696.
2. Nahm WK, Paiva C, Golomb C, et al. Asymmetric periflexural exanthema of childhood: a case involving a 4-month-old infant. Pediatr Dermatol. 2002;19:461-462.
3. Chan PK, To KF, Zawar V, et al. Asymmetric periflexural exanthema in an adult. Clin Exp Dermatol. 2004;29:320-321.
4. McCuaig CC, Russo P, Powell J, et al. Unilateral laterothoracic exanthem. a clinicopathologic study of forty-eight patients. J Am Acad Dermatol. 1996;34:979-984.
5. Taieb A, Megraud F, Legrain V, et al. Asymmetric periflexural exanthem of childhood. J Am Acad Dermatol. 1993;29:391-393.
6. Coustou D, Léauté-Labrèze C, Bioulac-Sage P, et al. Asymmetric periflexural exanthem of childhood. a clinical, pathologic, and epidemiologic prospective study. Arch Dermatol. 1999;135:799-803.
7. Guimerá-Martín-Neda F, Fagundo E, Rodríguez F, et al. Asymmetric periflexural exanthem of childhood: report of two cases with parvovirus B19. J Eur Acad Dermatol Venereol. 2006;20:461-462.
8. Pauluzzi P, Festini G, Gelmetti C. Asymmetric periflexural exanthem of childhood in an adult patient with parvovirus B19. J Eur Acad Dermatol Venereol. 2001;15:372-374.
1. Bodemer C, de Prost Y. Unilateral laterothoracic exanthem in children: a new disease? J Am Acad Dermatol. 1992;27(5, pt 1):693-696.
2. Nahm WK, Paiva C, Golomb C, et al. Asymmetric periflexural exanthema of childhood: a case involving a 4-month-old infant. Pediatr Dermatol. 2002;19:461-462.
3. Chan PK, To KF, Zawar V, et al. Asymmetric periflexural exanthema in an adult. Clin Exp Dermatol. 2004;29:320-321.
4. McCuaig CC, Russo P, Powell J, et al. Unilateral laterothoracic exanthem. a clinicopathologic study of forty-eight patients. J Am Acad Dermatol. 1996;34:979-984.
5. Taieb A, Megraud F, Legrain V, et al. Asymmetric periflexural exanthem of childhood. J Am Acad Dermatol. 1993;29:391-393.
6. Coustou D, Léauté-Labrèze C, Bioulac-Sage P, et al. Asymmetric periflexural exanthem of childhood. a clinical, pathologic, and epidemiologic prospective study. Arch Dermatol. 1999;135:799-803.
7. Guimerá-Martín-Neda F, Fagundo E, Rodríguez F, et al. Asymmetric periflexural exanthem of childhood: report of two cases with parvovirus B19. J Eur Acad Dermatol Venereol. 2006;20:461-462.
8. Pauluzzi P, Festini G, Gelmetti C. Asymmetric periflexural exanthem of childhood in an adult patient with parvovirus B19. J Eur Acad Dermatol Venereol. 2001;15:372-374.
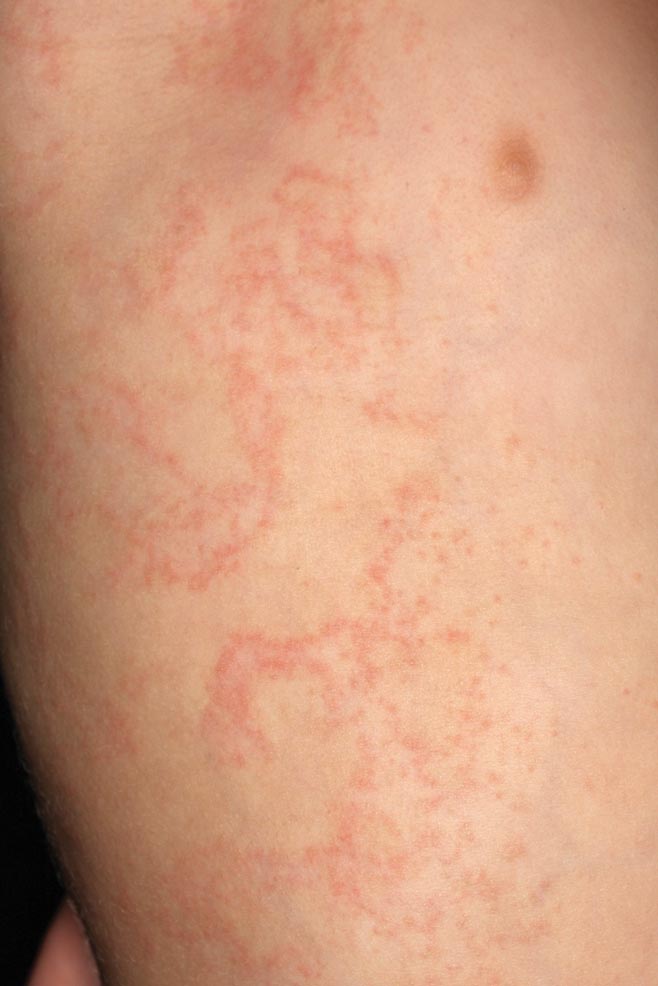
A 2-year-old girl presented with a mildly pruritic rash on the right flank and axilla of 3 weeks’ duration. Her pediatrician prescribed triamcinolone cream 0.1% daily, which was applied for the last week without much improvement. Her mother reported a history of upper respiratory tract infection approximately 1 to 2 weeks prior to onset of the rash.
Young girl with lower leg rash
An 8-year-old girl was brought into our clinic for evaluation of a leg rash on her right lower leg that had been bothering her for 2 months. Another physician had performed a biopsy and diagnosed subacute spongiotic dermatitis, but the rash did not respond to treatment with triamcinolone cream 0.1% twice daily.
The rash was mildly tender and markedly pruritic. The girl had no history of trauma, prior skin conditions, or other areas with a similar rash. Physical examination revealed concentric annular lesions on the right lower leg (FIGURE). The central areas demonstrated a bruised appearance that did not resolve with diascopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: tinea corporis
A potassium hydroxide (KOH) preparation was performed. It showed septate hyphae and confirmed a diagnosis of tinea corporis. We ordered a periodic acid-Schiff (PAS) stain on the previous biopsy specimen, and it revealed septate hyphae in the stratum corneum that were not apparent on the original hematoxylin and eosin (H&E) stained sections.
Dermatophyte infections of the skin are known as tinea corporis or “ringworm.” Ringworm fungi belong to 3 genera, Microsporum, Trichophyton, and Epidermophyton. These infections occur at any age and are more common in warmer climates.1 The classic lesion is an annular scaly patch, sometimes with the concentric rings, as seen in our patient (FIGURE). The bruising was almost certainly caused by rubbing and scratching.
We suspected tinea coporis based on the physical characteristics of the rash and the fact that it did not respond promptly to topical steroids. Our suspicions were confirmed by the KOH prep. Inked KOH using chlorazol black E stain turns fungal hyphae black, which makes them easier to distinguish from keratinocyte cell walls.2
Differential of a nonspecific rash should include infections
The initial misdiagnosis was based on the histopathologic diagnosis of spongiotic dermatitis. Subacute spongiotic dermatitis is associated with intracellular and intercellular edema of the keratinocytes in the epidermis. This is a nonspecific finding seen in eczematous dermatitis and can be etiologically associated with a wide variety of clinical conditions, including allergic contact dermatitis, atopic dermatitis, nummular eczema, and, in this case, dermatophytosis.3
If a biopsy is performed for a nonspecific rash, the pathologist should be advised of the possibility of superficial fungal infection. Providing a history and the physical characteristics of the rash or a differential diagnosis will prompt the performance of a PAS stain. Otherwise, the diagnosis can be missed because fungal elements are often not visible on routine H&E stains.
Proper treatment
provides speedy relief
Tinea corporis on a non-hair-bearing area is readily cleared with a topical azole antifungal agent, such as ketoconazole cream 2% twice daily for 2 weeks or a topical allylamine, such as terbinafine cream 1%, twice daily for 2 weeks. Topical allylamines may be more effective than topical azoles for tinea corporis4 (strength of recommendation [SOR]: A). Hair-bearing areas such as the scalp, fingers, and toes are unlikely to respond to topically applied medications and require an oral anti-fungal medication, such as griseofulvin 15 to 20 mg/kg/d.5
Relief for our patient
Our patient’s rash was treated with griseofulvin oral suspension 20 mg/kg/d (with milk to enhance absorption) for 6 weeks. There was complete clearing and the condition did not recur.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
1. Shrum JP, Millikan LE, Bataineh O. Superficial fungal infections in the tropics. Dermatol Clin. 1994;12:687-693.
2. Burke WA, Jones BE. A simple stain for rapid office diagnosis of fungus infections of the skin. Arch Dermatol. 1984;120:1519-1520.
3. Alsaad KO, Ghazarian D. My approach to superficial inflammatory dermatoses. J Clin Pathol. 2005;58:1233-1241.
4. Rotta I, Otuki MF, Sanches AC, et al. Efficacy of topical antifungal drugs in different dermatomycoses: a systematic review with meta-analysis. Rev Assoc Med Bras. 2012;58:308-318.
5. Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174,177-178.
An 8-year-old girl was brought into our clinic for evaluation of a leg rash on her right lower leg that had been bothering her for 2 months. Another physician had performed a biopsy and diagnosed subacute spongiotic dermatitis, but the rash did not respond to treatment with triamcinolone cream 0.1% twice daily.
The rash was mildly tender and markedly pruritic. The girl had no history of trauma, prior skin conditions, or other areas with a similar rash. Physical examination revealed concentric annular lesions on the right lower leg (FIGURE). The central areas demonstrated a bruised appearance that did not resolve with diascopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: tinea corporis
A potassium hydroxide (KOH) preparation was performed. It showed septate hyphae and confirmed a diagnosis of tinea corporis. We ordered a periodic acid-Schiff (PAS) stain on the previous biopsy specimen, and it revealed septate hyphae in the stratum corneum that were not apparent on the original hematoxylin and eosin (H&E) stained sections.
Dermatophyte infections of the skin are known as tinea corporis or “ringworm.” Ringworm fungi belong to 3 genera, Microsporum, Trichophyton, and Epidermophyton. These infections occur at any age and are more common in warmer climates.1 The classic lesion is an annular scaly patch, sometimes with the concentric rings, as seen in our patient (FIGURE). The bruising was almost certainly caused by rubbing and scratching.
We suspected tinea coporis based on the physical characteristics of the rash and the fact that it did not respond promptly to topical steroids. Our suspicions were confirmed by the KOH prep. Inked KOH using chlorazol black E stain turns fungal hyphae black, which makes them easier to distinguish from keratinocyte cell walls.2
Differential of a nonspecific rash should include infections
The initial misdiagnosis was based on the histopathologic diagnosis of spongiotic dermatitis. Subacute spongiotic dermatitis is associated with intracellular and intercellular edema of the keratinocytes in the epidermis. This is a nonspecific finding seen in eczematous dermatitis and can be etiologically associated with a wide variety of clinical conditions, including allergic contact dermatitis, atopic dermatitis, nummular eczema, and, in this case, dermatophytosis.3
If a biopsy is performed for a nonspecific rash, the pathologist should be advised of the possibility of superficial fungal infection. Providing a history and the physical characteristics of the rash or a differential diagnosis will prompt the performance of a PAS stain. Otherwise, the diagnosis can be missed because fungal elements are often not visible on routine H&E stains.
Proper treatment
provides speedy relief
Tinea corporis on a non-hair-bearing area is readily cleared with a topical azole antifungal agent, such as ketoconazole cream 2% twice daily for 2 weeks or a topical allylamine, such as terbinafine cream 1%, twice daily for 2 weeks. Topical allylamines may be more effective than topical azoles for tinea corporis4 (strength of recommendation [SOR]: A). Hair-bearing areas such as the scalp, fingers, and toes are unlikely to respond to topically applied medications and require an oral anti-fungal medication, such as griseofulvin 15 to 20 mg/kg/d.5
Relief for our patient
Our patient’s rash was treated with griseofulvin oral suspension 20 mg/kg/d (with milk to enhance absorption) for 6 weeks. There was complete clearing and the condition did not recur.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
An 8-year-old girl was brought into our clinic for evaluation of a leg rash on her right lower leg that had been bothering her for 2 months. Another physician had performed a biopsy and diagnosed subacute spongiotic dermatitis, but the rash did not respond to treatment with triamcinolone cream 0.1% twice daily.
The rash was mildly tender and markedly pruritic. The girl had no history of trauma, prior skin conditions, or other areas with a similar rash. Physical examination revealed concentric annular lesions on the right lower leg (FIGURE). The central areas demonstrated a bruised appearance that did not resolve with diascopy.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: tinea corporis
A potassium hydroxide (KOH) preparation was performed. It showed septate hyphae and confirmed a diagnosis of tinea corporis. We ordered a periodic acid-Schiff (PAS) stain on the previous biopsy specimen, and it revealed septate hyphae in the stratum corneum that were not apparent on the original hematoxylin and eosin (H&E) stained sections.
Dermatophyte infections of the skin are known as tinea corporis or “ringworm.” Ringworm fungi belong to 3 genera, Microsporum, Trichophyton, and Epidermophyton. These infections occur at any age and are more common in warmer climates.1 The classic lesion is an annular scaly patch, sometimes with the concentric rings, as seen in our patient (FIGURE). The bruising was almost certainly caused by rubbing and scratching.
We suspected tinea coporis based on the physical characteristics of the rash and the fact that it did not respond promptly to topical steroids. Our suspicions were confirmed by the KOH prep. Inked KOH using chlorazol black E stain turns fungal hyphae black, which makes them easier to distinguish from keratinocyte cell walls.2
Differential of a nonspecific rash should include infections
The initial misdiagnosis was based on the histopathologic diagnosis of spongiotic dermatitis. Subacute spongiotic dermatitis is associated with intracellular and intercellular edema of the keratinocytes in the epidermis. This is a nonspecific finding seen in eczematous dermatitis and can be etiologically associated with a wide variety of clinical conditions, including allergic contact dermatitis, atopic dermatitis, nummular eczema, and, in this case, dermatophytosis.3
If a biopsy is performed for a nonspecific rash, the pathologist should be advised of the possibility of superficial fungal infection. Providing a history and the physical characteristics of the rash or a differential diagnosis will prompt the performance of a PAS stain. Otherwise, the diagnosis can be missed because fungal elements are often not visible on routine H&E stains.
Proper treatment
provides speedy relief
Tinea corporis on a non-hair-bearing area is readily cleared with a topical azole antifungal agent, such as ketoconazole cream 2% twice daily for 2 weeks or a topical allylamine, such as terbinafine cream 1%, twice daily for 2 weeks. Topical allylamines may be more effective than topical azoles for tinea corporis4 (strength of recommendation [SOR]: A). Hair-bearing areas such as the scalp, fingers, and toes are unlikely to respond to topically applied medications and require an oral anti-fungal medication, such as griseofulvin 15 to 20 mg/kg/d.5
Relief for our patient
Our patient’s rash was treated with griseofulvin oral suspension 20 mg/kg/d (with milk to enhance absorption) for 6 weeks. There was complete clearing and the condition did not recur.
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
1. Shrum JP, Millikan LE, Bataineh O. Superficial fungal infections in the tropics. Dermatol Clin. 1994;12:687-693.
2. Burke WA, Jones BE. A simple stain for rapid office diagnosis of fungus infections of the skin. Arch Dermatol. 1984;120:1519-1520.
3. Alsaad KO, Ghazarian D. My approach to superficial inflammatory dermatoses. J Clin Pathol. 2005;58:1233-1241.
4. Rotta I, Otuki MF, Sanches AC, et al. Efficacy of topical antifungal drugs in different dermatomycoses: a systematic review with meta-analysis. Rev Assoc Med Bras. 2012;58:308-318.
5. Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174,177-178.
1. Shrum JP, Millikan LE, Bataineh O. Superficial fungal infections in the tropics. Dermatol Clin. 1994;12:687-693.
2. Burke WA, Jones BE. A simple stain for rapid office diagnosis of fungus infections of the skin. Arch Dermatol. 1984;120:1519-1520.
3. Alsaad KO, Ghazarian D. My approach to superficial inflammatory dermatoses. J Clin Pathol. 2005;58:1233-1241.
4. Rotta I, Otuki MF, Sanches AC, et al. Efficacy of topical antifungal drugs in different dermatomycoses: a systematic review with meta-analysis. Rev Assoc Med Bras. 2012;58:308-318.
5. Noble SL, Forbes RC, Stamm PL. Diagnosis and management of common tinea infections. Am Fam Physician. 1998;58:163-174,177-178.