User login
Secukinumab brings high PASI 75 results in 6- to 17-year-olds with psoriasis
at 24 weeks of follow-up in an ongoing 4-year phase 2 clinical trial, Adam Reich, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
Secukinumab (Cosentyx), a fully human monoclonal antibody that inhibits interleukin-17A, is widely approved for treatment of psoriasis in adults. In August 2020, the biologic received an expanded indication in Europe for treatment of 6- to 17-year-olds. Two phase 3 clinical trials are underway in an effort to gain a similar broadened indication in the United States to help address the high unmet need for new treatments for psoriasis in the pediatric population, said Dr. Reich, professor and head of the department of dermatology at the University of Rzeszow (Poland).
He reported on 84 pediatric patients participating in the open-label, phase 2, international study. They were randomized to one of two weight-based dosing regimens. Those in the low-dose arm received secukinumab dosed at 75 mg if they weighed less than 50 kg and 150 mg if they weighed more. In the high-dose arm, patients got secukinumab 75 mg if they weighed less than 25 kg, 150 mg if they weighed 25-50 kg, and 300 mg if they tipped the scales in excess of 50 kg.
The primary endpoint in the study was the week-12 rate of at least a 75% improvement from baseline in the Psoriasis Area and Severity Index score, or PASI 75. The rates were similar: 92.9% of patients in the high-dose arm achieved this endpoint, as did 90.5% in the low-dose arm. The PASI 90 rates were 83.3% and 78%, the PASI 100 rates were 61.9% and 54.8%, and clear or almost clear skin, as measured by the Investigator Global Assessment, was achieved in 88.7% of the high- and 85.7% of the low-dose groups. In addition,61.9% of those in the high-dose secukinumab group and 50% in the low-dose group had a score of 0 or 1 on the Children’s Dermatology Life Quality Index – indicating psoriasis has no impact on daily quality of life, he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
At week 24, roughly 95% of patients in both the low- and high-dose secukinumab groups had achieved PASI 75s, 88% reached a PASI 90 response, and 67% were at PASI 100. Nearly 60% of the low-dose and 70% of the high-dose groups had a score of 0 or 1 on the Children’s Dermatology Life Quality Index.
Treatment-emergent adverse event rates were similar in the two study arms. Of note, there was one case of new-onset inflammatory bowel disease in the high-dose group, and one case of vulvovaginal candidiasis as well.
Discussant Bruce E. Strober, MD, PhD, said that, if secukinumab gets a pediatric indication from the Food and Drug Administration, as seems likely, it won’t alter his biologic treatment hierarchy.
“I treat a lot of kids with psoriasis. We have three approved drugs now in etanercept [Enbrel], ustekinumab [Stelara], and ixekizumab [Taltz]. My bias is still towards ustekinumab because it’s infrequently dosed and that’s a huge issue for children. You want to expose them to as few injections as possible, for obvious reasons: It’s easier for parents and other caregivers,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn.
“The other issue is in IL-17 inhibition there has been a slight signal of inflammatory bowel disease popping up in children getting these drugs, and therefore you need to screen patients in this age group very carefully – not only the patients themselves, but their family – for IBD risk. If there is any sign of that I would move the IL-17 inhibitors to the back of the line, compared to ustekinumab and etanercept. Ustekinumab is still clearly the one that I think has to be used first line,” he said.
Dr. Strober offered a final word of advice for his colleagues: “You can’t be afraid to treat children with biologic therapies. In fact, preferentially I would use a biologic therapy over methotrexate or light therapy, which is really difficult for children.”
Dr. Reich and Dr. Strober reported receiving research grants from and serving as a consultant to numerous pharmaceutical companies, including Novartis, which markets secukinumab and funded the study.
MedscapeLIVE! and this news organization are owned by the same parent company.
at 24 weeks of follow-up in an ongoing 4-year phase 2 clinical trial, Adam Reich, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
Secukinumab (Cosentyx), a fully human monoclonal antibody that inhibits interleukin-17A, is widely approved for treatment of psoriasis in adults. In August 2020, the biologic received an expanded indication in Europe for treatment of 6- to 17-year-olds. Two phase 3 clinical trials are underway in an effort to gain a similar broadened indication in the United States to help address the high unmet need for new treatments for psoriasis in the pediatric population, said Dr. Reich, professor and head of the department of dermatology at the University of Rzeszow (Poland).
He reported on 84 pediatric patients participating in the open-label, phase 2, international study. They were randomized to one of two weight-based dosing regimens. Those in the low-dose arm received secukinumab dosed at 75 mg if they weighed less than 50 kg and 150 mg if they weighed more. In the high-dose arm, patients got secukinumab 75 mg if they weighed less than 25 kg, 150 mg if they weighed 25-50 kg, and 300 mg if they tipped the scales in excess of 50 kg.
The primary endpoint in the study was the week-12 rate of at least a 75% improvement from baseline in the Psoriasis Area and Severity Index score, or PASI 75. The rates were similar: 92.9% of patients in the high-dose arm achieved this endpoint, as did 90.5% in the low-dose arm. The PASI 90 rates were 83.3% and 78%, the PASI 100 rates were 61.9% and 54.8%, and clear or almost clear skin, as measured by the Investigator Global Assessment, was achieved in 88.7% of the high- and 85.7% of the low-dose groups. In addition,61.9% of those in the high-dose secukinumab group and 50% in the low-dose group had a score of 0 or 1 on the Children’s Dermatology Life Quality Index – indicating psoriasis has no impact on daily quality of life, he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
At week 24, roughly 95% of patients in both the low- and high-dose secukinumab groups had achieved PASI 75s, 88% reached a PASI 90 response, and 67% were at PASI 100. Nearly 60% of the low-dose and 70% of the high-dose groups had a score of 0 or 1 on the Children’s Dermatology Life Quality Index.
Treatment-emergent adverse event rates were similar in the two study arms. Of note, there was one case of new-onset inflammatory bowel disease in the high-dose group, and one case of vulvovaginal candidiasis as well.
Discussant Bruce E. Strober, MD, PhD, said that, if secukinumab gets a pediatric indication from the Food and Drug Administration, as seems likely, it won’t alter his biologic treatment hierarchy.
“I treat a lot of kids with psoriasis. We have three approved drugs now in etanercept [Enbrel], ustekinumab [Stelara], and ixekizumab [Taltz]. My bias is still towards ustekinumab because it’s infrequently dosed and that’s a huge issue for children. You want to expose them to as few injections as possible, for obvious reasons: It’s easier for parents and other caregivers,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn.
“The other issue is in IL-17 inhibition there has been a slight signal of inflammatory bowel disease popping up in children getting these drugs, and therefore you need to screen patients in this age group very carefully – not only the patients themselves, but their family – for IBD risk. If there is any sign of that I would move the IL-17 inhibitors to the back of the line, compared to ustekinumab and etanercept. Ustekinumab is still clearly the one that I think has to be used first line,” he said.
Dr. Strober offered a final word of advice for his colleagues: “You can’t be afraid to treat children with biologic therapies. In fact, preferentially I would use a biologic therapy over methotrexate or light therapy, which is really difficult for children.”
Dr. Reich and Dr. Strober reported receiving research grants from and serving as a consultant to numerous pharmaceutical companies, including Novartis, which markets secukinumab and funded the study.
MedscapeLIVE! and this news organization are owned by the same parent company.
at 24 weeks of follow-up in an ongoing 4-year phase 2 clinical trial, Adam Reich, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
Secukinumab (Cosentyx), a fully human monoclonal antibody that inhibits interleukin-17A, is widely approved for treatment of psoriasis in adults. In August 2020, the biologic received an expanded indication in Europe for treatment of 6- to 17-year-olds. Two phase 3 clinical trials are underway in an effort to gain a similar broadened indication in the United States to help address the high unmet need for new treatments for psoriasis in the pediatric population, said Dr. Reich, professor and head of the department of dermatology at the University of Rzeszow (Poland).
He reported on 84 pediatric patients participating in the open-label, phase 2, international study. They were randomized to one of two weight-based dosing regimens. Those in the low-dose arm received secukinumab dosed at 75 mg if they weighed less than 50 kg and 150 mg if they weighed more. In the high-dose arm, patients got secukinumab 75 mg if they weighed less than 25 kg, 150 mg if they weighed 25-50 kg, and 300 mg if they tipped the scales in excess of 50 kg.
The primary endpoint in the study was the week-12 rate of at least a 75% improvement from baseline in the Psoriasis Area and Severity Index score, or PASI 75. The rates were similar: 92.9% of patients in the high-dose arm achieved this endpoint, as did 90.5% in the low-dose arm. The PASI 90 rates were 83.3% and 78%, the PASI 100 rates were 61.9% and 54.8%, and clear or almost clear skin, as measured by the Investigator Global Assessment, was achieved in 88.7% of the high- and 85.7% of the low-dose groups. In addition,61.9% of those in the high-dose secukinumab group and 50% in the low-dose group had a score of 0 or 1 on the Children’s Dermatology Life Quality Index – indicating psoriasis has no impact on daily quality of life, he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
At week 24, roughly 95% of patients in both the low- and high-dose secukinumab groups had achieved PASI 75s, 88% reached a PASI 90 response, and 67% were at PASI 100. Nearly 60% of the low-dose and 70% of the high-dose groups had a score of 0 or 1 on the Children’s Dermatology Life Quality Index.
Treatment-emergent adverse event rates were similar in the two study arms. Of note, there was one case of new-onset inflammatory bowel disease in the high-dose group, and one case of vulvovaginal candidiasis as well.
Discussant Bruce E. Strober, MD, PhD, said that, if secukinumab gets a pediatric indication from the Food and Drug Administration, as seems likely, it won’t alter his biologic treatment hierarchy.
“I treat a lot of kids with psoriasis. We have three approved drugs now in etanercept [Enbrel], ustekinumab [Stelara], and ixekizumab [Taltz]. My bias is still towards ustekinumab because it’s infrequently dosed and that’s a huge issue for children. You want to expose them to as few injections as possible, for obvious reasons: It’s easier for parents and other caregivers,” explained Dr. Strober, a dermatologist at Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn.
“The other issue is in IL-17 inhibition there has been a slight signal of inflammatory bowel disease popping up in children getting these drugs, and therefore you need to screen patients in this age group very carefully – not only the patients themselves, but their family – for IBD risk. If there is any sign of that I would move the IL-17 inhibitors to the back of the line, compared to ustekinumab and etanercept. Ustekinumab is still clearly the one that I think has to be used first line,” he said.
Dr. Strober offered a final word of advice for his colleagues: “You can’t be afraid to treat children with biologic therapies. In fact, preferentially I would use a biologic therapy over methotrexate or light therapy, which is really difficult for children.”
Dr. Reich and Dr. Strober reported receiving research grants from and serving as a consultant to numerous pharmaceutical companies, including Novartis, which markets secukinumab and funded the study.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Enzymatic injections show durable improvement in buttock cellulite
follow-up in an ongoing, 5-year, phase 3b, open-label extension study, Michael H. Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
However, outcomes in that study, as well as in the earlier pivotal trials, were assessed via physician and patient subjective assessments of aesthetic appearance. In a separate presentation at the conference, Michael S. Kaminer, MD, presented a different study evaluating the objective quantifiable effects of CCH on buttock cellulite dimple volume using three-dimensional imaging. The results, indicating that smaller cellulite dimples responded better than larger dimples, he noted, were unexpected.
Discussant Zoe D. Draelos, MD, who practices in High Point, N.C., and is a consulting professor of dermatology at Duke University, Durham, N.C., put the two studies in perspective, explaining that there are multiple challenges associated with the use of CCH to treat buttock cellulite, and dermatologists need to understand them in order to maximize the benefit.
“There’s definitely a market for this therapy,” she observed, noting the plethora of over-the-counter products and devices sold for removal of cellulite. “I think if you manage patient expectations, this will be a very, very successful procedure.”
In 2020, the Food and Drug Administration approved subcutaneous injections of CCH (marketed under the brand name QWO) for treatment of cellulite in women’s buttocks on the basis of the randomized RELEASE-1 and -2 trials. But while this is a new indication for CCH, it is not a new drug. The medication has been approved for years for treatment of fibrotic band contracture disorders, namely Dupuytren’s contracture and Peyronie’s disease. The mechanism of action for treatment of cellulite involves a process dubbed enzymatic subcision, in which CCH breaks down mature collagen and stimulates new collagen formation and fat redistribution in an effort to achieve smoother skin contour.
“This adds a whole new wrinkle to injectables available in dermatology. We have fillers, we have toxins, and now we have enzymatic subcision,” Dr. Draelos commented.
Durability of effects
Dr. Gold, founder of the Gold Skin Care Center and at the Tennessee Clinical Research Center, Nashville, reported on 483 women with moderate to severe buttock cellulitis who completed the 71-day, randomized, double-blind, phase 3 RELEASE-1 or RELEASE-2 studies and then enrolled in the open-label extension study. At the end of the randomized trial, 61.7% of women experienced at least a 1-level improvement on the Patient-Reported Photonumeric Cellulite Severity Scale (PR-PCSS), compared with 36.7% of placebo controls. The key finding in the interim analysis of the extension study: After the first 6 months, during which no one received any additional therapy, 52.7% of the CCH group still had at least a 1-level improvement in PR-PCSS, compared with the randomized trial baseline, as did 32.6% of controls.
Similarly, 63% of CCH-treated patients showed at least a 1-level improvement in the Clinician-Reported Photonumeric Cellulite Severity Scale (CR-PCSS) from baseline to the end of the randomized trial, and 52.7% met that standard after 6 months off treatment in the open-label extension. In contrast, the control group had response rates of 36.7% and 32.6%. There were no long-term safety concerns, according to Dr. Gold.
Measuring cellulite dimple volume shrinkage
Dr. Kaminer and coinvestigators measured the change in cellulite dimple volume from baseline to 30 days after the final injection of 33 buttock dimples in 27 women in order to get a quantifiable sense of the effectiveness of the CCH injection. To their surprise, smaller-volume dimples up to 118 mm3 showed a mean 43% reduction in volume, a significantly better result than the 15.8% reduction seen in dimples greater than 118 mm3.
“That’s almost counterintuitive, right? You’d think that larger dimples would have a bigger improvement, but it turns out that the smaller dimples do better,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also, cellulite dimples in women age 40 and under responded significantly better than those in older women, added Dr. Kaminer, a dermatologist in private practice in Chestnut Hill, Mass., who is also on the faculty at Yale University, New Haven, Conn., and Brown University, Providence, R.I.
Challenges in using CCH therapy
Dr. Draelos, who is familiar with CCH, having worked on some earlier studies of the product, commented that “this is really the first medical treatment for cellulite that’s been proven to work.”
That being said, there are challenges with this therapy. While roughly 53% of women rated themselves as having at least a 1-level improvement after 6 months of follow-up, so did 33% of placebo-treated controls, for a placebo-subtracted 20% response.
“Is a 1-grade improvement going to be enough for women to engage in this procedure? You do need to remember that it takes multiple injections: most need at least three injections to see durable impact. And there’s discomfort during the procedure and afterwards during the healing process because the mechanism of action is enzymatic. You’re breaking down fibrous bands, and that’s a proinflammatory process. Many women who undergo this procedure may have discomfort and bruising, and they should be warned that this is not a procedure to do before taking a cruise or wearing a bikini. Also, it’s important to note that many women will have discomfort in the area where they sit, so if they have a job where they need to be sitting for long periods of time they need to plan their activities around this particular procedure,” the dermatologist said.
Another consideration: “The area they actually studied – the buttocks – is an area where I’m not sure a lot of women would expose their skin in public. I think thigh dimpling is more bothersome because it shows in shorts and other types of clothing. We need to figure out if the injections work on the posterior thighs, the most common place most postpubertal women get cellulite,” Dr. Draelos noted.
She wasn’t surprised that smaller cellulite dimples did better. Larger dimples presumably have a broader fibrous attachment and tighter fibrous band. She found the less robust outcomes in women over age 40 similarly unsurprising, since cellulitis seems to worsen with age. Cellulitis can’t really be called a disease, anyway, since it occurs in about 90% of postpubertal women.
One last tip about managing patient expectations: “Let a woman know that it’ll be better, but it won’t be gone,” she said.
Dr. Gold and Dr. Kaminer reported serving as paid investigators for and consultants to Endo Pharmaceuticals, the study sponsor and manufacturer of CCH, as well as for several other pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
follow-up in an ongoing, 5-year, phase 3b, open-label extension study, Michael H. Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
However, outcomes in that study, as well as in the earlier pivotal trials, were assessed via physician and patient subjective assessments of aesthetic appearance. In a separate presentation at the conference, Michael S. Kaminer, MD, presented a different study evaluating the objective quantifiable effects of CCH on buttock cellulite dimple volume using three-dimensional imaging. The results, indicating that smaller cellulite dimples responded better than larger dimples, he noted, were unexpected.
Discussant Zoe D. Draelos, MD, who practices in High Point, N.C., and is a consulting professor of dermatology at Duke University, Durham, N.C., put the two studies in perspective, explaining that there are multiple challenges associated with the use of CCH to treat buttock cellulite, and dermatologists need to understand them in order to maximize the benefit.
“There’s definitely a market for this therapy,” she observed, noting the plethora of over-the-counter products and devices sold for removal of cellulite. “I think if you manage patient expectations, this will be a very, very successful procedure.”
In 2020, the Food and Drug Administration approved subcutaneous injections of CCH (marketed under the brand name QWO) for treatment of cellulite in women’s buttocks on the basis of the randomized RELEASE-1 and -2 trials. But while this is a new indication for CCH, it is not a new drug. The medication has been approved for years for treatment of fibrotic band contracture disorders, namely Dupuytren’s contracture and Peyronie’s disease. The mechanism of action for treatment of cellulite involves a process dubbed enzymatic subcision, in which CCH breaks down mature collagen and stimulates new collagen formation and fat redistribution in an effort to achieve smoother skin contour.
“This adds a whole new wrinkle to injectables available in dermatology. We have fillers, we have toxins, and now we have enzymatic subcision,” Dr. Draelos commented.
Durability of effects
Dr. Gold, founder of the Gold Skin Care Center and at the Tennessee Clinical Research Center, Nashville, reported on 483 women with moderate to severe buttock cellulitis who completed the 71-day, randomized, double-blind, phase 3 RELEASE-1 or RELEASE-2 studies and then enrolled in the open-label extension study. At the end of the randomized trial, 61.7% of women experienced at least a 1-level improvement on the Patient-Reported Photonumeric Cellulite Severity Scale (PR-PCSS), compared with 36.7% of placebo controls. The key finding in the interim analysis of the extension study: After the first 6 months, during which no one received any additional therapy, 52.7% of the CCH group still had at least a 1-level improvement in PR-PCSS, compared with the randomized trial baseline, as did 32.6% of controls.
Similarly, 63% of CCH-treated patients showed at least a 1-level improvement in the Clinician-Reported Photonumeric Cellulite Severity Scale (CR-PCSS) from baseline to the end of the randomized trial, and 52.7% met that standard after 6 months off treatment in the open-label extension. In contrast, the control group had response rates of 36.7% and 32.6%. There were no long-term safety concerns, according to Dr. Gold.
Measuring cellulite dimple volume shrinkage
Dr. Kaminer and coinvestigators measured the change in cellulite dimple volume from baseline to 30 days after the final injection of 33 buttock dimples in 27 women in order to get a quantifiable sense of the effectiveness of the CCH injection. To their surprise, smaller-volume dimples up to 118 mm3 showed a mean 43% reduction in volume, a significantly better result than the 15.8% reduction seen in dimples greater than 118 mm3.
“That’s almost counterintuitive, right? You’d think that larger dimples would have a bigger improvement, but it turns out that the smaller dimples do better,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also, cellulite dimples in women age 40 and under responded significantly better than those in older women, added Dr. Kaminer, a dermatologist in private practice in Chestnut Hill, Mass., who is also on the faculty at Yale University, New Haven, Conn., and Brown University, Providence, R.I.
Challenges in using CCH therapy
Dr. Draelos, who is familiar with CCH, having worked on some earlier studies of the product, commented that “this is really the first medical treatment for cellulite that’s been proven to work.”
That being said, there are challenges with this therapy. While roughly 53% of women rated themselves as having at least a 1-level improvement after 6 months of follow-up, so did 33% of placebo-treated controls, for a placebo-subtracted 20% response.
“Is a 1-grade improvement going to be enough for women to engage in this procedure? You do need to remember that it takes multiple injections: most need at least three injections to see durable impact. And there’s discomfort during the procedure and afterwards during the healing process because the mechanism of action is enzymatic. You’re breaking down fibrous bands, and that’s a proinflammatory process. Many women who undergo this procedure may have discomfort and bruising, and they should be warned that this is not a procedure to do before taking a cruise or wearing a bikini. Also, it’s important to note that many women will have discomfort in the area where they sit, so if they have a job where they need to be sitting for long periods of time they need to plan their activities around this particular procedure,” the dermatologist said.
Another consideration: “The area they actually studied – the buttocks – is an area where I’m not sure a lot of women would expose their skin in public. I think thigh dimpling is more bothersome because it shows in shorts and other types of clothing. We need to figure out if the injections work on the posterior thighs, the most common place most postpubertal women get cellulite,” Dr. Draelos noted.
She wasn’t surprised that smaller cellulite dimples did better. Larger dimples presumably have a broader fibrous attachment and tighter fibrous band. She found the less robust outcomes in women over age 40 similarly unsurprising, since cellulitis seems to worsen with age. Cellulitis can’t really be called a disease, anyway, since it occurs in about 90% of postpubertal women.
One last tip about managing patient expectations: “Let a woman know that it’ll be better, but it won’t be gone,” she said.
Dr. Gold and Dr. Kaminer reported serving as paid investigators for and consultants to Endo Pharmaceuticals, the study sponsor and manufacturer of CCH, as well as for several other pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
follow-up in an ongoing, 5-year, phase 3b, open-label extension study, Michael H. Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
However, outcomes in that study, as well as in the earlier pivotal trials, were assessed via physician and patient subjective assessments of aesthetic appearance. In a separate presentation at the conference, Michael S. Kaminer, MD, presented a different study evaluating the objective quantifiable effects of CCH on buttock cellulite dimple volume using three-dimensional imaging. The results, indicating that smaller cellulite dimples responded better than larger dimples, he noted, were unexpected.
Discussant Zoe D. Draelos, MD, who practices in High Point, N.C., and is a consulting professor of dermatology at Duke University, Durham, N.C., put the two studies in perspective, explaining that there are multiple challenges associated with the use of CCH to treat buttock cellulite, and dermatologists need to understand them in order to maximize the benefit.
“There’s definitely a market for this therapy,” she observed, noting the plethora of over-the-counter products and devices sold for removal of cellulite. “I think if you manage patient expectations, this will be a very, very successful procedure.”
In 2020, the Food and Drug Administration approved subcutaneous injections of CCH (marketed under the brand name QWO) for treatment of cellulite in women’s buttocks on the basis of the randomized RELEASE-1 and -2 trials. But while this is a new indication for CCH, it is not a new drug. The medication has been approved for years for treatment of fibrotic band contracture disorders, namely Dupuytren’s contracture and Peyronie’s disease. The mechanism of action for treatment of cellulite involves a process dubbed enzymatic subcision, in which CCH breaks down mature collagen and stimulates new collagen formation and fat redistribution in an effort to achieve smoother skin contour.
“This adds a whole new wrinkle to injectables available in dermatology. We have fillers, we have toxins, and now we have enzymatic subcision,” Dr. Draelos commented.
Durability of effects
Dr. Gold, founder of the Gold Skin Care Center and at the Tennessee Clinical Research Center, Nashville, reported on 483 women with moderate to severe buttock cellulitis who completed the 71-day, randomized, double-blind, phase 3 RELEASE-1 or RELEASE-2 studies and then enrolled in the open-label extension study. At the end of the randomized trial, 61.7% of women experienced at least a 1-level improvement on the Patient-Reported Photonumeric Cellulite Severity Scale (PR-PCSS), compared with 36.7% of placebo controls. The key finding in the interim analysis of the extension study: After the first 6 months, during which no one received any additional therapy, 52.7% of the CCH group still had at least a 1-level improvement in PR-PCSS, compared with the randomized trial baseline, as did 32.6% of controls.
Similarly, 63% of CCH-treated patients showed at least a 1-level improvement in the Clinician-Reported Photonumeric Cellulite Severity Scale (CR-PCSS) from baseline to the end of the randomized trial, and 52.7% met that standard after 6 months off treatment in the open-label extension. In contrast, the control group had response rates of 36.7% and 32.6%. There were no long-term safety concerns, according to Dr. Gold.
Measuring cellulite dimple volume shrinkage
Dr. Kaminer and coinvestigators measured the change in cellulite dimple volume from baseline to 30 days after the final injection of 33 buttock dimples in 27 women in order to get a quantifiable sense of the effectiveness of the CCH injection. To their surprise, smaller-volume dimples up to 118 mm3 showed a mean 43% reduction in volume, a significantly better result than the 15.8% reduction seen in dimples greater than 118 mm3.
“That’s almost counterintuitive, right? You’d think that larger dimples would have a bigger improvement, but it turns out that the smaller dimples do better,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also, cellulite dimples in women age 40 and under responded significantly better than those in older women, added Dr. Kaminer, a dermatologist in private practice in Chestnut Hill, Mass., who is also on the faculty at Yale University, New Haven, Conn., and Brown University, Providence, R.I.
Challenges in using CCH therapy
Dr. Draelos, who is familiar with CCH, having worked on some earlier studies of the product, commented that “this is really the first medical treatment for cellulite that’s been proven to work.”
That being said, there are challenges with this therapy. While roughly 53% of women rated themselves as having at least a 1-level improvement after 6 months of follow-up, so did 33% of placebo-treated controls, for a placebo-subtracted 20% response.
“Is a 1-grade improvement going to be enough for women to engage in this procedure? You do need to remember that it takes multiple injections: most need at least three injections to see durable impact. And there’s discomfort during the procedure and afterwards during the healing process because the mechanism of action is enzymatic. You’re breaking down fibrous bands, and that’s a proinflammatory process. Many women who undergo this procedure may have discomfort and bruising, and they should be warned that this is not a procedure to do before taking a cruise or wearing a bikini. Also, it’s important to note that many women will have discomfort in the area where they sit, so if they have a job where they need to be sitting for long periods of time they need to plan their activities around this particular procedure,” the dermatologist said.
Another consideration: “The area they actually studied – the buttocks – is an area where I’m not sure a lot of women would expose their skin in public. I think thigh dimpling is more bothersome because it shows in shorts and other types of clothing. We need to figure out if the injections work on the posterior thighs, the most common place most postpubertal women get cellulite,” Dr. Draelos noted.
She wasn’t surprised that smaller cellulite dimples did better. Larger dimples presumably have a broader fibrous attachment and tighter fibrous band. She found the less robust outcomes in women over age 40 similarly unsurprising, since cellulitis seems to worsen with age. Cellulitis can’t really be called a disease, anyway, since it occurs in about 90% of postpubertal women.
One last tip about managing patient expectations: “Let a woman know that it’ll be better, but it won’t be gone,” she said.
Dr. Gold and Dr. Kaminer reported serving as paid investigators for and consultants to Endo Pharmaceuticals, the study sponsor and manufacturer of CCH, as well as for several other pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
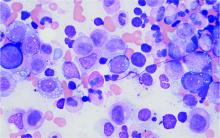
VEXAS: A novel rheumatologic, hematologic syndrome that’s making waves
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Older men with a novel adult-onset, severe autoinflammatory syndrome known by the acronym VEXAS are likely hiding in plain sight in many adult rheumatology, hematology, and dermatology practices. New clinical features are being described to fill out the clinical profile of such patients who may be currently misdiagnosed with other conditions, according to researchers who first described the syndrome in the last quarter of 2020.

VEXAS is often misdiagnosed as treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, or giant cell arteritis. These seemingly unrelated disorders are actually tied together by a single thread recently unraveled by David B. Beck, MD, PhD, a clinical fellow at the National Human Genome Research Institute, and colleagues, including rheumatologist Marcela Ferrada, MD, and others at institutes of the National Institutes of Health, Bethesda, Md. The connection between these disparate clinical presentations lies in somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation. VEXAS appears primarily limited to men because the UBA1 gene lies on the X chromosome, although it may be possible for women to have it because of an acquired loss of X chromosome.
VEXAS is an acronym for:
- Vacuoles in bone marrow cells
- E-1 activating enzyme, which is what UBA1 encodes for
- X-linked
- Autoinflammatory
- Somatic mutation featuring hematologic mosaicism
Dr. Beck said that VEXAS is “probably affecting thousands of Americans,” but it is tough to say this early in the understanding of the disease. He estimated that the prevalence of VEXAS could be 1 per 20,000-30,000 individuals.
A new way of looking for disease
VEXAS has caused a major stir among geneticists because of the novel manner in which Dr. Beck and his coinvestigators made their discovery. Instead of starting out in the traditional path to discovery of a new genetic disease – that is, by looking for clinical similarities among patients with undiagnosed diseases and then conducting a search for a gene or genes that might explain the shared patient symptoms – the investigators took a genotype-first approach. They scanned the mapped genomic sequences of patients in the National Institutes of Health Undiagnosed Diseases Network, which led them to zero in on mutations in UBA1 as their top candidate.
“We targeted the ubiquitin-proteasome pathway, because it has been implicated in many autoinflammatory diseases – for example, HA20 [A20 haploinsufficiency] and CANDLE syndrome [Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature]. Many of these recurrent inflammatory diseases are caused by mutations within this pathway,” Dr. Beck said in an interview.
Next, they analyzed the genomes of patients in other NIH databases and patients from other study populations at the University College London and Leeds Teaching Hospitals NHS Trust in the United Kingdom in a search for UBA1 somatic mutations, eventually identifying 25 men with the shared features they called VEXAS. These 25 formed the basis for their initial report on the syndrome in the New England Journal of Medicine.
Most autoinflammatory diseases appear in childhood because they stem from germline mutations. VEXAS syndrome, because of somatic mutations with mosaicism, appears to manifest later in life: The median age of the initial 25-man cohort was 64 years, ranging from 45 to 80 years. It’s a severe disorder. By the time the investigators were preparing their paper for publication, 10 of the 25 patients, or 40%, had died.
“I think that somatic mutations may account for a significant percentage of severe. adult-onset rheumatologic diseases, and it may change the way we think about treating them based on having a genetic diagnosis,” Dr. Beck said.
“This approach could be expanded to look at other pathways we know are important in inflammation, or alternatively, it could be completely unbiased and look for any shared variation that occurs across undiagnosed patients with inflammatory diseases. I think that one thing that’s important about our study is that previously we had been looking for mutations that really in most cases were the same sort of germline mutations present in [pediatric] patients who have disease at early onset, but now we’re thinking about things differently. There may be a different type of genetics that drives adult-onset rheumatologic disease, and this would be somatic mutations which are not present in every cell of the body, just in the blood, and that’s why there’s just this blood-based disease.”
When to suspect VEXAS syndrome
Consider the possibility of VEXAS in middle-aged or older men in a rheumatology clinic with characteristics suggestive of treatment-refractory relapsing polychondritis, giant cell arteritis, polyarteritis nodosa, or Sweet syndrome. In the original series of 25 men, 15 were diagnosed with relapsing polychondritis, 8 with Sweet syndrome, 3 with polyarteritis nodosa, and 1 with giant cell arteritis.
Men with VEXAS often have periodic fevers, pulmonary infiltrates, a history of unprovoked venous thromboembolic events, neutrophilic dermatoses, and/or hematologic abnormalities such as myelodysplastic syndrome, multiple myeloma, or monoclonal gammopathy of unknown origin.
Bone marrow biopsy will show vacuoles in myeloid and erythroid precursor cells. Inflammatory marker levels are very high: In the NIH series, the median C-reactive protein was 73 mg/L and median erythrocyte sedimentation rate was 97 mm/hr. The diagnosis of VEXAS can be confirmed by genetic testing performed by Dr. Beck and his NIH coworkers ([email protected]).
In interviews, Dr. Beck and Dr. Ferrada emphasized that management of VEXAS requires a multidisciplinary team of clinicians including rheumatologists, hematologists, and dermatologists.
Dr. Ferrada said that rheumatologists could suspect VEXAS in patients who have very high inflammatory markers and do not have a clear diagnosis or do not meet all criteria for other rheumatologic diseases, particularly in older men, but it’s possible in younger men as well. Hematologists could also consider VEXAS in patients with macrocytic anemia or macrocytosis without an explanation and inflammatory features, she said.
Dr. Ferrada, Dr. Beck, and colleagues also published a study in Arthritis & Rheumatology that presents a useful clinical algorithm for deciding whether to order genetic screening for VEXAS in patients with relapsing polychondritis.
First off, Dr. Ferrada and colleagues performed whole-exome sequencing and testing for UBA1 variants in an observational cohort of 92 relapsing polychondritis patients to determine the prevalence of VEXAS, which turned out to be 8%. They added an additional 6 patients with relapsing polychondritis and VEXAS from other cohorts, for a total of 13. The investigators determined that patients with VEXAS were older at disease onset, and more likely to have fever, ear chondritis, DVT, pulmonary infiltrates, skin involvement, and periorbital edema. In contrast, the RP cohort had a significantly higher prevalence of airway chondritis, joint involvement, and vestibular symptoms.
Dr. Ferrada’s algorithm for picking out VEXAS in patients who meet diagnostic criteria for relapsing polychondritis is based upon a few simple factors readily apparent in screening patient charts: male sex; age at onset older than 50 years; macrocytic anemia; and thrombocytopenia. Those four variables, when present, identify VEXAS within an RP cohort with 100% sensitivity and 96% specificity. “As we learn more about [VEXAS] and how it presents earlier, I think we are going to be able to find different manifestations or laboratory data that are going to allow us to diagnose these patients earlier,” she said. “The whole role of that algorithm was to guide clinicians who see patients with relapsing polychondritis to test these patients for the mutation, but I think over time that is going to evolve.”
Researchers are taking similar approaches for other clinical diagnoses to see which should be referred for UBA1 testing, Dr. Beck said.
Myelodysplastic syndrome and hematologic abnormalities
While patients with both myelodysplastic syndrome and relapsing polychondritis have been known in the literature for many years, it’s not until now that researchers are seeing a connection between the two, Dr. Ferrada said.
A majority of the VEXAS patients in the NEJM study had a workup for myelodysplastic syndrome, but only 24% met criteria. However, many were within the spectrum of myelodysplastic disease and some did not meet criteria because their anemia was attributed to a rheumatologic diagnosis and they did not have a known genetic driver of myelodysplastic syndrome, Dr. Beck said. It also fits with this new evidence that UBA1 is probably a driver of myelodysplastic syndrome in and of itself, and that anemia and hematologic involvement are not secondary to the rheumatologic disease; they are linked to the same disease process.
Dr. Beck said that there may be a subset of patients who present with primarily hematologic manifestations, noting the NEJM study could have ascertainment bias because the researchers analyzed mainly patients presenting to their clinic with relapsing polychondritis and severe inflammation. NIH researchers also are still looking in their cohort for any association with hematologic malignancies that preceded clinical manifestations, he said.
More cases reported
As of early April, another 27 cases had been reported in the literature as more researchers have begun to look for patients with UBA1 mutations, some with additional presenting clinical features associated with VEXAS, including chronic progressive inflammatory arthritis, Kikuchi-Fujimoto disease, spondyloarthritis, and bacterial pneumonia.
“Many times with rare diseases, we can’t get enough patients to understand the full spectrum of the disease, but this disease seems to be far more common than we would have expected. We’re actually getting many referrals,” Dr. Beck said.
It appears so far that the range of somatic UBA1 mutations that have been discovered in VEXAS patients does make a difference in the severity of clinical presentation and could potentially be useful in prognosis, Dr. Beck said.
Right now, NIH researchers are asking patients about their natural clinical course, assessing disease activity, and determining which treatments get a response, with the ultimate goal of a treatment trial at the NIH.
Treatment
Developing better treatments for VEXAS syndrome is a priority. In the initial report on VEXAS, the researchers found that the only reliably effective therapy is high-dose corticosteroids. Dr. Ferrada said that NIH investigators have begun thinking about agents that target both the hematologic and inflammatory features of VEXAS. “Most patients get exposed to treatments that are targeted to decrease the inflammatory process, and some of these treatments help partially but not completely to decrease the amount of steroids that patients are taking. For example, one of the medications is tocilizumab. [It was used in] patients who had previous diagnosis of relapsing polychondritis, but they still had to take steroids and their hematologic manifestations keep progressing. We’re in the process of figuring out medications that may help in treating both.” Dr. Ferrada added that because the source of the mutation is in the bone marrow, transplantation may be an effective option.
Laboratory work to identify potential treatments for VEXAS in studies of model organisms could identify treatments outside of the classic anti-inflammatory agents, such as targeting certain cell types in the bone marrow or the ubiquitin-proteasome pathway, Dr. Beck said. “We think that however UBA1 works to initiate inflammation may be important not just in VEXAS but in other diseases. Rare diseases may be informing the mechanisms in common diseases.”
The VEXAS NEJM study was sponsored by the NIH Intramural Research Programs and by an EU Horizon 2020 Research and Innovation Program grant. Dr. Beck reported a patent pending on “Diagnosis and Treatment of VEXAS with Mosaic Missense Mutations in UBA1.”
Bimekizumab superior to adalimumab in head-to-head psoriasis study
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Hedgehog inhibitor alternative dosing advantageous for BCC
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Coffee could be the secret weapon against NAFLD
Treatment of obesity through exercise and diet is unquestionably the foundation of care for patients with nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). But drinking at least several cups of coffee a day makes for additional powerful medicine, said Manal F. Abdelmalek, MD, MPH, at the Gastroenterology Updates, IBD, Liver Disease Conference.
“I do recommend at least two to three cups of coffee per day for my patients with NAFLD,” said Dr. Abdelmalek, professor of medicine and a gastroenterologist at Duke University, Durham, N.C.
Her thinking on this recommendation has been influenced by a meta-analysis of 16 studies including more than 3,000 coffee drinkers and 132,000 nonconsumers; the meta-analysis concluded that coffee drinkers were 39% less likely to develop cirrhosis. There was evidence of a dose-response effect: Consumers of two or more cups daily had a 47% reduction in the risk of cirrhosis, compared with the nondrinkers, while more modest consumption was associated with a 34% reduction. Moreover, the investigators found that coffee consumption was also associated with a 27% reduction in the likelihood of developing advanced hepatic fibrosis, compared with that of non–coffee drinkers.
“What’s even more provocative is the evidence that coffee decreases risk of hepatocellular carcinoma,” the gastroenterologist said.
She highlighted a U.K. meta-analysis of 18 cohort studies with 2.27 million participants and 2,905 cases, along with 8 case-control studies featuring a collective 1,825 cases and 4,652 controls. The investigators reported that drinking at least two cups of coffee per day was associated with a 35% reduction in the risk of hepatocellular carcinoma independent of a patient’s stage of liver disease or the presence or absence of high alcohol consumption, smoking, obesity, type 2 diabetes, or hepatitis B or C infection.
“This is very impressive data and certainly not something you should ignore,” according to Dr. Abdelmalek.
There is also “fairly strong” data that coffee reduces the risk of developing type 2 diabetes, she continued. The mechanism of these benefits is unclear.
“It’s not known if it’s caffeine or some other constituent of the bean; a phenol, for example. The story behind tea is not as compelling as for coffee, so it may be something beyond caffeine,” according to Dr. Abdelmalek.
Session moderator Norah A. Terrault, MD, MPH, noted that drinking at least two cups of coffee per day has also been associated with reduced risk of cirrhosis in patients with hepatitis B or hepatitis C infection. So she too is on board the coffee express.
“I’m also a big proponent of recommending coffee. We take so much away from the patients, it’s nice to give them back something, right?” said Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
Diet and exercise
Most of the major gastroenterology professional societies emphasize in their practice guidelines for NAFLD that diet and routine physical activity are mandatory. If sustained, these lifestyle modifications can improve NASH and hepatic fibrosis, as well as reduce the risk of portal hypertension and liver cancer. Dr. Abdelmalek counsels her patients to aim for at least 150 minutes per week of moderate or vigorous aerobic and/or resistance exercise. She doesn’t care about the exercise intensity or type, noting that what she considers to be “a beautifully done intervention trial” in 220 patients over the course of 12 months concluded that both moderate and vigorous exercise achieved a significant reduction in intrahepatic triglyceride content.
“Tailor exercise to what patients can do, what they enjoy, and what they can sustain,” she advised.
She identifies and addresses all modifiable risk factors for NAFLD, including hypertension, diabetes, abdominal obesity, smoking, excessive alcohol intake, obstructive sleep apnea, and an unhealthy diet high in fat, red meat, and fructose.
“The primary message I tell my patients interested in dieting is: I want you to find the right approach for you. There is no right or wrong answer. For some of my patients, it’s intermittent fasting and having their first meal at 2 or 3 o’clock in the afternoon. For others it’s a Weight Watchers approach, or a Mediterranean diet, or it’s high protein. The bottom line of my approach is a gravitation away from excess carbohydrates and fats, and beyond that if I can achieve weight loss through caloric restriction or intermittent fasting, I try to tailor that to my patients’ preferences. I do send them to nutritionists,” the gastroenterologist said.
A 7%-10% weight loss has been shown to result in resolution of NASH in 64%-90% of patients. However, only about 10% of patients who achieve clinically meaningful weight loss short term are able to maintain it at 1 year, so ongoing follow-up is essential.
At present there is no FDA-approved therapy for NAFLD/NASH. Beyond diet and exercise – and coffee – there is the option of antiobesity weight-loss drug therapy, which is about as effective as successful lifestyle modification, and bariatric surgery, which is dramatically effective. French surgeons recently reported in a prospective single-center study of 180 severely obese patients with NASH who underwent bariatric surgery that, at 5 years’ follow-up, 84% of them had resolution of NASH with no worsening of liver fibrosis. Indeed, 63% of patients with mild fibrosis at baseline experienced complete resolution of their fibrosis at follow-up, as did 46% of those with more severe baseline bridging fibrosis.
Dr. Abdelmalek reported having no financial conflicts of interest regarding her presentation.
Treatment of obesity through exercise and diet is unquestionably the foundation of care for patients with nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). But drinking at least several cups of coffee a day makes for additional powerful medicine, said Manal F. Abdelmalek, MD, MPH, at the Gastroenterology Updates, IBD, Liver Disease Conference.
“I do recommend at least two to three cups of coffee per day for my patients with NAFLD,” said Dr. Abdelmalek, professor of medicine and a gastroenterologist at Duke University, Durham, N.C.
Her thinking on this recommendation has been influenced by a meta-analysis of 16 studies including more than 3,000 coffee drinkers and 132,000 nonconsumers; the meta-analysis concluded that coffee drinkers were 39% less likely to develop cirrhosis. There was evidence of a dose-response effect: Consumers of two or more cups daily had a 47% reduction in the risk of cirrhosis, compared with the nondrinkers, while more modest consumption was associated with a 34% reduction. Moreover, the investigators found that coffee consumption was also associated with a 27% reduction in the likelihood of developing advanced hepatic fibrosis, compared with that of non–coffee drinkers.
“What’s even more provocative is the evidence that coffee decreases risk of hepatocellular carcinoma,” the gastroenterologist said.
She highlighted a U.K. meta-analysis of 18 cohort studies with 2.27 million participants and 2,905 cases, along with 8 case-control studies featuring a collective 1,825 cases and 4,652 controls. The investigators reported that drinking at least two cups of coffee per day was associated with a 35% reduction in the risk of hepatocellular carcinoma independent of a patient’s stage of liver disease or the presence or absence of high alcohol consumption, smoking, obesity, type 2 diabetes, or hepatitis B or C infection.
“This is very impressive data and certainly not something you should ignore,” according to Dr. Abdelmalek.
There is also “fairly strong” data that coffee reduces the risk of developing type 2 diabetes, she continued. The mechanism of these benefits is unclear.
“It’s not known if it’s caffeine or some other constituent of the bean; a phenol, for example. The story behind tea is not as compelling as for coffee, so it may be something beyond caffeine,” according to Dr. Abdelmalek.
Session moderator Norah A. Terrault, MD, MPH, noted that drinking at least two cups of coffee per day has also been associated with reduced risk of cirrhosis in patients with hepatitis B or hepatitis C infection. So she too is on board the coffee express.
“I’m also a big proponent of recommending coffee. We take so much away from the patients, it’s nice to give them back something, right?” said Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
Diet and exercise
Most of the major gastroenterology professional societies emphasize in their practice guidelines for NAFLD that diet and routine physical activity are mandatory. If sustained, these lifestyle modifications can improve NASH and hepatic fibrosis, as well as reduce the risk of portal hypertension and liver cancer. Dr. Abdelmalek counsels her patients to aim for at least 150 minutes per week of moderate or vigorous aerobic and/or resistance exercise. She doesn’t care about the exercise intensity or type, noting that what she considers to be “a beautifully done intervention trial” in 220 patients over the course of 12 months concluded that both moderate and vigorous exercise achieved a significant reduction in intrahepatic triglyceride content.
“Tailor exercise to what patients can do, what they enjoy, and what they can sustain,” she advised.
She identifies and addresses all modifiable risk factors for NAFLD, including hypertension, diabetes, abdominal obesity, smoking, excessive alcohol intake, obstructive sleep apnea, and an unhealthy diet high in fat, red meat, and fructose.
“The primary message I tell my patients interested in dieting is: I want you to find the right approach for you. There is no right or wrong answer. For some of my patients, it’s intermittent fasting and having their first meal at 2 or 3 o’clock in the afternoon. For others it’s a Weight Watchers approach, or a Mediterranean diet, or it’s high protein. The bottom line of my approach is a gravitation away from excess carbohydrates and fats, and beyond that if I can achieve weight loss through caloric restriction or intermittent fasting, I try to tailor that to my patients’ preferences. I do send them to nutritionists,” the gastroenterologist said.
A 7%-10% weight loss has been shown to result in resolution of NASH in 64%-90% of patients. However, only about 10% of patients who achieve clinically meaningful weight loss short term are able to maintain it at 1 year, so ongoing follow-up is essential.
At present there is no FDA-approved therapy for NAFLD/NASH. Beyond diet and exercise – and coffee – there is the option of antiobesity weight-loss drug therapy, which is about as effective as successful lifestyle modification, and bariatric surgery, which is dramatically effective. French surgeons recently reported in a prospective single-center study of 180 severely obese patients with NASH who underwent bariatric surgery that, at 5 years’ follow-up, 84% of them had resolution of NASH with no worsening of liver fibrosis. Indeed, 63% of patients with mild fibrosis at baseline experienced complete resolution of their fibrosis at follow-up, as did 46% of those with more severe baseline bridging fibrosis.
Dr. Abdelmalek reported having no financial conflicts of interest regarding her presentation.
Treatment of obesity through exercise and diet is unquestionably the foundation of care for patients with nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH). But drinking at least several cups of coffee a day makes for additional powerful medicine, said Manal F. Abdelmalek, MD, MPH, at the Gastroenterology Updates, IBD, Liver Disease Conference.
“I do recommend at least two to three cups of coffee per day for my patients with NAFLD,” said Dr. Abdelmalek, professor of medicine and a gastroenterologist at Duke University, Durham, N.C.
Her thinking on this recommendation has been influenced by a meta-analysis of 16 studies including more than 3,000 coffee drinkers and 132,000 nonconsumers; the meta-analysis concluded that coffee drinkers were 39% less likely to develop cirrhosis. There was evidence of a dose-response effect: Consumers of two or more cups daily had a 47% reduction in the risk of cirrhosis, compared with the nondrinkers, while more modest consumption was associated with a 34% reduction. Moreover, the investigators found that coffee consumption was also associated with a 27% reduction in the likelihood of developing advanced hepatic fibrosis, compared with that of non–coffee drinkers.
“What’s even more provocative is the evidence that coffee decreases risk of hepatocellular carcinoma,” the gastroenterologist said.
She highlighted a U.K. meta-analysis of 18 cohort studies with 2.27 million participants and 2,905 cases, along with 8 case-control studies featuring a collective 1,825 cases and 4,652 controls. The investigators reported that drinking at least two cups of coffee per day was associated with a 35% reduction in the risk of hepatocellular carcinoma independent of a patient’s stage of liver disease or the presence or absence of high alcohol consumption, smoking, obesity, type 2 diabetes, or hepatitis B or C infection.
“This is very impressive data and certainly not something you should ignore,” according to Dr. Abdelmalek.
There is also “fairly strong” data that coffee reduces the risk of developing type 2 diabetes, she continued. The mechanism of these benefits is unclear.
“It’s not known if it’s caffeine or some other constituent of the bean; a phenol, for example. The story behind tea is not as compelling as for coffee, so it may be something beyond caffeine,” according to Dr. Abdelmalek.
Session moderator Norah A. Terrault, MD, MPH, noted that drinking at least two cups of coffee per day has also been associated with reduced risk of cirrhosis in patients with hepatitis B or hepatitis C infection. So she too is on board the coffee express.
“I’m also a big proponent of recommending coffee. We take so much away from the patients, it’s nice to give them back something, right?” said Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
Diet and exercise
Most of the major gastroenterology professional societies emphasize in their practice guidelines for NAFLD that diet and routine physical activity are mandatory. If sustained, these lifestyle modifications can improve NASH and hepatic fibrosis, as well as reduce the risk of portal hypertension and liver cancer. Dr. Abdelmalek counsels her patients to aim for at least 150 minutes per week of moderate or vigorous aerobic and/or resistance exercise. She doesn’t care about the exercise intensity or type, noting that what she considers to be “a beautifully done intervention trial” in 220 patients over the course of 12 months concluded that both moderate and vigorous exercise achieved a significant reduction in intrahepatic triglyceride content.
“Tailor exercise to what patients can do, what they enjoy, and what they can sustain,” she advised.
She identifies and addresses all modifiable risk factors for NAFLD, including hypertension, diabetes, abdominal obesity, smoking, excessive alcohol intake, obstructive sleep apnea, and an unhealthy diet high in fat, red meat, and fructose.
“The primary message I tell my patients interested in dieting is: I want you to find the right approach for you. There is no right or wrong answer. For some of my patients, it’s intermittent fasting and having their first meal at 2 or 3 o’clock in the afternoon. For others it’s a Weight Watchers approach, or a Mediterranean diet, or it’s high protein. The bottom line of my approach is a gravitation away from excess carbohydrates and fats, and beyond that if I can achieve weight loss through caloric restriction or intermittent fasting, I try to tailor that to my patients’ preferences. I do send them to nutritionists,” the gastroenterologist said.
A 7%-10% weight loss has been shown to result in resolution of NASH in 64%-90% of patients. However, only about 10% of patients who achieve clinically meaningful weight loss short term are able to maintain it at 1 year, so ongoing follow-up is essential.
At present there is no FDA-approved therapy for NAFLD/NASH. Beyond diet and exercise – and coffee – there is the option of antiobesity weight-loss drug therapy, which is about as effective as successful lifestyle modification, and bariatric surgery, which is dramatically effective. French surgeons recently reported in a prospective single-center study of 180 severely obese patients with NASH who underwent bariatric surgery that, at 5 years’ follow-up, 84% of them had resolution of NASH with no worsening of liver fibrosis. Indeed, 63% of patients with mild fibrosis at baseline experienced complete resolution of their fibrosis at follow-up, as did 46% of those with more severe baseline bridging fibrosis.
Dr. Abdelmalek reported having no financial conflicts of interest regarding her presentation.
FROM GUILD 2021
AI system beats endoscopists for detecting early neoplasia in Barrett’s
One of the top publications in gastroenterology in 2020 was a Dutch study demonstrating that a computer-aided system suitable for real-time use in clinical practice detected early neoplasia in patients with Barrett’s esophagus with impressively greater accuracy than did a group of general endoscopists, according to Douglas A. Corley, MD, PhD.
It’s not just his personal opinion that this was one of the major studies of the past year, either. Analytic tools showed the Dutch report was one of the most frequently downloaded studies in 2020 by both clinical gastroenterologists and researchers, said Dr. Corley, director of delivery science and applied research at Kaiser Permanente of Northern California, Oakland, and a faculty gastroenterologist at the University of California, San Francisco.
The deep-learning system developed, evaluated, and externally validated by the Dutch investigators is designed to reduce the rate of failed detection of high-grade dysplasia and early adenocarcinoma in patients undergoing surveillance by general practice gastrointestinal endoscopists. The false-negative rate in looking for the sometimes subtle mucosal surface abnormalities indicative of early neoplasia is known to be higher among these general endoscopists than that among expert endoscopists, and yet it’s the general endoscopists who perform the majority of cancer surveillance in patients with Barrett’s esophagus.
The Dutch group developed the computer-aided detection system by applying artificial intelligence methods to analyze nearly one half-million endoscopic images of confirmed early neoplasia. Once the system was ready to go, they compared its diagnostic accuracy in 80 patients to that of 53 general, nonexpert endoscopists. The deep-learning system had 93% sensitivity and 83% specificity for identification of early neoplasia, significantly better than the 72% sensitivity and 74% specificity for the general endoscopists. The overall accuracy of the computer-assisted detection system was 88%, compared to 73% for the general endoscopists. Moreover, the deep-learning system achieved greater accuracy than did any single one of the endoscopists.
“I think this will be a really helpful addition, the equivalent of a second endoscopist raising a yellow flag to take a closer look at a particular area. It’ll be complementary,” Dr. Corley said at the Gastroenterology Updates, IBD, Liver Disease Conference.
An audience member said he’s aware that other computer-assisted detection systems have also shown outstanding performance for the detection of early neoplasia in Barrett’s esophagus. He asked, why aren’t these being deployed yet in routine clinical practice?
Two reasons, Dr. Corley replied. One is that some of those systems aren’t capable of working during real-time endoscopy. Also, industry seems to be taking a wait-and-see approach. The field of applied artificial intelligence is moving incredibly rapidly, and none of the endoscopic equipment manufacturers wants to incorporate a computer-assisted detection system into their gear when rumor has it that an even better system is going to be announced 6 months later. The manufacturers want to make sure they’re operationalizing the right one.
He suspects the major players in the endoscopic imaging industry are waiting to find a computer-assisted detection system that’s been published and widely accepted as clearly a winner. Then they’ll introduce it into their equipment.
“I do think we’re probably going to be seeing these increasingly. Some computer-assisted detection systems for colon cancer are starting to be put into equipment,” he observed.
Dr. Corley reported having no financial conflicts regarding his presentation.
Help your patients better understand the risks, testing, and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center: www.gastro.org/BE.
One of the top publications in gastroenterology in 2020 was a Dutch study demonstrating that a computer-aided system suitable for real-time use in clinical practice detected early neoplasia in patients with Barrett’s esophagus with impressively greater accuracy than did a group of general endoscopists, according to Douglas A. Corley, MD, PhD.
It’s not just his personal opinion that this was one of the major studies of the past year, either. Analytic tools showed the Dutch report was one of the most frequently downloaded studies in 2020 by both clinical gastroenterologists and researchers, said Dr. Corley, director of delivery science and applied research at Kaiser Permanente of Northern California, Oakland, and a faculty gastroenterologist at the University of California, San Francisco.
The deep-learning system developed, evaluated, and externally validated by the Dutch investigators is designed to reduce the rate of failed detection of high-grade dysplasia and early adenocarcinoma in patients undergoing surveillance by general practice gastrointestinal endoscopists. The false-negative rate in looking for the sometimes subtle mucosal surface abnormalities indicative of early neoplasia is known to be higher among these general endoscopists than that among expert endoscopists, and yet it’s the general endoscopists who perform the majority of cancer surveillance in patients with Barrett’s esophagus.
The Dutch group developed the computer-aided detection system by applying artificial intelligence methods to analyze nearly one half-million endoscopic images of confirmed early neoplasia. Once the system was ready to go, they compared its diagnostic accuracy in 80 patients to that of 53 general, nonexpert endoscopists. The deep-learning system had 93% sensitivity and 83% specificity for identification of early neoplasia, significantly better than the 72% sensitivity and 74% specificity for the general endoscopists. The overall accuracy of the computer-assisted detection system was 88%, compared to 73% for the general endoscopists. Moreover, the deep-learning system achieved greater accuracy than did any single one of the endoscopists.
“I think this will be a really helpful addition, the equivalent of a second endoscopist raising a yellow flag to take a closer look at a particular area. It’ll be complementary,” Dr. Corley said at the Gastroenterology Updates, IBD, Liver Disease Conference.
An audience member said he’s aware that other computer-assisted detection systems have also shown outstanding performance for the detection of early neoplasia in Barrett’s esophagus. He asked, why aren’t these being deployed yet in routine clinical practice?
Two reasons, Dr. Corley replied. One is that some of those systems aren’t capable of working during real-time endoscopy. Also, industry seems to be taking a wait-and-see approach. The field of applied artificial intelligence is moving incredibly rapidly, and none of the endoscopic equipment manufacturers wants to incorporate a computer-assisted detection system into their gear when rumor has it that an even better system is going to be announced 6 months later. The manufacturers want to make sure they’re operationalizing the right one.
He suspects the major players in the endoscopic imaging industry are waiting to find a computer-assisted detection system that’s been published and widely accepted as clearly a winner. Then they’ll introduce it into their equipment.
“I do think we’re probably going to be seeing these increasingly. Some computer-assisted detection systems for colon cancer are starting to be put into equipment,” he observed.
Dr. Corley reported having no financial conflicts regarding his presentation.
Help your patients better understand the risks, testing, and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center: www.gastro.org/BE.
One of the top publications in gastroenterology in 2020 was a Dutch study demonstrating that a computer-aided system suitable for real-time use in clinical practice detected early neoplasia in patients with Barrett’s esophagus with impressively greater accuracy than did a group of general endoscopists, according to Douglas A. Corley, MD, PhD.
It’s not just his personal opinion that this was one of the major studies of the past year, either. Analytic tools showed the Dutch report was one of the most frequently downloaded studies in 2020 by both clinical gastroenterologists and researchers, said Dr. Corley, director of delivery science and applied research at Kaiser Permanente of Northern California, Oakland, and a faculty gastroenterologist at the University of California, San Francisco.
The deep-learning system developed, evaluated, and externally validated by the Dutch investigators is designed to reduce the rate of failed detection of high-grade dysplasia and early adenocarcinoma in patients undergoing surveillance by general practice gastrointestinal endoscopists. The false-negative rate in looking for the sometimes subtle mucosal surface abnormalities indicative of early neoplasia is known to be higher among these general endoscopists than that among expert endoscopists, and yet it’s the general endoscopists who perform the majority of cancer surveillance in patients with Barrett’s esophagus.
The Dutch group developed the computer-aided detection system by applying artificial intelligence methods to analyze nearly one half-million endoscopic images of confirmed early neoplasia. Once the system was ready to go, they compared its diagnostic accuracy in 80 patients to that of 53 general, nonexpert endoscopists. The deep-learning system had 93% sensitivity and 83% specificity for identification of early neoplasia, significantly better than the 72% sensitivity and 74% specificity for the general endoscopists. The overall accuracy of the computer-assisted detection system was 88%, compared to 73% for the general endoscopists. Moreover, the deep-learning system achieved greater accuracy than did any single one of the endoscopists.
“I think this will be a really helpful addition, the equivalent of a second endoscopist raising a yellow flag to take a closer look at a particular area. It’ll be complementary,” Dr. Corley said at the Gastroenterology Updates, IBD, Liver Disease Conference.
An audience member said he’s aware that other computer-assisted detection systems have also shown outstanding performance for the detection of early neoplasia in Barrett’s esophagus. He asked, why aren’t these being deployed yet in routine clinical practice?
Two reasons, Dr. Corley replied. One is that some of those systems aren’t capable of working during real-time endoscopy. Also, industry seems to be taking a wait-and-see approach. The field of applied artificial intelligence is moving incredibly rapidly, and none of the endoscopic equipment manufacturers wants to incorporate a computer-assisted detection system into their gear when rumor has it that an even better system is going to be announced 6 months later. The manufacturers want to make sure they’re operationalizing the right one.
He suspects the major players in the endoscopic imaging industry are waiting to find a computer-assisted detection system that’s been published and widely accepted as clearly a winner. Then they’ll introduce it into their equipment.
“I do think we’re probably going to be seeing these increasingly. Some computer-assisted detection systems for colon cancer are starting to be put into equipment,” he observed.
Dr. Corley reported having no financial conflicts regarding his presentation.
Help your patients better understand the risks, testing, and treatment options for Barrett’s esophagus by sharing education from the AGA GI Patient Center: www.gastro.org/BE.
FROM GUILD 2021
Treatment paradigm for chronic HBV in flux
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
These days deciding when to stop targeted treatment for chronic hepatitis B is a bigger challenge than knowing when to start, Norah A. Terrault, MD, MPH, observed at the Gastroenterology Updates, IBD, Liver Disease Conference.
That’s because the treatment paradigm is in flux. The strategy is shifting from achieving hepatitis B virus (HBV) DNA suppression through indefinite use of nucleoside analogues to striving for functional cure, which means eliminating hepatitis B surface antigen (HBsAg) and sustained inactive chronic hepatitis B off therapy. It’s a goal that recognizes that, while suppression is worthwhile because it reduces a patient’s risk of hepatocellular carcinoma, HBsAg clearance is better because it’s associated with an even lower risk of the malignancy, explained Dr. Terrault, professor of medicine and chief of gastroenterology and liver diseases at the University of Southern California, Los Angeles.
The current strategy in patients who are hepatitis B e antigen (HBeAg) positive at the outset is to treat with a nucleoside analogue until seroconversion, followed by a further year or more of consolidation therapy then treatment withdrawal. It’s a rational approach whose primary benefit is it allows identification of the roughly 50% of patients who can remain off treatment with inactive chronic hepatitis B. The other 50% – those who experience clinical relapse – will need retreatment.
Factors predictive of increased likelihood of a sustained off-treatment response include age younger than 40 years at the time of seroconversion, more than 1 year of consolidation therapy, and undetectable HBV DNA at cessation of treatment.
“In my own practice now, I actually extend the consolidation period for 2 years before I consider stopping, and I really favor doing a trial of stopping treatment in those who are younger,” Dr. Terrault said.
The biggest change in thinking involves the duration of therapy in patients who are HBeAg negative. The strategy has been to treat indefinitely unless there is a compelling reason to stop, such as toxicity, cost, or patient preference. However, it has now been demonstrated in at least nine published studies that withdrawal of therapy has a favorable immunologic effect in noncirrhotic patients with HBeAg-negative chronic hepatitis B who have been HBV DNA negative on nucleoside analogues for at least 3 years. This trial off therapy can bring major benefits because roughly 50% of patients will have sustained inactive chronic hepatitis B off-treatment and 20% of patients will become HbsAg negative with functional cure at 3-5 years of follow-up.
“This is what’s impressive: that 20% of patients have lost surface antigen, because if you continue HbeAg-negative patients on nucleoside analogue therapy, essentially none of them lose surface antigen. This is an impressive number, and you’re also able to identify about 50% of patients who didn’t need to be on treatment because they now have immune control and can remain inactive carriers off treatment,” the gastroenterologist commented.
Treatment withdrawal in HBeAg-negative patients usually is followed by disease flares 8-12 weeks later because of host immune clearance, and therein lies a problem.
“The challenge with the withdrawal strategy is these flares that appear to be necessary and important, can be good or bad, and we’re really not very good at predicting what the flare is going to look like and how severe it’s going to be,” according to Dr. Terrault, first author of the current American Association for the Study of Liver Diseases guidance on prevention, diagnosis, and treatment of chronic hepatitis B.
The good flares are accompanied by a reductions in HBV DNA and viral proteins, loss of HbsAg, and preserved liver function. The bad flares entail excessive host immune clearance leading to liver dysfunction or failure, with no reduction in viral proteins. The search is on for predictors of response to treatment withdrawal in HbeAg-negative patients. Potential differences in outcomes with the three available nucleoside analogues are being looked at, as are duration of viral suppression on treatment and differences in patient characteristics. A low quantitative HbsAg level at the time of drug withdrawal may also be important as a predictor of a higher likelihood of HBsAg loss over time off treatment.
“The studies that have been done are basically withdrawing everyone and then seeing what happens. I think we want to have a more refined approach,” she said.
This is an unfolding story. The encouraging news is that the drug development pipeline is rich with agents with a variety of mechanisms aimed at achieving HbsAg loss with finite therapy. Some of the studies are now in phase 2 and 3.
“We should be extremely excited,” Dr. Terrault said. “I think in the future we’re very likely to have curative therapies in a much greater proportion of our patients.”
When to start nucleoside analogues
Three antiviral oral nucleoside analogues are available as preferred therapies for chronic HBV: entecavir (Baraclude), tenofovir alafenamide (Vemlidy), and tenofovir disoproxil (Viread). All three provide high antiviral efficacy and low risk for resistance. The treatment goal is to prevent disease progression and HBV complications, including hepatocellular carcinoma, in individuals with active chronic hepatitis B.
The major liver disease medical societies differ only slightly on the criteria for starting treatment. Broadly, they recommend starting therapy in all patients with cirrhosis, as well as in patients without cirrhosis who have both a serum ALT level more than twice the upper limit of normal and elevated HBV DNA levels. The treatment threshold for HBV DNA levels is higher in patients who are HBeAg positive than it is for patients who are HBeAg negative; for example, the American Association for the Study of Liver Diseases recommends that an HbeAg-positive patient should have a HBV DNA titer greater than 20,000 IU/mL, which is a level 10 times higher than the group’s treatment threshold in HBeAg-negative patients. However, these thresholds are intended as guidance, not absolute rules, Dr. Terrault emphasized. Nearly 40% of patients don’t meet the dual ALT and HBV DNA thresholds, and serial monitoring of such patients for 6-12 months is recommended because they may be in transition.
The choice of nucleoside analogue is largely based on comorbidities. Any of the three preferred antivirals can be used when there are none. Tenofovir disoproxil is preferred in pregnancy because of its safety profile in that setting. In patients who are aged over 60 years or have bone disease or renal impairment, tenofovir alafenamide and entecavir are preferred. Entecavir should be avoided in favor of either form of tenofovir in patients who are HIV positive or have prior exposure to lamivudine.
Regarding treatment with these drugs, the recommendations target those whose liver disease is being driven by active HBV rather than fatty liver disease or some other cause. That’s the reason for the reserving treatment for patients with both high HBV DNA and high serum ALT.
“There’s definitely a camp that feels these are safe drugs, easy to use, and we should treat more people. I have to say I’m not hanging out in that camp. I still feel we should do targeted treatment, especially since there are many new drugs coming where we’re going to be able to offer cure to more people. So I feel like putting everybody on suppressive therapy isn’t the answer,” she said.
Dr. Terrault receives research grants from and/or serves as a consultant to numerous pharmaceutical companies.
FROM GUILD 2021
Oral sarecycline promising for papulopustular rosacea
Linda Stein Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The oral broad-spectrum second-generation tetracyclines doxycycline and minocycline have long been considered first-line therapy for papulopustular rosacea that isn’t cleared using topical agents. But the widespread use of these oral tetracyclines has encouraged the development of antimicrobial resistance. In contrast, sarecycline (Seysara) is a third-generation, narrow-spectrum tetracycline designed to minimize antibiotic resistance. The Food and Drug Administration approved the drug for treatment of moderate to severe acne vulgaris in 2018.
At the meeting, Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit, highlighted a recent pilot study of oral sarecycline for papulopustular rosacea carried out by James Q. Del Rosso, DO, of Las Vegas and coinvestigators. Although she wasn’t involved in the study, she is a veteran clinical trialist with vast experience leading studies of new therapies for rosacea, acne, and other major dermatologic disorders.
The 12-week, prospective, investigator-blinded study included 97 adults with moderate to severe papulopustular rosacea; 72 were randomized to weight-based dosing of once-daily sarecycline, while the 25 controls took a daily oral vitamin.
One coprimary endpoint was achievement of an Investigator Global Assessment score of 0 or 1, meaning clear or almost clear skin, at week 12. The rates were 75% in the sarecycline group and 16% in controls. The other coprimary endpoint was the percent reduction from baseline to week 12 in inflammatory lesion count. Here again, there was a statistically significant difference in favor of the third-generation tetracycline derivative, which achieved an 80% reduction, compared with 50% in the control group.
Of note, the difference was already significant at the first evaluation at week 4, with a 58% reduction in inflammatory lesions in the sarecycline group versus 31% decrease in controls, Dr. Stein Gold observed at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also at week 12, 96% of patients on sarecycline reported having no or only trace symptoms of facial burning, 63% had no or only trace facial erythema, and 94% had no or trace facial itch, compared with 76%, 12%, and 76% of controls, respectively. The sarecycline group was also significantly more likely to report no or trace skin dryness and oiliness.
The side-effect profile was favorable and the same as encountered with the use of sarecycline for acne: no major photosensitivity issues, no serious adverse events, and only 2 of the original 75 patients in the active-treatment arm discontinued sarecycline for treatment-emergent headache or gastroenteritis considered “probably” related to the study drug. The investigators deemed further studies of sarecycline for rosacea to be warranted as a potential expanded indication.
Aiming for clear skin rather than ‘almost clear’
Dr. Stein Gold shared her mantra for rosacea therapy: “Always aim for clear skin.”
She cited a study led by Guy Webster, MD, professor of dermatology, Thomas Jefferson University, Philadelphia, in which he and his coinvestigators looked at the durability of treatment response in a pooled analysis of 1,366 rosacea patients in four clinical trials. If patients improved to “almost clear” after treatment, their median time to relapse was 3 months; if they reached “clear,” it was more than 8 months. Also, more clear patients rated their outcomes as excellent and reported that their skin disease no longer had any effect on their quality of life.
“That’s more than a 5-month difference,” Dr. Stein Gold noted. “It shows the importance of really striving to get that skin completely clear.”
The sarecycline study was funded by Almirall, which markets the antibiotic. Dr. Stein Gold, who has no financial relationship with Almirall, has received research funding from and/or served as a consultant to roughly a dozen other pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
Linda Stein Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The oral broad-spectrum second-generation tetracyclines doxycycline and minocycline have long been considered first-line therapy for papulopustular rosacea that isn’t cleared using topical agents. But the widespread use of these oral tetracyclines has encouraged the development of antimicrobial resistance. In contrast, sarecycline (Seysara) is a third-generation, narrow-spectrum tetracycline designed to minimize antibiotic resistance. The Food and Drug Administration approved the drug for treatment of moderate to severe acne vulgaris in 2018.
At the meeting, Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit, highlighted a recent pilot study of oral sarecycline for papulopustular rosacea carried out by James Q. Del Rosso, DO, of Las Vegas and coinvestigators. Although she wasn’t involved in the study, she is a veteran clinical trialist with vast experience leading studies of new therapies for rosacea, acne, and other major dermatologic disorders.
The 12-week, prospective, investigator-blinded study included 97 adults with moderate to severe papulopustular rosacea; 72 were randomized to weight-based dosing of once-daily sarecycline, while the 25 controls took a daily oral vitamin.
One coprimary endpoint was achievement of an Investigator Global Assessment score of 0 or 1, meaning clear or almost clear skin, at week 12. The rates were 75% in the sarecycline group and 16% in controls. The other coprimary endpoint was the percent reduction from baseline to week 12 in inflammatory lesion count. Here again, there was a statistically significant difference in favor of the third-generation tetracycline derivative, which achieved an 80% reduction, compared with 50% in the control group.
Of note, the difference was already significant at the first evaluation at week 4, with a 58% reduction in inflammatory lesions in the sarecycline group versus 31% decrease in controls, Dr. Stein Gold observed at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also at week 12, 96% of patients on sarecycline reported having no or only trace symptoms of facial burning, 63% had no or only trace facial erythema, and 94% had no or trace facial itch, compared with 76%, 12%, and 76% of controls, respectively. The sarecycline group was also significantly more likely to report no or trace skin dryness and oiliness.
The side-effect profile was favorable and the same as encountered with the use of sarecycline for acne: no major photosensitivity issues, no serious adverse events, and only 2 of the original 75 patients in the active-treatment arm discontinued sarecycline for treatment-emergent headache or gastroenteritis considered “probably” related to the study drug. The investigators deemed further studies of sarecycline for rosacea to be warranted as a potential expanded indication.
Aiming for clear skin rather than ‘almost clear’
Dr. Stein Gold shared her mantra for rosacea therapy: “Always aim for clear skin.”
She cited a study led by Guy Webster, MD, professor of dermatology, Thomas Jefferson University, Philadelphia, in which he and his coinvestigators looked at the durability of treatment response in a pooled analysis of 1,366 rosacea patients in four clinical trials. If patients improved to “almost clear” after treatment, their median time to relapse was 3 months; if they reached “clear,” it was more than 8 months. Also, more clear patients rated their outcomes as excellent and reported that their skin disease no longer had any effect on their quality of life.
“That’s more than a 5-month difference,” Dr. Stein Gold noted. “It shows the importance of really striving to get that skin completely clear.”
The sarecycline study was funded by Almirall, which markets the antibiotic. Dr. Stein Gold, who has no financial relationship with Almirall, has received research funding from and/or served as a consultant to roughly a dozen other pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
Linda Stein Gold, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The oral broad-spectrum second-generation tetracyclines doxycycline and minocycline have long been considered first-line therapy for papulopustular rosacea that isn’t cleared using topical agents. But the widespread use of these oral tetracyclines has encouraged the development of antimicrobial resistance. In contrast, sarecycline (Seysara) is a third-generation, narrow-spectrum tetracycline designed to minimize antibiotic resistance. The Food and Drug Administration approved the drug for treatment of moderate to severe acne vulgaris in 2018.
At the meeting, Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit, highlighted a recent pilot study of oral sarecycline for papulopustular rosacea carried out by James Q. Del Rosso, DO, of Las Vegas and coinvestigators. Although she wasn’t involved in the study, she is a veteran clinical trialist with vast experience leading studies of new therapies for rosacea, acne, and other major dermatologic disorders.
The 12-week, prospective, investigator-blinded study included 97 adults with moderate to severe papulopustular rosacea; 72 were randomized to weight-based dosing of once-daily sarecycline, while the 25 controls took a daily oral vitamin.
One coprimary endpoint was achievement of an Investigator Global Assessment score of 0 or 1, meaning clear or almost clear skin, at week 12. The rates were 75% in the sarecycline group and 16% in controls. The other coprimary endpoint was the percent reduction from baseline to week 12 in inflammatory lesion count. Here again, there was a statistically significant difference in favor of the third-generation tetracycline derivative, which achieved an 80% reduction, compared with 50% in the control group.
Of note, the difference was already significant at the first evaluation at week 4, with a 58% reduction in inflammatory lesions in the sarecycline group versus 31% decrease in controls, Dr. Stein Gold observed at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
Also at week 12, 96% of patients on sarecycline reported having no or only trace symptoms of facial burning, 63% had no or only trace facial erythema, and 94% had no or trace facial itch, compared with 76%, 12%, and 76% of controls, respectively. The sarecycline group was also significantly more likely to report no or trace skin dryness and oiliness.
The side-effect profile was favorable and the same as encountered with the use of sarecycline for acne: no major photosensitivity issues, no serious adverse events, and only 2 of the original 75 patients in the active-treatment arm discontinued sarecycline for treatment-emergent headache or gastroenteritis considered “probably” related to the study drug. The investigators deemed further studies of sarecycline for rosacea to be warranted as a potential expanded indication.
Aiming for clear skin rather than ‘almost clear’
Dr. Stein Gold shared her mantra for rosacea therapy: “Always aim for clear skin.”
She cited a study led by Guy Webster, MD, professor of dermatology, Thomas Jefferson University, Philadelphia, in which he and his coinvestigators looked at the durability of treatment response in a pooled analysis of 1,366 rosacea patients in four clinical trials. If patients improved to “almost clear” after treatment, their median time to relapse was 3 months; if they reached “clear,” it was more than 8 months. Also, more clear patients rated their outcomes as excellent and reported that their skin disease no longer had any effect on their quality of life.
“That’s more than a 5-month difference,” Dr. Stein Gold noted. “It shows the importance of really striving to get that skin completely clear.”
The sarecycline study was funded by Almirall, which markets the antibiotic. Dr. Stein Gold, who has no financial relationship with Almirall, has received research funding from and/or served as a consultant to roughly a dozen other pharmaceutical companies. MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
National Psoriasis Foundation recommends some stop methotrexate for 2 weeks after J&J vaccine
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
The , Joel M. Gelfand, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
The new guidance states: “Patients 60 or older who have at least one comorbidity associated with an increased risk for poor COVID-19 outcomes, and who are taking methotrexate with well-controlled psoriatic disease, may, in consultation with their prescriber, consider holding it for 2 weeks after receiving the Ad26.COV2.S [Johnson & Johnson] vaccine in order to potentially improve vaccine response.”
The key word here is “potentially.” There is no hard evidence that a 2-week hold on methotrexate after receiving the killed adenovirus vaccine will actually provide a clinically meaningful benefit. But it’s a hypothetical possibility. The rationale stems from a small randomized trial conducted in South Korea several years ago in which patients with rheumatoid arthritis were assigned to hold or continue their methotrexate for the first 2 weeks after receiving an inactivated-virus influenza vaccine. The antibody response to the vaccine was better in those who temporarily halted their methotrexate, explained Dr. Gelfand, cochair of the NPF COVID-19 Task Force and professor of dermatology and of epidemiology at the University of Pennsylvania, Philadelphia.
“If you have a patient on methotrexate who’s 60 or older and whose psoriasis is completely controlled and quiescent and the patient is concerned about how well the vaccine is going to work, this is a reasonable thing to consider in someone who’s at higher risk for poor outcomes if they get infected,” he said.
If the informed patient wants to continue on methotrexate without interruption, that’s fine, too, in light of the lack of compelling evidence on this issue, the dermatologist added at the conference, sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The NPF task force does not extend the recommendation to consider holding methotrexate in recipients of the mRNA-based Moderna and Pfizer vaccines because of their very different mechanisms of action. Nor is it recommended to hold biologic agents after receiving any of the available COVID-19 vaccines. Studies have shown no altered immunologic response to influenza or pneumococcal vaccines in patients who continued on tumor necrosis factor inhibitors or interleukin-17 inhibitors. The interleukin-23 inhibitors haven’t been studied in this regard.
The task force recommends that most psoriasis patients should continue on treatment throughout the pandemic, and newly diagnosed patients should commence appropriate therapy as if there was no pandemic.
“We’ve learned that many patients who stopped their treatment for psoriatic disease early in the pandemic came to regret that decision because their psoriasis flared and got worse and required reinstitution of therapy,” Dr. Gelfand said. “The current data is largely reassuring that if there is an effect of our therapies on the risk of COVID, it must be rather small and therefore unlikely to be clinically meaningful for our patients.”
Dr. Gelfand reported serving as a consultant to and recipient of institutional research grants from Pfizer and numerous other pharmaceutical companies.
MedscapeLIVE and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY