User login
Catherine Cooper Nellist is editor of Pediatric News and Ob. Gyn. News. She has more than 30 years of experience reporting, writing, and editing stories about clinical medicine and the U.S. health care industry. Prior to taking the helm of these award-winning publications, Catherine covered major medical research meetings throughout the United States and Canada, and had been editor of Clinical Psychiatry News, and Dermatology News. She joined the company in 1984 after graduating magna cum laude from Dickinson College, Carlisle, Pa., with a BA in English.
Go metric: No more liquid medication via teaspoons, the AAP says
Children should no longer be prescribed liquid medications that use teaspoon or tablespoon measurements but should get their liquid medications in syringes using metric-based dosing, the American Academy of Pediatrics Committee on Drugs said in a policy statement.
“Spoons come in many different sizes and are not precise enough to measure a child’s medication,” lead author Dr. Ian M. Paul, professor of pediatrics and public health sciences at Penn State University, Hershey, said in a prepared statement. “For infants and toddlers, a small error – especially if repeated for multiple doses – can quickly become toxic.”
The committee made other specific recommendations about clinical practice and advocacy in that policy statement (Pediatrics 2015 [doi:10.1542/peds.2015-0072]), including:
• Dose orally administered liquid medications using metric-based dosing with milliliters (mL) only, to “avoid confusion and dosing errors associated with common kitchen spoons.” Avoid using alternative abbreviations to mL (such as ml, ML, or cc).
• Dose to the nearest 0.1, 0.5, or 1 mL. Avoid dosing to the hundredth of a milliliter.
• Include leading zeros preceding doses less than 1 mL (such as 0.5 mL) to avoid 10-fold dosing errors, but avoid trailing zeros after decimals.
• Clearly note the concentration (strength) of all orally administered liquid medication (such as milligrams per milliliter [mg/mL]) so the dose can be accurately calculated.
• Review milliliter-based doses with patients and families when you administer or prescribe orally administered liquid to be sure they understand metric dosing units.
• Encourage electronic health record vendors to incorporate use of metric units for orally administered liquid medications; this would “eliminate the ability of providers to prescribe medications using non-milliliter–based dosing regimens,” the statement said. Likewise, advocacy efforts should target pharmacies, hospitals, and health centers so they dispense only orally administered liquid medications with metric dosing on the label.
“Syringes are the preferred dosing device for administering oral liquid medications,” according to the statement, but acceptable alternatives are “cups and spoons calibrated and marked in milliliters.”
The AAP also encourages manufacturers to make changes so “dosing devices” have no “extraneous or unnecessary liquid measure markings that may be confusing to caregivers,” and “should not be significantly larger than the dose described in the labeled dosage to avoid twofold dosing errors.” The academy also wants manufacturers to “eliminate labeling, instructions, and dosing devices that contain units other than metric units.”
“We are calling for a simple, universally recognized standard that will influence how doctors write prescriptions, how pharmacists dispense liquid medications and dosing cups, and how manufacturers print labels on their products,” Dr. Paul said in the statement.
Children should no longer be prescribed liquid medications that use teaspoon or tablespoon measurements but should get their liquid medications in syringes using metric-based dosing, the American Academy of Pediatrics Committee on Drugs said in a policy statement.
“Spoons come in many different sizes and are not precise enough to measure a child’s medication,” lead author Dr. Ian M. Paul, professor of pediatrics and public health sciences at Penn State University, Hershey, said in a prepared statement. “For infants and toddlers, a small error – especially if repeated for multiple doses – can quickly become toxic.”
The committee made other specific recommendations about clinical practice and advocacy in that policy statement (Pediatrics 2015 [doi:10.1542/peds.2015-0072]), including:
• Dose orally administered liquid medications using metric-based dosing with milliliters (mL) only, to “avoid confusion and dosing errors associated with common kitchen spoons.” Avoid using alternative abbreviations to mL (such as ml, ML, or cc).
• Dose to the nearest 0.1, 0.5, or 1 mL. Avoid dosing to the hundredth of a milliliter.
• Include leading zeros preceding doses less than 1 mL (such as 0.5 mL) to avoid 10-fold dosing errors, but avoid trailing zeros after decimals.
• Clearly note the concentration (strength) of all orally administered liquid medication (such as milligrams per milliliter [mg/mL]) so the dose can be accurately calculated.
• Review milliliter-based doses with patients and families when you administer or prescribe orally administered liquid to be sure they understand metric dosing units.
• Encourage electronic health record vendors to incorporate use of metric units for orally administered liquid medications; this would “eliminate the ability of providers to prescribe medications using non-milliliter–based dosing regimens,” the statement said. Likewise, advocacy efforts should target pharmacies, hospitals, and health centers so they dispense only orally administered liquid medications with metric dosing on the label.
“Syringes are the preferred dosing device for administering oral liquid medications,” according to the statement, but acceptable alternatives are “cups and spoons calibrated and marked in milliliters.”
The AAP also encourages manufacturers to make changes so “dosing devices” have no “extraneous or unnecessary liquid measure markings that may be confusing to caregivers,” and “should not be significantly larger than the dose described in the labeled dosage to avoid twofold dosing errors.” The academy also wants manufacturers to “eliminate labeling, instructions, and dosing devices that contain units other than metric units.”
“We are calling for a simple, universally recognized standard that will influence how doctors write prescriptions, how pharmacists dispense liquid medications and dosing cups, and how manufacturers print labels on their products,” Dr. Paul said in the statement.
Children should no longer be prescribed liquid medications that use teaspoon or tablespoon measurements but should get their liquid medications in syringes using metric-based dosing, the American Academy of Pediatrics Committee on Drugs said in a policy statement.
“Spoons come in many different sizes and are not precise enough to measure a child’s medication,” lead author Dr. Ian M. Paul, professor of pediatrics and public health sciences at Penn State University, Hershey, said in a prepared statement. “For infants and toddlers, a small error – especially if repeated for multiple doses – can quickly become toxic.”
The committee made other specific recommendations about clinical practice and advocacy in that policy statement (Pediatrics 2015 [doi:10.1542/peds.2015-0072]), including:
• Dose orally administered liquid medications using metric-based dosing with milliliters (mL) only, to “avoid confusion and dosing errors associated with common kitchen spoons.” Avoid using alternative abbreviations to mL (such as ml, ML, or cc).
• Dose to the nearest 0.1, 0.5, or 1 mL. Avoid dosing to the hundredth of a milliliter.
• Include leading zeros preceding doses less than 1 mL (such as 0.5 mL) to avoid 10-fold dosing errors, but avoid trailing zeros after decimals.
• Clearly note the concentration (strength) of all orally administered liquid medication (such as milligrams per milliliter [mg/mL]) so the dose can be accurately calculated.
• Review milliliter-based doses with patients and families when you administer or prescribe orally administered liquid to be sure they understand metric dosing units.
• Encourage electronic health record vendors to incorporate use of metric units for orally administered liquid medications; this would “eliminate the ability of providers to prescribe medications using non-milliliter–based dosing regimens,” the statement said. Likewise, advocacy efforts should target pharmacies, hospitals, and health centers so they dispense only orally administered liquid medications with metric dosing on the label.
“Syringes are the preferred dosing device for administering oral liquid medications,” according to the statement, but acceptable alternatives are “cups and spoons calibrated and marked in milliliters.”
The AAP also encourages manufacturers to make changes so “dosing devices” have no “extraneous or unnecessary liquid measure markings that may be confusing to caregivers,” and “should not be significantly larger than the dose described in the labeled dosage to avoid twofold dosing errors.” The academy also wants manufacturers to “eliminate labeling, instructions, and dosing devices that contain units other than metric units.”
“We are calling for a simple, universally recognized standard that will influence how doctors write prescriptions, how pharmacists dispense liquid medications and dosing cups, and how manufacturers print labels on their products,” Dr. Paul said in the statement.
Welcome to three new Editorial Advisory Board members!
We are pleased to welcome Dr. Joseph B. Domachowske, Dr. Howard Smart, and Dr. Francis E. Rushton Jr. to the Pediatric News Editorial Advisory Board.
Dr. Domachowske is professor of pediatrics and professor of microbiology and immunology at the State University of New York Upstate Medical University in Syracuse. He serves on the New York State American Academy of Pediatrics Chapter 1 executive committee, volunteers as his district’s immunization champion, and is an appointed member of the New York State Immunization Advisory Council. He also enjoys his work as an AAP PREP-ID editorial board member. His overlapping clinical and research interests include immunization advocacy and studies related to the treatment and prevention of viral respiratory tract infections, particularly respiratory syncytial virus. He has published more than 120 papers and book chapters in these areas. Dr. Domachowske has had the privilege of presenting on his global vaccine advocacy efforts with funding through AAP’s Shot@Life program.
Dr. Rushton Jr. is a clinical professor of pediatrics at the University of South Carolina, Columbia, and medical director of the Quality Through Innovation in Pediatrics (QTIP) network. He has practiced pediatrics in Beaufort, S.C., for 32 years and is the author of “Family Support in Community Pediatrics, Confronting the Challenge. “Dr. Rushton’s academic interests include quality improvement, community pediatrics, early brain development, home visitation, and group well child care.
Dr. Smart practices general pediatrics and adolescent medicine as a member of the Sharp Rees-Stealy Medical Group in San Diego. He is a voluntary assistant clinical professor of pediatrics at the University of California, San Diego, and is currently chief of pediatrics at Sharp Mary Birch Hospital for Women & Newborns. Dr. Smart’s interests include medical informatics, health care IT, and specifically clinical decision support and the use of data to drive clinical quality improvement.
We are pleased to welcome Dr. Joseph B. Domachowske, Dr. Howard Smart, and Dr. Francis E. Rushton Jr. to the Pediatric News Editorial Advisory Board.
Dr. Domachowske is professor of pediatrics and professor of microbiology and immunology at the State University of New York Upstate Medical University in Syracuse. He serves on the New York State American Academy of Pediatrics Chapter 1 executive committee, volunteers as his district’s immunization champion, and is an appointed member of the New York State Immunization Advisory Council. He also enjoys his work as an AAP PREP-ID editorial board member. His overlapping clinical and research interests include immunization advocacy and studies related to the treatment and prevention of viral respiratory tract infections, particularly respiratory syncytial virus. He has published more than 120 papers and book chapters in these areas. Dr. Domachowske has had the privilege of presenting on his global vaccine advocacy efforts with funding through AAP’s Shot@Life program.
Dr. Rushton Jr. is a clinical professor of pediatrics at the University of South Carolina, Columbia, and medical director of the Quality Through Innovation in Pediatrics (QTIP) network. He has practiced pediatrics in Beaufort, S.C., for 32 years and is the author of “Family Support in Community Pediatrics, Confronting the Challenge. “Dr. Rushton’s academic interests include quality improvement, community pediatrics, early brain development, home visitation, and group well child care.
Dr. Smart practices general pediatrics and adolescent medicine as a member of the Sharp Rees-Stealy Medical Group in San Diego. He is a voluntary assistant clinical professor of pediatrics at the University of California, San Diego, and is currently chief of pediatrics at Sharp Mary Birch Hospital for Women & Newborns. Dr. Smart’s interests include medical informatics, health care IT, and specifically clinical decision support and the use of data to drive clinical quality improvement.
We are pleased to welcome Dr. Joseph B. Domachowske, Dr. Howard Smart, and Dr. Francis E. Rushton Jr. to the Pediatric News Editorial Advisory Board.
Dr. Domachowske is professor of pediatrics and professor of microbiology and immunology at the State University of New York Upstate Medical University in Syracuse. He serves on the New York State American Academy of Pediatrics Chapter 1 executive committee, volunteers as his district’s immunization champion, and is an appointed member of the New York State Immunization Advisory Council. He also enjoys his work as an AAP PREP-ID editorial board member. His overlapping clinical and research interests include immunization advocacy and studies related to the treatment and prevention of viral respiratory tract infections, particularly respiratory syncytial virus. He has published more than 120 papers and book chapters in these areas. Dr. Domachowske has had the privilege of presenting on his global vaccine advocacy efforts with funding through AAP’s Shot@Life program.
Dr. Rushton Jr. is a clinical professor of pediatrics at the University of South Carolina, Columbia, and medical director of the Quality Through Innovation in Pediatrics (QTIP) network. He has practiced pediatrics in Beaufort, S.C., for 32 years and is the author of “Family Support in Community Pediatrics, Confronting the Challenge. “Dr. Rushton’s academic interests include quality improvement, community pediatrics, early brain development, home visitation, and group well child care.
Dr. Smart practices general pediatrics and adolescent medicine as a member of the Sharp Rees-Stealy Medical Group in San Diego. He is a voluntary assistant clinical professor of pediatrics at the University of California, San Diego, and is currently chief of pediatrics at Sharp Mary Birch Hospital for Women & Newborns. Dr. Smart’s interests include medical informatics, health care IT, and specifically clinical decision support and the use of data to drive clinical quality improvement.
Legislators, advocates call for paid family and medical leave
Parents’ ability to take paid family or medical leave has a real impact on child development, according to legislators and advocates who support the Family and Medical Insurance Leave Act (H.R. 3712/S. 1810).
Advocacy groups Zero to Three, MomsRising, and the National Partnership for Women & Families held a briefing on Capitol Hill to inform legislators and their staffs about the importance of paid medical leave. They were joined by Rep. Rosa L. DeLauro (D-Conn.) and Sen. Kirsten E. Gillibrand (D-N.Y.), the bill’s sponsors.
"Paid family and medical leave is an urgent imperative in this country. Workers, families, communities, and our economy are suffering because federal lawmakers have not put in place this basic protection to help families when babies are born or adopted, or illness strikes. It has been more than 20 years since the Family and Medical Leave Act became law, and it’s past time to take the next step and guarantee hardworking families paid leave," Debra L. Ness, president of the National Partnership for Women & Families, said in a statement.
Parents’ ability to take paid family or medical leave has a real impact on child development, according to legislators and advocates who support the Family and Medical Insurance Leave Act (H.R. 3712/S. 1810).
Advocacy groups Zero to Three, MomsRising, and the National Partnership for Women & Families held a briefing on Capitol Hill to inform legislators and their staffs about the importance of paid medical leave. They were joined by Rep. Rosa L. DeLauro (D-Conn.) and Sen. Kirsten E. Gillibrand (D-N.Y.), the bill’s sponsors.
"Paid family and medical leave is an urgent imperative in this country. Workers, families, communities, and our economy are suffering because federal lawmakers have not put in place this basic protection to help families when babies are born or adopted, or illness strikes. It has been more than 20 years since the Family and Medical Leave Act became law, and it’s past time to take the next step and guarantee hardworking families paid leave," Debra L. Ness, president of the National Partnership for Women & Families, said in a statement.
Parents’ ability to take paid family or medical leave has a real impact on child development, according to legislators and advocates who support the Family and Medical Insurance Leave Act (H.R. 3712/S. 1810).
Advocacy groups Zero to Three, MomsRising, and the National Partnership for Women & Families held a briefing on Capitol Hill to inform legislators and their staffs about the importance of paid medical leave. They were joined by Rep. Rosa L. DeLauro (D-Conn.) and Sen. Kirsten E. Gillibrand (D-N.Y.), the bill’s sponsors.
"Paid family and medical leave is an urgent imperative in this country. Workers, families, communities, and our economy are suffering because federal lawmakers have not put in place this basic protection to help families when babies are born or adopted, or illness strikes. It has been more than 20 years since the Family and Medical Leave Act became law, and it’s past time to take the next step and guarantee hardworking families paid leave," Debra L. Ness, president of the National Partnership for Women & Families, said in a statement.
Clinical prediction rule identifies risk for rheumatoid arthritis
A cut point of 8 or higher on the Leiden clinical prediction rule is linked to a good predictive value in determining which patients with undifferentiated arthritis are at risk for developing rheumatoid arthritis, according to a meta-analysis of data sets examined with the tool.
A number of research studies have indicated that early aggressive treatment of patients with the first signs of rheumatoid arthritis (RA) can delay or diminish joint damage and functional disability from disease progression. An estimated one-third of patients with undifferentiated arthritis (UA) will progress to RA, while approximately half will remit their disease; the remainder tend to develop other conditions, such as osteoarthritis, psoriatic arthritis, and reactive arthritis. Thus, it is important to identify which patients will progress to RA so they can benefit from early treatment, as well as prevent unnecessary treatment in those who won’t develop RA, the investigators said (Semin. Arthritis Rheum. 2013 Oct. 17 [doi:10.1016/j.semarthrit.2013.08.005]).
Emma McNally of the Royal College of Surgeons in Ireland, Dublin, and her associates performed this meta-analysis, using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and the QUADAS (Quality Assessment of Diagnostic Accuracy Studies). Their search of the MEDLINE, Cochrane Library, EMBASE, Cinahl, and Google Scholar databases identified more than 5,000 papers; they also performed a hand search of references found in the retrieved articles. They winnowed the papers down to four articles containing six data sets. A total of 1,084 patients were included in the analysis of the Leiden clinical prediction rule (CPR), which showed that as the total Leiden CPR score increased, the sensitivity decreased and the specificity increased.
"The pooled data indicate that a cut-point of greater than or equal to 8 is better for ruling in RA, with a higher specificity (0.95) than sensitivity (0.49). However, the cut points of greater than or equal to 9 and greater than or equal to 10 were associated with higher specificities (0.99 [for both])," Ms. McNally and her associates wrote, and the latter "may offer a more optimal marker for ruling in RA and determining initiation of treatment."
The duration of follow-up in the studies in the meta-analysis varied from a minimum of 6 months to 30 months; three data sets followed patients for 1 year, as did the study from which the Leiden CPR was derived. The percentage of UA patients who developed RA ranged from 31% to 76% across the six data sets. Four data sets used a modified version of the Leiden CPR, replacing the variable "severity of morning stiffness" with "duration of morning stiffness."
In two studies, some patients started receiving disease-modifying antirheumatic drugs (DMARDs), and in one study, some patients also received glucocorticoids.
In addition to the fact that some patients were treated during the studies, which would likely increase the discriminative ability of the CPR, other considerations to make when evaluating this meta-analysis include the fact that revised classification criteria have been developed for the diagnosis of RA since the Leiden CPR was developed. The American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 criteria were developed to "facilitate the identification of RA at an earlier stage (prior to the development of bone damage), compared with the 1987 ACR criteria," the investigators explained. A review of the two criteria showed that the new 2010 criteria had a higher sensitivity but lower specificity, compared with the 1987 criteria. That and other factors led the authors to conclude that use of the 1987 criteria in the studies in this meta-analysis was appropriate.
Nonetheless, Ms. McNally and her associates said that no impact-analysis studies of the Leiden CPR have been performed, and assessing the clinical effect of a CPR is key. Such studies, usually randomized, controlled trials, would explore the effect of the rule on "patient outcomes such as progression to RA, prescription of DMARDs, prevention of joint destruction, and disease remission."
"Further studies also need to be conducted to determine the predictive value of the Leiden CPR in patients with UA, if the new ACR/EULAR 2010 criteria for the diagnosis of RA are implemented in clinical practice," they wrote. The results of one study so far suggest that in this situation, the rule might be more useful as a marker of disease persistence of UA, unless the CPR was modified to accommodate the new criteria.
This research was funded by the Health Research Board in Ireland. The authors listed no relevant financial disclosures.
A cut point of 8 or higher on the Leiden clinical prediction rule is linked to a good predictive value in determining which patients with undifferentiated arthritis are at risk for developing rheumatoid arthritis, according to a meta-analysis of data sets examined with the tool.
A number of research studies have indicated that early aggressive treatment of patients with the first signs of rheumatoid arthritis (RA) can delay or diminish joint damage and functional disability from disease progression. An estimated one-third of patients with undifferentiated arthritis (UA) will progress to RA, while approximately half will remit their disease; the remainder tend to develop other conditions, such as osteoarthritis, psoriatic arthritis, and reactive arthritis. Thus, it is important to identify which patients will progress to RA so they can benefit from early treatment, as well as prevent unnecessary treatment in those who won’t develop RA, the investigators said (Semin. Arthritis Rheum. 2013 Oct. 17 [doi:10.1016/j.semarthrit.2013.08.005]).
Emma McNally of the Royal College of Surgeons in Ireland, Dublin, and her associates performed this meta-analysis, using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and the QUADAS (Quality Assessment of Diagnostic Accuracy Studies). Their search of the MEDLINE, Cochrane Library, EMBASE, Cinahl, and Google Scholar databases identified more than 5,000 papers; they also performed a hand search of references found in the retrieved articles. They winnowed the papers down to four articles containing six data sets. A total of 1,084 patients were included in the analysis of the Leiden clinical prediction rule (CPR), which showed that as the total Leiden CPR score increased, the sensitivity decreased and the specificity increased.
"The pooled data indicate that a cut-point of greater than or equal to 8 is better for ruling in RA, with a higher specificity (0.95) than sensitivity (0.49). However, the cut points of greater than or equal to 9 and greater than or equal to 10 were associated with higher specificities (0.99 [for both])," Ms. McNally and her associates wrote, and the latter "may offer a more optimal marker for ruling in RA and determining initiation of treatment."
The duration of follow-up in the studies in the meta-analysis varied from a minimum of 6 months to 30 months; three data sets followed patients for 1 year, as did the study from which the Leiden CPR was derived. The percentage of UA patients who developed RA ranged from 31% to 76% across the six data sets. Four data sets used a modified version of the Leiden CPR, replacing the variable "severity of morning stiffness" with "duration of morning stiffness."
In two studies, some patients started receiving disease-modifying antirheumatic drugs (DMARDs), and in one study, some patients also received glucocorticoids.
In addition to the fact that some patients were treated during the studies, which would likely increase the discriminative ability of the CPR, other considerations to make when evaluating this meta-analysis include the fact that revised classification criteria have been developed for the diagnosis of RA since the Leiden CPR was developed. The American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 criteria were developed to "facilitate the identification of RA at an earlier stage (prior to the development of bone damage), compared with the 1987 ACR criteria," the investigators explained. A review of the two criteria showed that the new 2010 criteria had a higher sensitivity but lower specificity, compared with the 1987 criteria. That and other factors led the authors to conclude that use of the 1987 criteria in the studies in this meta-analysis was appropriate.
Nonetheless, Ms. McNally and her associates said that no impact-analysis studies of the Leiden CPR have been performed, and assessing the clinical effect of a CPR is key. Such studies, usually randomized, controlled trials, would explore the effect of the rule on "patient outcomes such as progression to RA, prescription of DMARDs, prevention of joint destruction, and disease remission."
"Further studies also need to be conducted to determine the predictive value of the Leiden CPR in patients with UA, if the new ACR/EULAR 2010 criteria for the diagnosis of RA are implemented in clinical practice," they wrote. The results of one study so far suggest that in this situation, the rule might be more useful as a marker of disease persistence of UA, unless the CPR was modified to accommodate the new criteria.
This research was funded by the Health Research Board in Ireland. The authors listed no relevant financial disclosures.
A cut point of 8 or higher on the Leiden clinical prediction rule is linked to a good predictive value in determining which patients with undifferentiated arthritis are at risk for developing rheumatoid arthritis, according to a meta-analysis of data sets examined with the tool.
A number of research studies have indicated that early aggressive treatment of patients with the first signs of rheumatoid arthritis (RA) can delay or diminish joint damage and functional disability from disease progression. An estimated one-third of patients with undifferentiated arthritis (UA) will progress to RA, while approximately half will remit their disease; the remainder tend to develop other conditions, such as osteoarthritis, psoriatic arthritis, and reactive arthritis. Thus, it is important to identify which patients will progress to RA so they can benefit from early treatment, as well as prevent unnecessary treatment in those who won’t develop RA, the investigators said (Semin. Arthritis Rheum. 2013 Oct. 17 [doi:10.1016/j.semarthrit.2013.08.005]).
Emma McNally of the Royal College of Surgeons in Ireland, Dublin, and her associates performed this meta-analysis, using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and the QUADAS (Quality Assessment of Diagnostic Accuracy Studies). Their search of the MEDLINE, Cochrane Library, EMBASE, Cinahl, and Google Scholar databases identified more than 5,000 papers; they also performed a hand search of references found in the retrieved articles. They winnowed the papers down to four articles containing six data sets. A total of 1,084 patients were included in the analysis of the Leiden clinical prediction rule (CPR), which showed that as the total Leiden CPR score increased, the sensitivity decreased and the specificity increased.
"The pooled data indicate that a cut-point of greater than or equal to 8 is better for ruling in RA, with a higher specificity (0.95) than sensitivity (0.49). However, the cut points of greater than or equal to 9 and greater than or equal to 10 were associated with higher specificities (0.99 [for both])," Ms. McNally and her associates wrote, and the latter "may offer a more optimal marker for ruling in RA and determining initiation of treatment."
The duration of follow-up in the studies in the meta-analysis varied from a minimum of 6 months to 30 months; three data sets followed patients for 1 year, as did the study from which the Leiden CPR was derived. The percentage of UA patients who developed RA ranged from 31% to 76% across the six data sets. Four data sets used a modified version of the Leiden CPR, replacing the variable "severity of morning stiffness" with "duration of morning stiffness."
In two studies, some patients started receiving disease-modifying antirheumatic drugs (DMARDs), and in one study, some patients also received glucocorticoids.
In addition to the fact that some patients were treated during the studies, which would likely increase the discriminative ability of the CPR, other considerations to make when evaluating this meta-analysis include the fact that revised classification criteria have been developed for the diagnosis of RA since the Leiden CPR was developed. The American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) 2010 criteria were developed to "facilitate the identification of RA at an earlier stage (prior to the development of bone damage), compared with the 1987 ACR criteria," the investigators explained. A review of the two criteria showed that the new 2010 criteria had a higher sensitivity but lower specificity, compared with the 1987 criteria. That and other factors led the authors to conclude that use of the 1987 criteria in the studies in this meta-analysis was appropriate.
Nonetheless, Ms. McNally and her associates said that no impact-analysis studies of the Leiden CPR have been performed, and assessing the clinical effect of a CPR is key. Such studies, usually randomized, controlled trials, would explore the effect of the rule on "patient outcomes such as progression to RA, prescription of DMARDs, prevention of joint destruction, and disease remission."
"Further studies also need to be conducted to determine the predictive value of the Leiden CPR in patients with UA, if the new ACR/EULAR 2010 criteria for the diagnosis of RA are implemented in clinical practice," they wrote. The results of one study so far suggest that in this situation, the rule might be more useful as a marker of disease persistence of UA, unless the CPR was modified to accommodate the new criteria.
This research was funded by the Health Research Board in Ireland. The authors listed no relevant financial disclosures.
FROM SEMINARS IN ARTHRITIS AND RHEUMATISM
Major finding: A cut-point of 8 or higher is good for ruling in RA, with a higher specificity (0.95) than sensitivity (0.49).
Data source: Meta-analysis of four articles with six data sets including a total of 1,084 patients in the analysis of the Leiden clinical prediction rule.
Disclosures: This research was funded by the Health Research Board in Ireland. The authors listed no relevant financial disclosures.
Bone mineral density identifies fracture risk in women over 65
Bone mineral density testing is an effective means of identifying women aged 65 years and older who are at high risk of fracture, according to Jane A. Cauley, Dr.P.H., an epidemiologist at the University of Pittsburgh.
All women aged 65 and over should have a BMD test. In a presentation at the annual meeting of the North American Menopause Society, she related the findings of a 2012 study conducted to determine when the test should be repeated. In that study of 4,957 women aged 67 years and older at baseline who underwent baseline T-score testing, the mean interval until 2% of participants had a hip or clinical vertebral fracture was 25 years for women whose baseline bone mineral density (BMD) T-score was greater than or equal to –1.00, 15.4 years for those whose T-score range was –1.01 to –1.49, 5.5 years for those whose T-score range was –1.50 to –1.99, and 5.4 years for those whose T-score was –2.00 to –2.49 (N. Engl. J. Med. 2012;366:225-33).
Also, in women with BMD T-scores greater than –1.50, osteoporosis developed in less than 10% during 15 years of follow-up.
Based on these findings, Dr. Cauley suggested that an initial BMD test should be recommended for all women aged 65 years and older, and that physicians should reassess the testing interval based on the initial result. If the T-score declines, so should the length of the testing interval.
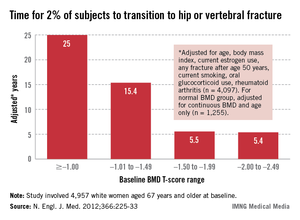
Bone mineral density testing is an effective means of identifying women aged 65 years and older who are at high risk of fracture, according to Jane A. Cauley, Dr.P.H., an epidemiologist at the University of Pittsburgh.
All women aged 65 and over should have a BMD test. In a presentation at the annual meeting of the North American Menopause Society, she related the findings of a 2012 study conducted to determine when the test should be repeated. In that study of 4,957 women aged 67 years and older at baseline who underwent baseline T-score testing, the mean interval until 2% of participants had a hip or clinical vertebral fracture was 25 years for women whose baseline bone mineral density (BMD) T-score was greater than or equal to –1.00, 15.4 years for those whose T-score range was –1.01 to –1.49, 5.5 years for those whose T-score range was –1.50 to –1.99, and 5.4 years for those whose T-score was –2.00 to –2.49 (N. Engl. J. Med. 2012;366:225-33).
Also, in women with BMD T-scores greater than –1.50, osteoporosis developed in less than 10% during 15 years of follow-up.
Based on these findings, Dr. Cauley suggested that an initial BMD test should be recommended for all women aged 65 years and older, and that physicians should reassess the testing interval based on the initial result. If the T-score declines, so should the length of the testing interval.

Bone mineral density testing is an effective means of identifying women aged 65 years and older who are at high risk of fracture, according to Jane A. Cauley, Dr.P.H., an epidemiologist at the University of Pittsburgh.
All women aged 65 and over should have a BMD test. In a presentation at the annual meeting of the North American Menopause Society, she related the findings of a 2012 study conducted to determine when the test should be repeated. In that study of 4,957 women aged 67 years and older at baseline who underwent baseline T-score testing, the mean interval until 2% of participants had a hip or clinical vertebral fracture was 25 years for women whose baseline bone mineral density (BMD) T-score was greater than or equal to –1.00, 15.4 years for those whose T-score range was –1.01 to –1.49, 5.5 years for those whose T-score range was –1.50 to –1.99, and 5.4 years for those whose T-score was –2.00 to –2.49 (N. Engl. J. Med. 2012;366:225-33).
Also, in women with BMD T-scores greater than –1.50, osteoporosis developed in less than 10% during 15 years of follow-up.
Based on these findings, Dr. Cauley suggested that an initial BMD test should be recommended for all women aged 65 years and older, and that physicians should reassess the testing interval based on the initial result. If the T-score declines, so should the length of the testing interval.

Dr. Meadow Maze Good joins Ob.Gyn. News board
Ob.Gyn. News is pleased to welcome Meadow Maze Good, D.O., to its editorial advisory board.
Dr. Good is completing a fellowship in Female Pelvic Medicine and Reconstructive Surgery (FPMRS) at the University of Texas Southwestern Medical Center at Dallas. Dr. Good graduated from the Arizona College of Osteopathic Medicine in Glendale in 2007, and in 2011 she completed her obstetrics and gynecology internship and residency at the University of Texas Southwestern Parkland Memorial Hospital in Dallas.
Dr. Good serves as the American Congress of Obstetricians and Gynecologists (ACOG) Junior Fellow Congress Advisory Council (JFCAC) Chair, representing young ob.gyns. as a voting member on the ACOG National Executive Board. She leads the ACOG Junior Fellow Council on a national initiative relevant to all medical professionals, Social Media Professionalism in the Medical Community. Dr. Good is the narrator in the ACOG video, "Social Media Professionalism," used by institutions and residency programs across the country to educate medical providers, including nurses, medical students, and resident physicians. Additionally, she serves on the ACOG National Patient Safety Council.
Dr. Good has presented and published research on topics relating to FPMRS including: prevention of L5-S1 discitis associated with sacrocolpopexy; vascular and ureteral anatomy; and prolapse-related knowledge and attitudes toward the uterus in women with pelvic organ prolapse.
A recipient of the American Urogynecologic Society Foundation June Allyson Memorial Fund Research Award in 2013, Dr. Good also received the ACOG Excellence in Medical Student Recruitment Award the previous year. In 2011, she was lauded for excellence in medical student teaching by the University of Texas Southwestern Medical Center. In 2011, she also was a fellow at the ACOG Robert C. Cefalo, M.D. National Leadership Institute.
Ob.Gyn. News is pleased to welcome Meadow Maze Good, D.O., to its editorial advisory board.
Dr. Good is completing a fellowship in Female Pelvic Medicine and Reconstructive Surgery (FPMRS) at the University of Texas Southwestern Medical Center at Dallas. Dr. Good graduated from the Arizona College of Osteopathic Medicine in Glendale in 2007, and in 2011 she completed her obstetrics and gynecology internship and residency at the University of Texas Southwestern Parkland Memorial Hospital in Dallas.
Dr. Good serves as the American Congress of Obstetricians and Gynecologists (ACOG) Junior Fellow Congress Advisory Council (JFCAC) Chair, representing young ob.gyns. as a voting member on the ACOG National Executive Board. She leads the ACOG Junior Fellow Council on a national initiative relevant to all medical professionals, Social Media Professionalism in the Medical Community. Dr. Good is the narrator in the ACOG video, "Social Media Professionalism," used by institutions and residency programs across the country to educate medical providers, including nurses, medical students, and resident physicians. Additionally, she serves on the ACOG National Patient Safety Council.
Dr. Good has presented and published research on topics relating to FPMRS including: prevention of L5-S1 discitis associated with sacrocolpopexy; vascular and ureteral anatomy; and prolapse-related knowledge and attitudes toward the uterus in women with pelvic organ prolapse.
A recipient of the American Urogynecologic Society Foundation June Allyson Memorial Fund Research Award in 2013, Dr. Good also received the ACOG Excellence in Medical Student Recruitment Award the previous year. In 2011, she was lauded for excellence in medical student teaching by the University of Texas Southwestern Medical Center. In 2011, she also was a fellow at the ACOG Robert C. Cefalo, M.D. National Leadership Institute.
Ob.Gyn. News is pleased to welcome Meadow Maze Good, D.O., to its editorial advisory board.
Dr. Good is completing a fellowship in Female Pelvic Medicine and Reconstructive Surgery (FPMRS) at the University of Texas Southwestern Medical Center at Dallas. Dr. Good graduated from the Arizona College of Osteopathic Medicine in Glendale in 2007, and in 2011 she completed her obstetrics and gynecology internship and residency at the University of Texas Southwestern Parkland Memorial Hospital in Dallas.
Dr. Good serves as the American Congress of Obstetricians and Gynecologists (ACOG) Junior Fellow Congress Advisory Council (JFCAC) Chair, representing young ob.gyns. as a voting member on the ACOG National Executive Board. She leads the ACOG Junior Fellow Council on a national initiative relevant to all medical professionals, Social Media Professionalism in the Medical Community. Dr. Good is the narrator in the ACOG video, "Social Media Professionalism," used by institutions and residency programs across the country to educate medical providers, including nurses, medical students, and resident physicians. Additionally, she serves on the ACOG National Patient Safety Council.
Dr. Good has presented and published research on topics relating to FPMRS including: prevention of L5-S1 discitis associated with sacrocolpopexy; vascular and ureteral anatomy; and prolapse-related knowledge and attitudes toward the uterus in women with pelvic organ prolapse.
A recipient of the American Urogynecologic Society Foundation June Allyson Memorial Fund Research Award in 2013, Dr. Good also received the ACOG Excellence in Medical Student Recruitment Award the previous year. In 2011, she was lauded for excellence in medical student teaching by the University of Texas Southwestern Medical Center. In 2011, she also was a fellow at the ACOG Robert C. Cefalo, M.D. National Leadership Institute.
Immunization schedule, catch-up plan combined for kids, teens
The recommended childhood and adolescent immunization schedules for 2013 have been approved by the American Academy of Pediatrics, the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention, and the American Academy of Family Physicians.
There was a change in design this year because the complexity of the schedules has increased and there has not been enough space for new information in the footnotes. Thus, there will be a single schedule for children and teens aged 0-18 years rather than divide the schedule into one for children under age 6 years and one for those aged 7-18 years. The catch-up schedule for children and adolescents who start late or are more than 1 month behind is unchanged. Footnotes for all the schedules have been combined (MMWR 2013;62:1-19).
Specific changes from last year include:
• The rotavirus vaccine footnote specifies the number of doses for RV1 and RV5.
• The CDC recommends Tdap for pregnant adolescents and adults during each pregnancy in response to rising cases of pertussis nationally and the appreciation that the greatest burden of disease, morbidity, and mortality occurs in infants before they are protected by their primary series with DTaP.
• The Haemophilus influenzae type b footnote states that only one dose should be given to unvaccinated children 15 months of age or older.
A pocket version of the combined schedule can be found here.
The recommended childhood and adolescent immunization schedules for 2013 have been approved by the American Academy of Pediatrics, the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention, and the American Academy of Family Physicians.
There was a change in design this year because the complexity of the schedules has increased and there has not been enough space for new information in the footnotes. Thus, there will be a single schedule for children and teens aged 0-18 years rather than divide the schedule into one for children under age 6 years and one for those aged 7-18 years. The catch-up schedule for children and adolescents who start late or are more than 1 month behind is unchanged. Footnotes for all the schedules have been combined (MMWR 2013;62:1-19).
Specific changes from last year include:
• The rotavirus vaccine footnote specifies the number of doses for RV1 and RV5.
• The CDC recommends Tdap for pregnant adolescents and adults during each pregnancy in response to rising cases of pertussis nationally and the appreciation that the greatest burden of disease, morbidity, and mortality occurs in infants before they are protected by their primary series with DTaP.
• The Haemophilus influenzae type b footnote states that only one dose should be given to unvaccinated children 15 months of age or older.
A pocket version of the combined schedule can be found here.
The recommended childhood and adolescent immunization schedules for 2013 have been approved by the American Academy of Pediatrics, the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention, and the American Academy of Family Physicians.
There was a change in design this year because the complexity of the schedules has increased and there has not been enough space for new information in the footnotes. Thus, there will be a single schedule for children and teens aged 0-18 years rather than divide the schedule into one for children under age 6 years and one for those aged 7-18 years. The catch-up schedule for children and adolescents who start late or are more than 1 month behind is unchanged. Footnotes for all the schedules have been combined (MMWR 2013;62:1-19).
Specific changes from last year include:
• The rotavirus vaccine footnote specifies the number of doses for RV1 and RV5.
• The CDC recommends Tdap for pregnant adolescents and adults during each pregnancy in response to rising cases of pertussis nationally and the appreciation that the greatest burden of disease, morbidity, and mortality occurs in infants before they are protected by their primary series with DTaP.
• The Haemophilus influenzae type b footnote states that only one dose should be given to unvaccinated children 15 months of age or older.
A pocket version of the combined schedule can be found here.
Pop Warner Football Aims to Limit Concussions
Pop Warner youth football programs have issued new rules to restrict the amount of contact players can have during practice in an attempt to limit head injuries to young players.
Recent research from Virginia Tech led to this change as the organization – which has more than 250,000 children ages 5 to 15 in its leagues – tries to limit concussions.
The study headed by Stefan Duma placed sensors in the helmets of seven youth football players aged 6-8 years during their 2011 season. He found that 95% of impacts were between 15-20 g’s, what he likened to an “aggressive pillow fight.” But the other 5% were 50-100 g’s, what he said was force like a “car accident,” and the majority of these hits took place during practice.
Research has shown that damage from concussions can be cumulative, so Pop Warner is trying to lessen the number of impacts by reducing incidents in practice.
Read the news story in the NY Times.
Pop Warner youth football programs have issued new rules to restrict the amount of contact players can have during practice in an attempt to limit head injuries to young players.
Recent research from Virginia Tech led to this change as the organization – which has more than 250,000 children ages 5 to 15 in its leagues – tries to limit concussions.
The study headed by Stefan Duma placed sensors in the helmets of seven youth football players aged 6-8 years during their 2011 season. He found that 95% of impacts were between 15-20 g’s, what he likened to an “aggressive pillow fight.” But the other 5% were 50-100 g’s, what he said was force like a “car accident,” and the majority of these hits took place during practice.
Research has shown that damage from concussions can be cumulative, so Pop Warner is trying to lessen the number of impacts by reducing incidents in practice.
Read the news story in the NY Times.
Pop Warner youth football programs have issued new rules to restrict the amount of contact players can have during practice in an attempt to limit head injuries to young players.
Recent research from Virginia Tech led to this change as the organization – which has more than 250,000 children ages 5 to 15 in its leagues – tries to limit concussions.
The study headed by Stefan Duma placed sensors in the helmets of seven youth football players aged 6-8 years during their 2011 season. He found that 95% of impacts were between 15-20 g’s, what he likened to an “aggressive pillow fight.” But the other 5% were 50-100 g’s, what he said was force like a “car accident,” and the majority of these hits took place during practice.
Research has shown that damage from concussions can be cumulative, so Pop Warner is trying to lessen the number of impacts by reducing incidents in practice.
Read the news story in the NY Times.
As CT Scans Increase, Concern of Radiation Risk Rises
As the use of CT scans in children – especially multiple scans – is increasing, so is concern about the long-term risks of CT radiation exposure.
There are approximately 7 million CT studies performed in children every year in the United States, and the number is increasing approximately 10% per year, according to the Alliance for Radiation Safety in Pediatric Imaging, a consortium of 63 professional societies representing the fields of radiology, pediatrics, and medical physics and radiation safety.
In a 2012 British study of children from birth to age 21 years who underwent multiple CT scans in 1985-2002, the researchers found a small increased risk of leukemia and brain tumors a decade after the first scan. They estimated that for every 10,000 head CT scans performed on children 10 years of age or younger, one case of leukemia and one brain tumor would occur in the decade following the first CT beyond what would have been expected if the children had no CT scans.
But the physicians involved in this study and others are quick to emphasize the benefits of CT scans: that these scans are extremely important in early diagnosis, clinical decision-making, and for saving lives. But much needs to be done to make sure all CT scans are justified and to reduce the doses of radiation associated with them.
The Food and Drug Administration is working to reduce unnecessary radiation exposure for children on several fronts, through recommending manufacturers design new X-ray imaging devices – including CT scanners – to address pediatric safety issues or by writing new safety training materials that emphasize dose reduction for children on existing equipment.
Read a news article in the Washington Post.
As the use of CT scans in children – especially multiple scans – is increasing, so is concern about the long-term risks of CT radiation exposure.
There are approximately 7 million CT studies performed in children every year in the United States, and the number is increasing approximately 10% per year, according to the Alliance for Radiation Safety in Pediatric Imaging, a consortium of 63 professional societies representing the fields of radiology, pediatrics, and medical physics and radiation safety.
In a 2012 British study of children from birth to age 21 years who underwent multiple CT scans in 1985-2002, the researchers found a small increased risk of leukemia and brain tumors a decade after the first scan. They estimated that for every 10,000 head CT scans performed on children 10 years of age or younger, one case of leukemia and one brain tumor would occur in the decade following the first CT beyond what would have been expected if the children had no CT scans.
But the physicians involved in this study and others are quick to emphasize the benefits of CT scans: that these scans are extremely important in early diagnosis, clinical decision-making, and for saving lives. But much needs to be done to make sure all CT scans are justified and to reduce the doses of radiation associated with them.
The Food and Drug Administration is working to reduce unnecessary radiation exposure for children on several fronts, through recommending manufacturers design new X-ray imaging devices – including CT scanners – to address pediatric safety issues or by writing new safety training materials that emphasize dose reduction for children on existing equipment.
Read a news article in the Washington Post.
As the use of CT scans in children – especially multiple scans – is increasing, so is concern about the long-term risks of CT radiation exposure.
There are approximately 7 million CT studies performed in children every year in the United States, and the number is increasing approximately 10% per year, according to the Alliance for Radiation Safety in Pediatric Imaging, a consortium of 63 professional societies representing the fields of radiology, pediatrics, and medical physics and radiation safety.
In a 2012 British study of children from birth to age 21 years who underwent multiple CT scans in 1985-2002, the researchers found a small increased risk of leukemia and brain tumors a decade after the first scan. They estimated that for every 10,000 head CT scans performed on children 10 years of age or younger, one case of leukemia and one brain tumor would occur in the decade following the first CT beyond what would have been expected if the children had no CT scans.
But the physicians involved in this study and others are quick to emphasize the benefits of CT scans: that these scans are extremely important in early diagnosis, clinical decision-making, and for saving lives. But much needs to be done to make sure all CT scans are justified and to reduce the doses of radiation associated with them.
The Food and Drug Administration is working to reduce unnecessary radiation exposure for children on several fronts, through recommending manufacturers design new X-ray imaging devices – including CT scanners – to address pediatric safety issues or by writing new safety training materials that emphasize dose reduction for children on existing equipment.
Read a news article in the Washington Post.
National Marfan Foundation Launches Mobile Web Site
The National Marfan Foundation is launching a new mobile Web site, www.MarfanDX.org
The site is compatible with Droid and iPhone smartphones.
The mobile site, which is also viewable on a desktop computer, is compatible with the Safari and Firefox browsers.
The site content is based on the new criteria for Marfan syndrome, and it also provides differential diagnosis and management for related conditions.
The site includes:
▸ Seven simple formulae for diagnosing Marfan syndrome.
▸ An interactive z-score calculator, which can be used to determine the size of the aorta compared with body surface area.
▸ Key points about the role of genetic testing and family history.
The National Marfan Foundation is launching a new mobile Web site, www.MarfanDX.org
The site is compatible with Droid and iPhone smartphones.
The mobile site, which is also viewable on a desktop computer, is compatible with the Safari and Firefox browsers.
The site content is based on the new criteria for Marfan syndrome, and it also provides differential diagnosis and management for related conditions.
The site includes:
▸ Seven simple formulae for diagnosing Marfan syndrome.
▸ An interactive z-score calculator, which can be used to determine the size of the aorta compared with body surface area.
▸ Key points about the role of genetic testing and family history.
The National Marfan Foundation is launching a new mobile Web site, www.MarfanDX.org
The site is compatible with Droid and iPhone smartphones.
The mobile site, which is also viewable on a desktop computer, is compatible with the Safari and Firefox browsers.
The site content is based on the new criteria for Marfan syndrome, and it also provides differential diagnosis and management for related conditions.
The site includes:
▸ Seven simple formulae for diagnosing Marfan syndrome.
▸ An interactive z-score calculator, which can be used to determine the size of the aorta compared with body surface area.
▸ Key points about the role of genetic testing and family history.