User login
Catherine Cooper Nellist is editor of Pediatric News and Ob. Gyn. News. She has more than 30 years of experience reporting, writing, and editing stories about clinical medicine and the U.S. health care industry. Prior to taking the helm of these award-winning publications, Catherine covered major medical research meetings throughout the United States and Canada, and had been editor of Clinical Psychiatry News, and Dermatology News. She joined the company in 1984 after graduating magna cum laude from Dickinson College, Carlisle, Pa., with a BA in English.
Teen tanning bed bans keep patients from phototherapy
said Daniel J. Lewis and Madeleine Duvic, MD, of the University of Texas MD Anderson Cancer Center, Houston.
To prevent skin cancer, many states and the District of Columbia have set in place age restrictions on the use of commercial tanning beds and banned minors from indoor tanning; another 12 states put in place bans at younger ages. The Food and Drug Administration proposed a policy in 2015 that would keep all minors from indoor tanning, according to Mr. Lewis and Dr. Duvic.
Phototherapy is known to be an effective treatment for psoriasis, mycosis fungoides, and vitiligo. Phototherapy usually is given in physician offices and administered in ultraviolet B for treatment of psoriasis, but studies have found that tanning beds, which emit primarily ultraviolet A, can produce a clinical response in 80% of psoriasis patients, and 53% of patients report using tanning beds for psoriasis treatment, Mr. Lewis and Dr. Duvic said in a letter to the editor in Clinics in Dermatology.
The demanding schedule of visiting a physician’s office three times a week for phototherapy can be hard for some patients, especially children attending school during regular office hours and patients in rural areas. High copayments also can limit access, the authors said.
Despite the risks, making tanning beds “uniformly illegal for minors would be a disservice to patients with limited access to phototherapy. A more effective approach might be to collaborate with the tanning industry to achieve reasonable limits on age and exposure as opposed to an outright ban. At the very least, patients who receive a prescription from a dermatologist should be exempted from a universal ban,” they concluded.
SOURCE: Lewis, DJ and Duvic, M. Clin Dermatol. 2018 Jan-Feb;36(1):104-5
said Daniel J. Lewis and Madeleine Duvic, MD, of the University of Texas MD Anderson Cancer Center, Houston.
To prevent skin cancer, many states and the District of Columbia have set in place age restrictions on the use of commercial tanning beds and banned minors from indoor tanning; another 12 states put in place bans at younger ages. The Food and Drug Administration proposed a policy in 2015 that would keep all minors from indoor tanning, according to Mr. Lewis and Dr. Duvic.
Phototherapy is known to be an effective treatment for psoriasis, mycosis fungoides, and vitiligo. Phototherapy usually is given in physician offices and administered in ultraviolet B for treatment of psoriasis, but studies have found that tanning beds, which emit primarily ultraviolet A, can produce a clinical response in 80% of psoriasis patients, and 53% of patients report using tanning beds for psoriasis treatment, Mr. Lewis and Dr. Duvic said in a letter to the editor in Clinics in Dermatology.
The demanding schedule of visiting a physician’s office three times a week for phototherapy can be hard for some patients, especially children attending school during regular office hours and patients in rural areas. High copayments also can limit access, the authors said.
Despite the risks, making tanning beds “uniformly illegal for minors would be a disservice to patients with limited access to phototherapy. A more effective approach might be to collaborate with the tanning industry to achieve reasonable limits on age and exposure as opposed to an outright ban. At the very least, patients who receive a prescription from a dermatologist should be exempted from a universal ban,” they concluded.
SOURCE: Lewis, DJ and Duvic, M. Clin Dermatol. 2018 Jan-Feb;36(1):104-5
said Daniel J. Lewis and Madeleine Duvic, MD, of the University of Texas MD Anderson Cancer Center, Houston.
To prevent skin cancer, many states and the District of Columbia have set in place age restrictions on the use of commercial tanning beds and banned minors from indoor tanning; another 12 states put in place bans at younger ages. The Food and Drug Administration proposed a policy in 2015 that would keep all minors from indoor tanning, according to Mr. Lewis and Dr. Duvic.
Phototherapy is known to be an effective treatment for psoriasis, mycosis fungoides, and vitiligo. Phototherapy usually is given in physician offices and administered in ultraviolet B for treatment of psoriasis, but studies have found that tanning beds, which emit primarily ultraviolet A, can produce a clinical response in 80% of psoriasis patients, and 53% of patients report using tanning beds for psoriasis treatment, Mr. Lewis and Dr. Duvic said in a letter to the editor in Clinics in Dermatology.
The demanding schedule of visiting a physician’s office three times a week for phototherapy can be hard for some patients, especially children attending school during regular office hours and patients in rural areas. High copayments also can limit access, the authors said.
Despite the risks, making tanning beds “uniformly illegal for minors would be a disservice to patients with limited access to phototherapy. A more effective approach might be to collaborate with the tanning industry to achieve reasonable limits on age and exposure as opposed to an outright ban. At the very least, patients who receive a prescription from a dermatologist should be exempted from a universal ban,” they concluded.
SOURCE: Lewis, DJ and Duvic, M. Clin Dermatol. 2018 Jan-Feb;36(1):104-5
FROM CLINICS IN DERMATOLOGY
Topical steroid reduces atopic dermatitis relapse in children
according to Elena Rubio-Gomis of the Consorcio Hospital General Universitario de Valencia, Spain, and her associates.
Most atopic disease management is based on managing active disease, but atopic dermatitis is a chronic relapsing disease for which long-term maintenance therapies are being sought.
“Fluticasone 0.05% cream is a potent topical corticosteroid with a low systemic absorption and rapid metabolism and clearance,” which argues for low likelihood of causing systemic side effects and possible use in preventing atopic dermatitis relapse, the investigators wrote. And it is not very expensive.
In a randomized, double-blind, placebo-controlled study of 54 children with mild to moderate atopic dermatitis treated with twice-daily fluticasone propionate 0.05% cream up to 2 weeks, 49 children aged 2-10 years entered the second phase of the study. These children were randomly assigned to receive fluticasone propionate or vehicle twice weekly on consecutive days for 16 weeks; treatment was stopped then or at relapse.
Children treated with fluticasone propionate had a 2.7-fold lower risk of relapse than that of children treated with vehicle; 7 of 26 (27%) in the fluticasone propionate group and 13 of 23 patients (57%) in the vehicle group experienced relapse, the investigators wrote. The report was published in Allergologia et Immunopathologia. The median time to relapse with fluticasone propionate exceeded 16 weeks (the duration of the study), while in the vehicle group it was 65 days (a little over 9 weeks).
The treatment with fluticasone propionate and vehicle both were well tolerated. During the second phase of the study, 9 patients (35%) reported at least one adverse event in the fluticasone propionate group and 11 (48%) in the vehicle group. None of these were believed to be related to the drug studied, the investigators said.
SOURCE: Rubio-Gomis E et al. Allergol Immunopathol (Madr). 2018. doi: 10.1016/j.aller.2017.12.001.
according to Elena Rubio-Gomis of the Consorcio Hospital General Universitario de Valencia, Spain, and her associates.
Most atopic disease management is based on managing active disease, but atopic dermatitis is a chronic relapsing disease for which long-term maintenance therapies are being sought.
“Fluticasone 0.05% cream is a potent topical corticosteroid with a low systemic absorption and rapid metabolism and clearance,” which argues for low likelihood of causing systemic side effects and possible use in preventing atopic dermatitis relapse, the investigators wrote. And it is not very expensive.
In a randomized, double-blind, placebo-controlled study of 54 children with mild to moderate atopic dermatitis treated with twice-daily fluticasone propionate 0.05% cream up to 2 weeks, 49 children aged 2-10 years entered the second phase of the study. These children were randomly assigned to receive fluticasone propionate or vehicle twice weekly on consecutive days for 16 weeks; treatment was stopped then or at relapse.
Children treated with fluticasone propionate had a 2.7-fold lower risk of relapse than that of children treated with vehicle; 7 of 26 (27%) in the fluticasone propionate group and 13 of 23 patients (57%) in the vehicle group experienced relapse, the investigators wrote. The report was published in Allergologia et Immunopathologia. The median time to relapse with fluticasone propionate exceeded 16 weeks (the duration of the study), while in the vehicle group it was 65 days (a little over 9 weeks).
The treatment with fluticasone propionate and vehicle both were well tolerated. During the second phase of the study, 9 patients (35%) reported at least one adverse event in the fluticasone propionate group and 11 (48%) in the vehicle group. None of these were believed to be related to the drug studied, the investigators said.
SOURCE: Rubio-Gomis E et al. Allergol Immunopathol (Madr). 2018. doi: 10.1016/j.aller.2017.12.001.
according to Elena Rubio-Gomis of the Consorcio Hospital General Universitario de Valencia, Spain, and her associates.
Most atopic disease management is based on managing active disease, but atopic dermatitis is a chronic relapsing disease for which long-term maintenance therapies are being sought.
“Fluticasone 0.05% cream is a potent topical corticosteroid with a low systemic absorption and rapid metabolism and clearance,” which argues for low likelihood of causing systemic side effects and possible use in preventing atopic dermatitis relapse, the investigators wrote. And it is not very expensive.
In a randomized, double-blind, placebo-controlled study of 54 children with mild to moderate atopic dermatitis treated with twice-daily fluticasone propionate 0.05% cream up to 2 weeks, 49 children aged 2-10 years entered the second phase of the study. These children were randomly assigned to receive fluticasone propionate or vehicle twice weekly on consecutive days for 16 weeks; treatment was stopped then or at relapse.
Children treated with fluticasone propionate had a 2.7-fold lower risk of relapse than that of children treated with vehicle; 7 of 26 (27%) in the fluticasone propionate group and 13 of 23 patients (57%) in the vehicle group experienced relapse, the investigators wrote. The report was published in Allergologia et Immunopathologia. The median time to relapse with fluticasone propionate exceeded 16 weeks (the duration of the study), while in the vehicle group it was 65 days (a little over 9 weeks).
The treatment with fluticasone propionate and vehicle both were well tolerated. During the second phase of the study, 9 patients (35%) reported at least one adverse event in the fluticasone propionate group and 11 (48%) in the vehicle group. None of these were believed to be related to the drug studied, the investigators said.
SOURCE: Rubio-Gomis E et al. Allergol Immunopathol (Madr). 2018. doi: 10.1016/j.aller.2017.12.001.
FROM ALLERGOLOGIA ET IMMUNOPATHOLOGIA
Antihistamines still are prescribed inappropriately for atopic dermatitis
said Alice He, MD, at the Center for Dermatology Research, Wake Forest University, Winston-Salem, N.C., and her associates.
Results of double-blind, randomized trials have found that oral antihistamines do not effectively treat itch associated with atopic dermatitis (AD), and American Academy of Dermatology guidelines state that intermittent short-term use of sedating antihistamines may help insomnia secondary to itch, but should not be substituted for topical treatments in atopic dermatitis management (J Am Acad Dermatol. 2014;71[2]:327-49), the investigators said. Nonetheless, physicians continue to prescribe antihistamines to AD patients.
Sedating antihistamines accounted for the majority of antihistamines prescribed by pediatricians (58%) and dermatologists (70%) for AD. Nonsedating antihistamines accounted for the majority of the antihistamines prescribed by family physicians (84%), internists (100%), and other specialists (55%), the investigators said.
(The sedating antihistamines prescribed included hydroxyzine, diphenhydramine, chlorpheniramine, brompheniramine, and cyproheptadine; nonsedating antihistamines include cetirizine, desloratadine, levocetirizine, loratadine, and fexofenadine.)
Although the AAD guidelines allow intermittent, short-term use of sedating antihistamines to treat AD-associated itch, these medications may be harmful to sleep in terms of reduced sleep quality and decreased REM sleep. Also, studies have shown that their effects can persist into the daytime and have the potential to impair cognitive function, including learning and memory.
“Physicians should consider the potential risks when prescribing sedating antihistamines as adjunctive treatment for atopic dermatitis,” Dr. He and her associates warned in the article in the Journal of the American Academy of Dermatology.
The researchers took a look at the top ten comorbidities reported among visits for AD, because nonsedating antihistamines might have been prescribed for a different diagnosis. There were three conditions for which antihistamines may be indicated: allergic rhinitis (13%), dermatitis attributable to food (8.5%), and conjunctivitis (3%). This “validates antihistamine prescriptions for a maximum of only 24.5% of patients who received antihistamines, indicating that a large proportion of antihistamine prescriptions were prescribed specifically for AD,” they said.
The authors said that, “while histamine does not play a role in AD via H1R [histamine-1 receptor], it may still be relevant in AD pathogenesis through actions on its most recently described receptor, histamine-4 receptor (H4R).”
“The advent of new targeted systemic therapies, such as T-helper 2 cytokine antagonists and H4R antagonists,” may one day help fill “the unmet need for effective treatments for atopic dermatitis,” Dr. He and her associates concluded.
The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories. Dr. He listed no other relevant financial disclosures. Her coauthors disclosed research, speaking, and/or consulting support from a variety of pharmaceutical companies; one author is an employee of Abbvie.
SOURCE: He A et al. J Amer Acad of Dermatol. 2008. doi: 10.1016/j.jaad.2017.12.077.
said Alice He, MD, at the Center for Dermatology Research, Wake Forest University, Winston-Salem, N.C., and her associates.
Results of double-blind, randomized trials have found that oral antihistamines do not effectively treat itch associated with atopic dermatitis (AD), and American Academy of Dermatology guidelines state that intermittent short-term use of sedating antihistamines may help insomnia secondary to itch, but should not be substituted for topical treatments in atopic dermatitis management (J Am Acad Dermatol. 2014;71[2]:327-49), the investigators said. Nonetheless, physicians continue to prescribe antihistamines to AD patients.
Sedating antihistamines accounted for the majority of antihistamines prescribed by pediatricians (58%) and dermatologists (70%) for AD. Nonsedating antihistamines accounted for the majority of the antihistamines prescribed by family physicians (84%), internists (100%), and other specialists (55%), the investigators said.
(The sedating antihistamines prescribed included hydroxyzine, diphenhydramine, chlorpheniramine, brompheniramine, and cyproheptadine; nonsedating antihistamines include cetirizine, desloratadine, levocetirizine, loratadine, and fexofenadine.)
Although the AAD guidelines allow intermittent, short-term use of sedating antihistamines to treat AD-associated itch, these medications may be harmful to sleep in terms of reduced sleep quality and decreased REM sleep. Also, studies have shown that their effects can persist into the daytime and have the potential to impair cognitive function, including learning and memory.
“Physicians should consider the potential risks when prescribing sedating antihistamines as adjunctive treatment for atopic dermatitis,” Dr. He and her associates warned in the article in the Journal of the American Academy of Dermatology.
The researchers took a look at the top ten comorbidities reported among visits for AD, because nonsedating antihistamines might have been prescribed for a different diagnosis. There were three conditions for which antihistamines may be indicated: allergic rhinitis (13%), dermatitis attributable to food (8.5%), and conjunctivitis (3%). This “validates antihistamine prescriptions for a maximum of only 24.5% of patients who received antihistamines, indicating that a large proportion of antihistamine prescriptions were prescribed specifically for AD,” they said.
The authors said that, “while histamine does not play a role in AD via H1R [histamine-1 receptor], it may still be relevant in AD pathogenesis through actions on its most recently described receptor, histamine-4 receptor (H4R).”
“The advent of new targeted systemic therapies, such as T-helper 2 cytokine antagonists and H4R antagonists,” may one day help fill “the unmet need for effective treatments for atopic dermatitis,” Dr. He and her associates concluded.
The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories. Dr. He listed no other relevant financial disclosures. Her coauthors disclosed research, speaking, and/or consulting support from a variety of pharmaceutical companies; one author is an employee of Abbvie.
SOURCE: He A et al. J Amer Acad of Dermatol. 2008. doi: 10.1016/j.jaad.2017.12.077.
said Alice He, MD, at the Center for Dermatology Research, Wake Forest University, Winston-Salem, N.C., and her associates.
Results of double-blind, randomized trials have found that oral antihistamines do not effectively treat itch associated with atopic dermatitis (AD), and American Academy of Dermatology guidelines state that intermittent short-term use of sedating antihistamines may help insomnia secondary to itch, but should not be substituted for topical treatments in atopic dermatitis management (J Am Acad Dermatol. 2014;71[2]:327-49), the investigators said. Nonetheless, physicians continue to prescribe antihistamines to AD patients.
Sedating antihistamines accounted for the majority of antihistamines prescribed by pediatricians (58%) and dermatologists (70%) for AD. Nonsedating antihistamines accounted for the majority of the antihistamines prescribed by family physicians (84%), internists (100%), and other specialists (55%), the investigators said.
(The sedating antihistamines prescribed included hydroxyzine, diphenhydramine, chlorpheniramine, brompheniramine, and cyproheptadine; nonsedating antihistamines include cetirizine, desloratadine, levocetirizine, loratadine, and fexofenadine.)
Although the AAD guidelines allow intermittent, short-term use of sedating antihistamines to treat AD-associated itch, these medications may be harmful to sleep in terms of reduced sleep quality and decreased REM sleep. Also, studies have shown that their effects can persist into the daytime and have the potential to impair cognitive function, including learning and memory.
“Physicians should consider the potential risks when prescribing sedating antihistamines as adjunctive treatment for atopic dermatitis,” Dr. He and her associates warned in the article in the Journal of the American Academy of Dermatology.
The researchers took a look at the top ten comorbidities reported among visits for AD, because nonsedating antihistamines might have been prescribed for a different diagnosis. There were three conditions for which antihistamines may be indicated: allergic rhinitis (13%), dermatitis attributable to food (8.5%), and conjunctivitis (3%). This “validates antihistamine prescriptions for a maximum of only 24.5% of patients who received antihistamines, indicating that a large proportion of antihistamine prescriptions were prescribed specifically for AD,” they said.
The authors said that, “while histamine does not play a role in AD via H1R [histamine-1 receptor], it may still be relevant in AD pathogenesis through actions on its most recently described receptor, histamine-4 receptor (H4R).”
“The advent of new targeted systemic therapies, such as T-helper 2 cytokine antagonists and H4R antagonists,” may one day help fill “the unmet need for effective treatments for atopic dermatitis,” Dr. He and her associates concluded.
The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories. Dr. He listed no other relevant financial disclosures. Her coauthors disclosed research, speaking, and/or consulting support from a variety of pharmaceutical companies; one author is an employee of Abbvie.
SOURCE: He A et al. J Amer Acad of Dermatol. 2008. doi: 10.1016/j.jaad.2017.12.077.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Physicians should consider the potential risks when prescribing sedating antihistamines as adjunctive treatment for atopic dermatitis.
Major finding: Physicians from specialties other than dermatology prescribed antihistamines at a greater proportion of AD visits than dermatologists (26%-44% of visits vs. 22%), with the exception of pediatricians (16%).
Study details: Data from the National Ambulatory Medical Care Survey on 9.9 million physician visits for AD from 2003 to 2012.
Disclosures: The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories. Dr. He listed no other relevant financial disclosures. Her associates disclosed research, speaking and/or consulting support from a variety of pharmaceutical companies. One author was an employee of Abbvie.
Source: He A et al. J Amer Acad of Dermatol. 2008. doi: 10.1016/j.jaad.2017.12.077.
JAK inhibitors look good for severe alopecia areata treatment
said Lucy Yichu Liu, MD, and Brett Andrew King, MD, of Yale University, New Haven, Conn.
Standard medical therapies for alopecia areata – usually topical or injected corticosteroids and allergic contact sensitization – are not very effective for severe disease, particularly alopecia totalis and alopecia universalis. The Janus kinase (JAK) pathway recently has been suggested as a target for treatment.
Dr. Liu and Dr. King reviewed several studies, including a retrospective cohort study of 13 patients aged 12-17 years, in which 7 patients had 100% hair loss and 6 had 20%-70% scalp hair loss. The adolescents were treated with the JAK1/3 inhibitor tofacitinib citrate 5 mg twice daily for 2-16 months (median, 5 months). That led to 93% median improvement in Severity of Alopecia Tool (SALT) score (range, 1%-100%) from baseline. Nine patients experienced hair regrowth. There were mild adverse effects, such as upper respiratory infections and headaches.
In a retrospective cohort study of 90 adults taking tofacitinib at a dosage of 5-10 mg twice daily for 4 months or longer with or without prednisone (300 mg once monthly for three doses), patients were divided into those who were more or less likely to respond based on duration of disease. Of 65 patients with alopecia totalis, or alopecia universalis that had lasted 10 years or less, or alopecia areata, 77% had some hair regrowth; 58% had more than 50% improvement from baseline, and 20% achieved full regrowth of hair, Dr. Liu and Dr. King reported in the Journal of Investigative Dermatology Symposium Proceedings.
“Given the finding in adults that complete scalp hair loss for more than 10 years is less likely to respond to treatment, there may be merit to pursuing treatment, even if only intermittently, in adolescents or even younger patients with stable, severe alopecia areata, to prevent irreversible hair loss in the future,” they wrote.
A patient with alopecia universalis achieved partial scalp hair regrowth and complete eyebrow regrowth with compounded ruxolitinib, a topical JAK inhibitor, according to a 2016 case report. Dr. Liu and Dr. King reported that clinical trials with topical JAK inhibitors, including topical tofacitinib and topical ruxolitinib, currently are ongoing.
SOURCE: Liu LY et al. J Investig Dermatol Symp Proc. 2018 Jan. doi: 10.1016/j.jisp.2017.10.003.
said Lucy Yichu Liu, MD, and Brett Andrew King, MD, of Yale University, New Haven, Conn.
Standard medical therapies for alopecia areata – usually topical or injected corticosteroids and allergic contact sensitization – are not very effective for severe disease, particularly alopecia totalis and alopecia universalis. The Janus kinase (JAK) pathway recently has been suggested as a target for treatment.
Dr. Liu and Dr. King reviewed several studies, including a retrospective cohort study of 13 patients aged 12-17 years, in which 7 patients had 100% hair loss and 6 had 20%-70% scalp hair loss. The adolescents were treated with the JAK1/3 inhibitor tofacitinib citrate 5 mg twice daily for 2-16 months (median, 5 months). That led to 93% median improvement in Severity of Alopecia Tool (SALT) score (range, 1%-100%) from baseline. Nine patients experienced hair regrowth. There were mild adverse effects, such as upper respiratory infections and headaches.
In a retrospective cohort study of 90 adults taking tofacitinib at a dosage of 5-10 mg twice daily for 4 months or longer with or without prednisone (300 mg once monthly for three doses), patients were divided into those who were more or less likely to respond based on duration of disease. Of 65 patients with alopecia totalis, or alopecia universalis that had lasted 10 years or less, or alopecia areata, 77% had some hair regrowth; 58% had more than 50% improvement from baseline, and 20% achieved full regrowth of hair, Dr. Liu and Dr. King reported in the Journal of Investigative Dermatology Symposium Proceedings.
“Given the finding in adults that complete scalp hair loss for more than 10 years is less likely to respond to treatment, there may be merit to pursuing treatment, even if only intermittently, in adolescents or even younger patients with stable, severe alopecia areata, to prevent irreversible hair loss in the future,” they wrote.
A patient with alopecia universalis achieved partial scalp hair regrowth and complete eyebrow regrowth with compounded ruxolitinib, a topical JAK inhibitor, according to a 2016 case report. Dr. Liu and Dr. King reported that clinical trials with topical JAK inhibitors, including topical tofacitinib and topical ruxolitinib, currently are ongoing.
SOURCE: Liu LY et al. J Investig Dermatol Symp Proc. 2018 Jan. doi: 10.1016/j.jisp.2017.10.003.
said Lucy Yichu Liu, MD, and Brett Andrew King, MD, of Yale University, New Haven, Conn.
Standard medical therapies for alopecia areata – usually topical or injected corticosteroids and allergic contact sensitization – are not very effective for severe disease, particularly alopecia totalis and alopecia universalis. The Janus kinase (JAK) pathway recently has been suggested as a target for treatment.
Dr. Liu and Dr. King reviewed several studies, including a retrospective cohort study of 13 patients aged 12-17 years, in which 7 patients had 100% hair loss and 6 had 20%-70% scalp hair loss. The adolescents were treated with the JAK1/3 inhibitor tofacitinib citrate 5 mg twice daily for 2-16 months (median, 5 months). That led to 93% median improvement in Severity of Alopecia Tool (SALT) score (range, 1%-100%) from baseline. Nine patients experienced hair regrowth. There were mild adverse effects, such as upper respiratory infections and headaches.
In a retrospective cohort study of 90 adults taking tofacitinib at a dosage of 5-10 mg twice daily for 4 months or longer with or without prednisone (300 mg once monthly for three doses), patients were divided into those who were more or less likely to respond based on duration of disease. Of 65 patients with alopecia totalis, or alopecia universalis that had lasted 10 years or less, or alopecia areata, 77% had some hair regrowth; 58% had more than 50% improvement from baseline, and 20% achieved full regrowth of hair, Dr. Liu and Dr. King reported in the Journal of Investigative Dermatology Symposium Proceedings.
“Given the finding in adults that complete scalp hair loss for more than 10 years is less likely to respond to treatment, there may be merit to pursuing treatment, even if only intermittently, in adolescents or even younger patients with stable, severe alopecia areata, to prevent irreversible hair loss in the future,” they wrote.
A patient with alopecia universalis achieved partial scalp hair regrowth and complete eyebrow regrowth with compounded ruxolitinib, a topical JAK inhibitor, according to a 2016 case report. Dr. Liu and Dr. King reported that clinical trials with topical JAK inhibitors, including topical tofacitinib and topical ruxolitinib, currently are ongoing.
SOURCE: Liu LY et al. J Investig Dermatol Symp Proc. 2018 Jan. doi: 10.1016/j.jisp.2017.10.003.
FROM JOURNAL OF INVESTIGATIVE DERMATOLOGY SYMPOSIUM PROCEEDINGS
Lower residual RA activity after initial ETN-MTX, better remission chance
There are four factors at baseline in adults with moderately active rheumatoid arthritis that predict those who are most likely to achieve remission with full-dose combo etanercept-methotrexate (ETN-MTX) induction treatment, said Josef S. Smolen, MD, of the University of Vienna, and his associates.
The original PRESERVE study found that after 604 adults with moderately active rheumatoid arthritis achieved low disease activity after 36 weeks of full-dose etanercept (50 mg once weekly) plus methotrexate, subsequent full-dose or reduced-dose (25 mg once weekly) ETN-MTX combos were better at maintaining remission than was methotrexate alone. At the time of the original study, a Disease Activity Score in 28 joints (DAS28) less than 2.6 was considered to denote remission in practice and clinical trials; now that the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) Boolean definition of remission has been published, a DAS28 less than 2.6 no longer indicates remission according to ACR/EULAR criteria, but rather indicates minimal disease activity, the investigators explained.
This study aimed to determine predictors of remission, the researchers said in their article published in Arthritis Research & Therapy.
Those predictors at baseline are young age; body mass index less than 30 kg/m2; lower Health Assessment Questionnaire scores; and lower disease activity – as measured by DAS28, Simplified Disease Activity Index, and Clinical Disease Activity Index.
If the predictors are favorable and the patients have an “early, strong, and durable response to induction therapy, they are most likely to experience a sustained response after biologic tapering or withdrawal,” Dr. Smolen and his associates said.
In other words, the investigators found that, the lower the residual disease activity, the greater the chance of an enduring response.
“Our findings suggest that patients who did not achieve sustained remission in the open-label period and had higher DAS28 at week 36 were more likely to lose remission with maintenance therapy in the double-blind period. Not surprisingly, patients who achieved remission at only week 36, those who sustained remission at only weeks 28 and 36, and those who sustained remission at only weeks 20, 28, and 36, were at higher risk for loss of remission than patients who sustained remission from week 12 to week 36, indicating that depth of disease control is an important predictor of remission loss,” noted Dr. Smolen and his colleagues.
This study was sponsored by Pfizer. Dr. Smolen has received research grants and consulting fees from AbbVie, Pfizer, Roche, and other biopharmaceutical companies. Several of the investigators are employees of Pfizer and hold Pfizer stock. Another author is an employee of inVentiv Health and was contracted by Pfizer to provide statistical support.
SOURCE: Smolen JS et al. Arthritis Res Ther. doi: 10.1186/s13075-017-1484-9.
There are four factors at baseline in adults with moderately active rheumatoid arthritis that predict those who are most likely to achieve remission with full-dose combo etanercept-methotrexate (ETN-MTX) induction treatment, said Josef S. Smolen, MD, of the University of Vienna, and his associates.
The original PRESERVE study found that after 604 adults with moderately active rheumatoid arthritis achieved low disease activity after 36 weeks of full-dose etanercept (50 mg once weekly) plus methotrexate, subsequent full-dose or reduced-dose (25 mg once weekly) ETN-MTX combos were better at maintaining remission than was methotrexate alone. At the time of the original study, a Disease Activity Score in 28 joints (DAS28) less than 2.6 was considered to denote remission in practice and clinical trials; now that the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) Boolean definition of remission has been published, a DAS28 less than 2.6 no longer indicates remission according to ACR/EULAR criteria, but rather indicates minimal disease activity, the investigators explained.
This study aimed to determine predictors of remission, the researchers said in their article published in Arthritis Research & Therapy.
Those predictors at baseline are young age; body mass index less than 30 kg/m2; lower Health Assessment Questionnaire scores; and lower disease activity – as measured by DAS28, Simplified Disease Activity Index, and Clinical Disease Activity Index.
If the predictors are favorable and the patients have an “early, strong, and durable response to induction therapy, they are most likely to experience a sustained response after biologic tapering or withdrawal,” Dr. Smolen and his associates said.
In other words, the investigators found that, the lower the residual disease activity, the greater the chance of an enduring response.
“Our findings suggest that patients who did not achieve sustained remission in the open-label period and had higher DAS28 at week 36 were more likely to lose remission with maintenance therapy in the double-blind period. Not surprisingly, patients who achieved remission at only week 36, those who sustained remission at only weeks 28 and 36, and those who sustained remission at only weeks 20, 28, and 36, were at higher risk for loss of remission than patients who sustained remission from week 12 to week 36, indicating that depth of disease control is an important predictor of remission loss,” noted Dr. Smolen and his colleagues.
This study was sponsored by Pfizer. Dr. Smolen has received research grants and consulting fees from AbbVie, Pfizer, Roche, and other biopharmaceutical companies. Several of the investigators are employees of Pfizer and hold Pfizer stock. Another author is an employee of inVentiv Health and was contracted by Pfizer to provide statistical support.
SOURCE: Smolen JS et al. Arthritis Res Ther. doi: 10.1186/s13075-017-1484-9.
There are four factors at baseline in adults with moderately active rheumatoid arthritis that predict those who are most likely to achieve remission with full-dose combo etanercept-methotrexate (ETN-MTX) induction treatment, said Josef S. Smolen, MD, of the University of Vienna, and his associates.
The original PRESERVE study found that after 604 adults with moderately active rheumatoid arthritis achieved low disease activity after 36 weeks of full-dose etanercept (50 mg once weekly) plus methotrexate, subsequent full-dose or reduced-dose (25 mg once weekly) ETN-MTX combos were better at maintaining remission than was methotrexate alone. At the time of the original study, a Disease Activity Score in 28 joints (DAS28) less than 2.6 was considered to denote remission in practice and clinical trials; now that the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) Boolean definition of remission has been published, a DAS28 less than 2.6 no longer indicates remission according to ACR/EULAR criteria, but rather indicates minimal disease activity, the investigators explained.
This study aimed to determine predictors of remission, the researchers said in their article published in Arthritis Research & Therapy.
Those predictors at baseline are young age; body mass index less than 30 kg/m2; lower Health Assessment Questionnaire scores; and lower disease activity – as measured by DAS28, Simplified Disease Activity Index, and Clinical Disease Activity Index.
If the predictors are favorable and the patients have an “early, strong, and durable response to induction therapy, they are most likely to experience a sustained response after biologic tapering or withdrawal,” Dr. Smolen and his associates said.
In other words, the investigators found that, the lower the residual disease activity, the greater the chance of an enduring response.
“Our findings suggest that patients who did not achieve sustained remission in the open-label period and had higher DAS28 at week 36 were more likely to lose remission with maintenance therapy in the double-blind period. Not surprisingly, patients who achieved remission at only week 36, those who sustained remission at only weeks 28 and 36, and those who sustained remission at only weeks 20, 28, and 36, were at higher risk for loss of remission than patients who sustained remission from week 12 to week 36, indicating that depth of disease control is an important predictor of remission loss,” noted Dr. Smolen and his colleagues.
This study was sponsored by Pfizer. Dr. Smolen has received research grants and consulting fees from AbbVie, Pfizer, Roche, and other biopharmaceutical companies. Several of the investigators are employees of Pfizer and hold Pfizer stock. Another author is an employee of inVentiv Health and was contracted by Pfizer to provide statistical support.
SOURCE: Smolen JS et al. Arthritis Res Ther. doi: 10.1186/s13075-017-1484-9.
FROM ARTHRITIS RESEARCH & THERAPY
Key clinical point:
Major finding: Predictors of remission at baseline are young age; BMI less than 30 kg/m2; lower HAQ scores; and lower disease activity – as measured by DAS28, SDAI, and CDAI.
Study details: Post hoc analysis of 604 patients with moderately active rheumatoid arthritis from the PRESERVE trial.
Disclosures: This study was sponsored by Pfizer. Dr. Smolen has received research grants and consulting fees from AbbVie, Pfizer, Roche, and other biopharmaceutical companies. Several of the investigators are employees of Pfizer and hold Pfizer stock. Another author is an employee of inVentiv Health and was contracted by Pfizer to provide statistical support.
Source: Smolen JS et al. Arthritis Res Ther. doi: 10.1186/s13075-017-1484-9.
Arthritis treatment costs per person held steady from 2008 to 2014
but the rise in proportion of the arthritis patients in the U.S. population led to billions more dollars being spent on this population, reported Amit D. Raval, PhD, and Ami Vyas, PhD, of the University of Rhode Island, Kingston.
Aggregate expenditures for patients with arthritis rose from $584.8 billion in 2008 to $645 billion in 2014, accounting for about 4% of the U.S. gross domestic product in these years, the investigators reported in the Journal of Rheumatology. “The increase in aggregate unadjusted total direct health care expenditures was mainly driven through an increase in weighted population of individuals with arthritis,” which rose from 56.1 million adults with arthritis in 2008 to 65.1 million in 2014. Nonetheless, there was a “slowdown in the intensity of adjusted incremental expenditures and [out of pocket expenditures] specifically from 2013, which led to a huge decline in the aggregate direct healthcare expenditures in 2014.”
During this time period, there was an increase in the percentage of Hispanics, poor, obese, and individuals with activity limitations and mental health disorders among both those with and without arthritis. This is consistent with the study findings that incremental health care expenditures in persons with arthritis were largely driven by difference in age, health status, and chronic conditions, such as hypertension, hyperlipidemia, and heart disease, among those with and without arthritis, Dr. Raval and Dr. Vyas said.
Annual total health care expenditures in persons with arthritis fell from $10,424 in 2008 to $9,910 in 2014. The average annual total out-of-pocket expenditures in 2008 was $1,493, which was 14% of total health care expenditures that year; in 2014, this fell to $1,099. There were similar trends in persons without arthritis, Dr. Raval and Dr. Vyas reported.
The top three expenditure categories in 2008 were outpatient (32.6%), inpatient (29.0%), and prescription drug costs (24.7%); in 2014, these changed to outpatient (33.8%), prescription drug (26.8%), and inpatient costs (26.4%). There are a number of ways to explain these trends, the researchers said. It is likely that outpatient services, such as outpatient orthopedic surgeries, are becoming “a common management option for arthritis.” Also, outpatient medication services now may include administration of injectables such as biologics. There also were “relatively stable” inpatient expenditures from 2008 to 2013 and a decline in 2014. “Our findings are consistent with studies assessing the effect of the Affordable Care Act, such as introduction of the Hospital Readmission Reduction Program,” said Dr. Raval and Dr. Vyas.
SOURCE: Amit R et al. J Rheumatol. 2018 Jan 15. doi: 10.3899/jrheum.170368.
but the rise in proportion of the arthritis patients in the U.S. population led to billions more dollars being spent on this population, reported Amit D. Raval, PhD, and Ami Vyas, PhD, of the University of Rhode Island, Kingston.
Aggregate expenditures for patients with arthritis rose from $584.8 billion in 2008 to $645 billion in 2014, accounting for about 4% of the U.S. gross domestic product in these years, the investigators reported in the Journal of Rheumatology. “The increase in aggregate unadjusted total direct health care expenditures was mainly driven through an increase in weighted population of individuals with arthritis,” which rose from 56.1 million adults with arthritis in 2008 to 65.1 million in 2014. Nonetheless, there was a “slowdown in the intensity of adjusted incremental expenditures and [out of pocket expenditures] specifically from 2013, which led to a huge decline in the aggregate direct healthcare expenditures in 2014.”
During this time period, there was an increase in the percentage of Hispanics, poor, obese, and individuals with activity limitations and mental health disorders among both those with and without arthritis. This is consistent with the study findings that incremental health care expenditures in persons with arthritis were largely driven by difference in age, health status, and chronic conditions, such as hypertension, hyperlipidemia, and heart disease, among those with and without arthritis, Dr. Raval and Dr. Vyas said.
Annual total health care expenditures in persons with arthritis fell from $10,424 in 2008 to $9,910 in 2014. The average annual total out-of-pocket expenditures in 2008 was $1,493, which was 14% of total health care expenditures that year; in 2014, this fell to $1,099. There were similar trends in persons without arthritis, Dr. Raval and Dr. Vyas reported.
The top three expenditure categories in 2008 were outpatient (32.6%), inpatient (29.0%), and prescription drug costs (24.7%); in 2014, these changed to outpatient (33.8%), prescription drug (26.8%), and inpatient costs (26.4%). There are a number of ways to explain these trends, the researchers said. It is likely that outpatient services, such as outpatient orthopedic surgeries, are becoming “a common management option for arthritis.” Also, outpatient medication services now may include administration of injectables such as biologics. There also were “relatively stable” inpatient expenditures from 2008 to 2013 and a decline in 2014. “Our findings are consistent with studies assessing the effect of the Affordable Care Act, such as introduction of the Hospital Readmission Reduction Program,” said Dr. Raval and Dr. Vyas.
SOURCE: Amit R et al. J Rheumatol. 2018 Jan 15. doi: 10.3899/jrheum.170368.
but the rise in proportion of the arthritis patients in the U.S. population led to billions more dollars being spent on this population, reported Amit D. Raval, PhD, and Ami Vyas, PhD, of the University of Rhode Island, Kingston.
Aggregate expenditures for patients with arthritis rose from $584.8 billion in 2008 to $645 billion in 2014, accounting for about 4% of the U.S. gross domestic product in these years, the investigators reported in the Journal of Rheumatology. “The increase in aggregate unadjusted total direct health care expenditures was mainly driven through an increase in weighted population of individuals with arthritis,” which rose from 56.1 million adults with arthritis in 2008 to 65.1 million in 2014. Nonetheless, there was a “slowdown in the intensity of adjusted incremental expenditures and [out of pocket expenditures] specifically from 2013, which led to a huge decline in the aggregate direct healthcare expenditures in 2014.”
During this time period, there was an increase in the percentage of Hispanics, poor, obese, and individuals with activity limitations and mental health disorders among both those with and without arthritis. This is consistent with the study findings that incremental health care expenditures in persons with arthritis were largely driven by difference in age, health status, and chronic conditions, such as hypertension, hyperlipidemia, and heart disease, among those with and without arthritis, Dr. Raval and Dr. Vyas said.
Annual total health care expenditures in persons with arthritis fell from $10,424 in 2008 to $9,910 in 2014. The average annual total out-of-pocket expenditures in 2008 was $1,493, which was 14% of total health care expenditures that year; in 2014, this fell to $1,099. There were similar trends in persons without arthritis, Dr. Raval and Dr. Vyas reported.
The top three expenditure categories in 2008 were outpatient (32.6%), inpatient (29.0%), and prescription drug costs (24.7%); in 2014, these changed to outpatient (33.8%), prescription drug (26.8%), and inpatient costs (26.4%). There are a number of ways to explain these trends, the researchers said. It is likely that outpatient services, such as outpatient orthopedic surgeries, are becoming “a common management option for arthritis.” Also, outpatient medication services now may include administration of injectables such as biologics. There also were “relatively stable” inpatient expenditures from 2008 to 2013 and a decline in 2014. “Our findings are consistent with studies assessing the effect of the Affordable Care Act, such as introduction of the Hospital Readmission Reduction Program,” said Dr. Raval and Dr. Vyas.
SOURCE: Amit R et al. J Rheumatol. 2018 Jan 15. doi: 10.3899/jrheum.170368.
FROM JOURNAL OF RHEUMATOLOGY
Key clinical point: The annual direct health care expenditures per person with arthritis remained relatively stable over the years 2008 to 2014.
Major finding: Aggregate expenditures for patients with arthritis rose from $584.8 billion in 2008 to $645 billion in 2014.
Study details: Medical Expenditure Panel Survey (MEPS) data from approximately 5,000-6,000 persons aged 18 years and older with arthritis each year during 2008-2014 and approximately 17,000-20,000 persons without arthritis each year during that period.
Disclosures: No information on relevant financial disclosures was evident.
Source: Amit R et al. J Rheumatol. 2018 Jan 15. doi: 10.3899/jrheum.170368.
Topical retinoid plus benzoyl peroxide beats antibiotics for inflammatory acne
, according to the consensus of an international panel of dermatologists, led by Diane M. Thiboutot, MD, professor of dermatology, Penn State University, Hershey.
Dr. Thiboutot is the chair of the Global Alliance to Improve Outcomes in Acne, an international group of dermatologists interested in acne research and education; they have been meeting regularly since 2001 and continuously evaluate the literature on acne.
A Delphi panel and questionnaire method was used to reach consensus. A panel of 36 dermatologists from 27 countries who were members of the alliance answered a set of questionnaires on selected topics. An online questionnaire on various topics was developed, and panel members were asked to rate agreement with each statement on a 5-point Likert scale. If they selected disagree, strongly disagree, or unable to answer, they were asked to provide a written explanation of what they disagreed with. Ultimately, consensus was reached if 75% of more of the panel members agreed on a statement.
Assessing acne severity
The term “severe acne” currently is perceived to refer to nodular/conglobate acne, which is generally treated with oral isotretinoin. But development of added first-line treatment options means there may be a need for a system of classifying moderately severe, severe, and very severe acne. The 2016 European S3 Acne Guideline uses a 4-point classification system that might help: 1) comedonal acne, 2) mild-moderate papulopustular acne; 3) severe papulopustular acne, moderate nodular acne; and 4) severe nodular acne, conglobate acne. In a similar fashion, the U.S. Food and Drug Administration’s Investigator’s Global Assessment scale considers quality of lesions and quantity, including a grade of severe acne that is separate from nodular/conglobate acne.
“We propose that the designation “very severe” be reserved for cystic and conglobate acne,” Dr. Thiboutot and her associates wrote.
Strategic approach to acne therapy
First-line therapy for most patients with inflammatory acne, comedonal acne, or both, should be a topical retinoid plus topical benzoyl peroxide (BPO), the panelists agreed.
Topical or systemic antibiotics should not be used as monotherapy because of the rapid development of resistance. All strains of Propionibacterium acnes are sensitive to BPhO.
Topical retinoids (with or without BPO) or azelaic acid are the treatment of choice for maintenance, not topical antibiotics, they said.
Oral isotretinoin is indicated as first-line therapy for very severe (cystic and conglobate) acne. A small percentage of patients, perhaps 15%, experience acne flare on oral isotretinoin. This often can be managed by using low-dose therapy, say 0.5 mg/kg, although some panelists said that in some cases inflammatory flare occurs regardless of dose.
Oral tretinoin therapy should continue until full clearance of acne. More studies are needed to define the total cumulative dose that maintains remission, the alliance members said.
Determining the risk-benefit for using systemic antibiotics needs to balance individual need against public interest in preserving antibiotic effectiveness, the authors said. Antibiotics should be avoided when there are effective alternatives.
Oral antibiotics are indicated if inflammatory acne isn’t responding well to topical treatments, and acne involves the trunk or multiple bodily areas. Evaluate response to therapy at 6-8 weeks, and don’t treat for more than 3-4 months. After stopping antibiotics, use a topical retinoid and BPO or azelaic acid, they said.
The article, published in the Journal of the American Academy of Dermatology, provides an acne management algorithm that summarizes a treatment approach on the basis of these consensus recommendations.
Special populations
The panelists largely agreed that azelaic acid cream 20% or gel 15% can be useful to treat acne in pregnant women and patients with acne and postinflammatory hyperpigmention, although some said it can cause irritation and aggravate already inflamed skin.
In patients with inflammatory acne, devices such as laser, intense pulsed light, and photodynamic therapy should not be considered first-line treatment, they concluded.
The article also includes clinical pearls for treating acne and postinflammatory hyperpigmentation, questions to ask when taking an acne scar history, an atrophic acne scar risk-assessment tool, clinical pearls for preventing atrophic acne scars, interventions for treating facial atrophic acne scars, clinical pearls for preventing and managing hypertrophic or keloidal scars, and clinical pearls regarding acne in women.
The study is supported by an unrestricted educational grant from Galderma International SAS, Paris. All authors have served as advisory board members for Galderma and received honoraria. Dr. Thiboutot has received fees and research funding for serving as a consultant and investigator for Allergan, Mimetica, Novan, and Sebacia and as a consultant for Dermira, Galderma, Photosonix, and Xenon. Many of the other alliance members have financial associations with biopharmaceutical companies.
SOURCE: Thiboutot DM et al. J Am Acad Dermatol. 2018 Feb. doi: 10.1016/j.jaad.2017.09.078.
, according to the consensus of an international panel of dermatologists, led by Diane M. Thiboutot, MD, professor of dermatology, Penn State University, Hershey.
Dr. Thiboutot is the chair of the Global Alliance to Improve Outcomes in Acne, an international group of dermatologists interested in acne research and education; they have been meeting regularly since 2001 and continuously evaluate the literature on acne.
A Delphi panel and questionnaire method was used to reach consensus. A panel of 36 dermatologists from 27 countries who were members of the alliance answered a set of questionnaires on selected topics. An online questionnaire on various topics was developed, and panel members were asked to rate agreement with each statement on a 5-point Likert scale. If they selected disagree, strongly disagree, or unable to answer, they were asked to provide a written explanation of what they disagreed with. Ultimately, consensus was reached if 75% of more of the panel members agreed on a statement.
Assessing acne severity
The term “severe acne” currently is perceived to refer to nodular/conglobate acne, which is generally treated with oral isotretinoin. But development of added first-line treatment options means there may be a need for a system of classifying moderately severe, severe, and very severe acne. The 2016 European S3 Acne Guideline uses a 4-point classification system that might help: 1) comedonal acne, 2) mild-moderate papulopustular acne; 3) severe papulopustular acne, moderate nodular acne; and 4) severe nodular acne, conglobate acne. In a similar fashion, the U.S. Food and Drug Administration’s Investigator’s Global Assessment scale considers quality of lesions and quantity, including a grade of severe acne that is separate from nodular/conglobate acne.
“We propose that the designation “very severe” be reserved for cystic and conglobate acne,” Dr. Thiboutot and her associates wrote.
Strategic approach to acne therapy
First-line therapy for most patients with inflammatory acne, comedonal acne, or both, should be a topical retinoid plus topical benzoyl peroxide (BPO), the panelists agreed.
Topical or systemic antibiotics should not be used as monotherapy because of the rapid development of resistance. All strains of Propionibacterium acnes are sensitive to BPhO.
Topical retinoids (with or without BPO) or azelaic acid are the treatment of choice for maintenance, not topical antibiotics, they said.
Oral isotretinoin is indicated as first-line therapy for very severe (cystic and conglobate) acne. A small percentage of patients, perhaps 15%, experience acne flare on oral isotretinoin. This often can be managed by using low-dose therapy, say 0.5 mg/kg, although some panelists said that in some cases inflammatory flare occurs regardless of dose.
Oral tretinoin therapy should continue until full clearance of acne. More studies are needed to define the total cumulative dose that maintains remission, the alliance members said.
Determining the risk-benefit for using systemic antibiotics needs to balance individual need against public interest in preserving antibiotic effectiveness, the authors said. Antibiotics should be avoided when there are effective alternatives.
Oral antibiotics are indicated if inflammatory acne isn’t responding well to topical treatments, and acne involves the trunk or multiple bodily areas. Evaluate response to therapy at 6-8 weeks, and don’t treat for more than 3-4 months. After stopping antibiotics, use a topical retinoid and BPO or azelaic acid, they said.
The article, published in the Journal of the American Academy of Dermatology, provides an acne management algorithm that summarizes a treatment approach on the basis of these consensus recommendations.
Special populations
The panelists largely agreed that azelaic acid cream 20% or gel 15% can be useful to treat acne in pregnant women and patients with acne and postinflammatory hyperpigmention, although some said it can cause irritation and aggravate already inflamed skin.
In patients with inflammatory acne, devices such as laser, intense pulsed light, and photodynamic therapy should not be considered first-line treatment, they concluded.
The article also includes clinical pearls for treating acne and postinflammatory hyperpigmentation, questions to ask when taking an acne scar history, an atrophic acne scar risk-assessment tool, clinical pearls for preventing atrophic acne scars, interventions for treating facial atrophic acne scars, clinical pearls for preventing and managing hypertrophic or keloidal scars, and clinical pearls regarding acne in women.
The study is supported by an unrestricted educational grant from Galderma International SAS, Paris. All authors have served as advisory board members for Galderma and received honoraria. Dr. Thiboutot has received fees and research funding for serving as a consultant and investigator for Allergan, Mimetica, Novan, and Sebacia and as a consultant for Dermira, Galderma, Photosonix, and Xenon. Many of the other alliance members have financial associations with biopharmaceutical companies.
SOURCE: Thiboutot DM et al. J Am Acad Dermatol. 2018 Feb. doi: 10.1016/j.jaad.2017.09.078.
, according to the consensus of an international panel of dermatologists, led by Diane M. Thiboutot, MD, professor of dermatology, Penn State University, Hershey.
Dr. Thiboutot is the chair of the Global Alliance to Improve Outcomes in Acne, an international group of dermatologists interested in acne research and education; they have been meeting regularly since 2001 and continuously evaluate the literature on acne.
A Delphi panel and questionnaire method was used to reach consensus. A panel of 36 dermatologists from 27 countries who were members of the alliance answered a set of questionnaires on selected topics. An online questionnaire on various topics was developed, and panel members were asked to rate agreement with each statement on a 5-point Likert scale. If they selected disagree, strongly disagree, or unable to answer, they were asked to provide a written explanation of what they disagreed with. Ultimately, consensus was reached if 75% of more of the panel members agreed on a statement.
Assessing acne severity
The term “severe acne” currently is perceived to refer to nodular/conglobate acne, which is generally treated with oral isotretinoin. But development of added first-line treatment options means there may be a need for a system of classifying moderately severe, severe, and very severe acne. The 2016 European S3 Acne Guideline uses a 4-point classification system that might help: 1) comedonal acne, 2) mild-moderate papulopustular acne; 3) severe papulopustular acne, moderate nodular acne; and 4) severe nodular acne, conglobate acne. In a similar fashion, the U.S. Food and Drug Administration’s Investigator’s Global Assessment scale considers quality of lesions and quantity, including a grade of severe acne that is separate from nodular/conglobate acne.
“We propose that the designation “very severe” be reserved for cystic and conglobate acne,” Dr. Thiboutot and her associates wrote.
Strategic approach to acne therapy
First-line therapy for most patients with inflammatory acne, comedonal acne, or both, should be a topical retinoid plus topical benzoyl peroxide (BPO), the panelists agreed.
Topical or systemic antibiotics should not be used as monotherapy because of the rapid development of resistance. All strains of Propionibacterium acnes are sensitive to BPhO.
Topical retinoids (with or without BPO) or azelaic acid are the treatment of choice for maintenance, not topical antibiotics, they said.
Oral isotretinoin is indicated as first-line therapy for very severe (cystic and conglobate) acne. A small percentage of patients, perhaps 15%, experience acne flare on oral isotretinoin. This often can be managed by using low-dose therapy, say 0.5 mg/kg, although some panelists said that in some cases inflammatory flare occurs regardless of dose.
Oral tretinoin therapy should continue until full clearance of acne. More studies are needed to define the total cumulative dose that maintains remission, the alliance members said.
Determining the risk-benefit for using systemic antibiotics needs to balance individual need against public interest in preserving antibiotic effectiveness, the authors said. Antibiotics should be avoided when there are effective alternatives.
Oral antibiotics are indicated if inflammatory acne isn’t responding well to topical treatments, and acne involves the trunk or multiple bodily areas. Evaluate response to therapy at 6-8 weeks, and don’t treat for more than 3-4 months. After stopping antibiotics, use a topical retinoid and BPO or azelaic acid, they said.
The article, published in the Journal of the American Academy of Dermatology, provides an acne management algorithm that summarizes a treatment approach on the basis of these consensus recommendations.
Special populations
The panelists largely agreed that azelaic acid cream 20% or gel 15% can be useful to treat acne in pregnant women and patients with acne and postinflammatory hyperpigmention, although some said it can cause irritation and aggravate already inflamed skin.
In patients with inflammatory acne, devices such as laser, intense pulsed light, and photodynamic therapy should not be considered first-line treatment, they concluded.
The article also includes clinical pearls for treating acne and postinflammatory hyperpigmentation, questions to ask when taking an acne scar history, an atrophic acne scar risk-assessment tool, clinical pearls for preventing atrophic acne scars, interventions for treating facial atrophic acne scars, clinical pearls for preventing and managing hypertrophic or keloidal scars, and clinical pearls regarding acne in women.
The study is supported by an unrestricted educational grant from Galderma International SAS, Paris. All authors have served as advisory board members for Galderma and received honoraria. Dr. Thiboutot has received fees and research funding for serving as a consultant and investigator for Allergan, Mimetica, Novan, and Sebacia and as a consultant for Dermira, Galderma, Photosonix, and Xenon. Many of the other alliance members have financial associations with biopharmaceutical companies.
SOURCE: Thiboutot DM et al. J Am Acad Dermatol. 2018 Feb. doi: 10.1016/j.jaad.2017.09.078.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
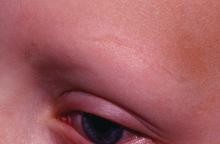
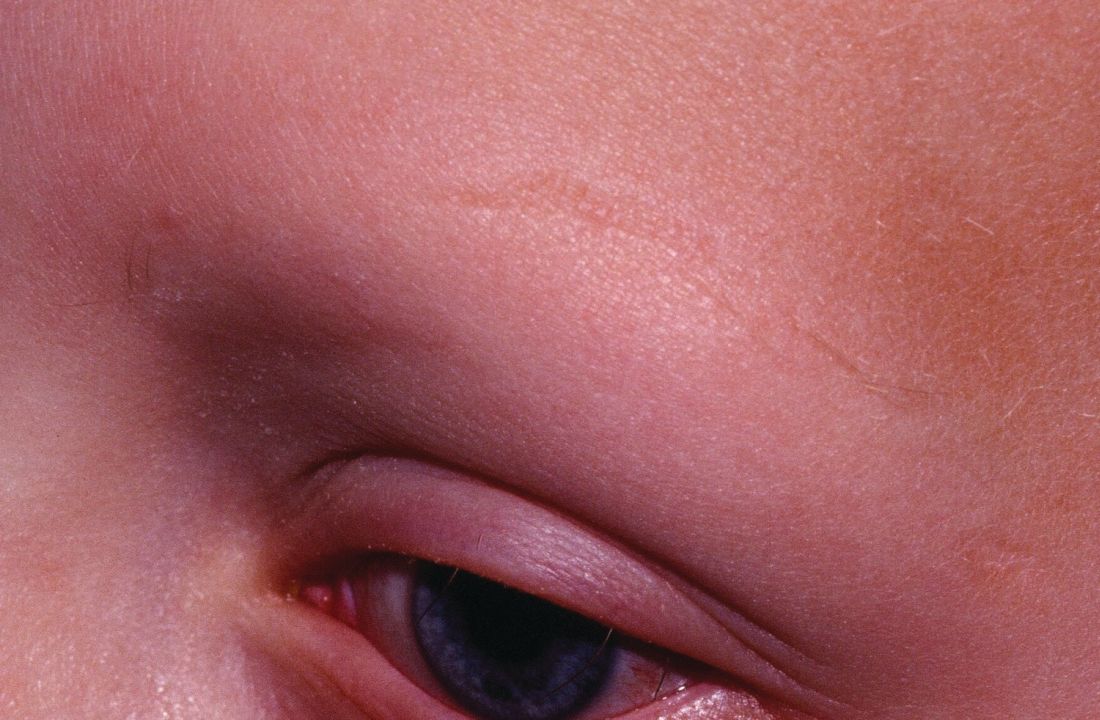
Mood changes reported in cases of methotrexate use for dermatologic disease
, said Trisha Bhat and Carrie C. Coughlin, MD, both of Washington University, St. Louis.
Neurotoxicity with low-dose methotrexate often has been described when used to treat rheumatologic disease, the investigators said, but not in the dermatologic literature – although CNS symptoms such as dizziness and headache have been described in both.
An 8-year-old girl with psoriasis vulgaris and no prior psychiatric history began weekly subcutaneous methotrexate 12.5 mg (0.23 mg/kg) with folic acid supplementation 6 days per week after failing topical therapy. Her parents noticed severe irritability right away and fluctuating mood changes over the next 2 months. At first, she was angry and unsettled, with depressed mood; her irritability became less frequent later, but she said she wanted to hurt someone else. After stopping methotrexate, her mood returned to normal within 2 weeks.
It is unclear how methotrexate affects mood, but recent evidence suggests that abnormalities in synaptic plasticity are involved in mood changes, and there is evidence that mice treated with methotrexate show changes in synaptic plasticity. Methotrexate also increases extracellular adenosine, which affects neuronal excitability and synaptic plasticity, Ms. Bhat and Dr. Coughlin observed. Methotrexate also affects glucose metabolism in rats, and regional metabolic disturbances occur in a number of psychiatric disorders.
Read more at Pediatric Dermatology (2018 Jan 9. doi: 10.1111/pde.13406).
, said Trisha Bhat and Carrie C. Coughlin, MD, both of Washington University, St. Louis.
Neurotoxicity with low-dose methotrexate often has been described when used to treat rheumatologic disease, the investigators said, but not in the dermatologic literature – although CNS symptoms such as dizziness and headache have been described in both.
An 8-year-old girl with psoriasis vulgaris and no prior psychiatric history began weekly subcutaneous methotrexate 12.5 mg (0.23 mg/kg) with folic acid supplementation 6 days per week after failing topical therapy. Her parents noticed severe irritability right away and fluctuating mood changes over the next 2 months. At first, she was angry and unsettled, with depressed mood; her irritability became less frequent later, but she said she wanted to hurt someone else. After stopping methotrexate, her mood returned to normal within 2 weeks.
It is unclear how methotrexate affects mood, but recent evidence suggests that abnormalities in synaptic plasticity are involved in mood changes, and there is evidence that mice treated with methotrexate show changes in synaptic plasticity. Methotrexate also increases extracellular adenosine, which affects neuronal excitability and synaptic plasticity, Ms. Bhat and Dr. Coughlin observed. Methotrexate also affects glucose metabolism in rats, and regional metabolic disturbances occur in a number of psychiatric disorders.
Read more at Pediatric Dermatology (2018 Jan 9. doi: 10.1111/pde.13406).
, said Trisha Bhat and Carrie C. Coughlin, MD, both of Washington University, St. Louis.
Neurotoxicity with low-dose methotrexate often has been described when used to treat rheumatologic disease, the investigators said, but not in the dermatologic literature – although CNS symptoms such as dizziness and headache have been described in both.
An 8-year-old girl with psoriasis vulgaris and no prior psychiatric history began weekly subcutaneous methotrexate 12.5 mg (0.23 mg/kg) with folic acid supplementation 6 days per week after failing topical therapy. Her parents noticed severe irritability right away and fluctuating mood changes over the next 2 months. At first, she was angry and unsettled, with depressed mood; her irritability became less frequent later, but she said she wanted to hurt someone else. After stopping methotrexate, her mood returned to normal within 2 weeks.
It is unclear how methotrexate affects mood, but recent evidence suggests that abnormalities in synaptic plasticity are involved in mood changes, and there is evidence that mice treated with methotrexate show changes in synaptic plasticity. Methotrexate also increases extracellular adenosine, which affects neuronal excitability and synaptic plasticity, Ms. Bhat and Dr. Coughlin observed. Methotrexate also affects glucose metabolism in rats, and regional metabolic disturbances occur in a number of psychiatric disorders.
Read more at Pediatric Dermatology (2018 Jan 9. doi: 10.1111/pde.13406).
FROM PEDIATRIC DERMATOLOGY
When cloth diapers cause diaper dermatitis, think calcineurin inhibitors
, a reemerging complication of diaper dermatitis associated with use of cloth reusable diapers, wrote Rita Ramos Pinheiro, MD, of Hospital Santo António dos Capuchos, Lisbon, and her associates.
An otherwise healthy 18-month-old girl was referred to the dermatology clinic with a 9-month history of severe relapsing diaper dermatitis, which responded to topical clotrimazole and zinc oxide cream. Nondisposable cloth diapers were used. She returned with a rash unresponsive to barrier creams, topical antibiotics and antifungals, and a variety of topical corticosteroids.
Clinically, a diagnosis of granuloma gluteale infantum was suspected; this was confirmed by a skin biopsy of one of the nodules.
The parents declined to stop using cloth diapers, so general measures to alleviate diaper dermatitis were tried, including frequent diaper-free periods. All previous topical treatments were stopped. A 0.1% pimecrolimus cream applied daily for 1 month was well tolerated, and a more potent 0.03% tacrolimus cream then was applied; both medications are topical calcineurin inhibitors.
At 4 weeks, there was complete regression of the ulcerated lesions, with later thinning of the lesions. At the last examination, there remained only slightly hypopigmented residual patches.
The same general measures were continued, including use of barrier creams. The patient had a relapse at her 9-month follow-up, with three nodules occurring on the gluteal region; these resolved with application of topical tacrolimus cream for 1 week.
Although reusable cloth diapers are considered better for the environment and cheaper than disposable diapers, “purportedly” they are less absorbent than disposable diapers, the investigators said.
Characteristics of the clinical diagnosis of granuloma gluteale infantum are “red-purple to red-brown, round to oval, deep, firm nodules with central ulceration,” with a “classic distribution over the convexities of the gluteal region, sparing the inguinal folds,” Dr. Pinheiro and her associates observed. The case study is the first reporting the use of topical calcineurin inhibitors for treating granuloma gluteale infantum, the researchers asserted.
Read more at Pediatrics (2017 Jan 3. doi: 10.1542/peds.2016-2064).
, a reemerging complication of diaper dermatitis associated with use of cloth reusable diapers, wrote Rita Ramos Pinheiro, MD, of Hospital Santo António dos Capuchos, Lisbon, and her associates.
An otherwise healthy 18-month-old girl was referred to the dermatology clinic with a 9-month history of severe relapsing diaper dermatitis, which responded to topical clotrimazole and zinc oxide cream. Nondisposable cloth diapers were used. She returned with a rash unresponsive to barrier creams, topical antibiotics and antifungals, and a variety of topical corticosteroids.
Clinically, a diagnosis of granuloma gluteale infantum was suspected; this was confirmed by a skin biopsy of one of the nodules.
The parents declined to stop using cloth diapers, so general measures to alleviate diaper dermatitis were tried, including frequent diaper-free periods. All previous topical treatments were stopped. A 0.1% pimecrolimus cream applied daily for 1 month was well tolerated, and a more potent 0.03% tacrolimus cream then was applied; both medications are topical calcineurin inhibitors.
At 4 weeks, there was complete regression of the ulcerated lesions, with later thinning of the lesions. At the last examination, there remained only slightly hypopigmented residual patches.
The same general measures were continued, including use of barrier creams. The patient had a relapse at her 9-month follow-up, with three nodules occurring on the gluteal region; these resolved with application of topical tacrolimus cream for 1 week.
Although reusable cloth diapers are considered better for the environment and cheaper than disposable diapers, “purportedly” they are less absorbent than disposable diapers, the investigators said.
Characteristics of the clinical diagnosis of granuloma gluteale infantum are “red-purple to red-brown, round to oval, deep, firm nodules with central ulceration,” with a “classic distribution over the convexities of the gluteal region, sparing the inguinal folds,” Dr. Pinheiro and her associates observed. The case study is the first reporting the use of topical calcineurin inhibitors for treating granuloma gluteale infantum, the researchers asserted.
Read more at Pediatrics (2017 Jan 3. doi: 10.1542/peds.2016-2064).
, a reemerging complication of diaper dermatitis associated with use of cloth reusable diapers, wrote Rita Ramos Pinheiro, MD, of Hospital Santo António dos Capuchos, Lisbon, and her associates.
An otherwise healthy 18-month-old girl was referred to the dermatology clinic with a 9-month history of severe relapsing diaper dermatitis, which responded to topical clotrimazole and zinc oxide cream. Nondisposable cloth diapers were used. She returned with a rash unresponsive to barrier creams, topical antibiotics and antifungals, and a variety of topical corticosteroids.
Clinically, a diagnosis of granuloma gluteale infantum was suspected; this was confirmed by a skin biopsy of one of the nodules.
The parents declined to stop using cloth diapers, so general measures to alleviate diaper dermatitis were tried, including frequent diaper-free periods. All previous topical treatments were stopped. A 0.1% pimecrolimus cream applied daily for 1 month was well tolerated, and a more potent 0.03% tacrolimus cream then was applied; both medications are topical calcineurin inhibitors.
At 4 weeks, there was complete regression of the ulcerated lesions, with later thinning of the lesions. At the last examination, there remained only slightly hypopigmented residual patches.
The same general measures were continued, including use of barrier creams. The patient had a relapse at her 9-month follow-up, with three nodules occurring on the gluteal region; these resolved with application of topical tacrolimus cream for 1 week.
Although reusable cloth diapers are considered better for the environment and cheaper than disposable diapers, “purportedly” they are less absorbent than disposable diapers, the investigators said.
Characteristics of the clinical diagnosis of granuloma gluteale infantum are “red-purple to red-brown, round to oval, deep, firm nodules with central ulceration,” with a “classic distribution over the convexities of the gluteal region, sparing the inguinal folds,” Dr. Pinheiro and her associates observed. The case study is the first reporting the use of topical calcineurin inhibitors for treating granuloma gluteale infantum, the researchers asserted.
Read more at Pediatrics (2017 Jan 3. doi: 10.1542/peds.2016-2064).
FROM PEDIATRICS
Cases link vitiligoid lichen sclerosus and darker skin
, said Margaret H. Dennin, of the University of Chicago, and her associates.
Vitiligoid lichen sclerosus is a superficial variant of lichen sclerosus (LS), in which the lesion clinically appears to be vitiligo, but histologically is consistent with LS.
Seven dark-skinned girls aged 3-9 years had symptomatic (pruritus, pain, bleeding, constipation) depigmented patches of the vulvar or perianal region; three had purpuric lesions. None of the patients had atrophy or scarring, and they had no depigmentation anywhere else on their bodies. Follow-up was an average 2 years (range 3 months to 4 years).
Treatment with high-potency topical steroids, calcineurin inhibitors, or both resulted in improvement or resolution of their symptoms in all cases, but there was mild or no improvement in the depigmentation. Biopsies were not performed because of the patients’ young age and the location of the lesions, the investigators said.
The term vitiligoid lichen sclerosus was first coined in 1961 by Borda et al. when depigmented patches, as seen in both conditions, constituted the clinical appearance, but lacked the inflammation, atrophy, and sclerosis of typical LS. Histologically, these lesions were like LS, “based on the presence of a thin band of papillary dermal sclerosis,” Ms. Dennin and her associates said. Borda et al. suggested that vitiligoid lichen sclerosus might be limited to dark-skinned people, and recent reports support this. Alternatively, it may be that the depigmentation simply is more obvious on dark-skinned people, and asymptomatic cases go unnoticed on lighter-skinned people, the investigators surmised.
Both vitiligo and LS are autoimmune cutaneous disorders, and they both often affect the anogenital region. The conditions “may be linked through a common autoimmune response from exposed intracellular or altered cell surface antigens on damaged melanocytes,” the investigators said. “Histologic evidence demonstrates that development of vitiligo involves a preceding lichenoid inflammatory reaction that may trigger an autoimmune reaction to melanocytes, decreasing their number. Evolving vitiligo with a lichenoid reaction may result in epitope spreading and the development of LS.”
The study is limited by its retrospective nature, small sample size, and lack of biopsies, the researchers noted. Larger studies are needed to look at the overlap of the conditions, and “understand the true prevalence of vitiligoid lichen sclerosus,” Ms. Dennin and her associates said.
Read more in Pediatric Dermatology (2018. doi: 10.1111/pde.13399).
, said Margaret H. Dennin, of the University of Chicago, and her associates.
Vitiligoid lichen sclerosus is a superficial variant of lichen sclerosus (LS), in which the lesion clinically appears to be vitiligo, but histologically is consistent with LS.
Seven dark-skinned girls aged 3-9 years had symptomatic (pruritus, pain, bleeding, constipation) depigmented patches of the vulvar or perianal region; three had purpuric lesions. None of the patients had atrophy or scarring, and they had no depigmentation anywhere else on their bodies. Follow-up was an average 2 years (range 3 months to 4 years).
Treatment with high-potency topical steroids, calcineurin inhibitors, or both resulted in improvement or resolution of their symptoms in all cases, but there was mild or no improvement in the depigmentation. Biopsies were not performed because of the patients’ young age and the location of the lesions, the investigators said.
The term vitiligoid lichen sclerosus was first coined in 1961 by Borda et al. when depigmented patches, as seen in both conditions, constituted the clinical appearance, but lacked the inflammation, atrophy, and sclerosis of typical LS. Histologically, these lesions were like LS, “based on the presence of a thin band of papillary dermal sclerosis,” Ms. Dennin and her associates said. Borda et al. suggested that vitiligoid lichen sclerosus might be limited to dark-skinned people, and recent reports support this. Alternatively, it may be that the depigmentation simply is more obvious on dark-skinned people, and asymptomatic cases go unnoticed on lighter-skinned people, the investigators surmised.
Both vitiligo and LS are autoimmune cutaneous disorders, and they both often affect the anogenital region. The conditions “may be linked through a common autoimmune response from exposed intracellular or altered cell surface antigens on damaged melanocytes,” the investigators said. “Histologic evidence demonstrates that development of vitiligo involves a preceding lichenoid inflammatory reaction that may trigger an autoimmune reaction to melanocytes, decreasing their number. Evolving vitiligo with a lichenoid reaction may result in epitope spreading and the development of LS.”
The study is limited by its retrospective nature, small sample size, and lack of biopsies, the researchers noted. Larger studies are needed to look at the overlap of the conditions, and “understand the true prevalence of vitiligoid lichen sclerosus,” Ms. Dennin and her associates said.
Read more in Pediatric Dermatology (2018. doi: 10.1111/pde.13399).
, said Margaret H. Dennin, of the University of Chicago, and her associates.
Vitiligoid lichen sclerosus is a superficial variant of lichen sclerosus (LS), in which the lesion clinically appears to be vitiligo, but histologically is consistent with LS.
Seven dark-skinned girls aged 3-9 years had symptomatic (pruritus, pain, bleeding, constipation) depigmented patches of the vulvar or perianal region; three had purpuric lesions. None of the patients had atrophy or scarring, and they had no depigmentation anywhere else on their bodies. Follow-up was an average 2 years (range 3 months to 4 years).
Treatment with high-potency topical steroids, calcineurin inhibitors, or both resulted in improvement or resolution of their symptoms in all cases, but there was mild or no improvement in the depigmentation. Biopsies were not performed because of the patients’ young age and the location of the lesions, the investigators said.
The term vitiligoid lichen sclerosus was first coined in 1961 by Borda et al. when depigmented patches, as seen in both conditions, constituted the clinical appearance, but lacked the inflammation, atrophy, and sclerosis of typical LS. Histologically, these lesions were like LS, “based on the presence of a thin band of papillary dermal sclerosis,” Ms. Dennin and her associates said. Borda et al. suggested that vitiligoid lichen sclerosus might be limited to dark-skinned people, and recent reports support this. Alternatively, it may be that the depigmentation simply is more obvious on dark-skinned people, and asymptomatic cases go unnoticed on lighter-skinned people, the investigators surmised.
Both vitiligo and LS are autoimmune cutaneous disorders, and they both often affect the anogenital region. The conditions “may be linked through a common autoimmune response from exposed intracellular or altered cell surface antigens on damaged melanocytes,” the investigators said. “Histologic evidence demonstrates that development of vitiligo involves a preceding lichenoid inflammatory reaction that may trigger an autoimmune reaction to melanocytes, decreasing their number. Evolving vitiligo with a lichenoid reaction may result in epitope spreading and the development of LS.”
The study is limited by its retrospective nature, small sample size, and lack of biopsies, the researchers noted. Larger studies are needed to look at the overlap of the conditions, and “understand the true prevalence of vitiligoid lichen sclerosus,” Ms. Dennin and her associates said.
Read more in Pediatric Dermatology (2018. doi: 10.1111/pde.13399).
FROM PEDIATRIC DERMATOLOGY