User login
The evolving pulmonary landscape in HIV
Chronic pulmonary disease continues to be a major cause of morbidity and mortality in individuals living with the human immunodeficiency virus, even with optimal HIV control. And this is independent, as seen in many studies, of age, smoking, and pulmonary infections.
Both chronic pulmonary obstructive disease (COPD) and lung cancer occur more frequently in people living with HIV than in the general population, and at earlier ages, and with worse outcomes. The risk for emphysema and interstitial lung abnormalities also appears to be higher, research has shown. And asthma has also recently emerged as another important lung disease in people with HIV (PWH).
“There is evidence that the severity of immunocompromise associated with HIV infection is linked with chronic lung diseases. People who have a lower CD4 cell count or a higher viral load do have an increased risk of COPD and emphysema as well as potentially lung cancer. ,” said Kristina Crothers, MD, professor in the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle.
Research has evolved from a focus on the epidemiology of HIV-related chronic lung diseases to a current emphasis on “trying to understand further the mechanisms [behind the heightened risk] through more benchwork and corollary translational studies, and then to the next level of trying to understand what this means for how we should manage people with HIV who have chronic lung diseases,” Dr. Crothers said. “Should management be tailored for people with HIV infection?”
Impairments in immune pathways, local and systemic inflammation, oxidative stress, dysbiosis, and accelerated cellular senescence are among potential mechanisms, but until ongoing mechanistic research yields more answers, pulmonologists should simply – but importantly – be aware of the increased risk and have a low threshold for investigating respiratory symptoms, she and other experts said in interviews. Referral of eligible patients for lung cancer screening is also a priority, as is smoking cessation, they said.
Notably, while spirometry has been the most commonly studied lung function measure in PWH, another noninvasive measure, diffusing capacity for carbon monoxide (DLCO), has garnered attention in the past decade and thus far appears to be the more frequent lung function abnormality.
In an analysis published in 2020 from the longitudinal Multicenter AIDS Cohort Study (MACS) – a study of a subcohort of 591 men with HIV and 476 without HIV – those with HIV were found to have a 1.6-fold increased risk of mild DLCO impairment (< 80% of predicted normal) and a 3-fold higher risk of more severe DLCO impairment (< 60% of predicted normal). There was no significant difference in spirometry findings by HIV status.
Such findings on DLCO are worthy of consideration in clinical practice, even in the absence of HIV-specific screening guidelines for noncommunicable lung diseases, Dr. Crothers said. “In thinking about screening and diagnosing chronic lung diseases in these patients, I’d not only consider spirometry, but also diffusing capacity” when possible, she said. Impaired DLCO is seen with emphysema and pulmonary vascular diseases like pulmonary hypertension and also interstitial lung diseases.
Key chronic lung diseases
Ken M. Kunisaki, MD, MS, associate professor of medicine at the University of Minnesota, Minneapolis, and the first author of the MACS analysis of lung function – one of the most recent and largest reports of DLCO impairment – points out that studies of chest computed tomography (CT) have also documented higher rates of emphysema and interstitial lung abnormalities.
A chest CT analysis from a cohort in Denmark (the Copenhagen Comorbidity in HIV Infection [COCOMO] cohort) found interstitial lung abnormalities in 10.9% of more than 700 PWH which represented a 1.8-fold increased risk compared to HIV-negative controls. And a study from an Italian sample of never-smoking PWH and controls reported emphysema in 18% and 4%, respectively. These studies, which did not measure DLCO, are among those discussed in a 2021 review by Dr. Kunisaki of advances in HIV-associated chronic lung disease research.
COPD is the best studied and most commonly encountered chronic lung disease in PWH. “Particularly for COPD, what’s both interesting and unfortunate is that we haven’t really seen any changes in the epidemiology with ART (antiretroviral therapy) – we’re still seeing the same findings, like the association of HIV with worse COPD at younger ages,” said Alison Morris, MD, MS, professor of medicine, immunology, and clinical and translational research at the University of Pittsburgh. “It doesn’t seem to have improved.”
Its prevalence has varied widely from cohort to cohort, from as low as 3% (similar to the general population) to over 40%, Dr. Kunisaki said, emphasizing that many studies, including studies showing higher rates, have controlled for current and past smoking. In evaluating patients with low or no smoking burden, “don’t discount respiratory symptoms as possibly reflecting underlying lung disease because COPD can develop with low to no smoking history in those with HIV,” he advised.
A better understanding of how a chronic viral infection like HIV leads to heightened COPD risk will not only help those with HIV, he notes, but also people without HIV who have COPD but have never smoked – a woefully underappreciated and understudied population. Ongoing research, he said, “should help us understand COPD pathogenesis generally.”
Research on asthma is relatively limited thus far, but it does appear that PWH may be more prone to developing severe asthma, just as with COPD, said Dr. Kunisaki, also a staff physician at the Minneapolis Veterans Administration Health Care System. Research has shown, for instance, that people with HIV more frequently needed aggressive respiratory support when hospitalized for asthma exacerbations.
It’s unclear how much of this potentially increased severity is attributable to the biology of HIV’s impact on the body and how much relates to social factors like disparities in income and access to care, Dr. Kunisaki said, noting that the same questions apply to the more frequent COPD exacerbations documented in PWH.
Dr. Crothers points out that, while most studies do not suggest a difference in the incidence of asthma in PWH, “there is some data from researchers looking at asthma profiles [suggesting] that the biomarkers associated with asthma may be different in people with and without HIV,” signaling potentially different molecular or biologic underpinnings of the disease.
Incidence rates of lung cancer in PWH, meanwhile, have declined over the last 2 decades, but lung cancer remains the leading cause of cancer-related mortality in PWH and occurs at a rate that is 2-2.5 times higher than that of individuals not infected with HIV, according to
Janice Leung, MD, of the division of respiratory medicine at the University of British Columbia and the Centre for Heart Lung Innovation at St. Paul’s Hospital in Vancouver.
Patients with HIV have “worse outcomes overall and a higher risk of mortality, even when presenting at the same stage,” said Dr. Leung, who reviewed trends in COPD and lung cancer in a recently published opinion piece.
Potential drivers
A bird’s eye view of potential – and likely interrelated – mechanisms for chronic lung disease includes chronic immune activation that impairs innate and adaptive immune pathways; chronic inflammation systemically and in the lung despite viral suppression; persistence of the virus in latent reservoirs in the lung, particularly in alveolar macrophages and T cells; HIV-related proteins contributing to oxidative stress; accelerated cellular aging; dysbiosis; and ongoing injury from inhaled toxins.
All are described in the literature and are being further explored. “It’s likely that multiple pathways are playing a role,” said Dr. Crothers, “and it could be that the balance of one to another leads to different manifestations of disease.”
Biomarkers that have been elevated and associated with different features of chronic lung disease – such as airflow obstruction, low DLCO, and emphysema – include markers of inflammation (e.g., C-reactive protein, interleukin-6), monocyte activation (e.g., soluble CD14), and markers of endothelial dysfunction, she noted in a 2021 commentary marking 40 years since the first reported cases of acquired immunodeficiency syndrome.
In her laboratory, Dr. Leung is using new epigenetic markers to look at the pathogenesis of accelerated aging in the lung. By profiling bronchial epithelial brushings for DNA methylation and gene expression, they have found that “people living with both HIV and COPD have the fastest epigenetic age acceleration in their airway epithelium,” she said. The findings “suggest that the HIV lung is aging faster.”
They reported their findings in 2022, describing methylation disruptions along age-related pathways such as cellular senescence, longevity regulation, and insulin signaling.
Dr. Leung and her team have also studied the lung microbiome and found lower microbial diversity in the airway epithelium in patients with HIV than those without, especially in those with HIV and COPD. The National Institutes of Health–sponsored Lung HIV Microbiome Project found that changes in the lung microbiome are most pronounced in patients who haven’t yet initiated ART, but research in her lab suggests ongoing suppression of microbial diversity even after ART, she said.
Dr. Morris is particularly interested in the oral microbiome, having found through her research that changes in the oral microbiome in PWH were more related to impaired lung function than alterations in the lung and gut microbiome. “That may be in part because of the way we measure things,” she said. “But we also think that the oral microbiome probably seeds the lung [through micro-aspiration].” A study published in 2020 from the Pittsburgh site of the MACS described alterations in oral microbial communities in PWH with abnormal lung function.
Preliminary research suggests that improved dental cleaning and periodontal work in PWH and COPD may influence the severity of COPD, she noted.
“We don’t see as much of a signal with the gut microbiome [and HIV status or lung function], though there could still be ways in which gut microbiome influences the lung,” through systemic inflammation, the release of metabolites into the bloodstream, or microbial translocation, for instance, she said.
The potential role of translocation of members of the microbiome, in fact, is an area of active research for Dr. Morris. Members of the microbiome – viruses and fungi in addition to bacteria – “can get into the bloodstream from the mouth, from the lung, from the gut, to stimulate inflammation and worsen lung disease,” she said.
Key questions in an evolving research landscape
Dr. Kunisaki looks forward to research providing a more longitudinal look at lung function decline– a move beyond a dominance of cross-sectional studies – as well as research that is more comprehensive, with simultaneous collection of various functional measures (eg., DLCO with chest imaging and fractional excretion of nitric oxide (FENO – a standardized breath measure of Th2 airway inflammation).
The several-year-old NIH-supported MACS/WIHS (Women’s Interagency HIV Study) Combined Cohort study, in which Dr. Kunisaki and Dr. Morris participate, aims in part to identity biomarkers of increased risk for chronic lung disease and other chronic disorders and to develop strategies for more effective interventions and treatments.
Researchers will also share biospecimens, “which will allow more mechanistic work,” Dr. Kunisaki noted. (The combined cohort study includes participants from the earlier, separate MACS and WIHS studies.)
Questions about treatment strategies include the risks versus benefits of inhaled corticosteroids, which may increase an already elevated risk of respiratory infections like bacterial pneumonia in PWH, Dr. Kunisaki said.
[An aside: Inhaled corticosteroids also have well-described interactions with ART regimens that contain CYP3A4 inhibitors (e.g., ritonavir and cobicistat) that can lead to hypercortisolism. In patients who require both types of drugs, he said, beclomethasone has the least interactions and is the preferred inhaled corticosteroid.]
For Dr. Crothers, unanswered critical questions include – as she wrote in her 2021 commentary – the question of how guidelines for the management of COPD and asthma should be adapted for PWH. Is COPD in PWH more or less responsive to inhaled corticosteroids, for instance? And are antifibrotic treatments for interstitial lung disease and immunotherapies for asthma or lung cancer similarly effective, and are there any increased risks for harms in people with HIV?
There’s also the question of whether PWH should be screened for lung cancer earlier and with a lower smoking exposure than is advised under current guidelines for the general population, she said in the interview. “And should the approach to shared decision-making be modified for people with HIV?” she said. “We’re doing some work on these questions” right now.
None of the researchers interviewed reported any conflicts of interest relevant to the story. Dr. Kunisaki reported that he has no relevant disclosures, and said that his comments are his personal views and not official views of the U.S. Government, Department of Veterans Affairs, the Minneapolis VA, or the University of Minnesota.
Chronic pulmonary disease continues to be a major cause of morbidity and mortality in individuals living with the human immunodeficiency virus, even with optimal HIV control. And this is independent, as seen in many studies, of age, smoking, and pulmonary infections.
Both chronic pulmonary obstructive disease (COPD) and lung cancer occur more frequently in people living with HIV than in the general population, and at earlier ages, and with worse outcomes. The risk for emphysema and interstitial lung abnormalities also appears to be higher, research has shown. And asthma has also recently emerged as another important lung disease in people with HIV (PWH).
“There is evidence that the severity of immunocompromise associated with HIV infection is linked with chronic lung diseases. People who have a lower CD4 cell count or a higher viral load do have an increased risk of COPD and emphysema as well as potentially lung cancer. ,” said Kristina Crothers, MD, professor in the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle.
Research has evolved from a focus on the epidemiology of HIV-related chronic lung diseases to a current emphasis on “trying to understand further the mechanisms [behind the heightened risk] through more benchwork and corollary translational studies, and then to the next level of trying to understand what this means for how we should manage people with HIV who have chronic lung diseases,” Dr. Crothers said. “Should management be tailored for people with HIV infection?”
Impairments in immune pathways, local and systemic inflammation, oxidative stress, dysbiosis, and accelerated cellular senescence are among potential mechanisms, but until ongoing mechanistic research yields more answers, pulmonologists should simply – but importantly – be aware of the increased risk and have a low threshold for investigating respiratory symptoms, she and other experts said in interviews. Referral of eligible patients for lung cancer screening is also a priority, as is smoking cessation, they said.
Notably, while spirometry has been the most commonly studied lung function measure in PWH, another noninvasive measure, diffusing capacity for carbon monoxide (DLCO), has garnered attention in the past decade and thus far appears to be the more frequent lung function abnormality.
In an analysis published in 2020 from the longitudinal Multicenter AIDS Cohort Study (MACS) – a study of a subcohort of 591 men with HIV and 476 without HIV – those with HIV were found to have a 1.6-fold increased risk of mild DLCO impairment (< 80% of predicted normal) and a 3-fold higher risk of more severe DLCO impairment (< 60% of predicted normal). There was no significant difference in spirometry findings by HIV status.
Such findings on DLCO are worthy of consideration in clinical practice, even in the absence of HIV-specific screening guidelines for noncommunicable lung diseases, Dr. Crothers said. “In thinking about screening and diagnosing chronic lung diseases in these patients, I’d not only consider spirometry, but also diffusing capacity” when possible, she said. Impaired DLCO is seen with emphysema and pulmonary vascular diseases like pulmonary hypertension and also interstitial lung diseases.
Key chronic lung diseases
Ken M. Kunisaki, MD, MS, associate professor of medicine at the University of Minnesota, Minneapolis, and the first author of the MACS analysis of lung function – one of the most recent and largest reports of DLCO impairment – points out that studies of chest computed tomography (CT) have also documented higher rates of emphysema and interstitial lung abnormalities.
A chest CT analysis from a cohort in Denmark (the Copenhagen Comorbidity in HIV Infection [COCOMO] cohort) found interstitial lung abnormalities in 10.9% of more than 700 PWH which represented a 1.8-fold increased risk compared to HIV-negative controls. And a study from an Italian sample of never-smoking PWH and controls reported emphysema in 18% and 4%, respectively. These studies, which did not measure DLCO, are among those discussed in a 2021 review by Dr. Kunisaki of advances in HIV-associated chronic lung disease research.
COPD is the best studied and most commonly encountered chronic lung disease in PWH. “Particularly for COPD, what’s both interesting and unfortunate is that we haven’t really seen any changes in the epidemiology with ART (antiretroviral therapy) – we’re still seeing the same findings, like the association of HIV with worse COPD at younger ages,” said Alison Morris, MD, MS, professor of medicine, immunology, and clinical and translational research at the University of Pittsburgh. “It doesn’t seem to have improved.”
Its prevalence has varied widely from cohort to cohort, from as low as 3% (similar to the general population) to over 40%, Dr. Kunisaki said, emphasizing that many studies, including studies showing higher rates, have controlled for current and past smoking. In evaluating patients with low or no smoking burden, “don’t discount respiratory symptoms as possibly reflecting underlying lung disease because COPD can develop with low to no smoking history in those with HIV,” he advised.
A better understanding of how a chronic viral infection like HIV leads to heightened COPD risk will not only help those with HIV, he notes, but also people without HIV who have COPD but have never smoked – a woefully underappreciated and understudied population. Ongoing research, he said, “should help us understand COPD pathogenesis generally.”
Research on asthma is relatively limited thus far, but it does appear that PWH may be more prone to developing severe asthma, just as with COPD, said Dr. Kunisaki, also a staff physician at the Minneapolis Veterans Administration Health Care System. Research has shown, for instance, that people with HIV more frequently needed aggressive respiratory support when hospitalized for asthma exacerbations.
It’s unclear how much of this potentially increased severity is attributable to the biology of HIV’s impact on the body and how much relates to social factors like disparities in income and access to care, Dr. Kunisaki said, noting that the same questions apply to the more frequent COPD exacerbations documented in PWH.
Dr. Crothers points out that, while most studies do not suggest a difference in the incidence of asthma in PWH, “there is some data from researchers looking at asthma profiles [suggesting] that the biomarkers associated with asthma may be different in people with and without HIV,” signaling potentially different molecular or biologic underpinnings of the disease.
Incidence rates of lung cancer in PWH, meanwhile, have declined over the last 2 decades, but lung cancer remains the leading cause of cancer-related mortality in PWH and occurs at a rate that is 2-2.5 times higher than that of individuals not infected with HIV, according to
Janice Leung, MD, of the division of respiratory medicine at the University of British Columbia and the Centre for Heart Lung Innovation at St. Paul’s Hospital in Vancouver.
Patients with HIV have “worse outcomes overall and a higher risk of mortality, even when presenting at the same stage,” said Dr. Leung, who reviewed trends in COPD and lung cancer in a recently published opinion piece.
Potential drivers
A bird’s eye view of potential – and likely interrelated – mechanisms for chronic lung disease includes chronic immune activation that impairs innate and adaptive immune pathways; chronic inflammation systemically and in the lung despite viral suppression; persistence of the virus in latent reservoirs in the lung, particularly in alveolar macrophages and T cells; HIV-related proteins contributing to oxidative stress; accelerated cellular aging; dysbiosis; and ongoing injury from inhaled toxins.
All are described in the literature and are being further explored. “It’s likely that multiple pathways are playing a role,” said Dr. Crothers, “and it could be that the balance of one to another leads to different manifestations of disease.”
Biomarkers that have been elevated and associated with different features of chronic lung disease – such as airflow obstruction, low DLCO, and emphysema – include markers of inflammation (e.g., C-reactive protein, interleukin-6), monocyte activation (e.g., soluble CD14), and markers of endothelial dysfunction, she noted in a 2021 commentary marking 40 years since the first reported cases of acquired immunodeficiency syndrome.
In her laboratory, Dr. Leung is using new epigenetic markers to look at the pathogenesis of accelerated aging in the lung. By profiling bronchial epithelial brushings for DNA methylation and gene expression, they have found that “people living with both HIV and COPD have the fastest epigenetic age acceleration in their airway epithelium,” she said. The findings “suggest that the HIV lung is aging faster.”
They reported their findings in 2022, describing methylation disruptions along age-related pathways such as cellular senescence, longevity regulation, and insulin signaling.
Dr. Leung and her team have also studied the lung microbiome and found lower microbial diversity in the airway epithelium in patients with HIV than those without, especially in those with HIV and COPD. The National Institutes of Health–sponsored Lung HIV Microbiome Project found that changes in the lung microbiome are most pronounced in patients who haven’t yet initiated ART, but research in her lab suggests ongoing suppression of microbial diversity even after ART, she said.
Dr. Morris is particularly interested in the oral microbiome, having found through her research that changes in the oral microbiome in PWH were more related to impaired lung function than alterations in the lung and gut microbiome. “That may be in part because of the way we measure things,” she said. “But we also think that the oral microbiome probably seeds the lung [through micro-aspiration].” A study published in 2020 from the Pittsburgh site of the MACS described alterations in oral microbial communities in PWH with abnormal lung function.
Preliminary research suggests that improved dental cleaning and periodontal work in PWH and COPD may influence the severity of COPD, she noted.
“We don’t see as much of a signal with the gut microbiome [and HIV status or lung function], though there could still be ways in which gut microbiome influences the lung,” through systemic inflammation, the release of metabolites into the bloodstream, or microbial translocation, for instance, she said.
The potential role of translocation of members of the microbiome, in fact, is an area of active research for Dr. Morris. Members of the microbiome – viruses and fungi in addition to bacteria – “can get into the bloodstream from the mouth, from the lung, from the gut, to stimulate inflammation and worsen lung disease,” she said.
Key questions in an evolving research landscape
Dr. Kunisaki looks forward to research providing a more longitudinal look at lung function decline– a move beyond a dominance of cross-sectional studies – as well as research that is more comprehensive, with simultaneous collection of various functional measures (eg., DLCO with chest imaging and fractional excretion of nitric oxide (FENO – a standardized breath measure of Th2 airway inflammation).
The several-year-old NIH-supported MACS/WIHS (Women’s Interagency HIV Study) Combined Cohort study, in which Dr. Kunisaki and Dr. Morris participate, aims in part to identity biomarkers of increased risk for chronic lung disease and other chronic disorders and to develop strategies for more effective interventions and treatments.
Researchers will also share biospecimens, “which will allow more mechanistic work,” Dr. Kunisaki noted. (The combined cohort study includes participants from the earlier, separate MACS and WIHS studies.)
Questions about treatment strategies include the risks versus benefits of inhaled corticosteroids, which may increase an already elevated risk of respiratory infections like bacterial pneumonia in PWH, Dr. Kunisaki said.
[An aside: Inhaled corticosteroids also have well-described interactions with ART regimens that contain CYP3A4 inhibitors (e.g., ritonavir and cobicistat) that can lead to hypercortisolism. In patients who require both types of drugs, he said, beclomethasone has the least interactions and is the preferred inhaled corticosteroid.]
For Dr. Crothers, unanswered critical questions include – as she wrote in her 2021 commentary – the question of how guidelines for the management of COPD and asthma should be adapted for PWH. Is COPD in PWH more or less responsive to inhaled corticosteroids, for instance? And are antifibrotic treatments for interstitial lung disease and immunotherapies for asthma or lung cancer similarly effective, and are there any increased risks for harms in people with HIV?
There’s also the question of whether PWH should be screened for lung cancer earlier and with a lower smoking exposure than is advised under current guidelines for the general population, she said in the interview. “And should the approach to shared decision-making be modified for people with HIV?” she said. “We’re doing some work on these questions” right now.
None of the researchers interviewed reported any conflicts of interest relevant to the story. Dr. Kunisaki reported that he has no relevant disclosures, and said that his comments are his personal views and not official views of the U.S. Government, Department of Veterans Affairs, the Minneapolis VA, or the University of Minnesota.
Chronic pulmonary disease continues to be a major cause of morbidity and mortality in individuals living with the human immunodeficiency virus, even with optimal HIV control. And this is independent, as seen in many studies, of age, smoking, and pulmonary infections.
Both chronic pulmonary obstructive disease (COPD) and lung cancer occur more frequently in people living with HIV than in the general population, and at earlier ages, and with worse outcomes. The risk for emphysema and interstitial lung abnormalities also appears to be higher, research has shown. And asthma has also recently emerged as another important lung disease in people with HIV (PWH).
“There is evidence that the severity of immunocompromise associated with HIV infection is linked with chronic lung diseases. People who have a lower CD4 cell count or a higher viral load do have an increased risk of COPD and emphysema as well as potentially lung cancer. ,” said Kristina Crothers, MD, professor in the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle.
Research has evolved from a focus on the epidemiology of HIV-related chronic lung diseases to a current emphasis on “trying to understand further the mechanisms [behind the heightened risk] through more benchwork and corollary translational studies, and then to the next level of trying to understand what this means for how we should manage people with HIV who have chronic lung diseases,” Dr. Crothers said. “Should management be tailored for people with HIV infection?”
Impairments in immune pathways, local and systemic inflammation, oxidative stress, dysbiosis, and accelerated cellular senescence are among potential mechanisms, but until ongoing mechanistic research yields more answers, pulmonologists should simply – but importantly – be aware of the increased risk and have a low threshold for investigating respiratory symptoms, she and other experts said in interviews. Referral of eligible patients for lung cancer screening is also a priority, as is smoking cessation, they said.
Notably, while spirometry has been the most commonly studied lung function measure in PWH, another noninvasive measure, diffusing capacity for carbon monoxide (DLCO), has garnered attention in the past decade and thus far appears to be the more frequent lung function abnormality.
In an analysis published in 2020 from the longitudinal Multicenter AIDS Cohort Study (MACS) – a study of a subcohort of 591 men with HIV and 476 without HIV – those with HIV were found to have a 1.6-fold increased risk of mild DLCO impairment (< 80% of predicted normal) and a 3-fold higher risk of more severe DLCO impairment (< 60% of predicted normal). There was no significant difference in spirometry findings by HIV status.
Such findings on DLCO are worthy of consideration in clinical practice, even in the absence of HIV-specific screening guidelines for noncommunicable lung diseases, Dr. Crothers said. “In thinking about screening and diagnosing chronic lung diseases in these patients, I’d not only consider spirometry, but also diffusing capacity” when possible, she said. Impaired DLCO is seen with emphysema and pulmonary vascular diseases like pulmonary hypertension and also interstitial lung diseases.
Key chronic lung diseases
Ken M. Kunisaki, MD, MS, associate professor of medicine at the University of Minnesota, Minneapolis, and the first author of the MACS analysis of lung function – one of the most recent and largest reports of DLCO impairment – points out that studies of chest computed tomography (CT) have also documented higher rates of emphysema and interstitial lung abnormalities.
A chest CT analysis from a cohort in Denmark (the Copenhagen Comorbidity in HIV Infection [COCOMO] cohort) found interstitial lung abnormalities in 10.9% of more than 700 PWH which represented a 1.8-fold increased risk compared to HIV-negative controls. And a study from an Italian sample of never-smoking PWH and controls reported emphysema in 18% and 4%, respectively. These studies, which did not measure DLCO, are among those discussed in a 2021 review by Dr. Kunisaki of advances in HIV-associated chronic lung disease research.
COPD is the best studied and most commonly encountered chronic lung disease in PWH. “Particularly for COPD, what’s both interesting and unfortunate is that we haven’t really seen any changes in the epidemiology with ART (antiretroviral therapy) – we’re still seeing the same findings, like the association of HIV with worse COPD at younger ages,” said Alison Morris, MD, MS, professor of medicine, immunology, and clinical and translational research at the University of Pittsburgh. “It doesn’t seem to have improved.”
Its prevalence has varied widely from cohort to cohort, from as low as 3% (similar to the general population) to over 40%, Dr. Kunisaki said, emphasizing that many studies, including studies showing higher rates, have controlled for current and past smoking. In evaluating patients with low or no smoking burden, “don’t discount respiratory symptoms as possibly reflecting underlying lung disease because COPD can develop with low to no smoking history in those with HIV,” he advised.
A better understanding of how a chronic viral infection like HIV leads to heightened COPD risk will not only help those with HIV, he notes, but also people without HIV who have COPD but have never smoked – a woefully underappreciated and understudied population. Ongoing research, he said, “should help us understand COPD pathogenesis generally.”
Research on asthma is relatively limited thus far, but it does appear that PWH may be more prone to developing severe asthma, just as with COPD, said Dr. Kunisaki, also a staff physician at the Minneapolis Veterans Administration Health Care System. Research has shown, for instance, that people with HIV more frequently needed aggressive respiratory support when hospitalized for asthma exacerbations.
It’s unclear how much of this potentially increased severity is attributable to the biology of HIV’s impact on the body and how much relates to social factors like disparities in income and access to care, Dr. Kunisaki said, noting that the same questions apply to the more frequent COPD exacerbations documented in PWH.
Dr. Crothers points out that, while most studies do not suggest a difference in the incidence of asthma in PWH, “there is some data from researchers looking at asthma profiles [suggesting] that the biomarkers associated with asthma may be different in people with and without HIV,” signaling potentially different molecular or biologic underpinnings of the disease.
Incidence rates of lung cancer in PWH, meanwhile, have declined over the last 2 decades, but lung cancer remains the leading cause of cancer-related mortality in PWH and occurs at a rate that is 2-2.5 times higher than that of individuals not infected with HIV, according to
Janice Leung, MD, of the division of respiratory medicine at the University of British Columbia and the Centre for Heart Lung Innovation at St. Paul’s Hospital in Vancouver.
Patients with HIV have “worse outcomes overall and a higher risk of mortality, even when presenting at the same stage,” said Dr. Leung, who reviewed trends in COPD and lung cancer in a recently published opinion piece.
Potential drivers
A bird’s eye view of potential – and likely interrelated – mechanisms for chronic lung disease includes chronic immune activation that impairs innate and adaptive immune pathways; chronic inflammation systemically and in the lung despite viral suppression; persistence of the virus in latent reservoirs in the lung, particularly in alveolar macrophages and T cells; HIV-related proteins contributing to oxidative stress; accelerated cellular aging; dysbiosis; and ongoing injury from inhaled toxins.
All are described in the literature and are being further explored. “It’s likely that multiple pathways are playing a role,” said Dr. Crothers, “and it could be that the balance of one to another leads to different manifestations of disease.”
Biomarkers that have been elevated and associated with different features of chronic lung disease – such as airflow obstruction, low DLCO, and emphysema – include markers of inflammation (e.g., C-reactive protein, interleukin-6), monocyte activation (e.g., soluble CD14), and markers of endothelial dysfunction, she noted in a 2021 commentary marking 40 years since the first reported cases of acquired immunodeficiency syndrome.
In her laboratory, Dr. Leung is using new epigenetic markers to look at the pathogenesis of accelerated aging in the lung. By profiling bronchial epithelial brushings for DNA methylation and gene expression, they have found that “people living with both HIV and COPD have the fastest epigenetic age acceleration in their airway epithelium,” she said. The findings “suggest that the HIV lung is aging faster.”
They reported their findings in 2022, describing methylation disruptions along age-related pathways such as cellular senescence, longevity regulation, and insulin signaling.
Dr. Leung and her team have also studied the lung microbiome and found lower microbial diversity in the airway epithelium in patients with HIV than those without, especially in those with HIV and COPD. The National Institutes of Health–sponsored Lung HIV Microbiome Project found that changes in the lung microbiome are most pronounced in patients who haven’t yet initiated ART, but research in her lab suggests ongoing suppression of microbial diversity even after ART, she said.
Dr. Morris is particularly interested in the oral microbiome, having found through her research that changes in the oral microbiome in PWH were more related to impaired lung function than alterations in the lung and gut microbiome. “That may be in part because of the way we measure things,” she said. “But we also think that the oral microbiome probably seeds the lung [through micro-aspiration].” A study published in 2020 from the Pittsburgh site of the MACS described alterations in oral microbial communities in PWH with abnormal lung function.
Preliminary research suggests that improved dental cleaning and periodontal work in PWH and COPD may influence the severity of COPD, she noted.
“We don’t see as much of a signal with the gut microbiome [and HIV status or lung function], though there could still be ways in which gut microbiome influences the lung,” through systemic inflammation, the release of metabolites into the bloodstream, or microbial translocation, for instance, she said.
The potential role of translocation of members of the microbiome, in fact, is an area of active research for Dr. Morris. Members of the microbiome – viruses and fungi in addition to bacteria – “can get into the bloodstream from the mouth, from the lung, from the gut, to stimulate inflammation and worsen lung disease,” she said.
Key questions in an evolving research landscape
Dr. Kunisaki looks forward to research providing a more longitudinal look at lung function decline– a move beyond a dominance of cross-sectional studies – as well as research that is more comprehensive, with simultaneous collection of various functional measures (eg., DLCO with chest imaging and fractional excretion of nitric oxide (FENO – a standardized breath measure of Th2 airway inflammation).
The several-year-old NIH-supported MACS/WIHS (Women’s Interagency HIV Study) Combined Cohort study, in which Dr. Kunisaki and Dr. Morris participate, aims in part to identity biomarkers of increased risk for chronic lung disease and other chronic disorders and to develop strategies for more effective interventions and treatments.
Researchers will also share biospecimens, “which will allow more mechanistic work,” Dr. Kunisaki noted. (The combined cohort study includes participants from the earlier, separate MACS and WIHS studies.)
Questions about treatment strategies include the risks versus benefits of inhaled corticosteroids, which may increase an already elevated risk of respiratory infections like bacterial pneumonia in PWH, Dr. Kunisaki said.
[An aside: Inhaled corticosteroids also have well-described interactions with ART regimens that contain CYP3A4 inhibitors (e.g., ritonavir and cobicistat) that can lead to hypercortisolism. In patients who require both types of drugs, he said, beclomethasone has the least interactions and is the preferred inhaled corticosteroid.]
For Dr. Crothers, unanswered critical questions include – as she wrote in her 2021 commentary – the question of how guidelines for the management of COPD and asthma should be adapted for PWH. Is COPD in PWH more or less responsive to inhaled corticosteroids, for instance? And are antifibrotic treatments for interstitial lung disease and immunotherapies for asthma or lung cancer similarly effective, and are there any increased risks for harms in people with HIV?
There’s also the question of whether PWH should be screened for lung cancer earlier and with a lower smoking exposure than is advised under current guidelines for the general population, she said in the interview. “And should the approach to shared decision-making be modified for people with HIV?” she said. “We’re doing some work on these questions” right now.
None of the researchers interviewed reported any conflicts of interest relevant to the story. Dr. Kunisaki reported that he has no relevant disclosures, and said that his comments are his personal views and not official views of the U.S. Government, Department of Veterans Affairs, the Minneapolis VA, or the University of Minnesota.
Advising patients on AD treatment options: Expert pearls
WASHINGTON –
The question was top of mind for experts who shared their advice during a “Tips and Tricks” session at the Revolutionizing Atopic Dermatitis meeting. Dupilumab dosing and dupilumab-associated facial redness and ocular disease, self-image issues, topical regimen adherence, and the quantification of disease were among the other topics raised by the experts.
Here are some of their practice pearls.
Treatment decisions, safety concerns
Deciding on a treatment is “kind of confusing ... particularly in the last year ... and it will only get more complicated,” said Raj J. Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago. “We’re all about some version of shared decision-making, but if all else is equal, sometimes it pays to explicitly ask the patient, what do you want to do?”
Questions about how long a systemic treatment should be tried, both initially and in the long run, are common. “I think that oftentimes we all get antsy about making changes when we’re not getting to the endpoint we want to. And at least in my real-world experience, late responders are a real thing. Sometimes 3-4 months ... isn’t enough,” he said.
Trial extension data show that patients who were nonresponders for various endpoints at 16 weeks are “captured continuously as you go further and further out,” Dr. Chovatiya said. Regarding the long term, “realistically, there’s no perfect time to call it quits.”
Addressing fears about Janus kinase inhibitors can be challenging, he said. “When you’ve identified the right patient and labs are done ... have them take the medication in front of you and hang out,” he advised. “It may sound ridiculous, but for the extremely anxious person it can be a big stress reducer for everyone involved.”
Regarding treatment fears more generally, “asking patients ‘what is the biggest risk of not treating your disease?’ sometimes gets people thinking,” Dr. Chovatiya said.
For parents of children with AD, said Robert Sidbury, MD, MPH, risks of not treating can become apparent once treatments are started and benefits are realized. “It’s so easy to focus on the risks of any treatment because they’re right there in black and white, and the risks of not treating are not always as apparent, even though – or maybe because – they live with them every day.”
When treatment is underway, “they see [how] everyone sleeps better, how school performance gets better, how concentration gets better,” said Dr. Sidbury, professor in the department of pediatrics at the University of Washington. Seattle, and chief of dermatology at Seattle Children’s Hospital.
“Always contextualize,” he advised. “As dermatologists, we’re savvy with navigating boxed warnings.”
David Rosmarin, MD, chair of dermatology, at Indiana University, Indianapolis, and formerly vice chair for research and education at Tufts Medical Center, Boston, said he addresses questions about the length of systemic treatment by advising patients: “Why don’t we start taking [the medication] for 3 months and then we’ll take it from there.”
In some pediatric cases, Dr. Rosmarin said, having the child express “what their AD means to them – how it affects them,” and then acknowledging and validating what the child says, is helpful to parents who are concerned about systemic treatments.
Dupilumab in the real world
Some patients on dupilumab do not have a complete response with dosing every 2 weeks and may benefit from more frequent dosing, said Dr. Rosmarin.
“We know from the SOLO-1 and SOLO-2 studies that dupilumab weekly dosing was evaluated. It was only the every-other-week dosing that was approved, and we can see why – in terms of the changes in EASI [Eczema Area and Severity Index] score they’re close to overlapping,” he said.
In real life, however, “some patients benefit from different dosing. It’s important to realize that. I think we all have some patients who may dose more frequently and some who may dose less frequently,” Dr. Rosmarin said.
For a patient who “gets absolutely no response from dupilumab after 3-4 months, I’d switch them to something else. But for those who are partial responders, particularly those who tell me they’re getting itchy before their next dose, they’re the ones who benefit most from doubling the dose to dupilumab weekly,” he said.
For patients who experience dupilumab-associated head and neck dermatitis, itraconazole may help, Dr. Rosmarin added. “We’re using 200 mg daily for 2 weeks and weekly thereafter, and it helps some of our patients.” The average self-reported improvement was 52% for patients with dupilumab-associated facial redness treated with itraconazole in a retrospective medical record review that he and his colleagues published in 2022.
Dr. Rosmarin pointed to a multicenter prospective cohort study also published in 2022 showing that baseline/pretreatment levels of Malassezia-specific IgE were associated with the development of dupilumab-associated head and neck dermatitis. The median levels of Malassezia-specific IgE were 32 kUL–1 versus 2.3 kUL–1 in patients who experienced dupilumab-associated facial redness, compared with those who did not.
He said that, while there “may be multiple reasons” for dupilumab-associated head and neck dermatitis and that “plenty of patients” who don’t have Malassezia-specific IgE develop head and neck dermatitis, “this could be one cause.”
Itraconazole has been shown in his practice to be superior to fluconazole, likely because it has greater anti-inflammatory effects and provides better coverage of Malassezia because it is more lipophilic, said Dr. Rosmarin, who does not test for Malassezia-specific IgE before trying itraconazole.
For dupilumab-associated ocular surface disease, Elaine C. Siegfried, MD, offered her first-line suggestions: warm compresses (such as a microwaved bean bag), bland ocular lubricant (such as preservative-free artificial tears), oral hydration, and if needed and accessible, the prescription ophthalmic solution lifitegrast.
“It’s become an issue – what the dermatologist can do first line,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital, St. Louis.
“If these don’t work, then I’ll identify an ophthalmologist who’s knowledgeable about Dupixent-related ocular surface disease,” she said. Selection is “important because they’re not all knowledgeable ... corneal specialists typically have the most knowledge.”
Topical adherence with diffuse xerosis and mild-moderate AD
For patients with diffuse xerosis and mild-moderate AD, especially those who are older and having difficulty with topical regimens, Anna De Benedetto, MD, said she tries to enhance adherence by simplifying the regimen. She asks patients to buy a pound jar of base cream (ceramide base) – “whatever emollient they like” – and mixes into it a high-potency steroid solution. They’re instructed to apply the combined cream once daily for 1-2 weeks, and then three times a week alternating with a nonmedicated cream.
“This way they’re using one [cream] to target the immune system and the skin barrier,” said Dr. De Benedetto, associate professor of dermatology and director of the dermatology clinical trial unit at the University of Rochester (N.Y.) Medical Center.
‘Wet wrap’ pajamas; self-image for children, teens
Dermatologist Melinda Gooderham, MSc, MD, assistant professor at Queen’s University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology, said that, for widespread and troublesome AD, she advises patients or parents to wet a thin cotton pajama top and bottom and spin it in the dryer “so it’s almost dry but still moist.” Dry, looser pajamas or a light track suit can then be worn over the damp pajamas. “I usually tell [patients] to buy one size up,” she said.
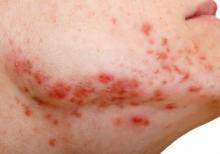
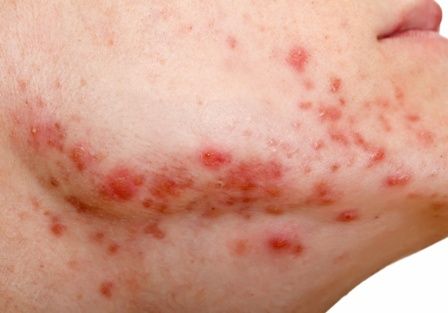
Body dysmorphia is common with skin disease, and its incidence is six times higher in people with eczema than those without the disease, said Dr. Siegfried. “I’ve found that, for patients subjected to AD for a long time,” this is still an issue, “even when you clear their skin.”
For children, teens and their families, the nonprofit organization Made a Masterpiece can be valuable, Dr. Siegfried said. It offers resources from parents, children, psychologists, dermatologists, and others to help manage the emotional, social and spiritual aspects of living with a skin condition.
To use or not to use BSA; environmental counseling
“I think [assessing] body surface area [BSA] is very important in pediatrics and for adolescents [especially in those with moderate to severe disease] because it quantifies the disease for the family,” said Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego.
“Families live with the disease, but quantification really matters” for understanding the extent and impact of the disease and for motivating families to treat, said Dr. Eichenfield.
(When the disease is markedly diminished in follow-up, knowing the BSA then “helps families to register the improvement and gives positive reinforcement,” Dr. Eichenfeld said after the meeting.)
Young patients can participate, he noted at the meeting. “When I do telemedicine visits, kids can tell me how many hands of eczema they have.”
Dr. Eichenfield also said that he now routinely counsels on the environmental impacts on eczema. For example, “I explain to people that we’re probably going to have a bad wildfire season in California, and it’s the kind of environmental perturbation that may impact some eczema patients,” he said, noting the 2021 study documenting an association of wildfire air pollution from the 2018 California Camp fire with an increase in dermatology clinic visits for AD and itch in San Francisco.
“It helps to keep an eye out for that, and also to be aware of some of the environmental changes,” he said.
Dr. Chovatiya reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others. Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, and Lilly, among others. Dr. Rosmarin reported ties with AbbVie, Incyte, Lilly, Pfizer, Regeneron, and Sanofi, among others. Dr. Siegfried reported ties with Regeneron, Sanofi Genzyme, AbbVie, Incyte, Leo, and Pfizer, among others. Dr. De Benedetto reported ties with Incyte, Pfizer, AbbVie, and Sanofi Advent, among others. Dr. Gooderham reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, among others. Dr. Eichenfield disclosed ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others.
WASHINGTON –
The question was top of mind for experts who shared their advice during a “Tips and Tricks” session at the Revolutionizing Atopic Dermatitis meeting. Dupilumab dosing and dupilumab-associated facial redness and ocular disease, self-image issues, topical regimen adherence, and the quantification of disease were among the other topics raised by the experts.
Here are some of their practice pearls.
Treatment decisions, safety concerns
Deciding on a treatment is “kind of confusing ... particularly in the last year ... and it will only get more complicated,” said Raj J. Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago. “We’re all about some version of shared decision-making, but if all else is equal, sometimes it pays to explicitly ask the patient, what do you want to do?”
Questions about how long a systemic treatment should be tried, both initially and in the long run, are common. “I think that oftentimes we all get antsy about making changes when we’re not getting to the endpoint we want to. And at least in my real-world experience, late responders are a real thing. Sometimes 3-4 months ... isn’t enough,” he said.
Trial extension data show that patients who were nonresponders for various endpoints at 16 weeks are “captured continuously as you go further and further out,” Dr. Chovatiya said. Regarding the long term, “realistically, there’s no perfect time to call it quits.”
Addressing fears about Janus kinase inhibitors can be challenging, he said. “When you’ve identified the right patient and labs are done ... have them take the medication in front of you and hang out,” he advised. “It may sound ridiculous, but for the extremely anxious person it can be a big stress reducer for everyone involved.”
Regarding treatment fears more generally, “asking patients ‘what is the biggest risk of not treating your disease?’ sometimes gets people thinking,” Dr. Chovatiya said.
For parents of children with AD, said Robert Sidbury, MD, MPH, risks of not treating can become apparent once treatments are started and benefits are realized. “It’s so easy to focus on the risks of any treatment because they’re right there in black and white, and the risks of not treating are not always as apparent, even though – or maybe because – they live with them every day.”
When treatment is underway, “they see [how] everyone sleeps better, how school performance gets better, how concentration gets better,” said Dr. Sidbury, professor in the department of pediatrics at the University of Washington. Seattle, and chief of dermatology at Seattle Children’s Hospital.
“Always contextualize,” he advised. “As dermatologists, we’re savvy with navigating boxed warnings.”
David Rosmarin, MD, chair of dermatology, at Indiana University, Indianapolis, and formerly vice chair for research and education at Tufts Medical Center, Boston, said he addresses questions about the length of systemic treatment by advising patients: “Why don’t we start taking [the medication] for 3 months and then we’ll take it from there.”
In some pediatric cases, Dr. Rosmarin said, having the child express “what their AD means to them – how it affects them,” and then acknowledging and validating what the child says, is helpful to parents who are concerned about systemic treatments.
Dupilumab in the real world
Some patients on dupilumab do not have a complete response with dosing every 2 weeks and may benefit from more frequent dosing, said Dr. Rosmarin.
“We know from the SOLO-1 and SOLO-2 studies that dupilumab weekly dosing was evaluated. It was only the every-other-week dosing that was approved, and we can see why – in terms of the changes in EASI [Eczema Area and Severity Index] score they’re close to overlapping,” he said.
In real life, however, “some patients benefit from different dosing. It’s important to realize that. I think we all have some patients who may dose more frequently and some who may dose less frequently,” Dr. Rosmarin said.
For a patient who “gets absolutely no response from dupilumab after 3-4 months, I’d switch them to something else. But for those who are partial responders, particularly those who tell me they’re getting itchy before their next dose, they’re the ones who benefit most from doubling the dose to dupilumab weekly,” he said.
For patients who experience dupilumab-associated head and neck dermatitis, itraconazole may help, Dr. Rosmarin added. “We’re using 200 mg daily for 2 weeks and weekly thereafter, and it helps some of our patients.” The average self-reported improvement was 52% for patients with dupilumab-associated facial redness treated with itraconazole in a retrospective medical record review that he and his colleagues published in 2022.
Dr. Rosmarin pointed to a multicenter prospective cohort study also published in 2022 showing that baseline/pretreatment levels of Malassezia-specific IgE were associated with the development of dupilumab-associated head and neck dermatitis. The median levels of Malassezia-specific IgE were 32 kUL–1 versus 2.3 kUL–1 in patients who experienced dupilumab-associated facial redness, compared with those who did not.
He said that, while there “may be multiple reasons” for dupilumab-associated head and neck dermatitis and that “plenty of patients” who don’t have Malassezia-specific IgE develop head and neck dermatitis, “this could be one cause.”
Itraconazole has been shown in his practice to be superior to fluconazole, likely because it has greater anti-inflammatory effects and provides better coverage of Malassezia because it is more lipophilic, said Dr. Rosmarin, who does not test for Malassezia-specific IgE before trying itraconazole.
For dupilumab-associated ocular surface disease, Elaine C. Siegfried, MD, offered her first-line suggestions: warm compresses (such as a microwaved bean bag), bland ocular lubricant (such as preservative-free artificial tears), oral hydration, and if needed and accessible, the prescription ophthalmic solution lifitegrast.
“It’s become an issue – what the dermatologist can do first line,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital, St. Louis.
“If these don’t work, then I’ll identify an ophthalmologist who’s knowledgeable about Dupixent-related ocular surface disease,” she said. Selection is “important because they’re not all knowledgeable ... corneal specialists typically have the most knowledge.”
Topical adherence with diffuse xerosis and mild-moderate AD
For patients with diffuse xerosis and mild-moderate AD, especially those who are older and having difficulty with topical regimens, Anna De Benedetto, MD, said she tries to enhance adherence by simplifying the regimen. She asks patients to buy a pound jar of base cream (ceramide base) – “whatever emollient they like” – and mixes into it a high-potency steroid solution. They’re instructed to apply the combined cream once daily for 1-2 weeks, and then three times a week alternating with a nonmedicated cream.
“This way they’re using one [cream] to target the immune system and the skin barrier,” said Dr. De Benedetto, associate professor of dermatology and director of the dermatology clinical trial unit at the University of Rochester (N.Y.) Medical Center.
‘Wet wrap’ pajamas; self-image for children, teens
Dermatologist Melinda Gooderham, MSc, MD, assistant professor at Queen’s University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology, said that, for widespread and troublesome AD, she advises patients or parents to wet a thin cotton pajama top and bottom and spin it in the dryer “so it’s almost dry but still moist.” Dry, looser pajamas or a light track suit can then be worn over the damp pajamas. “I usually tell [patients] to buy one size up,” she said.
Body dysmorphia is common with skin disease, and its incidence is six times higher in people with eczema than those without the disease, said Dr. Siegfried. “I’ve found that, for patients subjected to AD for a long time,” this is still an issue, “even when you clear their skin.”
For children, teens and their families, the nonprofit organization Made a Masterpiece can be valuable, Dr. Siegfried said. It offers resources from parents, children, psychologists, dermatologists, and others to help manage the emotional, social and spiritual aspects of living with a skin condition.
To use or not to use BSA; environmental counseling
“I think [assessing] body surface area [BSA] is very important in pediatrics and for adolescents [especially in those with moderate to severe disease] because it quantifies the disease for the family,” said Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego.
“Families live with the disease, but quantification really matters” for understanding the extent and impact of the disease and for motivating families to treat, said Dr. Eichenfield.
(When the disease is markedly diminished in follow-up, knowing the BSA then “helps families to register the improvement and gives positive reinforcement,” Dr. Eichenfeld said after the meeting.)
Young patients can participate, he noted at the meeting. “When I do telemedicine visits, kids can tell me how many hands of eczema they have.”
Dr. Eichenfield also said that he now routinely counsels on the environmental impacts on eczema. For example, “I explain to people that we’re probably going to have a bad wildfire season in California, and it’s the kind of environmental perturbation that may impact some eczema patients,” he said, noting the 2021 study documenting an association of wildfire air pollution from the 2018 California Camp fire with an increase in dermatology clinic visits for AD and itch in San Francisco.
“It helps to keep an eye out for that, and also to be aware of some of the environmental changes,” he said.
Dr. Chovatiya reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others. Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, and Lilly, among others. Dr. Rosmarin reported ties with AbbVie, Incyte, Lilly, Pfizer, Regeneron, and Sanofi, among others. Dr. Siegfried reported ties with Regeneron, Sanofi Genzyme, AbbVie, Incyte, Leo, and Pfizer, among others. Dr. De Benedetto reported ties with Incyte, Pfizer, AbbVie, and Sanofi Advent, among others. Dr. Gooderham reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, among others. Dr. Eichenfield disclosed ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others.
WASHINGTON –
The question was top of mind for experts who shared their advice during a “Tips and Tricks” session at the Revolutionizing Atopic Dermatitis meeting. Dupilumab dosing and dupilumab-associated facial redness and ocular disease, self-image issues, topical regimen adherence, and the quantification of disease were among the other topics raised by the experts.
Here are some of their practice pearls.
Treatment decisions, safety concerns
Deciding on a treatment is “kind of confusing ... particularly in the last year ... and it will only get more complicated,” said Raj J. Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago. “We’re all about some version of shared decision-making, but if all else is equal, sometimes it pays to explicitly ask the patient, what do you want to do?”
Questions about how long a systemic treatment should be tried, both initially and in the long run, are common. “I think that oftentimes we all get antsy about making changes when we’re not getting to the endpoint we want to. And at least in my real-world experience, late responders are a real thing. Sometimes 3-4 months ... isn’t enough,” he said.
Trial extension data show that patients who were nonresponders for various endpoints at 16 weeks are “captured continuously as you go further and further out,” Dr. Chovatiya said. Regarding the long term, “realistically, there’s no perfect time to call it quits.”
Addressing fears about Janus kinase inhibitors can be challenging, he said. “When you’ve identified the right patient and labs are done ... have them take the medication in front of you and hang out,” he advised. “It may sound ridiculous, but for the extremely anxious person it can be a big stress reducer for everyone involved.”
Regarding treatment fears more generally, “asking patients ‘what is the biggest risk of not treating your disease?’ sometimes gets people thinking,” Dr. Chovatiya said.
For parents of children with AD, said Robert Sidbury, MD, MPH, risks of not treating can become apparent once treatments are started and benefits are realized. “It’s so easy to focus on the risks of any treatment because they’re right there in black and white, and the risks of not treating are not always as apparent, even though – or maybe because – they live with them every day.”
When treatment is underway, “they see [how] everyone sleeps better, how school performance gets better, how concentration gets better,” said Dr. Sidbury, professor in the department of pediatrics at the University of Washington. Seattle, and chief of dermatology at Seattle Children’s Hospital.
“Always contextualize,” he advised. “As dermatologists, we’re savvy with navigating boxed warnings.”
David Rosmarin, MD, chair of dermatology, at Indiana University, Indianapolis, and formerly vice chair for research and education at Tufts Medical Center, Boston, said he addresses questions about the length of systemic treatment by advising patients: “Why don’t we start taking [the medication] for 3 months and then we’ll take it from there.”
In some pediatric cases, Dr. Rosmarin said, having the child express “what their AD means to them – how it affects them,” and then acknowledging and validating what the child says, is helpful to parents who are concerned about systemic treatments.
Dupilumab in the real world
Some patients on dupilumab do not have a complete response with dosing every 2 weeks and may benefit from more frequent dosing, said Dr. Rosmarin.
“We know from the SOLO-1 and SOLO-2 studies that dupilumab weekly dosing was evaluated. It was only the every-other-week dosing that was approved, and we can see why – in terms of the changes in EASI [Eczema Area and Severity Index] score they’re close to overlapping,” he said.
In real life, however, “some patients benefit from different dosing. It’s important to realize that. I think we all have some patients who may dose more frequently and some who may dose less frequently,” Dr. Rosmarin said.
For a patient who “gets absolutely no response from dupilumab after 3-4 months, I’d switch them to something else. But for those who are partial responders, particularly those who tell me they’re getting itchy before their next dose, they’re the ones who benefit most from doubling the dose to dupilumab weekly,” he said.
For patients who experience dupilumab-associated head and neck dermatitis, itraconazole may help, Dr. Rosmarin added. “We’re using 200 mg daily for 2 weeks and weekly thereafter, and it helps some of our patients.” The average self-reported improvement was 52% for patients with dupilumab-associated facial redness treated with itraconazole in a retrospective medical record review that he and his colleagues published in 2022.
Dr. Rosmarin pointed to a multicenter prospective cohort study also published in 2022 showing that baseline/pretreatment levels of Malassezia-specific IgE were associated with the development of dupilumab-associated head and neck dermatitis. The median levels of Malassezia-specific IgE were 32 kUL–1 versus 2.3 kUL–1 in patients who experienced dupilumab-associated facial redness, compared with those who did not.
He said that, while there “may be multiple reasons” for dupilumab-associated head and neck dermatitis and that “plenty of patients” who don’t have Malassezia-specific IgE develop head and neck dermatitis, “this could be one cause.”
Itraconazole has been shown in his practice to be superior to fluconazole, likely because it has greater anti-inflammatory effects and provides better coverage of Malassezia because it is more lipophilic, said Dr. Rosmarin, who does not test for Malassezia-specific IgE before trying itraconazole.
For dupilumab-associated ocular surface disease, Elaine C. Siegfried, MD, offered her first-line suggestions: warm compresses (such as a microwaved bean bag), bland ocular lubricant (such as preservative-free artificial tears), oral hydration, and if needed and accessible, the prescription ophthalmic solution lifitegrast.
“It’s become an issue – what the dermatologist can do first line,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital, St. Louis.
“If these don’t work, then I’ll identify an ophthalmologist who’s knowledgeable about Dupixent-related ocular surface disease,” she said. Selection is “important because they’re not all knowledgeable ... corneal specialists typically have the most knowledge.”
Topical adherence with diffuse xerosis and mild-moderate AD
For patients with diffuse xerosis and mild-moderate AD, especially those who are older and having difficulty with topical regimens, Anna De Benedetto, MD, said she tries to enhance adherence by simplifying the regimen. She asks patients to buy a pound jar of base cream (ceramide base) – “whatever emollient they like” – and mixes into it a high-potency steroid solution. They’re instructed to apply the combined cream once daily for 1-2 weeks, and then three times a week alternating with a nonmedicated cream.
“This way they’re using one [cream] to target the immune system and the skin barrier,” said Dr. De Benedetto, associate professor of dermatology and director of the dermatology clinical trial unit at the University of Rochester (N.Y.) Medical Center.
‘Wet wrap’ pajamas; self-image for children, teens
Dermatologist Melinda Gooderham, MSc, MD, assistant professor at Queen’s University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology, said that, for widespread and troublesome AD, she advises patients or parents to wet a thin cotton pajama top and bottom and spin it in the dryer “so it’s almost dry but still moist.” Dry, looser pajamas or a light track suit can then be worn over the damp pajamas. “I usually tell [patients] to buy one size up,” she said.
Body dysmorphia is common with skin disease, and its incidence is six times higher in people with eczema than those without the disease, said Dr. Siegfried. “I’ve found that, for patients subjected to AD for a long time,” this is still an issue, “even when you clear their skin.”
For children, teens and their families, the nonprofit organization Made a Masterpiece can be valuable, Dr. Siegfried said. It offers resources from parents, children, psychologists, dermatologists, and others to help manage the emotional, social and spiritual aspects of living with a skin condition.
To use or not to use BSA; environmental counseling
“I think [assessing] body surface area [BSA] is very important in pediatrics and for adolescents [especially in those with moderate to severe disease] because it quantifies the disease for the family,” said Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego.
“Families live with the disease, but quantification really matters” for understanding the extent and impact of the disease and for motivating families to treat, said Dr. Eichenfield.
(When the disease is markedly diminished in follow-up, knowing the BSA then “helps families to register the improvement and gives positive reinforcement,” Dr. Eichenfeld said after the meeting.)
Young patients can participate, he noted at the meeting. “When I do telemedicine visits, kids can tell me how many hands of eczema they have.”
Dr. Eichenfield also said that he now routinely counsels on the environmental impacts on eczema. For example, “I explain to people that we’re probably going to have a bad wildfire season in California, and it’s the kind of environmental perturbation that may impact some eczema patients,” he said, noting the 2021 study documenting an association of wildfire air pollution from the 2018 California Camp fire with an increase in dermatology clinic visits for AD and itch in San Francisco.
“It helps to keep an eye out for that, and also to be aware of some of the environmental changes,” he said.
Dr. Chovatiya reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others. Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, and Lilly, among others. Dr. Rosmarin reported ties with AbbVie, Incyte, Lilly, Pfizer, Regeneron, and Sanofi, among others. Dr. Siegfried reported ties with Regeneron, Sanofi Genzyme, AbbVie, Incyte, Leo, and Pfizer, among others. Dr. De Benedetto reported ties with Incyte, Pfizer, AbbVie, and Sanofi Advent, among others. Dr. Gooderham reported ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi Genzyme, among others. Dr. Eichenfield disclosed ties with AbbVie, Eli Lilly, Incyte, Leo Pharma, Pfizer, Regeneron, and Sanofi, among others.
AT RAD 2023
Abrocitinib remains effective at 96 weeks, in older as well as younger adults
WASHINGTON – A substantial proportion of , Andrew F. Alexis, MD, MPH, reported in a late-breaker abstract session at the annual Revolutionizing Atopic Dermatitis conference.
The analysis stratified patients by age – 18-50 and over 50 years – and found that the sustained improvement with the JAK-1 selective inhibitor as monotherapy was seen regardless of age. “In practice, patients who are older tend to have had AD for a longer period of time and tend to be more difficult to treat so it’s reassuring to see that even in the over-50 age group, they show substantial responses, even with more stringent endpoints,” said Dr. Alexis, professor of clinical dermatology at Weill Cornell Medical College, New York.
At week 96, for instance, the proportion of patients who achieved at least a 75% improvement from baseline on the Eczema Area and Severity Index (EASI-75) was 73% with the 100-mg dose and 85% with the 200-mg dose in the younger age group, and 86% and 89%, respectively, in the older age group.
An EASI-90 response – one of the more stringent outcomes – was achieved by 45% and 58% in the 18-50 group and 58% and 73% in the over 50 group (for 100-mg and 200-mg doses, respectively), Dr. Alexis reported.
The interim analysis also showed dose-dependent efficacy overall up to 96 weeks in the younger age group but only up to 48 weeks in the older age group. Response to some outcome measures in patients over age 50 years was “less clearly dose dependent after week 48” than earlier, Dr. Alexis said.
The ongoing JADE EXTEND trial enrolled patients who had participated in the phase 3 JADE clinical trials. This analysis covered 1,309 patients who were enrolled by a September 2021 cutoff. The patient population leaned young: Eighty percent (1,046) were aged 18-50, and 20% (263) were over 50.
Patients who were randomly assigned to abrocitinib 200 mg or 100 mg in the parent trials continued to receive the same dose in JADE EXTEND with blinding maintained. Those who received placebo in the qualifying trial were randomly assigned to abrocitinib 200 mg or 100 mg. And patients from JADE DARE continued with their dosing of 200 mg. Grouping by age for the analysis was made based on the age recorded at the screening visit of the qualifying trial.
IGA, PP-NRS, and DLQI results
At week 96, the proportion of patients 18-50 years of age who achieved the Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with at least a 2-grade improvement from baseline was 44% in the 100-mg group and 55% in the 200-mg group. Among patients over 50, these proportions were 51% and 58%, respectively.
The proportion of patients who achieved at least a 4-point improvement from baseline in the Peak Pruritus Numerical Rating Scale (PP-NRS) score was 54% and 66% (on 100 mg and 200 mg, respectively) among those aged 18-50, and 79% and 80%, respectively, among those over 50.
Looking at more stringent outcomes, 26% and 38% in the 18-50 group on 100 mg and 200 mg, respectively, achieved a PP-NRS of 0/1, as did 54% and 44% in the over-50 group.
Lastly, a score of less than 2 on the Dermatology Life Quality Index (DLQI 0/1) was achieved by 32% and 41% of patients aged 18-50 and by 51% and 48% of patients over 50, for the 100-mg and 200-mg doses, respectively.
The decline in dose-dependent efficacy in the older age group after 48 weeks may be due to the smaller sample of older patients and/or the fact that a higher proportion of older patients had moderate baseline disease per their IGA score, versus severe disease, compared with the younger patients, Dr. Alexis said. “We see a skewing toward a bit more severe [disease] in the younger age group compared to the older,” he noted.
Abrocitinib (Cibinqo) is approved for the treatment of moderate to severe AD in adolescents aged 12 and up and adults whose disease is not adequately controlled with other systemic treatments or those for whom the use of these drugs is not advised. It is available in a 50-mg dose for dose adjustments in special populations, but this dose was not studied in the clinical trials, Dr. Alexis noted. The interim analysis did not include safety data.
In a separate presentation in which he reviewed long-term data on AD medications, Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that most patients who meet defined endpoints at week 12 of treatment with abrocitinib maintain that response over time. “By and large, there’s a steep initial rise that flattens over the long run, which is what you want to see. People getting that response are generally staying there over the course of treatment,” he said, referring to the JADE EXTEND data up to week 48.
It’s important to also appreciate, however, that the proportion of patients meeting efficacy outcomes in the trials of abrocitinib has grown well beyond 12 weeks, Dr. Chovatiya said.
Pointing to data presented at a 2021 RAD meeting depicting the proportion of 12-week nonresponders achieving a response at weeks 24 and 48 on IGA 0/1, EASI-75, and PP-NRS, Dr. Chovatiya said the level of response grew at both time points. “You’re capturing a chunk of people well beyond the primary endpoint if you keep them on therapy continuously, suggesting that ... we may need to reframe how we’re thinking about oral JAK inhibitors,” he said. “Not only are they rapidly acting, but they are medications that can provide good control and changes in the long run.”
Dr. Alexis and Dr. Chovatiya disclosed ties with Pfizer, which funded the study.
WASHINGTON – A substantial proportion of , Andrew F. Alexis, MD, MPH, reported in a late-breaker abstract session at the annual Revolutionizing Atopic Dermatitis conference.
The analysis stratified patients by age – 18-50 and over 50 years – and found that the sustained improvement with the JAK-1 selective inhibitor as monotherapy was seen regardless of age. “In practice, patients who are older tend to have had AD for a longer period of time and tend to be more difficult to treat so it’s reassuring to see that even in the over-50 age group, they show substantial responses, even with more stringent endpoints,” said Dr. Alexis, professor of clinical dermatology at Weill Cornell Medical College, New York.
At week 96, for instance, the proportion of patients who achieved at least a 75% improvement from baseline on the Eczema Area and Severity Index (EASI-75) was 73% with the 100-mg dose and 85% with the 200-mg dose in the younger age group, and 86% and 89%, respectively, in the older age group.
An EASI-90 response – one of the more stringent outcomes – was achieved by 45% and 58% in the 18-50 group and 58% and 73% in the over 50 group (for 100-mg and 200-mg doses, respectively), Dr. Alexis reported.
The interim analysis also showed dose-dependent efficacy overall up to 96 weeks in the younger age group but only up to 48 weeks in the older age group. Response to some outcome measures in patients over age 50 years was “less clearly dose dependent after week 48” than earlier, Dr. Alexis said.
The ongoing JADE EXTEND trial enrolled patients who had participated in the phase 3 JADE clinical trials. This analysis covered 1,309 patients who were enrolled by a September 2021 cutoff. The patient population leaned young: Eighty percent (1,046) were aged 18-50, and 20% (263) were over 50.
Patients who were randomly assigned to abrocitinib 200 mg or 100 mg in the parent trials continued to receive the same dose in JADE EXTEND with blinding maintained. Those who received placebo in the qualifying trial were randomly assigned to abrocitinib 200 mg or 100 mg. And patients from JADE DARE continued with their dosing of 200 mg. Grouping by age for the analysis was made based on the age recorded at the screening visit of the qualifying trial.
IGA, PP-NRS, and DLQI results
At week 96, the proportion of patients 18-50 years of age who achieved the Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with at least a 2-grade improvement from baseline was 44% in the 100-mg group and 55% in the 200-mg group. Among patients over 50, these proportions were 51% and 58%, respectively.
The proportion of patients who achieved at least a 4-point improvement from baseline in the Peak Pruritus Numerical Rating Scale (PP-NRS) score was 54% and 66% (on 100 mg and 200 mg, respectively) among those aged 18-50, and 79% and 80%, respectively, among those over 50.
Looking at more stringent outcomes, 26% and 38% in the 18-50 group on 100 mg and 200 mg, respectively, achieved a PP-NRS of 0/1, as did 54% and 44% in the over-50 group.
Lastly, a score of less than 2 on the Dermatology Life Quality Index (DLQI 0/1) was achieved by 32% and 41% of patients aged 18-50 and by 51% and 48% of patients over 50, for the 100-mg and 200-mg doses, respectively.
The decline in dose-dependent efficacy in the older age group after 48 weeks may be due to the smaller sample of older patients and/or the fact that a higher proportion of older patients had moderate baseline disease per their IGA score, versus severe disease, compared with the younger patients, Dr. Alexis said. “We see a skewing toward a bit more severe [disease] in the younger age group compared to the older,” he noted.
Abrocitinib (Cibinqo) is approved for the treatment of moderate to severe AD in adolescents aged 12 and up and adults whose disease is not adequately controlled with other systemic treatments or those for whom the use of these drugs is not advised. It is available in a 50-mg dose for dose adjustments in special populations, but this dose was not studied in the clinical trials, Dr. Alexis noted. The interim analysis did not include safety data.
In a separate presentation in which he reviewed long-term data on AD medications, Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that most patients who meet defined endpoints at week 12 of treatment with abrocitinib maintain that response over time. “By and large, there’s a steep initial rise that flattens over the long run, which is what you want to see. People getting that response are generally staying there over the course of treatment,” he said, referring to the JADE EXTEND data up to week 48.
It’s important to also appreciate, however, that the proportion of patients meeting efficacy outcomes in the trials of abrocitinib has grown well beyond 12 weeks, Dr. Chovatiya said.
Pointing to data presented at a 2021 RAD meeting depicting the proportion of 12-week nonresponders achieving a response at weeks 24 and 48 on IGA 0/1, EASI-75, and PP-NRS, Dr. Chovatiya said the level of response grew at both time points. “You’re capturing a chunk of people well beyond the primary endpoint if you keep them on therapy continuously, suggesting that ... we may need to reframe how we’re thinking about oral JAK inhibitors,” he said. “Not only are they rapidly acting, but they are medications that can provide good control and changes in the long run.”
Dr. Alexis and Dr. Chovatiya disclosed ties with Pfizer, which funded the study.
WASHINGTON – A substantial proportion of , Andrew F. Alexis, MD, MPH, reported in a late-breaker abstract session at the annual Revolutionizing Atopic Dermatitis conference.
The analysis stratified patients by age – 18-50 and over 50 years – and found that the sustained improvement with the JAK-1 selective inhibitor as monotherapy was seen regardless of age. “In practice, patients who are older tend to have had AD for a longer period of time and tend to be more difficult to treat so it’s reassuring to see that even in the over-50 age group, they show substantial responses, even with more stringent endpoints,” said Dr. Alexis, professor of clinical dermatology at Weill Cornell Medical College, New York.
At week 96, for instance, the proportion of patients who achieved at least a 75% improvement from baseline on the Eczema Area and Severity Index (EASI-75) was 73% with the 100-mg dose and 85% with the 200-mg dose in the younger age group, and 86% and 89%, respectively, in the older age group.
An EASI-90 response – one of the more stringent outcomes – was achieved by 45% and 58% in the 18-50 group and 58% and 73% in the over 50 group (for 100-mg and 200-mg doses, respectively), Dr. Alexis reported.
The interim analysis also showed dose-dependent efficacy overall up to 96 weeks in the younger age group but only up to 48 weeks in the older age group. Response to some outcome measures in patients over age 50 years was “less clearly dose dependent after week 48” than earlier, Dr. Alexis said.
The ongoing JADE EXTEND trial enrolled patients who had participated in the phase 3 JADE clinical trials. This analysis covered 1,309 patients who were enrolled by a September 2021 cutoff. The patient population leaned young: Eighty percent (1,046) were aged 18-50, and 20% (263) were over 50.
Patients who were randomly assigned to abrocitinib 200 mg or 100 mg in the parent trials continued to receive the same dose in JADE EXTEND with blinding maintained. Those who received placebo in the qualifying trial were randomly assigned to abrocitinib 200 mg or 100 mg. And patients from JADE DARE continued with their dosing of 200 mg. Grouping by age for the analysis was made based on the age recorded at the screening visit of the qualifying trial.
IGA, PP-NRS, and DLQI results
At week 96, the proportion of patients 18-50 years of age who achieved the Investigator’s Global Assessment (IGA) score of 0 or 1 (clear or almost clear) with at least a 2-grade improvement from baseline was 44% in the 100-mg group and 55% in the 200-mg group. Among patients over 50, these proportions were 51% and 58%, respectively.
The proportion of patients who achieved at least a 4-point improvement from baseline in the Peak Pruritus Numerical Rating Scale (PP-NRS) score was 54% and 66% (on 100 mg and 200 mg, respectively) among those aged 18-50, and 79% and 80%, respectively, among those over 50.
Looking at more stringent outcomes, 26% and 38% in the 18-50 group on 100 mg and 200 mg, respectively, achieved a PP-NRS of 0/1, as did 54% and 44% in the over-50 group.
Lastly, a score of less than 2 on the Dermatology Life Quality Index (DLQI 0/1) was achieved by 32% and 41% of patients aged 18-50 and by 51% and 48% of patients over 50, for the 100-mg and 200-mg doses, respectively.
The decline in dose-dependent efficacy in the older age group after 48 weeks may be due to the smaller sample of older patients and/or the fact that a higher proportion of older patients had moderate baseline disease per their IGA score, versus severe disease, compared with the younger patients, Dr. Alexis said. “We see a skewing toward a bit more severe [disease] in the younger age group compared to the older,” he noted.
Abrocitinib (Cibinqo) is approved for the treatment of moderate to severe AD in adolescents aged 12 and up and adults whose disease is not adequately controlled with other systemic treatments or those for whom the use of these drugs is not advised. It is available in a 50-mg dose for dose adjustments in special populations, but this dose was not studied in the clinical trials, Dr. Alexis noted. The interim analysis did not include safety data.
In a separate presentation in which he reviewed long-term data on AD medications, Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, said that most patients who meet defined endpoints at week 12 of treatment with abrocitinib maintain that response over time. “By and large, there’s a steep initial rise that flattens over the long run, which is what you want to see. People getting that response are generally staying there over the course of treatment,” he said, referring to the JADE EXTEND data up to week 48.
It’s important to also appreciate, however, that the proportion of patients meeting efficacy outcomes in the trials of abrocitinib has grown well beyond 12 weeks, Dr. Chovatiya said.
Pointing to data presented at a 2021 RAD meeting depicting the proportion of 12-week nonresponders achieving a response at weeks 24 and 48 on IGA 0/1, EASI-75, and PP-NRS, Dr. Chovatiya said the level of response grew at both time points. “You’re capturing a chunk of people well beyond the primary endpoint if you keep them on therapy continuously, suggesting that ... we may need to reframe how we’re thinking about oral JAK inhibitors,” he said. “Not only are they rapidly acting, but they are medications that can provide good control and changes in the long run.”
Dr. Alexis and Dr. Chovatiya disclosed ties with Pfizer, which funded the study.
AT RAD 2023
Dupilumab outcomes stable at end of open label atopic dermatitis study
WASHINGTON – The and no new emergent side effects, Lisa Beck, MD, reported during a late-breaking session at the annual Revolutionizing Atopic Dermatitis conference.
Other recent research on the biologic has shown that it improves lesional skin barrier function and rapidly reduces the abundance of Staphylococcus aureus on lesional skin, Dr. Beck, professor of dermatology at the University of Rochester (N.Y.), said during another session at the meeting on long-term control of AD. Dr. Beck directs a laboratory at the University of Rochester Medical Center that focuses on understanding AD and is involved in the National Institute of Allergy and Infectious Diseases (NIAID)-funded Atopic Dermatitis Research Network (ADRN).
The LIBERTY AD open-label extension (OLE) study was a phase 3 trial of 2,677 adults with moderate to severe AD who had participated in previous dupilumab clinical trials and were treated with 300 mg dupilumab weekly or every other week. Concomitant treatments were permitted, including topical corticosteroids and topical calcineurin inhibitors. (The proportion of patients dosed on an every-other-week or weekly dosing schedule was not available.)
Of 334 patients (12.5%) who remained in the trial at week 260, or 5 years, 88.9% achieved at least a 75% improvement in lesion extent and severity (Eczema Area and Severity Index [EASI]-75), and 76.2% achieved an EASI-90. The proportion achieving at least a 4-point reduction in the Peak Pruritus Numerical Rating Scale (NRS) or a score of 0 was 66.5%. At 5 years, improvements “seem very stable,” with “no loss in efficacy,” Dr. Beck said.
The majority of patients who withdrew from the open-label extension trial did so because the study was terminated at their site or because of the drug’s approval and commercialization – not for a medical reason, Dr. Beck said. Over the course of the extension trial, 4% of those enrolled withdrew because of adverse events and about 2% withdrew because of lack of efficacy.
Safety of dupilumab
The extension trial lacked a control arm, so Dr. Beck and her colleagues compared safety results to those in the final data set for patients in the LIBERTY AD CHRONOS study who received dupilumab 300 mg weekly with concomitant corticosteroids. The CHRONOS study was a 1-year randomized, double-blinded placebo-controlled phase 3 trial.
The exposure-adjusted incidence rate of severe treatment-emergent adverse events (TEAE) was lower at the close of the extension trial (5 patients/100 patient years [PY]) than at the end of the CHRONOS study (5.9 patients/100 PY). The incidence of serious adverse events related to treatment was 0.6 patients/100 PY in the final open label extension study data set, compared with 0.7 patients/100 PY in the CHRONOS final data set.
Adverse event rates “are really, if anything, slightly less in the OLE study versus the CHRONOS study, which was 1 year of treatment,” Dr. Beck said. And “no new adverse events have emerged.”
During a question and answer period, Dr. Beck pointed out that existing and future “real world” registries of patients on dupilumab and other new therapies will better inform dermatologists of adverse events than clinical trials have done.
Ocular surface disease
In a separate presentation on the safety of biologics, Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, said that in routine care, ocular surface disease is the most predominant side effect associated with dupilumab. “We don’t know the mechanism of action. But it’s not infectious, it’s not pink eye, and importantly, it’s not allergic conjunctivitis,” he said, noting that the spectrum of disease ranges from dry eye and eye itching to “frank conjunctivitis” and keratitis.
Most cases are mild to moderate and can often be managed with lubricating eye drops and periodic use of corticosteroid eye drops. Co-management with an ophthalmologist is often advisable, he said.
Dupilumab-associated erythema/eczema of the face was “not seen much” in clinical trials but is also being reported in the literature, largely by European researchers, Dr. Blauvelt said. “We hear a lot about red face, but I don’t think it’s much of an issue,” he said. “Most of the time, in my experience, it will [reflect] breakthrough residual AD, and I like to treat it with non-steroidal topicals.”
Occasionally, the withdrawal of steroids or allergic contact dermatitis are at play, Dr. Blauvelt said. “If you see red face in a person on dupilumab, use your clinical prowess, do a differential diagnosis, and treat accordingly.”
Effect on S. aureus
The vast majority of adults with moderate to severe AD have skin colonization with S. aureus, Dr. Beck said during the session on long-term control of AD. The presence of S. aureus in skin cultures correlates strongly with AD severity, type 2 immunity polarization, skin barrier disruption, and allergen sensitization, she said.
“So if we could do something to get rid of the staph and keep it away, one might imagine that would help” control the AD disease process, she said.
An ADRN study evaluated S. aureus in the skin of 71 patients who were randomized to receive dupilumab or placebo and found a “profound” effect of the biologic. “We were truly shocked by how quickly we saw a reduction in Staph aureus ... in lesional skin as early as 3 days” into treatment with dupilumab, she said of the unpublished findings. “And there is a pretty nice association with improvement in disease severity.”
Dr. Beck reported consultancy/advisory board work with Regeneron, Sanofi/Genzyme, among other disclosures. Dr. Blauvelt reported consultancy/advisory board work for Regeneron and Sanofi Genzyme and has received speakers bureau/honoraria for non-CME work for Regeneron and Sanofi, among other disclosures.
WASHINGTON – The and no new emergent side effects, Lisa Beck, MD, reported during a late-breaking session at the annual Revolutionizing Atopic Dermatitis conference.
Other recent research on the biologic has shown that it improves lesional skin barrier function and rapidly reduces the abundance of Staphylococcus aureus on lesional skin, Dr. Beck, professor of dermatology at the University of Rochester (N.Y.), said during another session at the meeting on long-term control of AD. Dr. Beck directs a laboratory at the University of Rochester Medical Center that focuses on understanding AD and is involved in the National Institute of Allergy and Infectious Diseases (NIAID)-funded Atopic Dermatitis Research Network (ADRN).
The LIBERTY AD open-label extension (OLE) study was a phase 3 trial of 2,677 adults with moderate to severe AD who had participated in previous dupilumab clinical trials and were treated with 300 mg dupilumab weekly or every other week. Concomitant treatments were permitted, including topical corticosteroids and topical calcineurin inhibitors. (The proportion of patients dosed on an every-other-week or weekly dosing schedule was not available.)
Of 334 patients (12.5%) who remained in the trial at week 260, or 5 years, 88.9% achieved at least a 75% improvement in lesion extent and severity (Eczema Area and Severity Index [EASI]-75), and 76.2% achieved an EASI-90. The proportion achieving at least a 4-point reduction in the Peak Pruritus Numerical Rating Scale (NRS) or a score of 0 was 66.5%. At 5 years, improvements “seem very stable,” with “no loss in efficacy,” Dr. Beck said.
The majority of patients who withdrew from the open-label extension trial did so because the study was terminated at their site or because of the drug’s approval and commercialization – not for a medical reason, Dr. Beck said. Over the course of the extension trial, 4% of those enrolled withdrew because of adverse events and about 2% withdrew because of lack of efficacy.
Safety of dupilumab
The extension trial lacked a control arm, so Dr. Beck and her colleagues compared safety results to those in the final data set for patients in the LIBERTY AD CHRONOS study who received dupilumab 300 mg weekly with concomitant corticosteroids. The CHRONOS study was a 1-year randomized, double-blinded placebo-controlled phase 3 trial.
The exposure-adjusted incidence rate of severe treatment-emergent adverse events (TEAE) was lower at the close of the extension trial (5 patients/100 patient years [PY]) than at the end of the CHRONOS study (5.9 patients/100 PY). The incidence of serious adverse events related to treatment was 0.6 patients/100 PY in the final open label extension study data set, compared with 0.7 patients/100 PY in the CHRONOS final data set.
Adverse event rates “are really, if anything, slightly less in the OLE study versus the CHRONOS study, which was 1 year of treatment,” Dr. Beck said. And “no new adverse events have emerged.”
During a question and answer period, Dr. Beck pointed out that existing and future “real world” registries of patients on dupilumab and other new therapies will better inform dermatologists of adverse events than clinical trials have done.
Ocular surface disease
In a separate presentation on the safety of biologics, Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, said that in routine care, ocular surface disease is the most predominant side effect associated with dupilumab. “We don’t know the mechanism of action. But it’s not infectious, it’s not pink eye, and importantly, it’s not allergic conjunctivitis,” he said, noting that the spectrum of disease ranges from dry eye and eye itching to “frank conjunctivitis” and keratitis.
Most cases are mild to moderate and can often be managed with lubricating eye drops and periodic use of corticosteroid eye drops. Co-management with an ophthalmologist is often advisable, he said.
Dupilumab-associated erythema/eczema of the face was “not seen much” in clinical trials but is also being reported in the literature, largely by European researchers, Dr. Blauvelt said. “We hear a lot about red face, but I don’t think it’s much of an issue,” he said. “Most of the time, in my experience, it will [reflect] breakthrough residual AD, and I like to treat it with non-steroidal topicals.”
Occasionally, the withdrawal of steroids or allergic contact dermatitis are at play, Dr. Blauvelt said. “If you see red face in a person on dupilumab, use your clinical prowess, do a differential diagnosis, and treat accordingly.”
Effect on S. aureus
The vast majority of adults with moderate to severe AD have skin colonization with S. aureus, Dr. Beck said during the session on long-term control of AD. The presence of S. aureus in skin cultures correlates strongly with AD severity, type 2 immunity polarization, skin barrier disruption, and allergen sensitization, she said.
“So if we could do something to get rid of the staph and keep it away, one might imagine that would help” control the AD disease process, she said.
An ADRN study evaluated S. aureus in the skin of 71 patients who were randomized to receive dupilumab or placebo and found a “profound” effect of the biologic. “We were truly shocked by how quickly we saw a reduction in Staph aureus ... in lesional skin as early as 3 days” into treatment with dupilumab, she said of the unpublished findings. “And there is a pretty nice association with improvement in disease severity.”
Dr. Beck reported consultancy/advisory board work with Regeneron, Sanofi/Genzyme, among other disclosures. Dr. Blauvelt reported consultancy/advisory board work for Regeneron and Sanofi Genzyme and has received speakers bureau/honoraria for non-CME work for Regeneron and Sanofi, among other disclosures.
WASHINGTON – The and no new emergent side effects, Lisa Beck, MD, reported during a late-breaking session at the annual Revolutionizing Atopic Dermatitis conference.
Other recent research on the biologic has shown that it improves lesional skin barrier function and rapidly reduces the abundance of Staphylococcus aureus on lesional skin, Dr. Beck, professor of dermatology at the University of Rochester (N.Y.), said during another session at the meeting on long-term control of AD. Dr. Beck directs a laboratory at the University of Rochester Medical Center that focuses on understanding AD and is involved in the National Institute of Allergy and Infectious Diseases (NIAID)-funded Atopic Dermatitis Research Network (ADRN).
The LIBERTY AD open-label extension (OLE) study was a phase 3 trial of 2,677 adults with moderate to severe AD who had participated in previous dupilumab clinical trials and were treated with 300 mg dupilumab weekly or every other week. Concomitant treatments were permitted, including topical corticosteroids and topical calcineurin inhibitors. (The proportion of patients dosed on an every-other-week or weekly dosing schedule was not available.)
Of 334 patients (12.5%) who remained in the trial at week 260, or 5 years, 88.9% achieved at least a 75% improvement in lesion extent and severity (Eczema Area and Severity Index [EASI]-75), and 76.2% achieved an EASI-90. The proportion achieving at least a 4-point reduction in the Peak Pruritus Numerical Rating Scale (NRS) or a score of 0 was 66.5%. At 5 years, improvements “seem very stable,” with “no loss in efficacy,” Dr. Beck said.
The majority of patients who withdrew from the open-label extension trial did so because the study was terminated at their site or because of the drug’s approval and commercialization – not for a medical reason, Dr. Beck said. Over the course of the extension trial, 4% of those enrolled withdrew because of adverse events and about 2% withdrew because of lack of efficacy.
Safety of dupilumab
The extension trial lacked a control arm, so Dr. Beck and her colleagues compared safety results to those in the final data set for patients in the LIBERTY AD CHRONOS study who received dupilumab 300 mg weekly with concomitant corticosteroids. The CHRONOS study was a 1-year randomized, double-blinded placebo-controlled phase 3 trial.
The exposure-adjusted incidence rate of severe treatment-emergent adverse events (TEAE) was lower at the close of the extension trial (5 patients/100 patient years [PY]) than at the end of the CHRONOS study (5.9 patients/100 PY). The incidence of serious adverse events related to treatment was 0.6 patients/100 PY in the final open label extension study data set, compared with 0.7 patients/100 PY in the CHRONOS final data set.
Adverse event rates “are really, if anything, slightly less in the OLE study versus the CHRONOS study, which was 1 year of treatment,” Dr. Beck said. And “no new adverse events have emerged.”
During a question and answer period, Dr. Beck pointed out that existing and future “real world” registries of patients on dupilumab and other new therapies will better inform dermatologists of adverse events than clinical trials have done.
Ocular surface disease
In a separate presentation on the safety of biologics, Andrew Blauvelt, MD, MBA, of the Oregon Medical Research Center, Portland, said that in routine care, ocular surface disease is the most predominant side effect associated with dupilumab. “We don’t know the mechanism of action. But it’s not infectious, it’s not pink eye, and importantly, it’s not allergic conjunctivitis,” he said, noting that the spectrum of disease ranges from dry eye and eye itching to “frank conjunctivitis” and keratitis.
Most cases are mild to moderate and can often be managed with lubricating eye drops and periodic use of corticosteroid eye drops. Co-management with an ophthalmologist is often advisable, he said.
Dupilumab-associated erythema/eczema of the face was “not seen much” in clinical trials but is also being reported in the literature, largely by European researchers, Dr. Blauvelt said. “We hear a lot about red face, but I don’t think it’s much of an issue,” he said. “Most of the time, in my experience, it will [reflect] breakthrough residual AD, and I like to treat it with non-steroidal topicals.”
Occasionally, the withdrawal of steroids or allergic contact dermatitis are at play, Dr. Blauvelt said. “If you see red face in a person on dupilumab, use your clinical prowess, do a differential diagnosis, and treat accordingly.”
Effect on S. aureus
The vast majority of adults with moderate to severe AD have skin colonization with S. aureus, Dr. Beck said during the session on long-term control of AD. The presence of S. aureus in skin cultures correlates strongly with AD severity, type 2 immunity polarization, skin barrier disruption, and allergen sensitization, she said.
“So if we could do something to get rid of the staph and keep it away, one might imagine that would help” control the AD disease process, she said.
An ADRN study evaluated S. aureus in the skin of 71 patients who were randomized to receive dupilumab or placebo and found a “profound” effect of the biologic. “We were truly shocked by how quickly we saw a reduction in Staph aureus ... in lesional skin as early as 3 days” into treatment with dupilumab, she said of the unpublished findings. “And there is a pretty nice association with improvement in disease severity.”
Dr. Beck reported consultancy/advisory board work with Regeneron, Sanofi/Genzyme, among other disclosures. Dr. Blauvelt reported consultancy/advisory board work for Regeneron and Sanofi Genzyme and has received speakers bureau/honoraria for non-CME work for Regeneron and Sanofi, among other disclosures.
AT RAD 2023
JAK-inhibitor safety in adolescents with AD: Long-term analyses reported
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
WASHINGTON – and over 1,000 patient-years of exposure, Lawrence F. Eichenfield, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
In March 2023, the oral Janus kinase 1 (JAK1) inhibitor was approved by the Food and Drug Administration for treating adolescents aged 12-17 with refractory moderate to severe AD – an expanded indication from the approval in adults in 2022.
The new analysis evaluated data from patients who participated in the phase 3 JADE clinical trials – MONO-1, MONO-2, TEEN, and REGIMEN – and were subsequently enrolled in the ongoing phase 3 extension trial JADE EXTEND. Compared with a previous post hoc analysis in which adolescent patients had approximately 1 year of exposure, this updated analysis includes a sizable portion of patients with more than 96 weeks of exposure.
“We’re starting to get good numbers of [adolescents] who’ve had about 2 years of exposure,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of the department of dermatology at the University of California, San Diego, during a late-breaking research session.
With a data cut for this analysis of September 2021, “we haven’t seen additive long-term [adverse] effects” with longer exposures, he said. In addition, “there were no unique safety concerns related to adolescents compared to the findings observed [in an] integrated safety analysis using the same data cut in which most patients were adults.”
(The analysis in adults covered 3,802 patients with over 5,000 patient-years of exposure, and was presented at the annual American Academy of Dermatology meeting in March 2023.)
Also presented in the late-breaking abstract session at RAD 2023 was a long-term safety study of upadacitinib (Rinvoq), the other JAK1 inhibitor approved for adolescents with AD – approved by the FDA for both adolescents and adults with moderate to severe AD in 2022. The new analysis captures exposure of up to 4 years and shows no “worsening or accumulation of events,” compared with 1-year data, reported Christopher G. Bunick, MD, PhD, of the department of dermatology and the program in translational biomedicine at Yale University, New Haven, Conn.
Abrocitinib in adolescents
For the safety analysis of abrocitinib (Cibinqo), data were pooled into two cohorts: A consistent-dose cohort of 490 adolescents who received the same dose (200 mg or 100 mg) during the entire duration of the qualifying JADE trials, and a variable-dose cohort of 145 adolescents who received different doses (200 mg or 100 mg) during the JADE REGIMEN qualifying trial.
Duration of exposure was 96 weeks or more in 37%-38% of the consistent-dose cohort and 68% of the variable-dose cohort.
In the consistent-dose cohort, adverse events occurred in 243 (84%) and 153 (76%) of patients receiving 200-mg doses and 100-mg doses, respectively. Incidence rates for severe adverse events were 5.87 per 100 patient-years at both doses, and rates for adverse events leading to study discontinuation were 6.96/100 patient-years at 200 mg and 5.13/100 patient-years at 100 mg.
“No meaningful dose-response relationship was observed for serious adverse events, or adverse events leading to discontinuation, or adverse events of special interest,” said Dr. Eichenfield, also chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.
The IRs of adverse events of special interest were 1.84/100 patient-years and 1.28/100 patient-years for serious infection; 2.11/100 patient-years, and 1.62/100 patient-years for all herpes zoster infections; and 0.69/100 patient-years and 0.32/100 patient-years for opportunistic herpes zoster infections in the 200-mg and 100-mg arms, respectively.
“Other than herpes zoster, there were no opportunistic infections observed and no tuberculosis cases,” he said. “There was one nonfatal venous thromboembolism in an adolescent who had a very strong family history of [pulmonary embolism], one retinal detachment [with a concurrent diagnosis of cataracts and of left eyebrow folliculitis], and no events of nonmelanoma skin cancer or other malignancies, major adverse cardiovascular events, or deaths.” The thromboembolism case was reported in the previous post hoc analysis.
In the variable-dose cohort, data were similar, Dr. Eichenfield said. The IRs for severe adverse events, adverse events leading to study withdrawal, and adverse events of special interest were consistent with those in the other cohort. And similarly, there were no reports of tuberculosis or other opportunistic infections (excluding herpes zoster), and no reports of nonmelanoma skin cancer (NMSC) or other malignancies, major adverse cardiovascular events (MACE), or death. In this cohort, there were no venous thromboembolism (VTE) reports.
Upadacitinib in adolescents, adults
The new analysis looked at up to 4 years of upadacitinib treatment in almost 2,700 adolescents and adults– and over 6,200 patient-years – using integrated data from three ongoing pivotal phase 3 studies: Measure Up 1, Measure Up 2, and AD Up. (Of these patients, 539 were adolescents, Dr. Bunick said after the meeting.)
In the Measure Up studies, patients were randomized 1:1:1 to receive a 15-mg dose, a 30-mg dose, or placebo once daily. In AD Up, patients in each arm received concomitant topical corticosteroids. At week 16, patients receiving the drug continued their assigned treatment during the ongoing blinded extension period, and those receiving placebo were rerandomized to upadacitinib 15 mg or 30 mg.
The exposure-adjusted event rates for any adverse event leading to discontinuation were 4.1/100 patient-years and 4.7/100 patient-years in patients receiving 15 mg and 30 mg, respectively, and the rates of any serious adverse event were 6.5/100 patient-years and 7.5/100 patient-years, Dr. Bunick reported. Three deaths occurred in the 30-mg group; all deaths were related to COVID infection and occurred in adults with cardiovascular risk factors.
Incidence rates of adverse events of special interest were similar to those in a previous 1-year analysis. The rate of serious infections per 100 patient years, for instance, was 2.3 and 2.8 in the 15-mg and 30-mg groups, respectively, compared with 2.2 and 2.8 in the 1-year analysis.
The rate of opportunistic infections, including eczema herpeticum (and excluding TB and herpes zoster), saw a slight bump in the new analysis to 2.4/100 patient-years with the 30-mg dose. Other event rates, across both dosages and durations, were less than 0.1/100 patient-years for active TB; 0.3-0.4/100 patient-years for NMSC, and 0.1/100 patient-years or below for other malignancies, MACE, and VTE. Herpes zoster had the highest event rate in both the 1- and 4-year analyses of between 3.1/100 patient-years and 5.8/100 patient-years, Dr. Bunick reported.
The adverse event rates for adolescents and adults “show consistency and are very low,” Dr. Bunick said. At 4 years, no new safety risks were identified.
‘The more data ... the better’
Data on the safety of new medications in children and adolescents is always important, and with systemic JAK inhibitors in particular, “the more data we can accumulate in [younger] patients with AD ... the better,” said Robert Sidbury, MD, MPH, professor in the department of pediatrics at the University of Washington, Seattle, and chief of the division of dermatology at Seattle Children’s Hospital, who was asked to comment on the two studies.
Dermatologists have taken comfort in the fact that the “daunting” boxed warning on JAK inhibitors “was generated in a very different population than we generally propose to treat, certainly when talking about children and adolescents,” said Dr. Sidbury, who was not involved in either of the new safety analyses.
The JAK inhibitor boxed warning “reflects a study of tofacitinib – a different JAK inhibitor with arguably more risk of adverse effects – in adults over the age of 50 with rheumatoid arthritis and multiple risk factors for comorbidities included in the boxed warning,” he said.
“This allows dermatologists to reasonably conclude that the boxed warning – while critical to discuss and consider in every patient – is likely less concerning than might otherwise by implied.”
With more patient experience, “the more our assessment of risk, and of the ‘legitimacy’ of the boxed warning in our patient population, becomes evidence-based as opposed to extrapolation,” Dr. Sidbury said.
The two studies reported, he said, “detail an experience that is not adverse effect free –I have yet to find that medication – but is a reasonable profile considering the robust efficacy results they accompany.”
The abrocitinib safety analysis was sponsored by Pfizer. Regarding the study of upadacitinib, AbbVie contributed to the design of the safety analysis and participated in data collection. No honoria or payments were made to the authors, according to the study abstract. Dr. Eichenfield is a consultant/advisory board member for Pfizer and other companies, and has served on the speakers bureau/received honoria for Pfizer and other companies. Dr. Bunick is a consultant for AbbVie and other companies, and has served as an speaker/received honoraria or served as an investigator for several companies. Dr. Sidbury disclosed being a consultant/advisory board member for Lilly and Leo and serving on the speakers bureau/honoraria for Beiersdorf. All reported receiving grant/research support from various companies.
AT RAD 2023
AD in infancy: Diagnostic advice and treatment tips
WASHINGTON – mean it is often “woefully undertreated,” Robert Sidbury, MD, MPH, said at the annual Revolutionizing Atopic Dermatitis conference.
Identifying and mitigating triggers – such as irritation, contact allergy, and infection – is a cornerstone of treatment in infants, but tailored therapy with topical corticosteroids, topical calcineurin inhibitors (TCIs), and topical phosphodiesterase 4 (PDE4) inhibitors also have roles to play, said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital and professor in the department of pediatrics at the University of Washington, Seattle.
Views on the use of dupilumab as a systemic agent for severe infantile AD, meanwhile, have shifted significantly in the past year with the Food and Drug Administration approval of the biologic for children aged 6 months to 5 years and with extended experience with the biologic in all ages, including children, Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the meeting.
The pediatric dermatologists spoke during a session devoted to AD in infants, during which the diagnosis of AD and the role – and risks – of food allergy testing were also discussed. Diagnosis, said Elaine C. Siegfried, MD, who also spoke during the session, requires careful consideration of mimicking conditions and a broader list of differential diagnoses in those infants with poor growth or frequent infections.
Here are some of the experts’ pearls for practice.
Diagnosing AD in infants
Among infants who are growing well and otherwise healthy, the infantile eczema phenotype encompasses AD, seborrheic dermatitis, contact dermatitis, psoriasis – and overlap of more than one of these conditions. “Overlap is common,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital.
(Initial topical treatment for all these conditions is similar, but optimal treatment may differ for young children with moderate to severe disease that requires systemic treatment, she said in an interview after the meeting.)
Sparing of the diaper area that reflects skin barrier integrity is a classic feature of AD in infants and can be a useful diagnostic sign. In addition, “hypopigmentation is more characteristic of psoriasis” than AD, whereas AD tends to be hyperpigmented, which is most obvious in skin-of-color patients, Dr. Siegfried said.
Disease-specific pigment changes may be related to microbial colonization – such as Malassezia-associated hypopigmentation – or cell turnover, which is faster in psoriasis and slower in AD – with corresponding differences in pigment retention, and may be more obvious in children than adults, she said.
A less common scenario is dermatitis in infants who are not growing well. For these patients, she noted, the differential diagnosis includes metabolic or immune deficiency dermatitis as well as a variety of genodermatoses.
Generalized redness and scaling present on the first day of life is suggestive of non-atopic dermatitis. “If you’re born with red scaly skin, that’s very different than if you develop red skin in the first month or two of life,” Dr. Siegfried said.
When there is diaper area involvement with AD, contact dermatitis, impetigo, and Candida may be complicating factors. And in infants with other morbidities – especially those who are not growing well – diaper area involvement suggests a broader differential diagnosis. “I implore you, if you see children, make sure you weigh and measure them at every appointment,” she said.
Dr. Siegfried has seen infants with Netherton syndrome, and those with cystic fibrosis with zinc deficiency, for instance, presenting with “an eczematous-like picture,” diaper-area involvement, and other morbidities.
For infants with AD, she maintains a high index of suspicion for secondary infections such as molluscum, herpes simplex virus (HSV) with or without streptococci, scabies, tinea, and group A streptococci. “Secondary infections ... may be incognito,” she said. “Look for subtle signs. Even molluscum can be very subtle.”
Secondary allergic contact dermatitis is also common although it’s “technically difficult to confirm the diagnosis,” she said. Patch testing in infants is technically challenging, sensitivity is low, and monosensitization is uncommon. “So I do initial empiric topical allergen avoidance,” she said, keeping in mind ubiquitous and avoidable topical allergens such as Kathon, cocamidopropyl betaine, propylene glycol, disperse blue, and adhesives.
Treating AD in infancy
Irritation “is probably one of the biggest triggers” of AD in infants, and the often “pristine” diaper area compared with inflamed eczema elsewhere can demonstrate the importance of moisturization for healthy skin in atopic infants, Dr. Sidbury said.
Among treatments that “punch above their weight” for AD in infants is an ointment-based barrier applied around the mouth, chin, and chest – where the wet/dry impact of drooling is maximal – before and after meals, he said.
Another is hydrocortisone 2.5% mixed 1:1 with mupirocin for those infants who have secondary infections and “that exudative, weepy-looking appearance on the face,” he said. The topical antibiotic in the combination cream “lessens the potency of the steroid and oftentimes by synergy, makes it more effective” by simultaneously treating inflammation, he said. He cautions against products containing neomycin, which can be an allergen.
A combination antibiotic-steroid-emollient cream (the Aron Regimen) can also “sometimes punch above its weight,” Dr. Sidbury said.
Infections typically involve Staphylococcus aureus, but in up to 16% of cases Streptococcus is involved. And notably, lurking underneath the honey-colored crusting of S. aureus infections may be the grouped vesicles that characterize eczema herpeticum, Dr. Sidbury said.
“Counsel [parents] preemptively to treat cold sores immediately [in order to] decrease HSV shedding and minimize risk to their baby,” Dr. Sidbury said.
For treating AD-associated inflammation in skin not affected by secondary infections, over-the-counter 1% hydrocortisone cream is often sufficient, and “for very young babies and preemies in particular, I generally don’t use anything stronger because their skin barrier isn’t fully complete yet, so they absorb more than an older child does,” he said, referring to ages 2 months corrected as a marker for considering a stronger formulation if needed.
Many parents are “very concerned” about topical corticosteroid (TCS) use and pediatricians are also “often concerned,” Dr. Sidbury said. Addressing this concern, he tries to provide context and promote adherence by pointing out that infants have an easily visible vein at the temple area where the skin is naturally thin. If parents were to see this appearance for the first time in other areas while using topical steroids, he tells them, it may be the first sign of skin thinning, but “it’s entirely reversible at that stage.”
He also stressed the cost of not treating. It’s unknown whether “treating aggressively early on prevents any future development or manifestation of eczema, or future comorbidities, but we don’t know that it doesn’t,” Dr. Sidbury said. “And we certainly know how miserable that baby with eczema can be in the short term. So we need to use these medicines.”
Dr. Sidbury utilizes tacrolimus 0.03% ointment, a topical calcineurin inhibitor (TCI), only if he is worried about overuse of steroids, and uses a regimen that alternates the TCI (used in infants off-label because it is approved for ages 2 years and older) with TCS in periods of similar duration (for example, treatment with TCS for 1 week and TCI for 1 week, or rotations of 2 weeks each or 3 days each). “And these rotations may be dynamic depending on severity of the flare at any given time,” he said after the meeting.
Preapproval data from the pivotal trials of tacrolimus are reassuring and can be shared with parents. “Two-year-olds had 90% of BSA [body surface area] treated for 12 weeks” with no signs of systemic risks, Dr. Sidbury said at the meeting.
Crisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor approved for AD down to age 3 months, does not, like tacrolimus, have a boxed warning about a possible risk for cancer, and may also be alternated with TCSs. It will cause stinging in some children, but TCSs and TCIs can also sting in some children, he said, noting that samples can be helpful to predict what will or won’t sting each infant.
Systemic treatment in infants
The Liberty AD PRESCHOOL phase 3 trial that supported the FDA’s approval of dupilumab down to 6 months of age, published in 2022 in The Lancet, covered ages 6 months to 5 years but included only six children under the age of 2, “leaving us with a very limited dataset in this age group,” Dr. Eichenfield said at the meeting.
Other data and analyses that have provided reassurance, such as a laboratory safety analysis published online in 2022 showing no meaningful changes in laboratory safety parameters in children as young as 6 months, and pediatric data (not including infants) presented at a RAD meeting in 2022 showing that dupilumab, an interleukin-4 receptor alpha antagonist, may have positive effects on bone mineral density.
Data from the Liberty AD PRESCHOOL open-label extension study presented at the American Academy of Dermatology meeting in 2023, meanwhile, show that “the adverse event profile is not looking much different than what we see in older children,” Dr. Eichenfield said. “There are low rates of severe adverse events and a very low rate of discontinuations.”
At Rady Children’s Hospital, where Dr. Eichenfield is chief of pediatric and adolescent dermatology, dupilumab has become a first-line systemic agent for severe infantile AD, supplanting prior traditional but little used systemic agents such as oral corticosteroids, cyclosporine, methotrexate, azathioprine, or mycophenolate, he said after the meeting.
The decision to use systemics in the first 2 years of life is “a comprehensive one,” requiring knowledge of the child’s history, disease course, and assessment of response to prior therapies, comorbidities and severity, he said.
Food allergy in infants with AD
Food allergy is common in children with moderate to severe AD, but true food-triggered AD, with AD being the only symptom of food allergy, is rare, said Anne Marie Singh, MD, associate professor in the division of allergy and immunology and rheumatology at the University of Wisconsin, Madison, who focuses on pediatrics.
Over the years, studies of double-blind placebo-controlled food challenge tests in children with AD have tended to conflate immediate IgE hypersensitivity (and skin symptoms like urticaria) with AD, said Dr. Singh, who directs the university’s Food Allergy Research and Education Center of Excellence. In a recently published study she led involving 374 children with AD referred to allergy and/or dermatology subspecialty clinics at the University of Wisconsin, Madison, 55% had a food allergy but only 2% had food-triggered AD “where eczema is the only symptom and removal of the food cleared up the eczema and its return brought it back,” Dr. Singh said at the meeting. Another 4% had combined IgE-mediated food allergy and food-triggered AD. Almost half of the children with food-triggered AD were under 1 year of age, and egg was the most common trigger, she noted.
Food should be implicated largely by history, Dr. Singh emphasized.
Food allergy testing in the context of AD can be done but is challenging, with the clinical relevance of skin prick testing and food-specific immunoglobulin E (sIgE) difficult to predict. Predictive values of sIgE levels are established for immediate IgE mediated food allergy, but “cut-offs” for food-triggered AD are not established, she explained, noting that “cut-offs are likely higher for our children with AD.”
Elimination diets, moreover, pose significant risks of future oral tolerance and risks of nutritional deficiencies and poor growth, Dr. Singh said. New and immediate reactions to foods that are reintroduced after an elimination diet are common, and research has shown that 20% or more of such reactions involve anaphylaxis. “If an elimination diet is undertaken, you need emergency action plans, injectable epinephrine, and nutrition counseling,” she said.
A recent systematic review and meta-analysis conditionally recommended against elimination diets for the treatment of AD, Dr. Singh noted.
Asked by Dr. Sidbury whether there “is a sweet spot where you can eliminate [foods] without going all the way,” Dr. Singh said she will sometimes do a “diagnostic elimination trial” with food elimination for 2-4 weeks only – a time period after which “I’ll feel really comfortable reintroducing the food.”
Dr. Singh urged dermatologists to “know your allergist” because “patients respond best with a consistent message.”
Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, Lilly, and Beiersdorf. Dr. Siegfried reported ties with Pfizer, Regeneron, Sanofi Genzyme, Pfizer, UCB, Novan and Leo Pharma. Dr. Singh reported ties with Incyte and Siolta Therapeutics. Dr. Eichenfield reported ties with Pfizer, Regeneron, Sanofi Genzyme, Incyte, and Pfizer.
WASHINGTON – mean it is often “woefully undertreated,” Robert Sidbury, MD, MPH, said at the annual Revolutionizing Atopic Dermatitis conference.
Identifying and mitigating triggers – such as irritation, contact allergy, and infection – is a cornerstone of treatment in infants, but tailored therapy with topical corticosteroids, topical calcineurin inhibitors (TCIs), and topical phosphodiesterase 4 (PDE4) inhibitors also have roles to play, said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital and professor in the department of pediatrics at the University of Washington, Seattle.
Views on the use of dupilumab as a systemic agent for severe infantile AD, meanwhile, have shifted significantly in the past year with the Food and Drug Administration approval of the biologic for children aged 6 months to 5 years and with extended experience with the biologic in all ages, including children, Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the meeting.
The pediatric dermatologists spoke during a session devoted to AD in infants, during which the diagnosis of AD and the role – and risks – of food allergy testing were also discussed. Diagnosis, said Elaine C. Siegfried, MD, who also spoke during the session, requires careful consideration of mimicking conditions and a broader list of differential diagnoses in those infants with poor growth or frequent infections.
Here are some of the experts’ pearls for practice.
Diagnosing AD in infants
Among infants who are growing well and otherwise healthy, the infantile eczema phenotype encompasses AD, seborrheic dermatitis, contact dermatitis, psoriasis – and overlap of more than one of these conditions. “Overlap is common,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital.
(Initial topical treatment for all these conditions is similar, but optimal treatment may differ for young children with moderate to severe disease that requires systemic treatment, she said in an interview after the meeting.)
Sparing of the diaper area that reflects skin barrier integrity is a classic feature of AD in infants and can be a useful diagnostic sign. In addition, “hypopigmentation is more characteristic of psoriasis” than AD, whereas AD tends to be hyperpigmented, which is most obvious in skin-of-color patients, Dr. Siegfried said.
Disease-specific pigment changes may be related to microbial colonization – such as Malassezia-associated hypopigmentation – or cell turnover, which is faster in psoriasis and slower in AD – with corresponding differences in pigment retention, and may be more obvious in children than adults, she said.
A less common scenario is dermatitis in infants who are not growing well. For these patients, she noted, the differential diagnosis includes metabolic or immune deficiency dermatitis as well as a variety of genodermatoses.
Generalized redness and scaling present on the first day of life is suggestive of non-atopic dermatitis. “If you’re born with red scaly skin, that’s very different than if you develop red skin in the first month or two of life,” Dr. Siegfried said.
When there is diaper area involvement with AD, contact dermatitis, impetigo, and Candida may be complicating factors. And in infants with other morbidities – especially those who are not growing well – diaper area involvement suggests a broader differential diagnosis. “I implore you, if you see children, make sure you weigh and measure them at every appointment,” she said.
Dr. Siegfried has seen infants with Netherton syndrome, and those with cystic fibrosis with zinc deficiency, for instance, presenting with “an eczematous-like picture,” diaper-area involvement, and other morbidities.
For infants with AD, she maintains a high index of suspicion for secondary infections such as molluscum, herpes simplex virus (HSV) with or without streptococci, scabies, tinea, and group A streptococci. “Secondary infections ... may be incognito,” she said. “Look for subtle signs. Even molluscum can be very subtle.”
Secondary allergic contact dermatitis is also common although it’s “technically difficult to confirm the diagnosis,” she said. Patch testing in infants is technically challenging, sensitivity is low, and monosensitization is uncommon. “So I do initial empiric topical allergen avoidance,” she said, keeping in mind ubiquitous and avoidable topical allergens such as Kathon, cocamidopropyl betaine, propylene glycol, disperse blue, and adhesives.
Treating AD in infancy
Irritation “is probably one of the biggest triggers” of AD in infants, and the often “pristine” diaper area compared with inflamed eczema elsewhere can demonstrate the importance of moisturization for healthy skin in atopic infants, Dr. Sidbury said.
Among treatments that “punch above their weight” for AD in infants is an ointment-based barrier applied around the mouth, chin, and chest – where the wet/dry impact of drooling is maximal – before and after meals, he said.
Another is hydrocortisone 2.5% mixed 1:1 with mupirocin for those infants who have secondary infections and “that exudative, weepy-looking appearance on the face,” he said. The topical antibiotic in the combination cream “lessens the potency of the steroid and oftentimes by synergy, makes it more effective” by simultaneously treating inflammation, he said. He cautions against products containing neomycin, which can be an allergen.
A combination antibiotic-steroid-emollient cream (the Aron Regimen) can also “sometimes punch above its weight,” Dr. Sidbury said.
Infections typically involve Staphylococcus aureus, but in up to 16% of cases Streptococcus is involved. And notably, lurking underneath the honey-colored crusting of S. aureus infections may be the grouped vesicles that characterize eczema herpeticum, Dr. Sidbury said.
“Counsel [parents] preemptively to treat cold sores immediately [in order to] decrease HSV shedding and minimize risk to their baby,” Dr. Sidbury said.
For treating AD-associated inflammation in skin not affected by secondary infections, over-the-counter 1% hydrocortisone cream is often sufficient, and “for very young babies and preemies in particular, I generally don’t use anything stronger because their skin barrier isn’t fully complete yet, so they absorb more than an older child does,” he said, referring to ages 2 months corrected as a marker for considering a stronger formulation if needed.
Many parents are “very concerned” about topical corticosteroid (TCS) use and pediatricians are also “often concerned,” Dr. Sidbury said. Addressing this concern, he tries to provide context and promote adherence by pointing out that infants have an easily visible vein at the temple area where the skin is naturally thin. If parents were to see this appearance for the first time in other areas while using topical steroids, he tells them, it may be the first sign of skin thinning, but “it’s entirely reversible at that stage.”
He also stressed the cost of not treating. It’s unknown whether “treating aggressively early on prevents any future development or manifestation of eczema, or future comorbidities, but we don’t know that it doesn’t,” Dr. Sidbury said. “And we certainly know how miserable that baby with eczema can be in the short term. So we need to use these medicines.”
Dr. Sidbury utilizes tacrolimus 0.03% ointment, a topical calcineurin inhibitor (TCI), only if he is worried about overuse of steroids, and uses a regimen that alternates the TCI (used in infants off-label because it is approved for ages 2 years and older) with TCS in periods of similar duration (for example, treatment with TCS for 1 week and TCI for 1 week, or rotations of 2 weeks each or 3 days each). “And these rotations may be dynamic depending on severity of the flare at any given time,” he said after the meeting.
Preapproval data from the pivotal trials of tacrolimus are reassuring and can be shared with parents. “Two-year-olds had 90% of BSA [body surface area] treated for 12 weeks” with no signs of systemic risks, Dr. Sidbury said at the meeting.
Crisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor approved for AD down to age 3 months, does not, like tacrolimus, have a boxed warning about a possible risk for cancer, and may also be alternated with TCSs. It will cause stinging in some children, but TCSs and TCIs can also sting in some children, he said, noting that samples can be helpful to predict what will or won’t sting each infant.
Systemic treatment in infants
The Liberty AD PRESCHOOL phase 3 trial that supported the FDA’s approval of dupilumab down to 6 months of age, published in 2022 in The Lancet, covered ages 6 months to 5 years but included only six children under the age of 2, “leaving us with a very limited dataset in this age group,” Dr. Eichenfield said at the meeting.
Other data and analyses that have provided reassurance, such as a laboratory safety analysis published online in 2022 showing no meaningful changes in laboratory safety parameters in children as young as 6 months, and pediatric data (not including infants) presented at a RAD meeting in 2022 showing that dupilumab, an interleukin-4 receptor alpha antagonist, may have positive effects on bone mineral density.
Data from the Liberty AD PRESCHOOL open-label extension study presented at the American Academy of Dermatology meeting in 2023, meanwhile, show that “the adverse event profile is not looking much different than what we see in older children,” Dr. Eichenfield said. “There are low rates of severe adverse events and a very low rate of discontinuations.”
At Rady Children’s Hospital, where Dr. Eichenfield is chief of pediatric and adolescent dermatology, dupilumab has become a first-line systemic agent for severe infantile AD, supplanting prior traditional but little used systemic agents such as oral corticosteroids, cyclosporine, methotrexate, azathioprine, or mycophenolate, he said after the meeting.
The decision to use systemics in the first 2 years of life is “a comprehensive one,” requiring knowledge of the child’s history, disease course, and assessment of response to prior therapies, comorbidities and severity, he said.
Food allergy in infants with AD
Food allergy is common in children with moderate to severe AD, but true food-triggered AD, with AD being the only symptom of food allergy, is rare, said Anne Marie Singh, MD, associate professor in the division of allergy and immunology and rheumatology at the University of Wisconsin, Madison, who focuses on pediatrics.
Over the years, studies of double-blind placebo-controlled food challenge tests in children with AD have tended to conflate immediate IgE hypersensitivity (and skin symptoms like urticaria) with AD, said Dr. Singh, who directs the university’s Food Allergy Research and Education Center of Excellence. In a recently published study she led involving 374 children with AD referred to allergy and/or dermatology subspecialty clinics at the University of Wisconsin, Madison, 55% had a food allergy but only 2% had food-triggered AD “where eczema is the only symptom and removal of the food cleared up the eczema and its return brought it back,” Dr. Singh said at the meeting. Another 4% had combined IgE-mediated food allergy and food-triggered AD. Almost half of the children with food-triggered AD were under 1 year of age, and egg was the most common trigger, she noted.
Food should be implicated largely by history, Dr. Singh emphasized.
Food allergy testing in the context of AD can be done but is challenging, with the clinical relevance of skin prick testing and food-specific immunoglobulin E (sIgE) difficult to predict. Predictive values of sIgE levels are established for immediate IgE mediated food allergy, but “cut-offs” for food-triggered AD are not established, she explained, noting that “cut-offs are likely higher for our children with AD.”
Elimination diets, moreover, pose significant risks of future oral tolerance and risks of nutritional deficiencies and poor growth, Dr. Singh said. New and immediate reactions to foods that are reintroduced after an elimination diet are common, and research has shown that 20% or more of such reactions involve anaphylaxis. “If an elimination diet is undertaken, you need emergency action plans, injectable epinephrine, and nutrition counseling,” she said.
A recent systematic review and meta-analysis conditionally recommended against elimination diets for the treatment of AD, Dr. Singh noted.
Asked by Dr. Sidbury whether there “is a sweet spot where you can eliminate [foods] without going all the way,” Dr. Singh said she will sometimes do a “diagnostic elimination trial” with food elimination for 2-4 weeks only – a time period after which “I’ll feel really comfortable reintroducing the food.”
Dr. Singh urged dermatologists to “know your allergist” because “patients respond best with a consistent message.”
Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, Lilly, and Beiersdorf. Dr. Siegfried reported ties with Pfizer, Regeneron, Sanofi Genzyme, Pfizer, UCB, Novan and Leo Pharma. Dr. Singh reported ties with Incyte and Siolta Therapeutics. Dr. Eichenfield reported ties with Pfizer, Regeneron, Sanofi Genzyme, Incyte, and Pfizer.
WASHINGTON – mean it is often “woefully undertreated,” Robert Sidbury, MD, MPH, said at the annual Revolutionizing Atopic Dermatitis conference.
Identifying and mitigating triggers – such as irritation, contact allergy, and infection – is a cornerstone of treatment in infants, but tailored therapy with topical corticosteroids, topical calcineurin inhibitors (TCIs), and topical phosphodiesterase 4 (PDE4) inhibitors also have roles to play, said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital and professor in the department of pediatrics at the University of Washington, Seattle.
Views on the use of dupilumab as a systemic agent for severe infantile AD, meanwhile, have shifted significantly in the past year with the Food and Drug Administration approval of the biologic for children aged 6 months to 5 years and with extended experience with the biologic in all ages, including children, Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the meeting.
The pediatric dermatologists spoke during a session devoted to AD in infants, during which the diagnosis of AD and the role – and risks – of food allergy testing were also discussed. Diagnosis, said Elaine C. Siegfried, MD, who also spoke during the session, requires careful consideration of mimicking conditions and a broader list of differential diagnoses in those infants with poor growth or frequent infections.
Here are some of the experts’ pearls for practice.
Diagnosing AD in infants
Among infants who are growing well and otherwise healthy, the infantile eczema phenotype encompasses AD, seborrheic dermatitis, contact dermatitis, psoriasis – and overlap of more than one of these conditions. “Overlap is common,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital.
(Initial topical treatment for all these conditions is similar, but optimal treatment may differ for young children with moderate to severe disease that requires systemic treatment, she said in an interview after the meeting.)
Sparing of the diaper area that reflects skin barrier integrity is a classic feature of AD in infants and can be a useful diagnostic sign. In addition, “hypopigmentation is more characteristic of psoriasis” than AD, whereas AD tends to be hyperpigmented, which is most obvious in skin-of-color patients, Dr. Siegfried said.
Disease-specific pigment changes may be related to microbial colonization – such as Malassezia-associated hypopigmentation – or cell turnover, which is faster in psoriasis and slower in AD – with corresponding differences in pigment retention, and may be more obvious in children than adults, she said.
A less common scenario is dermatitis in infants who are not growing well. For these patients, she noted, the differential diagnosis includes metabolic or immune deficiency dermatitis as well as a variety of genodermatoses.
Generalized redness and scaling present on the first day of life is suggestive of non-atopic dermatitis. “If you’re born with red scaly skin, that’s very different than if you develop red skin in the first month or two of life,” Dr. Siegfried said.
When there is diaper area involvement with AD, contact dermatitis, impetigo, and Candida may be complicating factors. And in infants with other morbidities – especially those who are not growing well – diaper area involvement suggests a broader differential diagnosis. “I implore you, if you see children, make sure you weigh and measure them at every appointment,” she said.
Dr. Siegfried has seen infants with Netherton syndrome, and those with cystic fibrosis with zinc deficiency, for instance, presenting with “an eczematous-like picture,” diaper-area involvement, and other morbidities.
For infants with AD, she maintains a high index of suspicion for secondary infections such as molluscum, herpes simplex virus (HSV) with or without streptococci, scabies, tinea, and group A streptococci. “Secondary infections ... may be incognito,” she said. “Look for subtle signs. Even molluscum can be very subtle.”
Secondary allergic contact dermatitis is also common although it’s “technically difficult to confirm the diagnosis,” she said. Patch testing in infants is technically challenging, sensitivity is low, and monosensitization is uncommon. “So I do initial empiric topical allergen avoidance,” she said, keeping in mind ubiquitous and avoidable topical allergens such as Kathon, cocamidopropyl betaine, propylene glycol, disperse blue, and adhesives.
Treating AD in infancy
Irritation “is probably one of the biggest triggers” of AD in infants, and the often “pristine” diaper area compared with inflamed eczema elsewhere can demonstrate the importance of moisturization for healthy skin in atopic infants, Dr. Sidbury said.
Among treatments that “punch above their weight” for AD in infants is an ointment-based barrier applied around the mouth, chin, and chest – where the wet/dry impact of drooling is maximal – before and after meals, he said.
Another is hydrocortisone 2.5% mixed 1:1 with mupirocin for those infants who have secondary infections and “that exudative, weepy-looking appearance on the face,” he said. The topical antibiotic in the combination cream “lessens the potency of the steroid and oftentimes by synergy, makes it more effective” by simultaneously treating inflammation, he said. He cautions against products containing neomycin, which can be an allergen.
A combination antibiotic-steroid-emollient cream (the Aron Regimen) can also “sometimes punch above its weight,” Dr. Sidbury said.
Infections typically involve Staphylococcus aureus, but in up to 16% of cases Streptococcus is involved. And notably, lurking underneath the honey-colored crusting of S. aureus infections may be the grouped vesicles that characterize eczema herpeticum, Dr. Sidbury said.
“Counsel [parents] preemptively to treat cold sores immediately [in order to] decrease HSV shedding and minimize risk to their baby,” Dr. Sidbury said.
For treating AD-associated inflammation in skin not affected by secondary infections, over-the-counter 1% hydrocortisone cream is often sufficient, and “for very young babies and preemies in particular, I generally don’t use anything stronger because their skin barrier isn’t fully complete yet, so they absorb more than an older child does,” he said, referring to ages 2 months corrected as a marker for considering a stronger formulation if needed.
Many parents are “very concerned” about topical corticosteroid (TCS) use and pediatricians are also “often concerned,” Dr. Sidbury said. Addressing this concern, he tries to provide context and promote adherence by pointing out that infants have an easily visible vein at the temple area where the skin is naturally thin. If parents were to see this appearance for the first time in other areas while using topical steroids, he tells them, it may be the first sign of skin thinning, but “it’s entirely reversible at that stage.”
He also stressed the cost of not treating. It’s unknown whether “treating aggressively early on prevents any future development or manifestation of eczema, or future comorbidities, but we don’t know that it doesn’t,” Dr. Sidbury said. “And we certainly know how miserable that baby with eczema can be in the short term. So we need to use these medicines.”
Dr. Sidbury utilizes tacrolimus 0.03% ointment, a topical calcineurin inhibitor (TCI), only if he is worried about overuse of steroids, and uses a regimen that alternates the TCI (used in infants off-label because it is approved for ages 2 years and older) with TCS in periods of similar duration (for example, treatment with TCS for 1 week and TCI for 1 week, or rotations of 2 weeks each or 3 days each). “And these rotations may be dynamic depending on severity of the flare at any given time,” he said after the meeting.
Preapproval data from the pivotal trials of tacrolimus are reassuring and can be shared with parents. “Two-year-olds had 90% of BSA [body surface area] treated for 12 weeks” with no signs of systemic risks, Dr. Sidbury said at the meeting.
Crisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor approved for AD down to age 3 months, does not, like tacrolimus, have a boxed warning about a possible risk for cancer, and may also be alternated with TCSs. It will cause stinging in some children, but TCSs and TCIs can also sting in some children, he said, noting that samples can be helpful to predict what will or won’t sting each infant.
Systemic treatment in infants
The Liberty AD PRESCHOOL phase 3 trial that supported the FDA’s approval of dupilumab down to 6 months of age, published in 2022 in The Lancet, covered ages 6 months to 5 years but included only six children under the age of 2, “leaving us with a very limited dataset in this age group,” Dr. Eichenfield said at the meeting.
Other data and analyses that have provided reassurance, such as a laboratory safety analysis published online in 2022 showing no meaningful changes in laboratory safety parameters in children as young as 6 months, and pediatric data (not including infants) presented at a RAD meeting in 2022 showing that dupilumab, an interleukin-4 receptor alpha antagonist, may have positive effects on bone mineral density.
Data from the Liberty AD PRESCHOOL open-label extension study presented at the American Academy of Dermatology meeting in 2023, meanwhile, show that “the adverse event profile is not looking much different than what we see in older children,” Dr. Eichenfield said. “There are low rates of severe adverse events and a very low rate of discontinuations.”
At Rady Children’s Hospital, where Dr. Eichenfield is chief of pediatric and adolescent dermatology, dupilumab has become a first-line systemic agent for severe infantile AD, supplanting prior traditional but little used systemic agents such as oral corticosteroids, cyclosporine, methotrexate, azathioprine, or mycophenolate, he said after the meeting.
The decision to use systemics in the first 2 years of life is “a comprehensive one,” requiring knowledge of the child’s history, disease course, and assessment of response to prior therapies, comorbidities and severity, he said.
Food allergy in infants with AD
Food allergy is common in children with moderate to severe AD, but true food-triggered AD, with AD being the only symptom of food allergy, is rare, said Anne Marie Singh, MD, associate professor in the division of allergy and immunology and rheumatology at the University of Wisconsin, Madison, who focuses on pediatrics.
Over the years, studies of double-blind placebo-controlled food challenge tests in children with AD have tended to conflate immediate IgE hypersensitivity (and skin symptoms like urticaria) with AD, said Dr. Singh, who directs the university’s Food Allergy Research and Education Center of Excellence. In a recently published study she led involving 374 children with AD referred to allergy and/or dermatology subspecialty clinics at the University of Wisconsin, Madison, 55% had a food allergy but only 2% had food-triggered AD “where eczema is the only symptom and removal of the food cleared up the eczema and its return brought it back,” Dr. Singh said at the meeting. Another 4% had combined IgE-mediated food allergy and food-triggered AD. Almost half of the children with food-triggered AD were under 1 year of age, and egg was the most common trigger, she noted.
Food should be implicated largely by history, Dr. Singh emphasized.
Food allergy testing in the context of AD can be done but is challenging, with the clinical relevance of skin prick testing and food-specific immunoglobulin E (sIgE) difficult to predict. Predictive values of sIgE levels are established for immediate IgE mediated food allergy, but “cut-offs” for food-triggered AD are not established, she explained, noting that “cut-offs are likely higher for our children with AD.”
Elimination diets, moreover, pose significant risks of future oral tolerance and risks of nutritional deficiencies and poor growth, Dr. Singh said. New and immediate reactions to foods that are reintroduced after an elimination diet are common, and research has shown that 20% or more of such reactions involve anaphylaxis. “If an elimination diet is undertaken, you need emergency action plans, injectable epinephrine, and nutrition counseling,” she said.
A recent systematic review and meta-analysis conditionally recommended against elimination diets for the treatment of AD, Dr. Singh noted.
Asked by Dr. Sidbury whether there “is a sweet spot where you can eliminate [foods] without going all the way,” Dr. Singh said she will sometimes do a “diagnostic elimination trial” with food elimination for 2-4 weeks only – a time period after which “I’ll feel really comfortable reintroducing the food.”
Dr. Singh urged dermatologists to “know your allergist” because “patients respond best with a consistent message.”
Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, Lilly, and Beiersdorf. Dr. Siegfried reported ties with Pfizer, Regeneron, Sanofi Genzyme, Pfizer, UCB, Novan and Leo Pharma. Dr. Singh reported ties with Incyte and Siolta Therapeutics. Dr. Eichenfield reported ties with Pfizer, Regeneron, Sanofi Genzyme, Incyte, and Pfizer.
AT RAD 2023
Facial, hand, and foot dermatitis: Lebrikizumab and dupilumab show efficacy in new studies
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
AT RAD 2023
Study documents link between preadolescent acne and elevated BMI
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
The that used age- and sex-matched controls.
The investigators also identified “a potential association” with precocious puberty that they said “should be considered, especially among those presenting [with acne] under 8 or 9 years old.” The study was published in Pediatric Dermatology .
Senior author Megha M. Tollefson, MD, and coauthors used resources of the Rochester Epidemiology Project to identify all residents of Olmstead County, Minn., who were diagnosed with acne between the ages of 7 and 12 years during 2010-2018. They then randomly selected two age and sex-matched community controls in order to evaluate the relationship of preadolescent acne and BMI.
They confirmed 643 acne cases, and calculated an annual age- and sex-adjusted incidence rate for ages 7-12 of 58 per 10,000 person-years (95% confidence interval, 53.5-62.5). The incidence rate was significantly higher in females than males (89.2 vs. 28.2 per 10,000 person-years; P < .001), and it significantly increased with age (incidence rates of 4.3, 24.4, and 144.3 per 10,000 person-years among those ages 7-8, 9-10, and 11-12 years, respectively).
The median BMI percentile among children with acne was significantly higher than those without an acne diagnosis (75.0 vs. 65.0; P <.001). They also were much more likely to be obese: 16.7% of the children with acne had a BMI in at least the 95th percentile, compared with 12.2% among controls with no acne diagnosis (P = .01). (The qualifying 581 acne cases for this analysis had BMIs recorded within 8 months of the index data, in addition to not having pre-existing acne-relevant endocrine disorders.)
“High BMI is a strong risk factor for acne development and severity in adults, but until now pediatric studies have revealed mixed information ... [and have been] largely retrospective reviews without controls,” Dr. Tollefson, professor of pediatrics and dermatology at the Mayo Clinic, Rochester, Minn., and colleagues wrote.
‘Valuable’ data
Leah Lalor, MD, a pediatric dermatologist not involved with the research, said she is happy to see it. “It’s really valuable,” she said in an interview. “It’s actually the first study that gives us incidence data for preadolescent acne. We all have [had our estimates], but this study quantifies it ... and it will set the stage for further studies of preadolescents in the future.”
The study also documents that “girls are more likely to present to the clinic with acne, and to do so at younger ages, which we’ve suspected and which makes physiologic sense since girls tend to go through puberty earlier than boys,” said Dr. Lalor, assistant professor of dermatology and pediatrics at the Medical College of Wisconsin and the Children’s Wisconsin Clinics, both in Milwaukee. “And most interestingly, it really reveals that BMI is higher among preadolescents with acne than those without.”
The important caveat, she emphasized, is that the study population in Olmstead County, Minn. has a relatively higher level of education, wealth, and employment than the rest of the United States.
The investigators also found that use of systemic acne medications increased with increasing BMI (odds ratio, 1.43 per 5 kg/m2 increase in BMI; 95% CI, 1.07-1.92; P = .015). Approximately 5% of underweight or normal children were prescribed systemic acne medications, compared with 8.1% of overweight children, and 10.3% of those who were obese – data that suggest that most preadolescents with acne had mild to moderate disease and that more severe acne may be associated with increasing BMI percentiles, the authors wrote.
Approximately 4% of the 643 preadolescents with acne were diagnosed with an acne-relevant endocrine disorder prior to or at the time of acne diagnosis – most commonly precocious puberty. Of the 24 diagnoses of precocious puberty, 22 were in females, with a mean age at diagnosis of 7.3 years.
Puberty before age 8 in girls and 9 in boys is classified as precocious puberty. “Thus, a thorough review of systems and exam should be done in this population [with acne] to look for precocious puberty with a low threshold for systemic evaluation if indicated,” the authors wrote, also noting that 19 or the 482 female patients with acne were subsequently diagnosed with polycystic ovary syndrome.
Dr. Lalor said she “automatically” refers children with acne who are younger than 7 for an endocrine workup, but not necessarily children ages 7, 8, or 9 because “that’s considered within the normal realm of starting to get some acne.” Acne in the context of other symptoms such as body odor, hair, or thelarche may prompt referral in these ages, however, she said.
Future research
Obesity may influence preadolescent acne development through its effect on puberty, as overweight and obese girls achieve puberty earlier than those with normal BMI. And “insulin resistance, which may be related to obesity, has been implicated with inducing or worsening acne potentially related to shifts in IGF-1 [insulin-like growth factor 1] signaling and hyperandrogenemia,” Dr. Tollefson and colleagues wrote. Nutrition is also a possible confounder in the study.
“Patients and families have long felt that certain foods or practices contribute to acne, though this has been difficult to prove,” Dr. Lalor said. “We know that excess skim milk seems to contribute ... and there’s a correlation between high glycemic load diets [and acne].”
Assessing dietary habits in conjunction with BMI, and acne incidence and severity, would be valuable. So would research to determine “if decreasing the BMI percentile [in children with acne] would improve or prevent acne, without doing any acne treatments,” she said.
The study was supported by the National Institute on Aging and the Rochester Epidemiology Project. The authors reported no conflicts of interest. Dr. Lalor also reported no conflicts of interest.
FROM PEDIATRIC DERMATOLOGY
Large cohort study finds isotretinoin not associated with IBD
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Small study finds high dose vitamin D relieved toxic erythema of chemotherapy
seen on an inpatient dermatology consultative service.
Currently, chemotherapy cessation, delay, or dose modification are the “only reliable methods of resolving TEC,” and supportive agents such as topical corticosteroids, topical keratolytics, and pain control are associated with variable and “relatively slow improvement involving 2 to 4 weeks of recovery after chemotherapy interruption,” Cuong V. Nguyen, MD, of the department of dermatology at Northwestern University, Chicago, and colleagues, wrote in a research letter.
Onset of TEC in the six patients occurred a mean of 8.5 days after chemotherapy. Vitamin D – 50,000 IU for one patient and 100,000 IU for the others – was administered a mean of 4.3 days from rash onset and again in 7 days. Triamcinolone, 0.1%, or clobetasol, 0.05%, ointments were also prescribed.
All patients experienced symptomatic improvement in pain, pruritus, or swelling within a day of the first vitamin D treatment, and improvement in redness within 1 to 4 days, the authors said. The second treatment was administered for residual symptoms.
Adam Friedman, MD, professor and chair of dermatology and director of the supportive oncodermatology clinic at George Washington University, Washington, said that supporting patients through the “expected, disabling and often treatment-limiting side effects of oncologic therapies” is an area that is “in its infancy” and is characterized by limited evidence-based approaches.
“Creativity is therefore a must,” he said, commenting on the research letter. “Practice starts with anecdote, and this is certainly an exciting finding ... I look forward to trialing this with our patients at GW.”
Five of the six patients had a hematologic condition that required induction chemotherapy before hematopoietic stem cell transplant, and one was receiving regorafenib for treatment of glioblastoma multiforme. Diagnosis of TEC was established by clinical presentation, and five of the six patients underwent a biopsy. Biopsy findings were consistent with a TEC diagnosis in three patients, and showed nonspecific perivascular dermatitis in two, the investigators reported.
Further research is needed to determine optimal dosing, “delineate safety concerns and potential role in cancer treatment, and establish whether a durable response in patients with continuous chemotherapy, such as in an outpatient setting, is possible,” they said.
Dr. Nguyen and his coauthors reported no conflict of interest disclosures.
seen on an inpatient dermatology consultative service.
Currently, chemotherapy cessation, delay, or dose modification are the “only reliable methods of resolving TEC,” and supportive agents such as topical corticosteroids, topical keratolytics, and pain control are associated with variable and “relatively slow improvement involving 2 to 4 weeks of recovery after chemotherapy interruption,” Cuong V. Nguyen, MD, of the department of dermatology at Northwestern University, Chicago, and colleagues, wrote in a research letter.
Onset of TEC in the six patients occurred a mean of 8.5 days after chemotherapy. Vitamin D – 50,000 IU for one patient and 100,000 IU for the others – was administered a mean of 4.3 days from rash onset and again in 7 days. Triamcinolone, 0.1%, or clobetasol, 0.05%, ointments were also prescribed.
All patients experienced symptomatic improvement in pain, pruritus, or swelling within a day of the first vitamin D treatment, and improvement in redness within 1 to 4 days, the authors said. The second treatment was administered for residual symptoms.
Adam Friedman, MD, professor and chair of dermatology and director of the supportive oncodermatology clinic at George Washington University, Washington, said that supporting patients through the “expected, disabling and often treatment-limiting side effects of oncologic therapies” is an area that is “in its infancy” and is characterized by limited evidence-based approaches.
“Creativity is therefore a must,” he said, commenting on the research letter. “Practice starts with anecdote, and this is certainly an exciting finding ... I look forward to trialing this with our patients at GW.”
Five of the six patients had a hematologic condition that required induction chemotherapy before hematopoietic stem cell transplant, and one was receiving regorafenib for treatment of glioblastoma multiforme. Diagnosis of TEC was established by clinical presentation, and five of the six patients underwent a biopsy. Biopsy findings were consistent with a TEC diagnosis in three patients, and showed nonspecific perivascular dermatitis in two, the investigators reported.
Further research is needed to determine optimal dosing, “delineate safety concerns and potential role in cancer treatment, and establish whether a durable response in patients with continuous chemotherapy, such as in an outpatient setting, is possible,” they said.
Dr. Nguyen and his coauthors reported no conflict of interest disclosures.
seen on an inpatient dermatology consultative service.
Currently, chemotherapy cessation, delay, or dose modification are the “only reliable methods of resolving TEC,” and supportive agents such as topical corticosteroids, topical keratolytics, and pain control are associated with variable and “relatively slow improvement involving 2 to 4 weeks of recovery after chemotherapy interruption,” Cuong V. Nguyen, MD, of the department of dermatology at Northwestern University, Chicago, and colleagues, wrote in a research letter.
Onset of TEC in the six patients occurred a mean of 8.5 days after chemotherapy. Vitamin D – 50,000 IU for one patient and 100,000 IU for the others – was administered a mean of 4.3 days from rash onset and again in 7 days. Triamcinolone, 0.1%, or clobetasol, 0.05%, ointments were also prescribed.
All patients experienced symptomatic improvement in pain, pruritus, or swelling within a day of the first vitamin D treatment, and improvement in redness within 1 to 4 days, the authors said. The second treatment was administered for residual symptoms.
Adam Friedman, MD, professor and chair of dermatology and director of the supportive oncodermatology clinic at George Washington University, Washington, said that supporting patients through the “expected, disabling and often treatment-limiting side effects of oncologic therapies” is an area that is “in its infancy” and is characterized by limited evidence-based approaches.
“Creativity is therefore a must,” he said, commenting on the research letter. “Practice starts with anecdote, and this is certainly an exciting finding ... I look forward to trialing this with our patients at GW.”
Five of the six patients had a hematologic condition that required induction chemotherapy before hematopoietic stem cell transplant, and one was receiving regorafenib for treatment of glioblastoma multiforme. Diagnosis of TEC was established by clinical presentation, and five of the six patients underwent a biopsy. Biopsy findings were consistent with a TEC diagnosis in three patients, and showed nonspecific perivascular dermatitis in two, the investigators reported.
Further research is needed to determine optimal dosing, “delineate safety concerns and potential role in cancer treatment, and establish whether a durable response in patients with continuous chemotherapy, such as in an outpatient setting, is possible,” they said.
Dr. Nguyen and his coauthors reported no conflict of interest disclosures.
FROM JAMA DERMATOLOGY