User login
New PDT therapy for CTCL to be reviewed by FDA
based on phase 3 findings published in JAMA Dermatology.
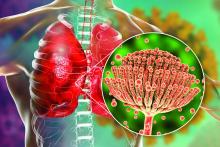
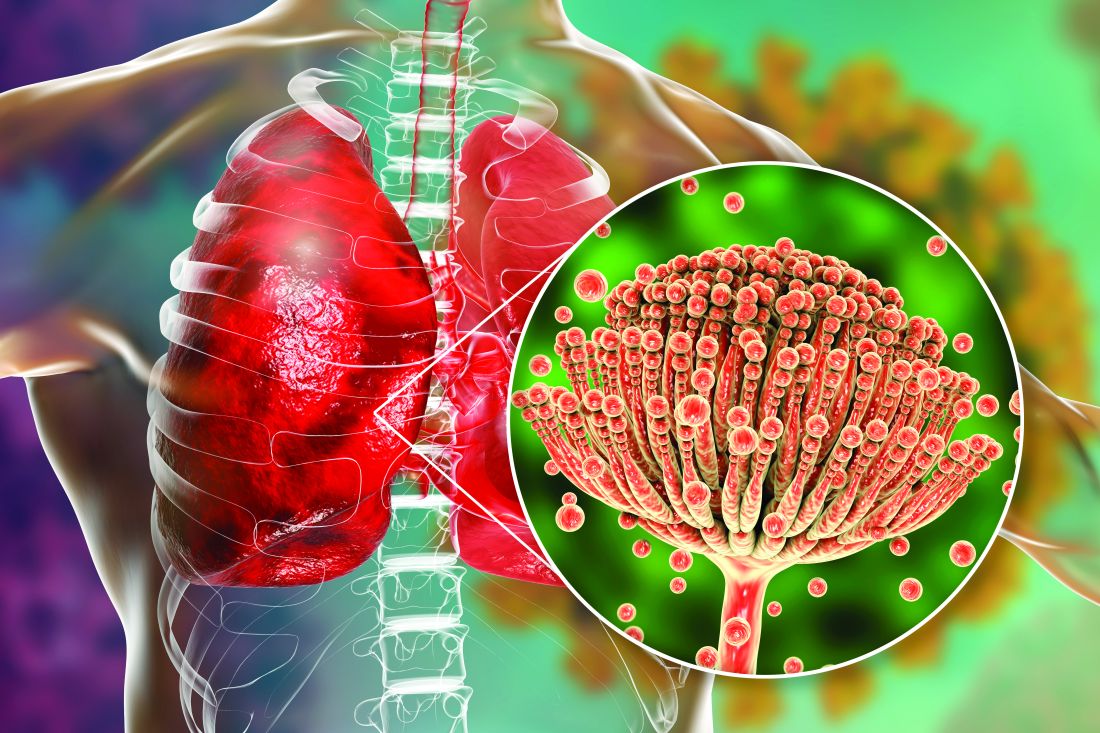
The treatment employs an ointment formulation of synthetic hypericin (HyBryte), a photosensitizer, that is preferentially absorbed into malignant cells and activated with visible light – rather than ultraviolet light – approximately 24 hours later. Investigators saw significant clinical responses in both patch and plaque type lesions and across races during the 24-week placebo-controlled, double-blinded, phase 3, randomized clinical trial.
“Traditional phototherapy, ultraviolet B phototherapy, has a limited depth of penetration, so patients with thicker plaque lesions don’t respond as well ... and UVB phototherapy typically is less effective in penetrating pigmented skin,” Ellen J. Kim, MD, lead author of the FLASH phase 3 trial, said in an interview.
Visible light in the yellow-red spectrum (500-650 nm) “penetrates deeper into the skin” and is nonmutagenic in vitro, so “theoretically it should have a much more favorable long-term safety profile,” said Dr. Kim, a dermatologist at the University of Pennsylvania, Philadelphia.
Currently, she said, the risk of secondary malignancies inherent with UV PDT, including melanoma, is a deterrent for some patients, especially “patients with really fair skin and a history of skin cancer.”
Hypericin PDT also seems well suited for use with an at-home light unit. “In our field, it’s not about which therapy is [universally] better or best, but a matter of what works best for each patient at that moment in time, depending on the side-effect profile and other issues such as access,” Dr. Kim said. “It will be great to have another option for an incurable disease that requires chronic management.”
Mycosis fungoides (MF)/CTCL is considered an orphan disease, and the treatment has received orphan drug and fast track designations from the FDA, and orphan designation from the European Medicines Agency, according to a press release from its developer, Soligenix. The company is anticipating potential approval in the second half of 2023 and is targeting early 2024 for a U.S. launch, the statement said.
Phase 3 results
The pivotal trial involved 169 patients at 39 academic and community-based U.S. medical centers and consisted of several 6-week cycles of twice-weekly treatment punctuated by 2-week breaks. In cycle 1, patients were randomized 2:1 to receive hypericin or placebo treatment of three index lesions. Cycle 2 involved the crossover of placebo patients to active treatment of index lesions, and cycle 3 (optional) involved open-label treatment of all desired lesions (index and nonindex).
The trial defined the primary endpoint in phase 1 as 50% or greater improvement in the modified Composite Assessment of Index Lesion Severity score – a tool that’s endorsed by U.S. and international MF/CTCL specialty group consensus guidelines. For cycles 2 and 3, open-label response rates were secondary endpoints. Responses were assessed after 2-week rest periods to allow for treatment-induced skin reactions to subside.
After one cycle of treatment, topical hypericin PDT was more effective than placebo (an index lesion response rate of 16% vs. 4%; P =.04). The index lesion response rate with treatment increased to 40% after two cycles and 49% after three cycles. All were statistically significant changes.
Response rates were similar in patch and plaque-type lesions and regardless of age, sex, race, stage IA versus IB, time since diagnosis, and number of prior therapies. Adverse events were primarily mild application-site skin reactions. No serious drug-related adverse events occurred, Dr. Kim said, and “we had a low drop-out rate overall.”
Into the real world
The 24-week phase 3 trial duration is short, considering that “typically, phototherapy takes between 4 to 24 months [to achieve] full responses in CTCL,” Dr. Kim said in the interview.
So with real-world application, she said, “we’ll want to see where the overall response peaks with longer treatment, what the effects are of continuous treatment without any built-in breaks, and whether we will indeed see less skin cancer development in patients who are at higher risk of developing skin cancers from light treatment.”
Such questions will be explored as part of a new 4-year, 50-patient, open-label, multicenter study with the primary aim of investigating home-based hypericin PDT therapy in a supervised setting, said Dr. Kim, principal investigator of this study. Patients who are doing well after 6 weeks of twice-weekly therapy will be given at-home light units to continue therapy and achieve 1 year of treatment with no breaks. They will be monitored with video-based telemedicine.
“Long term, having a home unit should really improve patient access and compliance and hopefully effectiveness,” Dr. Kim said. Based on the phase 3 experience, “we think that continuous treatment will be well tolerated and that we may see greater responses.”
On Dec. 19, Soligenix announced that enrollment had begun in a phase 2a study of synthetic hypericin for treating patients with mild to moderate psoriasis.
Dr. Kim reported to JAMA Dermatology grants from Innate Pharma and Galderma; consulting/advisory fees from Almirall, Galderma, and Helsinn; and honoraria from Ology and UptoDate.
based on phase 3 findings published in JAMA Dermatology.
The treatment employs an ointment formulation of synthetic hypericin (HyBryte), a photosensitizer, that is preferentially absorbed into malignant cells and activated with visible light – rather than ultraviolet light – approximately 24 hours later. Investigators saw significant clinical responses in both patch and plaque type lesions and across races during the 24-week placebo-controlled, double-blinded, phase 3, randomized clinical trial.
“Traditional phototherapy, ultraviolet B phototherapy, has a limited depth of penetration, so patients with thicker plaque lesions don’t respond as well ... and UVB phototherapy typically is less effective in penetrating pigmented skin,” Ellen J. Kim, MD, lead author of the FLASH phase 3 trial, said in an interview.
Visible light in the yellow-red spectrum (500-650 nm) “penetrates deeper into the skin” and is nonmutagenic in vitro, so “theoretically it should have a much more favorable long-term safety profile,” said Dr. Kim, a dermatologist at the University of Pennsylvania, Philadelphia.
Currently, she said, the risk of secondary malignancies inherent with UV PDT, including melanoma, is a deterrent for some patients, especially “patients with really fair skin and a history of skin cancer.”
Hypericin PDT also seems well suited for use with an at-home light unit. “In our field, it’s not about which therapy is [universally] better or best, but a matter of what works best for each patient at that moment in time, depending on the side-effect profile and other issues such as access,” Dr. Kim said. “It will be great to have another option for an incurable disease that requires chronic management.”
Mycosis fungoides (MF)/CTCL is considered an orphan disease, and the treatment has received orphan drug and fast track designations from the FDA, and orphan designation from the European Medicines Agency, according to a press release from its developer, Soligenix. The company is anticipating potential approval in the second half of 2023 and is targeting early 2024 for a U.S. launch, the statement said.
Phase 3 results
The pivotal trial involved 169 patients at 39 academic and community-based U.S. medical centers and consisted of several 6-week cycles of twice-weekly treatment punctuated by 2-week breaks. In cycle 1, patients were randomized 2:1 to receive hypericin or placebo treatment of three index lesions. Cycle 2 involved the crossover of placebo patients to active treatment of index lesions, and cycle 3 (optional) involved open-label treatment of all desired lesions (index and nonindex).
The trial defined the primary endpoint in phase 1 as 50% or greater improvement in the modified Composite Assessment of Index Lesion Severity score – a tool that’s endorsed by U.S. and international MF/CTCL specialty group consensus guidelines. For cycles 2 and 3, open-label response rates were secondary endpoints. Responses were assessed after 2-week rest periods to allow for treatment-induced skin reactions to subside.
After one cycle of treatment, topical hypericin PDT was more effective than placebo (an index lesion response rate of 16% vs. 4%; P =.04). The index lesion response rate with treatment increased to 40% after two cycles and 49% after three cycles. All were statistically significant changes.
Response rates were similar in patch and plaque-type lesions and regardless of age, sex, race, stage IA versus IB, time since diagnosis, and number of prior therapies. Adverse events were primarily mild application-site skin reactions. No serious drug-related adverse events occurred, Dr. Kim said, and “we had a low drop-out rate overall.”
Into the real world
The 24-week phase 3 trial duration is short, considering that “typically, phototherapy takes between 4 to 24 months [to achieve] full responses in CTCL,” Dr. Kim said in the interview.
So with real-world application, she said, “we’ll want to see where the overall response peaks with longer treatment, what the effects are of continuous treatment without any built-in breaks, and whether we will indeed see less skin cancer development in patients who are at higher risk of developing skin cancers from light treatment.”
Such questions will be explored as part of a new 4-year, 50-patient, open-label, multicenter study with the primary aim of investigating home-based hypericin PDT therapy in a supervised setting, said Dr. Kim, principal investigator of this study. Patients who are doing well after 6 weeks of twice-weekly therapy will be given at-home light units to continue therapy and achieve 1 year of treatment with no breaks. They will be monitored with video-based telemedicine.
“Long term, having a home unit should really improve patient access and compliance and hopefully effectiveness,” Dr. Kim said. Based on the phase 3 experience, “we think that continuous treatment will be well tolerated and that we may see greater responses.”
On Dec. 19, Soligenix announced that enrollment had begun in a phase 2a study of synthetic hypericin for treating patients with mild to moderate psoriasis.
Dr. Kim reported to JAMA Dermatology grants from Innate Pharma and Galderma; consulting/advisory fees from Almirall, Galderma, and Helsinn; and honoraria from Ology and UptoDate.
based on phase 3 findings published in JAMA Dermatology.
The treatment employs an ointment formulation of synthetic hypericin (HyBryte), a photosensitizer, that is preferentially absorbed into malignant cells and activated with visible light – rather than ultraviolet light – approximately 24 hours later. Investigators saw significant clinical responses in both patch and plaque type lesions and across races during the 24-week placebo-controlled, double-blinded, phase 3, randomized clinical trial.
“Traditional phototherapy, ultraviolet B phototherapy, has a limited depth of penetration, so patients with thicker plaque lesions don’t respond as well ... and UVB phototherapy typically is less effective in penetrating pigmented skin,” Ellen J. Kim, MD, lead author of the FLASH phase 3 trial, said in an interview.
Visible light in the yellow-red spectrum (500-650 nm) “penetrates deeper into the skin” and is nonmutagenic in vitro, so “theoretically it should have a much more favorable long-term safety profile,” said Dr. Kim, a dermatologist at the University of Pennsylvania, Philadelphia.
Currently, she said, the risk of secondary malignancies inherent with UV PDT, including melanoma, is a deterrent for some patients, especially “patients with really fair skin and a history of skin cancer.”
Hypericin PDT also seems well suited for use with an at-home light unit. “In our field, it’s not about which therapy is [universally] better or best, but a matter of what works best for each patient at that moment in time, depending on the side-effect profile and other issues such as access,” Dr. Kim said. “It will be great to have another option for an incurable disease that requires chronic management.”
Mycosis fungoides (MF)/CTCL is considered an orphan disease, and the treatment has received orphan drug and fast track designations from the FDA, and orphan designation from the European Medicines Agency, according to a press release from its developer, Soligenix. The company is anticipating potential approval in the second half of 2023 and is targeting early 2024 for a U.S. launch, the statement said.
Phase 3 results
The pivotal trial involved 169 patients at 39 academic and community-based U.S. medical centers and consisted of several 6-week cycles of twice-weekly treatment punctuated by 2-week breaks. In cycle 1, patients were randomized 2:1 to receive hypericin or placebo treatment of three index lesions. Cycle 2 involved the crossover of placebo patients to active treatment of index lesions, and cycle 3 (optional) involved open-label treatment of all desired lesions (index and nonindex).
The trial defined the primary endpoint in phase 1 as 50% or greater improvement in the modified Composite Assessment of Index Lesion Severity score – a tool that’s endorsed by U.S. and international MF/CTCL specialty group consensus guidelines. For cycles 2 and 3, open-label response rates were secondary endpoints. Responses were assessed after 2-week rest periods to allow for treatment-induced skin reactions to subside.
After one cycle of treatment, topical hypericin PDT was more effective than placebo (an index lesion response rate of 16% vs. 4%; P =.04). The index lesion response rate with treatment increased to 40% after two cycles and 49% after three cycles. All were statistically significant changes.
Response rates were similar in patch and plaque-type lesions and regardless of age, sex, race, stage IA versus IB, time since diagnosis, and number of prior therapies. Adverse events were primarily mild application-site skin reactions. No serious drug-related adverse events occurred, Dr. Kim said, and “we had a low drop-out rate overall.”
Into the real world
The 24-week phase 3 trial duration is short, considering that “typically, phototherapy takes between 4 to 24 months [to achieve] full responses in CTCL,” Dr. Kim said in the interview.
So with real-world application, she said, “we’ll want to see where the overall response peaks with longer treatment, what the effects are of continuous treatment without any built-in breaks, and whether we will indeed see less skin cancer development in patients who are at higher risk of developing skin cancers from light treatment.”
Such questions will be explored as part of a new 4-year, 50-patient, open-label, multicenter study with the primary aim of investigating home-based hypericin PDT therapy in a supervised setting, said Dr. Kim, principal investigator of this study. Patients who are doing well after 6 weeks of twice-weekly therapy will be given at-home light units to continue therapy and achieve 1 year of treatment with no breaks. They will be monitored with video-based telemedicine.
“Long term, having a home unit should really improve patient access and compliance and hopefully effectiveness,” Dr. Kim said. Based on the phase 3 experience, “we think that continuous treatment will be well tolerated and that we may see greater responses.”
On Dec. 19, Soligenix announced that enrollment had begun in a phase 2a study of synthetic hypericin for treating patients with mild to moderate psoriasis.
Dr. Kim reported to JAMA Dermatology grants from Innate Pharma and Galderma; consulting/advisory fees from Almirall, Galderma, and Helsinn; and honoraria from Ology and UptoDate.
Rise of the fungi: Pandemic tied to increasing fungal infections
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
Rosacea and the gut: Looking into SIBO
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
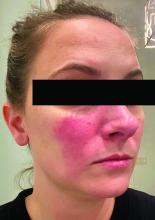
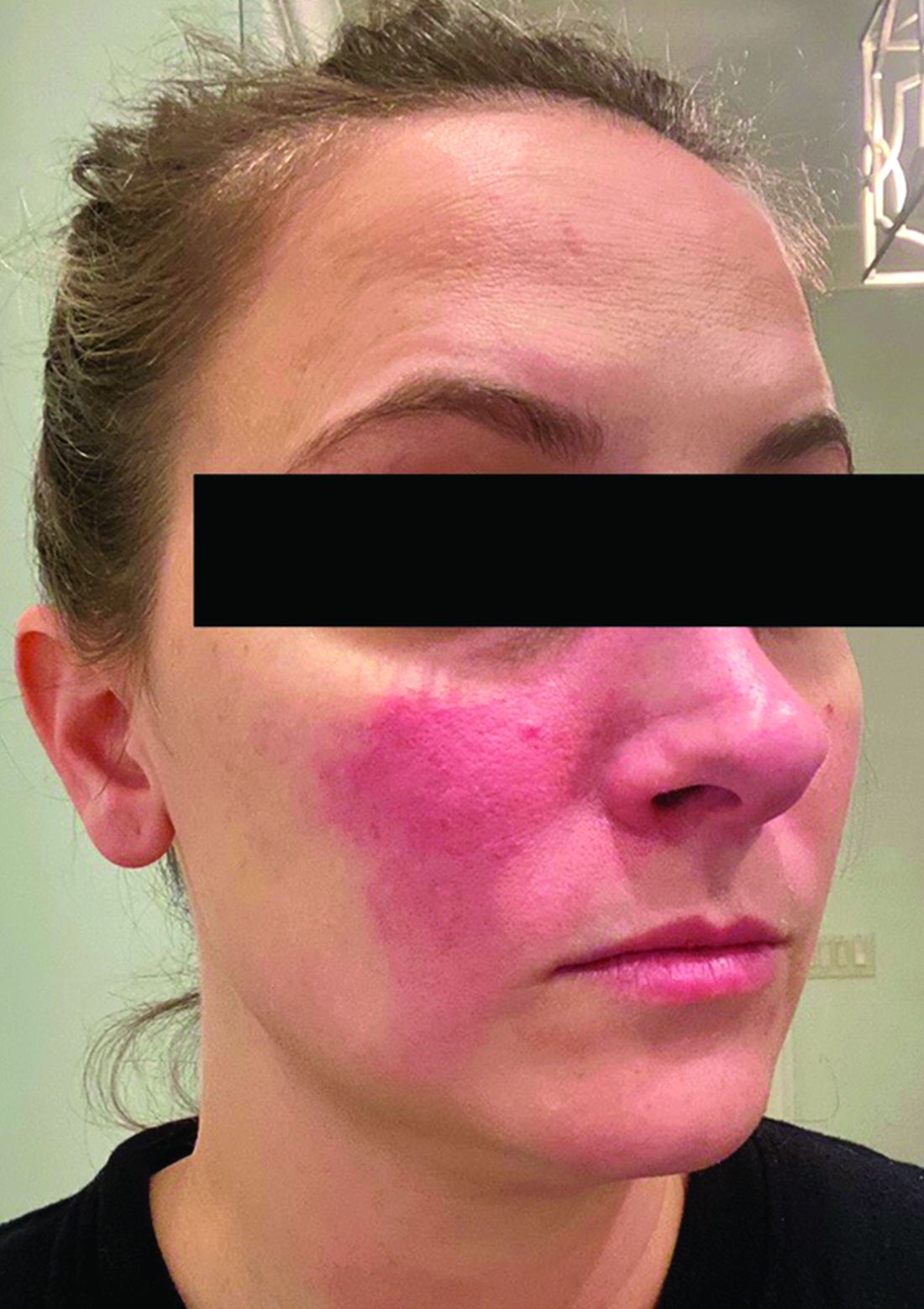
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
REPORTING FROM IDS 2022
Advanced practice providers – an evolving role in pulmonary medicine
The integration of advanced practice providers (APPs) into pulmonology practice is in flux and deepening across numerous settings, from outpatient clinics to intensive care and inpatient pulmonary consult services – and as it evolves, so are issues of training.
Some institutions are developing pulmonary fellowship programs for APPs. This is a good indication that team-based pulmonology may be moving toward a time in the future when nurse practitioners (NPs) and physician assistants (PAs) join pulmonologists in practice after having undergone formal education in the subspecialty, rather than learning solely on the job from dedicated mentors.
Neither NPs nor PAs, who comprise almost all of the APP workforce in pulmonology, currently have a pulmonary tract for training. “Weight falls on the employer’s shoulders to train and educate their APPs,” said Corinne R. Young, MSN, FNP-C, FCCP, director of APP and clinical services at Colorado Springs Pulmonary Consultants and founder and president of the Association of Pulmonary Advanced Practice Providers, which launched in 2018.
The role that an APP plays and their scope of practice is determined not only by state policies and regulations – and by their prior experience, knowledge and motivation – but by “how much work a practice puts into [education and training],” she said.
An estimated 3,000-8,000 APPs are working in pulmonology, according to an analysis done by a marketing agency that has worked for the American College of Chest Physicians, Ms. Young said.
A 2021 APAPP survey of its several hundred members at the time showed them working in hospital systems (41%), private practice (28%), university systems (10%), and other health care systems (21%). They indicated practicing in pulmonary medicine, sleep medicine, or critical care – or some combination of these areas – and the vast majority (82%) indicated they were seeing both new and established patients in their roles.
“Nobody knows exactly how many of us are out there,” Ms. Young said. “But CHEST and APAPP are making great efforts to be beacons to APPs working in this realm and to bring them together to have a voice.”
The APAPP also wants to “close the education gap” and to “eventually develop a certification program to vet our knowledge in this area,” she said. “Right now, the closest we can get to vetting our knowledge is to become an FCCP through CHEST.”
Earning trust, seeking training
Omar Hussain, DO, has been practicing with an NP for over a decade in his role as an intensivist and knows what it’s like to train, supervise, and grow together. He and his private practice colleagues have a contract with Advocate Condell Hospital in Libertyville, Ill., to cover its ICU, and they hired their NP primarily to help care for shorter-stay, non–critically ill patients in the ICU (for example, patients receiving postoperative monitoring).
The NP has been invaluable. “We literally sit next to each other and in the mornings we make a game plan of which patients she will tackle first and which ones I’ll see first,” Dr. Hussain said. “When we’re called by the nurse for an ICU evaluation [on the floor], we’ll decide in real time who goes.”
The NP ensures that all guidelines and quality measures are followed in the ICU and, with a Monday-Friday schedule, she provides valuable continuity when there are handoffs from one intensivist to another, said Dr. Hussain, who serves as cochair of the joint CHEST/American Thoracic Society clinical practice committee, which deals with issues of physician-APP collaboration.
After working collaboratively for some time, Dr. Hussain and his partners decided to teach the NP how to intubate. It was a thoughtful and deliberate process, and “we used the same kind of mindset we’d used when we’ve supervised residents at other institutions,” he said.
Dr. Hussain and his partners have been fortunate in having such a long-term relationship with an APP. Their NP had worked as a nurse in the ICU before training as an adult gerontology–acute care NP and joining Dr. Hussain’s practice, so she was also “well known to us,” he added.
Rachel Adney, CPNP-PC, a certified pediatric NP in the division of pediatric pulmonology at Stanford (Calif.) Medicine Children’s Health, is an APP who actively sought advanced training. She joined Stanford in 2011 to provide ambulatory care, primarily, and having years of prior experience in asthma management and education, she fast became known as “the asthma person.”
After a physician colleague one day objected to her caring for a patient without asthma, Ms. Adney, the first APP in the division, approached John D. Mark, MD, program director of the pediatric fellowship program at Stanford, and inquired about training “so I could have more breadth and depth across the whole pulmonary milieu.”
Together they designed a “mini pediatric pulmonary fellowship” for Ms. Adney, incorporating elements of the first year of Stanford’s pediatric fellowship program as well as training materials from the University of Arizona’s Pediatric Pulmonary Center, Tucson, one of six federally funded PCCs that train various health care providers to care for pediatric patients with chronic pulmonary conditions. (Dr. Mark had previously been an educator at the center while serving on the University of Arizona faculty.)
Her curriculum consisted of 1,000 total hours of training, including 125 hours of didactic learning and 400 hours of both inpatient and outpatient clinical training in areas such as cystic fibrosis, sleep medicine, bronchopulmonary dysplasia (BPD), neuromuscular disorders, and general pulmonary medicine. “Rachel rotated through clinics, first as an observer, then as a trainee ... and she attended lectures that my fellows attended,” said Dr. Mark, who has long been a preceptor for APPs. “She became like a 1-year fellow in my division.”
Today, Ms. Adney sees patients independently in four outreach clinics along California’s central coast. “She sees very complicated pediatric pulmonary patients now” overall, and has become integral to Stanford’s interdisciplinary CRIB program (cardiac and respiratory care for infants with BPD), Dr. Mark said. “She follows these patients at Stanford along with the whole CRIB group, then sees them on her own for follow-up.”
As a result of her training, Ms. Adney said, “knowing that I have the knowledge and experience to take on more complex patients, my colleagues now trust me and are confident in my skills. They feel comfortable sending [patients] to me much earlier. ... And they know that if there’s something I need help with I will go to them instantly.”
Pulmonology “really spoke to my heart,” she said, recalling her pre-Stanford journey as an in-hospital medical-surgical nurse, and then, after her NP training, as a outpatient primary care PNP. “For the most part, it’s like putting a puzzle together, and being able to really impact the quality of life these patients have,” said Ms. Adney, who serves on the APAPP’s pediatric subcommittee.
It’s clear, Dr. Mark said, that “things are changing around the country” with increasing institutional interest in developing formal APP specialty training programs. “There’s no way [for an APP] to walk into a specialty and play an active role without additional training,” and institutions are frustrated with turnover and the loss of APPs who decide after 6-9 months of on-the-job training that they’re not interested in the field.
Stanford Medicine Children’s Health, in fact, has launched an internal Pediatric APP Fellowship Program that is training its first cohort of six newly graduated NPs and PAs in two clinical tracks, including a medical/surgical track that incorporates rotations in pulmonary medicine, said Raji Koppolu, CPNP-PC/AC, manager of advanced practice professional development for Stanford Medicine Children’s Health.
APP fellowship programs have been in existence since 2007 in a variety of clinical settings, she said, but more institutions are developing them as a way of recruiting and retaining APPs in areas of high need and of equipping them for successful transitions to their APP roles. Various national bodies accredit APP fellowship programs.
Most pulmonary fellowship programs, Ms. Young said, are also internal programs providing postgraduate education to their own newly hired APPs or recent NP/PA graduates. This limits their reach, but “it’s a step in the right direction toward standardizing education for pulmonary APPs.”
Defining APP competencies
In interventional pulmonology, training may soon be guided by newly defined “core clinical competencies” for APPs. The soon-to-be published and distributed competencies – the first such national APP competencies in pulmonology – were developed by an APP Leadership Council within the American Association of Bronchology and Interventional Pulmonology and cover the most common disease processes and practices in IP, from COPD and bronchoscopic lung volume reduction to pleural effusion and lung cancer screening.
Rebecca Priebe, ACNP-BC, who cochairs the AABIP’s APP chapter, organized the effort several years ago, bringing together a group of APPs and physician experts in advanced bronchoscopy and IP (some but not all of whom have worked with APPs), after fielding questions from pulmonologists at AABIP meetings about what to look for in an AAP and how to train them.
Physicians and institutions who are hiring and training APPs for IP can use any or all of the 11 core competencies to personalize and evaluate the training process for each APP’s needs, she said. “Someone looking to hire an APP for pleural disease, for instance, can pull up the content on plural effusion.”
APP interest in interventional pulmonology is growing rapidly, Ms. Priebe said, noting growth in the AABIP’s APP chapter from about 7-8 APPs 5 years ago to at least 60 currently.
Ms. Priebe was hired by Henry Ford Health in Detroit about 5 years ago to help establish and run an inpatient IP consult service, and more recently, she helped establish their inpatient pleural disease service and a bronchoscopic lung volume reduction program.
For the inpatient IP service, after several months of side-by-side training with an IP fellow and attending physicians, she began independently evaluating new patients, writing notes, and making recommendations.
For patients with pleural disease, she performs ultrasound examinations, chest tube insertions, and bedside thoracentesis independently. And for the bronchoscopic lung volume reduction program, she evaluates patients for candidate status, participates in valve placement, and sees patients independently through a year of follow-up.
“Physician colleagues often aren’t sure what an APP’s education and scope of practice is,” said Ms. Priebe, who was an ICU nurse before training as an acute care NP and then worked first with a private practice inpatient service and then with the University of Michigan, Ann Arbor, where she established and grew an APP-run program managing lung transplant patients and a step-down ICU unit.
“It’s a matter of knowing [your state’s policies], treating them like a fellow you would train, and then using them to the fullest extent of their education and training. If they’re given an opportunity to learn a subspecialty skill set, they can be an asset to any pulmonary program.”
‘We’re here to support,’ not replace
In her own practice, Ms. Young is one of seven APPs who work with nine physicians on a full range of inpatient care, outpatient care, critical care, sleep medicine, and procedures. Many new patients are seen first by the APP, who does the workup and orders tests, and by the physician on a follow-up visit. Most patients needing routine management of asthma and COPD are seen by the physician every third or fourth visit, she said.
Ms. Young also directs a 24-hour in-house APP service recently established by the practice, and she participates in research. In a practice across town, she noted, APPs see mainly established patients and do not practice as autonomously as the state permits. “Part of that difference may [stem from] the lack of a standard of education and variable amounts of work the practice puts into their APPs.”
The American Medical Association’s #StopScopeCreep social media messaging feels divisive and “sheds a negative light on APPs working in any area,” Ms. Young said. “One of the biggest things we want to convey [at APAPP] is that we’re not here for [physicians’] jobs.”
“We’re here to support those who are practicing, to support underserved populations, and to help bridge gaps” created by an aging pulmonologist workforce and real and projected physician shortages, Ms. Young said, referring to a 2016 report from the Health Resources and Services Administration and a 2017 report from Merritt Hawkins indicating that 73% of U.S. pulmonologists (the largest percentage of all subspecialties) were at least 55 years old.
Dr. Hussain said he has “seen scope creep” first-hand in his hospitals, in the form of noncollaborative practices and tasks performed by APPs without adequate training – most likely often stemming from poor decisions and oversight by physicians. But when constructed thoughtfully, APP-physician teams are “serving great needs” in many types of care, he said, from follow-up care and management of chronic conditions to inpatient rounding. “My [colleagues] are having great success,” he said.
He is watching with interest – and some concern – pending reimbursement changes from the Centers for Medicare & Medicaid Services that will make time the only defining feature of the “substantive” portion of a split/shared visit involving physicians and APPs in a facility setting. Medical decision-making will no longer be applicable.
For time-based services like critical care, time alone is currently the metric. (And in the nonfacility setting, physician-APP teams may still apply “incident to” billing practices). But in the facility setting, said Amy M. Ahasic, MD, MPH, a pulmonologist in Norwalk, Conn., who coauthored a 2022 commentary on the issue, the change (now planned for 2024) could be problematic for employed physicians whose contracts are based on productivity, and could create tension and possibly lead to reduced use of APPs rather than supporting collaborative care.
“The team model has been evolving so well over the past 10-15 years,” said Dr. Ahasic, who serves on the CHEST Health Policy and Advocacy Reimbursement Workgroup and cochairs the CHEST/American Thoracic Society clinical practice committee with Dr. Hussain. “It’s good for patient safety to have more [providers] involved ... and because APP salaries are lower health systems could do it and be able to have better care and better coverage.”
The pulmonology culture, said Dr. Hussain, has been increasingly embracing APPs and “it’s collegial.” Pulmonologists are “coming to CHEST meetings with their APPs. They’re learning the same things we’re learning, to manage the same patients we manage.”
The article sources reported that they had no relevant financial conflicts of interest to disclose.
The integration of advanced practice providers (APPs) into pulmonology practice is in flux and deepening across numerous settings, from outpatient clinics to intensive care and inpatient pulmonary consult services – and as it evolves, so are issues of training.
Some institutions are developing pulmonary fellowship programs for APPs. This is a good indication that team-based pulmonology may be moving toward a time in the future when nurse practitioners (NPs) and physician assistants (PAs) join pulmonologists in practice after having undergone formal education in the subspecialty, rather than learning solely on the job from dedicated mentors.
Neither NPs nor PAs, who comprise almost all of the APP workforce in pulmonology, currently have a pulmonary tract for training. “Weight falls on the employer’s shoulders to train and educate their APPs,” said Corinne R. Young, MSN, FNP-C, FCCP, director of APP and clinical services at Colorado Springs Pulmonary Consultants and founder and president of the Association of Pulmonary Advanced Practice Providers, which launched in 2018.
The role that an APP plays and their scope of practice is determined not only by state policies and regulations – and by their prior experience, knowledge and motivation – but by “how much work a practice puts into [education and training],” she said.
An estimated 3,000-8,000 APPs are working in pulmonology, according to an analysis done by a marketing agency that has worked for the American College of Chest Physicians, Ms. Young said.
A 2021 APAPP survey of its several hundred members at the time showed them working in hospital systems (41%), private practice (28%), university systems (10%), and other health care systems (21%). They indicated practicing in pulmonary medicine, sleep medicine, or critical care – or some combination of these areas – and the vast majority (82%) indicated they were seeing both new and established patients in their roles.
“Nobody knows exactly how many of us are out there,” Ms. Young said. “But CHEST and APAPP are making great efforts to be beacons to APPs working in this realm and to bring them together to have a voice.”
The APAPP also wants to “close the education gap” and to “eventually develop a certification program to vet our knowledge in this area,” she said. “Right now, the closest we can get to vetting our knowledge is to become an FCCP through CHEST.”
Earning trust, seeking training
Omar Hussain, DO, has been practicing with an NP for over a decade in his role as an intensivist and knows what it’s like to train, supervise, and grow together. He and his private practice colleagues have a contract with Advocate Condell Hospital in Libertyville, Ill., to cover its ICU, and they hired their NP primarily to help care for shorter-stay, non–critically ill patients in the ICU (for example, patients receiving postoperative monitoring).
The NP has been invaluable. “We literally sit next to each other and in the mornings we make a game plan of which patients she will tackle first and which ones I’ll see first,” Dr. Hussain said. “When we’re called by the nurse for an ICU evaluation [on the floor], we’ll decide in real time who goes.”
The NP ensures that all guidelines and quality measures are followed in the ICU and, with a Monday-Friday schedule, she provides valuable continuity when there are handoffs from one intensivist to another, said Dr. Hussain, who serves as cochair of the joint CHEST/American Thoracic Society clinical practice committee, which deals with issues of physician-APP collaboration.
After working collaboratively for some time, Dr. Hussain and his partners decided to teach the NP how to intubate. It was a thoughtful and deliberate process, and “we used the same kind of mindset we’d used when we’ve supervised residents at other institutions,” he said.
Dr. Hussain and his partners have been fortunate in having such a long-term relationship with an APP. Their NP had worked as a nurse in the ICU before training as an adult gerontology–acute care NP and joining Dr. Hussain’s practice, so she was also “well known to us,” he added.
Rachel Adney, CPNP-PC, a certified pediatric NP in the division of pediatric pulmonology at Stanford (Calif.) Medicine Children’s Health, is an APP who actively sought advanced training. She joined Stanford in 2011 to provide ambulatory care, primarily, and having years of prior experience in asthma management and education, she fast became known as “the asthma person.”
After a physician colleague one day objected to her caring for a patient without asthma, Ms. Adney, the first APP in the division, approached John D. Mark, MD, program director of the pediatric fellowship program at Stanford, and inquired about training “so I could have more breadth and depth across the whole pulmonary milieu.”
Together they designed a “mini pediatric pulmonary fellowship” for Ms. Adney, incorporating elements of the first year of Stanford’s pediatric fellowship program as well as training materials from the University of Arizona’s Pediatric Pulmonary Center, Tucson, one of six federally funded PCCs that train various health care providers to care for pediatric patients with chronic pulmonary conditions. (Dr. Mark had previously been an educator at the center while serving on the University of Arizona faculty.)
Her curriculum consisted of 1,000 total hours of training, including 125 hours of didactic learning and 400 hours of both inpatient and outpatient clinical training in areas such as cystic fibrosis, sleep medicine, bronchopulmonary dysplasia (BPD), neuromuscular disorders, and general pulmonary medicine. “Rachel rotated through clinics, first as an observer, then as a trainee ... and she attended lectures that my fellows attended,” said Dr. Mark, who has long been a preceptor for APPs. “She became like a 1-year fellow in my division.”
Today, Ms. Adney sees patients independently in four outreach clinics along California’s central coast. “She sees very complicated pediatric pulmonary patients now” overall, and has become integral to Stanford’s interdisciplinary CRIB program (cardiac and respiratory care for infants with BPD), Dr. Mark said. “She follows these patients at Stanford along with the whole CRIB group, then sees them on her own for follow-up.”
As a result of her training, Ms. Adney said, “knowing that I have the knowledge and experience to take on more complex patients, my colleagues now trust me and are confident in my skills. They feel comfortable sending [patients] to me much earlier. ... And they know that if there’s something I need help with I will go to them instantly.”
Pulmonology “really spoke to my heart,” she said, recalling her pre-Stanford journey as an in-hospital medical-surgical nurse, and then, after her NP training, as a outpatient primary care PNP. “For the most part, it’s like putting a puzzle together, and being able to really impact the quality of life these patients have,” said Ms. Adney, who serves on the APAPP’s pediatric subcommittee.
It’s clear, Dr. Mark said, that “things are changing around the country” with increasing institutional interest in developing formal APP specialty training programs. “There’s no way [for an APP] to walk into a specialty and play an active role without additional training,” and institutions are frustrated with turnover and the loss of APPs who decide after 6-9 months of on-the-job training that they’re not interested in the field.
Stanford Medicine Children’s Health, in fact, has launched an internal Pediatric APP Fellowship Program that is training its first cohort of six newly graduated NPs and PAs in two clinical tracks, including a medical/surgical track that incorporates rotations in pulmonary medicine, said Raji Koppolu, CPNP-PC/AC, manager of advanced practice professional development for Stanford Medicine Children’s Health.
APP fellowship programs have been in existence since 2007 in a variety of clinical settings, she said, but more institutions are developing them as a way of recruiting and retaining APPs in areas of high need and of equipping them for successful transitions to their APP roles. Various national bodies accredit APP fellowship programs.
Most pulmonary fellowship programs, Ms. Young said, are also internal programs providing postgraduate education to their own newly hired APPs or recent NP/PA graduates. This limits their reach, but “it’s a step in the right direction toward standardizing education for pulmonary APPs.”
Defining APP competencies
In interventional pulmonology, training may soon be guided by newly defined “core clinical competencies” for APPs. The soon-to-be published and distributed competencies – the first such national APP competencies in pulmonology – were developed by an APP Leadership Council within the American Association of Bronchology and Interventional Pulmonology and cover the most common disease processes and practices in IP, from COPD and bronchoscopic lung volume reduction to pleural effusion and lung cancer screening.
Rebecca Priebe, ACNP-BC, who cochairs the AABIP’s APP chapter, organized the effort several years ago, bringing together a group of APPs and physician experts in advanced bronchoscopy and IP (some but not all of whom have worked with APPs), after fielding questions from pulmonologists at AABIP meetings about what to look for in an AAP and how to train them.
Physicians and institutions who are hiring and training APPs for IP can use any or all of the 11 core competencies to personalize and evaluate the training process for each APP’s needs, she said. “Someone looking to hire an APP for pleural disease, for instance, can pull up the content on plural effusion.”
APP interest in interventional pulmonology is growing rapidly, Ms. Priebe said, noting growth in the AABIP’s APP chapter from about 7-8 APPs 5 years ago to at least 60 currently.
Ms. Priebe was hired by Henry Ford Health in Detroit about 5 years ago to help establish and run an inpatient IP consult service, and more recently, she helped establish their inpatient pleural disease service and a bronchoscopic lung volume reduction program.
For the inpatient IP service, after several months of side-by-side training with an IP fellow and attending physicians, she began independently evaluating new patients, writing notes, and making recommendations.
For patients with pleural disease, she performs ultrasound examinations, chest tube insertions, and bedside thoracentesis independently. And for the bronchoscopic lung volume reduction program, she evaluates patients for candidate status, participates in valve placement, and sees patients independently through a year of follow-up.
“Physician colleagues often aren’t sure what an APP’s education and scope of practice is,” said Ms. Priebe, who was an ICU nurse before training as an acute care NP and then worked first with a private practice inpatient service and then with the University of Michigan, Ann Arbor, where she established and grew an APP-run program managing lung transplant patients and a step-down ICU unit.
“It’s a matter of knowing [your state’s policies], treating them like a fellow you would train, and then using them to the fullest extent of their education and training. If they’re given an opportunity to learn a subspecialty skill set, they can be an asset to any pulmonary program.”
‘We’re here to support,’ not replace
In her own practice, Ms. Young is one of seven APPs who work with nine physicians on a full range of inpatient care, outpatient care, critical care, sleep medicine, and procedures. Many new patients are seen first by the APP, who does the workup and orders tests, and by the physician on a follow-up visit. Most patients needing routine management of asthma and COPD are seen by the physician every third or fourth visit, she said.
Ms. Young also directs a 24-hour in-house APP service recently established by the practice, and she participates in research. In a practice across town, she noted, APPs see mainly established patients and do not practice as autonomously as the state permits. “Part of that difference may [stem from] the lack of a standard of education and variable amounts of work the practice puts into their APPs.”
The American Medical Association’s #StopScopeCreep social media messaging feels divisive and “sheds a negative light on APPs working in any area,” Ms. Young said. “One of the biggest things we want to convey [at APAPP] is that we’re not here for [physicians’] jobs.”
“We’re here to support those who are practicing, to support underserved populations, and to help bridge gaps” created by an aging pulmonologist workforce and real and projected physician shortages, Ms. Young said, referring to a 2016 report from the Health Resources and Services Administration and a 2017 report from Merritt Hawkins indicating that 73% of U.S. pulmonologists (the largest percentage of all subspecialties) were at least 55 years old.
Dr. Hussain said he has “seen scope creep” first-hand in his hospitals, in the form of noncollaborative practices and tasks performed by APPs without adequate training – most likely often stemming from poor decisions and oversight by physicians. But when constructed thoughtfully, APP-physician teams are “serving great needs” in many types of care, he said, from follow-up care and management of chronic conditions to inpatient rounding. “My [colleagues] are having great success,” he said.
He is watching with interest – and some concern – pending reimbursement changes from the Centers for Medicare & Medicaid Services that will make time the only defining feature of the “substantive” portion of a split/shared visit involving physicians and APPs in a facility setting. Medical decision-making will no longer be applicable.
For time-based services like critical care, time alone is currently the metric. (And in the nonfacility setting, physician-APP teams may still apply “incident to” billing practices). But in the facility setting, said Amy M. Ahasic, MD, MPH, a pulmonologist in Norwalk, Conn., who coauthored a 2022 commentary on the issue, the change (now planned for 2024) could be problematic for employed physicians whose contracts are based on productivity, and could create tension and possibly lead to reduced use of APPs rather than supporting collaborative care.
“The team model has been evolving so well over the past 10-15 years,” said Dr. Ahasic, who serves on the CHEST Health Policy and Advocacy Reimbursement Workgroup and cochairs the CHEST/American Thoracic Society clinical practice committee with Dr. Hussain. “It’s good for patient safety to have more [providers] involved ... and because APP salaries are lower health systems could do it and be able to have better care and better coverage.”
The pulmonology culture, said Dr. Hussain, has been increasingly embracing APPs and “it’s collegial.” Pulmonologists are “coming to CHEST meetings with their APPs. They’re learning the same things we’re learning, to manage the same patients we manage.”
The article sources reported that they had no relevant financial conflicts of interest to disclose.
The integration of advanced practice providers (APPs) into pulmonology practice is in flux and deepening across numerous settings, from outpatient clinics to intensive care and inpatient pulmonary consult services – and as it evolves, so are issues of training.
Some institutions are developing pulmonary fellowship programs for APPs. This is a good indication that team-based pulmonology may be moving toward a time in the future when nurse practitioners (NPs) and physician assistants (PAs) join pulmonologists in practice after having undergone formal education in the subspecialty, rather than learning solely on the job from dedicated mentors.
Neither NPs nor PAs, who comprise almost all of the APP workforce in pulmonology, currently have a pulmonary tract for training. “Weight falls on the employer’s shoulders to train and educate their APPs,” said Corinne R. Young, MSN, FNP-C, FCCP, director of APP and clinical services at Colorado Springs Pulmonary Consultants and founder and president of the Association of Pulmonary Advanced Practice Providers, which launched in 2018.
The role that an APP plays and their scope of practice is determined not only by state policies and regulations – and by their prior experience, knowledge and motivation – but by “how much work a practice puts into [education and training],” she said.
An estimated 3,000-8,000 APPs are working in pulmonology, according to an analysis done by a marketing agency that has worked for the American College of Chest Physicians, Ms. Young said.
A 2021 APAPP survey of its several hundred members at the time showed them working in hospital systems (41%), private practice (28%), university systems (10%), and other health care systems (21%). They indicated practicing in pulmonary medicine, sleep medicine, or critical care – or some combination of these areas – and the vast majority (82%) indicated they were seeing both new and established patients in their roles.
“Nobody knows exactly how many of us are out there,” Ms. Young said. “But CHEST and APAPP are making great efforts to be beacons to APPs working in this realm and to bring them together to have a voice.”
The APAPP also wants to “close the education gap” and to “eventually develop a certification program to vet our knowledge in this area,” she said. “Right now, the closest we can get to vetting our knowledge is to become an FCCP through CHEST.”
Earning trust, seeking training
Omar Hussain, DO, has been practicing with an NP for over a decade in his role as an intensivist and knows what it’s like to train, supervise, and grow together. He and his private practice colleagues have a contract with Advocate Condell Hospital in Libertyville, Ill., to cover its ICU, and they hired their NP primarily to help care for shorter-stay, non–critically ill patients in the ICU (for example, patients receiving postoperative monitoring).
The NP has been invaluable. “We literally sit next to each other and in the mornings we make a game plan of which patients she will tackle first and which ones I’ll see first,” Dr. Hussain said. “When we’re called by the nurse for an ICU evaluation [on the floor], we’ll decide in real time who goes.”
The NP ensures that all guidelines and quality measures are followed in the ICU and, with a Monday-Friday schedule, she provides valuable continuity when there are handoffs from one intensivist to another, said Dr. Hussain, who serves as cochair of the joint CHEST/American Thoracic Society clinical practice committee, which deals with issues of physician-APP collaboration.
After working collaboratively for some time, Dr. Hussain and his partners decided to teach the NP how to intubate. It was a thoughtful and deliberate process, and “we used the same kind of mindset we’d used when we’ve supervised residents at other institutions,” he said.
Dr. Hussain and his partners have been fortunate in having such a long-term relationship with an APP. Their NP had worked as a nurse in the ICU before training as an adult gerontology–acute care NP and joining Dr. Hussain’s practice, so she was also “well known to us,” he added.
Rachel Adney, CPNP-PC, a certified pediatric NP in the division of pediatric pulmonology at Stanford (Calif.) Medicine Children’s Health, is an APP who actively sought advanced training. She joined Stanford in 2011 to provide ambulatory care, primarily, and having years of prior experience in asthma management and education, she fast became known as “the asthma person.”
After a physician colleague one day objected to her caring for a patient without asthma, Ms. Adney, the first APP in the division, approached John D. Mark, MD, program director of the pediatric fellowship program at Stanford, and inquired about training “so I could have more breadth and depth across the whole pulmonary milieu.”
Together they designed a “mini pediatric pulmonary fellowship” for Ms. Adney, incorporating elements of the first year of Stanford’s pediatric fellowship program as well as training materials from the University of Arizona’s Pediatric Pulmonary Center, Tucson, one of six federally funded PCCs that train various health care providers to care for pediatric patients with chronic pulmonary conditions. (Dr. Mark had previously been an educator at the center while serving on the University of Arizona faculty.)
Her curriculum consisted of 1,000 total hours of training, including 125 hours of didactic learning and 400 hours of both inpatient and outpatient clinical training in areas such as cystic fibrosis, sleep medicine, bronchopulmonary dysplasia (BPD), neuromuscular disorders, and general pulmonary medicine. “Rachel rotated through clinics, first as an observer, then as a trainee ... and she attended lectures that my fellows attended,” said Dr. Mark, who has long been a preceptor for APPs. “She became like a 1-year fellow in my division.”
Today, Ms. Adney sees patients independently in four outreach clinics along California’s central coast. “She sees very complicated pediatric pulmonary patients now” overall, and has become integral to Stanford’s interdisciplinary CRIB program (cardiac and respiratory care for infants with BPD), Dr. Mark said. “She follows these patients at Stanford along with the whole CRIB group, then sees them on her own for follow-up.”
As a result of her training, Ms. Adney said, “knowing that I have the knowledge and experience to take on more complex patients, my colleagues now trust me and are confident in my skills. They feel comfortable sending [patients] to me much earlier. ... And they know that if there’s something I need help with I will go to them instantly.”
Pulmonology “really spoke to my heart,” she said, recalling her pre-Stanford journey as an in-hospital medical-surgical nurse, and then, after her NP training, as a outpatient primary care PNP. “For the most part, it’s like putting a puzzle together, and being able to really impact the quality of life these patients have,” said Ms. Adney, who serves on the APAPP’s pediatric subcommittee.
It’s clear, Dr. Mark said, that “things are changing around the country” with increasing institutional interest in developing formal APP specialty training programs. “There’s no way [for an APP] to walk into a specialty and play an active role without additional training,” and institutions are frustrated with turnover and the loss of APPs who decide after 6-9 months of on-the-job training that they’re not interested in the field.
Stanford Medicine Children’s Health, in fact, has launched an internal Pediatric APP Fellowship Program that is training its first cohort of six newly graduated NPs and PAs in two clinical tracks, including a medical/surgical track that incorporates rotations in pulmonary medicine, said Raji Koppolu, CPNP-PC/AC, manager of advanced practice professional development for Stanford Medicine Children’s Health.
APP fellowship programs have been in existence since 2007 in a variety of clinical settings, she said, but more institutions are developing them as a way of recruiting and retaining APPs in areas of high need and of equipping them for successful transitions to their APP roles. Various national bodies accredit APP fellowship programs.
Most pulmonary fellowship programs, Ms. Young said, are also internal programs providing postgraduate education to their own newly hired APPs or recent NP/PA graduates. This limits their reach, but “it’s a step in the right direction toward standardizing education for pulmonary APPs.”
Defining APP competencies
In interventional pulmonology, training may soon be guided by newly defined “core clinical competencies” for APPs. The soon-to-be published and distributed competencies – the first such national APP competencies in pulmonology – were developed by an APP Leadership Council within the American Association of Bronchology and Interventional Pulmonology and cover the most common disease processes and practices in IP, from COPD and bronchoscopic lung volume reduction to pleural effusion and lung cancer screening.
Rebecca Priebe, ACNP-BC, who cochairs the AABIP’s APP chapter, organized the effort several years ago, bringing together a group of APPs and physician experts in advanced bronchoscopy and IP (some but not all of whom have worked with APPs), after fielding questions from pulmonologists at AABIP meetings about what to look for in an AAP and how to train them.
Physicians and institutions who are hiring and training APPs for IP can use any or all of the 11 core competencies to personalize and evaluate the training process for each APP’s needs, she said. “Someone looking to hire an APP for pleural disease, for instance, can pull up the content on plural effusion.”
APP interest in interventional pulmonology is growing rapidly, Ms. Priebe said, noting growth in the AABIP’s APP chapter from about 7-8 APPs 5 years ago to at least 60 currently.
Ms. Priebe was hired by Henry Ford Health in Detroit about 5 years ago to help establish and run an inpatient IP consult service, and more recently, she helped establish their inpatient pleural disease service and a bronchoscopic lung volume reduction program.
For the inpatient IP service, after several months of side-by-side training with an IP fellow and attending physicians, she began independently evaluating new patients, writing notes, and making recommendations.
For patients with pleural disease, she performs ultrasound examinations, chest tube insertions, and bedside thoracentesis independently. And for the bronchoscopic lung volume reduction program, she evaluates patients for candidate status, participates in valve placement, and sees patients independently through a year of follow-up.
“Physician colleagues often aren’t sure what an APP’s education and scope of practice is,” said Ms. Priebe, who was an ICU nurse before training as an acute care NP and then worked first with a private practice inpatient service and then with the University of Michigan, Ann Arbor, where she established and grew an APP-run program managing lung transplant patients and a step-down ICU unit.
“It’s a matter of knowing [your state’s policies], treating them like a fellow you would train, and then using them to the fullest extent of their education and training. If they’re given an opportunity to learn a subspecialty skill set, they can be an asset to any pulmonary program.”
‘We’re here to support,’ not replace
In her own practice, Ms. Young is one of seven APPs who work with nine physicians on a full range of inpatient care, outpatient care, critical care, sleep medicine, and procedures. Many new patients are seen first by the APP, who does the workup and orders tests, and by the physician on a follow-up visit. Most patients needing routine management of asthma and COPD are seen by the physician every third or fourth visit, she said.
Ms. Young also directs a 24-hour in-house APP service recently established by the practice, and she participates in research. In a practice across town, she noted, APPs see mainly established patients and do not practice as autonomously as the state permits. “Part of that difference may [stem from] the lack of a standard of education and variable amounts of work the practice puts into their APPs.”
The American Medical Association’s #StopScopeCreep social media messaging feels divisive and “sheds a negative light on APPs working in any area,” Ms. Young said. “One of the biggest things we want to convey [at APAPP] is that we’re not here for [physicians’] jobs.”
“We’re here to support those who are practicing, to support underserved populations, and to help bridge gaps” created by an aging pulmonologist workforce and real and projected physician shortages, Ms. Young said, referring to a 2016 report from the Health Resources and Services Administration and a 2017 report from Merritt Hawkins indicating that 73% of U.S. pulmonologists (the largest percentage of all subspecialties) were at least 55 years old.
Dr. Hussain said he has “seen scope creep” first-hand in his hospitals, in the form of noncollaborative practices and tasks performed by APPs without adequate training – most likely often stemming from poor decisions and oversight by physicians. But when constructed thoughtfully, APP-physician teams are “serving great needs” in many types of care, he said, from follow-up care and management of chronic conditions to inpatient rounding. “My [colleagues] are having great success,” he said.
He is watching with interest – and some concern – pending reimbursement changes from the Centers for Medicare & Medicaid Services that will make time the only defining feature of the “substantive” portion of a split/shared visit involving physicians and APPs in a facility setting. Medical decision-making will no longer be applicable.
For time-based services like critical care, time alone is currently the metric. (And in the nonfacility setting, physician-APP teams may still apply “incident to” billing practices). But in the facility setting, said Amy M. Ahasic, MD, MPH, a pulmonologist in Norwalk, Conn., who coauthored a 2022 commentary on the issue, the change (now planned for 2024) could be problematic for employed physicians whose contracts are based on productivity, and could create tension and possibly lead to reduced use of APPs rather than supporting collaborative care.
“The team model has been evolving so well over the past 10-15 years,” said Dr. Ahasic, who serves on the CHEST Health Policy and Advocacy Reimbursement Workgroup and cochairs the CHEST/American Thoracic Society clinical practice committee with Dr. Hussain. “It’s good for patient safety to have more [providers] involved ... and because APP salaries are lower health systems could do it and be able to have better care and better coverage.”
The pulmonology culture, said Dr. Hussain, has been increasingly embracing APPs and “it’s collegial.” Pulmonologists are “coming to CHEST meetings with their APPs. They’re learning the same things we’re learning, to manage the same patients we manage.”
The article sources reported that they had no relevant financial conflicts of interest to disclose.
Breaking the itch-scratch cycle with mindfulness
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
Apple A. Bodemer, MD, a dermatologist at the University of Wisconsin, Madison, teaches patients how to breathe mindfully. So does Kathy Farah, MD, an integrative family physician who practices in Roberts, Wis.
, they said at the annual Integrative Dermatology Symposium.
“As with any integrative modality, if it’s safe and effective, then let’s use it,” Dr. Farah said in a presentation on the mind-body approach to pain and itch.
“A breathwork session can literally take 1 minute,” said Dr. Bodemer, associate professor of dermatology at the University of Wisconsin and director of an integrative dermatology clinic. Dr. Bodemer, who completed a fellowship in integrative medicine at the Andrew Weil Center for Integrative Medicine at the University of Arizona and sits on the American Board of Integrative Medicine, spoke on a mindfulness panel at the meeting.
Her favorite breathing practice is the “4-7-8” breath taught by Andrew Weil, MD, founder and director of the center. This involves inhaling through the nose for a count of 4, holding for 7, and exhaling through the mouth for a count of 8. “It doesn’t matter how slow or fast, it’s the tempo that matters ... On exhale, squeeze your abs in to engage your core and get air out of your lungs as much as you can,” she said, advising a cycle of three at a time.
A technique known as “square breathing” (breath in 4, hold for 4, breath out for 4, hold for 4) is another helpful technique to “reset the nervous system” said Dr. Farah, who worked for many years in a children’s hospital. With children, she said, “I often do five finger breathing.”
For five finger breathing, the children spread their fingers apart in front of them or on the ground and use the pointer finger of the opposite hand to trace each finger, inhaling while tracing upward, and exhaling while tracing down.
Dr. Farah, associate clinical director of The Center for Mind-Body Medicine in Washington, DC, said her commitment to mindfulness was influenced by a “seminal” study published over 20 years ago showing that patients with moderate to severe psoriasis who used a meditation-based, audiotape-guided stress reduction intervention during phototherapy sessions had more rapid resolution of psoriatic lesions than did patients who didn’t use the mindfulness exercise.
Among more recent findings: A cross-sectional study of 120 adult dermatology patients, published in the British Journal of Dermatology in 2016, assessed skin shame, social anxiety, anxiety, depression, dermatological quality of life, and levels of mindfulness, and found that higher levels of mindfulness were associated with lower levels of psychosocial distress.
Another cross-sectional questionnaire study looked at mindfulness and “itch catastrophizing” in 155 adult patients with atopic dermatitis. Higher levels of a specific facet of mindfulness termed “acting with awareness” were associated with lower levels of itch catastrophizing, the researchers found. “Catastrophizing is a negative way of thinking, this itching will never stop,” Dr. Farah explained. The study shows that “mindfulness can actually help reduce some of the automatic scratching and response to itch. So it’s a great adjunct to pharmaceuticals.”
Affirmations – phrases and statements that are repeated to oneself to help challenge negative thoughts – can also help reverse itch catastrophizing. Statements such as “I can breathe through this feeling of itching,” or “I can move to feel comfortable and relaxed” encourage positive change, she said.
“I teach [mindfulness skills like breathing] a lot, without any expectations. I’ll say ‘give it a try and see what you think.’ If patients feel even a micron better, then they’re invested” and can then find numerous tools online, Dr. Farah said. “Can I do this [in a busy schedule] with every patient? Absolutely not. But can I do it with every 10th patient? Maybe.”
Dr. Bodemer’s experience has shown her that “breathing with your patient builds rapport,” she said. “There’s something very powerful in that in terms of building trust. ... I’ll just do it [during a visit, to show them] and almost always, patients start breathing with me, with an invitation or without.”
For her own health, 4-7-8 breathing has “been a gateway to meditation and deeper practices,” she said. “But even without going very deep, it has a long history of being able to modulate the stress response. It’s the parasympathetic-sympathetic rebalancing I’m interested in.”
Mindful breathing and other mind-body practices also can be helpful for parents of children with eczema, she and Dr. Farah said.
Dr. Bodemer and Dr. Farah reported no financial relationships to disclose.
FROM IDS 2022
An integrative approach to atopic dermatitis features a long list of options
to probiotics and acupressure – that he encourages patients to try as they use the big guns, or as they attempt to wean off of them or avoid their use altogether.
During a presentation at the annual Integrative Dermatology Symposium, Dr. Lio said that he uses “5 pillars” to guide his integrative treatment plans: The skin barrier, the psyche, the microbiome, inflammation, and itch. “I try to flag approaches that predominantly address the categories that I think need the most help,” he said. “And I tell patients [which pillar or pillars] each treatment is addressing.”
Most commonly, the greatest challenge with AD – and the “single biggest weakness of conventional Western medicine” – lies not with getting patients clear in the first place, but in keeping them clear safely, he said. “I don’t think that using immunosuppressive [medications] is okay for the long-term unless there is no other choice,” said Dr. Lio, who cofounded the Chicago Integrative Eczema Center about 6 years ago and is clinical assistant professor of dermatology and pediatrics at Northwestern University, Chicago. Oftentimes, he said, complementary approaches, including dietary changes, can also serve as supportive adjunctive therapy to biologics and JAK inhibitors.
He has three main criteria, or “filters,” for evaluating these treatments before recommending them to patients: At least some clinical evidence for efficacy (preferably randomized trials but not necessarily), safety, and practicality. The “only way we’re going to move things forward [for AD and other conditions] is to try out less tested treatments ... to open up to them,” Dr. Lio said in an interview after the meeting. And in doing so, he said, dermatologists “can connect with a lot of patients whom naysayers can’t connect with.”
An integrative menu
Dr. Lio individualizes plans, suggesting treatments after “listening to patients’ stories” and considering their age, history, symptoms and skin presentation, and other factors. He said he “goes little by little,” telling a patient, for instance, “I’d love for us to try adding a little hemp oil to your diet.”
If patients aren’t pleased with or are tired of treatments, he said in the interview, “we move on and try something else.”
At the meeting, he described some of the treatments on his menu and the supporting evidence for those treatments:
Oral hempseed oil. A randomized crossover study of 20 adult patients with AD found that daily consumption of 2 tablespoons of hempseed oil decreased skin dryness, itchiness, and use of topical medications compared with consumption of olive oil. “It was statistically significant and seemed clinically meaningful,” likely resulting from the high concentration of polyunsaturated fatty acids in the oil, Dr. Lio said.
Topical vitamin B12. In a phase 3 randomized controlled trial of topical B12 applied twice a day for 8 weeks, patients experienced significant improvements in the extent and severity of AD compared with placebo. Another study in children with AD aged 6 months to 18 years found significant improvement in as early as 2 weeks of use. “It really does help, and is very gentle in babies,” Dr. Lio said.
Black tea compresses. “It’s absolutely my favorite kind of compress,” he said. “It was studied on the face and eyelids but I use it all over the body for adults and kids.” A German study of 22 patients with AD or contact facial dermatitis showed significant improvements in facial dermatitis within the first 3 days of treatment with application of black tea dressings plus an emollient cream, with significant reductions in four disease activity scores (the Facial Eczema Area and Severity Index, visual analog scale for pruritus, Investigator’s Global Assessment score, and Patient’s Self-Assessment Score) that continued through day 6.
Oolong tea. In a 2001 study, after 1 month of drinking oolong tea after each meal, 64% of patients with recalcitrant AD who continued with their regular treatment showed marked to moderate improvements in AD, with a beneficial effect first noticed after 1-2 weeks. At 6 months, 54% still had a good response to treatment. “It’s super cheap and accessible,” Dr. Lio said.
Coconut oil. One of the greatest benefits of coconut oil is on the microbiome and the dysbiosis that can result from a disrupted, or “leaky,” skin barrier – especially overgrowth of Staphylococcus aureus, which “drives AD,” Dr. Lio said. In a study of adults with AD from the Philippines, topically applied coconut oil decreased S. aureus colonization by 95% when applied twice daily for 4 weeks, compared with a 50% decrease in an olive oil control group. Other research has shown coconut oil to be superior to mineral oil as a moisturizer, he said at the meeting.
Acupressure. After a pilot study conducted by Dr. Lio and colleagues showed greater decreases in itch (per the visual analogue scale) in adults with AD who applied an acupressure bead at the LI11 point (near the elbow) for 3 minutes three times a week for 4 weeks, than among those who did not use the acupressure tool, Dr. Lio began trying it with some of his patients. “Now I use it broadly,” he added in the interview. “Kids over 10 can figure out how to use it and teenagers love it [to relief itch]. Some don’t use the beads anymore, they just use their fingertips.”
Advice on diet, vitamin D, and probiotics
AD severity is “powerfully” correlated with IgE food allergy, but Dr. Lio said at the meeting that he currently takes a cautious approach toward strict elimination diets.
There is a growing school of thought among allergists, he said, that positive IgE tests without evidence of acute reactions may not indicate true allergy, but rather sensitivity – and may not warrant food eliminations. And as has been shown with peanuts, there can be a serious downside to elimination, as food avoidance can lead to serious allergy later on, he said.
“More and more people are thinking that if you can tolerate [a food], continue it,” he added in the interview. In the absence of clear reactions, the only way to really know if a food is making eczema worse is to do a double-blind, placebo-controlled food challenge test, he noted.
Patients often come to see him believing that food is the “root cause” of their eczema and feeling frustrated, even anxious, about strict dietary restrictions they’ve implemented. But for many of these patients, the right question “would be to ask, why is my eczema causing my food allergy?” he said at the meeting, referring to the epithelial barrier hypothesis, which posits that skin barrier dysfunction can lead to asthma, allergic rhinitis, and food allergy.
Dr. Lio often recommends the Autoimmune Protocol (AIP) diet, a “close cousin” of the paleo diet for patients with AD, as general guidance to be followed “holistically” and often without the strict eliminations it prescribes. Minimizing processed foods and dairy and grains, which “can be inflammatory in some people,” and focusing on whole, nutrient-rich foods – all in keeping with the AIP principles – should have positive effects on the microbiome, overall health, and likely AD as well, he said.
Across the board, Dr. Lio recommends vitamin D (at nationally recommended dosages) and probiotics. Vitamin D has been shown to significantly help a small percentage of patients with eczema, he said, so he advises patients that it’s worth a trial. “I tell patients that I don’t know how to pick that small group out, so let’s try for a few months and see,” he said. “Inevitably, a percentage of patients come back and say it makes a huge difference.”
Dr. Lio’s understanding and use of probiotics has been “dynamic” over the years. “The “best, most reliable evidence” that probiotics can improve AD symptoms comes with the use of multiple probiotic strains together, he said. Based on limited but growing literature, he ensures that recommended formulations for babies include Lactobacillus rhamnosus, and that formulations for adults include Lactobacillus salivarius.
Dr. Lio works closely with dietitians, hypnotherapists, and psychologists – and will occasionally refer interested patients with AD to a Chinese medicine practitioner who personalizes the use of herbal formulations.
He reported no relevant disclosures.
to probiotics and acupressure – that he encourages patients to try as they use the big guns, or as they attempt to wean off of them or avoid their use altogether.
During a presentation at the annual Integrative Dermatology Symposium, Dr. Lio said that he uses “5 pillars” to guide his integrative treatment plans: The skin barrier, the psyche, the microbiome, inflammation, and itch. “I try to flag approaches that predominantly address the categories that I think need the most help,” he said. “And I tell patients [which pillar or pillars] each treatment is addressing.”
Most commonly, the greatest challenge with AD – and the “single biggest weakness of conventional Western medicine” – lies not with getting patients clear in the first place, but in keeping them clear safely, he said. “I don’t think that using immunosuppressive [medications] is okay for the long-term unless there is no other choice,” said Dr. Lio, who cofounded the Chicago Integrative Eczema Center about 6 years ago and is clinical assistant professor of dermatology and pediatrics at Northwestern University, Chicago. Oftentimes, he said, complementary approaches, including dietary changes, can also serve as supportive adjunctive therapy to biologics and JAK inhibitors.
He has three main criteria, or “filters,” for evaluating these treatments before recommending them to patients: At least some clinical evidence for efficacy (preferably randomized trials but not necessarily), safety, and practicality. The “only way we’re going to move things forward [for AD and other conditions] is to try out less tested treatments ... to open up to them,” Dr. Lio said in an interview after the meeting. And in doing so, he said, dermatologists “can connect with a lot of patients whom naysayers can’t connect with.”
An integrative menu
Dr. Lio individualizes plans, suggesting treatments after “listening to patients’ stories” and considering their age, history, symptoms and skin presentation, and other factors. He said he “goes little by little,” telling a patient, for instance, “I’d love for us to try adding a little hemp oil to your diet.”
If patients aren’t pleased with or are tired of treatments, he said in the interview, “we move on and try something else.”
At the meeting, he described some of the treatments on his menu and the supporting evidence for those treatments:
Oral hempseed oil. A randomized crossover study of 20 adult patients with AD found that daily consumption of 2 tablespoons of hempseed oil decreased skin dryness, itchiness, and use of topical medications compared with consumption of olive oil. “It was statistically significant and seemed clinically meaningful,” likely resulting from the high concentration of polyunsaturated fatty acids in the oil, Dr. Lio said.
Topical vitamin B12. In a phase 3 randomized controlled trial of topical B12 applied twice a day for 8 weeks, patients experienced significant improvements in the extent and severity of AD compared with placebo. Another study in children with AD aged 6 months to 18 years found significant improvement in as early as 2 weeks of use. “It really does help, and is very gentle in babies,” Dr. Lio said.
Black tea compresses. “It’s absolutely my favorite kind of compress,” he said. “It was studied on the face and eyelids but I use it all over the body for adults and kids.” A German study of 22 patients with AD or contact facial dermatitis showed significant improvements in facial dermatitis within the first 3 days of treatment with application of black tea dressings plus an emollient cream, with significant reductions in four disease activity scores (the Facial Eczema Area and Severity Index, visual analog scale for pruritus, Investigator’s Global Assessment score, and Patient’s Self-Assessment Score) that continued through day 6.
Oolong tea. In a 2001 study, after 1 month of drinking oolong tea after each meal, 64% of patients with recalcitrant AD who continued with their regular treatment showed marked to moderate improvements in AD, with a beneficial effect first noticed after 1-2 weeks. At 6 months, 54% still had a good response to treatment. “It’s super cheap and accessible,” Dr. Lio said.
Coconut oil. One of the greatest benefits of coconut oil is on the microbiome and the dysbiosis that can result from a disrupted, or “leaky,” skin barrier – especially overgrowth of Staphylococcus aureus, which “drives AD,” Dr. Lio said. In a study of adults with AD from the Philippines, topically applied coconut oil decreased S. aureus colonization by 95% when applied twice daily for 4 weeks, compared with a 50% decrease in an olive oil control group. Other research has shown coconut oil to be superior to mineral oil as a moisturizer, he said at the meeting.
Acupressure. After a pilot study conducted by Dr. Lio and colleagues showed greater decreases in itch (per the visual analogue scale) in adults with AD who applied an acupressure bead at the LI11 point (near the elbow) for 3 minutes three times a week for 4 weeks, than among those who did not use the acupressure tool, Dr. Lio began trying it with some of his patients. “Now I use it broadly,” he added in the interview. “Kids over 10 can figure out how to use it and teenagers love it [to relief itch]. Some don’t use the beads anymore, they just use their fingertips.”
Advice on diet, vitamin D, and probiotics
AD severity is “powerfully” correlated with IgE food allergy, but Dr. Lio said at the meeting that he currently takes a cautious approach toward strict elimination diets.
There is a growing school of thought among allergists, he said, that positive IgE tests without evidence of acute reactions may not indicate true allergy, but rather sensitivity – and may not warrant food eliminations. And as has been shown with peanuts, there can be a serious downside to elimination, as food avoidance can lead to serious allergy later on, he said.
“More and more people are thinking that if you can tolerate [a food], continue it,” he added in the interview. In the absence of clear reactions, the only way to really know if a food is making eczema worse is to do a double-blind, placebo-controlled food challenge test, he noted.
Patients often come to see him believing that food is the “root cause” of their eczema and feeling frustrated, even anxious, about strict dietary restrictions they’ve implemented. But for many of these patients, the right question “would be to ask, why is my eczema causing my food allergy?” he said at the meeting, referring to the epithelial barrier hypothesis, which posits that skin barrier dysfunction can lead to asthma, allergic rhinitis, and food allergy.
Dr. Lio often recommends the Autoimmune Protocol (AIP) diet, a “close cousin” of the paleo diet for patients with AD, as general guidance to be followed “holistically” and often without the strict eliminations it prescribes. Minimizing processed foods and dairy and grains, which “can be inflammatory in some people,” and focusing on whole, nutrient-rich foods – all in keeping with the AIP principles – should have positive effects on the microbiome, overall health, and likely AD as well, he said.
Across the board, Dr. Lio recommends vitamin D (at nationally recommended dosages) and probiotics. Vitamin D has been shown to significantly help a small percentage of patients with eczema, he said, so he advises patients that it’s worth a trial. “I tell patients that I don’t know how to pick that small group out, so let’s try for a few months and see,” he said. “Inevitably, a percentage of patients come back and say it makes a huge difference.”
Dr. Lio’s understanding and use of probiotics has been “dynamic” over the years. “The “best, most reliable evidence” that probiotics can improve AD symptoms comes with the use of multiple probiotic strains together, he said. Based on limited but growing literature, he ensures that recommended formulations for babies include Lactobacillus rhamnosus, and that formulations for adults include Lactobacillus salivarius.
Dr. Lio works closely with dietitians, hypnotherapists, and psychologists – and will occasionally refer interested patients with AD to a Chinese medicine practitioner who personalizes the use of herbal formulations.
He reported no relevant disclosures.
to probiotics and acupressure – that he encourages patients to try as they use the big guns, or as they attempt to wean off of them or avoid their use altogether.
During a presentation at the annual Integrative Dermatology Symposium, Dr. Lio said that he uses “5 pillars” to guide his integrative treatment plans: The skin barrier, the psyche, the microbiome, inflammation, and itch. “I try to flag approaches that predominantly address the categories that I think need the most help,” he said. “And I tell patients [which pillar or pillars] each treatment is addressing.”
Most commonly, the greatest challenge with AD – and the “single biggest weakness of conventional Western medicine” – lies not with getting patients clear in the first place, but in keeping them clear safely, he said. “I don’t think that using immunosuppressive [medications] is okay for the long-term unless there is no other choice,” said Dr. Lio, who cofounded the Chicago Integrative Eczema Center about 6 years ago and is clinical assistant professor of dermatology and pediatrics at Northwestern University, Chicago. Oftentimes, he said, complementary approaches, including dietary changes, can also serve as supportive adjunctive therapy to biologics and JAK inhibitors.
He has three main criteria, or “filters,” for evaluating these treatments before recommending them to patients: At least some clinical evidence for efficacy (preferably randomized trials but not necessarily), safety, and practicality. The “only way we’re going to move things forward [for AD and other conditions] is to try out less tested treatments ... to open up to them,” Dr. Lio said in an interview after the meeting. And in doing so, he said, dermatologists “can connect with a lot of patients whom naysayers can’t connect with.”
An integrative menu
Dr. Lio individualizes plans, suggesting treatments after “listening to patients’ stories” and considering their age, history, symptoms and skin presentation, and other factors. He said he “goes little by little,” telling a patient, for instance, “I’d love for us to try adding a little hemp oil to your diet.”
If patients aren’t pleased with or are tired of treatments, he said in the interview, “we move on and try something else.”
At the meeting, he described some of the treatments on his menu and the supporting evidence for those treatments:
Oral hempseed oil. A randomized crossover study of 20 adult patients with AD found that daily consumption of 2 tablespoons of hempseed oil decreased skin dryness, itchiness, and use of topical medications compared with consumption of olive oil. “It was statistically significant and seemed clinically meaningful,” likely resulting from the high concentration of polyunsaturated fatty acids in the oil, Dr. Lio said.
Topical vitamin B12. In a phase 3 randomized controlled trial of topical B12 applied twice a day for 8 weeks, patients experienced significant improvements in the extent and severity of AD compared with placebo. Another study in children with AD aged 6 months to 18 years found significant improvement in as early as 2 weeks of use. “It really does help, and is very gentle in babies,” Dr. Lio said.
Black tea compresses. “It’s absolutely my favorite kind of compress,” he said. “It was studied on the face and eyelids but I use it all over the body for adults and kids.” A German study of 22 patients with AD or contact facial dermatitis showed significant improvements in facial dermatitis within the first 3 days of treatment with application of black tea dressings plus an emollient cream, with significant reductions in four disease activity scores (the Facial Eczema Area and Severity Index, visual analog scale for pruritus, Investigator’s Global Assessment score, and Patient’s Self-Assessment Score) that continued through day 6.
Oolong tea. In a 2001 study, after 1 month of drinking oolong tea after each meal, 64% of patients with recalcitrant AD who continued with their regular treatment showed marked to moderate improvements in AD, with a beneficial effect first noticed after 1-2 weeks. At 6 months, 54% still had a good response to treatment. “It’s super cheap and accessible,” Dr. Lio said.
Coconut oil. One of the greatest benefits of coconut oil is on the microbiome and the dysbiosis that can result from a disrupted, or “leaky,” skin barrier – especially overgrowth of Staphylococcus aureus, which “drives AD,” Dr. Lio said. In a study of adults with AD from the Philippines, topically applied coconut oil decreased S. aureus colonization by 95% when applied twice daily for 4 weeks, compared with a 50% decrease in an olive oil control group. Other research has shown coconut oil to be superior to mineral oil as a moisturizer, he said at the meeting.
Acupressure. After a pilot study conducted by Dr. Lio and colleagues showed greater decreases in itch (per the visual analogue scale) in adults with AD who applied an acupressure bead at the LI11 point (near the elbow) for 3 minutes three times a week for 4 weeks, than among those who did not use the acupressure tool, Dr. Lio began trying it with some of his patients. “Now I use it broadly,” he added in the interview. “Kids over 10 can figure out how to use it and teenagers love it [to relief itch]. Some don’t use the beads anymore, they just use their fingertips.”
Advice on diet, vitamin D, and probiotics
AD severity is “powerfully” correlated with IgE food allergy, but Dr. Lio said at the meeting that he currently takes a cautious approach toward strict elimination diets.
There is a growing school of thought among allergists, he said, that positive IgE tests without evidence of acute reactions may not indicate true allergy, but rather sensitivity – and may not warrant food eliminations. And as has been shown with peanuts, there can be a serious downside to elimination, as food avoidance can lead to serious allergy later on, he said.
“More and more people are thinking that if you can tolerate [a food], continue it,” he added in the interview. In the absence of clear reactions, the only way to really know if a food is making eczema worse is to do a double-blind, placebo-controlled food challenge test, he noted.
Patients often come to see him believing that food is the “root cause” of their eczema and feeling frustrated, even anxious, about strict dietary restrictions they’ve implemented. But for many of these patients, the right question “would be to ask, why is my eczema causing my food allergy?” he said at the meeting, referring to the epithelial barrier hypothesis, which posits that skin barrier dysfunction can lead to asthma, allergic rhinitis, and food allergy.
Dr. Lio often recommends the Autoimmune Protocol (AIP) diet, a “close cousin” of the paleo diet for patients with AD, as general guidance to be followed “holistically” and often without the strict eliminations it prescribes. Minimizing processed foods and dairy and grains, which “can be inflammatory in some people,” and focusing on whole, nutrient-rich foods – all in keeping with the AIP principles – should have positive effects on the microbiome, overall health, and likely AD as well, he said.
Across the board, Dr. Lio recommends vitamin D (at nationally recommended dosages) and probiotics. Vitamin D has been shown to significantly help a small percentage of patients with eczema, he said, so he advises patients that it’s worth a trial. “I tell patients that I don’t know how to pick that small group out, so let’s try for a few months and see,” he said. “Inevitably, a percentage of patients come back and say it makes a huge difference.”
Dr. Lio’s understanding and use of probiotics has been “dynamic” over the years. “The “best, most reliable evidence” that probiotics can improve AD symptoms comes with the use of multiple probiotic strains together, he said. Based on limited but growing literature, he ensures that recommended formulations for babies include Lactobacillus rhamnosus, and that formulations for adults include Lactobacillus salivarius.
Dr. Lio works closely with dietitians, hypnotherapists, and psychologists – and will occasionally refer interested patients with AD to a Chinese medicine practitioner who personalizes the use of herbal formulations.
He reported no relevant disclosures.
FROM IDS 2022
Climate change: Commentary in four dermatology journals calls for emergency action
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
“moving beyond merely discussing skin-related impacts” and toward prioritizing both patient and planetary health.
Dermatologists must make emissions-saving changes in everyday practice, for instance, and the specialty must enlist key stakeholders in public health, nonprofits, and industry – that is, pharmaceutical and medical supply companies – in finding solutions to help mitigate and adapt to climate change, wrote Eva Rawlings Parker, MD, and Markus D. Boos, MD, PhD.
“We have an ethical imperative to act,” they wrote. “The time is now for dermatologists and our medical societies to collectively rise to meet this crisis.”
Their commentary was published online in the International Journal of Dermatology , Journal of the European Academy of Dermatology and Venereology, British Journal of Dermatology, and Pediatric Dermatology.
In an interview, Dr. Parker, assistant professor of dermatology at Vanderbilt University, Nashville, Tenn., said that she and Dr. Boos, associate professor in the division of dermatology and department of pediatrics at the University of Washington, Seattle, were motivated to write the editorial upon finding that dermatology was not represented among more than 230 medical journals that published an editorial in September 2021 calling for emergency action to limit global warming and protect health. In addition to the New England Journal of Medicine and The Lancet, the copublishing journals represented numerous specialties, from nursing and pediatrics, to cardiology, rheumatology, and gastroenterology.
The editorial was not published in any dermatology journals, Dr. Parker said. “It was incredibly disappointing for me along with many of my colleagues who advocate for climate action because we realized it was a missed opportunity for dermatology to align with other medical specialties and be on the forefront of leading climate action to protect health.”
‘A threat multiplier’
The impact of climate change on skin disease is “an incredibly important part of our conversation as dermatologists because many cutaneous diseases are climate sensitive and we’re often seeing the effects of climate change every day in our clinical practices,” Dr. Parker said.
In fact, the impact on skin disease needs to be explored much further through more robust research funding, so that dermatology can better understand not only the incidence and severity of climate-induced changes in skin diseases – including and beyond atopic dermatitis, acne, and psoriasis – but also the mechanisms and pathophysiology involved, she said.
However, the impacts are much broader, she and Dr. Boos, a pediatric dermatologist at Seattle Children’s Hospital, maintain in their commentary. “An essential concept to broker among dermatologists is that the impacts of climate change extend well beyond skin disease by also placing broad pressure” on infrastructure, the economy, financial markets, global supply chains, food and water insecurity, and more, they wrote, noting the deep inequities of climate change.
Climate change is a “threat multiplier for public health, equity, and health systems,” the commentary says. “The confluence of these climate-related pressures should sound alarm bells as they place enormous jeopardy on the practice of dermatology across all scales and regions.”
Health care is among the most carbon-intensive service sectors worldwide, contributing to almost 5% of greenhouse gas emissions globally, the commentary says. And nationally, of the estimated greenhouse gas emissions from the United States, the health care sector contributes 10%, Dr. Parker said in the interview, referring to a 2016 report.
In addition, according to a 2019 report, the United States is the top contributor to health care’s global climate footprint, contributing 27% of health care’s global emissions, Dr. Parker noted.
In their commentary, she and Dr. Boos wrote that individually and practice wide, dermatologists can impact decarbonization through measures such as virtual attendance at medical meetings and greater utilization of telehealth services. Reductions in carbon emissions were demonstrated for virtual isotretinoin follow-up visits in a recent study, and these savings could be extrapolated to other routine follow-up visits for conditions such as rosacea, monitoring of biologics in patients with well-controlled disease, and postoperative wound checks, they said.
But when it comes to measures such as significantly reducing packaging and waste and “curating supply chains to make them more sustainable,” it is medical societies that have the “larger voice and broader relationship with the pharmaceutical industry” and with medical supply manufacturers and distributors, Dr. Parker explained in the interview, noting the potential for reducing the extensive amount of packaging used for drug samples.
Dr. Parker cochairs the American Academy of Dermatology’s Expert Resource Group for Climate Change and Environmental Issues, which was established several years ago, and Dr. Boos is a member of the group’s executive committee.
AAD actions
In its 2018 Position Statement on Climate and Health, the American Academy of Dermatology resolved to raise awareness of the effects of climate change on the skin and educate patients about this, and to “work with other medical societies in ongoing and future efforts to educate the public and mitigate the effects of climate change on global health.”
Asked about the commentary’s call for more collaboration with industry and other stakeholders – and the impact that organized dermatology can have on planetary health – Mark D. Kaufmann, MD, president of the AAD, said in an email that the AAD is “first and foremost an organization focused on providing gold-standard educational resources for dermatologists.”
The academy recognizes that “there are many dermatologic consequences of climate change that will increasingly affect our patients and challenge our membership,” and it has provided education on climate change in forums such as articles, podcasts, and sessions at AAD meetings, said Dr. Kaufmann, clinical professor in the department of dermatology, Icahn School of Medicine at Mount Sinai, New York.
Regarding collaboration with other societies, he said that the AAD’s “focus to date has been on how to provide our members with educational resources to understand and prepare for how climate change may impact their practices and the dermatologic health of their patients,” he said.
The AAD has also sought to address its own carbon footprint and improve sustainability of its operations, including taking steps to reduce plastic and paper waste at its educational events, and to eliminate plastic waste associated with mailing resources like its member magazine, Dr. Kaufmann noted.
And in keeping with the Academy pledge – also articulated in the 2018 position statement – to support and facilitate dermatologists’ efforts to decrease their carbon footprint “in a cost effective (or cost-saving) manner,” Dr. Kaufmann said that the AAD has been offering a program called My Green Doctor as a free benefit of membership.
‘Be part of the solution’
In an interview, Mary E. Maloney, MD, professor of medicine and director of dermatologic surgery at the University of Massachusetts, Worcester, said her practice did an audit of their surgical area and found ways to increase the use of paper-packaged gauze – and decrease use of gauze in hard plastic containers – and otherwise decrease the amount of disposables, all of which take “huge amounts of resources” to create.
In the process, “we found significant savings,” she said. “Little things can turn out, in the long run, to be big things.”
Asked about the commentary, Dr. Maloney, who is involved in the AAD’s climate change resource group, said “the message is that yes, we need to be aware of the diseases affected by climate change. But our greater imperative is to be part of the solution and not part of the problem as far as doing things that affect climate change.”
Organized dermatology needs to broaden its advocacy, she said. “I don’t want us to stop advocating for things for our patients, but I do want us to start advocating for the world ... If we don’t try to [mitigate] climate change, we won’t have patients to advocate for.”
Dr. Parker, an associate editor of The Journal of Climate Change and Health, and Dr. Boos declared no conflicts of interest and no funding source for their commentary. Dr. Maloney said she has no conflicts of interest.
Newer 3D lung models starting to remake research
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.
Pulmonologist-scientist Veena B. Antony, MD, professor of medicine at the University of Alabama in Birmingham, grows “pulmospheres” in her lab. The tiny spheres, about 1 mL in diameter, contain cells representing all of the cell types in a lung struck with pulmonary fibrosis.
They are a three-dimensional model of idiopathic pulmonary fibrosis (IPF) that can be used to study the behavior of invasive myofibroblasts and to predict in vivo responsiveness to antifibrotic drugs;
“The utility is extensive, including looking at the impact of early-life exposures on mid-life lung disease. We can ask all kinds of questions and answer them much faster, and with more accuracy, than with any 2D model,” said Dr. Antony, also professor of environmental health sciences and director of UAB’s program for environmental and translational medicine.
“The future of 3D modeling of the lung will happen step by step ... but we’re right at the edge of a prime explosion of information coming from these models, in all kinds of lung diseases,” she said.
Two-dimensional model systems – mainly monolayer cell cultures where cells adhere to and grow on a plate – cannot approximate the variety of cell types and architecture found in tissue, nor can they recapitulate cell-cell communication, biochemical cues, and other factors that are key to lung development and the pathogenesis of disease.
Dr. Antony’s pulmospheres resemble what have come to be known as organoids – 3D tissue cultures emanating from induced pluripotent stem cells (iPSC) or adult stem cells, in which multiple cell types self-organize, usually while suspended in natural or synthetic extracellular matrix (with or without a scaffold of some kind).
Lung-on-a-chip
In lung-on-a-chip (LOC) models, multiple cell types are seeded into miniature chambers, or “chips,” that contain networks of microfabricated channels designed to deliver and remove fluids, chemical cues, oxygen, and biomechanical forces. LOCs and other organs-on-chips – also called tissues-on-chips – can be continuously perfused and are highly structured and precisely controlled.
It’s the organs-on-chip model – or potential fusions of the organoid and organs-on-chip models – that will likely impact drug development. Almost 9 out of 10 investigational drugs fail in clinical trials – approximately 60% because of lack of efficacy and 30% because of toxicity. More reliable and predictive preclinical investigation is key, said Danilo A. Tagle, PhD, director of the Office of Special Initiatives in the National Center for Advancing Translational Sciences, of the National Institutes of Health.
“We have so many candidate drugs that go through preclinical safety testing, and that do relatively well in animal studies of efficacy, but then fail in clinical trials,” Dr. Tagle said. “We need better preclinical models.”
In its 10 years of life, the Tissue Chip for Drug Screening Program led by the NCATS – and funded by the NIH and Defense Advanced Research Projects Agency – has shown that organs-on-chips can be used to model disease and to predict both the safety and efficacy of clinical compounds, he said.
Lung organoids
Dr. Antony’s pulmospheres emanate not from stem cells but from primary tissue obtained from diseased lung. “We reconstitute the lung cells in single-cell suspensions, and then we allow them to come back together to form lung tissue,” she said. The pulmospheres take about 3 days to grow.
In a study published 5 years ago of pulmospheres of 20 patients with IPF and 9 control subjects, Dr. Antony and colleagues quantitated invasiveness and found “remarkable” differences in the invasiveness of IPF pulmospheres following exposure to the Food and Drug Administration–approved antifibrotic drugs nintedanib and pirfenidone. Some pulmospheres responded to one or the other drug, some to both, and two to neither – findings that Dr. Antony said offer hope for the goals of personalizing therapy and assessing new drugs.
Moreover, clinical disease progression correlated with invasiveness of the pulmospheres, showing that the organoid-like structures “do give us a model that [reflects] what’s happening in the clinical setting,” she said. (Lung tissue for the study was obtained via video-assisted thoracic surgery biopsy of IPF patients and from failed donor lung explants, but bronchoscopic forceps biopsies have become a useful method for obtaining tissue.)
The pulmospheres are not yet in clinical use, Dr. Antony said, but her lab is testing other fibrosis modifiers and continuing to use the model as a research tool.
One state to the east, at Vanderbilt University, Nashville, Tenn., Amanda Linkous, PhD, grows “branching lung organoids” and brain organoids to study the biology of small cell lung cancer (SCLC).
“We want to understand how [SCLC] cells change in the primary organ site, compared with metastatic sites like the brain. ... Are different transcription factors expressed [for instance] depending on where the tumor is growing?” said Dr. Linkous, scientific center manager of the National Cancer Institute’s Center for Systems Biology of SCLC at Vanderbilt. “Then we hope to start drug screening within the next year.”
Her lung organoids take shape from either human embryonic stem cells or iPSCs. Within commercially available media, the cells mature through several stages of differentiation, forming definitive endoderm, anterior foregut endoderm, and then circular lung bud structures – the latter of which are then placed into droplets of Matrigel, an extracellular matrix gel.
“In the Matrigel droplets, the lung bud cells will develop proximal and distal-like branching structures that express things like EPCAM, MUC1, SOX2, SOX9, and NKX2.1 – key markers that you should see in a more mature lung microenvironment,” she said. Tumor cells from established SCLC cell lines will then easily invade the branching lung organoid.
Dr. Linkous said she has found her organoid models highly reproducible and values their long-lasting nature – especially for future drug screening. “We can keep organoids going for months at a time,” said Dr. Linkous, a research associate professor in Vanderbilt’s department of biochemistry.
Like Dr. Antony, she envisions personalizing treatment in the future. “SCLC is a very heterogeneous tumor with many different cell types, so what works for one patient may not work well at all for another patient,” she said.
As recently as 5 years ago, “many in the cancer field would have been resistant to moving away from mouse models,” Dr. Linkous noted. “But preclinical studies in mice often don’t pan out in the clinic ... so we’re moving toward a human microenvironment to study human disease.”
The greatest challenge, Dr. Linkous and Dr. Antony said, lies in integrating both vascular blood flow and air into these models. “We just don’t have that combination as of yet,” Dr. Antony said.
LOC models
One of the first LOC models – and a galvanizing event for organs-on-chips more broadly – was a 1- to 2-cm–long model of the alveolar-capillary interface developed at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, Boston.
Microchannels ran alongside a porous membrane coated with extracellular matrix, with alveolar cells seeded on one side and lung endothelial cells on the other side. When a vacuum was applied rhythmically to the channels, the cell-lined membrane stretched and relaxed, mimicking breathing movements.
Lead investigator Dongeun (Dan) Huh, PhD, then a postdoctoral student working with Donald E. Ingber, MD, PhD, founding director of the institute, ran tests showing that the model could reproduce organ-level responses to bacteria and inflammatory cytokines, as well as to silica nanoparticles. The widely cited paper was published in 2010 (Science. 2010;328[5986]:1662-8), and was followed by another study published in 2012 (Sci Transl Med. 2012;4[159]:159ra147) that used the LOC device to reproduce drug toxicity–induced pulmonary edema. “Here we were demonstrating for the first time that we could use the lung-on-chip to model human lung disease,” said Dr. Huh, who started his own lab at the University of Pennsylvania, Philadelphia, in 2013.
Since then, “as a field we’ve come a long way in modeling the complexity of human lung tissues ... with more advanced devices that can be used to mimic different parts of the lung and different processes, like immune responses in asthma and viral infections,” said Dr. Huh, “and with several studies using primary human cells taken from lung disease patients.”
Among Dr. Huh’s latest devices, built with NIH funding, is an asthma-on-a-chip device. Lung cells isolated from asthma patients are grown in a microfabricated device to create multilayered airway tissue, with airspace, that contains a fully differentiated epithelium and a vascularized stroma. “We can compress the entire engineered area of asthmatic human tissue in a lateral direction to mimic bronchoconstriction that happens during an asthma attack,” he said.
A paper soon to be published will describe how “abnormal pathophysiologic compressive forces due to bronchoconstriction in asthmatic lungs can make the lungs fibrotic, and how those mechanical forces also can induce increased vascularity,” said Dr. Huh, associate professor in the university’s department of bioengineering. “The increased vascular density can also change the phenotype of blood vessels in asthmatic airways.”
Dr. Huh also has an $8.3 million contract with the government’s Biomedical Advanced Research and Development Authority to study how chlorine gas damages lung tissues and identify biomarkers of chlorine gas–induced lung injury, with the goal of developing therapeutics.
Dr. Ingber and associates have developed a device modeling cystic fibrosis (CF). The chip is lined with primary human CF bronchial epithelial cells grown under an air-liquid interface and interfaced with primary lung microvascular endothelium that are exposed to fluid flow.
The chip reproduced, “with high fidelity, many of the structural, biochemical, and pathophysiological features of the human CF lung airway and its response to pathogens and circulating immune cells in vitro,” Dr. Ingber and colleagues reported (J Cyst Fibros. 2022;21:605-15).
Government investment in tissue chips
Efforts to commercialize organs-on-chip platforms and translate them for nonengineers have also picked in recent years. Several companies in the United States (including Emulate, a Wyss start-up) and in Europe now offer microengineered lung tissue models that can be used for research and drug testing. And some large pharmaceutical companies, said Dr. Tagle, have begun integrating tissue chip technology into their drug development programs.
The FDA, meanwhile, “has come to embrace the technology and see its promise,” Dr. Tagle said. An FDA pilot program announced in 2021 – called ISTAND (Innovative Science and Technology Approaches for New Drugs) – allows for tissue chip data to be submitted, as standalone data, for some drug applications.
The first 5 years of the government’s Tissue Chip for Drug Screening Program focused on safety and toxicity, and it “was successful in that model organ systems were able to capture the human response that [had been missed in] animal models,” he said.
For example, when a liver-tissue model was used to test several compounds that had passed animal testing for toxicity/safety but then failed in human clinical trials – killing some of the participants – the model showed a 100% sensitivity and a 87% specificity in predicting the human response, said Dr. Tagle, who recently coauthored a review on the future of organs-on-chips (Nature Reviews I Drug Discovery. 2021;20:345-61).
The second 5 years of the program, currently winding down, have focused on efficacy – the ability of organs-on-chip models to recreate the pathophysiology of chronic obstructive pulmonary disease, influenza, and other diseases, so that potential drugs can be assessed. In 2020, with extra support from the Coronavirus Aid, Relief, and Economic Security Act, NCATS funded academic labs to use organs-on-chip technology to evaluate SARS-CoV-2 and potential therapeutics.
Dr. Ingbar was one of the grantees. His team screened a number of FDA-approved drugs for potential repurposing using a bronchial-airway-on-a-chip and compared results with 2D model systems (Nat Biomed Eng. 2021;5:815-29). Amodiaquine inhibited infection in the 3D model and is now in phase 2 COVID trials. Several other drugs showed effectiveness in a 2D model but not in the chip.
Now, in a next phase of study at NCATS, coined Clinical Trials on a Chip, the center has awarded $35.5 million for investigators to test candidate therapies, often in parallel to ongoing clinical trials. The hope is that organs-on-chips can improve clinical trial design, from enrollment criteria and patient stratification to endpoints and the use of biomarkers. And in his lab, Dr. Huh is now engineering a shift to “organoids-on-a-chip” that combines the best features of each approach. “The idea,” he said, “is to grow organoids, and maintain the organoids in the microengineered systems where we can control their environment better ... and apply cues to allow them to develop into even more realistic tissues.”
Drs. Antony, Linkous, and Tagle reported no relevant disclosures. Dr. Huh is a co-founder of Vivodyne Inc, and owns shares in Vivodyne Inc. and Emulate Inc.
Brodalumab suicide risk similar to other biologics, postmarket study finds
.
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
.
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
.
The Food and Drug Administration approved brodalumab (Siliq) in 2017 for treatment of moderate to severe plaque psoriasis with a boxed warning for suicidal ideation and behavior and an associated Risk Evaluation and Mitigation Strategies (REMS) program indicating an increased risk of suicidality.
Half a decade later, “the available worldwide data do not support the notion that brodalumab has a unique risk of increased suicides,” senior investigator John Koo, MD, and coinvestigators at the University of California, San Francisco, wrote in a preproof article in JAAD International, noting that postmarketing data are “often considered a better reflection of real-world outcomes than clinical trials.”
The researchers extracted data through the end of 2021 on the number of completed suicides for brodalumab and ten other biologics approved for psoriasis from the FDA’s Adverse Events Reporting System (FAERS), an international publicly available database. The researchers included suicide data on the biologics for all indications.
The authors contacted pharmaceutical companies to determine the total number of patients prescribed each drug, securing mostly “best estimates” data on 5 of the 11 biologics available for psoriasis. The researchers then calculated the number of completed suicides per total number of prescribed patients.
For brodalumab, across 20,871 total prescriptions, there was only one verifiable suicide. It occurred in a Japanese man with terminal cancer and no nearby relatives 36 days after his first dose. The suicide rate for brodalumab was similar to that of ixekizumab, secukinumab, infliximab, and adalimumab.
“Brodalumab is a very efficacious agent and may have the fastest onset of action, yet its usage is minimal compared to the other agents because of this ‘black box’ warning ... despite the fact that it’s the least expensive of any biologic,” Dr. Koo, professor of dermatology and director of the Psoriasis and Skin Treatment Center, University of California, San Francisco, said in an interview.
Dr. Koo, who is board-certified in both dermatology and psychiatry, said he believes the boxed warning was never warranted. All three of the verified completed suicides that occurred during clinical trials of brodalumab for psoriasis were in people who had underlying psychiatric disorders or significant stressors, such as going to jail in one case, and depression and significant isolation in another, he said.
(An analysis of psychiatric adverse events during the psoriasis clinical trials, involving more than 4,000 patients, was published online Oct. 4, 2017, in the Journal of the American Academy of Dermatology.
George Han, MD, PhD, associate professor and director of research and teledermatology at the Zucker School of Medicine at Hofstra/Northwell, New Hyde Park, N.Y., who was not involved in the research, said the new data is reassuring.
“We sometimes put it into context [in thinking and counseling about risk] that in the trials for brodalumab, the number of suicide attempts [versus completed suicides] was not an outlier,” he said. “But it’s hard to know what to make of that, so this piece of knowledge that the postmarketing data show there’s no safety signal should give people a lot of reassurance.”
Dr. Han said he has used the medication, a fully human anti-interleukin 17 receptor A monoclonal antibody, in many patients who “have not done so well on other biologics and it’s been a lifesaver ... a couple who have switched over have maintained the longest level of clearance they’ve had with anything. It’s quite striking.”
The efficacy stems at least partly from its mechanism of blocking all cytokines in the IL-17 family – including those involved in the “feedback loops that perpetuate psoriasis” – rather than just one as other biologics do, Dr. Han said.
Usage of the drug has been hindered by the black box warning and REMS program, not only because of the extra steps required and hesitation potentially evoked, but because samples are not available, and because the “formulary access is not what it could have been otherwise,” he noted.
The Siliq REMS patient enrollment form requires patients to pledge awareness of the fact that suicidal thoughts and behaviors have occurred in treated patients and that they should seek medical attention if they experience suicidal thoughts or new or worsening depression, anxiety, or other mood changes. Prescribers must be certified with the program and must pledge on each enrollment form that they have counseled their patients.
The box warning states that there is no established causal association between treatment with brodalumab and increased risk for suicidal ideation and behaviors (SIB).
Individuals with psoriasis are an “already vulnerable population” who have been shown in reviews and meta-analyses to have a higher prevalence of depression and a higher risk of SIB than those without the disease, Dr. Koo and colleagues wrote in a narrative review published in Cutis .
Regardless of therapy, they wrote in the review, dermatologists should assess for any history of depression and SIB, and evaluate for signs and symptoms of current depression and SIB, referring patients as necessary to primary care or mental health care.
In the psoriasis trials, brodalumab treatment appeared to improve symptoms of depression and anxiety – a finding consistent with the effects reported for other biologic therapies, they wrote.
The first author on the newly published preproof is Samuel Yeroushalmi, BS, a fourth-year medical student at George Washington University, Washington.
Siliq is marketed by Valeant Pharmaceuticals.
Dr. Koo disclosed that he is an adviser/consultant/speaker for numerous pharmaceutical companies, but not those that were involved in the development of brodalumab. Dr. Han said he has relationships with numerous companies, including those that have developed brodalumab and other biologic agents used for psoriasis. The authors declared funding sources as none.
Litifilimab meets primary endpoint in phase 2 lupus trial
Treatment with the humanized monoclonal antibody litifilimab for patients with systemic lupus erythematosus (SLE) led to greater improvements in joint manifestations than did placebo in an international phase 2 trial that reflects keen interest in targeting type 1 interferon and the innate immune system.
Litifilimab was associated with an approximately three-joint reduction in the number of swollen and tender joints, compared with placebo, over 24 weeks in the study, which was published in The New England Journal of Medicine.
The study was the first part of the LILAC trial, a two-part, phase 2 study. The second part involved cutaneous lupus erythematosus (CLE) with or without systemic manifestations. Treatment led to improvements in skin disease, as measured by Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores. It was published in the New England Journal of Medicine.
The investigational drug targets blood dendritic cell antigen 2 (BDCA2). The antigen is expressed solely on plasmacytoid dendritic cells (pDCs), which accumulate in skin lesions and organs of patients with SLE. When the antibody binds to BDCA2, “the synthesis of a variety of cytokines is shut down – type 1 interferons, type 3 interferons, TNF [tumor necrosis factor], and [other cytokines and chemokines] made by the pDCs,” Richard A. Furie, MD, lead author of the article, said in an interview.
In a phase 1 trial involving patients with SLE and CLE, the drug’s biologic activity was shown by a dampened interferon signature in blood and modulated type 1 interferon-induced proteins in the skin, he and his coinvestigators noted.
Dr. Furie is chief of rheumatology at Northwell Health and professor of medicine at the Feinstein Institutes for Medical Research at Northwell and at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York.
Impact on the joints
The primary analysis in the SLE trial involved 102 patients who had SLE, arthritis, and active skin disease. The patients received litifilimab 450 mg or placebo, administered subcutaneously, at weeks 0, 2, 4, 8, 12, 16, and 20. The patients were required to have at least four tender joints and at least four swollen joints, and these active joints had to be those classically involved in lupus arthritis.
The mean (± standard deviation) baseline number of active joints was 19 ± 8.4 in the litifilimab group and 21.6 ± 8.5 in placebo group. From baseline to week 24, the least-squares mean (± standard deviation) change in the total number of active joints was –15.0 ± 1.2 with litifilimab and –11.6 ± 1.3 with placebo (mean difference, –3.4; 95% confidence interval, –6.7 to -0.2; P = .04).
Most of the secondary endpoints did not support the results of the primary analysis. However, improvement was seen in the SLE Responder Index (SRI-4) – a three-component global index that Dr. Furie and others developed in 2009 using data from the phase 2 SLE trial of belimumab (Benlysta).
The composite index, used in the phase 3 trial of belimumab, captures improvement in disease activity without a worsening of the condition overall or new significant disease activity in other domains. “It’s a dichotomous measure – either you’re a responder or not,” Dr. Furie said in the interview.
Response on the SRI-4 was defined as a reduction of at least 4 points from baseline in the SLEDAI-2K score (the Systemic Lupus Erythematosus Disease Activity Index), no new disease activity as measured by one score of A (severe) or more than one score of B (moderate) on the BILAG (British Isles Lupus Assessment Group) index, and no increase of 0.3 points or more on the Physician’s Global Assessment.
A total of 56% of the patients in the litifilimab group showed responses on the SRI-4 at week 24, compared with 29% in the placebo group (least-squares mean difference, 26.4%; 95% confidence interval [CI], 9.5-43.2). This is “a robust response” that is much greater than the effect size seen in the phase 3 trial of belimumab or in research on anifrolumab (Saphnelo). Both of those drugs are approved for SLE, Dr. Furie said. “We’ll need to see if it’s reproduced in phase 3.”
There’s “little question that litifilimab works for the skin,” Dr. Furie noted. In the second part of the LILAC study, which focused on CLE, litifilimab demonstrated efficacy, and the SLE trial lends more support. Among several secondary endpoints evaluating skin-related disease activity, a reduction of at least 7 points from baseline in the CLASI-A score (a clinically relevant threshold) occurred in 56% of the litifilimab group and 34% of the placebo group.
The trial was conducted at 55 centers in Asia, Europe, Latin America, and the United States. The SLE part of the study began as a dose-ranging study aimed at evaluating cutaneous lupus activity, but owing to “slow enrollment and to allow an assessment of the effect of litifilimab on arthritis in SLE,” the protocol and primary endpoint were amended before the trial data were unblinded to evaluate only the 450-mg dose among participants with active arthritis and skin disease (at least one active skin lesion), the investigators explained.
Background therapy for SLE was allowed if the therapy was initiated at least 12 weeks before randomization and if dose levels were stable through the trial period. Glucocorticoids had to be tapered to ≤ 10 mg/day according to a specified regimen.
Making progress for lupus
Jane E. Salmon, MD, director of the Lupus and APS Center of Excellence and codirector of the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery in New York, who was not involved in the research, said in an email that she is “cautiously optimistic, because in SLE, successful phase 2 trials too often are followed by unsuccessful phase 3 trials.”
Blocking the production of type 1 interferon by pDCs implicated in SLE pathogenesis has the theoretical advantage of preserving type 1 interferon critical to protection from viruses, she noted. Herpes infections were reported among patients who received litifilimab, but rates were not increased, compared with placebo.
Diversity is an important priority in further research, Dr. Salmon said.
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center in Los Angeles, similarly pointed out in an editorial that accompanied the SLE phase 2 trial that while Black patients make up one-third of the U.S. population with lupus, only about 10% of study participants whose race and ethnicity was reported were Black). (Race was not reported by sites in Europe.)
The results of the LILAC trials “encourage further exploration of interventions that affect upstream lupus inflammatory pathways in the innate immune system in lupus,” Dr. Wallace wrote. He noted that lupus has “lagged behind its rheumatic cousins,” such as rheumatoid arthritis and vasculitis, in drug development.
Developing endpoints and study designs for SLE trials has been challenging, at least partly because it is a multisystem disease, Dr. Furie said. “But we’re making progress.”
Anifrolumab, a type 1 interferon receptor monoclonal antibody that was approved for SLE in July 2021, “may have a broader effect on type 1 interferons,” he noted, while litifilimab “may have a broader effect on proinflammatory cytokines, at least those expressed by pDCs.”
Biogen, the sponsor of the LILAC trial, is currently enrolling patients in phase 3 studies – TOPAZ-1 and TOPAZ-2 – to evaluate litifilimab in SLE over a 52-week period. The company also plans to start a pivotal study of the drug in CLE later this year, according to a press release.
Six coauthors are employees of Biogen; five, including Dr. Furie, reported serving as a consultant to the company; one served on a data and safety monitoring board for Biogen; and Dr. Salmon owns stock in the company.
A version of this article first appeared on Medscape.com.
Treatment with the humanized monoclonal antibody litifilimab for patients with systemic lupus erythematosus (SLE) led to greater improvements in joint manifestations than did placebo in an international phase 2 trial that reflects keen interest in targeting type 1 interferon and the innate immune system.
Litifilimab was associated with an approximately three-joint reduction in the number of swollen and tender joints, compared with placebo, over 24 weeks in the study, which was published in The New England Journal of Medicine.
The study was the first part of the LILAC trial, a two-part, phase 2 study. The second part involved cutaneous lupus erythematosus (CLE) with or without systemic manifestations. Treatment led to improvements in skin disease, as measured by Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores. It was published in the New England Journal of Medicine.
The investigational drug targets blood dendritic cell antigen 2 (BDCA2). The antigen is expressed solely on plasmacytoid dendritic cells (pDCs), which accumulate in skin lesions and organs of patients with SLE. When the antibody binds to BDCA2, “the synthesis of a variety of cytokines is shut down – type 1 interferons, type 3 interferons, TNF [tumor necrosis factor], and [other cytokines and chemokines] made by the pDCs,” Richard A. Furie, MD, lead author of the article, said in an interview.
In a phase 1 trial involving patients with SLE and CLE, the drug’s biologic activity was shown by a dampened interferon signature in blood and modulated type 1 interferon-induced proteins in the skin, he and his coinvestigators noted.
Dr. Furie is chief of rheumatology at Northwell Health and professor of medicine at the Feinstein Institutes for Medical Research at Northwell and at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York.
Impact on the joints
The primary analysis in the SLE trial involved 102 patients who had SLE, arthritis, and active skin disease. The patients received litifilimab 450 mg or placebo, administered subcutaneously, at weeks 0, 2, 4, 8, 12, 16, and 20. The patients were required to have at least four tender joints and at least four swollen joints, and these active joints had to be those classically involved in lupus arthritis.
The mean (± standard deviation) baseline number of active joints was 19 ± 8.4 in the litifilimab group and 21.6 ± 8.5 in placebo group. From baseline to week 24, the least-squares mean (± standard deviation) change in the total number of active joints was –15.0 ± 1.2 with litifilimab and –11.6 ± 1.3 with placebo (mean difference, –3.4; 95% confidence interval, –6.7 to -0.2; P = .04).
Most of the secondary endpoints did not support the results of the primary analysis. However, improvement was seen in the SLE Responder Index (SRI-4) – a three-component global index that Dr. Furie and others developed in 2009 using data from the phase 2 SLE trial of belimumab (Benlysta).
The composite index, used in the phase 3 trial of belimumab, captures improvement in disease activity without a worsening of the condition overall or new significant disease activity in other domains. “It’s a dichotomous measure – either you’re a responder or not,” Dr. Furie said in the interview.
Response on the SRI-4 was defined as a reduction of at least 4 points from baseline in the SLEDAI-2K score (the Systemic Lupus Erythematosus Disease Activity Index), no new disease activity as measured by one score of A (severe) or more than one score of B (moderate) on the BILAG (British Isles Lupus Assessment Group) index, and no increase of 0.3 points or more on the Physician’s Global Assessment.
A total of 56% of the patients in the litifilimab group showed responses on the SRI-4 at week 24, compared with 29% in the placebo group (least-squares mean difference, 26.4%; 95% confidence interval [CI], 9.5-43.2). This is “a robust response” that is much greater than the effect size seen in the phase 3 trial of belimumab or in research on anifrolumab (Saphnelo). Both of those drugs are approved for SLE, Dr. Furie said. “We’ll need to see if it’s reproduced in phase 3.”
There’s “little question that litifilimab works for the skin,” Dr. Furie noted. In the second part of the LILAC study, which focused on CLE, litifilimab demonstrated efficacy, and the SLE trial lends more support. Among several secondary endpoints evaluating skin-related disease activity, a reduction of at least 7 points from baseline in the CLASI-A score (a clinically relevant threshold) occurred in 56% of the litifilimab group and 34% of the placebo group.
The trial was conducted at 55 centers in Asia, Europe, Latin America, and the United States. The SLE part of the study began as a dose-ranging study aimed at evaluating cutaneous lupus activity, but owing to “slow enrollment and to allow an assessment of the effect of litifilimab on arthritis in SLE,” the protocol and primary endpoint were amended before the trial data were unblinded to evaluate only the 450-mg dose among participants with active arthritis and skin disease (at least one active skin lesion), the investigators explained.
Background therapy for SLE was allowed if the therapy was initiated at least 12 weeks before randomization and if dose levels were stable through the trial period. Glucocorticoids had to be tapered to ≤ 10 mg/day according to a specified regimen.
Making progress for lupus
Jane E. Salmon, MD, director of the Lupus and APS Center of Excellence and codirector of the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery in New York, who was not involved in the research, said in an email that she is “cautiously optimistic, because in SLE, successful phase 2 trials too often are followed by unsuccessful phase 3 trials.”
Blocking the production of type 1 interferon by pDCs implicated in SLE pathogenesis has the theoretical advantage of preserving type 1 interferon critical to protection from viruses, she noted. Herpes infections were reported among patients who received litifilimab, but rates were not increased, compared with placebo.
Diversity is an important priority in further research, Dr. Salmon said.
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center in Los Angeles, similarly pointed out in an editorial that accompanied the SLE phase 2 trial that while Black patients make up one-third of the U.S. population with lupus, only about 10% of study participants whose race and ethnicity was reported were Black). (Race was not reported by sites in Europe.)
The results of the LILAC trials “encourage further exploration of interventions that affect upstream lupus inflammatory pathways in the innate immune system in lupus,” Dr. Wallace wrote. He noted that lupus has “lagged behind its rheumatic cousins,” such as rheumatoid arthritis and vasculitis, in drug development.
Developing endpoints and study designs for SLE trials has been challenging, at least partly because it is a multisystem disease, Dr. Furie said. “But we’re making progress.”
Anifrolumab, a type 1 interferon receptor monoclonal antibody that was approved for SLE in July 2021, “may have a broader effect on type 1 interferons,” he noted, while litifilimab “may have a broader effect on proinflammatory cytokines, at least those expressed by pDCs.”
Biogen, the sponsor of the LILAC trial, is currently enrolling patients in phase 3 studies – TOPAZ-1 and TOPAZ-2 – to evaluate litifilimab in SLE over a 52-week period. The company also plans to start a pivotal study of the drug in CLE later this year, according to a press release.
Six coauthors are employees of Biogen; five, including Dr. Furie, reported serving as a consultant to the company; one served on a data and safety monitoring board for Biogen; and Dr. Salmon owns stock in the company.
A version of this article first appeared on Medscape.com.
Treatment with the humanized monoclonal antibody litifilimab for patients with systemic lupus erythematosus (SLE) led to greater improvements in joint manifestations than did placebo in an international phase 2 trial that reflects keen interest in targeting type 1 interferon and the innate immune system.
Litifilimab was associated with an approximately three-joint reduction in the number of swollen and tender joints, compared with placebo, over 24 weeks in the study, which was published in The New England Journal of Medicine.
The study was the first part of the LILAC trial, a two-part, phase 2 study. The second part involved cutaneous lupus erythematosus (CLE) with or without systemic manifestations. Treatment led to improvements in skin disease, as measured by Cutaneous Lupus Erythematosus Disease Area and Severity Index–Activity (CLASI-A) scores. It was published in the New England Journal of Medicine.
The investigational drug targets blood dendritic cell antigen 2 (BDCA2). The antigen is expressed solely on plasmacytoid dendritic cells (pDCs), which accumulate in skin lesions and organs of patients with SLE. When the antibody binds to BDCA2, “the synthesis of a variety of cytokines is shut down – type 1 interferons, type 3 interferons, TNF [tumor necrosis factor], and [other cytokines and chemokines] made by the pDCs,” Richard A. Furie, MD, lead author of the article, said in an interview.
In a phase 1 trial involving patients with SLE and CLE, the drug’s biologic activity was shown by a dampened interferon signature in blood and modulated type 1 interferon-induced proteins in the skin, he and his coinvestigators noted.
Dr. Furie is chief of rheumatology at Northwell Health and professor of medicine at the Feinstein Institutes for Medical Research at Northwell and at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York.
Impact on the joints
The primary analysis in the SLE trial involved 102 patients who had SLE, arthritis, and active skin disease. The patients received litifilimab 450 mg or placebo, administered subcutaneously, at weeks 0, 2, 4, 8, 12, 16, and 20. The patients were required to have at least four tender joints and at least four swollen joints, and these active joints had to be those classically involved in lupus arthritis.
The mean (± standard deviation) baseline number of active joints was 19 ± 8.4 in the litifilimab group and 21.6 ± 8.5 in placebo group. From baseline to week 24, the least-squares mean (± standard deviation) change in the total number of active joints was –15.0 ± 1.2 with litifilimab and –11.6 ± 1.3 with placebo (mean difference, –3.4; 95% confidence interval, –6.7 to -0.2; P = .04).
Most of the secondary endpoints did not support the results of the primary analysis. However, improvement was seen in the SLE Responder Index (SRI-4) – a three-component global index that Dr. Furie and others developed in 2009 using data from the phase 2 SLE trial of belimumab (Benlysta).
The composite index, used in the phase 3 trial of belimumab, captures improvement in disease activity without a worsening of the condition overall or new significant disease activity in other domains. “It’s a dichotomous measure – either you’re a responder or not,” Dr. Furie said in the interview.
Response on the SRI-4 was defined as a reduction of at least 4 points from baseline in the SLEDAI-2K score (the Systemic Lupus Erythematosus Disease Activity Index), no new disease activity as measured by one score of A (severe) or more than one score of B (moderate) on the BILAG (British Isles Lupus Assessment Group) index, and no increase of 0.3 points or more on the Physician’s Global Assessment.
A total of 56% of the patients in the litifilimab group showed responses on the SRI-4 at week 24, compared with 29% in the placebo group (least-squares mean difference, 26.4%; 95% confidence interval [CI], 9.5-43.2). This is “a robust response” that is much greater than the effect size seen in the phase 3 trial of belimumab or in research on anifrolumab (Saphnelo). Both of those drugs are approved for SLE, Dr. Furie said. “We’ll need to see if it’s reproduced in phase 3.”
There’s “little question that litifilimab works for the skin,” Dr. Furie noted. In the second part of the LILAC study, which focused on CLE, litifilimab demonstrated efficacy, and the SLE trial lends more support. Among several secondary endpoints evaluating skin-related disease activity, a reduction of at least 7 points from baseline in the CLASI-A score (a clinically relevant threshold) occurred in 56% of the litifilimab group and 34% of the placebo group.
The trial was conducted at 55 centers in Asia, Europe, Latin America, and the United States. The SLE part of the study began as a dose-ranging study aimed at evaluating cutaneous lupus activity, but owing to “slow enrollment and to allow an assessment of the effect of litifilimab on arthritis in SLE,” the protocol and primary endpoint were amended before the trial data were unblinded to evaluate only the 450-mg dose among participants with active arthritis and skin disease (at least one active skin lesion), the investigators explained.
Background therapy for SLE was allowed if the therapy was initiated at least 12 weeks before randomization and if dose levels were stable through the trial period. Glucocorticoids had to be tapered to ≤ 10 mg/day according to a specified regimen.
Making progress for lupus
Jane E. Salmon, MD, director of the Lupus and APS Center of Excellence and codirector of the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery in New York, who was not involved in the research, said in an email that she is “cautiously optimistic, because in SLE, successful phase 2 trials too often are followed by unsuccessful phase 3 trials.”
Blocking the production of type 1 interferon by pDCs implicated in SLE pathogenesis has the theoretical advantage of preserving type 1 interferon critical to protection from viruses, she noted. Herpes infections were reported among patients who received litifilimab, but rates were not increased, compared with placebo.
Diversity is an important priority in further research, Dr. Salmon said.
Daniel J. Wallace, MD, of Cedars-Sinai Medical Center in Los Angeles, similarly pointed out in an editorial that accompanied the SLE phase 2 trial that while Black patients make up one-third of the U.S. population with lupus, only about 10% of study participants whose race and ethnicity was reported were Black). (Race was not reported by sites in Europe.)
The results of the LILAC trials “encourage further exploration of interventions that affect upstream lupus inflammatory pathways in the innate immune system in lupus,” Dr. Wallace wrote. He noted that lupus has “lagged behind its rheumatic cousins,” such as rheumatoid arthritis and vasculitis, in drug development.
Developing endpoints and study designs for SLE trials has been challenging, at least partly because it is a multisystem disease, Dr. Furie said. “But we’re making progress.”
Anifrolumab, a type 1 interferon receptor monoclonal antibody that was approved for SLE in July 2021, “may have a broader effect on type 1 interferons,” he noted, while litifilimab “may have a broader effect on proinflammatory cytokines, at least those expressed by pDCs.”
Biogen, the sponsor of the LILAC trial, is currently enrolling patients in phase 3 studies – TOPAZ-1 and TOPAZ-2 – to evaluate litifilimab in SLE over a 52-week period. The company also plans to start a pivotal study of the drug in CLE later this year, according to a press release.
Six coauthors are employees of Biogen; five, including Dr. Furie, reported serving as a consultant to the company; one served on a data and safety monitoring board for Biogen; and Dr. Salmon owns stock in the company.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE