User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Survey: Long Surgical Career Raises Likelihood of Lawsuit
SAN DIEGO – The most experienced bariatric surgeons are those who are most likely to be sued, judging by responses to a survey from more than 300 members of the American Society for Metabolic and Bariatric Surgery.
"There are no resources, national registries, or easily accessible databases to analyze bariatric-specific medical malpractice claims in the United States," Dr. Ramsey M. Dallal said at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "Without the ability to analyze aggregate data, surgeons cannot easily study common causes of medical malpractice litigation and develop patient safety improvements. Nor do there exist easily accessible measures of trends in bariatric surgery litigation."
In an effort to obtain a snapshot of the liability landscape in bariatric surgery, Dr. Dallal and other members of the ASMBS Patient Safety Committee e-mailed a survey to 1,672 surgeon members of the ASMBS in July 2011. A total of 330 surgeons in 46 states responded, for a response rate of 20%. Their mean number of years in practice was 15, which represented 5,042 years of bariatric surgery–specific liability exposure. Most respondents (38%) practiced in a hospital or academic group, 26% in a single specialty group, 20% in solo practice, 13% in a multispecialty group, and 3% in other settings.
Nine respondents chose not to have malpractice insurance. Those who were insured reported a mean yearly cost of malpractice insurance of $59,200.
Nearly half of respondents (48%) reported having no malpractice insurance cases since their careers began, but the average number of lifetime cases reported by their counterparts was 1.5.
Of the 464 lawsuits reported by 156 surgeons, 54% were dropped or dismissed, 27% were settled out of court, and 19% went to trial or arbitration.
Of those cases that went to trial, 72% were found in favor of the surgeon-defendant. The mean lifetime amount paid in lawsuits was $250,000, including one settlement for $7,000,000. The total amount paid by the respondents was $70,871,998.
Using multivariate logistic regression analysis, the researchers determined that the probability of reporting at least one lawsuit independently increased with the number of years in practice (odds ratio, 1.03; P = .03) and among those who have performed more than 1,000 cases (OR, 8.5%; P = .01). "In essence, our most experienced surgeons are the ones being sued the most," said Dr. Dallal, chief of bariatric/minimally invasive surgery at Einstein Healthcare Network, Philadelphia.
The odds of having lost a malpractice case that resulted in monetary compensation independently increased with the number of years in practice (OR, 1.09; P less than .0005), and the number of lawsuits experienced (OR, 1.42; P = .02). "The type of practice and the lack of a bariatric surgeon expert witness did not independently predict a payout," Dr. Dallal said.
Nearly 7% of survey respondents reported that the primary expert witness in determining the standard of bariatric surgery care was not a bariatric surgeon. In such cases, the surgeon-defendant had an 11-fold increased risk of having a lawsuit (P = .018). However, the use of an expert witness who was not a bariatric surgeon was not associated with the chance of settlement or the case’s going to trial.
Dr. Dallal noted that many lawsuits are filed about 2 years after the alleged injury, and another 1-2 years may pass before resolution of that lawsuit occurs. "So, there is a built-in bias against surgeons who have been in practice longer," he said.
He noted that successful lawsuits that are based on patient harm "do occur and are devastating to all involved. Improving the patient safety culture is the mainstay of reducing liability risk."
Dr. Dallal said that he had no relevant financial conflicts to disclose.
SAN DIEGO – The most experienced bariatric surgeons are those who are most likely to be sued, judging by responses to a survey from more than 300 members of the American Society for Metabolic and Bariatric Surgery.
"There are no resources, national registries, or easily accessible databases to analyze bariatric-specific medical malpractice claims in the United States," Dr. Ramsey M. Dallal said at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "Without the ability to analyze aggregate data, surgeons cannot easily study common causes of medical malpractice litigation and develop patient safety improvements. Nor do there exist easily accessible measures of trends in bariatric surgery litigation."
In an effort to obtain a snapshot of the liability landscape in bariatric surgery, Dr. Dallal and other members of the ASMBS Patient Safety Committee e-mailed a survey to 1,672 surgeon members of the ASMBS in July 2011. A total of 330 surgeons in 46 states responded, for a response rate of 20%. Their mean number of years in practice was 15, which represented 5,042 years of bariatric surgery–specific liability exposure. Most respondents (38%) practiced in a hospital or academic group, 26% in a single specialty group, 20% in solo practice, 13% in a multispecialty group, and 3% in other settings.
Nine respondents chose not to have malpractice insurance. Those who were insured reported a mean yearly cost of malpractice insurance of $59,200.
Nearly half of respondents (48%) reported having no malpractice insurance cases since their careers began, but the average number of lifetime cases reported by their counterparts was 1.5.
Of the 464 lawsuits reported by 156 surgeons, 54% were dropped or dismissed, 27% were settled out of court, and 19% went to trial or arbitration.
Of those cases that went to trial, 72% were found in favor of the surgeon-defendant. The mean lifetime amount paid in lawsuits was $250,000, including one settlement for $7,000,000. The total amount paid by the respondents was $70,871,998.
Using multivariate logistic regression analysis, the researchers determined that the probability of reporting at least one lawsuit independently increased with the number of years in practice (odds ratio, 1.03; P = .03) and among those who have performed more than 1,000 cases (OR, 8.5%; P = .01). "In essence, our most experienced surgeons are the ones being sued the most," said Dr. Dallal, chief of bariatric/minimally invasive surgery at Einstein Healthcare Network, Philadelphia.
The odds of having lost a malpractice case that resulted in monetary compensation independently increased with the number of years in practice (OR, 1.09; P less than .0005), and the number of lawsuits experienced (OR, 1.42; P = .02). "The type of practice and the lack of a bariatric surgeon expert witness did not independently predict a payout," Dr. Dallal said.
Nearly 7% of survey respondents reported that the primary expert witness in determining the standard of bariatric surgery care was not a bariatric surgeon. In such cases, the surgeon-defendant had an 11-fold increased risk of having a lawsuit (P = .018). However, the use of an expert witness who was not a bariatric surgeon was not associated with the chance of settlement or the case’s going to trial.
Dr. Dallal noted that many lawsuits are filed about 2 years after the alleged injury, and another 1-2 years may pass before resolution of that lawsuit occurs. "So, there is a built-in bias against surgeons who have been in practice longer," he said.
He noted that successful lawsuits that are based on patient harm "do occur and are devastating to all involved. Improving the patient safety culture is the mainstay of reducing liability risk."
Dr. Dallal said that he had no relevant financial conflicts to disclose.
SAN DIEGO – The most experienced bariatric surgeons are those who are most likely to be sued, judging by responses to a survey from more than 300 members of the American Society for Metabolic and Bariatric Surgery.
"There are no resources, national registries, or easily accessible databases to analyze bariatric-specific medical malpractice claims in the United States," Dr. Ramsey M. Dallal said at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "Without the ability to analyze aggregate data, surgeons cannot easily study common causes of medical malpractice litigation and develop patient safety improvements. Nor do there exist easily accessible measures of trends in bariatric surgery litigation."
In an effort to obtain a snapshot of the liability landscape in bariatric surgery, Dr. Dallal and other members of the ASMBS Patient Safety Committee e-mailed a survey to 1,672 surgeon members of the ASMBS in July 2011. A total of 330 surgeons in 46 states responded, for a response rate of 20%. Their mean number of years in practice was 15, which represented 5,042 years of bariatric surgery–specific liability exposure. Most respondents (38%) practiced in a hospital or academic group, 26% in a single specialty group, 20% in solo practice, 13% in a multispecialty group, and 3% in other settings.
Nine respondents chose not to have malpractice insurance. Those who were insured reported a mean yearly cost of malpractice insurance of $59,200.
Nearly half of respondents (48%) reported having no malpractice insurance cases since their careers began, but the average number of lifetime cases reported by their counterparts was 1.5.
Of the 464 lawsuits reported by 156 surgeons, 54% were dropped or dismissed, 27% were settled out of court, and 19% went to trial or arbitration.
Of those cases that went to trial, 72% were found in favor of the surgeon-defendant. The mean lifetime amount paid in lawsuits was $250,000, including one settlement for $7,000,000. The total amount paid by the respondents was $70,871,998.
Using multivariate logistic regression analysis, the researchers determined that the probability of reporting at least one lawsuit independently increased with the number of years in practice (odds ratio, 1.03; P = .03) and among those who have performed more than 1,000 cases (OR, 8.5%; P = .01). "In essence, our most experienced surgeons are the ones being sued the most," said Dr. Dallal, chief of bariatric/minimally invasive surgery at Einstein Healthcare Network, Philadelphia.
The odds of having lost a malpractice case that resulted in monetary compensation independently increased with the number of years in practice (OR, 1.09; P less than .0005), and the number of lawsuits experienced (OR, 1.42; P = .02). "The type of practice and the lack of a bariatric surgeon expert witness did not independently predict a payout," Dr. Dallal said.
Nearly 7% of survey respondents reported that the primary expert witness in determining the standard of bariatric surgery care was not a bariatric surgeon. In such cases, the surgeon-defendant had an 11-fold increased risk of having a lawsuit (P = .018). However, the use of an expert witness who was not a bariatric surgeon was not associated with the chance of settlement or the case’s going to trial.
Dr. Dallal noted that many lawsuits are filed about 2 years after the alleged injury, and another 1-2 years may pass before resolution of that lawsuit occurs. "So, there is a built-in bias against surgeons who have been in practice longer," he said.
He noted that successful lawsuits that are based on patient harm "do occur and are devastating to all involved. Improving the patient safety culture is the mainstay of reducing liability risk."
Dr. Dallal said that he had no relevant financial conflicts to disclose.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR METABOLIC AND BARIATRIC SURGERY
Major Finding: The probability of reporting at least one lawsuit independently increased with the number of years in practice (OR, 1.03; P = .03) and among those who have performed more than 1,000 cases (OR, 8.5%; P = .01).
Data Source: The findings are based on responses to a survey from 330 members of the American Society of Metabolic and Bariatric Surgery.
Disclosures: Dr. Dallal said that he had no relevant financial conflicts to disclose.
Endoscopy Exposure Generally Low Among Surgical Trainees
SAN DIEGO – Exposure to endoscopy is generally low among surgical trainees, yet most bariatric surgeons continue to perform endoscopy and manage complications, responses to a survey indicate.
"There is a varying amount of endoscopic training during surgical residency and fellowship, and endoscopic practices are not standardized," Dr. Bipan Chand said at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "Surgeons today perform varying degrees of endoscopy, pre- and postoperatively, as well as diagnostic and therapeutic procedures."
Dr. Chand and his associates sent a 19-question online survey to 1,670 active surgeon members of the ASMBS during a 1-month time frame in an effort to "obtain a better understanding on the current amount of exposure to endoscopy, to obtain information on the amount of diagnostic and therapeutic procedures being performed, by whom and [what the] the comfort level [is]."
Of the 1,670 members, 291 (17%) completed the survey, said Dr. Chand, director of metabolic surgery and bariatric care at Loyola University, Chicago. The largest proportion of respondents (30.9%) were more than 15 years removed from surgical training, whereas 18.9% were 11-15 years removed, 23.4% were 6-10 years removed, 23.4% were 1-5 years removed, and 3.4% were less than 1 year removed.
Nearly 60% of respondents completed a postresidency fellowship. Of these, 38% completed a fellowship in bariatric surgery with an emphasis in minimally invasive surgery (MIS), and 31% completed a fellowship in general MIS.
Dr. Chand, who chairs the ASMBS Emerging Technology and Procedures Committee, reported that during their combined residency and fellowship training, 25% of respondents performed fewer than 25 diagnostic procedures and 55% performed fewer than 5 therapeutic procedures such as dilation and treatment of bleeding.
After completing their training, 41% of respondents reported performing routine preoperative endoscopy for primary bariatric procedures, and 90% reported performing routine preoperative upper endoscopy for revisional bariatric procedures. More than 70% of these procedures were being done by the respondents or by their surgical partners.
Overall, 49% of respondents said they felt "very comfortable" with endoscopic management of bariatric complications, including bleeding, dilation of strictures, removal of foreign bodies, and stent placement.
More than three-quarters of respondents (78%) expressed interest in additional endoscopy training via postgraduate courses, particularly those related to advanced training techniques, whereas 53% and 56%, respectively, predicted that endoscopic procedures will play a role as primary and revisional endoluminal bariatric therapies.
More than two-thirds of surgeons (69%) said that they plan to increase the amount of endoscopy in their practice. "The ASMBS will continue to offer training labs to surgeons to help [them] acquire and refine these important skills," Dr. Chand said. "One such lab was offered at this year’s annual meeting."
Dr. Chand said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Exposure to endoscopy is generally low among surgical trainees, yet most bariatric surgeons continue to perform endoscopy and manage complications, responses to a survey indicate.
"There is a varying amount of endoscopic training during surgical residency and fellowship, and endoscopic practices are not standardized," Dr. Bipan Chand said at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "Surgeons today perform varying degrees of endoscopy, pre- and postoperatively, as well as diagnostic and therapeutic procedures."
Dr. Chand and his associates sent a 19-question online survey to 1,670 active surgeon members of the ASMBS during a 1-month time frame in an effort to "obtain a better understanding on the current amount of exposure to endoscopy, to obtain information on the amount of diagnostic and therapeutic procedures being performed, by whom and [what the] the comfort level [is]."
Of the 1,670 members, 291 (17%) completed the survey, said Dr. Chand, director of metabolic surgery and bariatric care at Loyola University, Chicago. The largest proportion of respondents (30.9%) were more than 15 years removed from surgical training, whereas 18.9% were 11-15 years removed, 23.4% were 6-10 years removed, 23.4% were 1-5 years removed, and 3.4% were less than 1 year removed.
Nearly 60% of respondents completed a postresidency fellowship. Of these, 38% completed a fellowship in bariatric surgery with an emphasis in minimally invasive surgery (MIS), and 31% completed a fellowship in general MIS.
Dr. Chand, who chairs the ASMBS Emerging Technology and Procedures Committee, reported that during their combined residency and fellowship training, 25% of respondents performed fewer than 25 diagnostic procedures and 55% performed fewer than 5 therapeutic procedures such as dilation and treatment of bleeding.
After completing their training, 41% of respondents reported performing routine preoperative endoscopy for primary bariatric procedures, and 90% reported performing routine preoperative upper endoscopy for revisional bariatric procedures. More than 70% of these procedures were being done by the respondents or by their surgical partners.
Overall, 49% of respondents said they felt "very comfortable" with endoscopic management of bariatric complications, including bleeding, dilation of strictures, removal of foreign bodies, and stent placement.
More than three-quarters of respondents (78%) expressed interest in additional endoscopy training via postgraduate courses, particularly those related to advanced training techniques, whereas 53% and 56%, respectively, predicted that endoscopic procedures will play a role as primary and revisional endoluminal bariatric therapies.
More than two-thirds of surgeons (69%) said that they plan to increase the amount of endoscopy in their practice. "The ASMBS will continue to offer training labs to surgeons to help [them] acquire and refine these important skills," Dr. Chand said. "One such lab was offered at this year’s annual meeting."
Dr. Chand said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Exposure to endoscopy is generally low among surgical trainees, yet most bariatric surgeons continue to perform endoscopy and manage complications, responses to a survey indicate.
"There is a varying amount of endoscopic training during surgical residency and fellowship, and endoscopic practices are not standardized," Dr. Bipan Chand said at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "Surgeons today perform varying degrees of endoscopy, pre- and postoperatively, as well as diagnostic and therapeutic procedures."
Dr. Chand and his associates sent a 19-question online survey to 1,670 active surgeon members of the ASMBS during a 1-month time frame in an effort to "obtain a better understanding on the current amount of exposure to endoscopy, to obtain information on the amount of diagnostic and therapeutic procedures being performed, by whom and [what the] the comfort level [is]."
Of the 1,670 members, 291 (17%) completed the survey, said Dr. Chand, director of metabolic surgery and bariatric care at Loyola University, Chicago. The largest proportion of respondents (30.9%) were more than 15 years removed from surgical training, whereas 18.9% were 11-15 years removed, 23.4% were 6-10 years removed, 23.4% were 1-5 years removed, and 3.4% were less than 1 year removed.
Nearly 60% of respondents completed a postresidency fellowship. Of these, 38% completed a fellowship in bariatric surgery with an emphasis in minimally invasive surgery (MIS), and 31% completed a fellowship in general MIS.
Dr. Chand, who chairs the ASMBS Emerging Technology and Procedures Committee, reported that during their combined residency and fellowship training, 25% of respondents performed fewer than 25 diagnostic procedures and 55% performed fewer than 5 therapeutic procedures such as dilation and treatment of bleeding.
After completing their training, 41% of respondents reported performing routine preoperative endoscopy for primary bariatric procedures, and 90% reported performing routine preoperative upper endoscopy for revisional bariatric procedures. More than 70% of these procedures were being done by the respondents or by their surgical partners.
Overall, 49% of respondents said they felt "very comfortable" with endoscopic management of bariatric complications, including bleeding, dilation of strictures, removal of foreign bodies, and stent placement.
More than three-quarters of respondents (78%) expressed interest in additional endoscopy training via postgraduate courses, particularly those related to advanced training techniques, whereas 53% and 56%, respectively, predicted that endoscopic procedures will play a role as primary and revisional endoluminal bariatric therapies.
More than two-thirds of surgeons (69%) said that they plan to increase the amount of endoscopy in their practice. "The ASMBS will continue to offer training labs to surgeons to help [them] acquire and refine these important skills," Dr. Chand said. "One such lab was offered at this year’s annual meeting."
Dr. Chand said that he had no relevant financial conflicts to disclose.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR METABOLIC AND BARIATRIC SURGERY
Major Finding: During their combined residency and fellowship training, 25% of surgeons performed fewer than 25 diagnostic endoscopic procedures and 55% performed fewer than 5 endoscopic therapeutic procedures such as dilation and treatment of bleeding.
Data Source: The study was based on responses from 291 surgeons to an online survey sent to members of the American Society for Metabolic and Bariatric Surgery.
Disclosures: Dr. Chand said he had no relevant financial conflicts to disclose.
Men Benefit Most From Combined Weight-Loss Procedure
SAN DIEGO – Significantly greater reductions in weight, body mass index, and adipose tissue were seen in men vs. women who underwent biliopancreatic diversion with duodenal switch surgery, a single-center study indicated.
The study, which was conducted at the Institute of Pneumology and Cardiology at Laval University in Quebec City, was designed to assess the impact of sex on weight loss along with changes in adiposity and skeletal muscle in 42 severely obese men and women who underwent biliopancreatic diversion with duodenal switch (BPD-DS) surgery. Audrey Auclair, a Ph.D. student in pharmacy at the university, presented the study results on behalf of her colleagues at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
At baseline and 6 months, the 12 men and 30 women (mean age, 46 years) underwent anthropometric measurements and a midthigh CT scan. At baseline, the men and women were similar in terms of thickness of total, deep, and subcutaneous adipose tissue. However, the men weighed significantly more(mean, 156 vs. 118 kg, respectively), and had a higher body mass index (51.2 vs. 46.2 kg/m2) as well as greater midthigh composition in terms of total muscle (193 vs. 130 cm2), normal-density muscle (115 vs. 79 cm2), and low-density muscle (54 vs. 37 cm2), compared with the women (all P less than.001).
At 6 months, after adjustment for baseline weight, the researchers found that the men had a significantly greater reduction in weight (–30% vs. –26%, respectively), BMI (–30% vs. –26%), subcutaneous adipose tissue (–45% vs. –31%), and deep adipose tissue (–50% vs. –31%) than did the women (all P less than or equal to .05). There were no significant differences between the sexes in the percent decline at 6 months in total muscle, normal-density muscle, and low-density muscle.
"The BPD-DS has a major impact on weight and on both thigh muscle and fat mass in both sexes," Ms. Auclair concluded.
In a later interview, she speculated that the outcome differences between sexes may be attributable to greater physical activity among men compared with women, which would explain a similar loss in midthigh muscle between the sexes, despite a greater reduction in body weight and midthigh adipose tissue among men.
"In order to confirm this hypothesis, we plan to begin a new study to determine the effectiveness of a supervised exercise program on the maintenance of muscle mass in months after the BPD-DS," she said.
Ms. Auclair said that she had no relevant financial conflicts to disclose.
SAN DIEGO – Significantly greater reductions in weight, body mass index, and adipose tissue were seen in men vs. women who underwent biliopancreatic diversion with duodenal switch surgery, a single-center study indicated.
The study, which was conducted at the Institute of Pneumology and Cardiology at Laval University in Quebec City, was designed to assess the impact of sex on weight loss along with changes in adiposity and skeletal muscle in 42 severely obese men and women who underwent biliopancreatic diversion with duodenal switch (BPD-DS) surgery. Audrey Auclair, a Ph.D. student in pharmacy at the university, presented the study results on behalf of her colleagues at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
At baseline and 6 months, the 12 men and 30 women (mean age, 46 years) underwent anthropometric measurements and a midthigh CT scan. At baseline, the men and women were similar in terms of thickness of total, deep, and subcutaneous adipose tissue. However, the men weighed significantly more(mean, 156 vs. 118 kg, respectively), and had a higher body mass index (51.2 vs. 46.2 kg/m2) as well as greater midthigh composition in terms of total muscle (193 vs. 130 cm2), normal-density muscle (115 vs. 79 cm2), and low-density muscle (54 vs. 37 cm2), compared with the women (all P less than.001).
At 6 months, after adjustment for baseline weight, the researchers found that the men had a significantly greater reduction in weight (–30% vs. –26%, respectively), BMI (–30% vs. –26%), subcutaneous adipose tissue (–45% vs. –31%), and deep adipose tissue (–50% vs. –31%) than did the women (all P less than or equal to .05). There were no significant differences between the sexes in the percent decline at 6 months in total muscle, normal-density muscle, and low-density muscle.
"The BPD-DS has a major impact on weight and on both thigh muscle and fat mass in both sexes," Ms. Auclair concluded.
In a later interview, she speculated that the outcome differences between sexes may be attributable to greater physical activity among men compared with women, which would explain a similar loss in midthigh muscle between the sexes, despite a greater reduction in body weight and midthigh adipose tissue among men.
"In order to confirm this hypothesis, we plan to begin a new study to determine the effectiveness of a supervised exercise program on the maintenance of muscle mass in months after the BPD-DS," she said.
Ms. Auclair said that she had no relevant financial conflicts to disclose.
SAN DIEGO – Significantly greater reductions in weight, body mass index, and adipose tissue were seen in men vs. women who underwent biliopancreatic diversion with duodenal switch surgery, a single-center study indicated.
The study, which was conducted at the Institute of Pneumology and Cardiology at Laval University in Quebec City, was designed to assess the impact of sex on weight loss along with changes in adiposity and skeletal muscle in 42 severely obese men and women who underwent biliopancreatic diversion with duodenal switch (BPD-DS) surgery. Audrey Auclair, a Ph.D. student in pharmacy at the university, presented the study results on behalf of her colleagues at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
At baseline and 6 months, the 12 men and 30 women (mean age, 46 years) underwent anthropometric measurements and a midthigh CT scan. At baseline, the men and women were similar in terms of thickness of total, deep, and subcutaneous adipose tissue. However, the men weighed significantly more(mean, 156 vs. 118 kg, respectively), and had a higher body mass index (51.2 vs. 46.2 kg/m2) as well as greater midthigh composition in terms of total muscle (193 vs. 130 cm2), normal-density muscle (115 vs. 79 cm2), and low-density muscle (54 vs. 37 cm2), compared with the women (all P less than.001).
At 6 months, after adjustment for baseline weight, the researchers found that the men had a significantly greater reduction in weight (–30% vs. –26%, respectively), BMI (–30% vs. –26%), subcutaneous adipose tissue (–45% vs. –31%), and deep adipose tissue (–50% vs. –31%) than did the women (all P less than or equal to .05). There were no significant differences between the sexes in the percent decline at 6 months in total muscle, normal-density muscle, and low-density muscle.
"The BPD-DS has a major impact on weight and on both thigh muscle and fat mass in both sexes," Ms. Auclair concluded.
In a later interview, she speculated that the outcome differences between sexes may be attributable to greater physical activity among men compared with women, which would explain a similar loss in midthigh muscle between the sexes, despite a greater reduction in body weight and midthigh adipose tissue among men.
"In order to confirm this hypothesis, we plan to begin a new study to determine the effectiveness of a supervised exercise program on the maintenance of muscle mass in months after the BPD-DS," she said.
Ms. Auclair said that she had no relevant financial conflicts to disclose.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR METABOLIC AND BARIATRIC SURGERY
Major Finding: At 6 months after undergoing BPD-DS surgery, men had a significantly greater reduction in weight (–-30% vs. –26%, respectively), body mass index (–30% vs. –26%), subcutaneous adipose tissue (–45% vs. –31%), and deep adipose tissue (–50% vs. –31%) than did women.
Data Source: The results are based on a single-center study of 42 severely obese patients who underwent BPD-DS.
Disclosures: Ms. Auclair said that she had no relevant financial conflicts to disclose.
Largest Series of Robotic-Assisted Gastric Bypass Reported
SAN DIEGO – At 30 days, the mortality rate from robotic-assisted gastric bypass surgery was zero and the rate of leak or abscess was just 0.3%, a multicenter study showed.
"Complications are few and may be less than with conventional laparoscopic techniques, even in different centers," Dr. Erik B. Wilson said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
In what he said is the largest reported series of its kind, Dr. Wilson and his associates reviewed 1,695 robotic-assisted Roux-en-Y gastric bypass procedures performed with the da Vinci Surgical System (Intuitive Surgical) between February 2003 and September 2011. The operations were performed at three centers: the University of Texas Health Science Center at Houston (578 procedures), Eastern Maine Medical Center, Bangor (708 procedures), and Florida Hospital Celebration Health (409). The mean body mass index of patients was 48.9 kg/m2, and the researchers evaluated complications and outcomes that occurred within the first 30 days of surgery.
Dr. Wilson, associate professor of surgery at the University of Texas Health Science Center at Houston, reported that the average length of stay was 2.2 days. Within the first 30 days of surgery there were 81 readmissions (4.8%), "which is not too different from what you’d expect in most populations," he said. Of these, 49 (2.9%) were for dehydration, 27 (1.6%) were for nausea/vomiting, and 5 (0.3%) were for stricture requiring dilation.
There were 46 reoperations (2.7%) within the first 30 days of surgery. Of these, 18 (1.06%) were for bowel obstruction/hernia, 17 (1%) were for bleeding/hematoma, 6 (0.35%) were for negative exploration of patients the surgeons were concerned about, and 5 (0.29%) were for abscess/leak.
There were 26 early major complications (1.5%). Of these, 14 (0.83%) were bleeding requiring transfusion, 5 (0.29%) were stricture requiring dilation, 3 (0.18%) were abscesses, 2 (0.12%) were anastomotic leaks, and 2 (0.12%) were cases of pulmonary embolism/infarct. There was no mortality, "which we think is very favorable," Dr. Wilson said.
Average operating times varied by center: 156 minutes in Houston, 128 minutes in Florida, and 104 minutes in Maine. "As time has gone on, and as we engage each other on how we do things, these operative times have continued to decrease, with current times approaching 90 minutes," Dr. Wilson said. "So long operative times are not necessary when you do robotic surgery."
He concluded by describing robotic-assisted bypass surgery as "an enabling technology that allows for excellent reproducible outcomes, because we have multiple centers doing it well. Future studies should focus on revisional and more complex procedures such as biliopancreatic conversion."
Dr. Wilson disclosed that he is a consultant for Intuitive Surgical, Ethicon Endo-Surgery, Apollo Endosurgery, and EndoGastric Solutions. He is also a proctor for Intuitive Surgical, and has received an educational grant from Gore.
SAN DIEGO – At 30 days, the mortality rate from robotic-assisted gastric bypass surgery was zero and the rate of leak or abscess was just 0.3%, a multicenter study showed.
"Complications are few and may be less than with conventional laparoscopic techniques, even in different centers," Dr. Erik B. Wilson said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
In what he said is the largest reported series of its kind, Dr. Wilson and his associates reviewed 1,695 robotic-assisted Roux-en-Y gastric bypass procedures performed with the da Vinci Surgical System (Intuitive Surgical) between February 2003 and September 2011. The operations were performed at three centers: the University of Texas Health Science Center at Houston (578 procedures), Eastern Maine Medical Center, Bangor (708 procedures), and Florida Hospital Celebration Health (409). The mean body mass index of patients was 48.9 kg/m2, and the researchers evaluated complications and outcomes that occurred within the first 30 days of surgery.
Dr. Wilson, associate professor of surgery at the University of Texas Health Science Center at Houston, reported that the average length of stay was 2.2 days. Within the first 30 days of surgery there were 81 readmissions (4.8%), "which is not too different from what you’d expect in most populations," he said. Of these, 49 (2.9%) were for dehydration, 27 (1.6%) were for nausea/vomiting, and 5 (0.3%) were for stricture requiring dilation.
There were 46 reoperations (2.7%) within the first 30 days of surgery. Of these, 18 (1.06%) were for bowel obstruction/hernia, 17 (1%) were for bleeding/hematoma, 6 (0.35%) were for negative exploration of patients the surgeons were concerned about, and 5 (0.29%) were for abscess/leak.
There were 26 early major complications (1.5%). Of these, 14 (0.83%) were bleeding requiring transfusion, 5 (0.29%) were stricture requiring dilation, 3 (0.18%) were abscesses, 2 (0.12%) were anastomotic leaks, and 2 (0.12%) were cases of pulmonary embolism/infarct. There was no mortality, "which we think is very favorable," Dr. Wilson said.
Average operating times varied by center: 156 minutes in Houston, 128 minutes in Florida, and 104 minutes in Maine. "As time has gone on, and as we engage each other on how we do things, these operative times have continued to decrease, with current times approaching 90 minutes," Dr. Wilson said. "So long operative times are not necessary when you do robotic surgery."
He concluded by describing robotic-assisted bypass surgery as "an enabling technology that allows for excellent reproducible outcomes, because we have multiple centers doing it well. Future studies should focus on revisional and more complex procedures such as biliopancreatic conversion."
Dr. Wilson disclosed that he is a consultant for Intuitive Surgical, Ethicon Endo-Surgery, Apollo Endosurgery, and EndoGastric Solutions. He is also a proctor for Intuitive Surgical, and has received an educational grant from Gore.
SAN DIEGO – At 30 days, the mortality rate from robotic-assisted gastric bypass surgery was zero and the rate of leak or abscess was just 0.3%, a multicenter study showed.
"Complications are few and may be less than with conventional laparoscopic techniques, even in different centers," Dr. Erik B. Wilson said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
In what he said is the largest reported series of its kind, Dr. Wilson and his associates reviewed 1,695 robotic-assisted Roux-en-Y gastric bypass procedures performed with the da Vinci Surgical System (Intuitive Surgical) between February 2003 and September 2011. The operations were performed at three centers: the University of Texas Health Science Center at Houston (578 procedures), Eastern Maine Medical Center, Bangor (708 procedures), and Florida Hospital Celebration Health (409). The mean body mass index of patients was 48.9 kg/m2, and the researchers evaluated complications and outcomes that occurred within the first 30 days of surgery.
Dr. Wilson, associate professor of surgery at the University of Texas Health Science Center at Houston, reported that the average length of stay was 2.2 days. Within the first 30 days of surgery there were 81 readmissions (4.8%), "which is not too different from what you’d expect in most populations," he said. Of these, 49 (2.9%) were for dehydration, 27 (1.6%) were for nausea/vomiting, and 5 (0.3%) were for stricture requiring dilation.
There were 46 reoperations (2.7%) within the first 30 days of surgery. Of these, 18 (1.06%) were for bowel obstruction/hernia, 17 (1%) were for bleeding/hematoma, 6 (0.35%) were for negative exploration of patients the surgeons were concerned about, and 5 (0.29%) were for abscess/leak.
There were 26 early major complications (1.5%). Of these, 14 (0.83%) were bleeding requiring transfusion, 5 (0.29%) were stricture requiring dilation, 3 (0.18%) were abscesses, 2 (0.12%) were anastomotic leaks, and 2 (0.12%) were cases of pulmonary embolism/infarct. There was no mortality, "which we think is very favorable," Dr. Wilson said.
Average operating times varied by center: 156 minutes in Houston, 128 minutes in Florida, and 104 minutes in Maine. "As time has gone on, and as we engage each other on how we do things, these operative times have continued to decrease, with current times approaching 90 minutes," Dr. Wilson said. "So long operative times are not necessary when you do robotic surgery."
He concluded by describing robotic-assisted bypass surgery as "an enabling technology that allows for excellent reproducible outcomes, because we have multiple centers doing it well. Future studies should focus on revisional and more complex procedures such as biliopancreatic conversion."
Dr. Wilson disclosed that he is a consultant for Intuitive Surgical, Ethicon Endo-Surgery, Apollo Endosurgery, and EndoGastric Solutions. He is also a proctor for Intuitive Surgical, and has received an educational grant from Gore.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR METABOLIC AND BARIATRIC SURGERY
Esophageal Cancer Survival Benefit Linked to Lymphadenectomy
SAN DIEGO – Removing 12-20 lymph nodes for node-negative patients and 8-25 lymph nodes for node-positive patients confers a survival advantage in esophageal cancer, according to a data analysis of more than 2,100 patients.
"The maximum survival advantage was seen when a minimum of 15 lymph nodes were removed in node-negative patients and 20 in the node-positive patients," Dr. Kenneth L. Meredith said.
The Surveillance Epidemiology and End Results (SEER) analysis also revealed that the benefit of adjuvant radiation therapy on survival in esophageal cancer is limited to those with node-positive disease, suggesting that the management of esophageal cancer remains a work in progress, Dr. Meredith said at the annual Digestive Disease Week.
"Currently the treatment for these patients includes esophagectomy with or without neoadjuvant therapy," Dr. Meredith said. "There are many approaches to esophagectomy, and there are a multitude of recommendations for nodal clearance of these patients. If you look at single and multi-institutional database reviews, their recommendation for nodal harvest is anywhere from 6 to 40. We decided to perform a more recent analysis of the SEER database."
Dr. Meredith, chief of esophagogastric oncology and director of esophageal research at Moffitt Cancer Center, Tampa, Fla., and his associates queried the database for patients who underwent esophagectomy for cancer between 2004 and 2008. They identified 2,109 patients and categorized them by nodal harvest: greater than or less than 5, 8, 10, 12, 15, 20, 25, and 30.
Of the 2,109 patients, 467 were treated with adjuvant radiation and 1,642 were not. Patients treated with neoadjuvant radiation were excluded from the analysis, as were those who had histologic subtypes of cancer that were not adenocarcinoma or squamous cell carcinoma.
Dr. Meredith reported that use of adjuvant radiation was associated with decreased survival in patients with stage I disease (hazard ratio, 2.73; P less than .0001), no benefit in stage II (P = .075), increased survival in stage III (HR, 0.71; P = .005), and no benefit in stage IV (P = .913).
The median number of lymph nodes retracted from all patients was nine, "which is a little low by most standards," said Dr. Meredith.
Multivariate analysis revealed that among node-positive patients, the median survival with and without adjuvant radiation was 23 months and 20 months, respectively, and the 3-year survival rates were 34% and 26.7%, respectively (P = .023). Among node-negative patients, the 3-year survival with and without adjuvant radiation was 48.8% and 68.8%, respectively.
"The only lymph node cutoff we found was significant for all patients was that if you had more than five lymph nodes resected," Dr. Meredith said. "As you [removed more], lymph node harvesting did not translate into a survival benefit. However, when you subclassified whether they were node negative or node positive, a cutoff of 12 and 15, respectively, did translate into a survival benefit. In node-positive patients, those who had more than 8, 10, 12, 15, and 20 lymph nodes did translate into a survival benefit." He added that with regard to extended lymphadenectomy, or more than 20 lymph nodes resected in either cohort, no additional survival benefit was seen.
Dr. Meredith acknowledged certain limitations of the study, including its retrospective design and the fact that SEER lacks information on the nutritional status and performance status of patients. "There is also no information on margin status, chemotherapy, radiation dose, field design, and treatment technique," he said.
Dr. Meredith said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Removing 12-20 lymph nodes for node-negative patients and 8-25 lymph nodes for node-positive patients confers a survival advantage in esophageal cancer, according to a data analysis of more than 2,100 patients.
"The maximum survival advantage was seen when a minimum of 15 lymph nodes were removed in node-negative patients and 20 in the node-positive patients," Dr. Kenneth L. Meredith said.
The Surveillance Epidemiology and End Results (SEER) analysis also revealed that the benefit of adjuvant radiation therapy on survival in esophageal cancer is limited to those with node-positive disease, suggesting that the management of esophageal cancer remains a work in progress, Dr. Meredith said at the annual Digestive Disease Week.
"Currently the treatment for these patients includes esophagectomy with or without neoadjuvant therapy," Dr. Meredith said. "There are many approaches to esophagectomy, and there are a multitude of recommendations for nodal clearance of these patients. If you look at single and multi-institutional database reviews, their recommendation for nodal harvest is anywhere from 6 to 40. We decided to perform a more recent analysis of the SEER database."
Dr. Meredith, chief of esophagogastric oncology and director of esophageal research at Moffitt Cancer Center, Tampa, Fla., and his associates queried the database for patients who underwent esophagectomy for cancer between 2004 and 2008. They identified 2,109 patients and categorized them by nodal harvest: greater than or less than 5, 8, 10, 12, 15, 20, 25, and 30.
Of the 2,109 patients, 467 were treated with adjuvant radiation and 1,642 were not. Patients treated with neoadjuvant radiation were excluded from the analysis, as were those who had histologic subtypes of cancer that were not adenocarcinoma or squamous cell carcinoma.
Dr. Meredith reported that use of adjuvant radiation was associated with decreased survival in patients with stage I disease (hazard ratio, 2.73; P less than .0001), no benefit in stage II (P = .075), increased survival in stage III (HR, 0.71; P = .005), and no benefit in stage IV (P = .913).
The median number of lymph nodes retracted from all patients was nine, "which is a little low by most standards," said Dr. Meredith.
Multivariate analysis revealed that among node-positive patients, the median survival with and without adjuvant radiation was 23 months and 20 months, respectively, and the 3-year survival rates were 34% and 26.7%, respectively (P = .023). Among node-negative patients, the 3-year survival with and without adjuvant radiation was 48.8% and 68.8%, respectively.
"The only lymph node cutoff we found was significant for all patients was that if you had more than five lymph nodes resected," Dr. Meredith said. "As you [removed more], lymph node harvesting did not translate into a survival benefit. However, when you subclassified whether they were node negative or node positive, a cutoff of 12 and 15, respectively, did translate into a survival benefit. In node-positive patients, those who had more than 8, 10, 12, 15, and 20 lymph nodes did translate into a survival benefit." He added that with regard to extended lymphadenectomy, or more than 20 lymph nodes resected in either cohort, no additional survival benefit was seen.
Dr. Meredith acknowledged certain limitations of the study, including its retrospective design and the fact that SEER lacks information on the nutritional status and performance status of patients. "There is also no information on margin status, chemotherapy, radiation dose, field design, and treatment technique," he said.
Dr. Meredith said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Removing 12-20 lymph nodes for node-negative patients and 8-25 lymph nodes for node-positive patients confers a survival advantage in esophageal cancer, according to a data analysis of more than 2,100 patients.
"The maximum survival advantage was seen when a minimum of 15 lymph nodes were removed in node-negative patients and 20 in the node-positive patients," Dr. Kenneth L. Meredith said.
The Surveillance Epidemiology and End Results (SEER) analysis also revealed that the benefit of adjuvant radiation therapy on survival in esophageal cancer is limited to those with node-positive disease, suggesting that the management of esophageal cancer remains a work in progress, Dr. Meredith said at the annual Digestive Disease Week.
"Currently the treatment for these patients includes esophagectomy with or without neoadjuvant therapy," Dr. Meredith said. "There are many approaches to esophagectomy, and there are a multitude of recommendations for nodal clearance of these patients. If you look at single and multi-institutional database reviews, their recommendation for nodal harvest is anywhere from 6 to 40. We decided to perform a more recent analysis of the SEER database."
Dr. Meredith, chief of esophagogastric oncology and director of esophageal research at Moffitt Cancer Center, Tampa, Fla., and his associates queried the database for patients who underwent esophagectomy for cancer between 2004 and 2008. They identified 2,109 patients and categorized them by nodal harvest: greater than or less than 5, 8, 10, 12, 15, 20, 25, and 30.
Of the 2,109 patients, 467 were treated with adjuvant radiation and 1,642 were not. Patients treated with neoadjuvant radiation were excluded from the analysis, as were those who had histologic subtypes of cancer that were not adenocarcinoma or squamous cell carcinoma.
Dr. Meredith reported that use of adjuvant radiation was associated with decreased survival in patients with stage I disease (hazard ratio, 2.73; P less than .0001), no benefit in stage II (P = .075), increased survival in stage III (HR, 0.71; P = .005), and no benefit in stage IV (P = .913).
The median number of lymph nodes retracted from all patients was nine, "which is a little low by most standards," said Dr. Meredith.
Multivariate analysis revealed that among node-positive patients, the median survival with and without adjuvant radiation was 23 months and 20 months, respectively, and the 3-year survival rates were 34% and 26.7%, respectively (P = .023). Among node-negative patients, the 3-year survival with and without adjuvant radiation was 48.8% and 68.8%, respectively.
"The only lymph node cutoff we found was significant for all patients was that if you had more than five lymph nodes resected," Dr. Meredith said. "As you [removed more], lymph node harvesting did not translate into a survival benefit. However, when you subclassified whether they were node negative or node positive, a cutoff of 12 and 15, respectively, did translate into a survival benefit. In node-positive patients, those who had more than 8, 10, 12, 15, and 20 lymph nodes did translate into a survival benefit." He added that with regard to extended lymphadenectomy, or more than 20 lymph nodes resected in either cohort, no additional survival benefit was seen.
Dr. Meredith acknowledged certain limitations of the study, including its retrospective design and the fact that SEER lacks information on the nutritional status and performance status of patients. "There is also no information on margin status, chemotherapy, radiation dose, field design, and treatment technique," he said.
Dr. Meredith said that he had no relevant financial conflicts to disclose.
AT THE ANNUAL DIGESTIVE DISEASE WEEK
Bariatric Surgery Yields Durable Results for Diabetic Nephropathy
SAN DIEGO – Bariatric surgery induced a significant and durable improvement in diabetic nephropathy after 5 years of follow-up, results from a single-center study showed.
"In addition to significant weight loss, [bariatric surgery] achieves profound metabolic effects, including improvements in glycemic control and insulin sensitivity, as well as a decrease in cardiovascular disease risk and mortality," lead author Dr. Helen M. Heneghan said at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "We hypothesized that improving diabetic control with bariatric surgery may have positive effects on the end-organ complications of this disease, such as diabetic nephropathy. We also wanted to address one of the prevailing questions in this field: whether or not the effects of bariatric surgery on diabetes and its complications are durable."
Dr. Heneghan, a bariatric surgery fellow at the Cleveland Clinic Bariatric and Metabolic Institute, and her associates identified 52 patients who underwent bariatric surgery at the institute and had completed the 5-year follow-up. At baseline, the mean age of patients was 51 years, and 75% were women. Their preoperative mean body mass index was 49 kg/m2, 84% had hypertension, and 71% had hyperlipidemia. Preoperatively, the mean duration of diabetes was 8.6 years, and 29% were already taking insulin. Their mean hemoglobin A1c level was 7.7%, and 38% had diabetic nephropathy as indicated by microalbuminuria (30-299 mg of albumin per g of creatinine) or macroalbuminura (greater than 300 mg/g), and 22% of patients were prescribed an ACE inhibitor or angiotensin receptor blocker.
The majority of patients (69%) underwent gastric bypass; 25% had laparoscopic gastric banding and 6% had sleeve gastrectomy. Dr. Heneghan reported that 5 years after their surgery, 44% of patients had sustained remission of their type 2 diabetes, 33% had a significant improvement, and 23% had no change or worsening of their disease. This latter cohort "had the least amount of weight loss and were those who had the longest standing duration of diabetes preoperatively."
The rates of patients with remission, improvement, or change in hypertension were 16%, 50%, and 34%, respectively, whereas the rates for patients with dyslipidemia were 39%, 20%, and 41%.
Only 25% of patients who did not have diabetic nephropathy at the time of surgery went on to develop the condition. Among patients with preoperative microalbuminuria, 42% remained stable whereas 58% regressed and had no albuminuria 5 years after surgery. Similarly, among patients with preoperative macroalbuminuria, 50% remained stable and 50% regressed and had no albuminuria at 5 years.*
There were no preoperative differences in the mean urinary albumin to creatinine ratio (ACR) between patients who were and patients who were not prescribed a renoprotective agent. However, postoperatively, patients who were not on a renoprotective agent had a significantly lower urinary ACR, compared with those who remained on a renoprotective agent (P = .039). "This probably reflects the fact that patients who had improvement of their diabetes and regression or nonprogression of their nephropathy status also had a significant improvement in – or remission of – hypertension, and were no longer prescribed an antihypertensive medication," Dr. Heneghan explained.
She characterized the study’s overall findings as "remarkable, considering that diabetes is a chronic, progressive disease, and certainly warrant further investigation in the form of a prospective and larger study."
Dr. Heneghan said that she had no relevant financial conflicts to disclose.
*CORRECTION 8/28/12: The original sentence contained an error in describing the patients. The sentence should read" "Similarly, among patients with preoperative macroalbuminuria, 50% remained stable and 50% regressed and had no albuminuria at 5 years."
SAN DIEGO – Bariatric surgery induced a significant and durable improvement in diabetic nephropathy after 5 years of follow-up, results from a single-center study showed.
"In addition to significant weight loss, [bariatric surgery] achieves profound metabolic effects, including improvements in glycemic control and insulin sensitivity, as well as a decrease in cardiovascular disease risk and mortality," lead author Dr. Helen M. Heneghan said at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "We hypothesized that improving diabetic control with bariatric surgery may have positive effects on the end-organ complications of this disease, such as diabetic nephropathy. We also wanted to address one of the prevailing questions in this field: whether or not the effects of bariatric surgery on diabetes and its complications are durable."
Dr. Heneghan, a bariatric surgery fellow at the Cleveland Clinic Bariatric and Metabolic Institute, and her associates identified 52 patients who underwent bariatric surgery at the institute and had completed the 5-year follow-up. At baseline, the mean age of patients was 51 years, and 75% were women. Their preoperative mean body mass index was 49 kg/m2, 84% had hypertension, and 71% had hyperlipidemia. Preoperatively, the mean duration of diabetes was 8.6 years, and 29% were already taking insulin. Their mean hemoglobin A1c level was 7.7%, and 38% had diabetic nephropathy as indicated by microalbuminuria (30-299 mg of albumin per g of creatinine) or macroalbuminura (greater than 300 mg/g), and 22% of patients were prescribed an ACE inhibitor or angiotensin receptor blocker.
The majority of patients (69%) underwent gastric bypass; 25% had laparoscopic gastric banding and 6% had sleeve gastrectomy. Dr. Heneghan reported that 5 years after their surgery, 44% of patients had sustained remission of their type 2 diabetes, 33% had a significant improvement, and 23% had no change or worsening of their disease. This latter cohort "had the least amount of weight loss and were those who had the longest standing duration of diabetes preoperatively."
The rates of patients with remission, improvement, or change in hypertension were 16%, 50%, and 34%, respectively, whereas the rates for patients with dyslipidemia were 39%, 20%, and 41%.
Only 25% of patients who did not have diabetic nephropathy at the time of surgery went on to develop the condition. Among patients with preoperative microalbuminuria, 42% remained stable whereas 58% regressed and had no albuminuria 5 years after surgery. Similarly, among patients with preoperative macroalbuminuria, 50% remained stable and 50% regressed and had no albuminuria at 5 years.*
There were no preoperative differences in the mean urinary albumin to creatinine ratio (ACR) between patients who were and patients who were not prescribed a renoprotective agent. However, postoperatively, patients who were not on a renoprotective agent had a significantly lower urinary ACR, compared with those who remained on a renoprotective agent (P = .039). "This probably reflects the fact that patients who had improvement of their diabetes and regression or nonprogression of their nephropathy status also had a significant improvement in – or remission of – hypertension, and were no longer prescribed an antihypertensive medication," Dr. Heneghan explained.
She characterized the study’s overall findings as "remarkable, considering that diabetes is a chronic, progressive disease, and certainly warrant further investigation in the form of a prospective and larger study."
Dr. Heneghan said that she had no relevant financial conflicts to disclose.
*CORRECTION 8/28/12: The original sentence contained an error in describing the patients. The sentence should read" "Similarly, among patients with preoperative macroalbuminuria, 50% remained stable and 50% regressed and had no albuminuria at 5 years."
SAN DIEGO – Bariatric surgery induced a significant and durable improvement in diabetic nephropathy after 5 years of follow-up, results from a single-center study showed.
"In addition to significant weight loss, [bariatric surgery] achieves profound metabolic effects, including improvements in glycemic control and insulin sensitivity, as well as a decrease in cardiovascular disease risk and mortality," lead author Dr. Helen M. Heneghan said at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "We hypothesized that improving diabetic control with bariatric surgery may have positive effects on the end-organ complications of this disease, such as diabetic nephropathy. We also wanted to address one of the prevailing questions in this field: whether or not the effects of bariatric surgery on diabetes and its complications are durable."
Dr. Heneghan, a bariatric surgery fellow at the Cleveland Clinic Bariatric and Metabolic Institute, and her associates identified 52 patients who underwent bariatric surgery at the institute and had completed the 5-year follow-up. At baseline, the mean age of patients was 51 years, and 75% were women. Their preoperative mean body mass index was 49 kg/m2, 84% had hypertension, and 71% had hyperlipidemia. Preoperatively, the mean duration of diabetes was 8.6 years, and 29% were already taking insulin. Their mean hemoglobin A1c level was 7.7%, and 38% had diabetic nephropathy as indicated by microalbuminuria (30-299 mg of albumin per g of creatinine) or macroalbuminura (greater than 300 mg/g), and 22% of patients were prescribed an ACE inhibitor or angiotensin receptor blocker.
The majority of patients (69%) underwent gastric bypass; 25% had laparoscopic gastric banding and 6% had sleeve gastrectomy. Dr. Heneghan reported that 5 years after their surgery, 44% of patients had sustained remission of their type 2 diabetes, 33% had a significant improvement, and 23% had no change or worsening of their disease. This latter cohort "had the least amount of weight loss and were those who had the longest standing duration of diabetes preoperatively."
The rates of patients with remission, improvement, or change in hypertension were 16%, 50%, and 34%, respectively, whereas the rates for patients with dyslipidemia were 39%, 20%, and 41%.
Only 25% of patients who did not have diabetic nephropathy at the time of surgery went on to develop the condition. Among patients with preoperative microalbuminuria, 42% remained stable whereas 58% regressed and had no albuminuria 5 years after surgery. Similarly, among patients with preoperative macroalbuminuria, 50% remained stable and 50% regressed and had no albuminuria at 5 years.*
There were no preoperative differences in the mean urinary albumin to creatinine ratio (ACR) between patients who were and patients who were not prescribed a renoprotective agent. However, postoperatively, patients who were not on a renoprotective agent had a significantly lower urinary ACR, compared with those who remained on a renoprotective agent (P = .039). "This probably reflects the fact that patients who had improvement of their diabetes and regression or nonprogression of their nephropathy status also had a significant improvement in – or remission of – hypertension, and were no longer prescribed an antihypertensive medication," Dr. Heneghan explained.
She characterized the study’s overall findings as "remarkable, considering that diabetes is a chronic, progressive disease, and certainly warrant further investigation in the form of a prospective and larger study."
Dr. Heneghan said that she had no relevant financial conflicts to disclose.
*CORRECTION 8/28/12: The original sentence contained an error in describing the patients. The sentence should read" "Similarly, among patients with preoperative macroalbuminuria, 50% remained stable and 50% regressed and had no albuminuria at 5 years."
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR METABOLIC AND BARIATRIC SURGERY
Major Finding: Among patients with preoperative microalbuminuria, 42% remained stable 5 years after their bariatric surgery, whereas 58% regressed and had no albuminuria. Similarly, among patients with preoperative macroalbuminuria, 50% remained stable, and 50% regressed and had no albuminuria at 5 years.
Data Source: The study included 52 patients who underwent bariatric surgery at the Cleveland Clinic and had completed the 5-year follow-up.
Disclosures: Dr. Heneghan said that she had no relevant financial conflicts to disclose.
BOLD Analysis Backs Safety of Sleeve Gastrectomy
SAN DIEGO – Laparoscopic sleeve gastrectomy is positioned between gastric banding and the laparoscopic gastric bypass for both safety and efficacy, results from the largest comparative study of its kind demonstrated.
The finding comes at a time when the Centers for Medicare and Medicaid Services is reviewing evidence to consider including sleeve gastrectomy as a covered benefit. Currently, gastric bypass, vertical banded gastroplasty, duodenal switch, and gastric banding are the only CMS-sanctioned bariatric procedures.
The study, which involved nearly 300,000 patients, "shows that across the board, regardless of the procedure, bariatric surgery is safe and effective," Dr. John M. Morton said in an interview at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "The emerging new procedure, the sleeve gastrectomy, is shown to be right between the bypass and the band. As a result, we have seen more interest from payers to cover it. In fact there are about 100 million lives that are covered. Our only outlier is CMS in deciding to cover. We hope that these data will help influence CMS in granting coverage for the sleeve gastrectomy."
Dr. Morton, associate professor of surgery and director of bariatric surgery at Stanford Hospitals and Clinics at Stanford (Calif.) University, and his associates examined BOLD (Bariatric Outcomes Longitudinal Database) to identify patients who had undergone laparoscopic Roux-en-Y gastric bypass (LRNYGB), gastric banding (LAGB), and sleeve gastrectomy (LSG) from June 2007 to December 2010. BOLD, the largest bariatric-specific database, is maintained by the ASMBS Bariatric Surgery Center of Excellence program, and includes more than 1,200 surgeons at 540 hospitals. Dr. Morton described the data as a "clinically rich" variable set that includes age, gender, race, insurance status, body mass index, excess body weight, and comorbidities.
"There is a definite need for more data around comparison of different procedures," Dr. Morton said at the meeting. "Our study hypothesis is very straightforward: Do demographics and outcomes for bariatric surgery vary by procedure?"
The primary outcomes were 30-day mortality, serious complications, and readmissions. The definitions of serious complications included death, anastomotic leakage, cardiac arrest, deep venous thrombosis, evisceration, heart failure and/or pulmonary edema, liver failure, and bleeding requiring transfusion.
Dr. Morton reported outcomes from 117,365 patients in the LAGB group, 138,222 in the LRNYGB group, and 16,139 in the LSG group. Patients in each group were generally the same age (a mean of 45, 46, and 45 years, respectively), mostly female (78%, 79%, and 74%), and mostly white (72%, 73%, and 72%). "The one area where there was a sizable difference was around self-pay," Dr. Morton said. About 21% of patients in the LSG group paid out-of-pocket, compared with 6% of those in the LAGB group and 2% of those in the LRNYGB group.
The proportion of preoperative comorbidities was similar among the three groups, with two exceptions. The prevalence of diabetes was highest in the LRNYGB group (37%, compared with 30% in the LSG group and 28% in the LAGB group; P less than .0001). A similar association was seen in the proportion of patients with five or more preoperative comorbidities (62%, 55%, and 52%, respectively; P less than .0001).
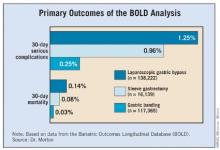
The mean length of stay was 0.7 days for the LAGB group, 1.9 days for the LSG group, and 2.3 days for the LRNYGB group. The percent change in BMI at 12 months was 7.6%, 13.4%, and 16.4%, respectively; the rate of 30-day serious complications was 0.25%, 0.96%, and 1.25%; and the rate of 30-day mortality was 0.03%, 0.08%, and 0.14%. All differences between the groups were significant (P less than .0001).
"If you look at the remainder of the safety outcomes – everything from 30-day readmission to 30-day reoperation – it’s pretty much the same order, with the band group having the lowest [percentage], and the bypass having the highest, and the sleeve being right in between," Dr. Morton said. "When we looked at age greater than 65 in isolation, we found that the order of safety remains, with the banding having the least amount of mortality and the sleeve being right between the band procedure and the bypass."
Logistic regression analysis revealed several significant factors that predicted serious adverse events at 30 days: male gender (odds ratio, 1.67), having nonprivate insurance (OR, 1.15), stepwise progression with increasing age (for example, an OR of 1.27 for those aged 26-35 years and an OR of 4.42 for those above age 65), and stepwise progression with increasing BMI (for example, an OR of 1.37 for those with a BMI of 46-55 kg/m2 and an OR of 3.03 for those with a BMI greater than 65).
The invited discussant, Dr. Matthew M. Hutter, of Massachusetts General Hospital, Boston, described the size of the overall study cohort as remarkable. "What I find most interesting about this study is that it shows that sleeve gastrectomy – a brand-new, very complex procedure – can be introduced safely and effectively when performed under the standards of a bariatric accreditation program," Dr. Hutter said. "Other surgical procedures such as laparoscopic cholecystectomy or laparoscopic colectomy had very high morbidity and conversion rates when they were first implemented. However, this new complex procedure has been safe and effective from the get-go, and that is really quite impressive. The other remarkable finding is how consistent this is with all of the other major [bariatric surgery] data collection programs."
Dr. Morton acknowledged certain limitations of the study, including the fact that 1-year follow-up was available in only 60% of patients, while 30-day follow-up was available in 98% of the cohort. "Potentially, patients could have been admitted to other hospitals," he added. "These are research-consented patients, so about 70% consented. Some of this is surgeon-directed reporting."
Dr. Morton said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Laparoscopic sleeve gastrectomy is positioned between gastric banding and the laparoscopic gastric bypass for both safety and efficacy, results from the largest comparative study of its kind demonstrated.
The finding comes at a time when the Centers for Medicare and Medicaid Services is reviewing evidence to consider including sleeve gastrectomy as a covered benefit. Currently, gastric bypass, vertical banded gastroplasty, duodenal switch, and gastric banding are the only CMS-sanctioned bariatric procedures.
The study, which involved nearly 300,000 patients, "shows that across the board, regardless of the procedure, bariatric surgery is safe and effective," Dr. John M. Morton said in an interview at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "The emerging new procedure, the sleeve gastrectomy, is shown to be right between the bypass and the band. As a result, we have seen more interest from payers to cover it. In fact there are about 100 million lives that are covered. Our only outlier is CMS in deciding to cover. We hope that these data will help influence CMS in granting coverage for the sleeve gastrectomy."
Dr. Morton, associate professor of surgery and director of bariatric surgery at Stanford Hospitals and Clinics at Stanford (Calif.) University, and his associates examined BOLD (Bariatric Outcomes Longitudinal Database) to identify patients who had undergone laparoscopic Roux-en-Y gastric bypass (LRNYGB), gastric banding (LAGB), and sleeve gastrectomy (LSG) from June 2007 to December 2010. BOLD, the largest bariatric-specific database, is maintained by the ASMBS Bariatric Surgery Center of Excellence program, and includes more than 1,200 surgeons at 540 hospitals. Dr. Morton described the data as a "clinically rich" variable set that includes age, gender, race, insurance status, body mass index, excess body weight, and comorbidities.
"There is a definite need for more data around comparison of different procedures," Dr. Morton said at the meeting. "Our study hypothesis is very straightforward: Do demographics and outcomes for bariatric surgery vary by procedure?"
The primary outcomes were 30-day mortality, serious complications, and readmissions. The definitions of serious complications included death, anastomotic leakage, cardiac arrest, deep venous thrombosis, evisceration, heart failure and/or pulmonary edema, liver failure, and bleeding requiring transfusion.
Dr. Morton reported outcomes from 117,365 patients in the LAGB group, 138,222 in the LRNYGB group, and 16,139 in the LSG group. Patients in each group were generally the same age (a mean of 45, 46, and 45 years, respectively), mostly female (78%, 79%, and 74%), and mostly white (72%, 73%, and 72%). "The one area where there was a sizable difference was around self-pay," Dr. Morton said. About 21% of patients in the LSG group paid out-of-pocket, compared with 6% of those in the LAGB group and 2% of those in the LRNYGB group.
The proportion of preoperative comorbidities was similar among the three groups, with two exceptions. The prevalence of diabetes was highest in the LRNYGB group (37%, compared with 30% in the LSG group and 28% in the LAGB group; P less than .0001). A similar association was seen in the proportion of patients with five or more preoperative comorbidities (62%, 55%, and 52%, respectively; P less than .0001).
The mean length of stay was 0.7 days for the LAGB group, 1.9 days for the LSG group, and 2.3 days for the LRNYGB group. The percent change in BMI at 12 months was 7.6%, 13.4%, and 16.4%, respectively; the rate of 30-day serious complications was 0.25%, 0.96%, and 1.25%; and the rate of 30-day mortality was 0.03%, 0.08%, and 0.14%. All differences between the groups were significant (P less than .0001).
"If you look at the remainder of the safety outcomes – everything from 30-day readmission to 30-day reoperation – it’s pretty much the same order, with the band group having the lowest [percentage], and the bypass having the highest, and the sleeve being right in between," Dr. Morton said. "When we looked at age greater than 65 in isolation, we found that the order of safety remains, with the banding having the least amount of mortality and the sleeve being right between the band procedure and the bypass."
Logistic regression analysis revealed several significant factors that predicted serious adverse events at 30 days: male gender (odds ratio, 1.67), having nonprivate insurance (OR, 1.15), stepwise progression with increasing age (for example, an OR of 1.27 for those aged 26-35 years and an OR of 4.42 for those above age 65), and stepwise progression with increasing BMI (for example, an OR of 1.37 for those with a BMI of 46-55 kg/m2 and an OR of 3.03 for those with a BMI greater than 65).
The invited discussant, Dr. Matthew M. Hutter, of Massachusetts General Hospital, Boston, described the size of the overall study cohort as remarkable. "What I find most interesting about this study is that it shows that sleeve gastrectomy – a brand-new, very complex procedure – can be introduced safely and effectively when performed under the standards of a bariatric accreditation program," Dr. Hutter said. "Other surgical procedures such as laparoscopic cholecystectomy or laparoscopic colectomy had very high morbidity and conversion rates when they were first implemented. However, this new complex procedure has been safe and effective from the get-go, and that is really quite impressive. The other remarkable finding is how consistent this is with all of the other major [bariatric surgery] data collection programs."
Dr. Morton acknowledged certain limitations of the study, including the fact that 1-year follow-up was available in only 60% of patients, while 30-day follow-up was available in 98% of the cohort. "Potentially, patients could have been admitted to other hospitals," he added. "These are research-consented patients, so about 70% consented. Some of this is surgeon-directed reporting."
Dr. Morton said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Laparoscopic sleeve gastrectomy is positioned between gastric banding and the laparoscopic gastric bypass for both safety and efficacy, results from the largest comparative study of its kind demonstrated.
The finding comes at a time when the Centers for Medicare and Medicaid Services is reviewing evidence to consider including sleeve gastrectomy as a covered benefit. Currently, gastric bypass, vertical banded gastroplasty, duodenal switch, and gastric banding are the only CMS-sanctioned bariatric procedures.
The study, which involved nearly 300,000 patients, "shows that across the board, regardless of the procedure, bariatric surgery is safe and effective," Dr. John M. Morton said in an interview at the annual meeting of the American Society for Metabolic and Bariatric Surgery. "The emerging new procedure, the sleeve gastrectomy, is shown to be right between the bypass and the band. As a result, we have seen more interest from payers to cover it. In fact there are about 100 million lives that are covered. Our only outlier is CMS in deciding to cover. We hope that these data will help influence CMS in granting coverage for the sleeve gastrectomy."
Dr. Morton, associate professor of surgery and director of bariatric surgery at Stanford Hospitals and Clinics at Stanford (Calif.) University, and his associates examined BOLD (Bariatric Outcomes Longitudinal Database) to identify patients who had undergone laparoscopic Roux-en-Y gastric bypass (LRNYGB), gastric banding (LAGB), and sleeve gastrectomy (LSG) from June 2007 to December 2010. BOLD, the largest bariatric-specific database, is maintained by the ASMBS Bariatric Surgery Center of Excellence program, and includes more than 1,200 surgeons at 540 hospitals. Dr. Morton described the data as a "clinically rich" variable set that includes age, gender, race, insurance status, body mass index, excess body weight, and comorbidities.
"There is a definite need for more data around comparison of different procedures," Dr. Morton said at the meeting. "Our study hypothesis is very straightforward: Do demographics and outcomes for bariatric surgery vary by procedure?"
The primary outcomes were 30-day mortality, serious complications, and readmissions. The definitions of serious complications included death, anastomotic leakage, cardiac arrest, deep venous thrombosis, evisceration, heart failure and/or pulmonary edema, liver failure, and bleeding requiring transfusion.
Dr. Morton reported outcomes from 117,365 patients in the LAGB group, 138,222 in the LRNYGB group, and 16,139 in the LSG group. Patients in each group were generally the same age (a mean of 45, 46, and 45 years, respectively), mostly female (78%, 79%, and 74%), and mostly white (72%, 73%, and 72%). "The one area where there was a sizable difference was around self-pay," Dr. Morton said. About 21% of patients in the LSG group paid out-of-pocket, compared with 6% of those in the LAGB group and 2% of those in the LRNYGB group.
The proportion of preoperative comorbidities was similar among the three groups, with two exceptions. The prevalence of diabetes was highest in the LRNYGB group (37%, compared with 30% in the LSG group and 28% in the LAGB group; P less than .0001). A similar association was seen in the proportion of patients with five or more preoperative comorbidities (62%, 55%, and 52%, respectively; P less than .0001).
The mean length of stay was 0.7 days for the LAGB group, 1.9 days for the LSG group, and 2.3 days for the LRNYGB group. The percent change in BMI at 12 months was 7.6%, 13.4%, and 16.4%, respectively; the rate of 30-day serious complications was 0.25%, 0.96%, and 1.25%; and the rate of 30-day mortality was 0.03%, 0.08%, and 0.14%. All differences between the groups were significant (P less than .0001).
"If you look at the remainder of the safety outcomes – everything from 30-day readmission to 30-day reoperation – it’s pretty much the same order, with the band group having the lowest [percentage], and the bypass having the highest, and the sleeve being right in between," Dr. Morton said. "When we looked at age greater than 65 in isolation, we found that the order of safety remains, with the banding having the least amount of mortality and the sleeve being right between the band procedure and the bypass."
Logistic regression analysis revealed several significant factors that predicted serious adverse events at 30 days: male gender (odds ratio, 1.67), having nonprivate insurance (OR, 1.15), stepwise progression with increasing age (for example, an OR of 1.27 for those aged 26-35 years and an OR of 4.42 for those above age 65), and stepwise progression with increasing BMI (for example, an OR of 1.37 for those with a BMI of 46-55 kg/m2 and an OR of 3.03 for those with a BMI greater than 65).
The invited discussant, Dr. Matthew M. Hutter, of Massachusetts General Hospital, Boston, described the size of the overall study cohort as remarkable. "What I find most interesting about this study is that it shows that sleeve gastrectomy – a brand-new, very complex procedure – can be introduced safely and effectively when performed under the standards of a bariatric accreditation program," Dr. Hutter said. "Other surgical procedures such as laparoscopic cholecystectomy or laparoscopic colectomy had very high morbidity and conversion rates when they were first implemented. However, this new complex procedure has been safe and effective from the get-go, and that is really quite impressive. The other remarkable finding is how consistent this is with all of the other major [bariatric surgery] data collection programs."
Dr. Morton acknowledged certain limitations of the study, including the fact that 1-year follow-up was available in only 60% of patients, while 30-day follow-up was available in 98% of the cohort. "Potentially, patients could have been admitted to other hospitals," he added. "These are research-consented patients, so about 70% consented. Some of this is surgeon-directed reporting."
Dr. Morton said that he had no relevant financial conflicts to disclose.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR METABOLIC AND BARIATRIC SURGERY
Major Finding: The rate of 30-day serious complications was 0.25% among those who underwent laparoscopic gastric banding, 0.96% among those who underwent laparoscopic sleeve gastrectomy, and 1.25% among those who underwent laparoscopic Roux-en-Y gastric bypass, while the rate of 30-day mortality was 0.03%, 0.08%, and 0.14%, respectively.
Data Source: The data analysis was based on 271,726 patients from the Bariatric Outcomes Longitudinal Database who underwent bariatric surgery from June 2007 to December 2010.
Disclosures: Dr. Morton said that he had no relevant financial conflicts to disclose.
Race, Sex Factor Into Weight Loss After Gastric Bypass
SAN DIEGO – Being black, male, or older significantly raised the risk for weight-loss failure after gastric bypass in a single-center study of more than 1,200 patients.
"Long-term treatments of obesity are hampered by the fixed behaviors that induce obesity, the possibility of weight set points, and the ever-present exposure to high-calorie foods. The treatments of obesity all have great variability in outcome," Dr. Ramsey M. Dallal said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
To determine predictors of weight-loss failure after gastric bypass surgery, Dr. Dallal and his associate at the department of surgery at Einstein Healthcare Network, Philadelphia, reviewed the medical records of 1,256 gastric bypass patients who had a least 1 year of follow-up. They separated patients into two groups: those who were above the 75th percentile in weight loss (success) and those who were below the 25th percentile in weight loss (failure). Multivariate logistic regression was performed to examine the impact of sex, race, age, initial weight, initial glycosylated hemoglobin (HbA1c) level, and insurance type (Medicare/Medicaid vs. private insurance).
The mean preoperative body mass index of the 1,256 patients was 48.3 kg/m2, their mean age was 42 years, and 82% were women. More than one-quarter of patients (27%) had diabetes, and the mean HbA1c level was 6.6% in blacks and 6.3% in whites. The majority of patients (75%) had private insurance, 19% were on Medicare, and 6% were on Medicaid.
Dr. Dallal reported that after a mean follow-up period of 665 days, the mean excess weight loss among all patients was 70%, and was significantly different between whites and blacks (72% vs. 63%, respectively), between those aged 65 years and older and those younger than age 40 (61% vs. 71%), and between men and women (62% vs. 71%). The calculated threshold estimated weight loss for the upper 75th and lower 25th percentiles was 82% vs. 57%, respectively.
Multivariate logistic regression analysis revealed the following independent predictors of weight-loss failure: being black (odds ratio, 3.1; P = .002), older (OR, 0.97; P = .001), or male (OR, 0.30; P less than .0005), and having a higher initial body weight (OR, 0.86; P less than .0005). Initial HbA1c and insurance type were not independent predictors of weight-loss failure.
Dr. Dallal acknowledged certain limitations of the study, including the fact that the ideal body weight calculations used "may not necessarily be valid for all ethnicities. Also, we did not distinguish between primary weight-loss failures (those who never reached adequate weight loss) and those [who had] a secondary weight-loss failure (regain of lost weight)."
Dr. Dallal said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Being black, male, or older significantly raised the risk for weight-loss failure after gastric bypass in a single-center study of more than 1,200 patients.
"Long-term treatments of obesity are hampered by the fixed behaviors that induce obesity, the possibility of weight set points, and the ever-present exposure to high-calorie foods. The treatments of obesity all have great variability in outcome," Dr. Ramsey M. Dallal said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
To determine predictors of weight-loss failure after gastric bypass surgery, Dr. Dallal and his associate at the department of surgery at Einstein Healthcare Network, Philadelphia, reviewed the medical records of 1,256 gastric bypass patients who had a least 1 year of follow-up. They separated patients into two groups: those who were above the 75th percentile in weight loss (success) and those who were below the 25th percentile in weight loss (failure). Multivariate logistic regression was performed to examine the impact of sex, race, age, initial weight, initial glycosylated hemoglobin (HbA1c) level, and insurance type (Medicare/Medicaid vs. private insurance).
The mean preoperative body mass index of the 1,256 patients was 48.3 kg/m2, their mean age was 42 years, and 82% were women. More than one-quarter of patients (27%) had diabetes, and the mean HbA1c level was 6.6% in blacks and 6.3% in whites. The majority of patients (75%) had private insurance, 19% were on Medicare, and 6% were on Medicaid.
Dr. Dallal reported that after a mean follow-up period of 665 days, the mean excess weight loss among all patients was 70%, and was significantly different between whites and blacks (72% vs. 63%, respectively), between those aged 65 years and older and those younger than age 40 (61% vs. 71%), and between men and women (62% vs. 71%). The calculated threshold estimated weight loss for the upper 75th and lower 25th percentiles was 82% vs. 57%, respectively.
Multivariate logistic regression analysis revealed the following independent predictors of weight-loss failure: being black (odds ratio, 3.1; P = .002), older (OR, 0.97; P = .001), or male (OR, 0.30; P less than .0005), and having a higher initial body weight (OR, 0.86; P less than .0005). Initial HbA1c and insurance type were not independent predictors of weight-loss failure.
Dr. Dallal acknowledged certain limitations of the study, including the fact that the ideal body weight calculations used "may not necessarily be valid for all ethnicities. Also, we did not distinguish between primary weight-loss failures (those who never reached adequate weight loss) and those [who had] a secondary weight-loss failure (regain of lost weight)."
Dr. Dallal said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Being black, male, or older significantly raised the risk for weight-loss failure after gastric bypass in a single-center study of more than 1,200 patients.
"Long-term treatments of obesity are hampered by the fixed behaviors that induce obesity, the possibility of weight set points, and the ever-present exposure to high-calorie foods. The treatments of obesity all have great variability in outcome," Dr. Ramsey M. Dallal said at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
To determine predictors of weight-loss failure after gastric bypass surgery, Dr. Dallal and his associate at the department of surgery at Einstein Healthcare Network, Philadelphia, reviewed the medical records of 1,256 gastric bypass patients who had a least 1 year of follow-up. They separated patients into two groups: those who were above the 75th percentile in weight loss (success) and those who were below the 25th percentile in weight loss (failure). Multivariate logistic regression was performed to examine the impact of sex, race, age, initial weight, initial glycosylated hemoglobin (HbA1c) level, and insurance type (Medicare/Medicaid vs. private insurance).
The mean preoperative body mass index of the 1,256 patients was 48.3 kg/m2, their mean age was 42 years, and 82% were women. More than one-quarter of patients (27%) had diabetes, and the mean HbA1c level was 6.6% in blacks and 6.3% in whites. The majority of patients (75%) had private insurance, 19% were on Medicare, and 6% were on Medicaid.
Dr. Dallal reported that after a mean follow-up period of 665 days, the mean excess weight loss among all patients was 70%, and was significantly different between whites and blacks (72% vs. 63%, respectively), between those aged 65 years and older and those younger than age 40 (61% vs. 71%), and between men and women (62% vs. 71%). The calculated threshold estimated weight loss for the upper 75th and lower 25th percentiles was 82% vs. 57%, respectively.
Multivariate logistic regression analysis revealed the following independent predictors of weight-loss failure: being black (odds ratio, 3.1; P = .002), older (OR, 0.97; P = .001), or male (OR, 0.30; P less than .0005), and having a higher initial body weight (OR, 0.86; P less than .0005). Initial HbA1c and insurance type were not independent predictors of weight-loss failure.
Dr. Dallal acknowledged certain limitations of the study, including the fact that the ideal body weight calculations used "may not necessarily be valid for all ethnicities. Also, we did not distinguish between primary weight-loss failures (those who never reached adequate weight loss) and those [who had] a secondary weight-loss failure (regain of lost weight)."
Dr. Dallal said that he had no relevant financial conflicts to disclose.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR METABOLIC AND BARIATRIC SURGERY
Major Finding: Following gastric bypass surgery, independent significant predictors of weight-loss failure were being black (OR, 3.1; P = .002), older (OR, 0.97; P = .001), or male (OR, 0.30; P less than .0005), and having higher initial body weight (OR, 0.86; P less than .0005).
Data Source: This single-center study comprised 1,256 gastric bypass patients who had a least 1 year of follow-up.
Disclosures: Dr. Dallal said that he had no relevant financial conflicts to disclose.
Ventral Hernia Repair Incurs Overall Financial Losses
SAN DIEGO – Ventral hernia repair is associated with overall financial losses reaching the thousands of dollars in some cases, a single-center study demonstrated.
"These financial losses are just simply not sustainable," Dr. Drew Reynolds said in an interview prior to the annual Digestive Disease Week, where the study was presented.
In what is believed to be the first study of its kind, he and his associates set out to systematically evaluate hospital finances with respect to open ventral incisional hernia repair in the tertiary care environment.
"There is limited cost data currently available in the literature," he said. "These patients are complex and often require management in tertiary referral centers. Biologic meshes have a legitimate role in certain clinical scenarios, especially in those encountered in the tertiary care environment. Reimbursement strategies need to be reevaluated with more appropriate adjustment for preoperative risk factors and operative complexity."
Hospital costs associated with complex hernia repairs include direct costs (mesh materials, supplies, and non–surgeon labor), and indirect costs (facility fees, equipment depreciation, and unallocated labor), said Dr. Reynolds, a fellow in minimally invasive surgery at the University of Kentucky, Lexington. "Operative supplies including mesh represent a significant component of direct costs, especially in an era of proprietary synthetic meshes and biologic grafts," the researchers wrote in their abstract.
Dr. Reynolds and his associates evaluated cost data on 415 consecutive open ventral hernia repairs performed at the university’s hospital between July 1, 2008, and May 31, 2011, using CPT codes 49560, 49561, 49565, and 49566. They analyzed data based on hospital status (inpatient vs. outpatient) and whether the hernia repair was a primary or secondary procedure. The primary end points were hospital revenue and costs. Revenue calculations were adjusted for comorbid conditions/diagnosis-related groups, and readmission costs were not included.
Of the 415 patients, 353 were inpatients and 62 were outpatients. Among the 353 inpatients, ventral hernia repair was the primary procedure performed on 173 patients and was the secondary procedure for 180 patients. The median net revenue was significantly greater for those who underwent hernia repair as a secondary procedure compared with those who had hernia repair as a primary procedure ($17,310 vs. $10,360, P less than .01), as were net losses ($3,340 vs. $1,700, P less than .01).
Among the inpatient primary ventral hernia repairs, 46 were repaired without mesh, 79 were repaired with synthetic mesh, and 48 were repaired with biologic mesh, for median direct costs of $5,432, $7,590, and $16,970, respectively (P less than .01).
Dr. Reynolds also reported that among all inpatient ventral hernia repairs, the median net losses for repairs without mesh were $500, while synthetic mesh–based repairs yielded a median net profit of $60. The median contribution margin for cases involving biologic mesh was –$4,560, and the median net financial loss was $8,370.
Among patients who underwent outpatient ventral hernia repairs, median net losses among those performed with and without synthetic mesh reached $1,560 and $230, respectively.
"It was surprising to note that the vast majority of open ventral incisional hernia repairs are performed at an overall financial loss for the hospital," Dr. Reynolds said. "Further, it was surprising to note that inpatient biologic mesh–based ventral hernia repairs resulted in such a sizable negative median contribution margin ($4,560), and the striking median net financial loss of $8,370."
He acknowledged certain limitations of the study, including the fact that data used in the analysis were retrieved by CPT code search. "Hospital readmission costs were not included, which leads to underestimation of the costs," he added.
Dr. Reynolds said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Ventral hernia repair is associated with overall financial losses reaching the thousands of dollars in some cases, a single-center study demonstrated.
"These financial losses are just simply not sustainable," Dr. Drew Reynolds said in an interview prior to the annual Digestive Disease Week, where the study was presented.
In what is believed to be the first study of its kind, he and his associates set out to systematically evaluate hospital finances with respect to open ventral incisional hernia repair in the tertiary care environment.
"There is limited cost data currently available in the literature," he said. "These patients are complex and often require management in tertiary referral centers. Biologic meshes have a legitimate role in certain clinical scenarios, especially in those encountered in the tertiary care environment. Reimbursement strategies need to be reevaluated with more appropriate adjustment for preoperative risk factors and operative complexity."
Hospital costs associated with complex hernia repairs include direct costs (mesh materials, supplies, and non–surgeon labor), and indirect costs (facility fees, equipment depreciation, and unallocated labor), said Dr. Reynolds, a fellow in minimally invasive surgery at the University of Kentucky, Lexington. "Operative supplies including mesh represent a significant component of direct costs, especially in an era of proprietary synthetic meshes and biologic grafts," the researchers wrote in their abstract.
Dr. Reynolds and his associates evaluated cost data on 415 consecutive open ventral hernia repairs performed at the university’s hospital between July 1, 2008, and May 31, 2011, using CPT codes 49560, 49561, 49565, and 49566. They analyzed data based on hospital status (inpatient vs. outpatient) and whether the hernia repair was a primary or secondary procedure. The primary end points were hospital revenue and costs. Revenue calculations were adjusted for comorbid conditions/diagnosis-related groups, and readmission costs were not included.
Of the 415 patients, 353 were inpatients and 62 were outpatients. Among the 353 inpatients, ventral hernia repair was the primary procedure performed on 173 patients and was the secondary procedure for 180 patients. The median net revenue was significantly greater for those who underwent hernia repair as a secondary procedure compared with those who had hernia repair as a primary procedure ($17,310 vs. $10,360, P less than .01), as were net losses ($3,340 vs. $1,700, P less than .01).
Among the inpatient primary ventral hernia repairs, 46 were repaired without mesh, 79 were repaired with synthetic mesh, and 48 were repaired with biologic mesh, for median direct costs of $5,432, $7,590, and $16,970, respectively (P less than .01).
Dr. Reynolds also reported that among all inpatient ventral hernia repairs, the median net losses for repairs without mesh were $500, while synthetic mesh–based repairs yielded a median net profit of $60. The median contribution margin for cases involving biologic mesh was –$4,560, and the median net financial loss was $8,370.
Among patients who underwent outpatient ventral hernia repairs, median net losses among those performed with and without synthetic mesh reached $1,560 and $230, respectively.
"It was surprising to note that the vast majority of open ventral incisional hernia repairs are performed at an overall financial loss for the hospital," Dr. Reynolds said. "Further, it was surprising to note that inpatient biologic mesh–based ventral hernia repairs resulted in such a sizable negative median contribution margin ($4,560), and the striking median net financial loss of $8,370."
He acknowledged certain limitations of the study, including the fact that data used in the analysis were retrieved by CPT code search. "Hospital readmission costs were not included, which leads to underestimation of the costs," he added.
Dr. Reynolds said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Ventral hernia repair is associated with overall financial losses reaching the thousands of dollars in some cases, a single-center study demonstrated.
"These financial losses are just simply not sustainable," Dr. Drew Reynolds said in an interview prior to the annual Digestive Disease Week, where the study was presented.
In what is believed to be the first study of its kind, he and his associates set out to systematically evaluate hospital finances with respect to open ventral incisional hernia repair in the tertiary care environment.
"There is limited cost data currently available in the literature," he said. "These patients are complex and often require management in tertiary referral centers. Biologic meshes have a legitimate role in certain clinical scenarios, especially in those encountered in the tertiary care environment. Reimbursement strategies need to be reevaluated with more appropriate adjustment for preoperative risk factors and operative complexity."
Hospital costs associated with complex hernia repairs include direct costs (mesh materials, supplies, and non–surgeon labor), and indirect costs (facility fees, equipment depreciation, and unallocated labor), said Dr. Reynolds, a fellow in minimally invasive surgery at the University of Kentucky, Lexington. "Operative supplies including mesh represent a significant component of direct costs, especially in an era of proprietary synthetic meshes and biologic grafts," the researchers wrote in their abstract.
Dr. Reynolds and his associates evaluated cost data on 415 consecutive open ventral hernia repairs performed at the university’s hospital between July 1, 2008, and May 31, 2011, using CPT codes 49560, 49561, 49565, and 49566. They analyzed data based on hospital status (inpatient vs. outpatient) and whether the hernia repair was a primary or secondary procedure. The primary end points were hospital revenue and costs. Revenue calculations were adjusted for comorbid conditions/diagnosis-related groups, and readmission costs were not included.
Of the 415 patients, 353 were inpatients and 62 were outpatients. Among the 353 inpatients, ventral hernia repair was the primary procedure performed on 173 patients and was the secondary procedure for 180 patients. The median net revenue was significantly greater for those who underwent hernia repair as a secondary procedure compared with those who had hernia repair as a primary procedure ($17,310 vs. $10,360, P less than .01), as were net losses ($3,340 vs. $1,700, P less than .01).
Among the inpatient primary ventral hernia repairs, 46 were repaired without mesh, 79 were repaired with synthetic mesh, and 48 were repaired with biologic mesh, for median direct costs of $5,432, $7,590, and $16,970, respectively (P less than .01).
Dr. Reynolds also reported that among all inpatient ventral hernia repairs, the median net losses for repairs without mesh were $500, while synthetic mesh–based repairs yielded a median net profit of $60. The median contribution margin for cases involving biologic mesh was –$4,560, and the median net financial loss was $8,370.
Among patients who underwent outpatient ventral hernia repairs, median net losses among those performed with and without synthetic mesh reached $1,560 and $230, respectively.
"It was surprising to note that the vast majority of open ventral incisional hernia repairs are performed at an overall financial loss for the hospital," Dr. Reynolds said. "Further, it was surprising to note that inpatient biologic mesh–based ventral hernia repairs resulted in such a sizable negative median contribution margin ($4,560), and the striking median net financial loss of $8,370."
He acknowledged certain limitations of the study, including the fact that data used in the analysis were retrieved by CPT code search. "Hospital readmission costs were not included, which leads to underestimation of the costs," he added.
Dr. Reynolds said that he had no relevant financial conflicts to disclose.
AT THE ANNUAL DIGESTIVE DISEASE WEEK
ATX-101 Nearing End of Pipeline
DANA POINT, CALIF. – No longer just a pipe dream, a novel injectable treatment effective at reducing fat is approaching the end of the drug development process.
The product is a non–animal derived, pharmaceutical grade sodium deoxycholate that "acts much like a detergent. It attacks the cell membranes of fat, disrupting and disintegrating its membranes. Thereafter, fat cell contents are released and the subsequent host response, including inflammation, macrophage scavenging, and subclinical fibrosis, may confer a long-lasting fat-removal effect. It doesn’t shrink the fat cell; it ablates the fat it encounters," said, Dr. Adam M. Rotunda.
Phase III trials of an injectable form of sodium deoxycholate for the reduction of submental fat have begun in the United States, building on the previous success of four phase I, three phase II, and two phase III European studies of the product, known as ATX-101, noted Dr. Rotunda at the Summit in Aesthetic Medicine sponsored by the Skin Disease Education Foundation (SDEF).
In the 1990s, compounded phosphatidylcholine/deoxycholate injections (PC/DC), or lipodissolve, caused harm to some patients because "essentially everything about that experience was wrong: the wrong dose (too much volume, too high concentration); wrong depth (injections at times were too superficial. For example, cellulite, which was erroneously believed to improve from the injections, was injected intradermally); wrong indication (injections in places that shouldn’t have been injected); and wrong formulation," said Dr. Rotunda, who practices dermatology in Newport Beach, Calif. "These compounds were [derived] from animal sources and their sterility and purity could be called into question."
As a result, the FDA and other regulatory agencies around the world warned against or banned the use of unapproved PC/DC for aesthetic purposes. But in 2003, sodium deoxycholate was identified as the major component producing fat cell lysis in compounded PC/DC formulations, said Dr. Rotunda, who is also with the department of dermatology at the University of California, Los Angeles. "It’s taken many years to get that message across," he said.
ATX-101, which is being developed by Kythera Biopharmaceuticals, "is very different from the DC in compounded formulations, which was derived from cow bile," he said. "ATX-101 is being studied for very small volumes of fat relatively small for the submental area, which is really an ideal area to observe the desired effect of this product."
Phase I histology studies revealed that ATX-101 causes rapid adipocytolysis on day 1. On day 28 "there’s septal thickening and macrophage infiltration, which removes cellular debris and triglycerides, cleared via the lymphatic system," Dr. Rotunda said, adding that before and after MRIs have quantitatively confirmed the volume reductions.
Tissue surrounding the fatty treatment area, he continued, "has relatively high protein content. This protein (such as albumin) binds and inactivates deoxycholate, making subcutaneous injections relatively safe and fat specific. There’s an inverse relationship: the higher the protein content of certain tissue, such as muscle, tendon, and dermis, the lower the lytic activity of the deoxycholate. Less activity means less damage to that tissue. In fat, however, we want maximal damage, and it works out well because fat has relatively low protein content."
The administration of ATX-101 involves the use of a 30g needle, a 1-mL syringe, and placement of a temporary tattoo grid to the submental fat to control spacing of injections. "The distribution of the grid and how much is injected depends on the patient’s configuration and neck fat volume," Dr. Rotunda said. During the clinical trials, up to 10 mL of medication was used in each session, with up to four monthly treatments.
Most adverse events in clinical trials to date have been mild to moderate. "This is not a lunchtime procedure," he said. "It will be associated with local swelling and tenderness that may last days to several weeks, depending on the amount of ATX-101 injected or the volume of fat treated."
He concluded his remarks by noting that ATX-101 may become a novel approach that complements but does not compete with other fat-reduction therapies.
Dr. Rotunda disclosed that he, along with Dr. Michael S. Kolodney, are coinventors of ATX-101. He is a consultant to Kythera and holds stock in the company. He also is a consultant to Lithera and Allergan.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – No longer just a pipe dream, a novel injectable treatment effective at reducing fat is approaching the end of the drug development process.
The product is a non–animal derived, pharmaceutical grade sodium deoxycholate that "acts much like a detergent. It attacks the cell membranes of fat, disrupting and disintegrating its membranes. Thereafter, fat cell contents are released and the subsequent host response, including inflammation, macrophage scavenging, and subclinical fibrosis, may confer a long-lasting fat-removal effect. It doesn’t shrink the fat cell; it ablates the fat it encounters," said, Dr. Adam M. Rotunda.
Phase III trials of an injectable form of sodium deoxycholate for the reduction of submental fat have begun in the United States, building on the previous success of four phase I, three phase II, and two phase III European studies of the product, known as ATX-101, noted Dr. Rotunda at the Summit in Aesthetic Medicine sponsored by the Skin Disease Education Foundation (SDEF).
In the 1990s, compounded phosphatidylcholine/deoxycholate injections (PC/DC), or lipodissolve, caused harm to some patients because "essentially everything about that experience was wrong: the wrong dose (too much volume, too high concentration); wrong depth (injections at times were too superficial. For example, cellulite, which was erroneously believed to improve from the injections, was injected intradermally); wrong indication (injections in places that shouldn’t have been injected); and wrong formulation," said Dr. Rotunda, who practices dermatology in Newport Beach, Calif. "These compounds were [derived] from animal sources and their sterility and purity could be called into question."
As a result, the FDA and other regulatory agencies around the world warned against or banned the use of unapproved PC/DC for aesthetic purposes. But in 2003, sodium deoxycholate was identified as the major component producing fat cell lysis in compounded PC/DC formulations, said Dr. Rotunda, who is also with the department of dermatology at the University of California, Los Angeles. "It’s taken many years to get that message across," he said.
ATX-101, which is being developed by Kythera Biopharmaceuticals, "is very different from the DC in compounded formulations, which was derived from cow bile," he said. "ATX-101 is being studied for very small volumes of fat relatively small for the submental area, which is really an ideal area to observe the desired effect of this product."
Phase I histology studies revealed that ATX-101 causes rapid adipocytolysis on day 1. On day 28 "there’s septal thickening and macrophage infiltration, which removes cellular debris and triglycerides, cleared via the lymphatic system," Dr. Rotunda said, adding that before and after MRIs have quantitatively confirmed the volume reductions.
Tissue surrounding the fatty treatment area, he continued, "has relatively high protein content. This protein (such as albumin) binds and inactivates deoxycholate, making subcutaneous injections relatively safe and fat specific. There’s an inverse relationship: the higher the protein content of certain tissue, such as muscle, tendon, and dermis, the lower the lytic activity of the deoxycholate. Less activity means less damage to that tissue. In fat, however, we want maximal damage, and it works out well because fat has relatively low protein content."
The administration of ATX-101 involves the use of a 30g needle, a 1-mL syringe, and placement of a temporary tattoo grid to the submental fat to control spacing of injections. "The distribution of the grid and how much is injected depends on the patient’s configuration and neck fat volume," Dr. Rotunda said. During the clinical trials, up to 10 mL of medication was used in each session, with up to four monthly treatments.
Most adverse events in clinical trials to date have been mild to moderate. "This is not a lunchtime procedure," he said. "It will be associated with local swelling and tenderness that may last days to several weeks, depending on the amount of ATX-101 injected or the volume of fat treated."
He concluded his remarks by noting that ATX-101 may become a novel approach that complements but does not compete with other fat-reduction therapies.
Dr. Rotunda disclosed that he, along with Dr. Michael S. Kolodney, are coinventors of ATX-101. He is a consultant to Kythera and holds stock in the company. He also is a consultant to Lithera and Allergan.
SDEF and this news organization are owned by Elsevier.
DANA POINT, CALIF. – No longer just a pipe dream, a novel injectable treatment effective at reducing fat is approaching the end of the drug development process.
The product is a non–animal derived, pharmaceutical grade sodium deoxycholate that "acts much like a detergent. It attacks the cell membranes of fat, disrupting and disintegrating its membranes. Thereafter, fat cell contents are released and the subsequent host response, including inflammation, macrophage scavenging, and subclinical fibrosis, may confer a long-lasting fat-removal effect. It doesn’t shrink the fat cell; it ablates the fat it encounters," said, Dr. Adam M. Rotunda.
Phase III trials of an injectable form of sodium deoxycholate for the reduction of submental fat have begun in the United States, building on the previous success of four phase I, three phase II, and two phase III European studies of the product, known as ATX-101, noted Dr. Rotunda at the Summit in Aesthetic Medicine sponsored by the Skin Disease Education Foundation (SDEF).
In the 1990s, compounded phosphatidylcholine/deoxycholate injections (PC/DC), or lipodissolve, caused harm to some patients because "essentially everything about that experience was wrong: the wrong dose (too much volume, too high concentration); wrong depth (injections at times were too superficial. For example, cellulite, which was erroneously believed to improve from the injections, was injected intradermally); wrong indication (injections in places that shouldn’t have been injected); and wrong formulation," said Dr. Rotunda, who practices dermatology in Newport Beach, Calif. "These compounds were [derived] from animal sources and their sterility and purity could be called into question."
As a result, the FDA and other regulatory agencies around the world warned against or banned the use of unapproved PC/DC for aesthetic purposes. But in 2003, sodium deoxycholate was identified as the major component producing fat cell lysis in compounded PC/DC formulations, said Dr. Rotunda, who is also with the department of dermatology at the University of California, Los Angeles. "It’s taken many years to get that message across," he said.
ATX-101, which is being developed by Kythera Biopharmaceuticals, "is very different from the DC in compounded formulations, which was derived from cow bile," he said. "ATX-101 is being studied for very small volumes of fat relatively small for the submental area, which is really an ideal area to observe the desired effect of this product."
Phase I histology studies revealed that ATX-101 causes rapid adipocytolysis on day 1. On day 28 "there’s septal thickening and macrophage infiltration, which removes cellular debris and triglycerides, cleared via the lymphatic system," Dr. Rotunda said, adding that before and after MRIs have quantitatively confirmed the volume reductions.
Tissue surrounding the fatty treatment area, he continued, "has relatively high protein content. This protein (such as albumin) binds and inactivates deoxycholate, making subcutaneous injections relatively safe and fat specific. There’s an inverse relationship: the higher the protein content of certain tissue, such as muscle, tendon, and dermis, the lower the lytic activity of the deoxycholate. Less activity means less damage to that tissue. In fat, however, we want maximal damage, and it works out well because fat has relatively low protein content."
The administration of ATX-101 involves the use of a 30g needle, a 1-mL syringe, and placement of a temporary tattoo grid to the submental fat to control spacing of injections. "The distribution of the grid and how much is injected depends on the patient’s configuration and neck fat volume," Dr. Rotunda said. During the clinical trials, up to 10 mL of medication was used in each session, with up to four monthly treatments.
Most adverse events in clinical trials to date have been mild to moderate. "This is not a lunchtime procedure," he said. "It will be associated with local swelling and tenderness that may last days to several weeks, depending on the amount of ATX-101 injected or the volume of fat treated."
He concluded his remarks by noting that ATX-101 may become a novel approach that complements but does not compete with other fat-reduction therapies.
Dr. Rotunda disclosed that he, along with Dr. Michael S. Kolodney, are coinventors of ATX-101. He is a consultant to Kythera and holds stock in the company. He also is a consultant to Lithera and Allergan.
SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF SUMMIT IN AESTHETIC MEDICINE