User login
Translating the 2019 AAD-NPF Guidelines of Care for Psoriasis With Attention to Comorbidities
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
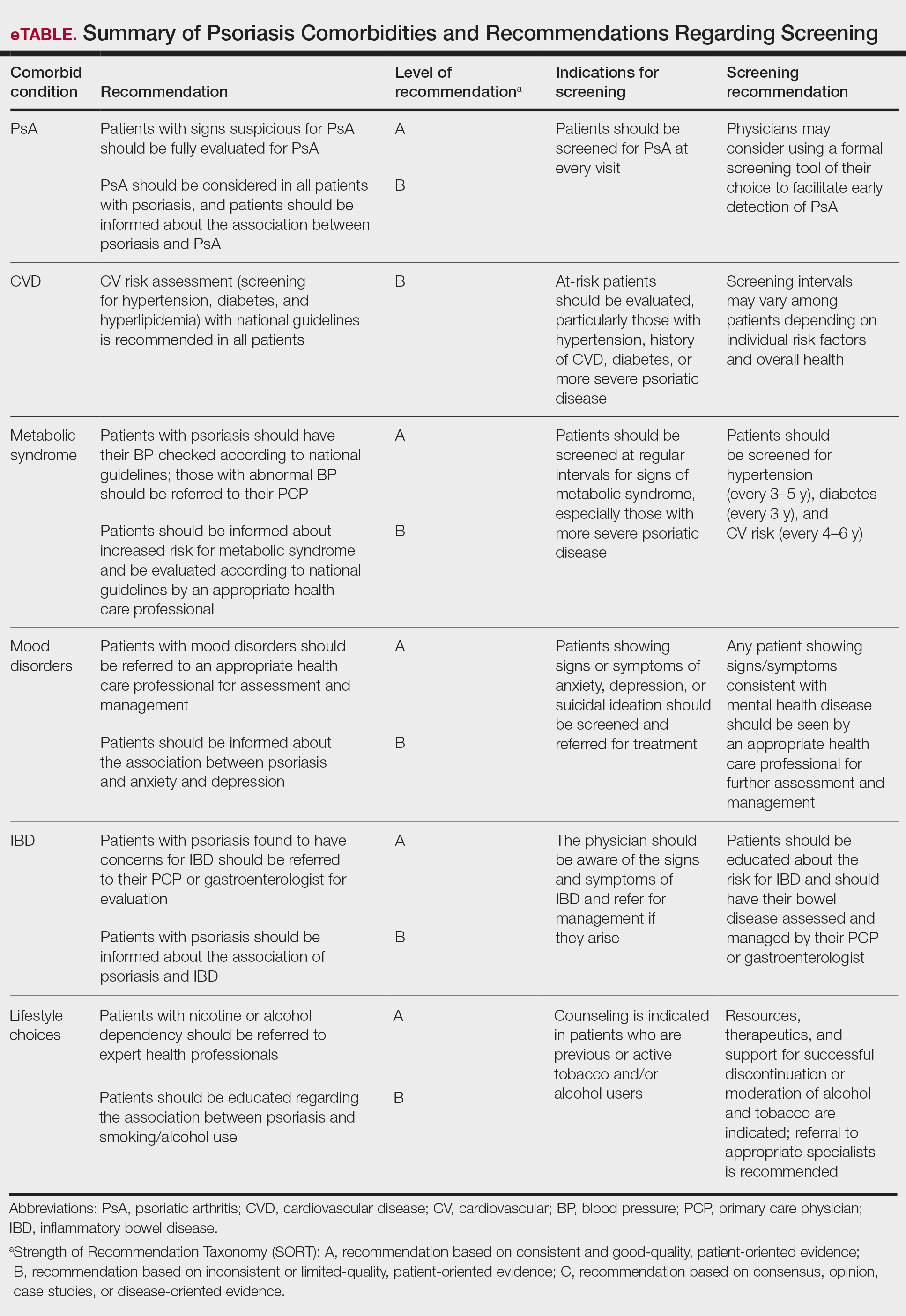
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.

Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Practice Points
- Educating patients about psoriasis and its extracutaneous manifestations, available treatment options, and the impact of lifestyle choices is advised to maximize their patient’s disease awareness and to promote a collaborative physician-patient partnership.
- Physicians are strongly recommended to screen patients with psoriasis for the presence of disease comorbidities to ensure comprehensive management of their disease.
- Managing psoriasis as a multisystem inflammatory disorder requires the combined effort of dermatologists and other specialists to prevent and treat disease comorbidities and enhance patients’ quality of life.
Update on Biologics for Psoriasis in Clinical Practice
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
Practice Points
- Choosing a biologic best fit for each patient’s individual health profile can reduce psoriasis disease burden.
- Clinicians should educate psoriasis patients that biologics are safe for most comorbidities, and conditions such as obesity have been associated with poorer therapeutic response.
- It is important to discuss possible side effects of biologics with patients and reassure them that mild side effects are the most common during therapy.
Home Phototherapy During the COVID-19 Pandemic
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
Practice Points
- Home phototherapy is a safe and effective option for patients with psoriasis during the coronavirus disease 2019 (COVID-19) pandemic.
- Although a consensus has not been reached with systemic immunosuppressive therapies for patients with psoriasis and the risk of COVID-19, we continue to recommend caution and careful monitoring of clinical outcomes for patients.
Translating the 2020 AAD-NPF Guidelines of Care for the Management of Psoriasis With Systemic Nonbiologics to Clinical Practice
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2
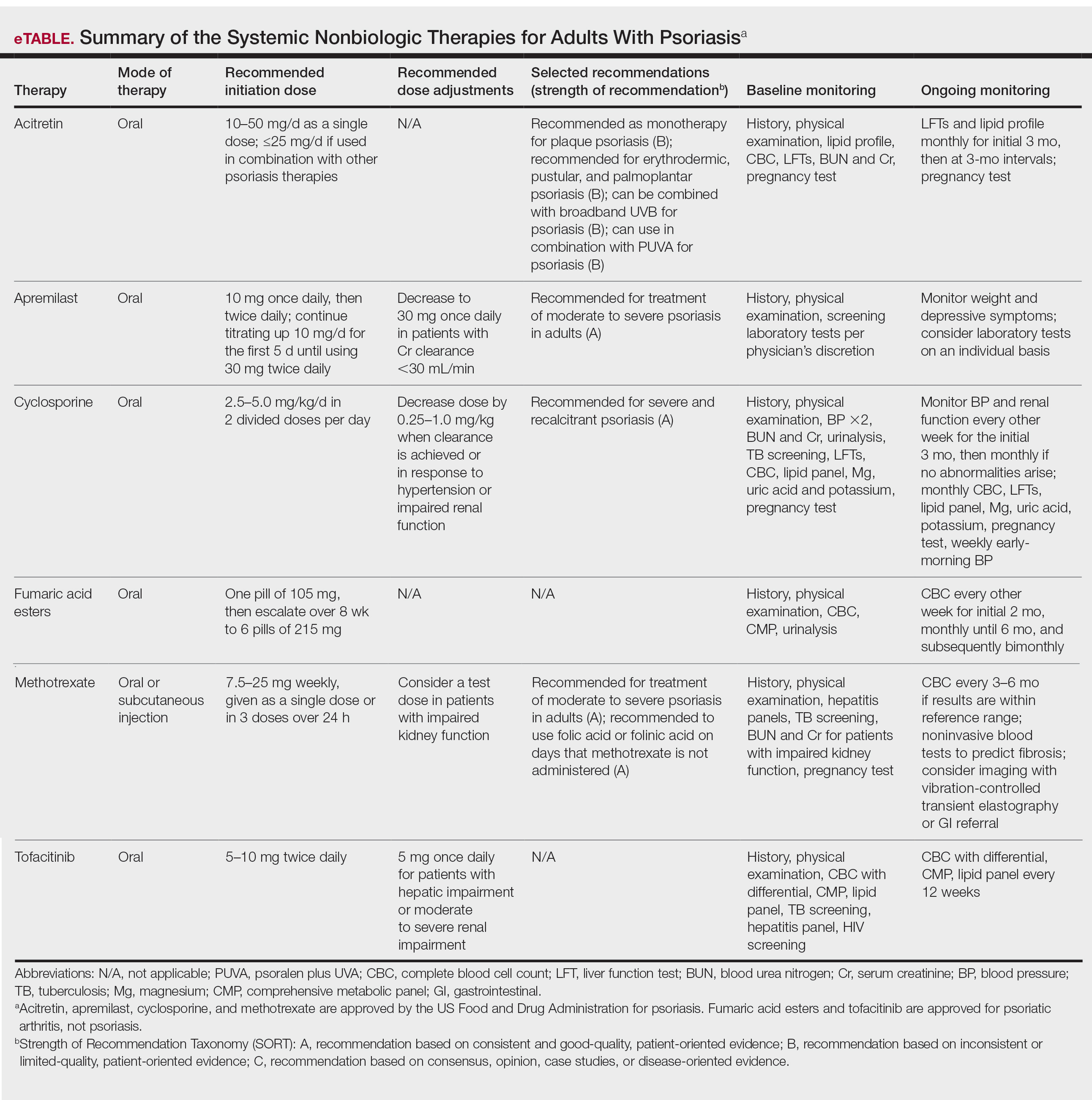
Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2

Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
Psoriasis is a chronic relapsing skin condition characterized by keratinocyte hyperproliferation and a chronic inflammatory cascade. Therefore, controlling inflammatory responses with systemic medications is beneficial in managing psoriatic lesions and their accompanying symptoms, especially in disease inadequately controlled by topicals. Ease of drug administration and treatment availability are benefits that systemic nonbiologic therapies may have over biologic therapies.
In 2020, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) published guidelines for managing psoriasis in adults with systemic nonbiologic therapies.1 Dosing, efficacy, toxicity, drug-related interactions, and contraindications are addressed alongside evidence-based treatment recommendations. This review addresses current recommendations for systemic nonbiologics in psoriasis with a focus on the treatments approved by the US Food and Drug Administration (FDA): acitretin, apremilast, cyclosporine, and methotrexate (eTable). Fumaric acid esters and tofacitinib are FDA approved for psoriatic arthritis but not for plaque psoriasis. Additional long-term safety analyses of tofacitinib for plaque psoriasis were requested by the FDA. Dimethyl fumarate is approved by the European Medicines Agency for treatment of psoriasis and is among the first-line systemic treatments used in Germany.2

Selecting a Systemic Nonbiologic Agent
Methotrexate and apremilast have a strength level A recommendation for treating moderate to severe psoriasis in adults. However, methotrexate is less effective than biologic agents, including adalimumab and infliximab, for cutaneous psoriasis. Methotrexate is believed to improve psoriasis because of its direct immunosuppressive effect and inhibition of lymphoid cell proliferation. It typically is administered orally but can be administered subcutaneously for decreased gastrointestinal (GI) adverse effects. Compliance with close laboratory monitoring and lifestyle modifications, such as contraceptive use (because of teratogenicity) and alcohol cessation (because of the risk of liver damage) are essential in patients using methotrexate.
Apremilast, the most recently FDA-approved oral systemic medication for psoriasis, inhibits phosphodiesterase 4, subsequently decreasing inflammatory responses involving helper T cells TH1 and TH17 as well as type 1 interferon pathways. Apremilast is particularly effective in treating psoriasis with scalp and palmoplantar involvement.3 Additionally, it has an encouraging safety profile and is favorable in patients with multiple comorbidities.
Among the 4 oral agents, cyclosporine has the quickest onset of effect and has a strength level A recommendation for treating severe and recalcitrant psoriasis. Because of its high-risk profile, it is recommended for short periods of time, acute flares, or during transitions to safer long-term treatment. Patients with multiple comorbidities should avoid cyclosporine as a treatment option.
Acitretin, an FDA-approved oral retinoid, is an optimal treatment option for immunosuppressed patients or patients with HIV on antiretroviral therapy because it is not immunosuppressive.4 Unlike cyclosporine, acitretin is less helpful for acute flares because it takes 3 to 6 months to reach peak therapeutic response for treating plaque psoriasis. Similar to cyclosporine, acitretin can be recommended for severe psoriatic variants of erythrodermic, generalized pustular, and palmoplantar psoriasis. Acitretin has been reported to be more effective and have a more rapid onset of action in erythrodermic and pustular psoriasis than in plaque psoriasis.5
Patient Comorbidities
Psoriatic arthritis (PsA) is a common comorbidity that affects treatment choice. Patients with coexisting PsA could be treated with apremilast, as it is approved for both psoriasis and PsA. In a phase 3 randomized, controlled trial, American College of Rheumatology (ACR) 20 response at weeks 16 and 52 was achieved by significantly more patients on apremilast at 20 mg twice daily (BID)(P=.0166) or 30 mg BID (P=.0001) than placebo.6 Although not FDA approved for PsA, methotrexate has been shown to improve concomitant PsA of the peripheral joints in patients with psoriasis. Furthermore, a trial of methotrexate has shown considerable improvements in PsA symptoms in patients with psoriasis—a 62.7% decrease in proportion of patients with dactylitis, 25.7% decrease in enthesitis, and improvements in ACR outcomes (ACR20 in 40.8%, ACR50 in 18.8%, and ACR70 in 8.6%, with 22.4% achieving minimal disease activity).7
Prior to starting a systemic medication for psoriasis, it is necessary to discuss effects on pregnancy and fertility. Pregnancy is an absolute contraindication for methotrexate and acitretin use because of the drugs’ teratogenicity. Fetal death and fetal abnormalities have been reported with methotrexate use in pregnant women.8 Bone, central nervous system, auditory, ocular, and cardiovascular fetal abnormalities have been reported with maternal acitretin use.9 Breastfeeding also is an absolute contraindication for methotrexate use, as methotrexate passes into breastmilk in small quantities. Patients taking acitretin also are strongly discouraged from nursing because of the long half-life (168 days) of etretinate, a reverse metabolism product of acitretin that is increased in the presence of alcohol. Women should wait 3 months after discontinuing methotrexate for complete drug clearance before conceiving compared to 3 years in women who have discontinued acitretin.8,10 Men also are recommended to wait 3 months after discontinuing methotrexate before attempting to conceive, as its effect on male spermatogenesis and teratogenicity is unclear. Acitretin has no documented teratogenic effect in men. For women planning to become pregnant, apremilast and cyclosporine can be continued throughout pregnancy on an individual basis. The benefit of apremilast should be weighed against its potential risk to the fetus. There is no evidence of teratogenicity of apremilast at doses of 20 mg/kg daily.11 Current research regarding cyclosporine use in pregnancy only exists in transplant patients and has revealed higher rates of prematurity and lower birth weight without teratogenic effects.10,12 The risks and benefits of continuing cyclosporine while nursing should be evaluated, as cyclosporine (and ethanol-methanol components used in some formulations) is detectable in breast milk.
Drug Contraindications
Hypersensitivity to a specific systemic nonbiologic medication is a contraindication to its use and is an absolute contraindication for methotrexate. Other absolute contraindications to methotrexate are pregnancy and nursing, alcoholism, alcoholic liver disease, chronic liver disease, immunodeficiency, and cytopenia. Contraindications to acitretin include pregnancy, severely impaired liver and kidney function, and chronic abnormally elevated lipid levels. There are no additional contraindications for apremilast, but patients must be informed of the risk for depression before initiating therapy. Cyclosporine is contraindicated in patients with prior psoralen plus UVA (PUVA) treatment or radiation therapy, abnormal renal function, uncontrolled hypertension, uncontrolled and active infections, and a history of systemic malignancy. Live vaccines should be avoided in patients on cyclosporine, and caution is advised when cyclosporine is prescribed for patients with poorly controlled diabetes.
Pretreatment Screening
Because of drug interactions, a detailed medication history is essential prior to starting any systemic medication for psoriasis. Apremilast and cyclosporine are metabolized by cytochrome P450 and therefore are more susceptible to drug-related interactions. Cyclosporine use can affect levels of other medications that are metabolized by cytochrome P450, such as statins, calcium channel blockers, and warfarin. Similarly, acitretin’s metabolism is affected by drugs that interfere with cytochrome P450. Additionally, screening laboratory tests are needed before initiating systemic nonbiologic agents for psoriasis, with the exception of apremilast.
Prior to initiating methotrexate treatment, patients may require tuberculosis (TB), hepatitis B, and hepatitis C screening tests, depending on their risk factors. A baseline liver fibrosis assessment is recommended because of the potential of hepatotoxicity in patients receiving methotrexate. Noninvasive serology tests utilized to evaluate the presence of pre-existing liver disease include Fibrosis-4, FibroMeter, FibroSure, and Hepascore. Patients with impaired renal function have an increased predisposition to methotrexate-induced hematologic toxicity. Thus, it is necessary to administer a test dose of methotrexate in these patients followed by a complete blood cell count (CBC) 5 to 7 days later. An unremarkable CBC after the test dose suggests the absence of myelosuppression, and methotrexate dosage can be increased weekly. Patients on methotrexate also must receive folate supplementation to reduce the risk for adverse effects during treatment.
Patients considering cyclosporine must undergo screening for family and personal history of renal disease. Prior to initiating treatment, patients require 2 blood pressure measurements, hepatitis screening, TB screening, urinalysis, serum creatinine (Cr), blood urea nitrogen (BUN), CBC, potassium and magnesium levels, uric acid levels, lipid profile, bilirubin, and liver function tests (LFTs). A pregnancy test also is warranted for women of childbearing potential (WOCP).
Patients receiving acitretin should receive screening laboratory tests consisting of fasting cholesterol and triglycerides, CBC, renal function tests, LFTs, and a pregnancy test, if applicable.
After baseline evaluations, the selected oral systemic can be initiated using specific dosing regimens to ensure optimal drug efficacy and reduce incidence of adverse effects (eTable).
Monitoring During Active Treatment
Physicians need to counsel patients on potential adverse effects of their medications. Because of its relatively safe profile among the systemic nonbiologic agents, apremilast requires the least monitoring during treatment. There is no required routine laboratory monitoring for patients using apremilast, though testing may be pursued at the clinician’s discretion. However, weight should be regularly measured in patients on apremilast. In a phase 3 clinical trial of patients with psoriasis, 12% of patients on apremilast experienced a 5% to 10% weight loss compared to 5% of patients on placebo.11,13 Thus, it is recommended that physicians consider discontinuing apremilast in patients with a weight loss of more than 5% from baseline, especially if it may lead to other unfavorable health effects. Because depression is reported among 1% of patients on apremilast, close monitoring for new or worsening symptoms of depression should be performed during treatment.11,13 To avoid common GI side effects, apremilast is initiated at 10 mg/d and is increased by 10 mg/d over the first 5 days to a final dose of 30 mg BID. Elderly patients in particular should be cautioned about the risk of dehydration associated with GI side effects. Patients with severe renal impairment (Cr clearance, <30 mL/min) should use apremilast at a dosage of 30 mg once daily.
For patients on methotrexate, laboratory monitoring is essential after each dose increase. It also is important for physicians to obtain regular blood work to assess for hematologic abnormalities and hepatoxicity. Patients with risk factors such as renal insufficiency, increased age, hypoalbuminemia, alcohol abuse and alcoholic liver disease, and methotrexate dosing errors, as well as those prone to drug-related interactions, must be monitored closely for pancytopenia.14,15 The protocol for screening for methotrexate-induced hepatotoxicity during treatment depends on patient risk factors. Risk factors for hepatoxicity include history of or current alcohol abuse, abnormal LFTs, personal or family history of liver disease, diabetes, obesity, use of other hepatotoxic drugs, and hyperlipidemia.16 In patients without blood work abnormalities, CBC and LFTs can be performed every 3 to 6 months. Patients with abnormally elevated LFTs require repeat blood work every 2 to 4 weeks. Persistent elevations in LFTs require further evaluation by a GI specialist. After a cumulative dose of 3.5 to 4 g, patients should receive a GI referral and further studies (such as vibration-controlled transient elastography or liver biopsy) to assess for liver fibrosis. Patients with signs of stage 3 liver fibrosis are recommended to discontinue methotrexate and switch to another medication for psoriasis. For patients with impaired renal function, periodic BUN and Cr monitoring are needed. Common adverse effects of methotrexate include diarrhea, nausea, and anorexia, which can be mitigated by taking methotrexate with food or lowering the dosage.8 Patients on methotrexate should be monitored for rare but potential risks of infection and reactivation of latent TB, hepatitis, and lymphoma. To reduce the incidence of methotrexate toxicity from drug interactions, a review of current medications at each follow-up visit is recommended.
Nephrotoxicity and hypertension are the most common adverse effects of cyclosporine. It is important to monitor BUN and Cr biweekly for the initial 3 months, then at monthly intervals if there are no persistent abnormalities. Patients also must receive monthly CBC, potassium and magnesium levels, uric acid levels, lipid panel, serum bilirubin, and LFTs to monitor for adverse effects.17 Physicians should obtain regular pregnancy tests in WOCP. Weekly monitoring of early-morning blood pressure is recommended for patients on cyclosporine to detect early cyclosporine-induced nephrotoxicity. Hypertension on 2 separate occasions warrants a reduction in cyclosporine dosage or an addition of a calcium channel blocker for blood pressure control. Dose reduction also should be performed in patients with an increase in Cr above baseline greater than 25%.17 If Cr level is persistently elevated or if blood pressure does not normalize to lower than 140/90 after dose reduction, cyclosporine should be immediately discontinued. Patients on cyclosporine for more than a year warrant an annual estimation of glomerular filtration rate because of irreversible kidney damage associated with long-term use. A systematic review of patients treated with cyclosporine for more than 2 years found that at least 50% of patients experienced a 30% increase in Cr above baseline.18
Patients taking acitretin should be monitored for hyperlipidemia, the most common laboratory abnormality seen in 25% to 50% of patients.19 Fasting lipid panel and LFTs should be performed monthly for the initial 3 months on acitretin, then at 3-month intervals. Lifestyle changes should be encouraged to reduce hyperlipidemia, and fibrates may be given to treat elevated triglyceride levels, the most common type of hyperlipidemia seen with acitretin. Acitretin-induced toxic hepatitis is a rare occurrence that warrants immediate discontinuation of the medication.20 Monthly pregnancy tests must be performed in WOCP.
Combination Therapy
For apremilast, there is anecdotal evidence supporting its use in conjunction with phototherapy or biologics in some cases, but no high-quality data.21 On the other hand, using combination therapy with other systemic therapies can reduce adverse effects and decrease the amount of medication needed to achieve psoriasis clearance. Methotrexate used with etanercept, for example, has been more effective than methotrexate monotherapy in treating psoriasis, which has been attributed to a methotrexate-mediated reduction in the production of antidrug antibodies.22,23
Methotrexate, cyclosporine, and acitretin have synergistic effects when used with phototherapy. Narrowband UVB (NB-UVB) phototherapy combined with methotrexate is more effective in clearing psoriasis than methotrexate or NB-UVB phototherapy alone. Similarly, acitretin and PUVA combination therapy is more effective than acitretin or PUVA phototherapy alone. Combination regimens of acitretin and broadband UVB phototherapy, acitretin and NB-UVB phototherapy, and acitretin and PUVA phototherapy also have been more effective than individual modalities alone. Combination therapy reduces the cumulative doses of both therapies and reduces the frequency and duration of phototherapy needed for psoriatic clearance.24 In acitretin combination therapy with UVB phototherapy, the recommended regimen is 2 weeks of acitretin monotherapy followed by UVB phototherapy. For patients with an inadequate response to UVB phototherapy, the UVB dose can be reduced by 30% to 50%, and acitretin 25 mg/d can be added to phototherapy treatment. Acitretin-UVB combination therapy has been shown to reduce the risk of UVB-induced erythema seen in UVB monotherapy. Similarly, the risk of squamous cell carcinoma is reduced in acitretin-PUVA combination therapy compared to PUVA monotherapy.25
The timing of phototherapy in combination with systemic nonbiologic agents is critical. Phototherapy used simultaneously with cyclosporine is contraindicated owing to increased risk of photocarcinogenesis, whereas phototherapy used in sequence with cyclosporine is well tolerated and effective. Furthermore, cyclosporine 3 mg/kg/d for 4 weeks followed by a rapid cyclosporine taper and initiation of NB-UVB phototherapy demonstrated resolution of psoriasis with fewer NB-UVB treatments and less UVB exposure than NB-UVB therapy alone.26
Final Thoughts
The FDA-approved systemic nonbiologic agents are accessible and effective treatment options for adults with widespread or inadequately controlled psoriasis. Selecting the ideal therapy requires careful consideration of medication toxicity, contraindications, monitoring requirements, and patient comorbidities. The AAD-NPF guidelines guide dermatologists in prescribing systemic nonbiologic treatments in adults with psoriasis. Utilizing these recommendations in combination with clinician judgment will help patients achieve safe and optimal psoriasis clearance.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486.
- Mrowietz U, Barker J, Boehncke WH, et al. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: a European expert consensus. J Eur Acad Dermatol Venereol. 2018;32(suppl 3):3-14.
- Van Voorhees AS, Gold LS, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83:96-103.
- Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
- Ormerod AD, Campalani E, Goodfield MJD. British Association of Dermatologists guidelines on the efficacy and use of acitretin in dermatology. Br J Dermatol. 2010;162:952-963.
- Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42:479-488.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Antares Pharma, Inc. Otrexup PFS (methotrexate) [package insert]. US Food and Drug Administration website. Revised June 2019. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204824s009lbl.pdf
- David M, Hodak E, Lowe NJ. Adverse effects of retinoids. Med Toxicol Adverse Drug Exp. 1988;3:273-288.
- Stiefel Laboratories, Inc. Soriatane (acitretin) [package insert]. US Food and Drug Administration website. Revised September 2017. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019821s028lbl.pdf
- Celgene Corporation. Otezla (apremilast) [package insert]. US Food and Drug Administration website. Revised March 2014. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205437s000lbl.pdf
- Ghanem ME, El-Baghdadi LA, Badawy AM, et al. Pregnancy outcome after renal allograft transplantation: 15 years experience. Eur J Obstet Gynecol Reprod Biol. 2005;121:178-181.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Kivity S, Zafrir Y, Loebstein R, et al. Clinical characteristics and risk factors for low dose methotrexate toxicity: a cohort of 28 patients. Autoimmun Rev. 2014;13:1109-1113.
- Boffa MJ, Chalmers RJ. Methotrexate for psoriasis. Clin Exp Dermatol. 1996;21:399-408.
- Rosenberg P, Urwitz H, Johannesson A, et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J Hepatol. 2007;46:1111-1118.
- Novartis Pharmaceuticals Corporation. Sandimmune (cyclosporine) [package insert]. US Food and Drug Administration website. Published 2015. Accessed February 28, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/050573s041,050574s051,050625s055lbl.pdf
- Maza A, Montaudie H, Sbidian E, et al. Oral cyclosporin in psoriasis: a systematic review on treatment modalities, risk of kidney toxicity and evidence for use in non-plaque psoriasis. J Eur Acad Dermatol Venereol. 2011;25(suppl 2):19-27.
- Yamauchi PS, Rizk D, Kormilli T, et al. Systemic retinoids. In: Weinstein GD, Gottlieb AB, eds. Therapy of Moderate-to-Severe Psoriasis. Marcel Dekker; 2003:137-150.
- van Ditzhuijsen TJ, van Haelst UJ, van Dooren-Greebe RJ, et al. Severe hepatotoxic reaction with progression to cirrhosis after use of a novel retinoid (acitretin). J Hepatol. 1990;11:185-188.
- AbuHilal M, Walsh S, Shear N. Use of apremilast in combination with other therapies for treatment of chronic plaque psoriasis: a retrospective study. J Cutan Med Surg. 2016;20:313-316.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167:649-657.
- Cronstein BN. Methotrexate BAFFles anti-drug antibodies. Nat Rev Rheumatol. 2018;14:505-506.
- Lebwohl M, Drake L, Menter A, et al. Consensus conference: acitretin in combination with UVB or PUVA in the treatment of psoriasis. J Am Acad Dermatol. 2001;45:544-553.
- Nijsten TE, Stern RS. Oral retinoid use reduces cutaneous squamous cell carcinoma risk in patients with psoriasis treated with psoralen-UVA: a nested cohort study. J Am Acad Dermatol. 2003;49:644-650.
- Calzavara-Pinton P, Leone G, Venturini M, et al. A comparative non randomized study of narrow-band (NB) (312 +/- 2 nm) UVB phototherapy versus sequential therapy with oral administration of low-dose cyclosporin A and NB-UVB phototherapy in patients with severe psoriasis vulgaris. Eur J Dermatol. 2005;15:470-473.
Practice Points
- Systemic nonbiologic therapies are effective treatments for adults with psoriasis. The benefits of these treatments include ease of administration and the ability to control widespread disease.
- When selecting a therapy, a thorough evaluation of patient characteristics and commitment to lifestyle adjustments is necessary, including careful consideration in women of childbearing potential and those with plans of starting a family.
- Regular drug monitoring and patient follow-up is crucial to ensure safe dosing adjustments and to mitigate potential adverse effects.
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis in Pediatric Patients
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).
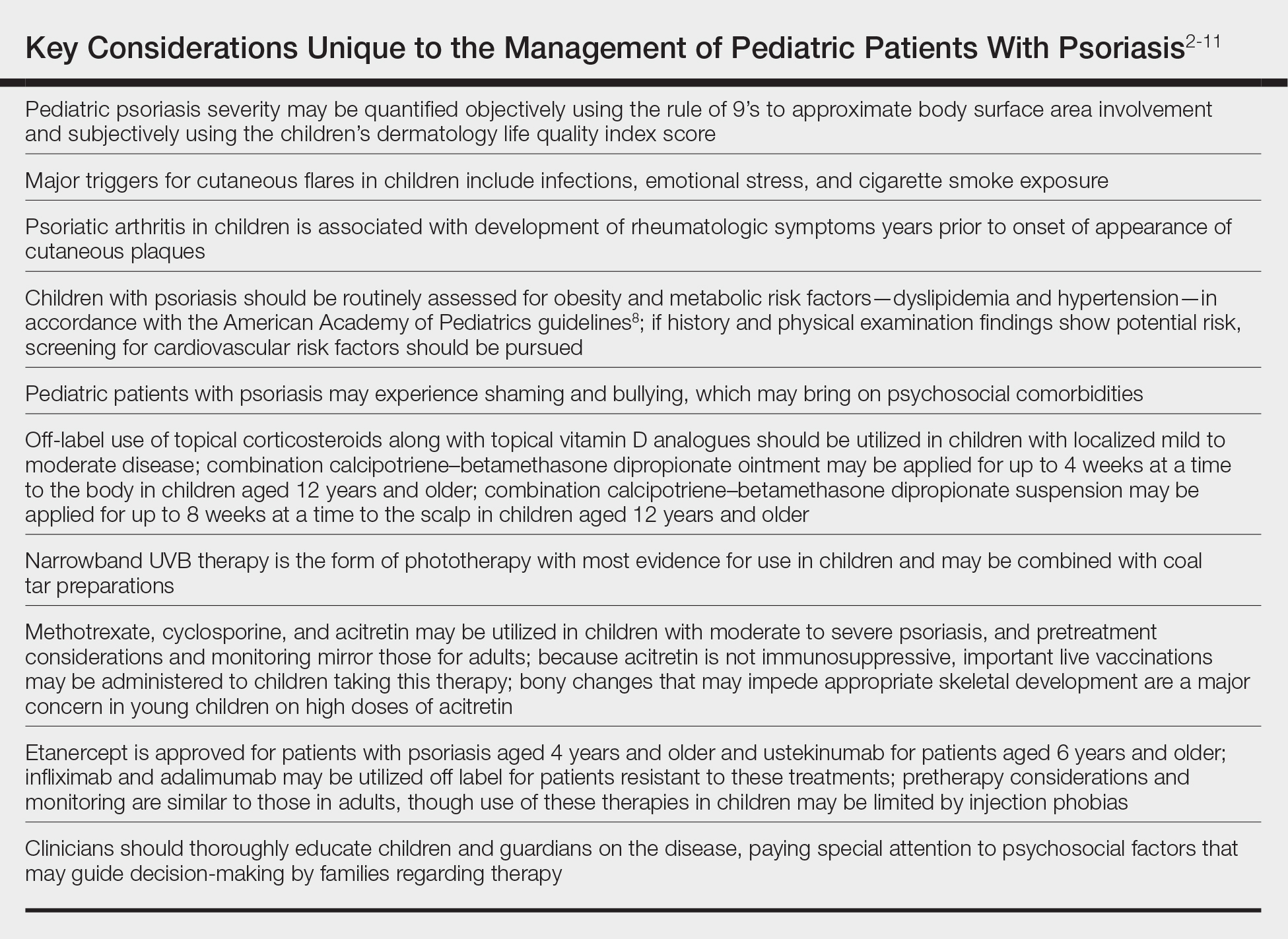
Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.
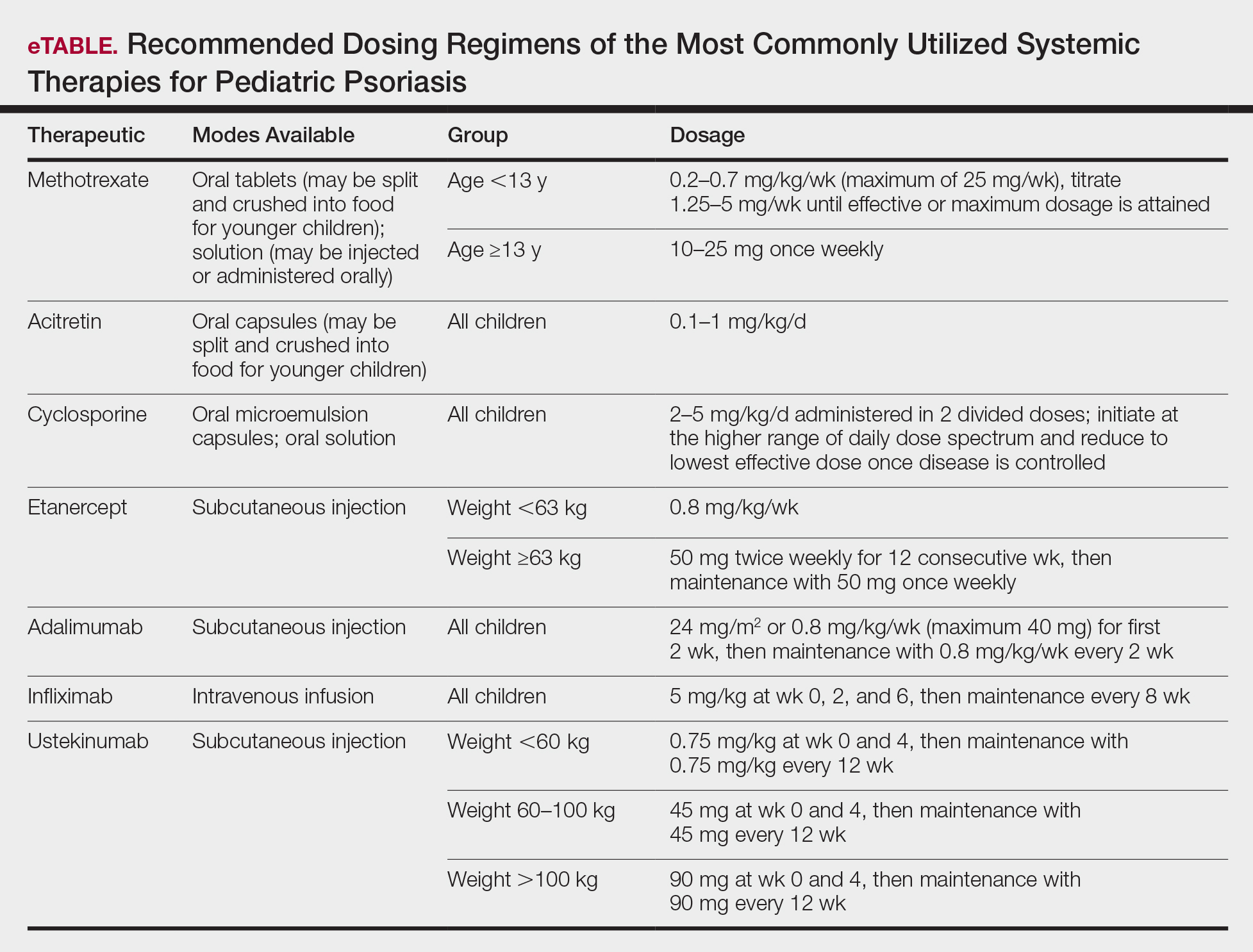
Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).

Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.

Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
In November 2019, the American Academy of Dermatology (AAD) and the National Psoriasis Foundation (NPF) released their first set of recommendations for the management of pediatric psoriasis.1 The pediatric guidelines discuss methods of quantifying disease severity in children, triggers and comorbidities, and the efficacy and safety of various therapeutic agents. This review aims to discuss, in a condensed form, special considerations unique to the management of children with psoriasis as presented in the guidelines as well as grade A– and grade B–level treatment recommendations (Table).

Quantifying Psoriasis Severity in Children
Percentage body surface area (BSA) involvement is the most common mode of grading psoriasis severity, with less than 3% BSA involvement being considered mild, 3% to 10% BSA moderate, and more than 10% severe disease. In children, the standard method of measuring BSA is the rule of 9’s: the head and each arm make up 9% of the total BSA, each leg and the front and back of the torso respectively each make up 18%, and the genitalia make up 1%. It also is important to consider impact on quality of life, which may be remarkable in spite of limited BSA involvement. The children’s dermatology life quality index score may be utilized in combination with affected BSA to determine the burden of psoriasis in context of impact on daily life. This metric is available in both written and cartoon form, and it consists of 10 questions that include variables such as severity of itch, impact on social life, and effects on sleep. Most notably, this tool incorporates pruritus,2 which generally is addressed inadequately in pediatric psoriasis.
Triggers and Comorbidities in Pediatric Patients
In children, it is important to identify and eliminate modifiable factors that may prompt psoriasis flares. Infections, particularly group A beta-hemolytic streptococcal infections, are a major trigger in neonates and infants. Other exacerbating factors in children include emotional stress, secondhand cigarette smoke, Kawasaki disease, and withdrawal from systemic corticosteroids.
Psoriatic arthritis (PsA) is a burdensome comorbidity affecting children with psoriasis. The prevalence of joint disease is 15-times greater in children with psoriasis vs those without,3 and 80% of children with PsA develop rheumatologic symptoms, which typically include oligoarticular disease and dactylitis in infants and girls and enthesitis and axial joint involvement in boys and older children, years prior to the onset of cutaneous disease.4 Uveitis often occurs in children with psoriasis and PsA but not in those with isolated cutaneous disease.
Compared to unaffected children, pediatric patients with psoriasis have greater prevalence of metabolic and cardiovascular risk factors during childhood, including central obesity, hypertension, hypertriglyceridemia, hypercholesterolemia, insulin resistance, atherosclerosis, arrythmia, and valvular heart disease. Family history of obesity increases the risk for early-onset development of cutaneous lesions,5,6 and weight reduction may alleviate severity of psoriasis lesions.7 In the United States, many of the metabolic associations observed are particularly robust in Black and Hispanic children vs those of other races. Furthermore, the prevalence of inflammatory bowel disease is 3- to 4-times higher in children with psoriasis compared to those without.
As with other cutaneous diseases, it is important to be aware of social and mental health concerns in children with psoriasis. The majority of pediatric patients with psoriasis experience name-calling, shaming, or bullying, and many have concerns from skin shedding and malodor. Independent risk for depression after the onset of psoriasis is high. Affected older children and adolescents are at increased risk for alcohol and drug abuse as well as eating disorders.
Despite these identified comorbidities, there are no unique screening recommendations for arthritis, ophthalmologic disease, metabolic disease, cardiovascular disease, gastrointestinal tract disease, or mental health issues in children with psoriasis. Rather, these patients should be monitored according to the American Academy of Pediatrics or American Diabetes Association guidelines for all pediatric patients.8,9 Nonetheless, educating patients and guardians about these potential issues may be warranted.
Topical Therapies
For children with mild to moderate psoriasis, topical therapies are first line. Despite being off label, topical corticosteroids are the mainstay of therapy for localized psoriatic plaques in children. Topical vitamin D analogues—calcitriol and calcipotriol/calcipotriene—are highly effective and well tolerated, and they frequently are used in combination with topical corticosteroids. Topical calcineurin inhibitors, namely tacrolimus, also are used off label but are considered first line for sensitive regions of the skin in children, including the face, genitalia, and body folds. There currently is limited evidence for supporting the use of the topical vitamin A analogue tazarotene in children with psoriasis, though some consider its off-label use effective for pediatric nail psoriasis. It also may be used as an adjunct to topical corticosteroids to minimize irritation.
Although there is no gold standard topical regimen, combination therapy with a high-potency topical steroid and topical vitamin D analogue commonly is used to minimize steroid-induced side effects. For the first 2 weeks of treatment, they each may be applied once daily or mixed together and applied twice daily. For subsequent maintenance, topical calcipotriene may be applied on weekdays and topical steroids only on weekends. Combination calcipotriol–betamethasone dipropionate also is available as cream, ointment, foam, and suspension vehicles for use on the body and scalp in children aged 12 years and older. Tacrolimus ointment 0.1% may be applied in a thin layer up to twice daily. Concurrent emollient use also is recommended with these therapies.
Health care providers should educate patients and guardians about the potential side effects of topical therapies. They also should provide explicit instructions for amount, site, frequency, and duration of application. Topical corticosteroids commonly result in burning on application and may potentially cause skin thinning and striae with overuse. Topical vitamin D analogues may result in local irritation that may be improved by concurrent emollient use, and they generally should be avoided on sensitive sites. Topical calcineurin inhibitors are associated with burning, stinging, and pruritus, and the US Food and Drug Administration has issued a black-box warning related to risk for lymphoma with their chronic intermittent use. However, it was based on rare reports of lymphoma in transplant patients taking oral calcineurin inhibitors; no clinical trials to date in humans have demonstrated an increased risk for malignancy with topical calcineurin inhibitors.10 Tazarotene should be used cautiously in females of childbearing age given its teratogenic potential.
Children younger than 7 years are especially prone to suppression of the hypothalamic-pituitary-adrenal axis from topical corticosteroid therapy and theoretically hypercalcemia and hypervitaminosis D from topical vitamin D analogues, as their high BSA-to-volume ratio increases potential for systemic absorption. Children should avoid occlusive application of topical vitamin D analogues to large areas of the skin. Monitoring of vitamin D metabolites in the serum may be considered if calcipotriene or calcipotriol application to a large BSA is warranted.
Light-Based Therapy
In children with widespread psoriasis or those refractory to topical therapy, phototherapy may be considered. Narrowband UVB (311- to 313-nm wavelength) therapy is considered a first-line form of phototherapy in pediatric psoriasis. Mineral oil or emollient pretreatment to affected areas may augment the efficacy of UV-based treatments.11 Excimer laser and UVA also may be efficacious, though evidence is limited in children. Treatment is recommended to start at 3 days a week, and once improvement is seen, the frequency can be decreased to 2 days a week. Once desired clearance is achieved, maintenance therapy can be continued at even longer intervals. Adjunctive use of tar preparations may potentiate the efficacy of phototherapy, though there is a theoretical increased risk for carcinogenicity with prolonged use of coal tar. Side effects of phototherapy include erythema, blistering hyperpigmentation, and pruritus. Psoralen is contraindicated in children younger than 12 years. All forms of phototherapy are contraindicated in children with generalized erythroderma and cutaneous cancer syndromes. Other important pediatric-specific considerations include anxiety that may be provoked by UV light machines and inconvenience of frequent appointments.
Nonbiologic Systemic Therapies
Systemic therapies may be considered in children with recalcitrant, widespread, or rapidly progressing psoriasis, particularly if the disease is accompanied by severe emotional and psychological burden. These drugs, which include methotrexate, cyclosporine, and acitretin (see eTable for recommended dosing), are advantageous in that they may be combined with other therapies; however, they have potential for dangerous toxicities.

Methotrexate is the most frequently utilized systemic therapy for psoriasis worldwide in children because of its low cost, once-weekly dosing, and the substantial amount of long-term efficacy and safety data available in the pediatric population. It is slow acting initially but has excellent long-term efficacy for nearly every subtype of psoriasis. The most common side effect of methotrexate is gastrointestinal tract intolerance. Nonetheless, adverse events are rare in children without prior history, with 1 large study (N=289) reporting no adverse events in more than 90% of patients aged 9 to 14 years treated with methotrexate.12 Current guidelines recommend monitoring for bone marrow suppression and elevated transaminase levels 4 to 6 days after initiating treatment.1 The absolute contraindications for methotrexate are pregnancy and liver disease, and caution should be taken in children with metabolic risk factors. Adolescents must be counseled regarding the elevated risk for hepatotoxicity associated with alcohol ingestion. Methotrexate therapy also requires 1 mg folic acid supplementation 6 to 7 days a week, which decreases the risk for developing folic acid deficiency and may decrease gastrointestinal tract intolerance and hepatic side effects that may result from therapy.
Cyclosporine is an effective and well-tolerated option for rapid control of severe psoriasis in children. It is useful for various types of psoriasis but generally is reserved for more severe subtypes, such as generalized pustular psoriasis, erythrodermic psoriasis, and uncontrolled plaque psoriasis. Long-term use of cyclosporine may result in renal toxicity and hypertension, and this therapy is absolutely contraindicated in children with kidney disease or hypertension at baseline. It is strongly recommended to evaluate blood pressure every week for the first month of therapy and at every subsequent follow-up visit, which may occur at variable intervals based on the judgement of the provider. Evaluation before and during treatment with cyclosporine also should include a complete blood cell count, complete metabolic panel, and lipid panel.
Systemic retinoids have a unique advantage over methotrexate and cyclosporine in that they are not immunosuppressive and therefore are not contraindicated in children who are very young or immunosuppressed. Children receiving systemic retinoids also can receive routine live vaccines—measles-mumps-rubella, varicella zoster, and rotavirus—that are contraindicated with other systemic therapies. Acitretin is particularly effective in pediatric patients with diffuse guttate psoriasis, pustular psoriasis, and palmoplantar psoriasis. Narrowband UVB therapy has been shown to augment the effectiveness of acitretin in children, which may allow for reduced acitretin dosing. Pustular psoriasis may respond as quickly as 3 weeks after initiation, whereas it may take 2 to 3 months before improvement is noticed in plaque psoriasis. Side effects of retinoids include skin dryness, hyperlipidemia, and gastrointestinal tract upset. The most severe long-term concern is skeletal toxicity, including premature epiphyseal closure, hyperostosis, periosteal bone formation, and decreased bone mineral density.1 Vitamin A derivatives also are known teratogens and should be avoided in females of childbearing potential. Lipids and transaminases should be monitored routinely, and screening for depression and psychiatric symptoms should be performed frequently.1
When utilizing systemic therapies, the objective should be to control the disease, maintain stability, and ultimately taper to the lowest effective dose or transition to a topical therapy, if feasible. Although no particular systemic therapy is recommended as first line for children with psoriasis, it is important to consider comorbidities, contraindications, monitoring frequency, mode of administration (injectable therapies elicit more psychological trauma in children than oral therapies), and expense when determining the best choice.
Biologics
Biologic agents are associated with very high to total psoriatic plaque clearance rates and require infrequent dosing and monitoring. However, their use may be limited by cost and injection phobias in children as well as limited evidence for their efficacy and safety in pediatric psoriasis. Several studies have established the safety and effectiveness of biologics in children with plaque psoriasis (see eTable for recommended dosing), whereas the evidence supporting their use in treating pustular and erythrodermic variants are limited to case reports and case series. The tumor necrosis factor α (TNF-α) inhibitor etanercept has been approved for use in children aged 4 years and older, and the IL-12/IL-23 inhibitor ustekinumab is approved in children aged 6 years and older. Other TNF-α inhibitors, namely infliximab and adalimumab, commonly are utilized off label for pediatric psoriasis. The most common side effect of biologic therapies in pediatric patients is injection-site reactions.1 Prior to initiating therapy, children must undergo tuberculosis screening either by purified protein derivative testing or IFN-γ release assay. Testing should be repeated annually in individuals taking TNF-α inhibitors, though the utility of repeat testing when taking biologics in other classes is not clear. High-risk patients also should be screened for human immunodeficiency virus and hepatitis. Follow-up frequency may range from every 3 months to annually, based on judgement of the provider. In children who develop loss of response to biologics, methotrexate can be added to the regimen to attenuate formation of efficacy-reducing antidrug antibodies.
Final Thoughts
When managing children with psoriasis, it is important for dermatologists to appropriately educate guardians and children on the disease course, as well as consider the psychological, emotional, social, and financial factors that may direct decision-making regarding optimal therapeutics. Dermatologists should consider collaboration with the child’s primary care physician and other specialists to ensure that all needs are met.
These guidelines provide a framework agreed upon by numerous experts in pediatric psoriasis, but they are limited by gaps in the research. There still is much to be learned regarding the pathophysiology of psoriasis; the risk for developing comorbidities during adulthood; and the efficacy and safety of certain therapeutics, particularly biologics, in pediatric patients with psoriasis.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients [published online November 5, 2019]. J Am Acad Dermatol. 2020;82:161-201.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942-949.
- Augustin M, Radtke MA, Glaeske G, et al. Epidemiology and comorbidity in children with psoriasis and atopic eczema. Dermatology. 2015;231:35-40.
- Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153:698-704.
- Boccardi D, Menni S, La Vecchia C, et al. Overweight and childhood psoriasis. Br J Dermatol. 2009;161:484-486.
- Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
- Alotaibi HA. Effects of weight loss on psoriasis: a review of clinical trials. Cureus. 2018;10:E3491.
- Guidelines summaries—American Academy of Pediatrics. Guideline Central
website. https://www.guidelinecentral.com/summaries/organizations/american-academy-of-pediatrics/2019. Accessed October 27, 2020. - Standards of Medical Care in Diabetes. American Diabetes Association website. https://care.diabetesjournals.org/content/43/Supplement_1. Published January 1, 2020. Accessed May 8, 2020.
- Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14:163-178.
- Jain VK, Bansal A, Aggarwal K, et al. Enhanced response of childhood psoriasis to narrow-band UV-B phototherapy with preirradiation use of mineral oil. Pediatr Dermatol. 2008;25:559-564.
- Ergun T, Seckin Gencosmanoglu D, Alpsoy E, et al. Efficacy, safety and drug survival of conventional agents in pediatric psoriasis: a multicenter, cohort study. J Dermatol. 2017;44:630-634.
Practice Points
- For children, several environmental factors may prompt psoriasis flares, and it is critical to identify and eliminate these triggers.
- Although the use of biologics may be limited by cost and injection phobias in children, they may be an appropriate option for children with moderate to severe psoriasis when other therapies have failed. A growing body of literature is establishing the safety and effectiveness of biologics in children.
- Clinicians should thoroughly educate parents/ guardians on the course of psoriasis and treatment options as well as pay special attention to treatment goals and psychosocial factors that may guide decision-making regarding therapy.
Dermatologists as Social Media Contributors During the COVID-19 Pandemic
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
On December 31, 2019, cases of a severe pneumonia in patients in Wuhan, Hubei Province, China, were reported to the World Health Organization.1,2 The novel coronavirus—severe acute respiratory syndrome coronavirus 2—was identified, and the coronavirus disease 2019 (COVID-19) became a public health emergency of international concern.1 In March 2020, the World Health Organization officially characterized COVID-19 as a pandemic.3 As of October 2020, more than 42.3 million cases and 1.1 million deaths from COVID-19 have been confirmed worldwide.4
As more understanding of severe acute respiratory syndrome coronavirus 2 develops, various cutaneous manifestations of COVID-19 are being uncovered.5 The most common cutaneous manifestations of COVID-19 reported in the literature are maculopapular or morbilliform exanthem (36.1% of cutaneous manifestations), papulovesicular rash (34.7%), painful acral red purple papules (15.3%), urticaria (9.7%), livedo reticularis lesions (2.8%), and petechiae (1.4%).5
Interestingly, a series of unique cases was identified in April 2020 by a group of dermatologists in Spain. Most patients were children (median age, 13 years) or young adults (median age, 31 years; average age, 36 years; adult age range, 18–91 years).1 Reporting on a representative sample of 6 patients in that series, the group noted that lesions, initially reddish, papular, and resembling chilblains (pernio), progressively became purpuric and flattened in the course of 1 week. Although the lesions presented with some referred discomfort or pain with palpation, they were not highly symptomatic, and no signs of ischemia or Raynaud syndrome were identified. Over time, lesions self-resolved without intervention. Most patients also did not present with what are considered classic COVID-19 signs or symptoms. Only the oldest patient (aged 91 years) presented with a notable respiratory condition; the remaining patients generally were in good health.1 Dermatologists in Italy, France, and the United States also have witnessed these COVID-19–associated cutaneous manifestations.
Scientific understanding of COVID-19 and its associated dermatologic symptoms is evolving. Attention has turned to social media to inform and provide possible health solutions during this unprecedented medical crisis.6 Strict physical distancing measures have made patients and providers alike reliant on global digital social networks, such as Instagram, Twitter, and Facebook, to facilitate information sharing about COVID-19.7 The abundance of nonexpert advice and misinformation on social media makes communication of unbiased expert information difficult.8,9 Furthermore, there is a need for dermatologists to provide medical information to patients regarding COVID-19, such as dermatologic manifestations, and clear guidance on immunobiologic or systemic medications during this unprecedented time.9
In recent years, dermatologists have established a growing presence on social media, with many recognized as social media influencers with the ability to affect patients’ health-related behavior.10 Social media frequently has been used by patients to solicit advice regarding skin concerns.9,10 Many individuals, in fact, never see a physician after consulting social media for medical concerns or professional advice.9
In addition, as of March 2020, more than 61% of health care workers were found to use social media as a source of COVID-19–related information.11 Therefore, dermatologists should utilize social media as a platform to share evidence-based information with the public and other health care workers.
Through social media, dermatologists can post high-quality images with clear descriptions to fully characterize skin manifestations in patients with COVID-19. The process of capturing and posting images to the virtual world using a smartphone allows practitioners to gain advice from peers and consultants, share findings with colleagues, and inform the public.12 Social media posts of many deidentified clinical images of rashes in COVID-19–infected patients already have enabled rapid recognition of skin signs by dermatologists.13
Social media sites also are resources where organizations can post updated, evidence-based findings from academic journals. For example, the American Academy of Dermatology and its official journal, the Journal of the American Academy of Dermatology, had more than 22,000 and 27,000 Instagram followers, respectively, as of a March 2020 analysis.14 Recent online forums and social media posts contain accessible, graphical, patient-friendly images and information on evidence-based treatments for skin disease during the COVID-19 pandemic.13
We should consider initiatives that empower dermatologists to use social media to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during this medical crisis. We hope that dermatologists will help lead the global response to the COVID-19 pandemic and contribute to the evolving knowledge base by characterizing COVID-19–related rashes, understanding their implications, and determining the best evidence for treatment.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
- Landa N, Mendieta-Eckert M, Fonda-Pascual P, et al. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739-743.
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709-710.
- World Health Organization. Coronavirus disease (COVID-19) Situation Report – 133. WHO Website. June 1, 2020. www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed October 14, 2020.
- COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at John Hopkins University. John Hopkins Coronavirus Resource Center website. https://coronavirus.jhu.edu/map.html. Accessed October 24, 2020.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatolog Sci. 2020;98:75-81.
- Kapoor A, Guha S, Kanti Das M, et al. Digital healthcare: the only solution for better healthcare during COVID-19 pandemic? Indian Heart J. 2020;72:61-64.
- Limaye RJ, Sauer M, Ali J, et al. Building trust while influencing online COVID-19 content in the social media world. Lancet Digit Health. 2020;2:E277-E278.
- Chawla S. COVID-19: challenges and opportunities for dermatology response. J Dermatolog Treat. 2020;31:326-326.
- Schoenberg E, Shalabi D, Wang JV, et al. Public social media consultations for dermatologic conditions: an online survey. Dermatol Online J. 2020;26:6.
- DeBord LC, Patel V, Braun TL, et al. Social media in dermatology: clinical relevance, academic value, and trends across platforms. J Dermatolog Treat. 2019;30:511-518.
- Bhagavathula AS, Aldhaleei WA, Rahmani J, et al. Knowledge and perceptions of COVID-19 among health care workers: cross-sectional study. JMIR Public Health Surveill. 2020;6:E19160.
- Ashique KT, Kaliyadan F, Aurangabadkar SJ. Clinical photography in dermatology using smartphones: an overview. Indian Dermatol Online J. 2015;6:158-163.
- Madigan LM, Micheletti RG, Shinkai K. How dermatologists can learn and contribute at the leading edge of the COVID-19 global pandemic. JAMA Dermatol. 2020;156:733-734.
- Guzman AK, Barbieri JS. Response to: “Dermatologists in social media: a study on top influencers, posts, and user engagement” [published online April 20, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.118.
Practice Points
- With the coronavirus disease 2019 (COVID-19) pandemic, strict physical distancing measures have made patients and providers alike reliant on global digital social networks such as Instagram, Twitter, and Facebook to facilitate information sharing about COVID-19.
- Dermatologists should utilize social media as a platform to post unbiased, evidence-based information regarding manifestations of COVID-19 and guidelines for treatment of skin disease during the global pandemic.
Risk for Deep Fungal Infections During IL-17 and IL-23 Inhibitor Therapy for Psoriasis
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.
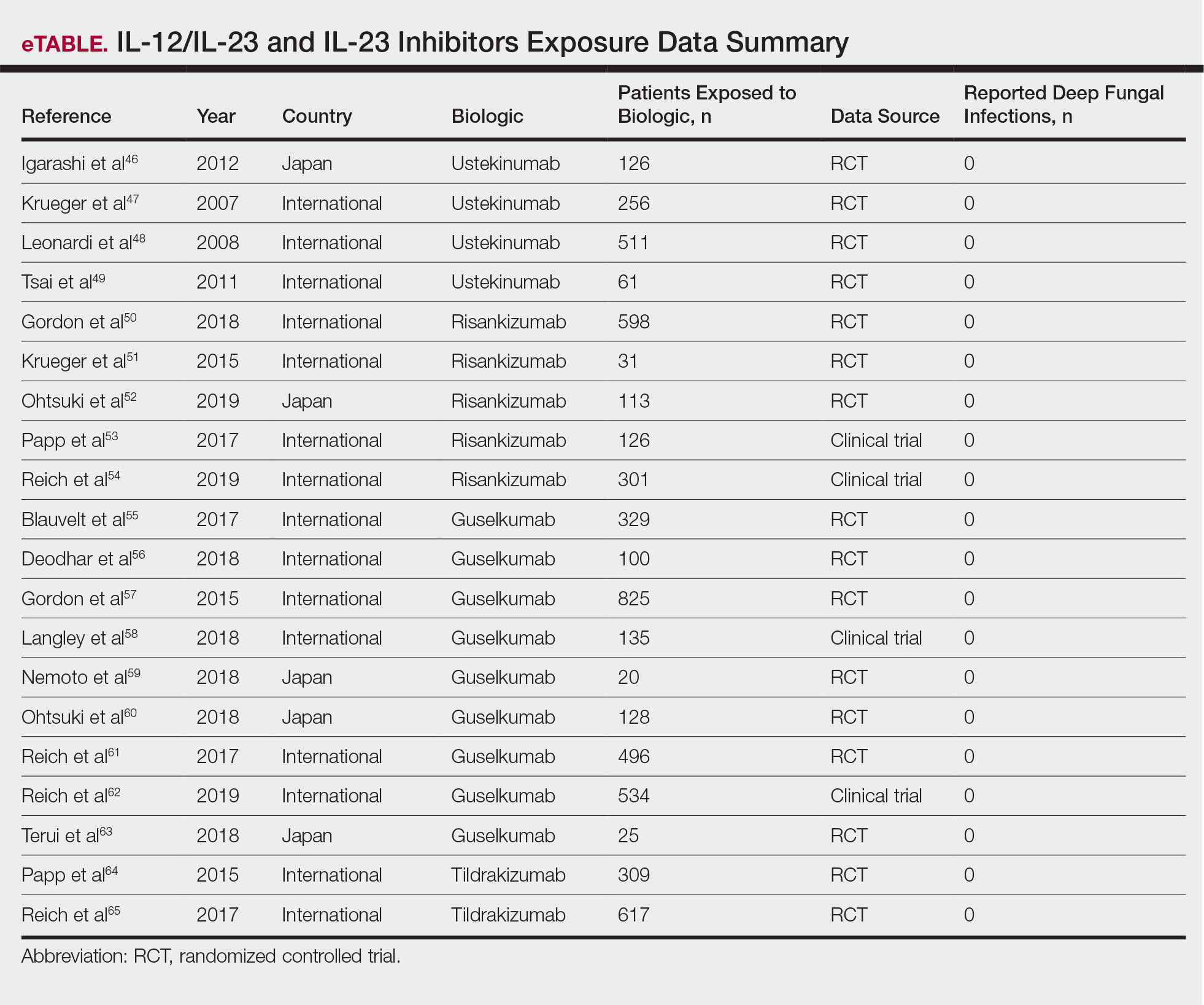
IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.

IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
Psoriasis is a common chronic, multisystem, inflammatory disease with predominantly skin and joint manifestations that affects approximately 2% of the world’s population.1 It occurs in a variety of clinical forms, from a few well-demarcated, erythematous plaques with a silvery scale to involvement of almost the entire body surface area. Beyond the debilitating physical ailments of the disease, psoriasis also may have psychosocial effects on quality of life.2 The pathogenesis of psoriasis is not fully understood but represents a complex multifactorial disease with both immune-mediated and genetic components. Characterized by hyperplasia of epidermal keratinocytes, psoriasis is shown to be mediated by infiltration of T-cell lymphocytes with an increase of various inflammatory cytokines, including
With the growing understanding of the pathophysiology of psoriasis, focused biologics have been developed to target specific cytokines implicated in the disease process and have been increasingly utilized. Tumor necrosis factor α inhibitors, including adalimumab, infliximab, and etanercept, along with the IL-12/IL-23 inhibitor ustekinumab, have been revolutionary in psoriasis treatment by providing safe and effective long-term therapy; however, there is concern of life-threatening infections with biologics because of the immunosuppressive effects and mechanisms of action.6 Specifically, there have been reported cases of deep fungal infections associated with TNF-α inhibitor use.7
Recently, the advent of IL-17 and IL-23 inhibitors has garnered notable interest in these biologics as promising treatments for psoriasis. With IL-17 and IL-23 supported to have a major role in the pathogenesis of psoriasis, targeting the cytokine is not only logical but also has proven to be effacacious.8-10 Secukinumab, ixekizumab, and brodalumab are IL-17 inhibitors that have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis. Secukinumab and ixekizumab are anti–IL-17A monoclonal antibodies, whereas brodalumab is an anti–IL-17 receptor antibody. Risankizumab, guselkumab, and tildrakizumab are IL-23 inhibitors that also have been approved by the FDA for the treatment of psoriasis. As with older biologics, there is concern over the safety of these inhibitors because of the central role of IL-17 and IL-23 in both innate and adaptive immune responses, particularly against fungi.11 Therefore, use of biologics targeting IL-17 and IL-23 may increase susceptibility to deep fungal infections.
Safety data and discussion of the risk for deep fungal infections from IL-17, IL-12/IL-23, and IL-23 inhibitor use for psoriasis treatment currently are lacking. Given the knowledge gap, we sought to synthesize and review the current evidence on risks for deep fungal infections during biologic therapy in patients with psoriasis, with a focus on IL-17 inhibitor therapies.
METHODS
A PubMed search of articles indexed for MEDLINE from database inception to 2019 (1946-2019) was performed to find randomized controlled trials (RCTs), including extended trials and clinical trials, for IL-17, IL-12/IL-23, and IL-23 inhibitors approved by the FDA for psoriasis treatment. The following keywords were used: psoriasis or inflammatory disease and secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Studies were restricted to the English-language literature, and those that did not provide adequate safety data on the specific types of infections that occurred were excluded.
RESULTSIL-17 Inhibitors
Our search yielded RCTs, some including extension trials, and clinical trials of IL-17 inhibitors used for psoriatic disease and other nonpsoriatic conditions (Table).

Risk for Deep Fungal Infection With Secukinumab
The queried studies included 20 RCTs or clinical trials along with extension trials of 3746 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In a 3-year extension study of SCULPTURE, Bissonnette et al12 reported no new safety concerns for the 340 patients with moderate to severe psoriasis treated with secukinumab. Common adverse events (AEs) included nasopharyngitis, upper respiratory tract infections, and headache, but there were no reports of deep fungal infections.12 In a subsequent 5-year analysis of 168 patients that focused on the 300-mg fixed interval treatment with secukinumab, the safety profile remained favorable, with 0 reports of invasive fungal infections.13 A study (FEATURE) of 118 patients with psoriasis treated with a prefilled syringe of 300 or 150 mg of secukinumab also described an acceptable safety profile and reported no deep fungal infections.14 JUNCTURE, another study utilizing autoinjectors, also found that treatment with 300 or 150 mg of secukinumab was well tolerated in 121 patients, with no deep fungal infections.15 Common AEs for both studies included nasopharyngitis and headache.14,15 A 24-week phase 3 study for scalp psoriasis treated with secukinumab also reported 0 deep fungal infections in 51 patients.16 In an RCT comparing secukinumab and ustekinumab for moderate to severe plaque psoriasis, Blauvelt et al17 demonstrated that the incidence of serious AEs was comparable between the 2 groups, with no reports of invasive fungal infections in the 334 patients exposed to secukinumab. The CLEAR study, which compared secukinumab and ustekinumab, also found no reported deep fungal disease in the 335 patients exposed to secukinumab.18 Secukinumab exhibited a similar safety profile to ustekinumab in both studies, with common AEs being headache and nasopharyngitis.17,18 The GESTURE study investigated the efficacy of secukinumab in 137 patients with palmoplantar psoriasis and reported a favorable profile with no reports of deep fungal disease.19 In a subanalysis of the phase 3 study ERASURE, secukinumab was shown to have a robust and sustainable efficacy in 58 Japanese patients with moderate to severe plaque psoriasis, and there were no reports of invasive fungal infections.20 Another subanalysis of 36 Taiwanese patients from the ERASURE study also had similar findings, with no dose relationship observed for AEs.21 In a phase 2 study of 103 patients with psoriasis, Papp et al22 demonstrated AE rates that were similar across different doses of secukinumab—3×150 mg, 3×75 mg, 3×25 mg, and 1×25 mg—and described no incidences of invasive fungal disease. In a phase 2 regimen-finding study of 337 patients conducted by Rich et al,23 the most commonly reported AEs included nasopharyngitis, worsening psoriasis, and upper respiratory tract infections, but there were no reported deep fungal infections.
Our search also resulted in studies specific to the treatment of psoriatic arthritis (PsA) with secukinumab. McInnes et al9 conducted a phase 2 proof-of-concept trial for patients with PsA and reported no deep fungal infections in 28 patients exposed to 10 mg/kg of secukinumab. A 2-year follow-up with the cohort from FUTURE 1, a phase 3 clinical trial, also showed no new or unexpected safety signals in 404 patients exposed to 150 or 75 mg of secukinumab, including no reports of invasive fungal disease.24 FUTURE 2, a phase 3 clinical trial, demonstrated that the most common AE was upper respiratory tract infection in the 299 patients treatedwith secukinumab, but there were no recorded invasive fungal infections.25 In FUTURE 3, 277 patients were treated with secukinumab, with 14 nonserious candida infections but no observed deep fungal infections.26 A study comparing secukinumab to fumaric acid esters reported that 6 of 105 patients treated with secukinumab also experienced superficial candidiasis, but there were no reports of deep fungal disease.27
Secukinumab also has been used in the treatment of ankylosing spondylitis in a phase 3 RCT (MEASURE 1) in which 4 cases of superficial candidiasis were reported (0.7 cases per 100 patient-years of secukinumab) that were all resolved with standard antifungal therapy.28 In MEASURE 2, a 5-year phase 3 RCT, 145 patients were treated with secukinumab for ankylosing spondylitis, with common AEs including nasopharyngitis, diarrhea, and upper respiratory tract infection, but there were no reports of any invasive fungal infections.29 MEASURE 3 also demonstrated similar results in which no invasive fungal infections were observed.30
Risk for Deep Fungal Infection With Ixekizumab
The queried studies included 7 RCTs or clinical trials of 3523 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 52 weeks. In UNCOVER-A, a phase 3 RCT of the pharmacokinetics and safety of ixekizumab, 204 patients were randomized to a prefilled syringe or autoinjector; 48% of patients experienced AEs, but no invasive fungal infections were observed.31 In an analysis of 3 phase 3 trials of ixekizumab including a total 2334 patients treated with ixekizumab from UNCOVER-1, UNCOVER-2, and UNCOVER-3, oral candidiasis frequently was reported, but no candidal infections met criteria for serious invasive infection.32 In UNCOVER-J, a 52-week phase 3 open-label trial of Japanese patients, 91 patients were treated for plaque psoriasis, erythrodermic psoriasis, or generalized pustular psoriasis using ixekizumab; the most common AEs included allergic reactions and injection-site reactions. One case of oral candidiasis was reported, but there were no reported cases of invasive fungal infections.33 A comparison of ixekizumab vs ustekinumab from the IXORA-S trial demonstrated no substantial differences in AEs between the two, and no cases of deep fungal infections were reported. The most common AE between the 2 groups was nasopharyngitis.34 An open-label extension over 4 years of a phase 2 RCT treated 211 patients with either 120 or 80 mg of ixekizumab; 87% of patients had experienced at least 1 AE, and all AEs were considered mild or moderate in severity, with no invasive fungal disease.35
Our search also resulted in 1 study specific to the treatment of PsA with ixekizumab. A phase 3, 52-week study of patients treated with ixekizumab for PsA observed 2 incidences of oral candidiasis and nail candida infections, but no invasive fungal infections were reported.36
We also found 1 study of ixekizumab used in the treatment of ankylosing spondylitis. COAST-V was a phase 3 RCT of patients treated for ankylosing spondylitis in which 164 patients were treated with ixekizumab; no serious AEs were recorded, including 0 deep fungal infections. The most common AEs observed were nasopharyngitis and upper respiratory tract infections.37
Risk for Deep Fungal Infection With Brodalumab
The queried studies included 9 RCTs and 3 clinical trials along with extension trials of 1599 patients with psoriasis or other inflammatory conditions, with follow-up ranging from 12 to 120 weeks. In a phase 2 RCT of Japanese patients with moderate to severe plaque psoriasis, 113 patients were treated with 70, 140, or 210 mg of brodalumab, and the most common AEs were nasopharyngitis, diarrhea, and upper respiratory tract inflammation. There were no reported cases of fungal infections in the study.38 In an open-label extension study of Japanese patients that evaluated the long-term clinical safety of brodalumab, 145 patients were enrolled and observed similar AEs to the RCT, with 7 patients experiencing oral candidiasis and 1 patient having skin candidiasis, but there were no observed deep fungal infections.39 In AMG 827, which evaluated the efficacy and safety of brodalumab, 320 patients were treated, and only 2 serious AEs were reported, neither of which were deep fungal disease.10 A phase 3 RCT conducted by Papp et al40 (AMAGINE-1) also treated 441 patients with moderate to severe plaque psoriasis with brodalumab and observed candida infections in 9 patients that were mild to moderate and responsive to treatment, with no patients discontinuing the study. In a 120-week open-label extension study of 181 patients, Papp et al41 reported 8% of patients experienced serious AEs, with 1 case of latent tuberculosis that led to withdrawal of treatment. A study also investigated the efficacy and safety of brodalumab in 30 patients with generalized pustular psoriasis or psoriatic erythroderma and observed 2 cases of mild candida infections that resolved with treatment. There were no reports of invasive fungal disease.42
Our search also resulted in studies of brodalumab used in the treatment of PsA and nonpsoriatic diseases. In one phase 2 RCT, 113 patients with PsA were treated with 140 mg, 280 mg, or combined doses of brodalumab, with the most common AEs being nasopharyngitis, upper respiratory tract infection, and diarrhea, but there were no reports of deep fungal infection.43 In a phase 1b trial of patients with methotrexate-resistant rheumatoid arthritis treated with brodalumab, common AEs reported included headache, cough, and abdominal pain, with only 1 case of oral candidiasis that was determined not to be drug related.44 Finally, an RCT of patients with moderate to severe asthma treated 226 patients with brodalumab and reported a greater incidence of oral candidiasis in treatment groups compared with placebo (3.5% vs 0%) but saw no instances of invasive fungal infection.45
IL-12/IL-23 Inhibitor
Risk for Deep Fungal Infection With Ustekinumab
The queried studies included 4 RCTs of 954 patients with psoriasis treated with ustekinumab (eTable).46-49 Within these trials, there were no reported cases of serious infections involving deep fungal organisms during the stated follow-up period. The literature search also found long-term safety data from the ACCEPT and PHOENIX trials that included 5437 patients with psoriasis treated with ustekinumab.66,67 There also were no demonstrated incidences of invasive fungal disease in these studies, with most cases of infection being common bacterial or viral infections.

IL-23 Inhibitors
Risk for Deep Fungal Infection With Risankizumab, Guselkumab, and Tildrakizumab
The queried studies included 16 RCTs or clinical trials for psoriatic patients treated with IL-23 inhibitors, including 5 with risankizumab,50-54 9 with guselkumab,55-63 and 2 with tildrakizumab.64,65 Within these trials there were no observed cases of serious infections with deep fungal disease.
COMMENT
Our literature review has demonstrated that there does not appear to be an increased incidence of deep fungal infections for patients treated with IL-17, IL-12/IL-23, or IL-23 inhibitors for psoriatic disease. All of the reviewed studies found no cases of invasive fungal infections for patients with psoriasis treated with secukinumab, ixekizumab, brodalumab, ustekinumab, risankizumab, guselkumab, or tildrakizumab. Patients with other inflammatory conditions, such as ankylosing spondylitis, rheumatoid arthritis, and asthma, also did not appear to show an increased incidence of deep fungal disease.
Although these results show promising safety data for the use of these biologic therapies in treating inflammatory conditions, caution still is warranted, as these medications still are relatively new, with FDA approvals within the last 5 years. Safety data among different study populations also cannot be derived without further investigation, and much of the available literature is limited in long-term data. More extended trials or registry data from a large, broadly representative cohort are necessary to establish the long-term safety and risk for deep fungal infections with IL-17 and especially the newer IL-23 inhibitors.
A small percentage of patients from the reviewed literature did develop superficial candidiasis. This outcome can be expected, as the central role of IL-17 and IL-23 has been recognized in immunologic protection against infections, specifically against fungi.11 Because all of the fungal infections reported for patients on IL-17 inhibitors were superficial candidiasis, guides for practical management and treatment should be implemented to standardize future research and care. A proposed screening algorithm for patients on these biologic therapies involves safety monitoring, including inspection of the oral cavity, folds, and genitals, along with inquiring about symptoms such as burning, dysgeusia, and dysuria.68 If infection is suspected, confirmation by culture, molecular method, or optimally with esophagoscopy can be performed, and appropriate treatment may be initiated.68 Patients with candida infections of the oral cavity, folds, or genitals can be placed on topical therapy such as nystatin, amphotericin B, ciclopirox, or other azoles, while those with infections of the esophagus can be started on oral fluconazole.68
Although there were no reported cases of deep fungal infections, the theoretical risk for developing one while on IL-17 and IL-23 inhibitors may warrant further screening prior to beginning therapy. The TNF inhibitors approved for the treatment of psoriasis currently contain a black box warning for risk for disseminated and extrapulmonary histoplasmosis, coccidioidomycosis, blastomycosis, and other invasive fungal infections, which may highlight the importance of thorough evaluation and awareness of endemic areas for patients on biologics. Prior to initiating treatment with TNF inhibitors, current suggestions involve performing a thorough examination along with keeping a high index of suspicion for invasive fungal infections in patients who live in or have traveled to endemic regions.69
Screening for invasive fungal infections for patients on TNF inhibitors involves questioning about potential exposures, such as demolition of old buildings, bird roosts, or spelunking.70 Serologies or antigen testing can be used routinely, but as these tests are insensitive, empiric antifungal therapy should be initiated if there is high enough clinical suspicion.71 Currently, there are no clinical guidelines regarding fungal screening and initiation of IL-17 and IL-23 inhibitors for treatment of psoriasis and other inflammatory conditions, but careful stewardship over using these effective medications should still be practiced.
Upon review of the available safety data on the use of IL-17 and IL-23 inhibitors for the treatment of psoriasis and other inflammatory conditions, there does not appear to be an increased incidence of deep fungal infections. Physicians, however, should still be cautiously optimistic in prescribing these medications, as there is a theoretical risk for infection for all patients on biologics. A high index of suspicion for patients presenting with symptoms of fungal infections should be maintained, and appropriate diagnosis and management should be initiated if they do occur.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
- Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Koo J, Marangell LB, Nakamura M, et al. Depression and suicidality in psoriasis: review of the literature including the cytokine theory of depression. J Eur Acad Dermatol Venereol. 2017;31:1999-2009.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 (suppl 2):ii30-36.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Shear NH. Fulfilling an unmet need in psoriasis: do biologicals hold the key to improved tolerability? Drug Saf. 2006;29:49-66.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565-2570.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2014;73:349-356.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181-1189.
- Isailovic N, Daigo K, Mantovani A, et al. Interleukin-17 and innate immunity in infections and chronic inflammation. J Autoimmun. 2015;60:1-11.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033-1042.
- Bissonnette R, Luger T, Thaci D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172:484-493.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29:1082-1090.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: Results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77:667-674.
- Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60.e9-69.e9.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400-409.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76:70-80.
- Ohtsuki M, Morita A, Abe M, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039-1046.
- Wu NL, Hsu CJ, Sun FJ, et al. Efficacy and safety of secukinumab in Taiwanese patients with moderate to severe plaque psoriasis: subanalysis from ERASURE phase III study. J Dermatol. 2017;44:1129-1137.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168:412-421.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402-411.
- Kavanaugh A, Mease PJ, Reimold AM, et al. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res (Hoboken). 2017;69:347-355.
- McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137-1146.
- Nash P, Mease PJ, McInnes IB, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20:47.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177:1024-1032.
- Braun J, Baraliakos X, Deodhar A, et al. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2017;76:1070-1077.
- Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3:e000592.
- Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19:285.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31:107-113.
- Gordon KB, Colombel JF, Hardin DS. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:2102.
- Saeki H, Nakagawa H, Nakajo K, et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017;44:355-362.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177:1014-1023.
- Zachariae C, Gordon K, Kimball AB, et al. Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis. J Am Acad Dermatol. 2018;79:294.e6-301.e6.
- van der Heijde D, Gladman DD, Kishimoto M, et al. Efficacy and safety of ixekizumab in patients with active psoriatic arthritis: 52-week results from a phase III study (SPIRIT-P1). J Rheumatol. 2018;45:367-377.
- van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392:2441-2451.
- Nakagawa H, Niiro H, Ootaki K, et al. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of Japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44-52.
- Umezawa Y, Nakagawa H, Niiro H, et al. Long-term clinical safety and efficacy of brodalumab in the treatment of Japanese patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2016;30:1957-1960.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183.e3-1190.e3.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370:2295-2306.
- Martin DA, Churchill M, Flores-Suarez L, et al. A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther. 2013;15:R164.
- Busse WW, Holgate S, Kerwin E, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294-1302.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242-252.
- Krueger GG, Langley RG, Leonardi C, et al. A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356:580-592.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in Taiwanese and Korean patients (PEARL). J Dermatol Sci. 2011;63:154-163.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116.e7-124.e7.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in Japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46:686-694.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Deodhar A, Gottlieb AB, Boehncke WH, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2018;391:2213-2224.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in Japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178:689-696.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in Japanese patients: Efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45:1053-1062.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394:831-839.
- Terui T, Kobayashi S, Okubo Y, et al. Efficacy and safety of guselkumab, an anti-interleukin 23 monoclonal antibody, for palmoplantar pustulosis: a randomized clinical trial. JAMA Dermatol. 2018;154:309-316.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Papp KA, Griffiths CE, Gordon K, et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br J Dermatol. 2013;168:844-854.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Lis K, Kuzawinska O, Balkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Hage CA, Bowyer S, Tarvin SE, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85-92
- Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448-454.
Practice Points
- The use of IL-17, IL-12/IL-23, and IL-23 inhibitors for psoriasis and other inflammatory conditions does not appear to increase the risk for deep fungal infections.
- Physicians should still be cautiously optimistic in prescribing these medications, as IL-17 and IL-23 play a central role in immunologic defenses, particularly against fungi.
- A high index of suspicion should be maintained for patients from endemic areas who are being treated with biologics.
Management of Psoriasis With Biologics in Clinical Practice: An Update for 2020
The advent of biologic therapy over the last 2 decades has transformed the treatment of psoriasis; patients who either are not good candidates for or have an inadequate response to traditional treatments (topicals and/or phototherapy) now have numerous options for treatment.1 Patients burdened by extensive disease, recurrent flares, and stubborn treatment areas are ideal candidates for biologics. There are 11 biologics approved by the US Food and Drug Administration (FDA)(Table) for treating moderate to severe plaque psoriasis as supported by grade A evidence. The FDA has authorized 1 new biologic—risankizumab—since the joint guidelines from the American Academy of Dermatology and National Psoriasis Foundation were released for the treatment of psoriasis with biologics.2 This article aims to address updates on recent clinical trial findings (April 2019 to April 2020) regarding biologic therapy initiation and maintenance for adult patients. Prescribers should use this update as guidance for determining the appropriate biologic class based on patient characteristics and for approaching biologic-experienced patients with refractory psoriasis. This update also may serve as a reference for the recommended dosing regimens of the 11 approved biologics.
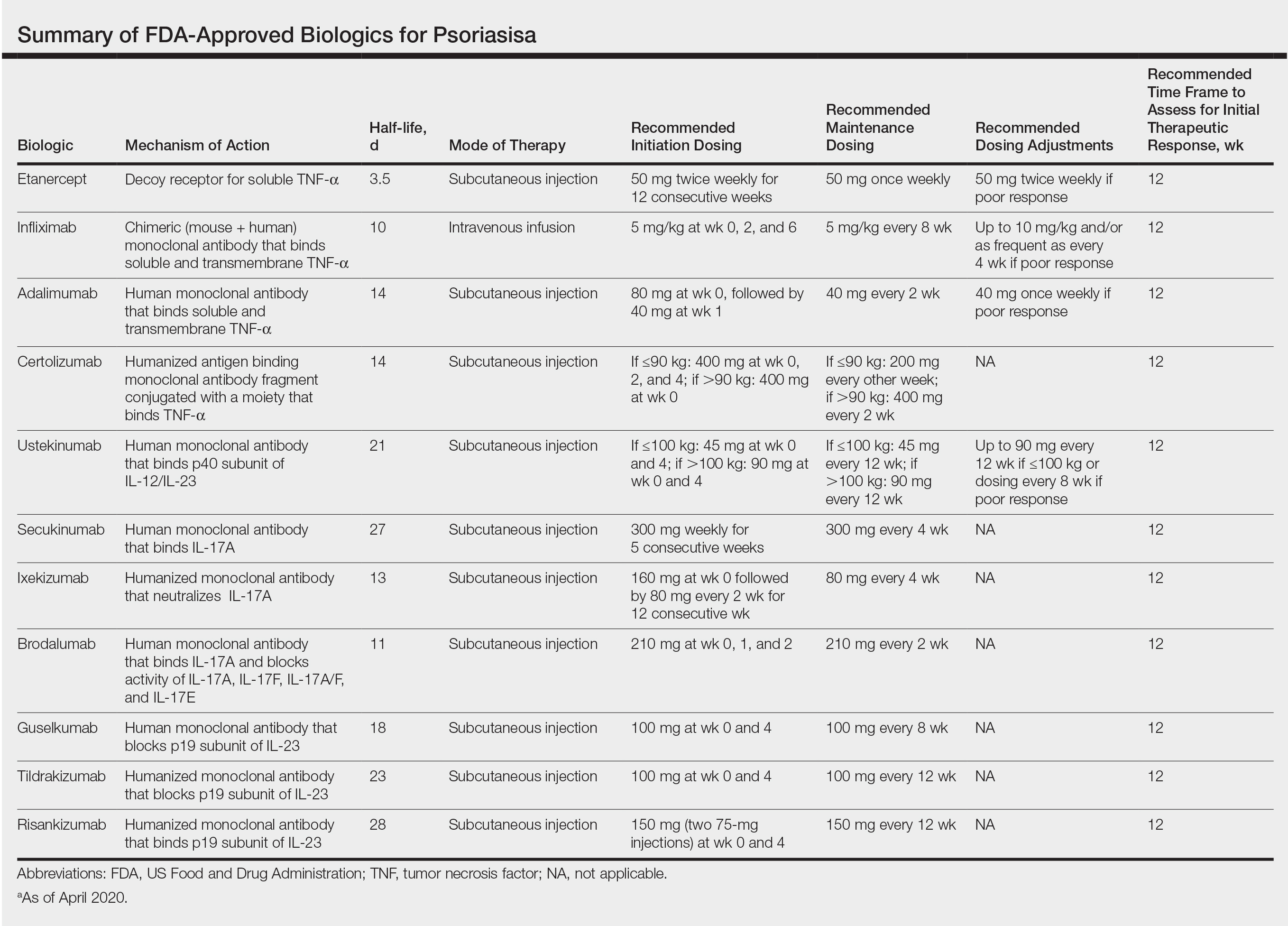
Using Risankizumab
Risankizumab is a new biologic that selectively targets the IL-23 pathway by binding the p19 subunit of IL-23. It was approved by the FDA in April 2019. Two recent studies have demonstrated the efficacy of risankizumab in disease management.3,4
IMMvent was a double-blind, 2-part, phase 3, randomized controlled trial (RCT) of participants 18 years and older (N=605) with moderate to severe psoriasis (with or without psoriatic arthritis) across 11 countries.3 Inclusion criteria consisted of psoriasis involving at least 10% of the body surface area (BSA), absolute psoriasis area and severity index (PASI) score of 12 or higher, and static physician global assessment (sPGA) score of 3 or higher. Prior biologic treatment did not preclude study entry (excluding risankizumab or adalimumab), and nearly 40% of participants previously had been on a different biologic. Notably, this trial allowed for inclusion of patients with prior malignancy (>5 years prior) and patients who tested positive for exposure to tuberculosis (TB) but were not shown to have active TB (provided appropriate treatment for latent TB was started). Study participants identified as white (81%), Asian (14%), black/African American (4%), or other ethnicity (1%). Part A involved administration of 150 mg risankizumab (n=301) at weeks 0 and 4 or 80 mg adalimumab (n=304) loading dose at week 0 followed by 40 mg at week 1 and 40 mg every other week thereafter until the end of week 15. At week 16 there was a significant difference in proportion of participants achieving 90% or more improvement (PASI-90) with risankizumab (72%) vs adalimumab (47%)(P<.0001) and achieving an sPGA score of 0 or 1 (clear or almost clear) with risankizumab (84%) vs adalimumab (60%)(P<.0001). In part B (weeks 16–44), adalimumab immediate responder (PASI ≥50 to PASI <90) participants were re-randomized to continue adalimumab 40 mg every other week (starting from week 17 and stopping at week 44) or switch to 150 mg risankizumab administered at weeks 16, 20, and 32. Patients taking risankizumab in part A continued the drug, administered at weeks 16 and 28. At week 44, there was a significant difference in percentage of participants achieving PASI-90 with risankizumab (66%) vs adalimumab (21%)(P<.0001).3
IMMhance was another double-blind phase 3 RCT with 2 parts that assessed the clinical efficacy of risankizumab compared to placebo in patients 18 years or older (N=507) across 9 countries with the same inclusion criteria for patients as IMMvent.4 Part A involved administration of 150 mg risankizumab (n=407) or placebo (n=100) at weeks 0 and 4 using a 4:1 random allocation ratio. At week 16, regardless of initial treatment, all participants received 150 mg risankizumab. Treatment results at week 16 showed a significant difference in percentage of participants achieving PASI-90 with risankizumab (73.2%) vs placebo (2.0%)(P<.001) and sPGA score of 0 or 1 with risankizumab (83.5%) vs placebo (7.0%)(P<.001). Furthermore, in part B (weeks 16–104), at week 28 participants on risankizumab with an sPGA score of 0 or 1 were randomized with a 1:2 allocation ratio to continue 150 mg risankizumab or switch to placebo to produce a treatment withdrawal effect. Part B results showed a significant difference in the proportion of participants achieving an sPGA score of 0 or 1 with risankizumab (87.4%) vs placebo (61.3%)(P<.001) at week 52 and at week 104 with risankizumab (81.1%) vs placebo (7.1%)(P<.001). Risankizumab was well tolerated, with the most common adverse events (AEs) being nasopharyngitis (23.4%), upper respiratory tract infection (15.4%), and headache (6.8%). Serious AEs included cancer (2.6%; 2.2 events per 100 patient-years), hepatic events (4.6%) including hepatic cirrhosis (0.2%), and serious infections (1.8%; 1.4 events per 100 patient-years).4
Overall, the strengths of risankizumab with regard to its clinical efficacy and utility in biologic-experienced patients were confirmed in these studies. The inclusion of patients with prior treated malignancy and positive TB tests also was more in line with what one might encounter with real-world practice and, as such, provided valuable data to help aid treatment decisions. These 2 studies provided valuable evidence for the therapeutic benefit and relatively mild safety profile of risankizumab in treatment of moderate to severe psoriasis for patients with and without prior biologic therapy.
Choosing a Biologic
Refractory psoriasis involves nonresponse (primary failure) or return of disease symptoms after initial improvement (secondary failure) with a biologic. Selecting a biologic for patients who have experienced prior biologic failure is difficult. It is still unknown whether it is more efficacious for patients to try a same-class drug or a biologic targeting a different inflammatory pathway or cytokine. Studies have shown mixed results regarding how to manage patients with biologic failure, with both approaches demonstrating positive outcomes.
One analysis of the Corrona Psoriasis Registry included 144 patients, the majority of whom (89.8%) were biologic experienced, who began secukinumab treatment and returned for a 6-month follow-up (5–9 months).5 Patients enrolled in the registry were 18 years or older, had been diagnosed with psoriasis by a dermatologist, and initiated or switched an FDA-approved systemic agent or biologic within the last 12 months. Of biologic-experienced participants, 37.7% had used 3 or more biologics. More than half of included participants were either male (55%) or obese (53.4%). Comorbidities included hypertension (43.2%), hyperlipidemia (33.9%), anxiety (20.3%), diabetes mellitus (15.3%), cardiovascular disease (14.4%), and depression (13.6%). After 6 months of treatment, there was significant improvement in the involvement of BSA (mean difference, −12.1), investigator global assessment score (−1.5), dermatology life quality index (DLQI)(−4.8), pain (−23.2), itch (−30.8), fatigue (−8.8), and work productivity (−9.2)(P<.01). Secukinumab therapy displayed notable reduction in symptom severity in this population with difficult-to-treat psoriasis. Its relative success in this cohort provides support for its use in treating patients who have failed other classes of biologics.5
Evidence supporting reduction of pruritus and pain with secukinumab also was notable. The CLEAR phase 3 RCT involved participants treated with 300 mg secukinumab every week for the first 4 weeks and then every 4 weeks thereafter for 48 weeks (n=312), up to 100 weeks (n=277).6 Participants had complete relief of pain (score 0), itching, and scaling at week 16 (69.4%, 49.7%, and 61.2%, respectively), week 52 (67.1%, 48.9%, and 53.3%, respectively), and week 104 (70.9%, 47.4%, and 54.8%, respectively). Reported AEs included candida infections (7.2%), malignant or unspecified tumors (1.5%), and neutropenia (<1%).6
Researchers investigated intraclass switching to brodalumab with prior failure of IL-17 inhibitors. An open-label study involved participants (n=39) with prior failure with secukinumab or ixekizumab therapy.7 Participants were administered 210 mg brodalumab with standard dosing at weeks 0, 1, and 2, and then every 2 weeks thereafter. At week 16, 69% of participants achieved PASI-75, 44% achieved PASI-90, 28% achieved PASI-100, and 62% achieved an sPGA score of 0 or 1. The authors attributed the relative success of brodalumab compared to prior anti–IL-17 agents to inhibition of the IL-17 receptor with brodalumab rather than the IL-17A ligand.7 Brodalumab may be a useful alternative biologic for patients with nonresponse to and secondary failure with biologics, including the IL-17A inhibitors.
Recent findings support effective skin clearance and improved symptom management with ixekizumab and ustekinumab. Of note, ixekizumab was reported to provide rapid improvement in skin lesions and quality of life to a greater extent than guselkumab.
The IXORA-R double-blinded RCT compared the clinical benefit of participants 18 years and older taking standard approved dosages of ixekizumab (n=520) or guselkumab (n=507).8 Patients were included if they had plaque psoriasis for at least 6 months before baseline, an sPGA score of at least 3, PASI score of 12 or higher, 10% or greater BSA, no prior IL-17 inhibitor failure, no use of IL-23 p19 inhibitors, and no use of any biologic within the specified period prior to baseline. At week 12, ixekizumab showed superior clinical improvement measured by the proportion of participants achieving complete skin clearance (ie, PASI-100)(41%) compared to guselkumab (25%)(P<.001). There were more participants taking ixekizumab who reported DLQI of 0 or 1 (no impact of disease on quality of life)(34%) compared to guselkumab (21%)(P<.001) as early on as week 4. The most common AE was upper respiratory tract infection (7%) in both groups. The risk of treatment-emergent AEs (56%), discontinuation because of AEs (2%), and serious AEs (3%) were comparable in both groups. The number of injection-site reactions was higher with ixekizumab (13%) vs guselkumab (3%). The authors concluded that ixekizumab offers the ability to provide rapid relief of symptoms, which is associated with improved DLQI.8
Response to ustekinumab therapy was assessed in a patient cohort enrolled in the Corrona Psoriasis Registry. This study involved 178 participants 18 years and older with psoriasis involvement of 3% or greater BSA who were treated with ustekinumab.9 By their 6-month follow-up visit, 55.6% of participants achieved adequate treatment response (BSA improving to <3% or 75% from enrollment). Increasing patient age was significantly associated with decreased likelihood of achieving a response (odds ratio, 0.981 [95% confidence interval, 0.962-0.999]; P=.049). Ustekinumab is a practical option for psoriasis treatment that seems to yield better results in younger patients.9 This evidence reveals that increased patient age is a characteristic that may contribute to poor treatment response and should be considered when choosing the best fit for biologic therapy.
Final Thoughts
Using evidence-based interventions to treat patients is the cornerstone of ethical and high-quality medical care. This guide sought to provide relevant updates in a variety of both comparator and pivotal trials, with the goal of summarizing clinically relevant information that may be extracted from these trials to guide patient care. It is not an exhaustive review but may be utilized as a reference tool to fine-tune selection criteria in choosing 1 of 11 biologics for the treatment of psoriasis.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31:333-341.
- Thaçi D, Puig L, Reich K, et al. Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: results from the CLEAR study. J Am Acad Dermatol. 2019;81:1405-1409.
- Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81:857-859.
- Blauvelt A, Papp K, Gottlieb A, et al. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182:1348-1358.
- Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to ustekinumab in patients with moderate-to-severe psoriasis in the US Corrona Psoriasis Registry [published online February 27, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1720586.
The advent of biologic therapy over the last 2 decades has transformed the treatment of psoriasis; patients who either are not good candidates for or have an inadequate response to traditional treatments (topicals and/or phototherapy) now have numerous options for treatment.1 Patients burdened by extensive disease, recurrent flares, and stubborn treatment areas are ideal candidates for biologics. There are 11 biologics approved by the US Food and Drug Administration (FDA)(Table) for treating moderate to severe plaque psoriasis as supported by grade A evidence. The FDA has authorized 1 new biologic—risankizumab—since the joint guidelines from the American Academy of Dermatology and National Psoriasis Foundation were released for the treatment of psoriasis with biologics.2 This article aims to address updates on recent clinical trial findings (April 2019 to April 2020) regarding biologic therapy initiation and maintenance for adult patients. Prescribers should use this update as guidance for determining the appropriate biologic class based on patient characteristics and for approaching biologic-experienced patients with refractory psoriasis. This update also may serve as a reference for the recommended dosing regimens of the 11 approved biologics.

Using Risankizumab
Risankizumab is a new biologic that selectively targets the IL-23 pathway by binding the p19 subunit of IL-23. It was approved by the FDA in April 2019. Two recent studies have demonstrated the efficacy of risankizumab in disease management.3,4
IMMvent was a double-blind, 2-part, phase 3, randomized controlled trial (RCT) of participants 18 years and older (N=605) with moderate to severe psoriasis (with or without psoriatic arthritis) across 11 countries.3 Inclusion criteria consisted of psoriasis involving at least 10% of the body surface area (BSA), absolute psoriasis area and severity index (PASI) score of 12 or higher, and static physician global assessment (sPGA) score of 3 or higher. Prior biologic treatment did not preclude study entry (excluding risankizumab or adalimumab), and nearly 40% of participants previously had been on a different biologic. Notably, this trial allowed for inclusion of patients with prior malignancy (>5 years prior) and patients who tested positive for exposure to tuberculosis (TB) but were not shown to have active TB (provided appropriate treatment for latent TB was started). Study participants identified as white (81%), Asian (14%), black/African American (4%), or other ethnicity (1%). Part A involved administration of 150 mg risankizumab (n=301) at weeks 0 and 4 or 80 mg adalimumab (n=304) loading dose at week 0 followed by 40 mg at week 1 and 40 mg every other week thereafter until the end of week 15. At week 16 there was a significant difference in proportion of participants achieving 90% or more improvement (PASI-90) with risankizumab (72%) vs adalimumab (47%)(P<.0001) and achieving an sPGA score of 0 or 1 (clear or almost clear) with risankizumab (84%) vs adalimumab (60%)(P<.0001). In part B (weeks 16–44), adalimumab immediate responder (PASI ≥50 to PASI <90) participants were re-randomized to continue adalimumab 40 mg every other week (starting from week 17 and stopping at week 44) or switch to 150 mg risankizumab administered at weeks 16, 20, and 32. Patients taking risankizumab in part A continued the drug, administered at weeks 16 and 28. At week 44, there was a significant difference in percentage of participants achieving PASI-90 with risankizumab (66%) vs adalimumab (21%)(P<.0001).3
IMMhance was another double-blind phase 3 RCT with 2 parts that assessed the clinical efficacy of risankizumab compared to placebo in patients 18 years or older (N=507) across 9 countries with the same inclusion criteria for patients as IMMvent.4 Part A involved administration of 150 mg risankizumab (n=407) or placebo (n=100) at weeks 0 and 4 using a 4:1 random allocation ratio. At week 16, regardless of initial treatment, all participants received 150 mg risankizumab. Treatment results at week 16 showed a significant difference in percentage of participants achieving PASI-90 with risankizumab (73.2%) vs placebo (2.0%)(P<.001) and sPGA score of 0 or 1 with risankizumab (83.5%) vs placebo (7.0%)(P<.001). Furthermore, in part B (weeks 16–104), at week 28 participants on risankizumab with an sPGA score of 0 or 1 were randomized with a 1:2 allocation ratio to continue 150 mg risankizumab or switch to placebo to produce a treatment withdrawal effect. Part B results showed a significant difference in the proportion of participants achieving an sPGA score of 0 or 1 with risankizumab (87.4%) vs placebo (61.3%)(P<.001) at week 52 and at week 104 with risankizumab (81.1%) vs placebo (7.1%)(P<.001). Risankizumab was well tolerated, with the most common adverse events (AEs) being nasopharyngitis (23.4%), upper respiratory tract infection (15.4%), and headache (6.8%). Serious AEs included cancer (2.6%; 2.2 events per 100 patient-years), hepatic events (4.6%) including hepatic cirrhosis (0.2%), and serious infections (1.8%; 1.4 events per 100 patient-years).4
Overall, the strengths of risankizumab with regard to its clinical efficacy and utility in biologic-experienced patients were confirmed in these studies. The inclusion of patients with prior treated malignancy and positive TB tests also was more in line with what one might encounter with real-world practice and, as such, provided valuable data to help aid treatment decisions. These 2 studies provided valuable evidence for the therapeutic benefit and relatively mild safety profile of risankizumab in treatment of moderate to severe psoriasis for patients with and without prior biologic therapy.
Choosing a Biologic
Refractory psoriasis involves nonresponse (primary failure) or return of disease symptoms after initial improvement (secondary failure) with a biologic. Selecting a biologic for patients who have experienced prior biologic failure is difficult. It is still unknown whether it is more efficacious for patients to try a same-class drug or a biologic targeting a different inflammatory pathway or cytokine. Studies have shown mixed results regarding how to manage patients with biologic failure, with both approaches demonstrating positive outcomes.
One analysis of the Corrona Psoriasis Registry included 144 patients, the majority of whom (89.8%) were biologic experienced, who began secukinumab treatment and returned for a 6-month follow-up (5–9 months).5 Patients enrolled in the registry were 18 years or older, had been diagnosed with psoriasis by a dermatologist, and initiated or switched an FDA-approved systemic agent or biologic within the last 12 months. Of biologic-experienced participants, 37.7% had used 3 or more biologics. More than half of included participants were either male (55%) or obese (53.4%). Comorbidities included hypertension (43.2%), hyperlipidemia (33.9%), anxiety (20.3%), diabetes mellitus (15.3%), cardiovascular disease (14.4%), and depression (13.6%). After 6 months of treatment, there was significant improvement in the involvement of BSA (mean difference, −12.1), investigator global assessment score (−1.5), dermatology life quality index (DLQI)(−4.8), pain (−23.2), itch (−30.8), fatigue (−8.8), and work productivity (−9.2)(P<.01). Secukinumab therapy displayed notable reduction in symptom severity in this population with difficult-to-treat psoriasis. Its relative success in this cohort provides support for its use in treating patients who have failed other classes of biologics.5
Evidence supporting reduction of pruritus and pain with secukinumab also was notable. The CLEAR phase 3 RCT involved participants treated with 300 mg secukinumab every week for the first 4 weeks and then every 4 weeks thereafter for 48 weeks (n=312), up to 100 weeks (n=277).6 Participants had complete relief of pain (score 0), itching, and scaling at week 16 (69.4%, 49.7%, and 61.2%, respectively), week 52 (67.1%, 48.9%, and 53.3%, respectively), and week 104 (70.9%, 47.4%, and 54.8%, respectively). Reported AEs included candida infections (7.2%), malignant or unspecified tumors (1.5%), and neutropenia (<1%).6
Researchers investigated intraclass switching to brodalumab with prior failure of IL-17 inhibitors. An open-label study involved participants (n=39) with prior failure with secukinumab or ixekizumab therapy.7 Participants were administered 210 mg brodalumab with standard dosing at weeks 0, 1, and 2, and then every 2 weeks thereafter. At week 16, 69% of participants achieved PASI-75, 44% achieved PASI-90, 28% achieved PASI-100, and 62% achieved an sPGA score of 0 or 1. The authors attributed the relative success of brodalumab compared to prior anti–IL-17 agents to inhibition of the IL-17 receptor with brodalumab rather than the IL-17A ligand.7 Brodalumab may be a useful alternative biologic for patients with nonresponse to and secondary failure with biologics, including the IL-17A inhibitors.
Recent findings support effective skin clearance and improved symptom management with ixekizumab and ustekinumab. Of note, ixekizumab was reported to provide rapid improvement in skin lesions and quality of life to a greater extent than guselkumab.
The IXORA-R double-blinded RCT compared the clinical benefit of participants 18 years and older taking standard approved dosages of ixekizumab (n=520) or guselkumab (n=507).8 Patients were included if they had plaque psoriasis for at least 6 months before baseline, an sPGA score of at least 3, PASI score of 12 or higher, 10% or greater BSA, no prior IL-17 inhibitor failure, no use of IL-23 p19 inhibitors, and no use of any biologic within the specified period prior to baseline. At week 12, ixekizumab showed superior clinical improvement measured by the proportion of participants achieving complete skin clearance (ie, PASI-100)(41%) compared to guselkumab (25%)(P<.001). There were more participants taking ixekizumab who reported DLQI of 0 or 1 (no impact of disease on quality of life)(34%) compared to guselkumab (21%)(P<.001) as early on as week 4. The most common AE was upper respiratory tract infection (7%) in both groups. The risk of treatment-emergent AEs (56%), discontinuation because of AEs (2%), and serious AEs (3%) were comparable in both groups. The number of injection-site reactions was higher with ixekizumab (13%) vs guselkumab (3%). The authors concluded that ixekizumab offers the ability to provide rapid relief of symptoms, which is associated with improved DLQI.8
Response to ustekinumab therapy was assessed in a patient cohort enrolled in the Corrona Psoriasis Registry. This study involved 178 participants 18 years and older with psoriasis involvement of 3% or greater BSA who were treated with ustekinumab.9 By their 6-month follow-up visit, 55.6% of participants achieved adequate treatment response (BSA improving to <3% or 75% from enrollment). Increasing patient age was significantly associated with decreased likelihood of achieving a response (odds ratio, 0.981 [95% confidence interval, 0.962-0.999]; P=.049). Ustekinumab is a practical option for psoriasis treatment that seems to yield better results in younger patients.9 This evidence reveals that increased patient age is a characteristic that may contribute to poor treatment response and should be considered when choosing the best fit for biologic therapy.
Final Thoughts
Using evidence-based interventions to treat patients is the cornerstone of ethical and high-quality medical care. This guide sought to provide relevant updates in a variety of both comparator and pivotal trials, with the goal of summarizing clinically relevant information that may be extracted from these trials to guide patient care. It is not an exhaustive review but may be utilized as a reference tool to fine-tune selection criteria in choosing 1 of 11 biologics for the treatment of psoriasis.
The advent of biologic therapy over the last 2 decades has transformed the treatment of psoriasis; patients who either are not good candidates for or have an inadequate response to traditional treatments (topicals and/or phototherapy) now have numerous options for treatment.1 Patients burdened by extensive disease, recurrent flares, and stubborn treatment areas are ideal candidates for biologics. There are 11 biologics approved by the US Food and Drug Administration (FDA)(Table) for treating moderate to severe plaque psoriasis as supported by grade A evidence. The FDA has authorized 1 new biologic—risankizumab—since the joint guidelines from the American Academy of Dermatology and National Psoriasis Foundation were released for the treatment of psoriasis with biologics.2 This article aims to address updates on recent clinical trial findings (April 2019 to April 2020) regarding biologic therapy initiation and maintenance for adult patients. Prescribers should use this update as guidance for determining the appropriate biologic class based on patient characteristics and for approaching biologic-experienced patients with refractory psoriasis. This update also may serve as a reference for the recommended dosing regimens of the 11 approved biologics.

Using Risankizumab
Risankizumab is a new biologic that selectively targets the IL-23 pathway by binding the p19 subunit of IL-23. It was approved by the FDA in April 2019. Two recent studies have demonstrated the efficacy of risankizumab in disease management.3,4
IMMvent was a double-blind, 2-part, phase 3, randomized controlled trial (RCT) of participants 18 years and older (N=605) with moderate to severe psoriasis (with or without psoriatic arthritis) across 11 countries.3 Inclusion criteria consisted of psoriasis involving at least 10% of the body surface area (BSA), absolute psoriasis area and severity index (PASI) score of 12 or higher, and static physician global assessment (sPGA) score of 3 or higher. Prior biologic treatment did not preclude study entry (excluding risankizumab or adalimumab), and nearly 40% of participants previously had been on a different biologic. Notably, this trial allowed for inclusion of patients with prior malignancy (>5 years prior) and patients who tested positive for exposure to tuberculosis (TB) but were not shown to have active TB (provided appropriate treatment for latent TB was started). Study participants identified as white (81%), Asian (14%), black/African American (4%), or other ethnicity (1%). Part A involved administration of 150 mg risankizumab (n=301) at weeks 0 and 4 or 80 mg adalimumab (n=304) loading dose at week 0 followed by 40 mg at week 1 and 40 mg every other week thereafter until the end of week 15. At week 16 there was a significant difference in proportion of participants achieving 90% or more improvement (PASI-90) with risankizumab (72%) vs adalimumab (47%)(P<.0001) and achieving an sPGA score of 0 or 1 (clear or almost clear) with risankizumab (84%) vs adalimumab (60%)(P<.0001). In part B (weeks 16–44), adalimumab immediate responder (PASI ≥50 to PASI <90) participants were re-randomized to continue adalimumab 40 mg every other week (starting from week 17 and stopping at week 44) or switch to 150 mg risankizumab administered at weeks 16, 20, and 32. Patients taking risankizumab in part A continued the drug, administered at weeks 16 and 28. At week 44, there was a significant difference in percentage of participants achieving PASI-90 with risankizumab (66%) vs adalimumab (21%)(P<.0001).3
IMMhance was another double-blind phase 3 RCT with 2 parts that assessed the clinical efficacy of risankizumab compared to placebo in patients 18 years or older (N=507) across 9 countries with the same inclusion criteria for patients as IMMvent.4 Part A involved administration of 150 mg risankizumab (n=407) or placebo (n=100) at weeks 0 and 4 using a 4:1 random allocation ratio. At week 16, regardless of initial treatment, all participants received 150 mg risankizumab. Treatment results at week 16 showed a significant difference in percentage of participants achieving PASI-90 with risankizumab (73.2%) vs placebo (2.0%)(P<.001) and sPGA score of 0 or 1 with risankizumab (83.5%) vs placebo (7.0%)(P<.001). Furthermore, in part B (weeks 16–104), at week 28 participants on risankizumab with an sPGA score of 0 or 1 were randomized with a 1:2 allocation ratio to continue 150 mg risankizumab or switch to placebo to produce a treatment withdrawal effect. Part B results showed a significant difference in the proportion of participants achieving an sPGA score of 0 or 1 with risankizumab (87.4%) vs placebo (61.3%)(P<.001) at week 52 and at week 104 with risankizumab (81.1%) vs placebo (7.1%)(P<.001). Risankizumab was well tolerated, with the most common adverse events (AEs) being nasopharyngitis (23.4%), upper respiratory tract infection (15.4%), and headache (6.8%). Serious AEs included cancer (2.6%; 2.2 events per 100 patient-years), hepatic events (4.6%) including hepatic cirrhosis (0.2%), and serious infections (1.8%; 1.4 events per 100 patient-years).4
Overall, the strengths of risankizumab with regard to its clinical efficacy and utility in biologic-experienced patients were confirmed in these studies. The inclusion of patients with prior treated malignancy and positive TB tests also was more in line with what one might encounter with real-world practice and, as such, provided valuable data to help aid treatment decisions. These 2 studies provided valuable evidence for the therapeutic benefit and relatively mild safety profile of risankizumab in treatment of moderate to severe psoriasis for patients with and without prior biologic therapy.
Choosing a Biologic
Refractory psoriasis involves nonresponse (primary failure) or return of disease symptoms after initial improvement (secondary failure) with a biologic. Selecting a biologic for patients who have experienced prior biologic failure is difficult. It is still unknown whether it is more efficacious for patients to try a same-class drug or a biologic targeting a different inflammatory pathway or cytokine. Studies have shown mixed results regarding how to manage patients with biologic failure, with both approaches demonstrating positive outcomes.
One analysis of the Corrona Psoriasis Registry included 144 patients, the majority of whom (89.8%) were biologic experienced, who began secukinumab treatment and returned for a 6-month follow-up (5–9 months).5 Patients enrolled in the registry were 18 years or older, had been diagnosed with psoriasis by a dermatologist, and initiated or switched an FDA-approved systemic agent or biologic within the last 12 months. Of biologic-experienced participants, 37.7% had used 3 or more biologics. More than half of included participants were either male (55%) or obese (53.4%). Comorbidities included hypertension (43.2%), hyperlipidemia (33.9%), anxiety (20.3%), diabetes mellitus (15.3%), cardiovascular disease (14.4%), and depression (13.6%). After 6 months of treatment, there was significant improvement in the involvement of BSA (mean difference, −12.1), investigator global assessment score (−1.5), dermatology life quality index (DLQI)(−4.8), pain (−23.2), itch (−30.8), fatigue (−8.8), and work productivity (−9.2)(P<.01). Secukinumab therapy displayed notable reduction in symptom severity in this population with difficult-to-treat psoriasis. Its relative success in this cohort provides support for its use in treating patients who have failed other classes of biologics.5
Evidence supporting reduction of pruritus and pain with secukinumab also was notable. The CLEAR phase 3 RCT involved participants treated with 300 mg secukinumab every week for the first 4 weeks and then every 4 weeks thereafter for 48 weeks (n=312), up to 100 weeks (n=277).6 Participants had complete relief of pain (score 0), itching, and scaling at week 16 (69.4%, 49.7%, and 61.2%, respectively), week 52 (67.1%, 48.9%, and 53.3%, respectively), and week 104 (70.9%, 47.4%, and 54.8%, respectively). Reported AEs included candida infections (7.2%), malignant or unspecified tumors (1.5%), and neutropenia (<1%).6
Researchers investigated intraclass switching to brodalumab with prior failure of IL-17 inhibitors. An open-label study involved participants (n=39) with prior failure with secukinumab or ixekizumab therapy.7 Participants were administered 210 mg brodalumab with standard dosing at weeks 0, 1, and 2, and then every 2 weeks thereafter. At week 16, 69% of participants achieved PASI-75, 44% achieved PASI-90, 28% achieved PASI-100, and 62% achieved an sPGA score of 0 or 1. The authors attributed the relative success of brodalumab compared to prior anti–IL-17 agents to inhibition of the IL-17 receptor with brodalumab rather than the IL-17A ligand.7 Brodalumab may be a useful alternative biologic for patients with nonresponse to and secondary failure with biologics, including the IL-17A inhibitors.
Recent findings support effective skin clearance and improved symptom management with ixekizumab and ustekinumab. Of note, ixekizumab was reported to provide rapid improvement in skin lesions and quality of life to a greater extent than guselkumab.
The IXORA-R double-blinded RCT compared the clinical benefit of participants 18 years and older taking standard approved dosages of ixekizumab (n=520) or guselkumab (n=507).8 Patients were included if they had plaque psoriasis for at least 6 months before baseline, an sPGA score of at least 3, PASI score of 12 or higher, 10% or greater BSA, no prior IL-17 inhibitor failure, no use of IL-23 p19 inhibitors, and no use of any biologic within the specified period prior to baseline. At week 12, ixekizumab showed superior clinical improvement measured by the proportion of participants achieving complete skin clearance (ie, PASI-100)(41%) compared to guselkumab (25%)(P<.001). There were more participants taking ixekizumab who reported DLQI of 0 or 1 (no impact of disease on quality of life)(34%) compared to guselkumab (21%)(P<.001) as early on as week 4. The most common AE was upper respiratory tract infection (7%) in both groups. The risk of treatment-emergent AEs (56%), discontinuation because of AEs (2%), and serious AEs (3%) were comparable in both groups. The number of injection-site reactions was higher with ixekizumab (13%) vs guselkumab (3%). The authors concluded that ixekizumab offers the ability to provide rapid relief of symptoms, which is associated with improved DLQI.8
Response to ustekinumab therapy was assessed in a patient cohort enrolled in the Corrona Psoriasis Registry. This study involved 178 participants 18 years and older with psoriasis involvement of 3% or greater BSA who were treated with ustekinumab.9 By their 6-month follow-up visit, 55.6% of participants achieved adequate treatment response (BSA improving to <3% or 75% from enrollment). Increasing patient age was significantly associated with decreased likelihood of achieving a response (odds ratio, 0.981 [95% confidence interval, 0.962-0.999]; P=.049). Ustekinumab is a practical option for psoriasis treatment that seems to yield better results in younger patients.9 This evidence reveals that increased patient age is a characteristic that may contribute to poor treatment response and should be considered when choosing the best fit for biologic therapy.
Final Thoughts
Using evidence-based interventions to treat patients is the cornerstone of ethical and high-quality medical care. This guide sought to provide relevant updates in a variety of both comparator and pivotal trials, with the goal of summarizing clinically relevant information that may be extracted from these trials to guide patient care. It is not an exhaustive review but may be utilized as a reference tool to fine-tune selection criteria in choosing 1 of 11 biologics for the treatment of psoriasis.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31:333-341.
- Thaçi D, Puig L, Reich K, et al. Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: results from the CLEAR study. J Am Acad Dermatol. 2019;81:1405-1409.
- Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81:857-859.
- Blauvelt A, Papp K, Gottlieb A, et al. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182:1348-1358.
- Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to ustekinumab in patients with moderate-to-severe psoriasis in the US Corrona Psoriasis Registry [published online February 27, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1720586.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Reich K, Gooderham M, Thaçi D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394:576-586.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658.
- Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31:333-341.
- Thaçi D, Puig L, Reich K, et al. Secukinumab demonstrates sustained efficacy in clearing skin and improving patient-reported outcomes in patients with moderate-to-severe psoriasis through 2 years of treatment: results from the CLEAR study. J Am Acad Dermatol. 2019;81:1405-1409.
- Kimmel G, Chima M, Kim HJ, et al. Brodalumab in the treatment of moderate to severe psoriasis in patients when previous anti-interleukin 17A therapies have failed. J Am Acad Dermatol. 2019;81:857-859.
- Blauvelt A, Papp K, Gottlieb A, et al. A head‐to‐head comparison of ixekizumab vs. guselkumab in patients with moderate‐to‐severe plaque psoriasis: 12‐week efficacy, safety and speed of response from a randomized, double‐blinded trial. Br J Dermatol. 2020;182:1348-1358.
- Van Voorhees AS, Mason MA, Harrold LR, et al. Characterization of insufficient responders to ustekinumab in patients with moderate-to-severe psoriasis in the US Corrona Psoriasis Registry [published online February 27, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1720586.
Practice Points
- Inform patients about current data guiding treatment from clinical trials of biologics.
- Explain to patients that finding the treatment that is the best fit for them may require trial and error, as everyone responds to treatments differently.
- Consult with patients about misconceptions and potential fears about biologics and what the protocol is for monitoring safety during treatment.
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Phototherapy
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and
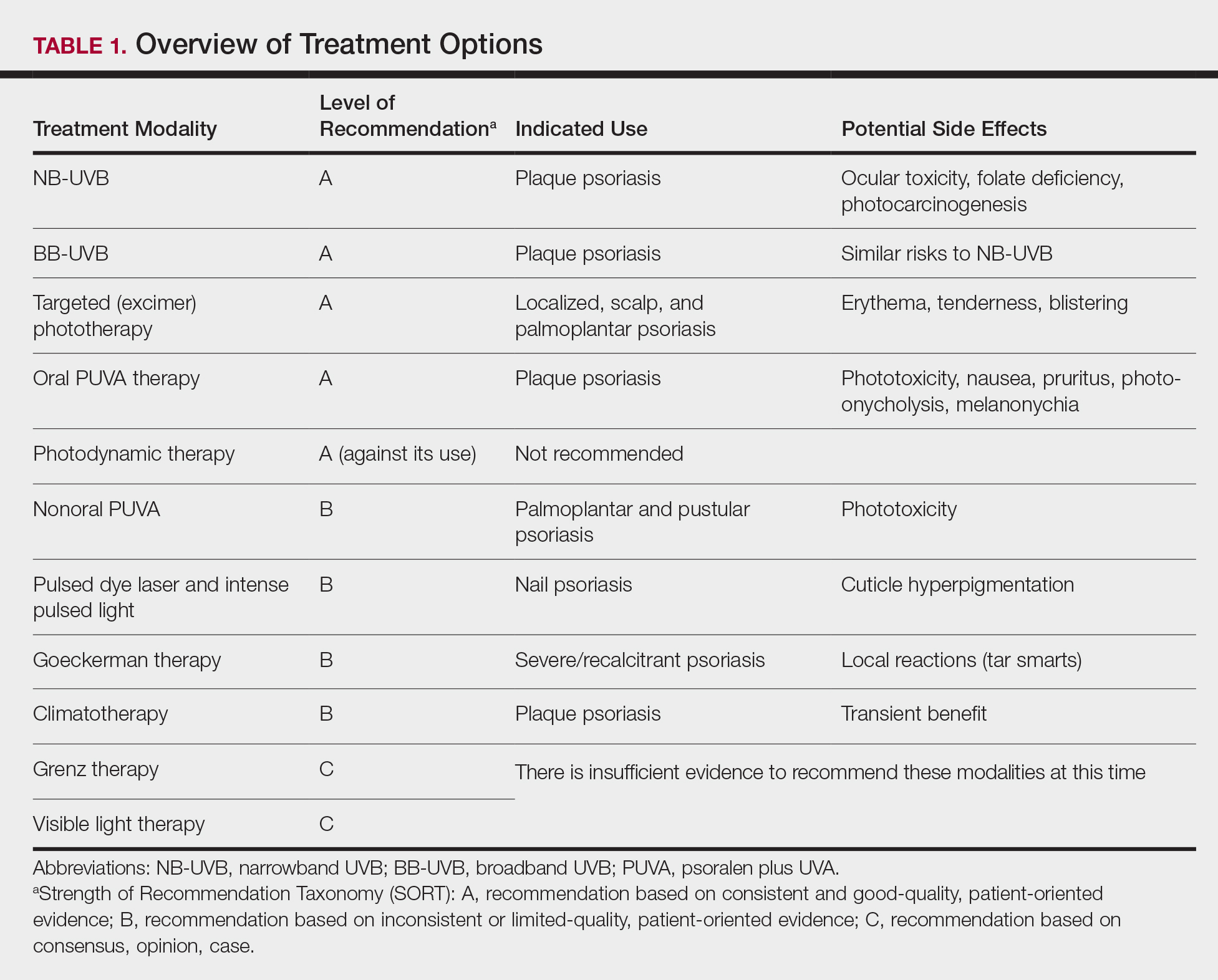
Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.
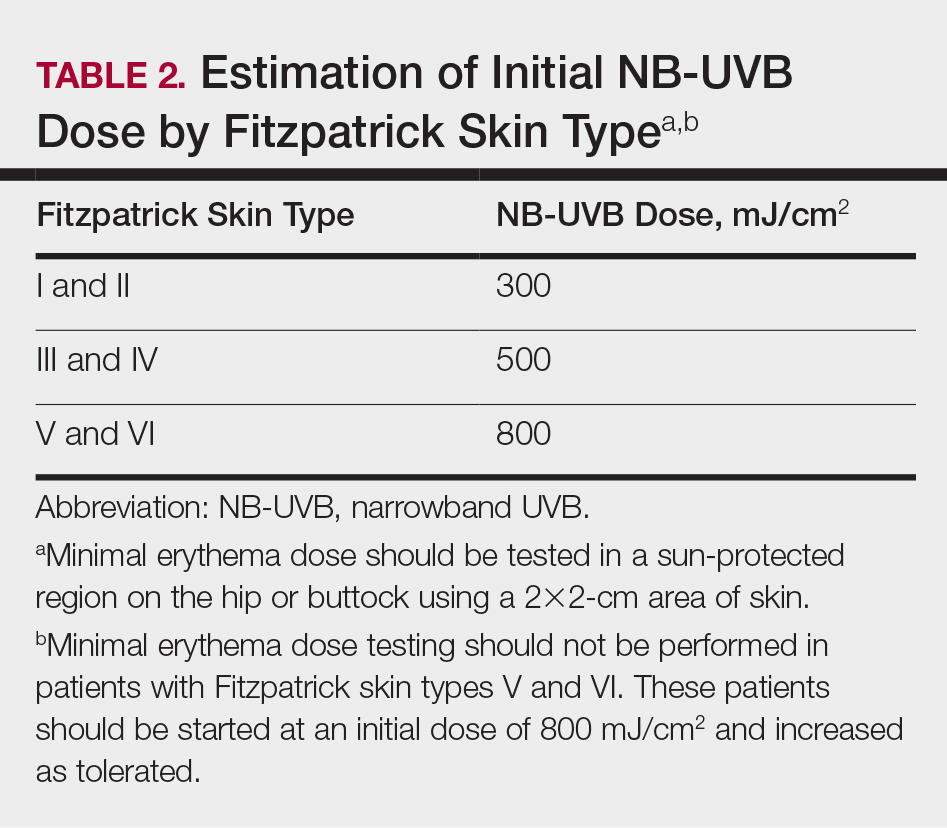
For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).
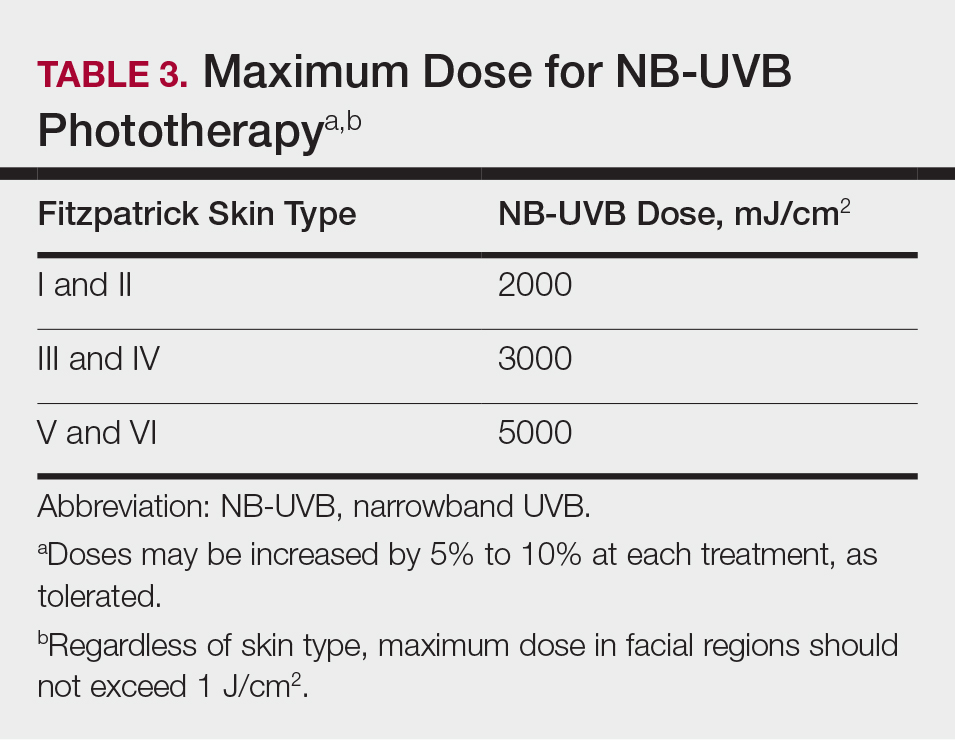
Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and

Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.

For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).

Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 Although topical therapies often are the first-line treatment of mild to moderate psoriasis, approximately 1 in 6 individuals has moderate to severe disease that requires systemic treatment such as biologics or phototherapy.2 In patients with localized disease that is refractory to treatment or who have moderate to severe psoriasis requiring systemic treatment, phototherapy should be considered as a potential low-risk treatment option.
In July 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of phototherapy in treating adult patients with psoriasis.3 Since the prior guidelines were released in 2010, there have been numerous studies affirming the efficacy of phototherapy, with several large meta-analyses helping to refine clinical recommendations.4,5 Each treatment was ranked using Strength of Recommendation Taxonomy, with a score of A, B, or C based on the strength of the evidence supporting the given modality. With the ever-increasing number of treatment options for patients with psoriasis, these guidelines inform dermatologists of the recommendations for the initiation, maintenance, and optimization of phototherapy in the treatment of psoriasis.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and frequency of adverse events of 10 commonly used phototherapy/photochemotherapy modalities. They also address dosing regimens, the potential to combine phototherapy with other therapies, and the efficacy of treatment modalities for different types of psoriasis.3 The purpose of this discussion is to present these guidelines in a condensed form for prescribers of phototherapy and to review the most clinically significant considerations during each step of treatment. Of note, we only highlight the treatment of adult patients and do not discuss information relevant to pediatric patients with psoriasis.
Choosing a Phototherapy Modality
Phototherapy may be considered for patients with psoriasis that affects more than 3% body surface area or for localized disease refractory to conventional treatments. UV light is believed to provide relief from psoriasis via multiple mechanisms, such as through favorable alterations in cytokine profiles, initiation of apoptosis, and local immunosupression.6 There is no single first-line phototherapeutic modality recommended for all patients with psoriasis. Rather, the decision to implement a particular modality should be individualized to the patient, considering factors such as percentage of body surface area affected by disease, quality-of-life assessment, comorbidities, lifestyle, and cost of treatment.
Of the 10 phototherapy modalities reviewed in these guidelines, 4 were ranked by the AAD and NPF as having grade A evidence for efficacy in the treatment of moderate to severe plaque psoriasis. Treatments with a grade A level of recommendation included narrowband UVB (NB-UVB), broadband UVB (BB-UVB), targeted phototherapy (excimer laser and excimer lamp), and

Studies have shown that the ideal wavelength needed to produce a therapeutic effect (ie, clearance of psoriatic plaques) is 304 to 313 nm. Wavelengths of 290 to 300 nm were found to be less therapeutic and more harmful, as they contributed to the development of sunburns.7 Broadband UVB phototherapy, with wavelengths ranging from 270 to 390 nm, exposes patients to a greater spectrum of radiation, thus making it more likely to cause sunburn and any theoretical form of sun-related damage, such as dysplasia and cancer. Compared with NB-UVB phototherapy, BB-UVB phototherapy is associated with a greater degree of sun damage–related side effects. Narrowband UVB, with a wavelength range of 311 to 313 nm, carries a grade A level of recommendation and should be considered as first-line monotherapy in patients with generalized plaque psoriasis, given its efficacy and promising safety profile. Multiple studies have shown that NB-UVB phototherapy is superior to BB-UVB phototherapy in the treatment of moderate to severe psoriasis in adults.8,9 In facilities where access to NB-UVB is limited, BB-UVB monotherapy is recommended as the treatment of generalized plaque psoriasis.
Psoralen plus UVA, which may be used topically (ie, bathwater PUVA) or taken orally, refers to treatment with photosensitizing psoralens. Psoralens are agents that intercalate with DNA and enhance the efficacy of phototherapy.10 Topical PUVA, with a grade B level of recommendation, is an effective treatment option for patients with localized disease and has been shown to be particularly efficacious in the treatment of palmoplantar pustular psoriasis. Oral PUVA is an effective option for psoriasis with a grade A recommendation, while bathwater PUVA has a grade B level of recommendation for moderate to severe plaque psoriasis. Oral PUVA is associated with greater systemic side effects (both acute and subacute) compared with NB-UVB and also is associated with photocarcinogenesis, particularly squamous cell carcinoma in white patients.11 Other side effects from PUVA include pigmented macules in sun-protected areas (known as PUVA lentigines), which may make evaluation of skin lesions challenging. Because of the increased risk for cancer with oral PUVA, NB-UVB is preferable as a first-line treatment vs PUVA, especially in patients with a history of skin cancer.12,13
Goeckerman therapy, which involves the synergistic combination of UVB and crude coal tar, is an older treatment that has shown efficacy in the treatment of severe or recalcitrant psoriasis (grade B level of recommendation). One prior case-control study comparing the efficacy of Goeckerman therapy with newer treatments, such as biologic therapies, steroids, and oral immunosuppressants, found a similar reduction in symptoms among both treatment groups, with longer disease-free periods in patients who received Goeckerman therapy than those who received newer therapies (22.3 years vs 4.6 months).14 However, Goeckerman therapy is utilized less frequently than more modern therapies because of the time required for treatment and declining insurance reimbursements for it. Climatotherapy, another older established therapy, involves the temporary or permanent relocation of patients to an environment that is favorable for disease control (grade B level of recommendation). Locations such as the Dead Sea and Canary Islands have been studied and shown to provide both subjective and objective improvement in patients’ psoriasis disease course. Patients had notable improvement in both their psoriasis area and severity index score and quality of life after a 3- to 4-week relocation to these areas.15,16 Access to climatotherapy and the transient nature of disease relief are apparent limitations of this treatment modality.
Grenz ray is a type of phototherapy that uses longer-wavelength ionizing radiation, which has low penetrance into surrounding tissues and a 95% absorption rate within the first 3 mm of the skin depth. This treatment has been used less frequently since the development of newer alternatives but should still be considered as a second line to UV therapy, especially in cases of recalcitrant disease and palmoplantar psoriasis, and when access to other treatment options is limited. Grenz ray has a grade C level of recommendation due to the paucity of evidence that supports its efficacy. Thus, it is not recommended as first-line therapy for the treatment of moderate to severe psoriasis. Visible light therapy is another treatment option that uses light in the visible wavelength spectrum but predominantly utilizes blue and red light. Psoriatic lesions are sensitive to light therapy because of the elevated levels of naturally occurring photosensitizing agents, called protoporphyrins, in these lesions.17 Several small studies have shown improvement in psoriatic lesions treated with visible light therapy, with blue light showing greater efficacy in lesional clearance than red light.18,19
Pulsed dye laser is a phototherapy modality that has shown efficacy in the treatment of nail psoriasis (grade B level of recommendation). One study comparing the effects of tazarotene cream 0.1% with pulsed dye laser and tazarotene cream 0.1% alone showed that patients receiving combination therapy had a greater decrease in
Initiation of Phototherapy
Prior to initiating phototherapy, it is important to assess the patient for any personal or family history of skin cancer, as phototherapy carries an increased risk for cutaneous malignancy, especially in patients with a history of skin cancer.22,23 All patients also should be evaluated for their Fitzpatrick skin type, and the minimal erythema dose should be defined for those initiating UVB treatment. These classifications can be useful for the initial determination of treatment dose and the prevention of treatment-related adverse events (TRAEs). A careful drug history also should be taken before the initiation of phototherapy to avoid photosensitizing reactions. Thiazide diuretics and tetracyclines are 2 such examples of medications commonly associated with photosensitizing reactions.24
Fitzpatrick skin type and/or minimal erythema dose testing also are essential in determining the appropriate initial NB-UVB dose required for treatment initiation (Table 2). Patient response to the initial NB-UVB trial will determine the optimal dosage for subsequent maintenance treatments.

For patients unable or unwilling to commit to office-based or institution-based treatments, home NB-UVB is another therapeutic option. One study comparing patients with moderate to severe psoriasis who received home NB-UVB vs in-office treatment showed comparable psoriasis area and severity index scores and quality-of-life indices and no difference in the frequency of TRAE indices. It is important to note that patients who received home treatment had a significantly lower treatment burden (P≤.001) and greater treatment satisfaction than those receiving treatment in an office-based setting (P=.001).25
Assessment and Optimization of Phototherapy
After an appropriate starting dosage has been established, patients should be evaluated at each subsequent visit for the degree of treatment response. Excessive erythema (lasting more than 48 hours) or adverse effects, such as itching, stinging, or burning, are indications that the patient should have their dose adjusted back to the last dose without such adverse responses. Because tolerance to treatment develops over time, patients who miss a scheduled dose of NB-UVB phototherapy require their dose to be temporarily lowered. Targeted dosage of UVB phototherapy at a frequency of 2 to 3 times weekly is preferred over treatment 1 to 2 times weekly; however, consideration should be given toward patient preference.26 Dosing may be increased at a rate of 5% to 10% after each treatment, as tolerated, if there is no clearance of skin lesions with the original treatment dose. Patient skin type also is helpful in dictating the maximum phototherapy dose for each patient (Table 3).

Once a patient’s psoriatic lesions have cleared, the patient has the option to taper or indefinitely continue maintenance therapy. The established protocol for patients who choose to taper therapy is treatment twice weekly for 4 weeks, followed by once-weekly treatment for the second month. The maintenance dosage is held constant during the taper. For patients who prefer indefinite maintenance therapy, treatment is administered every 1 to 2 weeks, with a maintenance dosage that is approximately 25% lower than the original maintenance dosage.
Treatment Considerations
Efforts should be made to ensure that the long-term sequalae of phototherapy are minimized (Table 1). Development of cataracts is a recognized consequence of prolonged UVB exposure; therefore, eye protection is recommended during all UVB treatment sessions to reduce the risk for ocular toxicity. Although pregnancy is not a contraindication to phototherapy, except for PUVA, there is a dose-dependent degradation of folate with NB-UVB treatment, so folate supplementation (0.8 mg) is recommended during NB-UVB treatment to prevent development of neural tube defects in fetuses of patients who are pregnant or who may become pregnant.27
Although phototherapy carries the theoretical risk for photocarcinogenesis, multiple studies have shown no increased risk for malignancy with either NB-UVB or BB-UVB phototherapy.22,23 Regardless, patients who develop new-onset skin cancer while receiving any phototherapeutic treatment should discuss the potential risks and benefits of continued treatment with their physician. Providers should take extra caution prior to initiating treatment, especially in patients with a history of cutaneous malignancy. Because oral PUVA is a systemic therapy, it is associated with a greater risk for photocarcinogenesis than any other modality, particularly in fair-skinned individuals. Patients younger than 10 years; pregnant or nursing patients; and those with a history of lupus, xeroderma pigmentosum, or melanoma should not receive PUVA therapy because of their increased risk for photocarcinogenesis and TRAEs.
The decision to switch patients between different phototherapy modalities during treatment should be individualized to each patient based on factors such as disease severity, quality of life, and treatment burden. Other factors to consider include dosing frequency, treatment cost, and logistical factors, such as proximity to a treatment facility. Physicians should have a detailed discussion about the risks and benefits of continuing therapy for patients who develop new-onset skin cancer during phototherapy.
Final Thoughts
Phototherapy is an effective and safe treatment for patients with psoriasis who have localized and systemic disease. There are several treatment modalities that can be tailored to patient needs and preferences, such as home NB-UVB for patients who are unable or unwilling to undergo office-based treatments. Phototherapy has few absolute contraindications; however, relative contraindications to phototherapy exist. Patients with a history of skin cancer, photosensitivity disorders, and autoimmune diseases (eg, lupus) carry greater risks for adverse events, such as sun-related damage, cancer, and dysplasia. Because these conditions may preclude patients from pursuing phototherapy as a safe and effective approach to treating moderate to severe psoriasis, these patients should be considered for other therapies, such as biologic medications, which may carry a more favorable risk-benefit ratio given that individual’s background.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173-1179.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):11-21.
- Chen X, Yang M, Cheng Y, et al. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013;10:CD009481.
- Wong T, Hsu L, Liao W. Phototherapy in psoriasis: a review of mechanisms of action. J Cutan Med Surg. 2013;17:6-12.
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. J Invest Dermatol. 1981;76:359-362.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- El-Mofty M, Mostafa WZ, Bosseila M, et al. A large scale analytical study on efficacy of different photo(chemo)therapeutic modalities in the treatment of psoriasis, vitiligo and mycosis fungoides. Dermatol Ther. 2010;23:428-434.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
- Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
- Bruynzeel I, Bergman W, Hartevelt HM, et al. 'High single-dose' European PUVA regimen also causes an excess of non-melanoma skin cancer. Br J Dermatol. 1991;124:49-55.
- Lindelöf B, Sigurgeirsson B, Tegner E, et al. PUVA and cancer risk: the Swedish follow-up study. Br J Dermatol. 1999;141:108-112.
- Chern E, Yau D, Ho JC, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Harari M, Czarnowicki T, Fluss R, et al. Patients with early-onset psoriasis achieve better results following Dead Sea climatotherapy. J Eur Acad Dermatol Venereol. 2012;26:554-559.
- Wahl AK, Langeland E, Larsen MH, et al. Positive changes in self-management and disease severity following climate therapy in people with psoriasis. Acta Dermatol Venereol. 2015;95:317-321.
- Bissonnette R, Zeng H, McLean DI, et al. Psoriatic plaques exhibit red autofluorescence that is due to protoporphyrin IX. J Invest Dermatol. 1998;111:586-591.
- Kleinpenning MM, Otero ME, van Erp PE, et al. Efficacy of blue light vs. red light in the treatment of psoriasis: a double-blind, randomized comparative study. J Eur Acad Dermatol Venereol. 2012;26:219-225.
- Weinstabl A, Hoff-Lesch S, Merk HF, et al. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology. 2011;223:251-259.
- Huang YC, Chou CL, Chiang YY. Efficacy of pulsed dye laser plus topical tazarotene versus topical tazarotene alone in psoriatic nail disease: a single-blind, intrapatient left-to-right controlled study. Lasers Surg Med. 2013;45:102-107.
- Tawfik AA. Novel treatment of nail psoriasis using the intense pulsed light: a one-year follow-up study. Dermatol Surg. 2014;40:763-768.
- Archier E, Devaux S, Castela E, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):22-31.
- Osmancevic A, Gillstedt M, Wennberg AM, et al. The risk of skin cancer in psoriasis patients treated with UVB therapy. Acta Dermatol Venereol. 2014;94:425-430.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:B1542.
- Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:5-14.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
Practice Points
- Phototherapy should be considered as an effective and low-risk treatment of psoriasis.
- Narrowband UVB, broadband UVB, targeted phototherapy (excimer laser and excimer lamp), and oral psoralen plus UVA have all received a grade A level of recommendation for the treatment of psoriasis in adults.
Tumor Necrosis Factor Inhibitors May Reduce Cardiovascular Morbidity in Patients With Psoriasis
The connection between psoriasis and increased major adverse cardiovascular events (MACEs) has been well studied. 1,2 Although treatment of psoriasis can improve skin and joint symptoms, it is less clear whether therapies may mitigate the increased risk for cardiovascular comorbidities. Tumor necrosis factor (TNF) inhibitors in particular have been studied with great interest given the role of TNF in vascular and metabolic functions. 3 Using a retrospective cohort design, Wu and colleagues 4 examined if treatment with TNF inhibitors in patients with psoriasis would be associated with a lower risk for MACEs compared to phototherapy. Results suggested a significantly lower hazard of MACEs in patients using TNF inhibitors vs patients treated with phototherapy (adjusted hazard ratio, 0.77; P = .046). Moreover, based on these findings, they calculated that treating approximately 161 patients with TNF inhibitors rather than phototherapy would result in 1 less MACE per year overall. 4
Patients with psoriasis have been shown to have a greater noncalcified coronary plaque burden and prevalence of high-risk plaque compared to healthy patients.5 Lerman and colleagues5 measured the coronary plaque burden of 105 patients with psoriasis and 25 healthy volunteers using coronary computed tomography angiography. Although the patients were on average 10 years younger and had lower cardiovascular risk as measured by traditional risk scores, patients with psoriasis were found to have a greater noncalcified coronary plaque burden compared to 100 patients with hyperlipidemia. This burden was associated with an increased prevalence of high-risk plaques. Furthermore, in patients followed for 1 year, improvements in psoriasis severity were associated with reductions in noncalcified coronary plaque burden, though this finding was across all treatment modalities. However, there was no significant difference in calcified coronary plaque burden associated with reduced psoriasis severity.5
Moreover, Pina et al6 conducted a prospective study evaluating the use of the TNF inhibitor adalimumab to improve endothelial function and arterial stiffness in patients with moderate to severe psoriasis. Among 29 patients, they found a significant improvement in endothelial function as measured by flow-mediated dilatation after 6 months of adalimumab therapy, with a mean increase from 6.19% to 7.46% (P=.008). They also reported decreases in arterial stiffness by pulse wave velocity (P=.03). Despite a small sample size, these findings provide 2 potential mechanisms by which TNF inhibitor therapy may reduce the risk for cardiovascular events.6
A retrospective cohort study evaluating data from the Kaiser Permanente Southern California health plan assessed whether TNF inhibitor therapy was associated with a lower risk for MACE in patients with psoriasis.7 A total of 18,194 patients were included; of these, 1463 received TNF inhibitor therapy for at least 2 months. After controlling for other variables, including age at psoriasis diagnosis, sex, race/ethnicity, and other cardiovascular risk factors (eg, history of smoking or alcohol use; use of clopidogrel, antihypertensive agents, antihyperlipidemics, or anticoagulants), patients in the TNF inhibitor cohort demonstrated a significantly lower MACE hazard ratio compared to patients treated with topicals (hazard ratio, 0.80; 95% confidence interval, 0.66-0.98; P<.05).7
Conversely, a randomized, placebo-controlled trial of 107 patients found no difference in vascular inflammation of the ascending aorta and the carotids after 16 weeks of adalimumab treatment vs placebo. In this study, however, most patients had only moderate psoriasis based on a mean psoriasis area and severity index score of 9.8.8 Given studies finding higher risk burden in patients with more severe skin disease,2 it is possible that the effect of TNF inhibitor therapy may not be as pronounced in patients with less skin involvement. There was a significant effect on C-reactive protein levels in patients receiving TNF inhibitor therapy compared to placebo at 16 weeks (P=.012), suggesting TNF does play some role in systemic inflammation, and it is possible it may exert cardiovascular effects through a mechanism other than vascular inflammation.8
A second double-blind, randomized trial reported similar results.9 Among 97 patients randomized to receive adalimumab, placebo, or phototherapy, no significant difference in vascular inflammation was found after 12 weeks of therapy. In contrast, levels of C-reactive protein, IL-6, and glycoprotein acetylation were markedly reduced. The authors also reported adverse effects of adalimumab therapy on lipid metabolism with reduced cholesterol efflux capacity, a marker of ability of high-density-lipoprotein particles to perform reverse cholesterol transport, and high-density-lipoprotein particles, suggesting these effects may counteract some of the anti-inflammatory effects of TNF inhibitors.9
A growing body of data regarding the effect of TNF inhibitors on cardiovascular morbidity in patients with psoriasis is being collected, but no strong conclusions can be made. Given the disconnect of findings across these studies, it is possible that we have yet to elucidate the full mechanism by which TNF inhibitors may affect cardiovascular health. However, there may be additional confounding factors or patient characteristics at play. More large, prospective, randomized, controlled studies are needed to further understand this relationship.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Kölliker Frers RA, Bisoendial RJ, Montoya SF, et al. Psoriasis and cardiovascular risk: immune-mediated crosstalk between metabolic, vascular, and autoimmune inflammation. Int J Cardiol Metab Endocr. 2015;6:43-54.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-α therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6 month prospective study. J Dermatol. 2016;43:1267-1272.
- Wu JJ, Joshi AA, Reddy SP, et al. Anti-inflammatory therapy with tumor necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis [published online March 24, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14951.
- Bissonnette R, Harel F, Krueger JG, et al. TNF-α antagonist and vascular inflammation patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137:1638-1645 .
- Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11:e007394.
The connection between psoriasis and increased major adverse cardiovascular events (MACEs) has been well studied. 1,2 Although treatment of psoriasis can improve skin and joint symptoms, it is less clear whether therapies may mitigate the increased risk for cardiovascular comorbidities. Tumor necrosis factor (TNF) inhibitors in particular have been studied with great interest given the role of TNF in vascular and metabolic functions. 3 Using a retrospective cohort design, Wu and colleagues 4 examined if treatment with TNF inhibitors in patients with psoriasis would be associated with a lower risk for MACEs compared to phototherapy. Results suggested a significantly lower hazard of MACEs in patients using TNF inhibitors vs patients treated with phototherapy (adjusted hazard ratio, 0.77; P = .046). Moreover, based on these findings, they calculated that treating approximately 161 patients with TNF inhibitors rather than phototherapy would result in 1 less MACE per year overall. 4
Patients with psoriasis have been shown to have a greater noncalcified coronary plaque burden and prevalence of high-risk plaque compared to healthy patients.5 Lerman and colleagues5 measured the coronary plaque burden of 105 patients with psoriasis and 25 healthy volunteers using coronary computed tomography angiography. Although the patients were on average 10 years younger and had lower cardiovascular risk as measured by traditional risk scores, patients with psoriasis were found to have a greater noncalcified coronary plaque burden compared to 100 patients with hyperlipidemia. This burden was associated with an increased prevalence of high-risk plaques. Furthermore, in patients followed for 1 year, improvements in psoriasis severity were associated with reductions in noncalcified coronary plaque burden, though this finding was across all treatment modalities. However, there was no significant difference in calcified coronary plaque burden associated with reduced psoriasis severity.5
Moreover, Pina et al6 conducted a prospective study evaluating the use of the TNF inhibitor adalimumab to improve endothelial function and arterial stiffness in patients with moderate to severe psoriasis. Among 29 patients, they found a significant improvement in endothelial function as measured by flow-mediated dilatation after 6 months of adalimumab therapy, with a mean increase from 6.19% to 7.46% (P=.008). They also reported decreases in arterial stiffness by pulse wave velocity (P=.03). Despite a small sample size, these findings provide 2 potential mechanisms by which TNF inhibitor therapy may reduce the risk for cardiovascular events.6
A retrospective cohort study evaluating data from the Kaiser Permanente Southern California health plan assessed whether TNF inhibitor therapy was associated with a lower risk for MACE in patients with psoriasis.7 A total of 18,194 patients were included; of these, 1463 received TNF inhibitor therapy for at least 2 months. After controlling for other variables, including age at psoriasis diagnosis, sex, race/ethnicity, and other cardiovascular risk factors (eg, history of smoking or alcohol use; use of clopidogrel, antihypertensive agents, antihyperlipidemics, or anticoagulants), patients in the TNF inhibitor cohort demonstrated a significantly lower MACE hazard ratio compared to patients treated with topicals (hazard ratio, 0.80; 95% confidence interval, 0.66-0.98; P<.05).7
Conversely, a randomized, placebo-controlled trial of 107 patients found no difference in vascular inflammation of the ascending aorta and the carotids after 16 weeks of adalimumab treatment vs placebo. In this study, however, most patients had only moderate psoriasis based on a mean psoriasis area and severity index score of 9.8.8 Given studies finding higher risk burden in patients with more severe skin disease,2 it is possible that the effect of TNF inhibitor therapy may not be as pronounced in patients with less skin involvement. There was a significant effect on C-reactive protein levels in patients receiving TNF inhibitor therapy compared to placebo at 16 weeks (P=.012), suggesting TNF does play some role in systemic inflammation, and it is possible it may exert cardiovascular effects through a mechanism other than vascular inflammation.8
A second double-blind, randomized trial reported similar results.9 Among 97 patients randomized to receive adalimumab, placebo, or phototherapy, no significant difference in vascular inflammation was found after 12 weeks of therapy. In contrast, levels of C-reactive protein, IL-6, and glycoprotein acetylation were markedly reduced. The authors also reported adverse effects of adalimumab therapy on lipid metabolism with reduced cholesterol efflux capacity, a marker of ability of high-density-lipoprotein particles to perform reverse cholesterol transport, and high-density-lipoprotein particles, suggesting these effects may counteract some of the anti-inflammatory effects of TNF inhibitors.9
A growing body of data regarding the effect of TNF inhibitors on cardiovascular morbidity in patients with psoriasis is being collected, but no strong conclusions can be made. Given the disconnect of findings across these studies, it is possible that we have yet to elucidate the full mechanism by which TNF inhibitors may affect cardiovascular health. However, there may be additional confounding factors or patient characteristics at play. More large, prospective, randomized, controlled studies are needed to further understand this relationship.
The connection between psoriasis and increased major adverse cardiovascular events (MACEs) has been well studied. 1,2 Although treatment of psoriasis can improve skin and joint symptoms, it is less clear whether therapies may mitigate the increased risk for cardiovascular comorbidities. Tumor necrosis factor (TNF) inhibitors in particular have been studied with great interest given the role of TNF in vascular and metabolic functions. 3 Using a retrospective cohort design, Wu and colleagues 4 examined if treatment with TNF inhibitors in patients with psoriasis would be associated with a lower risk for MACEs compared to phototherapy. Results suggested a significantly lower hazard of MACEs in patients using TNF inhibitors vs patients treated with phototherapy (adjusted hazard ratio, 0.77; P = .046). Moreover, based on these findings, they calculated that treating approximately 161 patients with TNF inhibitors rather than phototherapy would result in 1 less MACE per year overall. 4
Patients with psoriasis have been shown to have a greater noncalcified coronary plaque burden and prevalence of high-risk plaque compared to healthy patients.5 Lerman and colleagues5 measured the coronary plaque burden of 105 patients with psoriasis and 25 healthy volunteers using coronary computed tomography angiography. Although the patients were on average 10 years younger and had lower cardiovascular risk as measured by traditional risk scores, patients with psoriasis were found to have a greater noncalcified coronary plaque burden compared to 100 patients with hyperlipidemia. This burden was associated with an increased prevalence of high-risk plaques. Furthermore, in patients followed for 1 year, improvements in psoriasis severity were associated with reductions in noncalcified coronary plaque burden, though this finding was across all treatment modalities. However, there was no significant difference in calcified coronary plaque burden associated with reduced psoriasis severity.5
Moreover, Pina et al6 conducted a prospective study evaluating the use of the TNF inhibitor adalimumab to improve endothelial function and arterial stiffness in patients with moderate to severe psoriasis. Among 29 patients, they found a significant improvement in endothelial function as measured by flow-mediated dilatation after 6 months of adalimumab therapy, with a mean increase from 6.19% to 7.46% (P=.008). They also reported decreases in arterial stiffness by pulse wave velocity (P=.03). Despite a small sample size, these findings provide 2 potential mechanisms by which TNF inhibitor therapy may reduce the risk for cardiovascular events.6
A retrospective cohort study evaluating data from the Kaiser Permanente Southern California health plan assessed whether TNF inhibitor therapy was associated with a lower risk for MACE in patients with psoriasis.7 A total of 18,194 patients were included; of these, 1463 received TNF inhibitor therapy for at least 2 months. After controlling for other variables, including age at psoriasis diagnosis, sex, race/ethnicity, and other cardiovascular risk factors (eg, history of smoking or alcohol use; use of clopidogrel, antihypertensive agents, antihyperlipidemics, or anticoagulants), patients in the TNF inhibitor cohort demonstrated a significantly lower MACE hazard ratio compared to patients treated with topicals (hazard ratio, 0.80; 95% confidence interval, 0.66-0.98; P<.05).7
Conversely, a randomized, placebo-controlled trial of 107 patients found no difference in vascular inflammation of the ascending aorta and the carotids after 16 weeks of adalimumab treatment vs placebo. In this study, however, most patients had only moderate psoriasis based on a mean psoriasis area and severity index score of 9.8.8 Given studies finding higher risk burden in patients with more severe skin disease,2 it is possible that the effect of TNF inhibitor therapy may not be as pronounced in patients with less skin involvement. There was a significant effect on C-reactive protein levels in patients receiving TNF inhibitor therapy compared to placebo at 16 weeks (P=.012), suggesting TNF does play some role in systemic inflammation, and it is possible it may exert cardiovascular effects through a mechanism other than vascular inflammation.8
A second double-blind, randomized trial reported similar results.9 Among 97 patients randomized to receive adalimumab, placebo, or phototherapy, no significant difference in vascular inflammation was found after 12 weeks of therapy. In contrast, levels of C-reactive protein, IL-6, and glycoprotein acetylation were markedly reduced. The authors also reported adverse effects of adalimumab therapy on lipid metabolism with reduced cholesterol efflux capacity, a marker of ability of high-density-lipoprotein particles to perform reverse cholesterol transport, and high-density-lipoprotein particles, suggesting these effects may counteract some of the anti-inflammatory effects of TNF inhibitors.9
A growing body of data regarding the effect of TNF inhibitors on cardiovascular morbidity in patients with psoriasis is being collected, but no strong conclusions can be made. Given the disconnect of findings across these studies, it is possible that we have yet to elucidate the full mechanism by which TNF inhibitors may affect cardiovascular health. However, there may be additional confounding factors or patient characteristics at play. More large, prospective, randomized, controlled studies are needed to further understand this relationship.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Kölliker Frers RA, Bisoendial RJ, Montoya SF, et al. Psoriasis and cardiovascular risk: immune-mediated crosstalk between metabolic, vascular, and autoimmune inflammation. Int J Cardiol Metab Endocr. 2015;6:43-54.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-α therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6 month prospective study. J Dermatol. 2016;43:1267-1272.
- Wu JJ, Joshi AA, Reddy SP, et al. Anti-inflammatory therapy with tumor necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis [published online March 24, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14951.
- Bissonnette R, Harel F, Krueger JG, et al. TNF-α antagonist and vascular inflammation patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137:1638-1645 .
- Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11:e007394.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Kölliker Frers RA, Bisoendial RJ, Montoya SF, et al. Psoriasis and cardiovascular risk: immune-mediated crosstalk between metabolic, vascular, and autoimmune inflammation. Int J Cardiol Metab Endocr. 2015;6:43-54.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-α therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6 month prospective study. J Dermatol. 2016;43:1267-1272.
- Wu JJ, Joshi AA, Reddy SP, et al. Anti-inflammatory therapy with tumor necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis [published online March 24, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14951.
- Bissonnette R, Harel F, Krueger JG, et al. TNF-α antagonist and vascular inflammation patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137:1638-1645 .
- Mehta NN, Shin DB, Joshi AA, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11:e007394.


