User login
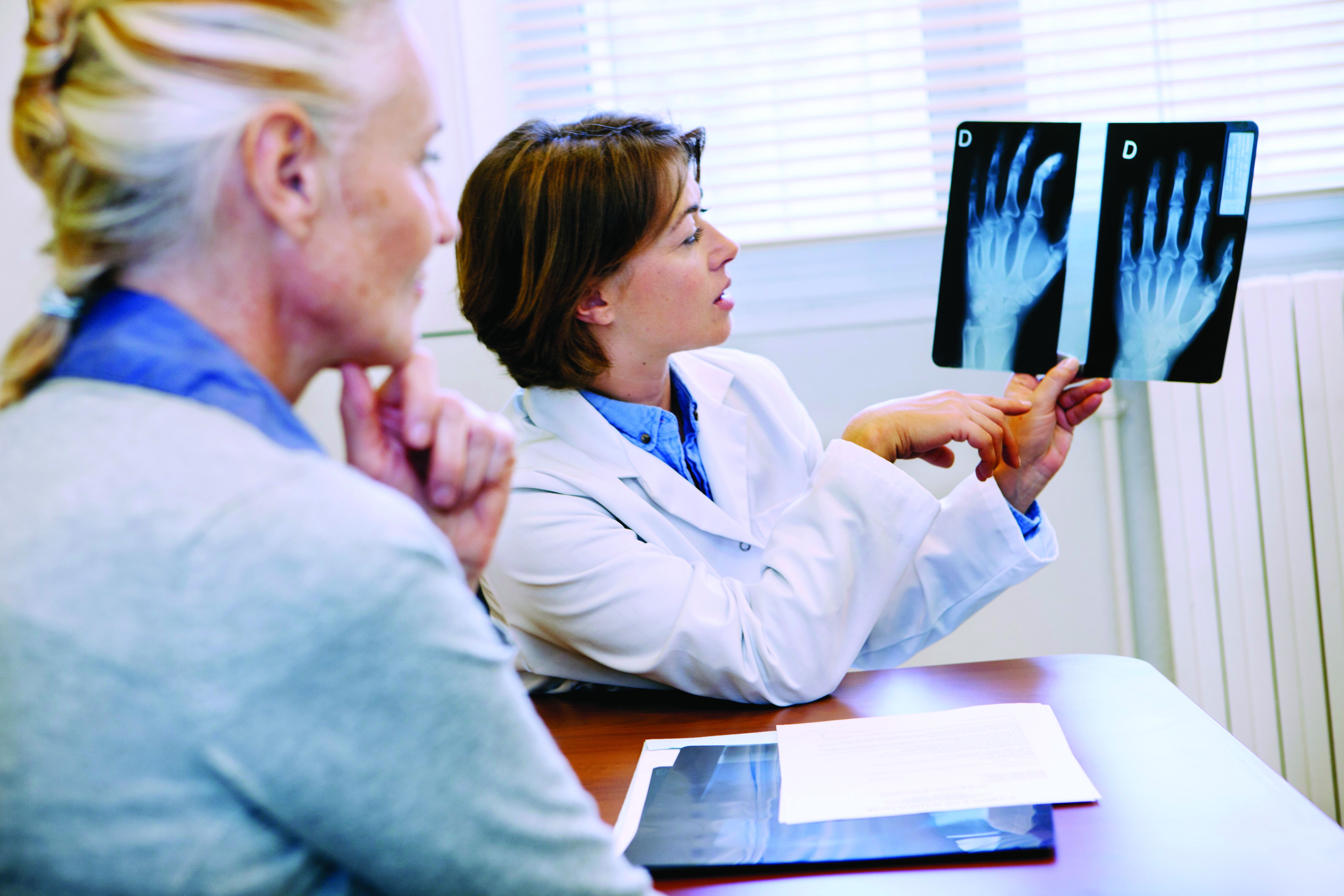
Experts share real-world experience prescribing voclosporin, belimumab for lupus nephritis
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Although patients with lupus nephritis recently gained two new add-on treatment options in voclosporin (Lupkynis) and belimumab (Benlysta), there have been little data published with real-world experience in using these drugs.
Voclosporin, a calcineurin inhibitor, was approved by the Food and Drug Administration in January 2021 to treat lupus nephritis in combination with immunosuppressive medication. Belimumab, a human monoclonal antibody and B-lymphocyte stimulator, was approved in December 2020 in the United States as an add-on treatment for lupus nephritis in adults and later in July 2022 for children who are already receiving standard therapy.
How the two drugs are prescribed for patients with lupus nephritis so far appears to be influenced by presence of extrarenal manifestations of lupus, proteinuria level, clinicians’ prior experience with belimumab, costs of the drugs, and patient preference, experts said.
Voclosporin’s approval was based on data from the phase 3 AURORA 1 trial and phase 2 AURA-LV trial. AURORA 1 evaluated 357 patients with systemic lupus erythematosus (SLE) and lupus nephritis who were randomized to receive voclosporin or placebo with mycophenolate mofetil and tapered low-dose oral steroids. In the voclosporin group, the results showed a significantly higher complete renal response at 52 weeks, compared with the placebo group, while having a similar adverse event profile. The AURA-LV trial, evaluating efficacy and safety of 179 patients with lupus nephritis, showed adding low-dose voclosporin to induction therapy improved renal response, compared with placebo. AURORA 2, a continuation of the AURORA trial, showed patients with lupus nephritis receiving voclosporin have a stable estimated glomerular filtration rate and reductions in proteinuria up to 3 years of follow-up.
Results from the phase 3 BLISS-LN trial of 448 patients with confirmed lupus nephritis were the basis for belimumab’s approval and showed a significantly higher proportion of patients who received belimumab had a primary efficacy renal response, complete renal response, and significantly lower risk of a renal-related adverse event or death, compared with the placebo group.
Lack of real-world data
The lack of real-world data on either of these treatments can be attributed to lupus nephritis being a rare disease, and the approvals happening fairly recently, experts said.
“This is really due to the recency of the approvals for both of these medications for lupus nephritis,” Amit Saxena, MD, a rheumatologist and assistant professor of medicine in the division of rheumatology at NYU Langone Health in New York, said in an interview.
“It’s too soon for any appreciable data to be collected.”
Ashira D. Blazer, MD, MSCI, a rheumatologist at Hospital for Special Surgery and assistant professor of medicine at Weill Cornell Medical College, both in New York, said that rheumatologists “are a little bit hesitant” to use newer agents rather than existing therapies, and have existing guidance from the American College of Rheumatology (ACR) on treating the condition.
“I think when someone has something like lupus nephritis that’s so serious, rheumatologists pull for the tried-and-true drugs that we know will affect the inflammation quickly and get that patient to remission,” she said.
Donald E. Thomas Jr., MD, of Arthritis and Pain Associates of P.G. County in Greenbelt, Md., said he was surprised there was a lack of case studies on voclosporin or belimumab for lupus nephritis, but pointed to the time and cost of publishing a case report and the rheumatologist shortage as potential reasons.
“Most community-based rheumatologists such as myself are too busy,” he said. “Why we are not getting case series from major medical centers, I am not sure.”
When this news organization asked GlaxoSmithKline (GSK) if the company tracked data on real-world use of belimumab, a spokesperson responded that the drug “has extensive clinical efficacy and safety data, and 12 years of postapproval experience, demonstrating its efficacy in SLE to reduce disease activity in multiple organ systems, reduce severe flares, and enabling some patients to taper steroid use over time.”
The spokesperson also referenced published data where belimumab “showed improvement in lupus nephritis when compared to standard therapy alone,” and that the drug “has an established safety profile that has shown to be consistent in diverse patient populations across multiple clinical trials.”
Aurinia Pharmaceuticals did not respond when sent an inquiry on whether the company tracked similar real-world data on voclosporin use.
Prescribing experience
Despite the lack of published data on real-world use, the drugs are being prescribed, Dr. Thomas said.
“I have quite a few patients on these drugs,” he said, citing one patient with severe membranoproliferative lupus nephritis not in remission who is receiving a combination of voclosporin, belimumab, and hydroxychloroquine.
“I have had absolutely no problems getting either drug. The indications for the medicines are crystal clear,” he said.
Irene Blanco, MD, MS, professor in the department of medicine-rheumatology at Northwestern University, Chicago, said that in her experience, both voclosporin and belimumab have been easy to get for patients.
However, she noted she was seeing mostly patients with government-based insurance in the Bronx, N.Y., prior to moving to Northwestern in September 2022. Belimumab had been available from the New York State Medicaid program for indications other than lupus nephritis for some time, and the program was quick to add voclosporin once it became available. “It wasn’t hard to get at all,” she said.
Dr. Saxena noted the respective pharmaceutical companies have provided help in prescribing voclosporin and belimumab through offering patient assistance programs and navigating insurers’ prior authorization hurdles. As belimumab has been available for many years, its availability hasn’t changed, he noted. “Voclosporin has seen more formulary restrictions, but in my experience, I have been able to get the drug utilizing authorization procedures,” he said.
One issue Dr. Blazer said that she encounters is cost. According to prices obtained from drugs.com in March 2023, belimumab has an estimated annual price of $58.389.96 per patient, and voclosporin has an estimated annual price of $86,506.20 per patient.
“I tend to treat patients who can have some socioeconomic challenges, and so I think very long and hard before prescribing either of them,” she explained. “[C]ertainly in the case of voclosporin, when there are older, cheaper calcineurin inhibitors and I think I need one, I’m more likely to reach for one of the others.”
While GSK offers a patient assistance program for belimumab, which Dr. Blazer said she has used, physicians may not be aware of the program or have the resources in their offices to provide social work support for their patients.
“I have had patients who started it and ... continued to have a flare and needed to go on disability or leave their jobs, and they were just too concerned with the ongoing cost burden, and so I ended up taking them off the medication for that reason at their request,” she said.
The fact that Black patients have lupus nephritis more often than White patients do, as well as greater socioeconomic barriers, points to access to care and cost as major factors in why new drugs are not being used, Dr. Blazer said. “I think that understanding how we can improve access is going to be extremely important in getting more real-world data and getting more patients treated,” she said.
Treatment preference
A chart audit recently released by market research firm Spherix Global Insights highlighted a potential treatment preference for lupus nephritis. Use of voclosporin increased among rheumatologists and nephrologists, but patients with lupus nephritis under the care of rheumatologists were more likely to be treated with belimumab than voclosporin.
Dr. Saxena said he has experience with both and doesn’t have a preference, instead using factors other than experience when deciding the best treatment for patients. “For example, if there are nonrenal manifestations such as arthritis or rashes, I may lean towards belimumab, but if a more rapid reduction in proteinuria is important, I may lean towards voclosporin,” he said.
Dr. Thomas weighs the pros and cons of voclosporin and belimumab with the patient. “With many lupus nephritis scenarios, either drug may be a good choice and it comes down to patient preference. The main scenario where I would choose [voclosporin] over [belimumab] is in patients with [proteinuria of] 3 g protein/day or more,” he said, while belimumab would be the choice for a patient with “nonrenal manifestations of SLE in addition to their nephritis.”
For other rheumatologists, comfort level with belimumab may play a role. “We always had [belimumab] and we were always using [belimumab], and so it would make sense that like we would go for a med, again, that we’re really familiar with and we use,” Dr. Blanco said.
Dr. Blanco has prescribed belimumab, but had been using tacrolimus until recently. “I’ve been using tacrolimus since 2016. I’m probably going to lean on the [tacrolimus] rather than going to [belimumab], which works, but maybe it’s not the end-all, be-all in terms of lupus,” she said.
Although she hasn’t yet prescribed voclosporin, Dr. Blazer said she had “much more experience with belimumab.
“I’ve prescribed other calcineurin inhibitors in the past, and usually for a patient who’s very proteinuric and as an adjunct to that standard of care to try to bring down the proteinuria,” she said.
With belimumab, she would consider adding it to a patient with severe disease who has failed treatment with mycophenolate mofetil or cyclophosphamide and has a recurrent lupus nephritis flare. “It’s something I can use as an adjunct, and I think that I can get some extra benefit from it, and it also tends to be well tolerated,” Dr. Blazer said.
How patients are responding
Dr. Thomas’ patients have been responding well on voclosporin and belimumab. “I was an early adopter of [belimumab] and had patients with lupus nephritis do great on it, way before the FDA approval,” he said.
For voclosporin, Dr. Thomas highlighted the “incredibly rapid” proteinuria response. “I had a patient have marked reduction in proteinuria in just 2 weeks. Proteinuria reduction is the number one predictor of long-term better outcomes,” he said.
Many patients receiving mycophenolate and cyclophosphamide do not go into complete remission, while the clinical trials for voclosporin and belimumab had significantly higher rates of complete response and faster response rates, compared with older therapies. “That is what we need,” he said.
“These drugs are game changers in the treatment of lupus nephritis. In my mind, belimumab and voclosporin should be considered the standard of medical care treating lupus nephritis patients,” he added.
Dr. Blanco said her patients appear to like and are tolerating voclosporin and belimumab well, but because there are no pregnancy data on voclosporin, she may choose belimumab or tacrolimus for patients of reproductive age who are considering starting a family.
Patients with extrarenal symptoms tend to do particularly well with belimumab, such as those with arthritis and skin rash, Dr. Blazer said. “In my experience, as an adjunct with those standard of care medications, I have been able to maintain remission in my patients,” she said.
Dr. Saxena said both medications are “important options” for lupus nephritis in patients who don’t respond to standard therapy. “As more doctors utilize each medication and additional data is published, I’d expect an increase uptake in both medications in the future,” he said.
Dr. Blazer reported being a contributor to GSK’s SLE Educators’ Network and has been a consultant for Aurinia. Dr. Saxena reported being a consultant for GSK and Aurinia. Dr. Thomas reported being on the speakers bureau for GSK and Aurinia. Dr. Blanco reported having no relevant financial relationships with pharmaceutical companies.
Expert offers caveats to perioperative antirheumatic drug guideline
The latest guideline for perioperative management of antirheumatic medication in patients undergoing total hip (THA) and total knee arthroplasty (TKA) offers recommendations based on the latest evidence, but many of those recommendations are based on a low level of evidence, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Martin Bergman, MD, clinical professor of medicine at Drexel University, Philadelphia, said the development of the American College of Rheumatology/American Association of Hip and Knee Surgeons guideline was necessary because there was a lack of consensus on when to stop treatments prior to patients with rheumatologic disease undergoing THA and TKA, and when it was appropriate to restart those treatments.
“We all were having the same problem, and I think everybody recognized that just stopping medicines forever didn’t make sense, but maybe continuing medicines also didn’t make sense,” Dr. Bergman said.
While the 2017 ACR/AAHKS perioperative management guideline contained good recommendations, the “explosion” of new medications in rheumatology made it necessary to update the guideline with the latest data on new medications such as immunosuppressants.
2022 guideline recommendations
In the 2022 guideline, which covers disease-modifying treatments taken by patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis, the authors reaffirmed their recommendations to continue methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, and apremilast through total joint arthroplasty.
Where the 2022 guideline differs from the 2017 guideline is in which biologics are covered and under what circumstances they should be withheld and restarted around surgery. The 2022 guideline includes recommendations for abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, guselkumab, infliximab, ixekizumab, rituximab, secukinumab, tocilizumab, and ustekinumab. Each biologic has its own recommended stop and restart times based around the dosing interval and respective method of administration. Dr. Bergman said a general rule with biologics under the new guideline is that the timing of surgery should occur approximately 1 week after the first missed dose of the medication. The only biologic that does not follow this pattern is rituximab, where surgery should be planned for 1 month after the last missed dose.
Dr. Bergman noted that how the guidelines handle interval dosing with infliximab may present a problem. The guideline provides recommendations for patients receiving infliximab every 4 weeks, every 6 weeks, and every 8 weeks. However, Dr. Bergman said this can create a scenario where a patient receiving infliximab at a dose of 3 mg/kg every 8 weeks has surgery at 9 weeks, a patient receiving 5 mg/kg every 6 weeks has surgery at 7 weeks, and a patient receiving 10 mg/kg every 4 weeks has surgery at 5 weeks. “There is some intellectual problem with it,” he said.
Another change from the 2017 guideline is how long to wait for surgery after stopping Janus kinase inhibitors. While the 2017 guideline recommended withholding JAK inhibitors 7 days before surgery, the 2022 guideline lowered that waiting period to 3 days, Dr. Bergman explained.
Concerning use of steroids around THA and TKA surgery, “the days of stress steroid dosing are done,” Dr. Bergman said. “You don’t have to stress dose them. You just follow them, and you keep them on their steroid dose.”
The new guideline recommends restarting therapy once the wound is healed and there is no physical evidence of infection at approximately 2 weeks. “There’s no data to support this,” he said, and his concern is that patients who have stopped a tumor necrosis factor inhibitor may flare if they don’t restart their medication.
While the guideline also covered recommendations for systemic lupus erythematosus, they are “very similar” to the recommendations for inflammatory arthritis, Dr. Bergman noted. “If you have somebody who is not very sick, you stop the medications,” he said, “but try to stop anything else about a week before the surgery. If they’re sick, you basically have to keep them on their medications.”
Caveats in guideline
The recommendations in the 2022 guideline come with a number of caveats, Dr. Bergman noted. For instance, the authors acknowledged limitations in the guideline regarding providing recommendations for only THA and TKA, the “paucity of evidence” around direct infection risk resulting from medications in the perioperative period for THA and TKA, the nonseparation of biologics when assessing infection risk, and the use of dosing interval as a metric for stopping the drug without considering the drug’s half-life.
A “crucial caveat,” Dr. Bergman said, was that the guideline focused on infection risk based on a statement from a panel of patients prior to the development of the 2017 guideline, which “stated very clearly any risk of infection, while rare, was more significant to them than the possibility of postoperative flares, despite flares being reported in over 60% of patients after surgery.
“For the patients, the paramount question was infection, infection, infection, infection. That’s all they cared about, and that is the basis behind a lot of the decision-making here,” Dr. Bergman said.
Another caveat came from a communication Dr. Bergman received from one of the panel members. The panel member noted there were no conclusions or recommendations provided in the guideline for how to manage perioperative flares, such as restarting a corticosteroid or biologic agent. “There was a lot of discussion about what to do with steroids if patients flare, or what to do with [other] medications if they flare, and they just couldn’t come to a consensus,” Dr. Bergman said. “It’s just not discussed.”
Dr. Bergman said he is “somewhat critical” of the ACR/AAHKS guideline, but noted it is an “ambitious project” given the lack of evidence for the recommendations. “The alternative was stop the medications forever and having people really flare, or at least try to get some semblance of rationality behind what we’re going to do,” he said.
Response from attendees
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, took issue with the new recommendations surrounding stopping infliximab. When giving a patient infliximab every 8 weeks at 3 mg/kg, “you’re giving [it] at the nadir of the drug,” he said.
Rather than drug half-life, “it’s about inflammation,” he emphasized. “Inflammation is dominant in causing infection. It drives risk more than anything. The worst thing you can do is wash someone out.
“If you’re going beyond 8 weeks on infliximab, you’re getting closer to washing them out,” he pointed out. “I think it’s a really bad idea.”
Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at Hospital for Special Surgery, both in New York, explained that the guideline is not standard of care, which would be subject to malpractice if not implemented properly.
“When you have guidelines, you follow them unless there are clinical situations which would necessitate another approach to the patient,” he said. “Professional institutions and associations will never put forth rules, they will put forth guidelines so you have the opportunity to deviate from them when the appropriate clinical situation dictates.”
Dr. Bergman reported being a speaker and consultant for AbbVie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Regeneron; he holds stock in Johnson & Johnson and Merck.
The latest guideline for perioperative management of antirheumatic medication in patients undergoing total hip (THA) and total knee arthroplasty (TKA) offers recommendations based on the latest evidence, but many of those recommendations are based on a low level of evidence, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Martin Bergman, MD, clinical professor of medicine at Drexel University, Philadelphia, said the development of the American College of Rheumatology/American Association of Hip and Knee Surgeons guideline was necessary because there was a lack of consensus on when to stop treatments prior to patients with rheumatologic disease undergoing THA and TKA, and when it was appropriate to restart those treatments.
“We all were having the same problem, and I think everybody recognized that just stopping medicines forever didn’t make sense, but maybe continuing medicines also didn’t make sense,” Dr. Bergman said.
While the 2017 ACR/AAHKS perioperative management guideline contained good recommendations, the “explosion” of new medications in rheumatology made it necessary to update the guideline with the latest data on new medications such as immunosuppressants.
2022 guideline recommendations
In the 2022 guideline, which covers disease-modifying treatments taken by patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis, the authors reaffirmed their recommendations to continue methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, and apremilast through total joint arthroplasty.
Where the 2022 guideline differs from the 2017 guideline is in which biologics are covered and under what circumstances they should be withheld and restarted around surgery. The 2022 guideline includes recommendations for abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, guselkumab, infliximab, ixekizumab, rituximab, secukinumab, tocilizumab, and ustekinumab. Each biologic has its own recommended stop and restart times based around the dosing interval and respective method of administration. Dr. Bergman said a general rule with biologics under the new guideline is that the timing of surgery should occur approximately 1 week after the first missed dose of the medication. The only biologic that does not follow this pattern is rituximab, where surgery should be planned for 1 month after the last missed dose.
Dr. Bergman noted that how the guidelines handle interval dosing with infliximab may present a problem. The guideline provides recommendations for patients receiving infliximab every 4 weeks, every 6 weeks, and every 8 weeks. However, Dr. Bergman said this can create a scenario where a patient receiving infliximab at a dose of 3 mg/kg every 8 weeks has surgery at 9 weeks, a patient receiving 5 mg/kg every 6 weeks has surgery at 7 weeks, and a patient receiving 10 mg/kg every 4 weeks has surgery at 5 weeks. “There is some intellectual problem with it,” he said.
Another change from the 2017 guideline is how long to wait for surgery after stopping Janus kinase inhibitors. While the 2017 guideline recommended withholding JAK inhibitors 7 days before surgery, the 2022 guideline lowered that waiting period to 3 days, Dr. Bergman explained.
Concerning use of steroids around THA and TKA surgery, “the days of stress steroid dosing are done,” Dr. Bergman said. “You don’t have to stress dose them. You just follow them, and you keep them on their steroid dose.”
The new guideline recommends restarting therapy once the wound is healed and there is no physical evidence of infection at approximately 2 weeks. “There’s no data to support this,” he said, and his concern is that patients who have stopped a tumor necrosis factor inhibitor may flare if they don’t restart their medication.
While the guideline also covered recommendations for systemic lupus erythematosus, they are “very similar” to the recommendations for inflammatory arthritis, Dr. Bergman noted. “If you have somebody who is not very sick, you stop the medications,” he said, “but try to stop anything else about a week before the surgery. If they’re sick, you basically have to keep them on their medications.”
Caveats in guideline
The recommendations in the 2022 guideline come with a number of caveats, Dr. Bergman noted. For instance, the authors acknowledged limitations in the guideline regarding providing recommendations for only THA and TKA, the “paucity of evidence” around direct infection risk resulting from medications in the perioperative period for THA and TKA, the nonseparation of biologics when assessing infection risk, and the use of dosing interval as a metric for stopping the drug without considering the drug’s half-life.
A “crucial caveat,” Dr. Bergman said, was that the guideline focused on infection risk based on a statement from a panel of patients prior to the development of the 2017 guideline, which “stated very clearly any risk of infection, while rare, was more significant to them than the possibility of postoperative flares, despite flares being reported in over 60% of patients after surgery.
“For the patients, the paramount question was infection, infection, infection, infection. That’s all they cared about, and that is the basis behind a lot of the decision-making here,” Dr. Bergman said.
Another caveat came from a communication Dr. Bergman received from one of the panel members. The panel member noted there were no conclusions or recommendations provided in the guideline for how to manage perioperative flares, such as restarting a corticosteroid or biologic agent. “There was a lot of discussion about what to do with steroids if patients flare, or what to do with [other] medications if they flare, and they just couldn’t come to a consensus,” Dr. Bergman said. “It’s just not discussed.”
Dr. Bergman said he is “somewhat critical” of the ACR/AAHKS guideline, but noted it is an “ambitious project” given the lack of evidence for the recommendations. “The alternative was stop the medications forever and having people really flare, or at least try to get some semblance of rationality behind what we’re going to do,” he said.
Response from attendees
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, took issue with the new recommendations surrounding stopping infliximab. When giving a patient infliximab every 8 weeks at 3 mg/kg, “you’re giving [it] at the nadir of the drug,” he said.
Rather than drug half-life, “it’s about inflammation,” he emphasized. “Inflammation is dominant in causing infection. It drives risk more than anything. The worst thing you can do is wash someone out.
“If you’re going beyond 8 weeks on infliximab, you’re getting closer to washing them out,” he pointed out. “I think it’s a really bad idea.”
Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at Hospital for Special Surgery, both in New York, explained that the guideline is not standard of care, which would be subject to malpractice if not implemented properly.
“When you have guidelines, you follow them unless there are clinical situations which would necessitate another approach to the patient,” he said. “Professional institutions and associations will never put forth rules, they will put forth guidelines so you have the opportunity to deviate from them when the appropriate clinical situation dictates.”
Dr. Bergman reported being a speaker and consultant for AbbVie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Regeneron; he holds stock in Johnson & Johnson and Merck.
The latest guideline for perioperative management of antirheumatic medication in patients undergoing total hip (THA) and total knee arthroplasty (TKA) offers recommendations based on the latest evidence, but many of those recommendations are based on a low level of evidence, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Martin Bergman, MD, clinical professor of medicine at Drexel University, Philadelphia, said the development of the American College of Rheumatology/American Association of Hip and Knee Surgeons guideline was necessary because there was a lack of consensus on when to stop treatments prior to patients with rheumatologic disease undergoing THA and TKA, and when it was appropriate to restart those treatments.
“We all were having the same problem, and I think everybody recognized that just stopping medicines forever didn’t make sense, but maybe continuing medicines also didn’t make sense,” Dr. Bergman said.
While the 2017 ACR/AAHKS perioperative management guideline contained good recommendations, the “explosion” of new medications in rheumatology made it necessary to update the guideline with the latest data on new medications such as immunosuppressants.
2022 guideline recommendations
In the 2022 guideline, which covers disease-modifying treatments taken by patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis, the authors reaffirmed their recommendations to continue methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, and apremilast through total joint arthroplasty.
Where the 2022 guideline differs from the 2017 guideline is in which biologics are covered and under what circumstances they should be withheld and restarted around surgery. The 2022 guideline includes recommendations for abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, guselkumab, infliximab, ixekizumab, rituximab, secukinumab, tocilizumab, and ustekinumab. Each biologic has its own recommended stop and restart times based around the dosing interval and respective method of administration. Dr. Bergman said a general rule with biologics under the new guideline is that the timing of surgery should occur approximately 1 week after the first missed dose of the medication. The only biologic that does not follow this pattern is rituximab, where surgery should be planned for 1 month after the last missed dose.
Dr. Bergman noted that how the guidelines handle interval dosing with infliximab may present a problem. The guideline provides recommendations for patients receiving infliximab every 4 weeks, every 6 weeks, and every 8 weeks. However, Dr. Bergman said this can create a scenario where a patient receiving infliximab at a dose of 3 mg/kg every 8 weeks has surgery at 9 weeks, a patient receiving 5 mg/kg every 6 weeks has surgery at 7 weeks, and a patient receiving 10 mg/kg every 4 weeks has surgery at 5 weeks. “There is some intellectual problem with it,” he said.
Another change from the 2017 guideline is how long to wait for surgery after stopping Janus kinase inhibitors. While the 2017 guideline recommended withholding JAK inhibitors 7 days before surgery, the 2022 guideline lowered that waiting period to 3 days, Dr. Bergman explained.
Concerning use of steroids around THA and TKA surgery, “the days of stress steroid dosing are done,” Dr. Bergman said. “You don’t have to stress dose them. You just follow them, and you keep them on their steroid dose.”
The new guideline recommends restarting therapy once the wound is healed and there is no physical evidence of infection at approximately 2 weeks. “There’s no data to support this,” he said, and his concern is that patients who have stopped a tumor necrosis factor inhibitor may flare if they don’t restart their medication.
While the guideline also covered recommendations for systemic lupus erythematosus, they are “very similar” to the recommendations for inflammatory arthritis, Dr. Bergman noted. “If you have somebody who is not very sick, you stop the medications,” he said, “but try to stop anything else about a week before the surgery. If they’re sick, you basically have to keep them on their medications.”
Caveats in guideline
The recommendations in the 2022 guideline come with a number of caveats, Dr. Bergman noted. For instance, the authors acknowledged limitations in the guideline regarding providing recommendations for only THA and TKA, the “paucity of evidence” around direct infection risk resulting from medications in the perioperative period for THA and TKA, the nonseparation of biologics when assessing infection risk, and the use of dosing interval as a metric for stopping the drug without considering the drug’s half-life.
A “crucial caveat,” Dr. Bergman said, was that the guideline focused on infection risk based on a statement from a panel of patients prior to the development of the 2017 guideline, which “stated very clearly any risk of infection, while rare, was more significant to them than the possibility of postoperative flares, despite flares being reported in over 60% of patients after surgery.
“For the patients, the paramount question was infection, infection, infection, infection. That’s all they cared about, and that is the basis behind a lot of the decision-making here,” Dr. Bergman said.
Another caveat came from a communication Dr. Bergman received from one of the panel members. The panel member noted there were no conclusions or recommendations provided in the guideline for how to manage perioperative flares, such as restarting a corticosteroid or biologic agent. “There was a lot of discussion about what to do with steroids if patients flare, or what to do with [other] medications if they flare, and they just couldn’t come to a consensus,” Dr. Bergman said. “It’s just not discussed.”
Dr. Bergman said he is “somewhat critical” of the ACR/AAHKS guideline, but noted it is an “ambitious project” given the lack of evidence for the recommendations. “The alternative was stop the medications forever and having people really flare, or at least try to get some semblance of rationality behind what we’re going to do,” he said.
Response from attendees
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, took issue with the new recommendations surrounding stopping infliximab. When giving a patient infliximab every 8 weeks at 3 mg/kg, “you’re giving [it] at the nadir of the drug,” he said.
Rather than drug half-life, “it’s about inflammation,” he emphasized. “Inflammation is dominant in causing infection. It drives risk more than anything. The worst thing you can do is wash someone out.
“If you’re going beyond 8 weeks on infliximab, you’re getting closer to washing them out,” he pointed out. “I think it’s a really bad idea.”
Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at Hospital for Special Surgery, both in New York, explained that the guideline is not standard of care, which would be subject to malpractice if not implemented properly.
“When you have guidelines, you follow them unless there are clinical situations which would necessitate another approach to the patient,” he said. “Professional institutions and associations will never put forth rules, they will put forth guidelines so you have the opportunity to deviate from them when the appropriate clinical situation dictates.”
Dr. Bergman reported being a speaker and consultant for AbbVie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Regeneron; he holds stock in Johnson & Johnson and Merck.
FROM RWCS 2023
Adaptations to education, training vital to alleviating rheumatologist shortage
To address the growing workforce shortage in rheumatology, medical educators will have to adapt and learn how to train a new generation of rheumatologists, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, told attendees she is “heavily invested in the training of our future rheumatologists” and strives to help them “operate at the top of the level across the spectrum.”
“They’re carrying forward our field,” Dr. Dua said. “We need to propagate our field and we need them to go out and serve and continue to make rheumatology awesome.”
The American College of Rheumatology’s 2015 workforce study estimates that by 2030, there will be a shortage of more than 4,000 rheumatologists in the United States.
Rheumatology may have inadvertently created the problem through rheumatologists diagnosing disease earlier and prescribing better treatments, with patients subsequently living longer with disease, she noted. Compounding the problem is an increasing number of rheumatologists looking to retire over the next decade and the continued need for care in rural areas where there are few practicing rheumatologists.
Interest in rheumatology is increasing
The good news is there is increasing interest in the field. “This has really shifted, I would say, from about 10 years ago when I was looking at fellowships,” Dr. Dua said. “It’s not really an interest problem. But the issue is that the training programs and slots don’t necessarily exist to fill the gap of the people who are leaving the field.”
The key to bringing more people into rheumatology is to understand how Millennials and Generation Z differ from generations that came before them. In general, members of Generation Z “tend to prefer an à la carte approach to education” with hands-on experiences, and they prefer customized feedback that is actionable, Dr. Dua explained.
“As a medical educator, there are different demands, and these are changing over time, so we have to figure out how we can best serve them and educate them,” she said.
This also means connecting with younger generations on social media. A research letter published in JAMA Network Open in 2021 found a minority of 650 physicians across 14 specialties had a presence on social media platforms, with 44.9% of physicians surveyed present on LinkedIn, 23.4% on Facebook, 18.6% of on Twitter, and 14.9% on ResearchGate. “There is a lot of room to grow, and this is where some of our future teaching is headed,” Dr. Dua said.
Future of rheumatology education
Does this mean rheumatologists should start dancing in TikTok videos? Maybe not, but Dr. Dua noted there are ways to bring understanding, recall, comprehension, and behavioral change through active learning, spaced learning, case-based modules, podcasts, videos, and other educational strategies.
“We need to find ways to engage our learners and connect with them and teach them,” she said.
Rheumatologists are already bringing innovation to the education space with initiatives like educational podcasts, remote learning developed during the COVID-19 pandemic, development of rheumatology Objective Structured Clinical Examinations using challenging patient scenarios, and other virtual learning opportunities. “We really have been forced to push the envelope,” Dr. Dua said.
“The future of medical education is here. It’s exciting. Embrace it,” she said.
Training nurse practitioners and physician assistants?
Commenting on the shortage of rheumatologists, Philip J. Mease, MD, clinical professor at the University of Washington and director of rheumatology research at Swedish Medical Center, both in Seattle, said one answer to the problem may be training more nurse practitioners (NPs) and physician assistants (PAs) to bridge the gap.
“Some are suggesting that part of the answer to the deficiency of rheumatologists will be having two NPs or PAs to every single rheumatologist that there is out there,” he said. “I work with three, and the issue of ... getting access to them when they are in school to demonstrate how sexy rheumatology is, is something that is deficient, way deficient.” Rheumatologists should be putting themselves out there with preceptorships and lectures to recruit more NPs and PAs to rheumatology, he explained. “That’s a 24/7 process.”
Dr. Dua, who is cochair of the E-Learning Subcommittee within the ACR Workforce Solutions Steering Committee, said the subcomittee’s focus has been connecting with primary care doctors, pediatricians, NPs, and PAs to “expand who can provide some rheumatologic care.”
Lindsay Orme, MD, a family medicine doctor from Caldwell, Idaho, shared her experience serving as faculty for a family medicine residency program, training family medicine doctors in rural areas.
“Our curriculum hasn’t had a section for what trainees are expected to learn in rheumatology. When I did the same program years ago in Idaho, it was very well defined: What I should know how to do without consulting a cardiologist, what I should know how to do without consulting an obstetrician, what I should be able to manage in terms of [chronic kidney disease] before referring to nephrology,” she explained. “No one ever taught me what I could manage in rheumatology.
“I do think we need to find some defined areas that we’re more comfortable teaching primary care doctors to manage because there is no one – there are no rheumatologists in Boise or any of the surrounding towns that accept Medicaid patients now. They are all expected to go 250 miles away,” she said.
“That’s a major, major problem,” Dr. Dua acknowledged. “Really, for me, the goal is to develop resources that you can tap into to be able to at least figure out where things stand, and at least bide time until they can get in with that rheumatologist 250 miles away and make sure that you’re getting the training, or feel comfortable with whatever it is you’re forced to manage from a rheumatologic sense.”
More engagement, more adaptation
Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said one thing he’s noticed over the years is that, as time spent in the hospital has decreased, the time residents and fellows spend with practitioners in front of patients has also decreased. “It just isn’t there, and that’s where you really learn,” he said.
“You are 100% correct the two generations are different. What I think is important in life is very different than what the fellows think is different in life at this point, and how much work I’m willing to put in or how much work they’re willing to put in, in the same way, is very different,” he explained. “What they want to spend their time on, I don’t, and vice versa. We do have to adapt, but I do think that they need more time in front of patients with very experienced physicians.”
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said if education is to move forward, “it’s got to change dramatically.”
“The competencies aren’t always knowledge,” he said. “Knowledge has now been replaced by everything at your fingertips. I don’t need to know all the formulas and everything right now.”
Engagement should be the “main statistic that we need to be striving for,” Dr. Cush explained. “Engagement as the measure of ... education’s value, I think, is where it has to go.”
Dr. Dua reported being a consultant and serving on an advisory board for Sanofi, Novartis, AbbVie, and Chemocentryx/Amgen.
To address the growing workforce shortage in rheumatology, medical educators will have to adapt and learn how to train a new generation of rheumatologists, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, told attendees she is “heavily invested in the training of our future rheumatologists” and strives to help them “operate at the top of the level across the spectrum.”
“They’re carrying forward our field,” Dr. Dua said. “We need to propagate our field and we need them to go out and serve and continue to make rheumatology awesome.”
The American College of Rheumatology’s 2015 workforce study estimates that by 2030, there will be a shortage of more than 4,000 rheumatologists in the United States.
Rheumatology may have inadvertently created the problem through rheumatologists diagnosing disease earlier and prescribing better treatments, with patients subsequently living longer with disease, she noted. Compounding the problem is an increasing number of rheumatologists looking to retire over the next decade and the continued need for care in rural areas where there are few practicing rheumatologists.
Interest in rheumatology is increasing
The good news is there is increasing interest in the field. “This has really shifted, I would say, from about 10 years ago when I was looking at fellowships,” Dr. Dua said. “It’s not really an interest problem. But the issue is that the training programs and slots don’t necessarily exist to fill the gap of the people who are leaving the field.”
The key to bringing more people into rheumatology is to understand how Millennials and Generation Z differ from generations that came before them. In general, members of Generation Z “tend to prefer an à la carte approach to education” with hands-on experiences, and they prefer customized feedback that is actionable, Dr. Dua explained.
“As a medical educator, there are different demands, and these are changing over time, so we have to figure out how we can best serve them and educate them,” she said.
This also means connecting with younger generations on social media. A research letter published in JAMA Network Open in 2021 found a minority of 650 physicians across 14 specialties had a presence on social media platforms, with 44.9% of physicians surveyed present on LinkedIn, 23.4% on Facebook, 18.6% of on Twitter, and 14.9% on ResearchGate. “There is a lot of room to grow, and this is where some of our future teaching is headed,” Dr. Dua said.
Future of rheumatology education
Does this mean rheumatologists should start dancing in TikTok videos? Maybe not, but Dr. Dua noted there are ways to bring understanding, recall, comprehension, and behavioral change through active learning, spaced learning, case-based modules, podcasts, videos, and other educational strategies.
“We need to find ways to engage our learners and connect with them and teach them,” she said.
Rheumatologists are already bringing innovation to the education space with initiatives like educational podcasts, remote learning developed during the COVID-19 pandemic, development of rheumatology Objective Structured Clinical Examinations using challenging patient scenarios, and other virtual learning opportunities. “We really have been forced to push the envelope,” Dr. Dua said.
“The future of medical education is here. It’s exciting. Embrace it,” she said.
Training nurse practitioners and physician assistants?
Commenting on the shortage of rheumatologists, Philip J. Mease, MD, clinical professor at the University of Washington and director of rheumatology research at Swedish Medical Center, both in Seattle, said one answer to the problem may be training more nurse practitioners (NPs) and physician assistants (PAs) to bridge the gap.
“Some are suggesting that part of the answer to the deficiency of rheumatologists will be having two NPs or PAs to every single rheumatologist that there is out there,” he said. “I work with three, and the issue of ... getting access to them when they are in school to demonstrate how sexy rheumatology is, is something that is deficient, way deficient.” Rheumatologists should be putting themselves out there with preceptorships and lectures to recruit more NPs and PAs to rheumatology, he explained. “That’s a 24/7 process.”
Dr. Dua, who is cochair of the E-Learning Subcommittee within the ACR Workforce Solutions Steering Committee, said the subcomittee’s focus has been connecting with primary care doctors, pediatricians, NPs, and PAs to “expand who can provide some rheumatologic care.”
Lindsay Orme, MD, a family medicine doctor from Caldwell, Idaho, shared her experience serving as faculty for a family medicine residency program, training family medicine doctors in rural areas.
“Our curriculum hasn’t had a section for what trainees are expected to learn in rheumatology. When I did the same program years ago in Idaho, it was very well defined: What I should know how to do without consulting a cardiologist, what I should know how to do without consulting an obstetrician, what I should be able to manage in terms of [chronic kidney disease] before referring to nephrology,” she explained. “No one ever taught me what I could manage in rheumatology.
“I do think we need to find some defined areas that we’re more comfortable teaching primary care doctors to manage because there is no one – there are no rheumatologists in Boise or any of the surrounding towns that accept Medicaid patients now. They are all expected to go 250 miles away,” she said.
“That’s a major, major problem,” Dr. Dua acknowledged. “Really, for me, the goal is to develop resources that you can tap into to be able to at least figure out where things stand, and at least bide time until they can get in with that rheumatologist 250 miles away and make sure that you’re getting the training, or feel comfortable with whatever it is you’re forced to manage from a rheumatologic sense.”
More engagement, more adaptation
Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said one thing he’s noticed over the years is that, as time spent in the hospital has decreased, the time residents and fellows spend with practitioners in front of patients has also decreased. “It just isn’t there, and that’s where you really learn,” he said.
“You are 100% correct the two generations are different. What I think is important in life is very different than what the fellows think is different in life at this point, and how much work I’m willing to put in or how much work they’re willing to put in, in the same way, is very different,” he explained. “What they want to spend their time on, I don’t, and vice versa. We do have to adapt, but I do think that they need more time in front of patients with very experienced physicians.”
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said if education is to move forward, “it’s got to change dramatically.”
“The competencies aren’t always knowledge,” he said. “Knowledge has now been replaced by everything at your fingertips. I don’t need to know all the formulas and everything right now.”
Engagement should be the “main statistic that we need to be striving for,” Dr. Cush explained. “Engagement as the measure of ... education’s value, I think, is where it has to go.”
Dr. Dua reported being a consultant and serving on an advisory board for Sanofi, Novartis, AbbVie, and Chemocentryx/Amgen.
To address the growing workforce shortage in rheumatology, medical educators will have to adapt and learn how to train a new generation of rheumatologists, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Anisha B. Dua, MD, an associate professor of rheumatology at Northwestern University, Chicago, told attendees she is “heavily invested in the training of our future rheumatologists” and strives to help them “operate at the top of the level across the spectrum.”
“They’re carrying forward our field,” Dr. Dua said. “We need to propagate our field and we need them to go out and serve and continue to make rheumatology awesome.”
The American College of Rheumatology’s 2015 workforce study estimates that by 2030, there will be a shortage of more than 4,000 rheumatologists in the United States.
Rheumatology may have inadvertently created the problem through rheumatologists diagnosing disease earlier and prescribing better treatments, with patients subsequently living longer with disease, she noted. Compounding the problem is an increasing number of rheumatologists looking to retire over the next decade and the continued need for care in rural areas where there are few practicing rheumatologists.
Interest in rheumatology is increasing
The good news is there is increasing interest in the field. “This has really shifted, I would say, from about 10 years ago when I was looking at fellowships,” Dr. Dua said. “It’s not really an interest problem. But the issue is that the training programs and slots don’t necessarily exist to fill the gap of the people who are leaving the field.”
The key to bringing more people into rheumatology is to understand how Millennials and Generation Z differ from generations that came before them. In general, members of Generation Z “tend to prefer an à la carte approach to education” with hands-on experiences, and they prefer customized feedback that is actionable, Dr. Dua explained.
“As a medical educator, there are different demands, and these are changing over time, so we have to figure out how we can best serve them and educate them,” she said.
This also means connecting with younger generations on social media. A research letter published in JAMA Network Open in 2021 found a minority of 650 physicians across 14 specialties had a presence on social media platforms, with 44.9% of physicians surveyed present on LinkedIn, 23.4% on Facebook, 18.6% of on Twitter, and 14.9% on ResearchGate. “There is a lot of room to grow, and this is where some of our future teaching is headed,” Dr. Dua said.
Future of rheumatology education
Does this mean rheumatologists should start dancing in TikTok videos? Maybe not, but Dr. Dua noted there are ways to bring understanding, recall, comprehension, and behavioral change through active learning, spaced learning, case-based modules, podcasts, videos, and other educational strategies.
“We need to find ways to engage our learners and connect with them and teach them,” she said.
Rheumatologists are already bringing innovation to the education space with initiatives like educational podcasts, remote learning developed during the COVID-19 pandemic, development of rheumatology Objective Structured Clinical Examinations using challenging patient scenarios, and other virtual learning opportunities. “We really have been forced to push the envelope,” Dr. Dua said.
“The future of medical education is here. It’s exciting. Embrace it,” she said.
Training nurse practitioners and physician assistants?
Commenting on the shortage of rheumatologists, Philip J. Mease, MD, clinical professor at the University of Washington and director of rheumatology research at Swedish Medical Center, both in Seattle, said one answer to the problem may be training more nurse practitioners (NPs) and physician assistants (PAs) to bridge the gap.
“Some are suggesting that part of the answer to the deficiency of rheumatologists will be having two NPs or PAs to every single rheumatologist that there is out there,” he said. “I work with three, and the issue of ... getting access to them when they are in school to demonstrate how sexy rheumatology is, is something that is deficient, way deficient.” Rheumatologists should be putting themselves out there with preceptorships and lectures to recruit more NPs and PAs to rheumatology, he explained. “That’s a 24/7 process.”
Dr. Dua, who is cochair of the E-Learning Subcommittee within the ACR Workforce Solutions Steering Committee, said the subcomittee’s focus has been connecting with primary care doctors, pediatricians, NPs, and PAs to “expand who can provide some rheumatologic care.”
Lindsay Orme, MD, a family medicine doctor from Caldwell, Idaho, shared her experience serving as faculty for a family medicine residency program, training family medicine doctors in rural areas.
“Our curriculum hasn’t had a section for what trainees are expected to learn in rheumatology. When I did the same program years ago in Idaho, it was very well defined: What I should know how to do without consulting a cardiologist, what I should know how to do without consulting an obstetrician, what I should be able to manage in terms of [chronic kidney disease] before referring to nephrology,” she explained. “No one ever taught me what I could manage in rheumatology.
“I do think we need to find some defined areas that we’re more comfortable teaching primary care doctors to manage because there is no one – there are no rheumatologists in Boise or any of the surrounding towns that accept Medicaid patients now. They are all expected to go 250 miles away,” she said.
“That’s a major, major problem,” Dr. Dua acknowledged. “Really, for me, the goal is to develop resources that you can tap into to be able to at least figure out where things stand, and at least bide time until they can get in with that rheumatologist 250 miles away and make sure that you’re getting the training, or feel comfortable with whatever it is you’re forced to manage from a rheumatologic sense.”
More engagement, more adaptation
Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said one thing he’s noticed over the years is that, as time spent in the hospital has decreased, the time residents and fellows spend with practitioners in front of patients has also decreased. “It just isn’t there, and that’s where you really learn,” he said.
“You are 100% correct the two generations are different. What I think is important in life is very different than what the fellows think is different in life at this point, and how much work I’m willing to put in or how much work they’re willing to put in, in the same way, is very different,” he explained. “What they want to spend their time on, I don’t, and vice versa. We do have to adapt, but I do think that they need more time in front of patients with very experienced physicians.”
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said if education is to move forward, “it’s got to change dramatically.”
“The competencies aren’t always knowledge,” he said. “Knowledge has now been replaced by everything at your fingertips. I don’t need to know all the formulas and everything right now.”
Engagement should be the “main statistic that we need to be striving for,” Dr. Cush explained. “Engagement as the measure of ... education’s value, I think, is where it has to go.”
Dr. Dua reported being a consultant and serving on an advisory board for Sanofi, Novartis, AbbVie, and Chemocentryx/Amgen.
FROM RWCS 2023
Telemedicine usage still high among rheumatologists as interest wanes in other specialties
There was an explosion in the use of telemedicine during the COVID-19 pandemic, but usage has stabilized and varies between specialties. However, telemedicine use is still somewhat high among rheumatologists, according to speakers at the 2023 Rheumatology Winter Clinical Symposium.
Speaking in general about the future of rheumatology, Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said it is up to rheumatologists to adapt to the changing winds in the specialty.
“The future is going to happen no matter what, so the question is, are you going to go along with it? Are you going to be a part of it? Are you going to be resistant to it?” Dr. Cush asked attendees. “Your recent experience with COVID would tell you maybe what your path is going to be if you’re dying to get back to the way it once was.”
Rheumatologists can expect changes in where they work, how they’re paid, increases in their workload, and new innovations in connecting with patients, he said.
“You’re going to be integrating a new style of medicine, you’re going to be digitally connected,” he explained. “All these networks are going to be working together to make you supposedly better at what you do, or maybe they’re working together to make you obsolete – and I think you better start protecting your space.”
One major area of change, telemedicine, already occurred as a result of the COVID-19 pandemic and will “begin to dominate” over the next decade, Dr. Cush said. An analysis conducted by consulting firm McKinsey & Company found telehealth usage increased 78-fold between February and April 2020 before leveling off at a 38-fold higher rate, compared with prepandemic levels. In the same analysis, rheumatology ranked third in terms of telehealth usage claims behind psychiatry and substance use disorder treatment, Dr. Cush observed, as other specialties have “fallen off quite a bit.”
“The common denominators are chronic care, cognitive care, nonprocedural care, pattern recognition, and monitoring, and this is what you do,” he said. “This is why, in many ways, for you to abandon telemedicine I think is a gigantic mistake.”
Changes to telemedicine
The most immediate change to telemedicine will come when the Biden administration officially ends the COVID-19 public health emergency in May 2023, and temporary telehealth services will be extended for approximately 5 months after the end of the public health emergency. Legislation passed by Congress will ensure some of the flexibilities in telemedicine will be extended until the end of December 2024.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said he sees telemedicine as persisting even after the official COVID-19 public health emergency ends. “There’s a lot of push from the American Medical Association, from the American College of Physicians. You’re going to see people – this will not go away because [there’s] also going to be that demand.”
Despite decreased usage since April 2020, telehealth was estimated to be a $60 billion industry in 2022 and will likely increase over the next decade, Dr. Cush noted. “I question [the decline] because I think it still is a major part of your [future in] 2033.”
The number of physicians who have at least three licenses to practice in other U.S. states increased from 50,454 in 2010 to 72,752 in 2020, and that trend will continue, Dr. Wells explained. It is now becoming easier for physicians to become licensed in other states with companies like CompHealth that offer services to simplify obtaining medical licenses with states that participate in the Interstate Medical Licensure Compact.
“It’s a telemedicine easy pass,” Dr. Cush said.
Concerns in telemedicine
Commenting on the presentation, Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at the Hospital for Special Surgery (HSS), both in New York, pointed out that because telemedicine is governed by U.S. states, rather than the federal government, a physician needs to be licensed in the state where the patient is located. While many states relaxed their restrictions during COVID-19, as states began tightening their restrictions later, “many physicians didn’t want to have three licenses,” he said.
“There’s an expense in getting three licenses. There’s an expense in obtaining it and maintaining it, and the reimbursement for the telemedicine visit did not reach that expectation,” Dr. Gibofsky explained. With the exception of the orthopedic surgeons at HSS who practice in New York and a satellite office in Florida, none of the surgeons at his center have obtained more than one license to practice telemedicine in other states.
“Our volume of telemedicine at HSS has remained about the same at 30%, but fewer physicians are doing it because they don’t want to maintain multiple licensures,” he said. “So don’t overlook the role of legal concerns in terms of who’s going to be allowed to do what where. Your talk was great in terms of an exuberance of what’s going to be available, but it’s not going to relieve the physician from the burden of being responsible for their use.”
Eric Ruderman, MD, professor of rheumatology at Northwestern University, Chicago, asked the presenters about the balance between seeing patients for virtual and in-person visits. “The question is what’s the sweet spot? Are there people you’re willing to see virtually forever?” he asked, noting that he has patients scheduling telemedicine visits that he hasn’t seen since before the COVID-19 pandemic.
“That’s not going to work for me. At some point, you have to lay hands on people,” he said.
Dr. Wells said his current practice is 40% virtual, and his staff converts potential no-shows into a telemedicine consultation over the phone. “My no-show rate has gone down to zero. Somebody’s scheduled for a visit, they don’t show up, my [medical assistants] get them on the phone, they put them on hold, tee up the refills. I turn them into a telephone call,” he said. “We don’t accept the no-show at all because we can do a telephone [consultation].”
In Dr. Cush’s practice, he alternates telemedicine visits with in-person visits. “If you come back for two videos in a row, you’re catching hell from me for that,” he said. Responding to how Dr. Wells incorporates telemedicine into his practice, Dr. Cush said many rheumatologists “don’t have the setups to support the care, and that’s why it’s hard to do and that’s why we’re not as great as we could be.”
“This is the way we were trained. We’re used to seeing these patients in the clinic that often. Not every single patient needs to be seen that frequently if they’re stable and doing fine,” Dr. Wells countered.
Dr. Cush and Dr. Wells reported having financial relationships with numerous pharmaceutical companies.
There was an explosion in the use of telemedicine during the COVID-19 pandemic, but usage has stabilized and varies between specialties. However, telemedicine use is still somewhat high among rheumatologists, according to speakers at the 2023 Rheumatology Winter Clinical Symposium.
Speaking in general about the future of rheumatology, Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said it is up to rheumatologists to adapt to the changing winds in the specialty.
“The future is going to happen no matter what, so the question is, are you going to go along with it? Are you going to be a part of it? Are you going to be resistant to it?” Dr. Cush asked attendees. “Your recent experience with COVID would tell you maybe what your path is going to be if you’re dying to get back to the way it once was.”
Rheumatologists can expect changes in where they work, how they’re paid, increases in their workload, and new innovations in connecting with patients, he said.
“You’re going to be integrating a new style of medicine, you’re going to be digitally connected,” he explained. “All these networks are going to be working together to make you supposedly better at what you do, or maybe they’re working together to make you obsolete – and I think you better start protecting your space.”
One major area of change, telemedicine, already occurred as a result of the COVID-19 pandemic and will “begin to dominate” over the next decade, Dr. Cush said. An analysis conducted by consulting firm McKinsey & Company found telehealth usage increased 78-fold between February and April 2020 before leveling off at a 38-fold higher rate, compared with prepandemic levels. In the same analysis, rheumatology ranked third in terms of telehealth usage claims behind psychiatry and substance use disorder treatment, Dr. Cush observed, as other specialties have “fallen off quite a bit.”
“The common denominators are chronic care, cognitive care, nonprocedural care, pattern recognition, and monitoring, and this is what you do,” he said. “This is why, in many ways, for you to abandon telemedicine I think is a gigantic mistake.”
Changes to telemedicine
The most immediate change to telemedicine will come when the Biden administration officially ends the COVID-19 public health emergency in May 2023, and temporary telehealth services will be extended for approximately 5 months after the end of the public health emergency. Legislation passed by Congress will ensure some of the flexibilities in telemedicine will be extended until the end of December 2024.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said he sees telemedicine as persisting even after the official COVID-19 public health emergency ends. “There’s a lot of push from the American Medical Association, from the American College of Physicians. You’re going to see people – this will not go away because [there’s] also going to be that demand.”
Despite decreased usage since April 2020, telehealth was estimated to be a $60 billion industry in 2022 and will likely increase over the next decade, Dr. Cush noted. “I question [the decline] because I think it still is a major part of your [future in] 2033.”
The number of physicians who have at least three licenses to practice in other U.S. states increased from 50,454 in 2010 to 72,752 in 2020, and that trend will continue, Dr. Wells explained. It is now becoming easier for physicians to become licensed in other states with companies like CompHealth that offer services to simplify obtaining medical licenses with states that participate in the Interstate Medical Licensure Compact.
“It’s a telemedicine easy pass,” Dr. Cush said.
Concerns in telemedicine
Commenting on the presentation, Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at the Hospital for Special Surgery (HSS), both in New York, pointed out that because telemedicine is governed by U.S. states, rather than the federal government, a physician needs to be licensed in the state where the patient is located. While many states relaxed their restrictions during COVID-19, as states began tightening their restrictions later, “many physicians didn’t want to have three licenses,” he said.
“There’s an expense in getting three licenses. There’s an expense in obtaining it and maintaining it, and the reimbursement for the telemedicine visit did not reach that expectation,” Dr. Gibofsky explained. With the exception of the orthopedic surgeons at HSS who practice in New York and a satellite office in Florida, none of the surgeons at his center have obtained more than one license to practice telemedicine in other states.
“Our volume of telemedicine at HSS has remained about the same at 30%, but fewer physicians are doing it because they don’t want to maintain multiple licensures,” he said. “So don’t overlook the role of legal concerns in terms of who’s going to be allowed to do what where. Your talk was great in terms of an exuberance of what’s going to be available, but it’s not going to relieve the physician from the burden of being responsible for their use.”
Eric Ruderman, MD, professor of rheumatology at Northwestern University, Chicago, asked the presenters about the balance between seeing patients for virtual and in-person visits. “The question is what’s the sweet spot? Are there people you’re willing to see virtually forever?” he asked, noting that he has patients scheduling telemedicine visits that he hasn’t seen since before the COVID-19 pandemic.
“That’s not going to work for me. At some point, you have to lay hands on people,” he said.
Dr. Wells said his current practice is 40% virtual, and his staff converts potential no-shows into a telemedicine consultation over the phone. “My no-show rate has gone down to zero. Somebody’s scheduled for a visit, they don’t show up, my [medical assistants] get them on the phone, they put them on hold, tee up the refills. I turn them into a telephone call,” he said. “We don’t accept the no-show at all because we can do a telephone [consultation].”
In Dr. Cush’s practice, he alternates telemedicine visits with in-person visits. “If you come back for two videos in a row, you’re catching hell from me for that,” he said. Responding to how Dr. Wells incorporates telemedicine into his practice, Dr. Cush said many rheumatologists “don’t have the setups to support the care, and that’s why it’s hard to do and that’s why we’re not as great as we could be.”
“This is the way we were trained. We’re used to seeing these patients in the clinic that often. Not every single patient needs to be seen that frequently if they’re stable and doing fine,” Dr. Wells countered.
Dr. Cush and Dr. Wells reported having financial relationships with numerous pharmaceutical companies.
There was an explosion in the use of telemedicine during the COVID-19 pandemic, but usage has stabilized and varies between specialties. However, telemedicine use is still somewhat high among rheumatologists, according to speakers at the 2023 Rheumatology Winter Clinical Symposium.
Speaking in general about the future of rheumatology, Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, said it is up to rheumatologists to adapt to the changing winds in the specialty.
“The future is going to happen no matter what, so the question is, are you going to go along with it? Are you going to be a part of it? Are you going to be resistant to it?” Dr. Cush asked attendees. “Your recent experience with COVID would tell you maybe what your path is going to be if you’re dying to get back to the way it once was.”
Rheumatologists can expect changes in where they work, how they’re paid, increases in their workload, and new innovations in connecting with patients, he said.
“You’re going to be integrating a new style of medicine, you’re going to be digitally connected,” he explained. “All these networks are going to be working together to make you supposedly better at what you do, or maybe they’re working together to make you obsolete – and I think you better start protecting your space.”
One major area of change, telemedicine, already occurred as a result of the COVID-19 pandemic and will “begin to dominate” over the next decade, Dr. Cush said. An analysis conducted by consulting firm McKinsey & Company found telehealth usage increased 78-fold between February and April 2020 before leveling off at a 38-fold higher rate, compared with prepandemic levels. In the same analysis, rheumatology ranked third in terms of telehealth usage claims behind psychiatry and substance use disorder treatment, Dr. Cush observed, as other specialties have “fallen off quite a bit.”
“The common denominators are chronic care, cognitive care, nonprocedural care, pattern recognition, and monitoring, and this is what you do,” he said. “This is why, in many ways, for you to abandon telemedicine I think is a gigantic mistake.”
Changes to telemedicine
The most immediate change to telemedicine will come when the Biden administration officially ends the COVID-19 public health emergency in May 2023, and temporary telehealth services will be extended for approximately 5 months after the end of the public health emergency. Legislation passed by Congress will ensure some of the flexibilities in telemedicine will be extended until the end of December 2024.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said he sees telemedicine as persisting even after the official COVID-19 public health emergency ends. “There’s a lot of push from the American Medical Association, from the American College of Physicians. You’re going to see people – this will not go away because [there’s] also going to be that demand.”
Despite decreased usage since April 2020, telehealth was estimated to be a $60 billion industry in 2022 and will likely increase over the next decade, Dr. Cush noted. “I question [the decline] because I think it still is a major part of your [future in] 2033.”
The number of physicians who have at least three licenses to practice in other U.S. states increased from 50,454 in 2010 to 72,752 in 2020, and that trend will continue, Dr. Wells explained. It is now becoming easier for physicians to become licensed in other states with companies like CompHealth that offer services to simplify obtaining medical licenses with states that participate in the Interstate Medical Licensure Compact.
“It’s a telemedicine easy pass,” Dr. Cush said.
Concerns in telemedicine
Commenting on the presentation, Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at the Hospital for Special Surgery (HSS), both in New York, pointed out that because telemedicine is governed by U.S. states, rather than the federal government, a physician needs to be licensed in the state where the patient is located. While many states relaxed their restrictions during COVID-19, as states began tightening their restrictions later, “many physicians didn’t want to have three licenses,” he said.
“There’s an expense in getting three licenses. There’s an expense in obtaining it and maintaining it, and the reimbursement for the telemedicine visit did not reach that expectation,” Dr. Gibofsky explained. With the exception of the orthopedic surgeons at HSS who practice in New York and a satellite office in Florida, none of the surgeons at his center have obtained more than one license to practice telemedicine in other states.
“Our volume of telemedicine at HSS has remained about the same at 30%, but fewer physicians are doing it because they don’t want to maintain multiple licensures,” he said. “So don’t overlook the role of legal concerns in terms of who’s going to be allowed to do what where. Your talk was great in terms of an exuberance of what’s going to be available, but it’s not going to relieve the physician from the burden of being responsible for their use.”
Eric Ruderman, MD, professor of rheumatology at Northwestern University, Chicago, asked the presenters about the balance between seeing patients for virtual and in-person visits. “The question is what’s the sweet spot? Are there people you’re willing to see virtually forever?” he asked, noting that he has patients scheduling telemedicine visits that he hasn’t seen since before the COVID-19 pandemic.
“That’s not going to work for me. At some point, you have to lay hands on people,” he said.
Dr. Wells said his current practice is 40% virtual, and his staff converts potential no-shows into a telemedicine consultation over the phone. “My no-show rate has gone down to zero. Somebody’s scheduled for a visit, they don’t show up, my [medical assistants] get them on the phone, they put them on hold, tee up the refills. I turn them into a telephone call,” he said. “We don’t accept the no-show at all because we can do a telephone [consultation].”
In Dr. Cush’s practice, he alternates telemedicine visits with in-person visits. “If you come back for two videos in a row, you’re catching hell from me for that,” he said. Responding to how Dr. Wells incorporates telemedicine into his practice, Dr. Cush said many rheumatologists “don’t have the setups to support the care, and that’s why it’s hard to do and that’s why we’re not as great as we could be.”
“This is the way we were trained. We’re used to seeing these patients in the clinic that often. Not every single patient needs to be seen that frequently if they’re stable and doing fine,” Dr. Wells countered.
Dr. Cush and Dr. Wells reported having financial relationships with numerous pharmaceutical companies.
FROM RWCS 2023
Are repeat radiographs necessary in rheumatoid and psoriatic arthritis?
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
Follow-up radiographs after an initial baseline reading in patients with rheumatoid arthritis or psoriatic arthritis may still show radiographic progression despite treatment with current therapies, but it’s unclear if they will affect treatment decisions between patients and doctors that may take place regardless of the radiographic information, according to arguments made for and against their usefulness in a point-counterpoint session at the 2023 Rheumatology Winter Clinical Symposium.
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Health in Franklin, Wisc., said that x-rays “reflect the history of joint pathology” and can get worse over time, correlating with disease activity and severity.
While RA does not yet have the “holy grail” of complete or true remission, Dr. Wells argued, the combination of clinical remission, laboratory remission, and imaging remission gets patients with RA close to the ideal when measured over time. “You need to continue to monitor these patients as you follow them along,” he said.
The BARFOT study, which evaluated 1,938 patients with early RA in two cohorts during 1992-1999 and again between 2000 and 2006, showed that more active treatments in the 2000s did not result in improvements in Health Assessment Questionnaire (HAQ) and pain scores, compared with patients treated in the 1990s. “You can see in some of those patients those scores do increase, and that even despite aggressive therapies that we had in 2006, you can still see some of those patients still have progression of the disease,” Dr. Wells explained. “How did they know? Because they looked.”
He also cited a study from researchers at the Mayo Clinic who examined 586 patients with RA that showed a higher prevalence of functional disability in patients with RA who also had radiographic changes, compared with patients without RA. “Radiographic changes correlate with disease severity and functional disability as well,” Dr. Wells said.
Just as prostate-specific antigen levels are used in prostate cancer screening and hemoglobin A1c is measured in diabetes management, radiographs should be used to track progression of disease in RA and PsA, Dr. Wells argued. “[I]f you don’t know, you can’t treat,” he said.
Some patients near remission may have radiographic progression even though disease activity measurements such as C-reactive protein (CRP) values do not show presence of active disease. In a study analyzing 1,184 patients with RA in the ASPIRE, ERA, Leflunomide, PREMIER and TEMPO trials, swollen joint count (SJC) was a better predictor of radiographic progression than CRP in patients near remission.
“[E]ven where you don’t see smoke, there still could be fire,” Dr. Wells said. “Some of these patients still progress and these are outliers, and the way they saw that [was] because they followed those patients along. If you don’t look, you don’t know.”
Radiographic progression can also be seen among nonswollen joints in patients with RA and PsA. In a study of 1,207 joints in 55 patients with RA and 352 joints in 18 patients with PsA, researchers in Austria found tenderness in nonswollen joints was associated with radiographic progression.
Despite having effective treatments in RA and PsA, “none of our therapies show that they’re able to prevent progression,” Dr. Wells said.
When it comes to hitting the treatment target in RA, some rheumatologists may think they can accomplish it without use of repeated radiographs. “I have a different perspective on that – that you really do indeed need to do the x-rays today and follow those x-rays along, especially if it’s going to change your treatment paradigm and what your treatment decision would be for the patient,” he said.
Counterpoint: Repeat radiographs aren’t helpful
Almost all rheumatologists would likely order an initial radiograph for their patients with RA or PsA, Roy M. Fleischmann, MD, clinical professor of medicine at the University of Texas and codirector of the Metroplex Clinical Research Center, both in Dallas, said in his presentation.
“If you see erosions when you start, chances are you’re going to be much more aggressive,” Dr. Fleischmann said. “So it is justification for early, more aggressive treatment of disease.”
In recent decades, radiographic progression in RA has decreased as more effective antirheumatic treatments have come into use, Dr. Fleischmann argued.
“We had x-ray progression in virtually everybody, and it was consistent no matter what we treated with, which was gold or penicillamine or any of the NSAIDs or sulfasalazine,” he said. “With methotrexate ... about 60% of patients actually have no x-ray progression, and that was a major change, and that’s one of the reasons why methotrexate has become the keystone of therapy. But even with methotrexate, [we] still had many patients who progressed.”
After the introduction of tumor necrosis factor inhibitors and other mechanisms in the late 1990s, “all of a sudden, you don’t see x-ray progression – mean x-ray progression – in a group of patients,” he noted.
Many rheumatologists now use a treat-to-target strategy, and if the patient achieves true clinical remission or sustained very low disease activity as measured by Boolean remission, Simple Disease Activity Index, or Clinical Disease Activity Index, they have “very little chance of radiographic progression and functional decline,” he said.
“If a patient doesn’t achieve remission or very low disease activity, obtaining a radiograph doesn’t change what you do because the patient’s not where they want to be, where you want them to be; you’re going to make a change anyway,” Dr. Fleischmann explained. “The radiograph isn’t going to help you do that.”
If a patient is in sustained remission but a radiograph is ordered and shows disease progression, he questioned what the rheumatologist would do in that situation.
“Now the patient’s in, let’s say, a Boolean remission. They have no tender joints. They have no swollen joints ... their pain assessment is zero, their CRP is zero, and they do have some x-ray progression. Where are you going to change?” Dr. Fleischmann asked. “There’s no data that anything else would work. I don’t know what you would do. So, in conclusion, I would say you really don’t need to repeat an x-ray.”
AI reading x-rays?
Commenting on the point-counterpoint session, Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego, and director of RWCS, asked Dr. Fleischmann and Dr. Wells how they address the issue of how many radiologists seem to be unfamiliar with reading hand radiographs and RA progression.
Dr. Fleischmann said he was trained in how to read hand radiographs in medical school, but that training no longer appears to be occurring. “If you have a good bone radiologist, of which there are not a lot, you’re great. But if you don’t have a really good bone radiologist, it’s difficult,” he said.
Dr. Kavanaugh alluded to the advancement of artificial intelligence (AI) in radiology and posed the question of how both rheumatologists felt about AI reading and interpreting their radiographs. “If you could reliably submit x-rays and they would say what the Sharp score was and where the differences were, would that change anything?” he asked.
“I think having artificial intelligence read the x-ray or an MRI is really, really good. It’ll be better than the radiologists,” Dr. Fleischmann responded. “But I don’t think that you really need to repeat the x-ray. I mean, I really don’t think you need to repeat it. You need to treat the patient.”
Dr. Wells reported having financial relationships with numerous pharmaceutical companies. Dr. Fleischmann reported no relevant financial relationships.
FROM RWCS 2023
Remote electrical neuromodulation device helps reduce migraine days
, according to recent research published in the journal Headache.
The prospective, randomized, double-blind, placebo-controlled, multicenter trial showed that remote electrical neuromodulation (REN) with Nerivio (Theranica Bio-Electronics Ltd.; Bridgewater, N.J.) found a mean reduction/decrease in the number of migraine days by an average of 4.0 days per month, according to Stewart J. Tepper MD, of the Geisel School of Medicine at Dartmouth in Hanover, N.H., and colleagues.*
“The statistically significant results were maintained in separate subanalyses of the chronic and episodic subsamples, as well as in the separate subanalyses of participants who used and did not use migraine prophylaxis,” Dr. Tepper and colleagues wrote.
A nonpharmacological alternative
Researchers randomized 248 participants into active and placebo groups, with 95 participants in the active group and 84 participants in the placebo group meeting the criteria for a modified intention-to-treat (mITT) analysis. Most of the participants in the ITT dataset were women (85.9%) with an average age of 41.7 years, and a baseline average of 12.2 migraine days and 15.6 headache days. Overall, 52.4% of participants in the ITT dataset had chronic migraine, 25.0% had migraine with aura, and 41.1% were taking preventative medication.
Dr. Tepper and colleagues followed participants for 4 weeks at baseline for observation followed by 8 weeks of participants using the REN device every other day for 45 minutes, or a placebo device that “produces electrical pulses of the same maximum intensity (34 mA) and overall energy, but with different pulse durations and much lower frequencies compared with the active device.” Participants completed a daily diary where they recorded their symptoms.
Researchers assessed the mean change in number of migraine days per month as a primary outcome, and evaluated participants who experienced episodic and chronic migraines separately in subgroup analyses. Secondary outcome measures included mean change in number of moderate or severe headache days, 50% reduction in mean number of headache days compared with baseline, Headache Impact Test short form (HIT-6) and Migraine Specific Quality of Life Questionnaire (MSQ) Role Function Domain total score mean change at 12 weeks compared with week 1, and reduction in mean number of days taking acute headache or migraine medication.
Participants receiving REN treatment had a significant reduction in mean migraine days per month compared with the placebo group (4.0 days vs. 1.3 days; 95% confidence interval, –3.9 days to –1.5 days; P < .001). In subgroup analyses, a significant reduction in migraine days was seen in participants receiving REN treatment with episodic migraine (3.2 days vs. 1.0 days; P = .003) and chronic migraine (4.7 days vs. 1.6 days; P = .001) compared with placebo.
Dr. Tepper and colleagues found a significant reduction in moderate and/or severe headache days among participants receiving REN treatment compared with placebo (3.8 days vs. 2.2 days; P = .005), a significant reduction in headache days overall compared with placebo (4.5 days vs. 1.8 days; P < .001), a significant percentage of patients who experienced 50% reduction in moderate and/or severe headache days compared with placebo (51.6% vs. 35.7%; P = .033), and a significant reduction in acute medication days compared with placebo (3.5 days vs. 1.4 days; P = .001). Dr. Tepper and colleagues found no serious device-related adverse events in either group.
The researchers noted that REN therapy is a “much-needed nonpharmacological alternative” to other preventive and acute treatments for migraine. “Given the previously well-established clinical efficacy and high safety profile in acute treatment of migraine, REN can cover the entire treatment spectrum of migraine, including both acute and preventive treatments,” they said.
‘A good place to start’
Commenting on the study, Alan M. Rapoport, MD, clinical professor of neurology at University of California, Los Angeles; past president of the International Headache Society; and editor-in-chief of Neurology Reviews, said the study was well designed, but acknowledged the 8-week follow-up time for participants as one potential area where he would have wanted to see more data.
As a medical device cleared for use by the Food and Drug Administration for acute treatment of migraine, the REM device also appears to be effective as a migraine preventative based on the results of the study with “virtually no adverse events,” he noted.
“I think this is a great treatment. I think it’s a good place to start,” Dr. Rapoport said. Given the low adverse event rate, he said he would be willing to offer the device to patients as a first option for preventing migraine and either switch to another preventative option or add an additional medication in combination based on how the patient responds. However, at the moment, he noted that this device is not covered by insurance.
Now that a REN device has been shown to work in the acute setting and as a preventative, Dr. Rapoport said he is interested in seeing other devices that have been cleared by the FDA as migraine treatments evaluated in migraine prevention. “I think we need more patients tried on the devices so we get an idea of which ones work acutely, which ones work preventively,” he said.
The authors reported personal and institutional relationships in the form of advisory board positions, consultancies, grants, research principal investigator roles, royalties, speakers bureau positions, and stockholders for a variety of pharmaceutical companies, agencies, and other organizations. Several authors disclosed ties with Theranica, the manufacturer of the REN device used in the study. Dr. Rapoport is editor-in-chief of Neurology Reviews and a consultant for Theranica, but was not involved in studies associated with the REN device.
Correction, 2/10/23: An earlier version of this article misstated the reduction in number of migraine days.
, according to recent research published in the journal Headache.
The prospective, randomized, double-blind, placebo-controlled, multicenter trial showed that remote electrical neuromodulation (REN) with Nerivio (Theranica Bio-Electronics Ltd.; Bridgewater, N.J.) found a mean reduction/decrease in the number of migraine days by an average of 4.0 days per month, according to Stewart J. Tepper MD, of the Geisel School of Medicine at Dartmouth in Hanover, N.H., and colleagues.*
“The statistically significant results were maintained in separate subanalyses of the chronic and episodic subsamples, as well as in the separate subanalyses of participants who used and did not use migraine prophylaxis,” Dr. Tepper and colleagues wrote.
A nonpharmacological alternative
Researchers randomized 248 participants into active and placebo groups, with 95 participants in the active group and 84 participants in the placebo group meeting the criteria for a modified intention-to-treat (mITT) analysis. Most of the participants in the ITT dataset were women (85.9%) with an average age of 41.7 years, and a baseline average of 12.2 migraine days and 15.6 headache days. Overall, 52.4% of participants in the ITT dataset had chronic migraine, 25.0% had migraine with aura, and 41.1% were taking preventative medication.
Dr. Tepper and colleagues followed participants for 4 weeks at baseline for observation followed by 8 weeks of participants using the REN device every other day for 45 minutes, or a placebo device that “produces electrical pulses of the same maximum intensity (34 mA) and overall energy, but with different pulse durations and much lower frequencies compared with the active device.” Participants completed a daily diary where they recorded their symptoms.
Researchers assessed the mean change in number of migraine days per month as a primary outcome, and evaluated participants who experienced episodic and chronic migraines separately in subgroup analyses. Secondary outcome measures included mean change in number of moderate or severe headache days, 50% reduction in mean number of headache days compared with baseline, Headache Impact Test short form (HIT-6) and Migraine Specific Quality of Life Questionnaire (MSQ) Role Function Domain total score mean change at 12 weeks compared with week 1, and reduction in mean number of days taking acute headache or migraine medication.
Participants receiving REN treatment had a significant reduction in mean migraine days per month compared with the placebo group (4.0 days vs. 1.3 days; 95% confidence interval, –3.9 days to –1.5 days; P < .001). In subgroup analyses, a significant reduction in migraine days was seen in participants receiving REN treatment with episodic migraine (3.2 days vs. 1.0 days; P = .003) and chronic migraine (4.7 days vs. 1.6 days; P = .001) compared with placebo.
Dr. Tepper and colleagues found a significant reduction in moderate and/or severe headache days among participants receiving REN treatment compared with placebo (3.8 days vs. 2.2 days; P = .005), a significant reduction in headache days overall compared with placebo (4.5 days vs. 1.8 days; P < .001), a significant percentage of patients who experienced 50% reduction in moderate and/or severe headache days compared with placebo (51.6% vs. 35.7%; P = .033), and a significant reduction in acute medication days compared with placebo (3.5 days vs. 1.4 days; P = .001). Dr. Tepper and colleagues found no serious device-related adverse events in either group.
The researchers noted that REN therapy is a “much-needed nonpharmacological alternative” to other preventive and acute treatments for migraine. “Given the previously well-established clinical efficacy and high safety profile in acute treatment of migraine, REN can cover the entire treatment spectrum of migraine, including both acute and preventive treatments,” they said.
‘A good place to start’
Commenting on the study, Alan M. Rapoport, MD, clinical professor of neurology at University of California, Los Angeles; past president of the International Headache Society; and editor-in-chief of Neurology Reviews, said the study was well designed, but acknowledged the 8-week follow-up time for participants as one potential area where he would have wanted to see more data.
As a medical device cleared for use by the Food and Drug Administration for acute treatment of migraine, the REM device also appears to be effective as a migraine preventative based on the results of the study with “virtually no adverse events,” he noted.
“I think this is a great treatment. I think it’s a good place to start,” Dr. Rapoport said. Given the low adverse event rate, he said he would be willing to offer the device to patients as a first option for preventing migraine and either switch to another preventative option or add an additional medication in combination based on how the patient responds. However, at the moment, he noted that this device is not covered by insurance.
Now that a REN device has been shown to work in the acute setting and as a preventative, Dr. Rapoport said he is interested in seeing other devices that have been cleared by the FDA as migraine treatments evaluated in migraine prevention. “I think we need more patients tried on the devices so we get an idea of which ones work acutely, which ones work preventively,” he said.
The authors reported personal and institutional relationships in the form of advisory board positions, consultancies, grants, research principal investigator roles, royalties, speakers bureau positions, and stockholders for a variety of pharmaceutical companies, agencies, and other organizations. Several authors disclosed ties with Theranica, the manufacturer of the REN device used in the study. Dr. Rapoport is editor-in-chief of Neurology Reviews and a consultant for Theranica, but was not involved in studies associated with the REN device.
Correction, 2/10/23: An earlier version of this article misstated the reduction in number of migraine days.
, according to recent research published in the journal Headache.
The prospective, randomized, double-blind, placebo-controlled, multicenter trial showed that remote electrical neuromodulation (REN) with Nerivio (Theranica Bio-Electronics Ltd.; Bridgewater, N.J.) found a mean reduction/decrease in the number of migraine days by an average of 4.0 days per month, according to Stewart J. Tepper MD, of the Geisel School of Medicine at Dartmouth in Hanover, N.H., and colleagues.*
“The statistically significant results were maintained in separate subanalyses of the chronic and episodic subsamples, as well as in the separate subanalyses of participants who used and did not use migraine prophylaxis,” Dr. Tepper and colleagues wrote.
A nonpharmacological alternative
Researchers randomized 248 participants into active and placebo groups, with 95 participants in the active group and 84 participants in the placebo group meeting the criteria for a modified intention-to-treat (mITT) analysis. Most of the participants in the ITT dataset were women (85.9%) with an average age of 41.7 years, and a baseline average of 12.2 migraine days and 15.6 headache days. Overall, 52.4% of participants in the ITT dataset had chronic migraine, 25.0% had migraine with aura, and 41.1% were taking preventative medication.
Dr. Tepper and colleagues followed participants for 4 weeks at baseline for observation followed by 8 weeks of participants using the REN device every other day for 45 minutes, or a placebo device that “produces electrical pulses of the same maximum intensity (34 mA) and overall energy, but with different pulse durations and much lower frequencies compared with the active device.” Participants completed a daily diary where they recorded their symptoms.
Researchers assessed the mean change in number of migraine days per month as a primary outcome, and evaluated participants who experienced episodic and chronic migraines separately in subgroup analyses. Secondary outcome measures included mean change in number of moderate or severe headache days, 50% reduction in mean number of headache days compared with baseline, Headache Impact Test short form (HIT-6) and Migraine Specific Quality of Life Questionnaire (MSQ) Role Function Domain total score mean change at 12 weeks compared with week 1, and reduction in mean number of days taking acute headache or migraine medication.
Participants receiving REN treatment had a significant reduction in mean migraine days per month compared with the placebo group (4.0 days vs. 1.3 days; 95% confidence interval, –3.9 days to –1.5 days; P < .001). In subgroup analyses, a significant reduction in migraine days was seen in participants receiving REN treatment with episodic migraine (3.2 days vs. 1.0 days; P = .003) and chronic migraine (4.7 days vs. 1.6 days; P = .001) compared with placebo.
Dr. Tepper and colleagues found a significant reduction in moderate and/or severe headache days among participants receiving REN treatment compared with placebo (3.8 days vs. 2.2 days; P = .005), a significant reduction in headache days overall compared with placebo (4.5 days vs. 1.8 days; P < .001), a significant percentage of patients who experienced 50% reduction in moderate and/or severe headache days compared with placebo (51.6% vs. 35.7%; P = .033), and a significant reduction in acute medication days compared with placebo (3.5 days vs. 1.4 days; P = .001). Dr. Tepper and colleagues found no serious device-related adverse events in either group.
The researchers noted that REN therapy is a “much-needed nonpharmacological alternative” to other preventive and acute treatments for migraine. “Given the previously well-established clinical efficacy and high safety profile in acute treatment of migraine, REN can cover the entire treatment spectrum of migraine, including both acute and preventive treatments,” they said.
‘A good place to start’
Commenting on the study, Alan M. Rapoport, MD, clinical professor of neurology at University of California, Los Angeles; past president of the International Headache Society; and editor-in-chief of Neurology Reviews, said the study was well designed, but acknowledged the 8-week follow-up time for participants as one potential area where he would have wanted to see more data.
As a medical device cleared for use by the Food and Drug Administration for acute treatment of migraine, the REM device also appears to be effective as a migraine preventative based on the results of the study with “virtually no adverse events,” he noted.
“I think this is a great treatment. I think it’s a good place to start,” Dr. Rapoport said. Given the low adverse event rate, he said he would be willing to offer the device to patients as a first option for preventing migraine and either switch to another preventative option or add an additional medication in combination based on how the patient responds. However, at the moment, he noted that this device is not covered by insurance.
Now that a REN device has been shown to work in the acute setting and as a preventative, Dr. Rapoport said he is interested in seeing other devices that have been cleared by the FDA as migraine treatments evaluated in migraine prevention. “I think we need more patients tried on the devices so we get an idea of which ones work acutely, which ones work preventively,” he said.
The authors reported personal and institutional relationships in the form of advisory board positions, consultancies, grants, research principal investigator roles, royalties, speakers bureau positions, and stockholders for a variety of pharmaceutical companies, agencies, and other organizations. Several authors disclosed ties with Theranica, the manufacturer of the REN device used in the study. Dr. Rapoport is editor-in-chief of Neurology Reviews and a consultant for Theranica, but was not involved in studies associated with the REN device.
Correction, 2/10/23: An earlier version of this article misstated the reduction in number of migraine days.
FROM HEADACHE
Brepocitinib improves symptoms of mild to moderate AD in phase 2b trial
compared with a group that received vehicle, in a recently published study..
The investigators said that brepocitinib, an investigational dual tyrosine kinase 2 (TYK2) and Janus kinase 1 (JAK1) inhibitor, was effective and well tolerated in patients with mild to moderate AD based on improvements in multiple measures, including Eczema Area and Severity Index (EASI) total score and Investigator Global Assessment (IGA) responder rates. Brepocitinib also reduced pruritus symptoms as early as 2 days after the start of treatment, they noted.
“This study supports the further evaluation of topical brepocitinib as a novel treatment for mild to moderate AD,” Megan N. Landis, MD, of the department of medicine at the University of Louisville (Ky.) and colleagues wrote in the study published in the British Journal of Dermatology.
They evaluated brepocitinib in a phase 2b, double-blind, dose-ranging study where 292 patients were randomized to receive brepocitinib once daily (brepocitinib 0.1%, 0.3%, 1.0%, 3.0%) or twice daily (brepocitinib 0.3%, 1.0%), or vehicle for 6 weeks. At 6 weeks, the researchers assessed EASI total score as a primary outcome, an IGA score of 0 or 1 as a secondary outcome. The mean age of the patients was 40 years (range, 13-74), almost 60% were White, 17.5% were Black, and about 20% were Asian.
Compared with the corresponding once-daily vehicle group (least squares mean reduction of –44.4; 90% confidence interval, –57.3 to –31.6) and the twice-daily vehicle group (LSM, –47.6; 90% CI, –57.5 to –37.7) , the brepocitinib 1% once-daily group (LSM, –70.1; 90% CI, –82.1 to –58.0) and twice-daily group (LSM, –75.0; 90% CI, –83.8 to –66.2) had significant percentage reductions in EASI total score compared with baseline at 6 weeks. Patients in the other brepocitinib dose groups had nonsignificant reductions in EASI from baseline.
Regarding secondary outcomes, a significantly higher percentage of patients in five of the six active treatment groups achieved an IGA score of 0 or 1 and at least a 2-point reduction in IGA score in the once-daily brepocitinib 0.1% group (29.7%; 90% CI, 18.5%-43.3%), 0.3% group (33.3%; 90% CI, 21.3%-47.0%), 1.0% group (40.5%; 90% CI, 28.0%-54.4%), 3.0% group (44.4%; 90% CI, 30.2%-59.1%), and brepocitinib 0.3% twice-daily group (33.3%; 90% CI, 21.3%-47.0%) compared with the once-daily (10.8%; 90% CI, 4.8%-22.2%) and twice-daily (13.9%; 90% CI, 6.9%-25.4%) vehicle groups.
The study authors noted that 37.0% of patients overall experienced treatment-emergent adverse events (TEAEs), with most TEAEs occurring in the once-daily vehicle (48.6%), twice-daily vehicle (47.2%), and brepocitinib 0.1% (45.9%) groups. Adverse events were not considered dose dependent, and no group had any serious TEAEs or deaths.
Nasopharyngitis and worsening AD were the most common TEAEs reported, with about 8% of those in the vehicle groups experiencing worsening AD.
Brepocitinib is also currently being developed as a treatment for dermatomyositis, systemic lupus erythematosus, hidradenitis suppurativa, and noninfectious uveitis by Priovant Therapeutics, a company founded by Pfizer and Roivant Sciences.
In September 2021, the Food and Drug Administration approved topical ruxolitinib cream for the treatment of patients with mild to moderate atopic dermatitis aged 12 years and older, the first topical JAK inhibitor approved for AD.
This study was sponsored by Pfizer. The authors reported personal and institutional relationships in the form of investigator positions, fees, honoraria, research grants, employee positions, and holding stock or shares for a variety of pharmaceutical, life science, and biotechnology companies.
compared with a group that received vehicle, in a recently published study..
The investigators said that brepocitinib, an investigational dual tyrosine kinase 2 (TYK2) and Janus kinase 1 (JAK1) inhibitor, was effective and well tolerated in patients with mild to moderate AD based on improvements in multiple measures, including Eczema Area and Severity Index (EASI) total score and Investigator Global Assessment (IGA) responder rates. Brepocitinib also reduced pruritus symptoms as early as 2 days after the start of treatment, they noted.
“This study supports the further evaluation of topical brepocitinib as a novel treatment for mild to moderate AD,” Megan N. Landis, MD, of the department of medicine at the University of Louisville (Ky.) and colleagues wrote in the study published in the British Journal of Dermatology.
They evaluated brepocitinib in a phase 2b, double-blind, dose-ranging study where 292 patients were randomized to receive brepocitinib once daily (brepocitinib 0.1%, 0.3%, 1.0%, 3.0%) or twice daily (brepocitinib 0.3%, 1.0%), or vehicle for 6 weeks. At 6 weeks, the researchers assessed EASI total score as a primary outcome, an IGA score of 0 or 1 as a secondary outcome. The mean age of the patients was 40 years (range, 13-74), almost 60% were White, 17.5% were Black, and about 20% were Asian.
Compared with the corresponding once-daily vehicle group (least squares mean reduction of –44.4; 90% confidence interval, –57.3 to –31.6) and the twice-daily vehicle group (LSM, –47.6; 90% CI, –57.5 to –37.7) , the brepocitinib 1% once-daily group (LSM, –70.1; 90% CI, –82.1 to –58.0) and twice-daily group (LSM, –75.0; 90% CI, –83.8 to –66.2) had significant percentage reductions in EASI total score compared with baseline at 6 weeks. Patients in the other brepocitinib dose groups had nonsignificant reductions in EASI from baseline.
Regarding secondary outcomes, a significantly higher percentage of patients in five of the six active treatment groups achieved an IGA score of 0 or 1 and at least a 2-point reduction in IGA score in the once-daily brepocitinib 0.1% group (29.7%; 90% CI, 18.5%-43.3%), 0.3% group (33.3%; 90% CI, 21.3%-47.0%), 1.0% group (40.5%; 90% CI, 28.0%-54.4%), 3.0% group (44.4%; 90% CI, 30.2%-59.1%), and brepocitinib 0.3% twice-daily group (33.3%; 90% CI, 21.3%-47.0%) compared with the once-daily (10.8%; 90% CI, 4.8%-22.2%) and twice-daily (13.9%; 90% CI, 6.9%-25.4%) vehicle groups.
The study authors noted that 37.0% of patients overall experienced treatment-emergent adverse events (TEAEs), with most TEAEs occurring in the once-daily vehicle (48.6%), twice-daily vehicle (47.2%), and brepocitinib 0.1% (45.9%) groups. Adverse events were not considered dose dependent, and no group had any serious TEAEs or deaths.
Nasopharyngitis and worsening AD were the most common TEAEs reported, with about 8% of those in the vehicle groups experiencing worsening AD.
Brepocitinib is also currently being developed as a treatment for dermatomyositis, systemic lupus erythematosus, hidradenitis suppurativa, and noninfectious uveitis by Priovant Therapeutics, a company founded by Pfizer and Roivant Sciences.
In September 2021, the Food and Drug Administration approved topical ruxolitinib cream for the treatment of patients with mild to moderate atopic dermatitis aged 12 years and older, the first topical JAK inhibitor approved for AD.
This study was sponsored by Pfizer. The authors reported personal and institutional relationships in the form of investigator positions, fees, honoraria, research grants, employee positions, and holding stock or shares for a variety of pharmaceutical, life science, and biotechnology companies.
compared with a group that received vehicle, in a recently published study..
The investigators said that brepocitinib, an investigational dual tyrosine kinase 2 (TYK2) and Janus kinase 1 (JAK1) inhibitor, was effective and well tolerated in patients with mild to moderate AD based on improvements in multiple measures, including Eczema Area and Severity Index (EASI) total score and Investigator Global Assessment (IGA) responder rates. Brepocitinib also reduced pruritus symptoms as early as 2 days after the start of treatment, they noted.
“This study supports the further evaluation of topical brepocitinib as a novel treatment for mild to moderate AD,” Megan N. Landis, MD, of the department of medicine at the University of Louisville (Ky.) and colleagues wrote in the study published in the British Journal of Dermatology.
They evaluated brepocitinib in a phase 2b, double-blind, dose-ranging study where 292 patients were randomized to receive brepocitinib once daily (brepocitinib 0.1%, 0.3%, 1.0%, 3.0%) or twice daily (brepocitinib 0.3%, 1.0%), or vehicle for 6 weeks. At 6 weeks, the researchers assessed EASI total score as a primary outcome, an IGA score of 0 or 1 as a secondary outcome. The mean age of the patients was 40 years (range, 13-74), almost 60% were White, 17.5% were Black, and about 20% were Asian.
Compared with the corresponding once-daily vehicle group (least squares mean reduction of –44.4; 90% confidence interval, –57.3 to –31.6) and the twice-daily vehicle group (LSM, –47.6; 90% CI, –57.5 to –37.7) , the brepocitinib 1% once-daily group (LSM, –70.1; 90% CI, –82.1 to –58.0) and twice-daily group (LSM, –75.0; 90% CI, –83.8 to –66.2) had significant percentage reductions in EASI total score compared with baseline at 6 weeks. Patients in the other brepocitinib dose groups had nonsignificant reductions in EASI from baseline.
Regarding secondary outcomes, a significantly higher percentage of patients in five of the six active treatment groups achieved an IGA score of 0 or 1 and at least a 2-point reduction in IGA score in the once-daily brepocitinib 0.1% group (29.7%; 90% CI, 18.5%-43.3%), 0.3% group (33.3%; 90% CI, 21.3%-47.0%), 1.0% group (40.5%; 90% CI, 28.0%-54.4%), 3.0% group (44.4%; 90% CI, 30.2%-59.1%), and brepocitinib 0.3% twice-daily group (33.3%; 90% CI, 21.3%-47.0%) compared with the once-daily (10.8%; 90% CI, 4.8%-22.2%) and twice-daily (13.9%; 90% CI, 6.9%-25.4%) vehicle groups.
The study authors noted that 37.0% of patients overall experienced treatment-emergent adverse events (TEAEs), with most TEAEs occurring in the once-daily vehicle (48.6%), twice-daily vehicle (47.2%), and brepocitinib 0.1% (45.9%) groups. Adverse events were not considered dose dependent, and no group had any serious TEAEs or deaths.
Nasopharyngitis and worsening AD were the most common TEAEs reported, with about 8% of those in the vehicle groups experiencing worsening AD.
Brepocitinib is also currently being developed as a treatment for dermatomyositis, systemic lupus erythematosus, hidradenitis suppurativa, and noninfectious uveitis by Priovant Therapeutics, a company founded by Pfizer and Roivant Sciences.
In September 2021, the Food and Drug Administration approved topical ruxolitinib cream for the treatment of patients with mild to moderate atopic dermatitis aged 12 years and older, the first topical JAK inhibitor approved for AD.
This study was sponsored by Pfizer. The authors reported personal and institutional relationships in the form of investigator positions, fees, honoraria, research grants, employee positions, and holding stock or shares for a variety of pharmaceutical, life science, and biotechnology companies.
FROM BRITISH JOURNAL OF DERMATOLOGY
ICD-10 code can identify patients with melasma for future study
To better understand melasma, it is important for researchers to find groups of patients with confirmed disease for future clinical study. A recent for researchers interested in conducting retrospective studies of this patient population.
“Overall, our results support the validity of using the ICD-10 code for melasma to identify patients with a diagnosis of melasma for future studies,” Nicholas Theodosakis, MD, PhD, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues wrote in their research letter. “Despite some variability in diagnostic confidence, most patients were ultimately classified as moderately or highly likely to have a true diagnosis of melasma.”
Dr. Theodosakis and colleagues evaluated data from 5,322 adult patients in the Mass General Brigham Research Patient Data Registry between October 2015 and January 2021 who had an encounter that used the ICD-10 code for melasma (L81.1). The researchers then validated the ICD-10 code by examining the medical records of 300 patients (5.6%), confirming that melasma was the clinician’s favored diagnosis and that the patient met secondary diagnostic criteria. Confidence was rated in categories of “low confidence,” “moderate confidence,” “high confidence,” and “maximum confidence” based on secondary criteria such as hyperpigmentation of the face and upper body, hormone-related therapy exposure before diagnosis, pregnancy history, and dermatologist-confirmed diagnosis.
The patients who had their medical records examined for confirmed melasma were primarily women (285 patients; 95.0%) and were a mean 48.4 years old at diagnosis.
Of those in the validation cohort, melasma was the preferred diagnosis for clinicians of 291 patients (97.0%), while 274 patients (91.3%) had secondary diagnostic criteria of hyperpigmentation of the face and upper body and 252 patients (84.0%) had received a diagnosis from a dermatologist. Other less common secondary diagnostic criteria of the patient group were a history of having received hormone-related therapy before a melasma diagnosis (148 patients; 49.3%) and a history of pregnancy (168 patients; 56.0%). Based on identification of secondary diagnostic criteria, confidence in melasma diagnosis was high for 208 patients (69.3%), moderate for 61 patients (20.3%), and low for 31 patients (10.3%).
Dr. Theodosakis and colleagues noted their study was limited by its retrospective nature and the presence of a small validation cohort. “Despite these limitations, our findings provide a framework for identifying cohorts to evaluate the clinical course and treatment of melasma,” the authors concluded.
One of the authors reported relationships with companies including AbbVie, Acom, Boehringer Ingelheim, Concert, Digital Diagnostics, and Eli Lilly in the form of personal fees, equity, royalties and/or licensing, or medical advisory board positions outside the submitted work; another author reported being an advisory board member and consultant for and receiving honoraria from Incyte, Castle Biosciences, Galderma, and Sanofi outside the submitted work. The other authors reported no relevant conflicts of interest.
To better understand melasma, it is important for researchers to find groups of patients with confirmed disease for future clinical study. A recent for researchers interested in conducting retrospective studies of this patient population.
“Overall, our results support the validity of using the ICD-10 code for melasma to identify patients with a diagnosis of melasma for future studies,” Nicholas Theodosakis, MD, PhD, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues wrote in their research letter. “Despite some variability in diagnostic confidence, most patients were ultimately classified as moderately or highly likely to have a true diagnosis of melasma.”
Dr. Theodosakis and colleagues evaluated data from 5,322 adult patients in the Mass General Brigham Research Patient Data Registry between October 2015 and January 2021 who had an encounter that used the ICD-10 code for melasma (L81.1). The researchers then validated the ICD-10 code by examining the medical records of 300 patients (5.6%), confirming that melasma was the clinician’s favored diagnosis and that the patient met secondary diagnostic criteria. Confidence was rated in categories of “low confidence,” “moderate confidence,” “high confidence,” and “maximum confidence” based on secondary criteria such as hyperpigmentation of the face and upper body, hormone-related therapy exposure before diagnosis, pregnancy history, and dermatologist-confirmed diagnosis.
The patients who had their medical records examined for confirmed melasma were primarily women (285 patients; 95.0%) and were a mean 48.4 years old at diagnosis.
Of those in the validation cohort, melasma was the preferred diagnosis for clinicians of 291 patients (97.0%), while 274 patients (91.3%) had secondary diagnostic criteria of hyperpigmentation of the face and upper body and 252 patients (84.0%) had received a diagnosis from a dermatologist. Other less common secondary diagnostic criteria of the patient group were a history of having received hormone-related therapy before a melasma diagnosis (148 patients; 49.3%) and a history of pregnancy (168 patients; 56.0%). Based on identification of secondary diagnostic criteria, confidence in melasma diagnosis was high for 208 patients (69.3%), moderate for 61 patients (20.3%), and low for 31 patients (10.3%).
Dr. Theodosakis and colleagues noted their study was limited by its retrospective nature and the presence of a small validation cohort. “Despite these limitations, our findings provide a framework for identifying cohorts to evaluate the clinical course and treatment of melasma,” the authors concluded.
One of the authors reported relationships with companies including AbbVie, Acom, Boehringer Ingelheim, Concert, Digital Diagnostics, and Eli Lilly in the form of personal fees, equity, royalties and/or licensing, or medical advisory board positions outside the submitted work; another author reported being an advisory board member and consultant for and receiving honoraria from Incyte, Castle Biosciences, Galderma, and Sanofi outside the submitted work. The other authors reported no relevant conflicts of interest.
To better understand melasma, it is important for researchers to find groups of patients with confirmed disease for future clinical study. A recent for researchers interested in conducting retrospective studies of this patient population.
“Overall, our results support the validity of using the ICD-10 code for melasma to identify patients with a diagnosis of melasma for future studies,” Nicholas Theodosakis, MD, PhD, of the department of dermatology at Massachusetts General Hospital, Boston, and colleagues wrote in their research letter. “Despite some variability in diagnostic confidence, most patients were ultimately classified as moderately or highly likely to have a true diagnosis of melasma.”
Dr. Theodosakis and colleagues evaluated data from 5,322 adult patients in the Mass General Brigham Research Patient Data Registry between October 2015 and January 2021 who had an encounter that used the ICD-10 code for melasma (L81.1). The researchers then validated the ICD-10 code by examining the medical records of 300 patients (5.6%), confirming that melasma was the clinician’s favored diagnosis and that the patient met secondary diagnostic criteria. Confidence was rated in categories of “low confidence,” “moderate confidence,” “high confidence,” and “maximum confidence” based on secondary criteria such as hyperpigmentation of the face and upper body, hormone-related therapy exposure before diagnosis, pregnancy history, and dermatologist-confirmed diagnosis.
The patients who had their medical records examined for confirmed melasma were primarily women (285 patients; 95.0%) and were a mean 48.4 years old at diagnosis.
Of those in the validation cohort, melasma was the preferred diagnosis for clinicians of 291 patients (97.0%), while 274 patients (91.3%) had secondary diagnostic criteria of hyperpigmentation of the face and upper body and 252 patients (84.0%) had received a diagnosis from a dermatologist. Other less common secondary diagnostic criteria of the patient group were a history of having received hormone-related therapy before a melasma diagnosis (148 patients; 49.3%) and a history of pregnancy (168 patients; 56.0%). Based on identification of secondary diagnostic criteria, confidence in melasma diagnosis was high for 208 patients (69.3%), moderate for 61 patients (20.3%), and low for 31 patients (10.3%).
Dr. Theodosakis and colleagues noted their study was limited by its retrospective nature and the presence of a small validation cohort. “Despite these limitations, our findings provide a framework for identifying cohorts to evaluate the clinical course and treatment of melasma,” the authors concluded.
One of the authors reported relationships with companies including AbbVie, Acom, Boehringer Ingelheim, Concert, Digital Diagnostics, and Eli Lilly in the form of personal fees, equity, royalties and/or licensing, or medical advisory board positions outside the submitted work; another author reported being an advisory board member and consultant for and receiving honoraria from Incyte, Castle Biosciences, Galderma, and Sanofi outside the submitted work. The other authors reported no relevant conflicts of interest.
FROM JAMA DERMATOLOGY
Immediate skin-to-skin contact after cesarean section improves outcomes for parent, newborn
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
Birth parents are typically separated from their newborns following a cesarean section. However, a recent study published in the journal Nursing Open suggests immediate skin-to-skin contact may accelerate uterine contractions, reduce maternal blood loss, reduce newborn crying, improve patient satisfaction and comfort, and increase the rate of breastfeeding.
“[O]ur study contributes to scientific knowledge with key information to reduce maternal morbidity and mortality rates in mothers who have undergone scheduled cesarean sections,” José Miguel Pérez-Jiménez, MD, of the faculty of nursing, physiotherapy, and podiatry at Hospital Universitario Virgen Macarena, University of Sevilla, Spain, and colleagues wrote in their study. It promotes greater stability in the mothers by reducing the risk of postpartum hemorrhage, making it better to not separate mother and child in the first hours after this surgery, he said.
Dr. Pérez-Jiménez and colleagues evaluated 83 women who underwent a scheduled cesarean section in an unblinded, randomized controlled trial. The women were randomized to receive skin-to-skin contact in the operating room that continued in the postpartum unit, or the normal protocol after cesarean section that consisted of having the mother transferred to the postanesthesia recovery room while the newborn was sent to a maternity room with a parent or companion. Researchers assessed variables such as plasma hemoglobin, uterine contractions, breastfeeding, and postoperative pain, as well as subjective measures such as maternal satisfaction, comfort, previous cesarean section experience, and newborn crying.
Women who received usual care following cesarean section were more likely to have uterine contractions at the umbilical level compared with the skin-to-skin contact group (70% vs. 3%; P ≤ .0001), while the skin-to-skin group was more likely to have uterine contractions at the infraumbilical level (92.5% vs. 22.5%; P ≤ .0001). There was a statistically significant decrease in predischarge hemoglobin in the control group compared with the skin-to-skin group (10.522 vs. 11.075 g/dL; P ≤ .017); the level of hemoglobin reduction favored the skin-to-skin group (1.01 vs. 2.265 g/dL; P ≤ .0001). Women in the skin-to-skin group were more likely to report mild pain on a 10-point visual analog scale (VAS) after being transferred to the recovery room (1.48 vs. 6.23 points; P ≤ .0001) and being transferred to a maternity room or room in the postpartum unit (0.60 vs. 5.23 points; P ≤ .0001). Breastfeeding at birth was significantly higher among patients with immediate skin-to-skin contact compared with the control group (92.5% vs. 32.5%; P ≤ .0001), and continued at 1 month after birth (92.5% vs. 12.5%; P ≤ .0001). Newborns of mothers in the skin-to-skin group were significantly less likely to cry compared with newborns in the control group (90% vs. 55%; P ≤ .001).
When asked to rate their satisfaction on a 10-point Likert scale, women in the skin-to-skin contact group rated their experience significantly higher than did the control group (9.98 vs. 6.5; P ≤ .0001), and all women who had previously had a cesarean section in the skin-to-skin group (30%) rated their experience at 10 points compared with their previous cesarean section without skin-to-skin contact.
Implementing skin-to-skin contact after cesarean section
Betsy M. Collins, MD, MPH, assistant professor of obstetrics and gynecology at Emory University, Atlanta, said in an interview that while some of the findings were largely unsurprising and “confirmed a lot of the things that we already know about skin-to-skin [contact],” one major finding was the “stark difference” in the percentage of new birth parents who started breastfeeding after skin-to-skin contact and were still breastfeeding at 1 month postpartum compared with birth parents in the control group. She was not involved with the study and noted that the results complement recommendations from the World Health Organization on starting breastfeeding within the first hour after birth and continuing breastfeeding through the first 6 months of life.
“That was likely one of the greatest take-home points from the study ... that early skin-to-skin really promoted initiation of breastfeeding,” Dr. Collins said.
Two reasons why skin-to-skin contact after cesarean section isn’t regularly provided is that it can be difficult for personnel and safety reasons to have an extra nurse to continue monitoring the health of the newborn in the operating room, and there is a lack of culture supporting of skin-to-skin contact in the OR, Dr. Collins explained.
“Just like anything else, if it’s built into your standard operating procedure, then you have everything set up in place to do that initial assessment of the infant and then get the baby skin-to-skin as quickly as possible,” she said. If it’s your standard operating procedure to not provide skin-to-skin contact, she said, then there is a little bit more inertia to overcome to start providing it as a standard procedure.
At her center, Dr. Collins said skin-to-skin contact is initiated as soon as possible after birth, even in the operating room. The steps to implementing that policy involved getting the anesthesiology department on board with supporting the policy in the OR and training the circulating nursing staff to ensure a that nurse is available to monitor the newborn.
“I think the most important thing to know is that it’s absolutely doable and that you just have to have a champion just like any other quality initiative,” she said. One of the best ways to do that is to have the patients themselves request it, she noted, compared with its being requested by a physician or nurse.
“I think some patients are disappointed when they have to undergo cesarean delivery or feel like they’re missing out if they can’t have a vaginal delivery,” Dr. Collins said. Immediate skin-to-skin contact is “very good for not only physiology, as we read about in this paper – all the things they said about the benefits of skin-to-skin [contact] are true – but it’s really good for mental health. That bonding begins right away.”
As a birth parent, being separated from your newborn for several hours after a cesarean section, on the other hand, can be “pretty devastating,” Dr. Collins said.
“I think this is something that, once it becomes a standard of care, it will be expected that most hospitals should be doing this,” she said.
The authors and Dr. Collins report no relevant conflicts of interest.
FROM NURSING OPEN
Health care providers should have higher suspicion for rare diseases
The number of cataloged rare diseases continues to grow every day. According to the National Human Genome Research Institute, more than 6,800 rare diseases have been identified and between 25 million and 30 million Americans are living with rare diseases today.
Rare diseases have collectively emerged as a unique field of medicine with an “entirely new generation of conditions,” said Marshall L. Summar, MD, chief of the division of genetics and metabolism at Children’s National Hospital in Washington, DC. He places the number of rare diseases closer to 8,000, and said it is “growing by a rate of 10 to 12 a week.”
Although the field has made significant advancements in health care providers’ ability to diagnose rare diseases, it has also highlighted what isn’t known as well, said Dr. Summar, who is also past president and a former scientific advisory board member with the National Organization for Rare Disorders (NORD).
Keeping up to date on the latest rare diseases may seem like a daunting task to the average health care professional. However, while rare diseases remain the domain of the subspecialists, the generalist “can make a tremendous impact for their patients” early in the process by having a higher suspicion for rare diseases in their practice, said Dr. Summar.
Thinking of rare diseases in categories
Many patients with undiagnosed rare diseases undergo what’s commonly referred to as a “diagnostic odyssey,” moving from one provider to another to try to find an explanation for a condition they may or may not know is rare. For some patients, this process can take many years or even decades. From the patient’s perspective, the main challenges are recognizing they have a problem that doesn’t fit a common disease model. Once they recognize they have a potential rare disease, working with a provider to find the right diagnosis among the “vast number of disease diagnoses and designations, and actually sifting through it to find the one that’s right for that patient” is the next challenge, said Dr. Summar.
However, knowledge of rare diseases among health care professionals is low, according to a 2019 paper published in the Orphanet Journal of Rare Diseases. In a survey from that paper asking general practitioners, pediatricians, specialists caring for adults, and specialists caring for children to evaluate their own knowledge of rare diseases, 42% of general practitioners said they had poor knowledge and 44% said they had a substandard understanding of rare diseases.
From a clinician’s standpoint, diagnosing rare diseases in their patients can be challenging, with the potential for overreferral or overdiagnosis. However, it is also easy to underdiagnose rare diseases by missing them, noted Dr. Summar. This issue can vary based on the experience of the provider, he said, because while general practitioners who recently began practicing may have had more exposure to rare diseases, for health care professionals who have been practicing for decades, “this is a new arrival in their field.”
During a busy day finding that extra time in an appointment to stop and question whether a patient might have a rare disease is another problem generalists face. “It is really tough for those general practitioners, because if you see 40 or 50 patients per day, how do you know which one of those [patients] were the ones that had something that wasn’t quite fitting or wasn’t quite ordinary?” he said.
When it comes to considering rare diseases in their patients, health care professionals in general practice should think in categories, rather than a particular rare disease, according to Dr. Summar. As the generalist is typically on the front lines of patient care, they don’t necessarily need to know everything about the rare disease they suspect a patient of having to help them. “You don’t need to know the specific gene and the specific mutation to make the diagnosis, or to even move the patient forward in the process,” he said.
The first steps a clinician can take include noticing when something with a patient is amiss, thinking about the disease category, and then creating a plan to move forward, such as referring the patient to a subspecialist. Learning to recognize when a cluster of symptoms doesn’t fit a pattern is important, as patients and their providers tend to gravitate toward diagnoses they are used to seeing, rather than suspecting a disease outside a usual pattern.
The framing of rare diseases as categories is a change in thinking over the last decade, said Dr. Summar. Whereas rare disease diagnoses previously focused on fitting certain criteria, the development of more refined genetic sequencing has allowed specialists to focus on categories and genes that affect rare diseases. “Getting at a diagnosis has really been turned up on its head, so that by employing both next-generation sequencing, newborn screening, and other [tools], we can actually get to diagnoses faster than we could before,” he said.
In terms of assessing for symptoms, health care professionals should be aware that “common” symptoms can be a sign of rare disease. What to look out for are the uncommon symptoms that create an “aha moment.” Having a “good filter” for sensing when something isn’t quite right with a patient is key. “It’s like any time when you’re screening things: You want high sensitivity, but you also have to have high specificity,” he said.
Another clinical pearl is that good communication between patient and provider is paramount. “We’re not always good listeners. Sometimes we hear what we expect to hear,” said Dr. Summar.
Rare disease warning signs
Within the context of rare neurological diseases, Dr. Summar noted one major category is delays in neurological development, which is typically identified in children or adolescents. As the most complex organ in the body, “the brain probably expresses more genes than any other tissue on a regular basis, both in its formation and its function,” said Dr. Summar. He said the single disease that rare disease specialists see most often is Down syndrome.
Another separate but overlapping major category is autism, identified in younger children through trouble with social interaction, lack of eye contact, and delays in speech and communication skills. A third major category is physical manifestations of neurological problems, such as in patients who have epilepsy.
A telltale sign in identifying a child with a potential rare neurological disease is when they are “not thriving in their development or not doing the things on track that you would expect, and you don’t have a really good answer for it,” said Dr. Summar. Generalists are normally on watch for developmental delays in newborns born premature or with a rough course in the nursery, but they should also be aware of delays in children born under otherwise typical circumstances. “If I have a patient who had normal pregnancy, normal labor and delivery, no real illnesses or anything like that, and yet wasn’t meeting those milestones, that’s a patient I would pay attention to,” he said.
Another clue general practitioners can use for suspecting rare diseases is when a patient is much sicker than usual during a routine illness like a cold or flu. “Those are patients we should be paying attention to because it may be there’s an underlying biochemical disorder or some disorder in how they’re responding to stress that’s just not quite right,” said Dr. Summar. How a patient responds to stressful situations can be a warning sign “because that can often unmask more severe symptoms in that rare disease and make it a little more apparent,” he said.
Learning more about rare diseases
Dr. Summar said he and his colleagues in the rare disease field have spent a lot of time working with medical schools to teach this mindset in their curricula. The concept is introduced in basic medical science courses and then reinforced in clinical rotations in the third or fourth year, he explained.
“One of the best places is during the pediatrics rotations in medical school,” he said. “Remember, kids are basically healthy. If a child has a chronic illness or a chronic disease, more often than not, it is probably a rare disease.”
For medical professionals not in pediatric practice, the concept is applied the same way for adult medicine. “You just want to make sure everyone takes a second when they have a patient and try not to assume. Don’t assume it’s exactly what it seems. Look at it carefully and make sure there’s not something else going on,” he said.
Health care professionals in general practice looking to learn more about rare diseases can increasingly find rare disease topics in their CME programs. Rare disease topics in CME programs are “one of the best places” to learn about the latest developments in the field, said Dr. Summar.
Will rare disease screening tools come to primary care?
Asking more doctors to refer out to rare disease specialists raises an issue: There simply aren’t enough rare disease specialists in the field to go around.
Dr. Summar said partnering testing – where a general practitioner contacts a specialist to begin the process of testing based on the suspected condition – is a good stopgap solution. Telemedicine, which rose in popularity during the COVID-19 pandemic, can also play an important role in connecting patients and their providers with rare disease specialists, especially for generalists in remote communities. Dr. Summar noted he continues to see approximately 30% of his patients this way today. Telemedicine appointments can take place in the patient’s home or at the provider’s office.
“It actually provides access to folks who otherwise might not be able to either take off from work for a day – particularly some of our single parent households – or have a child who just doesn’t travel well, or can’t really get there, even if it’s the patient themselves,” he explained. “We can see patients that historically would have had trouble or difficulty coming in, so for me, that’s been a good thing.”
Telemedicine also helps give access to care for more medically fragile patients, many of whom have rare diseases, he added. While some aspects of care need to occur in person, “it’s a good 80% or 90% solution for a lot of these things,” he said.
Sharing educational videos is another way for health care providers in general practice to inform patients and their families about rare diseases. Children’s National Medical Center has created a collection of these videos in a free app called GeneClips, which is available on major smartphone app stores. However, Dr. Summar emphasized that genetic counseling should still be performed by a rare disease specialist prior to testing.
“We’re still at the point where I think having genetic counseling for a family before they’re going into testing is really advisable, since a lot of the results have a probability assigned to them,” he said. “I don’t think we’re really at the level where a practitioner is going to, first of all, have the time to do those, and I don’t think there’s enough general public awareness of what these things mean.”
Although primary care providers may one day be able to perform more generalized sequencing in their own practice, that time has not yet come – but it is closer than you think. “The technology is there, and actually the cost has come down a lot,” said Dr. Summar.
One potential issue this would create is an additional discussion to manage expectations of test results with family when the results are unclear, which “actually takes more time than counseling about a yes or no, or even an outcome that is unexpected,” explained Dr. Summar.
“[W]e’re in a midlife period right now where we’re bringing forward this new technology, but we’ve got to continually prepare the field for it first,” he said. “I think in the future we’ll see that it has much greater utility in the general setting,” he said.
Jeff Craven is a freelance journalist specializing in medicine and health.
Suggested reading
Vandeborne L et al. Information needs of physicians regarding the diagnosis of rare diseases: A questionnaire-based study in Belgium. Orphanet J Rare Dis. 2019;14(1):99.
The number of cataloged rare diseases continues to grow every day. According to the National Human Genome Research Institute, more than 6,800 rare diseases have been identified and between 25 million and 30 million Americans are living with rare diseases today.
Rare diseases have collectively emerged as a unique field of medicine with an “entirely new generation of conditions,” said Marshall L. Summar, MD, chief of the division of genetics and metabolism at Children’s National Hospital in Washington, DC. He places the number of rare diseases closer to 8,000, and said it is “growing by a rate of 10 to 12 a week.”
Although the field has made significant advancements in health care providers’ ability to diagnose rare diseases, it has also highlighted what isn’t known as well, said Dr. Summar, who is also past president and a former scientific advisory board member with the National Organization for Rare Disorders (NORD).
Keeping up to date on the latest rare diseases may seem like a daunting task to the average health care professional. However, while rare diseases remain the domain of the subspecialists, the generalist “can make a tremendous impact for their patients” early in the process by having a higher suspicion for rare diseases in their practice, said Dr. Summar.
Thinking of rare diseases in categories
Many patients with undiagnosed rare diseases undergo what’s commonly referred to as a “diagnostic odyssey,” moving from one provider to another to try to find an explanation for a condition they may or may not know is rare. For some patients, this process can take many years or even decades. From the patient’s perspective, the main challenges are recognizing they have a problem that doesn’t fit a common disease model. Once they recognize they have a potential rare disease, working with a provider to find the right diagnosis among the “vast number of disease diagnoses and designations, and actually sifting through it to find the one that’s right for that patient” is the next challenge, said Dr. Summar.
However, knowledge of rare diseases among health care professionals is low, according to a 2019 paper published in the Orphanet Journal of Rare Diseases. In a survey from that paper asking general practitioners, pediatricians, specialists caring for adults, and specialists caring for children to evaluate their own knowledge of rare diseases, 42% of general practitioners said they had poor knowledge and 44% said they had a substandard understanding of rare diseases.
From a clinician’s standpoint, diagnosing rare diseases in their patients can be challenging, with the potential for overreferral or overdiagnosis. However, it is also easy to underdiagnose rare diseases by missing them, noted Dr. Summar. This issue can vary based on the experience of the provider, he said, because while general practitioners who recently began practicing may have had more exposure to rare diseases, for health care professionals who have been practicing for decades, “this is a new arrival in their field.”
During a busy day finding that extra time in an appointment to stop and question whether a patient might have a rare disease is another problem generalists face. “It is really tough for those general practitioners, because if you see 40 or 50 patients per day, how do you know which one of those [patients] were the ones that had something that wasn’t quite fitting or wasn’t quite ordinary?” he said.
When it comes to considering rare diseases in their patients, health care professionals in general practice should think in categories, rather than a particular rare disease, according to Dr. Summar. As the generalist is typically on the front lines of patient care, they don’t necessarily need to know everything about the rare disease they suspect a patient of having to help them. “You don’t need to know the specific gene and the specific mutation to make the diagnosis, or to even move the patient forward in the process,” he said.
The first steps a clinician can take include noticing when something with a patient is amiss, thinking about the disease category, and then creating a plan to move forward, such as referring the patient to a subspecialist. Learning to recognize when a cluster of symptoms doesn’t fit a pattern is important, as patients and their providers tend to gravitate toward diagnoses they are used to seeing, rather than suspecting a disease outside a usual pattern.
The framing of rare diseases as categories is a change in thinking over the last decade, said Dr. Summar. Whereas rare disease diagnoses previously focused on fitting certain criteria, the development of more refined genetic sequencing has allowed specialists to focus on categories and genes that affect rare diseases. “Getting at a diagnosis has really been turned up on its head, so that by employing both next-generation sequencing, newborn screening, and other [tools], we can actually get to diagnoses faster than we could before,” he said.
In terms of assessing for symptoms, health care professionals should be aware that “common” symptoms can be a sign of rare disease. What to look out for are the uncommon symptoms that create an “aha moment.” Having a “good filter” for sensing when something isn’t quite right with a patient is key. “It’s like any time when you’re screening things: You want high sensitivity, but you also have to have high specificity,” he said.
Another clinical pearl is that good communication between patient and provider is paramount. “We’re not always good listeners. Sometimes we hear what we expect to hear,” said Dr. Summar.
Rare disease warning signs
Within the context of rare neurological diseases, Dr. Summar noted one major category is delays in neurological development, which is typically identified in children or adolescents. As the most complex organ in the body, “the brain probably expresses more genes than any other tissue on a regular basis, both in its formation and its function,” said Dr. Summar. He said the single disease that rare disease specialists see most often is Down syndrome.
Another separate but overlapping major category is autism, identified in younger children through trouble with social interaction, lack of eye contact, and delays in speech and communication skills. A third major category is physical manifestations of neurological problems, such as in patients who have epilepsy.
A telltale sign in identifying a child with a potential rare neurological disease is when they are “not thriving in their development or not doing the things on track that you would expect, and you don’t have a really good answer for it,” said Dr. Summar. Generalists are normally on watch for developmental delays in newborns born premature or with a rough course in the nursery, but they should also be aware of delays in children born under otherwise typical circumstances. “If I have a patient who had normal pregnancy, normal labor and delivery, no real illnesses or anything like that, and yet wasn’t meeting those milestones, that’s a patient I would pay attention to,” he said.
Another clue general practitioners can use for suspecting rare diseases is when a patient is much sicker than usual during a routine illness like a cold or flu. “Those are patients we should be paying attention to because it may be there’s an underlying biochemical disorder or some disorder in how they’re responding to stress that’s just not quite right,” said Dr. Summar. How a patient responds to stressful situations can be a warning sign “because that can often unmask more severe symptoms in that rare disease and make it a little more apparent,” he said.
Learning more about rare diseases
Dr. Summar said he and his colleagues in the rare disease field have spent a lot of time working with medical schools to teach this mindset in their curricula. The concept is introduced in basic medical science courses and then reinforced in clinical rotations in the third or fourth year, he explained.
“One of the best places is during the pediatrics rotations in medical school,” he said. “Remember, kids are basically healthy. If a child has a chronic illness or a chronic disease, more often than not, it is probably a rare disease.”
For medical professionals not in pediatric practice, the concept is applied the same way for adult medicine. “You just want to make sure everyone takes a second when they have a patient and try not to assume. Don’t assume it’s exactly what it seems. Look at it carefully and make sure there’s not something else going on,” he said.
Health care professionals in general practice looking to learn more about rare diseases can increasingly find rare disease topics in their CME programs. Rare disease topics in CME programs are “one of the best places” to learn about the latest developments in the field, said Dr. Summar.
Will rare disease screening tools come to primary care?
Asking more doctors to refer out to rare disease specialists raises an issue: There simply aren’t enough rare disease specialists in the field to go around.
Dr. Summar said partnering testing – where a general practitioner contacts a specialist to begin the process of testing based on the suspected condition – is a good stopgap solution. Telemedicine, which rose in popularity during the COVID-19 pandemic, can also play an important role in connecting patients and their providers with rare disease specialists, especially for generalists in remote communities. Dr. Summar noted he continues to see approximately 30% of his patients this way today. Telemedicine appointments can take place in the patient’s home or at the provider’s office.
“It actually provides access to folks who otherwise might not be able to either take off from work for a day – particularly some of our single parent households – or have a child who just doesn’t travel well, or can’t really get there, even if it’s the patient themselves,” he explained. “We can see patients that historically would have had trouble or difficulty coming in, so for me, that’s been a good thing.”
Telemedicine also helps give access to care for more medically fragile patients, many of whom have rare diseases, he added. While some aspects of care need to occur in person, “it’s a good 80% or 90% solution for a lot of these things,” he said.
Sharing educational videos is another way for health care providers in general practice to inform patients and their families about rare diseases. Children’s National Medical Center has created a collection of these videos in a free app called GeneClips, which is available on major smartphone app stores. However, Dr. Summar emphasized that genetic counseling should still be performed by a rare disease specialist prior to testing.
“We’re still at the point where I think having genetic counseling for a family before they’re going into testing is really advisable, since a lot of the results have a probability assigned to them,” he said. “I don’t think we’re really at the level where a practitioner is going to, first of all, have the time to do those, and I don’t think there’s enough general public awareness of what these things mean.”
Although primary care providers may one day be able to perform more generalized sequencing in their own practice, that time has not yet come – but it is closer than you think. “The technology is there, and actually the cost has come down a lot,” said Dr. Summar.
One potential issue this would create is an additional discussion to manage expectations of test results with family when the results are unclear, which “actually takes more time than counseling about a yes or no, or even an outcome that is unexpected,” explained Dr. Summar.
“[W]e’re in a midlife period right now where we’re bringing forward this new technology, but we’ve got to continually prepare the field for it first,” he said. “I think in the future we’ll see that it has much greater utility in the general setting,” he said.
Jeff Craven is a freelance journalist specializing in medicine and health.
Suggested reading
Vandeborne L et al. Information needs of physicians regarding the diagnosis of rare diseases: A questionnaire-based study in Belgium. Orphanet J Rare Dis. 2019;14(1):99.
The number of cataloged rare diseases continues to grow every day. According to the National Human Genome Research Institute, more than 6,800 rare diseases have been identified and between 25 million and 30 million Americans are living with rare diseases today.
Rare diseases have collectively emerged as a unique field of medicine with an “entirely new generation of conditions,” said Marshall L. Summar, MD, chief of the division of genetics and metabolism at Children’s National Hospital in Washington, DC. He places the number of rare diseases closer to 8,000, and said it is “growing by a rate of 10 to 12 a week.”
Although the field has made significant advancements in health care providers’ ability to diagnose rare diseases, it has also highlighted what isn’t known as well, said Dr. Summar, who is also past president and a former scientific advisory board member with the National Organization for Rare Disorders (NORD).
Keeping up to date on the latest rare diseases may seem like a daunting task to the average health care professional. However, while rare diseases remain the domain of the subspecialists, the generalist “can make a tremendous impact for their patients” early in the process by having a higher suspicion for rare diseases in their practice, said Dr. Summar.
Thinking of rare diseases in categories
Many patients with undiagnosed rare diseases undergo what’s commonly referred to as a “diagnostic odyssey,” moving from one provider to another to try to find an explanation for a condition they may or may not know is rare. For some patients, this process can take many years or even decades. From the patient’s perspective, the main challenges are recognizing they have a problem that doesn’t fit a common disease model. Once they recognize they have a potential rare disease, working with a provider to find the right diagnosis among the “vast number of disease diagnoses and designations, and actually sifting through it to find the one that’s right for that patient” is the next challenge, said Dr. Summar.
However, knowledge of rare diseases among health care professionals is low, according to a 2019 paper published in the Orphanet Journal of Rare Diseases. In a survey from that paper asking general practitioners, pediatricians, specialists caring for adults, and specialists caring for children to evaluate their own knowledge of rare diseases, 42% of general practitioners said they had poor knowledge and 44% said they had a substandard understanding of rare diseases.
From a clinician’s standpoint, diagnosing rare diseases in their patients can be challenging, with the potential for overreferral or overdiagnosis. However, it is also easy to underdiagnose rare diseases by missing them, noted Dr. Summar. This issue can vary based on the experience of the provider, he said, because while general practitioners who recently began practicing may have had more exposure to rare diseases, for health care professionals who have been practicing for decades, “this is a new arrival in their field.”
During a busy day finding that extra time in an appointment to stop and question whether a patient might have a rare disease is another problem generalists face. “It is really tough for those general practitioners, because if you see 40 or 50 patients per day, how do you know which one of those [patients] were the ones that had something that wasn’t quite fitting or wasn’t quite ordinary?” he said.
When it comes to considering rare diseases in their patients, health care professionals in general practice should think in categories, rather than a particular rare disease, according to Dr. Summar. As the generalist is typically on the front lines of patient care, they don’t necessarily need to know everything about the rare disease they suspect a patient of having to help them. “You don’t need to know the specific gene and the specific mutation to make the diagnosis, or to even move the patient forward in the process,” he said.
The first steps a clinician can take include noticing when something with a patient is amiss, thinking about the disease category, and then creating a plan to move forward, such as referring the patient to a subspecialist. Learning to recognize when a cluster of symptoms doesn’t fit a pattern is important, as patients and their providers tend to gravitate toward diagnoses they are used to seeing, rather than suspecting a disease outside a usual pattern.
The framing of rare diseases as categories is a change in thinking over the last decade, said Dr. Summar. Whereas rare disease diagnoses previously focused on fitting certain criteria, the development of more refined genetic sequencing has allowed specialists to focus on categories and genes that affect rare diseases. “Getting at a diagnosis has really been turned up on its head, so that by employing both next-generation sequencing, newborn screening, and other [tools], we can actually get to diagnoses faster than we could before,” he said.
In terms of assessing for symptoms, health care professionals should be aware that “common” symptoms can be a sign of rare disease. What to look out for are the uncommon symptoms that create an “aha moment.” Having a “good filter” for sensing when something isn’t quite right with a patient is key. “It’s like any time when you’re screening things: You want high sensitivity, but you also have to have high specificity,” he said.
Another clinical pearl is that good communication between patient and provider is paramount. “We’re not always good listeners. Sometimes we hear what we expect to hear,” said Dr. Summar.
Rare disease warning signs
Within the context of rare neurological diseases, Dr. Summar noted one major category is delays in neurological development, which is typically identified in children or adolescents. As the most complex organ in the body, “the brain probably expresses more genes than any other tissue on a regular basis, both in its formation and its function,” said Dr. Summar. He said the single disease that rare disease specialists see most often is Down syndrome.
Another separate but overlapping major category is autism, identified in younger children through trouble with social interaction, lack of eye contact, and delays in speech and communication skills. A third major category is physical manifestations of neurological problems, such as in patients who have epilepsy.
A telltale sign in identifying a child with a potential rare neurological disease is when they are “not thriving in their development or not doing the things on track that you would expect, and you don’t have a really good answer for it,” said Dr. Summar. Generalists are normally on watch for developmental delays in newborns born premature or with a rough course in the nursery, but they should also be aware of delays in children born under otherwise typical circumstances. “If I have a patient who had normal pregnancy, normal labor and delivery, no real illnesses or anything like that, and yet wasn’t meeting those milestones, that’s a patient I would pay attention to,” he said.
Another clue general practitioners can use for suspecting rare diseases is when a patient is much sicker than usual during a routine illness like a cold or flu. “Those are patients we should be paying attention to because it may be there’s an underlying biochemical disorder or some disorder in how they’re responding to stress that’s just not quite right,” said Dr. Summar. How a patient responds to stressful situations can be a warning sign “because that can often unmask more severe symptoms in that rare disease and make it a little more apparent,” he said.
Learning more about rare diseases
Dr. Summar said he and his colleagues in the rare disease field have spent a lot of time working with medical schools to teach this mindset in their curricula. The concept is introduced in basic medical science courses and then reinforced in clinical rotations in the third or fourth year, he explained.
“One of the best places is during the pediatrics rotations in medical school,” he said. “Remember, kids are basically healthy. If a child has a chronic illness or a chronic disease, more often than not, it is probably a rare disease.”
For medical professionals not in pediatric practice, the concept is applied the same way for adult medicine. “You just want to make sure everyone takes a second when they have a patient and try not to assume. Don’t assume it’s exactly what it seems. Look at it carefully and make sure there’s not something else going on,” he said.
Health care professionals in general practice looking to learn more about rare diseases can increasingly find rare disease topics in their CME programs. Rare disease topics in CME programs are “one of the best places” to learn about the latest developments in the field, said Dr. Summar.
Will rare disease screening tools come to primary care?
Asking more doctors to refer out to rare disease specialists raises an issue: There simply aren’t enough rare disease specialists in the field to go around.
Dr. Summar said partnering testing – where a general practitioner contacts a specialist to begin the process of testing based on the suspected condition – is a good stopgap solution. Telemedicine, which rose in popularity during the COVID-19 pandemic, can also play an important role in connecting patients and their providers with rare disease specialists, especially for generalists in remote communities. Dr. Summar noted he continues to see approximately 30% of his patients this way today. Telemedicine appointments can take place in the patient’s home or at the provider’s office.
“It actually provides access to folks who otherwise might not be able to either take off from work for a day – particularly some of our single parent households – or have a child who just doesn’t travel well, or can’t really get there, even if it’s the patient themselves,” he explained. “We can see patients that historically would have had trouble or difficulty coming in, so for me, that’s been a good thing.”
Telemedicine also helps give access to care for more medically fragile patients, many of whom have rare diseases, he added. While some aspects of care need to occur in person, “it’s a good 80% or 90% solution for a lot of these things,” he said.
Sharing educational videos is another way for health care providers in general practice to inform patients and their families about rare diseases. Children’s National Medical Center has created a collection of these videos in a free app called GeneClips, which is available on major smartphone app stores. However, Dr. Summar emphasized that genetic counseling should still be performed by a rare disease specialist prior to testing.
“We’re still at the point where I think having genetic counseling for a family before they’re going into testing is really advisable, since a lot of the results have a probability assigned to them,” he said. “I don’t think we’re really at the level where a practitioner is going to, first of all, have the time to do those, and I don’t think there’s enough general public awareness of what these things mean.”
Although primary care providers may one day be able to perform more generalized sequencing in their own practice, that time has not yet come – but it is closer than you think. “The technology is there, and actually the cost has come down a lot,” said Dr. Summar.
One potential issue this would create is an additional discussion to manage expectations of test results with family when the results are unclear, which “actually takes more time than counseling about a yes or no, or even an outcome that is unexpected,” explained Dr. Summar.
“[W]e’re in a midlife period right now where we’re bringing forward this new technology, but we’ve got to continually prepare the field for it first,” he said. “I think in the future we’ll see that it has much greater utility in the general setting,” he said.
Jeff Craven is a freelance journalist specializing in medicine and health.
Suggested reading
Vandeborne L et al. Information needs of physicians regarding the diagnosis of rare diseases: A questionnaire-based study in Belgium. Orphanet J Rare Dis. 2019;14(1):99.