User login
Polyglutamine diseases are rare, but not the mutations that cause them
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Gardiner et al. describe the results of an appraisal of polyglutamine expansion variants in more than 14,000 individuals from the Netherlands, Scotland, and Ireland. Given the relative rarity of polyglutamine repeat disease, the first question that comes to mind is why were so many individuals identified with repeats in the pathogenic range? Based on our understanding of disease prevalence, it is unlikely that each of these individuals will become affected; therefore, this work suggests a reduced penetrance of these mutations. The findings are illustrative of a growing theme in human disease genetics: There are a very large number of apparently healthy individuals in the general population who carry mutations associated with various diseases. The phenomenon of reduced penetrance, where mutations cause disease in some but not all carriers, overlaps and arguably may be the same as that of variable expressivity, where the same mutation can lead to very different disease outcomes in different individuals. It is extremely likely that second-generation sequencing and population-scale screening will continue to reveal similar themes. We continue to appreciate the increasing complexity of the human genome and its relationship to disease, even those diseases we thought of previously as simple “single-gene” disorders.
Monia B. Hammer, PhD, and Andrew B. Singleton, PhD, are with the National Institute on Aging, National Institutes of Health, Bethesda, Md. Dr. Hammer and Dr. Singleton report no financial conflicts of interest related to their editorial.
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
Polyglutamine diseases are a group of hereditary neurodegenerative disorders caused by mutations in which a trinucleotide repeat expands pathologically on a disease-associated gene. The diseases are rare, with the most common among them – Huntington disease – affecting between 10 and 14 per 100,000 people in Western countries, where prevalence is highest.
In polyglutamine diseases, which include the spinocerebellar ataxias, dentatorubral-pallidoluysian atrophy, and spinal bulbar muscular atrophy, higher CAG (cytosine-adenine-guanine) repeat numbers are associated with greater disease severity, faster progression, or earlier age at onset.
In research published April 1 in JAMA Neurology, investigators report that more than one-tenth of the European population carries CAG expansions that fall short of the repeats needed to cause any of 9 polyglutamine diseases – but are enough to put them at risk of having children who develop one. A smaller number of people – about 1% – carry enough CAG repeats to cause one of the diseases late in life.
For their research, Sarah L. Gardiner, MD, of Leiden (the Netherlands) University, and her colleagues looked at polyglutamine expansion variants for nine diseases in samples from 14,196 adults (56% of whom were women) from the Netherlands, Scotland, and Ireland. The samples were taken from five population-based cohort studies conducted between 1997 and 2012, and all subjects were without a history of polyglutamine disease or major depression.
Of these, 10.7% had a CAG repeat number on a disease-associated gene that was in the intermediate range, defined as a number of repeats that cannot cause disease but for which “expansion into the fully pathological range has been observed on intergenerational transmission,” Dr. Gardiner and her colleagues wrote. And some 1.3% of subjects were found to have CAG repeats within the disease-causing range, “mostly in the lower range associated with elderly onset.”
The investigators found no differences in sex, age, or body mass index between individuals with CAG repeat numbers within the pathological range and individuals with CAG repeat numbers within the normal or intermediate range.
Whether carriers of immediate or lower-range pathological CAG repeats went on to develop disease could not be measured, as follow-up data were not available. Another limitation of the study, the investigators acknowledged, was that the genotyping method used “did not allow us to determine the presence of trinucleotide interruptions,” which can affect disease penetrance.
“A late age at onset, a reduced penetrance, or the presence of interruptions could all explain the asymptomatic status of our carriers of intermediate and pathological polyglutamine disease–associated alleles at the time of assessment,” Dr. Gardiner and her colleagues wrote.
This study was funded by the European Union and Dutch government agencies; one of the population-based cohort studies from which the study sample was taken received some support from Bristol-Myers-Squibb. One of Dr. Gardiner’s coauthors, Raymund A. C. Roos, MD, PhD, disclosed being an adviser for UniQure, a gene-therapy firm, and no other conflicts of interest were reported.
SOURCE: Gardiner et al. JAMA Neurol. 2019 Apr 1. doi: 10.001/jamaneurol.2019.042.
FROM JAMA NEUROLOGY
Non-TB mycobacteria infections rising in COPD patients
Veterans with chronic obstructive pulmonary disease (COPD) have seen a sharp increase since 2012 in rates of non-TB mycobacteria infections, which carry a significantly higher risk of death in COPD patients, according to findings from a nationwide study.
For their research, published in Frontiers of Medicine, Fahim Pyarali, MD, and colleagues at the University of Miami, reviewed data from Veterans Affairs hospitals to identify non-TB mycobacteria (NTM) infections among more than 2 million COPD patients seen between 2000 and 2015. Incidence of NTM infections was 34.2 per 100,000 COPD patients in 2001, a rate that remained steady until 2012, when it began climbing sharply through 2015 to reach 70.3 per 100,000 (P = .035). Dr. Pyarali and colleagues also found that, during the study period, prevalence of NTM climbed from 93.1 infections per 100,000 population in 2001 to 277.6 per 100,000 in 2015.
Hotspots for NTM infections included Puerto Rico, which had the highest prevalence seen in the study at 370 infections per 100,000 COPD population; Florida, with 351 per 100,000; and Washington, D.C., with 309 per 100,000. Additional hotspots were identified around Lake Michigan, in coastal Louisiana, and in parts of the Southwest.
Dr. Pyarali and colleagues noted that the geographical concentration of cases near oceans and lakes was “supported by previous findings that warmer temperatures, lower dissolved oxygen, and lower pH in the soils and waters provide a major environmental source for NTM organisms;” however, the study is the first to identify Puerto Rico as having exceptionally high prevalence. The reasons for this should be extensively investigated, the investigators argued.
The mortality risk was 43% higher among NTM-infected patients than in COPD patients without an NTM diagnosis (95% confidence interval, 1.31-1.58; P less than .001), independent of other comorbidities.
Though rates of NTM infection were seen rising steeply in men and women alike, Dr. Pyarali and colleagues noted as a limitation of their study its use of an overwhelmingly male population, writing that this may obscure “the true reach of NTM disease and mortality” in the general population. The average age of NTM diagnosis remained steady throughout the study period, suggesting that rising incidence is not attributable to earlier diagnosis.
Dr. Pyarali and colleagues reported no outside sources of funding or financial conflicts of interest.
SOURCE: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
Veterans with chronic obstructive pulmonary disease (COPD) have seen a sharp increase since 2012 in rates of non-TB mycobacteria infections, which carry a significantly higher risk of death in COPD patients, according to findings from a nationwide study.
For their research, published in Frontiers of Medicine, Fahim Pyarali, MD, and colleagues at the University of Miami, reviewed data from Veterans Affairs hospitals to identify non-TB mycobacteria (NTM) infections among more than 2 million COPD patients seen between 2000 and 2015. Incidence of NTM infections was 34.2 per 100,000 COPD patients in 2001, a rate that remained steady until 2012, when it began climbing sharply through 2015 to reach 70.3 per 100,000 (P = .035). Dr. Pyarali and colleagues also found that, during the study period, prevalence of NTM climbed from 93.1 infections per 100,000 population in 2001 to 277.6 per 100,000 in 2015.
Hotspots for NTM infections included Puerto Rico, which had the highest prevalence seen in the study at 370 infections per 100,000 COPD population; Florida, with 351 per 100,000; and Washington, D.C., with 309 per 100,000. Additional hotspots were identified around Lake Michigan, in coastal Louisiana, and in parts of the Southwest.
Dr. Pyarali and colleagues noted that the geographical concentration of cases near oceans and lakes was “supported by previous findings that warmer temperatures, lower dissolved oxygen, and lower pH in the soils and waters provide a major environmental source for NTM organisms;” however, the study is the first to identify Puerto Rico as having exceptionally high prevalence. The reasons for this should be extensively investigated, the investigators argued.
The mortality risk was 43% higher among NTM-infected patients than in COPD patients without an NTM diagnosis (95% confidence interval, 1.31-1.58; P less than .001), independent of other comorbidities.
Though rates of NTM infection were seen rising steeply in men and women alike, Dr. Pyarali and colleagues noted as a limitation of their study its use of an overwhelmingly male population, writing that this may obscure “the true reach of NTM disease and mortality” in the general population. The average age of NTM diagnosis remained steady throughout the study period, suggesting that rising incidence is not attributable to earlier diagnosis.
Dr. Pyarali and colleagues reported no outside sources of funding or financial conflicts of interest.
SOURCE: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
Veterans with chronic obstructive pulmonary disease (COPD) have seen a sharp increase since 2012 in rates of non-TB mycobacteria infections, which carry a significantly higher risk of death in COPD patients, according to findings from a nationwide study.
For their research, published in Frontiers of Medicine, Fahim Pyarali, MD, and colleagues at the University of Miami, reviewed data from Veterans Affairs hospitals to identify non-TB mycobacteria (NTM) infections among more than 2 million COPD patients seen between 2000 and 2015. Incidence of NTM infections was 34.2 per 100,000 COPD patients in 2001, a rate that remained steady until 2012, when it began climbing sharply through 2015 to reach 70.3 per 100,000 (P = .035). Dr. Pyarali and colleagues also found that, during the study period, prevalence of NTM climbed from 93.1 infections per 100,000 population in 2001 to 277.6 per 100,000 in 2015.
Hotspots for NTM infections included Puerto Rico, which had the highest prevalence seen in the study at 370 infections per 100,000 COPD population; Florida, with 351 per 100,000; and Washington, D.C., with 309 per 100,000. Additional hotspots were identified around Lake Michigan, in coastal Louisiana, and in parts of the Southwest.
Dr. Pyarali and colleagues noted that the geographical concentration of cases near oceans and lakes was “supported by previous findings that warmer temperatures, lower dissolved oxygen, and lower pH in the soils and waters provide a major environmental source for NTM organisms;” however, the study is the first to identify Puerto Rico as having exceptionally high prevalence. The reasons for this should be extensively investigated, the investigators argued.
The mortality risk was 43% higher among NTM-infected patients than in COPD patients without an NTM diagnosis (95% confidence interval, 1.31-1.58; P less than .001), independent of other comorbidities.
Though rates of NTM infection were seen rising steeply in men and women alike, Dr. Pyarali and colleagues noted as a limitation of their study its use of an overwhelmingly male population, writing that this may obscure “the true reach of NTM disease and mortality” in the general population. The average age of NTM diagnosis remained steady throughout the study period, suggesting that rising incidence is not attributable to earlier diagnosis.
Dr. Pyarali and colleagues reported no outside sources of funding or financial conflicts of interest.
SOURCE: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
FROM FRONTIERS IN MEDICINE
Key clinical point: Incidence and prevalence of non-TB mycobacteria infections rose sharply in a national veterans population with chronic obstructive pulmonary disease after 2012.
Major finding: Incidence of non-TB mycobacteria infections doubled in chronic obstructive pulmonary disease patients between 2001 and 2015, with most of the increase seen after 2012
Study details: A retrospective, cross-sectional study using records from over 2 million, mostly male chronic obstructive pulmonary disease patients in a Veterans Affairs database.
Disclosures: The study authors reported no outside sources of funding or financial conflicts of interest.
Source: Pyarali F et al. Front Med. 2018 Nov 6. doi: 10.3389/fmed2018.00311.
Perioperative M&M similar for lobar, sublobar surgeries in early lung cancer
Though lobectomy is the long-held standard of care for people with early stage non–small cell lung cancer, a noninferiority study shows little difference in perioperative morbidity and mortality outcomes when sublobar resections are performed instead.
The study, published online in The Lancet Respiratory Medicine, compared results from 697 functionally and physically fit patients with stage I cancer randomized over a 10-year period to lobar resection (n = 357) or sublobar resection (n = 340). Patients were analyzed for morbidity and mortality outcomes at 30 and 90 days post surgery. Nasser K. Altorki, MD, of Weill Cornell Medicine–New York Presbyterian Hospital, led the study as a post hoc, exploratory analysis of CALGB/Alliance 140503, a multinational phase 3 trial whose primary outcome – still pending – is disease-free survival associated with the two different surgeries.
Dr. Altorki and his colleagues found 30- and 90-day survival to be comparable between surgery types. At 30 days, six patients in the study had died; four in the lobar resection group and two in the sublobar group (1.1% and 0.6%). At 90 days, 10 patients had died, or 1.4% of the cohort; 6 following lobar resection and 4 following sublobar resection. The between-group difference at 30 days was 0.5% (95% confidence interval, –1.1 to 2.3) and at 90 days remained 0.5% (95% CI, –1.5 to 2.6).
Similar rates of serious (grade 3 or worse) adverse advents were seen between surgery groups at 15% and 14%, respectively, and no differences were seen for cardiac or pulmonary complications. In the study, the type of sublobar approach was left to the surgeon’s discretion, and a majority of the sublobar procedures (59%) were found to comprise wedge resections, with the rest segmentectomies. Dr. Altorki and colleagues noted the high rate of wedge resections as striking, because “conventional wisdom … holds that an anatomical segmentectomy, involving individual ligation of segmental vessels and bronchi and wider parenchymal resection, is oncologically superior to nonanatomical wedge resections.” In their analysis the researchers conceded that a three-arm trial allocating patients to lobectomy, segmentectomy, or wedge resection “would have answered more precisely the posited research question,” but said that the sample size needed would have been too large.
The study was funded by the National Cancer Institute. Dr. Altorki reported a research grant from AstraZeneca unrelated to the study; two more coauthors disclosed funding from pharmaceutical or device manufacturers, and an additional 17 coauthors listed no competing interests.
SOURCE: Altorki NK et al. Lancet Respir Med. 2018 Nov 12. doi: 10.1016/S2213-2600(18)30411-9 .
Though lobectomy is the long-held standard of care for people with early stage non–small cell lung cancer, a noninferiority study shows little difference in perioperative morbidity and mortality outcomes when sublobar resections are performed instead.
The study, published online in The Lancet Respiratory Medicine, compared results from 697 functionally and physically fit patients with stage I cancer randomized over a 10-year period to lobar resection (n = 357) or sublobar resection (n = 340). Patients were analyzed for morbidity and mortality outcomes at 30 and 90 days post surgery. Nasser K. Altorki, MD, of Weill Cornell Medicine–New York Presbyterian Hospital, led the study as a post hoc, exploratory analysis of CALGB/Alliance 140503, a multinational phase 3 trial whose primary outcome – still pending – is disease-free survival associated with the two different surgeries.
Dr. Altorki and his colleagues found 30- and 90-day survival to be comparable between surgery types. At 30 days, six patients in the study had died; four in the lobar resection group and two in the sublobar group (1.1% and 0.6%). At 90 days, 10 patients had died, or 1.4% of the cohort; 6 following lobar resection and 4 following sublobar resection. The between-group difference at 30 days was 0.5% (95% confidence interval, –1.1 to 2.3) and at 90 days remained 0.5% (95% CI, –1.5 to 2.6).
Similar rates of serious (grade 3 or worse) adverse advents were seen between surgery groups at 15% and 14%, respectively, and no differences were seen for cardiac or pulmonary complications. In the study, the type of sublobar approach was left to the surgeon’s discretion, and a majority of the sublobar procedures (59%) were found to comprise wedge resections, with the rest segmentectomies. Dr. Altorki and colleagues noted the high rate of wedge resections as striking, because “conventional wisdom … holds that an anatomical segmentectomy, involving individual ligation of segmental vessels and bronchi and wider parenchymal resection, is oncologically superior to nonanatomical wedge resections.” In their analysis the researchers conceded that a three-arm trial allocating patients to lobectomy, segmentectomy, or wedge resection “would have answered more precisely the posited research question,” but said that the sample size needed would have been too large.
The study was funded by the National Cancer Institute. Dr. Altorki reported a research grant from AstraZeneca unrelated to the study; two more coauthors disclosed funding from pharmaceutical or device manufacturers, and an additional 17 coauthors listed no competing interests.
SOURCE: Altorki NK et al. Lancet Respir Med. 2018 Nov 12. doi: 10.1016/S2213-2600(18)30411-9 .
Though lobectomy is the long-held standard of care for people with early stage non–small cell lung cancer, a noninferiority study shows little difference in perioperative morbidity and mortality outcomes when sublobar resections are performed instead.
The study, published online in The Lancet Respiratory Medicine, compared results from 697 functionally and physically fit patients with stage I cancer randomized over a 10-year period to lobar resection (n = 357) or sublobar resection (n = 340). Patients were analyzed for morbidity and mortality outcomes at 30 and 90 days post surgery. Nasser K. Altorki, MD, of Weill Cornell Medicine–New York Presbyterian Hospital, led the study as a post hoc, exploratory analysis of CALGB/Alliance 140503, a multinational phase 3 trial whose primary outcome – still pending – is disease-free survival associated with the two different surgeries.
Dr. Altorki and his colleagues found 30- and 90-day survival to be comparable between surgery types. At 30 days, six patients in the study had died; four in the lobar resection group and two in the sublobar group (1.1% and 0.6%). At 90 days, 10 patients had died, or 1.4% of the cohort; 6 following lobar resection and 4 following sublobar resection. The between-group difference at 30 days was 0.5% (95% confidence interval, –1.1 to 2.3) and at 90 days remained 0.5% (95% CI, –1.5 to 2.6).
Similar rates of serious (grade 3 or worse) adverse advents were seen between surgery groups at 15% and 14%, respectively, and no differences were seen for cardiac or pulmonary complications. In the study, the type of sublobar approach was left to the surgeon’s discretion, and a majority of the sublobar procedures (59%) were found to comprise wedge resections, with the rest segmentectomies. Dr. Altorki and colleagues noted the high rate of wedge resections as striking, because “conventional wisdom … holds that an anatomical segmentectomy, involving individual ligation of segmental vessels and bronchi and wider parenchymal resection, is oncologically superior to nonanatomical wedge resections.” In their analysis the researchers conceded that a three-arm trial allocating patients to lobectomy, segmentectomy, or wedge resection “would have answered more precisely the posited research question,” but said that the sample size needed would have been too large.
The study was funded by the National Cancer Institute. Dr. Altorki reported a research grant from AstraZeneca unrelated to the study; two more coauthors disclosed funding from pharmaceutical or device manufacturers, and an additional 17 coauthors listed no competing interests.
SOURCE: Altorki NK et al. Lancet Respir Med. 2018 Nov 12. doi: 10.1016/S2213-2600(18)30411-9 .
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: Patients with 30 and 90 days post surgery.
Major finding: Mortality at 30 days and 90 days was 0.5% for both trial groups and serious adverse advents were similar between groups.
Study details: A post hoc analysis from a multinational trial randomizing about 700 stage I NSCLC patients to lobar or sublobar surgery
Disclosures: National Cancer Institute sponsored the study; three authors including the lead author reported financial ties to manufacturers.
Source: Altorki et al. Lancet Respir Med. 2018 Nov 12. doi: 10.1016/S2213-2600(18)30411-9.
Huntington’s research returns to Latin America, as scientists tread with care
BARRANQUILLA, COLOMBIA – “We don’t like to call them brigades. That sounds militant,” said neuropsychologist Johan Acosta-López, PhD.
Dr. Acosta-López, the head of cognitive neurosciences at Simón Bolivar University in this city on Colombia’s Atlantic coast, was among five Colombian clinicians – neurologists, psychiatrists, and neuropsychologists – stuffed into a car on their way to a conference hotel in July 2018.

The following day they would be joined by clinicians and researchers from North America, other Latin American countries, and Europe for a first-of-its kind meeting on Huntington’s disease (HD) in the region, sponsored by Factor-H, an HD charity working in Latin America.
Once the talks wrapped up, the researchers – clinicians and basic scientists – were invited to see patients at a hospital in a town an hour inland with a large concentration of HD families, most of them extremely poor. For some, the Factor-H–sponsored “brigade” would be their first hands-on experience with HD patients in a developing country.
There was some debate in the car about what to call such events: brigades, “integrated health days,” or clinics. Around here – where HD abounded but patients were weary of researchers – terminology mattered.
“We’ve had so many investigators arrive in this area – foreigners and Colombians – telling people ‘we’ve got this huge, great project that you’ll benefit from.’ And they take blood samples and never return,” Dr. Acosta-López said.

Even as a local investigator, Dr. Acosta-López has faced challenges getting a new study off the ground. Dr. Acosta-López and his colleagues are working under a grant from the Colombian government to recruit 241 presymptomatic subjects with confirmed genetic markers for HD, and evaluate them for cognitive and neurologic changes preceding disease onset.
It’s a cross-sectional study, and such studies are usually funded for a year. But the investigators knew it would take much more than a year to recruit patients here, and planned their study for 3 years. As of July, the team had been engaging with the community for 6 months but still didn’t have a single blood sample.
“We’ve had to convince everyone that this time is different,” he said, “and that means focusing on the social aspect” – setting up a legal-assistance program through the university to help families claim health benefits and a job-training program sponsored by local businesses.
It’s unusual for researchers to find themselves playing such extensive roles in coordinating social and economic support for their subjects. But with HD, it’s happening across Latin America, where researchers speak frequently of a “debt” owed to HD families in this region.
Huntington’s disease is a neurodegenerative disease caused by a genetic mutation in the huntingtin (HTT) gene, changing the normal protein it expresses in the body to a toxic form that damages cortical and basal ganglia neurons. It affects between 0.5 to 1 in 10,000 people worldwide, with higher prevalence in the United States, Europe, and Australia.
HD is inherited in an autosomal dominant pattern; a child of a parent with the mutation has a 50% chance of developing the disease. Patients develop cognitive symptoms that progress to dementia, along with the debilitating involuntary, dancelike movements that gave the disease the name by which it was formerly known: Huntington’s chorea.
In the 1980s and 1990s, several generations of Latin American HD families provided data that allowed for some of the greatest research advances in the disease – and they may represent a large share of the world’s HD cases. Yet, they continue to live in extreme poverty and have benefited little from the findings of the past 3 decades.
Without recognizing this and working to improve the families’ well-being, the researchers at the conference said it’s unlikely that promising therapies in the pipeline will ever reach the populations that need them the most.
Discovery in Venezuela
Some 8 hours by car from Barranquilla sits Lake Maracaibo, Venezuela, home to the largest known clusters of HD patients worldwide. The disease is believed to have come to the shores of Lake Maracaibo with a lone European immigrant – a Spanish sailor, many claim – at the end of the 18th century. Cases were first described in the 1950s by a young Venezuelan physician named Américo Negrette, MD.
Dr. Negrette’s findings were ignored by health officials in Venezuela and went unnoticed in the international research community until 1972, when a student of Dr. Negrette’s presented at an HD conference in Ohio. There he drew the attention of the American neuropsychologist Nancy S. Wexler, PhD. Dr. Wexler’s own mother had died of HD, and her father Milton, a noted psychoanalyst, founded the first research foundation dedicated to the disease.

While prevalence of HD in North America, Australia, and Europe is about 1 in 10,000, the region around Lake Maracaibo saw 70 times that rate at the time, thanks to high birth rates, geographic isolation, and extensive intermarriage within a handful of families. The families comprised mostly poor fishermen who lived in makeshift homes in towns ringing the lake.
In 1979, Dr. Wexler, with funding from the U.S. National Institutes of Health, began making annual research visits to Lake Maracaibo, and in 1983, the research group she coordinated, using data from blood and tissue samples donated by affected families, identified the location of the huntingtin gene on chromosome 4 (Nature. 1983 Nov 17;306[5940]:234-8). A decade later, the researchers isolated the mutant version of the gene and found it to be a triplet (CAG) expansion mutation, with more CAG repetitions associated with earlier age at disease onset (Cell. 1993 Mar 26;72[6]:971-83). Dr. Wexler and her colleagues’ findings led to the first genetic tests for HD.
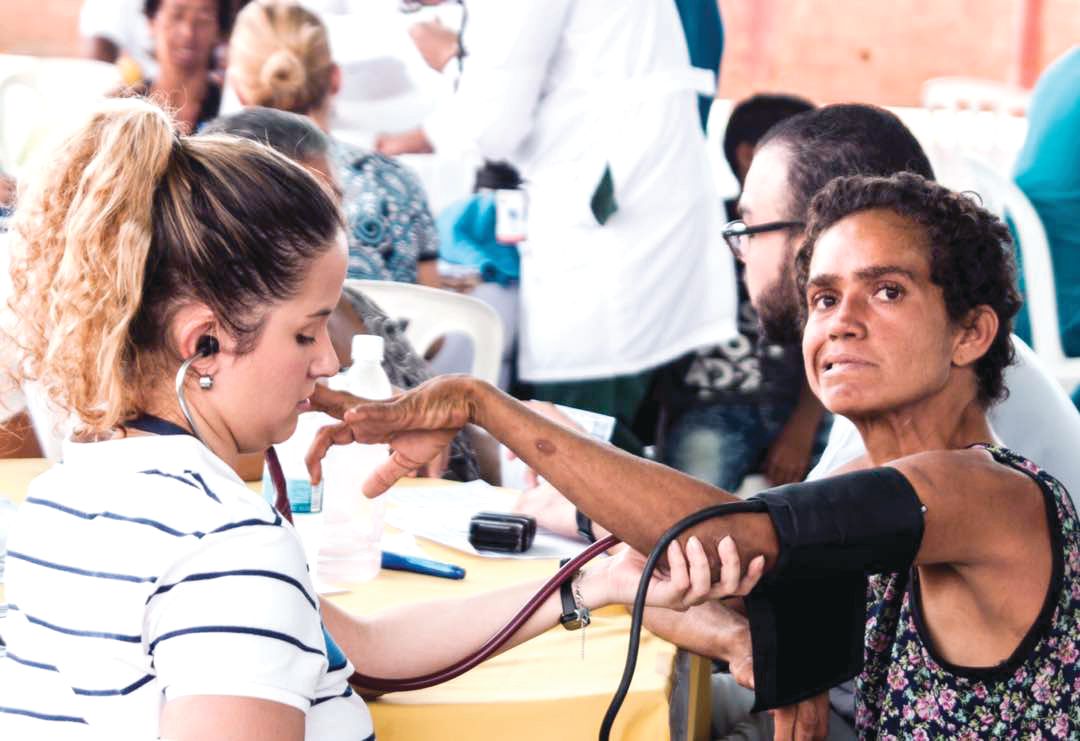
Nationalist policies in Venezuela ended Dr. Wexler and her colleagues’ annual visits to Lake Maracaibo in 2002, along with the food, clothing, and medicines that the group routinely distributed to the families when they came.
Over 23 years, the researchers obtained data from some 18,000 individuals, but the families did not benefit in any durable way from the research. Local investigators with whom Dr. Wexler’s group collaborated lacked the resources and training to continue independently.
Access to medications is limited in Venezuela, and there is no institutional support for hundreds of HD patients living in extreme poverty, many of them descendants of the patients who contributed to the research and generation of these samples. The families’ biological material was sent to labs abroad, where investigators continue to derive findings from it today. Though genetic testing was performed on thousands at risk for the disease, few received access to their results through genetic counseling. A hospice established by Dr. Wexler’s foundation limped along until 2014, when it was finally shuttered.
Rebuilding bridges
A handful of families from the Lake Maracaibo towns attended the conference in Barranquilla. Their travel costs were picked up by Factor-H, which sponsored the event.
Ignacio Muñoz-Sanjuán, PhD, Factor-H’s founder and president, knew the families personally. He’s visited them regularly for years. In 2017, Dr. Muñoz-Sanjuán, a molecular biologist known affectionately in the HD community as “Nacho,” invited several to Rome for a meeting with Pope Francis, as part of an effort to raise awareness of HD and to request support from the Catholic Church for the Latin American families.
Humanitarian work is relatively new to Dr. Muñoz-Sanjuán, who’s spent his career in drug development. In addition to his unpaid work with Factor-H, he is vice president of biology with the CHDI Foundation, a Los Angeles–based nonprofit that funds drug research in HD. CHDI is reported to have about $100 million in annual funding – about triple the NIH budget in recent years for HD research. Its major donors are a group of investors who for years have remained anonymous and do not publicly discuss their philanthropy.
The Spanish-born Dr. Muñoz-Sanjuán had little direct experience with HD populations in Latin America until a few years ago, he said.
At a CHDI meeting in Brazil, he said, “I was talking with physicians and patient advocates from Latin America, telling them they had to be willing to be involved, that these communities with high prevalence had a lot to offer science,” Dr. Muñoz-Sanjuán said in an interview. “I was told that it was me who needed to understand the conditions in which HD patients lived. It completely put me on the spot.”
HD tends to strike during the most productive years of a person’s life, from the late 30s onward, keeping them from working and obliging family members to stop working to care for them. In a poor community, it can condemn a family to a state of extreme poverty for generations. Tetrabenazine (Xenazine), a medication to quiet chorea symptoms, is costly enough that many patients must do without it. Ensuring adequate calorie intake is difficult in HD patients, whose constant movements cause them to lose weight.
Dr. Muñoz-Sanjuán traveled to Colombia, Venezuela, and Brazil, meeting HD families and doctors like neurologist Gustavo Barrios, MD, of Hospital Occidente de Kennedy in Bogotá, Colombia. In a talk at the Barranquilla conference, Dr. Barrios related the experience of his first visit to El Dificil, a community in northern Colombia where some large HD families are forced to survive on the equivalent of $5 a day. “I had to confront not only the fact that these families were living with a terrible disease but in conditions of extreme deprivation,” he said. “My life as a doctor changed that day.”
Dr. Muñoz-Sanjuán helped form a Latin American HD network to involve clinicians like Dr. Barrios who worked with HD clusters, most of them poorly studied. “These are all neglected communities that share similar features,” Dr. Muñoz-Sanjuán said.
On Colombia’s Caribbean coast, for example, HD had been documented since the early 1990s, but genotyping was not performed until recently. Prevalence data are “virtually nonexistent” in Colombia, said Sonia Moreno, PhD, a neuropsychologist at the University of Antioquia in Medellin. In a pilot study presented this year at the CHDI Foundation’s Enroll-HD Congress, Dr. Moreno and her colleagues mined Colombian public health data for likely HD cases, and argued for the creation of a national registry.
In 2012, Dr. Muñoz-Sanjuán founded Factor-H with the aim of improving living conditions for Latin American communities with HD.

Factor-H does not receive funds from CHDI Foundation and instead relies on donations from individuals and companies; its annual budget is less than $200,000. But through contracts with local nongovernmental organizations, it has sponsored health clinics and ongoing food assistance, delivered shipments of medicines and clothing, and started a sponsorship program for young people in HD families, whose studies often are interrupted caring for sick parents. It hopes to build permanent support centers in Colombia and Venezuela where HD families can get their food and medical needs met.
“The traditional thinking in the HD research community is that we’re helping people by doing the legwork to make medicine – and that’s not necessarily enough. You need a more holistic approach,” Dr. Muñoz-Sanjuán said.
Lennie Pineda, MSc, who recently retired as a geneticist with the University of Zulia in Maracaibo, Venezuela, said that Dr. Muñoz-Sanjuán was viewed skeptically when he first visited, in part because of his biomedical research background.
Ms. Pineda, who worked with the region’s HD families her whole career, has been wary of past research efforts in Venezuela. In 2010, she published a paper critical of Dr. Wexler’s and his colleagues’ approach (Revista Redbioética/UNESCO. 2010;1[2]:50-61), particularly regarding issues of informed consent.
“I was very cold to Nacho,” she laughed. “We all looked at him suspiciously.”
Ms. Pineda said Dr. Muñoz-Sanjuán won her over with his interest and creativity in finding concrete ways improve the lives of families in the Lake Maracaibo towns.
In a talk at the conference, Edison Soto, a young man from San Luis, a town on Lake Maracaibo that is a key cluster of HD, said Dr. Muñoz-Sanjuán’s visits had reawakened hope among the families there. “For years, no one thought about us, and because of the situation in the country it’s been hard, really hard,” he said.
“Nacho’s smart,” Ms. Pineda said. “He’s not coming to build a research cohort, he’s coming with genuine intention to help. But if one day conditions are adequate to support investigation, and the people here are well informed and volunteer for a study with full consent, well, all the better,” she said.
Dr. Muñoz-Sanjuán acknowledged that his humanitarian work could be perceived as preparing the ground for future clinical trials.
“I’m not doing anything research oriented with Latin America,” he said. “I would never approach these communities and recommend they take part in a study or give samples, unless their conditions change significantly. But the idea of cross-contamination is a problem I might need to fix. There may come a day where I need to depersonalize Factor-H from me.”
A research platform, a novel agent
Though HD research in Latin America remains rife with challenges, a number of investigators at the conference talked optimistically about planned and ongoing HD studies in Latin America.
The biggest of these is ENROLL-HD, a long-term global observational study of families with HD that uses a standardized approach to data collection. The platform, launched in 2013, aims to enroll 20,000 participants for yearly (or more frequent) assessment. Data from ENROLL-HD will support a diverse range of studies on everything from biomarkers to genetic modifiers to quality of life measures in HD.
ENROLL-HD has opened study sites in Argentina, Chile, and Colombia, and plans to launch a site near Lima, Peru, that is home to an HD cluster. Venezuela is considered out of reach, at least for now.
In Barranquilla, Claudia Perandones, MD, PhD, a genetics researcher in Argentina who manages ENROLL-HD for Latin America and is a cofounder of Factor-H, explained why the kind of clusters seen in Latin America are so valuable scientifically.
The extended family groups share a disease haplotype, eat the same foods, and live in similar environments, Dr. Perandones noted. Because not all the variation in HD can be explained by the number of CAG repeats a patient has, having a large sample with a common haplotype would help researchers pinpoint other environmental and genetic factors that can modify the onset or progress of the disease.
Another key goal of ENROLL-HD, investigators say, is to speed recruitment into clinical trials as they arise. And for the first time in history, potentially game-changing therapies are being developed specifically for HD.
For the past 5 years the Swiss pharmaceutical giant Roche has worked with a smaller biotech firm, Ionis Pharmaceuticals, on an agent called RG6042, which was known until recently as IONIS-HTTRx. CHDI was extensively involved in the agent’s preclinical development, contributing some $10 million to get it off the ground.
RG6042 is an antisense oligonucleotide, delivered by spinal injection, which works by interrupting an mRNA signaling pathway to suppress production of mutant HTT (mHTT) protein in the brain. Antisense oligonucleotides, sometimes called gene silencing therapies, are a new and promising approach in neurodegenerative diseases. Two have received FDA approval to treat spinal muscular atrophy and Duchenne muscular dystrophy.
In April 2018, Roche announced positive results from phase 1/2a study in 46 HD patients in Europe and North America. Patients in that 13-week study saw significant (up to 60%) dose-dependent reductions of the mHTT in their cerebrospinal fluid; a post hoc analysis also found some evidence of functional improvement (Neurology. 2018;90[15 Supplement]:CT.002).
These encouraging findings led to Roche’s announcement of a global phase 3 randomized, controlled trial that is scheduled to begin enrolling in 2019. Roche hopes to randomize 660 patients with mild HD across 15 countries for the 2-year trial, called GENERATION-HD1.
Sites in Latin America are expected to include Argentina, Chile, and Colombia.
At the Barranquilla meeting, Daniel Ciriano, MD, Roche’s Argentina-based medical director for Latin America, extolled the company’s commitment to ethics and social welfare in the region. In recent years, Roche has increased its humanitarian commitments across Latin America, including helping rebuild a Chilean village after an earthquake and offering free breast cancer and kidney disease treatments.
RG6042 is only one of a number of promising approaches to HD. Other therapies in the pipeline include gene silencing delivered by viral vectors instead of repeated spinal injections, an oral drug that interrupts mHTT production, immunotherapies, and even CRISPR gene–editing techniques.
Little was said at the conference, however, about how Latin American HD communities might be able to afford RG6042 or any other therapy that emerges from the pipeline.
Dr. Muñoz-Sanjuán called the issue “a theme for future discussion.”
“This is an area that has to be handled carefully and not one we are heavily invested in yet, although it’s very important,” he said.
On the ground
Several of the European and North American scientists who presented in Barranquilla took pains to express their concern with the well-being of HD patients in Latin America and to demonstrate goodwill toward the local researchers and clinicians.
Hilal A. Lashuel, PhD, a molecular biologist working on the structure and behavior of the HTT protein, said his participation in the Factor-H event at the Vatican the year before had awakened him to “the real human part of HD,” and changed the way he does science.
Normally, Dr. Lashuel said, “we do research disconnected from the realities of the diseases we work with.”
“We need to not just to do research but [to ensure] that research is done right,” he said, which means also focusing on improving patients’ standard of living.
The room broke out in applause when Dr. Lashuel announced new internships for investigators from developing countries. He also presented a parting video from his research team at the École Polytechnique Fédérale de Lausanne (Switzerland), complete with music and affectionate messages in Spanish.
Pharmacologist Elena Cattaneo, PhD, a stem cell researcher long active in the HD community, and also a senator in Italy’s parliament, delivered a similarly warm, carefully choreographed video message from her laboratory at the University of Milan.
Just days later in the town of Juan de Acosta, an hour inland of Barranquilla, the same researchers sat down with patients and families who crowded the waiting room of the town’s only hospital, as the sun beat in through the windows and as mule carts, stray dogs, and buses passed by on the main drag outside.
The event had been titled a “brigade” after all, but the HD families did not seem to mind – and indeed so many showed up that a sign had to be placed on the door saying that no one who arrived after noon could be seen. Consults were not limited to HD-related matters, so families could be seen for any complaint.
HD was first documented in this town in the early 1990s, but much remains to be understood about the size of the cluster, the haplotype, and its relation to other clusters in Colombia or Venezuela. The families here share a handful of last names and likely share a common ancestor. In the early 19th century, the Barranquilla region was flooded with European migrants who reached the city by ship. (HD clusters in Latin America tend to be concentrated in coastal regions, possibly because of migration patterns.)
The waiting room of the hospital was loud with chatter. Small children played as their relatives waited for consults. Some showed the characteristic restless movements and emaciated bodies of people with advanced HD.
The foreign scientists were barred from taking any patient data out of the hospital or asking for samples. Even picture taking was prohibited. Instead they performed genetic counseling and neuropsychological tests; they sorted out differential diagnoses and advised on medications. Visiting Colombian and Venezuelan physicians did the same, while their assistants met with families in the waiting room, taking medical histories and sketching out basic genealogies.
Some of the foreign researchers reported fruitful interactions with patients, while others seemed perplexed by what they’d experienced. Alba di Pardo, PhD, a genetic epidemiologist at the Istituto Neurologico Mediterraneo Pozzilli (Italy), said she’d spent the morning doing genetic counseling with families and going over genealogies to assess risk. Yet, despite the fact that anyone with an HD parent has a 50% chance of developing the disease, some family members acted uninterested, she said.
Dr. di Pardo’s colleague at the Istituto, biologist Vittorio Maglione, PhD, reported having a similar experience. As he was counseling a young woman about her risk for HD, she scrolled indifferently through Facebook posts on her phone, he said.
On some level, Dr. Maglione said, he could understand patients’ reluctance to engage noting that, while there were many potential HD therapies to try, any new treatment paradigm for HD was many years away from a place like this – and potentially very costly. Dr. Maglione – along with Dr. di Pardo – is researching the SP1 axis, a sphingolipid pathway implicated in neurodegenerative such diseases as HD and which has potential as a drug target (Trends Pharmacol Sci. 2018;39[5]:468-80).
Psychologist Pedro Puentes Rozo of Simón Bolivar University, who is working with Dr. Acosta-López on the local cohort study of presymptomatic HD patients, said that, for most of the families in the clinic that day, any seeming indifference probably masked deeper fears. People already were well aware of their risk. “They’ve known about it forever, said Dr. Puentes Rozo, who has been working with this HD population for a decade. “But this is a catastrophic illness and can generate a lot of anxiety.”
Dr. Puentes Rozo said the group’s planned study, unlike studies in the past, would be conducted under strict “international ethical norms and standards.” Subjects would receive ongoing psychological support, and the researchers were working to establish a genetic counseling center so that people who want to know their status “can be prepared,” he said, and plan for their lives and families.
By fall 2018, the cohort study was underway. The group had sponsored several more hospital brigades – or “integrated health days” as they preferred to call them, at the hospital in Juan de Acosta, giving them a chance to work face to face with families.
They drew no blood during the clinics, as investigators in the past had done. Instead, they explained the study to patients, performed the initial screenings, and invited them to designated study appointments at the university. Legal assistance was up and running, and the jobs program would start in 2019.
Enrollment was climbing. And the group was steadily accumulating data.
BARRANQUILLA, COLOMBIA – “We don’t like to call them brigades. That sounds militant,” said neuropsychologist Johan Acosta-López, PhD.
Dr. Acosta-López, the head of cognitive neurosciences at Simón Bolivar University in this city on Colombia’s Atlantic coast, was among five Colombian clinicians – neurologists, psychiatrists, and neuropsychologists – stuffed into a car on their way to a conference hotel in July 2018.

The following day they would be joined by clinicians and researchers from North America, other Latin American countries, and Europe for a first-of-its kind meeting on Huntington’s disease (HD) in the region, sponsored by Factor-H, an HD charity working in Latin America.
Once the talks wrapped up, the researchers – clinicians and basic scientists – were invited to see patients at a hospital in a town an hour inland with a large concentration of HD families, most of them extremely poor. For some, the Factor-H–sponsored “brigade” would be their first hands-on experience with HD patients in a developing country.
There was some debate in the car about what to call such events: brigades, “integrated health days,” or clinics. Around here – where HD abounded but patients were weary of researchers – terminology mattered.
“We’ve had so many investigators arrive in this area – foreigners and Colombians – telling people ‘we’ve got this huge, great project that you’ll benefit from.’ And they take blood samples and never return,” Dr. Acosta-López said.

Even as a local investigator, Dr. Acosta-López has faced challenges getting a new study off the ground. Dr. Acosta-López and his colleagues are working under a grant from the Colombian government to recruit 241 presymptomatic subjects with confirmed genetic markers for HD, and evaluate them for cognitive and neurologic changes preceding disease onset.
It’s a cross-sectional study, and such studies are usually funded for a year. But the investigators knew it would take much more than a year to recruit patients here, and planned their study for 3 years. As of July, the team had been engaging with the community for 6 months but still didn’t have a single blood sample.
“We’ve had to convince everyone that this time is different,” he said, “and that means focusing on the social aspect” – setting up a legal-assistance program through the university to help families claim health benefits and a job-training program sponsored by local businesses.
It’s unusual for researchers to find themselves playing such extensive roles in coordinating social and economic support for their subjects. But with HD, it’s happening across Latin America, where researchers speak frequently of a “debt” owed to HD families in this region.
Huntington’s disease is a neurodegenerative disease caused by a genetic mutation in the huntingtin (HTT) gene, changing the normal protein it expresses in the body to a toxic form that damages cortical and basal ganglia neurons. It affects between 0.5 to 1 in 10,000 people worldwide, with higher prevalence in the United States, Europe, and Australia.
HD is inherited in an autosomal dominant pattern; a child of a parent with the mutation has a 50% chance of developing the disease. Patients develop cognitive symptoms that progress to dementia, along with the debilitating involuntary, dancelike movements that gave the disease the name by which it was formerly known: Huntington’s chorea.
In the 1980s and 1990s, several generations of Latin American HD families provided data that allowed for some of the greatest research advances in the disease – and they may represent a large share of the world’s HD cases. Yet, they continue to live in extreme poverty and have benefited little from the findings of the past 3 decades.
Without recognizing this and working to improve the families’ well-being, the researchers at the conference said it’s unlikely that promising therapies in the pipeline will ever reach the populations that need them the most.
Discovery in Venezuela
Some 8 hours by car from Barranquilla sits Lake Maracaibo, Venezuela, home to the largest known clusters of HD patients worldwide. The disease is believed to have come to the shores of Lake Maracaibo with a lone European immigrant – a Spanish sailor, many claim – at the end of the 18th century. Cases were first described in the 1950s by a young Venezuelan physician named Américo Negrette, MD.
Dr. Negrette’s findings were ignored by health officials in Venezuela and went unnoticed in the international research community until 1972, when a student of Dr. Negrette’s presented at an HD conference in Ohio. There he drew the attention of the American neuropsychologist Nancy S. Wexler, PhD. Dr. Wexler’s own mother had died of HD, and her father Milton, a noted psychoanalyst, founded the first research foundation dedicated to the disease.

While prevalence of HD in North America, Australia, and Europe is about 1 in 10,000, the region around Lake Maracaibo saw 70 times that rate at the time, thanks to high birth rates, geographic isolation, and extensive intermarriage within a handful of families. The families comprised mostly poor fishermen who lived in makeshift homes in towns ringing the lake.
In 1979, Dr. Wexler, with funding from the U.S. National Institutes of Health, began making annual research visits to Lake Maracaibo, and in 1983, the research group she coordinated, using data from blood and tissue samples donated by affected families, identified the location of the huntingtin gene on chromosome 4 (Nature. 1983 Nov 17;306[5940]:234-8). A decade later, the researchers isolated the mutant version of the gene and found it to be a triplet (CAG) expansion mutation, with more CAG repetitions associated with earlier age at disease onset (Cell. 1993 Mar 26;72[6]:971-83). Dr. Wexler and her colleagues’ findings led to the first genetic tests for HD.
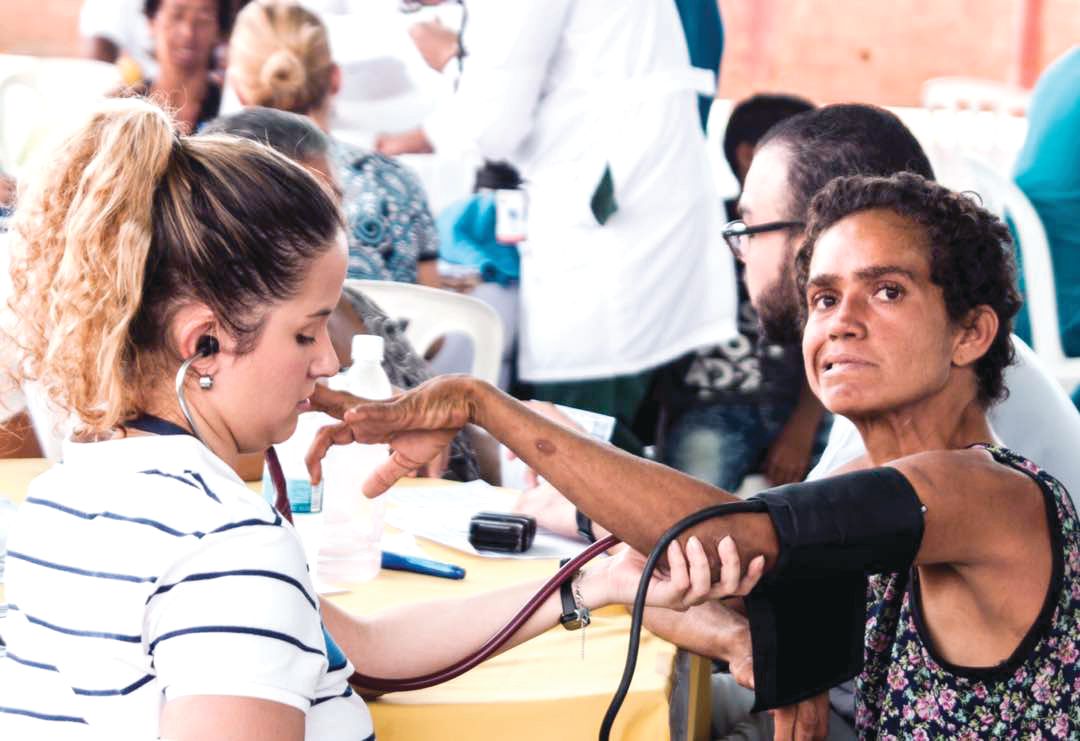
Nationalist policies in Venezuela ended Dr. Wexler and her colleagues’ annual visits to Lake Maracaibo in 2002, along with the food, clothing, and medicines that the group routinely distributed to the families when they came.
Over 23 years, the researchers obtained data from some 18,000 individuals, but the families did not benefit in any durable way from the research. Local investigators with whom Dr. Wexler’s group collaborated lacked the resources and training to continue independently.
Access to medications is limited in Venezuela, and there is no institutional support for hundreds of HD patients living in extreme poverty, many of them descendants of the patients who contributed to the research and generation of these samples. The families’ biological material was sent to labs abroad, where investigators continue to derive findings from it today. Though genetic testing was performed on thousands at risk for the disease, few received access to their results through genetic counseling. A hospice established by Dr. Wexler’s foundation limped along until 2014, when it was finally shuttered.
Rebuilding bridges
A handful of families from the Lake Maracaibo towns attended the conference in Barranquilla. Their travel costs were picked up by Factor-H, which sponsored the event.
Ignacio Muñoz-Sanjuán, PhD, Factor-H’s founder and president, knew the families personally. He’s visited them regularly for years. In 2017, Dr. Muñoz-Sanjuán, a molecular biologist known affectionately in the HD community as “Nacho,” invited several to Rome for a meeting with Pope Francis, as part of an effort to raise awareness of HD and to request support from the Catholic Church for the Latin American families.
Humanitarian work is relatively new to Dr. Muñoz-Sanjuán, who’s spent his career in drug development. In addition to his unpaid work with Factor-H, he is vice president of biology with the CHDI Foundation, a Los Angeles–based nonprofit that funds drug research in HD. CHDI is reported to have about $100 million in annual funding – about triple the NIH budget in recent years for HD research. Its major donors are a group of investors who for years have remained anonymous and do not publicly discuss their philanthropy.
The Spanish-born Dr. Muñoz-Sanjuán had little direct experience with HD populations in Latin America until a few years ago, he said.
At a CHDI meeting in Brazil, he said, “I was talking with physicians and patient advocates from Latin America, telling them they had to be willing to be involved, that these communities with high prevalence had a lot to offer science,” Dr. Muñoz-Sanjuán said in an interview. “I was told that it was me who needed to understand the conditions in which HD patients lived. It completely put me on the spot.”
HD tends to strike during the most productive years of a person’s life, from the late 30s onward, keeping them from working and obliging family members to stop working to care for them. In a poor community, it can condemn a family to a state of extreme poverty for generations. Tetrabenazine (Xenazine), a medication to quiet chorea symptoms, is costly enough that many patients must do without it. Ensuring adequate calorie intake is difficult in HD patients, whose constant movements cause them to lose weight.
Dr. Muñoz-Sanjuán traveled to Colombia, Venezuela, and Brazil, meeting HD families and doctors like neurologist Gustavo Barrios, MD, of Hospital Occidente de Kennedy in Bogotá, Colombia. In a talk at the Barranquilla conference, Dr. Barrios related the experience of his first visit to El Dificil, a community in northern Colombia where some large HD families are forced to survive on the equivalent of $5 a day. “I had to confront not only the fact that these families were living with a terrible disease but in conditions of extreme deprivation,” he said. “My life as a doctor changed that day.”
Dr. Muñoz-Sanjuán helped form a Latin American HD network to involve clinicians like Dr. Barrios who worked with HD clusters, most of them poorly studied. “These are all neglected communities that share similar features,” Dr. Muñoz-Sanjuán said.
On Colombia’s Caribbean coast, for example, HD had been documented since the early 1990s, but genotyping was not performed until recently. Prevalence data are “virtually nonexistent” in Colombia, said Sonia Moreno, PhD, a neuropsychologist at the University of Antioquia in Medellin. In a pilot study presented this year at the CHDI Foundation’s Enroll-HD Congress, Dr. Moreno and her colleagues mined Colombian public health data for likely HD cases, and argued for the creation of a national registry.
In 2012, Dr. Muñoz-Sanjuán founded Factor-H with the aim of improving living conditions for Latin American communities with HD.

Factor-H does not receive funds from CHDI Foundation and instead relies on donations from individuals and companies; its annual budget is less than $200,000. But through contracts with local nongovernmental organizations, it has sponsored health clinics and ongoing food assistance, delivered shipments of medicines and clothing, and started a sponsorship program for young people in HD families, whose studies often are interrupted caring for sick parents. It hopes to build permanent support centers in Colombia and Venezuela where HD families can get their food and medical needs met.
“The traditional thinking in the HD research community is that we’re helping people by doing the legwork to make medicine – and that’s not necessarily enough. You need a more holistic approach,” Dr. Muñoz-Sanjuán said.
Lennie Pineda, MSc, who recently retired as a geneticist with the University of Zulia in Maracaibo, Venezuela, said that Dr. Muñoz-Sanjuán was viewed skeptically when he first visited, in part because of his biomedical research background.
Ms. Pineda, who worked with the region’s HD families her whole career, has been wary of past research efforts in Venezuela. In 2010, she published a paper critical of Dr. Wexler’s and his colleagues’ approach (Revista Redbioética/UNESCO. 2010;1[2]:50-61), particularly regarding issues of informed consent.
“I was very cold to Nacho,” she laughed. “We all looked at him suspiciously.”
Ms. Pineda said Dr. Muñoz-Sanjuán won her over with his interest and creativity in finding concrete ways improve the lives of families in the Lake Maracaibo towns.
In a talk at the conference, Edison Soto, a young man from San Luis, a town on Lake Maracaibo that is a key cluster of HD, said Dr. Muñoz-Sanjuán’s visits had reawakened hope among the families there. “For years, no one thought about us, and because of the situation in the country it’s been hard, really hard,” he said.
“Nacho’s smart,” Ms. Pineda said. “He’s not coming to build a research cohort, he’s coming with genuine intention to help. But if one day conditions are adequate to support investigation, and the people here are well informed and volunteer for a study with full consent, well, all the better,” she said.
Dr. Muñoz-Sanjuán acknowledged that his humanitarian work could be perceived as preparing the ground for future clinical trials.
“I’m not doing anything research oriented with Latin America,” he said. “I would never approach these communities and recommend they take part in a study or give samples, unless their conditions change significantly. But the idea of cross-contamination is a problem I might need to fix. There may come a day where I need to depersonalize Factor-H from me.”
A research platform, a novel agent
Though HD research in Latin America remains rife with challenges, a number of investigators at the conference talked optimistically about planned and ongoing HD studies in Latin America.
The biggest of these is ENROLL-HD, a long-term global observational study of families with HD that uses a standardized approach to data collection. The platform, launched in 2013, aims to enroll 20,000 participants for yearly (or more frequent) assessment. Data from ENROLL-HD will support a diverse range of studies on everything from biomarkers to genetic modifiers to quality of life measures in HD.
ENROLL-HD has opened study sites in Argentina, Chile, and Colombia, and plans to launch a site near Lima, Peru, that is home to an HD cluster. Venezuela is considered out of reach, at least for now.
In Barranquilla, Claudia Perandones, MD, PhD, a genetics researcher in Argentina who manages ENROLL-HD for Latin America and is a cofounder of Factor-H, explained why the kind of clusters seen in Latin America are so valuable scientifically.
The extended family groups share a disease haplotype, eat the same foods, and live in similar environments, Dr. Perandones noted. Because not all the variation in HD can be explained by the number of CAG repeats a patient has, having a large sample with a common haplotype would help researchers pinpoint other environmental and genetic factors that can modify the onset or progress of the disease.
Another key goal of ENROLL-HD, investigators say, is to speed recruitment into clinical trials as they arise. And for the first time in history, potentially game-changing therapies are being developed specifically for HD.
For the past 5 years the Swiss pharmaceutical giant Roche has worked with a smaller biotech firm, Ionis Pharmaceuticals, on an agent called RG6042, which was known until recently as IONIS-HTTRx. CHDI was extensively involved in the agent’s preclinical development, contributing some $10 million to get it off the ground.
RG6042 is an antisense oligonucleotide, delivered by spinal injection, which works by interrupting an mRNA signaling pathway to suppress production of mutant HTT (mHTT) protein in the brain. Antisense oligonucleotides, sometimes called gene silencing therapies, are a new and promising approach in neurodegenerative diseases. Two have received FDA approval to treat spinal muscular atrophy and Duchenne muscular dystrophy.
In April 2018, Roche announced positive results from phase 1/2a study in 46 HD patients in Europe and North America. Patients in that 13-week study saw significant (up to 60%) dose-dependent reductions of the mHTT in their cerebrospinal fluid; a post hoc analysis also found some evidence of functional improvement (Neurology. 2018;90[15 Supplement]:CT.002).
These encouraging findings led to Roche’s announcement of a global phase 3 randomized, controlled trial that is scheduled to begin enrolling in 2019. Roche hopes to randomize 660 patients with mild HD across 15 countries for the 2-year trial, called GENERATION-HD1.
Sites in Latin America are expected to include Argentina, Chile, and Colombia.
At the Barranquilla meeting, Daniel Ciriano, MD, Roche’s Argentina-based medical director for Latin America, extolled the company’s commitment to ethics and social welfare in the region. In recent years, Roche has increased its humanitarian commitments across Latin America, including helping rebuild a Chilean village after an earthquake and offering free breast cancer and kidney disease treatments.
RG6042 is only one of a number of promising approaches to HD. Other therapies in the pipeline include gene silencing delivered by viral vectors instead of repeated spinal injections, an oral drug that interrupts mHTT production, immunotherapies, and even CRISPR gene–editing techniques.
Little was said at the conference, however, about how Latin American HD communities might be able to afford RG6042 or any other therapy that emerges from the pipeline.
Dr. Muñoz-Sanjuán called the issue “a theme for future discussion.”
“This is an area that has to be handled carefully and not one we are heavily invested in yet, although it’s very important,” he said.
On the ground
Several of the European and North American scientists who presented in Barranquilla took pains to express their concern with the well-being of HD patients in Latin America and to demonstrate goodwill toward the local researchers and clinicians.
Hilal A. Lashuel, PhD, a molecular biologist working on the structure and behavior of the HTT protein, said his participation in the Factor-H event at the Vatican the year before had awakened him to “the real human part of HD,” and changed the way he does science.
Normally, Dr. Lashuel said, “we do research disconnected from the realities of the diseases we work with.”
“We need to not just to do research but [to ensure] that research is done right,” he said, which means also focusing on improving patients’ standard of living.
The room broke out in applause when Dr. Lashuel announced new internships for investigators from developing countries. He also presented a parting video from his research team at the École Polytechnique Fédérale de Lausanne (Switzerland), complete with music and affectionate messages in Spanish.
Pharmacologist Elena Cattaneo, PhD, a stem cell researcher long active in the HD community, and also a senator in Italy’s parliament, delivered a similarly warm, carefully choreographed video message from her laboratory at the University of Milan.
Just days later in the town of Juan de Acosta, an hour inland of Barranquilla, the same researchers sat down with patients and families who crowded the waiting room of the town’s only hospital, as the sun beat in through the windows and as mule carts, stray dogs, and buses passed by on the main drag outside.
The event had been titled a “brigade” after all, but the HD families did not seem to mind – and indeed so many showed up that a sign had to be placed on the door saying that no one who arrived after noon could be seen. Consults were not limited to HD-related matters, so families could be seen for any complaint.
HD was first documented in this town in the early 1990s, but much remains to be understood about the size of the cluster, the haplotype, and its relation to other clusters in Colombia or Venezuela. The families here share a handful of last names and likely share a common ancestor. In the early 19th century, the Barranquilla region was flooded with European migrants who reached the city by ship. (HD clusters in Latin America tend to be concentrated in coastal regions, possibly because of migration patterns.)
The waiting room of the hospital was loud with chatter. Small children played as their relatives waited for consults. Some showed the characteristic restless movements and emaciated bodies of people with advanced HD.
The foreign scientists were barred from taking any patient data out of the hospital or asking for samples. Even picture taking was prohibited. Instead they performed genetic counseling and neuropsychological tests; they sorted out differential diagnoses and advised on medications. Visiting Colombian and Venezuelan physicians did the same, while their assistants met with families in the waiting room, taking medical histories and sketching out basic genealogies.
Some of the foreign researchers reported fruitful interactions with patients, while others seemed perplexed by what they’d experienced. Alba di Pardo, PhD, a genetic epidemiologist at the Istituto Neurologico Mediterraneo Pozzilli (Italy), said she’d spent the morning doing genetic counseling with families and going over genealogies to assess risk. Yet, despite the fact that anyone with an HD parent has a 50% chance of developing the disease, some family members acted uninterested, she said.
Dr. di Pardo’s colleague at the Istituto, biologist Vittorio Maglione, PhD, reported having a similar experience. As he was counseling a young woman about her risk for HD, she scrolled indifferently through Facebook posts on her phone, he said.
On some level, Dr. Maglione said, he could understand patients’ reluctance to engage noting that, while there were many potential HD therapies to try, any new treatment paradigm for HD was many years away from a place like this – and potentially very costly. Dr. Maglione – along with Dr. di Pardo – is researching the SP1 axis, a sphingolipid pathway implicated in neurodegenerative such diseases as HD and which has potential as a drug target (Trends Pharmacol Sci. 2018;39[5]:468-80).
Psychologist Pedro Puentes Rozo of Simón Bolivar University, who is working with Dr. Acosta-López on the local cohort study of presymptomatic HD patients, said that, for most of the families in the clinic that day, any seeming indifference probably masked deeper fears. People already were well aware of their risk. “They’ve known about it forever, said Dr. Puentes Rozo, who has been working with this HD population for a decade. “But this is a catastrophic illness and can generate a lot of anxiety.”
Dr. Puentes Rozo said the group’s planned study, unlike studies in the past, would be conducted under strict “international ethical norms and standards.” Subjects would receive ongoing psychological support, and the researchers were working to establish a genetic counseling center so that people who want to know their status “can be prepared,” he said, and plan for their lives and families.
By fall 2018, the cohort study was underway. The group had sponsored several more hospital brigades – or “integrated health days” as they preferred to call them, at the hospital in Juan de Acosta, giving them a chance to work face to face with families.
They drew no blood during the clinics, as investigators in the past had done. Instead, they explained the study to patients, performed the initial screenings, and invited them to designated study appointments at the university. Legal assistance was up and running, and the jobs program would start in 2019.
Enrollment was climbing. And the group was steadily accumulating data.
BARRANQUILLA, COLOMBIA – “We don’t like to call them brigades. That sounds militant,” said neuropsychologist Johan Acosta-López, PhD.
Dr. Acosta-López, the head of cognitive neurosciences at Simón Bolivar University in this city on Colombia’s Atlantic coast, was among five Colombian clinicians – neurologists, psychiatrists, and neuropsychologists – stuffed into a car on their way to a conference hotel in July 2018.

The following day they would be joined by clinicians and researchers from North America, other Latin American countries, and Europe for a first-of-its kind meeting on Huntington’s disease (HD) in the region, sponsored by Factor-H, an HD charity working in Latin America.
Once the talks wrapped up, the researchers – clinicians and basic scientists – were invited to see patients at a hospital in a town an hour inland with a large concentration of HD families, most of them extremely poor. For some, the Factor-H–sponsored “brigade” would be their first hands-on experience with HD patients in a developing country.
There was some debate in the car about what to call such events: brigades, “integrated health days,” or clinics. Around here – where HD abounded but patients were weary of researchers – terminology mattered.
“We’ve had so many investigators arrive in this area – foreigners and Colombians – telling people ‘we’ve got this huge, great project that you’ll benefit from.’ And they take blood samples and never return,” Dr. Acosta-López said.

Even as a local investigator, Dr. Acosta-López has faced challenges getting a new study off the ground. Dr. Acosta-López and his colleagues are working under a grant from the Colombian government to recruit 241 presymptomatic subjects with confirmed genetic markers for HD, and evaluate them for cognitive and neurologic changes preceding disease onset.
It’s a cross-sectional study, and such studies are usually funded for a year. But the investigators knew it would take much more than a year to recruit patients here, and planned their study for 3 years. As of July, the team had been engaging with the community for 6 months but still didn’t have a single blood sample.
“We’ve had to convince everyone that this time is different,” he said, “and that means focusing on the social aspect” – setting up a legal-assistance program through the university to help families claim health benefits and a job-training program sponsored by local businesses.
It’s unusual for researchers to find themselves playing such extensive roles in coordinating social and economic support for their subjects. But with HD, it’s happening across Latin America, where researchers speak frequently of a “debt” owed to HD families in this region.
Huntington’s disease is a neurodegenerative disease caused by a genetic mutation in the huntingtin (HTT) gene, changing the normal protein it expresses in the body to a toxic form that damages cortical and basal ganglia neurons. It affects between 0.5 to 1 in 10,000 people worldwide, with higher prevalence in the United States, Europe, and Australia.
HD is inherited in an autosomal dominant pattern; a child of a parent with the mutation has a 50% chance of developing the disease. Patients develop cognitive symptoms that progress to dementia, along with the debilitating involuntary, dancelike movements that gave the disease the name by which it was formerly known: Huntington’s chorea.
In the 1980s and 1990s, several generations of Latin American HD families provided data that allowed for some of the greatest research advances in the disease – and they may represent a large share of the world’s HD cases. Yet, they continue to live in extreme poverty and have benefited little from the findings of the past 3 decades.
Without recognizing this and working to improve the families’ well-being, the researchers at the conference said it’s unlikely that promising therapies in the pipeline will ever reach the populations that need them the most.
Discovery in Venezuela
Some 8 hours by car from Barranquilla sits Lake Maracaibo, Venezuela, home to the largest known clusters of HD patients worldwide. The disease is believed to have come to the shores of Lake Maracaibo with a lone European immigrant – a Spanish sailor, many claim – at the end of the 18th century. Cases were first described in the 1950s by a young Venezuelan physician named Américo Negrette, MD.
Dr. Negrette’s findings were ignored by health officials in Venezuela and went unnoticed in the international research community until 1972, when a student of Dr. Negrette’s presented at an HD conference in Ohio. There he drew the attention of the American neuropsychologist Nancy S. Wexler, PhD. Dr. Wexler’s own mother had died of HD, and her father Milton, a noted psychoanalyst, founded the first research foundation dedicated to the disease.

While prevalence of HD in North America, Australia, and Europe is about 1 in 10,000, the region around Lake Maracaibo saw 70 times that rate at the time, thanks to high birth rates, geographic isolation, and extensive intermarriage within a handful of families. The families comprised mostly poor fishermen who lived in makeshift homes in towns ringing the lake.
In 1979, Dr. Wexler, with funding from the U.S. National Institutes of Health, began making annual research visits to Lake Maracaibo, and in 1983, the research group she coordinated, using data from blood and tissue samples donated by affected families, identified the location of the huntingtin gene on chromosome 4 (Nature. 1983 Nov 17;306[5940]:234-8). A decade later, the researchers isolated the mutant version of the gene and found it to be a triplet (CAG) expansion mutation, with more CAG repetitions associated with earlier age at disease onset (Cell. 1993 Mar 26;72[6]:971-83). Dr. Wexler and her colleagues’ findings led to the first genetic tests for HD.
Nationalist policies in Venezuela ended Dr. Wexler and her colleagues’ annual visits to Lake Maracaibo in 2002, along with the food, clothing, and medicines that the group routinely distributed to the families when they came.
Over 23 years, the researchers obtained data from some 18,000 individuals, but the families did not benefit in any durable way from the research. Local investigators with whom Dr. Wexler’s group collaborated lacked the resources and training to continue independently.
Access to medications is limited in Venezuela, and there is no institutional support for hundreds of HD patients living in extreme poverty, many of them descendants of the patients who contributed to the research and generation of these samples. The families’ biological material was sent to labs abroad, where investigators continue to derive findings from it today. Though genetic testing was performed on thousands at risk for the disease, few received access to their results through genetic counseling. A hospice established by Dr. Wexler’s foundation limped along until 2014, when it was finally shuttered.
Rebuilding bridges
A handful of families from the Lake Maracaibo towns attended the conference in Barranquilla. Their travel costs were picked up by Factor-H, which sponsored the event.
Ignacio Muñoz-Sanjuán, PhD, Factor-H’s founder and president, knew the families personally. He’s visited them regularly for years. In 2017, Dr. Muñoz-Sanjuán, a molecular biologist known affectionately in the HD community as “Nacho,” invited several to Rome for a meeting with Pope Francis, as part of an effort to raise awareness of HD and to request support from the Catholic Church for the Latin American families.
Humanitarian work is relatively new to Dr. Muñoz-Sanjuán, who’s spent his career in drug development. In addition to his unpaid work with Factor-H, he is vice president of biology with the CHDI Foundation, a Los Angeles–based nonprofit that funds drug research in HD. CHDI is reported to have about $100 million in annual funding – about triple the NIH budget in recent years for HD research. Its major donors are a group of investors who for years have remained anonymous and do not publicly discuss their philanthropy.
The Spanish-born Dr. Muñoz-Sanjuán had little direct experience with HD populations in Latin America until a few years ago, he said.
At a CHDI meeting in Brazil, he said, “I was talking with physicians and patient advocates from Latin America, telling them they had to be willing to be involved, that these communities with high prevalence had a lot to offer science,” Dr. Muñoz-Sanjuán said in an interview. “I was told that it was me who needed to understand the conditions in which HD patients lived. It completely put me on the spot.”
HD tends to strike during the most productive years of a person’s life, from the late 30s onward, keeping them from working and obliging family members to stop working to care for them. In a poor community, it can condemn a family to a state of extreme poverty for generations. Tetrabenazine (Xenazine), a medication to quiet chorea symptoms, is costly enough that many patients must do without it. Ensuring adequate calorie intake is difficult in HD patients, whose constant movements cause them to lose weight.
Dr. Muñoz-Sanjuán traveled to Colombia, Venezuela, and Brazil, meeting HD families and doctors like neurologist Gustavo Barrios, MD, of Hospital Occidente de Kennedy in Bogotá, Colombia. In a talk at the Barranquilla conference, Dr. Barrios related the experience of his first visit to El Dificil, a community in northern Colombia where some large HD families are forced to survive on the equivalent of $5 a day. “I had to confront not only the fact that these families were living with a terrible disease but in conditions of extreme deprivation,” he said. “My life as a doctor changed that day.”
Dr. Muñoz-Sanjuán helped form a Latin American HD network to involve clinicians like Dr. Barrios who worked with HD clusters, most of them poorly studied. “These are all neglected communities that share similar features,” Dr. Muñoz-Sanjuán said.
On Colombia’s Caribbean coast, for example, HD had been documented since the early 1990s, but genotyping was not performed until recently. Prevalence data are “virtually nonexistent” in Colombia, said Sonia Moreno, PhD, a neuropsychologist at the University of Antioquia in Medellin. In a pilot study presented this year at the CHDI Foundation’s Enroll-HD Congress, Dr. Moreno and her colleagues mined Colombian public health data for likely HD cases, and argued for the creation of a national registry.
In 2012, Dr. Muñoz-Sanjuán founded Factor-H with the aim of improving living conditions for Latin American communities with HD.

Factor-H does not receive funds from CHDI Foundation and instead relies on donations from individuals and companies; its annual budget is less than $200,000. But through contracts with local nongovernmental organizations, it has sponsored health clinics and ongoing food assistance, delivered shipments of medicines and clothing, and started a sponsorship program for young people in HD families, whose studies often are interrupted caring for sick parents. It hopes to build permanent support centers in Colombia and Venezuela where HD families can get their food and medical needs met.
“The traditional thinking in the HD research community is that we’re helping people by doing the legwork to make medicine – and that’s not necessarily enough. You need a more holistic approach,” Dr. Muñoz-Sanjuán said.
Lennie Pineda, MSc, who recently retired as a geneticist with the University of Zulia in Maracaibo, Venezuela, said that Dr. Muñoz-Sanjuán was viewed skeptically when he first visited, in part because of his biomedical research background.
Ms. Pineda, who worked with the region’s HD families her whole career, has been wary of past research efforts in Venezuela. In 2010, she published a paper critical of Dr. Wexler’s and his colleagues’ approach (Revista Redbioética/UNESCO. 2010;1[2]:50-61), particularly regarding issues of informed consent.
“I was very cold to Nacho,” she laughed. “We all looked at him suspiciously.”
Ms. Pineda said Dr. Muñoz-Sanjuán won her over with his interest and creativity in finding concrete ways improve the lives of families in the Lake Maracaibo towns.
In a talk at the conference, Edison Soto, a young man from San Luis, a town on Lake Maracaibo that is a key cluster of HD, said Dr. Muñoz-Sanjuán’s visits had reawakened hope among the families there. “For years, no one thought about us, and because of the situation in the country it’s been hard, really hard,” he said.
“Nacho’s smart,” Ms. Pineda said. “He’s not coming to build a research cohort, he’s coming with genuine intention to help. But if one day conditions are adequate to support investigation, and the people here are well informed and volunteer for a study with full consent, well, all the better,” she said.
Dr. Muñoz-Sanjuán acknowledged that his humanitarian work could be perceived as preparing the ground for future clinical trials.
“I’m not doing anything research oriented with Latin America,” he said. “I would never approach these communities and recommend they take part in a study or give samples, unless their conditions change significantly. But the idea of cross-contamination is a problem I might need to fix. There may come a day where I need to depersonalize Factor-H from me.”
A research platform, a novel agent
Though HD research in Latin America remains rife with challenges, a number of investigators at the conference talked optimistically about planned and ongoing HD studies in Latin America.
The biggest of these is ENROLL-HD, a long-term global observational study of families with HD that uses a standardized approach to data collection. The platform, launched in 2013, aims to enroll 20,000 participants for yearly (or more frequent) assessment. Data from ENROLL-HD will support a diverse range of studies on everything from biomarkers to genetic modifiers to quality of life measures in HD.
ENROLL-HD has opened study sites in Argentina, Chile, and Colombia, and plans to launch a site near Lima, Peru, that is home to an HD cluster. Venezuela is considered out of reach, at least for now.
In Barranquilla, Claudia Perandones, MD, PhD, a genetics researcher in Argentina who manages ENROLL-HD for Latin America and is a cofounder of Factor-H, explained why the kind of clusters seen in Latin America are so valuable scientifically.
The extended family groups share a disease haplotype, eat the same foods, and live in similar environments, Dr. Perandones noted. Because not all the variation in HD can be explained by the number of CAG repeats a patient has, having a large sample with a common haplotype would help researchers pinpoint other environmental and genetic factors that can modify the onset or progress of the disease.
Another key goal of ENROLL-HD, investigators say, is to speed recruitment into clinical trials as they arise. And for the first time in history, potentially game-changing therapies are being developed specifically for HD.
For the past 5 years the Swiss pharmaceutical giant Roche has worked with a smaller biotech firm, Ionis Pharmaceuticals, on an agent called RG6042, which was known until recently as IONIS-HTTRx. CHDI was extensively involved in the agent’s preclinical development, contributing some $10 million to get it off the ground.
RG6042 is an antisense oligonucleotide, delivered by spinal injection, which works by interrupting an mRNA signaling pathway to suppress production of mutant HTT (mHTT) protein in the brain. Antisense oligonucleotides, sometimes called gene silencing therapies, are a new and promising approach in neurodegenerative diseases. Two have received FDA approval to treat spinal muscular atrophy and Duchenne muscular dystrophy.
In April 2018, Roche announced positive results from phase 1/2a study in 46 HD patients in Europe and North America. Patients in that 13-week study saw significant (up to 60%) dose-dependent reductions of the mHTT in their cerebrospinal fluid; a post hoc analysis also found some evidence of functional improvement (Neurology. 2018;90[15 Supplement]:CT.002).
These encouraging findings led to Roche’s announcement of a global phase 3 randomized, controlled trial that is scheduled to begin enrolling in 2019. Roche hopes to randomize 660 patients with mild HD across 15 countries for the 2-year trial, called GENERATION-HD1.
Sites in Latin America are expected to include Argentina, Chile, and Colombia.
At the Barranquilla meeting, Daniel Ciriano, MD, Roche’s Argentina-based medical director for Latin America, extolled the company’s commitment to ethics and social welfare in the region. In recent years, Roche has increased its humanitarian commitments across Latin America, including helping rebuild a Chilean village after an earthquake and offering free breast cancer and kidney disease treatments.
RG6042 is only one of a number of promising approaches to HD. Other therapies in the pipeline include gene silencing delivered by viral vectors instead of repeated spinal injections, an oral drug that interrupts mHTT production, immunotherapies, and even CRISPR gene–editing techniques.
Little was said at the conference, however, about how Latin American HD communities might be able to afford RG6042 or any other therapy that emerges from the pipeline.
Dr. Muñoz-Sanjuán called the issue “a theme for future discussion.”
“This is an area that has to be handled carefully and not one we are heavily invested in yet, although it’s very important,” he said.
On the ground
Several of the European and North American scientists who presented in Barranquilla took pains to express their concern with the well-being of HD patients in Latin America and to demonstrate goodwill toward the local researchers and clinicians.
Hilal A. Lashuel, PhD, a molecular biologist working on the structure and behavior of the HTT protein, said his participation in the Factor-H event at the Vatican the year before had awakened him to “the real human part of HD,” and changed the way he does science.
Normally, Dr. Lashuel said, “we do research disconnected from the realities of the diseases we work with.”
“We need to not just to do research but [to ensure] that research is done right,” he said, which means also focusing on improving patients’ standard of living.
The room broke out in applause when Dr. Lashuel announced new internships for investigators from developing countries. He also presented a parting video from his research team at the École Polytechnique Fédérale de Lausanne (Switzerland), complete with music and affectionate messages in Spanish.
Pharmacologist Elena Cattaneo, PhD, a stem cell researcher long active in the HD community, and also a senator in Italy’s parliament, delivered a similarly warm, carefully choreographed video message from her laboratory at the University of Milan.
Just days later in the town of Juan de Acosta, an hour inland of Barranquilla, the same researchers sat down with patients and families who crowded the waiting room of the town’s only hospital, as the sun beat in through the windows and as mule carts, stray dogs, and buses passed by on the main drag outside.
The event had been titled a “brigade” after all, but the HD families did not seem to mind – and indeed so many showed up that a sign had to be placed on the door saying that no one who arrived after noon could be seen. Consults were not limited to HD-related matters, so families could be seen for any complaint.
HD was first documented in this town in the early 1990s, but much remains to be understood about the size of the cluster, the haplotype, and its relation to other clusters in Colombia or Venezuela. The families here share a handful of last names and likely share a common ancestor. In the early 19th century, the Barranquilla region was flooded with European migrants who reached the city by ship. (HD clusters in Latin America tend to be concentrated in coastal regions, possibly because of migration patterns.)
The waiting room of the hospital was loud with chatter. Small children played as their relatives waited for consults. Some showed the characteristic restless movements and emaciated bodies of people with advanced HD.
The foreign scientists were barred from taking any patient data out of the hospital or asking for samples. Even picture taking was prohibited. Instead they performed genetic counseling and neuropsychological tests; they sorted out differential diagnoses and advised on medications. Visiting Colombian and Venezuelan physicians did the same, while their assistants met with families in the waiting room, taking medical histories and sketching out basic genealogies.
Some of the foreign researchers reported fruitful interactions with patients, while others seemed perplexed by what they’d experienced. Alba di Pardo, PhD, a genetic epidemiologist at the Istituto Neurologico Mediterraneo Pozzilli (Italy), said she’d spent the morning doing genetic counseling with families and going over genealogies to assess risk. Yet, despite the fact that anyone with an HD parent has a 50% chance of developing the disease, some family members acted uninterested, she said.
Dr. di Pardo’s colleague at the Istituto, biologist Vittorio Maglione, PhD, reported having a similar experience. As he was counseling a young woman about her risk for HD, she scrolled indifferently through Facebook posts on her phone, he said.
On some level, Dr. Maglione said, he could understand patients’ reluctance to engage noting that, while there were many potential HD therapies to try, any new treatment paradigm for HD was many years away from a place like this – and potentially very costly. Dr. Maglione – along with Dr. di Pardo – is researching the SP1 axis, a sphingolipid pathway implicated in neurodegenerative such diseases as HD and which has potential as a drug target (Trends Pharmacol Sci. 2018;39[5]:468-80).
Psychologist Pedro Puentes Rozo of Simón Bolivar University, who is working with Dr. Acosta-López on the local cohort study of presymptomatic HD patients, said that, for most of the families in the clinic that day, any seeming indifference probably masked deeper fears. People already were well aware of their risk. “They’ve known about it forever, said Dr. Puentes Rozo, who has been working with this HD population for a decade. “But this is a catastrophic illness and can generate a lot of anxiety.”
Dr. Puentes Rozo said the group’s planned study, unlike studies in the past, would be conducted under strict “international ethical norms and standards.” Subjects would receive ongoing psychological support, and the researchers were working to establish a genetic counseling center so that people who want to know their status “can be prepared,” he said, and plan for their lives and families.
By fall 2018, the cohort study was underway. The group had sponsored several more hospital brigades – or “integrated health days” as they preferred to call them, at the hospital in Juan de Acosta, giving them a chance to work face to face with families.
They drew no blood during the clinics, as investigators in the past had done. Instead, they explained the study to patients, performed the initial screenings, and invited them to designated study appointments at the university. Legal assistance was up and running, and the jobs program would start in 2019.
Enrollment was climbing. And the group was steadily accumulating data.
New PCNSL guidelines emphasize importance of patient fitness
New guidelines on the diagnosis and management of patients with primary central nervous system lymphoma (PCNSL) emphasize prompt diagnosis, aggressive treatment whenever possible, and multidisciplinary team support.
A unique aspect for hematologic cancers, the guidelines note, is that appropriate treatment for PCNSL requires input from neurology specialists.
And the guidelines recommend methotrexate-based treatment only be administered at centers experienced in delivering intensive chemotherapy.
Christopher P. Fox, MD, of the Nottingham University Hospitals NHS Trust in Nottingham, U.K., and his colleagues on behalf of the British Society for Haematology published the guidelines in BJH.
The authors incorporated findings from studies published since the society’s last comprehensive PCNSL guidelines were issued more than a decade ago.
The new guidelines provide recommendations for diagnosis and imaging, primary treatment of PCNSL, consolidation chemotherapy, follow-up, management of relapsed/refractory disease, and neuropsychological assessments.
Highlights include:
- People with suspected PCNSL must receive quick and coordinated attention from a multidisciplinary team of neurologists, hematologist-oncologists, and ocular specialists
- Histological diagnoses in addition to imaging findings should be performed
- Corticosteroids should be avoided or discontinued before biopsy, as even a short course of steroids can impede diagnosis
- Aggressive induction treatment should be chosen based on the patient’s fitness
- Patients should be offered entry into clinical trials whenever possible
- Universal screening for eye involvement should be conducted.
Primary treatment
Dr. Fox and his colleagues say definitive treatment for PCNSL—induction of remission followed by consolidation—should start within 2 weeks of diagnosis, and a treatment regimen should be chosen according to a patient’s physiological fitness, not age.
The fittest patients, who have better organ function and fewer comorbidities, should be eligible for intensive combination immuno-chemotherapy incorporating high-dose methotrexate (HD-MTX)—optimally, four cycles of HD-MTX, cytarabine, thiotepa, and rituximab.
Those deemed unfit for this regimen should be offered induction treatment with HD-MTX, rituximab, and procarbazine, the guidelines say.
If patients cannot tolerate HD-MTX, oral chemotherapy, whole-brain radiotherapy (WBRT), or corticosteroids may be used.
The authors do not recommend intrathecal chemotherapy alongside systemic CNS-directed therapy.
Response should be assessed with contrast-enhanced magnetic resonance imaging (MRI) routinely after every two cycles of HD-MTX-based therapy and at the end of remission induction.
Consolidation chemotherapy
Consolidation therapy should be initiated after induction for all patients with non-progressive disease. High-dose thiotepa-based chemotherapy with autologous stem cell transplant (ASCT) is the recommended first-line option for consolidation.
Patients ineligible for high-dose therapy followed by ASCT who have residual disease after induction therapy should be considered for WBRT. This is also the case for patients with residual disease after thiotepa-based ASCT.
However, Dr. Fox and his colleagues say WBRT consolidation is “contentious” for patients in complete response after HD-MTX regimens but ineligible for ASCT. The authors suggest carefully balancing potential improvement in progression-free survival against risks of neurocognitive toxicity.
Response to consolidation, again measured with contrast-enhanced MRI, should be carried out between 1 and 2 months after therapy is completed, and patients should be referred for neuropsychological testing to assess cognitive function.
Patients with relapsed or refractory disease should be approached with maximum urgency—the guidelines offer an algorithm for retreatment options—and offered clinical trial entry wherever possible.
Some coauthors, including the lead author, disclosed receiving fees from pharmaceutical manufacturers Adienne and/or F. Hoffman-La Roche.
New guidelines on the diagnosis and management of patients with primary central nervous system lymphoma (PCNSL) emphasize prompt diagnosis, aggressive treatment whenever possible, and multidisciplinary team support.
A unique aspect for hematologic cancers, the guidelines note, is that appropriate treatment for PCNSL requires input from neurology specialists.
And the guidelines recommend methotrexate-based treatment only be administered at centers experienced in delivering intensive chemotherapy.
Christopher P. Fox, MD, of the Nottingham University Hospitals NHS Trust in Nottingham, U.K., and his colleagues on behalf of the British Society for Haematology published the guidelines in BJH.
The authors incorporated findings from studies published since the society’s last comprehensive PCNSL guidelines were issued more than a decade ago.
The new guidelines provide recommendations for diagnosis and imaging, primary treatment of PCNSL, consolidation chemotherapy, follow-up, management of relapsed/refractory disease, and neuropsychological assessments.
Highlights include:
- People with suspected PCNSL must receive quick and coordinated attention from a multidisciplinary team of neurologists, hematologist-oncologists, and ocular specialists
- Histological diagnoses in addition to imaging findings should be performed
- Corticosteroids should be avoided or discontinued before biopsy, as even a short course of steroids can impede diagnosis
- Aggressive induction treatment should be chosen based on the patient’s fitness
- Patients should be offered entry into clinical trials whenever possible
- Universal screening for eye involvement should be conducted.
Primary treatment
Dr. Fox and his colleagues say definitive treatment for PCNSL—induction of remission followed by consolidation—should start within 2 weeks of diagnosis, and a treatment regimen should be chosen according to a patient’s physiological fitness, not age.
The fittest patients, who have better organ function and fewer comorbidities, should be eligible for intensive combination immuno-chemotherapy incorporating high-dose methotrexate (HD-MTX)—optimally, four cycles of HD-MTX, cytarabine, thiotepa, and rituximab.
Those deemed unfit for this regimen should be offered induction treatment with HD-MTX, rituximab, and procarbazine, the guidelines say.
If patients cannot tolerate HD-MTX, oral chemotherapy, whole-brain radiotherapy (WBRT), or corticosteroids may be used.
The authors do not recommend intrathecal chemotherapy alongside systemic CNS-directed therapy.
Response should be assessed with contrast-enhanced magnetic resonance imaging (MRI) routinely after every two cycles of HD-MTX-based therapy and at the end of remission induction.
Consolidation chemotherapy
Consolidation therapy should be initiated after induction for all patients with non-progressive disease. High-dose thiotepa-based chemotherapy with autologous stem cell transplant (ASCT) is the recommended first-line option for consolidation.
Patients ineligible for high-dose therapy followed by ASCT who have residual disease after induction therapy should be considered for WBRT. This is also the case for patients with residual disease after thiotepa-based ASCT.
However, Dr. Fox and his colleagues say WBRT consolidation is “contentious” for patients in complete response after HD-MTX regimens but ineligible for ASCT. The authors suggest carefully balancing potential improvement in progression-free survival against risks of neurocognitive toxicity.
Response to consolidation, again measured with contrast-enhanced MRI, should be carried out between 1 and 2 months after therapy is completed, and patients should be referred for neuropsychological testing to assess cognitive function.
Patients with relapsed or refractory disease should be approached with maximum urgency—the guidelines offer an algorithm for retreatment options—and offered clinical trial entry wherever possible.
Some coauthors, including the lead author, disclosed receiving fees from pharmaceutical manufacturers Adienne and/or F. Hoffman-La Roche.
New guidelines on the diagnosis and management of patients with primary central nervous system lymphoma (PCNSL) emphasize prompt diagnosis, aggressive treatment whenever possible, and multidisciplinary team support.
A unique aspect for hematologic cancers, the guidelines note, is that appropriate treatment for PCNSL requires input from neurology specialists.
And the guidelines recommend methotrexate-based treatment only be administered at centers experienced in delivering intensive chemotherapy.
Christopher P. Fox, MD, of the Nottingham University Hospitals NHS Trust in Nottingham, U.K., and his colleagues on behalf of the British Society for Haematology published the guidelines in BJH.
The authors incorporated findings from studies published since the society’s last comprehensive PCNSL guidelines were issued more than a decade ago.
The new guidelines provide recommendations for diagnosis and imaging, primary treatment of PCNSL, consolidation chemotherapy, follow-up, management of relapsed/refractory disease, and neuropsychological assessments.
Highlights include:
- People with suspected PCNSL must receive quick and coordinated attention from a multidisciplinary team of neurologists, hematologist-oncologists, and ocular specialists
- Histological diagnoses in addition to imaging findings should be performed
- Corticosteroids should be avoided or discontinued before biopsy, as even a short course of steroids can impede diagnosis
- Aggressive induction treatment should be chosen based on the patient’s fitness
- Patients should be offered entry into clinical trials whenever possible
- Universal screening for eye involvement should be conducted.
Primary treatment
Dr. Fox and his colleagues say definitive treatment for PCNSL—induction of remission followed by consolidation—should start within 2 weeks of diagnosis, and a treatment regimen should be chosen according to a patient’s physiological fitness, not age.
The fittest patients, who have better organ function and fewer comorbidities, should be eligible for intensive combination immuno-chemotherapy incorporating high-dose methotrexate (HD-MTX)—optimally, four cycles of HD-MTX, cytarabine, thiotepa, and rituximab.
Those deemed unfit for this regimen should be offered induction treatment with HD-MTX, rituximab, and procarbazine, the guidelines say.
If patients cannot tolerate HD-MTX, oral chemotherapy, whole-brain radiotherapy (WBRT), or corticosteroids may be used.
The authors do not recommend intrathecal chemotherapy alongside systemic CNS-directed therapy.
Response should be assessed with contrast-enhanced magnetic resonance imaging (MRI) routinely after every two cycles of HD-MTX-based therapy and at the end of remission induction.
Consolidation chemotherapy
Consolidation therapy should be initiated after induction for all patients with non-progressive disease. High-dose thiotepa-based chemotherapy with autologous stem cell transplant (ASCT) is the recommended first-line option for consolidation.
Patients ineligible for high-dose therapy followed by ASCT who have residual disease after induction therapy should be considered for WBRT. This is also the case for patients with residual disease after thiotepa-based ASCT.
However, Dr. Fox and his colleagues say WBRT consolidation is “contentious” for patients in complete response after HD-MTX regimens but ineligible for ASCT. The authors suggest carefully balancing potential improvement in progression-free survival against risks of neurocognitive toxicity.
Response to consolidation, again measured with contrast-enhanced MRI, should be carried out between 1 and 2 months after therapy is completed, and patients should be referred for neuropsychological testing to assess cognitive function.
Patients with relapsed or refractory disease should be approached with maximum urgency—the guidelines offer an algorithm for retreatment options—and offered clinical trial entry wherever possible.
Some coauthors, including the lead author, disclosed receiving fees from pharmaceutical manufacturers Adienne and/or F. Hoffman-La Roche.
CNS lymphoma guidelines stress patient fitness, not age, in choosing treatment
for the diagnosis and management of primary central nervous system diffuse large B‐cell lymphoma.
PCNSL, implicated in some 3% of all brain tumors, is complex to diagnose and treat. People with suspected PCNSL must receive quick and coordinated attention from a multidisciplinary team of neurologists, hematologist-oncologists, and ocular specialists, according to the guidelines, published in the British Journal of Haematology.
Christopher P. Fox, MD, of the Nottingham (England) University Hospitals NHS Trust, and his colleagues, stress the importance of early multidisciplinary attention, aggressive induction treatment, helping patients into trials, universal screening for eye involvement, attaining histological diagnoses in addition to imaging findings, and avoidance or discontinuation of any corticosteroids before biopsy, as even a short course of steroids can impede diagnosis.
The guidelines incorporate findings from studies published since the society’s last comprehensive PCNSL guideline was issued more than a decade ago.
Dr. Fox and his colleagues say definitive treatment for PCNSL – induction of remission followed by consolidation – should start within 2 weeks of diagnosis and that a treatment regimen should be chosen according to a patient’s physiological fitness, not age. The fittest patients, who have better organ function and fewer comorbidities, should be eligible for intensive combination immunochemotherapy incorporating high-dose methotrexate (optimally four cycles of HD-MTX, cytarabine, thiotepa, and rituximab). Those deemed unfit for this regimen should be offered induction treatment with HD-MTX, rituximab and procarbazine, the guidelines’ authors say.
If patients cannot tolerate HD-MTX, oral chemotherapy and/or whole-brain radiotherapy may be offered. Response should be assessed with contrast-enhanced magnetic resonance imaging.
Consolidation therapy should be initiated after induction for all patients with nonprogressive disease, and high-dose thiotepa-based chemotherapy with autologous stem cell transplant is the recommended first-line option for consolidation. Response to consolidation, again measured with contrast-enhanced MRI, should be carried out at between 1 and 2 months after therapy is completed, and patients should be referred for neuropsychological testing to assess cognitive function.
Patients with relapsed or refractory disease should be approached with maximum urgency – the guidelines offer an algorithm for retreatment options – and offered clinical trial entry wherever possible.
The PCNSL guideline writing process was sponsored by the British Society for Haematology, and some coauthors, including the lead author, disclosed receiving fees from pharmaceutical manufacturers Adienne or F. Hoffman-La Roche.
SOURCE: Fox et al. Br J Haematol. 2018 Nov 23 doi: 10.1111/bjh.15661.
for the diagnosis and management of primary central nervous system diffuse large B‐cell lymphoma.
PCNSL, implicated in some 3% of all brain tumors, is complex to diagnose and treat. People with suspected PCNSL must receive quick and coordinated attention from a multidisciplinary team of neurologists, hematologist-oncologists, and ocular specialists, according to the guidelines, published in the British Journal of Haematology.
Christopher P. Fox, MD, of the Nottingham (England) University Hospitals NHS Trust, and his colleagues, stress the importance of early multidisciplinary attention, aggressive induction treatment, helping patients into trials, universal screening for eye involvement, attaining histological diagnoses in addition to imaging findings, and avoidance or discontinuation of any corticosteroids before biopsy, as even a short course of steroids can impede diagnosis.
The guidelines incorporate findings from studies published since the society’s last comprehensive PCNSL guideline was issued more than a decade ago.
Dr. Fox and his colleagues say definitive treatment for PCNSL – induction of remission followed by consolidation – should start within 2 weeks of diagnosis and that a treatment regimen should be chosen according to a patient’s physiological fitness, not age. The fittest patients, who have better organ function and fewer comorbidities, should be eligible for intensive combination immunochemotherapy incorporating high-dose methotrexate (optimally four cycles of HD-MTX, cytarabine, thiotepa, and rituximab). Those deemed unfit for this regimen should be offered induction treatment with HD-MTX, rituximab and procarbazine, the guidelines’ authors say.
If patients cannot tolerate HD-MTX, oral chemotherapy and/or whole-brain radiotherapy may be offered. Response should be assessed with contrast-enhanced magnetic resonance imaging.
Consolidation therapy should be initiated after induction for all patients with nonprogressive disease, and high-dose thiotepa-based chemotherapy with autologous stem cell transplant is the recommended first-line option for consolidation. Response to consolidation, again measured with contrast-enhanced MRI, should be carried out at between 1 and 2 months after therapy is completed, and patients should be referred for neuropsychological testing to assess cognitive function.
Patients with relapsed or refractory disease should be approached with maximum urgency – the guidelines offer an algorithm for retreatment options – and offered clinical trial entry wherever possible.
The PCNSL guideline writing process was sponsored by the British Society for Haematology, and some coauthors, including the lead author, disclosed receiving fees from pharmaceutical manufacturers Adienne or F. Hoffman-La Roche.
SOURCE: Fox et al. Br J Haematol. 2018 Nov 23 doi: 10.1111/bjh.15661.
for the diagnosis and management of primary central nervous system diffuse large B‐cell lymphoma.
PCNSL, implicated in some 3% of all brain tumors, is complex to diagnose and treat. People with suspected PCNSL must receive quick and coordinated attention from a multidisciplinary team of neurologists, hematologist-oncologists, and ocular specialists, according to the guidelines, published in the British Journal of Haematology.
Christopher P. Fox, MD, of the Nottingham (England) University Hospitals NHS Trust, and his colleagues, stress the importance of early multidisciplinary attention, aggressive induction treatment, helping patients into trials, universal screening for eye involvement, attaining histological diagnoses in addition to imaging findings, and avoidance or discontinuation of any corticosteroids before biopsy, as even a short course of steroids can impede diagnosis.
The guidelines incorporate findings from studies published since the society’s last comprehensive PCNSL guideline was issued more than a decade ago.
Dr. Fox and his colleagues say definitive treatment for PCNSL – induction of remission followed by consolidation – should start within 2 weeks of diagnosis and that a treatment regimen should be chosen according to a patient’s physiological fitness, not age. The fittest patients, who have better organ function and fewer comorbidities, should be eligible for intensive combination immunochemotherapy incorporating high-dose methotrexate (optimally four cycles of HD-MTX, cytarabine, thiotepa, and rituximab). Those deemed unfit for this regimen should be offered induction treatment with HD-MTX, rituximab and procarbazine, the guidelines’ authors say.
If patients cannot tolerate HD-MTX, oral chemotherapy and/or whole-brain radiotherapy may be offered. Response should be assessed with contrast-enhanced magnetic resonance imaging.
Consolidation therapy should be initiated after induction for all patients with nonprogressive disease, and high-dose thiotepa-based chemotherapy with autologous stem cell transplant is the recommended first-line option for consolidation. Response to consolidation, again measured with contrast-enhanced MRI, should be carried out at between 1 and 2 months after therapy is completed, and patients should be referred for neuropsychological testing to assess cognitive function.
Patients with relapsed or refractory disease should be approached with maximum urgency – the guidelines offer an algorithm for retreatment options – and offered clinical trial entry wherever possible.
The PCNSL guideline writing process was sponsored by the British Society for Haematology, and some coauthors, including the lead author, disclosed receiving fees from pharmaceutical manufacturers Adienne or F. Hoffman-La Roche.
SOURCE: Fox et al. Br J Haematol. 2018 Nov 23 doi: 10.1111/bjh.15661.
FROM THE BRITISH JOURNAL OF HAEMATOLOGY
RFM awareness program not effective at preventing stillbirths
Because reduced fetal movement is associated with higher stillbirth risk, asking women to be alert to RFM and report it immediately has emerged as a potential intervention to prevent stillbirth. But a large, randomized trial of one reporting and management protocol showed no reduction in stillbirths, only a rise in C-sections and preterm inductions.
Jane E. Norman, MD, of the University of Edinburgh, and her colleagues published results from a trial in the Lancet, in which 409,175 pregnant women (mean age, 30 years) across 33 hospitals in the United Kingdom and Ireland received either standard care or the experimental RFM care intervention before delivery. Women were seen during an experimental 3-month period, in which all were treated according to the protocol, or the 3-month control period that preceded it. A 2-month washout period occurred between allocations as institutions adapted to the study protocol.
The trial intervention consisted of training clinical staff on the implications and management of RFM, distributing written information on RFM to women at about 20 weeks’ gestation, and a management protocol aimed at quick action following a report of RFM at 24 or more weeks’ gestation. The protocol included cardiotocography within 2 hours of presentation followed by measurement of liquor volume and a growth scan, along with umbilical artery Doppler where available. Delivery was recommended for women at 37 or more weeks’ gestation with estimated fetal weight below the 10th centile, abdominal circumference below the 10th centile, a low liquor volume, an abnormal cardiotocograph, or recurrent RFM.
Incidence of stillbirth at or beyond 24 weeks was 4.40 per 1,000 births during the control period and 4.06 per 1,000 births in the intervention period (adjusted odds ratio, 0.90; 95% confidence interval, 0.75-1.07; P = .23), the researchers found. No differences were seen when stratifying for different gestational ages.
Meanwhile, induction of labor before 39 weeks was more frequent during intervention period at 40% of deliveries, compared with 34% during the control time (P less than .0001), and at term (41% vs. 36%; P = .0015). C-section was higher in the intervention group at 28% versus 26% (P = .0001). Neonatal ICU stays were not more common but were likely to be longer in the intervention period, with stays of 2 days or longer occurring in 6.7% of deliveries versus 6.2% (P =.0001).
The investigators concluded that their protocol, in its current design, was not effective and could not be recommended because of the significant increase in interventions.
Dr. Norman and her colleagues wrote that the findings would “reignite the controversy about the efficacy of RFM awareness to reduce stillbirth and the underlying mechanisms linking RFM and stillbirth.” However, the results do not mean that RFM is a sign of inevitable fetal death or that there is no role for RFM awareness as a stillbirth-prevention strategy. Other large trials testing RFM-based interventions are still underway, they noted.
“Further research to identify better predictive tests for stillbirth [to enable targeting of the only current treatment of earlier delivery] is urgently needed,” the investigators added.
In a related study also published in the Lancet, Lucy K. Smith, PhD, of the University of Leicester (England), and her colleagues reported that the real burden of stillbirth in Europe, while much lower than in the developing world, is still a third higher than reported using the current international cutoff established by the World Health Organization.
Dr. Smith and her colleagues examined national cohort data from 19 European countries between 2004 and 2015 for pregnancy outcomes from 22 completed weeks’ gestation. In 2015, they found more than 9,000 stillbirths occurred among more than 25 million births, and 3,022 of these (32%) occurred between 22 and 28 weeks’ gestation.
The WHO officially defines stillbirth as any baby born without life at 28 weeks or beyond, although it recommends that countries collect data on fetal death from 22 weeks. However, discrepancies between and even within countries in reporting laws and their implementation “inhibit reliable international comparisons” at those earlier gestational ages, Dr. Smith and her colleagues wrote.
The researchers, pooling data from the 19 countries, found that the stillbirth rate at 24-28 weeks’ gestation declined from 0.97 per 1,000 births (95% CI, 0.80-1.14) to 0.70 per 1,000 births (95% CI, 0.57-0.82) between 2004 and 2015, a reduction of 25% (risk ratio; 0.75; 95% CI, 0.65-0.85).
“The decrease of 25% in stillbirths at 24 weeks to less than 28 weeks is very similar to that seen globally for stillbirths of 28 weeks of gestation [25.5% worldwide and 24.5% in developed regions] and above for a similar time period of 2000-2015, suggesting consistent improvements over time in the reduction of stillbirths from 24 completed weeks of gestation,” the researchers wrote in their analysis.
Data from France, Spain, and Cyprus was not included in the analysis as these countries did not collect fetal death reports for the gestational periods in the study. Also, for a few countries in the study, late terminations of pregnancy could not be distinguished from spontaneous fetal death.
“The consistency in reporting of births over time at 24 weeks to less than 28 weeks of gestation and the similarity of reduction in the rate of stillbirth over time to births at 28 completed weeks of gestation and above suggest that stillbirths at 24 weeks to less than 28 weeks of gestation can be routinely included in rates of stillbirth for international comparisons from now on,” at least in high-income countries, the investigators wrote.
The study by Norman et al. was funded by the Scottish government, Tommy’s Health Center, and Sands, a U.K. stillbirth charity. The article presents research funded in part by the National Institute for Health Research. Several investigators, including the lead author, reported financial support from these entities. One author reported salary from National Health Service Lothian. All other authors reported no relevant financial disclosures. The study by Smith et al. was funded by the European Union and National Institute for Health Research. Dr. Smith received funding from a National Institute for Health Research Career Development Fellowship. All other authors reported no financial conflicts of interest.
SOURCES: Norman JE et al. Lancet. 2018;392:1629-38; Smith LK et al. Lancet. 2018;392:1639-46.
The well-conducted, randomized trial by Norman et al. enrolled over 400,000 women at 33 trials to see if reduced fetal movement reporting would significantly reduce stillbirths. It did not, but it did increase C-sections, Kate F. Walker, PhD, and Jim G. Thornton wrote in an invited commentary.
“Repeated episodes of reduced fetal movement can be so stressful to the mother that some doctors are persuaded to induce, even if further tests are normal. There also are anecdotes of women feigning reduced fetal movements to attain an ultrasound scan or induction of labor. The prevalence of women falsifying RFM is important because, although induction of birth at full term is unlikely to seriously harm the mother or the baby, preterm induction has risks,” they wrote.
“Failure of health care providers to respond to reported changes to fetal movement is probably impossible. However, discouraging campaigns that promote awareness preterm, improving induction guidelines, and not inducing delivery in response to perception of altered movement alone would seem to be sensible first steps,” Dr. Walker and Mr. Thornton concluded.
Dr. Walker and Mr. Thornton are with the division of child health, obstetrics, and gynecology at the University of Nottingham (England). They reported no financial interests related to their commentary (Lancet. 2018 Nov 3. doi: 10.1016/S0140-6736[18]31720-3).
The well-conducted, randomized trial by Norman et al. enrolled over 400,000 women at 33 trials to see if reduced fetal movement reporting would significantly reduce stillbirths. It did not, but it did increase C-sections, Kate F. Walker, PhD, and Jim G. Thornton wrote in an invited commentary.
“Repeated episodes of reduced fetal movement can be so stressful to the mother that some doctors are persuaded to induce, even if further tests are normal. There also are anecdotes of women feigning reduced fetal movements to attain an ultrasound scan or induction of labor. The prevalence of women falsifying RFM is important because, although induction of birth at full term is unlikely to seriously harm the mother or the baby, preterm induction has risks,” they wrote.
“Failure of health care providers to respond to reported changes to fetal movement is probably impossible. However, discouraging campaigns that promote awareness preterm, improving induction guidelines, and not inducing delivery in response to perception of altered movement alone would seem to be sensible first steps,” Dr. Walker and Mr. Thornton concluded.
Dr. Walker and Mr. Thornton are with the division of child health, obstetrics, and gynecology at the University of Nottingham (England). They reported no financial interests related to their commentary (Lancet. 2018 Nov 3. doi: 10.1016/S0140-6736[18]31720-3).
The well-conducted, randomized trial by Norman et al. enrolled over 400,000 women at 33 trials to see if reduced fetal movement reporting would significantly reduce stillbirths. It did not, but it did increase C-sections, Kate F. Walker, PhD, and Jim G. Thornton wrote in an invited commentary.
“Repeated episodes of reduced fetal movement can be so stressful to the mother that some doctors are persuaded to induce, even if further tests are normal. There also are anecdotes of women feigning reduced fetal movements to attain an ultrasound scan or induction of labor. The prevalence of women falsifying RFM is important because, although induction of birth at full term is unlikely to seriously harm the mother or the baby, preterm induction has risks,” they wrote.
“Failure of health care providers to respond to reported changes to fetal movement is probably impossible. However, discouraging campaigns that promote awareness preterm, improving induction guidelines, and not inducing delivery in response to perception of altered movement alone would seem to be sensible first steps,” Dr. Walker and Mr. Thornton concluded.
Dr. Walker and Mr. Thornton are with the division of child health, obstetrics, and gynecology at the University of Nottingham (England). They reported no financial interests related to their commentary (Lancet. 2018 Nov 3. doi: 10.1016/S0140-6736[18]31720-3).
Because reduced fetal movement is associated with higher stillbirth risk, asking women to be alert to RFM and report it immediately has emerged as a potential intervention to prevent stillbirth. But a large, randomized trial of one reporting and management protocol showed no reduction in stillbirths, only a rise in C-sections and preterm inductions.
Jane E. Norman, MD, of the University of Edinburgh, and her colleagues published results from a trial in the Lancet, in which 409,175 pregnant women (mean age, 30 years) across 33 hospitals in the United Kingdom and Ireland received either standard care or the experimental RFM care intervention before delivery. Women were seen during an experimental 3-month period, in which all were treated according to the protocol, or the 3-month control period that preceded it. A 2-month washout period occurred between allocations as institutions adapted to the study protocol.
The trial intervention consisted of training clinical staff on the implications and management of RFM, distributing written information on RFM to women at about 20 weeks’ gestation, and a management protocol aimed at quick action following a report of RFM at 24 or more weeks’ gestation. The protocol included cardiotocography within 2 hours of presentation followed by measurement of liquor volume and a growth scan, along with umbilical artery Doppler where available. Delivery was recommended for women at 37 or more weeks’ gestation with estimated fetal weight below the 10th centile, abdominal circumference below the 10th centile, a low liquor volume, an abnormal cardiotocograph, or recurrent RFM.
Incidence of stillbirth at or beyond 24 weeks was 4.40 per 1,000 births during the control period and 4.06 per 1,000 births in the intervention period (adjusted odds ratio, 0.90; 95% confidence interval, 0.75-1.07; P = .23), the researchers found. No differences were seen when stratifying for different gestational ages.
Meanwhile, induction of labor before 39 weeks was more frequent during intervention period at 40% of deliveries, compared with 34% during the control time (P less than .0001), and at term (41% vs. 36%; P = .0015). C-section was higher in the intervention group at 28% versus 26% (P = .0001). Neonatal ICU stays were not more common but were likely to be longer in the intervention period, with stays of 2 days or longer occurring in 6.7% of deliveries versus 6.2% (P =.0001).
The investigators concluded that their protocol, in its current design, was not effective and could not be recommended because of the significant increase in interventions.
Dr. Norman and her colleagues wrote that the findings would “reignite the controversy about the efficacy of RFM awareness to reduce stillbirth and the underlying mechanisms linking RFM and stillbirth.” However, the results do not mean that RFM is a sign of inevitable fetal death or that there is no role for RFM awareness as a stillbirth-prevention strategy. Other large trials testing RFM-based interventions are still underway, they noted.
“Further research to identify better predictive tests for stillbirth [to enable targeting of the only current treatment of earlier delivery] is urgently needed,” the investigators added.
In a related study also published in the Lancet, Lucy K. Smith, PhD, of the University of Leicester (England), and her colleagues reported that the real burden of stillbirth in Europe, while much lower than in the developing world, is still a third higher than reported using the current international cutoff established by the World Health Organization.
Dr. Smith and her colleagues examined national cohort data from 19 European countries between 2004 and 2015 for pregnancy outcomes from 22 completed weeks’ gestation. In 2015, they found more than 9,000 stillbirths occurred among more than 25 million births, and 3,022 of these (32%) occurred between 22 and 28 weeks’ gestation.
The WHO officially defines stillbirth as any baby born without life at 28 weeks or beyond, although it recommends that countries collect data on fetal death from 22 weeks. However, discrepancies between and even within countries in reporting laws and their implementation “inhibit reliable international comparisons” at those earlier gestational ages, Dr. Smith and her colleagues wrote.
The researchers, pooling data from the 19 countries, found that the stillbirth rate at 24-28 weeks’ gestation declined from 0.97 per 1,000 births (95% CI, 0.80-1.14) to 0.70 per 1,000 births (95% CI, 0.57-0.82) between 2004 and 2015, a reduction of 25% (risk ratio; 0.75; 95% CI, 0.65-0.85).
“The decrease of 25% in stillbirths at 24 weeks to less than 28 weeks is very similar to that seen globally for stillbirths of 28 weeks of gestation [25.5% worldwide and 24.5% in developed regions] and above for a similar time period of 2000-2015, suggesting consistent improvements over time in the reduction of stillbirths from 24 completed weeks of gestation,” the researchers wrote in their analysis.
Data from France, Spain, and Cyprus was not included in the analysis as these countries did not collect fetal death reports for the gestational periods in the study. Also, for a few countries in the study, late terminations of pregnancy could not be distinguished from spontaneous fetal death.
“The consistency in reporting of births over time at 24 weeks to less than 28 weeks of gestation and the similarity of reduction in the rate of stillbirth over time to births at 28 completed weeks of gestation and above suggest that stillbirths at 24 weeks to less than 28 weeks of gestation can be routinely included in rates of stillbirth for international comparisons from now on,” at least in high-income countries, the investigators wrote.
The study by Norman et al. was funded by the Scottish government, Tommy’s Health Center, and Sands, a U.K. stillbirth charity. The article presents research funded in part by the National Institute for Health Research. Several investigators, including the lead author, reported financial support from these entities. One author reported salary from National Health Service Lothian. All other authors reported no relevant financial disclosures. The study by Smith et al. was funded by the European Union and National Institute for Health Research. Dr. Smith received funding from a National Institute for Health Research Career Development Fellowship. All other authors reported no financial conflicts of interest.
SOURCES: Norman JE et al. Lancet. 2018;392:1629-38; Smith LK et al. Lancet. 2018;392:1639-46.
Because reduced fetal movement is associated with higher stillbirth risk, asking women to be alert to RFM and report it immediately has emerged as a potential intervention to prevent stillbirth. But a large, randomized trial of one reporting and management protocol showed no reduction in stillbirths, only a rise in C-sections and preterm inductions.
Jane E. Norman, MD, of the University of Edinburgh, and her colleagues published results from a trial in the Lancet, in which 409,175 pregnant women (mean age, 30 years) across 33 hospitals in the United Kingdom and Ireland received either standard care or the experimental RFM care intervention before delivery. Women were seen during an experimental 3-month period, in which all were treated according to the protocol, or the 3-month control period that preceded it. A 2-month washout period occurred between allocations as institutions adapted to the study protocol.
The trial intervention consisted of training clinical staff on the implications and management of RFM, distributing written information on RFM to women at about 20 weeks’ gestation, and a management protocol aimed at quick action following a report of RFM at 24 or more weeks’ gestation. The protocol included cardiotocography within 2 hours of presentation followed by measurement of liquor volume and a growth scan, along with umbilical artery Doppler where available. Delivery was recommended for women at 37 or more weeks’ gestation with estimated fetal weight below the 10th centile, abdominal circumference below the 10th centile, a low liquor volume, an abnormal cardiotocograph, or recurrent RFM.
Incidence of stillbirth at or beyond 24 weeks was 4.40 per 1,000 births during the control period and 4.06 per 1,000 births in the intervention period (adjusted odds ratio, 0.90; 95% confidence interval, 0.75-1.07; P = .23), the researchers found. No differences were seen when stratifying for different gestational ages.
Meanwhile, induction of labor before 39 weeks was more frequent during intervention period at 40% of deliveries, compared with 34% during the control time (P less than .0001), and at term (41% vs. 36%; P = .0015). C-section was higher in the intervention group at 28% versus 26% (P = .0001). Neonatal ICU stays were not more common but were likely to be longer in the intervention period, with stays of 2 days or longer occurring in 6.7% of deliveries versus 6.2% (P =.0001).
The investigators concluded that their protocol, in its current design, was not effective and could not be recommended because of the significant increase in interventions.
Dr. Norman and her colleagues wrote that the findings would “reignite the controversy about the efficacy of RFM awareness to reduce stillbirth and the underlying mechanisms linking RFM and stillbirth.” However, the results do not mean that RFM is a sign of inevitable fetal death or that there is no role for RFM awareness as a stillbirth-prevention strategy. Other large trials testing RFM-based interventions are still underway, they noted.
“Further research to identify better predictive tests for stillbirth [to enable targeting of the only current treatment of earlier delivery] is urgently needed,” the investigators added.
In a related study also published in the Lancet, Lucy K. Smith, PhD, of the University of Leicester (England), and her colleagues reported that the real burden of stillbirth in Europe, while much lower than in the developing world, is still a third higher than reported using the current international cutoff established by the World Health Organization.
Dr. Smith and her colleagues examined national cohort data from 19 European countries between 2004 and 2015 for pregnancy outcomes from 22 completed weeks’ gestation. In 2015, they found more than 9,000 stillbirths occurred among more than 25 million births, and 3,022 of these (32%) occurred between 22 and 28 weeks’ gestation.
The WHO officially defines stillbirth as any baby born without life at 28 weeks or beyond, although it recommends that countries collect data on fetal death from 22 weeks. However, discrepancies between and even within countries in reporting laws and their implementation “inhibit reliable international comparisons” at those earlier gestational ages, Dr. Smith and her colleagues wrote.
The researchers, pooling data from the 19 countries, found that the stillbirth rate at 24-28 weeks’ gestation declined from 0.97 per 1,000 births (95% CI, 0.80-1.14) to 0.70 per 1,000 births (95% CI, 0.57-0.82) between 2004 and 2015, a reduction of 25% (risk ratio; 0.75; 95% CI, 0.65-0.85).
“The decrease of 25% in stillbirths at 24 weeks to less than 28 weeks is very similar to that seen globally for stillbirths of 28 weeks of gestation [25.5% worldwide and 24.5% in developed regions] and above for a similar time period of 2000-2015, suggesting consistent improvements over time in the reduction of stillbirths from 24 completed weeks of gestation,” the researchers wrote in their analysis.
Data from France, Spain, and Cyprus was not included in the analysis as these countries did not collect fetal death reports for the gestational periods in the study. Also, for a few countries in the study, late terminations of pregnancy could not be distinguished from spontaneous fetal death.
“The consistency in reporting of births over time at 24 weeks to less than 28 weeks of gestation and the similarity of reduction in the rate of stillbirth over time to births at 28 completed weeks of gestation and above suggest that stillbirths at 24 weeks to less than 28 weeks of gestation can be routinely included in rates of stillbirth for international comparisons from now on,” at least in high-income countries, the investigators wrote.
The study by Norman et al. was funded by the Scottish government, Tommy’s Health Center, and Sands, a U.K. stillbirth charity. The article presents research funded in part by the National Institute for Health Research. Several investigators, including the lead author, reported financial support from these entities. One author reported salary from National Health Service Lothian. All other authors reported no relevant financial disclosures. The study by Smith et al. was funded by the European Union and National Institute for Health Research. Dr. Smith received funding from a National Institute for Health Research Career Development Fellowship. All other authors reported no financial conflicts of interest.
SOURCES: Norman JE et al. Lancet. 2018;392:1629-38; Smith LK et al. Lancet. 2018;392:1639-46.
FROM THE LANCET
Key clinical point:
Major finding: Incidence of stillbirth at 24 weeks’ gestation or later was 4.06 per 1,000 in the intervention group and 4.40 per 1,000 with standard care (adjusted odds ratio, 0.90; 95% confidence interval, 0.75-1.07; P = .23).
Study details: Data from more than 400,000 pregnancies across 33 hospitals in the United Kingdom and Ireland; women were seen during a 3-month period of standard care or a 3-month intervention period.
Disclosures: The study by Norman et al. was funded by the Scottish government, Tommy’s Health Center, and Sands, a U.K. stillbirth charity. The article presents research funded in part by the National Institute for Health Research. Several investigators, including the lead author, reported financial support from these entities. One author reported salary from National Health Service Lothian. All other authors reported no relevant financial disclosures. The study by Smith et al. was funded by the European Union and National Institute for Health Research. Dr. Smith received funding from a National Institute for Health Research Career Development Fellowship. All other authors reported no financial conflicts of interest.
Sources: Norman JE et al. Lancet. 2018;392:1629-38; Smith LK et al. Lancet. 2018;392:1639-46.
FDA warning shines light on vaginal rejuvenation
The Food and Drug Administration has issued a stern warning to several manufacturers and a statement of caution to the public concerning “vaginal rejuvenation,” an umbrella term for a host of procedures to alter vaginal tissue for therapeutic or cosmetic purposes.
Lasers and other energy-based devices have been approved to treat abnormal or precancerous cervical or vaginal tissue and general warts, but the FDA has not approved any to treat vaginal atrophy, urinary incontinence, or reduced sexual function.
Device manufacturers claim lasers can address these conditions despite limited scientific evidence for their safety or efficacy. Insurers do not reimburse the procedures, considering them to be cosmetic.
In a July 30 statement, FDA Commissioner Scott Gottlieb, MD, slammed “deceptive” marketing practices on the part of manufacturers.
The FDA has reviewed 12 complaints since December 2015 of adverse effects related to vaginal procedures using the devices. Two were from manufacturers reporting pain and bleeding in patients following treatment, FDA spokeswoman Deborah Kotz said in an interview. “The FDA has also received voluntary MedWatch reports from individual patients who experienced significant pain and discomfort from procedures performed with these devices.”
The agency has targeted seven firms: Alma Lasers, BTL Aesthetics, BTL Industries, Cynosure, InMode, Sciton, and ThermiGen, with letters demanding evidence of FDA approval, clearance, or intent to seek clearance for use of their products on female genitalia. They also asked for evidence backing specific claims.
In a July 26 letter to BTL Industries, for example, the FDA demanded to know why the firm was marketing its Exilis laser device, approved for the treatment of facial wrinkles, as “Ultra Femme 360,” which it called “a whole new approach to women’s intimate health.” The device, according to the manufacturer, “provides the shortest noninvasive radio-frequency treatment available for female intimate parts” and “is proven to increase elastin and collagen in the treatment area.”
The FDA asked Cynosure, the maker of the Mona Lisa Touch, a system marketed as an FDA-approved treatment for vaginal atrophy, for evidence to support its claims that Mona Lisa Touch “is the only technology for vaginal and vulvar health with over 18+ published clinical studies” and is clinically proven to treat “painful symptoms of menopause, including intimacy.” They also asked for information about a modification to the originally approved device that was not brought to the FDA’s attention.
In a letter to Alma Lasers, whose Pixel CO2 Laser System was approved for use in a broad use of surgical applications including gynecologic surgery, the FDA noted that the device was being marketed as “FEMILIFT,” a laser procedure designed to “improve vaginal irregularities” and to assist “in vaginal mucosa revitalization.” The FDA demanded evidence for those claims.
The manufacturers have 30 days to respond to the FDA, which has not ruled out seeking enforcement action against firms with unsatisfactory responses.
For more than a decade, researchers have shown that healthy vaginal morphology is exceptionally wide ranging, including a recent study in more than 650 women, the largest to date (BJOG. 2018 Jun 25. doi: 10.1111/1471-0528.15387). Nonetheless, interest in elective vaginal procedures has only increased, with an industry report from the International Society of Aesthetic and Plastic Surgery describing a 45% increase in the use of one surgery, vaginal labiaplasty, between 2014 and 2016. Most procedures were performed in Brazil and the United States.
While plastic surgery societies support vaginal rejuvenation procedures, the American College of Obstetricians and Gynecologists has long frowned on them, with its first critique issued in a 2007 committee opinion. “No adequate studies have been published assessing the long-term satisfaction, safety, and complication rates for these procedures,” the association said last year in its most recent update on the subject.
Gynecologist David M. Jaspan, DO, of the Einstein Healthcare Network in Philadelphia, echoed ACOG’s views and said he welcomed FDA interest in vaginal rejuvenation.
The practice has “never been endorsed by the College or a board. It’s been considered a cosmetic procedure and it’s been under scrutiny for at least a decade,” Dr. Jaspan said. “I have reservations about the clinical outcomes and the training surrounds these procedures and I anxiously await randomized controlled trials to further evaluate them.”
Gynecologists who offer the procedures caution that they may have a role, and that randomized trials are underway to determine which groups of women might be best helped.
Marie Paraiso, MD, professor of obstetrics and gynecology at the Cleveland Clinic, said she uses the Mona Lisa Touch, a CO2 fractionated laser, to treat patients with genitourinary syndrome of menopause (GSM). These patients, Dr. Paraiso said, “complain of vaginal dryness and are unable to have intercourse or experience significant pain during or after intercourse. Some of them also may have irritative voiding, urinary frequency and urgency, or mild stress incontinence.”
Dr. Paraiso’s group has performed some 300 treatments with the laser and “we have fortunately not had patients complaining of persistent vaginal pain or scarring.” About 80%-90% of patients respond, she said, with some 20%-25% seeking retreatment within a year. “I believe for women who have contraindications to hormonal therapy or do not tolerate or cannot afford prolonged hormonal therapy, the CO2 fractional vaginal laser has been effective.”
Dr. Paraiso is also leading a multisite clinical trial randomizing about 200 patients with GSM to the laser treatment or estrogen-based vaginal creams, and following them for 6 months; thus far, she said, 6 of 89 patients, half in the laser arm, have reported mild to moderate adverse events.
Dr. Paraiso said she does not have a financial relationship with the manufacturer of Mona Lisa Touch, and that the trial was funded by the Foundation for Female Health Awareness, which receives unrestricted research grants from some device makers. “Our institute owns the laser and I have never been paid to train anyone to perform these procedures,” Dr. Paraiso added. “Our onus was to study the laser in order to improve the lives of our patients.”
Other trials comparing vaginal lasers with sham treatment are currently underway or in planning.
The North American Menopause Society struck a cautious note in response to the FDA criticism. In a statement issued August 1, JoAnn Pinkerton, MD, the society’s executive director, said the field needed prospective, randomized, sham-controlled trials of the laser and energy therapies. The therapies “may turn out to be an appropriate choice for many women, particularly for those concerned about breast cancer risk” associated with hormonal treatments. But until more robust data are available, doctors should “discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, and FDA-approved vaginal therapies such as vaginal estrogen and intravaginal dehydroepiandrosterone and oral therapies such as hormone therapy and ospemifene to determine the best treatment for women with GSM.”
Any discussion of vaginal energy-based therapies, should include the disclosure that these have not been approved for the specific indication, Dr. Pinkerton cautioned.
The term “vaginal rejuvenation,” coined by cosmetic gynecologists, incorporates surgeries designed to modify the appearance of the vulva, reduce the redundancy of vaginal tissue, and improve vaginal tone.
Endorsed by some well-known academic gynecologists, these devices have been promoted as “safe and effective” without any prospective, randomized studies and without accountability for conflicts of interest including “educational stipends” from device manufacturers and clinicians’ need to recoup the high cost of the devices themselves.
Studies have generally been limited to fewer than 100 patients followed for 12 weeks or less. Companies are not informing doctors that the devices may not be FDA approved for the purposes advertised, nor are they providing adverse effects reports. Laser and radio-frequency procedures at best demonstrate temporary, marginal improvement in vaginal tone and dyspareunia, and at worst are associated with increased pelvic pain and dyspareunia, as well as vaginal, rectal, and bladder thermal burns. For those of us who specialize in cosmetic surgery, they have very limited benefit with a significant risk of injury to the patient even when properly used.
Julio Cesar Novoa, MD, is a private practice ob.gyn from El Paso, Tex. He reported no relevant conflicts of interest.
The term “vaginal rejuvenation,” coined by cosmetic gynecologists, incorporates surgeries designed to modify the appearance of the vulva, reduce the redundancy of vaginal tissue, and improve vaginal tone.
Endorsed by some well-known academic gynecologists, these devices have been promoted as “safe and effective” without any prospective, randomized studies and without accountability for conflicts of interest including “educational stipends” from device manufacturers and clinicians’ need to recoup the high cost of the devices themselves.
Studies have generally been limited to fewer than 100 patients followed for 12 weeks or less. Companies are not informing doctors that the devices may not be FDA approved for the purposes advertised, nor are they providing adverse effects reports. Laser and radio-frequency procedures at best demonstrate temporary, marginal improvement in vaginal tone and dyspareunia, and at worst are associated with increased pelvic pain and dyspareunia, as well as vaginal, rectal, and bladder thermal burns. For those of us who specialize in cosmetic surgery, they have very limited benefit with a significant risk of injury to the patient even when properly used.
Julio Cesar Novoa, MD, is a private practice ob.gyn from El Paso, Tex. He reported no relevant conflicts of interest.
The term “vaginal rejuvenation,” coined by cosmetic gynecologists, incorporates surgeries designed to modify the appearance of the vulva, reduce the redundancy of vaginal tissue, and improve vaginal tone.
Endorsed by some well-known academic gynecologists, these devices have been promoted as “safe and effective” without any prospective, randomized studies and without accountability for conflicts of interest including “educational stipends” from device manufacturers and clinicians’ need to recoup the high cost of the devices themselves.
Studies have generally been limited to fewer than 100 patients followed for 12 weeks or less. Companies are not informing doctors that the devices may not be FDA approved for the purposes advertised, nor are they providing adverse effects reports. Laser and radio-frequency procedures at best demonstrate temporary, marginal improvement in vaginal tone and dyspareunia, and at worst are associated with increased pelvic pain and dyspareunia, as well as vaginal, rectal, and bladder thermal burns. For those of us who specialize in cosmetic surgery, they have very limited benefit with a significant risk of injury to the patient even when properly used.
Julio Cesar Novoa, MD, is a private practice ob.gyn from El Paso, Tex. He reported no relevant conflicts of interest.
The Food and Drug Administration has issued a stern warning to several manufacturers and a statement of caution to the public concerning “vaginal rejuvenation,” an umbrella term for a host of procedures to alter vaginal tissue for therapeutic or cosmetic purposes.
Lasers and other energy-based devices have been approved to treat abnormal or precancerous cervical or vaginal tissue and general warts, but the FDA has not approved any to treat vaginal atrophy, urinary incontinence, or reduced sexual function.
Device manufacturers claim lasers can address these conditions despite limited scientific evidence for their safety or efficacy. Insurers do not reimburse the procedures, considering them to be cosmetic.
In a July 30 statement, FDA Commissioner Scott Gottlieb, MD, slammed “deceptive” marketing practices on the part of manufacturers.
The FDA has reviewed 12 complaints since December 2015 of adverse effects related to vaginal procedures using the devices. Two were from manufacturers reporting pain and bleeding in patients following treatment, FDA spokeswoman Deborah Kotz said in an interview. “The FDA has also received voluntary MedWatch reports from individual patients who experienced significant pain and discomfort from procedures performed with these devices.”
The agency has targeted seven firms: Alma Lasers, BTL Aesthetics, BTL Industries, Cynosure, InMode, Sciton, and ThermiGen, with letters demanding evidence of FDA approval, clearance, or intent to seek clearance for use of their products on female genitalia. They also asked for evidence backing specific claims.
In a July 26 letter to BTL Industries, for example, the FDA demanded to know why the firm was marketing its Exilis laser device, approved for the treatment of facial wrinkles, as “Ultra Femme 360,” which it called “a whole new approach to women’s intimate health.” The device, according to the manufacturer, “provides the shortest noninvasive radio-frequency treatment available for female intimate parts” and “is proven to increase elastin and collagen in the treatment area.”
The FDA asked Cynosure, the maker of the Mona Lisa Touch, a system marketed as an FDA-approved treatment for vaginal atrophy, for evidence to support its claims that Mona Lisa Touch “is the only technology for vaginal and vulvar health with over 18+ published clinical studies” and is clinically proven to treat “painful symptoms of menopause, including intimacy.” They also asked for information about a modification to the originally approved device that was not brought to the FDA’s attention.
In a letter to Alma Lasers, whose Pixel CO2 Laser System was approved for use in a broad use of surgical applications including gynecologic surgery, the FDA noted that the device was being marketed as “FEMILIFT,” a laser procedure designed to “improve vaginal irregularities” and to assist “in vaginal mucosa revitalization.” The FDA demanded evidence for those claims.
The manufacturers have 30 days to respond to the FDA, which has not ruled out seeking enforcement action against firms with unsatisfactory responses.
For more than a decade, researchers have shown that healthy vaginal morphology is exceptionally wide ranging, including a recent study in more than 650 women, the largest to date (BJOG. 2018 Jun 25. doi: 10.1111/1471-0528.15387). Nonetheless, interest in elective vaginal procedures has only increased, with an industry report from the International Society of Aesthetic and Plastic Surgery describing a 45% increase in the use of one surgery, vaginal labiaplasty, between 2014 and 2016. Most procedures were performed in Brazil and the United States.
While plastic surgery societies support vaginal rejuvenation procedures, the American College of Obstetricians and Gynecologists has long frowned on them, with its first critique issued in a 2007 committee opinion. “No adequate studies have been published assessing the long-term satisfaction, safety, and complication rates for these procedures,” the association said last year in its most recent update on the subject.
Gynecologist David M. Jaspan, DO, of the Einstein Healthcare Network in Philadelphia, echoed ACOG’s views and said he welcomed FDA interest in vaginal rejuvenation.
The practice has “never been endorsed by the College or a board. It’s been considered a cosmetic procedure and it’s been under scrutiny for at least a decade,” Dr. Jaspan said. “I have reservations about the clinical outcomes and the training surrounds these procedures and I anxiously await randomized controlled trials to further evaluate them.”
Gynecologists who offer the procedures caution that they may have a role, and that randomized trials are underway to determine which groups of women might be best helped.
Marie Paraiso, MD, professor of obstetrics and gynecology at the Cleveland Clinic, said she uses the Mona Lisa Touch, a CO2 fractionated laser, to treat patients with genitourinary syndrome of menopause (GSM). These patients, Dr. Paraiso said, “complain of vaginal dryness and are unable to have intercourse or experience significant pain during or after intercourse. Some of them also may have irritative voiding, urinary frequency and urgency, or mild stress incontinence.”
Dr. Paraiso’s group has performed some 300 treatments with the laser and “we have fortunately not had patients complaining of persistent vaginal pain or scarring.” About 80%-90% of patients respond, she said, with some 20%-25% seeking retreatment within a year. “I believe for women who have contraindications to hormonal therapy or do not tolerate or cannot afford prolonged hormonal therapy, the CO2 fractional vaginal laser has been effective.”
Dr. Paraiso is also leading a multisite clinical trial randomizing about 200 patients with GSM to the laser treatment or estrogen-based vaginal creams, and following them for 6 months; thus far, she said, 6 of 89 patients, half in the laser arm, have reported mild to moderate adverse events.
Dr. Paraiso said she does not have a financial relationship with the manufacturer of Mona Lisa Touch, and that the trial was funded by the Foundation for Female Health Awareness, which receives unrestricted research grants from some device makers. “Our institute owns the laser and I have never been paid to train anyone to perform these procedures,” Dr. Paraiso added. “Our onus was to study the laser in order to improve the lives of our patients.”
Other trials comparing vaginal lasers with sham treatment are currently underway or in planning.
The North American Menopause Society struck a cautious note in response to the FDA criticism. In a statement issued August 1, JoAnn Pinkerton, MD, the society’s executive director, said the field needed prospective, randomized, sham-controlled trials of the laser and energy therapies. The therapies “may turn out to be an appropriate choice for many women, particularly for those concerned about breast cancer risk” associated with hormonal treatments. But until more robust data are available, doctors should “discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, and FDA-approved vaginal therapies such as vaginal estrogen and intravaginal dehydroepiandrosterone and oral therapies such as hormone therapy and ospemifene to determine the best treatment for women with GSM.”
Any discussion of vaginal energy-based therapies, should include the disclosure that these have not been approved for the specific indication, Dr. Pinkerton cautioned.
The Food and Drug Administration has issued a stern warning to several manufacturers and a statement of caution to the public concerning “vaginal rejuvenation,” an umbrella term for a host of procedures to alter vaginal tissue for therapeutic or cosmetic purposes.
Lasers and other energy-based devices have been approved to treat abnormal or precancerous cervical or vaginal tissue and general warts, but the FDA has not approved any to treat vaginal atrophy, urinary incontinence, or reduced sexual function.
Device manufacturers claim lasers can address these conditions despite limited scientific evidence for their safety or efficacy. Insurers do not reimburse the procedures, considering them to be cosmetic.
In a July 30 statement, FDA Commissioner Scott Gottlieb, MD, slammed “deceptive” marketing practices on the part of manufacturers.
The FDA has reviewed 12 complaints since December 2015 of adverse effects related to vaginal procedures using the devices. Two were from manufacturers reporting pain and bleeding in patients following treatment, FDA spokeswoman Deborah Kotz said in an interview. “The FDA has also received voluntary MedWatch reports from individual patients who experienced significant pain and discomfort from procedures performed with these devices.”
The agency has targeted seven firms: Alma Lasers, BTL Aesthetics, BTL Industries, Cynosure, InMode, Sciton, and ThermiGen, with letters demanding evidence of FDA approval, clearance, or intent to seek clearance for use of their products on female genitalia. They also asked for evidence backing specific claims.
In a July 26 letter to BTL Industries, for example, the FDA demanded to know why the firm was marketing its Exilis laser device, approved for the treatment of facial wrinkles, as “Ultra Femme 360,” which it called “a whole new approach to women’s intimate health.” The device, according to the manufacturer, “provides the shortest noninvasive radio-frequency treatment available for female intimate parts” and “is proven to increase elastin and collagen in the treatment area.”
The FDA asked Cynosure, the maker of the Mona Lisa Touch, a system marketed as an FDA-approved treatment for vaginal atrophy, for evidence to support its claims that Mona Lisa Touch “is the only technology for vaginal and vulvar health with over 18+ published clinical studies” and is clinically proven to treat “painful symptoms of menopause, including intimacy.” They also asked for information about a modification to the originally approved device that was not brought to the FDA’s attention.
In a letter to Alma Lasers, whose Pixel CO2 Laser System was approved for use in a broad use of surgical applications including gynecologic surgery, the FDA noted that the device was being marketed as “FEMILIFT,” a laser procedure designed to “improve vaginal irregularities” and to assist “in vaginal mucosa revitalization.” The FDA demanded evidence for those claims.
The manufacturers have 30 days to respond to the FDA, which has not ruled out seeking enforcement action against firms with unsatisfactory responses.
For more than a decade, researchers have shown that healthy vaginal morphology is exceptionally wide ranging, including a recent study in more than 650 women, the largest to date (BJOG. 2018 Jun 25. doi: 10.1111/1471-0528.15387). Nonetheless, interest in elective vaginal procedures has only increased, with an industry report from the International Society of Aesthetic and Plastic Surgery describing a 45% increase in the use of one surgery, vaginal labiaplasty, between 2014 and 2016. Most procedures were performed in Brazil and the United States.
While plastic surgery societies support vaginal rejuvenation procedures, the American College of Obstetricians and Gynecologists has long frowned on them, with its first critique issued in a 2007 committee opinion. “No adequate studies have been published assessing the long-term satisfaction, safety, and complication rates for these procedures,” the association said last year in its most recent update on the subject.
Gynecologist David M. Jaspan, DO, of the Einstein Healthcare Network in Philadelphia, echoed ACOG’s views and said he welcomed FDA interest in vaginal rejuvenation.
The practice has “never been endorsed by the College or a board. It’s been considered a cosmetic procedure and it’s been under scrutiny for at least a decade,” Dr. Jaspan said. “I have reservations about the clinical outcomes and the training surrounds these procedures and I anxiously await randomized controlled trials to further evaluate them.”
Gynecologists who offer the procedures caution that they may have a role, and that randomized trials are underway to determine which groups of women might be best helped.
Marie Paraiso, MD, professor of obstetrics and gynecology at the Cleveland Clinic, said she uses the Mona Lisa Touch, a CO2 fractionated laser, to treat patients with genitourinary syndrome of menopause (GSM). These patients, Dr. Paraiso said, “complain of vaginal dryness and are unable to have intercourse or experience significant pain during or after intercourse. Some of them also may have irritative voiding, urinary frequency and urgency, or mild stress incontinence.”
Dr. Paraiso’s group has performed some 300 treatments with the laser and “we have fortunately not had patients complaining of persistent vaginal pain or scarring.” About 80%-90% of patients respond, she said, with some 20%-25% seeking retreatment within a year. “I believe for women who have contraindications to hormonal therapy or do not tolerate or cannot afford prolonged hormonal therapy, the CO2 fractional vaginal laser has been effective.”
Dr. Paraiso is also leading a multisite clinical trial randomizing about 200 patients with GSM to the laser treatment or estrogen-based vaginal creams, and following them for 6 months; thus far, she said, 6 of 89 patients, half in the laser arm, have reported mild to moderate adverse events.
Dr. Paraiso said she does not have a financial relationship with the manufacturer of Mona Lisa Touch, and that the trial was funded by the Foundation for Female Health Awareness, which receives unrestricted research grants from some device makers. “Our institute owns the laser and I have never been paid to train anyone to perform these procedures,” Dr. Paraiso added. “Our onus was to study the laser in order to improve the lives of our patients.”
Other trials comparing vaginal lasers with sham treatment are currently underway or in planning.
The North American Menopause Society struck a cautious note in response to the FDA criticism. In a statement issued August 1, JoAnn Pinkerton, MD, the society’s executive director, said the field needed prospective, randomized, sham-controlled trials of the laser and energy therapies. The therapies “may turn out to be an appropriate choice for many women, particularly for those concerned about breast cancer risk” associated with hormonal treatments. But until more robust data are available, doctors should “discuss the benefits and risks of all available treatment options for vaginal symptoms, including over-the-counter lubricants, vaginal moisturizers, and FDA-approved vaginal therapies such as vaginal estrogen and intravaginal dehydroepiandrosterone and oral therapies such as hormone therapy and ospemifene to determine the best treatment for women with GSM.”
Any discussion of vaginal energy-based therapies, should include the disclosure that these have not been approved for the specific indication, Dr. Pinkerton cautioned.
Oral drug seen preventing angioedema attacks
An experimental agent reduced swelling episodes markedly in patients with hereditary angioedema, according to results from a phase 2 randomized, dose-ranging, placebo-controlled trial.
The drug BCX7353, developed by BioCryst Pharmaceuticals, is taken orally and works by inhibiting plasma kallikrein, an enzyme overexpressed in hereditary angioedema, a rare genetic disease that causes severe tissue swelling. In research published July 26 in the New England Journal of Medicine (N Engl J Med. 2018;379:352-62), Emel Aygören-Pürsün, MD, of Goethe University in Frankfurt, Germany, and colleagues, randomized 77 patients with type 1 or II hereditary angioedema and a pattern of frequent attacks to one of four doses of daily BCX7353, or placebo, for 28 days.
Dr. Aygören-Pürsün’s group found significant reductions in the number of monthly attacks for the three doses used in the study, with the best response seen in the group receiving the second-lowest dose of 125 mg. These patients saw a reduction of 73.8% (P less than .001) in monthly attacks from baseline, and 43% of patients receiving that dose had no attacks during the study period. The higher-dose groups saw more adverse events and apparently less efficacy, with the 250-mg dose associated with a reduction in attacks of 44.6% (P = .01), and for the 350-mg group, a 45.5% reduction (P = .006).
Patients receiving the lowest dose in the study, 62.5 mg, saw a small (about 10%) reduction in attacks that did not reach statistical significance. Gastrointestinal adverse events were reported in the two highest dose groups, and three patients in the 350-mg group dropped out after reporting serious adverse events, including one liver disorder considered likely related to the trial regimen.
The efficacy of the highest doses “was probably masked by gastrointestinal adverse events that may have been misattributed as early symptoms of abdominal angioedema attacks,” the investigators wrote in their analysis. Improvements in angioedema-related quality of life scores, a secondary trial endpoint, reached statistical significance for the 125- and 250-mg doses.
The authors cautioned that the safety of long-term dosing would need to be studied in longer-term trials.
The study was sponsored by the drug manufacturer, BioCryst Pharmaceuticals. All of the study’s authors, including the lead author, disclosed financial relationships in the form of grant support, fees, or employment with the study sponsor.
SOURCE: Aygören-Pürsün et al. N Engl J Med. 2018;379:352-62.
An experimental agent reduced swelling episodes markedly in patients with hereditary angioedema, according to results from a phase 2 randomized, dose-ranging, placebo-controlled trial.
The drug BCX7353, developed by BioCryst Pharmaceuticals, is taken orally and works by inhibiting plasma kallikrein, an enzyme overexpressed in hereditary angioedema, a rare genetic disease that causes severe tissue swelling. In research published July 26 in the New England Journal of Medicine (N Engl J Med. 2018;379:352-62), Emel Aygören-Pürsün, MD, of Goethe University in Frankfurt, Germany, and colleagues, randomized 77 patients with type 1 or II hereditary angioedema and a pattern of frequent attacks to one of four doses of daily BCX7353, or placebo, for 28 days.
Dr. Aygören-Pürsün’s group found significant reductions in the number of monthly attacks for the three doses used in the study, with the best response seen in the group receiving the second-lowest dose of 125 mg. These patients saw a reduction of 73.8% (P less than .001) in monthly attacks from baseline, and 43% of patients receiving that dose had no attacks during the study period. The higher-dose groups saw more adverse events and apparently less efficacy, with the 250-mg dose associated with a reduction in attacks of 44.6% (P = .01), and for the 350-mg group, a 45.5% reduction (P = .006).
Patients receiving the lowest dose in the study, 62.5 mg, saw a small (about 10%) reduction in attacks that did not reach statistical significance. Gastrointestinal adverse events were reported in the two highest dose groups, and three patients in the 350-mg group dropped out after reporting serious adverse events, including one liver disorder considered likely related to the trial regimen.
The efficacy of the highest doses “was probably masked by gastrointestinal adverse events that may have been misattributed as early symptoms of abdominal angioedema attacks,” the investigators wrote in their analysis. Improvements in angioedema-related quality of life scores, a secondary trial endpoint, reached statistical significance for the 125- and 250-mg doses.
The authors cautioned that the safety of long-term dosing would need to be studied in longer-term trials.
The study was sponsored by the drug manufacturer, BioCryst Pharmaceuticals. All of the study’s authors, including the lead author, disclosed financial relationships in the form of grant support, fees, or employment with the study sponsor.
SOURCE: Aygören-Pürsün et al. N Engl J Med. 2018;379:352-62.
An experimental agent reduced swelling episodes markedly in patients with hereditary angioedema, according to results from a phase 2 randomized, dose-ranging, placebo-controlled trial.
The drug BCX7353, developed by BioCryst Pharmaceuticals, is taken orally and works by inhibiting plasma kallikrein, an enzyme overexpressed in hereditary angioedema, a rare genetic disease that causes severe tissue swelling. In research published July 26 in the New England Journal of Medicine (N Engl J Med. 2018;379:352-62), Emel Aygören-Pürsün, MD, of Goethe University in Frankfurt, Germany, and colleagues, randomized 77 patients with type 1 or II hereditary angioedema and a pattern of frequent attacks to one of four doses of daily BCX7353, or placebo, for 28 days.
Dr. Aygören-Pürsün’s group found significant reductions in the number of monthly attacks for the three doses used in the study, with the best response seen in the group receiving the second-lowest dose of 125 mg. These patients saw a reduction of 73.8% (P less than .001) in monthly attacks from baseline, and 43% of patients receiving that dose had no attacks during the study period. The higher-dose groups saw more adverse events and apparently less efficacy, with the 250-mg dose associated with a reduction in attacks of 44.6% (P = .01), and for the 350-mg group, a 45.5% reduction (P = .006).
Patients receiving the lowest dose in the study, 62.5 mg, saw a small (about 10%) reduction in attacks that did not reach statistical significance. Gastrointestinal adverse events were reported in the two highest dose groups, and three patients in the 350-mg group dropped out after reporting serious adverse events, including one liver disorder considered likely related to the trial regimen.
The efficacy of the highest doses “was probably masked by gastrointestinal adverse events that may have been misattributed as early symptoms of abdominal angioedema attacks,” the investigators wrote in their analysis. Improvements in angioedema-related quality of life scores, a secondary trial endpoint, reached statistical significance for the 125- and 250-mg doses.
The authors cautioned that the safety of long-term dosing would need to be studied in longer-term trials.
The study was sponsored by the drug manufacturer, BioCryst Pharmaceuticals. All of the study’s authors, including the lead author, disclosed financial relationships in the form of grant support, fees, or employment with the study sponsor.
SOURCE: Aygören-Pürsün et al. N Engl J Med. 2018;379:352-62.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: An experimental kallikrein inhibitor, taken daily, prevented swelling attacks in people with hereditary angioedema.
Major finding: Patients in a moderate-dose group saw a 73.8% reduction in monthly swelling attacks from baseline, and 43% of saw no attacks.
Study details: A phase 2 study randomizing 77 patients with hereditary angioedema in three countries to one of four escalating doses or placebo.
Disclosures: All authors disclosed some relationship (current or past grant or fee support, or employment) with sponsor, BioCryst Pharmaceuticals.
Source: Aygören-Pürsün et. al. N Engl J Med. 2018;379:352-62.
Fibromyalgia should be seen as one, not two disorders
The symptomatology and severity of fibromyalgia is virtually identical among people with primary and secondary forms of the disorder, researchers say, and the two should therefore be considered the same.
Currently, patients classified as having primary fibromyalgia have a defined set of pain, fatigue, cognitive, and psychological symptoms in the absence of a clinically important inflammatory disorder, while secondary fibromyalgia occurs in the context of a disease such as rheumatoid arthritis.
In research published online in the Journal of Rheumatology, investigators led by Frederick Wolfe, MD, of the National Data Bank for Rheumatic Diseases and the University of Kansas in Wichita, sought to understand whether patients with primary and secondary diagnoses “had the same level of outcomes, symptoms, and characteristics” at different points across the polysymptomatic distress (PSD) scale, a measure to assess severity in fibromyalgia.
The PSD is calculated by combining two measurements used in fibromyalgia: the widespread pain index (WPI), which counts the number of painful regions in the body, and the somatic symptom scale (SSS), which measures fatigue, sleep, emotional and cognitive problems, and the extent of symptom reporting. Higher PSD scores correlate to worse outcomes, including for disability and health-related quality of life.
Dr. Wolfe and his colleagues compared records from 1,525 patients (mean age 57, 95% women) in the National Data Bank who were diagnosed with fibromyalgia only, with those from 12,037 people with rheumatoid arthritis (mean age 61, 82% women) and not evaluated for fibromyalgia. They also looked at data on fibromyalgia symptoms from a general population sample in Germany.
A total of 22% of the RA patients in the cohort met the criteria for a fibromyalgia diagnosis under current (2016) ACR criteria, while 53% of the patients in the primary fibromyalgia group and 2.0% of the general population sample did. Symptom magnitude and severity differed only slightly between the RA and fibromyalgia-only groups, the investigators found. Those without RA had a mean PSD score of 21.9 (of a possible 31) and those with RA and who met the fibromyalgia criteria had a mean score of 20.7.
The researchers found that the disease behaved similarly whether it occurred alone or with RA. Patients with higher PSD scores experienced worse outcomes across symptom domains regardless of RA status. When controlled for PSD, pain, patient global, and health-related quality of life, scores were similar between groups. However, disability scores and painful joint counts were slightly higher in the RA group.
“Fibromyalgia can exist whether or not there’s another disorder present,” Dr. Wolfe said in an interview, “but we don’t tend to think of it that way.” Clinicians, Dr. Wolfe said, “have to be able to identify [fibromyalgia] within other disorders. It can’t be only something that you find once you’ve ruled out everything else.”
Clinicians can look to PSD scores to inform management choices, the researchers said, instead of whether the fibromyalgia is considered primary or secondary.
Dr. Wolfe said that the current bifurcated concept of the disease also creates difficulties for research into fibromyalgia treatments. For example, using RA patients as a comparator group in a clinical trial is problematic because RA patients, as this study demonstrates, also have a substantial burden of fibromyalgia. And the use of so-called healthy controls in fibromyalgia studies is also a problem, Dr. Wolfe said.
“People say they’ve tried this drug or treatment in people with fibromyalgia and on normal controls. But there’s no such thing as normal controls for fibromyalgia. Does normal mean people with zero fatigue or pain? Or do we look at what’s normal among people who have multiple sclerosis or RA? If you’re going to test things in people with fibromyalgia, you never really have normal controls because it depends on where in this whole continuum you are.”
But what this new research also shows, he said, is that while up to now “you couldn’t easily study people with fibromyalgia [in other disorders], it doesn’t matter if you have RA or another disorder in addition to fibromyalgia. You get the full spectrum or severity regardless. The underlying disease wouldn’t affect your identification of the fibromyalgia symptoms.”
Dr. Wolfe and his colleagues described as a limitation of their study the lack of comparator groups besides RA patients. Secondary fibromyalgia is also commonly reported with osteoarthritis, they noted.
The study had no outside funding and investigators reported no conflicts of interest.
SOURCE: Wolfe F et al. J Rheumatol. 2018 Jul 15. doi: 10.3899/jrheum.180083.
The symptomatology and severity of fibromyalgia is virtually identical among people with primary and secondary forms of the disorder, researchers say, and the two should therefore be considered the same.
Currently, patients classified as having primary fibromyalgia have a defined set of pain, fatigue, cognitive, and psychological symptoms in the absence of a clinically important inflammatory disorder, while secondary fibromyalgia occurs in the context of a disease such as rheumatoid arthritis.
In research published online in the Journal of Rheumatology, investigators led by Frederick Wolfe, MD, of the National Data Bank for Rheumatic Diseases and the University of Kansas in Wichita, sought to understand whether patients with primary and secondary diagnoses “had the same level of outcomes, symptoms, and characteristics” at different points across the polysymptomatic distress (PSD) scale, a measure to assess severity in fibromyalgia.
The PSD is calculated by combining two measurements used in fibromyalgia: the widespread pain index (WPI), which counts the number of painful regions in the body, and the somatic symptom scale (SSS), which measures fatigue, sleep, emotional and cognitive problems, and the extent of symptom reporting. Higher PSD scores correlate to worse outcomes, including for disability and health-related quality of life.
Dr. Wolfe and his colleagues compared records from 1,525 patients (mean age 57, 95% women) in the National Data Bank who were diagnosed with fibromyalgia only, with those from 12,037 people with rheumatoid arthritis (mean age 61, 82% women) and not evaluated for fibromyalgia. They also looked at data on fibromyalgia symptoms from a general population sample in Germany.
A total of 22% of the RA patients in the cohort met the criteria for a fibromyalgia diagnosis under current (2016) ACR criteria, while 53% of the patients in the primary fibromyalgia group and 2.0% of the general population sample did. Symptom magnitude and severity differed only slightly between the RA and fibromyalgia-only groups, the investigators found. Those without RA had a mean PSD score of 21.9 (of a possible 31) and those with RA and who met the fibromyalgia criteria had a mean score of 20.7.
The researchers found that the disease behaved similarly whether it occurred alone or with RA. Patients with higher PSD scores experienced worse outcomes across symptom domains regardless of RA status. When controlled for PSD, pain, patient global, and health-related quality of life, scores were similar between groups. However, disability scores and painful joint counts were slightly higher in the RA group.
“Fibromyalgia can exist whether or not there’s another disorder present,” Dr. Wolfe said in an interview, “but we don’t tend to think of it that way.” Clinicians, Dr. Wolfe said, “have to be able to identify [fibromyalgia] within other disorders. It can’t be only something that you find once you’ve ruled out everything else.”
Clinicians can look to PSD scores to inform management choices, the researchers said, instead of whether the fibromyalgia is considered primary or secondary.
Dr. Wolfe said that the current bifurcated concept of the disease also creates difficulties for research into fibromyalgia treatments. For example, using RA patients as a comparator group in a clinical trial is problematic because RA patients, as this study demonstrates, also have a substantial burden of fibromyalgia. And the use of so-called healthy controls in fibromyalgia studies is also a problem, Dr. Wolfe said.
“People say they’ve tried this drug or treatment in people with fibromyalgia and on normal controls. But there’s no such thing as normal controls for fibromyalgia. Does normal mean people with zero fatigue or pain? Or do we look at what’s normal among people who have multiple sclerosis or RA? If you’re going to test things in people with fibromyalgia, you never really have normal controls because it depends on where in this whole continuum you are.”
But what this new research also shows, he said, is that while up to now “you couldn’t easily study people with fibromyalgia [in other disorders], it doesn’t matter if you have RA or another disorder in addition to fibromyalgia. You get the full spectrum or severity regardless. The underlying disease wouldn’t affect your identification of the fibromyalgia symptoms.”
Dr. Wolfe and his colleagues described as a limitation of their study the lack of comparator groups besides RA patients. Secondary fibromyalgia is also commonly reported with osteoarthritis, they noted.
The study had no outside funding and investigators reported no conflicts of interest.
SOURCE: Wolfe F et al. J Rheumatol. 2018 Jul 15. doi: 10.3899/jrheum.180083.
The symptomatology and severity of fibromyalgia is virtually identical among people with primary and secondary forms of the disorder, researchers say, and the two should therefore be considered the same.
Currently, patients classified as having primary fibromyalgia have a defined set of pain, fatigue, cognitive, and psychological symptoms in the absence of a clinically important inflammatory disorder, while secondary fibromyalgia occurs in the context of a disease such as rheumatoid arthritis.
In research published online in the Journal of Rheumatology, investigators led by Frederick Wolfe, MD, of the National Data Bank for Rheumatic Diseases and the University of Kansas in Wichita, sought to understand whether patients with primary and secondary diagnoses “had the same level of outcomes, symptoms, and characteristics” at different points across the polysymptomatic distress (PSD) scale, a measure to assess severity in fibromyalgia.
The PSD is calculated by combining two measurements used in fibromyalgia: the widespread pain index (WPI), which counts the number of painful regions in the body, and the somatic symptom scale (SSS), which measures fatigue, sleep, emotional and cognitive problems, and the extent of symptom reporting. Higher PSD scores correlate to worse outcomes, including for disability and health-related quality of life.
Dr. Wolfe and his colleagues compared records from 1,525 patients (mean age 57, 95% women) in the National Data Bank who were diagnosed with fibromyalgia only, with those from 12,037 people with rheumatoid arthritis (mean age 61, 82% women) and not evaluated for fibromyalgia. They also looked at data on fibromyalgia symptoms from a general population sample in Germany.
A total of 22% of the RA patients in the cohort met the criteria for a fibromyalgia diagnosis under current (2016) ACR criteria, while 53% of the patients in the primary fibromyalgia group and 2.0% of the general population sample did. Symptom magnitude and severity differed only slightly between the RA and fibromyalgia-only groups, the investigators found. Those without RA had a mean PSD score of 21.9 (of a possible 31) and those with RA and who met the fibromyalgia criteria had a mean score of 20.7.
The researchers found that the disease behaved similarly whether it occurred alone or with RA. Patients with higher PSD scores experienced worse outcomes across symptom domains regardless of RA status. When controlled for PSD, pain, patient global, and health-related quality of life, scores were similar between groups. However, disability scores and painful joint counts were slightly higher in the RA group.
“Fibromyalgia can exist whether or not there’s another disorder present,” Dr. Wolfe said in an interview, “but we don’t tend to think of it that way.” Clinicians, Dr. Wolfe said, “have to be able to identify [fibromyalgia] within other disorders. It can’t be only something that you find once you’ve ruled out everything else.”
Clinicians can look to PSD scores to inform management choices, the researchers said, instead of whether the fibromyalgia is considered primary or secondary.
Dr. Wolfe said that the current bifurcated concept of the disease also creates difficulties for research into fibromyalgia treatments. For example, using RA patients as a comparator group in a clinical trial is problematic because RA patients, as this study demonstrates, also have a substantial burden of fibromyalgia. And the use of so-called healthy controls in fibromyalgia studies is also a problem, Dr. Wolfe said.
“People say they’ve tried this drug or treatment in people with fibromyalgia and on normal controls. But there’s no such thing as normal controls for fibromyalgia. Does normal mean people with zero fatigue or pain? Or do we look at what’s normal among people who have multiple sclerosis or RA? If you’re going to test things in people with fibromyalgia, you never really have normal controls because it depends on where in this whole continuum you are.”
But what this new research also shows, he said, is that while up to now “you couldn’t easily study people with fibromyalgia [in other disorders], it doesn’t matter if you have RA or another disorder in addition to fibromyalgia. You get the full spectrum or severity regardless. The underlying disease wouldn’t affect your identification of the fibromyalgia symptoms.”
Dr. Wolfe and his colleagues described as a limitation of their study the lack of comparator groups besides RA patients. Secondary fibromyalgia is also commonly reported with osteoarthritis, they noted.
The study had no outside funding and investigators reported no conflicts of interest.
SOURCE: Wolfe F et al. J Rheumatol. 2018 Jul 15. doi: 10.3899/jrheum.180083.
FROM THE JOURNAL OF RHEUMATOLOGY
Key clinical point: Fibromyalgia behaves similarly whether it occurs alone or in the context of another disease.
Major finding: Patients with RA plus fibromyalgia had mean fibromyalgia severity scores of 21.9 while those with fibromyalgia alone had 20.7, and results were similar across 17 clinical variables measured in fibromyalgia.
Study details: Investigators compared records from 1,525 subjects with a diagnosis of fibromyalgia and another 12,037 with a diagnosis of RA.
Disclosures: The study had no outside funding and the investigators reported no conflicts of interest.
Source: Wolfe F et al. J Rheumatol. 2018 Jul 15. doi: 10.3899/jrheum.180083.