User login
Irritated Pigmented Plaque on the Scalp
The Diagnosis: Clonal Melanoacanthoma
Melanoacanthoma (MA) is an extremely rare, benign, epidermal tumor histologically characterized by keratinocytes and large, pigmented, dendritic melanocytes. These lesions are loosely related to seborrheic keratoses, and the term was first coined by Mishima and Pinkus1 in 1960. It is estimated that the lesion occurs in only 5 of 500,000 individuals and tends to occur in older, light-skinned individuals.2 The majority are slow growing and are present on the head, neck, or upper extremities; however, similar lesions also have been reported on the oral mucosa.3 Melanoacanthomas range in size from 2×2 to 15×15 cm; are clinically pigmented; and present as either a papule, plaque, nodule, or horn.2
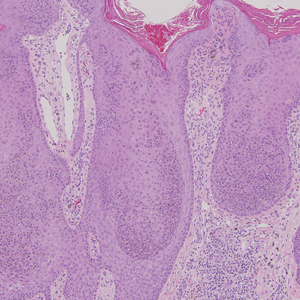
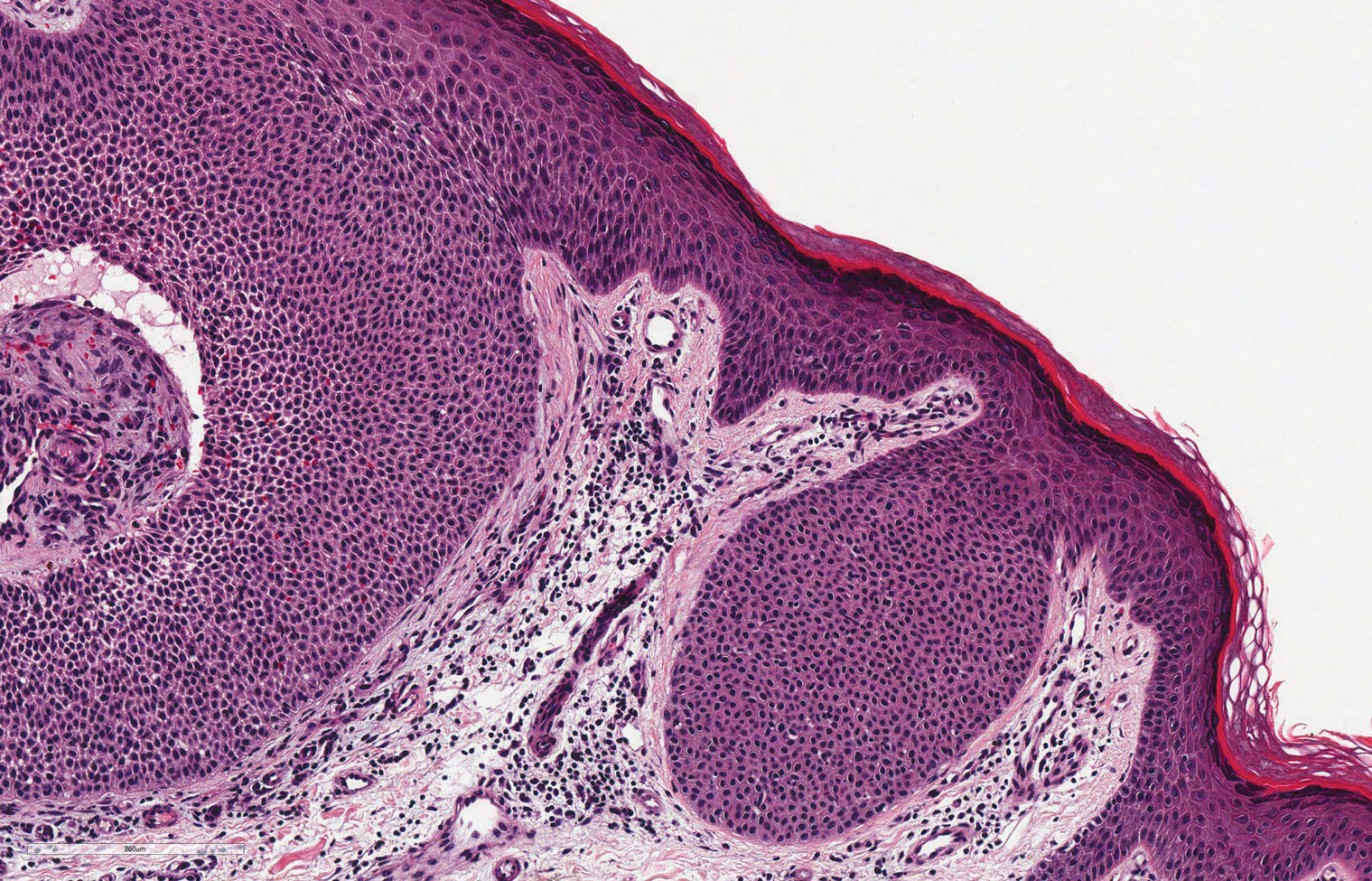
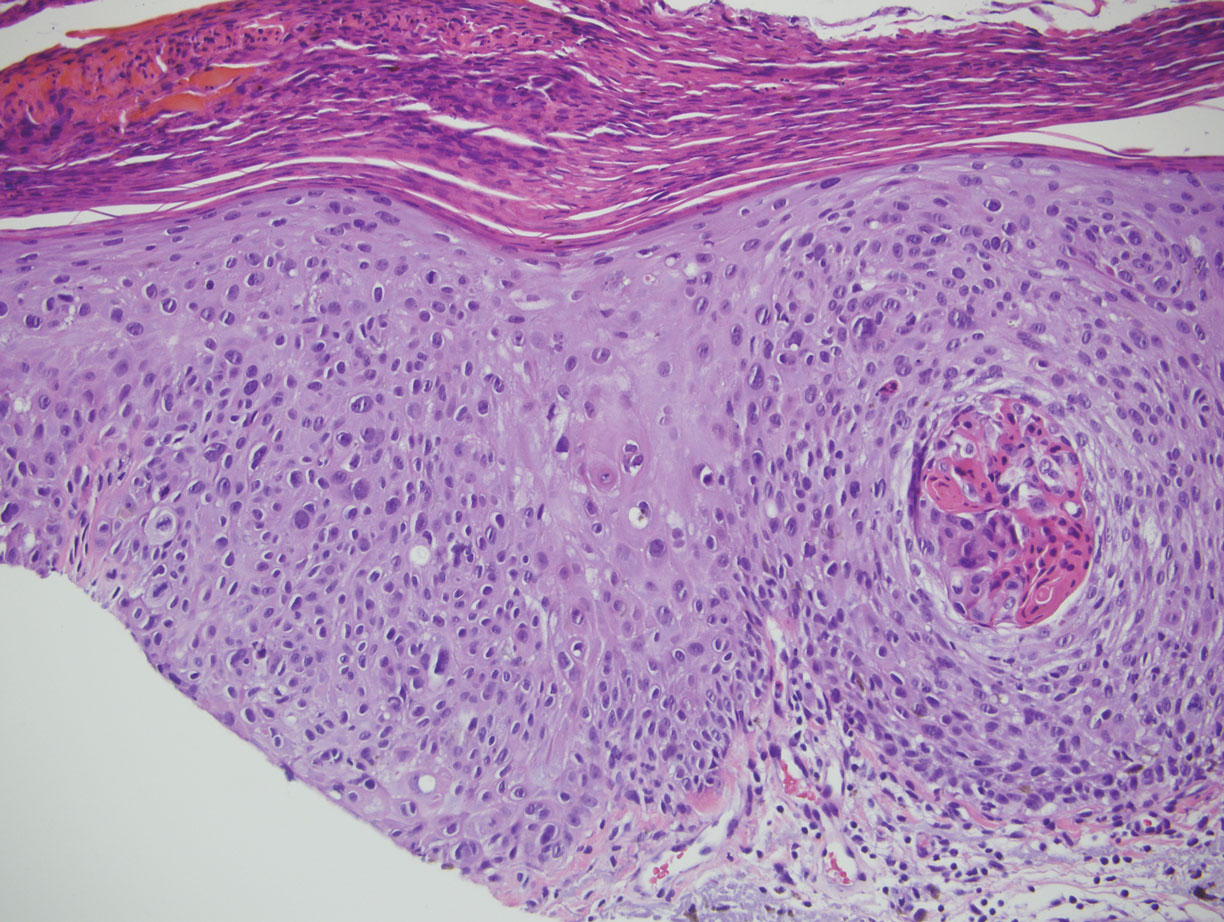
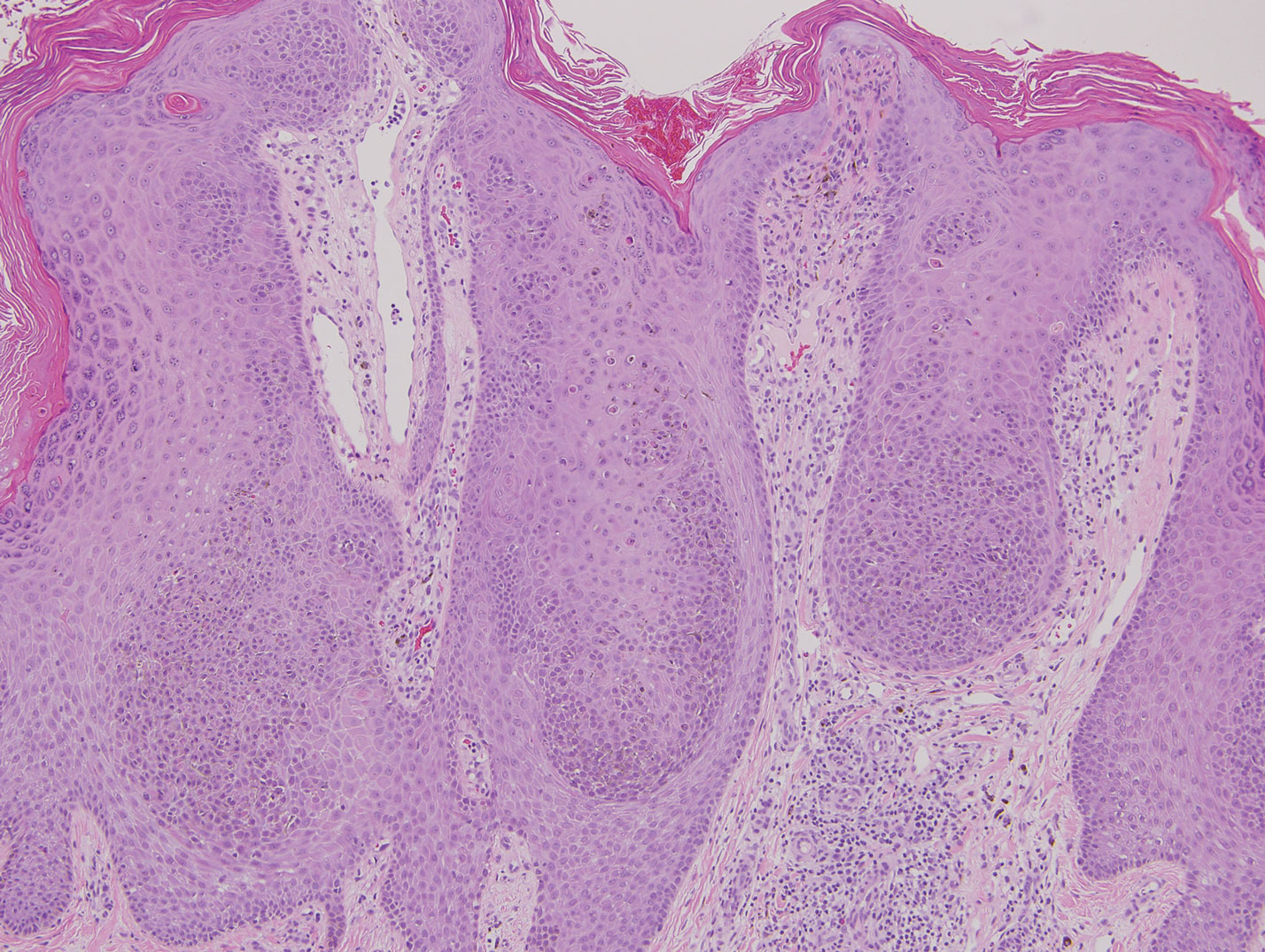
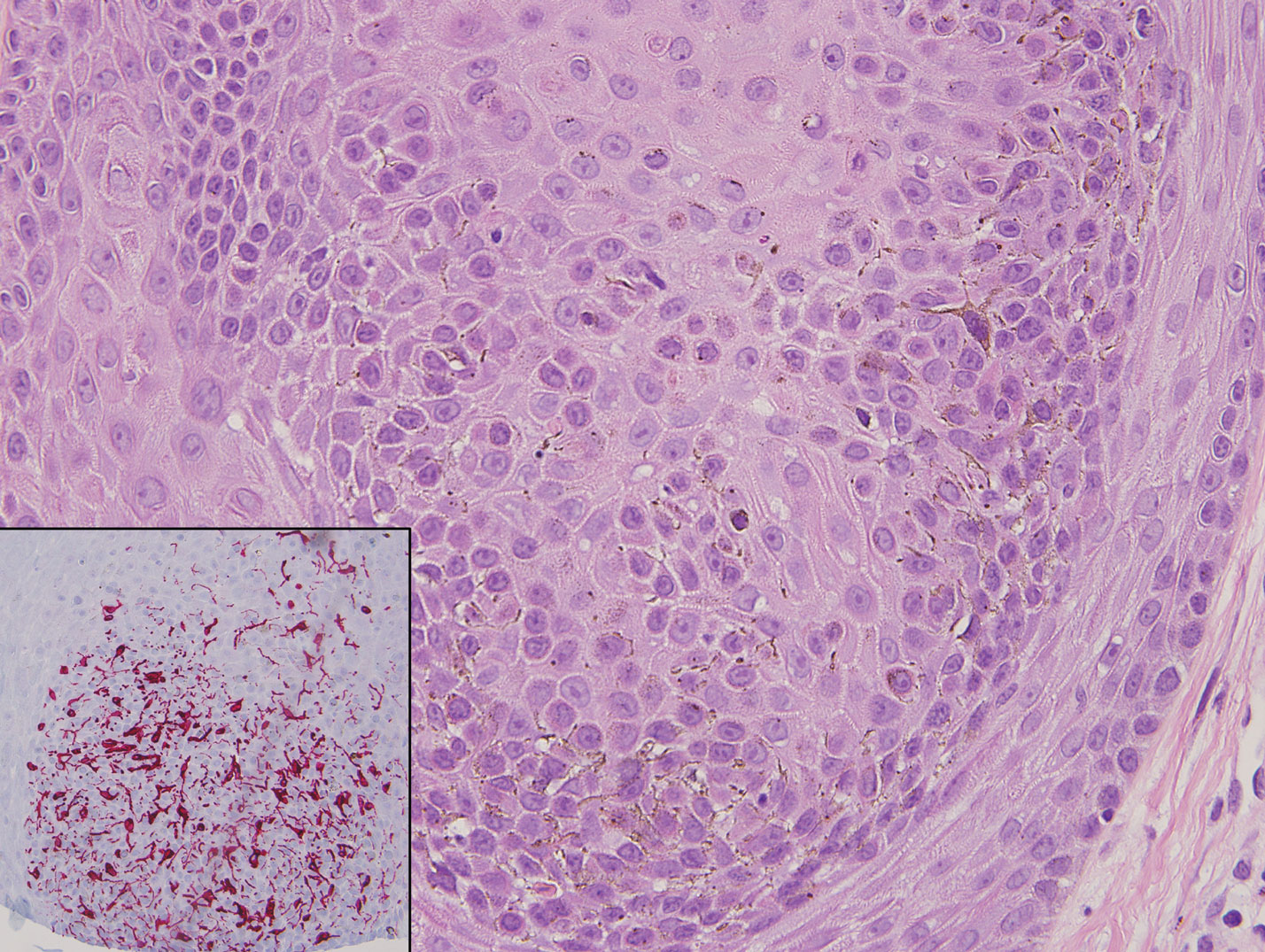
Classic histologic findings of MA include papillomatosis, acanthosis, and hyperkeratosis with heavily pigmented dendritic melanocytes diffusely dispersed throughout all layers of the seborrheic keratosis-like epidermis.3 Other features include keratin-filled pseudocysts, Langerhans cells, reactive spindling of keratinocytes, and an inflammatory infiltrate. In our case, the classic histologic findings also were architecturally arranged in oval to round clones within the epidermis (quiz images 1 and 2). A MART-1 (melanoma antigen recognized by T cells) immunostain was obtained that highlighted the numerous but benign-appearing, dendritic melanocytes (quiz image 2 [inset]). A dual MART-1/Ki67 immunostain later was obtained and demonstrated a negligible proliferation index within the dendritic melanocytes. Therefore, the diagnosis of clonal MA was rendered. This formation of epidermal clones also is called the Borst-Jadassohn phenomenon, which rarely occurs in MAs. This subtype is important to recognize because the clonal pattern can more closely mimic malignant neoplasms such as melanoma.
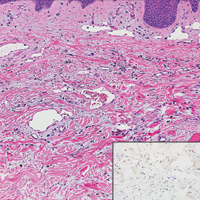
Hidroacanthoma simplex is an intraepidermal variant of eccrine poroma. It is a rare entity that typically occurs in the extremities of women as a hyperkeratotic plaque. These typically clonal epidermal tumors may be heavily pigmented and rarely contain dendritic melanocytes; therefore, they may be confused with MA. However, classic histology will reveal an intraepidermal clonal proliferation of bland, monotonous, cuboidal cells with ample pink cytoplasm, as well as occasional cuticle-lined ducts (Figure 1).4 These ducts will highlight with carcinoembryonic antigen and epithelial membrane antigen immunostaining.
Malignant melanoma typically presents as a growing pigmented lesion and therefore can clinically mimic MA. Histologically, MA could be confused with melanoma due to the increased number of melanocytes plus the appearance of pagetoid spread resulting from the diffuse presence of melanocytes throughout the neoplasm. However, histologic assessment of melanoma should reveal cytologic atypia such as nuclear enlargement, hyperchromasia, molding, pleomorphism, and mitotic activity (Figure 2). Architectural atypia such as poor lateral circumscription of melanocytes, confluence and pagetoid spread of nondendritic atypical junctional melanocytes, production of pigment in deep dermal nests of melanocytes, and lack of maturation and dispersion of dermal melanocytes also should be seen.5 Unlike a melanocytic neoplasm, true melanocytic nests are not seen in MA, and the melanocytes are bland, normal-appearing but heavily pigmented, dendritic melanocytes. Electron microscopy has shown a defect in the transfer of melanin from these highly dendritic melanocytes to the keratinocytes.6

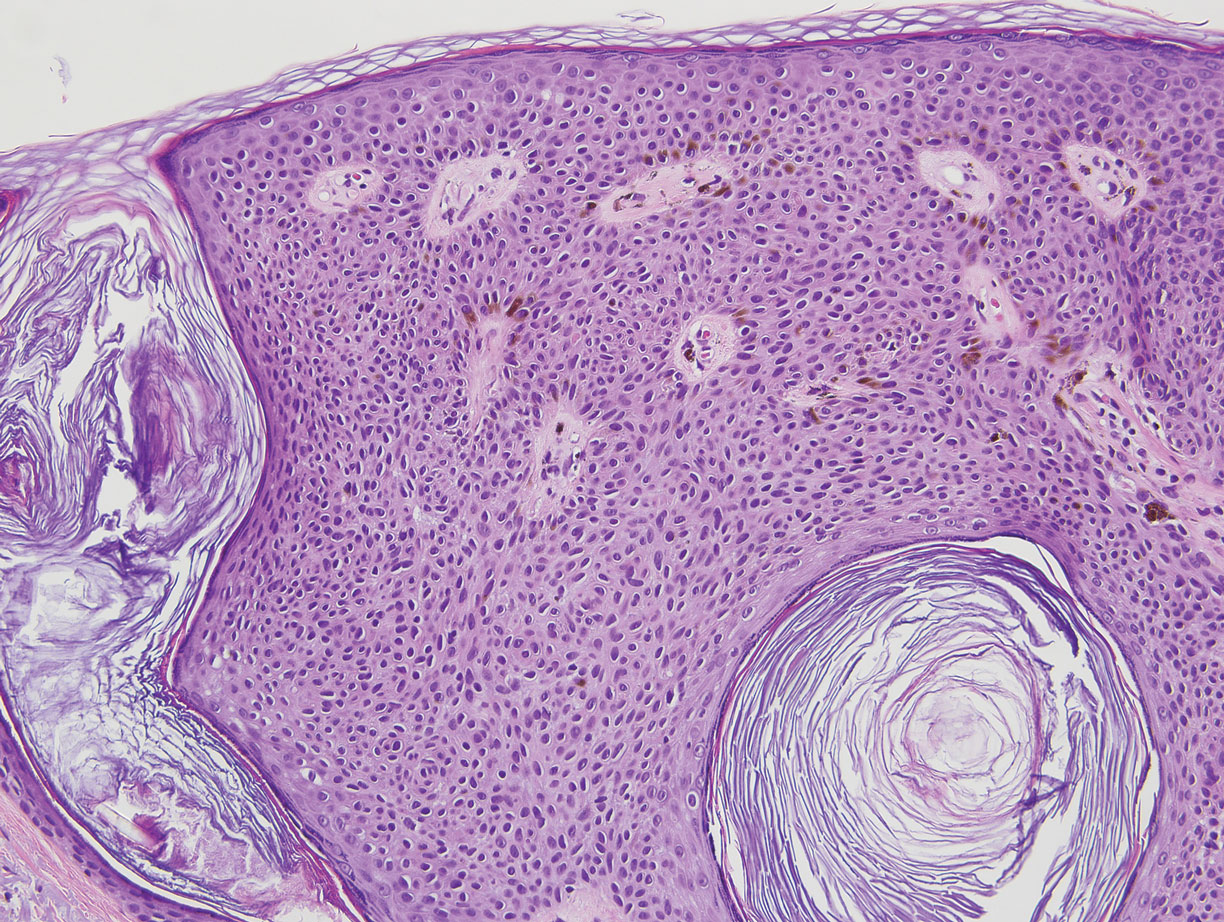
Similar to melanoma, seborrheic keratosis presents as a pigmented growing lesion; therefore, definitive diagnosis often is achieved via skin biopsy. Classic histologic findings include acanthotic or exophytic epidermal growth with a dome-shaped configuration containing multiple cornified hornlike cysts (Figure 3).7 Multiple keratin plugs and variably sized concentric keratin islands are common features. There may be varying degrees of melanin pigment deposition among the proliferating cells, and clonal formation may occur. Melanocyte-specific special stains and immunostains can be used to differentiate MA from seborrheic keratosis by highlighting numerous dendritic melanocytes diffusely spread throughout the epidermis in MA vs a normal distribution of occasional junctional melanocytes in seborrheic keratosis.2,8
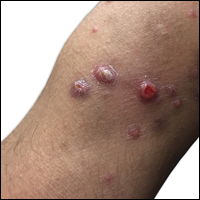
Squamous cell carcinoma in situ presents histologically with cytologically atypical keratinocytes encompassing the full thickness of the epidermis and sometimes crushing the basement membrane zone (Figure 4). There is a loss of the granular layer and overlying parakeratosis that often spares the adnexal ostial epithelium.9 Clonal formation can occur as well as increased pigment production. In comparison, bland keratinocytes are seen in MA.
Establishing the diagnosis of MA based on clinical features alone can be difficult. Dermoscopy can prove to be useful and typically will show a sunburst pattern with ridges and fissures.2 However, seborrheic keratoses and melanomas can have similar dermoscopic findings10; therefore, a biopsy often is necessary to establish the diagnosis.
- Mishima Y, Pinkus H. Benign mixed tumor of melanocytes and malpighian cells: melanoacanthoma: its relationship to Bloch's benign non-nevoid melanoepithelioma. Arch Dermatol. 1960;81:539-550.
- Gutierrez N, Erickson C P, Calame A, et al. Melanoacanthoma masquerading as melanoma: case reports and literature review. Cureus. 2019;11:E4998.
- Fornatora ML, Reich RF, Haber S, et al. Oral melanoacanthoma: a report of 10 cases, review of literature, and immunohistochemical analysis for HMB-45 reactivity. Am J Dermatopathol. 2003;25:12-15.
- Rahbari H. Hidroacanthoma simplex--a review of 15 cases. Br J Dermatol. 1983;109:219-225.
- Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Mod Pathol. 2006;19:S34-S40.
- Mishra DK, Jakati S, Dave TV, et al. A rare pigmented lesion of the eyelid. Int J Trichol. 2019;11:167-169.
- Greco MJ, Mahabadi N, Gossman W. Seborrheic keratosis. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK545285/. Accessed September 18, 2020.
- Kihiczak G, Centurion SA, Schwartz RA, et al. Giant cutaneous melanoacanthoma. Int J Dermatol. 2004;43:936-937.
- Morais P, Schettini A, Junior R. Pigmented squamous cell carcinoma: a case report and importance of differential diagnosis. An Bras Dermatol. 2018;93:96-98.
- Chung E, Marqhoob A, Carrera C, et al. Clinical and dermoscopic features of cutaneous melanoacanthoma. JAMA Dermatol. 2015;151:1129-1130.
The Diagnosis: Clonal Melanoacanthoma
Melanoacanthoma (MA) is an extremely rare, benign, epidermal tumor histologically characterized by keratinocytes and large, pigmented, dendritic melanocytes. These lesions are loosely related to seborrheic keratoses, and the term was first coined by Mishima and Pinkus1 in 1960. It is estimated that the lesion occurs in only 5 of 500,000 individuals and tends to occur in older, light-skinned individuals.2 The majority are slow growing and are present on the head, neck, or upper extremities; however, similar lesions also have been reported on the oral mucosa.3 Melanoacanthomas range in size from 2×2 to 15×15 cm; are clinically pigmented; and present as either a papule, plaque, nodule, or horn.2
Classic histologic findings of MA include papillomatosis, acanthosis, and hyperkeratosis with heavily pigmented dendritic melanocytes diffusely dispersed throughout all layers of the seborrheic keratosis-like epidermis.3 Other features include keratin-filled pseudocysts, Langerhans cells, reactive spindling of keratinocytes, and an inflammatory infiltrate. In our case, the classic histologic findings also were architecturally arranged in oval to round clones within the epidermis (quiz images 1 and 2). A MART-1 (melanoma antigen recognized by T cells) immunostain was obtained that highlighted the numerous but benign-appearing, dendritic melanocytes (quiz image 2 [inset]). A dual MART-1/Ki67 immunostain later was obtained and demonstrated a negligible proliferation index within the dendritic melanocytes. Therefore, the diagnosis of clonal MA was rendered. This formation of epidermal clones also is called the Borst-Jadassohn phenomenon, which rarely occurs in MAs. This subtype is important to recognize because the clonal pattern can more closely mimic malignant neoplasms such as melanoma.
Hidroacanthoma simplex is an intraepidermal variant of eccrine poroma. It is a rare entity that typically occurs in the extremities of women as a hyperkeratotic plaque. These typically clonal epidermal tumors may be heavily pigmented and rarely contain dendritic melanocytes; therefore, they may be confused with MA. However, classic histology will reveal an intraepidermal clonal proliferation of bland, monotonous, cuboidal cells with ample pink cytoplasm, as well as occasional cuticle-lined ducts (Figure 1).4 These ducts will highlight with carcinoembryonic antigen and epithelial membrane antigen immunostaining.
Malignant melanoma typically presents as a growing pigmented lesion and therefore can clinically mimic MA. Histologically, MA could be confused with melanoma due to the increased number of melanocytes plus the appearance of pagetoid spread resulting from the diffuse presence of melanocytes throughout the neoplasm. However, histologic assessment of melanoma should reveal cytologic atypia such as nuclear enlargement, hyperchromasia, molding, pleomorphism, and mitotic activity (Figure 2). Architectural atypia such as poor lateral circumscription of melanocytes, confluence and pagetoid spread of nondendritic atypical junctional melanocytes, production of pigment in deep dermal nests of melanocytes, and lack of maturation and dispersion of dermal melanocytes also should be seen.5 Unlike a melanocytic neoplasm, true melanocytic nests are not seen in MA, and the melanocytes are bland, normal-appearing but heavily pigmented, dendritic melanocytes. Electron microscopy has shown a defect in the transfer of melanin from these highly dendritic melanocytes to the keratinocytes.6


Similar to melanoma, seborrheic keratosis presents as a pigmented growing lesion; therefore, definitive diagnosis often is achieved via skin biopsy. Classic histologic findings include acanthotic or exophytic epidermal growth with a dome-shaped configuration containing multiple cornified hornlike cysts (Figure 3).7 Multiple keratin plugs and variably sized concentric keratin islands are common features. There may be varying degrees of melanin pigment deposition among the proliferating cells, and clonal formation may occur. Melanocyte-specific special stains and immunostains can be used to differentiate MA from seborrheic keratosis by highlighting numerous dendritic melanocytes diffusely spread throughout the epidermis in MA vs a normal distribution of occasional junctional melanocytes in seborrheic keratosis.2,8

Squamous cell carcinoma in situ presents histologically with cytologically atypical keratinocytes encompassing the full thickness of the epidermis and sometimes crushing the basement membrane zone (Figure 4). There is a loss of the granular layer and overlying parakeratosis that often spares the adnexal ostial epithelium.9 Clonal formation can occur as well as increased pigment production. In comparison, bland keratinocytes are seen in MA.
Establishing the diagnosis of MA based on clinical features alone can be difficult. Dermoscopy can prove to be useful and typically will show a sunburst pattern with ridges and fissures.2 However, seborrheic keratoses and melanomas can have similar dermoscopic findings10; therefore, a biopsy often is necessary to establish the diagnosis.

The Diagnosis: Clonal Melanoacanthoma
Melanoacanthoma (MA) is an extremely rare, benign, epidermal tumor histologically characterized by keratinocytes and large, pigmented, dendritic melanocytes. These lesions are loosely related to seborrheic keratoses, and the term was first coined by Mishima and Pinkus1 in 1960. It is estimated that the lesion occurs in only 5 of 500,000 individuals and tends to occur in older, light-skinned individuals.2 The majority are slow growing and are present on the head, neck, or upper extremities; however, similar lesions also have been reported on the oral mucosa.3 Melanoacanthomas range in size from 2×2 to 15×15 cm; are clinically pigmented; and present as either a papule, plaque, nodule, or horn.2
Classic histologic findings of MA include papillomatosis, acanthosis, and hyperkeratosis with heavily pigmented dendritic melanocytes diffusely dispersed throughout all layers of the seborrheic keratosis-like epidermis.3 Other features include keratin-filled pseudocysts, Langerhans cells, reactive spindling of keratinocytes, and an inflammatory infiltrate. In our case, the classic histologic findings also were architecturally arranged in oval to round clones within the epidermis (quiz images 1 and 2). A MART-1 (melanoma antigen recognized by T cells) immunostain was obtained that highlighted the numerous but benign-appearing, dendritic melanocytes (quiz image 2 [inset]). A dual MART-1/Ki67 immunostain later was obtained and demonstrated a negligible proliferation index within the dendritic melanocytes. Therefore, the diagnosis of clonal MA was rendered. This formation of epidermal clones also is called the Borst-Jadassohn phenomenon, which rarely occurs in MAs. This subtype is important to recognize because the clonal pattern can more closely mimic malignant neoplasms such as melanoma.
Hidroacanthoma simplex is an intraepidermal variant of eccrine poroma. It is a rare entity that typically occurs in the extremities of women as a hyperkeratotic plaque. These typically clonal epidermal tumors may be heavily pigmented and rarely contain dendritic melanocytes; therefore, they may be confused with MA. However, classic histology will reveal an intraepidermal clonal proliferation of bland, monotonous, cuboidal cells with ample pink cytoplasm, as well as occasional cuticle-lined ducts (Figure 1).4 These ducts will highlight with carcinoembryonic antigen and epithelial membrane antigen immunostaining.
Malignant melanoma typically presents as a growing pigmented lesion and therefore can clinically mimic MA. Histologically, MA could be confused with melanoma due to the increased number of melanocytes plus the appearance of pagetoid spread resulting from the diffuse presence of melanocytes throughout the neoplasm. However, histologic assessment of melanoma should reveal cytologic atypia such as nuclear enlargement, hyperchromasia, molding, pleomorphism, and mitotic activity (Figure 2). Architectural atypia such as poor lateral circumscription of melanocytes, confluence and pagetoid spread of nondendritic atypical junctional melanocytes, production of pigment in deep dermal nests of melanocytes, and lack of maturation and dispersion of dermal melanocytes also should be seen.5 Unlike a melanocytic neoplasm, true melanocytic nests are not seen in MA, and the melanocytes are bland, normal-appearing but heavily pigmented, dendritic melanocytes. Electron microscopy has shown a defect in the transfer of melanin from these highly dendritic melanocytes to the keratinocytes.6


Similar to melanoma, seborrheic keratosis presents as a pigmented growing lesion; therefore, definitive diagnosis often is achieved via skin biopsy. Classic histologic findings include acanthotic or exophytic epidermal growth with a dome-shaped configuration containing multiple cornified hornlike cysts (Figure 3).7 Multiple keratin plugs and variably sized concentric keratin islands are common features. There may be varying degrees of melanin pigment deposition among the proliferating cells, and clonal formation may occur. Melanocyte-specific special stains and immunostains can be used to differentiate MA from seborrheic keratosis by highlighting numerous dendritic melanocytes diffusely spread throughout the epidermis in MA vs a normal distribution of occasional junctional melanocytes in seborrheic keratosis.2,8

Squamous cell carcinoma in situ presents histologically with cytologically atypical keratinocytes encompassing the full thickness of the epidermis and sometimes crushing the basement membrane zone (Figure 4). There is a loss of the granular layer and overlying parakeratosis that often spares the adnexal ostial epithelium.9 Clonal formation can occur as well as increased pigment production. In comparison, bland keratinocytes are seen in MA.
Establishing the diagnosis of MA based on clinical features alone can be difficult. Dermoscopy can prove to be useful and typically will show a sunburst pattern with ridges and fissures.2 However, seborrheic keratoses and melanomas can have similar dermoscopic findings10; therefore, a biopsy often is necessary to establish the diagnosis.

- Mishima Y, Pinkus H. Benign mixed tumor of melanocytes and malpighian cells: melanoacanthoma: its relationship to Bloch's benign non-nevoid melanoepithelioma. Arch Dermatol. 1960;81:539-550.
- Gutierrez N, Erickson C P, Calame A, et al. Melanoacanthoma masquerading as melanoma: case reports and literature review. Cureus. 2019;11:E4998.
- Fornatora ML, Reich RF, Haber S, et al. Oral melanoacanthoma: a report of 10 cases, review of literature, and immunohistochemical analysis for HMB-45 reactivity. Am J Dermatopathol. 2003;25:12-15.
- Rahbari H. Hidroacanthoma simplex--a review of 15 cases. Br J Dermatol. 1983;109:219-225.
- Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Mod Pathol. 2006;19:S34-S40.
- Mishra DK, Jakati S, Dave TV, et al. A rare pigmented lesion of the eyelid. Int J Trichol. 2019;11:167-169.
- Greco MJ, Mahabadi N, Gossman W. Seborrheic keratosis. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK545285/. Accessed September 18, 2020.
- Kihiczak G, Centurion SA, Schwartz RA, et al. Giant cutaneous melanoacanthoma. Int J Dermatol. 2004;43:936-937.
- Morais P, Schettini A, Junior R. Pigmented squamous cell carcinoma: a case report and importance of differential diagnosis. An Bras Dermatol. 2018;93:96-98.
- Chung E, Marqhoob A, Carrera C, et al. Clinical and dermoscopic features of cutaneous melanoacanthoma. JAMA Dermatol. 2015;151:1129-1130.
- Mishima Y, Pinkus H. Benign mixed tumor of melanocytes and malpighian cells: melanoacanthoma: its relationship to Bloch's benign non-nevoid melanoepithelioma. Arch Dermatol. 1960;81:539-550.
- Gutierrez N, Erickson C P, Calame A, et al. Melanoacanthoma masquerading as melanoma: case reports and literature review. Cureus. 2019;11:E4998.
- Fornatora ML, Reich RF, Haber S, et al. Oral melanoacanthoma: a report of 10 cases, review of literature, and immunohistochemical analysis for HMB-45 reactivity. Am J Dermatopathol. 2003;25:12-15.
- Rahbari H. Hidroacanthoma simplex--a review of 15 cases. Br J Dermatol. 1983;109:219-225.
- Smoller BR. Histologic criteria for diagnosing primary cutaneous malignant melanoma. Mod Pathol. 2006;19:S34-S40.
- Mishra DK, Jakati S, Dave TV, et al. A rare pigmented lesion of the eyelid. Int J Trichol. 2019;11:167-169.
- Greco MJ, Mahabadi N, Gossman W. Seborrheic keratosis. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK545285/. Accessed September 18, 2020.
- Kihiczak G, Centurion SA, Schwartz RA, et al. Giant cutaneous melanoacanthoma. Int J Dermatol. 2004;43:936-937.
- Morais P, Schettini A, Junior R. Pigmented squamous cell carcinoma: a case report and importance of differential diagnosis. An Bras Dermatol. 2018;93:96-98.
- Chung E, Marqhoob A, Carrera C, et al. Clinical and dermoscopic features of cutaneous melanoacanthoma. JAMA Dermatol. 2015;151:1129-1130.
A 49-year-old man with light brown skin and no history of skin cancer presented with a pruritic lesion on the scalp of 3 years’ duration. Physical examination revealed a 7×3-cm, brown, mammillated plaque on the left parietal scalp. A shave biopsy of the scalp lesion was performed.
Risk Factors and Management of Skin Cancer Among Active-Duty Servicemembers and Veterans
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
Streaked Discoloration on the Upper Body
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.

Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.

Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
The Diagnosis: Bleomycin-Induced Flagellate Hyperpigmentation
Histopathology of the affected skin demonstrated a slight increase in collagen bundle thickness, a chronic dermal perivascular inflammation, and associated pigment incontinence with dermal melanophages compared to unaffected skin (Figure). CD34 was faintly decreased, and dermal mucin increased in affected skin. This postinflammatory pigmentary alteration with subtle dermal sclerosis had persisted unchanged for more than 5 years after cessation of bleomycin therapy. Topical hydroquinone, physical blocker photoprotection, and laser modalities such as the Q-switched alexandrite (755-nm)/Nd:YAG (1064-nm) and ablative CO2 resurfacing lasers were attempted with minimal overall impact on cosmesis.

Bleomycin is a chemotherapeutic antibiotic that has been commonly used to treat Hodgkin lymphoma, germ cell tumors, and recurrent malignant pleural effusions.1 The drug is inactivated in most tissues by the enzyme bleomycin hydrolase. This enzyme is not present in skin and lung tissue; as a result, these organs are the most common sites of bleomycin toxicity.1 There are a variety of cutaneous effects associated with bleomycin including alopecia, hyperpigmentation, acral erythema, Raynaud phenomenon, and nail dystrophy.2 Flagellate hyperpigmentation is a less common cutaneous toxicity. It is an unusual eruption that appears as whiplike linear streaks on the upper chest and back, limbs, and flanks.3 This cutaneous manifestation was once thought to be specific to bleomycin use; however, it also has been described in dermatomyositis, adult-onset Still disease, and after the ingestion of uncooked or undercooked shiitake mushrooms.4 Flagellate hyperpigmentation also was once thought to be dose dependent; however, it has been described in even very small doses.5 The eruption has been described as independent of the route of drug administration, appearing with intravenous, subcutaneous, and intramuscular bleomycin.2 The association of bleomycin and flagellate hyperpigmentation has been reported since 1970; however, it is less commonly seen in clinical practice with the declining use of bleomycin.1
The exact mechanism for the hyperpigmentation is unknown. It has been proposed that the linear lesions are related to areas of pruritus and subsequent excoriations.1 Dermatographism may be present to a limited extent, but it is unlikely to be a chief cause of flagellate hyperpigmentation, as linear streaks have been reported in the absence of trauma. It also has been proposed that bleomycin has a direct toxic effect on the melanocytes, which stimulates increased melanin secretion.2 The hyperpigmentation also may be due to pigmentary incontinence secondary to inflammation.5 Histopathologic findings usually are varied and nonspecific.2 There may be a deep perivascular lymphocytic infiltrate, which is nonspecific but can be associated with drug-induced pathology.4 Bleomycin also is used to induce localized scleroderma in mouse-model research6 and has been reported to cause localized scleroderma at an infusion site or after an intralesional injection,7,8 which is not typically reported in flagellate erythema, but bleomycin's sclerosing effects may have played a role in the visible and sclerosing atrophy noted in our patient. Yamamoto et al9 reported a similar case of dermal sclerosis induced by bleomycin.
Flagellate hyperpigmentation typically lasts for up to 6 months.3 Patients with cutaneous manifestations from bleomycin therapy usually respond to steroid therapy and discontinuation of the drug. Bleomycin re-exposure should be avoided, as it may cause extension or widespread recurrence of flagellate hyperpigmentation.3 Postinflammatory pigment alteration may persist in patients with darker skin types and in patients with dramatic inciting inflammation.
Atrophoderma of Pasini and Pierini is a form of dermal atrophy that presents with 1 or more sharply demarcated depressed patches. There is some debate whether it is a distinct entity or a primary atrophic morphea.10 Linear atrophoderma of Moulin has a similar morphology with hyperpigmented depressions and "cliff-drop" borders, but these lesions follow the lines of Blaschko.11 Linear morphea initially can present as a linear erythematous streak but more commonly appears as a plaque-type morphea lesion that forms a scarlike band.12 Erythema dyschromicum perstans is an ashy dermatosis characterized by gray or blue-brown macules seen in Fitzpatrick skin types III through V and typically is chronic and progressive.13
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.
- Lee HY, Lim KH, Ryu Y, et al. Bleomycininduced flagellate erythema: a case report and review of the literature. Oncol Lett. 2014;8:933-935.
- Simpson RC, Da Forno P, Nagarajan C, et al. A pruritic rash in a patient with Hodgkin lymphoma. Clin Exp Dermatol. 2011;36:680-682.
- Fyfe AJ, McKay P. Toxicities associated with bleomycin. J R Coll Physicians Edinb. 2010;40:213-215.
- Lu CC, Lu YY, Wang QR, et al. Bleomycin-induced flagellate erythema. Balkan Med J. 2014;31:189-190.
- Abess A, Keel DM, Graham BS. Flagellate hyperpigmentation following intralesional bleomycin treatment of verruca plantaris. Arch Dermatol. 2003;139:337-339.
- Yamamoto T. The bleomycin-induced scleroderma model: what have we learned for scleroderma pathogenesis? Arch Dermatol Res. 2006;297:333-344.
- Kim KH, Yoon TJ, Oh CW, et al. A case of bleomycin-induced scleroderma. J Korean Med Sci. 1996;11:454-456.
- Kerr LD, Spiera H. Scleroderma in association with the use of bleomycin: a report of 3 cases. J Rheumatol. 1992;19:294-296.
- Yamamoto T, Yokozeki H, Nishioka K. Dermal sclerosis in the lesional skin of 'flagellate' erythema (scratch dermatitis) induced by bleomycin. Dermatology. 1998;197:399-400.
- Kencka D, Blaszczyk M, Jablońska S. Atrophoderma Pasini-Pierini is a primary atrophic abortive morphea. Dermatology. 1995;190:203-206.
- Moulin G, Hill MP, Guillaud V, et al. Acquired atrophic pigmented band-like lesions following Blaschko's lines. Ann Dermatol Venereol. 1992;119:729-736.
- Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
- Zaynoun S, Rubeiz N, Kibbi AG. Ashy dermatosis--a critical review of literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542-544.

An 18-year-old woman presented to our dermatology clinic with persistent diffuse discoloration on the upper body of more than 5 years’ duration. Her medical history was notable for primary mediastinal classical Hodgkin lymphoma treated with ABVE-PC (doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide) chemotherapy and 22 Gy radiation therapy to the chest 5 years prior. She reported the initial onset of diffuse pruritus with associated scratching and persistent skin discoloration while receiving a course of chemotherapy. Physical examination revealed numerous thin, flagellate, faintly hyperpigmented streaks with subtle atrophy in a parallel configuration on the bilateral shoulders (top), upper back (bottom), and abdomen. Punch biopsies (5 mm) of both affected and unaffected skin on the left side of the lateral upper back were performed.
Acute-Onset Alopecia
The Diagnosis: Thallium-Induced Alopecia
At the time of presentation, a punch biopsy specimen of the scalp revealed nonscarring alopecia with increased catagen hairs; follicular miniaturization; peribulbar lymphoid infiltrates; and fibrous tract remnants containing melanin, lymphocytes, and occasional mast cells (Figure 1). The differential diagnosis included alopecia areata, syphilis, and toxin-mediated anagen effluvium (AE). Given the abrupt onset affecting multiple individuals in an industrial environment, heavy metal poisoning was suspected. Blood and urine testing was negative, but a few months had elapsed since exposure. Several months after his initial presentation, the patient reported problems with his teeth, thin brittle nails, and resolution of the visual changes. Photographs sent by the patient revealed darkening and degeneration of the gingival margin (Figure 2).
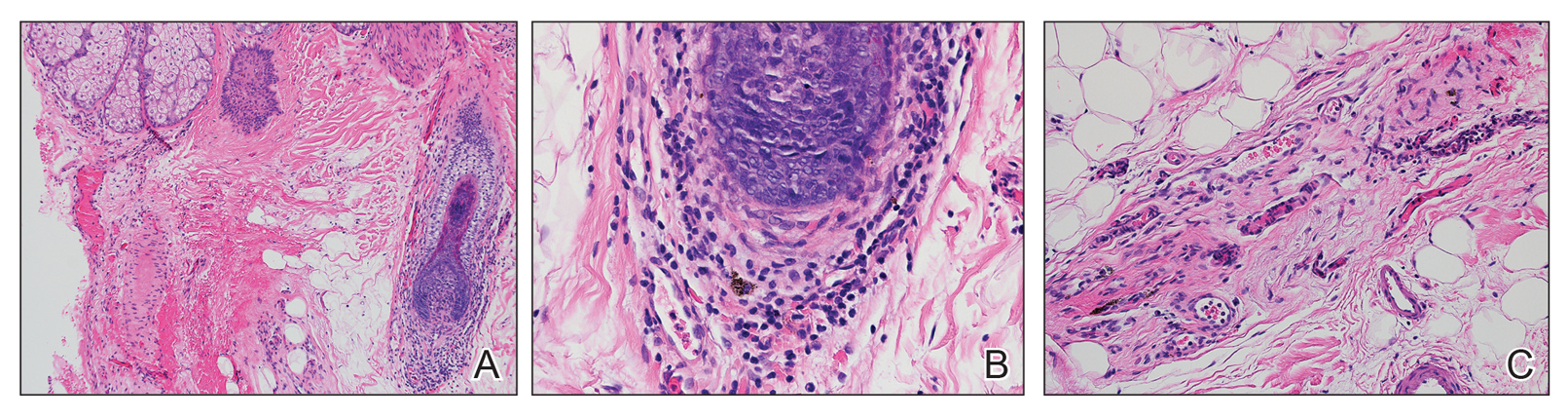
Environmental review revealed the patient was working on a demolition site of a 150-year-old electrical plant near a river. Inundation of rainfall caused a river swell and subsequent flooding of the work site. The patient reported working for more than 2 months in knee-deep muddy water, and he noted that water for consumption and showers was procured on-site from a well-based source that may have been contaminated by the floodwaters.
Acute nonscarring alopecia can be an AE or telogen effluvium (TE), also known as telogen defluvium. The key distinguishing factor is the mode of injury.1 In TE, medications, stress, hormonal shifts, or inflammation induce a synchronized and abrupt transition of hairs from anagen phase to catagen phase, a committed step that then must fully cycle through the telogen phase, culminating in the simultaneous shedding of numerous telogen hairs approximately 3 to 4 months later. Conversely, AE is caused by a sudden insult to the metabolic machinery of the hair matrix. Affected follicles rapidly produce thinner weaker shafts yielding Pohl-Pinkus constrictions or pencil point-shaped fractures that shed approximately 1 to 2 months after injury. The 10% of scalp hairs in the resting telogen phase have no matrix and thus are unaffected. Some etiologies can cause either AE or TE, depending on the dose and intensity of the insult. Common causes of AE include alopecia areata and syphilis, both consisting of abrupt severe bulbar inflammation.1 Other causes include chemotherapy, particularly antimetabolites, alkylating agents, and mitotic inhibitors; radiation; medications (eg, isoniazid); severe protein malnutrition; toxic chemicals (eg, boron/boric acid); and heavy metals (eg, thallium, mercury).
Thallium is one of the most common causes of heavy metal poisoning and is particularly dangerous due to its colorless, tasteless, and odorless characteristics. Although its common use as a rodenticide has dramatically decreased in the United States after it was banned in 1965, it is still used in this fashion in other countries and has a notable industrial presence, particularly in electronics, superconductors, and low-temperature thermometers. Accidental poisoning of a graduate chemistry student during copper research has been reported,2 highlighting that thallium can be inhaled, ingested, or absorbed through the skin. Thallium is even present in mycoplasma agar plates, the ingestion of which has resulted in poisoning.3
Systemic symptoms of thallium poisoning include somnolence, weakness, nausea, vomiting, stomatitis, abdominal pain, diarrhea, tachycardia, hypertension, and polyneuropathy.4-7 Neuropathy often manifests as painful acral dysesthesia and paresthesia, perioral numbness, optic neuropathy causing visual changes, and encephalopathy. Cutaneous findings include diffuse alopecia of the scalp and eyebrows, perioral dermatitis, glossitis, diffuse hyperpigmentation, oral hyperpigmentation (often as a stippled lead line along the gingival margin with subsequent alveolar damage and resorption), melanonychia, palmoplantar keratoderma, acneform or pustular eruption, and nail changes including Mees lines.2,4,5,7-9 Rarely, major organ failure and death may result.10
Toxin panels may not include thallium, and urine and serum tests may be negative if too much time has transpired since the acute exposure. Hair or nail analysis has proved useful in subacute cases11; however, most laboratories require a pencil-thick segment of hair cut at the roots and bundled, weighing at least 500 mg. Thallium poisoning is treated with activated charcoal, Prussian blue, and blood purification therapies (eg, hemodialysis, hemoperfusion, hemofiltration).4,7 Cutaneous findings typically resolve, but neuropathic changes may persist.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology With Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Campbell C, Bahrami S, Owen C. Anagen effluvium caused by thallium poisoning. JAMA Dermatol. 2016;152:724-726.
- Puschner B, Basso MM. Graham TW. Thallium toxicosis in a dog consequent to ingestion of Mycoplasma agar plates. J Vet Diagn Invest. 2012;24:227-230.
- Sojáková M, Zigrai M, Karaman A, et al. Thallium intoxication: case report. Neuro Endocrinol Lett. 2015;36:311-315.
- Lu Cl, Huang CC, Chang YC, et al. Short-term thallium intoxication: dermatological findings correlated with thallium concentration. Arch Dermatol. 2007;143:93-98.
- Liu EM, Rajagopal R, Grand MG. Optic nerve atrophy and hair loss in a young man. JAMA Ophthalmol. 2015;133:1469-1470.
- Zhang HT, Qiao BP, Liu BP, et al. Study on the treatment of acute thallium poisoning. Am J Med Sci. 2014;347:377-381.
- Misra UK, Kalita J, Yadav RK, et al. Thallium poisoning: emphasis on early diagnosis and response to haemodialysis. Postgrad Med J. 2003;79:103-105.
- Tromme I, Van Neste D, Dobbelaere F, et al. Skin signs in the diagnosis of thallium poisoning. Br J Dermatol. 1998;138:321-325.
- Li S, Huang W, Duan Y, et al. Human fatality due to thallium poisoning: autopsy, microscopy, and mass spectrometry assays. J Forensic Sci. 2015;60:247-251.
- Daniel CR 3rd, Piraccini BM, Tosti A. The nail and hair in forensic science. J Am Acad Dermatol. 2004;50:258-261.
The Diagnosis: Thallium-Induced Alopecia
At the time of presentation, a punch biopsy specimen of the scalp revealed nonscarring alopecia with increased catagen hairs; follicular miniaturization; peribulbar lymphoid infiltrates; and fibrous tract remnants containing melanin, lymphocytes, and occasional mast cells (Figure 1). The differential diagnosis included alopecia areata, syphilis, and toxin-mediated anagen effluvium (AE). Given the abrupt onset affecting multiple individuals in an industrial environment, heavy metal poisoning was suspected. Blood and urine testing was negative, but a few months had elapsed since exposure. Several months after his initial presentation, the patient reported problems with his teeth, thin brittle nails, and resolution of the visual changes. Photographs sent by the patient revealed darkening and degeneration of the gingival margin (Figure 2).


Environmental review revealed the patient was working on a demolition site of a 150-year-old electrical plant near a river. Inundation of rainfall caused a river swell and subsequent flooding of the work site. The patient reported working for more than 2 months in knee-deep muddy water, and he noted that water for consumption and showers was procured on-site from a well-based source that may have been contaminated by the floodwaters.
Acute nonscarring alopecia can be an AE or telogen effluvium (TE), also known as telogen defluvium. The key distinguishing factor is the mode of injury.1 In TE, medications, stress, hormonal shifts, or inflammation induce a synchronized and abrupt transition of hairs from anagen phase to catagen phase, a committed step that then must fully cycle through the telogen phase, culminating in the simultaneous shedding of numerous telogen hairs approximately 3 to 4 months later. Conversely, AE is caused by a sudden insult to the metabolic machinery of the hair matrix. Affected follicles rapidly produce thinner weaker shafts yielding Pohl-Pinkus constrictions or pencil point-shaped fractures that shed approximately 1 to 2 months after injury. The 10% of scalp hairs in the resting telogen phase have no matrix and thus are unaffected. Some etiologies can cause either AE or TE, depending on the dose and intensity of the insult. Common causes of AE include alopecia areata and syphilis, both consisting of abrupt severe bulbar inflammation.1 Other causes include chemotherapy, particularly antimetabolites, alkylating agents, and mitotic inhibitors; radiation; medications (eg, isoniazid); severe protein malnutrition; toxic chemicals (eg, boron/boric acid); and heavy metals (eg, thallium, mercury).
Thallium is one of the most common causes of heavy metal poisoning and is particularly dangerous due to its colorless, tasteless, and odorless characteristics. Although its common use as a rodenticide has dramatically decreased in the United States after it was banned in 1965, it is still used in this fashion in other countries and has a notable industrial presence, particularly in electronics, superconductors, and low-temperature thermometers. Accidental poisoning of a graduate chemistry student during copper research has been reported,2 highlighting that thallium can be inhaled, ingested, or absorbed through the skin. Thallium is even present in mycoplasma agar plates, the ingestion of which has resulted in poisoning.3
Systemic symptoms of thallium poisoning include somnolence, weakness, nausea, vomiting, stomatitis, abdominal pain, diarrhea, tachycardia, hypertension, and polyneuropathy.4-7 Neuropathy often manifests as painful acral dysesthesia and paresthesia, perioral numbness, optic neuropathy causing visual changes, and encephalopathy. Cutaneous findings include diffuse alopecia of the scalp and eyebrows, perioral dermatitis, glossitis, diffuse hyperpigmentation, oral hyperpigmentation (often as a stippled lead line along the gingival margin with subsequent alveolar damage and resorption), melanonychia, palmoplantar keratoderma, acneform or pustular eruption, and nail changes including Mees lines.2,4,5,7-9 Rarely, major organ failure and death may result.10
Toxin panels may not include thallium, and urine and serum tests may be negative if too much time has transpired since the acute exposure. Hair or nail analysis has proved useful in subacute cases11; however, most laboratories require a pencil-thick segment of hair cut at the roots and bundled, weighing at least 500 mg. Thallium poisoning is treated with activated charcoal, Prussian blue, and blood purification therapies (eg, hemodialysis, hemoperfusion, hemofiltration).4,7 Cutaneous findings typically resolve, but neuropathic changes may persist.
The Diagnosis: Thallium-Induced Alopecia
At the time of presentation, a punch biopsy specimen of the scalp revealed nonscarring alopecia with increased catagen hairs; follicular miniaturization; peribulbar lymphoid infiltrates; and fibrous tract remnants containing melanin, lymphocytes, and occasional mast cells (Figure 1). The differential diagnosis included alopecia areata, syphilis, and toxin-mediated anagen effluvium (AE). Given the abrupt onset affecting multiple individuals in an industrial environment, heavy metal poisoning was suspected. Blood and urine testing was negative, but a few months had elapsed since exposure. Several months after his initial presentation, the patient reported problems with his teeth, thin brittle nails, and resolution of the visual changes. Photographs sent by the patient revealed darkening and degeneration of the gingival margin (Figure 2).


Environmental review revealed the patient was working on a demolition site of a 150-year-old electrical plant near a river. Inundation of rainfall caused a river swell and subsequent flooding of the work site. The patient reported working for more than 2 months in knee-deep muddy water, and he noted that water for consumption and showers was procured on-site from a well-based source that may have been contaminated by the floodwaters.
Acute nonscarring alopecia can be an AE or telogen effluvium (TE), also known as telogen defluvium. The key distinguishing factor is the mode of injury.1 In TE, medications, stress, hormonal shifts, or inflammation induce a synchronized and abrupt transition of hairs from anagen phase to catagen phase, a committed step that then must fully cycle through the telogen phase, culminating in the simultaneous shedding of numerous telogen hairs approximately 3 to 4 months later. Conversely, AE is caused by a sudden insult to the metabolic machinery of the hair matrix. Affected follicles rapidly produce thinner weaker shafts yielding Pohl-Pinkus constrictions or pencil point-shaped fractures that shed approximately 1 to 2 months after injury. The 10% of scalp hairs in the resting telogen phase have no matrix and thus are unaffected. Some etiologies can cause either AE or TE, depending on the dose and intensity of the insult. Common causes of AE include alopecia areata and syphilis, both consisting of abrupt severe bulbar inflammation.1 Other causes include chemotherapy, particularly antimetabolites, alkylating agents, and mitotic inhibitors; radiation; medications (eg, isoniazid); severe protein malnutrition; toxic chemicals (eg, boron/boric acid); and heavy metals (eg, thallium, mercury).
Thallium is one of the most common causes of heavy metal poisoning and is particularly dangerous due to its colorless, tasteless, and odorless characteristics. Although its common use as a rodenticide has dramatically decreased in the United States after it was banned in 1965, it is still used in this fashion in other countries and has a notable industrial presence, particularly in electronics, superconductors, and low-temperature thermometers. Accidental poisoning of a graduate chemistry student during copper research has been reported,2 highlighting that thallium can be inhaled, ingested, or absorbed through the skin. Thallium is even present in mycoplasma agar plates, the ingestion of which has resulted in poisoning.3
Systemic symptoms of thallium poisoning include somnolence, weakness, nausea, vomiting, stomatitis, abdominal pain, diarrhea, tachycardia, hypertension, and polyneuropathy.4-7 Neuropathy often manifests as painful acral dysesthesia and paresthesia, perioral numbness, optic neuropathy causing visual changes, and encephalopathy. Cutaneous findings include diffuse alopecia of the scalp and eyebrows, perioral dermatitis, glossitis, diffuse hyperpigmentation, oral hyperpigmentation (often as a stippled lead line along the gingival margin with subsequent alveolar damage and resorption), melanonychia, palmoplantar keratoderma, acneform or pustular eruption, and nail changes including Mees lines.2,4,5,7-9 Rarely, major organ failure and death may result.10
Toxin panels may not include thallium, and urine and serum tests may be negative if too much time has transpired since the acute exposure. Hair or nail analysis has proved useful in subacute cases11; however, most laboratories require a pencil-thick segment of hair cut at the roots and bundled, weighing at least 500 mg. Thallium poisoning is treated with activated charcoal, Prussian blue, and blood purification therapies (eg, hemodialysis, hemoperfusion, hemofiltration).4,7 Cutaneous findings typically resolve, but neuropathic changes may persist.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology With Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Campbell C, Bahrami S, Owen C. Anagen effluvium caused by thallium poisoning. JAMA Dermatol. 2016;152:724-726.
- Puschner B, Basso MM. Graham TW. Thallium toxicosis in a dog consequent to ingestion of Mycoplasma agar plates. J Vet Diagn Invest. 2012;24:227-230.
- Sojáková M, Zigrai M, Karaman A, et al. Thallium intoxication: case report. Neuro Endocrinol Lett. 2015;36:311-315.
- Lu Cl, Huang CC, Chang YC, et al. Short-term thallium intoxication: dermatological findings correlated with thallium concentration. Arch Dermatol. 2007;143:93-98.
- Liu EM, Rajagopal R, Grand MG. Optic nerve atrophy and hair loss in a young man. JAMA Ophthalmol. 2015;133:1469-1470.
- Zhang HT, Qiao BP, Liu BP, et al. Study on the treatment of acute thallium poisoning. Am J Med Sci. 2014;347:377-381.
- Misra UK, Kalita J, Yadav RK, et al. Thallium poisoning: emphasis on early diagnosis and response to haemodialysis. Postgrad Med J. 2003;79:103-105.
- Tromme I, Van Neste D, Dobbelaere F, et al. Skin signs in the diagnosis of thallium poisoning. Br J Dermatol. 1998;138:321-325.
- Li S, Huang W, Duan Y, et al. Human fatality due to thallium poisoning: autopsy, microscopy, and mass spectrometry assays. J Forensic Sci. 2015;60:247-251.
- Daniel CR 3rd, Piraccini BM, Tosti A. The nail and hair in forensic science. J Am Acad Dermatol. 2004;50:258-261.
- Sperling LC, Cowper SE, Knopp EA. An Atlas of Hair Pathology With Clinical Correlations. 2nd ed. Boca Raton, FL: CRC Press; 2012.
- Campbell C, Bahrami S, Owen C. Anagen effluvium caused by thallium poisoning. JAMA Dermatol. 2016;152:724-726.
- Puschner B, Basso MM. Graham TW. Thallium toxicosis in a dog consequent to ingestion of Mycoplasma agar plates. J Vet Diagn Invest. 2012;24:227-230.
- Sojáková M, Zigrai M, Karaman A, et al. Thallium intoxication: case report. Neuro Endocrinol Lett. 2015;36:311-315.
- Lu Cl, Huang CC, Chang YC, et al. Short-term thallium intoxication: dermatological findings correlated with thallium concentration. Arch Dermatol. 2007;143:93-98.
- Liu EM, Rajagopal R, Grand MG. Optic nerve atrophy and hair loss in a young man. JAMA Ophthalmol. 2015;133:1469-1470.
- Zhang HT, Qiao BP, Liu BP, et al. Study on the treatment of acute thallium poisoning. Am J Med Sci. 2014;347:377-381.
- Misra UK, Kalita J, Yadav RK, et al. Thallium poisoning: emphasis on early diagnosis and response to haemodialysis. Postgrad Med J. 2003;79:103-105.
- Tromme I, Van Neste D, Dobbelaere F, et al. Skin signs in the diagnosis of thallium poisoning. Br J Dermatol. 1998;138:321-325.
- Li S, Huang W, Duan Y, et al. Human fatality due to thallium poisoning: autopsy, microscopy, and mass spectrometry assays. J Forensic Sci. 2015;60:247-251.
- Daniel CR 3rd, Piraccini BM, Tosti A. The nail and hair in forensic science. J Am Acad Dermatol. 2004;50:258-261.

A previously healthy 45-year-old man presented to the dermatology department with abrupt onset of patchy, progressively worsening alopecia of the scalp as well as nausea with emesis and blurry vision of a few weeks' duration. All symptoms were temporally associated with a new demolition job the patient had started at an industrial site. He reported 10 other contractors were similarly affected. The patient denied paresthesia or other skin changes. On physical examination, large patches of smooth alopecia without erythema, scale, scarring, tenderness, or edema that coalesced to involve the majority of the scalp, eyebrows, and eyelashes (inset) were noted.
Acquired Hypertrichosis of the Periorbital Area and Malar Cheek
The Diagnosis: Bimatoprost-Induced Hypertrichosis
Latanoprost, a prostaglandin analogue, typically is prescribed by ophthalmologists as eye drops to reduce intraocular pressure in open-angle glaucoma.1 Common adverse reactions of latanoprost drops include blurred vision, ocular irritation, darkening of the eyelid skin, and pigmentation of the iris.
In 1997, Johnstone2 reported hypertrichosis and increased pigmentation of the eyelashes of both eyes and adjacent skin after latanoprost drops were used in glaucoma patients. Subsequently, topical latanoprost and bimatoprost, a similar analogue, are now utilized for the cosmetic purpose of thickening and lengthening the eyelashes due to the hypertrichosis effect. Travoprost, another prostaglandin analogue used to treat glaucoma, also has been associated with periocular hypertrichosis.3 Concomitant poliosis of the eyelashes with hypertrichosis from latanoprost also has been reported.4 Our patient specifically purchased the eye drops (marketed as generic bimatoprost) to lengthen her eyelashes and had noticed an increase in length. She denied a family history of increased facial hair in females.
Along with gingival hyperplasia, systemic cyclosporine may cause generalized hypertrichosis consisting of terminal hair growth, particularly on the face and forearms. However, hypertrichosis from cyclosporine ophthalmic emulsion 0.05% rarely has been reported5 but would be more likely to occur in a patient reporting a history of chronic dry eye. Oral acetazolamide, not eye drops, is prescribed for glaucoma and typically is not associated with hypertrichosis. Betamethasone and timolol eye drops may cause burning, stinging, redness, or watering of the eyes, but they do not typically cause hypertrichosis.
Other systemic medications (eg, zidovudine, phenytoin, minoxidil, danazol, anabolic steroids) may cause hypertrichosis but not typically localized to the periocular area. Phenytoin usually causes hair growth on the limbs but not on the face and trunk. Oral minoxidil causes hypertrichosis, predominately on the face, lower legs, and forearms.
Systemic conditions such as endocrine abnormalities or porphyria cutanea tarda also may cause hypertrichosis; however, it typically does not present in small focal areas, and other stigmata often are present such as signs of virilization in hirsutism (ie, deepening of voice, pattern alopecia, acne) or liver disease with photosensitive erosions and bullae that leave scars and milia in porphyria cutanea tarda. Acquired hypertrichosis lanuginosa deserves consideration, in part due to its association with lung and colon cancers; however, it consists of softer, downy, nonterminal hairs (malignant down) and is more generalized on the face. Malnutrition from anorexia nervosa may similarly induce hypertrichosis lanuginose.
The molecular mechanism for latanoprost-induced hypertrichosis is unknown; however, it may promote anagen growth as well as hypertrophic changes in the affected follicles.6 Patients should use extreme caution when purchasing unregulated medications due to the risk for impurities, less stable formulation, or inaccurate concentrations. Comparison between brand name and approved generic latanoprost has found notable differences, including variations in active-ingredient concentration, poor stability in warmer temperatures, and higher levels of particulate matter.7 Some cosmetic eyelash enhancers sold over-the-counter or online may contain prostaglandin analogues, but they may not be listed as ingredients.8 One report noted a bimatoprost product with a concentration level double that of brand-name bimatoprost that was discovered using high-performance liquid chromatography-tandem mass spectrometry.9
Treatment options for eliminating the excess hairs include discontinuing the prostaglandin analogue or applying it only to the eyelid margin with an appropriate applicator. Waxing, manual extraction, laser hair removal, electrolysis, and depilatory creams are alternative treatments.
- Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967-1985.
- Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544-547.
- Ortiz-Perez S, Olver JM. Hypertrichosis of the upper cheek area associated with travoprost treatment of glaucoma. Ophthalmic Plast Reconstr Surg. 2010;26:376-377.
- Özyurt S, Çetinkaya GS. Hypertrichosis of the malar areas and poliosis of the eyelashes caused by latanoprost. Actas Dermosifiliogr. 2015;106:74-75.
- Lei HL, Ku WC, Sun MH, et al. Cyclosporine A eye drop-induced elongated eyelashes: a case report. Case Rep Ophthalmol. 2011;2:398-400.
- Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47(suppl 1):S185-S202.
- Kahook MY, Fechtner RD, Katz LJ, et al. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37:101-108.
- Swedish Medical Products Agency. Pharmaceutical ingredients in one out of three eyelash serums. https://www.dr-jetskeultee.nl/jetskeultee/download/common/artikel-wimpers-ingredients.pdf. Published April 15, 2013. Accessed April 11, 2019.
- Marchei E, De Orsi D, Guarino C, et al. High performance liquid chromatography tandem mass spectrometry measurement of bimatoprost, latanoprost and travoprost in eyelash enhancing cosmetic serums. Cosmetics. 2016;3:4.
The Diagnosis: Bimatoprost-Induced Hypertrichosis
Latanoprost, a prostaglandin analogue, typically is prescribed by ophthalmologists as eye drops to reduce intraocular pressure in open-angle glaucoma.1 Common adverse reactions of latanoprost drops include blurred vision, ocular irritation, darkening of the eyelid skin, and pigmentation of the iris.
In 1997, Johnstone2 reported hypertrichosis and increased pigmentation of the eyelashes of both eyes and adjacent skin after latanoprost drops were used in glaucoma patients. Subsequently, topical latanoprost and bimatoprost, a similar analogue, are now utilized for the cosmetic purpose of thickening and lengthening the eyelashes due to the hypertrichosis effect. Travoprost, another prostaglandin analogue used to treat glaucoma, also has been associated with periocular hypertrichosis.3 Concomitant poliosis of the eyelashes with hypertrichosis from latanoprost also has been reported.4 Our patient specifically purchased the eye drops (marketed as generic bimatoprost) to lengthen her eyelashes and had noticed an increase in length. She denied a family history of increased facial hair in females.
Along with gingival hyperplasia, systemic cyclosporine may cause generalized hypertrichosis consisting of terminal hair growth, particularly on the face and forearms. However, hypertrichosis from cyclosporine ophthalmic emulsion 0.05% rarely has been reported5 but would be more likely to occur in a patient reporting a history of chronic dry eye. Oral acetazolamide, not eye drops, is prescribed for glaucoma and typically is not associated with hypertrichosis. Betamethasone and timolol eye drops may cause burning, stinging, redness, or watering of the eyes, but they do not typically cause hypertrichosis.
Other systemic medications (eg, zidovudine, phenytoin, minoxidil, danazol, anabolic steroids) may cause hypertrichosis but not typically localized to the periocular area. Phenytoin usually causes hair growth on the limbs but not on the face and trunk. Oral minoxidil causes hypertrichosis, predominately on the face, lower legs, and forearms.
Systemic conditions such as endocrine abnormalities or porphyria cutanea tarda also may cause hypertrichosis; however, it typically does not present in small focal areas, and other stigmata often are present such as signs of virilization in hirsutism (ie, deepening of voice, pattern alopecia, acne) or liver disease with photosensitive erosions and bullae that leave scars and milia in porphyria cutanea tarda. Acquired hypertrichosis lanuginosa deserves consideration, in part due to its association with lung and colon cancers; however, it consists of softer, downy, nonterminal hairs (malignant down) and is more generalized on the face. Malnutrition from anorexia nervosa may similarly induce hypertrichosis lanuginose.
The molecular mechanism for latanoprost-induced hypertrichosis is unknown; however, it may promote anagen growth as well as hypertrophic changes in the affected follicles.6 Patients should use extreme caution when purchasing unregulated medications due to the risk for impurities, less stable formulation, or inaccurate concentrations. Comparison between brand name and approved generic latanoprost has found notable differences, including variations in active-ingredient concentration, poor stability in warmer temperatures, and higher levels of particulate matter.7 Some cosmetic eyelash enhancers sold over-the-counter or online may contain prostaglandin analogues, but they may not be listed as ingredients.8 One report noted a bimatoprost product with a concentration level double that of brand-name bimatoprost that was discovered using high-performance liquid chromatography-tandem mass spectrometry.9
Treatment options for eliminating the excess hairs include discontinuing the prostaglandin analogue or applying it only to the eyelid margin with an appropriate applicator. Waxing, manual extraction, laser hair removal, electrolysis, and depilatory creams are alternative treatments.
The Diagnosis: Bimatoprost-Induced Hypertrichosis
Latanoprost, a prostaglandin analogue, typically is prescribed by ophthalmologists as eye drops to reduce intraocular pressure in open-angle glaucoma.1 Common adverse reactions of latanoprost drops include blurred vision, ocular irritation, darkening of the eyelid skin, and pigmentation of the iris.
In 1997, Johnstone2 reported hypertrichosis and increased pigmentation of the eyelashes of both eyes and adjacent skin after latanoprost drops were used in glaucoma patients. Subsequently, topical latanoprost and bimatoprost, a similar analogue, are now utilized for the cosmetic purpose of thickening and lengthening the eyelashes due to the hypertrichosis effect. Travoprost, another prostaglandin analogue used to treat glaucoma, also has been associated with periocular hypertrichosis.3 Concomitant poliosis of the eyelashes with hypertrichosis from latanoprost also has been reported.4 Our patient specifically purchased the eye drops (marketed as generic bimatoprost) to lengthen her eyelashes and had noticed an increase in length. She denied a family history of increased facial hair in females.
Along with gingival hyperplasia, systemic cyclosporine may cause generalized hypertrichosis consisting of terminal hair growth, particularly on the face and forearms. However, hypertrichosis from cyclosporine ophthalmic emulsion 0.05% rarely has been reported5 but would be more likely to occur in a patient reporting a history of chronic dry eye. Oral acetazolamide, not eye drops, is prescribed for glaucoma and typically is not associated with hypertrichosis. Betamethasone and timolol eye drops may cause burning, stinging, redness, or watering of the eyes, but they do not typically cause hypertrichosis.
Other systemic medications (eg, zidovudine, phenytoin, minoxidil, danazol, anabolic steroids) may cause hypertrichosis but not typically localized to the periocular area. Phenytoin usually causes hair growth on the limbs but not on the face and trunk. Oral minoxidil causes hypertrichosis, predominately on the face, lower legs, and forearms.
Systemic conditions such as endocrine abnormalities or porphyria cutanea tarda also may cause hypertrichosis; however, it typically does not present in small focal areas, and other stigmata often are present such as signs of virilization in hirsutism (ie, deepening of voice, pattern alopecia, acne) or liver disease with photosensitive erosions and bullae that leave scars and milia in porphyria cutanea tarda. Acquired hypertrichosis lanuginosa deserves consideration, in part due to its association with lung and colon cancers; however, it consists of softer, downy, nonterminal hairs (malignant down) and is more generalized on the face. Malnutrition from anorexia nervosa may similarly induce hypertrichosis lanuginose.
The molecular mechanism for latanoprost-induced hypertrichosis is unknown; however, it may promote anagen growth as well as hypertrophic changes in the affected follicles.6 Patients should use extreme caution when purchasing unregulated medications due to the risk for impurities, less stable formulation, or inaccurate concentrations. Comparison between brand name and approved generic latanoprost has found notable differences, including variations in active-ingredient concentration, poor stability in warmer temperatures, and higher levels of particulate matter.7 Some cosmetic eyelash enhancers sold over-the-counter or online may contain prostaglandin analogues, but they may not be listed as ingredients.8 One report noted a bimatoprost product with a concentration level double that of brand-name bimatoprost that was discovered using high-performance liquid chromatography-tandem mass spectrometry.9
Treatment options for eliminating the excess hairs include discontinuing the prostaglandin analogue or applying it only to the eyelid margin with an appropriate applicator. Waxing, manual extraction, laser hair removal, electrolysis, and depilatory creams are alternative treatments.
- Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967-1985.
- Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544-547.
- Ortiz-Perez S, Olver JM. Hypertrichosis of the upper cheek area associated with travoprost treatment of glaucoma. Ophthalmic Plast Reconstr Surg. 2010;26:376-377.
- Özyurt S, Çetinkaya GS. Hypertrichosis of the malar areas and poliosis of the eyelashes caused by latanoprost. Actas Dermosifiliogr. 2015;106:74-75.
- Lei HL, Ku WC, Sun MH, et al. Cyclosporine A eye drop-induced elongated eyelashes: a case report. Case Rep Ophthalmol. 2011;2:398-400.
- Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47(suppl 1):S185-S202.
- Kahook MY, Fechtner RD, Katz LJ, et al. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37:101-108.
- Swedish Medical Products Agency. Pharmaceutical ingredients in one out of three eyelash serums. https://www.dr-jetskeultee.nl/jetskeultee/download/common/artikel-wimpers-ingredients.pdf. Published April 15, 2013. Accessed April 11, 2019.
- Marchei E, De Orsi D, Guarino C, et al. High performance liquid chromatography tandem mass spectrometry measurement of bimatoprost, latanoprost and travoprost in eyelash enhancing cosmetic serums. Cosmetics. 2016;3:4.
- Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967-1985.
- Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124:544-547.
- Ortiz-Perez S, Olver JM. Hypertrichosis of the upper cheek area associated with travoprost treatment of glaucoma. Ophthalmic Plast Reconstr Surg. 2010;26:376-377.
- Özyurt S, Çetinkaya GS. Hypertrichosis of the malar areas and poliosis of the eyelashes caused by latanoprost. Actas Dermosifiliogr. 2015;106:74-75.
- Lei HL, Ku WC, Sun MH, et al. Cyclosporine A eye drop-induced elongated eyelashes: a case report. Case Rep Ophthalmol. 2011;2:398-400.
- Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47(suppl 1):S185-S202.
- Kahook MY, Fechtner RD, Katz LJ, et al. A comparison of active ingredients and preservatives between brand name and generic topical glaucoma medications using liquid chromatography-tandem mass spectrometry. Curr Eye Res. 2012;37:101-108.
- Swedish Medical Products Agency. Pharmaceutical ingredients in one out of three eyelash serums. https://www.dr-jetskeultee.nl/jetskeultee/download/common/artikel-wimpers-ingredients.pdf. Published April 15, 2013. Accessed April 11, 2019.
- Marchei E, De Orsi D, Guarino C, et al. High performance liquid chromatography tandem mass spectrometry measurement of bimatoprost, latanoprost and travoprost in eyelash enhancing cosmetic serums. Cosmetics. 2016;3:4.
An otherwise healthy woman in her late 50s with Fitzpatrick skin type II presented to the dermatology department for a scheduled cosmetic botulinum toxin injection. Her medical history was notable only for periodic nonsurgical cosmetic procedures including botulinum toxin and dermal fillers, and she was not taking any daily systemic medications. During the preoperative assessment, subtle bilateral and symmetric hypertrichosis with darker terminal hair formation was noted on the periorbital skin and zygomatic cheek. Upon inquiry, the patient admitted to purchasing a “special eye drop” from Mexico and using it regularly. After instillation of 2 to 3 drops per eye, she would laterally wipe the resulting excess drops away from the eyes with her hands and then wash her hands. She denied a change in eye color from their natural brown but did report using blue color contact lenses. She denied an increase in hair growth elsewhere including the upper lip, chin, upper chest, forearms, and hands. She denied deepening of her voice, acne, or hair thinning.
Purpuric Macule of the Right Axilla
The Diagnosis: Atypical Vascular Lesion
Atypical vascular lesion (AVL)(quiz image), named by Fineberg and Rosen,1 is a vascular lesion that arises on mammary skin with a history of radiation exposure. Clinically, AVL can present as a papule or erythematous patch that manifests 3 to 7 years after radiation therapy.2,3 There are 2 histologic subtypes of AVL: lymphatic and vascular.2,4 Lymphatic-type AVL is comprised of a symmetric distribution of thin, dilated, and anastomosing vessels usually found in the superficial and mid dermis. The vessels are lined by flat or hobnail protuberant endothelial cells that lack nuclear irregularity or pleomorphism; however, hyperchromatism of endothelial cell nuclei is a common finding. Vascular-type AVL is morphologically similar to a capillary hemangioma, and histologic features include irregular growth of capillary-sized vessels that extend to the dermis and subcutis.2,4 Atypical vascular lesions are benign lesions but may be a precursor to angiosarcoma. Along with vascular markers, D2-40 typically is positive. Surgical excision with clear margins is recommended when the lesion is small.4,5 Observation is more appropriate for extensive lesions.
Angiosarcoma can arise spontaneously or in association with radiation or chronic lymphedema. Given the shared risk factors and presentation with AVL, it is essential to differentiate angiosarcoma from AVL. Primary cutaneous angiosarcoma usually presents on the head of elderly patients as an ecchymotic patch or plaque with ulceration.4 Secondary angiosarcoma may arise following radiation or chronic lymphedema (Stewart-Treves syndrome); however, some authors now prefer to consider lymphangiosarcoma arising in chronic lymphedematous limbs a distinct entity.6 Surgical excision with wide margins is the mainstay of therapy, but angiosarcoma has high recurrence rates, and the 5-year survival rate has been reported to be as low as 35%.7 Histologic overlap with AVL includes dissecting anastomosing vessels lined by hyperchromatic nuclei; however, angiosarcoma is distinguished by endothelial cell layering, nuclear pleomorphism, and prominent nucleoli (Figure 1).4,8 Increased positivity for Ki-67 immunostain, which indicates cell proliferation, may be used to distinguish angiosarcoma from an AVL (Figure 1 [inset]).9 Further, in contrast to AVL, radiation-induced angiosarcoma is characterized by amplification of C-MYC, a regulator gene, and FLT4 (FMS-related tyrosine kinase 4), a gene encoding vascular endothelial growth factor receptor 3. Gene amplification may be detected through immunohistochemistry or fluorescence in situ hybridization.10 Ki-67 labeling showed less than 10% staining in endothelial cells in our case (quiz image [inset]), and fluorescence in situ hybridization was negative for C-MYC amplification, supporting the diagnosis of AVL.
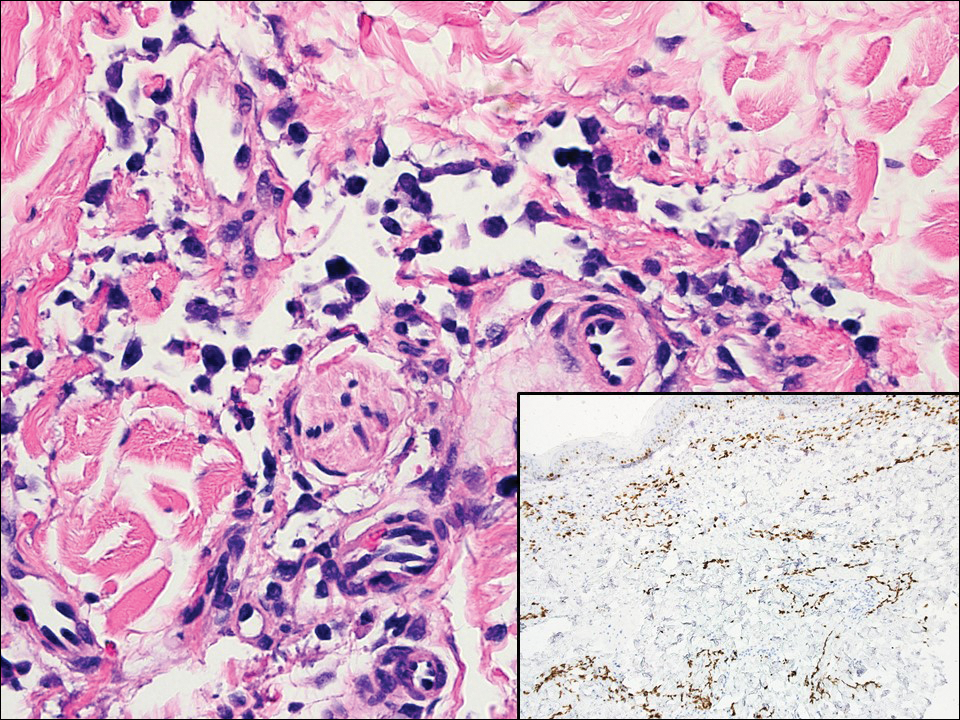
Lymphangioma circumscriptum, the most common superficial lymphangioma, is a hamartomatous malformation that usually occurs at the axillary folds, neck, and trunk. It clinically presents as small agminated vesicles with a characteristic frog spawn appearance.11 Dermoscopic features include yellow lacunae that may alternate with a dark red color secondary to extravasation of erythrocytes.12 These clinical features often lead to a differential diagnosis of verrucae, angiokeratoma, and angiosarcoma. Lymphangioma circumscriptum histologically is characterized by an overgrowth of dilated lymphatic vessels that fill the papillary dermis. The vessels are composed of flat endothelial cells typically filled with acellular proteinaceous debris and occasional erythrocytes (Figure 2). As the lesion traverses deeper into the dermis, the caliber of the lymphatic channel becomes narrower. The presence of deep lymphatic cisterns with surrounding smooth muscle is helpful to differentiate lymphangioma circumscriptum from other lymphatic malformations such as acquired lymphangiectasia. Treatment options include surgical excision, sclerosing agents, and destructive modalities such as cryotherapy.
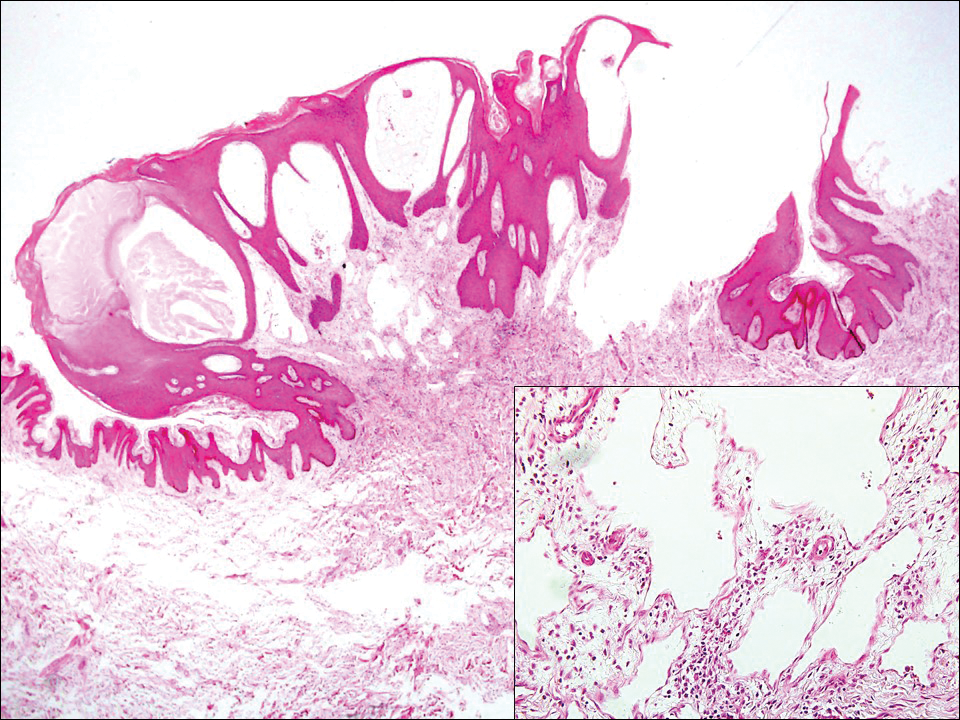
Hobnail hemangioma, originally termed targetoid hemosiderotic hemangioma by Santa Cruz and Aronberg,13 presents as a violaceous papule or nodule surrounded by a characteristic brown halo on the leg. Trauma has been proposed as the inciting factor for the clinical appearance of hobnail hemangioma.14 Microscopically, the lesion shows vessels in a wedge shape. The superficial component has telangiectatic vessels with focal areas of papillary projections lined by endothelial cells. Although the endothelial nuclei typically project into the lumen, the nuclei are small, bland, and without mitotic activity.15 Deeper components show slit-shaped vasculature with dermal collagen dissection. Hemosiderin, extravasated red blood cells, and inflammation are found adjacent to the vessels (Figure 3). Given the benign nature, hobnail hemangiomas may be monitored.
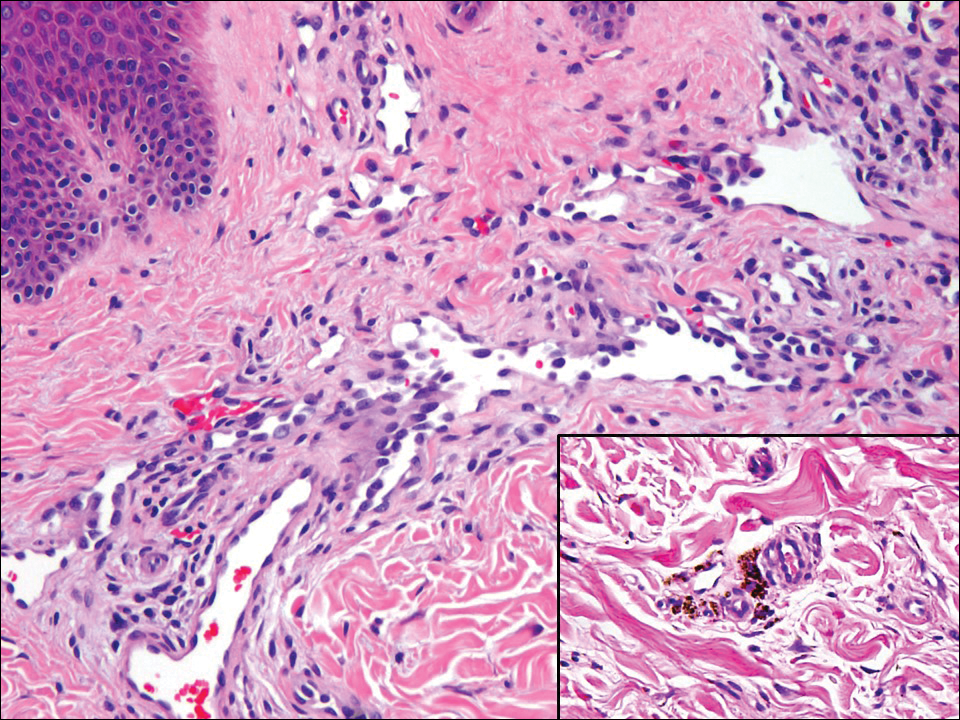
Kaposi sarcoma (KS) is a low-grade vascular neoplasm associated with human herpesvirus 8 that arises in multiple clinical settings, especially in immunosuppression secondary to human immunodeficiency virus. There are 3 distinct clinical stages: patch, plaque, and tumor. The patch stage appears as red macules that blend into larger plaques; the tumor stage is defined as larger nodules developing from plaques. Histologic features differ by stage. Similar to angiosarcoma, KS is comprised of anastomosing vessels that dissect collagen bundles; endothelial cell atypia is minimal. A useful feature of KS is its propensity to involve adnexa and display the promontory sign, which involves the tumor growing into normal vasculature (Figure 4).16 Positive immunohistochemistry for human herpesvirus 8 aids in confirmation of the diagnosis. Treatment options for KS are numerous but include destructive modalities, chemotherapeutic agents such as doxorubicin, or highly active antiretroviral therapy for AIDS-related KS.17
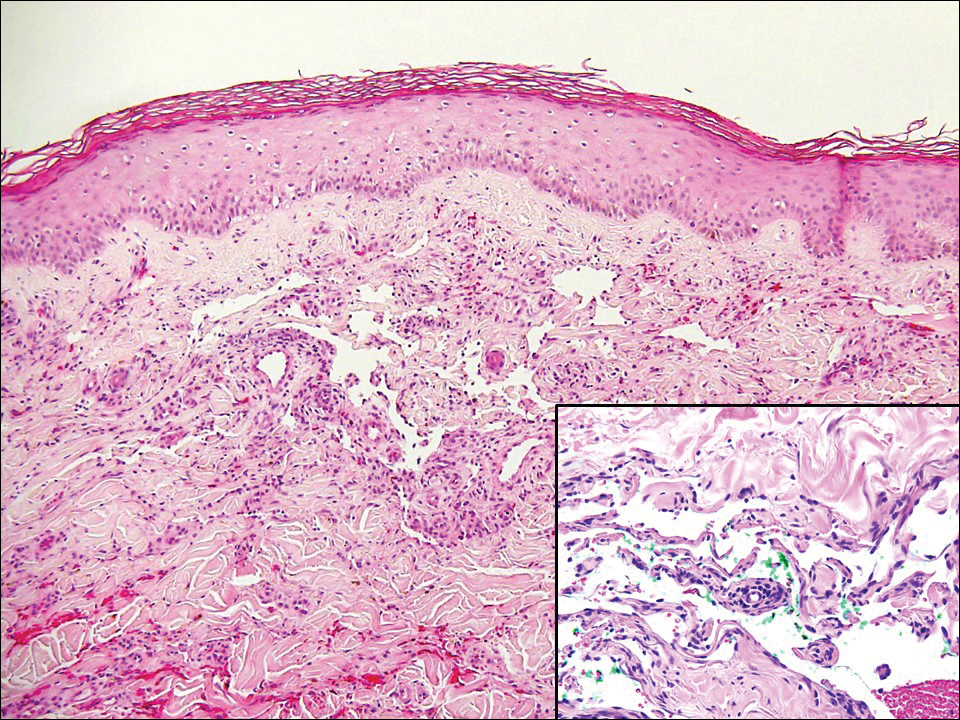
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Billings SD, McKenney JK, Folpe AL, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Udager AM, Ishikawa MK, Lucas DR, et al. MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology. 2016;48:697-704.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Elsevier; 2016:1069-1115.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fraga-Guedes C, Gobbi H, Mastropasqua MG, et al. Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat. 2014;146:347-354.
- Shin SJ, Lesser M, Rosen PP. Hemangiomas and angiosarcomas of the breast: diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch Pathol Lab Med. 2007;131:538-544.
- Cornejo KM, Deng A, Wu H, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. 2015;46:868-875.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
- Massa AF, Menezes N, Baptista A, et al. Cutaneous lymphangioma circumscriptum--dermoscopic features. An Bras Dermatol. 2015;90:262-264.
- Santa Cruz DJ, Aronberg J. Targetoid hemosiderotic hemangioma. J Am Acad Dermatol. 1988;19:550-558.
- Christenson LJ, Stone MS. Trauma-induced simulator of targetoid hemosiderotic hemangioma. Am J Dermatopathol. 2001;23:221-223.
- Trindade F, Kutzner H, Tellechea O, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Di Lorenzo G, Di Trolio R, Montesarchio V, et al. Pegylated liposomal doxorubicin as second-line therapy in the treatment of patients with advanced classic Kaposi sarcoma: a retrospective study. Cancer. 2008;112:1147-1152.
The Diagnosis: Atypical Vascular Lesion
Atypical vascular lesion (AVL)(quiz image), named by Fineberg and Rosen,1 is a vascular lesion that arises on mammary skin with a history of radiation exposure. Clinically, AVL can present as a papule or erythematous patch that manifests 3 to 7 years after radiation therapy.2,3 There are 2 histologic subtypes of AVL: lymphatic and vascular.2,4 Lymphatic-type AVL is comprised of a symmetric distribution of thin, dilated, and anastomosing vessels usually found in the superficial and mid dermis. The vessels are lined by flat or hobnail protuberant endothelial cells that lack nuclear irregularity or pleomorphism; however, hyperchromatism of endothelial cell nuclei is a common finding. Vascular-type AVL is morphologically similar to a capillary hemangioma, and histologic features include irregular growth of capillary-sized vessels that extend to the dermis and subcutis.2,4 Atypical vascular lesions are benign lesions but may be a precursor to angiosarcoma. Along with vascular markers, D2-40 typically is positive. Surgical excision with clear margins is recommended when the lesion is small.4,5 Observation is more appropriate for extensive lesions.
Angiosarcoma can arise spontaneously or in association with radiation or chronic lymphedema. Given the shared risk factors and presentation with AVL, it is essential to differentiate angiosarcoma from AVL. Primary cutaneous angiosarcoma usually presents on the head of elderly patients as an ecchymotic patch or plaque with ulceration.4 Secondary angiosarcoma may arise following radiation or chronic lymphedema (Stewart-Treves syndrome); however, some authors now prefer to consider lymphangiosarcoma arising in chronic lymphedematous limbs a distinct entity.6 Surgical excision with wide margins is the mainstay of therapy, but angiosarcoma has high recurrence rates, and the 5-year survival rate has been reported to be as low as 35%.7 Histologic overlap with AVL includes dissecting anastomosing vessels lined by hyperchromatic nuclei; however, angiosarcoma is distinguished by endothelial cell layering, nuclear pleomorphism, and prominent nucleoli (Figure 1).4,8 Increased positivity for Ki-67 immunostain, which indicates cell proliferation, may be used to distinguish angiosarcoma from an AVL (Figure 1 [inset]).9 Further, in contrast to AVL, radiation-induced angiosarcoma is characterized by amplification of C-MYC, a regulator gene, and FLT4 (FMS-related tyrosine kinase 4), a gene encoding vascular endothelial growth factor receptor 3. Gene amplification may be detected through immunohistochemistry or fluorescence in situ hybridization.10 Ki-67 labeling showed less than 10% staining in endothelial cells in our case (quiz image [inset]), and fluorescence in situ hybridization was negative for C-MYC amplification, supporting the diagnosis of AVL.

Lymphangioma circumscriptum, the most common superficial lymphangioma, is a hamartomatous malformation that usually occurs at the axillary folds, neck, and trunk. It clinically presents as small agminated vesicles with a characteristic frog spawn appearance.11 Dermoscopic features include yellow lacunae that may alternate with a dark red color secondary to extravasation of erythrocytes.12 These clinical features often lead to a differential diagnosis of verrucae, angiokeratoma, and angiosarcoma. Lymphangioma circumscriptum histologically is characterized by an overgrowth of dilated lymphatic vessels that fill the papillary dermis. The vessels are composed of flat endothelial cells typically filled with acellular proteinaceous debris and occasional erythrocytes (Figure 2). As the lesion traverses deeper into the dermis, the caliber of the lymphatic channel becomes narrower. The presence of deep lymphatic cisterns with surrounding smooth muscle is helpful to differentiate lymphangioma circumscriptum from other lymphatic malformations such as acquired lymphangiectasia. Treatment options include surgical excision, sclerosing agents, and destructive modalities such as cryotherapy.

Hobnail hemangioma, originally termed targetoid hemosiderotic hemangioma by Santa Cruz and Aronberg,13 presents as a violaceous papule or nodule surrounded by a characteristic brown halo on the leg. Trauma has been proposed as the inciting factor for the clinical appearance of hobnail hemangioma.14 Microscopically, the lesion shows vessels in a wedge shape. The superficial component has telangiectatic vessels with focal areas of papillary projections lined by endothelial cells. Although the endothelial nuclei typically project into the lumen, the nuclei are small, bland, and without mitotic activity.15 Deeper components show slit-shaped vasculature with dermal collagen dissection. Hemosiderin, extravasated red blood cells, and inflammation are found adjacent to the vessels (Figure 3). Given the benign nature, hobnail hemangiomas may be monitored.

Kaposi sarcoma (KS) is a low-grade vascular neoplasm associated with human herpesvirus 8 that arises in multiple clinical settings, especially in immunosuppression secondary to human immunodeficiency virus. There are 3 distinct clinical stages: patch, plaque, and tumor. The patch stage appears as red macules that blend into larger plaques; the tumor stage is defined as larger nodules developing from plaques. Histologic features differ by stage. Similar to angiosarcoma, KS is comprised of anastomosing vessels that dissect collagen bundles; endothelial cell atypia is minimal. A useful feature of KS is its propensity to involve adnexa and display the promontory sign, which involves the tumor growing into normal vasculature (Figure 4).16 Positive immunohistochemistry for human herpesvirus 8 aids in confirmation of the diagnosis. Treatment options for KS are numerous but include destructive modalities, chemotherapeutic agents such as doxorubicin, or highly active antiretroviral therapy for AIDS-related KS.17

The Diagnosis: Atypical Vascular Lesion
Atypical vascular lesion (AVL)(quiz image), named by Fineberg and Rosen,1 is a vascular lesion that arises on mammary skin with a history of radiation exposure. Clinically, AVL can present as a papule or erythematous patch that manifests 3 to 7 years after radiation therapy.2,3 There are 2 histologic subtypes of AVL: lymphatic and vascular.2,4 Lymphatic-type AVL is comprised of a symmetric distribution of thin, dilated, and anastomosing vessels usually found in the superficial and mid dermis. The vessels are lined by flat or hobnail protuberant endothelial cells that lack nuclear irregularity or pleomorphism; however, hyperchromatism of endothelial cell nuclei is a common finding. Vascular-type AVL is morphologically similar to a capillary hemangioma, and histologic features include irregular growth of capillary-sized vessels that extend to the dermis and subcutis.2,4 Atypical vascular lesions are benign lesions but may be a precursor to angiosarcoma. Along with vascular markers, D2-40 typically is positive. Surgical excision with clear margins is recommended when the lesion is small.4,5 Observation is more appropriate for extensive lesions.
Angiosarcoma can arise spontaneously or in association with radiation or chronic lymphedema. Given the shared risk factors and presentation with AVL, it is essential to differentiate angiosarcoma from AVL. Primary cutaneous angiosarcoma usually presents on the head of elderly patients as an ecchymotic patch or plaque with ulceration.4 Secondary angiosarcoma may arise following radiation or chronic lymphedema (Stewart-Treves syndrome); however, some authors now prefer to consider lymphangiosarcoma arising in chronic lymphedematous limbs a distinct entity.6 Surgical excision with wide margins is the mainstay of therapy, but angiosarcoma has high recurrence rates, and the 5-year survival rate has been reported to be as low as 35%.7 Histologic overlap with AVL includes dissecting anastomosing vessels lined by hyperchromatic nuclei; however, angiosarcoma is distinguished by endothelial cell layering, nuclear pleomorphism, and prominent nucleoli (Figure 1).4,8 Increased positivity for Ki-67 immunostain, which indicates cell proliferation, may be used to distinguish angiosarcoma from an AVL (Figure 1 [inset]).9 Further, in contrast to AVL, radiation-induced angiosarcoma is characterized by amplification of C-MYC, a regulator gene, and FLT4 (FMS-related tyrosine kinase 4), a gene encoding vascular endothelial growth factor receptor 3. Gene amplification may be detected through immunohistochemistry or fluorescence in situ hybridization.10 Ki-67 labeling showed less than 10% staining in endothelial cells in our case (quiz image [inset]), and fluorescence in situ hybridization was negative for C-MYC amplification, supporting the diagnosis of AVL.

Lymphangioma circumscriptum, the most common superficial lymphangioma, is a hamartomatous malformation that usually occurs at the axillary folds, neck, and trunk. It clinically presents as small agminated vesicles with a characteristic frog spawn appearance.11 Dermoscopic features include yellow lacunae that may alternate with a dark red color secondary to extravasation of erythrocytes.12 These clinical features often lead to a differential diagnosis of verrucae, angiokeratoma, and angiosarcoma. Lymphangioma circumscriptum histologically is characterized by an overgrowth of dilated lymphatic vessels that fill the papillary dermis. The vessels are composed of flat endothelial cells typically filled with acellular proteinaceous debris and occasional erythrocytes (Figure 2). As the lesion traverses deeper into the dermis, the caliber of the lymphatic channel becomes narrower. The presence of deep lymphatic cisterns with surrounding smooth muscle is helpful to differentiate lymphangioma circumscriptum from other lymphatic malformations such as acquired lymphangiectasia. Treatment options include surgical excision, sclerosing agents, and destructive modalities such as cryotherapy.

Hobnail hemangioma, originally termed targetoid hemosiderotic hemangioma by Santa Cruz and Aronberg,13 presents as a violaceous papule or nodule surrounded by a characteristic brown halo on the leg. Trauma has been proposed as the inciting factor for the clinical appearance of hobnail hemangioma.14 Microscopically, the lesion shows vessels in a wedge shape. The superficial component has telangiectatic vessels with focal areas of papillary projections lined by endothelial cells. Although the endothelial nuclei typically project into the lumen, the nuclei are small, bland, and without mitotic activity.15 Deeper components show slit-shaped vasculature with dermal collagen dissection. Hemosiderin, extravasated red blood cells, and inflammation are found adjacent to the vessels (Figure 3). Given the benign nature, hobnail hemangiomas may be monitored.

Kaposi sarcoma (KS) is a low-grade vascular neoplasm associated with human herpesvirus 8 that arises in multiple clinical settings, especially in immunosuppression secondary to human immunodeficiency virus. There are 3 distinct clinical stages: patch, plaque, and tumor. The patch stage appears as red macules that blend into larger plaques; the tumor stage is defined as larger nodules developing from plaques. Histologic features differ by stage. Similar to angiosarcoma, KS is comprised of anastomosing vessels that dissect collagen bundles; endothelial cell atypia is minimal. A useful feature of KS is its propensity to involve adnexa and display the promontory sign, which involves the tumor growing into normal vasculature (Figure 4).16 Positive immunohistochemistry for human herpesvirus 8 aids in confirmation of the diagnosis. Treatment options for KS are numerous but include destructive modalities, chemotherapeutic agents such as doxorubicin, or highly active antiretroviral therapy for AIDS-related KS.17

- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Billings SD, McKenney JK, Folpe AL, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Udager AM, Ishikawa MK, Lucas DR, et al. MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology. 2016;48:697-704.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Elsevier; 2016:1069-1115.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fraga-Guedes C, Gobbi H, Mastropasqua MG, et al. Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat. 2014;146:347-354.
- Shin SJ, Lesser M, Rosen PP. Hemangiomas and angiosarcomas of the breast: diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch Pathol Lab Med. 2007;131:538-544.
- Cornejo KM, Deng A, Wu H, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. 2015;46:868-875.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
- Massa AF, Menezes N, Baptista A, et al. Cutaneous lymphangioma circumscriptum--dermoscopic features. An Bras Dermatol. 2015;90:262-264.
- Santa Cruz DJ, Aronberg J. Targetoid hemosiderotic hemangioma. J Am Acad Dermatol. 1988;19:550-558.
- Christenson LJ, Stone MS. Trauma-induced simulator of targetoid hemosiderotic hemangioma. Am J Dermatopathol. 2001;23:221-223.
- Trindade F, Kutzner H, Tellechea O, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Di Lorenzo G, Di Trolio R, Montesarchio V, et al. Pegylated liposomal doxorubicin as second-line therapy in the treatment of patients with advanced classic Kaposi sarcoma: a retrospective study. Cancer. 2008;112:1147-1152.
- Fineberg S, Rosen PP. Cutaneous angiosarcoma and atypical vascular lesions of the skin and breast after radiation therapy for breast carcinoma. Am J Clin Pathol. 1994;102:757-763.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Billings SD, McKenney JK, Folpe AL, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
- Udager AM, Ishikawa MK, Lucas DR, et al. MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology. 2016;48:697-704.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Elsevier; 2016:1069-1115.
- Shin JY, Roh SG, Lee NH, et al. Predisposing factors for poor prognosis of angiosarcoma of the scalp and face: systematic review and meta-analysis. Head Neck. 2017;39:380-386.
- Fraga-Guedes C, Gobbi H, Mastropasqua MG, et al. Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat. 2014;146:347-354.
- Shin SJ, Lesser M, Rosen PP. Hemangiomas and angiosarcomas of the breast: diagnostic utility of cell cycle markers with emphasis on Ki-67. Arch Pathol Lab Med. 2007;131:538-544.
- Cornejo KM, Deng A, Wu H, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol. 2015;46:868-875.
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295.
- Massa AF, Menezes N, Baptista A, et al. Cutaneous lymphangioma circumscriptum--dermoscopic features. An Bras Dermatol. 2015;90:262-264.
- Santa Cruz DJ, Aronberg J. Targetoid hemosiderotic hemangioma. J Am Acad Dermatol. 1988;19:550-558.
- Christenson LJ, Stone MS. Trauma-induced simulator of targetoid hemosiderotic hemangioma. Am J Dermatopathol. 2001;23:221-223.
- Trindade F, Kutzner H, Tellechea O, et al. Hobnail hemangioma reclassified as superficial lymphatic malformation: a study of 52 cases. J Am Acad Dermatol. 2012;66:112-115.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Di Lorenzo G, Di Trolio R, Montesarchio V, et al. Pegylated liposomal doxorubicin as second-line therapy in the treatment of patients with advanced classic Kaposi sarcoma: a retrospective study. Cancer. 2008;112:1147-1152.
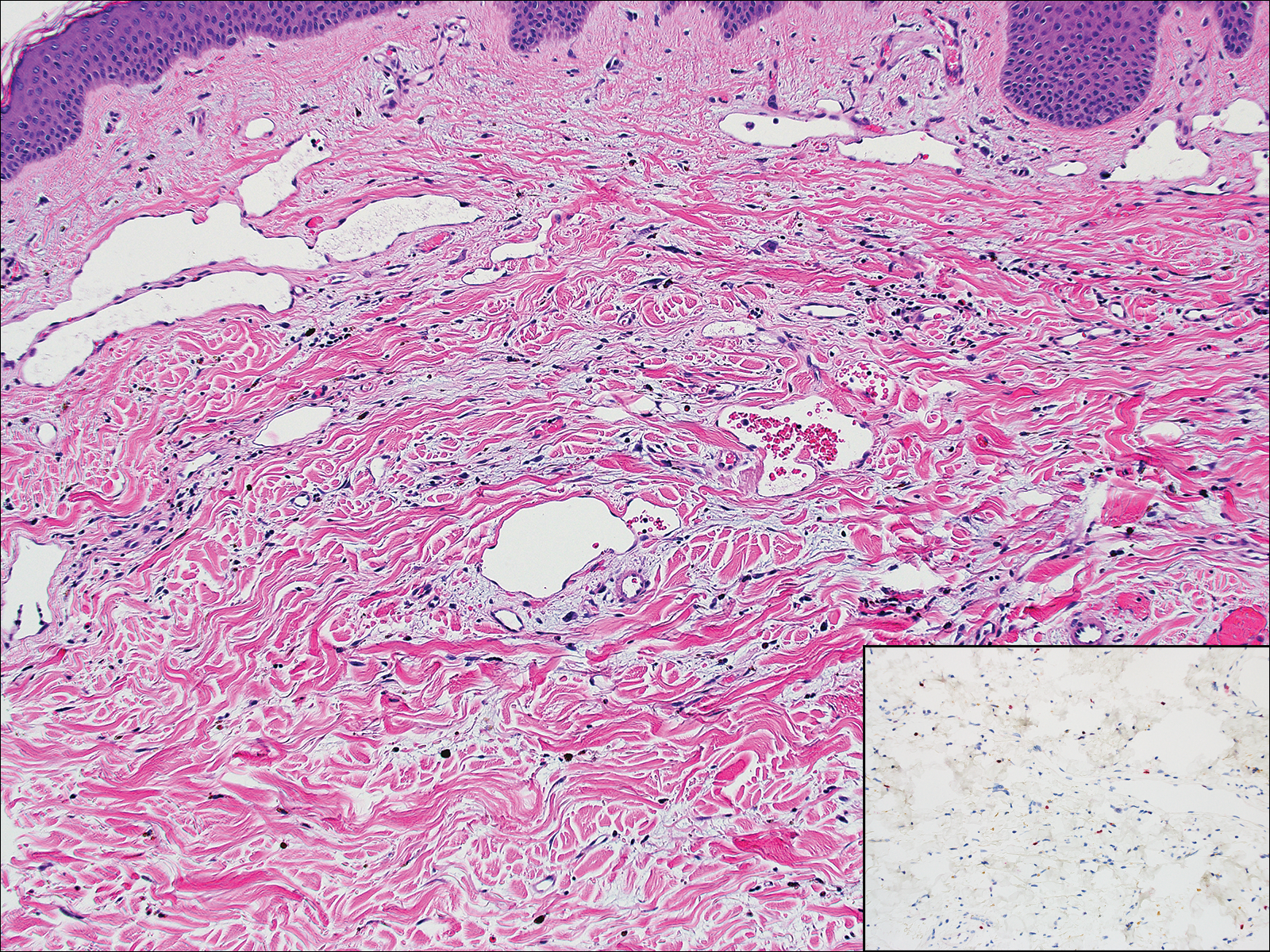
A 67-year-old woman presented with a lesion on the medial aspect of the right axilla of 2 weeks' duration. The patient had a history of cancer of the right breast treated with a mastectomy and adjuvant radiation. She denied pain, bleeding, pruritus, or rapid growth, as well as any changes in medication or recent trauma. Physical examination revealed a 5-mm purpuric macule of the right axilla. A punch biopsy was performed. Amplification for the C-MYC gene was negative by fluorescence in situ hybridization.
Chronic Diffuse Erythematous Papulonodules
The Diagnosis: Lymphomatoid Papulosis
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).

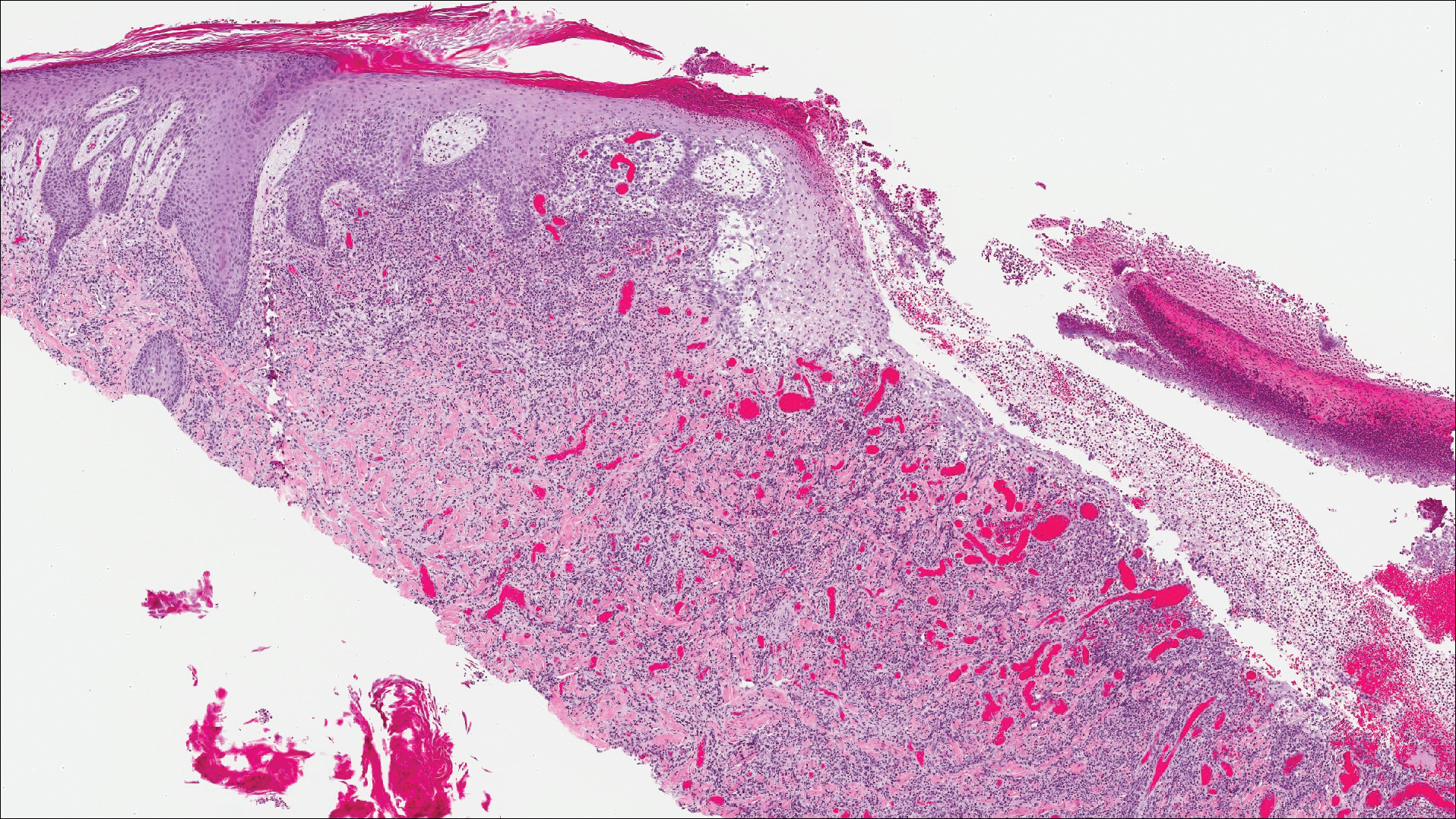
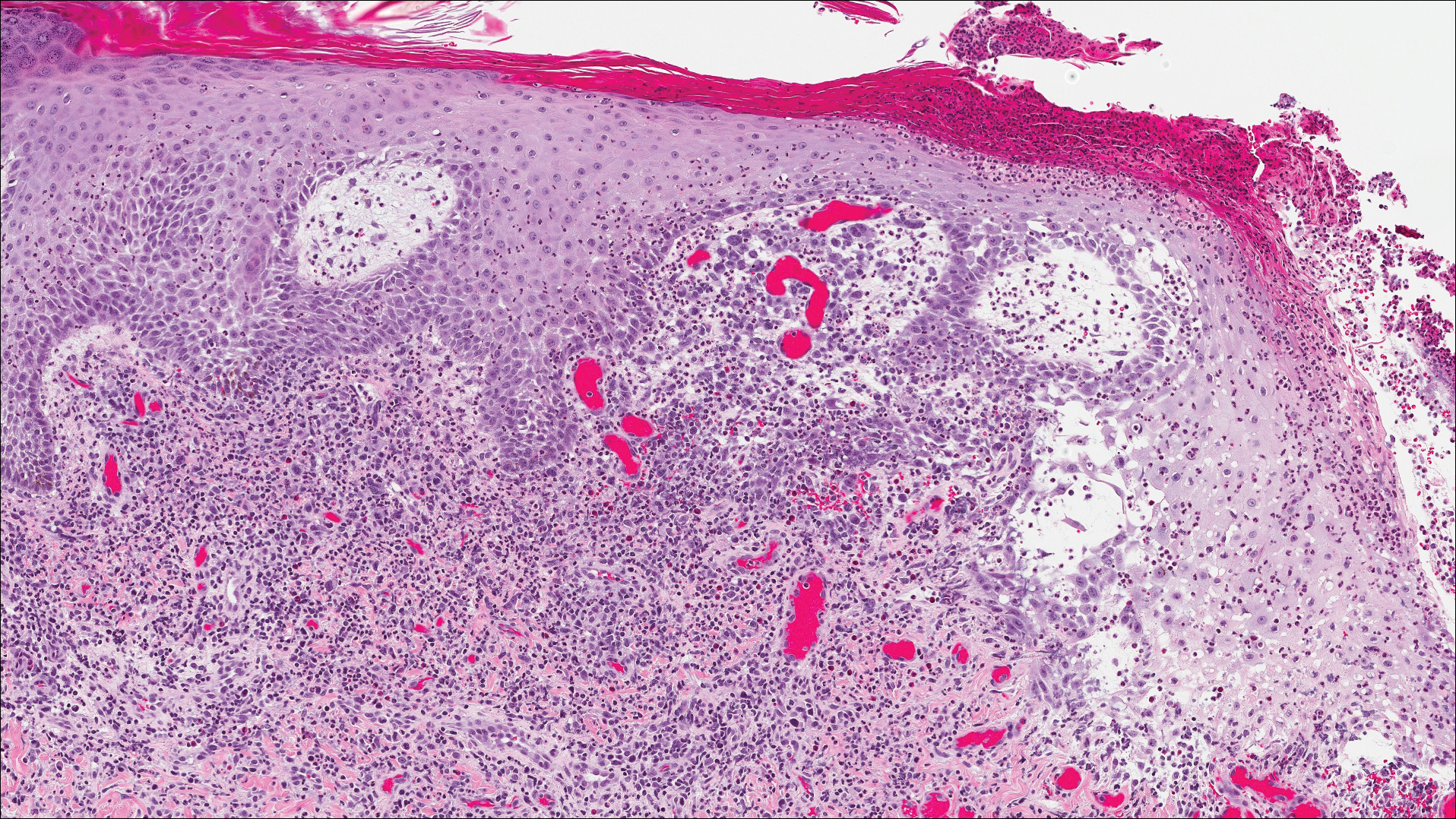
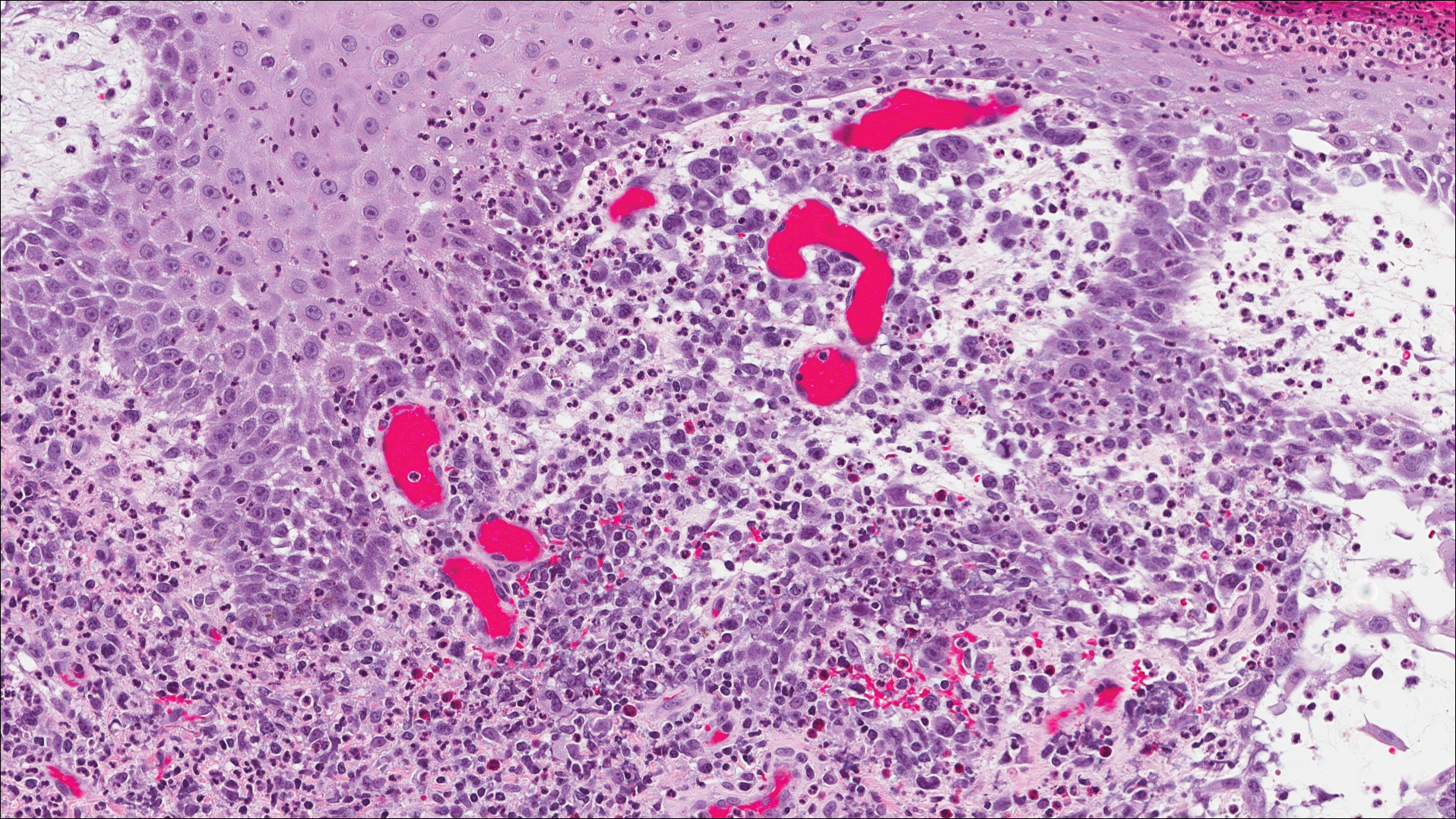
Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
The Diagnosis: Lymphomatoid Papulosis
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).




Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
The Diagnosis: Lymphomatoid Papulosis
A shave biopsy of an established lesion on the volar aspect of the left wrist was performed (Figure 1). The biopsy showed an ulcerated nodular lesion characterized by a dense mixed inflammatory cell infiltrate in the dermis composed of lymphocytes, histiocytes, scattered neutrophils, and numerous eosinophils (Figure 2). Notably there was a minor population of large atypical cells with immunoblastic and anaplastic morphology present individually and in small clusters most prominently within the upper dermis (Figures 3 and 4). Immunohistochemistry of the anaplastic cells revealed a CD30+, CD3−, CD4+, CD5−, CD8−, CD2−, CD7−, CD56−, ALK1− (anaplastic lymphoma kinase-1), PAX5− (paired box protein-5), CD20−, and CD15− phenotype. These morphologic and immunohistochemical features suggested a CD30+ cutaneous lymphoproliferative disorder. The clinical history of recurrent self-healing papulonodules in an otherwise-healthy patient established the diagnosis of lymphomatoid papulosis (LyP).




Lymphomatoid papulosis is a lymphoproliferative disorder characterized by recurrent crops of self-resolving eruptive papulonodular skin lesions that may show a variety of histologic features including a CD30+ malignant T-cell lymphoma.1 Lymphomatoid papulosis was first described in 19681 but debate continues whether the condition should be considered malignant or benign.2 Although the prognosis is excellent, LyP is characterized by a protracted course, often lasting many years. Additionally, these patients have a lifelong increased risk for development of a second cutaneous or systemic lymphoma such as mycosis fungoides (MF), cutaneous or nodal anaplastic large cell lymphoma (ALCL), or Hodgkin lymphoma, among others.
Lymphomatoid papulosis is a rare disease occurring in all ethnic groups and at any age, though most commonly presenting in the fifth decade of life. Finding large atypical T cells expressing CD30 in recurring skin lesions is highly suggestive of LyP; however, large CD30+ cells also can be seen in numerous benign reactive processes such as arthropod assault, drug eruption, viral skin infections, and other dermatoses, thus clinical correlation is always paramount. The cause of LyP is largely unknown; however, spontaneous regression may be explained by CD30-CD30 ligand interaction3 as well as an increased proapoptotic milieu.4 Specific translocations such as interferon regulatory factor-4 have been hypothesized as a risk factor for malignant progression.5-7 Additionally, an inactivating gene mutation resulting in loss of transforming growth factor β1 receptor expression and subsequent unresponsiveness to the growth inhibitory effect of transforming growth factor β may play a role in progression of LyP to ALCL.8
Clinically, LyP consists of red-brown papules and nodules generally smaller than 2 cm, often with central hemorrhage, necrosis, and crusting. Lesions are at different stages of eruption and resolution. They are often grouped but may be disseminated. Spontaneous regression typically occurs within 3 to 8 weeks. Pruritus or mild tenderness may occur as well as residual hyperpigmentation or scarring. Systemic symptoms are notably absent.
The histologic features of LyP vary according to the age of the lesion and subtype.2 Early lesions may only show a few inflammatory cells, but as lesions evolve, larger immunoblastlike CD30+ atypical cells accumulate that may resemble the Reed-Sternberg cells of Hodgkin lymphoma. Of the 5 subtypes, the most common is type A. It is characterized by a wedge-shaped infiltrate with a mixed population of scattered or clustered, large, atypical CD30+ cells, lymphocytes, neutrophils, eosinophils, and histiocytes.9 Frequent mitoses often are seen. Type B appears similar to MF due to a predominantly epidermotropic infiltrate of CD3+ and often CD30− atypical cells. Spontaneously regressing papules favor LyP, whereas persistent patches or plaques favor MF. Type C appears identical to ALCL with diffuse sheets of large atypical CD30+ cells and relatively few inflammatory cells, but spontaneously regressing lesions again favor LyP, whereas persistent tumors favor ALCL. Type D appears similar to primary cutaneous aggressive epidermotropic CD8+ cytotoxic T cell lymphoma due to a markedly epidermotropic infiltrate of small atypical CD8+ and CD30+ lymphocytes, often TIA-1+ (T-cell intracytoplasmic antigen-1) or granzyme B+, but CD30 positivity and self-resolving lesions favor LyP. Type E mimics extranodal natural killer/T cell lymphoma (nasal type) due to angioinvasive CD30+ and beta F1+ T lymphocytes, often CD8+ and/or TIA-1+, but self-resolving lesions again favor LyP, as well as absence of Epstein-Barr virus and CD56−.9
The most common therapeutic approaches to LyP include topical steroids, phototherapy, and low-dose methotrexate.10 However, treatment does not change overall disease course or reduce the future risk for developing an associated lymphoma. Accordingly, abstaining from active therapeutic intervention is reasonable, especially in patients with only a few asymptomatic lesions.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
- Macaulay WL. Lymphomatoid papulosis: a continuing self-healing eruption, clinically benign--histologically malignant. Arch Dermatol. 1968;97:23-30.
- Slater DN. The new World Health Organization-European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas: a practical marriage of two giants. Br J Dermatol. 2005;153:874-880.
- Mori M, Manuelli C, Pimpinelli N, et al. CD30-CD30 ligand interaction in primary cutaneous CD30(+) T-cell lymphomas: a clue to the pathophysiology of clinical regression. Blood. 1999;94:3077-3083.
- Greisser J, Doebbeling U, Roos M, et al. Apoptosis in CD30-positive lymphoproliferative disorders of the skin. Exp Dermatol. 2005;14:380-385.
- Kiran T, Demirkesen C, Eker C, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37:396-400.
- Wada DA, Law ME, Hsi ED, et al. Specificity of IRF4 translocations for primary cutaneous anaplastic large cell lymphoma: a multicenter study of 204 skin biopsies. Mod Pathol. 2011;24:596-605.
- Pham-Ledard A, Prochazkova-Carlotti M, Laharanne E, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130:816-825.
- Schiemann WP, Pfeifer WM, Levi E, et al. A deletion in the gene for transforming growth factor β type I receptor abolishes growth regulation by transforming growth factor β in a cutaneous T-cell lymphoma. Blood. 1999;94:2854-2861.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
A 29-year-old man from Saudi Arabia presented with slightly tender skin lesions occurring in crops every few months over the last 7 years. The lesions typically would occur on the inguinal area, lower abdomen, buttocks, thighs, or arms, resolving within a few weeks despite no treatment. The patient denied having systemic symptoms such as fevers, chills, sweats, chest pain, shortness of breath, or unexpected weight loss. Physical examination revealed multiple erythematous papulonodules, some ulcerated with a superficial crust, grouped predominantly on the medial aspect of the right upper arm and left lower inguinal region. Isolated lesions also were present on the forearms, dorsal aspects of the hands, abdomen, and thighs. The grouped papulonodules were intermixed with faint hyperpigmented macules indicative of prior lesions. No oral lesions were noted, and there was no marked axillary or inguinal lymphadenopathy.