User login
Failure to Reduce: Small Bowel Obstruction Hidden Within a Chronic Umbilical Hernia Sac
Strangulated hernias are a medical emergency that can lead to small bowel obstruction (SBO), bowel necrosis, and death. Practitioners look for signs of strangulation on examination to guide the urgency of management. If the hernia is soft and reducible without overlying skin changes or signs of obstruction, patients may be monitored for years.1 However, there is increasing evidence that even asymptomatic hernias should be repaired rather than monitored to avoid the need for emergent surgical intervention.1
We present a case of a patient with a chronic umbilical hernia who experienced acute worsening of pain at the site of her hernia but with few additional objective signs of strangulation. Prior to this presentation, she had been recently evaluated at our ED for the “same” pain, which included a computed tomography (CT) scan that was negative for an acute surgical emergency. The patient’s second ED visit led to a diagnostic dilemma: Practitioners are encouraged to avoid “unnecessary” radiation—especially in cases of chronic pain—and to rely on history, physical examination findings, and prior recent imaging studies, as appropriate. In this case, repeat imaging ultimately revealed a surgical emergency with an unusual underlying pathology likely related to the chronicity of the patient’s hernia, and explained her repeat presentation to the ED.
Case
A 45-year-old obese woman (body mass index, 46 kg/m2) with a medical history of an umbilical hernia, tubal ligation, and chronic pelvic pain presented a second time to our ED with pain at the site of her hernia, which she stated began 5 hours prior to presentation. Although the pain was associated with nausea and vomiting, the patient said her bowel movements were normal. She first noticed the hernia more than 5 years ago, but experienced her first episode of acute pain related to the hernia with associated nausea and vomiting 3 weeks earlier, which prompted her initial presentation. During this first ED visit, a CT scan of the abdomen/pelvis was obtained as part of her evaluation and was significant for umbilical herniation of bowel without evidence of strangulation. Bedside reduction was successful, and the patient was discharged home and informed of the need to follow-up with a surgeon for an elective repair. She returned to the ED prior to her scheduled operation due to recurrent pain of similar character, but increased severity.
On physical examination, the patient was hemodynamically stable and afebrile. Her vital signs were: heart rate, 84 beats/min; blood pressure, 113/68 mm Hg; and respiratory rate, 20 breaths/min. Oxygen saturation was 100% on room air.
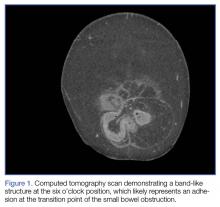
The abdomen was soft with tenderness to palpation over a 13-cm x 8-cm soft hernia to the left of the umbilicus without overlying skin changes. The patient’s pain was controlled with 1 mg of intravenous hydromorphone, after which bedside reduction was attempted. During reduction attempts, there was palpable bowel within the hernia sac, and a periumbilical defect was appreciated. Although the defect in the abdominal wall was estimated to be large enough to allow reduction, the hernia reduced only partially. Because imaging studies from the patient’s previous ED visit showed no visualized strangulation or obstruction, we deliberated over the need for a repeat CT scan prior to further attempts at reduction by general surgery services. Ultimately, we ordered a repeat CT scan, which was significant for a “mechanical small bowel obstruction with focal transition zone located within the hernia sac itself, not the neck of the umbilical hernia.”
Small bowel obstruction is commonly caused by strangulation at the neck of a hernia. In this case, however, the patient had developed an adhesion within the hernia sac itself, which caused the obstruction. This explains why none of the overlying skin changes commonly found in strangulation were visible, and why we were unable to reduce the bowel even though we could palpate the large abdominal wall defect.
Following evaluation by general surgery services, the patient was admitted for laparoscopic hernia repair. Her case was transitioned to an open repair due to extensive intra-abdominal adhesions. The hernia was closed with mesh, and the patient recovered appropriately postoperatively.
Discussion
Abdominal wall hernias are a common pathology, with more than 700,000 repairs performed every year in the United States.2 Patients most commonly present to the ED with abdominal pain, nausea, and vomiting. Less frequently, they present with obstruction, incarceration, strangulation, or rupture.3 Umbilical hernias are caused by increasing intra-abdominal pressure. As the incidence of obesity in the United States has continued to increase, the proportion of hernias that are umbilical or periumbilical has also increased.2,4 Unfortunately, even though umbilical hernias are becoming more common, they are often given less attention than other types of hernias.5 The practice of solely monitoring umbilical hernias can lead to serious outcomes. For example, in a case presentation from Spain, a morbidly obese patient died due to a strangulated umbilical hernia that had progressed over a 15-year period without treatment.6
Compared to elective surgery, emergent operative repair is associated with a higher rate of postoperative complications,1 and a growing body of evidence suggests that patients with symptomatic hernias should be encouraged to undergo operative repair.1,6
Conclusion
Umbilical hernias have become more common with increasing rates of obesity. These hernias have the potential to lead to serious medical emergencies, and the common practice of monitoring chronic hernias may increase the patient’s risk of serious complications. Emergency physicians use the physical examination to help determine the urgency of repair; however, imaging should be considered to assess hernias that cannot easily be reduced to evaluate for obstructed, strangulated, or incarcerated bowel and to help determine the urgency of surgical repair.
1. Davies M, Davies C, Morris-Stiff G, Shute K. Emergency presentation of abdominal hernias: outcome and reasons for delay in treatment-a prospective study. Ann R Coll Surg Engl. 2007;89(1):47-50.
2. Dabbas N, Adams K, Pearson K, Royle G. Frequency of abdominal wall hernias: is classical teaching out of date? JRSM Short Rep. 2011;2(1):5. doi:10.1258/shorts.2010.010071.
3. Rodriguez JA, Hinder RA. Surgical management of umbilical hernia. Operat Tech Gen Surg. 2004;6(3):156-164.
4. Aslani N, Brown CJ. Does mesh offer an advantage over tissue in the open repair of umbilical hernias? A systematic review and meta-analysis. Hernia. 2010;14(5):455-462. doi:10.1016/j.amjsurg.2011.11.015.
5. Arroyo A, García P, Pérez F, Andreu J, Candela F, Calpena R. Randomized clinical trial comparing suture and mesh repair of umbilical hernia in adults. Br J Surgery. 2001;8(10):1321-1323.
6. Rodríguez-Hermosa JI, Codina-Cazador A, Ruiz-Feliú B, Roig-García J, Albiol-Quer M, Planellas-Giné P. Incarcerated umbilical hernia in a super-super-obese patient. Obes Surg. 2008;18(7):893-895. doi:10.1007/s11695-007-9397-3.
Strangulated hernias are a medical emergency that can lead to small bowel obstruction (SBO), bowel necrosis, and death. Practitioners look for signs of strangulation on examination to guide the urgency of management. If the hernia is soft and reducible without overlying skin changes or signs of obstruction, patients may be monitored for years.1 However, there is increasing evidence that even asymptomatic hernias should be repaired rather than monitored to avoid the need for emergent surgical intervention.1
We present a case of a patient with a chronic umbilical hernia who experienced acute worsening of pain at the site of her hernia but with few additional objective signs of strangulation. Prior to this presentation, she had been recently evaluated at our ED for the “same” pain, which included a computed tomography (CT) scan that was negative for an acute surgical emergency. The patient’s second ED visit led to a diagnostic dilemma: Practitioners are encouraged to avoid “unnecessary” radiation—especially in cases of chronic pain—and to rely on history, physical examination findings, and prior recent imaging studies, as appropriate. In this case, repeat imaging ultimately revealed a surgical emergency with an unusual underlying pathology likely related to the chronicity of the patient’s hernia, and explained her repeat presentation to the ED.
Case
A 45-year-old obese woman (body mass index, 46 kg/m2) with a medical history of an umbilical hernia, tubal ligation, and chronic pelvic pain presented a second time to our ED with pain at the site of her hernia, which she stated began 5 hours prior to presentation. Although the pain was associated with nausea and vomiting, the patient said her bowel movements were normal. She first noticed the hernia more than 5 years ago, but experienced her first episode of acute pain related to the hernia with associated nausea and vomiting 3 weeks earlier, which prompted her initial presentation. During this first ED visit, a CT scan of the abdomen/pelvis was obtained as part of her evaluation and was significant for umbilical herniation of bowel without evidence of strangulation. Bedside reduction was successful, and the patient was discharged home and informed of the need to follow-up with a surgeon for an elective repair. She returned to the ED prior to her scheduled operation due to recurrent pain of similar character, but increased severity.
On physical examination, the patient was hemodynamically stable and afebrile. Her vital signs were: heart rate, 84 beats/min; blood pressure, 113/68 mm Hg; and respiratory rate, 20 breaths/min. Oxygen saturation was 100% on room air.
The abdomen was soft with tenderness to palpation over a 13-cm x 8-cm soft hernia to the left of the umbilicus without overlying skin changes. The patient’s pain was controlled with 1 mg of intravenous hydromorphone, after which bedside reduction was attempted. During reduction attempts, there was palpable bowel within the hernia sac, and a periumbilical defect was appreciated. Although the defect in the abdominal wall was estimated to be large enough to allow reduction, the hernia reduced only partially. Because imaging studies from the patient’s previous ED visit showed no visualized strangulation or obstruction, we deliberated over the need for a repeat CT scan prior to further attempts at reduction by general surgery services. Ultimately, we ordered a repeat CT scan, which was significant for a “mechanical small bowel obstruction with focal transition zone located within the hernia sac itself, not the neck of the umbilical hernia.”
Small bowel obstruction is commonly caused by strangulation at the neck of a hernia. In this case, however, the patient had developed an adhesion within the hernia sac itself, which caused the obstruction. This explains why none of the overlying skin changes commonly found in strangulation were visible, and why we were unable to reduce the bowel even though we could palpate the large abdominal wall defect.
Following evaluation by general surgery services, the patient was admitted for laparoscopic hernia repair. Her case was transitioned to an open repair due to extensive intra-abdominal adhesions. The hernia was closed with mesh, and the patient recovered appropriately postoperatively.
Discussion
Abdominal wall hernias are a common pathology, with more than 700,000 repairs performed every year in the United States.2 Patients most commonly present to the ED with abdominal pain, nausea, and vomiting. Less frequently, they present with obstruction, incarceration, strangulation, or rupture.3 Umbilical hernias are caused by increasing intra-abdominal pressure. As the incidence of obesity in the United States has continued to increase, the proportion of hernias that are umbilical or periumbilical has also increased.2,4 Unfortunately, even though umbilical hernias are becoming more common, they are often given less attention than other types of hernias.5 The practice of solely monitoring umbilical hernias can lead to serious outcomes. For example, in a case presentation from Spain, a morbidly obese patient died due to a strangulated umbilical hernia that had progressed over a 15-year period without treatment.6
Compared to elective surgery, emergent operative repair is associated with a higher rate of postoperative complications,1 and a growing body of evidence suggests that patients with symptomatic hernias should be encouraged to undergo operative repair.1,6
Conclusion
Umbilical hernias have become more common with increasing rates of obesity. These hernias have the potential to lead to serious medical emergencies, and the common practice of monitoring chronic hernias may increase the patient’s risk of serious complications. Emergency physicians use the physical examination to help determine the urgency of repair; however, imaging should be considered to assess hernias that cannot easily be reduced to evaluate for obstructed, strangulated, or incarcerated bowel and to help determine the urgency of surgical repair.
Strangulated hernias are a medical emergency that can lead to small bowel obstruction (SBO), bowel necrosis, and death. Practitioners look for signs of strangulation on examination to guide the urgency of management. If the hernia is soft and reducible without overlying skin changes or signs of obstruction, patients may be monitored for years.1 However, there is increasing evidence that even asymptomatic hernias should be repaired rather than monitored to avoid the need for emergent surgical intervention.1
We present a case of a patient with a chronic umbilical hernia who experienced acute worsening of pain at the site of her hernia but with few additional objective signs of strangulation. Prior to this presentation, she had been recently evaluated at our ED for the “same” pain, which included a computed tomography (CT) scan that was negative for an acute surgical emergency. The patient’s second ED visit led to a diagnostic dilemma: Practitioners are encouraged to avoid “unnecessary” radiation—especially in cases of chronic pain—and to rely on history, physical examination findings, and prior recent imaging studies, as appropriate. In this case, repeat imaging ultimately revealed a surgical emergency with an unusual underlying pathology likely related to the chronicity of the patient’s hernia, and explained her repeat presentation to the ED.
Case
A 45-year-old obese woman (body mass index, 46 kg/m2) with a medical history of an umbilical hernia, tubal ligation, and chronic pelvic pain presented a second time to our ED with pain at the site of her hernia, which she stated began 5 hours prior to presentation. Although the pain was associated with nausea and vomiting, the patient said her bowel movements were normal. She first noticed the hernia more than 5 years ago, but experienced her first episode of acute pain related to the hernia with associated nausea and vomiting 3 weeks earlier, which prompted her initial presentation. During this first ED visit, a CT scan of the abdomen/pelvis was obtained as part of her evaluation and was significant for umbilical herniation of bowel without evidence of strangulation. Bedside reduction was successful, and the patient was discharged home and informed of the need to follow-up with a surgeon for an elective repair. She returned to the ED prior to her scheduled operation due to recurrent pain of similar character, but increased severity.
On physical examination, the patient was hemodynamically stable and afebrile. Her vital signs were: heart rate, 84 beats/min; blood pressure, 113/68 mm Hg; and respiratory rate, 20 breaths/min. Oxygen saturation was 100% on room air.
The abdomen was soft with tenderness to palpation over a 13-cm x 8-cm soft hernia to the left of the umbilicus without overlying skin changes. The patient’s pain was controlled with 1 mg of intravenous hydromorphone, after which bedside reduction was attempted. During reduction attempts, there was palpable bowel within the hernia sac, and a periumbilical defect was appreciated. Although the defect in the abdominal wall was estimated to be large enough to allow reduction, the hernia reduced only partially. Because imaging studies from the patient’s previous ED visit showed no visualized strangulation or obstruction, we deliberated over the need for a repeat CT scan prior to further attempts at reduction by general surgery services. Ultimately, we ordered a repeat CT scan, which was significant for a “mechanical small bowel obstruction with focal transition zone located within the hernia sac itself, not the neck of the umbilical hernia.”
Small bowel obstruction is commonly caused by strangulation at the neck of a hernia. In this case, however, the patient had developed an adhesion within the hernia sac itself, which caused the obstruction. This explains why none of the overlying skin changes commonly found in strangulation were visible, and why we were unable to reduce the bowel even though we could palpate the large abdominal wall defect.
Following evaluation by general surgery services, the patient was admitted for laparoscopic hernia repair. Her case was transitioned to an open repair due to extensive intra-abdominal adhesions. The hernia was closed with mesh, and the patient recovered appropriately postoperatively.
Discussion
Abdominal wall hernias are a common pathology, with more than 700,000 repairs performed every year in the United States.2 Patients most commonly present to the ED with abdominal pain, nausea, and vomiting. Less frequently, they present with obstruction, incarceration, strangulation, or rupture.3 Umbilical hernias are caused by increasing intra-abdominal pressure. As the incidence of obesity in the United States has continued to increase, the proportion of hernias that are umbilical or periumbilical has also increased.2,4 Unfortunately, even though umbilical hernias are becoming more common, they are often given less attention than other types of hernias.5 The practice of solely monitoring umbilical hernias can lead to serious outcomes. For example, in a case presentation from Spain, a morbidly obese patient died due to a strangulated umbilical hernia that had progressed over a 15-year period without treatment.6
Compared to elective surgery, emergent operative repair is associated with a higher rate of postoperative complications,1 and a growing body of evidence suggests that patients with symptomatic hernias should be encouraged to undergo operative repair.1,6
Conclusion
Umbilical hernias have become more common with increasing rates of obesity. These hernias have the potential to lead to serious medical emergencies, and the common practice of monitoring chronic hernias may increase the patient’s risk of serious complications. Emergency physicians use the physical examination to help determine the urgency of repair; however, imaging should be considered to assess hernias that cannot easily be reduced to evaluate for obstructed, strangulated, or incarcerated bowel and to help determine the urgency of surgical repair.
1. Davies M, Davies C, Morris-Stiff G, Shute K. Emergency presentation of abdominal hernias: outcome and reasons for delay in treatment-a prospective study. Ann R Coll Surg Engl. 2007;89(1):47-50.
2. Dabbas N, Adams K, Pearson K, Royle G. Frequency of abdominal wall hernias: is classical teaching out of date? JRSM Short Rep. 2011;2(1):5. doi:10.1258/shorts.2010.010071.
3. Rodriguez JA, Hinder RA. Surgical management of umbilical hernia. Operat Tech Gen Surg. 2004;6(3):156-164.
4. Aslani N, Brown CJ. Does mesh offer an advantage over tissue in the open repair of umbilical hernias? A systematic review and meta-analysis. Hernia. 2010;14(5):455-462. doi:10.1016/j.amjsurg.2011.11.015.
5. Arroyo A, García P, Pérez F, Andreu J, Candela F, Calpena R. Randomized clinical trial comparing suture and mesh repair of umbilical hernia in adults. Br J Surgery. 2001;8(10):1321-1323.
6. Rodríguez-Hermosa JI, Codina-Cazador A, Ruiz-Feliú B, Roig-García J, Albiol-Quer M, Planellas-Giné P. Incarcerated umbilical hernia in a super-super-obese patient. Obes Surg. 2008;18(7):893-895. doi:10.1007/s11695-007-9397-3.
1. Davies M, Davies C, Morris-Stiff G, Shute K. Emergency presentation of abdominal hernias: outcome and reasons for delay in treatment-a prospective study. Ann R Coll Surg Engl. 2007;89(1):47-50.
2. Dabbas N, Adams K, Pearson K, Royle G. Frequency of abdominal wall hernias: is classical teaching out of date? JRSM Short Rep. 2011;2(1):5. doi:10.1258/shorts.2010.010071.
3. Rodriguez JA, Hinder RA. Surgical management of umbilical hernia. Operat Tech Gen Surg. 2004;6(3):156-164.
4. Aslani N, Brown CJ. Does mesh offer an advantage over tissue in the open repair of umbilical hernias? A systematic review and meta-analysis. Hernia. 2010;14(5):455-462. doi:10.1016/j.amjsurg.2011.11.015.
5. Arroyo A, García P, Pérez F, Andreu J, Candela F, Calpena R. Randomized clinical trial comparing suture and mesh repair of umbilical hernia in adults. Br J Surgery. 2001;8(10):1321-1323.
6. Rodríguez-Hermosa JI, Codina-Cazador A, Ruiz-Feliú B, Roig-García J, Albiol-Quer M, Planellas-Giné P. Incarcerated umbilical hernia in a super-super-obese patient. Obes Surg. 2008;18(7):893-895. doi:10.1007/s11695-007-9397-3.
The Burden of COPD
Case Scenario
A 62-year-old man who regularly presented to the ED for exacerbations of chronic obstructive pulmonary disease (COPD) after running out of his medications presented again for evaluation and treatment. His outpatient care had been poorly coordinated, and he relied on the ED to provide him with the support he needed. This presentation represented his fifth visit to the ED over the past 3 months.
The patient’s medical history was positive for asthma since childhood, tobacco use, hypertension, and a recent diagnosis of congestive heart failure (CHF). Over the past year, he had four hospital admissions, and was currently unable to walk from his bedroom to another room without becoming short of breath. He also had recently experienced a 20-lb weight loss.
At this visit, the patient complained of chest pain and lightheadedness, which he described as suffocating. Prior to these recent symptoms, he enjoyed walking in his neighborhood and talking with friends. He was an avid reader and sports fan, but admitted that he now had trouble focusing on reading and following games on television. He lived alone, and his family lived across the country. The patient further admitted that although he had attempted to quit cigarette smoking, he was unable to give up his 50-pack per year habit. He had no completed advance health care directive and had significant challenges tending to his basic needs.
The Trajectory of COPD
Chronic obstructive pulmonary disease is a common chronic illness that causes significant morbidity and mortality. A 2016 National Health Services report cited respiratory illness, primarily from COPD, as the third leading cause of death in the United States in 2014.1The trajectory of this disease is marked by frequent exacerbations with partial recovery to baseline function. The burden of those living with COPD is significant and marked by a poor overall health-related quality of life (QOL). The ED has become a staging area for patients seeking care for exacerbations of COPD.2
The World Health Organization (WHO) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) have defined COPD as a spectrum of diseases including emphysema, chronic bronchitis, and chronic obstructive asthma characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response to noxious particles or gases in the airways and lungs.3 Exacerbations and comorbidities contribute to the overall severity of COPD in individual patients.4
The case presented in this article illustrates the common scenario of a patient whose COPD has become severe and highly symptomatic with declining function to the point where he requires home support. His physical decline had been rapid and resulted in many unmet needs. When a patient such as this presents for emergent care, he must first be stabilized; then a care plan will need to be developed prior to discharge.
Management Goals
The overall goals of treating COPD are based on preserving function and are not curative in nature. Chronic obstructive pulmonary disease is a progressive illness that will intensify over time.5 As such, palliative care services are warranted. However, many patients with COPD do not receive palliative care services compared to patients with such other serious and life-limiting disease as cancer and heart disease.
Acute Exacerbations of COPD
Incidence
The frequency of acute exacerbations of COPD (AECOPD) increases with age, productive cough, long-standing COPD, previous hospitalizations related to COPD, eosinophilia, and comorbidities (eg, CHF). Patients with moderate to severe COPD and a history of prior exacerbations were found to have a higher likelihood of future exacerbations. From a quality and cost perspective, it may be useful to identify high-risk patients and strengthen their outpatient program to lessen the need for ED care and more intensive support.6,7
In our case scenario, the patient could have been stabilized at home with a well-controlled plan and home support, which would have resulted in an improved QOL and more time free from his high symptom burden.
Causes
Bacterial and viral respiratory infections are the most likely cause of AECOPD. Environmental pollution and pulmonary embolism are also triggers. Typically, patients with AECOPD present to the ED up to several times a year2 and represent the third most common cause of 30-day readmissions to the hospital.8 Prior exacerbations, dyspnea, and other medical comorbidities are also risk factors for more frequent hospital visits.
Presenting Signs and Symptoms
Each occurrence of AECOPD represents a worsening of a patient’s respiratory symptoms beyond normal variations. This might include increases in cough, sputum production, and dyspnea. The goal in caring for a person with an AECOPD is to stabilize the acute event and provide a treatment plan. The range of acuity for moderate to severe disease makes devising an appropriate treatment plan challenging, and after implementing the best plans, the patient’s course may be characterized by a prolonged cycle of admissions and readmissions without substantial return to baseline.
Management
In practice, ED management of AECOPD in older adults typically differs significantly from published guideline recommendations,9 which may result in pooroutcomes related to shortcomings in quality of care. Better adherence to guideline recommendations when caring for elderly patients with COPD may lead to improved clinical outcomes and better resource usage.
Risk Stratification
Complicating ED management is the challenge of determining the severity of illness and degree of the exacerbation. Airflow obstruction alone is not sufficient to predict outcomes, as any particular measure of obstruction is associated with a spectrum of forced expiratory volume in the first second (FEV1) and varying performance. Moreover, peak-flow measurements are not useful in the setting of AECOPD, as opposed to their use in acute asthma exacerbations, and are not predictive of changes in clinical status.
GOLD and NICE Criteria
Guidelines have been developed and widely promoted to assist ED and hospital and community clinicians in providing evidence-based management for COPD patients. The GOLD Criteria and the National Institute for Clinical Excellence (NICE) are both clinical guidelines on management of COPD.10
Although well recognized and commonly used, the original GOLD criteria did not take into account the frequency and importance of the extrapulmonary manifestations of COPD in predicting outcome. Typically, those with severe or very severe COPD have an average of 12 co-occurring symptoms, an even greater number of signs and symptoms than those occurring in patients with cancer or heart or renal disease.11
BODE Criteria
The body mass index, airflow obstruction, dyspnea and exercise capacity (BODE) criteria assess and predict the health-related QOL and mortality risk for patients with COPD. Risk is adjusted based on four factors—weight, airway obstruction, dyspnea, and exercise capacity (ie, 6-minute walk distance).13
Initial Evaluation and Work-Up
As previously noted, when an AECOPD patient arrives to the ED, the first priority is to stabilize the patient and initiate treatment. In this respect, initial identification of the patient’s pulse oxygen saturation (SpO2) is important.
Laboratory Evaluation
In cases of respiratory failure, obtaining arterial blood gas (ABG) values are critical. The ABG test will assist in determining acute exacerbations of chronic hypercapnia and the need for ventilatory support. When considering CHF, a plasma B-type natriuretic peptide is useful to assess for CHF.
Imaging Studies
A chest radiograph may be useful in the initial evaluation to identify abnormalities, including barotrauma (ie, pneumothorax) and infiltrates. Additionally, in patients with comorbidities, it is important to assess cardiac status, and a chest X-ray may assist in identification of pulmonary edema, pleural effusions, and cardiomegaly. If the radiograph does show a pulmonary infiltrate (ie, pneumonia), it will help identify the probable triggers, but even in these instances, a sputum gram stain will not assist in the diagnosis.
Treatment
Relieving airflow obstruction is achieved with inhaled short-acting bronchodilators and systemic glucocorticoids, by treating infection, and by providing supplemental oxygen and ventilatory support.
Bronchodilators
The short-acting beta-adrenergic agonists (eg, albuterol) act rapidly and are effective in producing bronchodilation. Nebulized therapy may be most comfortable for the acutely ill patient. Typical dosing is 2.5 mg albuterol diluted to 3 cc by nebulizer every hour. Higher doses are not more effective, and there is no evidence of a higher response rate from constant nebulized therapy, which can cause anxiety and tachycardia in patients.14 Anticholinergic agents (eg, ipratropium) are often added despite unclear data regarding clinical advantage. In one study evaluating the effectiveness of adding ipratropium to albuterol, patients receiving a combination had the same improvement in FEV1 at 90 minutes.15 Patients receiving ipratropium alone had the lowest rate of reported side effects.15
Systemic Glucocorticoids
Short-course systemic glucocorticoids are an important addition to treatment and have been found to improve spirometry and decrease relapse rate. The oral and intravenous (IV) routes provide the same benefit. For the acutely ill patient with challenges swallowing, the IV route is preferred. The optimal dose is not clear, but hydrocortisone doses of 100 mg to 125 mg every 6 hours for 3 days are effective, as is oral prednisone 30 mg per day for 14 days, or 60 mg per day for 3 days with a taper.
Antibiotic Therapy
Antibiotics are indicated for patients with moderate to severe AECOPD who are ill enough to be admitted to the hospital. Empiric broad spectrum treatment is recommended. The initial antibiotic regimen should target likely bacterial pathogens (Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in most patients) and take into account local patterns of antibiotic resistance. Flouroquinolones or third-generation cephalosporins generally provide sufficient coverage. For patients experiencing only a mild exacerbation, antibiotics are not warranted.
Magnesium Sulfate
Other supplemental medications that have been evaluated include magnesium sulfate for bronchial smooth muscle relaxation. Studies have found that while magnesium is helpful in asthma, results are mixed with COPD.16
Supplemental Oxygen
Oxygen therapy is important during an AECOPD episode. Often, concerns arise about decreasing respiratory drive, which is typically driven by hypoxia in patients who have chronic hypercapnia. Arterial blood gas determinations are important in managing a patient’s respiratory status and will assist in determining actual oxygenation and any coexistent metabolic disturbances.
Noninvasive Ventilation. Oxygen can be administered efficiently by a venturi mask, which delivers precise fractions of oxygen, or by nasal cannula. A facemask is less comfortable, but is available for higher oxygen requirements, providing up to 55% oxygen, while a nonrebreather mask delivers up to 90% oxygen.
When necessary, noninvasive positive pressure ventilation (NPPV) improves outcomes for those with severe dyspnea and signs of respiratory fatigue manifested as increased work of breathing. Noninvasive positive pressure ventilation can improve clinical outcomes and is the ventilator mode of choice for those patients with COPD. Indications include severe dyspnea with signs of increased work of breathing and respiratory acidosis (arterial pH <7.35) and partial pressure of arterial carbon dioxide (PaCO2) >45 mm Hg.
Whenever possible, NPPV should be initiated with a triggered mode to allow spontaneous breaths. Inspiratory pressure of 8 cm to 12 cm H2O and expiratory pressure of 3 cm to 5 cm of H2 are recommended.
Mechanical Ventilation. Mechanical ventilation is often undesirable because it may be extraordinarily difficult to wean a patient off the device and permit safe extubation. However, if a patient cannot be stabilized with NPPV, intubation and mechanical ventilation must be considered. Typically, this occurs when there is severe respiratory distress, tachypnea >30 breaths/min, accessory muscle use, and altered mentation.
Goals of intubation/mechanical respiration include correcting oxygenation and severe respiratory acidosis as well as reducing the work of breathing. Barotrauma is a significant risk when patients with COPD require mechanical ventilation. Volume-limited modes of ventilation are commonly used, while pressure support or pressure-limited modes are less suitable for patients with airflow limitation. Again, invasive ventilation should only be administered if a patient cannot tolerate NPPV.
Palliative Care in the ED
Palliative care is an approach that improves the QOL of patients and their families facing the issues associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and accurate assessment and treatment of pain and physical, psychosocial, and spiritual problems.3 This approach to care is warranted for COPD patients given the myriad of burdensome symptoms and functional decline that occurs.17
Palliative care expands traditional treatment goals to include enhancing QOL; helping with medical decision making; and identifying the goals of care. Palliative care is provided by board-certified physicians for the most complex of cases. However, the primary practice of palliative care must be delivered at the bedside by the treating provider. Managing pain, dyspnea, nausea, vomiting, and changes in bowel habits, as well as discussing goals of care, are among the basic palliative care skills all providers need to have and apply when indicated.
Palliative Care for Dyspnea
Opioids. Primary palliative care in the ED includes the appropriate use of low-dose oral and parenteral opioids to treat dyspnea in AECOPD. The use of a low-dose opioid, such as morphine 2 mg IV, titrated up to a desired response, is a safe and effective practice.18 Note the 2-mg starting dose is considered low-dose.19
With respect to managing dyspnea in AECOPD patients, nebulized opioids have not been found to be better than nebulized saline. More specific data regarding the use of oral opioids for managing refractory dyspnea in patients with predominantly COPD have been recently published: Long-acting morphine 20 mg once daily provides symptomatic relief in refractory dyspnea in the community setting. For the opioid-naïve patient, a lower dose is recommended.20
Oxygenation. There is no hard evidence of the effectiveness of oxygen in the palliation of breathlessness. Humidified air is effective initially, as is providing a fan at the bedside. Short-burst oxygen therapy should only be considered for episodes of severe breathlessness in patients whose COPD is not relieved by other treatments. Oxygen should continue to be prescribed only if an improvement in breathlessness following therapy has been documented. The American Thoracic Society recommends continuous oxygen therapy in patients with COPD who have severe resting hypoxemia (PaCO2 ≤55 mm Hg or SpO2 ≤88%).21
POLST Form
The Physicians Order for Life-Sustaining Treatment (POLST) form is a set of medical orders, similar to the “do not resuscitate” (allow natural death) order. A POLST form is not an advance directive and does not serve as a substitute for a patient’s assignation of a health care agent or durable power of attorney for health care.22
The POLST form enables physicians to order treatments patients would want, identify those treatments that patients would not want, and not provide those the patient considers “extraordinary” and excessively burdensome. A POLST form does not allow for active euthanasia or physician-assisted suicide.
Identifying treatment preferences is an important part of the initial evaluation of all patients. When dealing with an airway issue in a COPD patient, management can become complex. Ideally, the POLST form should arrive with the patient in the ED and list preferences regarding possible intensive interventions such as intubation and chest compressions. Discussing these issues with a patient in extreme distress is difficult or impossible, and in these cases, access to pertinent medical records, discussing preferences with family caregivers, and availability of a POLST form are much better ways to determine therapy.
Palliative Home Care
Patient Safety Considerations
Weight loss and associated muscle wasting are common features in patients with severe COPD, creating a high-risk situation for falls and a need for assistance with activities of daily living. The patient who is frail when discharged home from the ED requires a home-care plan before leaving the ED, and strict follow-up with the patient’s primary care provider will typically be needed within 2 to 4 weeks.
Psychological Considerations
Being mindful of the anxiety and depression that accompany the physical limitations of those with COPD is important. Mood disturbances serve as risk factors for re-hospitalization and mortality.13Multiple palliative care interventions provide patients assistance with these issues, including the use of antidepressants that may aid sleep, stabilize mood, and stimulate appetite.
Early referral to the palliative care team will provide improved care for the patient and family. Palliative care referral will provide continued management of the physical symptoms and evaluation and treatment of the psychosocial issues that accompany COPD. Additionally, the palliative care team can assist with safe discharge planning and follow-up, including the provision of the patient’s home needs as well as the family’s ability to cope with the home setting.
Prognosis
Predicting prognosis is difficult for the COPD patient due to the highly variable illness trajectory. Some patients have a low FEV1 and yet are very functional. However, assessment of severity of lung function impairment, frequency of exacerbations, and need for long-term oxygen therapy helps identify those patients who are entering the final 12 months of life. Evaluating symptom burden and impact on activities of daily living for patients with COPD is comparable to those of cancer patients, and in both cases, palliative care approaches are necessary.
Predicting Morbidity and Mortality
A profile developed from observational studies can help predict 6- to 12-month morbidity and mortality in patients with advanced COPD. This profile includes the following criteria:
- Significant dyspnea;
- FEV1 <30%;
- Number of exacerbations;
- Left heart failure or other comorbidities;
- Weight loss or cachexia;
- Decreasing performance status;
- Age older than 70 years; and
- Depression.
Although additional research is required to refine and verify this profile, reviewing these data points can prompt providers to initiate discussions with patients about treatment preferences and end-of-life care.23,24
Palliative Performance Scale
The Palliative Performance Scale (PPS) is another scale used to predict prognosis and eligibility for hospice care.25 This score provides a patient’s estimated survival.25 For a patient with a PPS score of 50%, hospice education may be appropriate.
Case Scenario Continued
Both the BODE and GOLD criteria scores assisted in determining prognosis and risk profiles of the patient in our case scenario. By applying the BODE criteria, our patient had a 4-year survival benefit of under 18%. The GOLD criteria results for this patient also were consistent with the BODE criteria and reflected end-stage COPD. Since this patient also had a PPS score of 50%, hospice education and care were discussed and initiated.
Conclusion
Patients with AECOPD commonly present to the ED. Such patients suffer with a high burden of illness and a need for immediate symptom management. However, after these measures have been instituted, strong evidence suggests that these patients typically do not receive palliative care with the same frequency compared to cancer or heart disease patients.
Management of AECOPD in the ED must include rapid treatment of dyspnea and pain, but also a determination of treatment preferences and an understanding of the prognosis. Several criteria are available to guide prognostic awareness and may help further the goals of care and disposition. Primary palliative care should be started by the ED provider for appropriate patients, with early referral to the palliative care team.
1. National Center for Health Statistics. Health, United States 2015 With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: US Dept. Health and Human Services; 2016. http://www.cdc.gov/nchs/hus/. Accessed October 17, 2016.
2. Khialani B, Sivakumaran P, Keijzers G, Sriram KB. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease and factors associated with hospitalization. J Res Med Sci . 2014;19(4):297-303.
3. World Health Organization Web site. Chronic respiratory diseases. COPD: Definition. http://www.who.int/respiratory/copd/definition/en/. Accessed October 17, 2016.
4. Rabe KF, Hurd S, Anzueto A, et al; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med . 2007;176(6):532-555.
5. Fan VS, Ramsey SD, Make BJ, Martinez FJ. Physiologic variables and functional status independently predict COPD hospitalizations and emergency department visits in patients with severe COPD. COPD . 2007;4(1):29-39.
6. Cydulka RK, Rowe BH, Clark S, Emerman CL, Camargo CA Jr; MARC Investigators. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: the Multicenter Airway Research Collaboration. J Am Geriatr Soc . 2003;51(7):908-916.
7. Strassels SA, Smith DH, Sullivan SD, et al. The costs of treating COPD in the United States. Chest . 2001;119:3.
8. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med . 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
9. Rowe BH, Bhutani M, Stickland MK, Cydulka R. Assessment and management of chronic obstructive pulmonary disease in the emergency department and beyond. Expert Rev Respir Med . 2011;5(4):549-559. doi:10.1586/ers.11.43.
10. National Institute for Clinical Excellence Web site. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Clinical Guideline CG101. https://www.nice.org.uk/Guidance/cg101. Published June 2010. Accessed October 17, 2016.
11. Christensen VL, Holm AM, Cooper B, Paul SM, Miaskowski C, Rustøen T. Differences in symptom burden among patients with moderate, severe, or very severe chronic obstructive pulmonary disease. J Pain Symptom Manage . 2016;51(5):849-859. doi:10.1016/j.jpainsymman.2015.12.324.
12. GOLD Reports. Global Initiative for Chronic Obstructive Lung Disease Web site. http://goldcopd.org/gold-reports/. Accessed October 17, 2016.
13. Funk GC, Kirchheiner K, Burghuber OC, Hartl S. BODE index versus GOLD classification for explaining anxious and depressive symptoms in patients with COPD—a cross-sectional study. Respir Res . 2009;10:1. doi:10.1186/1465-9921-10-1.
14. Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians-American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med . 2001;134(7):600-620.
15. McCrory DC, Brown CD. Inhaled short-acting beta 2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2001;(2):CD002984.
16. Shivanthan MC, Rajapakse S. Magnesium for acute exacerbation of chronic obstructive pulmonary disease: A systematic review of randomised trials. Ann Thorac Med . 2014;9(2):77-80. doi:10.4103/1817-1737.128844.
17. Curtis JR. Palliative and end of life care for patients with severe COPD. Eur Respir J . 2008;32(3):796-803.
18. Rocker GM, Simpson AC, Young J, et al. Opioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: patients’ experiences and outcomes. CMAJ Open . 2013;1(1):E27-E36.
19. Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnea. Thorax . 2002;57(11):939-944.
20. Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ . 2003;327(7414):523-528.
21. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med . 2011;155(3):179-191. doi:10.7326/0003-4819-155-3-201108020-00008.
22. National POLST Paradigm. http://polst.org/professionals-page/?pro=1. Accessed October 17, 2016.
23. Hansen-Flaschen J. Chronic obstructive pulmonary disease: the last year of life. Respir Care. 2004;49(1):90-97; discussion 97-98.
24. Spathis A, Booth S. End of life care in chronic obstructive pulmonary disease: in search of a good death. Int J Chron Obstruct Pulmon Dis . 2008;3(1):11-29.
25. Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care . 1996;12(1):5-11.
Case Scenario
A 62-year-old man who regularly presented to the ED for exacerbations of chronic obstructive pulmonary disease (COPD) after running out of his medications presented again for evaluation and treatment. His outpatient care had been poorly coordinated, and he relied on the ED to provide him with the support he needed. This presentation represented his fifth visit to the ED over the past 3 months.
The patient’s medical history was positive for asthma since childhood, tobacco use, hypertension, and a recent diagnosis of congestive heart failure (CHF). Over the past year, he had four hospital admissions, and was currently unable to walk from his bedroom to another room without becoming short of breath. He also had recently experienced a 20-lb weight loss.
At this visit, the patient complained of chest pain and lightheadedness, which he described as suffocating. Prior to these recent symptoms, he enjoyed walking in his neighborhood and talking with friends. He was an avid reader and sports fan, but admitted that he now had trouble focusing on reading and following games on television. He lived alone, and his family lived across the country. The patient further admitted that although he had attempted to quit cigarette smoking, he was unable to give up his 50-pack per year habit. He had no completed advance health care directive and had significant challenges tending to his basic needs.
The Trajectory of COPD
Chronic obstructive pulmonary disease is a common chronic illness that causes significant morbidity and mortality. A 2016 National Health Services report cited respiratory illness, primarily from COPD, as the third leading cause of death in the United States in 2014.1The trajectory of this disease is marked by frequent exacerbations with partial recovery to baseline function. The burden of those living with COPD is significant and marked by a poor overall health-related quality of life (QOL). The ED has become a staging area for patients seeking care for exacerbations of COPD.2
The World Health Organization (WHO) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) have defined COPD as a spectrum of diseases including emphysema, chronic bronchitis, and chronic obstructive asthma characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response to noxious particles or gases in the airways and lungs.3 Exacerbations and comorbidities contribute to the overall severity of COPD in individual patients.4
The case presented in this article illustrates the common scenario of a patient whose COPD has become severe and highly symptomatic with declining function to the point where he requires home support. His physical decline had been rapid and resulted in many unmet needs. When a patient such as this presents for emergent care, he must first be stabilized; then a care plan will need to be developed prior to discharge.
Management Goals
The overall goals of treating COPD are based on preserving function and are not curative in nature. Chronic obstructive pulmonary disease is a progressive illness that will intensify over time.5 As such, palliative care services are warranted. However, many patients with COPD do not receive palliative care services compared to patients with such other serious and life-limiting disease as cancer and heart disease.
Acute Exacerbations of COPD
Incidence
The frequency of acute exacerbations of COPD (AECOPD) increases with age, productive cough, long-standing COPD, previous hospitalizations related to COPD, eosinophilia, and comorbidities (eg, CHF). Patients with moderate to severe COPD and a history of prior exacerbations were found to have a higher likelihood of future exacerbations. From a quality and cost perspective, it may be useful to identify high-risk patients and strengthen their outpatient program to lessen the need for ED care and more intensive support.6,7
In our case scenario, the patient could have been stabilized at home with a well-controlled plan and home support, which would have resulted in an improved QOL and more time free from his high symptom burden.
Causes
Bacterial and viral respiratory infections are the most likely cause of AECOPD. Environmental pollution and pulmonary embolism are also triggers. Typically, patients with AECOPD present to the ED up to several times a year2 and represent the third most common cause of 30-day readmissions to the hospital.8 Prior exacerbations, dyspnea, and other medical comorbidities are also risk factors for more frequent hospital visits.
Presenting Signs and Symptoms
Each occurrence of AECOPD represents a worsening of a patient’s respiratory symptoms beyond normal variations. This might include increases in cough, sputum production, and dyspnea. The goal in caring for a person with an AECOPD is to stabilize the acute event and provide a treatment plan. The range of acuity for moderate to severe disease makes devising an appropriate treatment plan challenging, and after implementing the best plans, the patient’s course may be characterized by a prolonged cycle of admissions and readmissions without substantial return to baseline.
Management
In practice, ED management of AECOPD in older adults typically differs significantly from published guideline recommendations,9 which may result in pooroutcomes related to shortcomings in quality of care. Better adherence to guideline recommendations when caring for elderly patients with COPD may lead to improved clinical outcomes and better resource usage.
Risk Stratification
Complicating ED management is the challenge of determining the severity of illness and degree of the exacerbation. Airflow obstruction alone is not sufficient to predict outcomes, as any particular measure of obstruction is associated with a spectrum of forced expiratory volume in the first second (FEV1) and varying performance. Moreover, peak-flow measurements are not useful in the setting of AECOPD, as opposed to their use in acute asthma exacerbations, and are not predictive of changes in clinical status.
GOLD and NICE Criteria
Guidelines have been developed and widely promoted to assist ED and hospital and community clinicians in providing evidence-based management for COPD patients. The GOLD Criteria and the National Institute for Clinical Excellence (NICE) are both clinical guidelines on management of COPD.10
Although well recognized and commonly used, the original GOLD criteria did not take into account the frequency and importance of the extrapulmonary manifestations of COPD in predicting outcome. Typically, those with severe or very severe COPD have an average of 12 co-occurring symptoms, an even greater number of signs and symptoms than those occurring in patients with cancer or heart or renal disease.11
BODE Criteria
The body mass index, airflow obstruction, dyspnea and exercise capacity (BODE) criteria assess and predict the health-related QOL and mortality risk for patients with COPD. Risk is adjusted based on four factors—weight, airway obstruction, dyspnea, and exercise capacity (ie, 6-minute walk distance).13
Initial Evaluation and Work-Up
As previously noted, when an AECOPD patient arrives to the ED, the first priority is to stabilize the patient and initiate treatment. In this respect, initial identification of the patient’s pulse oxygen saturation (SpO2) is important.
Laboratory Evaluation
In cases of respiratory failure, obtaining arterial blood gas (ABG) values are critical. The ABG test will assist in determining acute exacerbations of chronic hypercapnia and the need for ventilatory support. When considering CHF, a plasma B-type natriuretic peptide is useful to assess for CHF.
Imaging Studies
A chest radiograph may be useful in the initial evaluation to identify abnormalities, including barotrauma (ie, pneumothorax) and infiltrates. Additionally, in patients with comorbidities, it is important to assess cardiac status, and a chest X-ray may assist in identification of pulmonary edema, pleural effusions, and cardiomegaly. If the radiograph does show a pulmonary infiltrate (ie, pneumonia), it will help identify the probable triggers, but even in these instances, a sputum gram stain will not assist in the diagnosis.
Treatment
Relieving airflow obstruction is achieved with inhaled short-acting bronchodilators and systemic glucocorticoids, by treating infection, and by providing supplemental oxygen and ventilatory support.
Bronchodilators
The short-acting beta-adrenergic agonists (eg, albuterol) act rapidly and are effective in producing bronchodilation. Nebulized therapy may be most comfortable for the acutely ill patient. Typical dosing is 2.5 mg albuterol diluted to 3 cc by nebulizer every hour. Higher doses are not more effective, and there is no evidence of a higher response rate from constant nebulized therapy, which can cause anxiety and tachycardia in patients.14 Anticholinergic agents (eg, ipratropium) are often added despite unclear data regarding clinical advantage. In one study evaluating the effectiveness of adding ipratropium to albuterol, patients receiving a combination had the same improvement in FEV1 at 90 minutes.15 Patients receiving ipratropium alone had the lowest rate of reported side effects.15
Systemic Glucocorticoids
Short-course systemic glucocorticoids are an important addition to treatment and have been found to improve spirometry and decrease relapse rate. The oral and intravenous (IV) routes provide the same benefit. For the acutely ill patient with challenges swallowing, the IV route is preferred. The optimal dose is not clear, but hydrocortisone doses of 100 mg to 125 mg every 6 hours for 3 days are effective, as is oral prednisone 30 mg per day for 14 days, or 60 mg per day for 3 days with a taper.
Antibiotic Therapy
Antibiotics are indicated for patients with moderate to severe AECOPD who are ill enough to be admitted to the hospital. Empiric broad spectrum treatment is recommended. The initial antibiotic regimen should target likely bacterial pathogens (Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in most patients) and take into account local patterns of antibiotic resistance. Flouroquinolones or third-generation cephalosporins generally provide sufficient coverage. For patients experiencing only a mild exacerbation, antibiotics are not warranted.
Magnesium Sulfate
Other supplemental medications that have been evaluated include magnesium sulfate for bronchial smooth muscle relaxation. Studies have found that while magnesium is helpful in asthma, results are mixed with COPD.16
Supplemental Oxygen
Oxygen therapy is important during an AECOPD episode. Often, concerns arise about decreasing respiratory drive, which is typically driven by hypoxia in patients who have chronic hypercapnia. Arterial blood gas determinations are important in managing a patient’s respiratory status and will assist in determining actual oxygenation and any coexistent metabolic disturbances.
Noninvasive Ventilation. Oxygen can be administered efficiently by a venturi mask, which delivers precise fractions of oxygen, or by nasal cannula. A facemask is less comfortable, but is available for higher oxygen requirements, providing up to 55% oxygen, while a nonrebreather mask delivers up to 90% oxygen.
When necessary, noninvasive positive pressure ventilation (NPPV) improves outcomes for those with severe dyspnea and signs of respiratory fatigue manifested as increased work of breathing. Noninvasive positive pressure ventilation can improve clinical outcomes and is the ventilator mode of choice for those patients with COPD. Indications include severe dyspnea with signs of increased work of breathing and respiratory acidosis (arterial pH <7.35) and partial pressure of arterial carbon dioxide (PaCO2) >45 mm Hg.
Whenever possible, NPPV should be initiated with a triggered mode to allow spontaneous breaths. Inspiratory pressure of 8 cm to 12 cm H2O and expiratory pressure of 3 cm to 5 cm of H2 are recommended.
Mechanical Ventilation. Mechanical ventilation is often undesirable because it may be extraordinarily difficult to wean a patient off the device and permit safe extubation. However, if a patient cannot be stabilized with NPPV, intubation and mechanical ventilation must be considered. Typically, this occurs when there is severe respiratory distress, tachypnea >30 breaths/min, accessory muscle use, and altered mentation.
Goals of intubation/mechanical respiration include correcting oxygenation and severe respiratory acidosis as well as reducing the work of breathing. Barotrauma is a significant risk when patients with COPD require mechanical ventilation. Volume-limited modes of ventilation are commonly used, while pressure support or pressure-limited modes are less suitable for patients with airflow limitation. Again, invasive ventilation should only be administered if a patient cannot tolerate NPPV.
Palliative Care in the ED
Palliative care is an approach that improves the QOL of patients and their families facing the issues associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and accurate assessment and treatment of pain and physical, psychosocial, and spiritual problems.3 This approach to care is warranted for COPD patients given the myriad of burdensome symptoms and functional decline that occurs.17
Palliative care expands traditional treatment goals to include enhancing QOL; helping with medical decision making; and identifying the goals of care. Palliative care is provided by board-certified physicians for the most complex of cases. However, the primary practice of palliative care must be delivered at the bedside by the treating provider. Managing pain, dyspnea, nausea, vomiting, and changes in bowel habits, as well as discussing goals of care, are among the basic palliative care skills all providers need to have and apply when indicated.
Palliative Care for Dyspnea
Opioids. Primary palliative care in the ED includes the appropriate use of low-dose oral and parenteral opioids to treat dyspnea in AECOPD. The use of a low-dose opioid, such as morphine 2 mg IV, titrated up to a desired response, is a safe and effective practice.18 Note the 2-mg starting dose is considered low-dose.19
With respect to managing dyspnea in AECOPD patients, nebulized opioids have not been found to be better than nebulized saline. More specific data regarding the use of oral opioids for managing refractory dyspnea in patients with predominantly COPD have been recently published: Long-acting morphine 20 mg once daily provides symptomatic relief in refractory dyspnea in the community setting. For the opioid-naïve patient, a lower dose is recommended.20
Oxygenation. There is no hard evidence of the effectiveness of oxygen in the palliation of breathlessness. Humidified air is effective initially, as is providing a fan at the bedside. Short-burst oxygen therapy should only be considered for episodes of severe breathlessness in patients whose COPD is not relieved by other treatments. Oxygen should continue to be prescribed only if an improvement in breathlessness following therapy has been documented. The American Thoracic Society recommends continuous oxygen therapy in patients with COPD who have severe resting hypoxemia (PaCO2 ≤55 mm Hg or SpO2 ≤88%).21
POLST Form
The Physicians Order for Life-Sustaining Treatment (POLST) form is a set of medical orders, similar to the “do not resuscitate” (allow natural death) order. A POLST form is not an advance directive and does not serve as a substitute for a patient’s assignation of a health care agent or durable power of attorney for health care.22
The POLST form enables physicians to order treatments patients would want, identify those treatments that patients would not want, and not provide those the patient considers “extraordinary” and excessively burdensome. A POLST form does not allow for active euthanasia or physician-assisted suicide.
Identifying treatment preferences is an important part of the initial evaluation of all patients. When dealing with an airway issue in a COPD patient, management can become complex. Ideally, the POLST form should arrive with the patient in the ED and list preferences regarding possible intensive interventions such as intubation and chest compressions. Discussing these issues with a patient in extreme distress is difficult or impossible, and in these cases, access to pertinent medical records, discussing preferences with family caregivers, and availability of a POLST form are much better ways to determine therapy.
Palliative Home Care
Patient Safety Considerations
Weight loss and associated muscle wasting are common features in patients with severe COPD, creating a high-risk situation for falls and a need for assistance with activities of daily living. The patient who is frail when discharged home from the ED requires a home-care plan before leaving the ED, and strict follow-up with the patient’s primary care provider will typically be needed within 2 to 4 weeks.
Psychological Considerations
Being mindful of the anxiety and depression that accompany the physical limitations of those with COPD is important. Mood disturbances serve as risk factors for re-hospitalization and mortality.13Multiple palliative care interventions provide patients assistance with these issues, including the use of antidepressants that may aid sleep, stabilize mood, and stimulate appetite.
Early referral to the palliative care team will provide improved care for the patient and family. Palliative care referral will provide continued management of the physical symptoms and evaluation and treatment of the psychosocial issues that accompany COPD. Additionally, the palliative care team can assist with safe discharge planning and follow-up, including the provision of the patient’s home needs as well as the family’s ability to cope with the home setting.
Prognosis
Predicting prognosis is difficult for the COPD patient due to the highly variable illness trajectory. Some patients have a low FEV1 and yet are very functional. However, assessment of severity of lung function impairment, frequency of exacerbations, and need for long-term oxygen therapy helps identify those patients who are entering the final 12 months of life. Evaluating symptom burden and impact on activities of daily living for patients with COPD is comparable to those of cancer patients, and in both cases, palliative care approaches are necessary.
Predicting Morbidity and Mortality
A profile developed from observational studies can help predict 6- to 12-month morbidity and mortality in patients with advanced COPD. This profile includes the following criteria:
- Significant dyspnea;
- FEV1 <30%;
- Number of exacerbations;
- Left heart failure or other comorbidities;
- Weight loss or cachexia;
- Decreasing performance status;
- Age older than 70 years; and
- Depression.
Although additional research is required to refine and verify this profile, reviewing these data points can prompt providers to initiate discussions with patients about treatment preferences and end-of-life care.23,24
Palliative Performance Scale
The Palliative Performance Scale (PPS) is another scale used to predict prognosis and eligibility for hospice care.25 This score provides a patient’s estimated survival.25 For a patient with a PPS score of 50%, hospice education may be appropriate.
Case Scenario Continued
Both the BODE and GOLD criteria scores assisted in determining prognosis and risk profiles of the patient in our case scenario. By applying the BODE criteria, our patient had a 4-year survival benefit of under 18%. The GOLD criteria results for this patient also were consistent with the BODE criteria and reflected end-stage COPD. Since this patient also had a PPS score of 50%, hospice education and care were discussed and initiated.
Conclusion
Patients with AECOPD commonly present to the ED. Such patients suffer with a high burden of illness and a need for immediate symptom management. However, after these measures have been instituted, strong evidence suggests that these patients typically do not receive palliative care with the same frequency compared to cancer or heart disease patients.
Management of AECOPD in the ED must include rapid treatment of dyspnea and pain, but also a determination of treatment preferences and an understanding of the prognosis. Several criteria are available to guide prognostic awareness and may help further the goals of care and disposition. Primary palliative care should be started by the ED provider for appropriate patients, with early referral to the palliative care team.
Case Scenario
A 62-year-old man who regularly presented to the ED for exacerbations of chronic obstructive pulmonary disease (COPD) after running out of his medications presented again for evaluation and treatment. His outpatient care had been poorly coordinated, and he relied on the ED to provide him with the support he needed. This presentation represented his fifth visit to the ED over the past 3 months.
The patient’s medical history was positive for asthma since childhood, tobacco use, hypertension, and a recent diagnosis of congestive heart failure (CHF). Over the past year, he had four hospital admissions, and was currently unable to walk from his bedroom to another room without becoming short of breath. He also had recently experienced a 20-lb weight loss.
At this visit, the patient complained of chest pain and lightheadedness, which he described as suffocating. Prior to these recent symptoms, he enjoyed walking in his neighborhood and talking with friends. He was an avid reader and sports fan, but admitted that he now had trouble focusing on reading and following games on television. He lived alone, and his family lived across the country. The patient further admitted that although he had attempted to quit cigarette smoking, he was unable to give up his 50-pack per year habit. He had no completed advance health care directive and had significant challenges tending to his basic needs.
The Trajectory of COPD
Chronic obstructive pulmonary disease is a common chronic illness that causes significant morbidity and mortality. A 2016 National Health Services report cited respiratory illness, primarily from COPD, as the third leading cause of death in the United States in 2014.1The trajectory of this disease is marked by frequent exacerbations with partial recovery to baseline function. The burden of those living with COPD is significant and marked by a poor overall health-related quality of life (QOL). The ED has become a staging area for patients seeking care for exacerbations of COPD.2
The World Health Organization (WHO) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) have defined COPD as a spectrum of diseases including emphysema, chronic bronchitis, and chronic obstructive asthma characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response to noxious particles or gases in the airways and lungs.3 Exacerbations and comorbidities contribute to the overall severity of COPD in individual patients.4
The case presented in this article illustrates the common scenario of a patient whose COPD has become severe and highly symptomatic with declining function to the point where he requires home support. His physical decline had been rapid and resulted in many unmet needs. When a patient such as this presents for emergent care, he must first be stabilized; then a care plan will need to be developed prior to discharge.
Management Goals
The overall goals of treating COPD are based on preserving function and are not curative in nature. Chronic obstructive pulmonary disease is a progressive illness that will intensify over time.5 As such, palliative care services are warranted. However, many patients with COPD do not receive palliative care services compared to patients with such other serious and life-limiting disease as cancer and heart disease.
Acute Exacerbations of COPD
Incidence
The frequency of acute exacerbations of COPD (AECOPD) increases with age, productive cough, long-standing COPD, previous hospitalizations related to COPD, eosinophilia, and comorbidities (eg, CHF). Patients with moderate to severe COPD and a history of prior exacerbations were found to have a higher likelihood of future exacerbations. From a quality and cost perspective, it may be useful to identify high-risk patients and strengthen their outpatient program to lessen the need for ED care and more intensive support.6,7
In our case scenario, the patient could have been stabilized at home with a well-controlled plan and home support, which would have resulted in an improved QOL and more time free from his high symptom burden.
Causes
Bacterial and viral respiratory infections are the most likely cause of AECOPD. Environmental pollution and pulmonary embolism are also triggers. Typically, patients with AECOPD present to the ED up to several times a year2 and represent the third most common cause of 30-day readmissions to the hospital.8 Prior exacerbations, dyspnea, and other medical comorbidities are also risk factors for more frequent hospital visits.
Presenting Signs and Symptoms
Each occurrence of AECOPD represents a worsening of a patient’s respiratory symptoms beyond normal variations. This might include increases in cough, sputum production, and dyspnea. The goal in caring for a person with an AECOPD is to stabilize the acute event and provide a treatment plan. The range of acuity for moderate to severe disease makes devising an appropriate treatment plan challenging, and after implementing the best plans, the patient’s course may be characterized by a prolonged cycle of admissions and readmissions without substantial return to baseline.
Management
In practice, ED management of AECOPD in older adults typically differs significantly from published guideline recommendations,9 which may result in pooroutcomes related to shortcomings in quality of care. Better adherence to guideline recommendations when caring for elderly patients with COPD may lead to improved clinical outcomes and better resource usage.
Risk Stratification
Complicating ED management is the challenge of determining the severity of illness and degree of the exacerbation. Airflow obstruction alone is not sufficient to predict outcomes, as any particular measure of obstruction is associated with a spectrum of forced expiratory volume in the first second (FEV1) and varying performance. Moreover, peak-flow measurements are not useful in the setting of AECOPD, as opposed to their use in acute asthma exacerbations, and are not predictive of changes in clinical status.
GOLD and NICE Criteria
Guidelines have been developed and widely promoted to assist ED and hospital and community clinicians in providing evidence-based management for COPD patients. The GOLD Criteria and the National Institute for Clinical Excellence (NICE) are both clinical guidelines on management of COPD.10
Although well recognized and commonly used, the original GOLD criteria did not take into account the frequency and importance of the extrapulmonary manifestations of COPD in predicting outcome. Typically, those with severe or very severe COPD have an average of 12 co-occurring symptoms, an even greater number of signs and symptoms than those occurring in patients with cancer or heart or renal disease.11
BODE Criteria
The body mass index, airflow obstruction, dyspnea and exercise capacity (BODE) criteria assess and predict the health-related QOL and mortality risk for patients with COPD. Risk is adjusted based on four factors—weight, airway obstruction, dyspnea, and exercise capacity (ie, 6-minute walk distance).13
Initial Evaluation and Work-Up
As previously noted, when an AECOPD patient arrives to the ED, the first priority is to stabilize the patient and initiate treatment. In this respect, initial identification of the patient’s pulse oxygen saturation (SpO2) is important.
Laboratory Evaluation
In cases of respiratory failure, obtaining arterial blood gas (ABG) values are critical. The ABG test will assist in determining acute exacerbations of chronic hypercapnia and the need for ventilatory support. When considering CHF, a plasma B-type natriuretic peptide is useful to assess for CHF.
Imaging Studies
A chest radiograph may be useful in the initial evaluation to identify abnormalities, including barotrauma (ie, pneumothorax) and infiltrates. Additionally, in patients with comorbidities, it is important to assess cardiac status, and a chest X-ray may assist in identification of pulmonary edema, pleural effusions, and cardiomegaly. If the radiograph does show a pulmonary infiltrate (ie, pneumonia), it will help identify the probable triggers, but even in these instances, a sputum gram stain will not assist in the diagnosis.
Treatment
Relieving airflow obstruction is achieved with inhaled short-acting bronchodilators and systemic glucocorticoids, by treating infection, and by providing supplemental oxygen and ventilatory support.
Bronchodilators
The short-acting beta-adrenergic agonists (eg, albuterol) act rapidly and are effective in producing bronchodilation. Nebulized therapy may be most comfortable for the acutely ill patient. Typical dosing is 2.5 mg albuterol diluted to 3 cc by nebulizer every hour. Higher doses are not more effective, and there is no evidence of a higher response rate from constant nebulized therapy, which can cause anxiety and tachycardia in patients.14 Anticholinergic agents (eg, ipratropium) are often added despite unclear data regarding clinical advantage. In one study evaluating the effectiveness of adding ipratropium to albuterol, patients receiving a combination had the same improvement in FEV1 at 90 minutes.15 Patients receiving ipratropium alone had the lowest rate of reported side effects.15
Systemic Glucocorticoids
Short-course systemic glucocorticoids are an important addition to treatment and have been found to improve spirometry and decrease relapse rate. The oral and intravenous (IV) routes provide the same benefit. For the acutely ill patient with challenges swallowing, the IV route is preferred. The optimal dose is not clear, but hydrocortisone doses of 100 mg to 125 mg every 6 hours for 3 days are effective, as is oral prednisone 30 mg per day for 14 days, or 60 mg per day for 3 days with a taper.
Antibiotic Therapy
Antibiotics are indicated for patients with moderate to severe AECOPD who are ill enough to be admitted to the hospital. Empiric broad spectrum treatment is recommended. The initial antibiotic regimen should target likely bacterial pathogens (Haemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae in most patients) and take into account local patterns of antibiotic resistance. Flouroquinolones or third-generation cephalosporins generally provide sufficient coverage. For patients experiencing only a mild exacerbation, antibiotics are not warranted.
Magnesium Sulfate
Other supplemental medications that have been evaluated include magnesium sulfate for bronchial smooth muscle relaxation. Studies have found that while magnesium is helpful in asthma, results are mixed with COPD.16
Supplemental Oxygen
Oxygen therapy is important during an AECOPD episode. Often, concerns arise about decreasing respiratory drive, which is typically driven by hypoxia in patients who have chronic hypercapnia. Arterial blood gas determinations are important in managing a patient’s respiratory status and will assist in determining actual oxygenation and any coexistent metabolic disturbances.
Noninvasive Ventilation. Oxygen can be administered efficiently by a venturi mask, which delivers precise fractions of oxygen, or by nasal cannula. A facemask is less comfortable, but is available for higher oxygen requirements, providing up to 55% oxygen, while a nonrebreather mask delivers up to 90% oxygen.
When necessary, noninvasive positive pressure ventilation (NPPV) improves outcomes for those with severe dyspnea and signs of respiratory fatigue manifested as increased work of breathing. Noninvasive positive pressure ventilation can improve clinical outcomes and is the ventilator mode of choice for those patients with COPD. Indications include severe dyspnea with signs of increased work of breathing and respiratory acidosis (arterial pH <7.35) and partial pressure of arterial carbon dioxide (PaCO2) >45 mm Hg.
Whenever possible, NPPV should be initiated with a triggered mode to allow spontaneous breaths. Inspiratory pressure of 8 cm to 12 cm H2O and expiratory pressure of 3 cm to 5 cm of H2 are recommended.
Mechanical Ventilation. Mechanical ventilation is often undesirable because it may be extraordinarily difficult to wean a patient off the device and permit safe extubation. However, if a patient cannot be stabilized with NPPV, intubation and mechanical ventilation must be considered. Typically, this occurs when there is severe respiratory distress, tachypnea >30 breaths/min, accessory muscle use, and altered mentation.
Goals of intubation/mechanical respiration include correcting oxygenation and severe respiratory acidosis as well as reducing the work of breathing. Barotrauma is a significant risk when patients with COPD require mechanical ventilation. Volume-limited modes of ventilation are commonly used, while pressure support or pressure-limited modes are less suitable for patients with airflow limitation. Again, invasive ventilation should only be administered if a patient cannot tolerate NPPV.
Palliative Care in the ED
Palliative care is an approach that improves the QOL of patients and their families facing the issues associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and accurate assessment and treatment of pain and physical, psychosocial, and spiritual problems.3 This approach to care is warranted for COPD patients given the myriad of burdensome symptoms and functional decline that occurs.17
Palliative care expands traditional treatment goals to include enhancing QOL; helping with medical decision making; and identifying the goals of care. Palliative care is provided by board-certified physicians for the most complex of cases. However, the primary practice of palliative care must be delivered at the bedside by the treating provider. Managing pain, dyspnea, nausea, vomiting, and changes in bowel habits, as well as discussing goals of care, are among the basic palliative care skills all providers need to have and apply when indicated.
Palliative Care for Dyspnea
Opioids. Primary palliative care in the ED includes the appropriate use of low-dose oral and parenteral opioids to treat dyspnea in AECOPD. The use of a low-dose opioid, such as morphine 2 mg IV, titrated up to a desired response, is a safe and effective practice.18 Note the 2-mg starting dose is considered low-dose.19
With respect to managing dyspnea in AECOPD patients, nebulized opioids have not been found to be better than nebulized saline. More specific data regarding the use of oral opioids for managing refractory dyspnea in patients with predominantly COPD have been recently published: Long-acting morphine 20 mg once daily provides symptomatic relief in refractory dyspnea in the community setting. For the opioid-naïve patient, a lower dose is recommended.20
Oxygenation. There is no hard evidence of the effectiveness of oxygen in the palliation of breathlessness. Humidified air is effective initially, as is providing a fan at the bedside. Short-burst oxygen therapy should only be considered for episodes of severe breathlessness in patients whose COPD is not relieved by other treatments. Oxygen should continue to be prescribed only if an improvement in breathlessness following therapy has been documented. The American Thoracic Society recommends continuous oxygen therapy in patients with COPD who have severe resting hypoxemia (PaCO2 ≤55 mm Hg or SpO2 ≤88%).21
POLST Form
The Physicians Order for Life-Sustaining Treatment (POLST) form is a set of medical orders, similar to the “do not resuscitate” (allow natural death) order. A POLST form is not an advance directive and does not serve as a substitute for a patient’s assignation of a health care agent or durable power of attorney for health care.22
The POLST form enables physicians to order treatments patients would want, identify those treatments that patients would not want, and not provide those the patient considers “extraordinary” and excessively burdensome. A POLST form does not allow for active euthanasia or physician-assisted suicide.
Identifying treatment preferences is an important part of the initial evaluation of all patients. When dealing with an airway issue in a COPD patient, management can become complex. Ideally, the POLST form should arrive with the patient in the ED and list preferences regarding possible intensive interventions such as intubation and chest compressions. Discussing these issues with a patient in extreme distress is difficult or impossible, and in these cases, access to pertinent medical records, discussing preferences with family caregivers, and availability of a POLST form are much better ways to determine therapy.
Palliative Home Care
Patient Safety Considerations
Weight loss and associated muscle wasting are common features in patients with severe COPD, creating a high-risk situation for falls and a need for assistance with activities of daily living. The patient who is frail when discharged home from the ED requires a home-care plan before leaving the ED, and strict follow-up with the patient’s primary care provider will typically be needed within 2 to 4 weeks.
Psychological Considerations
Being mindful of the anxiety and depression that accompany the physical limitations of those with COPD is important. Mood disturbances serve as risk factors for re-hospitalization and mortality.13Multiple palliative care interventions provide patients assistance with these issues, including the use of antidepressants that may aid sleep, stabilize mood, and stimulate appetite.
Early referral to the palliative care team will provide improved care for the patient and family. Palliative care referral will provide continued management of the physical symptoms and evaluation and treatment of the psychosocial issues that accompany COPD. Additionally, the palliative care team can assist with safe discharge planning and follow-up, including the provision of the patient’s home needs as well as the family’s ability to cope with the home setting.
Prognosis
Predicting prognosis is difficult for the COPD patient due to the highly variable illness trajectory. Some patients have a low FEV1 and yet are very functional. However, assessment of severity of lung function impairment, frequency of exacerbations, and need for long-term oxygen therapy helps identify those patients who are entering the final 12 months of life. Evaluating symptom burden and impact on activities of daily living for patients with COPD is comparable to those of cancer patients, and in both cases, palliative care approaches are necessary.
Predicting Morbidity and Mortality
A profile developed from observational studies can help predict 6- to 12-month morbidity and mortality in patients with advanced COPD. This profile includes the following criteria:
- Significant dyspnea;
- FEV1 <30%;
- Number of exacerbations;
- Left heart failure or other comorbidities;
- Weight loss or cachexia;
- Decreasing performance status;
- Age older than 70 years; and
- Depression.
Although additional research is required to refine and verify this profile, reviewing these data points can prompt providers to initiate discussions with patients about treatment preferences and end-of-life care.23,24
Palliative Performance Scale
The Palliative Performance Scale (PPS) is another scale used to predict prognosis and eligibility for hospice care.25 This score provides a patient’s estimated survival.25 For a patient with a PPS score of 50%, hospice education may be appropriate.
Case Scenario Continued
Both the BODE and GOLD criteria scores assisted in determining prognosis and risk profiles of the patient in our case scenario. By applying the BODE criteria, our patient had a 4-year survival benefit of under 18%. The GOLD criteria results for this patient also were consistent with the BODE criteria and reflected end-stage COPD. Since this patient also had a PPS score of 50%, hospice education and care were discussed and initiated.
Conclusion
Patients with AECOPD commonly present to the ED. Such patients suffer with a high burden of illness and a need for immediate symptom management. However, after these measures have been instituted, strong evidence suggests that these patients typically do not receive palliative care with the same frequency compared to cancer or heart disease patients.
Management of AECOPD in the ED must include rapid treatment of dyspnea and pain, but also a determination of treatment preferences and an understanding of the prognosis. Several criteria are available to guide prognostic awareness and may help further the goals of care and disposition. Primary palliative care should be started by the ED provider for appropriate patients, with early referral to the palliative care team.
1. National Center for Health Statistics. Health, United States 2015 With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: US Dept. Health and Human Services; 2016. http://www.cdc.gov/nchs/hus/. Accessed October 17, 2016.
2. Khialani B, Sivakumaran P, Keijzers G, Sriram KB. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease and factors associated with hospitalization. J Res Med Sci . 2014;19(4):297-303.
3. World Health Organization Web site. Chronic respiratory diseases. COPD: Definition. http://www.who.int/respiratory/copd/definition/en/. Accessed October 17, 2016.
4. Rabe KF, Hurd S, Anzueto A, et al; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med . 2007;176(6):532-555.
5. Fan VS, Ramsey SD, Make BJ, Martinez FJ. Physiologic variables and functional status independently predict COPD hospitalizations and emergency department visits in patients with severe COPD. COPD . 2007;4(1):29-39.
6. Cydulka RK, Rowe BH, Clark S, Emerman CL, Camargo CA Jr; MARC Investigators. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: the Multicenter Airway Research Collaboration. J Am Geriatr Soc . 2003;51(7):908-916.
7. Strassels SA, Smith DH, Sullivan SD, et al. The costs of treating COPD in the United States. Chest . 2001;119:3.
8. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med . 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
9. Rowe BH, Bhutani M, Stickland MK, Cydulka R. Assessment and management of chronic obstructive pulmonary disease in the emergency department and beyond. Expert Rev Respir Med . 2011;5(4):549-559. doi:10.1586/ers.11.43.
10. National Institute for Clinical Excellence Web site. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Clinical Guideline CG101. https://www.nice.org.uk/Guidance/cg101. Published June 2010. Accessed October 17, 2016.
11. Christensen VL, Holm AM, Cooper B, Paul SM, Miaskowski C, Rustøen T. Differences in symptom burden among patients with moderate, severe, or very severe chronic obstructive pulmonary disease. J Pain Symptom Manage . 2016;51(5):849-859. doi:10.1016/j.jpainsymman.2015.12.324.
12. GOLD Reports. Global Initiative for Chronic Obstructive Lung Disease Web site. http://goldcopd.org/gold-reports/. Accessed October 17, 2016.
13. Funk GC, Kirchheiner K, Burghuber OC, Hartl S. BODE index versus GOLD classification for explaining anxious and depressive symptoms in patients with COPD—a cross-sectional study. Respir Res . 2009;10:1. doi:10.1186/1465-9921-10-1.
14. Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians-American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med . 2001;134(7):600-620.
15. McCrory DC, Brown CD. Inhaled short-acting beta 2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2001;(2):CD002984.
16. Shivanthan MC, Rajapakse S. Magnesium for acute exacerbation of chronic obstructive pulmonary disease: A systematic review of randomised trials. Ann Thorac Med . 2014;9(2):77-80. doi:10.4103/1817-1737.128844.
17. Curtis JR. Palliative and end of life care for patients with severe COPD. Eur Respir J . 2008;32(3):796-803.
18. Rocker GM, Simpson AC, Young J, et al. Opioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: patients’ experiences and outcomes. CMAJ Open . 2013;1(1):E27-E36.
19. Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnea. Thorax . 2002;57(11):939-944.
20. Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ . 2003;327(7414):523-528.
21. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med . 2011;155(3):179-191. doi:10.7326/0003-4819-155-3-201108020-00008.
22. National POLST Paradigm. http://polst.org/professionals-page/?pro=1. Accessed October 17, 2016.
23. Hansen-Flaschen J. Chronic obstructive pulmonary disease: the last year of life. Respir Care. 2004;49(1):90-97; discussion 97-98.
24. Spathis A, Booth S. End of life care in chronic obstructive pulmonary disease: in search of a good death. Int J Chron Obstruct Pulmon Dis . 2008;3(1):11-29.
25. Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care . 1996;12(1):5-11.
1. National Center for Health Statistics. Health, United States 2015 With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: US Dept. Health and Human Services; 2016. http://www.cdc.gov/nchs/hus/. Accessed October 17, 2016.
2. Khialani B, Sivakumaran P, Keijzers G, Sriram KB. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease and factors associated with hospitalization. J Res Med Sci . 2014;19(4):297-303.
3. World Health Organization Web site. Chronic respiratory diseases. COPD: Definition. http://www.who.int/respiratory/copd/definition/en/. Accessed October 17, 2016.
4. Rabe KF, Hurd S, Anzueto A, et al; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med . 2007;176(6):532-555.
5. Fan VS, Ramsey SD, Make BJ, Martinez FJ. Physiologic variables and functional status independently predict COPD hospitalizations and emergency department visits in patients with severe COPD. COPD . 2007;4(1):29-39.
6. Cydulka RK, Rowe BH, Clark S, Emerman CL, Camargo CA Jr; MARC Investigators. Emergency department management of acute exacerbations of chronic obstructive pulmonary disease in the elderly: the Multicenter Airway Research Collaboration. J Am Geriatr Soc . 2003;51(7):908-916.
7. Strassels SA, Smith DH, Sullivan SD, et al. The costs of treating COPD in the United States. Chest . 2001;119:3.
8. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med . 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
9. Rowe BH, Bhutani M, Stickland MK, Cydulka R. Assessment and management of chronic obstructive pulmonary disease in the emergency department and beyond. Expert Rev Respir Med . 2011;5(4):549-559. doi:10.1586/ers.11.43.
10. National Institute for Clinical Excellence Web site. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Clinical Guideline CG101. https://www.nice.org.uk/Guidance/cg101. Published June 2010. Accessed October 17, 2016.
11. Christensen VL, Holm AM, Cooper B, Paul SM, Miaskowski C, Rustøen T. Differences in symptom burden among patients with moderate, severe, or very severe chronic obstructive pulmonary disease. J Pain Symptom Manage . 2016;51(5):849-859. doi:10.1016/j.jpainsymman.2015.12.324.
12. GOLD Reports. Global Initiative for Chronic Obstructive Lung Disease Web site. http://goldcopd.org/gold-reports/. Accessed October 17, 2016.
13. Funk GC, Kirchheiner K, Burghuber OC, Hartl S. BODE index versus GOLD classification for explaining anxious and depressive symptoms in patients with COPD—a cross-sectional study. Respir Res . 2009;10:1. doi:10.1186/1465-9921-10-1.
14. Bach PB, Brown C, Gelfand SE, McCrory DC; American College of Physicians-American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med . 2001;134(7):600-620.
15. McCrory DC, Brown CD. Inhaled short-acting beta 2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2001;(2):CD002984.
16. Shivanthan MC, Rajapakse S. Magnesium for acute exacerbation of chronic obstructive pulmonary disease: A systematic review of randomised trials. Ann Thorac Med . 2014;9(2):77-80. doi:10.4103/1817-1737.128844.
17. Curtis JR. Palliative and end of life care for patients with severe COPD. Eur Respir J . 2008;32(3):796-803.
18. Rocker GM, Simpson AC, Young J, et al. Opioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: patients’ experiences and outcomes. CMAJ Open . 2013;1(1):E27-E36.
19. Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnea. Thorax . 2002;57(11):939-944.
20. Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ . 2003;327(7414):523-528.
21. Qaseem A, Wilt TJ, Weinberger SE, et al; American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med . 2011;155(3):179-191. doi:10.7326/0003-4819-155-3-201108020-00008.
22. National POLST Paradigm. http://polst.org/professionals-page/?pro=1. Accessed October 17, 2016.
23. Hansen-Flaschen J. Chronic obstructive pulmonary disease: the last year of life. Respir Care. 2004;49(1):90-97; discussion 97-98.
24. Spathis A, Booth S. End of life care in chronic obstructive pulmonary disease: in search of a good death. Int J Chron Obstruct Pulmon Dis . 2008;3(1):11-29.
25. Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care . 1996;12(1):5-11.
Case Studies in Toxicology: Somehow…It’s Always Lupus
Case
A 14-year-old girl with no known medical history presented to the ED via emergency medical services (EMS) approximately 1.5 hours after intentionally ingesting what she described as “a handful or two” of her mother’s lupus prescription medication in a suicide attempt. Initial vital signs and physical examination were normal, and her only complaint was nausea.
Thirty minutes after presentation, the patient suffered acute cardiovascular (CV) collapse: blood pressure, 57/39 mm Hg; heart rate, 90 beats/min. An initial electrocardiogram (ECG) revealed QRS duration of 123 milliseconds and QTc duration of 510 milliseconds, along with nonspecific T-wave abnormalities. A 150-mEq intravenous (IV) bolus of sodium bicarbonate and a 40-mEq potassium chloride IV infusion were administered, and both epinephrine and norepinephrine IV infusions were also initiated. A basic metabolic panel obtained prior to medication administration showed a potassium concentration of 1.9 mmol/L.
What is the differential diagnosis of toxicological hypokalemia?
Hypokalemia may be reflective of diminished whole body potassium stores or a transient alteration of intravascular potassium concentrations. In acute ingestions and overdose, the etiology of the hypokalemia is often electrolyte redistribution through either blockade of constitutive outward potassium leakage (eg, barium, insulin, quinine) or through increased activity of the Na+/K+-ATPase pump (eg, catecholamines, insulin, methylxanthines). This activity has little effect on whole body potassium stores, but can result in a profound fall in the serum potassium. While mild hypokalemia is generally well tolerated, more severe potassium abnormalities can cause muscular weakness, areflexic paralysis, respiratory failure, and life-threatening dysrhythmias. Common ECG findings include decreased T-wave amplitudes, ST-segment depression, and the presence or amplification of U waves.
Case Continuation
While the emergency physicians were stabilizing the patient, her mother provided additional information. Approximately 30 minutes after the exposure, the patient had become nauseated and told her mother what she had done. Her mother called EMS, and the patient was transported to the hospital, where she rapidly became symptomatic. Despite CV decompensation, she remained neurologically intact. On further questioning, the patient admitted to ingesting 6 g of her mother’s prescription of hydroxychloroquine (HCQ) in a suicide attempt but denied taking any other medications. She was stabilized on vasopressors and admitted to the intensive care unit.
What characterizes hydroxychloroquine toxicity?
Hydroxychloroquine is an aminoquinoline antibiotic that is classically used as an antimalarial to treat infection with Plasmodium vivax, P ovale, P malariae, and susceptible strains of P falciparum. In the United States, it is more commonly used to manage both rheumatoid arthritis and systemic lupus erythematosus (SLE), debilitating diseases which are estimated to affect anywhere from 161,000 to 322,000 Americans.1 Hydroxychloroquine is considered first-line therapy for SLE, but its mechanism of action in treating SLE-associated arthralgias is unclear.
Hydroxychloroquine is structurally similar to quinine and chloroquine (CQ), and not surprisingly exerts quinidine-like effects on the CV system with resultant negative inotropy and vasodilation. Its toxicity is characterized by rapid onset of clinical effects including central nervous system depression, seizures, apnea, hypotension, and arrhythmia. After large overdoses, cardiac arrest and death can occur within hours.
Hypokalemia is another hallmark of HCQ toxicity. It is thought to develop secondary to potassium channel blockade, which slows the constitutive release of potassium from the myocytes.2 As noted, the hypokalemia is transient and does not reflect whole-body depletion. With CQ, which is considered more toxic, there appears to be a correlation between the quantity of CQ ingested and both the degree of hypokalemia and the severity of the outcome. It is reasonable to assume the same for HCQ. There are little data to support that hypokalemia itself causes cardiotoxicity in patients with CQ or HCQ overdose.
Although lethal doses are not well established, animal studies suggest that HCQ is much less toxic than CQ, for which the clinical toxicity is better understood due to its more widespread use in overdose abroad.3 In children, the reported therapeutic dose is 10 mg/kg, but the minimum reported lethal dose was a single 300-mg tablet (30 mg/kg in a toddler). In adults, the toxic dose is reported as 20 mg/kg with lethal doses suggested to be as low as 30 mg/kg.
What are the treatment modalities for patients with hydroxychloroquine toxicity?
By analogy with the treatment of CQ poisoning, the mainstay of HCQ therapy is supportive care, including early intubation and ventilation to minimize metabolic demand. Direct-acting inotropes and vasopressors should be administered after saline to treat hypotension. Because of its large volume of distribution, extracorporeal removal has not proved to be of clinical value.4,5 Though data are sparse to determine its efficacy, there may be a role for giving activated charcoal, particularly following large overdoses—if it is given early after exposure and the patient has normal consciousness. If the patient is intubated and aspiration risk is minimized, gastric lavage may also be beneficial—especially when performed within an hour of the overdose. Syrup of ipecac should not be used.
High-dose diazepam is typically recommended, again by analogy with CQ, although there is no clear mechanism of action and its use remains controversial. Its protective effect in patients with CQ overdose is based on swine and rat models that demonstrated dose dependent relationships between diazepam and survival.6,7 A prospective study of CQ toxicity in humans reported improved survival rates when high-dose diazepam was given in combination with epinephrine.8 However, this study is limited by its comparison of prospectively studied patients with a retrospective control. A subsequent prospective study of moderately CQ-intoxicated patients did not find a benefit from treatment with diazepam.9 Furthermore, it remains unclear if the proposed benefit from high-dose diazepam in CQ toxicity may be extrapolated to HCQ, and cases of even massive HCQ ingestions report good outcomes without the use of high-dose diazepam.10
How aggressively should hypokalemia in hydroxychloroquine toxicity be treated?
As noted earlier, hypokalemia resulting from HCQ toxicity is transient, and aggressive repletion may result in rebound hyperkalemia once toxicity resolves. However, these dangers should be balanced with risks of hypokalemia-induced ventricular arrhythmias. Additionally, hypokalemia may be worsened by sodium bicarbonate that is administered to correct QRS prolongations, increasing the risk of dysrhythmia. Correction of hypokalemia in these cases is necessary but should be done with care and monitoring of serum potassium concentrations to minimize risk of hyperkalemia-induced ventricular arrhythmia.11
Case Conclusion
Throughout treatment, the patient remained neurologically intact. She did not receive benzodiazepines. The epinephrine and norepinephrine infusions were weaned, and she was discharged on hospital day 3 with no neurological or cardiac sequelae. She received an inpatient psychiatric evaluation and was referred to outpatient services for ongoing care.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008;58(1):15-25. doi:10.1002/art.23177.
2. Clemessy JL, Favier C, Borron SW, Hantson PE, Vicaut E, Baud FJ. Hypokalaemia related to acute chloroquine ingestion. Lancet. 1995;3469(8979):877-880.
3. McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med. 1983;75(suppl 1A):11-18.
4. Carmichael SJ, Charles B, Tett SE. Population pharmacokinetics of hydroxychloroquine in patients with rheumatoid arthritis. Ther Drug Monit. 2003;25(6):671-681.
5. Marquardt K, Albertson TE. Treatment of hydroxychloroquine overdose. Am J Emerg Med. 2001;19(5):420-424.
6. Crouzette J, Vicaut E, Palombo S, Girre C, Fournier PE. Experimental assessment of the protective activity of diazepam on the acute toxicity of chloroquine. J Toxicol Clin Toxicol. 1983;20(3):271-279.
7. Riou B, Lecarpentier Y, Barriot P, Viars P. Diazepam does not improve the mechanical performance of rat cardiac papillary muscle exposed to chloroquine in vitro. Intensive Care Med. 1989;15:390-3955.
8. Riou B, Barriot P, Rimailho A, Baud FJ. Treatment of severe chloroquine poisoning. N Engl J Med. 1988;318(1):1-6.
9. Clemessy JL, Angel G, Borron SW, et al. Therapeutic trial of diazepam versus placebo in acute chloroquine intoxications of moderate gravity. Intensive Care Med. 1996;22:1400-1405.
10. Yanturali S. Diazepam for treatment of massive chloroquine intoxication. Resuscitation. 2004;63(3):347-348.
11. Ling Ngan Wong A, Tsz Fung Cheung I, Graham CA. Hydroxychloroquine overdose: case report and recommendations for management. Eur J Emerg Med. 2008;15(1):16-8. doi:10.1097/MEJ.0b013e3280adcb56.
Case
A 14-year-old girl with no known medical history presented to the ED via emergency medical services (EMS) approximately 1.5 hours after intentionally ingesting what she described as “a handful or two” of her mother’s lupus prescription medication in a suicide attempt. Initial vital signs and physical examination were normal, and her only complaint was nausea.
Thirty minutes after presentation, the patient suffered acute cardiovascular (CV) collapse: blood pressure, 57/39 mm Hg; heart rate, 90 beats/min. An initial electrocardiogram (ECG) revealed QRS duration of 123 milliseconds and QTc duration of 510 milliseconds, along with nonspecific T-wave abnormalities. A 150-mEq intravenous (IV) bolus of sodium bicarbonate and a 40-mEq potassium chloride IV infusion were administered, and both epinephrine and norepinephrine IV infusions were also initiated. A basic metabolic panel obtained prior to medication administration showed a potassium concentration of 1.9 mmol/L.
What is the differential diagnosis of toxicological hypokalemia?
Hypokalemia may be reflective of diminished whole body potassium stores or a transient alteration of intravascular potassium concentrations. In acute ingestions and overdose, the etiology of the hypokalemia is often electrolyte redistribution through either blockade of constitutive outward potassium leakage (eg, barium, insulin, quinine) or through increased activity of the Na+/K+-ATPase pump (eg, catecholamines, insulin, methylxanthines). This activity has little effect on whole body potassium stores, but can result in a profound fall in the serum potassium. While mild hypokalemia is generally well tolerated, more severe potassium abnormalities can cause muscular weakness, areflexic paralysis, respiratory failure, and life-threatening dysrhythmias. Common ECG findings include decreased T-wave amplitudes, ST-segment depression, and the presence or amplification of U waves.
Case Continuation
While the emergency physicians were stabilizing the patient, her mother provided additional information. Approximately 30 minutes after the exposure, the patient had become nauseated and told her mother what she had done. Her mother called EMS, and the patient was transported to the hospital, where she rapidly became symptomatic. Despite CV decompensation, she remained neurologically intact. On further questioning, the patient admitted to ingesting 6 g of her mother’s prescription of hydroxychloroquine (HCQ) in a suicide attempt but denied taking any other medications. She was stabilized on vasopressors and admitted to the intensive care unit.
What characterizes hydroxychloroquine toxicity?
Hydroxychloroquine is an aminoquinoline antibiotic that is classically used as an antimalarial to treat infection with Plasmodium vivax, P ovale, P malariae, and susceptible strains of P falciparum. In the United States, it is more commonly used to manage both rheumatoid arthritis and systemic lupus erythematosus (SLE), debilitating diseases which are estimated to affect anywhere from 161,000 to 322,000 Americans.1 Hydroxychloroquine is considered first-line therapy for SLE, but its mechanism of action in treating SLE-associated arthralgias is unclear.
Hydroxychloroquine is structurally similar to quinine and chloroquine (CQ), and not surprisingly exerts quinidine-like effects on the CV system with resultant negative inotropy and vasodilation. Its toxicity is characterized by rapid onset of clinical effects including central nervous system depression, seizures, apnea, hypotension, and arrhythmia. After large overdoses, cardiac arrest and death can occur within hours.
Hypokalemia is another hallmark of HCQ toxicity. It is thought to develop secondary to potassium channel blockade, which slows the constitutive release of potassium from the myocytes.2 As noted, the hypokalemia is transient and does not reflect whole-body depletion. With CQ, which is considered more toxic, there appears to be a correlation between the quantity of CQ ingested and both the degree of hypokalemia and the severity of the outcome. It is reasonable to assume the same for HCQ. There are little data to support that hypokalemia itself causes cardiotoxicity in patients with CQ or HCQ overdose.
Although lethal doses are not well established, animal studies suggest that HCQ is much less toxic than CQ, for which the clinical toxicity is better understood due to its more widespread use in overdose abroad.3 In children, the reported therapeutic dose is 10 mg/kg, but the minimum reported lethal dose was a single 300-mg tablet (30 mg/kg in a toddler). In adults, the toxic dose is reported as 20 mg/kg with lethal doses suggested to be as low as 30 mg/kg.
What are the treatment modalities for patients with hydroxychloroquine toxicity?
By analogy with the treatment of CQ poisoning, the mainstay of HCQ therapy is supportive care, including early intubation and ventilation to minimize metabolic demand. Direct-acting inotropes and vasopressors should be administered after saline to treat hypotension. Because of its large volume of distribution, extracorporeal removal has not proved to be of clinical value.4,5 Though data are sparse to determine its efficacy, there may be a role for giving activated charcoal, particularly following large overdoses—if it is given early after exposure and the patient has normal consciousness. If the patient is intubated and aspiration risk is minimized, gastric lavage may also be beneficial—especially when performed within an hour of the overdose. Syrup of ipecac should not be used.
High-dose diazepam is typically recommended, again by analogy with CQ, although there is no clear mechanism of action and its use remains controversial. Its protective effect in patients with CQ overdose is based on swine and rat models that demonstrated dose dependent relationships between diazepam and survival.6,7 A prospective study of CQ toxicity in humans reported improved survival rates when high-dose diazepam was given in combination with epinephrine.8 However, this study is limited by its comparison of prospectively studied patients with a retrospective control. A subsequent prospective study of moderately CQ-intoxicated patients did not find a benefit from treatment with diazepam.9 Furthermore, it remains unclear if the proposed benefit from high-dose diazepam in CQ toxicity may be extrapolated to HCQ, and cases of even massive HCQ ingestions report good outcomes without the use of high-dose diazepam.10
How aggressively should hypokalemia in hydroxychloroquine toxicity be treated?
As noted earlier, hypokalemia resulting from HCQ toxicity is transient, and aggressive repletion may result in rebound hyperkalemia once toxicity resolves. However, these dangers should be balanced with risks of hypokalemia-induced ventricular arrhythmias. Additionally, hypokalemia may be worsened by sodium bicarbonate that is administered to correct QRS prolongations, increasing the risk of dysrhythmia. Correction of hypokalemia in these cases is necessary but should be done with care and monitoring of serum potassium concentrations to minimize risk of hyperkalemia-induced ventricular arrhythmia.11
Case Conclusion
Throughout treatment, the patient remained neurologically intact. She did not receive benzodiazepines. The epinephrine and norepinephrine infusions were weaned, and she was discharged on hospital day 3 with no neurological or cardiac sequelae. She received an inpatient psychiatric evaluation and was referred to outpatient services for ongoing care.
Case
A 14-year-old girl with no known medical history presented to the ED via emergency medical services (EMS) approximately 1.5 hours after intentionally ingesting what she described as “a handful or two” of her mother’s lupus prescription medication in a suicide attempt. Initial vital signs and physical examination were normal, and her only complaint was nausea.
Thirty minutes after presentation, the patient suffered acute cardiovascular (CV) collapse: blood pressure, 57/39 mm Hg; heart rate, 90 beats/min. An initial electrocardiogram (ECG) revealed QRS duration of 123 milliseconds and QTc duration of 510 milliseconds, along with nonspecific T-wave abnormalities. A 150-mEq intravenous (IV) bolus of sodium bicarbonate and a 40-mEq potassium chloride IV infusion were administered, and both epinephrine and norepinephrine IV infusions were also initiated. A basic metabolic panel obtained prior to medication administration showed a potassium concentration of 1.9 mmol/L.
What is the differential diagnosis of toxicological hypokalemia?
Hypokalemia may be reflective of diminished whole body potassium stores or a transient alteration of intravascular potassium concentrations. In acute ingestions and overdose, the etiology of the hypokalemia is often electrolyte redistribution through either blockade of constitutive outward potassium leakage (eg, barium, insulin, quinine) or through increased activity of the Na+/K+-ATPase pump (eg, catecholamines, insulin, methylxanthines). This activity has little effect on whole body potassium stores, but can result in a profound fall in the serum potassium. While mild hypokalemia is generally well tolerated, more severe potassium abnormalities can cause muscular weakness, areflexic paralysis, respiratory failure, and life-threatening dysrhythmias. Common ECG findings include decreased T-wave amplitudes, ST-segment depression, and the presence or amplification of U waves.
Case Continuation
While the emergency physicians were stabilizing the patient, her mother provided additional information. Approximately 30 minutes after the exposure, the patient had become nauseated and told her mother what she had done. Her mother called EMS, and the patient was transported to the hospital, where she rapidly became symptomatic. Despite CV decompensation, she remained neurologically intact. On further questioning, the patient admitted to ingesting 6 g of her mother’s prescription of hydroxychloroquine (HCQ) in a suicide attempt but denied taking any other medications. She was stabilized on vasopressors and admitted to the intensive care unit.
What characterizes hydroxychloroquine toxicity?
Hydroxychloroquine is an aminoquinoline antibiotic that is classically used as an antimalarial to treat infection with Plasmodium vivax, P ovale, P malariae, and susceptible strains of P falciparum. In the United States, it is more commonly used to manage both rheumatoid arthritis and systemic lupus erythematosus (SLE), debilitating diseases which are estimated to affect anywhere from 161,000 to 322,000 Americans.1 Hydroxychloroquine is considered first-line therapy for SLE, but its mechanism of action in treating SLE-associated arthralgias is unclear.
Hydroxychloroquine is structurally similar to quinine and chloroquine (CQ), and not surprisingly exerts quinidine-like effects on the CV system with resultant negative inotropy and vasodilation. Its toxicity is characterized by rapid onset of clinical effects including central nervous system depression, seizures, apnea, hypotension, and arrhythmia. After large overdoses, cardiac arrest and death can occur within hours.
Hypokalemia is another hallmark of HCQ toxicity. It is thought to develop secondary to potassium channel blockade, which slows the constitutive release of potassium from the myocytes.2 As noted, the hypokalemia is transient and does not reflect whole-body depletion. With CQ, which is considered more toxic, there appears to be a correlation between the quantity of CQ ingested and both the degree of hypokalemia and the severity of the outcome. It is reasonable to assume the same for HCQ. There are little data to support that hypokalemia itself causes cardiotoxicity in patients with CQ or HCQ overdose.
Although lethal doses are not well established, animal studies suggest that HCQ is much less toxic than CQ, for which the clinical toxicity is better understood due to its more widespread use in overdose abroad.3 In children, the reported therapeutic dose is 10 mg/kg, but the minimum reported lethal dose was a single 300-mg tablet (30 mg/kg in a toddler). In adults, the toxic dose is reported as 20 mg/kg with lethal doses suggested to be as low as 30 mg/kg.
What are the treatment modalities for patients with hydroxychloroquine toxicity?
By analogy with the treatment of CQ poisoning, the mainstay of HCQ therapy is supportive care, including early intubation and ventilation to minimize metabolic demand. Direct-acting inotropes and vasopressors should be administered after saline to treat hypotension. Because of its large volume of distribution, extracorporeal removal has not proved to be of clinical value.4,5 Though data are sparse to determine its efficacy, there may be a role for giving activated charcoal, particularly following large overdoses—if it is given early after exposure and the patient has normal consciousness. If the patient is intubated and aspiration risk is minimized, gastric lavage may also be beneficial—especially when performed within an hour of the overdose. Syrup of ipecac should not be used.
High-dose diazepam is typically recommended, again by analogy with CQ, although there is no clear mechanism of action and its use remains controversial. Its protective effect in patients with CQ overdose is based on swine and rat models that demonstrated dose dependent relationships between diazepam and survival.6,7 A prospective study of CQ toxicity in humans reported improved survival rates when high-dose diazepam was given in combination with epinephrine.8 However, this study is limited by its comparison of prospectively studied patients with a retrospective control. A subsequent prospective study of moderately CQ-intoxicated patients did not find a benefit from treatment with diazepam.9 Furthermore, it remains unclear if the proposed benefit from high-dose diazepam in CQ toxicity may be extrapolated to HCQ, and cases of even massive HCQ ingestions report good outcomes without the use of high-dose diazepam.10
How aggressively should hypokalemia in hydroxychloroquine toxicity be treated?
As noted earlier, hypokalemia resulting from HCQ toxicity is transient, and aggressive repletion may result in rebound hyperkalemia once toxicity resolves. However, these dangers should be balanced with risks of hypokalemia-induced ventricular arrhythmias. Additionally, hypokalemia may be worsened by sodium bicarbonate that is administered to correct QRS prolongations, increasing the risk of dysrhythmia. Correction of hypokalemia in these cases is necessary but should be done with care and monitoring of serum potassium concentrations to minimize risk of hyperkalemia-induced ventricular arrhythmia.11
Case Conclusion
Throughout treatment, the patient remained neurologically intact. She did not receive benzodiazepines. The epinephrine and norepinephrine infusions were weaned, and she was discharged on hospital day 3 with no neurological or cardiac sequelae. She received an inpatient psychiatric evaluation and was referred to outpatient services for ongoing care.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008;58(1):15-25. doi:10.1002/art.23177.
2. Clemessy JL, Favier C, Borron SW, Hantson PE, Vicaut E, Baud FJ. Hypokalaemia related to acute chloroquine ingestion. Lancet. 1995;3469(8979):877-880.
3. McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med. 1983;75(suppl 1A):11-18.
4. Carmichael SJ, Charles B, Tett SE. Population pharmacokinetics of hydroxychloroquine in patients with rheumatoid arthritis. Ther Drug Monit. 2003;25(6):671-681.
5. Marquardt K, Albertson TE. Treatment of hydroxychloroquine overdose. Am J Emerg Med. 2001;19(5):420-424.
6. Crouzette J, Vicaut E, Palombo S, Girre C, Fournier PE. Experimental assessment of the protective activity of diazepam on the acute toxicity of chloroquine. J Toxicol Clin Toxicol. 1983;20(3):271-279.
7. Riou B, Lecarpentier Y, Barriot P, Viars P. Diazepam does not improve the mechanical performance of rat cardiac papillary muscle exposed to chloroquine in vitro. Intensive Care Med. 1989;15:390-3955.
8. Riou B, Barriot P, Rimailho A, Baud FJ. Treatment of severe chloroquine poisoning. N Engl J Med. 1988;318(1):1-6.
9. Clemessy JL, Angel G, Borron SW, et al. Therapeutic trial of diazepam versus placebo in acute chloroquine intoxications of moderate gravity. Intensive Care Med. 1996;22:1400-1405.
10. Yanturali S. Diazepam for treatment of massive chloroquine intoxication. Resuscitation. 2004;63(3):347-348.
11. Ling Ngan Wong A, Tsz Fung Cheung I, Graham CA. Hydroxychloroquine overdose: case report and recommendations for management. Eur J Emerg Med. 2008;15(1):16-8. doi:10.1097/MEJ.0b013e3280adcb56.
1. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008;58(1):15-25. doi:10.1002/art.23177.
2. Clemessy JL, Favier C, Borron SW, Hantson PE, Vicaut E, Baud FJ. Hypokalaemia related to acute chloroquine ingestion. Lancet. 1995;3469(8979):877-880.
3. McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med. 1983;75(suppl 1A):11-18.
4. Carmichael SJ, Charles B, Tett SE. Population pharmacokinetics of hydroxychloroquine in patients with rheumatoid arthritis. Ther Drug Monit. 2003;25(6):671-681.
5. Marquardt K, Albertson TE. Treatment of hydroxychloroquine overdose. Am J Emerg Med. 2001;19(5):420-424.
6. Crouzette J, Vicaut E, Palombo S, Girre C, Fournier PE. Experimental assessment of the protective activity of diazepam on the acute toxicity of chloroquine. J Toxicol Clin Toxicol. 1983;20(3):271-279.
7. Riou B, Lecarpentier Y, Barriot P, Viars P. Diazepam does not improve the mechanical performance of rat cardiac papillary muscle exposed to chloroquine in vitro. Intensive Care Med. 1989;15:390-3955.
8. Riou B, Barriot P, Rimailho A, Baud FJ. Treatment of severe chloroquine poisoning. N Engl J Med. 1988;318(1):1-6.
9. Clemessy JL, Angel G, Borron SW, et al. Therapeutic trial of diazepam versus placebo in acute chloroquine intoxications of moderate gravity. Intensive Care Med. 1996;22:1400-1405.
10. Yanturali S. Diazepam for treatment of massive chloroquine intoxication. Resuscitation. 2004;63(3):347-348.
11. Ling Ngan Wong A, Tsz Fung Cheung I, Graham CA. Hydroxychloroquine overdose: case report and recommendations for management. Eur J Emerg Med. 2008;15(1):16-8. doi:10.1097/MEJ.0b013e3280adcb56.
Bertrand M. Bell, MD: An Iconoclast Who Became an Icon
Bertrand M. Bell, MD, Distinguished University Professor Emeritus at Albert Einstein College of Medicine, died at the age of 86 in his Manhattan home on October 4, 2016. For decades, Dr Bell was the Director of Ambulatory Care, which included the ED at Einstein’s teaching hospital, the Bronx Municipal (Jacobi) Hospital Center. But, as Dr Bell was aware for the last 25 years of his life, he would always be remembered for a committee he chaired the year he was on sabbatical from Einstein and Jacobi in 1987.
After the death of 18-year-old Libby Zion from a dangerous drug interaction, the New York State Commissioner of Health asked Dr Bell to chair an ad hoc committee to investigate the care of hospitalized patients by residents and to make recommendations regarding medication ordering and administration, the use of patient restraints, attending supervision, and resident work hours. The “Bell Commission,” as it came to be known, recommended that residents not be allowed to work more than 80 hours a week or more than 24 consecutive hours, and that attending physicians be present in the hospital 24/7. These recommendations were made part of the New York State Health Code in 1989 and adopted nationwide by the Accreditation Council for Graduate Medical Education in 1993.
The Bell Commission changes in resident work hours were not enthusiastically received by all, with most of the criticism centering on a perceived lack of continuity in resident education resulting from the shortened work hours. Largely ignored, however, was the committee’s call for 24/7 attending supervision, which would have provided both continuity in patient care and enhanced resident education and experiences. Dr Bell was outspoken in defending his committee’s recommendations and his views on the inadequacies of graduate medical education (GME), occasionally infuriating those who disagreed with him.
Ironically, though the formal name of the Bell Commission was the “Ad Hoc Advisory Committee on Emergency Services,” the recommendations did not address prehospital care issues and probably affected emergency medicine (EM) residents less than they did residents from other specialties. Both the work-hour rules and mandated attending presence had already been implemented by many EM residency training programs from the time EM became a specialty in 1979, and were required of all EM programs by the end of the 1980s. (See “’My Patient’—More Than Ever,” Emerg Med. 2013;45[4]:1.)
Yet, Bert Bell may have had as profound an effect on the birth and survival of academic EM on the East Coast as did his committee’s recommendations on GME nationwide. Together with his ED Director, Sheldon Jacobson, MD, Bert secured for Einstein/Jacobi the first federally funded paramedic training program in New York State in 1974, followed a year later by the first EM residency in New York State, and one of the earliest in the nation. Bert also hired and trained nurse practitioners and physician assistants to care for patients in the ED and clinics, realizing their potential and the value of their contributions to patient care, years before others did.
The group of emergency physicians that Bert and Shelly assembled at Einstein/Jacobi in the 1970s included John Gallagher, Peter Moyer, Mark Henry, Gregg Husk, Paul Gennis, a young Wallace Carter, me, and several other EM pionee
At Dr Bell’s funeral on October 7, the rabbi alluded to a description of the prophet Elijah, in describing Bert as a “holy troublemaker.” Bert Bell was a larger-than-life iconoclast whose name became an icon for graduate medical education reforms and whose patient care values will survive in future generations of physicians.
Bertrand M. Bell, MD, Distinguished University Professor Emeritus at Albert Einstein College of Medicine, died at the age of 86 in his Manhattan home on October 4, 2016. For decades, Dr Bell was the Director of Ambulatory Care, which included the ED at Einstein’s teaching hospital, the Bronx Municipal (Jacobi) Hospital Center. But, as Dr Bell was aware for the last 25 years of his life, he would always be remembered for a committee he chaired the year he was on sabbatical from Einstein and Jacobi in 1987.
After the death of 18-year-old Libby Zion from a dangerous drug interaction, the New York State Commissioner of Health asked Dr Bell to chair an ad hoc committee to investigate the care of hospitalized patients by residents and to make recommendations regarding medication ordering and administration, the use of patient restraints, attending supervision, and resident work hours. The “Bell Commission,” as it came to be known, recommended that residents not be allowed to work more than 80 hours a week or more than 24 consecutive hours, and that attending physicians be present in the hospital 24/7. These recommendations were made part of the New York State Health Code in 1989 and adopted nationwide by the Accreditation Council for Graduate Medical Education in 1993.
The Bell Commission changes in resident work hours were not enthusiastically received by all, with most of the criticism centering on a perceived lack of continuity in resident education resulting from the shortened work hours. Largely ignored, however, was the committee’s call for 24/7 attending supervision, which would have provided both continuity in patient care and enhanced resident education and experiences. Dr Bell was outspoken in defending his committee’s recommendations and his views on the inadequacies of graduate medical education (GME), occasionally infuriating those who disagreed with him.
Ironically, though the formal name of the Bell Commission was the “Ad Hoc Advisory Committee on Emergency Services,” the recommendations did not address prehospital care issues and probably affected emergency medicine (EM) residents less than they did residents from other specialties. Both the work-hour rules and mandated attending presence had already been implemented by many EM residency training programs from the time EM became a specialty in 1979, and were required of all EM programs by the end of the 1980s. (See “’My Patient’—More Than Ever,” Emerg Med. 2013;45[4]:1.)
Yet, Bert Bell may have had as profound an effect on the birth and survival of academic EM on the East Coast as did his committee’s recommendations on GME nationwide. Together with his ED Director, Sheldon Jacobson, MD, Bert secured for Einstein/Jacobi the first federally funded paramedic training program in New York State in 1974, followed a year later by the first EM residency in New York State, and one of the earliest in the nation. Bert also hired and trained nurse practitioners and physician assistants to care for patients in the ED and clinics, realizing their potential and the value of their contributions to patient care, years before others did.
The group of emergency physicians that Bert and Shelly assembled at Einstein/Jacobi in the 1970s included John Gallagher, Peter Moyer, Mark Henry, Gregg Husk, Paul Gennis, a young Wallace Carter, me, and several other EM pionee
At Dr Bell’s funeral on October 7, the rabbi alluded to a description of the prophet Elijah, in describing Bert as a “holy troublemaker.” Bert Bell was a larger-than-life iconoclast whose name became an icon for graduate medical education reforms and whose patient care values will survive in future generations of physicians.
Bertrand M. Bell, MD, Distinguished University Professor Emeritus at Albert Einstein College of Medicine, died at the age of 86 in his Manhattan home on October 4, 2016. For decades, Dr Bell was the Director of Ambulatory Care, which included the ED at Einstein’s teaching hospital, the Bronx Municipal (Jacobi) Hospital Center. But, as Dr Bell was aware for the last 25 years of his life, he would always be remembered for a committee he chaired the year he was on sabbatical from Einstein and Jacobi in 1987.
After the death of 18-year-old Libby Zion from a dangerous drug interaction, the New York State Commissioner of Health asked Dr Bell to chair an ad hoc committee to investigate the care of hospitalized patients by residents and to make recommendations regarding medication ordering and administration, the use of patient restraints, attending supervision, and resident work hours. The “Bell Commission,” as it came to be known, recommended that residents not be allowed to work more than 80 hours a week or more than 24 consecutive hours, and that attending physicians be present in the hospital 24/7. These recommendations were made part of the New York State Health Code in 1989 and adopted nationwide by the Accreditation Council for Graduate Medical Education in 1993.
The Bell Commission changes in resident work hours were not enthusiastically received by all, with most of the criticism centering on a perceived lack of continuity in resident education resulting from the shortened work hours. Largely ignored, however, was the committee’s call for 24/7 attending supervision, which would have provided both continuity in patient care and enhanced resident education and experiences. Dr Bell was outspoken in defending his committee’s recommendations and his views on the inadequacies of graduate medical education (GME), occasionally infuriating those who disagreed with him.
Ironically, though the formal name of the Bell Commission was the “Ad Hoc Advisory Committee on Emergency Services,” the recommendations did not address prehospital care issues and probably affected emergency medicine (EM) residents less than they did residents from other specialties. Both the work-hour rules and mandated attending presence had already been implemented by many EM residency training programs from the time EM became a specialty in 1979, and were required of all EM programs by the end of the 1980s. (See “’My Patient’—More Than Ever,” Emerg Med. 2013;45[4]:1.)
Yet, Bert Bell may have had as profound an effect on the birth and survival of academic EM on the East Coast as did his committee’s recommendations on GME nationwide. Together with his ED Director, Sheldon Jacobson, MD, Bert secured for Einstein/Jacobi the first federally funded paramedic training program in New York State in 1974, followed a year later by the first EM residency in New York State, and one of the earliest in the nation. Bert also hired and trained nurse practitioners and physician assistants to care for patients in the ED and clinics, realizing their potential and the value of their contributions to patient care, years before others did.
The group of emergency physicians that Bert and Shelly assembled at Einstein/Jacobi in the 1970s included John Gallagher, Peter Moyer, Mark Henry, Gregg Husk, Paul Gennis, a young Wallace Carter, me, and several other EM pionee
At Dr Bell’s funeral on October 7, the rabbi alluded to a description of the prophet Elijah, in describing Bert as a “holy troublemaker.” Bert Bell was a larger-than-life iconoclast whose name became an icon for graduate medical education reforms and whose patient care values will survive in future generations of physicians.
This Month in CHEST
Editor’s Picks
Giants in Chest Medicine: Bartolome Celli, MD, FCCP. By Dr. G. J. Criner
Elevated Plasma Levels of sRAGE Are Associated With Nonfocal CT-Based Lung Imaging in Patients With ARDS: A Prospective Multicenter Study. By Dr. S. Mrozek, et al.
A Case-Control Study Assessing the Impact of Nonventilated Hospital-Acquired Pneumonia on Patient Outcomes. By Dr. S. T. Micek, et al.
Low Prevalence of High-Grade Lesions Detected With Autofluorescence Bronchoscopy in the Setting of Lung Cancer Screening in the Pan-Canadian Lung Cancer Screening Study. By Dr. A. Tremblay, et al.
Editor’s Picks
Giants in Chest Medicine: Bartolome Celli, MD, FCCP. By Dr. G. J. Criner
Elevated Plasma Levels of sRAGE Are Associated With Nonfocal CT-Based Lung Imaging in Patients With ARDS: A Prospective Multicenter Study. By Dr. S. Mrozek, et al.
A Case-Control Study Assessing the Impact of Nonventilated Hospital-Acquired Pneumonia on Patient Outcomes. By Dr. S. T. Micek, et al.
Low Prevalence of High-Grade Lesions Detected With Autofluorescence Bronchoscopy in the Setting of Lung Cancer Screening in the Pan-Canadian Lung Cancer Screening Study. By Dr. A. Tremblay, et al.
Editor’s Picks
Giants in Chest Medicine: Bartolome Celli, MD, FCCP. By Dr. G. J. Criner
Elevated Plasma Levels of sRAGE Are Associated With Nonfocal CT-Based Lung Imaging in Patients With ARDS: A Prospective Multicenter Study. By Dr. S. Mrozek, et al.
A Case-Control Study Assessing the Impact of Nonventilated Hospital-Acquired Pneumonia on Patient Outcomes. By Dr. S. T. Micek, et al.
Low Prevalence of High-Grade Lesions Detected With Autofluorescence Bronchoscopy in the Setting of Lung Cancer Screening in the Pan-Canadian Lung Cancer Screening Study. By Dr. A. Tremblay, et al.
Treating agitation in schizophrenia
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Consider Rx metformin to prevent metabolic syndrome
Many atypical antipsychotics, particularly clozapine and olanzapine, are associated with weight gain, insulin resistance, and metabolic syndrome. Metabolic syndrome is associated with type 2 diabetes mellitus (T2DM) and cardiovascular disease, which are among the leading causes of morbidity and mortality in persons with severe mental illness.1
Clinicians should take measures to prevent T2DM and weight gain in individuals taking antipsychotics before these conditions develop. Metformin re-sensitizes the body to insulin and is a first-line treatment for T2DM. Adding metformin when patients start metabolically high-risk antipsychotics or shortly after they begin gaining weight is an evidence-based strategy to prevent metabolic syndrome.
Evaluate the evidence
In randomized controlled trials, metformin was associated with modest weight loss and improvement in metabolic parameters (eg, fasting blood glucose, serum triglycerides, and total cholesterol) in patients with schizophrenia receiving antipsychotics.1,2 Metformin is effective for preventing metabolic syndrome and as a treatment intervention; therefore, it may prove most beneficial early in treatment before weight gain or insulin resistance develop.
Importantly, weight gain and metabolic syndrome are risk factors for cardiovascular disease, but the number needed to treat for metformin to prevent cardiovascular outcomes, such as myocardial infarction, is not known. Also, metformin is not FDA-approved for this indication. Clinicians should discuss with the patient the risks and benefits of prophylactic metformin, and consider his (her) treatment preferences.
Tolerability and adverse effects
Metformin generally is well-tolerated. Gastrointestinal (GI) symptoms, including nausea and vomiting (14%) and diarrhea (7%), are the most common adverse effects.2 Lactic acidosis is rare and is associated with alcohol use disorders and impaired renal, hepatic, or cardiopulmonary function.3 Because metformin is excreted renally, toxicity could occur in patients with impaired renal function.
Before initiating prophylactic metformin, confirm that the patient does not have T2DM (eg, hemoglobin A1c <6.5%). A thorough medical history, including alcohol use and kidney and liver function tests, are needed to reduce the risk of lactic acidosis.3
Dosing
Although metformin has been studied at many dosages,2 we recommend gradual titration to 1,000 mg, twice daily, taken with meals to reduce the risk of GI effects.
Additional interventions
Metformin alone is not sufficient to mitigate metabolic risk. Providers should address dietary interventions, exercise, and smoking cessation at each visit, and communicate actively with other providers to create a comprehensive treatment plan.
1. Jarskog LF, Hamer RF, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight patients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
2. Zheng W, Li X-B, Tang Y-L, et al. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35(5):499-509.
3. Wang M, Tong J-H, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
Many atypical antipsychotics, particularly clozapine and olanzapine, are associated with weight gain, insulin resistance, and metabolic syndrome. Metabolic syndrome is associated with type 2 diabetes mellitus (T2DM) and cardiovascular disease, which are among the leading causes of morbidity and mortality in persons with severe mental illness.1
Clinicians should take measures to prevent T2DM and weight gain in individuals taking antipsychotics before these conditions develop. Metformin re-sensitizes the body to insulin and is a first-line treatment for T2DM. Adding metformin when patients start metabolically high-risk antipsychotics or shortly after they begin gaining weight is an evidence-based strategy to prevent metabolic syndrome.
Evaluate the evidence
In randomized controlled trials, metformin was associated with modest weight loss and improvement in metabolic parameters (eg, fasting blood glucose, serum triglycerides, and total cholesterol) in patients with schizophrenia receiving antipsychotics.1,2 Metformin is effective for preventing metabolic syndrome and as a treatment intervention; therefore, it may prove most beneficial early in treatment before weight gain or insulin resistance develop.
Importantly, weight gain and metabolic syndrome are risk factors for cardiovascular disease, but the number needed to treat for metformin to prevent cardiovascular outcomes, such as myocardial infarction, is not known. Also, metformin is not FDA-approved for this indication. Clinicians should discuss with the patient the risks and benefits of prophylactic metformin, and consider his (her) treatment preferences.
Tolerability and adverse effects
Metformin generally is well-tolerated. Gastrointestinal (GI) symptoms, including nausea and vomiting (14%) and diarrhea (7%), are the most common adverse effects.2 Lactic acidosis is rare and is associated with alcohol use disorders and impaired renal, hepatic, or cardiopulmonary function.3 Because metformin is excreted renally, toxicity could occur in patients with impaired renal function.
Before initiating prophylactic metformin, confirm that the patient does not have T2DM (eg, hemoglobin A1c <6.5%). A thorough medical history, including alcohol use and kidney and liver function tests, are needed to reduce the risk of lactic acidosis.3
Dosing
Although metformin has been studied at many dosages,2 we recommend gradual titration to 1,000 mg, twice daily, taken with meals to reduce the risk of GI effects.
Additional interventions
Metformin alone is not sufficient to mitigate metabolic risk. Providers should address dietary interventions, exercise, and smoking cessation at each visit, and communicate actively with other providers to create a comprehensive treatment plan.
Many atypical antipsychotics, particularly clozapine and olanzapine, are associated with weight gain, insulin resistance, and metabolic syndrome. Metabolic syndrome is associated with type 2 diabetes mellitus (T2DM) and cardiovascular disease, which are among the leading causes of morbidity and mortality in persons with severe mental illness.1
Clinicians should take measures to prevent T2DM and weight gain in individuals taking antipsychotics before these conditions develop. Metformin re-sensitizes the body to insulin and is a first-line treatment for T2DM. Adding metformin when patients start metabolically high-risk antipsychotics or shortly after they begin gaining weight is an evidence-based strategy to prevent metabolic syndrome.
Evaluate the evidence
In randomized controlled trials, metformin was associated with modest weight loss and improvement in metabolic parameters (eg, fasting blood glucose, serum triglycerides, and total cholesterol) in patients with schizophrenia receiving antipsychotics.1,2 Metformin is effective for preventing metabolic syndrome and as a treatment intervention; therefore, it may prove most beneficial early in treatment before weight gain or insulin resistance develop.
Importantly, weight gain and metabolic syndrome are risk factors for cardiovascular disease, but the number needed to treat for metformin to prevent cardiovascular outcomes, such as myocardial infarction, is not known. Also, metformin is not FDA-approved for this indication. Clinicians should discuss with the patient the risks and benefits of prophylactic metformin, and consider his (her) treatment preferences.
Tolerability and adverse effects
Metformin generally is well-tolerated. Gastrointestinal (GI) symptoms, including nausea and vomiting (14%) and diarrhea (7%), are the most common adverse effects.2 Lactic acidosis is rare and is associated with alcohol use disorders and impaired renal, hepatic, or cardiopulmonary function.3 Because metformin is excreted renally, toxicity could occur in patients with impaired renal function.
Before initiating prophylactic metformin, confirm that the patient does not have T2DM (eg, hemoglobin A1c <6.5%). A thorough medical history, including alcohol use and kidney and liver function tests, are needed to reduce the risk of lactic acidosis.3
Dosing
Although metformin has been studied at many dosages,2 we recommend gradual titration to 1,000 mg, twice daily, taken with meals to reduce the risk of GI effects.
Additional interventions
Metformin alone is not sufficient to mitigate metabolic risk. Providers should address dietary interventions, exercise, and smoking cessation at each visit, and communicate actively with other providers to create a comprehensive treatment plan.
1. Jarskog LF, Hamer RF, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight patients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
2. Zheng W, Li X-B, Tang Y-L, et al. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35(5):499-509.
3. Wang M, Tong J-H, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
1. Jarskog LF, Hamer RF, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight patients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
2. Zheng W, Li X-B, Tang Y-L, et al. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35(5):499-509.
3. Wang M, Tong J-H, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
Lip Augmentation With Juvéderm Ultra XC



Ultrasound-Guided Percutaneous Reconstruction of the Anterolateral Ligament: Surgical Technique
Risk Factors for Early Readmission After Anatomical or Reverse Total Shoulder Arthroplasty
Hospital readmissions are undesirable and expensive.1 The Centers for Medicare & Medicaid Services (CMS) use hospital readmission rates as one measure of healthcare quality and hospital performance.2 In addition, the Patient Protection and Affordable Care Act of 2010 established a provision that decreases payments to hospitals with above-average readmission rates.3 Total knee arthroplasties (TKAs) and total hip arthroplasties (THAs) are among the most common surgical procedures leading to readmission and cost almost $20 billion dollars annually in the Medicare population alone.1 Identifying factors that lead to readmissions after certain popular procedures may be a way to improve healthcare quality and outcomes while decreasing costs.
One such operation is shoulder arthroplasty (SA), which has surged in popularity over the past decade and is projected to increase faster than TKAs and THAs.4-6 SA is used to treat a variety of shoulder conditions, including osteoarthritis, inflammatory arthritis, severe proximal humeral fracture, avascular necrosis, and rotator cuff tear arthropathy.7-12 Much as with knee and hip arthroplasty, good outcomes have been reported with SA: decreased pain, improved range of motion, and high patient satisfaction.10,13 However, there have been few studies of rates of readmission after SA and the associated risk factors.3,14,15 The reported rates of early readmission after SA have ranged from 5.6% to 7.3%.3,14,15 These rates are comparable to rates of readmission after TKA (4.0%-6.6%) and THA (3.5%-8.4%).15-17Recently, CMS introduced legislation to void payments for hospital-acquired conditions (HACs), preventable medical conditions that patients develop during or as a result of their hospital care and that were not present on admission.18 Although many factors contribute to readmission, a recent study regarding all-cause readmission during the first 30 days after discharge found that almost 50% of 30-day readmissions after knee and hip replacements were potentially preventable.19 HACs resulting in readmission after SAs make up 9.3% to 34.5% of all readmissions, after anatomical total shoulder arthroplasties (ATSAs) and reverse total shoulder arthroplasties (RTSAs).3,14 The most common HACs include retained foreign body after surgery, air embolism, falls and trauma, catheter-associated urinary tract infection (CAUTI), surgical-site infection, deep vein thrombosis (DVT), and pulmonary embolism (PE).18 Raines and colleagues16 found that HACs accounted for 41.7% of all complications in knee or hip arthroplasty and that HACs were the greatest predictors of early readmission after both procedures.
We conducted a study to evaluate rates of readmission within 30 days after ATSA and RTSA and to describe the independent risk factors for readmission. We hypothesized that the rate of readmission after SA would be similar to the rate after knee and hip arthroplasty and that readmission risk factors would be similar. Elucidating these rates and associated risk factors may ultimately help to minimize the burden of disability on patients and the burden of financial costs on healthcare institutions.
Materials and Methods
Institutional Review Board approval was not required for this study, and all data used were de-identified to Health Insurance Portability and Accountability Act (HIPAA) standards. We used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for this study. The NSQIP was developed in the 1990s to improve surgical quality in the Veterans Health Administration and was later adapted by the ACS.20 NSQIP follows patients for 30 days after operations and provides clinical data and outcome measures that are closely regulated and internally audited.21 The program has continued to expand and now includes more than 400 institutions. The NSQIP database has been validated as a reliable source of surgical outcomes data, including outcomes data for orthopedic procedures, and has been used in other studies of readmissions.17,22
In the present study, the ACS-NSQIP files for the period 2011-2013 were queried for all total shoulder arthroplasties (TSAs) (Current Procedural Terminology [CPT] code 23472, which includes ATSA and RTSA). Descriptive analysis was performed to determine the overall readmission rate as well as the percentages of readmissions for medical and surgical complications. Reasons for readmission were collected from 2012 and 2013 (information from 2011 was absent).
The various patient parameters compiled within the database were examined in a review of ATSAs and RTSAs. Demographics, comorbidities, operative characteristics, and predischarge complications were amassed from these data. Demographics included age, sex, race, body mass index, smoking status, preoperative functional health status, and American Society of Anesthesiologists (ASA) score. Comorbidities included diabetes mellitus, hypertension, chronic corticosteroid use, coagulation disorder, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), cardiac comorbidity (including congestive heart failure, history of myocardial infarction, previous coronary intervention or cardiac surgery, and angina), renal comorbidity (including acute renal failure and preoperative dialysis), neurologic comorbidity (including impaired sensorium, hemiplegia, history of transient ischemic attack, and history of cerebrovascular accident with or without residual deficit), and preoperative blood transfusion. Operative characteristics included resident involvement, operative time more than 1 SD from the mean (>164.4 minutes), intraoperative blood transfusion, and revision surgery. Predischarge complications included pneumonia, CAUTI, DVT, PE, postoperative bleeding that required transfusion, cerebrovascular accident, myocardial infarction, and sepsis. Surgical-site infection, CAUTI, DVT, and PE were selected for analysis because these HACs are common in our cohort.
After the data on these characteristics were collected, univariate analysis was performed to determine association with any readmission. Factors with P < .20 were then entered into multivariate analysis to determine independent risk factors for readmission. This P value was selected to make the model inclusive of any potentially important predictor. Univariate analysis was performed using the Fisher exact test. Multivariate analysis was performed using backward conditional binary logistic regression. Statistical significance was set at P < .05. All analysis was performed with SPSS Version 22.0 (SPSS).
Results
This study included a combined total of 3501 ATSAs and RTSAs performed between 2011 and 2013. The overall readmission rate was 2.7%. The associated diagnosis for readmission was available for 54% of the readmitted patients. Of the known readmission diagnoses, 33% were secondary to HACs.
Of the 51 readmissions, 34 (67%) were for medical complications, and 17 (33%) were for surgical complications. Pneumonia was the most common medical complication (11.8%), followed by UTI (7.8%), DVT (5.9%), PE (5.9%), and renal insufficiency (3.9%). Surgical-site infection was the most common surgical complication (13.7%), followed by prosthetic joint dislocation (9.8%) and hematoma (3.9%).
Other risk factors significantly (P < .05) associated with readmission were age over 75 years, dependent functional status, ASA score of 4 or higher, cardiac comorbidity, 2 or more comorbidities, postoperative CAUTI, extended LOS, and revision surgery (Table 3).
Discussion
Hospital readmissions are important because they represent quality of care and play a role in patient outcomes. Arthroplasty research has focused mainly on readmissions after primary knee and hip replacements.23-25
Historical rates of early readmission after SA14 are comparable to those found in our study. Previously identified risk factors have included increasing age, Medicaid insurance status, low-volume surgical centers, and SA type.3 Mahoney and colleagues14 reported a 90-day readmission rate of 5.9%, but, when they removed hemiarthroplasty replacement from the analysis and shortened the readmission timeline to 30 days, the readmission rate was identical to the 2.7% rate in the present study. In their series from a single high-volume institution, the highest 90-day readmission rate was found for hemiarthroplasty (8.8%), followed by RTSA (6.6%) and ATSA (4.5%). In a study by Schairer and colleagues,3 the readmission rate was also influenced by replacement type, but their results differed from those of Mahoney and colleagues.14 Schairer and colleagues3 analyzed data from 7 state inpatient databases and found that the highest readmission rate was associated with RTSA (11.2%), followed by hemiarthroplasty (8.2%) and ATSA (6.0%). In both series, RTSA readmission rates were higher than ATSA readmission rates—consistent with the complication profiles of these procedures, with RTSA often provided as a surgery of last resort, after failure of other procedures, including ATSA.26 The lower 30-day readmission rate in the present study may be attributable to the fact that some surgical and medical complications may not have developed within this short time. Nonetheless, the majority of readmissions typically present within the first 30 days after SA.14,15 Other factors, including hospital volume, surgeon volume, race, and hospital type, may also influence readmission rates but could not be compared between
The present study found that revision surgery, 3 or more comorbidities, and extended LOS (>4.3 days) more than doubled the risk of readmission. Published SA revision rates range from 5% to 42%, with most revisions performed for instability, dislocation, infection, and component loosening.6,29 Complication rates are higher for revision SA than for primary SA, which may explain why revisions predispose patients to readmission.30 Compared with primary SAs, revision SAs are also more likely to be RTSAs, and these salvage procedures have been found to have high complication rates.31 In the present study, the most common comorbidities were hypertension, diabetes, and COPD; the literature supports these as some of the most common comorbid medical conditions in patients who undergo ATSA or RTSA.5,26,32 Furthermore, all 3 of these comorbidities have been shown to be independent predictors of increased postoperative complications in patients who undergo SA, which ultimately would increase the risk of readmission.3,26,33,34 Last, extended LOS has also been shown to increase the risk of unplanned readmissions after orthopedic procedures.35 Risk factors associated with increased LOS after ATSA or RTSA include female sex, advanced age, multiple comorbidities, and postoperative complications.32Several other factors must be noted with respect to individual risk for readmission. In the present study, age over 75 years, dependent functional status, ASA score of 4 or higher, and cardiac comorbidity were found to have a significant association with readmission. Increased age is a risk factor for increased postoperative complications, more medical comorbidities, and increased LOS.34,36 Older people are at higher risk of developing osteoarthritis and rotator cuff tear arthropathy and are more likely to undergo SA.5,6 Older people also are more likely to be dependent, which itself is a risk factor for readmission.19 An ASA score of 3 or 4 has been found to be associated with increased LOS and complications after SA, and cardiac comorbidities predispose patients to a variety of complications.34,36,37In studies that have combined surgical and medical factors, rates of complications early after ATSA and RTSA have ranged from 3.6% to 17.8%.26,38,39 After SAs, medical complications (80%) are more common than surgical complications (20%).39 In the present cohort, many more readmissions were for medical complications (67%) than for surgical complications (33%). In addition, Schairer and colleagues3 found medical complications associated with more than 80% of readmissions after SA.3 Infection was the most common medical reason (pneumonia) and surgical reason (surgical-site infection) for readmission—consistent with findings of other studies.3,35,40 Infection has accounted for 9.4% to 41.4% of readmissions after ATSA and RTSA.3,14In joint arthroplasty, infection occurs more often in patients with coexisting medical comorbidities, leading to higher mortality and increased LOS.41 Prosthetic joint dislocation was common as well—similar to findings in other studies.3,10In the present study, 33% of known readmission diagnoses were secondary to HACs. Surgical-site infection was the most common, followed by CAUTI, DVT, and PE. In another study, of knee and hip arthroplasties, HACs accounted for more than 40% of all complications and were the strongest predictor of early readmission.16 In SA studies, HACs were responsible for 9.3% to 34.5% of readmissions after ATSA and RTSA.3,14 Our finding (33%) is more in line with Mahoney and colleagues14 (34.5%) than Schairer and colleagues3 (9.3%). One explanation for the large discrepancy with Schairer and colleagues3 is that UTI was not among the medical reasons for readmission in their study, but it was in ours. Another difference is that we used a database that included data from multiple institutions. Last, Schairer and colleagues3 excluded revision SAs from their analysis (complication rates are higher for revision SAs than for primary SAs30). They also excluded cases of SA used for fracture (shown to increase the risk for PE42). The US Department of Health and Human Services estimated that patients experienced 1.3 million fewer HACs during the period 2010-2013, corresponding to a 17% decline over the 3 years.43 This translates to an estimated 50,000 fewer mortalities, and $12 billion saved in healthcare costs, over the same period.43 Preventing HACs helps reduce readmission rates while improving patient outcomes and decreasing healthcare costs.
This study had several limitations. We could not differentiate between ATSA and RTSA readmission rates because, for the study period, these procedures are collectively organized under a common CPT code in the NSQIP database. Readmission and complication rates are higher for RTSAs than for ATSAs.3,14 In addition, our data were limited to hospitals that were participating in NSQIP, which could lead to selection bias. We studied rates of only those readmissions and complications that occurred within 30 days, but many complications develop after 30 days, and these increase the readmission rate. Last, reasons for readmission were not recorded for 2011, so this information was available only for the final 2 years of the study. Despite these limitations, NSQIP still allows for a powerful study, as it includes multiple institutions and a very large cohort.
Conclusion
With medical costs increasing, focus has shifted to quality care and good outcomes with the goal of reducing readmissions and complications after various procedures. SA has recently become more popular because of its multiple indications, and this trend will continue. In the present study, the rate of readmission within 30 days after ATSA or RTSA was 2.7%. Revision surgery, 3 or more comorbidities, and extended LOS were independent risk factors that more than doubled the risk of readmission. Understanding the risk factors for short-term readmission will allow for better patient care and decreased costs, and will benefit the healthcare system as a whole.
Am J Orthop. 2016;45(6):E386-E392. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
2. Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305(5):504-505.
3. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
4. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
5. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
6. Jain NB, Yamaguchi K. The contribution of reverse shoulder arthroplasty to utilization of primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1905-1912.
7. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction–internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
10. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
11. Fevang BT, Lygre SH, Bertelsen G, Skredderstuen A, Havelin LI, Furnes O. Good function after shoulder arthroplasty. Acta Orthop. 2012;83(5):467-473.
12. Orfaly RM, Rockwood CA Jr, Esenyel CZ, Wirth MA. Shoulder arthroplasty in cases with avascular necrosis of the humeral head. J Shoulder Elbow Surg. 2007;16(3 suppl):S27-S32.
13. Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13(6):604-613.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722.
16. Raines BT, Ponce BA, Reed RD, Richman JS, Hawn MT. Hospital acquired conditions are the strongest predictor for early readmission: an analysis of 26,710 arthroplasties. J Arthroplasty. 2015;30(8):1299-1307.
17. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
18. Centers for Medicare & Medicaid Services. Hospital-Acquired Conditions. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions.html. Published 2014. Accessed May 21, 2015.
19. Feigenbaum P, Neuwirth E, Trowbridge L, et al. Factors contributing to all-cause 30-day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599-605.
20. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250(3):363-376.
21. American College of Surgeons. About ACS NSQIP. http://www.facs.org/quality-programs/acs-nsqip/about. Published 2015. Accessed June 14, 2015.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
24. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
25. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
26. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467.
27. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
28. Tsai TC, Orav EJ, Joynt KE. Disparities in surgical 30-day readmission rates for Medicare beneficiaries by race and site of care. Ann Surg. 2014;259(6):1086-1090.
29. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
30. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
31. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJ. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042.
32. Menendez ME, Baker DK, Fryberger CT, Ponce BA. Predictors of extended length of stay after elective shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1527-1533.
33. Jain NB, Guller U, Pietrobon R, Bond TK, Higgins LD. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;(435):232-238.
34. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1.
35. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
36. Dunn JC, Lanzi J, Kusnezov N, Bader J, Waterman BR, Belmont PJ Jr. Predictors of length of stay after elective total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(5):754-759.
37. Maile MD, Engoren MC, Tremper KK, Jewell E, Kheterpal S. Worsening preoperative heart failure is associated with mortality and noncardiac complications, but not myocardial infarction after noncardiac surgery: a retrospective cohort study. Anesth Analg. 2014;119(3):522-532.
38. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
39. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
40. Kassin MT, Owen RM, Perez SD, et al. Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg. 2012;215(3):322-330.
41. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
42. Young BL, Menendez ME, Baker DK, Ponce BA. Factors associated with in-hospital pulmonary embolism after shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):e271-e278.
43. US Department of Health and Human Services. Efforts to improve patient safety result in 1.3 million fewer patient harms, 50,000 lives saved and $12 billion in health spending avoided [press release]. http://www.hhs.gov/news/press/2014pres/12/20141202a.html. Published December 2, 2014. Accessed May 25, 2015.
Hospital readmissions are undesirable and expensive.1 The Centers for Medicare & Medicaid Services (CMS) use hospital readmission rates as one measure of healthcare quality and hospital performance.2 In addition, the Patient Protection and Affordable Care Act of 2010 established a provision that decreases payments to hospitals with above-average readmission rates.3 Total knee arthroplasties (TKAs) and total hip arthroplasties (THAs) are among the most common surgical procedures leading to readmission and cost almost $20 billion dollars annually in the Medicare population alone.1 Identifying factors that lead to readmissions after certain popular procedures may be a way to improve healthcare quality and outcomes while decreasing costs.
One such operation is shoulder arthroplasty (SA), which has surged in popularity over the past decade and is projected to increase faster than TKAs and THAs.4-6 SA is used to treat a variety of shoulder conditions, including osteoarthritis, inflammatory arthritis, severe proximal humeral fracture, avascular necrosis, and rotator cuff tear arthropathy.7-12 Much as with knee and hip arthroplasty, good outcomes have been reported with SA: decreased pain, improved range of motion, and high patient satisfaction.10,13 However, there have been few studies of rates of readmission after SA and the associated risk factors.3,14,15 The reported rates of early readmission after SA have ranged from 5.6% to 7.3%.3,14,15 These rates are comparable to rates of readmission after TKA (4.0%-6.6%) and THA (3.5%-8.4%).15-17Recently, CMS introduced legislation to void payments for hospital-acquired conditions (HACs), preventable medical conditions that patients develop during or as a result of their hospital care and that were not present on admission.18 Although many factors contribute to readmission, a recent study regarding all-cause readmission during the first 30 days after discharge found that almost 50% of 30-day readmissions after knee and hip replacements were potentially preventable.19 HACs resulting in readmission after SAs make up 9.3% to 34.5% of all readmissions, after anatomical total shoulder arthroplasties (ATSAs) and reverse total shoulder arthroplasties (RTSAs).3,14 The most common HACs include retained foreign body after surgery, air embolism, falls and trauma, catheter-associated urinary tract infection (CAUTI), surgical-site infection, deep vein thrombosis (DVT), and pulmonary embolism (PE).18 Raines and colleagues16 found that HACs accounted for 41.7% of all complications in knee or hip arthroplasty and that HACs were the greatest predictors of early readmission after both procedures.
We conducted a study to evaluate rates of readmission within 30 days after ATSA and RTSA and to describe the independent risk factors for readmission. We hypothesized that the rate of readmission after SA would be similar to the rate after knee and hip arthroplasty and that readmission risk factors would be similar. Elucidating these rates and associated risk factors may ultimately help to minimize the burden of disability on patients and the burden of financial costs on healthcare institutions.
Materials and Methods
Institutional Review Board approval was not required for this study, and all data used were de-identified to Health Insurance Portability and Accountability Act (HIPAA) standards. We used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for this study. The NSQIP was developed in the 1990s to improve surgical quality in the Veterans Health Administration and was later adapted by the ACS.20 NSQIP follows patients for 30 days after operations and provides clinical data and outcome measures that are closely regulated and internally audited.21 The program has continued to expand and now includes more than 400 institutions. The NSQIP database has been validated as a reliable source of surgical outcomes data, including outcomes data for orthopedic procedures, and has been used in other studies of readmissions.17,22
In the present study, the ACS-NSQIP files for the period 2011-2013 were queried for all total shoulder arthroplasties (TSAs) (Current Procedural Terminology [CPT] code 23472, which includes ATSA and RTSA). Descriptive analysis was performed to determine the overall readmission rate as well as the percentages of readmissions for medical and surgical complications. Reasons for readmission were collected from 2012 and 2013 (information from 2011 was absent).
The various patient parameters compiled within the database were examined in a review of ATSAs and RTSAs. Demographics, comorbidities, operative characteristics, and predischarge complications were amassed from these data. Demographics included age, sex, race, body mass index, smoking status, preoperative functional health status, and American Society of Anesthesiologists (ASA) score. Comorbidities included diabetes mellitus, hypertension, chronic corticosteroid use, coagulation disorder, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), cardiac comorbidity (including congestive heart failure, history of myocardial infarction, previous coronary intervention or cardiac surgery, and angina), renal comorbidity (including acute renal failure and preoperative dialysis), neurologic comorbidity (including impaired sensorium, hemiplegia, history of transient ischemic attack, and history of cerebrovascular accident with or without residual deficit), and preoperative blood transfusion. Operative characteristics included resident involvement, operative time more than 1 SD from the mean (>164.4 minutes), intraoperative blood transfusion, and revision surgery. Predischarge complications included pneumonia, CAUTI, DVT, PE, postoperative bleeding that required transfusion, cerebrovascular accident, myocardial infarction, and sepsis. Surgical-site infection, CAUTI, DVT, and PE were selected for analysis because these HACs are common in our cohort.
After the data on these characteristics were collected, univariate analysis was performed to determine association with any readmission. Factors with P < .20 were then entered into multivariate analysis to determine independent risk factors for readmission. This P value was selected to make the model inclusive of any potentially important predictor. Univariate analysis was performed using the Fisher exact test. Multivariate analysis was performed using backward conditional binary logistic regression. Statistical significance was set at P < .05. All analysis was performed with SPSS Version 22.0 (SPSS).
Results
This study included a combined total of 3501 ATSAs and RTSAs performed between 2011 and 2013. The overall readmission rate was 2.7%. The associated diagnosis for readmission was available for 54% of the readmitted patients. Of the known readmission diagnoses, 33% were secondary to HACs.
Of the 51 readmissions, 34 (67%) were for medical complications, and 17 (33%) were for surgical complications. Pneumonia was the most common medical complication (11.8%), followed by UTI (7.8%), DVT (5.9%), PE (5.9%), and renal insufficiency (3.9%). Surgical-site infection was the most common surgical complication (13.7%), followed by prosthetic joint dislocation (9.8%) and hematoma (3.9%).
Other risk factors significantly (P < .05) associated with readmission were age over 75 years, dependent functional status, ASA score of 4 or higher, cardiac comorbidity, 2 or more comorbidities, postoperative CAUTI, extended LOS, and revision surgery (Table 3).
Discussion
Hospital readmissions are important because they represent quality of care and play a role in patient outcomes. Arthroplasty research has focused mainly on readmissions after primary knee and hip replacements.23-25
Historical rates of early readmission after SA14 are comparable to those found in our study. Previously identified risk factors have included increasing age, Medicaid insurance status, low-volume surgical centers, and SA type.3 Mahoney and colleagues14 reported a 90-day readmission rate of 5.9%, but, when they removed hemiarthroplasty replacement from the analysis and shortened the readmission timeline to 30 days, the readmission rate was identical to the 2.7% rate in the present study. In their series from a single high-volume institution, the highest 90-day readmission rate was found for hemiarthroplasty (8.8%), followed by RTSA (6.6%) and ATSA (4.5%). In a study by Schairer and colleagues,3 the readmission rate was also influenced by replacement type, but their results differed from those of Mahoney and colleagues.14 Schairer and colleagues3 analyzed data from 7 state inpatient databases and found that the highest readmission rate was associated with RTSA (11.2%), followed by hemiarthroplasty (8.2%) and ATSA (6.0%). In both series, RTSA readmission rates were higher than ATSA readmission rates—consistent with the complication profiles of these procedures, with RTSA often provided as a surgery of last resort, after failure of other procedures, including ATSA.26 The lower 30-day readmission rate in the present study may be attributable to the fact that some surgical and medical complications may not have developed within this short time. Nonetheless, the majority of readmissions typically present within the first 30 days after SA.14,15 Other factors, including hospital volume, surgeon volume, race, and hospital type, may also influence readmission rates but could not be compared between
The present study found that revision surgery, 3 or more comorbidities, and extended LOS (>4.3 days) more than doubled the risk of readmission. Published SA revision rates range from 5% to 42%, with most revisions performed for instability, dislocation, infection, and component loosening.6,29 Complication rates are higher for revision SA than for primary SA, which may explain why revisions predispose patients to readmission.30 Compared with primary SAs, revision SAs are also more likely to be RTSAs, and these salvage procedures have been found to have high complication rates.31 In the present study, the most common comorbidities were hypertension, diabetes, and COPD; the literature supports these as some of the most common comorbid medical conditions in patients who undergo ATSA or RTSA.5,26,32 Furthermore, all 3 of these comorbidities have been shown to be independent predictors of increased postoperative complications in patients who undergo SA, which ultimately would increase the risk of readmission.3,26,33,34 Last, extended LOS has also been shown to increase the risk of unplanned readmissions after orthopedic procedures.35 Risk factors associated with increased LOS after ATSA or RTSA include female sex, advanced age, multiple comorbidities, and postoperative complications.32Several other factors must be noted with respect to individual risk for readmission. In the present study, age over 75 years, dependent functional status, ASA score of 4 or higher, and cardiac comorbidity were found to have a significant association with readmission. Increased age is a risk factor for increased postoperative complications, more medical comorbidities, and increased LOS.34,36 Older people are at higher risk of developing osteoarthritis and rotator cuff tear arthropathy and are more likely to undergo SA.5,6 Older people also are more likely to be dependent, which itself is a risk factor for readmission.19 An ASA score of 3 or 4 has been found to be associated with increased LOS and complications after SA, and cardiac comorbidities predispose patients to a variety of complications.34,36,37In studies that have combined surgical and medical factors, rates of complications early after ATSA and RTSA have ranged from 3.6% to 17.8%.26,38,39 After SAs, medical complications (80%) are more common than surgical complications (20%).39 In the present cohort, many more readmissions were for medical complications (67%) than for surgical complications (33%). In addition, Schairer and colleagues3 found medical complications associated with more than 80% of readmissions after SA.3 Infection was the most common medical reason (pneumonia) and surgical reason (surgical-site infection) for readmission—consistent with findings of other studies.3,35,40 Infection has accounted for 9.4% to 41.4% of readmissions after ATSA and RTSA.3,14In joint arthroplasty, infection occurs more often in patients with coexisting medical comorbidities, leading to higher mortality and increased LOS.41 Prosthetic joint dislocation was common as well—similar to findings in other studies.3,10In the present study, 33% of known readmission diagnoses were secondary to HACs. Surgical-site infection was the most common, followed by CAUTI, DVT, and PE. In another study, of knee and hip arthroplasties, HACs accounted for more than 40% of all complications and were the strongest predictor of early readmission.16 In SA studies, HACs were responsible for 9.3% to 34.5% of readmissions after ATSA and RTSA.3,14 Our finding (33%) is more in line with Mahoney and colleagues14 (34.5%) than Schairer and colleagues3 (9.3%). One explanation for the large discrepancy with Schairer and colleagues3 is that UTI was not among the medical reasons for readmission in their study, but it was in ours. Another difference is that we used a database that included data from multiple institutions. Last, Schairer and colleagues3 excluded revision SAs from their analysis (complication rates are higher for revision SAs than for primary SAs30). They also excluded cases of SA used for fracture (shown to increase the risk for PE42). The US Department of Health and Human Services estimated that patients experienced 1.3 million fewer HACs during the period 2010-2013, corresponding to a 17% decline over the 3 years.43 This translates to an estimated 50,000 fewer mortalities, and $12 billion saved in healthcare costs, over the same period.43 Preventing HACs helps reduce readmission rates while improving patient outcomes and decreasing healthcare costs.
This study had several limitations. We could not differentiate between ATSA and RTSA readmission rates because, for the study period, these procedures are collectively organized under a common CPT code in the NSQIP database. Readmission and complication rates are higher for RTSAs than for ATSAs.3,14 In addition, our data were limited to hospitals that were participating in NSQIP, which could lead to selection bias. We studied rates of only those readmissions and complications that occurred within 30 days, but many complications develop after 30 days, and these increase the readmission rate. Last, reasons for readmission were not recorded for 2011, so this information was available only for the final 2 years of the study. Despite these limitations, NSQIP still allows for a powerful study, as it includes multiple institutions and a very large cohort.
Conclusion
With medical costs increasing, focus has shifted to quality care and good outcomes with the goal of reducing readmissions and complications after various procedures. SA has recently become more popular because of its multiple indications, and this trend will continue. In the present study, the rate of readmission within 30 days after ATSA or RTSA was 2.7%. Revision surgery, 3 or more comorbidities, and extended LOS were independent risk factors that more than doubled the risk of readmission. Understanding the risk factors for short-term readmission will allow for better patient care and decreased costs, and will benefit the healthcare system as a whole.
Am J Orthop. 2016;45(6):E386-E392. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Hospital readmissions are undesirable and expensive.1 The Centers for Medicare & Medicaid Services (CMS) use hospital readmission rates as one measure of healthcare quality and hospital performance.2 In addition, the Patient Protection and Affordable Care Act of 2010 established a provision that decreases payments to hospitals with above-average readmission rates.3 Total knee arthroplasties (TKAs) and total hip arthroplasties (THAs) are among the most common surgical procedures leading to readmission and cost almost $20 billion dollars annually in the Medicare population alone.1 Identifying factors that lead to readmissions after certain popular procedures may be a way to improve healthcare quality and outcomes while decreasing costs.
One such operation is shoulder arthroplasty (SA), which has surged in popularity over the past decade and is projected to increase faster than TKAs and THAs.4-6 SA is used to treat a variety of shoulder conditions, including osteoarthritis, inflammatory arthritis, severe proximal humeral fracture, avascular necrosis, and rotator cuff tear arthropathy.7-12 Much as with knee and hip arthroplasty, good outcomes have been reported with SA: decreased pain, improved range of motion, and high patient satisfaction.10,13 However, there have been few studies of rates of readmission after SA and the associated risk factors.3,14,15 The reported rates of early readmission after SA have ranged from 5.6% to 7.3%.3,14,15 These rates are comparable to rates of readmission after TKA (4.0%-6.6%) and THA (3.5%-8.4%).15-17Recently, CMS introduced legislation to void payments for hospital-acquired conditions (HACs), preventable medical conditions that patients develop during or as a result of their hospital care and that were not present on admission.18 Although many factors contribute to readmission, a recent study regarding all-cause readmission during the first 30 days after discharge found that almost 50% of 30-day readmissions after knee and hip replacements were potentially preventable.19 HACs resulting in readmission after SAs make up 9.3% to 34.5% of all readmissions, after anatomical total shoulder arthroplasties (ATSAs) and reverse total shoulder arthroplasties (RTSAs).3,14 The most common HACs include retained foreign body after surgery, air embolism, falls and trauma, catheter-associated urinary tract infection (CAUTI), surgical-site infection, deep vein thrombosis (DVT), and pulmonary embolism (PE).18 Raines and colleagues16 found that HACs accounted for 41.7% of all complications in knee or hip arthroplasty and that HACs were the greatest predictors of early readmission after both procedures.
We conducted a study to evaluate rates of readmission within 30 days after ATSA and RTSA and to describe the independent risk factors for readmission. We hypothesized that the rate of readmission after SA would be similar to the rate after knee and hip arthroplasty and that readmission risk factors would be similar. Elucidating these rates and associated risk factors may ultimately help to minimize the burden of disability on patients and the burden of financial costs on healthcare institutions.
Materials and Methods
Institutional Review Board approval was not required for this study, and all data used were de-identified to Health Insurance Portability and Accountability Act (HIPAA) standards. We used the American College of Surgeons (ACS) National Surgical Quality Improvement Program (NSQIP) database for this study. The NSQIP was developed in the 1990s to improve surgical quality in the Veterans Health Administration and was later adapted by the ACS.20 NSQIP follows patients for 30 days after operations and provides clinical data and outcome measures that are closely regulated and internally audited.21 The program has continued to expand and now includes more than 400 institutions. The NSQIP database has been validated as a reliable source of surgical outcomes data, including outcomes data for orthopedic procedures, and has been used in other studies of readmissions.17,22
In the present study, the ACS-NSQIP files for the period 2011-2013 were queried for all total shoulder arthroplasties (TSAs) (Current Procedural Terminology [CPT] code 23472, which includes ATSA and RTSA). Descriptive analysis was performed to determine the overall readmission rate as well as the percentages of readmissions for medical and surgical complications. Reasons for readmission were collected from 2012 and 2013 (information from 2011 was absent).
The various patient parameters compiled within the database were examined in a review of ATSAs and RTSAs. Demographics, comorbidities, operative characteristics, and predischarge complications were amassed from these data. Demographics included age, sex, race, body mass index, smoking status, preoperative functional health status, and American Society of Anesthesiologists (ASA) score. Comorbidities included diabetes mellitus, hypertension, chronic corticosteroid use, coagulation disorder, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), cardiac comorbidity (including congestive heart failure, history of myocardial infarction, previous coronary intervention or cardiac surgery, and angina), renal comorbidity (including acute renal failure and preoperative dialysis), neurologic comorbidity (including impaired sensorium, hemiplegia, history of transient ischemic attack, and history of cerebrovascular accident with or without residual deficit), and preoperative blood transfusion. Operative characteristics included resident involvement, operative time more than 1 SD from the mean (>164.4 minutes), intraoperative blood transfusion, and revision surgery. Predischarge complications included pneumonia, CAUTI, DVT, PE, postoperative bleeding that required transfusion, cerebrovascular accident, myocardial infarction, and sepsis. Surgical-site infection, CAUTI, DVT, and PE were selected for analysis because these HACs are common in our cohort.
After the data on these characteristics were collected, univariate analysis was performed to determine association with any readmission. Factors with P < .20 were then entered into multivariate analysis to determine independent risk factors for readmission. This P value was selected to make the model inclusive of any potentially important predictor. Univariate analysis was performed using the Fisher exact test. Multivariate analysis was performed using backward conditional binary logistic regression. Statistical significance was set at P < .05. All analysis was performed with SPSS Version 22.0 (SPSS).
Results
This study included a combined total of 3501 ATSAs and RTSAs performed between 2011 and 2013. The overall readmission rate was 2.7%. The associated diagnosis for readmission was available for 54% of the readmitted patients. Of the known readmission diagnoses, 33% were secondary to HACs.
Of the 51 readmissions, 34 (67%) were for medical complications, and 17 (33%) were for surgical complications. Pneumonia was the most common medical complication (11.8%), followed by UTI (7.8%), DVT (5.9%), PE (5.9%), and renal insufficiency (3.9%). Surgical-site infection was the most common surgical complication (13.7%), followed by prosthetic joint dislocation (9.8%) and hematoma (3.9%).
Other risk factors significantly (P < .05) associated with readmission were age over 75 years, dependent functional status, ASA score of 4 or higher, cardiac comorbidity, 2 or more comorbidities, postoperative CAUTI, extended LOS, and revision surgery (Table 3).
Discussion
Hospital readmissions are important because they represent quality of care and play a role in patient outcomes. Arthroplasty research has focused mainly on readmissions after primary knee and hip replacements.23-25
Historical rates of early readmission after SA14 are comparable to those found in our study. Previously identified risk factors have included increasing age, Medicaid insurance status, low-volume surgical centers, and SA type.3 Mahoney and colleagues14 reported a 90-day readmission rate of 5.9%, but, when they removed hemiarthroplasty replacement from the analysis and shortened the readmission timeline to 30 days, the readmission rate was identical to the 2.7% rate in the present study. In their series from a single high-volume institution, the highest 90-day readmission rate was found for hemiarthroplasty (8.8%), followed by RTSA (6.6%) and ATSA (4.5%). In a study by Schairer and colleagues,3 the readmission rate was also influenced by replacement type, but their results differed from those of Mahoney and colleagues.14 Schairer and colleagues3 analyzed data from 7 state inpatient databases and found that the highest readmission rate was associated with RTSA (11.2%), followed by hemiarthroplasty (8.2%) and ATSA (6.0%). In both series, RTSA readmission rates were higher than ATSA readmission rates—consistent with the complication profiles of these procedures, with RTSA often provided as a surgery of last resort, after failure of other procedures, including ATSA.26 The lower 30-day readmission rate in the present study may be attributable to the fact that some surgical and medical complications may not have developed within this short time. Nonetheless, the majority of readmissions typically present within the first 30 days after SA.14,15 Other factors, including hospital volume, surgeon volume, race, and hospital type, may also influence readmission rates but could not be compared between
The present study found that revision surgery, 3 or more comorbidities, and extended LOS (>4.3 days) more than doubled the risk of readmission. Published SA revision rates range from 5% to 42%, with most revisions performed for instability, dislocation, infection, and component loosening.6,29 Complication rates are higher for revision SA than for primary SA, which may explain why revisions predispose patients to readmission.30 Compared with primary SAs, revision SAs are also more likely to be RTSAs, and these salvage procedures have been found to have high complication rates.31 In the present study, the most common comorbidities were hypertension, diabetes, and COPD; the literature supports these as some of the most common comorbid medical conditions in patients who undergo ATSA or RTSA.5,26,32 Furthermore, all 3 of these comorbidities have been shown to be independent predictors of increased postoperative complications in patients who undergo SA, which ultimately would increase the risk of readmission.3,26,33,34 Last, extended LOS has also been shown to increase the risk of unplanned readmissions after orthopedic procedures.35 Risk factors associated with increased LOS after ATSA or RTSA include female sex, advanced age, multiple comorbidities, and postoperative complications.32Several other factors must be noted with respect to individual risk for readmission. In the present study, age over 75 years, dependent functional status, ASA score of 4 or higher, and cardiac comorbidity were found to have a significant association with readmission. Increased age is a risk factor for increased postoperative complications, more medical comorbidities, and increased LOS.34,36 Older people are at higher risk of developing osteoarthritis and rotator cuff tear arthropathy and are more likely to undergo SA.5,6 Older people also are more likely to be dependent, which itself is a risk factor for readmission.19 An ASA score of 3 or 4 has been found to be associated with increased LOS and complications after SA, and cardiac comorbidities predispose patients to a variety of complications.34,36,37In studies that have combined surgical and medical factors, rates of complications early after ATSA and RTSA have ranged from 3.6% to 17.8%.26,38,39 After SAs, medical complications (80%) are more common than surgical complications (20%).39 In the present cohort, many more readmissions were for medical complications (67%) than for surgical complications (33%). In addition, Schairer and colleagues3 found medical complications associated with more than 80% of readmissions after SA.3 Infection was the most common medical reason (pneumonia) and surgical reason (surgical-site infection) for readmission—consistent with findings of other studies.3,35,40 Infection has accounted for 9.4% to 41.4% of readmissions after ATSA and RTSA.3,14In joint arthroplasty, infection occurs more often in patients with coexisting medical comorbidities, leading to higher mortality and increased LOS.41 Prosthetic joint dislocation was common as well—similar to findings in other studies.3,10In the present study, 33% of known readmission diagnoses were secondary to HACs. Surgical-site infection was the most common, followed by CAUTI, DVT, and PE. In another study, of knee and hip arthroplasties, HACs accounted for more than 40% of all complications and were the strongest predictor of early readmission.16 In SA studies, HACs were responsible for 9.3% to 34.5% of readmissions after ATSA and RTSA.3,14 Our finding (33%) is more in line with Mahoney and colleagues14 (34.5%) than Schairer and colleagues3 (9.3%). One explanation for the large discrepancy with Schairer and colleagues3 is that UTI was not among the medical reasons for readmission in their study, but it was in ours. Another difference is that we used a database that included data from multiple institutions. Last, Schairer and colleagues3 excluded revision SAs from their analysis (complication rates are higher for revision SAs than for primary SAs30). They also excluded cases of SA used for fracture (shown to increase the risk for PE42). The US Department of Health and Human Services estimated that patients experienced 1.3 million fewer HACs during the period 2010-2013, corresponding to a 17% decline over the 3 years.43 This translates to an estimated 50,000 fewer mortalities, and $12 billion saved in healthcare costs, over the same period.43 Preventing HACs helps reduce readmission rates while improving patient outcomes and decreasing healthcare costs.
This study had several limitations. We could not differentiate between ATSA and RTSA readmission rates because, for the study period, these procedures are collectively organized under a common CPT code in the NSQIP database. Readmission and complication rates are higher for RTSAs than for ATSAs.3,14 In addition, our data were limited to hospitals that were participating in NSQIP, which could lead to selection bias. We studied rates of only those readmissions and complications that occurred within 30 days, but many complications develop after 30 days, and these increase the readmission rate. Last, reasons for readmission were not recorded for 2011, so this information was available only for the final 2 years of the study. Despite these limitations, NSQIP still allows for a powerful study, as it includes multiple institutions and a very large cohort.
Conclusion
With medical costs increasing, focus has shifted to quality care and good outcomes with the goal of reducing readmissions and complications after various procedures. SA has recently become more popular because of its multiple indications, and this trend will continue. In the present study, the rate of readmission within 30 days after ATSA or RTSA was 2.7%. Revision surgery, 3 or more comorbidities, and extended LOS were independent risk factors that more than doubled the risk of readmission. Understanding the risk factors for short-term readmission will allow for better patient care and decreased costs, and will benefit the healthcare system as a whole.
Am J Orthop. 2016;45(6):E386-E392. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
2. Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305(5):504-505.
3. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
4. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
5. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
6. Jain NB, Yamaguchi K. The contribution of reverse shoulder arthroplasty to utilization of primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1905-1912.
7. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction–internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
10. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
11. Fevang BT, Lygre SH, Bertelsen G, Skredderstuen A, Havelin LI, Furnes O. Good function after shoulder arthroplasty. Acta Orthop. 2012;83(5):467-473.
12. Orfaly RM, Rockwood CA Jr, Esenyel CZ, Wirth MA. Shoulder arthroplasty in cases with avascular necrosis of the humeral head. J Shoulder Elbow Surg. 2007;16(3 suppl):S27-S32.
13. Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13(6):604-613.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722.
16. Raines BT, Ponce BA, Reed RD, Richman JS, Hawn MT. Hospital acquired conditions are the strongest predictor for early readmission: an analysis of 26,710 arthroplasties. J Arthroplasty. 2015;30(8):1299-1307.
17. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
18. Centers for Medicare & Medicaid Services. Hospital-Acquired Conditions. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions.html. Published 2014. Accessed May 21, 2015.
19. Feigenbaum P, Neuwirth E, Trowbridge L, et al. Factors contributing to all-cause 30-day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599-605.
20. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250(3):363-376.
21. American College of Surgeons. About ACS NSQIP. http://www.facs.org/quality-programs/acs-nsqip/about. Published 2015. Accessed June 14, 2015.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
24. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
25. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
26. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467.
27. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
28. Tsai TC, Orav EJ, Joynt KE. Disparities in surgical 30-day readmission rates for Medicare beneficiaries by race and site of care. Ann Surg. 2014;259(6):1086-1090.
29. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
30. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
31. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJ. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042.
32. Menendez ME, Baker DK, Fryberger CT, Ponce BA. Predictors of extended length of stay after elective shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1527-1533.
33. Jain NB, Guller U, Pietrobon R, Bond TK, Higgins LD. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;(435):232-238.
34. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1.
35. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
36. Dunn JC, Lanzi J, Kusnezov N, Bader J, Waterman BR, Belmont PJ Jr. Predictors of length of stay after elective total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(5):754-759.
37. Maile MD, Engoren MC, Tremper KK, Jewell E, Kheterpal S. Worsening preoperative heart failure is associated with mortality and noncardiac complications, but not myocardial infarction after noncardiac surgery: a retrospective cohort study. Anesth Analg. 2014;119(3):522-532.
38. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
39. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
40. Kassin MT, Owen RM, Perez SD, et al. Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg. 2012;215(3):322-330.
41. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
42. Young BL, Menendez ME, Baker DK, Ponce BA. Factors associated with in-hospital pulmonary embolism after shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):e271-e278.
43. US Department of Health and Human Services. Efforts to improve patient safety result in 1.3 million fewer patient harms, 50,000 lives saved and $12 billion in health spending avoided [press release]. http://www.hhs.gov/news/press/2014pres/12/20141202a.html. Published December 2, 2014. Accessed May 25, 2015.
1. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
2. Axon RN, Williams MV. Hospital readmission as an accountability measure. JAMA. 2011;305(5):504-505.
3. Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355.
4. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120.
5. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
6. Jain NB, Yamaguchi K. The contribution of reverse shoulder arthroplasty to utilization of primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1905-1912.
7. Bartelt R, Sperling JW, Schleck CD, Cofield RH. Shoulder arthroplasty in patients aged fifty-five years or younger with osteoarthritis. J Shoulder Elbow Surg. 2011;20(1):123-130.
8. Chalmers PN, Slikker W 3rd, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction–internal fixation and hemiarthroplasty. J Shoulder Elbow Surg. 2014;23(2):197-204.
9. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
10. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
11. Fevang BT, Lygre SH, Bertelsen G, Skredderstuen A, Havelin LI, Furnes O. Good function after shoulder arthroplasty. Acta Orthop. 2012;83(5):467-473.
12. Orfaly RM, Rockwood CA Jr, Esenyel CZ, Wirth MA. Shoulder arthroplasty in cases with avascular necrosis of the humeral head. J Shoulder Elbow Surg. 2007;16(3 suppl):S27-S32.
13. Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13(6):604-613.
14. Mahoney A, Bosco JA 3rd, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381.
15. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722.
16. Raines BT, Ponce BA, Reed RD, Richman JS, Hawn MT. Hospital acquired conditions are the strongest predictor for early readmission: an analysis of 26,710 arthroplasties. J Arthroplasty. 2015;30(8):1299-1307.
17. Pugely AJ, Callaghan JJ, Martin CT, Cram P, Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499-1504.
18. Centers for Medicare & Medicaid Services. Hospital-Acquired Conditions. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/Hospital-Acquired_Conditions.html. Published 2014. Accessed May 21, 2015.
19. Feigenbaum P, Neuwirth E, Trowbridge L, et al. Factors contributing to all-cause 30-day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50(7):599-605.
20. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250(3):363-376.
21. American College of Surgeons. About ACS NSQIP. http://www.facs.org/quality-programs/acs-nsqip/about. Published 2015. Accessed June 14, 2015.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Bini SA, Fithian DC, Paxton LW, Khatod MX, Inacio MC, Namba RS. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25(1):114-117.
24. Husted H, Otte KS, Kristensen BB, Orsnes T, Kehlet H. Readmissions after fast-track hip and knee arthroplasty. Arch Orthop Trauma Surg. 2010;130(9):1185-1191.
25. Vorhies JS, Wang Y, Herndon J, Maloney WJ, Huddleston JI. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 suppl):119-123.
26. Ponce BA, Oladeji LO, Rogers ME, Menendez ME. Comparative analysis of anatomic and reverse total shoulder arthroplasty: in-hospital outcomes and costs. J Shoulder Elbow Surg. 2015;24(3):460-467.
27. Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92(16):2643-2652.
28. Tsai TC, Orav EJ, Joynt KE. Disparities in surgical 30-day readmission rates for Medicare beneficiaries by race and site of care. Ann Surg. 2014;259(6):1086-1090.
29. Bohsali KI, Wirth MA, Rockwood CA Jr. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292.
30. Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(11):1647-1654.
31. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJ. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042.
32. Menendez ME, Baker DK, Fryberger CT, Ponce BA. Predictors of extended length of stay after elective shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1527-1533.
33. Jain NB, Guller U, Pietrobon R, Bond TK, Higgins LD. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res. 2005;(435):232-238.
34. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1.
35. Dailey EA, Cizik A, Kasten J, Chapman JR, Lee MJ. Risk factors for readmission of orthopaedic surgical patients. J Bone Joint Surg Am. 2013;95(11):1012-1019.
36. Dunn JC, Lanzi J, Kusnezov N, Bader J, Waterman BR, Belmont PJ Jr. Predictors of length of stay after elective total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2015;24(5):754-759.
37. Maile MD, Engoren MC, Tremper KK, Jewell E, Kheterpal S. Worsening preoperative heart failure is associated with mortality and noncardiac complications, but not myocardial infarction after noncardiac surgery: a retrospective cohort study. Anesth Analg. 2014;119(3):522-532.
38. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
39. Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30.
40. Kassin MT, Owen RM, Perez SD, et al. Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg. 2012;215(3):322-330.
41. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In-hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385-389.
42. Young BL, Menendez ME, Baker DK, Ponce BA. Factors associated with in-hospital pulmonary embolism after shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):e271-e278.
43. US Department of Health and Human Services. Efforts to improve patient safety result in 1.3 million fewer patient harms, 50,000 lives saved and $12 billion in health spending avoided [press release]. http://www.hhs.gov/news/press/2014pres/12/20141202a.html. Published December 2, 2014. Accessed May 25, 2015.