User login
Application of Amniotic Tissue in Orthopedic Surgery
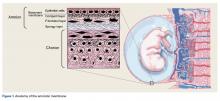
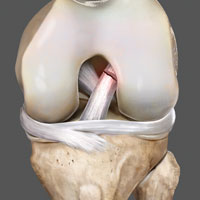
The amniotic membrane is a multilayer tissue forming the innermost layer of the amniotic sac that surrounds the developing fetus. It is comprised of 5 layers, from the inside out: a single layer of epithelial cells, a thick basement membrane, a compact layer, a fibroblast layer, and a spongy layer that abuts the surrounding chorion (Figure 1).1
Amniotic epithelial cells are derived from the pluripotent epiblast at approximately day 8 of gestation. This is well before gastrulation occurs at days 15 to 17, considered the “tipping point” when pluripotent cells differentiate into ectoderm, mesoderm, and endoderm.3 These cells express Oct-4 and Nanog, 2 molecular markers that are indicative of pluripotency.3 Two cell types have been identified in amniotic tissues that possess stem cell-like characteristics: human amniotic epithelial cells and human amniotic mesenchymal stromal cells.4 Both of these cell types have demonstrated the ability to differentiate into various cell lineages, including endothelial cells, adipocytes, myogenic cells, neurogenic cells, chondrocytes, tenocytes, and osteogenic cells.5-7 These previously reported findings indicate that amniotic cells and tissue have the capability to generate mesenchymal tissues.
FDA Classification and Available Forms
The US Food and Drug Administration (FDA) classifies amnion as an allograft tissue under Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) 361. To meet criteria, the tissue needs to be minimally manipulated. It is to be for homologous use and cannot be combined with other cells or tissues. There can be no systemic effect or dependence on the metabolic activity of living cells to achieve its primary function. The tissue has to have a localized effect in vivo. Therefore, amnion allograft tissue can be commercialized, provided it is not marketed as a stem cell product or to contain viable cells.
Amniotic tissue is commercially available in several forms.
Safety
Amniotic tissue has been used for over 100 years in burn, ophthalmology, and chronic wound patients with favorable outcomes and no adverse effects reported in the literature. Unlike embryonic stem cells, which may be tumorigenic,8 amniotic cells do not possess any known tumorigenicity.9 In one study, 50 immunodeficient mice were injected with 1 to 2 million amniotic epithelial cells and observed for a maximum of 516 days with no tumorigenicity observed in any of the animals.10 In another study, amniotic epithelial cells were implanted into the forearms of healthy volunteers and no immunologic response was observed in any of the recipients.11 Furthermore, viable amniotic cells were recovered via biopsy 7 weeks following transplantation, demonstrating viability of the transplanted cells.11 The lack of tumorigenicity and immunologic response in hosts is due in part to the fact that amniotic cells do not express human leukocyte antigen class II antigens and only express class I antigens in small amounts.3
Advantages of Amnion Tissue
Amniotic tissue is readily available, as it is often discarded after childbirth. The use of this tissue poses no added risk to the fetus or mother, eliminating the ethical concerns associated with obtaining embryonic stem cells. Amniotic tissue is comprised of an extracellular matrix, which acts as a natural scaffold for cellular attachment and structural support for cells as well as collagen types I, III, IV, V, and VI, hyaluronic acid, and a host of growth factors.12 In addition, it possesses antimicrobial properties, including beta-defensins.13
Amniotic tissue has been shown to exert an anti-inflammatory effect by inhibiting the inflammatory cascade. Specifically, it has been shown to inhibit cytokines such as tumor necrosis factor-alpha in the presence of dendritic cells,14 as well as inhibiting transforming growth factor-beta, interleukin-8, and fibroblast proliferation.15 These findings indicate that amniotic tissue has the ability to dampen the “cytokine storm” that occurs after an injury in an adult, which would lead to beneficial impacts on healing and scar formation in patients.16
Basic Science and Animal Studies
Several studies have demonstrated promising outcomes for orthopedic applications in vitro. A comparison of osteogenic potential found that amniotic fluid-derived cells were able to produce approximately 5 times more mineralized matrix than bone marrow-derived mesenchymal stem cells.17 More recently, Si and colleagues18 compared the osteogenic potential of human amniotic epithelial cells, amniotic cells, and human bone marrow-derived mesenchymal stem cells. They found that all 3 cell lines were osteogenic, though the amniotic epithelial cells had better immunomodulatory properties and marginally less osteogenic potential than the other 2 cell types. Furthermore, several in vivo animal studies have demonstrated the ability of human amniotic cells to stimulate bone growth in rats,19,20 rabbits,21 and sheep.22
Amniotic tissue also possesses potential for chondrogenesis. Cryopreserved human amniotic membrane cells used for in vitro human osteoarthritis tissue scaffolds did not differentiate in culture, and they integrated and repaired damaged articular cartilage.23 Various in vitro24,25 and animal in vivo26,27 studies have reported similar supportive findings. Kunisaki and colleagues28 used sheep amniotic fluid mesenchymal stem cells to reconstruct lamb tracheal cartilage in utero, concluding that cells obtained from the amniotic fluid possess chondrogenic capabilities. Further in utero lamb studies of cartilage artificial defects, given 7 days to settle before adding a hypocellular matrix as a scaffold, showed chondrocyte density and cell architecture was restored at the defect site after 28 days without the formation of an inflammatory response or scar tissue.29
Amniotic tissue has had similar success in tendon repair studies in vivo.9,30,31 Barboni and colleagues32 implanted amniotic epithelial cells (AECs) into artificially created sheep Achilles tendon defects in situ, inducing superior structural and mechanical recovery in the defects at a faster rate compared to controls not receiving AECs. Healing via AECs started at the healthy tissue around the borders of the defect and progressed centrally, suggesting recruitment of native progenitor cells to the lesion.32 Kueckelhaus and colleagues33 investigated the role of amnion-derived cellular cytokine solution in the healing of transections of rat Achilles tendons, reporting improved mechanical properties of healing tendons at early time points compared to controls. Beredjiklian and colleagues34 compared the healing of transected extensor tendons of pregnant ewes and of their fetus in utero, reporting a reparative form of healing with scar formation in adult subjects and regenerative form of healing without scar formation or inflammation in fetal subjects.
Amniotic tissue has properties that prevent adhesion formation around tendons following injury and reconstruction.35 Ozgenel36 investigated the effects of hyaluronic acid and amniotic membrane alone and in combination on the presence of adhesions and the rate of healing following chicken flexor tendon repair. The study found amniotic membrane wrapped around the repaired tendon was superior in preventing adhesion formation. Kim and colleagues37 report a similar reduction in fibrosis and adhesion following application of a human amniotic membrane wrap to rabbit ulnar neurorrhaphy sites.
This barrier function of amniotic tissue has also been investigated in the prevention of surgical scarring and peridural fibrosis in animal models following spinal discectomy. A study in canine models showed a reduction of scarring following the application of cross-linked amniotic membrane compared to freeze dried amniotic membrane.38 Similar reductions in scarring in rat models with the application of freeze-dried amniotic membrane compared to negative controls have been reported.39
Human Studies
A randomized trial investigated the outcomes of prenatal vs postnatal repair of myelomeningocele in humans, finding a reduced need for implanted shunts and improved functional outcomes at 30 months of life in the prenatal intervention group compared to the postnatal group.40 This study was concluded early due to the efficacy of prenatal surgery and the benefit of nervous system repair in utero in the presence of amniotic growth factors.
Vines and colleagues41 performed a 6-patient feasibility study using amnion injections to treat symptomatic knee osteoarthritis. Each patient received a single intra-articular cryopreserved amniotic suspension allograft (ASA) injection and was followed for 1 year. No adverse outcomes were reported, with the only abnormal finding being a small increase in serum immunoglobulin G and immunoglobulin E levels. Intra-articular ASA injection was found to be safe, but a large-scale trial investigating symptomatic relief was recommended.41
Most of the human studies using amnion pertain to foot and ankle surgery. Its use as a treatment for diabetic foot ulcers and recalcitrant plantar fasciitis was one of the early-recognized successes.42-45 Zelen and colleagues46 investigated the applications of injectable micronized dehydrated human amniotic/chorionic membrane as an alternative to surgical intervention in the treatment of refractory plantar fasciitis. This prospective, randomized trial with 45 patients showed significant improvement in plantar fasciitis symptoms at 8 weeks compared to controls (saline injections). A similar study compared the use of cryopreserved human amniotic membrane (c-hAM) injections to corticosteroid injections in plantar fasciitis patients.47 The results indicated that c-hAM is safe and comparable to corticosteroids, with the authors noting that pain improvement was greatest in patients receiving 2 injections of c-hAM at 18 weeks.
Tendon wrapping, in which the amniotic membrane is laid over a tendon repair, has been reported with success. Amniotic membrane is superior to collagen for tendon wrapping as it actively contributes to healing while minimizing adhesions, which collagen alone cannot do.48 The membrane serves as a protective sheath around repaired tendons with anti-inflammatory, anti-adhesive, immunomodulatory, and antimicrobial benefits. A 124-patient study demonstrated the safety of using amnion in this manner, and the authors reported a decreased rate of complication compared to previously published data.49 Another study of 14 patients undergoing foot and ankle surgery with tendon wrapping reported clinical improvement with reduced pain and greater functional outcomes postoperatively compared to preoperative measurements.50
Conclusion
Amniotic membrane-derived tissues are safe and non-tumorigenic, producing an abundance of growth factors that have shown promise as tissue scaffolds and as aids in the regeneration of human bone and soft tissues. Amnion applications in orthopedic surgery may be numerous, but development is ongoing. Given the vast array of in vitro and in vivo animal data supporting the benefits of amnion in tissue regeneration, orthopedic surgeons and researchers should place emphasis on conducting clinical studies to validate the safety and efficacy of amniotic cells in the treatment of orthopedic conditions.
Am J Orthop. 2016;45(7):E421-E425. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Benirschke K, Kaufman P. Anatomy and pathology of the placental membranes. In: Pathology of the Human Placent., 4th ed. New York, NY: Springer-Verlag; 2000:281-334.
2. Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447-458.
3. Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2(2):133-142.
4. Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26(2):300-311.
5. Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577-588.
6. Alviano F, Fossati V, Marchionni C, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11.
7. Barboni B, Curini V, Russo V, et al. Indirect co-culture with tendons or tenocytes can program amniotic epithelial cells towards stepwise tenogenic differentiation. PLoS One. 2012;7(2):e30974.
8. Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature Reviews Cancer. 2011;11(4):268-277.
9. Lange-Consiglio A, Rossi D, Tassan S, Perego R, Cremonesi F, Parolini O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013;22(22):3015-3024.
10. Miki T. Amnion-derived stem cells: in quest of clinical applications. Stem Cell Res Ther. 2011;2(3):25.
11. Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2(8254):1003-1035.
12. Gupta A, Kedige SD, Jain K. Amnion and chorion membranes: potential stem cell reservoir with wide applications in periodontics. Int J Biomater. 2015;2015:274082.
13. Buhimschi IA, Jabr M, Buhimschi CS, Petkova AP, Weiner CP, Saed GM. The novel antimicrobial peptide beta3-defensin is produced by the amnion: a possible role of the fetal membranes in innate immunity of the amniotic cavity. Am J Obstet Gynecol. 2004;191(5):1678-1687.
14. Magatti M, De Munari S, Vertua E, et al. Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant. 2009;18(8):899-914.
15. Solomon A, Wajngarten M, Alviano F, et al. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy. 2005;35(7):941-948.
16. Silini A, Parolini O, Huppertz B, Lang I. Soluble factors of amnion-derived cells in treatment of inflammatory and fibrotic pathologies. Curr Stem Cell Res Ther. 2013;8(1):6-14.
17. Peister A, Woodruff MA, Prince JJ, Gray DP, Hutmacher DW, Guldberg RE. Cell sourcing for bone tissue engineering: amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res. 2011;7(1):17-27.
18. Si J, Dai J, Zhang J, et al. Comparative investigation of human amniotic epithelial cells and mesenchymal stem cells for application in bone tissue engineering. Stem Cells Int. 2015;2015:565732.
19. Starecki M, Schwartz JA, Grande DA. Evaluation of amniotic-derived membrane biomaterial as an adjunct for repair of critical sized bone defects. Advances in Orthopedic Surgery. 2014;2014:572586.
20. Kerimoglu S, Livaoglu M, Sönmez B, et al. Effects of human amniotic fluid on fracture healing in rat tibia. J Surg Res. 2009;152(2):281-287.
21. Karaçal N, Kocucu P, Cobanglu U, Kutlu N. Effect of human amniotic fluid on bone healing. J Surg Res. 2005;129(2):283-287.
22. Barboni B, Mangano C, Valbonetti L, et al. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS One. 2013;8(5):e63256.
23. Díaz-Prado S, Rendal-Vázquez ME, Muiños-Lopez E, et al. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010;11(2):183-195.
24. Krishnamurithy G, Shilpa PN, Ahmad RE, Sulaiman S, Ng CL, Kamarul T. Human amniotic membrane as a chondrocyte carrier vehicle/substrate: in vitro study. J Biomed Mater Res A. 2011;99(3):500-506.
25. Tan SL, Sulaiman S, Pingguan-Murphy B, Selvaratnam L, Tai CC, Kamarul T. Human amnion as a novel cell delivery vehicle for chondrogenic mesenchymal stem cells. Cell Tissue Bank. 2011;12(1):59-70.
26. Jin CZ, Park SR, Choi BH, Lee KY, Kang CK, Min BH. Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng. 2007;13(4):693-702.
27. Garcia D, Longo UG, Vaquero J, et al. Amniotic membrane transplant for articular cartilage repair: an experimental study in sheep. Curr Stem Cell Res Ther. 2014;10(1):77-83.
28. Kunisaki SM, Freedman DA, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg. 2006;41(4):675-682.
29. Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80(1):4-10.
30. Philip J, Hackl F, Canseco JA, et al. Amnion-derived multipotent progenitor cells improve achilles tendon repair in rats. Eplasty. 2013;13:e31.
31. Lange-Consiglio A, Tassan S, Corradetti B, et al. Investigating the efficacy of amnion-derived compared with bone marrow–derived mesenchymal stromal cells in equine tendon and ligament injuries. Cytotherapy. 2013;15(8):1011-1020.
32. Barboni B, Russo V, Curini V, et al. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012;21(11):2377-2395.
33. Kueckelhaus M, Philip J, Kamel RA, et al. Sustained release of amnion-derived cellular cytokine solution facilitates achilles tendon healing in rats. Eplasty. 2014;14:e29.
34. Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowski LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31(10):1143-1152.
35. Demirkan F, Colakoglu N, Herek O, Erkula G. The use of amniotic membrane in flexor tendon repair: an experimental model. Arch Orthop Trauma Surg. 2002;122(7):396-369.
36. Ozgenel GY. The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J Bone Joint Surg Br. 2004;86(2):301-307.
37. Kim SS, Sohn SK, Lee KY, Lee MJ, Roh MS, Kim CH. Use of human amniotic membrane wrap in reducing perineural adhesions in a rabbit model of ulnar nerve neurorrhaphy. J Hand Surg Eur Vol. 2010;35(3):214-219.
38. Tao H, Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18(8):1202-1212.
39. Choi HJ, Kim KB, Kwon YM. Effect of amniotic membrane to reduce postlaminectomy epidural adhesion on a rat model. J Korean Neurosurg Soc. 2011;49(6):323-328.
40. Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993-1004.
41. Vines JB, Aliprantis AO, Gomoll AH, Farr J. Cryopreserved amniotic suspension for the treatment of knee osteoarthritis. J Knee Surg. 2016;29(6):443-450.
42. Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22(7):347-348,350-351.
43. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507.
44. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J. 2014;11(2):122-128.
45. Zelen CM, Snyder RJ, Serena TE, Li WW. The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: a review. Clin Podiatr Med Surg. 2015;32(1):135-146.
46. Zelen CM, Poka A, Andrews J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis: a feasibility study. Foot Ankle Int. 2013;34(10):1332-1339.
47. Hanselman AE, Tidwell JE, Santrock RD. Cryopreserved human amniotic membrane injection for plantar fasciitis: a randomized, controlled, double-blind pilot study. Foot Ankle Int. 2015;36(2):151-158.
48. Jay RM. Initial clinical experience with the use of human amniotic membrane tissue during repair of posterior tibial and achilles tendons. 2009. http://encompassbiologics.com/wp-content/uploads/2015/07/DrJayClinicalExperience.pdf. Accessed September 29, 2016.
49. DeMill SL, Granata JD, McAlister JE, Berlet GC, Hyer CF. Safety analysis of cryopreserved amniotic membrane/umbilical cord tissue in foot and ankle surgery: a consecutive case series of 124 patients. Surg Technol Int. 2014;25:257-261.
50. Warner M, Lasyone L. An open-label, single-center, retrospective study of cryopreserved amniotic membrane and umbilical cord tissue as an adjunct for foot and ankle surgery. Surg Technol Int. 2014;25:251-255.
The amniotic membrane is a multilayer tissue forming the innermost layer of the amniotic sac that surrounds the developing fetus. It is comprised of 5 layers, from the inside out: a single layer of epithelial cells, a thick basement membrane, a compact layer, a fibroblast layer, and a spongy layer that abuts the surrounding chorion (Figure 1).1
Amniotic epithelial cells are derived from the pluripotent epiblast at approximately day 8 of gestation. This is well before gastrulation occurs at days 15 to 17, considered the “tipping point” when pluripotent cells differentiate into ectoderm, mesoderm, and endoderm.3 These cells express Oct-4 and Nanog, 2 molecular markers that are indicative of pluripotency.3 Two cell types have been identified in amniotic tissues that possess stem cell-like characteristics: human amniotic epithelial cells and human amniotic mesenchymal stromal cells.4 Both of these cell types have demonstrated the ability to differentiate into various cell lineages, including endothelial cells, adipocytes, myogenic cells, neurogenic cells, chondrocytes, tenocytes, and osteogenic cells.5-7 These previously reported findings indicate that amniotic cells and tissue have the capability to generate mesenchymal tissues.
FDA Classification and Available Forms
The US Food and Drug Administration (FDA) classifies amnion as an allograft tissue under Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) 361. To meet criteria, the tissue needs to be minimally manipulated. It is to be for homologous use and cannot be combined with other cells or tissues. There can be no systemic effect or dependence on the metabolic activity of living cells to achieve its primary function. The tissue has to have a localized effect in vivo. Therefore, amnion allograft tissue can be commercialized, provided it is not marketed as a stem cell product or to contain viable cells.
Amniotic tissue is commercially available in several forms.
Safety
Amniotic tissue has been used for over 100 years in burn, ophthalmology, and chronic wound patients with favorable outcomes and no adverse effects reported in the literature. Unlike embryonic stem cells, which may be tumorigenic,8 amniotic cells do not possess any known tumorigenicity.9 In one study, 50 immunodeficient mice were injected with 1 to 2 million amniotic epithelial cells and observed for a maximum of 516 days with no tumorigenicity observed in any of the animals.10 In another study, amniotic epithelial cells were implanted into the forearms of healthy volunteers and no immunologic response was observed in any of the recipients.11 Furthermore, viable amniotic cells were recovered via biopsy 7 weeks following transplantation, demonstrating viability of the transplanted cells.11 The lack of tumorigenicity and immunologic response in hosts is due in part to the fact that amniotic cells do not express human leukocyte antigen class II antigens and only express class I antigens in small amounts.3
Advantages of Amnion Tissue
Amniotic tissue is readily available, as it is often discarded after childbirth. The use of this tissue poses no added risk to the fetus or mother, eliminating the ethical concerns associated with obtaining embryonic stem cells. Amniotic tissue is comprised of an extracellular matrix, which acts as a natural scaffold for cellular attachment and structural support for cells as well as collagen types I, III, IV, V, and VI, hyaluronic acid, and a host of growth factors.12 In addition, it possesses antimicrobial properties, including beta-defensins.13
Amniotic tissue has been shown to exert an anti-inflammatory effect by inhibiting the inflammatory cascade. Specifically, it has been shown to inhibit cytokines such as tumor necrosis factor-alpha in the presence of dendritic cells,14 as well as inhibiting transforming growth factor-beta, interleukin-8, and fibroblast proliferation.15 These findings indicate that amniotic tissue has the ability to dampen the “cytokine storm” that occurs after an injury in an adult, which would lead to beneficial impacts on healing and scar formation in patients.16
Basic Science and Animal Studies
Several studies have demonstrated promising outcomes for orthopedic applications in vitro. A comparison of osteogenic potential found that amniotic fluid-derived cells were able to produce approximately 5 times more mineralized matrix than bone marrow-derived mesenchymal stem cells.17 More recently, Si and colleagues18 compared the osteogenic potential of human amniotic epithelial cells, amniotic cells, and human bone marrow-derived mesenchymal stem cells. They found that all 3 cell lines were osteogenic, though the amniotic epithelial cells had better immunomodulatory properties and marginally less osteogenic potential than the other 2 cell types. Furthermore, several in vivo animal studies have demonstrated the ability of human amniotic cells to stimulate bone growth in rats,19,20 rabbits,21 and sheep.22
Amniotic tissue also possesses potential for chondrogenesis. Cryopreserved human amniotic membrane cells used for in vitro human osteoarthritis tissue scaffolds did not differentiate in culture, and they integrated and repaired damaged articular cartilage.23 Various in vitro24,25 and animal in vivo26,27 studies have reported similar supportive findings. Kunisaki and colleagues28 used sheep amniotic fluid mesenchymal stem cells to reconstruct lamb tracheal cartilage in utero, concluding that cells obtained from the amniotic fluid possess chondrogenic capabilities. Further in utero lamb studies of cartilage artificial defects, given 7 days to settle before adding a hypocellular matrix as a scaffold, showed chondrocyte density and cell architecture was restored at the defect site after 28 days without the formation of an inflammatory response or scar tissue.29
Amniotic tissue has had similar success in tendon repair studies in vivo.9,30,31 Barboni and colleagues32 implanted amniotic epithelial cells (AECs) into artificially created sheep Achilles tendon defects in situ, inducing superior structural and mechanical recovery in the defects at a faster rate compared to controls not receiving AECs. Healing via AECs started at the healthy tissue around the borders of the defect and progressed centrally, suggesting recruitment of native progenitor cells to the lesion.32 Kueckelhaus and colleagues33 investigated the role of amnion-derived cellular cytokine solution in the healing of transections of rat Achilles tendons, reporting improved mechanical properties of healing tendons at early time points compared to controls. Beredjiklian and colleagues34 compared the healing of transected extensor tendons of pregnant ewes and of their fetus in utero, reporting a reparative form of healing with scar formation in adult subjects and regenerative form of healing without scar formation or inflammation in fetal subjects.
Amniotic tissue has properties that prevent adhesion formation around tendons following injury and reconstruction.35 Ozgenel36 investigated the effects of hyaluronic acid and amniotic membrane alone and in combination on the presence of adhesions and the rate of healing following chicken flexor tendon repair. The study found amniotic membrane wrapped around the repaired tendon was superior in preventing adhesion formation. Kim and colleagues37 report a similar reduction in fibrosis and adhesion following application of a human amniotic membrane wrap to rabbit ulnar neurorrhaphy sites.
This barrier function of amniotic tissue has also been investigated in the prevention of surgical scarring and peridural fibrosis in animal models following spinal discectomy. A study in canine models showed a reduction of scarring following the application of cross-linked amniotic membrane compared to freeze dried amniotic membrane.38 Similar reductions in scarring in rat models with the application of freeze-dried amniotic membrane compared to negative controls have been reported.39
Human Studies
A randomized trial investigated the outcomes of prenatal vs postnatal repair of myelomeningocele in humans, finding a reduced need for implanted shunts and improved functional outcomes at 30 months of life in the prenatal intervention group compared to the postnatal group.40 This study was concluded early due to the efficacy of prenatal surgery and the benefit of nervous system repair in utero in the presence of amniotic growth factors.
Vines and colleagues41 performed a 6-patient feasibility study using amnion injections to treat symptomatic knee osteoarthritis. Each patient received a single intra-articular cryopreserved amniotic suspension allograft (ASA) injection and was followed for 1 year. No adverse outcomes were reported, with the only abnormal finding being a small increase in serum immunoglobulin G and immunoglobulin E levels. Intra-articular ASA injection was found to be safe, but a large-scale trial investigating symptomatic relief was recommended.41
Most of the human studies using amnion pertain to foot and ankle surgery. Its use as a treatment for diabetic foot ulcers and recalcitrant plantar fasciitis was one of the early-recognized successes.42-45 Zelen and colleagues46 investigated the applications of injectable micronized dehydrated human amniotic/chorionic membrane as an alternative to surgical intervention in the treatment of refractory plantar fasciitis. This prospective, randomized trial with 45 patients showed significant improvement in plantar fasciitis symptoms at 8 weeks compared to controls (saline injections). A similar study compared the use of cryopreserved human amniotic membrane (c-hAM) injections to corticosteroid injections in plantar fasciitis patients.47 The results indicated that c-hAM is safe and comparable to corticosteroids, with the authors noting that pain improvement was greatest in patients receiving 2 injections of c-hAM at 18 weeks.
Tendon wrapping, in which the amniotic membrane is laid over a tendon repair, has been reported with success. Amniotic membrane is superior to collagen for tendon wrapping as it actively contributes to healing while minimizing adhesions, which collagen alone cannot do.48 The membrane serves as a protective sheath around repaired tendons with anti-inflammatory, anti-adhesive, immunomodulatory, and antimicrobial benefits. A 124-patient study demonstrated the safety of using amnion in this manner, and the authors reported a decreased rate of complication compared to previously published data.49 Another study of 14 patients undergoing foot and ankle surgery with tendon wrapping reported clinical improvement with reduced pain and greater functional outcomes postoperatively compared to preoperative measurements.50
Conclusion
Amniotic membrane-derived tissues are safe and non-tumorigenic, producing an abundance of growth factors that have shown promise as tissue scaffolds and as aids in the regeneration of human bone and soft tissues. Amnion applications in orthopedic surgery may be numerous, but development is ongoing. Given the vast array of in vitro and in vivo animal data supporting the benefits of amnion in tissue regeneration, orthopedic surgeons and researchers should place emphasis on conducting clinical studies to validate the safety and efficacy of amniotic cells in the treatment of orthopedic conditions.
Am J Orthop. 2016;45(7):E421-E425. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The amniotic membrane is a multilayer tissue forming the innermost layer of the amniotic sac that surrounds the developing fetus. It is comprised of 5 layers, from the inside out: a single layer of epithelial cells, a thick basement membrane, a compact layer, a fibroblast layer, and a spongy layer that abuts the surrounding chorion (Figure 1).1
Amniotic epithelial cells are derived from the pluripotent epiblast at approximately day 8 of gestation. This is well before gastrulation occurs at days 15 to 17, considered the “tipping point” when pluripotent cells differentiate into ectoderm, mesoderm, and endoderm.3 These cells express Oct-4 and Nanog, 2 molecular markers that are indicative of pluripotency.3 Two cell types have been identified in amniotic tissues that possess stem cell-like characteristics: human amniotic epithelial cells and human amniotic mesenchymal stromal cells.4 Both of these cell types have demonstrated the ability to differentiate into various cell lineages, including endothelial cells, adipocytes, myogenic cells, neurogenic cells, chondrocytes, tenocytes, and osteogenic cells.5-7 These previously reported findings indicate that amniotic cells and tissue have the capability to generate mesenchymal tissues.
FDA Classification and Available Forms
The US Food and Drug Administration (FDA) classifies amnion as an allograft tissue under Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) 361. To meet criteria, the tissue needs to be minimally manipulated. It is to be for homologous use and cannot be combined with other cells or tissues. There can be no systemic effect or dependence on the metabolic activity of living cells to achieve its primary function. The tissue has to have a localized effect in vivo. Therefore, amnion allograft tissue can be commercialized, provided it is not marketed as a stem cell product or to contain viable cells.
Amniotic tissue is commercially available in several forms.
Safety
Amniotic tissue has been used for over 100 years in burn, ophthalmology, and chronic wound patients with favorable outcomes and no adverse effects reported in the literature. Unlike embryonic stem cells, which may be tumorigenic,8 amniotic cells do not possess any known tumorigenicity.9 In one study, 50 immunodeficient mice were injected with 1 to 2 million amniotic epithelial cells and observed for a maximum of 516 days with no tumorigenicity observed in any of the animals.10 In another study, amniotic epithelial cells were implanted into the forearms of healthy volunteers and no immunologic response was observed in any of the recipients.11 Furthermore, viable amniotic cells were recovered via biopsy 7 weeks following transplantation, demonstrating viability of the transplanted cells.11 The lack of tumorigenicity and immunologic response in hosts is due in part to the fact that amniotic cells do not express human leukocyte antigen class II antigens and only express class I antigens in small amounts.3
Advantages of Amnion Tissue
Amniotic tissue is readily available, as it is often discarded after childbirth. The use of this tissue poses no added risk to the fetus or mother, eliminating the ethical concerns associated with obtaining embryonic stem cells. Amniotic tissue is comprised of an extracellular matrix, which acts as a natural scaffold for cellular attachment and structural support for cells as well as collagen types I, III, IV, V, and VI, hyaluronic acid, and a host of growth factors.12 In addition, it possesses antimicrobial properties, including beta-defensins.13
Amniotic tissue has been shown to exert an anti-inflammatory effect by inhibiting the inflammatory cascade. Specifically, it has been shown to inhibit cytokines such as tumor necrosis factor-alpha in the presence of dendritic cells,14 as well as inhibiting transforming growth factor-beta, interleukin-8, and fibroblast proliferation.15 These findings indicate that amniotic tissue has the ability to dampen the “cytokine storm” that occurs after an injury in an adult, which would lead to beneficial impacts on healing and scar formation in patients.16
Basic Science and Animal Studies
Several studies have demonstrated promising outcomes for orthopedic applications in vitro. A comparison of osteogenic potential found that amniotic fluid-derived cells were able to produce approximately 5 times more mineralized matrix than bone marrow-derived mesenchymal stem cells.17 More recently, Si and colleagues18 compared the osteogenic potential of human amniotic epithelial cells, amniotic cells, and human bone marrow-derived mesenchymal stem cells. They found that all 3 cell lines were osteogenic, though the amniotic epithelial cells had better immunomodulatory properties and marginally less osteogenic potential than the other 2 cell types. Furthermore, several in vivo animal studies have demonstrated the ability of human amniotic cells to stimulate bone growth in rats,19,20 rabbits,21 and sheep.22
Amniotic tissue also possesses potential for chondrogenesis. Cryopreserved human amniotic membrane cells used for in vitro human osteoarthritis tissue scaffolds did not differentiate in culture, and they integrated and repaired damaged articular cartilage.23 Various in vitro24,25 and animal in vivo26,27 studies have reported similar supportive findings. Kunisaki and colleagues28 used sheep amniotic fluid mesenchymal stem cells to reconstruct lamb tracheal cartilage in utero, concluding that cells obtained from the amniotic fluid possess chondrogenic capabilities. Further in utero lamb studies of cartilage artificial defects, given 7 days to settle before adding a hypocellular matrix as a scaffold, showed chondrocyte density and cell architecture was restored at the defect site after 28 days without the formation of an inflammatory response or scar tissue.29
Amniotic tissue has had similar success in tendon repair studies in vivo.9,30,31 Barboni and colleagues32 implanted amniotic epithelial cells (AECs) into artificially created sheep Achilles tendon defects in situ, inducing superior structural and mechanical recovery in the defects at a faster rate compared to controls not receiving AECs. Healing via AECs started at the healthy tissue around the borders of the defect and progressed centrally, suggesting recruitment of native progenitor cells to the lesion.32 Kueckelhaus and colleagues33 investigated the role of amnion-derived cellular cytokine solution in the healing of transections of rat Achilles tendons, reporting improved mechanical properties of healing tendons at early time points compared to controls. Beredjiklian and colleagues34 compared the healing of transected extensor tendons of pregnant ewes and of their fetus in utero, reporting a reparative form of healing with scar formation in adult subjects and regenerative form of healing without scar formation or inflammation in fetal subjects.
Amniotic tissue has properties that prevent adhesion formation around tendons following injury and reconstruction.35 Ozgenel36 investigated the effects of hyaluronic acid and amniotic membrane alone and in combination on the presence of adhesions and the rate of healing following chicken flexor tendon repair. The study found amniotic membrane wrapped around the repaired tendon was superior in preventing adhesion formation. Kim and colleagues37 report a similar reduction in fibrosis and adhesion following application of a human amniotic membrane wrap to rabbit ulnar neurorrhaphy sites.
This barrier function of amniotic tissue has also been investigated in the prevention of surgical scarring and peridural fibrosis in animal models following spinal discectomy. A study in canine models showed a reduction of scarring following the application of cross-linked amniotic membrane compared to freeze dried amniotic membrane.38 Similar reductions in scarring in rat models with the application of freeze-dried amniotic membrane compared to negative controls have been reported.39
Human Studies
A randomized trial investigated the outcomes of prenatal vs postnatal repair of myelomeningocele in humans, finding a reduced need for implanted shunts and improved functional outcomes at 30 months of life in the prenatal intervention group compared to the postnatal group.40 This study was concluded early due to the efficacy of prenatal surgery and the benefit of nervous system repair in utero in the presence of amniotic growth factors.
Vines and colleagues41 performed a 6-patient feasibility study using amnion injections to treat symptomatic knee osteoarthritis. Each patient received a single intra-articular cryopreserved amniotic suspension allograft (ASA) injection and was followed for 1 year. No adverse outcomes were reported, with the only abnormal finding being a small increase in serum immunoglobulin G and immunoglobulin E levels. Intra-articular ASA injection was found to be safe, but a large-scale trial investigating symptomatic relief was recommended.41
Most of the human studies using amnion pertain to foot and ankle surgery. Its use as a treatment for diabetic foot ulcers and recalcitrant plantar fasciitis was one of the early-recognized successes.42-45 Zelen and colleagues46 investigated the applications of injectable micronized dehydrated human amniotic/chorionic membrane as an alternative to surgical intervention in the treatment of refractory plantar fasciitis. This prospective, randomized trial with 45 patients showed significant improvement in plantar fasciitis symptoms at 8 weeks compared to controls (saline injections). A similar study compared the use of cryopreserved human amniotic membrane (c-hAM) injections to corticosteroid injections in plantar fasciitis patients.47 The results indicated that c-hAM is safe and comparable to corticosteroids, with the authors noting that pain improvement was greatest in patients receiving 2 injections of c-hAM at 18 weeks.
Tendon wrapping, in which the amniotic membrane is laid over a tendon repair, has been reported with success. Amniotic membrane is superior to collagen for tendon wrapping as it actively contributes to healing while minimizing adhesions, which collagen alone cannot do.48 The membrane serves as a protective sheath around repaired tendons with anti-inflammatory, anti-adhesive, immunomodulatory, and antimicrobial benefits. A 124-patient study demonstrated the safety of using amnion in this manner, and the authors reported a decreased rate of complication compared to previously published data.49 Another study of 14 patients undergoing foot and ankle surgery with tendon wrapping reported clinical improvement with reduced pain and greater functional outcomes postoperatively compared to preoperative measurements.50
Conclusion
Amniotic membrane-derived tissues are safe and non-tumorigenic, producing an abundance of growth factors that have shown promise as tissue scaffolds and as aids in the regeneration of human bone and soft tissues. Amnion applications in orthopedic surgery may be numerous, but development is ongoing. Given the vast array of in vitro and in vivo animal data supporting the benefits of amnion in tissue regeneration, orthopedic surgeons and researchers should place emphasis on conducting clinical studies to validate the safety and efficacy of amniotic cells in the treatment of orthopedic conditions.
Am J Orthop. 2016;45(7):E421-E425. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Benirschke K, Kaufman P. Anatomy and pathology of the placental membranes. In: Pathology of the Human Placent., 4th ed. New York, NY: Springer-Verlag; 2000:281-334.
2. Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447-458.
3. Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2(2):133-142.
4. Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26(2):300-311.
5. Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577-588.
6. Alviano F, Fossati V, Marchionni C, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11.
7. Barboni B, Curini V, Russo V, et al. Indirect co-culture with tendons or tenocytes can program amniotic epithelial cells towards stepwise tenogenic differentiation. PLoS One. 2012;7(2):e30974.
8. Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature Reviews Cancer. 2011;11(4):268-277.
9. Lange-Consiglio A, Rossi D, Tassan S, Perego R, Cremonesi F, Parolini O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013;22(22):3015-3024.
10. Miki T. Amnion-derived stem cells: in quest of clinical applications. Stem Cell Res Ther. 2011;2(3):25.
11. Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2(8254):1003-1035.
12. Gupta A, Kedige SD, Jain K. Amnion and chorion membranes: potential stem cell reservoir with wide applications in periodontics. Int J Biomater. 2015;2015:274082.
13. Buhimschi IA, Jabr M, Buhimschi CS, Petkova AP, Weiner CP, Saed GM. The novel antimicrobial peptide beta3-defensin is produced by the amnion: a possible role of the fetal membranes in innate immunity of the amniotic cavity. Am J Obstet Gynecol. 2004;191(5):1678-1687.
14. Magatti M, De Munari S, Vertua E, et al. Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant. 2009;18(8):899-914.
15. Solomon A, Wajngarten M, Alviano F, et al. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy. 2005;35(7):941-948.
16. Silini A, Parolini O, Huppertz B, Lang I. Soluble factors of amnion-derived cells in treatment of inflammatory and fibrotic pathologies. Curr Stem Cell Res Ther. 2013;8(1):6-14.
17. Peister A, Woodruff MA, Prince JJ, Gray DP, Hutmacher DW, Guldberg RE. Cell sourcing for bone tissue engineering: amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res. 2011;7(1):17-27.
18. Si J, Dai J, Zhang J, et al. Comparative investigation of human amniotic epithelial cells and mesenchymal stem cells for application in bone tissue engineering. Stem Cells Int. 2015;2015:565732.
19. Starecki M, Schwartz JA, Grande DA. Evaluation of amniotic-derived membrane biomaterial as an adjunct for repair of critical sized bone defects. Advances in Orthopedic Surgery. 2014;2014:572586.
20. Kerimoglu S, Livaoglu M, Sönmez B, et al. Effects of human amniotic fluid on fracture healing in rat tibia. J Surg Res. 2009;152(2):281-287.
21. Karaçal N, Kocucu P, Cobanglu U, Kutlu N. Effect of human amniotic fluid on bone healing. J Surg Res. 2005;129(2):283-287.
22. Barboni B, Mangano C, Valbonetti L, et al. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS One. 2013;8(5):e63256.
23. Díaz-Prado S, Rendal-Vázquez ME, Muiños-Lopez E, et al. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010;11(2):183-195.
24. Krishnamurithy G, Shilpa PN, Ahmad RE, Sulaiman S, Ng CL, Kamarul T. Human amniotic membrane as a chondrocyte carrier vehicle/substrate: in vitro study. J Biomed Mater Res A. 2011;99(3):500-506.
25. Tan SL, Sulaiman S, Pingguan-Murphy B, Selvaratnam L, Tai CC, Kamarul T. Human amnion as a novel cell delivery vehicle for chondrogenic mesenchymal stem cells. Cell Tissue Bank. 2011;12(1):59-70.
26. Jin CZ, Park SR, Choi BH, Lee KY, Kang CK, Min BH. Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng. 2007;13(4):693-702.
27. Garcia D, Longo UG, Vaquero J, et al. Amniotic membrane transplant for articular cartilage repair: an experimental study in sheep. Curr Stem Cell Res Ther. 2014;10(1):77-83.
28. Kunisaki SM, Freedman DA, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg. 2006;41(4):675-682.
29. Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80(1):4-10.
30. Philip J, Hackl F, Canseco JA, et al. Amnion-derived multipotent progenitor cells improve achilles tendon repair in rats. Eplasty. 2013;13:e31.
31. Lange-Consiglio A, Tassan S, Corradetti B, et al. Investigating the efficacy of amnion-derived compared with bone marrow–derived mesenchymal stromal cells in equine tendon and ligament injuries. Cytotherapy. 2013;15(8):1011-1020.
32. Barboni B, Russo V, Curini V, et al. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012;21(11):2377-2395.
33. Kueckelhaus M, Philip J, Kamel RA, et al. Sustained release of amnion-derived cellular cytokine solution facilitates achilles tendon healing in rats. Eplasty. 2014;14:e29.
34. Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowski LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31(10):1143-1152.
35. Demirkan F, Colakoglu N, Herek O, Erkula G. The use of amniotic membrane in flexor tendon repair: an experimental model. Arch Orthop Trauma Surg. 2002;122(7):396-369.
36. Ozgenel GY. The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J Bone Joint Surg Br. 2004;86(2):301-307.
37. Kim SS, Sohn SK, Lee KY, Lee MJ, Roh MS, Kim CH. Use of human amniotic membrane wrap in reducing perineural adhesions in a rabbit model of ulnar nerve neurorrhaphy. J Hand Surg Eur Vol. 2010;35(3):214-219.
38. Tao H, Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18(8):1202-1212.
39. Choi HJ, Kim KB, Kwon YM. Effect of amniotic membrane to reduce postlaminectomy epidural adhesion on a rat model. J Korean Neurosurg Soc. 2011;49(6):323-328.
40. Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993-1004.
41. Vines JB, Aliprantis AO, Gomoll AH, Farr J. Cryopreserved amniotic suspension for the treatment of knee osteoarthritis. J Knee Surg. 2016;29(6):443-450.
42. Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22(7):347-348,350-351.
43. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507.
44. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J. 2014;11(2):122-128.
45. Zelen CM, Snyder RJ, Serena TE, Li WW. The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: a review. Clin Podiatr Med Surg. 2015;32(1):135-146.
46. Zelen CM, Poka A, Andrews J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis: a feasibility study. Foot Ankle Int. 2013;34(10):1332-1339.
47. Hanselman AE, Tidwell JE, Santrock RD. Cryopreserved human amniotic membrane injection for plantar fasciitis: a randomized, controlled, double-blind pilot study. Foot Ankle Int. 2015;36(2):151-158.
48. Jay RM. Initial clinical experience with the use of human amniotic membrane tissue during repair of posterior tibial and achilles tendons. 2009. http://encompassbiologics.com/wp-content/uploads/2015/07/DrJayClinicalExperience.pdf. Accessed September 29, 2016.
49. DeMill SL, Granata JD, McAlister JE, Berlet GC, Hyer CF. Safety analysis of cryopreserved amniotic membrane/umbilical cord tissue in foot and ankle surgery: a consecutive case series of 124 patients. Surg Technol Int. 2014;25:257-261.
50. Warner M, Lasyone L. An open-label, single-center, retrospective study of cryopreserved amniotic membrane and umbilical cord tissue as an adjunct for foot and ankle surgery. Surg Technol Int. 2014;25:251-255.
1. Benirschke K, Kaufman P. Anatomy and pathology of the placental membranes. In: Pathology of the Human Placent., 4th ed. New York, NY: Springer-Verlag; 2000:281-334.
2. Mamede AC, Carvalho MJ, Abrantes AM, Laranjo M, Maia CJ, Botelho MF. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012;349(2):447-458.
3. Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2(2):133-142.
4. Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international workshop on placenta derived stem cells. Stem Cells. 2008;26(2):300-311.
5. Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M, Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77(3):577-588.
6. Alviano F, Fossati V, Marchionni C, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11.
7. Barboni B, Curini V, Russo V, et al. Indirect co-culture with tendons or tenocytes can program amniotic epithelial cells towards stepwise tenogenic differentiation. PLoS One. 2012;7(2):e30974.
8. Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nature Reviews Cancer. 2011;11(4):268-277.
9. Lange-Consiglio A, Rossi D, Tassan S, Perego R, Cremonesi F, Parolini O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013;22(22):3015-3024.
10. Miki T. Amnion-derived stem cells: in quest of clinical applications. Stem Cell Res Ther. 2011;2(3):25.
11. Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2(8254):1003-1035.
12. Gupta A, Kedige SD, Jain K. Amnion and chorion membranes: potential stem cell reservoir with wide applications in periodontics. Int J Biomater. 2015;2015:274082.
13. Buhimschi IA, Jabr M, Buhimschi CS, Petkova AP, Weiner CP, Saed GM. The novel antimicrobial peptide beta3-defensin is produced by the amnion: a possible role of the fetal membranes in innate immunity of the amniotic cavity. Am J Obstet Gynecol. 2004;191(5):1678-1687.
14. Magatti M, De Munari S, Vertua E, et al. Amniotic mesenchymal tissue cells inhibit dendritic cell differentiation of peripheral blood and amnion resident monocytes. Cell Transplant. 2009;18(8):899-914.
15. Solomon A, Wajngarten M, Alviano F, et al. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin Exp Allergy. 2005;35(7):941-948.
16. Silini A, Parolini O, Huppertz B, Lang I. Soluble factors of amnion-derived cells in treatment of inflammatory and fibrotic pathologies. Curr Stem Cell Res Ther. 2013;8(1):6-14.
17. Peister A, Woodruff MA, Prince JJ, Gray DP, Hutmacher DW, Guldberg RE. Cell sourcing for bone tissue engineering: amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res. 2011;7(1):17-27.
18. Si J, Dai J, Zhang J, et al. Comparative investigation of human amniotic epithelial cells and mesenchymal stem cells for application in bone tissue engineering. Stem Cells Int. 2015;2015:565732.
19. Starecki M, Schwartz JA, Grande DA. Evaluation of amniotic-derived membrane biomaterial as an adjunct for repair of critical sized bone defects. Advances in Orthopedic Surgery. 2014;2014:572586.
20. Kerimoglu S, Livaoglu M, Sönmez B, et al. Effects of human amniotic fluid on fracture healing in rat tibia. J Surg Res. 2009;152(2):281-287.
21. Karaçal N, Kocucu P, Cobanglu U, Kutlu N. Effect of human amniotic fluid on bone healing. J Surg Res. 2005;129(2):283-287.
22. Barboni B, Mangano C, Valbonetti L, et al. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS One. 2013;8(5):e63256.
23. Díaz-Prado S, Rendal-Vázquez ME, Muiños-Lopez E, et al. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010;11(2):183-195.
24. Krishnamurithy G, Shilpa PN, Ahmad RE, Sulaiman S, Ng CL, Kamarul T. Human amniotic membrane as a chondrocyte carrier vehicle/substrate: in vitro study. J Biomed Mater Res A. 2011;99(3):500-506.
25. Tan SL, Sulaiman S, Pingguan-Murphy B, Selvaratnam L, Tai CC, Kamarul T. Human amnion as a novel cell delivery vehicle for chondrogenic mesenchymal stem cells. Cell Tissue Bank. 2011;12(1):59-70.
26. Jin CZ, Park SR, Choi BH, Lee KY, Kang CK, Min BH. Human amniotic membrane as a delivery matrix for articular cartilage repair. Tissue Eng. 2007;13(4):693-702.
27. Garcia D, Longo UG, Vaquero J, et al. Amniotic membrane transplant for articular cartilage repair: an experimental study in sheep. Curr Stem Cell Res Ther. 2014;10(1):77-83.
28. Kunisaki SM, Freedman DA, Fauza DO. Fetal tracheal reconstruction with cartilaginous grafts engineered from mesenchymal amniocytes. J Pediatr Surg. 2006;41(4):675-682.
29. Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80(1):4-10.
30. Philip J, Hackl F, Canseco JA, et al. Amnion-derived multipotent progenitor cells improve achilles tendon repair in rats. Eplasty. 2013;13:e31.
31. Lange-Consiglio A, Tassan S, Corradetti B, et al. Investigating the efficacy of amnion-derived compared with bone marrow–derived mesenchymal stromal cells in equine tendon and ligament injuries. Cytotherapy. 2013;15(8):1011-1020.
32. Barboni B, Russo V, Curini V, et al. Achilles tendon regeneration can be improved by amniotic epithelial cell allotransplantation. Cell Transplant. 2012;21(11):2377-2395.
33. Kueckelhaus M, Philip J, Kamel RA, et al. Sustained release of amnion-derived cellular cytokine solution facilitates achilles tendon healing in rats. Eplasty. 2014;14:e29.
34. Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowski LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31(10):1143-1152.
35. Demirkan F, Colakoglu N, Herek O, Erkula G. The use of amniotic membrane in flexor tendon repair: an experimental model. Arch Orthop Trauma Surg. 2002;122(7):396-369.
36. Ozgenel GY. The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J Bone Joint Surg Br. 2004;86(2):301-307.
37. Kim SS, Sohn SK, Lee KY, Lee MJ, Roh MS, Kim CH. Use of human amniotic membrane wrap in reducing perineural adhesions in a rabbit model of ulnar nerve neurorrhaphy. J Hand Surg Eur Vol. 2010;35(3):214-219.
38. Tao H, Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18(8):1202-1212.
39. Choi HJ, Kim KB, Kwon YM. Effect of amniotic membrane to reduce postlaminectomy epidural adhesion on a rat model. J Korean Neurosurg Soc. 2011;49(6):323-328.
40. Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364(11):993-1004.
41. Vines JB, Aliprantis AO, Gomoll AH, Farr J. Cryopreserved amniotic suspension for the treatment of knee osteoarthritis. J Knee Surg. 2016;29(6):443-450.
42. Zelen CM. An evaluation of dehydrated human amniotic membrane allografts in patients with DFUs. J Wound Care. 2013;22(7):347-348,350-351.
43. Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502-507.
44. Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers. Int Wound J. 2014;11(2):122-128.
45. Zelen CM, Snyder RJ, Serena TE, Li WW. The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: a review. Clin Podiatr Med Surg. 2015;32(1):135-146.
46. Zelen CM, Poka A, Andrews J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis: a feasibility study. Foot Ankle Int. 2013;34(10):1332-1339.
47. Hanselman AE, Tidwell JE, Santrock RD. Cryopreserved human amniotic membrane injection for plantar fasciitis: a randomized, controlled, double-blind pilot study. Foot Ankle Int. 2015;36(2):151-158.
48. Jay RM. Initial clinical experience with the use of human amniotic membrane tissue during repair of posterior tibial and achilles tendons. 2009. http://encompassbiologics.com/wp-content/uploads/2015/07/DrJayClinicalExperience.pdf. Accessed September 29, 2016.
49. DeMill SL, Granata JD, McAlister JE, Berlet GC, Hyer CF. Safety analysis of cryopreserved amniotic membrane/umbilical cord tissue in foot and ankle surgery: a consecutive case series of 124 patients. Surg Technol Int. 2014;25:257-261.
50. Warner M, Lasyone L. An open-label, single-center, retrospective study of cryopreserved amniotic membrane and umbilical cord tissue as an adjunct for foot and ankle surgery. Surg Technol Int. 2014;25:251-255.
Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality
Injury of the anterior cruciate ligament (ACL) is very common with over 200,000 annual injuries in the United Status.1,2 There is a general consensus that these injuries should not be treated conservatively in patients that are younger, or who wish to remain active.3,4 Reconstructive surgery is currently the preferred treatment in these patients, and anatomic single-bundle reconstruction with autografts is considered the gold standard.5,6
Reconstruction of the ACL is, however, not a perfect treatment. Following single-bundle autograft reconstruction, revision rates of 3% to 8%,6-9 contralateral injury rates of 3% to 8%,10,11 and infection rates of 0.5% to 3%7,12,13 have been reported. Furthermore, due to the invasive nature of graft harvesting and the surgical procedure, 10% to 25% of the patients are not satisfied following ACL reconstruction.14,15 This can often be explained by common complaints, such as anterior knee pain (13%-43%), kneeling pain (12%-54%), quadriceps muscle atrophy (20%-30%),16,17 and loss of range of motion (ROM) (12%-23%).7,9,18,19 Furthermore, as a result of the invasive nature of reconstructive surgery, revisions can be difficult due to complications, such as tunnel widening, tunnel malpositioning, and preexisting hardware.20-22 This can lead to inferior outcomes and higher rates (13%) of revision surgery compared to primary reconstruction.23-26 Finally, reconstructive surgery does not restore native kinematics of the ACL,27-29 which may partially explain why reconstructive surgery has not been shown to prevent osteoarthritis.28-31
Over the past decades, there has been an increasing interest in the preservation of the ACL in an attempt to ameliorate these issues.32-37 Ligament preservation focuses on preserving the native tissues and biology, while minimizing the surgical morbidity to the patients.
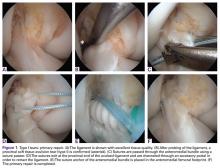
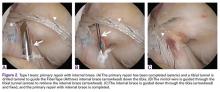
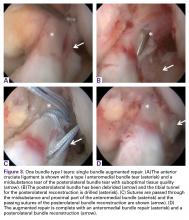
Some authors have recently reported on arthroscopic primary repair of proximal ACL tears in which the ligament is reattached onto the femoral wall using modern-day suture anchor technology.32,38 Others have augmented this repair technique with an internal brace39,40 or with a synthetic device.33,41 When performing primary repair, it is believed that proprioception is maintained,42-44 while experimental studies have suggested that primary repair also restores the native kinematics,45 and may prevent osteoarthritis.46 Furthermore, primary repair is a conservative approach in that no grafts need to be harvested, no tunnels need to be drilled, and revision surgery, if necessary, is more analogous to primary reconstructions.32In patients with partial tears, some surgeons have advocated preserving the anteromedial (AM) or posterolateral (PL) bundle and performing selective single-bundle augmentation.34,35 In addition, several authors have used remnant tensioning36,47 or remnant preservation37,48 in combination with reconstructive surgery in order to benefit from the biological characteristics of the remnant. These techniques lead to better proprioceptive function,44,49,50 vascularization and ligamentization of the graft,50-52 provide an optical guide for anatomic tunnel placement,53 and decrease the incidence of tunnel widening.54,55The feasibility and applicability of these surgical techniques mainly depends on the tear type and tissue quality of the torn ligament. In this article we (I) discuss the history of ACL preservation, (II) discuss how modern advances alter the risk-benefit ratio for ACL preservation, and (III) propose a treatment algorithm for ACL injuries that is based on tear location and tissue quality.
History of ACL Preservation
The history of the surgical treatment of ACL injuries started in 1895 when Robson56 treated a 41-year-old male who tore both cruciate ligaments from the femoral wall. Performing primary repair with catgut ligatures, both cruciate ligaments were preserved and the patients had resolution of pain symptoms and full function at 6-year follow-up. Over the following decades, Palmer57,58 and O’Donoghue59,60 further popularized open primary repair for the treatment of ACL injuries, and this technique was the most commonly performed treatment in the 1970s and early 1980s.61-65 The initial short-term results of primary repair were excellent,61,62 but Feagin and Curl66 were the first to note that the results deteriorated at mid-term follow-up. Despite improvements in the surgical technique of repairing the ACL, such as the usage of nonabsorbable sutures and directly tying the sutures over bone,63,67 the results remained disappointing at longer-term follow-up.68-70
In response to these disappointing results, surgeons sought to improve the surgical treatment by either augmenting the primary repair with a semitendinosus, a patella tendon graft or an augmentation device,71-74 or by performing primary reconstruction.75-77 At the end of the 1980s and early 1990s, several randomized and prospective clinical trials were performed in order to compare the outcomes of these techniques.74,78-82 Many studies showed that results of augmented repair were more reliable when compared to primary repair, which led to the abandonment of primary repair in favor of augmented repair, and eventually primary reconstruction.65
The Important Role of Tear Location in Ligament Preservation
When taking a closer look at the outcomes of primary repair and augmented repair, it seems that the results of these preservation techniques were not as disappointing as was suggested. This can be explained, in large part, by the fact that the important roles of tear location and tissue quality were not widely recognized.
Sherman and colleagues70 reported in 1991 their mid-term results of open primary repair. Similar to others, they noted a deterioration of their results at mid-term follow-up. However, they uniquely performed an extensive subgroup analysis in order to find an explanation for this. In their study, considered a landmark paper on primary repair,65,70 they concluded that, “poor tissue quality is typical for midsubstance tears and that a repair of these injuries will predictably fail while type I tears (proximal), with better tissue quality, show a definite trend towards better results.”70 With these findings, they confirmed the findings of others that had recognized a trend of better outcomes with proximal tears.64,67,83-85
A majority of the historical studies that were published before 1991 had not considered the role of tear location and tissue quality on outcomes of open primary repair. This was also true for the aforementioned randomized studies that compared primary repair with augmented repair and primary reconstruction. Because these studies randomized patients and did not take tear location into account, it can be expected that patients with midsubstance tears were included in the cohorts of primary repair and the outcomes of these studies were therefore confounded.74,78-82 If these studies would have been aware of the role that tear location plays on primary repair outcomes, different outcomes may have been found and different conclusions on the optimal treatment for different tear types may have been drawn.86
Open Primary ACL Repair Outcomes Stratified by Tear Location
When reviewing the literature of open primary repair outcomes stratified by tear location, it is noted that multiple studies reported excellent outcomes following primary repair of proximal ACL tears.73,83,84,87-90 Weaver and colleagues64 were among the first to stratify their results by tear location, and they found that more patients with proximal tears (52 of 66; 79%) were satisfied after the procedure when compared to patients with midsubstance tears (3 of 13; 23%) at 3.5-year follow-up. They concluded that, “selection can be made with some predictability of the type of injury to the ligament as to which patients will do better.”64 Kühne and colleagues89 reported the outcomes of 75 patients with proximal tears treated with open primary repair and noted no failures, negative pivot shift in 88% of patients, stable or nearly stable Lachman test in 87% of patients, and 89% return to sports rate at 4-year follow-up. Raunest and colleagues91 reported a negative pivot shift and negative anterior drawer test in 84%, return to sports in 71%, and satisfaction in 75% of 51 patients that underwent open primary repair of proximal tears at 3.5-year follow-up.
Interestingly, and in contrast to the findings of Feagin and Curl,66 no deterioration of the outcomes at mid-term follow-up was noted in patients with proximal tears. Genelin and colleagues88 reported their results of 42 patients with proximal tears treated with open primary repair at 5- to 7-year follow-up. They found a negative pivot shift in 81%, stable or nearly stable Lachman test in 81%, and patient satisfaction in 86% of patients. Similarly, Bräm and colleagues87 found good results at mid-term follow-up with a good-excellent Lysholm score in 79%, return to a similar level of sports in 76%, stable or nearly stable Lachman test in 91%, and anterior drawer test in 94% of patients, along with an 88% satisfaction rate and 7% failure rate in patients who underwent open primary repair of proximal tears.
On the contrary, when the outcomes of studies that performed open primary repair in mainly, or only, patients with midsubstance tears are reviewed, significantly inferior results are found. Frank and colleagues92 reported outcomes in 42 patients with midsubstance tears at 4-year follow-up. They reported that 56% had a stable or nearly stable anterior drawer test, 78% had a positive pivot shift, and that only 61% were satisfied with the procedure. Odensten and colleagues78 reported outcomes of open primary repair in a subgroup of 22 patients with midsubstance tears at 1.5-year follow-up, and noted a 14% failure rate.
When reviewing the mid-term results in patients with midsubstance tears, it seems that there was more deterioration in outcomes.69,70 Firstly, the aforementioned study by Sherman and colleagues70 showed poor results in the patients with (type IV) midsubstance tears at mid-term follow-up. Furthermore, Kaplan and colleagues69 reported the mid-term outcomes of 70 patients, of which 56 patients had midsubstance tears. After having reported good outcomes at short-term follow-up,63,67 they noted that 42% of patients had >3 mm anteroposterior stability when compared to the contralateral leg, only a 62% return to sport rate, and a 17% failure rate. They concluded that, “Although … primary repair of the anterior cruciate may work in some patients, it is an unpredictable operative procedure.”
These studies showed that the outcomes of open primary repair were significantly better in patients with proximal ACL tears and sufficient tissue quality when compared to midsubstance tears. This suggests that open primary ACL repair may have been prematurely abandoned as a treatment option for patients with proximal tears.
Augmented ACL Repair
There were several reasons why augmented repair became the preferred treatment in the early and mid 1990s. First of all, the results of augmented repair were more consistent compared to primary repair in the aforementioned randomized and prospective studies,74,78-82 which is not surprising given the fact that the role of tear location was not widely recognized at the time. Secondly, in the 1970s and early 1980s, patients were treated postoperatively in a cast for 6 weeks, which led to problems, such as loss of ROM, pain, and decreased function.93,94 At the end of the1980s and 1990s, the focus shifted from prolonged joint immobilization towards early postoperative ROM.95-97 Since many authors believed that primary repair of the ACL was not strong enough to tolerate early mobilization, an augmentation was added to the technique in order to fortify the repair and enable early ROM.98
Interestingly, augmented repair, which is essentially a combination of primary ACL repair and ACL reconstruction, was mainly performed in the 1990s and many surgeons did recognize the role of tear location in this treatment at this point.73,98-103 In these years, the treatment algorithm consisted of augmented ACL repair in patients with proximal tears in the acute setting and ACL reconstruction in patients with midsubstance or chronic tears. Several different augmentation techniques were used to reinforce the primary repair in these years including autograft tissues (semitendinosus tendon,102-104 patellar tendon,100 or iliotibial band [ITB]105) synthetic materials (polydioxanone [PDS],101,102,106 carbon fibre,74 and polyester [Trevira]97), augmentation devices (Kennedy Ligament Augmentation Device [LAD]98-100) and extra-articular augmentations.73
When reviewing the outcomes of augmented repair of the ACL, good to excellent results can be found in studies that used this technique in patients with proximal tears.73,98-106 Kdolsky and colleagues98 were in one of the first groups that reported their results of augmented repair in only patients with proximal tears. In 1993, they reported their mid-term outcomes (5 to 8 years) in 66 patients who underwent primary repair and augmentation with the Kennedy LAD and found that 97% of patients had stable knees (<3 mm on KT-1000 examination), 98% had a negative pivot shift, and 76% returned to previous level of sports. However, often-reported problems with the augmentation devices were found in this study with rupture of the device (12%) and decreased ROM (14%).98 In 1995, Grøntvedt and Engebretsen100 compared augmentation with the Kennedy LAD to patellar tendon augmentation in a randomized study of patients with acute proximal tears. They noted that 50% of the patients in the Kennedy LAD group had a positive pivot shift compared to 23% in the patellar tendon group. Furthermore, they found KT-1000 leg differences of <3 mm in 92% of the patellar tendon group and 54% of the Kennedy LAD group. Because the authors found significant differences between both groups at 1- and 2-year follow-up, they stopped the clinical trial.
Several authors in the following years reported good results of augmented repair using autograft tissues. Natri and colleagues105 reported the outcomes of 72 patients treated with primary repair of proximal tears augmented with the ITB at 3.5-year follow-up. They found 89% negative pivot shift rate, 93% stable or nearly stable Lachman test, 99% stable or nearly stable anterior drawer test, 79% satisfaction rate, and 91% return to previous level of sports rate. Krueger-Franke and colleagues104 reported the outcomes of primary repair of proximal tears with augmentation using the semitendinosus tendon. In a retrospective study of 76 patients, they noted that 96% of patients had a negative pivot shift, 75% of patients had stable or nearly stable Lachman test, 93% were satisfied with the procedure, a mean Lysholm score of 92, a Tegner score that only decreased from 7.2 to 7.1, and KT-1000 testing with 78% <4 mm leg difference with the contralateral leg. The authors concluded that patients with femoral ruptures could be treated with augmented repair when performed in the acute setting. As this study was published in 1998, they stated that magnetic resonance imaging and arthroscopy could be helpful in identifying the tear location.
Final Abandonment of ACL Preservation
Reviewing these outcomes raises the question as to why these techniques were ultimately abandoned in the treatment algorithm of proximal ACL injuries, especially given the aforementioned advantages of ACL preservation. One of the possible answers can be found in a landmark study on ACL reconstruction and rehabilitation published by Shelbourne and colleagues107 in 1991. At that time, arthrofibrosis and knee stiffness were frequently reported problems following ACL surgery, which could partially be explained by the standard conservative rehabilitation using postoperative joint immobilization.67,70,80,88
Shelbourne and colleagues107 aimed to assess the cause of arthrofibrosis and knee stiffness, and divided the patients into groups by number of days between injury and surgery (<7, 7 to 21 days, and >21 days between injury and surgery). Furthermore, patients within these groups underwent either a conventional or accelerated rehabilitation program. The authors not only found that patients undergoing accelerated rehabilitation had less arthrofibrosis, but they also noted that less arthrofibrosis was seen when surgery was delayed. These findings, however, contrasted with the general perception that the ACL should be repaired in the first 3 weeks postinjury to ensure optimal tissue quality with an augmented approach. As a result, the treatment of ACL injuries shifted towards ACL reconstruction after these findings. Krueger-Franke and colleagues104 commented on the trend after the study of Shelbourne and colleagues:107 “Less consideration has been given to the importance of the proprioceptive receptors in the tibial remnants of the torn ACL and the value of their preservation as part of a primary reconstruction.”
In addition to the trend away from an augmented repair approach due to the novel understanding of the importance of early mobilization, some discussion should focus on the technical limitations of arthroscopy at that time. While arthroscopy had been around for several decades, fluid management and arthroscopic instrumentation was slow to develop. All of the repair and augmentation techniques previously discussed had been performed via an open arthrotomy. Arthroscopic technologies of the time were not refined enough to enable surgeons to perform such complex, intra-articular techniques that would enable suturing of the ligament remnant. In this regard, arthroscopic ACL reconstruction was a much simpler technique to accomplish, and this also likely contributed to the final abandonment of the ligament preservation approach.
Role for ACL Preservation with Modern Advances
As stated in the introduction, there has been a recent resurgence of interest in preservation of the native ligament.32-37 With the passage of time, many technologic advances have been made, which has allowed surgeons to reconsider the concept of ligament preservation.
First of all, appropriate patient selection was not applied historically, as the critical factors of tear location and tissue quality were not recognized in the era of open primary repair. In modern days, however, advances such as MRI have been developed, which can give the surgeon an idea of the status, and tear type of the ACL pre-operatively.108 This may help the orthopaedic surgeon to plan the surgery and make an assessment as to whether ACL preservation is possible. Secondly, in the historic literature the postoperative regimen consisted of casting for 5 or 6 weeks,67,70,80,88 while the focus later shifted towards early ROM.95-97Modern day ACL rehabilitation focuses on immediate ROM to avoid the complications stiffness, pain and decreased function that plagued the outcomes when immobilization was used.93,94 Thirdly, historically small tunnels were drilled with primary repair and sutures had to be tied over bone,57,67 whereas currently suture anchors are available that prevent the need for tunnel drilling and enable direct suture tensioning.32,38 Finally, and most importantly, in the historic literature patients were treated with an invasive arthrotomy technique, while modern day arthroscopic techniques readily enable the surgeon to effectively suture the remnant arthroscopically. Interestingly, in 2005, in their 20-year follow-up of primary repair surgeries, Strand and colleagues109 stated, “if the same results could be accomplished by a smaller, arthroscopic procedure, primary repair might reduce the number of patients needing later reconstructions with small ‘costs’ in the way of risk and inconvenience for the patients. We therefore believe that further research and development of methods for closed (arthroscopic) repair are justified.”
Altered Risk-Benefit Ratio
Historically, the treatments of open primary repair and open ACL reconstruction were both invasive surgeries with an arthrotomy, drilling of bone tunnels, and postoperative joint immobilization for 4 to 6 weeks. However, with the modern-day advances, the risk-benefit ratio of both treatments has changed, as Strand and colleagues109 had already suggested. Although ACL reconstruction can be performed arthroscopically, it remains an invasive procedure, in which tunnels are drilled, patellar tendons or hamstring tendons are harvested, and complications, such as knee pain and quadriceps atrophy, are common. The surgery of primary ACL repair, however, has benefited significantly from the modern developments.32,38 Primary ACL repair can now be performed arthroscopically, and by using suture anchors no tunnels need to be drilled and the remnant can be tensioned directly. An additional benefit of the use of suture anchors is that revision surgery of a failed primary repair is analogous to primary reconstruction, whereas revision surgery of a failed ACL reconstruction can be problematic due to tunnel widening, tunnel malpositioning, and preexisting hardware.20-22
Reviewing the differences between arthroscopic primary ACL repair and ACL reconstruction, it becomes clear that primary repair has benefited significantly from the modern advances and that the risk-benefit ratio for primary repair has been altered. This means that patients with proximal tears can be treated with a relatively straight forward, minimally invasive surgery, which has been shown to be effective in 85% to 90% of patients.32,38
Treatment Algorithm Based on Tear Location
Since 2008, in the practice of the senior author (GSD), the surgical treatment algorithm for ACL injuries is completely based on the tear location and tissue quality of the ligament.110,111 To describe the different tear types, we use the modified Sherman classification in which we extended his classification towards the tibial side whereas Sherman and colleagues70 only described the femoral side of the tears (Figures A-F, Table).
Type I Tears: Primary Repair
Type I tears are soft tissue avulsion type tears that can be easily treated with arthroscopic primary repair.107 The length of the distal remnant has to be at least 90% and the tissue quality has to be good to excellent in order to approximate the remnant towards the femoral wall (Table).112 The incidence of type I tears was 26% in the study of Sherman and colleagues,70 although recent studies showed a lower incidence (6% to 10%) in a larger population.32,38 Certainly, individual practices will see different percentages of type I tears based upon the mix of injury mechanisms they see most frequently. Over the last 2 years, with the recognition of the importance of tear type and tissue quality, there has been a renewed interest in arthroscopic primary ACL repair.32,38
DiFelice and colleagues32 were the first to arthroscopically perform primary repair of the ACL in proximal tears using suture anchors. They reported the outcomes of the first 11 consecutive patients that underwent primary repair in a previously described technique.113 At mean 3.5-year follow-up, they noted only 1 failure (9%) due to re-injury; mean Lysholm score of 93.2; mean modified Cincinnati score of 91.5; pre- and postoperative Tegner score of 7.3 and 6.9, respectively; SANE score of 91.8; and subjective International Knee Documentation Committee (IKDC) score of 86.4. Of the patients with an intact repair, 9 patients had an objective IKDC rating A and 1 patient had B and all patients had KT-1000 leg differences of <3 mm with the contralateral side (three patients were not available for KT-1000 testing). The authors concluded that arthroscopic primary ACL repair could achieve short-term clinical success in a selected group of patients with proximal avulsion tears and excellent tissue quality. They further noted that mid-term outcomes are necessary given that the results of open primary repair deteriorated at longer-term follow-up in the historical literature. Recently, the senior author (GSD) has added an Internal Brace (Arthrex) to the primary repair with the goal of protecting the ligament in the first weeks to further promote healing of the ligament.39,40,114
More recently, Achtnich and colleagues38 compared the treatment of arthroscopic primary ACL repair with primary ACL reconstruction in 41 patients with type I tears at 2.3-years follow-up. Twenty-one patients consented for primary repair while 20 patients declined this procedure and underwent primary reconstruction. They noted no significant differences in Lachman test, pivot shift test, objective IKDC score, and KT-1000 scores. Although not significant, the clinical failure rate in the primary repair group (15%) was higher than the reconstruction group (0%). Interestingly, despite the higher failure rate in the repair group, the authors concluded that primary ACL repair is recommended in a carefully selected group of patients with type I tears and excellent tissue quality, which can likely be explained by the differences in the risk-benefit ratio between both procedures.
Over the last decade, the research group led by Murray46,115,116 has performed experimental research on primary repair with a biological scaffold and reported many interesting findings that could be extrapolated to primary ACL repair. First of all, they compared bioenhanced primary repair with bioenhanced primary reconstruction in 64 Yucutan pigs and noted that there was significantly less macroscopic cartilage damage in the primary repair group at 1-year follow-up.46 They concluded that bioenhanced ACL repair may provide a new, less invasive treatment option that reduces cartilage damage following joint injury. This may suggest that primary repair may have a lower incidence of osteoarthritis when compared to ACL reconstruction, which is interesting as osteoarthritis is very common after ACL reconstruction. Further research in this area is certainly warranted.
In another study they compared bioenhanced primary repair in juvenile, adolescent and mature Yucutan pigs and noted that functional healing depended on the level of skeletal maturity with immature animals having a more productive healing response.116 This indicates that primary repair might be a good treatment option in skeletally immature patients, especially since reconstruction increases the risk of premature closure of the epiphysis117,118 and delaying treatment increases the risk of meniscus injury.119 Interestingly, a recent meta-analysis showed indeed that the risk of epiphysis closure was lower in primary repair when compared to ACL reconstruction and the rupture rate was also lower.118 Primary repair may be a good treatment option in children as the procedure has all the attributes that should be applicable to children: it is minimally morbid, tissue sparing, and it is a conservative approach that does not burn any surgical bridges for future reconstructive surgery if necessary.
Finally, the research group of Murray115 assessed the effect of surgical delay of primary repair following injury in Yucutan pigs and noted that better biomechanical outcomes were noted after delaying surgery for 2 weeks when compared to 6 weeks. This suggests that primary repair should preferably be performed in the acute setting, which has also been shown in historical studies since the ligament in the acute setting has optimal tissue quality and the ligament is less likely to be retracted or reabsorbed.59,60,115
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the tear locations of the AM and PL bundle are not at the same location and Zantop and colleagues120 reported in an arthroscopic study that this could be as frequent as in 30% of all complete tears. In some of these tears, one of the bundles can be avulsed of the femoral wall (type I tear) while the other bundle is not directly repairable (non-type I tear). In these cases, the senior author (GSD) will repair the type I tear bundle, whereas a hamstring augmentation is placed at the location of the other bundle. When reviewing the literature, a combination of primary repair of one bundle and reconstruction of the second bundle has not been described before. However, over the last decade several surgeons have performed augmentation of one bundle in the setting of partial tears.34,35,121-124
Buda and colleagues34 were the first to perform selective AM or PL bundle reconstruction in the setting of partial tears.34 At 5-year follow-up, they reported no reruptures and only 1 patient with an IKDC C-score, although reoperation was necessary in 4 out of 47 patients (9%). Following this publication, many others reported on selective bundle reconstruction.35,121-124 However, with partial tears, the knee is often stable and a selective augmentation technique is utilized to prevent complete rupture of the ligament. The application of this technique is essentially different from reconstruction for complete ACL tears in which the knee is unstable, there is a giving way sensation and patients have problems participating in sports.
Type II Tears: Augmented Repair
Type II tears often have good or excellent tissue quality and can be pulled up towards the femoral footprint, but are too short to be firmly attached. Sherman and colleagues70 reported that approximately 22% patients had a type II tear, which corresponds to a tear located in the proximal part of the ligament. With this technique, multiple suture passes are used to stitch the remnant and, in addition, a smaller hamstring autograft or allograft is passed through the middle of the tibial remnant. A suture button is used proximally for the graft, and the tensioning repair sutures through the remnant are also passed through the suture button. The suture button is passed through the femoral tunnel and flipped so that the graft is proximally fixed. Then, the repair sutures of the remnant are tensioned, and the ligament is pulled towards the femoral wall as a sleeve around the graft. When the ligament is approximated to the femoral wall, the sutures are tied over the suture button. The graft is then tensioned distally to complete the augmented repair.
In the recent literature, the technique of augmentation of a primary repair using autograft tissue has not been reported. However, augmented repair using an internal brace39,40 or augmentation devices33,41 have been recently performed. MacKay and colleagues39 reported good outcomes of arthroscopic primary repair of proximal tears using an internal brace. Eggli and colleagues33 reported the results of the first 10 patients treated with ACL preservation using primary repair of the ligament with the addition of a dynamic screw-spring mechanism. The authors reported good preliminary results with one failure (10%) and good objective and subjective outcomes. In a next study, they reported the outcomes of 278 patients and although they reported good clinical outcomes and a revision rate of 4%, the reoperation rate for removal of the screw-spring mechanism was high (24%).41 This is not surprising when reviewing the historical literature in which high complication rates of the augmentation devices were reported.99,100 We were unable to identify any other studies reporting surgical techniques of augmenting primary repair in the literature.
Type III Tears: Reconstruction With Remnant Tensioning
In patients with type III tears, the ligament cannot be approximated to the wall and reconstruction is necessary in order to restore knee stability. However, in these cases the ligament has sufficient length (25%-75%) and can be tensioned along or around the graft. Preservation of the ligament remnant has several (theoretical) advantages, such as better proprioceptive function,42,49,50 vascularization and ligamentization of the graft,50-52 an optical guide for anatomic tunnel placement,53 and a decreased incidence of tunnel widening.54,55 Furthermore, tensioning of the remnant is thought to lower the risk of cyclops lesions when compared to remnant preservation.125 Although the difference between augmented repair and remnant tensioning seems small, the purpose of surgery is different. With augmented repair, the ligament can be approximated close to the femoral wall and the goal of surgery is to use the healing capacity that the ACL has in the proximal part of the ligament,126 while with remnant tensioning the goal is only to benefit from some of the aforementioned advantages. Ahn and colleagues36 were the first to perform this technique and stated, “Our concept is that the remnant tissue has only an additive effect.” Furthermore, with augmented repair multiple sutures are passed through the AM and PL bundle in order to sufficiently approximate the ligament to the femoral wall, while with the remnant tensioning technique generally one or a few sutures or lasso loop are passed through the proximal part to tension the ligament, prevent sagging of the remnant, and decrease the risk of cyclops lesions.127,128
Several authors have recently performed remnant tensioning during ACL.36,47,125-127 Ahn and colleagues47 reported excellent objective and subjective outcomes following this procedure and found that with re-arthroscopy nearly all patients had fair synovialization of the graft. Others have reported similarly good outcomes of these techniques.125,129,130 However, studies comparing this treatment with normal ACL reconstruction and assessing outcomes, failure rates and proprioception are lacking.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some patients the ligament is torn distally or the tissue quality is not optimal. In these patients, the remnant can be debrided to the part of good tissue quality in order to preserve the biology and minimize the risk for cyclops lesions. A standard reconstruction needs to be performed to restore the instability, but by preserving the remnant, advantages, such as proprioception,44,49,50 graft vascularization,50-52 an optical guide for tibial tunnel placement,53 and a decreased incidence of tunnel widening54,55 can be expected.
Lee and colleagues37 presented the tibial remnant technique in which standard reconstruction was performed, and the tibial tunnel was drilled through the center of the remnant. In a later study, they compared remnant preservation with a remnant of <20% of the total ACL length with >20% of the length and found that proprioception was better with more remnant volume.48 Similarly, Muneta and colleagues131 assessed the role of remnant length and found that remnant length is positively correlated with better stability measured on KT-1000 anteroposterior stability.
Several studies compared ACL reconstruction with remnant preservation vs conventional ACL reconstruction.52,54,129 Takazawa and colleagues52 performed a retrospective study of 183 patients and found that patients in the remnant preservation group had significantly better KT-2000 stability, while they also reported a significantly lower graft rupture rate in this group (1.1% vs 7.1%) at 2-year follow-up. Hong and colleagues129 performed a randomized clinical trial of 80 patients and did not find these differences, although there was a trend towards higher Lysholm scores in the remnant preservation group. Finally, Zhang and colleagues54 performed a randomized clinical trial and found a lower incidence and amount of tibial tunnel widening in the preserving-remnant group when compared to the removing-remnant group. These studies show that there is likely a role for remnant preservation.
Type V Tears: Primary Repair
In some patients, the ligament is torn in the distal 10% of the ligament, which can occur as a distal avulsion tear or as a distal bony avulsion fracture.132 Bony avulsion fractures are most commonly seen in children whereas true distal soft tissue avulsion tears are very rare.132
Treatments of these tear types include antegrade screw fixation, pullout sutures or the use of suture anchors in case of bony avulsion fractures and pullout sutures with tying over a bony bridge or ligament button in case of soft tissue avulsions. Leeberg and colleagues132 recently performed a systematic review of all studies reporting on treatment of distal avulsion fractures.They noted that most treatments were currently performed arthroscopically and that outcomes were generally good. Another recent biomechanical study compared antegrade screw fixation with suture anchor fixation and pullout suture fixation.133 The authors noted that suture anchor fixation has slightly less displacement of the bony fragment when compared to screw fixation and pull-out sutures, and that the strength to failure was higher in the suture anchor fixation when compared to the pullout suture fixation. The outcomes of this study suggest that screw fixation and suture anchor fixation might be superior to pullout suture fixation, which might be interesting as with pullout suture fixation the ligament cannot be directly tensioned to the tibial footprint, which can lead to anteroposterior laxity.132 Clinical studies are necessary to assess the preferred treatment in these tear types but it seems that screw fixation is preferred in large bony avulsion fractures, while suture anchor fixation or pullout suture fixation can be used for soft tissue avulsion tears.
Complex Tears or Poor Tissue Quality: Reconstruction
If the tear is complex, multiple tears are present, or the tissue quality is poor, then preservation of the ligament is not possible, and in these cases a standard reconstruction should be performed.
Conclusion
When reviewing the literature of ACL preservation, it becomes clear that the evolution of surgical treatment of ACL injuries was biased. Preservation of the native ligament has many advantages, such as better proprioception, graft vascularization, an optical guide for tibial tunnel placement, and a decreased incidence of tunnel widening that can be expected. Furthermore, arthroscopic primary ACL repair is minimally invasive and does not burn any bridges for future reconstructions, if necessary. This is in addition to the other (theoretical) advantages of primary repair, such as restoration of native kinematics and a decreased risk of osteoarthritis. Modern advances have significantly changed the risk-benefit ratio that should make us reconsider ACL preservation approaches. Certainly, further research in this area is warranted. In this article we have presented a treatment algorithm for ACL preservation, which is based on tear location and remnant tissue quality.
Am J Orthop. 2016;45(7):E393-E405. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370.
2. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502-1507.
3. Ciccotti MG, Lombardo SJ, Nonweiler B, Pink M. Non-operative treatment of ruptures of the anterior cruciate ligament in middle-aged patients. Results after long-term follow-up. J Bone Joint Surg Am. 1994;76(9):1315-1321.
4. Sanders TL, Pareek A, Kremers HM, et al. Long-term follow-up of isolated ACL tears treated without ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016 May 24. [Epub ahead of print]
5. Irarrázaval S, Kurosaka M, Cohen M, Fu FH. Anterior cruciate ligament reconstruction. J ISAKOS. 2016;1(1):38-52.
6. Gabler CM, Jacobs CA, Howard JS, Mattacola CG, Johnson DL. Comparison of graft failure rate between autografts placed via an anatomic anterior cruciate ligament reconstruction technique: a systematic review, meta-analysis, and meta-regression. Am J Sports Med. 2016;44(4):1069-1079.
7. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287-1297.
8. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind M. Comparison of hamstring tendon and patellar tendon grafts in anterior cruciate ligament reconstruction in a nationwide population-based cohort study: results from the danish registry of knee ligament reconstruction. Am J Sports Med. 2014;42(2):278-284.
9. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100-110.
10. Andernord D, Desai N, Björnsson H, Gillén S, Karlsson J, Samuelsson K. Predictors of contralateral anterior cruciate ligament reconstruction: a cohort study of 9061 patients with 5-year follow-up. Am J Sports Med. 2015;43(2):295-302.
11. Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43(3):641-647.
12. Kim SJ, Postigo R, Koo S, Kim JH. Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics. 2014;37(7):477-484.
13. Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS. Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1392-1401.
14. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84-A(9):1560-1572.
15. Ardern CL, Österberg A, Sonesson S, Gauffin H, Webster KE, Kvist J. Satisfaction with knee function after primary anterior cruciate ligament reconstruction is associated with self-efficacy, quality of life, and returning to the preinjury physical activity. Arthroscopy. 2016;32(8):1631-1638.e3.
16. Grant JA, Mohtadi NG, Maitland ME, Zernicke RF. Comparison of home versus physical therapy-supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. Am J Sports Med. 2005;33(9):1288-1297.
17. Lindström M, Strandberg S, Wredmark T, Fell änder-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431-442.
18. Biau DJ, Tournoux C, Katsahian S, Schranz PJ, Nizard RS. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta-analysis. BMJ. 2006;332(7548):995-1001.
19. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
20. Aga C, Wilson KJ, Johansen S, Dornan G, La Prade RF, Engebretsen L. Tunnel widening in single- versus double-bundle anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2016 Jun 21. [Epub ahead of print]
21. Maak TG, Voos JE, Wickiewicz TL, Warren RF. Tunnel widening in revision anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2010;18(11):695-706.
22. Cheatham SA, Johnson DL. Anticipating problems unique to revision ACL surgery. Sports Med Arthrosc. 2013;21(2):129-134.
23. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
24. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
25. Andriolo L, Filardo G, Kon E, et al. Revision anterior cruciate ligament reconstruction: clinical outcome and evidence for return to sport. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2825-2845.
26. Grassi A, Ardern CL, Marcheggiani Muccioli GM, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br J Sports Med. 2016;50(12):716-724.
27. Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1323-1329.
28. Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447-457.
29. Imhauser C, Mauro C, Choi D, et al. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013;41(4):815-825.
30. Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242-2252.
31. Chalmers PN, Mall NA, Moric M, et al. Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292-300.
32. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
33. Eggli S, Kohlhof H, Zumstein M, et al. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1215-1221.
34. Buda R, Ferruzzi A, Vannini F, Zambelli L, Di Caprio F. Augmentation technique with semitendinosus and gracilis tendons in chronic partial lesions of the ACL: clinical and arthrometric analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1101-1107.
35. Ochi M, Adachi N, Uchio Y, et al. A minimum 2-year follow-up after selective anteromedial or posterolateral bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(2):117-122.
36. Ahn JH, Lee YS, Ha HC. Anterior cruciate ligament reconstruction with preservation of remnant bundle using hamstring autograft: technical note. Arch Orthop Trauma Surg. 2009;129(8):1011-1015.
37. Lee BI, Min KD, Choi HS, Kim JB, Kim ST. Arthroscopic anterior cruciate ligament reconstruction with the tibial-remnant preserving technique using a hamstring graft. Arthroscopy. 2006;22(3):340.e1-e7.
38. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016 Jun 17. [Epub ahead of print]
39. MacKay G, Anthony IC, Jenkins PJ, Blyth M. Anterior cruciate ligament repair revisited. Preliminary results of primary repair with internal brace ligament augmentation: a case series. Orthop Muscul Syst. 2015;4:188.
40. Mackay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
41. Henle P, Röder C, Perler G, Heitkemper S, Eggli S. Dynamic intraligamentary stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord. 2015;16:27.
42. Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002;73(3):330-334.
43. Gao F, Zhou J, He C, et al. A morphologic and quantitative study of mechanoreceptors in the remnant stump of the human anterior cruciate ligament. Arthroscopy. 2016;32(2):273-280.
44. Georgoulis AD, Pappa L, Moebius U, et al. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg Sports Traumatol Arthrosc. 2001;9(6):364-368.
45. Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500-1505.
46. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762-1770.
47. Ahn JH, Wang JH, Lee YS, Kim JG, Kang JH, Koh KH. Anterior cruciate ligament reconstruction using remnant preservation and a femoral tensioning technique: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(8):1079-1089.
48. Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008;24(5):560-568.
49. Lee BI, Min KD, Choi HS, et al. Immunohistochemical study of mechanoreceptors in the tibial remnant of the ruptured anterior cruciate ligament in human knees. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1095-1101.
50. Takahashi T, Kondo E, Yasuda K, et al. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: a biomechanical and histological study. Am J Sports Med. 2016;44(7):1708-1716.
51. Dong S, Xie G, Zhang Y, Shen P, Huangfu X, Zhao J. Ligamentization of autogenous hamstring grafts after anterior cruciate ligament reconstruction: midterm versus long-term results. Am J Sports Med. 2015;43(8):1908-1917.
52. Takazawa Y, Ikeda H, Kawasaki T, et al. ACL reconstruction preserving the ACL remnant achieves good clinical outcomes and can reduce subsequent graft rupture. Orthop J Sports Med. 2013;1(4):2325967113505076.
53. Shimodaira H, Tensho K, Akaoka Y, Takanashi S, Kato H, Saito N. Remnant-preserving tibial tunnel positioning using anatomic landmarks in double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2016;32(9):1822-1830.
54. Zhang Q, Zhang S, Cao X, Liu L, Liu Y, Li R. The effect of remnant preservation on tibial tunnel enlargement in ACL reconstruction with hamstring autograft: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):166-173.
55. Tie K, Chen L, Hu D, Wang H. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: A meta-analysis. Knee. 2016;23(4):566-574.
56. Robson AW. VI. Ruptured crucial ligaments and their repair by operation. Ann Surg. 1903;37(5):716-718.
57. Palmer I. On the injuries to the ligaments of the knee joint. Acta Orthop Scand. 1938;53.
58. Palmer I. On the injuries to the ligaments of the knee joint: a clinical study. 1938. Clin Orthop Relat Res. 2007;454:17-22.
59 O’Donoghue DH. An analysis of end results of surgical treatment of major injuries to the ligaments of the knee. J Bone Joint Surg Am. 1955;37-A(1):1-13.
60. O’Donoghue DH. Surgical treatment of fresh injuries to the major ligaments of the knee. J Bone Joint Surg Am. 1950;32 A(4):721-738.
61. Feagin JA, Abbott HG, Rokous JR. The isolated tear of the anterior cruciate ligament. J Bone Joint Surg Am. 1972;54-A:1340-1341.
62. England RL. Repair of the ligaments about the knee. Orthop Clin North Am. 1976;7(1):195-204.
63. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
64. Weaver JK, Derkash RS, Freeman JR, Kirk RE, Oden RR, Matyas J. Primary knee ligament repair--revisited. Clin Orthop Relat Res. 1985;(199):185-191.
65. Nogalski MP, Bach BR Jr. A review of early anterior cruciate ligament surgical repair or reconstruction. Results and caveats. Orthop Rev. 1993;22(11):1213-1223.
66. Feagin JA Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4(3):95-100.
67. Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10(2):103-107.
68. Straub T, Hunter RE. Acute anterior cruciate ligament repair. Clin Orthop Relat Res. 1988;227:238-250.
69. Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med. 1990;18(4):354-358.
70. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
71. Paar O. Use of semitendinosus tendon to strengthen a freshly repaired anterior cruciate ligament. Chirurg. 1985;56(11):728-734.
72. Aglietti P, Buzzi R, Pisaneschi A, Salvi M. Comparison between suture and augmentation with the semitendinosus tendon in the repair of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1986;8(4):217-231.
73. Higgins RW, Steadman JR. Anterior cruciate ligament repairs in world class skiers. Am J Sports Med. 1987;15(5):439-447.
74. Harilainen A, Myllynen P. Treatment of fresh tears of the anterior cruciate ligament. A comparison of primary suture and augmentation with carbon fibre. Injury. 1987;18(6):396-400.
75. Jones KG. Results of use of the central one-third of the patellar ligament to compensate for anterior cruciate ligament deficiency. Clin Orthop Relat Res. 1980;(147):39-44.
76. Puddu G. Method for reconstruction of the anterior cruciate ligament using the semitendinosus tendon. Am J Sports Med. 1980;8(6):402-404.
77. Hefti F, Gächter A, Jenny H, Morscher E. Replacement of the anterior cruciate ligament. a comparative study of four different methods of reconstruction. Arch Orthop Trauma Surg. 1982;100(2):83-94.
78. Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament. A randomized study with short-term follow-up observations. Clin Orthop Relat Res. 1985;(198):87-93.
79. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
80. Engebretsen L, Benum P, Fasting O, Mølster A, Strand T. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med. 1990;18(6):585-590.
81. Jonsson T, Peterson L, Renström P. Anterior cruciate ligament repair with and without augmentation. A prospective 7-year study of 51 patients. Acta Orthop Scand. 1990;61(6):562-566.
82. Andersson C, Odensten M, Gillquist J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: a randomized study with a long-term follow-up period. Clin Orthop Relat Res. 1991;(264):255-263.
83. Heim U, Bachmann B, Infanger K. Reinsertion of the anterior cruciate ligament or primary ligamentous plasty? Helv Chir Acta. 1982;48(5):703-708.
84. Strand T, Engesaeter LB, Mølster AO, et al. Knee function following suture of fresh tear of the anterior cruciate ligament. Acta Orthop Scand. 1984;55(2):181-184.
85. Marcacci M, Spinelli M, Chiellini F, Buccolieri V. Notes on 53 cases of immediate suture of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1985;7(2):69-79.
86. van der List JP, DiFelice GS. Primary repair of the anterior cruciate ligament: a paradigm shift. Surgeon. 2016 Oct 6. [Epub ahead of print]
87. Bräm J, Plaschy S, Lütolf M, Leutenegger A. [The primary cruciate ligament suture--is the method outdated? Results in follow-up of 58 patients]. Z Unfallchir Versicherungsmed. 1994;87(2):91-109.
88. Genelin F, Trost A, Primavesi C, Knoll P. Late results following proximal reinsertion of isolated ruptured ACL ligaments. Knee Surg Sports Traumatol Arthrosc. 1993;1(1):17-19.
89. Kühne JH, Theermann R, Neumann R, Sagasser J. [Acute uncomplicated anterior knee instability. 2-5 year follow-up of surgical treatment]. Unfallchirurg. 1991;94(2):81-87.
90. Simonet WT, Sim FH. Repair and reconstruction of rotatory instability of the knee. Am J Sports Med. 1984;12(2):89-97.
91. Raunest J, Derra E, Ohmann C. [Clinical results of Palmer’s primary cruciate ligament insertion without augmentation]. Unfallchirurgie. 1991;17(3):166-174.
92. Frank C, Beaver P, Rademaker F, Becker K, Schachar N, Edwards G. A computerized study of knee-ligament injuries: repair versus removal of the torn anterior cruciate ligament. Can J Surg. 1982;25(4):454-458.
93. Enneking WF, Horowitz M. The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am. 1972;54(5):973-985.
94. Millett PJ, Wickiewicz TL, Warren RF. Motion loss after ligament injuries to the knee. Part I: causes. Am J Sports Med. 2001;29(5):664-675.
95. Bilko TE, Paulos LE, Feagin JA Jr, Lambert KL, Cunningham HR. Current trends in repair and rehabilitation of complete (acute) anterior cruciate ligament injuries. Analysis of 1984 questionnaire completed by ACL Study Group. Am J Sports Med. 1986;14(2):143-147.
96. Paulos L, Noyes FR, Grood E, Butler DL. Knee rehabilitation after anterior cruciate ligament reconstruction and repair. J Orthop Sports Phys Ther. 1991;13(2):60-70.
97. Paessler HH, Deneke J, Dahners LE. Augmented repair and early mobilization of acute anterior cruciate ligament injuries. Am J Sports Med. 1992;20(6):667-674.
98.
99. Kdolsky RK, Gibbons DF, Kwasny O, Schabus R, Plenk H Jr. Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res. 1997;15(1):1-10.
100. Grøntvedt T, Engebretsen L. Comparison between two techniques for surgical repair of the acutely torn anterior cruciate ligament. A prospective, randomized follow-up study of 48 patients. Scand J Med Sci Sports. 1995;5(6):358-363.
101. Hehl G, Strecker W, Richter M, Kiefer H, Wissmeyer T. Clinical experience with PDS II augmentation for operative treatment of acute proximal ACL ruptures--2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):102-106.
102. Schenk S, Landsiedl F, Enenkel M. Arthroscopic single-stranded semitendinosus tendon- versus PDS-augmentation of reinserted acute femoral anterior cruciate ligament tears: 7 year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):318-324.
103. Zysk SP, Refior HJ. Operative or conservative treatment of the acutely torn anterior cruciate ligament in middle-aged patients. A follow-up study of 133 patients between the ages of 40 and 59 years. Arch Orthop Trauma Surg. 2000;120(1-2):59-64.
104. Krueger-Franke M, Siebert CH, Schupp A. Refixation of femoral anterior cruciate ligament tears combined with a semitendinosus tendon augmentation. Technique and results. Arch Orthop Trauma Surg. 1998;117(1-2):68-72.
105. Natri A, Järvinen M, Kannus P. Primary repair plus intra-articular iliotibial band augmentation in the treatment of an acute anterior cruciate ligament rupture. A follow-up study of 70 patients. Arch Orthop Trauma Surg. 1996;115(1):22-27.
106. Träger D, Pohle K, Tschirner W. Anterior cruciate ligament suture in comparison with plasty. A 5-year follow-up study. Arch Orthop Trauma Surg. 1995;114(5):278-280.
107. Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332-336.
108. Volokhina YV, Syed HM, Pham PH, Blackburn AK. Two helpful MRI signs for evaluation of posterolateral bundle tears of the anterior cruciate ligament: a pilot study. Orthop J Sports Med. 2015;3(8):2325967115597641.
109. Strand T, Mølster A, Hordvik M, Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years postoperatively. Arch Orthop Trauma Surg. 2005;125(4):217-221.
110. van der List JP, DiFelice GS. Successful arthroscopic primary repair of a chronic anterior cruciate ligament tear 11 years following injury. HSS J. 2016. In press.
111. van der List JP, DiFelice GS. The role of ligament repair in anterior cruciate ligament surgery. In: Mascarenhas R, Bhatia S, Lowe WR, eds. Ligamentous Injuries of the Knee. 1st ed. Houston: Nova Science Publishers; 2016:199-220.
112. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
113. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
114. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
115. Magarian EM, Fleming BC, Harrison SL, Mastrangelo AN, Badger GJ, Murray MM. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med. 2010;38(12):2528-2534.
116. Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039-2049.
117. Werner BC, Yang S, Looney AM, Gwathmey FW Jr. Trends in pediatric and adolescent anterior cruciate ligament iInjury and reconstruction. J Pediatr Orthop. 2016;36(5):447-452.
118. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
119. Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ. Anterior cruciate ligament tears in children and adolescents: a meta-analysis of nonoperative versus operative treatment. Am J Sports Med. 2014;42(11):2769-2776.
120. Zantop T, Brucker PU, Vidal A, Zelle BA, Fu FH. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;454:48-53.
121. Yoon KH, Bae DK, Cho SM, Park SY, Lee JH. Standard anterior cruciate ligament reconstruction versus isolated single-bundle augmentation with hamstring autograft. Arthroscopy. 2009;25(11):1265-1274.
122 Demirağ B, Ermutlu C, Aydemir F, Durak K. A comparison of clinical outcome of augmentation and standard reconstruction techniques for partial anterior cruciate ligament tears. Eklem Hastalik Cerrahisi. 2012;23(3):140-144.
123. Sonnery-Cottet B, Zayni R, Conteduca J, et al. Posterolateral bundle reconstruction with anteromedial bundle remnant preservation in ACL tears: clinical and MRI evaluation of 39 patients with 24-month follow-up. Orthop J Sports Med. 2013;1(3):2325967113501624.
124. Sabat D, Kumar V. Partial tears of anterior cruciate ligament: results of single bundle augmentation. Indian J Orthop. 2015;49(2):129-135.
125. Jung YB, Jung HJ, Siti HT, et al. Comparison of anterior cruciate ligament reconstruction with preservation only versus remnant tensioning technique. Arthroscopy. 2011;27(9):1252-1258.
126. Nguyen DT, Ramwadhdoebe TH, van der Hart CP, Blankevoort L, Tak PP, van Dijk CN. Intrinsic healing response of the human anterior cruciate ligament: an histological study of reattached ACL remnants. J Orthop Res. 2014;32(2):296-301.
127. Boutsiadis A, Karampalis C, Tzavelas A, Vraggalas V, Christodoulou P, Bisbinas I. Anterior cruciate ligament remnant-preserving reconstruction using a “lasso-loop” knot configuration. Arthrosc Tech. 2015;4(6):e741-e746.
128. Noh JH, Yoon KH, Song SJ, Roh YH. Re-tensioning technique to cover the graft with remnant in anterior cruciate ligament reconstruction. Arthrosc Tech. 2014;3(6):e679-e682.
129. Hong L, Li X, Zhang H, et al. Anterior cruciate ligament reconstruction with remnant preservation: a prospective, randomized controlled study. Am J Sports Med. 2012;40(12):2747-2755.
130. Noh JH, Kyung HS, Roh YH, Kang TS. Remnant-preserving and re-tensioning technique to cover the graft in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 12. [Epub ahead of print]
131. Muneta T, Koga H, Ju YJ, Horie M, Nakamura T, Sekiya I. Remnant volume of anterior cruciate ligament correlates preoperative patients’ status and postoperative outcome. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):906-913.
132. Leeberg V, Lekdorf J, Wong C, Sonne-Holm S. Tibial eminentia avulsion fracture in children - a systematic review of the current literature. Dan Med J. 2014;61(3):A4792.
133. In Y, Kwak DS, Moon CW, Han SH, Choi NY. Biomechanical comparison of three techniques for fixation of tibial avulsion fractures of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1470-1478.
Injury of the anterior cruciate ligament (ACL) is very common with over 200,000 annual injuries in the United Status.1,2 There is a general consensus that these injuries should not be treated conservatively in patients that are younger, or who wish to remain active.3,4 Reconstructive surgery is currently the preferred treatment in these patients, and anatomic single-bundle reconstruction with autografts is considered the gold standard.5,6
Reconstruction of the ACL is, however, not a perfect treatment. Following single-bundle autograft reconstruction, revision rates of 3% to 8%,6-9 contralateral injury rates of 3% to 8%,10,11 and infection rates of 0.5% to 3%7,12,13 have been reported. Furthermore, due to the invasive nature of graft harvesting and the surgical procedure, 10% to 25% of the patients are not satisfied following ACL reconstruction.14,15 This can often be explained by common complaints, such as anterior knee pain (13%-43%), kneeling pain (12%-54%), quadriceps muscle atrophy (20%-30%),16,17 and loss of range of motion (ROM) (12%-23%).7,9,18,19 Furthermore, as a result of the invasive nature of reconstructive surgery, revisions can be difficult due to complications, such as tunnel widening, tunnel malpositioning, and preexisting hardware.20-22 This can lead to inferior outcomes and higher rates (13%) of revision surgery compared to primary reconstruction.23-26 Finally, reconstructive surgery does not restore native kinematics of the ACL,27-29 which may partially explain why reconstructive surgery has not been shown to prevent osteoarthritis.28-31
Over the past decades, there has been an increasing interest in the preservation of the ACL in an attempt to ameliorate these issues.32-37 Ligament preservation focuses on preserving the native tissues and biology, while minimizing the surgical morbidity to the patients.
Some authors have recently reported on arthroscopic primary repair of proximal ACL tears in which the ligament is reattached onto the femoral wall using modern-day suture anchor technology.32,38 Others have augmented this repair technique with an internal brace39,40 or with a synthetic device.33,41 When performing primary repair, it is believed that proprioception is maintained,42-44 while experimental studies have suggested that primary repair also restores the native kinematics,45 and may prevent osteoarthritis.46 Furthermore, primary repair is a conservative approach in that no grafts need to be harvested, no tunnels need to be drilled, and revision surgery, if necessary, is more analogous to primary reconstructions.32In patients with partial tears, some surgeons have advocated preserving the anteromedial (AM) or posterolateral (PL) bundle and performing selective single-bundle augmentation.34,35 In addition, several authors have used remnant tensioning36,47 or remnant preservation37,48 in combination with reconstructive surgery in order to benefit from the biological characteristics of the remnant. These techniques lead to better proprioceptive function,44,49,50 vascularization and ligamentization of the graft,50-52 provide an optical guide for anatomic tunnel placement,53 and decrease the incidence of tunnel widening.54,55The feasibility and applicability of these surgical techniques mainly depends on the tear type and tissue quality of the torn ligament. In this article we (I) discuss the history of ACL preservation, (II) discuss how modern advances alter the risk-benefit ratio for ACL preservation, and (III) propose a treatment algorithm for ACL injuries that is based on tear location and tissue quality.
History of ACL Preservation
The history of the surgical treatment of ACL injuries started in 1895 when Robson56 treated a 41-year-old male who tore both cruciate ligaments from the femoral wall. Performing primary repair with catgut ligatures, both cruciate ligaments were preserved and the patients had resolution of pain symptoms and full function at 6-year follow-up. Over the following decades, Palmer57,58 and O’Donoghue59,60 further popularized open primary repair for the treatment of ACL injuries, and this technique was the most commonly performed treatment in the 1970s and early 1980s.61-65 The initial short-term results of primary repair were excellent,61,62 but Feagin and Curl66 were the first to note that the results deteriorated at mid-term follow-up. Despite improvements in the surgical technique of repairing the ACL, such as the usage of nonabsorbable sutures and directly tying the sutures over bone,63,67 the results remained disappointing at longer-term follow-up.68-70
In response to these disappointing results, surgeons sought to improve the surgical treatment by either augmenting the primary repair with a semitendinosus, a patella tendon graft or an augmentation device,71-74 or by performing primary reconstruction.75-77 At the end of the 1980s and early 1990s, several randomized and prospective clinical trials were performed in order to compare the outcomes of these techniques.74,78-82 Many studies showed that results of augmented repair were more reliable when compared to primary repair, which led to the abandonment of primary repair in favor of augmented repair, and eventually primary reconstruction.65
The Important Role of Tear Location in Ligament Preservation
When taking a closer look at the outcomes of primary repair and augmented repair, it seems that the results of these preservation techniques were not as disappointing as was suggested. This can be explained, in large part, by the fact that the important roles of tear location and tissue quality were not widely recognized.
Sherman and colleagues70 reported in 1991 their mid-term results of open primary repair. Similar to others, they noted a deterioration of their results at mid-term follow-up. However, they uniquely performed an extensive subgroup analysis in order to find an explanation for this. In their study, considered a landmark paper on primary repair,65,70 they concluded that, “poor tissue quality is typical for midsubstance tears and that a repair of these injuries will predictably fail while type I tears (proximal), with better tissue quality, show a definite trend towards better results.”70 With these findings, they confirmed the findings of others that had recognized a trend of better outcomes with proximal tears.64,67,83-85
A majority of the historical studies that were published before 1991 had not considered the role of tear location and tissue quality on outcomes of open primary repair. This was also true for the aforementioned randomized studies that compared primary repair with augmented repair and primary reconstruction. Because these studies randomized patients and did not take tear location into account, it can be expected that patients with midsubstance tears were included in the cohorts of primary repair and the outcomes of these studies were therefore confounded.74,78-82 If these studies would have been aware of the role that tear location plays on primary repair outcomes, different outcomes may have been found and different conclusions on the optimal treatment for different tear types may have been drawn.86
Open Primary ACL Repair Outcomes Stratified by Tear Location
When reviewing the literature of open primary repair outcomes stratified by tear location, it is noted that multiple studies reported excellent outcomes following primary repair of proximal ACL tears.73,83,84,87-90 Weaver and colleagues64 were among the first to stratify their results by tear location, and they found that more patients with proximal tears (52 of 66; 79%) were satisfied after the procedure when compared to patients with midsubstance tears (3 of 13; 23%) at 3.5-year follow-up. They concluded that, “selection can be made with some predictability of the type of injury to the ligament as to which patients will do better.”64 Kühne and colleagues89 reported the outcomes of 75 patients with proximal tears treated with open primary repair and noted no failures, negative pivot shift in 88% of patients, stable or nearly stable Lachman test in 87% of patients, and 89% return to sports rate at 4-year follow-up. Raunest and colleagues91 reported a negative pivot shift and negative anterior drawer test in 84%, return to sports in 71%, and satisfaction in 75% of 51 patients that underwent open primary repair of proximal tears at 3.5-year follow-up.
Interestingly, and in contrast to the findings of Feagin and Curl,66 no deterioration of the outcomes at mid-term follow-up was noted in patients with proximal tears. Genelin and colleagues88 reported their results of 42 patients with proximal tears treated with open primary repair at 5- to 7-year follow-up. They found a negative pivot shift in 81%, stable or nearly stable Lachman test in 81%, and patient satisfaction in 86% of patients. Similarly, Bräm and colleagues87 found good results at mid-term follow-up with a good-excellent Lysholm score in 79%, return to a similar level of sports in 76%, stable or nearly stable Lachman test in 91%, and anterior drawer test in 94% of patients, along with an 88% satisfaction rate and 7% failure rate in patients who underwent open primary repair of proximal tears.
On the contrary, when the outcomes of studies that performed open primary repair in mainly, or only, patients with midsubstance tears are reviewed, significantly inferior results are found. Frank and colleagues92 reported outcomes in 42 patients with midsubstance tears at 4-year follow-up. They reported that 56% had a stable or nearly stable anterior drawer test, 78% had a positive pivot shift, and that only 61% were satisfied with the procedure. Odensten and colleagues78 reported outcomes of open primary repair in a subgroup of 22 patients with midsubstance tears at 1.5-year follow-up, and noted a 14% failure rate.
When reviewing the mid-term results in patients with midsubstance tears, it seems that there was more deterioration in outcomes.69,70 Firstly, the aforementioned study by Sherman and colleagues70 showed poor results in the patients with (type IV) midsubstance tears at mid-term follow-up. Furthermore, Kaplan and colleagues69 reported the mid-term outcomes of 70 patients, of which 56 patients had midsubstance tears. After having reported good outcomes at short-term follow-up,63,67 they noted that 42% of patients had >3 mm anteroposterior stability when compared to the contralateral leg, only a 62% return to sport rate, and a 17% failure rate. They concluded that, “Although … primary repair of the anterior cruciate may work in some patients, it is an unpredictable operative procedure.”
These studies showed that the outcomes of open primary repair were significantly better in patients with proximal ACL tears and sufficient tissue quality when compared to midsubstance tears. This suggests that open primary ACL repair may have been prematurely abandoned as a treatment option for patients with proximal tears.
Augmented ACL Repair
There were several reasons why augmented repair became the preferred treatment in the early and mid 1990s. First of all, the results of augmented repair were more consistent compared to primary repair in the aforementioned randomized and prospective studies,74,78-82 which is not surprising given the fact that the role of tear location was not widely recognized at the time. Secondly, in the 1970s and early 1980s, patients were treated postoperatively in a cast for 6 weeks, which led to problems, such as loss of ROM, pain, and decreased function.93,94 At the end of the1980s and 1990s, the focus shifted from prolonged joint immobilization towards early postoperative ROM.95-97 Since many authors believed that primary repair of the ACL was not strong enough to tolerate early mobilization, an augmentation was added to the technique in order to fortify the repair and enable early ROM.98
Interestingly, augmented repair, which is essentially a combination of primary ACL repair and ACL reconstruction, was mainly performed in the 1990s and many surgeons did recognize the role of tear location in this treatment at this point.73,98-103 In these years, the treatment algorithm consisted of augmented ACL repair in patients with proximal tears in the acute setting and ACL reconstruction in patients with midsubstance or chronic tears. Several different augmentation techniques were used to reinforce the primary repair in these years including autograft tissues (semitendinosus tendon,102-104 patellar tendon,100 or iliotibial band [ITB]105) synthetic materials (polydioxanone [PDS],101,102,106 carbon fibre,74 and polyester [Trevira]97), augmentation devices (Kennedy Ligament Augmentation Device [LAD]98-100) and extra-articular augmentations.73
When reviewing the outcomes of augmented repair of the ACL, good to excellent results can be found in studies that used this technique in patients with proximal tears.73,98-106 Kdolsky and colleagues98 were in one of the first groups that reported their results of augmented repair in only patients with proximal tears. In 1993, they reported their mid-term outcomes (5 to 8 years) in 66 patients who underwent primary repair and augmentation with the Kennedy LAD and found that 97% of patients had stable knees (<3 mm on KT-1000 examination), 98% had a negative pivot shift, and 76% returned to previous level of sports. However, often-reported problems with the augmentation devices were found in this study with rupture of the device (12%) and decreased ROM (14%).98 In 1995, Grøntvedt and Engebretsen100 compared augmentation with the Kennedy LAD to patellar tendon augmentation in a randomized study of patients with acute proximal tears. They noted that 50% of the patients in the Kennedy LAD group had a positive pivot shift compared to 23% in the patellar tendon group. Furthermore, they found KT-1000 leg differences of <3 mm in 92% of the patellar tendon group and 54% of the Kennedy LAD group. Because the authors found significant differences between both groups at 1- and 2-year follow-up, they stopped the clinical trial.
Several authors in the following years reported good results of augmented repair using autograft tissues. Natri and colleagues105 reported the outcomes of 72 patients treated with primary repair of proximal tears augmented with the ITB at 3.5-year follow-up. They found 89% negative pivot shift rate, 93% stable or nearly stable Lachman test, 99% stable or nearly stable anterior drawer test, 79% satisfaction rate, and 91% return to previous level of sports rate. Krueger-Franke and colleagues104 reported the outcomes of primary repair of proximal tears with augmentation using the semitendinosus tendon. In a retrospective study of 76 patients, they noted that 96% of patients had a negative pivot shift, 75% of patients had stable or nearly stable Lachman test, 93% were satisfied with the procedure, a mean Lysholm score of 92, a Tegner score that only decreased from 7.2 to 7.1, and KT-1000 testing with 78% <4 mm leg difference with the contralateral leg. The authors concluded that patients with femoral ruptures could be treated with augmented repair when performed in the acute setting. As this study was published in 1998, they stated that magnetic resonance imaging and arthroscopy could be helpful in identifying the tear location.
Final Abandonment of ACL Preservation
Reviewing these outcomes raises the question as to why these techniques were ultimately abandoned in the treatment algorithm of proximal ACL injuries, especially given the aforementioned advantages of ACL preservation. One of the possible answers can be found in a landmark study on ACL reconstruction and rehabilitation published by Shelbourne and colleagues107 in 1991. At that time, arthrofibrosis and knee stiffness were frequently reported problems following ACL surgery, which could partially be explained by the standard conservative rehabilitation using postoperative joint immobilization.67,70,80,88
Shelbourne and colleagues107 aimed to assess the cause of arthrofibrosis and knee stiffness, and divided the patients into groups by number of days between injury and surgery (<7, 7 to 21 days, and >21 days between injury and surgery). Furthermore, patients within these groups underwent either a conventional or accelerated rehabilitation program. The authors not only found that patients undergoing accelerated rehabilitation had less arthrofibrosis, but they also noted that less arthrofibrosis was seen when surgery was delayed. These findings, however, contrasted with the general perception that the ACL should be repaired in the first 3 weeks postinjury to ensure optimal tissue quality with an augmented approach. As a result, the treatment of ACL injuries shifted towards ACL reconstruction after these findings. Krueger-Franke and colleagues104 commented on the trend after the study of Shelbourne and colleagues:107 “Less consideration has been given to the importance of the proprioceptive receptors in the tibial remnants of the torn ACL and the value of their preservation as part of a primary reconstruction.”
In addition to the trend away from an augmented repair approach due to the novel understanding of the importance of early mobilization, some discussion should focus on the technical limitations of arthroscopy at that time. While arthroscopy had been around for several decades, fluid management and arthroscopic instrumentation was slow to develop. All of the repair and augmentation techniques previously discussed had been performed via an open arthrotomy. Arthroscopic technologies of the time were not refined enough to enable surgeons to perform such complex, intra-articular techniques that would enable suturing of the ligament remnant. In this regard, arthroscopic ACL reconstruction was a much simpler technique to accomplish, and this also likely contributed to the final abandonment of the ligament preservation approach.
Role for ACL Preservation with Modern Advances
As stated in the introduction, there has been a recent resurgence of interest in preservation of the native ligament.32-37 With the passage of time, many technologic advances have been made, which has allowed surgeons to reconsider the concept of ligament preservation.
First of all, appropriate patient selection was not applied historically, as the critical factors of tear location and tissue quality were not recognized in the era of open primary repair. In modern days, however, advances such as MRI have been developed, which can give the surgeon an idea of the status, and tear type of the ACL pre-operatively.108 This may help the orthopaedic surgeon to plan the surgery and make an assessment as to whether ACL preservation is possible. Secondly, in the historic literature the postoperative regimen consisted of casting for 5 or 6 weeks,67,70,80,88 while the focus later shifted towards early ROM.95-97Modern day ACL rehabilitation focuses on immediate ROM to avoid the complications stiffness, pain and decreased function that plagued the outcomes when immobilization was used.93,94 Thirdly, historically small tunnels were drilled with primary repair and sutures had to be tied over bone,57,67 whereas currently suture anchors are available that prevent the need for tunnel drilling and enable direct suture tensioning.32,38 Finally, and most importantly, in the historic literature patients were treated with an invasive arthrotomy technique, while modern day arthroscopic techniques readily enable the surgeon to effectively suture the remnant arthroscopically. Interestingly, in 2005, in their 20-year follow-up of primary repair surgeries, Strand and colleagues109 stated, “if the same results could be accomplished by a smaller, arthroscopic procedure, primary repair might reduce the number of patients needing later reconstructions with small ‘costs’ in the way of risk and inconvenience for the patients. We therefore believe that further research and development of methods for closed (arthroscopic) repair are justified.”
Altered Risk-Benefit Ratio
Historically, the treatments of open primary repair and open ACL reconstruction were both invasive surgeries with an arthrotomy, drilling of bone tunnels, and postoperative joint immobilization for 4 to 6 weeks. However, with the modern-day advances, the risk-benefit ratio of both treatments has changed, as Strand and colleagues109 had already suggested. Although ACL reconstruction can be performed arthroscopically, it remains an invasive procedure, in which tunnels are drilled, patellar tendons or hamstring tendons are harvested, and complications, such as knee pain and quadriceps atrophy, are common. The surgery of primary ACL repair, however, has benefited significantly from the modern developments.32,38 Primary ACL repair can now be performed arthroscopically, and by using suture anchors no tunnels need to be drilled and the remnant can be tensioned directly. An additional benefit of the use of suture anchors is that revision surgery of a failed primary repair is analogous to primary reconstruction, whereas revision surgery of a failed ACL reconstruction can be problematic due to tunnel widening, tunnel malpositioning, and preexisting hardware.20-22
Reviewing the differences between arthroscopic primary ACL repair and ACL reconstruction, it becomes clear that primary repair has benefited significantly from the modern advances and that the risk-benefit ratio for primary repair has been altered. This means that patients with proximal tears can be treated with a relatively straight forward, minimally invasive surgery, which has been shown to be effective in 85% to 90% of patients.32,38
Treatment Algorithm Based on Tear Location
Since 2008, in the practice of the senior author (GSD), the surgical treatment algorithm for ACL injuries is completely based on the tear location and tissue quality of the ligament.110,111 To describe the different tear types, we use the modified Sherman classification in which we extended his classification towards the tibial side whereas Sherman and colleagues70 only described the femoral side of the tears (Figures A-F, Table).
Type I Tears: Primary Repair
Type I tears are soft tissue avulsion type tears that can be easily treated with arthroscopic primary repair.107 The length of the distal remnant has to be at least 90% and the tissue quality has to be good to excellent in order to approximate the remnant towards the femoral wall (Table).112 The incidence of type I tears was 26% in the study of Sherman and colleagues,70 although recent studies showed a lower incidence (6% to 10%) in a larger population.32,38 Certainly, individual practices will see different percentages of type I tears based upon the mix of injury mechanisms they see most frequently. Over the last 2 years, with the recognition of the importance of tear type and tissue quality, there has been a renewed interest in arthroscopic primary ACL repair.32,38
DiFelice and colleagues32 were the first to arthroscopically perform primary repair of the ACL in proximal tears using suture anchors. They reported the outcomes of the first 11 consecutive patients that underwent primary repair in a previously described technique.113 At mean 3.5-year follow-up, they noted only 1 failure (9%) due to re-injury; mean Lysholm score of 93.2; mean modified Cincinnati score of 91.5; pre- and postoperative Tegner score of 7.3 and 6.9, respectively; SANE score of 91.8; and subjective International Knee Documentation Committee (IKDC) score of 86.4. Of the patients with an intact repair, 9 patients had an objective IKDC rating A and 1 patient had B and all patients had KT-1000 leg differences of <3 mm with the contralateral side (three patients were not available for KT-1000 testing). The authors concluded that arthroscopic primary ACL repair could achieve short-term clinical success in a selected group of patients with proximal avulsion tears and excellent tissue quality. They further noted that mid-term outcomes are necessary given that the results of open primary repair deteriorated at longer-term follow-up in the historical literature. Recently, the senior author (GSD) has added an Internal Brace (Arthrex) to the primary repair with the goal of protecting the ligament in the first weeks to further promote healing of the ligament.39,40,114
More recently, Achtnich and colleagues38 compared the treatment of arthroscopic primary ACL repair with primary ACL reconstruction in 41 patients with type I tears at 2.3-years follow-up. Twenty-one patients consented for primary repair while 20 patients declined this procedure and underwent primary reconstruction. They noted no significant differences in Lachman test, pivot shift test, objective IKDC score, and KT-1000 scores. Although not significant, the clinical failure rate in the primary repair group (15%) was higher than the reconstruction group (0%). Interestingly, despite the higher failure rate in the repair group, the authors concluded that primary ACL repair is recommended in a carefully selected group of patients with type I tears and excellent tissue quality, which can likely be explained by the differences in the risk-benefit ratio between both procedures.
Over the last decade, the research group led by Murray46,115,116 has performed experimental research on primary repair with a biological scaffold and reported many interesting findings that could be extrapolated to primary ACL repair. First of all, they compared bioenhanced primary repair with bioenhanced primary reconstruction in 64 Yucutan pigs and noted that there was significantly less macroscopic cartilage damage in the primary repair group at 1-year follow-up.46 They concluded that bioenhanced ACL repair may provide a new, less invasive treatment option that reduces cartilage damage following joint injury. This may suggest that primary repair may have a lower incidence of osteoarthritis when compared to ACL reconstruction, which is interesting as osteoarthritis is very common after ACL reconstruction. Further research in this area is certainly warranted.
In another study they compared bioenhanced primary repair in juvenile, adolescent and mature Yucutan pigs and noted that functional healing depended on the level of skeletal maturity with immature animals having a more productive healing response.116 This indicates that primary repair might be a good treatment option in skeletally immature patients, especially since reconstruction increases the risk of premature closure of the epiphysis117,118 and delaying treatment increases the risk of meniscus injury.119 Interestingly, a recent meta-analysis showed indeed that the risk of epiphysis closure was lower in primary repair when compared to ACL reconstruction and the rupture rate was also lower.118 Primary repair may be a good treatment option in children as the procedure has all the attributes that should be applicable to children: it is minimally morbid, tissue sparing, and it is a conservative approach that does not burn any surgical bridges for future reconstructive surgery if necessary.
Finally, the research group of Murray115 assessed the effect of surgical delay of primary repair following injury in Yucutan pigs and noted that better biomechanical outcomes were noted after delaying surgery for 2 weeks when compared to 6 weeks. This suggests that primary repair should preferably be performed in the acute setting, which has also been shown in historical studies since the ligament in the acute setting has optimal tissue quality and the ligament is less likely to be retracted or reabsorbed.59,60,115
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the tear locations of the AM and PL bundle are not at the same location and Zantop and colleagues120 reported in an arthroscopic study that this could be as frequent as in 30% of all complete tears. In some of these tears, one of the bundles can be avulsed of the femoral wall (type I tear) while the other bundle is not directly repairable (non-type I tear). In these cases, the senior author (GSD) will repair the type I tear bundle, whereas a hamstring augmentation is placed at the location of the other bundle. When reviewing the literature, a combination of primary repair of one bundle and reconstruction of the second bundle has not been described before. However, over the last decade several surgeons have performed augmentation of one bundle in the setting of partial tears.34,35,121-124
Buda and colleagues34 were the first to perform selective AM or PL bundle reconstruction in the setting of partial tears.34 At 5-year follow-up, they reported no reruptures and only 1 patient with an IKDC C-score, although reoperation was necessary in 4 out of 47 patients (9%). Following this publication, many others reported on selective bundle reconstruction.35,121-124 However, with partial tears, the knee is often stable and a selective augmentation technique is utilized to prevent complete rupture of the ligament. The application of this technique is essentially different from reconstruction for complete ACL tears in which the knee is unstable, there is a giving way sensation and patients have problems participating in sports.
Type II Tears: Augmented Repair
Type II tears often have good or excellent tissue quality and can be pulled up towards the femoral footprint, but are too short to be firmly attached. Sherman and colleagues70 reported that approximately 22% patients had a type II tear, which corresponds to a tear located in the proximal part of the ligament. With this technique, multiple suture passes are used to stitch the remnant and, in addition, a smaller hamstring autograft or allograft is passed through the middle of the tibial remnant. A suture button is used proximally for the graft, and the tensioning repair sutures through the remnant are also passed through the suture button. The suture button is passed through the femoral tunnel and flipped so that the graft is proximally fixed. Then, the repair sutures of the remnant are tensioned, and the ligament is pulled towards the femoral wall as a sleeve around the graft. When the ligament is approximated to the femoral wall, the sutures are tied over the suture button. The graft is then tensioned distally to complete the augmented repair.
In the recent literature, the technique of augmentation of a primary repair using autograft tissue has not been reported. However, augmented repair using an internal brace39,40 or augmentation devices33,41 have been recently performed. MacKay and colleagues39 reported good outcomes of arthroscopic primary repair of proximal tears using an internal brace. Eggli and colleagues33 reported the results of the first 10 patients treated with ACL preservation using primary repair of the ligament with the addition of a dynamic screw-spring mechanism. The authors reported good preliminary results with one failure (10%) and good objective and subjective outcomes. In a next study, they reported the outcomes of 278 patients and although they reported good clinical outcomes and a revision rate of 4%, the reoperation rate for removal of the screw-spring mechanism was high (24%).41 This is not surprising when reviewing the historical literature in which high complication rates of the augmentation devices were reported.99,100 We were unable to identify any other studies reporting surgical techniques of augmenting primary repair in the literature.
Type III Tears: Reconstruction With Remnant Tensioning
In patients with type III tears, the ligament cannot be approximated to the wall and reconstruction is necessary in order to restore knee stability. However, in these cases the ligament has sufficient length (25%-75%) and can be tensioned along or around the graft. Preservation of the ligament remnant has several (theoretical) advantages, such as better proprioceptive function,42,49,50 vascularization and ligamentization of the graft,50-52 an optical guide for anatomic tunnel placement,53 and a decreased incidence of tunnel widening.54,55 Furthermore, tensioning of the remnant is thought to lower the risk of cyclops lesions when compared to remnant preservation.125 Although the difference between augmented repair and remnant tensioning seems small, the purpose of surgery is different. With augmented repair, the ligament can be approximated close to the femoral wall and the goal of surgery is to use the healing capacity that the ACL has in the proximal part of the ligament,126 while with remnant tensioning the goal is only to benefit from some of the aforementioned advantages. Ahn and colleagues36 were the first to perform this technique and stated, “Our concept is that the remnant tissue has only an additive effect.” Furthermore, with augmented repair multiple sutures are passed through the AM and PL bundle in order to sufficiently approximate the ligament to the femoral wall, while with the remnant tensioning technique generally one or a few sutures or lasso loop are passed through the proximal part to tension the ligament, prevent sagging of the remnant, and decrease the risk of cyclops lesions.127,128
Several authors have recently performed remnant tensioning during ACL.36,47,125-127 Ahn and colleagues47 reported excellent objective and subjective outcomes following this procedure and found that with re-arthroscopy nearly all patients had fair synovialization of the graft. Others have reported similarly good outcomes of these techniques.125,129,130 However, studies comparing this treatment with normal ACL reconstruction and assessing outcomes, failure rates and proprioception are lacking.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some patients the ligament is torn distally or the tissue quality is not optimal. In these patients, the remnant can be debrided to the part of good tissue quality in order to preserve the biology and minimize the risk for cyclops lesions. A standard reconstruction needs to be performed to restore the instability, but by preserving the remnant, advantages, such as proprioception,44,49,50 graft vascularization,50-52 an optical guide for tibial tunnel placement,53 and a decreased incidence of tunnel widening54,55 can be expected.
Lee and colleagues37 presented the tibial remnant technique in which standard reconstruction was performed, and the tibial tunnel was drilled through the center of the remnant. In a later study, they compared remnant preservation with a remnant of <20% of the total ACL length with >20% of the length and found that proprioception was better with more remnant volume.48 Similarly, Muneta and colleagues131 assessed the role of remnant length and found that remnant length is positively correlated with better stability measured on KT-1000 anteroposterior stability.
Several studies compared ACL reconstruction with remnant preservation vs conventional ACL reconstruction.52,54,129 Takazawa and colleagues52 performed a retrospective study of 183 patients and found that patients in the remnant preservation group had significantly better KT-2000 stability, while they also reported a significantly lower graft rupture rate in this group (1.1% vs 7.1%) at 2-year follow-up. Hong and colleagues129 performed a randomized clinical trial of 80 patients and did not find these differences, although there was a trend towards higher Lysholm scores in the remnant preservation group. Finally, Zhang and colleagues54 performed a randomized clinical trial and found a lower incidence and amount of tibial tunnel widening in the preserving-remnant group when compared to the removing-remnant group. These studies show that there is likely a role for remnant preservation.
Type V Tears: Primary Repair
In some patients, the ligament is torn in the distal 10% of the ligament, which can occur as a distal avulsion tear or as a distal bony avulsion fracture.132 Bony avulsion fractures are most commonly seen in children whereas true distal soft tissue avulsion tears are very rare.132
Treatments of these tear types include antegrade screw fixation, pullout sutures or the use of suture anchors in case of bony avulsion fractures and pullout sutures with tying over a bony bridge or ligament button in case of soft tissue avulsions. Leeberg and colleagues132 recently performed a systematic review of all studies reporting on treatment of distal avulsion fractures.They noted that most treatments were currently performed arthroscopically and that outcomes were generally good. Another recent biomechanical study compared antegrade screw fixation with suture anchor fixation and pullout suture fixation.133 The authors noted that suture anchor fixation has slightly less displacement of the bony fragment when compared to screw fixation and pull-out sutures, and that the strength to failure was higher in the suture anchor fixation when compared to the pullout suture fixation. The outcomes of this study suggest that screw fixation and suture anchor fixation might be superior to pullout suture fixation, which might be interesting as with pullout suture fixation the ligament cannot be directly tensioned to the tibial footprint, which can lead to anteroposterior laxity.132 Clinical studies are necessary to assess the preferred treatment in these tear types but it seems that screw fixation is preferred in large bony avulsion fractures, while suture anchor fixation or pullout suture fixation can be used for soft tissue avulsion tears.
Complex Tears or Poor Tissue Quality: Reconstruction
If the tear is complex, multiple tears are present, or the tissue quality is poor, then preservation of the ligament is not possible, and in these cases a standard reconstruction should be performed.
Conclusion
When reviewing the literature of ACL preservation, it becomes clear that the evolution of surgical treatment of ACL injuries was biased. Preservation of the native ligament has many advantages, such as better proprioception, graft vascularization, an optical guide for tibial tunnel placement, and a decreased incidence of tunnel widening that can be expected. Furthermore, arthroscopic primary ACL repair is minimally invasive and does not burn any bridges for future reconstructions, if necessary. This is in addition to the other (theoretical) advantages of primary repair, such as restoration of native kinematics and a decreased risk of osteoarthritis. Modern advances have significantly changed the risk-benefit ratio that should make us reconsider ACL preservation approaches. Certainly, further research in this area is warranted. In this article we have presented a treatment algorithm for ACL preservation, which is based on tear location and remnant tissue quality.
Am J Orthop. 2016;45(7):E393-E405. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Injury of the anterior cruciate ligament (ACL) is very common with over 200,000 annual injuries in the United Status.1,2 There is a general consensus that these injuries should not be treated conservatively in patients that are younger, or who wish to remain active.3,4 Reconstructive surgery is currently the preferred treatment in these patients, and anatomic single-bundle reconstruction with autografts is considered the gold standard.5,6
Reconstruction of the ACL is, however, not a perfect treatment. Following single-bundle autograft reconstruction, revision rates of 3% to 8%,6-9 contralateral injury rates of 3% to 8%,10,11 and infection rates of 0.5% to 3%7,12,13 have been reported. Furthermore, due to the invasive nature of graft harvesting and the surgical procedure, 10% to 25% of the patients are not satisfied following ACL reconstruction.14,15 This can often be explained by common complaints, such as anterior knee pain (13%-43%), kneeling pain (12%-54%), quadriceps muscle atrophy (20%-30%),16,17 and loss of range of motion (ROM) (12%-23%).7,9,18,19 Furthermore, as a result of the invasive nature of reconstructive surgery, revisions can be difficult due to complications, such as tunnel widening, tunnel malpositioning, and preexisting hardware.20-22 This can lead to inferior outcomes and higher rates (13%) of revision surgery compared to primary reconstruction.23-26 Finally, reconstructive surgery does not restore native kinematics of the ACL,27-29 which may partially explain why reconstructive surgery has not been shown to prevent osteoarthritis.28-31
Over the past decades, there has been an increasing interest in the preservation of the ACL in an attempt to ameliorate these issues.32-37 Ligament preservation focuses on preserving the native tissues and biology, while minimizing the surgical morbidity to the patients.
Some authors have recently reported on arthroscopic primary repair of proximal ACL tears in which the ligament is reattached onto the femoral wall using modern-day suture anchor technology.32,38 Others have augmented this repair technique with an internal brace39,40 or with a synthetic device.33,41 When performing primary repair, it is believed that proprioception is maintained,42-44 while experimental studies have suggested that primary repair also restores the native kinematics,45 and may prevent osteoarthritis.46 Furthermore, primary repair is a conservative approach in that no grafts need to be harvested, no tunnels need to be drilled, and revision surgery, if necessary, is more analogous to primary reconstructions.32In patients with partial tears, some surgeons have advocated preserving the anteromedial (AM) or posterolateral (PL) bundle and performing selective single-bundle augmentation.34,35 In addition, several authors have used remnant tensioning36,47 or remnant preservation37,48 in combination with reconstructive surgery in order to benefit from the biological characteristics of the remnant. These techniques lead to better proprioceptive function,44,49,50 vascularization and ligamentization of the graft,50-52 provide an optical guide for anatomic tunnel placement,53 and decrease the incidence of tunnel widening.54,55The feasibility and applicability of these surgical techniques mainly depends on the tear type and tissue quality of the torn ligament. In this article we (I) discuss the history of ACL preservation, (II) discuss how modern advances alter the risk-benefit ratio for ACL preservation, and (III) propose a treatment algorithm for ACL injuries that is based on tear location and tissue quality.
History of ACL Preservation
The history of the surgical treatment of ACL injuries started in 1895 when Robson56 treated a 41-year-old male who tore both cruciate ligaments from the femoral wall. Performing primary repair with catgut ligatures, both cruciate ligaments were preserved and the patients had resolution of pain symptoms and full function at 6-year follow-up. Over the following decades, Palmer57,58 and O’Donoghue59,60 further popularized open primary repair for the treatment of ACL injuries, and this technique was the most commonly performed treatment in the 1970s and early 1980s.61-65 The initial short-term results of primary repair were excellent,61,62 but Feagin and Curl66 were the first to note that the results deteriorated at mid-term follow-up. Despite improvements in the surgical technique of repairing the ACL, such as the usage of nonabsorbable sutures and directly tying the sutures over bone,63,67 the results remained disappointing at longer-term follow-up.68-70
In response to these disappointing results, surgeons sought to improve the surgical treatment by either augmenting the primary repair with a semitendinosus, a patella tendon graft or an augmentation device,71-74 or by performing primary reconstruction.75-77 At the end of the 1980s and early 1990s, several randomized and prospective clinical trials were performed in order to compare the outcomes of these techniques.74,78-82 Many studies showed that results of augmented repair were more reliable when compared to primary repair, which led to the abandonment of primary repair in favor of augmented repair, and eventually primary reconstruction.65
The Important Role of Tear Location in Ligament Preservation
When taking a closer look at the outcomes of primary repair and augmented repair, it seems that the results of these preservation techniques were not as disappointing as was suggested. This can be explained, in large part, by the fact that the important roles of tear location and tissue quality were not widely recognized.
Sherman and colleagues70 reported in 1991 their mid-term results of open primary repair. Similar to others, they noted a deterioration of their results at mid-term follow-up. However, they uniquely performed an extensive subgroup analysis in order to find an explanation for this. In their study, considered a landmark paper on primary repair,65,70 they concluded that, “poor tissue quality is typical for midsubstance tears and that a repair of these injuries will predictably fail while type I tears (proximal), with better tissue quality, show a definite trend towards better results.”70 With these findings, they confirmed the findings of others that had recognized a trend of better outcomes with proximal tears.64,67,83-85
A majority of the historical studies that were published before 1991 had not considered the role of tear location and tissue quality on outcomes of open primary repair. This was also true for the aforementioned randomized studies that compared primary repair with augmented repair and primary reconstruction. Because these studies randomized patients and did not take tear location into account, it can be expected that patients with midsubstance tears were included in the cohorts of primary repair and the outcomes of these studies were therefore confounded.74,78-82 If these studies would have been aware of the role that tear location plays on primary repair outcomes, different outcomes may have been found and different conclusions on the optimal treatment for different tear types may have been drawn.86
Open Primary ACL Repair Outcomes Stratified by Tear Location
When reviewing the literature of open primary repair outcomes stratified by tear location, it is noted that multiple studies reported excellent outcomes following primary repair of proximal ACL tears.73,83,84,87-90 Weaver and colleagues64 were among the first to stratify their results by tear location, and they found that more patients with proximal tears (52 of 66; 79%) were satisfied after the procedure when compared to patients with midsubstance tears (3 of 13; 23%) at 3.5-year follow-up. They concluded that, “selection can be made with some predictability of the type of injury to the ligament as to which patients will do better.”64 Kühne and colleagues89 reported the outcomes of 75 patients with proximal tears treated with open primary repair and noted no failures, negative pivot shift in 88% of patients, stable or nearly stable Lachman test in 87% of patients, and 89% return to sports rate at 4-year follow-up. Raunest and colleagues91 reported a negative pivot shift and negative anterior drawer test in 84%, return to sports in 71%, and satisfaction in 75% of 51 patients that underwent open primary repair of proximal tears at 3.5-year follow-up.
Interestingly, and in contrast to the findings of Feagin and Curl,66 no deterioration of the outcomes at mid-term follow-up was noted in patients with proximal tears. Genelin and colleagues88 reported their results of 42 patients with proximal tears treated with open primary repair at 5- to 7-year follow-up. They found a negative pivot shift in 81%, stable or nearly stable Lachman test in 81%, and patient satisfaction in 86% of patients. Similarly, Bräm and colleagues87 found good results at mid-term follow-up with a good-excellent Lysholm score in 79%, return to a similar level of sports in 76%, stable or nearly stable Lachman test in 91%, and anterior drawer test in 94% of patients, along with an 88% satisfaction rate and 7% failure rate in patients who underwent open primary repair of proximal tears.
On the contrary, when the outcomes of studies that performed open primary repair in mainly, or only, patients with midsubstance tears are reviewed, significantly inferior results are found. Frank and colleagues92 reported outcomes in 42 patients with midsubstance tears at 4-year follow-up. They reported that 56% had a stable or nearly stable anterior drawer test, 78% had a positive pivot shift, and that only 61% were satisfied with the procedure. Odensten and colleagues78 reported outcomes of open primary repair in a subgroup of 22 patients with midsubstance tears at 1.5-year follow-up, and noted a 14% failure rate.
When reviewing the mid-term results in patients with midsubstance tears, it seems that there was more deterioration in outcomes.69,70 Firstly, the aforementioned study by Sherman and colleagues70 showed poor results in the patients with (type IV) midsubstance tears at mid-term follow-up. Furthermore, Kaplan and colleagues69 reported the mid-term outcomes of 70 patients, of which 56 patients had midsubstance tears. After having reported good outcomes at short-term follow-up,63,67 they noted that 42% of patients had >3 mm anteroposterior stability when compared to the contralateral leg, only a 62% return to sport rate, and a 17% failure rate. They concluded that, “Although … primary repair of the anterior cruciate may work in some patients, it is an unpredictable operative procedure.”
These studies showed that the outcomes of open primary repair were significantly better in patients with proximal ACL tears and sufficient tissue quality when compared to midsubstance tears. This suggests that open primary ACL repair may have been prematurely abandoned as a treatment option for patients with proximal tears.
Augmented ACL Repair
There were several reasons why augmented repair became the preferred treatment in the early and mid 1990s. First of all, the results of augmented repair were more consistent compared to primary repair in the aforementioned randomized and prospective studies,74,78-82 which is not surprising given the fact that the role of tear location was not widely recognized at the time. Secondly, in the 1970s and early 1980s, patients were treated postoperatively in a cast for 6 weeks, which led to problems, such as loss of ROM, pain, and decreased function.93,94 At the end of the1980s and 1990s, the focus shifted from prolonged joint immobilization towards early postoperative ROM.95-97 Since many authors believed that primary repair of the ACL was not strong enough to tolerate early mobilization, an augmentation was added to the technique in order to fortify the repair and enable early ROM.98
Interestingly, augmented repair, which is essentially a combination of primary ACL repair and ACL reconstruction, was mainly performed in the 1990s and many surgeons did recognize the role of tear location in this treatment at this point.73,98-103 In these years, the treatment algorithm consisted of augmented ACL repair in patients with proximal tears in the acute setting and ACL reconstruction in patients with midsubstance or chronic tears. Several different augmentation techniques were used to reinforce the primary repair in these years including autograft tissues (semitendinosus tendon,102-104 patellar tendon,100 or iliotibial band [ITB]105) synthetic materials (polydioxanone [PDS],101,102,106 carbon fibre,74 and polyester [Trevira]97), augmentation devices (Kennedy Ligament Augmentation Device [LAD]98-100) and extra-articular augmentations.73
When reviewing the outcomes of augmented repair of the ACL, good to excellent results can be found in studies that used this technique in patients with proximal tears.73,98-106 Kdolsky and colleagues98 were in one of the first groups that reported their results of augmented repair in only patients with proximal tears. In 1993, they reported their mid-term outcomes (5 to 8 years) in 66 patients who underwent primary repair and augmentation with the Kennedy LAD and found that 97% of patients had stable knees (<3 mm on KT-1000 examination), 98% had a negative pivot shift, and 76% returned to previous level of sports. However, often-reported problems with the augmentation devices were found in this study with rupture of the device (12%) and decreased ROM (14%).98 In 1995, Grøntvedt and Engebretsen100 compared augmentation with the Kennedy LAD to patellar tendon augmentation in a randomized study of patients with acute proximal tears. They noted that 50% of the patients in the Kennedy LAD group had a positive pivot shift compared to 23% in the patellar tendon group. Furthermore, they found KT-1000 leg differences of <3 mm in 92% of the patellar tendon group and 54% of the Kennedy LAD group. Because the authors found significant differences between both groups at 1- and 2-year follow-up, they stopped the clinical trial.
Several authors in the following years reported good results of augmented repair using autograft tissues. Natri and colleagues105 reported the outcomes of 72 patients treated with primary repair of proximal tears augmented with the ITB at 3.5-year follow-up. They found 89% negative pivot shift rate, 93% stable or nearly stable Lachman test, 99% stable or nearly stable anterior drawer test, 79% satisfaction rate, and 91% return to previous level of sports rate. Krueger-Franke and colleagues104 reported the outcomes of primary repair of proximal tears with augmentation using the semitendinosus tendon. In a retrospective study of 76 patients, they noted that 96% of patients had a negative pivot shift, 75% of patients had stable or nearly stable Lachman test, 93% were satisfied with the procedure, a mean Lysholm score of 92, a Tegner score that only decreased from 7.2 to 7.1, and KT-1000 testing with 78% <4 mm leg difference with the contralateral leg. The authors concluded that patients with femoral ruptures could be treated with augmented repair when performed in the acute setting. As this study was published in 1998, they stated that magnetic resonance imaging and arthroscopy could be helpful in identifying the tear location.
Final Abandonment of ACL Preservation
Reviewing these outcomes raises the question as to why these techniques were ultimately abandoned in the treatment algorithm of proximal ACL injuries, especially given the aforementioned advantages of ACL preservation. One of the possible answers can be found in a landmark study on ACL reconstruction and rehabilitation published by Shelbourne and colleagues107 in 1991. At that time, arthrofibrosis and knee stiffness were frequently reported problems following ACL surgery, which could partially be explained by the standard conservative rehabilitation using postoperative joint immobilization.67,70,80,88
Shelbourne and colleagues107 aimed to assess the cause of arthrofibrosis and knee stiffness, and divided the patients into groups by number of days between injury and surgery (<7, 7 to 21 days, and >21 days between injury and surgery). Furthermore, patients within these groups underwent either a conventional or accelerated rehabilitation program. The authors not only found that patients undergoing accelerated rehabilitation had less arthrofibrosis, but they also noted that less arthrofibrosis was seen when surgery was delayed. These findings, however, contrasted with the general perception that the ACL should be repaired in the first 3 weeks postinjury to ensure optimal tissue quality with an augmented approach. As a result, the treatment of ACL injuries shifted towards ACL reconstruction after these findings. Krueger-Franke and colleagues104 commented on the trend after the study of Shelbourne and colleagues:107 “Less consideration has been given to the importance of the proprioceptive receptors in the tibial remnants of the torn ACL and the value of their preservation as part of a primary reconstruction.”
In addition to the trend away from an augmented repair approach due to the novel understanding of the importance of early mobilization, some discussion should focus on the technical limitations of arthroscopy at that time. While arthroscopy had been around for several decades, fluid management and arthroscopic instrumentation was slow to develop. All of the repair and augmentation techniques previously discussed had been performed via an open arthrotomy. Arthroscopic technologies of the time were not refined enough to enable surgeons to perform such complex, intra-articular techniques that would enable suturing of the ligament remnant. In this regard, arthroscopic ACL reconstruction was a much simpler technique to accomplish, and this also likely contributed to the final abandonment of the ligament preservation approach.
Role for ACL Preservation with Modern Advances
As stated in the introduction, there has been a recent resurgence of interest in preservation of the native ligament.32-37 With the passage of time, many technologic advances have been made, which has allowed surgeons to reconsider the concept of ligament preservation.
First of all, appropriate patient selection was not applied historically, as the critical factors of tear location and tissue quality were not recognized in the era of open primary repair. In modern days, however, advances such as MRI have been developed, which can give the surgeon an idea of the status, and tear type of the ACL pre-operatively.108 This may help the orthopaedic surgeon to plan the surgery and make an assessment as to whether ACL preservation is possible. Secondly, in the historic literature the postoperative regimen consisted of casting for 5 or 6 weeks,67,70,80,88 while the focus later shifted towards early ROM.95-97Modern day ACL rehabilitation focuses on immediate ROM to avoid the complications stiffness, pain and decreased function that plagued the outcomes when immobilization was used.93,94 Thirdly, historically small tunnels were drilled with primary repair and sutures had to be tied over bone,57,67 whereas currently suture anchors are available that prevent the need for tunnel drilling and enable direct suture tensioning.32,38 Finally, and most importantly, in the historic literature patients were treated with an invasive arthrotomy technique, while modern day arthroscopic techniques readily enable the surgeon to effectively suture the remnant arthroscopically. Interestingly, in 2005, in their 20-year follow-up of primary repair surgeries, Strand and colleagues109 stated, “if the same results could be accomplished by a smaller, arthroscopic procedure, primary repair might reduce the number of patients needing later reconstructions with small ‘costs’ in the way of risk and inconvenience for the patients. We therefore believe that further research and development of methods for closed (arthroscopic) repair are justified.”
Altered Risk-Benefit Ratio
Historically, the treatments of open primary repair and open ACL reconstruction were both invasive surgeries with an arthrotomy, drilling of bone tunnels, and postoperative joint immobilization for 4 to 6 weeks. However, with the modern-day advances, the risk-benefit ratio of both treatments has changed, as Strand and colleagues109 had already suggested. Although ACL reconstruction can be performed arthroscopically, it remains an invasive procedure, in which tunnels are drilled, patellar tendons or hamstring tendons are harvested, and complications, such as knee pain and quadriceps atrophy, are common. The surgery of primary ACL repair, however, has benefited significantly from the modern developments.32,38 Primary ACL repair can now be performed arthroscopically, and by using suture anchors no tunnels need to be drilled and the remnant can be tensioned directly. An additional benefit of the use of suture anchors is that revision surgery of a failed primary repair is analogous to primary reconstruction, whereas revision surgery of a failed ACL reconstruction can be problematic due to tunnel widening, tunnel malpositioning, and preexisting hardware.20-22
Reviewing the differences between arthroscopic primary ACL repair and ACL reconstruction, it becomes clear that primary repair has benefited significantly from the modern advances and that the risk-benefit ratio for primary repair has been altered. This means that patients with proximal tears can be treated with a relatively straight forward, minimally invasive surgery, which has been shown to be effective in 85% to 90% of patients.32,38
Treatment Algorithm Based on Tear Location
Since 2008, in the practice of the senior author (GSD), the surgical treatment algorithm for ACL injuries is completely based on the tear location and tissue quality of the ligament.110,111 To describe the different tear types, we use the modified Sherman classification in which we extended his classification towards the tibial side whereas Sherman and colleagues70 only described the femoral side of the tears (Figures A-F, Table).
Type I Tears: Primary Repair
Type I tears are soft tissue avulsion type tears that can be easily treated with arthroscopic primary repair.107 The length of the distal remnant has to be at least 90% and the tissue quality has to be good to excellent in order to approximate the remnant towards the femoral wall (Table).112 The incidence of type I tears was 26% in the study of Sherman and colleagues,70 although recent studies showed a lower incidence (6% to 10%) in a larger population.32,38 Certainly, individual practices will see different percentages of type I tears based upon the mix of injury mechanisms they see most frequently. Over the last 2 years, with the recognition of the importance of tear type and tissue quality, there has been a renewed interest in arthroscopic primary ACL repair.32,38
DiFelice and colleagues32 were the first to arthroscopically perform primary repair of the ACL in proximal tears using suture anchors. They reported the outcomes of the first 11 consecutive patients that underwent primary repair in a previously described technique.113 At mean 3.5-year follow-up, they noted only 1 failure (9%) due to re-injury; mean Lysholm score of 93.2; mean modified Cincinnati score of 91.5; pre- and postoperative Tegner score of 7.3 and 6.9, respectively; SANE score of 91.8; and subjective International Knee Documentation Committee (IKDC) score of 86.4. Of the patients with an intact repair, 9 patients had an objective IKDC rating A and 1 patient had B and all patients had KT-1000 leg differences of <3 mm with the contralateral side (three patients were not available for KT-1000 testing). The authors concluded that arthroscopic primary ACL repair could achieve short-term clinical success in a selected group of patients with proximal avulsion tears and excellent tissue quality. They further noted that mid-term outcomes are necessary given that the results of open primary repair deteriorated at longer-term follow-up in the historical literature. Recently, the senior author (GSD) has added an Internal Brace (Arthrex) to the primary repair with the goal of protecting the ligament in the first weeks to further promote healing of the ligament.39,40,114
More recently, Achtnich and colleagues38 compared the treatment of arthroscopic primary ACL repair with primary ACL reconstruction in 41 patients with type I tears at 2.3-years follow-up. Twenty-one patients consented for primary repair while 20 patients declined this procedure and underwent primary reconstruction. They noted no significant differences in Lachman test, pivot shift test, objective IKDC score, and KT-1000 scores. Although not significant, the clinical failure rate in the primary repair group (15%) was higher than the reconstruction group (0%). Interestingly, despite the higher failure rate in the repair group, the authors concluded that primary ACL repair is recommended in a carefully selected group of patients with type I tears and excellent tissue quality, which can likely be explained by the differences in the risk-benefit ratio between both procedures.
Over the last decade, the research group led by Murray46,115,116 has performed experimental research on primary repair with a biological scaffold and reported many interesting findings that could be extrapolated to primary ACL repair. First of all, they compared bioenhanced primary repair with bioenhanced primary reconstruction in 64 Yucutan pigs and noted that there was significantly less macroscopic cartilage damage in the primary repair group at 1-year follow-up.46 They concluded that bioenhanced ACL repair may provide a new, less invasive treatment option that reduces cartilage damage following joint injury. This may suggest that primary repair may have a lower incidence of osteoarthritis when compared to ACL reconstruction, which is interesting as osteoarthritis is very common after ACL reconstruction. Further research in this area is certainly warranted.
In another study they compared bioenhanced primary repair in juvenile, adolescent and mature Yucutan pigs and noted that functional healing depended on the level of skeletal maturity with immature animals having a more productive healing response.116 This indicates that primary repair might be a good treatment option in skeletally immature patients, especially since reconstruction increases the risk of premature closure of the epiphysis117,118 and delaying treatment increases the risk of meniscus injury.119 Interestingly, a recent meta-analysis showed indeed that the risk of epiphysis closure was lower in primary repair when compared to ACL reconstruction and the rupture rate was also lower.118 Primary repair may be a good treatment option in children as the procedure has all the attributes that should be applicable to children: it is minimally morbid, tissue sparing, and it is a conservative approach that does not burn any surgical bridges for future reconstructive surgery if necessary.
Finally, the research group of Murray115 assessed the effect of surgical delay of primary repair following injury in Yucutan pigs and noted that better biomechanical outcomes were noted after delaying surgery for 2 weeks when compared to 6 weeks. This suggests that primary repair should preferably be performed in the acute setting, which has also been shown in historical studies since the ligament in the acute setting has optimal tissue quality and the ligament is less likely to be retracted or reabsorbed.59,60,115
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the tear locations of the AM and PL bundle are not at the same location and Zantop and colleagues120 reported in an arthroscopic study that this could be as frequent as in 30% of all complete tears. In some of these tears, one of the bundles can be avulsed of the femoral wall (type I tear) while the other bundle is not directly repairable (non-type I tear). In these cases, the senior author (GSD) will repair the type I tear bundle, whereas a hamstring augmentation is placed at the location of the other bundle. When reviewing the literature, a combination of primary repair of one bundle and reconstruction of the second bundle has not been described before. However, over the last decade several surgeons have performed augmentation of one bundle in the setting of partial tears.34,35,121-124
Buda and colleagues34 were the first to perform selective AM or PL bundle reconstruction in the setting of partial tears.34 At 5-year follow-up, they reported no reruptures and only 1 patient with an IKDC C-score, although reoperation was necessary in 4 out of 47 patients (9%). Following this publication, many others reported on selective bundle reconstruction.35,121-124 However, with partial tears, the knee is often stable and a selective augmentation technique is utilized to prevent complete rupture of the ligament. The application of this technique is essentially different from reconstruction for complete ACL tears in which the knee is unstable, there is a giving way sensation and patients have problems participating in sports.
Type II Tears: Augmented Repair
Type II tears often have good or excellent tissue quality and can be pulled up towards the femoral footprint, but are too short to be firmly attached. Sherman and colleagues70 reported that approximately 22% patients had a type II tear, which corresponds to a tear located in the proximal part of the ligament. With this technique, multiple suture passes are used to stitch the remnant and, in addition, a smaller hamstring autograft or allograft is passed through the middle of the tibial remnant. A suture button is used proximally for the graft, and the tensioning repair sutures through the remnant are also passed through the suture button. The suture button is passed through the femoral tunnel and flipped so that the graft is proximally fixed. Then, the repair sutures of the remnant are tensioned, and the ligament is pulled towards the femoral wall as a sleeve around the graft. When the ligament is approximated to the femoral wall, the sutures are tied over the suture button. The graft is then tensioned distally to complete the augmented repair.
In the recent literature, the technique of augmentation of a primary repair using autograft tissue has not been reported. However, augmented repair using an internal brace39,40 or augmentation devices33,41 have been recently performed. MacKay and colleagues39 reported good outcomes of arthroscopic primary repair of proximal tears using an internal brace. Eggli and colleagues33 reported the results of the first 10 patients treated with ACL preservation using primary repair of the ligament with the addition of a dynamic screw-spring mechanism. The authors reported good preliminary results with one failure (10%) and good objective and subjective outcomes. In a next study, they reported the outcomes of 278 patients and although they reported good clinical outcomes and a revision rate of 4%, the reoperation rate for removal of the screw-spring mechanism was high (24%).41 This is not surprising when reviewing the historical literature in which high complication rates of the augmentation devices were reported.99,100 We were unable to identify any other studies reporting surgical techniques of augmenting primary repair in the literature.
Type III Tears: Reconstruction With Remnant Tensioning
In patients with type III tears, the ligament cannot be approximated to the wall and reconstruction is necessary in order to restore knee stability. However, in these cases the ligament has sufficient length (25%-75%) and can be tensioned along or around the graft. Preservation of the ligament remnant has several (theoretical) advantages, such as better proprioceptive function,42,49,50 vascularization and ligamentization of the graft,50-52 an optical guide for anatomic tunnel placement,53 and a decreased incidence of tunnel widening.54,55 Furthermore, tensioning of the remnant is thought to lower the risk of cyclops lesions when compared to remnant preservation.125 Although the difference between augmented repair and remnant tensioning seems small, the purpose of surgery is different. With augmented repair, the ligament can be approximated close to the femoral wall and the goal of surgery is to use the healing capacity that the ACL has in the proximal part of the ligament,126 while with remnant tensioning the goal is only to benefit from some of the aforementioned advantages. Ahn and colleagues36 were the first to perform this technique and stated, “Our concept is that the remnant tissue has only an additive effect.” Furthermore, with augmented repair multiple sutures are passed through the AM and PL bundle in order to sufficiently approximate the ligament to the femoral wall, while with the remnant tensioning technique generally one or a few sutures or lasso loop are passed through the proximal part to tension the ligament, prevent sagging of the remnant, and decrease the risk of cyclops lesions.127,128
Several authors have recently performed remnant tensioning during ACL.36,47,125-127 Ahn and colleagues47 reported excellent objective and subjective outcomes following this procedure and found that with re-arthroscopy nearly all patients had fair synovialization of the graft. Others have reported similarly good outcomes of these techniques.125,129,130 However, studies comparing this treatment with normal ACL reconstruction and assessing outcomes, failure rates and proprioception are lacking.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some patients the ligament is torn distally or the tissue quality is not optimal. In these patients, the remnant can be debrided to the part of good tissue quality in order to preserve the biology and minimize the risk for cyclops lesions. A standard reconstruction needs to be performed to restore the instability, but by preserving the remnant, advantages, such as proprioception,44,49,50 graft vascularization,50-52 an optical guide for tibial tunnel placement,53 and a decreased incidence of tunnel widening54,55 can be expected.
Lee and colleagues37 presented the tibial remnant technique in which standard reconstruction was performed, and the tibial tunnel was drilled through the center of the remnant. In a later study, they compared remnant preservation with a remnant of <20% of the total ACL length with >20% of the length and found that proprioception was better with more remnant volume.48 Similarly, Muneta and colleagues131 assessed the role of remnant length and found that remnant length is positively correlated with better stability measured on KT-1000 anteroposterior stability.
Several studies compared ACL reconstruction with remnant preservation vs conventional ACL reconstruction.52,54,129 Takazawa and colleagues52 performed a retrospective study of 183 patients and found that patients in the remnant preservation group had significantly better KT-2000 stability, while they also reported a significantly lower graft rupture rate in this group (1.1% vs 7.1%) at 2-year follow-up. Hong and colleagues129 performed a randomized clinical trial of 80 patients and did not find these differences, although there was a trend towards higher Lysholm scores in the remnant preservation group. Finally, Zhang and colleagues54 performed a randomized clinical trial and found a lower incidence and amount of tibial tunnel widening in the preserving-remnant group when compared to the removing-remnant group. These studies show that there is likely a role for remnant preservation.
Type V Tears: Primary Repair
In some patients, the ligament is torn in the distal 10% of the ligament, which can occur as a distal avulsion tear or as a distal bony avulsion fracture.132 Bony avulsion fractures are most commonly seen in children whereas true distal soft tissue avulsion tears are very rare.132
Treatments of these tear types include antegrade screw fixation, pullout sutures or the use of suture anchors in case of bony avulsion fractures and pullout sutures with tying over a bony bridge or ligament button in case of soft tissue avulsions. Leeberg and colleagues132 recently performed a systematic review of all studies reporting on treatment of distal avulsion fractures.They noted that most treatments were currently performed arthroscopically and that outcomes were generally good. Another recent biomechanical study compared antegrade screw fixation with suture anchor fixation and pullout suture fixation.133 The authors noted that suture anchor fixation has slightly less displacement of the bony fragment when compared to screw fixation and pull-out sutures, and that the strength to failure was higher in the suture anchor fixation when compared to the pullout suture fixation. The outcomes of this study suggest that screw fixation and suture anchor fixation might be superior to pullout suture fixation, which might be interesting as with pullout suture fixation the ligament cannot be directly tensioned to the tibial footprint, which can lead to anteroposterior laxity.132 Clinical studies are necessary to assess the preferred treatment in these tear types but it seems that screw fixation is preferred in large bony avulsion fractures, while suture anchor fixation or pullout suture fixation can be used for soft tissue avulsion tears.
Complex Tears or Poor Tissue Quality: Reconstruction
If the tear is complex, multiple tears are present, or the tissue quality is poor, then preservation of the ligament is not possible, and in these cases a standard reconstruction should be performed.
Conclusion
When reviewing the literature of ACL preservation, it becomes clear that the evolution of surgical treatment of ACL injuries was biased. Preservation of the native ligament has many advantages, such as better proprioception, graft vascularization, an optical guide for tibial tunnel placement, and a decreased incidence of tunnel widening that can be expected. Furthermore, arthroscopic primary ACL repair is minimally invasive and does not burn any bridges for future reconstructions, if necessary. This is in addition to the other (theoretical) advantages of primary repair, such as restoration of native kinematics and a decreased risk of osteoarthritis. Modern advances have significantly changed the risk-benefit ratio that should make us reconsider ACL preservation approaches. Certainly, further research in this area is warranted. In this article we have presented a treatment algorithm for ACL preservation, which is based on tear location and remnant tissue quality.
Am J Orthop. 2016;45(7):E393-E405. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370.
2. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502-1507.
3. Ciccotti MG, Lombardo SJ, Nonweiler B, Pink M. Non-operative treatment of ruptures of the anterior cruciate ligament in middle-aged patients. Results after long-term follow-up. J Bone Joint Surg Am. 1994;76(9):1315-1321.
4. Sanders TL, Pareek A, Kremers HM, et al. Long-term follow-up of isolated ACL tears treated without ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016 May 24. [Epub ahead of print]
5. Irarrázaval S, Kurosaka M, Cohen M, Fu FH. Anterior cruciate ligament reconstruction. J ISAKOS. 2016;1(1):38-52.
6. Gabler CM, Jacobs CA, Howard JS, Mattacola CG, Johnson DL. Comparison of graft failure rate between autografts placed via an anatomic anterior cruciate ligament reconstruction technique: a systematic review, meta-analysis, and meta-regression. Am J Sports Med. 2016;44(4):1069-1079.
7. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287-1297.
8. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind M. Comparison of hamstring tendon and patellar tendon grafts in anterior cruciate ligament reconstruction in a nationwide population-based cohort study: results from the danish registry of knee ligament reconstruction. Am J Sports Med. 2014;42(2):278-284.
9. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100-110.
10. Andernord D, Desai N, Björnsson H, Gillén S, Karlsson J, Samuelsson K. Predictors of contralateral anterior cruciate ligament reconstruction: a cohort study of 9061 patients with 5-year follow-up. Am J Sports Med. 2015;43(2):295-302.
11. Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43(3):641-647.
12. Kim SJ, Postigo R, Koo S, Kim JH. Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics. 2014;37(7):477-484.
13. Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS. Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1392-1401.
14. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84-A(9):1560-1572.
15. Ardern CL, Österberg A, Sonesson S, Gauffin H, Webster KE, Kvist J. Satisfaction with knee function after primary anterior cruciate ligament reconstruction is associated with self-efficacy, quality of life, and returning to the preinjury physical activity. Arthroscopy. 2016;32(8):1631-1638.e3.
16. Grant JA, Mohtadi NG, Maitland ME, Zernicke RF. Comparison of home versus physical therapy-supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. Am J Sports Med. 2005;33(9):1288-1297.
17. Lindström M, Strandberg S, Wredmark T, Fell änder-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431-442.
18. Biau DJ, Tournoux C, Katsahian S, Schranz PJ, Nizard RS. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta-analysis. BMJ. 2006;332(7548):995-1001.
19. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
20. Aga C, Wilson KJ, Johansen S, Dornan G, La Prade RF, Engebretsen L. Tunnel widening in single- versus double-bundle anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2016 Jun 21. [Epub ahead of print]
21. Maak TG, Voos JE, Wickiewicz TL, Warren RF. Tunnel widening in revision anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2010;18(11):695-706.
22. Cheatham SA, Johnson DL. Anticipating problems unique to revision ACL surgery. Sports Med Arthrosc. 2013;21(2):129-134.
23. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
24. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
25. Andriolo L, Filardo G, Kon E, et al. Revision anterior cruciate ligament reconstruction: clinical outcome and evidence for return to sport. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2825-2845.
26. Grassi A, Ardern CL, Marcheggiani Muccioli GM, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br J Sports Med. 2016;50(12):716-724.
27. Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1323-1329.
28. Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447-457.
29. Imhauser C, Mauro C, Choi D, et al. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013;41(4):815-825.
30. Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242-2252.
31. Chalmers PN, Mall NA, Moric M, et al. Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292-300.
32. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
33. Eggli S, Kohlhof H, Zumstein M, et al. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1215-1221.
34. Buda R, Ferruzzi A, Vannini F, Zambelli L, Di Caprio F. Augmentation technique with semitendinosus and gracilis tendons in chronic partial lesions of the ACL: clinical and arthrometric analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1101-1107.
35. Ochi M, Adachi N, Uchio Y, et al. A minimum 2-year follow-up after selective anteromedial or posterolateral bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(2):117-122.
36. Ahn JH, Lee YS, Ha HC. Anterior cruciate ligament reconstruction with preservation of remnant bundle using hamstring autograft: technical note. Arch Orthop Trauma Surg. 2009;129(8):1011-1015.
37. Lee BI, Min KD, Choi HS, Kim JB, Kim ST. Arthroscopic anterior cruciate ligament reconstruction with the tibial-remnant preserving technique using a hamstring graft. Arthroscopy. 2006;22(3):340.e1-e7.
38. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016 Jun 17. [Epub ahead of print]
39. MacKay G, Anthony IC, Jenkins PJ, Blyth M. Anterior cruciate ligament repair revisited. Preliminary results of primary repair with internal brace ligament augmentation: a case series. Orthop Muscul Syst. 2015;4:188.
40. Mackay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
41. Henle P, Röder C, Perler G, Heitkemper S, Eggli S. Dynamic intraligamentary stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord. 2015;16:27.
42. Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002;73(3):330-334.
43. Gao F, Zhou J, He C, et al. A morphologic and quantitative study of mechanoreceptors in the remnant stump of the human anterior cruciate ligament. Arthroscopy. 2016;32(2):273-280.
44. Georgoulis AD, Pappa L, Moebius U, et al. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg Sports Traumatol Arthrosc. 2001;9(6):364-368.
45. Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500-1505.
46. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762-1770.
47. Ahn JH, Wang JH, Lee YS, Kim JG, Kang JH, Koh KH. Anterior cruciate ligament reconstruction using remnant preservation and a femoral tensioning technique: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(8):1079-1089.
48. Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008;24(5):560-568.
49. Lee BI, Min KD, Choi HS, et al. Immunohistochemical study of mechanoreceptors in the tibial remnant of the ruptured anterior cruciate ligament in human knees. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1095-1101.
50. Takahashi T, Kondo E, Yasuda K, et al. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: a biomechanical and histological study. Am J Sports Med. 2016;44(7):1708-1716.
51. Dong S, Xie G, Zhang Y, Shen P, Huangfu X, Zhao J. Ligamentization of autogenous hamstring grafts after anterior cruciate ligament reconstruction: midterm versus long-term results. Am J Sports Med. 2015;43(8):1908-1917.
52. Takazawa Y, Ikeda H, Kawasaki T, et al. ACL reconstruction preserving the ACL remnant achieves good clinical outcomes and can reduce subsequent graft rupture. Orthop J Sports Med. 2013;1(4):2325967113505076.
53. Shimodaira H, Tensho K, Akaoka Y, Takanashi S, Kato H, Saito N. Remnant-preserving tibial tunnel positioning using anatomic landmarks in double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2016;32(9):1822-1830.
54. Zhang Q, Zhang S, Cao X, Liu L, Liu Y, Li R. The effect of remnant preservation on tibial tunnel enlargement in ACL reconstruction with hamstring autograft: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):166-173.
55. Tie K, Chen L, Hu D, Wang H. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: A meta-analysis. Knee. 2016;23(4):566-574.
56. Robson AW. VI. Ruptured crucial ligaments and their repair by operation. Ann Surg. 1903;37(5):716-718.
57. Palmer I. On the injuries to the ligaments of the knee joint. Acta Orthop Scand. 1938;53.
58. Palmer I. On the injuries to the ligaments of the knee joint: a clinical study. 1938. Clin Orthop Relat Res. 2007;454:17-22.
59 O’Donoghue DH. An analysis of end results of surgical treatment of major injuries to the ligaments of the knee. J Bone Joint Surg Am. 1955;37-A(1):1-13.
60. O’Donoghue DH. Surgical treatment of fresh injuries to the major ligaments of the knee. J Bone Joint Surg Am. 1950;32 A(4):721-738.
61. Feagin JA, Abbott HG, Rokous JR. The isolated tear of the anterior cruciate ligament. J Bone Joint Surg Am. 1972;54-A:1340-1341.
62. England RL. Repair of the ligaments about the knee. Orthop Clin North Am. 1976;7(1):195-204.
63. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
64. Weaver JK, Derkash RS, Freeman JR, Kirk RE, Oden RR, Matyas J. Primary knee ligament repair--revisited. Clin Orthop Relat Res. 1985;(199):185-191.
65. Nogalski MP, Bach BR Jr. A review of early anterior cruciate ligament surgical repair or reconstruction. Results and caveats. Orthop Rev. 1993;22(11):1213-1223.
66. Feagin JA Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4(3):95-100.
67. Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10(2):103-107.
68. Straub T, Hunter RE. Acute anterior cruciate ligament repair. Clin Orthop Relat Res. 1988;227:238-250.
69. Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med. 1990;18(4):354-358.
70. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
71. Paar O. Use of semitendinosus tendon to strengthen a freshly repaired anterior cruciate ligament. Chirurg. 1985;56(11):728-734.
72. Aglietti P, Buzzi R, Pisaneschi A, Salvi M. Comparison between suture and augmentation with the semitendinosus tendon in the repair of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1986;8(4):217-231.
73. Higgins RW, Steadman JR. Anterior cruciate ligament repairs in world class skiers. Am J Sports Med. 1987;15(5):439-447.
74. Harilainen A, Myllynen P. Treatment of fresh tears of the anterior cruciate ligament. A comparison of primary suture and augmentation with carbon fibre. Injury. 1987;18(6):396-400.
75. Jones KG. Results of use of the central one-third of the patellar ligament to compensate for anterior cruciate ligament deficiency. Clin Orthop Relat Res. 1980;(147):39-44.
76. Puddu G. Method for reconstruction of the anterior cruciate ligament using the semitendinosus tendon. Am J Sports Med. 1980;8(6):402-404.
77. Hefti F, Gächter A, Jenny H, Morscher E. Replacement of the anterior cruciate ligament. a comparative study of four different methods of reconstruction. Arch Orthop Trauma Surg. 1982;100(2):83-94.
78. Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament. A randomized study with short-term follow-up observations. Clin Orthop Relat Res. 1985;(198):87-93.
79. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
80. Engebretsen L, Benum P, Fasting O, Mølster A, Strand T. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med. 1990;18(6):585-590.
81. Jonsson T, Peterson L, Renström P. Anterior cruciate ligament repair with and without augmentation. A prospective 7-year study of 51 patients. Acta Orthop Scand. 1990;61(6):562-566.
82. Andersson C, Odensten M, Gillquist J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: a randomized study with a long-term follow-up period. Clin Orthop Relat Res. 1991;(264):255-263.
83. Heim U, Bachmann B, Infanger K. Reinsertion of the anterior cruciate ligament or primary ligamentous plasty? Helv Chir Acta. 1982;48(5):703-708.
84. Strand T, Engesaeter LB, Mølster AO, et al. Knee function following suture of fresh tear of the anterior cruciate ligament. Acta Orthop Scand. 1984;55(2):181-184.
85. Marcacci M, Spinelli M, Chiellini F, Buccolieri V. Notes on 53 cases of immediate suture of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1985;7(2):69-79.
86. van der List JP, DiFelice GS. Primary repair of the anterior cruciate ligament: a paradigm shift. Surgeon. 2016 Oct 6. [Epub ahead of print]
87. Bräm J, Plaschy S, Lütolf M, Leutenegger A. [The primary cruciate ligament suture--is the method outdated? Results in follow-up of 58 patients]. Z Unfallchir Versicherungsmed. 1994;87(2):91-109.
88. Genelin F, Trost A, Primavesi C, Knoll P. Late results following proximal reinsertion of isolated ruptured ACL ligaments. Knee Surg Sports Traumatol Arthrosc. 1993;1(1):17-19.
89. Kühne JH, Theermann R, Neumann R, Sagasser J. [Acute uncomplicated anterior knee instability. 2-5 year follow-up of surgical treatment]. Unfallchirurg. 1991;94(2):81-87.
90. Simonet WT, Sim FH. Repair and reconstruction of rotatory instability of the knee. Am J Sports Med. 1984;12(2):89-97.
91. Raunest J, Derra E, Ohmann C. [Clinical results of Palmer’s primary cruciate ligament insertion without augmentation]. Unfallchirurgie. 1991;17(3):166-174.
92. Frank C, Beaver P, Rademaker F, Becker K, Schachar N, Edwards G. A computerized study of knee-ligament injuries: repair versus removal of the torn anterior cruciate ligament. Can J Surg. 1982;25(4):454-458.
93. Enneking WF, Horowitz M. The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am. 1972;54(5):973-985.
94. Millett PJ, Wickiewicz TL, Warren RF. Motion loss after ligament injuries to the knee. Part I: causes. Am J Sports Med. 2001;29(5):664-675.
95. Bilko TE, Paulos LE, Feagin JA Jr, Lambert KL, Cunningham HR. Current trends in repair and rehabilitation of complete (acute) anterior cruciate ligament injuries. Analysis of 1984 questionnaire completed by ACL Study Group. Am J Sports Med. 1986;14(2):143-147.
96. Paulos L, Noyes FR, Grood E, Butler DL. Knee rehabilitation after anterior cruciate ligament reconstruction and repair. J Orthop Sports Phys Ther. 1991;13(2):60-70.
97. Paessler HH, Deneke J, Dahners LE. Augmented repair and early mobilization of acute anterior cruciate ligament injuries. Am J Sports Med. 1992;20(6):667-674.
98.
99. Kdolsky RK, Gibbons DF, Kwasny O, Schabus R, Plenk H Jr. Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res. 1997;15(1):1-10.
100. Grøntvedt T, Engebretsen L. Comparison between two techniques for surgical repair of the acutely torn anterior cruciate ligament. A prospective, randomized follow-up study of 48 patients. Scand J Med Sci Sports. 1995;5(6):358-363.
101. Hehl G, Strecker W, Richter M, Kiefer H, Wissmeyer T. Clinical experience with PDS II augmentation for operative treatment of acute proximal ACL ruptures--2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):102-106.
102. Schenk S, Landsiedl F, Enenkel M. Arthroscopic single-stranded semitendinosus tendon- versus PDS-augmentation of reinserted acute femoral anterior cruciate ligament tears: 7 year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):318-324.
103. Zysk SP, Refior HJ. Operative or conservative treatment of the acutely torn anterior cruciate ligament in middle-aged patients. A follow-up study of 133 patients between the ages of 40 and 59 years. Arch Orthop Trauma Surg. 2000;120(1-2):59-64.
104. Krueger-Franke M, Siebert CH, Schupp A. Refixation of femoral anterior cruciate ligament tears combined with a semitendinosus tendon augmentation. Technique and results. Arch Orthop Trauma Surg. 1998;117(1-2):68-72.
105. Natri A, Järvinen M, Kannus P. Primary repair plus intra-articular iliotibial band augmentation in the treatment of an acute anterior cruciate ligament rupture. A follow-up study of 70 patients. Arch Orthop Trauma Surg. 1996;115(1):22-27.
106. Träger D, Pohle K, Tschirner W. Anterior cruciate ligament suture in comparison with plasty. A 5-year follow-up study. Arch Orthop Trauma Surg. 1995;114(5):278-280.
107. Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332-336.
108. Volokhina YV, Syed HM, Pham PH, Blackburn AK. Two helpful MRI signs for evaluation of posterolateral bundle tears of the anterior cruciate ligament: a pilot study. Orthop J Sports Med. 2015;3(8):2325967115597641.
109. Strand T, Mølster A, Hordvik M, Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years postoperatively. Arch Orthop Trauma Surg. 2005;125(4):217-221.
110. van der List JP, DiFelice GS. Successful arthroscopic primary repair of a chronic anterior cruciate ligament tear 11 years following injury. HSS J. 2016. In press.
111. van der List JP, DiFelice GS. The role of ligament repair in anterior cruciate ligament surgery. In: Mascarenhas R, Bhatia S, Lowe WR, eds. Ligamentous Injuries of the Knee. 1st ed. Houston: Nova Science Publishers; 2016:199-220.
112. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
113. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
114. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
115. Magarian EM, Fleming BC, Harrison SL, Mastrangelo AN, Badger GJ, Murray MM. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med. 2010;38(12):2528-2534.
116. Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039-2049.
117. Werner BC, Yang S, Looney AM, Gwathmey FW Jr. Trends in pediatric and adolescent anterior cruciate ligament iInjury and reconstruction. J Pediatr Orthop. 2016;36(5):447-452.
118. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
119. Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ. Anterior cruciate ligament tears in children and adolescents: a meta-analysis of nonoperative versus operative treatment. Am J Sports Med. 2014;42(11):2769-2776.
120. Zantop T, Brucker PU, Vidal A, Zelle BA, Fu FH. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;454:48-53.
121. Yoon KH, Bae DK, Cho SM, Park SY, Lee JH. Standard anterior cruciate ligament reconstruction versus isolated single-bundle augmentation with hamstring autograft. Arthroscopy. 2009;25(11):1265-1274.
122 Demirağ B, Ermutlu C, Aydemir F, Durak K. A comparison of clinical outcome of augmentation and standard reconstruction techniques for partial anterior cruciate ligament tears. Eklem Hastalik Cerrahisi. 2012;23(3):140-144.
123. Sonnery-Cottet B, Zayni R, Conteduca J, et al. Posterolateral bundle reconstruction with anteromedial bundle remnant preservation in ACL tears: clinical and MRI evaluation of 39 patients with 24-month follow-up. Orthop J Sports Med. 2013;1(3):2325967113501624.
124. Sabat D, Kumar V. Partial tears of anterior cruciate ligament: results of single bundle augmentation. Indian J Orthop. 2015;49(2):129-135.
125. Jung YB, Jung HJ, Siti HT, et al. Comparison of anterior cruciate ligament reconstruction with preservation only versus remnant tensioning technique. Arthroscopy. 2011;27(9):1252-1258.
126. Nguyen DT, Ramwadhdoebe TH, van der Hart CP, Blankevoort L, Tak PP, van Dijk CN. Intrinsic healing response of the human anterior cruciate ligament: an histological study of reattached ACL remnants. J Orthop Res. 2014;32(2):296-301.
127. Boutsiadis A, Karampalis C, Tzavelas A, Vraggalas V, Christodoulou P, Bisbinas I. Anterior cruciate ligament remnant-preserving reconstruction using a “lasso-loop” knot configuration. Arthrosc Tech. 2015;4(6):e741-e746.
128. Noh JH, Yoon KH, Song SJ, Roh YH. Re-tensioning technique to cover the graft with remnant in anterior cruciate ligament reconstruction. Arthrosc Tech. 2014;3(6):e679-e682.
129. Hong L, Li X, Zhang H, et al. Anterior cruciate ligament reconstruction with remnant preservation: a prospective, randomized controlled study. Am J Sports Med. 2012;40(12):2747-2755.
130. Noh JH, Kyung HS, Roh YH, Kang TS. Remnant-preserving and re-tensioning technique to cover the graft in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 12. [Epub ahead of print]
131. Muneta T, Koga H, Ju YJ, Horie M, Nakamura T, Sekiya I. Remnant volume of anterior cruciate ligament correlates preoperative patients’ status and postoperative outcome. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):906-913.
132. Leeberg V, Lekdorf J, Wong C, Sonne-Holm S. Tibial eminentia avulsion fracture in children - a systematic review of the current literature. Dan Med J. 2014;61(3):A4792.
133. In Y, Kwak DS, Moon CW, Han SH, Choi NY. Biomechanical comparison of three techniques for fixation of tibial avulsion fractures of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1470-1478.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370.
2. Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med. 2016;44(6):1502-1507.
3. Ciccotti MG, Lombardo SJ, Nonweiler B, Pink M. Non-operative treatment of ruptures of the anterior cruciate ligament in middle-aged patients. Results after long-term follow-up. J Bone Joint Surg Am. 1994;76(9):1315-1321.
4. Sanders TL, Pareek A, Kremers HM, et al. Long-term follow-up of isolated ACL tears treated without ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2016 May 24. [Epub ahead of print]
5. Irarrázaval S, Kurosaka M, Cohen M, Fu FH. Anterior cruciate ligament reconstruction. J ISAKOS. 2016;1(1):38-52.
6. Gabler CM, Jacobs CA, Howard JS, Mattacola CG, Johnson DL. Comparison of graft failure rate between autografts placed via an anatomic anterior cruciate ligament reconstruction technique: a systematic review, meta-analysis, and meta-regression. Am J Sports Med. 2016;44(4):1069-1079.
7. Li S, Chen Y, Lin Z, Cui W, Zhao J, Su W. A systematic review of randomized controlled clinical trials comparing hamstring autografts versus bone-patellar tendon-bone autografts for the reconstruction of the anterior cruciate ligament. Arch Orthop Trauma Surg. 2012;132(9):1287-1297.
8. Rahr-Wagner L, Thillemann TM, Pedersen AB, Lind M. Comparison of hamstring tendon and patellar tendon grafts in anterior cruciate ligament reconstruction in a nationwide population-based cohort study: results from the danish registry of knee ligament reconstruction. Am J Sports Med. 2014;42(2):278-284.
9. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100-110.
10. Andernord D, Desai N, Björnsson H, Gillén S, Karlsson J, Samuelsson K. Predictors of contralateral anterior cruciate ligament reconstruction: a cohort study of 9061 patients with 5-year follow-up. Am J Sports Med. 2015;43(2):295-302.
11. Maletis GB, Inacio MC, Funahashi TT. Risk factors associated with revision and contralateral anterior cruciate ligament reconstructions in the Kaiser Permanente ACLR registry. Am J Sports Med. 2015;43(3):641-647.
12. Kim SJ, Postigo R, Koo S, Kim JH. Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics. 2014;37(7):477-484.
13. Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS. Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(7):1392-1401.
14. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84-A(9):1560-1572.
15. Ardern CL, Österberg A, Sonesson S, Gauffin H, Webster KE, Kvist J. Satisfaction with knee function after primary anterior cruciate ligament reconstruction is associated with self-efficacy, quality of life, and returning to the preinjury physical activity. Arthroscopy. 2016;32(8):1631-1638.e3.
16. Grant JA, Mohtadi NG, Maitland ME, Zernicke RF. Comparison of home versus physical therapy-supervised rehabilitation programs after anterior cruciate ligament reconstruction: a randomized clinical trial. Am J Sports Med. 2005;33(9):1288-1297.
17. Lindström M, Strandberg S, Wredmark T, Fell änder-Tsai L, Henriksson M. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports. 2013;23(4):431-442.
18. Biau DJ, Tournoux C, Katsahian S, Schranz PJ, Nizard RS. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: meta-analysis. BMJ. 2006;332(7548):995-1001.
19. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
20. Aga C, Wilson KJ, Johansen S, Dornan G, La Prade RF, Engebretsen L. Tunnel widening in single- versus double-bundle anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2016 Jun 21. [Epub ahead of print]
21. Maak TG, Voos JE, Wickiewicz TL, Warren RF. Tunnel widening in revision anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2010;18(11):695-706.
22. Cheatham SA, Johnson DL. Anticipating problems unique to revision ACL surgery. Sports Med Arthrosc. 2013;21(2):129-134.
23. Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199-217.
24. Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94(6):531-536.
25. Andriolo L, Filardo G, Kon E, et al. Revision anterior cruciate ligament reconstruction: clinical outcome and evidence for return to sport. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2825-2845.
26. Grassi A, Ardern CL, Marcheggiani Muccioli GM, Neri MP, Marcacci M, Zaffagnini S. Does revision ACL reconstruction measure up to primary surgery? A meta-analysis comparing patient-reported and clinician-reported outcomes, and radiographic results. Br J Sports Med. 2016;50(12):716-724.
27. Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1323-1329.
28. Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447-457.
29. Imhauser C, Mauro C, Choi D, et al. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013;41(4):815-825.
30. Ajuied A, Wong F, Smith C, et al. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42(9):2242-2252.
31. Chalmers PN, Mall NA, Moric M, et al. Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292-300.
32. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
33. Eggli S, Kohlhof H, Zumstein M, et al. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1215-1221.
34. Buda R, Ferruzzi A, Vannini F, Zambelli L, Di Caprio F. Augmentation technique with semitendinosus and gracilis tendons in chronic partial lesions of the ACL: clinical and arthrometric analysis. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1101-1107.
35. Ochi M, Adachi N, Uchio Y, et al. A minimum 2-year follow-up after selective anteromedial or posterolateral bundle anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(2):117-122.
36. Ahn JH, Lee YS, Ha HC. Anterior cruciate ligament reconstruction with preservation of remnant bundle using hamstring autograft: technical note. Arch Orthop Trauma Surg. 2009;129(8):1011-1015.
37. Lee BI, Min KD, Choi HS, Kim JB, Kim ST. Arthroscopic anterior cruciate ligament reconstruction with the tibial-remnant preserving technique using a hamstring graft. Arthroscopy. 2006;22(3):340.e1-e7.
38. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016 Jun 17. [Epub ahead of print]
39. MacKay G, Anthony IC, Jenkins PJ, Blyth M. Anterior cruciate ligament repair revisited. Preliminary results of primary repair with internal brace ligament augmentation: a case series. Orthop Muscul Syst. 2015;4:188.
40. Mackay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
41. Henle P, Röder C, Perler G, Heitkemper S, Eggli S. Dynamic intraligamentary stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord. 2015;16:27.
42. Adachi N, Ochi M, Uchio Y, Iwasa J, Ryoke K, Kuriwaka M. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand. 2002;73(3):330-334.
43. Gao F, Zhou J, He C, et al. A morphologic and quantitative study of mechanoreceptors in the remnant stump of the human anterior cruciate ligament. Arthroscopy. 2016;32(2):273-280.
44. Georgoulis AD, Pappa L, Moebius U, et al. The presence of proprioceptive mechanoreceptors in the remnants of the ruptured ACL as a possible source of re-innervation of the ACL autograft. Knee Surg Sports Traumatol Arthrosc. 2001;9(6):364-368.
45. Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500-1505.
46. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762-1770.
47. Ahn JH, Wang JH, Lee YS, Kim JG, Kang JH, Koh KH. Anterior cruciate ligament reconstruction using remnant preservation and a femoral tensioning technique: clinical and magnetic resonance imaging results. Arthroscopy. 2011;27(8):1079-1089.
48. Lee BI, Kwon SW, Kim JB, Choi HS, Min KD. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy. 2008;24(5):560-568.
49. Lee BI, Min KD, Choi HS, et al. Immunohistochemical study of mechanoreceptors in the tibial remnant of the ruptured anterior cruciate ligament in human knees. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1095-1101.
50. Takahashi T, Kondo E, Yasuda K, et al. Effects of remnant tissue preservation on the tendon graft in anterior cruciate ligament reconstruction: a biomechanical and histological study. Am J Sports Med. 2016;44(7):1708-1716.
51. Dong S, Xie G, Zhang Y, Shen P, Huangfu X, Zhao J. Ligamentization of autogenous hamstring grafts after anterior cruciate ligament reconstruction: midterm versus long-term results. Am J Sports Med. 2015;43(8):1908-1917.
52. Takazawa Y, Ikeda H, Kawasaki T, et al. ACL reconstruction preserving the ACL remnant achieves good clinical outcomes and can reduce subsequent graft rupture. Orthop J Sports Med. 2013;1(4):2325967113505076.
53. Shimodaira H, Tensho K, Akaoka Y, Takanashi S, Kato H, Saito N. Remnant-preserving tibial tunnel positioning using anatomic landmarks in double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2016;32(9):1822-1830.
54. Zhang Q, Zhang S, Cao X, Liu L, Liu Y, Li R. The effect of remnant preservation on tibial tunnel enlargement in ACL reconstruction with hamstring autograft: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):166-173.
55. Tie K, Chen L, Hu D, Wang H. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: A meta-analysis. Knee. 2016;23(4):566-574.
56. Robson AW. VI. Ruptured crucial ligaments and their repair by operation. Ann Surg. 1903;37(5):716-718.
57. Palmer I. On the injuries to the ligaments of the knee joint. Acta Orthop Scand. 1938;53.
58. Palmer I. On the injuries to the ligaments of the knee joint: a clinical study. 1938. Clin Orthop Relat Res. 2007;454:17-22.
59 O’Donoghue DH. An analysis of end results of surgical treatment of major injuries to the ligaments of the knee. J Bone Joint Surg Am. 1955;37-A(1):1-13.
60. O’Donoghue DH. Surgical treatment of fresh injuries to the major ligaments of the knee. J Bone Joint Surg Am. 1950;32 A(4):721-738.
61. Feagin JA, Abbott HG, Rokous JR. The isolated tear of the anterior cruciate ligament. J Bone Joint Surg Am. 1972;54-A:1340-1341.
62. England RL. Repair of the ligaments about the knee. Orthop Clin North Am. 1976;7(1):195-204.
63. Marshall JL, Warren RF, Wickiewicz TL, Reider B. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res. 1979;(143):97-106.
64. Weaver JK, Derkash RS, Freeman JR, Kirk RE, Oden RR, Matyas J. Primary knee ligament repair--revisited. Clin Orthop Relat Res. 1985;(199):185-191.
65. Nogalski MP, Bach BR Jr. A review of early anterior cruciate ligament surgical repair or reconstruction. Results and caveats. Orthop Rev. 1993;22(11):1213-1223.
66. Feagin JA Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4(3):95-100.
67. Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10(2):103-107.
68. Straub T, Hunter RE. Acute anterior cruciate ligament repair. Clin Orthop Relat Res. 1988;227:238-250.
69. Kaplan N, Wickiewicz TL, Warren RF. Primary surgical treatment of anterior cruciate ligament ruptures. A long-term follow-up study. Am J Sports Med. 1990;18(4):354-358.
70. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
71. Paar O. Use of semitendinosus tendon to strengthen a freshly repaired anterior cruciate ligament. Chirurg. 1985;56(11):728-734.
72. Aglietti P, Buzzi R, Pisaneschi A, Salvi M. Comparison between suture and augmentation with the semitendinosus tendon in the repair of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1986;8(4):217-231.
73. Higgins RW, Steadman JR. Anterior cruciate ligament repairs in world class skiers. Am J Sports Med. 1987;15(5):439-447.
74. Harilainen A, Myllynen P. Treatment of fresh tears of the anterior cruciate ligament. A comparison of primary suture and augmentation with carbon fibre. Injury. 1987;18(6):396-400.
75. Jones KG. Results of use of the central one-third of the patellar ligament to compensate for anterior cruciate ligament deficiency. Clin Orthop Relat Res. 1980;(147):39-44.
76. Puddu G. Method for reconstruction of the anterior cruciate ligament using the semitendinosus tendon. Am J Sports Med. 1980;8(6):402-404.
77. Hefti F, Gächter A, Jenny H, Morscher E. Replacement of the anterior cruciate ligament. a comparative study of four different methods of reconstruction. Arch Orthop Trauma Surg. 1982;100(2):83-94.
78. Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament. A randomized study with short-term follow-up observations. Clin Orthop Relat Res. 1985;(198):87-93.
79. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
80. Engebretsen L, Benum P, Fasting O, Mølster A, Strand T. A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med. 1990;18(6):585-590.
81. Jonsson T, Peterson L, Renström P. Anterior cruciate ligament repair with and without augmentation. A prospective 7-year study of 51 patients. Acta Orthop Scand. 1990;61(6):562-566.
82. Andersson C, Odensten M, Gillquist J. Knee function after surgical or nonsurgical treatment of acute rupture of the anterior cruciate ligament: a randomized study with a long-term follow-up period. Clin Orthop Relat Res. 1991;(264):255-263.
83. Heim U, Bachmann B, Infanger K. Reinsertion of the anterior cruciate ligament or primary ligamentous plasty? Helv Chir Acta. 1982;48(5):703-708.
84. Strand T, Engesaeter LB, Mølster AO, et al. Knee function following suture of fresh tear of the anterior cruciate ligament. Acta Orthop Scand. 1984;55(2):181-184.
85. Marcacci M, Spinelli M, Chiellini F, Buccolieri V. Notes on 53 cases of immediate suture of acute lesions of the anterior cruciate ligament. Ital J Orthop Traumatol. 1985;7(2):69-79.
86. van der List JP, DiFelice GS. Primary repair of the anterior cruciate ligament: a paradigm shift. Surgeon. 2016 Oct 6. [Epub ahead of print]
87. Bräm J, Plaschy S, Lütolf M, Leutenegger A. [The primary cruciate ligament suture--is the method outdated? Results in follow-up of 58 patients]. Z Unfallchir Versicherungsmed. 1994;87(2):91-109.
88. Genelin F, Trost A, Primavesi C, Knoll P. Late results following proximal reinsertion of isolated ruptured ACL ligaments. Knee Surg Sports Traumatol Arthrosc. 1993;1(1):17-19.
89. Kühne JH, Theermann R, Neumann R, Sagasser J. [Acute uncomplicated anterior knee instability. 2-5 year follow-up of surgical treatment]. Unfallchirurg. 1991;94(2):81-87.
90. Simonet WT, Sim FH. Repair and reconstruction of rotatory instability of the knee. Am J Sports Med. 1984;12(2):89-97.
91. Raunest J, Derra E, Ohmann C. [Clinical results of Palmer’s primary cruciate ligament insertion without augmentation]. Unfallchirurgie. 1991;17(3):166-174.
92. Frank C, Beaver P, Rademaker F, Becker K, Schachar N, Edwards G. A computerized study of knee-ligament injuries: repair versus removal of the torn anterior cruciate ligament. Can J Surg. 1982;25(4):454-458.
93. Enneking WF, Horowitz M. The intra-articular effects of immobilization on the human knee. J Bone Joint Surg Am. 1972;54(5):973-985.
94. Millett PJ, Wickiewicz TL, Warren RF. Motion loss after ligament injuries to the knee. Part I: causes. Am J Sports Med. 2001;29(5):664-675.
95. Bilko TE, Paulos LE, Feagin JA Jr, Lambert KL, Cunningham HR. Current trends in repair and rehabilitation of complete (acute) anterior cruciate ligament injuries. Analysis of 1984 questionnaire completed by ACL Study Group. Am J Sports Med. 1986;14(2):143-147.
96. Paulos L, Noyes FR, Grood E, Butler DL. Knee rehabilitation after anterior cruciate ligament reconstruction and repair. J Orthop Sports Phys Ther. 1991;13(2):60-70.
97. Paessler HH, Deneke J, Dahners LE. Augmented repair and early mobilization of acute anterior cruciate ligament injuries. Am J Sports Med. 1992;20(6):667-674.
98.
99. Kdolsky RK, Gibbons DF, Kwasny O, Schabus R, Plenk H Jr. Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res. 1997;15(1):1-10.
100. Grøntvedt T, Engebretsen L. Comparison between two techniques for surgical repair of the acutely torn anterior cruciate ligament. A prospective, randomized follow-up study of 48 patients. Scand J Med Sci Sports. 1995;5(6):358-363.
101. Hehl G, Strecker W, Richter M, Kiefer H, Wissmeyer T. Clinical experience with PDS II augmentation for operative treatment of acute proximal ACL ruptures--2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(2):102-106.
102. Schenk S, Landsiedl F, Enenkel M. Arthroscopic single-stranded semitendinosus tendon- versus PDS-augmentation of reinserted acute femoral anterior cruciate ligament tears: 7 year follow-up study. Knee Surg Sports Traumatol Arthrosc. 2006;14(4):318-324.
103. Zysk SP, Refior HJ. Operative or conservative treatment of the acutely torn anterior cruciate ligament in middle-aged patients. A follow-up study of 133 patients between the ages of 40 and 59 years. Arch Orthop Trauma Surg. 2000;120(1-2):59-64.
104. Krueger-Franke M, Siebert CH, Schupp A. Refixation of femoral anterior cruciate ligament tears combined with a semitendinosus tendon augmentation. Technique and results. Arch Orthop Trauma Surg. 1998;117(1-2):68-72.
105. Natri A, Järvinen M, Kannus P. Primary repair plus intra-articular iliotibial band augmentation in the treatment of an acute anterior cruciate ligament rupture. A follow-up study of 70 patients. Arch Orthop Trauma Surg. 1996;115(1):22-27.
106. Träger D, Pohle K, Tschirner W. Anterior cruciate ligament suture in comparison with plasty. A 5-year follow-up study. Arch Orthop Trauma Surg. 1995;114(5):278-280.
107. Shelbourne KD, Wilckens JH, Mollabashy A, DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19(4):332-336.
108. Volokhina YV, Syed HM, Pham PH, Blackburn AK. Two helpful MRI signs for evaluation of posterolateral bundle tears of the anterior cruciate ligament: a pilot study. Orthop J Sports Med. 2015;3(8):2325967115597641.
109. Strand T, Mølster A, Hordvik M, Krukhaug Y. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years postoperatively. Arch Orthop Trauma Surg. 2005;125(4):217-221.
110. van der List JP, DiFelice GS. Successful arthroscopic primary repair of a chronic anterior cruciate ligament tear 11 years following injury. HSS J. 2016. In press.
111. van der List JP, DiFelice GS. The role of ligament repair in anterior cruciate ligament surgery. In: Mascarenhas R, Bhatia S, Lowe WR, eds. Ligamentous Injuries of the Knee. 1st ed. Houston: Nova Science Publishers; 2016:199-220.
112. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
113. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
114. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
115. Magarian EM, Fleming BC, Harrison SL, Mastrangelo AN, Badger GJ, Murray MM. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med. 2010;38(12):2528-2534.
116. Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039-2049.
117. Werner BC, Yang S, Looney AM, Gwathmey FW Jr. Trends in pediatric and adolescent anterior cruciate ligament iInjury and reconstruction. J Pediatr Orthop. 2016;36(5):447-452.
118. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
119. Ramski DE, Kanj WW, Franklin CC, Baldwin KD, Ganley TJ. Anterior cruciate ligament tears in children and adolescents: a meta-analysis of nonoperative versus operative treatment. Am J Sports Med. 2014;42(11):2769-2776.
120. Zantop T, Brucker PU, Vidal A, Zelle BA, Fu FH. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;454:48-53.
121. Yoon KH, Bae DK, Cho SM, Park SY, Lee JH. Standard anterior cruciate ligament reconstruction versus isolated single-bundle augmentation with hamstring autograft. Arthroscopy. 2009;25(11):1265-1274.
122 Demirağ B, Ermutlu C, Aydemir F, Durak K. A comparison of clinical outcome of augmentation and standard reconstruction techniques for partial anterior cruciate ligament tears. Eklem Hastalik Cerrahisi. 2012;23(3):140-144.
123. Sonnery-Cottet B, Zayni R, Conteduca J, et al. Posterolateral bundle reconstruction with anteromedial bundle remnant preservation in ACL tears: clinical and MRI evaluation of 39 patients with 24-month follow-up. Orthop J Sports Med. 2013;1(3):2325967113501624.
124. Sabat D, Kumar V. Partial tears of anterior cruciate ligament: results of single bundle augmentation. Indian J Orthop. 2015;49(2):129-135.
125. Jung YB, Jung HJ, Siti HT, et al. Comparison of anterior cruciate ligament reconstruction with preservation only versus remnant tensioning technique. Arthroscopy. 2011;27(9):1252-1258.
126. Nguyen DT, Ramwadhdoebe TH, van der Hart CP, Blankevoort L, Tak PP, van Dijk CN. Intrinsic healing response of the human anterior cruciate ligament: an histological study of reattached ACL remnants. J Orthop Res. 2014;32(2):296-301.
127. Boutsiadis A, Karampalis C, Tzavelas A, Vraggalas V, Christodoulou P, Bisbinas I. Anterior cruciate ligament remnant-preserving reconstruction using a “lasso-loop” knot configuration. Arthrosc Tech. 2015;4(6):e741-e746.
128. Noh JH, Yoon KH, Song SJ, Roh YH. Re-tensioning technique to cover the graft with remnant in anterior cruciate ligament reconstruction. Arthrosc Tech. 2014;3(6):e679-e682.
129. Hong L, Li X, Zhang H, et al. Anterior cruciate ligament reconstruction with remnant preservation: a prospective, randomized controlled study. Am J Sports Med. 2012;40(12):2747-2755.
130. Noh JH, Kyung HS, Roh YH, Kang TS. Remnant-preserving and re-tensioning technique to cover the graft in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 12. [Epub ahead of print]
131. Muneta T, Koga H, Ju YJ, Horie M, Nakamura T, Sekiya I. Remnant volume of anterior cruciate ligament correlates preoperative patients’ status and postoperative outcome. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):906-913.
132. Leeberg V, Lekdorf J, Wong C, Sonne-Holm S. Tibial eminentia avulsion fracture in children - a systematic review of the current literature. Dan Med J. 2014;61(3):A4792.
133. In Y, Kwak DS, Moon CW, Han SH, Choi NY. Biomechanical comparison of three techniques for fixation of tibial avulsion fractures of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1470-1478.
Preservation of the Anterior Cruciate Ligament: Surgical Techniques
In the first part of this series, “Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality” we discussed the history of anterior cruciate ligament (ACL) preservation, and the historical outcomes of both open primary repair and augmented repair. We also presented our surgical treatment algorithm for ACL preservation, which is based on the tear location and tissue quality of the ligament remnant. In this article, we propose a modification of the Sherman classification1 to identify the different tear types, and we will discuss the different surgical techniques that can be used for each one. Furthermore, we aim to provide an overview of the variations of these techniques that are seen in the literature. It is important to emphasize that these tear types and corresponding surgical techniques are to be seen as guidelines, rather than strict criteria, and that significant overlap between these tear types and surgical indications exist.
Assessment of Tear Type and Tissue Quality
The first assessment of the tear location and tissue quality is made using magnetic resonance imaging (MRI). Although MRI can give you an idea of where the tear is located, the final assessment for eligibility of each specific preservation technique is made during arthroscopy. Therefore, the routine preoperative discussion and informed consent process with the patient should encompass the gamut of surgical possibilities ranging from repair to reconstruction.
The Table shows our tear type classification, along with the corresponding preservation surgical techniques.
Surgical Preparation
In the operating room, the patient is placed in supine position on a standard operative table, such that the knee can be moved freely through its range of motion (ROM). The operative leg is then prepped and draped in standard fashion for knee arthroscopy. Standard knee arthroscopy equipment and implants are used, although some instruments from the standard shoulder set are also utilized. Anteromedial and anterolateral portals are created, and a general inspection of the knee is performed. By pulling the remnant ligament proximally using a broad tissue gasper, the available length of the remnant can be assessed. It is important to reduce possible anterior tibial subluxation in the sagittal plane in order to prevent “false” shortening of the distal ligament remnant. Once the length of the remnant tissue is assessed and the tissue quality is determined, the surgical preservation technique can be chosen (Table).
Type I Tears: Primary Repair
In order to be a candidate for arthroscopic primary repair, sufficient tissue length and tissue quality are necessary (Figures 1A and 1B, Table).
Sutures are then passed through the anteromedial bundle using the Scorpion Suture Passer (Arthrex) with a No. 2 FiberWire suture (Arthrex) (Figure 1C). Suturing is commenced at the intact distal end of the anteromedial bundle and is advanced in an alternating, interlocking Bunnell-type pattern towards the avulsed proximal end with approximately 4 mm to 5 mm between each pass. In general, 3 to 4 passes can be made before the final pass exits via the avulsed end of the ligament towards the femur (Figure 1D). The same process is then repeated for the posterolateral bundle of the ACL remnant with a No. 2 TigerWire suture (Arthrex) to optimize suture management. With each subsequent pass of the sutures, it is important to assess tissue resistance to prevent perforation of a previous stitch. Mild resistance is normal, but the suture-passing device should be repositioned when notably increased resistance is encountered. In addition, placing all of the bites in the same plane should be avoided since this can allow the sutures to “cheese cut” along the collagen fibers of the ligament remnant rather than holding firm.
After passing the sutures through both bundles, the sutures are guided outside the knee using an accessory stab incision situated just above the medial portal. Using this portal, the ligament can be retracted away from the femoral footprint for optimal visibility. The femoral footprint is then roughed using a shaver or burr, and bleeding is induced to stimulate a local healing response,2 while the sutures and the ACL are protected via the portal. With the knee in flexion, an accessory inferomedial portal is then created under direct visualization using a spinal needle for localization. Care should be taken to enable the appropriate trajectory for anchor placement to be achieved.
Many different techniques can be used to provide fixation of the ACL repair to the femoral footprint; the 2 most straightforward techniques are presented here. The first technique provides fixation with knotless suture anchors,3,4 whereas in the second technique the sutures are transosseously passed, and tied over a bone bridge, as was performed in the 1970s and 1980s.
Suture Anchor Fixation
With the suture anchor fixation technique, the knee is flexed in 90°, the anteromedial bundle origin within the femoral footprint is identified, and a 4.5-mm x 20-mm hole is drilled, punched, or tapped, in the case of high bone density. The FiberWire sutures are then retrieved through the accessory portal and passed through a 4.75-mm Vented BioComposite SwiveLock suture anchor (Arthrex). The suture anchor for the anteromedial bundle is then deployed into the hole within the anteromedial footprint, while tensioning the ACL remnant to the wall with a visual gap of <1 mm (Figure 1E).5 The procedure is then repeated using another suture anchor with TigerWire sutures for the posterolateral bundle with the knee flexed at 110° to 115°. This ensures an optimal angle of approach and avoids perforating the posterior condyle with the anchor. The drill hole and anchor are placed into the origin of the posterolateral bundle within the femoral footprint. The order of bundle tensioning and repair may be varied depending on the particulars of each case.
Once the anchors are fully deployed and flush with the femoral footprint, the handle is removed and the additional core stitches are unloaded. Occasionally, the core stitches can be passed from lateral to medial through the proximal ligament remnant and tied down with an arthroscopic knot pusher to add extra compression of the remnant to the origin. The free ends of the repair sutures are cut with an Open Ended Suture Cutter (Arthrex) so that they are flush with the notch. The repair is now complete (Figure 1F). Using a probe, the ACL remnant is tested for tension and stiffness. Finally, cycling of the knee through the full ROM confirms anatomic positioning without impingement of the graft. Manual laxity testing should reveal minimal anteroposterior translation with a firm endpoint on Lachman examination intraoperatively.
Bone Bridge Fixation
With this technique, parallel drill holes are created exiting at each bundle origin. The repair stitches can then be retrieved and tensioned proximally. One way to accomplish this is by using an ACL femoral guide (Arthrex) that is placed via the anterolateral portal and is centered on the anteromedial bundle insertion. This device guides a cannulated RetroDrill (Arthrex) to drill through the lateral femoral condyle towards the anteromedial footprint. A passing wire can then be delivered through the cannulation and used to retrieve that anteromedial bundle repair stitches. This process can then be repeated for the posterolateral bundle and the associated repair stitches. Drill holes can also be made retrograde from a low anteromedial accessory portal using a slotted pit that can be used to shuttle the repair stitches. When all the repair sutures are passed, the ligament is tensioned while being visualized arthroscopically. The knee is held at 20° of flexion and a posterior drawer force can be applied, if necessary, to reduce the tibia to its anatomic position. The suture limbs are then tensioned and can be fixated using any of a multitude of techniques, including tying over a bony bridge, tying over a 4-hole ligament button, and tying to a post.
One disadvantage of the bone bridge fixation technique, however, is the suspensory fixation that is not as stiff as tensioning and fixating with suture anchors. Despite this disadvantage, however, the senior author (GSD) has achieved excellent results with this technique at longer-term follow-up in a small group of patients. One advantage of the bone bridge fixation technique is that the procedure has lower costs than fixation with suture anchors.
One Anchor Repair Fixation
Achtnich and colleagues6 recently published a slightly different technique for repairing type I tears. The authors passed a No. 2 FiberWire suture through the midsubstance of both bundles of the ACL remnant to create a modified Mason-Allen stitch configuration. Subsequently, they tensioned the remnant towards the middle of the ACL footprint (between the anteromedial and posterolateral footprint) using one PushLock suture anchor (Arthrex). They hypothesized that using 1 anchor would be enough fixation for tears amenable to repair, and that doing so would minimize the invasion of the bone.
The preference of the senior author (GSD) is, however, to use 2 suture anchors for each bundle in order to more anatomically and biomechanically repair the remnant, since both bundles have different biomechanical characteristics.7 Similarly, the preference of the senior author is to commence the suturing as distal as possible and pass multiple sutures towards the proximal end. This ensures that the last suture pass is exited very proximally, and ensures that the proximal end is approximated towards the femoral wall. One suture passed at the midsubstance portion of the remnant might cause a different tension pattern and prevent optimal re-approximation of the most proximal part towards the femoral wall. Future studies are necessary to assess the efficacy of different suture and fixation techniques as these are currently lacking.
Addition of Internal Brace
Over the last few years, the senior author has added an internal brace (FiberTape, Arthrex) to the repair technique, which was first performed by MacKay and colleagues.8 The added internal brace protects the repair and the healing process in the first few weeks and enables early ROM.
With this technique, the previously described arthroscopic primary repair technique is performed with suturing of both bundles. However, after punching, tapping, or drilling a hole in the anteromedial origin of the femoral footprint, the anteromedial anchor is first loaded with the FiberTape in addition to the repair stitches. After placing the anteromedial suture anchor in the femoral footprint, the internal brace is fixated proximally with the suture anchor into the femoral wall.
Others, however, have advocated fixing the internal brace independently of the repaired ligament and suture anchors.9 With this technique, tunnels are drilled in the femur and tibia and the internal brace construct is fixed proximally using a RetroButton (Arthrex) and fixed distally in the tibial metaphysis using a suture anchor. A disadvantage of this technique is that an extra femoral tunnel needs to be drilled, which is especially important in pediatric patients with the increased risk for growth disturbances.10
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the anteromedial or posterolateral bundle is a type I tear with good or excellent tissue quality, whereas the other bundle is not a type I tear or has poor tissue quality (Figure 3A). In these cases, a primary repair of one bundle is performed with a hamstring reconstruction of the other bundle.
First, a No. 2 FiberWire is used to make 4 to 5 passes from distal to proximal, as previously described. Then, the remnants of the irreparable bundle are debrided (Figure 3B). Subsequently, the semitendinosus tendon is harvested in standard fashion, or soft tissue allografts can be used.
Type II Tears: Augmented Repair
In patients with type II tears, primary repair is not possible as the length of the remnant is too short to firmly approximate the remnant towards the femoral wall (75%-90% of native tissue length) (Figure 4A). In these patients, an augmented repair of the entire ACL is performed using hamstring autograft or soft tissue allograft.
With this technique, repair stitches are passed into the anteromedial bundle of the remnant as previously described (Figure 4B). Keeping the repair stitches anteriorly in the anteromedial bundle tends to prevent entanglement during graft passage later in the case.
Once the repair stitches are in place, a small accessory stab incision is made just above the medial portal. The repair stitches are parked here to keep them out of harms way. Traction on the repair stitches will retract the ACL away from the lateral wall of the notch and allow work to be performed here. A small opening notchplasty is generally performed to enhance visualization and to add a bleeding surface for enhanced healing. Next, the arthroscope is placed into the medial portal, which allows the femoral guide to be placed into the lateral portal. The femoral guide is positioned to optimize the femoral tunnel location in the center of the footprint. A small incision is made laterally over the condyle and through the iliotibial band to allow access to the lateral cortex of the lateral femoral condyle. The FlipCutter is then used to back-cut the femoral socket as described above. A FiberStick (Arthrex) passing suture is then placed in the femoral tunnel and brought out through the anteromedial portal.
Next, the tibial tunnel is drilled with a tibial guide at 55° inclination. The pin is drilled up into the center of the tibial footprint and this is over-reamed with a reamer. The reaming is stopped precisely upon breaking to proximal tibial cortex so as to minimize soft tissue damage of the ACL insertion fibers that are typically pristine. Then, a grasper is passed up and through the tunnel to retrieve the repair stitches and bring them out distally for later use. At the same time, the passing suture in the femoral is also retrieved distally. The soft tissue graft is proximally prepared with a TightRope RT button, and the repair stitches are passed through the button. The passing suture from the femoral socket is then used to shuttle the draw sutures and repair stitches up through the tibia, through the ACL remnant, and out the femoral socket (Figure 4C). The TightRope RT button is then engaged on the lateral femoral cortex in standard fashion. Using the cinch stitches, the graft is delivered through the tibia, up and through the center of the ACL remnant, and into the femoral socket. The knee is then cycled and the graft is tensioned distally in standard fashion, and fixed using a BioComposite interference screw. Finally, the repair stitches can be tensioned pulling the ligament remnant up as a sleeve around the hamstring graft (Figure 4D). They are then tied over the TightRope RT button using alternating half hitches tied with a knot pusher from laterally.
Type III Tears: Reconstruction With Remnant Tensioning
The previously discussed techniques have the goals of preserving as much native ligament remnant as possible, approximating the ligament remnant towards the femoral wall, and promoting healing of the ligament. In some cases, however, the ligament remnant is too short for healing (Figure 5A). Although the ligament cannot be approximated to the femoral wall in these cases, there is still an argument for ACL preservation, as was discussed in the first article of this series.
If the ligament length is between 25% and 75% of the native tissue length, the senior author performs a remnant tensioning technique.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some cases, the distal remnant is small or the tissue quality in the largest part of the remnant is poor, and after debriding back to good tissue quality, only 10% to 25% of the native tissue length is left (Figure 6A). In these cases, the remnant is preserved, however, tensioning of the remnant with sutures is usually not necessary for the prevention of cyclops lesions. Nonetheless, it is important to debride the parts of the remnant ligament with poor tissue quality as mop-end patterns of the remnant may increase the chance of these lesions (Figure 6B).
In this situation, any of the standard ACL reconstruction techniques can be performed with simple attention being paid to preserving what is left of the tibial insertion site. At the very least, the small insertion remnant guides the anatomic placement of the graft, and prevents egress of joint fluid into the tibial tunnel and could minimize tunnel widening.
Type V Tears: Primary Repair
Finally, in some patients a soft tissue avulsion (Figure 7A) or bony avulsion of the distal attachment of the ACL can be seen. Both injuries are relatively rare, although bony avulsions are frequently seen in children, especially those younger than 12 years old. In these cases, the same techniques and theory that are applied to proximal avulsion type tears can be used and applied to distal avulsion type tears.
First, No. 2 FiberWire sutures are passed from proximal towards the distal end of the ligament in the anteromedial bundle, and the same process is then repeated for No. 2 TigerWire sutures for the posterolateral bundle. Then both sutures are exited at the distal avulsed end at the locations of the anteromedial and posterolateral footprints (Figure 7B). A 2.4-mm ACL guide wire and a Ninitol wire are used to drill 2 tunnels from the tibia towards the tibial footprint. The repair sutures are then retrieved through both tunnels (Figure 7C) and the sutures are tied distally over a ligament button after cycling of the knee (Figure 7D). This technique is very useful for soft tissue avulsions, or when there are only small flecks of bone or when the avulsed bone is significantly comminuted. If a large bony avulsion fragment is present, this technique can also be applied with some modification, although there have been multiple other techniques described in the literature that work well in this situation including fixation with screw and washer, or with suture anchors.
Complex Tear or Poor Tissue Quality: Reconstruction
In some cases, the tissue quality is poor, or the ligament has complex or multiple tears. Essentially, in these cases, there is nothing to preserve and a standard reconstruction approach is performed in these cases.
Conclusion
The uniform gold standard for all ACL tear types is currently primary reconstruction. However, several disadvantages of ACL reconstruction exist, while there are multiple advantages to the concept of ACL preservation. In this surgical technique article, we have discussed our tear type classification and the recommended surgical techniques for each. With this treatment algorithm, which is based on tear location and tissue quality, an optimal and minimally invasive treatment can be chosen for each individual patient. Future studies are needed to compare and contrast these treatments with the current gold standard of ACL reconstruction.
Am J Orthop. 2016;45(7):E406-E414. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
2. Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25(3):255-260.
3. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
4. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
5. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
6. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016. [Epub ahead of print]
7. Amis AA. The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):613-620.
8. MacKay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
9. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
10. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
11. Crain EH, Fithian DC, Paxton EW, Luetzow WF. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy. 2005;21(1):19-24.
In the first part of this series, “Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality” we discussed the history of anterior cruciate ligament (ACL) preservation, and the historical outcomes of both open primary repair and augmented repair. We also presented our surgical treatment algorithm for ACL preservation, which is based on the tear location and tissue quality of the ligament remnant. In this article, we propose a modification of the Sherman classification1 to identify the different tear types, and we will discuss the different surgical techniques that can be used for each one. Furthermore, we aim to provide an overview of the variations of these techniques that are seen in the literature. It is important to emphasize that these tear types and corresponding surgical techniques are to be seen as guidelines, rather than strict criteria, and that significant overlap between these tear types and surgical indications exist.
Assessment of Tear Type and Tissue Quality
The first assessment of the tear location and tissue quality is made using magnetic resonance imaging (MRI). Although MRI can give you an idea of where the tear is located, the final assessment for eligibility of each specific preservation technique is made during arthroscopy. Therefore, the routine preoperative discussion and informed consent process with the patient should encompass the gamut of surgical possibilities ranging from repair to reconstruction.
The Table shows our tear type classification, along with the corresponding preservation surgical techniques.
Surgical Preparation
In the operating room, the patient is placed in supine position on a standard operative table, such that the knee can be moved freely through its range of motion (ROM). The operative leg is then prepped and draped in standard fashion for knee arthroscopy. Standard knee arthroscopy equipment and implants are used, although some instruments from the standard shoulder set are also utilized. Anteromedial and anterolateral portals are created, and a general inspection of the knee is performed. By pulling the remnant ligament proximally using a broad tissue gasper, the available length of the remnant can be assessed. It is important to reduce possible anterior tibial subluxation in the sagittal plane in order to prevent “false” shortening of the distal ligament remnant. Once the length of the remnant tissue is assessed and the tissue quality is determined, the surgical preservation technique can be chosen (Table).
Type I Tears: Primary Repair
In order to be a candidate for arthroscopic primary repair, sufficient tissue length and tissue quality are necessary (Figures 1A and 1B, Table).
Sutures are then passed through the anteromedial bundle using the Scorpion Suture Passer (Arthrex) with a No. 2 FiberWire suture (Arthrex) (Figure 1C). Suturing is commenced at the intact distal end of the anteromedial bundle and is advanced in an alternating, interlocking Bunnell-type pattern towards the avulsed proximal end with approximately 4 mm to 5 mm between each pass. In general, 3 to 4 passes can be made before the final pass exits via the avulsed end of the ligament towards the femur (Figure 1D). The same process is then repeated for the posterolateral bundle of the ACL remnant with a No. 2 TigerWire suture (Arthrex) to optimize suture management. With each subsequent pass of the sutures, it is important to assess tissue resistance to prevent perforation of a previous stitch. Mild resistance is normal, but the suture-passing device should be repositioned when notably increased resistance is encountered. In addition, placing all of the bites in the same plane should be avoided since this can allow the sutures to “cheese cut” along the collagen fibers of the ligament remnant rather than holding firm.
After passing the sutures through both bundles, the sutures are guided outside the knee using an accessory stab incision situated just above the medial portal. Using this portal, the ligament can be retracted away from the femoral footprint for optimal visibility. The femoral footprint is then roughed using a shaver or burr, and bleeding is induced to stimulate a local healing response,2 while the sutures and the ACL are protected via the portal. With the knee in flexion, an accessory inferomedial portal is then created under direct visualization using a spinal needle for localization. Care should be taken to enable the appropriate trajectory for anchor placement to be achieved.
Many different techniques can be used to provide fixation of the ACL repair to the femoral footprint; the 2 most straightforward techniques are presented here. The first technique provides fixation with knotless suture anchors,3,4 whereas in the second technique the sutures are transosseously passed, and tied over a bone bridge, as was performed in the 1970s and 1980s.
Suture Anchor Fixation
With the suture anchor fixation technique, the knee is flexed in 90°, the anteromedial bundle origin within the femoral footprint is identified, and a 4.5-mm x 20-mm hole is drilled, punched, or tapped, in the case of high bone density. The FiberWire sutures are then retrieved through the accessory portal and passed through a 4.75-mm Vented BioComposite SwiveLock suture anchor (Arthrex). The suture anchor for the anteromedial bundle is then deployed into the hole within the anteromedial footprint, while tensioning the ACL remnant to the wall with a visual gap of <1 mm (Figure 1E).5 The procedure is then repeated using another suture anchor with TigerWire sutures for the posterolateral bundle with the knee flexed at 110° to 115°. This ensures an optimal angle of approach and avoids perforating the posterior condyle with the anchor. The drill hole and anchor are placed into the origin of the posterolateral bundle within the femoral footprint. The order of bundle tensioning and repair may be varied depending on the particulars of each case.
Once the anchors are fully deployed and flush with the femoral footprint, the handle is removed and the additional core stitches are unloaded. Occasionally, the core stitches can be passed from lateral to medial through the proximal ligament remnant and tied down with an arthroscopic knot pusher to add extra compression of the remnant to the origin. The free ends of the repair sutures are cut with an Open Ended Suture Cutter (Arthrex) so that they are flush with the notch. The repair is now complete (Figure 1F). Using a probe, the ACL remnant is tested for tension and stiffness. Finally, cycling of the knee through the full ROM confirms anatomic positioning without impingement of the graft. Manual laxity testing should reveal minimal anteroposterior translation with a firm endpoint on Lachman examination intraoperatively.
Bone Bridge Fixation
With this technique, parallel drill holes are created exiting at each bundle origin. The repair stitches can then be retrieved and tensioned proximally. One way to accomplish this is by using an ACL femoral guide (Arthrex) that is placed via the anterolateral portal and is centered on the anteromedial bundle insertion. This device guides a cannulated RetroDrill (Arthrex) to drill through the lateral femoral condyle towards the anteromedial footprint. A passing wire can then be delivered through the cannulation and used to retrieve that anteromedial bundle repair stitches. This process can then be repeated for the posterolateral bundle and the associated repair stitches. Drill holes can also be made retrograde from a low anteromedial accessory portal using a slotted pit that can be used to shuttle the repair stitches. When all the repair sutures are passed, the ligament is tensioned while being visualized arthroscopically. The knee is held at 20° of flexion and a posterior drawer force can be applied, if necessary, to reduce the tibia to its anatomic position. The suture limbs are then tensioned and can be fixated using any of a multitude of techniques, including tying over a bony bridge, tying over a 4-hole ligament button, and tying to a post.
One disadvantage of the bone bridge fixation technique, however, is the suspensory fixation that is not as stiff as tensioning and fixating with suture anchors. Despite this disadvantage, however, the senior author (GSD) has achieved excellent results with this technique at longer-term follow-up in a small group of patients. One advantage of the bone bridge fixation technique is that the procedure has lower costs than fixation with suture anchors.
One Anchor Repair Fixation
Achtnich and colleagues6 recently published a slightly different technique for repairing type I tears. The authors passed a No. 2 FiberWire suture through the midsubstance of both bundles of the ACL remnant to create a modified Mason-Allen stitch configuration. Subsequently, they tensioned the remnant towards the middle of the ACL footprint (between the anteromedial and posterolateral footprint) using one PushLock suture anchor (Arthrex). They hypothesized that using 1 anchor would be enough fixation for tears amenable to repair, and that doing so would minimize the invasion of the bone.
The preference of the senior author (GSD) is, however, to use 2 suture anchors for each bundle in order to more anatomically and biomechanically repair the remnant, since both bundles have different biomechanical characteristics.7 Similarly, the preference of the senior author is to commence the suturing as distal as possible and pass multiple sutures towards the proximal end. This ensures that the last suture pass is exited very proximally, and ensures that the proximal end is approximated towards the femoral wall. One suture passed at the midsubstance portion of the remnant might cause a different tension pattern and prevent optimal re-approximation of the most proximal part towards the femoral wall. Future studies are necessary to assess the efficacy of different suture and fixation techniques as these are currently lacking.
Addition of Internal Brace
Over the last few years, the senior author has added an internal brace (FiberTape, Arthrex) to the repair technique, which was first performed by MacKay and colleagues.8 The added internal brace protects the repair and the healing process in the first few weeks and enables early ROM.
With this technique, the previously described arthroscopic primary repair technique is performed with suturing of both bundles. However, after punching, tapping, or drilling a hole in the anteromedial origin of the femoral footprint, the anteromedial anchor is first loaded with the FiberTape in addition to the repair stitches. After placing the anteromedial suture anchor in the femoral footprint, the internal brace is fixated proximally with the suture anchor into the femoral wall.
Others, however, have advocated fixing the internal brace independently of the repaired ligament and suture anchors.9 With this technique, tunnels are drilled in the femur and tibia and the internal brace construct is fixed proximally using a RetroButton (Arthrex) and fixed distally in the tibial metaphysis using a suture anchor. A disadvantage of this technique is that an extra femoral tunnel needs to be drilled, which is especially important in pediatric patients with the increased risk for growth disturbances.10
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the anteromedial or posterolateral bundle is a type I tear with good or excellent tissue quality, whereas the other bundle is not a type I tear or has poor tissue quality (Figure 3A). In these cases, a primary repair of one bundle is performed with a hamstring reconstruction of the other bundle.
First, a No. 2 FiberWire is used to make 4 to 5 passes from distal to proximal, as previously described. Then, the remnants of the irreparable bundle are debrided (Figure 3B). Subsequently, the semitendinosus tendon is harvested in standard fashion, or soft tissue allografts can be used.
Type II Tears: Augmented Repair
In patients with type II tears, primary repair is not possible as the length of the remnant is too short to firmly approximate the remnant towards the femoral wall (75%-90% of native tissue length) (Figure 4A). In these patients, an augmented repair of the entire ACL is performed using hamstring autograft or soft tissue allograft.
With this technique, repair stitches are passed into the anteromedial bundle of the remnant as previously described (Figure 4B). Keeping the repair stitches anteriorly in the anteromedial bundle tends to prevent entanglement during graft passage later in the case.
Once the repair stitches are in place, a small accessory stab incision is made just above the medial portal. The repair stitches are parked here to keep them out of harms way. Traction on the repair stitches will retract the ACL away from the lateral wall of the notch and allow work to be performed here. A small opening notchplasty is generally performed to enhance visualization and to add a bleeding surface for enhanced healing. Next, the arthroscope is placed into the medial portal, which allows the femoral guide to be placed into the lateral portal. The femoral guide is positioned to optimize the femoral tunnel location in the center of the footprint. A small incision is made laterally over the condyle and through the iliotibial band to allow access to the lateral cortex of the lateral femoral condyle. The FlipCutter is then used to back-cut the femoral socket as described above. A FiberStick (Arthrex) passing suture is then placed in the femoral tunnel and brought out through the anteromedial portal.
Next, the tibial tunnel is drilled with a tibial guide at 55° inclination. The pin is drilled up into the center of the tibial footprint and this is over-reamed with a reamer. The reaming is stopped precisely upon breaking to proximal tibial cortex so as to minimize soft tissue damage of the ACL insertion fibers that are typically pristine. Then, a grasper is passed up and through the tunnel to retrieve the repair stitches and bring them out distally for later use. At the same time, the passing suture in the femoral is also retrieved distally. The soft tissue graft is proximally prepared with a TightRope RT button, and the repair stitches are passed through the button. The passing suture from the femoral socket is then used to shuttle the draw sutures and repair stitches up through the tibia, through the ACL remnant, and out the femoral socket (Figure 4C). The TightRope RT button is then engaged on the lateral femoral cortex in standard fashion. Using the cinch stitches, the graft is delivered through the tibia, up and through the center of the ACL remnant, and into the femoral socket. The knee is then cycled and the graft is tensioned distally in standard fashion, and fixed using a BioComposite interference screw. Finally, the repair stitches can be tensioned pulling the ligament remnant up as a sleeve around the hamstring graft (Figure 4D). They are then tied over the TightRope RT button using alternating half hitches tied with a knot pusher from laterally.
Type III Tears: Reconstruction With Remnant Tensioning
The previously discussed techniques have the goals of preserving as much native ligament remnant as possible, approximating the ligament remnant towards the femoral wall, and promoting healing of the ligament. In some cases, however, the ligament remnant is too short for healing (Figure 5A). Although the ligament cannot be approximated to the femoral wall in these cases, there is still an argument for ACL preservation, as was discussed in the first article of this series.
If the ligament length is between 25% and 75% of the native tissue length, the senior author performs a remnant tensioning technique.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some cases, the distal remnant is small or the tissue quality in the largest part of the remnant is poor, and after debriding back to good tissue quality, only 10% to 25% of the native tissue length is left (Figure 6A). In these cases, the remnant is preserved, however, tensioning of the remnant with sutures is usually not necessary for the prevention of cyclops lesions. Nonetheless, it is important to debride the parts of the remnant ligament with poor tissue quality as mop-end patterns of the remnant may increase the chance of these lesions (Figure 6B).
In this situation, any of the standard ACL reconstruction techniques can be performed with simple attention being paid to preserving what is left of the tibial insertion site. At the very least, the small insertion remnant guides the anatomic placement of the graft, and prevents egress of joint fluid into the tibial tunnel and could minimize tunnel widening.
Type V Tears: Primary Repair
Finally, in some patients a soft tissue avulsion (Figure 7A) or bony avulsion of the distal attachment of the ACL can be seen. Both injuries are relatively rare, although bony avulsions are frequently seen in children, especially those younger than 12 years old. In these cases, the same techniques and theory that are applied to proximal avulsion type tears can be used and applied to distal avulsion type tears.
First, No. 2 FiberWire sutures are passed from proximal towards the distal end of the ligament in the anteromedial bundle, and the same process is then repeated for No. 2 TigerWire sutures for the posterolateral bundle. Then both sutures are exited at the distal avulsed end at the locations of the anteromedial and posterolateral footprints (Figure 7B). A 2.4-mm ACL guide wire and a Ninitol wire are used to drill 2 tunnels from the tibia towards the tibial footprint. The repair sutures are then retrieved through both tunnels (Figure 7C) and the sutures are tied distally over a ligament button after cycling of the knee (Figure 7D). This technique is very useful for soft tissue avulsions, or when there are only small flecks of bone or when the avulsed bone is significantly comminuted. If a large bony avulsion fragment is present, this technique can also be applied with some modification, although there have been multiple other techniques described in the literature that work well in this situation including fixation with screw and washer, or with suture anchors.
Complex Tear or Poor Tissue Quality: Reconstruction
In some cases, the tissue quality is poor, or the ligament has complex or multiple tears. Essentially, in these cases, there is nothing to preserve and a standard reconstruction approach is performed in these cases.
Conclusion
The uniform gold standard for all ACL tear types is currently primary reconstruction. However, several disadvantages of ACL reconstruction exist, while there are multiple advantages to the concept of ACL preservation. In this surgical technique article, we have discussed our tear type classification and the recommended surgical techniques for each. With this treatment algorithm, which is based on tear location and tissue quality, an optimal and minimally invasive treatment can be chosen for each individual patient. Future studies are needed to compare and contrast these treatments with the current gold standard of ACL reconstruction.
Am J Orthop. 2016;45(7):E406-E414. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
In the first part of this series, “Preservation of the Anterior Cruciate Ligament: A Treatment Algorithm Based on Tear Location and Tissue Quality” we discussed the history of anterior cruciate ligament (ACL) preservation, and the historical outcomes of both open primary repair and augmented repair. We also presented our surgical treatment algorithm for ACL preservation, which is based on the tear location and tissue quality of the ligament remnant. In this article, we propose a modification of the Sherman classification1 to identify the different tear types, and we will discuss the different surgical techniques that can be used for each one. Furthermore, we aim to provide an overview of the variations of these techniques that are seen in the literature. It is important to emphasize that these tear types and corresponding surgical techniques are to be seen as guidelines, rather than strict criteria, and that significant overlap between these tear types and surgical indications exist.
Assessment of Tear Type and Tissue Quality
The first assessment of the tear location and tissue quality is made using magnetic resonance imaging (MRI). Although MRI can give you an idea of where the tear is located, the final assessment for eligibility of each specific preservation technique is made during arthroscopy. Therefore, the routine preoperative discussion and informed consent process with the patient should encompass the gamut of surgical possibilities ranging from repair to reconstruction.
The Table shows our tear type classification, along with the corresponding preservation surgical techniques.
Surgical Preparation
In the operating room, the patient is placed in supine position on a standard operative table, such that the knee can be moved freely through its range of motion (ROM). The operative leg is then prepped and draped in standard fashion for knee arthroscopy. Standard knee arthroscopy equipment and implants are used, although some instruments from the standard shoulder set are also utilized. Anteromedial and anterolateral portals are created, and a general inspection of the knee is performed. By pulling the remnant ligament proximally using a broad tissue gasper, the available length of the remnant can be assessed. It is important to reduce possible anterior tibial subluxation in the sagittal plane in order to prevent “false” shortening of the distal ligament remnant. Once the length of the remnant tissue is assessed and the tissue quality is determined, the surgical preservation technique can be chosen (Table).
Type I Tears: Primary Repair
In order to be a candidate for arthroscopic primary repair, sufficient tissue length and tissue quality are necessary (Figures 1A and 1B, Table).
Sutures are then passed through the anteromedial bundle using the Scorpion Suture Passer (Arthrex) with a No. 2 FiberWire suture (Arthrex) (Figure 1C). Suturing is commenced at the intact distal end of the anteromedial bundle and is advanced in an alternating, interlocking Bunnell-type pattern towards the avulsed proximal end with approximately 4 mm to 5 mm between each pass. In general, 3 to 4 passes can be made before the final pass exits via the avulsed end of the ligament towards the femur (Figure 1D). The same process is then repeated for the posterolateral bundle of the ACL remnant with a No. 2 TigerWire suture (Arthrex) to optimize suture management. With each subsequent pass of the sutures, it is important to assess tissue resistance to prevent perforation of a previous stitch. Mild resistance is normal, but the suture-passing device should be repositioned when notably increased resistance is encountered. In addition, placing all of the bites in the same plane should be avoided since this can allow the sutures to “cheese cut” along the collagen fibers of the ligament remnant rather than holding firm.
After passing the sutures through both bundles, the sutures are guided outside the knee using an accessory stab incision situated just above the medial portal. Using this portal, the ligament can be retracted away from the femoral footprint for optimal visibility. The femoral footprint is then roughed using a shaver or burr, and bleeding is induced to stimulate a local healing response,2 while the sutures and the ACL are protected via the portal. With the knee in flexion, an accessory inferomedial portal is then created under direct visualization using a spinal needle for localization. Care should be taken to enable the appropriate trajectory for anchor placement to be achieved.
Many different techniques can be used to provide fixation of the ACL repair to the femoral footprint; the 2 most straightforward techniques are presented here. The first technique provides fixation with knotless suture anchors,3,4 whereas in the second technique the sutures are transosseously passed, and tied over a bone bridge, as was performed in the 1970s and 1980s.
Suture Anchor Fixation
With the suture anchor fixation technique, the knee is flexed in 90°, the anteromedial bundle origin within the femoral footprint is identified, and a 4.5-mm x 20-mm hole is drilled, punched, or tapped, in the case of high bone density. The FiberWire sutures are then retrieved through the accessory portal and passed through a 4.75-mm Vented BioComposite SwiveLock suture anchor (Arthrex). The suture anchor for the anteromedial bundle is then deployed into the hole within the anteromedial footprint, while tensioning the ACL remnant to the wall with a visual gap of <1 mm (Figure 1E).5 The procedure is then repeated using another suture anchor with TigerWire sutures for the posterolateral bundle with the knee flexed at 110° to 115°. This ensures an optimal angle of approach and avoids perforating the posterior condyle with the anchor. The drill hole and anchor are placed into the origin of the posterolateral bundle within the femoral footprint. The order of bundle tensioning and repair may be varied depending on the particulars of each case.
Once the anchors are fully deployed and flush with the femoral footprint, the handle is removed and the additional core stitches are unloaded. Occasionally, the core stitches can be passed from lateral to medial through the proximal ligament remnant and tied down with an arthroscopic knot pusher to add extra compression of the remnant to the origin. The free ends of the repair sutures are cut with an Open Ended Suture Cutter (Arthrex) so that they are flush with the notch. The repair is now complete (Figure 1F). Using a probe, the ACL remnant is tested for tension and stiffness. Finally, cycling of the knee through the full ROM confirms anatomic positioning without impingement of the graft. Manual laxity testing should reveal minimal anteroposterior translation with a firm endpoint on Lachman examination intraoperatively.
Bone Bridge Fixation
With this technique, parallel drill holes are created exiting at each bundle origin. The repair stitches can then be retrieved and tensioned proximally. One way to accomplish this is by using an ACL femoral guide (Arthrex) that is placed via the anterolateral portal and is centered on the anteromedial bundle insertion. This device guides a cannulated RetroDrill (Arthrex) to drill through the lateral femoral condyle towards the anteromedial footprint. A passing wire can then be delivered through the cannulation and used to retrieve that anteromedial bundle repair stitches. This process can then be repeated for the posterolateral bundle and the associated repair stitches. Drill holes can also be made retrograde from a low anteromedial accessory portal using a slotted pit that can be used to shuttle the repair stitches. When all the repair sutures are passed, the ligament is tensioned while being visualized arthroscopically. The knee is held at 20° of flexion and a posterior drawer force can be applied, if necessary, to reduce the tibia to its anatomic position. The suture limbs are then tensioned and can be fixated using any of a multitude of techniques, including tying over a bony bridge, tying over a 4-hole ligament button, and tying to a post.
One disadvantage of the bone bridge fixation technique, however, is the suspensory fixation that is not as stiff as tensioning and fixating with suture anchors. Despite this disadvantage, however, the senior author (GSD) has achieved excellent results with this technique at longer-term follow-up in a small group of patients. One advantage of the bone bridge fixation technique is that the procedure has lower costs than fixation with suture anchors.
One Anchor Repair Fixation
Achtnich and colleagues6 recently published a slightly different technique for repairing type I tears. The authors passed a No. 2 FiberWire suture through the midsubstance of both bundles of the ACL remnant to create a modified Mason-Allen stitch configuration. Subsequently, they tensioned the remnant towards the middle of the ACL footprint (between the anteromedial and posterolateral footprint) using one PushLock suture anchor (Arthrex). They hypothesized that using 1 anchor would be enough fixation for tears amenable to repair, and that doing so would minimize the invasion of the bone.
The preference of the senior author (GSD) is, however, to use 2 suture anchors for each bundle in order to more anatomically and biomechanically repair the remnant, since both bundles have different biomechanical characteristics.7 Similarly, the preference of the senior author is to commence the suturing as distal as possible and pass multiple sutures towards the proximal end. This ensures that the last suture pass is exited very proximally, and ensures that the proximal end is approximated towards the femoral wall. One suture passed at the midsubstance portion of the remnant might cause a different tension pattern and prevent optimal re-approximation of the most proximal part towards the femoral wall. Future studies are necessary to assess the efficacy of different suture and fixation techniques as these are currently lacking.
Addition of Internal Brace
Over the last few years, the senior author has added an internal brace (FiberTape, Arthrex) to the repair technique, which was first performed by MacKay and colleagues.8 The added internal brace protects the repair and the healing process in the first few weeks and enables early ROM.
With this technique, the previously described arthroscopic primary repair technique is performed with suturing of both bundles. However, after punching, tapping, or drilling a hole in the anteromedial origin of the femoral footprint, the anteromedial anchor is first loaded with the FiberTape in addition to the repair stitches. After placing the anteromedial suture anchor in the femoral footprint, the internal brace is fixated proximally with the suture anchor into the femoral wall.
Others, however, have advocated fixing the internal brace independently of the repaired ligament and suture anchors.9 With this technique, tunnels are drilled in the femur and tibia and the internal brace construct is fixed proximally using a RetroButton (Arthrex) and fixed distally in the tibial metaphysis using a suture anchor. A disadvantage of this technique is that an extra femoral tunnel needs to be drilled, which is especially important in pediatric patients with the increased risk for growth disturbances.10
One Bundle Type I Tears: Single Bundle Augmented Repair
In some cases, the anteromedial or posterolateral bundle is a type I tear with good or excellent tissue quality, whereas the other bundle is not a type I tear or has poor tissue quality (Figure 3A). In these cases, a primary repair of one bundle is performed with a hamstring reconstruction of the other bundle.
First, a No. 2 FiberWire is used to make 4 to 5 passes from distal to proximal, as previously described. Then, the remnants of the irreparable bundle are debrided (Figure 3B). Subsequently, the semitendinosus tendon is harvested in standard fashion, or soft tissue allografts can be used.
Type II Tears: Augmented Repair
In patients with type II tears, primary repair is not possible as the length of the remnant is too short to firmly approximate the remnant towards the femoral wall (75%-90% of native tissue length) (Figure 4A). In these patients, an augmented repair of the entire ACL is performed using hamstring autograft or soft tissue allograft.
With this technique, repair stitches are passed into the anteromedial bundle of the remnant as previously described (Figure 4B). Keeping the repair stitches anteriorly in the anteromedial bundle tends to prevent entanglement during graft passage later in the case.
Once the repair stitches are in place, a small accessory stab incision is made just above the medial portal. The repair stitches are parked here to keep them out of harms way. Traction on the repair stitches will retract the ACL away from the lateral wall of the notch and allow work to be performed here. A small opening notchplasty is generally performed to enhance visualization and to add a bleeding surface for enhanced healing. Next, the arthroscope is placed into the medial portal, which allows the femoral guide to be placed into the lateral portal. The femoral guide is positioned to optimize the femoral tunnel location in the center of the footprint. A small incision is made laterally over the condyle and through the iliotibial band to allow access to the lateral cortex of the lateral femoral condyle. The FlipCutter is then used to back-cut the femoral socket as described above. A FiberStick (Arthrex) passing suture is then placed in the femoral tunnel and brought out through the anteromedial portal.
Next, the tibial tunnel is drilled with a tibial guide at 55° inclination. The pin is drilled up into the center of the tibial footprint and this is over-reamed with a reamer. The reaming is stopped precisely upon breaking to proximal tibial cortex so as to minimize soft tissue damage of the ACL insertion fibers that are typically pristine. Then, a grasper is passed up and through the tunnel to retrieve the repair stitches and bring them out distally for later use. At the same time, the passing suture in the femoral is also retrieved distally. The soft tissue graft is proximally prepared with a TightRope RT button, and the repair stitches are passed through the button. The passing suture from the femoral socket is then used to shuttle the draw sutures and repair stitches up through the tibia, through the ACL remnant, and out the femoral socket (Figure 4C). The TightRope RT button is then engaged on the lateral femoral cortex in standard fashion. Using the cinch stitches, the graft is delivered through the tibia, up and through the center of the ACL remnant, and into the femoral socket. The knee is then cycled and the graft is tensioned distally in standard fashion, and fixed using a BioComposite interference screw. Finally, the repair stitches can be tensioned pulling the ligament remnant up as a sleeve around the hamstring graft (Figure 4D). They are then tied over the TightRope RT button using alternating half hitches tied with a knot pusher from laterally.
Type III Tears: Reconstruction With Remnant Tensioning
The previously discussed techniques have the goals of preserving as much native ligament remnant as possible, approximating the ligament remnant towards the femoral wall, and promoting healing of the ligament. In some cases, however, the ligament remnant is too short for healing (Figure 5A). Although the ligament cannot be approximated to the femoral wall in these cases, there is still an argument for ACL preservation, as was discussed in the first article of this series.
If the ligament length is between 25% and 75% of the native tissue length, the senior author performs a remnant tensioning technique.
Type IV Tears: Reconstruction With Remnant Preservation
Finally, in some cases, the distal remnant is small or the tissue quality in the largest part of the remnant is poor, and after debriding back to good tissue quality, only 10% to 25% of the native tissue length is left (Figure 6A). In these cases, the remnant is preserved, however, tensioning of the remnant with sutures is usually not necessary for the prevention of cyclops lesions. Nonetheless, it is important to debride the parts of the remnant ligament with poor tissue quality as mop-end patterns of the remnant may increase the chance of these lesions (Figure 6B).
In this situation, any of the standard ACL reconstruction techniques can be performed with simple attention being paid to preserving what is left of the tibial insertion site. At the very least, the small insertion remnant guides the anatomic placement of the graft, and prevents egress of joint fluid into the tibial tunnel and could minimize tunnel widening.
Type V Tears: Primary Repair
Finally, in some patients a soft tissue avulsion (Figure 7A) or bony avulsion of the distal attachment of the ACL can be seen. Both injuries are relatively rare, although bony avulsions are frequently seen in children, especially those younger than 12 years old. In these cases, the same techniques and theory that are applied to proximal avulsion type tears can be used and applied to distal avulsion type tears.
First, No. 2 FiberWire sutures are passed from proximal towards the distal end of the ligament in the anteromedial bundle, and the same process is then repeated for No. 2 TigerWire sutures for the posterolateral bundle. Then both sutures are exited at the distal avulsed end at the locations of the anteromedial and posterolateral footprints (Figure 7B). A 2.4-mm ACL guide wire and a Ninitol wire are used to drill 2 tunnels from the tibia towards the tibial footprint. The repair sutures are then retrieved through both tunnels (Figure 7C) and the sutures are tied distally over a ligament button after cycling of the knee (Figure 7D). This technique is very useful for soft tissue avulsions, or when there are only small flecks of bone or when the avulsed bone is significantly comminuted. If a large bony avulsion fragment is present, this technique can also be applied with some modification, although there have been multiple other techniques described in the literature that work well in this situation including fixation with screw and washer, or with suture anchors.
Complex Tear or Poor Tissue Quality: Reconstruction
In some cases, the tissue quality is poor, or the ligament has complex or multiple tears. Essentially, in these cases, there is nothing to preserve and a standard reconstruction approach is performed in these cases.
Conclusion
The uniform gold standard for all ACL tear types is currently primary reconstruction. However, several disadvantages of ACL reconstruction exist, while there are multiple advantages to the concept of ACL preservation. In this surgical technique article, we have discussed our tear type classification and the recommended surgical techniques for each. With this treatment algorithm, which is based on tear location and tissue quality, an optimal and minimally invasive treatment can be chosen for each individual patient. Future studies are needed to compare and contrast these treatments with the current gold standard of ACL reconstruction.
Am J Orthop. 2016;45(7):E406-E414. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
2. Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25(3):255-260.
3. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
4. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
5. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
6. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016. [Epub ahead of print]
7. Amis AA. The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):613-620.
8. MacKay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
9. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
10. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
11. Crain EH, Fithian DC, Paxton EW, Luetzow WF. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy. 2005;21(1):19-24.
1. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair. Defining a rationale for augmentation. Am J Sports Med. 1991;19(3):243-255.
2. Steadman JR, Matheny LM, Briggs KK, Rodkey WG, Carreira DS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg. 2012;25(3):255-260.
3. DiFelice GS, Villegas C, Taylor SA. Anterior cruciate ligament preservation: early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy. 2015;31(11):2162-2171.
4. DiFelice GS, van der List JP. Arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthrosc Tech. 2016. In press.
5. van der List JP, DiFelice GS. Gap formation following primary anterior cruciate ligament repair: a biomechanical study. Knee. 2016. In press.
6. Achtnich A, Herbst E, Forkel P, et al. Acute proximal anterior cruciate ligament tears: outcomes after arthroscopic suture anchor repair versus anatomic single-bundle reconstruction. Arthroscopy. 2016. [Epub ahead of print]
7. Amis AA. The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):613-620.
8. MacKay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ. A review of ligament augmentation with the InternalBrace™: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int. 2015;26:239-255.
9. Smith JO, Yasen SK, Palmer HC, Lord BR, Britton EM, Wilson AJ. Paediatric ACL repair reinforced with temporary internal bracing. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1845-1851.
10. Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy. 2010;26(11):1539-1550.
11. Crain EH, Fithian DC, Paxton EW, Luetzow WF. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy. 2005;21(1):19-24.
Allografts for Ligament Reconstruction: Where Are We Now?
Musculoskeletal allografts are becoming increasingly accepted as a viable alternative to autografts in a variety of orthopedic procedures. A 2006 American Orthopaedic Society for Sports Medicine (AOSSM) survey indicated that 86% of the participating 365 orthopedic surgeons use allografts in their practice.1 Although the overwhelming majority of orthopedic surgeons use allografts, they share common concerns, including safety, tissue integrity, and biologic incorporation. It is essential for the orthopedic surgeon to understand the current standards of tissue banking, risks and benefits related to the use of allografts, and common indications for safe use in clinical practice. This article reviews the current status of musculoskeletal allografts, including tissue procurement and processing, infections, complications, and specific uses tailored to ligament reconstruction.
Donor Bank, Processing, Sterilization, and Regulation
In the United States, the American Association of Tissue Banks (AATB) is responsible for establishing the standards for more than 100 accredited tissue banks. These tissue banks recover tissue from approximately 30,000 donors annually and account for an estimated 90% of the available musculoskeletal allografts used in the United States. While not all tissue banks are accredited by the AATB, all are required to register with the Food and Drug Administration (FDA), which allows for unannounced inspections of any facility. Facilities are required to abide by the FDA-implemented Current Good Tissue Practices (CGTP), which encompasses regulations on all donor tissue collected after May 2005 to help prevent the transmission of communicable diseases. The FDA released an updated draft in January 2009 that emphasizes safe practices and regulations spanning from environmental control to specific equipment.2
The safety of a transplanted allograft tissue begins within the tissue bank. Donor screening and testing is the first step in reducing the risk of transmission. Screening consists of collecting medical and social history from the family and any healthcare resources to assess the eligibility of the donor. If prior blood donations or autopsy information is available, that information is scrutinized. Donor tissue undergoes nucleic acid testing (NAT), which is required by both the AATB and FDA. All donor tissue must be screened for both types of human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), treponema pallidum, and human transmissible spongiform encephalopathies.3 NAT of donor tissue effectively reduces the risk of viral transmission. Additionally, routine preprocessing swabs for bacterial and fungal cultures are performed, although the sensitivity of these cultures ranges from 78% to 92%.4
After donor screening and testing, allograft tissues are usually obtained under aseptic conditions, though this is not FDA-required.5 Once procured, the tissue undergoes sterilization. Currently, there is no standard method ubiquitous to all tissue banks, nor does the FDA require a specific method. Rather, the FDA and AATB require tissue banks to validate their sterilization process and provide supporting data. The goal of sterilization is to inactivate viruses and eradicate bacteria while maintaining the biological and mechanical properties of the tissue. The AATB requires a Sterility Assurance Level (SAL) of 10-6, meaning there is no more than one in a million chance that a nonviral viable microbe exists on or within the tissue. Sterilization techniques may include both radiation and a variety of chemical reagents. Gamma irradiation is a commonly used method of sterilizing soft tissue allografts, although some studies indicate that it is detrimental to tissue biology.6 Newer methods of sterilization are being tested, one of which includes carbon dioxide in combination with antioxidants and irradiation. Bui and colleagues7 directly compared the biomechanical and histological properties of allograft tissue after either the standard 25 kGy gamma irradiation or supercritical carbon dioxide techniques. Although there is no histological difference, the samples treated with supercritical carbon dioxide had less biomechanical damage.7 Finally, the terminally sterilized allograft tissue is frozen to temperatures between -40°C and -80°C.5
Infections
One major concern of allografts is the risk of disease transmission. While numerous studies have investigated the incidence of bacterial infection following transplantation of allograft tissue, there are challenges associated with differentiating common postoperative infections from ones directly associated with the transmission of bacteria within the graft. There is a wide array of reported incidences of infection in the literature, from the Tomford and colleagues8 1981 study that reported a 6.9% rateto the 2001 study by Munting and colleagues,9 who reported 0% in their series. Multiple confounding variables exist, such as possible contamination during handling of an otherwise noncontaminated or properly sterilized allograft with inappropriate inclusion of all postoperative infections. In contrast, recognizing viral transmission has been somewhat easier, although reporting of these incidences has been variable in the past. In either case, there is no accredited reporting system for infections related to allografts.
Bacterial Transmission
Clostridium species. Clostridium species are commonly found among intestinal flora. There is a general consensus that between 24 to 48 hours after death intestinal flora transmigrates into the surrounding tissue and blood. Therefore, a commonly accepted recommendation is that cadaveric tissue needs to be excised prior to 24 hours postmortem.10
In 2001, a 23-year-old man underwent reconstructive knee surgery with a femoral condyle allograft. A few days after surgery, he became septic and ultimately died from the infection. Clostridium sordellii was cultured from the tissue. Several days later, a 17-year-old boy underwent reconstructive knee surgery with a fresh femoral condyle and frozen meniscus from the same donor. Twenty-four hours after surgery, he developed a fever and was readmitted a week later for presumed infection and treated effectively with penicillin and ampicillin/sulbactam. Tissue from the same cadaveric donor had been transplanted into 7 other patients without reports of infection. In a 2002 Centers for Disease Control and Prevention (CDC) update report,11 there were 26 total bacterial cases from allografts and 13 cases were attributed to Clostridium. Malinin and colleagues10 reviewed 795 consecutive cadaveric donors and found that 64 (8.1%) had positive cultures for Clostridia. Of all the positive cultures for Clostridia, 81.3% had positive blood cultures, 57.8% had positive bone marrow aspirate cultures, and 46.9% had positive tissue cultures. They concluded that multiple cultures are required for cadaveric tissue donors in order to reach a higher sensitivity for Clostridial contamination, and these should be done routinely to guide the sterilization process.
Strep species. In 2003, a 17-year-old boy underwent anterior cruciate ligament (ACL) reconstruction with a patellar tendon allograft.12 About 1 week later, he was admitted for signs of infection and received intravenous antibiotics. He required surgical debridement, and intraoperative cultures grew Group A Streptococcus (GAS) that was also identified in the postmortem donor cultures. The tissues underwent processing in an antimicrobial solution and postprocessing cultures were negative for bacteria, but they were not sterilized. Tissues from this donor had been implanted in 5 other patients without report of infection. Following this event, recommendations have been made for prompt rejection of tissue with cultures positive for GAS, unless a sterilizing procedure is used.
Other bacteria. According to the 2002 CDC update, 11 of the 26 cases of bacterial infection reported to the agency were a combination of gram-negative bacilli, polymicrobial flora, or culture negative.11
Viral Transmission
The most effective way to prevent transmission of a viral disease from allografts is thorough donor screening. Since the AATB implemented NAT in 2005 for HIV and HCV, there have been no reported cases of transmission.3 Even prior to this, regular blood screening along with social questionnaires completed by donors or donor families eliminated high-risk donors and significantly decreased the rate of transmission.
Human Immunodeficiency Virus. The first reported case of HIV transmission via implantation of allograft was in 1988. Further investigation revealed that there were 8 transmissions between 1984 and 1986, when routine screening of donors had not yet been implemented. The last reported case of HIV transmission occurred in 1996 with an untested donor.13Hepatitis C Virus. There are several reported cases of HCV transmission that occurred where the donors initially tested negative for HCV. In one case, 40 allografts from the same donor were transplanted over a period of nearly 2 years. This resulted in at least 8 patients being infected with HCV.14 Another case of HCV transmission was reported in 2005 after a patient developed acute HCV 6 weeks after transplantation of a patellar tendon allograft. Further investigation revealed that there had been 3 additional cases over a year from the same donor. Researchers determined that if the initial case had been reported, at least 3 transmissions could have been prevented.15Human T-cell Lymphotropic Virus (HTLV).The first reported transmission of HTLV was in 1991. This was reported in an asymptomatic patient who received a femoral head allograft from a donor who had been previously infected via a blood transfusion.16Zika virus. With recent outbreaks of the Zika virus, the FDA recently released recommendations regarding the screening and deferral of donors, mainly for blood transfusion. Orthopedists should take into consideration the potential for transmission through allografts. The FDA states that all potential donors should be screened for Zika virus using questionnaires and whole blood tests. Symptomatic donors are deferred at least 4 weeks following resolution of symptoms. While this is a recent recommendation from the FDA, orthopedists must be cognizant of the potential harms from this unfamiliar and evolving situation.17
Graft Specifics
Anterior Cruciate Ligament
ACL reconstruction is one of the most commonly performed surgeries by orthopedic surgeons, with an estimated 200,000 reconstructions per year.18Despite the popularity of this surgery, controversies remain regarding the optimal graft for reconstruction.19,20 One would provide adequate strength, be readily available, not elicit an immunologic response from the host, rapidly incorporate, elicit low morbidity, and vascularize early. Current options include both autografts and allografts. Common autograft options include patellar bone-tendon-bone (PBTB), hamstrings tendon, quadriceps tendon, and iliotibial band. PBTB autograft remains a common choice among orthopedic surgeons, as it allows early incorporation of the graft into bone and eliminates immune rejection. However, donor site morbidity, including anterior knee pain, weakness of knee extension, joint stiffness, increased postoperative pain, and iatrogenic patella fractures, have been reported in the literature.21 Commonly used allograft options include donor bone-patellar tendon-bone, quadriceps tendon, Achilles tendon, anterior and posterior tibialis tendons, hamstring tendons, and iliotibial band. Allografts provide the advantage of avoiding donor site morbidity, being readily available, allowing for shorter operative times, and providing lower postoperative pain compared to autografts, although they carry the risk of disease transmission, rejection, and slower incorporation into bone.22-27
Autograft donor site morbidities. One of the general disadvantages of autografts is the donor site morbidity associated with harvesting the grafts. In specific, PBTB grafts allow for bony blocks on both ends of the graft to incorporate into the host bone. However, this technique comes with the risk of disrupting the extensor mechanism.28,29 Milankov and colleagues30 published a retrospective review of over 2000 ACLs using autologous PBTB graft. They noted a 0.45% incidence of patella fracture and 0.18% patellar tendon rupture.30 Others have reported that intraoperative repair of the patellar tendon after tendon harvesting can increase infrapatellar fibrosis, thus increasing the risk for stiffness.31-33
Hamstring autografts include the semitendinosus and the gracilis tendons. The harvesting process is technically demanding and can be complicated by inadvertent amputation of the tendons, making the graft unsuitable for reconstructive purposes.34 Additionally, several reports have identified persistent numbness and hyperesthesia following hamstring harvesting due to iatrogenic injury to the prepatellar branches of the saphenous nerve.35,36A comprehensive review by Slone and colleagues37 reported comparable functional outcomes with quadriceps tendon autograft compared to PBTB; however, this comes with the risk of postoperative hematoma formation and the potential for thigh compartment syndrome.
Biology and Biomechanics of Allografts
One of the major disadvantages of allografts is the reduced ability to incorporate into the host tissue. Several in vitro and animal studies have suggested that allografts incorporate in the host slower than autografts.24,26,38 Early studies by Jackson and colleagues24 on goat models demonstrated that allografts and autografts have similar structural and biological properties initially, but allografts display significantly slower incorporation into the host tissue at 6 months. Histologically, allografts demonstrated lower revascularization, a smaller cross-sectional area, and a prolonged inflammatory response at 6 months postoperatively.24,39,40 Muramatsu and colleagues41 further showed through the use of magnetic resonance imaging a slower rate of revascularization of allografts over 2 years post-reconstruction.
Acknowledging these limitations, one should use caution when choosing to use an allograft or starting aggressive early rehabilitation after an allograft reconstruction, especially in athletes and young patients.
Clinical Outcomes
Although in vitro studies demonstrate inferior strength and delayed incorporation of allografts in the early postoperative period, there is still controversy surrounding the clinical and functional outcomes. Numerous studies have identified allografts as a viable option for ACL reconstruction, with similar reported patient satisfaction scores compared to autografts.43,44
The MOON Consortium recently published a prospective study of nearly 2500 subjects looking to identify risk factors for failure of ACL reconstruction. The study found that allografts had an odds ratio for failure 5.2 times that of PBTB autografts, correlating this factor to an increased re-tear rate of 6.9% in the allograft group compared to 3.2% in the PBTB group (P < .01).45 The elevated risk is more prevalent in younger patients, especially athletic teenagers. This issue has been reiterated in multiple studies.45-50A meta-analysis by Hu and colleagues23 identified 9 studies, either randomized control trials or prospective cohort studies, that looked at clinical outcomes between the different graft choices. They showed there was no significant difference between graft options in terms of instrumental laxity (P = .59), Lachman test (P = .41), pivot shift test (P = .88), and multiple functional outcome scores, including the International Knee Documentation Committee (IKDC), Lysholm, and Tegner scores.23,51-59Processing and sterilization techniques are thought to play a role in allograft failure. Guo and other researchers have demonstrated a significantly higher rate of failure for patients who received gamma-irradiated allografts compared to fresh frozen allografts.23,58-64 With improved sterilization techniques and a strict selection process of donors, gamma radiation has fallen out of favor to protect the biological characteristics of the tissue graft.5,65,66Several factors need to be considered when selecting between allograft or autograft tissue for ligamentous reconstruction. The selection must be balanced between the surgeon’s experience, patient and surgeon preferences, age of the patient, level of physical activity, primary or revision surgical setting, multiligamentous failure, geographical availability of donor grafts, and economical factors.
Medial Patellofemoral Ligament Reconstruction
Another relatively recent application for allografts has been described for the reconstruction of the medial patellofemoral ligament (MPFL) in recurrent lateral patellar dislocations.67-74
Typically, MPFL reconstructions make use of autografts, including quadriceps tendon, patellar tendon, and hamstring ligaments. However, allografts have the potential to limit postoperative donor site morbidity and to allow a faster rehabilitation.75,76 Allografts include semitendinosus, gracilis, anterior tibialis, posterior tibialis, and quadriceps tendons.
Calvo Rodríguez and colleagues76 performed a retrospective review in 2015 comparing allografts to autografts for MPFL reconstruction with respect to postoperative knee function and re-dislocation rates. Among the collective 28 patients, there was no difference in overall functional scores or dislocation rates between the grafts. Although this was a retrospective review and had a small number of subjects, the findings identify allografts as a reliable graft option for MPFL reconstruction.76While there has been a surge of interest in techniques for MPFL reconstruction, there is limited research available regarding the superiority of allografts compared to autografts. For this specific application, it seems that clinical outcomes correlate more to adequate stabilization of the patellofemoral joint than to the type of graft used.77,78 Future research should be dedicated to prospective randomized control trials to delineate any disadvantages to using allografts for MPFL reconstruction.
Discussion
Musculoskeletal allografts are gaining popularity for ligamentous reconstruction as their safety and efficacy continue to improve. With the great majority of tissue banks being accredited by the AATB and specific regulations such as NAT screening becoming common practice, infection rates and transmission of diseases have become incredibly rare. However, a thorough consideration needs to be taken into account when choosing between autograft and allograft on a case-by-case basis (Table).
Am J Orthop. 2016;45(7):446-453. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. The American Orthopaedic Society for Sports Medicine. Allografts for ACL Reconstruction Survey Report. 2013. http://www.sportsmed.org/AOSSMIMIS/members/downloads/research/AllograftACLReconstructionSurveyReport.pdf. Accessed October 21, 2016.
2. US Department of Health and Human Services, Food and Drug Administration. Guidance for industry: Current good tissue practice (CGTP) and additional requirements for manufacturers of human cells, tissues, and cellular and tissue-based products (HCT/Ps). http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM285223.pdf. Published December 2011. Accessed August 17, 2015.
3. Vaishnav S, Thomas Vangsness C Jr, Dellamaggiora R. New techniques in allograft tissue processing. Clin Sports Med. 2009;28(1):127-141.
4. Veen MR, Bloem RM, Petit PL. Sensitivity and negative predictive value of swab cultures in musculoskeletal allograft procurement. Clin Orthop Relat Res. 1994;(300):259-263.
5. McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35(12):2148-2158.
6. Mickiewicz P, Binkowski M, Bursig H, Wróbel Z. Preservation and sterilization methods of the meniscal allografts: literature review. Cell Tissue Bank. 2014;15(3):307-317.
7. Bui D, Lovric V, Oliver R, Bertollo N, Broe D, Walsh WR. Meniscal allograft sterilisation: effect on biomechanical and histological properties. Cell Tissue Bank. 2015;16(3):467-475.
8. Tomford WW, Starkweather RJ, Goldman MH. A study of the clinical incidence of infection in the use of banked allograft bone. J Bone Joint Surg Am. 1981;63(2):244-248.
9. Munting E, Faundez A, Manche E. Vertebral reconstruction with cortical allograft: long-term evaluation. Eur Spine J. 2001;10 Suppl 2:S153-S157.
10. Malinin TI, Buck BE, Temple HT, Martinez OV, Fox WP. Incidence of clostridial contamination in donors’ musculoskeletal tissue. J Bone Joint Surg Br. 2003;85(7):1051-1054.
11. Centers for Disease Control and Prevention (CDC). Update: allograft-associated bacterial infections--United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(10):207-210.
12. Centers for Disease Control and Prevention (CDC). Invasive Streptococcus pyogenes after allograft implantation--Colorado, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(48):1174-1176.
13. Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noël L, Strong DM. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation project NOTIFY. Int Orthop. 2012;36(3):633-641.
14. Schratt HE, Regel G, Kiesewetter B, Tscherne H. HIV infection caused by cold preserved bone transplants. Unfallchirurg. 1996;99(9):679-684.
15. Tugwell BD, Patel PR, Williams IT, et al. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med. 2005;143(9):648-654.
16. Sanzén L, Carlsson A. Transmission of human T-cell lymphotrophic virus type 1 by a deep-frozen bone allograft. Acta Orthop Scand. 1997;68(1):72-74.
17. US Department of Health and Human Services, Food and Drug Administration. Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Published February 2016. Accessed August 10, 2016.
18. Gottlob CA, Baker CL Jr, Pellissier JM, Colvin L. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res. 1999;(367):272-282.
19. Fu F, Christel P, Miller MD, Johnson DL. Graft selection for anterior cruciate ligament reconstruction. Instr Course Lect. 2009;58:337-354.
20. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
21. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
22. Harner CD, Irrgang JJ, Paul J, Dearwater S, Fu FH. Loss of motion after anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):499-506.
23. Hu J, Qu J, Xu D, Zhou J, Lu H. Allograft versus autograft for anterior cruciate ligament reconstruction: an up-to-date meta-analysis of prospective studies. Int Orthop. 2013;37(2):311-320.
24. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
25. Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16(10):559-565.
26. Malinin TI, Levitt RL, Bashore C, Temple HT, Mnaymneh W. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002;18(2):163-170.
27. Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. Am J Sports Med. 2010;38(1):189-199.
28. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
29. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
30 Milankov M, Kecojević V, Rasović P, Kovacević N, Gvozdenović N, Obradović M. Disruption of the knee extensor apparatus complicating anterior cruciate ligament reconstruction. Acta Chir Iugosl. 2013;60(2):13-21.
31. Atkinson TS, Atkinson PJ, Mendenhall HV, Haut RC. Patellar tendon and infrapatellar fat pad healing after harvest of an ACL graft. J Surg Res. 1998;79(1):25-30.
32. Tang G, Niitsu M, Ikeda K, Endo H, Itai Y. Fibrous scar in the infrapatellar fat pad after arthroscopy: MR imaging. Radiat Med. 2000;18(1):1-5.
33. Unterhauser FN, Bosch U, Zeichen J, Weiler A. Alpha-smooth muscle actin containing contractile fibroblastic cells in human knee arthrofibrosis tissue. Winner of the AGA-DonJoy Award 2003. Arch Orthop Trauma Surg. 2004;124(9):585-591.
34. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
35. Sabat D, Kumar V. Nerve injury during hamstring graft harvest: a prospective comparative study of three different incisions. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2089-2095.
36. Kjaergaard J, Faunø LZ, Faunø P. Sensibility loss after ACL reconstruction with hamstring graft. Int J Sports Med. 2008;29(6):507-511.
37. Slone HS, Romine SE, Premkumar A, Xerogeanes JW. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015;31(3):541-554.
38. Nikolaou PK, Seaber AV, Glisson RR, Ribbeck BM, Bassett FH 3rd. Anterior cruciate ligament allograft transplantation. Long-term function, histology, revascularization, and operative technique. Am J Sports Med. 1986;14(5):348-360.
39. Arnoczky SP, Warren RF, Ashlock MA. Replacement of the anterior cruciate ligament using a patellar tendon allograft. An experimental study. J Bone Joint Surg Am. 1986;68(3):376-385.
40. Scheffler SU, Schmidt T, Gangéy I, Dustmann M, Unterhauser F, Weiler A. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24(4):448-458.
41. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24(9):1038-1044.
42. Jackson DW, Grood ES, Arnoczky SP, Butler DL, Simon TM. Freeze dried anterior cruciate ligament allografts. Preliminary studies in a goat model. Am J Sports Med. 1987;15(4):295-303.
43. Chang SK, Egami DK, Shaieb MD, Kan DM, Richardson AB. Anterior cruciate ligament reconstruction: allograft versus autograft. Arthroscopy. 2003;19(5):453-462.
44. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
45. Kaeding CC, Pedroza AD, Reinke EK, Huston LJ; MOON Consortium, Spindler KP. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am J Sports Med. 2015;43(7):1583-1590.
46. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81.
47. Lynch TS, Parker RD, Patel RM, et al. The impact of the Multicenter Orthopaedic Outcomes Network (MOON) research on anterior cruciate ligament reconstruction and orthopaedic practice. J Am Acad Orthop Surg. 2015;23(3):154-163.
48. Hettrich CM, Dunn WR, Reinke EK; MOON Group, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534-1540.
49. Steadman JR, Matheny LM, Hurst JM, Briggs KK. Patient-centered outcomes and revision rate in patients undergoing ACL reconstruction using bone-patellar tendon-bone autograft compared with bone-patellar tendon-bone allograft: a matched case-control study. Arthroscopy. 2015;31(12):2320-2326.
50. Lenehan EA, Payne WB, Askam BM, Grana WA, Farrow LD. Long-term outcomes of allograft reconstruction of the anterior cruciate ligament. Am J Orthop. 2015;44(5):217-222.
51. Noh JH, Yi SR, Song SJ, Kim SW, Kim W. Comparison between hamstring autograft and free tendon achilles allograft: minimum 2-year follow-up after anterior cruciate ligament reconstruction using EndoButton and Intrafix. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):816-822.
52. Victor J, Bellemans J, Witvrouw E, Govaers K, Fabry G. Graft selection in anterior cruciate ligament reconstruction--prospective analysis of patellar tendon autografts compared with allografts. Int Orthop. 1997;21(2):93-97.
53. Kleipool AE, Zijl JA, Willems WJ. Arthroscopic anterior cruciate ligament reconstruction with bone-patellar tendon-bone allograft or autograft. A prospective study with an average follow up of 4 years. Knee Surg Sports Traumatol Arthrosc. 1998;6(4):224-230.
54. Peterson RK, Shelton WR, Bomboy AL. Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: a 5-year follow-up. Arthroscopy. 2001;17(1):9-13.
55. Edgar CM, Zimmer S, Kakar S, Jones H, Schepsis AA. Prospective comparison of auto and allograft hamstring tendon constructs for ACL reconstruction. Clin Orthop Relat Res. 2008;466(9):2238-2246.
56. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
57. Leal-Blanquet J, Alentorn-Geli E, Tuneu J, Valentí JR, Maestro A. Anterior cruciate ligament reconstruction: a multicenter prospective cohort study evaluating 3 different grafts using same bone drilling method. Clin J Sport Med. 2011;21(4):294-300.
58. Sun K, Zhang J, Wang Y, et al. Arthroscopic reconstruction of the anterior cruciate ligament with hamstring tendon autograft and fresh-frozen allograft: a prospective, randomized controlled study. Am J Sports Med. 2011;39(7):1430-1438.
59. Lawhorn KW, Howell SM, Traina SM, Gottlieb JE, Meade TD, Freedberg HI. The effect of graft tissue on anterior cruciate ligament outcomes: a multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28(8):1079-1086.
60. Guo L, Yang L, Duan XJ, et al. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft: comparison of autograft, fresh-frozen allograft, and γ-irradiated allograft. Arthroscopy. 2012;28(2):211-217.
61. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
62. Mayr HO, Willkomm D, Stoehr A, et al. Revision of anterior cruciate ligament reconstruction with patellar tendon allograft and autograft: 2- and 5-year results. Arch Orthop Trauma Surg. 2012;132(6):867-874.
63. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2014;42(2):492-499.
64. Mehta VM, Mandala C, Foster D, Petsche TS. Comparison of revision rates in bone-patella tendon-bone autograft and allograft anterior cruciate ligament reconstruction. Orthopedics. 2010;33(1):12.
65. Vangsness CT Jr, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31(3):474-481.
66. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
67. Reagan J, Kullar R, Burks R. MPFL reconstruction: technique and results. Clin Sports Med. 2014;33(3):501-516.
68. Christiansen SE, Jacobsen BW, Lund B, Lind M. Reconstruction of the medial patellofemoral ligament with gracilis tendon autograft in transverse patellar drill holes. Arthroscopy. 2008;24(1):82-87.
69. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521.
70. Deie M, Ochi M, Sumen Y, Adachi N, Kobayashi K, Yasumoto M. A long-term follow-up study after medial patellofemoral ligament reconstruction using the transferred semitendinosus tendon for patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):522-528.
71. Nomura E, Inoue M. Hybrid medial patellofemoral ligament reconstruction using the semitendinous tendon for recurrent patellar dislocation: minimum 3 years’ follow-up. Arthroscopy. 2006;22(7):787-793.
72. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):E47.
73. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
74. Drez D Jr, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthroscopy. 2001;17(3):298-306.
75. Fink C, Veselko M, Herbort M, Hoser C. MPFL reconstruction using a quadriceps tendon graft: part 2: operative technique and short term clinical results. Knee. 2014;21(6):1175-1179.
76. Calvo Rodríguez R, Figueroa Poblete D, Anastasiadis Le Roy Z, Etchegaray Bascur F, Vaisman Burucker A, Calvo Mena R. Reconstruction of the medial patellofemoral ligament: evaluation of the clinical results of autografts versus allografts. Rev Esp Cir Ortop Traumatol. 2015;59(5):348-353.
77. Becher C, Kley K, Lobenhoffer P, Ezechieli M, Smith T, Ostermeier S. Dynamic versus static reconstruction of the medial patellofemoral ligament for recurrent lateral patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2452-2457.
78. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435.
Musculoskeletal allografts are becoming increasingly accepted as a viable alternative to autografts in a variety of orthopedic procedures. A 2006 American Orthopaedic Society for Sports Medicine (AOSSM) survey indicated that 86% of the participating 365 orthopedic surgeons use allografts in their practice.1 Although the overwhelming majority of orthopedic surgeons use allografts, they share common concerns, including safety, tissue integrity, and biologic incorporation. It is essential for the orthopedic surgeon to understand the current standards of tissue banking, risks and benefits related to the use of allografts, and common indications for safe use in clinical practice. This article reviews the current status of musculoskeletal allografts, including tissue procurement and processing, infections, complications, and specific uses tailored to ligament reconstruction.
Donor Bank, Processing, Sterilization, and Regulation
In the United States, the American Association of Tissue Banks (AATB) is responsible for establishing the standards for more than 100 accredited tissue banks. These tissue banks recover tissue from approximately 30,000 donors annually and account for an estimated 90% of the available musculoskeletal allografts used in the United States. While not all tissue banks are accredited by the AATB, all are required to register with the Food and Drug Administration (FDA), which allows for unannounced inspections of any facility. Facilities are required to abide by the FDA-implemented Current Good Tissue Practices (CGTP), which encompasses regulations on all donor tissue collected after May 2005 to help prevent the transmission of communicable diseases. The FDA released an updated draft in January 2009 that emphasizes safe practices and regulations spanning from environmental control to specific equipment.2
The safety of a transplanted allograft tissue begins within the tissue bank. Donor screening and testing is the first step in reducing the risk of transmission. Screening consists of collecting medical and social history from the family and any healthcare resources to assess the eligibility of the donor. If prior blood donations or autopsy information is available, that information is scrutinized. Donor tissue undergoes nucleic acid testing (NAT), which is required by both the AATB and FDA. All donor tissue must be screened for both types of human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), treponema pallidum, and human transmissible spongiform encephalopathies.3 NAT of donor tissue effectively reduces the risk of viral transmission. Additionally, routine preprocessing swabs for bacterial and fungal cultures are performed, although the sensitivity of these cultures ranges from 78% to 92%.4
After donor screening and testing, allograft tissues are usually obtained under aseptic conditions, though this is not FDA-required.5 Once procured, the tissue undergoes sterilization. Currently, there is no standard method ubiquitous to all tissue banks, nor does the FDA require a specific method. Rather, the FDA and AATB require tissue banks to validate their sterilization process and provide supporting data. The goal of sterilization is to inactivate viruses and eradicate bacteria while maintaining the biological and mechanical properties of the tissue. The AATB requires a Sterility Assurance Level (SAL) of 10-6, meaning there is no more than one in a million chance that a nonviral viable microbe exists on or within the tissue. Sterilization techniques may include both radiation and a variety of chemical reagents. Gamma irradiation is a commonly used method of sterilizing soft tissue allografts, although some studies indicate that it is detrimental to tissue biology.6 Newer methods of sterilization are being tested, one of which includes carbon dioxide in combination with antioxidants and irradiation. Bui and colleagues7 directly compared the biomechanical and histological properties of allograft tissue after either the standard 25 kGy gamma irradiation or supercritical carbon dioxide techniques. Although there is no histological difference, the samples treated with supercritical carbon dioxide had less biomechanical damage.7 Finally, the terminally sterilized allograft tissue is frozen to temperatures between -40°C and -80°C.5
Infections
One major concern of allografts is the risk of disease transmission. While numerous studies have investigated the incidence of bacterial infection following transplantation of allograft tissue, there are challenges associated with differentiating common postoperative infections from ones directly associated with the transmission of bacteria within the graft. There is a wide array of reported incidences of infection in the literature, from the Tomford and colleagues8 1981 study that reported a 6.9% rateto the 2001 study by Munting and colleagues,9 who reported 0% in their series. Multiple confounding variables exist, such as possible contamination during handling of an otherwise noncontaminated or properly sterilized allograft with inappropriate inclusion of all postoperative infections. In contrast, recognizing viral transmission has been somewhat easier, although reporting of these incidences has been variable in the past. In either case, there is no accredited reporting system for infections related to allografts.
Bacterial Transmission
Clostridium species. Clostridium species are commonly found among intestinal flora. There is a general consensus that between 24 to 48 hours after death intestinal flora transmigrates into the surrounding tissue and blood. Therefore, a commonly accepted recommendation is that cadaveric tissue needs to be excised prior to 24 hours postmortem.10
In 2001, a 23-year-old man underwent reconstructive knee surgery with a femoral condyle allograft. A few days after surgery, he became septic and ultimately died from the infection. Clostridium sordellii was cultured from the tissue. Several days later, a 17-year-old boy underwent reconstructive knee surgery with a fresh femoral condyle and frozen meniscus from the same donor. Twenty-four hours after surgery, he developed a fever and was readmitted a week later for presumed infection and treated effectively with penicillin and ampicillin/sulbactam. Tissue from the same cadaveric donor had been transplanted into 7 other patients without reports of infection. In a 2002 Centers for Disease Control and Prevention (CDC) update report,11 there were 26 total bacterial cases from allografts and 13 cases were attributed to Clostridium. Malinin and colleagues10 reviewed 795 consecutive cadaveric donors and found that 64 (8.1%) had positive cultures for Clostridia. Of all the positive cultures for Clostridia, 81.3% had positive blood cultures, 57.8% had positive bone marrow aspirate cultures, and 46.9% had positive tissue cultures. They concluded that multiple cultures are required for cadaveric tissue donors in order to reach a higher sensitivity for Clostridial contamination, and these should be done routinely to guide the sterilization process.
Strep species. In 2003, a 17-year-old boy underwent anterior cruciate ligament (ACL) reconstruction with a patellar tendon allograft.12 About 1 week later, he was admitted for signs of infection and received intravenous antibiotics. He required surgical debridement, and intraoperative cultures grew Group A Streptococcus (GAS) that was also identified in the postmortem donor cultures. The tissues underwent processing in an antimicrobial solution and postprocessing cultures were negative for bacteria, but they were not sterilized. Tissues from this donor had been implanted in 5 other patients without report of infection. Following this event, recommendations have been made for prompt rejection of tissue with cultures positive for GAS, unless a sterilizing procedure is used.
Other bacteria. According to the 2002 CDC update, 11 of the 26 cases of bacterial infection reported to the agency were a combination of gram-negative bacilli, polymicrobial flora, or culture negative.11
Viral Transmission
The most effective way to prevent transmission of a viral disease from allografts is thorough donor screening. Since the AATB implemented NAT in 2005 for HIV and HCV, there have been no reported cases of transmission.3 Even prior to this, regular blood screening along with social questionnaires completed by donors or donor families eliminated high-risk donors and significantly decreased the rate of transmission.
Human Immunodeficiency Virus. The first reported case of HIV transmission via implantation of allograft was in 1988. Further investigation revealed that there were 8 transmissions between 1984 and 1986, when routine screening of donors had not yet been implemented. The last reported case of HIV transmission occurred in 1996 with an untested donor.13Hepatitis C Virus. There are several reported cases of HCV transmission that occurred where the donors initially tested negative for HCV. In one case, 40 allografts from the same donor were transplanted over a period of nearly 2 years. This resulted in at least 8 patients being infected with HCV.14 Another case of HCV transmission was reported in 2005 after a patient developed acute HCV 6 weeks after transplantation of a patellar tendon allograft. Further investigation revealed that there had been 3 additional cases over a year from the same donor. Researchers determined that if the initial case had been reported, at least 3 transmissions could have been prevented.15Human T-cell Lymphotropic Virus (HTLV).The first reported transmission of HTLV was in 1991. This was reported in an asymptomatic patient who received a femoral head allograft from a donor who had been previously infected via a blood transfusion.16Zika virus. With recent outbreaks of the Zika virus, the FDA recently released recommendations regarding the screening and deferral of donors, mainly for blood transfusion. Orthopedists should take into consideration the potential for transmission through allografts. The FDA states that all potential donors should be screened for Zika virus using questionnaires and whole blood tests. Symptomatic donors are deferred at least 4 weeks following resolution of symptoms. While this is a recent recommendation from the FDA, orthopedists must be cognizant of the potential harms from this unfamiliar and evolving situation.17
Graft Specifics
Anterior Cruciate Ligament
ACL reconstruction is one of the most commonly performed surgeries by orthopedic surgeons, with an estimated 200,000 reconstructions per year.18Despite the popularity of this surgery, controversies remain regarding the optimal graft for reconstruction.19,20 One would provide adequate strength, be readily available, not elicit an immunologic response from the host, rapidly incorporate, elicit low morbidity, and vascularize early. Current options include both autografts and allografts. Common autograft options include patellar bone-tendon-bone (PBTB), hamstrings tendon, quadriceps tendon, and iliotibial band. PBTB autograft remains a common choice among orthopedic surgeons, as it allows early incorporation of the graft into bone and eliminates immune rejection. However, donor site morbidity, including anterior knee pain, weakness of knee extension, joint stiffness, increased postoperative pain, and iatrogenic patella fractures, have been reported in the literature.21 Commonly used allograft options include donor bone-patellar tendon-bone, quadriceps tendon, Achilles tendon, anterior and posterior tibialis tendons, hamstring tendons, and iliotibial band. Allografts provide the advantage of avoiding donor site morbidity, being readily available, allowing for shorter operative times, and providing lower postoperative pain compared to autografts, although they carry the risk of disease transmission, rejection, and slower incorporation into bone.22-27
Autograft donor site morbidities. One of the general disadvantages of autografts is the donor site morbidity associated with harvesting the grafts. In specific, PBTB grafts allow for bony blocks on both ends of the graft to incorporate into the host bone. However, this technique comes with the risk of disrupting the extensor mechanism.28,29 Milankov and colleagues30 published a retrospective review of over 2000 ACLs using autologous PBTB graft. They noted a 0.45% incidence of patella fracture and 0.18% patellar tendon rupture.30 Others have reported that intraoperative repair of the patellar tendon after tendon harvesting can increase infrapatellar fibrosis, thus increasing the risk for stiffness.31-33
Hamstring autografts include the semitendinosus and the gracilis tendons. The harvesting process is technically demanding and can be complicated by inadvertent amputation of the tendons, making the graft unsuitable for reconstructive purposes.34 Additionally, several reports have identified persistent numbness and hyperesthesia following hamstring harvesting due to iatrogenic injury to the prepatellar branches of the saphenous nerve.35,36A comprehensive review by Slone and colleagues37 reported comparable functional outcomes with quadriceps tendon autograft compared to PBTB; however, this comes with the risk of postoperative hematoma formation and the potential for thigh compartment syndrome.
Biology and Biomechanics of Allografts
One of the major disadvantages of allografts is the reduced ability to incorporate into the host tissue. Several in vitro and animal studies have suggested that allografts incorporate in the host slower than autografts.24,26,38 Early studies by Jackson and colleagues24 on goat models demonstrated that allografts and autografts have similar structural and biological properties initially, but allografts display significantly slower incorporation into the host tissue at 6 months. Histologically, allografts demonstrated lower revascularization, a smaller cross-sectional area, and a prolonged inflammatory response at 6 months postoperatively.24,39,40 Muramatsu and colleagues41 further showed through the use of magnetic resonance imaging a slower rate of revascularization of allografts over 2 years post-reconstruction.
Acknowledging these limitations, one should use caution when choosing to use an allograft or starting aggressive early rehabilitation after an allograft reconstruction, especially in athletes and young patients.
Clinical Outcomes
Although in vitro studies demonstrate inferior strength and delayed incorporation of allografts in the early postoperative period, there is still controversy surrounding the clinical and functional outcomes. Numerous studies have identified allografts as a viable option for ACL reconstruction, with similar reported patient satisfaction scores compared to autografts.43,44
The MOON Consortium recently published a prospective study of nearly 2500 subjects looking to identify risk factors for failure of ACL reconstruction. The study found that allografts had an odds ratio for failure 5.2 times that of PBTB autografts, correlating this factor to an increased re-tear rate of 6.9% in the allograft group compared to 3.2% in the PBTB group (P < .01).45 The elevated risk is more prevalent in younger patients, especially athletic teenagers. This issue has been reiterated in multiple studies.45-50A meta-analysis by Hu and colleagues23 identified 9 studies, either randomized control trials or prospective cohort studies, that looked at clinical outcomes between the different graft choices. They showed there was no significant difference between graft options in terms of instrumental laxity (P = .59), Lachman test (P = .41), pivot shift test (P = .88), and multiple functional outcome scores, including the International Knee Documentation Committee (IKDC), Lysholm, and Tegner scores.23,51-59Processing and sterilization techniques are thought to play a role in allograft failure. Guo and other researchers have demonstrated a significantly higher rate of failure for patients who received gamma-irradiated allografts compared to fresh frozen allografts.23,58-64 With improved sterilization techniques and a strict selection process of donors, gamma radiation has fallen out of favor to protect the biological characteristics of the tissue graft.5,65,66Several factors need to be considered when selecting between allograft or autograft tissue for ligamentous reconstruction. The selection must be balanced between the surgeon’s experience, patient and surgeon preferences, age of the patient, level of physical activity, primary or revision surgical setting, multiligamentous failure, geographical availability of donor grafts, and economical factors.
Medial Patellofemoral Ligament Reconstruction
Another relatively recent application for allografts has been described for the reconstruction of the medial patellofemoral ligament (MPFL) in recurrent lateral patellar dislocations.67-74
Typically, MPFL reconstructions make use of autografts, including quadriceps tendon, patellar tendon, and hamstring ligaments. However, allografts have the potential to limit postoperative donor site morbidity and to allow a faster rehabilitation.75,76 Allografts include semitendinosus, gracilis, anterior tibialis, posterior tibialis, and quadriceps tendons.
Calvo Rodríguez and colleagues76 performed a retrospective review in 2015 comparing allografts to autografts for MPFL reconstruction with respect to postoperative knee function and re-dislocation rates. Among the collective 28 patients, there was no difference in overall functional scores or dislocation rates between the grafts. Although this was a retrospective review and had a small number of subjects, the findings identify allografts as a reliable graft option for MPFL reconstruction.76While there has been a surge of interest in techniques for MPFL reconstruction, there is limited research available regarding the superiority of allografts compared to autografts. For this specific application, it seems that clinical outcomes correlate more to adequate stabilization of the patellofemoral joint than to the type of graft used.77,78 Future research should be dedicated to prospective randomized control trials to delineate any disadvantages to using allografts for MPFL reconstruction.
Discussion
Musculoskeletal allografts are gaining popularity for ligamentous reconstruction as their safety and efficacy continue to improve. With the great majority of tissue banks being accredited by the AATB and specific regulations such as NAT screening becoming common practice, infection rates and transmission of diseases have become incredibly rare. However, a thorough consideration needs to be taken into account when choosing between autograft and allograft on a case-by-case basis (Table).
Am J Orthop. 2016;45(7):446-453. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Musculoskeletal allografts are becoming increasingly accepted as a viable alternative to autografts in a variety of orthopedic procedures. A 2006 American Orthopaedic Society for Sports Medicine (AOSSM) survey indicated that 86% of the participating 365 orthopedic surgeons use allografts in their practice.1 Although the overwhelming majority of orthopedic surgeons use allografts, they share common concerns, including safety, tissue integrity, and biologic incorporation. It is essential for the orthopedic surgeon to understand the current standards of tissue banking, risks and benefits related to the use of allografts, and common indications for safe use in clinical practice. This article reviews the current status of musculoskeletal allografts, including tissue procurement and processing, infections, complications, and specific uses tailored to ligament reconstruction.
Donor Bank, Processing, Sterilization, and Regulation
In the United States, the American Association of Tissue Banks (AATB) is responsible for establishing the standards for more than 100 accredited tissue banks. These tissue banks recover tissue from approximately 30,000 donors annually and account for an estimated 90% of the available musculoskeletal allografts used in the United States. While not all tissue banks are accredited by the AATB, all are required to register with the Food and Drug Administration (FDA), which allows for unannounced inspections of any facility. Facilities are required to abide by the FDA-implemented Current Good Tissue Practices (CGTP), which encompasses regulations on all donor tissue collected after May 2005 to help prevent the transmission of communicable diseases. The FDA released an updated draft in January 2009 that emphasizes safe practices and regulations spanning from environmental control to specific equipment.2
The safety of a transplanted allograft tissue begins within the tissue bank. Donor screening and testing is the first step in reducing the risk of transmission. Screening consists of collecting medical and social history from the family and any healthcare resources to assess the eligibility of the donor. If prior blood donations or autopsy information is available, that information is scrutinized. Donor tissue undergoes nucleic acid testing (NAT), which is required by both the AATB and FDA. All donor tissue must be screened for both types of human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), treponema pallidum, and human transmissible spongiform encephalopathies.3 NAT of donor tissue effectively reduces the risk of viral transmission. Additionally, routine preprocessing swabs for bacterial and fungal cultures are performed, although the sensitivity of these cultures ranges from 78% to 92%.4
After donor screening and testing, allograft tissues are usually obtained under aseptic conditions, though this is not FDA-required.5 Once procured, the tissue undergoes sterilization. Currently, there is no standard method ubiquitous to all tissue banks, nor does the FDA require a specific method. Rather, the FDA and AATB require tissue banks to validate their sterilization process and provide supporting data. The goal of sterilization is to inactivate viruses and eradicate bacteria while maintaining the biological and mechanical properties of the tissue. The AATB requires a Sterility Assurance Level (SAL) of 10-6, meaning there is no more than one in a million chance that a nonviral viable microbe exists on or within the tissue. Sterilization techniques may include both radiation and a variety of chemical reagents. Gamma irradiation is a commonly used method of sterilizing soft tissue allografts, although some studies indicate that it is detrimental to tissue biology.6 Newer methods of sterilization are being tested, one of which includes carbon dioxide in combination with antioxidants and irradiation. Bui and colleagues7 directly compared the biomechanical and histological properties of allograft tissue after either the standard 25 kGy gamma irradiation or supercritical carbon dioxide techniques. Although there is no histological difference, the samples treated with supercritical carbon dioxide had less biomechanical damage.7 Finally, the terminally sterilized allograft tissue is frozen to temperatures between -40°C and -80°C.5
Infections
One major concern of allografts is the risk of disease transmission. While numerous studies have investigated the incidence of bacterial infection following transplantation of allograft tissue, there are challenges associated with differentiating common postoperative infections from ones directly associated with the transmission of bacteria within the graft. There is a wide array of reported incidences of infection in the literature, from the Tomford and colleagues8 1981 study that reported a 6.9% rateto the 2001 study by Munting and colleagues,9 who reported 0% in their series. Multiple confounding variables exist, such as possible contamination during handling of an otherwise noncontaminated or properly sterilized allograft with inappropriate inclusion of all postoperative infections. In contrast, recognizing viral transmission has been somewhat easier, although reporting of these incidences has been variable in the past. In either case, there is no accredited reporting system for infections related to allografts.
Bacterial Transmission
Clostridium species. Clostridium species are commonly found among intestinal flora. There is a general consensus that between 24 to 48 hours after death intestinal flora transmigrates into the surrounding tissue and blood. Therefore, a commonly accepted recommendation is that cadaveric tissue needs to be excised prior to 24 hours postmortem.10
In 2001, a 23-year-old man underwent reconstructive knee surgery with a femoral condyle allograft. A few days after surgery, he became septic and ultimately died from the infection. Clostridium sordellii was cultured from the tissue. Several days later, a 17-year-old boy underwent reconstructive knee surgery with a fresh femoral condyle and frozen meniscus from the same donor. Twenty-four hours after surgery, he developed a fever and was readmitted a week later for presumed infection and treated effectively with penicillin and ampicillin/sulbactam. Tissue from the same cadaveric donor had been transplanted into 7 other patients without reports of infection. In a 2002 Centers for Disease Control and Prevention (CDC) update report,11 there were 26 total bacterial cases from allografts and 13 cases were attributed to Clostridium. Malinin and colleagues10 reviewed 795 consecutive cadaveric donors and found that 64 (8.1%) had positive cultures for Clostridia. Of all the positive cultures for Clostridia, 81.3% had positive blood cultures, 57.8% had positive bone marrow aspirate cultures, and 46.9% had positive tissue cultures. They concluded that multiple cultures are required for cadaveric tissue donors in order to reach a higher sensitivity for Clostridial contamination, and these should be done routinely to guide the sterilization process.
Strep species. In 2003, a 17-year-old boy underwent anterior cruciate ligament (ACL) reconstruction with a patellar tendon allograft.12 About 1 week later, he was admitted for signs of infection and received intravenous antibiotics. He required surgical debridement, and intraoperative cultures grew Group A Streptococcus (GAS) that was also identified in the postmortem donor cultures. The tissues underwent processing in an antimicrobial solution and postprocessing cultures were negative for bacteria, but they were not sterilized. Tissues from this donor had been implanted in 5 other patients without report of infection. Following this event, recommendations have been made for prompt rejection of tissue with cultures positive for GAS, unless a sterilizing procedure is used.
Other bacteria. According to the 2002 CDC update, 11 of the 26 cases of bacterial infection reported to the agency were a combination of gram-negative bacilli, polymicrobial flora, or culture negative.11
Viral Transmission
The most effective way to prevent transmission of a viral disease from allografts is thorough donor screening. Since the AATB implemented NAT in 2005 for HIV and HCV, there have been no reported cases of transmission.3 Even prior to this, regular blood screening along with social questionnaires completed by donors or donor families eliminated high-risk donors and significantly decreased the rate of transmission.
Human Immunodeficiency Virus. The first reported case of HIV transmission via implantation of allograft was in 1988. Further investigation revealed that there were 8 transmissions between 1984 and 1986, when routine screening of donors had not yet been implemented. The last reported case of HIV transmission occurred in 1996 with an untested donor.13Hepatitis C Virus. There are several reported cases of HCV transmission that occurred where the donors initially tested negative for HCV. In one case, 40 allografts from the same donor were transplanted over a period of nearly 2 years. This resulted in at least 8 patients being infected with HCV.14 Another case of HCV transmission was reported in 2005 after a patient developed acute HCV 6 weeks after transplantation of a patellar tendon allograft. Further investigation revealed that there had been 3 additional cases over a year from the same donor. Researchers determined that if the initial case had been reported, at least 3 transmissions could have been prevented.15Human T-cell Lymphotropic Virus (HTLV).The first reported transmission of HTLV was in 1991. This was reported in an asymptomatic patient who received a femoral head allograft from a donor who had been previously infected via a blood transfusion.16Zika virus. With recent outbreaks of the Zika virus, the FDA recently released recommendations regarding the screening and deferral of donors, mainly for blood transfusion. Orthopedists should take into consideration the potential for transmission through allografts. The FDA states that all potential donors should be screened for Zika virus using questionnaires and whole blood tests. Symptomatic donors are deferred at least 4 weeks following resolution of symptoms. While this is a recent recommendation from the FDA, orthopedists must be cognizant of the potential harms from this unfamiliar and evolving situation.17
Graft Specifics
Anterior Cruciate Ligament
ACL reconstruction is one of the most commonly performed surgeries by orthopedic surgeons, with an estimated 200,000 reconstructions per year.18Despite the popularity of this surgery, controversies remain regarding the optimal graft for reconstruction.19,20 One would provide adequate strength, be readily available, not elicit an immunologic response from the host, rapidly incorporate, elicit low morbidity, and vascularize early. Current options include both autografts and allografts. Common autograft options include patellar bone-tendon-bone (PBTB), hamstrings tendon, quadriceps tendon, and iliotibial band. PBTB autograft remains a common choice among orthopedic surgeons, as it allows early incorporation of the graft into bone and eliminates immune rejection. However, donor site morbidity, including anterior knee pain, weakness of knee extension, joint stiffness, increased postoperative pain, and iatrogenic patella fractures, have been reported in the literature.21 Commonly used allograft options include donor bone-patellar tendon-bone, quadriceps tendon, Achilles tendon, anterior and posterior tibialis tendons, hamstring tendons, and iliotibial band. Allografts provide the advantage of avoiding donor site morbidity, being readily available, allowing for shorter operative times, and providing lower postoperative pain compared to autografts, although they carry the risk of disease transmission, rejection, and slower incorporation into bone.22-27
Autograft donor site morbidities. One of the general disadvantages of autografts is the donor site morbidity associated with harvesting the grafts. In specific, PBTB grafts allow for bony blocks on both ends of the graft to incorporate into the host bone. However, this technique comes with the risk of disrupting the extensor mechanism.28,29 Milankov and colleagues30 published a retrospective review of over 2000 ACLs using autologous PBTB graft. They noted a 0.45% incidence of patella fracture and 0.18% patellar tendon rupture.30 Others have reported that intraoperative repair of the patellar tendon after tendon harvesting can increase infrapatellar fibrosis, thus increasing the risk for stiffness.31-33
Hamstring autografts include the semitendinosus and the gracilis tendons. The harvesting process is technically demanding and can be complicated by inadvertent amputation of the tendons, making the graft unsuitable for reconstructive purposes.34 Additionally, several reports have identified persistent numbness and hyperesthesia following hamstring harvesting due to iatrogenic injury to the prepatellar branches of the saphenous nerve.35,36A comprehensive review by Slone and colleagues37 reported comparable functional outcomes with quadriceps tendon autograft compared to PBTB; however, this comes with the risk of postoperative hematoma formation and the potential for thigh compartment syndrome.
Biology and Biomechanics of Allografts
One of the major disadvantages of allografts is the reduced ability to incorporate into the host tissue. Several in vitro and animal studies have suggested that allografts incorporate in the host slower than autografts.24,26,38 Early studies by Jackson and colleagues24 on goat models demonstrated that allografts and autografts have similar structural and biological properties initially, but allografts display significantly slower incorporation into the host tissue at 6 months. Histologically, allografts demonstrated lower revascularization, a smaller cross-sectional area, and a prolonged inflammatory response at 6 months postoperatively.24,39,40 Muramatsu and colleagues41 further showed through the use of magnetic resonance imaging a slower rate of revascularization of allografts over 2 years post-reconstruction.
Acknowledging these limitations, one should use caution when choosing to use an allograft or starting aggressive early rehabilitation after an allograft reconstruction, especially in athletes and young patients.
Clinical Outcomes
Although in vitro studies demonstrate inferior strength and delayed incorporation of allografts in the early postoperative period, there is still controversy surrounding the clinical and functional outcomes. Numerous studies have identified allografts as a viable option for ACL reconstruction, with similar reported patient satisfaction scores compared to autografts.43,44
The MOON Consortium recently published a prospective study of nearly 2500 subjects looking to identify risk factors for failure of ACL reconstruction. The study found that allografts had an odds ratio for failure 5.2 times that of PBTB autografts, correlating this factor to an increased re-tear rate of 6.9% in the allograft group compared to 3.2% in the PBTB group (P < .01).45 The elevated risk is more prevalent in younger patients, especially athletic teenagers. This issue has been reiterated in multiple studies.45-50A meta-analysis by Hu and colleagues23 identified 9 studies, either randomized control trials or prospective cohort studies, that looked at clinical outcomes between the different graft choices. They showed there was no significant difference between graft options in terms of instrumental laxity (P = .59), Lachman test (P = .41), pivot shift test (P = .88), and multiple functional outcome scores, including the International Knee Documentation Committee (IKDC), Lysholm, and Tegner scores.23,51-59Processing and sterilization techniques are thought to play a role in allograft failure. Guo and other researchers have demonstrated a significantly higher rate of failure for patients who received gamma-irradiated allografts compared to fresh frozen allografts.23,58-64 With improved sterilization techniques and a strict selection process of donors, gamma radiation has fallen out of favor to protect the biological characteristics of the tissue graft.5,65,66Several factors need to be considered when selecting between allograft or autograft tissue for ligamentous reconstruction. The selection must be balanced between the surgeon’s experience, patient and surgeon preferences, age of the patient, level of physical activity, primary or revision surgical setting, multiligamentous failure, geographical availability of donor grafts, and economical factors.
Medial Patellofemoral Ligament Reconstruction
Another relatively recent application for allografts has been described for the reconstruction of the medial patellofemoral ligament (MPFL) in recurrent lateral patellar dislocations.67-74
Typically, MPFL reconstructions make use of autografts, including quadriceps tendon, patellar tendon, and hamstring ligaments. However, allografts have the potential to limit postoperative donor site morbidity and to allow a faster rehabilitation.75,76 Allografts include semitendinosus, gracilis, anterior tibialis, posterior tibialis, and quadriceps tendons.
Calvo Rodríguez and colleagues76 performed a retrospective review in 2015 comparing allografts to autografts for MPFL reconstruction with respect to postoperative knee function and re-dislocation rates. Among the collective 28 patients, there was no difference in overall functional scores or dislocation rates between the grafts. Although this was a retrospective review and had a small number of subjects, the findings identify allografts as a reliable graft option for MPFL reconstruction.76While there has been a surge of interest in techniques for MPFL reconstruction, there is limited research available regarding the superiority of allografts compared to autografts. For this specific application, it seems that clinical outcomes correlate more to adequate stabilization of the patellofemoral joint than to the type of graft used.77,78 Future research should be dedicated to prospective randomized control trials to delineate any disadvantages to using allografts for MPFL reconstruction.
Discussion
Musculoskeletal allografts are gaining popularity for ligamentous reconstruction as their safety and efficacy continue to improve. With the great majority of tissue banks being accredited by the AATB and specific regulations such as NAT screening becoming common practice, infection rates and transmission of diseases have become incredibly rare. However, a thorough consideration needs to be taken into account when choosing between autograft and allograft on a case-by-case basis (Table).
Am J Orthop. 2016;45(7):446-453. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. The American Orthopaedic Society for Sports Medicine. Allografts for ACL Reconstruction Survey Report. 2013. http://www.sportsmed.org/AOSSMIMIS/members/downloads/research/AllograftACLReconstructionSurveyReport.pdf. Accessed October 21, 2016.
2. US Department of Health and Human Services, Food and Drug Administration. Guidance for industry: Current good tissue practice (CGTP) and additional requirements for manufacturers of human cells, tissues, and cellular and tissue-based products (HCT/Ps). http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM285223.pdf. Published December 2011. Accessed August 17, 2015.
3. Vaishnav S, Thomas Vangsness C Jr, Dellamaggiora R. New techniques in allograft tissue processing. Clin Sports Med. 2009;28(1):127-141.
4. Veen MR, Bloem RM, Petit PL. Sensitivity and negative predictive value of swab cultures in musculoskeletal allograft procurement. Clin Orthop Relat Res. 1994;(300):259-263.
5. McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35(12):2148-2158.
6. Mickiewicz P, Binkowski M, Bursig H, Wróbel Z. Preservation and sterilization methods of the meniscal allografts: literature review. Cell Tissue Bank. 2014;15(3):307-317.
7. Bui D, Lovric V, Oliver R, Bertollo N, Broe D, Walsh WR. Meniscal allograft sterilisation: effect on biomechanical and histological properties. Cell Tissue Bank. 2015;16(3):467-475.
8. Tomford WW, Starkweather RJ, Goldman MH. A study of the clinical incidence of infection in the use of banked allograft bone. J Bone Joint Surg Am. 1981;63(2):244-248.
9. Munting E, Faundez A, Manche E. Vertebral reconstruction with cortical allograft: long-term evaluation. Eur Spine J. 2001;10 Suppl 2:S153-S157.
10. Malinin TI, Buck BE, Temple HT, Martinez OV, Fox WP. Incidence of clostridial contamination in donors’ musculoskeletal tissue. J Bone Joint Surg Br. 2003;85(7):1051-1054.
11. Centers for Disease Control and Prevention (CDC). Update: allograft-associated bacterial infections--United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(10):207-210.
12. Centers for Disease Control and Prevention (CDC). Invasive Streptococcus pyogenes after allograft implantation--Colorado, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(48):1174-1176.
13. Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noël L, Strong DM. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation project NOTIFY. Int Orthop. 2012;36(3):633-641.
14. Schratt HE, Regel G, Kiesewetter B, Tscherne H. HIV infection caused by cold preserved bone transplants. Unfallchirurg. 1996;99(9):679-684.
15. Tugwell BD, Patel PR, Williams IT, et al. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med. 2005;143(9):648-654.
16. Sanzén L, Carlsson A. Transmission of human T-cell lymphotrophic virus type 1 by a deep-frozen bone allograft. Acta Orthop Scand. 1997;68(1):72-74.
17. US Department of Health and Human Services, Food and Drug Administration. Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Published February 2016. Accessed August 10, 2016.
18. Gottlob CA, Baker CL Jr, Pellissier JM, Colvin L. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res. 1999;(367):272-282.
19. Fu F, Christel P, Miller MD, Johnson DL. Graft selection for anterior cruciate ligament reconstruction. Instr Course Lect. 2009;58:337-354.
20. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
21. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
22. Harner CD, Irrgang JJ, Paul J, Dearwater S, Fu FH. Loss of motion after anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):499-506.
23. Hu J, Qu J, Xu D, Zhou J, Lu H. Allograft versus autograft for anterior cruciate ligament reconstruction: an up-to-date meta-analysis of prospective studies. Int Orthop. 2013;37(2):311-320.
24. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
25. Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16(10):559-565.
26. Malinin TI, Levitt RL, Bashore C, Temple HT, Mnaymneh W. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002;18(2):163-170.
27. Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. Am J Sports Med. 2010;38(1):189-199.
28. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
29. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
30 Milankov M, Kecojević V, Rasović P, Kovacević N, Gvozdenović N, Obradović M. Disruption of the knee extensor apparatus complicating anterior cruciate ligament reconstruction. Acta Chir Iugosl. 2013;60(2):13-21.
31. Atkinson TS, Atkinson PJ, Mendenhall HV, Haut RC. Patellar tendon and infrapatellar fat pad healing after harvest of an ACL graft. J Surg Res. 1998;79(1):25-30.
32. Tang G, Niitsu M, Ikeda K, Endo H, Itai Y. Fibrous scar in the infrapatellar fat pad after arthroscopy: MR imaging. Radiat Med. 2000;18(1):1-5.
33. Unterhauser FN, Bosch U, Zeichen J, Weiler A. Alpha-smooth muscle actin containing contractile fibroblastic cells in human knee arthrofibrosis tissue. Winner of the AGA-DonJoy Award 2003. Arch Orthop Trauma Surg. 2004;124(9):585-591.
34. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
35. Sabat D, Kumar V. Nerve injury during hamstring graft harvest: a prospective comparative study of three different incisions. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2089-2095.
36. Kjaergaard J, Faunø LZ, Faunø P. Sensibility loss after ACL reconstruction with hamstring graft. Int J Sports Med. 2008;29(6):507-511.
37. Slone HS, Romine SE, Premkumar A, Xerogeanes JW. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015;31(3):541-554.
38. Nikolaou PK, Seaber AV, Glisson RR, Ribbeck BM, Bassett FH 3rd. Anterior cruciate ligament allograft transplantation. Long-term function, histology, revascularization, and operative technique. Am J Sports Med. 1986;14(5):348-360.
39. Arnoczky SP, Warren RF, Ashlock MA. Replacement of the anterior cruciate ligament using a patellar tendon allograft. An experimental study. J Bone Joint Surg Am. 1986;68(3):376-385.
40. Scheffler SU, Schmidt T, Gangéy I, Dustmann M, Unterhauser F, Weiler A. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24(4):448-458.
41. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24(9):1038-1044.
42. Jackson DW, Grood ES, Arnoczky SP, Butler DL, Simon TM. Freeze dried anterior cruciate ligament allografts. Preliminary studies in a goat model. Am J Sports Med. 1987;15(4):295-303.
43. Chang SK, Egami DK, Shaieb MD, Kan DM, Richardson AB. Anterior cruciate ligament reconstruction: allograft versus autograft. Arthroscopy. 2003;19(5):453-462.
44. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
45. Kaeding CC, Pedroza AD, Reinke EK, Huston LJ; MOON Consortium, Spindler KP. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am J Sports Med. 2015;43(7):1583-1590.
46. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81.
47. Lynch TS, Parker RD, Patel RM, et al. The impact of the Multicenter Orthopaedic Outcomes Network (MOON) research on anterior cruciate ligament reconstruction and orthopaedic practice. J Am Acad Orthop Surg. 2015;23(3):154-163.
48. Hettrich CM, Dunn WR, Reinke EK; MOON Group, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534-1540.
49. Steadman JR, Matheny LM, Hurst JM, Briggs KK. Patient-centered outcomes and revision rate in patients undergoing ACL reconstruction using bone-patellar tendon-bone autograft compared with bone-patellar tendon-bone allograft: a matched case-control study. Arthroscopy. 2015;31(12):2320-2326.
50. Lenehan EA, Payne WB, Askam BM, Grana WA, Farrow LD. Long-term outcomes of allograft reconstruction of the anterior cruciate ligament. Am J Orthop. 2015;44(5):217-222.
51. Noh JH, Yi SR, Song SJ, Kim SW, Kim W. Comparison between hamstring autograft and free tendon achilles allograft: minimum 2-year follow-up after anterior cruciate ligament reconstruction using EndoButton and Intrafix. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):816-822.
52. Victor J, Bellemans J, Witvrouw E, Govaers K, Fabry G. Graft selection in anterior cruciate ligament reconstruction--prospective analysis of patellar tendon autografts compared with allografts. Int Orthop. 1997;21(2):93-97.
53. Kleipool AE, Zijl JA, Willems WJ. Arthroscopic anterior cruciate ligament reconstruction with bone-patellar tendon-bone allograft or autograft. A prospective study with an average follow up of 4 years. Knee Surg Sports Traumatol Arthrosc. 1998;6(4):224-230.
54. Peterson RK, Shelton WR, Bomboy AL. Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: a 5-year follow-up. Arthroscopy. 2001;17(1):9-13.
55. Edgar CM, Zimmer S, Kakar S, Jones H, Schepsis AA. Prospective comparison of auto and allograft hamstring tendon constructs for ACL reconstruction. Clin Orthop Relat Res. 2008;466(9):2238-2246.
56. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
57. Leal-Blanquet J, Alentorn-Geli E, Tuneu J, Valentí JR, Maestro A. Anterior cruciate ligament reconstruction: a multicenter prospective cohort study evaluating 3 different grafts using same bone drilling method. Clin J Sport Med. 2011;21(4):294-300.
58. Sun K, Zhang J, Wang Y, et al. Arthroscopic reconstruction of the anterior cruciate ligament with hamstring tendon autograft and fresh-frozen allograft: a prospective, randomized controlled study. Am J Sports Med. 2011;39(7):1430-1438.
59. Lawhorn KW, Howell SM, Traina SM, Gottlieb JE, Meade TD, Freedberg HI. The effect of graft tissue on anterior cruciate ligament outcomes: a multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28(8):1079-1086.
60. Guo L, Yang L, Duan XJ, et al. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft: comparison of autograft, fresh-frozen allograft, and γ-irradiated allograft. Arthroscopy. 2012;28(2):211-217.
61. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
62. Mayr HO, Willkomm D, Stoehr A, et al. Revision of anterior cruciate ligament reconstruction with patellar tendon allograft and autograft: 2- and 5-year results. Arch Orthop Trauma Surg. 2012;132(6):867-874.
63. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2014;42(2):492-499.
64. Mehta VM, Mandala C, Foster D, Petsche TS. Comparison of revision rates in bone-patella tendon-bone autograft and allograft anterior cruciate ligament reconstruction. Orthopedics. 2010;33(1):12.
65. Vangsness CT Jr, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31(3):474-481.
66. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
67. Reagan J, Kullar R, Burks R. MPFL reconstruction: technique and results. Clin Sports Med. 2014;33(3):501-516.
68. Christiansen SE, Jacobsen BW, Lund B, Lind M. Reconstruction of the medial patellofemoral ligament with gracilis tendon autograft in transverse patellar drill holes. Arthroscopy. 2008;24(1):82-87.
69. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521.
70. Deie M, Ochi M, Sumen Y, Adachi N, Kobayashi K, Yasumoto M. A long-term follow-up study after medial patellofemoral ligament reconstruction using the transferred semitendinosus tendon for patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):522-528.
71. Nomura E, Inoue M. Hybrid medial patellofemoral ligament reconstruction using the semitendinous tendon for recurrent patellar dislocation: minimum 3 years’ follow-up. Arthroscopy. 2006;22(7):787-793.
72. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):E47.
73. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
74. Drez D Jr, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthroscopy. 2001;17(3):298-306.
75. Fink C, Veselko M, Herbort M, Hoser C. MPFL reconstruction using a quadriceps tendon graft: part 2: operative technique and short term clinical results. Knee. 2014;21(6):1175-1179.
76. Calvo Rodríguez R, Figueroa Poblete D, Anastasiadis Le Roy Z, Etchegaray Bascur F, Vaisman Burucker A, Calvo Mena R. Reconstruction of the medial patellofemoral ligament: evaluation of the clinical results of autografts versus allografts. Rev Esp Cir Ortop Traumatol. 2015;59(5):348-353.
77. Becher C, Kley K, Lobenhoffer P, Ezechieli M, Smith T, Ostermeier S. Dynamic versus static reconstruction of the medial patellofemoral ligament for recurrent lateral patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2452-2457.
78. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435.
1. The American Orthopaedic Society for Sports Medicine. Allografts for ACL Reconstruction Survey Report. 2013. http://www.sportsmed.org/AOSSMIMIS/members/downloads/research/AllograftACLReconstructionSurveyReport.pdf. Accessed October 21, 2016.
2. US Department of Health and Human Services, Food and Drug Administration. Guidance for industry: Current good tissue practice (CGTP) and additional requirements for manufacturers of human cells, tissues, and cellular and tissue-based products (HCT/Ps). http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM285223.pdf. Published December 2011. Accessed August 17, 2015.
3. Vaishnav S, Thomas Vangsness C Jr, Dellamaggiora R. New techniques in allograft tissue processing. Clin Sports Med. 2009;28(1):127-141.
4. Veen MR, Bloem RM, Petit PL. Sensitivity and negative predictive value of swab cultures in musculoskeletal allograft procurement. Clin Orthop Relat Res. 1994;(300):259-263.
5. McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35(12):2148-2158.
6. Mickiewicz P, Binkowski M, Bursig H, Wróbel Z. Preservation and sterilization methods of the meniscal allografts: literature review. Cell Tissue Bank. 2014;15(3):307-317.
7. Bui D, Lovric V, Oliver R, Bertollo N, Broe D, Walsh WR. Meniscal allograft sterilisation: effect on biomechanical and histological properties. Cell Tissue Bank. 2015;16(3):467-475.
8. Tomford WW, Starkweather RJ, Goldman MH. A study of the clinical incidence of infection in the use of banked allograft bone. J Bone Joint Surg Am. 1981;63(2):244-248.
9. Munting E, Faundez A, Manche E. Vertebral reconstruction with cortical allograft: long-term evaluation. Eur Spine J. 2001;10 Suppl 2:S153-S157.
10. Malinin TI, Buck BE, Temple HT, Martinez OV, Fox WP. Incidence of clostridial contamination in donors’ musculoskeletal tissue. J Bone Joint Surg Br. 2003;85(7):1051-1054.
11. Centers for Disease Control and Prevention (CDC). Update: allograft-associated bacterial infections--United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(10):207-210.
12. Centers for Disease Control and Prevention (CDC). Invasive Streptococcus pyogenes after allograft implantation--Colorado, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(48):1174-1176.
13. Hinsenkamp M, Muylle L, Eastlund T, Fehily D, Noël L, Strong DM. Adverse reactions and events related to musculoskeletal allografts: reviewed by the World Health Organisation project NOTIFY. Int Orthop. 2012;36(3):633-641.
14. Schratt HE, Regel G, Kiesewetter B, Tscherne H. HIV infection caused by cold preserved bone transplants. Unfallchirurg. 1996;99(9):679-684.
15. Tugwell BD, Patel PR, Williams IT, et al. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med. 2005;143(9):648-654.
16. Sanzén L, Carlsson A. Transmission of human T-cell lymphotrophic virus type 1 by a deep-frozen bone allograft. Acta Orthop Scand. 1997;68(1):72-74.
17. US Department of Health and Human Services, Food and Drug Administration. Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Published February 2016. Accessed August 10, 2016.
18. Gottlob CA, Baker CL Jr, Pellissier JM, Colvin L. Cost effectiveness of anterior cruciate ligament reconstruction in young adults. Clin Orthop Relat Res. 1999;(367):272-282.
19. Fu F, Christel P, Miller MD, Johnson DL. Graft selection for anterior cruciate ligament reconstruction. Instr Course Lect. 2009;58:337-354.
20. Chechik O, Amar E, Khashan M, Lador R, Eyal G, Gold A. An international survey on anterior cruciate ligament reconstruction practices. Int Orthop. 2013;37(2):201-206.
21. Spindler KP, Kuhn JE, Freedman KB, Matthews CE, Dittus RS, Harrell FE Jr. Anterior cruciate ligament reconstruction autograft choice: bone-tendon-bone versus hamstring: does it really matter? A systematic review. Am J Sports Med. 2004;32(8):1986-1995.
22. Harner CD, Irrgang JJ, Paul J, Dearwater S, Fu FH. Loss of motion after anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20(5):499-506.
23. Hu J, Qu J, Xu D, Zhou J, Lu H. Allograft versus autograft for anterior cruciate ligament reconstruction: an up-to-date meta-analysis of prospective studies. Int Orthop. 2013;37(2):311-320.
24. Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176-185.
25. Mroz TE, Joyce MJ, Steinmetz MP, Lieberman IH, Wang JC. Musculoskeletal allograft risks and recalls in the United States. J Am Acad Orthop Surg. 2008;16(10):559-565.
26. Malinin TI, Levitt RL, Bashore C, Temple HT, Mnaymneh W. A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy. 2002;18(2):163-170.
27. Foster TE, Wolfe BL, Ryan S, Silvestri L, Kaye EK. Does the graft source really matter in the outcome of patients undergoing anterior cruciate ligament reconstruction? An evaluation of autograft versus allograft reconstruction results: a systematic review. Am J Sports Med. 2010;38(1):189-199.
28. Lee GH, McCulloch P, Cole BJ, Bush-Joseph CA, Bach BR Jr. The incidence of acute patellar tendon harvest complications for anterior cruciate ligament reconstruction. Arthroscopy. 2008;24(2):162-166.
29. Marumoto JM, Mitsunaga MM, Richardson AB, Medoff RJ, Mayfield GW. Late patellar tendon ruptures after removal of the central third for anterior cruciate ligament reconstruction. A report of two cases. Am J Sports Med. 1996;24(5):698-701.
30 Milankov M, Kecojević V, Rasović P, Kovacević N, Gvozdenović N, Obradović M. Disruption of the knee extensor apparatus complicating anterior cruciate ligament reconstruction. Acta Chir Iugosl. 2013;60(2):13-21.
31. Atkinson TS, Atkinson PJ, Mendenhall HV, Haut RC. Patellar tendon and infrapatellar fat pad healing after harvest of an ACL graft. J Surg Res. 1998;79(1):25-30.
32. Tang G, Niitsu M, Ikeda K, Endo H, Itai Y. Fibrous scar in the infrapatellar fat pad after arthroscopy: MR imaging. Radiat Med. 2000;18(1):1-5.
33. Unterhauser FN, Bosch U, Zeichen J, Weiler A. Alpha-smooth muscle actin containing contractile fibroblastic cells in human knee arthrofibrosis tissue. Winner of the AGA-DonJoy Award 2003. Arch Orthop Trauma Surg. 2004;124(9):585-591.
34. Prodromos CC, Fu FH, Howell SM, Johnson DH, Lawhorn K. Controversies in soft-tissue anterior cruciate ligament reconstruction: grafts, bundles, tunnels, fixation, and harvest. J Am Acad Orthop Surg. 2008;16(7):376-384.
35. Sabat D, Kumar V. Nerve injury during hamstring graft harvest: a prospective comparative study of three different incisions. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2089-2095.
36. Kjaergaard J, Faunø LZ, Faunø P. Sensibility loss after ACL reconstruction with hamstring graft. Int J Sports Med. 2008;29(6):507-511.
37. Slone HS, Romine SE, Premkumar A, Xerogeanes JW. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015;31(3):541-554.
38. Nikolaou PK, Seaber AV, Glisson RR, Ribbeck BM, Bassett FH 3rd. Anterior cruciate ligament allograft transplantation. Long-term function, histology, revascularization, and operative technique. Am J Sports Med. 1986;14(5):348-360.
39. Arnoczky SP, Warren RF, Ashlock MA. Replacement of the anterior cruciate ligament using a patellar tendon allograft. An experimental study. J Bone Joint Surg Am. 1986;68(3):376-385.
40. Scheffler SU, Schmidt T, Gangéy I, Dustmann M, Unterhauser F, Weiler A. Fresh-frozen free-tendon allografts versus autografts in anterior cruciate ligament reconstruction: delayed remodeling and inferior mechanical function during long-term healing in sheep. Arthroscopy. 2008;24(4):448-458.
41. Muramatsu K, Hachiya Y, Izawa H. Serial evaluation of human anterior cruciate ligament grafts by contrast-enhanced magnetic resonance imaging: comparison of allografts and autografts. Arthroscopy. 2008;24(9):1038-1044.
42. Jackson DW, Grood ES, Arnoczky SP, Butler DL, Simon TM. Freeze dried anterior cruciate ligament allografts. Preliminary studies in a goat model. Am J Sports Med. 1987;15(4):295-303.
43. Chang SK, Egami DK, Shaieb MD, Kan DM, Richardson AB. Anterior cruciate ligament reconstruction: allograft versus autograft. Arthroscopy. 2003;19(5):453-462.
44. Poehling GG, Curl WW, Lee CA, et al. Analysis of outcomes of anterior cruciate ligament repair with 5-year follow-up: allograft versus autograft. Arthroscopy. 2005;21(7):774-785.
45. Kaeding CC, Pedroza AD, Reinke EK, Huston LJ; MOON Consortium, Spindler KP. Risk factors and predictors of subsequent ACL injury in either knee after ACL reconstruction: prospective analysis of 2488 primary ACL reconstructions from the MOON cohort. Am J Sports Med. 2015;43(7):1583-1590.
46. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81.
47. Lynch TS, Parker RD, Patel RM, et al. The impact of the Multicenter Orthopaedic Outcomes Network (MOON) research on anterior cruciate ligament reconstruction and orthopaedic practice. J Am Acad Orthop Surg. 2015;23(3):154-163.
48. Hettrich CM, Dunn WR, Reinke EK; MOON Group, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534-1540.
49. Steadman JR, Matheny LM, Hurst JM, Briggs KK. Patient-centered outcomes and revision rate in patients undergoing ACL reconstruction using bone-patellar tendon-bone autograft compared with bone-patellar tendon-bone allograft: a matched case-control study. Arthroscopy. 2015;31(12):2320-2326.
50. Lenehan EA, Payne WB, Askam BM, Grana WA, Farrow LD. Long-term outcomes of allograft reconstruction of the anterior cruciate ligament. Am J Orthop. 2015;44(5):217-222.
51. Noh JH, Yi SR, Song SJ, Kim SW, Kim W. Comparison between hamstring autograft and free tendon achilles allograft: minimum 2-year follow-up after anterior cruciate ligament reconstruction using EndoButton and Intrafix. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):816-822.
52. Victor J, Bellemans J, Witvrouw E, Govaers K, Fabry G. Graft selection in anterior cruciate ligament reconstruction--prospective analysis of patellar tendon autografts compared with allografts. Int Orthop. 1997;21(2):93-97.
53. Kleipool AE, Zijl JA, Willems WJ. Arthroscopic anterior cruciate ligament reconstruction with bone-patellar tendon-bone allograft or autograft. A prospective study with an average follow up of 4 years. Knee Surg Sports Traumatol Arthrosc. 1998;6(4):224-230.
54. Peterson RK, Shelton WR, Bomboy AL. Allograft versus autograft patellar tendon anterior cruciate ligament reconstruction: a 5-year follow-up. Arthroscopy. 2001;17(1):9-13.
55. Edgar CM, Zimmer S, Kakar S, Jones H, Schepsis AA. Prospective comparison of auto and allograft hamstring tendon constructs for ACL reconstruction. Clin Orthop Relat Res. 2008;466(9):2238-2246.
56. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
57. Leal-Blanquet J, Alentorn-Geli E, Tuneu J, Valentí JR, Maestro A. Anterior cruciate ligament reconstruction: a multicenter prospective cohort study evaluating 3 different grafts using same bone drilling method. Clin J Sport Med. 2011;21(4):294-300.
58. Sun K, Zhang J, Wang Y, et al. Arthroscopic reconstruction of the anterior cruciate ligament with hamstring tendon autograft and fresh-frozen allograft: a prospective, randomized controlled study. Am J Sports Med. 2011;39(7):1430-1438.
59. Lawhorn KW, Howell SM, Traina SM, Gottlieb JE, Meade TD, Freedberg HI. The effect of graft tissue on anterior cruciate ligament outcomes: a multicenter, prospective, randomized controlled trial comparing autograft hamstrings with fresh-frozen anterior tibialis allograft. Arthroscopy. 2012;28(8):1079-1086.
60. Guo L, Yang L, Duan XJ, et al. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft: comparison of autograft, fresh-frozen allograft, and γ-irradiated allograft. Arthroscopy. 2012;28(2):211-217.
61. Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy. 2013;29(6):1113-1122.
62. Mayr HO, Willkomm D, Stoehr A, et al. Revision of anterior cruciate ligament reconstruction with patellar tendon allograft and autograft: 2- and 5-year results. Arch Orthop Trauma Surg. 2012;132(6):867-874.
63. Mariscalco MW, Magnussen RA, Mehta D, Hewett TE, Flanigan DC, Kaeding CC. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: a systematic review. Am J Sports Med. 2014;42(2):492-499.
64. Mehta VM, Mandala C, Foster D, Petsche TS. Comparison of revision rates in bone-patella tendon-bone autograft and allograft anterior cruciate ligament reconstruction. Orthopedics. 2010;33(1):12.
65. Vangsness CT Jr, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31(3):474-481.
66. Barrett GR, Luber K, Replogle WH, Manley JL. Allograft anterior cruciate ligament reconstruction in the young, active patient: tegner activity level and failure rate. Arthroscopy. 2010;26(12):1593-1601.
67. Reagan J, Kullar R, Burks R. MPFL reconstruction: technique and results. Clin Sports Med. 2014;33(3):501-516.
68. Christiansen SE, Jacobsen BW, Lund B, Lind M. Reconstruction of the medial patellofemoral ligament with gracilis tendon autograft in transverse patellar drill holes. Arthroscopy. 2008;24(1):82-87.
69. Schöttle PB, Fucentese SF, Romero J. Clinical and radiological outcome of medial patellofemoral ligament reconstruction with a semitendinosus autograft for patella instability. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):516-521.
70. Deie M, Ochi M, Sumen Y, Adachi N, Kobayashi K, Yasumoto M. A long-term follow-up study after medial patellofemoral ligament reconstruction using the transferred semitendinosus tendon for patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):522-528.
71. Nomura E, Inoue M. Hybrid medial patellofemoral ligament reconstruction using the semitendinous tendon for recurrent patellar dislocation: minimum 3 years’ follow-up. Arthroscopy. 2006;22(7):787-793.
72. Nomura E, Inoue M. Surgical technique and rationale for medial patellofemoral ligament reconstruction for recurrent patellar dislocation. Arthroscopy. 2003;19(5):E47.
73. Hautamaa PV, Fithian DC, Kaufman KR, Daniel DM, Pohlmeyer AM. Medial soft tissue restraints in lateral patellar instability and repair. Clin Orthop Relat Res. 1998;(349):174-182.
74. Drez D Jr, Edwards TB, Williams CS. Results of medial patellofemoral ligament reconstruction in the treatment of patellar dislocation. Arthroscopy. 2001;17(3):298-306.
75. Fink C, Veselko M, Herbort M, Hoser C. MPFL reconstruction using a quadriceps tendon graft: part 2: operative technique and short term clinical results. Knee. 2014;21(6):1175-1179.
76. Calvo Rodríguez R, Figueroa Poblete D, Anastasiadis Le Roy Z, Etchegaray Bascur F, Vaisman Burucker A, Calvo Mena R. Reconstruction of the medial patellofemoral ligament: evaluation of the clinical results of autografts versus allografts. Rev Esp Cir Ortop Traumatol. 2015;59(5):348-353.
77. Becher C, Kley K, Lobenhoffer P, Ezechieli M, Smith T, Ostermeier S. Dynamic versus static reconstruction of the medial patellofemoral ligament for recurrent lateral patellar dislocation. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2452-2457.
78. Gomes JE. Comparison between a static and a dynamic technique for medial patellofemoral ligament reconstruction. Arthroscopy. 2008;24(4):430-435.
Why Do Lateral Unicompartmental Knee Arthroplasties Fail Today?
In 1975, Skolnick and colleagues1 introduced unicompartmental knee arthroplasty (UKA) for patients with isolated unicompartmental osteoarthritis (OA). They reported a study of 14 UKA procedures, of which 12 were at the medial and 2 at the lateral side. Forty years since this procedure was introduced, UKA is used in 8% to 12% of all knee arthroplasties.2-6 A minority of these procedures are performed at the lateral side (5%-10%).6-8
The considerable anatomical and kinematical differences between compartments9-14 make it impossible to directly compare outcomes of medial and lateral UKA. For example, a greater degree of femoral roll and more posterior translation at the lateral side in flexion9,10,13 can contribute to different pattern and volume differences of cartilage wear.15 Because of these differences, and because of implant design factors and lower surgical volume, lateral UKA is considered a technically more challenging surgery compared to medial UKA.12,16,17
Since isolated lateral compartment OA is relatively scarce, current literature on lateral UKA is limited, and most studies combine medial and lateral outcomes to report UKA outcomes and failure modes.3,4,18-20 However, as the UKA has grown in popularity over the last decade,2,21-25 the number of reports about the lateral UKA also has increased. Recent studies reported excellent short-term survivorship results of the lateral UKA (96%-99%)26,27 and smaller lateral UKA studies reported the 10-year survivorship with varying outcomes from good (84%)14,28-30 to excellent (94%-100%).8,31,32 Indeed, a recent systematic review showed survivorship of lateral UKA at 5, 10, and 15 years of 93%, 91%, and 89%, respectively.33Because of the differences between the medial and lateral compartment, it is important to know the failure modes of lateral UKA in order to improve clinical outcomes and revision rates. We performed a systematic review of cohort studies and registry-based studies that reported lateral UKA failure to assess the causes of lateral UKA failure. In addition, we compared the failure modes in cohort studies with those found in registry-based studies.
Patients and Methods
Search Strategy and Criteria
Databases of PubMed, Embase, and Cochrane (Cochrane Central Register of Clinical Trials) were searched with the terms “knee, arthroplasty, replacement,” “unicompartmental,” “unicondylar,” “partial,” “UKA,” “UKR,” “UCA,” “UCR,” “PKA,” “PKR,” “PCA,” “prosthesis failure,” “reoperation,” “survivorship,” and “treatment failure.” After removal of duplicates, 2 authors (JPvdL and HAZ) scanned the articles for their title and abstract to assess eligibility for the study.
Inclusion criteria were: (I) English language articles describing studies in humans published in the last 25 years, (II) retrospective and prospective studies, (III) featured lateral UKA, (IV) OA was indication for surgery, and (V) included failure modes data. The exclusion criteria were studies that featured: (I) only a specific group of failure (eg, bearing dislocations only), (II) previous surgery in ipsilateral knee (high tibial osteotomy, medial UKA), (III) acute concurrent knee diagnoses (acute anterior cruciate ligament rupture, acute meniscal tear), (IV) combined reporting of medial and lateral UKA, or (V) multiple studies with the same patient database.
Data Collection
All studies that reported modes of failure were used in this study and these failure modes were noted in a datasheet in Microsoft Excel 2011 (Microsoft).
Statistical Analysis
For this systematic review, statistical analysis was performed with IBM SPSS Statistics 22 (SPSS Inc.). We performed chi square tests and Fisher’s exact tests to assess a difference between cohort studies and registry-based studies with the null hypothesis of no difference between both groups. A difference was considered significant when P < .05.
Results
Through the search of the databases, 1294 studies were identified and 26 handpicked studies were added. Initially, based on the title and abstract, 184 of these studies were found eligible.
A total of 366 lateral UKA failures were included. The most common failure modes were progression of OA (29%), aseptic loosening (23%), and bearing dislocation (10%). Infection (6%), instability (6%), unexplained pain (6%), and fractures (4%) were less common causes of failure of lateral UKA (Table 2).
One hundred fifty-five of these failures were reported in the cohort studies. The most common modes of failure were OA progression (36%), bearing dislocation (17%) and aseptic loosening (16%). Less common were infection (10%), fractures (5%), pain (5%), and other causes (6%). In registry-based studies, with 211 lateral UKA failures, the most common modes of failure were aseptic loosening (28%), OA progression (24%), other causes (12%), instability (10%), pain (7%), bearing dislocation (5%), and polyethylene wear (4%) (Table 2).
When pooling cohort and registry-based studies, progression of OA was significantly more common than aseptic loosening (29% vs 23%, respectively; P < .01). It was also significantly more common in the cohort studies (36% vs 16%, respectively; P < .01) but no significant difference was found between progression of OA and aseptic loosening in registry-based studies (24% and 28%, respectively; P = .16) (Table 2).
When comparing cohort with registry-based studies, progression of OA was higher in cohort studies (36% vs. 24%; P < .01). Other failures modes that were more common in cohort studies compared with registry-based studies were bearing dislocation (17% vs 5%, respectively; P < .01) and infections (10% vs 3%, P < .01). Failure modes that were more common in registry-based studies than cohort studies were aseptic loosening (28% vs 16%, respectively; P < .01), other causes (12% vs 6%, respectively, P = .02), and instability (10% vs 1%, respectively, P < .01) (Table 2).
Discussion
In this systematic review, the most common failure modes in lateral UKA review were OA progression (29%), aseptic loosening (23%), and bearing dislocation (10%). Progression of OA and bearing dislocation were the most common modes of failure in cohort studies (36% and 17%, respectively), while aseptic loosening and OA progression were the most common failure modes in registry-based studies (28% and 24%, respectively).
As mentioned above, there are differences in anatomy and kinematics between the medial and lateral compartment. When the lateral UKA failure modes are compared with studies reporting medial UKA failure modes, differences in failure modes are seen.34 Siddiqui and Ahmad35 performed a systematic review of outcomes after UKA revision and presented a table with the failure modes of included studies. Unfortunately they did not report the ratio of medial and lateral UKA. However, when assuming an average percentage of 90% to 95% of medial UKA,6,7,36 the main failure mode in their review in 17 out of 21 studies was aseptic loosening. Indeed, a recent systematic review on medial UKA failure modes showed that aseptic loosening is the most common cause of failure following this procedure.34 Similarly, a search through registry-based studies6,7 and large cohort studies37-40 that only reported medial UKA failures showed that the majority of these studies7,37-39 also reported aseptic loosening as the main cause of failure in medial UKA. When comparing the results of our systematic review of lateral UKA failures with the results of these studies of medial UKA failures, it seems that OA progression seems to play a more dominant role in failures of lateral UKA, while aseptic loosening seems to be more common in medial UKA.
Differences in anatomy and kinematics of the medial and lateral compartment can explain this. Malalignment of the joint is an important factor in the etiology of OA41,42 and biomechanical studies showed that this malalignment can cause decreased viability and further degenerative changes of cartilage of the knee.43 Hernigou and Deschamps44 showed that the alignment of the knee after medial UKA is an important factor in postoperative joint changes. They found that overcorrection of varus deformity during medial UKA surgery, measured by the hip-knee-ankle (HKA) angle, was associated with increased OA at the lateral condyle and less tibial wear of the medial UKA. Undercorrection of the varus caused an increase in tibial wear of polyethylene. Chatellard and colleagues45 found the same results in the correction of varus, measured by HKA. In addition, they found that when the prosthetic (medial) joint space was smaller than healthy (lateral) joint space, this was correlated with lower prosthesis survival. A smaller joint space at the healthy side was correlated with OA progression at the lateral compartment and tibial component wear.
These studies explain the mechanism of progression of OA and aseptic loosening. Harrington46 assessed the load in patients with valgus and varus deformity. Patients with a valgus deformity have high mechanical load on the lateral condyle during the static phase, but during the dynamic phase, a major part of this load shifts to the medial condyle. In the patients with varus deformity, the mechanical load was noted on the medial condyle during both the static and dynamic phase. Ohdera and colleagues47 advised, based on this biomechanical study and their own experiences, to correct the knee during lateral UKA to a slight valgus angle (5°-7°) to prevent OA progression at the medial side. van der List and colleagues48 similarly showed that undercorrection of 3° to 7° was correlated with better functional outcomes when compared to more neutral alignment. Moreover, Khamaisy and colleagues49 recently showed that overcorrection during UKA surgery is more common in lateral than medial UKA.
These studies are important to understanding why OA progression is more common as a failure mode in lateral UKA. The shift of mechanical load from the lateral to medial epicondyle during the dynamic phase also could explain why aseptic loosening is less common in lateral UKA. As Hernigou and Deschamps44 and Chatellard and colleagues45 stated, undercorrection of varus deformity in medial UKA is associated with higher mechanical load on the medial prosthesis side and smaller joint space width. These factors are correlated with mechanical failure of medial UKA. We think this process can be applied to lateral UKA, with the addition that the mechanical load is higher on the healthy medial compartment during the dynamic phase. This causes more forces on the healthy (medial) side in lateral UKA, and in medial UKA more forces on the prosthesis (medial) side, which results in more OA progression in lateral UKA and more aseptic loosening in medial UKA. This finding is consistent with the results of our review of more OA progression and less aseptic loosening in lateral UKA. This study also suggests that medial and lateral UKA should not be reported together in studies that present survivorship, failure modes, or clinical outcomes.
A large discrepancy was seen in bearing dislocation between cohort studies (17%) and registry-based studies (5%). When we take a closer look to the bearing dislocation failures in the cohort studies, most of the failures were reported in only 2 cohort studies.50,51 In a study by Pandit and colleagues,50 3 different prosthesis designs were used in 3 different time periods. In the first series of lateral UKA (1983-1991), 6 out of 51 (12%) bearings dislocated. In the second series (1998-2004), a modified technique was used and 3 out of 65 (5%) bearings dislocated. In the third series (2004-2008), a modified technique and a domed tibial component was used and only 1 out of 68 bearings dislocated (1%). In a study published in 1996, Gunther and colleagues51 also used surgical techniques and implants that were modified over the course of the study period. Because of these modified techniques, different implant designs, and year of publication, bearing dislocation most likely plays a smaller role than the 17% reported in the cohort studies. This discrepancy is a good example of the important role for the registries and registry-based studies in reporting failure modes and survivorship, especially in lateral UKA due to the low surgical frequency. Pabinger and colleagues52 recently performed a systematic review of cohort studies and registry-based studies in which they stated that the reliability in non-registry-based studies should be questioned and they considered registry-based studies superior in reporting UKA outcomes and revision rates. Furthermore, given the differences in anatomic and kinematic differences between the medial and lateral compartment and different failure modes between medial and lateral UKA, it would be better if future studies presented the medial and lateral failures separately. As stated above, most large cohort studies and especially annual registries currently do not report modes of failure of medial and lateral UKA separately.3,4,18-20
There are limitations in this study. First, this systematic review is not a full meta-analysis but a pooled analysis of collected study series and retrospective studies. Therefore, we cannot exclude sampling bias, confounders, and selection bias from the literature. We included all studies reporting failure modes of lateral UKA and excluded all case reports. We made a conscious choice about including all lateral UKA failures because this is the first systematic review of lateral UKA failure modes. Another limitation is that the follow-up period of the studies differed (Table 1) and we did not correct for the follow-up period. As stated in the example of bearing dislocations, some of these studies reported old or different techniques, while other, more recently published studies used more modified techniques11,29,53-56 Unfortunately, most studies did not report the time of arthroplasty survival and therefore we could not correct for the follow-up period.
In conclusion, progression of OA is the most common failure mode in lateral UKA, followed by aseptic loosening. Anatomic and kinematic factors such as alignment, mechanical forces during dynamic phase, and correction of valgus seem to play important roles in failure modes of lateral UKA. In the future, failure modes of medial and lateral UKA should be reported separately.
Am J Orthop. 2016;45(7):432-438, 462. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Skolnick MD, Bryan RS, Peterson LFA. Unicompartmental polycentric knee arthroplasty. Description and preliminary results. Clin Orthop Relat Res. 1975;(112):208-214.
2. Riddle DL, Jiranek WA, McGlynn FJ. Yearly Incidence of Unicompartmental Knee Arthroplasty in the United States. J Arthroplasty. 2008;23(3):408-412.
3. Australian Orthopaedic Association. Hip and Knee Arthroplasty 2014 Annual Report. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed June 3, 2015.
4. Swedish Knee Arthroplasty Register. 2013 Annual Report.http://myknee.se/pdf/SKAR2013_Eng.pdf. Accessed June 3, 2015.
5. The New Zealand Joint Registry. Fourteen Year Report. January 1999 to December 2012. 2013. http://nzoa.org.nz/system/files/NJR 14 Year Report.pdf. Accessed June 3, 2015.
6. Baker PN, Jameson SS, Deehan DJ, Gregg PJ, Porter M, Tucker K. Mid-term equivalent survival of medial and lateral unicondylar knee replacement: an analysis of data from a National Joint Registry. J Bone Joint Surg Br. 2012;94(12):1641-1648.
7. Lewold S, Robertsson O, Knutson K, Lidgren L. Revision of unicompartmental knee arthroplasty: outcome in 1,135 cases from the Swedish Knee Arthroplasty study. Acta Orthop Scand. 1998;69(5):469-474.
8. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Lateral unicompartmental knee arthroplasty: survivorship and technical considerations at an average follow-up of 12.4 years. J Arthroplasty. 2006;21(1):13-17.
9. Hill PF, Vedi V, Williams A, Iwaki H, Pinskerova V, Freeman MA. Tibiofemoral movement 2: the loaded and unloaded living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1196-1198.
10. Nakagawa S, Kadoya Y, Todo S, et al. Tibiofemoral movement 3: full flexion in the living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1199-1200.
11. Ashraf T, Newman JH, Evans RL, Ackroyd CE. Lateral unicompartmental knee replacement survivorship and clinical experience over 21 years. J Bone Joint Surg Br. 2002;84(8):1126-1130.
12. Scott RD. Lateral unicompartmental replacement: a road less traveled. Orthopedics. 2005;28(9):983-984.
13. Sah AP, Scott RD. Lateral unicompartmental knee arthroplasty through a medial approach. Study with an average five-year follow-up. J Bone Joint Surg Am. 2007;89(9):1948-1954.
14. Argenson JN, Parratte S, Bertani A, Flecher X, Aubaniac JM. Long-term results with a lateral unicondylar replacement. Clin Orthop Relat Res. 2008;466(11):2686-2693.
15. Weidow J, Pak J, Karrholm J. Different patterns of cartilage wear in medial and lateral gonarthrosis. Acta Orthop Scand. 2002;73(3):326-329.
16. Ollivier M, Abdel MP, Parratte S, Argenson JN. Lateral unicondylar knee arthroplasty (UKA): contemporary indications, surgical technique, and results. Int Orthop. 2014;38(2):449-455.
17. Demange MK, Von Keudell A, Probst C, Yoshioka H, Gomoll AH. Patient-specific implants for lateral unicompartmental knee arthroplasty. Int Orthop. 2015;39(8):1519-1526.
18. Khan Z, Nawaz SZ, Kahane S, Esler C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
19. Epinette JA, Brunschweiler B, Mertl P, et al. Unicompartmental knee arthroplasty modes of failure: wear is not the main reason for failure: a multicentre study of 418 failed knees. Orthop Traumatol Surg Res. 2012;98(6 Suppl):S124-S130.
20. Bordini B, Stea S, Falcioni S, Ancarani C, Toni A. Unicompartmental knee arthroplasty: 11-year experience from 3929 implants in RIPO register. Knee. 2014;21(6):1275-1279.
21. Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among medicare beneficiaries, 2000 to 2009. J Bone Joint Surg Am. 2013;95(22):e174.
22. Nwachukwu BU, McCormick FM, Schairer WW, Frank RM, Provencher MT, Roche MW. Unicompartmental knee arthroplasty versus high tibial osteotomy: United States practice patterns for the surgical treatment of unicompartmental arthritis. J Arthroplasty. 2014;29(8):1586-1589.
23. van der List JP, Chawla H, Pearle AD. Robotic-assisted knee arthroplasty: an overview. Am J Orthop. 2016;45(4):202-211.
24. van der List JP, Chawla H, Joskowicz L, Pearle AD. Current state of computer navigation and robotics in unicompartmental and total knee arthroplasty: a systematic review with meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016 Sep 6. [Epub ahead of print]
25. Zuiderbaan HA, van der List JP, Kleeblad LJ, et al. Modern indications, results and global trends in the use of unicompartmental knee arthroplasty and high tibial osteotomy for the treatment of medial unicondylar knee osteoarthritis. Am J Orthop. 2016;45(6):E355-E361.
26. Smith JR, Robinson JR, Porteous AJ, et al. Fixed bearing lateral unicompartmental knee arthroplasty--short to midterm survivorship and knee scores for 101 prostheses. Knee. 2014;21(4):843-847.
27. Berend KR, Kolczun MC 2nd, George JW Jr, Lombardi AV Jr. Lateral unicompartmental knee arthroplasty through a lateral parapatellar approach has high early survivorship. Clin Orthop Relat Res. 2012;470(1):77-83.
28. Keblish PA, Briard JL. Mobile-bearing unicompartmental knee arthroplasty: a 2-center study with an 11-year (mean) follow-up. J Arthroplasty. 2004;19(7 Suppl 2):87-94.
29. Bertani A, Flecher X, Parratte S, Aubaniac JM, Argenson JN. Unicompartmental-knee arthroplasty for treatment of lateral gonarthrosis: about 30 cases. Midterm results. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(8):763-770.
30. Sebilo A, Casin C, Lebel B, et al. Clinical and technical factors influencing outcomes of unicompartmental knee arthroplasty: Retrospective multicentre study of 944 knees. Orthop Traumatol Surg Res. 2013;99(4 Suppl):S227-S234.
31. Cartier P, Khefacha A, Sanouiller JL, Frederick K. Unicondylar knee arthroplasty in middle-aged patients: A minimum 5-year follow-up. Orthopedics. 2007;30(8 Suppl):62-65.
32. Lustig S, Paillot JL, Servien E, Henry J, Ait Si Selmi T, Neyret P. Cemented all polyethylene tibial insert unicompartimental knee arthroplasty: a long term follow-up study. Orthop Traumatol Surg Res. 2009;95(1):12-21.
33. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
34. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2016;31(5):1016-1021.
35. Siddiqui NA, Ahmad ZM. Revision of unicondylar to total knee arthroplasty: a systematic review. Open Orthop J. 2012;6:268-275.
36. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Lateral unicompartmental knee arthroplasty: survivorship and technical considerations at an average follow-up of 12.4 years. J Arthroplasty. 2006;21(1):13-17.
37. Kalra S, Smith TO, Berko B, Walton NP. Assessment of radiolucent lines around the Oxford unicompartmental knee replacement: sensitivity and specificity for loosening. J Bone Joint Surg Br. 2011;93(6):777-781.
38. Wynn Jones H, Chan W, Harrison T, Smith TO, Masonda P, Walton NP. Revision of medial Oxford unicompartmental knee replacement to a total knee replacement: similar to a primary? Knee. 2012;19(4):339-343.
39. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
40. Citak M, Dersch K, Kamath AF, Haasper C, Gehrke T, Kendoff D. Common causes of failed unicompartmental knee arthroplasty: a single-centre analysis of four hundred and seventy one cases. Int Orthop. 2014;38(5):961-965.
41. Hunter DJ, Wilson DR. Role of alignment and biomechanics in osteoarthritis and implications for imaging. Radiol Clin North Am. 2009;47(4):553-566.
42. Hunter DJ, Sharma L, Skaife T. Alignment and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91 Suppl 1:85-89.
43. Roemhildt ML, Beynnon BD, Gauthier AE, Gardner-Morse M, Ertem F, Badger GJ. Chronic in vivo load alteration induces degenerative changes in the rat tibiofemoral joint. Osteoarthritis Cartilage. 2013;21(2):346-357.
44. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
45. Chatellard R, Sauleau V, Colmar M, et al. Medial unicompartmental knee arthroplasty: does tibial component position influence clinical outcomes and arthroplasty survival? Orthop Traumatol Surg Res. 2013;99(4 Suppl):S219-S225.
46. Harrington IJ. Static and dynamic loading patterns in knee joints with deformities. J Bone Joint Surg Am. 1983;65(2):247-259.
47. Ohdera T, Tokunaga J, Kobayashi A. Unicompartmental knee arthroplasty for lateral gonarthrosis: midterm results. J Arthroplasty. 2001;16(2):196-200.
48. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 26. [Epub ahead of print]
49. Khamaisy S, Gladnick BP, Nam D, Reinhardt KR, Heyse TJ, Pearle AD. Lower limb alignment control: Is it more challenging in lateral compared to medial unicondylar knee arthroplasty? Knee. 2015;22(4):347-350.
50. Pandit H, Jenkins C, Beard DJ, et al. Mobile bearing dislocation in lateral unicompartmental knee replacement. Knee. 2010;17(6):392-397.
51. Gunther TV, Murray DW, Miller R, et al. Lateral unicompartmental arthroplasty with the Oxford meniscal knee. Knee. 1996;3(1):33-39.
52. Pabinger C, Lumenta DB, Cupak D, Berghold A, Boehler N, Labek G. Quality of outcome data in knee arthroplasty: Comparison of registry data and worldwide non-registry studies from 4 decades. Acta Orthopaedica. 2015;86(1):58-62.
53. Lustig S, Elguindy A, Servien E, et al. 5- to 16-year follow-up of 54 consecutive lateral unicondylar knee arthroplasties with a fixed-all polyethylene bearing. J Arthroplasty. 2011;26(8):1318-1325.
54. Walton MJ, Weale AE, Newman JH. The progression of arthritis following lateral unicompartmental knee replacement. Knee. 2006;13(5):374-377.
55. Lustig S, Lording T, Frank F, Debette C, Servien E, Neyret P. Progression of medial osteoarthritis and long term results of lateral unicompartmental arthroplasty: 10 to 18 year follow-up of 54 consecutive implants. Knee. 2014;21(S1):S26-S32.
56. O’Rourke MR, Gardner JJ, Callaghan JJ, et al. Unicompartmental knee replacement: a minimum twenty-one-year followup, end-result study. Clin Orthop Relat Res. 2005;440:27-37.
57. Citak M, Cross MB, Gehrke T, Dersch K, Kendoff D. Modes of failure and revision of failed lateral unicompartmental knee arthroplasties. Knee. 2015;22(4):338-340.
58. Liebs TR, Herzberg W. Better quality of life after medial versus lateral unicondylar knee arthroplasty knee. Clin Orthop Relat Res. 2013;471(8):2629-2640.
59. Weston-Simons JS, Pandit H, Kendrick BJ, et al. The mid-term outcomes of the Oxford Domed Lateral unicompartmental knee replacement. Bone Joint J. 2014;96-B(1):59-64.
60. Thompson SA, Liabaud B, Nellans KW, Geller JA. Factors associated with poor outcomes following unicompartmental knee arthroplasty: redefining the “classic” indications for surgery. J Arthroplasty. 2013;28(9):1561-1564.
61. Saxler G, Temmen D, Bontemps G. Medium-term results of the AMC-unicompartmental knee arthroplasty. Knee. 2004;11(5):349-355.
62. Forster MC, Bauze AJ, Keene GCR. Lateral unicompartmental knee replacement: Fixed or mobile bearing? Knee Surg Sports Traumatol Arthrosc. 2007;15(9):1107-1111.
63. Streit MR, Walker T, Bruckner T, et al. Mobile-bearing lateral unicompartmental knee replacement with the Oxford domed tibial component: an independent series. J Bone Joint Surg Br. 2012;94(10):1356-1361.
64. Altuntas AO, Alsop H, Cobb JP. Early results of a domed tibia, mobile bearing lateral unicompartmental knee arthroplasty from an independent centre. Knee. 2013;20(6):466-470.
65. Ashraf T, Newman JH, Desai VV, Beard D, Nevelos JE. Polyethylene wear in a non-congruous unicompartmental knee replacement: a retrieval analysis. Knee. 2004;11(3):177-181.
66. Schelfaut S, Beckers L, Verdonk P, Bellemans J, Victor J. The risk of bearing dislocation in lateral unicompartmental knee arthroplasty using a mobile biconcave design. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2487-2494.
67. Marson B, Prasad N, Jenkins R, Lewis M. Lateral unicompartmental knee replacements: Early results from a District General Hospital. Eur J Orthop Surg Traumatol. 2014;24(6):987-991.
68. Walker T, Gotterbarm T, Bruckner T, Merle C, Streit MR. Total versus unicompartmental knee replacement for isolated lateral osteoarthritis: a matched-pairs study. Int Orthop. 2014;38(11):2259-2264.
In 1975, Skolnick and colleagues1 introduced unicompartmental knee arthroplasty (UKA) for patients with isolated unicompartmental osteoarthritis (OA). They reported a study of 14 UKA procedures, of which 12 were at the medial and 2 at the lateral side. Forty years since this procedure was introduced, UKA is used in 8% to 12% of all knee arthroplasties.2-6 A minority of these procedures are performed at the lateral side (5%-10%).6-8
The considerable anatomical and kinematical differences between compartments9-14 make it impossible to directly compare outcomes of medial and lateral UKA. For example, a greater degree of femoral roll and more posterior translation at the lateral side in flexion9,10,13 can contribute to different pattern and volume differences of cartilage wear.15 Because of these differences, and because of implant design factors and lower surgical volume, lateral UKA is considered a technically more challenging surgery compared to medial UKA.12,16,17
Since isolated lateral compartment OA is relatively scarce, current literature on lateral UKA is limited, and most studies combine medial and lateral outcomes to report UKA outcomes and failure modes.3,4,18-20 However, as the UKA has grown in popularity over the last decade,2,21-25 the number of reports about the lateral UKA also has increased. Recent studies reported excellent short-term survivorship results of the lateral UKA (96%-99%)26,27 and smaller lateral UKA studies reported the 10-year survivorship with varying outcomes from good (84%)14,28-30 to excellent (94%-100%).8,31,32 Indeed, a recent systematic review showed survivorship of lateral UKA at 5, 10, and 15 years of 93%, 91%, and 89%, respectively.33Because of the differences between the medial and lateral compartment, it is important to know the failure modes of lateral UKA in order to improve clinical outcomes and revision rates. We performed a systematic review of cohort studies and registry-based studies that reported lateral UKA failure to assess the causes of lateral UKA failure. In addition, we compared the failure modes in cohort studies with those found in registry-based studies.
Patients and Methods
Search Strategy and Criteria
Databases of PubMed, Embase, and Cochrane (Cochrane Central Register of Clinical Trials) were searched with the terms “knee, arthroplasty, replacement,” “unicompartmental,” “unicondylar,” “partial,” “UKA,” “UKR,” “UCA,” “UCR,” “PKA,” “PKR,” “PCA,” “prosthesis failure,” “reoperation,” “survivorship,” and “treatment failure.” After removal of duplicates, 2 authors (JPvdL and HAZ) scanned the articles for their title and abstract to assess eligibility for the study.
Inclusion criteria were: (I) English language articles describing studies in humans published in the last 25 years, (II) retrospective and prospective studies, (III) featured lateral UKA, (IV) OA was indication for surgery, and (V) included failure modes data. The exclusion criteria were studies that featured: (I) only a specific group of failure (eg, bearing dislocations only), (II) previous surgery in ipsilateral knee (high tibial osteotomy, medial UKA), (III) acute concurrent knee diagnoses (acute anterior cruciate ligament rupture, acute meniscal tear), (IV) combined reporting of medial and lateral UKA, or (V) multiple studies with the same patient database.
Data Collection
All studies that reported modes of failure were used in this study and these failure modes were noted in a datasheet in Microsoft Excel 2011 (Microsoft).
Statistical Analysis
For this systematic review, statistical analysis was performed with IBM SPSS Statistics 22 (SPSS Inc.). We performed chi square tests and Fisher’s exact tests to assess a difference between cohort studies and registry-based studies with the null hypothesis of no difference between both groups. A difference was considered significant when P < .05.
Results
Through the search of the databases, 1294 studies were identified and 26 handpicked studies were added. Initially, based on the title and abstract, 184 of these studies were found eligible.
A total of 366 lateral UKA failures were included. The most common failure modes were progression of OA (29%), aseptic loosening (23%), and bearing dislocation (10%). Infection (6%), instability (6%), unexplained pain (6%), and fractures (4%) were less common causes of failure of lateral UKA (Table 2).
One hundred fifty-five of these failures were reported in the cohort studies. The most common modes of failure were OA progression (36%), bearing dislocation (17%) and aseptic loosening (16%). Less common were infection (10%), fractures (5%), pain (5%), and other causes (6%). In registry-based studies, with 211 lateral UKA failures, the most common modes of failure were aseptic loosening (28%), OA progression (24%), other causes (12%), instability (10%), pain (7%), bearing dislocation (5%), and polyethylene wear (4%) (Table 2).
When pooling cohort and registry-based studies, progression of OA was significantly more common than aseptic loosening (29% vs 23%, respectively; P < .01). It was also significantly more common in the cohort studies (36% vs 16%, respectively; P < .01) but no significant difference was found between progression of OA and aseptic loosening in registry-based studies (24% and 28%, respectively; P = .16) (Table 2).
When comparing cohort with registry-based studies, progression of OA was higher in cohort studies (36% vs. 24%; P < .01). Other failures modes that were more common in cohort studies compared with registry-based studies were bearing dislocation (17% vs 5%, respectively; P < .01) and infections (10% vs 3%, P < .01). Failure modes that were more common in registry-based studies than cohort studies were aseptic loosening (28% vs 16%, respectively; P < .01), other causes (12% vs 6%, respectively, P = .02), and instability (10% vs 1%, respectively, P < .01) (Table 2).
Discussion
In this systematic review, the most common failure modes in lateral UKA review were OA progression (29%), aseptic loosening (23%), and bearing dislocation (10%). Progression of OA and bearing dislocation were the most common modes of failure in cohort studies (36% and 17%, respectively), while aseptic loosening and OA progression were the most common failure modes in registry-based studies (28% and 24%, respectively).
As mentioned above, there are differences in anatomy and kinematics between the medial and lateral compartment. When the lateral UKA failure modes are compared with studies reporting medial UKA failure modes, differences in failure modes are seen.34 Siddiqui and Ahmad35 performed a systematic review of outcomes after UKA revision and presented a table with the failure modes of included studies. Unfortunately they did not report the ratio of medial and lateral UKA. However, when assuming an average percentage of 90% to 95% of medial UKA,6,7,36 the main failure mode in their review in 17 out of 21 studies was aseptic loosening. Indeed, a recent systematic review on medial UKA failure modes showed that aseptic loosening is the most common cause of failure following this procedure.34 Similarly, a search through registry-based studies6,7 and large cohort studies37-40 that only reported medial UKA failures showed that the majority of these studies7,37-39 also reported aseptic loosening as the main cause of failure in medial UKA. When comparing the results of our systematic review of lateral UKA failures with the results of these studies of medial UKA failures, it seems that OA progression seems to play a more dominant role in failures of lateral UKA, while aseptic loosening seems to be more common in medial UKA.
Differences in anatomy and kinematics of the medial and lateral compartment can explain this. Malalignment of the joint is an important factor in the etiology of OA41,42 and biomechanical studies showed that this malalignment can cause decreased viability and further degenerative changes of cartilage of the knee.43 Hernigou and Deschamps44 showed that the alignment of the knee after medial UKA is an important factor in postoperative joint changes. They found that overcorrection of varus deformity during medial UKA surgery, measured by the hip-knee-ankle (HKA) angle, was associated with increased OA at the lateral condyle and less tibial wear of the medial UKA. Undercorrection of the varus caused an increase in tibial wear of polyethylene. Chatellard and colleagues45 found the same results in the correction of varus, measured by HKA. In addition, they found that when the prosthetic (medial) joint space was smaller than healthy (lateral) joint space, this was correlated with lower prosthesis survival. A smaller joint space at the healthy side was correlated with OA progression at the lateral compartment and tibial component wear.
These studies explain the mechanism of progression of OA and aseptic loosening. Harrington46 assessed the load in patients with valgus and varus deformity. Patients with a valgus deformity have high mechanical load on the lateral condyle during the static phase, but during the dynamic phase, a major part of this load shifts to the medial condyle. In the patients with varus deformity, the mechanical load was noted on the medial condyle during both the static and dynamic phase. Ohdera and colleagues47 advised, based on this biomechanical study and their own experiences, to correct the knee during lateral UKA to a slight valgus angle (5°-7°) to prevent OA progression at the medial side. van der List and colleagues48 similarly showed that undercorrection of 3° to 7° was correlated with better functional outcomes when compared to more neutral alignment. Moreover, Khamaisy and colleagues49 recently showed that overcorrection during UKA surgery is more common in lateral than medial UKA.
These studies are important to understanding why OA progression is more common as a failure mode in lateral UKA. The shift of mechanical load from the lateral to medial epicondyle during the dynamic phase also could explain why aseptic loosening is less common in lateral UKA. As Hernigou and Deschamps44 and Chatellard and colleagues45 stated, undercorrection of varus deformity in medial UKA is associated with higher mechanical load on the medial prosthesis side and smaller joint space width. These factors are correlated with mechanical failure of medial UKA. We think this process can be applied to lateral UKA, with the addition that the mechanical load is higher on the healthy medial compartment during the dynamic phase. This causes more forces on the healthy (medial) side in lateral UKA, and in medial UKA more forces on the prosthesis (medial) side, which results in more OA progression in lateral UKA and more aseptic loosening in medial UKA. This finding is consistent with the results of our review of more OA progression and less aseptic loosening in lateral UKA. This study also suggests that medial and lateral UKA should not be reported together in studies that present survivorship, failure modes, or clinical outcomes.
A large discrepancy was seen in bearing dislocation between cohort studies (17%) and registry-based studies (5%). When we take a closer look to the bearing dislocation failures in the cohort studies, most of the failures were reported in only 2 cohort studies.50,51 In a study by Pandit and colleagues,50 3 different prosthesis designs were used in 3 different time periods. In the first series of lateral UKA (1983-1991), 6 out of 51 (12%) bearings dislocated. In the second series (1998-2004), a modified technique was used and 3 out of 65 (5%) bearings dislocated. In the third series (2004-2008), a modified technique and a domed tibial component was used and only 1 out of 68 bearings dislocated (1%). In a study published in 1996, Gunther and colleagues51 also used surgical techniques and implants that were modified over the course of the study period. Because of these modified techniques, different implant designs, and year of publication, bearing dislocation most likely plays a smaller role than the 17% reported in the cohort studies. This discrepancy is a good example of the important role for the registries and registry-based studies in reporting failure modes and survivorship, especially in lateral UKA due to the low surgical frequency. Pabinger and colleagues52 recently performed a systematic review of cohort studies and registry-based studies in which they stated that the reliability in non-registry-based studies should be questioned and they considered registry-based studies superior in reporting UKA outcomes and revision rates. Furthermore, given the differences in anatomic and kinematic differences between the medial and lateral compartment and different failure modes between medial and lateral UKA, it would be better if future studies presented the medial and lateral failures separately. As stated above, most large cohort studies and especially annual registries currently do not report modes of failure of medial and lateral UKA separately.3,4,18-20
There are limitations in this study. First, this systematic review is not a full meta-analysis but a pooled analysis of collected study series and retrospective studies. Therefore, we cannot exclude sampling bias, confounders, and selection bias from the literature. We included all studies reporting failure modes of lateral UKA and excluded all case reports. We made a conscious choice about including all lateral UKA failures because this is the first systematic review of lateral UKA failure modes. Another limitation is that the follow-up period of the studies differed (Table 1) and we did not correct for the follow-up period. As stated in the example of bearing dislocations, some of these studies reported old or different techniques, while other, more recently published studies used more modified techniques11,29,53-56 Unfortunately, most studies did not report the time of arthroplasty survival and therefore we could not correct for the follow-up period.
In conclusion, progression of OA is the most common failure mode in lateral UKA, followed by aseptic loosening. Anatomic and kinematic factors such as alignment, mechanical forces during dynamic phase, and correction of valgus seem to play important roles in failure modes of lateral UKA. In the future, failure modes of medial and lateral UKA should be reported separately.
Am J Orthop. 2016;45(7):432-438, 462. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
In 1975, Skolnick and colleagues1 introduced unicompartmental knee arthroplasty (UKA) for patients with isolated unicompartmental osteoarthritis (OA). They reported a study of 14 UKA procedures, of which 12 were at the medial and 2 at the lateral side. Forty years since this procedure was introduced, UKA is used in 8% to 12% of all knee arthroplasties.2-6 A minority of these procedures are performed at the lateral side (5%-10%).6-8
The considerable anatomical and kinematical differences between compartments9-14 make it impossible to directly compare outcomes of medial and lateral UKA. For example, a greater degree of femoral roll and more posterior translation at the lateral side in flexion9,10,13 can contribute to different pattern and volume differences of cartilage wear.15 Because of these differences, and because of implant design factors and lower surgical volume, lateral UKA is considered a technically more challenging surgery compared to medial UKA.12,16,17
Since isolated lateral compartment OA is relatively scarce, current literature on lateral UKA is limited, and most studies combine medial and lateral outcomes to report UKA outcomes and failure modes.3,4,18-20 However, as the UKA has grown in popularity over the last decade,2,21-25 the number of reports about the lateral UKA also has increased. Recent studies reported excellent short-term survivorship results of the lateral UKA (96%-99%)26,27 and smaller lateral UKA studies reported the 10-year survivorship with varying outcomes from good (84%)14,28-30 to excellent (94%-100%).8,31,32 Indeed, a recent systematic review showed survivorship of lateral UKA at 5, 10, and 15 years of 93%, 91%, and 89%, respectively.33Because of the differences between the medial and lateral compartment, it is important to know the failure modes of lateral UKA in order to improve clinical outcomes and revision rates. We performed a systematic review of cohort studies and registry-based studies that reported lateral UKA failure to assess the causes of lateral UKA failure. In addition, we compared the failure modes in cohort studies with those found in registry-based studies.
Patients and Methods
Search Strategy and Criteria
Databases of PubMed, Embase, and Cochrane (Cochrane Central Register of Clinical Trials) were searched with the terms “knee, arthroplasty, replacement,” “unicompartmental,” “unicondylar,” “partial,” “UKA,” “UKR,” “UCA,” “UCR,” “PKA,” “PKR,” “PCA,” “prosthesis failure,” “reoperation,” “survivorship,” and “treatment failure.” After removal of duplicates, 2 authors (JPvdL and HAZ) scanned the articles for their title and abstract to assess eligibility for the study.
Inclusion criteria were: (I) English language articles describing studies in humans published in the last 25 years, (II) retrospective and prospective studies, (III) featured lateral UKA, (IV) OA was indication for surgery, and (V) included failure modes data. The exclusion criteria were studies that featured: (I) only a specific group of failure (eg, bearing dislocations only), (II) previous surgery in ipsilateral knee (high tibial osteotomy, medial UKA), (III) acute concurrent knee diagnoses (acute anterior cruciate ligament rupture, acute meniscal tear), (IV) combined reporting of medial and lateral UKA, or (V) multiple studies with the same patient database.
Data Collection
All studies that reported modes of failure were used in this study and these failure modes were noted in a datasheet in Microsoft Excel 2011 (Microsoft).
Statistical Analysis
For this systematic review, statistical analysis was performed with IBM SPSS Statistics 22 (SPSS Inc.). We performed chi square tests and Fisher’s exact tests to assess a difference between cohort studies and registry-based studies with the null hypothesis of no difference between both groups. A difference was considered significant when P < .05.
Results
Through the search of the databases, 1294 studies were identified and 26 handpicked studies were added. Initially, based on the title and abstract, 184 of these studies were found eligible.
A total of 366 lateral UKA failures were included. The most common failure modes were progression of OA (29%), aseptic loosening (23%), and bearing dislocation (10%). Infection (6%), instability (6%), unexplained pain (6%), and fractures (4%) were less common causes of failure of lateral UKA (Table 2).
One hundred fifty-five of these failures were reported in the cohort studies. The most common modes of failure were OA progression (36%), bearing dislocation (17%) and aseptic loosening (16%). Less common were infection (10%), fractures (5%), pain (5%), and other causes (6%). In registry-based studies, with 211 lateral UKA failures, the most common modes of failure were aseptic loosening (28%), OA progression (24%), other causes (12%), instability (10%), pain (7%), bearing dislocation (5%), and polyethylene wear (4%) (Table 2).
When pooling cohort and registry-based studies, progression of OA was significantly more common than aseptic loosening (29% vs 23%, respectively; P < .01). It was also significantly more common in the cohort studies (36% vs 16%, respectively; P < .01) but no significant difference was found between progression of OA and aseptic loosening in registry-based studies (24% and 28%, respectively; P = .16) (Table 2).
When comparing cohort with registry-based studies, progression of OA was higher in cohort studies (36% vs. 24%; P < .01). Other failures modes that were more common in cohort studies compared with registry-based studies were bearing dislocation (17% vs 5%, respectively; P < .01) and infections (10% vs 3%, P < .01). Failure modes that were more common in registry-based studies than cohort studies were aseptic loosening (28% vs 16%, respectively; P < .01), other causes (12% vs 6%, respectively, P = .02), and instability (10% vs 1%, respectively, P < .01) (Table 2).
Discussion
In this systematic review, the most common failure modes in lateral UKA review were OA progression (29%), aseptic loosening (23%), and bearing dislocation (10%). Progression of OA and bearing dislocation were the most common modes of failure in cohort studies (36% and 17%, respectively), while aseptic loosening and OA progression were the most common failure modes in registry-based studies (28% and 24%, respectively).
As mentioned above, there are differences in anatomy and kinematics between the medial and lateral compartment. When the lateral UKA failure modes are compared with studies reporting medial UKA failure modes, differences in failure modes are seen.34 Siddiqui and Ahmad35 performed a systematic review of outcomes after UKA revision and presented a table with the failure modes of included studies. Unfortunately they did not report the ratio of medial and lateral UKA. However, when assuming an average percentage of 90% to 95% of medial UKA,6,7,36 the main failure mode in their review in 17 out of 21 studies was aseptic loosening. Indeed, a recent systematic review on medial UKA failure modes showed that aseptic loosening is the most common cause of failure following this procedure.34 Similarly, a search through registry-based studies6,7 and large cohort studies37-40 that only reported medial UKA failures showed that the majority of these studies7,37-39 also reported aseptic loosening as the main cause of failure in medial UKA. When comparing the results of our systematic review of lateral UKA failures with the results of these studies of medial UKA failures, it seems that OA progression seems to play a more dominant role in failures of lateral UKA, while aseptic loosening seems to be more common in medial UKA.
Differences in anatomy and kinematics of the medial and lateral compartment can explain this. Malalignment of the joint is an important factor in the etiology of OA41,42 and biomechanical studies showed that this malalignment can cause decreased viability and further degenerative changes of cartilage of the knee.43 Hernigou and Deschamps44 showed that the alignment of the knee after medial UKA is an important factor in postoperative joint changes. They found that overcorrection of varus deformity during medial UKA surgery, measured by the hip-knee-ankle (HKA) angle, was associated with increased OA at the lateral condyle and less tibial wear of the medial UKA. Undercorrection of the varus caused an increase in tibial wear of polyethylene. Chatellard and colleagues45 found the same results in the correction of varus, measured by HKA. In addition, they found that when the prosthetic (medial) joint space was smaller than healthy (lateral) joint space, this was correlated with lower prosthesis survival. A smaller joint space at the healthy side was correlated with OA progression at the lateral compartment and tibial component wear.
These studies explain the mechanism of progression of OA and aseptic loosening. Harrington46 assessed the load in patients with valgus and varus deformity. Patients with a valgus deformity have high mechanical load on the lateral condyle during the static phase, but during the dynamic phase, a major part of this load shifts to the medial condyle. In the patients with varus deformity, the mechanical load was noted on the medial condyle during both the static and dynamic phase. Ohdera and colleagues47 advised, based on this biomechanical study and their own experiences, to correct the knee during lateral UKA to a slight valgus angle (5°-7°) to prevent OA progression at the medial side. van der List and colleagues48 similarly showed that undercorrection of 3° to 7° was correlated with better functional outcomes when compared to more neutral alignment. Moreover, Khamaisy and colleagues49 recently showed that overcorrection during UKA surgery is more common in lateral than medial UKA.
These studies are important to understanding why OA progression is more common as a failure mode in lateral UKA. The shift of mechanical load from the lateral to medial epicondyle during the dynamic phase also could explain why aseptic loosening is less common in lateral UKA. As Hernigou and Deschamps44 and Chatellard and colleagues45 stated, undercorrection of varus deformity in medial UKA is associated with higher mechanical load on the medial prosthesis side and smaller joint space width. These factors are correlated with mechanical failure of medial UKA. We think this process can be applied to lateral UKA, with the addition that the mechanical load is higher on the healthy medial compartment during the dynamic phase. This causes more forces on the healthy (medial) side in lateral UKA, and in medial UKA more forces on the prosthesis (medial) side, which results in more OA progression in lateral UKA and more aseptic loosening in medial UKA. This finding is consistent with the results of our review of more OA progression and less aseptic loosening in lateral UKA. This study also suggests that medial and lateral UKA should not be reported together in studies that present survivorship, failure modes, or clinical outcomes.
A large discrepancy was seen in bearing dislocation between cohort studies (17%) and registry-based studies (5%). When we take a closer look to the bearing dislocation failures in the cohort studies, most of the failures were reported in only 2 cohort studies.50,51 In a study by Pandit and colleagues,50 3 different prosthesis designs were used in 3 different time periods. In the first series of lateral UKA (1983-1991), 6 out of 51 (12%) bearings dislocated. In the second series (1998-2004), a modified technique was used and 3 out of 65 (5%) bearings dislocated. In the third series (2004-2008), a modified technique and a domed tibial component was used and only 1 out of 68 bearings dislocated (1%). In a study published in 1996, Gunther and colleagues51 also used surgical techniques and implants that were modified over the course of the study period. Because of these modified techniques, different implant designs, and year of publication, bearing dislocation most likely plays a smaller role than the 17% reported in the cohort studies. This discrepancy is a good example of the important role for the registries and registry-based studies in reporting failure modes and survivorship, especially in lateral UKA due to the low surgical frequency. Pabinger and colleagues52 recently performed a systematic review of cohort studies and registry-based studies in which they stated that the reliability in non-registry-based studies should be questioned and they considered registry-based studies superior in reporting UKA outcomes and revision rates. Furthermore, given the differences in anatomic and kinematic differences between the medial and lateral compartment and different failure modes between medial and lateral UKA, it would be better if future studies presented the medial and lateral failures separately. As stated above, most large cohort studies and especially annual registries currently do not report modes of failure of medial and lateral UKA separately.3,4,18-20
There are limitations in this study. First, this systematic review is not a full meta-analysis but a pooled analysis of collected study series and retrospective studies. Therefore, we cannot exclude sampling bias, confounders, and selection bias from the literature. We included all studies reporting failure modes of lateral UKA and excluded all case reports. We made a conscious choice about including all lateral UKA failures because this is the first systematic review of lateral UKA failure modes. Another limitation is that the follow-up period of the studies differed (Table 1) and we did not correct for the follow-up period. As stated in the example of bearing dislocations, some of these studies reported old or different techniques, while other, more recently published studies used more modified techniques11,29,53-56 Unfortunately, most studies did not report the time of arthroplasty survival and therefore we could not correct for the follow-up period.
In conclusion, progression of OA is the most common failure mode in lateral UKA, followed by aseptic loosening. Anatomic and kinematic factors such as alignment, mechanical forces during dynamic phase, and correction of valgus seem to play important roles in failure modes of lateral UKA. In the future, failure modes of medial and lateral UKA should be reported separately.
Am J Orthop. 2016;45(7):432-438, 462. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Skolnick MD, Bryan RS, Peterson LFA. Unicompartmental polycentric knee arthroplasty. Description and preliminary results. Clin Orthop Relat Res. 1975;(112):208-214.
2. Riddle DL, Jiranek WA, McGlynn FJ. Yearly Incidence of Unicompartmental Knee Arthroplasty in the United States. J Arthroplasty. 2008;23(3):408-412.
3. Australian Orthopaedic Association. Hip and Knee Arthroplasty 2014 Annual Report. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed June 3, 2015.
4. Swedish Knee Arthroplasty Register. 2013 Annual Report.http://myknee.se/pdf/SKAR2013_Eng.pdf. Accessed June 3, 2015.
5. The New Zealand Joint Registry. Fourteen Year Report. January 1999 to December 2012. 2013. http://nzoa.org.nz/system/files/NJR 14 Year Report.pdf. Accessed June 3, 2015.
6. Baker PN, Jameson SS, Deehan DJ, Gregg PJ, Porter M, Tucker K. Mid-term equivalent survival of medial and lateral unicondylar knee replacement: an analysis of data from a National Joint Registry. J Bone Joint Surg Br. 2012;94(12):1641-1648.
7. Lewold S, Robertsson O, Knutson K, Lidgren L. Revision of unicompartmental knee arthroplasty: outcome in 1,135 cases from the Swedish Knee Arthroplasty study. Acta Orthop Scand. 1998;69(5):469-474.
8. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Lateral unicompartmental knee arthroplasty: survivorship and technical considerations at an average follow-up of 12.4 years. J Arthroplasty. 2006;21(1):13-17.
9. Hill PF, Vedi V, Williams A, Iwaki H, Pinskerova V, Freeman MA. Tibiofemoral movement 2: the loaded and unloaded living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1196-1198.
10. Nakagawa S, Kadoya Y, Todo S, et al. Tibiofemoral movement 3: full flexion in the living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1199-1200.
11. Ashraf T, Newman JH, Evans RL, Ackroyd CE. Lateral unicompartmental knee replacement survivorship and clinical experience over 21 years. J Bone Joint Surg Br. 2002;84(8):1126-1130.
12. Scott RD. Lateral unicompartmental replacement: a road less traveled. Orthopedics. 2005;28(9):983-984.
13. Sah AP, Scott RD. Lateral unicompartmental knee arthroplasty through a medial approach. Study with an average five-year follow-up. J Bone Joint Surg Am. 2007;89(9):1948-1954.
14. Argenson JN, Parratte S, Bertani A, Flecher X, Aubaniac JM. Long-term results with a lateral unicondylar replacement. Clin Orthop Relat Res. 2008;466(11):2686-2693.
15. Weidow J, Pak J, Karrholm J. Different patterns of cartilage wear in medial and lateral gonarthrosis. Acta Orthop Scand. 2002;73(3):326-329.
16. Ollivier M, Abdel MP, Parratte S, Argenson JN. Lateral unicondylar knee arthroplasty (UKA): contemporary indications, surgical technique, and results. Int Orthop. 2014;38(2):449-455.
17. Demange MK, Von Keudell A, Probst C, Yoshioka H, Gomoll AH. Patient-specific implants for lateral unicompartmental knee arthroplasty. Int Orthop. 2015;39(8):1519-1526.
18. Khan Z, Nawaz SZ, Kahane S, Esler C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
19. Epinette JA, Brunschweiler B, Mertl P, et al. Unicompartmental knee arthroplasty modes of failure: wear is not the main reason for failure: a multicentre study of 418 failed knees. Orthop Traumatol Surg Res. 2012;98(6 Suppl):S124-S130.
20. Bordini B, Stea S, Falcioni S, Ancarani C, Toni A. Unicompartmental knee arthroplasty: 11-year experience from 3929 implants in RIPO register. Knee. 2014;21(6):1275-1279.
21. Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among medicare beneficiaries, 2000 to 2009. J Bone Joint Surg Am. 2013;95(22):e174.
22. Nwachukwu BU, McCormick FM, Schairer WW, Frank RM, Provencher MT, Roche MW. Unicompartmental knee arthroplasty versus high tibial osteotomy: United States practice patterns for the surgical treatment of unicompartmental arthritis. J Arthroplasty. 2014;29(8):1586-1589.
23. van der List JP, Chawla H, Pearle AD. Robotic-assisted knee arthroplasty: an overview. Am J Orthop. 2016;45(4):202-211.
24. van der List JP, Chawla H, Joskowicz L, Pearle AD. Current state of computer navigation and robotics in unicompartmental and total knee arthroplasty: a systematic review with meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016 Sep 6. [Epub ahead of print]
25. Zuiderbaan HA, van der List JP, Kleeblad LJ, et al. Modern indications, results and global trends in the use of unicompartmental knee arthroplasty and high tibial osteotomy for the treatment of medial unicondylar knee osteoarthritis. Am J Orthop. 2016;45(6):E355-E361.
26. Smith JR, Robinson JR, Porteous AJ, et al. Fixed bearing lateral unicompartmental knee arthroplasty--short to midterm survivorship and knee scores for 101 prostheses. Knee. 2014;21(4):843-847.
27. Berend KR, Kolczun MC 2nd, George JW Jr, Lombardi AV Jr. Lateral unicompartmental knee arthroplasty through a lateral parapatellar approach has high early survivorship. Clin Orthop Relat Res. 2012;470(1):77-83.
28. Keblish PA, Briard JL. Mobile-bearing unicompartmental knee arthroplasty: a 2-center study with an 11-year (mean) follow-up. J Arthroplasty. 2004;19(7 Suppl 2):87-94.
29. Bertani A, Flecher X, Parratte S, Aubaniac JM, Argenson JN. Unicompartmental-knee arthroplasty for treatment of lateral gonarthrosis: about 30 cases. Midterm results. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(8):763-770.
30. Sebilo A, Casin C, Lebel B, et al. Clinical and technical factors influencing outcomes of unicompartmental knee arthroplasty: Retrospective multicentre study of 944 knees. Orthop Traumatol Surg Res. 2013;99(4 Suppl):S227-S234.
31. Cartier P, Khefacha A, Sanouiller JL, Frederick K. Unicondylar knee arthroplasty in middle-aged patients: A minimum 5-year follow-up. Orthopedics. 2007;30(8 Suppl):62-65.
32. Lustig S, Paillot JL, Servien E, Henry J, Ait Si Selmi T, Neyret P. Cemented all polyethylene tibial insert unicompartimental knee arthroplasty: a long term follow-up study. Orthop Traumatol Surg Res. 2009;95(1):12-21.
33. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
34. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2016;31(5):1016-1021.
35. Siddiqui NA, Ahmad ZM. Revision of unicondylar to total knee arthroplasty: a systematic review. Open Orthop J. 2012;6:268-275.
36. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Lateral unicompartmental knee arthroplasty: survivorship and technical considerations at an average follow-up of 12.4 years. J Arthroplasty. 2006;21(1):13-17.
37. Kalra S, Smith TO, Berko B, Walton NP. Assessment of radiolucent lines around the Oxford unicompartmental knee replacement: sensitivity and specificity for loosening. J Bone Joint Surg Br. 2011;93(6):777-781.
38. Wynn Jones H, Chan W, Harrison T, Smith TO, Masonda P, Walton NP. Revision of medial Oxford unicompartmental knee replacement to a total knee replacement: similar to a primary? Knee. 2012;19(4):339-343.
39. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
40. Citak M, Dersch K, Kamath AF, Haasper C, Gehrke T, Kendoff D. Common causes of failed unicompartmental knee arthroplasty: a single-centre analysis of four hundred and seventy one cases. Int Orthop. 2014;38(5):961-965.
41. Hunter DJ, Wilson DR. Role of alignment and biomechanics in osteoarthritis and implications for imaging. Radiol Clin North Am. 2009;47(4):553-566.
42. Hunter DJ, Sharma L, Skaife T. Alignment and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91 Suppl 1:85-89.
43. Roemhildt ML, Beynnon BD, Gauthier AE, Gardner-Morse M, Ertem F, Badger GJ. Chronic in vivo load alteration induces degenerative changes in the rat tibiofemoral joint. Osteoarthritis Cartilage. 2013;21(2):346-357.
44. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
45. Chatellard R, Sauleau V, Colmar M, et al. Medial unicompartmental knee arthroplasty: does tibial component position influence clinical outcomes and arthroplasty survival? Orthop Traumatol Surg Res. 2013;99(4 Suppl):S219-S225.
46. Harrington IJ. Static and dynamic loading patterns in knee joints with deformities. J Bone Joint Surg Am. 1983;65(2):247-259.
47. Ohdera T, Tokunaga J, Kobayashi A. Unicompartmental knee arthroplasty for lateral gonarthrosis: midterm results. J Arthroplasty. 2001;16(2):196-200.
48. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 26. [Epub ahead of print]
49. Khamaisy S, Gladnick BP, Nam D, Reinhardt KR, Heyse TJ, Pearle AD. Lower limb alignment control: Is it more challenging in lateral compared to medial unicondylar knee arthroplasty? Knee. 2015;22(4):347-350.
50. Pandit H, Jenkins C, Beard DJ, et al. Mobile bearing dislocation in lateral unicompartmental knee replacement. Knee. 2010;17(6):392-397.
51. Gunther TV, Murray DW, Miller R, et al. Lateral unicompartmental arthroplasty with the Oxford meniscal knee. Knee. 1996;3(1):33-39.
52. Pabinger C, Lumenta DB, Cupak D, Berghold A, Boehler N, Labek G. Quality of outcome data in knee arthroplasty: Comparison of registry data and worldwide non-registry studies from 4 decades. Acta Orthopaedica. 2015;86(1):58-62.
53. Lustig S, Elguindy A, Servien E, et al. 5- to 16-year follow-up of 54 consecutive lateral unicondylar knee arthroplasties with a fixed-all polyethylene bearing. J Arthroplasty. 2011;26(8):1318-1325.
54. Walton MJ, Weale AE, Newman JH. The progression of arthritis following lateral unicompartmental knee replacement. Knee. 2006;13(5):374-377.
55. Lustig S, Lording T, Frank F, Debette C, Servien E, Neyret P. Progression of medial osteoarthritis and long term results of lateral unicompartmental arthroplasty: 10 to 18 year follow-up of 54 consecutive implants. Knee. 2014;21(S1):S26-S32.
56. O’Rourke MR, Gardner JJ, Callaghan JJ, et al. Unicompartmental knee replacement: a minimum twenty-one-year followup, end-result study. Clin Orthop Relat Res. 2005;440:27-37.
57. Citak M, Cross MB, Gehrke T, Dersch K, Kendoff D. Modes of failure and revision of failed lateral unicompartmental knee arthroplasties. Knee. 2015;22(4):338-340.
58. Liebs TR, Herzberg W. Better quality of life after medial versus lateral unicondylar knee arthroplasty knee. Clin Orthop Relat Res. 2013;471(8):2629-2640.
59. Weston-Simons JS, Pandit H, Kendrick BJ, et al. The mid-term outcomes of the Oxford Domed Lateral unicompartmental knee replacement. Bone Joint J. 2014;96-B(1):59-64.
60. Thompson SA, Liabaud B, Nellans KW, Geller JA. Factors associated with poor outcomes following unicompartmental knee arthroplasty: redefining the “classic” indications for surgery. J Arthroplasty. 2013;28(9):1561-1564.
61. Saxler G, Temmen D, Bontemps G. Medium-term results of the AMC-unicompartmental knee arthroplasty. Knee. 2004;11(5):349-355.
62. Forster MC, Bauze AJ, Keene GCR. Lateral unicompartmental knee replacement: Fixed or mobile bearing? Knee Surg Sports Traumatol Arthrosc. 2007;15(9):1107-1111.
63. Streit MR, Walker T, Bruckner T, et al. Mobile-bearing lateral unicompartmental knee replacement with the Oxford domed tibial component: an independent series. J Bone Joint Surg Br. 2012;94(10):1356-1361.
64. Altuntas AO, Alsop H, Cobb JP. Early results of a domed tibia, mobile bearing lateral unicompartmental knee arthroplasty from an independent centre. Knee. 2013;20(6):466-470.
65. Ashraf T, Newman JH, Desai VV, Beard D, Nevelos JE. Polyethylene wear in a non-congruous unicompartmental knee replacement: a retrieval analysis. Knee. 2004;11(3):177-181.
66. Schelfaut S, Beckers L, Verdonk P, Bellemans J, Victor J. The risk of bearing dislocation in lateral unicompartmental knee arthroplasty using a mobile biconcave design. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2487-2494.
67. Marson B, Prasad N, Jenkins R, Lewis M. Lateral unicompartmental knee replacements: Early results from a District General Hospital. Eur J Orthop Surg Traumatol. 2014;24(6):987-991.
68. Walker T, Gotterbarm T, Bruckner T, Merle C, Streit MR. Total versus unicompartmental knee replacement for isolated lateral osteoarthritis: a matched-pairs study. Int Orthop. 2014;38(11):2259-2264.
1. Skolnick MD, Bryan RS, Peterson LFA. Unicompartmental polycentric knee arthroplasty. Description and preliminary results. Clin Orthop Relat Res. 1975;(112):208-214.
2. Riddle DL, Jiranek WA, McGlynn FJ. Yearly Incidence of Unicompartmental Knee Arthroplasty in the United States. J Arthroplasty. 2008;23(3):408-412.
3. Australian Orthopaedic Association. Hip and Knee Arthroplasty 2014 Annual Report. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed June 3, 2015.
4. Swedish Knee Arthroplasty Register. 2013 Annual Report.http://myknee.se/pdf/SKAR2013_Eng.pdf. Accessed June 3, 2015.
5. The New Zealand Joint Registry. Fourteen Year Report. January 1999 to December 2012. 2013. http://nzoa.org.nz/system/files/NJR 14 Year Report.pdf. Accessed June 3, 2015.
6. Baker PN, Jameson SS, Deehan DJ, Gregg PJ, Porter M, Tucker K. Mid-term equivalent survival of medial and lateral unicondylar knee replacement: an analysis of data from a National Joint Registry. J Bone Joint Surg Br. 2012;94(12):1641-1648.
7. Lewold S, Robertsson O, Knutson K, Lidgren L. Revision of unicompartmental knee arthroplasty: outcome in 1,135 cases from the Swedish Knee Arthroplasty study. Acta Orthop Scand. 1998;69(5):469-474.
8. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Lateral unicompartmental knee arthroplasty: survivorship and technical considerations at an average follow-up of 12.4 years. J Arthroplasty. 2006;21(1):13-17.
9. Hill PF, Vedi V, Williams A, Iwaki H, Pinskerova V, Freeman MA. Tibiofemoral movement 2: the loaded and unloaded living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1196-1198.
10. Nakagawa S, Kadoya Y, Todo S, et al. Tibiofemoral movement 3: full flexion in the living knee studied by MRI. J Bone Joint Surg Br. 2000;82(8):1199-1200.
11. Ashraf T, Newman JH, Evans RL, Ackroyd CE. Lateral unicompartmental knee replacement survivorship and clinical experience over 21 years. J Bone Joint Surg Br. 2002;84(8):1126-1130.
12. Scott RD. Lateral unicompartmental replacement: a road less traveled. Orthopedics. 2005;28(9):983-984.
13. Sah AP, Scott RD. Lateral unicompartmental knee arthroplasty through a medial approach. Study with an average five-year follow-up. J Bone Joint Surg Am. 2007;89(9):1948-1954.
14. Argenson JN, Parratte S, Bertani A, Flecher X, Aubaniac JM. Long-term results with a lateral unicondylar replacement. Clin Orthop Relat Res. 2008;466(11):2686-2693.
15. Weidow J, Pak J, Karrholm J. Different patterns of cartilage wear in medial and lateral gonarthrosis. Acta Orthop Scand. 2002;73(3):326-329.
16. Ollivier M, Abdel MP, Parratte S, Argenson JN. Lateral unicondylar knee arthroplasty (UKA): contemporary indications, surgical technique, and results. Int Orthop. 2014;38(2):449-455.
17. Demange MK, Von Keudell A, Probst C, Yoshioka H, Gomoll AH. Patient-specific implants for lateral unicompartmental knee arthroplasty. Int Orthop. 2015;39(8):1519-1526.
18. Khan Z, Nawaz SZ, Kahane S, Esler C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
19. Epinette JA, Brunschweiler B, Mertl P, et al. Unicompartmental knee arthroplasty modes of failure: wear is not the main reason for failure: a multicentre study of 418 failed knees. Orthop Traumatol Surg Res. 2012;98(6 Suppl):S124-S130.
20. Bordini B, Stea S, Falcioni S, Ancarani C, Toni A. Unicompartmental knee arthroplasty: 11-year experience from 3929 implants in RIPO register. Knee. 2014;21(6):1275-1279.
21. Bolognesi MP, Greiner MA, Attarian DE, et al. Unicompartmental knee arthroplasty and total knee arthroplasty among medicare beneficiaries, 2000 to 2009. J Bone Joint Surg Am. 2013;95(22):e174.
22. Nwachukwu BU, McCormick FM, Schairer WW, Frank RM, Provencher MT, Roche MW. Unicompartmental knee arthroplasty versus high tibial osteotomy: United States practice patterns for the surgical treatment of unicompartmental arthritis. J Arthroplasty. 2014;29(8):1586-1589.
23. van der List JP, Chawla H, Pearle AD. Robotic-assisted knee arthroplasty: an overview. Am J Orthop. 2016;45(4):202-211.
24. van der List JP, Chawla H, Joskowicz L, Pearle AD. Current state of computer navigation and robotics in unicompartmental and total knee arthroplasty: a systematic review with meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016 Sep 6. [Epub ahead of print]
25. Zuiderbaan HA, van der List JP, Kleeblad LJ, et al. Modern indications, results and global trends in the use of unicompartmental knee arthroplasty and high tibial osteotomy for the treatment of medial unicondylar knee osteoarthritis. Am J Orthop. 2016;45(6):E355-E361.
26. Smith JR, Robinson JR, Porteous AJ, et al. Fixed bearing lateral unicompartmental knee arthroplasty--short to midterm survivorship and knee scores for 101 prostheses. Knee. 2014;21(4):843-847.
27. Berend KR, Kolczun MC 2nd, George JW Jr, Lombardi AV Jr. Lateral unicompartmental knee arthroplasty through a lateral parapatellar approach has high early survivorship. Clin Orthop Relat Res. 2012;470(1):77-83.
28. Keblish PA, Briard JL. Mobile-bearing unicompartmental knee arthroplasty: a 2-center study with an 11-year (mean) follow-up. J Arthroplasty. 2004;19(7 Suppl 2):87-94.
29. Bertani A, Flecher X, Parratte S, Aubaniac JM, Argenson JN. Unicompartmental-knee arthroplasty for treatment of lateral gonarthrosis: about 30 cases. Midterm results. Rev Chir Orthop Reparatrice Appar Mot. 2008;94(8):763-770.
30. Sebilo A, Casin C, Lebel B, et al. Clinical and technical factors influencing outcomes of unicompartmental knee arthroplasty: Retrospective multicentre study of 944 knees. Orthop Traumatol Surg Res. 2013;99(4 Suppl):S227-S234.
31. Cartier P, Khefacha A, Sanouiller JL, Frederick K. Unicondylar knee arthroplasty in middle-aged patients: A minimum 5-year follow-up. Orthopedics. 2007;30(8 Suppl):62-65.
32. Lustig S, Paillot JL, Servien E, Henry J, Ait Si Selmi T, Neyret P. Cemented all polyethylene tibial insert unicompartimental knee arthroplasty: a long term follow-up study. Orthop Traumatol Surg Res. 2009;95(1):12-21.
33. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
34. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2016;31(5):1016-1021.
35. Siddiqui NA, Ahmad ZM. Revision of unicondylar to total knee arthroplasty: a systematic review. Open Orthop J. 2012;6:268-275.
36. Pennington DW, Swienckowski JJ, Lutes WB, Drake GN. Lateral unicompartmental knee arthroplasty: survivorship and technical considerations at an average follow-up of 12.4 years. J Arthroplasty. 2006;21(1):13-17.
37. Kalra S, Smith TO, Berko B, Walton NP. Assessment of radiolucent lines around the Oxford unicompartmental knee replacement: sensitivity and specificity for loosening. J Bone Joint Surg Br. 2011;93(6):777-781.
38. Wynn Jones H, Chan W, Harrison T, Smith TO, Masonda P, Walton NP. Revision of medial Oxford unicompartmental knee replacement to a total knee replacement: similar to a primary? Knee. 2012;19(4):339-343.
39. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
40. Citak M, Dersch K, Kamath AF, Haasper C, Gehrke T, Kendoff D. Common causes of failed unicompartmental knee arthroplasty: a single-centre analysis of four hundred and seventy one cases. Int Orthop. 2014;38(5):961-965.
41. Hunter DJ, Wilson DR. Role of alignment and biomechanics in osteoarthritis and implications for imaging. Radiol Clin North Am. 2009;47(4):553-566.
42. Hunter DJ, Sharma L, Skaife T. Alignment and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91 Suppl 1:85-89.
43. Roemhildt ML, Beynnon BD, Gauthier AE, Gardner-Morse M, Ertem F, Badger GJ. Chronic in vivo load alteration induces degenerative changes in the rat tibiofemoral joint. Osteoarthritis Cartilage. 2013;21(2):346-357.
44. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
45. Chatellard R, Sauleau V, Colmar M, et al. Medial unicompartmental knee arthroplasty: does tibial component position influence clinical outcomes and arthroplasty survival? Orthop Traumatol Surg Res. 2013;99(4 Suppl):S219-S225.
46. Harrington IJ. Static and dynamic loading patterns in knee joints with deformities. J Bone Joint Surg Am. 1983;65(2):247-259.
47. Ohdera T, Tokunaga J, Kobayashi A. Unicompartmental knee arthroplasty for lateral gonarthrosis: midterm results. J Arthroplasty. 2001;16(2):196-200.
48. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015 Nov 26. [Epub ahead of print]
49. Khamaisy S, Gladnick BP, Nam D, Reinhardt KR, Heyse TJ, Pearle AD. Lower limb alignment control: Is it more challenging in lateral compared to medial unicondylar knee arthroplasty? Knee. 2015;22(4):347-350.
50. Pandit H, Jenkins C, Beard DJ, et al. Mobile bearing dislocation in lateral unicompartmental knee replacement. Knee. 2010;17(6):392-397.
51. Gunther TV, Murray DW, Miller R, et al. Lateral unicompartmental arthroplasty with the Oxford meniscal knee. Knee. 1996;3(1):33-39.
52. Pabinger C, Lumenta DB, Cupak D, Berghold A, Boehler N, Labek G. Quality of outcome data in knee arthroplasty: Comparison of registry data and worldwide non-registry studies from 4 decades. Acta Orthopaedica. 2015;86(1):58-62.
53. Lustig S, Elguindy A, Servien E, et al. 5- to 16-year follow-up of 54 consecutive lateral unicondylar knee arthroplasties with a fixed-all polyethylene bearing. J Arthroplasty. 2011;26(8):1318-1325.
54. Walton MJ, Weale AE, Newman JH. The progression of arthritis following lateral unicompartmental knee replacement. Knee. 2006;13(5):374-377.
55. Lustig S, Lording T, Frank F, Debette C, Servien E, Neyret P. Progression of medial osteoarthritis and long term results of lateral unicompartmental arthroplasty: 10 to 18 year follow-up of 54 consecutive implants. Knee. 2014;21(S1):S26-S32.
56. O’Rourke MR, Gardner JJ, Callaghan JJ, et al. Unicompartmental knee replacement: a minimum twenty-one-year followup, end-result study. Clin Orthop Relat Res. 2005;440:27-37.
57. Citak M, Cross MB, Gehrke T, Dersch K, Kendoff D. Modes of failure and revision of failed lateral unicompartmental knee arthroplasties. Knee. 2015;22(4):338-340.
58. Liebs TR, Herzberg W. Better quality of life after medial versus lateral unicondylar knee arthroplasty knee. Clin Orthop Relat Res. 2013;471(8):2629-2640.
59. Weston-Simons JS, Pandit H, Kendrick BJ, et al. The mid-term outcomes of the Oxford Domed Lateral unicompartmental knee replacement. Bone Joint J. 2014;96-B(1):59-64.
60. Thompson SA, Liabaud B, Nellans KW, Geller JA. Factors associated with poor outcomes following unicompartmental knee arthroplasty: redefining the “classic” indications for surgery. J Arthroplasty. 2013;28(9):1561-1564.
61. Saxler G, Temmen D, Bontemps G. Medium-term results of the AMC-unicompartmental knee arthroplasty. Knee. 2004;11(5):349-355.
62. Forster MC, Bauze AJ, Keene GCR. Lateral unicompartmental knee replacement: Fixed or mobile bearing? Knee Surg Sports Traumatol Arthrosc. 2007;15(9):1107-1111.
63. Streit MR, Walker T, Bruckner T, et al. Mobile-bearing lateral unicompartmental knee replacement with the Oxford domed tibial component: an independent series. J Bone Joint Surg Br. 2012;94(10):1356-1361.
64. Altuntas AO, Alsop H, Cobb JP. Early results of a domed tibia, mobile bearing lateral unicompartmental knee arthroplasty from an independent centre. Knee. 2013;20(6):466-470.
65. Ashraf T, Newman JH, Desai VV, Beard D, Nevelos JE. Polyethylene wear in a non-congruous unicompartmental knee replacement: a retrieval analysis. Knee. 2004;11(3):177-181.
66. Schelfaut S, Beckers L, Verdonk P, Bellemans J, Victor J. The risk of bearing dislocation in lateral unicompartmental knee arthroplasty using a mobile biconcave design. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2487-2494.
67. Marson B, Prasad N, Jenkins R, Lewis M. Lateral unicompartmental knee replacements: Early results from a District General Hospital. Eur J Orthop Surg Traumatol. 2014;24(6):987-991.
68. Walker T, Gotterbarm T, Bruckner T, Merle C, Streit MR. Total versus unicompartmental knee replacement for isolated lateral osteoarthritis: a matched-pairs study. Int Orthop. 2014;38(11):2259-2264.
Liposomal Bupivacaine vs Interscalene Nerve Block for Pain Control After Shoulder Arthroplasty: A Retrospective Cohort Analysis
The annual number of total shoulder arthroplasties (TSAs) is rising with the growing elderly population and development of new technologies such as reverse shoulder arthroplasty.1 In 2008, 47,000 shoulder arthroplasties were performed in the US compared with 19,000 in 1998.1 As of 2011, there were 53,000 shoulder arthroplasties performed annually.2 Pain control after shoulder procedures, particularly TSA, is challenging. 3
Several modalities exist to manage pain after shoulder arthroplasty. The interscalene brachial plexus nerve block is considered the “gold standard” for shoulder analgesia. A new approach is the periarticular injection method, in which the surgeon administers a local anesthetic intraoperatively. Liposomal bupivacaine (Exparel, Pacira Pharmaceuticals, Inc.) is a nonopioid anesthetic that has been shown to improve pain control, shorten hospital stays, and decrease costs for total knee and hip arthroplasty compared with nerve blocks.4-6 Patients who were treated with liposomal bupivacaine consumed less opioid medication than a placebo group.7
Our purpose was to compare intraoperative local liposomal bupivacaine injection with preoperative single-shot interscalene nerve block (ISNB) in terms of pain control, opioid use, and length of hospital stay (LOS) after shoulder arthroplasty. We hypothesized that patients in the liposomal bupivacaine group would have lower pain scores, less opioid use, and shorter LOS compared with patients in the ISNB group.
Methods
A retrospective cohort analysis was conducted with 58 patients who underwent shoulder arthroplasty by 1 surgeon at our academically affiliated community hospital from January 2012 through January 2015. ISNBs were the standard at the beginning of the study period and were used until Exparel became available on the hospital formulary in 2013. We began using Exparel for all shoulder arthroplasties in November 2013. No other changes were made in the perioperative management of our arthroplasty patients during this period. Patients who underwent TSA, reverse TSA, or hemiarthroplasty of the shoulder were included. Patients who underwent revision TSA were excluded. Twenty-one patients received ISNBs and 37 received liposomal bupivacaine injections. This study was approved by our Institutional Review Board.
Baseline data for each patient were age, sex, body mass index, and the American Society of Anesthesiologists (ASA) Physical Status Classification. The primary outcome measure was the numeric rating scale (NRS) pain score at 4 post-operative time intervals. The NRS pain score has a range of 0 to 10, with 10 representing severe pain. Data were gathered from nursing and physical therapy notes in patient charts. The postoperative time intervals were 0 to 1 hour, 8 to 14 hours, 18 to 24 hours, and 27 to 36 hours. Available NRS scores for these time intervals were averaged. Patients were included if they had pain scores for at least 3 of the postoperative time intervals documented in their charts. Secondary outcome measures were LOS and opioid consumption during hospital admission. Intravenous acetaminophen use was also measured in both groups. All data on opioids were converted to oral morphine equivalents using the method described by Schneider and colleagues.8
A board-certified, fellowship-trained anesthesiologist, experienced in regional anesthesia, administered the single-shot ISNB before surgery. The block was administered under ultrasound guidance using a 44-mm, 22-gauge needle with the patient in the supine position. No indwelling catheter was used. The medication consisted of 30 mL of 5% ropivacaine (5 mg/mL). The surgeon injected liposomal bupivacaine (266 mg diluted into 40 mL of injectable saline) near the end of the procedure throughout the pericapsular area and multiple layers of the wound, per manufacturer guidelines.9 A 60-mL syringe with a 20-gauge needle was used. All operations were performed by 1 board-certified, fellowship-trained surgeon using a standard deltopectoral approach with the same surgical equipment. The same postoperative pain protocol was used for all patients, including intravenous acetaminophen and patient-controlled analgesia. Additional oral pain medication was provided as needed for all patients. Physical therapy protocols were identical between groups.
Statistical Analysis
Mean patient ages in the 2 treatment groups were compared using the Student t test. Sex distribution and the ASA scores were compared using a χ2 test and a Fisher exact test, respectively. Arthroplasty types were compared using a Fisher exact test. The medians and interquartile ranges of the NRS scores at each time point measured were tabulated by treatment group, and at each time point the difference between groups was tested using nonparametric rank sum tests.
We tested the longitudinal trajectory of NRS scores over time, accounting for repeated measurements in the same patients using linear mixed model analysis. Treatment group, time period as a categorical variable, and the interaction between treatment and time period were included as fixed effects, and patient identification number was included as the random effect. An initial omnibus test was performed for all treatment and treatment-by-time interaction effects. Subsequently, the treatment-by-time interaction was tested for each of the time periods. The association of day of discharge (as a categorical variable) with treatment was tested using the Fisher exact test. All analyses were conducted using Stata, version 13, software (StataCorp LP). P values <.05 were considered significant.
Sample Size Analysis
We calculated the minimum detectable effect size with 80% power at an alpha level of 0.05 for the nonparametric rank sum test in terms of the proportion of every possible pair of patients treated with the 2 treatments, where the patient treated with liposomal bupivacaine has a lower pain score than the patient treated with ISNB. For pain score at 18 to 24 hours, the sample sizes of 33 patients treated with liposomal bupivacaine and 20 treated with ISNB, the minimum detectable effect size is 73%.
Results
Fifty-eight patient charts (21 in the ISNB group and 37 in the liposomal bupivacaine group) were reviewed for the study. Patient sex distribution, mean age, mean body mass index, and mean baseline ASA scores were not statistically different (Table 1).
The primary outcome measure, NRS pain score, showed no significant differences between groups at 0 to 1 hour after surgery (P = .99) or 8 to 14 hours after surgery (P = .208).
There was no difference in the amount of intravenous acetaminophen given during the hospital stay between groups. There was no significant difference in opioid consumption on postoperative day 1 in the hospital (P = .59) (Figure 2). However, there were significant differences between groups on postoperative days 2 and 3.
Sixteen of 37 patients in the liposomal bupivacaine group and 2 of 21 in the ISNB group were discharged on the day after surgery (P = .010) (Table 3).
There were no major cardiac or respiratory events in either group. No long-term paresthesias or neuropathies were noted. There were no readmissions for either group.
Discussion
Postoperative pain control after shoulder arthroplasty can be challenging, and several modalities have been tried in various combinations to minimize pain and decrease adverse effects of opioid medications. The most common method for pain relief after shoulder arthroplasty is the ISNB. Several studies of ISNBs have shown improved pain control after shoulder arthroplasty with associated decreased opioid consumption and related side effects.10 Patient rehabilitation and satisfaction have improved with the increasing use of peripheral nerve blocks.11
Despite the well-established benefits of ISNBs, several limitations exist. Although the superior portion of the shoulder is well covered by an ISNB, the inferior portion of the brachial plexus can remain uncovered or only partially covered.12 Complications of ISNBs include hemidiaphragmatic paresis, rebound pain 24 hours after surgery,13 chronic neurologic complications,14 and substantial respiratory and cardiovascular events.15 Nerve blocks also require additional time and resources in the perioperative period, including an anesthesiologist with specialized training, assistants, and ultrasonography or nerve stimulation equipment contraindicated in patients taking blood thinners.16
Periarticular injections of local anesthetics have also shown promise in reducing pain after arthroplasty.4 Benefits include an enhanced safety profile because local injection avoids the concurrent blockade of the phrenic nerve and recurrent laryngeal nerve and has not been associated with the risk of peripheral neuropathies. Further, local injection is a simple technique that can be performed during surgery without additional personnel or expertise. A limitation of this approach is the relatively short duration of effectiveness of the local anesthetic and uncertainty regarding the best agent and the ideal volume of injection.6 Liposomal bupivacaine is a new agent (approved by the US Food and Drug Administration in 201117) with a sustained release over 72 to 96 hours.18 The most common adverse effects of liposomal bupivacaine are nausea, vomiting, constipation, pyrexia, dizziness, and headache.19 Chondrotoxicity and granulomatous inflammation are more serious, yet rare, complications of liposomal bupivacaine.20
We found that liposomal bupivacaine injections were associated with lower pain scores compared with ISNB at 18 to 24 hours after surgery. This correlated with less opioid consumption in the liposomal bupivacaine group than in the ISNB group on the second postoperative day. These differences in pain values are consistent with the known pharmacokinetics of liposomal bupivacaine.18 Peak plasma levels normally occur approximately 24 hours after injection, leaving the early postoperative period relatively uncovered by anesthetic agent. This finding of relatively poor pain control early after surgery has also been noted in patients undergoing knee arthroplasty.5 On the basis of the findings of this study, we have added standard bupivacaine injections to our separate liposomal bupivacaine injection to cover early postoperative pain. Opioid consumption was significantly lower in the liposomal bupivacaine group than in the ISNB group on postoperative days 2 and 3. We did not measure adverse events related to opioid consumption, so we cannot comment on whether the decreased opioid consumption was associated with the rate of adverse events. However, other studies21,22 have established this relationship.
We found the liposomal bupivacaine group to have earlier discharges to home. Sixteen of 37 patients in the liposomal bupivacaine group compared with 2 of 21 patients in the ISNB group were discharged on the day after surgery. A mean reduction in LOS of 18 hours for the liposomal bupivacaine group was statistically significant (P = .012). This reduction in LOS has important implications for hospitals and value analysis committees considering whether to keep a new, more expensive local anesthetic on formulary. Savings from reduced LOS and improvements in patient satisfaction may justify the expense (approximately $300 per 266-mg vial) of Exparel.
From a societal cost perspective, liposomal bupivacaine is more economical compared with ISNB, which adds approximately $1500 to the cost of anesthesia per patient.23 Eliminating the costs associated with ISNB administration in shoulder arthroplasties could result in substantial savings to our healthcare system. More research examining time savings and exact costs of each procedure is needed to determine the true cost effectiveness of each approach.
Limitations of our study include the retrospective design, relatively small numbers of patients in each group, missing data for some patients at various time points, variation in the types of procedures in each group, and lack of long-term outcome measures. It is important to note that we did not confirm the success of the nerve block after administration. However, this study reflects the effectiveness of each of the modalities in actual clinical conditions (as opposed to a controlled experimental setting). The actual effectiveness of a nerve block varies, even when performed by an experienced anesthesiologist with ultrasound guidance. Furthermore, immediate postoperative pain scores in the nerve block group are consistent with those of prior research reporting pain values ranging from 4 to 5 and a mean duration of effect ranging from 9 to 14 hours.23,24 Additionally, the patients, surgeon, and nursing team were not blinded to the treatment group. Although we did note a significant difference in the types of procedures between groups, this finding is related to the greater number of hemiarthroplasties performed in the ISNB group (N = 5) compared with the liposomal group (N = 1). Because of this variation and the decreased invasiveness of hemiarthroplasties, the bias is against the liposomal group. Finally, our primary outcome variable was pain, which is a subjective, self-reported measure. However, our opioid consumption data and LOS data corroborate the improved pain scores in the liposomal bupivacaine group.
Limiting the study to a single surgeon may limit external validity. Another limitation is the lack of data on adverse events related to opioid medication use. There was no additional experimental group to determine whether less expensive local anesthetics injected locally would perform similarly to liposomal bupivacaine. In total knee arthroplasty, periarticular injections of liposomal bupivacaine were not as effective as less expensive periarticular injections.25 It is unclear which agents (and in what doses or combinations) should be used for periarticular injections. Finally, we acknowledge that our retrospective study design cannot account for all potential factors affecting discharge time.
This is the first comparative study of liposomal bupivacaine and ISNB in TSA. The study design allowed us to control for variables such as surgical technique, postoperative protocols (including use and type of sling), and use of other pain modalities such as patient-controlled analgesia and intravenous acetaminophen that are likely to affect postoperative pain and LOS. This study provides preliminary data that confirm relative equipoise between liposomal bupivacaine and ISNB, which is needed for the ethical conduct of a randomized controlled trial. Such a trial would allow for a more robust comparison, and this retrospective study provides appropriate pilot data on which to base this design and the clinical information needed to counsel patients during enrollment.
Our results suggest that liposomal bupivacaine may provide superior or similar pain relief compared with ISNB after shoulder arthroplasty. Additionally, the use of liposomal bupivacaine was associated with decreased opioid consumption and earlier discharge to home compared with ISNB. These findings have important implications for pain control after TSA because pain represents a major concern for patients and providers after surgery. In addition to clinical improvements, use of liposomal bupivacaine may save time and eliminate costs associated with administering nerve blocks. Local injection may also be used in patients who are contraindicated for ISNB such as those with obesity, pulmonary disease, or peripheral neuropathy. Although we cannot definitively suggest that liposomal bupivacaine is superior to the current gold standard ISNB for pain control after shoulder arthroplasty, our results suggest a relative clinical equipoise between these modalities. Larger analytical studies, including randomized trials, should be performed to explore the potential benefits of liposomal bupivacaine injections for pain control after shoulder arthroplasty.
Am J Orthop. 2016;45(7):424-430. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. American Academy of Orthopaedic Surgeons. Shoulder joint replacement. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Accessed June 3, 2015.
3. Desai VN, Cheung EV. Postoperative pain associated with orthopedic shoulder and elbow surgery: a prospective study. J Shoulder Elbow Surg. 2012;21(4):441-450.
4. Springer BD. Transition from nerve blocks to periarticular injections and emerging techniques in total joint arthroplasty. Am J Orthop. 2014;43(10 Suppl):S6-S9.
5. Surdam JW, Licini DJ, Baynes NT, Arce BR. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J Arthroplasty. 2015;30(2):325-329.
6. Tong YC, Kaye AD, Urman RD. Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol. 2014;28(1):15-27.
7. Chahar P, Cummings KC 3rd. Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res. 2012;5:257-264.
8. Schneider C, Yale SH, Larson M. Principles of pain management. Clin Med Res. 2003;1(4):337-340.
9. Pacira Pharmaceuticals, Inc. Highlights of prescribing information. http://www.exparel.com/pdf/EXPAREL_Prescribing_Information.pdf. Accessed May 7, 2015.
10. Gohl MR, Moeller RK, Olson RL, Vacchiano CA. The addition of interscalene block to general anesthesia for patients undergoing open shoulder procedures. AANA J. 2001;69(2):105-109.
11. Ironfield CM, Barrington MJ, Kluger R, Sites B. Are patients satisfied after peripheral nerve blockade? Results from an International Registry of Regional Anesthesia. Reg Anesth Pain Med. 2014;39(1):48-55.
12. Srikumaran U, Stein BE, Tan EW, Freehill MT, Wilckens JH. Upper-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(24):e197(1-13).
13. DeMarco JR, Componovo R, Barfield WR, Liles L, Nietert P. Efficacy of augmenting a subacromial continuous-infusion pump with a preoperative interscalene block in outpatient arthroscopic shoulder surgery: a prospective, randomized, blinded, and placebo-controlled study. Arthroscopy. 2011;27(5):603-610.
14. Misamore G, Webb B, McMurray S, Sallay P. A prospective analysis of interscalene brachial plexus blocks performed under general anesthesia. J Shoulder Elbow Surg. 2011;20(2):308-314.
15. Lenters TR, Davies J, Matsen FA 3rd. The types and severity of complications associated with interscalene brachial plexus block anesthesia: local and national evidence. J Shoulder Elbow Surg. 2007;16(4):379-387.
16. Park SK, Choi YS, Choi SW, Song SW. A comparison of three methods for postoperative pain control in patients undergoing arthroscopic shoulder surgery. Korean J Pain. 2015;28(1):45-51.
17. Pacira Pharmaceuticals, Inc. Pacira Pharmaceuticals, Inc. announces U.S. FDA approval of EXPAREL™ for postsurgical pain management. http://investor.pacira.com/phoenix.zhtml?c=220759&p=irol-newsArticle_print&ID=1623529. Published October 31, 2011. Accessed June 3, 2015.
18. White PF, Ardeleanu M, Schooley G, Burch RM. Pharmocokinetics of depobupivacaine following infiltration in patients undergoing two types of surgery and in normal volunteers. Paper presented at: Annual Meeting of the International Anesthesia Research Society; March 14, 2009; San Diego, CA.
19. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
20. Lambrechts M, O’Brien MJ, Savoie FH, You Z. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer Adherence. 2013;7:885-890.
21. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
22. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res Clin Exp. 2013;76:1-6.
23. Weber SC, Jain R. Scalene regional anesthesia for shoulder surgery in a community setting: an assessment of risk. J Bone Joint Surg Am. 2002;84-A(5):775-779.
24. Beaudet V, Williams SR, Tétreault P, Perrault MA. Perioperative interscalene block versus intra-articular injection of local anesthetics for postoperative analgesia in shoulder surgery. Reg Anesth Pain Med. 2008;33(2):134-138.
25. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
The annual number of total shoulder arthroplasties (TSAs) is rising with the growing elderly population and development of new technologies such as reverse shoulder arthroplasty.1 In 2008, 47,000 shoulder arthroplasties were performed in the US compared with 19,000 in 1998.1 As of 2011, there were 53,000 shoulder arthroplasties performed annually.2 Pain control after shoulder procedures, particularly TSA, is challenging. 3
Several modalities exist to manage pain after shoulder arthroplasty. The interscalene brachial plexus nerve block is considered the “gold standard” for shoulder analgesia. A new approach is the periarticular injection method, in which the surgeon administers a local anesthetic intraoperatively. Liposomal bupivacaine (Exparel, Pacira Pharmaceuticals, Inc.) is a nonopioid anesthetic that has been shown to improve pain control, shorten hospital stays, and decrease costs for total knee and hip arthroplasty compared with nerve blocks.4-6 Patients who were treated with liposomal bupivacaine consumed less opioid medication than a placebo group.7
Our purpose was to compare intraoperative local liposomal bupivacaine injection with preoperative single-shot interscalene nerve block (ISNB) in terms of pain control, opioid use, and length of hospital stay (LOS) after shoulder arthroplasty. We hypothesized that patients in the liposomal bupivacaine group would have lower pain scores, less opioid use, and shorter LOS compared with patients in the ISNB group.
Methods
A retrospective cohort analysis was conducted with 58 patients who underwent shoulder arthroplasty by 1 surgeon at our academically affiliated community hospital from January 2012 through January 2015. ISNBs were the standard at the beginning of the study period and were used until Exparel became available on the hospital formulary in 2013. We began using Exparel for all shoulder arthroplasties in November 2013. No other changes were made in the perioperative management of our arthroplasty patients during this period. Patients who underwent TSA, reverse TSA, or hemiarthroplasty of the shoulder were included. Patients who underwent revision TSA were excluded. Twenty-one patients received ISNBs and 37 received liposomal bupivacaine injections. This study was approved by our Institutional Review Board.
Baseline data for each patient were age, sex, body mass index, and the American Society of Anesthesiologists (ASA) Physical Status Classification. The primary outcome measure was the numeric rating scale (NRS) pain score at 4 post-operative time intervals. The NRS pain score has a range of 0 to 10, with 10 representing severe pain. Data were gathered from nursing and physical therapy notes in patient charts. The postoperative time intervals were 0 to 1 hour, 8 to 14 hours, 18 to 24 hours, and 27 to 36 hours. Available NRS scores for these time intervals were averaged. Patients were included if they had pain scores for at least 3 of the postoperative time intervals documented in their charts. Secondary outcome measures were LOS and opioid consumption during hospital admission. Intravenous acetaminophen use was also measured in both groups. All data on opioids were converted to oral morphine equivalents using the method described by Schneider and colleagues.8
A board-certified, fellowship-trained anesthesiologist, experienced in regional anesthesia, administered the single-shot ISNB before surgery. The block was administered under ultrasound guidance using a 44-mm, 22-gauge needle with the patient in the supine position. No indwelling catheter was used. The medication consisted of 30 mL of 5% ropivacaine (5 mg/mL). The surgeon injected liposomal bupivacaine (266 mg diluted into 40 mL of injectable saline) near the end of the procedure throughout the pericapsular area and multiple layers of the wound, per manufacturer guidelines.9 A 60-mL syringe with a 20-gauge needle was used. All operations were performed by 1 board-certified, fellowship-trained surgeon using a standard deltopectoral approach with the same surgical equipment. The same postoperative pain protocol was used for all patients, including intravenous acetaminophen and patient-controlled analgesia. Additional oral pain medication was provided as needed for all patients. Physical therapy protocols were identical between groups.
Statistical Analysis
Mean patient ages in the 2 treatment groups were compared using the Student t test. Sex distribution and the ASA scores were compared using a χ2 test and a Fisher exact test, respectively. Arthroplasty types were compared using a Fisher exact test. The medians and interquartile ranges of the NRS scores at each time point measured were tabulated by treatment group, and at each time point the difference between groups was tested using nonparametric rank sum tests.
We tested the longitudinal trajectory of NRS scores over time, accounting for repeated measurements in the same patients using linear mixed model analysis. Treatment group, time period as a categorical variable, and the interaction between treatment and time period were included as fixed effects, and patient identification number was included as the random effect. An initial omnibus test was performed for all treatment and treatment-by-time interaction effects. Subsequently, the treatment-by-time interaction was tested for each of the time periods. The association of day of discharge (as a categorical variable) with treatment was tested using the Fisher exact test. All analyses were conducted using Stata, version 13, software (StataCorp LP). P values <.05 were considered significant.
Sample Size Analysis
We calculated the minimum detectable effect size with 80% power at an alpha level of 0.05 for the nonparametric rank sum test in terms of the proportion of every possible pair of patients treated with the 2 treatments, where the patient treated with liposomal bupivacaine has a lower pain score than the patient treated with ISNB. For pain score at 18 to 24 hours, the sample sizes of 33 patients treated with liposomal bupivacaine and 20 treated with ISNB, the minimum detectable effect size is 73%.
Results
Fifty-eight patient charts (21 in the ISNB group and 37 in the liposomal bupivacaine group) were reviewed for the study. Patient sex distribution, mean age, mean body mass index, and mean baseline ASA scores were not statistically different (Table 1).
The primary outcome measure, NRS pain score, showed no significant differences between groups at 0 to 1 hour after surgery (P = .99) or 8 to 14 hours after surgery (P = .208).
There was no difference in the amount of intravenous acetaminophen given during the hospital stay between groups. There was no significant difference in opioid consumption on postoperative day 1 in the hospital (P = .59) (Figure 2). However, there were significant differences between groups on postoperative days 2 and 3.
Sixteen of 37 patients in the liposomal bupivacaine group and 2 of 21 in the ISNB group were discharged on the day after surgery (P = .010) (Table 3).
There were no major cardiac or respiratory events in either group. No long-term paresthesias or neuropathies were noted. There were no readmissions for either group.
Discussion
Postoperative pain control after shoulder arthroplasty can be challenging, and several modalities have been tried in various combinations to minimize pain and decrease adverse effects of opioid medications. The most common method for pain relief after shoulder arthroplasty is the ISNB. Several studies of ISNBs have shown improved pain control after shoulder arthroplasty with associated decreased opioid consumption and related side effects.10 Patient rehabilitation and satisfaction have improved with the increasing use of peripheral nerve blocks.11
Despite the well-established benefits of ISNBs, several limitations exist. Although the superior portion of the shoulder is well covered by an ISNB, the inferior portion of the brachial plexus can remain uncovered or only partially covered.12 Complications of ISNBs include hemidiaphragmatic paresis, rebound pain 24 hours after surgery,13 chronic neurologic complications,14 and substantial respiratory and cardiovascular events.15 Nerve blocks also require additional time and resources in the perioperative period, including an anesthesiologist with specialized training, assistants, and ultrasonography or nerve stimulation equipment contraindicated in patients taking blood thinners.16
Periarticular injections of local anesthetics have also shown promise in reducing pain after arthroplasty.4 Benefits include an enhanced safety profile because local injection avoids the concurrent blockade of the phrenic nerve and recurrent laryngeal nerve and has not been associated with the risk of peripheral neuropathies. Further, local injection is a simple technique that can be performed during surgery without additional personnel or expertise. A limitation of this approach is the relatively short duration of effectiveness of the local anesthetic and uncertainty regarding the best agent and the ideal volume of injection.6 Liposomal bupivacaine is a new agent (approved by the US Food and Drug Administration in 201117) with a sustained release over 72 to 96 hours.18 The most common adverse effects of liposomal bupivacaine are nausea, vomiting, constipation, pyrexia, dizziness, and headache.19 Chondrotoxicity and granulomatous inflammation are more serious, yet rare, complications of liposomal bupivacaine.20
We found that liposomal bupivacaine injections were associated with lower pain scores compared with ISNB at 18 to 24 hours after surgery. This correlated with less opioid consumption in the liposomal bupivacaine group than in the ISNB group on the second postoperative day. These differences in pain values are consistent with the known pharmacokinetics of liposomal bupivacaine.18 Peak plasma levels normally occur approximately 24 hours after injection, leaving the early postoperative period relatively uncovered by anesthetic agent. This finding of relatively poor pain control early after surgery has also been noted in patients undergoing knee arthroplasty.5 On the basis of the findings of this study, we have added standard bupivacaine injections to our separate liposomal bupivacaine injection to cover early postoperative pain. Opioid consumption was significantly lower in the liposomal bupivacaine group than in the ISNB group on postoperative days 2 and 3. We did not measure adverse events related to opioid consumption, so we cannot comment on whether the decreased opioid consumption was associated with the rate of adverse events. However, other studies21,22 have established this relationship.
We found the liposomal bupivacaine group to have earlier discharges to home. Sixteen of 37 patients in the liposomal bupivacaine group compared with 2 of 21 patients in the ISNB group were discharged on the day after surgery. A mean reduction in LOS of 18 hours for the liposomal bupivacaine group was statistically significant (P = .012). This reduction in LOS has important implications for hospitals and value analysis committees considering whether to keep a new, more expensive local anesthetic on formulary. Savings from reduced LOS and improvements in patient satisfaction may justify the expense (approximately $300 per 266-mg vial) of Exparel.
From a societal cost perspective, liposomal bupivacaine is more economical compared with ISNB, which adds approximately $1500 to the cost of anesthesia per patient.23 Eliminating the costs associated with ISNB administration in shoulder arthroplasties could result in substantial savings to our healthcare system. More research examining time savings and exact costs of each procedure is needed to determine the true cost effectiveness of each approach.
Limitations of our study include the retrospective design, relatively small numbers of patients in each group, missing data for some patients at various time points, variation in the types of procedures in each group, and lack of long-term outcome measures. It is important to note that we did not confirm the success of the nerve block after administration. However, this study reflects the effectiveness of each of the modalities in actual clinical conditions (as opposed to a controlled experimental setting). The actual effectiveness of a nerve block varies, even when performed by an experienced anesthesiologist with ultrasound guidance. Furthermore, immediate postoperative pain scores in the nerve block group are consistent with those of prior research reporting pain values ranging from 4 to 5 and a mean duration of effect ranging from 9 to 14 hours.23,24 Additionally, the patients, surgeon, and nursing team were not blinded to the treatment group. Although we did note a significant difference in the types of procedures between groups, this finding is related to the greater number of hemiarthroplasties performed in the ISNB group (N = 5) compared with the liposomal group (N = 1). Because of this variation and the decreased invasiveness of hemiarthroplasties, the bias is against the liposomal group. Finally, our primary outcome variable was pain, which is a subjective, self-reported measure. However, our opioid consumption data and LOS data corroborate the improved pain scores in the liposomal bupivacaine group.
Limiting the study to a single surgeon may limit external validity. Another limitation is the lack of data on adverse events related to opioid medication use. There was no additional experimental group to determine whether less expensive local anesthetics injected locally would perform similarly to liposomal bupivacaine. In total knee arthroplasty, periarticular injections of liposomal bupivacaine were not as effective as less expensive periarticular injections.25 It is unclear which agents (and in what doses or combinations) should be used for periarticular injections. Finally, we acknowledge that our retrospective study design cannot account for all potential factors affecting discharge time.
This is the first comparative study of liposomal bupivacaine and ISNB in TSA. The study design allowed us to control for variables such as surgical technique, postoperative protocols (including use and type of sling), and use of other pain modalities such as patient-controlled analgesia and intravenous acetaminophen that are likely to affect postoperative pain and LOS. This study provides preliminary data that confirm relative equipoise between liposomal bupivacaine and ISNB, which is needed for the ethical conduct of a randomized controlled trial. Such a trial would allow for a more robust comparison, and this retrospective study provides appropriate pilot data on which to base this design and the clinical information needed to counsel patients during enrollment.
Our results suggest that liposomal bupivacaine may provide superior or similar pain relief compared with ISNB after shoulder arthroplasty. Additionally, the use of liposomal bupivacaine was associated with decreased opioid consumption and earlier discharge to home compared with ISNB. These findings have important implications for pain control after TSA because pain represents a major concern for patients and providers after surgery. In addition to clinical improvements, use of liposomal bupivacaine may save time and eliminate costs associated with administering nerve blocks. Local injection may also be used in patients who are contraindicated for ISNB such as those with obesity, pulmonary disease, or peripheral neuropathy. Although we cannot definitively suggest that liposomal bupivacaine is superior to the current gold standard ISNB for pain control after shoulder arthroplasty, our results suggest a relative clinical equipoise between these modalities. Larger analytical studies, including randomized trials, should be performed to explore the potential benefits of liposomal bupivacaine injections for pain control after shoulder arthroplasty.
Am J Orthop. 2016;45(7):424-430. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
The annual number of total shoulder arthroplasties (TSAs) is rising with the growing elderly population and development of new technologies such as reverse shoulder arthroplasty.1 In 2008, 47,000 shoulder arthroplasties were performed in the US compared with 19,000 in 1998.1 As of 2011, there were 53,000 shoulder arthroplasties performed annually.2 Pain control after shoulder procedures, particularly TSA, is challenging. 3
Several modalities exist to manage pain after shoulder arthroplasty. The interscalene brachial plexus nerve block is considered the “gold standard” for shoulder analgesia. A new approach is the periarticular injection method, in which the surgeon administers a local anesthetic intraoperatively. Liposomal bupivacaine (Exparel, Pacira Pharmaceuticals, Inc.) is a nonopioid anesthetic that has been shown to improve pain control, shorten hospital stays, and decrease costs for total knee and hip arthroplasty compared with nerve blocks.4-6 Patients who were treated with liposomal bupivacaine consumed less opioid medication than a placebo group.7
Our purpose was to compare intraoperative local liposomal bupivacaine injection with preoperative single-shot interscalene nerve block (ISNB) in terms of pain control, opioid use, and length of hospital stay (LOS) after shoulder arthroplasty. We hypothesized that patients in the liposomal bupivacaine group would have lower pain scores, less opioid use, and shorter LOS compared with patients in the ISNB group.
Methods
A retrospective cohort analysis was conducted with 58 patients who underwent shoulder arthroplasty by 1 surgeon at our academically affiliated community hospital from January 2012 through January 2015. ISNBs were the standard at the beginning of the study period and were used until Exparel became available on the hospital formulary in 2013. We began using Exparel for all shoulder arthroplasties in November 2013. No other changes were made in the perioperative management of our arthroplasty patients during this period. Patients who underwent TSA, reverse TSA, or hemiarthroplasty of the shoulder were included. Patients who underwent revision TSA were excluded. Twenty-one patients received ISNBs and 37 received liposomal bupivacaine injections. This study was approved by our Institutional Review Board.
Baseline data for each patient were age, sex, body mass index, and the American Society of Anesthesiologists (ASA) Physical Status Classification. The primary outcome measure was the numeric rating scale (NRS) pain score at 4 post-operative time intervals. The NRS pain score has a range of 0 to 10, with 10 representing severe pain. Data were gathered from nursing and physical therapy notes in patient charts. The postoperative time intervals were 0 to 1 hour, 8 to 14 hours, 18 to 24 hours, and 27 to 36 hours. Available NRS scores for these time intervals were averaged. Patients were included if they had pain scores for at least 3 of the postoperative time intervals documented in their charts. Secondary outcome measures were LOS and opioid consumption during hospital admission. Intravenous acetaminophen use was also measured in both groups. All data on opioids were converted to oral morphine equivalents using the method described by Schneider and colleagues.8
A board-certified, fellowship-trained anesthesiologist, experienced in regional anesthesia, administered the single-shot ISNB before surgery. The block was administered under ultrasound guidance using a 44-mm, 22-gauge needle with the patient in the supine position. No indwelling catheter was used. The medication consisted of 30 mL of 5% ropivacaine (5 mg/mL). The surgeon injected liposomal bupivacaine (266 mg diluted into 40 mL of injectable saline) near the end of the procedure throughout the pericapsular area and multiple layers of the wound, per manufacturer guidelines.9 A 60-mL syringe with a 20-gauge needle was used. All operations were performed by 1 board-certified, fellowship-trained surgeon using a standard deltopectoral approach with the same surgical equipment. The same postoperative pain protocol was used for all patients, including intravenous acetaminophen and patient-controlled analgesia. Additional oral pain medication was provided as needed for all patients. Physical therapy protocols were identical between groups.
Statistical Analysis
Mean patient ages in the 2 treatment groups were compared using the Student t test. Sex distribution and the ASA scores were compared using a χ2 test and a Fisher exact test, respectively. Arthroplasty types were compared using a Fisher exact test. The medians and interquartile ranges of the NRS scores at each time point measured were tabulated by treatment group, and at each time point the difference between groups was tested using nonparametric rank sum tests.
We tested the longitudinal trajectory of NRS scores over time, accounting for repeated measurements in the same patients using linear mixed model analysis. Treatment group, time period as a categorical variable, and the interaction between treatment and time period were included as fixed effects, and patient identification number was included as the random effect. An initial omnibus test was performed for all treatment and treatment-by-time interaction effects. Subsequently, the treatment-by-time interaction was tested for each of the time periods. The association of day of discharge (as a categorical variable) with treatment was tested using the Fisher exact test. All analyses were conducted using Stata, version 13, software (StataCorp LP). P values <.05 were considered significant.
Sample Size Analysis
We calculated the minimum detectable effect size with 80% power at an alpha level of 0.05 for the nonparametric rank sum test in terms of the proportion of every possible pair of patients treated with the 2 treatments, where the patient treated with liposomal bupivacaine has a lower pain score than the patient treated with ISNB. For pain score at 18 to 24 hours, the sample sizes of 33 patients treated with liposomal bupivacaine and 20 treated with ISNB, the minimum detectable effect size is 73%.
Results
Fifty-eight patient charts (21 in the ISNB group and 37 in the liposomal bupivacaine group) were reviewed for the study. Patient sex distribution, mean age, mean body mass index, and mean baseline ASA scores were not statistically different (Table 1).
The primary outcome measure, NRS pain score, showed no significant differences between groups at 0 to 1 hour after surgery (P = .99) or 8 to 14 hours after surgery (P = .208).
There was no difference in the amount of intravenous acetaminophen given during the hospital stay between groups. There was no significant difference in opioid consumption on postoperative day 1 in the hospital (P = .59) (Figure 2). However, there were significant differences between groups on postoperative days 2 and 3.
Sixteen of 37 patients in the liposomal bupivacaine group and 2 of 21 in the ISNB group were discharged on the day after surgery (P = .010) (Table 3).
There were no major cardiac or respiratory events in either group. No long-term paresthesias or neuropathies were noted. There were no readmissions for either group.
Discussion
Postoperative pain control after shoulder arthroplasty can be challenging, and several modalities have been tried in various combinations to minimize pain and decrease adverse effects of opioid medications. The most common method for pain relief after shoulder arthroplasty is the ISNB. Several studies of ISNBs have shown improved pain control after shoulder arthroplasty with associated decreased opioid consumption and related side effects.10 Patient rehabilitation and satisfaction have improved with the increasing use of peripheral nerve blocks.11
Despite the well-established benefits of ISNBs, several limitations exist. Although the superior portion of the shoulder is well covered by an ISNB, the inferior portion of the brachial plexus can remain uncovered or only partially covered.12 Complications of ISNBs include hemidiaphragmatic paresis, rebound pain 24 hours after surgery,13 chronic neurologic complications,14 and substantial respiratory and cardiovascular events.15 Nerve blocks also require additional time and resources in the perioperative period, including an anesthesiologist with specialized training, assistants, and ultrasonography or nerve stimulation equipment contraindicated in patients taking blood thinners.16
Periarticular injections of local anesthetics have also shown promise in reducing pain after arthroplasty.4 Benefits include an enhanced safety profile because local injection avoids the concurrent blockade of the phrenic nerve and recurrent laryngeal nerve and has not been associated with the risk of peripheral neuropathies. Further, local injection is a simple technique that can be performed during surgery without additional personnel or expertise. A limitation of this approach is the relatively short duration of effectiveness of the local anesthetic and uncertainty regarding the best agent and the ideal volume of injection.6 Liposomal bupivacaine is a new agent (approved by the US Food and Drug Administration in 201117) with a sustained release over 72 to 96 hours.18 The most common adverse effects of liposomal bupivacaine are nausea, vomiting, constipation, pyrexia, dizziness, and headache.19 Chondrotoxicity and granulomatous inflammation are more serious, yet rare, complications of liposomal bupivacaine.20
We found that liposomal bupivacaine injections were associated with lower pain scores compared with ISNB at 18 to 24 hours after surgery. This correlated with less opioid consumption in the liposomal bupivacaine group than in the ISNB group on the second postoperative day. These differences in pain values are consistent with the known pharmacokinetics of liposomal bupivacaine.18 Peak plasma levels normally occur approximately 24 hours after injection, leaving the early postoperative period relatively uncovered by anesthetic agent. This finding of relatively poor pain control early after surgery has also been noted in patients undergoing knee arthroplasty.5 On the basis of the findings of this study, we have added standard bupivacaine injections to our separate liposomal bupivacaine injection to cover early postoperative pain. Opioid consumption was significantly lower in the liposomal bupivacaine group than in the ISNB group on postoperative days 2 and 3. We did not measure adverse events related to opioid consumption, so we cannot comment on whether the decreased opioid consumption was associated with the rate of adverse events. However, other studies21,22 have established this relationship.
We found the liposomal bupivacaine group to have earlier discharges to home. Sixteen of 37 patients in the liposomal bupivacaine group compared with 2 of 21 patients in the ISNB group were discharged on the day after surgery. A mean reduction in LOS of 18 hours for the liposomal bupivacaine group was statistically significant (P = .012). This reduction in LOS has important implications for hospitals and value analysis committees considering whether to keep a new, more expensive local anesthetic on formulary. Savings from reduced LOS and improvements in patient satisfaction may justify the expense (approximately $300 per 266-mg vial) of Exparel.
From a societal cost perspective, liposomal bupivacaine is more economical compared with ISNB, which adds approximately $1500 to the cost of anesthesia per patient.23 Eliminating the costs associated with ISNB administration in shoulder arthroplasties could result in substantial savings to our healthcare system. More research examining time savings and exact costs of each procedure is needed to determine the true cost effectiveness of each approach.
Limitations of our study include the retrospective design, relatively small numbers of patients in each group, missing data for some patients at various time points, variation in the types of procedures in each group, and lack of long-term outcome measures. It is important to note that we did not confirm the success of the nerve block after administration. However, this study reflects the effectiveness of each of the modalities in actual clinical conditions (as opposed to a controlled experimental setting). The actual effectiveness of a nerve block varies, even when performed by an experienced anesthesiologist with ultrasound guidance. Furthermore, immediate postoperative pain scores in the nerve block group are consistent with those of prior research reporting pain values ranging from 4 to 5 and a mean duration of effect ranging from 9 to 14 hours.23,24 Additionally, the patients, surgeon, and nursing team were not blinded to the treatment group. Although we did note a significant difference in the types of procedures between groups, this finding is related to the greater number of hemiarthroplasties performed in the ISNB group (N = 5) compared with the liposomal group (N = 1). Because of this variation and the decreased invasiveness of hemiarthroplasties, the bias is against the liposomal group. Finally, our primary outcome variable was pain, which is a subjective, self-reported measure. However, our opioid consumption data and LOS data corroborate the improved pain scores in the liposomal bupivacaine group.
Limiting the study to a single surgeon may limit external validity. Another limitation is the lack of data on adverse events related to opioid medication use. There was no additional experimental group to determine whether less expensive local anesthetics injected locally would perform similarly to liposomal bupivacaine. In total knee arthroplasty, periarticular injections of liposomal bupivacaine were not as effective as less expensive periarticular injections.25 It is unclear which agents (and in what doses or combinations) should be used for periarticular injections. Finally, we acknowledge that our retrospective study design cannot account for all potential factors affecting discharge time.
This is the first comparative study of liposomal bupivacaine and ISNB in TSA. The study design allowed us to control for variables such as surgical technique, postoperative protocols (including use and type of sling), and use of other pain modalities such as patient-controlled analgesia and intravenous acetaminophen that are likely to affect postoperative pain and LOS. This study provides preliminary data that confirm relative equipoise between liposomal bupivacaine and ISNB, which is needed for the ethical conduct of a randomized controlled trial. Such a trial would allow for a more robust comparison, and this retrospective study provides appropriate pilot data on which to base this design and the clinical information needed to counsel patients during enrollment.
Our results suggest that liposomal bupivacaine may provide superior or similar pain relief compared with ISNB after shoulder arthroplasty. Additionally, the use of liposomal bupivacaine was associated with decreased opioid consumption and earlier discharge to home compared with ISNB. These findings have important implications for pain control after TSA because pain represents a major concern for patients and providers after surgery. In addition to clinical improvements, use of liposomal bupivacaine may save time and eliminate costs associated with administering nerve blocks. Local injection may also be used in patients who are contraindicated for ISNB such as those with obesity, pulmonary disease, or peripheral neuropathy. Although we cannot definitively suggest that liposomal bupivacaine is superior to the current gold standard ISNB for pain control after shoulder arthroplasty, our results suggest a relative clinical equipoise between these modalities. Larger analytical studies, including randomized trials, should be performed to explore the potential benefits of liposomal bupivacaine injections for pain control after shoulder arthroplasty.
Am J Orthop. 2016;45(7):424-430. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. American Academy of Orthopaedic Surgeons. Shoulder joint replacement. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Accessed June 3, 2015.
3. Desai VN, Cheung EV. Postoperative pain associated with orthopedic shoulder and elbow surgery: a prospective study. J Shoulder Elbow Surg. 2012;21(4):441-450.
4. Springer BD. Transition from nerve blocks to periarticular injections and emerging techniques in total joint arthroplasty. Am J Orthop. 2014;43(10 Suppl):S6-S9.
5. Surdam JW, Licini DJ, Baynes NT, Arce BR. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J Arthroplasty. 2015;30(2):325-329.
6. Tong YC, Kaye AD, Urman RD. Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol. 2014;28(1):15-27.
7. Chahar P, Cummings KC 3rd. Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res. 2012;5:257-264.
8. Schneider C, Yale SH, Larson M. Principles of pain management. Clin Med Res. 2003;1(4):337-340.
9. Pacira Pharmaceuticals, Inc. Highlights of prescribing information. http://www.exparel.com/pdf/EXPAREL_Prescribing_Information.pdf. Accessed May 7, 2015.
10. Gohl MR, Moeller RK, Olson RL, Vacchiano CA. The addition of interscalene block to general anesthesia for patients undergoing open shoulder procedures. AANA J. 2001;69(2):105-109.
11. Ironfield CM, Barrington MJ, Kluger R, Sites B. Are patients satisfied after peripheral nerve blockade? Results from an International Registry of Regional Anesthesia. Reg Anesth Pain Med. 2014;39(1):48-55.
12. Srikumaran U, Stein BE, Tan EW, Freehill MT, Wilckens JH. Upper-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(24):e197(1-13).
13. DeMarco JR, Componovo R, Barfield WR, Liles L, Nietert P. Efficacy of augmenting a subacromial continuous-infusion pump with a preoperative interscalene block in outpatient arthroscopic shoulder surgery: a prospective, randomized, blinded, and placebo-controlled study. Arthroscopy. 2011;27(5):603-610.
14. Misamore G, Webb B, McMurray S, Sallay P. A prospective analysis of interscalene brachial plexus blocks performed under general anesthesia. J Shoulder Elbow Surg. 2011;20(2):308-314.
15. Lenters TR, Davies J, Matsen FA 3rd. The types and severity of complications associated with interscalene brachial plexus block anesthesia: local and national evidence. J Shoulder Elbow Surg. 2007;16(4):379-387.
16. Park SK, Choi YS, Choi SW, Song SW. A comparison of three methods for postoperative pain control in patients undergoing arthroscopic shoulder surgery. Korean J Pain. 2015;28(1):45-51.
17. Pacira Pharmaceuticals, Inc. Pacira Pharmaceuticals, Inc. announces U.S. FDA approval of EXPAREL™ for postsurgical pain management. http://investor.pacira.com/phoenix.zhtml?c=220759&p=irol-newsArticle_print&ID=1623529. Published October 31, 2011. Accessed June 3, 2015.
18. White PF, Ardeleanu M, Schooley G, Burch RM. Pharmocokinetics of depobupivacaine following infiltration in patients undergoing two types of surgery and in normal volunteers. Paper presented at: Annual Meeting of the International Anesthesia Research Society; March 14, 2009; San Diego, CA.
19. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
20. Lambrechts M, O’Brien MJ, Savoie FH, You Z. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer Adherence. 2013;7:885-890.
21. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
22. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res Clin Exp. 2013;76:1-6.
23. Weber SC, Jain R. Scalene regional anesthesia for shoulder surgery in a community setting: an assessment of risk. J Bone Joint Surg Am. 2002;84-A(5):775-779.
24. Beaudet V, Williams SR, Tétreault P, Perrault MA. Perioperative interscalene block versus intra-articular injection of local anesthetics for postoperative analgesia in shoulder surgery. Reg Anesth Pain Med. 2008;33(2):134-138.
25. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
2. American Academy of Orthopaedic Surgeons. Shoulder joint replacement. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Accessed June 3, 2015.
3. Desai VN, Cheung EV. Postoperative pain associated with orthopedic shoulder and elbow surgery: a prospective study. J Shoulder Elbow Surg. 2012;21(4):441-450.
4. Springer BD. Transition from nerve blocks to periarticular injections and emerging techniques in total joint arthroplasty. Am J Orthop. 2014;43(10 Suppl):S6-S9.
5. Surdam JW, Licini DJ, Baynes NT, Arce BR. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J Arthroplasty. 2015;30(2):325-329.
6. Tong YC, Kaye AD, Urman RD. Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol. 2014;28(1):15-27.
7. Chahar P, Cummings KC 3rd. Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res. 2012;5:257-264.
8. Schneider C, Yale SH, Larson M. Principles of pain management. Clin Med Res. 2003;1(4):337-340.
9. Pacira Pharmaceuticals, Inc. Highlights of prescribing information. http://www.exparel.com/pdf/EXPAREL_Prescribing_Information.pdf. Accessed May 7, 2015.
10. Gohl MR, Moeller RK, Olson RL, Vacchiano CA. The addition of interscalene block to general anesthesia for patients undergoing open shoulder procedures. AANA J. 2001;69(2):105-109.
11. Ironfield CM, Barrington MJ, Kluger R, Sites B. Are patients satisfied after peripheral nerve blockade? Results from an International Registry of Regional Anesthesia. Reg Anesth Pain Med. 2014;39(1):48-55.
12. Srikumaran U, Stein BE, Tan EW, Freehill MT, Wilckens JH. Upper-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(24):e197(1-13).
13. DeMarco JR, Componovo R, Barfield WR, Liles L, Nietert P. Efficacy of augmenting a subacromial continuous-infusion pump with a preoperative interscalene block in outpatient arthroscopic shoulder surgery: a prospective, randomized, blinded, and placebo-controlled study. Arthroscopy. 2011;27(5):603-610.
14. Misamore G, Webb B, McMurray S, Sallay P. A prospective analysis of interscalene brachial plexus blocks performed under general anesthesia. J Shoulder Elbow Surg. 2011;20(2):308-314.
15. Lenters TR, Davies J, Matsen FA 3rd. The types and severity of complications associated with interscalene brachial plexus block anesthesia: local and national evidence. J Shoulder Elbow Surg. 2007;16(4):379-387.
16. Park SK, Choi YS, Choi SW, Song SW. A comparison of three methods for postoperative pain control in patients undergoing arthroscopic shoulder surgery. Korean J Pain. 2015;28(1):45-51.
17. Pacira Pharmaceuticals, Inc. Pacira Pharmaceuticals, Inc. announces U.S. FDA approval of EXPAREL™ for postsurgical pain management. http://investor.pacira.com/phoenix.zhtml?c=220759&p=irol-newsArticle_print&ID=1623529. Published October 31, 2011. Accessed June 3, 2015.
18. White PF, Ardeleanu M, Schooley G, Burch RM. Pharmocokinetics of depobupivacaine following infiltration in patients undergoing two types of surgery and in normal volunteers. Paper presented at: Annual Meeting of the International Anesthesia Research Society; March 14, 2009; San Diego, CA.
19. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19(5):530-536.
20. Lambrechts M, O’Brien MJ, Savoie FH, You Z. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer Adherence. 2013;7:885-890.
21. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273.
22. Candiotti KA, Sands LR, Lee E, et al. Liposome bupivacaine for postsurgical analgesia in adult patients undergoing laparoscopic colectomy: results from prospective phase IV sequential cohort studies assessing health economic outcomes. Curr Ther Res Clin Exp. 2013;76:1-6.
23. Weber SC, Jain R. Scalene regional anesthesia for shoulder surgery in a community setting: an assessment of risk. J Bone Joint Surg Am. 2002;84-A(5):775-779.
24. Beaudet V, Williams SR, Tétreault P, Perrault MA. Perioperative interscalene block versus intra-articular injection of local anesthetics for postoperative analgesia in shoulder surgery. Reg Anesth Pain Med. 2008;33(2):134-138.
25. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
Ultrasound-Guided Percutaneous Reconstruction of the Anterolateral Ligament: Surgical Technique and Case Report
Restoring native kinematics of the knee has been a primary goal of anterior cruciate ligament (ACL) procedures. Double-bundle ACL reconstruction, compared to single-bundle, has been hypothesized to more effectively re-establish rotational stability by re-creating the anatomic ACL, but has not yet proven to result in better clinical outcomes.1
In 1879, Dr. Paul Segond described a “fibrous, pearly band” at the lateral aspect of the knee that avulsed off the anterolateral proximal tibia during many ACL injuries.2 The role of the lateral tissues in knee stability and their relationship with ACL pathology has attracted noteworthy attention in recent time. There have been multiple studies presenting an anatomical description of a structure at the anterolateral portion of the knee with definitive femoral, meniscal, and tibial attachments, which helps control internal rotational forces.3-7 Claes and colleagues4 later found that band of tissue to be the anterolateral ligament (ALL) and determined its injury to be pathognomonic with ACL ruptures.
The ALL is a vital static stabilizer of the tibio-femoral joint, especially during internal tibial rotation.8-10 In their report on ALL and ACL reconstruction, Helito and colleagues11 acknowledge the necessity of accurate assessment of the lateral structures through imaging to determine the presence of extra-articular injury. Musculoskeletal diagnostic ultrasound has been established as an appropriate means to identify the ALL.12
Ultrasound can accurately determine the exact anatomic location of the origin and insertion of the ALL. Reconstruction of the ALL could yield better patient outcomes for those who experience concurrent ACL/ALL injury. Here we present an innovative technique for an ultrasound-guided percutaneous method for reconstruction of the ALL and report on a patient who had underwent ALL reconstruction.
Surgical Indications
All patients undergo an ultrasound evaluation preoperatively to determine if the ALL is intact or injured. Our experience has shown that when ultrasound evaluation reveals an intact ALL, the pivot shift has never been a grade III.
Surgical Technique
For a demonstration of this technique, see the video that accompanies this article.
The pivot shift test is conducted under anesthesia to determine whether an ALL reconstruction is required. The patient is placed in a supine position with the knee flexed at 30o, at neutral rotation, and without any varus or valgus stress.
A No. 15 blade is used to make a small incision centered on each spinal needle. The spinal needle is replaced with a 2.4-mm drill pin (Figure 2).
The graft and FiberTape are then passed under the IT band to the distal incision. Using the length of the BioComposite SwiveLock anchor as a guide, a mark is made on the graft after tensioning the construct in line with the leg, distal to the tibial drill pin (Table 2, Figure 4).
Rehabilitation
Rehabilitation following an ALL procedure is similar to traditional ACL rehabilitation with an added emphasis on minimizing rotational torque of the tibia in the early stages.
Case Report
In January 2013, a 17-year-old male soccer player suffered an ACL rupture of his right knee. Later that spring, he had an ACL reconstruction with an allograft. Twelve months postoperatively, the patient returned, saying that he felt much better; however, anytime he tried to plant his foot and rotate over that fixed foot, his knee felt unstable. The physical examination revealed both negative Lachman and anterior drawer tests but a I+ pivot shift test. A magnetic resonance imaging (MRI) examination revealed an intact ACL graft. A diagnostic ultrasound evaluation revealed a distal ALL injury. After discussing the risks, benefits, and goals with the patient, we opted for a diagnostic arthroscopy and a percutaneous, ultrasound-guided reconstruction of the ALL.
Postoperatively, the patient did very well. One week after surgery, he returned, saying he felt completely stable and demonstrated by repeating the rotation of his knee. The patient continued to have no issues until he returned 13 months post-ALL surgery, complaining of a recent injury that had caused the return of his feelings of instability. An MRI evaluation showed an intact ACL graft and the possibility of a ruptured ALL. Fifteen months after the initial ALL reconstruction, we proceeded with surgery. At arthroscopy, the patient was found to have a pivot shift of I+ and an intact ACL graft. The ALL was reconstructed again using an allograft, internal brace, and bone marrow concentrate. At 13 months post-ALL reconstruction revision, the patient had no complaints.
Discussion
Reconstruction of the ALL is aimed to restore anatomic rotational kinematics. Sonnery-Cottet and colleagues14 have reported promising initial results in their 2-year follow-up study of combined ACL and ALL reconstruction outcomes. This surgical technique includes use of an internal brace, which negates the necessity for external support devices and allows for earlier mobilization of the joint. A reconstruction of the ALL, performed concurrently with the ACL, does not add recovery time, but could prevent postsurgical complications and improve rehabilitation by eliminating rotational instability that presents in some ACL-reconstructed patients.
Sonnery-Cottet and colleagues15 state that their arthroscopic identification of the ALL can help to cultivate a “less invasive and more anatomic” reconstruction. The use of musculoskeletal ultrasound allows our technique to utilize a completely noninvasive imaging tool that allows proper establishment of ALL anatomy prior to the procedure. The entirety of the ALL is easily identifiable,4,12 which has proven to be shortcoming of MRI evaluation.15-17 Accurate preoperative assessment of the lateral structures is necessary in ACL-deficient individuals.11,15 Sonography also provides a means of accurate guidance and socket creation, without generating large incisions.
If the ALL is responsible for internal rotatory stability as asserted, the structure should exhibit biomechanical properties during movement. In their study on the function of the ligament, Parsons and colleagues9 established the inverse relationship between the ALL and ACL during internal rotation. As their cadaveric knees were subjected to an internal rotatory force through increasing angles of flexion, the contribution of the ALL towards stability significantly increased while the ACL declined. Helito and colleagues8 and Zens and colleagues10 have demonstrated length changes of the ligament through varying degrees of flexion and internal rotation. Their reports indicate greater tension during knee movements, coinciding with the description of increasing ALL stability contribution by Parsons and colleagues.9 Kennedy and colleagues7 conducted a pull-to-failure test on the ALL. The average failure load was 175 N with a stiffness of 20 N/mm, illustrating the structure is a candidate for most traditional soft tissue grafts. The biomechanical evidence of the structural properties of the ALL confirms its importance in knee function and the necessity for its reconstruction.
With the understanding that ACL contributes to rotatory stability to some extent, the notion begs the question of how a centrally located ligament is able to prevent excessive rotation in a structure with a large relative radius. Biomechanically, with such a small moment arm, the ACL would experience tremendous stress when a rotatory force is applied. The same torque applied to a more superficial structure, with a greater moment, would sustain a large reduction in the applied force. The concept of a wheel and an axle should be considered. The equation is F1 × R1 = F2 × R2. We measured on a cadaveric knee the distance from the center of rotation to the ACL and the ALL, finding the radii were 5 mm and 30 mm, respectively. Taking these measurements, we would then expect the force experienced on the axle (ACL) to be 6 times greater than what would be experienced on the periphery of the wheel (ALL). The ALL (wheel) has a significant biomechanical advantage over the ACL (axle) in controlling and enduring internal rotatory forces of the knee. This would imply that if the ALL were damaged and not re-established, the ACL would experience a 6 times greater force trying to control internal rotation, which would result in a significantly increased chance of failure and rupture.
While there is a degree of dissent on the presence of the ALL, a number of studies have classified the tissue as an independent ligamentous structure.3-7 While there is disagreement on the precise location of the femoral attachment, there is a consensus on the location of the tibial and meniscal attachments. Claes and colleagues4 originally outlined the femoral attachment as anterior and distal to the origin of the fibular collateral ligament (FCL), which is the description this technique follows. Since Claes and colleagues’4 report, many have investigated the ligament’s femoral origin with delineations ranging from posterior and proximal3,5,7 to anterior and distal.6,16-18
The accurate, noninvasive nature of the musculoskeletal ultrasound prior to any incisions being made makes this technique innovative and superior to other open surgical techniques or those that require fluoroscopy.
Conclusion
The ALL has been determined to play an integral role in the rotational stability of the knee. In the setting of instability and insufficiency, reconstruction will lead to better patient outcomes for concurrent ACL/ALL injuries and postsurgical rotatory instability following ACL procedures. This innovative technique utilizes ultrasound to ascertain the precise anatomical attachments of the ALL prior to the operation. The novel nature of this ultrasound-guided reconstruction has the potential to be applicable in many other surgical procedures.
1. Suomalainen P, Järvelä T, Paakkala A, Kannus P, Järvinen M. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: A prospective randomized study with 5-year results. Am J Sports Med. 2012;40(7):1511-1518.
2. Segond P. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Progrés Médical. 1879;6(6):1-85. French.
3. Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Athrosc. 2015;23(11):3186-3195.
4. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321-328.
5. Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: Anatomy, length changes and association with the segond fracture. Bone Joint J. 2014;96-B(3):325-331.
6. Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546.
7. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: An anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606-1615.
8. Helito CP, Helito PV, Bonadio MB, et al. Evaluation of the length and isometric pattern of the anterolateral ligament with serial computer tomography. Orthop J Sports Med. 2014;2(12):2325967114562205.
9. Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(3):669-674.
10. Zens M, Niemeyer P, Ruhhamer J, et al. Length changes of the anterolateral ligament during passive knee motion: A human cadaveric study. Am J Sports Med. 2015;43(10):2545-2552.
11. Helito CP, Bonadio MB, Gobbi RG, et al. Combined intra- and extra-articular reconstruction of the anterior cruciate ligament: the reconstruction of the knee anterolateral ligament. Arthrosc Tech. 2015;4(3):e239-e244.
12. Cianca J, John J, Pandit S, Chiou-Tan FY. Musculoskeletal ultrasound imaging of the recently described anterolateral ligament of the knee. Am J Phys Med Rehabil. 2014;93(2):186
13. Adams JE, Zobitz ME, Reach JS, et al. Rotator cuff repair using an acellular dermal matrix graft: An in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
14. Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BHB, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598-1605.
15. Sonnery-Cottet B, Archbold P, Rezende FC, Neto AM, Fayard JM, Thaunat M. Arthroscopic identification of the anterolateral ligament of the knee. Arthrosc Tech. 2014;3(3):e389-e392.
16. Helito CP, Helito PV, Costa HP, et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol. 2014;43(10):1421-1427.
17. Helito CP, Demange MK, Helito PV, et al. Evaluation of the anterolateral ligament of the knee by means of magnetic resonance examination. Rev Bras Orthop. 2015;50(2):214-219.
18. Helito CP, Demange MK, Bonadio MB, et al. Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am J Sports Med. 2014;42(10):2356-2362.
Restoring native kinematics of the knee has been a primary goal of anterior cruciate ligament (ACL) procedures. Double-bundle ACL reconstruction, compared to single-bundle, has been hypothesized to more effectively re-establish rotational stability by re-creating the anatomic ACL, but has not yet proven to result in better clinical outcomes.1
In 1879, Dr. Paul Segond described a “fibrous, pearly band” at the lateral aspect of the knee that avulsed off the anterolateral proximal tibia during many ACL injuries.2 The role of the lateral tissues in knee stability and their relationship with ACL pathology has attracted noteworthy attention in recent time. There have been multiple studies presenting an anatomical description of a structure at the anterolateral portion of the knee with definitive femoral, meniscal, and tibial attachments, which helps control internal rotational forces.3-7 Claes and colleagues4 later found that band of tissue to be the anterolateral ligament (ALL) and determined its injury to be pathognomonic with ACL ruptures.
The ALL is a vital static stabilizer of the tibio-femoral joint, especially during internal tibial rotation.8-10 In their report on ALL and ACL reconstruction, Helito and colleagues11 acknowledge the necessity of accurate assessment of the lateral structures through imaging to determine the presence of extra-articular injury. Musculoskeletal diagnostic ultrasound has been established as an appropriate means to identify the ALL.12
Ultrasound can accurately determine the exact anatomic location of the origin and insertion of the ALL. Reconstruction of the ALL could yield better patient outcomes for those who experience concurrent ACL/ALL injury. Here we present an innovative technique for an ultrasound-guided percutaneous method for reconstruction of the ALL and report on a patient who had underwent ALL reconstruction.
Surgical Indications
All patients undergo an ultrasound evaluation preoperatively to determine if the ALL is intact or injured. Our experience has shown that when ultrasound evaluation reveals an intact ALL, the pivot shift has never been a grade III.
Surgical Technique
For a demonstration of this technique, see the video that accompanies this article.
The pivot shift test is conducted under anesthesia to determine whether an ALL reconstruction is required. The patient is placed in a supine position with the knee flexed at 30o, at neutral rotation, and without any varus or valgus stress.
A No. 15 blade is used to make a small incision centered on each spinal needle. The spinal needle is replaced with a 2.4-mm drill pin (Figure 2).
The graft and FiberTape are then passed under the IT band to the distal incision. Using the length of the BioComposite SwiveLock anchor as a guide, a mark is made on the graft after tensioning the construct in line with the leg, distal to the tibial drill pin (Table 2, Figure 4).
Rehabilitation
Rehabilitation following an ALL procedure is similar to traditional ACL rehabilitation with an added emphasis on minimizing rotational torque of the tibia in the early stages.
Case Report
In January 2013, a 17-year-old male soccer player suffered an ACL rupture of his right knee. Later that spring, he had an ACL reconstruction with an allograft. Twelve months postoperatively, the patient returned, saying that he felt much better; however, anytime he tried to plant his foot and rotate over that fixed foot, his knee felt unstable. The physical examination revealed both negative Lachman and anterior drawer tests but a I+ pivot shift test. A magnetic resonance imaging (MRI) examination revealed an intact ACL graft. A diagnostic ultrasound evaluation revealed a distal ALL injury. After discussing the risks, benefits, and goals with the patient, we opted for a diagnostic arthroscopy and a percutaneous, ultrasound-guided reconstruction of the ALL.
Postoperatively, the patient did very well. One week after surgery, he returned, saying he felt completely stable and demonstrated by repeating the rotation of his knee. The patient continued to have no issues until he returned 13 months post-ALL surgery, complaining of a recent injury that had caused the return of his feelings of instability. An MRI evaluation showed an intact ACL graft and the possibility of a ruptured ALL. Fifteen months after the initial ALL reconstruction, we proceeded with surgery. At arthroscopy, the patient was found to have a pivot shift of I+ and an intact ACL graft. The ALL was reconstructed again using an allograft, internal brace, and bone marrow concentrate. At 13 months post-ALL reconstruction revision, the patient had no complaints.
Discussion
Reconstruction of the ALL is aimed to restore anatomic rotational kinematics. Sonnery-Cottet and colleagues14 have reported promising initial results in their 2-year follow-up study of combined ACL and ALL reconstruction outcomes. This surgical technique includes use of an internal brace, which negates the necessity for external support devices and allows for earlier mobilization of the joint. A reconstruction of the ALL, performed concurrently with the ACL, does not add recovery time, but could prevent postsurgical complications and improve rehabilitation by eliminating rotational instability that presents in some ACL-reconstructed patients.
Sonnery-Cottet and colleagues15 state that their arthroscopic identification of the ALL can help to cultivate a “less invasive and more anatomic” reconstruction. The use of musculoskeletal ultrasound allows our technique to utilize a completely noninvasive imaging tool that allows proper establishment of ALL anatomy prior to the procedure. The entirety of the ALL is easily identifiable,4,12 which has proven to be shortcoming of MRI evaluation.15-17 Accurate preoperative assessment of the lateral structures is necessary in ACL-deficient individuals.11,15 Sonography also provides a means of accurate guidance and socket creation, without generating large incisions.
If the ALL is responsible for internal rotatory stability as asserted, the structure should exhibit biomechanical properties during movement. In their study on the function of the ligament, Parsons and colleagues9 established the inverse relationship between the ALL and ACL during internal rotation. As their cadaveric knees were subjected to an internal rotatory force through increasing angles of flexion, the contribution of the ALL towards stability significantly increased while the ACL declined. Helito and colleagues8 and Zens and colleagues10 have demonstrated length changes of the ligament through varying degrees of flexion and internal rotation. Their reports indicate greater tension during knee movements, coinciding with the description of increasing ALL stability contribution by Parsons and colleagues.9 Kennedy and colleagues7 conducted a pull-to-failure test on the ALL. The average failure load was 175 N with a stiffness of 20 N/mm, illustrating the structure is a candidate for most traditional soft tissue grafts. The biomechanical evidence of the structural properties of the ALL confirms its importance in knee function and the necessity for its reconstruction.
With the understanding that ACL contributes to rotatory stability to some extent, the notion begs the question of how a centrally located ligament is able to prevent excessive rotation in a structure with a large relative radius. Biomechanically, with such a small moment arm, the ACL would experience tremendous stress when a rotatory force is applied. The same torque applied to a more superficial structure, with a greater moment, would sustain a large reduction in the applied force. The concept of a wheel and an axle should be considered. The equation is F1 × R1 = F2 × R2. We measured on a cadaveric knee the distance from the center of rotation to the ACL and the ALL, finding the radii were 5 mm and 30 mm, respectively. Taking these measurements, we would then expect the force experienced on the axle (ACL) to be 6 times greater than what would be experienced on the periphery of the wheel (ALL). The ALL (wheel) has a significant biomechanical advantage over the ACL (axle) in controlling and enduring internal rotatory forces of the knee. This would imply that if the ALL were damaged and not re-established, the ACL would experience a 6 times greater force trying to control internal rotation, which would result in a significantly increased chance of failure and rupture.
While there is a degree of dissent on the presence of the ALL, a number of studies have classified the tissue as an independent ligamentous structure.3-7 While there is disagreement on the precise location of the femoral attachment, there is a consensus on the location of the tibial and meniscal attachments. Claes and colleagues4 originally outlined the femoral attachment as anterior and distal to the origin of the fibular collateral ligament (FCL), which is the description this technique follows. Since Claes and colleagues’4 report, many have investigated the ligament’s femoral origin with delineations ranging from posterior and proximal3,5,7 to anterior and distal.6,16-18
The accurate, noninvasive nature of the musculoskeletal ultrasound prior to any incisions being made makes this technique innovative and superior to other open surgical techniques or those that require fluoroscopy.
Conclusion
The ALL has been determined to play an integral role in the rotational stability of the knee. In the setting of instability and insufficiency, reconstruction will lead to better patient outcomes for concurrent ACL/ALL injuries and postsurgical rotatory instability following ACL procedures. This innovative technique utilizes ultrasound to ascertain the precise anatomical attachments of the ALL prior to the operation. The novel nature of this ultrasound-guided reconstruction has the potential to be applicable in many other surgical procedures.
Restoring native kinematics of the knee has been a primary goal of anterior cruciate ligament (ACL) procedures. Double-bundle ACL reconstruction, compared to single-bundle, has been hypothesized to more effectively re-establish rotational stability by re-creating the anatomic ACL, but has not yet proven to result in better clinical outcomes.1
In 1879, Dr. Paul Segond described a “fibrous, pearly band” at the lateral aspect of the knee that avulsed off the anterolateral proximal tibia during many ACL injuries.2 The role of the lateral tissues in knee stability and their relationship with ACL pathology has attracted noteworthy attention in recent time. There have been multiple studies presenting an anatomical description of a structure at the anterolateral portion of the knee with definitive femoral, meniscal, and tibial attachments, which helps control internal rotational forces.3-7 Claes and colleagues4 later found that band of tissue to be the anterolateral ligament (ALL) and determined its injury to be pathognomonic with ACL ruptures.
The ALL is a vital static stabilizer of the tibio-femoral joint, especially during internal tibial rotation.8-10 In their report on ALL and ACL reconstruction, Helito and colleagues11 acknowledge the necessity of accurate assessment of the lateral structures through imaging to determine the presence of extra-articular injury. Musculoskeletal diagnostic ultrasound has been established as an appropriate means to identify the ALL.12
Ultrasound can accurately determine the exact anatomic location of the origin and insertion of the ALL. Reconstruction of the ALL could yield better patient outcomes for those who experience concurrent ACL/ALL injury. Here we present an innovative technique for an ultrasound-guided percutaneous method for reconstruction of the ALL and report on a patient who had underwent ALL reconstruction.
Surgical Indications
All patients undergo an ultrasound evaluation preoperatively to determine if the ALL is intact or injured. Our experience has shown that when ultrasound evaluation reveals an intact ALL, the pivot shift has never been a grade III.
Surgical Technique
For a demonstration of this technique, see the video that accompanies this article.
The pivot shift test is conducted under anesthesia to determine whether an ALL reconstruction is required. The patient is placed in a supine position with the knee flexed at 30o, at neutral rotation, and without any varus or valgus stress.
A No. 15 blade is used to make a small incision centered on each spinal needle. The spinal needle is replaced with a 2.4-mm drill pin (Figure 2).
The graft and FiberTape are then passed under the IT band to the distal incision. Using the length of the BioComposite SwiveLock anchor as a guide, a mark is made on the graft after tensioning the construct in line with the leg, distal to the tibial drill pin (Table 2, Figure 4).
Rehabilitation
Rehabilitation following an ALL procedure is similar to traditional ACL rehabilitation with an added emphasis on minimizing rotational torque of the tibia in the early stages.
Case Report
In January 2013, a 17-year-old male soccer player suffered an ACL rupture of his right knee. Later that spring, he had an ACL reconstruction with an allograft. Twelve months postoperatively, the patient returned, saying that he felt much better; however, anytime he tried to plant his foot and rotate over that fixed foot, his knee felt unstable. The physical examination revealed both negative Lachman and anterior drawer tests but a I+ pivot shift test. A magnetic resonance imaging (MRI) examination revealed an intact ACL graft. A diagnostic ultrasound evaluation revealed a distal ALL injury. After discussing the risks, benefits, and goals with the patient, we opted for a diagnostic arthroscopy and a percutaneous, ultrasound-guided reconstruction of the ALL.
Postoperatively, the patient did very well. One week after surgery, he returned, saying he felt completely stable and demonstrated by repeating the rotation of his knee. The patient continued to have no issues until he returned 13 months post-ALL surgery, complaining of a recent injury that had caused the return of his feelings of instability. An MRI evaluation showed an intact ACL graft and the possibility of a ruptured ALL. Fifteen months after the initial ALL reconstruction, we proceeded with surgery. At arthroscopy, the patient was found to have a pivot shift of I+ and an intact ACL graft. The ALL was reconstructed again using an allograft, internal brace, and bone marrow concentrate. At 13 months post-ALL reconstruction revision, the patient had no complaints.
Discussion
Reconstruction of the ALL is aimed to restore anatomic rotational kinematics. Sonnery-Cottet and colleagues14 have reported promising initial results in their 2-year follow-up study of combined ACL and ALL reconstruction outcomes. This surgical technique includes use of an internal brace, which negates the necessity for external support devices and allows for earlier mobilization of the joint. A reconstruction of the ALL, performed concurrently with the ACL, does not add recovery time, but could prevent postsurgical complications and improve rehabilitation by eliminating rotational instability that presents in some ACL-reconstructed patients.
Sonnery-Cottet and colleagues15 state that their arthroscopic identification of the ALL can help to cultivate a “less invasive and more anatomic” reconstruction. The use of musculoskeletal ultrasound allows our technique to utilize a completely noninvasive imaging tool that allows proper establishment of ALL anatomy prior to the procedure. The entirety of the ALL is easily identifiable,4,12 which has proven to be shortcoming of MRI evaluation.15-17 Accurate preoperative assessment of the lateral structures is necessary in ACL-deficient individuals.11,15 Sonography also provides a means of accurate guidance and socket creation, without generating large incisions.
If the ALL is responsible for internal rotatory stability as asserted, the structure should exhibit biomechanical properties during movement. In their study on the function of the ligament, Parsons and colleagues9 established the inverse relationship between the ALL and ACL during internal rotation. As their cadaveric knees were subjected to an internal rotatory force through increasing angles of flexion, the contribution of the ALL towards stability significantly increased while the ACL declined. Helito and colleagues8 and Zens and colleagues10 have demonstrated length changes of the ligament through varying degrees of flexion and internal rotation. Their reports indicate greater tension during knee movements, coinciding with the description of increasing ALL stability contribution by Parsons and colleagues.9 Kennedy and colleagues7 conducted a pull-to-failure test on the ALL. The average failure load was 175 N with a stiffness of 20 N/mm, illustrating the structure is a candidate for most traditional soft tissue grafts. The biomechanical evidence of the structural properties of the ALL confirms its importance in knee function and the necessity for its reconstruction.
With the understanding that ACL contributes to rotatory stability to some extent, the notion begs the question of how a centrally located ligament is able to prevent excessive rotation in a structure with a large relative radius. Biomechanically, with such a small moment arm, the ACL would experience tremendous stress when a rotatory force is applied. The same torque applied to a more superficial structure, with a greater moment, would sustain a large reduction in the applied force. The concept of a wheel and an axle should be considered. The equation is F1 × R1 = F2 × R2. We measured on a cadaveric knee the distance from the center of rotation to the ACL and the ALL, finding the radii were 5 mm and 30 mm, respectively. Taking these measurements, we would then expect the force experienced on the axle (ACL) to be 6 times greater than what would be experienced on the periphery of the wheel (ALL). The ALL (wheel) has a significant biomechanical advantage over the ACL (axle) in controlling and enduring internal rotatory forces of the knee. This would imply that if the ALL were damaged and not re-established, the ACL would experience a 6 times greater force trying to control internal rotation, which would result in a significantly increased chance of failure and rupture.
While there is a degree of dissent on the presence of the ALL, a number of studies have classified the tissue as an independent ligamentous structure.3-7 While there is disagreement on the precise location of the femoral attachment, there is a consensus on the location of the tibial and meniscal attachments. Claes and colleagues4 originally outlined the femoral attachment as anterior and distal to the origin of the fibular collateral ligament (FCL), which is the description this technique follows. Since Claes and colleagues’4 report, many have investigated the ligament’s femoral origin with delineations ranging from posterior and proximal3,5,7 to anterior and distal.6,16-18
The accurate, noninvasive nature of the musculoskeletal ultrasound prior to any incisions being made makes this technique innovative and superior to other open surgical techniques or those that require fluoroscopy.
Conclusion
The ALL has been determined to play an integral role in the rotational stability of the knee. In the setting of instability and insufficiency, reconstruction will lead to better patient outcomes for concurrent ACL/ALL injuries and postsurgical rotatory instability following ACL procedures. This innovative technique utilizes ultrasound to ascertain the precise anatomical attachments of the ALL prior to the operation. The novel nature of this ultrasound-guided reconstruction has the potential to be applicable in many other surgical procedures.
1. Suomalainen P, Järvelä T, Paakkala A, Kannus P, Järvinen M. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: A prospective randomized study with 5-year results. Am J Sports Med. 2012;40(7):1511-1518.
2. Segond P. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Progrés Médical. 1879;6(6):1-85. French.
3. Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Athrosc. 2015;23(11):3186-3195.
4. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321-328.
5. Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: Anatomy, length changes and association with the segond fracture. Bone Joint J. 2014;96-B(3):325-331.
6. Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546.
7. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: An anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606-1615.
8. Helito CP, Helito PV, Bonadio MB, et al. Evaluation of the length and isometric pattern of the anterolateral ligament with serial computer tomography. Orthop J Sports Med. 2014;2(12):2325967114562205.
9. Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(3):669-674.
10. Zens M, Niemeyer P, Ruhhamer J, et al. Length changes of the anterolateral ligament during passive knee motion: A human cadaveric study. Am J Sports Med. 2015;43(10):2545-2552.
11. Helito CP, Bonadio MB, Gobbi RG, et al. Combined intra- and extra-articular reconstruction of the anterior cruciate ligament: the reconstruction of the knee anterolateral ligament. Arthrosc Tech. 2015;4(3):e239-e244.
12. Cianca J, John J, Pandit S, Chiou-Tan FY. Musculoskeletal ultrasound imaging of the recently described anterolateral ligament of the knee. Am J Phys Med Rehabil. 2014;93(2):186
13. Adams JE, Zobitz ME, Reach JS, et al. Rotator cuff repair using an acellular dermal matrix graft: An in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
14. Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BHB, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598-1605.
15. Sonnery-Cottet B, Archbold P, Rezende FC, Neto AM, Fayard JM, Thaunat M. Arthroscopic identification of the anterolateral ligament of the knee. Arthrosc Tech. 2014;3(3):e389-e392.
16. Helito CP, Helito PV, Costa HP, et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol. 2014;43(10):1421-1427.
17. Helito CP, Demange MK, Helito PV, et al. Evaluation of the anterolateral ligament of the knee by means of magnetic resonance examination. Rev Bras Orthop. 2015;50(2):214-219.
18. Helito CP, Demange MK, Bonadio MB, et al. Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am J Sports Med. 2014;42(10):2356-2362.
1. Suomalainen P, Järvelä T, Paakkala A, Kannus P, Järvinen M. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: A prospective randomized study with 5-year results. Am J Sports Med. 2012;40(7):1511-1518.
2. Segond P. Recherches cliniques et expérimentales sur les épanchements sanguins du genou par entorse. Progrés Médical. 1879;6(6):1-85. French.
3. Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Athrosc. 2015;23(11):3186-3195.
4. Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321-328.
5. Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: Anatomy, length changes and association with the segond fracture. Bone Joint J. 2014;96-B(3):325-331.
6. Helito CP, Demange MK, Bonadio MB, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546.
7. Kennedy MI, Claes S, Fuso FA, et al. The anterolateral ligament: An anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606-1615.
8. Helito CP, Helito PV, Bonadio MB, et al. Evaluation of the length and isometric pattern of the anterolateral ligament with serial computer tomography. Orthop J Sports Med. 2014;2(12):2325967114562205.
9. Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(3):669-674.
10. Zens M, Niemeyer P, Ruhhamer J, et al. Length changes of the anterolateral ligament during passive knee motion: A human cadaveric study. Am J Sports Med. 2015;43(10):2545-2552.
11. Helito CP, Bonadio MB, Gobbi RG, et al. Combined intra- and extra-articular reconstruction of the anterior cruciate ligament: the reconstruction of the knee anterolateral ligament. Arthrosc Tech. 2015;4(3):e239-e244.
12. Cianca J, John J, Pandit S, Chiou-Tan FY. Musculoskeletal ultrasound imaging of the recently described anterolateral ligament of the knee. Am J Phys Med Rehabil. 2014;93(2):186
13. Adams JE, Zobitz ME, Reach JS, et al. Rotator cuff repair using an acellular dermal matrix graft: An in vivo study in a canine model. Arthroscopy. 2006;22(7):700-709.
14. Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BHB, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598-1605.
15. Sonnery-Cottet B, Archbold P, Rezende FC, Neto AM, Fayard JM, Thaunat M. Arthroscopic identification of the anterolateral ligament of the knee. Arthrosc Tech. 2014;3(3):e389-e392.
16. Helito CP, Helito PV, Costa HP, et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol. 2014;43(10):1421-1427.
17. Helito CP, Demange MK, Helito PV, et al. Evaluation of the anterolateral ligament of the knee by means of magnetic resonance examination. Rev Bras Orthop. 2015;50(2):214-219.
18. Helito CP, Demange MK, Bonadio MB, et al. Radiographic landmarks for locating the femoral origin and tibial insertion of the knee anterolateral ligament. Am J Sports Med. 2014;42(10):2356-2362.
Malpractice Counsel: Missed Nodule
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
Case
A 48-year-old man presented to the ED with a 2-day history of cough and congestion. He described the cough as gradual in onset and, though initially nonproductive, it was now productive of green sputum. He denied fevers or chills, chest pain, nausea, vomiting, or diarrhea, and complained of only mild shortness of breath. His medical history was significant for hypertension, which was well managed with daily lisinopril-hydrochlorothiazide. He admitted to smoking one pack of cigarettes per day for the past 25 years, but denied alcohol or illicit drug use.
On physical examination, the patient’s vital signs were: blood pressure, 112/64 mm Hg; heart rate, 84 beats/min; respiratory rate, 20 breaths/min; and temperature, 98oF. Oxygen saturation was 97% on room air. The head, eyes, ears, nose, and throat examination was normal. Auscultation of the lungs revealed bilateral breath sounds with scattered, faint expiratory wheezing; the heart had a regular rate and rhythm, without murmurs, rubs, or gallops.
The emergency physician (EP) ordered posteroanterior and lateral chest X-rays (CXR), which he interpreted as normal. He also ordered an albuterol handheld nebulizer treatment for the patient. After the albuterol treatment, the patient felt he was breathing more easily. The frequency of his cough had also decreased following treatment and, on re-examination, he exhibited no wheezing and was given azithromycin 500 mg orally in the ED. The EP diagnosed the patient with acute bronchitis and discharged him home with an albuterol metered dose inhaler with a spacer, and a 4-day course of azithromycin. He also encouraged the patient to quit smoking.
The next day the radiologist’s official reading of the patient’s radiographs included the finding of a very small pulmonary nodule, which was seen only on the lateral X-ray. The radiologist recommended a repeat CXR or a computed tomography (CT) scan of the chest in 6 months.
Unfortunately, the EP never saw this information, and the patient was not contacted regarding the abnormal radiology finding and the need for follow-up. Approximately 20 months later, the patient was diagnosed with lung cancer with metastasis to the thoracic spine and liver. Despite chemotherapy and radiation treatment, he died from the cancer.
The patient’s family brought a malpractice suit against the EP, stating that the cancer could have been successfully treated prior to any metastasis if the patient had been informed of the abnormal radiology findings at his ED visit 20 months prior. The EP argued that he never saw the official radiology report, and therefore had no knowledge of the need for follow-up. At trial, a jury verdict was returned in favor of the defendant.
Discussion
Unfortunately, some version of this scenario occurs on a frequent basis. While imaging studies account for the majority of such cases, the same situation can occur with abnormal laboratory results, body-fluid cultures, or pathology reports in which an abnormality is identified (eg, positive blood culture, missed fracture) but, for a myriad of reasons, the critical information does not get related to the patient.
Because of the episodic nature of the practice of emergency medicine (EM), a process must be in place to ensure any “positive” test results or findings discovered after patient discharge are reviewed and compared to the ED diagnosis, and that any “misses” result in notifying the patient and/or his or her primary care physician and arranging follow-up. In cases such as the one presented here, a system issue existed—one that was not due to any fault or oversight of the EP. Ideally, EM leadership should work closely with leadership from radiology and laboratory services and hospital risk management to develop such a process—one that will be effective every day, including weekends and holidays.
Missed fractures on radiographs are a common cause of malpractice litigation against EPs. In one review by Kachalia et al1 examining malpractice claims involving EPs, missed fractures on radiographs accounted for 19% (the most common) of the 79 missed diagnoses identified in their study.In a similar study by Karcz et al,2 missed fractures ranked second in frequency and dollars lost in malpractice cases against EPs in Massachusetts.
While missed lesions on CXR do not occur with the same frequency as missed fractures, the results are much more devastating when the lesion turns out to be malignant. Three common areas where such lesions are missed on CXR include: the apex of the lung, obscured by overlying clavicle and ribs; the retrocardiac region (as in the patient in this case); and the lung bases obscured by the diaphragm.
Emergency physicians are neither trained nor expected to identify every single abnormality—especially subtle radiographic abnormalities. This is why there are radiology overreads, and a system or process must be in place to ensure patients are informed of any positive findings and to arrange proper follow-up.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
1. Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196-205.
2. Karcz A, Korn R, Burke MC, et al. Malpractice claims against emergency physicians in Massachusetts: 1975-1993. Am J Emerg Med. 1996;14(4):341-345.
Emergency Ultrasound: Ultrasound-Guided Femoral Nerve Block
Case Scenario
A young man presented to the ED for evaluation of a large laceration to the anterior thigh that resulted from an industrial accident (Figure 1).
Femoral nerve blocks are useful in a variety of clinical scenarios, including fractures of the femur or hip1 and laceration repairs (Figure 2).
Identifying the Femoral Nerve on Ultrasound
To perform this nerve block, one must recall the anatomy of femoral central-line placement. The femoral nerve lies lateral to the femoral artery and vein. The high-frequency probe should be placed over the femoral crease (Figure 3).
Performing the Block
An ultrasound-guided femoral nerve block can be performed using a 22-gauge blunt tip spinal needle, and an in-plane or out-of-plane technique can be employed. We prefer using an in-plane technique because the entire shaft of the needle can be visualized as it approaches the nerve. Anatomically, the femoral nerve lies in a separate fascial plane from the artery and vein, beneath the fascia iliaca (Figure 4). You can use this anatomic location of the femoral nerve to your advantage when performing the block. The needle can be advanced to a target slightly lateral to the nerve until it pops beneath the fascia iliaca. On the ultrasound, you can monitor the spread of anesthetic as it is injected. If the needle is in the right location, the hypoechoic fluid will spread medially toward the nerve, but will not track around the artery or vein. At least 15 cc to 20 cc of local anesthetic is typically required.2,3 If you prefer, the anesthetic can be diluted in normal saline, in a 1:1 ratio, to achieve adequate volume.
If you do not see the anesthetic spread during the injection, you should stop and check the needle placement, as it may be intravascular. Using a more lateral approach, targeting the injection at the fascial plane, rather than the nerve, helps to avoid direct intraneural injection or contact with the nerve—and it keeps the needle far away from the femoral vascular bundle.
Safety Considerations
As with any technique, prior to the procedure, aseptic measures should be taken, including the use of a sterile probe cover and sterile gloves. All patients undergoing ultrasound-guided nerve blocks proximal to the wrist or ankle should be placed on a cardiac monitor. In addition, intralipid emulsion should be readily available for administration in the unlikely event there is inadvertent intravascular injection of local anesthetic and cardiovascular collapse occurs.
Summary
With practice, ultrasound guidance can improve the procedural success of femoral nerve blocks and decrease the risk of nerve injury compared to blind nerve blocks
1. Dickman E, Pushkar I, Likourezos A, et al. Ultrasound-guided nerve blocks for intracapsular and extracapsular hip fractures. Am J Emerg Med. 2016;34(3):586-589.
2. Femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:267-279.
3. Ultrasound-guided femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:397-404.
Case Scenario
A young man presented to the ED for evaluation of a large laceration to the anterior thigh that resulted from an industrial accident (Figure 1).
Femoral nerve blocks are useful in a variety of clinical scenarios, including fractures of the femur or hip1 and laceration repairs (Figure 2).
Identifying the Femoral Nerve on Ultrasound
To perform this nerve block, one must recall the anatomy of femoral central-line placement. The femoral nerve lies lateral to the femoral artery and vein. The high-frequency probe should be placed over the femoral crease (Figure 3).
Performing the Block
An ultrasound-guided femoral nerve block can be performed using a 22-gauge blunt tip spinal needle, and an in-plane or out-of-plane technique can be employed. We prefer using an in-plane technique because the entire shaft of the needle can be visualized as it approaches the nerve. Anatomically, the femoral nerve lies in a separate fascial plane from the artery and vein, beneath the fascia iliaca (Figure 4). You can use this anatomic location of the femoral nerve to your advantage when performing the block. The needle can be advanced to a target slightly lateral to the nerve until it pops beneath the fascia iliaca. On the ultrasound, you can monitor the spread of anesthetic as it is injected. If the needle is in the right location, the hypoechoic fluid will spread medially toward the nerve, but will not track around the artery or vein. At least 15 cc to 20 cc of local anesthetic is typically required.2,3 If you prefer, the anesthetic can be diluted in normal saline, in a 1:1 ratio, to achieve adequate volume.
If you do not see the anesthetic spread during the injection, you should stop and check the needle placement, as it may be intravascular. Using a more lateral approach, targeting the injection at the fascial plane, rather than the nerve, helps to avoid direct intraneural injection or contact with the nerve—and it keeps the needle far away from the femoral vascular bundle.
Safety Considerations
As with any technique, prior to the procedure, aseptic measures should be taken, including the use of a sterile probe cover and sterile gloves. All patients undergoing ultrasound-guided nerve blocks proximal to the wrist or ankle should be placed on a cardiac monitor. In addition, intralipid emulsion should be readily available for administration in the unlikely event there is inadvertent intravascular injection of local anesthetic and cardiovascular collapse occurs.
Summary
With practice, ultrasound guidance can improve the procedural success of femoral nerve blocks and decrease the risk of nerve injury compared to blind nerve blocks
Case Scenario
A young man presented to the ED for evaluation of a large laceration to the anterior thigh that resulted from an industrial accident (Figure 1).
Femoral nerve blocks are useful in a variety of clinical scenarios, including fractures of the femur or hip1 and laceration repairs (Figure 2).
Identifying the Femoral Nerve on Ultrasound
To perform this nerve block, one must recall the anatomy of femoral central-line placement. The femoral nerve lies lateral to the femoral artery and vein. The high-frequency probe should be placed over the femoral crease (Figure 3).
Performing the Block
An ultrasound-guided femoral nerve block can be performed using a 22-gauge blunt tip spinal needle, and an in-plane or out-of-plane technique can be employed. We prefer using an in-plane technique because the entire shaft of the needle can be visualized as it approaches the nerve. Anatomically, the femoral nerve lies in a separate fascial plane from the artery and vein, beneath the fascia iliaca (Figure 4). You can use this anatomic location of the femoral nerve to your advantage when performing the block. The needle can be advanced to a target slightly lateral to the nerve until it pops beneath the fascia iliaca. On the ultrasound, you can monitor the spread of anesthetic as it is injected. If the needle is in the right location, the hypoechoic fluid will spread medially toward the nerve, but will not track around the artery or vein. At least 15 cc to 20 cc of local anesthetic is typically required.2,3 If you prefer, the anesthetic can be diluted in normal saline, in a 1:1 ratio, to achieve adequate volume.
If you do not see the anesthetic spread during the injection, you should stop and check the needle placement, as it may be intravascular. Using a more lateral approach, targeting the injection at the fascial plane, rather than the nerve, helps to avoid direct intraneural injection or contact with the nerve—and it keeps the needle far away from the femoral vascular bundle.
Safety Considerations
As with any technique, prior to the procedure, aseptic measures should be taken, including the use of a sterile probe cover and sterile gloves. All patients undergoing ultrasound-guided nerve blocks proximal to the wrist or ankle should be placed on a cardiac monitor. In addition, intralipid emulsion should be readily available for administration in the unlikely event there is inadvertent intravascular injection of local anesthetic and cardiovascular collapse occurs.
Summary
With practice, ultrasound guidance can improve the procedural success of femoral nerve blocks and decrease the risk of nerve injury compared to blind nerve blocks
1. Dickman E, Pushkar I, Likourezos A, et al. Ultrasound-guided nerve blocks for intracapsular and extracapsular hip fractures. Am J Emerg Med. 2016;34(3):586-589.
2. Femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:267-279.
3. Ultrasound-guided femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:397-404.
1. Dickman E, Pushkar I, Likourezos A, et al. Ultrasound-guided nerve blocks for intracapsular and extracapsular hip fractures. Am J Emerg Med. 2016;34(3):586-589.
2. Femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:267-279.
3. Ultrasound-guided femoral nerve block. In: Hadzic A, Carrera A, Clark T, et al, eds. Hadzic’s Peripheral Nerve Blocks: An Anatomy for Ultrasound-Guided Regional Anesthesia. 2nd ed. New York, NY: The McGraw Hill Companies, Inc; 2012:397-404.
Diagnosis at a Glance: Debilitating Thigh Mass in an Obese Patient
Case
A 60-year-old morbidly obese man presented to the ED with a painless mass on his left thigh (Figure 1), which he stated had formed over a several day period 2 months earlier.
On examination, the patient appeared well, with normal vital signs and a body mass index of 56 kg/m2. A computed tomography (CT) scan was obtained to further evaluate the mass (Figure 2), and dermatology services were consulted.
Discussion
Massive localized lymphedema is a complication associated with morbid obesity. First described in 1998 by Farshid and Weiss,1 MLL is characterized by a benign pedunculated mass primarily of the lower extremity that slowly enlarges over years.2 The pathogenesis of MLL is currently unknown. Histologically, MLL contains lobules of mature fat with expanded connective tissue septa without the degree of cellular atypia in well-differentiated liposarcoma (WDL). Though similar to WDL, MLL can be differentiated by the clinical history of a slowly growing mass in a morbidly obese patient and examination findings of overlying reactive skin and soft-tissue changes associated with chronic lymphedema (eg, thickened peau d’orange skin).1,2
The diagnosis of MLL may be made clinically, and if there is no evidence of infection, the patient may be referred to a surgeon. If diagnostic uncertainty remains, biopsy and further CT imaging studies should be considered. The treatment for MLL is a direct excision if the mass is interfering with the patient’s gait. If left untreated, MLL can progress to angiosarcoma. Recurrence is possible, even after surgical excision.3
1. Farshid G, Weiss SW. Massive localized lymphedema in the morbidly obese: a histologically distinct reactive lesion simulating liposarcoma. Am J Surg Pathol. 1998;22(10):1277-1283.
2. Evans RJ, Scilley C. Massive localized lymphedema: A case series and literature review. Can J Plast Surg. 2011;19(3):e30-e31.
3. Moon Y, Pyon JK. A rare case of massive localized lymphedema in a morbidly obese patient. Arch of Plast Surg. 2016;43(1):125-127. doi:10.5999/aps.2016.43.1.125.
Case
A 60-year-old morbidly obese man presented to the ED with a painless mass on his left thigh (Figure 1), which he stated had formed over a several day period 2 months earlier.
On examination, the patient appeared well, with normal vital signs and a body mass index of 56 kg/m2. A computed tomography (CT) scan was obtained to further evaluate the mass (Figure 2), and dermatology services were consulted.
Discussion
Massive localized lymphedema is a complication associated with morbid obesity. First described in 1998 by Farshid and Weiss,1 MLL is characterized by a benign pedunculated mass primarily of the lower extremity that slowly enlarges over years.2 The pathogenesis of MLL is currently unknown. Histologically, MLL contains lobules of mature fat with expanded connective tissue septa without the degree of cellular atypia in well-differentiated liposarcoma (WDL). Though similar to WDL, MLL can be differentiated by the clinical history of a slowly growing mass in a morbidly obese patient and examination findings of overlying reactive skin and soft-tissue changes associated with chronic lymphedema (eg, thickened peau d’orange skin).1,2
The diagnosis of MLL may be made clinically, and if there is no evidence of infection, the patient may be referred to a surgeon. If diagnostic uncertainty remains, biopsy and further CT imaging studies should be considered. The treatment for MLL is a direct excision if the mass is interfering with the patient’s gait. If left untreated, MLL can progress to angiosarcoma. Recurrence is possible, even after surgical excision.3
Case
A 60-year-old morbidly obese man presented to the ED with a painless mass on his left thigh (Figure 1), which he stated had formed over a several day period 2 months earlier.
On examination, the patient appeared well, with normal vital signs and a body mass index of 56 kg/m2. A computed tomography (CT) scan was obtained to further evaluate the mass (Figure 2), and dermatology services were consulted.
Discussion
Massive localized lymphedema is a complication associated with morbid obesity. First described in 1998 by Farshid and Weiss,1 MLL is characterized by a benign pedunculated mass primarily of the lower extremity that slowly enlarges over years.2 The pathogenesis of MLL is currently unknown. Histologically, MLL contains lobules of mature fat with expanded connective tissue septa without the degree of cellular atypia in well-differentiated liposarcoma (WDL). Though similar to WDL, MLL can be differentiated by the clinical history of a slowly growing mass in a morbidly obese patient and examination findings of overlying reactive skin and soft-tissue changes associated with chronic lymphedema (eg, thickened peau d’orange skin).1,2
The diagnosis of MLL may be made clinically, and if there is no evidence of infection, the patient may be referred to a surgeon. If diagnostic uncertainty remains, biopsy and further CT imaging studies should be considered. The treatment for MLL is a direct excision if the mass is interfering with the patient’s gait. If left untreated, MLL can progress to angiosarcoma. Recurrence is possible, even after surgical excision.3
1. Farshid G, Weiss SW. Massive localized lymphedema in the morbidly obese: a histologically distinct reactive lesion simulating liposarcoma. Am J Surg Pathol. 1998;22(10):1277-1283.
2. Evans RJ, Scilley C. Massive localized lymphedema: A case series and literature review. Can J Plast Surg. 2011;19(3):e30-e31.
3. Moon Y, Pyon JK. A rare case of massive localized lymphedema in a morbidly obese patient. Arch of Plast Surg. 2016;43(1):125-127. doi:10.5999/aps.2016.43.1.125.
1. Farshid G, Weiss SW. Massive localized lymphedema in the morbidly obese: a histologically distinct reactive lesion simulating liposarcoma. Am J Surg Pathol. 1998;22(10):1277-1283.
2. Evans RJ, Scilley C. Massive localized lymphedema: A case series and literature review. Can J Plast Surg. 2011;19(3):e30-e31.
3. Moon Y, Pyon JK. A rare case of massive localized lymphedema in a morbidly obese patient. Arch of Plast Surg. 2016;43(1):125-127. doi:10.5999/aps.2016.43.1.125.