User login
Mohs Micrographic Surgery in the VHA (FULL)
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
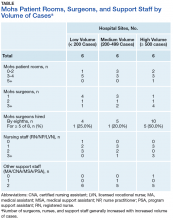
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
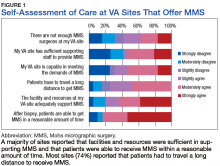
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
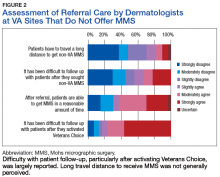
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
Skin cancer is one of the most prevalent conditions among VHA patients.1 One of the largest U.S. health care systems, the VHA serves more than 9 million veterans.2 In 2012, 4% of VHA patients had a diagnosis of keratinocyte carcinoma or actinic keratosis; 49,229 cases of basal cell carcinoma and 26,310 cases of squamous cell carcinoma were diagnosed.1 With an aging veteran population and the incidence of skin cancers expected to increase, the development of cost-effective ways to provide easily accessible skin cancer treatments has become a priority for the VHA.
National Comprehensive Cancer Network (NCCN) guidelines recommend 3 types of surgical treatment for localized keratinocyte carcinoma: local destruction, wide local excision (WLE), and Mohs micrographic surgery (MMS). Tumors at low risk for recurrence may be treated with local destruction or WLE, and tumors at high risk may be treated with WLE or MMS.3
Mohs micrographic surgery involves staged narrow-margin excision with intraoperative tumor mapping and complete circumferential peripheral and deep margin assessment (CCPDMA). With the Mohs surgeon acting as both surgeon and dermatopathologist, it is possible to provide intraoperative correlation with the tissue bed and immediate additional margin resection precisely where needed. Relative to WLE, MMS yields improved histopathologic clearance rates and lower 5-year recurrence rates. It also provides improved preservation of normal tissue, optimized aesthetic outcomes, and high patient satisfaction.4-7 All this is achieved in an outpatient setting with the patient under local anesthesia; therefore the cost of ambulatory surgical centers or hospital operating rooms are avoided.5,8,9
The NCCN recommends WLE for high-risk tumors only if CCPDMA can be achieved. However, CCPDMA requires specialized surgical technique, tissue orientation, and pathology and is not equivalent to standard WLE with routine surgical pathology. Even with intraoperative bread-loafed frozen section analysis, WLE does not achieve the 100% margin assessment obtained with MMS.
In 2012, the American Academy of Dermatology in collaboration with the American College of Mohs Surgery, the American Society for Dermatologic Surgery, and the American Society for Mohs Surgery developed the Mohs Appropriate Use Criteria,which are now widely used as part of the standard of care to determine which cases of skin cancer should be treated with MMS over other modalities.10 These criteria, which are based on both evidence and expert consensus, take into account tumor size, histology, location, and patient factors, such as immunosuppression.
Despite its established benefits, MMS has not been uniformly accessible to veterans seeking VHA care. In 2007, Karen and colleagues surveyed dermatology chiefs and staff dermatologists from 101 VHA hospitals to characterize veterans’ access to MMS and found MMS available at only 11 VHA sites in 9 states.11 Further, access within the VHA was not evenly distributed across the U.S.
The VHA often makes payments, under “non-VA medical care” or “fee-basis care,” to providers in the community for services that the VHA is otherwise unable to provide. In 2014, Congress passed the Veterans Access, Choice, and Accountability Act and established the Veterans Choice program.2,12 This program allows veterans to obtain medical services from providers outside the VHA, based on veteran wait time and place of residence.12 The goal is to improve access. The present authors distinguish between 2 types of care: there are fee-based referrals managed and tracked by the VHA physician and the Veterans Choice for care without the diagnosing physician involvement or knowledge. In addition to expanding treatment options, the act called for reform within the VHA to improve resources and infrastructure needed to provide the best care for the veteran patient population.2
The authors conducted a study to identify current availability of MMS within the VHA and to provide a 10-year update to the survey findings of Karen and colleagues.11 VHA facilities that offer MMS were surveyed to determine available resources and what is needed to provide MMS within the VHA. Also surveyed were VHA facilities that do not offer MMS to determine how VHA patients with skin cancer receive surgical care from non-VA providers or from other surgical specialties.
Related: Nivolumab Linked to Nephritis in Melanoma
Methods
This study, deemed exempt from review by the University of California San Francisco Institutional Review Board, was a survey of dermatology section and service chiefs across the VHA. Subjects were identified through conference calls with VHA dermatologists, searches of individual VHA websites, and requests on dermatology e-mail listservs and were invited by email to participate in the survey.
The Research Electronic Data Capture platform (REDCap; Vanderbilt University Medical Center) was used for survey creation, implementation, dissemination, and data storage. The survey had 6 sections: site information; MMS availability; Mohs surgeon, Mohs laboratory, and support staff; MMS care; patient referral; and Mohs surgeon recruitment.
Data were collected between June 20 and August 1, 2016. Collected VHA site information included name, location, description, and MMS availability. If MMS was available, data were collected on surgeon training and background, number of MMS cases in 2015, and facility and support staff. In addition, subjects rated statements about various aspects of care provided (eg, patient wait time, patient distance traveled) on a 6-point Likert scale: strongly disagree, moderately disagree, slightly disagree, slightly agree, moderately agree, or strongly agree. This section included both positive and negative statements.
If MMS was not available at the VHA site, data were collected on patient referrals, including location within or outside the VHA and patient use of the Veterans Choice program. Subjects also rated positive and negative statements about referral experiences on a Likert scale (eg, patient wait time, patient distance traveled).
Categorical data were summarized, means and standard deviations were calculated for nominal data, and data analysis was performed with Microsoft Excel (Redmond, WA).
Results
The authors identified and surveyed 74 dermatology service and section chiefs across the VHA. Of these chiefs, 52 (70.3%) completed the survey. Completed surveys represented 49 hospital sites and 3 community-based outpatient clinics (CBOCs), including an integrated community-based clinic-hospital.
Sites That Provided MMS
Of the 52 sites with a completed survey, 19 provided MMS. These 19 sites were in 13 states and the District of Columbia, and the majority were in major cities along the coasts. All 19 sites were hospital medical centers, not community-based outpatient clinics, and all provided MMS through the dermatology department. In 2015, an estimated 6,686 MMS cases were performed, or an average of 371 per site (range, 40-1,000 cases/site) or 4.9 MMS cases per day (range, 3-8). These 19 sites were divided by yearly volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y).
Physical Space. On average, each site used 2.89 patient rooms (SD, 1.1; range, 1-6) for MMS. The Table lists numbers of patient rooms based on case volume.
The MMS laboratory was adjacent to the surgical suite at 18 of the MMS sites and in the same building as the surgical suite, but not next to it, at 1 site. For their samples, 11 sites used an automated staining method, 7 used hand staining, and 2 used other methods (1 site used both automated and hand staining). Fourteen sites used hematoxlyin-eosin only, 1 used toluidine blue only, 3 used both hematoxlyin-eosin and toluidine blue, and 1 used MART-1 (melanoma antigen recognized by T cells 1) with hematoxlyin-eosin.
Related: Systemic Therapy in Metastatic Melanoma
Mohs Micrographic Surgeons. Sites with higher case volumes had more Mohs surgeons and more Mohs surgeons with VA appointments (captured as “eighths” or fraction of 8/8 full-time equivalent [FTE]). Information on fellowships and professional memberships was available for 30 Mohs surgeons: Ten (33.3%) were trained in fellowships accredited by both the American College of Mohs Surgery (ACMS) and the Accreditation Council for Graduate Medical Education (ACGME), 8 (26.7%) were trained in ACMS-recognized fellowships only, 7 (23.3%) were trained at ACGME-accredited fellowships only, 2 (6.7%) were trained elsewhere, and 3 (10.0%) had training listed as “uncertain.”
The majority of Mohs surgeons were members of professional societies, and many were members of more than one. Of the 30 Mohs surgeons, 24 (80.0%) were ACMS members, 5 (16.7%) were members of the American Society of Mohs Surgery, and 22 (73.3%) were members of the American Society of Dermatologic Surgery. Twenty-five (89.3%) were affiliated with an academic program.
Of the 30 surgeons, 19 (63.3%) were VHA employees hired by eighths, with an average eighths of 3.9 (SD, 2.7), or 49% of a FTE. Data on these surgeons’ pay tables and tiers were insufficient (only 3 provided the information). Of the other 11 surgeons, 10 (33.3%) were contracted, and 1 (3.3%) volunteered without compensation.
Support Staff. Of the 19 MMS sites, 17 (89.5%) used 1 histotechnician, and 2 (10.5%) used more than 1. Ten sites (52.6%) hired histotechnicians as contractors, 8 (42.1%) as employees, and 1 (5.3%) on a fee basis. In general, sites with higher case volumes had more nursing and support staff. Thirteen sites (68.4%) participated in the training of dermatology residents, and 5 sites (26.3%) trained Mohs fellows.
Wait Time Estimate. The survey also asked for estimates of the average amount of time patients waited for MMS. Of the 19 sites, 8 (42.1%) reported a wait time of less than 1 month, 10 (52.6%) reported 2 to 6 months, and 1 (5.3%) reported 7 months to 1 year. Seventeen (89.5%) of the 19 sites had a grading or triage system for expediting certain cancer types. At 7 sites, cases were prioritized on the basis of physician assessment; at 3 sites, aggressive or invasive squamous cell carcinoma received priority; other sites gave priority to patients with melanoma, patients with carcinoma near the nose or eye, organ transplant recipients, and other immunosuppressed patients.
Sites That Did Not Provide MMS
Of the 52 sites with a completed survey, 33 (63.5%) did not provide on-site MMS. Of these 33 sites, 28 (84.8%) used purchased care to refer patients to fee-basis non-VA dermatologists. In addition, 30 sites (90.9%) had patients activate Veterans Choice. Three sites referred patients to VA sites in another VISN.
Surgeon Recruitment
Five sites (9.6%) had an unfilled Mohs micrographic surgeon position. The average FTE of these unfilled positions was 0.6. One position had been open for less than 6 months, and the other 4 for more than 1 year. All 5 respondents with unfilled positions strongly agreed with the statement, “The position is unfilled because the salary is not competitive with the local market.”
Assessment of Care Provided
Respondents at sites that provided MMS rated various aspects of care (Figure 1).
Respondents from sites that purchased MMS care from non-VA medical care rated surgery availability and ease of patient follow-up (Figure 2).
Related: Getting a Better Picture of Skin Cancer
Discussion
Skin cancer is highly prevalent in the veteran patient population, and each year treatment by the VHA requires considerable spending.1 The results of this cross-sectional survey characterize veterans’ access to MMS within the VHA and provide a 10-year update to the survey findings of Karen and colleagues.11 Compared with their study, this survey offers a more granular description of practices and facilities as well as comparisons of VHA care with care purchased from outside sources. In outlining the state of MMS care within the VHA, this study highlights progress made and provides the updated data needed for continued efforts to optimize care and resource allocation for patients who require MMS within the VHA.
Although the number of VHA sites that provide MMS has increased over the past 10 years—from 11 sites in 9 states in 2007 to 19 sites in 13 states now—it is important to note that access to MMS care highly depends on geographic location.11 The VHA sites that provide MMS are clustered in major cities along the coasts. Four states (California, Florida, New York, and Texas) had > 1 MMS site, whereas most other states did not have any. In addition, only 1 MMS site served all of the northwest U.S. To ensure the anonymity of survey respondents, the authors did not further characterize the regional distribution of MMS sites.
Despite the increase in MMS sites, the number of MMS cases performed within the VHA seemed to have decreased. An estimated 8,310 cases were performed within the VHA in 2006,which decreased to 6,686 in 2015.11 Although these are estimates, the number of VHA cases likely decreased because of a rise in purchased care. Reviewing VHA electronic health records, Yoon and colleagues found that 19,681 MMS cases were performed either within the VHA or at non-VA medical care sites in 2012.1 Although the proportions of MMS cases performed within and outside the VHA were not reported, clearly many veterans had MMS performed through the VHA in recent years, and a high percentage of these cases were external referrals. More study is needed to further characterize MMS care within the VHA and MMS care purchased.
The 19 sites that provided MMS were evenly divided by volume: high (> 500 cases/y), medium (200-500 cases/y), and low (< 200 cases/y). Case volume correlated with the numbers of surgeons, nurses, and support staff at each site. Number of patient rooms dedicated to MMS at each site was not correlated with case volume; however, not ascertaining the number of days per week MMS was performed may have contributed to the lack of observed correlation.The majority of Mohs surgeons (25; 89.3%) within the VHA were affiliated with academic programs, which may partly explain the uneven geographic distribution of VHA sites that provide MMS (dermatology residency programs typically are in larger cities). The majority of Mohs surgeons were fellowship-trained through the ACMS or the ACGME. As the ACGME first began accrediting fellowship programs in 2003, younger surgeons were more likely to have completed this fellowship. According to respondents from sites that did not provide MMS, noncompetitive VHA salaries might be a barrier to Mohs surgeon recruitment. If a shift to providing more MMS care within the VHA were desired, an effective strategy could be to raise surgeon salaries. Higher salaries would bring in more Mohs surgeons and thereby yield higher MMS case volumes at VHA sites.
However, whether MMS is best provided for veterans within the VHA or at outside sites through referrals warrants further study. More than 60% of sites provided access to MMS through purchased care, either by fee-basis/non-VA medical care referrals or by the patient-elected Veterans Choice program. According to 84.2% of respondents at MMS sites and 66.7% of respondents at non-MMS sites, patients received care within a reasonable amount of time. In addition, respondents at MMS sites estimated longer patient travel distance for surgery. Respondents reported being concerned about coordination of care and follow-up for patients who received MMS outside the VHA. Other than referrals to outside sites for MMS, current triage practices include referral to other surgical specialties within the VHA, predominantly ear, nose, and throat and plastic surgery, for WLE. Given that access to on-site MMS varies significantly by geographic location, on-site MMS may be preferable in some locations, and external referrals in others. Based on this study's findings, on-site MMS seems superior to external referrals in all respects except patient travel distance. More research is needed to determine the most cost-effective triage practices. One option would be to have each VISN develop a skin cancer care center of excellence that would assist providers in appropriate triage and management.
Limitations
A decade has passed since Karen and colleagues conducted their study on MMS within the VHA.11 Data from this study suggest some progress has been made in improving veterans’ access to MMS. However, VHA sites that provide MMS are still predominantly located in large cities. In cases in which VHA providers refer patients to outside facilities, care coordination and follow-up are challenging. The present findings provide a basis for continuing VHA efforts to optimize resource allocation and improve longitudinal care for veterans who require MMS for skin cancer. Another area of interest is the comparative cost-effectiveness of MMS care provided within the VHA rather than at outside sites through purchased care. The answer may depend on geographic location, as MMS demand may be higher in some regions than that of others. For patients who receive MMS care outside the VHA, efforts should be made to improve communication and follow-up between VHA and external providers.
This study was limited in that it surveyed only those VHA sites with dermatology services or sections. It is possible, though unlikely, that MMS also was provided through nondermatology services. This study’s 70.3% response rate (52/74 dermatology chiefs) matched that of Karen and colleagues.11 Nevertheless, given that 30% of the surveyed chiefs did not respond and that analysis was performed separately for 2 small subgroups, (19 VHA sites that provided on-site MMS and 33 VHA sites that did not), the present findings may not be representative of the VHA as a whole.
Another limitation was that the survey captured respondent estimates of surgical caseloads and resources. Confirmation of these estimates would require a review of internal medical records and workforce analyses, which was beyond the scope of this study.
Conclusion
Although some progress has been made over the past 10 years, access to MMS within the VHA remains limited. About one-third of VHA sites provide on-site MMS; the other two-thirds refer patients with skin cancer to MMS sites outside the VHA. According to their dermatology chiefs, VHA sites that provide MMS have adequate resources and staffing and acceptable wait times for surgery; the challenge is in patients’ long travel distances. At sites that do not provide MMS, patients have access to MMS as well, and acceptable wait times and travel distances; the challenge is in follow-up, especially with activation of the Veterans Choice program. Studies should focus on standardizing veterans’ care and improving their access to MMS.
Click here to read the digital edition.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
1. Yoon J, Phibbs CS, Chow A, Pomerantz H, Weinstock MA. Costs of keratinocyte carcinoma (nonmelanoma skin cancer) and actinic keratosis treatment in the Veterans Health Administration. Dermatol Surg. 2016;42(9):1041-1047.
2. Giroir BP, Wilensky GR. Reforming the Veterans Health Administration—beyond palliation of symptoms. N Engl J Med. 2015;373(18):1693-1695.
3. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Basal Cell Skin Cancer 1.2018. https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf. Updated September 18, 2017. Accessed January 31, 2018.
4. Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351-1357.
5. Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39(5, pt 1):698-703.
6. Kauvar AN, Arpey CJ, Hruza G, Olbricht SM, Bennett R, Mahmoud BH. Consensus for nonmelanoma skin cancer treatment, part ii: squamous cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(11):1214-1240.
7. Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R; American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. Dermatol Surg. 2015;41(5):550-571.
8. Chen JT, Kempton SJ, Rao VK. The economics of skin cancer: an analysis of Medicare payment data. Plast Reconstr Surg Glob Open. 2016;4(9):e868.
9. Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8(10):914-922.
10. Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550.
11. Karen JK, Hale EK, Nehal KS, Levine VJ. Use of Mohs surgery by the Veterans Affairs Health Care System. J Am Acad Dermatol. 2009;60(6):1069-1070.
12. U.S. Department of Veterans Affairs. Expanded access to non-VA care through the Veterans Choice program. Interim final rule. Fed Regist. 2015;80(230):74991-74996.
Acne Treatment: Analysis of Acne-Related Social Media Posts and the Impact on Patient Care
Social media has become a prominent source of medical information for patients, including those with dermatologic conditions.1,2 Physicians, patients, and pharmaceutical companies can use social media platforms to communicate with each other and share knowledge and advertisements related to conditions. Social media can influence patients’ perceptions of their disease and serve as a modality to acquire medical treatments.3 Furthermore, social media posts from illicit pharmacies can result in patients buying harmful medications without physician oversight.4,5 Examination of the content and sources of social media posts related to acne may be useful in determining those who are primarily utilizing social media and for what purpose. The goal of this systematic review was to identify sources of acne-related social media posts to determine communication trends to gain a better understanding of the potential impact social media may have on patient care.
Methods
Social media posts were identified (May 2008 to May 2016) using the search terms acne and treatment across all social media platforms available through a commercial social media data aggregating software (Crimson Hexagon). Information from relevant posts was extracted and compiled into a spreadsheet that included the content, post date, social media platform, and hyperlink. To further analyze the data, the first 100 posts on acne treatment from May 2008 to May 2016 were selected and manually classified by the following types of communication: (1) patient-to-patient (eg, testimonies of patients’ medical experiences); (2) professional-to-patient (eg, clinical knowledge or experience provided by a medical provider and/or cited article in reference to relevant treatments); (3) pharmaceutical company–to-patient (eg, information from reputable drug manufacturers regarding drug activity and adverse effects); (4) illicit pharmacy–to-patient (eg, pharmacies with advertisements calling patients to buy a drug online or offering discrete shipping without a prescription)4,5; or (5) other-to-patient (eg, posts that did not contain enough detail to be classified).
Results
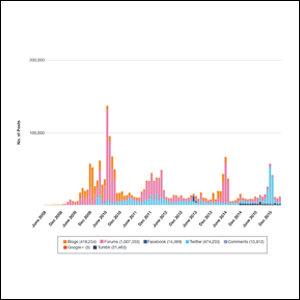
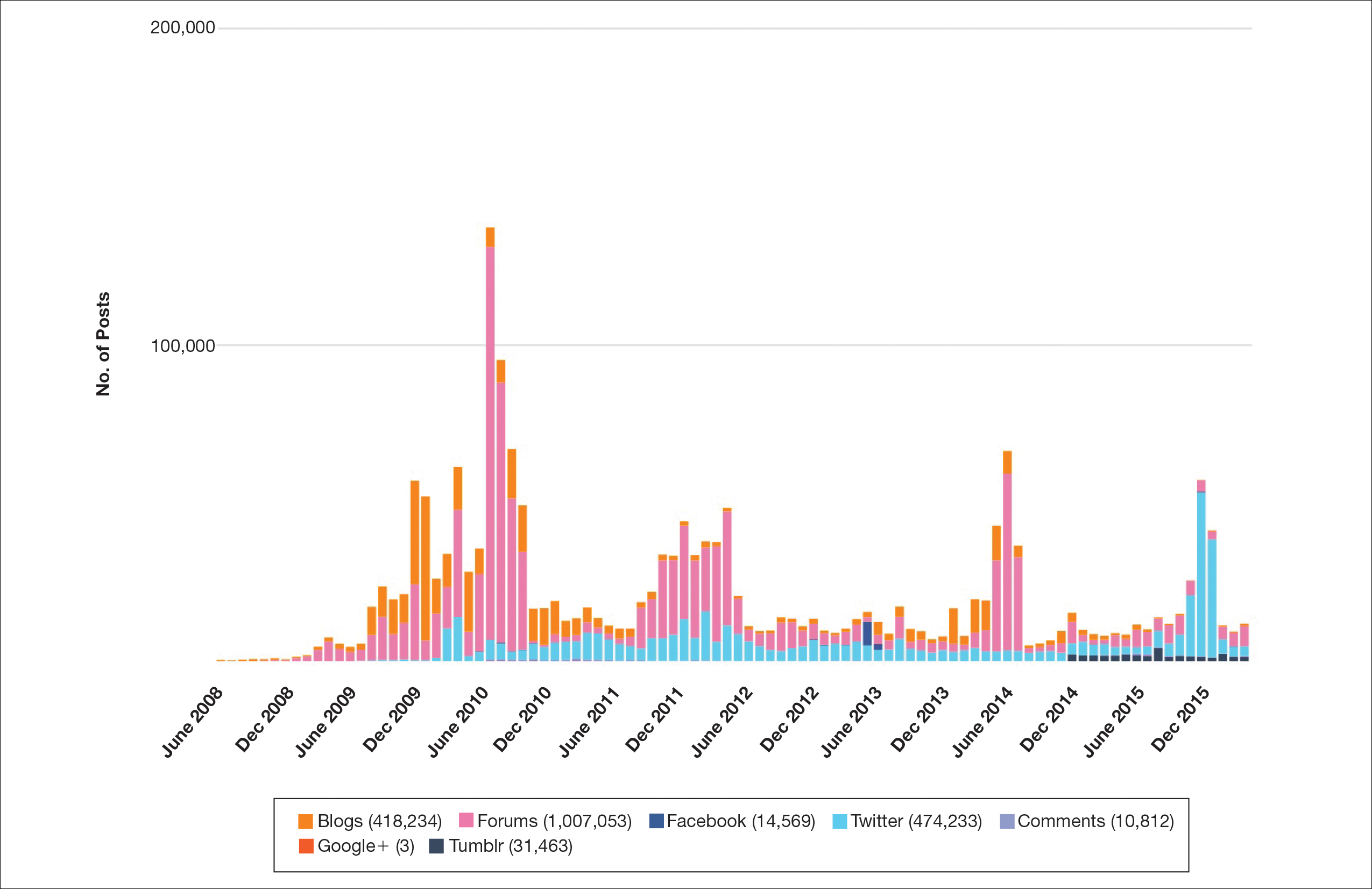
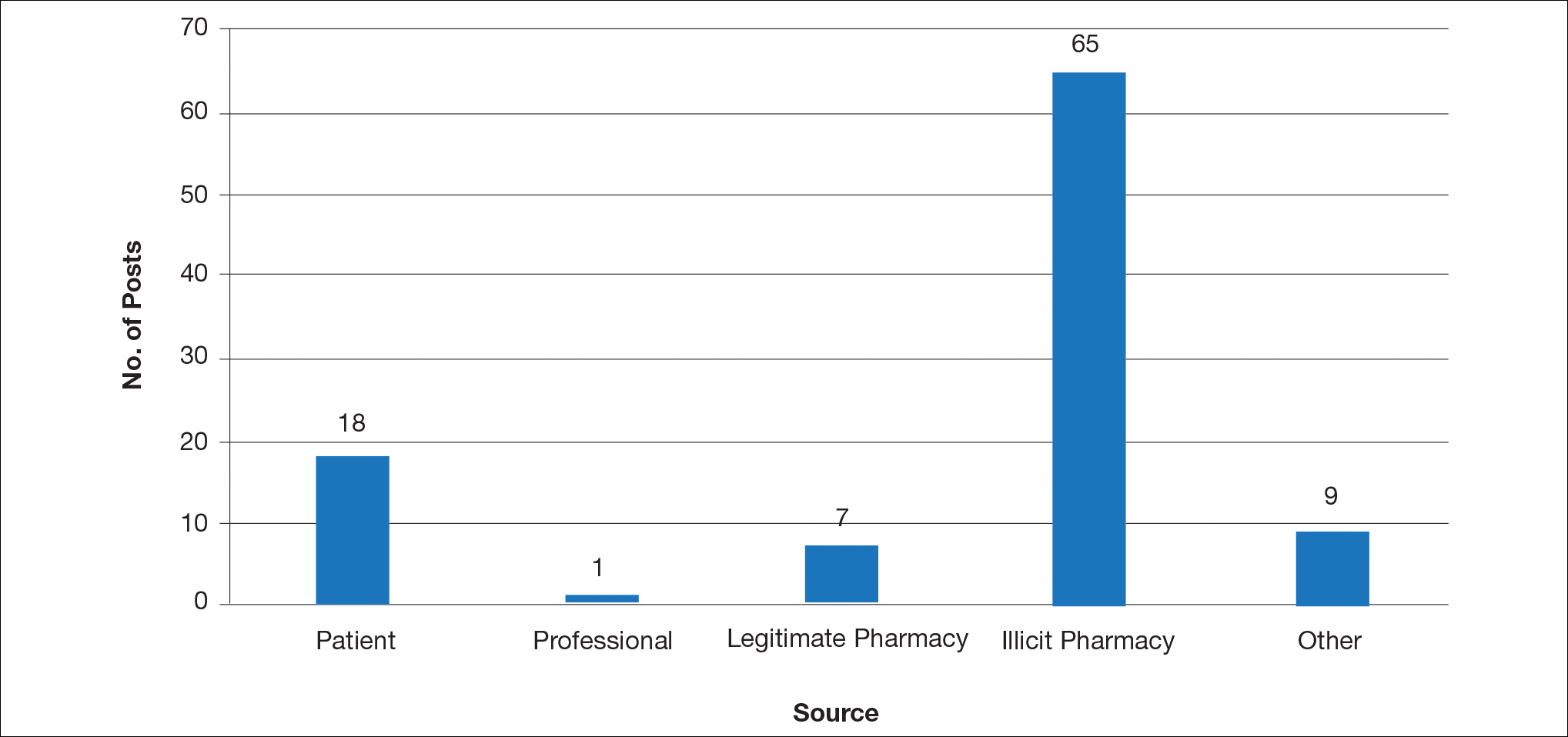
Hundreds of thousands of social media posts discussing acne treatment were identified over the 8-year study period (Figure 1). The social media data aggregator extracted posts from various blogs, website comment sections, and online forums, as well as major social media platforms (ie, Facebook, Twitter, Google+, Tumblr). The first 100 posts selected for further analysis included 0 from 2008, 6 from 2009, 36 from 2010, 15 from 2011, 7 from 2012, 8 from 2013, 12 from 2014, 11 from 2015, and 5 from 2016. From this sample, 65 posts were considered to have an illicit source; conversely, 18 posts were from patients and 7 posts were from pharmaceutical companies (Figure 2).
Comment
This study demonstrated that discussion of acne treatment is prevalent in social media. Although our research underrepresents the social media interest in specific acne treatments, as only posts mentioning the terms acne and treatment were evaluated to gain insights into how social media platforms are being used by individuals with cutaneous disease. As such, even with this potential underrepresentation, our study demonstrated a high incidence of illicit marketing of prescription acne medications across multiple social media platforms (Figure 2). The sale of dermatologic pharmaceuticals (eg, isotretinoin) without a prescription is recognized by the US Government as a problem that is rapidly growing.4,5 Illicit pharmacies pose as legitimate pharmacies that can provide prescription medications to consumers without a prescription.5,6 The fact that these illicit pharmacy–to-patient posts were the most abundant in our study may speak to their relative success on social media platforms in encouraging patients to purchase prescription medications without physician oversight. These findings should concern health care providers, as the procurement of prescription medications without a prescription may put patients at risk.
- Alinia H, Moradi Tuchayi S, Farhangian ME, et al. Rosacea patients seeking advice: qualitative analysis of patients’ posts on a rosacea support forum. J Dermatolog Treat. 2016;27:99-102.
- Karimkhani C, Connett J, Boyers L, et al. Dermatology on Instagram. Dermatology Online J. 2014:20. pii:13030/qt71g178w9.
- Smailhodzic E, Hooijsma W, Boonstra A, et al. Social media use in healthcare: a systematic review of effects on patients and on their relationship with healthcare professionals. BMC Health Serv Res. 2016;16:442.
- Lagan BM, Dolk H, White B, et al. Assessing the availability of the teratogenic drug isotretinoin outside the pregnancy prevention programme: a survey of e-pharmacies. Pharmacoepidemiol Drug Saf. 2014;23:411-418.
- Lott JP, Kovarik CL. Availability of oral isotretinoin and terbinafine on the Internet. J Am Acad Dermatol. 2010;62:153-154.
- Mahé E, Beauchet A. Dermatologists and the Internet. J Am Acad Dermatol. 2010;63:908.
Social media has become a prominent source of medical information for patients, including those with dermatologic conditions.1,2 Physicians, patients, and pharmaceutical companies can use social media platforms to communicate with each other and share knowledge and advertisements related to conditions. Social media can influence patients’ perceptions of their disease and serve as a modality to acquire medical treatments.3 Furthermore, social media posts from illicit pharmacies can result in patients buying harmful medications without physician oversight.4,5 Examination of the content and sources of social media posts related to acne may be useful in determining those who are primarily utilizing social media and for what purpose. The goal of this systematic review was to identify sources of acne-related social media posts to determine communication trends to gain a better understanding of the potential impact social media may have on patient care.
Methods
Social media posts were identified (May 2008 to May 2016) using the search terms acne and treatment across all social media platforms available through a commercial social media data aggregating software (Crimson Hexagon). Information from relevant posts was extracted and compiled into a spreadsheet that included the content, post date, social media platform, and hyperlink. To further analyze the data, the first 100 posts on acne treatment from May 2008 to May 2016 were selected and manually classified by the following types of communication: (1) patient-to-patient (eg, testimonies of patients’ medical experiences); (2) professional-to-patient (eg, clinical knowledge or experience provided by a medical provider and/or cited article in reference to relevant treatments); (3) pharmaceutical company–to-patient (eg, information from reputable drug manufacturers regarding drug activity and adverse effects); (4) illicit pharmacy–to-patient (eg, pharmacies with advertisements calling patients to buy a drug online or offering discrete shipping without a prescription)4,5; or (5) other-to-patient (eg, posts that did not contain enough detail to be classified).
Results
Hundreds of thousands of social media posts discussing acne treatment were identified over the 8-year study period (Figure 1). The social media data aggregator extracted posts from various blogs, website comment sections, and online forums, as well as major social media platforms (ie, Facebook, Twitter, Google+, Tumblr). The first 100 posts selected for further analysis included 0 from 2008, 6 from 2009, 36 from 2010, 15 from 2011, 7 from 2012, 8 from 2013, 12 from 2014, 11 from 2015, and 5 from 2016. From this sample, 65 posts were considered to have an illicit source; conversely, 18 posts were from patients and 7 posts were from pharmaceutical companies (Figure 2).


Comment
This study demonstrated that discussion of acne treatment is prevalent in social media. Although our research underrepresents the social media interest in specific acne treatments, as only posts mentioning the terms acne and treatment were evaluated to gain insights into how social media platforms are being used by individuals with cutaneous disease. As such, even with this potential underrepresentation, our study demonstrated a high incidence of illicit marketing of prescription acne medications across multiple social media platforms (Figure 2). The sale of dermatologic pharmaceuticals (eg, isotretinoin) without a prescription is recognized by the US Government as a problem that is rapidly growing.4,5 Illicit pharmacies pose as legitimate pharmacies that can provide prescription medications to consumers without a prescription.5,6 The fact that these illicit pharmacy–to-patient posts were the most abundant in our study may speak to their relative success on social media platforms in encouraging patients to purchase prescription medications without physician oversight. These findings should concern health care providers, as the procurement of prescription medications without a prescription may put patients at risk.
Social media has become a prominent source of medical information for patients, including those with dermatologic conditions.1,2 Physicians, patients, and pharmaceutical companies can use social media platforms to communicate with each other and share knowledge and advertisements related to conditions. Social media can influence patients’ perceptions of their disease and serve as a modality to acquire medical treatments.3 Furthermore, social media posts from illicit pharmacies can result in patients buying harmful medications without physician oversight.4,5 Examination of the content and sources of social media posts related to acne may be useful in determining those who are primarily utilizing social media and for what purpose. The goal of this systematic review was to identify sources of acne-related social media posts to determine communication trends to gain a better understanding of the potential impact social media may have on patient care.
Methods
Social media posts were identified (May 2008 to May 2016) using the search terms acne and treatment across all social media platforms available through a commercial social media data aggregating software (Crimson Hexagon). Information from relevant posts was extracted and compiled into a spreadsheet that included the content, post date, social media platform, and hyperlink. To further analyze the data, the first 100 posts on acne treatment from May 2008 to May 2016 were selected and manually classified by the following types of communication: (1) patient-to-patient (eg, testimonies of patients’ medical experiences); (2) professional-to-patient (eg, clinical knowledge or experience provided by a medical provider and/or cited article in reference to relevant treatments); (3) pharmaceutical company–to-patient (eg, information from reputable drug manufacturers regarding drug activity and adverse effects); (4) illicit pharmacy–to-patient (eg, pharmacies with advertisements calling patients to buy a drug online or offering discrete shipping without a prescription)4,5; or (5) other-to-patient (eg, posts that did not contain enough detail to be classified).
Results
Hundreds of thousands of social media posts discussing acne treatment were identified over the 8-year study period (Figure 1). The social media data aggregator extracted posts from various blogs, website comment sections, and online forums, as well as major social media platforms (ie, Facebook, Twitter, Google+, Tumblr). The first 100 posts selected for further analysis included 0 from 2008, 6 from 2009, 36 from 2010, 15 from 2011, 7 from 2012, 8 from 2013, 12 from 2014, 11 from 2015, and 5 from 2016. From this sample, 65 posts were considered to have an illicit source; conversely, 18 posts were from patients and 7 posts were from pharmaceutical companies (Figure 2).


Comment
This study demonstrated that discussion of acne treatment is prevalent in social media. Although our research underrepresents the social media interest in specific acne treatments, as only posts mentioning the terms acne and treatment were evaluated to gain insights into how social media platforms are being used by individuals with cutaneous disease. As such, even with this potential underrepresentation, our study demonstrated a high incidence of illicit marketing of prescription acne medications across multiple social media platforms (Figure 2). The sale of dermatologic pharmaceuticals (eg, isotretinoin) without a prescription is recognized by the US Government as a problem that is rapidly growing.4,5 Illicit pharmacies pose as legitimate pharmacies that can provide prescription medications to consumers without a prescription.5,6 The fact that these illicit pharmacy–to-patient posts were the most abundant in our study may speak to their relative success on social media platforms in encouraging patients to purchase prescription medications without physician oversight. These findings should concern health care providers, as the procurement of prescription medications without a prescription may put patients at risk.
- Alinia H, Moradi Tuchayi S, Farhangian ME, et al. Rosacea patients seeking advice: qualitative analysis of patients’ posts on a rosacea support forum. J Dermatolog Treat. 2016;27:99-102.
- Karimkhani C, Connett J, Boyers L, et al. Dermatology on Instagram. Dermatology Online J. 2014:20. pii:13030/qt71g178w9.
- Smailhodzic E, Hooijsma W, Boonstra A, et al. Social media use in healthcare: a systematic review of effects on patients and on their relationship with healthcare professionals. BMC Health Serv Res. 2016;16:442.
- Lagan BM, Dolk H, White B, et al. Assessing the availability of the teratogenic drug isotretinoin outside the pregnancy prevention programme: a survey of e-pharmacies. Pharmacoepidemiol Drug Saf. 2014;23:411-418.
- Lott JP, Kovarik CL. Availability of oral isotretinoin and terbinafine on the Internet. J Am Acad Dermatol. 2010;62:153-154.
- Mahé E, Beauchet A. Dermatologists and the Internet. J Am Acad Dermatol. 2010;63:908.
- Alinia H, Moradi Tuchayi S, Farhangian ME, et al. Rosacea patients seeking advice: qualitative analysis of patients’ posts on a rosacea support forum. J Dermatolog Treat. 2016;27:99-102.
- Karimkhani C, Connett J, Boyers L, et al. Dermatology on Instagram. Dermatology Online J. 2014:20. pii:13030/qt71g178w9.
- Smailhodzic E, Hooijsma W, Boonstra A, et al. Social media use in healthcare: a systematic review of effects on patients and on their relationship with healthcare professionals. BMC Health Serv Res. 2016;16:442.
- Lagan BM, Dolk H, White B, et al. Assessing the availability of the teratogenic drug isotretinoin outside the pregnancy prevention programme: a survey of e-pharmacies. Pharmacoepidemiol Drug Saf. 2014;23:411-418.
- Lott JP, Kovarik CL. Availability of oral isotretinoin and terbinafine on the Internet. J Am Acad Dermatol. 2010;62:153-154.
- Mahé E, Beauchet A. Dermatologists and the Internet. J Am Acad Dermatol. 2010;63:908.
Practice Points
- Social media content can influence patients’ perceptions of their disease and serve as a modality to acquire medical treatments, though the source often is unknown.
- This study aimed to identify sources of acne-related social media posts to determine communication trends to gain a better understanding of the potential impact social media may have on patient care.
- Due to the potential for illicit marketing of prescription acne medications across multiple social media platforms, it is important to ask your patients what resources they use to learn about acne and offer to answer any questions regarding acne and its treatment.
Perceptions of Tanning Risk Among Melanoma Patients With a History of Indoor Tanning
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).
Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).
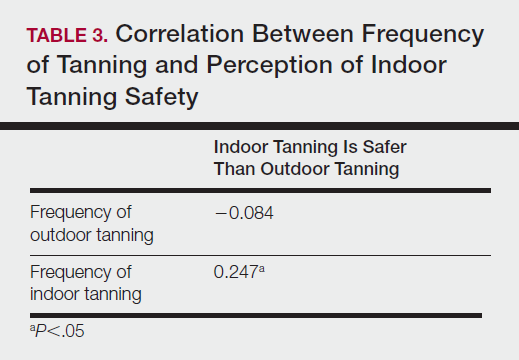
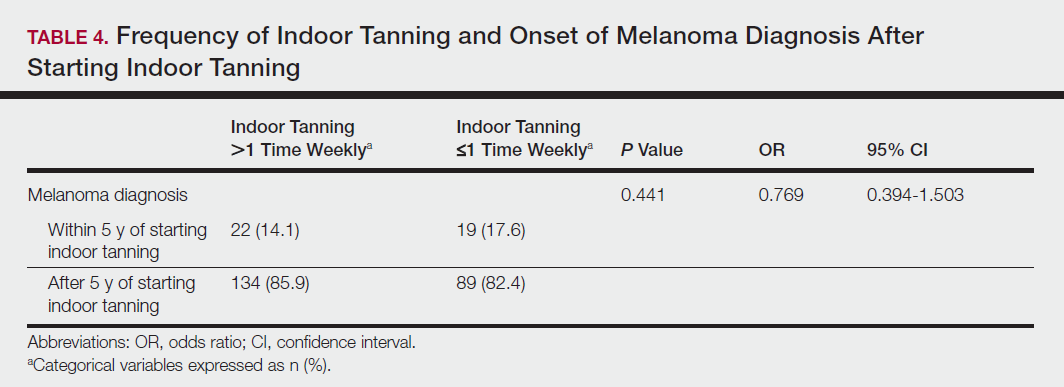
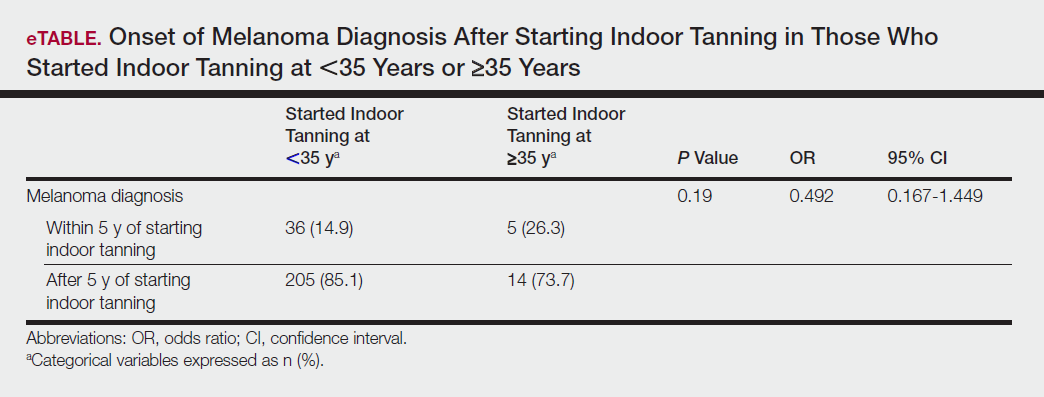
Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).
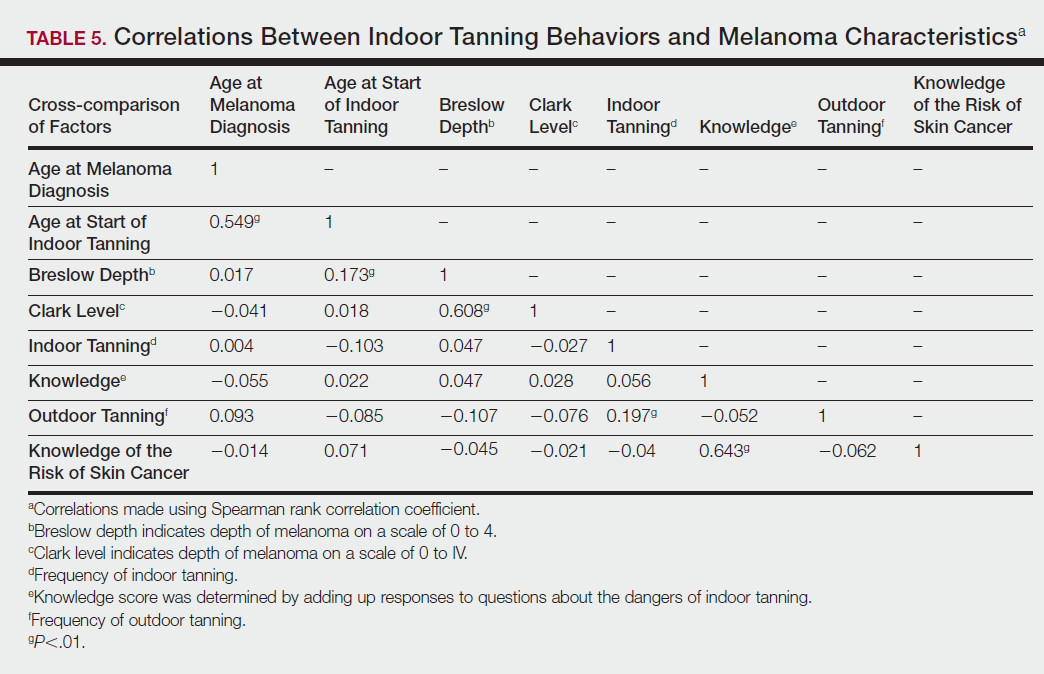
Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).


Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).



Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).

Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).


Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).



Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).

Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
Practice Points
- Despite US Food and Drug Administration reclassification and publicity of the risks of skin cancer, many patients continue to use sunbeds.
- It is important to assess how patients are obtaining information regarding sunbed safety, as indoor tanning companies are promoting sunbeds as “safe” tans.
- The increased combination of sunbed use and outdoor tanning is putting people at greater risk for the development of melanoma and nonmelanoma skin cancer.