User login
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.
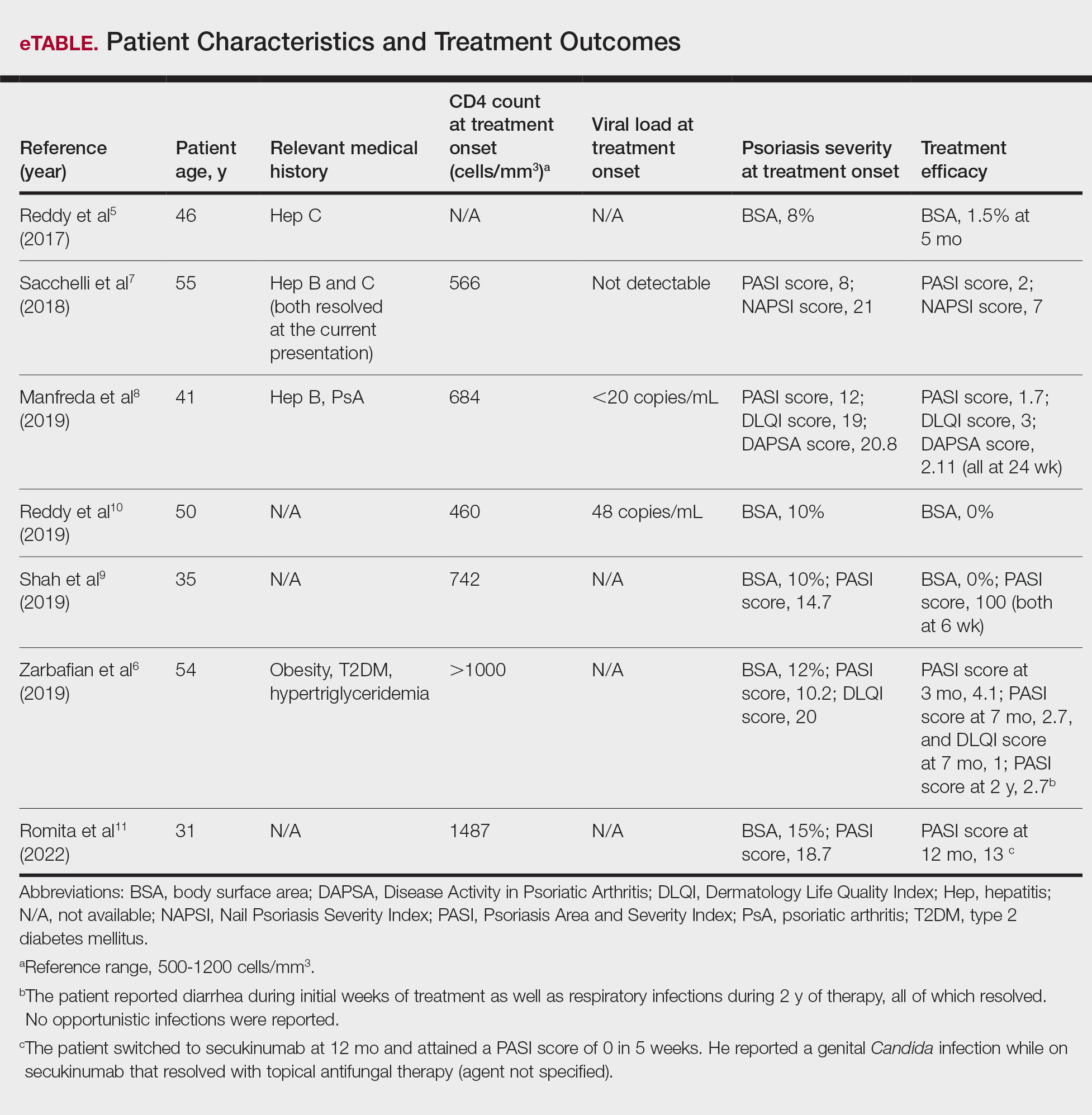
The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.

The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.

The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
PRACTICE POINT
- For patients with HIV who require systemic therapy for psoriasis, apremilast may provide an effective and safe therapeutic option, with minimal immunosuppressive adverse effects.
How Media Coverage of Oral Minoxidil for Hair Loss Has Impacted Prescribing Habits
Minoxidil, a potent vasodilator, was approved by the US Food and Drug Administration (FDA) in 1963 to treat high blood pressure. Its application as a hair loss treatment was discovered by accident—patients taking oral minoxidil for blood pressure noticed hair growth on their bodies as a side effect of the medication. In 1988, topical minoxidil (Rogaine [Johnson & Johnson Consumer Inc]) was approved by the FDA for the treatment of androgenetic alopecia in men, and then it was approved for the same indication in women in 1991. The mechanism of action by which minoxidil increases hair growth still has not been fully elucidated. When applied topically, it is thought to extend the anagen phase (or growth phase) of the hair cycle and increase hair follicle size. It also increases oxygen to the hair follicle through vasodilation and stimulates the production of vascular endothelial growth factor, which is thought to promote hair growth.1 Since its approval, topical minoxidil has become a first-line treatment of androgenetic alopecia in men and women.
In August 2022, The New York Times (NYT) published an article on dermatologists’ use of oral minoxidil at a fraction of the dose prescribed for blood pressure with profound results in hair regrowth.2 Several dermatologists quoted in the article endorsed that the decreased dose minimizes unwanted side effects such as hypertrichosis, hypotension, and other cardiac issues while still being effective for hair loss. Also, compared to topical minoxidil, low-dose oral minoxidil (LDOM) is relatively cheaper and easier to use; topicals are more cumbersome to apply and often leave the hair and scalp sticky, leading to noncompliance among patients.2 Currently, oral minoxidil is not approved by the FDA for use in hair loss, making it an off-label use.
Since the NYT article was published, we have observed an increase in patient questions and requests for LDOM as well as heightened use by fellow dermatologists in our community. As of November 2022, the NYT had approximately 9,330,000 total subscribers, solidifying its place as a newspaper of record in the United States and across the world.3 In April 2023, we conducted a survey of US-based board-certified dermatologists to investigate the impact of the NYT article on prescribing practices of LDOM for alopecia. The survey was conducted as a poll in a Facebook group for board-certified dermatologists and asked, “How did the NYT article on oral minoxidil for alopecia change your utilization of LDOM (low-dose oral minoxidil) for alopecia?” Three answer choices were given: (1) I started Rx’ing LDOM or increased the number of patients I manage with LDOM; (2) No change. I never Rx’d LDOM and/or no increase in utilization; and (3) I was already prescribing LDOM.
Of the 65 total respondents, 27 (42%) reported that the NYT article influenced their decision to start prescribing LDOM for alopecia. Nine respondents (14%) reported that the article did not influence their prescribing habits, and 27 (42%) responded that they were already prescribing the medication prior to the article’s publication.
Data from Epiphany Dermatology, a practice with more than 70 locations throughout the United States, showed that oral minoxidil was prescribed for alopecia 107 times in 2020 and 672 times in 2021 (Amy Hadley, Epiphany Dermatology, written communication, March 24, 2023). In 2022, prescriptions increased exponentially to 1626, and in the period of January 2023 to March 2023 alone, oral minoxidil was prescribed 510 times. Following publication of the NYT article in August 2022, LDOM was prescribed a total of 1377 times in the next 8 months.
Moreover, data from Summit Pharmacy, a retail pharmacy in Centennial, Colorado, showed an 1800% increase in LDOM prescriptions in the 7 months following the NYT article’s publication (August 2022 to March 2023) compared with the 7 months prior (January 2022 to August 2022)(Brandon Johnson, Summit Pharmacy, written communication, March 30, 2023). These data provide evidence for the influence of the NYT article on prescribing habits of dermatology providers in the United States.
The safety of oral minoxidil for use in hair loss has been established through several studies in the literature.4,5 These results show that LDOM may be a safe, readily accessible, and revolutionary treatment for hair loss. A retrospective multicenter study of 1404 patients treated with LDOM for any type of alopecia found that side effects were infrequent, and only 1.7% of patients discontinued treatment due to adverse effects. The most frequent adverse effect was hypertrichosis, occurring in 15.1% of patients but leading to treatment withdrawal in only 0.5% of patients.4 Similarly, Randolph and Tosti5 found that hypertrichosis of the face and body was the most common adverse effect observed, though it rarely resulted in discontinuation and likely was dose dependent: less than 10% of patients receiving 0.25 mg/d experienced hypertrichosis compared with more than 50% of those receiving 5 mg/d (N=634). They also described patients in whom topical minoxidil, though effective, posed major barriers to compliance due to the twice-daily application, changes to hair texture from the medication, and scalp irritation. A literature review of 17 studies with 634 patients on LDOM as a primary treatment for hair loss found that it was an effective, well-tolerated treatment and should be considered for healthy patients who have difficulty with topical formulations.5
In the age of media with data constantly at users’ fingertips, the art of practicing medicine also has changed. Although physicians pride themselves on evidence-based medicine, it appears that an NYT article had an impact on how physicians, particularly dermatologists, prescribe oral minoxidil. However, it is difficult to know if the article exposed dermatologists to another treatment in their armamentarium for hair loss or if it influenced patients to ask their health care provider about LDOM for hair loss. One thing is clear—since the article’s publication, the off-label use of LDOM for alopecia has produced what many may call “miracles” for patients with hair loss.5
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j.1365-2133.2004.05785.x
- Kolata G. An old medicine grows new hair for pennies a day, doctors say. The New York Times. August 18, 2022. Accessed May 20, 2024. https://www.nytimes.com/2022/08/18/health/minoxidil-hair-loss-pills.html
- The New York Times Company reports third-quarter 2022 results. Press release. The New York Times Company; November 2, 2022. Accessed May 20, 2024. https://nytco-assets.nytimes.com/2022/11/NYT-Press-Release-Q3-2022-Final-nM7GzWGr.pdf
- Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84:1644-1651. doi:10.1016/j.jaad.2021.02.054
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-746. doi:10.1016/j.jaad.2020.06.1009
Minoxidil, a potent vasodilator, was approved by the US Food and Drug Administration (FDA) in 1963 to treat high blood pressure. Its application as a hair loss treatment was discovered by accident—patients taking oral minoxidil for blood pressure noticed hair growth on their bodies as a side effect of the medication. In 1988, topical minoxidil (Rogaine [Johnson & Johnson Consumer Inc]) was approved by the FDA for the treatment of androgenetic alopecia in men, and then it was approved for the same indication in women in 1991. The mechanism of action by which minoxidil increases hair growth still has not been fully elucidated. When applied topically, it is thought to extend the anagen phase (or growth phase) of the hair cycle and increase hair follicle size. It also increases oxygen to the hair follicle through vasodilation and stimulates the production of vascular endothelial growth factor, which is thought to promote hair growth.1 Since its approval, topical minoxidil has become a first-line treatment of androgenetic alopecia in men and women.
In August 2022, The New York Times (NYT) published an article on dermatologists’ use of oral minoxidil at a fraction of the dose prescribed for blood pressure with profound results in hair regrowth.2 Several dermatologists quoted in the article endorsed that the decreased dose minimizes unwanted side effects such as hypertrichosis, hypotension, and other cardiac issues while still being effective for hair loss. Also, compared to topical minoxidil, low-dose oral minoxidil (LDOM) is relatively cheaper and easier to use; topicals are more cumbersome to apply and often leave the hair and scalp sticky, leading to noncompliance among patients.2 Currently, oral minoxidil is not approved by the FDA for use in hair loss, making it an off-label use.
Since the NYT article was published, we have observed an increase in patient questions and requests for LDOM as well as heightened use by fellow dermatologists in our community. As of November 2022, the NYT had approximately 9,330,000 total subscribers, solidifying its place as a newspaper of record in the United States and across the world.3 In April 2023, we conducted a survey of US-based board-certified dermatologists to investigate the impact of the NYT article on prescribing practices of LDOM for alopecia. The survey was conducted as a poll in a Facebook group for board-certified dermatologists and asked, “How did the NYT article on oral minoxidil for alopecia change your utilization of LDOM (low-dose oral minoxidil) for alopecia?” Three answer choices were given: (1) I started Rx’ing LDOM or increased the number of patients I manage with LDOM; (2) No change. I never Rx’d LDOM and/or no increase in utilization; and (3) I was already prescribing LDOM.
Of the 65 total respondents, 27 (42%) reported that the NYT article influenced their decision to start prescribing LDOM for alopecia. Nine respondents (14%) reported that the article did not influence their prescribing habits, and 27 (42%) responded that they were already prescribing the medication prior to the article’s publication.
Data from Epiphany Dermatology, a practice with more than 70 locations throughout the United States, showed that oral minoxidil was prescribed for alopecia 107 times in 2020 and 672 times in 2021 (Amy Hadley, Epiphany Dermatology, written communication, March 24, 2023). In 2022, prescriptions increased exponentially to 1626, and in the period of January 2023 to March 2023 alone, oral minoxidil was prescribed 510 times. Following publication of the NYT article in August 2022, LDOM was prescribed a total of 1377 times in the next 8 months.
Moreover, data from Summit Pharmacy, a retail pharmacy in Centennial, Colorado, showed an 1800% increase in LDOM prescriptions in the 7 months following the NYT article’s publication (August 2022 to March 2023) compared with the 7 months prior (January 2022 to August 2022)(Brandon Johnson, Summit Pharmacy, written communication, March 30, 2023). These data provide evidence for the influence of the NYT article on prescribing habits of dermatology providers in the United States.
The safety of oral minoxidil for use in hair loss has been established through several studies in the literature.4,5 These results show that LDOM may be a safe, readily accessible, and revolutionary treatment for hair loss. A retrospective multicenter study of 1404 patients treated with LDOM for any type of alopecia found that side effects were infrequent, and only 1.7% of patients discontinued treatment due to adverse effects. The most frequent adverse effect was hypertrichosis, occurring in 15.1% of patients but leading to treatment withdrawal in only 0.5% of patients.4 Similarly, Randolph and Tosti5 found that hypertrichosis of the face and body was the most common adverse effect observed, though it rarely resulted in discontinuation and likely was dose dependent: less than 10% of patients receiving 0.25 mg/d experienced hypertrichosis compared with more than 50% of those receiving 5 mg/d (N=634). They also described patients in whom topical minoxidil, though effective, posed major barriers to compliance due to the twice-daily application, changes to hair texture from the medication, and scalp irritation. A literature review of 17 studies with 634 patients on LDOM as a primary treatment for hair loss found that it was an effective, well-tolerated treatment and should be considered for healthy patients who have difficulty with topical formulations.5
In the age of media with data constantly at users’ fingertips, the art of practicing medicine also has changed. Although physicians pride themselves on evidence-based medicine, it appears that an NYT article had an impact on how physicians, particularly dermatologists, prescribe oral minoxidil. However, it is difficult to know if the article exposed dermatologists to another treatment in their armamentarium for hair loss or if it influenced patients to ask their health care provider about LDOM for hair loss. One thing is clear—since the article’s publication, the off-label use of LDOM for alopecia has produced what many may call “miracles” for patients with hair loss.5
Minoxidil, a potent vasodilator, was approved by the US Food and Drug Administration (FDA) in 1963 to treat high blood pressure. Its application as a hair loss treatment was discovered by accident—patients taking oral minoxidil for blood pressure noticed hair growth on their bodies as a side effect of the medication. In 1988, topical minoxidil (Rogaine [Johnson & Johnson Consumer Inc]) was approved by the FDA for the treatment of androgenetic alopecia in men, and then it was approved for the same indication in women in 1991. The mechanism of action by which minoxidil increases hair growth still has not been fully elucidated. When applied topically, it is thought to extend the anagen phase (or growth phase) of the hair cycle and increase hair follicle size. It also increases oxygen to the hair follicle through vasodilation and stimulates the production of vascular endothelial growth factor, which is thought to promote hair growth.1 Since its approval, topical minoxidil has become a first-line treatment of androgenetic alopecia in men and women.
In August 2022, The New York Times (NYT) published an article on dermatologists’ use of oral minoxidil at a fraction of the dose prescribed for blood pressure with profound results in hair regrowth.2 Several dermatologists quoted in the article endorsed that the decreased dose minimizes unwanted side effects such as hypertrichosis, hypotension, and other cardiac issues while still being effective for hair loss. Also, compared to topical minoxidil, low-dose oral minoxidil (LDOM) is relatively cheaper and easier to use; topicals are more cumbersome to apply and often leave the hair and scalp sticky, leading to noncompliance among patients.2 Currently, oral minoxidil is not approved by the FDA for use in hair loss, making it an off-label use.
Since the NYT article was published, we have observed an increase in patient questions and requests for LDOM as well as heightened use by fellow dermatologists in our community. As of November 2022, the NYT had approximately 9,330,000 total subscribers, solidifying its place as a newspaper of record in the United States and across the world.3 In April 2023, we conducted a survey of US-based board-certified dermatologists to investigate the impact of the NYT article on prescribing practices of LDOM for alopecia. The survey was conducted as a poll in a Facebook group for board-certified dermatologists and asked, “How did the NYT article on oral minoxidil for alopecia change your utilization of LDOM (low-dose oral minoxidil) for alopecia?” Three answer choices were given: (1) I started Rx’ing LDOM or increased the number of patients I manage with LDOM; (2) No change. I never Rx’d LDOM and/or no increase in utilization; and (3) I was already prescribing LDOM.
Of the 65 total respondents, 27 (42%) reported that the NYT article influenced their decision to start prescribing LDOM for alopecia. Nine respondents (14%) reported that the article did not influence their prescribing habits, and 27 (42%) responded that they were already prescribing the medication prior to the article’s publication.
Data from Epiphany Dermatology, a practice with more than 70 locations throughout the United States, showed that oral minoxidil was prescribed for alopecia 107 times in 2020 and 672 times in 2021 (Amy Hadley, Epiphany Dermatology, written communication, March 24, 2023). In 2022, prescriptions increased exponentially to 1626, and in the period of January 2023 to March 2023 alone, oral minoxidil was prescribed 510 times. Following publication of the NYT article in August 2022, LDOM was prescribed a total of 1377 times in the next 8 months.
Moreover, data from Summit Pharmacy, a retail pharmacy in Centennial, Colorado, showed an 1800% increase in LDOM prescriptions in the 7 months following the NYT article’s publication (August 2022 to March 2023) compared with the 7 months prior (January 2022 to August 2022)(Brandon Johnson, Summit Pharmacy, written communication, March 30, 2023). These data provide evidence for the influence of the NYT article on prescribing habits of dermatology providers in the United States.
The safety of oral minoxidil for use in hair loss has been established through several studies in the literature.4,5 These results show that LDOM may be a safe, readily accessible, and revolutionary treatment for hair loss. A retrospective multicenter study of 1404 patients treated with LDOM for any type of alopecia found that side effects were infrequent, and only 1.7% of patients discontinued treatment due to adverse effects. The most frequent adverse effect was hypertrichosis, occurring in 15.1% of patients but leading to treatment withdrawal in only 0.5% of patients.4 Similarly, Randolph and Tosti5 found that hypertrichosis of the face and body was the most common adverse effect observed, though it rarely resulted in discontinuation and likely was dose dependent: less than 10% of patients receiving 0.25 mg/d experienced hypertrichosis compared with more than 50% of those receiving 5 mg/d (N=634). They also described patients in whom topical minoxidil, though effective, posed major barriers to compliance due to the twice-daily application, changes to hair texture from the medication, and scalp irritation. A literature review of 17 studies with 634 patients on LDOM as a primary treatment for hair loss found that it was an effective, well-tolerated treatment and should be considered for healthy patients who have difficulty with topical formulations.5
In the age of media with data constantly at users’ fingertips, the art of practicing medicine also has changed. Although physicians pride themselves on evidence-based medicine, it appears that an NYT article had an impact on how physicians, particularly dermatologists, prescribe oral minoxidil. However, it is difficult to know if the article exposed dermatologists to another treatment in their armamentarium for hair loss or if it influenced patients to ask their health care provider about LDOM for hair loss. One thing is clear—since the article’s publication, the off-label use of LDOM for alopecia has produced what many may call “miracles” for patients with hair loss.5
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j.1365-2133.2004.05785.x
- Kolata G. An old medicine grows new hair for pennies a day, doctors say. The New York Times. August 18, 2022. Accessed May 20, 2024. https://www.nytimes.com/2022/08/18/health/minoxidil-hair-loss-pills.html
- The New York Times Company reports third-quarter 2022 results. Press release. The New York Times Company; November 2, 2022. Accessed May 20, 2024. https://nytco-assets.nytimes.com/2022/11/NYT-Press-Release-Q3-2022-Final-nM7GzWGr.pdf
- Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84:1644-1651. doi:10.1016/j.jaad.2021.02.054
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-746. doi:10.1016/j.jaad.2020.06.1009
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j.1365-2133.2004.05785.x
- Kolata G. An old medicine grows new hair for pennies a day, doctors say. The New York Times. August 18, 2022. Accessed May 20, 2024. https://www.nytimes.com/2022/08/18/health/minoxidil-hair-loss-pills.html
- The New York Times Company reports third-quarter 2022 results. Press release. The New York Times Company; November 2, 2022. Accessed May 20, 2024. https://nytco-assets.nytimes.com/2022/11/NYT-Press-Release-Q3-2022-Final-nM7GzWGr.pdf
- Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84:1644-1651. doi:10.1016/j.jaad.2021.02.054
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-746. doi:10.1016/j.jaad.2020.06.1009
Practice Points
- Low-dose oral minoxidil (LDOM) prescriptions have increased due to rising attention to its efficacy and safety.
- Media outlets can have a powerful effect on prescribing habits of physicians.
- Physicians should be aware of media trends to help direct patient education.
Cadaveric Split-Thickness Skin Graft With Partial Guiding Closure for Scalp Defects Extending to the Periosteum
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
Practice Gap
Scalp defects that extend to or below the periosteum often pose a reconstructive conundrum. Secondary-intention healing is challenging without an intact periosteum, and complex rotational flaps are required in these scenarios.1 For a tumor that is at high risk for recurrence or when adjuvant therapy is necessary, tissue distortion of flaps can make monitoring for recurrence difficult. Similarly, for patients in poor health or who are elderly and have substantial skin atrophy, extensive closure may be undesirable or more technically challenging with a higher risk for adverse events. In these scenarios, additional strategies are necessary to optimize wound healing and cosmesis. A cadaveric split-thickness skin graft (STSG) consisting of biologically active tissue can be used to expedite granulation.2

Technique
Following tumor clearance on the scalp (Figure 1), wide undermining is performed and 3-0 polyglactin 910 epidermal pulley sutures are placed to partially close the defect. A cadaveric STSG is placed over the remaining exposed periosteum and secured under the pulley sutures (Figure 2). The cadaveric STSG is replaced at 1-week intervals. At 4 weeks, sutures typically are removed. The cadaveric STSG is used until the exposed periosteum is fully granulated and the surgeon decides that granulation arrest is unlikely. The wound then heals by unassisted granulation. This approach provides an excellent final cosmetic outcome while avoiding extensive reconstruction (Figure 3).

Practice Implications
Scalp defects requiring closure are common for dermatologic surgeons. Several techniques to promote tissue granulation in defects that involve exposed periosteum have been reported, including (1) creation of small holes with a scalpel or chisel to access cortical circulation and (2) using laser modalities to stimulate granulation (eg, an erbium:YAG or CO2 laser).3,4 Although direct comparative studies are needed, the cadaveric STSG provides an approach that increases tissue granulation but does not require more invasive techniques or equipment.

Autologous STSGs need a wound bed and can fail with an exposed periosteum. Furthermore, an autologous STSG that survives may leave an unsightly, hypopigmented, depressed defect. When a defect involves the periosteum and a primary closure or flap is not ideal, a skin substitute may be an option.
Skin substitutes, including cadaveric STSG, generally are classified as bioengineered skin equivalents, amniotic tissue, or cadaveric bioproducts (Table). Unlike autologous grafts, these skin substitutes can provide rapid coverage of the defect and do not require a highly vascularized wound bed.6 They also minimize the inflammatory response and potentially improve the final cosmetic outcome by improving granulation rather than immediate STSG closure creating a step-off in deep wounds.6
Cadaveric STSGs also have been used in nonhealing ulcerations; diabetic foot ulcers; and ulcerations in which muscle, tendon, or bone are exposed, demonstrating induction of wound healing with superior scar quality and skin function.2,7,8 The utility of the cadaveric STSG is further highlighted by its potential to reduce costs9 compared to bioengineered skin substitutes, though considerable variability exists in pricing (Table).

Consider using a cadaveric STSG with a guiding closure in cases in which there is concern for delayed or absent tissue granulation or when monitoring for recurrence is essential.
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
- Jibbe A, Tolkachjov SN. An efficient single-layer suture technique for large scalp flaps. J Am Acad Dermatol. 2020;83:E395-E396. doi:10.1016/j.jaad.2019.07.062
- Mosti G, Mattaliano V, Magliaro A, et al. Cadaveric skin grafts may greatly increase the healing rate of recalcitrant ulcers when used both alone and in combination with split-thickness skin grafts. Dermatol Surg. 2020;46:169-179. doi:10.1097/dss.0000000000001990
- Valesky EM, Vogl T, Kaufmann R, et al. Trepanation or complete removal of the outer table of the calvarium for granulation induction: the erbium:YAG laser as an alternative to the rose head burr. Dermatology. 2015;230:276-281. doi:10.1159/000368749
- Drosou A, Trieu D, Goldberg LH. Scalpel-made holes on exposed scalp bone to promote second intention healing. J Am Acad Dermatol. 2014;71:387-388. doi:10.1016/j.jaad.2014.04.020
- Centers for Medicare & Medicaid Services. April 2023 ASP Pricing. Accessed August 25, 2023. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/asp-pricing-files
- Shores JT, Gabriel A, Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20(9 Pt 1):493-508. doi:10.1097/01.ASW.0000288217.83128.f3
- Li X, Meng X, Wang X, et al. Human acellular dermal matrix allograft: a randomized, controlled human trial for the long-term evaluation of patients with extensive burns. Burns. 2015;41:689-699. doi:10.1016/j.burns.2014.12.007
- Juhasz I, Kiss B, Lukacs L, et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract. 2010;2010:210150. doi:10.1155/2010/210150
- Towler MA, Rush EW, Richardson MK, et al. Randomized, prospective, blinded-enrollment, head-to-head venous leg ulcer healing trial comparing living, bioengineered skin graft substitute (Apligraf) with living, cryopreserved, human skin allograft (TheraSkin). Clin Podiatr Med Surg. 2018;35:357-365. doi:10.1016/j.cpm.2018.02.006
Treatment of Angiosarcoma of the Head and Neck: A Systematic Review
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51
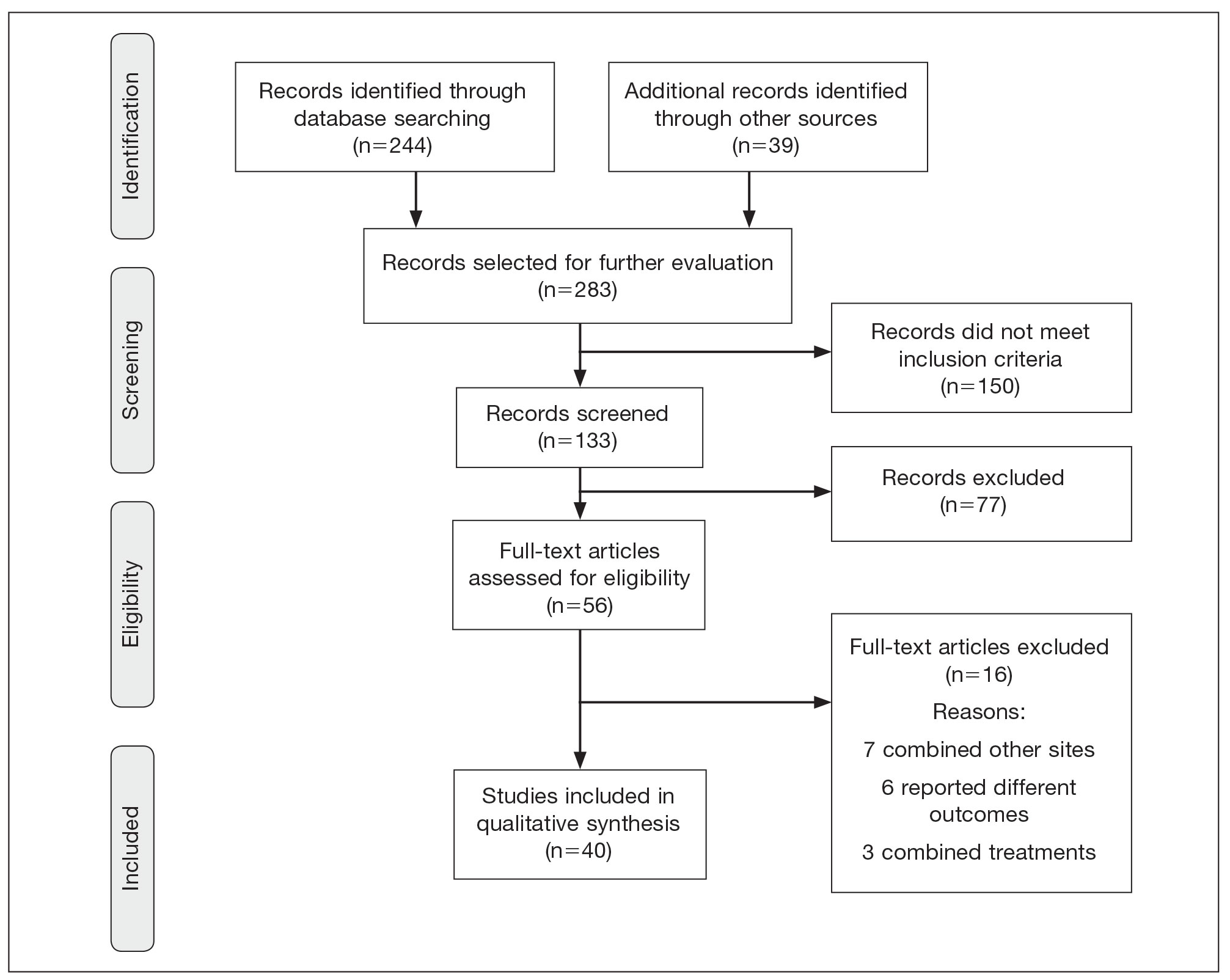
Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).
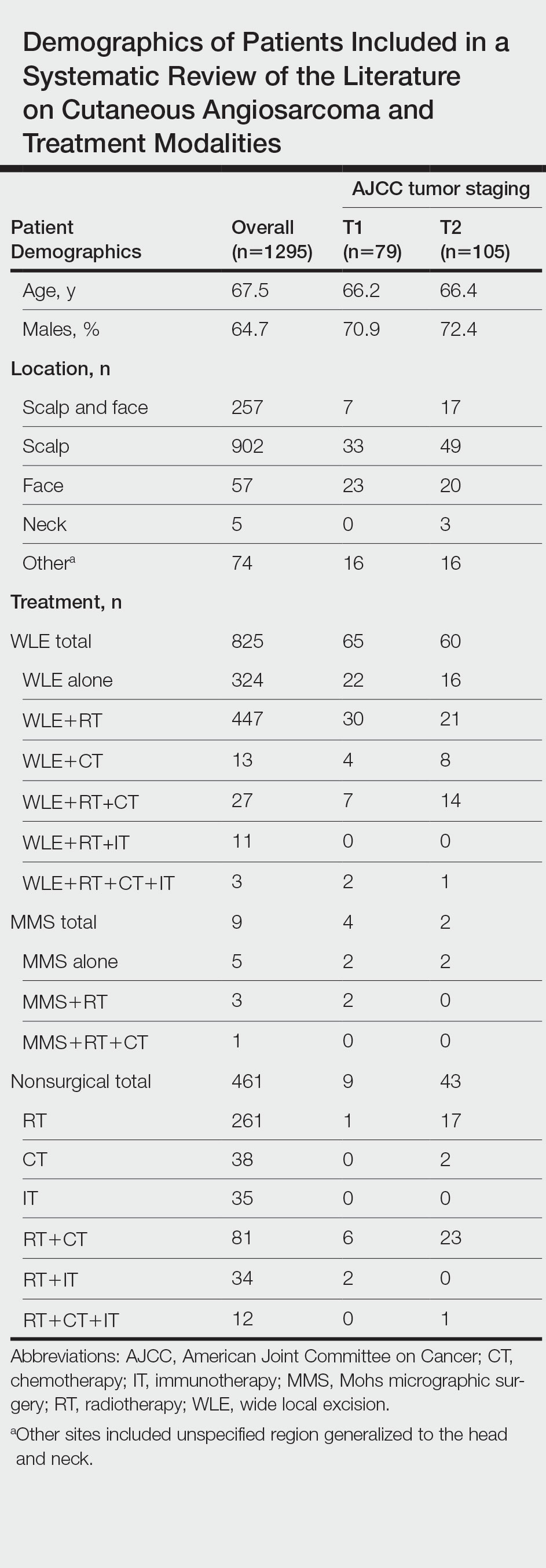
Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).
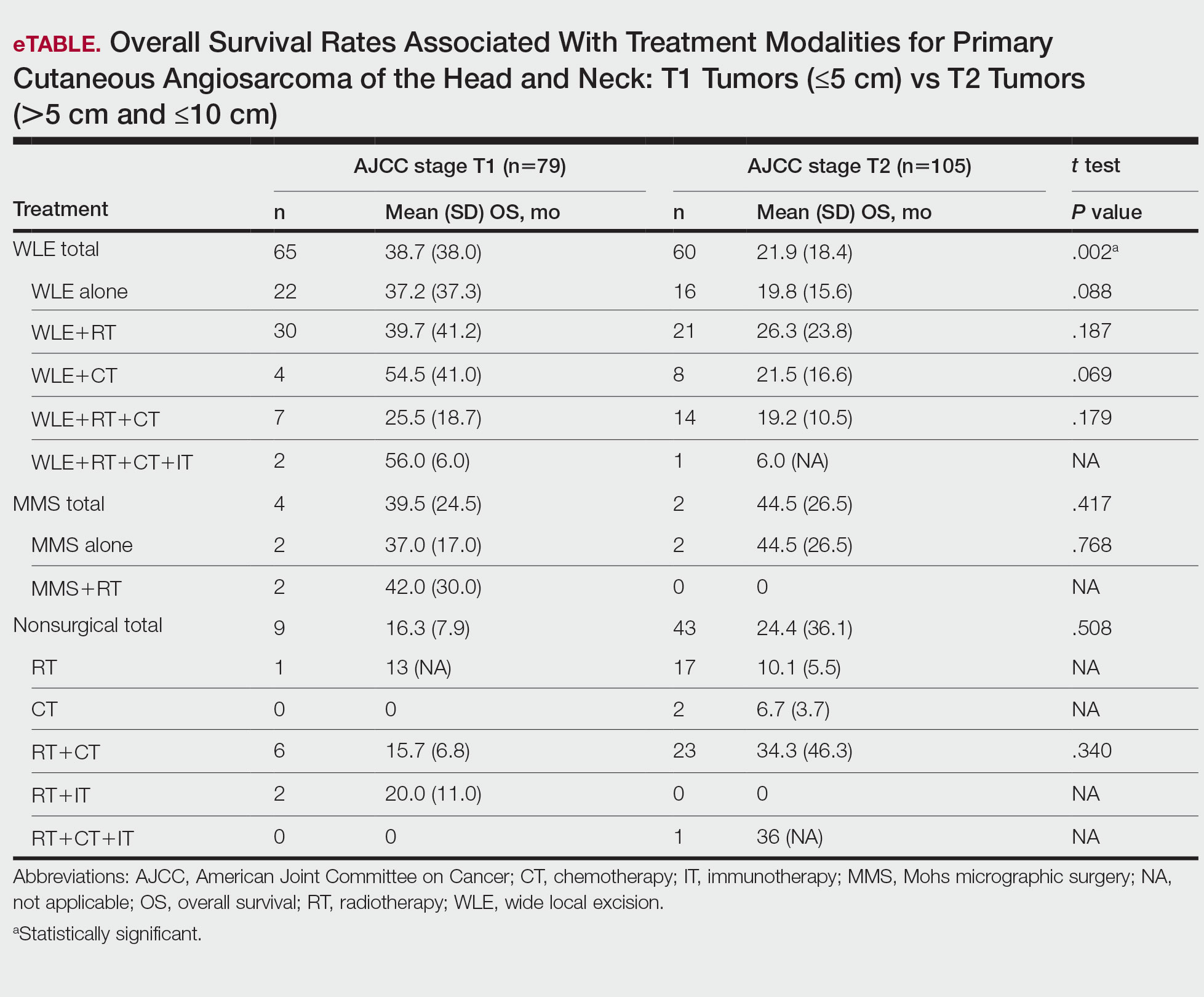
T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51

Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).

Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).

T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
Cutaneous angiosarcoma (cAS) is a rare malignancy arising from vascular or lymphatic tissue. It classically presents during the sixth or seventh decades of life as a raised purple papule or plaque on the head and neck areas.1 Primary cAS frequently mimics benign conditions, leading to delays in care. Such delays coupled with the aggressive nature of angiosarcomas leads to a poor prognosis. Five-year survival rates range from 11% to 50%, and more than half of patients die within 1 year of diagnosis.2-7
Currently, there is no consensus on the most effective treatments, as the rare nature of cAS has made the development of controlled clinical trials difficult. Wide local excision (WLE) is most frequently employed; however, the tumor’s infiltrative growth makes complete resection and negative surgical margins difficult to achieve.8 Recently, Mohs micrographic surgery (MMS) has been postulated as a treatment option. The tissue-sparing nature and intraoperative margin control of MMS may provide tumor eradication and cosmesis benefits reported with other cutaneous malignancies.9
Nearly all localized cASs are treated with surgical excision with or without adjuvant treatment modalities; however, it is unclear which of these modalities provide a survival benefit. We conducted a systematic review of the literature to compare treatment modalities for localized cAS of the head and neck regions and to compare treatments based on tumor stage.
METHODS
A literature search was performed to identify published studies indexed by MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, and PubMed from January 1, 1977, to May 8, 2020, reporting on cAS and treatment modalities used. The search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.5 Data extracted included patient demographics, tumor characteristics (including T1 [≤5 cm] and T2 [>5 cm and ≤10 cm] based on the American Joint Committee on Cancer soft tissue sarcoma staging criteria), treatments used, follow-up time, overall survival (OS) rates, and complications.10,11
Studies were required to (1) include participants with head and neck cAS; (2) report original patient data following cAS treatment with surgical (WLE or MMS) and/or nonsurgical modalities (chemotherapy [CT], radiotherapy [RT], immunotherapy [IT]); (3) report outcome data related to OS rates following treatment; and (4) have articles published in English. Given the rare nature of cAS, there was no limitation on the number of participants needed.
The Newcastle-Ottawa scale for observational studies was used to assess the quality of studies.12 Higher scores indicate low risk of bias, while lower scores represent high risk of bias.
Continuous data were reported with means and SDs, while categorical variables were reported as percentages. Overall survival means and SDs were compared between treatment modalities using an independent sample t test with P<.05 considered statistically significant. Due to the heterogeneity of the data, a meta-analysis was not reported.
RESULTS
Literature Search and Risk of Bias Assessment
There were 283 manuscripts identified, 56 articles read in full, and 40 articles included in the review (Figure). Among the 16 studies not meeting inclusion criteria, 7 did not provide enough data to isolate head and neck cAS cases,1,13-18 6 did not report outcomes related to the current review,19-24 and 3 did not provide enough data to isolate different treatment outcomes.25-27 Among the included studies, 32 reported use of WLE: WLE alone (n=21)2,7,11,28-45; WLE with RT (n=24)2,3,11,28-31,33-36,38-41,43-51; WLE with CT (n=7)2,31,35,39,41,48,52; WLE with RT and CT (n=11)2,29,31,33-35,39,40,48,52,53; WLE with RT and IT (n=3)35,54,55; and WLE with RT, CT, and IT (n=1).53 Nine studies reported MMS: MMS alone (n=5)39,56-59; MMS with RT (n=3)32,50,60,61; and MMS with RT and CT (n=1).51

Risk of bias assessment identified low risk in 3 articles. High risk was identified in 5 case reports,57-61 and 1 study did not describe patient selection.43 Clayton et al56 showed intermediate risk, given the study controlled for 1 factor.
Patient Demographics
A total of 1295 patients were included. The pooled mean age of the patients was 67.5 years (range, 3–88 years), and 64.7% were male. There were 79 cases identified as T1 and 105 as T2. A total of 825 cases were treated using WLE with or without adjuvant therapy, while a total of 9 cases were treated using MMS with and without adjuvant therapies (Table). There were 461 cases treated without surgical excision: RT alone (n=261), CT alone (n=38), IT alone (n=35), RT with CT (n=81), RT with IT (n=34), and RT with CT and IT (n=12)(Table). The median follow-up period across all studies was 23.5 months (range, 1–228 months).

Comparison Between Surgical and Nonsurgical Modalities
Wide Local Excision—Wide local excision (n=825; 63.7%) alone or in combination with other therapies was the most frequently used treatment modality. The mean (SD) OS was longest for WLE with RT, CT, and IT (n=3; 39.3 [24.1]), followed by WLE with RT (n=447; 35.9 [34.3] months), WLE with CT (n=13; 32.4 [30.2] months), WLE alone (n=324; 29.6 [34.1] months), WLE with RT and IT (n=11; 23.5 [4.9] months), and WLE with RT and CT (n=27; 20.7 [13.1] months).
Nonsurgical Modalities—Nonsurgical methods were used less frequently than surgical methods (n=461; 35.6%). The mean (SD) OS time in descending order was as follows: RT with CT and IT (n=12; 34.9 [1.2] months), RT with CT (n=81; 30.4 [37.8] months), IT alone (n=35; 25.7 [no SD reported] months), RT with IT (n=34; 20.5 [8.6] months), CT alone (n=38; 20.1 [15.9] months), and RT alone (n=261; 12.8 [8.3] months).
When comparing mean (SD) OS outcomes between surgical and nonsurgical treatment modalities, only the addition of WLE to RT significantly increased OS when compared with RT alone (WLE, 35.9 [34.3] months; RT alone, 12.8 [8.3] months; P=.001). When WLE was added to CT or both RT and CT, there was no significant difference with OS when compared with CT alone (WLE with CT, 32.4 [30.2] months; CT alone, 20.1 [15.9] months; P=.065); or both RT and CT in combination (WLE with RT and CT, 20.7 [13.1] months; RT and CT, 30.4 [37.8] months; P=.204).
Comparison Between T1 and T2 cAS
T1 Angiosarcoma—There were 79 patients identified as having T1 tumors across 16 studies.2,31,32,34,39-41,46,48-50,53,58-60,62 The mean (SD) OS was longest for WLE with RT, CT, and IT (n=2; 56.0 [6.0] months), followed by WLE with CT (n=4; 54.5 [41.0] months); WLE with RT (n=30; 39.7 [41.2] months); WLE alone (n=22; 37.2 [37.3] months); WLE with both RT and CT (n=7; 25.5 [18.7] months); RT with IT (n=2; 20.0 [11.0] months); RT with CT (n=6; 15.7 [6.8] months); and RT alone (n=1; 13 [no SD]) months)(eTable).

T2 Angiosarcoma—There were 105 patients with T2 tumors in 15 studies.2,31,32,34,39-41,46,48-50,52,53,57,62 The mean (SD) OS for each treatment modality in descending order was as follows: RT with CT and IT (n=1; 36 [no SD reported] months); RT with CT (n=23; 34.3 [46.3] months); WLE with RT (n=21; 26.3 [23.8] months); WLE with CT (n=8; 21.5 [16.6] months); WLE alone (n=16; 19.8 [15.6] months); WLE with RT and CT (n=14; 19.2 [10.5] months); RT alone (n=17; 10.1 [5.5] months); CT alone (n=2; 6.7 [3.7] months); and WLE with RT, CT, and IT (n=1; 6.0 [no SD] months)(eTable).
Mohs Micrographic Surgery—The use of MMS was only identified in case reports or small observational studies for a total of 9 patients. Five cASs were treated with MMS alone for a mean (SD) OS of 37 (21.5) months, with 4 reporting cAS staging: 2 were T158,59 (mean [SD] OS, 37.0 [17.0] months) and 2 were T2 tumors39,57 (mean [SD] OS, 44.5 [26.5] months). Mohs micrographic surgery with RT was used for 3 tumors (mean [SD] OS, 34.0 [26.9] months); 2 were T150,60 (mean [SD] OS, 42.0 [30.0] months) and 1 unreported staging (eTable).56 Mohs micrographic surgery with both RT and CT was used in 1 patient (unreported staging; OS, 82 months).51
Complications
Complications were rare and mainly associated with CT and RT. Four studies reported radiation dermatitis with RT.53,55,62,63 Two studies reported peripheral neuropathy and myelotoxicity with CT.35,51 Only 1 study reported poor wound healing due to surgical complications.29
COMMENT
Cutaneous angiosarcomas are rare and have limited treatment guidelines. Surgical excision does appear to be an effective adjunct to nonsurgical treatments, particularly WLE combined with RT, CT, and IT. Although MMS ultimately may be useful for cAS, the limited number and substantial heterogeneity of reported cases precludes definitive conclusions at this time.
Achieving margin control during WLE is associated with higher OS when treating angiosarcoma,36,46 which is particularly true for T1 tumors where margin control is imperative, and many cases are treated with a combination of WLE and RT. Overall survival times are lower for T2 tumors, as these tumors are larger and most likely have spread; therefore, more aggressive combination treatments were more prevalent. In these cases, complete margin control may be difficult to achieve and may not be as critical to the outcome if another form of adjuvant therapy can be administered promptly.24,64
When surgery is contraindicated, RT with or without CT was the most commonly reported treatment modality. However, these treatments were notably less effective than when used in combination with surgical resection. The use of RT alone has a recurrence rate reported up to 100% in certain studies, suggesting the need to utilize RT in combination with other modalities.23,39 It is important to note that RT often is used as monotherapy in palliative treatment, which may indirectly skew survival rates.2
Limitations of the study include a lack of randomized controlled trials. Most reports were retrospective reviews or case series, and tumor staging was sparsely reported. Finally, although MMS may provide utility in the treatment of cAS, the sample size of 9 precluded definitive conclusions from being formed about its efficacy.
CONCLUSION
Cutaneous angiosarcoma is rare and has limited data comparing different treatment modalities. The paucity of data currently limits definitive recommendations; however, both surgical and nonsurgical modalities have demonstrated potential efficacy in the treatment of cAS and may benefit from additional research. Clinicians should consider a multidisciplinary approach for patients with a diagnosis of cAS to tailor treatments on a case-by-case basis.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
- Rodríguez-Jiménez P, Jimenez YD, Reolid A, et al. State of the art of Mohs surgery for rare cutaneous tumors in the Spanish Registry of Mohs Surgery (REGESMOHS). Int J Dermatol. 2020;59:321-325.
- Alqumber NA, Choi JW, Kang MK. The management and prognosis of facial and scalp angiosarcoma: a retrospective analysis of 15 patients. Ann Plast Surg. 2019;83:55-62.
- Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Deyrup AT, McKenney JK, Tighiouart M, et al. Sporadic cutaneous angiosarcomas: a proposal for risk stratification based on 69 cases. Am J Surg Pathol. 2008;32:72-77.
- Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683-697.
- Harbour P, Song DH. The skin and subcutaneous tissue. In: Brunicardi FC, Andersen DK, Billiar TR, et al, eds. Schwartz’s Principles of Surgery. 11th ed. McGraw-Hill Education; 2019. Accessed April 24, 2023. https://accesssurgery.mhmedical.com/content.aspx?bookid=2576§ionid=216206374
- Oashi K, Namikawa K, Tsutsumida A, et al. Surgery with curative intent is associated with prolonged survival in patients with cutaneous angiosarcoma of the scalp and face—a retrospective study of 38 untreated cases in the Japanese population. Eur J Surg Oncol. 2018;44:823-829.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271.
- Amin M, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. Springer; 2017.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90:1067-1076.
- Lee BL, Chen CF, Chen PC, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: a retrospective study. Ann Plast Surg. 2017;78(3 suppl 2):S41-S46.
- Shen CJ, Parzuchowski AS, Kummerlowe MN, et al. Combined modality therapy improves overall survival for angiosarcoma. Acta Oncol. 2017;56:1235-1238.
- Breakey RW, Crowley TP, Anderson IB, et al. The surgical management of head and neck sarcoma: the Newcastle experience. J Plast Reconstr Aesthet Surg. 2017;70:78-84.
- Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg. 2014;208:254-259.
- Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52:1032-1040.
- Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer. 1995;75:989-996.
- DeMartelaere SL, Roberts D, Burgess MA, et al. Neoadjuvant chemotherapy-specific and overall treatment outcomes in patients with cutaneous angiosarcoma of the face with periorbital involvement. Head Neck. 2008;30:639-646.
- Ward JR, Feigenberg SJ, Mendenhall NP, et al. Radiation therapy for angiosarcoma. Head Neck. 2003;25:873-878.
- Letsa I, Benson C, Al-Muderis O, et al. Angiosarcoma of the face and scalp: effective systemic treatment in the older patient. J Geriatr Oncol. 2014;5:276-280.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg. 2015;141:335-340.
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck. 2011;33:661-667.
- Zhang Y, Yan Y, Zhu M, et al. Clinical outcomes in primary scalp angiosarcoma. Oncol Lett. 2019;18:5091-5096.
- Kamo R, Ishii M. Histological differentiation, histogenesis and prognosis of cutaneous angiosarcoma. Osaka City Med J. 2011;57:31-44.
- Ito T, Uchi H, Nakahara T, et al. Cutaneous angiosarcoma of the head and face: a single-center analysis of treatment outcomes in 43 patients in Japan. J Cancer Res Clin Oncol. 2016;142:1387-1394.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Buschmann A, Lehnhardt M, Toman N, et al. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61:399-403.
- Cassidy RJ, Switchenko JM, Yushak ML, et al. The importance of surgery in scalp angiosarcomas. Surg Oncol. 2018;27:A3-A8.
- Choi JH, Ahn KC, Chang H, et al. Surgical treatment and prognosis of angiosarcoma of the scalp: a retrospective analysis of 14 patients in a single institution. Biomed Res Int. 2015;2015:321896.
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J. 2018;24:25-31.
- Donghi D, Kerl K, Dummer R, et al. Cutaneous angiosarcoma: own experience over 13 years. clinical features, disease course and immunohistochemical profile. J Eur Acad Dermatol Venereol. 2010;24:1230-1234.
- Ferrari A, Casanova M, Bisogno G, et al. Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol. 2002;39:109-114.
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat. 2014;25:419-423.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Lim SY, Pyon JK, Mun GH, et al. Surgical treatment of angiosarcoma of the scalp with superficial parotidectomy. Ann Plast Surg. 2010;64:180-182.
- Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907-1921.
- Mark RJ, Tran LM, Sercarz J, et al. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993;119:973-978.
- Morgan MB, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015;19:191-195.
- Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients. Br J Radiol. 2012;85:E1127-E1133.
- Panje WR, Moran WJ, Bostwick DG, et al. Angiosarcoma of the head and neck: review of 11 cases. Laryngoscope. 1986;96:1381-1384.
- Perez MC, Padhya TA, Messina JL, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol. 2013;20:3391-3397.
- Veness M, Cooper S. Treatment of cutaneous angiosarcomas of the head and neck. Australas Radiol. 1995;39:277-281.
- Barttelbort SW, Stahl R, Ariyan S. Cutaneous angiosarcoma of the face and scalp. Plast Reconstr Surg. 1989;84:55-59.
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck. 2017;39:1205-1211.
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139:519-524.
- Morales PH, Lindberg RD, Barkley HT Jr. Soft tissue angiosarcomas. Int J Radiat Oncol Biol Phys. 1981;7:1655-1659.
- Wollina U, Hansel G, Schönlebe J, et al. Cutaneous angiosarcoma is a rare aggressive malignant vascular tumour of the skin. J Eur Acad Dermatol Venereol. 2011;25:964-968.
- Wollina U, Koch A, Hansel G, et al. A 10-year analysis of cutaneous mesenchymal tumors (sarcomas and related entities) in a skin cancer center. Int J Dermatol. 2013;52:1189-1197.
- Bien E, Stachowicz-Stencel T, Balcerska A, et al. Angiosarcoma in children - still uncontrollable oncological problem. The report of the Polish Paediatric Rare Tumours Study. Eur J Cancer Care (Engl). 2009;18:411-420.
- Suzuki G, Yamazaki H, Takenaka H, et al. Definitive radiation therapy for angiosarcoma of the face and scalp. In Vivo. 2016;30:921-926.
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol. 2013;86:20130439.
- Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1446-1453.
- Clayton BD, Leshin B, Hitchcock MG, et al. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26:671-678.
- Goldberg DJ, Kim YA. Angiosarcoma of the scalp treated with Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:156-158.
- Mikhail GR, Kelly AP Jr. Malignant angioendothelioma of the face. J Dermatol Surg Oncol. 1977;3:181-183.
- Muscarella VA. Angiosarcoma treated by Mohs micrographic surgery. J Dermatol Surg Oncol. 1993;19:1132-1133.
- Bullen R, Larson PO, Landeck AE, et al. Angiosarcoma of the head and neck managed by a combination of multiple biopsies to determine tumor margin and radiation therapy. report of three cases and review of the literature. Dermatol Surg. 1998;24:1105-1110.
- Wiwatwongwana D, White VA, Dolman PJ. Two cases of periocular cutaneous angiosarcoma. Ophthalmic Plast Reconstr Surg. 2010;26:365-366.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76:319-327.
- Hata M, Wada H, Ogino I, et al. Radiation therapy for angiosarcoma of the scalp: treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899-904.
- Hwang K, Kim MY, Lee SH. Recommendations for therapeutic decisions of angiosarcoma of the scalp and face. J Craniofac Surg. 2015;26:E253-E256.
Practice Points
- Angiosarcoma is a rare tumor that is difficult to treat, with multiple treatment options being utilized.
- Within this systematic review, wide local excision (WLE) combined with radiotherapy (RT), chemotherapy, and immunotherapy, as well as Mohs micrographic surgery (MMS), offered the longest mean (SD) overall survival time.
- When clinicians are tasked with treating primary cutaneous angiosarcoma of the head and neck, they should consider MMS or WLE combined with RT.
Erythematous Indurated Nodule on the Forehead
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.
Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1
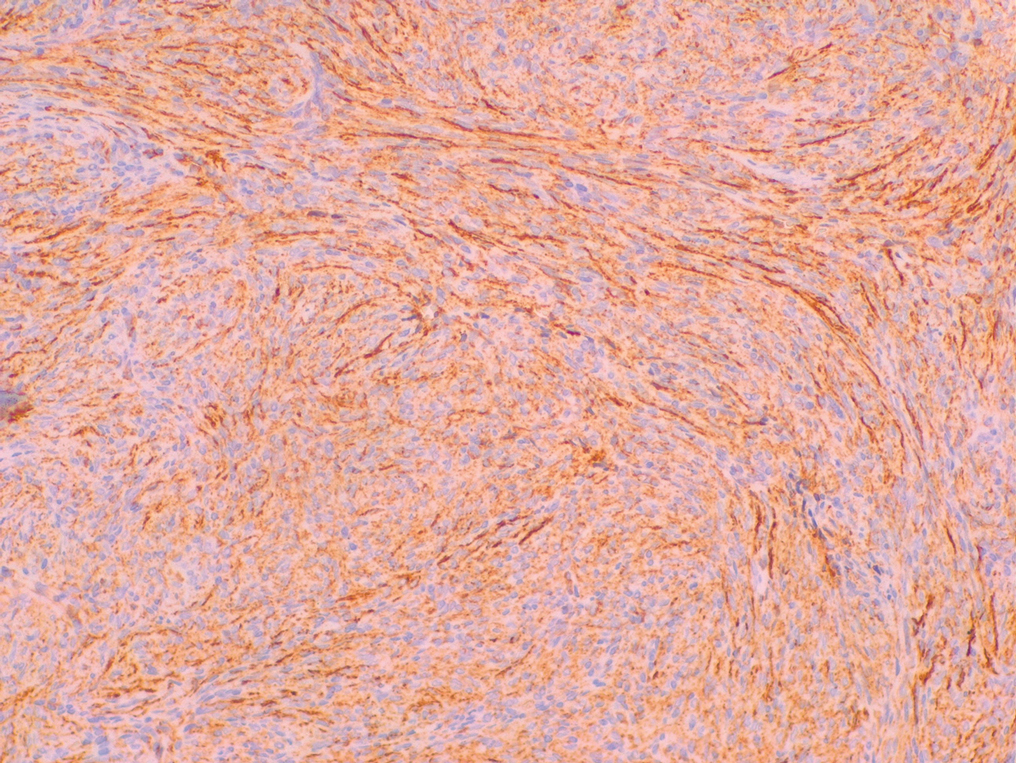
A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.

Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1

A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
The Diagnosis: Dermatofibrosarcoma Protuberans
Histopathologic examination showed a dermal tumor composed of spindle cells in a storiform arrangement (Figure 1). Immunohistochemistry demonstrated positive CD34 staining of the tumoral cells (Figure 2). Clinical review, histopathologic examination, and immunohistochemistry confirmed a diagnosis of dermatofibrosarcoma protuberans (DFSP). The patient underwent Mohs micrographic surgery (MMS) with clear margins after 3 stages, followed by repair with a rotation flap. No evidence of recurrence was found at 4-year follow-up.

Dermatofibrosarcoma protuberans is a rare low-grade sarcoma of fibroblast origin with an annual incidence of 0.8 to 5 cases per million individuals.1 It typically presents in patients aged 30 to 50 years on the trunk, scalp, or proximal extremities as an asymptomatic, flesh-colored, erythematous or brown, indurated plaque or nodule.2 Due to its variable presentation, these lesions often may be misdiagnosed as lipomas or epidermoid cysts, preventing proper targeted treatment. Therefore, suspicious enlarging indurated nodules require a lower threshold for biopsy.1

A definitive diagnosis of DFSP is achieved after a biopsy and histopathologic evaluation. Hematoxylin and eosin staining typically shows diffuse infiltration of the dermis and the subcutaneous fat by densely packed, cytologic, relatively uniform, spindle-shaped tumor cells arranged in a characteristic storiform shape. Tumor cells are spread along the septae of the subcutaneous fatty tissue.3 Immunohistochemistry is characterized by positive CD34 and negative factor XIIIa, with rare exceptions.
The differential diagnosis includes lipoma, epidermoid cyst, plexiform fibrohistiocytic tumor, and malignant peripheral nerve sheath tumor.3 Positive CD34 immunostaining, negative S-100 staining, and a storiform pattern of spindle cells can assist in differentiating DFSP from these possible differential diagnoses; lesions of these other entities are characterized by different pathologic findings. Lipomas are composed of fat tissue, epidermoid cysts have epithelial-lined cysts filled with keratin, plexiform fibrohistiocytic tumors have plexiform rays of fibrous tissue extending into fat with negative CD34 staining, and malignant peripheral nerve sheath tumors have fleshy variegated masses involving the peripheral nerve trunks with partial S-100 staining.4-7 Additional evaluation to confirm DFSP can be accomplished by analysis of tumor samples by fluorescence in situ hybridization or reverse transcriptase–polymerase chain reaction to detect chromosomal translocations and fusion gene transcripts, as chromosomal translocations may be found in more than 90% of cases.3
Early diagnosis of DFSP is beneficial, as it can help prevent recurrence as well as metastasis. Studies have attempted to document the risk for recurrence as well as metastasis based on characteristic features and treatment strategies of DFSP. In a study of 186 patients, 3 had metastatic disease to the lungs, the most common site of metastasis.8 These 3 patients had fibrosarcomatous transformation within DFSP, emphasizing the importance of detailing this finding early in the diagnosis, as it was characterized by a higher degree of cellularity, cytologic atypia, mitotic activity, and negative CD34 immunostaining.9 In patients with suspected metastasis, lymph node ultrasonography, chest radiography, and computed tomography may be utilized.3
When treating DFSP, the goal is complete removal of the tumor with clear margins. Mohs micrographic surgery, modified MMS, and wide local excision (WLE) with 2- to 4-cm margins are appropriate treatment options, though MMS is the treatment of choice. A study comparing MMS and WLE demonstrated 3% and 30.8% recurrence rates, respectively.8 In MMS, complete margin evaluation on microscopy is performed after each stage to ensure negative surgical margins. The presence of positive surgical margins elicits continued resection until the margins are clear.10,11
Other treatment modalities may be considered for patients with DFSP. Molecular therapy with imatinib, an oral tyrosine kinase inhibitor targeting platelet-derived growth factor–regulated expression, can be utilized for inoperable tumors; however, additional clinical trials are required to ensure efficacy.3 Surgical removal of the possible remaining tumor is still recommended after molecular therapy. Radiotherapy is an additional method of treatment that may be used for inoperable tumors.3
Dermatofibrosarcoma protuberans is a rare lowgrade sarcoma of fibroblast origin that typically does not metastasize but often has notable subclinical extension and recurrence. Differentiating DFSP from other tumors often may be difficult. A protuberant, flesh-colored, slowgrowing, and asymptomatic lesion often may be confused with lipomas or epidermoid cysts; therefore, biopsies with immunohistostaining for suspicious lesions is required.12 Mohs micrographic surgery has evolved as the treatment of choice for this tumor, though WLE and new targeted molecular therapies still are considered. Proper diagnosis and treatment of DFSP is paramount in preventing future morbidity.
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
- Benoit A, Aycock J, Milam D, et al. Dermatofibrosarcoma protuberans of the forehead with extensive subclinical spread. Dermatol Surg. 2016;42:261-264. doi:10.1097/DSS.0000000000000604
- Khachemoune A, Barkoe D, Braun M, et al. Dermatofibrosarcoma protuberans of the forehead and scalp with involvement of the outer calvarial plate: multistaged repair with the use of skin expanders. Dermatol Surg. 2005;31:115-119. doi:10.1111/j.1524-4725.2005.31021
- Saiag P, Grob J-J, Lebbe C, et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:2604-2608. doi:10.1016/j.ejca.2015.06.108
- Charifa A, Badri T. Lipomas, pathology. StatPearls. StatPearls Publishing; 2020.
- Zito PM, Scharf R. Cyst, epidermoid (sebaceous cyst). StatPearls. StatPearls Publishing; 2020.
- Taher A, Pushpanathan C. Plexiform fibrohistiocytic tumor: a brief review. Arch Pathol Lab Med. 2007;131:1135-1138. doi:10.5858 /2007-131-1135-PFTABR
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295-319. doi:10.1007 /s00401-012-0954-z
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106. doi:10.1097/DSS.0000000000000910
- Rouhani P, Fletcher CDM, Devesa SS, et al. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: an analysis of 12,114 cases. Cancer. 2008;113:616-627. doi:10.1002/cncr.23571
- Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37:600-613. doi:10.1016/s0190 -9622(97)70179-8
- Buck DW, Kim JYS, Alam M, et al. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2012;67:861-866. doi:10.1016/j.jaad.2012.01.039
- Shih P-Y, Chen C-H, Kuo T-T, et al. Deep dermatofibrosarcoma protuberans: a pitfall in the ultrasonographic diagnosis of lipoma -like subcutaneous lesions. Dermatologica Sinica. 2010;28:32-35. doi:10.1016/S1027-8117(10)60005-5
A 39-year-old man presented with an enlarging asymptomatic nodule on the forehead of more than 3 years’ duration. Physical examination revealed a 3.4×2.3-cm, indurated, firm, erythematous nodule on the frontotemporal scalp. The patient denied any history of trauma to the area.

Cutaneous Odontogenic Sinus: An Inflammatory Mimicker of Squamous Cell Carcinoma and Epidermal Cysts
Clinical Challenge
An
Practice Gap
It is estimated that half of patients with an extraoral fistula are treated with multiple dermatologic surgical operations, radiotherapy, antibiotic therapy, and chemotherapy before the correct diagnosis is made.1 Thus, proper identification of these lesions is crucial for prognosis and treatment. The most common locations for OCSTs are the mandibular, submandibular, and cervical skin.1,2 Given these locations, patients with OCSTs commonly present to the dermatology office for evaluation. Education regarding the clinical presentation, histopathology, and proper evaluation and further referral for treatment is essential for dermatologists.
Tools and Technique for Diagnosis
We present 2 patients with OCSTs who were referred for cutaneous surgery for an SCC and epidermal cyst, but the proper diagnosis was rendered after an index of suspicion and clinicopathologic correlation led to additional testing and eventual referral for imaging.
Patient 1
A 68-year-old woman presented for Mohs micrographic surgery (MMS) of a biopsy-proven SCC on the chin. The tumor cleared after 2 MMS stages (Figure 1A). Due to notable inflammation in each stage, the slides were sent to a pathologist who confirmed clear margins. Within 2 weeks of MMS, the wound began to dehisce (Figure 1B). The patient presented 4 months later with a crusted ulcerated nodule at the MMS site (Figure 1C). A biopsy showed likely recurrence of SCC. Upon presentation to the Mohs surgeon, the nodule felt fixed to the underlying jaw, and the patient was noted to have poor dentition. The patient was sent for computed tomography (CT), which showed focal thinning of the mandible, likely postsurgical, and clear maxillary sinuses. Due to the clinical appearance and anatomic location of the lesion, a request was made for a second read of the CT, specifically looking for an OCST at the prior surgical site. With this information, the radiologist noted an OCST extending from the mandible to the lesion, reported as a periapical lucency (representing a periapical abscess) at a mandibular tooth with a dental sinus draining into the soft tissues. The patient was started on antibiotics and referred to an oral surgeon for OCST excision.

Patient 2
A 62-year-old man presented with an inflamed subcutaneous nodule on the left anterior neck. A biopsy showed a ruptured cyst, and the patient was referred for excision. Clinical examination revealed a subcutaneous nodule fixed to the lower portion of the mandible (Figure 2A) that exhibited a rubbery retraction when pulled (Figure 2B). After a discussion about the atypical feel and appearance of this cyst, the patient preferred to undergo excision. During excision, the lesion felt deep and fixed with retraction (Figure 2C). With intraoperative re-evaluation of the clinical scenario and location, the patient was sent for CT. The initial read noted clear maxillary and ethmoid sinuses, with no mention of an OCST. After discussing the clinical history and suspicion specifically for an OCST with the radiologist, the re-read showed notable inflammation and decay of the tooth adjacent to the area of interest. An OCST was diagnosed, and the patient was sent to an oral surgeon for excision after antibiotics were prescribed.
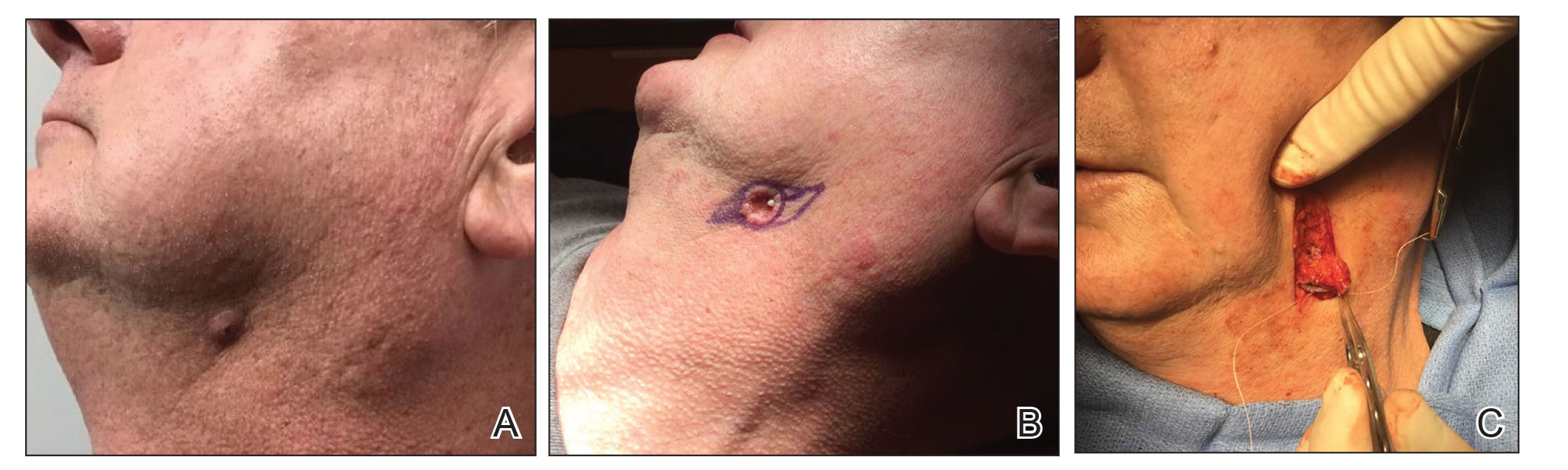
Practice Implications
Odontogenic cutaneous sinus tracts commonly are misdiagnosed due to variations in clinical presentations resembling more common cutaneous diagnoses, nonspecific histopathologic findings, and lack of dental symptoms or concerns about dentition. Clinically, an OCST presents as a fixed, red, crusty, nontender nodule with intermittent draining. With palpation of the involved area, the clinician may feel a cord of tissue connecting the skin lesion intraorally.2,4 A clinician should have a high index of suspicion for an OCST when evaluating fixed lesions of the lower face, jawline, and neck due to the possibility of a dental origin,1 which is important because an OCST can have similar clinical findings to lesions such as congenital fistulas, pustules, cysts, osteomyelitis, foreign-body granulomas, pyogenic granulomas, syphilis, metastatic carcinomas, basal cell carcinomas, and SCCs.2,4 A PubMed search of articles indexed for MEDLINE using the terms Mohs, MMS, chemosurgery, odontogenic sinus, odontogenic cutaneous sinus tract, and dental sinus yielded only 2 OCSTs that were referred for MMS in the last 30 years, both of which were in the nasolabial fold/medial malar cheek.2,4 Histopathologic findings of an OCST are nonspecific; a mixed or granulomatous inflammatory infiltrate, granulation tissue, and scarring can be seen.1 Pseudocarcinomatous/pseudoepitheliomatous hyperplasia of the epidermis can be seen and cause histologic misinterpretation for an SCC.2 Given that these findings are nonspecific without a clinical context, even with a histopathologic diagnosis of SCC or cyst, a clinical suspicion for an OCST should lead to an intraoral examination. Imaging can be ordered to look for an OCST in the area of interest. Although panoramic or periapical radiography with or without dental probes/radiopaque markers commonly have been used, more recent literature has suggested that CT may be superior to radiographs for making an OCST diagnosis.1,3 If imaging is not consistent with the clinically suspected OCST, we recommend directly contacting the radiologist to explain the clinical history and even refresh his/her suspicion for this diagnosis.
If a diagnosis of an OCST is made, oral antibiotics can be prescribed, though the use of antibiotics has been controversial. For severe odontogenic infections, typically beta-lactam antibiotics, cephalosporins, metronidazole, clindamycin, moxifloxacin, or erythromycin can be given for 7 days or until 3 days after symptoms have resolved.5 Although antibiotics can bring temporary resolution, it is imperative to treat the source of infection to prevent recurrence. It is crucial for these patients to be referred to an oral surgeon for evaluation and treatment of OCST by either a root canal or tooth extraction.
Final Thoughts
We present this pearl on the diagnosis and management of an OCST, also known as a dental sinus, to better assist clinicians in making this diagnosis. With an index of suspicion as well as intraoral and radiologic evaluations, a proper diagnosis may be rendered, potentially avoiding unnecessary cutaneous surgery. In addition, we highlight the importance of communication between the clinician and the radiologist to directly look for OCST in the area of concern and consider a re-read of the images when clinical suspicion does not correlate with the radiology report.
- Bai J, Ji AP, Huang MW. Submental cutaneous sinus tract of mandibular second molar origin. Int Endod J. 2014;47:1185-1191.
- Plast Reconstr Surg.
- Gregoire C. How are odontogenic infections best managed? J Can Dent Assoc. 2010;76:a37.
- Bodner L, Bar-Ziv J. Cutaneous sinus tract of dental origin—imaging with a dental CT software programme. Br J Oral Maxillofac Surg. 1998;36:311-313.
- Peermohamed S, Barber D, Kurwa H. Diagnostic challenges of cutaneous draining sinus tracts of odontogenic origin: a case report. Dermatol Surg. 2011;37:1525-1527.
Clinical Challenge
An
Practice Gap
It is estimated that half of patients with an extraoral fistula are treated with multiple dermatologic surgical operations, radiotherapy, antibiotic therapy, and chemotherapy before the correct diagnosis is made.1 Thus, proper identification of these lesions is crucial for prognosis and treatment. The most common locations for OCSTs are the mandibular, submandibular, and cervical skin.1,2 Given these locations, patients with OCSTs commonly present to the dermatology office for evaluation. Education regarding the clinical presentation, histopathology, and proper evaluation and further referral for treatment is essential for dermatologists.
Tools and Technique for Diagnosis
We present 2 patients with OCSTs who were referred for cutaneous surgery for an SCC and epidermal cyst, but the proper diagnosis was rendered after an index of suspicion and clinicopathologic correlation led to additional testing and eventual referral for imaging.
Patient 1
A 68-year-old woman presented for Mohs micrographic surgery (MMS) of a biopsy-proven SCC on the chin. The tumor cleared after 2 MMS stages (Figure 1A). Due to notable inflammation in each stage, the slides were sent to a pathologist who confirmed clear margins. Within 2 weeks of MMS, the wound began to dehisce (Figure 1B). The patient presented 4 months later with a crusted ulcerated nodule at the MMS site (Figure 1C). A biopsy showed likely recurrence of SCC. Upon presentation to the Mohs surgeon, the nodule felt fixed to the underlying jaw, and the patient was noted to have poor dentition. The patient was sent for computed tomography (CT), which showed focal thinning of the mandible, likely postsurgical, and clear maxillary sinuses. Due to the clinical appearance and anatomic location of the lesion, a request was made for a second read of the CT, specifically looking for an OCST at the prior surgical site. With this information, the radiologist noted an OCST extending from the mandible to the lesion, reported as a periapical lucency (representing a periapical abscess) at a mandibular tooth with a dental sinus draining into the soft tissues. The patient was started on antibiotics and referred to an oral surgeon for OCST excision.

Patient 2
A 62-year-old man presented with an inflamed subcutaneous nodule on the left anterior neck. A biopsy showed a ruptured cyst, and the patient was referred for excision. Clinical examination revealed a subcutaneous nodule fixed to the lower portion of the mandible (Figure 2A) that exhibited a rubbery retraction when pulled (Figure 2B). After a discussion about the atypical feel and appearance of this cyst, the patient preferred to undergo excision. During excision, the lesion felt deep and fixed with retraction (Figure 2C). With intraoperative re-evaluation of the clinical scenario and location, the patient was sent for CT. The initial read noted clear maxillary and ethmoid sinuses, with no mention of an OCST. After discussing the clinical history and suspicion specifically for an OCST with the radiologist, the re-read showed notable inflammation and decay of the tooth adjacent to the area of interest. An OCST was diagnosed, and the patient was sent to an oral surgeon for excision after antibiotics were prescribed.

Practice Implications
Odontogenic cutaneous sinus tracts commonly are misdiagnosed due to variations in clinical presentations resembling more common cutaneous diagnoses, nonspecific histopathologic findings, and lack of dental symptoms or concerns about dentition. Clinically, an OCST presents as a fixed, red, crusty, nontender nodule with intermittent draining. With palpation of the involved area, the clinician may feel a cord of tissue connecting the skin lesion intraorally.2,4 A clinician should have a high index of suspicion for an OCST when evaluating fixed lesions of the lower face, jawline, and neck due to the possibility of a dental origin,1 which is important because an OCST can have similar clinical findings to lesions such as congenital fistulas, pustules, cysts, osteomyelitis, foreign-body granulomas, pyogenic granulomas, syphilis, metastatic carcinomas, basal cell carcinomas, and SCCs.2,4 A PubMed search of articles indexed for MEDLINE using the terms Mohs, MMS, chemosurgery, odontogenic sinus, odontogenic cutaneous sinus tract, and dental sinus yielded only 2 OCSTs that were referred for MMS in the last 30 years, both of which were in the nasolabial fold/medial malar cheek.2,4 Histopathologic findings of an OCST are nonspecific; a mixed or granulomatous inflammatory infiltrate, granulation tissue, and scarring can be seen.1 Pseudocarcinomatous/pseudoepitheliomatous hyperplasia of the epidermis can be seen and cause histologic misinterpretation for an SCC.2 Given that these findings are nonspecific without a clinical context, even with a histopathologic diagnosis of SCC or cyst, a clinical suspicion for an OCST should lead to an intraoral examination. Imaging can be ordered to look for an OCST in the area of interest. Although panoramic or periapical radiography with or without dental probes/radiopaque markers commonly have been used, more recent literature has suggested that CT may be superior to radiographs for making an OCST diagnosis.1,3 If imaging is not consistent with the clinically suspected OCST, we recommend directly contacting the radiologist to explain the clinical history and even refresh his/her suspicion for this diagnosis.
If a diagnosis of an OCST is made, oral antibiotics can be prescribed, though the use of antibiotics has been controversial. For severe odontogenic infections, typically beta-lactam antibiotics, cephalosporins, metronidazole, clindamycin, moxifloxacin, or erythromycin can be given for 7 days or until 3 days after symptoms have resolved.5 Although antibiotics can bring temporary resolution, it is imperative to treat the source of infection to prevent recurrence. It is crucial for these patients to be referred to an oral surgeon for evaluation and treatment of OCST by either a root canal or tooth extraction.
Final Thoughts
We present this pearl on the diagnosis and management of an OCST, also known as a dental sinus, to better assist clinicians in making this diagnosis. With an index of suspicion as well as intraoral and radiologic evaluations, a proper diagnosis may be rendered, potentially avoiding unnecessary cutaneous surgery. In addition, we highlight the importance of communication between the clinician and the radiologist to directly look for OCST in the area of concern and consider a re-read of the images when clinical suspicion does not correlate with the radiology report.
Clinical Challenge
An
Practice Gap
It is estimated that half of patients with an extraoral fistula are treated with multiple dermatologic surgical operations, radiotherapy, antibiotic therapy, and chemotherapy before the correct diagnosis is made.1 Thus, proper identification of these lesions is crucial for prognosis and treatment. The most common locations for OCSTs are the mandibular, submandibular, and cervical skin.1,2 Given these locations, patients with OCSTs commonly present to the dermatology office for evaluation. Education regarding the clinical presentation, histopathology, and proper evaluation and further referral for treatment is essential for dermatologists.
Tools and Technique for Diagnosis
We present 2 patients with OCSTs who were referred for cutaneous surgery for an SCC and epidermal cyst, but the proper diagnosis was rendered after an index of suspicion and clinicopathologic correlation led to additional testing and eventual referral for imaging.
Patient 1
A 68-year-old woman presented for Mohs micrographic surgery (MMS) of a biopsy-proven SCC on the chin. The tumor cleared after 2 MMS stages (Figure 1A). Due to notable inflammation in each stage, the slides were sent to a pathologist who confirmed clear margins. Within 2 weeks of MMS, the wound began to dehisce (Figure 1B). The patient presented 4 months later with a crusted ulcerated nodule at the MMS site (Figure 1C). A biopsy showed likely recurrence of SCC. Upon presentation to the Mohs surgeon, the nodule felt fixed to the underlying jaw, and the patient was noted to have poor dentition. The patient was sent for computed tomography (CT), which showed focal thinning of the mandible, likely postsurgical, and clear maxillary sinuses. Due to the clinical appearance and anatomic location of the lesion, a request was made for a second read of the CT, specifically looking for an OCST at the prior surgical site. With this information, the radiologist noted an OCST extending from the mandible to the lesion, reported as a periapical lucency (representing a periapical abscess) at a mandibular tooth with a dental sinus draining into the soft tissues. The patient was started on antibiotics and referred to an oral surgeon for OCST excision.

Patient 2
A 62-year-old man presented with an inflamed subcutaneous nodule on the left anterior neck. A biopsy showed a ruptured cyst, and the patient was referred for excision. Clinical examination revealed a subcutaneous nodule fixed to the lower portion of the mandible (Figure 2A) that exhibited a rubbery retraction when pulled (Figure 2B). After a discussion about the atypical feel and appearance of this cyst, the patient preferred to undergo excision. During excision, the lesion felt deep and fixed with retraction (Figure 2C). With intraoperative re-evaluation of the clinical scenario and location, the patient was sent for CT. The initial read noted clear maxillary and ethmoid sinuses, with no mention of an OCST. After discussing the clinical history and suspicion specifically for an OCST with the radiologist, the re-read showed notable inflammation and decay of the tooth adjacent to the area of interest. An OCST was diagnosed, and the patient was sent to an oral surgeon for excision after antibiotics were prescribed.

Practice Implications
Odontogenic cutaneous sinus tracts commonly are misdiagnosed due to variations in clinical presentations resembling more common cutaneous diagnoses, nonspecific histopathologic findings, and lack of dental symptoms or concerns about dentition. Clinically, an OCST presents as a fixed, red, crusty, nontender nodule with intermittent draining. With palpation of the involved area, the clinician may feel a cord of tissue connecting the skin lesion intraorally.2,4 A clinician should have a high index of suspicion for an OCST when evaluating fixed lesions of the lower face, jawline, and neck due to the possibility of a dental origin,1 which is important because an OCST can have similar clinical findings to lesions such as congenital fistulas, pustules, cysts, osteomyelitis, foreign-body granulomas, pyogenic granulomas, syphilis, metastatic carcinomas, basal cell carcinomas, and SCCs.2,4 A PubMed search of articles indexed for MEDLINE using the terms Mohs, MMS, chemosurgery, odontogenic sinus, odontogenic cutaneous sinus tract, and dental sinus yielded only 2 OCSTs that were referred for MMS in the last 30 years, both of which were in the nasolabial fold/medial malar cheek.2,4 Histopathologic findings of an OCST are nonspecific; a mixed or granulomatous inflammatory infiltrate, granulation tissue, and scarring can be seen.1 Pseudocarcinomatous/pseudoepitheliomatous hyperplasia of the epidermis can be seen and cause histologic misinterpretation for an SCC.2 Given that these findings are nonspecific without a clinical context, even with a histopathologic diagnosis of SCC or cyst, a clinical suspicion for an OCST should lead to an intraoral examination. Imaging can be ordered to look for an OCST in the area of interest. Although panoramic or periapical radiography with or without dental probes/radiopaque markers commonly have been used, more recent literature has suggested that CT may be superior to radiographs for making an OCST diagnosis.1,3 If imaging is not consistent with the clinically suspected OCST, we recommend directly contacting the radiologist to explain the clinical history and even refresh his/her suspicion for this diagnosis.
If a diagnosis of an OCST is made, oral antibiotics can be prescribed, though the use of antibiotics has been controversial. For severe odontogenic infections, typically beta-lactam antibiotics, cephalosporins, metronidazole, clindamycin, moxifloxacin, or erythromycin can be given for 7 days or until 3 days after symptoms have resolved.5 Although antibiotics can bring temporary resolution, it is imperative to treat the source of infection to prevent recurrence. It is crucial for these patients to be referred to an oral surgeon for evaluation and treatment of OCST by either a root canal or tooth extraction.
Final Thoughts
We present this pearl on the diagnosis and management of an OCST, also known as a dental sinus, to better assist clinicians in making this diagnosis. With an index of suspicion as well as intraoral and radiologic evaluations, a proper diagnosis may be rendered, potentially avoiding unnecessary cutaneous surgery. In addition, we highlight the importance of communication between the clinician and the radiologist to directly look for OCST in the area of concern and consider a re-read of the images when clinical suspicion does not correlate with the radiology report.
- Bai J, Ji AP, Huang MW. Submental cutaneous sinus tract of mandibular second molar origin. Int Endod J. 2014;47:1185-1191.
- Plast Reconstr Surg.
- Gregoire C. How are odontogenic infections best managed? J Can Dent Assoc. 2010;76:a37.
- Bodner L, Bar-Ziv J. Cutaneous sinus tract of dental origin—imaging with a dental CT software programme. Br J Oral Maxillofac Surg. 1998;36:311-313.
- Peermohamed S, Barber D, Kurwa H. Diagnostic challenges of cutaneous draining sinus tracts of odontogenic origin: a case report. Dermatol Surg. 2011;37:1525-1527.
- Bai J, Ji AP, Huang MW. Submental cutaneous sinus tract of mandibular second molar origin. Int Endod J. 2014;47:1185-1191.
- Plast Reconstr Surg.
- Gregoire C. How are odontogenic infections best managed? J Can Dent Assoc. 2010;76:a37.
- Bodner L, Bar-Ziv J. Cutaneous sinus tract of dental origin—imaging with a dental CT software programme. Br J Oral Maxillofac Surg. 1998;36:311-313.
- Peermohamed S, Barber D, Kurwa H. Diagnostic challenges of cutaneous draining sinus tracts of odontogenic origin: a case report. Dermatol Surg. 2011;37:1525-1527.
Generalized pustular eruption
A 38-year-old man sought care in the emergency department for an acute, pruritic, generalized cutaneous eruption that manifested in the intertriginous areas of the inner thighs, antecubital fossae, and axilla (FIGURE 1A). He reported associated chills, a 15-pound weight gain, and swelling of his inner thighs. Two weeks before presentation, he had received azithromycin for an upper respiratory tract infection. He was unsure if the rash developed prior to or after taking the medication. He was not taking any other medications and had no history of skin conditions.
On examination, the patient was afebrile and had bilateral thigh edema. Skin examination revealed background erythema with morbilliform papules, plaques, and patches on the bilateral flanks, back, buttocks, arms, legs, and central neck. Pinpoint pustules were present in the intertriginous sites and on the low back and buttocks. The laboratory evaluation revealed leukocytosis (11.0 × 109 cells/L), increased levels of neutrophils and eosinophils, and an elevated C-reactive protein level (12.8 mg/L). The remaining laboratory results were unremarkable. The patient was referred to Dermatology.
An examination by the dermatologist 3 days later revealed small areas of annular desquamation with a few pinpoint pustules, mostly located on the inner thighs and buttocks (FIGURE 1B). Skin biopsies were taken from the anterior hip region. The histopathology revealed subacute dermatitis with mixed dermal inflammatory cells, including neutrophils and eosinophils, and discrete subcorneal spongiform pustules.
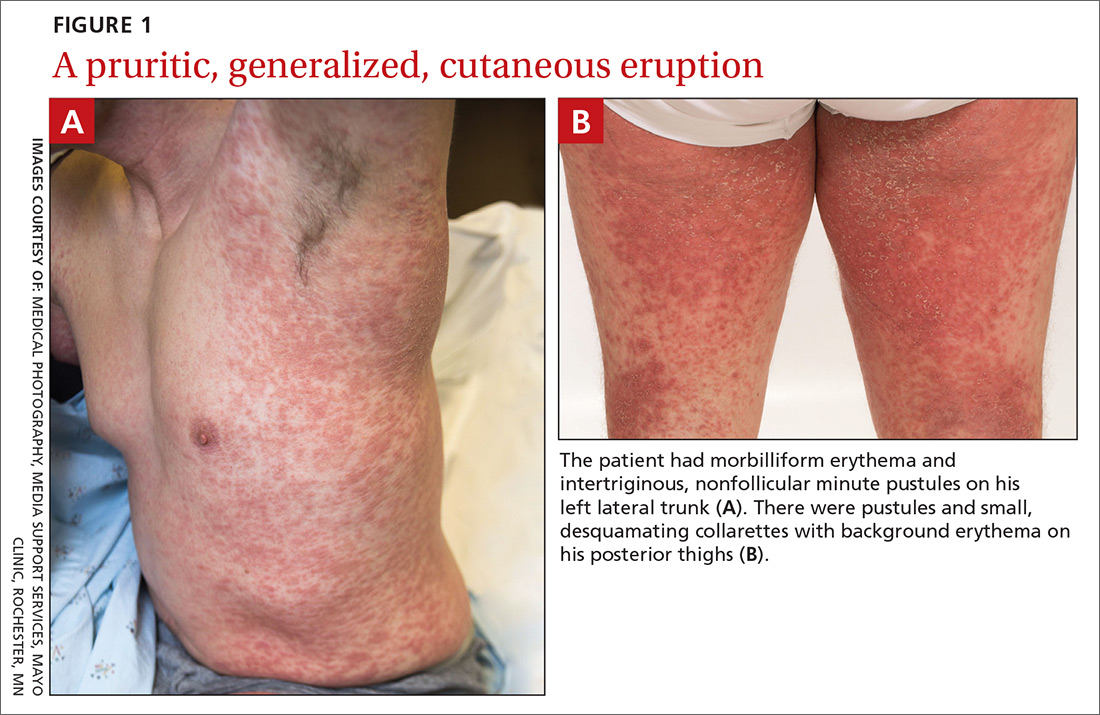
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute generalized exanthematous pustulosis (AGEP)
The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to an early diagnosis of AGEP, which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized.1,2 The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%); leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days.1 Although common, fever is not always documented in patients with AGEP. 3 (Our patient was a case in point.) While not a key characteristic of AGEP, our patient’s weight gain was likely explained by the severe edema secondary to his inflammatory skin eruption.
Medications are implicated, but pathophysiology is unknown
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis.1,4 In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days.5 In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs.6 A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men.7 While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.4,7
Distinguishing AGEP from some look-alikes
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include: drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome; Stevens-Johnson syndrome (SJS); toxic epidermal necrolysis (TEN); and pustular psoriasis.8-10 The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.4,8
DRESS occurs 2 to 6 weeks after drug exposure, rather than a few days, as is seen with AGEP. It often involves morbilliform erythema and facial edema with substantial eosinophilia and possible nephritis, pneumonitis, myocarditis, and thyroiditis.9 Unlike AGEP, DRESS does not have a predilection for intertriginous anatomic locations.
SJS and TEN occur 1 to 3 weeks after drug exposure. These conditions manifest with the development of bullae, atypical targetoid lesions, painful dusky erythema, epidermal necrosis, and mucosal involvement at multiple sites. Tubular nephritis, tracheobronchial necrosis, and multisystem organ failure can occur, with reported mortality rates of 5% to 35%.8,11
Pustular psoriasis is frequently confused with AGEP. However, AGEP usually develops fewer than 2 days after drug exposure, with pustules that begin in intertriginous sites, and there is associated neutrophilia and possible organ involvement.1,8 Patients who have AGEP typically do not have a history of psoriasis, while patients with pustular psoriasis often do.7 A history of drug reaction is uncommon with pustular psoriasis (although rapid tapering of systemic corticosteroids in patients with psoriasis can trigger the development of pustular psoriasis), whereas a previous history of drug reaction is common in AGEP.3,7
Discontinue medication, treat with corticosteroids
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated.6 A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.3
Our patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared, and only minimal residual erythema was noted (FIGURE 2). The patient was instructed to avoid azithromycin.
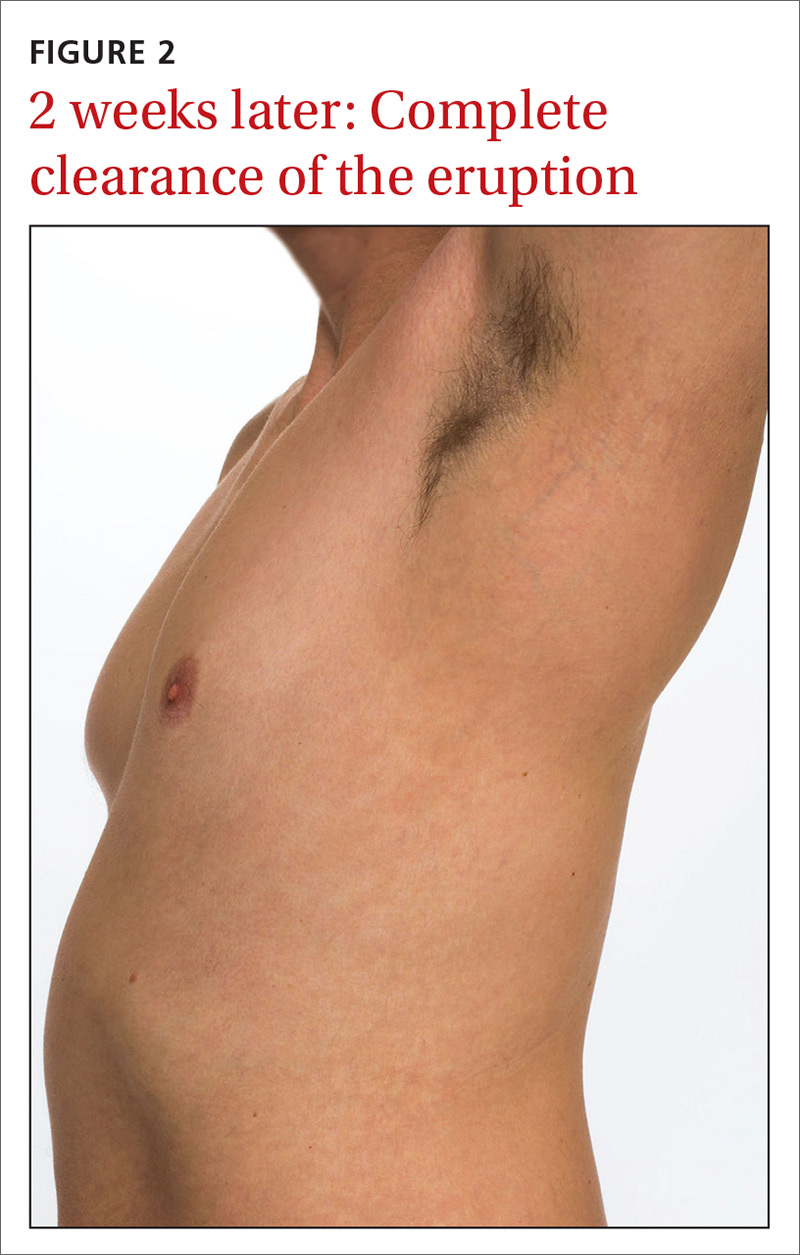
CORRESPONDENCE
David A. Wetter, MD, Department of Dermatology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected].
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010;49:507-512.
3. Alniemi DT, Wetter DA, Bridges AG, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996-2013. Int J Dermatol. 2017;56:405-414.
4. Bouvresse S, Valeyrie-Allanore L, Ortonne N, et al. Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist? Orphanet J Rare Dis. 2012;7:72.
5. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
6. Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
7. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
8. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
9. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
10. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92-96.
11. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994;102:28S-30S.
A 38-year-old man sought care in the emergency department for an acute, pruritic, generalized cutaneous eruption that manifested in the intertriginous areas of the inner thighs, antecubital fossae, and axilla (FIGURE 1A). He reported associated chills, a 15-pound weight gain, and swelling of his inner thighs. Two weeks before presentation, he had received azithromycin for an upper respiratory tract infection. He was unsure if the rash developed prior to or after taking the medication. He was not taking any other medications and had no history of skin conditions.
On examination, the patient was afebrile and had bilateral thigh edema. Skin examination revealed background erythema with morbilliform papules, plaques, and patches on the bilateral flanks, back, buttocks, arms, legs, and central neck. Pinpoint pustules were present in the intertriginous sites and on the low back and buttocks. The laboratory evaluation revealed leukocytosis (11.0 × 109 cells/L), increased levels of neutrophils and eosinophils, and an elevated C-reactive protein level (12.8 mg/L). The remaining laboratory results were unremarkable. The patient was referred to Dermatology.
An examination by the dermatologist 3 days later revealed small areas of annular desquamation with a few pinpoint pustules, mostly located on the inner thighs and buttocks (FIGURE 1B). Skin biopsies were taken from the anterior hip region. The histopathology revealed subacute dermatitis with mixed dermal inflammatory cells, including neutrophils and eosinophils, and discrete subcorneal spongiform pustules.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute generalized exanthematous pustulosis (AGEP)
The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to an early diagnosis of AGEP, which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized.1,2 The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%); leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days.1 Although common, fever is not always documented in patients with AGEP. 3 (Our patient was a case in point.) While not a key characteristic of AGEP, our patient’s weight gain was likely explained by the severe edema secondary to his inflammatory skin eruption.
Medications are implicated, but pathophysiology is unknown
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis.1,4 In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days.5 In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs.6 A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men.7 While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.4,7
Distinguishing AGEP from some look-alikes
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include: drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome; Stevens-Johnson syndrome (SJS); toxic epidermal necrolysis (TEN); and pustular psoriasis.8-10 The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.4,8
DRESS occurs 2 to 6 weeks after drug exposure, rather than a few days, as is seen with AGEP. It often involves morbilliform erythema and facial edema with substantial eosinophilia and possible nephritis, pneumonitis, myocarditis, and thyroiditis.9 Unlike AGEP, DRESS does not have a predilection for intertriginous anatomic locations.
SJS and TEN occur 1 to 3 weeks after drug exposure. These conditions manifest with the development of bullae, atypical targetoid lesions, painful dusky erythema, epidermal necrosis, and mucosal involvement at multiple sites. Tubular nephritis, tracheobronchial necrosis, and multisystem organ failure can occur, with reported mortality rates of 5% to 35%.8,11
Pustular psoriasis is frequently confused with AGEP. However, AGEP usually develops fewer than 2 days after drug exposure, with pustules that begin in intertriginous sites, and there is associated neutrophilia and possible organ involvement.1,8 Patients who have AGEP typically do not have a history of psoriasis, while patients with pustular psoriasis often do.7 A history of drug reaction is uncommon with pustular psoriasis (although rapid tapering of systemic corticosteroids in patients with psoriasis can trigger the development of pustular psoriasis), whereas a previous history of drug reaction is common in AGEP.3,7
Discontinue medication, treat with corticosteroids
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated.6 A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.3
Our patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared, and only minimal residual erythema was noted (FIGURE 2). The patient was instructed to avoid azithromycin.

CORRESPONDENCE
David A. Wetter, MD, Department of Dermatology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected].
A 38-year-old man sought care in the emergency department for an acute, pruritic, generalized cutaneous eruption that manifested in the intertriginous areas of the inner thighs, antecubital fossae, and axilla (FIGURE 1A). He reported associated chills, a 15-pound weight gain, and swelling of his inner thighs. Two weeks before presentation, he had received azithromycin for an upper respiratory tract infection. He was unsure if the rash developed prior to or after taking the medication. He was not taking any other medications and had no history of skin conditions.
On examination, the patient was afebrile and had bilateral thigh edema. Skin examination revealed background erythema with morbilliform papules, plaques, and patches on the bilateral flanks, back, buttocks, arms, legs, and central neck. Pinpoint pustules were present in the intertriginous sites and on the low back and buttocks. The laboratory evaluation revealed leukocytosis (11.0 × 109 cells/L), increased levels of neutrophils and eosinophils, and an elevated C-reactive protein level (12.8 mg/L). The remaining laboratory results were unremarkable. The patient was referred to Dermatology.
An examination by the dermatologist 3 days later revealed small areas of annular desquamation with a few pinpoint pustules, mostly located on the inner thighs and buttocks (FIGURE 1B). Skin biopsies were taken from the anterior hip region. The histopathology revealed subacute dermatitis with mixed dermal inflammatory cells, including neutrophils and eosinophils, and discrete subcorneal spongiform pustules.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute generalized exanthematous pustulosis (AGEP)
The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to an early diagnosis of AGEP, which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized.1,2 The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%); leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days.1 Although common, fever is not always documented in patients with AGEP. 3 (Our patient was a case in point.) While not a key characteristic of AGEP, our patient’s weight gain was likely explained by the severe edema secondary to his inflammatory skin eruption.
Medications are implicated, but pathophysiology is unknown
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis.1,4 In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days.5 In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs.6 A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men.7 While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.4,7
Distinguishing AGEP from some look-alikes
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include: drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome; Stevens-Johnson syndrome (SJS); toxic epidermal necrolysis (TEN); and pustular psoriasis.8-10 The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.4,8
DRESS occurs 2 to 6 weeks after drug exposure, rather than a few days, as is seen with AGEP. It often involves morbilliform erythema and facial edema with substantial eosinophilia and possible nephritis, pneumonitis, myocarditis, and thyroiditis.9 Unlike AGEP, DRESS does not have a predilection for intertriginous anatomic locations.
SJS and TEN occur 1 to 3 weeks after drug exposure. These conditions manifest with the development of bullae, atypical targetoid lesions, painful dusky erythema, epidermal necrosis, and mucosal involvement at multiple sites. Tubular nephritis, tracheobronchial necrosis, and multisystem organ failure can occur, with reported mortality rates of 5% to 35%.8,11
Pustular psoriasis is frequently confused with AGEP. However, AGEP usually develops fewer than 2 days after drug exposure, with pustules that begin in intertriginous sites, and there is associated neutrophilia and possible organ involvement.1,8 Patients who have AGEP typically do not have a history of psoriasis, while patients with pustular psoriasis often do.7 A history of drug reaction is uncommon with pustular psoriasis (although rapid tapering of systemic corticosteroids in patients with psoriasis can trigger the development of pustular psoriasis), whereas a previous history of drug reaction is common in AGEP.3,7
Discontinue medication, treat with corticosteroids
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated.6 A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.3
Our patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared, and only minimal residual erythema was noted (FIGURE 2). The patient was instructed to avoid azithromycin.

CORRESPONDENCE
David A. Wetter, MD, Department of Dermatology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; [email protected].
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010;49:507-512.
3. Alniemi DT, Wetter DA, Bridges AG, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996-2013. Int J Dermatol. 2017;56:405-414.
4. Bouvresse S, Valeyrie-Allanore L, Ortonne N, et al. Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist? Orphanet J Rare Dis. 2012;7:72.
5. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
6. Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
7. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
8. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
9. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
10. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92-96.
11. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994;102:28S-30S.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010;49:507-512.
3. Alniemi DT, Wetter DA, Bridges AG, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996-2013. Int J Dermatol. 2017;56:405-414.
4. Bouvresse S, Valeyrie-Allanore L, Ortonne N, et al. Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist? Orphanet J Rare Dis. 2012;7:72.
5. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
6. Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
7. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
8. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
9. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
10. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92-96.
11. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994;102:28S-30S.