User login
Treat-to-Target Outcomes With Tapinarof Cream 1% in Phase 3 Trials for Plaque Psoriasis
Psoriasis is a chronic inflammatory disease affecting approximately 8 million adults in the United States and 2% of the global population.1,2 Psoriasis causes pain, itching, and disfigurement and is associated with a physical, psychological, and economic burden that substantially affects health-related quality of life.3-5
Setting treatment goals and treating to target are evidence-based approaches that have been successfully applied to several chronic diseases to improve patient outcomes, including diabetes, hypertension, and rheumatoid arthritis.6-9 Treat-to-target strategies generally set low disease activity (or remission) as an overall goal and seek to achieve this using available therapeutic options as necessary. Introduced following the availability of biologics and targeted systemic therapies, treat-to-target strategies generally provide guidance on expectations of treatment but not specific treatments, as personalized treatment decisions depend on an assessment of individual patients and consider clinical and demographic features as well as preferences for available therapeutic options. If targets are not achieved in the assigned time span, adjustments can be made to the treatment approach in close consultation with the patient. If the target is reached, follow-up visits can be scheduled to ensure improvement is maintained or to establish if more aggressive goals could be selected.
Treat-to-target strategies for the management of psoriasis developed by the National Psoriasis Foundation (NPF) Medical Board include reducing the extent of psoriasis to 1% or lower total body surface area (BSA) after 3 months of treatment.10 Treatment targets endorsed by the European Academy of Dermatology and Venereology (EADV) in guidelines on the use of systemic therapies in psoriasis include achieving a 75% or greater reduction in Psoriasis Area and Severity Index (PASI) score within 3 to 4 months of treatment.11
In clinical practice, many patients do not achieve these treatment targets, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.12,13 Moreover, conventional topical treatments (eg, topical corticosteroids) used by most patients with psoriasis regardless of disease severity are associated with adverse events that can limit their use. Most topical corticosteroids have US Food and Drug Administration label restrictions relating to sites of application, duration and extent of use, and frequency of administration.14,15
Tapinarof cream 1% (VTAMA [Dermavant Sciences, Inc]) is a first-in-class topical nonsteroidal aryl hydrocarbon receptor agonist that was approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults16 and is being studied for the treatment of plaque psoriasis in children 2 years and older as well as for atopic dermatitis in adults and children 2 years and older. In PSOARING 1 (ClinicalTrials .gov identifier NCT03956355) and PSOARING 2 (NCT03983980)—identical 12-week pivotal phase 3 trials—monotherapy with tapinarof cream 1% once daily (QD) demonstrated statistically significant efficacy vs vehicle cream and was well tolerated in adults with mild to severe plaque psoriasis (Supplementary Figure S1).17 Lebwohl et al17 reported that significantly higher PASI75 responses were observed at week 12 with tapinarof cream vs vehicle in PSOARING 1 and PSOARING 2 (36% and 48% vs 10% and 7%, respectively; both P<.0001). A significantly higher PASI90 response of 19% and 21% at week 12 also was observed with tapinarof cream vs 2% and 3% with vehicle in PSOARING 1 and PSOARING 2, respectively (P=.0005 and P<.0001).17
In PSOARING 3 (NCT04053387)—the long-term extension trial (Supplementary Figure S1)—efficacy continued to improve or was maintained beyond the two 12-week trials, with improvements in total BSA affected and PASI scores for up to 52 weeks.18 Tapinarof cream 1% QD demonstrated positive, rapid, and durable outcomes in PSOARING 3, including high rates of complete disease clearance (Physician Global Assessment [PGA] score=0 [clear])(40.9% [312/763]), durability of response on treatment with no evidence of tachyphylaxis, and a remittive effect of approximately 4 months when off therapy (defined as maintenance of a PGA score of 0 [clear] or 1 [almost clear] after first achieving a PGA score of 0).18
Herein, we report absolute treatment targets for patients with plaque psoriasis who received tapinarof cream 1% QD in the PSOARING trials that are at least as stringent as the corresponding NPF and EADV targets of achieving a total BSA affected of 1% or lower or a PASI75 response within 3 to 4 months, respectively.
METHODS
Study Design
The pooled efficacy analyses included all patients with a baseline PGA score of 2 or higher (mild or worse) before treatment with tapinarof cream 1% QD in the PSOARING trials. This included patients who received tapinarof cream 1% in PSOARING 1 and PSOARING 2 who may or may not have continued into PSOARING 3, as well as those who received the vehicle in PSOARING 1 and PSOARING 2 who enrolled in PSOARING 3 and had a PGA score of 2 or higher before receiving tapinarof cream 1%.
Trial Participants
Full methods, including inclusion and exclusion criteria, for the PSOARING trials have been previously reported.17,18 Patients were aged 18 to 75 years and had chronic plaque psoriasis that was stable for at least 6 months before randomization; 3% to 20% total BSA affected (excluding the scalp, palms, fingernails, toenails, and soles); and a PGA score of 2 (mild), 3 (moderate), or 4 (severe) at baseline.
The clinical trials were conducted in compliance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Approval was obtained from local ethics committees or institutional review boards at each center. All patients provided written informed consent.
Trial Treatment
In PSOARING 1 and PSOARING 2, patients were randomized (2:1) to receive tapinarof cream 1% or vehicle QD for 12 weeks. In PSOARING 3 (the long-term extension trial), patients received up to 40 weeks of open-label tapinarof, followed by 4 weeks of follow-up off treatment. Patients received intermittent or continuous treatment with tapinarof cream 1% in PSOARING 3 based on PGA score: those entering the trial with a PGA score of 1 or higher received tapinarof cream 1% until complete disease clearance was achieved (defined as a PGA score of 0 [clear]). Those entering PSOARING 3 with or achieving a PGA score of 0 (clear) discontinued treatment and were observed for the duration of maintenance of a PGA score of 0 (clear) or 1 (almost clear) while off therapy (the protocol-defined “duration of remittive effect”). If disease worsening (defined as a PGA score 2 or higher) occurred, tapinarof cream 1% was restarted and continued until a PGA score of 0 (clear) was achieved. This pattern of treatment, discontinuation on achieving a PGA score of 0 (clear), and retreatment on disease worsening continued until the end of the trial. As a result, patients in PSOARING 3 could receive tapinarof cream 1% continuously or intermittently for 40 weeks.
Outcome Measures and Statistical Analyses
The assessment of total BSA affected by plaque psoriasis is an estimate of the total extent of disease as a percentage of total skin area. In the PSOARING trials, the skin surface of one hand (palm and digits) was assumed to be approximately equivalent to 1% BSA. The total BSA affected by psoriasis was evaluated from 0% to 100%, with greater total BSA affected being an indication of more extensive disease. The BSA efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved a 1% or lower or 0.5% or lower total BSA affected.
Psoriasis Area and Severity Index scores assess both the severity and extent of psoriasis. A PASI score lower than 5 often is considered indicative of mild psoriasis, a score of 5 to 10 indicates moderate disease, and a score higher than 10 indicates severe disease.19 The maximum PASI score is 72. The PASI efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved an absolute total PASI score of 3 or lower, 2 or lower, and 1 or lower.
Efficacy analyses were based on pooled data for all patients in the PSOARING trials who had a PGA score of 2 to 4 (mild to severe) before treatment with tapinarof cream 1% in the intention-to-treat population using observed cases. Time-to-target analyses were based on Kaplan-Meier (KM) estimates using observed cases.
Safety analyses included the incidence and frequency of adverse events and were based on all patients who received tapinarof cream 1% in the PSOARING trials.
RESULTS
Baseline Patient Demographics and Disease Characteristics
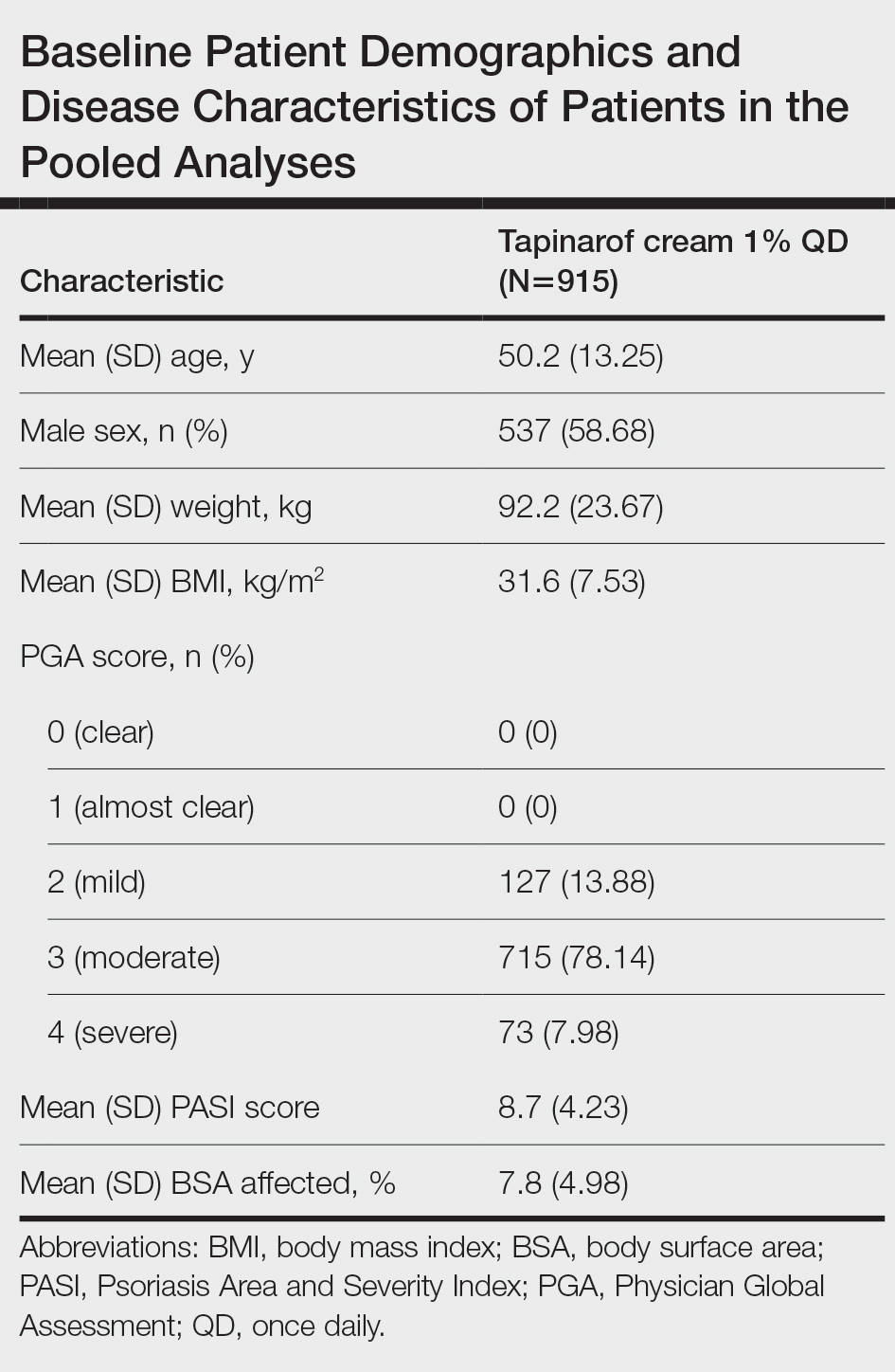
The pooled efficacy analyses included 915 eligible patients (Table). At baseline, the mean (SD) age was 50.2 (13.25) years, 58.7% were male, the mean (SD) weight was 92.2 (23.67) kg, and the mean (SD) body mass index was 31.6 (7.53) kg/m2. The percentage of patients with a PGA score of 2 (mild), 3 (moderate), or 4 (severe) was 13.9%, 78.1%, and 8.0%, respectively. The mean (SD) PASI score was 8.7 (4.23) and mean (SD) total BSA affected was 7.8% (4.98).
Efficacy
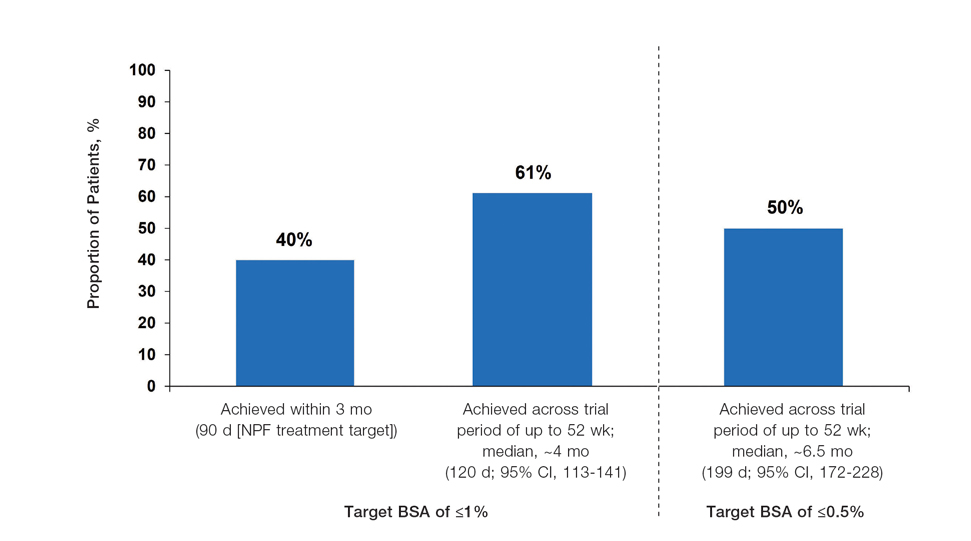
Achievement of BSA-Affected Targets—
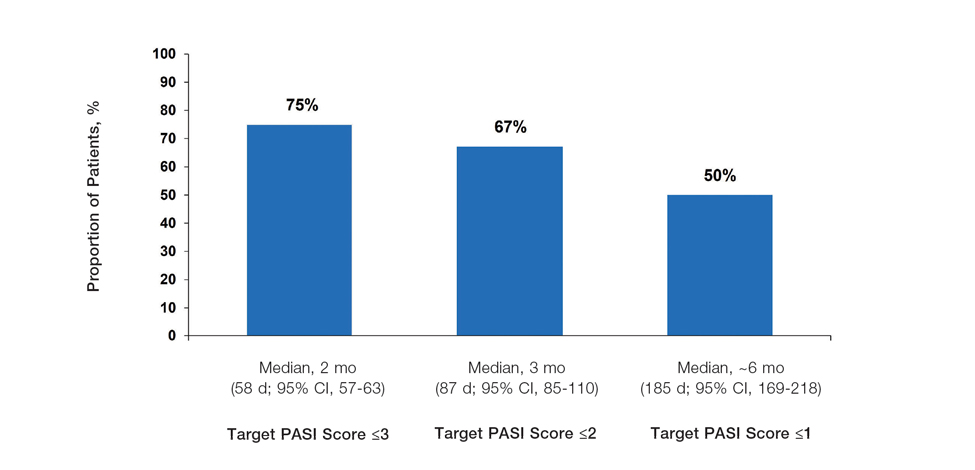
Achievement of Absolute PASI Targets—Across the total trial period (up to 52 weeks), an absolute total PASI score of 3 or lower was achieved by 75% of patients (686/915), with a median time to achieve this of 2 months (KM estimate: 58 days [95% CI, 57-63]); approximately 67% of patients (612/915) achieved a total PASI score of 2 or lower, with a median time to achieve of 3 months (KM estimate: 87 days [95% CI, 85-110])(Figure 2; Supplementary Figures S3a and S3b). A PASI score of 1 or lower was achieved by approximately 50% of patients (460/915), with a median time to achieve of approximately 6 months (KM estimate: 185 days [95% CI, 169-218])(Figure 2, Supplementary Figure S3c).
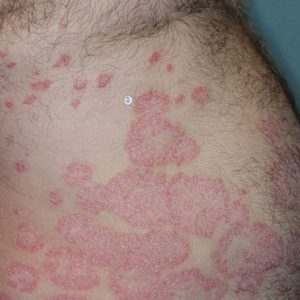
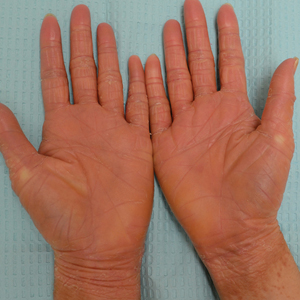
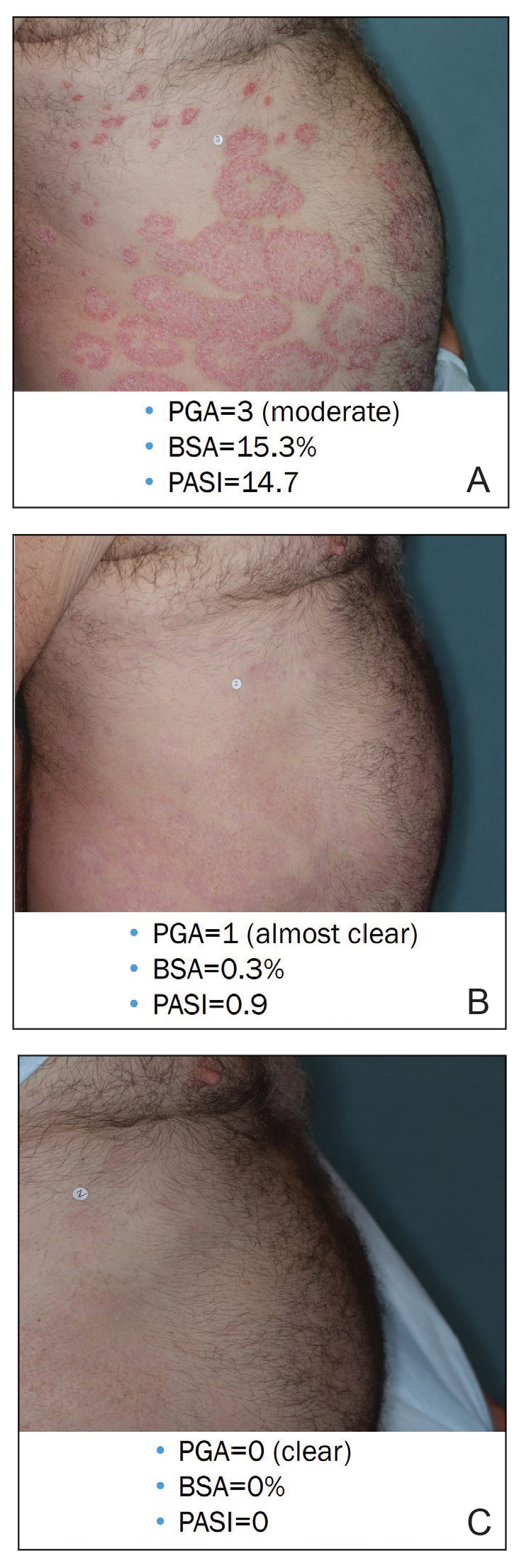
Illustrative Case—Case photography showing the clinical response in a 63-year-old man with moderate plaque psoriasis in PSOARING 2 is shown in Figure 3. After 12 weeks of treatment with tapinarof cream 1% QD, the patient achieved all primary and secondary efficacy end points. In addition to achieving the regulatory end point of a PGA score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points, achievement of 0% total BSA affected and a total PASI score of 0 at week 12 exceeded the NPF and EADV consensus treatment targets.10,11 Targets were achieved as early as week 4, with a total BSA affected of 0.5% or lower and a total PASI score of 1 or lower, illustrated by marked skin clearing and only faint residual erythema that completely resolved at week 12, with the absence of postinflammatory hyperpigmentation.
Safety
Safety data for the PSOARING trials have been previously reported.17,18 The most common treatment-emergent adverse events were folliculitis, contact dermatitis, upper respiratory tract infection, and nasopharyngitis. Treatment-emergent adverse events generally were mild or moderate in severity and did not lead to trial discontinuation.17,18
COMMENT
Treat-to-target management approaches aim to improve patient outcomes by striving to achieve optimal goals. The treat-to-target approach supports shared decision-making between clinicians and patients based on common expectations of what constitutes treatment success.
The findings of this analysis based on pooled data from a large cohort of patients demonstrate that a high proportion of patients can achieve or exceed recommended treatment targets with tapinarof cream 1% QD and maintain improvements long-term. The NPF-recommended treatment target of 1% or lower BSA affected within approximately 3 months (90 days) of treatment was achieved by 40% of tapinarof-treated patients. In addition, 1% or lower BSA affected at any time during the trials was achieved by 61% of patients (median, approximately 4 months). The analyses also indicated that PASI total scores of 3 or lower and 2 or lower were achieved by 75% and 67% of tapinarof-treated patients, respectively, within 2 to 3 months.
These findings support the previously reported efficacy of tapinarof cream, including high rates of complete disease clearance (40.9% [312/763]), durable response following treatment interruption, an off-therapy remittive effect of approximately 4 months, and good disease control on therapy with no evidence of tachyphylaxis.17,18
CONCLUSION
Taken together with previously reported tapinarof efficacy and safety results, our findings demonstrate that a high proportion of patients treated with tapinarof cream as monotherapy can achieve aggressive treatment targets set by both US and European guidelines developed for systemic and biologic therapies. Tapinarof cream 1% QD is an effective topical treatment option for patients with plaque psoriasis that has been approved without restrictions relating to severity or extent of disease treated, duration of use, or application sites, including application to sensitive and intertriginous skin.
Acknowledgments—Editorial and medical writing support under the guidance of the authors was provided by Melanie Govender, MSc (Med), ApotheCom (United Kingdom), and was funded by Dermavant Sciences, Inc, in accordance with Good Publication Practice (GPP) guidelines.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Pilon D, Teeple A, Zhdanava M, et al. The economic burden of psoriasis with high comorbidity among privately insured patients in the United States. J Med Econ. 2019;22:196-203.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425-440.e2.
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9:504-513.
- Ford JA, Solomon DH. Challenges in implementing treat-to-target strategies in rheumatology. Rheum Dis Clin North Am. 2019;45:101-112.
- Sitbon O, Galiè N. Treat-to-target strategies in pulmonary arterial hypertension: the importance of using multiple goals. Eur Respir Rev. 2010;19:272-278.
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631-637.
- Wangnoo SK, Sethi B, Sahay RK, et al. Treat-to-target trials in diabetes. Indian J Endocrinol Metab. 2014;18:166-174.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1-70.
- Strober BE, van der Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb). 2019; 9:5-18.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35 (2 Suppl 2):S36-S44; quiz S45.
- VTAMA® (tapinarof) cream. Prescribing information. Dermavant Sciences; 2022. Accessed September 13, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215272s000lbl.pdf
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229 and supplementary appendix.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Clinical Review Report: Guselkumab (Tremfya) [Internet]. Canadian Agency for Drugs and Technologies in Health; 2018. Accessed September 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534047/pdf/Bookshelf_NBK534047.pdf
Psoriasis is a chronic inflammatory disease affecting approximately 8 million adults in the United States and 2% of the global population.1,2 Psoriasis causes pain, itching, and disfigurement and is associated with a physical, psychological, and economic burden that substantially affects health-related quality of life.3-5
Setting treatment goals and treating to target are evidence-based approaches that have been successfully applied to several chronic diseases to improve patient outcomes, including diabetes, hypertension, and rheumatoid arthritis.6-9 Treat-to-target strategies generally set low disease activity (or remission) as an overall goal and seek to achieve this using available therapeutic options as necessary. Introduced following the availability of biologics and targeted systemic therapies, treat-to-target strategies generally provide guidance on expectations of treatment but not specific treatments, as personalized treatment decisions depend on an assessment of individual patients and consider clinical and demographic features as well as preferences for available therapeutic options. If targets are not achieved in the assigned time span, adjustments can be made to the treatment approach in close consultation with the patient. If the target is reached, follow-up visits can be scheduled to ensure improvement is maintained or to establish if more aggressive goals could be selected.
Treat-to-target strategies for the management of psoriasis developed by the National Psoriasis Foundation (NPF) Medical Board include reducing the extent of psoriasis to 1% or lower total body surface area (BSA) after 3 months of treatment.10 Treatment targets endorsed by the European Academy of Dermatology and Venereology (EADV) in guidelines on the use of systemic therapies in psoriasis include achieving a 75% or greater reduction in Psoriasis Area and Severity Index (PASI) score within 3 to 4 months of treatment.11
In clinical practice, many patients do not achieve these treatment targets, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.12,13 Moreover, conventional topical treatments (eg, topical corticosteroids) used by most patients with psoriasis regardless of disease severity are associated with adverse events that can limit their use. Most topical corticosteroids have US Food and Drug Administration label restrictions relating to sites of application, duration and extent of use, and frequency of administration.14,15
Tapinarof cream 1% (VTAMA [Dermavant Sciences, Inc]) is a first-in-class topical nonsteroidal aryl hydrocarbon receptor agonist that was approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults16 and is being studied for the treatment of plaque psoriasis in children 2 years and older as well as for atopic dermatitis in adults and children 2 years and older. In PSOARING 1 (ClinicalTrials .gov identifier NCT03956355) and PSOARING 2 (NCT03983980)—identical 12-week pivotal phase 3 trials—monotherapy with tapinarof cream 1% once daily (QD) demonstrated statistically significant efficacy vs vehicle cream and was well tolerated in adults with mild to severe plaque psoriasis (Supplementary Figure S1).17 Lebwohl et al17 reported that significantly higher PASI75 responses were observed at week 12 with tapinarof cream vs vehicle in PSOARING 1 and PSOARING 2 (36% and 48% vs 10% and 7%, respectively; both P<.0001). A significantly higher PASI90 response of 19% and 21% at week 12 also was observed with tapinarof cream vs 2% and 3% with vehicle in PSOARING 1 and PSOARING 2, respectively (P=.0005 and P<.0001).17
In PSOARING 3 (NCT04053387)—the long-term extension trial (Supplementary Figure S1)—efficacy continued to improve or was maintained beyond the two 12-week trials, with improvements in total BSA affected and PASI scores for up to 52 weeks.18 Tapinarof cream 1% QD demonstrated positive, rapid, and durable outcomes in PSOARING 3, including high rates of complete disease clearance (Physician Global Assessment [PGA] score=0 [clear])(40.9% [312/763]), durability of response on treatment with no evidence of tachyphylaxis, and a remittive effect of approximately 4 months when off therapy (defined as maintenance of a PGA score of 0 [clear] or 1 [almost clear] after first achieving a PGA score of 0).18
Herein, we report absolute treatment targets for patients with plaque psoriasis who received tapinarof cream 1% QD in the PSOARING trials that are at least as stringent as the corresponding NPF and EADV targets of achieving a total BSA affected of 1% or lower or a PASI75 response within 3 to 4 months, respectively.
METHODS
Study Design
The pooled efficacy analyses included all patients with a baseline PGA score of 2 or higher (mild or worse) before treatment with tapinarof cream 1% QD in the PSOARING trials. This included patients who received tapinarof cream 1% in PSOARING 1 and PSOARING 2 who may or may not have continued into PSOARING 3, as well as those who received the vehicle in PSOARING 1 and PSOARING 2 who enrolled in PSOARING 3 and had a PGA score of 2 or higher before receiving tapinarof cream 1%.
Trial Participants
Full methods, including inclusion and exclusion criteria, for the PSOARING trials have been previously reported.17,18 Patients were aged 18 to 75 years and had chronic plaque psoriasis that was stable for at least 6 months before randomization; 3% to 20% total BSA affected (excluding the scalp, palms, fingernails, toenails, and soles); and a PGA score of 2 (mild), 3 (moderate), or 4 (severe) at baseline.
The clinical trials were conducted in compliance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Approval was obtained from local ethics committees or institutional review boards at each center. All patients provided written informed consent.
Trial Treatment
In PSOARING 1 and PSOARING 2, patients were randomized (2:1) to receive tapinarof cream 1% or vehicle QD for 12 weeks. In PSOARING 3 (the long-term extension trial), patients received up to 40 weeks of open-label tapinarof, followed by 4 weeks of follow-up off treatment. Patients received intermittent or continuous treatment with tapinarof cream 1% in PSOARING 3 based on PGA score: those entering the trial with a PGA score of 1 or higher received tapinarof cream 1% until complete disease clearance was achieved (defined as a PGA score of 0 [clear]). Those entering PSOARING 3 with or achieving a PGA score of 0 (clear) discontinued treatment and were observed for the duration of maintenance of a PGA score of 0 (clear) or 1 (almost clear) while off therapy (the protocol-defined “duration of remittive effect”). If disease worsening (defined as a PGA score 2 or higher) occurred, tapinarof cream 1% was restarted and continued until a PGA score of 0 (clear) was achieved. This pattern of treatment, discontinuation on achieving a PGA score of 0 (clear), and retreatment on disease worsening continued until the end of the trial. As a result, patients in PSOARING 3 could receive tapinarof cream 1% continuously or intermittently for 40 weeks.
Outcome Measures and Statistical Analyses
The assessment of total BSA affected by plaque psoriasis is an estimate of the total extent of disease as a percentage of total skin area. In the PSOARING trials, the skin surface of one hand (palm and digits) was assumed to be approximately equivalent to 1% BSA. The total BSA affected by psoriasis was evaluated from 0% to 100%, with greater total BSA affected being an indication of more extensive disease. The BSA efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved a 1% or lower or 0.5% or lower total BSA affected.
Psoriasis Area and Severity Index scores assess both the severity and extent of psoriasis. A PASI score lower than 5 often is considered indicative of mild psoriasis, a score of 5 to 10 indicates moderate disease, and a score higher than 10 indicates severe disease.19 The maximum PASI score is 72. The PASI efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved an absolute total PASI score of 3 or lower, 2 or lower, and 1 or lower.
Efficacy analyses were based on pooled data for all patients in the PSOARING trials who had a PGA score of 2 to 4 (mild to severe) before treatment with tapinarof cream 1% in the intention-to-treat population using observed cases. Time-to-target analyses were based on Kaplan-Meier (KM) estimates using observed cases.
Safety analyses included the incidence and frequency of adverse events and were based on all patients who received tapinarof cream 1% in the PSOARING trials.
RESULTS
Baseline Patient Demographics and Disease Characteristics
The pooled efficacy analyses included 915 eligible patients (Table). At baseline, the mean (SD) age was 50.2 (13.25) years, 58.7% were male, the mean (SD) weight was 92.2 (23.67) kg, and the mean (SD) body mass index was 31.6 (7.53) kg/m2. The percentage of patients with a PGA score of 2 (mild), 3 (moderate), or 4 (severe) was 13.9%, 78.1%, and 8.0%, respectively. The mean (SD) PASI score was 8.7 (4.23) and mean (SD) total BSA affected was 7.8% (4.98).
Efficacy
Achievement of BSA-Affected Targets—



Achievement of Absolute PASI Targets—Across the total trial period (up to 52 weeks), an absolute total PASI score of 3 or lower was achieved by 75% of patients (686/915), with a median time to achieve this of 2 months (KM estimate: 58 days [95% CI, 57-63]); approximately 67% of patients (612/915) achieved a total PASI score of 2 or lower, with a median time to achieve of 3 months (KM estimate: 87 days [95% CI, 85-110])(Figure 2; Supplementary Figures S3a and S3b). A PASI score of 1 or lower was achieved by approximately 50% of patients (460/915), with a median time to achieve of approximately 6 months (KM estimate: 185 days [95% CI, 169-218])(Figure 2, Supplementary Figure S3c).
Illustrative Case—Case photography showing the clinical response in a 63-year-old man with moderate plaque psoriasis in PSOARING 2 is shown in Figure 3. After 12 weeks of treatment with tapinarof cream 1% QD, the patient achieved all primary and secondary efficacy end points. In addition to achieving the regulatory end point of a PGA score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points, achievement of 0% total BSA affected and a total PASI score of 0 at week 12 exceeded the NPF and EADV consensus treatment targets.10,11 Targets were achieved as early as week 4, with a total BSA affected of 0.5% or lower and a total PASI score of 1 or lower, illustrated by marked skin clearing and only faint residual erythema that completely resolved at week 12, with the absence of postinflammatory hyperpigmentation.
Safety
Safety data for the PSOARING trials have been previously reported.17,18 The most common treatment-emergent adverse events were folliculitis, contact dermatitis, upper respiratory tract infection, and nasopharyngitis. Treatment-emergent adverse events generally were mild or moderate in severity and did not lead to trial discontinuation.17,18

COMMENT
Treat-to-target management approaches aim to improve patient outcomes by striving to achieve optimal goals. The treat-to-target approach supports shared decision-making between clinicians and patients based on common expectations of what constitutes treatment success.
The findings of this analysis based on pooled data from a large cohort of patients demonstrate that a high proportion of patients can achieve or exceed recommended treatment targets with tapinarof cream 1% QD and maintain improvements long-term. The NPF-recommended treatment target of 1% or lower BSA affected within approximately 3 months (90 days) of treatment was achieved by 40% of tapinarof-treated patients. In addition, 1% or lower BSA affected at any time during the trials was achieved by 61% of patients (median, approximately 4 months). The analyses also indicated that PASI total scores of 3 or lower and 2 or lower were achieved by 75% and 67% of tapinarof-treated patients, respectively, within 2 to 3 months.
These findings support the previously reported efficacy of tapinarof cream, including high rates of complete disease clearance (40.9% [312/763]), durable response following treatment interruption, an off-therapy remittive effect of approximately 4 months, and good disease control on therapy with no evidence of tachyphylaxis.17,18
CONCLUSION
Taken together with previously reported tapinarof efficacy and safety results, our findings demonstrate that a high proportion of patients treated with tapinarof cream as monotherapy can achieve aggressive treatment targets set by both US and European guidelines developed for systemic and biologic therapies. Tapinarof cream 1% QD is an effective topical treatment option for patients with plaque psoriasis that has been approved without restrictions relating to severity or extent of disease treated, duration of use, or application sites, including application to sensitive and intertriginous skin.
Acknowledgments—Editorial and medical writing support under the guidance of the authors was provided by Melanie Govender, MSc (Med), ApotheCom (United Kingdom), and was funded by Dermavant Sciences, Inc, in accordance with Good Publication Practice (GPP) guidelines.
Psoriasis is a chronic inflammatory disease affecting approximately 8 million adults in the United States and 2% of the global population.1,2 Psoriasis causes pain, itching, and disfigurement and is associated with a physical, psychological, and economic burden that substantially affects health-related quality of life.3-5
Setting treatment goals and treating to target are evidence-based approaches that have been successfully applied to several chronic diseases to improve patient outcomes, including diabetes, hypertension, and rheumatoid arthritis.6-9 Treat-to-target strategies generally set low disease activity (or remission) as an overall goal and seek to achieve this using available therapeutic options as necessary. Introduced following the availability of biologics and targeted systemic therapies, treat-to-target strategies generally provide guidance on expectations of treatment but not specific treatments, as personalized treatment decisions depend on an assessment of individual patients and consider clinical and demographic features as well as preferences for available therapeutic options. If targets are not achieved in the assigned time span, adjustments can be made to the treatment approach in close consultation with the patient. If the target is reached, follow-up visits can be scheduled to ensure improvement is maintained or to establish if more aggressive goals could be selected.
Treat-to-target strategies for the management of psoriasis developed by the National Psoriasis Foundation (NPF) Medical Board include reducing the extent of psoriasis to 1% or lower total body surface area (BSA) after 3 months of treatment.10 Treatment targets endorsed by the European Academy of Dermatology and Venereology (EADV) in guidelines on the use of systemic therapies in psoriasis include achieving a 75% or greater reduction in Psoriasis Area and Severity Index (PASI) score within 3 to 4 months of treatment.11
In clinical practice, many patients do not achieve these treatment targets, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.12,13 Moreover, conventional topical treatments (eg, topical corticosteroids) used by most patients with psoriasis regardless of disease severity are associated with adverse events that can limit their use. Most topical corticosteroids have US Food and Drug Administration label restrictions relating to sites of application, duration and extent of use, and frequency of administration.14,15
Tapinarof cream 1% (VTAMA [Dermavant Sciences, Inc]) is a first-in-class topical nonsteroidal aryl hydrocarbon receptor agonist that was approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults16 and is being studied for the treatment of plaque psoriasis in children 2 years and older as well as for atopic dermatitis in adults and children 2 years and older. In PSOARING 1 (ClinicalTrials .gov identifier NCT03956355) and PSOARING 2 (NCT03983980)—identical 12-week pivotal phase 3 trials—monotherapy with tapinarof cream 1% once daily (QD) demonstrated statistically significant efficacy vs vehicle cream and was well tolerated in adults with mild to severe plaque psoriasis (Supplementary Figure S1).17 Lebwohl et al17 reported that significantly higher PASI75 responses were observed at week 12 with tapinarof cream vs vehicle in PSOARING 1 and PSOARING 2 (36% and 48% vs 10% and 7%, respectively; both P<.0001). A significantly higher PASI90 response of 19% and 21% at week 12 also was observed with tapinarof cream vs 2% and 3% with vehicle in PSOARING 1 and PSOARING 2, respectively (P=.0005 and P<.0001).17
In PSOARING 3 (NCT04053387)—the long-term extension trial (Supplementary Figure S1)—efficacy continued to improve or was maintained beyond the two 12-week trials, with improvements in total BSA affected and PASI scores for up to 52 weeks.18 Tapinarof cream 1% QD demonstrated positive, rapid, and durable outcomes in PSOARING 3, including high rates of complete disease clearance (Physician Global Assessment [PGA] score=0 [clear])(40.9% [312/763]), durability of response on treatment with no evidence of tachyphylaxis, and a remittive effect of approximately 4 months when off therapy (defined as maintenance of a PGA score of 0 [clear] or 1 [almost clear] after first achieving a PGA score of 0).18
Herein, we report absolute treatment targets for patients with plaque psoriasis who received tapinarof cream 1% QD in the PSOARING trials that are at least as stringent as the corresponding NPF and EADV targets of achieving a total BSA affected of 1% or lower or a PASI75 response within 3 to 4 months, respectively.
METHODS
Study Design
The pooled efficacy analyses included all patients with a baseline PGA score of 2 or higher (mild or worse) before treatment with tapinarof cream 1% QD in the PSOARING trials. This included patients who received tapinarof cream 1% in PSOARING 1 and PSOARING 2 who may or may not have continued into PSOARING 3, as well as those who received the vehicle in PSOARING 1 and PSOARING 2 who enrolled in PSOARING 3 and had a PGA score of 2 or higher before receiving tapinarof cream 1%.
Trial Participants
Full methods, including inclusion and exclusion criteria, for the PSOARING trials have been previously reported.17,18 Patients were aged 18 to 75 years and had chronic plaque psoriasis that was stable for at least 6 months before randomization; 3% to 20% total BSA affected (excluding the scalp, palms, fingernails, toenails, and soles); and a PGA score of 2 (mild), 3 (moderate), or 4 (severe) at baseline.
The clinical trials were conducted in compliance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. Approval was obtained from local ethics committees or institutional review boards at each center. All patients provided written informed consent.
Trial Treatment
In PSOARING 1 and PSOARING 2, patients were randomized (2:1) to receive tapinarof cream 1% or vehicle QD for 12 weeks. In PSOARING 3 (the long-term extension trial), patients received up to 40 weeks of open-label tapinarof, followed by 4 weeks of follow-up off treatment. Patients received intermittent or continuous treatment with tapinarof cream 1% in PSOARING 3 based on PGA score: those entering the trial with a PGA score of 1 or higher received tapinarof cream 1% until complete disease clearance was achieved (defined as a PGA score of 0 [clear]). Those entering PSOARING 3 with or achieving a PGA score of 0 (clear) discontinued treatment and were observed for the duration of maintenance of a PGA score of 0 (clear) or 1 (almost clear) while off therapy (the protocol-defined “duration of remittive effect”). If disease worsening (defined as a PGA score 2 or higher) occurred, tapinarof cream 1% was restarted and continued until a PGA score of 0 (clear) was achieved. This pattern of treatment, discontinuation on achieving a PGA score of 0 (clear), and retreatment on disease worsening continued until the end of the trial. As a result, patients in PSOARING 3 could receive tapinarof cream 1% continuously or intermittently for 40 weeks.
Outcome Measures and Statistical Analyses
The assessment of total BSA affected by plaque psoriasis is an estimate of the total extent of disease as a percentage of total skin area. In the PSOARING trials, the skin surface of one hand (palm and digits) was assumed to be approximately equivalent to 1% BSA. The total BSA affected by psoriasis was evaluated from 0% to 100%, with greater total BSA affected being an indication of more extensive disease. The BSA efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved a 1% or lower or 0.5% or lower total BSA affected.
Psoriasis Area and Severity Index scores assess both the severity and extent of psoriasis. A PASI score lower than 5 often is considered indicative of mild psoriasis, a score of 5 to 10 indicates moderate disease, and a score higher than 10 indicates severe disease.19 The maximum PASI score is 72. The PASI efficacy outcomes used in these analyses were based post hoc on the proportion of patients who achieved an absolute total PASI score of 3 or lower, 2 or lower, and 1 or lower.
Efficacy analyses were based on pooled data for all patients in the PSOARING trials who had a PGA score of 2 to 4 (mild to severe) before treatment with tapinarof cream 1% in the intention-to-treat population using observed cases. Time-to-target analyses were based on Kaplan-Meier (KM) estimates using observed cases.
Safety analyses included the incidence and frequency of adverse events and were based on all patients who received tapinarof cream 1% in the PSOARING trials.
RESULTS
Baseline Patient Demographics and Disease Characteristics
The pooled efficacy analyses included 915 eligible patients (Table). At baseline, the mean (SD) age was 50.2 (13.25) years, 58.7% were male, the mean (SD) weight was 92.2 (23.67) kg, and the mean (SD) body mass index was 31.6 (7.53) kg/m2. The percentage of patients with a PGA score of 2 (mild), 3 (moderate), or 4 (severe) was 13.9%, 78.1%, and 8.0%, respectively. The mean (SD) PASI score was 8.7 (4.23) and mean (SD) total BSA affected was 7.8% (4.98).
Efficacy
Achievement of BSA-Affected Targets—



Achievement of Absolute PASI Targets—Across the total trial period (up to 52 weeks), an absolute total PASI score of 3 or lower was achieved by 75% of patients (686/915), with a median time to achieve this of 2 months (KM estimate: 58 days [95% CI, 57-63]); approximately 67% of patients (612/915) achieved a total PASI score of 2 or lower, with a median time to achieve of 3 months (KM estimate: 87 days [95% CI, 85-110])(Figure 2; Supplementary Figures S3a and S3b). A PASI score of 1 or lower was achieved by approximately 50% of patients (460/915), with a median time to achieve of approximately 6 months (KM estimate: 185 days [95% CI, 169-218])(Figure 2, Supplementary Figure S3c).
Illustrative Case—Case photography showing the clinical response in a 63-year-old man with moderate plaque psoriasis in PSOARING 2 is shown in Figure 3. After 12 weeks of treatment with tapinarof cream 1% QD, the patient achieved all primary and secondary efficacy end points. In addition to achieving the regulatory end point of a PGA score of 0 (clear) or 1 (almost clear) and a decrease from baseline of at least 2 points, achievement of 0% total BSA affected and a total PASI score of 0 at week 12 exceeded the NPF and EADV consensus treatment targets.10,11 Targets were achieved as early as week 4, with a total BSA affected of 0.5% or lower and a total PASI score of 1 or lower, illustrated by marked skin clearing and only faint residual erythema that completely resolved at week 12, with the absence of postinflammatory hyperpigmentation.
Safety
Safety data for the PSOARING trials have been previously reported.17,18 The most common treatment-emergent adverse events were folliculitis, contact dermatitis, upper respiratory tract infection, and nasopharyngitis. Treatment-emergent adverse events generally were mild or moderate in severity and did not lead to trial discontinuation.17,18

COMMENT
Treat-to-target management approaches aim to improve patient outcomes by striving to achieve optimal goals. The treat-to-target approach supports shared decision-making between clinicians and patients based on common expectations of what constitutes treatment success.
The findings of this analysis based on pooled data from a large cohort of patients demonstrate that a high proportion of patients can achieve or exceed recommended treatment targets with tapinarof cream 1% QD and maintain improvements long-term. The NPF-recommended treatment target of 1% or lower BSA affected within approximately 3 months (90 days) of treatment was achieved by 40% of tapinarof-treated patients. In addition, 1% or lower BSA affected at any time during the trials was achieved by 61% of patients (median, approximately 4 months). The analyses also indicated that PASI total scores of 3 or lower and 2 or lower were achieved by 75% and 67% of tapinarof-treated patients, respectively, within 2 to 3 months.
These findings support the previously reported efficacy of tapinarof cream, including high rates of complete disease clearance (40.9% [312/763]), durable response following treatment interruption, an off-therapy remittive effect of approximately 4 months, and good disease control on therapy with no evidence of tachyphylaxis.17,18
CONCLUSION
Taken together with previously reported tapinarof efficacy and safety results, our findings demonstrate that a high proportion of patients treated with tapinarof cream as monotherapy can achieve aggressive treatment targets set by both US and European guidelines developed for systemic and biologic therapies. Tapinarof cream 1% QD is an effective topical treatment option for patients with plaque psoriasis that has been approved without restrictions relating to severity or extent of disease treated, duration of use, or application sites, including application to sensitive and intertriginous skin.
Acknowledgments—Editorial and medical writing support under the guidance of the authors was provided by Melanie Govender, MSc (Med), ApotheCom (United Kingdom), and was funded by Dermavant Sciences, Inc, in accordance with Good Publication Practice (GPP) guidelines.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Pilon D, Teeple A, Zhdanava M, et al. The economic burden of psoriasis with high comorbidity among privately insured patients in the United States. J Med Econ. 2019;22:196-203.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425-440.e2.
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9:504-513.
- Ford JA, Solomon DH. Challenges in implementing treat-to-target strategies in rheumatology. Rheum Dis Clin North Am. 2019;45:101-112.
- Sitbon O, Galiè N. Treat-to-target strategies in pulmonary arterial hypertension: the importance of using multiple goals. Eur Respir Rev. 2010;19:272-278.
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631-637.
- Wangnoo SK, Sethi B, Sahay RK, et al. Treat-to-target trials in diabetes. Indian J Endocrinol Metab. 2014;18:166-174.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1-70.
- Strober BE, van der Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb). 2019; 9:5-18.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35 (2 Suppl 2):S36-S44; quiz S45.
- VTAMA® (tapinarof) cream. Prescribing information. Dermavant Sciences; 2022. Accessed September 13, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215272s000lbl.pdf
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229 and supplementary appendix.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Clinical Review Report: Guselkumab (Tremfya) [Internet]. Canadian Agency for Drugs and Technologies in Health; 2018. Accessed September 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534047/pdf/Bookshelf_NBK534047.pdf
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Pilon D, Teeple A, Zhdanava M, et al. The economic burden of psoriasis with high comorbidity among privately insured patients in the United States. J Med Econ. 2019;22:196-203.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425-440.e2.
- Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9:504-513.
- Ford JA, Solomon DH. Challenges in implementing treat-to-target strategies in rheumatology. Rheum Dis Clin North Am. 2019;45:101-112.
- Sitbon O, Galiè N. Treat-to-target strategies in pulmonary arterial hypertension: the importance of using multiple goals. Eur Respir Rev. 2010;19:272-278.
- Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631-637.
- Wangnoo SK, Sethi B, Sahay RK, et al. Treat-to-target trials in diabetes. Indian J Endocrinol Metab. 2014;18:166-174.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23(Suppl 2):1-70.
- Strober BE, van der Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther (Heidelb). 2019; 9:5-18.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35 (2 Suppl 2):S36-S44; quiz S45.
- VTAMA® (tapinarof) cream. Prescribing information. Dermavant Sciences; 2022. Accessed September 13, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215272s000lbl.pdf
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229 and supplementary appendix.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Clinical Review Report: Guselkumab (Tremfya) [Internet]. Canadian Agency for Drugs and Technologies in Health; 2018. Accessed September 13, 2024. https://www.ncbi.nlm.nih.gov/books/NBK534047/pdf/Bookshelf_NBK534047.pdf
Practice Points
- In clinical practice, many patients with psoriasis do not achieve treatment targets set forth by the National Psoriasis Foundation and the European Academy of Dermatology and Venereology, and topical treatments alone generally are insufficient in achieving treatment goals for psoriasis.
- Tapinarof cream 1% is a nonsteroidal aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for the treatment of plaque psoriasis in adults; it also is being studied for the treatment of plaque psoriasis in children 2 years and older.
- Tapinarof cream 1% is an effective topical treatment option for patients with plaque psoriasis of any severity, with no limitations on treatment duration, total extent of use, or application sites, including intertriginous skin and sensitive areas.
The Potential Benefits of Dietary Changes in Psoriasis Patients
Psoriasis is a chronic inflammatory skin disease for which several lifestyle factors—smoking, alcohol use, and psychological stress—are associated with higher incidence and more severe disease.1-3 Diet also has been implicated as a factor that can affect psoriasis,4 and many patients have shown interest in possible dietary interventions to help their disease.5
In 2018, the National Psoriasis Foundation (NPF) presented dietary recommendations for patients based on results from a systematic review. From the available literature, only dietary weight reduction with hypocaloric diets in overweight or obese patients could be strongly recommended, and it has been proven that obesity is associated with worse psoriasis severity.6 Other more recent studies have shown that dietary modifications such as intermittent fasting and the ketogenic diet also led to weight loss and improved psoriasis severity in overweight patients; however, it is difficult to discern if the improvement was due to weight loss alone or if the dietary patterns themselves played a role.7,8 The paucity of well-designed studies evaluating the effects of other dietary changes has prevented further guidelines from being written. We propose that dietary patterns such as the Mediterranean diet (MeD) and vegan/vegetarian diets—even without strong data showing benefits in skin disease—may help to decrease systemic inflammation, improve gut dysbiosis, and help decrease the risk for cardiometabolic comorbidities that are associated with psoriasis.
Mediterranean Diet
The MeD is based on the dietary tendencies of inhabitants from the regions surrounding the Mediterranean Sea and is centered around nutrient-rich foods such as vegetables, olive oil, and legumes while limiting meat and dairy.9 The NPF recommended considering a trial of the MeD based on low-quality evidence.6 Observational studies have indicated that psoriasis patients are less likely to adhere to the MeD, but those who do have less severe disease.8 However, a search of PubMed articles indexed for MEDLINE using the terms Mediterranean diet and psoriasis yielded no prospective interventional studies. Given the association of the MeD with less severe disease, it is important to understand which specific foods in the MeD could be beneficial. Intake of omega-3 fatty acids, such as those found in fatty fish, are important for modulation of systemic inflammation.7 High intake of polyphenols—found in fruits and vegetables, extra-virgin olive oil, and wine—also have been implicated in improving inflammatory diseases due to potent antioxidant and anti-inflammatory properties. Individually, fruits, vegetables, whole grains, and sea fish have been associated with lowering C-reactive protein levels, which also is indicative of the benefits of these foods on systemic inflammation.7
Vegan/Vegetarian Diets
Although fruits, vegetables, legumes, and whole grains are a substantial component of the MeD, there are limited data on vegetarian or purely vegan plant-based diets. An observational study from the NPF found that only 48.4% (15/31) of patients on the MeD vs 69.0% (20/29) on a vegan diet reported a favorable skin response.5 Two case reports also have shown beneficial results of a strict vegan diet for psoriasis and psoriatic arthritis, where whole-food plant-based diets also improved joint symptoms.10-12 As with any diet, those who pursue a plant-based diet should strive to consume a variety of foods to avoid nutrient deficiencies. A recent systematic meta-analysis of 141 studies evaluated nutrient status of vegan and vegetarian diets compared to pescovegetarians and those who consume meat. All dietary patterns showed varying degrees of low levels of different nutrients.13 Of note, the researchers found that vitamin B12, vitamin D, iron, zinc, iodine, calcium, and docosahexaenoic acid were lower in plant-based diets. In contrast, folate; vitamins B1, B6, C, and E; polyunsaturated fatty acids; α-linolenic acid; and magnesium intake were higher. Those who consumed meat were at risk for inadequate intake of fiber, polyunsaturated fatty acids, α-linolenic acid, folate, vitamin E, calcium, magnesium, and vitamin D, though vitamin D intake was higher than in vegans/vegetarians.13 The results of this meta-analysis indicated the importance of educating patients on what constitutes a well-rounded, micronutrient-rich diet or appropriate supplementation for any diet.
Effects on Gut Microbiome
Any changes in diet can lead to alterations in the gut microbiome, which may impact skin disease, as evidence indicates a bidirectional relationship between gut and skin health.10 A metagenomic analysis of the gut microbiota in patients with untreated plaque psoriasis revealed a signature dysbiosis for which the researchers developed a psoriasis microbiota index, suggesting the gut microbiota may play a role in psoriasis pathophysiology.14 Research shows that both the MeD and vegan/vegetarian diets, which are relatively rich in fiber and omega-3 fatty acids and low in saturated fat and animal protein compared to many diets, cause increases in dietary fiber–metabolizing bacteria that produce short-chain fatty acids. These short-chain fatty acids improve gut epithelial integrity and alleviate both gut and systemic inflammation.10
The changes to the gut microbiome induced by a high-fat diet also are concerning. In contrast to the MeD or vegan/vegetarian diets, consumption of a high-fat diet induces alterations in the composition of the gut microbiota that in turn increase the release of proinflammatory cytokines and promote higher intestinal permeability.10 Similarly, high sugar consumption promotes increased intestinal permeability and shifts the gut microbiota to organisms that can rapidly utilize simple carbohydrates at the expense of other beneficial organisms, reducing bacterial diversity.15 The Western diet, which is notable for both high fat and high sugar content, is sometimes referred to as a proinflammatory diet and has been shown to worsen psoriasiformlike lesions in mice.16 Importantly, most research indicates that high fat and high sugar consumption appear to be more prevalent in psoriasis patients,8 but the type of fat consumed in the diet matters. The Western diet includes abundant saturated fat found in meat, dairy products, palm and coconut oils, and processed foods, as well as omega-6 fatty acids that are found in meat, poultry, and eggs. Saturated fat has been shown to promote helper T cell (TH17) accumulation in the skin, and omega-6 fatty acids serve as precursors to various inflammatory lipid mediators.4 This distinction of sources of fat between the Western diet and MeD is important in understanding the diets’ different effects on psoriasis and overall health. As previously discussed, the high intake of omega-3 acids in the MeD is one of the ways it may exert its anti-inflammatory benefits.7
Next Steps in Advising Psoriasis Patients
A major limitation of the data for MeD and vegan/vegetarian diets is limited randomized controlled trials evaluating the impact of these diets on psoriasis. Thus, dietary recommendations for psoriasis are not as strong as for other diseases for which more conclusive data exist.8 Although the data on diet and psoriasis are not definitive, perhaps dermatologists should shift the question from “Does this diet definitely improve psoriasis?” to “Does this diet definitely improve my patient’s health as a whole and maybe also their psoriasis?” For instance, the MeD has been shown to reduce the risk for type 2 diabetes mellitus and cardiovascular disease as well as to slow cognitive decline.17 Vegan/vegetarian diets focusing on whole vs processed foods have been shown to be highly effective in combatting obesity, type 2 diabetes mellitus, coronary artery disease including severe atherosclerosis, and hypertension.18 Psoriasis patients are at increased risk for many of the ailments that the MeD and plant-based diets protect against, making these diets potentially even more impactful than for someone without psoriasis.19 Dietary recommendations should still be made in conjunction with continuing traditional therapies for psoriasis and in consultation with the patient’s primary care physician and/or dietitian; however, rather than waiting for more randomized controlled trials before making health-promoting recommendations, what would be the downside of starting now? At worst, the dietary change decreases their risk for several metabolic conditions, and at best they may even see an improvement in their psoriasis.
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case–control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Armstrong AW, Harskamp CT, Dhillon JS, et al. Psoriasis and smoking: a systematic review and meta‐analysis. Br J Dermatol. 2014;170:304-314. doi:10.1111/bjd.12670
- Zhu K, Zhu C, Fan Y. Alcohol consumption and psoriatic risk: a meta‐analysis of case–control studies. J Dermatol. 2012;39:770-773. doi:10.1111/j.1346-8138.2012.01577.x
- Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci. 2020;21:5405. doi:10.3390/ijms21155405
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther. 2017;7:227-242. doi:10.1007/s13555-017-0183-4
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934. doi:10.1001/jamadermatol.2018.1412
- Duchnik E, Kruk J, Tuchowska A, et al. The impact of diet and physical activity on psoriasis: a narrative review of the current evidence. Nutrients. 2023;15:840. doi:10.3390/nu15040840
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis Targets Ther. 2022;12:151-176. doi:10.2147/PTT.S328581
- Mazza E, Ferro Y, Pujia R, et al. Mediterranean diet in healthy aging. J Nutr Health Aging. 2021;25:1076-1083. doi:10.1007/s12603-021-1675-6
- Flores-Balderas X, Peña-Peña M, Rada KM, et al. Beneficial effects of plant-based diets on skin health and inflammatory skin diseases. Nutrients. 2023;15:2842. doi:10.3390/nu15132842
- Bonjour M, Gabriel S, Valencia A, et al. Challenging case in clinical practice: prolonged water-only fasting followed by an exclusively whole-plant-food diet in the management of severe plaque psoriasis. Integr Complement Ther. 2022;28:85-87. doi:10.1089/ict.2022.29010.mbo
- Lewandowska M, Dunbar K, Kassam S. Managing psoriatic arthritis with a whole food plant-based diet: a case study. Am J Lifestyle Med. 2021;15:402-406. doi:10.1177/1559827621993435
- Neufingerl N, Eilander A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. 2021;14:29. doi:10.3390/nu14010029
- Dei-Cas I, Giliberto F, Luce L, et al. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new psoriasis-microbiome index. Sci Rep. 2020;10:12754. doi:10.1038/s41598-020-69537-3
- Satokari R. High intake of sugar and the balance between pro- and anti-inflammatory gut bacteria. Nutrients. 2020;12:1348. doi:10.3390/nu12051348
- Shi Z, Wu X, Santos Rocha C, et al. Short-term Western diet intake promotes IL-23–mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021;141:1780-1791. doi:10.1016/j.jid.2020.11.032
- Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222. doi:10.1097/NT.0000000000000228
- Tuso PJ, Ismail MH, Ha BP, et al. Nutritional update for physicians: plant-based diets. Perm J. 2013;17:61-66. doi:10.7812/TPP/12-085
- Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
Psoriasis is a chronic inflammatory skin disease for which several lifestyle factors—smoking, alcohol use, and psychological stress—are associated with higher incidence and more severe disease.1-3 Diet also has been implicated as a factor that can affect psoriasis,4 and many patients have shown interest in possible dietary interventions to help their disease.5
In 2018, the National Psoriasis Foundation (NPF) presented dietary recommendations for patients based on results from a systematic review. From the available literature, only dietary weight reduction with hypocaloric diets in overweight or obese patients could be strongly recommended, and it has been proven that obesity is associated with worse psoriasis severity.6 Other more recent studies have shown that dietary modifications such as intermittent fasting and the ketogenic diet also led to weight loss and improved psoriasis severity in overweight patients; however, it is difficult to discern if the improvement was due to weight loss alone or if the dietary patterns themselves played a role.7,8 The paucity of well-designed studies evaluating the effects of other dietary changes has prevented further guidelines from being written. We propose that dietary patterns such as the Mediterranean diet (MeD) and vegan/vegetarian diets—even without strong data showing benefits in skin disease—may help to decrease systemic inflammation, improve gut dysbiosis, and help decrease the risk for cardiometabolic comorbidities that are associated with psoriasis.
Mediterranean Diet
The MeD is based on the dietary tendencies of inhabitants from the regions surrounding the Mediterranean Sea and is centered around nutrient-rich foods such as vegetables, olive oil, and legumes while limiting meat and dairy.9 The NPF recommended considering a trial of the MeD based on low-quality evidence.6 Observational studies have indicated that psoriasis patients are less likely to adhere to the MeD, but those who do have less severe disease.8 However, a search of PubMed articles indexed for MEDLINE using the terms Mediterranean diet and psoriasis yielded no prospective interventional studies. Given the association of the MeD with less severe disease, it is important to understand which specific foods in the MeD could be beneficial. Intake of omega-3 fatty acids, such as those found in fatty fish, are important for modulation of systemic inflammation.7 High intake of polyphenols—found in fruits and vegetables, extra-virgin olive oil, and wine—also have been implicated in improving inflammatory diseases due to potent antioxidant and anti-inflammatory properties. Individually, fruits, vegetables, whole grains, and sea fish have been associated with lowering C-reactive protein levels, which also is indicative of the benefits of these foods on systemic inflammation.7
Vegan/Vegetarian Diets
Although fruits, vegetables, legumes, and whole grains are a substantial component of the MeD, there are limited data on vegetarian or purely vegan plant-based diets. An observational study from the NPF found that only 48.4% (15/31) of patients on the MeD vs 69.0% (20/29) on a vegan diet reported a favorable skin response.5 Two case reports also have shown beneficial results of a strict vegan diet for psoriasis and psoriatic arthritis, where whole-food plant-based diets also improved joint symptoms.10-12 As with any diet, those who pursue a plant-based diet should strive to consume a variety of foods to avoid nutrient deficiencies. A recent systematic meta-analysis of 141 studies evaluated nutrient status of vegan and vegetarian diets compared to pescovegetarians and those who consume meat. All dietary patterns showed varying degrees of low levels of different nutrients.13 Of note, the researchers found that vitamin B12, vitamin D, iron, zinc, iodine, calcium, and docosahexaenoic acid were lower in plant-based diets. In contrast, folate; vitamins B1, B6, C, and E; polyunsaturated fatty acids; α-linolenic acid; and magnesium intake were higher. Those who consumed meat were at risk for inadequate intake of fiber, polyunsaturated fatty acids, α-linolenic acid, folate, vitamin E, calcium, magnesium, and vitamin D, though vitamin D intake was higher than in vegans/vegetarians.13 The results of this meta-analysis indicated the importance of educating patients on what constitutes a well-rounded, micronutrient-rich diet or appropriate supplementation for any diet.
Effects on Gut Microbiome
Any changes in diet can lead to alterations in the gut microbiome, which may impact skin disease, as evidence indicates a bidirectional relationship between gut and skin health.10 A metagenomic analysis of the gut microbiota in patients with untreated plaque psoriasis revealed a signature dysbiosis for which the researchers developed a psoriasis microbiota index, suggesting the gut microbiota may play a role in psoriasis pathophysiology.14 Research shows that both the MeD and vegan/vegetarian diets, which are relatively rich in fiber and omega-3 fatty acids and low in saturated fat and animal protein compared to many diets, cause increases in dietary fiber–metabolizing bacteria that produce short-chain fatty acids. These short-chain fatty acids improve gut epithelial integrity and alleviate both gut and systemic inflammation.10
The changes to the gut microbiome induced by a high-fat diet also are concerning. In contrast to the MeD or vegan/vegetarian diets, consumption of a high-fat diet induces alterations in the composition of the gut microbiota that in turn increase the release of proinflammatory cytokines and promote higher intestinal permeability.10 Similarly, high sugar consumption promotes increased intestinal permeability and shifts the gut microbiota to organisms that can rapidly utilize simple carbohydrates at the expense of other beneficial organisms, reducing bacterial diversity.15 The Western diet, which is notable for both high fat and high sugar content, is sometimes referred to as a proinflammatory diet and has been shown to worsen psoriasiformlike lesions in mice.16 Importantly, most research indicates that high fat and high sugar consumption appear to be more prevalent in psoriasis patients,8 but the type of fat consumed in the diet matters. The Western diet includes abundant saturated fat found in meat, dairy products, palm and coconut oils, and processed foods, as well as omega-6 fatty acids that are found in meat, poultry, and eggs. Saturated fat has been shown to promote helper T cell (TH17) accumulation in the skin, and omega-6 fatty acids serve as precursors to various inflammatory lipid mediators.4 This distinction of sources of fat between the Western diet and MeD is important in understanding the diets’ different effects on psoriasis and overall health. As previously discussed, the high intake of omega-3 acids in the MeD is one of the ways it may exert its anti-inflammatory benefits.7
Next Steps in Advising Psoriasis Patients
A major limitation of the data for MeD and vegan/vegetarian diets is limited randomized controlled trials evaluating the impact of these diets on psoriasis. Thus, dietary recommendations for psoriasis are not as strong as for other diseases for which more conclusive data exist.8 Although the data on diet and psoriasis are not definitive, perhaps dermatologists should shift the question from “Does this diet definitely improve psoriasis?” to “Does this diet definitely improve my patient’s health as a whole and maybe also their psoriasis?” For instance, the MeD has been shown to reduce the risk for type 2 diabetes mellitus and cardiovascular disease as well as to slow cognitive decline.17 Vegan/vegetarian diets focusing on whole vs processed foods have been shown to be highly effective in combatting obesity, type 2 diabetes mellitus, coronary artery disease including severe atherosclerosis, and hypertension.18 Psoriasis patients are at increased risk for many of the ailments that the MeD and plant-based diets protect against, making these diets potentially even more impactful than for someone without psoriasis.19 Dietary recommendations should still be made in conjunction with continuing traditional therapies for psoriasis and in consultation with the patient’s primary care physician and/or dietitian; however, rather than waiting for more randomized controlled trials before making health-promoting recommendations, what would be the downside of starting now? At worst, the dietary change decreases their risk for several metabolic conditions, and at best they may even see an improvement in their psoriasis.
Psoriasis is a chronic inflammatory skin disease for which several lifestyle factors—smoking, alcohol use, and psychological stress—are associated with higher incidence and more severe disease.1-3 Diet also has been implicated as a factor that can affect psoriasis,4 and many patients have shown interest in possible dietary interventions to help their disease.5
In 2018, the National Psoriasis Foundation (NPF) presented dietary recommendations for patients based on results from a systematic review. From the available literature, only dietary weight reduction with hypocaloric diets in overweight or obese patients could be strongly recommended, and it has been proven that obesity is associated with worse psoriasis severity.6 Other more recent studies have shown that dietary modifications such as intermittent fasting and the ketogenic diet also led to weight loss and improved psoriasis severity in overweight patients; however, it is difficult to discern if the improvement was due to weight loss alone or if the dietary patterns themselves played a role.7,8 The paucity of well-designed studies evaluating the effects of other dietary changes has prevented further guidelines from being written. We propose that dietary patterns such as the Mediterranean diet (MeD) and vegan/vegetarian diets—even without strong data showing benefits in skin disease—may help to decrease systemic inflammation, improve gut dysbiosis, and help decrease the risk for cardiometabolic comorbidities that are associated with psoriasis.
Mediterranean Diet
The MeD is based on the dietary tendencies of inhabitants from the regions surrounding the Mediterranean Sea and is centered around nutrient-rich foods such as vegetables, olive oil, and legumes while limiting meat and dairy.9 The NPF recommended considering a trial of the MeD based on low-quality evidence.6 Observational studies have indicated that psoriasis patients are less likely to adhere to the MeD, but those who do have less severe disease.8 However, a search of PubMed articles indexed for MEDLINE using the terms Mediterranean diet and psoriasis yielded no prospective interventional studies. Given the association of the MeD with less severe disease, it is important to understand which specific foods in the MeD could be beneficial. Intake of omega-3 fatty acids, such as those found in fatty fish, are important for modulation of systemic inflammation.7 High intake of polyphenols—found in fruits and vegetables, extra-virgin olive oil, and wine—also have been implicated in improving inflammatory diseases due to potent antioxidant and anti-inflammatory properties. Individually, fruits, vegetables, whole grains, and sea fish have been associated with lowering C-reactive protein levels, which also is indicative of the benefits of these foods on systemic inflammation.7
Vegan/Vegetarian Diets
Although fruits, vegetables, legumes, and whole grains are a substantial component of the MeD, there are limited data on vegetarian or purely vegan plant-based diets. An observational study from the NPF found that only 48.4% (15/31) of patients on the MeD vs 69.0% (20/29) on a vegan diet reported a favorable skin response.5 Two case reports also have shown beneficial results of a strict vegan diet for psoriasis and psoriatic arthritis, where whole-food plant-based diets also improved joint symptoms.10-12 As with any diet, those who pursue a plant-based diet should strive to consume a variety of foods to avoid nutrient deficiencies. A recent systematic meta-analysis of 141 studies evaluated nutrient status of vegan and vegetarian diets compared to pescovegetarians and those who consume meat. All dietary patterns showed varying degrees of low levels of different nutrients.13 Of note, the researchers found that vitamin B12, vitamin D, iron, zinc, iodine, calcium, and docosahexaenoic acid were lower in plant-based diets. In contrast, folate; vitamins B1, B6, C, and E; polyunsaturated fatty acids; α-linolenic acid; and magnesium intake were higher. Those who consumed meat were at risk for inadequate intake of fiber, polyunsaturated fatty acids, α-linolenic acid, folate, vitamin E, calcium, magnesium, and vitamin D, though vitamin D intake was higher than in vegans/vegetarians.13 The results of this meta-analysis indicated the importance of educating patients on what constitutes a well-rounded, micronutrient-rich diet or appropriate supplementation for any diet.
Effects on Gut Microbiome
Any changes in diet can lead to alterations in the gut microbiome, which may impact skin disease, as evidence indicates a bidirectional relationship between gut and skin health.10 A metagenomic analysis of the gut microbiota in patients with untreated plaque psoriasis revealed a signature dysbiosis for which the researchers developed a psoriasis microbiota index, suggesting the gut microbiota may play a role in psoriasis pathophysiology.14 Research shows that both the MeD and vegan/vegetarian diets, which are relatively rich in fiber and omega-3 fatty acids and low in saturated fat and animal protein compared to many diets, cause increases in dietary fiber–metabolizing bacteria that produce short-chain fatty acids. These short-chain fatty acids improve gut epithelial integrity and alleviate both gut and systemic inflammation.10
The changes to the gut microbiome induced by a high-fat diet also are concerning. In contrast to the MeD or vegan/vegetarian diets, consumption of a high-fat diet induces alterations in the composition of the gut microbiota that in turn increase the release of proinflammatory cytokines and promote higher intestinal permeability.10 Similarly, high sugar consumption promotes increased intestinal permeability and shifts the gut microbiota to organisms that can rapidly utilize simple carbohydrates at the expense of other beneficial organisms, reducing bacterial diversity.15 The Western diet, which is notable for both high fat and high sugar content, is sometimes referred to as a proinflammatory diet and has been shown to worsen psoriasiformlike lesions in mice.16 Importantly, most research indicates that high fat and high sugar consumption appear to be more prevalent in psoriasis patients,8 but the type of fat consumed in the diet matters. The Western diet includes abundant saturated fat found in meat, dairy products, palm and coconut oils, and processed foods, as well as omega-6 fatty acids that are found in meat, poultry, and eggs. Saturated fat has been shown to promote helper T cell (TH17) accumulation in the skin, and omega-6 fatty acids serve as precursors to various inflammatory lipid mediators.4 This distinction of sources of fat between the Western diet and MeD is important in understanding the diets’ different effects on psoriasis and overall health. As previously discussed, the high intake of omega-3 acids in the MeD is one of the ways it may exert its anti-inflammatory benefits.7
Next Steps in Advising Psoriasis Patients
A major limitation of the data for MeD and vegan/vegetarian diets is limited randomized controlled trials evaluating the impact of these diets on psoriasis. Thus, dietary recommendations for psoriasis are not as strong as for other diseases for which more conclusive data exist.8 Although the data on diet and psoriasis are not definitive, perhaps dermatologists should shift the question from “Does this diet definitely improve psoriasis?” to “Does this diet definitely improve my patient’s health as a whole and maybe also their psoriasis?” For instance, the MeD has been shown to reduce the risk for type 2 diabetes mellitus and cardiovascular disease as well as to slow cognitive decline.17 Vegan/vegetarian diets focusing on whole vs processed foods have been shown to be highly effective in combatting obesity, type 2 diabetes mellitus, coronary artery disease including severe atherosclerosis, and hypertension.18 Psoriasis patients are at increased risk for many of the ailments that the MeD and plant-based diets protect against, making these diets potentially even more impactful than for someone without psoriasis.19 Dietary recommendations should still be made in conjunction with continuing traditional therapies for psoriasis and in consultation with the patient’s primary care physician and/or dietitian; however, rather than waiting for more randomized controlled trials before making health-promoting recommendations, what would be the downside of starting now? At worst, the dietary change decreases their risk for several metabolic conditions, and at best they may even see an improvement in their psoriasis.
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case–control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Armstrong AW, Harskamp CT, Dhillon JS, et al. Psoriasis and smoking: a systematic review and meta‐analysis. Br J Dermatol. 2014;170:304-314. doi:10.1111/bjd.12670
- Zhu K, Zhu C, Fan Y. Alcohol consumption and psoriatic risk: a meta‐analysis of case–control studies. J Dermatol. 2012;39:770-773. doi:10.1111/j.1346-8138.2012.01577.x
- Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci. 2020;21:5405. doi:10.3390/ijms21155405
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther. 2017;7:227-242. doi:10.1007/s13555-017-0183-4
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934. doi:10.1001/jamadermatol.2018.1412
- Duchnik E, Kruk J, Tuchowska A, et al. The impact of diet and physical activity on psoriasis: a narrative review of the current evidence. Nutrients. 2023;15:840. doi:10.3390/nu15040840
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis Targets Ther. 2022;12:151-176. doi:10.2147/PTT.S328581
- Mazza E, Ferro Y, Pujia R, et al. Mediterranean diet in healthy aging. J Nutr Health Aging. 2021;25:1076-1083. doi:10.1007/s12603-021-1675-6
- Flores-Balderas X, Peña-Peña M, Rada KM, et al. Beneficial effects of plant-based diets on skin health and inflammatory skin diseases. Nutrients. 2023;15:2842. doi:10.3390/nu15132842
- Bonjour M, Gabriel S, Valencia A, et al. Challenging case in clinical practice: prolonged water-only fasting followed by an exclusively whole-plant-food diet in the management of severe plaque psoriasis. Integr Complement Ther. 2022;28:85-87. doi:10.1089/ict.2022.29010.mbo
- Lewandowska M, Dunbar K, Kassam S. Managing psoriatic arthritis with a whole food plant-based diet: a case study. Am J Lifestyle Med. 2021;15:402-406. doi:10.1177/1559827621993435
- Neufingerl N, Eilander A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. 2021;14:29. doi:10.3390/nu14010029
- Dei-Cas I, Giliberto F, Luce L, et al. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new psoriasis-microbiome index. Sci Rep. 2020;10:12754. doi:10.1038/s41598-020-69537-3
- Satokari R. High intake of sugar and the balance between pro- and anti-inflammatory gut bacteria. Nutrients. 2020;12:1348. doi:10.3390/nu12051348
- Shi Z, Wu X, Santos Rocha C, et al. Short-term Western diet intake promotes IL-23–mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021;141:1780-1791. doi:10.1016/j.jid.2020.11.032
- Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222. doi:10.1097/NT.0000000000000228
- Tuso PJ, Ismail MH, Ha BP, et al. Nutritional update for physicians: plant-based diets. Perm J. 2013;17:61-66. doi:10.7812/TPP/12-085
- Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case–control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Armstrong AW, Harskamp CT, Dhillon JS, et al. Psoriasis and smoking: a systematic review and meta‐analysis. Br J Dermatol. 2014;170:304-314. doi:10.1111/bjd.12670
- Zhu K, Zhu C, Fan Y. Alcohol consumption and psoriatic risk: a meta‐analysis of case–control studies. J Dermatol. 2012;39:770-773. doi:10.1111/j.1346-8138.2012.01577.x
- Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci. 2020;21:5405. doi:10.3390/ijms21155405
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther. 2017;7:227-242. doi:10.1007/s13555-017-0183-4
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934. doi:10.1001/jamadermatol.2018.1412
- Duchnik E, Kruk J, Tuchowska A, et al. The impact of diet and physical activity on psoriasis: a narrative review of the current evidence. Nutrients. 2023;15:840. doi:10.3390/nu15040840
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis Targets Ther. 2022;12:151-176. doi:10.2147/PTT.S328581
- Mazza E, Ferro Y, Pujia R, et al. Mediterranean diet in healthy aging. J Nutr Health Aging. 2021;25:1076-1083. doi:10.1007/s12603-021-1675-6
- Flores-Balderas X, Peña-Peña M, Rada KM, et al. Beneficial effects of plant-based diets on skin health and inflammatory skin diseases. Nutrients. 2023;15:2842. doi:10.3390/nu15132842
- Bonjour M, Gabriel S, Valencia A, et al. Challenging case in clinical practice: prolonged water-only fasting followed by an exclusively whole-plant-food diet in the management of severe plaque psoriasis. Integr Complement Ther. 2022;28:85-87. doi:10.1089/ict.2022.29010.mbo
- Lewandowska M, Dunbar K, Kassam S. Managing psoriatic arthritis with a whole food plant-based diet: a case study. Am J Lifestyle Med. 2021;15:402-406. doi:10.1177/1559827621993435
- Neufingerl N, Eilander A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. 2021;14:29. doi:10.3390/nu14010029
- Dei-Cas I, Giliberto F, Luce L, et al. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new psoriasis-microbiome index. Sci Rep. 2020;10:12754. doi:10.1038/s41598-020-69537-3
- Satokari R. High intake of sugar and the balance between pro- and anti-inflammatory gut bacteria. Nutrients. 2020;12:1348. doi:10.3390/nu12051348
- Shi Z, Wu X, Santos Rocha C, et al. Short-term Western diet intake promotes IL-23–mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021;141:1780-1791. doi:10.1016/j.jid.2020.11.032
- Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222. doi:10.1097/NT.0000000000000228
- Tuso PJ, Ismail MH, Ha BP, et al. Nutritional update for physicians: plant-based diets. Perm J. 2013;17:61-66. doi:10.7812/TPP/12-085
- Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
Practice Points
- Psoriasis is affected by lifestyle factors such as diet, which is an area of interest for many patients.
- Low-calorie diets are strongly recommended for overweight/obese patients with psoriasis to improve their disease.
- Changes in dietary patterns, such as adopting a Mediterranean diet or a plant-based diet, also have shown promise.
Atypical Presentation of Pityriasis Rubra Pilaris: Challenges in Diagnosis and Management
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.1 In the United States, an incidence of 1 in 3500to 5000 patients presenting to dermatology clinics has been reported.2 Pityriasis rubra pilaris has several subtypes and variability in presentation that can make accurate and timely diagnosis challenging.3-5 Herein, we present a case of PRP with complex diagnostic and therapeutic challenges.
A 22-year-old woman presented with symmetrical, well-demarcated, hyperkeratotic, erythematous plaques with a carnauba wax–like appearance on the palms (Figure 1), soles, elbows, and trunk covering approximately 5% of the body surface area. Two weeks prior to presentation, she experienced an upper respiratory tract infection without any treatment and subsequently developed redness on the palms, which became very hard and scaly. The redness then spread to the elbows, soles, and trunk. She reported itching as well as pain in areas of fissuring. Hand mobility became restricted due to thick scale.
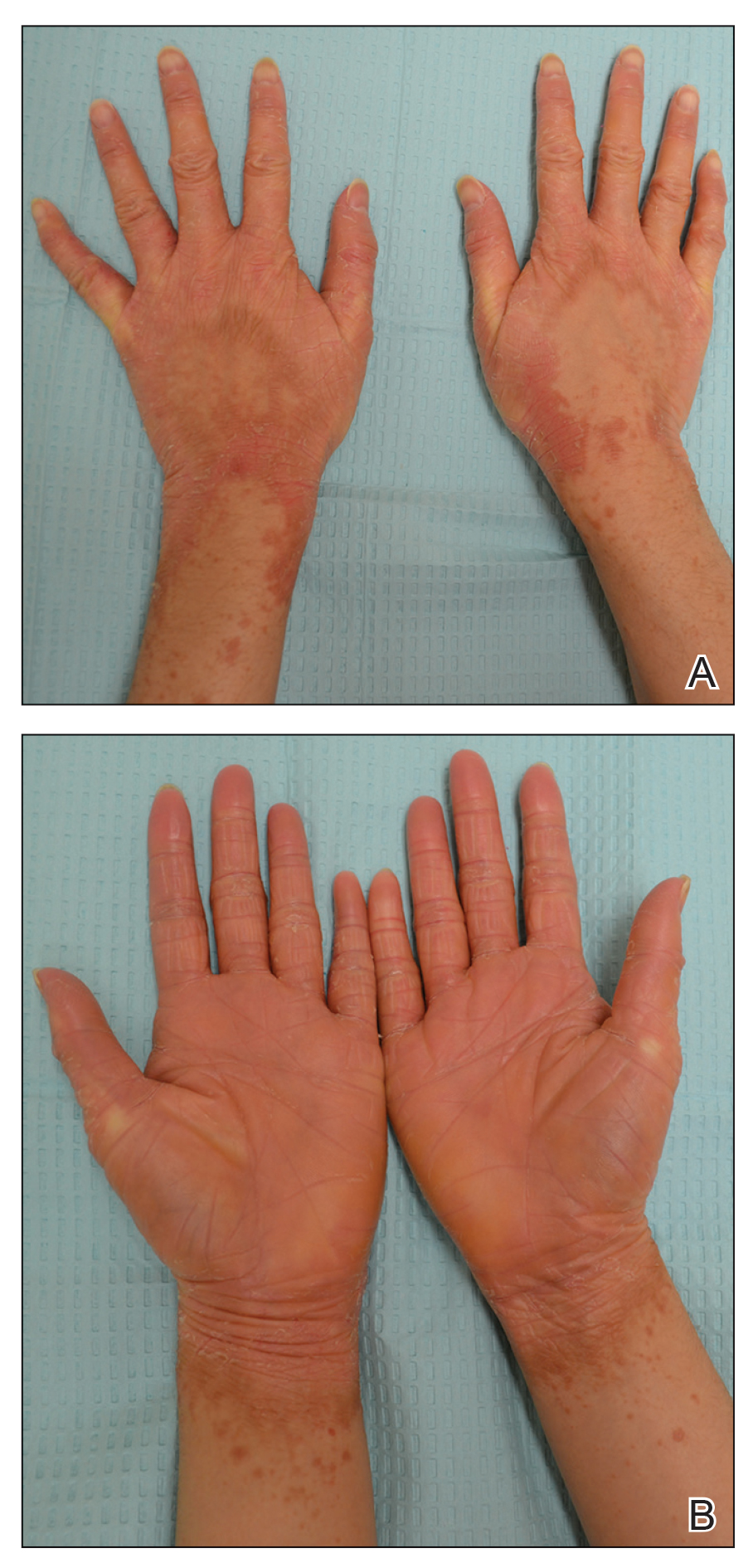
The patient’s medical history was notable for suspected psoriasis 9 years prior, but there were no records or biopsy reports that could be obtained to confirm the diagnosis. She also reported a similar skin condition in her father, which also was diagnosed as psoriasis, but this diagnosis could not be verified.
Although the morphology of the lesions was most consistent with localized PRP, atypical psoriasis, palmoplantar keratoderma (PPK), and erythroderma progressive symmetrica (EPS) also were considered given the personal and family history of suspected psoriasis. A biopsy could not be obtained due to an insurance issue. She was started on clobetasol cream 0.05% and ointment. At 2-week follow-up, her condition remained unchanged. Empiric systemic treatment was discussed, which would potentially work for diagnoses of both PRP and psoriasis. Due to the history of psoriasis and level of discomfort, cyclosporine 300 mg once daily was started to gain rapid control of the disease. Methotrexate also was considered due to its efficacy and economic considerations but was not selected due to patient concerns about the medication.
After 10 weeks of cyclosporine treatment, our patient showed some improvement of the skin with decreased scale and flattening of plaques but not complete resolution. At this point, a biopsy was able to be obtained with prior authorization. A 4-mm punch biopsy of the right flank demonstrated a psoriasiform and papillated epidermis with multifocally capped, compact parakeratosis and minimal lymphocytic infiltrate consistent with PRP. Although EPS also was on the histologic differential, clinical history was more consistent with a diagnosis of PRP. There was some minimal improvement with cyclosporine, but with the diagnosis of PRP confirmed, a systemic retinoid became the treatment of choice. Although acitretin is the preferred treatment for PRP, given that pregnancy would be contraindicated during and for 3 years following acitretin therapy, a trial of isotretinoin 40 mg once daily was started due to its shorter half-life compared to acitretin and was continued for 3 months (Figure 2).6,7
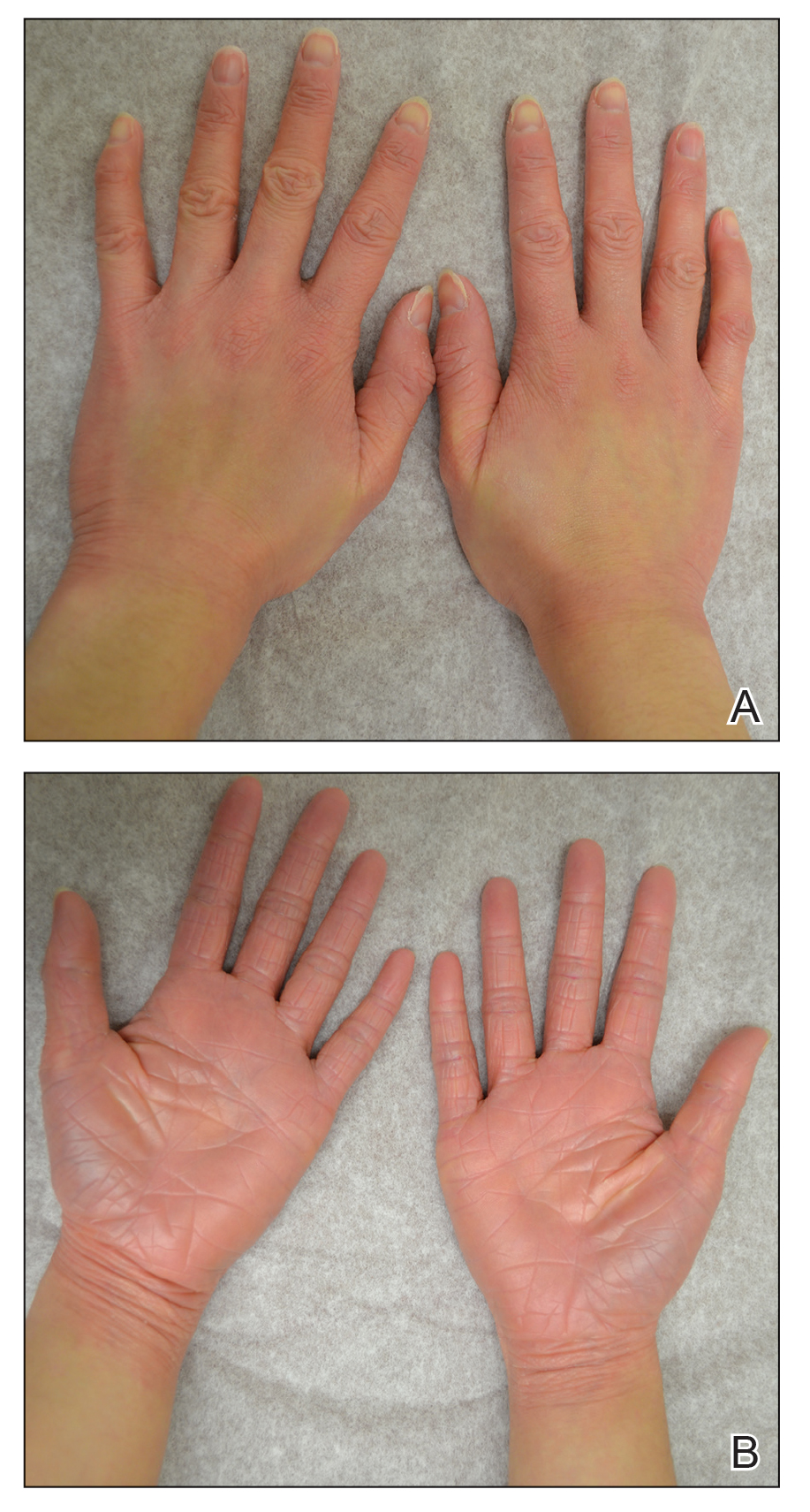
The diagnosis of PRP often can be challenging given the variety of clinical presentations. This case was an atypical presentation of PRP with several learning points, as our patient’s condition did not fit perfectly into any of the 6 types of PRP. The age of onset was atypical at 22 years old. Pityriasis rubra pilaris typically presents with a bimodal age distribution, appearing either in the first decade or the fifth to sixth decades of life.3,8 Her clinical presentation was atypical for adult-onset types I and II, which typically present with cephalocaudal progression or ichthyosiform dermatitis, respectively. Her presentation also was atypical for juvenile onset in types III, IV, and V, which tend to present in younger children and with different physical examination findings.3,8
The morphology of our patient’s lesions also was atypical for PRP, PPK, EPS, and psoriasis. The clinical presentation had features of these entities with erythema, fissuring, xerosis, carnauba wax–like appearance, symmetric scale, and well-demarcated plaques. Although these findings are not mutually exclusive, their combined presentation is atypical. Coupled with the ambiguous family history of similar skin disease in the patient’s father, the discussion of genodermatoses, particularly PPK, further confounded the diagnosis.4,9 When evaluating for PRP, especially with any family history of skin conditions, genodermatoses should be considered. Furthermore, our patient’s remote and unverifiable history of psoriasis serves as a cautionary reminder that prior diagnoses and medical history always should be reasonably scrutinized. Additionally, a drug-induced PRP eruption also should be considered. Although our patient received no medical treatment for the upper respiratory tract infection prior to the onset of PRP, there have been several reports of drug-induced PRP.10-12
The therapeutic challenge in this case is one that often is encountered in clinical practice. The health care system often may pose a barrier to diagnosis by inhibiting particular services required for adequate patient care. For our patient, diagnosis was delayed by several weeks due to difficulties obtaining a diagnostic skin biopsy. When faced with challenges from health care infrastructure, creativity with treatment options, such as finding an empiric treatment option (cyclosporine in this case), must be considered.
Systemic retinoids have been found to be efficacious treatment options for PRP, but when dealing with a woman of reproductive age, reproductive preferences must be discussed before identifying an appropriate treatment regimen.1,13-15 The half-life of acitretin compared to isotretinoin is 2 days vs 22 hours.6,16 With alcohol consumption, acitretin can be metabolized to etretinate, which has a half-life of 120 days.17 In our patient, isotretinoin was a more manageable option to allow for greater reproductive freedom upon treatment completion.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris: a review of diagnosis and treatment. Am J Clin Dermatol. 2010;11:157-170.
- Shenefelt PD. Pityriasis rubra pilaris. Medscape website. Updated September 11, 2020. Accessed September 28, 2021. https://reference.medscape.com/article/1107742-overview
- Griffiths WA. Pityriasis rubra pilaris. Clin Exp Dermatol. 1980;5:105-112.
- Itin PH, Lautenschlager S. Palmoplantar keratoderma and associated syndromes. Semin Dermatol. 1995;14:152-161.
- Guidelines of care for psoriasis. Committee on Guidelines of Care. Task Force on Psoriasis. J Am Acad Dermatol. 1993;28:632-637.
- Larsen FG, Jakobsen P, Eriksen H, et al. The pharmacokinetics of acitretin and its 13-cis-metabolite in psoriatic patients. J Clin Pharmacol. 1991;31:477-483.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Sørensen KB, Thestrup-Pedersen K. Pityriasis rubra pilaris: a retrospective analysis of 43 patients. Acta Derm Venereol. 1999;79:405-406.
- Lucker GP, Van de Kerkhof PC, Steijlen PM. The hereditary palmoplantar keratoses: an updated review and classification. Br J Dermatol. 1994;131:1-14.
- Cutaneous reactions to labetalol. Br Med J. 1978;1:987.
- Plana A, Carrascosa JM, Vilavella M. Pityriasis rubra pilaris‐like reaction induced by imatinib. Clin Exp Dermatol. 2013;38:520-522.
- Gajinov ZT, Matc´ MB, Duran VD, et al. Drug-related pityriasis rubra pilaris with acantholysis. Vojnosanit Pregl. 2013;70:871-873.
- Clayton BD, Jorizzo JL, Hitchcock MG, et al. Adult pityriasis rubra pilaris: a 10-year case series. J Am Acad Dermatol. 1997;36:959-964.
- Cohen PR, Prystowsky JH. Pityriasis rubra pilaris: a review of diagnosis and treatment. J Am Acad Dermatol. 1989;20:801-807.
- Dicken CH. Isotretinoin treatment of pityriasis rubra pilaris. J Am Acad Dermatol. 1987;16(2 pt 1):297-301.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Grønhøj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.1 In the United States, an incidence of 1 in 3500to 5000 patients presenting to dermatology clinics has been reported.2 Pityriasis rubra pilaris has several subtypes and variability in presentation that can make accurate and timely diagnosis challenging.3-5 Herein, we present a case of PRP with complex diagnostic and therapeutic challenges.
A 22-year-old woman presented with symmetrical, well-demarcated, hyperkeratotic, erythematous plaques with a carnauba wax–like appearance on the palms (Figure 1), soles, elbows, and trunk covering approximately 5% of the body surface area. Two weeks prior to presentation, she experienced an upper respiratory tract infection without any treatment and subsequently developed redness on the palms, which became very hard and scaly. The redness then spread to the elbows, soles, and trunk. She reported itching as well as pain in areas of fissuring. Hand mobility became restricted due to thick scale.

The patient’s medical history was notable for suspected psoriasis 9 years prior, but there were no records or biopsy reports that could be obtained to confirm the diagnosis. She also reported a similar skin condition in her father, which also was diagnosed as psoriasis, but this diagnosis could not be verified.
Although the morphology of the lesions was most consistent with localized PRP, atypical psoriasis, palmoplantar keratoderma (PPK), and erythroderma progressive symmetrica (EPS) also were considered given the personal and family history of suspected psoriasis. A biopsy could not be obtained due to an insurance issue. She was started on clobetasol cream 0.05% and ointment. At 2-week follow-up, her condition remained unchanged. Empiric systemic treatment was discussed, which would potentially work for diagnoses of both PRP and psoriasis. Due to the history of psoriasis and level of discomfort, cyclosporine 300 mg once daily was started to gain rapid control of the disease. Methotrexate also was considered due to its efficacy and economic considerations but was not selected due to patient concerns about the medication.
After 10 weeks of cyclosporine treatment, our patient showed some improvement of the skin with decreased scale and flattening of plaques but not complete resolution. At this point, a biopsy was able to be obtained with prior authorization. A 4-mm punch biopsy of the right flank demonstrated a psoriasiform and papillated epidermis with multifocally capped, compact parakeratosis and minimal lymphocytic infiltrate consistent with PRP. Although EPS also was on the histologic differential, clinical history was more consistent with a diagnosis of PRP. There was some minimal improvement with cyclosporine, but with the diagnosis of PRP confirmed, a systemic retinoid became the treatment of choice. Although acitretin is the preferred treatment for PRP, given that pregnancy would be contraindicated during and for 3 years following acitretin therapy, a trial of isotretinoin 40 mg once daily was started due to its shorter half-life compared to acitretin and was continued for 3 months (Figure 2).6,7

The diagnosis of PRP often can be challenging given the variety of clinical presentations. This case was an atypical presentation of PRP with several learning points, as our patient’s condition did not fit perfectly into any of the 6 types of PRP. The age of onset was atypical at 22 years old. Pityriasis rubra pilaris typically presents with a bimodal age distribution, appearing either in the first decade or the fifth to sixth decades of life.3,8 Her clinical presentation was atypical for adult-onset types I and II, which typically present with cephalocaudal progression or ichthyosiform dermatitis, respectively. Her presentation also was atypical for juvenile onset in types III, IV, and V, which tend to present in younger children and with different physical examination findings.3,8
The morphology of our patient’s lesions also was atypical for PRP, PPK, EPS, and psoriasis. The clinical presentation had features of these entities with erythema, fissuring, xerosis, carnauba wax–like appearance, symmetric scale, and well-demarcated plaques. Although these findings are not mutually exclusive, their combined presentation is atypical. Coupled with the ambiguous family history of similar skin disease in the patient’s father, the discussion of genodermatoses, particularly PPK, further confounded the diagnosis.4,9 When evaluating for PRP, especially with any family history of skin conditions, genodermatoses should be considered. Furthermore, our patient’s remote and unverifiable history of psoriasis serves as a cautionary reminder that prior diagnoses and medical history always should be reasonably scrutinized. Additionally, a drug-induced PRP eruption also should be considered. Although our patient received no medical treatment for the upper respiratory tract infection prior to the onset of PRP, there have been several reports of drug-induced PRP.10-12
The therapeutic challenge in this case is one that often is encountered in clinical practice. The health care system often may pose a barrier to diagnosis by inhibiting particular services required for adequate patient care. For our patient, diagnosis was delayed by several weeks due to difficulties obtaining a diagnostic skin biopsy. When faced with challenges from health care infrastructure, creativity with treatment options, such as finding an empiric treatment option (cyclosporine in this case), must be considered.
Systemic retinoids have been found to be efficacious treatment options for PRP, but when dealing with a woman of reproductive age, reproductive preferences must be discussed before identifying an appropriate treatment regimen.1,13-15 The half-life of acitretin compared to isotretinoin is 2 days vs 22 hours.6,16 With alcohol consumption, acitretin can be metabolized to etretinate, which has a half-life of 120 days.17 In our patient, isotretinoin was a more manageable option to allow for greater reproductive freedom upon treatment completion.
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.1 In the United States, an incidence of 1 in 3500to 5000 patients presenting to dermatology clinics has been reported.2 Pityriasis rubra pilaris has several subtypes and variability in presentation that can make accurate and timely diagnosis challenging.3-5 Herein, we present a case of PRP with complex diagnostic and therapeutic challenges.
A 22-year-old woman presented with symmetrical, well-demarcated, hyperkeratotic, erythematous plaques with a carnauba wax–like appearance on the palms (Figure 1), soles, elbows, and trunk covering approximately 5% of the body surface area. Two weeks prior to presentation, she experienced an upper respiratory tract infection without any treatment and subsequently developed redness on the palms, which became very hard and scaly. The redness then spread to the elbows, soles, and trunk. She reported itching as well as pain in areas of fissuring. Hand mobility became restricted due to thick scale.

The patient’s medical history was notable for suspected psoriasis 9 years prior, but there were no records or biopsy reports that could be obtained to confirm the diagnosis. She also reported a similar skin condition in her father, which also was diagnosed as psoriasis, but this diagnosis could not be verified.
Although the morphology of the lesions was most consistent with localized PRP, atypical psoriasis, palmoplantar keratoderma (PPK), and erythroderma progressive symmetrica (EPS) also were considered given the personal and family history of suspected psoriasis. A biopsy could not be obtained due to an insurance issue. She was started on clobetasol cream 0.05% and ointment. At 2-week follow-up, her condition remained unchanged. Empiric systemic treatment was discussed, which would potentially work for diagnoses of both PRP and psoriasis. Due to the history of psoriasis and level of discomfort, cyclosporine 300 mg once daily was started to gain rapid control of the disease. Methotrexate also was considered due to its efficacy and economic considerations but was not selected due to patient concerns about the medication.
After 10 weeks of cyclosporine treatment, our patient showed some improvement of the skin with decreased scale and flattening of plaques but not complete resolution. At this point, a biopsy was able to be obtained with prior authorization. A 4-mm punch biopsy of the right flank demonstrated a psoriasiform and papillated epidermis with multifocally capped, compact parakeratosis and minimal lymphocytic infiltrate consistent with PRP. Although EPS also was on the histologic differential, clinical history was more consistent with a diagnosis of PRP. There was some minimal improvement with cyclosporine, but with the diagnosis of PRP confirmed, a systemic retinoid became the treatment of choice. Although acitretin is the preferred treatment for PRP, given that pregnancy would be contraindicated during and for 3 years following acitretin therapy, a trial of isotretinoin 40 mg once daily was started due to its shorter half-life compared to acitretin and was continued for 3 months (Figure 2).6,7

The diagnosis of PRP often can be challenging given the variety of clinical presentations. This case was an atypical presentation of PRP with several learning points, as our patient’s condition did not fit perfectly into any of the 6 types of PRP. The age of onset was atypical at 22 years old. Pityriasis rubra pilaris typically presents with a bimodal age distribution, appearing either in the first decade or the fifth to sixth decades of life.3,8 Her clinical presentation was atypical for adult-onset types I and II, which typically present with cephalocaudal progression or ichthyosiform dermatitis, respectively. Her presentation also was atypical for juvenile onset in types III, IV, and V, which tend to present in younger children and with different physical examination findings.3,8
The morphology of our patient’s lesions also was atypical for PRP, PPK, EPS, and psoriasis. The clinical presentation had features of these entities with erythema, fissuring, xerosis, carnauba wax–like appearance, symmetric scale, and well-demarcated plaques. Although these findings are not mutually exclusive, their combined presentation is atypical. Coupled with the ambiguous family history of similar skin disease in the patient’s father, the discussion of genodermatoses, particularly PPK, further confounded the diagnosis.4,9 When evaluating for PRP, especially with any family history of skin conditions, genodermatoses should be considered. Furthermore, our patient’s remote and unverifiable history of psoriasis serves as a cautionary reminder that prior diagnoses and medical history always should be reasonably scrutinized. Additionally, a drug-induced PRP eruption also should be considered. Although our patient received no medical treatment for the upper respiratory tract infection prior to the onset of PRP, there have been several reports of drug-induced PRP.10-12
The therapeutic challenge in this case is one that often is encountered in clinical practice. The health care system often may pose a barrier to diagnosis by inhibiting particular services required for adequate patient care. For our patient, diagnosis was delayed by several weeks due to difficulties obtaining a diagnostic skin biopsy. When faced with challenges from health care infrastructure, creativity with treatment options, such as finding an empiric treatment option (cyclosporine in this case), must be considered.
Systemic retinoids have been found to be efficacious treatment options for PRP, but when dealing with a woman of reproductive age, reproductive preferences must be discussed before identifying an appropriate treatment regimen.1,13-15 The half-life of acitretin compared to isotretinoin is 2 days vs 22 hours.6,16 With alcohol consumption, acitretin can be metabolized to etretinate, which has a half-life of 120 days.17 In our patient, isotretinoin was a more manageable option to allow for greater reproductive freedom upon treatment completion.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris: a review of diagnosis and treatment. Am J Clin Dermatol. 2010;11:157-170.
- Shenefelt PD. Pityriasis rubra pilaris. Medscape website. Updated September 11, 2020. Accessed September 28, 2021. https://reference.medscape.com/article/1107742-overview
- Griffiths WA. Pityriasis rubra pilaris. Clin Exp Dermatol. 1980;5:105-112.
- Itin PH, Lautenschlager S. Palmoplantar keratoderma and associated syndromes. Semin Dermatol. 1995;14:152-161.
- Guidelines of care for psoriasis. Committee on Guidelines of Care. Task Force on Psoriasis. J Am Acad Dermatol. 1993;28:632-637.
- Larsen FG, Jakobsen P, Eriksen H, et al. The pharmacokinetics of acitretin and its 13-cis-metabolite in psoriatic patients. J Clin Pharmacol. 1991;31:477-483.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Sørensen KB, Thestrup-Pedersen K. Pityriasis rubra pilaris: a retrospective analysis of 43 patients. Acta Derm Venereol. 1999;79:405-406.
- Lucker GP, Van de Kerkhof PC, Steijlen PM. The hereditary palmoplantar keratoses: an updated review and classification. Br J Dermatol. 1994;131:1-14.
- Cutaneous reactions to labetalol. Br Med J. 1978;1:987.
- Plana A, Carrascosa JM, Vilavella M. Pityriasis rubra pilaris‐like reaction induced by imatinib. Clin Exp Dermatol. 2013;38:520-522.
- Gajinov ZT, Matc´ MB, Duran VD, et al. Drug-related pityriasis rubra pilaris with acantholysis. Vojnosanit Pregl. 2013;70:871-873.
- Clayton BD, Jorizzo JL, Hitchcock MG, et al. Adult pityriasis rubra pilaris: a 10-year case series. J Am Acad Dermatol. 1997;36:959-964.
- Cohen PR, Prystowsky JH. Pityriasis rubra pilaris: a review of diagnosis and treatment. J Am Acad Dermatol. 1989;20:801-807.
- Dicken CH. Isotretinoin treatment of pityriasis rubra pilaris. J Am Acad Dermatol. 1987;16(2 pt 1):297-301.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Grønhøj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
- Klein A, Landthaler M, Karrer S. Pityriasis rubra pilaris: a review of diagnosis and treatment. Am J Clin Dermatol. 2010;11:157-170.
- Shenefelt PD. Pityriasis rubra pilaris. Medscape website. Updated September 11, 2020. Accessed September 28, 2021. https://reference.medscape.com/article/1107742-overview
- Griffiths WA. Pityriasis rubra pilaris. Clin Exp Dermatol. 1980;5:105-112.
- Itin PH, Lautenschlager S. Palmoplantar keratoderma and associated syndromes. Semin Dermatol. 1995;14:152-161.
- Guidelines of care for psoriasis. Committee on Guidelines of Care. Task Force on Psoriasis. J Am Acad Dermatol. 1993;28:632-637.
- Larsen FG, Jakobsen P, Eriksen H, et al. The pharmacokinetics of acitretin and its 13-cis-metabolite in psoriatic patients. J Clin Pharmacol. 1991;31:477-483.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Sørensen KB, Thestrup-Pedersen K. Pityriasis rubra pilaris: a retrospective analysis of 43 patients. Acta Derm Venereol. 1999;79:405-406.
- Lucker GP, Van de Kerkhof PC, Steijlen PM. The hereditary palmoplantar keratoses: an updated review and classification. Br J Dermatol. 1994;131:1-14.
- Cutaneous reactions to labetalol. Br Med J. 1978;1:987.
- Plana A, Carrascosa JM, Vilavella M. Pityriasis rubra pilaris‐like reaction induced by imatinib. Clin Exp Dermatol. 2013;38:520-522.
- Gajinov ZT, Matc´ MB, Duran VD, et al. Drug-related pityriasis rubra pilaris with acantholysis. Vojnosanit Pregl. 2013;70:871-873.
- Clayton BD, Jorizzo JL, Hitchcock MG, et al. Adult pityriasis rubra pilaris: a 10-year case series. J Am Acad Dermatol. 1997;36:959-964.
- Cohen PR, Prystowsky JH. Pityriasis rubra pilaris: a review of diagnosis and treatment. J Am Acad Dermatol. 1989;20:801-807.
- Dicken CH. Isotretinoin treatment of pityriasis rubra pilaris. J Am Acad Dermatol. 1987;16(2 pt 1):297-301.
- Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1:162-169.
- Grønhøj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
Practice Points
- Pityriasis rubra pilaris (PRP) is a rare inflammatory dermatosis of unknown etiology characterized by erythematosquamous salmon-colored plaques with well-demarcated islands of unaffected skin and hyperkeratotic follicles.
- The diagnosis of PRP often can be challenging given the variety of clinical presentations.
Dupilumab-Induced Facial Flushing After Alcohol Consumption
Dupilumab is a fully humanized monoclonal antibody to the α subunit of the IL-4 receptor that inhibits the action of helper T cell (TH2)–type cytokines IL-4 and IL-13. Dupilumab was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe atopic dermatitis (AD). We report 2 patients with AD who were treated with dupilumab and subsequently developed facial flushing after consuming alcohol.
Case Report
Patient 1
A 24-year-old woman presented to the dermatology clinic with a lifelong history of moderate to severe AD. She had a medical history of asthma and seasonal allergies, which were treated with fexofenadine and an inhaler, as needed. The patient had an affected body surface area of approximately 70% and had achieved only partial relief with topical corticosteroids and topical calcineurin inhibitors.
Because her disease was severe, the patient was started on dupilumab at FDA-approved dosing for AD: a 600-mg subcutaneous (SC) loading dose, followed by 300 mg SC every 2 weeks. She reported rapid skin clearance within 2 weeks of the start of treatment. Her course was complicated by mild head and neck dermatitis.
Seven months after starting treatment, the patient began to acutely experience erythema and warmth over the entire face that was triggered by drinking alcohol (Figure). Before starting dupilumab, she had consumed alcohol on multiple occasions without a flushing effect. This new finding was distinguishable from her facial dermatitis. Onset was within a few minutes after drinking alcohol; flushing self-resolved in 15 to 30 minutes. Although diffuse, erythema and warmth were concentrated around the jawline, eyebrows, and ears and occurred every time the patient drank alcohol. Moreover, she reported that consumption of hard (ie, distilled) liquor, specifically tequila, caused a more severe presentation. She denied other symptoms associated with dupilumab.
Patient 2
A 32-year-old man presented to the dermatology clinic with a 10-year history of moderate to severe AD. He had a medical history of asthma (treated with albuterol, montelukast, and fluticasone); allergic rhinitis; and severe environmental allergies, including sensitivity to dust mites, dogs, trees, and grass.
For AD, the patient had been treated with topical corticosteroids and the Goeckerman regimen (a combination of phototherapy and crude coal tar). He experienced only partial relief with topical corticosteroids; the Goeckerman regimen cleared his skin, but he had quick recurrence after approximately 1 month. Given his work schedule, the patient was unable to resume phototherapy.
Because of symptoms related to the patient’s severe allergies, his allergist prescribed dupilumab: a 600-mg SC loading dose, followed by 300 mg SC every 2 weeks. The patient reported near-complete resolution of AD symptoms approximately 2 months after initiating treatment. He reported a few episodes of mild conjunctivitis that self-resolved after the first month of treatment.
Three weeks after initiating dupilumab, the patient noticed new-onset facial flushing in response to consuming alcohol. He described flushing as sudden immediate redness and warmth concentrated around the forehead, eyes, and cheeks. He reported that flushing was worse with hard liquor than with beer. Flushing would slowly subside over approximately 30 minutes despite continued alcohol consumption.
Comment
Two other single-patient case reports have discussed similar findings of alcohol-induced flushing associated with dupilumab.1,2 Both of those patients—a 19-year-old woman and a 26-year-old woman—had not experienced flushing before beginning treatment with dupilumab for AD. Both experienced onset of facial flushing months after beginning dupilumab even though both had consumed alcohol before starting dupilumab, similar to the cases presented here. One patient had a history of asthma; the other had a history of seasonal and environmental allergies.
Possible Mechanism of Action
Acute alcohol ingestion causes dermal vasodilation of the skin (ie, flushing).3 A proposed mechanism is that flushing results from direct action on central vascular-control mechanisms. This theory results from observations that individuals with quadriplegia lack notable ethanol-induced vasodilation, suggesting that ethanol has a central neural site of action.Although some research has indicated that ethanol might induce these effects by altering the action of certain hormones (eg, angiotensin, vasopressin, and catecholamines), the precise mechanism by which ethanol alters vascular function in humans remains unexplained.3
Deficiencies in alcohol dehydrogenase (ADH), aldehyde dehydrogenase 2, and certain cytochrome P450 enzymes also might contribute to facial flushing. People of Asian, especially East Asian, descent often respond to an acute dose of ethanol with symptoms of facial flushing—predominantly the result of an elevated blood level of acetaldehyde caused by an inherited deficiency of aldehyde dehydrogenase 2,4 which is downstream from ADH in the metabolic pathway of alcohol. The major enzyme system responsible for metabolism of ethanol is ADH; however, the cytochrome P450–dependent ethanol-oxidizing system—including major CYP450 isoforms CYP3A, CYP2C19, CYP2C9, CYP1A2, and CYP2D6, as well as minor CYP450 isoforms, such as CYP2E1— also are involved, to a lesser extent.5
A Role for Dupilumab?
A recent pharmacokinetic study found that dupilumab appears to have little effect on the activity of the major CYP450 isoforms. However, the drug’s effect on ADH and minor CYP450 minor isoforms is unknown. Prior drug-drug interaction studies have shown that certain cytokines and cytokine modulators can markedly influence the expression, stability, and activity of specific CYP450 enzymes.6 For example, IL-6 causes a reduction in messenger RNA for CYP3A4 and, to a lesser extent, for other isoforms.7 Whether dupilumab influences enzymes involved in processing alcohol requires further study.
Conclusion
We describe 2 cases of dupilumab-induced facial flushing after alcohol consumption. The mechanism of this dupilumab-associated flushing is unknown and requires further research.
- Herz S, Petri M, Sondermann W. New alcohol flushing in a patient with atopic dermatitis under therapy with dupilumab. Dermatol Ther. 2019;32:e12762. doi:10.1111/dth.12762
- Igelman SJ, Na C, Simpson EL. Alcohol-induced facial flushing in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020;6:139-140. doi:10.1016/j.jdcr.2019.12.002
- Malpas SC, Robinson BJ, Maling TJ. Mechanism of ethanol-induced vasodilation. J Appl Physiol (1985). 1990;68:731-734. doi:10.1152/jappl.1990.68.2.731
- Brooks PJ, Enoch M-A, Goldman D, et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi:10.1371/journal.pmed.1000050
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667-685. doi:10.1016/j.cld.2012.08.002
- Davis JD, Bansal A, Hassman D, et al. Evaluation of potential disease-mediated drug-drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146-1154. doi:10.1002/cpt.1058
- Mimura H, Kobayashi K, Xu L, et al. Effects of cytokines on CYP3A4 expression and reversal of the effects by anti-cytokine agents in the three-dimensionally cultured human hepatoma cell line FLC-4. Drug Metab Pharmacokinet. 2015;30:105-110. doi:10.1016/j.dmpk.2014.09.004
Dupilumab is a fully humanized monoclonal antibody to the α subunit of the IL-4 receptor that inhibits the action of helper T cell (TH2)–type cytokines IL-4 and IL-13. Dupilumab was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe atopic dermatitis (AD). We report 2 patients with AD who were treated with dupilumab and subsequently developed facial flushing after consuming alcohol.
Case Report
Patient 1
A 24-year-old woman presented to the dermatology clinic with a lifelong history of moderate to severe AD. She had a medical history of asthma and seasonal allergies, which were treated with fexofenadine and an inhaler, as needed. The patient had an affected body surface area of approximately 70% and had achieved only partial relief with topical corticosteroids and topical calcineurin inhibitors.
Because her disease was severe, the patient was started on dupilumab at FDA-approved dosing for AD: a 600-mg subcutaneous (SC) loading dose, followed by 300 mg SC every 2 weeks. She reported rapid skin clearance within 2 weeks of the start of treatment. Her course was complicated by mild head and neck dermatitis.
Seven months after starting treatment, the patient began to acutely experience erythema and warmth over the entire face that was triggered by drinking alcohol (Figure). Before starting dupilumab, she had consumed alcohol on multiple occasions without a flushing effect. This new finding was distinguishable from her facial dermatitis. Onset was within a few minutes after drinking alcohol; flushing self-resolved in 15 to 30 minutes. Although diffuse, erythema and warmth were concentrated around the jawline, eyebrows, and ears and occurred every time the patient drank alcohol. Moreover, she reported that consumption of hard (ie, distilled) liquor, specifically tequila, caused a more severe presentation. She denied other symptoms associated with dupilumab.

Patient 2
A 32-year-old man presented to the dermatology clinic with a 10-year history of moderate to severe AD. He had a medical history of asthma (treated with albuterol, montelukast, and fluticasone); allergic rhinitis; and severe environmental allergies, including sensitivity to dust mites, dogs, trees, and grass.
For AD, the patient had been treated with topical corticosteroids and the Goeckerman regimen (a combination of phototherapy and crude coal tar). He experienced only partial relief with topical corticosteroids; the Goeckerman regimen cleared his skin, but he had quick recurrence after approximately 1 month. Given his work schedule, the patient was unable to resume phototherapy.
Because of symptoms related to the patient’s severe allergies, his allergist prescribed dupilumab: a 600-mg SC loading dose, followed by 300 mg SC every 2 weeks. The patient reported near-complete resolution of AD symptoms approximately 2 months after initiating treatment. He reported a few episodes of mild conjunctivitis that self-resolved after the first month of treatment.
Three weeks after initiating dupilumab, the patient noticed new-onset facial flushing in response to consuming alcohol. He described flushing as sudden immediate redness and warmth concentrated around the forehead, eyes, and cheeks. He reported that flushing was worse with hard liquor than with beer. Flushing would slowly subside over approximately 30 minutes despite continued alcohol consumption.
Comment
Two other single-patient case reports have discussed similar findings of alcohol-induced flushing associated with dupilumab.1,2 Both of those patients—a 19-year-old woman and a 26-year-old woman—had not experienced flushing before beginning treatment with dupilumab for AD. Both experienced onset of facial flushing months after beginning dupilumab even though both had consumed alcohol before starting dupilumab, similar to the cases presented here. One patient had a history of asthma; the other had a history of seasonal and environmental allergies.
Possible Mechanism of Action
Acute alcohol ingestion causes dermal vasodilation of the skin (ie, flushing).3 A proposed mechanism is that flushing results from direct action on central vascular-control mechanisms. This theory results from observations that individuals with quadriplegia lack notable ethanol-induced vasodilation, suggesting that ethanol has a central neural site of action.Although some research has indicated that ethanol might induce these effects by altering the action of certain hormones (eg, angiotensin, vasopressin, and catecholamines), the precise mechanism by which ethanol alters vascular function in humans remains unexplained.3
Deficiencies in alcohol dehydrogenase (ADH), aldehyde dehydrogenase 2, and certain cytochrome P450 enzymes also might contribute to facial flushing. People of Asian, especially East Asian, descent often respond to an acute dose of ethanol with symptoms of facial flushing—predominantly the result of an elevated blood level of acetaldehyde caused by an inherited deficiency of aldehyde dehydrogenase 2,4 which is downstream from ADH in the metabolic pathway of alcohol. The major enzyme system responsible for metabolism of ethanol is ADH; however, the cytochrome P450–dependent ethanol-oxidizing system—including major CYP450 isoforms CYP3A, CYP2C19, CYP2C9, CYP1A2, and CYP2D6, as well as minor CYP450 isoforms, such as CYP2E1— also are involved, to a lesser extent.5
A Role for Dupilumab?
A recent pharmacokinetic study found that dupilumab appears to have little effect on the activity of the major CYP450 isoforms. However, the drug’s effect on ADH and minor CYP450 minor isoforms is unknown. Prior drug-drug interaction studies have shown that certain cytokines and cytokine modulators can markedly influence the expression, stability, and activity of specific CYP450 enzymes.6 For example, IL-6 causes a reduction in messenger RNA for CYP3A4 and, to a lesser extent, for other isoforms.7 Whether dupilumab influences enzymes involved in processing alcohol requires further study.
Conclusion
We describe 2 cases of dupilumab-induced facial flushing after alcohol consumption. The mechanism of this dupilumab-associated flushing is unknown and requires further research.
Dupilumab is a fully humanized monoclonal antibody to the α subunit of the IL-4 receptor that inhibits the action of helper T cell (TH2)–type cytokines IL-4 and IL-13. Dupilumab was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe atopic dermatitis (AD). We report 2 patients with AD who were treated with dupilumab and subsequently developed facial flushing after consuming alcohol.
Case Report
Patient 1
A 24-year-old woman presented to the dermatology clinic with a lifelong history of moderate to severe AD. She had a medical history of asthma and seasonal allergies, which were treated with fexofenadine and an inhaler, as needed. The patient had an affected body surface area of approximately 70% and had achieved only partial relief with topical corticosteroids and topical calcineurin inhibitors.
Because her disease was severe, the patient was started on dupilumab at FDA-approved dosing for AD: a 600-mg subcutaneous (SC) loading dose, followed by 300 mg SC every 2 weeks. She reported rapid skin clearance within 2 weeks of the start of treatment. Her course was complicated by mild head and neck dermatitis.
Seven months after starting treatment, the patient began to acutely experience erythema and warmth over the entire face that was triggered by drinking alcohol (Figure). Before starting dupilumab, she had consumed alcohol on multiple occasions without a flushing effect. This new finding was distinguishable from her facial dermatitis. Onset was within a few minutes after drinking alcohol; flushing self-resolved in 15 to 30 minutes. Although diffuse, erythema and warmth were concentrated around the jawline, eyebrows, and ears and occurred every time the patient drank alcohol. Moreover, she reported that consumption of hard (ie, distilled) liquor, specifically tequila, caused a more severe presentation. She denied other symptoms associated with dupilumab.

Patient 2
A 32-year-old man presented to the dermatology clinic with a 10-year history of moderate to severe AD. He had a medical history of asthma (treated with albuterol, montelukast, and fluticasone); allergic rhinitis; and severe environmental allergies, including sensitivity to dust mites, dogs, trees, and grass.
For AD, the patient had been treated with topical corticosteroids and the Goeckerman regimen (a combination of phototherapy and crude coal tar). He experienced only partial relief with topical corticosteroids; the Goeckerman regimen cleared his skin, but he had quick recurrence after approximately 1 month. Given his work schedule, the patient was unable to resume phototherapy.
Because of symptoms related to the patient’s severe allergies, his allergist prescribed dupilumab: a 600-mg SC loading dose, followed by 300 mg SC every 2 weeks. The patient reported near-complete resolution of AD symptoms approximately 2 months after initiating treatment. He reported a few episodes of mild conjunctivitis that self-resolved after the first month of treatment.
Three weeks after initiating dupilumab, the patient noticed new-onset facial flushing in response to consuming alcohol. He described flushing as sudden immediate redness and warmth concentrated around the forehead, eyes, and cheeks. He reported that flushing was worse with hard liquor than with beer. Flushing would slowly subside over approximately 30 minutes despite continued alcohol consumption.
Comment
Two other single-patient case reports have discussed similar findings of alcohol-induced flushing associated with dupilumab.1,2 Both of those patients—a 19-year-old woman and a 26-year-old woman—had not experienced flushing before beginning treatment with dupilumab for AD. Both experienced onset of facial flushing months after beginning dupilumab even though both had consumed alcohol before starting dupilumab, similar to the cases presented here. One patient had a history of asthma; the other had a history of seasonal and environmental allergies.
Possible Mechanism of Action
Acute alcohol ingestion causes dermal vasodilation of the skin (ie, flushing).3 A proposed mechanism is that flushing results from direct action on central vascular-control mechanisms. This theory results from observations that individuals with quadriplegia lack notable ethanol-induced vasodilation, suggesting that ethanol has a central neural site of action.Although some research has indicated that ethanol might induce these effects by altering the action of certain hormones (eg, angiotensin, vasopressin, and catecholamines), the precise mechanism by which ethanol alters vascular function in humans remains unexplained.3
Deficiencies in alcohol dehydrogenase (ADH), aldehyde dehydrogenase 2, and certain cytochrome P450 enzymes also might contribute to facial flushing. People of Asian, especially East Asian, descent often respond to an acute dose of ethanol with symptoms of facial flushing—predominantly the result of an elevated blood level of acetaldehyde caused by an inherited deficiency of aldehyde dehydrogenase 2,4 which is downstream from ADH in the metabolic pathway of alcohol. The major enzyme system responsible for metabolism of ethanol is ADH; however, the cytochrome P450–dependent ethanol-oxidizing system—including major CYP450 isoforms CYP3A, CYP2C19, CYP2C9, CYP1A2, and CYP2D6, as well as minor CYP450 isoforms, such as CYP2E1— also are involved, to a lesser extent.5
A Role for Dupilumab?
A recent pharmacokinetic study found that dupilumab appears to have little effect on the activity of the major CYP450 isoforms. However, the drug’s effect on ADH and minor CYP450 minor isoforms is unknown. Prior drug-drug interaction studies have shown that certain cytokines and cytokine modulators can markedly influence the expression, stability, and activity of specific CYP450 enzymes.6 For example, IL-6 causes a reduction in messenger RNA for CYP3A4 and, to a lesser extent, for other isoforms.7 Whether dupilumab influences enzymes involved in processing alcohol requires further study.
Conclusion
We describe 2 cases of dupilumab-induced facial flushing after alcohol consumption. The mechanism of this dupilumab-associated flushing is unknown and requires further research.
- Herz S, Petri M, Sondermann W. New alcohol flushing in a patient with atopic dermatitis under therapy with dupilumab. Dermatol Ther. 2019;32:e12762. doi:10.1111/dth.12762
- Igelman SJ, Na C, Simpson EL. Alcohol-induced facial flushing in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020;6:139-140. doi:10.1016/j.jdcr.2019.12.002
- Malpas SC, Robinson BJ, Maling TJ. Mechanism of ethanol-induced vasodilation. J Appl Physiol (1985). 1990;68:731-734. doi:10.1152/jappl.1990.68.2.731
- Brooks PJ, Enoch M-A, Goldman D, et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi:10.1371/journal.pmed.1000050
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667-685. doi:10.1016/j.cld.2012.08.002
- Davis JD, Bansal A, Hassman D, et al. Evaluation of potential disease-mediated drug-drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146-1154. doi:10.1002/cpt.1058
- Mimura H, Kobayashi K, Xu L, et al. Effects of cytokines on CYP3A4 expression and reversal of the effects by anti-cytokine agents in the three-dimensionally cultured human hepatoma cell line FLC-4. Drug Metab Pharmacokinet. 2015;30:105-110. doi:10.1016/j.dmpk.2014.09.004
- Herz S, Petri M, Sondermann W. New alcohol flushing in a patient with atopic dermatitis under therapy with dupilumab. Dermatol Ther. 2019;32:e12762. doi:10.1111/dth.12762
- Igelman SJ, Na C, Simpson EL. Alcohol-induced facial flushing in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020;6:139-140. doi:10.1016/j.jdcr.2019.12.002
- Malpas SC, Robinson BJ, Maling TJ. Mechanism of ethanol-induced vasodilation. J Appl Physiol (1985). 1990;68:731-734. doi:10.1152/jappl.1990.68.2.731
- Brooks PJ, Enoch M-A, Goldman D, et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi:10.1371/journal.pmed.1000050
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667-685. doi:10.1016/j.cld.2012.08.002
- Davis JD, Bansal A, Hassman D, et al. Evaluation of potential disease-mediated drug-drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146-1154. doi:10.1002/cpt.1058
- Mimura H, Kobayashi K, Xu L, et al. Effects of cytokines on CYP3A4 expression and reversal of the effects by anti-cytokine agents in the three-dimensionally cultured human hepatoma cell line FLC-4. Drug Metab Pharmacokinet. 2015;30:105-110. doi:10.1016/j.dmpk.2014.09.004
Practice Points
- Dupilumab is a fully humanized monoclonal antibody that inhibits the action of IL-4 and IL-13. It was approved by the US Food and Drug Administration in 2017 for treatment of moderate to severe atopic dermatitis.
- Facial flushing after alcohol consumption may be an emerging side effect of dupilumab.
- Whether dupilumab influences enzymes involved in processing alcohol requires further study.
Psoriasis Risk Factors and Triggers
Psoriasis is a chronic autoimmune skin disease affecting approximately 6.7 million adults in the United States.1 Although its pathogenesis is not yet clear, risk factors and triggers provide insight into potential pathways by which psoriasis can occur. There is notable overlap between risk factors and triggers of psoriasis; perceived risk factors might, in fact, be triggers causing manifestation of disease in predisposed persons. In this review, we summarize the key factors contributing to onset and exacerbation of psoriasis. When learning to manage this chronic disease, it also may be helpful to educate patients about how these elements may affect the course of psoriasis.
Genetics
The pathogenesis of psoriasis has a strong genetic component, with approximately 70% and 20% concordance rates in monozygotic and dizygotic twins, respectively.2 Moreover, studies have shown a positive family history in approximately 35% of patients.3,4 Family-based studies have found a 50% risk of developing psoriasis in patients with 2 affected parents.5 However, the genetics of psoriasis are complex and are attributed to many different genes. Thus far, genes involving antigen presentation, T-cell receptor development and polarization, and the nuclear factor κβ (NF-κβ) pathway have been identified.6
HLA-Cw6
The most well-studied gene implicated in psoriasis is HLA-Cw6, which encodes a major histocompatibility complex class I allele supporting psoriasis as a T cell–mediated reaction to an autoantigen.6 Two potential antigens for HLA-Cw6 recently have been identified: LL-37, a cathelicidin-related antimicrobial peptide, and the A disintegrin and metalloproteinase with thrombospondin motifs-like protein 5 (ADAMTSL5), found on melanocytes and keratinocytes.7 The percentage of psoriasis patients with HLA-Cw6 ranges from 10.5% to 77.2%, with higher frequency in white individuals than in Asians.7
HLA-Cw6 manifests as specific features in psoriasis, including onset of disease before 21 years of age.8 It also is more strongly associated with guttate-type psoriasis, greater body surface area involvement, and higher incidence of Köbner phenomenon. Patients with positive HLA-Cw6 also reported worsening of psoriasis during and after throat infection.9
Caspase Recruitment Domain Family Member 14
Another gene mutation implicated in psoriasis pathogenesis is caspase recruitment domain family member 14, CARD14 (formerly PSORS2), a gene encoding a scaffolding protein important in the activation of NF-κβ.10,11 Missense CARD14 mutations cause upregulation of NF-κβ through formation of a complex with adapter protein B-cell lymphoma 10 (BCL10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1),12 which, in turn, causes increased transcription of cytokines IL-8, C-C motif chemokine ligand 20 (CCL-20), and IL-36 gamma in the keratinocyte.13 Mutations in CARD14 alone lead to psoriasiform skin in mice through amplified activation of the IL-23/IL-17 axis.14,15 Patients with a mutation in a CARD14 variant (p.Arg820Trp) have demonstrated better response to tumor necrosis factor (TNF) inhibitors.16
Further characterization of the genetic pathogenesis of psoriasis might lead to better targeted therapies, including the possibility of MALT1 inhibitors as a treatment option.12
Infection
Streptococcus
The association between streptococcal infection and psoriasis was first documented more than 100 years ago, specifically the onset of acute guttate psoriasis.17,18 Although classically described following throat infection, psoriasis also occurs following streptococcal vulvovaginitis and perianal streptococcal infection.19,20
This type of psoriasis is typically self-limited but can recur with subsequent streptococcal infections or initiate a more chronic plaque psoriasis. Patients have a 1 in 3 risk of developing chronic psoriasis within 10 years of a single episode of acute guttate psoriasis.21 Moreover, in many patients with existing plaque psoriasis, throat infection exacerbates psoriatic symptoms.22 The mechanism of exacerbation is likely due to cross-reactivity between streptococcal M surface antigen and human keratinocytes and might also be influenced by inherited abnormalities in immune response.23-26 Therefore, tonsillectomy has been studied as a possible treatment of psoriasis but is likely helpful only in patients with exacerbations of disease that are closely associated with recurrent tonsillitis.27
Human Immunodeficiency Virus
The prevalence of psoriasis in human immunodeficiency virus (HIV) patients is similar to or greater than the general population.28 Human immunodeficiency virus infection causes new onset of psoriasis and exacerbation of existing psoriasis; severity often is correlated with worsening immune function.28,29
The clinical subtypes of psoriasis that occur most frequently with HIV include guttate, inverse, and erythrodermic, though patients may present with any subtype.28 The mechanism is puzzling because HIV is primarily mediated by helper T cell 2 (TH2) cytokines, whereas psoriasis is mainly driven by helper T cell 1 (TH1) cytokines.30 Furthermore, despite increased severity with lower CD4+ counts, treatments further lowering T-cell counts paradoxically improve symptoms.31 Current literature suggests that expansion of CD8+ memory T cells might be the primary mechanism in the exacerbation of psoriasis in HIV-mediated immunosuppression.30
Treatment of HIV-associated psoriasis presents challenges because many therapeutics cause further immunosuppression. The National Psoriasis Foundation recommends topical preparations as first-line agents for mild to moderate psoriasis.32 For moderate to severe psoriasis, retroviral agents may be effective as first-line monotherapy or when supplemented by phototherapy with UVB or psoralen plus UVA. Retinoids can be used as second-line agents.32 For cases of severe refractory psoriasis, cyclosporine, methotrexate, TNF inhibitors, or hydroxyurea can be considered. There also is evidence that apremilast is effective without risk for worsening immune function.33
Other Infections
Other bacteria associated with triggering or exacerbating psoriasis include Staphylococcus aureus and Helicobacter pylori.34,35 Fungi, such as species of the genera Malassezia and Candida, and other viruses, including papillomaviruses and retroviruses, also have been implicated.34
Medications
Numerous medications can trigger psoriasis, including lithium, nonsteroidal anti-inflammatory drugs, antimalarials, beta-blockers, and angiotensin-converting enzyme inhibitors.34 More recent literature suggests that TNF inhibitors also can paradoxically induce psoriasis in rare cases.35
Lithium
Psoriasis is the most common cutaneous adverse effect of lithium.34 It is more likely to exacerbate existing disease but also can induce onset of psoriasis; it also can cause disease that is more refractory to treatment.34,36 Current literature hypothesizes that lithium triggers psoriasis by interference of intracellular calcium channels through reduction of inositol, thereby affecting keratinocyte proliferation and differentiation.34 Lithium also inhibits glycogen synthase kinase-3 (GSK-3), a serine threonine kinase, which, in turn, induces human keratinocyte proliferation.37 However, it is unlikely lithium alone can induce psoriasis; genetic predisposition is necessary.
TNF Inhibitors
Tumor necrosis factor inhibitors such as adalimumab, etanercept, certolizumab pegol, golimumab, and infliximab are used in various inflammatory diseases, including psoriasis. Interestingly, there have been more than 200 reported cases of suspected TNF inhibitor–induced or –exacerbated psoriasis.38 This phenomenon appears to occur more frequently with infliximab and is most likely to occur in the first year of treatment of Crohn disease and rheumatoid arthritis.38 Plaque psoriasis is the most common form, but 15% to 26% of cases presented with 2 or more morphologies.38,39
Treatment options include discontinuing therapy, though many patients experience resolution while continuing treatment or switching to another TNF inhibitor.38-40 Traditional topical therapies also have been used with success.40 The pathogenesis of this phenomenon is still unclear but is thought to involve both the IL-23/helper T cell 17 (TH17) axis and dysregulation of IFN-α in the setting of TNF suppression.38
Lifestyle
Obesity is a chronic low-grade inflammatory state that can contribute to the onset of psoriasis or exacerbation of exist
The relationship between psoriasis and alcohol consumption is less clear than it is between psoriasis and obesity or smoking; greater consumption is found in psoriasis patients, but evidence is insufficient to deem alcohol a risk factor.44
Conclusion
Various factors, including genetics, infection, pharmacotherapeutic, and lifestyle, can all contribute to the induction or exacerbation of psoriasis. These factors can provide clues to the pathogenesis of psoriasis as well as help clinicians better counsel patients about their disease.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
- Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93-122.
- Swanbeck G, Inerot A, Martinsson T, et al. A population genetic study of psoriasis. Br J Dermatol. 1994;131:32-39.
- Kimberling W, Dobson RL. The inheritance of psoriasis. J Invest Dermatol. 1973;60:538-540.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun. 2015;64:66-73.
- Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018;178:854-862.
- Enerbäck C, Martinsson T, Ineraot A, et al. Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SSP). Acta Derm Venereol. 1997;77:273-276.
- Gudjónsson JE, Kárason A, Antonsdóttir EH, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118:362-365.
- Tomfohrde J, Silverman A, Barnes R, et al. Gene for familial psoriasis susceptibility mapped to distal end of human chromosome 17q. Science. 1994;264:1141-1145.
- Blonska M, Lin X. NF-κB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55-70.
- Van Nuffel E, Schmitt A, Afonina IS, et al. CARD14-mediated activation of paracaspase MALT1 in keratinocytes: implications for psoriasis. J Invest Dermatol. 2017;137:569-575.
- Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784-795.
- Wang M, Zhang S, Zheng G, et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity. 2018;49:66-79.
- Mellet M, Meier B, Mohanan D, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol. 2018;138:2010-2023.
- Coto-Segura P, González-Fernández D, Batalla A, et al. Common and rare CARD14 gene variants affect the antitumour necrosis factor response among patients with psoriasis. Br J Dermatol. 2016;175:134-141.
- Winfield JM. Psoriasis as a sequel to acute inflammations of the tonsils: a clinical note. J Cutan Dis. 1916;34:441-443.
- Telfer NR, Chalmers RJG, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Hernandez M, Simms-Cendan J, Zendell K. Guttate psoriasis following streptococcal vulvovaginitis in a five-year-old girl. J Pediatr Adolesc Gynecol. 2015;28:e127-e129.
- Herbst RA, Hoch O, Kapp A, et al. Guttate psoriasis triggered by perianal streptococcal dermatitis in a four-year-old boy. J Am Acad Dermatol. 2000;42(5, pt 2):885-887.
- Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
- Thorleifsdottir RH, Eysteinsdóttir, Olafsson JH, et al. Throat infections are associated with exacerbation in a substantial proportion of patients with chronic plaque psoriasis. Acta Derm Venereol. 2016;96:788-791.
- McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443-447.
- Validmarsson H, Thorleifsdottir RH, Sigurdardottir SL, et al. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494-501.
- Muto M, Fujikara Y, Hamamoto Y, et al. Immune response to Streptococcus pyogenes and the susceptibility to psoriasis. Australas J Dermatol. 1996;37(suppl 1):S54-S55.
- Weisenseel P, Laumbacher B, Besgen P, et al. Streptococcal infection distinguishes different types of psoriasis. J Med Genet. 2002;39:767-768.
- Rachakonda TD, Dhillon JS, Florek AG, et al. Effect of tonsillectomy on psoriasis: a systematic review. J Am Acad Dermatol. 2015;72:261-275.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Duvic M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632.
- Fife DJ, Waller JM, Jeffes EW, et al. Unraveling the paradoxes of HIV-associated psoriasis: a review of T-cell subsets and cytokine profiles. Dermatol Online J. 2007;13:4.
- Ortonne JP, Lebwohl M, Em Griffiths C; Alefacept Clinical Study Group. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. Eur J Dermatol. 2003;13:117-123.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Yeung CK, Chan HH. Cutaneous adverse effects of lithium: epidemiology and management. Am J Clin Dermatol. 2004;5:3-8.
- Hampton PJ, Jans R, Flockhart RJ, et al. Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT 2 (nuclear factor of activated T cells 2). J Cell Physiol. 2012;227:1529-1537.
- Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Collamer AN, Guerrero KT, Henning JS, et al. Psoriatic skin lesions induced by tumor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996-1001.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13:743.
- Lee EJ, Han KD, Han JH, et al. Smoking and risk of psoriasis: a nationwide cohort study. J Am Acad Dermatol. 2017;77:573-575.
- Brenaut E, Horreau C, Pouplard C, et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):30-35.
Psoriasis is a chronic autoimmune skin disease affecting approximately 6.7 million adults in the United States.1 Although its pathogenesis is not yet clear, risk factors and triggers provide insight into potential pathways by which psoriasis can occur. There is notable overlap between risk factors and triggers of psoriasis; perceived risk factors might, in fact, be triggers causing manifestation of disease in predisposed persons. In this review, we summarize the key factors contributing to onset and exacerbation of psoriasis. When learning to manage this chronic disease, it also may be helpful to educate patients about how these elements may affect the course of psoriasis.
Genetics
The pathogenesis of psoriasis has a strong genetic component, with approximately 70% and 20% concordance rates in monozygotic and dizygotic twins, respectively.2 Moreover, studies have shown a positive family history in approximately 35% of patients.3,4 Family-based studies have found a 50% risk of developing psoriasis in patients with 2 affected parents.5 However, the genetics of psoriasis are complex and are attributed to many different genes. Thus far, genes involving antigen presentation, T-cell receptor development and polarization, and the nuclear factor κβ (NF-κβ) pathway have been identified.6
HLA-Cw6
The most well-studied gene implicated in psoriasis is HLA-Cw6, which encodes a major histocompatibility complex class I allele supporting psoriasis as a T cell–mediated reaction to an autoantigen.6 Two potential antigens for HLA-Cw6 recently have been identified: LL-37, a cathelicidin-related antimicrobial peptide, and the A disintegrin and metalloproteinase with thrombospondin motifs-like protein 5 (ADAMTSL5), found on melanocytes and keratinocytes.7 The percentage of psoriasis patients with HLA-Cw6 ranges from 10.5% to 77.2%, with higher frequency in white individuals than in Asians.7
HLA-Cw6 manifests as specific features in psoriasis, including onset of disease before 21 years of age.8 It also is more strongly associated with guttate-type psoriasis, greater body surface area involvement, and higher incidence of Köbner phenomenon. Patients with positive HLA-Cw6 also reported worsening of psoriasis during and after throat infection.9
Caspase Recruitment Domain Family Member 14
Another gene mutation implicated in psoriasis pathogenesis is caspase recruitment domain family member 14, CARD14 (formerly PSORS2), a gene encoding a scaffolding protein important in the activation of NF-κβ.10,11 Missense CARD14 mutations cause upregulation of NF-κβ through formation of a complex with adapter protein B-cell lymphoma 10 (BCL10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1),12 which, in turn, causes increased transcription of cytokines IL-8, C-C motif chemokine ligand 20 (CCL-20), and IL-36 gamma in the keratinocyte.13 Mutations in CARD14 alone lead to psoriasiform skin in mice through amplified activation of the IL-23/IL-17 axis.14,15 Patients with a mutation in a CARD14 variant (p.Arg820Trp) have demonstrated better response to tumor necrosis factor (TNF) inhibitors.16
Further characterization of the genetic pathogenesis of psoriasis might lead to better targeted therapies, including the possibility of MALT1 inhibitors as a treatment option.12
Infection
Streptococcus
The association between streptococcal infection and psoriasis was first documented more than 100 years ago, specifically the onset of acute guttate psoriasis.17,18 Although classically described following throat infection, psoriasis also occurs following streptococcal vulvovaginitis and perianal streptococcal infection.19,20
This type of psoriasis is typically self-limited but can recur with subsequent streptococcal infections or initiate a more chronic plaque psoriasis. Patients have a 1 in 3 risk of developing chronic psoriasis within 10 years of a single episode of acute guttate psoriasis.21 Moreover, in many patients with existing plaque psoriasis, throat infection exacerbates psoriatic symptoms.22 The mechanism of exacerbation is likely due to cross-reactivity between streptococcal M surface antigen and human keratinocytes and might also be influenced by inherited abnormalities in immune response.23-26 Therefore, tonsillectomy has been studied as a possible treatment of psoriasis but is likely helpful only in patients with exacerbations of disease that are closely associated with recurrent tonsillitis.27
Human Immunodeficiency Virus
The prevalence of psoriasis in human immunodeficiency virus (HIV) patients is similar to or greater than the general population.28 Human immunodeficiency virus infection causes new onset of psoriasis and exacerbation of existing psoriasis; severity often is correlated with worsening immune function.28,29
The clinical subtypes of psoriasis that occur most frequently with HIV include guttate, inverse, and erythrodermic, though patients may present with any subtype.28 The mechanism is puzzling because HIV is primarily mediated by helper T cell 2 (TH2) cytokines, whereas psoriasis is mainly driven by helper T cell 1 (TH1) cytokines.30 Furthermore, despite increased severity with lower CD4+ counts, treatments further lowering T-cell counts paradoxically improve symptoms.31 Current literature suggests that expansion of CD8+ memory T cells might be the primary mechanism in the exacerbation of psoriasis in HIV-mediated immunosuppression.30
Treatment of HIV-associated psoriasis presents challenges because many therapeutics cause further immunosuppression. The National Psoriasis Foundation recommends topical preparations as first-line agents for mild to moderate psoriasis.32 For moderate to severe psoriasis, retroviral agents may be effective as first-line monotherapy or when supplemented by phototherapy with UVB or psoralen plus UVA. Retinoids can be used as second-line agents.32 For cases of severe refractory psoriasis, cyclosporine, methotrexate, TNF inhibitors, or hydroxyurea can be considered. There also is evidence that apremilast is effective without risk for worsening immune function.33
Other Infections
Other bacteria associated with triggering or exacerbating psoriasis include Staphylococcus aureus and Helicobacter pylori.34,35 Fungi, such as species of the genera Malassezia and Candida, and other viruses, including papillomaviruses and retroviruses, also have been implicated.34
Medications
Numerous medications can trigger psoriasis, including lithium, nonsteroidal anti-inflammatory drugs, antimalarials, beta-blockers, and angiotensin-converting enzyme inhibitors.34 More recent literature suggests that TNF inhibitors also can paradoxically induce psoriasis in rare cases.35
Lithium
Psoriasis is the most common cutaneous adverse effect of lithium.34 It is more likely to exacerbate existing disease but also can induce onset of psoriasis; it also can cause disease that is more refractory to treatment.34,36 Current literature hypothesizes that lithium triggers psoriasis by interference of intracellular calcium channels through reduction of inositol, thereby affecting keratinocyte proliferation and differentiation.34 Lithium also inhibits glycogen synthase kinase-3 (GSK-3), a serine threonine kinase, which, in turn, induces human keratinocyte proliferation.37 However, it is unlikely lithium alone can induce psoriasis; genetic predisposition is necessary.
TNF Inhibitors
Tumor necrosis factor inhibitors such as adalimumab, etanercept, certolizumab pegol, golimumab, and infliximab are used in various inflammatory diseases, including psoriasis. Interestingly, there have been more than 200 reported cases of suspected TNF inhibitor–induced or –exacerbated psoriasis.38 This phenomenon appears to occur more frequently with infliximab and is most likely to occur in the first year of treatment of Crohn disease and rheumatoid arthritis.38 Plaque psoriasis is the most common form, but 15% to 26% of cases presented with 2 or more morphologies.38,39
Treatment options include discontinuing therapy, though many patients experience resolution while continuing treatment or switching to another TNF inhibitor.38-40 Traditional topical therapies also have been used with success.40 The pathogenesis of this phenomenon is still unclear but is thought to involve both the IL-23/helper T cell 17 (TH17) axis and dysregulation of IFN-α in the setting of TNF suppression.38
Lifestyle
Obesity is a chronic low-grade inflammatory state that can contribute to the onset of psoriasis or exacerbation of exist
The relationship between psoriasis and alcohol consumption is less clear than it is between psoriasis and obesity or smoking; greater consumption is found in psoriasis patients, but evidence is insufficient to deem alcohol a risk factor.44
Conclusion
Various factors, including genetics, infection, pharmacotherapeutic, and lifestyle, can all contribute to the induction or exacerbation of psoriasis. These factors can provide clues to the pathogenesis of psoriasis as well as help clinicians better counsel patients about their disease.
Psoriasis is a chronic autoimmune skin disease affecting approximately 6.7 million adults in the United States.1 Although its pathogenesis is not yet clear, risk factors and triggers provide insight into potential pathways by which psoriasis can occur. There is notable overlap between risk factors and triggers of psoriasis; perceived risk factors might, in fact, be triggers causing manifestation of disease in predisposed persons. In this review, we summarize the key factors contributing to onset and exacerbation of psoriasis. When learning to manage this chronic disease, it also may be helpful to educate patients about how these elements may affect the course of psoriasis.
Genetics
The pathogenesis of psoriasis has a strong genetic component, with approximately 70% and 20% concordance rates in monozygotic and dizygotic twins, respectively.2 Moreover, studies have shown a positive family history in approximately 35% of patients.3,4 Family-based studies have found a 50% risk of developing psoriasis in patients with 2 affected parents.5 However, the genetics of psoriasis are complex and are attributed to many different genes. Thus far, genes involving antigen presentation, T-cell receptor development and polarization, and the nuclear factor κβ (NF-κβ) pathway have been identified.6
HLA-Cw6
The most well-studied gene implicated in psoriasis is HLA-Cw6, which encodes a major histocompatibility complex class I allele supporting psoriasis as a T cell–mediated reaction to an autoantigen.6 Two potential antigens for HLA-Cw6 recently have been identified: LL-37, a cathelicidin-related antimicrobial peptide, and the A disintegrin and metalloproteinase with thrombospondin motifs-like protein 5 (ADAMTSL5), found on melanocytes and keratinocytes.7 The percentage of psoriasis patients with HLA-Cw6 ranges from 10.5% to 77.2%, with higher frequency in white individuals than in Asians.7
HLA-Cw6 manifests as specific features in psoriasis, including onset of disease before 21 years of age.8 It also is more strongly associated with guttate-type psoriasis, greater body surface area involvement, and higher incidence of Köbner phenomenon. Patients with positive HLA-Cw6 also reported worsening of psoriasis during and after throat infection.9
Caspase Recruitment Domain Family Member 14
Another gene mutation implicated in psoriasis pathogenesis is caspase recruitment domain family member 14, CARD14 (formerly PSORS2), a gene encoding a scaffolding protein important in the activation of NF-κβ.10,11 Missense CARD14 mutations cause upregulation of NF-κβ through formation of a complex with adapter protein B-cell lymphoma 10 (BCL10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1),12 which, in turn, causes increased transcription of cytokines IL-8, C-C motif chemokine ligand 20 (CCL-20), and IL-36 gamma in the keratinocyte.13 Mutations in CARD14 alone lead to psoriasiform skin in mice through amplified activation of the IL-23/IL-17 axis.14,15 Patients with a mutation in a CARD14 variant (p.Arg820Trp) have demonstrated better response to tumor necrosis factor (TNF) inhibitors.16
Further characterization of the genetic pathogenesis of psoriasis might lead to better targeted therapies, including the possibility of MALT1 inhibitors as a treatment option.12
Infection
Streptococcus
The association between streptococcal infection and psoriasis was first documented more than 100 years ago, specifically the onset of acute guttate psoriasis.17,18 Although classically described following throat infection, psoriasis also occurs following streptococcal vulvovaginitis and perianal streptococcal infection.19,20
This type of psoriasis is typically self-limited but can recur with subsequent streptococcal infections or initiate a more chronic plaque psoriasis. Patients have a 1 in 3 risk of developing chronic psoriasis within 10 years of a single episode of acute guttate psoriasis.21 Moreover, in many patients with existing plaque psoriasis, throat infection exacerbates psoriatic symptoms.22 The mechanism of exacerbation is likely due to cross-reactivity between streptococcal M surface antigen and human keratinocytes and might also be influenced by inherited abnormalities in immune response.23-26 Therefore, tonsillectomy has been studied as a possible treatment of psoriasis but is likely helpful only in patients with exacerbations of disease that are closely associated with recurrent tonsillitis.27
Human Immunodeficiency Virus
The prevalence of psoriasis in human immunodeficiency virus (HIV) patients is similar to or greater than the general population.28 Human immunodeficiency virus infection causes new onset of psoriasis and exacerbation of existing psoriasis; severity often is correlated with worsening immune function.28,29
The clinical subtypes of psoriasis that occur most frequently with HIV include guttate, inverse, and erythrodermic, though patients may present with any subtype.28 The mechanism is puzzling because HIV is primarily mediated by helper T cell 2 (TH2) cytokines, whereas psoriasis is mainly driven by helper T cell 1 (TH1) cytokines.30 Furthermore, despite increased severity with lower CD4+ counts, treatments further lowering T-cell counts paradoxically improve symptoms.31 Current literature suggests that expansion of CD8+ memory T cells might be the primary mechanism in the exacerbation of psoriasis in HIV-mediated immunosuppression.30
Treatment of HIV-associated psoriasis presents challenges because many therapeutics cause further immunosuppression. The National Psoriasis Foundation recommends topical preparations as first-line agents for mild to moderate psoriasis.32 For moderate to severe psoriasis, retroviral agents may be effective as first-line monotherapy or when supplemented by phototherapy with UVB or psoralen plus UVA. Retinoids can be used as second-line agents.32 For cases of severe refractory psoriasis, cyclosporine, methotrexate, TNF inhibitors, or hydroxyurea can be considered. There also is evidence that apremilast is effective without risk for worsening immune function.33
Other Infections
Other bacteria associated with triggering or exacerbating psoriasis include Staphylococcus aureus and Helicobacter pylori.34,35 Fungi, such as species of the genera Malassezia and Candida, and other viruses, including papillomaviruses and retroviruses, also have been implicated.34
Medications
Numerous medications can trigger psoriasis, including lithium, nonsteroidal anti-inflammatory drugs, antimalarials, beta-blockers, and angiotensin-converting enzyme inhibitors.34 More recent literature suggests that TNF inhibitors also can paradoxically induce psoriasis in rare cases.35
Lithium
Psoriasis is the most common cutaneous adverse effect of lithium.34 It is more likely to exacerbate existing disease but also can induce onset of psoriasis; it also can cause disease that is more refractory to treatment.34,36 Current literature hypothesizes that lithium triggers psoriasis by interference of intracellular calcium channels through reduction of inositol, thereby affecting keratinocyte proliferation and differentiation.34 Lithium also inhibits glycogen synthase kinase-3 (GSK-3), a serine threonine kinase, which, in turn, induces human keratinocyte proliferation.37 However, it is unlikely lithium alone can induce psoriasis; genetic predisposition is necessary.
TNF Inhibitors
Tumor necrosis factor inhibitors such as adalimumab, etanercept, certolizumab pegol, golimumab, and infliximab are used in various inflammatory diseases, including psoriasis. Interestingly, there have been more than 200 reported cases of suspected TNF inhibitor–induced or –exacerbated psoriasis.38 This phenomenon appears to occur more frequently with infliximab and is most likely to occur in the first year of treatment of Crohn disease and rheumatoid arthritis.38 Plaque psoriasis is the most common form, but 15% to 26% of cases presented with 2 or more morphologies.38,39
Treatment options include discontinuing therapy, though many patients experience resolution while continuing treatment or switching to another TNF inhibitor.38-40 Traditional topical therapies also have been used with success.40 The pathogenesis of this phenomenon is still unclear but is thought to involve both the IL-23/helper T cell 17 (TH17) axis and dysregulation of IFN-α in the setting of TNF suppression.38
Lifestyle
Obesity is a chronic low-grade inflammatory state that can contribute to the onset of psoriasis or exacerbation of exist
The relationship between psoriasis and alcohol consumption is less clear than it is between psoriasis and obesity or smoking; greater consumption is found in psoriasis patients, but evidence is insufficient to deem alcohol a risk factor.44
Conclusion
Various factors, including genetics, infection, pharmacotherapeutic, and lifestyle, can all contribute to the induction or exacerbation of psoriasis. These factors can provide clues to the pathogenesis of psoriasis as well as help clinicians better counsel patients about their disease.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
- Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93-122.
- Swanbeck G, Inerot A, Martinsson T, et al. A population genetic study of psoriasis. Br J Dermatol. 1994;131:32-39.
- Kimberling W, Dobson RL. The inheritance of psoriasis. J Invest Dermatol. 1973;60:538-540.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun. 2015;64:66-73.
- Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018;178:854-862.
- Enerbäck C, Martinsson T, Ineraot A, et al. Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SSP). Acta Derm Venereol. 1997;77:273-276.
- Gudjónsson JE, Kárason A, Antonsdóttir EH, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118:362-365.
- Tomfohrde J, Silverman A, Barnes R, et al. Gene for familial psoriasis susceptibility mapped to distal end of human chromosome 17q. Science. 1994;264:1141-1145.
- Blonska M, Lin X. NF-κB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55-70.
- Van Nuffel E, Schmitt A, Afonina IS, et al. CARD14-mediated activation of paracaspase MALT1 in keratinocytes: implications for psoriasis. J Invest Dermatol. 2017;137:569-575.
- Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784-795.
- Wang M, Zhang S, Zheng G, et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity. 2018;49:66-79.
- Mellet M, Meier B, Mohanan D, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol. 2018;138:2010-2023.
- Coto-Segura P, González-Fernández D, Batalla A, et al. Common and rare CARD14 gene variants affect the antitumour necrosis factor response among patients with psoriasis. Br J Dermatol. 2016;175:134-141.
- Winfield JM. Psoriasis as a sequel to acute inflammations of the tonsils: a clinical note. J Cutan Dis. 1916;34:441-443.
- Telfer NR, Chalmers RJG, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Hernandez M, Simms-Cendan J, Zendell K. Guttate psoriasis following streptococcal vulvovaginitis in a five-year-old girl. J Pediatr Adolesc Gynecol. 2015;28:e127-e129.
- Herbst RA, Hoch O, Kapp A, et al. Guttate psoriasis triggered by perianal streptococcal dermatitis in a four-year-old boy. J Am Acad Dermatol. 2000;42(5, pt 2):885-887.
- Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
- Thorleifsdottir RH, Eysteinsdóttir, Olafsson JH, et al. Throat infections are associated with exacerbation in a substantial proportion of patients with chronic plaque psoriasis. Acta Derm Venereol. 2016;96:788-791.
- McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443-447.
- Validmarsson H, Thorleifsdottir RH, Sigurdardottir SL, et al. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494-501.
- Muto M, Fujikara Y, Hamamoto Y, et al. Immune response to Streptococcus pyogenes and the susceptibility to psoriasis. Australas J Dermatol. 1996;37(suppl 1):S54-S55.
- Weisenseel P, Laumbacher B, Besgen P, et al. Streptococcal infection distinguishes different types of psoriasis. J Med Genet. 2002;39:767-768.
- Rachakonda TD, Dhillon JS, Florek AG, et al. Effect of tonsillectomy on psoriasis: a systematic review. J Am Acad Dermatol. 2015;72:261-275.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Duvic M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632.
- Fife DJ, Waller JM, Jeffes EW, et al. Unraveling the paradoxes of HIV-associated psoriasis: a review of T-cell subsets and cytokine profiles. Dermatol Online J. 2007;13:4.
- Ortonne JP, Lebwohl M, Em Griffiths C; Alefacept Clinical Study Group. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. Eur J Dermatol. 2003;13:117-123.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Yeung CK, Chan HH. Cutaneous adverse effects of lithium: epidemiology and management. Am J Clin Dermatol. 2004;5:3-8.
- Hampton PJ, Jans R, Flockhart RJ, et al. Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT 2 (nuclear factor of activated T cells 2). J Cell Physiol. 2012;227:1529-1537.
- Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Collamer AN, Guerrero KT, Henning JS, et al. Psoriatic skin lesions induced by tumor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996-1001.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13:743.
- Lee EJ, Han KD, Han JH, et al. Smoking and risk of psoriasis: a nationwide cohort study. J Am Acad Dermatol. 2017;77:573-575.
- Brenaut E, Horreau C, Pouplard C, et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):30-35.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
- Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93-122.
- Swanbeck G, Inerot A, Martinsson T, et al. A population genetic study of psoriasis. Br J Dermatol. 1994;131:32-39.
- Kimberling W, Dobson RL. The inheritance of psoriasis. J Invest Dermatol. 1973;60:538-540.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun. 2015;64:66-73.
- Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018;178:854-862.
- Enerbäck C, Martinsson T, Ineraot A, et al. Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SSP). Acta Derm Venereol. 1997;77:273-276.
- Gudjónsson JE, Kárason A, Antonsdóttir EH, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118:362-365.
- Tomfohrde J, Silverman A, Barnes R, et al. Gene for familial psoriasis susceptibility mapped to distal end of human chromosome 17q. Science. 1994;264:1141-1145.
- Blonska M, Lin X. NF-κB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55-70.
- Van Nuffel E, Schmitt A, Afonina IS, et al. CARD14-mediated activation of paracaspase MALT1 in keratinocytes: implications for psoriasis. J Invest Dermatol. 2017;137:569-575.
- Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784-795.
- Wang M, Zhang S, Zheng G, et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity. 2018;49:66-79.
- Mellet M, Meier B, Mohanan D, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol. 2018;138:2010-2023.
- Coto-Segura P, González-Fernández D, Batalla A, et al. Common and rare CARD14 gene variants affect the antitumour necrosis factor response among patients with psoriasis. Br J Dermatol. 2016;175:134-141.
- Winfield JM. Psoriasis as a sequel to acute inflammations of the tonsils: a clinical note. J Cutan Dis. 1916;34:441-443.
- Telfer NR, Chalmers RJG, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Hernandez M, Simms-Cendan J, Zendell K. Guttate psoriasis following streptococcal vulvovaginitis in a five-year-old girl. J Pediatr Adolesc Gynecol. 2015;28:e127-e129.
- Herbst RA, Hoch O, Kapp A, et al. Guttate psoriasis triggered by perianal streptococcal dermatitis in a four-year-old boy. J Am Acad Dermatol. 2000;42(5, pt 2):885-887.
- Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
- Thorleifsdottir RH, Eysteinsdóttir, Olafsson JH, et al. Throat infections are associated with exacerbation in a substantial proportion of patients with chronic plaque psoriasis. Acta Derm Venereol. 2016;96:788-791.
- McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443-447.
- Validmarsson H, Thorleifsdottir RH, Sigurdardottir SL, et al. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494-501.
- Muto M, Fujikara Y, Hamamoto Y, et al. Immune response to Streptococcus pyogenes and the susceptibility to psoriasis. Australas J Dermatol. 1996;37(suppl 1):S54-S55.
- Weisenseel P, Laumbacher B, Besgen P, et al. Streptococcal infection distinguishes different types of psoriasis. J Med Genet. 2002;39:767-768.
- Rachakonda TD, Dhillon JS, Florek AG, et al. Effect of tonsillectomy on psoriasis: a systematic review. J Am Acad Dermatol. 2015;72:261-275.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Duvic M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632.
- Fife DJ, Waller JM, Jeffes EW, et al. Unraveling the paradoxes of HIV-associated psoriasis: a review of T-cell subsets and cytokine profiles. Dermatol Online J. 2007;13:4.
- Ortonne JP, Lebwohl M, Em Griffiths C; Alefacept Clinical Study Group. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. Eur J Dermatol. 2003;13:117-123.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Yeung CK, Chan HH. Cutaneous adverse effects of lithium: epidemiology and management. Am J Clin Dermatol. 2004;5:3-8.
- Hampton PJ, Jans R, Flockhart RJ, et al. Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT 2 (nuclear factor of activated T cells 2). J Cell Physiol. 2012;227:1529-1537.
- Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Collamer AN, Guerrero KT, Henning JS, et al. Psoriatic skin lesions induced by tumor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996-1001.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13:743.
- Lee EJ, Han KD, Han JH, et al. Smoking and risk of psoriasis: a nationwide cohort study. J Am Acad Dermatol. 2017;77:573-575.
- Brenaut E, Horreau C, Pouplard C, et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):30-35.
Practice Points
- HLA-Cw6 and CARD14 are genetic factors associated with psoriasis.
- Psoriasis in the setting of human immunodeficiency virus infection may be treated with topical steroids, phototherapy, systemic retinoids, or apremilast.
- Psoriasis is a potential adverse effect in patients taking lithium or tumor necrosis factor inhibitors.
- Patients should be counseled about the role of obesity and smoking on psoriasis.
Emerging Therapies In Psoriasis: A Systematic Review
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.
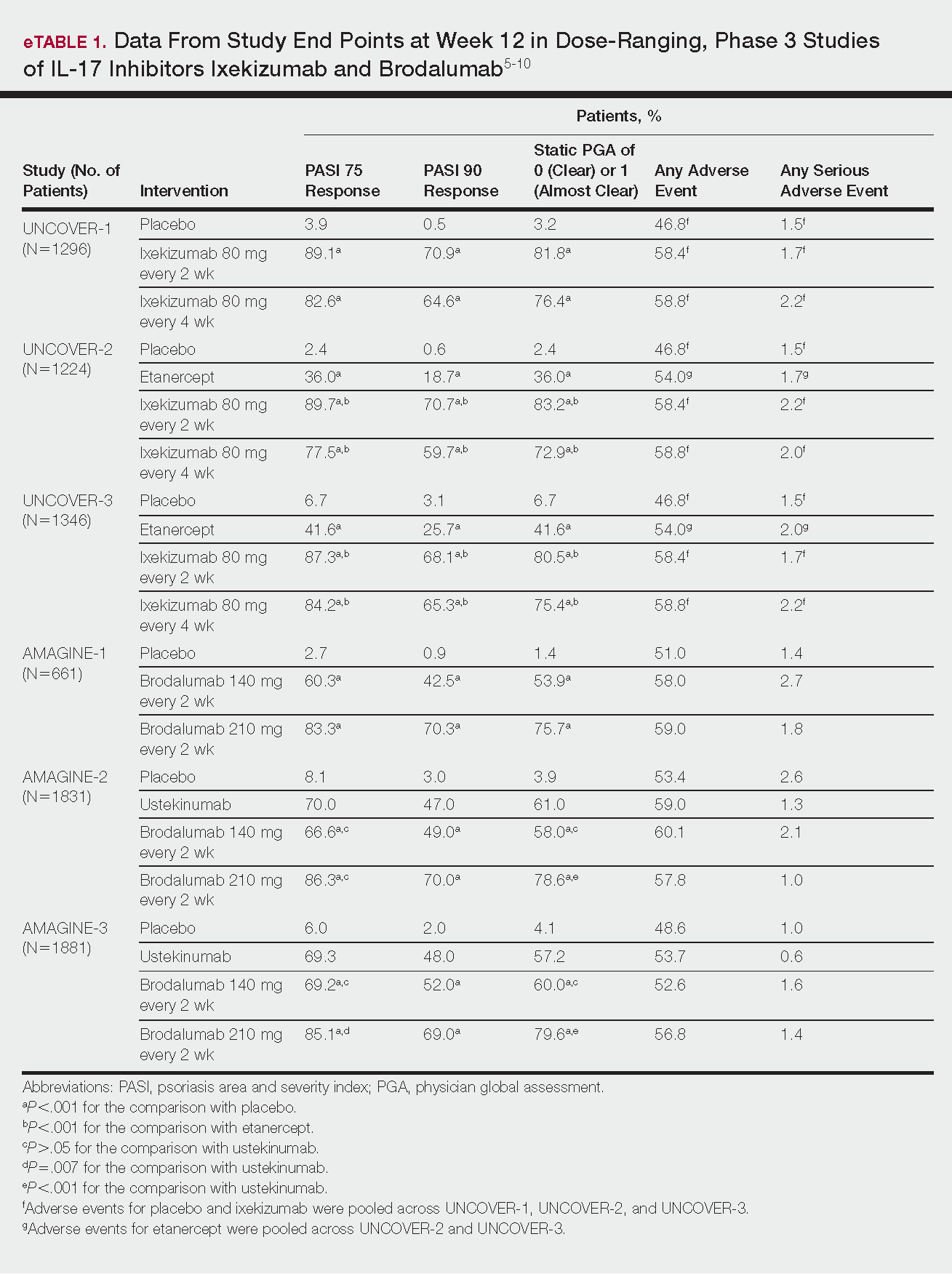
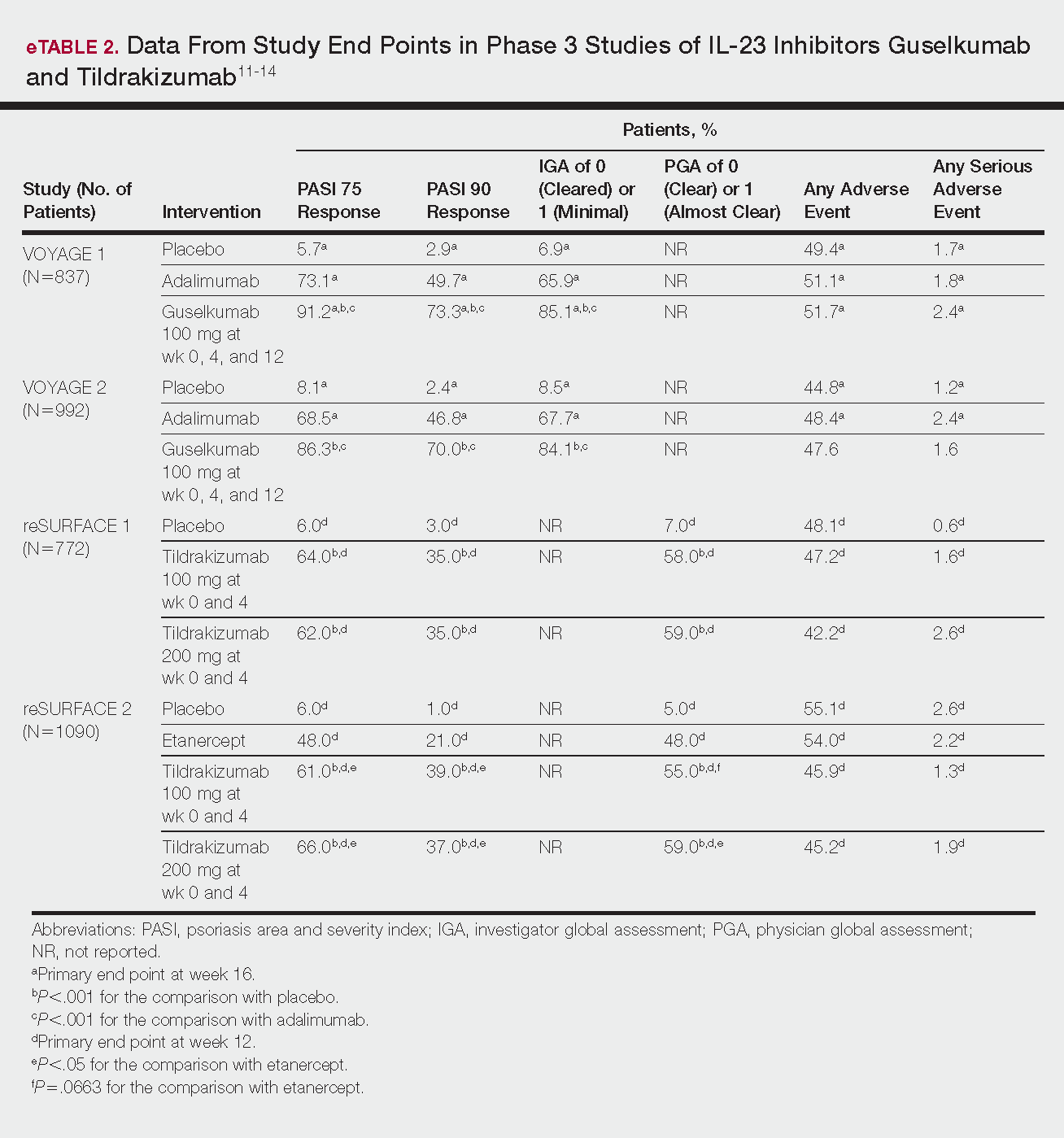
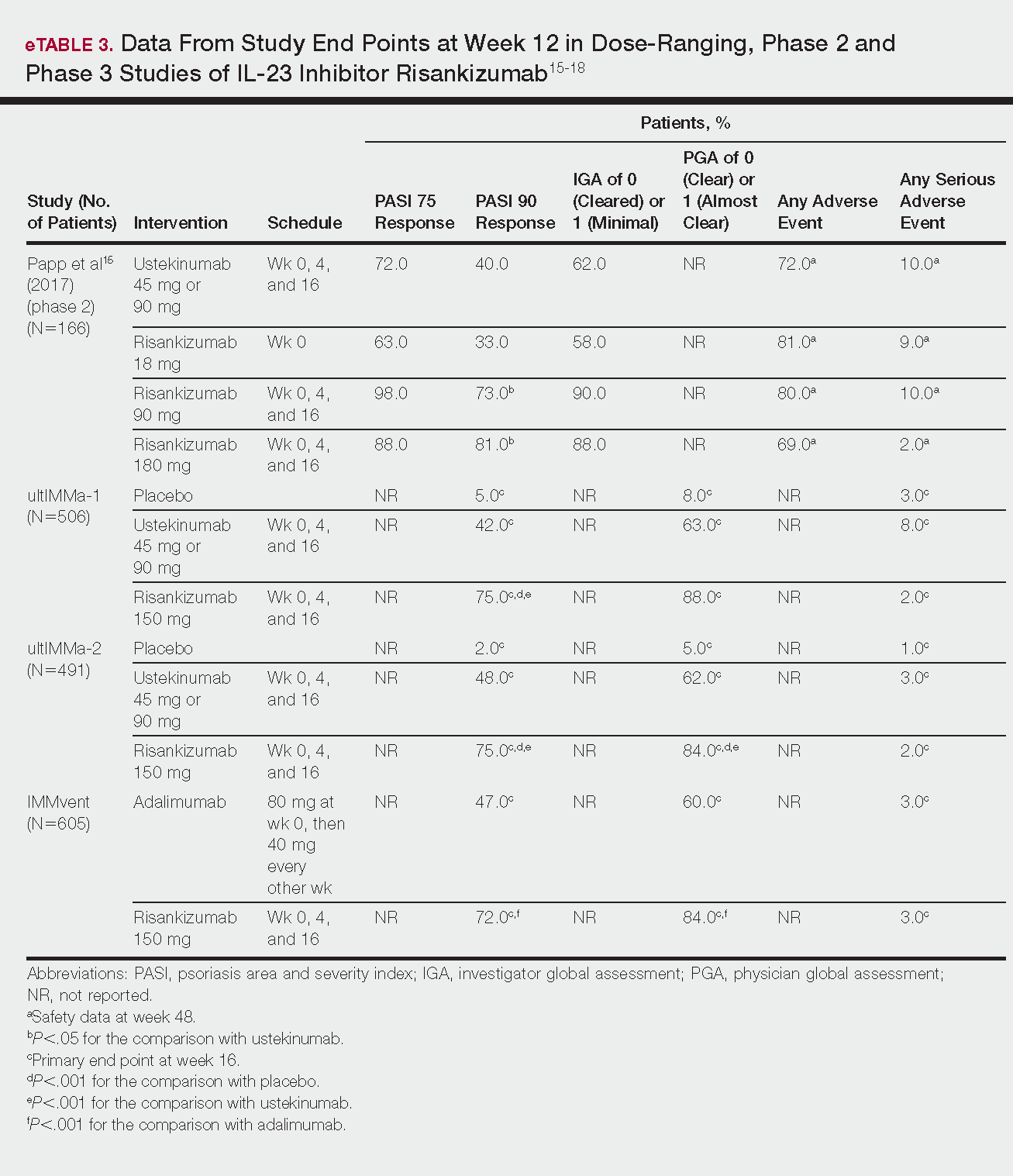
IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.




IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
Psoriasis is a chronic, autoimmune-mediated disease estimated to affect 2.8% of the US population.1 The pathogenesis of psoriasis is thought to involve a complex process triggered by a combination of genetic and environmental factors that induce tumor necrosis factor (TNF) α secretion by keratinocytes, which in turn activates dendritic cells. Activated dendritic cells produce IL-23, leading to helper T cell (TH17) differentiation.2,3 TH17 cells secrete IL-17A, which has been shown to promote psoriatic skin changes.4 Therefore, TNF-α, IL-23, and IL-17A have been recognized as key targets for psoriasis therapy.
The newest biologic agents targeting IL-17–mediated pathways include ixekizumab, brodalumab, and bimekizumab. Secukinumab, the first US Food and Drug Administration (FDA)–approved IL-17 inhibitor, has been available since 2015 and therefore is not included in this review. IL-23 inhibitors that are FDA approved or being evaluated in clinical trials include guselkumab, tildrakizumab, and risankizumab. In addition, certolizumab pegol, a TNF-α inhibitor, is being studied for use in psoriasis.
METHODS
We reviewed the published results of phase 3 clinical trials for ixekizumab, brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol. We performed an English-language literature search (January 1, 2012 to October 15, 2017) of articles indexed for PubMed/MEDLINE using the following combinations of keywords: IL-23 and psoriasis; IL-17 and psoriasis; tumor necrosis factor and psoriasis; [drug name] and psoriasis. If data from phase 3 clinical trials were not yet available, data from phase 2 clinical trials were incorporated in our analysis. We also reviewed citations within articles to identify relevant sources.
RESULTS
Phase 3 clinical trial design, efficacy, and adverse events (AEs) for ixekizumab and brodalumab are reported in eTable 15-10 and for guselkumab and tildrakizumab in eTable 2.11-14 Phase 2 clinical trial design, efficacy, and AEs are presented for risankizumab in eTable 315-18 and for certolizumab pegol in eTable 4.17,19 No published clinical trial data were found for bimekizumab.




IL-17 Inhibitors
Ixekizumab
This recombinant, high-affinity IgG4κ antibody selectively binds and neutralizes IL-17A.5,6 Three phase 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—evaluated ixekizumab for moderate to severe plaque psoriasis.7
The 3 UNCOVER trials were randomized, double-blind, phase 3 trials of 1296, 1224, and 1346 patients, respectively, assigned to a placebo group; a group treated with ixekizumab 80 mg every 2 weeks; and a group treated with ixekizumab 80 mg every 4 weeks. Both ixekizumab groups received a loading dose of 160 mg at week 0.5,6 UNCOVER-2 and UNCOVER-3 also included a comparator group of patients on etanercept 50 mg.5 Co-primary end points included the percentage of patients reaching a psoriasis area and severity index (PASI) of 75 and with a static physician global assessment (PGA) score of clear (0) or almost clear (1) at week 12.5,6
Ixekizumab achieved greater efficacy than placebo: 89.1%, 89.7%, and 87.3% of patients achieved PASI 75 in the every 2-week dosing group, and 82.6%, 77.5% and 84.2% achieved PASI 75 in the every 4-week dosing group in UNCOVER-1, UNCOVER-2, and UNCOVER-3, respectively (P<.001 for both treatment arms compared to placebo in all trials). The percentage of patients achieving a static PGA score of 0 or 1 also was higher in the ixekizumab groups in the 2-week and 4-week dosing groups in all UNCOVER trials—81.8% and 76.4% in UNCOVER-1, 83.2% and 72.9% in UNCOVER-2, and 80.5% and 75.4% in UNCOVER-3—compared to 3.2%, 2.4%, and 6.7% in the placebo groups of the 3 trials (P<.001 for both ixekizumab groups compared to placebo in all trials).5,6 Ixekizumab also was found to be more effective than etanercept for both co-primary end points in both UNCOVER-2 and UNCOVER-3 (eTable 1).5
Safety data for all UNCOVER trials were pooled and reported.6 At week 12 the rate of at least 1 AE was 58.4% in patients on ixekizumab every 2 weeks and 58.8% in patients on ixekizumab every 4 weeks compared to 54.0% in the etanercept group in UNCOVER-2 and UNCOVER-3 and 46.8% in the placebo group. At week 12, 72 nonfatal serious AEs were reported: 12 in the placebo group, 14 in the etanercept group, 20 in the ixekizumab every 2 weeks group, and 26 in the ixekizumab every 4 weeks group.6
The most common AE across all groups was nasopharyngitis. Overall, infections were more frequent in patients treated with ixekizumab than in patients treated with placebo or etanercept. Specifically, oral candidiasis occurred more frequently in the ixekizumab groups, with a higher rate in the 2-week dosing group than in the 4-week dosing group.6 Two myocardial infarctions (MIs) occurred: 1 in the etanercept group and 1 in the placebo group.5
Brodalumab
This human monoclonal antibody binds to IL-17ra.8,9 Three double-blind, placebo-controlled, phase 3 trials—AMAGINE-1, AMAGINE-2, and AMAGINE-3—evaluated its use for plaque psoriasis.10
In AMAGINE-1 (N=661), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), or placebo.8 In AMAGINE-2 (N=1831) and AMAGINE-3 (N=1881), patients were randomized to receive brodalumab 140 mg or 210 mg (every 2 weeks for 12 weeks), ustekinumab 45 mg or 90 mg by weight (at weeks 0 and 4, then every 12 weeks thereafter), or placebo. In all trials, patients on brodalumab received a dose at week 0 and week 1. Co-primary end points were PASI 75 and a static PGA score of 0 or 1 at 12 weeks compared to placebo and to ustekinumab (in AMAGINE-2 and AMAGINE-3 only).8
At week 12, 83.3%, 86.3%, and 85.1% of patients on brodalumab 210 mg, and 60.3%, 66.6%, and 69.2% of patients on brodalumab 140 mg, achieved PASI 75 in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively, compared to 2.7%, 8.1%, and 6.0% in the placebo groups (P<.001 between both brodalumab groups and placebo in all trials).8 Both brodalumab groups were noninferior but not significantly superior to ustekinumab, which achieved a PASI 75 of 70.0% in AMAGINE-2 and 69.3% in AMAGINE-3. The PASI 90 rate was higher, however, in both brodalumab groups compared to ustekinumab but significance was not reported (eTable 1).9 For both brodalumab groups, significantly more patients achieved a static PGA value of 0 or 1 compared to placebo (P<.001 across all trials). However, only the brodalumab 210-mg group achieved a significantly higher rate of static PGA 0 or 1 compared to ustekinumab in AMAGINE-2 and AMAGINE-3 (P<.001).9
After 12 weeks, the percentage of patients reporting at least 1 AE was 59.0%, 57.8%, and 56.8% in the brodalumab 210-mg group in AMAGINE-1, AMAGINE-2, and AMAGINE-3, respectively; 58.0%, 60.1%, and 52.6% in the brodalumab 140-mg group; and 51.0%, 53.4%, and 48.6% in the placebo group. Patients taking ustekinumab had an AE rate of 59.0% in AMAGINE-2 and 53.7% in AMAGINE-3. The most common AE was nasopharyngitis, followed by upper respiratory infection (URI) and headache across all trials.8,9 Serious AEs were rare: 10 in AMAGINE-1, 31 in AMAGINE-2, and 24 in AMAGINE-3 across all groups. One death occurred from stroke in the brodalumab 210-mg group in AMAGINE-2.9
IL-23 Inhibitors
Guselkumab
This drug is a human IgG1κ antibody that binds to the p19 subunit of IL-23, thereby inhibiting IL-23 signaling.11,12 Guselkumab was approved by the FDA in July 2017 for moderate to severe plaque psoriasis.13
VOYAGE 1 and VOYAGE 2 were phase 3, double-blind, placebo- and active comparator–controlled trials of 837 and 992 patients, respectively, randomized to receive adalimumab (80 mg at week 0 and 40 mg at week 1, then at 40 mg every 2 weeks thereafter), guselkumab 100 mg at weeks 0, 4, and 12, or placebo.11 Co-primary end points for both trials were the percentage of patients reaching PASI 90 and an investigator global assessment (IGA) score of cleared (0) or minimal (1) at week 16.11
By week 16 of both trials, PASI 90 values were statistically superior for guselkumab (VOYAGE 1, 73.3%; VOYAGE 2, 70.0%) compared to adalimumab (VOYAGE 1, 49.7%; VOYAGE 2, 46.8%) and placebo (VOYAGE 1, 2.9%; VOYAGE 2, 2.4%)(P<.001). Moreover, patients on guselkumab achieved a higher rate of IGA values of 0 and 1 at week 12 (85.1% in VOYAGE 1 and 84.1% in VOYAGE 2) than patients on adalimumab (65.9% in VOYAGE 1 and 67.7% in VOYAGE 2) and placebo (6.9% in VOYAGE 1 and 8.5% in VOYAGE 2)(P<.001).11,12
The frequency of AEs was comparable across all groups in both trials.11,12 During the 16-week treatment period, 51.7% and 47.6% of the guselkumab groups in VOYAGE 1 and VOYAGE 2, respectively; 51.1% and 48.4% of the adalimumab groups; and 49.4% and 44.8% of the placebo groups reported at least 1 AE. The most common AEs in all groups were nasopharyngitis, headache, and URI.11,12
Serious AEs also occurred at similar rates: 2.4% and 1.6% in the guselkumab group in VOYAGE 1 and VOYAGE 2, respectively; 2.4% and 1.8% in the adalimumab group; and 1.7% and 1.2% in the placebo group.11,12 One case of malignancy occurred in the VOYAGE 1 trial: basal cell carcinoma in the guselkumab group.11 Three major cardiovascular events occurred across both trials: 1 MI in the guselkumab group in each trial and 1 MI in the adalimumab group in VOYAGE 1.11,12
Tildrakizumab
A high-affinity, humanized IgG1κ antibody, tildrakizumab targets the p19 subunit of IL-23. As of February 2018, 2 double-blind, randomized phase 3 trials have studied tildrakizumab with published results: reSURFACE 1 and reSURFACE 2.14
reSURFACE 1 (N=772) and reSURFACE 2 (N=1090) randomized patients to receive tildrakizumab 100 or 200 mg (at weeks 0 and 4), etanercept 50 mg (twice weekly) for 12 weeks (reSURFACE 2 only), or placebo. Co-primary end points were the percentage of patients achieving PASI 75 and the percentage of patients achieving a PGA score of 0 or 1 at week 12.14
In reSURFACE 1, significantly more patients receiving tildrakizumab attained PASI 75 at week 12 compared to placebo: 200 mg, 62.0%; 100 mg, 64.0%; and placebo, 6.0% (P<.001 for tildrakizumab groups compared to placebo). Moreover, significantly proportionally more patients received a PGA score of 0 or 1 compared to placebo: 100 mg, 59%; 200 mg, 58.0%; placebo, 7.0% (P<.001 for both tildrakizumab groups compared to placebo).14
In reSURFACE 2, significantly more patients receiving tildrakizumab achieved PASI 75 compared to etanercept and placebo at week 12: 200 mg, 66.0%; 100mg, 61.0%; etanercept, 48.0%; placebo, 6.0% (P<.001 for both tildrakizumab groups compared to placebo; P<.05 for both tildrakizumab groups compared to etanercept). Additionally, significantly more patients in the tildrakizumab groups experienced a PGA score of 0 or 1 at week 12 compared to placebo: 200 mg, 59%; 100 mg, 55.0%; placebo, 5% (P<.001 for both tildrakizumab groups compared to placebo).14
Adverse events were reported at a similar rate across all groups. For reSURFACE 1 and reSURFACE 2, at least 1 AE by week 12 was reported by 42.2% and 45.2% of patients in the 200-mg group; 47.2% and 45.9% in the 100-mg group; and 48.1% and 55.1% in the placebo groups.14The most common AEs were nasopharyngitis, URI (reSURFACE 1), and erythema at the injection site (reSURFACE 2). One case of serious infection was reported in each of the tildrakizumab groups: 1 case of drug-related hypersensitivity reaction in the 200-mg group, and 1 major cardiovascular event in the 100-mg group of reSURFACE 1. There was 1 serious AE in reSURFACE 2 that led to death in which the cause was undetermined.14
Risankizumab
This humanized IgG1 antibody binds the p19 unit of IL-23.15,16 The drug is undergoing 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—for which only preliminary data have been published and are reported here.16,17 There is 1 phase 2 randomized, dose-ranging trial with published data.15
ultIMMa-1 and ultIMMa-2 comprised 506 and 491 patients, respectively, randomized to receive risankizumab (150 mg at weeks 0, 4, and 16), ustekinumab (45 mg or 90 mg, by weight, at weeks 0, 4, and 16), or placebo. Co-primary end points were PASI 90 and a PGA score of 0 or 1 at week 16.17
In ultIMMa-1 and ultIMMa-2, 75.0% and 75.0% of patients on risankizumab 150 mg achieved PASI 90 compared to 42.0% and 48.0% on ustekinumab and 5.0% and 2.0% on placebo at 16 weeks (P<.001 between both placebo and ustekinumab in both trials).17 In both trials, patients receiving risankizumab achieved higher rates of a static PGA score of 0 or 1 (88.0% and 84.0%) compared to ustekinumab (63.0% and 62.0%) and placebo (8.0% and 5.0%) at 16 weeks (P<.001 for both trials).18
At week 16, 2.0% of patients on risankizumab reported a serious AE in both trials, compared to 8.0% and 3.0% of patients on ustekinumab and 3.0% and 1.0% on placebo. No new safety concerns were noted.17
In the phase 3 IMMvent trial, 605 patients were randomized to receive risankizumab (150 mg at weeks 0, 4, and 16) or adalimumab (80 mg at week 0, 40 mg at week 1, then 40 mg every 2 weeks). Co-primary end points were PASI 90 and a static PGA score of 0 or 1 at week 16.17
In IMMvent, risankizumab was significantly more effective than adalimumab for PASI 75 (risankizumab, 72.0%; adalimumab, 47.0%) and a static PGA score of 0 or 1 (risankizumab 84.0%; adalimumab, 60.0%) (P<.001 risankizumab compared to adalimumab for both end points).17
At week 16, serious AEs were reported in 3.0% of patients on risankizumab and 3.0% of patients on adalimumab. One patient receiving risankizumab died of an acute MI during the treatment phase.17
TNF Inhibitor
Certolizumab Pegol
Certolizumab pegol is a human PEGylated anti-TNF agent. In vitro studies have shown that certolizumab binds to soluble and membrane-bound TNF.19 Unlike other TNF inhibitors, certolizumab pegol is a Fab‘ portion of anti-TNF conjugated to a molecule of polyethylene glycol.19 The drug is approved in the United States for treating psoriatic arthritis, Crohn disease, and rheumatoid arthritis; its potential for treating psoriasis has been confirmed. Results of 1 phase 2 trial have been published19; data from 3 phase 3 trials are forthcoming.
This randomized, placebo-controlled, double-blind phase 2 study comprised 176 patients who received certolizumab 200 mg, certolizumab 400 mg, or placebo. The dosing schedule was 400 mg at week 0, followed by either 200 or 400 mg every other week until week 10. Co-primary end points were PASI 75 and a PGA score of 0 or 1 at week 12.19
Certolizumab was significantly more effective than placebo at week 12: 74.6% of the 200-mg group and 82.8% of the 400-mg group achieved PASI 75 compared to 6.8% of the placebo group (P<.001). Certolizumab also performed better for the PGA score: 52.5% and 72.4% of patients attained a score of 0 or 1 in the 200-mg and 400-mg groups compared to 1.7% in the placebo group.19
Adverse events were reported equally across all groups: 72% of patients in the 200-mg group, 70% in the 400-mg group, and 71% in the placebo group reported at least 1 AE, most commonly nasopharyngitis, headache, and pruritis.19
COMMENT
With the development of new insights into the pathogenesis of psoriasis, therapies that are targeted toward key cytokines may contribute to improved management of the disease. The results of these clinical trials demonstrate numerous promising options for psoriatic patients.
IL-17 Inhibitors Ixekizumab and Brodalumab
When comparing these 2 biologics, it is important to consider that these studies were not performed head to head, thereby inhibiting direct comparisons. Moreover, dosage ranges of the investigative drugs were not identical, which also makes comparisons challenging. However, when looking at the highest dosages of ixekizumab and brodalumab, results indicate that ixekizumab may be slightly more effective than brodalumab based on the percentage of patients who achieved a PASI 75 and a static PGA score of 0 or 1 (eTable 1).
Phase 3 trials have shown ixekizumab to maintain efficacy over 60 weeks of treatment.6 Ixekizumab also has been shown to alleviate other symptoms of psoriasis, such as itching, pain, and nail involvement.20,21 Furthermore, ixekizumab appears to be equally effective in patients with or without prior exposure to biologics22; therefore, ixekizumab may benefit patients who have not experienced success with other biologics.
Across the UNCOVER trials, 11 cases of inflammatory bowel disease were reported in patients receiving ixekizumab (ulcerative colitis in 7; Crohn disease in 4)6; it appears that at least 3 of these cases were new diagnoses. In light of a study suggesting that IL-17A might have a protective function in the intestine,23 these findings may have important clinical implications and require follow-up studies.
Brodalumab also has been shown to maintain efficacy and acceptable safety for as long as 120 weeks.24 In the extension period of the AMAGINE-1 trial, patients who experienced a return of disease during a withdrawal period recaptured static PGA success with re-treatment for 12 weeks (re-treatment was successful in 97% of those given a dosage of 210 mg and in 84% of those given 140 mg).8
Furthermore, phase 2 trials also have shown that brodalumab is effective in patients with a history of biologic use.25 Across all AMAGINE trials, only 1 case of Crohn disease was reported in a patient taking brodalumab.9 There are concerns about depression, despite data from AMAGINE-1 stating patients on brodalumab actually had greater improvements in Hospital Anxiety and Depression Scale scores after 12 weeks of treatment (P<.001) for both brodalumab 140 mg and 210 mg compared to placebo.8 Regardless, brodalumab has a black-box warning for suicidal ideation and behavior, and availability is restricted through a Risk Evaluation and Mitigation Strategy (REMS) program.26
Bimekizumab
Although no phase 2 or phase 3 clinical trial data have been published for bimekizumab (phase 2 trials are underway), it has been shown in a phase 1 trial to be effective for psoriasis. Bimekizumab also is unique; it is the first dual inhibitor of IL-17A and IL-17F.18
IL-23 Inhibitors Guselkumab, Tildrakizumab, and Risankizumab
Making comparisons among the IL-23 inhibitors also is difficult; studies were not head-to-head comparison trials, and the VOYAGE and reSURFACE studies used different time points for primary end points. Furthermore, only phase 2 trial data are available for risankizumab. Despite these limitations, results of these trials suggest that guselkumab and risankizumab may be slightly more efficacious than tildrakizumab. However, future studies, including head-to-head studies, would ultimately provide further information on how these agents compare.
Guselkumab was shown to remain efficacious at 48 weeks, though patients on maintenance dosing had better results than those who were re-treated.12 Moreover, guselkumab was found to be effective in hard-to-treat areas, such as the scalp,11 and in patients who did not respond to adalimumab. Guselkumab may therefore benefit patients who have experienced limited clinical improvement on other biologics.12
Tildrakizumab was shown to improve PASI 75 and PGA scores through week 28 of treatment. Moreover, a higher percentage of patients taking tildrakizumab scored 0 or 1 on the dermatology life quality index, suggesting that the drug improves quality of life.14 No specific safety concerns arose in either reSURFACE trial; however, long-term studies are needed for further evaluation.
Risankizumab appears to be a promising new therapy based on phase 2 trial results. Improvements also were seen in dermatology life quality index scores, scalp and fingernail symptoms, and palmoplantar psoriasis.15 Of note, neutralizing antidrug antibodies were found in 3 patients during this study,15 which may present potential problems for long-term efficacy. However, preliminary data from 3 phase 3 trials—ultIMMa-1, ultIMMa-2, and IMMvent—are promising.17
CONCLUSION
Advances in the understanding of psoriasis have led to new targeted therapies. Ongoing clinical trials have shown encouraging results for treating physical and psychological symptoms of psoriasis. The findings of these trials support the idea that therapies targeting IL-23, specifically its p19 subunit, are effective against psoriasis while sparing IL-12. Long-term data from open-label extension studies would help guide clinical recommendations regarding the safety profiles of these agents and determine their long-term utility.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl 2):ii18-ii23; discussion, ii24, ii25.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Amin M, Darji K, No DJ, et al. Review of phase III trial data on IL-23 inhibitors tildrakizumab and guselkumab for psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1627-1632.
- Arican O, Aral M, Sasmaz S, et al. Levels of TNF-alpha, IFN-gamma, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:273-279.
- Griffiths CE, Reich K, Lebwohl M, et al; UNCOVER-2 and UNCOVER-3 investigators. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 study group, UNCOVER-2 study group, UNCOVER-3 study group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- FDA approves new psoriasis drug Taltz [news release]. Silver Spring, MD: US Food and Drug Administration; March 22, 2016. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm491872.htm. Accessed January 29, 2018.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Mentor A, et al. Phase 3 studies comparing brodalumab with ustekinumab for psoriasis. N Engl J Med. 2015;373:1318-1328.
- FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed January 29, 2018.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate-to-severe plaque psoriasis: results from the phase III, double-blinded placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76:418-431.
- Janssen announces U.S. FDA approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis [news release]. Horsham, PA: Johnson & Johnson; July 13, 2017. https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed January 29, 2018.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE1 and reSURFACE 2): results from two randomized controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Risankizumab. AbbVie Inc website. https://www.abbvie.com/our-science/pipeline/risankizumab.html. Accessed January 29, 2018.
- Risankizumab meets all co-primary and ranked secondary endpoints, achieving significantly greater efficacy versus standard biologic therapies in three pivotal phase 3 psoriasis studies [news release]. North Chicago, IL: AbbVie Inc; October 26, 2017. https://news.abbvie.com/news/risankizumab-meets-all-co-primary-and-ranked-secondary-endpoints-achieving-significantly-greater-efficacy-versus-standard-biologic-therapies-in-three-pivotal-phase-3-psoriasis-studies.htm. Accessed January 29, 2018.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab‘ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167:180-190.
- Kimball AB, Luger T, Gottlieb A, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156-1161.
- Dennehy EB, Zhang L, Amato D, et al. Ixekizumab is effective in subjects with moderate to severe plaque psoriasis with significant nail involvement: results from UNCOVER 3. J Drugs Dermatol. 2016;15:958-961.
- Gottlieb AB, Lacour JP, Korman N, et al. Treatment outcomes with ixekizumab in patients with moderate-to-severe psoriasis who have not received prior biological therapies: an integrated analysis of two phase III randomized studies. J Eur Acad Dermatol Venereol. 2017;31:679-685.
- Hueber W, Sands BE, Lewitsky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.
- Papp K, Menter A, Strober B, et al. Efficacy and safety of brodalumab in subpopulations of patients with difficult-to-treat moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2015;72:436-439.
- SILIQ [package insert]. Thousand Oaks, CA: Amgen, Inc; 2017.
Practice Points
- Tumor necrosis factor α, IL-23, and IL-17A are key targets for psoriasis therapy based on an understanding of the key role that these cytokines play in the pathophysiology of disease.
- The biologic agents secukinumab and ixekizumab are approved for use in the management of psoriasis. Other biologics—brodalumab, bimekizumab, guselkumab, tildrakizumab, risankizumab, and certolizumab pegol—have been (and some continue to be) the focus of phase 2 and phase 3 clinical trials.
- Findings of several of those trials support the idea that therapies targeting IL-23, specifically its p19 subunit, but that spare IL-12 are effective against psoriasis.
- Longer-term studies are needed to determine whether the agents reviewed here, including those approved for clinical use, are suitable for prolonged administration.
Do Psoriasis Patients Engage In Vigorous Physical Activity?
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
Psoriasis is a chronic inflammatory disease that affects approximately 2% to 3% of the US population.1 Patients with psoriasis are more likely to have cardiovascular risk factors (eg, obesity, metabolic syndrome) than individuals without psoriasis.2 In fact, recent evidence has suggested that a diagnosis of psoriasis is an independent risk factor for cardiometabolic diseases including diabetes, major adverse cardiovascular events, and obesity.3 Given the well-recognized health benefits of physical activity and the associated reduction in coronary heart disease risk,4 patients with psoriasis specifically may benefit from regular participation in physical activity. Thus, an enhanced understanding of the relationship between psoriasis and vigorous physical activity would help determine the role of initiating and recommending interventions that implement physical activity for patients with psoriasis. A review was conducted to determine the relationship between psoriasis and vigorous physical activity.
Methods
An English-language literature search of PubMed articles indexed for MEDLINE (January 1, 1946–October 15, 2017) as well as articles in the Embase database (January 1, 1947–October 15, 2017) and Cochrane Library (January 1, 1992–October 15, 2017) using the terms psoriasis and physical activity was performed. The search strategy was established based on a prior review of vigorous physical activity in eczema.5 The article titles and/or abstracts were reviewed, and the studies were excluded if they did not evaluate physical activity in patients with psoriasis. Studies without a control group also were excluded. Articles on patients with psoriatic arthritis and studies that involved modification of dietary intake also were excluded.
Two reviewers (M.A. and E.B.L.) independently extracted data from the studies and compiled the results. The following factors were included in the data extracted: study year, location, and design; method of diagnosis of psoriasis; total number of patients included in the study; and age, gender, and level of physical activity of the study patients. Level of physical activity was the exposure, and diagnosis of psoriasis was the dependent variable. Physical activity was defined differently across the studies that were evaluated. To determine study quality, we implemented the Newcastle–Ottawa Scale (NOS), a 9-star scoring system that includes items such as selection criteria, comparability, and study outcome.6 Studies with an NOS score of 7 or higher were included in the meta-analysis.
Results
The literature search generated 353 nonduplicate articles. A thorough review of the articles yielded 4 studies that were incorporated in the final analysis.7-10 We aimed to perform a meta-analysis; however, only 1 of the studies included in the final analysis had an NOS score of 7 or higher along with adequate data to be incorporated into our study.10 As a result, the meta-analysis was converted to a regular review.
The cross-sectional study we reviewed, which had an NOS score of 7, included males and females in the United States aged 20 to 59 years.10 Data were collected using the population-based National Health and Nutrition Examination Survey from 2003 to 2006. The survey measured the likelihood of participation in leisure-time moderate to vigorous physical activity (MVPA) and metabolic equivalent task (MET) minutes of MVPA in the past 30 days. Of 6549 participants, 385 were excluded from the analysis due to missing values for 1 or more of the study variables. Of the remaining 6164 participants, 84 (1.4%) reported having a diagnosis of psoriasis with few or no psoriasis patches at the time of the survey, and 71 (1.2%) reported having a diagnosis of psoriasis with few to extensive patches at the time of the survey.10
Participants with psoriasis were less likely to participate in MVPA in the previous 30 days compared to participants without psoriasis, but the association was not statistically significant.10 The study demonstrated that, on average, participants with psoriasis spent 31% (95% confidence interval [CI], −0.57 to −0.05) fewer MET minutes on leisure-time MVPA versus participants without psoriasis; however, this association was not statistically significant. It is important to note that the diagnosis of psoriasis was self-reported, and measures of disease duration or areas of involvement were not incorporated.
Comment
Our review revealed that vigorous physical activity may be reduced in patients with psoriasis compared to those without psoriasis. Initially, we aimed to perform a systematic review of the literature; however, only 1 study met the criteria for the systematic review, highlighting the need for more robust studies evaluating this subject.
Do et al10 demonstrated that psoriasis patients were less likely to participate in MVPA, but the findings were not statistically significant. Of those who participated in MVPA, MET minutes were fewer among patients with few to extensive skin lesions compared to those without psoriasis. The investigators suggested that psoriasis patients with more severe disease tend to exercise less and ultimately would benefit from regular vigorous physical activity.
Frankel et al7 performed a prospective cohort study in US women to evaluate the role of physical activity in preventing psoriasis. The investigators reported that the most physically active quintile had a lower multivariate relative risk of psoriasis (0.72; 95% CI, 0.59–0.89; P<.001 for trend) compared to the least active quintile.7 Additionally, vigorous physical activity, which was defined as 6 or more MET minutes, was associated with a significantly lower risk of incident psoriasis (0.66; 95% CI, 0.54–0.81; P<.001 for trend), which maintained significance after adjusting for body mass index (BMI). The investigators suggested that, by decreasing chronic inflammation and lowering levels of proinflammatory cytokines, vigorous physical activity may reduce the risk of psoriasis development in women.7 It is plausible that vigorous physical activity modifies the state of chronic inflammation, which could subsequently reduce the risk of developing psoriasis; however, further long-term, randomized, prospective studies are needed to verify the relationship between physical activity and development of psoriasis.
Torres et al8 performed a cross-sectional questionnaire study to assess physical activity in patients with severe psoriasis (defined as >10% body surface area involvement and/or disease requiring systemic therapy or phototherapy) versus healthy controls. Physical activity level was measured using the International Physical Activity Questionnaire. The odds ratio of low-level physical activity compared to non–low-level physical activity among psoriasis patients versus controls was 3.42 (95% CI, 1.47–7.91; P=.002). Additionally, the average total MET minutes of psoriasis patients were significantly reduced compared to those of the healthy controls (P=.001). Thus, the investigators suggested that vigorous physical activity is less likely in psoriasis patients, which may contribute to the increased risk of cardiovascular disease in this population.8 Vigorous physical activity would benefit patients with psoriasis to help lower the chronic state of inflammation and cardiometabolic comorbidities.
Demirel et al9 performed a study to compare aerobic exercise capacity and daily physical activity level in psoriasis patients (n=30) compared to controls (n=30). Daily physical activity, measured with an accelerometer, was significantly higher in male patients with psoriasis compared to controls (P=.021). No significant difference was reported in maximal aerobic capacity in both male and female psoriasis patients versus controls. The investigators suggested that the level of daily physical activity is not limited in psoriasis patients, yet the small sample size may limit the generalizability of the study.
The ability to dissipate heat during exercise seems to be diminished in patients with psoriasis. Specifically, it has been suggested that psoriasis lesions interfere with normal perspiration.11 Moreover, joint involvement in patients with psoriatic arthritis may lead to physical functional disabilities that can interfere with the ability of these patients to participate in regular physical activity.12-14 For this reason, our review excluded articles that evaluated patients with psoriatic arthritis. Despite this exclusion, it is important to consider that comorbid psoriatic arthritis in clinical practice may impede patients with psoriasis from participating in physical activity. Additionally, various social aspects also may limit physical activity in psoriasis patients; for instance, psoriasis patients often avoid activities that involve increased exposure of the skin (eg, communal showers, wearing sports attire).15
Furthermore, obese psoriasis patients are less likely to exercise compared to obese individuals without psoriasis.16 In patients with higher BMI, the risk of psoriasis is increased.17 A systematic review suggested that weight loss may improve psoriasis severity.18 Bariatric surgery also may improve psoriasis.19 Moreover, obesity may interfere with response to biologic therapies for psoriasis. Specifically, higher BMI is linked with lower response to fixed-dose biologic therapies compared to weight-based biologic options (eg, infliximab).20,21
Conclusion
Given the increased risk of myocardial infarction in patients with psoriasis, it is important to recognize the barriers to physical activity that psoriasis patients face.22 Due to the considerable health benefits associated with regular physical activity, physicians should encourage patients with psoriasis to participate in physical activity as tolerated. Of note, the studies included in this review varied in their definitions of psoriasis disease severity and measures of physical activity level. Long-term, randomized, prospective studies are needed to clarify the relationship between psoriasis and physical activity. Evidence from these studies would help guide clinical recommendations regarding the role of physical activity for patients with psoriasis.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
- Takeshita J, Gelfand JM, Li P, et al. Psoriasis in the US Medicare population: prevalence, treatment, and factors associated with biologic use. J Invest Dermatol. 2015;135:2955-2963.
- Prey S, Paul C, Bronsard V, et al. Cardiovascular risk factors in patients with plaque psoriasis: a systematic review of epidemiological studies. J Eur Acad Dermatol Venereol. 2010;24(suppl 2):23-30.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Leon AS. Biological mechanisms for the cardioprotective effects of aerobic exercise. Am J Lifestyle Med. 2009;3:32S-34S.
- Kim A, Silverberg JI. A systematic review of vigorous physical activity in eczema. Br J Dermatol. 2016;174:660-662.
- Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. The Ottawa Hospital Research Institute website. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed February 23, 2018.
- Frankel HC, Han J, Li T, et al. The association between physical activity and the risk of incident psoriasis. Arch Dermatol. 2012;148:918-924.
- Torres T, Alexandre JM, Mendonça D, et al. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129-135.
- Demirel R, Genc A, Ucok K, et al. Do patients with mild to moderate psoriasis really have a sedentary lifestyle? Int J Dermatol. 2013;52:1129-1134.
- Do YK, Lakhani N, Malhotra R, et al. Association between psoriasis and leisure‐time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol. 2015;42:148-153.
- Leibowitz E, Seidman DS, Laor A, et al. Are psoriatic patients at risk of heat intolerance? Br J Dermatol. 1991;124:439-442.
- Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: a longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53:404-409.
- Wilson FC, Icen M, Crowson CS, et al. Incidence and clinical predictors of psoriatic arthritis in patients with psoriasis: a population‐based study. Arthritis Rheum. 2009;61:233-239.
- Shih M, Hootman JM, Kruger J, et al. Physical activity in men and women with arthritis: National Health Interview Survey, 2002. Am J Prev Med. 2006;30:385-393.
- Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118:195-201.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Kumar S, Han J, Li T, et al. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293-1298.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Sako EY, Famenini S, Wu JJ. Bariatric surgery and psoriasis. J Am Acad Dermatol. 2014;70:774-779.
- Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol. 2008;58:443-446.
- Puig L. Obesity and psoriasis: body weight and body mass index influence the response to biological treatment. J Eur Acad Dermatol Venereol. 2011;25:1007-1011.
- Wu JJ, Choi YM, Bebchuk JD. Risk of myocardial infarction in psoriasis patients: a retrospective cohort study. J Dermatolog Treat. 2015;26:230-234.
Practice Points
- Psoriasis is associated with comorbid disease conditions, including cardiovascular disease.
- Regular physical activity is known to decrease the risk of developing cardiovascular disease.
- Patients with psoriasis would likely benefit from regular participation in vigorous physical activity to help reduce the risk of developing cardiovascular disease.
Psoriasis Treatment in HIV-Positive Patients: A Systematic Review of Systemic Immunosuppressive Therapies
The prevalence of psoriasis among human immunodeficiency virus (HIV)–positive patients in the United States is reported to be approximately 1% to 3%, which is similar to the rates reported for the general population.1 Recalcitrant cases of psoriasis in patients with no history of the condition can be the initial manifestation of HIV infection. In patients with preexisting psoriasis, a flare of their disease can be seen following infection, and progression of HIV correlates with worsening psoriasis.2 Psoriatic arthropathy also affects 23% to 50% of HIV-positive patients with psoriasis worldwide, which may be higher than the general population,1 with more severe joint disease.
The management of psoriatic disease in the HIV-positive population is challenging. The current first-line recommendations for treatment include topical therapies, phototherapy, and highly active antiretroviral therapy (HAART), followed by oral retinoids as second-line agents.3 However, the clinical course of psoriasis in HIV-positive patients often is progressive and refractory2; therefore, these therapies often are inadequate to control both skin and joint manifestations. Most other currently available systemic therapies for psoriatic disease are immunosuppressive, which poses a distinct clinical challenge because HIV-positive patients are already immunocompromised.
There currently are many systemic immunosuppressive agents used for the treatment of psoriatic disease, including oral agents (eg, methotrexate, hydroxyurea, cyclosporine), as well as newer biologic medications, including tumor necrosis factor (TNF) α inhibitors etanercept, adalimumab, infliximab, golimumab, and certolizumab pegol. Golimumab and certolizumab pegol currently are indicated for psoriatic arthritis only. Other newer biologic therapies include ustekinumab, which inhibits IL-12 and IL-23, and secukinumab, which inhibits IL-17A. The purpose of this systematic review is to evaluate the most current literature to explore the efficacy and safety data as they pertain to systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive individuals.
Methods
To investigate the efficacy and safety of systemic immunosuppressive therapies for psoriatic disease in HIV-positive individuals, a PubMed search of articles indexed for MEDLINE (1985-2015) was conducted using the terms psoriasis and HIV and psoriatic arthritis and HIV combined with each of the following systemic immunosuppressive agents: methotrexate, hydroxyurea, cyclosporine, etanercept, adalimumab, infliximab, golimumab, certolizumab pegol, ustekinumab, and secukinumab. Pediatric cases and articles that were not available in the English language were excluded.
For each case, patient demographic information (ie, age, sex), prior failed psoriasis treatments, and history of HAART were documented. The dosing regimen of the systemic agent was noted when different from the US Food and Drug administration–approved dosage for psoriasis or psoriatic arthritis. The duration of immunosuppressive therapy as well as pretreatment and posttreatment CD4 and viral counts (when available) were collected. The response to treatment and adverse effects were summarized.
Results
Our review of the literature yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients, including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab (Table). There were no reports of the use of hydroxyurea, golimumab, certolizumab pegol, or secukinumab to treat psoriatic disease in this patient population.
Methotrexate
Eight individual cases of methotrexate used to treat psoriasis and/or psoriatic arthritis in HIV-positive patients were reported.4-6 Duvic et al6 described 4 patients with psoriatic disease that was treated with methotrexate with varying efficacy. One patient developed toxic encephalopathy, which improved after discontinuation of methotrexate; however, he died 5 months later from pneumocystis pneumonia. In this early study, none of the 4 patients were on antiretroviral therapy for HIV.6
In the cases reported by Masson et al4 and Maurer et al,5 4 patients were treated with a single antiretroviral agent and received appropriate prophylaxis against opportunistic infections. In 1 case, methotrexate was given at a chemotherapeutic dose of 525 mg once weekly for Kaposi sarcoma.4 In 2 of 4 cases, the patients developed pneumocystis pneumonia.4,5
Cyclosporine
There were 2 case reports of successful treatment of psoriatic disease with cyclosporine in HIV-positive patients.7,8 Skin and joint manifestations improved rapidly without reports of infection for 27 and 8 years.8 Both patients were treated with one antiretroviral agent.7,8
Etanercept
There were 5 case reports of successful treatment of psoriatic disease with etanercept. In all 5 cases the patients were on HAART, and the CD4 count increased or remained stable and viral count became undetectable or remained stable following treatment.9-13 In 2 cases, the patient also had hepatitis C virus, which remained stable throughout the treatment period.9,12 The maximum duration of treatment was 6 years, with only 1 reported adverse event.13 In this case reported by Aboulafia et al,13 the patient experienced recurrent polymicrobial infections, including enterococcal cellulitis, cystitis, and bacteremia, as well as pseudomonas pneumonia and septic arthritis. Therapy was discontinued at 6 months. Four months after discontinuation of etanercept, the patient died from infectious causes.13
Adalimumab
There was 1 case of successful treatment of psoriatic disease with adalimumab in an HIV-positive patient. In this case, the patient was on HAART, and CD4 and viral counts improved substantially after 30 months of treatment.14
Infliximab
Six individual cases of successful treatment of psoriatic disease with infliximab were reported.15-17 In a report by Cepeda et al,15 HIV-positive patients with various rheumatologic diseases were chosen to receive etanercept followed by adalimumab and/or infliximab if clinical improvement was not observed on etanercept. In 3 patients with psoriasis and psoriatic arthritis, inadequate response was observed on etanercept. Two of these 3 patients received adalimumab with only partial response. All 3 were treated with infliximab in the end and showed excellent response. One of the patients experienced facial abscess responsive to antibiotics and was continued on infliximab therapy without further complications. In all 6 cases of infliximab therapy, the patients were on HAART, and CD4 and viral counts improved or remained stable.15
Ustekinumab
There were 3 case reports of successful treatment of psoriatic disease with ustekinumab in HIV-positive patients on HAART. CD4 and viral counts improved or remained stable.18-20
Comment
Currently, all of the systemic immunosuppressive therapies approved for psoriatic disease have a warning by the US Food and Drug Administration for increased risk of serious infection. Given such labels, these therapies are not routinely prescribed for HIV-positive patients who are already immunocompromised; however, many HIV-positive patients have severe psoriatic disease that cannot be adequately treated with first- and second-line therapies including topical agents, phototherapy, or oral retinoids.
Our comprehensive review yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab. Although data are limited to case reports and case series, some trends were observed.
Efficacy
In most of the cases reviewed, the patients had inadequate improvement of psoriatic disease with first- and second-line therapies, which included antiretrovirals alone, topical agents, phototherapy, and oral retinoids. Some cases reported poor response to methotrexate and cyclosporine.4-8 Biologic agents were effective in many such cases.
Safety
Overall, there were 11 cases in which the patient was not on adequate HAART while being treated with systemic immunosuppressive therapy for psoriatic disease.4-8,15 Of them, 3 were associated with serious infection while on methotrexate.5,6 There was only 1 report of serious infection13 of 14 cases in which the patient was on concomitant HAART. In this case, which reported polymicrobial infections and subsequent death of the patient, the infections continued after discontinuing etanercept; thus, the association is unclear. Interestingly, despite multiple infections, the CD4 and viral counts were stable throughout treatment with etanercept.13
From reviewing the 4 total cases5,6,13 of serious infection, HAART appears to be a valuable concomitant treatment during systemic immunosuppressive therapy for HIV-positive patients; however, it does not necessarily prevent serious infections from occurring, and thus the clinician’s diligence in monitoring for signs and symptoms of infection remains important.
CD4 and Viral Counts
Although reports of CD4 and viral counts were not available in earlier studies,4-8 there were 15 cases that reported consistent pretreatment and posttreatment CD4 and viral counts during treatment with etanercept, adalimumab, infliximab, and ustekinumab.9-20 In all cases, the CD4 count was stable or increased. Similarly, the viral count was stable or decreased. All patients, except 1 by Cepeda et al,15 were on concomitant HAART.9-14,16-20
Although data are limited, treatment of psoriatic disease with biologic agents when used in combination with HAART may have beneficial effects on CD4 and viral counts. Tumor necrosis factor has a role in HIV expression through the action of nuclear factor κβ.21 An increase in TNF levels is shown to be associated with increased viral count, decreased CD4 count, and increased symptoms of HIV progression, such as fever, fatigue, cachexia, and dementia.22 Although more studies are necessary, TNF-α inhibitors may have a positive effect on HIV while simultaneously treating psoriatic disease. Other cytokines (eg, IL-12, IL-23, IL-17) involved in the mechanism of action of other biologic agents (ustekinumab and secukinumab) have not been shown to be directly associated with HIV activity; however, studies have shown that IL-10 has a role in inhibiting HIV-1 replication and inhibits secretion of proinflammatory cytokines such as IL-12 and TNF-α.21 It may be speculated that the inhibition of IL-12 and TNF-α may create a positive feedback effect to increase IL-10, which in turn inhibits HIV replication.
Conclusion
Although there are limited data on the efficacy and safety of systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive patients, a review of 25 individual cases suggest that these treatments are not only required but also are sufficient to treat some of the most resistant cases. It is possible that with adequate concomitant HAART and monitoring for signs and symptoms of infection, the likelihood of serious infection may be low. Furthermore, biologic agents may have a positive effect over other systemic immunosuppressive agents, such as methotrexate and cyclosporine, in improving CD4 and viral counts when used in combination with HAART. Although randomized controlled trials are necessary, current biologic therapies such as etanercept, adalimumab, infliximab, and ustekinumab may be safe viable options as third-line treatment of severe psoriasis in the HIV-positive population.
- Mallon
E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246. - Montaz
eri A, Kanitakis J, Bazex J. Psoriasis and HIV infection. Int J Dermatol. 1996;35:475-479. - Menon
K, Van Vorhees AS, Bebo BF, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299. - Masso
n C, Chennebault JM, Leclech C. Is HIV infection contraindication to the use of methotrexate in psoriatic arthritis? J Rheumatol. 1995;22:2191. - Maurer
TA, Zackheim HS, Tuffanelli L, et al. The use of methotrexate for treatment of psoriasis in patients with HIV infection. J Am Acad Dermatol. 1994;31:372-375. - Duvic
M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632. - Tourne
L, Durez P, Van Vooren JP, et al. Alleviation of HIV-associated psoriasis and psoriatic arthritis with cyclosporine. J Am Acad Dermatol. 1997;37:501-502. - Allen
BR. Use of cyclosporine for psoriasis in HIV-positive patient. Lancet. 1992;339:686. - Di Ler
nia V, Zoboli G, Ficarelli E. Long-term management of HIV/hepatitis C virus associated psoriasis with etanercept. Indian J Dermatol Venereol Leprol. 2013;79:444. - Lee E
S, Heller MM, Kamangar F, et al. Long-term etanercept use for severe generalized psoriasis in an HIV-infected individual: a case study. J Drugs Dermatol. 2012;11:413-414. - Mikha
il M, Weinberg JM, Smith BL. Successful treatment with etanercept of von Zumbusch pustular psoriasis in a patient with human immunodeficiency virus. Arch Dermatol. 2008;144:453-456. - Linar
daki G, Katsarou O, Ioannidou P, et al. Effective etanercept treatment for psoriatic arthritis complicating concomitant human immunodeficiency virus and hepatitis C virus infection. J Rheumatol. 2007;34:1353-1355. - Aboul
afia DM, Bundow D, Wilske K, et al. Etanercept for the treatment of human immunodeficiency virus-associated psoriatic arthritis. Mayo Clin Proc. 2000;75:1093-1098. - Linds
ey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871. - Ceped
a EJ, Williams FM, Ishimori ML, et al. The use of anti-tumor necrosis factor therapy in HIV-positive individuals with rheumatic disease. Ann Rheum Dis. 2008;67:710-712. - Sella
m J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200. - Bartk
e U, Venten I, Kreuter A, et al. Human immunodeficiency virus-associated psoriasis and psoriatic arthritis treated with infliximab. Br J Dermatol. 2004;150:784-786. - Saeki
H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655. - Wiede
r S, Routt E, Levitt J, et al. Treatment of refractory psoriasis with ustekinumab in an HIV-positive patient: a case presentation and review of the biologic literature. Psoriasis Forum. 2014;20:96-102. - Papar
izos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399. - Kedzierska K, Crowe SM, Turville S, et al. The influence of cytokines, chemokines, and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13:39-56.
- Emer JJ. Is there a potential role for anti-tumor necrosis factor therapy in patients with human immunodeficiency virus? J Clin Aesthet Dermatol. 2009;2:29-35.
The prevalence of psoriasis among human immunodeficiency virus (HIV)–positive patients in the United States is reported to be approximately 1% to 3%, which is similar to the rates reported for the general population.1 Recalcitrant cases of psoriasis in patients with no history of the condition can be the initial manifestation of HIV infection. In patients with preexisting psoriasis, a flare of their disease can be seen following infection, and progression of HIV correlates with worsening psoriasis.2 Psoriatic arthropathy also affects 23% to 50% of HIV-positive patients with psoriasis worldwide, which may be higher than the general population,1 with more severe joint disease.
The management of psoriatic disease in the HIV-positive population is challenging. The current first-line recommendations for treatment include topical therapies, phototherapy, and highly active antiretroviral therapy (HAART), followed by oral retinoids as second-line agents.3 However, the clinical course of psoriasis in HIV-positive patients often is progressive and refractory2; therefore, these therapies often are inadequate to control both skin and joint manifestations. Most other currently available systemic therapies for psoriatic disease are immunosuppressive, which poses a distinct clinical challenge because HIV-positive patients are already immunocompromised.
There currently are many systemic immunosuppressive agents used for the treatment of psoriatic disease, including oral agents (eg, methotrexate, hydroxyurea, cyclosporine), as well as newer biologic medications, including tumor necrosis factor (TNF) α inhibitors etanercept, adalimumab, infliximab, golimumab, and certolizumab pegol. Golimumab and certolizumab pegol currently are indicated for psoriatic arthritis only. Other newer biologic therapies include ustekinumab, which inhibits IL-12 and IL-23, and secukinumab, which inhibits IL-17A. The purpose of this systematic review is to evaluate the most current literature to explore the efficacy and safety data as they pertain to systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive individuals.
Methods
To investigate the efficacy and safety of systemic immunosuppressive therapies for psoriatic disease in HIV-positive individuals, a PubMed search of articles indexed for MEDLINE (1985-2015) was conducted using the terms psoriasis and HIV and psoriatic arthritis and HIV combined with each of the following systemic immunosuppressive agents: methotrexate, hydroxyurea, cyclosporine, etanercept, adalimumab, infliximab, golimumab, certolizumab pegol, ustekinumab, and secukinumab. Pediatric cases and articles that were not available in the English language were excluded.
For each case, patient demographic information (ie, age, sex), prior failed psoriasis treatments, and history of HAART were documented. The dosing regimen of the systemic agent was noted when different from the US Food and Drug administration–approved dosage for psoriasis or psoriatic arthritis. The duration of immunosuppressive therapy as well as pretreatment and posttreatment CD4 and viral counts (when available) were collected. The response to treatment and adverse effects were summarized.
Results
Our review of the literature yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients, including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab (Table). There were no reports of the use of hydroxyurea, golimumab, certolizumab pegol, or secukinumab to treat psoriatic disease in this patient population.

Methotrexate
Eight individual cases of methotrexate used to treat psoriasis and/or psoriatic arthritis in HIV-positive patients were reported.4-6 Duvic et al6 described 4 patients with psoriatic disease that was treated with methotrexate with varying efficacy. One patient developed toxic encephalopathy, which improved after discontinuation of methotrexate; however, he died 5 months later from pneumocystis pneumonia. In this early study, none of the 4 patients were on antiretroviral therapy for HIV.6
In the cases reported by Masson et al4 and Maurer et al,5 4 patients were treated with a single antiretroviral agent and received appropriate prophylaxis against opportunistic infections. In 1 case, methotrexate was given at a chemotherapeutic dose of 525 mg once weekly for Kaposi sarcoma.4 In 2 of 4 cases, the patients developed pneumocystis pneumonia.4,5
Cyclosporine
There were 2 case reports of successful treatment of psoriatic disease with cyclosporine in HIV-positive patients.7,8 Skin and joint manifestations improved rapidly without reports of infection for 27 and 8 years.8 Both patients were treated with one antiretroviral agent.7,8
Etanercept
There were 5 case reports of successful treatment of psoriatic disease with etanercept. In all 5 cases the patients were on HAART, and the CD4 count increased or remained stable and viral count became undetectable or remained stable following treatment.9-13 In 2 cases, the patient also had hepatitis C virus, which remained stable throughout the treatment period.9,12 The maximum duration of treatment was 6 years, with only 1 reported adverse event.13 In this case reported by Aboulafia et al,13 the patient experienced recurrent polymicrobial infections, including enterococcal cellulitis, cystitis, and bacteremia, as well as pseudomonas pneumonia and septic arthritis. Therapy was discontinued at 6 months. Four months after discontinuation of etanercept, the patient died from infectious causes.13
Adalimumab
There was 1 case of successful treatment of psoriatic disease with adalimumab in an HIV-positive patient. In this case, the patient was on HAART, and CD4 and viral counts improved substantially after 30 months of treatment.14
Infliximab
Six individual cases of successful treatment of psoriatic disease with infliximab were reported.15-17 In a report by Cepeda et al,15 HIV-positive patients with various rheumatologic diseases were chosen to receive etanercept followed by adalimumab and/or infliximab if clinical improvement was not observed on etanercept. In 3 patients with psoriasis and psoriatic arthritis, inadequate response was observed on etanercept. Two of these 3 patients received adalimumab with only partial response. All 3 were treated with infliximab in the end and showed excellent response. One of the patients experienced facial abscess responsive to antibiotics and was continued on infliximab therapy without further complications. In all 6 cases of infliximab therapy, the patients were on HAART, and CD4 and viral counts improved or remained stable.15
Ustekinumab
There were 3 case reports of successful treatment of psoriatic disease with ustekinumab in HIV-positive patients on HAART. CD4 and viral counts improved or remained stable.18-20
Comment
Currently, all of the systemic immunosuppressive therapies approved for psoriatic disease have a warning by the US Food and Drug Administration for increased risk of serious infection. Given such labels, these therapies are not routinely prescribed for HIV-positive patients who are already immunocompromised; however, many HIV-positive patients have severe psoriatic disease that cannot be adequately treated with first- and second-line therapies including topical agents, phototherapy, or oral retinoids.
Our comprehensive review yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab. Although data are limited to case reports and case series, some trends were observed.
Efficacy
In most of the cases reviewed, the patients had inadequate improvement of psoriatic disease with first- and second-line therapies, which included antiretrovirals alone, topical agents, phototherapy, and oral retinoids. Some cases reported poor response to methotrexate and cyclosporine.4-8 Biologic agents were effective in many such cases.
Safety
Overall, there were 11 cases in which the patient was not on adequate HAART while being treated with systemic immunosuppressive therapy for psoriatic disease.4-8,15 Of them, 3 were associated with serious infection while on methotrexate.5,6 There was only 1 report of serious infection13 of 14 cases in which the patient was on concomitant HAART. In this case, which reported polymicrobial infections and subsequent death of the patient, the infections continued after discontinuing etanercept; thus, the association is unclear. Interestingly, despite multiple infections, the CD4 and viral counts were stable throughout treatment with etanercept.13
From reviewing the 4 total cases5,6,13 of serious infection, HAART appears to be a valuable concomitant treatment during systemic immunosuppressive therapy for HIV-positive patients; however, it does not necessarily prevent serious infections from occurring, and thus the clinician’s diligence in monitoring for signs and symptoms of infection remains important.
CD4 and Viral Counts
Although reports of CD4 and viral counts were not available in earlier studies,4-8 there were 15 cases that reported consistent pretreatment and posttreatment CD4 and viral counts during treatment with etanercept, adalimumab, infliximab, and ustekinumab.9-20 In all cases, the CD4 count was stable or increased. Similarly, the viral count was stable or decreased. All patients, except 1 by Cepeda et al,15 were on concomitant HAART.9-14,16-20
Although data are limited, treatment of psoriatic disease with biologic agents when used in combination with HAART may have beneficial effects on CD4 and viral counts. Tumor necrosis factor has a role in HIV expression through the action of nuclear factor κβ.21 An increase in TNF levels is shown to be associated with increased viral count, decreased CD4 count, and increased symptoms of HIV progression, such as fever, fatigue, cachexia, and dementia.22 Although more studies are necessary, TNF-α inhibitors may have a positive effect on HIV while simultaneously treating psoriatic disease. Other cytokines (eg, IL-12, IL-23, IL-17) involved in the mechanism of action of other biologic agents (ustekinumab and secukinumab) have not been shown to be directly associated with HIV activity; however, studies have shown that IL-10 has a role in inhibiting HIV-1 replication and inhibits secretion of proinflammatory cytokines such as IL-12 and TNF-α.21 It may be speculated that the inhibition of IL-12 and TNF-α may create a positive feedback effect to increase IL-10, which in turn inhibits HIV replication.
Conclusion
Although there are limited data on the efficacy and safety of systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive patients, a review of 25 individual cases suggest that these treatments are not only required but also are sufficient to treat some of the most resistant cases. It is possible that with adequate concomitant HAART and monitoring for signs and symptoms of infection, the likelihood of serious infection may be low. Furthermore, biologic agents may have a positive effect over other systemic immunosuppressive agents, such as methotrexate and cyclosporine, in improving CD4 and viral counts when used in combination with HAART. Although randomized controlled trials are necessary, current biologic therapies such as etanercept, adalimumab, infliximab, and ustekinumab may be safe viable options as third-line treatment of severe psoriasis in the HIV-positive population.
The prevalence of psoriasis among human immunodeficiency virus (HIV)–positive patients in the United States is reported to be approximately 1% to 3%, which is similar to the rates reported for the general population.1 Recalcitrant cases of psoriasis in patients with no history of the condition can be the initial manifestation of HIV infection. In patients with preexisting psoriasis, a flare of their disease can be seen following infection, and progression of HIV correlates with worsening psoriasis.2 Psoriatic arthropathy also affects 23% to 50% of HIV-positive patients with psoriasis worldwide, which may be higher than the general population,1 with more severe joint disease.
The management of psoriatic disease in the HIV-positive population is challenging. The current first-line recommendations for treatment include topical therapies, phototherapy, and highly active antiretroviral therapy (HAART), followed by oral retinoids as second-line agents.3 However, the clinical course of psoriasis in HIV-positive patients often is progressive and refractory2; therefore, these therapies often are inadequate to control both skin and joint manifestations. Most other currently available systemic therapies for psoriatic disease are immunosuppressive, which poses a distinct clinical challenge because HIV-positive patients are already immunocompromised.
There currently are many systemic immunosuppressive agents used for the treatment of psoriatic disease, including oral agents (eg, methotrexate, hydroxyurea, cyclosporine), as well as newer biologic medications, including tumor necrosis factor (TNF) α inhibitors etanercept, adalimumab, infliximab, golimumab, and certolizumab pegol. Golimumab and certolizumab pegol currently are indicated for psoriatic arthritis only. Other newer biologic therapies include ustekinumab, which inhibits IL-12 and IL-23, and secukinumab, which inhibits IL-17A. The purpose of this systematic review is to evaluate the most current literature to explore the efficacy and safety data as they pertain to systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive individuals.
Methods
To investigate the efficacy and safety of systemic immunosuppressive therapies for psoriatic disease in HIV-positive individuals, a PubMed search of articles indexed for MEDLINE (1985-2015) was conducted using the terms psoriasis and HIV and psoriatic arthritis and HIV combined with each of the following systemic immunosuppressive agents: methotrexate, hydroxyurea, cyclosporine, etanercept, adalimumab, infliximab, golimumab, certolizumab pegol, ustekinumab, and secukinumab. Pediatric cases and articles that were not available in the English language were excluded.
For each case, patient demographic information (ie, age, sex), prior failed psoriasis treatments, and history of HAART were documented. The dosing regimen of the systemic agent was noted when different from the US Food and Drug administration–approved dosage for psoriasis or psoriatic arthritis. The duration of immunosuppressive therapy as well as pretreatment and posttreatment CD4 and viral counts (when available) were collected. The response to treatment and adverse effects were summarized.
Results
Our review of the literature yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients, including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab (Table). There were no reports of the use of hydroxyurea, golimumab, certolizumab pegol, or secukinumab to treat psoriatic disease in this patient population.

Methotrexate
Eight individual cases of methotrexate used to treat psoriasis and/or psoriatic arthritis in HIV-positive patients were reported.4-6 Duvic et al6 described 4 patients with psoriatic disease that was treated with methotrexate with varying efficacy. One patient developed toxic encephalopathy, which improved after discontinuation of methotrexate; however, he died 5 months later from pneumocystis pneumonia. In this early study, none of the 4 patients were on antiretroviral therapy for HIV.6
In the cases reported by Masson et al4 and Maurer et al,5 4 patients were treated with a single antiretroviral agent and received appropriate prophylaxis against opportunistic infections. In 1 case, methotrexate was given at a chemotherapeutic dose of 525 mg once weekly for Kaposi sarcoma.4 In 2 of 4 cases, the patients developed pneumocystis pneumonia.4,5
Cyclosporine
There were 2 case reports of successful treatment of psoriatic disease with cyclosporine in HIV-positive patients.7,8 Skin and joint manifestations improved rapidly without reports of infection for 27 and 8 years.8 Both patients were treated with one antiretroviral agent.7,8
Etanercept
There were 5 case reports of successful treatment of psoriatic disease with etanercept. In all 5 cases the patients were on HAART, and the CD4 count increased or remained stable and viral count became undetectable or remained stable following treatment.9-13 In 2 cases, the patient also had hepatitis C virus, which remained stable throughout the treatment period.9,12 The maximum duration of treatment was 6 years, with only 1 reported adverse event.13 In this case reported by Aboulafia et al,13 the patient experienced recurrent polymicrobial infections, including enterococcal cellulitis, cystitis, and bacteremia, as well as pseudomonas pneumonia and septic arthritis. Therapy was discontinued at 6 months. Four months after discontinuation of etanercept, the patient died from infectious causes.13
Adalimumab
There was 1 case of successful treatment of psoriatic disease with adalimumab in an HIV-positive patient. In this case, the patient was on HAART, and CD4 and viral counts improved substantially after 30 months of treatment.14
Infliximab
Six individual cases of successful treatment of psoriatic disease with infliximab were reported.15-17 In a report by Cepeda et al,15 HIV-positive patients with various rheumatologic diseases were chosen to receive etanercept followed by adalimumab and/or infliximab if clinical improvement was not observed on etanercept. In 3 patients with psoriasis and psoriatic arthritis, inadequate response was observed on etanercept. Two of these 3 patients received adalimumab with only partial response. All 3 were treated with infliximab in the end and showed excellent response. One of the patients experienced facial abscess responsive to antibiotics and was continued on infliximab therapy without further complications. In all 6 cases of infliximab therapy, the patients were on HAART, and CD4 and viral counts improved or remained stable.15
Ustekinumab
There were 3 case reports of successful treatment of psoriatic disease with ustekinumab in HIV-positive patients on HAART. CD4 and viral counts improved or remained stable.18-20
Comment
Currently, all of the systemic immunosuppressive therapies approved for psoriatic disease have a warning by the US Food and Drug Administration for increased risk of serious infection. Given such labels, these therapies are not routinely prescribed for HIV-positive patients who are already immunocompromised; however, many HIV-positive patients have severe psoriatic disease that cannot be adequately treated with first- and second-line therapies including topical agents, phototherapy, or oral retinoids.
Our comprehensive review yielded a total of 25 reported cases of systemic immunosuppressive therapies used to treat psoriatic disease in HIV-positive patients including methotrexate, cyclosporine, etanercept, adalimumab, in-fliximab, and ustekinumab. Although data are limited to case reports and case series, some trends were observed.
Efficacy
In most of the cases reviewed, the patients had inadequate improvement of psoriatic disease with first- and second-line therapies, which included antiretrovirals alone, topical agents, phototherapy, and oral retinoids. Some cases reported poor response to methotrexate and cyclosporine.4-8 Biologic agents were effective in many such cases.
Safety
Overall, there were 11 cases in which the patient was not on adequate HAART while being treated with systemic immunosuppressive therapy for psoriatic disease.4-8,15 Of them, 3 were associated with serious infection while on methotrexate.5,6 There was only 1 report of serious infection13 of 14 cases in which the patient was on concomitant HAART. In this case, which reported polymicrobial infections and subsequent death of the patient, the infections continued after discontinuing etanercept; thus, the association is unclear. Interestingly, despite multiple infections, the CD4 and viral counts were stable throughout treatment with etanercept.13
From reviewing the 4 total cases5,6,13 of serious infection, HAART appears to be a valuable concomitant treatment during systemic immunosuppressive therapy for HIV-positive patients; however, it does not necessarily prevent serious infections from occurring, and thus the clinician’s diligence in monitoring for signs and symptoms of infection remains important.
CD4 and Viral Counts
Although reports of CD4 and viral counts were not available in earlier studies,4-8 there were 15 cases that reported consistent pretreatment and posttreatment CD4 and viral counts during treatment with etanercept, adalimumab, infliximab, and ustekinumab.9-20 In all cases, the CD4 count was stable or increased. Similarly, the viral count was stable or decreased. All patients, except 1 by Cepeda et al,15 were on concomitant HAART.9-14,16-20
Although data are limited, treatment of psoriatic disease with biologic agents when used in combination with HAART may have beneficial effects on CD4 and viral counts. Tumor necrosis factor has a role in HIV expression through the action of nuclear factor κβ.21 An increase in TNF levels is shown to be associated with increased viral count, decreased CD4 count, and increased symptoms of HIV progression, such as fever, fatigue, cachexia, and dementia.22 Although more studies are necessary, TNF-α inhibitors may have a positive effect on HIV while simultaneously treating psoriatic disease. Other cytokines (eg, IL-12, IL-23, IL-17) involved in the mechanism of action of other biologic agents (ustekinumab and secukinumab) have not been shown to be directly associated with HIV activity; however, studies have shown that IL-10 has a role in inhibiting HIV-1 replication and inhibits secretion of proinflammatory cytokines such as IL-12 and TNF-α.21 It may be speculated that the inhibition of IL-12 and TNF-α may create a positive feedback effect to increase IL-10, which in turn inhibits HIV replication.
Conclusion
Although there are limited data on the efficacy and safety of systemic immunosuppressive therapies for the treatment of psoriatic disease in HIV-positive patients, a review of 25 individual cases suggest that these treatments are not only required but also are sufficient to treat some of the most resistant cases. It is possible that with adequate concomitant HAART and monitoring for signs and symptoms of infection, the likelihood of serious infection may be low. Furthermore, biologic agents may have a positive effect over other systemic immunosuppressive agents, such as methotrexate and cyclosporine, in improving CD4 and viral counts when used in combination with HAART. Although randomized controlled trials are necessary, current biologic therapies such as etanercept, adalimumab, infliximab, and ustekinumab may be safe viable options as third-line treatment of severe psoriasis in the HIV-positive population.
- Mallon
E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246. - Montaz
eri A, Kanitakis J, Bazex J. Psoriasis and HIV infection. Int J Dermatol. 1996;35:475-479. - Menon
K, Van Vorhees AS, Bebo BF, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299. - Masso
n C, Chennebault JM, Leclech C. Is HIV infection contraindication to the use of methotrexate in psoriatic arthritis? J Rheumatol. 1995;22:2191. - Maurer
TA, Zackheim HS, Tuffanelli L, et al. The use of methotrexate for treatment of psoriasis in patients with HIV infection. J Am Acad Dermatol. 1994;31:372-375. - Duvic
M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632. - Tourne
L, Durez P, Van Vooren JP, et al. Alleviation of HIV-associated psoriasis and psoriatic arthritis with cyclosporine. J Am Acad Dermatol. 1997;37:501-502. - Allen
BR. Use of cyclosporine for psoriasis in HIV-positive patient. Lancet. 1992;339:686. - Di Ler
nia V, Zoboli G, Ficarelli E. Long-term management of HIV/hepatitis C virus associated psoriasis with etanercept. Indian J Dermatol Venereol Leprol. 2013;79:444. - Lee E
S, Heller MM, Kamangar F, et al. Long-term etanercept use for severe generalized psoriasis in an HIV-infected individual: a case study. J Drugs Dermatol. 2012;11:413-414. - Mikha
il M, Weinberg JM, Smith BL. Successful treatment with etanercept of von Zumbusch pustular psoriasis in a patient with human immunodeficiency virus. Arch Dermatol. 2008;144:453-456. - Linar
daki G, Katsarou O, Ioannidou P, et al. Effective etanercept treatment for psoriatic arthritis complicating concomitant human immunodeficiency virus and hepatitis C virus infection. J Rheumatol. 2007;34:1353-1355. - Aboul
afia DM, Bundow D, Wilske K, et al. Etanercept for the treatment of human immunodeficiency virus-associated psoriatic arthritis. Mayo Clin Proc. 2000;75:1093-1098. - Linds
ey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871. - Ceped
a EJ, Williams FM, Ishimori ML, et al. The use of anti-tumor necrosis factor therapy in HIV-positive individuals with rheumatic disease. Ann Rheum Dis. 2008;67:710-712. - Sella
m J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200. - Bartk
e U, Venten I, Kreuter A, et al. Human immunodeficiency virus-associated psoriasis and psoriatic arthritis treated with infliximab. Br J Dermatol. 2004;150:784-786. - Saeki
H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655. - Wiede
r S, Routt E, Levitt J, et al. Treatment of refractory psoriasis with ustekinumab in an HIV-positive patient: a case presentation and review of the biologic literature. Psoriasis Forum. 2014;20:96-102. - Papar
izos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399. - Kedzierska K, Crowe SM, Turville S, et al. The influence of cytokines, chemokines, and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13:39-56.
- Emer JJ. Is there a potential role for anti-tumor necrosis factor therapy in patients with human immunodeficiency virus? J Clin Aesthet Dermatol. 2009;2:29-35.
- Mallon
E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246. - Montaz
eri A, Kanitakis J, Bazex J. Psoriasis and HIV infection. Int J Dermatol. 1996;35:475-479. - Menon
K, Van Vorhees AS, Bebo BF, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299. - Masso
n C, Chennebault JM, Leclech C. Is HIV infection contraindication to the use of methotrexate in psoriatic arthritis? J Rheumatol. 1995;22:2191. - Maurer
TA, Zackheim HS, Tuffanelli L, et al. The use of methotrexate for treatment of psoriasis in patients with HIV infection. J Am Acad Dermatol. 1994;31:372-375. - Duvic
M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632. - Tourne
L, Durez P, Van Vooren JP, et al. Alleviation of HIV-associated psoriasis and psoriatic arthritis with cyclosporine. J Am Acad Dermatol. 1997;37:501-502. - Allen
BR. Use of cyclosporine for psoriasis in HIV-positive patient. Lancet. 1992;339:686. - Di Ler
nia V, Zoboli G, Ficarelli E. Long-term management of HIV/hepatitis C virus associated psoriasis with etanercept. Indian J Dermatol Venereol Leprol. 2013;79:444. - Lee E
S, Heller MM, Kamangar F, et al. Long-term etanercept use for severe generalized psoriasis in an HIV-infected individual: a case study. J Drugs Dermatol. 2012;11:413-414. - Mikha
il M, Weinberg JM, Smith BL. Successful treatment with etanercept of von Zumbusch pustular psoriasis in a patient with human immunodeficiency virus. Arch Dermatol. 2008;144:453-456. - Linar
daki G, Katsarou O, Ioannidou P, et al. Effective etanercept treatment for psoriatic arthritis complicating concomitant human immunodeficiency virus and hepatitis C virus infection. J Rheumatol. 2007;34:1353-1355. - Aboul
afia DM, Bundow D, Wilske K, et al. Etanercept for the treatment of human immunodeficiency virus-associated psoriatic arthritis. Mayo Clin Proc. 2000;75:1093-1098. - Linds
ey SF, Weiss J, Lee ES, et al. Treatment of severe psoriasis and psoriatic arthritis with adalimumab in an HIV-positive patient. J Drugs Dermatol. 2014;13:869-871. - Ceped
a EJ, Williams FM, Ishimori ML, et al. The use of anti-tumor necrosis factor therapy in HIV-positive individuals with rheumatic disease. Ann Rheum Dis. 2008;67:710-712. - Sella
m J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200. - Bartk
e U, Venten I, Kreuter A, et al. Human immunodeficiency virus-associated psoriasis and psoriatic arthritis treated with infliximab. Br J Dermatol. 2004;150:784-786. - Saeki
H, Ito T, Hayashi M, et al. Successful treatment of ustekinumab in a severe psoriasis patient with human immunodeficiency virus infection. J Eur Acad Dermatol Venereol. 2015;29:1653-1655. - Wiede
r S, Routt E, Levitt J, et al. Treatment of refractory psoriasis with ustekinumab in an HIV-positive patient: a case presentation and review of the biologic literature. Psoriasis Forum. 2014;20:96-102. - Papar
izos V, Rallis E, Kirsten L, et al. Ustekinumab for the treatment of HIV psoriasis. J Dermatol Treat. 2012;23:398-399. - Kedzierska K, Crowe SM, Turville S, et al. The influence of cytokines, chemokines, and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13:39-56.
- Emer JJ. Is there a potential role for anti-tumor necrosis factor therapy in patients with human immunodeficiency virus? J Clin Aesthet Dermatol. 2009;2:29-35.
Practice Points
- There are limited data on the use of systemic immunosuppressive therapies for the treatment of psoriatic disease in human immunodeficiency virus–positive patients.
- The limited data suggest that biologic therapies may be effective for cases of psoriasis recalcitrant to other systemic agents and may have a positive effect on CD4 and viral counts when used in combination with highly active antiretroviral therapy.
- Further research is needed.