User login
Beta-thalassemia: Benefits of gene therapy outweigh costs
Surveyed at 3 years, patients also reported ongoing benefits from treatment, including positive impacts on employment, school attendance, and physical activity, according to a second report.
The findings address a major question about betibeglogene autotemcel: Its durability. The therapy is priced at over $2 million per treatment, based on the premise that it will benefit patients in the long-term, in part by offsetting the cost of ongoing transfusions. Therefore, proof of long-standing benefit is important.
The Food and Drug Administration approved betibeglogene autotemcel in August 2022 for children and adults with transfusion dependent beta-thalassemia, a condition that causes patients to have absent or reduced levels of hemoglobin due to mutations in the beta-globin gene. Patients typically require transfusions every 2-5 weeks.
The treatment inserts functional copies of the mutated gene into the patients’ hematopoietic stem cells via a replication-defective lentivirus. The cells are then transfused back into the patient.
As of August 2021, 63 patients had undergone treatment and been followed for a median of 41.4 months. So far, durability looks solid.
“We now have up to 8 years efficacy and safety follow-up” with beti-cel. “Patients experience durable transfusion independence,” said Mark Walters, MD, a pediatric hematologist/oncologist at the University of California, San Francisco, who presented the long-term efficacy data at the meeting.
Overall, 89.5% of patients (34/38) in phase 3 testing achieved transfusion independence, meaning that they had hemoglobin levels of at least 9 g/dL without transfusions for a year or more.
The response rate was an improvement over phase 1/2 testing, in which 68% of subjects (15/22) became transfusion free. Improvements in the manufacturing process led to better outcomes in phase 3, Dr. Walters said.
As for quality of life (QoL), improvement “continues through 3 years following treatment,” said Franco Locatelli, MD, a pediatric hematologist/oncologist at Catholic University of the Sacred Heart, Rome, who led the QoL study.
When patients who achieved transfusion independence were surveyed 3 years after treatment, 93% of adults were employed or able to seek employment, up from 67% before treatment. School absences were down among children, almost half of subjects no longer needed symptom management, and 81% reported improvements in physical activity.
There were also improvements on various quality of life scales, including in physical functioning and mental health.
Patient age and underlying thalassemia genotype had no impact on the likelihood of transfusion independence. Those who achieved it also had reductions in markers of ineffective erythropoiesis and iron overload.
On multivariate analysis, the greatest predictor of transfusion independence was having at least 62% of cells transduced prior to reintroduction to the patient.
As for adverse events, seven subjects (11%) developed severe veno-occlusive liver disease that resolved with supportive care. Mucositis and febrile neutropenia are also a concern and related to the busulfan conditioning regimen.
No malignancies, insertional oncogenesis, or lentivirus replication have been observed.
The studies were funded by beti-cel maker Bluebird Bio, and many of the investigators are employees. Others reported ties to Bluebird and a range of other companies. Among his industry ties, Dr. Locatelli is a speaker for Bluebird. Dr. Walters also had industry relationships, but didn’t report any ties to Bluebird.
Surveyed at 3 years, patients also reported ongoing benefits from treatment, including positive impacts on employment, school attendance, and physical activity, according to a second report.
The findings address a major question about betibeglogene autotemcel: Its durability. The therapy is priced at over $2 million per treatment, based on the premise that it will benefit patients in the long-term, in part by offsetting the cost of ongoing transfusions. Therefore, proof of long-standing benefit is important.
The Food and Drug Administration approved betibeglogene autotemcel in August 2022 for children and adults with transfusion dependent beta-thalassemia, a condition that causes patients to have absent or reduced levels of hemoglobin due to mutations in the beta-globin gene. Patients typically require transfusions every 2-5 weeks.
The treatment inserts functional copies of the mutated gene into the patients’ hematopoietic stem cells via a replication-defective lentivirus. The cells are then transfused back into the patient.
As of August 2021, 63 patients had undergone treatment and been followed for a median of 41.4 months. So far, durability looks solid.
“We now have up to 8 years efficacy and safety follow-up” with beti-cel. “Patients experience durable transfusion independence,” said Mark Walters, MD, a pediatric hematologist/oncologist at the University of California, San Francisco, who presented the long-term efficacy data at the meeting.
Overall, 89.5% of patients (34/38) in phase 3 testing achieved transfusion independence, meaning that they had hemoglobin levels of at least 9 g/dL without transfusions for a year or more.
The response rate was an improvement over phase 1/2 testing, in which 68% of subjects (15/22) became transfusion free. Improvements in the manufacturing process led to better outcomes in phase 3, Dr. Walters said.
As for quality of life (QoL), improvement “continues through 3 years following treatment,” said Franco Locatelli, MD, a pediatric hematologist/oncologist at Catholic University of the Sacred Heart, Rome, who led the QoL study.
When patients who achieved transfusion independence were surveyed 3 years after treatment, 93% of adults were employed or able to seek employment, up from 67% before treatment. School absences were down among children, almost half of subjects no longer needed symptom management, and 81% reported improvements in physical activity.
There were also improvements on various quality of life scales, including in physical functioning and mental health.
Patient age and underlying thalassemia genotype had no impact on the likelihood of transfusion independence. Those who achieved it also had reductions in markers of ineffective erythropoiesis and iron overload.
On multivariate analysis, the greatest predictor of transfusion independence was having at least 62% of cells transduced prior to reintroduction to the patient.
As for adverse events, seven subjects (11%) developed severe veno-occlusive liver disease that resolved with supportive care. Mucositis and febrile neutropenia are also a concern and related to the busulfan conditioning regimen.
No malignancies, insertional oncogenesis, or lentivirus replication have been observed.
The studies were funded by beti-cel maker Bluebird Bio, and many of the investigators are employees. Others reported ties to Bluebird and a range of other companies. Among his industry ties, Dr. Locatelli is a speaker for Bluebird. Dr. Walters also had industry relationships, but didn’t report any ties to Bluebird.
Surveyed at 3 years, patients also reported ongoing benefits from treatment, including positive impacts on employment, school attendance, and physical activity, according to a second report.
The findings address a major question about betibeglogene autotemcel: Its durability. The therapy is priced at over $2 million per treatment, based on the premise that it will benefit patients in the long-term, in part by offsetting the cost of ongoing transfusions. Therefore, proof of long-standing benefit is important.
The Food and Drug Administration approved betibeglogene autotemcel in August 2022 for children and adults with transfusion dependent beta-thalassemia, a condition that causes patients to have absent or reduced levels of hemoglobin due to mutations in the beta-globin gene. Patients typically require transfusions every 2-5 weeks.
The treatment inserts functional copies of the mutated gene into the patients’ hematopoietic stem cells via a replication-defective lentivirus. The cells are then transfused back into the patient.
As of August 2021, 63 patients had undergone treatment and been followed for a median of 41.4 months. So far, durability looks solid.
“We now have up to 8 years efficacy and safety follow-up” with beti-cel. “Patients experience durable transfusion independence,” said Mark Walters, MD, a pediatric hematologist/oncologist at the University of California, San Francisco, who presented the long-term efficacy data at the meeting.
Overall, 89.5% of patients (34/38) in phase 3 testing achieved transfusion independence, meaning that they had hemoglobin levels of at least 9 g/dL without transfusions for a year or more.
The response rate was an improvement over phase 1/2 testing, in which 68% of subjects (15/22) became transfusion free. Improvements in the manufacturing process led to better outcomes in phase 3, Dr. Walters said.
As for quality of life (QoL), improvement “continues through 3 years following treatment,” said Franco Locatelli, MD, a pediatric hematologist/oncologist at Catholic University of the Sacred Heart, Rome, who led the QoL study.
When patients who achieved transfusion independence were surveyed 3 years after treatment, 93% of adults were employed or able to seek employment, up from 67% before treatment. School absences were down among children, almost half of subjects no longer needed symptom management, and 81% reported improvements in physical activity.
There were also improvements on various quality of life scales, including in physical functioning and mental health.
Patient age and underlying thalassemia genotype had no impact on the likelihood of transfusion independence. Those who achieved it also had reductions in markers of ineffective erythropoiesis and iron overload.
On multivariate analysis, the greatest predictor of transfusion independence was having at least 62% of cells transduced prior to reintroduction to the patient.
As for adverse events, seven subjects (11%) developed severe veno-occlusive liver disease that resolved with supportive care. Mucositis and febrile neutropenia are also a concern and related to the busulfan conditioning regimen.
No malignancies, insertional oncogenesis, or lentivirus replication have been observed.
The studies were funded by beti-cel maker Bluebird Bio, and many of the investigators are employees. Others reported ties to Bluebird and a range of other companies. Among his industry ties, Dr. Locatelli is a speaker for Bluebird. Dr. Walters also had industry relationships, but didn’t report any ties to Bluebird.
FROM ASH 2022
U.S. sees most flu hospitalizations in a decade
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.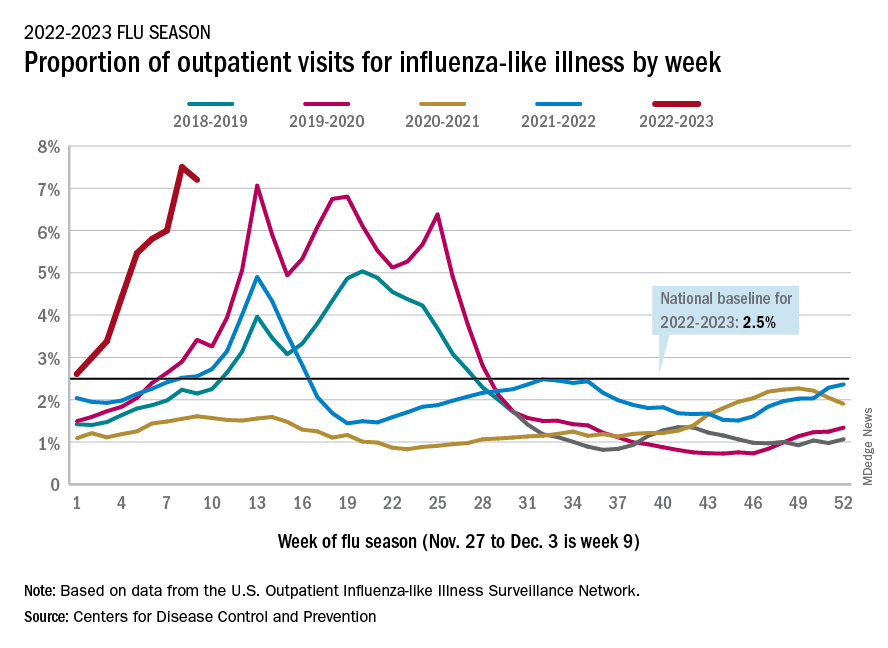
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
ADA issues 2023 ‘Standards of Care’ for diabetes: Focus on tight BP, lipids
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
New more aggressive targets for blood pressure and lipids are among the changes to the annual American Diabetes Association (ADA) Standards of Care in Diabetes – 2023.
The document, long considered the gold standard for care of the more than 100 million Americans living with diabetes and prediabetes, was published as a supplement in Diabetes Care. The guidelines are also accessible to doctors via an app; last year’s standards were accessed more than 4 million times.
The standards now advise a blood pressure target for people with diabetes of less than 130/80 mm Hg, and low-density lipoprotein (LDL) cholesterol targets of below 70 mg/dL or no greater than 55 mg/dL, depending on the individual’s cardiovascular risk.
“In this year’s version of the ADA Standards of Care – the longstanding guidelines for diabetes management globally – you’ll see information that really speaks to how we can more aggressively treat diabetes and reduce complications in a variety of different ways,” ADA Chief Scientific and Medical Officer Robert A. Gabbay, MD, PhD, said in an interview.
Other changes for 2023 include a new emphasis on weight loss as a goal of therapy for type 2 diabetes; guidance for screening and assessing peripheral arterial disease in an effort to prevent amputations; use of finerenone in people with diabetes and chronic kidney disease; use of approved point-of-care A1c tests; and guidance on screening for food insecurity, along with an elevated role for community health workers.
“The management of type 2 diabetes is not just about glucose,” Dr. Gabbay emphasized, noting that the ADA Standards have increasingly focused on cardiorenal risk as well as weight management. “We need to think about all those things, not just one. We have better tools now that have been helpful in being able to move forward with this.”
New targets in cardiovascular disease and risk management
As it has been for the past 6 years, the section on cardiovascular disease and risk management is also endorsed by the American College of Cardiology.
The new definition of hypertension in people with diabetes is ≥ 130 mm Hg systolic or ≥ 80 mm Hg diastolic blood pressure, repeated on two measurements at different times. Among individuals with established cardiovascular disease, hypertension can be diagnosed with one measurement of ≥ 180/110 mm Hg.
The goal of treatment is now less than 130/80 mm Hg if it can be reached safely.
In 2012, easing of the systolic target to 140 mm Hg by the ADA caused some controversy.
But, as Dr. Gabbay explained: “The evidence wasn’t there 10 years ago. We stuck to the evidence at that time, although there was a belief that lower was better. Over the past decade, a number of studies have made it quite clear that there is benefit to a lower target. That’s why we staked out the ground on this.”
The new Standards of Care also has new lipid targets. For people with diabetes aged 40-75 years at increased cardiovascular risk, including those with one or more atherosclerotic risk factors, high-intensity statin therapy is recommended to reduce LDL cholesterol by 50% or more from baseline and to a target of less than 70 mg/dL, in contrast to the previous target of 100 mg/dL.
To achieve that goal, the document advises to consider adding ezetimibe or a PCSK9 inhibitor to maximally tolerated statin therapy.
For people with diabetes aged 40-75 who have established cardiovascular disease, treatment with high-intensity statin therapy is recommended with the target of a 50% or greater reduction from baseline and an LDL cholesterol level of 55 mg/dL or lower, in contrast to the previous 70 mg/dL.
“That is a lower goal than previously recommended, and based on strong evidence in the literature,” Dr. Gabbay noted.
Here, a stronger recommendation is made for ezetimibe or a PCSK9 inhibitor added to maximal statins.
And for people with diabetes older than 75 years, those already on statins should continue taking them. For those who aren’t, it may be reasonable to initiate moderate-intensity statin therapy after discussion of the benefits and risks.
Another new recommendation based on recent trial data is use of a sodium–glucose cotransporter 2 (SGLT2) inhibitor in people with diabetes and heart failure with preserved, as well as reduced, ejection fraction.
Kidney disease guidance updated: SGLT2 inhibitors, finerenone
Another recommendation calls for the addition of finerenone for people with type 2 diabetes who have chronic kidney disease (CKD) with albuminuria and have been treated with the maximum tolerated doses of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) to improve cardiovascular outcomes as well as reduce the risk of CKD progression.
The threshold for initiating an SGLT2 inhibitor for kidney protection has changed to an estimated glomerular filtration rate (eGFR) ≥ 20 mL/min/1.73 m2 and urinary albumin ≥ 200 mg/g creatinine (previously ≥ 25 mL/min/1.73 m2 and ≥ 300 mg/g, respectively). An SGLT2 inhibitor may also be beneficial in people with a urinary albumin of normal to ≥ 200 mg/g creatinine, but supporting data have not yet been published.
Referral to a nephrologist is advised for individuals with increasing urinary albumin levels or continued decreasing eGFR or eGFR < 30 mL/min/1.73 m2.
Weight loss, point-of-care testing, food insecurity assessment
Other changes for 2023 include fresh emphasis on supporting weight loss of up to 15% with the new twincretin tirzepatide (Mounjaro) – approved in the United States in May for type 2 diabetes – added as a glucose-lowering drug with weight loss potential.
A novel section was added with guidance for peripheral arterial disease screening.
And a new recommendation advises use of point-of-care A1c testing for diabetes screening and diagnosis using only tests approved by the Food and Drug Administration.
Also introduced for 2023 is guidance to use community health workers to support the management of diabetes and cardiovascular risk factors, particularly in underserved areas and health systems.
“Community health workers can be a link to help people navigate and engage with the health system for better outcomes,” said Dr. Gabbay.
He added that these professionals are among those who can also assist with screening for food insecurity, another new recommendation. “We talk about screening for food insecurity and tools to use. That shouldn’t be something only dietitians do.”
Dr. Gabbay said he’d like to see more clinicians partner with community health workers. “We’d like to see more of that ... They should be considered part of the health care team,” he said.
Dr. Gabbay has reported serving on advisory boards for Lark, Health Reveal, Sweetch, StartUp Health, Vida Health, and Onduo.
A version of this article first appeared on Medscape.com.
As COVID treatments dwindle, are new ones waiting in the wings?
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three antiseizure medications join list for newborn risks
NASHVILLE, TENN. – A study of more than 4 million births over 20 years in five Scandinavian countries has reported that three antiseizure medications should be used with caution in women of child-bearing age because they were associated with low birth weights.
In results presented at the annual meeting of the American Epilepsy Society, Jakob Christensen, MD, DSc, PhD, a professor at Aarhus University Hospital in Denmark, said that the study found that
“Because we have this large data set we were able to confirm the suspicion that’s been raised in the past that these drugs may be associated with low birth weight,” Dr. Christensen said in an interview.
The study analyzed records from population-based registers of 4.5 million births in Denmark, Finland, Iceland, Norway, and Sweden between 1996 and 2017, known as the SCAN-AED project. The researchers analyzed the association between prenatal use of antiseizure medications and birth weight, defining low birth weight as less than 5.5 pounds and small for gestational age as being in the lowest 10th percentile for sex, country, and gestational weight at birth.
The antiseizure medications and adjusted odds ratios for risk of low birth rate were:
- Carbamazepine, 1.44 (95% confidence interval [CI], 1.21-1.71).
- Oxcarbazepine, 1.32 (95% CI, 1.03-1.69).
- Topiramate, 1.60 (95% CI, 1.15-2.24).
- Pregabalin, 1.23 (95% CI, 1.02-1.48).
- Clobazam, 4.36 (95% CI, 1.66-11.45).
The odds ratios for being born small for gestational age were:
- Carbamazepine, 1.25 (95% CI, 1.11-1.41).
- Oxcarbazepine, 1.48 (95% CI, 1.27-1.73).
- Topiramate, 1.52 (95% CI, 1.20-1.91).
“Prenatal exposure to carbamazepine, oxcarbazepine, and topiramate were associated with all estimates of adverse birth weight outcomes, thus confirming results from preclinical studies in animals and previous smaller studies in humans,” Dr. Christensen said.
He noted a lack of evidence for newer medications because their use was relatively low over the 20 years of the study. “However, for drugs like lamotrigine where we have a high number of exposed children, the finding of no association with low birth weight is reassuring, indicating the drug is safe,” Dr. Christensen said.
Use with caution
This study adds supportive evidence for expanding the list of antiseizure medications associated with small for gestational age infants, Elizabeth Gerard, MD, director of the Women with Epilepsy Program and associate professor of neurology at Northwestern University in Chicago, said in an interview.
“Previous clinical trials demonstrated that topiramate and zonisamide as well as phenobarbital were associated with small for gestational age,” she said. “This study added to the list carbamazepine and oxcarbazepine. Previously it wasn’t clear from clinical data but there were some hints that carbamazepine and oxcarbazepine might be associated with small for gestational age, but this is the first study to present robust data that carbamazepine and oxcarbazepine are associated with small for gestational age infants as well.”
She noted that these drugs can be used cautiously in women of child-bearing age and pregnant women. “I think these lines of evidence suggest that women with epilepsy should be more carefully monitored, at least with these high-quality, standard-of-care drugs, for fetal growth monitoring and perhaps most of them, especially those on at-risk drugs, should have detailed growth gradings,” Dr. Gerard said. Pregnant women on these antiseizure medications should have ultrasound beginning at 24 weeks gestation to monitor fetal growth, she said.
The NordForsk Nordic Program and Health and Welfare and the Independent Research Fund Denmark provided funding for the study. Dr. Christensen disclosed financial relationships with Union Chimique Belge Nordic and Eisai. Dr. Gerard disclosed relationships with Xenon Pharmaceuticals and Eisai.
NASHVILLE, TENN. – A study of more than 4 million births over 20 years in five Scandinavian countries has reported that three antiseizure medications should be used with caution in women of child-bearing age because they were associated with low birth weights.
In results presented at the annual meeting of the American Epilepsy Society, Jakob Christensen, MD, DSc, PhD, a professor at Aarhus University Hospital in Denmark, said that the study found that
“Because we have this large data set we were able to confirm the suspicion that’s been raised in the past that these drugs may be associated with low birth weight,” Dr. Christensen said in an interview.
The study analyzed records from population-based registers of 4.5 million births in Denmark, Finland, Iceland, Norway, and Sweden between 1996 and 2017, known as the SCAN-AED project. The researchers analyzed the association between prenatal use of antiseizure medications and birth weight, defining low birth weight as less than 5.5 pounds and small for gestational age as being in the lowest 10th percentile for sex, country, and gestational weight at birth.
The antiseizure medications and adjusted odds ratios for risk of low birth rate were:
- Carbamazepine, 1.44 (95% confidence interval [CI], 1.21-1.71).
- Oxcarbazepine, 1.32 (95% CI, 1.03-1.69).
- Topiramate, 1.60 (95% CI, 1.15-2.24).
- Pregabalin, 1.23 (95% CI, 1.02-1.48).
- Clobazam, 4.36 (95% CI, 1.66-11.45).
The odds ratios for being born small for gestational age were:
- Carbamazepine, 1.25 (95% CI, 1.11-1.41).
- Oxcarbazepine, 1.48 (95% CI, 1.27-1.73).
- Topiramate, 1.52 (95% CI, 1.20-1.91).
“Prenatal exposure to carbamazepine, oxcarbazepine, and topiramate were associated with all estimates of adverse birth weight outcomes, thus confirming results from preclinical studies in animals and previous smaller studies in humans,” Dr. Christensen said.
He noted a lack of evidence for newer medications because their use was relatively low over the 20 years of the study. “However, for drugs like lamotrigine where we have a high number of exposed children, the finding of no association with low birth weight is reassuring, indicating the drug is safe,” Dr. Christensen said.
Use with caution
This study adds supportive evidence for expanding the list of antiseizure medications associated with small for gestational age infants, Elizabeth Gerard, MD, director of the Women with Epilepsy Program and associate professor of neurology at Northwestern University in Chicago, said in an interview.
“Previous clinical trials demonstrated that topiramate and zonisamide as well as phenobarbital were associated with small for gestational age,” she said. “This study added to the list carbamazepine and oxcarbazepine. Previously it wasn’t clear from clinical data but there were some hints that carbamazepine and oxcarbazepine might be associated with small for gestational age, but this is the first study to present robust data that carbamazepine and oxcarbazepine are associated with small for gestational age infants as well.”
She noted that these drugs can be used cautiously in women of child-bearing age and pregnant women. “I think these lines of evidence suggest that women with epilepsy should be more carefully monitored, at least with these high-quality, standard-of-care drugs, for fetal growth monitoring and perhaps most of them, especially those on at-risk drugs, should have detailed growth gradings,” Dr. Gerard said. Pregnant women on these antiseizure medications should have ultrasound beginning at 24 weeks gestation to monitor fetal growth, she said.
The NordForsk Nordic Program and Health and Welfare and the Independent Research Fund Denmark provided funding for the study. Dr. Christensen disclosed financial relationships with Union Chimique Belge Nordic and Eisai. Dr. Gerard disclosed relationships with Xenon Pharmaceuticals and Eisai.
NASHVILLE, TENN. – A study of more than 4 million births over 20 years in five Scandinavian countries has reported that three antiseizure medications should be used with caution in women of child-bearing age because they were associated with low birth weights.
In results presented at the annual meeting of the American Epilepsy Society, Jakob Christensen, MD, DSc, PhD, a professor at Aarhus University Hospital in Denmark, said that the study found that
“Because we have this large data set we were able to confirm the suspicion that’s been raised in the past that these drugs may be associated with low birth weight,” Dr. Christensen said in an interview.
The study analyzed records from population-based registers of 4.5 million births in Denmark, Finland, Iceland, Norway, and Sweden between 1996 and 2017, known as the SCAN-AED project. The researchers analyzed the association between prenatal use of antiseizure medications and birth weight, defining low birth weight as less than 5.5 pounds and small for gestational age as being in the lowest 10th percentile for sex, country, and gestational weight at birth.
The antiseizure medications and adjusted odds ratios for risk of low birth rate were:
- Carbamazepine, 1.44 (95% confidence interval [CI], 1.21-1.71).
- Oxcarbazepine, 1.32 (95% CI, 1.03-1.69).
- Topiramate, 1.60 (95% CI, 1.15-2.24).
- Pregabalin, 1.23 (95% CI, 1.02-1.48).
- Clobazam, 4.36 (95% CI, 1.66-11.45).
The odds ratios for being born small for gestational age were:
- Carbamazepine, 1.25 (95% CI, 1.11-1.41).
- Oxcarbazepine, 1.48 (95% CI, 1.27-1.73).
- Topiramate, 1.52 (95% CI, 1.20-1.91).
“Prenatal exposure to carbamazepine, oxcarbazepine, and topiramate were associated with all estimates of adverse birth weight outcomes, thus confirming results from preclinical studies in animals and previous smaller studies in humans,” Dr. Christensen said.
He noted a lack of evidence for newer medications because their use was relatively low over the 20 years of the study. “However, for drugs like lamotrigine where we have a high number of exposed children, the finding of no association with low birth weight is reassuring, indicating the drug is safe,” Dr. Christensen said.
Use with caution
This study adds supportive evidence for expanding the list of antiseizure medications associated with small for gestational age infants, Elizabeth Gerard, MD, director of the Women with Epilepsy Program and associate professor of neurology at Northwestern University in Chicago, said in an interview.
“Previous clinical trials demonstrated that topiramate and zonisamide as well as phenobarbital were associated with small for gestational age,” she said. “This study added to the list carbamazepine and oxcarbazepine. Previously it wasn’t clear from clinical data but there were some hints that carbamazepine and oxcarbazepine might be associated with small for gestational age, but this is the first study to present robust data that carbamazepine and oxcarbazepine are associated with small for gestational age infants as well.”
She noted that these drugs can be used cautiously in women of child-bearing age and pregnant women. “I think these lines of evidence suggest that women with epilepsy should be more carefully monitored, at least with these high-quality, standard-of-care drugs, for fetal growth monitoring and perhaps most of them, especially those on at-risk drugs, should have detailed growth gradings,” Dr. Gerard said. Pregnant women on these antiseizure medications should have ultrasound beginning at 24 weeks gestation to monitor fetal growth, she said.
The NordForsk Nordic Program and Health and Welfare and the Independent Research Fund Denmark provided funding for the study. Dr. Christensen disclosed financial relationships with Union Chimique Belge Nordic and Eisai. Dr. Gerard disclosed relationships with Xenon Pharmaceuticals and Eisai.
AT AES 2022
‘Exciting’ responsiveness to talquetamab for r/r MM
“Talquetamab is a novel agent directed against a new antigen target in myeloma,” explained lead investigator Ajai Chari, MD, of Icahn School of Medicine at Mount Sinai in New York.
The product has demonstrated “a response rate of 73% to 74% with both weekly and every 2-week schedules in a heavily treated patient population. Even in those patients with prior T-cell redirection, we see a 63% response rate,” said Dr. Chari, who reported the data at the American Society of Hematology annual meeting.
It is encouraging to see high response rates among patients with disease that is refractory to multiple prior lines of therapy, and the results suggest that talquetamab may buy time for patients with few other options, said Stephanie Lee, MD, MPH, of the Fred Hutchinson Cancer Center in Seattle, and a former ASH president.
“It looks like there are responses even in people who are heavily pretreated and have had other agents similar to it, “ she said. “We hear about ‘penta-refractory’ [disease] and everything, and I think we’re going to start hearing about ‘octo-refractory’ and ‘deci-refractory,’ and those things. It’s really exciting [to see these responses],” she said. Dr. Lee moderated a media briefing prior to Dr. Chari’s discussion of the data.
Talquetamab is a bispecific antibody directed against a novel target with the prosaic name of “G protein–coupled receptor, family C, group 5, member D” or simply GPRC5D. The antigen is a so-called “orphan” receptor with an unidentified ligand. The receptor is expressed in malignant plasma cells, making it a particularly attractive target for the treatment of patients with multiple myeloma.
“It’s important to pick the right target for these [patients] and GPRC5D is a good candidate for that because it’s highly expressed on myeloma cells but spares normal tissues, in particular the hematopoietic stem cells for the precursors to their blood,” Dr. Chari said.
The new drug could be available next year. The manufacturer, Janssen, announced last week that it had submitted an approval application with the Food and Drug Administration for talquetamab use in the treatment of patients with relapsed or refractory multiple myeloma.
The clinical results so far “indicate the potential of this treatment for heavily pretreated patients who have exhausted currently approved therapies,” Dr. Chari said in a statement.
MonumenTAL study
Dr. Chari and colleagues recently reported results of the phase 1 MonumenTAL-1 study in The New England Journal of Medicine. At ASH 2022, they reported phase 2 results from the same study, including some patients carried over from phase 1, but also a subgroup of patients with prior exposure to either chimeric antigen receptor T-cell (CAR-T) therapy or other bispecific T cell-engaging antibodies.
In the phase 1 dose-escalation study published in NEJM, the response rates among patients who had a median of six prior lines of therapy ranged from 64% to 70%. The drug was delivered in this phase at a variety of dose levels and schedules, and both intravenously and subcutaneously.
Three cohorts, two doses
The phase 2 study enrolled patients who had a minimum of three prior lines of therapy including a proteasome inhibitor, immunomodulating agent, and anti-CD38 antibody, and who had good-to-fair performance status.
There were three cohorts. The first cohort comprised 21 patients from phase 1 and 122 from phase 2 who received talquetamab at a dose of 0.4 mg/kg subcutaneously once weekly. These patients were allowed to have received prior therapy with an antibody-drug conjugate (ADC) targeted against B-cell maturation antigen (BCMA), but could not have received a prior T-cell redirection therapy.
The second cohort comprised 36 patients in phase 1 and 109 in phase 2 who were treated with 0.8 mg/kg subcutaneously every 2 weeks. These patients had the same prior therapy allowances and restrictions as the first cohort.
The third cohort comprised 17 patients in phase 1 plus 34 patients in phase 2 who had received either CAR T-cell receptor therapy or a different bispecific T-cell engager. These patients received either 0.4 mg/kg weekly subcutaneous talquetamab, or 0.8 mg/kg every 2 weeks.
Phase 2 results
The overall response rate (ORR) among patients treated at 0.4 mg/kg weekly was 74.1%, including 23.8% stringent complete responses (sCR), 9.8% complete response (CR), 25.9% very good partial responses (VGPR) and 14.7% partial responses (PR).
The ORR among patients treated at the 0.8 mg/kg every 2 week dose was 73.1%, consisting of 20% sCR, 12.4% CR, 24,8% VGPR, and 15.9% PR.
The response rates were consistent across subgroups, including baseline International Staging System (ISS) stage III disease, baseline cytogenetic risk, number of prior therapies, degree of refractoriness to prior therapy, and prior exposure to the anti-BCMA antibody belantamab (except patients with baseline plasmacytomas).
The median durations of responses were 9.3 months and 13 months in the 0.4 and 0.8 mg/kg doses, respectively. The median duration of response was not reached among patients who had achieved a CR or better in either dosing group.
Safety profile
Most adverse events of grade 3 or 4 were cytopenias, including anemia, neutropenia, lymphopenia, and thrombocytopenia. These adverse events were generally limited to the first three cycles, and less than a third of all cytopenias were grade 3 or greater.
Infection occurred in 57.3% of patients treated at 0.4 mg/kg weekly and 50.3% treated at 0.8 mg/kg every 2 weeks. Of these infections, 16.8% and 11.7%, respectively, were grade 3 or 4.
Opportunistic infections were seen in 3.5% of patients treated at 0.4 mg/kg and 2.8% of those treated at 0.8 mg/kg.
Taste alterations (dysgeusia) occurred in nearly half of patients in each dosing group. Dysgeusia was managed with supportive care, including hydration, and in some cases with dose reductions.
Immune effector cell-associated neurotoxicity syndrome (ICANS) was observed in 10% to 11% of patients, but most of these events were grade 1 or 2.
What’s next?
At the briefing, this news organization asked Dr. Chari whether, given the evident efficacy and relative safety of this agent, it could be moved up higher in the therapeutic lines and combined with other agents such as proteasome inhibitors (bortezomib et al.), immunomodulators (lenalidomide and others) and CD38-directed antibodies (daratumumab, etc.)
Dr. Chari replied that several studies combining talquetamab with agents in all of these classes and with other bispecific T-cell engagers are currently underway.
Dr. Chari disclosed consulting fees from Janssen, which supported the study. Dr. Lee has received clinical trial research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, and Takeda and has served as a consultant to Incyte.
A version of this article first appeared on Medscape.com.
“Talquetamab is a novel agent directed against a new antigen target in myeloma,” explained lead investigator Ajai Chari, MD, of Icahn School of Medicine at Mount Sinai in New York.
The product has demonstrated “a response rate of 73% to 74% with both weekly and every 2-week schedules in a heavily treated patient population. Even in those patients with prior T-cell redirection, we see a 63% response rate,” said Dr. Chari, who reported the data at the American Society of Hematology annual meeting.
It is encouraging to see high response rates among patients with disease that is refractory to multiple prior lines of therapy, and the results suggest that talquetamab may buy time for patients with few other options, said Stephanie Lee, MD, MPH, of the Fred Hutchinson Cancer Center in Seattle, and a former ASH president.
“It looks like there are responses even in people who are heavily pretreated and have had other agents similar to it, “ she said. “We hear about ‘penta-refractory’ [disease] and everything, and I think we’re going to start hearing about ‘octo-refractory’ and ‘deci-refractory,’ and those things. It’s really exciting [to see these responses],” she said. Dr. Lee moderated a media briefing prior to Dr. Chari’s discussion of the data.
Talquetamab is a bispecific antibody directed against a novel target with the prosaic name of “G protein–coupled receptor, family C, group 5, member D” or simply GPRC5D. The antigen is a so-called “orphan” receptor with an unidentified ligand. The receptor is expressed in malignant plasma cells, making it a particularly attractive target for the treatment of patients with multiple myeloma.
“It’s important to pick the right target for these [patients] and GPRC5D is a good candidate for that because it’s highly expressed on myeloma cells but spares normal tissues, in particular the hematopoietic stem cells for the precursors to their blood,” Dr. Chari said.
The new drug could be available next year. The manufacturer, Janssen, announced last week that it had submitted an approval application with the Food and Drug Administration for talquetamab use in the treatment of patients with relapsed or refractory multiple myeloma.
The clinical results so far “indicate the potential of this treatment for heavily pretreated patients who have exhausted currently approved therapies,” Dr. Chari said in a statement.
MonumenTAL study
Dr. Chari and colleagues recently reported results of the phase 1 MonumenTAL-1 study in The New England Journal of Medicine. At ASH 2022, they reported phase 2 results from the same study, including some patients carried over from phase 1, but also a subgroup of patients with prior exposure to either chimeric antigen receptor T-cell (CAR-T) therapy or other bispecific T cell-engaging antibodies.
In the phase 1 dose-escalation study published in NEJM, the response rates among patients who had a median of six prior lines of therapy ranged from 64% to 70%. The drug was delivered in this phase at a variety of dose levels and schedules, and both intravenously and subcutaneously.
Three cohorts, two doses
The phase 2 study enrolled patients who had a minimum of three prior lines of therapy including a proteasome inhibitor, immunomodulating agent, and anti-CD38 antibody, and who had good-to-fair performance status.
There were three cohorts. The first cohort comprised 21 patients from phase 1 and 122 from phase 2 who received talquetamab at a dose of 0.4 mg/kg subcutaneously once weekly. These patients were allowed to have received prior therapy with an antibody-drug conjugate (ADC) targeted against B-cell maturation antigen (BCMA), but could not have received a prior T-cell redirection therapy.
The second cohort comprised 36 patients in phase 1 and 109 in phase 2 who were treated with 0.8 mg/kg subcutaneously every 2 weeks. These patients had the same prior therapy allowances and restrictions as the first cohort.
The third cohort comprised 17 patients in phase 1 plus 34 patients in phase 2 who had received either CAR T-cell receptor therapy or a different bispecific T-cell engager. These patients received either 0.4 mg/kg weekly subcutaneous talquetamab, or 0.8 mg/kg every 2 weeks.
Phase 2 results
The overall response rate (ORR) among patients treated at 0.4 mg/kg weekly was 74.1%, including 23.8% stringent complete responses (sCR), 9.8% complete response (CR), 25.9% very good partial responses (VGPR) and 14.7% partial responses (PR).
The ORR among patients treated at the 0.8 mg/kg every 2 week dose was 73.1%, consisting of 20% sCR, 12.4% CR, 24,8% VGPR, and 15.9% PR.
The response rates were consistent across subgroups, including baseline International Staging System (ISS) stage III disease, baseline cytogenetic risk, number of prior therapies, degree of refractoriness to prior therapy, and prior exposure to the anti-BCMA antibody belantamab (except patients with baseline plasmacytomas).
The median durations of responses were 9.3 months and 13 months in the 0.4 and 0.8 mg/kg doses, respectively. The median duration of response was not reached among patients who had achieved a CR or better in either dosing group.
Safety profile
Most adverse events of grade 3 or 4 were cytopenias, including anemia, neutropenia, lymphopenia, and thrombocytopenia. These adverse events were generally limited to the first three cycles, and less than a third of all cytopenias were grade 3 or greater.
Infection occurred in 57.3% of patients treated at 0.4 mg/kg weekly and 50.3% treated at 0.8 mg/kg every 2 weeks. Of these infections, 16.8% and 11.7%, respectively, were grade 3 or 4.
Opportunistic infections were seen in 3.5% of patients treated at 0.4 mg/kg and 2.8% of those treated at 0.8 mg/kg.
Taste alterations (dysgeusia) occurred in nearly half of patients in each dosing group. Dysgeusia was managed with supportive care, including hydration, and in some cases with dose reductions.
Immune effector cell-associated neurotoxicity syndrome (ICANS) was observed in 10% to 11% of patients, but most of these events were grade 1 or 2.
What’s next?
At the briefing, this news organization asked Dr. Chari whether, given the evident efficacy and relative safety of this agent, it could be moved up higher in the therapeutic lines and combined with other agents such as proteasome inhibitors (bortezomib et al.), immunomodulators (lenalidomide and others) and CD38-directed antibodies (daratumumab, etc.)
Dr. Chari replied that several studies combining talquetamab with agents in all of these classes and with other bispecific T-cell engagers are currently underway.
Dr. Chari disclosed consulting fees from Janssen, which supported the study. Dr. Lee has received clinical trial research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, and Takeda and has served as a consultant to Incyte.
A version of this article first appeared on Medscape.com.
“Talquetamab is a novel agent directed against a new antigen target in myeloma,” explained lead investigator Ajai Chari, MD, of Icahn School of Medicine at Mount Sinai in New York.
The product has demonstrated “a response rate of 73% to 74% with both weekly and every 2-week schedules in a heavily treated patient population. Even in those patients with prior T-cell redirection, we see a 63% response rate,” said Dr. Chari, who reported the data at the American Society of Hematology annual meeting.
It is encouraging to see high response rates among patients with disease that is refractory to multiple prior lines of therapy, and the results suggest that talquetamab may buy time for patients with few other options, said Stephanie Lee, MD, MPH, of the Fred Hutchinson Cancer Center in Seattle, and a former ASH president.
“It looks like there are responses even in people who are heavily pretreated and have had other agents similar to it, “ she said. “We hear about ‘penta-refractory’ [disease] and everything, and I think we’re going to start hearing about ‘octo-refractory’ and ‘deci-refractory,’ and those things. It’s really exciting [to see these responses],” she said. Dr. Lee moderated a media briefing prior to Dr. Chari’s discussion of the data.
Talquetamab is a bispecific antibody directed against a novel target with the prosaic name of “G protein–coupled receptor, family C, group 5, member D” or simply GPRC5D. The antigen is a so-called “orphan” receptor with an unidentified ligand. The receptor is expressed in malignant plasma cells, making it a particularly attractive target for the treatment of patients with multiple myeloma.
“It’s important to pick the right target for these [patients] and GPRC5D is a good candidate for that because it’s highly expressed on myeloma cells but spares normal tissues, in particular the hematopoietic stem cells for the precursors to their blood,” Dr. Chari said.
The new drug could be available next year. The manufacturer, Janssen, announced last week that it had submitted an approval application with the Food and Drug Administration for talquetamab use in the treatment of patients with relapsed or refractory multiple myeloma.
The clinical results so far “indicate the potential of this treatment for heavily pretreated patients who have exhausted currently approved therapies,” Dr. Chari said in a statement.
MonumenTAL study
Dr. Chari and colleagues recently reported results of the phase 1 MonumenTAL-1 study in The New England Journal of Medicine. At ASH 2022, they reported phase 2 results from the same study, including some patients carried over from phase 1, but also a subgroup of patients with prior exposure to either chimeric antigen receptor T-cell (CAR-T) therapy or other bispecific T cell-engaging antibodies.
In the phase 1 dose-escalation study published in NEJM, the response rates among patients who had a median of six prior lines of therapy ranged from 64% to 70%. The drug was delivered in this phase at a variety of dose levels and schedules, and both intravenously and subcutaneously.
Three cohorts, two doses
The phase 2 study enrolled patients who had a minimum of three prior lines of therapy including a proteasome inhibitor, immunomodulating agent, and anti-CD38 antibody, and who had good-to-fair performance status.
There were three cohorts. The first cohort comprised 21 patients from phase 1 and 122 from phase 2 who received talquetamab at a dose of 0.4 mg/kg subcutaneously once weekly. These patients were allowed to have received prior therapy with an antibody-drug conjugate (ADC) targeted against B-cell maturation antigen (BCMA), but could not have received a prior T-cell redirection therapy.
The second cohort comprised 36 patients in phase 1 and 109 in phase 2 who were treated with 0.8 mg/kg subcutaneously every 2 weeks. These patients had the same prior therapy allowances and restrictions as the first cohort.
The third cohort comprised 17 patients in phase 1 plus 34 patients in phase 2 who had received either CAR T-cell receptor therapy or a different bispecific T-cell engager. These patients received either 0.4 mg/kg weekly subcutaneous talquetamab, or 0.8 mg/kg every 2 weeks.
Phase 2 results
The overall response rate (ORR) among patients treated at 0.4 mg/kg weekly was 74.1%, including 23.8% stringent complete responses (sCR), 9.8% complete response (CR), 25.9% very good partial responses (VGPR) and 14.7% partial responses (PR).
The ORR among patients treated at the 0.8 mg/kg every 2 week dose was 73.1%, consisting of 20% sCR, 12.4% CR, 24,8% VGPR, and 15.9% PR.
The response rates were consistent across subgroups, including baseline International Staging System (ISS) stage III disease, baseline cytogenetic risk, number of prior therapies, degree of refractoriness to prior therapy, and prior exposure to the anti-BCMA antibody belantamab (except patients with baseline plasmacytomas).
The median durations of responses were 9.3 months and 13 months in the 0.4 and 0.8 mg/kg doses, respectively. The median duration of response was not reached among patients who had achieved a CR or better in either dosing group.
Safety profile
Most adverse events of grade 3 or 4 were cytopenias, including anemia, neutropenia, lymphopenia, and thrombocytopenia. These adverse events were generally limited to the first three cycles, and less than a third of all cytopenias were grade 3 or greater.
Infection occurred in 57.3% of patients treated at 0.4 mg/kg weekly and 50.3% treated at 0.8 mg/kg every 2 weeks. Of these infections, 16.8% and 11.7%, respectively, were grade 3 or 4.
Opportunistic infections were seen in 3.5% of patients treated at 0.4 mg/kg and 2.8% of those treated at 0.8 mg/kg.
Taste alterations (dysgeusia) occurred in nearly half of patients in each dosing group. Dysgeusia was managed with supportive care, including hydration, and in some cases with dose reductions.
Immune effector cell-associated neurotoxicity syndrome (ICANS) was observed in 10% to 11% of patients, but most of these events were grade 1 or 2.
What’s next?
At the briefing, this news organization asked Dr. Chari whether, given the evident efficacy and relative safety of this agent, it could be moved up higher in the therapeutic lines and combined with other agents such as proteasome inhibitors (bortezomib et al.), immunomodulators (lenalidomide and others) and CD38-directed antibodies (daratumumab, etc.)
Dr. Chari replied that several studies combining talquetamab with agents in all of these classes and with other bispecific T-cell engagers are currently underway.
Dr. Chari disclosed consulting fees from Janssen, which supported the study. Dr. Lee has received clinical trial research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, and Takeda and has served as a consultant to Incyte.
A version of this article first appeared on Medscape.com.
AT ASH 2022
Pricey gene therapy looks cost-effective for SCD
NEW ORLEANS –
Another gene therapy, a treatment for sickle cell anemia now in late clinical development, is expected to come on the market soon. It, too, is expected to bear an exorbitant price tag.
Such potentially curative therapies put financial pressure on publicly and privately funded health insurance.
However, investigators said that the new treatment for patients with sickle cell disease (SCD) in the United States has the potential to be cost-effective. Those who analyzed the costs used a novel method that takes historical health inequities into account.
“When faced with costs of innovative, one-time-administered therapies, budgetary constraints, as we all know too well, can and have driven therapy availability or lack thereof for patients,” said George Goshua, MD, from the Yale University, New Haven, Conn., speaking here at the annual meeting of the American Society of Hematology.
“We believe that quantitative consideration of health inequities, in addition to the important quality considerations, may be an additional helpful metric in this decision-making context,” he said.
He noted that SCD predominantly affects Black Americans, “who have historically been a very marginalized population when it comes to health care.
“Our study shows that, when we compare the costs of gene therapy and existing standard-of-care treatment for SCD using a technique that accounts for historical health disparities, gene therapy could be an equitable therapeutic strategy for all patients with SCD, whether their disease is mild, moderate, or severe,” he said.
Commenting on the study for this news organization, Bosula Oluwole, MD, from the University of Washington, Seattle, who studies sickle cell disease but was not involved in this study, said the cost-analysis approach taken by Dr. Goshua and colleagues is interesting, but she added: “I think we still have a way to go in trying to fully understand the issue.
“When you look over time at the cost for a patient to get gene therapy vs. the standard of care, it might actually be beneficial to have the gene therapy,” Dr. Oluwole said.
She noted, however, that some patients start gene therapy for SCD at older ages and that it’s important to analyze whether the treatment can still be cost-effective or the best therapeutic option for such patients.
Adding a D to CEA
Dr. Goshua and colleagues at Yale University and the Harvard T.H. Chan School of Public Health in Boston conducted what they believe is the first study in hematology to use distributional cost-effectiveness analysis (DCEA), developed at the University of York, England.
A University of York website explains that DCEA “is a general umbrella term for economic evaluation studies that provide information about equity in the distribution of costs and effects as well as efficiency in terms of aggregate costs and effects. DCEA can provide distributional breakdowns of who gains most and who bears the largest burdens (opportunity costs) by equity-relevant social variables (e.g., socioeconomic status, ethnicity, location) and disease categories (e.g., severity of illness, rarity, disability).”
The technique can also employ equity weight to evaluate trade-offs between equity and efficiency, the website says.
As Dr. Goshua put it, equity weighting is “a way of quantifying how much we prioritize health care equity.”
QALYs considered
Dr. Goshua and colleagues included equity weight in an analysis of 10 years of data on annual health care costs for patients with SCD who were covered by private insurance and were treated with medications (for example, hydroxyurea), antibiotics, blood transfusion, and hematopoietic stem cell transplants. Sex and the frequency of hospitalizations for acute pain crises were factors in the Markov model they created.
The model assumes that a single course of gene therapy for SCD would cost $2.1 million. The estimate was based on the cost of U.S. Food and Drug Administration–approved gene therapies, and it was assumed that the therapy would result in permanent disease remission for all patients.
In addition, the model assumed that all eligible patients in the United States with SCD who are aged 12 years and older would be offered the gene therapy.
In their base-case analysis, gene therapy starting at age 12 would yield 25.5 discounted lifetime quality-adjusted life-years (QALYs) at a cost of $2.4 million, compared with 16.0 discounted lifetime QALYs at a cost of $1.1 million for standard care.
Under traditional cost-effectiveness calculations, the upper limit of the incremental cost-effectiveness ratio (ICER) is estimated to be $100,000 per QALY. Under this scenario, the ICER of gene therapy for SCD at $144,000 per QALY would be considered by health economists or insurers to be too steep a price to pay.
However, applying equity weighting to the formula would bring the price of gene therapy into the $1.4 million to $3 million range.
Dr. Goshua acknowledged that the study is limited by the assumption that gene therapy would be a one-time cost and that patients would not need to undergo repeat therapy or treatment for relapses.
Stephanie Lee, MD, MPH, from the Fred Hutchinson Cancer Center in Seattle, and a former ASH president, who moderated a briefing the day before Dr. Goshua presented his data, recommended that he and his colleagues use their technique to explore other health inequities, such as in the care of patients with multiple myeloma.
“There’s some evidence that Black patients are not using even the agents we have as [are] some of the other groups, so there may be some distributional inequities there as well,” she said.
The study was funded by ASH and the Yale School of Medicine. Dr. Goshua, Dr. Oluwole, and Dr. Lee have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
Another gene therapy, a treatment for sickle cell anemia now in late clinical development, is expected to come on the market soon. It, too, is expected to bear an exorbitant price tag.
Such potentially curative therapies put financial pressure on publicly and privately funded health insurance.
However, investigators said that the new treatment for patients with sickle cell disease (SCD) in the United States has the potential to be cost-effective. Those who analyzed the costs used a novel method that takes historical health inequities into account.
“When faced with costs of innovative, one-time-administered therapies, budgetary constraints, as we all know too well, can and have driven therapy availability or lack thereof for patients,” said George Goshua, MD, from the Yale University, New Haven, Conn., speaking here at the annual meeting of the American Society of Hematology.
“We believe that quantitative consideration of health inequities, in addition to the important quality considerations, may be an additional helpful metric in this decision-making context,” he said.
He noted that SCD predominantly affects Black Americans, “who have historically been a very marginalized population when it comes to health care.
“Our study shows that, when we compare the costs of gene therapy and existing standard-of-care treatment for SCD using a technique that accounts for historical health disparities, gene therapy could be an equitable therapeutic strategy for all patients with SCD, whether their disease is mild, moderate, or severe,” he said.
Commenting on the study for this news organization, Bosula Oluwole, MD, from the University of Washington, Seattle, who studies sickle cell disease but was not involved in this study, said the cost-analysis approach taken by Dr. Goshua and colleagues is interesting, but she added: “I think we still have a way to go in trying to fully understand the issue.
“When you look over time at the cost for a patient to get gene therapy vs. the standard of care, it might actually be beneficial to have the gene therapy,” Dr. Oluwole said.
She noted, however, that some patients start gene therapy for SCD at older ages and that it’s important to analyze whether the treatment can still be cost-effective or the best therapeutic option for such patients.
Adding a D to CEA
Dr. Goshua and colleagues at Yale University and the Harvard T.H. Chan School of Public Health in Boston conducted what they believe is the first study in hematology to use distributional cost-effectiveness analysis (DCEA), developed at the University of York, England.
A University of York website explains that DCEA “is a general umbrella term for economic evaluation studies that provide information about equity in the distribution of costs and effects as well as efficiency in terms of aggregate costs and effects. DCEA can provide distributional breakdowns of who gains most and who bears the largest burdens (opportunity costs) by equity-relevant social variables (e.g., socioeconomic status, ethnicity, location) and disease categories (e.g., severity of illness, rarity, disability).”
The technique can also employ equity weight to evaluate trade-offs between equity and efficiency, the website says.
As Dr. Goshua put it, equity weighting is “a way of quantifying how much we prioritize health care equity.”
QALYs considered
Dr. Goshua and colleagues included equity weight in an analysis of 10 years of data on annual health care costs for patients with SCD who were covered by private insurance and were treated with medications (for example, hydroxyurea), antibiotics, blood transfusion, and hematopoietic stem cell transplants. Sex and the frequency of hospitalizations for acute pain crises were factors in the Markov model they created.
The model assumes that a single course of gene therapy for SCD would cost $2.1 million. The estimate was based on the cost of U.S. Food and Drug Administration–approved gene therapies, and it was assumed that the therapy would result in permanent disease remission for all patients.
In addition, the model assumed that all eligible patients in the United States with SCD who are aged 12 years and older would be offered the gene therapy.
In their base-case analysis, gene therapy starting at age 12 would yield 25.5 discounted lifetime quality-adjusted life-years (QALYs) at a cost of $2.4 million, compared with 16.0 discounted lifetime QALYs at a cost of $1.1 million for standard care.
Under traditional cost-effectiveness calculations, the upper limit of the incremental cost-effectiveness ratio (ICER) is estimated to be $100,000 per QALY. Under this scenario, the ICER of gene therapy for SCD at $144,000 per QALY would be considered by health economists or insurers to be too steep a price to pay.
However, applying equity weighting to the formula would bring the price of gene therapy into the $1.4 million to $3 million range.
Dr. Goshua acknowledged that the study is limited by the assumption that gene therapy would be a one-time cost and that patients would not need to undergo repeat therapy or treatment for relapses.
Stephanie Lee, MD, MPH, from the Fred Hutchinson Cancer Center in Seattle, and a former ASH president, who moderated a briefing the day before Dr. Goshua presented his data, recommended that he and his colleagues use their technique to explore other health inequities, such as in the care of patients with multiple myeloma.
“There’s some evidence that Black patients are not using even the agents we have as [are] some of the other groups, so there may be some distributional inequities there as well,” she said.
The study was funded by ASH and the Yale School of Medicine. Dr. Goshua, Dr. Oluwole, and Dr. Lee have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
Another gene therapy, a treatment for sickle cell anemia now in late clinical development, is expected to come on the market soon. It, too, is expected to bear an exorbitant price tag.
Such potentially curative therapies put financial pressure on publicly and privately funded health insurance.
However, investigators said that the new treatment for patients with sickle cell disease (SCD) in the United States has the potential to be cost-effective. Those who analyzed the costs used a novel method that takes historical health inequities into account.
“When faced with costs of innovative, one-time-administered therapies, budgetary constraints, as we all know too well, can and have driven therapy availability or lack thereof for patients,” said George Goshua, MD, from the Yale University, New Haven, Conn., speaking here at the annual meeting of the American Society of Hematology.
“We believe that quantitative consideration of health inequities, in addition to the important quality considerations, may be an additional helpful metric in this decision-making context,” he said.
He noted that SCD predominantly affects Black Americans, “who have historically been a very marginalized population when it comes to health care.
“Our study shows that, when we compare the costs of gene therapy and existing standard-of-care treatment for SCD using a technique that accounts for historical health disparities, gene therapy could be an equitable therapeutic strategy for all patients with SCD, whether their disease is mild, moderate, or severe,” he said.
Commenting on the study for this news organization, Bosula Oluwole, MD, from the University of Washington, Seattle, who studies sickle cell disease but was not involved in this study, said the cost-analysis approach taken by Dr. Goshua and colleagues is interesting, but she added: “I think we still have a way to go in trying to fully understand the issue.
“When you look over time at the cost for a patient to get gene therapy vs. the standard of care, it might actually be beneficial to have the gene therapy,” Dr. Oluwole said.
She noted, however, that some patients start gene therapy for SCD at older ages and that it’s important to analyze whether the treatment can still be cost-effective or the best therapeutic option for such patients.
Adding a D to CEA
Dr. Goshua and colleagues at Yale University and the Harvard T.H. Chan School of Public Health in Boston conducted what they believe is the first study in hematology to use distributional cost-effectiveness analysis (DCEA), developed at the University of York, England.
A University of York website explains that DCEA “is a general umbrella term for economic evaluation studies that provide information about equity in the distribution of costs and effects as well as efficiency in terms of aggregate costs and effects. DCEA can provide distributional breakdowns of who gains most and who bears the largest burdens (opportunity costs) by equity-relevant social variables (e.g., socioeconomic status, ethnicity, location) and disease categories (e.g., severity of illness, rarity, disability).”
The technique can also employ equity weight to evaluate trade-offs between equity and efficiency, the website says.
As Dr. Goshua put it, equity weighting is “a way of quantifying how much we prioritize health care equity.”
QALYs considered
Dr. Goshua and colleagues included equity weight in an analysis of 10 years of data on annual health care costs for patients with SCD who were covered by private insurance and were treated with medications (for example, hydroxyurea), antibiotics, blood transfusion, and hematopoietic stem cell transplants. Sex and the frequency of hospitalizations for acute pain crises were factors in the Markov model they created.
The model assumes that a single course of gene therapy for SCD would cost $2.1 million. The estimate was based on the cost of U.S. Food and Drug Administration–approved gene therapies, and it was assumed that the therapy would result in permanent disease remission for all patients.
In addition, the model assumed that all eligible patients in the United States with SCD who are aged 12 years and older would be offered the gene therapy.
In their base-case analysis, gene therapy starting at age 12 would yield 25.5 discounted lifetime quality-adjusted life-years (QALYs) at a cost of $2.4 million, compared with 16.0 discounted lifetime QALYs at a cost of $1.1 million for standard care.
Under traditional cost-effectiveness calculations, the upper limit of the incremental cost-effectiveness ratio (ICER) is estimated to be $100,000 per QALY. Under this scenario, the ICER of gene therapy for SCD at $144,000 per QALY would be considered by health economists or insurers to be too steep a price to pay.
However, applying equity weighting to the formula would bring the price of gene therapy into the $1.4 million to $3 million range.
Dr. Goshua acknowledged that the study is limited by the assumption that gene therapy would be a one-time cost and that patients would not need to undergo repeat therapy or treatment for relapses.
Stephanie Lee, MD, MPH, from the Fred Hutchinson Cancer Center in Seattle, and a former ASH president, who moderated a briefing the day before Dr. Goshua presented his data, recommended that he and his colleagues use their technique to explore other health inequities, such as in the care of patients with multiple myeloma.
“There’s some evidence that Black patients are not using even the agents we have as [are] some of the other groups, so there may be some distributional inequities there as well,” she said.
The study was funded by ASH and the Yale School of Medicine. Dr. Goshua, Dr. Oluwole, and Dr. Lee have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASH 2022
‘Striking’ rate of mental health comorbidities in epilepsy
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AES 2022
Significant racial disparities persist in status epilepticus
NASHVILLE, Tenn. – Investigators found that among Black patients with status epilepticus, the hospitalization rate was twice that of their White counterparts. Other findings reveal age and income disparities.
“The results suggest that racial minorities, those with a lower income, and the elderly are an appropriate target to improve health outcomes and reduce health inequality,” said Gabriela Tantillo Sepúlveda, MD, assistant professor of neurology, Baylor College of Medicine, Houston.
The findings were presented at the annual meeting of the American Epilepsy Society.
An examination of outcomes
Status epilepticus is associated with high rates of morbidity and mortality. Disparities in epilepsy care have previously been described, but little attention has been paid to the contribution of disparities to status epilepticus care and associated outcomes.
Researchers used 2010-2019 data from the Nationwide Inpatient Sample, a database covering a cross-section of hospitalizations in 48 states and the District of Columbia. From relevant diagnostic codes, they calculated status epilepticus prevalence as the rate per 10,000 hospitalizations and stratified this by demographics.
Over the study period, investigators identified 486,861 status epilepticus hospitalizations, most (71.3%) at urban teaching hospitals.
Status epilepticus prevalence was highest for non-Hispanic Black patients, at 27.3, followed by non-Hispanic others, at 16.1, Hispanic patients, at 15.8, and non-Hispanic-White patients, at 13.7 (P < .01).
The finding that Black patients had double the rate as White patients was “definitely surprising,” said Dr. Tantillo Sepúlveda.
Research over the past 20 years revealed similar disparities related to status epilepticus, “so it’s upsetting that these disparities have persisted. Unfortunately, we still have a lot of work to do to reduce health inequalities,” she said.
The investigators found that the prevalence of status epilepticus was higher in the lowest-income quartile, compared with the highest (18.7 vs. 14; P < .01).
Need for physician advocacy
Unlike previous studies, this research assessed various interventions in different age groups and showed that the likelihood of intubation, tracheostomy, gastrostomy, and in-hospital mortality increased with age.
For example, compared with the reference group (patients aged 18-39 years), the odds of intubation were 1.22 (95% confidence interval, 1.16-1.27) for those aged 40-59 years and 1.48 (95% CI, 1.42-1.54) for those aged 60-79. Those aged 80 and older were most likely to be intubated, at an odds ratio of 1.5 (95% CI, 1.43-1.58).
Elderly patients were most likely to undergo tracheostomy (OR, 2.0; 95% CI, 1.75-2.27), gastrostomy (OR, 3.37; 95% CI, 2.97-3.83), and to experience in-hospital mortality (OR, 6.51; 95% CI, 5.95-7.13), compared with the youngest patients.
These intervention rates also varied by racial/ethnic groups. Minority populations, particularly Black people, had higher odds of tracheostomy and gastrostomy, compared with non-Hispanic White persons.
The odds of undergoing electroencephalography monitoring progressively rose as income level increased (OR, 1.47; 95% CI, 1.34-1.62) for the highest income quartile versus the lowest quartile. The odds of undergoing EEG monitoring were also higher at urban teaching hospitals than at rural hospitals.
Tackling these disparities in this patient population include increasing resources, personnel, and health education aimed at minorities, low-income patients, and the elderly, said Dr. Tantillo Sepúlveda. She added that more research is needed “to determine the most effective ways of accomplishing this goal.”
The medical community can help reduce disparities, said Dr. Tantillo Sepúlveda, by working to improve health literacy, to reduce stigma associated with seizures, and to increase awareness of seizure risk factors.
They can also work to expand access to outpatient neurology clinics, epilepsy monitoring units, and epilepsy surgery. “Ethnic and racial minorities are less likely to receive epilepsy surgery for temporal lobe epilepsy, which has been shown to improve quality of life and reduce seizure burden,” Dr. Tantillo Sepúlveda noted.
Across-the-board problem
Commenting on the research, Daniel Lowenstein, MD, professor of neurology, University of California, San Francisco, said the findings aren’t at all surprising. “It’s yet another piece of evidence on what has now become a rather voluminous literature that documents the very significant disparities that exist in our health care system,” said Dr. Lowenstein. “There’s just a huge literature on ‘name your disease and you’ll see the disparities.’ ”
Disparities exist, for example, in diagnosing breast cancer and prostate cancer, in the treatment of stroke and in related outcomes, and there is a well-documented “big disparity” in the approach to pain control among patients presenting at the emergency department, said Dr. Lowenstein.
However, he doesn’t know how disparities in epilepsy and specifically in status epilepticus, compared with disparities regarding other diseases and disorders. He noted that in the case of epilepsy, the situation is likely exacerbated by the stigma associated with that disease.
Dr. Lowenstein agreed that clinicians should play a role in reversing disparities. “We as physicians have a responsibility to be a voice for change in our health care system.”
The study was supported by the Center of Excellence for health equity, training, and research at the Baylor College of Medicine. Dr. Tantillo Sepúlveda and Dr. Lowenstein report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, Tenn. – Investigators found that among Black patients with status epilepticus, the hospitalization rate was twice that of their White counterparts. Other findings reveal age and income disparities.
“The results suggest that racial minorities, those with a lower income, and the elderly are an appropriate target to improve health outcomes and reduce health inequality,” said Gabriela Tantillo Sepúlveda, MD, assistant professor of neurology, Baylor College of Medicine, Houston.
The findings were presented at the annual meeting of the American Epilepsy Society.
An examination of outcomes
Status epilepticus is associated with high rates of morbidity and mortality. Disparities in epilepsy care have previously been described, but little attention has been paid to the contribution of disparities to status epilepticus care and associated outcomes.
Researchers used 2010-2019 data from the Nationwide Inpatient Sample, a database covering a cross-section of hospitalizations in 48 states and the District of Columbia. From relevant diagnostic codes, they calculated status epilepticus prevalence as the rate per 10,000 hospitalizations and stratified this by demographics.
Over the study period, investigators identified 486,861 status epilepticus hospitalizations, most (71.3%) at urban teaching hospitals.
Status epilepticus prevalence was highest for non-Hispanic Black patients, at 27.3, followed by non-Hispanic others, at 16.1, Hispanic patients, at 15.8, and non-Hispanic-White patients, at 13.7 (P < .01).
The finding that Black patients had double the rate as White patients was “definitely surprising,” said Dr. Tantillo Sepúlveda.
Research over the past 20 years revealed similar disparities related to status epilepticus, “so it’s upsetting that these disparities have persisted. Unfortunately, we still have a lot of work to do to reduce health inequalities,” she said.
The investigators found that the prevalence of status epilepticus was higher in the lowest-income quartile, compared with the highest (18.7 vs. 14; P < .01).
Need for physician advocacy
Unlike previous studies, this research assessed various interventions in different age groups and showed that the likelihood of intubation, tracheostomy, gastrostomy, and in-hospital mortality increased with age.
For example, compared with the reference group (patients aged 18-39 years), the odds of intubation were 1.22 (95% confidence interval, 1.16-1.27) for those aged 40-59 years and 1.48 (95% CI, 1.42-1.54) for those aged 60-79. Those aged 80 and older were most likely to be intubated, at an odds ratio of 1.5 (95% CI, 1.43-1.58).
Elderly patients were most likely to undergo tracheostomy (OR, 2.0; 95% CI, 1.75-2.27), gastrostomy (OR, 3.37; 95% CI, 2.97-3.83), and to experience in-hospital mortality (OR, 6.51; 95% CI, 5.95-7.13), compared with the youngest patients.
These intervention rates also varied by racial/ethnic groups. Minority populations, particularly Black people, had higher odds of tracheostomy and gastrostomy, compared with non-Hispanic White persons.
The odds of undergoing electroencephalography monitoring progressively rose as income level increased (OR, 1.47; 95% CI, 1.34-1.62) for the highest income quartile versus the lowest quartile. The odds of undergoing EEG monitoring were also higher at urban teaching hospitals than at rural hospitals.
Tackling these disparities in this patient population include increasing resources, personnel, and health education aimed at minorities, low-income patients, and the elderly, said Dr. Tantillo Sepúlveda. She added that more research is needed “to determine the most effective ways of accomplishing this goal.”
The medical community can help reduce disparities, said Dr. Tantillo Sepúlveda, by working to improve health literacy, to reduce stigma associated with seizures, and to increase awareness of seizure risk factors.
They can also work to expand access to outpatient neurology clinics, epilepsy monitoring units, and epilepsy surgery. “Ethnic and racial minorities are less likely to receive epilepsy surgery for temporal lobe epilepsy, which has been shown to improve quality of life and reduce seizure burden,” Dr. Tantillo Sepúlveda noted.
Across-the-board problem
Commenting on the research, Daniel Lowenstein, MD, professor of neurology, University of California, San Francisco, said the findings aren’t at all surprising. “It’s yet another piece of evidence on what has now become a rather voluminous literature that documents the very significant disparities that exist in our health care system,” said Dr. Lowenstein. “There’s just a huge literature on ‘name your disease and you’ll see the disparities.’ ”
Disparities exist, for example, in diagnosing breast cancer and prostate cancer, in the treatment of stroke and in related outcomes, and there is a well-documented “big disparity” in the approach to pain control among patients presenting at the emergency department, said Dr. Lowenstein.
However, he doesn’t know how disparities in epilepsy and specifically in status epilepticus, compared with disparities regarding other diseases and disorders. He noted that in the case of epilepsy, the situation is likely exacerbated by the stigma associated with that disease.
Dr. Lowenstein agreed that clinicians should play a role in reversing disparities. “We as physicians have a responsibility to be a voice for change in our health care system.”
The study was supported by the Center of Excellence for health equity, training, and research at the Baylor College of Medicine. Dr. Tantillo Sepúlveda and Dr. Lowenstein report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, Tenn. – Investigators found that among Black patients with status epilepticus, the hospitalization rate was twice that of their White counterparts. Other findings reveal age and income disparities.
“The results suggest that racial minorities, those with a lower income, and the elderly are an appropriate target to improve health outcomes and reduce health inequality,” said Gabriela Tantillo Sepúlveda, MD, assistant professor of neurology, Baylor College of Medicine, Houston.
The findings were presented at the annual meeting of the American Epilepsy Society.
An examination of outcomes
Status epilepticus is associated with high rates of morbidity and mortality. Disparities in epilepsy care have previously been described, but little attention has been paid to the contribution of disparities to status epilepticus care and associated outcomes.
Researchers used 2010-2019 data from the Nationwide Inpatient Sample, a database covering a cross-section of hospitalizations in 48 states and the District of Columbia. From relevant diagnostic codes, they calculated status epilepticus prevalence as the rate per 10,000 hospitalizations and stratified this by demographics.
Over the study period, investigators identified 486,861 status epilepticus hospitalizations, most (71.3%) at urban teaching hospitals.
Status epilepticus prevalence was highest for non-Hispanic Black patients, at 27.3, followed by non-Hispanic others, at 16.1, Hispanic patients, at 15.8, and non-Hispanic-White patients, at 13.7 (P < .01).
The finding that Black patients had double the rate as White patients was “definitely surprising,” said Dr. Tantillo Sepúlveda.
Research over the past 20 years revealed similar disparities related to status epilepticus, “so it’s upsetting that these disparities have persisted. Unfortunately, we still have a lot of work to do to reduce health inequalities,” she said.
The investigators found that the prevalence of status epilepticus was higher in the lowest-income quartile, compared with the highest (18.7 vs. 14; P < .01).
Need for physician advocacy
Unlike previous studies, this research assessed various interventions in different age groups and showed that the likelihood of intubation, tracheostomy, gastrostomy, and in-hospital mortality increased with age.
For example, compared with the reference group (patients aged 18-39 years), the odds of intubation were 1.22 (95% confidence interval, 1.16-1.27) for those aged 40-59 years and 1.48 (95% CI, 1.42-1.54) for those aged 60-79. Those aged 80 and older were most likely to be intubated, at an odds ratio of 1.5 (95% CI, 1.43-1.58).
Elderly patients were most likely to undergo tracheostomy (OR, 2.0; 95% CI, 1.75-2.27), gastrostomy (OR, 3.37; 95% CI, 2.97-3.83), and to experience in-hospital mortality (OR, 6.51; 95% CI, 5.95-7.13), compared with the youngest patients.
These intervention rates also varied by racial/ethnic groups. Minority populations, particularly Black people, had higher odds of tracheostomy and gastrostomy, compared with non-Hispanic White persons.
The odds of undergoing electroencephalography monitoring progressively rose as income level increased (OR, 1.47; 95% CI, 1.34-1.62) for the highest income quartile versus the lowest quartile. The odds of undergoing EEG monitoring were also higher at urban teaching hospitals than at rural hospitals.
Tackling these disparities in this patient population include increasing resources, personnel, and health education aimed at minorities, low-income patients, and the elderly, said Dr. Tantillo Sepúlveda. She added that more research is needed “to determine the most effective ways of accomplishing this goal.”
The medical community can help reduce disparities, said Dr. Tantillo Sepúlveda, by working to improve health literacy, to reduce stigma associated with seizures, and to increase awareness of seizure risk factors.
They can also work to expand access to outpatient neurology clinics, epilepsy monitoring units, and epilepsy surgery. “Ethnic and racial minorities are less likely to receive epilepsy surgery for temporal lobe epilepsy, which has been shown to improve quality of life and reduce seizure burden,” Dr. Tantillo Sepúlveda noted.
Across-the-board problem
Commenting on the research, Daniel Lowenstein, MD, professor of neurology, University of California, San Francisco, said the findings aren’t at all surprising. “It’s yet another piece of evidence on what has now become a rather voluminous literature that documents the very significant disparities that exist in our health care system,” said Dr. Lowenstein. “There’s just a huge literature on ‘name your disease and you’ll see the disparities.’ ”
Disparities exist, for example, in diagnosing breast cancer and prostate cancer, in the treatment of stroke and in related outcomes, and there is a well-documented “big disparity” in the approach to pain control among patients presenting at the emergency department, said Dr. Lowenstein.
However, he doesn’t know how disparities in epilepsy and specifically in status epilepticus, compared with disparities regarding other diseases and disorders. He noted that in the case of epilepsy, the situation is likely exacerbated by the stigma associated with that disease.
Dr. Lowenstein agreed that clinicians should play a role in reversing disparities. “We as physicians have a responsibility to be a voice for change in our health care system.”
The study was supported by the Center of Excellence for health equity, training, and research at the Baylor College of Medicine. Dr. Tantillo Sepúlveda and Dr. Lowenstein report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AES 2022
MCL: Ibrutinib could become the ‘new standard’
First-line patients fared well on ibrutinib, a Bruton’s tyrosine kinase inhibitor, according to the findings.
“Based on the results so far ... at least for the majority of patients, ibrutinib early will become the new standard,” said study lead author Martin Dreyling, MD, associate professor of medicine at Ludwig Maximilian University (LMU) Munich. Dr. Dreyling spoke in a news briefing and at a separate presentation at the annual meeting of the American Society of Hematology. “It might well be that specific subsets of patients may benefit from autologous transplant.”
MCL is a rare form of non-Hodgkin’s lymphoma that strikes cells in the mantle zone of lymph nodes. It is usually diagnosed in older men and often presents at an advanced stage. Multiple available treatments include rituximab/bendamustine, CAR-T cell therapy, stem cell transplants, and Bruton’s tyrosine kinase inhibitors. Ibrutinib is approved by the Food and Drug Administration only for refractory/relapsed cases, however.
Dr. Dreyling was a pioneer in confirming benefit from stem-cell transplants for MCL. “However,” he said, “no one likes autologous transplant because it also has side effects.”
For the new open-label study, Dr. Dreyling and colleagues in the European MCL Network in 2016 began recruiting patients with newly diagnosed, advanced stage II-IV MCL. The patients were younger than 65.
The subjects were randomly assigned to three trial arms: Standard treatment (high-dose cytarabine followed by autologous stem cell transplant and rituximab maintenance, n = 288), the standard treatment plus ibrutinib (n = 292), and ibrutinib without stem cell transplant (n = 290). The median age was 57, and 76% of patients were male.
The primary endpoint was failure-free survival at 31 months. Standard therapy was not superior to the ibrutinib without transplant group (72% vs. 86%, respectively, P = .9979). However, standard therapy with ibrutinib was superior to the standard therapy group (88% vs. 72%, respectively, P = .0008). The researchers haven’t finished their analysis of standard therapy with ibrutinib vs. ibrutinib without transplant.
Subjects in the standard therapy plus ibrutinib arm had more grade 3-5 adverse events than did the standard therapy and ibrutinib without transplant groups: Neutropenia, 44%, 17%, and 23%, respectively; leukopenia, 4%, 2%, and 2%; febrile neutropenia, 6%, 3%, and 3%; infections and infestations, 25%, 13%, and 19%; and cardiac disorders, 3%, 1%, 4%. P values were not provided.
In an interview, Ohio State University hematologist Narendranath Epperla, MD, MS, who was not involved in the study, said that this research reflects efforts to understand how novel agents such as ibrutinib and cellular therapies fit into MCL treatment. “We are trying to incorporate them in the frontline setting with either chemo backbone or with other targeted agents to improve outcomes and minimize toxicity. We are also trying to understand in whom auto-HCT can be precluded.”
The results of the new study appear promising, Dr. Epperla said, but he questioned the primary endpoint (failure-free survival instead of progress-free survival) and the short duration of the trial.
“I would like to see how the patients with high-risk features such as TP53 mutation, complex cytogenetics, and blastoid/pleomorphic variants did on the three arms,” Dr. Epperla said. “And I would like to see longer follow-up data before adapting this – [addition] of ibrutinib to the chemotherapy backbone without auto-HCT – into clinical practice.”
What’s next? Dr. Dreyling said that upcoming data will provide further insight into ibrutinib vs. stem-cell transplantation. And “within the next half year or so,” he said, “there will be a next generation of studies challenging chemotherapy overall in mantle cell lymphoma and substituting targeted treatment, hopefully achieving much better tolerability.”
Funding information was not provided. Dr. Dreyling disclosed ties with Lilly/Loxo, AstraZeneca, Novartis, Amgen, Roche, Janssen, Gilead/Kite, BMS/Celgene, Bayer, Abbvie, and Beigene. The other study authors reported various disclosures. Dr. Epperla disclosed a relationship with Pharmacyclics.
First-line patients fared well on ibrutinib, a Bruton’s tyrosine kinase inhibitor, according to the findings.
“Based on the results so far ... at least for the majority of patients, ibrutinib early will become the new standard,” said study lead author Martin Dreyling, MD, associate professor of medicine at Ludwig Maximilian University (LMU) Munich. Dr. Dreyling spoke in a news briefing and at a separate presentation at the annual meeting of the American Society of Hematology. “It might well be that specific subsets of patients may benefit from autologous transplant.”
MCL is a rare form of non-Hodgkin’s lymphoma that strikes cells in the mantle zone of lymph nodes. It is usually diagnosed in older men and often presents at an advanced stage. Multiple available treatments include rituximab/bendamustine, CAR-T cell therapy, stem cell transplants, and Bruton’s tyrosine kinase inhibitors. Ibrutinib is approved by the Food and Drug Administration only for refractory/relapsed cases, however.
Dr. Dreyling was a pioneer in confirming benefit from stem-cell transplants for MCL. “However,” he said, “no one likes autologous transplant because it also has side effects.”
For the new open-label study, Dr. Dreyling and colleagues in the European MCL Network in 2016 began recruiting patients with newly diagnosed, advanced stage II-IV MCL. The patients were younger than 65.
The subjects were randomly assigned to three trial arms: Standard treatment (high-dose cytarabine followed by autologous stem cell transplant and rituximab maintenance, n = 288), the standard treatment plus ibrutinib (n = 292), and ibrutinib without stem cell transplant (n = 290). The median age was 57, and 76% of patients were male.
The primary endpoint was failure-free survival at 31 months. Standard therapy was not superior to the ibrutinib without transplant group (72% vs. 86%, respectively, P = .9979). However, standard therapy with ibrutinib was superior to the standard therapy group (88% vs. 72%, respectively, P = .0008). The researchers haven’t finished their analysis of standard therapy with ibrutinib vs. ibrutinib without transplant.
Subjects in the standard therapy plus ibrutinib arm had more grade 3-5 adverse events than did the standard therapy and ibrutinib without transplant groups: Neutropenia, 44%, 17%, and 23%, respectively; leukopenia, 4%, 2%, and 2%; febrile neutropenia, 6%, 3%, and 3%; infections and infestations, 25%, 13%, and 19%; and cardiac disorders, 3%, 1%, 4%. P values were not provided.
In an interview, Ohio State University hematologist Narendranath Epperla, MD, MS, who was not involved in the study, said that this research reflects efforts to understand how novel agents such as ibrutinib and cellular therapies fit into MCL treatment. “We are trying to incorporate them in the frontline setting with either chemo backbone or with other targeted agents to improve outcomes and minimize toxicity. We are also trying to understand in whom auto-HCT can be precluded.”
The results of the new study appear promising, Dr. Epperla said, but he questioned the primary endpoint (failure-free survival instead of progress-free survival) and the short duration of the trial.
“I would like to see how the patients with high-risk features such as TP53 mutation, complex cytogenetics, and blastoid/pleomorphic variants did on the three arms,” Dr. Epperla said. “And I would like to see longer follow-up data before adapting this – [addition] of ibrutinib to the chemotherapy backbone without auto-HCT – into clinical practice.”
What’s next? Dr. Dreyling said that upcoming data will provide further insight into ibrutinib vs. stem-cell transplantation. And “within the next half year or so,” he said, “there will be a next generation of studies challenging chemotherapy overall in mantle cell lymphoma and substituting targeted treatment, hopefully achieving much better tolerability.”
Funding information was not provided. Dr. Dreyling disclosed ties with Lilly/Loxo, AstraZeneca, Novartis, Amgen, Roche, Janssen, Gilead/Kite, BMS/Celgene, Bayer, Abbvie, and Beigene. The other study authors reported various disclosures. Dr. Epperla disclosed a relationship with Pharmacyclics.
First-line patients fared well on ibrutinib, a Bruton’s tyrosine kinase inhibitor, according to the findings.
“Based on the results so far ... at least for the majority of patients, ibrutinib early will become the new standard,” said study lead author Martin Dreyling, MD, associate professor of medicine at Ludwig Maximilian University (LMU) Munich. Dr. Dreyling spoke in a news briefing and at a separate presentation at the annual meeting of the American Society of Hematology. “It might well be that specific subsets of patients may benefit from autologous transplant.”
MCL is a rare form of non-Hodgkin’s lymphoma that strikes cells in the mantle zone of lymph nodes. It is usually diagnosed in older men and often presents at an advanced stage. Multiple available treatments include rituximab/bendamustine, CAR-T cell therapy, stem cell transplants, and Bruton’s tyrosine kinase inhibitors. Ibrutinib is approved by the Food and Drug Administration only for refractory/relapsed cases, however.
Dr. Dreyling was a pioneer in confirming benefit from stem-cell transplants for MCL. “However,” he said, “no one likes autologous transplant because it also has side effects.”
For the new open-label study, Dr. Dreyling and colleagues in the European MCL Network in 2016 began recruiting patients with newly diagnosed, advanced stage II-IV MCL. The patients were younger than 65.
The subjects were randomly assigned to three trial arms: Standard treatment (high-dose cytarabine followed by autologous stem cell transplant and rituximab maintenance, n = 288), the standard treatment plus ibrutinib (n = 292), and ibrutinib without stem cell transplant (n = 290). The median age was 57, and 76% of patients were male.
The primary endpoint was failure-free survival at 31 months. Standard therapy was not superior to the ibrutinib without transplant group (72% vs. 86%, respectively, P = .9979). However, standard therapy with ibrutinib was superior to the standard therapy group (88% vs. 72%, respectively, P = .0008). The researchers haven’t finished their analysis of standard therapy with ibrutinib vs. ibrutinib without transplant.
Subjects in the standard therapy plus ibrutinib arm had more grade 3-5 adverse events than did the standard therapy and ibrutinib without transplant groups: Neutropenia, 44%, 17%, and 23%, respectively; leukopenia, 4%, 2%, and 2%; febrile neutropenia, 6%, 3%, and 3%; infections and infestations, 25%, 13%, and 19%; and cardiac disorders, 3%, 1%, 4%. P values were not provided.
In an interview, Ohio State University hematologist Narendranath Epperla, MD, MS, who was not involved in the study, said that this research reflects efforts to understand how novel agents such as ibrutinib and cellular therapies fit into MCL treatment. “We are trying to incorporate them in the frontline setting with either chemo backbone or with other targeted agents to improve outcomes and minimize toxicity. We are also trying to understand in whom auto-HCT can be precluded.”
The results of the new study appear promising, Dr. Epperla said, but he questioned the primary endpoint (failure-free survival instead of progress-free survival) and the short duration of the trial.
“I would like to see how the patients with high-risk features such as TP53 mutation, complex cytogenetics, and blastoid/pleomorphic variants did on the three arms,” Dr. Epperla said. “And I would like to see longer follow-up data before adapting this – [addition] of ibrutinib to the chemotherapy backbone without auto-HCT – into clinical practice.”
What’s next? Dr. Dreyling said that upcoming data will provide further insight into ibrutinib vs. stem-cell transplantation. And “within the next half year or so,” he said, “there will be a next generation of studies challenging chemotherapy overall in mantle cell lymphoma and substituting targeted treatment, hopefully achieving much better tolerability.”
Funding information was not provided. Dr. Dreyling disclosed ties with Lilly/Loxo, AstraZeneca, Novartis, Amgen, Roche, Janssen, Gilead/Kite, BMS/Celgene, Bayer, Abbvie, and Beigene. The other study authors reported various disclosures. Dr. Epperla disclosed a relationship with Pharmacyclics.
AT ASH 2022





