User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
FFR not better, just different from IVUS for revascularizing intermediate stenoses
In a head-to-head comparison of fractional flow reserve (FFR) and intravenous ultrasound (IVUS) for guiding revascularization during percutaneous intervention (PCI), outcomes were noninferior at 2 years, but the approaches appear to have different strengths, according to results of the FLAVOUR trial.
For the primary composite outcome of death from any cause, myocardial infarction, or revascularization at 24 months, the approaches performed comparatively, but there were substantial differences in the number of revascularization procedures performed, reported Bon-Kwon Koo, MD, at the annual scientific sessions of the American College of Cardiology.
At 24 months, 8.1% of the FFR group and 8.5% of the IVUS group had a primary event. The 0.4% difference was not significantly different and fulfilled the definition of noninferiority (P = .015). When the components of the primary endpoint were compared along with rates of stroke, the rates were also similar and not significantly different.
However, the proportion of patients who received a stent (44.4% vs. 65.3%), the total number of stents per patient (0.6 vs. 0.9), and the total stent length per patient (16.5 vs. 25.2) were significantly lower (all P < .001) in the FFR group.
FLAVOUR (Fractional Flow Reserve And IVUS for Clinical Outcomes in Patients With Intermediate Stenosis) confirmed the investigators’ hypothesis that an FFR-guided strategy for intermediate coronary stenosis is noninferior to IVUS for outcomes. In addition, patient-reported angina outcomes on the Seattle Angina Questionnaire were nearly identical across domains, including angina frequency, physical limitations, and treatment satisfaction.
FFR vs. IVUS differences revealed
However, the more important value of this study might its role in showing how the two approaches differ in ways unrelated to the primary outcome, according to Dr. Koo, chair of cardiology at Seoul (South Korea) National University Hospital, as well as several experts that commented on the results.
Most notably, the fact that FFR-guided PCI provides similar outcomes at 2 years even though it was associated with a substantially reduced rate of revascularizations is telling about its role relative to IVUS.
“These data confirm how a lot of us are already approaching this,” said an ACC-invited expert, Frederick G. Welt, MD, director of the cardiac catheterization at the University of Utah, Salt Lake City. “FFR should be used to decide who should get an intervention, and IVUS should be use to optimize the intervention.”
Dr. Koo explained that FFR is an invasive tool that provides a physiological assessment of the degree to which a stenosis is causing ischemia. IVUS is a tool that permits visualization and measurement of plaque severity and characteristics to better optimize PCI. They can both help guide PCI, but they are not necessarily competing strategies. Often, the information they provide is complementary.
In this multicenter trial conducted at 18 centers in Korea and China, 1,682 candidates with de novo stenoses of intermediate severity, defined as 40%-70%, were randomized to FFR- or IVUS-guided PCI. At 24 months, outcomes could be assessed in 832 of the FFR patients and 836 of the IVUS patients, which represented more than 99% of both groups.
In the study, the indications for stent placement were predefined for the FFR-guided and IVUS-guided approaches. The criteria to define optimal outcomes post PCI were also predefined. For FFR, this included a postprocedure value of at least 0.88. For IVUS, the definition of optimal outcome included a plaque burden of 55% or less at the stent edge and a minimal stent area of at least 5.5 mm2.
The primary outcome for those with optimal versus suboptimal FFR-guided PCI were similar at all time points. For those with an optimal post-PCI result, the event rate was only slightly higher for those with an optimal relative to a suboptimal result (12.3% vs. 11.8%).
Suboptimal IVUS differs from suboptimal FFR
In contrast, the event rates over the course of follow-up were consistently higher among those with a suboptimal relative to an optimal IVUS-guided PCI. At the end of 2 years, the numerically greater rate of events among those with a suboptimal IVUS-guided PCI was not significant (9.8% vs. 8.5%; P = .212), but the gap was larger than that seen with FFR-guided PCI.
FFR-guided and IVUS-guided PCI performed similarly for the primary outcome across numerous stratifications. These included age older or younger than 65 years, male or female sex, presence or absence of multivessel disease, and presence of diabetes. They were also similar for those with acute coronary syndrome (ACS) as an indication for PCI, which accounted for about 30% of patients, relative to those without ACS.
“I would say that at least some interventionalists in the U.S. would be uncomfortable using FFR in ACS patients,” said Dr. Welt, pointing out a potential difference between how these tools are used to guide PCI. Still, because “there are not a lot of data to compare these technologies,” he expressed appreciation for a study looking at these tools side-by-side.
A similar point was made by Ajay Kirtane, MD, director of Cardiac Catheterization Laboratories at New York–Presbyterian/Columbia University Irving Medical Center. With the slightly lower rates of primary events in those treated optimally according to IVUS relative to those treated optimally by FFR (8.5% vs. 12.3%), he suggested IVUS appears better for evaluating the physiology of the stenosis.
Dr. Kirtane pointed out that two-thirds of the lesions were left behind in those guided by FFR versus only about half of the lesions when PCI was guided by IVUS, yet outcomes were similar. He indicated that the data support current practice in which FFR is most commonly used to select PCI patients with intermediate disease for stent placement.
Dr. Koo has financial relationships with Abbott, Boston Scientific, and Philips Volcano. Dr. Welt has financial relationships with Medtronic and Xenter. Dr. Kirtane has financial relationships with Abbott, Amgen, Boston Scientific, Chiesi, Cardiovascular Systems Incorporate, Medtronic, Philips/Spectranetics, Recor Medical, and Regeneron. The study received a research grant from Boston Scientific.
In a head-to-head comparison of fractional flow reserve (FFR) and intravenous ultrasound (IVUS) for guiding revascularization during percutaneous intervention (PCI), outcomes were noninferior at 2 years, but the approaches appear to have different strengths, according to results of the FLAVOUR trial.
For the primary composite outcome of death from any cause, myocardial infarction, or revascularization at 24 months, the approaches performed comparatively, but there were substantial differences in the number of revascularization procedures performed, reported Bon-Kwon Koo, MD, at the annual scientific sessions of the American College of Cardiology.
At 24 months, 8.1% of the FFR group and 8.5% of the IVUS group had a primary event. The 0.4% difference was not significantly different and fulfilled the definition of noninferiority (P = .015). When the components of the primary endpoint were compared along with rates of stroke, the rates were also similar and not significantly different.
However, the proportion of patients who received a stent (44.4% vs. 65.3%), the total number of stents per patient (0.6 vs. 0.9), and the total stent length per patient (16.5 vs. 25.2) were significantly lower (all P < .001) in the FFR group.
FLAVOUR (Fractional Flow Reserve And IVUS for Clinical Outcomes in Patients With Intermediate Stenosis) confirmed the investigators’ hypothesis that an FFR-guided strategy for intermediate coronary stenosis is noninferior to IVUS for outcomes. In addition, patient-reported angina outcomes on the Seattle Angina Questionnaire were nearly identical across domains, including angina frequency, physical limitations, and treatment satisfaction.
FFR vs. IVUS differences revealed
However, the more important value of this study might its role in showing how the two approaches differ in ways unrelated to the primary outcome, according to Dr. Koo, chair of cardiology at Seoul (South Korea) National University Hospital, as well as several experts that commented on the results.
Most notably, the fact that FFR-guided PCI provides similar outcomes at 2 years even though it was associated with a substantially reduced rate of revascularizations is telling about its role relative to IVUS.
“These data confirm how a lot of us are already approaching this,” said an ACC-invited expert, Frederick G. Welt, MD, director of the cardiac catheterization at the University of Utah, Salt Lake City. “FFR should be used to decide who should get an intervention, and IVUS should be use to optimize the intervention.”
Dr. Koo explained that FFR is an invasive tool that provides a physiological assessment of the degree to which a stenosis is causing ischemia. IVUS is a tool that permits visualization and measurement of plaque severity and characteristics to better optimize PCI. They can both help guide PCI, but they are not necessarily competing strategies. Often, the information they provide is complementary.
In this multicenter trial conducted at 18 centers in Korea and China, 1,682 candidates with de novo stenoses of intermediate severity, defined as 40%-70%, were randomized to FFR- or IVUS-guided PCI. At 24 months, outcomes could be assessed in 832 of the FFR patients and 836 of the IVUS patients, which represented more than 99% of both groups.
In the study, the indications for stent placement were predefined for the FFR-guided and IVUS-guided approaches. The criteria to define optimal outcomes post PCI were also predefined. For FFR, this included a postprocedure value of at least 0.88. For IVUS, the definition of optimal outcome included a plaque burden of 55% or less at the stent edge and a minimal stent area of at least 5.5 mm2.
The primary outcome for those with optimal versus suboptimal FFR-guided PCI were similar at all time points. For those with an optimal post-PCI result, the event rate was only slightly higher for those with an optimal relative to a suboptimal result (12.3% vs. 11.8%).
Suboptimal IVUS differs from suboptimal FFR
In contrast, the event rates over the course of follow-up were consistently higher among those with a suboptimal relative to an optimal IVUS-guided PCI. At the end of 2 years, the numerically greater rate of events among those with a suboptimal IVUS-guided PCI was not significant (9.8% vs. 8.5%; P = .212), but the gap was larger than that seen with FFR-guided PCI.
FFR-guided and IVUS-guided PCI performed similarly for the primary outcome across numerous stratifications. These included age older or younger than 65 years, male or female sex, presence or absence of multivessel disease, and presence of diabetes. They were also similar for those with acute coronary syndrome (ACS) as an indication for PCI, which accounted for about 30% of patients, relative to those without ACS.
“I would say that at least some interventionalists in the U.S. would be uncomfortable using FFR in ACS patients,” said Dr. Welt, pointing out a potential difference between how these tools are used to guide PCI. Still, because “there are not a lot of data to compare these technologies,” he expressed appreciation for a study looking at these tools side-by-side.
A similar point was made by Ajay Kirtane, MD, director of Cardiac Catheterization Laboratories at New York–Presbyterian/Columbia University Irving Medical Center. With the slightly lower rates of primary events in those treated optimally according to IVUS relative to those treated optimally by FFR (8.5% vs. 12.3%), he suggested IVUS appears better for evaluating the physiology of the stenosis.
Dr. Kirtane pointed out that two-thirds of the lesions were left behind in those guided by FFR versus only about half of the lesions when PCI was guided by IVUS, yet outcomes were similar. He indicated that the data support current practice in which FFR is most commonly used to select PCI patients with intermediate disease for stent placement.
Dr. Koo has financial relationships with Abbott, Boston Scientific, and Philips Volcano. Dr. Welt has financial relationships with Medtronic and Xenter. Dr. Kirtane has financial relationships with Abbott, Amgen, Boston Scientific, Chiesi, Cardiovascular Systems Incorporate, Medtronic, Philips/Spectranetics, Recor Medical, and Regeneron. The study received a research grant from Boston Scientific.
In a head-to-head comparison of fractional flow reserve (FFR) and intravenous ultrasound (IVUS) for guiding revascularization during percutaneous intervention (PCI), outcomes were noninferior at 2 years, but the approaches appear to have different strengths, according to results of the FLAVOUR trial.
For the primary composite outcome of death from any cause, myocardial infarction, or revascularization at 24 months, the approaches performed comparatively, but there were substantial differences in the number of revascularization procedures performed, reported Bon-Kwon Koo, MD, at the annual scientific sessions of the American College of Cardiology.
At 24 months, 8.1% of the FFR group and 8.5% of the IVUS group had a primary event. The 0.4% difference was not significantly different and fulfilled the definition of noninferiority (P = .015). When the components of the primary endpoint were compared along with rates of stroke, the rates were also similar and not significantly different.
However, the proportion of patients who received a stent (44.4% vs. 65.3%), the total number of stents per patient (0.6 vs. 0.9), and the total stent length per patient (16.5 vs. 25.2) were significantly lower (all P < .001) in the FFR group.
FLAVOUR (Fractional Flow Reserve And IVUS for Clinical Outcomes in Patients With Intermediate Stenosis) confirmed the investigators’ hypothesis that an FFR-guided strategy for intermediate coronary stenosis is noninferior to IVUS for outcomes. In addition, patient-reported angina outcomes on the Seattle Angina Questionnaire were nearly identical across domains, including angina frequency, physical limitations, and treatment satisfaction.
FFR vs. IVUS differences revealed
However, the more important value of this study might its role in showing how the two approaches differ in ways unrelated to the primary outcome, according to Dr. Koo, chair of cardiology at Seoul (South Korea) National University Hospital, as well as several experts that commented on the results.
Most notably, the fact that FFR-guided PCI provides similar outcomes at 2 years even though it was associated with a substantially reduced rate of revascularizations is telling about its role relative to IVUS.
“These data confirm how a lot of us are already approaching this,” said an ACC-invited expert, Frederick G. Welt, MD, director of the cardiac catheterization at the University of Utah, Salt Lake City. “FFR should be used to decide who should get an intervention, and IVUS should be use to optimize the intervention.”
Dr. Koo explained that FFR is an invasive tool that provides a physiological assessment of the degree to which a stenosis is causing ischemia. IVUS is a tool that permits visualization and measurement of plaque severity and characteristics to better optimize PCI. They can both help guide PCI, but they are not necessarily competing strategies. Often, the information they provide is complementary.
In this multicenter trial conducted at 18 centers in Korea and China, 1,682 candidates with de novo stenoses of intermediate severity, defined as 40%-70%, were randomized to FFR- or IVUS-guided PCI. At 24 months, outcomes could be assessed in 832 of the FFR patients and 836 of the IVUS patients, which represented more than 99% of both groups.
In the study, the indications for stent placement were predefined for the FFR-guided and IVUS-guided approaches. The criteria to define optimal outcomes post PCI were also predefined. For FFR, this included a postprocedure value of at least 0.88. For IVUS, the definition of optimal outcome included a plaque burden of 55% or less at the stent edge and a minimal stent area of at least 5.5 mm2.
The primary outcome for those with optimal versus suboptimal FFR-guided PCI were similar at all time points. For those with an optimal post-PCI result, the event rate was only slightly higher for those with an optimal relative to a suboptimal result (12.3% vs. 11.8%).
Suboptimal IVUS differs from suboptimal FFR
In contrast, the event rates over the course of follow-up were consistently higher among those with a suboptimal relative to an optimal IVUS-guided PCI. At the end of 2 years, the numerically greater rate of events among those with a suboptimal IVUS-guided PCI was not significant (9.8% vs. 8.5%; P = .212), but the gap was larger than that seen with FFR-guided PCI.
FFR-guided and IVUS-guided PCI performed similarly for the primary outcome across numerous stratifications. These included age older or younger than 65 years, male or female sex, presence or absence of multivessel disease, and presence of diabetes. They were also similar for those with acute coronary syndrome (ACS) as an indication for PCI, which accounted for about 30% of patients, relative to those without ACS.
“I would say that at least some interventionalists in the U.S. would be uncomfortable using FFR in ACS patients,” said Dr. Welt, pointing out a potential difference between how these tools are used to guide PCI. Still, because “there are not a lot of data to compare these technologies,” he expressed appreciation for a study looking at these tools side-by-side.
A similar point was made by Ajay Kirtane, MD, director of Cardiac Catheterization Laboratories at New York–Presbyterian/Columbia University Irving Medical Center. With the slightly lower rates of primary events in those treated optimally according to IVUS relative to those treated optimally by FFR (8.5% vs. 12.3%), he suggested IVUS appears better for evaluating the physiology of the stenosis.
Dr. Kirtane pointed out that two-thirds of the lesions were left behind in those guided by FFR versus only about half of the lesions when PCI was guided by IVUS, yet outcomes were similar. He indicated that the data support current practice in which FFR is most commonly used to select PCI patients with intermediate disease for stent placement.
Dr. Koo has financial relationships with Abbott, Boston Scientific, and Philips Volcano. Dr. Welt has financial relationships with Medtronic and Xenter. Dr. Kirtane has financial relationships with Abbott, Amgen, Boston Scientific, Chiesi, Cardiovascular Systems Incorporate, Medtronic, Philips/Spectranetics, Recor Medical, and Regeneron. The study received a research grant from Boston Scientific.
FROM ACC 2022
AHA statement addresses CVD risk in NAFLD
At least one in four adults worldwide is thought to have nonalcoholic fatty liver disease, a major risk factor for cardiovascular disease (CVD), which is the leading cause of death in NAFLD, but the condition is widely underdiagnosed, according to a new American Heart Association scientific statement on NAFLD and cardiovascular risks.
The statement, published in Arteriosclerosis, Thrombosis, and Vascular Biology, aims to increase awareness of NAFLD among cardiologists and other clinicians treating vulnerable patients. It pulls together the existing evidence for using imaging to diagnose NAFLD as well as the role of current and emerging treatments for managing the disease.
“NAFLD is common, but most patients are undiagnosed,” statement writing committee chair P. Barton Duell, MD, said in an interview. “The identification of normal liver enzyme levels does not exclude the diagnosis of NAFLD. Early diagnosis and treatment are necessary to improve the health of patients with established NAFLD, as well as preventing the development of NAFLD in patients who are at risk for the condition.”
Dr. Duell is a professor at the Knight Cardiovascular Institute and division of endocrinology, diabetes and clinical nutrition at Oregon Health & Science University, Portland.
This is the AHA’s first scientific statement on NAFLD. In 2021, the association issued a statement on obesity and CVD). Also in 2021, a multiorganization group headed by the American Gastroenterological Association published a “Call to Action” on nonalcoholic steatohepatitis (NASH) , a form of NAFLD that’s characterized by inflammation and scarring of the liver, and typically requires a liver biopsy for diagnosis.
Key take-homes
The AHA statement on NAFLD is sweeping. Among its key take-home messages:
- Calling into question the effectiveness of AST and ALT testing for diagnosing NAFLD and NASH.
- Providing context to the role of insulin resistance – either with or without diabetes – as well as obesity (particularly visceral adiposity), metabolic syndrome, and dyslipidemia in NAFLD.
- Advocating for lifestyle interventions – diet, exercise, weight loss and alcohol avoidance – as the key therapeutic intervention for NAFLD.
- Asserting that glucagonlike peptide–1 receptor agonists may modestly improve NAFLD.
The statement also tackles the differences in terminology different organizations use to describe NAFLD. “The terminology section is important to ensure everyone is using the right terminology in assessing patients, as well as choosing appropriate treatment interventions,” Dr. Duell said.
The statement also explores genetic factors that can predispose people to NAFLD, Dr. Duell pointed out, and it goes into detail about strategies for screening NAFLD and NASH. “It is not possible to diagnose NAFLD without understanding the pros and cons of various screening modalities, as well as the lack of sensitivity of some tests for detection of NAFLD We hope this information will increase success in screening for and early identification of NAFLD.”
Dr. Duell explained the rationale for issuing the statement. “Rates of NAFLD are increasing worldwide in association with rising rates of elevated body mass index and the metabolic syndrome, but the condition is commonly undiagnosed,” he said. “This allows patients to experience progression of disease, leading to hepatic and cardiovascular complications.”
Avoiding NAFLD risk factors along with early diagnosis and treatment “may have the potential to mitigate long-term complications from NAFLD,” Dr. Duell said.
“This is one of first times where we really look at cardiovascular risks associated with NAFLD and pinpoint the risk factors, the imaging tools that can be used for diagnosing fatty liver disease, and ultimately what potential treatments we can consider,” Tiffany M. Powell-Wiley, MD, MPH, author of the AHA statement on obesity and CV risk, said in an interview.
“NAFLD has not been at the forefront of cardiologists’ minds, but this statement highlights the importance of liver fat as a fat depot,” said Dr. Powell-Wiley, chief of the Social Determinants of Obesity and Cardiovascular Risk Laboratory at the National Heart, Lung, and Blood Institute in Bethesda, Md.
“It does provide greater clarity for us as cardiologists, especially when thinking about what is required for diagnosis and ultimately how this relates to cardiovascular disease for people with fatty liver disease,” she said.
Dr. Duell and Dr. Powell-Wiley have no relevant relationships to disclose.
At least one in four adults worldwide is thought to have nonalcoholic fatty liver disease, a major risk factor for cardiovascular disease (CVD), which is the leading cause of death in NAFLD, but the condition is widely underdiagnosed, according to a new American Heart Association scientific statement on NAFLD and cardiovascular risks.
The statement, published in Arteriosclerosis, Thrombosis, and Vascular Biology, aims to increase awareness of NAFLD among cardiologists and other clinicians treating vulnerable patients. It pulls together the existing evidence for using imaging to diagnose NAFLD as well as the role of current and emerging treatments for managing the disease.
“NAFLD is common, but most patients are undiagnosed,” statement writing committee chair P. Barton Duell, MD, said in an interview. “The identification of normal liver enzyme levels does not exclude the diagnosis of NAFLD. Early diagnosis and treatment are necessary to improve the health of patients with established NAFLD, as well as preventing the development of NAFLD in patients who are at risk for the condition.”
Dr. Duell is a professor at the Knight Cardiovascular Institute and division of endocrinology, diabetes and clinical nutrition at Oregon Health & Science University, Portland.
This is the AHA’s first scientific statement on NAFLD. In 2021, the association issued a statement on obesity and CVD). Also in 2021, a multiorganization group headed by the American Gastroenterological Association published a “Call to Action” on nonalcoholic steatohepatitis (NASH) , a form of NAFLD that’s characterized by inflammation and scarring of the liver, and typically requires a liver biopsy for diagnosis.
Key take-homes
The AHA statement on NAFLD is sweeping. Among its key take-home messages:
- Calling into question the effectiveness of AST and ALT testing for diagnosing NAFLD and NASH.
- Providing context to the role of insulin resistance – either with or without diabetes – as well as obesity (particularly visceral adiposity), metabolic syndrome, and dyslipidemia in NAFLD.
- Advocating for lifestyle interventions – diet, exercise, weight loss and alcohol avoidance – as the key therapeutic intervention for NAFLD.
- Asserting that glucagonlike peptide–1 receptor agonists may modestly improve NAFLD.
The statement also tackles the differences in terminology different organizations use to describe NAFLD. “The terminology section is important to ensure everyone is using the right terminology in assessing patients, as well as choosing appropriate treatment interventions,” Dr. Duell said.
The statement also explores genetic factors that can predispose people to NAFLD, Dr. Duell pointed out, and it goes into detail about strategies for screening NAFLD and NASH. “It is not possible to diagnose NAFLD without understanding the pros and cons of various screening modalities, as well as the lack of sensitivity of some tests for detection of NAFLD We hope this information will increase success in screening for and early identification of NAFLD.”
Dr. Duell explained the rationale for issuing the statement. “Rates of NAFLD are increasing worldwide in association with rising rates of elevated body mass index and the metabolic syndrome, but the condition is commonly undiagnosed,” he said. “This allows patients to experience progression of disease, leading to hepatic and cardiovascular complications.”
Avoiding NAFLD risk factors along with early diagnosis and treatment “may have the potential to mitigate long-term complications from NAFLD,” Dr. Duell said.
“This is one of first times where we really look at cardiovascular risks associated with NAFLD and pinpoint the risk factors, the imaging tools that can be used for diagnosing fatty liver disease, and ultimately what potential treatments we can consider,” Tiffany M. Powell-Wiley, MD, MPH, author of the AHA statement on obesity and CV risk, said in an interview.
“NAFLD has not been at the forefront of cardiologists’ minds, but this statement highlights the importance of liver fat as a fat depot,” said Dr. Powell-Wiley, chief of the Social Determinants of Obesity and Cardiovascular Risk Laboratory at the National Heart, Lung, and Blood Institute in Bethesda, Md.
“It does provide greater clarity for us as cardiologists, especially when thinking about what is required for diagnosis and ultimately how this relates to cardiovascular disease for people with fatty liver disease,” she said.
Dr. Duell and Dr. Powell-Wiley have no relevant relationships to disclose.
At least one in four adults worldwide is thought to have nonalcoholic fatty liver disease, a major risk factor for cardiovascular disease (CVD), which is the leading cause of death in NAFLD, but the condition is widely underdiagnosed, according to a new American Heart Association scientific statement on NAFLD and cardiovascular risks.
The statement, published in Arteriosclerosis, Thrombosis, and Vascular Biology, aims to increase awareness of NAFLD among cardiologists and other clinicians treating vulnerable patients. It pulls together the existing evidence for using imaging to diagnose NAFLD as well as the role of current and emerging treatments for managing the disease.
“NAFLD is common, but most patients are undiagnosed,” statement writing committee chair P. Barton Duell, MD, said in an interview. “The identification of normal liver enzyme levels does not exclude the diagnosis of NAFLD. Early diagnosis and treatment are necessary to improve the health of patients with established NAFLD, as well as preventing the development of NAFLD in patients who are at risk for the condition.”
Dr. Duell is a professor at the Knight Cardiovascular Institute and division of endocrinology, diabetes and clinical nutrition at Oregon Health & Science University, Portland.
This is the AHA’s first scientific statement on NAFLD. In 2021, the association issued a statement on obesity and CVD). Also in 2021, a multiorganization group headed by the American Gastroenterological Association published a “Call to Action” on nonalcoholic steatohepatitis (NASH) , a form of NAFLD that’s characterized by inflammation and scarring of the liver, and typically requires a liver biopsy for diagnosis.
Key take-homes
The AHA statement on NAFLD is sweeping. Among its key take-home messages:
- Calling into question the effectiveness of AST and ALT testing for diagnosing NAFLD and NASH.
- Providing context to the role of insulin resistance – either with or without diabetes – as well as obesity (particularly visceral adiposity), metabolic syndrome, and dyslipidemia in NAFLD.
- Advocating for lifestyle interventions – diet, exercise, weight loss and alcohol avoidance – as the key therapeutic intervention for NAFLD.
- Asserting that glucagonlike peptide–1 receptor agonists may modestly improve NAFLD.
The statement also tackles the differences in terminology different organizations use to describe NAFLD. “The terminology section is important to ensure everyone is using the right terminology in assessing patients, as well as choosing appropriate treatment interventions,” Dr. Duell said.
The statement also explores genetic factors that can predispose people to NAFLD, Dr. Duell pointed out, and it goes into detail about strategies for screening NAFLD and NASH. “It is not possible to diagnose NAFLD without understanding the pros and cons of various screening modalities, as well as the lack of sensitivity of some tests for detection of NAFLD We hope this information will increase success in screening for and early identification of NAFLD.”
Dr. Duell explained the rationale for issuing the statement. “Rates of NAFLD are increasing worldwide in association with rising rates of elevated body mass index and the metabolic syndrome, but the condition is commonly undiagnosed,” he said. “This allows patients to experience progression of disease, leading to hepatic and cardiovascular complications.”
Avoiding NAFLD risk factors along with early diagnosis and treatment “may have the potential to mitigate long-term complications from NAFLD,” Dr. Duell said.
“This is one of first times where we really look at cardiovascular risks associated with NAFLD and pinpoint the risk factors, the imaging tools that can be used for diagnosing fatty liver disease, and ultimately what potential treatments we can consider,” Tiffany M. Powell-Wiley, MD, MPH, author of the AHA statement on obesity and CV risk, said in an interview.
“NAFLD has not been at the forefront of cardiologists’ minds, but this statement highlights the importance of liver fat as a fat depot,” said Dr. Powell-Wiley, chief of the Social Determinants of Obesity and Cardiovascular Risk Laboratory at the National Heart, Lung, and Blood Institute in Bethesda, Md.
“It does provide greater clarity for us as cardiologists, especially when thinking about what is required for diagnosis and ultimately how this relates to cardiovascular disease for people with fatty liver disease,” she said.
Dr. Duell and Dr. Powell-Wiley have no relevant relationships to disclose.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Cardiac issues after COVID infection and vaccination: New data
The new information comes from the Centers for Disease Control and Prevention’s National Patient-Centered Clinical Research Network (PCORnet) and from a separate large international clinical study published online in Circulation.
CDC data
The CDC study analyzed electronic health record data from 40 U.S. health care systems from Jan. 1, 2021, to Jan. 31, 2022, on more than 15 million people aged 5 years or older.
It reports a rate of myocarditis or pericarditis after mRNA COVID-19 vaccination of 0-35.9 per 100,000 for males and 0-10.9 per 100,000 for females across different age groups and vaccine cohorts.
Rates of myocarditis or pericarditis after SARS-CoV-2 infection ranged from 12.6 to 114 per 100,000 for males and from 5.4 to 61.7 per 100,000 for females across different age groups.
Even among males aged 12-17 years, the group with the highest incidence of cardiac complications after receipt of a second mRNA COVID-19 vaccine dose, the risk was 1.8-5.6 times higher after SARS-CoV-2 infection than after vaccination, the CDC report notes.
“These findings provide important context for balancing risks and benefits of mRNA COVID-19 vaccination among eligible persons greater than or equal to 5 years,” the report states. They also “support the continued use of recommended mRNA vaccines among all eligible persons aged greater than or equal to 5 years,” it concludes.
International study
The international study focused on prevalence, clinical characteristics, and outcomes of clinically manifest acute myocarditis in patients with COVID-19 infection.
The study showed a rate of acute myocarditis of 2.4 per 1,000 patients hospitalized with COVID-19.
“A small study previously indicated acute myocarditis is a rare occurrence in people infected with COVID-19. Our analysis of international data offers better insight to the occurrence of acute myocarditis during COVID-19 hospitalization, particularly before the COVID-19 vaccines were widely available,” coauthor Enrico Ammirati, MD, PhD, Niguarda Hospital, Milan, commented.
“This analysis indicates that, although rare, hospitalized patients with acute myocarditis associated with COVID-19 infection have a much greater need for intensive care unit admission, in up to 70.5% of the cases, despite the average age of the individuals in the study being much younger than expected, at 38 years old,” added coauthor Marco Metra, MD, University of Brescia, Italy.
The researchers report that the use of corticosteroids in patients with acute myocarditis appeared safe, and, in most cases, a rapid increase in the left ventricular ejection fraction was observed. In addition, they say that discharged patients with acute myocarditis had “an excellent short-term prognosis without occurrence of cardiovascular events.”
The authors also point out that these data show much higher frequency and severity of acute myocarditis linked to COVID-19 infection, compared with myocarditis cases linked to the mRNA COVID-19 vaccines.
The international study examined health data on 56,963 patients who were hospitalized with COVID-19 at 23 hospitals across the United States and Europe from February 2020 through April 2021.
Among these patients, 97 with possible acute myocarditis were identified (4.1 per 1,000), of whom 54 (2.4 per 1,000) were classified as having “definite or probable” acute myocarditis supported by endomyocardial biopsy (31.5% of cases) or magnetic resonance imaging (92.6% of cases).
The median age of definite/probable acute myocarditis cases was 38 years, and 39% were female. On admission, chest pain and dyspnea were the most frequent symptoms (55.5% and 53.7%, respectively), and 31 cases (57.4%) occurred in the absence of COVID-19–associated pneumonia. A fulminant presentation requiring inotropic support or temporary mechanical circulatory support occurred in 21 cases (39%).
Overall, 38 patients (70.4%) were admitted to the intensive care unit for a median time of 6 days. Ten patients (18.5%) received temporary mechanical circulatory support for a median time of 5 days. Three patients died (5.5%) during the index hospitalization, all of whom also had pneumonia. At 120 days, estimated mortality was 6.6%. Patients with pneumonia were more likely to develop hemodynamic instability, require mechanical circulatory support, and die, compared with those without pneumonia.
The authors note that their reported prevalence of acute myocarditis associated with COVID-19 is lower, compared with studies that performed universal cardiac MRI screening during the convalescent COVID-19 period.
They say that underestimation of the prevalence of mild or subclinical acute myocarditis is likely in this study because of the retrospective nature of the registry, the lack of systematic cardiac MRI, and the possibility of missing some diagnoses, particularly during the first pandemic wave when cardiac MRI and endomyocardial biopsy were less frequently performed.
The authors also point out that data on myocarditis after COVID-19 vaccination suggest that vaccination-linked myocarditis is milder than that associated with the virus itself.
With regard to the prevalence of acute myocarditis after vaccination, they report that among 2.8 million doses of mRNA COVID-19 vaccine in the armed forces, 23 individuals had evidence of acute myocarditis, suggesting a prevalence of less than 1 case of acute myocarditis per 100,000 mRNA COVID-19 vaccine doses.
They note that the CDC has also reported 399 reports of myocarditis among 129 million fully vaccinated individuals with the mRNA COVID-19 vaccines.
“These figures appear reassuring, compared with the prevalence of clinically manifest acute myocarditis observed in this study among hospitalized patients with COVID-19,” they conclude.
A version of this article first appeared on Medscape.com.
The new information comes from the Centers for Disease Control and Prevention’s National Patient-Centered Clinical Research Network (PCORnet) and from a separate large international clinical study published online in Circulation.
CDC data
The CDC study analyzed electronic health record data from 40 U.S. health care systems from Jan. 1, 2021, to Jan. 31, 2022, on more than 15 million people aged 5 years or older.
It reports a rate of myocarditis or pericarditis after mRNA COVID-19 vaccination of 0-35.9 per 100,000 for males and 0-10.9 per 100,000 for females across different age groups and vaccine cohorts.
Rates of myocarditis or pericarditis after SARS-CoV-2 infection ranged from 12.6 to 114 per 100,000 for males and from 5.4 to 61.7 per 100,000 for females across different age groups.
Even among males aged 12-17 years, the group with the highest incidence of cardiac complications after receipt of a second mRNA COVID-19 vaccine dose, the risk was 1.8-5.6 times higher after SARS-CoV-2 infection than after vaccination, the CDC report notes.
“These findings provide important context for balancing risks and benefits of mRNA COVID-19 vaccination among eligible persons greater than or equal to 5 years,” the report states. They also “support the continued use of recommended mRNA vaccines among all eligible persons aged greater than or equal to 5 years,” it concludes.
International study
The international study focused on prevalence, clinical characteristics, and outcomes of clinically manifest acute myocarditis in patients with COVID-19 infection.
The study showed a rate of acute myocarditis of 2.4 per 1,000 patients hospitalized with COVID-19.
“A small study previously indicated acute myocarditis is a rare occurrence in people infected with COVID-19. Our analysis of international data offers better insight to the occurrence of acute myocarditis during COVID-19 hospitalization, particularly before the COVID-19 vaccines were widely available,” coauthor Enrico Ammirati, MD, PhD, Niguarda Hospital, Milan, commented.
“This analysis indicates that, although rare, hospitalized patients with acute myocarditis associated with COVID-19 infection have a much greater need for intensive care unit admission, in up to 70.5% of the cases, despite the average age of the individuals in the study being much younger than expected, at 38 years old,” added coauthor Marco Metra, MD, University of Brescia, Italy.
The researchers report that the use of corticosteroids in patients with acute myocarditis appeared safe, and, in most cases, a rapid increase in the left ventricular ejection fraction was observed. In addition, they say that discharged patients with acute myocarditis had “an excellent short-term prognosis without occurrence of cardiovascular events.”
The authors also point out that these data show much higher frequency and severity of acute myocarditis linked to COVID-19 infection, compared with myocarditis cases linked to the mRNA COVID-19 vaccines.
The international study examined health data on 56,963 patients who were hospitalized with COVID-19 at 23 hospitals across the United States and Europe from February 2020 through April 2021.
Among these patients, 97 with possible acute myocarditis were identified (4.1 per 1,000), of whom 54 (2.4 per 1,000) were classified as having “definite or probable” acute myocarditis supported by endomyocardial biopsy (31.5% of cases) or magnetic resonance imaging (92.6% of cases).
The median age of definite/probable acute myocarditis cases was 38 years, and 39% were female. On admission, chest pain and dyspnea were the most frequent symptoms (55.5% and 53.7%, respectively), and 31 cases (57.4%) occurred in the absence of COVID-19–associated pneumonia. A fulminant presentation requiring inotropic support or temporary mechanical circulatory support occurred in 21 cases (39%).
Overall, 38 patients (70.4%) were admitted to the intensive care unit for a median time of 6 days. Ten patients (18.5%) received temporary mechanical circulatory support for a median time of 5 days. Three patients died (5.5%) during the index hospitalization, all of whom also had pneumonia. At 120 days, estimated mortality was 6.6%. Patients with pneumonia were more likely to develop hemodynamic instability, require mechanical circulatory support, and die, compared with those without pneumonia.
The authors note that their reported prevalence of acute myocarditis associated with COVID-19 is lower, compared with studies that performed universal cardiac MRI screening during the convalescent COVID-19 period.
They say that underestimation of the prevalence of mild or subclinical acute myocarditis is likely in this study because of the retrospective nature of the registry, the lack of systematic cardiac MRI, and the possibility of missing some diagnoses, particularly during the first pandemic wave when cardiac MRI and endomyocardial biopsy were less frequently performed.
The authors also point out that data on myocarditis after COVID-19 vaccination suggest that vaccination-linked myocarditis is milder than that associated with the virus itself.
With regard to the prevalence of acute myocarditis after vaccination, they report that among 2.8 million doses of mRNA COVID-19 vaccine in the armed forces, 23 individuals had evidence of acute myocarditis, suggesting a prevalence of less than 1 case of acute myocarditis per 100,000 mRNA COVID-19 vaccine doses.
They note that the CDC has also reported 399 reports of myocarditis among 129 million fully vaccinated individuals with the mRNA COVID-19 vaccines.
“These figures appear reassuring, compared with the prevalence of clinically manifest acute myocarditis observed in this study among hospitalized patients with COVID-19,” they conclude.
A version of this article first appeared on Medscape.com.
The new information comes from the Centers for Disease Control and Prevention’s National Patient-Centered Clinical Research Network (PCORnet) and from a separate large international clinical study published online in Circulation.
CDC data
The CDC study analyzed electronic health record data from 40 U.S. health care systems from Jan. 1, 2021, to Jan. 31, 2022, on more than 15 million people aged 5 years or older.
It reports a rate of myocarditis or pericarditis after mRNA COVID-19 vaccination of 0-35.9 per 100,000 for males and 0-10.9 per 100,000 for females across different age groups and vaccine cohorts.
Rates of myocarditis or pericarditis after SARS-CoV-2 infection ranged from 12.6 to 114 per 100,000 for males and from 5.4 to 61.7 per 100,000 for females across different age groups.
Even among males aged 12-17 years, the group with the highest incidence of cardiac complications after receipt of a second mRNA COVID-19 vaccine dose, the risk was 1.8-5.6 times higher after SARS-CoV-2 infection than after vaccination, the CDC report notes.
“These findings provide important context for balancing risks and benefits of mRNA COVID-19 vaccination among eligible persons greater than or equal to 5 years,” the report states. They also “support the continued use of recommended mRNA vaccines among all eligible persons aged greater than or equal to 5 years,” it concludes.
International study
The international study focused on prevalence, clinical characteristics, and outcomes of clinically manifest acute myocarditis in patients with COVID-19 infection.
The study showed a rate of acute myocarditis of 2.4 per 1,000 patients hospitalized with COVID-19.
“A small study previously indicated acute myocarditis is a rare occurrence in people infected with COVID-19. Our analysis of international data offers better insight to the occurrence of acute myocarditis during COVID-19 hospitalization, particularly before the COVID-19 vaccines were widely available,” coauthor Enrico Ammirati, MD, PhD, Niguarda Hospital, Milan, commented.
“This analysis indicates that, although rare, hospitalized patients with acute myocarditis associated with COVID-19 infection have a much greater need for intensive care unit admission, in up to 70.5% of the cases, despite the average age of the individuals in the study being much younger than expected, at 38 years old,” added coauthor Marco Metra, MD, University of Brescia, Italy.
The researchers report that the use of corticosteroids in patients with acute myocarditis appeared safe, and, in most cases, a rapid increase in the left ventricular ejection fraction was observed. In addition, they say that discharged patients with acute myocarditis had “an excellent short-term prognosis without occurrence of cardiovascular events.”
The authors also point out that these data show much higher frequency and severity of acute myocarditis linked to COVID-19 infection, compared with myocarditis cases linked to the mRNA COVID-19 vaccines.
The international study examined health data on 56,963 patients who were hospitalized with COVID-19 at 23 hospitals across the United States and Europe from February 2020 through April 2021.
Among these patients, 97 with possible acute myocarditis were identified (4.1 per 1,000), of whom 54 (2.4 per 1,000) were classified as having “definite or probable” acute myocarditis supported by endomyocardial biopsy (31.5% of cases) or magnetic resonance imaging (92.6% of cases).
The median age of definite/probable acute myocarditis cases was 38 years, and 39% were female. On admission, chest pain and dyspnea were the most frequent symptoms (55.5% and 53.7%, respectively), and 31 cases (57.4%) occurred in the absence of COVID-19–associated pneumonia. A fulminant presentation requiring inotropic support or temporary mechanical circulatory support occurred in 21 cases (39%).
Overall, 38 patients (70.4%) were admitted to the intensive care unit for a median time of 6 days. Ten patients (18.5%) received temporary mechanical circulatory support for a median time of 5 days. Three patients died (5.5%) during the index hospitalization, all of whom also had pneumonia. At 120 days, estimated mortality was 6.6%. Patients with pneumonia were more likely to develop hemodynamic instability, require mechanical circulatory support, and die, compared with those without pneumonia.
The authors note that their reported prevalence of acute myocarditis associated with COVID-19 is lower, compared with studies that performed universal cardiac MRI screening during the convalescent COVID-19 period.
They say that underestimation of the prevalence of mild or subclinical acute myocarditis is likely in this study because of the retrospective nature of the registry, the lack of systematic cardiac MRI, and the possibility of missing some diagnoses, particularly during the first pandemic wave when cardiac MRI and endomyocardial biopsy were less frequently performed.
The authors also point out that data on myocarditis after COVID-19 vaccination suggest that vaccination-linked myocarditis is milder than that associated with the virus itself.
With regard to the prevalence of acute myocarditis after vaccination, they report that among 2.8 million doses of mRNA COVID-19 vaccine in the armed forces, 23 individuals had evidence of acute myocarditis, suggesting a prevalence of less than 1 case of acute myocarditis per 100,000 mRNA COVID-19 vaccine doses.
They note that the CDC has also reported 399 reports of myocarditis among 129 million fully vaccinated individuals with the mRNA COVID-19 vaccines.
“These figures appear reassuring, compared with the prevalence of clinically manifest acute myocarditis observed in this study among hospitalized patients with COVID-19,” they conclude.
A version of this article first appeared on Medscape.com.
Fourth Pfizer dose better for severe than symptomatic COVID: Study
A fourth dose of the Pfizer-BioNTech vaccine is effective in reducing the short-term risk for COVID-19 infection, hospitalization, and death in people who got a third dose at least 4 months before, a large study shows.
However, Paul Offit, MD, author of an editorial accompanying the study, told this news organization, “I would argue, without fear of contradiction, that this is going to have no impact on this pandemic.”
“We are still in the midst of a zero-tolerance policy for this virus. We don’t accept mild illness and if we’re not going to accept mild illness, we think we have to boost it away, which would mean probably about two doses every year. That’s not a reasonable public health strategy,” said Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Booster confusion
Results of the research out of Israel, published in the New England Journal of Medicine, make a case for a fourth booster for people 60 and over.
Researchers, led by Ori Magen, MD, Clalit Research Institute, innovation division, Clalit Health Services, Tel Aviv, analyzed data comparing 182,122 matched pairs recorded by the largest health care organization in Israel from Jan. 3 to Feb. 18, 2022. With more than 4.7 million members, Clalit Health Services covers more than half of the population of Israel.
The researchers compared outcomes in people 60 or older (average age, 72 years) who got a fourth dose with outcomes in those who had only a third dose. They individually matched people from the two groups, considering factors such as age, health status, and ethnicity.
Relative vaccine effectiveness in days 7-30 after the fourth dose was estimated to be 45% (95% confidence interval, 44%-47%) against confirmed SARS-CoV-2 infection, 55% (95% CI, 53%-58%) against symptomatic COVID-19, 68% (95% CI, 59%-74%) against hospitalization, 62% (95% CI, 50%-74%) against severe COVID, and 74% (95% CI, 50%-90%) against COVID-related death.
Several countries, including the United States, have begun offering a fourth vaccine dose for higher-risk populations in light of evidence of waning immunity after the third dose and waves of infection, driven by Omicron and its variants, in some parts of the world. But the recommended age groups differ considerably.
In the United States, for instance, the Food and Drug Administration in late March approved a fourth dose of the Pfizer or Moderna vaccine for anyone over 50 and people over 18 who have gotten a solid organ transplant or have a similar level of immune risk.
Dr. Offit pointed out that Israel offers the fourth vaccine for people 60 and over and the European Medical Association offers it for those over 80. No surprise that confusion over the fourth dose is rampant.
Booster advice
Dr. Offit offered this perspective: People who are immunocompromised could reasonably get a fourth dose, depending on the manner in which they are compromised.
“Someone who has a solid organ transplant is not the same as someone who is getting a monoclonal antibody for their rheumatoid arthritis,” Dr. Offit said, adding that people could also make a reasonable argument for the fourth dose if they are over 65 and have multiple comorbidities.
“I’m over 65,” Dr. Offit said. “I’m generally healthy. I’m not going to get a fourth dose.”
People with multiple comorbidities over age 12 could reasonably get a third dose, he said. “For everybody else – healthy people less than 65 – I would argue this is a two-dose vaccine.”
CHOP, he noted as an example, mandates the vaccine but doesn’t mandate three doses and he says that’s not unusual for hospital systems.
“How many lives are you really saving with that fourth dose? If you really want to have an effect on this pandemic, vaccinate the unvaccinated,” Dr. Offit said.
Focus on the memory cells
Dr. Offit wrote in the editorial: “Arguably, the most disappointing error surrounding the use of COVID-19 vaccines was the labeling of mild illnesses or asymptomatic infections after vaccination as ‘breakthroughs.’ As is true for all mucosal vaccines, the goal is to protect against serious illness – to keep people out of the hospital, intensive care unit, and morgue. The term ‘breakthrough,’ which implies failure, created unrealistic expectations and led to the adoption of a zero-tolerance strategy for this virus.”
Dr. Offit said that the focus should be on the memory cells, not the neutralizing antibodies.
Regarding mRNA vaccines, Dr. Offit said “the surprise of this vaccine – it surprised me and other vaccine researchers – is that with these two doses of mRNA separated by 3-4 weeks, you actually appear to have long-lived memory response.
“That’s not the history of vaccines. If you look at the inactivated polio vaccine or the inactivated hepatitis A vaccine, you really do need a 4- to 6-month interval between doses to get high frequencies of memory cells. That doesn’t appear to be the case here. It looks like two doses given close together do just that. Memory cells last for years if not, sometimes, decades.”
Neutralizing antibodies, on the other hand, protect against mild illness and their effectiveness wanes after months.
“At some point we are going to have to get used to mild illness,” Dr. Offit said.
The Centers for Disease Control and Prevention must now determine who will benefit most from booster dosing and educate the public about the limits of mucosal vaccines, Dr. Offit wrote in the editorial.
“Otherwise, a zero-tolerance strategy for mild or asymptomatic infection, which can be implemented only with frequent booster doses, will continue to mislead the public about what COVID-19 vaccines can and cannot do.”
The work was funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
A version of this article first appeared on Medscape.com.
A fourth dose of the Pfizer-BioNTech vaccine is effective in reducing the short-term risk for COVID-19 infection, hospitalization, and death in people who got a third dose at least 4 months before, a large study shows.
However, Paul Offit, MD, author of an editorial accompanying the study, told this news organization, “I would argue, without fear of contradiction, that this is going to have no impact on this pandemic.”
“We are still in the midst of a zero-tolerance policy for this virus. We don’t accept mild illness and if we’re not going to accept mild illness, we think we have to boost it away, which would mean probably about two doses every year. That’s not a reasonable public health strategy,” said Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Booster confusion
Results of the research out of Israel, published in the New England Journal of Medicine, make a case for a fourth booster for people 60 and over.
Researchers, led by Ori Magen, MD, Clalit Research Institute, innovation division, Clalit Health Services, Tel Aviv, analyzed data comparing 182,122 matched pairs recorded by the largest health care organization in Israel from Jan. 3 to Feb. 18, 2022. With more than 4.7 million members, Clalit Health Services covers more than half of the population of Israel.
The researchers compared outcomes in people 60 or older (average age, 72 years) who got a fourth dose with outcomes in those who had only a third dose. They individually matched people from the two groups, considering factors such as age, health status, and ethnicity.
Relative vaccine effectiveness in days 7-30 after the fourth dose was estimated to be 45% (95% confidence interval, 44%-47%) against confirmed SARS-CoV-2 infection, 55% (95% CI, 53%-58%) against symptomatic COVID-19, 68% (95% CI, 59%-74%) against hospitalization, 62% (95% CI, 50%-74%) against severe COVID, and 74% (95% CI, 50%-90%) against COVID-related death.
Several countries, including the United States, have begun offering a fourth vaccine dose for higher-risk populations in light of evidence of waning immunity after the third dose and waves of infection, driven by Omicron and its variants, in some parts of the world. But the recommended age groups differ considerably.
In the United States, for instance, the Food and Drug Administration in late March approved a fourth dose of the Pfizer or Moderna vaccine for anyone over 50 and people over 18 who have gotten a solid organ transplant or have a similar level of immune risk.
Dr. Offit pointed out that Israel offers the fourth vaccine for people 60 and over and the European Medical Association offers it for those over 80. No surprise that confusion over the fourth dose is rampant.
Booster advice
Dr. Offit offered this perspective: People who are immunocompromised could reasonably get a fourth dose, depending on the manner in which they are compromised.
“Someone who has a solid organ transplant is not the same as someone who is getting a monoclonal antibody for their rheumatoid arthritis,” Dr. Offit said, adding that people could also make a reasonable argument for the fourth dose if they are over 65 and have multiple comorbidities.
“I’m over 65,” Dr. Offit said. “I’m generally healthy. I’m not going to get a fourth dose.”
People with multiple comorbidities over age 12 could reasonably get a third dose, he said. “For everybody else – healthy people less than 65 – I would argue this is a two-dose vaccine.”
CHOP, he noted as an example, mandates the vaccine but doesn’t mandate three doses and he says that’s not unusual for hospital systems.
“How many lives are you really saving with that fourth dose? If you really want to have an effect on this pandemic, vaccinate the unvaccinated,” Dr. Offit said.
Focus on the memory cells
Dr. Offit wrote in the editorial: “Arguably, the most disappointing error surrounding the use of COVID-19 vaccines was the labeling of mild illnesses or asymptomatic infections after vaccination as ‘breakthroughs.’ As is true for all mucosal vaccines, the goal is to protect against serious illness – to keep people out of the hospital, intensive care unit, and morgue. The term ‘breakthrough,’ which implies failure, created unrealistic expectations and led to the adoption of a zero-tolerance strategy for this virus.”
Dr. Offit said that the focus should be on the memory cells, not the neutralizing antibodies.
Regarding mRNA vaccines, Dr. Offit said “the surprise of this vaccine – it surprised me and other vaccine researchers – is that with these two doses of mRNA separated by 3-4 weeks, you actually appear to have long-lived memory response.
“That’s not the history of vaccines. If you look at the inactivated polio vaccine or the inactivated hepatitis A vaccine, you really do need a 4- to 6-month interval between doses to get high frequencies of memory cells. That doesn’t appear to be the case here. It looks like two doses given close together do just that. Memory cells last for years if not, sometimes, decades.”
Neutralizing antibodies, on the other hand, protect against mild illness and their effectiveness wanes after months.
“At some point we are going to have to get used to mild illness,” Dr. Offit said.
The Centers for Disease Control and Prevention must now determine who will benefit most from booster dosing and educate the public about the limits of mucosal vaccines, Dr. Offit wrote in the editorial.
“Otherwise, a zero-tolerance strategy for mild or asymptomatic infection, which can be implemented only with frequent booster doses, will continue to mislead the public about what COVID-19 vaccines can and cannot do.”
The work was funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
A version of this article first appeared on Medscape.com.
A fourth dose of the Pfizer-BioNTech vaccine is effective in reducing the short-term risk for COVID-19 infection, hospitalization, and death in people who got a third dose at least 4 months before, a large study shows.
However, Paul Offit, MD, author of an editorial accompanying the study, told this news organization, “I would argue, without fear of contradiction, that this is going to have no impact on this pandemic.”
“We are still in the midst of a zero-tolerance policy for this virus. We don’t accept mild illness and if we’re not going to accept mild illness, we think we have to boost it away, which would mean probably about two doses every year. That’s not a reasonable public health strategy,” said Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Booster confusion
Results of the research out of Israel, published in the New England Journal of Medicine, make a case for a fourth booster for people 60 and over.
Researchers, led by Ori Magen, MD, Clalit Research Institute, innovation division, Clalit Health Services, Tel Aviv, analyzed data comparing 182,122 matched pairs recorded by the largest health care organization in Israel from Jan. 3 to Feb. 18, 2022. With more than 4.7 million members, Clalit Health Services covers more than half of the population of Israel.
The researchers compared outcomes in people 60 or older (average age, 72 years) who got a fourth dose with outcomes in those who had only a third dose. They individually matched people from the two groups, considering factors such as age, health status, and ethnicity.
Relative vaccine effectiveness in days 7-30 after the fourth dose was estimated to be 45% (95% confidence interval, 44%-47%) against confirmed SARS-CoV-2 infection, 55% (95% CI, 53%-58%) against symptomatic COVID-19, 68% (95% CI, 59%-74%) against hospitalization, 62% (95% CI, 50%-74%) against severe COVID, and 74% (95% CI, 50%-90%) against COVID-related death.
Several countries, including the United States, have begun offering a fourth vaccine dose for higher-risk populations in light of evidence of waning immunity after the third dose and waves of infection, driven by Omicron and its variants, in some parts of the world. But the recommended age groups differ considerably.
In the United States, for instance, the Food and Drug Administration in late March approved a fourth dose of the Pfizer or Moderna vaccine for anyone over 50 and people over 18 who have gotten a solid organ transplant or have a similar level of immune risk.
Dr. Offit pointed out that Israel offers the fourth vaccine for people 60 and over and the European Medical Association offers it for those over 80. No surprise that confusion over the fourth dose is rampant.
Booster advice
Dr. Offit offered this perspective: People who are immunocompromised could reasonably get a fourth dose, depending on the manner in which they are compromised.
“Someone who has a solid organ transplant is not the same as someone who is getting a monoclonal antibody for their rheumatoid arthritis,” Dr. Offit said, adding that people could also make a reasonable argument for the fourth dose if they are over 65 and have multiple comorbidities.
“I’m over 65,” Dr. Offit said. “I’m generally healthy. I’m not going to get a fourth dose.”
People with multiple comorbidities over age 12 could reasonably get a third dose, he said. “For everybody else – healthy people less than 65 – I would argue this is a two-dose vaccine.”
CHOP, he noted as an example, mandates the vaccine but doesn’t mandate three doses and he says that’s not unusual for hospital systems.
“How many lives are you really saving with that fourth dose? If you really want to have an effect on this pandemic, vaccinate the unvaccinated,” Dr. Offit said.
Focus on the memory cells
Dr. Offit wrote in the editorial: “Arguably, the most disappointing error surrounding the use of COVID-19 vaccines was the labeling of mild illnesses or asymptomatic infections after vaccination as ‘breakthroughs.’ As is true for all mucosal vaccines, the goal is to protect against serious illness – to keep people out of the hospital, intensive care unit, and morgue. The term ‘breakthrough,’ which implies failure, created unrealistic expectations and led to the adoption of a zero-tolerance strategy for this virus.”
Dr. Offit said that the focus should be on the memory cells, not the neutralizing antibodies.
Regarding mRNA vaccines, Dr. Offit said “the surprise of this vaccine – it surprised me and other vaccine researchers – is that with these two doses of mRNA separated by 3-4 weeks, you actually appear to have long-lived memory response.
“That’s not the history of vaccines. If you look at the inactivated polio vaccine or the inactivated hepatitis A vaccine, you really do need a 4- to 6-month interval between doses to get high frequencies of memory cells. That doesn’t appear to be the case here. It looks like two doses given close together do just that. Memory cells last for years if not, sometimes, decades.”
Neutralizing antibodies, on the other hand, protect against mild illness and their effectiveness wanes after months.
“At some point we are going to have to get used to mild illness,” Dr. Offit said.
The Centers for Disease Control and Prevention must now determine who will benefit most from booster dosing and educate the public about the limits of mucosal vaccines, Dr. Offit wrote in the editorial.
“Otherwise, a zero-tolerance strategy for mild or asymptomatic infection, which can be implemented only with frequent booster doses, will continue to mislead the public about what COVID-19 vaccines can and cannot do.”
The work was funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Woman who faked medical degree practiced for 3 years
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
No need to ‘guess what size horse you are’
Is COVID-19 warming up for yet another surge? Maybe. That means it’s also time for the return of its remora-like follower, ivermectin. Our thanks go out to the Tennessee state legislature for bringing the proven-to-be-ineffective treatment for COVID back into our hearts and minds and emergency rooms.
Both the state House and Senate have approved a bill that allows pharmacists to dispense the antiparasitic drug without a prescription while shielding them “from any liability that could arise from dispensing ivermectin,” Nashville Public Radio reported.
The drug’s manufacturer, Merck, said over a year ago that there is “no scientific basis for a potential therapeutic effect against COVID-19 from preclinical studies … and a concerning lack of safety data.” More recently, a study published in the New England Journal of Medicine showed that ivermectin treatment had no important benefits in patients with COVID.
Last week, the bill’s Senate sponsor, Frank Niceley of Strawberry Plains, said that it was all about safety, as he explained to NPR station WPLN: “It’s a lot safer to go to your pharmacist and let him tell you how much ivermectin to take than it is to go to the co-op and guess what size horse you are.”
And on that note, here are a few more items of business that just might end up on the legislature’s calendar:
- Horses will be allowed to “share” their unused ivermectin with humans and other mammals.
- An apple a day not only keeps the doctor away, but the IRS and the FDA as well.
- Colon cleansing is more fun than humans should be allowed to have.
- TikTok videos qualify as CME.
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
Speak louder, I can’t see you
With the introduction of FaceTime and the pandemic pushing work and social events to Zoom, video calls have become ubiquitous. Along the way, however, we’ve had to learn to adjust to technical difficulties. Often by yelling at the screen when the video quality is disrupted. Waving our hands and arms, speaking louder. Sound like you?
Well, a new study published in Royal Society Open Science shows that it sounds like a lot of us.
James Trujillo of the Max Planck Institute for Psycholinguistics in Nijmegen, the Netherlands, who was lead author of the paper, said on Eurekalert that “previous research has shown that speech and gestures are linked, but ours is the first to look into how visuals impact our behavior in those fields.”
He and his associates set up 40 participants in separate rooms to have conversations in pairs over a video chat. Over the course of 40 minutes, the video quality started to deteriorate from clear to extremely blurry. When the video quality was affected, participants started with gestures but as the quality continued to lessen the gestures increased and so did the decibels of their voices.
Even when the participants could barely see each other, they still gestured and their voices were even louder, positively supporting the idea that gestures and speech are a dynamically linked when it comes to communication. Even on regular phone calls, when we can’t see each other at all, people make small movements and gestures, Mr. Trujillo said.
So, the next time the Wifi is terrible and your video calls keep cutting out, don’t worry about looking foolish screaming at the computer. We’ve all been there.
Seek a doctor if standing at attention for more than 4 hours
Imbrochável. In Brazil, it means “unfloppable” or “flaccid proof.” It’s also a word that Brazilian president Jair Bolsonaro likes to use when referring to himself. Gives you a good idea of what he’s all about. Imagine his embarrassment when news recently broke about more than 30,000 pills of Viagra that had been secretly distributed to the Brazilian military.
The military offered a simple and plausible explanation: The Viagra had been prescribed to treat pulmonary hypertension. Fair, but when a Brazilian newspaper dug a little deeper, they found that this was not the case. The Viagra was, in general, being used for its, shall we say, traditional purpose.
Many Brazilians reacted poorly to the news that their tax dollars were being used to provide Brazilian soldiers with downstairs assistance, with the standard associated furor on social media. A rival politician, Ciro Gomes, who is planning on challenging the president in an upcoming election, had perhaps the best remark on the situation: “Unless they’re able to prove they’re developing some kind of secret weapon – capable of revolutionizing the international arms industry – it’ll be tough to justify the purchase of 35,000 units of a erectile dysfunction drug.”
Hmm, secret weapon. Well, a certain Russian fellow has made a bit of a thrust into world affairs recently. Does anyone know if Putin is sitting on a big Viagra stash?
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
No need to ‘guess what size horse you are’
Is COVID-19 warming up for yet another surge? Maybe. That means it’s also time for the return of its remora-like follower, ivermectin. Our thanks go out to the Tennessee state legislature for bringing the proven-to-be-ineffective treatment for COVID back into our hearts and minds and emergency rooms.
Both the state House and Senate have approved a bill that allows pharmacists to dispense the antiparasitic drug without a prescription while shielding them “from any liability that could arise from dispensing ivermectin,” Nashville Public Radio reported.
The drug’s manufacturer, Merck, said over a year ago that there is “no scientific basis for a potential therapeutic effect against COVID-19 from preclinical studies … and a concerning lack of safety data.” More recently, a study published in the New England Journal of Medicine showed that ivermectin treatment had no important benefits in patients with COVID.
Last week, the bill’s Senate sponsor, Frank Niceley of Strawberry Plains, said that it was all about safety, as he explained to NPR station WPLN: “It’s a lot safer to go to your pharmacist and let him tell you how much ivermectin to take than it is to go to the co-op and guess what size horse you are.”
And on that note, here are a few more items of business that just might end up on the legislature’s calendar:
- Horses will be allowed to “share” their unused ivermectin with humans and other mammals.
- An apple a day not only keeps the doctor away, but the IRS and the FDA as well.
- Colon cleansing is more fun than humans should be allowed to have.
- TikTok videos qualify as CME.
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
Speak louder, I can’t see you
With the introduction of FaceTime and the pandemic pushing work and social events to Zoom, video calls have become ubiquitous. Along the way, however, we’ve had to learn to adjust to technical difficulties. Often by yelling at the screen when the video quality is disrupted. Waving our hands and arms, speaking louder. Sound like you?
Well, a new study published in Royal Society Open Science shows that it sounds like a lot of us.
James Trujillo of the Max Planck Institute for Psycholinguistics in Nijmegen, the Netherlands, who was lead author of the paper, said on Eurekalert that “previous research has shown that speech and gestures are linked, but ours is the first to look into how visuals impact our behavior in those fields.”
He and his associates set up 40 participants in separate rooms to have conversations in pairs over a video chat. Over the course of 40 minutes, the video quality started to deteriorate from clear to extremely blurry. When the video quality was affected, participants started with gestures but as the quality continued to lessen the gestures increased and so did the decibels of their voices.
Even when the participants could barely see each other, they still gestured and their voices were even louder, positively supporting the idea that gestures and speech are a dynamically linked when it comes to communication. Even on regular phone calls, when we can’t see each other at all, people make small movements and gestures, Mr. Trujillo said.
So, the next time the Wifi is terrible and your video calls keep cutting out, don’t worry about looking foolish screaming at the computer. We’ve all been there.
Seek a doctor if standing at attention for more than 4 hours
Imbrochável. In Brazil, it means “unfloppable” or “flaccid proof.” It’s also a word that Brazilian president Jair Bolsonaro likes to use when referring to himself. Gives you a good idea of what he’s all about. Imagine his embarrassment when news recently broke about more than 30,000 pills of Viagra that had been secretly distributed to the Brazilian military.
The military offered a simple and plausible explanation: The Viagra had been prescribed to treat pulmonary hypertension. Fair, but when a Brazilian newspaper dug a little deeper, they found that this was not the case. The Viagra was, in general, being used for its, shall we say, traditional purpose.
Many Brazilians reacted poorly to the news that their tax dollars were being used to provide Brazilian soldiers with downstairs assistance, with the standard associated furor on social media. A rival politician, Ciro Gomes, who is planning on challenging the president in an upcoming election, had perhaps the best remark on the situation: “Unless they’re able to prove they’re developing some kind of secret weapon – capable of revolutionizing the international arms industry – it’ll be tough to justify the purchase of 35,000 units of a erectile dysfunction drug.”
Hmm, secret weapon. Well, a certain Russian fellow has made a bit of a thrust into world affairs recently. Does anyone know if Putin is sitting on a big Viagra stash?
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
No need to ‘guess what size horse you are’
Is COVID-19 warming up for yet another surge? Maybe. That means it’s also time for the return of its remora-like follower, ivermectin. Our thanks go out to the Tennessee state legislature for bringing the proven-to-be-ineffective treatment for COVID back into our hearts and minds and emergency rooms.
Both the state House and Senate have approved a bill that allows pharmacists to dispense the antiparasitic drug without a prescription while shielding them “from any liability that could arise from dispensing ivermectin,” Nashville Public Radio reported.
The drug’s manufacturer, Merck, said over a year ago that there is “no scientific basis for a potential therapeutic effect against COVID-19 from preclinical studies … and a concerning lack of safety data.” More recently, a study published in the New England Journal of Medicine showed that ivermectin treatment had no important benefits in patients with COVID.
Last week, the bill’s Senate sponsor, Frank Niceley of Strawberry Plains, said that it was all about safety, as he explained to NPR station WPLN: “It’s a lot safer to go to your pharmacist and let him tell you how much ivermectin to take than it is to go to the co-op and guess what size horse you are.”
And on that note, here are a few more items of business that just might end up on the legislature’s calendar:
- Horses will be allowed to “share” their unused ivermectin with humans and other mammals.
- An apple a day not only keeps the doctor away, but the IRS and the FDA as well.
- Colon cleansing is more fun than humans should be allowed to have.
- TikTok videos qualify as CME.
Who needs medical degrees anyway?
It’s no secret that doctors make a fair chunk of change. It’s a lucrative profession, but that big fat paycheck is siloed behind long, tough years of medical school and residency. It’s not an easy path doctors walk. Or at least, it’s not supposed to be. Anything’s easy if you’re willing to lie.
That brings us to Sonia, a 31-year-old woman from northern France with a bachelor’s degree in real estate management who wasn’t bringing in enough money for her three children, at least not to her satisfaction. Naturally, the only decision was to forge some diplomas from the University of Strasbourg, as well as a certificate from the French Order of Physicians. Sonia got hired as a general practitioner by using the identities of two doctors who shared her name. She had no experience, had no idea what she was doing, and was wearing a GPS tagging bracelet for an unrelated crime, so she was quickly caught and exposed in October 2021, after, um, 3 years of fake doctoring, according to France Live.
Not to be deterred by this temporary setback, Sonia proceeded to immediately find work as an ophthalmologist, a career that requires more than 10 years of training, continuing her fraudulent medical career until recently, when she was caught again and sentenced to 3 years in prison. She did make 70,000 euros a year as a fake doctor, which isn’t exactly huge money, but certainly not bad either.
We certainly hope she’s learned her lesson about impersonating a doctor, at this point, but maybe she should just go to medical school. If not, northern France might just end up with a new endocrinologist or oncologist floating around in 3 years.
Speak louder, I can’t see you
With the introduction of FaceTime and the pandemic pushing work and social events to Zoom, video calls have become ubiquitous. Along the way, however, we’ve had to learn to adjust to technical difficulties. Often by yelling at the screen when the video quality is disrupted. Waving our hands and arms, speaking louder. Sound like you?
Well, a new study published in Royal Society Open Science shows that it sounds like a lot of us.
James Trujillo of the Max Planck Institute for Psycholinguistics in Nijmegen, the Netherlands, who was lead author of the paper, said on Eurekalert that “previous research has shown that speech and gestures are linked, but ours is the first to look into how visuals impact our behavior in those fields.”
He and his associates set up 40 participants in separate rooms to have conversations in pairs over a video chat. Over the course of 40 minutes, the video quality started to deteriorate from clear to extremely blurry. When the video quality was affected, participants started with gestures but as the quality continued to lessen the gestures increased and so did the decibels of their voices.
Even when the participants could barely see each other, they still gestured and their voices were even louder, positively supporting the idea that gestures and speech are a dynamically linked when it comes to communication. Even on regular phone calls, when we can’t see each other at all, people make small movements and gestures, Mr. Trujillo said.
So, the next time the Wifi is terrible and your video calls keep cutting out, don’t worry about looking foolish screaming at the computer. We’ve all been there.
Seek a doctor if standing at attention for more than 4 hours
Imbrochável. In Brazil, it means “unfloppable” or “flaccid proof.” It’s also a word that Brazilian president Jair Bolsonaro likes to use when referring to himself. Gives you a good idea of what he’s all about. Imagine his embarrassment when news recently broke about more than 30,000 pills of Viagra that had been secretly distributed to the Brazilian military.
The military offered a simple and plausible explanation: The Viagra had been prescribed to treat pulmonary hypertension. Fair, but when a Brazilian newspaper dug a little deeper, they found that this was not the case. The Viagra was, in general, being used for its, shall we say, traditional purpose.
Many Brazilians reacted poorly to the news that their tax dollars were being used to provide Brazilian soldiers with downstairs assistance, with the standard associated furor on social media. A rival politician, Ciro Gomes, who is planning on challenging the president in an upcoming election, had perhaps the best remark on the situation: “Unless they’re able to prove they’re developing some kind of secret weapon – capable of revolutionizing the international arms industry – it’ll be tough to justify the purchase of 35,000 units of a erectile dysfunction drug.”
Hmm, secret weapon. Well, a certain Russian fellow has made a bit of a thrust into world affairs recently. Does anyone know if Putin is sitting on a big Viagra stash?
COVID-19 cardiovascular complications in children: AHA statement
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
High comorbidity rate seen before osteoarthritis diagnosis
presented at the OARSI 2022 World Congress.
“Some of the associations that we have found are previously known, such as of course, obesity, which is a known risk factor, but also other musculoskeletal conditions, depression, and reflux disease,” said Anne Kamps, an MD and PhD student at Erasmus University Medical Centre in Rotterdam, the Netherlands.
“But there are also some remarkable associations that we have found that are less well known, such as liver cirrhosis, thromboembolic disease, sinusitis, allergy, and migraine,” said Dr. Kamps during her presentation at the conference, sponsored by the Osteoarthritis Research Society International.
The results are “very interesting starting points for future research, because of course, this was an explorative study,” she added. Indeed, is still not known whether the comorbidities found share the same risk factors as OA, or if they have a causal effect and add to development of osteoarthritis.
Comorbidity and OA
One of the issues in managing osteoarthritis so far is that it’s often addressed as one disease, commented Andrea Dell’isola, PT, PhD, a postdoctoral researcher from Lund University who was not involved in the study.
“All of the treatments that have been developed and the treatment process are tailored to take care of one single disease,” he explained. However, “when we look at the characteristics of people with osteoarthritis, we see that roughly 70% of them have other conditions on top of their joint disease.” This high comorbidity rate is significantly higher than in “healthy” people of the same age and sex, he added.
“So, this means that either there is something linked to osteoarthritis that makes people frailer and more likely to develop other diseases, or there may be links between these other diseases, that we often call comorbidities, and osteoarthritis,” Dr. Dell’isola observed.
While the work Dr. Kamps presented looked at the rate of comorbidities that existed before the diagnosis of OA, some of Dr. Dell’isola’s recent research has considered the rate of developing comorbid disease in the years following an OA diagnosis. Associations were found between having hip or knee OA and an increased risk for coexisting depression, cardiovascular diseases, back pain, osteoporosis, and, in the case of knee OA only, diabetes. “It’s interesting to see that certain diseases seem to have a bidirectional association. This means that they can both precede and follow osteoarthritis,” said Dr. Dell’isola. These are just associations, not causation, he stressed, but they might help identify people visiting a doctor for other reasons who may be at risk for developing OA.
“One of the biggest challenges is that once a person develops osteoarthritis, there is not any treatment that can really change their disease,” he added.
Perhaps, “if we can target certain conditions that increase the risk of developing osteoarthritis, and maybe convince people to exercise earlier, or undergo some lifestyle changes early on, we can maybe prevent or delay the onset of the disease,” he suggested.
Results and perspective
Dr. Kamps and associates performed a nested case-control study using data from a large Dutch general practice database. All new cases of OA – which included hip, knee, and other peripheral OA – that were logged between the start of 2006 and the end of 2019 were considered and matched to one to four control subjects of a similar age, sex, and type of general practice. In all, there were just under 80,000 people with newly diagnosed OA who were matched to just over 318,000 controls; the mean age in both groups was 64 years.
Of 58 comorbidities that were assessed, 42 showed a positive association with OA and had odds ratios of 1 or more. The highest associations were found for fibromyalgia (OR, 1.9), obesity (1.8), polymyalgia rheumatica (1.5), spinal disc herniation (1.4), and gout (1.4). A further 13 comorbidities had an OR of about 1, and 3 (all neuropsychiatric conditions – dementia, schizophrenia, and multiple sclerosis) had an OR of below 1.
Dr. Kamps conceded that this type of research has its limitations, the two most important being the coding behavior of the GP and the consulting behavior of patients.
“It’s known that the prevalence of osteoarthritis is underestimated if you only use the diagnostic codes, because some GPs will write the diagnosis in free text or use symptom ICPC codes,” she said.
“We have matched on general practice, so the cases and controls were from the same general practice and therefore we hope that this potential underestimation is balanced and did not affect our odds ratios.”
One of the important outcomes for this research is that it will hopefully be used to inform future clinical practice guidelines, said Dr. Dell’isola.
“Guidelines in osteoarthritis report that is important to screen for comorbidities, but they give no indication on how to deal with the presence of multimorbidity,” he added. Looking at which comorbidities may be associated with OA diagnosis could potentially help to give a bit more of a prescriptive guide on what to look out for.
“Maybe people with a certain disease profile should be screened a bit more often by their doctor. For example, if someone has their blood pressure and diabetes under control, maybe there should be also a bit more attention to their joint health and encouragement to do exercise, not only for being active per se, but maybe also to reinforce their lower limbs,” he explained.
The study was funded by the Foundation for Research in Rheumatology (FOREUM). Dr. Kamps and Dr. Dell’isola, had no conflicts of interest to disclose.
presented at the OARSI 2022 World Congress.
“Some of the associations that we have found are previously known, such as of course, obesity, which is a known risk factor, but also other musculoskeletal conditions, depression, and reflux disease,” said Anne Kamps, an MD and PhD student at Erasmus University Medical Centre in Rotterdam, the Netherlands.
“But there are also some remarkable associations that we have found that are less well known, such as liver cirrhosis, thromboembolic disease, sinusitis, allergy, and migraine,” said Dr. Kamps during her presentation at the conference, sponsored by the Osteoarthritis Research Society International.
The results are “very interesting starting points for future research, because of course, this was an explorative study,” she added. Indeed, is still not known whether the comorbidities found share the same risk factors as OA, or if they have a causal effect and add to development of osteoarthritis.
Comorbidity and OA
One of the issues in managing osteoarthritis so far is that it’s often addressed as one disease, commented Andrea Dell’isola, PT, PhD, a postdoctoral researcher from Lund University who was not involved in the study.
“All of the treatments that have been developed and the treatment process are tailored to take care of one single disease,” he explained. However, “when we look at the characteristics of people with osteoarthritis, we see that roughly 70% of them have other conditions on top of their joint disease.” This high comorbidity rate is significantly higher than in “healthy” people of the same age and sex, he added.
“So, this means that either there is something linked to osteoarthritis that makes people frailer and more likely to develop other diseases, or there may be links between these other diseases, that we often call comorbidities, and osteoarthritis,” Dr. Dell’isola observed.
While the work Dr. Kamps presented looked at the rate of comorbidities that existed before the diagnosis of OA, some of Dr. Dell’isola’s recent research has considered the rate of developing comorbid disease in the years following an OA diagnosis. Associations were found between having hip or knee OA and an increased risk for coexisting depression, cardiovascular diseases, back pain, osteoporosis, and, in the case of knee OA only, diabetes. “It’s interesting to see that certain diseases seem to have a bidirectional association. This means that they can both precede and follow osteoarthritis,” said Dr. Dell’isola. These are just associations, not causation, he stressed, but they might help identify people visiting a doctor for other reasons who may be at risk for developing OA.
“One of the biggest challenges is that once a person develops osteoarthritis, there is not any treatment that can really change their disease,” he added.
Perhaps, “if we can target certain conditions that increase the risk of developing osteoarthritis, and maybe convince people to exercise earlier, or undergo some lifestyle changes early on, we can maybe prevent or delay the onset of the disease,” he suggested.
Results and perspective
Dr. Kamps and associates performed a nested case-control study using data from a large Dutch general practice database. All new cases of OA – which included hip, knee, and other peripheral OA – that were logged between the start of 2006 and the end of 2019 were considered and matched to one to four control subjects of a similar age, sex, and type of general practice. In all, there were just under 80,000 people with newly diagnosed OA who were matched to just over 318,000 controls; the mean age in both groups was 64 years.
Of 58 comorbidities that were assessed, 42 showed a positive association with OA and had odds ratios of 1 or more. The highest associations were found for fibromyalgia (OR, 1.9), obesity (1.8), polymyalgia rheumatica (1.5), spinal disc herniation (1.4), and gout (1.4). A further 13 comorbidities had an OR of about 1, and 3 (all neuropsychiatric conditions – dementia, schizophrenia, and multiple sclerosis) had an OR of below 1.
Dr. Kamps conceded that this type of research has its limitations, the two most important being the coding behavior of the GP and the consulting behavior of patients.
“It’s known that the prevalence of osteoarthritis is underestimated if you only use the diagnostic codes, because some GPs will write the diagnosis in free text or use symptom ICPC codes,” she said.
“We have matched on general practice, so the cases and controls were from the same general practice and therefore we hope that this potential underestimation is balanced and did not affect our odds ratios.”
One of the important outcomes for this research is that it will hopefully be used to inform future clinical practice guidelines, said Dr. Dell’isola.
“Guidelines in osteoarthritis report that is important to screen for comorbidities, but they give no indication on how to deal with the presence of multimorbidity,” he added. Looking at which comorbidities may be associated with OA diagnosis could potentially help to give a bit more of a prescriptive guide on what to look out for.
“Maybe people with a certain disease profile should be screened a bit more often by their doctor. For example, if someone has their blood pressure and diabetes under control, maybe there should be also a bit more attention to their joint health and encouragement to do exercise, not only for being active per se, but maybe also to reinforce their lower limbs,” he explained.
The study was funded by the Foundation for Research in Rheumatology (FOREUM). Dr. Kamps and Dr. Dell’isola, had no conflicts of interest to disclose.
presented at the OARSI 2022 World Congress.
“Some of the associations that we have found are previously known, such as of course, obesity, which is a known risk factor, but also other musculoskeletal conditions, depression, and reflux disease,” said Anne Kamps, an MD and PhD student at Erasmus University Medical Centre in Rotterdam, the Netherlands.
“But there are also some remarkable associations that we have found that are less well known, such as liver cirrhosis, thromboembolic disease, sinusitis, allergy, and migraine,” said Dr. Kamps during her presentation at the conference, sponsored by the Osteoarthritis Research Society International.
The results are “very interesting starting points for future research, because of course, this was an explorative study,” she added. Indeed, is still not known whether the comorbidities found share the same risk factors as OA, or if they have a causal effect and add to development of osteoarthritis.
Comorbidity and OA
One of the issues in managing osteoarthritis so far is that it’s often addressed as one disease, commented Andrea Dell’isola, PT, PhD, a postdoctoral researcher from Lund University who was not involved in the study.
“All of the treatments that have been developed and the treatment process are tailored to take care of one single disease,” he explained. However, “when we look at the characteristics of people with osteoarthritis, we see that roughly 70% of them have other conditions on top of their joint disease.” This high comorbidity rate is significantly higher than in “healthy” people of the same age and sex, he added.
“So, this means that either there is something linked to osteoarthritis that makes people frailer and more likely to develop other diseases, or there may be links between these other diseases, that we often call comorbidities, and osteoarthritis,” Dr. Dell’isola observed.
While the work Dr. Kamps presented looked at the rate of comorbidities that existed before the diagnosis of OA, some of Dr. Dell’isola’s recent research has considered the rate of developing comorbid disease in the years following an OA diagnosis. Associations were found between having hip or knee OA and an increased risk for coexisting depression, cardiovascular diseases, back pain, osteoporosis, and, in the case of knee OA only, diabetes. “It’s interesting to see that certain diseases seem to have a bidirectional association. This means that they can both precede and follow osteoarthritis,” said Dr. Dell’isola. These are just associations, not causation, he stressed, but they might help identify people visiting a doctor for other reasons who may be at risk for developing OA.
“One of the biggest challenges is that once a person develops osteoarthritis, there is not any treatment that can really change their disease,” he added.
Perhaps, “if we can target certain conditions that increase the risk of developing osteoarthritis, and maybe convince people to exercise earlier, or undergo some lifestyle changes early on, we can maybe prevent or delay the onset of the disease,” he suggested.
Results and perspective
Dr. Kamps and associates performed a nested case-control study using data from a large Dutch general practice database. All new cases of OA – which included hip, knee, and other peripheral OA – that were logged between the start of 2006 and the end of 2019 were considered and matched to one to four control subjects of a similar age, sex, and type of general practice. In all, there were just under 80,000 people with newly diagnosed OA who were matched to just over 318,000 controls; the mean age in both groups was 64 years.
Of 58 comorbidities that were assessed, 42 showed a positive association with OA and had odds ratios of 1 or more. The highest associations were found for fibromyalgia (OR, 1.9), obesity (1.8), polymyalgia rheumatica (1.5), spinal disc herniation (1.4), and gout (1.4). A further 13 comorbidities had an OR of about 1, and 3 (all neuropsychiatric conditions – dementia, schizophrenia, and multiple sclerosis) had an OR of below 1.
Dr. Kamps conceded that this type of research has its limitations, the two most important being the coding behavior of the GP and the consulting behavior of patients.
“It’s known that the prevalence of osteoarthritis is underestimated if you only use the diagnostic codes, because some GPs will write the diagnosis in free text or use symptom ICPC codes,” she said.
“We have matched on general practice, so the cases and controls were from the same general practice and therefore we hope that this potential underestimation is balanced and did not affect our odds ratios.”
One of the important outcomes for this research is that it will hopefully be used to inform future clinical practice guidelines, said Dr. Dell’isola.
“Guidelines in osteoarthritis report that is important to screen for comorbidities, but they give no indication on how to deal with the presence of multimorbidity,” he added. Looking at which comorbidities may be associated with OA diagnosis could potentially help to give a bit more of a prescriptive guide on what to look out for.
“Maybe people with a certain disease profile should be screened a bit more often by their doctor. For example, if someone has their blood pressure and diabetes under control, maybe there should be also a bit more attention to their joint health and encouragement to do exercise, not only for being active per se, but maybe also to reinforce their lower limbs,” he explained.
The study was funded by the Foundation for Research in Rheumatology (FOREUM). Dr. Kamps and Dr. Dell’isola, had no conflicts of interest to disclose.
FROM OARSI 2022
Empagliflozin rapidly improves acute heart failure symptoms in hospitalized patients
WASHINGTON – Treatment of patients acutely hospitalized for heart failure with the SGLT2 inhibitor empagliflozin led to a rapid incremental increase in patient well-being, compared with control patients who received placebo, that appeared after 2 weeks on treatment in a secondary analysis from 530 randomized patients in the EMPULSE trial.
To Mikhail N. Kosiborod, MD, a coinvestigator for EMPULSE who presented new analysis at the annual scientific sessions of the American College of Cardiology, the message from the quick response of acutely hospitalized patients to empagliflozin was clear: “Use these medications, SGLT2 [sodium-glucose cotransporter 2] inhibitors, as early as possible. We’ve seen with other medications that if they are not prescribed during hospitalization it’s unlikely to happen post discharge,” said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“To our knowledge, the very early improvement in the Kansas City Cardiomyopathy Questionnaire [KCCQ] score – a well-known predictor of cardiovascular death and heart failure readmissions – that we observed with empagliflozin at 15 days is the first such observation, and if corroborated by future studies would suggest that initiation of SGLT2 inhibitors during hospitalization for acute heart failure may be a tool for improving the quality of hospital-to-home transitions,” wrote Dr. Kosiborod and his associates in the published version of their report that appeared concurrently with his report at the meeting.
“These data really support initiation [of empagliflozin or another SGLT2 inhibitor] in hospital, presuming that the patient has no contraindications,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and designated discussant for the report.
“The fact that the benefit kicks in so early is really important, because there is a bit of a penalty to wait” to start treatment with an agent from the SGLT2-inhibitor class, added Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Health, in Boston.
In hospital creates a teachable moment
Starting treatment when a patient is hospitalized is also important as “a teachable moment,” added Dr. Bhatt in an interview. “A physician can say to a patient ‘take this drug, and it will prevent you from returning to the hospital,’ at a time when it’s more likely to be impactful, compared with when a patient is out of the hospital and feeling okay and adherence will likely be much lower.”
The results Dr. Kosiborod reported on quality-of-life parameters measured with the KCCQ expanded on what he and his coinvestigators first reported in 2021 with the primary results from EMPULSE, which enrolled 530 patients at 118 centers in 15 countries during June 2020–February 2021. The trial randomized patients hospitalized for acute heart failure after a brief period of stabilization regardless of their left ventricular ejection fraction or presence of diabetes to receive a single, daily dose of 10 mg of empagliflozin (Jardiance) or placebo starting a median of 3 days after admission. Enrolled patients averaged about 71 years of age, about two-thirds were men, 45% had diabetes, 32% had left ventricular ejection fraction greater than 40%, and about two-thirds had decompensated chronic heart failure, while a third had acute de novo heart failure.
The primary outcome for EMPULSE was a combined endpoint of “total clinical endpoints” that included all-cause mortality, heart failure events (heart failure hospitalizations, urgent heart failure visits, and unplanned outpatient heart failure visits) or at least a 5-point change from baseline in the KCCQ score. Using a “win ratio” method for analyzing the composite endpoint, the primary analysis showed that treatment with empagliflozin for 90 days boosted the win ratio by a significant 36% relative to placebo (Nature Med. 2022 Mar;28[3]: 568-74).
Benefit independent of baseline symptomatic impairment
Among the new secondary analyses that Dr. Kosiborod reported was a post-hoc calculation that divided the study cohort into tertiles of baseline KCCQ score. The results showed that the degree of improvement for the primary, 90-day outcome of “total clinical benefit” compared with placebo was consistent across all three KCCQ-score tertiles, showing that empagliflozin’s benefit was “independent of symptomatic impairment at baseline,” he said.
The degree of improvement was also similar across all the tested domains of the KCCQ, including the overall summary, clinical summary, the physical limitations, and quality-of-life scores. Average improvement in KCCQ total symptom score 15 days after treatment onset was 5.35 points, compared with control patients. On an individual-patient basis, a change in KCCQ score of 5 points or more was previously shown to represent a clinically meaningful change.
“Treatment of patients with heart failure is geared to making patients live longer and stay out of the hospital. Enabling patients to feel better is an equally important goal of management, but not all treatments for heart failure can do that. These data from EMPULSE show that, in addition to other clinical benefits, patients also feel better on an SGLT2 inhibitor after just 2 weeks,” Dr. Kosiborod said in an interview.
EMPULSE builds on SOLOIST-WHF
EMPULSE is the second trial to show that an SGLT2 inhibitor can safely and effectively treat patients hospitalized for acute heart failure. Previously, results from the SOLOIST-WHF pivotal trial, which enrolled 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure, showed that treatment with an investigational, combined SGLT2 and SGLT1 inhibitor, sotagliflozin, resulted in a significant, 33% relative reduction in the primary outcome compared with placebo after a median 9 months of treatment.
“It’s reassuring to see two different drugs and research groups get essentially the same result, showing that starting an SGLT2 inhibitor is safe and effective in selected patients with no contraindications,” said Dr. Bhatt, who was lead investigator for SOLOIST-WHF.
The accumulating evidence for the safety and value of starting an SGLT2 inhibitor when patients are hospitalized for acute heart failure is making this approach increasingly routine for patients who present with heart failure with reduced ejection fraction at Saint Luke’s-Mid America Heart Institute, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri, Kansas City.
“I think we’ll also gradually start using [an SGLT2 inhibitor] in patients hospitalized with heart failure with preserved ejection fraction [HFpEF],” he added, based on the findings from SOLOIST-WHF and EMPULSE, and also recent evidence showing safety and efficacy of empagliflozin in patients with chronic HFpEF in the EMPEROR-Preserved trial, and for dapagliflozin (Farxiga) in the PRESERVED-HF trial.
Empagliflozin recently received from the U.S. Food and Drug Administration an expanded label indication for treating patients with heart failure with no specification for a level of left ventricular ejection fraction. An outcome trial of dapagliflozin in more than 6,000 patients with HFpEF, DELIVER, is currently ongoing but is expected to report results soon.
“The evidence is already compelling that the benefits outweigh the risk. Results from both SOLOIST-WHF and EMPULSE show that there are no significant safety concerns” when these agents are used in patients with acute heart failure,” Dr. Kosiborod declared.
EMPULSE was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). SOLOIST-WHF was sponsored by Sanofi and Lexicon, the companies that have been developing sotagliflozin. Dr. Kosiborod has been a consultant to and received research funding from Boehringer Ingelheim and Eli Lilly, and he has been a consultant or adviser to or led trials on behalf of numerous other companies. Dr. Bhatt has been an adviser to Boehringer Ingelheim and numerous other companies, and he has received research funding from Sanofi, Lexicon, Boehringer Ingelheim, Eli Lilly, and numerous other companies.
WASHINGTON – Treatment of patients acutely hospitalized for heart failure with the SGLT2 inhibitor empagliflozin led to a rapid incremental increase in patient well-being, compared with control patients who received placebo, that appeared after 2 weeks on treatment in a secondary analysis from 530 randomized patients in the EMPULSE trial.
To Mikhail N. Kosiborod, MD, a coinvestigator for EMPULSE who presented new analysis at the annual scientific sessions of the American College of Cardiology, the message from the quick response of acutely hospitalized patients to empagliflozin was clear: “Use these medications, SGLT2 [sodium-glucose cotransporter 2] inhibitors, as early as possible. We’ve seen with other medications that if they are not prescribed during hospitalization it’s unlikely to happen post discharge,” said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“To our knowledge, the very early improvement in the Kansas City Cardiomyopathy Questionnaire [KCCQ] score – a well-known predictor of cardiovascular death and heart failure readmissions – that we observed with empagliflozin at 15 days is the first such observation, and if corroborated by future studies would suggest that initiation of SGLT2 inhibitors during hospitalization for acute heart failure may be a tool for improving the quality of hospital-to-home transitions,” wrote Dr. Kosiborod and his associates in the published version of their report that appeared concurrently with his report at the meeting.
“These data really support initiation [of empagliflozin or another SGLT2 inhibitor] in hospital, presuming that the patient has no contraindications,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and designated discussant for the report.
“The fact that the benefit kicks in so early is really important, because there is a bit of a penalty to wait” to start treatment with an agent from the SGLT2-inhibitor class, added Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Health, in Boston.
In hospital creates a teachable moment
Starting treatment when a patient is hospitalized is also important as “a teachable moment,” added Dr. Bhatt in an interview. “A physician can say to a patient ‘take this drug, and it will prevent you from returning to the hospital,’ at a time when it’s more likely to be impactful, compared with when a patient is out of the hospital and feeling okay and adherence will likely be much lower.”
The results Dr. Kosiborod reported on quality-of-life parameters measured with the KCCQ expanded on what he and his coinvestigators first reported in 2021 with the primary results from EMPULSE, which enrolled 530 patients at 118 centers in 15 countries during June 2020–February 2021. The trial randomized patients hospitalized for acute heart failure after a brief period of stabilization regardless of their left ventricular ejection fraction or presence of diabetes to receive a single, daily dose of 10 mg of empagliflozin (Jardiance) or placebo starting a median of 3 days after admission. Enrolled patients averaged about 71 years of age, about two-thirds were men, 45% had diabetes, 32% had left ventricular ejection fraction greater than 40%, and about two-thirds had decompensated chronic heart failure, while a third had acute de novo heart failure.
The primary outcome for EMPULSE was a combined endpoint of “total clinical endpoints” that included all-cause mortality, heart failure events (heart failure hospitalizations, urgent heart failure visits, and unplanned outpatient heart failure visits) or at least a 5-point change from baseline in the KCCQ score. Using a “win ratio” method for analyzing the composite endpoint, the primary analysis showed that treatment with empagliflozin for 90 days boosted the win ratio by a significant 36% relative to placebo (Nature Med. 2022 Mar;28[3]: 568-74).
Benefit independent of baseline symptomatic impairment
Among the new secondary analyses that Dr. Kosiborod reported was a post-hoc calculation that divided the study cohort into tertiles of baseline KCCQ score. The results showed that the degree of improvement for the primary, 90-day outcome of “total clinical benefit” compared with placebo was consistent across all three KCCQ-score tertiles, showing that empagliflozin’s benefit was “independent of symptomatic impairment at baseline,” he said.
The degree of improvement was also similar across all the tested domains of the KCCQ, including the overall summary, clinical summary, the physical limitations, and quality-of-life scores. Average improvement in KCCQ total symptom score 15 days after treatment onset was 5.35 points, compared with control patients. On an individual-patient basis, a change in KCCQ score of 5 points or more was previously shown to represent a clinically meaningful change.
“Treatment of patients with heart failure is geared to making patients live longer and stay out of the hospital. Enabling patients to feel better is an equally important goal of management, but not all treatments for heart failure can do that. These data from EMPULSE show that, in addition to other clinical benefits, patients also feel better on an SGLT2 inhibitor after just 2 weeks,” Dr. Kosiborod said in an interview.
EMPULSE builds on SOLOIST-WHF
EMPULSE is the second trial to show that an SGLT2 inhibitor can safely and effectively treat patients hospitalized for acute heart failure. Previously, results from the SOLOIST-WHF pivotal trial, which enrolled 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure, showed that treatment with an investigational, combined SGLT2 and SGLT1 inhibitor, sotagliflozin, resulted in a significant, 33% relative reduction in the primary outcome compared with placebo after a median 9 months of treatment.
“It’s reassuring to see two different drugs and research groups get essentially the same result, showing that starting an SGLT2 inhibitor is safe and effective in selected patients with no contraindications,” said Dr. Bhatt, who was lead investigator for SOLOIST-WHF.
The accumulating evidence for the safety and value of starting an SGLT2 inhibitor when patients are hospitalized for acute heart failure is making this approach increasingly routine for patients who present with heart failure with reduced ejection fraction at Saint Luke’s-Mid America Heart Institute, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri, Kansas City.
“I think we’ll also gradually start using [an SGLT2 inhibitor] in patients hospitalized with heart failure with preserved ejection fraction [HFpEF],” he added, based on the findings from SOLOIST-WHF and EMPULSE, and also recent evidence showing safety and efficacy of empagliflozin in patients with chronic HFpEF in the EMPEROR-Preserved trial, and for dapagliflozin (Farxiga) in the PRESERVED-HF trial.
Empagliflozin recently received from the U.S. Food and Drug Administration an expanded label indication for treating patients with heart failure with no specification for a level of left ventricular ejection fraction. An outcome trial of dapagliflozin in more than 6,000 patients with HFpEF, DELIVER, is currently ongoing but is expected to report results soon.
“The evidence is already compelling that the benefits outweigh the risk. Results from both SOLOIST-WHF and EMPULSE show that there are no significant safety concerns” when these agents are used in patients with acute heart failure,” Dr. Kosiborod declared.
EMPULSE was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). SOLOIST-WHF was sponsored by Sanofi and Lexicon, the companies that have been developing sotagliflozin. Dr. Kosiborod has been a consultant to and received research funding from Boehringer Ingelheim and Eli Lilly, and he has been a consultant or adviser to or led trials on behalf of numerous other companies. Dr. Bhatt has been an adviser to Boehringer Ingelheim and numerous other companies, and he has received research funding from Sanofi, Lexicon, Boehringer Ingelheim, Eli Lilly, and numerous other companies.
WASHINGTON – Treatment of patients acutely hospitalized for heart failure with the SGLT2 inhibitor empagliflozin led to a rapid incremental increase in patient well-being, compared with control patients who received placebo, that appeared after 2 weeks on treatment in a secondary analysis from 530 randomized patients in the EMPULSE trial.
To Mikhail N. Kosiborod, MD, a coinvestigator for EMPULSE who presented new analysis at the annual scientific sessions of the American College of Cardiology, the message from the quick response of acutely hospitalized patients to empagliflozin was clear: “Use these medications, SGLT2 [sodium-glucose cotransporter 2] inhibitors, as early as possible. We’ve seen with other medications that if they are not prescribed during hospitalization it’s unlikely to happen post discharge,” said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“To our knowledge, the very early improvement in the Kansas City Cardiomyopathy Questionnaire [KCCQ] score – a well-known predictor of cardiovascular death and heart failure readmissions – that we observed with empagliflozin at 15 days is the first such observation, and if corroborated by future studies would suggest that initiation of SGLT2 inhibitors during hospitalization for acute heart failure may be a tool for improving the quality of hospital-to-home transitions,” wrote Dr. Kosiborod and his associates in the published version of their report that appeared concurrently with his report at the meeting.
“These data really support initiation [of empagliflozin or another SGLT2 inhibitor] in hospital, presuming that the patient has no contraindications,” commented Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and designated discussant for the report.
“The fact that the benefit kicks in so early is really important, because there is a bit of a penalty to wait” to start treatment with an agent from the SGLT2-inhibitor class, added Dr. Bhatt, who is also executive director of interventional cardiovascular programs at Brigham and Women’s Health, in Boston.
In hospital creates a teachable moment
Starting treatment when a patient is hospitalized is also important as “a teachable moment,” added Dr. Bhatt in an interview. “A physician can say to a patient ‘take this drug, and it will prevent you from returning to the hospital,’ at a time when it’s more likely to be impactful, compared with when a patient is out of the hospital and feeling okay and adherence will likely be much lower.”
The results Dr. Kosiborod reported on quality-of-life parameters measured with the KCCQ expanded on what he and his coinvestigators first reported in 2021 with the primary results from EMPULSE, which enrolled 530 patients at 118 centers in 15 countries during June 2020–February 2021. The trial randomized patients hospitalized for acute heart failure after a brief period of stabilization regardless of their left ventricular ejection fraction or presence of diabetes to receive a single, daily dose of 10 mg of empagliflozin (Jardiance) or placebo starting a median of 3 days after admission. Enrolled patients averaged about 71 years of age, about two-thirds were men, 45% had diabetes, 32% had left ventricular ejection fraction greater than 40%, and about two-thirds had decompensated chronic heart failure, while a third had acute de novo heart failure.
The primary outcome for EMPULSE was a combined endpoint of “total clinical endpoints” that included all-cause mortality, heart failure events (heart failure hospitalizations, urgent heart failure visits, and unplanned outpatient heart failure visits) or at least a 5-point change from baseline in the KCCQ score. Using a “win ratio” method for analyzing the composite endpoint, the primary analysis showed that treatment with empagliflozin for 90 days boosted the win ratio by a significant 36% relative to placebo (Nature Med. 2022 Mar;28[3]: 568-74).
Benefit independent of baseline symptomatic impairment
Among the new secondary analyses that Dr. Kosiborod reported was a post-hoc calculation that divided the study cohort into tertiles of baseline KCCQ score. The results showed that the degree of improvement for the primary, 90-day outcome of “total clinical benefit” compared with placebo was consistent across all three KCCQ-score tertiles, showing that empagliflozin’s benefit was “independent of symptomatic impairment at baseline,” he said.
The degree of improvement was also similar across all the tested domains of the KCCQ, including the overall summary, clinical summary, the physical limitations, and quality-of-life scores. Average improvement in KCCQ total symptom score 15 days after treatment onset was 5.35 points, compared with control patients. On an individual-patient basis, a change in KCCQ score of 5 points or more was previously shown to represent a clinically meaningful change.
“Treatment of patients with heart failure is geared to making patients live longer and stay out of the hospital. Enabling patients to feel better is an equally important goal of management, but not all treatments for heart failure can do that. These data from EMPULSE show that, in addition to other clinical benefits, patients also feel better on an SGLT2 inhibitor after just 2 weeks,” Dr. Kosiborod said in an interview.
EMPULSE builds on SOLOIST-WHF
EMPULSE is the second trial to show that an SGLT2 inhibitor can safely and effectively treat patients hospitalized for acute heart failure. Previously, results from the SOLOIST-WHF pivotal trial, which enrolled 1,222 patients with type 2 diabetes recently hospitalized for worsening heart failure, showed that treatment with an investigational, combined SGLT2 and SGLT1 inhibitor, sotagliflozin, resulted in a significant, 33% relative reduction in the primary outcome compared with placebo after a median 9 months of treatment.
“It’s reassuring to see two different drugs and research groups get essentially the same result, showing that starting an SGLT2 inhibitor is safe and effective in selected patients with no contraindications,” said Dr. Bhatt, who was lead investigator for SOLOIST-WHF.
The accumulating evidence for the safety and value of starting an SGLT2 inhibitor when patients are hospitalized for acute heart failure is making this approach increasingly routine for patients who present with heart failure with reduced ejection fraction at Saint Luke’s-Mid America Heart Institute, said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri, Kansas City.
“I think we’ll also gradually start using [an SGLT2 inhibitor] in patients hospitalized with heart failure with preserved ejection fraction [HFpEF],” he added, based on the findings from SOLOIST-WHF and EMPULSE, and also recent evidence showing safety and efficacy of empagliflozin in patients with chronic HFpEF in the EMPEROR-Preserved trial, and for dapagliflozin (Farxiga) in the PRESERVED-HF trial.
Empagliflozin recently received from the U.S. Food and Drug Administration an expanded label indication for treating patients with heart failure with no specification for a level of left ventricular ejection fraction. An outcome trial of dapagliflozin in more than 6,000 patients with HFpEF, DELIVER, is currently ongoing but is expected to report results soon.
“The evidence is already compelling that the benefits outweigh the risk. Results from both SOLOIST-WHF and EMPULSE show that there are no significant safety concerns” when these agents are used in patients with acute heart failure,” Dr. Kosiborod declared.
EMPULSE was sponsored by Boehringer Ingelheim and Eli Lilly, the companies that jointly market empagliflozin (Jardiance). SOLOIST-WHF was sponsored by Sanofi and Lexicon, the companies that have been developing sotagliflozin. Dr. Kosiborod has been a consultant to and received research funding from Boehringer Ingelheim and Eli Lilly, and he has been a consultant or adviser to or led trials on behalf of numerous other companies. Dr. Bhatt has been an adviser to Boehringer Ingelheim and numerous other companies, and he has received research funding from Sanofi, Lexicon, Boehringer Ingelheim, Eli Lilly, and numerous other companies.
AT ACC 2022
Are all medical errors now crimes? The Nurse Vaught verdict
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome! I’m Dr Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have a distinguished panel joining us to discuss an important legal decision resulting in a criminal conviction, involving a medical error due to administration of the wrong medication by a critical care nurse that led to a patient’s death.
Joining us to discuss this case is Dr. Megan Ranney, professor of emergency medicine and the academic dean at Brown University School of Public Health. Also joining us is Dr. Jane Barnsteiner, emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. Welcome to both of you.
Jane Barnsteiner, PhD, RN: Thank you.
Megan L. Ranney, MD, MPH: Thank you. It’s a joy to be with you.
Dr. Glatter: Let’s discuss this very tragic case involving RaDonda Vaught, who was an ICU nurse who was recently convicted in Tennessee of criminally negligent homicide and gross neglect of an impaired adult. She accidentally administered a paralytic medication, vecuronium, instead of a sedative, Versed, which was ordered to sedate a 75-year-old patient who had a brain bleed and TBI. She was scheduled to have a PET scan. After receiving the wrong medication and not really being monitored in any true way, just being in the care of an MRI tech, she suffered cardiac arrest and subsequently died.
Dr. Ranney, I want to begin with you. I saw on Twitter that you had written something that really stuck with me. I’ll quote you. “A culture of safety is one in which the system that allowed the mistake to happen is changed, not one in which the individual is scapegoated. And a culture of safety correlates with better patient outcomes that we know. This verdict is the opposite.”
I’ll let you explain from here. The system issue is the medication dispensing cabinet, in my mind, and there was a medication override. The question is, how was this override allowed to occur in the first place?
Dr. Ranney: My goodness, overrides happen every single day across this country, dozens of times a day in any particular shift. I would think of the system as being much bigger than just the Pyxis or that kind of automated dispensing cabinet, but around the larger system of the verbal orders, the time pressures that the nurse is under, the fact that the nurses are with a trainee, the fact that they’re being asked to operate outside of their normal environment by going down to MRI. There’s a series of issues.
Just as we thought about the Swiss cheese model for COVID-19, that model originated when we talked about patient safety and medical errors. It is a Swiss cheese of circumstances that allows this type of tragic error to occur.
Many of us have worked for years on trying to change the system from one of punishing people, changing it from that punitive system, to rather a system where we can do root-cause analysis, allow people to disclose errors, and allow us to inquire as to what are those series of Swiss cheese holes that allowed this mistake or any other to happen.
When you punish people, you lead them to hide their mistakes instead of allowing them to disclose them and allowing that important inquiry to happen. That’s why this is just so harmful to that culture of safety that so many of us are trying to create.
Dr. Glatter: It’s a chilling verdict in so many ways. I’m right on the same page with you, having worked for so long in the emergency department and seeing nurses that are overtaxed, overburdened, but also on patient floors. This goes to an ICU-type environment where this woman was having a nonemergent head scan and required some sedation.
The question I want to get to is how the system allowed the nurse to dispense this medication —though she was distracted, she’ll admit that. Jane, I want to get to you on this. How can we avoid this? What are the system checks that can be done in some fashion to make this safer and to avoid this tragic error?
Dr. Barnsteiner: First of all, I would say that you do not put in a major change, as they were doing with their EPIC system, as a big bank where you do the change through the entire organization. You do it in one area where you get the whole system smoothed out and all the errors taken care of so that you’re not having a problem like they had through their entire organization, which required overrides multiple times a day.
One of the things that’s been recommended is that these systems, like the Pyxis system, require the first five letters of a medication to be entered into the system so that when you have multiple medications where the first two letters are the same, the chances of pulling out the wrong medication are much smaller.
There’s a question of whether this medication, vecuronium, should have even been in this machine. You can have high-alert medications like this in baggies that have written on the front of the bag, “This is a high-alert medication. It requires two independent double checks.” These are all the things that will help alert the fatigued or distracted nurse or physician and will make things safer. There are many things that can be put into place.
Dr. Glatter: It’s almost like a hard stop. This is a different class of medication. Even if the nurse had a lapse and didn’t realize that, there should have been a hard stop asking whether you want this class. A sedative and a paralytic are two very different medications.
I’m not trying to assign any blame here. I’m just trying to look at mechanics of what happened and how we can put in place methods to avoid these types of errors where a system clearly is overtaxed and overburdened. Is it an artificial intelligence alert? Is it a pharmacy alert that goes out? Is it a Vocera message that gets triggered? It’s something to stop the nurse from doing something where they know better.
She’s used Versed before, apparently, and knows it’s a liquid and doesn’t have to be reconstituted. In my mind, as a practicing doctor for a long time, I see this and I see how it can happen. There are ways I think we can address it. Megan, I want to bring you into this and get your viewpoint.
Dr. Ranney: We’re working in an environment right now — and obviously, this happened pre-COVID — where medicines are constantly in short supply and we’re constantly dealing with substitutions of one for another. This has worsened during COVID, but it existed in the pre-COVID era as well. We’d have time periods where, like today, we’re out of D50 and we have to use D10, or we have a different formulation of a common antibiotic.
I could totally imagine that this nurse had been exposed to multiple medication substitution and so they were rushing; they thought, well, they just put one thing in instead of another and didn’t make that kind of cognitive connection.
What we know so well from our studies of human factors, engineering, and the way that systems work is that when someone is cognitively overloaded and constantly having to think outside the box and make decisions, particularly when they’re exposed to a new system for ordering medicine, there’s only so much that the brain can do at a time. This person was set up for this type of error.
Again, not to say that they didn’t do something wrong. That’s why we have a civil system. That’s why we have licensing. That’s why we have malpractice. To call this a criminal error when they were working within a system that had all these other problems where they were constantly having to make do for system failures, it’s almost inevitable that at some point something really horrible happened.
I’m sorry that it was this nurse, and how horrible for the patient and the family. I’m not excusing that. You can totally imagine, as a practicing physician, nurse, or anyone else in the healthcare system, how this happened.
Dr. Barnsteiner: The other part of it was that they did not have in place, at this time, the barcoding system in this particular patient area. What nurses are used to doing is when they have to pull a medication, they’re using the barcoding system to coordinate with what’s in the electronic health record, with the medication, and with the person’s ID band.
Those are all well-known safety checks that obviously were used to being used by this nurse in the critical care unit but that weren’t available in this MRI area. That is something that absolutely is a system failure. Those kinds of safety systems have to be available at any place in a health system where medications are being delivered.
Dr. Glatter: I think that’s an important point. Here, we have a technology that can supersede the ability of a human to make a mistake, and to have that in place is very critical. I want to go back to the idea of medical malpractice vs homicide charges.
Megan, you made a point of this. This nurse is now an example of someone who went to trial and was convicted, and it could have a chilling effect on healthcare providers. Pre-COVID, post-COVID, it is just chilling. It makes people want to leave the field. It causes PTSD. The psychiatric downstream effects of such an error are just immense.
I don’t know how the district attorney went for criminal charges here. I’m not an attorney and we don’t have a legal expert with us. For this to have happened is just setting precedent that it’s okay to have the effect of making so many people leave the field.
Dr. Ranney: I’m not a lawyer, but I’ve certainly been on the front lines, not only for the past 2 years during COVID but for almost 20 years prior to that. I will say that these types of errors are never-events that sit with our colleagues and friends for their entire career. No one goes into medicine intending to hurt someone. The system fails us and fails the patient.
There are certainly examples of intentional harm, and those people deserve to be prosecuted. This type of thing where a system let them down, again, should require an inquiry of the system. Don’t punish the individuals to the point of putting them in jail.
I think about my last few months working in the emergency department and what my nurses, in particular, have said to me. They worry that they’re going to lose their license and their ability to practice because of the horrific circumstances that we’ve been working in — the understaffing, the lack of access to standard medications, the long wait times, and on and on. They’re not able to take care of patients the way that they’ve been taught to do.
They’re worried already about the downstream effects on their sense of self, as well as on their ability to maintain their livelihood. When you put something like this on top of it, where again, an unintentional error that was potentiated by a somewhat broken system or by a series of Swiss cheese holes that just happened to line up, what message does that send to my nursing colleagues who have stayed on the front lines and who know that they have not been able to provide the standard of care that they’re used to?
Dr. Barnsteiner: On Friday, I did a program on fair and just culture with three health systems and a university school of nursing. Already, some of the faculty reported that students are talking about transferring to another major outside of the School of Nursing because of their worry about this particular guilty verdict.
The other thing is that we already have a tremendous shortage of nurses. We’ve seen many people leave the profession or retire in the past couple of years, and this is only going to compound it further. It is a sobering message that the public can’t afford to have, actually, because this will impact the quality of care and the safety of care that can be delivered to people and families as a result of not having sufficient numbers of professionals to deliver care.
Dr. Glatter: That’s such an important point. In any high-reliability organization, a culture of safety is key. There are tenets we try to adhere to. When we have people leaving the field after seeing a case like this, it’s chilling. We have to re-educate the public and we need to have a realignment of how errors are handled.
This is just the beginning. Her sentencing is going to be in about a month, and we’ll see what happens on reckless homicide charges and neglect. I think there’s going to be a follow-up to this and we’re going to need to discuss this more.
I just wanted to get a couple of takeaways for our audience to just really sear in the brain what we can learn from such an event.
Dr. Ranney: The big takeaway, to me, is the importance of us both continuing to use our voices and working across professional boundaries to help to create this culture of safety, one in which we all feel safe and supported in advocating for systems that work for us. We cannot ask nurses, respiratory technicians, radiology technicians, physicians, or anyone else within the healthcare system to work unsupported, and we have to recognize the degree to which we are all interdependent. My biggest takeaway is for us to use our voices together.
Dr. Barnsteiner: The takeaway that I would have from this, and what I’m working with a number of health systems on, is to have the chair of the board, the CEO of the hospital, the chief medical officer, and the chief nursing officer together promulgate a statement that is sent out to all employees to discuss this verdict and to say what they’re doing to promote a high-reliability organization and a fair and just culture. They should also ask for open conversation and for employees to let the top leadership know any concerns that they have about vulnerabilities in the system. It’s extremely important right now with this verdict that the leaders in healthcare settings, as well as in education settings, let people know what they’ll be doing to protect their employees.
Dr. Glatter: Jane and Megan, I want to thank you so much for such an important discussion that was very informative. I think there’s going to be a follow-up to this that’ll be very, very important. Thanks again.
Robert D. Glatter, MD, is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Glatter has disclosed no relevant financial relationships.
Megan Ranney, MD, MPH, is professor of emergency medicine and the academic dean at Brown University School of Public Health in Providence, Rhode Island. She is the director and founder of the Brown Emergency Digital Health Innovation (eDHI) program. She is also chief research officer for the American Foundation for Firearm Injury Reduction in Medicine, the country’s only nonprofit committed to reducing firearm injury through the public health approach, and a founding partner of GetUsPPE.org, dedicated to matching donors to health systems in need of protective equipment. Dr. Ranney has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for: Medscape; Merck.
Jane Barnsteiner, PhD, RN, is an emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. In addition to her teaching responsibilities, she was director of translational research at the Hospital of the University of Pennsylvania. Jane was one of the developers of the Quality and Safety in Education for Nurses (QSEN) initiative and is co-editor of Quality and Safety in Nursing: A Competency Based Approach to Improving Outcomes, published by Wiley. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome! I’m Dr Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have a distinguished panel joining us to discuss an important legal decision resulting in a criminal conviction, involving a medical error due to administration of the wrong medication by a critical care nurse that led to a patient’s death.
Joining us to discuss this case is Dr. Megan Ranney, professor of emergency medicine and the academic dean at Brown University School of Public Health. Also joining us is Dr. Jane Barnsteiner, emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. Welcome to both of you.
Jane Barnsteiner, PhD, RN: Thank you.
Megan L. Ranney, MD, MPH: Thank you. It’s a joy to be with you.
Dr. Glatter: Let’s discuss this very tragic case involving RaDonda Vaught, who was an ICU nurse who was recently convicted in Tennessee of criminally negligent homicide and gross neglect of an impaired adult. She accidentally administered a paralytic medication, vecuronium, instead of a sedative, Versed, which was ordered to sedate a 75-year-old patient who had a brain bleed and TBI. She was scheduled to have a PET scan. After receiving the wrong medication and not really being monitored in any true way, just being in the care of an MRI tech, she suffered cardiac arrest and subsequently died.
Dr. Ranney, I want to begin with you. I saw on Twitter that you had written something that really stuck with me. I’ll quote you. “A culture of safety is one in which the system that allowed the mistake to happen is changed, not one in which the individual is scapegoated. And a culture of safety correlates with better patient outcomes that we know. This verdict is the opposite.”
I’ll let you explain from here. The system issue is the medication dispensing cabinet, in my mind, and there was a medication override. The question is, how was this override allowed to occur in the first place?
Dr. Ranney: My goodness, overrides happen every single day across this country, dozens of times a day in any particular shift. I would think of the system as being much bigger than just the Pyxis or that kind of automated dispensing cabinet, but around the larger system of the verbal orders, the time pressures that the nurse is under, the fact that the nurses are with a trainee, the fact that they’re being asked to operate outside of their normal environment by going down to MRI. There’s a series of issues.
Just as we thought about the Swiss cheese model for COVID-19, that model originated when we talked about patient safety and medical errors. It is a Swiss cheese of circumstances that allows this type of tragic error to occur.
Many of us have worked for years on trying to change the system from one of punishing people, changing it from that punitive system, to rather a system where we can do root-cause analysis, allow people to disclose errors, and allow us to inquire as to what are those series of Swiss cheese holes that allowed this mistake or any other to happen.
When you punish people, you lead them to hide their mistakes instead of allowing them to disclose them and allowing that important inquiry to happen. That’s why this is just so harmful to that culture of safety that so many of us are trying to create.
Dr. Glatter: It’s a chilling verdict in so many ways. I’m right on the same page with you, having worked for so long in the emergency department and seeing nurses that are overtaxed, overburdened, but also on patient floors. This goes to an ICU-type environment where this woman was having a nonemergent head scan and required some sedation.
The question I want to get to is how the system allowed the nurse to dispense this medication —though she was distracted, she’ll admit that. Jane, I want to get to you on this. How can we avoid this? What are the system checks that can be done in some fashion to make this safer and to avoid this tragic error?
Dr. Barnsteiner: First of all, I would say that you do not put in a major change, as they were doing with their EPIC system, as a big bank where you do the change through the entire organization. You do it in one area where you get the whole system smoothed out and all the errors taken care of so that you’re not having a problem like they had through their entire organization, which required overrides multiple times a day.
One of the things that’s been recommended is that these systems, like the Pyxis system, require the first five letters of a medication to be entered into the system so that when you have multiple medications where the first two letters are the same, the chances of pulling out the wrong medication are much smaller.
There’s a question of whether this medication, vecuronium, should have even been in this machine. You can have high-alert medications like this in baggies that have written on the front of the bag, “This is a high-alert medication. It requires two independent double checks.” These are all the things that will help alert the fatigued or distracted nurse or physician and will make things safer. There are many things that can be put into place.
Dr. Glatter: It’s almost like a hard stop. This is a different class of medication. Even if the nurse had a lapse and didn’t realize that, there should have been a hard stop asking whether you want this class. A sedative and a paralytic are two very different medications.
I’m not trying to assign any blame here. I’m just trying to look at mechanics of what happened and how we can put in place methods to avoid these types of errors where a system clearly is overtaxed and overburdened. Is it an artificial intelligence alert? Is it a pharmacy alert that goes out? Is it a Vocera message that gets triggered? It’s something to stop the nurse from doing something where they know better.
She’s used Versed before, apparently, and knows it’s a liquid and doesn’t have to be reconstituted. In my mind, as a practicing doctor for a long time, I see this and I see how it can happen. There are ways I think we can address it. Megan, I want to bring you into this and get your viewpoint.
Dr. Ranney: We’re working in an environment right now — and obviously, this happened pre-COVID — where medicines are constantly in short supply and we’re constantly dealing with substitutions of one for another. This has worsened during COVID, but it existed in the pre-COVID era as well. We’d have time periods where, like today, we’re out of D50 and we have to use D10, or we have a different formulation of a common antibiotic.
I could totally imagine that this nurse had been exposed to multiple medication substitution and so they were rushing; they thought, well, they just put one thing in instead of another and didn’t make that kind of cognitive connection.
What we know so well from our studies of human factors, engineering, and the way that systems work is that when someone is cognitively overloaded and constantly having to think outside the box and make decisions, particularly when they’re exposed to a new system for ordering medicine, there’s only so much that the brain can do at a time. This person was set up for this type of error.
Again, not to say that they didn’t do something wrong. That’s why we have a civil system. That’s why we have licensing. That’s why we have malpractice. To call this a criminal error when they were working within a system that had all these other problems where they were constantly having to make do for system failures, it’s almost inevitable that at some point something really horrible happened.
I’m sorry that it was this nurse, and how horrible for the patient and the family. I’m not excusing that. You can totally imagine, as a practicing physician, nurse, or anyone else in the healthcare system, how this happened.
Dr. Barnsteiner: The other part of it was that they did not have in place, at this time, the barcoding system in this particular patient area. What nurses are used to doing is when they have to pull a medication, they’re using the barcoding system to coordinate with what’s in the electronic health record, with the medication, and with the person’s ID band.
Those are all well-known safety checks that obviously were used to being used by this nurse in the critical care unit but that weren’t available in this MRI area. That is something that absolutely is a system failure. Those kinds of safety systems have to be available at any place in a health system where medications are being delivered.
Dr. Glatter: I think that’s an important point. Here, we have a technology that can supersede the ability of a human to make a mistake, and to have that in place is very critical. I want to go back to the idea of medical malpractice vs homicide charges.
Megan, you made a point of this. This nurse is now an example of someone who went to trial and was convicted, and it could have a chilling effect on healthcare providers. Pre-COVID, post-COVID, it is just chilling. It makes people want to leave the field. It causes PTSD. The psychiatric downstream effects of such an error are just immense.
I don’t know how the district attorney went for criminal charges here. I’m not an attorney and we don’t have a legal expert with us. For this to have happened is just setting precedent that it’s okay to have the effect of making so many people leave the field.
Dr. Ranney: I’m not a lawyer, but I’ve certainly been on the front lines, not only for the past 2 years during COVID but for almost 20 years prior to that. I will say that these types of errors are never-events that sit with our colleagues and friends for their entire career. No one goes into medicine intending to hurt someone. The system fails us and fails the patient.
There are certainly examples of intentional harm, and those people deserve to be prosecuted. This type of thing where a system let them down, again, should require an inquiry of the system. Don’t punish the individuals to the point of putting them in jail.
I think about my last few months working in the emergency department and what my nurses, in particular, have said to me. They worry that they’re going to lose their license and their ability to practice because of the horrific circumstances that we’ve been working in — the understaffing, the lack of access to standard medications, the long wait times, and on and on. They’re not able to take care of patients the way that they’ve been taught to do.
They’re worried already about the downstream effects on their sense of self, as well as on their ability to maintain their livelihood. When you put something like this on top of it, where again, an unintentional error that was potentiated by a somewhat broken system or by a series of Swiss cheese holes that just happened to line up, what message does that send to my nursing colleagues who have stayed on the front lines and who know that they have not been able to provide the standard of care that they’re used to?
Dr. Barnsteiner: On Friday, I did a program on fair and just culture with three health systems and a university school of nursing. Already, some of the faculty reported that students are talking about transferring to another major outside of the School of Nursing because of their worry about this particular guilty verdict.
The other thing is that we already have a tremendous shortage of nurses. We’ve seen many people leave the profession or retire in the past couple of years, and this is only going to compound it further. It is a sobering message that the public can’t afford to have, actually, because this will impact the quality of care and the safety of care that can be delivered to people and families as a result of not having sufficient numbers of professionals to deliver care.
Dr. Glatter: That’s such an important point. In any high-reliability organization, a culture of safety is key. There are tenets we try to adhere to. When we have people leaving the field after seeing a case like this, it’s chilling. We have to re-educate the public and we need to have a realignment of how errors are handled.
This is just the beginning. Her sentencing is going to be in about a month, and we’ll see what happens on reckless homicide charges and neglect. I think there’s going to be a follow-up to this and we’re going to need to discuss this more.
I just wanted to get a couple of takeaways for our audience to just really sear in the brain what we can learn from such an event.
Dr. Ranney: The big takeaway, to me, is the importance of us both continuing to use our voices and working across professional boundaries to help to create this culture of safety, one in which we all feel safe and supported in advocating for systems that work for us. We cannot ask nurses, respiratory technicians, radiology technicians, physicians, or anyone else within the healthcare system to work unsupported, and we have to recognize the degree to which we are all interdependent. My biggest takeaway is for us to use our voices together.
Dr. Barnsteiner: The takeaway that I would have from this, and what I’m working with a number of health systems on, is to have the chair of the board, the CEO of the hospital, the chief medical officer, and the chief nursing officer together promulgate a statement that is sent out to all employees to discuss this verdict and to say what they’re doing to promote a high-reliability organization and a fair and just culture. They should also ask for open conversation and for employees to let the top leadership know any concerns that they have about vulnerabilities in the system. It’s extremely important right now with this verdict that the leaders in healthcare settings, as well as in education settings, let people know what they’ll be doing to protect their employees.
Dr. Glatter: Jane and Megan, I want to thank you so much for such an important discussion that was very informative. I think there’s going to be a follow-up to this that’ll be very, very important. Thanks again.
Robert D. Glatter, MD, is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Glatter has disclosed no relevant financial relationships.
Megan Ranney, MD, MPH, is professor of emergency medicine and the academic dean at Brown University School of Public Health in Providence, Rhode Island. She is the director and founder of the Brown Emergency Digital Health Innovation (eDHI) program. She is also chief research officer for the American Foundation for Firearm Injury Reduction in Medicine, the country’s only nonprofit committed to reducing firearm injury through the public health approach, and a founding partner of GetUsPPE.org, dedicated to matching donors to health systems in need of protective equipment. Dr. Ranney has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for: Medscape; Merck.
Jane Barnsteiner, PhD, RN, is an emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. In addition to her teaching responsibilities, she was director of translational research at the Hospital of the University of Pennsylvania. Jane was one of the developers of the Quality and Safety in Education for Nurses (QSEN) initiative and is co-editor of Quality and Safety in Nursing: A Competency Based Approach to Improving Outcomes, published by Wiley. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome! I’m Dr Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have a distinguished panel joining us to discuss an important legal decision resulting in a criminal conviction, involving a medical error due to administration of the wrong medication by a critical care nurse that led to a patient’s death.
Joining us to discuss this case is Dr. Megan Ranney, professor of emergency medicine and the academic dean at Brown University School of Public Health. Also joining us is Dr. Jane Barnsteiner, emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. Welcome to both of you.
Jane Barnsteiner, PhD, RN: Thank you.
Megan L. Ranney, MD, MPH: Thank you. It’s a joy to be with you.
Dr. Glatter: Let’s discuss this very tragic case involving RaDonda Vaught, who was an ICU nurse who was recently convicted in Tennessee of criminally negligent homicide and gross neglect of an impaired adult. She accidentally administered a paralytic medication, vecuronium, instead of a sedative, Versed, which was ordered to sedate a 75-year-old patient who had a brain bleed and TBI. She was scheduled to have a PET scan. After receiving the wrong medication and not really being monitored in any true way, just being in the care of an MRI tech, she suffered cardiac arrest and subsequently died.
Dr. Ranney, I want to begin with you. I saw on Twitter that you had written something that really stuck with me. I’ll quote you. “A culture of safety is one in which the system that allowed the mistake to happen is changed, not one in which the individual is scapegoated. And a culture of safety correlates with better patient outcomes that we know. This verdict is the opposite.”
I’ll let you explain from here. The system issue is the medication dispensing cabinet, in my mind, and there was a medication override. The question is, how was this override allowed to occur in the first place?
Dr. Ranney: My goodness, overrides happen every single day across this country, dozens of times a day in any particular shift. I would think of the system as being much bigger than just the Pyxis or that kind of automated dispensing cabinet, but around the larger system of the verbal orders, the time pressures that the nurse is under, the fact that the nurses are with a trainee, the fact that they’re being asked to operate outside of their normal environment by going down to MRI. There’s a series of issues.
Just as we thought about the Swiss cheese model for COVID-19, that model originated when we talked about patient safety and medical errors. It is a Swiss cheese of circumstances that allows this type of tragic error to occur.
Many of us have worked for years on trying to change the system from one of punishing people, changing it from that punitive system, to rather a system where we can do root-cause analysis, allow people to disclose errors, and allow us to inquire as to what are those series of Swiss cheese holes that allowed this mistake or any other to happen.
When you punish people, you lead them to hide their mistakes instead of allowing them to disclose them and allowing that important inquiry to happen. That’s why this is just so harmful to that culture of safety that so many of us are trying to create.
Dr. Glatter: It’s a chilling verdict in so many ways. I’m right on the same page with you, having worked for so long in the emergency department and seeing nurses that are overtaxed, overburdened, but also on patient floors. This goes to an ICU-type environment where this woman was having a nonemergent head scan and required some sedation.
The question I want to get to is how the system allowed the nurse to dispense this medication —though she was distracted, she’ll admit that. Jane, I want to get to you on this. How can we avoid this? What are the system checks that can be done in some fashion to make this safer and to avoid this tragic error?
Dr. Barnsteiner: First of all, I would say that you do not put in a major change, as they were doing with their EPIC system, as a big bank where you do the change through the entire organization. You do it in one area where you get the whole system smoothed out and all the errors taken care of so that you’re not having a problem like they had through their entire organization, which required overrides multiple times a day.
One of the things that’s been recommended is that these systems, like the Pyxis system, require the first five letters of a medication to be entered into the system so that when you have multiple medications where the first two letters are the same, the chances of pulling out the wrong medication are much smaller.
There’s a question of whether this medication, vecuronium, should have even been in this machine. You can have high-alert medications like this in baggies that have written on the front of the bag, “This is a high-alert medication. It requires two independent double checks.” These are all the things that will help alert the fatigued or distracted nurse or physician and will make things safer. There are many things that can be put into place.
Dr. Glatter: It’s almost like a hard stop. This is a different class of medication. Even if the nurse had a lapse and didn’t realize that, there should have been a hard stop asking whether you want this class. A sedative and a paralytic are two very different medications.
I’m not trying to assign any blame here. I’m just trying to look at mechanics of what happened and how we can put in place methods to avoid these types of errors where a system clearly is overtaxed and overburdened. Is it an artificial intelligence alert? Is it a pharmacy alert that goes out? Is it a Vocera message that gets triggered? It’s something to stop the nurse from doing something where they know better.
She’s used Versed before, apparently, and knows it’s a liquid and doesn’t have to be reconstituted. In my mind, as a practicing doctor for a long time, I see this and I see how it can happen. There are ways I think we can address it. Megan, I want to bring you into this and get your viewpoint.
Dr. Ranney: We’re working in an environment right now — and obviously, this happened pre-COVID — where medicines are constantly in short supply and we’re constantly dealing with substitutions of one for another. This has worsened during COVID, but it existed in the pre-COVID era as well. We’d have time periods where, like today, we’re out of D50 and we have to use D10, or we have a different formulation of a common antibiotic.
I could totally imagine that this nurse had been exposed to multiple medication substitution and so they were rushing; they thought, well, they just put one thing in instead of another and didn’t make that kind of cognitive connection.
What we know so well from our studies of human factors, engineering, and the way that systems work is that when someone is cognitively overloaded and constantly having to think outside the box and make decisions, particularly when they’re exposed to a new system for ordering medicine, there’s only so much that the brain can do at a time. This person was set up for this type of error.
Again, not to say that they didn’t do something wrong. That’s why we have a civil system. That’s why we have licensing. That’s why we have malpractice. To call this a criminal error when they were working within a system that had all these other problems where they were constantly having to make do for system failures, it’s almost inevitable that at some point something really horrible happened.
I’m sorry that it was this nurse, and how horrible for the patient and the family. I’m not excusing that. You can totally imagine, as a practicing physician, nurse, or anyone else in the healthcare system, how this happened.
Dr. Barnsteiner: The other part of it was that they did not have in place, at this time, the barcoding system in this particular patient area. What nurses are used to doing is when they have to pull a medication, they’re using the barcoding system to coordinate with what’s in the electronic health record, with the medication, and with the person’s ID band.
Those are all well-known safety checks that obviously were used to being used by this nurse in the critical care unit but that weren’t available in this MRI area. That is something that absolutely is a system failure. Those kinds of safety systems have to be available at any place in a health system where medications are being delivered.
Dr. Glatter: I think that’s an important point. Here, we have a technology that can supersede the ability of a human to make a mistake, and to have that in place is very critical. I want to go back to the idea of medical malpractice vs homicide charges.
Megan, you made a point of this. This nurse is now an example of someone who went to trial and was convicted, and it could have a chilling effect on healthcare providers. Pre-COVID, post-COVID, it is just chilling. It makes people want to leave the field. It causes PTSD. The psychiatric downstream effects of such an error are just immense.
I don’t know how the district attorney went for criminal charges here. I’m not an attorney and we don’t have a legal expert with us. For this to have happened is just setting precedent that it’s okay to have the effect of making so many people leave the field.
Dr. Ranney: I’m not a lawyer, but I’ve certainly been on the front lines, not only for the past 2 years during COVID but for almost 20 years prior to that. I will say that these types of errors are never-events that sit with our colleagues and friends for their entire career. No one goes into medicine intending to hurt someone. The system fails us and fails the patient.
There are certainly examples of intentional harm, and those people deserve to be prosecuted. This type of thing where a system let them down, again, should require an inquiry of the system. Don’t punish the individuals to the point of putting them in jail.
I think about my last few months working in the emergency department and what my nurses, in particular, have said to me. They worry that they’re going to lose their license and their ability to practice because of the horrific circumstances that we’ve been working in — the understaffing, the lack of access to standard medications, the long wait times, and on and on. They’re not able to take care of patients the way that they’ve been taught to do.
They’re worried already about the downstream effects on their sense of self, as well as on their ability to maintain their livelihood. When you put something like this on top of it, where again, an unintentional error that was potentiated by a somewhat broken system or by a series of Swiss cheese holes that just happened to line up, what message does that send to my nursing colleagues who have stayed on the front lines and who know that they have not been able to provide the standard of care that they’re used to?
Dr. Barnsteiner: On Friday, I did a program on fair and just culture with three health systems and a university school of nursing. Already, some of the faculty reported that students are talking about transferring to another major outside of the School of Nursing because of their worry about this particular guilty verdict.
The other thing is that we already have a tremendous shortage of nurses. We’ve seen many people leave the profession or retire in the past couple of years, and this is only going to compound it further. It is a sobering message that the public can’t afford to have, actually, because this will impact the quality of care and the safety of care that can be delivered to people and families as a result of not having sufficient numbers of professionals to deliver care.
Dr. Glatter: That’s such an important point. In any high-reliability organization, a culture of safety is key. There are tenets we try to adhere to. When we have people leaving the field after seeing a case like this, it’s chilling. We have to re-educate the public and we need to have a realignment of how errors are handled.
This is just the beginning. Her sentencing is going to be in about a month, and we’ll see what happens on reckless homicide charges and neglect. I think there’s going to be a follow-up to this and we’re going to need to discuss this more.
I just wanted to get a couple of takeaways for our audience to just really sear in the brain what we can learn from such an event.
Dr. Ranney: The big takeaway, to me, is the importance of us both continuing to use our voices and working across professional boundaries to help to create this culture of safety, one in which we all feel safe and supported in advocating for systems that work for us. We cannot ask nurses, respiratory technicians, radiology technicians, physicians, or anyone else within the healthcare system to work unsupported, and we have to recognize the degree to which we are all interdependent. My biggest takeaway is for us to use our voices together.
Dr. Barnsteiner: The takeaway that I would have from this, and what I’m working with a number of health systems on, is to have the chair of the board, the CEO of the hospital, the chief medical officer, and the chief nursing officer together promulgate a statement that is sent out to all employees to discuss this verdict and to say what they’re doing to promote a high-reliability organization and a fair and just culture. They should also ask for open conversation and for employees to let the top leadership know any concerns that they have about vulnerabilities in the system. It’s extremely important right now with this verdict that the leaders in healthcare settings, as well as in education settings, let people know what they’ll be doing to protect their employees.
Dr. Glatter: Jane and Megan, I want to thank you so much for such an important discussion that was very informative. I think there’s going to be a follow-up to this that’ll be very, very important. Thanks again.
Robert D. Glatter, MD, is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Glatter has disclosed no relevant financial relationships.
Megan Ranney, MD, MPH, is professor of emergency medicine and the academic dean at Brown University School of Public Health in Providence, Rhode Island. She is the director and founder of the Brown Emergency Digital Health Innovation (eDHI) program. She is also chief research officer for the American Foundation for Firearm Injury Reduction in Medicine, the country’s only nonprofit committed to reducing firearm injury through the public health approach, and a founding partner of GetUsPPE.org, dedicated to matching donors to health systems in need of protective equipment. Dr. Ranney has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for: Medscape; Merck.
Jane Barnsteiner, PhD, RN, is an emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. In addition to her teaching responsibilities, she was director of translational research at the Hospital of the University of Pennsylvania. Jane was one of the developers of the Quality and Safety in Education for Nurses (QSEN) initiative and is co-editor of Quality and Safety in Nursing: A Competency Based Approach to Improving Outcomes, published by Wiley. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Erectile dysfunction drugs linked to ocular conditions
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).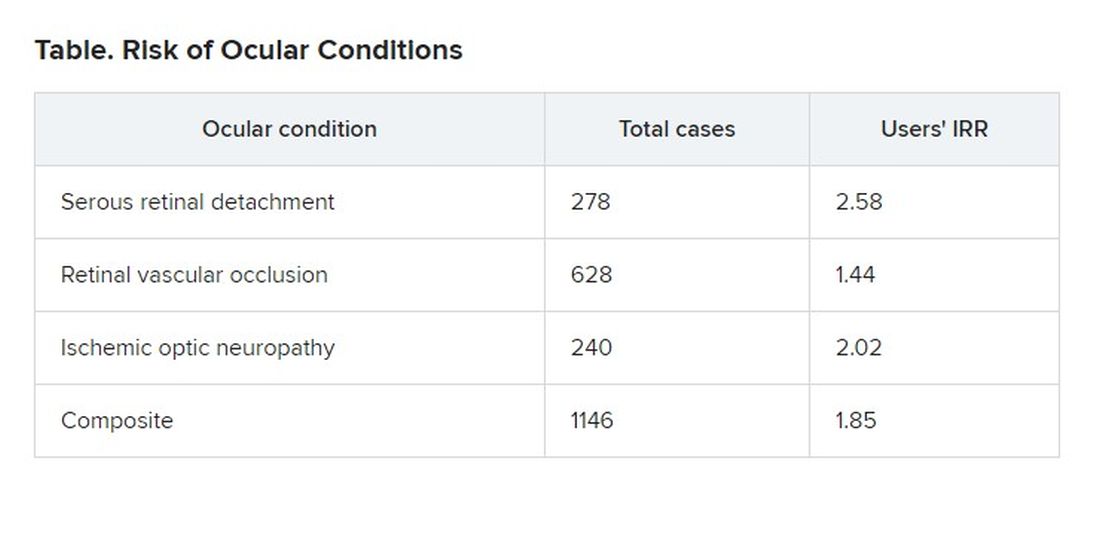
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com
, researchers say.
Patients in an insurance database who were prescribed sildenafil (Viagra), tadalafil (Cialis), vardenafil (Levitra), or avanafil (Stendra) were almost twice as likely as were patients not prescribed the drugs to have ischemic optic neuropathy, retinal vascular occlusion, or serous retinal detachment.
In 2020, physicians wrote about 20 million monthly prescriptions for PDE5Is in the United States alone, said Mahyar Etminan, PharmD, associate professor of ophthalmology at the University of British Columbia, Vancouver.
“We don’t want to alarm people taking them, but generally speaking, if they experience visual problems or changes in vision, then these drugs may be the culprits, and they should check it out,” he said in an interview.
The study was published in JAMA Ophthalmology.
Previous reports, including postmarketing studies by the drug makers, have documented ocular events. The monographs for sildenafil, tadalafil, vardenafil, and avanafil warn users about ischemic optic neuropathy, the researchers found.
The monographs for sildenafil, tadalafil, and vardenafil list retinal vascular occlusion as a potential adverse event but do not quantify the risk. None of the drug monographs mention serous retinal detachment.
Previous research has associated PDE5Is with compromised perfusion of the optic nerve. Some researchers have speculated that the choroid blood vessels can undergo smooth muscle relaxation through a cyclic guanosine monophosphate pathway that can lead to choroidal congestion.
To get a better handle on the ocular risks of PDE51s, Dr. Etminan and his colleagues analyzed health insurance claim records from the PharMetrics Plus database of 213,033 men who had not experienced any of the three ocular conditions in the year before they became regular users of the medications.
They identified 1,146 patients who had been diagnosed with at least one of the three conditions.
The overall number of conditions diagnosed was small relative to the size of the population, 15.5 cases per 10,000 person-years. “So that’s still relatively rare, but the problem is that these are very heavily used medications,” Dr. Etminan said.
For each man diagnosed with one of the ocular conditions, the researchers matched four control persons who were the same age and could be followed for the same length of time. There was a total of 4,584 control persons.
The researchers compared regular users of PDE5Is (those who had received at least one prescription for a PDE5I every 3 months in the year before the ocular diagnosis) with nonusers (those who had not received a PDE5I prescription during that time).
Patients with the ocular conditions were more likely than were those in the control group to have hypertension, diabetes, cardiovascular disease, or sleep apnea. After controlling for these covariates, the researchers found that the users were overall 85% more likely to be diagnosed with one or more of them (incidence rate ratio [IRR], 1.85).
The researchers also found that the risk was even greater for those patients who were given five or more prescriptions of PDE5Is, compared to those given fewer than five prescriptions, suggesting a dose response.
On the basis of these findings, Dr. Etminan thinks drug companies should add warnings about serous retinal detachment and retinal vascular occlusion to the drug monographs.
Asked to comment, Pfizer, which developed Viagra, referred questions to its spinoff company, Viatris, which did not respond. Eli Lilly, which makes Cialis, also did not respond to a request for comment. Vivus, which makes Stendra, could not be reached by press time.
Bayer, which makes Levitra, declined to provide anyone who could answer questions, but it provided a statement noting that the occurrence of ocular adverse events is already known among PDE5I users and that retinal vascular occlusion and ischemic optic neuropathy are mentioned in the product information.
“For example, non-arteritic anterior ischemic optic neuropathy (NAION) is a very rare condition which occurs with an overall higher risk in the population usually suffering from erectile dysfunction (ED) – that is, elderly men with concomitant diseases such as diabetes, dyslipidemia, and hypertension – compared to the general population,” the statement said.
Because of the retrospective nature of the analysis, Dr. Etminan acknowledged that researchers could not prove that the increased risk of ocular disease was associated with use of the drugs rather than some underlying condition. But in addition to adjusting for known risk factors, they also separately analyzed men without hypertension, diabetes, or coronary artery disease and still found that the risk of the ocular conditions was roughly double for men with PDE5I prescriptions.
Howard Pomeranz, MD, PhD, professor of ophthalmology at Northwell Health in Great Neck, N.Y., who was not involved in this study, said its findings confirmed similar research that he conducted on ischemic optic neuropathy.
He told this news organization that people taking PDE5Is should weigh the risk against the benefit, but added that the calculation might be different for people who use them to treat pulmonary hypertension rather than erectile dysfunction.
Although people taking the drugs should discuss any changes in their vision with their practitioners, he said they should not be concerned about a “bluish type of tint to the vision that may occur transiently for anywhere from a few minutes up to 40 or 45 minutes.”
Drug companies and regulators should consider changing the monographs in light of this new evidence, Dr. Pomeranz said. “Perhaps this data might drive the warning to be perhaps a little bit stronger, now that there’s more data to suggest maybe a bit of a stronger association and not just some chance association between using these drugs and these visual events.”
The study was funded by the University of British Columbia. Dr. Etminan and Dr. Pomeranz have disclosed no relevant financial interests.
A version of this article first appeared on Medscape.com