User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Infant’s COVID-19–related myocardial injury reversed
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
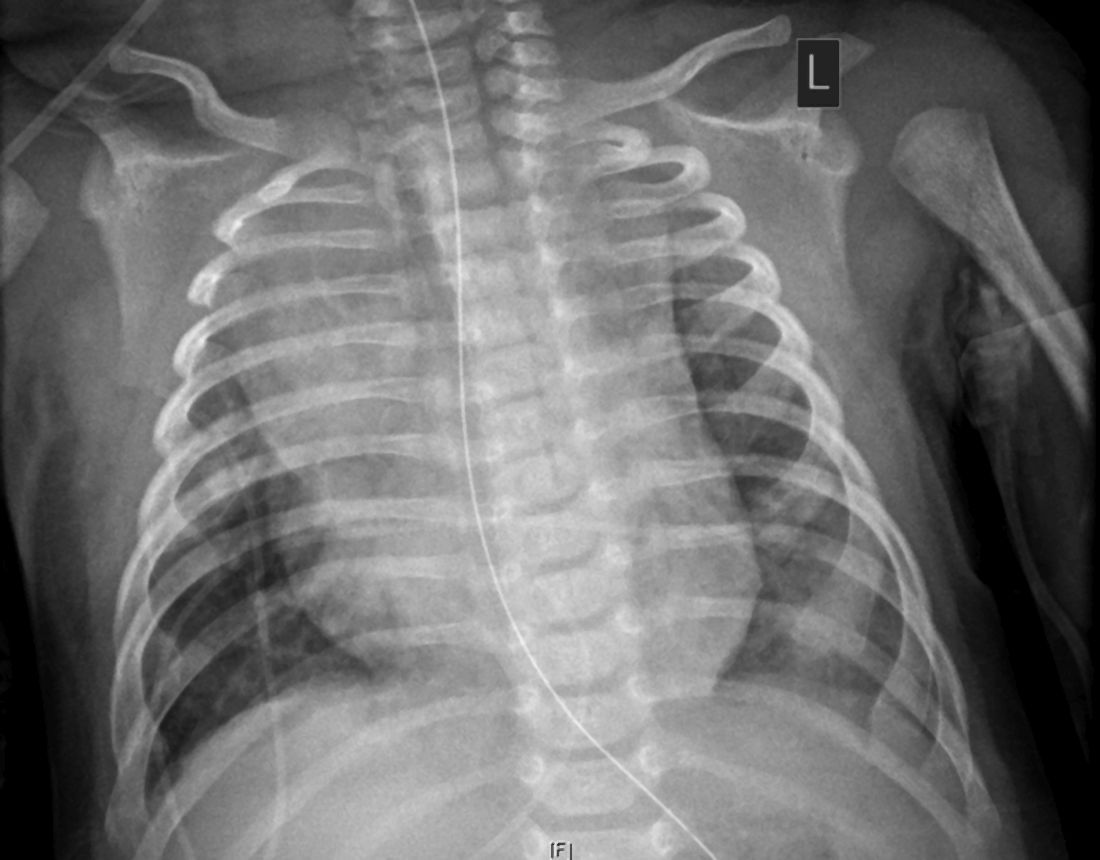
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
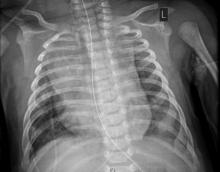
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
FROM JACC CASE REPORTS
Key clinical point: Children presenting with COVID-19 should be tested for heart failure.
Major finding: A 2-month-old infant with COVID-19 had acute but reversible myocardial injury.
Study details: Single case report.
Disclosures: Dr. Sharma, MD, has no relevant financial relationships to disclose.
Source: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
FDA clears first drug for rare genetic causes of severe obesity
The Food and Drug Administration has approved setmelanotide (Imcivree, Rhythm Pharmaceuticals) for weight management in adults and children as young as 6 years with obesity because of proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing.
Individuals with these rare genetic causes of severe obesity have a normal weight at birth but develop persistent severe obesity within months because of insatiable hunger (hyperphagia).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for these disorders.
“Many patients and families who live with these diseases face an often-burdensome stigma associated with severe obesity. To manage this obesity and control disruptive food-seeking behavior, caregivers often lock cabinets and refrigerators and significantly limit social activities,” said Jennifer Miller, MD, a pediatric endocrinologist at University of Florida Health, Gainesville, in a press release issued by the company.
“This FDA approval marks an important turning point, providing a much needed therapy and supporting the use of genetic testing to identify and properly diagnose patients with these rare genetic diseases of obesity,” she noted.
David Meeker, MD, chair, president, and CEO of Rhythm Pharmaceuticals, added: “We are advancing a first-in-class, precision medicine that is designed to directly address the underlying cause of obesities driven by genetic deficits in the MC4R pathway.”
Setmelanotide was evaluated in two phase 3 clinical trials. In one trial, 80% of patients with obesity caused by POMC or PCSK1 deficiency achieved greater than 10% weight loss after 1 year of treatment.
In the other trial, 45.5% of patients with obesity caused by LEPR deficiency achieved greater than 10% weight loss with 1 year of treatment.
Results for the two trials were recently published in The Lancet Diabetes & Endocrinology and discussed at the ObesityWeek Interactive 2020 meeting.
Setmelanotide was generally well tolerated in both trials. The most common adverse events were injection-site reactions, skin hyperpigmentation, and nausea.
The drug label notes that disturbances in sexual arousal, depression, and suicidal ideation; skin pigmentation; and darkening of preexisting nevi may occur with setmelanotide treatment.
The drug label also notes a risk for serious adverse reactions because of benzyl alcohol preservative in neonates and low-birth-weight infants. Setmelanotide is not approved for use in neonates or infants.
The company expects the drug to be commercially available in the United States in the first quarter of 2021.
Setmelanotide for the treatment of obesity associated with rare genetic defects had FDA breakthrough therapy designation as well as orphan drug designation.
The company is also evaluating setmelanotide for reduction in hunger and body weight in a pivotal phase 3 trial in people living with Bardet-Biedl or Alström syndrome, and top-line data are due soon.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved setmelanotide (Imcivree, Rhythm Pharmaceuticals) for weight management in adults and children as young as 6 years with obesity because of proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing.
Individuals with these rare genetic causes of severe obesity have a normal weight at birth but develop persistent severe obesity within months because of insatiable hunger (hyperphagia).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for these disorders.
“Many patients and families who live with these diseases face an often-burdensome stigma associated with severe obesity. To manage this obesity and control disruptive food-seeking behavior, caregivers often lock cabinets and refrigerators and significantly limit social activities,” said Jennifer Miller, MD, a pediatric endocrinologist at University of Florida Health, Gainesville, in a press release issued by the company.
“This FDA approval marks an important turning point, providing a much needed therapy and supporting the use of genetic testing to identify and properly diagnose patients with these rare genetic diseases of obesity,” she noted.
David Meeker, MD, chair, president, and CEO of Rhythm Pharmaceuticals, added: “We are advancing a first-in-class, precision medicine that is designed to directly address the underlying cause of obesities driven by genetic deficits in the MC4R pathway.”
Setmelanotide was evaluated in two phase 3 clinical trials. In one trial, 80% of patients with obesity caused by POMC or PCSK1 deficiency achieved greater than 10% weight loss after 1 year of treatment.
In the other trial, 45.5% of patients with obesity caused by LEPR deficiency achieved greater than 10% weight loss with 1 year of treatment.
Results for the two trials were recently published in The Lancet Diabetes & Endocrinology and discussed at the ObesityWeek Interactive 2020 meeting.
Setmelanotide was generally well tolerated in both trials. The most common adverse events were injection-site reactions, skin hyperpigmentation, and nausea.
The drug label notes that disturbances in sexual arousal, depression, and suicidal ideation; skin pigmentation; and darkening of preexisting nevi may occur with setmelanotide treatment.
The drug label also notes a risk for serious adverse reactions because of benzyl alcohol preservative in neonates and low-birth-weight infants. Setmelanotide is not approved for use in neonates or infants.
The company expects the drug to be commercially available in the United States in the first quarter of 2021.
Setmelanotide for the treatment of obesity associated with rare genetic defects had FDA breakthrough therapy designation as well as orphan drug designation.
The company is also evaluating setmelanotide for reduction in hunger and body weight in a pivotal phase 3 trial in people living with Bardet-Biedl or Alström syndrome, and top-line data are due soon.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved setmelanotide (Imcivree, Rhythm Pharmaceuticals) for weight management in adults and children as young as 6 years with obesity because of proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing.
Individuals with these rare genetic causes of severe obesity have a normal weight at birth but develop persistent severe obesity within months because of insatiable hunger (hyperphagia).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for these disorders.
“Many patients and families who live with these diseases face an often-burdensome stigma associated with severe obesity. To manage this obesity and control disruptive food-seeking behavior, caregivers often lock cabinets and refrigerators and significantly limit social activities,” said Jennifer Miller, MD, a pediatric endocrinologist at University of Florida Health, Gainesville, in a press release issued by the company.
“This FDA approval marks an important turning point, providing a much needed therapy and supporting the use of genetic testing to identify and properly diagnose patients with these rare genetic diseases of obesity,” she noted.
David Meeker, MD, chair, president, and CEO of Rhythm Pharmaceuticals, added: “We are advancing a first-in-class, precision medicine that is designed to directly address the underlying cause of obesities driven by genetic deficits in the MC4R pathway.”
Setmelanotide was evaluated in two phase 3 clinical trials. In one trial, 80% of patients with obesity caused by POMC or PCSK1 deficiency achieved greater than 10% weight loss after 1 year of treatment.
In the other trial, 45.5% of patients with obesity caused by LEPR deficiency achieved greater than 10% weight loss with 1 year of treatment.
Results for the two trials were recently published in The Lancet Diabetes & Endocrinology and discussed at the ObesityWeek Interactive 2020 meeting.
Setmelanotide was generally well tolerated in both trials. The most common adverse events were injection-site reactions, skin hyperpigmentation, and nausea.
The drug label notes that disturbances in sexual arousal, depression, and suicidal ideation; skin pigmentation; and darkening of preexisting nevi may occur with setmelanotide treatment.
The drug label also notes a risk for serious adverse reactions because of benzyl alcohol preservative in neonates and low-birth-weight infants. Setmelanotide is not approved for use in neonates or infants.
The company expects the drug to be commercially available in the United States in the first quarter of 2021.
Setmelanotide for the treatment of obesity associated with rare genetic defects had FDA breakthrough therapy designation as well as orphan drug designation.
The company is also evaluating setmelanotide for reduction in hunger and body weight in a pivotal phase 3 trial in people living with Bardet-Biedl or Alström syndrome, and top-line data are due soon.
A version of this article originally appeared on Medscape.com.
VTE prophylaxis is feasible, effective in some high-risk cancer patients
Primary thromboprophylaxis is feasible and worth considering for high-risk ambulatory patients with cancer who are initiating systemic chemotherapy, according to Marc Carrier, MD.
Risk scores can identify patients at high risk for venous thromboembolism (VTE), and treatments that are effective and associated with low bleeding risk are available, Dr. Carrier explained at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
However, caution is advised in patients with certain types of cancer, including some gastrointestinal and genitourinary cancers, because of the possibility of increased major and clinically relevant nonmajor bleeding risk, he said.
VTE and cancer
VTE is relatively rare in the general population, occurring in about 1 or 2 per 1,000 people annually. The risk increases 4.1-fold in patients with cancer, and 6.5-fold in patients with cancer receiving chemotherapy.
“So just putting these numbers together, we’re no longer talking about 1 in 1,000, but 1 in 200, so [this is] something that is very common among cancer patients,” said Dr. Carrier, a professor at the University of Ottawa and chief of the division of hematology at The Ottawa Hospital.
The mortality rate associated with cancer-associated thrombosis is about 9%, comparable to that associated with infection in the cancer outpatient setting, which underscores the importance of educating patients about the signs and symptoms of VTE so they can seek medical treatment quickly if necessary, he added.
It may also be useful to discuss prophylaxis or other ways to prevent venous thromboembolic complications with certain patients, he said, noting that in an observational cohort study of nearly 600 patients at the University of Ottawa, 25% of those initiating chemotherapy were identified as intermediate or high risk using the validated Khorana risk score, and thus would likely benefit from thromboprophylaxis.
Risk assessment
The Khorana risk score assesses VTE risk based on cancer site, blood counts, and body mass index. It is simple to use and has been validated in more than 20,000 people in multiple countries, Dr. Carrier said.
In a well-known validation study, Ay et al. showed a VTE complication rate of 10% in patients with a Khorana risk score of 2 or higher who were followed up to 6 months.
“This is huge,” Dr. Carrier stressed. “This is much higher than what we tolerate for all sorts of different populations for which we would recommend anticoagulation or thromboprophylaxis.”
The question is whether the risk score can be helpful in a real-world clinic setting, he said, adding: “I’d like to think the answer to that is yes.”
In the University of Ottawa cohort study, 11% of high-risk patients experienced a VTE complication, compared with 4% of those with lower risk, suggesting that the validation data for the Khorana risk score is not only accurate, it is “actually applicable in real-world practice, and you can use it in your own center,” he said.
Further, recent studies have demonstrated that treatment based on Khorana risk score assessment reduces VTE complications.
Prophylaxis options
Low-molecular-weight heparin (LMWH) has been shown in several studies to be associated with a significant relative VTE risk reduction in patients with cancer initiating chemotherapy – with only a slight, nonsignificant increase in the risk of major bleeding.
However, the absolute benefit was small, and LMWH is “parenteral, relatively costly, and, based on that, although we showed relatively good risk-benefit ratio, it never really got translated to clinical practice,” Dr. Carrier said.
In fact, a 2015 American Society of Clinical Oncology guidelines update recommended against routine thromboprophylaxis in this setting, but stated that it could be considered in select high-risk patients identified using a validated risk-assessment tool.
The guidelines noted that “individual risk factors such as biomarkers and cancer site don’t reliably identify high-risk patients.”
More recent data provide additional support for risk assessment and treatment based on Khorana risk score of 2 or higher.
The AVERT trial, for which Dr. Carrier was the first author, showed that the direct-acting oral anticoagulant (DOAC) apixaban reduced VTE incidence, compared with placebo, in patients with Khorana score of 2 or higher (4.2% vs. 10.2%; hazard ratio, 0.41 overall, and 1.0 vs. 7.3; HR, 0.14 on treatment), and the CASSINI trial showed that another DOAC, rivaroxaban, reduced VTE incidence, compared with placebo, in those with Khorana score of 2 or higher (5.9 vs. 6.7; HR, 0.6 overall, and 2.6 vs. 6.4; HR, 0.40 on treatment). The differences in the on-treatment populations were statistically significant.
The two trials, which included a variety of tumor types, showed similar rates of major bleeding, with an absolute difference of about 1% between treatment and placebo, which was not statistically significant in the on-treatment analyses (HR, 1.89 in AVERT and HR, 1.96 in CASSINI).
A systematic review of these trials showed an overall significant decrease in VTE complication risk with treatment in high-risk patients, and a nonstatistically significant major bleeding risk increase.
Based on these findings, ASCO guidelines were updated in 2020 to state that “routine thromboprophylaxis should not be offered to all patients with cancer. ... However, high-risk outpatients with cancer may be offered thromboprophylaxis with apixaban, rivaroxaban or LMWH, providing there are no significant risk factors for bleeding or drug-drug interactions, and after having a full discussion with patients ... to make sure they understand the risk-benefit ratio and the rationale for that particular recommendation,” he said.
Real-world implementation
Implementing this approach in the clinic setting requires a practical model, such as the Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic (VTEPACC) program, a prospective quality improvement research initiative developed in collaboration with the Jeffords Institute for Quality at the University of Vermont Medical Center and described in a recent report, Dr. Carrier said.
The “Vermont model” is “really a comprehensive model that includes identifying patients with the electronic medical records, gathering the formal education and insight from other health care providers like pharmacists and nurses in order to really come up with personalized care for your patients,” he explained.
In 918 outpatients with cancer who were included in the program, VTE awareness increased from less than 5% before VTEPACC to nearly 82% during the implementation phase and 94.7% after 2 years, with nearly 94% of high-risk patients receiving VTE prophylaxis at that time.
“So we can certainly do that in our own center.” he said. “It’s a matter of coming up with the model and making sure that the patients are seen at the right time.”
Given the high frequency of VTE in patients with cancer initiating chemotherapy, the usefulness of risk scores such as the Khorana risk score for identifying those at high risk, and the availability of safe and effective interventions for reducing risk, “we should probably use the data and incorporate them into clinical practice by implementation of programs for primary prevention,” he said.
A word of caution
Caution is warranted, however, when it comes to using DOACs in patients with higher-risk or potentially higher-risk tumor types, he added.
“It’s an important question we are facing as clinicians on a daily basis,” he said, responding to an attendee’s query, as shared by session moderator James Douketis, MD, professor of medicine at McMaster University, Hamilton, Ont., regarding possible bleeding risks in certain genitourinary cancers.
A recent meta-analysis published in Nature, for example, noted that, in the SELECT-D trial, rivaroxaban was associated with significantly higher incidence of clinically relevant nonmajor bleeding, most often in bladder and colorectal cancers, and most often at genitourinary and gastrointestinal sites.
Both Dr. Carrier and fellow panelist Michael Streiff, MD, professor of medicine at Johns Hopkins University and medical director at the Johns Hopkins Hospital Special Coagulation Laboratory, Baltimore, said they approach DOAC use cautiously, but don’t rule it out entirely, in patients with unresected genitourinary tumors that could pose a risk of bleeding.
“It’s worth mentioning and being cautious. In my own personal practice, I’m very careful with unresected urothelial-type tumors or, for example, bladder cancer, for the same reason as [with] unresected luminal GI tumors,” Dr. Carrier said, adding that he’s also mindful that patients with nephropathy were excluded from U.S. DOAC trials because of bleeding risk.
He said he sometimes tries a LMWH challenge first in higher-risk patients, and then might try a DOAC if no bleeding occurs.
“But it certainly is controversial,” he noted.
Dr. Streiff added that he also worries less with genitourinary cancers than with upper GI lesions because “the signals weren’t as big as in GI” cancers, but he noted that “the drugs are going out through the kidneys ... so I’m cautious in those populations.”
“So caution, but not complete exclusion, is the operative management,” Dr. Douketis said, summarizing the panelists’ consensus.
Dr. Carrier reported clinical trial or advisory board participation for Bayer, Pfizer, Servier, Leo Pharma, and/or BMS.
Primary thromboprophylaxis is feasible and worth considering for high-risk ambulatory patients with cancer who are initiating systemic chemotherapy, according to Marc Carrier, MD.
Risk scores can identify patients at high risk for venous thromboembolism (VTE), and treatments that are effective and associated with low bleeding risk are available, Dr. Carrier explained at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
However, caution is advised in patients with certain types of cancer, including some gastrointestinal and genitourinary cancers, because of the possibility of increased major and clinically relevant nonmajor bleeding risk, he said.
VTE and cancer
VTE is relatively rare in the general population, occurring in about 1 or 2 per 1,000 people annually. The risk increases 4.1-fold in patients with cancer, and 6.5-fold in patients with cancer receiving chemotherapy.
“So just putting these numbers together, we’re no longer talking about 1 in 1,000, but 1 in 200, so [this is] something that is very common among cancer patients,” said Dr. Carrier, a professor at the University of Ottawa and chief of the division of hematology at The Ottawa Hospital.
The mortality rate associated with cancer-associated thrombosis is about 9%, comparable to that associated with infection in the cancer outpatient setting, which underscores the importance of educating patients about the signs and symptoms of VTE so they can seek medical treatment quickly if necessary, he added.
It may also be useful to discuss prophylaxis or other ways to prevent venous thromboembolic complications with certain patients, he said, noting that in an observational cohort study of nearly 600 patients at the University of Ottawa, 25% of those initiating chemotherapy were identified as intermediate or high risk using the validated Khorana risk score, and thus would likely benefit from thromboprophylaxis.
Risk assessment
The Khorana risk score assesses VTE risk based on cancer site, blood counts, and body mass index. It is simple to use and has been validated in more than 20,000 people in multiple countries, Dr. Carrier said.
In a well-known validation study, Ay et al. showed a VTE complication rate of 10% in patients with a Khorana risk score of 2 or higher who were followed up to 6 months.
“This is huge,” Dr. Carrier stressed. “This is much higher than what we tolerate for all sorts of different populations for which we would recommend anticoagulation or thromboprophylaxis.”
The question is whether the risk score can be helpful in a real-world clinic setting, he said, adding: “I’d like to think the answer to that is yes.”
In the University of Ottawa cohort study, 11% of high-risk patients experienced a VTE complication, compared with 4% of those with lower risk, suggesting that the validation data for the Khorana risk score is not only accurate, it is “actually applicable in real-world practice, and you can use it in your own center,” he said.
Further, recent studies have demonstrated that treatment based on Khorana risk score assessment reduces VTE complications.
Prophylaxis options
Low-molecular-weight heparin (LMWH) has been shown in several studies to be associated with a significant relative VTE risk reduction in patients with cancer initiating chemotherapy – with only a slight, nonsignificant increase in the risk of major bleeding.
However, the absolute benefit was small, and LMWH is “parenteral, relatively costly, and, based on that, although we showed relatively good risk-benefit ratio, it never really got translated to clinical practice,” Dr. Carrier said.
In fact, a 2015 American Society of Clinical Oncology guidelines update recommended against routine thromboprophylaxis in this setting, but stated that it could be considered in select high-risk patients identified using a validated risk-assessment tool.
The guidelines noted that “individual risk factors such as biomarkers and cancer site don’t reliably identify high-risk patients.”
More recent data provide additional support for risk assessment and treatment based on Khorana risk score of 2 or higher.
The AVERT trial, for which Dr. Carrier was the first author, showed that the direct-acting oral anticoagulant (DOAC) apixaban reduced VTE incidence, compared with placebo, in patients with Khorana score of 2 or higher (4.2% vs. 10.2%; hazard ratio, 0.41 overall, and 1.0 vs. 7.3; HR, 0.14 on treatment), and the CASSINI trial showed that another DOAC, rivaroxaban, reduced VTE incidence, compared with placebo, in those with Khorana score of 2 or higher (5.9 vs. 6.7; HR, 0.6 overall, and 2.6 vs. 6.4; HR, 0.40 on treatment). The differences in the on-treatment populations were statistically significant.
The two trials, which included a variety of tumor types, showed similar rates of major bleeding, with an absolute difference of about 1% between treatment and placebo, which was not statistically significant in the on-treatment analyses (HR, 1.89 in AVERT and HR, 1.96 in CASSINI).
A systematic review of these trials showed an overall significant decrease in VTE complication risk with treatment in high-risk patients, and a nonstatistically significant major bleeding risk increase.
Based on these findings, ASCO guidelines were updated in 2020 to state that “routine thromboprophylaxis should not be offered to all patients with cancer. ... However, high-risk outpatients with cancer may be offered thromboprophylaxis with apixaban, rivaroxaban or LMWH, providing there are no significant risk factors for bleeding or drug-drug interactions, and after having a full discussion with patients ... to make sure they understand the risk-benefit ratio and the rationale for that particular recommendation,” he said.
Real-world implementation
Implementing this approach in the clinic setting requires a practical model, such as the Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic (VTEPACC) program, a prospective quality improvement research initiative developed in collaboration with the Jeffords Institute for Quality at the University of Vermont Medical Center and described in a recent report, Dr. Carrier said.
The “Vermont model” is “really a comprehensive model that includes identifying patients with the electronic medical records, gathering the formal education and insight from other health care providers like pharmacists and nurses in order to really come up with personalized care for your patients,” he explained.
In 918 outpatients with cancer who were included in the program, VTE awareness increased from less than 5% before VTEPACC to nearly 82% during the implementation phase and 94.7% after 2 years, with nearly 94% of high-risk patients receiving VTE prophylaxis at that time.
“So we can certainly do that in our own center.” he said. “It’s a matter of coming up with the model and making sure that the patients are seen at the right time.”
Given the high frequency of VTE in patients with cancer initiating chemotherapy, the usefulness of risk scores such as the Khorana risk score for identifying those at high risk, and the availability of safe and effective interventions for reducing risk, “we should probably use the data and incorporate them into clinical practice by implementation of programs for primary prevention,” he said.
A word of caution
Caution is warranted, however, when it comes to using DOACs in patients with higher-risk or potentially higher-risk tumor types, he added.
“It’s an important question we are facing as clinicians on a daily basis,” he said, responding to an attendee’s query, as shared by session moderator James Douketis, MD, professor of medicine at McMaster University, Hamilton, Ont., regarding possible bleeding risks in certain genitourinary cancers.
A recent meta-analysis published in Nature, for example, noted that, in the SELECT-D trial, rivaroxaban was associated with significantly higher incidence of clinically relevant nonmajor bleeding, most often in bladder and colorectal cancers, and most often at genitourinary and gastrointestinal sites.
Both Dr. Carrier and fellow panelist Michael Streiff, MD, professor of medicine at Johns Hopkins University and medical director at the Johns Hopkins Hospital Special Coagulation Laboratory, Baltimore, said they approach DOAC use cautiously, but don’t rule it out entirely, in patients with unresected genitourinary tumors that could pose a risk of bleeding.
“It’s worth mentioning and being cautious. In my own personal practice, I’m very careful with unresected urothelial-type tumors or, for example, bladder cancer, for the same reason as [with] unresected luminal GI tumors,” Dr. Carrier said, adding that he’s also mindful that patients with nephropathy were excluded from U.S. DOAC trials because of bleeding risk.
He said he sometimes tries a LMWH challenge first in higher-risk patients, and then might try a DOAC if no bleeding occurs.
“But it certainly is controversial,” he noted.
Dr. Streiff added that he also worries less with genitourinary cancers than with upper GI lesions because “the signals weren’t as big as in GI” cancers, but he noted that “the drugs are going out through the kidneys ... so I’m cautious in those populations.”
“So caution, but not complete exclusion, is the operative management,” Dr. Douketis said, summarizing the panelists’ consensus.
Dr. Carrier reported clinical trial or advisory board participation for Bayer, Pfizer, Servier, Leo Pharma, and/or BMS.
Primary thromboprophylaxis is feasible and worth considering for high-risk ambulatory patients with cancer who are initiating systemic chemotherapy, according to Marc Carrier, MD.
Risk scores can identify patients at high risk for venous thromboembolism (VTE), and treatments that are effective and associated with low bleeding risk are available, Dr. Carrier explained at the biennial summit of the Thrombosis & Hemostasis Societies of North America.
However, caution is advised in patients with certain types of cancer, including some gastrointestinal and genitourinary cancers, because of the possibility of increased major and clinically relevant nonmajor bleeding risk, he said.
VTE and cancer
VTE is relatively rare in the general population, occurring in about 1 or 2 per 1,000 people annually. The risk increases 4.1-fold in patients with cancer, and 6.5-fold in patients with cancer receiving chemotherapy.
“So just putting these numbers together, we’re no longer talking about 1 in 1,000, but 1 in 200, so [this is] something that is very common among cancer patients,” said Dr. Carrier, a professor at the University of Ottawa and chief of the division of hematology at The Ottawa Hospital.
The mortality rate associated with cancer-associated thrombosis is about 9%, comparable to that associated with infection in the cancer outpatient setting, which underscores the importance of educating patients about the signs and symptoms of VTE so they can seek medical treatment quickly if necessary, he added.
It may also be useful to discuss prophylaxis or other ways to prevent venous thromboembolic complications with certain patients, he said, noting that in an observational cohort study of nearly 600 patients at the University of Ottawa, 25% of those initiating chemotherapy were identified as intermediate or high risk using the validated Khorana risk score, and thus would likely benefit from thromboprophylaxis.
Risk assessment
The Khorana risk score assesses VTE risk based on cancer site, blood counts, and body mass index. It is simple to use and has been validated in more than 20,000 people in multiple countries, Dr. Carrier said.
In a well-known validation study, Ay et al. showed a VTE complication rate of 10% in patients with a Khorana risk score of 2 or higher who were followed up to 6 months.
“This is huge,” Dr. Carrier stressed. “This is much higher than what we tolerate for all sorts of different populations for which we would recommend anticoagulation or thromboprophylaxis.”
The question is whether the risk score can be helpful in a real-world clinic setting, he said, adding: “I’d like to think the answer to that is yes.”
In the University of Ottawa cohort study, 11% of high-risk patients experienced a VTE complication, compared with 4% of those with lower risk, suggesting that the validation data for the Khorana risk score is not only accurate, it is “actually applicable in real-world practice, and you can use it in your own center,” he said.
Further, recent studies have demonstrated that treatment based on Khorana risk score assessment reduces VTE complications.
Prophylaxis options
Low-molecular-weight heparin (LMWH) has been shown in several studies to be associated with a significant relative VTE risk reduction in patients with cancer initiating chemotherapy – with only a slight, nonsignificant increase in the risk of major bleeding.
However, the absolute benefit was small, and LMWH is “parenteral, relatively costly, and, based on that, although we showed relatively good risk-benefit ratio, it never really got translated to clinical practice,” Dr. Carrier said.
In fact, a 2015 American Society of Clinical Oncology guidelines update recommended against routine thromboprophylaxis in this setting, but stated that it could be considered in select high-risk patients identified using a validated risk-assessment tool.
The guidelines noted that “individual risk factors such as biomarkers and cancer site don’t reliably identify high-risk patients.”
More recent data provide additional support for risk assessment and treatment based on Khorana risk score of 2 or higher.
The AVERT trial, for which Dr. Carrier was the first author, showed that the direct-acting oral anticoagulant (DOAC) apixaban reduced VTE incidence, compared with placebo, in patients with Khorana score of 2 or higher (4.2% vs. 10.2%; hazard ratio, 0.41 overall, and 1.0 vs. 7.3; HR, 0.14 on treatment), and the CASSINI trial showed that another DOAC, rivaroxaban, reduced VTE incidence, compared with placebo, in those with Khorana score of 2 or higher (5.9 vs. 6.7; HR, 0.6 overall, and 2.6 vs. 6.4; HR, 0.40 on treatment). The differences in the on-treatment populations were statistically significant.
The two trials, which included a variety of tumor types, showed similar rates of major bleeding, with an absolute difference of about 1% between treatment and placebo, which was not statistically significant in the on-treatment analyses (HR, 1.89 in AVERT and HR, 1.96 in CASSINI).
A systematic review of these trials showed an overall significant decrease in VTE complication risk with treatment in high-risk patients, and a nonstatistically significant major bleeding risk increase.
Based on these findings, ASCO guidelines were updated in 2020 to state that “routine thromboprophylaxis should not be offered to all patients with cancer. ... However, high-risk outpatients with cancer may be offered thromboprophylaxis with apixaban, rivaroxaban or LMWH, providing there are no significant risk factors for bleeding or drug-drug interactions, and after having a full discussion with patients ... to make sure they understand the risk-benefit ratio and the rationale for that particular recommendation,” he said.
Real-world implementation
Implementing this approach in the clinic setting requires a practical model, such as the Venous Thromboembolism Prevention in the Ambulatory Cancer Clinic (VTEPACC) program, a prospective quality improvement research initiative developed in collaboration with the Jeffords Institute for Quality at the University of Vermont Medical Center and described in a recent report, Dr. Carrier said.
The “Vermont model” is “really a comprehensive model that includes identifying patients with the electronic medical records, gathering the formal education and insight from other health care providers like pharmacists and nurses in order to really come up with personalized care for your patients,” he explained.
In 918 outpatients with cancer who were included in the program, VTE awareness increased from less than 5% before VTEPACC to nearly 82% during the implementation phase and 94.7% after 2 years, with nearly 94% of high-risk patients receiving VTE prophylaxis at that time.
“So we can certainly do that in our own center.” he said. “It’s a matter of coming up with the model and making sure that the patients are seen at the right time.”
Given the high frequency of VTE in patients with cancer initiating chemotherapy, the usefulness of risk scores such as the Khorana risk score for identifying those at high risk, and the availability of safe and effective interventions for reducing risk, “we should probably use the data and incorporate them into clinical practice by implementation of programs for primary prevention,” he said.
A word of caution
Caution is warranted, however, when it comes to using DOACs in patients with higher-risk or potentially higher-risk tumor types, he added.
“It’s an important question we are facing as clinicians on a daily basis,” he said, responding to an attendee’s query, as shared by session moderator James Douketis, MD, professor of medicine at McMaster University, Hamilton, Ont., regarding possible bleeding risks in certain genitourinary cancers.
A recent meta-analysis published in Nature, for example, noted that, in the SELECT-D trial, rivaroxaban was associated with significantly higher incidence of clinically relevant nonmajor bleeding, most often in bladder and colorectal cancers, and most often at genitourinary and gastrointestinal sites.
Both Dr. Carrier and fellow panelist Michael Streiff, MD, professor of medicine at Johns Hopkins University and medical director at the Johns Hopkins Hospital Special Coagulation Laboratory, Baltimore, said they approach DOAC use cautiously, but don’t rule it out entirely, in patients with unresected genitourinary tumors that could pose a risk of bleeding.
“It’s worth mentioning and being cautious. In my own personal practice, I’m very careful with unresected urothelial-type tumors or, for example, bladder cancer, for the same reason as [with] unresected luminal GI tumors,” Dr. Carrier said, adding that he’s also mindful that patients with nephropathy were excluded from U.S. DOAC trials because of bleeding risk.
He said he sometimes tries a LMWH challenge first in higher-risk patients, and then might try a DOAC if no bleeding occurs.
“But it certainly is controversial,” he noted.
Dr. Streiff added that he also worries less with genitourinary cancers than with upper GI lesions because “the signals weren’t as big as in GI” cancers, but he noted that “the drugs are going out through the kidneys ... so I’m cautious in those populations.”
“So caution, but not complete exclusion, is the operative management,” Dr. Douketis said, summarizing the panelists’ consensus.
Dr. Carrier reported clinical trial or advisory board participation for Bayer, Pfizer, Servier, Leo Pharma, and/or BMS.
FROM THE THSNA BIENNIAL SUMMIT
Obesity, hypoxia predict severity in children with COVID-19
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
FROM THE JOURNAL OF PEDIATRICS
Age no barrier to weight loss in those with morbid obesity
Older adults should be recommended for hospital-based lifestyle interventions to reduce weight, say U.K. investigators after finding there was no difference in weight loss between older and younger individuals in their program for those with morbid obesity.
Thomas M. Barber, PhD, and colleagues looked back at nearly 250 randomly selected adults who attended their obesity service over an 11-year period.
Older individuals, defined as aged 60 years and over, had higher rates of type 2 diabetes but experienced a similar percentage weight loss and reduction in body mass index (BMI) as younger patients over the course of around 40 months.
“Age should be no barrier to lifestyle management of obesity,” said Dr. Barber, of University Hospitals Coventry (England) and Warwickshire, in a news release from his institution. “Rather than putting up barriers to older people accessing weight-loss programs, we should be proactively facilitating that process. To do otherwise would risk further and unnecessary neglect of older people through societal ageist misconceptions.”
He urged service providers and policy makers to “appreciate the importance of weight loss in older people with obesity for the maintenance of health and well-being and the facilitation of healthy aging. Furthermore, age per se should not contribute toward clinical decisions regarding the implementation of lifestyle management of older people.”
The research was published online Nov. 22 in Clinical Endocrinology.
Real-world data will inform clinical practice
Jason Halford, PhD, a professor of biological psychology and health behavior, said in an interview: “The fear is that older patients are perceived not to respond” to lifestyle interventions to control obesity, “and that’s clearly a fallacy, according to this study.”
The findings are strengthened by the fact that these are real-world data, “and so it will inform clinical practice,” he added.
And one of the “more interesting” findings was that [type 2] diabetes was “more prevalent” in the older group “but they’re still losing weight,” he noted.
“Traditionally it’s been thought that people with type 2 diabetes find it more difficult to lose weight because you’re trying to manage two conditions,” said Dr. Halford, of the University of Leeds (England), who is also president-elect of the European Association for the Study of Obesity.
Don’t discount older patients
The researchers note that many of the comorbidities associated with obesity “develop over time” and that “no one is immune to obesity,” regardless of their age, sex, ethnicity, and socioeconomic status.
Barber said there are “a number of reasons” why health care professionals “may discount weight loss in older people,” including “an ‘ageist’ perspective that weight-loss is not relevant to older people and misconceptions of reduced ability of older people to lose weight through dietary modification and increased exercise.”
And “older people may feel that hospital-based obesity services are not for them,” he noted.
To determine the effect of age on the ability to lose weight through lifestyle interventions, Dr. Barber and colleagues randomly selected 242 patients with morbid obesity who attended their hospital-based service between 2005 and 2016.
Of these, 167 were aged 18-60 years and 75 were aged 60 years and older. Most participants were women (75.4% of the younger patients and 60.0% of the older patients).
The proportion of patients with confirmed diabetes was markedly higher in the older group, compared with the younger group, at 62.7% versus 35.3%, although older patients had a significantly lower baseline BMI, at 46.9 versus 49.7 kg/m2 (P < .05).
The average duration of the lifestyle intervention was over 3 years (41.5 months) in the younger patients and 33.6 months in the older patients.
There was no significant difference in percentage weight loss between younger and older patients, at 6.9% and 7.3%, respectively, and no difference in percentage reduction in BMI, at 8.1% versus 7.8%.
Further analysis demonstrated that there was no significant correlation between age at referral to the hospital-based service and percentage weight loss (correlation coefficient, –0.13).
Dr. Halford said it would have been “useful” to know the proportion of patients achieving 5% and 10% weight loss because, if a third of patients lost more than 10% of their weight, “even in an elderly population, that would suggest there’d be real benefits in terms of things like type 2 diabetes,” he noted.
And he would like to have seen more data around how long participants had been struggling with obesity, as it’s “just an assumption that the second group is further down the path because they’re older, but we can’t be 100% sure.”
The team noted the study is limited by being retrospective and including a random selection of patients attending the service rather than the entire cohort.
Dr. Halford agreed but said the analysis is a “starting point” and could be used as a platform to conduct “much more systematic research on this area.”
No funding or relevant financial relationships were declared.
A version of this article originally appeared on Medscape.com.
Older adults should be recommended for hospital-based lifestyle interventions to reduce weight, say U.K. investigators after finding there was no difference in weight loss between older and younger individuals in their program for those with morbid obesity.
Thomas M. Barber, PhD, and colleagues looked back at nearly 250 randomly selected adults who attended their obesity service over an 11-year period.
Older individuals, defined as aged 60 years and over, had higher rates of type 2 diabetes but experienced a similar percentage weight loss and reduction in body mass index (BMI) as younger patients over the course of around 40 months.
“Age should be no barrier to lifestyle management of obesity,” said Dr. Barber, of University Hospitals Coventry (England) and Warwickshire, in a news release from his institution. “Rather than putting up barriers to older people accessing weight-loss programs, we should be proactively facilitating that process. To do otherwise would risk further and unnecessary neglect of older people through societal ageist misconceptions.”
He urged service providers and policy makers to “appreciate the importance of weight loss in older people with obesity for the maintenance of health and well-being and the facilitation of healthy aging. Furthermore, age per se should not contribute toward clinical decisions regarding the implementation of lifestyle management of older people.”
The research was published online Nov. 22 in Clinical Endocrinology.
Real-world data will inform clinical practice
Jason Halford, PhD, a professor of biological psychology and health behavior, said in an interview: “The fear is that older patients are perceived not to respond” to lifestyle interventions to control obesity, “and that’s clearly a fallacy, according to this study.”
The findings are strengthened by the fact that these are real-world data, “and so it will inform clinical practice,” he added.
And one of the “more interesting” findings was that [type 2] diabetes was “more prevalent” in the older group “but they’re still losing weight,” he noted.
“Traditionally it’s been thought that people with type 2 diabetes find it more difficult to lose weight because you’re trying to manage two conditions,” said Dr. Halford, of the University of Leeds (England), who is also president-elect of the European Association for the Study of Obesity.
Don’t discount older patients
The researchers note that many of the comorbidities associated with obesity “develop over time” and that “no one is immune to obesity,” regardless of their age, sex, ethnicity, and socioeconomic status.
Barber said there are “a number of reasons” why health care professionals “may discount weight loss in older people,” including “an ‘ageist’ perspective that weight-loss is not relevant to older people and misconceptions of reduced ability of older people to lose weight through dietary modification and increased exercise.”
And “older people may feel that hospital-based obesity services are not for them,” he noted.
To determine the effect of age on the ability to lose weight through lifestyle interventions, Dr. Barber and colleagues randomly selected 242 patients with morbid obesity who attended their hospital-based service between 2005 and 2016.
Of these, 167 were aged 18-60 years and 75 were aged 60 years and older. Most participants were women (75.4% of the younger patients and 60.0% of the older patients).
The proportion of patients with confirmed diabetes was markedly higher in the older group, compared with the younger group, at 62.7% versus 35.3%, although older patients had a significantly lower baseline BMI, at 46.9 versus 49.7 kg/m2 (P < .05).
The average duration of the lifestyle intervention was over 3 years (41.5 months) in the younger patients and 33.6 months in the older patients.
There was no significant difference in percentage weight loss between younger and older patients, at 6.9% and 7.3%, respectively, and no difference in percentage reduction in BMI, at 8.1% versus 7.8%.
Further analysis demonstrated that there was no significant correlation between age at referral to the hospital-based service and percentage weight loss (correlation coefficient, –0.13).
Dr. Halford said it would have been “useful” to know the proportion of patients achieving 5% and 10% weight loss because, if a third of patients lost more than 10% of their weight, “even in an elderly population, that would suggest there’d be real benefits in terms of things like type 2 diabetes,” he noted.
And he would like to have seen more data around how long participants had been struggling with obesity, as it’s “just an assumption that the second group is further down the path because they’re older, but we can’t be 100% sure.”
The team noted the study is limited by being retrospective and including a random selection of patients attending the service rather than the entire cohort.
Dr. Halford agreed but said the analysis is a “starting point” and could be used as a platform to conduct “much more systematic research on this area.”
No funding or relevant financial relationships were declared.
A version of this article originally appeared on Medscape.com.
Older adults should be recommended for hospital-based lifestyle interventions to reduce weight, say U.K. investigators after finding there was no difference in weight loss between older and younger individuals in their program for those with morbid obesity.
Thomas M. Barber, PhD, and colleagues looked back at nearly 250 randomly selected adults who attended their obesity service over an 11-year period.
Older individuals, defined as aged 60 years and over, had higher rates of type 2 diabetes but experienced a similar percentage weight loss and reduction in body mass index (BMI) as younger patients over the course of around 40 months.
“Age should be no barrier to lifestyle management of obesity,” said Dr. Barber, of University Hospitals Coventry (England) and Warwickshire, in a news release from his institution. “Rather than putting up barriers to older people accessing weight-loss programs, we should be proactively facilitating that process. To do otherwise would risk further and unnecessary neglect of older people through societal ageist misconceptions.”
He urged service providers and policy makers to “appreciate the importance of weight loss in older people with obesity for the maintenance of health and well-being and the facilitation of healthy aging. Furthermore, age per se should not contribute toward clinical decisions regarding the implementation of lifestyle management of older people.”
The research was published online Nov. 22 in Clinical Endocrinology.
Real-world data will inform clinical practice
Jason Halford, PhD, a professor of biological psychology and health behavior, said in an interview: “The fear is that older patients are perceived not to respond” to lifestyle interventions to control obesity, “and that’s clearly a fallacy, according to this study.”
The findings are strengthened by the fact that these are real-world data, “and so it will inform clinical practice,” he added.
And one of the “more interesting” findings was that [type 2] diabetes was “more prevalent” in the older group “but they’re still losing weight,” he noted.
“Traditionally it’s been thought that people with type 2 diabetes find it more difficult to lose weight because you’re trying to manage two conditions,” said Dr. Halford, of the University of Leeds (England), who is also president-elect of the European Association for the Study of Obesity.
Don’t discount older patients
The researchers note that many of the comorbidities associated with obesity “develop over time” and that “no one is immune to obesity,” regardless of their age, sex, ethnicity, and socioeconomic status.
Barber said there are “a number of reasons” why health care professionals “may discount weight loss in older people,” including “an ‘ageist’ perspective that weight-loss is not relevant to older people and misconceptions of reduced ability of older people to lose weight through dietary modification and increased exercise.”
And “older people may feel that hospital-based obesity services are not for them,” he noted.
To determine the effect of age on the ability to lose weight through lifestyle interventions, Dr. Barber and colleagues randomly selected 242 patients with morbid obesity who attended their hospital-based service between 2005 and 2016.
Of these, 167 were aged 18-60 years and 75 were aged 60 years and older. Most participants were women (75.4% of the younger patients and 60.0% of the older patients).
The proportion of patients with confirmed diabetes was markedly higher in the older group, compared with the younger group, at 62.7% versus 35.3%, although older patients had a significantly lower baseline BMI, at 46.9 versus 49.7 kg/m2 (P < .05).
The average duration of the lifestyle intervention was over 3 years (41.5 months) in the younger patients and 33.6 months in the older patients.
There was no significant difference in percentage weight loss between younger and older patients, at 6.9% and 7.3%, respectively, and no difference in percentage reduction in BMI, at 8.1% versus 7.8%.
Further analysis demonstrated that there was no significant correlation between age at referral to the hospital-based service and percentage weight loss (correlation coefficient, –0.13).
Dr. Halford said it would have been “useful” to know the proportion of patients achieving 5% and 10% weight loss because, if a third of patients lost more than 10% of their weight, “even in an elderly population, that would suggest there’d be real benefits in terms of things like type 2 diabetes,” he noted.
And he would like to have seen more data around how long participants had been struggling with obesity, as it’s “just an assumption that the second group is further down the path because they’re older, but we can’t be 100% sure.”
The team noted the study is limited by being retrospective and including a random selection of patients attending the service rather than the entire cohort.
Dr. Halford agreed but said the analysis is a “starting point” and could be used as a platform to conduct “much more systematic research on this area.”
No funding or relevant financial relationships were declared.
A version of this article originally appeared on Medscape.com.
Obesity phenotyping matches patients with more effective interventions
A phenotype-guided strategy for systematically matching weight-loss patients to their potentially ideal weight-loss drug roughly doubled treatment efficacy, compared with usual practice, in a single-center, randomized study with 268 patients.
In contrast, in 200 patients who received weight loss–drug therapy selected by routine means, 35% achieved greater than 10% loss compared with their starting weight, Andres J. Acosta, MD, said at the virtual ObesityWeek® Interactive 2020 meeting.
The phenotype-guided strategy also led to an average 16% weight loss from baseline after 12 months, compared with a 9% average loss among the usual-care controls, reported Dr. Acosta, a gastroenterologist at the Mayo Clinic in Rochester, Minn.
A “one-size-fits-all approach to weight loss treatment is not working,” he declared. “Our long-term goal is to develop a personalized approach to obesity management.”
Personalized weight loss treatment isn’t new
“The better we can match treatment to a patient’s needs, the more likely it will succeed. That’s not a brand new idea. They are trying to standardize the way that we classify the disorders that play a role in why a person gains weight or has trouble losing weight,” commented John D. Clark III, MD, an internal medicine physician and weight-management specialist at UT Southwestern Medical Center in Dallas.
The increased weight loss levels that Dr. Acosta reported in patients who underwent the study’s phenotyping protocol and received tailored treatment “are similar to the numbers we see when a patient’s treatment is the right fit for them. You see weight loss in these ranges,” Dr. Clark said in an interview.
The study run by Dr. Acosta and his associates consisted of two phases. First, they established normal and abnormal ranges for four different obesity phenotypes by studying 100 patients with obesity. The patients underwent an extensive and uniform workup designed to classify their obesity phenotype.
Four obesity phenotypes
The researchers categorized patients into one of four types:
- Disordered initial eating satiation, called ‘hungry brain,” and assessed by measuring food intake at a buffet, ad libidum meal.
- Disordered maintenance of satiety, called “hungry gut,” assessed by both a gastric-emptying study as well as patient self-assessment for postprandial fullness.
- “Emotional hunger,” assessed with two questionnaires.
- Disordered energy expenditure, called “slow burn,” assessed by measuring basal metabolic rate, and self-reports of both exercise and nonexercise activity.
Dr. Acosta estimated that the complete workup to assess all four potential phenotypes costs about $1,200.
The researchers then applied the 75th percentile value from each of these assessments to 450 patients with obesity in their clinic to see the prevalence of the four phenotypes. They identified a single phenotype in 58% of these patients, including 18% with hungry gut, 16% with hungry brain, 12% with emotional hunger, and 12% with slow burn. An additional 27% of the patients were positive for two or more phenotypes (including 9% who were positive for all four phenotypes), and 15% did not test positive for any of the four phenotypes.
Phenotype-guided treatments
They then applied their findings in a prospective randomized study that matched a drug intervention to each of the four phenotypes during a year-long, comprehensive weight-loss program at the Mayo Clinic’s Weight Management Clinic. The study randomized 100 patients to the phenotype-driven arm, with 68 of these patients receiving their assigned drug, and 200 patients served as controls. Patients averaged about 47 years old, and their average body mass index was about 41 kg/m2.
The investigational arm included 30 patients classified as having a hungry brain, with 20 of these patients treated with phentermine plus topiramate and 10 treated with lorcaserin (before it was withdrawn by the Food and Drug Administration); 12 with hungry gut and treated with liraglutide (Saxenda); 19 with emotional hunger who received naltrexone SR/bupropion SR (Contrave); and seven with slow burn who received phentermine.
The control arm included 200 patients seeking weight loss treatment at Mayo who did not undergo phenotyping and received their drug treatment based on their personal preference in consultation with their Mayo physician. In this group, drug treatment broke down as 106 patients (53%) on phentermine plus topiramate, 41 (21%) on liraglutide, 34 (17%) on phentermine alone, 14 (7%) on naltrexone SR/bupropion SR, and 5 patients (3%) on locaserin (percentages total 101% because of rounding).
Overall, phenotyping led to more patients treated with naltrexone SR/buproprion SR and lorcaserin and fewer treated with phentermine or phentermine and topiramate ER. All patients were eligible to also receive behavioral interventions as needed.
“We do a lot of testing to identify the phenotype,” in addition to gathering additional clues from a detailed history, said Dr. Acosta. Patients identified with more than one phenotype in routine practice at Mayo are often begun on more than one drug. When phenotyping fails to classify a patient, Dr. Acosta puts the patient on a low-calorie diet and then does a follow-up assessment “to see if the phenotype pops up as a metabolic adaptation.”
“This is something we’re all working toward” in the obesity management field. “How can we better identify the underlying causes in a way that can fit into the work flow. How can we move from research to things we can use daily in the clinic,” observed Dr. Clark. “We need a lot more investigation to determine how well this works in the real world. Are there other tools we can use that are not as expensive” as what Dr. Acosta used for this study?
“For this proof of concept study, it made sense to be very rigorous, but that probably is not realistic for every patient. What are other ways to get this information, or perhaps only use an extensive workup when initial weight loss attempts are unsuccessful,” Dr. Clark suggested.
A phenotype-guided strategy for systematically matching weight-loss patients to their potentially ideal weight-loss drug roughly doubled treatment efficacy, compared with usual practice, in a single-center, randomized study with 268 patients.
In contrast, in 200 patients who received weight loss–drug therapy selected by routine means, 35% achieved greater than 10% loss compared with their starting weight, Andres J. Acosta, MD, said at the virtual ObesityWeek® Interactive 2020 meeting.
The phenotype-guided strategy also led to an average 16% weight loss from baseline after 12 months, compared with a 9% average loss among the usual-care controls, reported Dr. Acosta, a gastroenterologist at the Mayo Clinic in Rochester, Minn.
A “one-size-fits-all approach to weight loss treatment is not working,” he declared. “Our long-term goal is to develop a personalized approach to obesity management.”
Personalized weight loss treatment isn’t new
“The better we can match treatment to a patient’s needs, the more likely it will succeed. That’s not a brand new idea. They are trying to standardize the way that we classify the disorders that play a role in why a person gains weight or has trouble losing weight,” commented John D. Clark III, MD, an internal medicine physician and weight-management specialist at UT Southwestern Medical Center in Dallas.
The increased weight loss levels that Dr. Acosta reported in patients who underwent the study’s phenotyping protocol and received tailored treatment “are similar to the numbers we see when a patient’s treatment is the right fit for them. You see weight loss in these ranges,” Dr. Clark said in an interview.
The study run by Dr. Acosta and his associates consisted of two phases. First, they established normal and abnormal ranges for four different obesity phenotypes by studying 100 patients with obesity. The patients underwent an extensive and uniform workup designed to classify their obesity phenotype.
Four obesity phenotypes
The researchers categorized patients into one of four types:
- Disordered initial eating satiation, called ‘hungry brain,” and assessed by measuring food intake at a buffet, ad libidum meal.
- Disordered maintenance of satiety, called “hungry gut,” assessed by both a gastric-emptying study as well as patient self-assessment for postprandial fullness.
- “Emotional hunger,” assessed with two questionnaires.
- Disordered energy expenditure, called “slow burn,” assessed by measuring basal metabolic rate, and self-reports of both exercise and nonexercise activity.
Dr. Acosta estimated that the complete workup to assess all four potential phenotypes costs about $1,200.
The researchers then applied the 75th percentile value from each of these assessments to 450 patients with obesity in their clinic to see the prevalence of the four phenotypes. They identified a single phenotype in 58% of these patients, including 18% with hungry gut, 16% with hungry brain, 12% with emotional hunger, and 12% with slow burn. An additional 27% of the patients were positive for two or more phenotypes (including 9% who were positive for all four phenotypes), and 15% did not test positive for any of the four phenotypes.
Phenotype-guided treatments
They then applied their findings in a prospective randomized study that matched a drug intervention to each of the four phenotypes during a year-long, comprehensive weight-loss program at the Mayo Clinic’s Weight Management Clinic. The study randomized 100 patients to the phenotype-driven arm, with 68 of these patients receiving their assigned drug, and 200 patients served as controls. Patients averaged about 47 years old, and their average body mass index was about 41 kg/m2.
The investigational arm included 30 patients classified as having a hungry brain, with 20 of these patients treated with phentermine plus topiramate and 10 treated with lorcaserin (before it was withdrawn by the Food and Drug Administration); 12 with hungry gut and treated with liraglutide (Saxenda); 19 with emotional hunger who received naltrexone SR/bupropion SR (Contrave); and seven with slow burn who received phentermine.
The control arm included 200 patients seeking weight loss treatment at Mayo who did not undergo phenotyping and received their drug treatment based on their personal preference in consultation with their Mayo physician. In this group, drug treatment broke down as 106 patients (53%) on phentermine plus topiramate, 41 (21%) on liraglutide, 34 (17%) on phentermine alone, 14 (7%) on naltrexone SR/bupropion SR, and 5 patients (3%) on locaserin (percentages total 101% because of rounding).
Overall, phenotyping led to more patients treated with naltrexone SR/buproprion SR and lorcaserin and fewer treated with phentermine or phentermine and topiramate ER. All patients were eligible to also receive behavioral interventions as needed.
“We do a lot of testing to identify the phenotype,” in addition to gathering additional clues from a detailed history, said Dr. Acosta. Patients identified with more than one phenotype in routine practice at Mayo are often begun on more than one drug. When phenotyping fails to classify a patient, Dr. Acosta puts the patient on a low-calorie diet and then does a follow-up assessment “to see if the phenotype pops up as a metabolic adaptation.”
“This is something we’re all working toward” in the obesity management field. “How can we better identify the underlying causes in a way that can fit into the work flow. How can we move from research to things we can use daily in the clinic,” observed Dr. Clark. “We need a lot more investigation to determine how well this works in the real world. Are there other tools we can use that are not as expensive” as what Dr. Acosta used for this study?
“For this proof of concept study, it made sense to be very rigorous, but that probably is not realistic for every patient. What are other ways to get this information, or perhaps only use an extensive workup when initial weight loss attempts are unsuccessful,” Dr. Clark suggested.
A phenotype-guided strategy for systematically matching weight-loss patients to their potentially ideal weight-loss drug roughly doubled treatment efficacy, compared with usual practice, in a single-center, randomized study with 268 patients.
In contrast, in 200 patients who received weight loss–drug therapy selected by routine means, 35% achieved greater than 10% loss compared with their starting weight, Andres J. Acosta, MD, said at the virtual ObesityWeek® Interactive 2020 meeting.
The phenotype-guided strategy also led to an average 16% weight loss from baseline after 12 months, compared with a 9% average loss among the usual-care controls, reported Dr. Acosta, a gastroenterologist at the Mayo Clinic in Rochester, Minn.
A “one-size-fits-all approach to weight loss treatment is not working,” he declared. “Our long-term goal is to develop a personalized approach to obesity management.”
Personalized weight loss treatment isn’t new
“The better we can match treatment to a patient’s needs, the more likely it will succeed. That’s not a brand new idea. They are trying to standardize the way that we classify the disorders that play a role in why a person gains weight or has trouble losing weight,” commented John D. Clark III, MD, an internal medicine physician and weight-management specialist at UT Southwestern Medical Center in Dallas.
The increased weight loss levels that Dr. Acosta reported in patients who underwent the study’s phenotyping protocol and received tailored treatment “are similar to the numbers we see when a patient’s treatment is the right fit for them. You see weight loss in these ranges,” Dr. Clark said in an interview.
The study run by Dr. Acosta and his associates consisted of two phases. First, they established normal and abnormal ranges for four different obesity phenotypes by studying 100 patients with obesity. The patients underwent an extensive and uniform workup designed to classify their obesity phenotype.
Four obesity phenotypes
The researchers categorized patients into one of four types:
- Disordered initial eating satiation, called ‘hungry brain,” and assessed by measuring food intake at a buffet, ad libidum meal.
- Disordered maintenance of satiety, called “hungry gut,” assessed by both a gastric-emptying study as well as patient self-assessment for postprandial fullness.
- “Emotional hunger,” assessed with two questionnaires.
- Disordered energy expenditure, called “slow burn,” assessed by measuring basal metabolic rate, and self-reports of both exercise and nonexercise activity.
Dr. Acosta estimated that the complete workup to assess all four potential phenotypes costs about $1,200.
The researchers then applied the 75th percentile value from each of these assessments to 450 patients with obesity in their clinic to see the prevalence of the four phenotypes. They identified a single phenotype in 58% of these patients, including 18% with hungry gut, 16% with hungry brain, 12% with emotional hunger, and 12% with slow burn. An additional 27% of the patients were positive for two or more phenotypes (including 9% who were positive for all four phenotypes), and 15% did not test positive for any of the four phenotypes.
Phenotype-guided treatments
They then applied their findings in a prospective randomized study that matched a drug intervention to each of the four phenotypes during a year-long, comprehensive weight-loss program at the Mayo Clinic’s Weight Management Clinic. The study randomized 100 patients to the phenotype-driven arm, with 68 of these patients receiving their assigned drug, and 200 patients served as controls. Patients averaged about 47 years old, and their average body mass index was about 41 kg/m2.
The investigational arm included 30 patients classified as having a hungry brain, with 20 of these patients treated with phentermine plus topiramate and 10 treated with lorcaserin (before it was withdrawn by the Food and Drug Administration); 12 with hungry gut and treated with liraglutide (Saxenda); 19 with emotional hunger who received naltrexone SR/bupropion SR (Contrave); and seven with slow burn who received phentermine.
The control arm included 200 patients seeking weight loss treatment at Mayo who did not undergo phenotyping and received their drug treatment based on their personal preference in consultation with their Mayo physician. In this group, drug treatment broke down as 106 patients (53%) on phentermine plus topiramate, 41 (21%) on liraglutide, 34 (17%) on phentermine alone, 14 (7%) on naltrexone SR/bupropion SR, and 5 patients (3%) on locaserin (percentages total 101% because of rounding).
Overall, phenotyping led to more patients treated with naltrexone SR/buproprion SR and lorcaserin and fewer treated with phentermine or phentermine and topiramate ER. All patients were eligible to also receive behavioral interventions as needed.
“We do a lot of testing to identify the phenotype,” in addition to gathering additional clues from a detailed history, said Dr. Acosta. Patients identified with more than one phenotype in routine practice at Mayo are often begun on more than one drug. When phenotyping fails to classify a patient, Dr. Acosta puts the patient on a low-calorie diet and then does a follow-up assessment “to see if the phenotype pops up as a metabolic adaptation.”
“This is something we’re all working toward” in the obesity management field. “How can we better identify the underlying causes in a way that can fit into the work flow. How can we move from research to things we can use daily in the clinic,” observed Dr. Clark. “We need a lot more investigation to determine how well this works in the real world. Are there other tools we can use that are not as expensive” as what Dr. Acosta used for this study?
“For this proof of concept study, it made sense to be very rigorous, but that probably is not realistic for every patient. What are other ways to get this information, or perhaps only use an extensive workup when initial weight loss attempts are unsuccessful,” Dr. Clark suggested.
FROM OBESITY WEEK 2020
Diabetic retinopathy may predict greater risk of COVID-19 severity
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
COVID-19 vaccine distribution could start in 2 weeks, Pence says
Initial doses of a coronavirus vaccine could be sent out as early as mid-December, Vice President Mike Pence told governors during a call on Monday.
The distribution process could start during the week of Dec. 14, according to audio of a White House Coronavirus Task Force call obtained by CBS News. The call focused on the timeline of vaccine approval and distribution.
“With this morning’s news that Moderna is joining Pfizer in submitting an emergency-use authorization [to the Food and Drug Administration], we continue to be on pace,” Pence said.
The FDA is scheduled to make a decision about Pfizer’s emergency use authorization after an advisory panel meets on Dec. 10 to review the company’s application. FDA Commissioner Stephen Hahn, MD, didn’t commit to the Dec. 14 date, CBS News reported.
“We do all the number crunching ourselves,” Dr. Hahn said. “We look line by line by line on all the data, on all the patients and manufacturing. We do statistical analyses and we come to our own conclusions to support a decision of either thumbs-up or thumbs-down.”
According to a meeting agenda, Pfizer vaccine deliveries should start on Dec. 15, followed by the Moderna vaccine on Dec. 22, CBS News reported.
Between Dec. 13-19, Pfizer is slated to deliver 6.4 million doses, which is enough to immunize about 3 million people with two shots. An “undetermined number” are reserved for backup doses, the news outlet reported.
During the next week, Pfizer and Moderna are scheduled to produce enough doses to vaccinate an additional 10 million people. By the end of the month, about 30 million people should receive doses.
As vaccines begin to roll out, Mr. Pence said “we have a ways to go” in reassuring the public about immunization. He urged governors to use their “bully pulpit” to educate their states and “develop public confidence” in the vaccines.
During the call, Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases, supported the safety and efficacy of the vaccines. Although the vaccine development and approval process was accelerated this year, he said, it “does not at all compromise safety, nor does it compromise scientific integrity.”
“Any misrepresentation that the vaccines had government interference or company interference is patently untrue,” he said.
This article first appeared on Medscape.com.
Initial doses of a coronavirus vaccine could be sent out as early as mid-December, Vice President Mike Pence told governors during a call on Monday.
The distribution process could start during the week of Dec. 14, according to audio of a White House Coronavirus Task Force call obtained by CBS News. The call focused on the timeline of vaccine approval and distribution.
“With this morning’s news that Moderna is joining Pfizer in submitting an emergency-use authorization [to the Food and Drug Administration], we continue to be on pace,” Pence said.
The FDA is scheduled to make a decision about Pfizer’s emergency use authorization after an advisory panel meets on Dec. 10 to review the company’s application. FDA Commissioner Stephen Hahn, MD, didn’t commit to the Dec. 14 date, CBS News reported.
“We do all the number crunching ourselves,” Dr. Hahn said. “We look line by line by line on all the data, on all the patients and manufacturing. We do statistical analyses and we come to our own conclusions to support a decision of either thumbs-up or thumbs-down.”
According to a meeting agenda, Pfizer vaccine deliveries should start on Dec. 15, followed by the Moderna vaccine on Dec. 22, CBS News reported.
Between Dec. 13-19, Pfizer is slated to deliver 6.4 million doses, which is enough to immunize about 3 million people with two shots. An “undetermined number” are reserved for backup doses, the news outlet reported.
During the next week, Pfizer and Moderna are scheduled to produce enough doses to vaccinate an additional 10 million people. By the end of the month, about 30 million people should receive doses.
As vaccines begin to roll out, Mr. Pence said “we have a ways to go” in reassuring the public about immunization. He urged governors to use their “bully pulpit” to educate their states and “develop public confidence” in the vaccines.
During the call, Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases, supported the safety and efficacy of the vaccines. Although the vaccine development and approval process was accelerated this year, he said, it “does not at all compromise safety, nor does it compromise scientific integrity.”
“Any misrepresentation that the vaccines had government interference or company interference is patently untrue,” he said.
This article first appeared on Medscape.com.
Initial doses of a coronavirus vaccine could be sent out as early as mid-December, Vice President Mike Pence told governors during a call on Monday.
The distribution process could start during the week of Dec. 14, according to audio of a White House Coronavirus Task Force call obtained by CBS News. The call focused on the timeline of vaccine approval and distribution.
“With this morning’s news that Moderna is joining Pfizer in submitting an emergency-use authorization [to the Food and Drug Administration], we continue to be on pace,” Pence said.
The FDA is scheduled to make a decision about Pfizer’s emergency use authorization after an advisory panel meets on Dec. 10 to review the company’s application. FDA Commissioner Stephen Hahn, MD, didn’t commit to the Dec. 14 date, CBS News reported.
“We do all the number crunching ourselves,” Dr. Hahn said. “We look line by line by line on all the data, on all the patients and manufacturing. We do statistical analyses and we come to our own conclusions to support a decision of either thumbs-up or thumbs-down.”
According to a meeting agenda, Pfizer vaccine deliveries should start on Dec. 15, followed by the Moderna vaccine on Dec. 22, CBS News reported.
Between Dec. 13-19, Pfizer is slated to deliver 6.4 million doses, which is enough to immunize about 3 million people with two shots. An “undetermined number” are reserved for backup doses, the news outlet reported.
During the next week, Pfizer and Moderna are scheduled to produce enough doses to vaccinate an additional 10 million people. By the end of the month, about 30 million people should receive doses.
As vaccines begin to roll out, Mr. Pence said “we have a ways to go” in reassuring the public about immunization. He urged governors to use their “bully pulpit” to educate their states and “develop public confidence” in the vaccines.
During the call, Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases, supported the safety and efficacy of the vaccines. Although the vaccine development and approval process was accelerated this year, he said, it “does not at all compromise safety, nor does it compromise scientific integrity.”
“Any misrepresentation that the vaccines had government interference or company interference is patently untrue,” he said.
This article first appeared on Medscape.com.
Medicare finalizes 2021 physician pay rule with E/M changes
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Colchicine a case study for what’s wrong with U.S. drug pricing
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.