User login
ECTRIMS and EAN Publish Recommendations for Treating MS
The European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN) have published a guideline to offer up-to-date, evidence-based recommendations for the treatment of adult patients with MS. The guideline is intended to fill a perceived need for a comprehensive document that includes information about recently approved MS therapies and helps clinicians and patients resolve difficulties in everyday clinical practice. It was published in the February issue of Multiple Sclerosis.
Authors Addressed 10 Questions
Xavier Montalban, MD, PhD, Chair and Director of the Department of Neurology and Neuroimmunology at Vall d’Hebron University Hospital in Barcelona, and colleagues agreed to investigate 10 questions related to treatment efficacy, response criteria, strategies to address suboptimal response and safety concerns, and treatment of MS in pregnancy. They developed the guideline following the recommendations of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group, along with EAN recommendations for writing a neurologic management guideline. Literature searches relied upon databases such as the Cochrane Central Register of Controlled Trials, Excerpta Medica, and Medical Literature Analysis and Retrieval System Online.
The authors evaluated data for all disease-modifying treatments (DMTs) approved by the European Medicines Agency at the time of publication. They did not consider combination therapies, complementary or alternative medicine, or symptomatic treatment. Although focused on Europe, the guideline does not account for regulatory or organizational issues specific to any European country.
Strong Recommendations
Dr. Montalban and colleagues agreed upon 21 recommendations and consensus statements. The recommendations were categorized as strong or weak, according to the quality of evidence and the risk–benefit balance. The authors formulated consensus statements on questions for which evidence was insufficient to support a formal recommendation.
The first of the guideline’s three strong recommendations is that neurologists offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS. The second is to offer early treatment with DMTs to patients with active relapsing-remitting MS, as defined by clinical relapses or MRI activity. The authors defined active lesions as contrast-enhancing lesions or new or unequivocally enlarging T2 lesions assessed at least annually. This recommendation also applies to patients with CIS who fulfill current diagnostic criteria for MS. The third strong recommendation is to offer a more efficacious drug to patients treated with interferon or glatiramer acetate who show evidence of disease activity.
Weak Recommendations
Nine of the guideline’s recommendations are categorized as weak. For example, neurologists should consider treating patients with active secondary progressive MS with interferon beta-1a or -1b, taking into account the treatments’ “dubious efficacy,” as well as their safety and tolerability, according to the authors. Neurologists should consider mitoxantrone, ocrelizumab, and cladribine for this population. The authors recommend considering ocrelizumab as a treatment for patients with primary progressive MS.
As a way of monitoring treatment response, the authors recommend that neurologists consider combining MRI with clinical measures when evaluating disease evolution in treated patients. When making treatment decisions in the event of safety concerns, neurologists should consider the possibility that disease activity may resume or rebound if treatment is stopped, particularly with natalizumab, said Dr. Montalban and colleagues. Continuation of DMT treatment should be considered for patients who are clinically stable, who have stable MRI, and who have no problems with safety or tolerability, according to the guideline.
“For women planning a pregnancy, if there is a high risk of disease reactivation, consider using interferon or glatiramer acetate until pregnancy is confirmed,” said the authors. “In some very specific (active) cases, continuing this treatment during pregnancy could also be considered.” Delaying pregnancy is advisable for women with persistent high disease activity. If such a woman decides to become pregnant or has an unplanned pregnancy, neurologists may consider treatment with natalizumab throughout pregnancy after a full discussion with the patient of the potential implications. “Treatment with alemtuzumab could be an alternative therapeutic option for planned pregnancy in very active cases, provided that a four-month interval is strictly observed from the latest infusion until conception,” said Dr. Montalban and colleagues.
Consensus Statements
Several of the guideline’s consensus statements relate to the monitoring of treatment response. For patients treated with DMTs, the authors recommend performing a standardized reference brain MRI within six months of treatment onset, and comparing it with a subsequent brain MRI performed 12 months after starting treatment. The measurement of new or unequivocally enlarging T2 lesions is the preferred MRI method of gauging treatment response, supplemented by gadolinium-enhancing lesions. To monitor treatment safety, the authors recommend performing a standardized reference brain MRI every year in patients at low risk for progressive multifocal leukoencephalopathy (PML), and every three to six months in patients at high risk for PML.
If the neurologist and patient have decided to switch therapies, they should consider the patient’s characteristics and comorbidities, drug safety profiles, and disease severity when choosing a new DMT. If treatment of a highly efficacious drug is stopped because of safety or efficacy problems, the neurologist should consider starting another highly efficacious drug, according to the guideline. Disease activity, the drug’s half-life and biologic activity, and the potential for resumed disease activity or rebound should be considered when choosing a new therapy, said Dr. Montalban and colleagues.
—Erik Greb
Suggested Reading
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96-120.
The European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN) have published a guideline to offer up-to-date, evidence-based recommendations for the treatment of adult patients with MS. The guideline is intended to fill a perceived need for a comprehensive document that includes information about recently approved MS therapies and helps clinicians and patients resolve difficulties in everyday clinical practice. It was published in the February issue of Multiple Sclerosis.
Authors Addressed 10 Questions
Xavier Montalban, MD, PhD, Chair and Director of the Department of Neurology and Neuroimmunology at Vall d’Hebron University Hospital in Barcelona, and colleagues agreed to investigate 10 questions related to treatment efficacy, response criteria, strategies to address suboptimal response and safety concerns, and treatment of MS in pregnancy. They developed the guideline following the recommendations of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group, along with EAN recommendations for writing a neurologic management guideline. Literature searches relied upon databases such as the Cochrane Central Register of Controlled Trials, Excerpta Medica, and Medical Literature Analysis and Retrieval System Online.
The authors evaluated data for all disease-modifying treatments (DMTs) approved by the European Medicines Agency at the time of publication. They did not consider combination therapies, complementary or alternative medicine, or symptomatic treatment. Although focused on Europe, the guideline does not account for regulatory or organizational issues specific to any European country.
Strong Recommendations
Dr. Montalban and colleagues agreed upon 21 recommendations and consensus statements. The recommendations were categorized as strong or weak, according to the quality of evidence and the risk–benefit balance. The authors formulated consensus statements on questions for which evidence was insufficient to support a formal recommendation.
The first of the guideline’s three strong recommendations is that neurologists offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS. The second is to offer early treatment with DMTs to patients with active relapsing-remitting MS, as defined by clinical relapses or MRI activity. The authors defined active lesions as contrast-enhancing lesions or new or unequivocally enlarging T2 lesions assessed at least annually. This recommendation also applies to patients with CIS who fulfill current diagnostic criteria for MS. The third strong recommendation is to offer a more efficacious drug to patients treated with interferon or glatiramer acetate who show evidence of disease activity.
Weak Recommendations
Nine of the guideline’s recommendations are categorized as weak. For example, neurologists should consider treating patients with active secondary progressive MS with interferon beta-1a or -1b, taking into account the treatments’ “dubious efficacy,” as well as their safety and tolerability, according to the authors. Neurologists should consider mitoxantrone, ocrelizumab, and cladribine for this population. The authors recommend considering ocrelizumab as a treatment for patients with primary progressive MS.
As a way of monitoring treatment response, the authors recommend that neurologists consider combining MRI with clinical measures when evaluating disease evolution in treated patients. When making treatment decisions in the event of safety concerns, neurologists should consider the possibility that disease activity may resume or rebound if treatment is stopped, particularly with natalizumab, said Dr. Montalban and colleagues. Continuation of DMT treatment should be considered for patients who are clinically stable, who have stable MRI, and who have no problems with safety or tolerability, according to the guideline.
“For women planning a pregnancy, if there is a high risk of disease reactivation, consider using interferon or glatiramer acetate until pregnancy is confirmed,” said the authors. “In some very specific (active) cases, continuing this treatment during pregnancy could also be considered.” Delaying pregnancy is advisable for women with persistent high disease activity. If such a woman decides to become pregnant or has an unplanned pregnancy, neurologists may consider treatment with natalizumab throughout pregnancy after a full discussion with the patient of the potential implications. “Treatment with alemtuzumab could be an alternative therapeutic option for planned pregnancy in very active cases, provided that a four-month interval is strictly observed from the latest infusion until conception,” said Dr. Montalban and colleagues.
Consensus Statements
Several of the guideline’s consensus statements relate to the monitoring of treatment response. For patients treated with DMTs, the authors recommend performing a standardized reference brain MRI within six months of treatment onset, and comparing it with a subsequent brain MRI performed 12 months after starting treatment. The measurement of new or unequivocally enlarging T2 lesions is the preferred MRI method of gauging treatment response, supplemented by gadolinium-enhancing lesions. To monitor treatment safety, the authors recommend performing a standardized reference brain MRI every year in patients at low risk for progressive multifocal leukoencephalopathy (PML), and every three to six months in patients at high risk for PML.
If the neurologist and patient have decided to switch therapies, they should consider the patient’s characteristics and comorbidities, drug safety profiles, and disease severity when choosing a new DMT. If treatment of a highly efficacious drug is stopped because of safety or efficacy problems, the neurologist should consider starting another highly efficacious drug, according to the guideline. Disease activity, the drug’s half-life and biologic activity, and the potential for resumed disease activity or rebound should be considered when choosing a new therapy, said Dr. Montalban and colleagues.
—Erik Greb
Suggested Reading
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96-120.
The European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the European Academy of Neurology (EAN) have published a guideline to offer up-to-date, evidence-based recommendations for the treatment of adult patients with MS. The guideline is intended to fill a perceived need for a comprehensive document that includes information about recently approved MS therapies and helps clinicians and patients resolve difficulties in everyday clinical practice. It was published in the February issue of Multiple Sclerosis.
Authors Addressed 10 Questions
Xavier Montalban, MD, PhD, Chair and Director of the Department of Neurology and Neuroimmunology at Vall d’Hebron University Hospital in Barcelona, and colleagues agreed to investigate 10 questions related to treatment efficacy, response criteria, strategies to address suboptimal response and safety concerns, and treatment of MS in pregnancy. They developed the guideline following the recommendations of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group, along with EAN recommendations for writing a neurologic management guideline. Literature searches relied upon databases such as the Cochrane Central Register of Controlled Trials, Excerpta Medica, and Medical Literature Analysis and Retrieval System Online.
The authors evaluated data for all disease-modifying treatments (DMTs) approved by the European Medicines Agency at the time of publication. They did not consider combination therapies, complementary or alternative medicine, or symptomatic treatment. Although focused on Europe, the guideline does not account for regulatory or organizational issues specific to any European country.
Strong Recommendations
Dr. Montalban and colleagues agreed upon 21 recommendations and consensus statements. The recommendations were categorized as strong or weak, according to the quality of evidence and the risk–benefit balance. The authors formulated consensus statements on questions for which evidence was insufficient to support a formal recommendation.
The first of the guideline’s three strong recommendations is that neurologists offer interferon or glatiramer acetate to patients with clinically isolated syndrome (CIS) and an abnormal MRI with lesions suggestive of MS who do not fulfill criteria for MS. The second is to offer early treatment with DMTs to patients with active relapsing-remitting MS, as defined by clinical relapses or MRI activity. The authors defined active lesions as contrast-enhancing lesions or new or unequivocally enlarging T2 lesions assessed at least annually. This recommendation also applies to patients with CIS who fulfill current diagnostic criteria for MS. The third strong recommendation is to offer a more efficacious drug to patients treated with interferon or glatiramer acetate who show evidence of disease activity.
Weak Recommendations
Nine of the guideline’s recommendations are categorized as weak. For example, neurologists should consider treating patients with active secondary progressive MS with interferon beta-1a or -1b, taking into account the treatments’ “dubious efficacy,” as well as their safety and tolerability, according to the authors. Neurologists should consider mitoxantrone, ocrelizumab, and cladribine for this population. The authors recommend considering ocrelizumab as a treatment for patients with primary progressive MS.
As a way of monitoring treatment response, the authors recommend that neurologists consider combining MRI with clinical measures when evaluating disease evolution in treated patients. When making treatment decisions in the event of safety concerns, neurologists should consider the possibility that disease activity may resume or rebound if treatment is stopped, particularly with natalizumab, said Dr. Montalban and colleagues. Continuation of DMT treatment should be considered for patients who are clinically stable, who have stable MRI, and who have no problems with safety or tolerability, according to the guideline.
“For women planning a pregnancy, if there is a high risk of disease reactivation, consider using interferon or glatiramer acetate until pregnancy is confirmed,” said the authors. “In some very specific (active) cases, continuing this treatment during pregnancy could also be considered.” Delaying pregnancy is advisable for women with persistent high disease activity. If such a woman decides to become pregnant or has an unplanned pregnancy, neurologists may consider treatment with natalizumab throughout pregnancy after a full discussion with the patient of the potential implications. “Treatment with alemtuzumab could be an alternative therapeutic option for planned pregnancy in very active cases, provided that a four-month interval is strictly observed from the latest infusion until conception,” said Dr. Montalban and colleagues.
Consensus Statements
Several of the guideline’s consensus statements relate to the monitoring of treatment response. For patients treated with DMTs, the authors recommend performing a standardized reference brain MRI within six months of treatment onset, and comparing it with a subsequent brain MRI performed 12 months after starting treatment. The measurement of new or unequivocally enlarging T2 lesions is the preferred MRI method of gauging treatment response, supplemented by gadolinium-enhancing lesions. To monitor treatment safety, the authors recommend performing a standardized reference brain MRI every year in patients at low risk for progressive multifocal leukoencephalopathy (PML), and every three to six months in patients at high risk for PML.
If the neurologist and patient have decided to switch therapies, they should consider the patient’s characteristics and comorbidities, drug safety profiles, and disease severity when choosing a new DMT. If treatment of a highly efficacious drug is stopped because of safety or efficacy problems, the neurologist should consider starting another highly efficacious drug, according to the guideline. Disease activity, the drug’s half-life and biologic activity, and the potential for resumed disease activity or rebound should be considered when choosing a new therapy, said Dr. Montalban and colleagues.
—Erik Greb
Suggested Reading
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96-120.
Guidelines update best practices for hemorrhoid treatment
Each year, more than 2.2 million patients in the United States undergo evaluations for symptoms of hemorrhoids, according to updated guidelines on the management of hemorrhoids issued by the American Society of Colon and Rectal Surgeons.
“As a result, it is important to identify symptomatic hemorrhoids as the underlying source of the anorectal symptom and to have a clear understanding of the evaluation and management of this disease process,” wrote Bradley R. Davis, MD, FACS, chief of colon and rectal surgery at the Carolinas Medical Center, Charlotte, N.C., and the fellow members of the Clinical Practice Guidelines Committee of the ASCRS.
The guidelines recommend evaluation of hemorrhoids based on a disease-specific history, and a physical that emphasizes the degree and duration of symptoms and identifies risk factors. But the guideline writers note that the recommendation is a grade 1C because the supporting data mainly come from observational or case studies.
“The cardinal signs of internal hemorrhoids are painless bleeding with bowel movements with intermittent protrusion,” the committee said, also emphasizing that patients should be evaluated for fecal incontinence, which could inform surgical decision making.
In addition, the guidelines call for a complete endoscopic evaluation of the colon for patients who present with symptomatic hemorrhoids and rectal bleeding; this recommendation is based on moderately strong evidence, and presented with a grade of 1B.
Medical management of hemorrhoids may include office-based procedures or surgery, according to the guidelines.
“Most patients with grade I and II and select patients with grade III internal hemorrhoidal disease who fail medical treatment can be effectively treated with office-based procedures, such as banding, sclerotherapy, and infrared coagulation,” the committee wrote, and medical office treatment received a strong grade 1A recommendation based on high-quality evidence. Although office procedures are generally well tolerated, the condition can recur. Bleeding is the most common complication, and it is more likely after rubber-band ligation than other office-based options, the guidelines state.
The guidelines offer a weak recommendation of 2C, based on the lack of quality evidence, for the use of early surgical excision to treat patients with thrombosed external hemorrhoids. “Although most patients treated nonoperatively will experience eventual resolution of their symptoms, excision of thrombosed external hemorrhoids may result in more rapid symptom resolution, lower incidence of recurrence, and longer remission intervals,” the committee noted.
Surgical hemorrhoidectomy received the strongest possible recommendation (1A, based on high-quality evidence) for the treatment of patients with external hemorrhoids or a combination of internal and external hemorrhoids with prolapse.
Surgical options described in the recommendations include surgical excision (hemorrhoidectomy), hemorrhoidopexy, and Doppler-guided hemorrhoidectomy, with citations of studies on each procedure. Data from a meta-analysis of 18 randomized prospective studies comparing hemorrhoidectomy with office-based procedures showed that hemorrhoidectomy was “the most effective treatment for patients with grade III hemorrhoids,” but it was associated with greater pain and complication rates, according to the guidelines.
However, complications in general are low after surgical hemorrhoidectomy, with reported complication rates of 1%-2% for the most common complication of postprocedure hemorrhage, the guidelines state. After surgery, the guidelines recommend with a 1B grade (moderate quality evidence) that patients use “a multimodality pain regimen to reduce narcotic usage and promote a faster recovery.”
The committee members had no financial conflicts to disclose.
SOURCE: Davis BR et al. Dis Colon Rectum. 2018; 61:284-92.
Each year, more than 2.2 million patients in the United States undergo evaluations for symptoms of hemorrhoids, according to updated guidelines on the management of hemorrhoids issued by the American Society of Colon and Rectal Surgeons.
“As a result, it is important to identify symptomatic hemorrhoids as the underlying source of the anorectal symptom and to have a clear understanding of the evaluation and management of this disease process,” wrote Bradley R. Davis, MD, FACS, chief of colon and rectal surgery at the Carolinas Medical Center, Charlotte, N.C., and the fellow members of the Clinical Practice Guidelines Committee of the ASCRS.
The guidelines recommend evaluation of hemorrhoids based on a disease-specific history, and a physical that emphasizes the degree and duration of symptoms and identifies risk factors. But the guideline writers note that the recommendation is a grade 1C because the supporting data mainly come from observational or case studies.
“The cardinal signs of internal hemorrhoids are painless bleeding with bowel movements with intermittent protrusion,” the committee said, also emphasizing that patients should be evaluated for fecal incontinence, which could inform surgical decision making.
In addition, the guidelines call for a complete endoscopic evaluation of the colon for patients who present with symptomatic hemorrhoids and rectal bleeding; this recommendation is based on moderately strong evidence, and presented with a grade of 1B.
Medical management of hemorrhoids may include office-based procedures or surgery, according to the guidelines.
“Most patients with grade I and II and select patients with grade III internal hemorrhoidal disease who fail medical treatment can be effectively treated with office-based procedures, such as banding, sclerotherapy, and infrared coagulation,” the committee wrote, and medical office treatment received a strong grade 1A recommendation based on high-quality evidence. Although office procedures are generally well tolerated, the condition can recur. Bleeding is the most common complication, and it is more likely after rubber-band ligation than other office-based options, the guidelines state.
The guidelines offer a weak recommendation of 2C, based on the lack of quality evidence, for the use of early surgical excision to treat patients with thrombosed external hemorrhoids. “Although most patients treated nonoperatively will experience eventual resolution of their symptoms, excision of thrombosed external hemorrhoids may result in more rapid symptom resolution, lower incidence of recurrence, and longer remission intervals,” the committee noted.
Surgical hemorrhoidectomy received the strongest possible recommendation (1A, based on high-quality evidence) for the treatment of patients with external hemorrhoids or a combination of internal and external hemorrhoids with prolapse.
Surgical options described in the recommendations include surgical excision (hemorrhoidectomy), hemorrhoidopexy, and Doppler-guided hemorrhoidectomy, with citations of studies on each procedure. Data from a meta-analysis of 18 randomized prospective studies comparing hemorrhoidectomy with office-based procedures showed that hemorrhoidectomy was “the most effective treatment for patients with grade III hemorrhoids,” but it was associated with greater pain and complication rates, according to the guidelines.
However, complications in general are low after surgical hemorrhoidectomy, with reported complication rates of 1%-2% for the most common complication of postprocedure hemorrhage, the guidelines state. After surgery, the guidelines recommend with a 1B grade (moderate quality evidence) that patients use “a multimodality pain regimen to reduce narcotic usage and promote a faster recovery.”
The committee members had no financial conflicts to disclose.
SOURCE: Davis BR et al. Dis Colon Rectum. 2018; 61:284-92.
Each year, more than 2.2 million patients in the United States undergo evaluations for symptoms of hemorrhoids, according to updated guidelines on the management of hemorrhoids issued by the American Society of Colon and Rectal Surgeons.
“As a result, it is important to identify symptomatic hemorrhoids as the underlying source of the anorectal symptom and to have a clear understanding of the evaluation and management of this disease process,” wrote Bradley R. Davis, MD, FACS, chief of colon and rectal surgery at the Carolinas Medical Center, Charlotte, N.C., and the fellow members of the Clinical Practice Guidelines Committee of the ASCRS.
The guidelines recommend evaluation of hemorrhoids based on a disease-specific history, and a physical that emphasizes the degree and duration of symptoms and identifies risk factors. But the guideline writers note that the recommendation is a grade 1C because the supporting data mainly come from observational or case studies.
“The cardinal signs of internal hemorrhoids are painless bleeding with bowel movements with intermittent protrusion,” the committee said, also emphasizing that patients should be evaluated for fecal incontinence, which could inform surgical decision making.
In addition, the guidelines call for a complete endoscopic evaluation of the colon for patients who present with symptomatic hemorrhoids and rectal bleeding; this recommendation is based on moderately strong evidence, and presented with a grade of 1B.
Medical management of hemorrhoids may include office-based procedures or surgery, according to the guidelines.
“Most patients with grade I and II and select patients with grade III internal hemorrhoidal disease who fail medical treatment can be effectively treated with office-based procedures, such as banding, sclerotherapy, and infrared coagulation,” the committee wrote, and medical office treatment received a strong grade 1A recommendation based on high-quality evidence. Although office procedures are generally well tolerated, the condition can recur. Bleeding is the most common complication, and it is more likely after rubber-band ligation than other office-based options, the guidelines state.
The guidelines offer a weak recommendation of 2C, based on the lack of quality evidence, for the use of early surgical excision to treat patients with thrombosed external hemorrhoids. “Although most patients treated nonoperatively will experience eventual resolution of their symptoms, excision of thrombosed external hemorrhoids may result in more rapid symptom resolution, lower incidence of recurrence, and longer remission intervals,” the committee noted.
Surgical hemorrhoidectomy received the strongest possible recommendation (1A, based on high-quality evidence) for the treatment of patients with external hemorrhoids or a combination of internal and external hemorrhoids with prolapse.
Surgical options described in the recommendations include surgical excision (hemorrhoidectomy), hemorrhoidopexy, and Doppler-guided hemorrhoidectomy, with citations of studies on each procedure. Data from a meta-analysis of 18 randomized prospective studies comparing hemorrhoidectomy with office-based procedures showed that hemorrhoidectomy was “the most effective treatment for patients with grade III hemorrhoids,” but it was associated with greater pain and complication rates, according to the guidelines.
However, complications in general are low after surgical hemorrhoidectomy, with reported complication rates of 1%-2% for the most common complication of postprocedure hemorrhage, the guidelines state. After surgery, the guidelines recommend with a 1B grade (moderate quality evidence) that patients use “a multimodality pain regimen to reduce narcotic usage and promote a faster recovery.”
The committee members had no financial conflicts to disclose.
SOURCE: Davis BR et al. Dis Colon Rectum. 2018; 61:284-92.
FROM DISEASES OF THE COLON & RECTUM
Screening for adolescent idiopathic scoliosis
The United States Preventive Services Task Force (USPSTF) has issued recommendations on screening for idiopathic scoliosis in asymptomatic children and adolescents aged 10-18 years.1 This recommendation concluded that the current evidence on the benefits and harms of screening is insufficient (I statement) and updated its 2004 recommendation against routine screening, in which it had concluded that the harms of screening exceeded the potential benefits (D recommendation).
Importance
Screening methods
The USPSTF concluded that currently available screening tests can accurately detect adolescent idiopathic scoliosis. Screening methods include visual inspection using the forward bend test, use of scoliometer measurement of the angle of trunk rotation during forward bend test with a rotation of 5 degrees–7 degrees recommended to be referred for radiography, and Moiré topography that enumerates asymmetric contour lines on the back (values greater than 2 are referred to radiography).
The USPSTF reviewed seven fair-quality observational studies (n = 447,243) and concluded that screening with a combination of forward bend test, scoliometer measurement and that Moiré topography had the highest sensitivity (93.8%) and specificity (99.2%), a low false-negative rate (6.2%), the lowest false-positive rate (0.8%), and the highest positive predictive value (81%). Sensitivity was lower when screening programs used only one or two screening tests, and single screening tests were associated with highest false-positive rates.
In general, the potential harms associated with false-positive results include psychological harm, chest radiation exposure, and other unnecessary treatment, but the USPSTF did not find evidence on the direct harms of screening.
Effectiveness of intervention or treatment
Bracing: The USPSTF found five studies (n = 651) that evaluated the effectiveness of treatment with three different types of braces. The average ages of participants ranged from 12 to 13 years, and their curvature severity varied from Cobb angle of 20 degrees to 30 degrees. The largest study (n = 242) was a good-quality, international, controlled clinical trial known as the Bracing in Adolescent Idiopathic Scoliosis Trial; it demonstrated significant benefit and quality-of-life outcomes associated with bracing for 18 hours/day. In this study, the rate of treatment success in the as-treated analysis was 72% in the intervention group and 48% in the control group. The rate of treatment success in the intention-to-treat analysis was 75% in the intervention group and 42% in the control group. The number needed to treat was three to prevent one case of curvature progression past 50%.
Exercise: The USPSTF found just two trials (n = 184) that evaluated the effectiveness of tailored physiotherapeutic, scoliosis-specific exercise treatments. The participants were older than 10 years and had Cobb angles ranging from 10 degrees to 25 degrees. At the 12-month follow-up, the studies showed significant improvement, including those in quality-of-life measures. In one of the trials, the intervention group had a Cobb angle reduction of 4.9 degrees while the control group had an increase of 2.8 degrees.
Harms: Only one good-quality study (n = 242) reported harms of bracing, which include skin problems, body pain, physical limitations, anxiety, and depression. The USPSTF did not find any studies that assessed the harms of treatment with exercise or surgery.
Association between spinal curvature severity and adult health outcomes
The USPSTF did not find any studies that directly addressed whether changes in the severity of spinal curvature in adolescence resulted in changes in adult health outcomes. The USPSTF did review two fair-quality retrospective, observational, long-term, follow-up analyses (n = 339) of adults diagnosed with idiopathic scoliosis in adolescence and treated with either bracing or surgery. Quality of life measurements, pulmonary consequences, and pregnancy outcomes were not significantly different between the two treatment groups or between those treated and those simply observed. However, those treated with bracing did report more negative treatment experience and body distortion.
Recommendation of others
The Scoliosis Research Society, American Academy of Orthopedic Surgeons, Pediatric Orthopedic Society of North America, and American Academy of Pediatrics issued a joint position statement in September 2015 recommending that screening examinations for scoliosis should be performed for females at ages 10 and 12 years and for males at either 13 or 14 years.2
Their rationale, articulated in the statement and in an editorial in JAMA accompanying the publication of the USPSTF statement, is primarily based on findings in the Bracing in Adolescent Idiopathic Scoliosis Trial that showed a 56% decrease in the rate of progression of moderate curves to greater than 50 degrees. The evidence that intervention works – along with concerns about costs, family burden, loss of school time, risks of surgical complications, and the 22% need for long-term revision surgery – makes avoidance of progression of curves in scoliosis a high-value issue. In addition, they reasoned, the screening trials from which the false-positive values were derived were primarily school-based screening and not done in physician offices.
The Bottom Line
Although the joint statement made by pediatric orthopedic societies and the American Academy of Pediatrics had recommended screening examinations, the USPSTF concluded that the current evidence is insufficient and that the balance of benefits and harms of screening for adolescent (aged 10-18 years) idiopathic scoliosis (Cobb angle greater than 10 degrees) cannot be determined, giving an “I” recommendation.
Dr. Aarisha Shrestha is a first-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
References
1. US Preventive Services Task Force. JAMA. 2018;319(2):165–72.
2. HreskoMT et al. SRS/POSNA/AAOS/AAP position statement: Screening for the early detection for idiopathic scoliosis in adolescents. 2015. Accessed December 8, 2017.
The United States Preventive Services Task Force (USPSTF) has issued recommendations on screening for idiopathic scoliosis in asymptomatic children and adolescents aged 10-18 years.1 This recommendation concluded that the current evidence on the benefits and harms of screening is insufficient (I statement) and updated its 2004 recommendation against routine screening, in which it had concluded that the harms of screening exceeded the potential benefits (D recommendation).
Importance
Screening methods
The USPSTF concluded that currently available screening tests can accurately detect adolescent idiopathic scoliosis. Screening methods include visual inspection using the forward bend test, use of scoliometer measurement of the angle of trunk rotation during forward bend test with a rotation of 5 degrees–7 degrees recommended to be referred for radiography, and Moiré topography that enumerates asymmetric contour lines on the back (values greater than 2 are referred to radiography).
The USPSTF reviewed seven fair-quality observational studies (n = 447,243) and concluded that screening with a combination of forward bend test, scoliometer measurement and that Moiré topography had the highest sensitivity (93.8%) and specificity (99.2%), a low false-negative rate (6.2%), the lowest false-positive rate (0.8%), and the highest positive predictive value (81%). Sensitivity was lower when screening programs used only one or two screening tests, and single screening tests were associated with highest false-positive rates.
In general, the potential harms associated with false-positive results include psychological harm, chest radiation exposure, and other unnecessary treatment, but the USPSTF did not find evidence on the direct harms of screening.
Effectiveness of intervention or treatment
Bracing: The USPSTF found five studies (n = 651) that evaluated the effectiveness of treatment with three different types of braces. The average ages of participants ranged from 12 to 13 years, and their curvature severity varied from Cobb angle of 20 degrees to 30 degrees. The largest study (n = 242) was a good-quality, international, controlled clinical trial known as the Bracing in Adolescent Idiopathic Scoliosis Trial; it demonstrated significant benefit and quality-of-life outcomes associated with bracing for 18 hours/day. In this study, the rate of treatment success in the as-treated analysis was 72% in the intervention group and 48% in the control group. The rate of treatment success in the intention-to-treat analysis was 75% in the intervention group and 42% in the control group. The number needed to treat was three to prevent one case of curvature progression past 50%.
Exercise: The USPSTF found just two trials (n = 184) that evaluated the effectiveness of tailored physiotherapeutic, scoliosis-specific exercise treatments. The participants were older than 10 years and had Cobb angles ranging from 10 degrees to 25 degrees. At the 12-month follow-up, the studies showed significant improvement, including those in quality-of-life measures. In one of the trials, the intervention group had a Cobb angle reduction of 4.9 degrees while the control group had an increase of 2.8 degrees.
Harms: Only one good-quality study (n = 242) reported harms of bracing, which include skin problems, body pain, physical limitations, anxiety, and depression. The USPSTF did not find any studies that assessed the harms of treatment with exercise or surgery.
Association between spinal curvature severity and adult health outcomes
The USPSTF did not find any studies that directly addressed whether changes in the severity of spinal curvature in adolescence resulted in changes in adult health outcomes. The USPSTF did review two fair-quality retrospective, observational, long-term, follow-up analyses (n = 339) of adults diagnosed with idiopathic scoliosis in adolescence and treated with either bracing or surgery. Quality of life measurements, pulmonary consequences, and pregnancy outcomes were not significantly different between the two treatment groups or between those treated and those simply observed. However, those treated with bracing did report more negative treatment experience and body distortion.
Recommendation of others
The Scoliosis Research Society, American Academy of Orthopedic Surgeons, Pediatric Orthopedic Society of North America, and American Academy of Pediatrics issued a joint position statement in September 2015 recommending that screening examinations for scoliosis should be performed for females at ages 10 and 12 years and for males at either 13 or 14 years.2
Their rationale, articulated in the statement and in an editorial in JAMA accompanying the publication of the USPSTF statement, is primarily based on findings in the Bracing in Adolescent Idiopathic Scoliosis Trial that showed a 56% decrease in the rate of progression of moderate curves to greater than 50 degrees. The evidence that intervention works – along with concerns about costs, family burden, loss of school time, risks of surgical complications, and the 22% need for long-term revision surgery – makes avoidance of progression of curves in scoliosis a high-value issue. In addition, they reasoned, the screening trials from which the false-positive values were derived were primarily school-based screening and not done in physician offices.
The Bottom Line
Although the joint statement made by pediatric orthopedic societies and the American Academy of Pediatrics had recommended screening examinations, the USPSTF concluded that the current evidence is insufficient and that the balance of benefits and harms of screening for adolescent (aged 10-18 years) idiopathic scoliosis (Cobb angle greater than 10 degrees) cannot be determined, giving an “I” recommendation.
Dr. Aarisha Shrestha is a first-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
References
1. US Preventive Services Task Force. JAMA. 2018;319(2):165–72.
2. HreskoMT et al. SRS/POSNA/AAOS/AAP position statement: Screening for the early detection for idiopathic scoliosis in adolescents. 2015. Accessed December 8, 2017.
The United States Preventive Services Task Force (USPSTF) has issued recommendations on screening for idiopathic scoliosis in asymptomatic children and adolescents aged 10-18 years.1 This recommendation concluded that the current evidence on the benefits and harms of screening is insufficient (I statement) and updated its 2004 recommendation against routine screening, in which it had concluded that the harms of screening exceeded the potential benefits (D recommendation).
Importance
Screening methods
The USPSTF concluded that currently available screening tests can accurately detect adolescent idiopathic scoliosis. Screening methods include visual inspection using the forward bend test, use of scoliometer measurement of the angle of trunk rotation during forward bend test with a rotation of 5 degrees–7 degrees recommended to be referred for radiography, and Moiré topography that enumerates asymmetric contour lines on the back (values greater than 2 are referred to radiography).
The USPSTF reviewed seven fair-quality observational studies (n = 447,243) and concluded that screening with a combination of forward bend test, scoliometer measurement and that Moiré topography had the highest sensitivity (93.8%) and specificity (99.2%), a low false-negative rate (6.2%), the lowest false-positive rate (0.8%), and the highest positive predictive value (81%). Sensitivity was lower when screening programs used only one or two screening tests, and single screening tests were associated with highest false-positive rates.
In general, the potential harms associated with false-positive results include psychological harm, chest radiation exposure, and other unnecessary treatment, but the USPSTF did not find evidence on the direct harms of screening.
Effectiveness of intervention or treatment
Bracing: The USPSTF found five studies (n = 651) that evaluated the effectiveness of treatment with three different types of braces. The average ages of participants ranged from 12 to 13 years, and their curvature severity varied from Cobb angle of 20 degrees to 30 degrees. The largest study (n = 242) was a good-quality, international, controlled clinical trial known as the Bracing in Adolescent Idiopathic Scoliosis Trial; it demonstrated significant benefit and quality-of-life outcomes associated with bracing for 18 hours/day. In this study, the rate of treatment success in the as-treated analysis was 72% in the intervention group and 48% in the control group. The rate of treatment success in the intention-to-treat analysis was 75% in the intervention group and 42% in the control group. The number needed to treat was three to prevent one case of curvature progression past 50%.
Exercise: The USPSTF found just two trials (n = 184) that evaluated the effectiveness of tailored physiotherapeutic, scoliosis-specific exercise treatments. The participants were older than 10 years and had Cobb angles ranging from 10 degrees to 25 degrees. At the 12-month follow-up, the studies showed significant improvement, including those in quality-of-life measures. In one of the trials, the intervention group had a Cobb angle reduction of 4.9 degrees while the control group had an increase of 2.8 degrees.
Harms: Only one good-quality study (n = 242) reported harms of bracing, which include skin problems, body pain, physical limitations, anxiety, and depression. The USPSTF did not find any studies that assessed the harms of treatment with exercise or surgery.
Association between spinal curvature severity and adult health outcomes
The USPSTF did not find any studies that directly addressed whether changes in the severity of spinal curvature in adolescence resulted in changes in adult health outcomes. The USPSTF did review two fair-quality retrospective, observational, long-term, follow-up analyses (n = 339) of adults diagnosed with idiopathic scoliosis in adolescence and treated with either bracing or surgery. Quality of life measurements, pulmonary consequences, and pregnancy outcomes were not significantly different between the two treatment groups or between those treated and those simply observed. However, those treated with bracing did report more negative treatment experience and body distortion.
Recommendation of others
The Scoliosis Research Society, American Academy of Orthopedic Surgeons, Pediatric Orthopedic Society of North America, and American Academy of Pediatrics issued a joint position statement in September 2015 recommending that screening examinations for scoliosis should be performed for females at ages 10 and 12 years and for males at either 13 or 14 years.2
Their rationale, articulated in the statement and in an editorial in JAMA accompanying the publication of the USPSTF statement, is primarily based on findings in the Bracing in Adolescent Idiopathic Scoliosis Trial that showed a 56% decrease in the rate of progression of moderate curves to greater than 50 degrees. The evidence that intervention works – along with concerns about costs, family burden, loss of school time, risks of surgical complications, and the 22% need for long-term revision surgery – makes avoidance of progression of curves in scoliosis a high-value issue. In addition, they reasoned, the screening trials from which the false-positive values were derived were primarily school-based screening and not done in physician offices.
The Bottom Line
Although the joint statement made by pediatric orthopedic societies and the American Academy of Pediatrics had recommended screening examinations, the USPSTF concluded that the current evidence is insufficient and that the balance of benefits and harms of screening for adolescent (aged 10-18 years) idiopathic scoliosis (Cobb angle greater than 10 degrees) cannot be determined, giving an “I” recommendation.
Dr. Aarisha Shrestha is a first-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Jefferson Health.
References
1. US Preventive Services Task Force. JAMA. 2018;319(2):165–72.
2. HreskoMT et al. SRS/POSNA/AAOS/AAP position statement: Screening for the early detection for idiopathic scoliosis in adolescents. 2015. Accessed December 8, 2017.
AAN Recommends Exercise for People With MCI
Neurologists should recommend twice-weekly exercise to patients diagnosed with mild cognitive impairment (MCI) as part of an overall approach to management, according to a practice guideline update from the American Academy of Neurology (AAN). The Level B recommendation is based on six-month studies that suggest that such exercise possibly improves cognition. The update was published online ahead of print December 27, 2017, in Neurology.
“Regular physical exercise has long been shown to have heart health benefits, and now we can say exercise also may help improve memory for people with MCI,” said Ronald Petersen, MD, PhD, Director of the Alzheimer’s Disease Research Center at the Mayo Clinic in Rochester, Minnesota, and lead author of the update. “What is good for your heart can be good for your brain.”
The update also states that clinicians may recommend cognitive training for people with MCI (Level C). The evidence, however, is insufficient “to support or refute the use of any individual cognitive intervention strategy,” according to the guideline. “When various cognitive interventions are considered as a group, for patients with MCI, cognitive interventions may improve select measures of cognitive function.”
Document Updates 2001 Practice Parameter
The current practice guideline update revises the AAN’s 2001 practice parameter that provided recommendations for the diagnosis and treatment of MCI. Dr. Petersen and colleagues based the update on a systematic review of articles about MCI prevalence, prognosis, and treatment. They classified evidence according to AAN criteria and based recommendations on modified Delphi consensus.
The authors found that the prevalence of MCI is 6.7% for people between ages 60 and 64, 8.4% for people between ages 65 and 69, 10.1% for people between ages 70 and 74, 14.8% for people between ages 75 and 79, and 25.2% for people between ages 80 and 84. Approximately 15% of people with MCI who are older than 65 develop dementia during two years of follow-up.
No Evidence for Pharmacologic Treatment
Evidence does not support a symptomatic cognitive benefit in MCI for any pharmacologic or dietary agents, according to the authors. The FDA has not approved any medication for treating MCI. If clinicians offer cholinesterase inhibitors to their patients with MCI, they must first discuss the fact that the treatment is off label and not backed by empirical evidence, according to the update. Gastrointestinal symptoms and cardiac concerns are common side effects of cholinesterase inhibitors.
Assessment for MCI is appropriate for patients who complain of impaired memory or cognition, as well as those who present for a Medicare Annual Wellness Visit, according to the update. Clinicians should evaluate patients with MCI for risk factors that are potentially modifiable. Patients with MCI also should undergo serial assessments over time so that clinicians can monitor them for changes in cognitive status.
—Erik Greb
Suggested Reading
Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312(23):2551-2561.
Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017 Dec 27 [Epub ahead of print].
Vega JN, Newhouse PA. Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments. Curr Psychiatry Rep. 2014;16(10):490.
Neurologists should recommend twice-weekly exercise to patients diagnosed with mild cognitive impairment (MCI) as part of an overall approach to management, according to a practice guideline update from the American Academy of Neurology (AAN). The Level B recommendation is based on six-month studies that suggest that such exercise possibly improves cognition. The update was published online ahead of print December 27, 2017, in Neurology.
“Regular physical exercise has long been shown to have heart health benefits, and now we can say exercise also may help improve memory for people with MCI,” said Ronald Petersen, MD, PhD, Director of the Alzheimer’s Disease Research Center at the Mayo Clinic in Rochester, Minnesota, and lead author of the update. “What is good for your heart can be good for your brain.”
The update also states that clinicians may recommend cognitive training for people with MCI (Level C). The evidence, however, is insufficient “to support or refute the use of any individual cognitive intervention strategy,” according to the guideline. “When various cognitive interventions are considered as a group, for patients with MCI, cognitive interventions may improve select measures of cognitive function.”
Document Updates 2001 Practice Parameter
The current practice guideline update revises the AAN’s 2001 practice parameter that provided recommendations for the diagnosis and treatment of MCI. Dr. Petersen and colleagues based the update on a systematic review of articles about MCI prevalence, prognosis, and treatment. They classified evidence according to AAN criteria and based recommendations on modified Delphi consensus.
The authors found that the prevalence of MCI is 6.7% for people between ages 60 and 64, 8.4% for people between ages 65 and 69, 10.1% for people between ages 70 and 74, 14.8% for people between ages 75 and 79, and 25.2% for people between ages 80 and 84. Approximately 15% of people with MCI who are older than 65 develop dementia during two years of follow-up.
No Evidence for Pharmacologic Treatment
Evidence does not support a symptomatic cognitive benefit in MCI for any pharmacologic or dietary agents, according to the authors. The FDA has not approved any medication for treating MCI. If clinicians offer cholinesterase inhibitors to their patients with MCI, they must first discuss the fact that the treatment is off label and not backed by empirical evidence, according to the update. Gastrointestinal symptoms and cardiac concerns are common side effects of cholinesterase inhibitors.
Assessment for MCI is appropriate for patients who complain of impaired memory or cognition, as well as those who present for a Medicare Annual Wellness Visit, according to the update. Clinicians should evaluate patients with MCI for risk factors that are potentially modifiable. Patients with MCI also should undergo serial assessments over time so that clinicians can monitor them for changes in cognitive status.
—Erik Greb
Suggested Reading
Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312(23):2551-2561.
Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017 Dec 27 [Epub ahead of print].
Vega JN, Newhouse PA. Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments. Curr Psychiatry Rep. 2014;16(10):490.
Neurologists should recommend twice-weekly exercise to patients diagnosed with mild cognitive impairment (MCI) as part of an overall approach to management, according to a practice guideline update from the American Academy of Neurology (AAN). The Level B recommendation is based on six-month studies that suggest that such exercise possibly improves cognition. The update was published online ahead of print December 27, 2017, in Neurology.
“Regular physical exercise has long been shown to have heart health benefits, and now we can say exercise also may help improve memory for people with MCI,” said Ronald Petersen, MD, PhD, Director of the Alzheimer’s Disease Research Center at the Mayo Clinic in Rochester, Minnesota, and lead author of the update. “What is good for your heart can be good for your brain.”
The update also states that clinicians may recommend cognitive training for people with MCI (Level C). The evidence, however, is insufficient “to support or refute the use of any individual cognitive intervention strategy,” according to the guideline. “When various cognitive interventions are considered as a group, for patients with MCI, cognitive interventions may improve select measures of cognitive function.”
Document Updates 2001 Practice Parameter
The current practice guideline update revises the AAN’s 2001 practice parameter that provided recommendations for the diagnosis and treatment of MCI. Dr. Petersen and colleagues based the update on a systematic review of articles about MCI prevalence, prognosis, and treatment. They classified evidence according to AAN criteria and based recommendations on modified Delphi consensus.
The authors found that the prevalence of MCI is 6.7% for people between ages 60 and 64, 8.4% for people between ages 65 and 69, 10.1% for people between ages 70 and 74, 14.8% for people between ages 75 and 79, and 25.2% for people between ages 80 and 84. Approximately 15% of people with MCI who are older than 65 develop dementia during two years of follow-up.
No Evidence for Pharmacologic Treatment
Evidence does not support a symptomatic cognitive benefit in MCI for any pharmacologic or dietary agents, according to the authors. The FDA has not approved any medication for treating MCI. If clinicians offer cholinesterase inhibitors to their patients with MCI, they must first discuss the fact that the treatment is off label and not backed by empirical evidence, according to the update. Gastrointestinal symptoms and cardiac concerns are common side effects of cholinesterase inhibitors.
Assessment for MCI is appropriate for patients who complain of impaired memory or cognition, as well as those who present for a Medicare Annual Wellness Visit, according to the update. Clinicians should evaluate patients with MCI for risk factors that are potentially modifiable. Patients with MCI also should undergo serial assessments over time so that clinicians can monitor them for changes in cognitive status.
—Erik Greb
Suggested Reading
Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312(23):2551-2561.
Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017 Dec 27 [Epub ahead of print].
Vega JN, Newhouse PA. Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments. Curr Psychiatry Rep. 2014;16(10):490.
nPEP for HIV: Updated CDC guidelines available for primary care physicians
In 2016, the Centers for Disease Control and Prevention provided health care providers with updated recommendations for nonoccupational postexposure prophylaxis (nPEP) with antiretroviral drugs to prevent transmission of HIV following sexual interaction, injection-drug use, or other nonoccupational exposures.1 The new recommendations include the use of more effective and more tolerable drug regimens that employ antiretroviral medications that were approved since the previous guidelines came out in 2005; they also provide updated guidance on exposure assessment, baseline and follow-up HIV testing, and longer-term prevention measures, such as pre-exposure prophylaxis (PrEP).
Screening for HIV infection has been expanding broadly in all health care settings over the past decade, so primary care physicians play an increasingly vital role in preventing HIV infection. Today, primary care physicians are also often the most likely “go-to” health care provider when patients think they may have been exposed to HIV. Clinically, this is an emergency situation, so time is of the essence: Treatment with three powerful antiretrovirals must be initiated within a few hours of – but no later than 72 hours after – an isolated exposure to blood, genital secretions, or other potentially infectious body fluids that may contain HIV.
The key issue for primary care physicians, especially those who have never prescribed PEP before, is advance planning. What you do up front, in terms of organizing materials and training staff, is worth the effort because there is so much at stake – for your patients and for society. The good news is that once you have an established nPEP protocol in place, it stays in place. When a patient asks for help, the protocol kicks in automatically.
Getting ready for nPEP
Prepare your staff:
- Educate your whole staff about the urgency of seeing potential nPEP patients immediately.
- Choose the staff person in your office who will submit requests for PEP medications to the pharmacy and/or pharmaceutical companies; your financial reimbursement staff person is likely a good candidate for this job.
- Learn about patient assistance programs (for uninsured or underinsured patients) and crime victims compensation programs (reimbursement or emergency awards for victims of violent crimes, including rape, for various out-of-pocket expenses including medical expenses).
Keep paperwork and materials on hand:
- Have information and forms for patient assistance programs for pharmaceutical companies supplying the drugs. Pharmaceutical companies are aware of the urgency for nPEP medications and are ready to respond immediately. They may mail the medication so it arrives the next day or, more likely, fax a voucher or other information for the patient to present to a local pharmacist who will fill the prescription.
- Have information on your state’s crime victims compensation program available.
- Consider keeping nPEP Starter Packs (with an initial 3-7 days’ worth of medication) readily available in your office.
Rapid evaluation of patients seeking care after potential exposure to HIV
Effective delivery of nPEP requires prompt initial evaluation of patients and assessment of HIV transmission risk. Take a methodical, step-by-step history of the exposure to address the following basic questions:
- Date and time of exposure? nPEP should be initiated as soon as possible after HIV exposure; it is unlikely to be effective if not initiated within 72 hours or less.
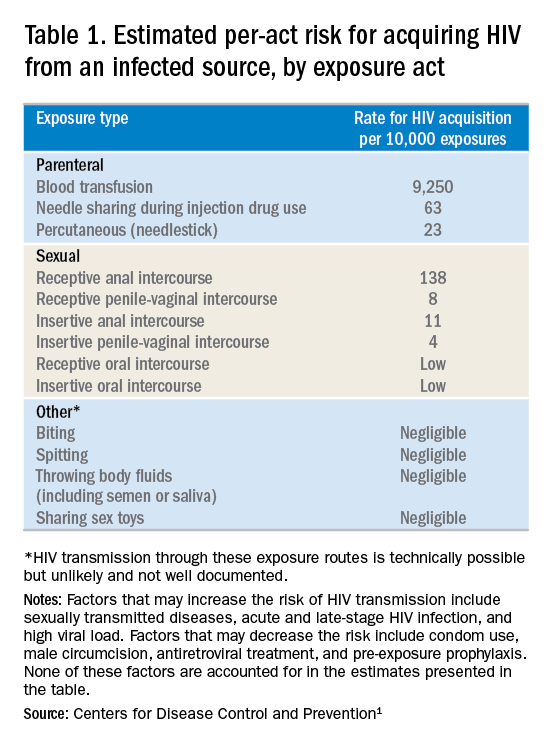
- Frequency of exposure? Type/route of exposure? nPEP is generally reserved for isolated or infrequent exposures that present a substantial risk for HIV acquisition (see Table 1 on HIV acquisition risk below).
- HIV status of exposure source? If the source is positive, is the source person on HIV treatment with antiretroviral therapy? If unknown, is the source person an injecting drug user or a man who has sex with men (MSM)?
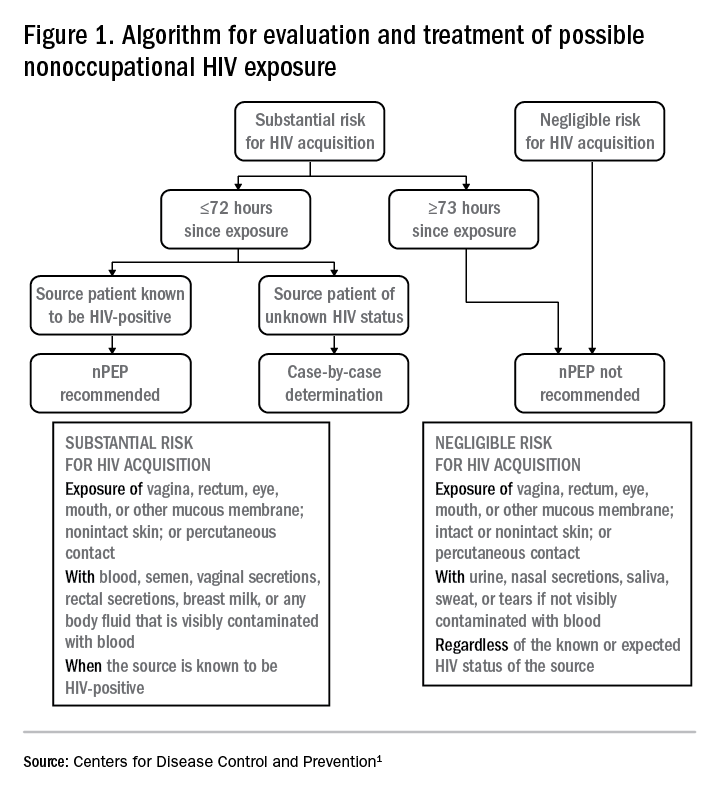
Based on the initial evaluation, is nPEP recommended?
Answers to the questions asked during the initial evaluation of the patient will determine whether nPEP is indicated. Along with its updated recommendations, the CDC provided an algorithm to help guide evaluation and treatment.
Preferred HIV test
Administer an HIV test to all patients considered for nPEP, preferably the rapid combined antigen and antibody test (Ag/Ab), or just the antibody test if the Ag/Ab test is not available. nPEP is indicated only for persons without HIV infections. However, if results are not available during the initial evaluation, assume the patient is not infected. If indicated and started, nPEP can be discontinued if tests later shown the patient already has an HIV infection.
Laboratory testing
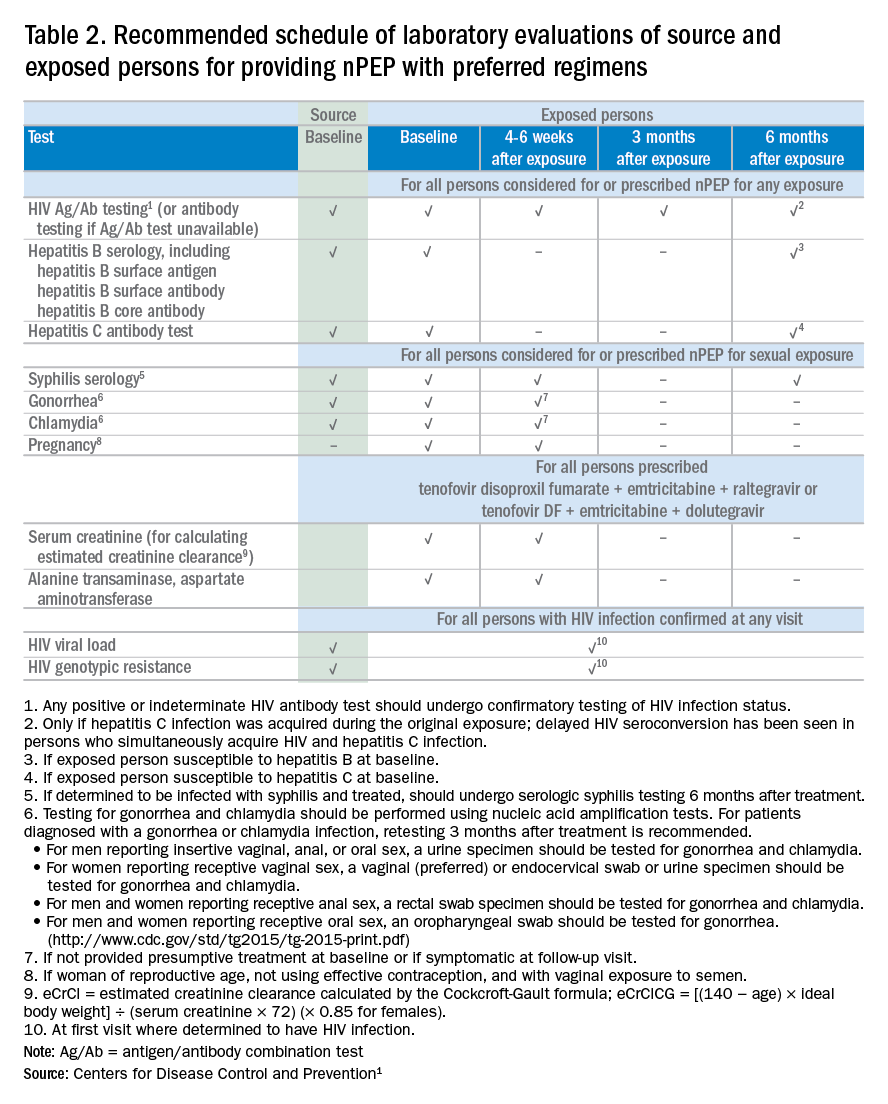
If nPEP is indicated, conduct laboratory testing. Lab testing is required to document the patient’s HIV status (and that of the source person, when available), identify and manage other conditions potentially resulting from exposure, identify conditions that may affect the nPEP medication regimen, and monitor safety or toxicities to the prescribed regimen.
nPEP treatment regimen for otherwise healthy adults and adolescents
In the absence of randomized clinical trials, data from a case/control study demonstrating an 81% reduction of HIV transmission after use of occupational PEP among hospital workers remains the strongest evidence for the benefit of nPEP.1,2 For patients offered nPEP, recommended treatment includes prescribing either of the following regimens for 28 days:
- Preferred regimen: tenofovir disoproxil fumarate (TDF) (300 mg) with emtricitabine (FTC) (200 mg) once daily plus either raltegravir (RAL) 400 mg twice daily or dolutegravir (DTG) 50 mg daily.
- Alternative regimen: TDF (300 mg) with FTC (200 mg) once daily plus darunavir (DRV) (800 mg) and ritonavir (RTV) (100 mg) once daily.
Additional considerations and nPEP treatment regimens for children, patients with decreased renal function, and pregnant women are included in the CDC guidelines.
Crucial Information for Patients on nPEP
Emphasize the importance of proper dosing and adherence.
Review the patient information for each drug in the regimen, specifically the black boxes, warnings, and side effects, and counsel your patients accordingly.
Transitioning from nPEP to PrEP or from PrEP to nPEP
If you have a patient who engages in behavior that places them at risk for frequent, recurrent exposures to HIV, consider transitioning them to PrEP (pre-exposure prophylaxis) following their 28-day course of nPEP.3 PrEP is a two-drug regimen taken daily on an ongoing basis.
Additionally, for patients who are already on PrEP but who have not taken their medications within a week before the possible exposure, consider initiating nPEP for 28 days and then reintroducing PrEP if their HIV status is negative and the problems with adherence can be addressed moving forward.
Raising Awareness About nPEP
Many people never expect to be exposed to HIV and may not know about the availability of PEP in an emergency situation. You can help raise awareness by making educational materials available in your waiting rooms and exam rooms. Brochures and other HIV/AIDS educational materials for patients are available from the CDC Act Against AIDS campaign.
Summary
Dr. Dominguez is a Captain, U.S. Public Health Service, epidemiology branch, division of HIV/AIDS prevention, CDC.
Additional resources
- The CDC recommends that everyone between the ages of 13 and 64 get tested for HIV at least once as part of routine health care. As part of its Act Against AIDS initiative, the CDC developed the HIV Screening Standard Care program, which provides free tools and resources to help clinicians and nurses incorporate routine HIV screening into primary care settings.
- HIV guidelines and recommendations .
- Postexposure prophylaxis (PEP)
- Pre-Exposure prophylaxis (PrEP)
References
1. Centers for Disease Control and Prevention. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV. United States, 2016. Accessed March 6, 2017.
2. Cardo DM et al. New Engl J Med. 1997;337(21):1485-90.
3. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States–2014: a clinical practice guideline. Accessed March 6, 2017.
In 2016, the Centers for Disease Control and Prevention provided health care providers with updated recommendations for nonoccupational postexposure prophylaxis (nPEP) with antiretroviral drugs to prevent transmission of HIV following sexual interaction, injection-drug use, or other nonoccupational exposures.1 The new recommendations include the use of more effective and more tolerable drug regimens that employ antiretroviral medications that were approved since the previous guidelines came out in 2005; they also provide updated guidance on exposure assessment, baseline and follow-up HIV testing, and longer-term prevention measures, such as pre-exposure prophylaxis (PrEP).
Screening for HIV infection has been expanding broadly in all health care settings over the past decade, so primary care physicians play an increasingly vital role in preventing HIV infection. Today, primary care physicians are also often the most likely “go-to” health care provider when patients think they may have been exposed to HIV. Clinically, this is an emergency situation, so time is of the essence: Treatment with three powerful antiretrovirals must be initiated within a few hours of – but no later than 72 hours after – an isolated exposure to blood, genital secretions, or other potentially infectious body fluids that may contain HIV.
The key issue for primary care physicians, especially those who have never prescribed PEP before, is advance planning. What you do up front, in terms of organizing materials and training staff, is worth the effort because there is so much at stake – for your patients and for society. The good news is that once you have an established nPEP protocol in place, it stays in place. When a patient asks for help, the protocol kicks in automatically.
Getting ready for nPEP
Prepare your staff:
- Educate your whole staff about the urgency of seeing potential nPEP patients immediately.
- Choose the staff person in your office who will submit requests for PEP medications to the pharmacy and/or pharmaceutical companies; your financial reimbursement staff person is likely a good candidate for this job.
- Learn about patient assistance programs (for uninsured or underinsured patients) and crime victims compensation programs (reimbursement or emergency awards for victims of violent crimes, including rape, for various out-of-pocket expenses including medical expenses).
Keep paperwork and materials on hand:
- Have information and forms for patient assistance programs for pharmaceutical companies supplying the drugs. Pharmaceutical companies are aware of the urgency for nPEP medications and are ready to respond immediately. They may mail the medication so it arrives the next day or, more likely, fax a voucher or other information for the patient to present to a local pharmacist who will fill the prescription.
- Have information on your state’s crime victims compensation program available.
- Consider keeping nPEP Starter Packs (with an initial 3-7 days’ worth of medication) readily available in your office.
Rapid evaluation of patients seeking care after potential exposure to HIV
Effective delivery of nPEP requires prompt initial evaluation of patients and assessment of HIV transmission risk. Take a methodical, step-by-step history of the exposure to address the following basic questions:
- Date and time of exposure? nPEP should be initiated as soon as possible after HIV exposure; it is unlikely to be effective if not initiated within 72 hours or less.
- Frequency of exposure? Type/route of exposure? nPEP is generally reserved for isolated or infrequent exposures that present a substantial risk for HIV acquisition (see Table 1 on HIV acquisition risk below).
- HIV status of exposure source? If the source is positive, is the source person on HIV treatment with antiretroviral therapy? If unknown, is the source person an injecting drug user or a man who has sex with men (MSM)?

Based on the initial evaluation, is nPEP recommended?
Answers to the questions asked during the initial evaluation of the patient will determine whether nPEP is indicated. Along with its updated recommendations, the CDC provided an algorithm to help guide evaluation and treatment.

Preferred HIV test
Administer an HIV test to all patients considered for nPEP, preferably the rapid combined antigen and antibody test (Ag/Ab), or just the antibody test if the Ag/Ab test is not available. nPEP is indicated only for persons without HIV infections. However, if results are not available during the initial evaluation, assume the patient is not infected. If indicated and started, nPEP can be discontinued if tests later shown the patient already has an HIV infection.
Laboratory testing
If nPEP is indicated, conduct laboratory testing. Lab testing is required to document the patient’s HIV status (and that of the source person, when available), identify and manage other conditions potentially resulting from exposure, identify conditions that may affect the nPEP medication regimen, and monitor safety or toxicities to the prescribed regimen.

nPEP treatment regimen for otherwise healthy adults and adolescents
In the absence of randomized clinical trials, data from a case/control study demonstrating an 81% reduction of HIV transmission after use of occupational PEP among hospital workers remains the strongest evidence for the benefit of nPEP.1,2 For patients offered nPEP, recommended treatment includes prescribing either of the following regimens for 28 days:
- Preferred regimen: tenofovir disoproxil fumarate (TDF) (300 mg) with emtricitabine (FTC) (200 mg) once daily plus either raltegravir (RAL) 400 mg twice daily or dolutegravir (DTG) 50 mg daily.
- Alternative regimen: TDF (300 mg) with FTC (200 mg) once daily plus darunavir (DRV) (800 mg) and ritonavir (RTV) (100 mg) once daily.
Additional considerations and nPEP treatment regimens for children, patients with decreased renal function, and pregnant women are included in the CDC guidelines.
Crucial Information for Patients on nPEP
Emphasize the importance of proper dosing and adherence.
Review the patient information for each drug in the regimen, specifically the black boxes, warnings, and side effects, and counsel your patients accordingly.
Transitioning from nPEP to PrEP or from PrEP to nPEP
If you have a patient who engages in behavior that places them at risk for frequent, recurrent exposures to HIV, consider transitioning them to PrEP (pre-exposure prophylaxis) following their 28-day course of nPEP.3 PrEP is a two-drug regimen taken daily on an ongoing basis.
Additionally, for patients who are already on PrEP but who have not taken their medications within a week before the possible exposure, consider initiating nPEP for 28 days and then reintroducing PrEP if their HIV status is negative and the problems with adherence can be addressed moving forward.
Raising Awareness About nPEP
Many people never expect to be exposed to HIV and may not know about the availability of PEP in an emergency situation. You can help raise awareness by making educational materials available in your waiting rooms and exam rooms. Brochures and other HIV/AIDS educational materials for patients are available from the CDC Act Against AIDS campaign.
Summary
Dr. Dominguez is a Captain, U.S. Public Health Service, epidemiology branch, division of HIV/AIDS prevention, CDC.
Additional resources
- The CDC recommends that everyone between the ages of 13 and 64 get tested for HIV at least once as part of routine health care. As part of its Act Against AIDS initiative, the CDC developed the HIV Screening Standard Care program, which provides free tools and resources to help clinicians and nurses incorporate routine HIV screening into primary care settings.
- HIV guidelines and recommendations .
- Postexposure prophylaxis (PEP)
- Pre-Exposure prophylaxis (PrEP)
References
1. Centers for Disease Control and Prevention. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV. United States, 2016. Accessed March 6, 2017.
2. Cardo DM et al. New Engl J Med. 1997;337(21):1485-90.
3. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States–2014: a clinical practice guideline. Accessed March 6, 2017.
In 2016, the Centers for Disease Control and Prevention provided health care providers with updated recommendations for nonoccupational postexposure prophylaxis (nPEP) with antiretroviral drugs to prevent transmission of HIV following sexual interaction, injection-drug use, or other nonoccupational exposures.1 The new recommendations include the use of more effective and more tolerable drug regimens that employ antiretroviral medications that were approved since the previous guidelines came out in 2005; they also provide updated guidance on exposure assessment, baseline and follow-up HIV testing, and longer-term prevention measures, such as pre-exposure prophylaxis (PrEP).
Screening for HIV infection has been expanding broadly in all health care settings over the past decade, so primary care physicians play an increasingly vital role in preventing HIV infection. Today, primary care physicians are also often the most likely “go-to” health care provider when patients think they may have been exposed to HIV. Clinically, this is an emergency situation, so time is of the essence: Treatment with three powerful antiretrovirals must be initiated within a few hours of – but no later than 72 hours after – an isolated exposure to blood, genital secretions, or other potentially infectious body fluids that may contain HIV.
The key issue for primary care physicians, especially those who have never prescribed PEP before, is advance planning. What you do up front, in terms of organizing materials and training staff, is worth the effort because there is so much at stake – for your patients and for society. The good news is that once you have an established nPEP protocol in place, it stays in place. When a patient asks for help, the protocol kicks in automatically.
Getting ready for nPEP
Prepare your staff:
- Educate your whole staff about the urgency of seeing potential nPEP patients immediately.
- Choose the staff person in your office who will submit requests for PEP medications to the pharmacy and/or pharmaceutical companies; your financial reimbursement staff person is likely a good candidate for this job.
- Learn about patient assistance programs (for uninsured or underinsured patients) and crime victims compensation programs (reimbursement or emergency awards for victims of violent crimes, including rape, for various out-of-pocket expenses including medical expenses).
Keep paperwork and materials on hand:
- Have information and forms for patient assistance programs for pharmaceutical companies supplying the drugs. Pharmaceutical companies are aware of the urgency for nPEP medications and are ready to respond immediately. They may mail the medication so it arrives the next day or, more likely, fax a voucher or other information for the patient to present to a local pharmacist who will fill the prescription.
- Have information on your state’s crime victims compensation program available.
- Consider keeping nPEP Starter Packs (with an initial 3-7 days’ worth of medication) readily available in your office.
Rapid evaluation of patients seeking care after potential exposure to HIV
Effective delivery of nPEP requires prompt initial evaluation of patients and assessment of HIV transmission risk. Take a methodical, step-by-step history of the exposure to address the following basic questions:
- Date and time of exposure? nPEP should be initiated as soon as possible after HIV exposure; it is unlikely to be effective if not initiated within 72 hours or less.
- Frequency of exposure? Type/route of exposure? nPEP is generally reserved for isolated or infrequent exposures that present a substantial risk for HIV acquisition (see Table 1 on HIV acquisition risk below).
- HIV status of exposure source? If the source is positive, is the source person on HIV treatment with antiretroviral therapy? If unknown, is the source person an injecting drug user or a man who has sex with men (MSM)?

Based on the initial evaluation, is nPEP recommended?
Answers to the questions asked during the initial evaluation of the patient will determine whether nPEP is indicated. Along with its updated recommendations, the CDC provided an algorithm to help guide evaluation and treatment.

Preferred HIV test
Administer an HIV test to all patients considered for nPEP, preferably the rapid combined antigen and antibody test (Ag/Ab), or just the antibody test if the Ag/Ab test is not available. nPEP is indicated only for persons without HIV infections. However, if results are not available during the initial evaluation, assume the patient is not infected. If indicated and started, nPEP can be discontinued if tests later shown the patient already has an HIV infection.
Laboratory testing
If nPEP is indicated, conduct laboratory testing. Lab testing is required to document the patient’s HIV status (and that of the source person, when available), identify and manage other conditions potentially resulting from exposure, identify conditions that may affect the nPEP medication regimen, and monitor safety or toxicities to the prescribed regimen.

nPEP treatment regimen for otherwise healthy adults and adolescents
In the absence of randomized clinical trials, data from a case/control study demonstrating an 81% reduction of HIV transmission after use of occupational PEP among hospital workers remains the strongest evidence for the benefit of nPEP.1,2 For patients offered nPEP, recommended treatment includes prescribing either of the following regimens for 28 days:
- Preferred regimen: tenofovir disoproxil fumarate (TDF) (300 mg) with emtricitabine (FTC) (200 mg) once daily plus either raltegravir (RAL) 400 mg twice daily or dolutegravir (DTG) 50 mg daily.
- Alternative regimen: TDF (300 mg) with FTC (200 mg) once daily plus darunavir (DRV) (800 mg) and ritonavir (RTV) (100 mg) once daily.
Additional considerations and nPEP treatment regimens for children, patients with decreased renal function, and pregnant women are included in the CDC guidelines.
Crucial Information for Patients on nPEP
Emphasize the importance of proper dosing and adherence.
Review the patient information for each drug in the regimen, specifically the black boxes, warnings, and side effects, and counsel your patients accordingly.
Transitioning from nPEP to PrEP or from PrEP to nPEP
If you have a patient who engages in behavior that places them at risk for frequent, recurrent exposures to HIV, consider transitioning them to PrEP (pre-exposure prophylaxis) following their 28-day course of nPEP.3 PrEP is a two-drug regimen taken daily on an ongoing basis.
Additionally, for patients who are already on PrEP but who have not taken their medications within a week before the possible exposure, consider initiating nPEP for 28 days and then reintroducing PrEP if their HIV status is negative and the problems with adherence can be addressed moving forward.
Raising Awareness About nPEP
Many people never expect to be exposed to HIV and may not know about the availability of PEP in an emergency situation. You can help raise awareness by making educational materials available in your waiting rooms and exam rooms. Brochures and other HIV/AIDS educational materials for patients are available from the CDC Act Against AIDS campaign.
Summary
Dr. Dominguez is a Captain, U.S. Public Health Service, epidemiology branch, division of HIV/AIDS prevention, CDC.
Additional resources
- The CDC recommends that everyone between the ages of 13 and 64 get tested for HIV at least once as part of routine health care. As part of its Act Against AIDS initiative, the CDC developed the HIV Screening Standard Care program, which provides free tools and resources to help clinicians and nurses incorporate routine HIV screening into primary care settings.
- HIV guidelines and recommendations .
- Postexposure prophylaxis (PEP)
- Pre-Exposure prophylaxis (PrEP)
References
1. Centers for Disease Control and Prevention. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV. United States, 2016. Accessed March 6, 2017.
2. Cardo DM et al. New Engl J Med. 1997;337(21):1485-90.
3. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States–2014: a clinical practice guideline. Accessed March 6, 2017.
AHA: Heart health helps optimize breast cancer outcomes
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
Breast cancer outcomes rely on “coexisting cardiovascular health” at every step in the patient journey, the American Heart Association has said in its first-ever scientific statement on the matter.
The statement, published in Circulation provides a comprehensive overview of cardiovascular disease and breast cancer prevalence and shared risk factors, as well as current recommendations on avoiding the cardiotoxic effects of some cancer treatments and strategies to prevent or treat CVD in breast cancer patients.
Cardiovascular disease and breast cancer are two entities that are frequently intertwined, Laxmi S. Mehta, MD, chair of the statement writing group, said in an interview.
“For any oncologic patient, it’s important to also consider their heart in the risk assessment because that also affects survival from the cancer,” said Dr. Mehta, the director of the Women’s Cardiovascular Health Program and an associate professor of medicine at the Ohio State University, Columbus.
Mortality risk attributable to CVD is higher in breast cancer survivors than in women who have no history of breast cancer, according to evidence Dr. Mehta and her colleagues cite in the statement.
Breast cancer and CVD share a number of common and sometimes modifiable risk factors, including dietary patterns, physical activity, and being overweight or obese, and tobacco use. There is “growing awareness” that modifying those risk factors may help prevent some cases of breast cancer.
Even in patients who have a breast cancer diagnosis, “from a cardiology standpoint, lifestyle is going to be key,” said Dr. Mehta. She said breast cancer patients can be encouraged to follow AHA recommendations to aim for 150 minutes of moderate aerobic exercise weekly and to follow an “overall healthy dietary pattern” that limits saturated and trans fats, sodium, red meat, sweets, and sugar-sweetened beverages.
Although left ventricular systolic dysfunction is the most common cardiovascular side effect associated with chemotherapy, other manifestations of early or delayed cardiotoxicity can include heart failure, hypertension, thromboembolic disease, pulmonary hypertension, pericarditis, and myocardial ischemia, according to the AHA scientific statement.
A wide range of standard breast cancer treatments cause cardiovascular adverse effects, including taxanes such as paclitaxel, anthracyclines such as doxorubicin, and alkylating agents such as cisplatin and cyclophosphamide, as outlined in the statement.
Targeted HER-2–directed therapies, including trastuzumab and pertuzumab, are associated with left ventricular dysfunction and heart failure, while emerging therapies, such as the cyclin-dependent kinase 4/6 inhibitors palbociclib and ribociclib, are associated with QTc prolongation, the statement also notes.
Current strategies for monitoring for cardiovascular toxicity, which typically involve myocardial strain imaging, assessing cardiac biomarkers, or a combination of imaging and biomarkers, are outlined in the report.
To mitigate the impact of cancer treatments on cardiovascular health, several oncologic strategies are useful, according to Dr. Mehta and colleagues.
In multiple clinical trials of breast cancer patients receiving doxorubicin or epirubicin, the iron-binding agent dexrazoxane reduced the combined endpoint of decreased left ventricular ejection fraction or development of heart failure, the authors said.
Likewise, clinical trial evidence has suggested cardiotoxicity associated with doxorubicin can be mitigated through administration via infusion as opposed to bolus and with longer versus shorter infusion durations, they continued.
Extreme cases or difficult-to-manage patients can be referred to a center that has an active program in cardio-oncology, a newer and rapidly expanding field, according to Dr. Mehta.
“That’s where there’s tight collaboration in terms of understanding the current treatments, and that’s also where research on how to best take care of these patients will be conducted,” she said.
Dr. Mehta reported no disclosures. Coauthors reported disclosures related to Amgen, Boehringer Ingelheim, Genentech, AstraZeneca, Lilly, Roche, Novartis, and others.
SOURCE: Mehta LS et al. Circulation. 2017 Jan 22. doi: 10.1161/CIR.0000000000000556.
FROM CIRCULATION
APA guideline backs naltrexone, acamprosate for alcohol use disorder
Naltrexone or acamprosate should be offered as first-line pharmacologic therapy to patients with moderate to severe alcohol use disorder (AUD) who do not respond to nonpharmacologic therapy alone, according to a practice guideline published by the American Psychiatric Association.
if naltrexone and acamprosate are not effective or well tolerated, the report said.
The APA guideline recommends against the use of antidepressants and benzodiazepines for patients with alcohol use disorder, except for situations where a co-occurring disorder requires treatment. In addition, the guideline recommends against the use of acamprosate in patients with renal impairment, and specifies that naltrexone should not be used by patients with acute hepatitis, hepatic failure, or opioid dependence.
“Naltrexone and acamprosate have the best available evidence as pharmacotherapy for patients with AUD,” wrote Victor I. Reus, MD, and his coauthors in the APA Guideline Writing Group, which formed the guideline using a systematic review of current literature in accordance with Institute of Medicine (now called the National Academy of Medicine) and Agency for Healthcare Research and Quality standards.
Naltrexone, an opioid receptor antagonist, is effective in treating both AUD and opioid use disorder. Studies have shown that it may decrease cravings, and is associated with fewer drinking days and a reduced likelihood of return to drinking, the authors reported. In patients with a history of renal impairment, serum creatinine should be measured, and results should be reviewed before initiating treatment with acamprosate – a synthetic amino acid.
Disulfiram breaks down acetaldehyde, an ethanol byproduct, and should be used only to treat patients with a goal of abstinence. It is not recommended as a first-line therapy because of the side effects of concurrent alcohol use, including tachycardia, flushing, headache, nausea, and vomiting, reported Dr. Reus of the psychiatry department at the University of California, San Francisco, and his coauthors.
“Patients should be fully informed of the physiological consequences of consuming alcohol while taking disulfiram and should agree to taking the medication,” the authors wrote. “They should be instructed to abstain from drinking alcohol for at least 12 hours before taking a dose of the medication and be advised that reactions with alcohol can occur up to 14 days after taking disulfiram.”
Lastly, topiramate and gabapentin may be used in patients for whom naltrexone and acamprosate are ineffective, though topiramate may have side effects of concern to the patient, including cognitive dysfunction and numbness, tingling, paresthesias, dizziness, taste abnormalities, and decreased appetite or weight loss, the report said.
Although the APA guideline acknowledges the importance of psychiatric evaluation and nonpharmacologic treatments such as cognitive-behavioral therapy and 12-step programs, it does not provide recommendations on those treatment options.
Further research on alcohol use disorder should include study of quantitative measures for longitudinal monitoring, co-occurring medical and psychiatric conditions, and the effectiveness of naltrexone versus combination therapy for patients with both AUD and opioid use disorder, the authors said.
“The overall goal of this guideline is to enhance the treatment of AUD for millions of affected individuals, thereby reducing the significant psychosocial and public health consequences of this important psychiatric condition,” the report concluded. An executive summary of the guideline was published in the American Journal of Psychiatry.
The guideline authors disclosed no conflicts of interest with their work on the guideline.
SOURCE: Reus VI et al. Am J Psychiatry. 2018;175:86-90.
Naltrexone or acamprosate should be offered as first-line pharmacologic therapy to patients with moderate to severe alcohol use disorder (AUD) who do not respond to nonpharmacologic therapy alone, according to a practice guideline published by the American Psychiatric Association.
if naltrexone and acamprosate are not effective or well tolerated, the report said.
The APA guideline recommends against the use of antidepressants and benzodiazepines for patients with alcohol use disorder, except for situations where a co-occurring disorder requires treatment. In addition, the guideline recommends against the use of acamprosate in patients with renal impairment, and specifies that naltrexone should not be used by patients with acute hepatitis, hepatic failure, or opioid dependence.
“Naltrexone and acamprosate have the best available evidence as pharmacotherapy for patients with AUD,” wrote Victor I. Reus, MD, and his coauthors in the APA Guideline Writing Group, which formed the guideline using a systematic review of current literature in accordance with Institute of Medicine (now called the National Academy of Medicine) and Agency for Healthcare Research and Quality standards.
Naltrexone, an opioid receptor antagonist, is effective in treating both AUD and opioid use disorder. Studies have shown that it may decrease cravings, and is associated with fewer drinking days and a reduced likelihood of return to drinking, the authors reported. In patients with a history of renal impairment, serum creatinine should be measured, and results should be reviewed before initiating treatment with acamprosate – a synthetic amino acid.
Disulfiram breaks down acetaldehyde, an ethanol byproduct, and should be used only to treat patients with a goal of abstinence. It is not recommended as a first-line therapy because of the side effects of concurrent alcohol use, including tachycardia, flushing, headache, nausea, and vomiting, reported Dr. Reus of the psychiatry department at the University of California, San Francisco, and his coauthors.
“Patients should be fully informed of the physiological consequences of consuming alcohol while taking disulfiram and should agree to taking the medication,” the authors wrote. “They should be instructed to abstain from drinking alcohol for at least 12 hours before taking a dose of the medication and be advised that reactions with alcohol can occur up to 14 days after taking disulfiram.”
Lastly, topiramate and gabapentin may be used in patients for whom naltrexone and acamprosate are ineffective, though topiramate may have side effects of concern to the patient, including cognitive dysfunction and numbness, tingling, paresthesias, dizziness, taste abnormalities, and decreased appetite or weight loss, the report said.
Although the APA guideline acknowledges the importance of psychiatric evaluation and nonpharmacologic treatments such as cognitive-behavioral therapy and 12-step programs, it does not provide recommendations on those treatment options.
Further research on alcohol use disorder should include study of quantitative measures for longitudinal monitoring, co-occurring medical and psychiatric conditions, and the effectiveness of naltrexone versus combination therapy for patients with both AUD and opioid use disorder, the authors said.
“The overall goal of this guideline is to enhance the treatment of AUD for millions of affected individuals, thereby reducing the significant psychosocial and public health consequences of this important psychiatric condition,” the report concluded. An executive summary of the guideline was published in the American Journal of Psychiatry.
The guideline authors disclosed no conflicts of interest with their work on the guideline.
SOURCE: Reus VI et al. Am J Psychiatry. 2018;175:86-90.
Naltrexone or acamprosate should be offered as first-line pharmacologic therapy to patients with moderate to severe alcohol use disorder (AUD) who do not respond to nonpharmacologic therapy alone, according to a practice guideline published by the American Psychiatric Association.
if naltrexone and acamprosate are not effective or well tolerated, the report said.
The APA guideline recommends against the use of antidepressants and benzodiazepines for patients with alcohol use disorder, except for situations where a co-occurring disorder requires treatment. In addition, the guideline recommends against the use of acamprosate in patients with renal impairment, and specifies that naltrexone should not be used by patients with acute hepatitis, hepatic failure, or opioid dependence.
“Naltrexone and acamprosate have the best available evidence as pharmacotherapy for patients with AUD,” wrote Victor I. Reus, MD, and his coauthors in the APA Guideline Writing Group, which formed the guideline using a systematic review of current literature in accordance with Institute of Medicine (now called the National Academy of Medicine) and Agency for Healthcare Research and Quality standards.
Naltrexone, an opioid receptor antagonist, is effective in treating both AUD and opioid use disorder. Studies have shown that it may decrease cravings, and is associated with fewer drinking days and a reduced likelihood of return to drinking, the authors reported. In patients with a history of renal impairment, serum creatinine should be measured, and results should be reviewed before initiating treatment with acamprosate – a synthetic amino acid.
Disulfiram breaks down acetaldehyde, an ethanol byproduct, and should be used only to treat patients with a goal of abstinence. It is not recommended as a first-line therapy because of the side effects of concurrent alcohol use, including tachycardia, flushing, headache, nausea, and vomiting, reported Dr. Reus of the psychiatry department at the University of California, San Francisco, and his coauthors.
“Patients should be fully informed of the physiological consequences of consuming alcohol while taking disulfiram and should agree to taking the medication,” the authors wrote. “They should be instructed to abstain from drinking alcohol for at least 12 hours before taking a dose of the medication and be advised that reactions with alcohol can occur up to 14 days after taking disulfiram.”
Lastly, topiramate and gabapentin may be used in patients for whom naltrexone and acamprosate are ineffective, though topiramate may have side effects of concern to the patient, including cognitive dysfunction and numbness, tingling, paresthesias, dizziness, taste abnormalities, and decreased appetite or weight loss, the report said.
Although the APA guideline acknowledges the importance of psychiatric evaluation and nonpharmacologic treatments such as cognitive-behavioral therapy and 12-step programs, it does not provide recommendations on those treatment options.
Further research on alcohol use disorder should include study of quantitative measures for longitudinal monitoring, co-occurring medical and psychiatric conditions, and the effectiveness of naltrexone versus combination therapy for patients with both AUD and opioid use disorder, the authors said.
“The overall goal of this guideline is to enhance the treatment of AUD for millions of affected individuals, thereby reducing the significant psychosocial and public health consequences of this important psychiatric condition,” the report concluded. An executive summary of the guideline was published in the American Journal of Psychiatry.
The guideline authors disclosed no conflicts of interest with their work on the guideline.
SOURCE: Reus VI et al. Am J Psychiatry. 2018;175:86-90.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
CDC provides advice on recent hepatitis A outbreaks
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3
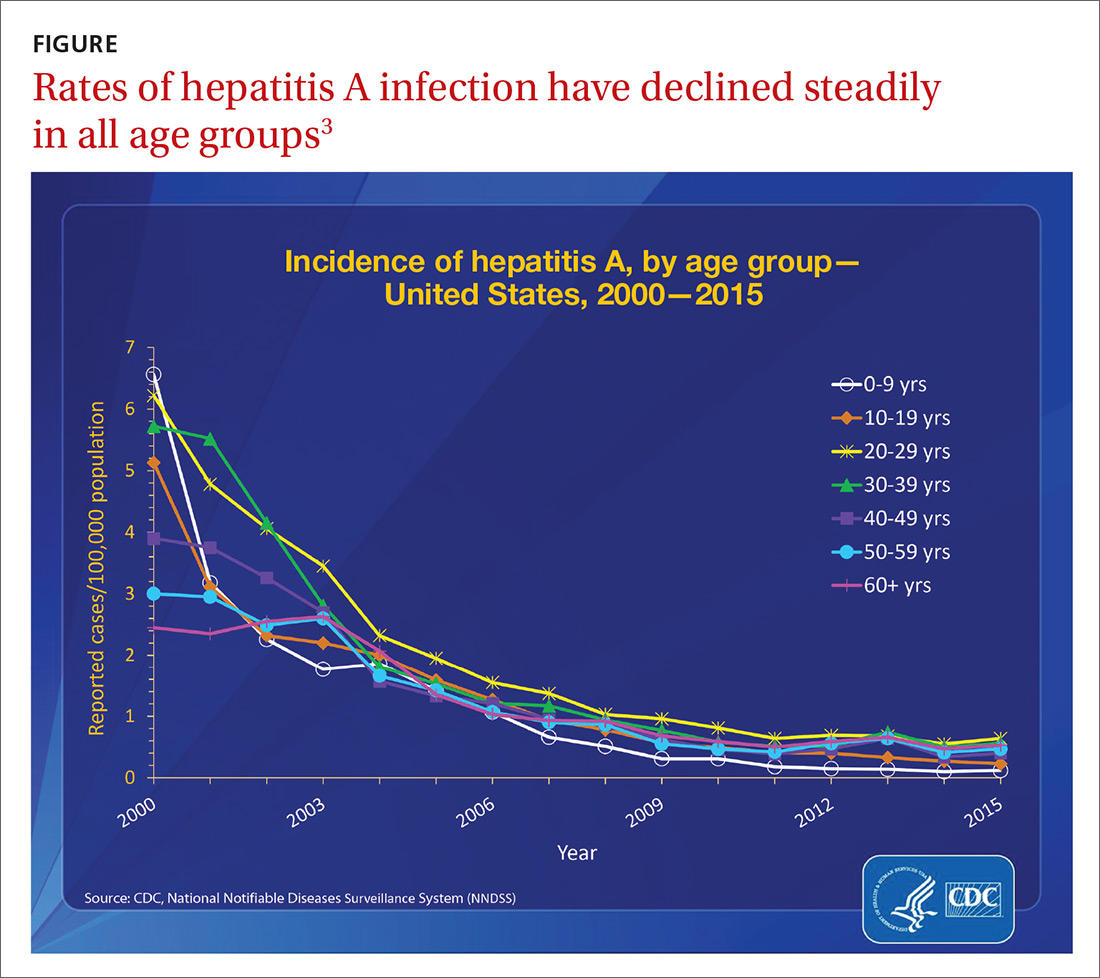
Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.
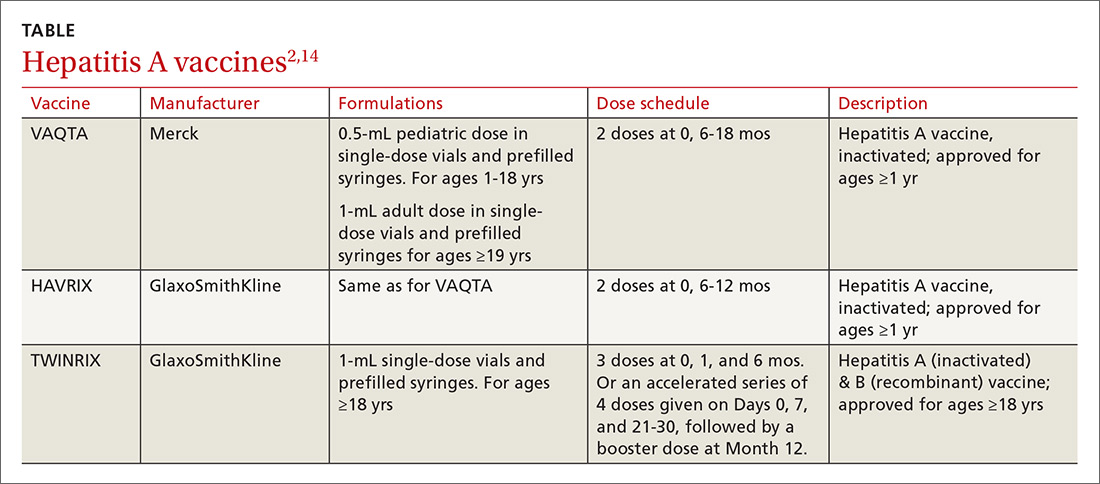
The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3

Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.

The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3

Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.

The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
ADA guidelines embrace heart health
Recent studies that confirm the cardiovascular benefit of some antihyperglycemic agents are shaping the newest therapeutic recommendations for patients with type 2 diabetes and comorbid atherosclerotic cardiovascular disease (ASCVD).
Treatment for these patients – as all with diabetes – should start with lifestyle modifications and metformin. But in its new position statement, the American Diabetes Association now recommends that clinicians consider adding agents proved to reduce major cardiovascular events and cardiovascular death – such as the sodium glucose cotransporter-2 (SGLT2) inhibitor empagliflozin or the glucagon-like peptide 1 (GLP-1) agonist liraglutide – to the regimens of patients with diabetes and ASCVD (Diabetes Care 2018;41(Suppl. 1):S86-S104. doi: 10.2337/dc18-S009).
The medications are indicated if, after being treated with lifestyle and metformin therapy, the patient isn’t meeting hemoglobin A1c goals, said Rita R. Kalyani, MD, who led the ADA’s 12-member writing committee. But clinicians may also consider adding these agents for cardiovascular benefit alone, even when glucose control is adequate on a regimen of lifestyle modification and metformin, with dose adjustments as appropriate, she said in an interview.
“A1c remains the main target of sequencing antihyperglycemic therapies, if it’s not reached after 3 months,” said Dr. Kalyani of Johns Hopkins University, Baltimore. “But, it could also be that the provider, after consulting with the patient, feels it’s appropriate to add one of these agents solely for cardioprotective benefit in patients with ASCVD.”
The recommendation to incorporate agents with cardiovascular benefit is related directly to data from two trials, LEADER and EMPA-REG, which support this recommendation. All of these cardiovascular outcome trials included a majority of patients who were already on metformin. “We developed these evidence-based recommendations based on these trials and to appropriately reflect the populations studied,” said Dr. Kalyani.
The 2018 update contains a number of new recommendations; more will be added as new data emerge, since the ADA intends it to be a continuously refreshed “living document.” This makes it especially clinically useful, Paul S. Jellinger, MD, said in an interview. A member of the writing committee of the American Association of Clinical Endocrinologists’ diabetes management guidelines, Dr. Jellinger feels ADA’s previous versions have not been as targeted as this new one and, he hopes, its subsequent iterations.
“This is a nice enhancement of previously published guidelines for diabetes therapy,” said Dr. Jellinger, professor of clinical medicine at the University of Miami. “For the first time, ADA is providing some guidance in terms of which agents to use. It’s definitely more prescriptive than it was in the past, when, unlike the AACE Diabetes Guidelines, it was a palette of choice for clinicians, but with very little guidance about which agent to pick. The guidance for patients with cardiovascular disease in particular is big news because these antihyperglycemic agents showed such a significant cardiovascular benefit in the trials.”
While the document gives a detailed algorithm of advancing therapy in patients with ASCVD, it doesn’t specify a preference for a specific drug class after metformin therapy in patients without ASCVD. Instead, it provides a detailed table listing the drug-specific effects and patient factors to consider when selecting from different classes of antihyperglycemic agents ( SGLT2 inhibitors, GLP-1 agonists, DPP-4 inhibitors, thiazolidinones, sulfonylureas, and insulins). The table notes the drugs’ general efficacy in diabetes, and their impact on hypoglycemia, weight gain, and cardiovascular and renal health. The table also includes the Food and Drug Administration black box warnings that are on some of these medications.
Another helpful feature is a cost comparison of antidiabetic agents, Dr. Kalyani noted. “Last year we added comprehensive cost tables for all the different insulins and noninsulins, and this year we added a second data set of cost information, to assist the provider when prescribing these agents.”
The pricing information is a very important addition to this guideline, and one that clinicians will appreciate, said Richard Hellman, MD, clinical professor of medicine at the University of Missouri–Kansas City.
“In this document, ADA is urging providers of care to ask about whether the cost of their diabetes care is more than they can deal with. They present tables which compare the costs of the current blood glucose lowering agents used in the U.S., and it is plain to see that many patients, without insurance coverage, will find some of the medications unaffordable,” said Dr. Hellman, a past president of AACE. “They also provide data that show half of all patients with diabetes have financial problems,” and he suspects that medication costs are an important component of their financial insecurity.
The document also notes data from the 2017 National Health and Nutrition Examination Survey, which found that 10% of people with diabetes have severe food insecurity and 20% have mild food insecurity (Diabetes Educ. 2017;43:260-71. doi: 10.1177/0145721717699890).
“Another thing the document points out is that two-thirds of the patients who don’t take all their medications due to cost don’t tell their doctor,” Dr. Hellman said. “The ADA is making the point that providers have a responsibility to ask if a patient is not taking certain medications because of the cost. We have so many better tools to manage this disease, but so many of these tools are unaffordable.”
While the treatment algorithm for patients with ASCVD will likely be embraced, another new recommendation may stir the pot a bit, Dr. Hellman noted. The section on cardiovascular disease and risk management sticks to a definition of hypertension as 140/90 mm Hg or higher – a striking diversion from the new 130/80 mm Hg limit set this fall by both the American Heart Association and the American College of Cardiology.
“This difference in recommendations is very important and will be controversial,” Dr. Hellman said, adding that he agrees with this clinical point.
Again, this recommendation is grounded in clinical trials, which suggest that people with diabetes don’t benefit from overly strict blood pressure control. The new AHA/ACC recommendations largely drew on data from the SPRINT trial, which was conducted in an entirely nondiabetic population. “These gave a clear signal that a lower BP target is beneficial to that group,” Dr. Hellman said.
But large well-designed randomized controlled trials of intensive blood pressure lowering in people with diabetes, such as ACCORD-BP, did not demonstrate that intensive blood-pressure lowering targeting a systolic less than120 mm Hg had a significant benefit on the composite primary cardiovascular endpoint. And while the ADVANCE BP trial found that the composite endpoint was improved with intensive blood pressure lowering, the blood pressure level achieved in the intervention group was 136/73 mm Hg.
“This recommendation is based on current evidence for people with diabetes,” Dr. Kalyani said. “We maintain our definition of hypertension as 140/90 mm Hg or higher based on the results of large clinical trials specifically in people with diabetes but emphasize that intensification of antihypertensive therapy to target lower blood pressures (less than 130/80 mm Hg) may be beneficial for high-risk patients with diabetes such as those with cardiovascular disease. We are constantly assessing the evidence and will continue to review the results of new studies for potential incorporation into recommendations in the future.”
Dr. Kalyani and Dr. Hellman had no financial disclosures. Dr. Jellinger has been a speaker for several pharmaceutical companies.
SOURCE: Kalyani R et al. Diabetes Care 2018;41(Suppl. 1):S86-S104 doi: 10.2337/dc18-S009
This article was updated 12/21/17.
Recent studies that confirm the cardiovascular benefit of some antihyperglycemic agents are shaping the newest therapeutic recommendations for patients with type 2 diabetes and comorbid atherosclerotic cardiovascular disease (ASCVD).
Treatment for these patients – as all with diabetes – should start with lifestyle modifications and metformin. But in its new position statement, the American Diabetes Association now recommends that clinicians consider adding agents proved to reduce major cardiovascular events and cardiovascular death – such as the sodium glucose cotransporter-2 (SGLT2) inhibitor empagliflozin or the glucagon-like peptide 1 (GLP-1) agonist liraglutide – to the regimens of patients with diabetes and ASCVD (Diabetes Care 2018;41(Suppl. 1):S86-S104. doi: 10.2337/dc18-S009).
The medications are indicated if, after being treated with lifestyle and metformin therapy, the patient isn’t meeting hemoglobin A1c goals, said Rita R. Kalyani, MD, who led the ADA’s 12-member writing committee. But clinicians may also consider adding these agents for cardiovascular benefit alone, even when glucose control is adequate on a regimen of lifestyle modification and metformin, with dose adjustments as appropriate, she said in an interview.
“A1c remains the main target of sequencing antihyperglycemic therapies, if it’s not reached after 3 months,” said Dr. Kalyani of Johns Hopkins University, Baltimore. “But, it could also be that the provider, after consulting with the patient, feels it’s appropriate to add one of these agents solely for cardioprotective benefit in patients with ASCVD.”
The recommendation to incorporate agents with cardiovascular benefit is related directly to data from two trials, LEADER and EMPA-REG, which support this recommendation. All of these cardiovascular outcome trials included a majority of patients who were already on metformin. “We developed these evidence-based recommendations based on these trials and to appropriately reflect the populations studied,” said Dr. Kalyani.
The 2018 update contains a number of new recommendations; more will be added as new data emerge, since the ADA intends it to be a continuously refreshed “living document.” This makes it especially clinically useful, Paul S. Jellinger, MD, said in an interview. A member of the writing committee of the American Association of Clinical Endocrinologists’ diabetes management guidelines, Dr. Jellinger feels ADA’s previous versions have not been as targeted as this new one and, he hopes, its subsequent iterations.
“This is a nice enhancement of previously published guidelines for diabetes therapy,” said Dr. Jellinger, professor of clinical medicine at the University of Miami. “For the first time, ADA is providing some guidance in terms of which agents to use. It’s definitely more prescriptive than it was in the past, when, unlike the AACE Diabetes Guidelines, it was a palette of choice for clinicians, but with very little guidance about which agent to pick. The guidance for patients with cardiovascular disease in particular is big news because these antihyperglycemic agents showed such a significant cardiovascular benefit in the trials.”
While the document gives a detailed algorithm of advancing therapy in patients with ASCVD, it doesn’t specify a preference for a specific drug class after metformin therapy in patients without ASCVD. Instead, it provides a detailed table listing the drug-specific effects and patient factors to consider when selecting from different classes of antihyperglycemic agents ( SGLT2 inhibitors, GLP-1 agonists, DPP-4 inhibitors, thiazolidinones, sulfonylureas, and insulins). The table notes the drugs’ general efficacy in diabetes, and their impact on hypoglycemia, weight gain, and cardiovascular and renal health. The table also includes the Food and Drug Administration black box warnings that are on some of these medications.
Another helpful feature is a cost comparison of antidiabetic agents, Dr. Kalyani noted. “Last year we added comprehensive cost tables for all the different insulins and noninsulins, and this year we added a second data set of cost information, to assist the provider when prescribing these agents.”
The pricing information is a very important addition to this guideline, and one that clinicians will appreciate, said Richard Hellman, MD, clinical professor of medicine at the University of Missouri–Kansas City.
“In this document, ADA is urging providers of care to ask about whether the cost of their diabetes care is more than they can deal with. They present tables which compare the costs of the current blood glucose lowering agents used in the U.S., and it is plain to see that many patients, without insurance coverage, will find some of the medications unaffordable,” said Dr. Hellman, a past president of AACE. “They also provide data that show half of all patients with diabetes have financial problems,” and he suspects that medication costs are an important component of their financial insecurity.
The document also notes data from the 2017 National Health and Nutrition Examination Survey, which found that 10% of people with diabetes have severe food insecurity and 20% have mild food insecurity (Diabetes Educ. 2017;43:260-71. doi: 10.1177/0145721717699890).
“Another thing the document points out is that two-thirds of the patients who don’t take all their medications due to cost don’t tell their doctor,” Dr. Hellman said. “The ADA is making the point that providers have a responsibility to ask if a patient is not taking certain medications because of the cost. We have so many better tools to manage this disease, but so many of these tools are unaffordable.”
While the treatment algorithm for patients with ASCVD will likely be embraced, another new recommendation may stir the pot a bit, Dr. Hellman noted. The section on cardiovascular disease and risk management sticks to a definition of hypertension as 140/90 mm Hg or higher – a striking diversion from the new 130/80 mm Hg limit set this fall by both the American Heart Association and the American College of Cardiology.
“This difference in recommendations is very important and will be controversial,” Dr. Hellman said, adding that he agrees with this clinical point.
Again, this recommendation is grounded in clinical trials, which suggest that people with diabetes don’t benefit from overly strict blood pressure control. The new AHA/ACC recommendations largely drew on data from the SPRINT trial, which was conducted in an entirely nondiabetic population. “These gave a clear signal that a lower BP target is beneficial to that group,” Dr. Hellman said.
But large well-designed randomized controlled trials of intensive blood pressure lowering in people with diabetes, such as ACCORD-BP, did not demonstrate that intensive blood-pressure lowering targeting a systolic less than120 mm Hg had a significant benefit on the composite primary cardiovascular endpoint. And while the ADVANCE BP trial found that the composite endpoint was improved with intensive blood pressure lowering, the blood pressure level achieved in the intervention group was 136/73 mm Hg.
“This recommendation is based on current evidence for people with diabetes,” Dr. Kalyani said. “We maintain our definition of hypertension as 140/90 mm Hg or higher based on the results of large clinical trials specifically in people with diabetes but emphasize that intensification of antihypertensive therapy to target lower blood pressures (less than 130/80 mm Hg) may be beneficial for high-risk patients with diabetes such as those with cardiovascular disease. We are constantly assessing the evidence and will continue to review the results of new studies for potential incorporation into recommendations in the future.”
Dr. Kalyani and Dr. Hellman had no financial disclosures. Dr. Jellinger has been a speaker for several pharmaceutical companies.
SOURCE: Kalyani R et al. Diabetes Care 2018;41(Suppl. 1):S86-S104 doi: 10.2337/dc18-S009
This article was updated 12/21/17.
Recent studies that confirm the cardiovascular benefit of some antihyperglycemic agents are shaping the newest therapeutic recommendations for patients with type 2 diabetes and comorbid atherosclerotic cardiovascular disease (ASCVD).
Treatment for these patients – as all with diabetes – should start with lifestyle modifications and metformin. But in its new position statement, the American Diabetes Association now recommends that clinicians consider adding agents proved to reduce major cardiovascular events and cardiovascular death – such as the sodium glucose cotransporter-2 (SGLT2) inhibitor empagliflozin or the glucagon-like peptide 1 (GLP-1) agonist liraglutide – to the regimens of patients with diabetes and ASCVD (Diabetes Care 2018;41(Suppl. 1):S86-S104. doi: 10.2337/dc18-S009).
The medications are indicated if, after being treated with lifestyle and metformin therapy, the patient isn’t meeting hemoglobin A1c goals, said Rita R. Kalyani, MD, who led the ADA’s 12-member writing committee. But clinicians may also consider adding these agents for cardiovascular benefit alone, even when glucose control is adequate on a regimen of lifestyle modification and metformin, with dose adjustments as appropriate, she said in an interview.
“A1c remains the main target of sequencing antihyperglycemic therapies, if it’s not reached after 3 months,” said Dr. Kalyani of Johns Hopkins University, Baltimore. “But, it could also be that the provider, after consulting with the patient, feels it’s appropriate to add one of these agents solely for cardioprotective benefit in patients with ASCVD.”
The recommendation to incorporate agents with cardiovascular benefit is related directly to data from two trials, LEADER and EMPA-REG, which support this recommendation. All of these cardiovascular outcome trials included a majority of patients who were already on metformin. “We developed these evidence-based recommendations based on these trials and to appropriately reflect the populations studied,” said Dr. Kalyani.
The 2018 update contains a number of new recommendations; more will be added as new data emerge, since the ADA intends it to be a continuously refreshed “living document.” This makes it especially clinically useful, Paul S. Jellinger, MD, said in an interview. A member of the writing committee of the American Association of Clinical Endocrinologists’ diabetes management guidelines, Dr. Jellinger feels ADA’s previous versions have not been as targeted as this new one and, he hopes, its subsequent iterations.
“This is a nice enhancement of previously published guidelines for diabetes therapy,” said Dr. Jellinger, professor of clinical medicine at the University of Miami. “For the first time, ADA is providing some guidance in terms of which agents to use. It’s definitely more prescriptive than it was in the past, when, unlike the AACE Diabetes Guidelines, it was a palette of choice for clinicians, but with very little guidance about which agent to pick. The guidance for patients with cardiovascular disease in particular is big news because these antihyperglycemic agents showed such a significant cardiovascular benefit in the trials.”
While the document gives a detailed algorithm of advancing therapy in patients with ASCVD, it doesn’t specify a preference for a specific drug class after metformin therapy in patients without ASCVD. Instead, it provides a detailed table listing the drug-specific effects and patient factors to consider when selecting from different classes of antihyperglycemic agents ( SGLT2 inhibitors, GLP-1 agonists, DPP-4 inhibitors, thiazolidinones, sulfonylureas, and insulins). The table notes the drugs’ general efficacy in diabetes, and their impact on hypoglycemia, weight gain, and cardiovascular and renal health. The table also includes the Food and Drug Administration black box warnings that are on some of these medications.
Another helpful feature is a cost comparison of antidiabetic agents, Dr. Kalyani noted. “Last year we added comprehensive cost tables for all the different insulins and noninsulins, and this year we added a second data set of cost information, to assist the provider when prescribing these agents.”
The pricing information is a very important addition to this guideline, and one that clinicians will appreciate, said Richard Hellman, MD, clinical professor of medicine at the University of Missouri–Kansas City.
“In this document, ADA is urging providers of care to ask about whether the cost of their diabetes care is more than they can deal with. They present tables which compare the costs of the current blood glucose lowering agents used in the U.S., and it is plain to see that many patients, without insurance coverage, will find some of the medications unaffordable,” said Dr. Hellman, a past president of AACE. “They also provide data that show half of all patients with diabetes have financial problems,” and he suspects that medication costs are an important component of their financial insecurity.
The document also notes data from the 2017 National Health and Nutrition Examination Survey, which found that 10% of people with diabetes have severe food insecurity and 20% have mild food insecurity (Diabetes Educ. 2017;43:260-71. doi: 10.1177/0145721717699890).
“Another thing the document points out is that two-thirds of the patients who don’t take all their medications due to cost don’t tell their doctor,” Dr. Hellman said. “The ADA is making the point that providers have a responsibility to ask if a patient is not taking certain medications because of the cost. We have so many better tools to manage this disease, but so many of these tools are unaffordable.”
While the treatment algorithm for patients with ASCVD will likely be embraced, another new recommendation may stir the pot a bit, Dr. Hellman noted. The section on cardiovascular disease and risk management sticks to a definition of hypertension as 140/90 mm Hg or higher – a striking diversion from the new 130/80 mm Hg limit set this fall by both the American Heart Association and the American College of Cardiology.
“This difference in recommendations is very important and will be controversial,” Dr. Hellman said, adding that he agrees with this clinical point.
Again, this recommendation is grounded in clinical trials, which suggest that people with diabetes don’t benefit from overly strict blood pressure control. The new AHA/ACC recommendations largely drew on data from the SPRINT trial, which was conducted in an entirely nondiabetic population. “These gave a clear signal that a lower BP target is beneficial to that group,” Dr. Hellman said.
But large well-designed randomized controlled trials of intensive blood pressure lowering in people with diabetes, such as ACCORD-BP, did not demonstrate that intensive blood-pressure lowering targeting a systolic less than120 mm Hg had a significant benefit on the composite primary cardiovascular endpoint. And while the ADVANCE BP trial found that the composite endpoint was improved with intensive blood pressure lowering, the blood pressure level achieved in the intervention group was 136/73 mm Hg.
“This recommendation is based on current evidence for people with diabetes,” Dr. Kalyani said. “We maintain our definition of hypertension as 140/90 mm Hg or higher based on the results of large clinical trials specifically in people with diabetes but emphasize that intensification of antihypertensive therapy to target lower blood pressures (less than 130/80 mm Hg) may be beneficial for high-risk patients with diabetes such as those with cardiovascular disease. We are constantly assessing the evidence and will continue to review the results of new studies for potential incorporation into recommendations in the future.”
Dr. Kalyani and Dr. Hellman had no financial disclosures. Dr. Jellinger has been a speaker for several pharmaceutical companies.
SOURCE: Kalyani R et al. Diabetes Care 2018;41(Suppl. 1):S86-S104 doi: 10.2337/dc18-S009
This article was updated 12/21/17.
EXPERT ANALYSIS FROM DIABETES CARE
Testing for latent tuberculosis infection
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.
While cases of active tuberculosis are relatively rare in the United States, TB is a major cause of morbidity and mortality worldwide. In the United States, there are an estimated 11 million individuals who have latent TB infection (LTBI). Without prophylactic treatment, somewhere between 4%-6% of individuals with LTBI will develop active disease during their lifetimes; roughly half of these cases will occur within a few years of the initial infection. Treatment of LTBI reduces – but does not eliminate – the risk for active disease, decreasing the consequences of active disease for the patient and the risk of transmitting infection to others.
Diagnostic tests for LTBI
The tuberculin skin test (TST) has been the standard method of diagnosing LTBI. It involves measuring induration caused by a delayed-type hypersensitivity reaction to Mycobacterium tuberculosis (Mtb) 2 or 3 days after injecting the reagent into the skin. The TST can result in false positives when detecting antibodies to BCG and nontuberculous mycobacteria, and false negatives when the patient does not demonstrate a robust immune response. A newer testing method is the Interferon Gamma Release Assay (IGRA), which involves phlebotomy, followed by a series of laboratory procedures that measure IFN-gamma release by T cells that have been sensitized to Mtb. The sensitivity of IGRA is similar to the TST, but it has better specificity; it is much less likely to react to antigens from BCG or nontuberculous mycobacteria. As detailed below, this guideline suggests a significantly more prominent role for IGRA, compared with previous recommendations.
Recommendation 1. Perform an IGRA, rather than a TST, in individuals 5 years or older who meet the following criteria: 1) are likely to be infected with Mtb; 2) have a low or intermediate risk of disease progression; 3) in whom it has been decided that testing for LTBI is warranted. A TST is an acceptable alternative, particularly if an IGRA is not available, is too costly, or is too burdensome. If an individual either has a history of BCG vaccination or is unlikely to return to have their TST read, then it is strongly recommended to use the IGRA as the test of choice.
Recommendation 2. There are insufficient data to recommend a preference for either a TST or an IGRA as the first-line diagnostic test in individuals 5 years or older who are likely to be infected with Mtb, who have a high risk of progression to active disease, and in whom it has been determined that diagnostic testing for LTBI infection is warranted; either test would be acceptable. In very high-risk patients, consider dual testing, with a positive result from either test (TST or IGRA) being considered positive.
Recommendation 3. Guidelines do not recommend testing for persons at low risk for Mtb infection. However, the authors recognize that testing in such persons may nevertheless be mandated in certain situations (for example in some school or child care settings). In these cases, the authors recommend performing an IGRA instead of a TST, to minimize the chance of a false-positive result, although a TST is an acceptable alternative. Furthermore, if the initial test is positive, they suggest performing a confirmatory test (either an IGRA or TST) and considering the person infected only if both tests are positive.
Recommendation 4. The authors suggest performing a TST rather than an IGRA in healthy children less than 5 years of age for whom it has been decided that diagnostic testing for LTBI is warranted. This recommendation reflects the limited body of evidence regarding IGRA testing in young children and the apparent decreased sensitivity (i.e. more false negatives) in this population, compared with TST use.
In the area of serial testing for TB infection, often done in health care and institutional settings, the guideline points out areas of uncertainty with IGRA testing. Specifically, the IGRA test is subject to variability in readings and boosting with antigen exposure that can complicate interpretation of apparent conversion on repeat testing. One longitudinal study showed conversion rates with IGRA to be six to nine times higher than that seen for the TST, and those conversions were thought to represent false positive tests. The guideline concludes that, “There is insufficient information available to guide the establishment of definitive criteria for the conversion.” The committee thought that a positive test in a low-risk individual was likely to be a false-positive result and recommended repeat testing. Because of the possibility of boosting with antigen exposure in situations where dual testing is anticipated, it may be preferable to obtain a specimen for IGRA prior to, or concurrently with TST placement.
Bottom line
Current guidelines suggest a more prominent role for IGRA in testing for LTBI, particularly when the likelihood of exposure is low and in situations where a person may have received BCG vaccination, or would be unlikely to return for TST reading.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Clark is associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
Reference
Lewisohn DM et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Inf Dis. 2017;64(2):111-5.