User login
Immune response detected in most IBD patients after COVID vaccines
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
Most patients with inflammatory bowel disease (IBD) develop a humoral immune response after completing an mRNA SARS-CoV-2 vaccine series, according to data from almost 800 patients.
Anti–receptor binding domain IgG antibodies specific to SARS-CoV-2 were detectable in 95% of patients, with “generally similar” results across vaccine type, age group, and medication class, apart from corticosteroid users, who had an 86% antibody detection rate, reported lead author Kimberly N. Weaver, MD, of the University of North Carolina at Chapel Hill, and colleagues.
“Patients with IBD on immunosuppressive medications have the potential for attenuated response to the SARS-CoV-2 vaccination,” Dr. Weaver said at the annual meeting of the American College of Gastroenterology.
In support of this possibility, Dr. Weaver cited two recent trials from earlier in 2021: one demonstrated blunted antibody responses in IBD patients taking infliximab, while the other showed that full vaccination was less effective at preventing SARS-CoV-2 infection among patients with IBD than nonimmunosuppressed individuals.
To better characterize antibody responses after receiving an mRNA vaccination series, Dr. Weaver and colleagues launched the PREVENT-COVID trial, including the present dataset of 787 patients with IBD older than 12 years, all of whom provided serum samples 8 weeks after completing an mRNA vaccine series. Patients with positive nucleocapsid antibody (indicating prior infection), and/or those who reported prior COVID-19 infection, were excluded. Most patients were White (95%) and female (73%), with an average age of 48 years. Slightly more patients received the BNT162b2 vaccine than the mRNA-1273 vaccine (58% vs. 42%).
At 8 weeks, 752 out of 787 patients had detectable antibodies (95%). Antibody rates were highest among patients receiving vedolizumab monotherapy (n = 83; 99%) or ustekinumab monotherapy (n = 102; 99%), followed by mercaptopurine, azathioprine, or methotrexate monotherapy (n = 67; 97%); anti–tumor necrosis factor monotherapy (n = 270; 96%); mesalamine, sulfasalazine, or budesonide monotherapy or no medication (n = 143; 95%); and finally anti-TNF/immunosuppressive combination therapy (n = 75; 86%). Median and mean antibody titers were lowest for anti-TNF combination therapy and highest for vedolizumab.
Thirty-five patients taking corticosteroids had an antibody detection rate of 85.7% (95% CI, 70.6-93.7), compared with 95.9% (95% CI, 94.2-97.1) among nonsteroid users. In contrast, antibody detection rates were not significantly affected by age or vaccine type.
“Reassuringly, most IBD medications do not prevent an initial antibody response after SARS-CoV-2 vaccination, and this is unlike other classes of immune suppression such as B-cell depletion therapy,” Dr. Weaver concluded. “Additional data are forthcoming on a larger subset of participants in the PREVENT-COVID study which will allow for analysis of factors associated with humoral immune response and potential optimization of immunization strategies.” She described a dataset of about 500 IBD patients in which booster vaccines overcame poor antibody responses to the initial vaccine series.
‘The data we need’
Serre-yu Wong, MD, PhD, of Icahn School of Medicine at Mount Sinai, New York, agreed that the findings should offer some reassurance to patients with IBD and their care providers.
“At the end of the day we have really nice seroconversion rates for the IBD population,” Dr. Wong said.
In April 2021, Dr. Wong and the ICARUS-IBD Working Group published a similar report of 48 patients with IBD receiving biologic therapies, among whom the seroconversion rate was 100%.
“A lot of the early data, including ours, are on infusion medications, and that’s sort of a practical thing because those were the only patients we could get samples from, but [Dr. Weaver and colleagues] were able to get samples from patients not on medications, on oral medications, and on other injection medications that people can take at home, and these are really the data we need for all of our other IBD patients,” Dr. Wong said.
Dr. Wong highlighted that both trials showed some IBD patients generating “very, very high” titers, many of them above the threshold needed for donating convalescent plasma for COVID-19 treatment; still, exact titer levels needed to protect against SARS-CoV-2 infection remain unclear.
“This is going to require longitudinal studies,” Dr. Wong said. “We can’t answer that perfectly right now. We don’t know the magic level of antibodies. I don’t know if you need a titer of 1:100 or 1:1,000.”
Although postvaccination antibody testing is not recommended by the Centers for Disease Control and Prevention, Dr. Wong said that “many patients” check their titers anyway, leading to anxiety if antibodies are low or undetectable.
“I know that it’s very disconcerting sometimes when you don’t see an antibody response, and this is one of the hardest things to try to explain to patients,” Dr. Wong said. “[It’s necessary] to have a frank discussion about the fact that we don’t know the magic level of antibodies, and that there are also other parts of the immune system that we haven’t tested with antibodies. We haven’t tested the T-cell response, and we do know you can have a T-cell response even if you don’t have a B-cell response.”
Dr. Wong suggested that more work is needed to determine the impact of the IBD disease process on susceptibility to SARS-CoV-2 infection, and the rates of antibody responses for the various other vaccines being used around the world.
The PREVENT-COVID study was supported by the Leona M. and Harry B. Helmsley Charitable Trust. The investigators disclosed additional relationships with AbbVie, Johnson & Johnson, Genentech, and others. Dr. Wong reported no relevant conflicts of interest.
This article was updated Oct. 28, 2021.
AT ACG 2021
Men occupy most leadership roles in medicine
Since the early 2000s, approximately half of medical students in the United States – and in many years, more than half – have been women, but according to an update provided at the virtual Pediatric Hospital Medicine.
In pediatrics, a specialty in which approximately 70% of physicians are now women, there has been progress, but still less than 30% of pediatric department chairs are female, said Vincent Chiang, MD, chief medical officer of Boston Children’s Hospital, during a presentation at the virtual meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Citing published data and a survey he personally conducted of the top children’s hospitals identified by the U.S. News and World Report, Dr. Chiang said a minority of division chiefs, chief medical officers, chief financial officers, and other leaders are female. At his institution, only 2 of 16 division chiefs are female.
“No matter how you slice it, women are underrepresented in leadership positions,” he noted.
The problem is certainly not confined to medicine. Dr. Chiang cited data showing that women and men have reached “near parity” in workforce participation in the United States even though the 20% earnings gap has changed little over time.
According to 2020 data from the World Economic Forum, the United States ranked 51 for the gender gap calculated on the basis of economic, political, educational, and health attainment. Even if this places the United States in the top third of the rankings, it is far behind Iceland and the Scandinavian countries that lead the list.
Efforts to reduce structural biases are part of the fix, but Dr. Chiang cautioned that fundamental changes might never occur if the plan is to wait for an approach based on meritocracy. He said that existing structural biases are “slanted away from women,” who are not necessarily granted the opportunities that are readily available to men.
“A meritocracy only works if the initial playing field was level. Otherwise, it just perpetuates the inequalities,” he said.
The problem is not a shortage of women with the skills to lead. In a study by Zenger/Folkman, a consulting company that works on leadership skill development, women performed better than men in 16 of 18 leadership categories, according to Dr. Chiang.
“There is certainly no shortage of capable women,” he noted.
Of the many issues, Dr. Chiang highlighted two. The first is the challenge of placing women on leadership pathways. This is likely to require proactive strategies, such as fast-track advancement programs that guide female candidates toward leadership roles.
The second is more nuanced. According to Dr. Chiang, women who want to assume a leadership role should think more actively about how and who is making decisions at their institution so they can position themselves appropriately. This is nuanced because “there is a certain amount of gamesmanship,” he said. The rise to leadership “has never been a pure meritocracy.”
Importantly, many of the key decisions in any institution involve money, according to Dr. Chiang. As a result, he advised those seeking leadership roles to join audit committees or otherwise take on responsibility for profit-and-loss management. Even in a nonprofit institution, “you need to make the numbers work,” he said, citing the common catchphrase: “No margin, no mission.”
However, Dr. Chiang acknowledged the many obstacles that prevent women from working their way into positions of leadership. For example, networking is important, but women are not necessarily attracted or invited to some of the social engagements, such as golf outings, where strong relationships are created.
In a survey of 100,000 people working at Fortune 500 companies, “82% of women say they feel excluded at work and much of that comes from that informal networking,” Dr. Chiang said. “Whereas 92% of men think they are not excluding women in their daily work.”
There is no single solution, but Dr. Chiang believes that concrete structural changes are needed. Female doctors remain grossly underrepresented in leadership roles even as they now represent more than half of the workforce for many specialties. Based on the need for proactive approaches outlined by Dr. Chiang, it appears unlikely that gender inequality will ever resolve itself.
Lisa S. Rotenstein, MD, who has written on fixing the gender imbalance in health care, including for the Harvard Business Review, said she agreed during an interview that structural changes are critical.
“In order to address current disparities, leaders should be thinking about how to remove both the formal and informal obstacles that prevent women and minorities from getting into the rooms where these decisions are being made,” said Dr. Rotenstein, who is an instructor in medicine at Brigham and Women’s Hospital, Harvard Medical School in Boston.
“This will need to involve sponsorship that gets women invited to the right committees or in positions with responsibility for profit-and-loss management,” she added.
Dr. Rotenstein spoke about improving “access to the pipeline” that leads to leadership roles. The ways in which women are excluded from opportunities is often subtle and difficult to penetrate without fundamental changes, she explained.
“Institutions need to understand the processes that lead to leadership roles and make the changes that allow women and minorities to participate,” she said. It is not enough to recognize the problem, according to Dr. Rotenstein.
Like Dr. Chiang, she noted that changes are needed in the methods that move underrepresented groups into leadership roles.
Dr. Chiang reported no potential conflicts of interest relevant to this study.
Since the early 2000s, approximately half of medical students in the United States – and in many years, more than half – have been women, but according to an update provided at the virtual Pediatric Hospital Medicine.
In pediatrics, a specialty in which approximately 70% of physicians are now women, there has been progress, but still less than 30% of pediatric department chairs are female, said Vincent Chiang, MD, chief medical officer of Boston Children’s Hospital, during a presentation at the virtual meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Citing published data and a survey he personally conducted of the top children’s hospitals identified by the U.S. News and World Report, Dr. Chiang said a minority of division chiefs, chief medical officers, chief financial officers, and other leaders are female. At his institution, only 2 of 16 division chiefs are female.
“No matter how you slice it, women are underrepresented in leadership positions,” he noted.
The problem is certainly not confined to medicine. Dr. Chiang cited data showing that women and men have reached “near parity” in workforce participation in the United States even though the 20% earnings gap has changed little over time.
According to 2020 data from the World Economic Forum, the United States ranked 51 for the gender gap calculated on the basis of economic, political, educational, and health attainment. Even if this places the United States in the top third of the rankings, it is far behind Iceland and the Scandinavian countries that lead the list.
Efforts to reduce structural biases are part of the fix, but Dr. Chiang cautioned that fundamental changes might never occur if the plan is to wait for an approach based on meritocracy. He said that existing structural biases are “slanted away from women,” who are not necessarily granted the opportunities that are readily available to men.
“A meritocracy only works if the initial playing field was level. Otherwise, it just perpetuates the inequalities,” he said.
The problem is not a shortage of women with the skills to lead. In a study by Zenger/Folkman, a consulting company that works on leadership skill development, women performed better than men in 16 of 18 leadership categories, according to Dr. Chiang.
“There is certainly no shortage of capable women,” he noted.
Of the many issues, Dr. Chiang highlighted two. The first is the challenge of placing women on leadership pathways. This is likely to require proactive strategies, such as fast-track advancement programs that guide female candidates toward leadership roles.
The second is more nuanced. According to Dr. Chiang, women who want to assume a leadership role should think more actively about how and who is making decisions at their institution so they can position themselves appropriately. This is nuanced because “there is a certain amount of gamesmanship,” he said. The rise to leadership “has never been a pure meritocracy.”
Importantly, many of the key decisions in any institution involve money, according to Dr. Chiang. As a result, he advised those seeking leadership roles to join audit committees or otherwise take on responsibility for profit-and-loss management. Even in a nonprofit institution, “you need to make the numbers work,” he said, citing the common catchphrase: “No margin, no mission.”
However, Dr. Chiang acknowledged the many obstacles that prevent women from working their way into positions of leadership. For example, networking is important, but women are not necessarily attracted or invited to some of the social engagements, such as golf outings, where strong relationships are created.
In a survey of 100,000 people working at Fortune 500 companies, “82% of women say they feel excluded at work and much of that comes from that informal networking,” Dr. Chiang said. “Whereas 92% of men think they are not excluding women in their daily work.”
There is no single solution, but Dr. Chiang believes that concrete structural changes are needed. Female doctors remain grossly underrepresented in leadership roles even as they now represent more than half of the workforce for many specialties. Based on the need for proactive approaches outlined by Dr. Chiang, it appears unlikely that gender inequality will ever resolve itself.
Lisa S. Rotenstein, MD, who has written on fixing the gender imbalance in health care, including for the Harvard Business Review, said she agreed during an interview that structural changes are critical.
“In order to address current disparities, leaders should be thinking about how to remove both the formal and informal obstacles that prevent women and minorities from getting into the rooms where these decisions are being made,” said Dr. Rotenstein, who is an instructor in medicine at Brigham and Women’s Hospital, Harvard Medical School in Boston.
“This will need to involve sponsorship that gets women invited to the right committees or in positions with responsibility for profit-and-loss management,” she added.
Dr. Rotenstein spoke about improving “access to the pipeline” that leads to leadership roles. The ways in which women are excluded from opportunities is often subtle and difficult to penetrate without fundamental changes, she explained.
“Institutions need to understand the processes that lead to leadership roles and make the changes that allow women and minorities to participate,” she said. It is not enough to recognize the problem, according to Dr. Rotenstein.
Like Dr. Chiang, she noted that changes are needed in the methods that move underrepresented groups into leadership roles.
Dr. Chiang reported no potential conflicts of interest relevant to this study.
Since the early 2000s, approximately half of medical students in the United States – and in many years, more than half – have been women, but according to an update provided at the virtual Pediatric Hospital Medicine.
In pediatrics, a specialty in which approximately 70% of physicians are now women, there has been progress, but still less than 30% of pediatric department chairs are female, said Vincent Chiang, MD, chief medical officer of Boston Children’s Hospital, during a presentation at the virtual meeting sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Citing published data and a survey he personally conducted of the top children’s hospitals identified by the U.S. News and World Report, Dr. Chiang said a minority of division chiefs, chief medical officers, chief financial officers, and other leaders are female. At his institution, only 2 of 16 division chiefs are female.
“No matter how you slice it, women are underrepresented in leadership positions,” he noted.
The problem is certainly not confined to medicine. Dr. Chiang cited data showing that women and men have reached “near parity” in workforce participation in the United States even though the 20% earnings gap has changed little over time.
According to 2020 data from the World Economic Forum, the United States ranked 51 for the gender gap calculated on the basis of economic, political, educational, and health attainment. Even if this places the United States in the top third of the rankings, it is far behind Iceland and the Scandinavian countries that lead the list.
Efforts to reduce structural biases are part of the fix, but Dr. Chiang cautioned that fundamental changes might never occur if the plan is to wait for an approach based on meritocracy. He said that existing structural biases are “slanted away from women,” who are not necessarily granted the opportunities that are readily available to men.
“A meritocracy only works if the initial playing field was level. Otherwise, it just perpetuates the inequalities,” he said.
The problem is not a shortage of women with the skills to lead. In a study by Zenger/Folkman, a consulting company that works on leadership skill development, women performed better than men in 16 of 18 leadership categories, according to Dr. Chiang.
“There is certainly no shortage of capable women,” he noted.
Of the many issues, Dr. Chiang highlighted two. The first is the challenge of placing women on leadership pathways. This is likely to require proactive strategies, such as fast-track advancement programs that guide female candidates toward leadership roles.
The second is more nuanced. According to Dr. Chiang, women who want to assume a leadership role should think more actively about how and who is making decisions at their institution so they can position themselves appropriately. This is nuanced because “there is a certain amount of gamesmanship,” he said. The rise to leadership “has never been a pure meritocracy.”
Importantly, many of the key decisions in any institution involve money, according to Dr. Chiang. As a result, he advised those seeking leadership roles to join audit committees or otherwise take on responsibility for profit-and-loss management. Even in a nonprofit institution, “you need to make the numbers work,” he said, citing the common catchphrase: “No margin, no mission.”
However, Dr. Chiang acknowledged the many obstacles that prevent women from working their way into positions of leadership. For example, networking is important, but women are not necessarily attracted or invited to some of the social engagements, such as golf outings, where strong relationships are created.
In a survey of 100,000 people working at Fortune 500 companies, “82% of women say they feel excluded at work and much of that comes from that informal networking,” Dr. Chiang said. “Whereas 92% of men think they are not excluding women in their daily work.”
There is no single solution, but Dr. Chiang believes that concrete structural changes are needed. Female doctors remain grossly underrepresented in leadership roles even as they now represent more than half of the workforce for many specialties. Based on the need for proactive approaches outlined by Dr. Chiang, it appears unlikely that gender inequality will ever resolve itself.
Lisa S. Rotenstein, MD, who has written on fixing the gender imbalance in health care, including for the Harvard Business Review, said she agreed during an interview that structural changes are critical.
“In order to address current disparities, leaders should be thinking about how to remove both the formal and informal obstacles that prevent women and minorities from getting into the rooms where these decisions are being made,” said Dr. Rotenstein, who is an instructor in medicine at Brigham and Women’s Hospital, Harvard Medical School in Boston.
“This will need to involve sponsorship that gets women invited to the right committees or in positions with responsibility for profit-and-loss management,” she added.
Dr. Rotenstein spoke about improving “access to the pipeline” that leads to leadership roles. The ways in which women are excluded from opportunities is often subtle and difficult to penetrate without fundamental changes, she explained.
“Institutions need to understand the processes that lead to leadership roles and make the changes that allow women and minorities to participate,” she said. It is not enough to recognize the problem, according to Dr. Rotenstein.
Like Dr. Chiang, she noted that changes are needed in the methods that move underrepresented groups into leadership roles.
Dr. Chiang reported no potential conflicts of interest relevant to this study.
FROM PHM20
Hepatitis vaccination update
One of the most important commitments family physicians can undertake in protecting the health of their patients and communities is to ensure that their patients are fully vaccinated. This task is increasingly complicated as new vaccines are approved every year and recommendations change regarding new and established vaccines. To assist primary care providers, the Centers for Disease Control and Prevention (CDC) annually updates 2 immunization schedules—one for children and adolescents, and one for adults. These schedules are available on the CDC Web site (https://www.cdc.gov/vaccines/schedules/index.html).
These updates originate from the Advisory Committee on Immunization Practices (ACIP), which meets 3 times a year to consider and adopt changes to the schedules. During 2018, relatively few new recommendations were adopted. The September 2018 Practice Alert1 in this journal covered the updated recommendations for influenza immunization, which included reinstating live attenuated influenza vaccine (LAIV) to the active list of influenza vaccines.
This current Practice Alert reviews 3 additional updates: 1) a new hepatitis B (HepB) vaccine; 2) updated recommendations for the use of hepatitis A (HepA) vaccine for post-exposure prevention and before travel; and 3) inclusion of the homeless among those who should be routinely vaccinated with HepA vaccine.
Hepatitis B: New 2-dose product
As of 2015, the annual incidence of new hepatitis B cases had declined by 88.5% since the first HepB vaccine was licensed in 1981 and recommendations for its routine use were issued in 1982.2 The HepB vaccine products available in the United States are 2 single-antigen products, Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck & Co.). Both can be used in all age groups, starting at birth, in a 3-dose series. HepB vaccine is also available in 2 combination products: Pediarix, containing HepB, diphtheria and tetanus toxoids, acellular pertussis, and inactivated poliovirus (GlaxoSmithKline), approved for use in children 6 weeks to 6 years old; and Twinrix (GlaxoSmithKline), which contains both HepB and HepA and is approved for use in adults 18 years and older.

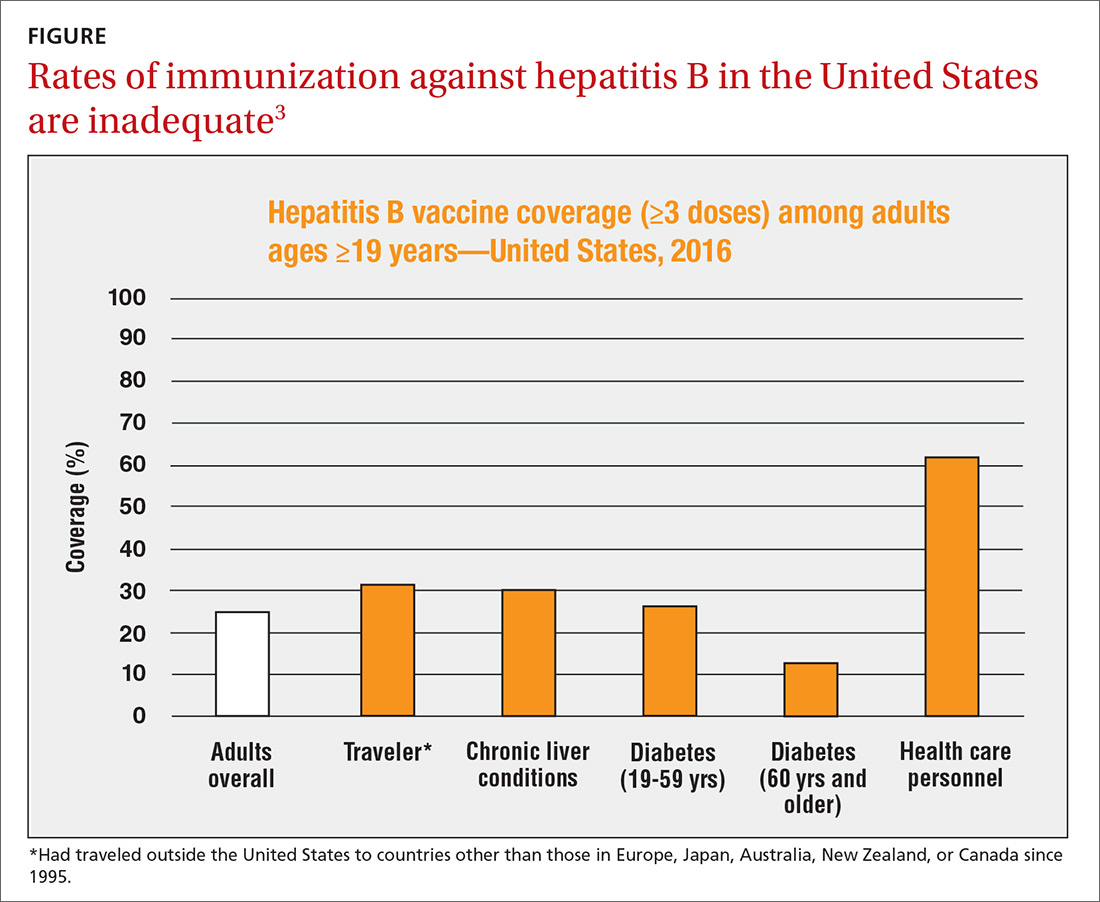
The HepB vaccine is recommended for all children and unvaccinated adolescents as part of the routine vaccination schedule. It is also recommended for unvaccinated adults with specific risks (TABLE 12). However, the rate of HepB vaccination in adults for whom it is recommended is suboptimal (FIGURE),3 and just a little more than half of adults who start a 3-dose series of HepB complete it.4A new vaccine against hepatitis B, HEPLISAV-B (Dynavax Technologies), was licensed by the US Food and Drug Administration in late 2017. ACIP now recommends it as an option along with other available HepB products. HEPLISAV-B is given in 2 doses separated by 1 month. It is hoped that this shortened 2-dose series will increase the number of adults who achieve full vaccination. In addition, it appears that HEPLISAV-B provides higher levels of protection in some high-risk groups—those with type 2 diabetes or chronic kidney disease.3 However, initial safety studies have shown a small absolute increase in cardiac events after vaccination with HEPLISAV-B. Post-marketing surveillance will be needed to show whether this is causal or coincidental.3
As with other HepB products, use of HEPLISAV-B should follow the latest CDC directives on who to test serologically for prior immunity, and on post-vaccination testing to ensure protective antibody levels were achieved.2 It is best to complete a HepB series with the same product, but, if necessary, a combination of products at different doses can be used to complete the HepB series. Any such combination should include 3 doses, even if one of the doses is HEPLISAV-B.
Hepatitis A: Vaccination assumes greater importance for more people
A Practice Alert in early 2018 described a series of outbreaks of hepatitis A around the country and the high rates of associated hospitalizations.5 These outbreaks have occurred primarily among the homeless and their contacts and those who use illicit drugs. This nationwide outbreak has now spread, resulting in more than 7500 cases since July 1, 2016.6 The progress of this epidemic can be viewed on the CDC Web site

Continue to: Remember that the current recommendation...
Remember that the current recommendation is to vaccinate all children 12 to 23 months old with HepA, in 2 separate doses. Two single-antigen HepA products are available: Havrix (GSK) and Vaqta (Merck). For the 2-dose sequence, Havrix is given at 0 and 6 to 12 months; Vaqta at 0 and 6 to 18 months. Even a single dose will provide protection for up to 11 years. In addition to these vaccines, there is the combination HepA and HepB vaccine (Twinrix) mentioned earlier.
Previous recommendations for preventing hepatitis A after exposure, made in 2007, stated that HepA vaccine was preferred for healthy individuals ages 12 months through 40 years, while immune globulin (IG) was preferred for adults older than 40, infants before their first birthday, immunocompromised individuals, those with chronic liver disease, and those for whom HepA vaccine is contraindicated.8 The 2007 recommendations also advised vaccinating individuals traveling to countries with intermediate to high hepatitis A endemicity.
A single dose of HepA vaccine was recommended for all those 12 months or older, although older adults, immunocompromised individuals, and those with chronic liver disease or other chronic medical conditions planning to visit an endemic area in ≤ 2 weeks were supposed to receive the initial dose of vaccine and could also receive IG (0.02 mL/kg) if their provider advised it. Travelers who declined vaccination, those younger than 12 months, or those allergic to a vaccine component could receive a single dose of IG (0.02 mL/kg), which provides protection up to 3 months.
Several factors influenced ACIP to reconsider both the pre- and post-exposure recommendations. Regarding IG, evidence of its decreased potency over time led the committee to increase the recommended dose (see below). IG also must be re-administered every 2 months, the supply of the product is questionable, and many health care facilities do not stock it. By comparison, HepA vaccine offers the advantages of easier administration, inducing active immunity, and providing longer protection. Another issue involved infants ages 6 to 11 months traveling to an area with endemic measles transmission and who must therefore receive the measles, mumps, and rubella (MMR) vaccine. MMR and IG should not be co-administered, and, for infants, the health risk from measles outweighs that from hepatitis A.
Updated recommendations. After considering all this information, ACIP made the following changes to its hepatitis A virus (HAV) prevention recommendations (in addition to adding homeless people to the list of HepA vaccine recipients)9:
- Administer HepA vaccine as post-exposure prophylaxis to all individuals 12 months and older.
- IG may be administered, in addition to HepA vaccine, to those older than 40 years, depending on the provider’s risk assessment (degree of exposure and medical conditions that might lead to severe complications from HAV infection). The recommended IG dose is now 0.1 mL/kg for post-exposure prevention; it is 0.1 to 0.2 mL/kg for pre-exposure prophylaxis for travelers, depending on the length of planned travel.
- Administer HepA vaccine alone to infants ages 6 to 11 months traveling outside the United States when protection against hepatitis A is recommended.
These recommendations have been published in the Morbidity and Mortality Weekly Report.9
1. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
3. CDC. Schillie S. HEPLISAV-B: considerations and proposed recommendations, vote. Presented at: meeting of the Hepatitis Work Group, Advisory Committee on Immunization Practices; February 21, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-02/Hepatitis-03-Schillie-508.pdf. Accessed January 19, 2019.
4. Nelson JC, Bittner RC, Bounds L, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009;99(Suppl 2):S389-S397.
5. Campos-Outcalt D. CDC provides advice on recent hepatitis A outbreaks. J Fam Pract. 2018;67:30-32.
6. CDC. Nelson N. Background – hepatitis A among the homeless. Presented at: meeting of the Advisory Committee on Immunization Practices; October 24, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-10/Hepatitis-02-Nelson-508.pdf. Accessed January 19, 2019.
7. CDC. 2017 – Outbreaks of hepatitis A in multiple states among people who use drugs and/or people who are homeless. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed January 19, 2019.
8. CDC. Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed February 9, 2019.
9. Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for postexposure prophylaxis and for preexposure prophylaxis for international travel. MMWR Morb Mortal Wkly Rep. 2018;67:1216-1220.
One of the most important commitments family physicians can undertake in protecting the health of their patients and communities is to ensure that their patients are fully vaccinated. This task is increasingly complicated as new vaccines are approved every year and recommendations change regarding new and established vaccines. To assist primary care providers, the Centers for Disease Control and Prevention (CDC) annually updates 2 immunization schedules—one for children and adolescents, and one for adults. These schedules are available on the CDC Web site (https://www.cdc.gov/vaccines/schedules/index.html).
These updates originate from the Advisory Committee on Immunization Practices (ACIP), which meets 3 times a year to consider and adopt changes to the schedules. During 2018, relatively few new recommendations were adopted. The September 2018 Practice Alert1 in this journal covered the updated recommendations for influenza immunization, which included reinstating live attenuated influenza vaccine (LAIV) to the active list of influenza vaccines.
This current Practice Alert reviews 3 additional updates: 1) a new hepatitis B (HepB) vaccine; 2) updated recommendations for the use of hepatitis A (HepA) vaccine for post-exposure prevention and before travel; and 3) inclusion of the homeless among those who should be routinely vaccinated with HepA vaccine.
Hepatitis B: New 2-dose product
As of 2015, the annual incidence of new hepatitis B cases had declined by 88.5% since the first HepB vaccine was licensed in 1981 and recommendations for its routine use were issued in 1982.2 The HepB vaccine products available in the United States are 2 single-antigen products, Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck & Co.). Both can be used in all age groups, starting at birth, in a 3-dose series. HepB vaccine is also available in 2 combination products: Pediarix, containing HepB, diphtheria and tetanus toxoids, acellular pertussis, and inactivated poliovirus (GlaxoSmithKline), approved for use in children 6 weeks to 6 years old; and Twinrix (GlaxoSmithKline), which contains both HepB and HepA and is approved for use in adults 18 years and older.

The HepB vaccine is recommended for all children and unvaccinated adolescents as part of the routine vaccination schedule. It is also recommended for unvaccinated adults with specific risks (TABLE 12). However, the rate of HepB vaccination in adults for whom it is recommended is suboptimal (FIGURE),3 and just a little more than half of adults who start a 3-dose series of HepB complete it.4A new vaccine against hepatitis B, HEPLISAV-B (Dynavax Technologies), was licensed by the US Food and Drug Administration in late 2017. ACIP now recommends it as an option along with other available HepB products. HEPLISAV-B is given in 2 doses separated by 1 month. It is hoped that this shortened 2-dose series will increase the number of adults who achieve full vaccination. In addition, it appears that HEPLISAV-B provides higher levels of protection in some high-risk groups—those with type 2 diabetes or chronic kidney disease.3 However, initial safety studies have shown a small absolute increase in cardiac events after vaccination with HEPLISAV-B. Post-marketing surveillance will be needed to show whether this is causal or coincidental.3

As with other HepB products, use of HEPLISAV-B should follow the latest CDC directives on who to test serologically for prior immunity, and on post-vaccination testing to ensure protective antibody levels were achieved.2 It is best to complete a HepB series with the same product, but, if necessary, a combination of products at different doses can be used to complete the HepB series. Any such combination should include 3 doses, even if one of the doses is HEPLISAV-B.
Hepatitis A: Vaccination assumes greater importance for more people
A Practice Alert in early 2018 described a series of outbreaks of hepatitis A around the country and the high rates of associated hospitalizations.5 These outbreaks have occurred primarily among the homeless and their contacts and those who use illicit drugs. This nationwide outbreak has now spread, resulting in more than 7500 cases since July 1, 2016.6 The progress of this epidemic can be viewed on the CDC Web site

Continue to: Remember that the current recommendation...
Remember that the current recommendation is to vaccinate all children 12 to 23 months old with HepA, in 2 separate doses. Two single-antigen HepA products are available: Havrix (GSK) and Vaqta (Merck). For the 2-dose sequence, Havrix is given at 0 and 6 to 12 months; Vaqta at 0 and 6 to 18 months. Even a single dose will provide protection for up to 11 years. In addition to these vaccines, there is the combination HepA and HepB vaccine (Twinrix) mentioned earlier.
Previous recommendations for preventing hepatitis A after exposure, made in 2007, stated that HepA vaccine was preferred for healthy individuals ages 12 months through 40 years, while immune globulin (IG) was preferred for adults older than 40, infants before their first birthday, immunocompromised individuals, those with chronic liver disease, and those for whom HepA vaccine is contraindicated.8 The 2007 recommendations also advised vaccinating individuals traveling to countries with intermediate to high hepatitis A endemicity.
A single dose of HepA vaccine was recommended for all those 12 months or older, although older adults, immunocompromised individuals, and those with chronic liver disease or other chronic medical conditions planning to visit an endemic area in ≤ 2 weeks were supposed to receive the initial dose of vaccine and could also receive IG (0.02 mL/kg) if their provider advised it. Travelers who declined vaccination, those younger than 12 months, or those allergic to a vaccine component could receive a single dose of IG (0.02 mL/kg), which provides protection up to 3 months.
Several factors influenced ACIP to reconsider both the pre- and post-exposure recommendations. Regarding IG, evidence of its decreased potency over time led the committee to increase the recommended dose (see below). IG also must be re-administered every 2 months, the supply of the product is questionable, and many health care facilities do not stock it. By comparison, HepA vaccine offers the advantages of easier administration, inducing active immunity, and providing longer protection. Another issue involved infants ages 6 to 11 months traveling to an area with endemic measles transmission and who must therefore receive the measles, mumps, and rubella (MMR) vaccine. MMR and IG should not be co-administered, and, for infants, the health risk from measles outweighs that from hepatitis A.
Updated recommendations. After considering all this information, ACIP made the following changes to its hepatitis A virus (HAV) prevention recommendations (in addition to adding homeless people to the list of HepA vaccine recipients)9:
- Administer HepA vaccine as post-exposure prophylaxis to all individuals 12 months and older.
- IG may be administered, in addition to HepA vaccine, to those older than 40 years, depending on the provider’s risk assessment (degree of exposure and medical conditions that might lead to severe complications from HAV infection). The recommended IG dose is now 0.1 mL/kg for post-exposure prevention; it is 0.1 to 0.2 mL/kg for pre-exposure prophylaxis for travelers, depending on the length of planned travel.
- Administer HepA vaccine alone to infants ages 6 to 11 months traveling outside the United States when protection against hepatitis A is recommended.
These recommendations have been published in the Morbidity and Mortality Weekly Report.9
One of the most important commitments family physicians can undertake in protecting the health of their patients and communities is to ensure that their patients are fully vaccinated. This task is increasingly complicated as new vaccines are approved every year and recommendations change regarding new and established vaccines. To assist primary care providers, the Centers for Disease Control and Prevention (CDC) annually updates 2 immunization schedules—one for children and adolescents, and one for adults. These schedules are available on the CDC Web site (https://www.cdc.gov/vaccines/schedules/index.html).
These updates originate from the Advisory Committee on Immunization Practices (ACIP), which meets 3 times a year to consider and adopt changes to the schedules. During 2018, relatively few new recommendations were adopted. The September 2018 Practice Alert1 in this journal covered the updated recommendations for influenza immunization, which included reinstating live attenuated influenza vaccine (LAIV) to the active list of influenza vaccines.
This current Practice Alert reviews 3 additional updates: 1) a new hepatitis B (HepB) vaccine; 2) updated recommendations for the use of hepatitis A (HepA) vaccine for post-exposure prevention and before travel; and 3) inclusion of the homeless among those who should be routinely vaccinated with HepA vaccine.
Hepatitis B: New 2-dose product
As of 2015, the annual incidence of new hepatitis B cases had declined by 88.5% since the first HepB vaccine was licensed in 1981 and recommendations for its routine use were issued in 1982.2 The HepB vaccine products available in the United States are 2 single-antigen products, Engerix-B (GlaxoSmithKline) and Recombivax HB (Merck & Co.). Both can be used in all age groups, starting at birth, in a 3-dose series. HepB vaccine is also available in 2 combination products: Pediarix, containing HepB, diphtheria and tetanus toxoids, acellular pertussis, and inactivated poliovirus (GlaxoSmithKline), approved for use in children 6 weeks to 6 years old; and Twinrix (GlaxoSmithKline), which contains both HepB and HepA and is approved for use in adults 18 years and older.

The HepB vaccine is recommended for all children and unvaccinated adolescents as part of the routine vaccination schedule. It is also recommended for unvaccinated adults with specific risks (TABLE 12). However, the rate of HepB vaccination in adults for whom it is recommended is suboptimal (FIGURE),3 and just a little more than half of adults who start a 3-dose series of HepB complete it.4A new vaccine against hepatitis B, HEPLISAV-B (Dynavax Technologies), was licensed by the US Food and Drug Administration in late 2017. ACIP now recommends it as an option along with other available HepB products. HEPLISAV-B is given in 2 doses separated by 1 month. It is hoped that this shortened 2-dose series will increase the number of adults who achieve full vaccination. In addition, it appears that HEPLISAV-B provides higher levels of protection in some high-risk groups—those with type 2 diabetes or chronic kidney disease.3 However, initial safety studies have shown a small absolute increase in cardiac events after vaccination with HEPLISAV-B. Post-marketing surveillance will be needed to show whether this is causal or coincidental.3

As with other HepB products, use of HEPLISAV-B should follow the latest CDC directives on who to test serologically for prior immunity, and on post-vaccination testing to ensure protective antibody levels were achieved.2 It is best to complete a HepB series with the same product, but, if necessary, a combination of products at different doses can be used to complete the HepB series. Any such combination should include 3 doses, even if one of the doses is HEPLISAV-B.
Hepatitis A: Vaccination assumes greater importance for more people
A Practice Alert in early 2018 described a series of outbreaks of hepatitis A around the country and the high rates of associated hospitalizations.5 These outbreaks have occurred primarily among the homeless and their contacts and those who use illicit drugs. This nationwide outbreak has now spread, resulting in more than 7500 cases since July 1, 2016.6 The progress of this epidemic can be viewed on the CDC Web site

Continue to: Remember that the current recommendation...
Remember that the current recommendation is to vaccinate all children 12 to 23 months old with HepA, in 2 separate doses. Two single-antigen HepA products are available: Havrix (GSK) and Vaqta (Merck). For the 2-dose sequence, Havrix is given at 0 and 6 to 12 months; Vaqta at 0 and 6 to 18 months. Even a single dose will provide protection for up to 11 years. In addition to these vaccines, there is the combination HepA and HepB vaccine (Twinrix) mentioned earlier.
Previous recommendations for preventing hepatitis A after exposure, made in 2007, stated that HepA vaccine was preferred for healthy individuals ages 12 months through 40 years, while immune globulin (IG) was preferred for adults older than 40, infants before their first birthday, immunocompromised individuals, those with chronic liver disease, and those for whom HepA vaccine is contraindicated.8 The 2007 recommendations also advised vaccinating individuals traveling to countries with intermediate to high hepatitis A endemicity.
A single dose of HepA vaccine was recommended for all those 12 months or older, although older adults, immunocompromised individuals, and those with chronic liver disease or other chronic medical conditions planning to visit an endemic area in ≤ 2 weeks were supposed to receive the initial dose of vaccine and could also receive IG (0.02 mL/kg) if their provider advised it. Travelers who declined vaccination, those younger than 12 months, or those allergic to a vaccine component could receive a single dose of IG (0.02 mL/kg), which provides protection up to 3 months.
Several factors influenced ACIP to reconsider both the pre- and post-exposure recommendations. Regarding IG, evidence of its decreased potency over time led the committee to increase the recommended dose (see below). IG also must be re-administered every 2 months, the supply of the product is questionable, and many health care facilities do not stock it. By comparison, HepA vaccine offers the advantages of easier administration, inducing active immunity, and providing longer protection. Another issue involved infants ages 6 to 11 months traveling to an area with endemic measles transmission and who must therefore receive the measles, mumps, and rubella (MMR) vaccine. MMR and IG should not be co-administered, and, for infants, the health risk from measles outweighs that from hepatitis A.
Updated recommendations. After considering all this information, ACIP made the following changes to its hepatitis A virus (HAV) prevention recommendations (in addition to adding homeless people to the list of HepA vaccine recipients)9:
- Administer HepA vaccine as post-exposure prophylaxis to all individuals 12 months and older.
- IG may be administered, in addition to HepA vaccine, to those older than 40 years, depending on the provider’s risk assessment (degree of exposure and medical conditions that might lead to severe complications from HAV infection). The recommended IG dose is now 0.1 mL/kg for post-exposure prevention; it is 0.1 to 0.2 mL/kg for pre-exposure prophylaxis for travelers, depending on the length of planned travel.
- Administer HepA vaccine alone to infants ages 6 to 11 months traveling outside the United States when protection against hepatitis A is recommended.
These recommendations have been published in the Morbidity and Mortality Weekly Report.9
1. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
3. CDC. Schillie S. HEPLISAV-B: considerations and proposed recommendations, vote. Presented at: meeting of the Hepatitis Work Group, Advisory Committee on Immunization Practices; February 21, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-02/Hepatitis-03-Schillie-508.pdf. Accessed January 19, 2019.
4. Nelson JC, Bittner RC, Bounds L, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009;99(Suppl 2):S389-S397.
5. Campos-Outcalt D. CDC provides advice on recent hepatitis A outbreaks. J Fam Pract. 2018;67:30-32.
6. CDC. Nelson N. Background – hepatitis A among the homeless. Presented at: meeting of the Advisory Committee on Immunization Practices; October 24, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-10/Hepatitis-02-Nelson-508.pdf. Accessed January 19, 2019.
7. CDC. 2017 – Outbreaks of hepatitis A in multiple states among people who use drugs and/or people who are homeless. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed January 19, 2019.
8. CDC. Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed February 9, 2019.
9. Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for postexposure prophylaxis and for preexposure prophylaxis for international travel. MMWR Morb Mortal Wkly Rep. 2018;67:1216-1220.
1. Campos-Outcalt D. CDC recommendations for the 2018-2019 influenza season. J Fam Pract. 2018;67:550-553.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31.
3. CDC. Schillie S. HEPLISAV-B: considerations and proposed recommendations, vote. Presented at: meeting of the Hepatitis Work Group, Advisory Committee on Immunization Practices; February 21, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-02/Hepatitis-03-Schillie-508.pdf. Accessed January 19, 2019.
4. Nelson JC, Bittner RC, Bounds L, et al. Compliance with multiple-dose vaccine schedules among older children, adolescents, and adults: results from a vaccine safety datalink study. Am J Public Health. 2009;99(Suppl 2):S389-S397.
5. Campos-Outcalt D. CDC provides advice on recent hepatitis A outbreaks. J Fam Pract. 2018;67:30-32.
6. CDC. Nelson N. Background – hepatitis A among the homeless. Presented at: meeting of the Advisory Committee on Immunization Practices; October 24, 2018; Atlanta, Ga. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-10/Hepatitis-02-Nelson-508.pdf. Accessed January 19, 2019.
7. CDC. 2017 – Outbreaks of hepatitis A in multiple states among people who use drugs and/or people who are homeless. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed January 19, 2019.
8. CDC. Update: Prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed February 9, 2019.
9. Nelson NP, Link-Gelles R, Hofmeister MG, et al. Update: recommendations of the Advisory Committee on Immunization Practices for use of hepatitis A vaccine for postexposure prophylaxis and for preexposure prophylaxis for international travel. MMWR Morb Mortal Wkly Rep. 2018;67:1216-1220.
A look at new guidelines for HIV treatment and prevention
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

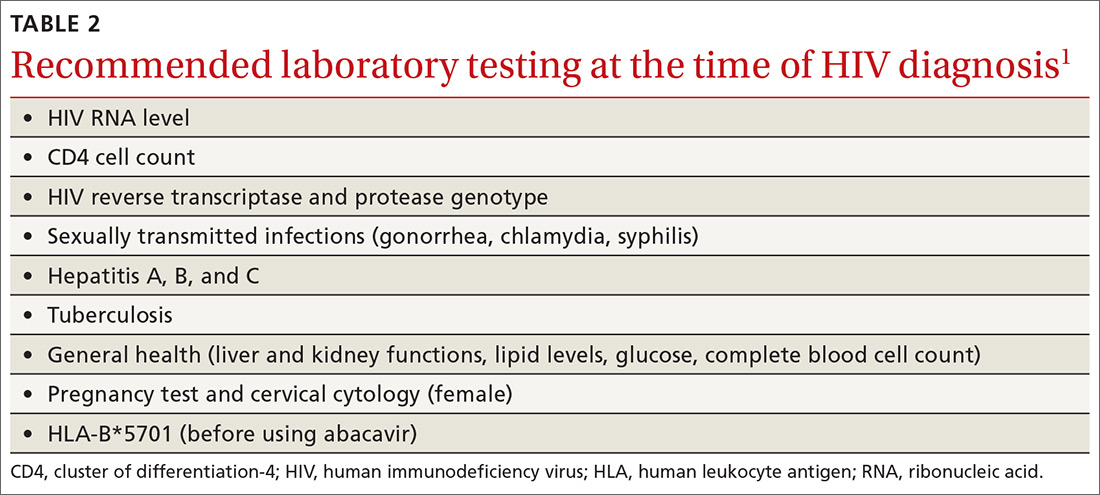
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
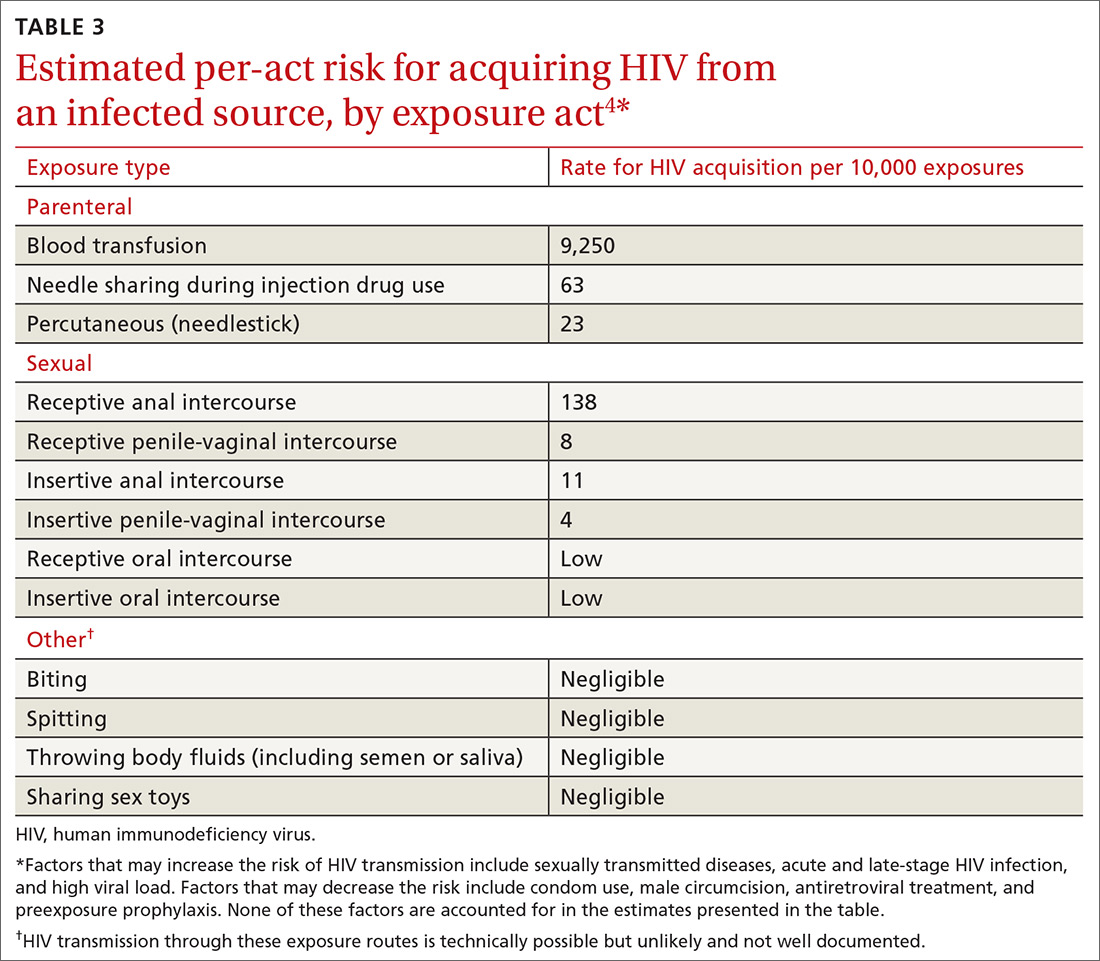
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
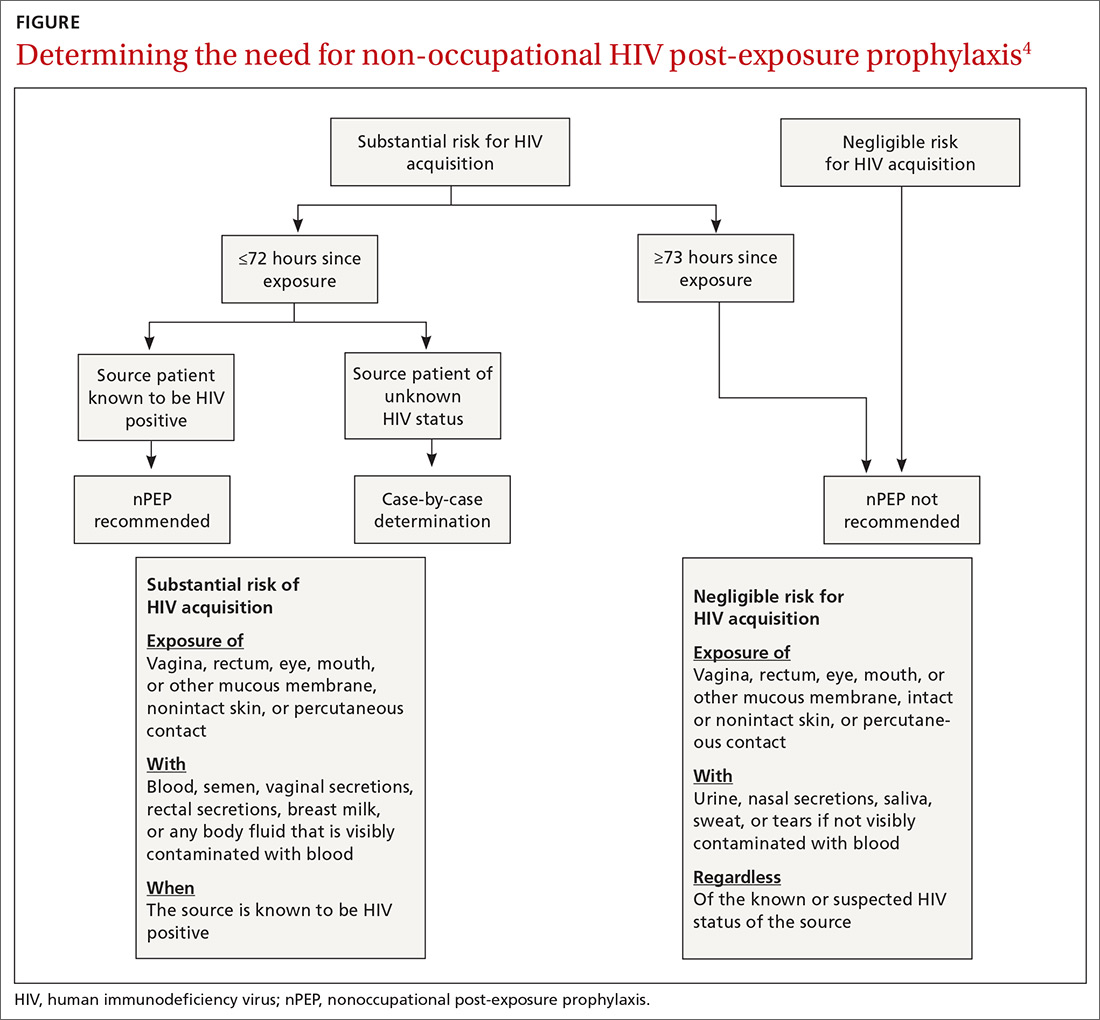
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.

Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.

nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).

Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1

An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.

Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.

nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).

Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.

Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
CDC recommendations for the 2018-2019 influenza season
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).
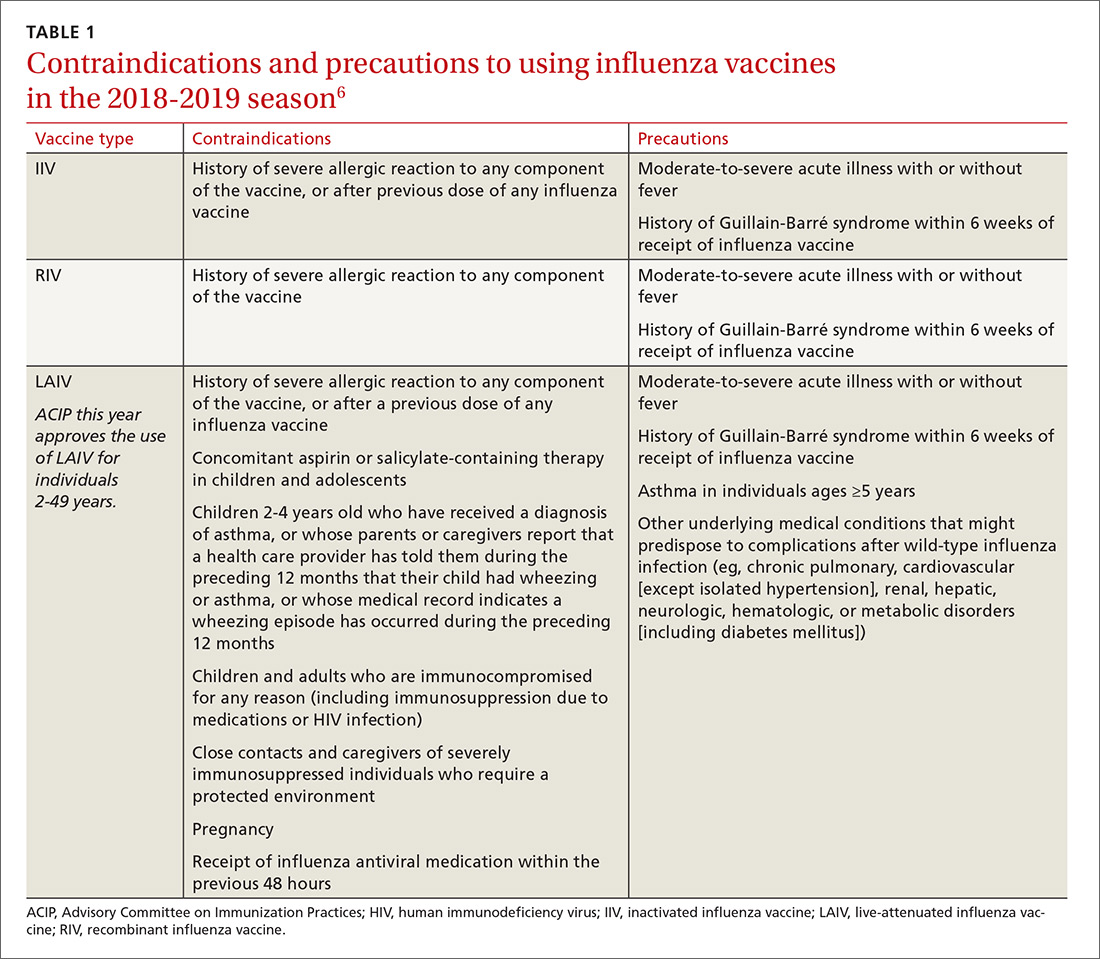
After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).

After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).

After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
Dietary recommendations for patients with diabetes
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2
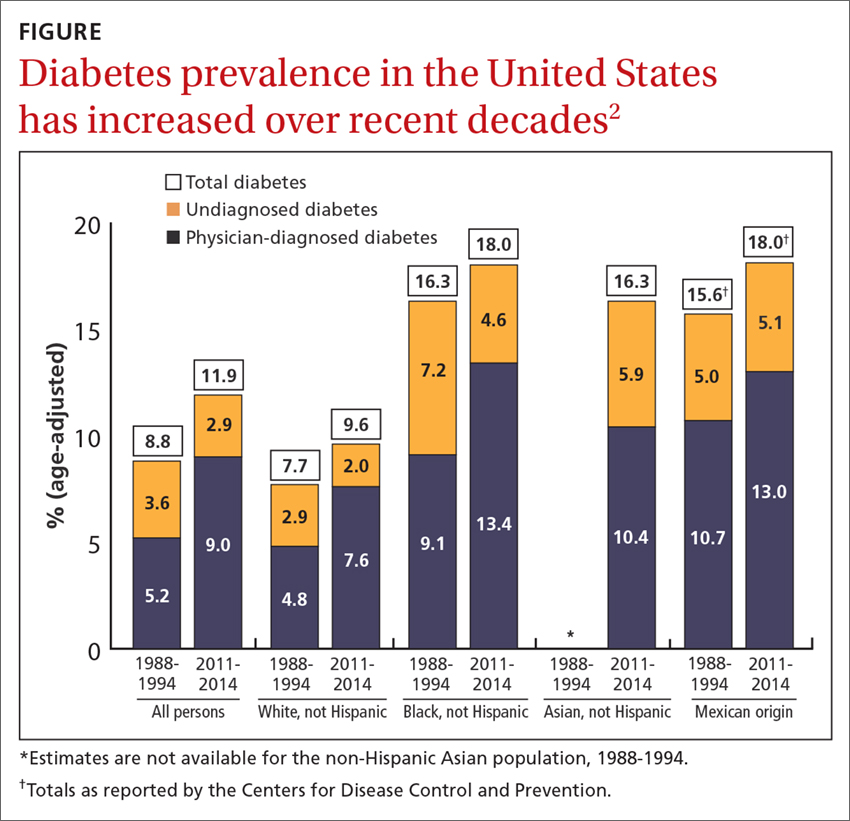
Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
Diabetes affects approximately 9.4% of the US population (more than 30 million people),1 and it is one of the most common conditions treated by family physicians. Additionally, more than 80 million Americans meet the criteria for prediabetes.1 The prevalence of diabetes has increased in adults between the time periods 1988-1994 and 2011-2014, and it varies by race and ethnicity, with the highest prevalence, 18%, among African Americans and Mexican Americans, and the lowest, 9.6%, among non-Hispanic whites (FIGURE).2

Diet is the cornerstone of diabetes treatment
The foundation of a comprehensive management plan for type 2 diabetes mellitus (T2DM) is an appropriate diet. A growing body of evidence shows that a well-structured diet is important in controlling diabetes, delaying or preventing the onset of diabetes, and, in some instances, contributing to its remission. Diabetes UK, the United Kingdom’s equivalent of the American Diabetes Association (ADA), recently updated its clinical guideline for physicians and patients on the role of nutrition in managing and preventing diabetes, and it is consistent with one published by the ADA in 2013.3,4
The Diabetes UK guideline is the result of an evidence-based process that meets the standards recommended by the National Academy of Medicine (previously the Institute of Medicine): a systematic review and formal assessment of the quality of the evidence, and recommendations based on the highest quality evidence available, with the level of evidence stated for each recommendation.5 Assessing the level of evidence and determining the strengths of recommendations were done using the Grades of Recommendation Assessment, Development, and Evaluation (GRADE) system, which uses an approach similar to that of the Strength of Recommendation Taxonomy (SORT).
What, and what not, to focus on. The first set of recommendations states that everyone with, or at risk for, diabetes should receive structured, personalized, and ongoing nutritional advice from a dietician who is coordinated with their clinical care. Nutritional advice should focus on the quality and quantity of food, not on specific nutrients (fat and carbohydrates), since there is no good evidence on what proportion of such nutrients is optimal. And it should be tailored to the culture and eating preferences of the patient.
The type of diet with the strongest evidence base for preventing T2DM is a Mediterranean diet, which is supported by level-4, high-quality evidence. Important aspects of a Mediterranean diet are the regular consumption of nuts, whole grains, fruits, and vegetables; use of olive oil instead of butter; and favoring fish over red meat.6 Other dietary patterns associated with reduced risk but supported only by level-2, low-quality evidence, include Dietary Approaches to Stop Hypertension (DASH), vegetarian, vegan, and Nordic healthy diets. Moderate carbohydrate restriction is supported only by level-1, very low-quality evidence.
The UK guideline, too, recommends preferentially eating whole grains, fruits, and green leafy vegetables, as well as yogurt, cheese, tea, and coffee. And it advises reducing consumption of red processed meats, potatoes (especially French fries), sugar-sweetened beverages, and refined carbohydrates. However, these specific food preferences are supported only by low-level evidence.
Plant stanols and plant sterols are found in a variety of plant foods such as cereals, vegetable oils, seeds, and nuts, and are now being added to some food products. (For more on plant stanols and plant sterols.) They have a chemical structure similar to cholesterol and reduce the intestinal absorption of cholesterol, thereby lowering total serum cholesterol and LDL-cholesterol. Both Diabetes UK and the ADA recommend 2 to 3 grams of stanols/sterols per day.
Continue to: Alcohol intake
Alcohol intake. And what about alcohol intake in those with T2DM? Once again, both guidelines are in concert by stating that alcohol use in those with diabetes should be moderate, defined by the ADA as one or fewer drinks/d for women and 2 or fewer for men.
Weight loss and exercise are important, too. Those who are overweight or obese with T2DM can improve glycemic control with a 5% weight loss achieved by reducing caloric intake and by increasing energy expenditure with 150 minutes of moderate physical activity per week over at least 3 days.3 This recommendation is supported by high-quality evidence.
A 15-kg weight loss is recommended for those attempting diabetes remission (supported by moderate-level evidence).3 One small study in the United Kingdom found that more than half of those with T2DM could achieve remission with weight loss of 10 kg or more; 86% with weight loss of 15 kg or more.7 The Diabetes UK guideline panel rated this as having moderate-level evidence.
The bottom line. Diet and exercise are key interventions for the prevention and treatment of diabetes and can lead to remission if sufficient weight loss is achieved. To achieve and maintain an optimal diet, patients need individualized professional advice and followup. The evidence base for nutritional advice is growing and can be used to improve the quality of these patient-provider interactions.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
1. America Diabetes Association. Statistics About Diabetes. http://www.diabetes.org/diabetes-basics/statistics/. Accessed May 13, 2018.
2. CDC. National Center for Health Statistics. Health, United States, 2016. Available at: https://www.cdc.gov/nchs/data/hus/hus16.pdf. Accessed May 21, 2018.
3. Dyson PA, Twenefour D, Breen C, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2018;35:541-547.
4. Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821-3842.
5. IOM (Institute of Medicine). 2011. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
6. Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222.
7. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551.
USPSTF update: New and revised recommendations
Over the past year the US Preventive Services Task Force made 14 recommendations on 12 conditions (TABLE 11-12). One of these pronouncements was the unusual reversal of a previous “D” recommendation against screening for scoliosis in adolescents, changing it to an “I” statement (insufficient evidence).
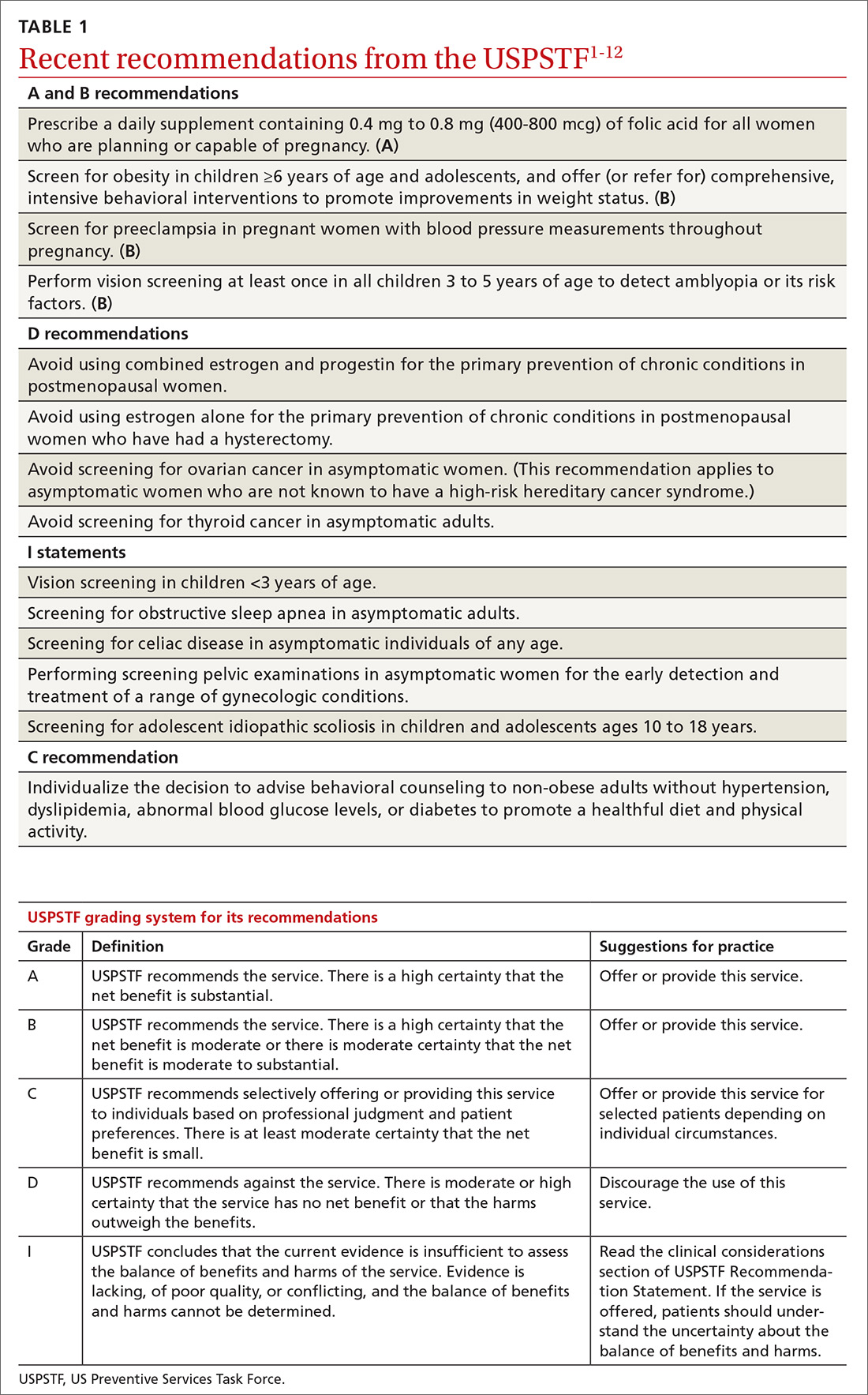
Affirmative recommendations
Four interventions were given an “A” or “B” recommendation this past year. Both grades signify a recommendation to perform the service, with “A” reflecting a higher level of certainty or a higher level of net benefit than “B.”
Recommend folic acid to prevent neural tube defects (A)
The evidence is very strong that folic acid intake prevents neural tube defects. In 2009 the Task Force recommended folic acid supplementation for women of childbearing age. In 2017 this recommendation was updated and slightly reworded to advise that all women who are planning a pregnancy or capable of becoming pregnant take a daily supplement containing 0.4 mg to 0.8 mg (400-800 mcg) of folic acid.
In the United States many grain products have been fortified with folic acid since 1996. This step has reduced the prevalence of neural tube defects from 10.7 cases per 10,000 live births to 7 cases per 10,000 live births in 2011.1 However, in spite of food fortification, most women in the United States do not consume the recommended daily amount of 0.4 mg (400 mcg) of folic acid. This supplementation is most important from one month before conception through the first 3 months of pregnancy.
Screen for obesity in children and adolescents (B)
Nearly 17% of children and adolescents ages 2 to 19 years in the United States are obese, and almost 32% are overweight or obese.2 Obesity is defined as a body mass index (BMI) ≥95th percentile, based on year-2000 growth charts published by the Centers for Disease Control and Prevention. Overweight is defined as a BMI between the 85th and 94th percentiles.
Obesity in children and adolescents is associated with many physical problems, including obstructive sleep apnea, orthopedic problems, high blood pressure, hyperlipidemia, and diabetes, as well as psychological harms from being teased and bullied. Obesity that continues into adulthood is associated with diabetes, cardiovascular disease, and orthopedic problems.
The Task Force found that intensive behavioral interventions for obesity in children ≥6 years of age and in adolescents can lead to moderate improvements in weight status for up to 12 months. Intensive behavioral interventions need to include at least 26 contact hours over 2 to 12 months. The recommendation statement includes a more detailed description of the types of programs that have evidence to support them.2
The Task Force did not recommend the use of either metformin or orlistat because of inadequate evidence on the harmful effects of metformin and because of sound evidence that orlistat causes moderate harms, such as abdominal pain, cramping, incontinence, and flatus.
Screen for preeclampsia (B), but dipstick testing is unreliable
Preeclampsia occurs in a little more than 3% of pregnancies in the United States.13 For the mother, this condition can lead to stroke, eclampsia, organ failure, and death; for the fetus, intrauterine growth retardation, preterm birth, low birth weight, and still birth. Preeclampsia is a leading cause of maternal mortality worldwide. Adverse health outcomes can be prevented by early detection of preeclampsia and by managing it appropriately.3
In 1996 the Task Force recommended screening for preeclampsia during pregnancy, and it reaffirmed that recommendation last year. The Task Force recommends taking blood pressure measurements at every prenatal visit, but does not recommend testing for urine protein with a dipstick because of the technique’s low accuracy.
Since 2014 the Task Force has also recommended using low-dose aspirin after Week 12 of pregnancy to prevent preeclampsia in women who are at high risk.14
Conduct vision screening in all children ages 3 to 5 years (B)
One of the more nuanced recommendations involves vision screening in children. The Task Force recently reaffirmed its 2011 recommendation to perform vision screening at least once in all children ages 3 to 5 years to detect amblyopia or its risk factors. But it found insufficient evidence to test children <3 years of age.
Amblyopia is a “functional reduction in visual acuity characterized by abnormal processing of visual images; [it is] established by the brain during a critical period of vision development.”4 Risk factors associated with the development of amblyopia include strabismus (ocular misalignment); vision loss caused by cataracts; refractive errors such as near and far sightedness, astigmatism (“blurred vision at any distance due to abnormal curvature of the cornea or lens”); and anisometropia (“asymmetric refractive error between the … eyes that causes image suppression in the eye with the larger error”). 4
Physical exam- and machine-based screening tests are available in the primary care setting (TABLE 2).4
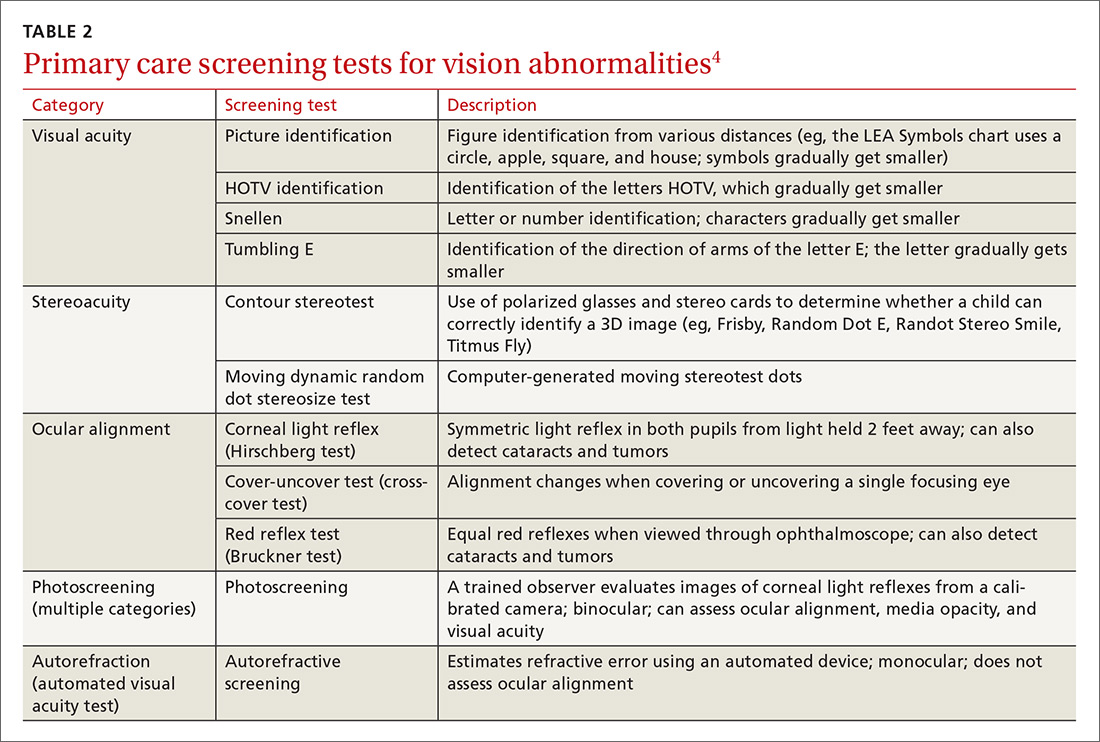
At first glance it appears that the Task Force recommends screening only for amblyopia, but the addition of “risk factors” implies a more comprehensive vision evaluation that would include visual acuity. This interpretation more closely aligns the Task Force recommendation with that of a joint report by the American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Certified Orthoptists, and American Academy of Ophthalmology, which recommends testing for a variety of vision problems in children.15 Nevertheless, the Task Force maintains that the evidence of benefit in testing more extensively before age 3 is insufficient, while the other organizations recommend starting testing at age 6 months.
Negative “D” recommendations
Equally as important as affirmative recommendations for effective interventions are the “D” recommendations advising against interventions that are ineffective or cause more harm than benefits. This past year, the Task Force recommended against 4 interventions. Two pertain to the use of estrogen or combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal women.5 This topic has been discussed in a recent JFP audiocast. Also receiving “D” recommendations were screening for ovarian cancer in asymptomatic women,6 discussed in another JFP audiocast, and screening for thyroid cancer in asymptomatic adults.7
The “D” recommendation for thyroid cancer screening was based on the low incidence of thyroid cancer, the evidence showing no change in mortality after the introduction of population-based screening, and the likelihood of overdiagnosis and overtreatment that would result from screening. The screening tests considered by the Task Force included neck palpation and ultrasound.7
Insufficient evidence
In addition to the previously mentioned “I” statement on vision screening for children <3 years of age,4 4 other interventions lacked sufficient evidence that the Task Force could use in determining relative levels of harms and benefits. These interventions were screening for obstructive sleep apnea in asymptomatic adults,8 screening for celiac disease in asymptomatic patients of all ages,9 screening with a pelvic examination in asymptomatic women,10 and screening for adolescent idiopathic scoliosis in children and adolescents ages 10 to 18 years.11
The lack of evidence regarding the value of a routine pelvic exam for asymptomatic women is surprising given how often this procedure is performed. The Task Force defined a pelvic exam as an “assessment of the external genitalia, internal speculum examination, bimanual palpation, and rectovaginal examination.”10 The Task Force found very little evidence on the accuracy and effectiveness of this exam for a range of gynecologic conditions other than cervical cancer, gonorrhea, and chlamydia, for which screening is recommended.10
The “I” statement on screening for adolescent idiopathic scoliosis in children and adolescents is an unusual revision of a “D” recommendation from 2004. At that time, the Task Force found that treatment of adolescent idiopathic scoliosis leads to health benefits in only a small proportion of individuals and that there are harms of treatment such as unnecessary bracing and referral to specialty care. For the most recent evidence report, the Task Force used a new methodology to assess treatment harms and concluded that the evidence is now inadequate. That finding, along with new evidence that “suggests that brace treatment can interrupt or slow scoliosis progression” led the Task Force to move away from a “D” recommendation.11
The enigmatic “C” recommendation
Perhaps the most difficult recommendation category to understand and implement is the “C” recommendation. With a “C” intervention, there is moderate certainty that the net benefit of universal implementation would be very small; but there are some individuals who might benefit from it, and physicians should offer it selectively.
The Task Force made one “C” recommendation over the past year: for adults who are not obese and who do not have other cardiovascular disease (CVD) risks, the net gain in referring them to behavioral counseling to promote a healthful diet and physical activity is small. However, the harms from such referrals are also small. Counseling interventions can result in healthier habits and in small improvements in intermediate outcomes, such as blood pressure, cholesterol levels, and weight. The effect on overall CVD mortality, though, has been minimal.12 The Task Force concluded that “[those] who are interested and ready to make behavioral changes may be most likely to benefit from behavioral counseling.”
1. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2018.
2. USPSTF. Obesity in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-children-and-adolescents-screening1. Accessed March 22, 2018.
3. USPSTF. Preeclampsia: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/preeclampsia-screening1. Accessed March 22, 2018.
4. USPSTF. Vision in children ages 6 months to 5 years: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/vision-in-children-ages-6-months-to-5-years-screening. Accessed March 22, 2018.
5. USPSTF. Hormone therapy in postmenopausal women: primary prevention of chronic conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/menopausal-hormone-therapy-preventive-medication1. Accessed March 24, 2018.
6. USPSTF. Ovarian cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ovarian-cancer-screening1. Accessed March 24, 2018.
7. USPSTF. Thyroid cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/thyroid-cancer-screening1. Accessed March 22, 2018.
8. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2018.
9. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/celiac-disease-screening. Accessed March 24, 2018.
10. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2018.
11. USPSTF. Adolescent idiopathic scoliosis: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 22, 2018.
12. USPSTF. Healthful diet and physical activity for cardiovascular disease prevention in adults without known risk factors: behavioral counseling. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/healthful-diet-and-physical-activity-for-cardiovascular-disease-prevention-in-adults-without-known-risk-factors-behavioral-counseling. Accessed March 22, 2018.
13. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
14. USPSTF. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed March 22, 2018.
15. Donahue SP, Baker CN; Committee on Practice and Ambulatory Medicine, American Academy of Pediatrics; Section on Ophthalmology, American Academy of Pediatrics; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Procedures for the evaluation of the visual system by pediatricians. Pediatrics. 2016;137.2015-3597.
Over the past year the US Preventive Services Task Force made 14 recommendations on 12 conditions (TABLE 11-12). One of these pronouncements was the unusual reversal of a previous “D” recommendation against screening for scoliosis in adolescents, changing it to an “I” statement (insufficient evidence).

Affirmative recommendations
Four interventions were given an “A” or “B” recommendation this past year. Both grades signify a recommendation to perform the service, with “A” reflecting a higher level of certainty or a higher level of net benefit than “B.”
Recommend folic acid to prevent neural tube defects (A)
The evidence is very strong that folic acid intake prevents neural tube defects. In 2009 the Task Force recommended folic acid supplementation for women of childbearing age. In 2017 this recommendation was updated and slightly reworded to advise that all women who are planning a pregnancy or capable of becoming pregnant take a daily supplement containing 0.4 mg to 0.8 mg (400-800 mcg) of folic acid.
In the United States many grain products have been fortified with folic acid since 1996. This step has reduced the prevalence of neural tube defects from 10.7 cases per 10,000 live births to 7 cases per 10,000 live births in 2011.1 However, in spite of food fortification, most women in the United States do not consume the recommended daily amount of 0.4 mg (400 mcg) of folic acid. This supplementation is most important from one month before conception through the first 3 months of pregnancy.
Screen for obesity in children and adolescents (B)
Nearly 17% of children and adolescents ages 2 to 19 years in the United States are obese, and almost 32% are overweight or obese.2 Obesity is defined as a body mass index (BMI) ≥95th percentile, based on year-2000 growth charts published by the Centers for Disease Control and Prevention. Overweight is defined as a BMI between the 85th and 94th percentiles.
Obesity in children and adolescents is associated with many physical problems, including obstructive sleep apnea, orthopedic problems, high blood pressure, hyperlipidemia, and diabetes, as well as psychological harms from being teased and bullied. Obesity that continues into adulthood is associated with diabetes, cardiovascular disease, and orthopedic problems.
The Task Force found that intensive behavioral interventions for obesity in children ≥6 years of age and in adolescents can lead to moderate improvements in weight status for up to 12 months. Intensive behavioral interventions need to include at least 26 contact hours over 2 to 12 months. The recommendation statement includes a more detailed description of the types of programs that have evidence to support them.2
The Task Force did not recommend the use of either metformin or orlistat because of inadequate evidence on the harmful effects of metformin and because of sound evidence that orlistat causes moderate harms, such as abdominal pain, cramping, incontinence, and flatus.
Screen for preeclampsia (B), but dipstick testing is unreliable
Preeclampsia occurs in a little more than 3% of pregnancies in the United States.13 For the mother, this condition can lead to stroke, eclampsia, organ failure, and death; for the fetus, intrauterine growth retardation, preterm birth, low birth weight, and still birth. Preeclampsia is a leading cause of maternal mortality worldwide. Adverse health outcomes can be prevented by early detection of preeclampsia and by managing it appropriately.3
In 1996 the Task Force recommended screening for preeclampsia during pregnancy, and it reaffirmed that recommendation last year. The Task Force recommends taking blood pressure measurements at every prenatal visit, but does not recommend testing for urine protein with a dipstick because of the technique’s low accuracy.
Since 2014 the Task Force has also recommended using low-dose aspirin after Week 12 of pregnancy to prevent preeclampsia in women who are at high risk.14
Conduct vision screening in all children ages 3 to 5 years (B)
One of the more nuanced recommendations involves vision screening in children. The Task Force recently reaffirmed its 2011 recommendation to perform vision screening at least once in all children ages 3 to 5 years to detect amblyopia or its risk factors. But it found insufficient evidence to test children <3 years of age.
Amblyopia is a “functional reduction in visual acuity characterized by abnormal processing of visual images; [it is] established by the brain during a critical period of vision development.”4 Risk factors associated with the development of amblyopia include strabismus (ocular misalignment); vision loss caused by cataracts; refractive errors such as near and far sightedness, astigmatism (“blurred vision at any distance due to abnormal curvature of the cornea or lens”); and anisometropia (“asymmetric refractive error between the … eyes that causes image suppression in the eye with the larger error”). 4
Physical exam- and machine-based screening tests are available in the primary care setting (TABLE 2).4

At first glance it appears that the Task Force recommends screening only for amblyopia, but the addition of “risk factors” implies a more comprehensive vision evaluation that would include visual acuity. This interpretation more closely aligns the Task Force recommendation with that of a joint report by the American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Certified Orthoptists, and American Academy of Ophthalmology, which recommends testing for a variety of vision problems in children.15 Nevertheless, the Task Force maintains that the evidence of benefit in testing more extensively before age 3 is insufficient, while the other organizations recommend starting testing at age 6 months.
Negative “D” recommendations
Equally as important as affirmative recommendations for effective interventions are the “D” recommendations advising against interventions that are ineffective or cause more harm than benefits. This past year, the Task Force recommended against 4 interventions. Two pertain to the use of estrogen or combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal women.5 This topic has been discussed in a recent JFP audiocast. Also receiving “D” recommendations were screening for ovarian cancer in asymptomatic women,6 discussed in another JFP audiocast, and screening for thyroid cancer in asymptomatic adults.7
The “D” recommendation for thyroid cancer screening was based on the low incidence of thyroid cancer, the evidence showing no change in mortality after the introduction of population-based screening, and the likelihood of overdiagnosis and overtreatment that would result from screening. The screening tests considered by the Task Force included neck palpation and ultrasound.7
Insufficient evidence
In addition to the previously mentioned “I” statement on vision screening for children <3 years of age,4 4 other interventions lacked sufficient evidence that the Task Force could use in determining relative levels of harms and benefits. These interventions were screening for obstructive sleep apnea in asymptomatic adults,8 screening for celiac disease in asymptomatic patients of all ages,9 screening with a pelvic examination in asymptomatic women,10 and screening for adolescent idiopathic scoliosis in children and adolescents ages 10 to 18 years.11
The lack of evidence regarding the value of a routine pelvic exam for asymptomatic women is surprising given how often this procedure is performed. The Task Force defined a pelvic exam as an “assessment of the external genitalia, internal speculum examination, bimanual palpation, and rectovaginal examination.”10 The Task Force found very little evidence on the accuracy and effectiveness of this exam for a range of gynecologic conditions other than cervical cancer, gonorrhea, and chlamydia, for which screening is recommended.10
The “I” statement on screening for adolescent idiopathic scoliosis in children and adolescents is an unusual revision of a “D” recommendation from 2004. At that time, the Task Force found that treatment of adolescent idiopathic scoliosis leads to health benefits in only a small proportion of individuals and that there are harms of treatment such as unnecessary bracing and referral to specialty care. For the most recent evidence report, the Task Force used a new methodology to assess treatment harms and concluded that the evidence is now inadequate. That finding, along with new evidence that “suggests that brace treatment can interrupt or slow scoliosis progression” led the Task Force to move away from a “D” recommendation.11
The enigmatic “C” recommendation
Perhaps the most difficult recommendation category to understand and implement is the “C” recommendation. With a “C” intervention, there is moderate certainty that the net benefit of universal implementation would be very small; but there are some individuals who might benefit from it, and physicians should offer it selectively.
The Task Force made one “C” recommendation over the past year: for adults who are not obese and who do not have other cardiovascular disease (CVD) risks, the net gain in referring them to behavioral counseling to promote a healthful diet and physical activity is small. However, the harms from such referrals are also small. Counseling interventions can result in healthier habits and in small improvements in intermediate outcomes, such as blood pressure, cholesterol levels, and weight. The effect on overall CVD mortality, though, has been minimal.12 The Task Force concluded that “[those] who are interested and ready to make behavioral changes may be most likely to benefit from behavioral counseling.”
Over the past year the US Preventive Services Task Force made 14 recommendations on 12 conditions (TABLE 11-12). One of these pronouncements was the unusual reversal of a previous “D” recommendation against screening for scoliosis in adolescents, changing it to an “I” statement (insufficient evidence).

Affirmative recommendations
Four interventions were given an “A” or “B” recommendation this past year. Both grades signify a recommendation to perform the service, with “A” reflecting a higher level of certainty or a higher level of net benefit than “B.”
Recommend folic acid to prevent neural tube defects (A)
The evidence is very strong that folic acid intake prevents neural tube defects. In 2009 the Task Force recommended folic acid supplementation for women of childbearing age. In 2017 this recommendation was updated and slightly reworded to advise that all women who are planning a pregnancy or capable of becoming pregnant take a daily supplement containing 0.4 mg to 0.8 mg (400-800 mcg) of folic acid.
In the United States many grain products have been fortified with folic acid since 1996. This step has reduced the prevalence of neural tube defects from 10.7 cases per 10,000 live births to 7 cases per 10,000 live births in 2011.1 However, in spite of food fortification, most women in the United States do not consume the recommended daily amount of 0.4 mg (400 mcg) of folic acid. This supplementation is most important from one month before conception through the first 3 months of pregnancy.
Screen for obesity in children and adolescents (B)
Nearly 17% of children and adolescents ages 2 to 19 years in the United States are obese, and almost 32% are overweight or obese.2 Obesity is defined as a body mass index (BMI) ≥95th percentile, based on year-2000 growth charts published by the Centers for Disease Control and Prevention. Overweight is defined as a BMI between the 85th and 94th percentiles.
Obesity in children and adolescents is associated with many physical problems, including obstructive sleep apnea, orthopedic problems, high blood pressure, hyperlipidemia, and diabetes, as well as psychological harms from being teased and bullied. Obesity that continues into adulthood is associated with diabetes, cardiovascular disease, and orthopedic problems.
The Task Force found that intensive behavioral interventions for obesity in children ≥6 years of age and in adolescents can lead to moderate improvements in weight status for up to 12 months. Intensive behavioral interventions need to include at least 26 contact hours over 2 to 12 months. The recommendation statement includes a more detailed description of the types of programs that have evidence to support them.2
The Task Force did not recommend the use of either metformin or orlistat because of inadequate evidence on the harmful effects of metformin and because of sound evidence that orlistat causes moderate harms, such as abdominal pain, cramping, incontinence, and flatus.
Screen for preeclampsia (B), but dipstick testing is unreliable
Preeclampsia occurs in a little more than 3% of pregnancies in the United States.13 For the mother, this condition can lead to stroke, eclampsia, organ failure, and death; for the fetus, intrauterine growth retardation, preterm birth, low birth weight, and still birth. Preeclampsia is a leading cause of maternal mortality worldwide. Adverse health outcomes can be prevented by early detection of preeclampsia and by managing it appropriately.3
In 1996 the Task Force recommended screening for preeclampsia during pregnancy, and it reaffirmed that recommendation last year. The Task Force recommends taking blood pressure measurements at every prenatal visit, but does not recommend testing for urine protein with a dipstick because of the technique’s low accuracy.
Since 2014 the Task Force has also recommended using low-dose aspirin after Week 12 of pregnancy to prevent preeclampsia in women who are at high risk.14
Conduct vision screening in all children ages 3 to 5 years (B)
One of the more nuanced recommendations involves vision screening in children. The Task Force recently reaffirmed its 2011 recommendation to perform vision screening at least once in all children ages 3 to 5 years to detect amblyopia or its risk factors. But it found insufficient evidence to test children <3 years of age.
Amblyopia is a “functional reduction in visual acuity characterized by abnormal processing of visual images; [it is] established by the brain during a critical period of vision development.”4 Risk factors associated with the development of amblyopia include strabismus (ocular misalignment); vision loss caused by cataracts; refractive errors such as near and far sightedness, astigmatism (“blurred vision at any distance due to abnormal curvature of the cornea or lens”); and anisometropia (“asymmetric refractive error between the … eyes that causes image suppression in the eye with the larger error”). 4
Physical exam- and machine-based screening tests are available in the primary care setting (TABLE 2).4

At first glance it appears that the Task Force recommends screening only for amblyopia, but the addition of “risk factors” implies a more comprehensive vision evaluation that would include visual acuity. This interpretation more closely aligns the Task Force recommendation with that of a joint report by the American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Certified Orthoptists, and American Academy of Ophthalmology, which recommends testing for a variety of vision problems in children.15 Nevertheless, the Task Force maintains that the evidence of benefit in testing more extensively before age 3 is insufficient, while the other organizations recommend starting testing at age 6 months.
Negative “D” recommendations
Equally as important as affirmative recommendations for effective interventions are the “D” recommendations advising against interventions that are ineffective or cause more harm than benefits. This past year, the Task Force recommended against 4 interventions. Two pertain to the use of estrogen or combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal women.5 This topic has been discussed in a recent JFP audiocast. Also receiving “D” recommendations were screening for ovarian cancer in asymptomatic women,6 discussed in another JFP audiocast, and screening for thyroid cancer in asymptomatic adults.7
The “D” recommendation for thyroid cancer screening was based on the low incidence of thyroid cancer, the evidence showing no change in mortality after the introduction of population-based screening, and the likelihood of overdiagnosis and overtreatment that would result from screening. The screening tests considered by the Task Force included neck palpation and ultrasound.7
Insufficient evidence
In addition to the previously mentioned “I” statement on vision screening for children <3 years of age,4 4 other interventions lacked sufficient evidence that the Task Force could use in determining relative levels of harms and benefits. These interventions were screening for obstructive sleep apnea in asymptomatic adults,8 screening for celiac disease in asymptomatic patients of all ages,9 screening with a pelvic examination in asymptomatic women,10 and screening for adolescent idiopathic scoliosis in children and adolescents ages 10 to 18 years.11
The lack of evidence regarding the value of a routine pelvic exam for asymptomatic women is surprising given how often this procedure is performed. The Task Force defined a pelvic exam as an “assessment of the external genitalia, internal speculum examination, bimanual palpation, and rectovaginal examination.”10 The Task Force found very little evidence on the accuracy and effectiveness of this exam for a range of gynecologic conditions other than cervical cancer, gonorrhea, and chlamydia, for which screening is recommended.10
The “I” statement on screening for adolescent idiopathic scoliosis in children and adolescents is an unusual revision of a “D” recommendation from 2004. At that time, the Task Force found that treatment of adolescent idiopathic scoliosis leads to health benefits in only a small proportion of individuals and that there are harms of treatment such as unnecessary bracing and referral to specialty care. For the most recent evidence report, the Task Force used a new methodology to assess treatment harms and concluded that the evidence is now inadequate. That finding, along with new evidence that “suggests that brace treatment can interrupt or slow scoliosis progression” led the Task Force to move away from a “D” recommendation.11
The enigmatic “C” recommendation
Perhaps the most difficult recommendation category to understand and implement is the “C” recommendation. With a “C” intervention, there is moderate certainty that the net benefit of universal implementation would be very small; but there are some individuals who might benefit from it, and physicians should offer it selectively.
The Task Force made one “C” recommendation over the past year: for adults who are not obese and who do not have other cardiovascular disease (CVD) risks, the net gain in referring them to behavioral counseling to promote a healthful diet and physical activity is small. However, the harms from such referrals are also small. Counseling interventions can result in healthier habits and in small improvements in intermediate outcomes, such as blood pressure, cholesterol levels, and weight. The effect on overall CVD mortality, though, has been minimal.12 The Task Force concluded that “[those] who are interested and ready to make behavioral changes may be most likely to benefit from behavioral counseling.”
1. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2018.
2. USPSTF. Obesity in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-children-and-adolescents-screening1. Accessed March 22, 2018.
3. USPSTF. Preeclampsia: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/preeclampsia-screening1. Accessed March 22, 2018.
4. USPSTF. Vision in children ages 6 months to 5 years: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/vision-in-children-ages-6-months-to-5-years-screening. Accessed March 22, 2018.
5. USPSTF. Hormone therapy in postmenopausal women: primary prevention of chronic conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/menopausal-hormone-therapy-preventive-medication1. Accessed March 24, 2018.
6. USPSTF. Ovarian cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ovarian-cancer-screening1. Accessed March 24, 2018.
7. USPSTF. Thyroid cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/thyroid-cancer-screening1. Accessed March 22, 2018.
8. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2018.
9. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/celiac-disease-screening. Accessed March 24, 2018.
10. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2018.
11. USPSTF. Adolescent idiopathic scoliosis: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 22, 2018.
12. USPSTF. Healthful diet and physical activity for cardiovascular disease prevention in adults without known risk factors: behavioral counseling. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/healthful-diet-and-physical-activity-for-cardiovascular-disease-prevention-in-adults-without-known-risk-factors-behavioral-counseling. Accessed March 22, 2018.
13. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
14. USPSTF. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed March 22, 2018.
15. Donahue SP, Baker CN; Committee on Practice and Ambulatory Medicine, American Academy of Pediatrics; Section on Ophthalmology, American Academy of Pediatrics; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Procedures for the evaluation of the visual system by pediatricians. Pediatrics. 2016;137.2015-3597.
1. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2018.
2. USPSTF. Obesity in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-children-and-adolescents-screening1. Accessed March 22, 2018.
3. USPSTF. Preeclampsia: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/preeclampsia-screening1. Accessed March 22, 2018.
4. USPSTF. Vision in children ages 6 months to 5 years: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/vision-in-children-ages-6-months-to-5-years-screening. Accessed March 22, 2018.
5. USPSTF. Hormone therapy in postmenopausal women: primary prevention of chronic conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/menopausal-hormone-therapy-preventive-medication1. Accessed March 24, 2018.
6. USPSTF. Ovarian cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ovarian-cancer-screening1. Accessed March 24, 2018.
7. USPSTF. Thyroid cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/thyroid-cancer-screening1. Accessed March 22, 2018.
8. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2018.
9. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/celiac-disease-screening. Accessed March 24, 2018.
10. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2018.
11. USPSTF. Adolescent idiopathic scoliosis: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 22, 2018.
12. USPSTF. Healthful diet and physical activity for cardiovascular disease prevention in adults without known risk factors: behavioral counseling. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/healthful-diet-and-physical-activity-for-cardiovascular-disease-prevention-in-adults-without-known-risk-factors-behavioral-counseling. Accessed March 22, 2018.
13. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
14. USPSTF. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed March 22, 2018.
15. Donahue SP, Baker CN; Committee on Practice and Ambulatory Medicine, American Academy of Pediatrics; Section on Ophthalmology, American Academy of Pediatrics; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Procedures for the evaluation of the visual system by pediatricians. Pediatrics. 2016;137.2015-3597.
From The Journal of Family Practice | 2018;67(5):294-296,298-299.
Guidelines May Help Reduce Stroke Risk in Patients With Diabetes
LOS ANGELES—Current guidelines can help physicians prevent stroke in patients with diabetes, according to a lecture delivered at the International Stroke Conference 2018. The prevalence of diabetes is expected to approximately double by 2050, and 16% of patients with diabetes die from stroke, said Philip B. Gorelick, MD, MPH, Professor of Translational Science and Molecular Medicine at Michigan State University College of Human Medicine in Grand Rapids. Patients with diabetes and ischemic stroke tend to have hypertension, thus controlling blood pressure in these patients could improve public health.
The ACC/AHA 2017 Hypertension Guideline
The 2017 hypertension guideline published by the American College of Cardiology (ACC) and the American Heart Association (AHA) recommends antihypertensive therapy for patients with diabetes and blood pressure of 130/80 mm Hg or higher. The treatment goal is blood pressure lower than 130/80 mm Hg. First-line treatment options include thiazide-like diuretics, ACE inhibitors, angiotensin receptor blockers (ARBs), and calcium-channel blockers. For patients with albuminuria, physicians may consider treatment with an ACE inhibitor or an ARB.
The ADA Position Statement
The American Diabetes Association (ADA) published an updated position statement on diabetes and hypertension in Diabetes Care in September 2017. One of its important recommendations is that orthostatic measurement of blood pressure be performed during the initial evaluation of hypertension and periodically at follow-up, said Dr. Gorelick. “Many of us are not doing that, I suspect,” he added.
Unlike the ACC and AHA, the ADA recommends a blood pressure target of less than 140/90 mm Hg and adds that lower blood pressure targets may be indicated for patients at high risk of cardiovascular disease. For patients with blood pressure greater than 120/80 mm Hg, the ADA recommends lifestyle management such as weight loss, the Dietary Approaches to Stop Hypertension diet, increased consumption of fruits and vegetables, moderate alcohol consumption, and increased physical activity.
Furthermore, the ADA recommends timely titration of pharmacologic therapy plus lifestyle management for patients with diabetes and blood pressure of 140/90 mm Hg or greater. For patients with blood pressure of 160/100 mm Hg or greater, the statement recommends prompt initiation and timely titration of two drugs or a single-pill combination, plus lifestyle intervention. Appropriate therapies include ACE inhibitors, ARBs, thiazide-like diuretics, and calcium-channel blockers, according to the statement. An ACE inhibitor or ARB is recommended for patients with a high ratio of urine albumin to creatinine.
AHA/ASA 2014 Guidelines
The ADA’s recommendations are similar to those of the guidelines for primary stroke prevention that the AHA and the American Stroke Association (ASA) issued in 2014. The latter guidance recommends control of blood pressure to a target of less than 140/90 mm Hg for patients with type 1 or type 2 diabetes. The associations recommend statin therapy to lower the risk of a first stroke and suggest that aspirin be considered for primary stroke prevention in patients with diabetes and a high 10-year risk of cardiovascular disease. Finally, the AHA and ASA recommend that physicians use the ADA’s guidance for glycemic control and management of cardiovascular risk factors.
Evidence That Informed Guidelines
The abovementioned guidelines incorporate evidence from various trials that examined the effect of antihypertensive treatment on stroke risk in patients with and without diabetes. One such trial is the Secondary Prevention of Small Subcortical Strokes study, the results of which were published by Benavente et al in 2013. They found that treatment to a target systolic blood pressure of less than 130 mm Hg reduced the risk of recurrent stroke by about 20%, compared with a target of between 130 mm Hg and 149 mm Hg, but the difference was not statistically significant. Treatment to the lower blood pressure target did, however, significantly reduce the risk of intracranial hemorrhage by about two-thirds.
In the ACCORD trial, which was published in 2010, investigators randomized participants with type 2 diabetes to intensive therapy (ie, a target systolic blood pressure of less than 120 mm Hg) or standard therapy (ie, a target systolic blood pressure of less than 140 mm Hg). Although intensive therapy did not reduce the composite risk of fatal and nonfatal major cardiovascular events, compared with standard therapy, it did reduce the risk of stroke.
—Erik Greb
Suggested Reading
ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585.
de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: A position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273-1284.
Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754-3832.
SPS3 Study Group, Benavente OR, Coffey CS, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507-515.
Whelton PK, Carey RM. The 2017 American College of Cardiology/American Heart Association clinical practice guideline for high blood pressure in adults. JAMA Cardiol. 2018 Feb 21 [Epub ahead of print].
LOS ANGELES—Current guidelines can help physicians prevent stroke in patients with diabetes, according to a lecture delivered at the International Stroke Conference 2018. The prevalence of diabetes is expected to approximately double by 2050, and 16% of patients with diabetes die from stroke, said Philip B. Gorelick, MD, MPH, Professor of Translational Science and Molecular Medicine at Michigan State University College of Human Medicine in Grand Rapids. Patients with diabetes and ischemic stroke tend to have hypertension, thus controlling blood pressure in these patients could improve public health.
The ACC/AHA 2017 Hypertension Guideline
The 2017 hypertension guideline published by the American College of Cardiology (ACC) and the American Heart Association (AHA) recommends antihypertensive therapy for patients with diabetes and blood pressure of 130/80 mm Hg or higher. The treatment goal is blood pressure lower than 130/80 mm Hg. First-line treatment options include thiazide-like diuretics, ACE inhibitors, angiotensin receptor blockers (ARBs), and calcium-channel blockers. For patients with albuminuria, physicians may consider treatment with an ACE inhibitor or an ARB.
The ADA Position Statement
The American Diabetes Association (ADA) published an updated position statement on diabetes and hypertension in Diabetes Care in September 2017. One of its important recommendations is that orthostatic measurement of blood pressure be performed during the initial evaluation of hypertension and periodically at follow-up, said Dr. Gorelick. “Many of us are not doing that, I suspect,” he added.
Unlike the ACC and AHA, the ADA recommends a blood pressure target of less than 140/90 mm Hg and adds that lower blood pressure targets may be indicated for patients at high risk of cardiovascular disease. For patients with blood pressure greater than 120/80 mm Hg, the ADA recommends lifestyle management such as weight loss, the Dietary Approaches to Stop Hypertension diet, increased consumption of fruits and vegetables, moderate alcohol consumption, and increased physical activity.
Furthermore, the ADA recommends timely titration of pharmacologic therapy plus lifestyle management for patients with diabetes and blood pressure of 140/90 mm Hg or greater. For patients with blood pressure of 160/100 mm Hg or greater, the statement recommends prompt initiation and timely titration of two drugs or a single-pill combination, plus lifestyle intervention. Appropriate therapies include ACE inhibitors, ARBs, thiazide-like diuretics, and calcium-channel blockers, according to the statement. An ACE inhibitor or ARB is recommended for patients with a high ratio of urine albumin to creatinine.
AHA/ASA 2014 Guidelines
The ADA’s recommendations are similar to those of the guidelines for primary stroke prevention that the AHA and the American Stroke Association (ASA) issued in 2014. The latter guidance recommends control of blood pressure to a target of less than 140/90 mm Hg for patients with type 1 or type 2 diabetes. The associations recommend statin therapy to lower the risk of a first stroke and suggest that aspirin be considered for primary stroke prevention in patients with diabetes and a high 10-year risk of cardiovascular disease. Finally, the AHA and ASA recommend that physicians use the ADA’s guidance for glycemic control and management of cardiovascular risk factors.
Evidence That Informed Guidelines
The abovementioned guidelines incorporate evidence from various trials that examined the effect of antihypertensive treatment on stroke risk in patients with and without diabetes. One such trial is the Secondary Prevention of Small Subcortical Strokes study, the results of which were published by Benavente et al in 2013. They found that treatment to a target systolic blood pressure of less than 130 mm Hg reduced the risk of recurrent stroke by about 20%, compared with a target of between 130 mm Hg and 149 mm Hg, but the difference was not statistically significant. Treatment to the lower blood pressure target did, however, significantly reduce the risk of intracranial hemorrhage by about two-thirds.
In the ACCORD trial, which was published in 2010, investigators randomized participants with type 2 diabetes to intensive therapy (ie, a target systolic blood pressure of less than 120 mm Hg) or standard therapy (ie, a target systolic blood pressure of less than 140 mm Hg). Although intensive therapy did not reduce the composite risk of fatal and nonfatal major cardiovascular events, compared with standard therapy, it did reduce the risk of stroke.
—Erik Greb
Suggested Reading
ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585.
de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: A position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273-1284.
Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754-3832.
SPS3 Study Group, Benavente OR, Coffey CS, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507-515.
Whelton PK, Carey RM. The 2017 American College of Cardiology/American Heart Association clinical practice guideline for high blood pressure in adults. JAMA Cardiol. 2018 Feb 21 [Epub ahead of print].
LOS ANGELES—Current guidelines can help physicians prevent stroke in patients with diabetes, according to a lecture delivered at the International Stroke Conference 2018. The prevalence of diabetes is expected to approximately double by 2050, and 16% of patients with diabetes die from stroke, said Philip B. Gorelick, MD, MPH, Professor of Translational Science and Molecular Medicine at Michigan State University College of Human Medicine in Grand Rapids. Patients with diabetes and ischemic stroke tend to have hypertension, thus controlling blood pressure in these patients could improve public health.
The ACC/AHA 2017 Hypertension Guideline
The 2017 hypertension guideline published by the American College of Cardiology (ACC) and the American Heart Association (AHA) recommends antihypertensive therapy for patients with diabetes and blood pressure of 130/80 mm Hg or higher. The treatment goal is blood pressure lower than 130/80 mm Hg. First-line treatment options include thiazide-like diuretics, ACE inhibitors, angiotensin receptor blockers (ARBs), and calcium-channel blockers. For patients with albuminuria, physicians may consider treatment with an ACE inhibitor or an ARB.
The ADA Position Statement
The American Diabetes Association (ADA) published an updated position statement on diabetes and hypertension in Diabetes Care in September 2017. One of its important recommendations is that orthostatic measurement of blood pressure be performed during the initial evaluation of hypertension and periodically at follow-up, said Dr. Gorelick. “Many of us are not doing that, I suspect,” he added.
Unlike the ACC and AHA, the ADA recommends a blood pressure target of less than 140/90 mm Hg and adds that lower blood pressure targets may be indicated for patients at high risk of cardiovascular disease. For patients with blood pressure greater than 120/80 mm Hg, the ADA recommends lifestyle management such as weight loss, the Dietary Approaches to Stop Hypertension diet, increased consumption of fruits and vegetables, moderate alcohol consumption, and increased physical activity.
Furthermore, the ADA recommends timely titration of pharmacologic therapy plus lifestyle management for patients with diabetes and blood pressure of 140/90 mm Hg or greater. For patients with blood pressure of 160/100 mm Hg or greater, the statement recommends prompt initiation and timely titration of two drugs or a single-pill combination, plus lifestyle intervention. Appropriate therapies include ACE inhibitors, ARBs, thiazide-like diuretics, and calcium-channel blockers, according to the statement. An ACE inhibitor or ARB is recommended for patients with a high ratio of urine albumin to creatinine.
AHA/ASA 2014 Guidelines
The ADA’s recommendations are similar to those of the guidelines for primary stroke prevention that the AHA and the American Stroke Association (ASA) issued in 2014. The latter guidance recommends control of blood pressure to a target of less than 140/90 mm Hg for patients with type 1 or type 2 diabetes. The associations recommend statin therapy to lower the risk of a first stroke and suggest that aspirin be considered for primary stroke prevention in patients with diabetes and a high 10-year risk of cardiovascular disease. Finally, the AHA and ASA recommend that physicians use the ADA’s guidance for glycemic control and management of cardiovascular risk factors.
Evidence That Informed Guidelines
The abovementioned guidelines incorporate evidence from various trials that examined the effect of antihypertensive treatment on stroke risk in patients with and without diabetes. One such trial is the Secondary Prevention of Small Subcortical Strokes study, the results of which were published by Benavente et al in 2013. They found that treatment to a target systolic blood pressure of less than 130 mm Hg reduced the risk of recurrent stroke by about 20%, compared with a target of between 130 mm Hg and 149 mm Hg, but the difference was not statistically significant. Treatment to the lower blood pressure target did, however, significantly reduce the risk of intracranial hemorrhage by about two-thirds.
In the ACCORD trial, which was published in 2010, investigators randomized participants with type 2 diabetes to intensive therapy (ie, a target systolic blood pressure of less than 120 mm Hg) or standard therapy (ie, a target systolic blood pressure of less than 140 mm Hg). Although intensive therapy did not reduce the composite risk of fatal and nonfatal major cardiovascular events, compared with standard therapy, it did reduce the risk of stroke.
—Erik Greb
Suggested Reading
ACCORD Study Group, Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585.
de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: A position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273-1284.
Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754-3832.
SPS3 Study Group, Benavente OR, Coffey CS, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507-515.
Whelton PK, Carey RM. The 2017 American College of Cardiology/American Heart Association clinical practice guideline for high blood pressure in adults. JAMA Cardiol. 2018 Feb 21 [Epub ahead of print].
Medical associations fight American College of Physicians HBA1c recommendations
in a statement released Friday, March 9.
The new guideline, which was published in the Annals of Internal Medicine on March 5 “could prevent many patients from receiving the full benefits of long-term glucose control,” according to a joint statement from the Endocrine Society, American Diabetes Association, American Association of Diabetes Educators, and American Association of Clinical Endocrinologists.
“The main concern here is this statement is too large to apply to most people with type 2 diabetes,” Grazia Aleppo, MD, chair of the Endocrine Society’s Clinical Affairs Core Committee, said in an interview. “We are all in agreement among the societies to include the ACP’s recommendation on individualizing treatment, but there is a big difference in a 1% HbA1c increase.
“A 7% HbA1c reflects the average of about 154 mg/dL on average, but 8% reflects the average of about 183 mg/dL.”
With patients without diabetes averaging between 70 and 99 mg/dL, a jump to 183 is concerning for Dr. Aleppo and her colleagues.
The ACP’s recommendations were determined from analysis of four drug therapy trials, which found aiming HbA1c levels at below 7% put patients at risk for hypoglycemia and cardiovascular events.
While the trials themselves were approved by the ADA, AADE, and AACE, associations expressed concern with the exclusion of important data, including the addition of newly diagnosed patients in the test population and more modern medications that reduce the adverse effects mentioned in the ACP’s reasoning for raising the target levels.
The patients participating in the trials were older and could have already had underlying cardiovascular disease, according to Dr. Aleppo. These factors plus a rapid change to their medication to get them below 6.5% could have instigated a cardiovascular response, which would not be as likely for patients in their 40s who are just being diagnosed.
“A great majority of patients who are seen in the primary care setting usually don’t have advanced diabetes and, in that case, if someone does not have an increased risk for hypoglycemia, which is the concern that the ACP has, they should be kept at the tightest possible control so that when they are much older they have a legacy effect of good control of their disease,” said Dr. Aleppo.** “Also, if you place someone on modern, lower-risk medications that are so much safer today than before, these actually have been shown not only to improve glucose level in high-risk patients, they can actually cause a very big improvement in cardiovascular disease outcomes.”
Sodium-glucose cotransporter 2 inhibitors and glucagonlike peptide-1 receptor agonists are both newer medications with signs of high success, according to Dr. Aleppo.*
At press time the American College of Physicians was unable to provide a statement.
Corrections, 3/12/18: *An earlier version of this article misstated one of the drug classes mentioned.
**An earlier version of this article misstated a reference to the patient group specified.
in a statement released Friday, March 9.
The new guideline, which was published in the Annals of Internal Medicine on March 5 “could prevent many patients from receiving the full benefits of long-term glucose control,” according to a joint statement from the Endocrine Society, American Diabetes Association, American Association of Diabetes Educators, and American Association of Clinical Endocrinologists.
“The main concern here is this statement is too large to apply to most people with type 2 diabetes,” Grazia Aleppo, MD, chair of the Endocrine Society’s Clinical Affairs Core Committee, said in an interview. “We are all in agreement among the societies to include the ACP’s recommendation on individualizing treatment, but there is a big difference in a 1% HbA1c increase.
“A 7% HbA1c reflects the average of about 154 mg/dL on average, but 8% reflects the average of about 183 mg/dL.”
With patients without diabetes averaging between 70 and 99 mg/dL, a jump to 183 is concerning for Dr. Aleppo and her colleagues.
The ACP’s recommendations were determined from analysis of four drug therapy trials, which found aiming HbA1c levels at below 7% put patients at risk for hypoglycemia and cardiovascular events.
While the trials themselves were approved by the ADA, AADE, and AACE, associations expressed concern with the exclusion of important data, including the addition of newly diagnosed patients in the test population and more modern medications that reduce the adverse effects mentioned in the ACP’s reasoning for raising the target levels.
The patients participating in the trials were older and could have already had underlying cardiovascular disease, according to Dr. Aleppo. These factors plus a rapid change to their medication to get them below 6.5% could have instigated a cardiovascular response, which would not be as likely for patients in their 40s who are just being diagnosed.
“A great majority of patients who are seen in the primary care setting usually don’t have advanced diabetes and, in that case, if someone does not have an increased risk for hypoglycemia, which is the concern that the ACP has, they should be kept at the tightest possible control so that when they are much older they have a legacy effect of good control of their disease,” said Dr. Aleppo.** “Also, if you place someone on modern, lower-risk medications that are so much safer today than before, these actually have been shown not only to improve glucose level in high-risk patients, they can actually cause a very big improvement in cardiovascular disease outcomes.”
Sodium-glucose cotransporter 2 inhibitors and glucagonlike peptide-1 receptor agonists are both newer medications with signs of high success, according to Dr. Aleppo.*
At press time the American College of Physicians was unable to provide a statement.
Corrections, 3/12/18: *An earlier version of this article misstated one of the drug classes mentioned.
**An earlier version of this article misstated a reference to the patient group specified.
in a statement released Friday, March 9.
The new guideline, which was published in the Annals of Internal Medicine on March 5 “could prevent many patients from receiving the full benefits of long-term glucose control,” according to a joint statement from the Endocrine Society, American Diabetes Association, American Association of Diabetes Educators, and American Association of Clinical Endocrinologists.
“The main concern here is this statement is too large to apply to most people with type 2 diabetes,” Grazia Aleppo, MD, chair of the Endocrine Society’s Clinical Affairs Core Committee, said in an interview. “We are all in agreement among the societies to include the ACP’s recommendation on individualizing treatment, but there is a big difference in a 1% HbA1c increase.
“A 7% HbA1c reflects the average of about 154 mg/dL on average, but 8% reflects the average of about 183 mg/dL.”
With patients without diabetes averaging between 70 and 99 mg/dL, a jump to 183 is concerning for Dr. Aleppo and her colleagues.
The ACP’s recommendations were determined from analysis of four drug therapy trials, which found aiming HbA1c levels at below 7% put patients at risk for hypoglycemia and cardiovascular events.
While the trials themselves were approved by the ADA, AADE, and AACE, associations expressed concern with the exclusion of important data, including the addition of newly diagnosed patients in the test population and more modern medications that reduce the adverse effects mentioned in the ACP’s reasoning for raising the target levels.
The patients participating in the trials were older and could have already had underlying cardiovascular disease, according to Dr. Aleppo. These factors plus a rapid change to their medication to get them below 6.5% could have instigated a cardiovascular response, which would not be as likely for patients in their 40s who are just being diagnosed.
“A great majority of patients who are seen in the primary care setting usually don’t have advanced diabetes and, in that case, if someone does not have an increased risk for hypoglycemia, which is the concern that the ACP has, they should be kept at the tightest possible control so that when they are much older they have a legacy effect of good control of their disease,” said Dr. Aleppo.** “Also, if you place someone on modern, lower-risk medications that are so much safer today than before, these actually have been shown not only to improve glucose level in high-risk patients, they can actually cause a very big improvement in cardiovascular disease outcomes.”
Sodium-glucose cotransporter 2 inhibitors and glucagonlike peptide-1 receptor agonists are both newer medications with signs of high success, according to Dr. Aleppo.*
At press time the American College of Physicians was unable to provide a statement.
Corrections, 3/12/18: *An earlier version of this article misstated one of the drug classes mentioned.
**An earlier version of this article misstated a reference to the patient group specified.
ACIP vaccine update
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.
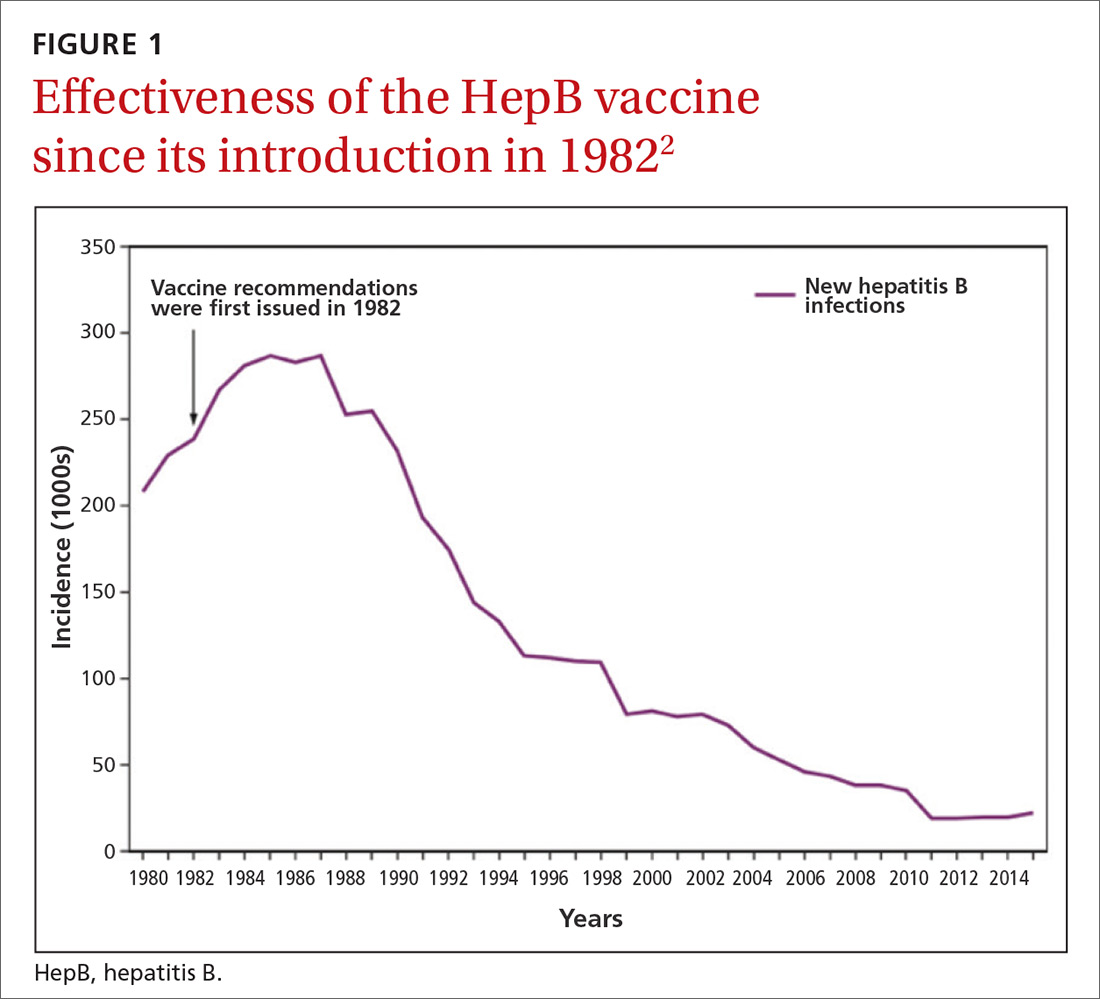
Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).
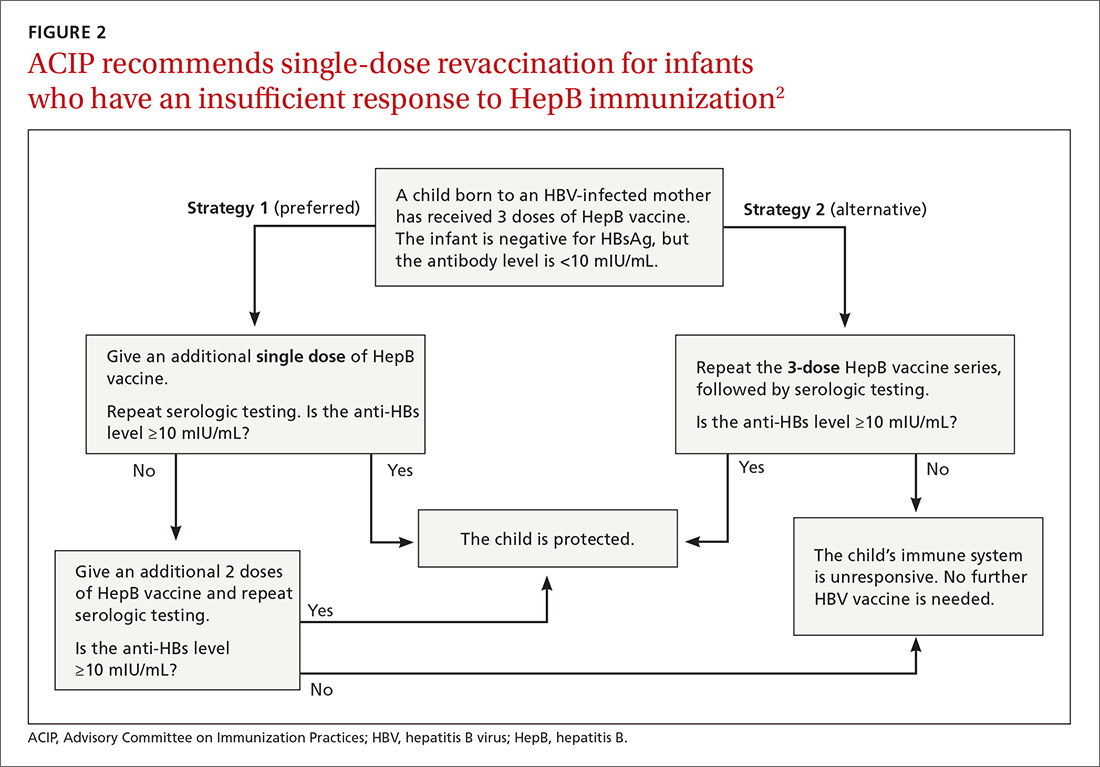
(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.

Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).

(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.

Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).

(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.