User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Should the Body Roundness Index Replace BMI?
In daily practice, physicians need a quick and simple way to assess whether a patient’s weight presents a health risk. For decades, the body mass index (BMI) has been used for this purpose, with calculations based on height and weight. Despite its convenience, BMI has faced increasing criticism.
According to experts, BRI may more accurately identify people with high levels of visceral fat than BMI. It’s well documented that abdominal fat is strongly linked to higher risks for obesity-related diseases.
Studies Support BRI
Several studies have suggested that BRI could be a valuable tool for assessing health risks. In June of this year, researchers from China reported a significant U-shaped association between BRI and overall mortality in a paper published in JAMA Network Open. People with very low or very high BRI had an increased risk for death, noted Xiaoqian Zhang, MD, from Beijing University of Chinese Medicine, Beijing, China, and his colleagues.
A study published in September in the Journal of the American Heart Association showed that elevated BRI over several years was associated with an increased risk for cardiovascular diseases. “The BRI can be included as a predictive factor for cardiovascular disease incidence,” stated the authors, led by Man Yang, MD, from Nanjing Medical University in Nanjing, China.
Why Replace BMI?
Why is a replacement for BMI necessary? When asked by this news organization, Manfred Müller, MD, senior professor at the Institute of Human Nutrition and Food Science at the University of Kiel, in Germany, explained: “BMI was designed to provide a simple value that was as independent of body size as possible, that could detect obesity and estimate related disease risks. But scientifically, BMI has always been a very crude measure to characterize disease risks.”
Müller was part of a research group led by US mathematician Diana Thomas, PhD, who, at the time, worked at Montclair State University, Montclair, New Jersey, and now holds a position at the US Military Academy at West Point, in New York. The group developed and published the BRI in 2013.
BMI Classifies Bodybuilders as Obese
The researchers justified their search for a “better” anthropometric measure with two aspects of BMI that still constitute the main points of criticism of the widely used index today:
BMI incorrectly classifies individuals with significant muscle mass, like bodybuilders, as obese, as it doesn’t distinguish between fat and muscle mass.
BMI provides no information about fat distribution in the body — whether it’s concentrated in the hips or the abdomen, for example.
In practice, this means that a person with a normal BMI could already have prediabetes, high blood pressure, and high cholesterol, which might go undetected if no further investigations are conducted based solely on their BMI.
The BRI aims to solve this problem. As the name suggests, this index seeks to capture a person’s “roundness.” The formula for calculating BRI includes waist circumference and height but excludes body weight:
BRI = 364.2 − 365.5 × √(1 − [Waist circumference in cm/2π]²/[0.5 × Height in cm]²)
In their 2013 article, Thomas, Müller, and colleagues wrote that it still needed to be proven whether their newly developed index correlated with mortality and the risk for cardiovascular and metabolic diseases — and whether it was sufficiently better than BMI to justify the more complex calculation.
Could BRI Replace BMI?
Opinions differ on whether the BRI should replace the BMI. Zhang’s team concluded that the BRI needs to be validated in additional independent cohorts. If it does, it could become a practical screening tool in patient care.
Yang’s research group is optimistic about the BRI’s future: “The longitudinal trajectory of the BRI could be used as a novel indicator of cardiovascular disease risk, which provides a new possibility for cardiovascular disease prevention,” they wrote.
However, even BRI Co-creator Thomas has concerns. “Our entire medical system has been built around the BMI,” she told JAMA, referring to factors such as children’s growth charts and dosage recommendations for medications. That cannot be changed overnight.
Any anthropometric measure intended to replace BMI would need to be rigorously validated across all age groups, genders, and ethnicities. The impact of interventions such as bariatric surgery, diet, and exercise on the new measure would also need to be demonstrated.
Anthropometric Measures Only for Clinical Use
Even if BRI proves to be a “better” metric than BMI for patient care, Müller believes it would be no more suitable for research than BMI. “Regardless of the anthropometric measure, these are practical tools for everyday use,” he stressed.
“A high BRI, like a high BMI, is a risk factor — similar to high blood pressure, high cholesterol levels, or smoking — but it is not a disease,” he added. “In practice, as a physician, I know that a patient with a high BMI or BRI has an increased risk. I need to pay attention to that patient.”
Problems arise when indices like BMI or BRI are used in research. “These ‘invented’ anthropometric measures have no biological basis, which can harm obesity research,” Müller emphasized.
He cited the example of genetic research into obesity, which seeks to identify associations between specific genetic patterns and BMI values. “Why should weight in kilograms divided by height in meters squared be genetically determined?” he asked. “These measures are human-made constructs that have nothing to do with biology.”
Müller believes that the use of BMI has created a “gray area in obesity research” that may account for many of the “unexplained” phenomena in this field.
The BMI Might Be Responsible for the ‘Healthy Obese’
One such phenomenon is the much-discussed “healthy obese,” referring to individuals with a BMI over 30 who do not have high blood sugar, high blood pressure, metabolic disorders, or elevated uric acid levels. “It’s speculated that it must be due to genetic factors, but in reality, the classification is simply wrong,” Müller said.
According to Müller, research should rely on other methods to determine obesity or relevant fat. For example, to assess diabetes risk, liver fat needs to be measured through enzyme tests, ultrasonography, CT, or MRI.
Visceral fat is also important in assessing cardiometabolic risk. “In the doctor’s office, it’s acceptable to estimate this by looking at waist circumference or even BRI. But for research, that’s inadequate,” noted Müller. Direct measurement of trunk fat with dual-energy x-ray absorptiometry or visceral fat with CT or MRI is needed.
“You always have to distinguish between research and patient care. In daily practice, measures like BRI or BMI are sufficient for assessing cardiometabolic risk. But in research, they are not,” Müller explained. To accurately study the disease risks associated with obesity, one must be aware that “with BMI, you cannot create scientifically valid patient or population groups because this value is far too imprecise.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In daily practice, physicians need a quick and simple way to assess whether a patient’s weight presents a health risk. For decades, the body mass index (BMI) has been used for this purpose, with calculations based on height and weight. Despite its convenience, BMI has faced increasing criticism.
According to experts, BRI may more accurately identify people with high levels of visceral fat than BMI. It’s well documented that abdominal fat is strongly linked to higher risks for obesity-related diseases.
Studies Support BRI
Several studies have suggested that BRI could be a valuable tool for assessing health risks. In June of this year, researchers from China reported a significant U-shaped association between BRI and overall mortality in a paper published in JAMA Network Open. People with very low or very high BRI had an increased risk for death, noted Xiaoqian Zhang, MD, from Beijing University of Chinese Medicine, Beijing, China, and his colleagues.
A study published in September in the Journal of the American Heart Association showed that elevated BRI over several years was associated with an increased risk for cardiovascular diseases. “The BRI can be included as a predictive factor for cardiovascular disease incidence,” stated the authors, led by Man Yang, MD, from Nanjing Medical University in Nanjing, China.
Why Replace BMI?
Why is a replacement for BMI necessary? When asked by this news organization, Manfred Müller, MD, senior professor at the Institute of Human Nutrition and Food Science at the University of Kiel, in Germany, explained: “BMI was designed to provide a simple value that was as independent of body size as possible, that could detect obesity and estimate related disease risks. But scientifically, BMI has always been a very crude measure to characterize disease risks.”
Müller was part of a research group led by US mathematician Diana Thomas, PhD, who, at the time, worked at Montclair State University, Montclair, New Jersey, and now holds a position at the US Military Academy at West Point, in New York. The group developed and published the BRI in 2013.
BMI Classifies Bodybuilders as Obese
The researchers justified their search for a “better” anthropometric measure with two aspects of BMI that still constitute the main points of criticism of the widely used index today:
BMI incorrectly classifies individuals with significant muscle mass, like bodybuilders, as obese, as it doesn’t distinguish between fat and muscle mass.
BMI provides no information about fat distribution in the body — whether it’s concentrated in the hips or the abdomen, for example.
In practice, this means that a person with a normal BMI could already have prediabetes, high blood pressure, and high cholesterol, which might go undetected if no further investigations are conducted based solely on their BMI.
The BRI aims to solve this problem. As the name suggests, this index seeks to capture a person’s “roundness.” The formula for calculating BRI includes waist circumference and height but excludes body weight:
BRI = 364.2 − 365.5 × √(1 − [Waist circumference in cm/2π]²/[0.5 × Height in cm]²)
In their 2013 article, Thomas, Müller, and colleagues wrote that it still needed to be proven whether their newly developed index correlated with mortality and the risk for cardiovascular and metabolic diseases — and whether it was sufficiently better than BMI to justify the more complex calculation.
Could BRI Replace BMI?
Opinions differ on whether the BRI should replace the BMI. Zhang’s team concluded that the BRI needs to be validated in additional independent cohorts. If it does, it could become a practical screening tool in patient care.
Yang’s research group is optimistic about the BRI’s future: “The longitudinal trajectory of the BRI could be used as a novel indicator of cardiovascular disease risk, which provides a new possibility for cardiovascular disease prevention,” they wrote.
However, even BRI Co-creator Thomas has concerns. “Our entire medical system has been built around the BMI,” she told JAMA, referring to factors such as children’s growth charts and dosage recommendations for medications. That cannot be changed overnight.
Any anthropometric measure intended to replace BMI would need to be rigorously validated across all age groups, genders, and ethnicities. The impact of interventions such as bariatric surgery, diet, and exercise on the new measure would also need to be demonstrated.
Anthropometric Measures Only for Clinical Use
Even if BRI proves to be a “better” metric than BMI for patient care, Müller believes it would be no more suitable for research than BMI. “Regardless of the anthropometric measure, these are practical tools for everyday use,” he stressed.
“A high BRI, like a high BMI, is a risk factor — similar to high blood pressure, high cholesterol levels, or smoking — but it is not a disease,” he added. “In practice, as a physician, I know that a patient with a high BMI or BRI has an increased risk. I need to pay attention to that patient.”
Problems arise when indices like BMI or BRI are used in research. “These ‘invented’ anthropometric measures have no biological basis, which can harm obesity research,” Müller emphasized.
He cited the example of genetic research into obesity, which seeks to identify associations between specific genetic patterns and BMI values. “Why should weight in kilograms divided by height in meters squared be genetically determined?” he asked. “These measures are human-made constructs that have nothing to do with biology.”
Müller believes that the use of BMI has created a “gray area in obesity research” that may account for many of the “unexplained” phenomena in this field.
The BMI Might Be Responsible for the ‘Healthy Obese’
One such phenomenon is the much-discussed “healthy obese,” referring to individuals with a BMI over 30 who do not have high blood sugar, high blood pressure, metabolic disorders, or elevated uric acid levels. “It’s speculated that it must be due to genetic factors, but in reality, the classification is simply wrong,” Müller said.
According to Müller, research should rely on other methods to determine obesity or relevant fat. For example, to assess diabetes risk, liver fat needs to be measured through enzyme tests, ultrasonography, CT, or MRI.
Visceral fat is also important in assessing cardiometabolic risk. “In the doctor’s office, it’s acceptable to estimate this by looking at waist circumference or even BRI. But for research, that’s inadequate,” noted Müller. Direct measurement of trunk fat with dual-energy x-ray absorptiometry or visceral fat with CT or MRI is needed.
“You always have to distinguish between research and patient care. In daily practice, measures like BRI or BMI are sufficient for assessing cardiometabolic risk. But in research, they are not,” Müller explained. To accurately study the disease risks associated with obesity, one must be aware that “with BMI, you cannot create scientifically valid patient or population groups because this value is far too imprecise.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In daily practice, physicians need a quick and simple way to assess whether a patient’s weight presents a health risk. For decades, the body mass index (BMI) has been used for this purpose, with calculations based on height and weight. Despite its convenience, BMI has faced increasing criticism.
According to experts, BRI may more accurately identify people with high levels of visceral fat than BMI. It’s well documented that abdominal fat is strongly linked to higher risks for obesity-related diseases.
Studies Support BRI
Several studies have suggested that BRI could be a valuable tool for assessing health risks. In June of this year, researchers from China reported a significant U-shaped association between BRI and overall mortality in a paper published in JAMA Network Open. People with very low or very high BRI had an increased risk for death, noted Xiaoqian Zhang, MD, from Beijing University of Chinese Medicine, Beijing, China, and his colleagues.
A study published in September in the Journal of the American Heart Association showed that elevated BRI over several years was associated with an increased risk for cardiovascular diseases. “The BRI can be included as a predictive factor for cardiovascular disease incidence,” stated the authors, led by Man Yang, MD, from Nanjing Medical University in Nanjing, China.
Why Replace BMI?
Why is a replacement for BMI necessary? When asked by this news organization, Manfred Müller, MD, senior professor at the Institute of Human Nutrition and Food Science at the University of Kiel, in Germany, explained: “BMI was designed to provide a simple value that was as independent of body size as possible, that could detect obesity and estimate related disease risks. But scientifically, BMI has always been a very crude measure to characterize disease risks.”
Müller was part of a research group led by US mathematician Diana Thomas, PhD, who, at the time, worked at Montclair State University, Montclair, New Jersey, and now holds a position at the US Military Academy at West Point, in New York. The group developed and published the BRI in 2013.
BMI Classifies Bodybuilders as Obese
The researchers justified their search for a “better” anthropometric measure with two aspects of BMI that still constitute the main points of criticism of the widely used index today:
BMI incorrectly classifies individuals with significant muscle mass, like bodybuilders, as obese, as it doesn’t distinguish between fat and muscle mass.
BMI provides no information about fat distribution in the body — whether it’s concentrated in the hips or the abdomen, for example.
In practice, this means that a person with a normal BMI could already have prediabetes, high blood pressure, and high cholesterol, which might go undetected if no further investigations are conducted based solely on their BMI.
The BRI aims to solve this problem. As the name suggests, this index seeks to capture a person’s “roundness.” The formula for calculating BRI includes waist circumference and height but excludes body weight:
BRI = 364.2 − 365.5 × √(1 − [Waist circumference in cm/2π]²/[0.5 × Height in cm]²)
In their 2013 article, Thomas, Müller, and colleagues wrote that it still needed to be proven whether their newly developed index correlated with mortality and the risk for cardiovascular and metabolic diseases — and whether it was sufficiently better than BMI to justify the more complex calculation.
Could BRI Replace BMI?
Opinions differ on whether the BRI should replace the BMI. Zhang’s team concluded that the BRI needs to be validated in additional independent cohorts. If it does, it could become a practical screening tool in patient care.
Yang’s research group is optimistic about the BRI’s future: “The longitudinal trajectory of the BRI could be used as a novel indicator of cardiovascular disease risk, which provides a new possibility for cardiovascular disease prevention,” they wrote.
However, even BRI Co-creator Thomas has concerns. “Our entire medical system has been built around the BMI,” she told JAMA, referring to factors such as children’s growth charts and dosage recommendations for medications. That cannot be changed overnight.
Any anthropometric measure intended to replace BMI would need to be rigorously validated across all age groups, genders, and ethnicities. The impact of interventions such as bariatric surgery, diet, and exercise on the new measure would also need to be demonstrated.
Anthropometric Measures Only for Clinical Use
Even if BRI proves to be a “better” metric than BMI for patient care, Müller believes it would be no more suitable for research than BMI. “Regardless of the anthropometric measure, these are practical tools for everyday use,” he stressed.
“A high BRI, like a high BMI, is a risk factor — similar to high blood pressure, high cholesterol levels, or smoking — but it is not a disease,” he added. “In practice, as a physician, I know that a patient with a high BMI or BRI has an increased risk. I need to pay attention to that patient.”
Problems arise when indices like BMI or BRI are used in research. “These ‘invented’ anthropometric measures have no biological basis, which can harm obesity research,” Müller emphasized.
He cited the example of genetic research into obesity, which seeks to identify associations between specific genetic patterns and BMI values. “Why should weight in kilograms divided by height in meters squared be genetically determined?” he asked. “These measures are human-made constructs that have nothing to do with biology.”
Müller believes that the use of BMI has created a “gray area in obesity research” that may account for many of the “unexplained” phenomena in this field.
The BMI Might Be Responsible for the ‘Healthy Obese’
One such phenomenon is the much-discussed “healthy obese,” referring to individuals with a BMI over 30 who do not have high blood sugar, high blood pressure, metabolic disorders, or elevated uric acid levels. “It’s speculated that it must be due to genetic factors, but in reality, the classification is simply wrong,” Müller said.
According to Müller, research should rely on other methods to determine obesity or relevant fat. For example, to assess diabetes risk, liver fat needs to be measured through enzyme tests, ultrasonography, CT, or MRI.
Visceral fat is also important in assessing cardiometabolic risk. “In the doctor’s office, it’s acceptable to estimate this by looking at waist circumference or even BRI. But for research, that’s inadequate,” noted Müller. Direct measurement of trunk fat with dual-energy x-ray absorptiometry or visceral fat with CT or MRI is needed.
“You always have to distinguish between research and patient care. In daily practice, measures like BRI or BMI are sufficient for assessing cardiometabolic risk. But in research, they are not,” Müller explained. To accurately study the disease risks associated with obesity, one must be aware that “with BMI, you cannot create scientifically valid patient or population groups because this value is far too imprecise.”
This story was translated from Medscape’s German edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Is Being ‘Manly’ a Threat to a Man’s Health?
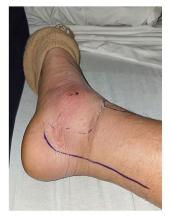
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here. 
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?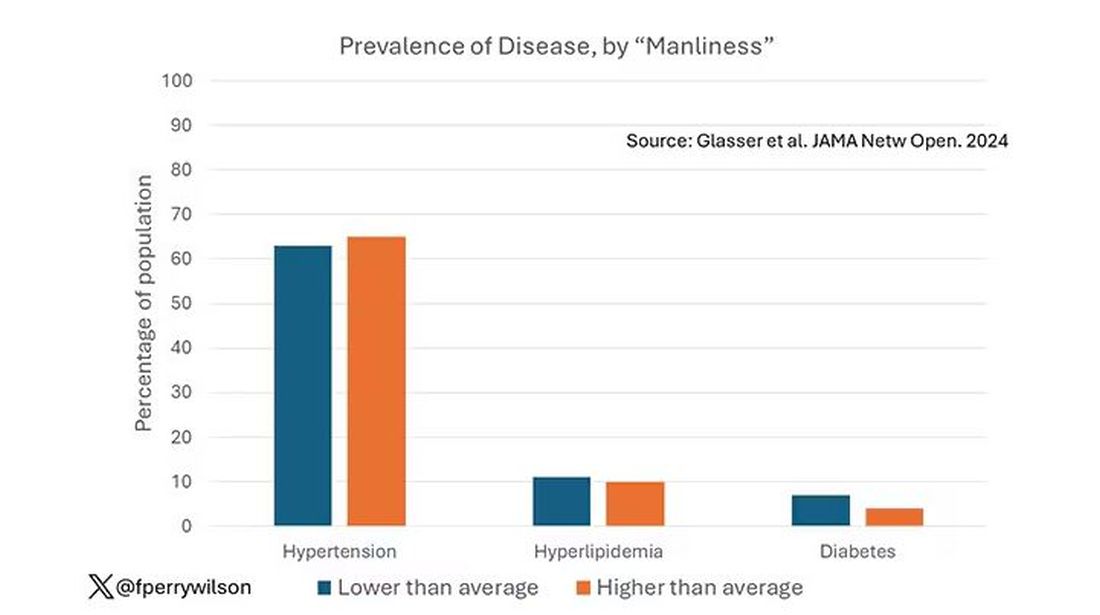
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.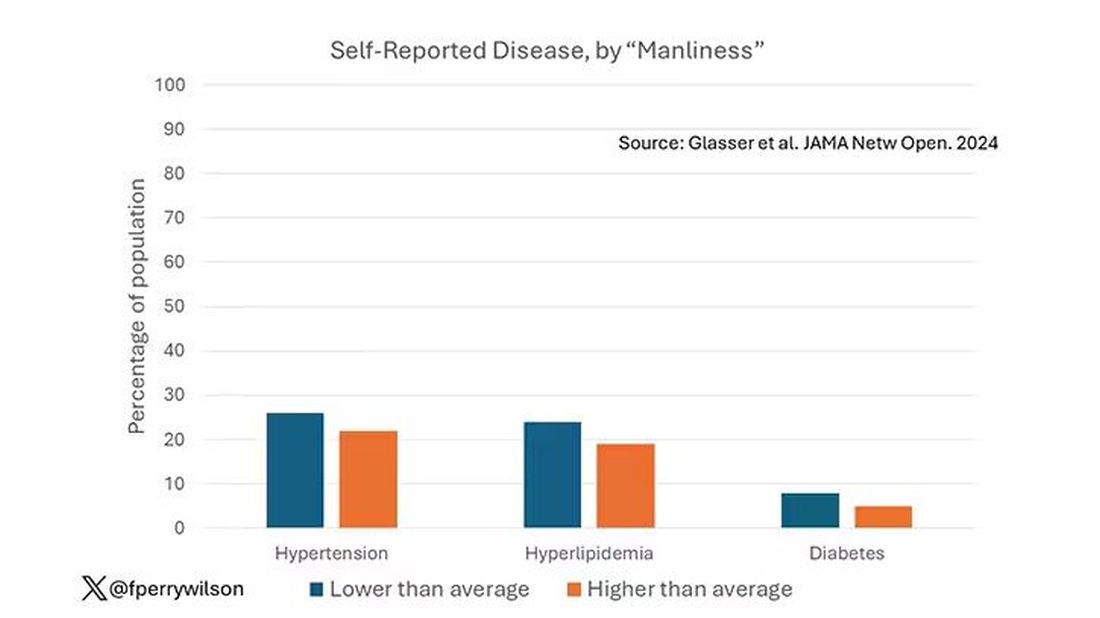
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.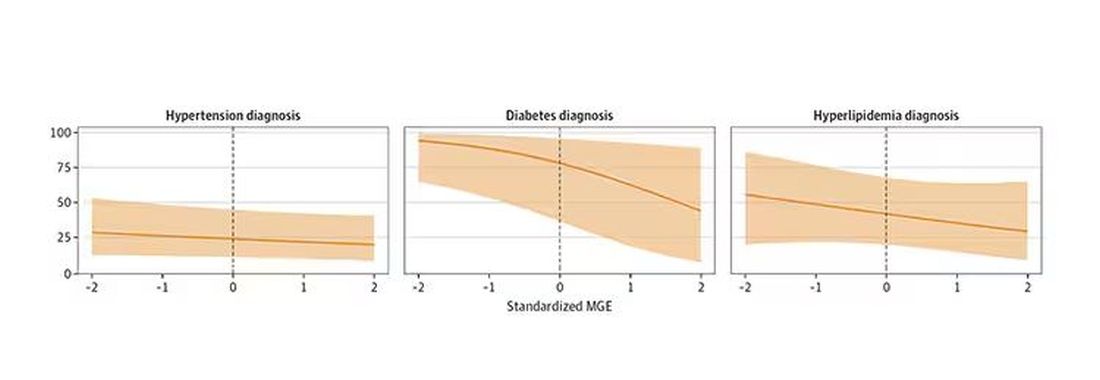
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here. 
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
When my normally adorable cat Biscuit bit my ankle in a playful stalking exercise gone wrong, I washed it with soap and some rubbing alcohol, slapped on a Band-Aid, and went about my day.
The next morning, when it was swollen, I told myself it was probably just a hematoma and went about my day.
The next day, when the swelling had increased and red lines started creeping up my leg, I called my doctor. Long story short, I ended up hospitalized for intravenous antibiotics.
This is all to say that, yes, I’m sort of an idiot, but also to introduce the idea that maybe I minimized my very obvious lymphangitis because I am a man.
This week, we have empirical evidence that men downplay their medical symptoms — and that manlier men downplay them even more.
I’m going to talk about a study that links manliness (or, scientifically speaking, “male gender expressivity”) to medical diagnoses that are based on hard evidence and medical diagnoses that are based on self-report. You see where this is going but I want to walk you through the methods here because they are fairly interesting.
This study used data from the US National Longitudinal Study of Adolescent to Adult Health. This study enrolled 20,000 adolescents who were in grades 7-12 in the 1994-1995 school year and has been following them ever since — about 30 years so far.
The authors wanted to link early gender roles to long-term outcomes, so they cut that 20,000 number down to the 4230 males in the group who had complete follow-up.
Now comes the first interesting question. How do you quantify the “male gender expressivity” of boys in 7th-12th grade? There was no survey item that asked them how masculine or manly they felt. What the authors did was look at the surveys that were administered and identify the questions on those surveys where boys and girls gave the most disparate answers. I have some examples here. 
Some of these questions make sense when it comes to gender expressivity: “How often do you cry?” for example, has a lot of validity for the social construct that is gender. But some questions where boys and girls gave very different answers — like “How often do you exercise?” — don’t quite fit that mold. Regardless, this structure allowed the researchers to take individual kids’ responses to these questions and combine them into what amounts to a manliness score — how much their answers aligned with the typical male answer.
The score was established in adolescence — which is interesting because I’m sure some of this stuff may change over time — but notable because adolescence is where many gender roles develop.
Now we can fast-forward 30 years and see how these manliness scores link to various outcomes. The authors were interested in fairly common diseases: diabetes, hypertension, and hyperlipidemia.
Let’s start simply. Are males with higher gender expressivity in adolescence more or less likely to have these diseases in the future?
Not really. Those above the average in male gender expressivity had similar rates of hypertension and hyperlipidemia as those below the median. They were actually a bit less likely to have diabetes.
But that’s not what’s really interesting here.
I told you that there was no difference in the rate of hypertension among those with high vs low male gender expressivity. But there was a significant difference in their answer to the question “Do you have hypertension?” The same was seen for hyperlipidemia. In other words, those with higher manliness scores are less likely to admit (or perhaps know) that they have a particular disease.
You can see the relationship across the manliness spectrum here in a series of adjusted models. The x-axis is the male gender expressivity score, and the y-axis is the percentage of people who report having the disease that we know they have based on the actual laboratory tests or vital sign measurements. As manliness increases, the self-report of a given disease decreases.
There are some important consequences of this systematic denial. Specifically, men with the diseases of interest who have higher male gender expressivity are less likely to get treatment. And, as we all know, the lack of treatment of something like hypertension puts people at risk for bad downstream outcomes.
Putting this all together, I’m not that surprised. Society trains boys from a young age to behave in certain ways: to hide emotions, to eschew vulnerability, to not complain when we are hurt. And those lessons can persist into later life. Whether the disease that strikes is hypertension or Pasteurella multocida from a slightly psychotic house cat, men are more likely to ignore it, to their detriment.
So, gents, be brave. Get your blood tests and check your blood pressure. If there’s something wrong, admit it, and fix it. After all, fixing problems — that’s a manly thing, right?
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Thyroid Cancer Overdiagnosis Continues Despite Cautions
according to a recently published global study.
The proportion of thyroid cancer cases attributable to overdiagnosis globally was higher in women (78%) than in men (68%), with this rate varying substantially across countries, wrote Mengmeng Li, PhD, of the Sun Yat-sen University Cancer Center, Guangzhou, China, and coauthors in an October paper in The Lancet Diabetes & Endocrinology.
Overdiagnosis refers to the diagnosis of lesions that would not cause symptoms and that would not progress, if left alone.
Increased testing for thyroid cancer, fueled in large part by the expansion of imaging technologies and progressively more intense and disorganized scrutiny of the thyroid, led many people to be treated for often indolent lesions, exposing them to potential side effects as well as financial and emotional distress.
Li and coauthors estimate that more than 1.7 million people might have been overdiagnosed between 2013 and 2017 in 63 countries.
“Overdiagnosis clearly emerged in some high-resource countries with private-based health systems in which access to healthcare overrules regulatory controls (eg, in the USA) and in some high-quality public health systems with easy and broad access to thyroid gland diagnostic examinations (eg, in Canada),” Li and coauthors wrote. “Conversely, thyroid cancer is less commonly diagnosed in those countries in which access to diagnosis is guided by strong regulatory rules (eg, in Nordic countries).”
Their study drew from almost 40 years of research, including the latest available data from the World Health Organization’s International Agency for Research on Cancer’s (IARC’s) Global Cancer Observatory. Li and coauthors examined patterns in the time trends of thyroid cancer, mortality data, and trends in diagnosis of thyroid cancer before testing became common in many nations.
This approach is needed in estimating overdiagnosis, where it’s not possible to see what’s happening on a case-by-case level, Salvatore Vaccarella, PhD, a scientist at IARC’s Cancer Surveillance Branch, said in an interview.
Researchers can’t tell whether an individual’s detected early-stage cancers would have remained indolent for years or eventually would have put their life at risk, he said. Instead, the patterns emerge through larger studies of the reported cases of cancer like thyroid tumors and then looking at separate datasets on mortality.
“We can only see that as a big phenomenon when we look at population-based data,” Vaccarella said.
Persisting Problem
Recognition of the harms of overdiagnosis has resulted in some reduction of the incidence of thyroid cancer in the United States, Li and coauthors wrote. After adjusting for age, incidence has fallen from 19 per 100,000 women in 2013 to 16 per 100,000 women in 2017. The proportion of thyroid cancer attributed to overdiagnosis has dropped from 76% to 68% in the country.
The paper adds to the evidence suggesting that the rise in screening has not changed mortality rates for thyroid cancer. For example, Li and coauthors reported seeing “a small decrease in thyroid cancer mortality rates over time in some European countries, but this decline (less than 1 per 100,000 women) is marginal compared with the increases in incidence (reaching around 100 per 100,000 women).”
“Moreover, previous data show that the downward mortality trends had begun before the wide use of ultrasonography for early detection and that period and birth cohort effects have been declining, probably due to treatment advances and reduced prevalence of risk factors, such as the reduction in iodine deficiency,” they wrote.
In an interview, Amanda Davis, MD, of AnMed, a nonprofit health system based in Anderson, South Carolina, said the new paper from Li and Vaccarella provides further evidence for a cautious approach to thyroid nodules given concerns about overdiagnosis.
If early detection of cancer via discovery of thyroid nodules actually helped patients, mortality rates would have dropped with expansion of screening and the resulting diagnoses, said Davis, who is an associate program director at AnMed’s family medicine residency program and affiliate professor at the Medical University of South Carolina, Charleston.
In many cases, people learn they have thyroid lesions after being tested for other conditions such as ultrasound done on carotid arteries to check for stroke risk. The most common form of thyroid cancer is the papillary form. Papillary thyroid cancer tends to be slow growing, carries a low risk for distant metastasis, and in many cases poses little risk. Some small (< 1 cm) papillary thyroid cancers can be monitored with active surveillance as opposed to thyroid lobectomy.
“So just finding more nodules incidentally or through screening ultrasound and even finding more papillary cancers via these methods does not make people healthier or decrease mortality,” Davis said.
“So just finding more things and even finding more papillary cancers does not increase our ability to treat people and keep them alive longer,” Davis said.
The 5-year survival rate for thyroid cancer overall is 98.1% and varies from 99.9% for localized disease to 55.3% for distant disease, the US Preventive Services Task Force (USPSTF) said in a 2017 publication in JAMA. The task force that year gave a “D” rating on screening of asymptomatic people for thyroid cancer. That means there’s moderate certainty that screening for thyroid cancer in asymptomatic persons results in harms that outweigh the benefits. The decision to give this “D” rating meant this screening is not recommended. That’s still the panel’s view.
“You can think of it as a “D” for ‘don’t screen for thyroid cancer,’ ” in people who present no symptoms of this illness, John Wong, MD, the vice chair of the USPSTF, said in an interview.
In primary care, the challenge is assessing thyroid nodules detected when people undergo testing for another reason, such as an ultrasound of the carotid artery to check for stroke risk.
Thyroid nodules can be detected by ultrasonography in up to 68% of the general population, reported a study in American Family Physician. Nodules with suspicious features or ≥ 1 cm require fine needle aspiration. The Bethesda System for Reporting Thyroid Cytopathology can be used to classify samples, with molecular testing applied to guide treatment when fine needle aspiration yields an indeterminate result.
New Thinking on Thyroid Cancer
There’s been a shift in recent years in the approach to how physicians should proceed if certain kinds of thyroid cancer are detected, Cari M. Kitahara, PhD, of the National Cancer Institute noted in a comment accompanying the Li paper.
“Clinicians need to be judicious in the use of thyroid ultrasonography, the diagnostic follow-up of incidentally detected thyroid nodules, and determining the optimal course of treatment,” Kitahara wrote. “For low-risk and incidentally detected tumors, strong consideration should be given to less intensive treatment options (eg, lobectomy, delayed treatment, and active surveillance).”
The American Thyroid Association guidelines encourage de-escalation of treatment for low-risk papillary thyroid carcinoma up to 4 cm.
Physicians often need to make clear to patients how a diagnosis of low-risk papillary thyroid cancer differs from other oncology diagnoses, R. Michael Tuttle, MD, of Memorial Sloan Kettering Cancer Center, New York City, said in an interview.
“I’ll frequently say that everything you’ve ever learned about cancer, you need to forget,” Tuttle said.
Some patients will mistakenly think any cancer diagnosis is a likely death sentence, meaning they should rush to get aggressive treatment. Tuttle has been a leader for many years in efforts in advancing active surveillance as an option for certain people with low-risk thyroid cancer.
“I often start my consultation by saying: ‘We’re going to choose between two right answers here. One right answer is watching right. One right answer is going to surgery,’ ” Tuttle said.
Patients with low-risk thyroid cancer tend to fall into two camps, with maximalists likely to seek quick treatment and minimalists more inclined for surveillance if that’s an option for them, Tuttle said. As opinions have shifted within the medical community about approaches to low-risk thyroid cancer, there’s also been some growing awareness among the public about thyroid overdiagnosis.
“Ten or 15 years ago, people thought we were crazy” to consider active surveillance as an option for low-risk thyroid cancers,” Tuttle said. “Now we have swung, at least in some of the public opinion, to this recognition that every little speck of cancer doesn’t need to be immediately taken out of your body.”
Some patients express regret about having learned that they have low-risk thyroid cancer, Tuttle said.
“Over the last 5 years, it’s not uncommon for patients to ask me, ‘Is this one of those that needs to be treated now, or is this one of those that we wish we would have never found?’ Or people will say, ‘My doctor talked me into an ultrasound, I didn’t want it’ or ‘I had a car wreck, and I found this nodule and I wished I had never found it.’ ”
This study from Li and coauthors was funded by the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Young Talents Program of Sun Yat-sen University Cancer Center, the Italian Association for Cancer Research, and the Italian Ministry of Health. Davis and Tuttle had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a recently published global study.
The proportion of thyroid cancer cases attributable to overdiagnosis globally was higher in women (78%) than in men (68%), with this rate varying substantially across countries, wrote Mengmeng Li, PhD, of the Sun Yat-sen University Cancer Center, Guangzhou, China, and coauthors in an October paper in The Lancet Diabetes & Endocrinology.
Overdiagnosis refers to the diagnosis of lesions that would not cause symptoms and that would not progress, if left alone.
Increased testing for thyroid cancer, fueled in large part by the expansion of imaging technologies and progressively more intense and disorganized scrutiny of the thyroid, led many people to be treated for often indolent lesions, exposing them to potential side effects as well as financial and emotional distress.
Li and coauthors estimate that more than 1.7 million people might have been overdiagnosed between 2013 and 2017 in 63 countries.
“Overdiagnosis clearly emerged in some high-resource countries with private-based health systems in which access to healthcare overrules regulatory controls (eg, in the USA) and in some high-quality public health systems with easy and broad access to thyroid gland diagnostic examinations (eg, in Canada),” Li and coauthors wrote. “Conversely, thyroid cancer is less commonly diagnosed in those countries in which access to diagnosis is guided by strong regulatory rules (eg, in Nordic countries).”
Their study drew from almost 40 years of research, including the latest available data from the World Health Organization’s International Agency for Research on Cancer’s (IARC’s) Global Cancer Observatory. Li and coauthors examined patterns in the time trends of thyroid cancer, mortality data, and trends in diagnosis of thyroid cancer before testing became common in many nations.
This approach is needed in estimating overdiagnosis, where it’s not possible to see what’s happening on a case-by-case level, Salvatore Vaccarella, PhD, a scientist at IARC’s Cancer Surveillance Branch, said in an interview.
Researchers can’t tell whether an individual’s detected early-stage cancers would have remained indolent for years or eventually would have put their life at risk, he said. Instead, the patterns emerge through larger studies of the reported cases of cancer like thyroid tumors and then looking at separate datasets on mortality.
“We can only see that as a big phenomenon when we look at population-based data,” Vaccarella said.
Persisting Problem
Recognition of the harms of overdiagnosis has resulted in some reduction of the incidence of thyroid cancer in the United States, Li and coauthors wrote. After adjusting for age, incidence has fallen from 19 per 100,000 women in 2013 to 16 per 100,000 women in 2017. The proportion of thyroid cancer attributed to overdiagnosis has dropped from 76% to 68% in the country.
The paper adds to the evidence suggesting that the rise in screening has not changed mortality rates for thyroid cancer. For example, Li and coauthors reported seeing “a small decrease in thyroid cancer mortality rates over time in some European countries, but this decline (less than 1 per 100,000 women) is marginal compared with the increases in incidence (reaching around 100 per 100,000 women).”
“Moreover, previous data show that the downward mortality trends had begun before the wide use of ultrasonography for early detection and that period and birth cohort effects have been declining, probably due to treatment advances and reduced prevalence of risk factors, such as the reduction in iodine deficiency,” they wrote.
In an interview, Amanda Davis, MD, of AnMed, a nonprofit health system based in Anderson, South Carolina, said the new paper from Li and Vaccarella provides further evidence for a cautious approach to thyroid nodules given concerns about overdiagnosis.
If early detection of cancer via discovery of thyroid nodules actually helped patients, mortality rates would have dropped with expansion of screening and the resulting diagnoses, said Davis, who is an associate program director at AnMed’s family medicine residency program and affiliate professor at the Medical University of South Carolina, Charleston.
In many cases, people learn they have thyroid lesions after being tested for other conditions such as ultrasound done on carotid arteries to check for stroke risk. The most common form of thyroid cancer is the papillary form. Papillary thyroid cancer tends to be slow growing, carries a low risk for distant metastasis, and in many cases poses little risk. Some small (< 1 cm) papillary thyroid cancers can be monitored with active surveillance as opposed to thyroid lobectomy.
“So just finding more nodules incidentally or through screening ultrasound and even finding more papillary cancers via these methods does not make people healthier or decrease mortality,” Davis said.
“So just finding more things and even finding more papillary cancers does not increase our ability to treat people and keep them alive longer,” Davis said.
The 5-year survival rate for thyroid cancer overall is 98.1% and varies from 99.9% for localized disease to 55.3% for distant disease, the US Preventive Services Task Force (USPSTF) said in a 2017 publication in JAMA. The task force that year gave a “D” rating on screening of asymptomatic people for thyroid cancer. That means there’s moderate certainty that screening for thyroid cancer in asymptomatic persons results in harms that outweigh the benefits. The decision to give this “D” rating meant this screening is not recommended. That’s still the panel’s view.
“You can think of it as a “D” for ‘don’t screen for thyroid cancer,’ ” in people who present no symptoms of this illness, John Wong, MD, the vice chair of the USPSTF, said in an interview.
In primary care, the challenge is assessing thyroid nodules detected when people undergo testing for another reason, such as an ultrasound of the carotid artery to check for stroke risk.
Thyroid nodules can be detected by ultrasonography in up to 68% of the general population, reported a study in American Family Physician. Nodules with suspicious features or ≥ 1 cm require fine needle aspiration. The Bethesda System for Reporting Thyroid Cytopathology can be used to classify samples, with molecular testing applied to guide treatment when fine needle aspiration yields an indeterminate result.
New Thinking on Thyroid Cancer
There’s been a shift in recent years in the approach to how physicians should proceed if certain kinds of thyroid cancer are detected, Cari M. Kitahara, PhD, of the National Cancer Institute noted in a comment accompanying the Li paper.
“Clinicians need to be judicious in the use of thyroid ultrasonography, the diagnostic follow-up of incidentally detected thyroid nodules, and determining the optimal course of treatment,” Kitahara wrote. “For low-risk and incidentally detected tumors, strong consideration should be given to less intensive treatment options (eg, lobectomy, delayed treatment, and active surveillance).”
The American Thyroid Association guidelines encourage de-escalation of treatment for low-risk papillary thyroid carcinoma up to 4 cm.
Physicians often need to make clear to patients how a diagnosis of low-risk papillary thyroid cancer differs from other oncology diagnoses, R. Michael Tuttle, MD, of Memorial Sloan Kettering Cancer Center, New York City, said in an interview.
“I’ll frequently say that everything you’ve ever learned about cancer, you need to forget,” Tuttle said.
Some patients will mistakenly think any cancer diagnosis is a likely death sentence, meaning they should rush to get aggressive treatment. Tuttle has been a leader for many years in efforts in advancing active surveillance as an option for certain people with low-risk thyroid cancer.
“I often start my consultation by saying: ‘We’re going to choose between two right answers here. One right answer is watching right. One right answer is going to surgery,’ ” Tuttle said.
Patients with low-risk thyroid cancer tend to fall into two camps, with maximalists likely to seek quick treatment and minimalists more inclined for surveillance if that’s an option for them, Tuttle said. As opinions have shifted within the medical community about approaches to low-risk thyroid cancer, there’s also been some growing awareness among the public about thyroid overdiagnosis.
“Ten or 15 years ago, people thought we were crazy” to consider active surveillance as an option for low-risk thyroid cancers,” Tuttle said. “Now we have swung, at least in some of the public opinion, to this recognition that every little speck of cancer doesn’t need to be immediately taken out of your body.”
Some patients express regret about having learned that they have low-risk thyroid cancer, Tuttle said.
“Over the last 5 years, it’s not uncommon for patients to ask me, ‘Is this one of those that needs to be treated now, or is this one of those that we wish we would have never found?’ Or people will say, ‘My doctor talked me into an ultrasound, I didn’t want it’ or ‘I had a car wreck, and I found this nodule and I wished I had never found it.’ ”
This study from Li and coauthors was funded by the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Young Talents Program of Sun Yat-sen University Cancer Center, the Italian Association for Cancer Research, and the Italian Ministry of Health. Davis and Tuttle had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
according to a recently published global study.
The proportion of thyroid cancer cases attributable to overdiagnosis globally was higher in women (78%) than in men (68%), with this rate varying substantially across countries, wrote Mengmeng Li, PhD, of the Sun Yat-sen University Cancer Center, Guangzhou, China, and coauthors in an October paper in The Lancet Diabetes & Endocrinology.
Overdiagnosis refers to the diagnosis of lesions that would not cause symptoms and that would not progress, if left alone.
Increased testing for thyroid cancer, fueled in large part by the expansion of imaging technologies and progressively more intense and disorganized scrutiny of the thyroid, led many people to be treated for often indolent lesions, exposing them to potential side effects as well as financial and emotional distress.
Li and coauthors estimate that more than 1.7 million people might have been overdiagnosed between 2013 and 2017 in 63 countries.
“Overdiagnosis clearly emerged in some high-resource countries with private-based health systems in which access to healthcare overrules regulatory controls (eg, in the USA) and in some high-quality public health systems with easy and broad access to thyroid gland diagnostic examinations (eg, in Canada),” Li and coauthors wrote. “Conversely, thyroid cancer is less commonly diagnosed in those countries in which access to diagnosis is guided by strong regulatory rules (eg, in Nordic countries).”
Their study drew from almost 40 years of research, including the latest available data from the World Health Organization’s International Agency for Research on Cancer’s (IARC’s) Global Cancer Observatory. Li and coauthors examined patterns in the time trends of thyroid cancer, mortality data, and trends in diagnosis of thyroid cancer before testing became common in many nations.
This approach is needed in estimating overdiagnosis, where it’s not possible to see what’s happening on a case-by-case level, Salvatore Vaccarella, PhD, a scientist at IARC’s Cancer Surveillance Branch, said in an interview.
Researchers can’t tell whether an individual’s detected early-stage cancers would have remained indolent for years or eventually would have put their life at risk, he said. Instead, the patterns emerge through larger studies of the reported cases of cancer like thyroid tumors and then looking at separate datasets on mortality.
“We can only see that as a big phenomenon when we look at population-based data,” Vaccarella said.
Persisting Problem
Recognition of the harms of overdiagnosis has resulted in some reduction of the incidence of thyroid cancer in the United States, Li and coauthors wrote. After adjusting for age, incidence has fallen from 19 per 100,000 women in 2013 to 16 per 100,000 women in 2017. The proportion of thyroid cancer attributed to overdiagnosis has dropped from 76% to 68% in the country.
The paper adds to the evidence suggesting that the rise in screening has not changed mortality rates for thyroid cancer. For example, Li and coauthors reported seeing “a small decrease in thyroid cancer mortality rates over time in some European countries, but this decline (less than 1 per 100,000 women) is marginal compared with the increases in incidence (reaching around 100 per 100,000 women).”
“Moreover, previous data show that the downward mortality trends had begun before the wide use of ultrasonography for early detection and that period and birth cohort effects have been declining, probably due to treatment advances and reduced prevalence of risk factors, such as the reduction in iodine deficiency,” they wrote.
In an interview, Amanda Davis, MD, of AnMed, a nonprofit health system based in Anderson, South Carolina, said the new paper from Li and Vaccarella provides further evidence for a cautious approach to thyroid nodules given concerns about overdiagnosis.
If early detection of cancer via discovery of thyroid nodules actually helped patients, mortality rates would have dropped with expansion of screening and the resulting diagnoses, said Davis, who is an associate program director at AnMed’s family medicine residency program and affiliate professor at the Medical University of South Carolina, Charleston.
In many cases, people learn they have thyroid lesions after being tested for other conditions such as ultrasound done on carotid arteries to check for stroke risk. The most common form of thyroid cancer is the papillary form. Papillary thyroid cancer tends to be slow growing, carries a low risk for distant metastasis, and in many cases poses little risk. Some small (< 1 cm) papillary thyroid cancers can be monitored with active surveillance as opposed to thyroid lobectomy.
“So just finding more nodules incidentally or through screening ultrasound and even finding more papillary cancers via these methods does not make people healthier or decrease mortality,” Davis said.
“So just finding more things and even finding more papillary cancers does not increase our ability to treat people and keep them alive longer,” Davis said.
The 5-year survival rate for thyroid cancer overall is 98.1% and varies from 99.9% for localized disease to 55.3% for distant disease, the US Preventive Services Task Force (USPSTF) said in a 2017 publication in JAMA. The task force that year gave a “D” rating on screening of asymptomatic people for thyroid cancer. That means there’s moderate certainty that screening for thyroid cancer in asymptomatic persons results in harms that outweigh the benefits. The decision to give this “D” rating meant this screening is not recommended. That’s still the panel’s view.
“You can think of it as a “D” for ‘don’t screen for thyroid cancer,’ ” in people who present no symptoms of this illness, John Wong, MD, the vice chair of the USPSTF, said in an interview.
In primary care, the challenge is assessing thyroid nodules detected when people undergo testing for another reason, such as an ultrasound of the carotid artery to check for stroke risk.
Thyroid nodules can be detected by ultrasonography in up to 68% of the general population, reported a study in American Family Physician. Nodules with suspicious features or ≥ 1 cm require fine needle aspiration. The Bethesda System for Reporting Thyroid Cytopathology can be used to classify samples, with molecular testing applied to guide treatment when fine needle aspiration yields an indeterminate result.
New Thinking on Thyroid Cancer
There’s been a shift in recent years in the approach to how physicians should proceed if certain kinds of thyroid cancer are detected, Cari M. Kitahara, PhD, of the National Cancer Institute noted in a comment accompanying the Li paper.
“Clinicians need to be judicious in the use of thyroid ultrasonography, the diagnostic follow-up of incidentally detected thyroid nodules, and determining the optimal course of treatment,” Kitahara wrote. “For low-risk and incidentally detected tumors, strong consideration should be given to less intensive treatment options (eg, lobectomy, delayed treatment, and active surveillance).”
The American Thyroid Association guidelines encourage de-escalation of treatment for low-risk papillary thyroid carcinoma up to 4 cm.
Physicians often need to make clear to patients how a diagnosis of low-risk papillary thyroid cancer differs from other oncology diagnoses, R. Michael Tuttle, MD, of Memorial Sloan Kettering Cancer Center, New York City, said in an interview.
“I’ll frequently say that everything you’ve ever learned about cancer, you need to forget,” Tuttle said.
Some patients will mistakenly think any cancer diagnosis is a likely death sentence, meaning they should rush to get aggressive treatment. Tuttle has been a leader for many years in efforts in advancing active surveillance as an option for certain people with low-risk thyroid cancer.
“I often start my consultation by saying: ‘We’re going to choose between two right answers here. One right answer is watching right. One right answer is going to surgery,’ ” Tuttle said.
Patients with low-risk thyroid cancer tend to fall into two camps, with maximalists likely to seek quick treatment and minimalists more inclined for surveillance if that’s an option for them, Tuttle said. As opinions have shifted within the medical community about approaches to low-risk thyroid cancer, there’s also been some growing awareness among the public about thyroid overdiagnosis.
“Ten or 15 years ago, people thought we were crazy” to consider active surveillance as an option for low-risk thyroid cancers,” Tuttle said. “Now we have swung, at least in some of the public opinion, to this recognition that every little speck of cancer doesn’t need to be immediately taken out of your body.”
Some patients express regret about having learned that they have low-risk thyroid cancer, Tuttle said.
“Over the last 5 years, it’s not uncommon for patients to ask me, ‘Is this one of those that needs to be treated now, or is this one of those that we wish we would have never found?’ Or people will say, ‘My doctor talked me into an ultrasound, I didn’t want it’ or ‘I had a car wreck, and I found this nodule and I wished I had never found it.’ ”
This study from Li and coauthors was funded by the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Young Talents Program of Sun Yat-sen University Cancer Center, the Italian Association for Cancer Research, and the Italian Ministry of Health. Davis and Tuttle had no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE LANCET DIABETES & ENDOCRINOLOGY
American Diabetes Association Advises on Hospital CGM Use
, based in part on data collected during the COVID-19 pandemic.
The statement, Consensus Considerations and Good Practice Points for Use of Continuous Glucose Monitoring Systems in Hospital Settings, was published on October 25, 2024, in Diabetes Care.
“This is something that requires close collaboration with many groups in the hospital ... There needs to be really good guidance within the hospital as to when it can be used, in which patients, and what checks and balances need to be in place,” statement lead author Julie L.V. Shaw, PhD, Laboratory Director at Renfrew Victoria Hospital and St. Francis Memorial Hospital, Ottawa, Ontario, Canada, told this news organization.
CGM use in the outpatient setting continues to grow, among people with type 2 as well as type 1 diabetes. The devices are worn on the body for up to 15 days via a subcutaneously-inserted sensor that detects glucose in interstitial fluid every 1-15 minutes. The readings generally track with blood glucose levels, although discrepancies can occur and may be even more relevant in hospital settings.
About 1 in 4 hospitalized patients have diabetes and/or hyperglycemia. During the COVID-19 pandemic, the US Food and Drug Administration (FDA) and Health Canada temporarily authorized the use of CGM systems in hospitals to supplement point-of-care glucose testing, as an emergency measure to reduce healthcare worker exposure and preserve personal protective equipment. That FDA authorization expired on November 7, 2023, and currently hospital CGM use in the United States is technically off-label, although it is often allowed for patients who already use CGM systems.
The new statement summarizes clinical study data and also addresses the potential benefits of CGM systems for inpatients, existing guidance, analytical and clinical evaluation of CGM performance, safety factors, staff training, clinical workflow, and hospital policies. Also covered are issues around quality assurance, integration of CGM data into electronic health records, cost considerations, and barriers to implementation.
The “good practice points for consideration” in the document are as follows:
- If healthcare professionals want to use CGM systems beyond their intended use, eg, to replace or reduce point-of-care glucose measurements, analytical and clinical performance should be assessed.
- The Clinical and Laboratory Standards Institute (CLSI) 2nd Edition of POCT05 — Performance Metrics for Continuous Interstitial Glucose Monitoring provides helpful guidance.
- Potential interferences that preclude patients from being eligible for CGM should be noted, and staff must be aware that CGM can’t be used for clinical decision-making in these patients.
- A CGM system and/or inpatient glycemia management committee should oversee the development and implementation of hospital-approved policies and procedures for CGM use in the hospital. This committee should have representatives from nursing leadership, physician leadership (e.g., endocrinologists, internal medicine specialists, hospitalists), laboratory, information services, hospital administration, pharmacy, and risk management/legal.
- Policies for patient-owned and hospital-owned CGM devices should be developed, and staff should be trained in their use.
“During the pandemic, there was a lot of research on CGM use in the hospital setting, so we could look at how it works and was it safe. I think we have some good data to show where it can be used,” said Shaw, who also heads the Division of Biochemistry at the Ottawa Hospital. She added, “There’s quite a bit we still don’t know, but I think with some guidance in place about when not to use it, there are certainly patient populations who could benefit from it in the hospital setting.”
Shaw had no disclosures. Another author is general manager and medical director of the Institute for Diabetes Technology (IfDT), which carries out clinical studies, eg, with medical devices for diabetes therapy, on its own initiative and on behalf of various companies. Another author is an IfDT employee. Other authors have received speakers’ honoraria or consulting fees in the last 3 years from Abbott, Berlin-Chemie, BOYDSense, Dexcom, Lilly Deutschland, Novo Nordisk, Perfood, PharmaSens, Roche, Sinocare, Terumo, and Ypsomed.
A version of this article appeared on Medscape.com.
, based in part on data collected during the COVID-19 pandemic.
The statement, Consensus Considerations and Good Practice Points for Use of Continuous Glucose Monitoring Systems in Hospital Settings, was published on October 25, 2024, in Diabetes Care.
“This is something that requires close collaboration with many groups in the hospital ... There needs to be really good guidance within the hospital as to when it can be used, in which patients, and what checks and balances need to be in place,” statement lead author Julie L.V. Shaw, PhD, Laboratory Director at Renfrew Victoria Hospital and St. Francis Memorial Hospital, Ottawa, Ontario, Canada, told this news organization.
CGM use in the outpatient setting continues to grow, among people with type 2 as well as type 1 diabetes. The devices are worn on the body for up to 15 days via a subcutaneously-inserted sensor that detects glucose in interstitial fluid every 1-15 minutes. The readings generally track with blood glucose levels, although discrepancies can occur and may be even more relevant in hospital settings.
About 1 in 4 hospitalized patients have diabetes and/or hyperglycemia. During the COVID-19 pandemic, the US Food and Drug Administration (FDA) and Health Canada temporarily authorized the use of CGM systems in hospitals to supplement point-of-care glucose testing, as an emergency measure to reduce healthcare worker exposure and preserve personal protective equipment. That FDA authorization expired on November 7, 2023, and currently hospital CGM use in the United States is technically off-label, although it is often allowed for patients who already use CGM systems.
The new statement summarizes clinical study data and also addresses the potential benefits of CGM systems for inpatients, existing guidance, analytical and clinical evaluation of CGM performance, safety factors, staff training, clinical workflow, and hospital policies. Also covered are issues around quality assurance, integration of CGM data into electronic health records, cost considerations, and barriers to implementation.
The “good practice points for consideration” in the document are as follows:
- If healthcare professionals want to use CGM systems beyond their intended use, eg, to replace or reduce point-of-care glucose measurements, analytical and clinical performance should be assessed.
- The Clinical and Laboratory Standards Institute (CLSI) 2nd Edition of POCT05 — Performance Metrics for Continuous Interstitial Glucose Monitoring provides helpful guidance.
- Potential interferences that preclude patients from being eligible for CGM should be noted, and staff must be aware that CGM can’t be used for clinical decision-making in these patients.
- A CGM system and/or inpatient glycemia management committee should oversee the development and implementation of hospital-approved policies and procedures for CGM use in the hospital. This committee should have representatives from nursing leadership, physician leadership (e.g., endocrinologists, internal medicine specialists, hospitalists), laboratory, information services, hospital administration, pharmacy, and risk management/legal.
- Policies for patient-owned and hospital-owned CGM devices should be developed, and staff should be trained in their use.
“During the pandemic, there was a lot of research on CGM use in the hospital setting, so we could look at how it works and was it safe. I think we have some good data to show where it can be used,” said Shaw, who also heads the Division of Biochemistry at the Ottawa Hospital. She added, “There’s quite a bit we still don’t know, but I think with some guidance in place about when not to use it, there are certainly patient populations who could benefit from it in the hospital setting.”
Shaw had no disclosures. Another author is general manager and medical director of the Institute for Diabetes Technology (IfDT), which carries out clinical studies, eg, with medical devices for diabetes therapy, on its own initiative and on behalf of various companies. Another author is an IfDT employee. Other authors have received speakers’ honoraria or consulting fees in the last 3 years from Abbott, Berlin-Chemie, BOYDSense, Dexcom, Lilly Deutschland, Novo Nordisk, Perfood, PharmaSens, Roche, Sinocare, Terumo, and Ypsomed.
A version of this article appeared on Medscape.com.
, based in part on data collected during the COVID-19 pandemic.
The statement, Consensus Considerations and Good Practice Points for Use of Continuous Glucose Monitoring Systems in Hospital Settings, was published on October 25, 2024, in Diabetes Care.
“This is something that requires close collaboration with many groups in the hospital ... There needs to be really good guidance within the hospital as to when it can be used, in which patients, and what checks and balances need to be in place,” statement lead author Julie L.V. Shaw, PhD, Laboratory Director at Renfrew Victoria Hospital and St. Francis Memorial Hospital, Ottawa, Ontario, Canada, told this news organization.
CGM use in the outpatient setting continues to grow, among people with type 2 as well as type 1 diabetes. The devices are worn on the body for up to 15 days via a subcutaneously-inserted sensor that detects glucose in interstitial fluid every 1-15 minutes. The readings generally track with blood glucose levels, although discrepancies can occur and may be even more relevant in hospital settings.
About 1 in 4 hospitalized patients have diabetes and/or hyperglycemia. During the COVID-19 pandemic, the US Food and Drug Administration (FDA) and Health Canada temporarily authorized the use of CGM systems in hospitals to supplement point-of-care glucose testing, as an emergency measure to reduce healthcare worker exposure and preserve personal protective equipment. That FDA authorization expired on November 7, 2023, and currently hospital CGM use in the United States is technically off-label, although it is often allowed for patients who already use CGM systems.
The new statement summarizes clinical study data and also addresses the potential benefits of CGM systems for inpatients, existing guidance, analytical and clinical evaluation of CGM performance, safety factors, staff training, clinical workflow, and hospital policies. Also covered are issues around quality assurance, integration of CGM data into electronic health records, cost considerations, and barriers to implementation.
The “good practice points for consideration” in the document are as follows:
- If healthcare professionals want to use CGM systems beyond their intended use, eg, to replace or reduce point-of-care glucose measurements, analytical and clinical performance should be assessed.
- The Clinical and Laboratory Standards Institute (CLSI) 2nd Edition of POCT05 — Performance Metrics for Continuous Interstitial Glucose Monitoring provides helpful guidance.
- Potential interferences that preclude patients from being eligible for CGM should be noted, and staff must be aware that CGM can’t be used for clinical decision-making in these patients.
- A CGM system and/or inpatient glycemia management committee should oversee the development and implementation of hospital-approved policies and procedures for CGM use in the hospital. This committee should have representatives from nursing leadership, physician leadership (e.g., endocrinologists, internal medicine specialists, hospitalists), laboratory, information services, hospital administration, pharmacy, and risk management/legal.
- Policies for patient-owned and hospital-owned CGM devices should be developed, and staff should be trained in their use.
“During the pandemic, there was a lot of research on CGM use in the hospital setting, so we could look at how it works and was it safe. I think we have some good data to show where it can be used,” said Shaw, who also heads the Division of Biochemistry at the Ottawa Hospital. She added, “There’s quite a bit we still don’t know, but I think with some guidance in place about when not to use it, there are certainly patient populations who could benefit from it in the hospital setting.”
Shaw had no disclosures. Another author is general manager and medical director of the Institute for Diabetes Technology (IfDT), which carries out clinical studies, eg, with medical devices for diabetes therapy, on its own initiative and on behalf of various companies. Another author is an IfDT employee. Other authors have received speakers’ honoraria or consulting fees in the last 3 years from Abbott, Berlin-Chemie, BOYDSense, Dexcom, Lilly Deutschland, Novo Nordisk, Perfood, PharmaSens, Roche, Sinocare, Terumo, and Ypsomed.
A version of this article appeared on Medscape.com.
AI in Medicine: Are Large Language Models Ready for the Exam Room?
In seconds, Ravi Parikh, MD, an oncologist at the Emory University School of Medicine in Atlanta, had a summary of his patient’s entire medical history. Normally, Parikh skimmed the cumbersome files before seeing a patient. However, the artificial intelligence (AI) tool his institution was testing could list the highlights he needed in a fraction of the time.
“On the whole, I like it ... it saves me time,” Parikh said of the tool. “But I’d be lying if I told you it was perfect all the time. It’s interpreting the [patient] history in some ways that may be inaccurate,” he said.
Within the first week of testing the tool, Parikh started to notice that the large language model (LLM) made a particular mistake in his patients with prostate cancer. If their prostate-specific antigen test results came back slightly elevated — which is part of normal variation — the LLM recorded it as disease progression. Because Parikh reviews all his notes — with or without using an AI tool — after a visit, he easily caught the mistake before it was added to the chart. “The problem, I think, is if these mistakes go under the hood,” he said.
In the data science world, these mistakes are called hallucinations. And a growing body of research suggests they’re happening more frequently than is safe for healthcare. The industry promised LLMs would alleviate administrative burden and reduce physician burnout. But so far, studies show these AI-tool mistakes often create more work for doctors, not less. To truly help physicians and be safe for patients, some experts say healthcare needs to build its own LLMs from the ground up. And all agree that the field desperately needs a way to vet these algorithms more thoroughly.
Prone to Error
Right now, “I think the industry is focused on taking existing LLMs and forcing them into usage for healthcare,” said Nigam H. Shah, MBBS, PhD, chief data scientist for Stanford Health. However, the value of deploying general LLMs in the healthcare space is questionable. “People are starting to wonder if we’re using these tools wrong,” he told this news organization.
In 2023, Shah and his colleagues evaluated seven LLMs on their ability to answer electronic health record–based questions. For realistic tasks, the error rate in the best cases was about 35%, he said. “To me, that rate seems a bit high ... to adopt for routine use.”
A study earlier this year by the UC San Diego School of Medicine showed that using LLMs to respond to patient messages increased the time doctors spent on messages. And this summer, a study by the clinical AI firm Mendel found that when GPT-4o or Llama-3 were used to summarize patient medical records, almost every summary contained at least one type of hallucination.
“We’ve seen cases where a patient does have drug allergies, but the system says ‘no known drug allergies’ ” in the medical history summary, said Wael Salloum, PhD, cofounder and chief science officer at Mendel. “That’s a serious hallucination.” And if physicians have to constantly verify what the system is telling them, that “defeats the purpose [of summarization],” he said.
A Higher Quality Diet
Part of the trouble with LLMs is that there’s just not enough high-quality information to feed them. The algorithms are insatiable, requiring vast swaths of data for training. GPT-3.5, for instance, was trained on 570 GB of data from the internet, more than 300 billion words. And to train GPT-4o, OpenAI reportedly transcribed more than 1 million hours of YouTube content.
However, the strategies that built these general LLMs don’t always translate well to healthcare. The internet is full of low-quality or misleading health information from wellness sites and supplement advertisements. And even data that are trustworthy, like the millions of clinical studies and the US Food and Drug Administration (FDA) statements, can be outdated, Salloum said. And “an LLM in training can’t distinguish good from bad,” he added.
The good news is that clinicians don’t rely on controversial information in the real world. Medical knowledge is standardized. “Healthcare is a domain rich with explicit knowledge,” Salloum said. So there’s potential to build a more reliable LLM that is guided by robust medical standards and guidelines.
It’s possible that healthcare could use small language models, which are LLM’s pocket-sized cousins, and perform tasks needing only bite-sized datasets requiring fewer resources and easier fine-tuning, according to Microsoft’s website. Shah said training these smaller models on real medical data might be an option, like an LLM meant to respond to patient messages that could be trained with real messages sent by physicians.
Several groups are already working on databases of standardized human medical knowledge or real physician responses. “Perhaps that will work better than using LLMs trained on the general internet. Those studies need to be done,” Shah said.
Jon Tamir, assistant professor of electrical and computer engineering and co-lead of the AI Health Lab at The University of Texas at Austin, said, “The community has recognized that we are entering a new era of AI where the dataset itself is the most important aspect. We need training sets that are highly curated and highly specialized.
“If the dataset is highly specialized, it will definitely help reduce hallucinations,” he said.
Cutting Overconfidence
A major problem with LLM mistakes is that they are often hard to detect. Hallucinations can be highly convincing even if they’re highly inaccurate, according to Tamir.
When Shah, for instance, was recently testing an LLM on de-identified patient data, he asked the LLM which blood test the patient last had. The model responded with “complete blood count [CBC].” But when he asked for the results, the model gave him white blood count and other values. “Turns out that record did not have a CBC done at all! The result was entirely made up,” he said.
Making healthcare LLMs safer and more reliable will mean training AI to acknowledge potential mistakes and uncertainty. Existing LLMs are trained to project confidence and produce a lot of answers, even when there isn’t one, Salloum said. They rarely respond with “I don’t know” even when their prediction has low confidence, he added.
Healthcare stands to benefit from a system that highlights uncertainty and potential errors. For instance, if a patient’s history shows they have smoked, stopped smoking, vaped, and started smoking again. The LLM might call them a smoker but flag the comment as uncertain because the chronology is complicated, Salloum said.
Tamir added that this strategy could improve LLM and doctor collaboration by honing in on where human expertise is needed most.
Too Little Evaluation
For any improvement strategy to work, LLMs — and all AI-assisted healthcare tools — first need a better evaluation framework. So far, LLMs have “been used in really exciting ways but not really well-vetted ways,” Tamir said.
While some AI-assisted tools, particularly in medical imaging, have undergone rigorous FDA evaluations and earned approval, most haven’t. And because the FDA only regulates algorithms that are considered medical devices, Parikh said that most LLMs used for administrative tasks and efficiency don’t fall under the regulatory agency’s purview.
But these algorithms still have access to patient information and can directly influence patient and doctor decisions. Third-party regulatory agencies are expected to emerge, but it’s still unclear who those will be. Before developers can build a safer and more efficient LLM for healthcare, they’ll need better guidelines and guardrails. “Unless we figure out evaluation, how would we know whether the healthcare-appropriate large language models are better or worse?” Shah asked.
A version of this article appeared on Medscape.com.
In seconds, Ravi Parikh, MD, an oncologist at the Emory University School of Medicine in Atlanta, had a summary of his patient’s entire medical history. Normally, Parikh skimmed the cumbersome files before seeing a patient. However, the artificial intelligence (AI) tool his institution was testing could list the highlights he needed in a fraction of the time.
“On the whole, I like it ... it saves me time,” Parikh said of the tool. “But I’d be lying if I told you it was perfect all the time. It’s interpreting the [patient] history in some ways that may be inaccurate,” he said.
Within the first week of testing the tool, Parikh started to notice that the large language model (LLM) made a particular mistake in his patients with prostate cancer. If their prostate-specific antigen test results came back slightly elevated — which is part of normal variation — the LLM recorded it as disease progression. Because Parikh reviews all his notes — with or without using an AI tool — after a visit, he easily caught the mistake before it was added to the chart. “The problem, I think, is if these mistakes go under the hood,” he said.
In the data science world, these mistakes are called hallucinations. And a growing body of research suggests they’re happening more frequently than is safe for healthcare. The industry promised LLMs would alleviate administrative burden and reduce physician burnout. But so far, studies show these AI-tool mistakes often create more work for doctors, not less. To truly help physicians and be safe for patients, some experts say healthcare needs to build its own LLMs from the ground up. And all agree that the field desperately needs a way to vet these algorithms more thoroughly.
Prone to Error
Right now, “I think the industry is focused on taking existing LLMs and forcing them into usage for healthcare,” said Nigam H. Shah, MBBS, PhD, chief data scientist for Stanford Health. However, the value of deploying general LLMs in the healthcare space is questionable. “People are starting to wonder if we’re using these tools wrong,” he told this news organization.
In 2023, Shah and his colleagues evaluated seven LLMs on their ability to answer electronic health record–based questions. For realistic tasks, the error rate in the best cases was about 35%, he said. “To me, that rate seems a bit high ... to adopt for routine use.”
A study earlier this year by the UC San Diego School of Medicine showed that using LLMs to respond to patient messages increased the time doctors spent on messages. And this summer, a study by the clinical AI firm Mendel found that when GPT-4o or Llama-3 were used to summarize patient medical records, almost every summary contained at least one type of hallucination.
“We’ve seen cases where a patient does have drug allergies, but the system says ‘no known drug allergies’ ” in the medical history summary, said Wael Salloum, PhD, cofounder and chief science officer at Mendel. “That’s a serious hallucination.” And if physicians have to constantly verify what the system is telling them, that “defeats the purpose [of summarization],” he said.
A Higher Quality Diet
Part of the trouble with LLMs is that there’s just not enough high-quality information to feed them. The algorithms are insatiable, requiring vast swaths of data for training. GPT-3.5, for instance, was trained on 570 GB of data from the internet, more than 300 billion words. And to train GPT-4o, OpenAI reportedly transcribed more than 1 million hours of YouTube content.
However, the strategies that built these general LLMs don’t always translate well to healthcare. The internet is full of low-quality or misleading health information from wellness sites and supplement advertisements. And even data that are trustworthy, like the millions of clinical studies and the US Food and Drug Administration (FDA) statements, can be outdated, Salloum said. And “an LLM in training can’t distinguish good from bad,” he added.
The good news is that clinicians don’t rely on controversial information in the real world. Medical knowledge is standardized. “Healthcare is a domain rich with explicit knowledge,” Salloum said. So there’s potential to build a more reliable LLM that is guided by robust medical standards and guidelines.
It’s possible that healthcare could use small language models, which are LLM’s pocket-sized cousins, and perform tasks needing only bite-sized datasets requiring fewer resources and easier fine-tuning, according to Microsoft’s website. Shah said training these smaller models on real medical data might be an option, like an LLM meant to respond to patient messages that could be trained with real messages sent by physicians.
Several groups are already working on databases of standardized human medical knowledge or real physician responses. “Perhaps that will work better than using LLMs trained on the general internet. Those studies need to be done,” Shah said.
Jon Tamir, assistant professor of electrical and computer engineering and co-lead of the AI Health Lab at The University of Texas at Austin, said, “The community has recognized that we are entering a new era of AI where the dataset itself is the most important aspect. We need training sets that are highly curated and highly specialized.
“If the dataset is highly specialized, it will definitely help reduce hallucinations,” he said.
Cutting Overconfidence
A major problem with LLM mistakes is that they are often hard to detect. Hallucinations can be highly convincing even if they’re highly inaccurate, according to Tamir.
When Shah, for instance, was recently testing an LLM on de-identified patient data, he asked the LLM which blood test the patient last had. The model responded with “complete blood count [CBC].” But when he asked for the results, the model gave him white blood count and other values. “Turns out that record did not have a CBC done at all! The result was entirely made up,” he said.
Making healthcare LLMs safer and more reliable will mean training AI to acknowledge potential mistakes and uncertainty. Existing LLMs are trained to project confidence and produce a lot of answers, even when there isn’t one, Salloum said. They rarely respond with “I don’t know” even when their prediction has low confidence, he added.
Healthcare stands to benefit from a system that highlights uncertainty and potential errors. For instance, if a patient’s history shows they have smoked, stopped smoking, vaped, and started smoking again. The LLM might call them a smoker but flag the comment as uncertain because the chronology is complicated, Salloum said.
Tamir added that this strategy could improve LLM and doctor collaboration by honing in on where human expertise is needed most.
Too Little Evaluation
For any improvement strategy to work, LLMs — and all AI-assisted healthcare tools — first need a better evaluation framework. So far, LLMs have “been used in really exciting ways but not really well-vetted ways,” Tamir said.
While some AI-assisted tools, particularly in medical imaging, have undergone rigorous FDA evaluations and earned approval, most haven’t. And because the FDA only regulates algorithms that are considered medical devices, Parikh said that most LLMs used for administrative tasks and efficiency don’t fall under the regulatory agency’s purview.
But these algorithms still have access to patient information and can directly influence patient and doctor decisions. Third-party regulatory agencies are expected to emerge, but it’s still unclear who those will be. Before developers can build a safer and more efficient LLM for healthcare, they’ll need better guidelines and guardrails. “Unless we figure out evaluation, how would we know whether the healthcare-appropriate large language models are better or worse?” Shah asked.
A version of this article appeared on Medscape.com.
In seconds, Ravi Parikh, MD, an oncologist at the Emory University School of Medicine in Atlanta, had a summary of his patient’s entire medical history. Normally, Parikh skimmed the cumbersome files before seeing a patient. However, the artificial intelligence (AI) tool his institution was testing could list the highlights he needed in a fraction of the time.
“On the whole, I like it ... it saves me time,” Parikh said of the tool. “But I’d be lying if I told you it was perfect all the time. It’s interpreting the [patient] history in some ways that may be inaccurate,” he said.
Within the first week of testing the tool, Parikh started to notice that the large language model (LLM) made a particular mistake in his patients with prostate cancer. If their prostate-specific antigen test results came back slightly elevated — which is part of normal variation — the LLM recorded it as disease progression. Because Parikh reviews all his notes — with or without using an AI tool — after a visit, he easily caught the mistake before it was added to the chart. “The problem, I think, is if these mistakes go under the hood,” he said.
In the data science world, these mistakes are called hallucinations. And a growing body of research suggests they’re happening more frequently than is safe for healthcare. The industry promised LLMs would alleviate administrative burden and reduce physician burnout. But so far, studies show these AI-tool mistakes often create more work for doctors, not less. To truly help physicians and be safe for patients, some experts say healthcare needs to build its own LLMs from the ground up. And all agree that the field desperately needs a way to vet these algorithms more thoroughly.
Prone to Error
Right now, “I think the industry is focused on taking existing LLMs and forcing them into usage for healthcare,” said Nigam H. Shah, MBBS, PhD, chief data scientist for Stanford Health. However, the value of deploying general LLMs in the healthcare space is questionable. “People are starting to wonder if we’re using these tools wrong,” he told this news organization.
In 2023, Shah and his colleagues evaluated seven LLMs on their ability to answer electronic health record–based questions. For realistic tasks, the error rate in the best cases was about 35%, he said. “To me, that rate seems a bit high ... to adopt for routine use.”
A study earlier this year by the UC San Diego School of Medicine showed that using LLMs to respond to patient messages increased the time doctors spent on messages. And this summer, a study by the clinical AI firm Mendel found that when GPT-4o or Llama-3 were used to summarize patient medical records, almost every summary contained at least one type of hallucination.
“We’ve seen cases where a patient does have drug allergies, but the system says ‘no known drug allergies’ ” in the medical history summary, said Wael Salloum, PhD, cofounder and chief science officer at Mendel. “That’s a serious hallucination.” And if physicians have to constantly verify what the system is telling them, that “defeats the purpose [of summarization],” he said.
A Higher Quality Diet
Part of the trouble with LLMs is that there’s just not enough high-quality information to feed them. The algorithms are insatiable, requiring vast swaths of data for training. GPT-3.5, for instance, was trained on 570 GB of data from the internet, more than 300 billion words. And to train GPT-4o, OpenAI reportedly transcribed more than 1 million hours of YouTube content.
However, the strategies that built these general LLMs don’t always translate well to healthcare. The internet is full of low-quality or misleading health information from wellness sites and supplement advertisements. And even data that are trustworthy, like the millions of clinical studies and the US Food and Drug Administration (FDA) statements, can be outdated, Salloum said. And “an LLM in training can’t distinguish good from bad,” he added.
The good news is that clinicians don’t rely on controversial information in the real world. Medical knowledge is standardized. “Healthcare is a domain rich with explicit knowledge,” Salloum said. So there’s potential to build a more reliable LLM that is guided by robust medical standards and guidelines.
It’s possible that healthcare could use small language models, which are LLM’s pocket-sized cousins, and perform tasks needing only bite-sized datasets requiring fewer resources and easier fine-tuning, according to Microsoft’s website. Shah said training these smaller models on real medical data might be an option, like an LLM meant to respond to patient messages that could be trained with real messages sent by physicians.
Several groups are already working on databases of standardized human medical knowledge or real physician responses. “Perhaps that will work better than using LLMs trained on the general internet. Those studies need to be done,” Shah said.
Jon Tamir, assistant professor of electrical and computer engineering and co-lead of the AI Health Lab at The University of Texas at Austin, said, “The community has recognized that we are entering a new era of AI where the dataset itself is the most important aspect. We need training sets that are highly curated and highly specialized.
“If the dataset is highly specialized, it will definitely help reduce hallucinations,” he said.
Cutting Overconfidence
A major problem with LLM mistakes is that they are often hard to detect. Hallucinations can be highly convincing even if they’re highly inaccurate, according to Tamir.
When Shah, for instance, was recently testing an LLM on de-identified patient data, he asked the LLM which blood test the patient last had. The model responded with “complete blood count [CBC].” But when he asked for the results, the model gave him white blood count and other values. “Turns out that record did not have a CBC done at all! The result was entirely made up,” he said.
Making healthcare LLMs safer and more reliable will mean training AI to acknowledge potential mistakes and uncertainty. Existing LLMs are trained to project confidence and produce a lot of answers, even when there isn’t one, Salloum said. They rarely respond with “I don’t know” even when their prediction has low confidence, he added.
Healthcare stands to benefit from a system that highlights uncertainty and potential errors. For instance, if a patient’s history shows they have smoked, stopped smoking, vaped, and started smoking again. The LLM might call them a smoker but flag the comment as uncertain because the chronology is complicated, Salloum said.
Tamir added that this strategy could improve LLM and doctor collaboration by honing in on where human expertise is needed most.
Too Little Evaluation
For any improvement strategy to work, LLMs — and all AI-assisted healthcare tools — first need a better evaluation framework. So far, LLMs have “been used in really exciting ways but not really well-vetted ways,” Tamir said.
While some AI-assisted tools, particularly in medical imaging, have undergone rigorous FDA evaluations and earned approval, most haven’t. And because the FDA only regulates algorithms that are considered medical devices, Parikh said that most LLMs used for administrative tasks and efficiency don’t fall under the regulatory agency’s purview.
But these algorithms still have access to patient information and can directly influence patient and doctor decisions. Third-party regulatory agencies are expected to emerge, but it’s still unclear who those will be. Before developers can build a safer and more efficient LLM for healthcare, they’ll need better guidelines and guardrails. “Unless we figure out evaluation, how would we know whether the healthcare-appropriate large language models are better or worse?” Shah asked.
A version of this article appeared on Medscape.com.
Cybersecurity Concerns Continue to Rise With Ransom, Data Manipulation, AI Risks
From the largest healthcare companies to solo practices, just every organization in medicine faces a risk for costly cyberattacks. In recent years, hackers have threatened to release the personal information of patients and employees — or paralyze online systems — unless they’re paid a ransom.
Should companies pay? It’s not an easy answer, a pair of experts told colleagues in an American Medical Association (AMA) cybersecurity webinar on October 18. It turns out that each choice — pay or don’t pay — can end up being costly.
This is just one of the new challenges facing the American medical system on the cybersecurity front, the speakers said. Others include the possibility that hackers will manipulate patient data — turning a medical test negative, for example, when it’s actually positive — and take advantage of the powers of artificial intelligence (AI).
The AMA held the webinar to educate physicians about cybersecurity risks and defenses, an especially hot topic in the wake of February’s Change Healthcare hack, which cost UnitedHealth Group an estimated $2.5 billion — so far — and deeply disrupted the American healthcare system.
Cautionary tales abound. Greg Garcia, executive director for cybersecurity of the Health Sector Coordinating Council, a coalition of medical industry organizations, pointed to a Pennsylvania clinic that refused to pay a ransom to prevent the release of hundreds of images of patients with breast cancer undressed from the waist up. Garcia told webinar participants that the ransom was $5 million.
Risky Choices
While the Federal Bureau of Investigation recommends against paying a ransom, this can be a risky choice, Garcia said. Hackers released the images, and the center has reportedly agreed to settle a class-action lawsuit for $65 million. “They traded $5 million for $60 million,” Garcia added, slightly misstating the settlement amount.
Health systems have been cagey about whether they’ve paid ransoms to prevent private data from being made public in cyberattacks. If a ransom is demanded, “it’s every organization for itself,” Garcia said.
He highlighted the case of a chain of psychiatry practices in Finland that suffered a ransomware attack in 2020. The hackers “contacted the patients and said: ‘Hey, call your clinic and tell them to pay the ransom. Otherwise, we’re going to release all your psychiatric notes to the public.’ ”
Cyberattacks continue. In October, Boston Children’s Health Physicians announced that it had suffered a “ recent security incident” involving data — possibly including Social Security numbers and treatment information — regarding patients and employees. A hacker group reportedly claimed responsibility and wants the system, which boasts more than 300 clinicians, to pay a ransom or else it will release the stolen information.
Should Paying Ransom Be a Crime?
Christian Dameff, MD, MS, an emergency medicine physician and director of the Center for Healthcare Cybersecurity at the University of California (UC), San Diego, noted that there are efforts to turn paying ransom into a crime. “If people aren’t paying ransoms, then ransomware operators will move to something else that makes them money.”
Dameff urged colleagues to understand we no longer live in a world where clinicians only bother to think of technology when they call the IT department to help them reset their password.
New challenges face clinicians, he said. “How do we develop better strategies, downtime procedures, and safe clinical care in an era where our vital technology may be gone, not just for an hour or 2, but as is the case with these ransomware attacks, sometimes weeks to months.”
Garcia said “cybersecurity is everybody’s responsibility, including frontline clinicians. Because you’re touching data, you’re touching technology, you’re touching patients, and all of those things combine to present some vulnerabilities in the digital world.”
Next Frontier: Hackers May Manipulate Patient Data
Dameff said future hackers may use AI to manipulate individual patient data in ways that threaten patient health. AI makes this easier to accomplish.
“What if I delete your allergies in your electronic health record, or I manipulate your chest x-ray, or I change your lab values so it looks like you’re in diabetic ketoacidosis when you’re not so a clinician gives you insulin when you don’t need it?”
Garcia highlighted another new threat: Phishing efforts that are harder to ignore thanks to AI.
“One of the most successful way that hackers get in, disrupt systems, and steal data is through email phishing, and it’s only going to get better because of artificial intelligence,” he said. “No longer are you going to have typos in that email written by a hacking group in Nigeria or in China. It’s going to be perfect looking.”
What can practices and healthcare systems do? Garcia highlighted federal health agency efforts to encourage organizations to adopt best practices in cybersecurity.
“If you’ve got a data breach, and you can show to the US Department of Health & Human Services [HHS] you have implemented generally recognized cybersecurity controls over the past year, that you have done your best, you did the right thing, and you still got hit, HHS is directed to essentially take it easy on you,” he said. “That’s a positive incentive.”
Ransomware Guide in the Works
Dameff said UC San Diego’s Center for Healthcare Cybersecurity plans to publish a free cybersecurity guide in 2025 that will include specific information about ransomware attacks for medical specialties such as cardiology, trauma surgery, and pediatrics.
“Then, should you ever be ransomed, you can pull out this guide. You’ll know what’s going to kind of happen, and you can better prepare for those effects.”
Will the future president prioritize healthcare cybersecurity? That remains to be seen, but crises do have the capacity to concentrate the mind, experts said.
The nation’s capital “has a very short memory, a short attention span. The policymakers tend to be reactive,” Dameff said. “All it takes is yet another Change Healthcare–like attack that disrupts 30% or more of the nation’s healthcare system for the policymakers to sit up, take notice, and try to come up with solutions.”
In addition, he said, an estimated two data breaches/ransomware attacks are occurring per day. “The fact is that we’re all patients, up to the President of the United States and every member of the Congress is a patient.”
There’s a “very existential, very palpable understanding that cyber safety is patient safety and cyber insecurity is patient insecurity,” Dameff said.
A version of this article appeared on Medscape.com.
From the largest healthcare companies to solo practices, just every organization in medicine faces a risk for costly cyberattacks. In recent years, hackers have threatened to release the personal information of patients and employees — or paralyze online systems — unless they’re paid a ransom.
Should companies pay? It’s not an easy answer, a pair of experts told colleagues in an American Medical Association (AMA) cybersecurity webinar on October 18. It turns out that each choice — pay or don’t pay — can end up being costly.
This is just one of the new challenges facing the American medical system on the cybersecurity front, the speakers said. Others include the possibility that hackers will manipulate patient data — turning a medical test negative, for example, when it’s actually positive — and take advantage of the powers of artificial intelligence (AI).
The AMA held the webinar to educate physicians about cybersecurity risks and defenses, an especially hot topic in the wake of February’s Change Healthcare hack, which cost UnitedHealth Group an estimated $2.5 billion — so far — and deeply disrupted the American healthcare system.
Cautionary tales abound. Greg Garcia, executive director for cybersecurity of the Health Sector Coordinating Council, a coalition of medical industry organizations, pointed to a Pennsylvania clinic that refused to pay a ransom to prevent the release of hundreds of images of patients with breast cancer undressed from the waist up. Garcia told webinar participants that the ransom was $5 million.
Risky Choices
While the Federal Bureau of Investigation recommends against paying a ransom, this can be a risky choice, Garcia said. Hackers released the images, and the center has reportedly agreed to settle a class-action lawsuit for $65 million. “They traded $5 million for $60 million,” Garcia added, slightly misstating the settlement amount.
Health systems have been cagey about whether they’ve paid ransoms to prevent private data from being made public in cyberattacks. If a ransom is demanded, “it’s every organization for itself,” Garcia said.
He highlighted the case of a chain of psychiatry practices in Finland that suffered a ransomware attack in 2020. The hackers “contacted the patients and said: ‘Hey, call your clinic and tell them to pay the ransom. Otherwise, we’re going to release all your psychiatric notes to the public.’ ”
Cyberattacks continue. In October, Boston Children’s Health Physicians announced that it had suffered a “ recent security incident” involving data — possibly including Social Security numbers and treatment information — regarding patients and employees. A hacker group reportedly claimed responsibility and wants the system, which boasts more than 300 clinicians, to pay a ransom or else it will release the stolen information.
Should Paying Ransom Be a Crime?
Christian Dameff, MD, MS, an emergency medicine physician and director of the Center for Healthcare Cybersecurity at the University of California (UC), San Diego, noted that there are efforts to turn paying ransom into a crime. “If people aren’t paying ransoms, then ransomware operators will move to something else that makes them money.”
Dameff urged colleagues to understand we no longer live in a world where clinicians only bother to think of technology when they call the IT department to help them reset their password.
New challenges face clinicians, he said. “How do we develop better strategies, downtime procedures, and safe clinical care in an era where our vital technology may be gone, not just for an hour or 2, but as is the case with these ransomware attacks, sometimes weeks to months.”
Garcia said “cybersecurity is everybody’s responsibility, including frontline clinicians. Because you’re touching data, you’re touching technology, you’re touching patients, and all of those things combine to present some vulnerabilities in the digital world.”
Next Frontier: Hackers May Manipulate Patient Data
Dameff said future hackers may use AI to manipulate individual patient data in ways that threaten patient health. AI makes this easier to accomplish.
“What if I delete your allergies in your electronic health record, or I manipulate your chest x-ray, or I change your lab values so it looks like you’re in diabetic ketoacidosis when you’re not so a clinician gives you insulin when you don’t need it?”
Garcia highlighted another new threat: Phishing efforts that are harder to ignore thanks to AI.
“One of the most successful way that hackers get in, disrupt systems, and steal data is through email phishing, and it’s only going to get better because of artificial intelligence,” he said. “No longer are you going to have typos in that email written by a hacking group in Nigeria or in China. It’s going to be perfect looking.”
What can practices and healthcare systems do? Garcia highlighted federal health agency efforts to encourage organizations to adopt best practices in cybersecurity.
“If you’ve got a data breach, and you can show to the US Department of Health & Human Services [HHS] you have implemented generally recognized cybersecurity controls over the past year, that you have done your best, you did the right thing, and you still got hit, HHS is directed to essentially take it easy on you,” he said. “That’s a positive incentive.”
Ransomware Guide in the Works
Dameff said UC San Diego’s Center for Healthcare Cybersecurity plans to publish a free cybersecurity guide in 2025 that will include specific information about ransomware attacks for medical specialties such as cardiology, trauma surgery, and pediatrics.
“Then, should you ever be ransomed, you can pull out this guide. You’ll know what’s going to kind of happen, and you can better prepare for those effects.”
Will the future president prioritize healthcare cybersecurity? That remains to be seen, but crises do have the capacity to concentrate the mind, experts said.
The nation’s capital “has a very short memory, a short attention span. The policymakers tend to be reactive,” Dameff said. “All it takes is yet another Change Healthcare–like attack that disrupts 30% or more of the nation’s healthcare system for the policymakers to sit up, take notice, and try to come up with solutions.”
In addition, he said, an estimated two data breaches/ransomware attacks are occurring per day. “The fact is that we’re all patients, up to the President of the United States and every member of the Congress is a patient.”
There’s a “very existential, very palpable understanding that cyber safety is patient safety and cyber insecurity is patient insecurity,” Dameff said.
A version of this article appeared on Medscape.com.
From the largest healthcare companies to solo practices, just every organization in medicine faces a risk for costly cyberattacks. In recent years, hackers have threatened to release the personal information of patients and employees — or paralyze online systems — unless they’re paid a ransom.
Should companies pay? It’s not an easy answer, a pair of experts told colleagues in an American Medical Association (AMA) cybersecurity webinar on October 18. It turns out that each choice — pay or don’t pay — can end up being costly.
This is just one of the new challenges facing the American medical system on the cybersecurity front, the speakers said. Others include the possibility that hackers will manipulate patient data — turning a medical test negative, for example, when it’s actually positive — and take advantage of the powers of artificial intelligence (AI).
The AMA held the webinar to educate physicians about cybersecurity risks and defenses, an especially hot topic in the wake of February’s Change Healthcare hack, which cost UnitedHealth Group an estimated $2.5 billion — so far — and deeply disrupted the American healthcare system.
Cautionary tales abound. Greg Garcia, executive director for cybersecurity of the Health Sector Coordinating Council, a coalition of medical industry organizations, pointed to a Pennsylvania clinic that refused to pay a ransom to prevent the release of hundreds of images of patients with breast cancer undressed from the waist up. Garcia told webinar participants that the ransom was $5 million.
Risky Choices
While the Federal Bureau of Investigation recommends against paying a ransom, this can be a risky choice, Garcia said. Hackers released the images, and the center has reportedly agreed to settle a class-action lawsuit for $65 million. “They traded $5 million for $60 million,” Garcia added, slightly misstating the settlement amount.
Health systems have been cagey about whether they’ve paid ransoms to prevent private data from being made public in cyberattacks. If a ransom is demanded, “it’s every organization for itself,” Garcia said.
He highlighted the case of a chain of psychiatry practices in Finland that suffered a ransomware attack in 2020. The hackers “contacted the patients and said: ‘Hey, call your clinic and tell them to pay the ransom. Otherwise, we’re going to release all your psychiatric notes to the public.’ ”
Cyberattacks continue. In October, Boston Children’s Health Physicians announced that it had suffered a “ recent security incident” involving data — possibly including Social Security numbers and treatment information — regarding patients and employees. A hacker group reportedly claimed responsibility and wants the system, which boasts more than 300 clinicians, to pay a ransom or else it will release the stolen information.
Should Paying Ransom Be a Crime?
Christian Dameff, MD, MS, an emergency medicine physician and director of the Center for Healthcare Cybersecurity at the University of California (UC), San Diego, noted that there are efforts to turn paying ransom into a crime. “If people aren’t paying ransoms, then ransomware operators will move to something else that makes them money.”
Dameff urged colleagues to understand we no longer live in a world where clinicians only bother to think of technology when they call the IT department to help them reset their password.
New challenges face clinicians, he said. “How do we develop better strategies, downtime procedures, and safe clinical care in an era where our vital technology may be gone, not just for an hour or 2, but as is the case with these ransomware attacks, sometimes weeks to months.”
Garcia said “cybersecurity is everybody’s responsibility, including frontline clinicians. Because you’re touching data, you’re touching technology, you’re touching patients, and all of those things combine to present some vulnerabilities in the digital world.”
Next Frontier: Hackers May Manipulate Patient Data
Dameff said future hackers may use AI to manipulate individual patient data in ways that threaten patient health. AI makes this easier to accomplish.
“What if I delete your allergies in your electronic health record, or I manipulate your chest x-ray, or I change your lab values so it looks like you’re in diabetic ketoacidosis when you’re not so a clinician gives you insulin when you don’t need it?”
Garcia highlighted another new threat: Phishing efforts that are harder to ignore thanks to AI.
“One of the most successful way that hackers get in, disrupt systems, and steal data is through email phishing, and it’s only going to get better because of artificial intelligence,” he said. “No longer are you going to have typos in that email written by a hacking group in Nigeria or in China. It’s going to be perfect looking.”
What can practices and healthcare systems do? Garcia highlighted federal health agency efforts to encourage organizations to adopt best practices in cybersecurity.
“If you’ve got a data breach, and you can show to the US Department of Health & Human Services [HHS] you have implemented generally recognized cybersecurity controls over the past year, that you have done your best, you did the right thing, and you still got hit, HHS is directed to essentially take it easy on you,” he said. “That’s a positive incentive.”
Ransomware Guide in the Works
Dameff said UC San Diego’s Center for Healthcare Cybersecurity plans to publish a free cybersecurity guide in 2025 that will include specific information about ransomware attacks for medical specialties such as cardiology, trauma surgery, and pediatrics.
“Then, should you ever be ransomed, you can pull out this guide. You’ll know what’s going to kind of happen, and you can better prepare for those effects.”
Will the future president prioritize healthcare cybersecurity? That remains to be seen, but crises do have the capacity to concentrate the mind, experts said.
The nation’s capital “has a very short memory, a short attention span. The policymakers tend to be reactive,” Dameff said. “All it takes is yet another Change Healthcare–like attack that disrupts 30% or more of the nation’s healthcare system for the policymakers to sit up, take notice, and try to come up with solutions.”
In addition, he said, an estimated two data breaches/ransomware attacks are occurring per day. “The fact is that we’re all patients, up to the President of the United States and every member of the Congress is a patient.”
There’s a “very existential, very palpable understanding that cyber safety is patient safety and cyber insecurity is patient insecurity,” Dameff said.
A version of this article appeared on Medscape.com.
Cardiovascular Disease 2050: No, GLP-1s Won’t Save the Day
This transcript has been edited for clarity .
Robert A. Harrington, MD: I’m here in London at the European Society of Cardiology meetings, at theheart.org | Medscape Cardiology booth, using the meetings as an opportunity to meet with colleagues to talk about recent things that they’ve been writing about.
Today I’m joined by a good friend and colleague, Dr. Dhruv Kazi from Beth Israel Deaconess in Boston. Thanks for joining us.
Dhruv S. Kazi, MD, MS: Thank you for having me.
Harrington: Dr. Kazi is an associate professor of medicine at Harvard Medical School. He’s also the associate director of the Smith Center, which is an outcomes research center at the Beth Israel Deaconess. Thanks for joining us.
Kazi: Excited to be here.
Harrington: The topic I think you know that I want to discuss is a really important paper. There are two papers. They’re part of the American Heart Association’s 100th anniversary celebration, if you will. Many of the papers looked back at where science taken us.
With your coauthor, Karen Joynt Maddox, your papers are looking forward. They’re about the burden of cardiovascular disease in 2050. One paper really focused on what I would call the clinical and public health issues. Yours is focused on the economics. Is that a good description?
Kazi: Perfect.
Harrington: Tell us what you, Karen, and the other writers set out to do. What were you asked to do?
Kazi: As you know, the American Heart Association is entering its second century. Part of this was an exercise to say, where will the country be in 2050, which is a long enough time horizon for us to start planning for the future.
We looked back and said, if prior trends remain the same, where will we be in 2050, accounting for changes in demographics, changes in the composition of the population, and knowing that some of the cardiovascular risk factors are getting worse?
Harrington: For me, what was really striking is that, when I first saw the title and read “2050,” I thought, Oh, that’s a long way away. Then as I started reading it, I realized that this is not so far away.
Kazi: Absolutely.
Harrington: If we’re going to make a difference, it might take us 25 years.
Kazi: Especially if we set ourselves ambitious goals, we›re going to have to dig deep. Business-as-usual is not going to get us there.
Harrington: No. What I think has happened is we›ve spent so much time taking care of acute illness. Case fatality rates are fantastic. I was actually making the comment yesterday to a colleague that when I was an intern, the 30-day death rate from acute myocardial infarction was about 20%.
Kazi: Oh, wow.
Harrington: Now it’s 5%. That’s a big difference in a career.
Trends in the Wrong Direction
Kazi: There are fundamental trends. The decline in case fatalities is a really positive development, and I would hope that, going forward, that would continue. Those are risk-adjusted death rates and what is happening is that risk is going up. This is a function of the fact that the US population is aging; 2030 will be the first year that all the baby boomers will be over the age of 65.
By the mid-2030s, we’ll have more adults over the age of 65 than kids. That aging of the population is going to increase risk. The second is — and this is a positive development — we are a more diverse population, but the populations that are minoritized have higher cardiovascular risk, for a variety of reasons.
As the population of Asian Americans increases and doubles, in fact, as the population of Hispanic Americans doubles, we’re going to see an increase in risk related to cardiovascular disease. The third is that, over the past decade, there are some risk factors that are going in the wrong direction.
Harrington: Let’s talk about that because that’s humbling. I’m involved, as you know, with the American Heart Association, as are you. Despite all the work on Life’s Simple 7 and now Life’s Essential 8, we still have some issues.
Kazi: The big ones that come to mind are hypertension, diabetes, and obesity, all of which are trending in the wrong direction. Hypertension, we were gaining traction; and then over the past decade, we’ve slipped again. As you know, national blood pressure control rates have declined in many populations.
Harrington: Rather substantially.
Kazi: Substantially so, which has implications, in particular, for stroke rates in the future and stroke rates in young adults in the future. Obesity is a problem that we have very little control over. We’re already at 40% on average, which means that some populations are already in the 60% range.
Harrington: We also have obesity in kids — the burden, I’ll call it, of obesity. It’s not that you become obese in your thirties or your forties; you›re becoming obese as a teenager or even younger.
Kazi: Exactly. Since the 1990s, obesity in US adults has doubled, but obesity in US children has quadrupled. It’s starting from a lower base, but it’s very much an escalating problem.
Harrington: Diabetes is tightly linked to it but not totally explained.
Kazi: Exactly. The increase in diabetes is largely driven by obesity, but it›s probably also driven by changes in diet and lifestyle that don›t go through obesity.
Harrington: Yeah, it’s interesting. I think I have this figure correctly. It used to be rare that you saw a child with type 2 diabetes or what we call type 2 diabetes.
Kazi: Yeah.
Harrington: Now, the vast majority of kids with diabetes have type 2 diabetes.
Kazi: In the adolescents/young adults age group, most of it is type 2.
Harrington: Diabetes going up, obesity up, hypertension not well controlled, smoking combustible cigarettes way down.
Kazi: Yeah.
Harrington: Cholesterol levels. I was surprised. Cholesterol looked better. You said — because I was at a meeting where somebody asked you — that’s not explained by treatment.
Kazi: No, it’s not, at least going back to the ‘70s, but likely even sooner. I think that can only be attributed to substantial dietary changes. We are consuming less fat and less trans-fat. It’s possible that those collectively are improving our cholesterol levels, possibly at the expense of our glucose levels, because we basically substituted fats in our diet with more carbs at a population level.
Cigarettes and Vaping
Harrington: Some things certainly trend in the right direction but others in a really difficult direction. It’s going to lead to pretty large changes in risk for coronary disease, atrial fibrillation, and heart failure.
Kazi: I want to go back to the tobacco point. There are definitely marked declines in tobacco, still tightly related to income in the country. You see much higher prevalence of tobacco use in lower-income populations, but it’s unclear to me where it’s going in kids. We know that combustible tobacco use is going down but e-cigarettes went up. What that leads to over the next 30 years is unclear to me.
Harrington: That is a really important comment that’s worth sidebarring. The vaping use has been a terrible epidemic among our high schoolers. What is that going to lead to? Is it going to lead to the use of combustible cigarettes and we’re going to see that go back up? It remains to be seen.
Kazi: Yes, it remains to be seen. Going back to your point about this change in risk factors and this change in demographics, both aging and becoming a more diverse population means that we have large increases in some healthcare conditions.
Coronary heart disease goes up some, there›s a big jump in stroke — nearly a doubling in stroke — which is related to hypertension, obesity, an aging population, and a more diverse population. There are changes in stroke in the young, and atrial fibrillation related to, again, hypertension. We’re seeing these projections, and with them come these pretty large projections in changes in healthcare spending.
Healthcare Spending Not Sustainable
Harrington: Big. I mean, it’s not sustainable. Give the audience the number — it’s pretty frightening.
Kazi: We’re talking about a quadrupling of healthcare costs related to cardiovascular disease over 25 years. We’ve gotten used to the narrative that healthcare in the US is expensive and drugs are expensive, but this is an enormous problem — an unsustainable problem, like you called it.
It’s a doubling as a proportion of the economy. I was looking this up this morning. If the US healthcare economy were its own economy, it would be the fourth largest economy in the world.
Harrington: Healthcare as it is today, is it 21% of our economy?
Kazi: It’s 17% now. If it were its own economy, it would be the fourth largest in the world. We are spending more on healthcare than all but two other countries’ total economies. It’s kind of crazy.
Harrington: We’re talking about a quadrupling.
Kazi: Within that, the cardiovascular piece is a big piece, and we›re talking about a quadrupling.
Harrington: That’s both direct and indirect costs.
Kazi: The quadrupling of costs is just the direct costs. Indirect costs, for the listeners, refer to costs unrelated to healthcare but changes in productivity, either because people are disabled and unable to participate fully in the workforce or they die early.
The productivity costs are also increased substantially as a result. If you look at both healthcare and productivity, that goes up threefold. These are very large changes.
Harrington: Let’s now get to what we can do about it. I made the comment to you when I first read the papers that I was very depressed. Then, after I went through my Kübler-Ross stages of depression, death, and dying, I came to acceptance.
What are we going to do about it? This is a focus on policy, but also a focus on how we deliver healthcare, how we think about healthcare, and how we develop drugs and devices.
The drug question is going to be the one the audience is thinking about. They say, well, what about GLP-1 agonists? Aren’t those going to save the day?
Kazi: Yes and no. I’ll say that, early in my career, I used to be very attracted to simple solutions to complex problems. I’ve come to realize that simple solutions are elegant, attractive, and wrong. We›re dealing with a very complex issue and I think we’re going to need a multipronged approach.
The way I think about it is that there was a group of people who are at very high risk today. How do we help those individuals? Then how do we help the future generation so that they’re not dealing with the projections that we’re talking about.
My colleague, Karen Joynt Maddox, who led one of the papers, as you mentioned, has an elegant line in the paper where she says projections are not destiny. These are things we can change.
Harrington: If nothing changes, this is what it’s going to look like.
Kazi: This is where we’re headed.
Harrington: We can change. We’ve got some time to change, but we don’t have forever.
Kazi: Yes, exactly. We picked the 25-year timeline instead of a “let’s plan for the next century” timeline because we want something concrete and actionable. It’s close enough to be meaningful but far enough to give us the runway we need to act.
Harrington: Give me two things from the policy perspective, because it’s mostly policy.
Kazi: There are policy and clinical interventions. From the policy perspective, if I had to list two things, one is expansion of access to care. As we talk about this big increase in the burden of disease and risk factors, if you have a large proportion of your population that has hypertension or diabetes, you’re going to have to expand access to care to ensure that people get treated so they can get access to this care before they develop the complications that we worry about, like stroke and heart disease, that are very expensive to treat downstream.
The second, more broadly related to access to care, is the access to medications that are effective. You bring up GLP-1s. I think we need a real strategy for how we can give people access to GLP-1s at a price that is affordable to individuals but also affordable to the health system, and to help them stay on the drugs.
GLP-1s are transformative in what they do for weight loss and for diabetes, but more than 50% of people who start one are off it at 12 months. There’s something fundamentally wrong about how we’re delivering GLP-1s today. It’s not just about the cost of the drugs but the support system people need to stay on.
Harrington: I’ve made the comment, in many forms now, that we know the drugs work. We have to figure out how to use them.
Kazi: Exactly, yes.
Harrington: Using them includes chronicity. This is a chronic condition. Some people can come off the drugs, but many can’t. We’re going to have to figure this out, and maybe the newer generations of drugs will help us address what people call the off-ramping. How are we going to do that? I think you’re spot-on. Those are critically important questions.
Kazi: As we looked at this modeling, I’ll tell you — I had a come-to-Jesus moment where I was like, there is no way to fix cardiovascular disease in the US without going through obesity and diabetes. We have to address obesity in the US. We can’t just treat our way out of it. Obesity is fundamentally a food problem and we’ve got to engage again with food policy in a meaningful way.
Harrington: As you know, with the American Heart Association, we›re doing a large amount of work now on food as medicine and food is medicine. We are trying to figure out what the levers are that we can pull to actually help people eat healthier diets.
Kazi: Yes. Rather than framing it as an individual choice that people are eating poorly, it’s, how do we make healthy diets the default in the environment?
Harrington: This is where you get to the children as well.
Kazi: Exactly.
Harrington: I could talk about this all day. I’ve had the benefit of reading the papers now a few times and talking to you on several occasions. Thank you for joining us.
Kazi: Thank you.
Dr. Harrington, Stephen and Suzanne Weiss Dean, Weill Cornell Medicine; Provost for Medical Affairs, Cornell University, New York, NY, disclosed ties with Baim Institute (DSMB); CSL (RCT Executive Committee); Janssen (RCT Char), NHLBI (RCT Executive Committee, DSMB Chair); PCORI (RCT Co-Chair); DCRI, Atropos Health; Bitterroot Bio; Bristol Myers Squibb; BridgeBio; Element Science; Edwards Lifesciences; Foresite Labs; Medscape/WebMD Board of Directors for: American Heart Association; College of the Holy Cross; and Cytokinetics. Dr. Kazi, Associate Director, Smith Center for Outcomes Research, Associate Professor, Department of Medicine (Cardiology), Harvard Medical School, Director, Department of Cardiac Critical Care Unit, Beth Israel Deaconess Medical Center, Boston, Massachusetts, has disclosed receiving a research grant from Boston Scientific (grant to examine the economics of stroke prevention).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity .
Robert A. Harrington, MD: I’m here in London at the European Society of Cardiology meetings, at theheart.org | Medscape Cardiology booth, using the meetings as an opportunity to meet with colleagues to talk about recent things that they’ve been writing about.
Today I’m joined by a good friend and colleague, Dr. Dhruv Kazi from Beth Israel Deaconess in Boston. Thanks for joining us.
Dhruv S. Kazi, MD, MS: Thank you for having me.
Harrington: Dr. Kazi is an associate professor of medicine at Harvard Medical School. He’s also the associate director of the Smith Center, which is an outcomes research center at the Beth Israel Deaconess. Thanks for joining us.
Kazi: Excited to be here.
Harrington: The topic I think you know that I want to discuss is a really important paper. There are two papers. They’re part of the American Heart Association’s 100th anniversary celebration, if you will. Many of the papers looked back at where science taken us.
With your coauthor, Karen Joynt Maddox, your papers are looking forward. They’re about the burden of cardiovascular disease in 2050. One paper really focused on what I would call the clinical and public health issues. Yours is focused on the economics. Is that a good description?
Kazi: Perfect.
Harrington: Tell us what you, Karen, and the other writers set out to do. What were you asked to do?
Kazi: As you know, the American Heart Association is entering its second century. Part of this was an exercise to say, where will the country be in 2050, which is a long enough time horizon for us to start planning for the future.
We looked back and said, if prior trends remain the same, where will we be in 2050, accounting for changes in demographics, changes in the composition of the population, and knowing that some of the cardiovascular risk factors are getting worse?
Harrington: For me, what was really striking is that, when I first saw the title and read “2050,” I thought, Oh, that’s a long way away. Then as I started reading it, I realized that this is not so far away.
Kazi: Absolutely.
Harrington: If we’re going to make a difference, it might take us 25 years.
Kazi: Especially if we set ourselves ambitious goals, we›re going to have to dig deep. Business-as-usual is not going to get us there.
Harrington: No. What I think has happened is we›ve spent so much time taking care of acute illness. Case fatality rates are fantastic. I was actually making the comment yesterday to a colleague that when I was an intern, the 30-day death rate from acute myocardial infarction was about 20%.
Kazi: Oh, wow.
Harrington: Now it’s 5%. That’s a big difference in a career.
Trends in the Wrong Direction
Kazi: There are fundamental trends. The decline in case fatalities is a really positive development, and I would hope that, going forward, that would continue. Those are risk-adjusted death rates and what is happening is that risk is going up. This is a function of the fact that the US population is aging; 2030 will be the first year that all the baby boomers will be over the age of 65.
By the mid-2030s, we’ll have more adults over the age of 65 than kids. That aging of the population is going to increase risk. The second is — and this is a positive development — we are a more diverse population, but the populations that are minoritized have higher cardiovascular risk, for a variety of reasons.
As the population of Asian Americans increases and doubles, in fact, as the population of Hispanic Americans doubles, we’re going to see an increase in risk related to cardiovascular disease. The third is that, over the past decade, there are some risk factors that are going in the wrong direction.
Harrington: Let’s talk about that because that’s humbling. I’m involved, as you know, with the American Heart Association, as are you. Despite all the work on Life’s Simple 7 and now Life’s Essential 8, we still have some issues.
Kazi: The big ones that come to mind are hypertension, diabetes, and obesity, all of which are trending in the wrong direction. Hypertension, we were gaining traction; and then over the past decade, we’ve slipped again. As you know, national blood pressure control rates have declined in many populations.
Harrington: Rather substantially.
Kazi: Substantially so, which has implications, in particular, for stroke rates in the future and stroke rates in young adults in the future. Obesity is a problem that we have very little control over. We’re already at 40% on average, which means that some populations are already in the 60% range.
Harrington: We also have obesity in kids — the burden, I’ll call it, of obesity. It’s not that you become obese in your thirties or your forties; you›re becoming obese as a teenager or even younger.
Kazi: Exactly. Since the 1990s, obesity in US adults has doubled, but obesity in US children has quadrupled. It’s starting from a lower base, but it’s very much an escalating problem.
Harrington: Diabetes is tightly linked to it but not totally explained.
Kazi: Exactly. The increase in diabetes is largely driven by obesity, but it›s probably also driven by changes in diet and lifestyle that don›t go through obesity.
Harrington: Yeah, it’s interesting. I think I have this figure correctly. It used to be rare that you saw a child with type 2 diabetes or what we call type 2 diabetes.
Kazi: Yeah.
Harrington: Now, the vast majority of kids with diabetes have type 2 diabetes.
Kazi: In the adolescents/young adults age group, most of it is type 2.
Harrington: Diabetes going up, obesity up, hypertension not well controlled, smoking combustible cigarettes way down.
Kazi: Yeah.
Harrington: Cholesterol levels. I was surprised. Cholesterol looked better. You said — because I was at a meeting where somebody asked you — that’s not explained by treatment.
Kazi: No, it’s not, at least going back to the ‘70s, but likely even sooner. I think that can only be attributed to substantial dietary changes. We are consuming less fat and less trans-fat. It’s possible that those collectively are improving our cholesterol levels, possibly at the expense of our glucose levels, because we basically substituted fats in our diet with more carbs at a population level.
Cigarettes and Vaping
Harrington: Some things certainly trend in the right direction but others in a really difficult direction. It’s going to lead to pretty large changes in risk for coronary disease, atrial fibrillation, and heart failure.
Kazi: I want to go back to the tobacco point. There are definitely marked declines in tobacco, still tightly related to income in the country. You see much higher prevalence of tobacco use in lower-income populations, but it’s unclear to me where it’s going in kids. We know that combustible tobacco use is going down but e-cigarettes went up. What that leads to over the next 30 years is unclear to me.
Harrington: That is a really important comment that’s worth sidebarring. The vaping use has been a terrible epidemic among our high schoolers. What is that going to lead to? Is it going to lead to the use of combustible cigarettes and we’re going to see that go back up? It remains to be seen.
Kazi: Yes, it remains to be seen. Going back to your point about this change in risk factors and this change in demographics, both aging and becoming a more diverse population means that we have large increases in some healthcare conditions.
Coronary heart disease goes up some, there›s a big jump in stroke — nearly a doubling in stroke — which is related to hypertension, obesity, an aging population, and a more diverse population. There are changes in stroke in the young, and atrial fibrillation related to, again, hypertension. We’re seeing these projections, and with them come these pretty large projections in changes in healthcare spending.
Healthcare Spending Not Sustainable
Harrington: Big. I mean, it’s not sustainable. Give the audience the number — it’s pretty frightening.
Kazi: We’re talking about a quadrupling of healthcare costs related to cardiovascular disease over 25 years. We’ve gotten used to the narrative that healthcare in the US is expensive and drugs are expensive, but this is an enormous problem — an unsustainable problem, like you called it.
It’s a doubling as a proportion of the economy. I was looking this up this morning. If the US healthcare economy were its own economy, it would be the fourth largest economy in the world.
Harrington: Healthcare as it is today, is it 21% of our economy?
Kazi: It’s 17% now. If it were its own economy, it would be the fourth largest in the world. We are spending more on healthcare than all but two other countries’ total economies. It’s kind of crazy.
Harrington: We’re talking about a quadrupling.
Kazi: Within that, the cardiovascular piece is a big piece, and we›re talking about a quadrupling.
Harrington: That’s both direct and indirect costs.
Kazi: The quadrupling of costs is just the direct costs. Indirect costs, for the listeners, refer to costs unrelated to healthcare but changes in productivity, either because people are disabled and unable to participate fully in the workforce or they die early.
The productivity costs are also increased substantially as a result. If you look at both healthcare and productivity, that goes up threefold. These are very large changes.
Harrington: Let’s now get to what we can do about it. I made the comment to you when I first read the papers that I was very depressed. Then, after I went through my Kübler-Ross stages of depression, death, and dying, I came to acceptance.
What are we going to do about it? This is a focus on policy, but also a focus on how we deliver healthcare, how we think about healthcare, and how we develop drugs and devices.
The drug question is going to be the one the audience is thinking about. They say, well, what about GLP-1 agonists? Aren’t those going to save the day?
Kazi: Yes and no. I’ll say that, early in my career, I used to be very attracted to simple solutions to complex problems. I’ve come to realize that simple solutions are elegant, attractive, and wrong. We›re dealing with a very complex issue and I think we’re going to need a multipronged approach.
The way I think about it is that there was a group of people who are at very high risk today. How do we help those individuals? Then how do we help the future generation so that they’re not dealing with the projections that we’re talking about.
My colleague, Karen Joynt Maddox, who led one of the papers, as you mentioned, has an elegant line in the paper where she says projections are not destiny. These are things we can change.
Harrington: If nothing changes, this is what it’s going to look like.
Kazi: This is where we’re headed.
Harrington: We can change. We’ve got some time to change, but we don’t have forever.
Kazi: Yes, exactly. We picked the 25-year timeline instead of a “let’s plan for the next century” timeline because we want something concrete and actionable. It’s close enough to be meaningful but far enough to give us the runway we need to act.
Harrington: Give me two things from the policy perspective, because it’s mostly policy.
Kazi: There are policy and clinical interventions. From the policy perspective, if I had to list two things, one is expansion of access to care. As we talk about this big increase in the burden of disease and risk factors, if you have a large proportion of your population that has hypertension or diabetes, you’re going to have to expand access to care to ensure that people get treated so they can get access to this care before they develop the complications that we worry about, like stroke and heart disease, that are very expensive to treat downstream.
The second, more broadly related to access to care, is the access to medications that are effective. You bring up GLP-1s. I think we need a real strategy for how we can give people access to GLP-1s at a price that is affordable to individuals but also affordable to the health system, and to help them stay on the drugs.
GLP-1s are transformative in what they do for weight loss and for diabetes, but more than 50% of people who start one are off it at 12 months. There’s something fundamentally wrong about how we’re delivering GLP-1s today. It’s not just about the cost of the drugs but the support system people need to stay on.
Harrington: I’ve made the comment, in many forms now, that we know the drugs work. We have to figure out how to use them.
Kazi: Exactly, yes.
Harrington: Using them includes chronicity. This is a chronic condition. Some people can come off the drugs, but many can’t. We’re going to have to figure this out, and maybe the newer generations of drugs will help us address what people call the off-ramping. How are we going to do that? I think you’re spot-on. Those are critically important questions.
Kazi: As we looked at this modeling, I’ll tell you — I had a come-to-Jesus moment where I was like, there is no way to fix cardiovascular disease in the US without going through obesity and diabetes. We have to address obesity in the US. We can’t just treat our way out of it. Obesity is fundamentally a food problem and we’ve got to engage again with food policy in a meaningful way.
Harrington: As you know, with the American Heart Association, we›re doing a large amount of work now on food as medicine and food is medicine. We are trying to figure out what the levers are that we can pull to actually help people eat healthier diets.
Kazi: Yes. Rather than framing it as an individual choice that people are eating poorly, it’s, how do we make healthy diets the default in the environment?
Harrington: This is where you get to the children as well.
Kazi: Exactly.
Harrington: I could talk about this all day. I’ve had the benefit of reading the papers now a few times and talking to you on several occasions. Thank you for joining us.
Kazi: Thank you.
Dr. Harrington, Stephen and Suzanne Weiss Dean, Weill Cornell Medicine; Provost for Medical Affairs, Cornell University, New York, NY, disclosed ties with Baim Institute (DSMB); CSL (RCT Executive Committee); Janssen (RCT Char), NHLBI (RCT Executive Committee, DSMB Chair); PCORI (RCT Co-Chair); DCRI, Atropos Health; Bitterroot Bio; Bristol Myers Squibb; BridgeBio; Element Science; Edwards Lifesciences; Foresite Labs; Medscape/WebMD Board of Directors for: American Heart Association; College of the Holy Cross; and Cytokinetics. Dr. Kazi, Associate Director, Smith Center for Outcomes Research, Associate Professor, Department of Medicine (Cardiology), Harvard Medical School, Director, Department of Cardiac Critical Care Unit, Beth Israel Deaconess Medical Center, Boston, Massachusetts, has disclosed receiving a research grant from Boston Scientific (grant to examine the economics of stroke prevention).
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity .
Robert A. Harrington, MD: I’m here in London at the European Society of Cardiology meetings, at theheart.org | Medscape Cardiology booth, using the meetings as an opportunity to meet with colleagues to talk about recent things that they’ve been writing about.
Today I’m joined by a good friend and colleague, Dr. Dhruv Kazi from Beth Israel Deaconess in Boston. Thanks for joining us.
Dhruv S. Kazi, MD, MS: Thank you for having me.
Harrington: Dr. Kazi is an associate professor of medicine at Harvard Medical School. He’s also the associate director of the Smith Center, which is an outcomes research center at the Beth Israel Deaconess. Thanks for joining us.
Kazi: Excited to be here.
Harrington: The topic I think you know that I want to discuss is a really important paper. There are two papers. They’re part of the American Heart Association’s 100th anniversary celebration, if you will. Many of the papers looked back at where science taken us.
With your coauthor, Karen Joynt Maddox, your papers are looking forward. They’re about the burden of cardiovascular disease in 2050. One paper really focused on what I would call the clinical and public health issues. Yours is focused on the economics. Is that a good description?
Kazi: Perfect.
Harrington: Tell us what you, Karen, and the other writers set out to do. What were you asked to do?
Kazi: As you know, the American Heart Association is entering its second century. Part of this was an exercise to say, where will the country be in 2050, which is a long enough time horizon for us to start planning for the future.
We looked back and said, if prior trends remain the same, where will we be in 2050, accounting for changes in demographics, changes in the composition of the population, and knowing that some of the cardiovascular risk factors are getting worse?
Harrington: For me, what was really striking is that, when I first saw the title and read “2050,” I thought, Oh, that’s a long way away. Then as I started reading it, I realized that this is not so far away.
Kazi: Absolutely.
Harrington: If we’re going to make a difference, it might take us 25 years.
Kazi: Especially if we set ourselves ambitious goals, we›re going to have to dig deep. Business-as-usual is not going to get us there.
Harrington: No. What I think has happened is we›ve spent so much time taking care of acute illness. Case fatality rates are fantastic. I was actually making the comment yesterday to a colleague that when I was an intern, the 30-day death rate from acute myocardial infarction was about 20%.
Kazi: Oh, wow.
Harrington: Now it’s 5%. That’s a big difference in a career.
Trends in the Wrong Direction
Kazi: There are fundamental trends. The decline in case fatalities is a really positive development, and I would hope that, going forward, that would continue. Those are risk-adjusted death rates and what is happening is that risk is going up. This is a function of the fact that the US population is aging; 2030 will be the first year that all the baby boomers will be over the age of 65.
By the mid-2030s, we’ll have more adults over the age of 65 than kids. That aging of the population is going to increase risk. The second is — and this is a positive development — we are a more diverse population, but the populations that are minoritized have higher cardiovascular risk, for a variety of reasons.
As the population of Asian Americans increases and doubles, in fact, as the population of Hispanic Americans doubles, we’re going to see an increase in risk related to cardiovascular disease. The third is that, over the past decade, there are some risk factors that are going in the wrong direction.
Harrington: Let’s talk about that because that’s humbling. I’m involved, as you know, with the American Heart Association, as are you. Despite all the work on Life’s Simple 7 and now Life’s Essential 8, we still have some issues.
Kazi: The big ones that come to mind are hypertension, diabetes, and obesity, all of which are trending in the wrong direction. Hypertension, we were gaining traction; and then over the past decade, we’ve slipped again. As you know, national blood pressure control rates have declined in many populations.
Harrington: Rather substantially.
Kazi: Substantially so, which has implications, in particular, for stroke rates in the future and stroke rates in young adults in the future. Obesity is a problem that we have very little control over. We’re already at 40% on average, which means that some populations are already in the 60% range.
Harrington: We also have obesity in kids — the burden, I’ll call it, of obesity. It’s not that you become obese in your thirties or your forties; you›re becoming obese as a teenager or even younger.
Kazi: Exactly. Since the 1990s, obesity in US adults has doubled, but obesity in US children has quadrupled. It’s starting from a lower base, but it’s very much an escalating problem.
Harrington: Diabetes is tightly linked to it but not totally explained.
Kazi: Exactly. The increase in diabetes is largely driven by obesity, but it›s probably also driven by changes in diet and lifestyle that don›t go through obesity.
Harrington: Yeah, it’s interesting. I think I have this figure correctly. It used to be rare that you saw a child with type 2 diabetes or what we call type 2 diabetes.
Kazi: Yeah.
Harrington: Now, the vast majority of kids with diabetes have type 2 diabetes.
Kazi: In the adolescents/young adults age group, most of it is type 2.
Harrington: Diabetes going up, obesity up, hypertension not well controlled, smoking combustible cigarettes way down.
Kazi: Yeah.
Harrington: Cholesterol levels. I was surprised. Cholesterol looked better. You said — because I was at a meeting where somebody asked you — that’s not explained by treatment.
Kazi: No, it’s not, at least going back to the ‘70s, but likely even sooner. I think that can only be attributed to substantial dietary changes. We are consuming less fat and less trans-fat. It’s possible that those collectively are improving our cholesterol levels, possibly at the expense of our glucose levels, because we basically substituted fats in our diet with more carbs at a population level.
Cigarettes and Vaping
Harrington: Some things certainly trend in the right direction but others in a really difficult direction. It’s going to lead to pretty large changes in risk for coronary disease, atrial fibrillation, and heart failure.
Kazi: I want to go back to the tobacco point. There are definitely marked declines in tobacco, still tightly related to income in the country. You see much higher prevalence of tobacco use in lower-income populations, but it’s unclear to me where it’s going in kids. We know that combustible tobacco use is going down but e-cigarettes went up. What that leads to over the next 30 years is unclear to me.
Harrington: That is a really important comment that’s worth sidebarring. The vaping use has been a terrible epidemic among our high schoolers. What is that going to lead to? Is it going to lead to the use of combustible cigarettes and we’re going to see that go back up? It remains to be seen.
Kazi: Yes, it remains to be seen. Going back to your point about this change in risk factors and this change in demographics, both aging and becoming a more diverse population means that we have large increases in some healthcare conditions.
Coronary heart disease goes up some, there›s a big jump in stroke — nearly a doubling in stroke — which is related to hypertension, obesity, an aging population, and a more diverse population. There are changes in stroke in the young, and atrial fibrillation related to, again, hypertension. We’re seeing these projections, and with them come these pretty large projections in changes in healthcare spending.
Healthcare Spending Not Sustainable
Harrington: Big. I mean, it’s not sustainable. Give the audience the number — it’s pretty frightening.
Kazi: We’re talking about a quadrupling of healthcare costs related to cardiovascular disease over 25 years. We’ve gotten used to the narrative that healthcare in the US is expensive and drugs are expensive, but this is an enormous problem — an unsustainable problem, like you called it.
It’s a doubling as a proportion of the economy. I was looking this up this morning. If the US healthcare economy were its own economy, it would be the fourth largest economy in the world.
Harrington: Healthcare as it is today, is it 21% of our economy?
Kazi: It’s 17% now. If it were its own economy, it would be the fourth largest in the world. We are spending more on healthcare than all but two other countries’ total economies. It’s kind of crazy.
Harrington: We’re talking about a quadrupling.
Kazi: Within that, the cardiovascular piece is a big piece, and we›re talking about a quadrupling.
Harrington: That’s both direct and indirect costs.
Kazi: The quadrupling of costs is just the direct costs. Indirect costs, for the listeners, refer to costs unrelated to healthcare but changes in productivity, either because people are disabled and unable to participate fully in the workforce or they die early.
The productivity costs are also increased substantially as a result. If you look at both healthcare and productivity, that goes up threefold. These are very large changes.
Harrington: Let’s now get to what we can do about it. I made the comment to you when I first read the papers that I was very depressed. Then, after I went through my Kübler-Ross stages of depression, death, and dying, I came to acceptance.
What are we going to do about it? This is a focus on policy, but also a focus on how we deliver healthcare, how we think about healthcare, and how we develop drugs and devices.
The drug question is going to be the one the audience is thinking about. They say, well, what about GLP-1 agonists? Aren’t those going to save the day?
Kazi: Yes and no. I’ll say that, early in my career, I used to be very attracted to simple solutions to complex problems. I’ve come to realize that simple solutions are elegant, attractive, and wrong. We›re dealing with a very complex issue and I think we’re going to need a multipronged approach.
The way I think about it is that there was a group of people who are at very high risk today. How do we help those individuals? Then how do we help the future generation so that they’re not dealing with the projections that we’re talking about.
My colleague, Karen Joynt Maddox, who led one of the papers, as you mentioned, has an elegant line in the paper where she says projections are not destiny. These are things we can change.
Harrington: If nothing changes, this is what it’s going to look like.
Kazi: This is where we’re headed.
Harrington: We can change. We’ve got some time to change, but we don’t have forever.
Kazi: Yes, exactly. We picked the 25-year timeline instead of a “let’s plan for the next century” timeline because we want something concrete and actionable. It’s close enough to be meaningful but far enough to give us the runway we need to act.
Harrington: Give me two things from the policy perspective, because it’s mostly policy.
Kazi: There are policy and clinical interventions. From the policy perspective, if I had to list two things, one is expansion of access to care. As we talk about this big increase in the burden of disease and risk factors, if you have a large proportion of your population that has hypertension or diabetes, you’re going to have to expand access to care to ensure that people get treated so they can get access to this care before they develop the complications that we worry about, like stroke and heart disease, that are very expensive to treat downstream.
The second, more broadly related to access to care, is the access to medications that are effective. You bring up GLP-1s. I think we need a real strategy for how we can give people access to GLP-1s at a price that is affordable to individuals but also affordable to the health system, and to help them stay on the drugs.
GLP-1s are transformative in what they do for weight loss and for diabetes, but more than 50% of people who start one are off it at 12 months. There’s something fundamentally wrong about how we’re delivering GLP-1s today. It’s not just about the cost of the drugs but the support system people need to stay on.
Harrington: I’ve made the comment, in many forms now, that we know the drugs work. We have to figure out how to use them.
Kazi: Exactly, yes.
Harrington: Using them includes chronicity. This is a chronic condition. Some people can come off the drugs, but many can’t. We’re going to have to figure this out, and maybe the newer generations of drugs will help us address what people call the off-ramping. How are we going to do that? I think you’re spot-on. Those are critically important questions.
Kazi: As we looked at this modeling, I’ll tell you — I had a come-to-Jesus moment where I was like, there is no way to fix cardiovascular disease in the US without going through obesity and diabetes. We have to address obesity in the US. We can’t just treat our way out of it. Obesity is fundamentally a food problem and we’ve got to engage again with food policy in a meaningful way.
Harrington: As you know, with the American Heart Association, we›re doing a large amount of work now on food as medicine and food is medicine. We are trying to figure out what the levers are that we can pull to actually help people eat healthier diets.
Kazi: Yes. Rather than framing it as an individual choice that people are eating poorly, it’s, how do we make healthy diets the default in the environment?
Harrington: This is where you get to the children as well.
Kazi: Exactly.
Harrington: I could talk about this all day. I’ve had the benefit of reading the papers now a few times and talking to you on several occasions. Thank you for joining us.
Kazi: Thank you.
Dr. Harrington, Stephen and Suzanne Weiss Dean, Weill Cornell Medicine; Provost for Medical Affairs, Cornell University, New York, NY, disclosed ties with Baim Institute (DSMB); CSL (RCT Executive Committee); Janssen (RCT Char), NHLBI (RCT Executive Committee, DSMB Chair); PCORI (RCT Co-Chair); DCRI, Atropos Health; Bitterroot Bio; Bristol Myers Squibb; BridgeBio; Element Science; Edwards Lifesciences; Foresite Labs; Medscape/WebMD Board of Directors for: American Heart Association; College of the Holy Cross; and Cytokinetics. Dr. Kazi, Associate Director, Smith Center for Outcomes Research, Associate Professor, Department of Medicine (Cardiology), Harvard Medical School, Director, Department of Cardiac Critical Care Unit, Beth Israel Deaconess Medical Center, Boston, Massachusetts, has disclosed receiving a research grant from Boston Scientific (grant to examine the economics of stroke prevention).
A version of this article appeared on Medscape.com.
ICD-10-CM Codes for CCCA, FFA Now Available
in the field of hair loss disorders.
“CCCA and FFA are conditions that require early diagnosis and intervention to prevent irreversible hair loss,” Maria Hordinsky, MD, professor of dermatology at the University of Minnesota, Minneapolis, and a member of the Board of Directors, Scarring Alopecia Foundation (SAF), said in an interview.
“The use of these new codes will make it easier for clinicians to identify affected patients and improve treatment outcomes. It also opens the door for more robust research efforts aimed at understanding the etiology and progression of CCCA and FFA, which could lead to new and more effective treatments in the future. Overall, this development represents a positive step toward improving care for individuals affected by these challenging conditions.”
The new codes — L66.81 for CCCA and L66.12 for FFA — were approved by the Centers for Disease Control and Prevention (CDC) on June 15, 2023, but not implemented until October 1, 2024.
Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, North Carolina, and a scientific advisor to SAF, told this news organization that Itisha Jefferson, a medical student at Loyola University Chicago’s Stritch School of Medicine, and her peers on the SAF’s Medical Student Executive Board, played a pivotal role in advocating for the codes.
In 2022, Jefferson, who has CCCA, and her fellow medical students helped create the proposals that were ultimately submitted to the CDC.
“They were critical in working with the CDC leaders to get the necessary information submitted and processed,” McMichael said. “They were also amazing at corralling our dermatologist group for the development of the necessary presentations and helped to shepherd us to the finish line for all logistic issues.”
On March 8, 2023, McMichael and Hordinsky made their pitch for the codes in person at the CDC’s ICD-10 Coordination and Maintenance Committee meeting, with McMichael discussing CCCA and Hordinsky discussing FFA.
“We also discussed the lack of standardized tracking, which has contributed to misdiagnoses and inadequate treatment options,” Hordinsky recalled. “We highlighted the importance of having distinct codes for these conditions to improve clinical outcomes, ensure that patients have access to appropriate care, better tracking of disease prevalence, and greater epidemiologic monitoring with access to electronic medical records and other large real-world evidence datasets and databases, the results of which could contribute to health policy decision-making.”
To spread the word about the new codes, McMichael, Hordinsky, and other members of the SAF are working with the original team of medical students, some of whom who are now dermatology residents, to develop an information guide to send to societies and organizations that were supportive of the codes. A publication in the dermatology literature is also planned.
For her part, Jefferson said that she will continue to advocate for patients with scarring alopecia as a medical student and when she becomes a physician. “I hope in the near future we will see an externally led FDA Patient-Focused Drug Development meeting for both CCCA and FFA, further advancing care and research for these conditions,” she said in an interview.
McMichael, Hordinsky, and Jefferson had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
in the field of hair loss disorders.
“CCCA and FFA are conditions that require early diagnosis and intervention to prevent irreversible hair loss,” Maria Hordinsky, MD, professor of dermatology at the University of Minnesota, Minneapolis, and a member of the Board of Directors, Scarring Alopecia Foundation (SAF), said in an interview.
“The use of these new codes will make it easier for clinicians to identify affected patients and improve treatment outcomes. It also opens the door for more robust research efforts aimed at understanding the etiology and progression of CCCA and FFA, which could lead to new and more effective treatments in the future. Overall, this development represents a positive step toward improving care for individuals affected by these challenging conditions.”
The new codes — L66.81 for CCCA and L66.12 for FFA — were approved by the Centers for Disease Control and Prevention (CDC) on June 15, 2023, but not implemented until October 1, 2024.
Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, North Carolina, and a scientific advisor to SAF, told this news organization that Itisha Jefferson, a medical student at Loyola University Chicago’s Stritch School of Medicine, and her peers on the SAF’s Medical Student Executive Board, played a pivotal role in advocating for the codes.
In 2022, Jefferson, who has CCCA, and her fellow medical students helped create the proposals that were ultimately submitted to the CDC.
“They were critical in working with the CDC leaders to get the necessary information submitted and processed,” McMichael said. “They were also amazing at corralling our dermatologist group for the development of the necessary presentations and helped to shepherd us to the finish line for all logistic issues.”
On March 8, 2023, McMichael and Hordinsky made their pitch for the codes in person at the CDC’s ICD-10 Coordination and Maintenance Committee meeting, with McMichael discussing CCCA and Hordinsky discussing FFA.
“We also discussed the lack of standardized tracking, which has contributed to misdiagnoses and inadequate treatment options,” Hordinsky recalled. “We highlighted the importance of having distinct codes for these conditions to improve clinical outcomes, ensure that patients have access to appropriate care, better tracking of disease prevalence, and greater epidemiologic monitoring with access to electronic medical records and other large real-world evidence datasets and databases, the results of which could contribute to health policy decision-making.”
To spread the word about the new codes, McMichael, Hordinsky, and other members of the SAF are working with the original team of medical students, some of whom who are now dermatology residents, to develop an information guide to send to societies and organizations that were supportive of the codes. A publication in the dermatology literature is also planned.
For her part, Jefferson said that she will continue to advocate for patients with scarring alopecia as a medical student and when she becomes a physician. “I hope in the near future we will see an externally led FDA Patient-Focused Drug Development meeting for both CCCA and FFA, further advancing care and research for these conditions,” she said in an interview.
McMichael, Hordinsky, and Jefferson had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
in the field of hair loss disorders.
“CCCA and FFA are conditions that require early diagnosis and intervention to prevent irreversible hair loss,” Maria Hordinsky, MD, professor of dermatology at the University of Minnesota, Minneapolis, and a member of the Board of Directors, Scarring Alopecia Foundation (SAF), said in an interview.
“The use of these new codes will make it easier for clinicians to identify affected patients and improve treatment outcomes. It also opens the door for more robust research efforts aimed at understanding the etiology and progression of CCCA and FFA, which could lead to new and more effective treatments in the future. Overall, this development represents a positive step toward improving care for individuals affected by these challenging conditions.”
The new codes — L66.81 for CCCA and L66.12 for FFA — were approved by the Centers for Disease Control and Prevention (CDC) on June 15, 2023, but not implemented until October 1, 2024.
Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, North Carolina, and a scientific advisor to SAF, told this news organization that Itisha Jefferson, a medical student at Loyola University Chicago’s Stritch School of Medicine, and her peers on the SAF’s Medical Student Executive Board, played a pivotal role in advocating for the codes.
In 2022, Jefferson, who has CCCA, and her fellow medical students helped create the proposals that were ultimately submitted to the CDC.
“They were critical in working with the CDC leaders to get the necessary information submitted and processed,” McMichael said. “They were also amazing at corralling our dermatologist group for the development of the necessary presentations and helped to shepherd us to the finish line for all logistic issues.”
On March 8, 2023, McMichael and Hordinsky made their pitch for the codes in person at the CDC’s ICD-10 Coordination and Maintenance Committee meeting, with McMichael discussing CCCA and Hordinsky discussing FFA.
“We also discussed the lack of standardized tracking, which has contributed to misdiagnoses and inadequate treatment options,” Hordinsky recalled. “We highlighted the importance of having distinct codes for these conditions to improve clinical outcomes, ensure that patients have access to appropriate care, better tracking of disease prevalence, and greater epidemiologic monitoring with access to electronic medical records and other large real-world evidence datasets and databases, the results of which could contribute to health policy decision-making.”
To spread the word about the new codes, McMichael, Hordinsky, and other members of the SAF are working with the original team of medical students, some of whom who are now dermatology residents, to develop an information guide to send to societies and organizations that were supportive of the codes. A publication in the dermatology literature is also planned.
For her part, Jefferson said that she will continue to advocate for patients with scarring alopecia as a medical student and when she becomes a physician. “I hope in the near future we will see an externally led FDA Patient-Focused Drug Development meeting for both CCCA and FFA, further advancing care and research for these conditions,” she said in an interview.
McMichael, Hordinsky, and Jefferson had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
Humans and Carbs: A Complicated 800,000-Year Relationship
Trying to reduce your carbohydrate intake means going against nearly a million years of evolution.
Humans are among a few species with multiple copies of certain genes that help us break down starch — carbs like potatoes, beans, corn, and grains — so that we can turn it into energy our bodies can use.
However, it’s been difficult for researchers to pinpoint when in human history we acquired multiple copies of these genes because they’re in a region of the genome that’s hard to sequence.
A recent study published in Science suggests that humans may have developed multiple copies of the gene for amylase — an enzyme that’s the first step in starch digestion — over 800,000 years ago, long before the agricultural revolution. This genetic change could have helped us adapt to eating starchy foods.
The study shows how “what your ancestors ate thousands of years ago could be affecting our genetics today,” said Kelsey Jorgensen, PhD, a biological anthropologist at The University of Kansas, Lawrence, who was not involved in the study.
The double-edged sword has sharpened over all those centuries. On one hand, the human body needs and craves carbs to function. On the other hand, our modern-day consumption of carbs, especially calorie-dense/nutritionally-barren processed carbs, has long since passed “healthy.”
How Researchers Found Our Carb-Lover Gene
The enzyme amylase turns complex carbs into maltose, a sweet-tasting sugar that is made of two glucose molecules linked together. We make two kinds of amylases: Salivary amylase that breaks down carbs in our mouths and pancreatic amylase that is secreted into our small intestines.
Modern humans have multiple copies of both amylases. Past research showed that human populations with diets high in starch can have up to nine copies of the gene for salivary amylase, called AMY1.
To pinpoint when in human history we acquired multiple copies of AMY1, the new study utilized novel techniques, called optical genome mapping and long-read sequencing, to sequence and analyze the genes. They sequenced 98 modern-day samples and 68 ancient DNA samples, including one from a Siberian person who lived 45,000 years ago.
The ancient DNA data in the study allowed the researchers to track how the number of amylase genes changed over time, said George Perry, PhD, an anthropological geneticist at The Pennsylvania State University-University Park (he was not involved in the study).
Based on the sequencing, the team analyzed changes in the genes in their samples to gauge evolutionary timelines. Perry noted that this was a “very clever approach to estimating the amylase copy number mutation rate, which in turn can really help in testing evolutionary hypotheses.”
The researchers found that even before farming, hunter-gatherers had between four and eight AMY1 genes in their cells. This suggests that people across Eurasia already had a number of these genes long before they started growing crops. (Recent research indicates that Neanderthals also ate starchy foods.)
“Even archaic hominins had these [genetic] variations and that indicates that they were consuming starch,” said Feyza Yilmaz, PhD, an associate computational scientist at The Jackson Laboratory in Bar Harbor, Maine, and a lead author of the study.
However, 4000 years ago, after the agricultural revolution, the research indicates that there were even more AMY1 copies acquired. Yilmaz noted, “with the advance of agriculture, we see an increase in high amylase copy number haplotypes. So genetic variation goes hand in hand with adaptation to the environment.”
A previous study showed that species that share an environment with humans, such as dogs and pigs, also have copy number variation of amylase genes, said Yilmaz, indicating a link between genome changes and an increase in starch consumption.
Potential Health Impacts on Modern Humans
The duplications in the AMY1 gene could have allowed humans to better digest starches. And it’s conceivable that having more copies of the gene means being able to break down starches even more efficiently, and those with more copies “may be more prone to having high blood sugar, prediabetes, that sort of thing,” Jorgensen said.
Whether those with more AMY1 genes have more health risks is an active area of research. “Researchers tested whether there’s a correlation between AMY1 gene copies and diabetes or BMI [body mass index]. And so far, some studies show that there is indeed correlation, but other studies show that there is no correlation at all,” said Yilmaz.
Yilmaz pointed out that only 5 or 10% of carb digestion happens in our mouths, the rest occurs in our small intestine, plus there are many other factors involved in eating and metabolism.
“I am really looking forward to seeing studies which truly figure out the connection between AMY1 copy number and metabolic health and also what type of factors play a role in metabolic health,” said Yilmaz.
It’s also possible that having more AMY1 copies could lead to more carb cravings as the enzyme creates a type of sugar in our mouths. “Previous studies show that there’s a correlation between AMY1 copy number and also the amylase enzyme levels, so the faster we process the starch, the taste [of starches] will be sweeter,” said Yilmaz.
However, the link between cravings and copy numbers isn’t clear. And we don’t exactly know what came first — did the starch in humans’ diet lead to more copies of amylase genes, or did the copies of the amylase genes drive cravings that lead us to cultivate more carbs? We’ll need more research to find out.
How Will Today’s Processed Carbs Affect Our Genes Tomorrow?
As our diet changes to increasingly include processed carbs, what will happen to our AMY1 genes is fuzzy. “I don’t know what this could do to our genomes in the next 1000 years or more than 1000 years,” Yilmaz noted, but she said from the evidence it seems as though we may have peaked in AMY1 copies.
Jorgensen noted that this research is focused on a European population. She wonders whether the pattern of AMY1 duplication will be repeated in other populations “because the rise of starch happened first in the Middle East and then Europe and then later in the Americas,” she said.
“There’s individual variation and then there’s population-wide variation,” Jorgensen pointed out. She speculates that the historical diet of different cultures could explain population-based variations in AMY1 genes — it’s something future research could investigate. Other populations may also experience genetic changes as much of the world shifts to a more carb-heavy Western diet.
Overall, this research adds to the growing evidence that humans have a long history of loving carbs — for better and, at least over our most recent history and immediate future, for worse.
A version of this article appeared on Medscape.com.
Trying to reduce your carbohydrate intake means going against nearly a million years of evolution.
Humans are among a few species with multiple copies of certain genes that help us break down starch — carbs like potatoes, beans, corn, and grains — so that we can turn it into energy our bodies can use.
However, it’s been difficult for researchers to pinpoint when in human history we acquired multiple copies of these genes because they’re in a region of the genome that’s hard to sequence.
A recent study published in Science suggests that humans may have developed multiple copies of the gene for amylase — an enzyme that’s the first step in starch digestion — over 800,000 years ago, long before the agricultural revolution. This genetic change could have helped us adapt to eating starchy foods.
The study shows how “what your ancestors ate thousands of years ago could be affecting our genetics today,” said Kelsey Jorgensen, PhD, a biological anthropologist at The University of Kansas, Lawrence, who was not involved in the study.
The double-edged sword has sharpened over all those centuries. On one hand, the human body needs and craves carbs to function. On the other hand, our modern-day consumption of carbs, especially calorie-dense/nutritionally-barren processed carbs, has long since passed “healthy.”
How Researchers Found Our Carb-Lover Gene
The enzyme amylase turns complex carbs into maltose, a sweet-tasting sugar that is made of two glucose molecules linked together. We make two kinds of amylases: Salivary amylase that breaks down carbs in our mouths and pancreatic amylase that is secreted into our small intestines.
Modern humans have multiple copies of both amylases. Past research showed that human populations with diets high in starch can have up to nine copies of the gene for salivary amylase, called AMY1.
To pinpoint when in human history we acquired multiple copies of AMY1, the new study utilized novel techniques, called optical genome mapping and long-read sequencing, to sequence and analyze the genes. They sequenced 98 modern-day samples and 68 ancient DNA samples, including one from a Siberian person who lived 45,000 years ago.
The ancient DNA data in the study allowed the researchers to track how the number of amylase genes changed over time, said George Perry, PhD, an anthropological geneticist at The Pennsylvania State University-University Park (he was not involved in the study).
Based on the sequencing, the team analyzed changes in the genes in their samples to gauge evolutionary timelines. Perry noted that this was a “very clever approach to estimating the amylase copy number mutation rate, which in turn can really help in testing evolutionary hypotheses.”
The researchers found that even before farming, hunter-gatherers had between four and eight AMY1 genes in their cells. This suggests that people across Eurasia already had a number of these genes long before they started growing crops. (Recent research indicates that Neanderthals also ate starchy foods.)
“Even archaic hominins had these [genetic] variations and that indicates that they were consuming starch,” said Feyza Yilmaz, PhD, an associate computational scientist at The Jackson Laboratory in Bar Harbor, Maine, and a lead author of the study.
However, 4000 years ago, after the agricultural revolution, the research indicates that there were even more AMY1 copies acquired. Yilmaz noted, “with the advance of agriculture, we see an increase in high amylase copy number haplotypes. So genetic variation goes hand in hand with adaptation to the environment.”
A previous study showed that species that share an environment with humans, such as dogs and pigs, also have copy number variation of amylase genes, said Yilmaz, indicating a link between genome changes and an increase in starch consumption.
Potential Health Impacts on Modern Humans
The duplications in the AMY1 gene could have allowed humans to better digest starches. And it’s conceivable that having more copies of the gene means being able to break down starches even more efficiently, and those with more copies “may be more prone to having high blood sugar, prediabetes, that sort of thing,” Jorgensen said.
Whether those with more AMY1 genes have more health risks is an active area of research. “Researchers tested whether there’s a correlation between AMY1 gene copies and diabetes or BMI [body mass index]. And so far, some studies show that there is indeed correlation, but other studies show that there is no correlation at all,” said Yilmaz.
Yilmaz pointed out that only 5 or 10% of carb digestion happens in our mouths, the rest occurs in our small intestine, plus there are many other factors involved in eating and metabolism.
“I am really looking forward to seeing studies which truly figure out the connection between AMY1 copy number and metabolic health and also what type of factors play a role in metabolic health,” said Yilmaz.
It’s also possible that having more AMY1 copies could lead to more carb cravings as the enzyme creates a type of sugar in our mouths. “Previous studies show that there’s a correlation between AMY1 copy number and also the amylase enzyme levels, so the faster we process the starch, the taste [of starches] will be sweeter,” said Yilmaz.
However, the link between cravings and copy numbers isn’t clear. And we don’t exactly know what came first — did the starch in humans’ diet lead to more copies of amylase genes, or did the copies of the amylase genes drive cravings that lead us to cultivate more carbs? We’ll need more research to find out.
How Will Today’s Processed Carbs Affect Our Genes Tomorrow?
As our diet changes to increasingly include processed carbs, what will happen to our AMY1 genes is fuzzy. “I don’t know what this could do to our genomes in the next 1000 years or more than 1000 years,” Yilmaz noted, but she said from the evidence it seems as though we may have peaked in AMY1 copies.
Jorgensen noted that this research is focused on a European population. She wonders whether the pattern of AMY1 duplication will be repeated in other populations “because the rise of starch happened first in the Middle East and then Europe and then later in the Americas,” she said.
“There’s individual variation and then there’s population-wide variation,” Jorgensen pointed out. She speculates that the historical diet of different cultures could explain population-based variations in AMY1 genes — it’s something future research could investigate. Other populations may also experience genetic changes as much of the world shifts to a more carb-heavy Western diet.
Overall, this research adds to the growing evidence that humans have a long history of loving carbs — for better and, at least over our most recent history and immediate future, for worse.
A version of this article appeared on Medscape.com.
Trying to reduce your carbohydrate intake means going against nearly a million years of evolution.
Humans are among a few species with multiple copies of certain genes that help us break down starch — carbs like potatoes, beans, corn, and grains — so that we can turn it into energy our bodies can use.
However, it’s been difficult for researchers to pinpoint when in human history we acquired multiple copies of these genes because they’re in a region of the genome that’s hard to sequence.
A recent study published in Science suggests that humans may have developed multiple copies of the gene for amylase — an enzyme that’s the first step in starch digestion — over 800,000 years ago, long before the agricultural revolution. This genetic change could have helped us adapt to eating starchy foods.
The study shows how “what your ancestors ate thousands of years ago could be affecting our genetics today,” said Kelsey Jorgensen, PhD, a biological anthropologist at The University of Kansas, Lawrence, who was not involved in the study.
The double-edged sword has sharpened over all those centuries. On one hand, the human body needs and craves carbs to function. On the other hand, our modern-day consumption of carbs, especially calorie-dense/nutritionally-barren processed carbs, has long since passed “healthy.”
How Researchers Found Our Carb-Lover Gene
The enzyme amylase turns complex carbs into maltose, a sweet-tasting sugar that is made of two glucose molecules linked together. We make two kinds of amylases: Salivary amylase that breaks down carbs in our mouths and pancreatic amylase that is secreted into our small intestines.
Modern humans have multiple copies of both amylases. Past research showed that human populations with diets high in starch can have up to nine copies of the gene for salivary amylase, called AMY1.
To pinpoint when in human history we acquired multiple copies of AMY1, the new study utilized novel techniques, called optical genome mapping and long-read sequencing, to sequence and analyze the genes. They sequenced 98 modern-day samples and 68 ancient DNA samples, including one from a Siberian person who lived 45,000 years ago.
The ancient DNA data in the study allowed the researchers to track how the number of amylase genes changed over time, said George Perry, PhD, an anthropological geneticist at The Pennsylvania State University-University Park (he was not involved in the study).
Based on the sequencing, the team analyzed changes in the genes in their samples to gauge evolutionary timelines. Perry noted that this was a “very clever approach to estimating the amylase copy number mutation rate, which in turn can really help in testing evolutionary hypotheses.”
The researchers found that even before farming, hunter-gatherers had between four and eight AMY1 genes in their cells. This suggests that people across Eurasia already had a number of these genes long before they started growing crops. (Recent research indicates that Neanderthals also ate starchy foods.)
“Even archaic hominins had these [genetic] variations and that indicates that they were consuming starch,” said Feyza Yilmaz, PhD, an associate computational scientist at The Jackson Laboratory in Bar Harbor, Maine, and a lead author of the study.
However, 4000 years ago, after the agricultural revolution, the research indicates that there were even more AMY1 copies acquired. Yilmaz noted, “with the advance of agriculture, we see an increase in high amylase copy number haplotypes. So genetic variation goes hand in hand with adaptation to the environment.”
A previous study showed that species that share an environment with humans, such as dogs and pigs, also have copy number variation of amylase genes, said Yilmaz, indicating a link between genome changes and an increase in starch consumption.
Potential Health Impacts on Modern Humans
The duplications in the AMY1 gene could have allowed humans to better digest starches. And it’s conceivable that having more copies of the gene means being able to break down starches even more efficiently, and those with more copies “may be more prone to having high blood sugar, prediabetes, that sort of thing,” Jorgensen said.
Whether those with more AMY1 genes have more health risks is an active area of research. “Researchers tested whether there’s a correlation between AMY1 gene copies and diabetes or BMI [body mass index]. And so far, some studies show that there is indeed correlation, but other studies show that there is no correlation at all,” said Yilmaz.
Yilmaz pointed out that only 5 or 10% of carb digestion happens in our mouths, the rest occurs in our small intestine, plus there are many other factors involved in eating and metabolism.
“I am really looking forward to seeing studies which truly figure out the connection between AMY1 copy number and metabolic health and also what type of factors play a role in metabolic health,” said Yilmaz.
It’s also possible that having more AMY1 copies could lead to more carb cravings as the enzyme creates a type of sugar in our mouths. “Previous studies show that there’s a correlation between AMY1 copy number and also the amylase enzyme levels, so the faster we process the starch, the taste [of starches] will be sweeter,” said Yilmaz.
However, the link between cravings and copy numbers isn’t clear. And we don’t exactly know what came first — did the starch in humans’ diet lead to more copies of amylase genes, or did the copies of the amylase genes drive cravings that lead us to cultivate more carbs? We’ll need more research to find out.
How Will Today’s Processed Carbs Affect Our Genes Tomorrow?
As our diet changes to increasingly include processed carbs, what will happen to our AMY1 genes is fuzzy. “I don’t know what this could do to our genomes in the next 1000 years or more than 1000 years,” Yilmaz noted, but she said from the evidence it seems as though we may have peaked in AMY1 copies.
Jorgensen noted that this research is focused on a European population. She wonders whether the pattern of AMY1 duplication will be repeated in other populations “because the rise of starch happened first in the Middle East and then Europe and then later in the Americas,” she said.
“There’s individual variation and then there’s population-wide variation,” Jorgensen pointed out. She speculates that the historical diet of different cultures could explain population-based variations in AMY1 genes — it’s something future research could investigate. Other populations may also experience genetic changes as much of the world shifts to a more carb-heavy Western diet.
Overall, this research adds to the growing evidence that humans have a long history of loving carbs — for better and, at least over our most recent history and immediate future, for worse.
A version of this article appeared on Medscape.com.
Six Tips for Media Interviews
As a physician, you might be contacted by the media to provide your professional opinion and advice. Or you might be looking for media interview opportunities to market your practice or side project. And if you do research, media interviews can be an effective way to spread the word. It’s important to prepare for a media interview so that you achieve the outcome you are looking for.
Keep your message simple. When you are a subject expert, you might think that the basics are obvious or even boring, and that the nuances are more important. However, most of the audience is looking for big-picture information that they can apply to their lives. Consider a few key takeaways, keeping in mind that your interview is likely to be edited to short sound bites or a few quotes. It may help to jot down notes so that you cover the fundamentals clearly. You could even write and rehearse a script beforehand. If there is something complicated or subtle that you want to convey, you can preface it by saying, “This is confusing but very important …” to let the audience know to give extra consideration to what you are about to say.
Avoid extremes and hyperbole. Sometimes, exaggerated statements make their way into medical discussions. Statements such as “it doesn’t matter how many calories you consume — it’s all about the quality” are common oversimplifications. But you might be upset to see your name next to a comment like this because it is not actually correct. Check the phrasing of your key takeaways to avoid being stuck defending or explaining an inaccurate statement when your patients ask you about it later.
Ask the interviewers what they are looking for. Many medical topics have some controversial element, so it is good to know what you’re getting into. Find out the purpose of the article or interview before you decide whether it is right for you. It could be about another doctor in town who is being sued; if you don’t want to be associated with that story, it might be best to decline the interview.
Explain your goals. You might accept or pursue an interview to raise awareness about an underrecognized condition. You might want the public to identify and get help for early symptoms, or you might want to create empathy for people coping with a disease you treat. Consider why you are participating in an interview, and communicate that to the interviewer to ensure that your objective can be part of the final product.
Know whom you’re dealing with. It is good to learn about the publication/media channel before you agree to participate. It may have a political bias, or perhaps the interview is intended to promote a specific product. If you agree with and support their purposes, then you may be happy to lend your opinion. But learning about the “voice” of the publication in advance allows you to make an informed decision about whether you want to be identified with a particular political ideology or product endorsement.
Ask to see your quotes before publication. It’s good to have the opportunity to make corrections in case you are accidentally misquoted or misunderstood. It is best to ask to see quotes before you agree to the interview. Some reporters may agree to (or even prefer) a written question-and-answer format so that they can directly quote your responses without rephrasing your words. You could suggest this, especially if you are too busy for a call or live meeting.
As a physician, your insights and advice can be highly beneficial to others. You can also use media interviews to propel your career forward. Doing your homework can ensure that you will be pleased with the final product and how your words were used.
Dr. Moawad, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
As a physician, you might be contacted by the media to provide your professional opinion and advice. Or you might be looking for media interview opportunities to market your practice or side project. And if you do research, media interviews can be an effective way to spread the word. It’s important to prepare for a media interview so that you achieve the outcome you are looking for.
Keep your message simple. When you are a subject expert, you might think that the basics are obvious or even boring, and that the nuances are more important. However, most of the audience is looking for big-picture information that they can apply to their lives. Consider a few key takeaways, keeping in mind that your interview is likely to be edited to short sound bites or a few quotes. It may help to jot down notes so that you cover the fundamentals clearly. You could even write and rehearse a script beforehand. If there is something complicated or subtle that you want to convey, you can preface it by saying, “This is confusing but very important …” to let the audience know to give extra consideration to what you are about to say.
Avoid extremes and hyperbole. Sometimes, exaggerated statements make their way into medical discussions. Statements such as “it doesn’t matter how many calories you consume — it’s all about the quality” are common oversimplifications. But you might be upset to see your name next to a comment like this because it is not actually correct. Check the phrasing of your key takeaways to avoid being stuck defending or explaining an inaccurate statement when your patients ask you about it later.
Ask the interviewers what they are looking for. Many medical topics have some controversial element, so it is good to know what you’re getting into. Find out the purpose of the article or interview before you decide whether it is right for you. It could be about another doctor in town who is being sued; if you don’t want to be associated with that story, it might be best to decline the interview.
Explain your goals. You might accept or pursue an interview to raise awareness about an underrecognized condition. You might want the public to identify and get help for early symptoms, or you might want to create empathy for people coping with a disease you treat. Consider why you are participating in an interview, and communicate that to the interviewer to ensure that your objective can be part of the final product.
Know whom you’re dealing with. It is good to learn about the publication/media channel before you agree to participate. It may have a political bias, or perhaps the interview is intended to promote a specific product. If you agree with and support their purposes, then you may be happy to lend your opinion. But learning about the “voice” of the publication in advance allows you to make an informed decision about whether you want to be identified with a particular political ideology or product endorsement.
Ask to see your quotes before publication. It’s good to have the opportunity to make corrections in case you are accidentally misquoted or misunderstood. It is best to ask to see quotes before you agree to the interview. Some reporters may agree to (or even prefer) a written question-and-answer format so that they can directly quote your responses without rephrasing your words. You could suggest this, especially if you are too busy for a call or live meeting.
As a physician, your insights and advice can be highly beneficial to others. You can also use media interviews to propel your career forward. Doing your homework can ensure that you will be pleased with the final product and how your words were used.
Dr. Moawad, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
As a physician, you might be contacted by the media to provide your professional opinion and advice. Or you might be looking for media interview opportunities to market your practice or side project. And if you do research, media interviews can be an effective way to spread the word. It’s important to prepare for a media interview so that you achieve the outcome you are looking for.
Keep your message simple. When you are a subject expert, you might think that the basics are obvious or even boring, and that the nuances are more important. However, most of the audience is looking for big-picture information that they can apply to their lives. Consider a few key takeaways, keeping in mind that your interview is likely to be edited to short sound bites or a few quotes. It may help to jot down notes so that you cover the fundamentals clearly. You could even write and rehearse a script beforehand. If there is something complicated or subtle that you want to convey, you can preface it by saying, “This is confusing but very important …” to let the audience know to give extra consideration to what you are about to say.
Avoid extremes and hyperbole. Sometimes, exaggerated statements make their way into medical discussions. Statements such as “it doesn’t matter how many calories you consume — it’s all about the quality” are common oversimplifications. But you might be upset to see your name next to a comment like this because it is not actually correct. Check the phrasing of your key takeaways to avoid being stuck defending or explaining an inaccurate statement when your patients ask you about it later.
Ask the interviewers what they are looking for. Many medical topics have some controversial element, so it is good to know what you’re getting into. Find out the purpose of the article or interview before you decide whether it is right for you. It could be about another doctor in town who is being sued; if you don’t want to be associated with that story, it might be best to decline the interview.
Explain your goals. You might accept or pursue an interview to raise awareness about an underrecognized condition. You might want the public to identify and get help for early symptoms, or you might want to create empathy for people coping with a disease you treat. Consider why you are participating in an interview, and communicate that to the interviewer to ensure that your objective can be part of the final product.
Know whom you’re dealing with. It is good to learn about the publication/media channel before you agree to participate. It may have a political bias, or perhaps the interview is intended to promote a specific product. If you agree with and support their purposes, then you may be happy to lend your opinion. But learning about the “voice” of the publication in advance allows you to make an informed decision about whether you want to be identified with a particular political ideology or product endorsement.
Ask to see your quotes before publication. It’s good to have the opportunity to make corrections in case you are accidentally misquoted or misunderstood. It is best to ask to see quotes before you agree to the interview. Some reporters may agree to (or even prefer) a written question-and-answer format so that they can directly quote your responses without rephrasing your words. You could suggest this, especially if you are too busy for a call or live meeting.
As a physician, your insights and advice can be highly beneficial to others. You can also use media interviews to propel your career forward. Doing your homework can ensure that you will be pleased with the final product and how your words were used.
Dr. Moawad, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio, has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.