User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Dramatic Increase in College Student Suicide Rates
TOPLINE:
, a new study by the National Collegiate Athletic Association (NCAA) found.
METHODOLOGY:
- Investigators analyzed deaths between 2002 and 2022, using Poisson regression models to assess changes in incidence rates over time.
- Data were drawn from the NCAA death database, which includes death from any cause, and included demographic characteristics such as age and race and sporting discipline.
- They utilized linear and quadratic fits between year and suicide incidence for men and women.
- Given the low incidence of suicide deaths per year, the incidence rate was multiplied by 100,000 to calculate the incidence per 100,000 athlete-years (AYs).
TAKEAWAY:
- Of 1102 total deaths, 11.6% were due to suicide (98 men, 30 women).
- Athletes who died by suicide ranged in age from 17 to 24 years (mean, 20 years) were predominantly men (77%) and White (59%), with the highest suicide incidence rate among male cross-country athletes (1:29 per 815 AYs).
- The overall incidence of suicide was 1:71 per 145 AYs.
- Over the last 10 years, suicide was the second most common cause of death after accidents, with the proportion of deaths by suicide doubling from the first to the second decades (7.6% to 15.3%).
- Among men, the suicide incidence rate increased in a linear fashion (5-year incidence rate ratio, 1.32; 95% CI, 1.14-1.53), while among women, a quadratic association was identified (P = .002), with the incidence rate reaching its lowest point in women from 2010 to 2011 and increasing thereafter.
IN PRACTICE:
“Athletes are generally thought of as one of the healthiest populations in our society, yet the pressures of school, internal and external performance expectations, time demands, injury, athletic identity, and physical fatigue can lead to depression, mental health problems, and suicide,” the authors wrote. “Although the rate of suicide among collegiate athletes remains lower than the general population, it is important to recognize the parallel increase to ensure this population is not overlooked when assessing for risk factors and implementing prevention strategies.”
SOURCE:
Bridget M. Whelan, MPH, research scientist in the Department of Family Medicine, Sports Medicine Section, University of Washington School of Medicine, Seattle, was the lead and corresponding author on the study, which was published online in the British Journal of Sports Medicine.
LIMITATIONS:
There is no mandatory reporting system for athlete deaths in the United States, and investigators’ search identified 16 deaths with unknown causes, suggesting reported suicide incidence rates may be underestimated. Additionally, in cases of overdose that were not clearly intentional, the death was listed as “overdose,” possibly resulting in underreporting of suicide.
DISCLOSURES:
No source of study funding was listed. The authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study by the National Collegiate Athletic Association (NCAA) found.
METHODOLOGY:
- Investigators analyzed deaths between 2002 and 2022, using Poisson regression models to assess changes in incidence rates over time.
- Data were drawn from the NCAA death database, which includes death from any cause, and included demographic characteristics such as age and race and sporting discipline.
- They utilized linear and quadratic fits between year and suicide incidence for men and women.
- Given the low incidence of suicide deaths per year, the incidence rate was multiplied by 100,000 to calculate the incidence per 100,000 athlete-years (AYs).
TAKEAWAY:
- Of 1102 total deaths, 11.6% were due to suicide (98 men, 30 women).
- Athletes who died by suicide ranged in age from 17 to 24 years (mean, 20 years) were predominantly men (77%) and White (59%), with the highest suicide incidence rate among male cross-country athletes (1:29 per 815 AYs).
- The overall incidence of suicide was 1:71 per 145 AYs.
- Over the last 10 years, suicide was the second most common cause of death after accidents, with the proportion of deaths by suicide doubling from the first to the second decades (7.6% to 15.3%).
- Among men, the suicide incidence rate increased in a linear fashion (5-year incidence rate ratio, 1.32; 95% CI, 1.14-1.53), while among women, a quadratic association was identified (P = .002), with the incidence rate reaching its lowest point in women from 2010 to 2011 and increasing thereafter.
IN PRACTICE:
“Athletes are generally thought of as one of the healthiest populations in our society, yet the pressures of school, internal and external performance expectations, time demands, injury, athletic identity, and physical fatigue can lead to depression, mental health problems, and suicide,” the authors wrote. “Although the rate of suicide among collegiate athletes remains lower than the general population, it is important to recognize the parallel increase to ensure this population is not overlooked when assessing for risk factors and implementing prevention strategies.”
SOURCE:
Bridget M. Whelan, MPH, research scientist in the Department of Family Medicine, Sports Medicine Section, University of Washington School of Medicine, Seattle, was the lead and corresponding author on the study, which was published online in the British Journal of Sports Medicine.
LIMITATIONS:
There is no mandatory reporting system for athlete deaths in the United States, and investigators’ search identified 16 deaths with unknown causes, suggesting reported suicide incidence rates may be underestimated. Additionally, in cases of overdose that were not clearly intentional, the death was listed as “overdose,” possibly resulting in underreporting of suicide.
DISCLOSURES:
No source of study funding was listed. The authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study by the National Collegiate Athletic Association (NCAA) found.
METHODOLOGY:
- Investigators analyzed deaths between 2002 and 2022, using Poisson regression models to assess changes in incidence rates over time.
- Data were drawn from the NCAA death database, which includes death from any cause, and included demographic characteristics such as age and race and sporting discipline.
- They utilized linear and quadratic fits between year and suicide incidence for men and women.
- Given the low incidence of suicide deaths per year, the incidence rate was multiplied by 100,000 to calculate the incidence per 100,000 athlete-years (AYs).
TAKEAWAY:
- Of 1102 total deaths, 11.6% were due to suicide (98 men, 30 women).
- Athletes who died by suicide ranged in age from 17 to 24 years (mean, 20 years) were predominantly men (77%) and White (59%), with the highest suicide incidence rate among male cross-country athletes (1:29 per 815 AYs).
- The overall incidence of suicide was 1:71 per 145 AYs.
- Over the last 10 years, suicide was the second most common cause of death after accidents, with the proportion of deaths by suicide doubling from the first to the second decades (7.6% to 15.3%).
- Among men, the suicide incidence rate increased in a linear fashion (5-year incidence rate ratio, 1.32; 95% CI, 1.14-1.53), while among women, a quadratic association was identified (P = .002), with the incidence rate reaching its lowest point in women from 2010 to 2011 and increasing thereafter.
IN PRACTICE:
“Athletes are generally thought of as one of the healthiest populations in our society, yet the pressures of school, internal and external performance expectations, time demands, injury, athletic identity, and physical fatigue can lead to depression, mental health problems, and suicide,” the authors wrote. “Although the rate of suicide among collegiate athletes remains lower than the general population, it is important to recognize the parallel increase to ensure this population is not overlooked when assessing for risk factors and implementing prevention strategies.”
SOURCE:
Bridget M. Whelan, MPH, research scientist in the Department of Family Medicine, Sports Medicine Section, University of Washington School of Medicine, Seattle, was the lead and corresponding author on the study, which was published online in the British Journal of Sports Medicine.
LIMITATIONS:
There is no mandatory reporting system for athlete deaths in the United States, and investigators’ search identified 16 deaths with unknown causes, suggesting reported suicide incidence rates may be underestimated. Additionally, in cases of overdose that were not clearly intentional, the death was listed as “overdose,” possibly resulting in underreporting of suicide.
DISCLOSURES:
No source of study funding was listed. The authors disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Girls Catching Up With Boys in Substance Use
, warned the authors of a new report detailing trends across several regions between 2018 and 2022. The latest 4-yearly Health Behaviour in School-Aged Children study, in collaboration with the World Health Organization (WHO) Regional Office for Europe, concluded that substance use remains “a crucial public health problem among adolescents” despite overall declines in smoking, alcohol, and cannabis use.
The new report: A focus on adolescent substance use in Europe, central Asia, and Canada, detailed substance use among adolescents aged 11, 13, and 15 years across 44 countries and regions in Europe, Central Asia, and Canada in the 2021-2022 school-based survey.
Principal findings included:
- Cigarette smoking: Lifetime smoking declined between 2018 and 2022, particularly among 13-year-old boys and 15-year-old boys and girls. There was also a small but significant decrease in current smoking among 15-year-old boys.
- Alcohol use: Lifetime use decreased overall in boys between 2018 and 2022, particularly among 15-year-olds. An increase was observed among 11- and 13-year-old girls but not 15-year-old girls. There was a small but significant decrease in the proportion of current drinkers among 15-year-old boys, with no change among 11- and 13-year-old boys. Current alcohol use increased among girls in all age groups.
- Cannabis use: Lifetime use among 15-year-olds decreased slightly from 14% to 12% between 2018 and 2022, while 6% of 15-year-olds reported having used cannabis in the previous 30 days.
- Vaping: In 2022 vapes (e-cigarettes) were more popular among adolescents than conventional tobacco cigarettes.
Traditional Gender Gap Narrowing or Reversing
Report coauthor Judith Brown from the University of Glasgow, Glasgow, Scotland, and a project manager for the Scottish survey, said that “there was an overall increase in current alcohol use and drunkenness among older girls” despite the overall decrease in boys’ alcohol use.
She explained: “Substance use has traditionally been more prevalent among boys, and the survey findings confirm a well-established gender difference, with higher prevalence in boys than in girls among 11-year-olds. By the age of 13, however, gender differences diminish or even disappear in many countries and regions.”
“Among 15-year-olds, girls often reported more frequent substance use than boys. While this pattern has been known for cigarette smoking in many countries and regions for about two decades, especially among 15-year-olds, it is a new phenomenon for behaviors related to other substances (such as alcohol consumption and drunkenness) in most countries and regions. Historically, prevalence for these behaviors has been higher among boys than girls.”
The new survey results highlight this gender reversal for several substances, she said. “Cannabis is the only substance for which both lifetime and current use is consistently higher in boys.”
Vaping Is an Emerging Public Health Concern
Dr. Brown added that the 2022 survey was the first time that vaping data had been collected from all countries. Although this is against the background of continuing decreases in smoking rates, “researchers suggest the transition to e-cigarettes, as a more popular choice than conventional cigarettes, highlights an urgent need for more targeted interventions to address this emerging public health concern.”
The report authors commented that because young people’s brains are still developing, they are “very sensitive to substances such as nicotine,” making it “easier for them to get hooked.”
Margreet de Looze, PhD, assistant professor of interdisciplinary social science at Utrecht University in Utrecht, the Netherlands, agreed with the authors’ concerns. “Vaping is extremely attractive for young people,” she said, “because the taste is more attractive than that of traditional cigarettes.” Until recently, many people were not aware of health hazards attached to vaping. “While more research is needed, vaping may function as a first step toward tobacco use and is hazardous for young people’s health. Therefore, it should be strongly discouraged.”
Substance Use Trends May Be Stabilizing or Rising Again
Increased awareness of the harmful effects of alcohol for adolescent development is also one postulated reason for declining adolescent alcohol consumption in both Europe and North America over the past two decades, which Dr. de Looze’s research has explored. Her work has also noted the “growing trend” of young people abstaining from alcohol altogether and some evidence of reductions in adolescent risk behaviors more generally, including early sexual initiation and juvenile crime.
“It may be good to realize that, in fact, the current generation of youth in many respects is healthier and reports less risky health behaviors as compared to previous generations,” she said.
However, “The declining trend in adolescent substance use that took place in many countries since the beginning of the 21st century seems to have stabilized, and moreover, in some countries and subgroups of adolescents, substance use appears to be on the rise again.” She cited particularly an overall increase in current alcohol use and drunkenness among older girls between 2018 and 2022. “It appears that, especially for girls, recent trends over time are less favorable as compared with boys.”
Multiple Influences on Adolescent Substance Abuse
Peer group influences are known to come to the fore during adolescence, and Dr. de Looze added that the early 21st century saw marked reductions in adolescent face-to-face contacts with their peers due to the rise in digital communications. “Adolescents typically use substances in the presence of peers (and in the absence of adults/parents), as it increases their status in their peer group.” Reduced in person interactions with friends may therefore have contributed to the earlier decline in substance use.
However, her team had found that adolescents who spend much time online with friends often also spend much time with friends offline. “They are what you could call the ‘social’ youth, who just spend much time with peers, be it offline or online,” she said. “More research is needed to disentangle exactly how, what kind, for whom the digital environment may be related to young people’s substance use,” she said.
“We also see that young people actively select their friends. So, if you are curious and a bit of a sensation-seeker yourself, you are more likely to become friends with youth who are just like you, and together, you may be more likely to try out substances.”
Factors underlying adolescent substance use and differences between countries are influenced by a complex interplay of factors, said Carina Ferreira-Borges, PhD, regional adviser for alcohol, illicit drugs, and prison health at the WHO Regional Office for Europe.
“Prevention measures definitely play a critical role in reducing substance use,” she said, “but other factors, such as cultural norms and socioeconomic conditions, also significantly impact these patterns.”
“Variations in substance use among countries can be attributed to different levels of implemented polices, public health initiatives, and the extent to which substance use is normalized or stigmatized within each society.”
Policy Efforts Must Be Targeted
“To address these disparities effectively, interventions and population-level policies need to be culturally adapted and target the specific environments where substance use is normalized among adolescents. By understanding and modifying the broader context in which young people make choices about substance use, we can better influence their behavior and health outcomes.”
Dr. de Looze cautioned, “In the past two decades, public health efforts in many countries have focused on reducing young people’s engagement in substance use. It is important that these efforts continue, as every year a new generation of youth is born. If public health efforts do not continue to focus on supporting a healthy lifestyle among young people, it should not come as a surprise that rates start or continue to rise again.”
A version of this article appeared on Medscape.com.
, warned the authors of a new report detailing trends across several regions between 2018 and 2022. The latest 4-yearly Health Behaviour in School-Aged Children study, in collaboration with the World Health Organization (WHO) Regional Office for Europe, concluded that substance use remains “a crucial public health problem among adolescents” despite overall declines in smoking, alcohol, and cannabis use.
The new report: A focus on adolescent substance use in Europe, central Asia, and Canada, detailed substance use among adolescents aged 11, 13, and 15 years across 44 countries and regions in Europe, Central Asia, and Canada in the 2021-2022 school-based survey.
Principal findings included:
- Cigarette smoking: Lifetime smoking declined between 2018 and 2022, particularly among 13-year-old boys and 15-year-old boys and girls. There was also a small but significant decrease in current smoking among 15-year-old boys.
- Alcohol use: Lifetime use decreased overall in boys between 2018 and 2022, particularly among 15-year-olds. An increase was observed among 11- and 13-year-old girls but not 15-year-old girls. There was a small but significant decrease in the proportion of current drinkers among 15-year-old boys, with no change among 11- and 13-year-old boys. Current alcohol use increased among girls in all age groups.
- Cannabis use: Lifetime use among 15-year-olds decreased slightly from 14% to 12% between 2018 and 2022, while 6% of 15-year-olds reported having used cannabis in the previous 30 days.
- Vaping: In 2022 vapes (e-cigarettes) were more popular among adolescents than conventional tobacco cigarettes.
Traditional Gender Gap Narrowing or Reversing
Report coauthor Judith Brown from the University of Glasgow, Glasgow, Scotland, and a project manager for the Scottish survey, said that “there was an overall increase in current alcohol use and drunkenness among older girls” despite the overall decrease in boys’ alcohol use.
She explained: “Substance use has traditionally been more prevalent among boys, and the survey findings confirm a well-established gender difference, with higher prevalence in boys than in girls among 11-year-olds. By the age of 13, however, gender differences diminish or even disappear in many countries and regions.”
“Among 15-year-olds, girls often reported more frequent substance use than boys. While this pattern has been known for cigarette smoking in many countries and regions for about two decades, especially among 15-year-olds, it is a new phenomenon for behaviors related to other substances (such as alcohol consumption and drunkenness) in most countries and regions. Historically, prevalence for these behaviors has been higher among boys than girls.”
The new survey results highlight this gender reversal for several substances, she said. “Cannabis is the only substance for which both lifetime and current use is consistently higher in boys.”
Vaping Is an Emerging Public Health Concern
Dr. Brown added that the 2022 survey was the first time that vaping data had been collected from all countries. Although this is against the background of continuing decreases in smoking rates, “researchers suggest the transition to e-cigarettes, as a more popular choice than conventional cigarettes, highlights an urgent need for more targeted interventions to address this emerging public health concern.”
The report authors commented that because young people’s brains are still developing, they are “very sensitive to substances such as nicotine,” making it “easier for them to get hooked.”
Margreet de Looze, PhD, assistant professor of interdisciplinary social science at Utrecht University in Utrecht, the Netherlands, agreed with the authors’ concerns. “Vaping is extremely attractive for young people,” she said, “because the taste is more attractive than that of traditional cigarettes.” Until recently, many people were not aware of health hazards attached to vaping. “While more research is needed, vaping may function as a first step toward tobacco use and is hazardous for young people’s health. Therefore, it should be strongly discouraged.”
Substance Use Trends May Be Stabilizing or Rising Again
Increased awareness of the harmful effects of alcohol for adolescent development is also one postulated reason for declining adolescent alcohol consumption in both Europe and North America over the past two decades, which Dr. de Looze’s research has explored. Her work has also noted the “growing trend” of young people abstaining from alcohol altogether and some evidence of reductions in adolescent risk behaviors more generally, including early sexual initiation and juvenile crime.
“It may be good to realize that, in fact, the current generation of youth in many respects is healthier and reports less risky health behaviors as compared to previous generations,” she said.
However, “The declining trend in adolescent substance use that took place in many countries since the beginning of the 21st century seems to have stabilized, and moreover, in some countries and subgroups of adolescents, substance use appears to be on the rise again.” She cited particularly an overall increase in current alcohol use and drunkenness among older girls between 2018 and 2022. “It appears that, especially for girls, recent trends over time are less favorable as compared with boys.”
Multiple Influences on Adolescent Substance Abuse
Peer group influences are known to come to the fore during adolescence, and Dr. de Looze added that the early 21st century saw marked reductions in adolescent face-to-face contacts with their peers due to the rise in digital communications. “Adolescents typically use substances in the presence of peers (and in the absence of adults/parents), as it increases their status in their peer group.” Reduced in person interactions with friends may therefore have contributed to the earlier decline in substance use.
However, her team had found that adolescents who spend much time online with friends often also spend much time with friends offline. “They are what you could call the ‘social’ youth, who just spend much time with peers, be it offline or online,” she said. “More research is needed to disentangle exactly how, what kind, for whom the digital environment may be related to young people’s substance use,” she said.
“We also see that young people actively select their friends. So, if you are curious and a bit of a sensation-seeker yourself, you are more likely to become friends with youth who are just like you, and together, you may be more likely to try out substances.”
Factors underlying adolescent substance use and differences between countries are influenced by a complex interplay of factors, said Carina Ferreira-Borges, PhD, regional adviser for alcohol, illicit drugs, and prison health at the WHO Regional Office for Europe.
“Prevention measures definitely play a critical role in reducing substance use,” she said, “but other factors, such as cultural norms and socioeconomic conditions, also significantly impact these patterns.”
“Variations in substance use among countries can be attributed to different levels of implemented polices, public health initiatives, and the extent to which substance use is normalized or stigmatized within each society.”
Policy Efforts Must Be Targeted
“To address these disparities effectively, interventions and population-level policies need to be culturally adapted and target the specific environments where substance use is normalized among adolescents. By understanding and modifying the broader context in which young people make choices about substance use, we can better influence their behavior and health outcomes.”
Dr. de Looze cautioned, “In the past two decades, public health efforts in many countries have focused on reducing young people’s engagement in substance use. It is important that these efforts continue, as every year a new generation of youth is born. If public health efforts do not continue to focus on supporting a healthy lifestyle among young people, it should not come as a surprise that rates start or continue to rise again.”
A version of this article appeared on Medscape.com.
, warned the authors of a new report detailing trends across several regions between 2018 and 2022. The latest 4-yearly Health Behaviour in School-Aged Children study, in collaboration with the World Health Organization (WHO) Regional Office for Europe, concluded that substance use remains “a crucial public health problem among adolescents” despite overall declines in smoking, alcohol, and cannabis use.
The new report: A focus on adolescent substance use in Europe, central Asia, and Canada, detailed substance use among adolescents aged 11, 13, and 15 years across 44 countries and regions in Europe, Central Asia, and Canada in the 2021-2022 school-based survey.
Principal findings included:
- Cigarette smoking: Lifetime smoking declined between 2018 and 2022, particularly among 13-year-old boys and 15-year-old boys and girls. There was also a small but significant decrease in current smoking among 15-year-old boys.
- Alcohol use: Lifetime use decreased overall in boys between 2018 and 2022, particularly among 15-year-olds. An increase was observed among 11- and 13-year-old girls but not 15-year-old girls. There was a small but significant decrease in the proportion of current drinkers among 15-year-old boys, with no change among 11- and 13-year-old boys. Current alcohol use increased among girls in all age groups.
- Cannabis use: Lifetime use among 15-year-olds decreased slightly from 14% to 12% between 2018 and 2022, while 6% of 15-year-olds reported having used cannabis in the previous 30 days.
- Vaping: In 2022 vapes (e-cigarettes) were more popular among adolescents than conventional tobacco cigarettes.
Traditional Gender Gap Narrowing or Reversing
Report coauthor Judith Brown from the University of Glasgow, Glasgow, Scotland, and a project manager for the Scottish survey, said that “there was an overall increase in current alcohol use and drunkenness among older girls” despite the overall decrease in boys’ alcohol use.
She explained: “Substance use has traditionally been more prevalent among boys, and the survey findings confirm a well-established gender difference, with higher prevalence in boys than in girls among 11-year-olds. By the age of 13, however, gender differences diminish or even disappear in many countries and regions.”
“Among 15-year-olds, girls often reported more frequent substance use than boys. While this pattern has been known for cigarette smoking in many countries and regions for about two decades, especially among 15-year-olds, it is a new phenomenon for behaviors related to other substances (such as alcohol consumption and drunkenness) in most countries and regions. Historically, prevalence for these behaviors has been higher among boys than girls.”
The new survey results highlight this gender reversal for several substances, she said. “Cannabis is the only substance for which both lifetime and current use is consistently higher in boys.”
Vaping Is an Emerging Public Health Concern
Dr. Brown added that the 2022 survey was the first time that vaping data had been collected from all countries. Although this is against the background of continuing decreases in smoking rates, “researchers suggest the transition to e-cigarettes, as a more popular choice than conventional cigarettes, highlights an urgent need for more targeted interventions to address this emerging public health concern.”
The report authors commented that because young people’s brains are still developing, they are “very sensitive to substances such as nicotine,” making it “easier for them to get hooked.”
Margreet de Looze, PhD, assistant professor of interdisciplinary social science at Utrecht University in Utrecht, the Netherlands, agreed with the authors’ concerns. “Vaping is extremely attractive for young people,” she said, “because the taste is more attractive than that of traditional cigarettes.” Until recently, many people were not aware of health hazards attached to vaping. “While more research is needed, vaping may function as a first step toward tobacco use and is hazardous for young people’s health. Therefore, it should be strongly discouraged.”
Substance Use Trends May Be Stabilizing or Rising Again
Increased awareness of the harmful effects of alcohol for adolescent development is also one postulated reason for declining adolescent alcohol consumption in both Europe and North America over the past two decades, which Dr. de Looze’s research has explored. Her work has also noted the “growing trend” of young people abstaining from alcohol altogether and some evidence of reductions in adolescent risk behaviors more generally, including early sexual initiation and juvenile crime.
“It may be good to realize that, in fact, the current generation of youth in many respects is healthier and reports less risky health behaviors as compared to previous generations,” she said.
However, “The declining trend in adolescent substance use that took place in many countries since the beginning of the 21st century seems to have stabilized, and moreover, in some countries and subgroups of adolescents, substance use appears to be on the rise again.” She cited particularly an overall increase in current alcohol use and drunkenness among older girls between 2018 and 2022. “It appears that, especially for girls, recent trends over time are less favorable as compared with boys.”
Multiple Influences on Adolescent Substance Abuse
Peer group influences are known to come to the fore during adolescence, and Dr. de Looze added that the early 21st century saw marked reductions in adolescent face-to-face contacts with their peers due to the rise in digital communications. “Adolescents typically use substances in the presence of peers (and in the absence of adults/parents), as it increases their status in their peer group.” Reduced in person interactions with friends may therefore have contributed to the earlier decline in substance use.
However, her team had found that adolescents who spend much time online with friends often also spend much time with friends offline. “They are what you could call the ‘social’ youth, who just spend much time with peers, be it offline or online,” she said. “More research is needed to disentangle exactly how, what kind, for whom the digital environment may be related to young people’s substance use,” she said.
“We also see that young people actively select their friends. So, if you are curious and a bit of a sensation-seeker yourself, you are more likely to become friends with youth who are just like you, and together, you may be more likely to try out substances.”
Factors underlying adolescent substance use and differences between countries are influenced by a complex interplay of factors, said Carina Ferreira-Borges, PhD, regional adviser for alcohol, illicit drugs, and prison health at the WHO Regional Office for Europe.
“Prevention measures definitely play a critical role in reducing substance use,” she said, “but other factors, such as cultural norms and socioeconomic conditions, also significantly impact these patterns.”
“Variations in substance use among countries can be attributed to different levels of implemented polices, public health initiatives, and the extent to which substance use is normalized or stigmatized within each society.”
Policy Efforts Must Be Targeted
“To address these disparities effectively, interventions and population-level policies need to be culturally adapted and target the specific environments where substance use is normalized among adolescents. By understanding and modifying the broader context in which young people make choices about substance use, we can better influence their behavior and health outcomes.”
Dr. de Looze cautioned, “In the past two decades, public health efforts in many countries have focused on reducing young people’s engagement in substance use. It is important that these efforts continue, as every year a new generation of youth is born. If public health efforts do not continue to focus on supporting a healthy lifestyle among young people, it should not come as a surprise that rates start or continue to rise again.”
A version of this article appeared on Medscape.com.
Antidepressants and Dementia Risk: Reassuring Data
TOPLINE:
, new research suggests.
METHODOLOGY:
- Investigators studied 5511 individuals (58% women; mean age, 71 years) from the Rotterdam study, an ongoing prospective population-based cohort study.
- Participants were free from dementia at baseline, and incident dementia was monitored from baseline until 2018 with repeated cognitive assessments using the Mini-Mental Status Examination (MMSE) and the Geriatric Mental Schedule, as well as MRIs.
- Information on participants’ antidepressant use was extracted from pharmacy records from 1992 until baseline (2002-2008).
- During a mean follow-up of 10 years, 12% of participants developed dementia.
TAKEAWAY:
- Overall, 17% of participants had used antidepressants during the roughly 10-year period prior to baseline, and 4.1% were still using antidepressants at baseline.
- Medication use at baseline was more common in women than in men (21% vs 18%), and use increased with age: From 2.1% in participants aged between 45 and 50 years to 4.5% in those older than 80 years.
- After adjustment for confounders, there was no association between antidepressant use and dementia risk (hazard ratio [HR], 1.14; 95% CI, 0.92-1.41), accelerated cognitive decline, or atrophy of white and gray matter.
- However, tricyclic antidepressant use was associated with increased dementia risk (HR, 1.36; 95% CI, 1.01-1.83) compared with the use of selective serotonin reuptake inhibitors (HR, 1.12; 95% CI, 0.81-1.54).
IN PRACTICE:
“Although prescription of antidepressant medication in older individuals, in particular those with some cognitive impairment, may have acute symptomatic anticholinergic effects that warrant consideration in clinical practice, our results show that long-term antidepressant use does not have lasting effects on cognition or brain health in older adults without indication of cognitive impairment,” the authors wrote.
SOURCE:
Frank J. Wolters, MD, of the Department of Epidemiology and the Department of Radiology and Nuclear Medicine and Alzheimer Center, Erasmus University Medical Center, Rotterdam, the Netherlands, was the senior author on this study that was published online in Alzheimer’s and Dementia.
LIMITATIONS:
Limitations included the concern that although exclusion of participants with MMSE < 26 at baseline prevented reversed causation (ie, antidepressant use in response to depression during the prodromal phase of dementia), it may have introduced selection bias by disregarding the effects of antidepressant use prior to baseline and excluding participants with lower education.
DISCLOSURES:
This study was conducted as part of the Netherlands Consortium of Dementia Cohorts, which receives funding in the context of Deltaplan Dementie from ZonMW Memorabel and Alzheimer Nederland. Further funding was also obtained from the Stichting Erasmus Trustfonds. This study was further supported by a 2020 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. The authors reported no conflicts of interest or relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, new research suggests.
METHODOLOGY:
- Investigators studied 5511 individuals (58% women; mean age, 71 years) from the Rotterdam study, an ongoing prospective population-based cohort study.
- Participants were free from dementia at baseline, and incident dementia was monitored from baseline until 2018 with repeated cognitive assessments using the Mini-Mental Status Examination (MMSE) and the Geriatric Mental Schedule, as well as MRIs.
- Information on participants’ antidepressant use was extracted from pharmacy records from 1992 until baseline (2002-2008).
- During a mean follow-up of 10 years, 12% of participants developed dementia.
TAKEAWAY:
- Overall, 17% of participants had used antidepressants during the roughly 10-year period prior to baseline, and 4.1% were still using antidepressants at baseline.
- Medication use at baseline was more common in women than in men (21% vs 18%), and use increased with age: From 2.1% in participants aged between 45 and 50 years to 4.5% in those older than 80 years.
- After adjustment for confounders, there was no association between antidepressant use and dementia risk (hazard ratio [HR], 1.14; 95% CI, 0.92-1.41), accelerated cognitive decline, or atrophy of white and gray matter.
- However, tricyclic antidepressant use was associated with increased dementia risk (HR, 1.36; 95% CI, 1.01-1.83) compared with the use of selective serotonin reuptake inhibitors (HR, 1.12; 95% CI, 0.81-1.54).
IN PRACTICE:
“Although prescription of antidepressant medication in older individuals, in particular those with some cognitive impairment, may have acute symptomatic anticholinergic effects that warrant consideration in clinical practice, our results show that long-term antidepressant use does not have lasting effects on cognition or brain health in older adults without indication of cognitive impairment,” the authors wrote.
SOURCE:
Frank J. Wolters, MD, of the Department of Epidemiology and the Department of Radiology and Nuclear Medicine and Alzheimer Center, Erasmus University Medical Center, Rotterdam, the Netherlands, was the senior author on this study that was published online in Alzheimer’s and Dementia.
LIMITATIONS:
Limitations included the concern that although exclusion of participants with MMSE < 26 at baseline prevented reversed causation (ie, antidepressant use in response to depression during the prodromal phase of dementia), it may have introduced selection bias by disregarding the effects of antidepressant use prior to baseline and excluding participants with lower education.
DISCLOSURES:
This study was conducted as part of the Netherlands Consortium of Dementia Cohorts, which receives funding in the context of Deltaplan Dementie from ZonMW Memorabel and Alzheimer Nederland. Further funding was also obtained from the Stichting Erasmus Trustfonds. This study was further supported by a 2020 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. The authors reported no conflicts of interest or relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, new research suggests.
METHODOLOGY:
- Investigators studied 5511 individuals (58% women; mean age, 71 years) from the Rotterdam study, an ongoing prospective population-based cohort study.
- Participants were free from dementia at baseline, and incident dementia was monitored from baseline until 2018 with repeated cognitive assessments using the Mini-Mental Status Examination (MMSE) and the Geriatric Mental Schedule, as well as MRIs.
- Information on participants’ antidepressant use was extracted from pharmacy records from 1992 until baseline (2002-2008).
- During a mean follow-up of 10 years, 12% of participants developed dementia.
TAKEAWAY:
- Overall, 17% of participants had used antidepressants during the roughly 10-year period prior to baseline, and 4.1% were still using antidepressants at baseline.
- Medication use at baseline was more common in women than in men (21% vs 18%), and use increased with age: From 2.1% in participants aged between 45 and 50 years to 4.5% in those older than 80 years.
- After adjustment for confounders, there was no association between antidepressant use and dementia risk (hazard ratio [HR], 1.14; 95% CI, 0.92-1.41), accelerated cognitive decline, or atrophy of white and gray matter.
- However, tricyclic antidepressant use was associated with increased dementia risk (HR, 1.36; 95% CI, 1.01-1.83) compared with the use of selective serotonin reuptake inhibitors (HR, 1.12; 95% CI, 0.81-1.54).
IN PRACTICE:
“Although prescription of antidepressant medication in older individuals, in particular those with some cognitive impairment, may have acute symptomatic anticholinergic effects that warrant consideration in clinical practice, our results show that long-term antidepressant use does not have lasting effects on cognition or brain health in older adults without indication of cognitive impairment,” the authors wrote.
SOURCE:
Frank J. Wolters, MD, of the Department of Epidemiology and the Department of Radiology and Nuclear Medicine and Alzheimer Center, Erasmus University Medical Center, Rotterdam, the Netherlands, was the senior author on this study that was published online in Alzheimer’s and Dementia.
LIMITATIONS:
Limitations included the concern that although exclusion of participants with MMSE < 26 at baseline prevented reversed causation (ie, antidepressant use in response to depression during the prodromal phase of dementia), it may have introduced selection bias by disregarding the effects of antidepressant use prior to baseline and excluding participants with lower education.
DISCLOSURES:
This study was conducted as part of the Netherlands Consortium of Dementia Cohorts, which receives funding in the context of Deltaplan Dementie from ZonMW Memorabel and Alzheimer Nederland. Further funding was also obtained from the Stichting Erasmus Trustfonds. This study was further supported by a 2020 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. The authors reported no conflicts of interest or relevant financial relationships.
A version of this article appeared on Medscape.com.
Mandatory DMV Reporting Tied to Dementia Underdiagnosis
, new research suggests.
Investigators found that primary care physicians (PCPs) in states with clinician reporting mandates had a 59% higher probability of underdiagnosing dementia compared with their counterparts in states that require patients to self-report or that have no reporting mandates.
“Our findings in this cross-sectional study raise concerns about potential adverse effects of mandatory clinician reporting for dementia diagnosis and underscore the need for careful consideration of the effect of such policies,” wrote the investigators, led by Soeren Mattke, MD, DSc, director of the USC Brain Health Observatory and research professor of economics at the University of Southern California, Los Angeles.
The study was published online in JAMA Network Open.
Lack of Guidance
As the US population ages, the number of older drivers is increasing, with 55.8 million drivers 65 years old or older. Approximately 7 million people in this age group have dementia — an estimate that is expected to increase to nearly 12 million by 2040.
The aging population raises a “critical policy question” about how to ensure road safety. Although the American Medical Association’s Code of Ethics outlines a physician’s obligation to identify drivers with medical impairments that impede safe driving, guidance restricting cognitively impaired drivers from driving is lacking.
In addition, evidence as to whether cognitive impairment indeed poses a threat to driving safety is mixed and has led to a lack of uniform policies with respect to reporting dementia.
Four states explicitly require clinicians to report dementia diagnoses to the DMV, which will then determine the patient’s fitness to drive, whereas 14 states require people with dementia to self-report. The remaining states have no explicit reporting requirements.
The issue of mandatory reporting is controversial, the researchers noted. On the one hand, physicians could protect patients and others by reporting potentially unsafe drivers.
On the other hand, evidence of an association with lower accident risks in patients with dementia is sparse and mandatory reporting may adversely affect physician-patient relationships. Empirical evidence for unintended consequences of reporting laws is lacking.
To examine the potential link between dementia underdiagnosis and mandatory reporting policies, the investigators analyzed the 100% data from the Medicare fee-for-service program and Medicare Advantage plans from 2017 to 2019, which included 223,036 PCPs with a panel of 25 or more Medicare patients.
The researchers examined dementia diagnosis rates in the patient panel of PCPs, rather than neurologists or gerontologists, regardless of who documented the diagnosis. Dr. Mattke said that it is possible that the diagnosis was established after referral to a specialist.
Each physician’s expected number of dementia cases was estimated using a predictive model based on patient characteristics. The researchers then compared the estimate with observed dementia diagnoses, thereby identifying clinicians who underdiagnosed dementia after sampling errors were accounted for.
‘Heavy-Handed Interference’
The researchers adjusted for several covariates potentially associated with a clinician’s probability of underdiagnosing dementia. These included sex, office location, practice specialty, racial/ethnic composition of the patient panel, and percentage of patients dually eligible for Medicare and Medicaid. The table shows PCP characteristics.
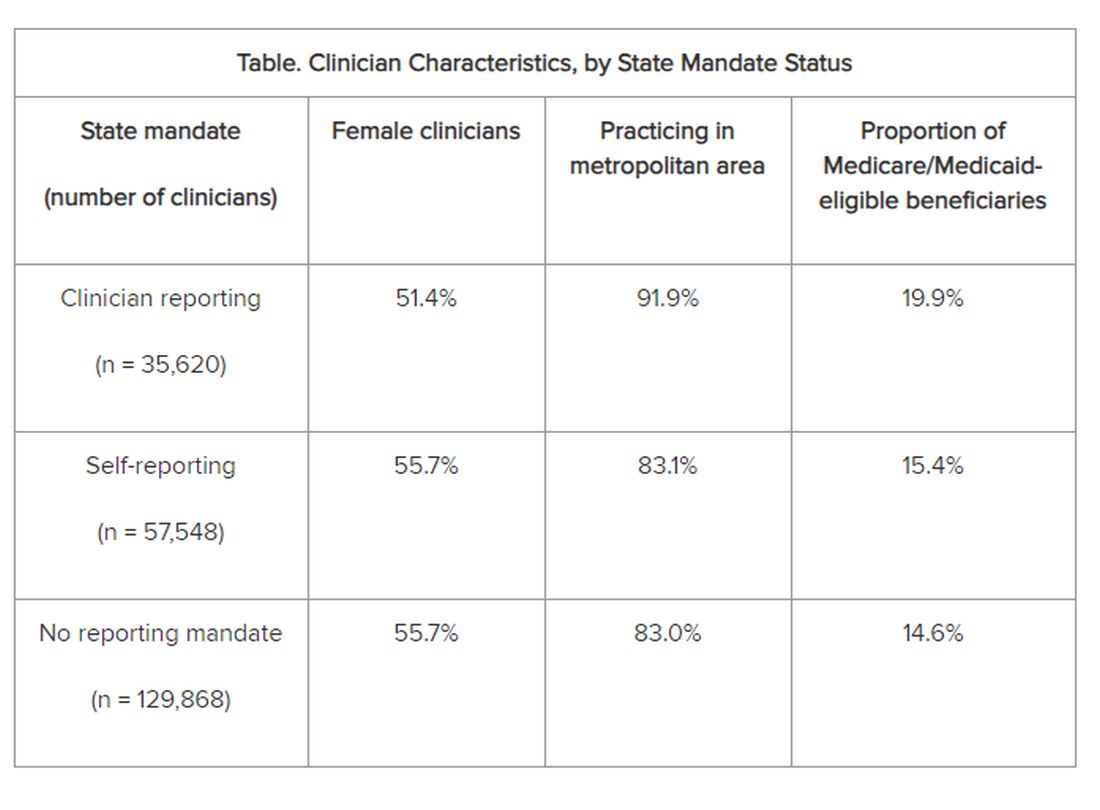
Adjusted results showed that PCPs practicing in states with clinician reporting mandates had a 12.4% (95% confidence interval [CI], 10.5%-14.2%) probability of underdiagnosing dementia versus 7.8% (95% CI, 6.9%-8.7%) in states with self-reporting and 7.7% (95% CI, 6.9%-8.4%) in states with no mandates, translating into a 4–percentage point difference (P < .001).
“Our study is the first to provide empirical evidence for the potential adverse effects of reporting policies,” the researchers noted. “Although we found that some clinicians underdiagnosed dementia regardless of state mandates, the key finding of this study reveals that primary care clinicians who practice in states with clinician reporting mandates were 59% more likely to do so…compared with those states with no reporting requirements…or driver self-reporting requirements.”
The investigators suggested that one potential explanation for underdiagnosis is patient resistance to cognitive testing. If patients were aware that the clinician was obligated by law to report their dementia diagnosis to the DMV, “they might be more inclined to conceal their symptoms or refuse further assessments, in addition to the general stigma and resistance to a formal assessment after a positive dementia screening result.”
“The findings suggest that policymakers might want to rethink those physician reporting mandates, since we also could not find conclusive evidence that they improve road safety,” Dr. Mattke said. “Maybe patients and their physicians can arrive at a sensible approach to determine driving fitness without such heavy-handed interference.”
However, he cautioned that the findings are not definitive and further study is needed before firm recommendations either for or against mandatory reporting.
In addition, the researchers noted several study limitations. One is that dementia underdiagnosis may also be associated with factors not captured in their model, including physician-patient relationships, health literacy, or language barriers.
However, Dr. Mattke noted, “ my sense is that those unobservable factors are not systematically related to state reporting policies and having omitted them would therefore not bias our results.”
Experts Weigh In
Commenting on the research, Morgan Daven, MA, the Alzheimer’s Association vice president of health systems, said that dementia is widely and significantly underdiagnosed, and not only in the states with dementia reporting mandates. Many factors may contribute to underdiagnosis, and although the study shows an association between reporting mandates and underdiagnosis, it does not demonstrate causation.
That said, Mr. Daven added, “fear and stigma related to dementia may inhibit the clinician, the patient, and their family from pursuing detection and diagnosis for dementia. As a society, we need to address dementia fear and stigma for all parties.”
He noted that useful tools include healthcare policies, workforce training, public awareness and education, and public policies to mitigate fear and stigma and their negative effects on diagnosis, care, support, and communication.
A potential study limitation is that it relied only on diagnoses by PCPs. Mr. Daven noted that the diagnosis of Alzheimer’ disease — the most common cause of dementia — is confirmation of amyloid buildup via a biomarker test, using PET or cerebrospinal fluid analysis.
“Both of these tests are extremely limited in their use and accessibility in a primary care setting. Inclusion of diagnoses by dementia specialists would provide a more complete picture,” he said.
Mr. Daven added that the Alzheimer’s Association encourages families to proactively discuss driving and other disease-related safety concerns as soon as possible. The Alzheimer’s Association Dementia and Driving webpage offers tips and strategies to discuss driving concerns with a family member.
In an accompanying editorial, Donald Redelmeier, MD, MS(HSR), and Vidhi Bhatt, BSc, both of the Department of Medicine, University of Toronto, differentiate the mandate for physicians to warn patients with dementia about traffic safety from the mandate for reporting child maltreatment, gunshot victims, or communicable diseases. They noted that mandated warnings “are not easy, can engender patient dissatisfaction, and need to be handled with tact.”
Yet, they pointed out, “breaking bad news is what practicing medicine entails.” They emphasized that, regardless of government mandates, “counseling patients for more road safety is an essential skill for clinicians in diverse states who hope to help their patients avoid becoming more traffic statistics.”
Research reported in this publication was supported by Genentech, a member of the Roche Group, and a grant from the National Institute on Aging of the National Institutes of Health. Dr. Mattke reported receiving grants from Genentech for a research contract with USC during the conduct of the study; personal fees from Eisai, Biogen, C2N, Novo Nordisk, Novartis, and Roche Genentech; and serving on the Senscio Systems board of directors, ALZpath scientific advisory board, AiCure scientific advisory board, and Boston Millennia Partners scientific advisory board outside the submitted work. The other authors’ disclosures are listed on the original paper. The editorial was supported by the Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, Kimel-Schatzky Traumatic Brain Injury Research Fund, and the Graduate Diploma Program in Health Research at the University of Toronto. The editorial authors report no other relevant financial relationships.
A version of this article appeared on Medscape.com.
, new research suggests.
Investigators found that primary care physicians (PCPs) in states with clinician reporting mandates had a 59% higher probability of underdiagnosing dementia compared with their counterparts in states that require patients to self-report or that have no reporting mandates.
“Our findings in this cross-sectional study raise concerns about potential adverse effects of mandatory clinician reporting for dementia diagnosis and underscore the need for careful consideration of the effect of such policies,” wrote the investigators, led by Soeren Mattke, MD, DSc, director of the USC Brain Health Observatory and research professor of economics at the University of Southern California, Los Angeles.
The study was published online in JAMA Network Open.
Lack of Guidance
As the US population ages, the number of older drivers is increasing, with 55.8 million drivers 65 years old or older. Approximately 7 million people in this age group have dementia — an estimate that is expected to increase to nearly 12 million by 2040.
The aging population raises a “critical policy question” about how to ensure road safety. Although the American Medical Association’s Code of Ethics outlines a physician’s obligation to identify drivers with medical impairments that impede safe driving, guidance restricting cognitively impaired drivers from driving is lacking.
In addition, evidence as to whether cognitive impairment indeed poses a threat to driving safety is mixed and has led to a lack of uniform policies with respect to reporting dementia.
Four states explicitly require clinicians to report dementia diagnoses to the DMV, which will then determine the patient’s fitness to drive, whereas 14 states require people with dementia to self-report. The remaining states have no explicit reporting requirements.
The issue of mandatory reporting is controversial, the researchers noted. On the one hand, physicians could protect patients and others by reporting potentially unsafe drivers.
On the other hand, evidence of an association with lower accident risks in patients with dementia is sparse and mandatory reporting may adversely affect physician-patient relationships. Empirical evidence for unintended consequences of reporting laws is lacking.
To examine the potential link between dementia underdiagnosis and mandatory reporting policies, the investigators analyzed the 100% data from the Medicare fee-for-service program and Medicare Advantage plans from 2017 to 2019, which included 223,036 PCPs with a panel of 25 or more Medicare patients.
The researchers examined dementia diagnosis rates in the patient panel of PCPs, rather than neurologists or gerontologists, regardless of who documented the diagnosis. Dr. Mattke said that it is possible that the diagnosis was established after referral to a specialist.
Each physician’s expected number of dementia cases was estimated using a predictive model based on patient characteristics. The researchers then compared the estimate with observed dementia diagnoses, thereby identifying clinicians who underdiagnosed dementia after sampling errors were accounted for.
‘Heavy-Handed Interference’
The researchers adjusted for several covariates potentially associated with a clinician’s probability of underdiagnosing dementia. These included sex, office location, practice specialty, racial/ethnic composition of the patient panel, and percentage of patients dually eligible for Medicare and Medicaid. The table shows PCP characteristics.

Adjusted results showed that PCPs practicing in states with clinician reporting mandates had a 12.4% (95% confidence interval [CI], 10.5%-14.2%) probability of underdiagnosing dementia versus 7.8% (95% CI, 6.9%-8.7%) in states with self-reporting and 7.7% (95% CI, 6.9%-8.4%) in states with no mandates, translating into a 4–percentage point difference (P < .001).
“Our study is the first to provide empirical evidence for the potential adverse effects of reporting policies,” the researchers noted. “Although we found that some clinicians underdiagnosed dementia regardless of state mandates, the key finding of this study reveals that primary care clinicians who practice in states with clinician reporting mandates were 59% more likely to do so…compared with those states with no reporting requirements…or driver self-reporting requirements.”
The investigators suggested that one potential explanation for underdiagnosis is patient resistance to cognitive testing. If patients were aware that the clinician was obligated by law to report their dementia diagnosis to the DMV, “they might be more inclined to conceal their symptoms or refuse further assessments, in addition to the general stigma and resistance to a formal assessment after a positive dementia screening result.”
“The findings suggest that policymakers might want to rethink those physician reporting mandates, since we also could not find conclusive evidence that they improve road safety,” Dr. Mattke said. “Maybe patients and their physicians can arrive at a sensible approach to determine driving fitness without such heavy-handed interference.”
However, he cautioned that the findings are not definitive and further study is needed before firm recommendations either for or against mandatory reporting.
In addition, the researchers noted several study limitations. One is that dementia underdiagnosis may also be associated with factors not captured in their model, including physician-patient relationships, health literacy, or language barriers.
However, Dr. Mattke noted, “ my sense is that those unobservable factors are not systematically related to state reporting policies and having omitted them would therefore not bias our results.”
Experts Weigh In
Commenting on the research, Morgan Daven, MA, the Alzheimer’s Association vice president of health systems, said that dementia is widely and significantly underdiagnosed, and not only in the states with dementia reporting mandates. Many factors may contribute to underdiagnosis, and although the study shows an association between reporting mandates and underdiagnosis, it does not demonstrate causation.
That said, Mr. Daven added, “fear and stigma related to dementia may inhibit the clinician, the patient, and their family from pursuing detection and diagnosis for dementia. As a society, we need to address dementia fear and stigma for all parties.”
He noted that useful tools include healthcare policies, workforce training, public awareness and education, and public policies to mitigate fear and stigma and their negative effects on diagnosis, care, support, and communication.
A potential study limitation is that it relied only on diagnoses by PCPs. Mr. Daven noted that the diagnosis of Alzheimer’ disease — the most common cause of dementia — is confirmation of amyloid buildup via a biomarker test, using PET or cerebrospinal fluid analysis.
“Both of these tests are extremely limited in their use and accessibility in a primary care setting. Inclusion of diagnoses by dementia specialists would provide a more complete picture,” he said.
Mr. Daven added that the Alzheimer’s Association encourages families to proactively discuss driving and other disease-related safety concerns as soon as possible. The Alzheimer’s Association Dementia and Driving webpage offers tips and strategies to discuss driving concerns with a family member.
In an accompanying editorial, Donald Redelmeier, MD, MS(HSR), and Vidhi Bhatt, BSc, both of the Department of Medicine, University of Toronto, differentiate the mandate for physicians to warn patients with dementia about traffic safety from the mandate for reporting child maltreatment, gunshot victims, or communicable diseases. They noted that mandated warnings “are not easy, can engender patient dissatisfaction, and need to be handled with tact.”
Yet, they pointed out, “breaking bad news is what practicing medicine entails.” They emphasized that, regardless of government mandates, “counseling patients for more road safety is an essential skill for clinicians in diverse states who hope to help their patients avoid becoming more traffic statistics.”
Research reported in this publication was supported by Genentech, a member of the Roche Group, and a grant from the National Institute on Aging of the National Institutes of Health. Dr. Mattke reported receiving grants from Genentech for a research contract with USC during the conduct of the study; personal fees from Eisai, Biogen, C2N, Novo Nordisk, Novartis, and Roche Genentech; and serving on the Senscio Systems board of directors, ALZpath scientific advisory board, AiCure scientific advisory board, and Boston Millennia Partners scientific advisory board outside the submitted work. The other authors’ disclosures are listed on the original paper. The editorial was supported by the Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, Kimel-Schatzky Traumatic Brain Injury Research Fund, and the Graduate Diploma Program in Health Research at the University of Toronto. The editorial authors report no other relevant financial relationships.
A version of this article appeared on Medscape.com.
, new research suggests.
Investigators found that primary care physicians (PCPs) in states with clinician reporting mandates had a 59% higher probability of underdiagnosing dementia compared with their counterparts in states that require patients to self-report or that have no reporting mandates.
“Our findings in this cross-sectional study raise concerns about potential adverse effects of mandatory clinician reporting for dementia diagnosis and underscore the need for careful consideration of the effect of such policies,” wrote the investigators, led by Soeren Mattke, MD, DSc, director of the USC Brain Health Observatory and research professor of economics at the University of Southern California, Los Angeles.
The study was published online in JAMA Network Open.
Lack of Guidance
As the US population ages, the number of older drivers is increasing, with 55.8 million drivers 65 years old or older. Approximately 7 million people in this age group have dementia — an estimate that is expected to increase to nearly 12 million by 2040.
The aging population raises a “critical policy question” about how to ensure road safety. Although the American Medical Association’s Code of Ethics outlines a physician’s obligation to identify drivers with medical impairments that impede safe driving, guidance restricting cognitively impaired drivers from driving is lacking.
In addition, evidence as to whether cognitive impairment indeed poses a threat to driving safety is mixed and has led to a lack of uniform policies with respect to reporting dementia.
Four states explicitly require clinicians to report dementia diagnoses to the DMV, which will then determine the patient’s fitness to drive, whereas 14 states require people with dementia to self-report. The remaining states have no explicit reporting requirements.
The issue of mandatory reporting is controversial, the researchers noted. On the one hand, physicians could protect patients and others by reporting potentially unsafe drivers.
On the other hand, evidence of an association with lower accident risks in patients with dementia is sparse and mandatory reporting may adversely affect physician-patient relationships. Empirical evidence for unintended consequences of reporting laws is lacking.
To examine the potential link between dementia underdiagnosis and mandatory reporting policies, the investigators analyzed the 100% data from the Medicare fee-for-service program and Medicare Advantage plans from 2017 to 2019, which included 223,036 PCPs with a panel of 25 or more Medicare patients.
The researchers examined dementia diagnosis rates in the patient panel of PCPs, rather than neurologists or gerontologists, regardless of who documented the diagnosis. Dr. Mattke said that it is possible that the diagnosis was established after referral to a specialist.
Each physician’s expected number of dementia cases was estimated using a predictive model based on patient characteristics. The researchers then compared the estimate with observed dementia diagnoses, thereby identifying clinicians who underdiagnosed dementia after sampling errors were accounted for.
‘Heavy-Handed Interference’
The researchers adjusted for several covariates potentially associated with a clinician’s probability of underdiagnosing dementia. These included sex, office location, practice specialty, racial/ethnic composition of the patient panel, and percentage of patients dually eligible for Medicare and Medicaid. The table shows PCP characteristics.

Adjusted results showed that PCPs practicing in states with clinician reporting mandates had a 12.4% (95% confidence interval [CI], 10.5%-14.2%) probability of underdiagnosing dementia versus 7.8% (95% CI, 6.9%-8.7%) in states with self-reporting and 7.7% (95% CI, 6.9%-8.4%) in states with no mandates, translating into a 4–percentage point difference (P < .001).
“Our study is the first to provide empirical evidence for the potential adverse effects of reporting policies,” the researchers noted. “Although we found that some clinicians underdiagnosed dementia regardless of state mandates, the key finding of this study reveals that primary care clinicians who practice in states with clinician reporting mandates were 59% more likely to do so…compared with those states with no reporting requirements…or driver self-reporting requirements.”
The investigators suggested that one potential explanation for underdiagnosis is patient resistance to cognitive testing. If patients were aware that the clinician was obligated by law to report their dementia diagnosis to the DMV, “they might be more inclined to conceal their symptoms or refuse further assessments, in addition to the general stigma and resistance to a formal assessment after a positive dementia screening result.”
“The findings suggest that policymakers might want to rethink those physician reporting mandates, since we also could not find conclusive evidence that they improve road safety,” Dr. Mattke said. “Maybe patients and their physicians can arrive at a sensible approach to determine driving fitness without such heavy-handed interference.”
However, he cautioned that the findings are not definitive and further study is needed before firm recommendations either for or against mandatory reporting.
In addition, the researchers noted several study limitations. One is that dementia underdiagnosis may also be associated with factors not captured in their model, including physician-patient relationships, health literacy, or language barriers.
However, Dr. Mattke noted, “ my sense is that those unobservable factors are not systematically related to state reporting policies and having omitted them would therefore not bias our results.”
Experts Weigh In
Commenting on the research, Morgan Daven, MA, the Alzheimer’s Association vice president of health systems, said that dementia is widely and significantly underdiagnosed, and not only in the states with dementia reporting mandates. Many factors may contribute to underdiagnosis, and although the study shows an association between reporting mandates and underdiagnosis, it does not demonstrate causation.
That said, Mr. Daven added, “fear and stigma related to dementia may inhibit the clinician, the patient, and their family from pursuing detection and diagnosis for dementia. As a society, we need to address dementia fear and stigma for all parties.”
He noted that useful tools include healthcare policies, workforce training, public awareness and education, and public policies to mitigate fear and stigma and their negative effects on diagnosis, care, support, and communication.
A potential study limitation is that it relied only on diagnoses by PCPs. Mr. Daven noted that the diagnosis of Alzheimer’ disease — the most common cause of dementia — is confirmation of amyloid buildup via a biomarker test, using PET or cerebrospinal fluid analysis.
“Both of these tests are extremely limited in their use and accessibility in a primary care setting. Inclusion of diagnoses by dementia specialists would provide a more complete picture,” he said.
Mr. Daven added that the Alzheimer’s Association encourages families to proactively discuss driving and other disease-related safety concerns as soon as possible. The Alzheimer’s Association Dementia and Driving webpage offers tips and strategies to discuss driving concerns with a family member.
In an accompanying editorial, Donald Redelmeier, MD, MS(HSR), and Vidhi Bhatt, BSc, both of the Department of Medicine, University of Toronto, differentiate the mandate for physicians to warn patients with dementia about traffic safety from the mandate for reporting child maltreatment, gunshot victims, or communicable diseases. They noted that mandated warnings “are not easy, can engender patient dissatisfaction, and need to be handled with tact.”
Yet, they pointed out, “breaking bad news is what practicing medicine entails.” They emphasized that, regardless of government mandates, “counseling patients for more road safety is an essential skill for clinicians in diverse states who hope to help their patients avoid becoming more traffic statistics.”
Research reported in this publication was supported by Genentech, a member of the Roche Group, and a grant from the National Institute on Aging of the National Institutes of Health. Dr. Mattke reported receiving grants from Genentech for a research contract with USC during the conduct of the study; personal fees from Eisai, Biogen, C2N, Novo Nordisk, Novartis, and Roche Genentech; and serving on the Senscio Systems board of directors, ALZpath scientific advisory board, AiCure scientific advisory board, and Boston Millennia Partners scientific advisory board outside the submitted work. The other authors’ disclosures are listed on the original paper. The editorial was supported by the Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, Kimel-Schatzky Traumatic Brain Injury Research Fund, and the Graduate Diploma Program in Health Research at the University of Toronto. The editorial authors report no other relevant financial relationships.
A version of this article appeared on Medscape.com.
From JAMA Network Open
Does ‘Brain Training’ Really Improve Cognition and Forestall Cognitive Decline?
The concept that cognitive health can be preserved or improved is often expressed as “use it or lose it.” Numerous modifiable risk factors are associated with “losing” cognitive abilities with age, and a cognitively active lifestyle may have a protective effect.
But what is a “cognitively active lifestyle” — do crosswords and Sudoku count?
One popular approach is “brain training.” While not a scientific term with an established definition, it “typically refers to tasks or drills that are designed to strengthen specific aspects of one’s cognitive function,” explained Yuko Hara, PhD, director of Aging and Alzheimer’s Prevention at the Alzheimer’s Drug Discovery Foundation.
Manuel Montero-Odasso, MD, PhD, director of the Gait and Brain Lab, Parkwood Institute, London, Ontario, Canada, elaborated: “Cognitive training involves performing a definitive task or set of tasks where you increase attentional demands to improve focus and concentration and memory. You try to execute the new things that you’ve learned and to remember them.”
In a commentary published by this news organization in 2022, neuroscientist Michael Merzenich, PhD, professor emeritus at University of California San Francisco, said that growing a person’s cognitive reserve and actively managing brain health can play an important role in preventing or delaying Alzheimer’s disease. Important components of this include brain training and physical exercise.
Brain Training: Mechanism of Action
Dr. Montero-Odasso, team leader at the Canadian Consortium on Neurodegeneration in Aging and team co-leader at the Ontario Neurodegenerative Research Initiative, explained that cognitive training creates new synapses in the brain, thus stimulating neuroplasticity.
“When we try to activate networks mainly in the frontal lobe, the prefrontal cortex, a key mechanism underlying this process is enhancement of the synaptic plasticity at excitatory synapses, which connect neurons into networks; in other words, we generate new synapses, and that’s how we enhance brain health and cognitive abilities.”
The more neural connections, the greater the processing speed of the brain, he continued. “Cognitive training creates an anatomical change in the brain.”
Executive functions, which include attention, inhibition, planning, and multitasking, are regulated predominantly by the prefrontal cortex. Damage in this region of the brain is also implicated in dementia. Alterations in the connectivity of this area are associated with cognitive impairment, independent of other structural pathological aberrations (eg, gray matter atrophy). These patterns may precede structural pathological changes associated with cognitive impairment and dementia.
Neuroplasticity changes have been corroborated through neuroimaging, which has demonstrated that after cognitive training, there is more activation in the prefrontal cortex that correlates with new synapses, Dr. Montero-Odasso said.
Henry Mahncke, PhD, CEO of the brain training company Posit Science/BrainHQ, explained that early research was conducted on rodents and monkeys, with Dr. Merzenich as one of the leading pioneers in developing the concept of brain plasticity. Dr. Merzenich cofounded Posit Science and is currently its chief scientific officer.
Dr. Mahncke recounted that as a graduate student, he had worked with Dr. Merzenich researching brain plasticity. When Dr. Merzenich founded Posit Science, he asked Dr. Mahncke to join the company to help develop approaches to enhance brain plasticity — building the brain-training exercises and running the clinical trials.
“It’s now well understood that the brain can rewire itself at any age and in almost any condition,” Dr. Mahncke said. “In kids and in younger and older adults, whether with healthy or unhealthy brains, the fundamental way the brain works is by continually rewiring and rebuilding itself, based on what we ask it to do.”
Dr. Mahncke said.
Unsubstantiated Claims and Controversy
Brain training is not without controversy, Dr. Hara pointed out. “Some manufacturers of brain games have been criticized and even fined for making unsubstantiated claims,” she said.
A 2016 review found that brain-training interventions do improve performance on specific trained tasks, but there is less evidence that they improve performance on closely related tasks and little evidence that training improves everyday cognitive performance. A 2017 review reached similar conclusions, calling evidence regarding prevention or delay of cognitive decline or dementia through brain games “insufficient,” although cognitive training could “improve cognition in the domain trained.”
“The general consensus is that for most brain-training programs, people may get better at specific tasks through practice, but these improvements don’t necessarily translate into improvement in other tasks that require other cognitive domains or prevention of dementia or age-related cognitive decline,” Dr. Hara said.
She noted that most brain-training programs “have not been rigorously tested in clinical trials” — although some, such as those featured in the ACTIVE trial, did show evidence of effectiveness.
Dr. Mahncke agreed. “Asking whether brain training works is like asking whether small molecules improve health,” he said noting that some brain-training programs are nonsense and not evidence based. He believes that his company’s product, BrainHQ, and some others are “backed by robust evidence in their ability to stave off, slow, or even reverse cognitive changes.”
BrainHQ is a web-based brain game suite that can be used independently as an app or in group settings (classes and webinars) and is covered by some Medicare Advantage insurance plans. It encompasses “dozens of individual brain-training exercises, linked by a common thread. Each one is intensively designed to make the brain faster and more accurate,” said Dr. Mahncke.
He explained that human brains “get noisy as people get older, like a radio which is wearing out, so there’s static in the background. This makes the music hard to hear, and in the case of the human brain, it makes it difficult to pay attention.” The exercises are “designed to tamp down the ‘noise,’ speed up the brain, and make information processing more accurate.”
Dr. Mahncke called this a “bottom-up” approach, in contrast to many previous cognitive-training approaches that come from the brain injury rehabilitation field. They teach “top-down” skills and strategies designed to compensate for deficits in specific domains, such as reading, concentration, or fine motor skills.
By contrast, the approach of BrainHQ is “to improve the overall processing system of the brain with speed, attention, working memory, and executive function, which will in turn impact all skills and activities.”
Supporting Evidence
Dr. Mahncke cited several supporting studies. For example, the IMPACT study randomized 487 adults (aged ≥ 65 years) to receive either a brain plasticity–based computerized cognitive training program (BrainHQ) or a novelty- and intensity-matched general cognitive stimulation treatment program (intervention and control group, respectively) for an 8-week period.
Those who underwent brain training showed significantly greater improvement in the repeatable Battery for the Assessment of Neuropsychological Status (RBANS Auditory Memory/Attention) compared with those in the control group (3.9 vs 1.8, respectively; P =.02). The intervention group also showed significant improvements on multiple secondary measures of attention and memory. The magnitude of the effect sizes suggests that the results are clinically significant, according to the authors.
The ACTIVE study tested the effects of different cognitive training programs on cognitive function and time to dementia. The researchers randomized 2802 healthy older adults (mean age, 74 years) to a control group with no cognitive training or one of three brain-training groups comprising:
1. In-person training on verbal memory skills
2. In-person training on reasoning and problem-solving
3. Computer-based speed-of-processing training on visual attention
Participants in the training groups completed 10 sessions, each lasting 60-75 minutes, over a 5- to 6-week period. A random subsample of each training group was selected to receive “booster” sessions, with four-session booster training delivered at 11 and 35 months. All study participants completed follow-up tests of cognition and function after 1, 2, 3, 5, and 10 years.
At the end of 10 years, those assigned to the speed-of-processing training, now part of BrainHQ, had a 29% lower risk for dementia than those in the control group who received no training. No reduction was found in the memory or reasoning training groups. Participants who completed the “booster” sessions had an even greater reduction: Each additional booster session was associated with a 10% lower risk for dementia.
Dr. Montero-Odasso was involved in the SYNERGIC study that randomized 175 participants with mild cognitive impairment (MCI; average age, 73 years) to one of five study arms:
1. Multidomain intervention with exercise, cognitive training, and vitamin D
2. Exercise, cognitive training, and placebo
3. Exercise, sham cognitive training, and vitamin D
4. Exercise, sham cognitive training, and placebo
5. Control group with balance-toning exercise, sham cognitive training, and placebo
“Sham” cognitive training consisted of alternating between two tasks (touristic search and video watching) performed on a tablet, with the same time exposure as the intervention training.
The researchers found that after 6 months of interventions, all active arms with aerobic-resistance exercise showed improvement in the ADAS-Cog-13, an established outcome to evaluate dementia treatments, when compared with the control group — regardless of the addition of cognitive training or vitamin D.
Compared with exercise alone (arms 3 and 4), those who did exercise plus cognitive training (arms 1 and 2) showed greater improvements in their ADAS-Cog-13l score, with a mean difference of −1.45 points (P = .02). The greatest improvement was seen in those who underwent the multidomain intervention in arm 1.
The authors noted that the mean 2.64-point improvement seen in the ADAS-Cog-13 for the multidomain intervention is actually larger than changes seen in previous pharmaceutical trials among individuals with MCI or mild dementia and “approaches” the three points considered clinically meaningful.
“We found that older adults with MCI who received aerobic-resistance exercise with sequential computerized cognitive training significantly improved cognition,” Dr. Montero-Odasso said. “The cognitive training we used was called Neuropeak, a multidomain lifestyle training delivered through a web-based platform developed by our co-leader Louis Bherer at Université de Montréal.”
He explained that the purpose “is to challenge your brain to the point where you need to make an effort to remember things, pay attention, and later to execute tasks. The evidence from clinical trials, including ours, shows this type of brain challenge is effective in slowing and even reversing cognitive decline.”
A follow-up study, SYNERGIC 2.0, is ongoing.
Puzzles, Board Games, and New Challenges
Formal brain-training programs aren’t the only way to improve brain plasticity, Dr. Hara said. Observational studies suggested an association between improved cognitive performance and/or lower dementia risk and engaging in number and word puzzles, such as crosswords, cards, or board games.
Some studies suggested that older adults who use technology might also protect their cognitive reserve. Dr. Hara cited a US longitudinal study of more than 18,000 older adults suggesting that regular Internet users had roughly half the risk for dementia compared to nonregular Internet users. Estimates of daily Internet use suggested a U-shaped relationship with dementia with 0.1-2.0 hours daily (excluding time spent watching television or movies online) associated with the lowest risk. Similar associations between Internet use and a lower risk for cognitive decline have been reported in the United Kingdom and Europe.
“Engaging in mentally stimulating activities can increase ‘cognitive reserve’ — meaning, capacity of the brain to resist the effects of age-related changes or disease-related pathology, such that one can maintain cognitive function for longer,” Dr. Hara said. “Cognitively stimulating activities, regardless of the type, may help delay the onset of cognitive decline.”
She listed several examples of activities that are stimulating to the brain, including learning a new game or puzzle, a new language, or a new dance, and learning how to play a musical instrument.
Dr. Montero-Odasso emphasized that the “newness” is key to increasing and preserving cognitive reserve. “Just surfing the Internet, playing word or board games, or doing crossword puzzles won’t be enough if you’ve been doing these things all your life,” he said. “It won’t hurt, of course, but it won’t necessarily increase your cognitive abilities.
“For example, a person who regularly engages in public speaking may not improve cognition by taking a public-speaking course, but someone who has never spoken before an audience might show cognitive improvements as a result of learning a new skill,” he said. “Or someone who knows several languages already might gain from learning a brand-new language.”
He cited research supporting the benefits of dancing, which he called “an ideal activity because it’s physical, so it provides the exercise that’s been associated with improved cognition. But it also requires learning new steps and moves, which builds the synapses in the brain. And the socialization of dance classes adds another component that can improve cognition.”
Dr. Mahncke hopes that beyond engaging in day-to-day new activities, seniors will participate in computerized brain training. “There’s no reason that evidence-based training can’t be offered in senior and community centers, as yoga and swimming are,” he said. “It doesn’t have to be simply something people do on their own virtually.”
Zoom classes and Medicare reimbursements are “good steps in the right direction, but it’s time to expand this potentially life-transformative intervention so that it reaches the ever-expanding population of seniors in the United States and beyond.”
Dr. Hara reported having no disclosures. Dr. Montero-Odasso reported having no commercial or financial interest related to this topic. He serves as the president of the Canadian Geriatrics Société and is team leader in the Canadian Consortium of Neurodegeneration in Aging. Dr. Mahncke is CEO of the brain training company Posit Science/BrainHQ.
A version of this article appeared on Medscape.com.
The concept that cognitive health can be preserved or improved is often expressed as “use it or lose it.” Numerous modifiable risk factors are associated with “losing” cognitive abilities with age, and a cognitively active lifestyle may have a protective effect.
But what is a “cognitively active lifestyle” — do crosswords and Sudoku count?
One popular approach is “brain training.” While not a scientific term with an established definition, it “typically refers to tasks or drills that are designed to strengthen specific aspects of one’s cognitive function,” explained Yuko Hara, PhD, director of Aging and Alzheimer’s Prevention at the Alzheimer’s Drug Discovery Foundation.
Manuel Montero-Odasso, MD, PhD, director of the Gait and Brain Lab, Parkwood Institute, London, Ontario, Canada, elaborated: “Cognitive training involves performing a definitive task or set of tasks where you increase attentional demands to improve focus and concentration and memory. You try to execute the new things that you’ve learned and to remember them.”
In a commentary published by this news organization in 2022, neuroscientist Michael Merzenich, PhD, professor emeritus at University of California San Francisco, said that growing a person’s cognitive reserve and actively managing brain health can play an important role in preventing or delaying Alzheimer’s disease. Important components of this include brain training and physical exercise.
Brain Training: Mechanism of Action
Dr. Montero-Odasso, team leader at the Canadian Consortium on Neurodegeneration in Aging and team co-leader at the Ontario Neurodegenerative Research Initiative, explained that cognitive training creates new synapses in the brain, thus stimulating neuroplasticity.
“When we try to activate networks mainly in the frontal lobe, the prefrontal cortex, a key mechanism underlying this process is enhancement of the synaptic plasticity at excitatory synapses, which connect neurons into networks; in other words, we generate new synapses, and that’s how we enhance brain health and cognitive abilities.”
The more neural connections, the greater the processing speed of the brain, he continued. “Cognitive training creates an anatomical change in the brain.”
Executive functions, which include attention, inhibition, planning, and multitasking, are regulated predominantly by the prefrontal cortex. Damage in this region of the brain is also implicated in dementia. Alterations in the connectivity of this area are associated with cognitive impairment, independent of other structural pathological aberrations (eg, gray matter atrophy). These patterns may precede structural pathological changes associated with cognitive impairment and dementia.
Neuroplasticity changes have been corroborated through neuroimaging, which has demonstrated that after cognitive training, there is more activation in the prefrontal cortex that correlates with new synapses, Dr. Montero-Odasso said.
Henry Mahncke, PhD, CEO of the brain training company Posit Science/BrainHQ, explained that early research was conducted on rodents and monkeys, with Dr. Merzenich as one of the leading pioneers in developing the concept of brain plasticity. Dr. Merzenich cofounded Posit Science and is currently its chief scientific officer.
Dr. Mahncke recounted that as a graduate student, he had worked with Dr. Merzenich researching brain plasticity. When Dr. Merzenich founded Posit Science, he asked Dr. Mahncke to join the company to help develop approaches to enhance brain plasticity — building the brain-training exercises and running the clinical trials.
“It’s now well understood that the brain can rewire itself at any age and in almost any condition,” Dr. Mahncke said. “In kids and in younger and older adults, whether with healthy or unhealthy brains, the fundamental way the brain works is by continually rewiring and rebuilding itself, based on what we ask it to do.”
Dr. Mahncke said.
Unsubstantiated Claims and Controversy
Brain training is not without controversy, Dr. Hara pointed out. “Some manufacturers of brain games have been criticized and even fined for making unsubstantiated claims,” she said.
A 2016 review found that brain-training interventions do improve performance on specific trained tasks, but there is less evidence that they improve performance on closely related tasks and little evidence that training improves everyday cognitive performance. A 2017 review reached similar conclusions, calling evidence regarding prevention or delay of cognitive decline or dementia through brain games “insufficient,” although cognitive training could “improve cognition in the domain trained.”
“The general consensus is that for most brain-training programs, people may get better at specific tasks through practice, but these improvements don’t necessarily translate into improvement in other tasks that require other cognitive domains or prevention of dementia or age-related cognitive decline,” Dr. Hara said.
She noted that most brain-training programs “have not been rigorously tested in clinical trials” — although some, such as those featured in the ACTIVE trial, did show evidence of effectiveness.
Dr. Mahncke agreed. “Asking whether brain training works is like asking whether small molecules improve health,” he said noting that some brain-training programs are nonsense and not evidence based. He believes that his company’s product, BrainHQ, and some others are “backed by robust evidence in their ability to stave off, slow, or even reverse cognitive changes.”
BrainHQ is a web-based brain game suite that can be used independently as an app or in group settings (classes and webinars) and is covered by some Medicare Advantage insurance plans. It encompasses “dozens of individual brain-training exercises, linked by a common thread. Each one is intensively designed to make the brain faster and more accurate,” said Dr. Mahncke.
He explained that human brains “get noisy as people get older, like a radio which is wearing out, so there’s static in the background. This makes the music hard to hear, and in the case of the human brain, it makes it difficult to pay attention.” The exercises are “designed to tamp down the ‘noise,’ speed up the brain, and make information processing more accurate.”
Dr. Mahncke called this a “bottom-up” approach, in contrast to many previous cognitive-training approaches that come from the brain injury rehabilitation field. They teach “top-down” skills and strategies designed to compensate for deficits in specific domains, such as reading, concentration, or fine motor skills.
By contrast, the approach of BrainHQ is “to improve the overall processing system of the brain with speed, attention, working memory, and executive function, which will in turn impact all skills and activities.”
Supporting Evidence
Dr. Mahncke cited several supporting studies. For example, the IMPACT study randomized 487 adults (aged ≥ 65 years) to receive either a brain plasticity–based computerized cognitive training program (BrainHQ) or a novelty- and intensity-matched general cognitive stimulation treatment program (intervention and control group, respectively) for an 8-week period.
Those who underwent brain training showed significantly greater improvement in the repeatable Battery for the Assessment of Neuropsychological Status (RBANS Auditory Memory/Attention) compared with those in the control group (3.9 vs 1.8, respectively; P =.02). The intervention group also showed significant improvements on multiple secondary measures of attention and memory. The magnitude of the effect sizes suggests that the results are clinically significant, according to the authors.
The ACTIVE study tested the effects of different cognitive training programs on cognitive function and time to dementia. The researchers randomized 2802 healthy older adults (mean age, 74 years) to a control group with no cognitive training or one of three brain-training groups comprising:
1. In-person training on verbal memory skills
2. In-person training on reasoning and problem-solving
3. Computer-based speed-of-processing training on visual attention
Participants in the training groups completed 10 sessions, each lasting 60-75 minutes, over a 5- to 6-week period. A random subsample of each training group was selected to receive “booster” sessions, with four-session booster training delivered at 11 and 35 months. All study participants completed follow-up tests of cognition and function after 1, 2, 3, 5, and 10 years.
At the end of 10 years, those assigned to the speed-of-processing training, now part of BrainHQ, had a 29% lower risk for dementia than those in the control group who received no training. No reduction was found in the memory or reasoning training groups. Participants who completed the “booster” sessions had an even greater reduction: Each additional booster session was associated with a 10% lower risk for dementia.
Dr. Montero-Odasso was involved in the SYNERGIC study that randomized 175 participants with mild cognitive impairment (MCI; average age, 73 years) to one of five study arms:
1. Multidomain intervention with exercise, cognitive training, and vitamin D
2. Exercise, cognitive training, and placebo
3. Exercise, sham cognitive training, and vitamin D
4. Exercise, sham cognitive training, and placebo
5. Control group with balance-toning exercise, sham cognitive training, and placebo
“Sham” cognitive training consisted of alternating between two tasks (touristic search and video watching) performed on a tablet, with the same time exposure as the intervention training.
The researchers found that after 6 months of interventions, all active arms with aerobic-resistance exercise showed improvement in the ADAS-Cog-13, an established outcome to evaluate dementia treatments, when compared with the control group — regardless of the addition of cognitive training or vitamin D.
Compared with exercise alone (arms 3 and 4), those who did exercise plus cognitive training (arms 1 and 2) showed greater improvements in their ADAS-Cog-13l score, with a mean difference of −1.45 points (P = .02). The greatest improvement was seen in those who underwent the multidomain intervention in arm 1.
The authors noted that the mean 2.64-point improvement seen in the ADAS-Cog-13 for the multidomain intervention is actually larger than changes seen in previous pharmaceutical trials among individuals with MCI or mild dementia and “approaches” the three points considered clinically meaningful.
“We found that older adults with MCI who received aerobic-resistance exercise with sequential computerized cognitive training significantly improved cognition,” Dr. Montero-Odasso said. “The cognitive training we used was called Neuropeak, a multidomain lifestyle training delivered through a web-based platform developed by our co-leader Louis Bherer at Université de Montréal.”
He explained that the purpose “is to challenge your brain to the point where you need to make an effort to remember things, pay attention, and later to execute tasks. The evidence from clinical trials, including ours, shows this type of brain challenge is effective in slowing and even reversing cognitive decline.”
A follow-up study, SYNERGIC 2.0, is ongoing.
Puzzles, Board Games, and New Challenges
Formal brain-training programs aren’t the only way to improve brain plasticity, Dr. Hara said. Observational studies suggested an association between improved cognitive performance and/or lower dementia risk and engaging in number and word puzzles, such as crosswords, cards, or board games.
Some studies suggested that older adults who use technology might also protect their cognitive reserve. Dr. Hara cited a US longitudinal study of more than 18,000 older adults suggesting that regular Internet users had roughly half the risk for dementia compared to nonregular Internet users. Estimates of daily Internet use suggested a U-shaped relationship with dementia with 0.1-2.0 hours daily (excluding time spent watching television or movies online) associated with the lowest risk. Similar associations between Internet use and a lower risk for cognitive decline have been reported in the United Kingdom and Europe.
“Engaging in mentally stimulating activities can increase ‘cognitive reserve’ — meaning, capacity of the brain to resist the effects of age-related changes or disease-related pathology, such that one can maintain cognitive function for longer,” Dr. Hara said. “Cognitively stimulating activities, regardless of the type, may help delay the onset of cognitive decline.”
She listed several examples of activities that are stimulating to the brain, including learning a new game or puzzle, a new language, or a new dance, and learning how to play a musical instrument.
Dr. Montero-Odasso emphasized that the “newness” is key to increasing and preserving cognitive reserve. “Just surfing the Internet, playing word or board games, or doing crossword puzzles won’t be enough if you’ve been doing these things all your life,” he said. “It won’t hurt, of course, but it won’t necessarily increase your cognitive abilities.
“For example, a person who regularly engages in public speaking may not improve cognition by taking a public-speaking course, but someone who has never spoken before an audience might show cognitive improvements as a result of learning a new skill,” he said. “Or someone who knows several languages already might gain from learning a brand-new language.”
He cited research supporting the benefits of dancing, which he called “an ideal activity because it’s physical, so it provides the exercise that’s been associated with improved cognition. But it also requires learning new steps and moves, which builds the synapses in the brain. And the socialization of dance classes adds another component that can improve cognition.”
Dr. Mahncke hopes that beyond engaging in day-to-day new activities, seniors will participate in computerized brain training. “There’s no reason that evidence-based training can’t be offered in senior and community centers, as yoga and swimming are,” he said. “It doesn’t have to be simply something people do on their own virtually.”
Zoom classes and Medicare reimbursements are “good steps in the right direction, but it’s time to expand this potentially life-transformative intervention so that it reaches the ever-expanding population of seniors in the United States and beyond.”
Dr. Hara reported having no disclosures. Dr. Montero-Odasso reported having no commercial or financial interest related to this topic. He serves as the president of the Canadian Geriatrics Société and is team leader in the Canadian Consortium of Neurodegeneration in Aging. Dr. Mahncke is CEO of the brain training company Posit Science/BrainHQ.
A version of this article appeared on Medscape.com.
The concept that cognitive health can be preserved or improved is often expressed as “use it or lose it.” Numerous modifiable risk factors are associated with “losing” cognitive abilities with age, and a cognitively active lifestyle may have a protective effect.
But what is a “cognitively active lifestyle” — do crosswords and Sudoku count?
One popular approach is “brain training.” While not a scientific term with an established definition, it “typically refers to tasks or drills that are designed to strengthen specific aspects of one’s cognitive function,” explained Yuko Hara, PhD, director of Aging and Alzheimer’s Prevention at the Alzheimer’s Drug Discovery Foundation.
Manuel Montero-Odasso, MD, PhD, director of the Gait and Brain Lab, Parkwood Institute, London, Ontario, Canada, elaborated: “Cognitive training involves performing a definitive task or set of tasks where you increase attentional demands to improve focus and concentration and memory. You try to execute the new things that you’ve learned and to remember them.”
In a commentary published by this news organization in 2022, neuroscientist Michael Merzenich, PhD, professor emeritus at University of California San Francisco, said that growing a person’s cognitive reserve and actively managing brain health can play an important role in preventing or delaying Alzheimer’s disease. Important components of this include brain training and physical exercise.
Brain Training: Mechanism of Action
Dr. Montero-Odasso, team leader at the Canadian Consortium on Neurodegeneration in Aging and team co-leader at the Ontario Neurodegenerative Research Initiative, explained that cognitive training creates new synapses in the brain, thus stimulating neuroplasticity.
“When we try to activate networks mainly in the frontal lobe, the prefrontal cortex, a key mechanism underlying this process is enhancement of the synaptic plasticity at excitatory synapses, which connect neurons into networks; in other words, we generate new synapses, and that’s how we enhance brain health and cognitive abilities.”
The more neural connections, the greater the processing speed of the brain, he continued. “Cognitive training creates an anatomical change in the brain.”
Executive functions, which include attention, inhibition, planning, and multitasking, are regulated predominantly by the prefrontal cortex. Damage in this region of the brain is also implicated in dementia. Alterations in the connectivity of this area are associated with cognitive impairment, independent of other structural pathological aberrations (eg, gray matter atrophy). These patterns may precede structural pathological changes associated with cognitive impairment and dementia.
Neuroplasticity changes have been corroborated through neuroimaging, which has demonstrated that after cognitive training, there is more activation in the prefrontal cortex that correlates with new synapses, Dr. Montero-Odasso said.
Henry Mahncke, PhD, CEO of the brain training company Posit Science/BrainHQ, explained that early research was conducted on rodents and monkeys, with Dr. Merzenich as one of the leading pioneers in developing the concept of brain plasticity. Dr. Merzenich cofounded Posit Science and is currently its chief scientific officer.
Dr. Mahncke recounted that as a graduate student, he had worked with Dr. Merzenich researching brain plasticity. When Dr. Merzenich founded Posit Science, he asked Dr. Mahncke to join the company to help develop approaches to enhance brain plasticity — building the brain-training exercises and running the clinical trials.
“It’s now well understood that the brain can rewire itself at any age and in almost any condition,” Dr. Mahncke said. “In kids and in younger and older adults, whether with healthy or unhealthy brains, the fundamental way the brain works is by continually rewiring and rebuilding itself, based on what we ask it to do.”
Dr. Mahncke said.
Unsubstantiated Claims and Controversy
Brain training is not without controversy, Dr. Hara pointed out. “Some manufacturers of brain games have been criticized and even fined for making unsubstantiated claims,” she said.
A 2016 review found that brain-training interventions do improve performance on specific trained tasks, but there is less evidence that they improve performance on closely related tasks and little evidence that training improves everyday cognitive performance. A 2017 review reached similar conclusions, calling evidence regarding prevention or delay of cognitive decline or dementia through brain games “insufficient,” although cognitive training could “improve cognition in the domain trained.”
“The general consensus is that for most brain-training programs, people may get better at specific tasks through practice, but these improvements don’t necessarily translate into improvement in other tasks that require other cognitive domains or prevention of dementia or age-related cognitive decline,” Dr. Hara said.
She noted that most brain-training programs “have not been rigorously tested in clinical trials” — although some, such as those featured in the ACTIVE trial, did show evidence of effectiveness.
Dr. Mahncke agreed. “Asking whether brain training works is like asking whether small molecules improve health,” he said noting that some brain-training programs are nonsense and not evidence based. He believes that his company’s product, BrainHQ, and some others are “backed by robust evidence in their ability to stave off, slow, or even reverse cognitive changes.”
BrainHQ is a web-based brain game suite that can be used independently as an app or in group settings (classes and webinars) and is covered by some Medicare Advantage insurance plans. It encompasses “dozens of individual brain-training exercises, linked by a common thread. Each one is intensively designed to make the brain faster and more accurate,” said Dr. Mahncke.
He explained that human brains “get noisy as people get older, like a radio which is wearing out, so there’s static in the background. This makes the music hard to hear, and in the case of the human brain, it makes it difficult to pay attention.” The exercises are “designed to tamp down the ‘noise,’ speed up the brain, and make information processing more accurate.”
Dr. Mahncke called this a “bottom-up” approach, in contrast to many previous cognitive-training approaches that come from the brain injury rehabilitation field. They teach “top-down” skills and strategies designed to compensate for deficits in specific domains, such as reading, concentration, or fine motor skills.
By contrast, the approach of BrainHQ is “to improve the overall processing system of the brain with speed, attention, working memory, and executive function, which will in turn impact all skills and activities.”
Supporting Evidence
Dr. Mahncke cited several supporting studies. For example, the IMPACT study randomized 487 adults (aged ≥ 65 years) to receive either a brain plasticity–based computerized cognitive training program (BrainHQ) or a novelty- and intensity-matched general cognitive stimulation treatment program (intervention and control group, respectively) for an 8-week period.
Those who underwent brain training showed significantly greater improvement in the repeatable Battery for the Assessment of Neuropsychological Status (RBANS Auditory Memory/Attention) compared with those in the control group (3.9 vs 1.8, respectively; P =.02). The intervention group also showed significant improvements on multiple secondary measures of attention and memory. The magnitude of the effect sizes suggests that the results are clinically significant, according to the authors.
The ACTIVE study tested the effects of different cognitive training programs on cognitive function and time to dementia. The researchers randomized 2802 healthy older adults (mean age, 74 years) to a control group with no cognitive training or one of three brain-training groups comprising:
1. In-person training on verbal memory skills
2. In-person training on reasoning and problem-solving
3. Computer-based speed-of-processing training on visual attention
Participants in the training groups completed 10 sessions, each lasting 60-75 minutes, over a 5- to 6-week period. A random subsample of each training group was selected to receive “booster” sessions, with four-session booster training delivered at 11 and 35 months. All study participants completed follow-up tests of cognition and function after 1, 2, 3, 5, and 10 years.
At the end of 10 years, those assigned to the speed-of-processing training, now part of BrainHQ, had a 29% lower risk for dementia than those in the control group who received no training. No reduction was found in the memory or reasoning training groups. Participants who completed the “booster” sessions had an even greater reduction: Each additional booster session was associated with a 10% lower risk for dementia.
Dr. Montero-Odasso was involved in the SYNERGIC study that randomized 175 participants with mild cognitive impairment (MCI; average age, 73 years) to one of five study arms:
1. Multidomain intervention with exercise, cognitive training, and vitamin D
2. Exercise, cognitive training, and placebo
3. Exercise, sham cognitive training, and vitamin D
4. Exercise, sham cognitive training, and placebo
5. Control group with balance-toning exercise, sham cognitive training, and placebo
“Sham” cognitive training consisted of alternating between two tasks (touristic search and video watching) performed on a tablet, with the same time exposure as the intervention training.
The researchers found that after 6 months of interventions, all active arms with aerobic-resistance exercise showed improvement in the ADAS-Cog-13, an established outcome to evaluate dementia treatments, when compared with the control group — regardless of the addition of cognitive training or vitamin D.
Compared with exercise alone (arms 3 and 4), those who did exercise plus cognitive training (arms 1 and 2) showed greater improvements in their ADAS-Cog-13l score, with a mean difference of −1.45 points (P = .02). The greatest improvement was seen in those who underwent the multidomain intervention in arm 1.
The authors noted that the mean 2.64-point improvement seen in the ADAS-Cog-13 for the multidomain intervention is actually larger than changes seen in previous pharmaceutical trials among individuals with MCI or mild dementia and “approaches” the three points considered clinically meaningful.
“We found that older adults with MCI who received aerobic-resistance exercise with sequential computerized cognitive training significantly improved cognition,” Dr. Montero-Odasso said. “The cognitive training we used was called Neuropeak, a multidomain lifestyle training delivered through a web-based platform developed by our co-leader Louis Bherer at Université de Montréal.”
He explained that the purpose “is to challenge your brain to the point where you need to make an effort to remember things, pay attention, and later to execute tasks. The evidence from clinical trials, including ours, shows this type of brain challenge is effective in slowing and even reversing cognitive decline.”
A follow-up study, SYNERGIC 2.0, is ongoing.
Puzzles, Board Games, and New Challenges
Formal brain-training programs aren’t the only way to improve brain plasticity, Dr. Hara said. Observational studies suggested an association between improved cognitive performance and/or lower dementia risk and engaging in number and word puzzles, such as crosswords, cards, or board games.
Some studies suggested that older adults who use technology might also protect their cognitive reserve. Dr. Hara cited a US longitudinal study of more than 18,000 older adults suggesting that regular Internet users had roughly half the risk for dementia compared to nonregular Internet users. Estimates of daily Internet use suggested a U-shaped relationship with dementia with 0.1-2.0 hours daily (excluding time spent watching television or movies online) associated with the lowest risk. Similar associations between Internet use and a lower risk for cognitive decline have been reported in the United Kingdom and Europe.
“Engaging in mentally stimulating activities can increase ‘cognitive reserve’ — meaning, capacity of the brain to resist the effects of age-related changes or disease-related pathology, such that one can maintain cognitive function for longer,” Dr. Hara said. “Cognitively stimulating activities, regardless of the type, may help delay the onset of cognitive decline.”
She listed several examples of activities that are stimulating to the brain, including learning a new game or puzzle, a new language, or a new dance, and learning how to play a musical instrument.
Dr. Montero-Odasso emphasized that the “newness” is key to increasing and preserving cognitive reserve. “Just surfing the Internet, playing word or board games, or doing crossword puzzles won’t be enough if you’ve been doing these things all your life,” he said. “It won’t hurt, of course, but it won’t necessarily increase your cognitive abilities.
“For example, a person who regularly engages in public speaking may not improve cognition by taking a public-speaking course, but someone who has never spoken before an audience might show cognitive improvements as a result of learning a new skill,” he said. “Or someone who knows several languages already might gain from learning a brand-new language.”
He cited research supporting the benefits of dancing, which he called “an ideal activity because it’s physical, so it provides the exercise that’s been associated with improved cognition. But it also requires learning new steps and moves, which builds the synapses in the brain. And the socialization of dance classes adds another component that can improve cognition.”
Dr. Mahncke hopes that beyond engaging in day-to-day new activities, seniors will participate in computerized brain training. “There’s no reason that evidence-based training can’t be offered in senior and community centers, as yoga and swimming are,” he said. “It doesn’t have to be simply something people do on their own virtually.”
Zoom classes and Medicare reimbursements are “good steps in the right direction, but it’s time to expand this potentially life-transformative intervention so that it reaches the ever-expanding population of seniors in the United States and beyond.”
Dr. Hara reported having no disclosures. Dr. Montero-Odasso reported having no commercial or financial interest related to this topic. He serves as the president of the Canadian Geriatrics Société and is team leader in the Canadian Consortium of Neurodegeneration in Aging. Dr. Mahncke is CEO of the brain training company Posit Science/BrainHQ.
A version of this article appeared on Medscape.com.
Working Hard or Work Addiction — Have You Crossed the Line?
When child psychiatrist Javeed Sukhera, MD, PhD, was a few years into his career, he found himself doing it all. “I was in a leadership role academically at the medical school, I had a leadership role at the hospital, and I was seeing as many patients as I could. I could work all day every day.”
“It still wouldn’t have been enough,” he said.
Whenever there was a shift available, Dr. Sukhera would take it. His job was stressful, but as a new physician with a young family, he saw this obsession with work as necessary. “I began to cope with the stress from work by doing extra work and feeling like I needed to be everywhere. It was like I became a hamster on a spinning wheel. I was just running, running, running.”
Things shifted for Dr. Sukhera when he realized that while he was emotionally available for the children who were his patients, at home, his own children weren’t getting the best of him. “There was a specific moment when I thought my son was afraid of me,” he said. “I just stopped and realized that there was something happening that I needed to break. I needed to make a change.”
Dr. Sukhera, now chair of psychiatry at the Institute of Living and chief of the Department of Psychiatry at Hartford Hospital, Hartford, Connecticut, believes what he experienced was a steep fall into work addiction.
What Does Work Addiction Look Like for Doctors?
Behavioral addictions are fairly new in the addiction space. When gambling disorder, the first and only behavioral addiction in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, was added in 2013, it was seen as a “breakthrough addiction,” said Mark D. Griffiths, PhD, a leading behavioral addiction researcher and a distinguished professor at Nottingham Trent University.
Because there is not enough evidence yet to classify work addiction as a formal diagnosis, there is no clear consensus on how to define it. To further complicate things, the terms “workaholism” and “work addiction” can be used interchangeably, and some experts say the two are not the same, though they can overlap.
That said, a 2018 review of literature from several countries found that work addiction “fits very well into recently postulated criteria for conceptualization of a behavioral addiction.
“If you accept that gambling can be genuinely addictive, then there’s no reason to think that something like work, exercise, or video game playing couldn’t be an addiction as well,” said Dr. Griffiths.
“The neurobiology of addiction is that we get drawn to something that gives us a dopamine hit,” Dr. Sukhera added. “But to do that all day, every day, has consequences. It drains our emotional reserves, and it can greatly impact our relationships.”
On top of that, work addiction has been linked with poor sleep, poor cardiovascular health, high blood pressure, burnout, the development of autoimmune disorders, and other health issues.
Physicians are particularly susceptible. Doctors, after all, are expected to work long hours and put their patients’ needs first, even at the expense of their own health and well-being.
“Workaholism is not just socially acceptable in medicine,” said Dr. Sukhera. “It’s baked into the system and built into the structures. The healthcare system has largely functioned on the emotional labor of health workers, whose tendency to show up and work harder can, at times, in certain organizations, be exploited.”
Dr. Griffiths agreed that with the limited amount of data available, work addiction does appear to exist at higher rates in medicine than in other fields. As early as the 1970s, medical literature describes work as a “socially acceptable” addiction among doctors. A 2014 study published in Occupational Medicine reported that of 445 physicians who took part in the research, nearly half exhibited some level of work addiction with 13% “highly work addicted.”
Of course, working hard or even meeting unreasonable demands from work is not the same as work addiction, as Dr. Griffiths clarified in a 2023 editorial in BMJ Quality & Safety. The difference, as with other behavioral addictions, is when people obsess about work and use it to cope with stress. It can be easier to stay distracted and busy to gain a sense of control rather than learning to deal with complex emotions.
A 2021 study that Dr. Sukhera conducted with resident physicians found that working harder was one of the main ways they dealt with stress during the COVID-19 pandemic. “This idea that we deal with the stress of being burnt out by doing more and more of what burns us out is fairly ubiquitous at all stages of medical professionals’ careers,” he said.
Financial incentives also can fuel work addiction, said Dr. Sukhera. In residency, there are some safeguards around overwork and duty hours. When you become an attending, those limits no longer exist. As a young physician, Dr. Sukhera had student debt to pay off and a family to support. When he found opportunities to earn more by working more, his answer was always “yes.”
Pressure to produce medical research also can pose issues. Some physicians can become addicted to publishing studies, fearing that they might lose their professional status or position if they stop. It’s a cycle that can force a doctor to not only work long hours doing their job but also practically take on a second one.
How Physicians Can Recognize Work Addiction in Themselves
Work addiction can look and feel different for every person, said Malissa Clark, PhD, associate professor at the University of Georgia and author of the recent book Never Not Working: Why the Always-On Culture Is Bad for Business—and How to Fix It.
Dr. Clark noted that people who are highly engaged in their work tend to be driven by intrinsic motivation: “You work because you love it.” With work addiction, “you work because you feel like you ought to be working all the time.”
Of course, it’s not always so cut and dried; you can experience both forms of motivation and not necessarily become addicted to work. But if you are solely driven by the feeling that you ought to be working all the time, that can be a red flag.
Dr. Griffiths said that while many people may have problematic work habits or work too much, true work addicts must meet six criteria that apply to all addictions:
1. Salience: Work is the single most important thing in your life, to the point of neglecting everything else. Even if you’re on vacation, your mind might be flooded with work thoughts.
2. Mood modification: You use work to modify your mood, either to get a “high” or to cope with stress.
3. Tolerance: Over time, you’ve gone from working 8 or 10 hours a day to 12 hours a day, to a point where you’re working all the time.
4. Withdrawal: On a physiological level, you will have symptoms such as anxiety, nausea, or headaches when unable to work.
5. Conflict: You feel conflicted with yourself (you know you’re working too much) or with others (partners, friends, and children) about work, but you can’t stop.
6. Relapse: If you manage to cut down your hours but can’t resist overworking 1 day, you wind up right back where you were.
When It’s Time to Address Work Addiction
The lack of a formal diagnosis for work addiction makes getting treatment difficult. But there are ways to seek help. Unlike the drug and alcohol literature, abstinence is not the goal. “The therapeutic goal is getting a behavior under control and looking for the triggers of why you’re compulsively working,” said Dr. Griffiths.
Practice self-compassion
Dr. Sukhera eventually realized that his work addiction stemmed from the fear of being somehow excluded or unworthy. He actively corrected much of this through self-compassion and self-kindness, which helped him set boundaries. “Self-compassion is the root of everything,” he said. “Reminding ourselves that we’re doing our best is an important ingredient in breaking the cycle.”
Slowly expose yourself to relaxation
Many workaholics find rest very difficult. “When I conducted interviews with people [who considered themselves workaholics], a very common thing I heard was, ‘I have a very hard time being idle,’ ” said Dr. Clark. If rest feels hard, Dr. Sukhera suggests practicing relaxation for 2 minutes to start. Even small periods of downtime can challenge the belief that you must be constantly productive.
Reframe your to-do list
For work addicts, to-do lists can seem like they must be finished, which prolongs work hours. Instead, use to-do lists to help prioritize what is urgent, identify what can wait, and delegate out tasks to others, Dr. Clark recommends.
Pick up a mastery experience
Research from professor Sabine Sonnentag, Dr. rer. nat., at the University of Mannheim, Mannheim, Germany, suggests that mastery experiences — leisure activities that require thought and focus like learning a new language or taking a woodworking class — can help you actively disengage from work.
Try cognitive behavioral therapy
Widely used for other forms of addiction, cognitive behavioral therapy centers around recognizing emotions, challenging thought patterns, and changing behaviors. However, Dr. Clark admits the research on its impact on work addiction, in particular, is “pretty nascent.”
Shift your mindset
It seems logical to think that detaching from your feelings will allow you to “do more,” but experts say that idea is both untrue and dangerous. “The safest hospitals are the hospitals where people are attuned to their humanness,” said Dr. Sukhera. “It’s normal to overwork in medicine, and if you’re challenging a norm, you really have to be thoughtful about how you frame that for yourself.”
Most importantly: Seek support
Today, there is increased awareness about work addiction and more resources for physicians who are struggling, including programs such as Workaholics Anonymous or Physicians Anonymous and workplace wellness initiatives. But try not to overwhelm yourself with choosing whom to talk to or what specific resource to utilize, Dr. Sukhera advised. “Just talk to someone about it. You don’t have to carry this on your own.”
A version of this article appeared on Medscape.com.
When child psychiatrist Javeed Sukhera, MD, PhD, was a few years into his career, he found himself doing it all. “I was in a leadership role academically at the medical school, I had a leadership role at the hospital, and I was seeing as many patients as I could. I could work all day every day.”
“It still wouldn’t have been enough,” he said.
Whenever there was a shift available, Dr. Sukhera would take it. His job was stressful, but as a new physician with a young family, he saw this obsession with work as necessary. “I began to cope with the stress from work by doing extra work and feeling like I needed to be everywhere. It was like I became a hamster on a spinning wheel. I was just running, running, running.”
Things shifted for Dr. Sukhera when he realized that while he was emotionally available for the children who were his patients, at home, his own children weren’t getting the best of him. “There was a specific moment when I thought my son was afraid of me,” he said. “I just stopped and realized that there was something happening that I needed to break. I needed to make a change.”
Dr. Sukhera, now chair of psychiatry at the Institute of Living and chief of the Department of Psychiatry at Hartford Hospital, Hartford, Connecticut, believes what he experienced was a steep fall into work addiction.
What Does Work Addiction Look Like for Doctors?
Behavioral addictions are fairly new in the addiction space. When gambling disorder, the first and only behavioral addiction in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, was added in 2013, it was seen as a “breakthrough addiction,” said Mark D. Griffiths, PhD, a leading behavioral addiction researcher and a distinguished professor at Nottingham Trent University.
Because there is not enough evidence yet to classify work addiction as a formal diagnosis, there is no clear consensus on how to define it. To further complicate things, the terms “workaholism” and “work addiction” can be used interchangeably, and some experts say the two are not the same, though they can overlap.
That said, a 2018 review of literature from several countries found that work addiction “fits very well into recently postulated criteria for conceptualization of a behavioral addiction.
“If you accept that gambling can be genuinely addictive, then there’s no reason to think that something like work, exercise, or video game playing couldn’t be an addiction as well,” said Dr. Griffiths.
“The neurobiology of addiction is that we get drawn to something that gives us a dopamine hit,” Dr. Sukhera added. “But to do that all day, every day, has consequences. It drains our emotional reserves, and it can greatly impact our relationships.”
On top of that, work addiction has been linked with poor sleep, poor cardiovascular health, high blood pressure, burnout, the development of autoimmune disorders, and other health issues.
Physicians are particularly susceptible. Doctors, after all, are expected to work long hours and put their patients’ needs first, even at the expense of their own health and well-being.
“Workaholism is not just socially acceptable in medicine,” said Dr. Sukhera. “It’s baked into the system and built into the structures. The healthcare system has largely functioned on the emotional labor of health workers, whose tendency to show up and work harder can, at times, in certain organizations, be exploited.”
Dr. Griffiths agreed that with the limited amount of data available, work addiction does appear to exist at higher rates in medicine than in other fields. As early as the 1970s, medical literature describes work as a “socially acceptable” addiction among doctors. A 2014 study published in Occupational Medicine reported that of 445 physicians who took part in the research, nearly half exhibited some level of work addiction with 13% “highly work addicted.”
Of course, working hard or even meeting unreasonable demands from work is not the same as work addiction, as Dr. Griffiths clarified in a 2023 editorial in BMJ Quality & Safety. The difference, as with other behavioral addictions, is when people obsess about work and use it to cope with stress. It can be easier to stay distracted and busy to gain a sense of control rather than learning to deal with complex emotions.
A 2021 study that Dr. Sukhera conducted with resident physicians found that working harder was one of the main ways they dealt with stress during the COVID-19 pandemic. “This idea that we deal with the stress of being burnt out by doing more and more of what burns us out is fairly ubiquitous at all stages of medical professionals’ careers,” he said.
Financial incentives also can fuel work addiction, said Dr. Sukhera. In residency, there are some safeguards around overwork and duty hours. When you become an attending, those limits no longer exist. As a young physician, Dr. Sukhera had student debt to pay off and a family to support. When he found opportunities to earn more by working more, his answer was always “yes.”
Pressure to produce medical research also can pose issues. Some physicians can become addicted to publishing studies, fearing that they might lose their professional status or position if they stop. It’s a cycle that can force a doctor to not only work long hours doing their job but also practically take on a second one.
How Physicians Can Recognize Work Addiction in Themselves
Work addiction can look and feel different for every person, said Malissa Clark, PhD, associate professor at the University of Georgia and author of the recent book Never Not Working: Why the Always-On Culture Is Bad for Business—and How to Fix It.
Dr. Clark noted that people who are highly engaged in their work tend to be driven by intrinsic motivation: “You work because you love it.” With work addiction, “you work because you feel like you ought to be working all the time.”
Of course, it’s not always so cut and dried; you can experience both forms of motivation and not necessarily become addicted to work. But if you are solely driven by the feeling that you ought to be working all the time, that can be a red flag.
Dr. Griffiths said that while many people may have problematic work habits or work too much, true work addicts must meet six criteria that apply to all addictions:
1. Salience: Work is the single most important thing in your life, to the point of neglecting everything else. Even if you’re on vacation, your mind might be flooded with work thoughts.
2. Mood modification: You use work to modify your mood, either to get a “high” or to cope with stress.
3. Tolerance: Over time, you’ve gone from working 8 or 10 hours a day to 12 hours a day, to a point where you’re working all the time.
4. Withdrawal: On a physiological level, you will have symptoms such as anxiety, nausea, or headaches when unable to work.
5. Conflict: You feel conflicted with yourself (you know you’re working too much) or with others (partners, friends, and children) about work, but you can’t stop.
6. Relapse: If you manage to cut down your hours but can’t resist overworking 1 day, you wind up right back where you were.
When It’s Time to Address Work Addiction
The lack of a formal diagnosis for work addiction makes getting treatment difficult. But there are ways to seek help. Unlike the drug and alcohol literature, abstinence is not the goal. “The therapeutic goal is getting a behavior under control and looking for the triggers of why you’re compulsively working,” said Dr. Griffiths.
Practice self-compassion
Dr. Sukhera eventually realized that his work addiction stemmed from the fear of being somehow excluded or unworthy. He actively corrected much of this through self-compassion and self-kindness, which helped him set boundaries. “Self-compassion is the root of everything,” he said. “Reminding ourselves that we’re doing our best is an important ingredient in breaking the cycle.”
Slowly expose yourself to relaxation
Many workaholics find rest very difficult. “When I conducted interviews with people [who considered themselves workaholics], a very common thing I heard was, ‘I have a very hard time being idle,’ ” said Dr. Clark. If rest feels hard, Dr. Sukhera suggests practicing relaxation for 2 minutes to start. Even small periods of downtime can challenge the belief that you must be constantly productive.
Reframe your to-do list
For work addicts, to-do lists can seem like they must be finished, which prolongs work hours. Instead, use to-do lists to help prioritize what is urgent, identify what can wait, and delegate out tasks to others, Dr. Clark recommends.
Pick up a mastery experience
Research from professor Sabine Sonnentag, Dr. rer. nat., at the University of Mannheim, Mannheim, Germany, suggests that mastery experiences — leisure activities that require thought and focus like learning a new language or taking a woodworking class — can help you actively disengage from work.
Try cognitive behavioral therapy
Widely used for other forms of addiction, cognitive behavioral therapy centers around recognizing emotions, challenging thought patterns, and changing behaviors. However, Dr. Clark admits the research on its impact on work addiction, in particular, is “pretty nascent.”
Shift your mindset
It seems logical to think that detaching from your feelings will allow you to “do more,” but experts say that idea is both untrue and dangerous. “The safest hospitals are the hospitals where people are attuned to their humanness,” said Dr. Sukhera. “It’s normal to overwork in medicine, and if you’re challenging a norm, you really have to be thoughtful about how you frame that for yourself.”
Most importantly: Seek support
Today, there is increased awareness about work addiction and more resources for physicians who are struggling, including programs such as Workaholics Anonymous or Physicians Anonymous and workplace wellness initiatives. But try not to overwhelm yourself with choosing whom to talk to or what specific resource to utilize, Dr. Sukhera advised. “Just talk to someone about it. You don’t have to carry this on your own.”
A version of this article appeared on Medscape.com.
When child psychiatrist Javeed Sukhera, MD, PhD, was a few years into his career, he found himself doing it all. “I was in a leadership role academically at the medical school, I had a leadership role at the hospital, and I was seeing as many patients as I could. I could work all day every day.”
“It still wouldn’t have been enough,” he said.
Whenever there was a shift available, Dr. Sukhera would take it. His job was stressful, but as a new physician with a young family, he saw this obsession with work as necessary. “I began to cope with the stress from work by doing extra work and feeling like I needed to be everywhere. It was like I became a hamster on a spinning wheel. I was just running, running, running.”
Things shifted for Dr. Sukhera when he realized that while he was emotionally available for the children who were his patients, at home, his own children weren’t getting the best of him. “There was a specific moment when I thought my son was afraid of me,” he said. “I just stopped and realized that there was something happening that I needed to break. I needed to make a change.”
Dr. Sukhera, now chair of psychiatry at the Institute of Living and chief of the Department of Psychiatry at Hartford Hospital, Hartford, Connecticut, believes what he experienced was a steep fall into work addiction.
What Does Work Addiction Look Like for Doctors?
Behavioral addictions are fairly new in the addiction space. When gambling disorder, the first and only behavioral addiction in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, was added in 2013, it was seen as a “breakthrough addiction,” said Mark D. Griffiths, PhD, a leading behavioral addiction researcher and a distinguished professor at Nottingham Trent University.
Because there is not enough evidence yet to classify work addiction as a formal diagnosis, there is no clear consensus on how to define it. To further complicate things, the terms “workaholism” and “work addiction” can be used interchangeably, and some experts say the two are not the same, though they can overlap.
That said, a 2018 review of literature from several countries found that work addiction “fits very well into recently postulated criteria for conceptualization of a behavioral addiction.
“If you accept that gambling can be genuinely addictive, then there’s no reason to think that something like work, exercise, or video game playing couldn’t be an addiction as well,” said Dr. Griffiths.
“The neurobiology of addiction is that we get drawn to something that gives us a dopamine hit,” Dr. Sukhera added. “But to do that all day, every day, has consequences. It drains our emotional reserves, and it can greatly impact our relationships.”
On top of that, work addiction has been linked with poor sleep, poor cardiovascular health, high blood pressure, burnout, the development of autoimmune disorders, and other health issues.
Physicians are particularly susceptible. Doctors, after all, are expected to work long hours and put their patients’ needs first, even at the expense of their own health and well-being.
“Workaholism is not just socially acceptable in medicine,” said Dr. Sukhera. “It’s baked into the system and built into the structures. The healthcare system has largely functioned on the emotional labor of health workers, whose tendency to show up and work harder can, at times, in certain organizations, be exploited.”
Dr. Griffiths agreed that with the limited amount of data available, work addiction does appear to exist at higher rates in medicine than in other fields. As early as the 1970s, medical literature describes work as a “socially acceptable” addiction among doctors. A 2014 study published in Occupational Medicine reported that of 445 physicians who took part in the research, nearly half exhibited some level of work addiction with 13% “highly work addicted.”
Of course, working hard or even meeting unreasonable demands from work is not the same as work addiction, as Dr. Griffiths clarified in a 2023 editorial in BMJ Quality & Safety. The difference, as with other behavioral addictions, is when people obsess about work and use it to cope with stress. It can be easier to stay distracted and busy to gain a sense of control rather than learning to deal with complex emotions.
A 2021 study that Dr. Sukhera conducted with resident physicians found that working harder was one of the main ways they dealt with stress during the COVID-19 pandemic. “This idea that we deal with the stress of being burnt out by doing more and more of what burns us out is fairly ubiquitous at all stages of medical professionals’ careers,” he said.
Financial incentives also can fuel work addiction, said Dr. Sukhera. In residency, there are some safeguards around overwork and duty hours. When you become an attending, those limits no longer exist. As a young physician, Dr. Sukhera had student debt to pay off and a family to support. When he found opportunities to earn more by working more, his answer was always “yes.”
Pressure to produce medical research also can pose issues. Some physicians can become addicted to publishing studies, fearing that they might lose their professional status or position if they stop. It’s a cycle that can force a doctor to not only work long hours doing their job but also practically take on a second one.
How Physicians Can Recognize Work Addiction in Themselves
Work addiction can look and feel different for every person, said Malissa Clark, PhD, associate professor at the University of Georgia and author of the recent book Never Not Working: Why the Always-On Culture Is Bad for Business—and How to Fix It.
Dr. Clark noted that people who are highly engaged in their work tend to be driven by intrinsic motivation: “You work because you love it.” With work addiction, “you work because you feel like you ought to be working all the time.”
Of course, it’s not always so cut and dried; you can experience both forms of motivation and not necessarily become addicted to work. But if you are solely driven by the feeling that you ought to be working all the time, that can be a red flag.
Dr. Griffiths said that while many people may have problematic work habits or work too much, true work addicts must meet six criteria that apply to all addictions:
1. Salience: Work is the single most important thing in your life, to the point of neglecting everything else. Even if you’re on vacation, your mind might be flooded with work thoughts.
2. Mood modification: You use work to modify your mood, either to get a “high” or to cope with stress.
3. Tolerance: Over time, you’ve gone from working 8 or 10 hours a day to 12 hours a day, to a point where you’re working all the time.
4. Withdrawal: On a physiological level, you will have symptoms such as anxiety, nausea, or headaches when unable to work.
5. Conflict: You feel conflicted with yourself (you know you’re working too much) or with others (partners, friends, and children) about work, but you can’t stop.
6. Relapse: If you manage to cut down your hours but can’t resist overworking 1 day, you wind up right back where you were.
When It’s Time to Address Work Addiction
The lack of a formal diagnosis for work addiction makes getting treatment difficult. But there are ways to seek help. Unlike the drug and alcohol literature, abstinence is not the goal. “The therapeutic goal is getting a behavior under control and looking for the triggers of why you’re compulsively working,” said Dr. Griffiths.
Practice self-compassion
Dr. Sukhera eventually realized that his work addiction stemmed from the fear of being somehow excluded or unworthy. He actively corrected much of this through self-compassion and self-kindness, which helped him set boundaries. “Self-compassion is the root of everything,” he said. “Reminding ourselves that we’re doing our best is an important ingredient in breaking the cycle.”
Slowly expose yourself to relaxation
Many workaholics find rest very difficult. “When I conducted interviews with people [who considered themselves workaholics], a very common thing I heard was, ‘I have a very hard time being idle,’ ” said Dr. Clark. If rest feels hard, Dr. Sukhera suggests practicing relaxation for 2 minutes to start. Even small periods of downtime can challenge the belief that you must be constantly productive.
Reframe your to-do list
For work addicts, to-do lists can seem like they must be finished, which prolongs work hours. Instead, use to-do lists to help prioritize what is urgent, identify what can wait, and delegate out tasks to others, Dr. Clark recommends.
Pick up a mastery experience
Research from professor Sabine Sonnentag, Dr. rer. nat., at the University of Mannheim, Mannheim, Germany, suggests that mastery experiences — leisure activities that require thought and focus like learning a new language or taking a woodworking class — can help you actively disengage from work.
Try cognitive behavioral therapy
Widely used for other forms of addiction, cognitive behavioral therapy centers around recognizing emotions, challenging thought patterns, and changing behaviors. However, Dr. Clark admits the research on its impact on work addiction, in particular, is “pretty nascent.”
Shift your mindset
It seems logical to think that detaching from your feelings will allow you to “do more,” but experts say that idea is both untrue and dangerous. “The safest hospitals are the hospitals where people are attuned to their humanness,” said Dr. Sukhera. “It’s normal to overwork in medicine, and if you’re challenging a norm, you really have to be thoughtful about how you frame that for yourself.”
Most importantly: Seek support
Today, there is increased awareness about work addiction and more resources for physicians who are struggling, including programs such as Workaholics Anonymous or Physicians Anonymous and workplace wellness initiatives. But try not to overwhelm yourself with choosing whom to talk to or what specific resource to utilize, Dr. Sukhera advised. “Just talk to someone about it. You don’t have to carry this on your own.”
A version of this article appeared on Medscape.com.
Oregon Physician Assistants Get Name Change
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
Device Uses Sleep Data to Pinpoint Stress Risk
TOPLINE:
Decreased total sleep time (TST) and increased resting heart rate (RHR), heart rate variability (HRV), and average nightly respiratory rate (ARR) as measured by a multisensor device worn during sleep accurately correlated with self-reported stress levels in college students, a new study suggests.
METHODOLOGY:
- First-semester college students (n = 525; aged 18-24 years) enrolled in the Lived Experiences measured Using Rings Study (LEMURS) provided continuous biometric data via a wearable device (Oura Ring; Oura Health) and answered weekly surveys regarding stress levels.
- The researchers used mixed-effects regression models to identify associations between perceived stress scores and average nightly TST, RHR, HRV, and ARR.
TAKEAWAY:
- Consistent associations were found between perceived stress scores and TST, RHR, HRV, and ARR, which persisted even after controlling for gender and week of the semester.
- Risk for moderate to high stress decreased by 38% with every additional hour of TST (P < .01) and by 1.2% with each millisecond increase in HRV (P < .05).
- Moderate to high stress risk increased by 3.6% with each beat-per-minute-increase in RHR (P < .01) and by 23% with each additional breath-per-minute increase in ARR (P < .01).
- Participants who identified as female, nonbinary, or transgender reported significantly higher stress throughout the study.
IN PRACTICE:
“The present work highlights the potential utility of monitoring sleep, suggesting that these measures may identify within individual changes that are concerning for stress. As the demand for mental health services grows, determining which wearable-derived sleep estimates provide information about well-being and can predict worsening mental health in young adults is an important area of study,” study authors wrote.
SOURCE:
The study, led by Laura S.P. Bloomfield, University of Vermont, Burlington, Vermont, was published online in PLOS Digital Health.
LIMITATIONS:
The study focused on raw sleep measures; the researchers suggest that future studies evaluate additional sleep variables (eg, daytime naps), which have been associated with mental health in college students. In addition, the researchers did not have stress or sleep data before participants started college, so they could not assess the impact of starting college on participants’ sleep.
DISCLOSURES:
Bloomfield was supported by the Gund Fellowship and received a partial salary from the Mass Mutual Insurance Wellness Initiative. Other authors’ funding is reported in the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
Decreased total sleep time (TST) and increased resting heart rate (RHR), heart rate variability (HRV), and average nightly respiratory rate (ARR) as measured by a multisensor device worn during sleep accurately correlated with self-reported stress levels in college students, a new study suggests.
METHODOLOGY:
- First-semester college students (n = 525; aged 18-24 years) enrolled in the Lived Experiences measured Using Rings Study (LEMURS) provided continuous biometric data via a wearable device (Oura Ring; Oura Health) and answered weekly surveys regarding stress levels.
- The researchers used mixed-effects regression models to identify associations between perceived stress scores and average nightly TST, RHR, HRV, and ARR.
TAKEAWAY:
- Consistent associations were found between perceived stress scores and TST, RHR, HRV, and ARR, which persisted even after controlling for gender and week of the semester.
- Risk for moderate to high stress decreased by 38% with every additional hour of TST (P < .01) and by 1.2% with each millisecond increase in HRV (P < .05).
- Moderate to high stress risk increased by 3.6% with each beat-per-minute-increase in RHR (P < .01) and by 23% with each additional breath-per-minute increase in ARR (P < .01).
- Participants who identified as female, nonbinary, or transgender reported significantly higher stress throughout the study.
IN PRACTICE:
“The present work highlights the potential utility of monitoring sleep, suggesting that these measures may identify within individual changes that are concerning for stress. As the demand for mental health services grows, determining which wearable-derived sleep estimates provide information about well-being and can predict worsening mental health in young adults is an important area of study,” study authors wrote.
SOURCE:
The study, led by Laura S.P. Bloomfield, University of Vermont, Burlington, Vermont, was published online in PLOS Digital Health.
LIMITATIONS:
The study focused on raw sleep measures; the researchers suggest that future studies evaluate additional sleep variables (eg, daytime naps), which have been associated with mental health in college students. In addition, the researchers did not have stress or sleep data before participants started college, so they could not assess the impact of starting college on participants’ sleep.
DISCLOSURES:
Bloomfield was supported by the Gund Fellowship and received a partial salary from the Mass Mutual Insurance Wellness Initiative. Other authors’ funding is reported in the original article.
A version of this article appeared on Medscape.com.
TOPLINE:
Decreased total sleep time (TST) and increased resting heart rate (RHR), heart rate variability (HRV), and average nightly respiratory rate (ARR) as measured by a multisensor device worn during sleep accurately correlated with self-reported stress levels in college students, a new study suggests.
METHODOLOGY:
- First-semester college students (n = 525; aged 18-24 years) enrolled in the Lived Experiences measured Using Rings Study (LEMURS) provided continuous biometric data via a wearable device (Oura Ring; Oura Health) and answered weekly surveys regarding stress levels.
- The researchers used mixed-effects regression models to identify associations between perceived stress scores and average nightly TST, RHR, HRV, and ARR.
TAKEAWAY:
- Consistent associations were found between perceived stress scores and TST, RHR, HRV, and ARR, which persisted even after controlling for gender and week of the semester.
- Risk for moderate to high stress decreased by 38% with every additional hour of TST (P < .01) and by 1.2% with each millisecond increase in HRV (P < .05).
- Moderate to high stress risk increased by 3.6% with each beat-per-minute-increase in RHR (P < .01) and by 23% with each additional breath-per-minute increase in ARR (P < .01).
- Participants who identified as female, nonbinary, or transgender reported significantly higher stress throughout the study.
IN PRACTICE:
“The present work highlights the potential utility of monitoring sleep, suggesting that these measures may identify within individual changes that are concerning for stress. As the demand for mental health services grows, determining which wearable-derived sleep estimates provide information about well-being and can predict worsening mental health in young adults is an important area of study,” study authors wrote.
SOURCE:
The study, led by Laura S.P. Bloomfield, University of Vermont, Burlington, Vermont, was published online in PLOS Digital Health.
LIMITATIONS:
The study focused on raw sleep measures; the researchers suggest that future studies evaluate additional sleep variables (eg, daytime naps), which have been associated with mental health in college students. In addition, the researchers did not have stress or sleep data before participants started college, so they could not assess the impact of starting college on participants’ sleep.
DISCLOSURES:
Bloomfield was supported by the Gund Fellowship and received a partial salary from the Mass Mutual Insurance Wellness Initiative. Other authors’ funding is reported in the original article.
A version of this article appeared on Medscape.com.
Novel Agent Curbs Alzheimer’s-Related Agitation
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
DENVER —
More than half of participants in the open-label extension period of the randomized clinical trial responded to the medication, which was associated with a 3.6-fold lower risk for relapse compared with placebo.
“The positive efficacy and favorable safety results with AXS-05 support its potential to fulfill a high unmet need for the treatment of Alzheimer’s disease agitation,” said Anton P. Porsteinsson, MD, director of the Alzheimer’s Disease Care, Research and Education Program, University of Rochester, New York.
The findings were presented at the 2024 annual meeting of the American Academy of Neurology.
Common and Disruptive
Agitation is reported in up to 70% of patients with Alzheimer’s disease and is characterized by emotional distress, aggressive behaviors, disruptive irritability, and disinhibition. Alzheimer’s disease-related agitation has been associated with increased caregiver burden, decreased functioning, accelerated cognitive decline, earlier nursing home placement, and increased mortality.
A previous phase 2/3 study of AXS-05 showed that the investigative agent led to rapid and significantly improvement in Alzheimer’s disease agitation, as measured by the Cohen-Mansfield Agitation Inventory (CMAI) total score, compared with placebo.
ACCORD was a phase 3, randomized, double-blind, placebo-controlled withdrawal trial evaluating the efficacy and safety of AXS-05 in patients with Alzheimer’s disease agitation.
In the open-label period, 178 adults with probable Alzheimer’s disease and clinically significant agitation received AXS-05 (titrated to 45 mg dextromethorphan/105 mg bupropion twice daily) for up to 9 weeks.
A total of 108 (61%) patients had a sustained response, with 30% or more improvement from baseline in the CMAI total score and improvement on the Patient Global Impression of Change that were both maintained for 4 or more consecutive weeks. These patients entered the double-blind phase and were randomly allocated to receive twice-daily AXS-05 or placebo for up to 26 weeks.
In the double-blind period, AXS-05 “substantially and statistically” increased the time to relapse of agitation symptoms compared with placebo (hazard ratio [HR], 0.275; P = .014).
“The risk of relapse was 3.6-fold lower with AXS-05 compared with placebo,” Dr. Porsteinsson reported.
AXS-05 was also associated with a significantly lower relapse rate compared with placebo (7.5% vs 25.9%; P = .018).
Rates of discontinuation in the double-blind period owing to adverse events (AEs) were low (0% for AXS-05 and 1.9% for placebo). Three serious AEs were reported: one in the AXS-05 group (fecaloma), which was not related to study medication, and two in the placebo group (cardiac arrest, femur fracture).
Falls were reported in four participants in the AXS-05 group, none of which were related to study medication or associated with serious AEs, and in two participants in the placebo group, one of which was associated with femur fracture.
One death was reported in the placebo group. There was no evidence of cognitive decline with AXS-05, and treatment was not associated with sedation.
Promising Agent
Commenting on this research, Glen R. Finney, MD, director of the Geisinger Memory and Cognition Clinic in Wilkes-Barre, Pennsylvania, said the data “look promising as a safe way to help address acute agitation and reduce agitation reoccurrence.
“Agitation is a common, distressing, and sometimes safety issue for people fighting Alzheimer’s disease, and there’s very little evidence for efficacy and significant side effect issues for current medical management of agitation in Alzheimer’s disease,” said Dr. Finney, who was not part of the study.
He noted that first-line strategies for addressing agitation involve behavioral and environmental interventions.
“See if there’s a reason for the agitation and address that. Look for triggers for agitation and avoid those. Find places, things, and interactions that help people with Alzheimer’s disease avoid agitation: familiar locations, music, simple engaging activities. Reassurance, redirection, and distraction can help de-escalate agitation. Provide a safe environment that reduces safety risks,” Dr. Finney explained.
The next step, when medically appropriate, is trying acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine, and then adding memantine, a weak N-methyl-D-aspartate receptor antagonist.
“These medications can help reduce the risk of agitation,” Dr. Finney said.
“Beyond that, the evidence becomes weaker for any specific treatments, and that is where treatments with emerging evidence of efficacy and safety like dextromethorphan-bupropion become important,” Dr. Finney added.
Last May, the US Food and Drug Administration (FDA) approved the antipsychotic brexpiprazole (Rexulti) for Alzheimer’s disease-related agitation, making it the first FDA-approved drug for this indication.
The drug includes a boxed warning for medications in this class that older patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk for death.
“There’s certainly a need to have multiple options for treating agitation in individuals with Alzheimer’s disease,” said Rebecca Edelmayer, PhD, senior director of scientific engagement for the Alzheimer’s Association.
Dr. Edelmayer, who was not part of the study, noted that in the ACCORD study, AXS-05 “significantly delayed the relapse or prevented the relapse with Alzheimer’s disease agitation compared with the placebo group and it was generally well tolerated, but it will be important to make sure that there’s more thorough review of the data overall to be sure that it’s both safe and effective.”
The study was funded by Axsome Therapeutics, the manufacturer of AXS-05. Dr. Porsteinsson has disclosed no relevant conflicts of interest. Dr. Finney and Dr. Edelmayer have no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM AAN 2024
Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.