User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
EoE: One-food elimination works as well as six-food elimination
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
according to a new report.
A one-food elimination diet (1FED) led to histologic remission in 34% of patients, as determined on the basis of eosinophil count at 6 weeks, and in 40% of patients who followed a six-food elimination diet (6FED) – a nonstatistical difference, the research team wrote.
“The takeaway message is that one-food (milk) elimination is an effective treatment and a reasonable first-line treatment for EoE,” senior study author Marc Rothenberg, MD, PhD, a professor of pediatrics and director of the allergy and immunology division at the Cincinnati Center for Eosinophilic Disorders at Cincinnati Children’s Hospital Medical Center, said in an interview.
“The study was designed by the Consortium of Eosinophilic Disease Researchers (CEGIR), which includes the nation’s top institutions working with patient advocacy groups, together with the National Institutes of Health,” he said. “The group, under advice from patients, determined that it was an important question to research if one-food elimination would be effective – and how effective – compared with six-food elimination.”
The study was published in The Lancet Gastroenterology and Hepatology.
Studying EOE and food elimination
Previous studies have found that eliminating six common foods that trigger esophageal injury – milk, eggs, wheat, soy, fish, and nuts – can substantially reduce EoE symptoms. The 6FED has become a common approach to managing the disease.
In recent years, however, researchers have conducted small, nonrandomized studies of the less restrictive 1FED and have found some success.
In a multisite, randomized trial, Dr. Rothenberg and colleagues compared the 6FED with the 1FED among 129 adults aged 18-60 years with a confirmed EoE diagnosis, active EoE symptoms, and a high number of eosinophils in esophageal tissue. The participants enrolled at 1 of 10 U.S. medical centers that participate in CEGIR, which is part of the NIH-funded Rare Diseases Clinical Research Network.
Between 2016 and 2019, 67 participants were assigned to the 1FED group, which eliminated only animal milk from the diet, and 62 participants were assigned to the 6FED group, which eliminated milk, eggs, wheat, soy, fish/shellfish, and peanuts/tree nuts. After following the diet for 6 weeks, participants underwent an upper endoscopy exam and esophageal tissue biopsy. The primary endpoint was the proportion of patients with histologic remission, or a peak count of less than 15 eosinophils per high-power field (eos/hpf).
If the number of eosinophils indicated that EoE was in remission, the participant exited the study. If EoE wasn’t in remission, those who were on 1FED could proceed to 6FED, and those who were on 6FED could take fluticasone propionate 880 mcg two times per day with an unrestricted diet. Both groups followed the protocols for 6 weeks and underwent another exam with tissue biopsy.
At 6 weeks, 25 patients (40%) on 6FED and 23 patients (34%) on 1FED achieved histologic remission. The difference was not statistically significant.
There were also no significant differences between the groups at stricter thresholds for partial remission, defined as peak counts of 10 eos/hpf or less and 6 eos/hpf or less. The rate of complete remission (at a peak count of ≤ 1 eos/hpf) favored 6FED, at 19% versus 6% among 1FED.
The two diets had a similar impact across several other measures, including reduction in peak eosinophil counts, reduction in EoE symptoms, and improvement in quality of life. For 6FED versus 1FED, the mean changes from baseline in the Eosinophilic Esophagitis Histology Scoring System were –0.23 versus –0.15. In addition, the mean changes in the Eosinophilic Esophagitis Endoscopic Reference Score were 1 versus –0.6, and in the Eosinophilic Esophagitis Activity Index, they were –8.2 versus –3. None of the differences were significant.
Among the patients who didn’t respond to 1FED, 21 opted to follow 6FED in the study’s second phase. Of those patients, nine (43%) attained remission after following the more restrictive diet. Among the 11 patients who didn’t initially respond to 6FED and who opted to receive fluticasone propionate, nine patients (82%) achieved remission.
“We examined a series of validated endpoints that have not previously been examined in diet trials,” Dr. Rothenberg said. “We are surprised to see that one food was equally effective as six foods.”
Incorporating food elimination therapy
Dr. Rothenberg and colleagues are continuing their research into EoE and food-elimination diets, with a strong focus on furthering diet therapy. In particular, the research team wants to understand how to potentially add milk – and other foods – back to the diet.
Wael Sayej, MD, associate professor of pediatrics at the University of Massachusetts Baystate Regional Campus, Springfield, has found success with the one-food elimination diet among children with EoE, he said in an interview.
In a retrospective study, Dr. Sayej and colleagues found that a one-food elimination diet was an effective first-line treatment option for pediatric patients.
“Once we get past the one-food or two-food elimination, it becomes much more difficult and cumbersome for patients to follow,” said Dr. Sayej, who is also a pediatric gastroenterologist with Baystate Health in Springfield and who wasn’t involved with the CEGIR study. “Obviously, I prefer my patients to follow a strict dairy-free diet as long-term therapy, rather than have them on a medication for the rest of their life.”
Dr. Sayej advises patients to follow the one-food elimination diet in his practice. If patients aren’t responsive, he offers options for additional dietary elimination or initiation of steroid therapy.
“The most important thing about initiating dietary elimination therapy is to take the time to educate the patient and family about the disease, the risks or complications associated with untreated disease, and the pros and cons of the treatment options,” he said.
The study was cofunded by the National Institute of Allergy and Infectious Diseases, the National Center for Advancing Translational Sciences, and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have research, consultant, and leadership relationships with several pharmaceutical companies and organizations not related to this study. Dr. Sayej disclosed no relevant financial relationships.
FROM THE LANCET GASTROENTEROLOGY AND HEPATOLOGY
Widespread flaky red skin
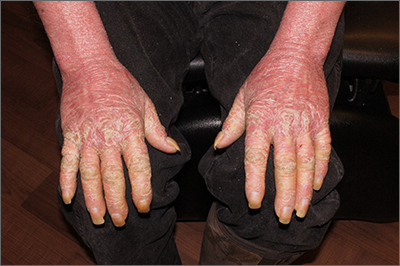
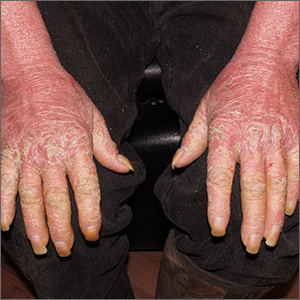
This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345

This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345
COORDINATEd effort boosts optimal therapy in patients with T2D and ASCVD
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
AT ACC 2023
Med center and top cardio surgeon must pay $8.5 million for fraud, concurrent surgeries
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
What happens if we sit for more than 8 hours per day?
according to a recent Latin American study published in BMC Public Health.
These data come from almost 8,000 people aged 20-65 years (half of whom are women) who participated in the Latin American Study on Nutrition and Health (ELANS). The cross-sectional survey included representative samples from urban populations in Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Peru, and Venezuela. The average time spent sitting was 420 min/d. Ecuador had the lowest time (300 min/day), and Argentina and Peru had the highest (480 min/day).
No amount of sitting time has been associated with a greater health risk, but the World Health Organization recommends that sitting time be minimal.
“We used to believe that any intense physical exercise could compensate for a sedentary life. But now we know that a sedentary lifestyle in general and sitting time in particular have a direct effect and are an independent risk factor for chronic diseases,” said study author Irina Kovalskys, PhD, a pediatric specialist in nutrition and a professor of nutrition at the Catholic University of Argentina, Buenos Aires, and a principal investigator of ELANS.
Dr. Kovalskys stated that the 420-min average sitting time is worrying in a population such as the one studied, in which 60% of adults are obese and there are high rates of cardiometabolic risk factors. She affirmed that it is important to raise awareness among the population and focus on adolescents.
Felipe Lobelo, PhD, is a Colombian physician, an associate professor of global health at Emory University and director of epidemiology at Kaiser Permanente Georgia, both in Atlanta. He did not participate in this study but promotes the concept of exercise in medicine. The activity of the patient must be included in a clinical setting, and improving the level of physical activity can have a positive impact on health prognosis, he said.
“To make public health recommendations or even advise patients, a cutoff point is needed. Guidelines recommend 150 minutes per week of moderate to vigorous physical activity, and some countries have started to indicate that we should be concerned about people’s sitting time. There is still no equivalent to the 150 minutes, therefore, these studies are important, especially in the Latin American population,” said Dr. Lobelo.
He explained that the concept of an increased risk of death or chronic disease because of a lack of physical activity arose in the past 50 years, but only in the past 2 decades have we started thinking about sitting time.
“Spending more than 8 hours sitting per day clearly causes a much higher risk of chronic diseases, including obesity and diabetes. It may be a continuous and progressive association, and the point at which this increase becomes exponential is clearly between 6 and 8 hours of sitting time,” Dr. Lobelo added.
The authors expected to find a linear association with risk for being overweight or obese after 4 hours, but they did not find one. “This study has limitations. Among them was that other indicators were not considered, such as health indicators. Collaborations are starting with other research groups, and other studies are being designed,” said study author Gerson Ferrari, PhD, an associate professor at Santiago de Chile University.
Comparing indicators
The Latin American study tried to establish a sitting cutoff time after which the risk of becoming overweight or obese increases. It used three indicators of excess weight: body mass index (BMI), waist circumference, and neck circumference.
Sitting for more than 8 hours increased the chances of excess weight by 10% when measured by BMI and by 13% when neck circumference was used.
Dr. Ferrari stated that the result obtained measuring BMI is the one that should be considered, because it is used in public policy. Neck circumference is a more recent measurement of detection and it is less studied, but it is a valid indicator, with good sensitivity and advantages over others, such as ease of measurement and lack of variation over time.
According to the results of this study, measuring neck circumference may be the most sensitive method of the three. Neck circumference was proportionally greater in people who sat for at least 4, at least 6, and at least 8 hours/day than in those who sat for less than 4, less than 6, and less than 8 hours/day. This relationship was not observed with the other indicators.
Broaching the topic
“What is important is uninterrupted sitting time. The recommendation is to break up those sitting times with active periods. Health professionals have already incorporated the concept of moderate to vigorous physical exercise, but nonintense activities are sufficient to reduce sitting time. Yoga may not be vigorous, but it is valuable at reducing sitting time,” said Dr. Kovalskys.
Dr. Ferrari recommended giving patients concrete messages so that they spend as little time possible sitting. “It is better to stand on the bus or the subway even when there is a place to sit. Are you going to talk on the phone? It is better to do it while walking or at least standing instead of sitting.”
A recent literature review conducted by investigators of the University of Birmingham (England) studied the possible molecular and physiologic mechanisms of inactivity time, health consequences, and protection strategies. It offers an evaluation of interventions that can compensate for the immediate negative consequences of inactivity.
Physical activity
Some studies suggest that more than 60 min/day of moderate-intensity exercise or more than 150 min/week of moderate to vigorous exercise may be effective at mitigating the increased risk for mortality associated with sitting time, but reduced intensity may not be enough.
Active pauses
Interrupting sitting every 30-60 min to walk or cycle (2-10 min), performing 3 minutes of simple resistance activities every 30 minutes, such as calf or knee lifts, performing intermittent leg movements (1 minute of activity for every 4 minutes of inactivity during a 3-hour protocol session), or pausing to climb stairs (5 minutes every hour) may be beneficial for vascular health. However, not all studies have demonstrated these positive effects, therefore, some populations may need exercise of greater intensity or duration to counteract the negative vascular effects of acute inactivity periods.
Standing workstations
Standing workstations are effective at reducing sitting time in offices but may be ineffective at reducing vascular alterations related to sitting time. Although some experimental studies indicate vascular benefits, epidemiologic studies suggest that long periods of standing can be harmful to vascular health, especially for venous diseases. Recommendations for use should be accompanied by specific regimens on the frequency and duration of the position to attain the maximum benefits and minimize other vascular complications.
One problem that Dr. Lobelo noted is that some doctors ask their patients how active they are, but they do so in a nonstandardized manner. This observation led him to publish, together with the American Heart Association, an article on the importance for health systems of considering physical activity as a vital sign and including it in records in a standardized manner.
He said that “one advantage of having physical activity as a vital sign in patient medical records is that it allows us to identify individuals who are at greater risk.”
Kaiser Permanente asks the following questions: how many minutes of physical activity do you perform regularly per week, and what is the average intensity of that activity? Patients can be classified into the following three groups: those who follow the recommendations, those with almost no activity, and those who perform some physical activity but do not meet the recommended 150 min/week of moderate to vigorous activity.
Recording sitting time is more difficult. Dr. Lobelo indicated that “it is easier for a person to remember how much time they spent running than how long they were sitting.” Regarding the use of technology, he commented that most watches provide a good estimate. Without technology, it can be estimated by asking how much time is spent in the car, on the bus, or in front of the computer or television and then adding up these times.
Dr. Lobelo emphasized that the two behaviors, lack of physical activity and excessive sitting time, have independent associations with health outcomes. But if both are combined, the risk of obesity, diabetes, and cardiovascular diseases is not just added but rather is multiplied. These behaviors contribute to the epidemic of obesity and diabetes, since most people do not follow either of the two recommendations.
“Studies show that of the two behaviors, the more negative for health would be not following the physical activity recommendations,” said Dr. Lobelo. “If the recommendation of 150 min/week of moderate to vigorous physical activity is followed, the associated risk of sitting too much declines by 80%-90%. Additionally, we can prevent, help to manage, and decrease the risk of complications in more than 100 diseases, including infections. During the pandemic, it was observed that more active people had a lower risk of dying or of being hospitalized due to COVID-19 than less active people, independently of other factors, such as hypertension, diabetes, and obesity.”
Moreover, Dr. Lobelo believes in “practicing what you preach” and advocates that doctors become healthy models.
Dr. Lobelo, Dr. Ferrari, and Dr. Kovalskys disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version appeared on Medscape.com.
according to a recent Latin American study published in BMC Public Health.
These data come from almost 8,000 people aged 20-65 years (half of whom are women) who participated in the Latin American Study on Nutrition and Health (ELANS). The cross-sectional survey included representative samples from urban populations in Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Peru, and Venezuela. The average time spent sitting was 420 min/d. Ecuador had the lowest time (300 min/day), and Argentina and Peru had the highest (480 min/day).
No amount of sitting time has been associated with a greater health risk, but the World Health Organization recommends that sitting time be minimal.
“We used to believe that any intense physical exercise could compensate for a sedentary life. But now we know that a sedentary lifestyle in general and sitting time in particular have a direct effect and are an independent risk factor for chronic diseases,” said study author Irina Kovalskys, PhD, a pediatric specialist in nutrition and a professor of nutrition at the Catholic University of Argentina, Buenos Aires, and a principal investigator of ELANS.
Dr. Kovalskys stated that the 420-min average sitting time is worrying in a population such as the one studied, in which 60% of adults are obese and there are high rates of cardiometabolic risk factors. She affirmed that it is important to raise awareness among the population and focus on adolescents.
Felipe Lobelo, PhD, is a Colombian physician, an associate professor of global health at Emory University and director of epidemiology at Kaiser Permanente Georgia, both in Atlanta. He did not participate in this study but promotes the concept of exercise in medicine. The activity of the patient must be included in a clinical setting, and improving the level of physical activity can have a positive impact on health prognosis, he said.
“To make public health recommendations or even advise patients, a cutoff point is needed. Guidelines recommend 150 minutes per week of moderate to vigorous physical activity, and some countries have started to indicate that we should be concerned about people’s sitting time. There is still no equivalent to the 150 minutes, therefore, these studies are important, especially in the Latin American population,” said Dr. Lobelo.
He explained that the concept of an increased risk of death or chronic disease because of a lack of physical activity arose in the past 50 years, but only in the past 2 decades have we started thinking about sitting time.
“Spending more than 8 hours sitting per day clearly causes a much higher risk of chronic diseases, including obesity and diabetes. It may be a continuous and progressive association, and the point at which this increase becomes exponential is clearly between 6 and 8 hours of sitting time,” Dr. Lobelo added.
The authors expected to find a linear association with risk for being overweight or obese after 4 hours, but they did not find one. “This study has limitations. Among them was that other indicators were not considered, such as health indicators. Collaborations are starting with other research groups, and other studies are being designed,” said study author Gerson Ferrari, PhD, an associate professor at Santiago de Chile University.
Comparing indicators
The Latin American study tried to establish a sitting cutoff time after which the risk of becoming overweight or obese increases. It used three indicators of excess weight: body mass index (BMI), waist circumference, and neck circumference.
Sitting for more than 8 hours increased the chances of excess weight by 10% when measured by BMI and by 13% when neck circumference was used.
Dr. Ferrari stated that the result obtained measuring BMI is the one that should be considered, because it is used in public policy. Neck circumference is a more recent measurement of detection and it is less studied, but it is a valid indicator, with good sensitivity and advantages over others, such as ease of measurement and lack of variation over time.
According to the results of this study, measuring neck circumference may be the most sensitive method of the three. Neck circumference was proportionally greater in people who sat for at least 4, at least 6, and at least 8 hours/day than in those who sat for less than 4, less than 6, and less than 8 hours/day. This relationship was not observed with the other indicators.
Broaching the topic
“What is important is uninterrupted sitting time. The recommendation is to break up those sitting times with active periods. Health professionals have already incorporated the concept of moderate to vigorous physical exercise, but nonintense activities are sufficient to reduce sitting time. Yoga may not be vigorous, but it is valuable at reducing sitting time,” said Dr. Kovalskys.
Dr. Ferrari recommended giving patients concrete messages so that they spend as little time possible sitting. “It is better to stand on the bus or the subway even when there is a place to sit. Are you going to talk on the phone? It is better to do it while walking or at least standing instead of sitting.”
A recent literature review conducted by investigators of the University of Birmingham (England) studied the possible molecular and physiologic mechanisms of inactivity time, health consequences, and protection strategies. It offers an evaluation of interventions that can compensate for the immediate negative consequences of inactivity.
Physical activity
Some studies suggest that more than 60 min/day of moderate-intensity exercise or more than 150 min/week of moderate to vigorous exercise may be effective at mitigating the increased risk for mortality associated with sitting time, but reduced intensity may not be enough.
Active pauses
Interrupting sitting every 30-60 min to walk or cycle (2-10 min), performing 3 minutes of simple resistance activities every 30 minutes, such as calf or knee lifts, performing intermittent leg movements (1 minute of activity for every 4 minutes of inactivity during a 3-hour protocol session), or pausing to climb stairs (5 minutes every hour) may be beneficial for vascular health. However, not all studies have demonstrated these positive effects, therefore, some populations may need exercise of greater intensity or duration to counteract the negative vascular effects of acute inactivity periods.
Standing workstations
Standing workstations are effective at reducing sitting time in offices but may be ineffective at reducing vascular alterations related to sitting time. Although some experimental studies indicate vascular benefits, epidemiologic studies suggest that long periods of standing can be harmful to vascular health, especially for venous diseases. Recommendations for use should be accompanied by specific regimens on the frequency and duration of the position to attain the maximum benefits and minimize other vascular complications.
One problem that Dr. Lobelo noted is that some doctors ask their patients how active they are, but they do so in a nonstandardized manner. This observation led him to publish, together with the American Heart Association, an article on the importance for health systems of considering physical activity as a vital sign and including it in records in a standardized manner.
He said that “one advantage of having physical activity as a vital sign in patient medical records is that it allows us to identify individuals who are at greater risk.”
Kaiser Permanente asks the following questions: how many minutes of physical activity do you perform regularly per week, and what is the average intensity of that activity? Patients can be classified into the following three groups: those who follow the recommendations, those with almost no activity, and those who perform some physical activity but do not meet the recommended 150 min/week of moderate to vigorous activity.
Recording sitting time is more difficult. Dr. Lobelo indicated that “it is easier for a person to remember how much time they spent running than how long they were sitting.” Regarding the use of technology, he commented that most watches provide a good estimate. Without technology, it can be estimated by asking how much time is spent in the car, on the bus, or in front of the computer or television and then adding up these times.
Dr. Lobelo emphasized that the two behaviors, lack of physical activity and excessive sitting time, have independent associations with health outcomes. But if both are combined, the risk of obesity, diabetes, and cardiovascular diseases is not just added but rather is multiplied. These behaviors contribute to the epidemic of obesity and diabetes, since most people do not follow either of the two recommendations.
“Studies show that of the two behaviors, the more negative for health would be not following the physical activity recommendations,” said Dr. Lobelo. “If the recommendation of 150 min/week of moderate to vigorous physical activity is followed, the associated risk of sitting too much declines by 80%-90%. Additionally, we can prevent, help to manage, and decrease the risk of complications in more than 100 diseases, including infections. During the pandemic, it was observed that more active people had a lower risk of dying or of being hospitalized due to COVID-19 than less active people, independently of other factors, such as hypertension, diabetes, and obesity.”
Moreover, Dr. Lobelo believes in “practicing what you preach” and advocates that doctors become healthy models.
Dr. Lobelo, Dr. Ferrari, and Dr. Kovalskys disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version appeared on Medscape.com.
according to a recent Latin American study published in BMC Public Health.
These data come from almost 8,000 people aged 20-65 years (half of whom are women) who participated in the Latin American Study on Nutrition and Health (ELANS). The cross-sectional survey included representative samples from urban populations in Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Peru, and Venezuela. The average time spent sitting was 420 min/d. Ecuador had the lowest time (300 min/day), and Argentina and Peru had the highest (480 min/day).
No amount of sitting time has been associated with a greater health risk, but the World Health Organization recommends that sitting time be minimal.
“We used to believe that any intense physical exercise could compensate for a sedentary life. But now we know that a sedentary lifestyle in general and sitting time in particular have a direct effect and are an independent risk factor for chronic diseases,” said study author Irina Kovalskys, PhD, a pediatric specialist in nutrition and a professor of nutrition at the Catholic University of Argentina, Buenos Aires, and a principal investigator of ELANS.
Dr. Kovalskys stated that the 420-min average sitting time is worrying in a population such as the one studied, in which 60% of adults are obese and there are high rates of cardiometabolic risk factors. She affirmed that it is important to raise awareness among the population and focus on adolescents.
Felipe Lobelo, PhD, is a Colombian physician, an associate professor of global health at Emory University and director of epidemiology at Kaiser Permanente Georgia, both in Atlanta. He did not participate in this study but promotes the concept of exercise in medicine. The activity of the patient must be included in a clinical setting, and improving the level of physical activity can have a positive impact on health prognosis, he said.
“To make public health recommendations or even advise patients, a cutoff point is needed. Guidelines recommend 150 minutes per week of moderate to vigorous physical activity, and some countries have started to indicate that we should be concerned about people’s sitting time. There is still no equivalent to the 150 minutes, therefore, these studies are important, especially in the Latin American population,” said Dr. Lobelo.
He explained that the concept of an increased risk of death or chronic disease because of a lack of physical activity arose in the past 50 years, but only in the past 2 decades have we started thinking about sitting time.
“Spending more than 8 hours sitting per day clearly causes a much higher risk of chronic diseases, including obesity and diabetes. It may be a continuous and progressive association, and the point at which this increase becomes exponential is clearly between 6 and 8 hours of sitting time,” Dr. Lobelo added.
The authors expected to find a linear association with risk for being overweight or obese after 4 hours, but they did not find one. “This study has limitations. Among them was that other indicators were not considered, such as health indicators. Collaborations are starting with other research groups, and other studies are being designed,” said study author Gerson Ferrari, PhD, an associate professor at Santiago de Chile University.
Comparing indicators
The Latin American study tried to establish a sitting cutoff time after which the risk of becoming overweight or obese increases. It used three indicators of excess weight: body mass index (BMI), waist circumference, and neck circumference.
Sitting for more than 8 hours increased the chances of excess weight by 10% when measured by BMI and by 13% when neck circumference was used.
Dr. Ferrari stated that the result obtained measuring BMI is the one that should be considered, because it is used in public policy. Neck circumference is a more recent measurement of detection and it is less studied, but it is a valid indicator, with good sensitivity and advantages over others, such as ease of measurement and lack of variation over time.
According to the results of this study, measuring neck circumference may be the most sensitive method of the three. Neck circumference was proportionally greater in people who sat for at least 4, at least 6, and at least 8 hours/day than in those who sat for less than 4, less than 6, and less than 8 hours/day. This relationship was not observed with the other indicators.
Broaching the topic
“What is important is uninterrupted sitting time. The recommendation is to break up those sitting times with active periods. Health professionals have already incorporated the concept of moderate to vigorous physical exercise, but nonintense activities are sufficient to reduce sitting time. Yoga may not be vigorous, but it is valuable at reducing sitting time,” said Dr. Kovalskys.
Dr. Ferrari recommended giving patients concrete messages so that they spend as little time possible sitting. “It is better to stand on the bus or the subway even when there is a place to sit. Are you going to talk on the phone? It is better to do it while walking or at least standing instead of sitting.”
A recent literature review conducted by investigators of the University of Birmingham (England) studied the possible molecular and physiologic mechanisms of inactivity time, health consequences, and protection strategies. It offers an evaluation of interventions that can compensate for the immediate negative consequences of inactivity.
Physical activity
Some studies suggest that more than 60 min/day of moderate-intensity exercise or more than 150 min/week of moderate to vigorous exercise may be effective at mitigating the increased risk for mortality associated with sitting time, but reduced intensity may not be enough.
Active pauses
Interrupting sitting every 30-60 min to walk or cycle (2-10 min), performing 3 minutes of simple resistance activities every 30 minutes, such as calf or knee lifts, performing intermittent leg movements (1 minute of activity for every 4 minutes of inactivity during a 3-hour protocol session), or pausing to climb stairs (5 minutes every hour) may be beneficial for vascular health. However, not all studies have demonstrated these positive effects, therefore, some populations may need exercise of greater intensity or duration to counteract the negative vascular effects of acute inactivity periods.
Standing workstations
Standing workstations are effective at reducing sitting time in offices but may be ineffective at reducing vascular alterations related to sitting time. Although some experimental studies indicate vascular benefits, epidemiologic studies suggest that long periods of standing can be harmful to vascular health, especially for venous diseases. Recommendations for use should be accompanied by specific regimens on the frequency and duration of the position to attain the maximum benefits and minimize other vascular complications.
One problem that Dr. Lobelo noted is that some doctors ask their patients how active they are, but they do so in a nonstandardized manner. This observation led him to publish, together with the American Heart Association, an article on the importance for health systems of considering physical activity as a vital sign and including it in records in a standardized manner.
He said that “one advantage of having physical activity as a vital sign in patient medical records is that it allows us to identify individuals who are at greater risk.”
Kaiser Permanente asks the following questions: how many minutes of physical activity do you perform regularly per week, and what is the average intensity of that activity? Patients can be classified into the following three groups: those who follow the recommendations, those with almost no activity, and those who perform some physical activity but do not meet the recommended 150 min/week of moderate to vigorous activity.
Recording sitting time is more difficult. Dr. Lobelo indicated that “it is easier for a person to remember how much time they spent running than how long they were sitting.” Regarding the use of technology, he commented that most watches provide a good estimate. Without technology, it can be estimated by asking how much time is spent in the car, on the bus, or in front of the computer or television and then adding up these times.
Dr. Lobelo emphasized that the two behaviors, lack of physical activity and excessive sitting time, have independent associations with health outcomes. But if both are combined, the risk of obesity, diabetes, and cardiovascular diseases is not just added but rather is multiplied. These behaviors contribute to the epidemic of obesity and diabetes, since most people do not follow either of the two recommendations.
“Studies show that of the two behaviors, the more negative for health would be not following the physical activity recommendations,” said Dr. Lobelo. “If the recommendation of 150 min/week of moderate to vigorous physical activity is followed, the associated risk of sitting too much declines by 80%-90%. Additionally, we can prevent, help to manage, and decrease the risk of complications in more than 100 diseases, including infections. During the pandemic, it was observed that more active people had a lower risk of dying or of being hospitalized due to COVID-19 than less active people, independently of other factors, such as hypertension, diabetes, and obesity.”
Moreover, Dr. Lobelo believes in “practicing what you preach” and advocates that doctors become healthy models.
Dr. Lobelo, Dr. Ferrari, and Dr. Kovalskys disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version appeared on Medscape.com.
FROM BMC PUBLIC HEALTH
Biomarkers linked to elevated T2D MACE risk in DECLARE-TIMI 58
The researchers found that N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hsTnT) levels helped identify a subset of T2D patients at higher risk of major adverse cardiovascular events who would benefit most from dapagliflozin.
“We’ve shown previously that these two biomarkers are very robust risk indicators for cardiovascular death and heart failure events,” senior study author David A. Morrow, MD, of Harvard University, Boston, said in an interview. “In this study, we now show that the two biomarkers also yield important prognostic information for MACE [major adverse cardiovascular events].”
Although NT-proBNP is typically measured to diagnose heart failure, and hsTnT to diagnose acute MI, Dr. Morrow pointed out that this analysis demonstrated the potential for using the two tests to evaluate risks in T2D patients.
Study results
The secondary analysis included 14,565 patients in the DECLARE-TIMI 58 trial. The patients had T2D and multiple risk factors for atherosclerotic cardiovascular disease (about 60%) or established ASCVD (about 40%). All patients had available blood samples and the data were collected from May 2013 to September 2018. The primary outcome was MACE, a composite of MI, ischemic stroke, and cardiovascular death. The results were reported online in JAMA Cardiology.
The analysis found that higher baseline concentrations of NT-proBNP increased MACE risks by 62% (95% confidence interval, 1.49-1.76) and hsTnT elevated those risks by 59% (95% CI, 1.46-1.74).
Among placebo patients, when divided into risk quartiles, those in the highest quartile had significantly higher risk with both elevated NT-proBNP and hsTnT, compared with those with low concentrations. For example, patients with established ASCVD had a 22.9% risk vs. 9.5% with elevated NT-proBNP (P < .001) and a 24.2% vs. 7.2% risk with elevated hsTnT (P < .001). The gap was similar for patients with multiple risk factors.
Dr. Morrow noted that the main DECLARE-TIMI 58 trial showed that dapagliflozin reduced the rates of cardiovascular death or hospitalization for heart failure in patients with T2D, when compared to placebo, but didn’t reach statistical significance for MACE (N Engl J Med. 2019;380:347-57).
“We have previously shown that among patients with T2D who have high risk indicators, such as prior MI or long-standing diabetes, dapagliflozin also appeared to reduce MACE,” Dr. Morrow said. “In this study, we find that these two widely available biomarkers also identify a high-risk group who may have even more potential benefits from treatment with an SGLT2i.”
Dr. Morrow noted that the study design – a nested prospective biomarker study within a randomized, double-blind, placebo-controlled clinical trial – “is a particular strength.”
Results clarify which patients will benefit
This secondary analysis of DECLARE-TIMI 58 brings more clarity to the types of T2D patients who will get the most cardiovascular benefits from dapagliflozin, said Matthew J. Budoff, MD, professor of medicine at University of California, Los Angeles, and Endowed Chair of Preventive Cardiology at the Lundquist Institute in Torrance, Calif.
“The big picture is, we’ve known for some time from epidemiologic studies that these biomarkers, when they’re elevated, mean that the patient is at higher risk of having a cardiovascular event,” he said, “but I think what it helps us with is in knowing in whom to use dapagliflozin for prevention of ASCVD. The effect in the DECLARE-TIMI 58 trial was quite modest, but if you can subgroup it, in these high-risk people there’s a more profound effect. It helps in risk stratification because the absolute benefit is larger.”
The specific biomarkers, NT-proBNP and hsTnT, “haven’t been explored very much in clinical trials,” Dr. Budoff said, “so I do think that it’s nice that in a randomized trial it plays out the way we might expect.”
He added that “for many clinicians this is novel, because I don’t think they were aware of the biomarker data, so I think that this does add some clinical benefit in that context.” The findings also strengthen the case to get T2D patients with higher ASCVD risk onto SGLT2 inhibitors if they’re not already, he said.
Dr. Morrow disclosed relationships with AstraZeneca, Roche Diagnostics, Abbott Laboratories, Anthos Therapeutics, ARCA Biopharma, Merck, Novartis, Pfizer, Regeneron, Siemens, and InCarda outside the reported work.
Dr. Budoff has no relevant disclosures.
The researchers found that N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hsTnT) levels helped identify a subset of T2D patients at higher risk of major adverse cardiovascular events who would benefit most from dapagliflozin.
“We’ve shown previously that these two biomarkers are very robust risk indicators for cardiovascular death and heart failure events,” senior study author David A. Morrow, MD, of Harvard University, Boston, said in an interview. “In this study, we now show that the two biomarkers also yield important prognostic information for MACE [major adverse cardiovascular events].”
Although NT-proBNP is typically measured to diagnose heart failure, and hsTnT to diagnose acute MI, Dr. Morrow pointed out that this analysis demonstrated the potential for using the two tests to evaluate risks in T2D patients.
Study results
The secondary analysis included 14,565 patients in the DECLARE-TIMI 58 trial. The patients had T2D and multiple risk factors for atherosclerotic cardiovascular disease (about 60%) or established ASCVD (about 40%). All patients had available blood samples and the data were collected from May 2013 to September 2018. The primary outcome was MACE, a composite of MI, ischemic stroke, and cardiovascular death. The results were reported online in JAMA Cardiology.
The analysis found that higher baseline concentrations of NT-proBNP increased MACE risks by 62% (95% confidence interval, 1.49-1.76) and hsTnT elevated those risks by 59% (95% CI, 1.46-1.74).
Among placebo patients, when divided into risk quartiles, those in the highest quartile had significantly higher risk with both elevated NT-proBNP and hsTnT, compared with those with low concentrations. For example, patients with established ASCVD had a 22.9% risk vs. 9.5% with elevated NT-proBNP (P < .001) and a 24.2% vs. 7.2% risk with elevated hsTnT (P < .001). The gap was similar for patients with multiple risk factors.
Dr. Morrow noted that the main DECLARE-TIMI 58 trial showed that dapagliflozin reduced the rates of cardiovascular death or hospitalization for heart failure in patients with T2D, when compared to placebo, but didn’t reach statistical significance for MACE (N Engl J Med. 2019;380:347-57).
“We have previously shown that among patients with T2D who have high risk indicators, such as prior MI or long-standing diabetes, dapagliflozin also appeared to reduce MACE,” Dr. Morrow said. “In this study, we find that these two widely available biomarkers also identify a high-risk group who may have even more potential benefits from treatment with an SGLT2i.”
Dr. Morrow noted that the study design – a nested prospective biomarker study within a randomized, double-blind, placebo-controlled clinical trial – “is a particular strength.”
Results clarify which patients will benefit
This secondary analysis of DECLARE-TIMI 58 brings more clarity to the types of T2D patients who will get the most cardiovascular benefits from dapagliflozin, said Matthew J. Budoff, MD, professor of medicine at University of California, Los Angeles, and Endowed Chair of Preventive Cardiology at the Lundquist Institute in Torrance, Calif.
“The big picture is, we’ve known for some time from epidemiologic studies that these biomarkers, when they’re elevated, mean that the patient is at higher risk of having a cardiovascular event,” he said, “but I think what it helps us with is in knowing in whom to use dapagliflozin for prevention of ASCVD. The effect in the DECLARE-TIMI 58 trial was quite modest, but if you can subgroup it, in these high-risk people there’s a more profound effect. It helps in risk stratification because the absolute benefit is larger.”
The specific biomarkers, NT-proBNP and hsTnT, “haven’t been explored very much in clinical trials,” Dr. Budoff said, “so I do think that it’s nice that in a randomized trial it plays out the way we might expect.”
He added that “for many clinicians this is novel, because I don’t think they were aware of the biomarker data, so I think that this does add some clinical benefit in that context.” The findings also strengthen the case to get T2D patients with higher ASCVD risk onto SGLT2 inhibitors if they’re not already, he said.
Dr. Morrow disclosed relationships with AstraZeneca, Roche Diagnostics, Abbott Laboratories, Anthos Therapeutics, ARCA Biopharma, Merck, Novartis, Pfizer, Regeneron, Siemens, and InCarda outside the reported work.
Dr. Budoff has no relevant disclosures.
The researchers found that N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hsTnT) levels helped identify a subset of T2D patients at higher risk of major adverse cardiovascular events who would benefit most from dapagliflozin.
“We’ve shown previously that these two biomarkers are very robust risk indicators for cardiovascular death and heart failure events,” senior study author David A. Morrow, MD, of Harvard University, Boston, said in an interview. “In this study, we now show that the two biomarkers also yield important prognostic information for MACE [major adverse cardiovascular events].”
Although NT-proBNP is typically measured to diagnose heart failure, and hsTnT to diagnose acute MI, Dr. Morrow pointed out that this analysis demonstrated the potential for using the two tests to evaluate risks in T2D patients.
Study results
The secondary analysis included 14,565 patients in the DECLARE-TIMI 58 trial. The patients had T2D and multiple risk factors for atherosclerotic cardiovascular disease (about 60%) or established ASCVD (about 40%). All patients had available blood samples and the data were collected from May 2013 to September 2018. The primary outcome was MACE, a composite of MI, ischemic stroke, and cardiovascular death. The results were reported online in JAMA Cardiology.
The analysis found that higher baseline concentrations of NT-proBNP increased MACE risks by 62% (95% confidence interval, 1.49-1.76) and hsTnT elevated those risks by 59% (95% CI, 1.46-1.74).
Among placebo patients, when divided into risk quartiles, those in the highest quartile had significantly higher risk with both elevated NT-proBNP and hsTnT, compared with those with low concentrations. For example, patients with established ASCVD had a 22.9% risk vs. 9.5% with elevated NT-proBNP (P < .001) and a 24.2% vs. 7.2% risk with elevated hsTnT (P < .001). The gap was similar for patients with multiple risk factors.
Dr. Morrow noted that the main DECLARE-TIMI 58 trial showed that dapagliflozin reduced the rates of cardiovascular death or hospitalization for heart failure in patients with T2D, when compared to placebo, but didn’t reach statistical significance for MACE (N Engl J Med. 2019;380:347-57).
“We have previously shown that among patients with T2D who have high risk indicators, such as prior MI or long-standing diabetes, dapagliflozin also appeared to reduce MACE,” Dr. Morrow said. “In this study, we find that these two widely available biomarkers also identify a high-risk group who may have even more potential benefits from treatment with an SGLT2i.”
Dr. Morrow noted that the study design – a nested prospective biomarker study within a randomized, double-blind, placebo-controlled clinical trial – “is a particular strength.”
Results clarify which patients will benefit
This secondary analysis of DECLARE-TIMI 58 brings more clarity to the types of T2D patients who will get the most cardiovascular benefits from dapagliflozin, said Matthew J. Budoff, MD, professor of medicine at University of California, Los Angeles, and Endowed Chair of Preventive Cardiology at the Lundquist Institute in Torrance, Calif.
“The big picture is, we’ve known for some time from epidemiologic studies that these biomarkers, when they’re elevated, mean that the patient is at higher risk of having a cardiovascular event,” he said, “but I think what it helps us with is in knowing in whom to use dapagliflozin for prevention of ASCVD. The effect in the DECLARE-TIMI 58 trial was quite modest, but if you can subgroup it, in these high-risk people there’s a more profound effect. It helps in risk stratification because the absolute benefit is larger.”
The specific biomarkers, NT-proBNP and hsTnT, “haven’t been explored very much in clinical trials,” Dr. Budoff said, “so I do think that it’s nice that in a randomized trial it plays out the way we might expect.”
He added that “for many clinicians this is novel, because I don’t think they were aware of the biomarker data, so I think that this does add some clinical benefit in that context.” The findings also strengthen the case to get T2D patients with higher ASCVD risk onto SGLT2 inhibitors if they’re not already, he said.
Dr. Morrow disclosed relationships with AstraZeneca, Roche Diagnostics, Abbott Laboratories, Anthos Therapeutics, ARCA Biopharma, Merck, Novartis, Pfizer, Regeneron, Siemens, and InCarda outside the reported work.
Dr. Budoff has no relevant disclosures.
FROM JAMA CARDIOLOGY
NUDGE-FLU: Electronic ‘nudges’ boost flu shot uptake in seniors
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
Two types of electronically delivered letter strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination and a repeat reminder letter – increased flu shot uptake, compared with usual care alone, in a national study of seniors in Denmark.
And in a prespecified subanalysis focusing on older adults with cardiovascular disease, these two strategies were also effective in boosting vaccine uptake in those with or without CVD.
The findings are from the Nationwide Utilization of Danish Government Electronic Letter System for Increasing Influenza Vaccine Uptake (NUDGE-FLU) trial, which compared usual care alone with one of nine different electronic letter “behavioral nudge” strategies during the 2022-2023 flu season in people aged 65 years and older.
Niklas Dyrby Johansen, MD, Hospital–Herlev and Gentofte and Copenhagen University, presented the main study findings in a late-breaking clinical trial session at the joint scientific sessions of the American College of Cardiology and the World Heart Federation, and the article was simultaneously published in The Lancet
The subanalysis in patients with CVD was published online March 5 in Circulation.
“Despite modest effect sizes, the results may have important implications when translated to a population level,” Dr. Dyrby Johansen concluded during his presentation. Still, the authors write, “the low-touch (no person-to-person interaction), inexpensive, and highly scalable nature of these electronic letters might have important population-level public health implications.”
They note that, among approximately 63 million Medicare beneficiaries in the United States, a 0.89–percentage point absolute increase in vaccination rate achieved through the most successful electronic letter in NUDGE-FLU, the one highlighting cardiovascular gain, would be expected to lead to 500,000 additional vaccinations and potentially prevent 7,849 illnesses, 4,395 medical visits, 714 hospitalizations, and 66 deaths each year.
Electronic letter systems similar to the one used in this trial are already in place in several European countries, including Sweden, Norway, and Ireland, the researchers note.
In countries such as the United States, where implementing a nationwide government electronic letter system might not be feasible, nudges could be done via email, text message, or other systems, but whether this would be as effective remains to be seen.
Commenting on the findings, David Cho, MD, UCLA Health and chair of the ACC Health Care Innovation Council, commended the researchers on engaging patients with more than a million separate nudges sent out during one flu season, and randomly assigning participants to 10 different types of nudges, calling it “impressive.”
“I think the concept that the nudge is to plant an idea that leads to an action is pretty much the basis of a lot of these health care interventions, which seems like a small way to have a big impact at outcome,” Dr. Cho noted. “The behavioral science aspects of the nudges are also fascinating to me personally, and I think to a lot of the cardiologists in the audience – about how you actually get people to act. I think it’s been a lifelong question for people in general, how do you get people to follow through on an action?”
“So I found the fact that secondary gain from a cardiovascular health standpoint, but also the repeated nudges were sort of simple ways that you could have people take ownership and get their flu vaccination,” he said.
“This is ACC, this is a cardiovascular conference, but the influence of vaccine is not just a primary care problem, it is also directly affecting cardiovascular disease,” Dr. Cho concluded.
‘Small but important effect’
In an accompanying editorial (Lancet. 2023 Mar 5. doi: 10.1016/S0140-6736(23)00453-1), Melissa Stockwell, MD, Columbia University, New York, writes, “The study by Johansen and colleagues highlights the small but still important effect of scalable, digital interventions across an entire at-risk population.”
A difference of 0.89% in the entire study population of over 960,000 adults age 65 years or older would be more than 8,500 additional adults protected, she notes. “That increase is important for a scalable intervention that has a low cost per letter.”
Moreover, “that the cardiovascular gain–framed messages worked best in those who had not been vaccinated in the previous season further highlights the potential impact on a more vaccine-hesitant population,” Dr. Stockwell notes.
However, with the mandatory government electronic notification system in Denmark, “notifications are sent via regular email and SMS message, and recipients log in through a portal or smartphone app to view the letter.” Similar studies in the United States that included this extra step of needing to sign in online have not been effective in older populations.
Another limitation is that the intervention may have a different effect in populations for which there is a digital divide between people with or without Internet access of sufficient data on their mobile phones.
First-of-its kind, nationwide pragmatic trial
The NUDGE-FLU protocol was previously published in the American Heart Journal. NUDGE-FLU is a first-of-its kind nationwide, pragmatic, registry-based, cluster-randomized implementation trial of electronically delivered nudges to increase influenza vaccination uptake, the researchers note.
They identified 964,870 individuals who were 65 years or older (or would turn 65 by Jan. 15, 2023) who lived in one of 691,820 households in Denmark.
This excluded individuals who lived in a nursing home or were exempt from the government’s mandatory electronic letter system that is used for official communications.
Households were randomly assigned 9:1:1:1:1:1:1:1:1:1 to receive usual care alone or to one of nine electronic letter strategies based on different behavioral science approaches to encourage influenza vaccination uptake:
- Standard electronic letter
- Standard electronic letter sent at randomization and again 14 days later (repeated letter)
- Depersonalized letter without the recipient’s name
- Gain-framing nudge (“Vaccinations help end pandemics, like COVID-19 and the flu. Protect yourself and your loved ones.”)
- Loss-framing nudge (“When too few people get vaccinated, pandemics from diseases like COVID-19 and the flu can spread and place you and your loved ones at risk.”)
- Collective-goal nudge (“78% of Danes 65 and above were vaccinated against influenza last year. Help us achieve an even higher goal this year.”)
- Active choice or implementation-intention prompt (“We encourage you to record your appointment time here.”)
- Cardiovascular gain–framing nudge (“In addition to its protection against influenza infection, influenza vaccination also seems to protect against cardiovascular disease such as heart attacks and heart failure.”)
- Expert-authority statement (“I recommend everyone over the age of 65 years to get vaccinated against influenza – Tyra Grove Krause, Executive Vice President, Statens Serum Institut.”)
The electronic letters were sent out Sept. 16, 2022, and the primary endpoint was vaccine receipt on or before Jan. 1, 2023.
All individuals received an informative vaccination encouragement letter from the Danish Health Authority (usual care) delivered via the same electronic letter system during Sept. 17 through Sept. 21, 2022.
The individuals had a mean age of 73.8 years, 51.5% were women, and 27.4% had chronic cardiovascular disease.
The analyses were done in one randomly selected individual per household.
Influenza vaccination rates were significantly higher in the cardiovascular gain–framing nudge group vs. usual care (81.00% vs. 80.12%; difference, 0.89 percentage points; P < .0001) and in the repeat-letter group vs. usual care (80.85% vs 80.12%; difference, 0.73 percentage points; P = .0006).
These two strategies also improved vaccination rates across major subgroups.
The cardiovascular gain–framed letter was particularly effective among participants who had not been vaccinated for influenza in the previous season.
The seven other letter strategies did not increase flu shot uptake.
Subanalysis in CVD
In the prespecified subanalysis of the NUDGE-FLU trial of patients aged 65 and older that focused on patients with CVD, Daniel Modin, MB, and colleagues report that 83.1% of patients with CVD vs. 79.2% of patients without CVD received influenza vaccination within the requested time (P < .0001).
The two nudging strategies – a letter highlighting potential cardiovascular benefits of influenza vaccination or a repeat letter – that were effective in boosting flu shot rates in the main analysis were also effective in all major CVD subgroups (ischemic heart disease, pulmonary heart disease, heart failure, atrial fibrillation, cerebrovascular disease, atherosclerotic CVD, embolic or thrombotic disease, and congenital heart disease).
Despite strong guideline endorsement, “influenza vaccination rates remain suboptimal in patients with high-risk cardiovascular disease,” Dr. Morin and colleagues write, possibly because of “insufficient knowledge among patients and providers of potential clinical benefits, concerns about vaccine safety, and other forms of vaccine hesitancy.”
Their findings suggest that “select digital behaviorally informed nudges delivered in advance of vaccine availability might be utilized to increase influenza vaccinate uptake in individuals with cardiovascular disease.”
NUDGE-HF was funded by Sanofi. Dr. Johansen and Dr. Modin have no disclosures. The disclosures of the other authors are listed with the articles. Dr. Stockwell has no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Atorvastatin cut anthracycline cardiac dysfunction in lymphoma
NEW ORLEANS – Atorvastatin treatment of patients with lymphoma undergoing treatment with an anthracycline significantly cut the incidence of incident cardiac dysfunction by about two-thirds during 12 months of treatment, in a multicenter, randomized trial with 300 enrolled patients.
“These data support the use of atorvastatin among patients with lymphoma being treated with anthracyclines where prevention of cardiac systolic dysfunction is important,” concluded Tomas G. Neilan, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
He highlighted that an important difference between the new study, STOP-CA, and a major prior study with a neutral effect published in 2022, was that STOP-CA “was powered for a major change” in cardiac function as the study’s primary outcome, a decline from baseline in left ventricular ejection fraction (LVEF) of at least 10% that also reduced ejection fraction to less than 55%.
“We can consider these medications [atorvastatin] for patients at higher risk for cardiac toxicity from anthracyclines, such as patients who receive a higher dose of an anthracycline, older patients, people with obesity, and women, commented Anita Deswal, MD, professor and chair of the department of cardiology at the University of Texas MD Anderson Cancer Center, Houston, who was not involved with the study.
A basis for an ‘important discussion’ with patients
“For patients receiving higher doses of anthracyclines, the STOP-CA trial says that whether to start a statin for cardiac protection is now an important discussion” for these patients to have with their treating clinicians. ”That was not the case before today,” commented Ronald M. Witteles, MD, a cardiologist and professor who specializes in cardio-oncology at Stanford (Calif.) University.
“For a patient being treated for lymphoma or for another cancer and treated with equal or higher anthracycline doses, such as patients with a sarcoma, this trial’s results at the very least warrant a discussion between physicians and patients to make the decision,” Dr. Witteles, who was not involved in the study, said in an interview. But he also cautioned that “whether an individual patient should take a statin in this scenario is still not a no-brainer. While the trial was positive, it was for an imaging rather than for a clinical endpoint.”
Experts noted that a similar study with the clinical endpoint of heart failure would require both many more randomized patients as well as much longer follow-up. STOP-CA was not powered for this endpoint. During its 12-month duration, a total of 11 patients developed heart failure, with no between group difference.
STOP-CA enrolled adults with lymphoma (Hodgkin or non-Hodgkin) and scheduled to undergo anthracycline treatment at eight U.S. centers and one in Canada, and excluded patients already on statin treatment or those for whom a statin was already indicated. Of the 300 enrolled patients, 286 had 12-month follow-up. Randomization assigned patients to receive either 40 mg daily of atorvastatin or placebo.
Their cumulative, median anthracycline dose was 300 mg/m2, which is typical for treating lymphoma, but higher than the typical dose use for patients with breast cancer. At baseline, average LVEF was 63%, and after 12 months this had declined to 59%. Forty-six of the 286 patients assessed after 12 months fulfilled the primary outcome of at least a 10–percentage point reduction from baseline in their LVEF and a decline in LVEF to less than 55%. Researchers used cardiac MR to assess LVEF at baseline, and in most patients at follow-up, but a minority of patients had their follow-up assessments by echocardiography because of logistical issues. Greater than 90% of patients were adherent to their assigned regimen.
Tripled incidence of cardiac dysfunction in placebo patients
The incidence of this outcome was 9% among the patients who received atorvastatin, and 22% among those on placebo, a significant difference. The calculated odds of the primary outcome was 2.9-fold more likely among the patients treated with placebo, compared with those who received atorvastatin, also a significant difference.
The study’s secondary outcome was patients who had at least a 5% drop from baseline in their LVEF and with a LVEF of less than 55% after 12 months. This outcome occurred in 13% of patients treated with atorvastatin and in 29% of those who received placebo, a significant difference.
The atorvastatin and placebo arms showed no significant differences in adverse events during the study, with roughly similar incidence rates for muscle pain, elevated liver enzymes, and renal failure. None of the enrolled patients developed myositis.
Atorvastatin treatment also produced an expected average 37% decline from baseline in levels of LDL cholesterol.
“This was a well-designed and important trial,” said Dr. Witteles. “Anthracyclines remain a mainstay of cancer therapies for a number of malignancies, such as lymphoma and sarcoma, and the cardiac side effects of development of cardiac dysfunction are unequivocally real.”
The importance of a clinically meaningful effect
The results especially contrast with the findings from the PREVENT study, published in 2022, which compared a daily, 40-mg atorvastatin treatment with placebo in 279 randomized patients with breast cancer and treated for 24 months. However, patients in PREVENT had a cumulative, median anthracycline dose of 240 mg/m2, and the study’s primary outcome was the average change from baseline in LVEF after 24 months of treatment, which was a reduction of 0.08 percentage points in the placebo arm, a nonsignificant difference.
In STOP-CA, the average change in LVEF from baseline was a 1–percentage point reduction in the placebo arm, compared with the atorvastatin-treated patients, a difference that was statistically significant, but “not clinically significant,” said Dr. Neilan, director of the cardio-oncology program at Massachusetts General Hospital, Boston. He cited the good fortune of the STOP-CA investigators when they received a recommendation from reviewers early on to design their study to track a clinically meaningful change in LVEF rather than just looking at the average overall change.
Dr. Deswal also noted that it is unlikely that future studies will examine the efficacy of a statin for preventing LVEF in patients across the range of cancers that are eligible for anthracycline treatment. As a result, she predicted that “we may have to extrapolate” the results from STOP-CA to patients with other cancer types.
STOP-CA received no commercial funding. Dr. Neilan has been a consultant for and received fees from Abbvie, Amgen, Bristol-Myers Squibb, CRC Oncology, Genentech, Roche, and Sanofi, and has received grant funding from AstraZeneca and Bristol Myers Squib. Dr. Deswal and Dr. Witteles had no relevant disclosures.
NEW ORLEANS – Atorvastatin treatment of patients with lymphoma undergoing treatment with an anthracycline significantly cut the incidence of incident cardiac dysfunction by about two-thirds during 12 months of treatment, in a multicenter, randomized trial with 300 enrolled patients.
“These data support the use of atorvastatin among patients with lymphoma being treated with anthracyclines where prevention of cardiac systolic dysfunction is important,” concluded Tomas G. Neilan, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
He highlighted that an important difference between the new study, STOP-CA, and a major prior study with a neutral effect published in 2022, was that STOP-CA “was powered for a major change” in cardiac function as the study’s primary outcome, a decline from baseline in left ventricular ejection fraction (LVEF) of at least 10% that also reduced ejection fraction to less than 55%.
“We can consider these medications [atorvastatin] for patients at higher risk for cardiac toxicity from anthracyclines, such as patients who receive a higher dose of an anthracycline, older patients, people with obesity, and women, commented Anita Deswal, MD, professor and chair of the department of cardiology at the University of Texas MD Anderson Cancer Center, Houston, who was not involved with the study.
A basis for an ‘important discussion’ with patients
“For patients receiving higher doses of anthracyclines, the STOP-CA trial says that whether to start a statin for cardiac protection is now an important discussion” for these patients to have with their treating clinicians. ”That was not the case before today,” commented Ronald M. Witteles, MD, a cardiologist and professor who specializes in cardio-oncology at Stanford (Calif.) University.
“For a patient being treated for lymphoma or for another cancer and treated with equal or higher anthracycline doses, such as patients with a sarcoma, this trial’s results at the very least warrant a discussion between physicians and patients to make the decision,” Dr. Witteles, who was not involved in the study, said in an interview. But he also cautioned that “whether an individual patient should take a statin in this scenario is still not a no-brainer. While the trial was positive, it was for an imaging rather than for a clinical endpoint.”
Experts noted that a similar study with the clinical endpoint of heart failure would require both many more randomized patients as well as much longer follow-up. STOP-CA was not powered for this endpoint. During its 12-month duration, a total of 11 patients developed heart failure, with no between group difference.
STOP-CA enrolled adults with lymphoma (Hodgkin or non-Hodgkin) and scheduled to undergo anthracycline treatment at eight U.S. centers and one in Canada, and excluded patients already on statin treatment or those for whom a statin was already indicated. Of the 300 enrolled patients, 286 had 12-month follow-up. Randomization assigned patients to receive either 40 mg daily of atorvastatin or placebo.
Their cumulative, median anthracycline dose was 300 mg/m2, which is typical for treating lymphoma, but higher than the typical dose use for patients with breast cancer. At baseline, average LVEF was 63%, and after 12 months this had declined to 59%. Forty-six of the 286 patients assessed after 12 months fulfilled the primary outcome of at least a 10–percentage point reduction from baseline in their LVEF and a decline in LVEF to less than 55%. Researchers used cardiac MR to assess LVEF at baseline, and in most patients at follow-up, but a minority of patients had their follow-up assessments by echocardiography because of logistical issues. Greater than 90% of patients were adherent to their assigned regimen.
Tripled incidence of cardiac dysfunction in placebo patients
The incidence of this outcome was 9% among the patients who received atorvastatin, and 22% among those on placebo, a significant difference. The calculated odds of the primary outcome was 2.9-fold more likely among the patients treated with placebo, compared with those who received atorvastatin, also a significant difference.
The study’s secondary outcome was patients who had at least a 5% drop from baseline in their LVEF and with a LVEF of less than 55% after 12 months. This outcome occurred in 13% of patients treated with atorvastatin and in 29% of those who received placebo, a significant difference.
The atorvastatin and placebo arms showed no significant differences in adverse events during the study, with roughly similar incidence rates for muscle pain, elevated liver enzymes, and renal failure. None of the enrolled patients developed myositis.
Atorvastatin treatment also produced an expected average 37% decline from baseline in levels of LDL cholesterol.
“This was a well-designed and important trial,” said Dr. Witteles. “Anthracyclines remain a mainstay of cancer therapies for a number of malignancies, such as lymphoma and sarcoma, and the cardiac side effects of development of cardiac dysfunction are unequivocally real.”
The importance of a clinically meaningful effect
The results especially contrast with the findings from the PREVENT study, published in 2022, which compared a daily, 40-mg atorvastatin treatment with placebo in 279 randomized patients with breast cancer and treated for 24 months. However, patients in PREVENT had a cumulative, median anthracycline dose of 240 mg/m2, and the study’s primary outcome was the average change from baseline in LVEF after 24 months of treatment, which was a reduction of 0.08 percentage points in the placebo arm, a nonsignificant difference.
In STOP-CA, the average change in LVEF from baseline was a 1–percentage point reduction in the placebo arm, compared with the atorvastatin-treated patients, a difference that was statistically significant, but “not clinically significant,” said Dr. Neilan, director of the cardio-oncology program at Massachusetts General Hospital, Boston. He cited the good fortune of the STOP-CA investigators when they received a recommendation from reviewers early on to design their study to track a clinically meaningful change in LVEF rather than just looking at the average overall change.
Dr. Deswal also noted that it is unlikely that future studies will examine the efficacy of a statin for preventing LVEF in patients across the range of cancers that are eligible for anthracycline treatment. As a result, she predicted that “we may have to extrapolate” the results from STOP-CA to patients with other cancer types.
STOP-CA received no commercial funding. Dr. Neilan has been a consultant for and received fees from Abbvie, Amgen, Bristol-Myers Squibb, CRC Oncology, Genentech, Roche, and Sanofi, and has received grant funding from AstraZeneca and Bristol Myers Squib. Dr. Deswal and Dr. Witteles had no relevant disclosures.
NEW ORLEANS – Atorvastatin treatment of patients with lymphoma undergoing treatment with an anthracycline significantly cut the incidence of incident cardiac dysfunction by about two-thirds during 12 months of treatment, in a multicenter, randomized trial with 300 enrolled patients.
“These data support the use of atorvastatin among patients with lymphoma being treated with anthracyclines where prevention of cardiac systolic dysfunction is important,” concluded Tomas G. Neilan, MD, at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
He highlighted that an important difference between the new study, STOP-CA, and a major prior study with a neutral effect published in 2022, was that STOP-CA “was powered for a major change” in cardiac function as the study’s primary outcome, a decline from baseline in left ventricular ejection fraction (LVEF) of at least 10% that also reduced ejection fraction to less than 55%.
“We can consider these medications [atorvastatin] for patients at higher risk for cardiac toxicity from anthracyclines, such as patients who receive a higher dose of an anthracycline, older patients, people with obesity, and women, commented Anita Deswal, MD, professor and chair of the department of cardiology at the University of Texas MD Anderson Cancer Center, Houston, who was not involved with the study.
A basis for an ‘important discussion’ with patients
“For patients receiving higher doses of anthracyclines, the STOP-CA trial says that whether to start a statin for cardiac protection is now an important discussion” for these patients to have with their treating clinicians. ”That was not the case before today,” commented Ronald M. Witteles, MD, a cardiologist and professor who specializes in cardio-oncology at Stanford (Calif.) University.
“For a patient being treated for lymphoma or for another cancer and treated with equal or higher anthracycline doses, such as patients with a sarcoma, this trial’s results at the very least warrant a discussion between physicians and patients to make the decision,” Dr. Witteles, who was not involved in the study, said in an interview. But he also cautioned that “whether an individual patient should take a statin in this scenario is still not a no-brainer. While the trial was positive, it was for an imaging rather than for a clinical endpoint.”
Experts noted that a similar study with the clinical endpoint of heart failure would require both many more randomized patients as well as much longer follow-up. STOP-CA was not powered for this endpoint. During its 12-month duration, a total of 11 patients developed heart failure, with no between group difference.
STOP-CA enrolled adults with lymphoma (Hodgkin or non-Hodgkin) and scheduled to undergo anthracycline treatment at eight U.S. centers and one in Canada, and excluded patients already on statin treatment or those for whom a statin was already indicated. Of the 300 enrolled patients, 286 had 12-month follow-up. Randomization assigned patients to receive either 40 mg daily of atorvastatin or placebo.
Their cumulative, median anthracycline dose was 300 mg/m2, which is typical for treating lymphoma, but higher than the typical dose use for patients with breast cancer. At baseline, average LVEF was 63%, and after 12 months this had declined to 59%. Forty-six of the 286 patients assessed after 12 months fulfilled the primary outcome of at least a 10–percentage point reduction from baseline in their LVEF and a decline in LVEF to less than 55%. Researchers used cardiac MR to assess LVEF at baseline, and in most patients at follow-up, but a minority of patients had their follow-up assessments by echocardiography because of logistical issues. Greater than 90% of patients were adherent to their assigned regimen.
Tripled incidence of cardiac dysfunction in placebo patients
The incidence of this outcome was 9% among the patients who received atorvastatin, and 22% among those on placebo, a significant difference. The calculated odds of the primary outcome was 2.9-fold more likely among the patients treated with placebo, compared with those who received atorvastatin, also a significant difference.
The study’s secondary outcome was patients who had at least a 5% drop from baseline in their LVEF and with a LVEF of less than 55% after 12 months. This outcome occurred in 13% of patients treated with atorvastatin and in 29% of those who received placebo, a significant difference.
The atorvastatin and placebo arms showed no significant differences in adverse events during the study, with roughly similar incidence rates for muscle pain, elevated liver enzymes, and renal failure. None of the enrolled patients developed myositis.
Atorvastatin treatment also produced an expected average 37% decline from baseline in levels of LDL cholesterol.
“This was a well-designed and important trial,” said Dr. Witteles. “Anthracyclines remain a mainstay of cancer therapies for a number of malignancies, such as lymphoma and sarcoma, and the cardiac side effects of development of cardiac dysfunction are unequivocally real.”
The importance of a clinically meaningful effect
The results especially contrast with the findings from the PREVENT study, published in 2022, which compared a daily, 40-mg atorvastatin treatment with placebo in 279 randomized patients with breast cancer and treated for 24 months. However, patients in PREVENT had a cumulative, median anthracycline dose of 240 mg/m2, and the study’s primary outcome was the average change from baseline in LVEF after 24 months of treatment, which was a reduction of 0.08 percentage points in the placebo arm, a nonsignificant difference.
In STOP-CA, the average change in LVEF from baseline was a 1–percentage point reduction in the placebo arm, compared with the atorvastatin-treated patients, a difference that was statistically significant, but “not clinically significant,” said Dr. Neilan, director of the cardio-oncology program at Massachusetts General Hospital, Boston. He cited the good fortune of the STOP-CA investigators when they received a recommendation from reviewers early on to design their study to track a clinically meaningful change in LVEF rather than just looking at the average overall change.
Dr. Deswal also noted that it is unlikely that future studies will examine the efficacy of a statin for preventing LVEF in patients across the range of cancers that are eligible for anthracycline treatment. As a result, she predicted that “we may have to extrapolate” the results from STOP-CA to patients with other cancer types.
STOP-CA received no commercial funding. Dr. Neilan has been a consultant for and received fees from Abbvie, Amgen, Bristol-Myers Squibb, CRC Oncology, Genentech, Roche, and Sanofi, and has received grant funding from AstraZeneca and Bristol Myers Squib. Dr. Deswal and Dr. Witteles had no relevant disclosures.
AT ACC 2023
Bempedoic acid cuts CV events in statin-intolerant patients: CLEAR Outcomes
A new approach to lowering cholesterol with the use of bempedoic acid (Nexletol, Esperion) brought about a significant reduction in cardiovascular events in patients intolerant to statins in the large phase 3, placebo-controlled CLEAR Outcomes trial.
The drug lowered LDL cholesterol by 21% in the study and reduced the composite primary endpoint, including cardiovascular death, MI, stroke, or coronary revascularization, by 13%; MI was reduced by 23% and coronary revascularization, by 19%.
The drug was also well tolerated in the mixed population of primary and secondary prevention patients unable or unwilling to take statins.
“These findings establish bempedoic acid as an effective approach to reduce major cardiovascular events in statin-intolerant patients,” study chair, Steven E. Nissen, MD, of the Cleveland Clinic concluded.
Dr. Nissen presented the CLEAR Outcomes trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The study was simultaneously published online in the New England Journal of Medicine. Top-line results were previously reported in December 2022.
Dr. Nissen pointed out that, while in the current study bempedoic acid was studied as monotherapy, he believes the drug will mainly be used in clinical practice in combination with ezetimibe, a combination shown to reduce LDL by 38%. “I think this is how it will be used in clinical practice. So, we can get an almost 40% LDL reduction – that’s about the same as 40 mg simvastatin or 20 mg atorvastatin – without giving a statin. And I think that’s where I see the potential of this therapy,” he said.
Dr. Nissen described statin intolerance as “a vexing problem” that prevents many patients from achieving LDL cholesterol levels associated with cardiovascular benefits.
He explained that bempedoic acid, an adenosine triphosphate citrate lyase inhibitor, inhibits hepatic cholesterol synthesis upstream of hydroxymethylglutaryl coenzyme A reductase, the enzyme inhibited by statins. Bempedoic acid is a prodrug activated in the liver, but not in peripheral tissues, resulting in a low incidence of muscle-related adverse events. Although bempedoic acid is approved for lowering LDL cholesterol, this is the first trial to assess its effects on cardiovascular outcomes.
CLEAR Outcomes
The CLEAR Outcomes trial included 13,970 patients (48% women) from 32 countries who were unable or unwilling to take statins owing to unacceptable adverse effects and who had, or were at high risk for, cardiovascular disease. They were randomly assigned to oral bempedoic acid, 180 mg daily, or placebo.
The mean LDL cholesterol level at baseline was 139 mg/dL in both groups, and after 6 months, the reduction in the level was greater with bempedoic acid than with placebo by 29.2 mg/dL (a 21.1% reduction).
The drug was also associated with a 22% reduction in high-sensitivity C-reactive protein.
After a median duration of follow-up of 40.6 months, the incidence of a primary endpoint (cardiovascular death, MI, stroke, or coronary revascularization) was significantly lower (by 13%) with bempedoic acid than with placebo (11.7% vs. 13.3%; hazard ratio, 0.87; P = .004).
The absolute risk reduction was 1.6 percentage points, and the number needed to treat for 40 months to prevent one event was 63.
The secondary composite endpoint of cardiovascular death/stroke/MI was reduced by 15% (8.2% vs. 9.5%; HR, 0.85; P = .006). Fatal or nonfatal MI was reduced by 23% (3.7% vs. 4.8%; HR, 0.77; P = .002), and coronary revascularization was reduced by 19% (6.2% vs. 7.6%; HR, 0.81; P = .001).
Bempedoic acid had no significant effects on fatal or nonfatal stroke, death from cardiovascular causes, and death from any cause.
Subgroup analysis showed similar results across all groups and no difference in treatment effect between men and women.
Adverse events were reported by 25% of patients in both groups, with adverse events leading to discontinuation reported by 10.8% of the bempedoic acid group and 10.4% of the placebo group.
Muscle disorders were reported in 15.0% of the bempedoic acid group versus 15.4% of the placebo group. And there was also no difference in new cases of diabetes (16.1% vs. 17.1%).
Bempedoic acid was associated with small increases in the incidence of gout (3.1% vs. 2.1%) and cholelithiasis (2.2% vs. 1.2%), and also small increases in serum creatinine, uric acid, and hepatic enzyme levels.
In the NEJM article, the authors pointed out that the concept of statin intolerance remains controversial. Some recent studies suggested that reported adverse effects represent an anticipation of harm, often described as the “nocebo” effect.
“Whether real or perceived, statin intolerance remains a vexing clinical problem that can prevent patients who are guideline eligible for statin treatment from reaching LDL cholesterol levels associated with clinical benefits. Accordingly, alternative nonstatin therapies are needed to manage the LDL cholesterol level in these patients,” they wrote.
“Management of patients unable or unwilling to take statins represents a challenging and frustrating clinical issue. Regardless whether this problem represents the ‘nocebo’ effect or actual intolerance, these high-risk patients need effective alternative therapies,” Dr. Nissen concluded. “The CLEAR Outcomes trial provides a sound rationale for use of bempedoic acid to reduce major adverse cardiovascular outcomes in patients intolerant to statins.”
‘Compelling findings’
Discussing the trial at the ACC late-breaking clinical trial session, Michelle O’Donoghue, MD, Brigham and Women’s Hospital, Boston, noted that this is the largest trial to date in statin-intolerant patients.
She pointed out that although the issue of statin intolerance remains controversial, adherence to statins is often not good, so this is an important patient population to study.
She said it was “quite remarkable” that 48% of the study were women, adding: “There is still much that we need to understand about why women appear to be less willing or able to tolerate statin therapy.”
Dr. O’Donoghue concluded that the study showed “compelling findings,” and the event reduction was in line with what would be expected from the LDL cholesterol reduction, further supporting the LDL cholesterol hypothesis.
She added: “Bempedoic acid is an important addition to our arsenal of nonstatin LDL-lowering therapies. And while it was overall well tolerated, it did not get a complete free pass, as there were some modest safety concerns.”
In an editorial accompanying the NEJM publication, John Alexander, MD, Duke Clinical Research Institute, Durham, N.C., wrote: “The compelling results of the CLEAR Outcomes trial will and should increase the use of bempedoic acid in patients with established atherosclerotic vascular disease and in those at high risk for vascular disease who are unable or unwilling to take statins.”
He warned, however, that it is premature to consider bempedoic acid as an alternative to statins. “Given the overwhelming evidence of the vascular benefits of statins, clinicians should continue their efforts to prescribe them at the maximum tolerated doses for appropriate patients, including those who may have discontinued statins because of presumed side effects.”.
Dr. Alexander also pointed out that although bempedoic acid also reduces the LDL cholesterol level in patients taking statins, the clinical benefits of bempedoic acid added to standard statin therapy are unknown.
On the observation that bempedoic acid had no observed effect on mortality, he noted that “Many individual trials of statins have also not shown an effect of the agent on mortality; it was only through the meta-analysis of multiple clinical trials that the effects of statins on mortality became clear.”
“Bempedoic acid has now entered the list of evidence-based alternatives to statins for primary and secondary prevention in patients at high cardiovascular risk,” Dr. Alexander concluded. “The benefits of bempedoic acid are now clearer, and it is now our responsibility to translate this information into better primary and secondary prevention for more at-risk patients, who will, as a result, benefit from fewer cardiovascular events.”
In a second editorial, John F. Keaney Jr., MD, Brigham and Women’s Hospital, said the lack of a clear association between bempedoic acid and muscle disorders, new-onset diabetes, or worsening hyperglycemia is “welcome news” for statin-intolerant patients.
But he cautioned that “these data must be interpreted cautiously, because bempedoic acid, when combined with a statin, appears to enhance the occurrence of muscle symptoms. Moreover, bempedoic acid has its own reported side effects, including tendon rupture, increased uric acid levels, gout, and reduced glomerular filtration rate, which are not seen with statin use.”
In terms of drug interactions, Dr. Keaney noted that bempedoic acid can increase the circulating levels of simvastatin and pravastatin, so it should not be used in patients who are receiving these agents at doses above 20 mg and 40 mg, respectively. Similarly, bempedoic acid should not be used with fibrates other than fenofibrate because of concerns regarding cholelithiasis.
“Available data clearly indicate that bempedoic acid can be used as an adjunct to statin and nonstatin therapies (except as noted above) to produce an additional 16%-26% reduction in the LDL cholesterol level,” he added. “However, it is not yet clear to what extent adjunctive bempedoic acid will further reduce the risk of cardiovascular events.”
The CLEAR Outcomes trial was supported by Esperion Therapeutics. Dr. Nissen reported receiving grants from AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Esperion, Novartis, and Silence Pharmaceuticals and consultancies with Amgen and Glenmark Pharmaceuticals.
A version of this article first appeared on Medscape.com.
A new approach to lowering cholesterol with the use of bempedoic acid (Nexletol, Esperion) brought about a significant reduction in cardiovascular events in patients intolerant to statins in the large phase 3, placebo-controlled CLEAR Outcomes trial.
The drug lowered LDL cholesterol by 21% in the study and reduced the composite primary endpoint, including cardiovascular death, MI, stroke, or coronary revascularization, by 13%; MI was reduced by 23% and coronary revascularization, by 19%.
The drug was also well tolerated in the mixed population of primary and secondary prevention patients unable or unwilling to take statins.
“These findings establish bempedoic acid as an effective approach to reduce major cardiovascular events in statin-intolerant patients,” study chair, Steven E. Nissen, MD, of the Cleveland Clinic concluded.
Dr. Nissen presented the CLEAR Outcomes trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The study was simultaneously published online in the New England Journal of Medicine. Top-line results were previously reported in December 2022.
Dr. Nissen pointed out that, while in the current study bempedoic acid was studied as monotherapy, he believes the drug will mainly be used in clinical practice in combination with ezetimibe, a combination shown to reduce LDL by 38%. “I think this is how it will be used in clinical practice. So, we can get an almost 40% LDL reduction – that’s about the same as 40 mg simvastatin or 20 mg atorvastatin – without giving a statin. And I think that’s where I see the potential of this therapy,” he said.
Dr. Nissen described statin intolerance as “a vexing problem” that prevents many patients from achieving LDL cholesterol levels associated with cardiovascular benefits.
He explained that bempedoic acid, an adenosine triphosphate citrate lyase inhibitor, inhibits hepatic cholesterol synthesis upstream of hydroxymethylglutaryl coenzyme A reductase, the enzyme inhibited by statins. Bempedoic acid is a prodrug activated in the liver, but not in peripheral tissues, resulting in a low incidence of muscle-related adverse events. Although bempedoic acid is approved for lowering LDL cholesterol, this is the first trial to assess its effects on cardiovascular outcomes.
CLEAR Outcomes
The CLEAR Outcomes trial included 13,970 patients (48% women) from 32 countries who were unable or unwilling to take statins owing to unacceptable adverse effects and who had, or were at high risk for, cardiovascular disease. They were randomly assigned to oral bempedoic acid, 180 mg daily, or placebo.
The mean LDL cholesterol level at baseline was 139 mg/dL in both groups, and after 6 months, the reduction in the level was greater with bempedoic acid than with placebo by 29.2 mg/dL (a 21.1% reduction).
The drug was also associated with a 22% reduction in high-sensitivity C-reactive protein.
After a median duration of follow-up of 40.6 months, the incidence of a primary endpoint (cardiovascular death, MI, stroke, or coronary revascularization) was significantly lower (by 13%) with bempedoic acid than with placebo (11.7% vs. 13.3%; hazard ratio, 0.87; P = .004).
The absolute risk reduction was 1.6 percentage points, and the number needed to treat for 40 months to prevent one event was 63.
The secondary composite endpoint of cardiovascular death/stroke/MI was reduced by 15% (8.2% vs. 9.5%; HR, 0.85; P = .006). Fatal or nonfatal MI was reduced by 23% (3.7% vs. 4.8%; HR, 0.77; P = .002), and coronary revascularization was reduced by 19% (6.2% vs. 7.6%; HR, 0.81; P = .001).
Bempedoic acid had no significant effects on fatal or nonfatal stroke, death from cardiovascular causes, and death from any cause.
Subgroup analysis showed similar results across all groups and no difference in treatment effect between men and women.
Adverse events were reported by 25% of patients in both groups, with adverse events leading to discontinuation reported by 10.8% of the bempedoic acid group and 10.4% of the placebo group.
Muscle disorders were reported in 15.0% of the bempedoic acid group versus 15.4% of the placebo group. And there was also no difference in new cases of diabetes (16.1% vs. 17.1%).
Bempedoic acid was associated with small increases in the incidence of gout (3.1% vs. 2.1%) and cholelithiasis (2.2% vs. 1.2%), and also small increases in serum creatinine, uric acid, and hepatic enzyme levels.
In the NEJM article, the authors pointed out that the concept of statin intolerance remains controversial. Some recent studies suggested that reported adverse effects represent an anticipation of harm, often described as the “nocebo” effect.
“Whether real or perceived, statin intolerance remains a vexing clinical problem that can prevent patients who are guideline eligible for statin treatment from reaching LDL cholesterol levels associated with clinical benefits. Accordingly, alternative nonstatin therapies are needed to manage the LDL cholesterol level in these patients,” they wrote.
“Management of patients unable or unwilling to take statins represents a challenging and frustrating clinical issue. Regardless whether this problem represents the ‘nocebo’ effect or actual intolerance, these high-risk patients need effective alternative therapies,” Dr. Nissen concluded. “The CLEAR Outcomes trial provides a sound rationale for use of bempedoic acid to reduce major adverse cardiovascular outcomes in patients intolerant to statins.”
‘Compelling findings’
Discussing the trial at the ACC late-breaking clinical trial session, Michelle O’Donoghue, MD, Brigham and Women’s Hospital, Boston, noted that this is the largest trial to date in statin-intolerant patients.
She pointed out that although the issue of statin intolerance remains controversial, adherence to statins is often not good, so this is an important patient population to study.
She said it was “quite remarkable” that 48% of the study were women, adding: “There is still much that we need to understand about why women appear to be less willing or able to tolerate statin therapy.”
Dr. O’Donoghue concluded that the study showed “compelling findings,” and the event reduction was in line with what would be expected from the LDL cholesterol reduction, further supporting the LDL cholesterol hypothesis.
She added: “Bempedoic acid is an important addition to our arsenal of nonstatin LDL-lowering therapies. And while it was overall well tolerated, it did not get a complete free pass, as there were some modest safety concerns.”
In an editorial accompanying the NEJM publication, John Alexander, MD, Duke Clinical Research Institute, Durham, N.C., wrote: “The compelling results of the CLEAR Outcomes trial will and should increase the use of bempedoic acid in patients with established atherosclerotic vascular disease and in those at high risk for vascular disease who are unable or unwilling to take statins.”
He warned, however, that it is premature to consider bempedoic acid as an alternative to statins. “Given the overwhelming evidence of the vascular benefits of statins, clinicians should continue their efforts to prescribe them at the maximum tolerated doses for appropriate patients, including those who may have discontinued statins because of presumed side effects.”.
Dr. Alexander also pointed out that although bempedoic acid also reduces the LDL cholesterol level in patients taking statins, the clinical benefits of bempedoic acid added to standard statin therapy are unknown.
On the observation that bempedoic acid had no observed effect on mortality, he noted that “Many individual trials of statins have also not shown an effect of the agent on mortality; it was only through the meta-analysis of multiple clinical trials that the effects of statins on mortality became clear.”
“Bempedoic acid has now entered the list of evidence-based alternatives to statins for primary and secondary prevention in patients at high cardiovascular risk,” Dr. Alexander concluded. “The benefits of bempedoic acid are now clearer, and it is now our responsibility to translate this information into better primary and secondary prevention for more at-risk patients, who will, as a result, benefit from fewer cardiovascular events.”
In a second editorial, John F. Keaney Jr., MD, Brigham and Women’s Hospital, said the lack of a clear association between bempedoic acid and muscle disorders, new-onset diabetes, or worsening hyperglycemia is “welcome news” for statin-intolerant patients.
But he cautioned that “these data must be interpreted cautiously, because bempedoic acid, when combined with a statin, appears to enhance the occurrence of muscle symptoms. Moreover, bempedoic acid has its own reported side effects, including tendon rupture, increased uric acid levels, gout, and reduced glomerular filtration rate, which are not seen with statin use.”
In terms of drug interactions, Dr. Keaney noted that bempedoic acid can increase the circulating levels of simvastatin and pravastatin, so it should not be used in patients who are receiving these agents at doses above 20 mg and 40 mg, respectively. Similarly, bempedoic acid should not be used with fibrates other than fenofibrate because of concerns regarding cholelithiasis.
“Available data clearly indicate that bempedoic acid can be used as an adjunct to statin and nonstatin therapies (except as noted above) to produce an additional 16%-26% reduction in the LDL cholesterol level,” he added. “However, it is not yet clear to what extent adjunctive bempedoic acid will further reduce the risk of cardiovascular events.”
The CLEAR Outcomes trial was supported by Esperion Therapeutics. Dr. Nissen reported receiving grants from AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Esperion, Novartis, and Silence Pharmaceuticals and consultancies with Amgen and Glenmark Pharmaceuticals.
A version of this article first appeared on Medscape.com.
A new approach to lowering cholesterol with the use of bempedoic acid (Nexletol, Esperion) brought about a significant reduction in cardiovascular events in patients intolerant to statins in the large phase 3, placebo-controlled CLEAR Outcomes trial.
The drug lowered LDL cholesterol by 21% in the study and reduced the composite primary endpoint, including cardiovascular death, MI, stroke, or coronary revascularization, by 13%; MI was reduced by 23% and coronary revascularization, by 19%.
The drug was also well tolerated in the mixed population of primary and secondary prevention patients unable or unwilling to take statins.
“These findings establish bempedoic acid as an effective approach to reduce major cardiovascular events in statin-intolerant patients,” study chair, Steven E. Nissen, MD, of the Cleveland Clinic concluded.
Dr. Nissen presented the CLEAR Outcomes trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The study was simultaneously published online in the New England Journal of Medicine. Top-line results were previously reported in December 2022.
Dr. Nissen pointed out that, while in the current study bempedoic acid was studied as monotherapy, he believes the drug will mainly be used in clinical practice in combination with ezetimibe, a combination shown to reduce LDL by 38%. “I think this is how it will be used in clinical practice. So, we can get an almost 40% LDL reduction – that’s about the same as 40 mg simvastatin or 20 mg atorvastatin – without giving a statin. And I think that’s where I see the potential of this therapy,” he said.
Dr. Nissen described statin intolerance as “a vexing problem” that prevents many patients from achieving LDL cholesterol levels associated with cardiovascular benefits.
He explained that bempedoic acid, an adenosine triphosphate citrate lyase inhibitor, inhibits hepatic cholesterol synthesis upstream of hydroxymethylglutaryl coenzyme A reductase, the enzyme inhibited by statins. Bempedoic acid is a prodrug activated in the liver, but not in peripheral tissues, resulting in a low incidence of muscle-related adverse events. Although bempedoic acid is approved for lowering LDL cholesterol, this is the first trial to assess its effects on cardiovascular outcomes.
CLEAR Outcomes
The CLEAR Outcomes trial included 13,970 patients (48% women) from 32 countries who were unable or unwilling to take statins owing to unacceptable adverse effects and who had, or were at high risk for, cardiovascular disease. They were randomly assigned to oral bempedoic acid, 180 mg daily, or placebo.
The mean LDL cholesterol level at baseline was 139 mg/dL in both groups, and after 6 months, the reduction in the level was greater with bempedoic acid than with placebo by 29.2 mg/dL (a 21.1% reduction).
The drug was also associated with a 22% reduction in high-sensitivity C-reactive protein.
After a median duration of follow-up of 40.6 months, the incidence of a primary endpoint (cardiovascular death, MI, stroke, or coronary revascularization) was significantly lower (by 13%) with bempedoic acid than with placebo (11.7% vs. 13.3%; hazard ratio, 0.87; P = .004).
The absolute risk reduction was 1.6 percentage points, and the number needed to treat for 40 months to prevent one event was 63.
The secondary composite endpoint of cardiovascular death/stroke/MI was reduced by 15% (8.2% vs. 9.5%; HR, 0.85; P = .006). Fatal or nonfatal MI was reduced by 23% (3.7% vs. 4.8%; HR, 0.77; P = .002), and coronary revascularization was reduced by 19% (6.2% vs. 7.6%; HR, 0.81; P = .001).
Bempedoic acid had no significant effects on fatal or nonfatal stroke, death from cardiovascular causes, and death from any cause.
Subgroup analysis showed similar results across all groups and no difference in treatment effect between men and women.
Adverse events were reported by 25% of patients in both groups, with adverse events leading to discontinuation reported by 10.8% of the bempedoic acid group and 10.4% of the placebo group.
Muscle disorders were reported in 15.0% of the bempedoic acid group versus 15.4% of the placebo group. And there was also no difference in new cases of diabetes (16.1% vs. 17.1%).
Bempedoic acid was associated with small increases in the incidence of gout (3.1% vs. 2.1%) and cholelithiasis (2.2% vs. 1.2%), and also small increases in serum creatinine, uric acid, and hepatic enzyme levels.
In the NEJM article, the authors pointed out that the concept of statin intolerance remains controversial. Some recent studies suggested that reported adverse effects represent an anticipation of harm, often described as the “nocebo” effect.
“Whether real or perceived, statin intolerance remains a vexing clinical problem that can prevent patients who are guideline eligible for statin treatment from reaching LDL cholesterol levels associated with clinical benefits. Accordingly, alternative nonstatin therapies are needed to manage the LDL cholesterol level in these patients,” they wrote.
“Management of patients unable or unwilling to take statins represents a challenging and frustrating clinical issue. Regardless whether this problem represents the ‘nocebo’ effect or actual intolerance, these high-risk patients need effective alternative therapies,” Dr. Nissen concluded. “The CLEAR Outcomes trial provides a sound rationale for use of bempedoic acid to reduce major adverse cardiovascular outcomes in patients intolerant to statins.”
‘Compelling findings’
Discussing the trial at the ACC late-breaking clinical trial session, Michelle O’Donoghue, MD, Brigham and Women’s Hospital, Boston, noted that this is the largest trial to date in statin-intolerant patients.
She pointed out that although the issue of statin intolerance remains controversial, adherence to statins is often not good, so this is an important patient population to study.
She said it was “quite remarkable” that 48% of the study were women, adding: “There is still much that we need to understand about why women appear to be less willing or able to tolerate statin therapy.”
Dr. O’Donoghue concluded that the study showed “compelling findings,” and the event reduction was in line with what would be expected from the LDL cholesterol reduction, further supporting the LDL cholesterol hypothesis.
She added: “Bempedoic acid is an important addition to our arsenal of nonstatin LDL-lowering therapies. And while it was overall well tolerated, it did not get a complete free pass, as there were some modest safety concerns.”
In an editorial accompanying the NEJM publication, John Alexander, MD, Duke Clinical Research Institute, Durham, N.C., wrote: “The compelling results of the CLEAR Outcomes trial will and should increase the use of bempedoic acid in patients with established atherosclerotic vascular disease and in those at high risk for vascular disease who are unable or unwilling to take statins.”
He warned, however, that it is premature to consider bempedoic acid as an alternative to statins. “Given the overwhelming evidence of the vascular benefits of statins, clinicians should continue their efforts to prescribe them at the maximum tolerated doses for appropriate patients, including those who may have discontinued statins because of presumed side effects.”.
Dr. Alexander also pointed out that although bempedoic acid also reduces the LDL cholesterol level in patients taking statins, the clinical benefits of bempedoic acid added to standard statin therapy are unknown.
On the observation that bempedoic acid had no observed effect on mortality, he noted that “Many individual trials of statins have also not shown an effect of the agent on mortality; it was only through the meta-analysis of multiple clinical trials that the effects of statins on mortality became clear.”
“Bempedoic acid has now entered the list of evidence-based alternatives to statins for primary and secondary prevention in patients at high cardiovascular risk,” Dr. Alexander concluded. “The benefits of bempedoic acid are now clearer, and it is now our responsibility to translate this information into better primary and secondary prevention for more at-risk patients, who will, as a result, benefit from fewer cardiovascular events.”
In a second editorial, John F. Keaney Jr., MD, Brigham and Women’s Hospital, said the lack of a clear association between bempedoic acid and muscle disorders, new-onset diabetes, or worsening hyperglycemia is “welcome news” for statin-intolerant patients.
But he cautioned that “these data must be interpreted cautiously, because bempedoic acid, when combined with a statin, appears to enhance the occurrence of muscle symptoms. Moreover, bempedoic acid has its own reported side effects, including tendon rupture, increased uric acid levels, gout, and reduced glomerular filtration rate, which are not seen with statin use.”
In terms of drug interactions, Dr. Keaney noted that bempedoic acid can increase the circulating levels of simvastatin and pravastatin, so it should not be used in patients who are receiving these agents at doses above 20 mg and 40 mg, respectively. Similarly, bempedoic acid should not be used with fibrates other than fenofibrate because of concerns regarding cholelithiasis.
“Available data clearly indicate that bempedoic acid can be used as an adjunct to statin and nonstatin therapies (except as noted above) to produce an additional 16%-26% reduction in the LDL cholesterol level,” he added. “However, it is not yet clear to what extent adjunctive bempedoic acid will further reduce the risk of cardiovascular events.”
The CLEAR Outcomes trial was supported by Esperion Therapeutics. Dr. Nissen reported receiving grants from AbbVie, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Esperion, Novartis, and Silence Pharmaceuticals and consultancies with Amgen and Glenmark Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Skin reactions from melanoma targeted and immune therapies range from pruritus to SJS
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
AT MELANOMA 2023