User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
New insight into preventing antipsychotic-induced weight gain
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.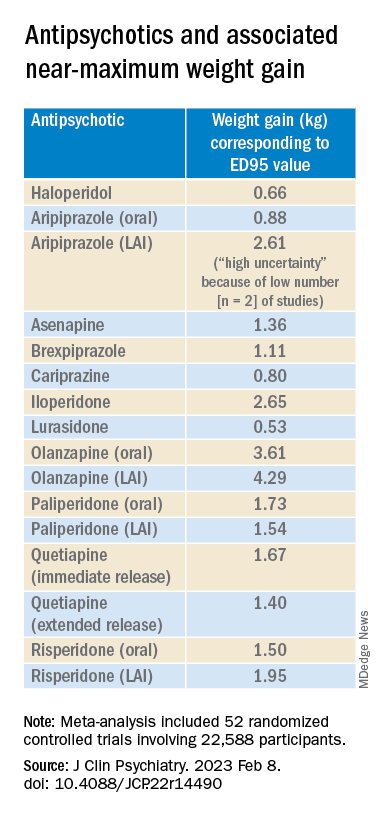
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
In the first dose-response meta-analysis focusing on antipsychotic-induced weight gain, researchers provide data on the trajectory of this risk associated with individual agents.
Investigators analyzed 52 randomized controlled trials (RCTs) encompassing more than 22,500 participants with schizophrenia treated with antipsychotics. They found that, with the exception of aripiprazole long-acting injectable (LAI), all of the other antipsychotics has significant dose-response effect on weight gain. Furthermore, weight gain occurred with some antipsychotics even at relatively low doses.
“We found significant dose-response associations for weight and metabolic variables, with a unique signature for each antipsychotic,” write the investigators, led by Michel Sabé, MD, of the division of adult psychiatry, department of psychiatry, Geneva University Hospitals.
“Despite several limitations, including the limited number of available studies, our results may provide useful information for preventing weight gain and metabolic disturbances by adapting antipsychotic doses,” they add.
The study was published online in The Journal of Clinical Psychiatry.
Balancing risks and benefits
Antipsychotics are first-line therapy for schizophrenia and are associated with weight gain, lipid disturbances, and glucose dysregulation – especially second-generation antipsychotics (SGAs), which can lead to obesity, type 2 diabetes, and metabolic syndrome.
Given that people with schizophrenia also tend to have lifestyle-related cardiovascular risk factors, it’s important to find “a balance between beneficial and adverse effects of antipsychotics,” the investigators note
The question of whether weight gain and metabolic dysregulation are dose-dependent “remains controversial.” The effect of specific SGAs on weight gain has been investigated, but only one study has been conducted using a dose-response meta-analysis, and that study did not address metabolic disturbance.
The investigators conducted a systematic review and a dose-response meta-analysis of fixed-dose randomized controlled trials (RCTs) investigating antipsychotic-induced weight gain and metabolic disturbance in adults with acute schizophrenia.
To be included in the analysis, RCTs had to focus on adult patients with schizophrenia or related disorders and include a placebo as a comparator to the drug.
Studies involved only short-term administration of antipsychotics (2-13 weeks) rather than maintenance therapy.
The mean (SD) change in weight (body weight and/or body mass index) between baseline and the study endpoint constituted the primary outcome, with secondary outcomes including changes in metabolic parameters.
The researchers characterized the dose-response relationship using a nonlinear restricted cubic spline model, with three “knots” located at the 10th, 50th, and 90th percentiles of overall dose distribution.
They also calculated dose-response curves and estimated 50% and 95% effective doses (ED50 and ED95, respectively), extracted from the estimated dose-response curves for each antipsychotic.
The researchers then calculated the weight gain at each effective dose (ED50 and ED95) in milligrams and the weight gain corresponding to the ED95 value in kilograms.
Shared decision-making
Of 6,812 citations, the researchers selected 52 RCTs that met inclusion criteria (n = 22,588 participants, with 16,311 receiving antipsychotics and 6,277 receiving placebo; mean age, 38.5 years, 69.2% male). The studies were conducted between1996 and 2021.
The risk for bias in most studies was “low,” although 21% of the studies “presented a high risk.”
With the exception of aripiprazole LAI, all of the other antipsychotics had a “significant dose-response” association with weight.
For example, oral aripiprazole exhibited a significant dose-response association for weight, but there was no significant association found for aripiprazole LAI (c2 = 8.744; P = .0126 vs. c2 = 3.107; P = .2115). However, both curves were still ascending at maximum doses, the authors note.
Metabolically neutral
Antipsychotics with a decreasing or quasi-parabolic dose-response curve for weight included brexpiprazole, cariprazine, haloperidol, lurasidone, and quetiapine ER: for these antipsychotics, the ED95 weight gain ranged from 0.53 kg to 1.40 kg.
These antipsychotics “reach their weight gain ED95 at relatively low median effective doses, and higher doses, which mostly correspond to near-maximum effective doses, may even be associated with less weight gain,” the authors note.
In addition, only doses higher than the near-maximum effective dose of brexpiprazole were associated with a small increase in total cholesterol. And cariprazine presented “significantly decreasing curves” at higher doses for LDL cholesterol.
With the exception of quetiapine, this group of medications might be regarded as “metabolically neutral” in terms of weight gain and metabolic disturbances.
Antipsychotics with a plateau-shaped curve were asenapine, iloperidone, paliperidone LAI, quetiapine IR, and risperidone, with a weight gain ED95 ranging from 1.36 to 2.65 kg.
Aripiprazole and olanzapine (oral and LAI formulations), as well as risperidone LAI and oral paliperidone, presented weight gain curves that continued climbing at higher doses (especially olanzapine). However, the drugs have different metabolic profiles, ranging from 0.88 kg ED95 for oral aripiprazole to 4.29 kg for olanzapine LAI.
Olanzapine had the most pronounced weight gain, in addition to associations with all metabolic outcomes.
For some drugs with important metabolic side effects, “a lower dose might provide a better combination of high efficacy and reduced metabolic side effects,” the authors write.
The findings might “provide additional information for clinicians aiming to determine the most suitable dose to prevent weight gain and metabolic disturbance in a shared decision-making process with their patients,” they note.
The results add to “existing concerns about the use of olanzapine as a first-line drug,” they add.
Lowest effective dose
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said clinicians “not infrequently increase doses to achieve better symptom control, [but] this decision should be informed by the additional observation herein that the increase in those could be accompanied by weight increase.”
Moreover, many patients “take concomitant medications that could possibly increase the bioavailability of antipsychotics, which may also increase the risk for weight gain,” said Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto. He was not involved with this study.
“These data provide a reason to believe that for many people antipsychotic-associated weight gain could be mitigated by using the lowest effective dose, and rather than censor the use of some medications out of concern for weight gain, perhaps using the lowest effective dose of the medication will provide the opportunity for mitigation,” he added. “So I think it really guides clinicians to provide the lowest effective dose as a potential therapeutic and preventive strategy.”
The study received no financial support. Dr. Sabé reports no relevant financial relationships. Three coauthors report relationships with industry; the full list is contained in the original article.
Dr. McIntyre is a CEO of Braxia Scientific Corp. He has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute; speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie, and Atai Life Sciences.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Two FDA clearances add diabetes technology options
Two diabetes management devices that aid in the precision of insulin delivery have been recently cleared by the Food and Drug Administration.
On March 2, the FDA cleared the Android version of Bigfoot Biomedical’s Unity Mobile App for use with its system of smart pen caps that are compatible with different disposable insulin pens for administering both long-acting and rapid-acting insulin.
The system, which has been compatible with iOS devices since May 2021, is “the first and only FDA-cleared smart injection system that turns CGM [continuous glucose monitoring] data into dosing recommendations displayed right on the pen cap for people using multiple daily [insulin] injection therapy,” according to a company statement.
The Bigfoot app allows users to input and review provider treatment recommendations, displays current glucose ranges, and delivers real-time alerts.
Once it is commercially launched, the Android phone application will be available via the Google Play Store. “Given that 41% of U.S. smartphone users choose Android devices, this clearance enables expanded access to a large group of people with diabetes,” the company said.
On March 6, the FDA cleared the Abbott FreeStyle Libre 2 and FreeStyle Libre 3 devices as “integrated” CGM sensors. This means that they can now be used as components in automated insulin delivery systems, along with insulin pumps and connectivity software.
Abbott is working with insulin pump manufacturers Insulet and Tandem in the United States for integration with the FreeStyle Libre versions 2 and 3. Outside the United States, the Libre 3 is already authorized to work with mylife Loop from Ypsomed and CamDiab in Germany. Further launches are expected in the United Kingdom, Switzerland, and the Netherlands later this year.
The modified FreeStyle Libre 2 and FreeStyle Libre 3 sensors have been cleared for use by patients as young as age 2 years and for up to 15 days, in contrast to the previous versions, which were available for patients as young as 4 years for use up to 14 days. The FDA has cleared all Libre sensors – 2 and 3, current and future versions – for use by pregnant women with any type of diabetes.
The modified sensors will be available in the United States later this year and will eventually replace the Libre sensors in current use, the company said in a statement.
“The FreeStyle Libre portfolio is still the most affordable CGM on the market,” an Abbott representative said in an interview.
A version of this article first appeared on Medscape.com.
Two diabetes management devices that aid in the precision of insulin delivery have been recently cleared by the Food and Drug Administration.
On March 2, the FDA cleared the Android version of Bigfoot Biomedical’s Unity Mobile App for use with its system of smart pen caps that are compatible with different disposable insulin pens for administering both long-acting and rapid-acting insulin.
The system, which has been compatible with iOS devices since May 2021, is “the first and only FDA-cleared smart injection system that turns CGM [continuous glucose monitoring] data into dosing recommendations displayed right on the pen cap for people using multiple daily [insulin] injection therapy,” according to a company statement.
The Bigfoot app allows users to input and review provider treatment recommendations, displays current glucose ranges, and delivers real-time alerts.
Once it is commercially launched, the Android phone application will be available via the Google Play Store. “Given that 41% of U.S. smartphone users choose Android devices, this clearance enables expanded access to a large group of people with diabetes,” the company said.
On March 6, the FDA cleared the Abbott FreeStyle Libre 2 and FreeStyle Libre 3 devices as “integrated” CGM sensors. This means that they can now be used as components in automated insulin delivery systems, along with insulin pumps and connectivity software.
Abbott is working with insulin pump manufacturers Insulet and Tandem in the United States for integration with the FreeStyle Libre versions 2 and 3. Outside the United States, the Libre 3 is already authorized to work with mylife Loop from Ypsomed and CamDiab in Germany. Further launches are expected in the United Kingdom, Switzerland, and the Netherlands later this year.
The modified FreeStyle Libre 2 and FreeStyle Libre 3 sensors have been cleared for use by patients as young as age 2 years and for up to 15 days, in contrast to the previous versions, which were available for patients as young as 4 years for use up to 14 days. The FDA has cleared all Libre sensors – 2 and 3, current and future versions – for use by pregnant women with any type of diabetes.
The modified sensors will be available in the United States later this year and will eventually replace the Libre sensors in current use, the company said in a statement.
“The FreeStyle Libre portfolio is still the most affordable CGM on the market,” an Abbott representative said in an interview.
A version of this article first appeared on Medscape.com.
Two diabetes management devices that aid in the precision of insulin delivery have been recently cleared by the Food and Drug Administration.
On March 2, the FDA cleared the Android version of Bigfoot Biomedical’s Unity Mobile App for use with its system of smart pen caps that are compatible with different disposable insulin pens for administering both long-acting and rapid-acting insulin.
The system, which has been compatible with iOS devices since May 2021, is “the first and only FDA-cleared smart injection system that turns CGM [continuous glucose monitoring] data into dosing recommendations displayed right on the pen cap for people using multiple daily [insulin] injection therapy,” according to a company statement.
The Bigfoot app allows users to input and review provider treatment recommendations, displays current glucose ranges, and delivers real-time alerts.
Once it is commercially launched, the Android phone application will be available via the Google Play Store. “Given that 41% of U.S. smartphone users choose Android devices, this clearance enables expanded access to a large group of people with diabetes,” the company said.
On March 6, the FDA cleared the Abbott FreeStyle Libre 2 and FreeStyle Libre 3 devices as “integrated” CGM sensors. This means that they can now be used as components in automated insulin delivery systems, along with insulin pumps and connectivity software.
Abbott is working with insulin pump manufacturers Insulet and Tandem in the United States for integration with the FreeStyle Libre versions 2 and 3. Outside the United States, the Libre 3 is already authorized to work with mylife Loop from Ypsomed and CamDiab in Germany. Further launches are expected in the United Kingdom, Switzerland, and the Netherlands later this year.
The modified FreeStyle Libre 2 and FreeStyle Libre 3 sensors have been cleared for use by patients as young as age 2 years and for up to 15 days, in contrast to the previous versions, which were available for patients as young as 4 years for use up to 14 days. The FDA has cleared all Libre sensors – 2 and 3, current and future versions – for use by pregnant women with any type of diabetes.
The modified sensors will be available in the United States later this year and will eventually replace the Libre sensors in current use, the company said in a statement.
“The FreeStyle Libre portfolio is still the most affordable CGM on the market,” an Abbott representative said in an interview.
A version of this article first appeared on Medscape.com.
‘Keto-like’ diet linked to doubling of heart disease risk
Consumption of a low-carbohydrate, high-fat diet, dubbed a “keto-like” diet, was associated with an increase in LDL levels and a twofold increase in the risk for future cardiovascular events, in a new observational study.
“To our knowledge this is the first study to demonstrate an association between a carbohydrate-restricted dietary platform and greater risk of atherosclerotic cardiovascular disease,” said study investigator Iulia Iatan, MD, PhD, University of British Columbia, Vancouver.
“Hypercholesterolemia occurring during a low-carb, high-fat diet should not be assumed to be benign,” she concluded.
Dr. Iatan presented the study March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The presentation received much media attention, with headlines implying a causal relationship with cardiac events based on these observational results. But lipid expert Steven Nissen, MD, of the Cleveland Clinic, warned against paying much attention to the headlines or to the study’s conclusions.
In an interview, Dr. Nissen pointed out that the LDL increase in the “keto-like” diet group was relatively small and “certainly not enough to produce a doubling in cardiovascular risk.
“The people who were on the ‘keto-like’ diet in this study were different than those who were on the standard diet,” he said. “Those on the ‘keto-like’ diet were on it for a reason – they were more overweight, they had a higher incidence of diabetes, so their risk profile was completely different. Even though the researchers tried to adjust for other cardiovascular risk factors, there will be unmeasured confounding in a study like this.”
He said he doesn’t think this study “answers any significant questions in a way that we want to have them answered. I’m not a big fan of this type of diet, but I don’t think it doubles the risk of adverse cardiovascular events, and I don’t think this study tells us one way or another.”
For the study, Dr. Iatan and colleagues defined a low-carbohydrate, high-fat diet as consisting of no more than 25% of total daily energy from carbohydrates and more than 45% of total daily calories from fat. This is somewhat higher in carbohydrates and lower in fat than a strict ketogenic diet but could be thought of as a ‘keto-like’ diet.
They analyzed data from the UK Biobank, a large-scale prospective database with health information from over half a million people living in the United Kingdom who were followed for at least 10 years.
On enrollment in the Biobank, participants completed a one-time, self-reported 24-hour diet questionnaire and, at the same time, had blood drawn to check their levels of cholesterol. The researchers identified 305 participants whose questionnaire responses indicated that they followed a low-carbohydrate, high-fat diet. These participants were matched by age and sex with 1,220 individuals who reported being on a standard diet.
Of the study population, 73% were women and the average age was 54 years. Those on a low carbohydrate/high fat diet had a higher average body mass index (27.7 vs. 26.7) and a higher incidence of diabetes (4.9% vs. 1.7%).
Results showed that compared with participants on a standard diet, those on the “keto-like” diet had significantly higher levels of both LDL cholesterol and apolipoprotein B (ApoB).
Levels of LDL were 3.80 mmol/L (147 mg/dL) in the keto-like group vs. 3.64 mmol/L (141 mg/dL) in the standard group (P = .004). Levels of ApoB were 1.09 g/L (109 mg/dL) in the keto-like group and 1.04 g/L (104 mg/dL) in the standard group (P < .001).
After an average of 11.8 years of follow-up, 9.8% of participants on the low-carbohydrate/high-fat diet vs. 4.3% in the standard diet group experienced one of the events included in the composite event endpoint: Angina, myocardial infarction, coronary artery disease, ischemic stroke, peripheral arterial disease, or coronary/carotid revascularization.
After adjustment for other risk factors for heart disease – diabetes, hypertension, obesity, and smoking – individuals on a low-carbohydrate, high-fat diet were found to have a twofold risk of having a cardiovascular event (HR, 2.18; P < .001).
‘Closer monitoring needed’
“Our results have shown, I think for the first time, that there is an association between this increasingly popular dietary pattern and high LDL cholesterol and an increased future risk of cardiovascular events,” senior author Liam Brunham, MD, of the University of British Columbia, said in an interview. “This is concerning as there are many people out there following this type of diet, and I think it suggests there is a need for closer monitoring of these people.”
He explained that while it would be expected for cholesterol levels to rise on a high-fat diet, “there has been a perception by some that this is not worrisome as it is reflecting certain metabolic changes. What we’ve shown in this study is that if your cholesterol does increase significantly on this diet then you should not assume that this is not a problem.
“For some people with diabetes this diet can help lower blood sugar and some people can lose weight on it,” he noted, “but what our data show is that there is a subgroup of people who experience high levels of LDL and ApoB and that seems to be driving the risk.”
He pointed out that overall the mean level of LDL was only slightly increased in the individuals on the low-carb/high-fat diet but severe high cholesterol (more than 5 mmol/L or 190 mg/dL) was about doubled in that group (10% vs. 5%). And these patients had a sixfold increase in risk of cardiovascular disease (P < .001).
“This suggests that there is a subgroup of people who are susceptible to this exacerbation of hypercholesterolemia in response to a low-carb/high-fat diet.”
Dr. Brunham said his advice would be that if people choose to follow this diet, they should have their cholesterol monitored, and manage their cardiovascular risk factors.
“I wouldn’t say it is not appropriate to follow this diet based on this study,” he added. “This is just an observational study. It is not definitive. But if people do want to follow this dietary pattern because they feel there would be some benefits, then they should be aware of the potential risks and take steps to mitigate those risks.”
Jury still out
Dr. Nissen said in his view “the jury was still out” on this type of diet. “I’m open to the possibility that, particularly in the short run, a ‘keto-like’ diet may help some people lose weight and that’s a good thing. But I do not generally recommend this type of diet.”
Rather, he advises patients to follow a Mediterranean diet, which has been proven to reduce cardiovascular events in a randomized study, the PREDIMED trial.
“We can’t make decisions on what type of diet to recommend to patients based on observational studies like this where there is a lot of subtlety missing. But when studies like this are reported, the mass media seize on it. That’s not the way the public needs to be educated,” Dr. Nissen said.
“We refer to this type of study as hypothesis-generating. It raises a hypothesis. It doesn’t answer the question. It is worth looking at the question of whether a ketogenic-like diet is harmful. We don’t know at present, and I don’t think we know any more after this study,” he added.
The authors of the study reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Consumption of a low-carbohydrate, high-fat diet, dubbed a “keto-like” diet, was associated with an increase in LDL levels and a twofold increase in the risk for future cardiovascular events, in a new observational study.
“To our knowledge this is the first study to demonstrate an association between a carbohydrate-restricted dietary platform and greater risk of atherosclerotic cardiovascular disease,” said study investigator Iulia Iatan, MD, PhD, University of British Columbia, Vancouver.
“Hypercholesterolemia occurring during a low-carb, high-fat diet should not be assumed to be benign,” she concluded.
Dr. Iatan presented the study March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The presentation received much media attention, with headlines implying a causal relationship with cardiac events based on these observational results. But lipid expert Steven Nissen, MD, of the Cleveland Clinic, warned against paying much attention to the headlines or to the study’s conclusions.
In an interview, Dr. Nissen pointed out that the LDL increase in the “keto-like” diet group was relatively small and “certainly not enough to produce a doubling in cardiovascular risk.
“The people who were on the ‘keto-like’ diet in this study were different than those who were on the standard diet,” he said. “Those on the ‘keto-like’ diet were on it for a reason – they were more overweight, they had a higher incidence of diabetes, so their risk profile was completely different. Even though the researchers tried to adjust for other cardiovascular risk factors, there will be unmeasured confounding in a study like this.”
He said he doesn’t think this study “answers any significant questions in a way that we want to have them answered. I’m not a big fan of this type of diet, but I don’t think it doubles the risk of adverse cardiovascular events, and I don’t think this study tells us one way or another.”
For the study, Dr. Iatan and colleagues defined a low-carbohydrate, high-fat diet as consisting of no more than 25% of total daily energy from carbohydrates and more than 45% of total daily calories from fat. This is somewhat higher in carbohydrates and lower in fat than a strict ketogenic diet but could be thought of as a ‘keto-like’ diet.
They analyzed data from the UK Biobank, a large-scale prospective database with health information from over half a million people living in the United Kingdom who were followed for at least 10 years.
On enrollment in the Biobank, participants completed a one-time, self-reported 24-hour diet questionnaire and, at the same time, had blood drawn to check their levels of cholesterol. The researchers identified 305 participants whose questionnaire responses indicated that they followed a low-carbohydrate, high-fat diet. These participants were matched by age and sex with 1,220 individuals who reported being on a standard diet.
Of the study population, 73% were women and the average age was 54 years. Those on a low carbohydrate/high fat diet had a higher average body mass index (27.7 vs. 26.7) and a higher incidence of diabetes (4.9% vs. 1.7%).
Results showed that compared with participants on a standard diet, those on the “keto-like” diet had significantly higher levels of both LDL cholesterol and apolipoprotein B (ApoB).
Levels of LDL were 3.80 mmol/L (147 mg/dL) in the keto-like group vs. 3.64 mmol/L (141 mg/dL) in the standard group (P = .004). Levels of ApoB were 1.09 g/L (109 mg/dL) in the keto-like group and 1.04 g/L (104 mg/dL) in the standard group (P < .001).
After an average of 11.8 years of follow-up, 9.8% of participants on the low-carbohydrate/high-fat diet vs. 4.3% in the standard diet group experienced one of the events included in the composite event endpoint: Angina, myocardial infarction, coronary artery disease, ischemic stroke, peripheral arterial disease, or coronary/carotid revascularization.
After adjustment for other risk factors for heart disease – diabetes, hypertension, obesity, and smoking – individuals on a low-carbohydrate, high-fat diet were found to have a twofold risk of having a cardiovascular event (HR, 2.18; P < .001).
‘Closer monitoring needed’
“Our results have shown, I think for the first time, that there is an association between this increasingly popular dietary pattern and high LDL cholesterol and an increased future risk of cardiovascular events,” senior author Liam Brunham, MD, of the University of British Columbia, said in an interview. “This is concerning as there are many people out there following this type of diet, and I think it suggests there is a need for closer monitoring of these people.”
He explained that while it would be expected for cholesterol levels to rise on a high-fat diet, “there has been a perception by some that this is not worrisome as it is reflecting certain metabolic changes. What we’ve shown in this study is that if your cholesterol does increase significantly on this diet then you should not assume that this is not a problem.
“For some people with diabetes this diet can help lower blood sugar and some people can lose weight on it,” he noted, “but what our data show is that there is a subgroup of people who experience high levels of LDL and ApoB and that seems to be driving the risk.”
He pointed out that overall the mean level of LDL was only slightly increased in the individuals on the low-carb/high-fat diet but severe high cholesterol (more than 5 mmol/L or 190 mg/dL) was about doubled in that group (10% vs. 5%). And these patients had a sixfold increase in risk of cardiovascular disease (P < .001).
“This suggests that there is a subgroup of people who are susceptible to this exacerbation of hypercholesterolemia in response to a low-carb/high-fat diet.”
Dr. Brunham said his advice would be that if people choose to follow this diet, they should have their cholesterol monitored, and manage their cardiovascular risk factors.
“I wouldn’t say it is not appropriate to follow this diet based on this study,” he added. “This is just an observational study. It is not definitive. But if people do want to follow this dietary pattern because they feel there would be some benefits, then they should be aware of the potential risks and take steps to mitigate those risks.”
Jury still out
Dr. Nissen said in his view “the jury was still out” on this type of diet. “I’m open to the possibility that, particularly in the short run, a ‘keto-like’ diet may help some people lose weight and that’s a good thing. But I do not generally recommend this type of diet.”
Rather, he advises patients to follow a Mediterranean diet, which has been proven to reduce cardiovascular events in a randomized study, the PREDIMED trial.
“We can’t make decisions on what type of diet to recommend to patients based on observational studies like this where there is a lot of subtlety missing. But when studies like this are reported, the mass media seize on it. That’s not the way the public needs to be educated,” Dr. Nissen said.
“We refer to this type of study as hypothesis-generating. It raises a hypothesis. It doesn’t answer the question. It is worth looking at the question of whether a ketogenic-like diet is harmful. We don’t know at present, and I don’t think we know any more after this study,” he added.
The authors of the study reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Consumption of a low-carbohydrate, high-fat diet, dubbed a “keto-like” diet, was associated with an increase in LDL levels and a twofold increase in the risk for future cardiovascular events, in a new observational study.
“To our knowledge this is the first study to demonstrate an association between a carbohydrate-restricted dietary platform and greater risk of atherosclerotic cardiovascular disease,” said study investigator Iulia Iatan, MD, PhD, University of British Columbia, Vancouver.
“Hypercholesterolemia occurring during a low-carb, high-fat diet should not be assumed to be benign,” she concluded.
Dr. Iatan presented the study March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The presentation received much media attention, with headlines implying a causal relationship with cardiac events based on these observational results. But lipid expert Steven Nissen, MD, of the Cleveland Clinic, warned against paying much attention to the headlines or to the study’s conclusions.
In an interview, Dr. Nissen pointed out that the LDL increase in the “keto-like” diet group was relatively small and “certainly not enough to produce a doubling in cardiovascular risk.
“The people who were on the ‘keto-like’ diet in this study were different than those who were on the standard diet,” he said. “Those on the ‘keto-like’ diet were on it for a reason – they were more overweight, they had a higher incidence of diabetes, so their risk profile was completely different. Even though the researchers tried to adjust for other cardiovascular risk factors, there will be unmeasured confounding in a study like this.”
He said he doesn’t think this study “answers any significant questions in a way that we want to have them answered. I’m not a big fan of this type of diet, but I don’t think it doubles the risk of adverse cardiovascular events, and I don’t think this study tells us one way or another.”
For the study, Dr. Iatan and colleagues defined a low-carbohydrate, high-fat diet as consisting of no more than 25% of total daily energy from carbohydrates and more than 45% of total daily calories from fat. This is somewhat higher in carbohydrates and lower in fat than a strict ketogenic diet but could be thought of as a ‘keto-like’ diet.
They analyzed data from the UK Biobank, a large-scale prospective database with health information from over half a million people living in the United Kingdom who were followed for at least 10 years.
On enrollment in the Biobank, participants completed a one-time, self-reported 24-hour diet questionnaire and, at the same time, had blood drawn to check their levels of cholesterol. The researchers identified 305 participants whose questionnaire responses indicated that they followed a low-carbohydrate, high-fat diet. These participants were matched by age and sex with 1,220 individuals who reported being on a standard diet.
Of the study population, 73% were women and the average age was 54 years. Those on a low carbohydrate/high fat diet had a higher average body mass index (27.7 vs. 26.7) and a higher incidence of diabetes (4.9% vs. 1.7%).
Results showed that compared with participants on a standard diet, those on the “keto-like” diet had significantly higher levels of both LDL cholesterol and apolipoprotein B (ApoB).
Levels of LDL were 3.80 mmol/L (147 mg/dL) in the keto-like group vs. 3.64 mmol/L (141 mg/dL) in the standard group (P = .004). Levels of ApoB were 1.09 g/L (109 mg/dL) in the keto-like group and 1.04 g/L (104 mg/dL) in the standard group (P < .001).
After an average of 11.8 years of follow-up, 9.8% of participants on the low-carbohydrate/high-fat diet vs. 4.3% in the standard diet group experienced one of the events included in the composite event endpoint: Angina, myocardial infarction, coronary artery disease, ischemic stroke, peripheral arterial disease, or coronary/carotid revascularization.
After adjustment for other risk factors for heart disease – diabetes, hypertension, obesity, and smoking – individuals on a low-carbohydrate, high-fat diet were found to have a twofold risk of having a cardiovascular event (HR, 2.18; P < .001).
‘Closer monitoring needed’
“Our results have shown, I think for the first time, that there is an association between this increasingly popular dietary pattern and high LDL cholesterol and an increased future risk of cardiovascular events,” senior author Liam Brunham, MD, of the University of British Columbia, said in an interview. “This is concerning as there are many people out there following this type of diet, and I think it suggests there is a need for closer monitoring of these people.”
He explained that while it would be expected for cholesterol levels to rise on a high-fat diet, “there has been a perception by some that this is not worrisome as it is reflecting certain metabolic changes. What we’ve shown in this study is that if your cholesterol does increase significantly on this diet then you should not assume that this is not a problem.
“For some people with diabetes this diet can help lower blood sugar and some people can lose weight on it,” he noted, “but what our data show is that there is a subgroup of people who experience high levels of LDL and ApoB and that seems to be driving the risk.”
He pointed out that overall the mean level of LDL was only slightly increased in the individuals on the low-carb/high-fat diet but severe high cholesterol (more than 5 mmol/L or 190 mg/dL) was about doubled in that group (10% vs. 5%). And these patients had a sixfold increase in risk of cardiovascular disease (P < .001).
“This suggests that there is a subgroup of people who are susceptible to this exacerbation of hypercholesterolemia in response to a low-carb/high-fat diet.”
Dr. Brunham said his advice would be that if people choose to follow this diet, they should have their cholesterol monitored, and manage their cardiovascular risk factors.
“I wouldn’t say it is not appropriate to follow this diet based on this study,” he added. “This is just an observational study. It is not definitive. But if people do want to follow this dietary pattern because they feel there would be some benefits, then they should be aware of the potential risks and take steps to mitigate those risks.”
Jury still out
Dr. Nissen said in his view “the jury was still out” on this type of diet. “I’m open to the possibility that, particularly in the short run, a ‘keto-like’ diet may help some people lose weight and that’s a good thing. But I do not generally recommend this type of diet.”
Rather, he advises patients to follow a Mediterranean diet, which has been proven to reduce cardiovascular events in a randomized study, the PREDIMED trial.
“We can’t make decisions on what type of diet to recommend to patients based on observational studies like this where there is a lot of subtlety missing. But when studies like this are reported, the mass media seize on it. That’s not the way the public needs to be educated,” Dr. Nissen said.
“We refer to this type of study as hypothesis-generating. It raises a hypothesis. It doesn’t answer the question. It is worth looking at the question of whether a ketogenic-like diet is harmful. We don’t know at present, and I don’t think we know any more after this study,” he added.
The authors of the study reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ACC 2023
FDA to review dupilumab for treating chronic spontaneous urticaria
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.
Heart-healthy actions promote longer, disease-free life
Adults who follow a heart-healthy lifestyle are more likely to live longer and to be free of chronic health conditions, based on data from a pair of related studies from the United States and United Kingdom involving nearly 200,000 individuals.
The studies, presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting in Boston, assessed the impact of cardiovascular health on life expectancy and freedom from chronic diseases. Cardiovascular health (CVH) was based on the Life’s Essential 8 (LE8) score, a composite of health metrics released by the American Heart Association in 2022. The LE8 was developed to guide research and assessment of cardiovascular health, and includes diet, physical activity, tobacco/nicotine exposure, sleep, body mass index, non-HDL cholesterol, blood glucose, and blood pressure.
In one study, Xuan Wang, MD, a postdoctoral fellow and biostatistician in the department of epidemiology at Tulane University, New Orleans, and colleagues reviewed data from 136,599 adults in the United Kingdom Biobank who were free of cardiovascular disease, diabetes, cancer, and dementia at baseline, and for whom complete LE8 data were available.
CVH was classified as poor, intermediate, and ideal, defined as LE8 scores of less than 50, 50 to 80, and 80 or higher, respectively.
The goal of the study was to examine the role of CVH based on LE8 scores on the percentage of life expectancy free of chronic diseases.
Men and women with ideal CVH averaged 5.2 years and 6.3 years more of total life expectancy at age 50 years, compared with those with poor CVH. Out of total life expectancy, the percentage of life expectancy free of chronic diseases was 75.9% and 83.4% for men and women, respectively, compared with 64.9% and 69.4%, respectively, for men and women with poor CVH.
The researchers also found that disparities in the percentage of disease-free years for both men and women were reduced in the high CVH groups.
The findings were limited by several factors including the use of only CVD, diabetes, cancer, and dementia in the definition of “disease-free life expectancy,” the researchers noted in a press release accompanying the study. Other limitations include the lack of data on e-cigarettes, and the homogeneous White study population. More research is needed in diverse populations who experience a stronger impact from negative social determinants of health, they said.
In a second study, Hao Ma, MD, and colleagues reviewed data from 23,003 adults who participated in the National Health and Nutrition Examination Survey (NHANES) between 2005 and 2018 with mortality linked to the National Death Index through Dec. 31, 2019. The goal of the second study was to examine the association between CVH based on LE8 scores and life expectancy.
Over a median follow-up of 7.8 years, deaths occurred in 772 men and 587 women, said Dr. Ma, a postdoctoral fellow and biostatistician in epidemiology at Tulane University and coauthor on Dr. Wang’s study.
The estimated life expectancies at age 50 years for men with poor, intermediate, and ideal cardiovascular health based on the LE8 were 25.5 years, 31.2 years, and 33.1 years, respectively.
For women, the corresponding life expectancies for women at age 50 with poor, intermediate, and ideal CVH were 29.5 years, 34.2 years, and 38.4 years, respectively.
Men and women had similar gains in life expectancy from adhering to a heart-healthy lifestyle as defined by the LE8 score that reduced their risk of death from cardiovascular disease (41.8% and 44.1%, respectively).
Associations of cardiovascular health and life expectancy were similar for non-Hispanic Whites and non-Hispanic Blacks, but not among people of Mexican heritage, and more research is needed in diverse populations, the researchers wrote.
The study was limited by several factors including potential changes in cardiovascular health during the follow-up period, and by the limited analysis of racial and ethnic groups to non-Hispanic white, non-Hispanic Black, and people of Mexican heritage because of small sample sizes for other racial/ethnic groups, the researchers noted in a press release accompanying the study.
The message for clinicians and their patients is that adherence to cardiovascular health as defined by the LE8 will help not only extend life, but enhance quality of life, Dr. Xang and Dr. Ma said in an interview. “If your overall CVH score is low, we might be able to focus on one element first and improve them one by one,” they said. Sedentary lifestyle and an unhealthy diet are barriers to improving LE8 metrics that can be addressed, they added.
More research is needed to examine the effects of LE8 on high-risk patients, the researchers told this news organization. “No studies have yet focused on these patients with chronic diseases. We suspect that LE8 will play a role even in these high-risk groups,” they said. Further studies should include diverse populations and evaluations of the association between CVH change and health outcomes, they added.
“Overall, we see this 7.5-year difference [in life expectancy] going from poor to high cardiovascular health,” said Donald M. Lloyd-Jones, MD, of Northwestern University, Chicago, in a video accompanying the presentation of the study findings. The impact on life expectancy is yet another reason to motivate people to improve their cardiovascular health, said Dr. Lloyd-Jones, immediate past president of the American Heart Association and lead author on the writing group for Life’s Essential 8. “The earlier we do this, the better, and the greater the gains in life expectancy we’re likely to see in the U.S. population,” he said.
People maintaining high cardiovascular health into midlife are avoiding not only cardiovascular disease, but other chronic diseases of aging, Dr. Lloyd-Jones added. These conditions are delayed until much later in the lifespan, which allows people to enjoy better quality of life for more of their remaining years, he said.
The meeting was sponsored by the American Heart Association.
Both studies were supported by the National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, part of the National Institutes of Health; the Fogarty International Center; and the Tulane Research Centers of Excellence Awards. The researchers had no financial conflicts to disclose.
Adults who follow a heart-healthy lifestyle are more likely to live longer and to be free of chronic health conditions, based on data from a pair of related studies from the United States and United Kingdom involving nearly 200,000 individuals.
The studies, presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting in Boston, assessed the impact of cardiovascular health on life expectancy and freedom from chronic diseases. Cardiovascular health (CVH) was based on the Life’s Essential 8 (LE8) score, a composite of health metrics released by the American Heart Association in 2022. The LE8 was developed to guide research and assessment of cardiovascular health, and includes diet, physical activity, tobacco/nicotine exposure, sleep, body mass index, non-HDL cholesterol, blood glucose, and blood pressure.
In one study, Xuan Wang, MD, a postdoctoral fellow and biostatistician in the department of epidemiology at Tulane University, New Orleans, and colleagues reviewed data from 136,599 adults in the United Kingdom Biobank who were free of cardiovascular disease, diabetes, cancer, and dementia at baseline, and for whom complete LE8 data were available.
CVH was classified as poor, intermediate, and ideal, defined as LE8 scores of less than 50, 50 to 80, and 80 or higher, respectively.
The goal of the study was to examine the role of CVH based on LE8 scores on the percentage of life expectancy free of chronic diseases.
Men and women with ideal CVH averaged 5.2 years and 6.3 years more of total life expectancy at age 50 years, compared with those with poor CVH. Out of total life expectancy, the percentage of life expectancy free of chronic diseases was 75.9% and 83.4% for men and women, respectively, compared with 64.9% and 69.4%, respectively, for men and women with poor CVH.
The researchers also found that disparities in the percentage of disease-free years for both men and women were reduced in the high CVH groups.
The findings were limited by several factors including the use of only CVD, diabetes, cancer, and dementia in the definition of “disease-free life expectancy,” the researchers noted in a press release accompanying the study. Other limitations include the lack of data on e-cigarettes, and the homogeneous White study population. More research is needed in diverse populations who experience a stronger impact from negative social determinants of health, they said.
In a second study, Hao Ma, MD, and colleagues reviewed data from 23,003 adults who participated in the National Health and Nutrition Examination Survey (NHANES) between 2005 and 2018 with mortality linked to the National Death Index through Dec. 31, 2019. The goal of the second study was to examine the association between CVH based on LE8 scores and life expectancy.
Over a median follow-up of 7.8 years, deaths occurred in 772 men and 587 women, said Dr. Ma, a postdoctoral fellow and biostatistician in epidemiology at Tulane University and coauthor on Dr. Wang’s study.
The estimated life expectancies at age 50 years for men with poor, intermediate, and ideal cardiovascular health based on the LE8 were 25.5 years, 31.2 years, and 33.1 years, respectively.
For women, the corresponding life expectancies for women at age 50 with poor, intermediate, and ideal CVH were 29.5 years, 34.2 years, and 38.4 years, respectively.
Men and women had similar gains in life expectancy from adhering to a heart-healthy lifestyle as defined by the LE8 score that reduced their risk of death from cardiovascular disease (41.8% and 44.1%, respectively).
Associations of cardiovascular health and life expectancy were similar for non-Hispanic Whites and non-Hispanic Blacks, but not among people of Mexican heritage, and more research is needed in diverse populations, the researchers wrote.
The study was limited by several factors including potential changes in cardiovascular health during the follow-up period, and by the limited analysis of racial and ethnic groups to non-Hispanic white, non-Hispanic Black, and people of Mexican heritage because of small sample sizes for other racial/ethnic groups, the researchers noted in a press release accompanying the study.
The message for clinicians and their patients is that adherence to cardiovascular health as defined by the LE8 will help not only extend life, but enhance quality of life, Dr. Xang and Dr. Ma said in an interview. “If your overall CVH score is low, we might be able to focus on one element first and improve them one by one,” they said. Sedentary lifestyle and an unhealthy diet are barriers to improving LE8 metrics that can be addressed, they added.
More research is needed to examine the effects of LE8 on high-risk patients, the researchers told this news organization. “No studies have yet focused on these patients with chronic diseases. We suspect that LE8 will play a role even in these high-risk groups,” they said. Further studies should include diverse populations and evaluations of the association between CVH change and health outcomes, they added.
“Overall, we see this 7.5-year difference [in life expectancy] going from poor to high cardiovascular health,” said Donald M. Lloyd-Jones, MD, of Northwestern University, Chicago, in a video accompanying the presentation of the study findings. The impact on life expectancy is yet another reason to motivate people to improve their cardiovascular health, said Dr. Lloyd-Jones, immediate past president of the American Heart Association and lead author on the writing group for Life’s Essential 8. “The earlier we do this, the better, and the greater the gains in life expectancy we’re likely to see in the U.S. population,” he said.
People maintaining high cardiovascular health into midlife are avoiding not only cardiovascular disease, but other chronic diseases of aging, Dr. Lloyd-Jones added. These conditions are delayed until much later in the lifespan, which allows people to enjoy better quality of life for more of their remaining years, he said.
The meeting was sponsored by the American Heart Association.
Both studies were supported by the National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, part of the National Institutes of Health; the Fogarty International Center; and the Tulane Research Centers of Excellence Awards. The researchers had no financial conflicts to disclose.
Adults who follow a heart-healthy lifestyle are more likely to live longer and to be free of chronic health conditions, based on data from a pair of related studies from the United States and United Kingdom involving nearly 200,000 individuals.
The studies, presented at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting in Boston, assessed the impact of cardiovascular health on life expectancy and freedom from chronic diseases. Cardiovascular health (CVH) was based on the Life’s Essential 8 (LE8) score, a composite of health metrics released by the American Heart Association in 2022. The LE8 was developed to guide research and assessment of cardiovascular health, and includes diet, physical activity, tobacco/nicotine exposure, sleep, body mass index, non-HDL cholesterol, blood glucose, and blood pressure.
In one study, Xuan Wang, MD, a postdoctoral fellow and biostatistician in the department of epidemiology at Tulane University, New Orleans, and colleagues reviewed data from 136,599 adults in the United Kingdom Biobank who were free of cardiovascular disease, diabetes, cancer, and dementia at baseline, and for whom complete LE8 data were available.
CVH was classified as poor, intermediate, and ideal, defined as LE8 scores of less than 50, 50 to 80, and 80 or higher, respectively.
The goal of the study was to examine the role of CVH based on LE8 scores on the percentage of life expectancy free of chronic diseases.
Men and women with ideal CVH averaged 5.2 years and 6.3 years more of total life expectancy at age 50 years, compared with those with poor CVH. Out of total life expectancy, the percentage of life expectancy free of chronic diseases was 75.9% and 83.4% for men and women, respectively, compared with 64.9% and 69.4%, respectively, for men and women with poor CVH.
The researchers also found that disparities in the percentage of disease-free years for both men and women were reduced in the high CVH groups.
The findings were limited by several factors including the use of only CVD, diabetes, cancer, and dementia in the definition of “disease-free life expectancy,” the researchers noted in a press release accompanying the study. Other limitations include the lack of data on e-cigarettes, and the homogeneous White study population. More research is needed in diverse populations who experience a stronger impact from negative social determinants of health, they said.
In a second study, Hao Ma, MD, and colleagues reviewed data from 23,003 adults who participated in the National Health and Nutrition Examination Survey (NHANES) between 2005 and 2018 with mortality linked to the National Death Index through Dec. 31, 2019. The goal of the second study was to examine the association between CVH based on LE8 scores and life expectancy.
Over a median follow-up of 7.8 years, deaths occurred in 772 men and 587 women, said Dr. Ma, a postdoctoral fellow and biostatistician in epidemiology at Tulane University and coauthor on Dr. Wang’s study.
The estimated life expectancies at age 50 years for men with poor, intermediate, and ideal cardiovascular health based on the LE8 were 25.5 years, 31.2 years, and 33.1 years, respectively.
For women, the corresponding life expectancies for women at age 50 with poor, intermediate, and ideal CVH were 29.5 years, 34.2 years, and 38.4 years, respectively.
Men and women had similar gains in life expectancy from adhering to a heart-healthy lifestyle as defined by the LE8 score that reduced their risk of death from cardiovascular disease (41.8% and 44.1%, respectively).
Associations of cardiovascular health and life expectancy were similar for non-Hispanic Whites and non-Hispanic Blacks, but not among people of Mexican heritage, and more research is needed in diverse populations, the researchers wrote.
The study was limited by several factors including potential changes in cardiovascular health during the follow-up period, and by the limited analysis of racial and ethnic groups to non-Hispanic white, non-Hispanic Black, and people of Mexican heritage because of small sample sizes for other racial/ethnic groups, the researchers noted in a press release accompanying the study.
The message for clinicians and their patients is that adherence to cardiovascular health as defined by the LE8 will help not only extend life, but enhance quality of life, Dr. Xang and Dr. Ma said in an interview. “If your overall CVH score is low, we might be able to focus on one element first and improve them one by one,” they said. Sedentary lifestyle and an unhealthy diet are barriers to improving LE8 metrics that can be addressed, they added.
More research is needed to examine the effects of LE8 on high-risk patients, the researchers told this news organization. “No studies have yet focused on these patients with chronic diseases. We suspect that LE8 will play a role even in these high-risk groups,” they said. Further studies should include diverse populations and evaluations of the association between CVH change and health outcomes, they added.
“Overall, we see this 7.5-year difference [in life expectancy] going from poor to high cardiovascular health,” said Donald M. Lloyd-Jones, MD, of Northwestern University, Chicago, in a video accompanying the presentation of the study findings. The impact on life expectancy is yet another reason to motivate people to improve their cardiovascular health, said Dr. Lloyd-Jones, immediate past president of the American Heart Association and lead author on the writing group for Life’s Essential 8. “The earlier we do this, the better, and the greater the gains in life expectancy we’re likely to see in the U.S. population,” he said.
People maintaining high cardiovascular health into midlife are avoiding not only cardiovascular disease, but other chronic diseases of aging, Dr. Lloyd-Jones added. These conditions are delayed until much later in the lifespan, which allows people to enjoy better quality of life for more of their remaining years, he said.
The meeting was sponsored by the American Heart Association.
Both studies were supported by the National Heart, Lung, and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, part of the National Institutes of Health; the Fogarty International Center; and the Tulane Research Centers of Excellence Awards. The researchers had no financial conflicts to disclose.
FROM EPI/LIFESTYLE 2023
Even mild COVID is hard on the brain
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
Do artificial sweeteners alter postmeal glucose, hunger hormones?
Drinking a sugar-sweetened beverage (SSB), however, had a different effect on postprandial levels of glucose and the hormones insulin, glucagonlike peptide–1 (GLP-1), gastric inhibitory polypeptide (GIP), peptide YY (PYY), ghrelin, leptin, and glucagon.
These findings are from a new meta-analysis by Roselyn Zhang and colleagues, supported by the nonprofit organization Institute for the Advancement of Food and Nutrition Sciences. The study was published recently in Nutrients.
“Nonnutritive sweeteners have no acute metabolic or endocrine effects and they are similar to water in that respect, and they show a different response from caloric sweeteners,” study author Tauseef Khan, MBBS, PhD, summarized in an interview following a press briefing from the IAFNS.
“Our study supports that nonnutritive sweeteners are a healthier alternative to sugar-sweetened beverages or caloric beverages,” said Dr. Khan, an epidemiologist in the department of nutritional sciences, University of Toronto.
Most participants in the 36 trials included in the meta-analysis were healthy, he noted. However, for certain types of NNS beverages, “we had enough studies for type 2 diabetes to also assess that separately, and the results were the same: Nonnutritive sweeteners were no different from water; however, they were different from caloric sweeteners.”
Of note, none of the studies included erythritol – a sugar alcohol (polyol) increasingly used as an artificial sweetener in keto and other types of foods – which was associated with a risk for adverse cardiac events in a paper in Nature Medicine.
Are these NNS drinks largely inert?
“This [meta-analysis] implies that sweeteners are largely inert,” in terms of acute postprandial glucose and hormone response, but the review did not include newer reports that differ, Duane Mellor, PhD, RD, RNutr, who was not involved with the research, noted in an email.
“This is possibly,” he said, because the study “only reviewed the literature up until January 2022 and therefore it missed the World Health Organization review ‘Health Effects of the Use of Non-Sugar Sweeteners’ published in April [2022], and a study published in August 2022 in the journal Cell suggesting that some nonnutritive sweeteners may have a minor effect on gut microbiome and glucose response.
“Although there is a place of nonnutritive sweeteners as a way to reduce sugar intake, they are a small part of dietary pattern and lifestyle which can help reduce risk of disease,” said Dr. Mellor, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England.
“So, although we are clear we need to reduce our intake of sugar-sweetened beverages, switching to non-nutritive sweetened beverages (such as diet sodas) is not necessarily the healthiest option, as unlike water, it seems that some nonnutritive sweeteners may influence glucose responses and levels of related hormones in more recent studies.”
NNS beverages ‘are similar to water’
Dr. Khan pointed out that the meta-analysis addressed two major concerns about NNS beverages.
First, the “sweet uncoupling hypothesis” proposes that low-calorie sweeteners affect sweet taste by separating sweet taste from calories. “The body is confused, and then there is hormonal change. Our study shows that actually that’s not true, and [NNS beverages] are similar to water.”
Second, when no-calorie or low-calorie sweeteners are taken with calories (coupling), a concern is that “then you eat more somehow, or your response is different. However, the results [in this meta-analysis] also show that that is not the case for glucose response, insulin response, and other hormonal markers.”
“The strength is not that low-calorie sweeteners have some benefit per se,” he elaborated. “The advantage is that they replace caloric beverages.
“We are not saying that anybody who is not taking low-calorie sweeteners should start taking [them],” he continued. “What we are saying is somebody who is taking sugar-sweetened beverages and has a problem of taking excess calories, if you replace those calories with low-calorie sweetener, replacement of calories itself may be beneficial, and also they should not be concerned of any [acute] issues with a moderate amount of low-calorie sweeteners.”
Postprandial effect of NNS beverages, SSBs, water
Eight NNS are currently approved by the Food and Drug Administration: aspartame, acesulfame potassium (ace-K), luo han guo (monkfruit) extract, neotame, saccharin, stevia, sucralose, and advantame, the researchers noted.
Ms. Zhang and colleagues searched the literature up until Jan. 15, 2022, for studies of NNS beverages and acute postprandial glycemic and endocrine responses.
Trials were excluded if they involved sugar alcohols (eg, erythritol) or rare sugars (eg, allulose), or if they were shorter than 2 hours, lacked a comparator arm, or did not provide suitable endpoint data.
They identified 36 randomized and nonrandomized clinical trials of 472 predominantly healthy participants: 21 trials (15 reports, n = 266) with NNS consumed alone (uncoupled), 3 trials (3 reports, n = 27) with NNS consumed in a solution containing a carbohydrate (coupled), and 12 trials (7 reports, n = 179) with NNS consumed up to 15 minutes before oral glucose carbohydrate load (delayed coupling).
The four types of beverages were single NNS (ace-K, aspartame, cyclamate, saccharin, stevia, and sucralose), NNS blends (ace-K + aspartame; ace-K + sucralose; ace-K + aspartame + cyclamate; and ace-K + aspartame + sucralose), SSBs (glucose, sucrose, and fructose), and water (control).
In the uncoupled interventions, NNS beverages (single or blends) had no effect on postprandial glucose, insulin, GLP-1, GIP, PYY, ghrelin, and glucagon, with responses similar to water.
In the uncoupled interventions, SSBs sweetened with caloric sugars (glucose and sucrose) increased postprandial glucose, insulin, GLP-1, and GIP responses, with no differences in postprandial ghrelin and glucagon responses.
In the coupled and delayed coupling interventions, NNS beverages had no postprandial glucose and endocrine effects, with responses similar to water.
The studies generally had low to moderate confidence.
The study was supported by an unrestricted grant from IAFNS. Dr. Khan has received research support from the Canadian Institutes of Health Research, the International Life Sciences Institute, and the National Honey Board. He has received honorariums for lectures from the International Food Information Council and the IAFNS. Dr. Mellor has no disclosures.
A version of this article first appeared on Medscape.com.
Drinking a sugar-sweetened beverage (SSB), however, had a different effect on postprandial levels of glucose and the hormones insulin, glucagonlike peptide–1 (GLP-1), gastric inhibitory polypeptide (GIP), peptide YY (PYY), ghrelin, leptin, and glucagon.
These findings are from a new meta-analysis by Roselyn Zhang and colleagues, supported by the nonprofit organization Institute for the Advancement of Food and Nutrition Sciences. The study was published recently in Nutrients.
“Nonnutritive sweeteners have no acute metabolic or endocrine effects and they are similar to water in that respect, and they show a different response from caloric sweeteners,” study author Tauseef Khan, MBBS, PhD, summarized in an interview following a press briefing from the IAFNS.
“Our study supports that nonnutritive sweeteners are a healthier alternative to sugar-sweetened beverages or caloric beverages,” said Dr. Khan, an epidemiologist in the department of nutritional sciences, University of Toronto.
Most participants in the 36 trials included in the meta-analysis were healthy, he noted. However, for certain types of NNS beverages, “we had enough studies for type 2 diabetes to also assess that separately, and the results were the same: Nonnutritive sweeteners were no different from water; however, they were different from caloric sweeteners.”
Of note, none of the studies included erythritol – a sugar alcohol (polyol) increasingly used as an artificial sweetener in keto and other types of foods – which was associated with a risk for adverse cardiac events in a paper in Nature Medicine.
Are these NNS drinks largely inert?
“This [meta-analysis] implies that sweeteners are largely inert,” in terms of acute postprandial glucose and hormone response, but the review did not include newer reports that differ, Duane Mellor, PhD, RD, RNutr, who was not involved with the research, noted in an email.
“This is possibly,” he said, because the study “only reviewed the literature up until January 2022 and therefore it missed the World Health Organization review ‘Health Effects of the Use of Non-Sugar Sweeteners’ published in April [2022], and a study published in August 2022 in the journal Cell suggesting that some nonnutritive sweeteners may have a minor effect on gut microbiome and glucose response.
“Although there is a place of nonnutritive sweeteners as a way to reduce sugar intake, they are a small part of dietary pattern and lifestyle which can help reduce risk of disease,” said Dr. Mellor, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England.
“So, although we are clear we need to reduce our intake of sugar-sweetened beverages, switching to non-nutritive sweetened beverages (such as diet sodas) is not necessarily the healthiest option, as unlike water, it seems that some nonnutritive sweeteners may influence glucose responses and levels of related hormones in more recent studies.”
NNS beverages ‘are similar to water’
Dr. Khan pointed out that the meta-analysis addressed two major concerns about NNS beverages.
First, the “sweet uncoupling hypothesis” proposes that low-calorie sweeteners affect sweet taste by separating sweet taste from calories. “The body is confused, and then there is hormonal change. Our study shows that actually that’s not true, and [NNS beverages] are similar to water.”
Second, when no-calorie or low-calorie sweeteners are taken with calories (coupling), a concern is that “then you eat more somehow, or your response is different. However, the results [in this meta-analysis] also show that that is not the case for glucose response, insulin response, and other hormonal markers.”
“The strength is not that low-calorie sweeteners have some benefit per se,” he elaborated. “The advantage is that they replace caloric beverages.
“We are not saying that anybody who is not taking low-calorie sweeteners should start taking [them],” he continued. “What we are saying is somebody who is taking sugar-sweetened beverages and has a problem of taking excess calories, if you replace those calories with low-calorie sweetener, replacement of calories itself may be beneficial, and also they should not be concerned of any [acute] issues with a moderate amount of low-calorie sweeteners.”
Postprandial effect of NNS beverages, SSBs, water
Eight NNS are currently approved by the Food and Drug Administration: aspartame, acesulfame potassium (ace-K), luo han guo (monkfruit) extract, neotame, saccharin, stevia, sucralose, and advantame, the researchers noted.
Ms. Zhang and colleagues searched the literature up until Jan. 15, 2022, for studies of NNS beverages and acute postprandial glycemic and endocrine responses.
Trials were excluded if they involved sugar alcohols (eg, erythritol) or rare sugars (eg, allulose), or if they were shorter than 2 hours, lacked a comparator arm, or did not provide suitable endpoint data.
They identified 36 randomized and nonrandomized clinical trials of 472 predominantly healthy participants: 21 trials (15 reports, n = 266) with NNS consumed alone (uncoupled), 3 trials (3 reports, n = 27) with NNS consumed in a solution containing a carbohydrate (coupled), and 12 trials (7 reports, n = 179) with NNS consumed up to 15 minutes before oral glucose carbohydrate load (delayed coupling).
The four types of beverages were single NNS (ace-K, aspartame, cyclamate, saccharin, stevia, and sucralose), NNS blends (ace-K + aspartame; ace-K + sucralose; ace-K + aspartame + cyclamate; and ace-K + aspartame + sucralose), SSBs (glucose, sucrose, and fructose), and water (control).
In the uncoupled interventions, NNS beverages (single or blends) had no effect on postprandial glucose, insulin, GLP-1, GIP, PYY, ghrelin, and glucagon, with responses similar to water.
In the uncoupled interventions, SSBs sweetened with caloric sugars (glucose and sucrose) increased postprandial glucose, insulin, GLP-1, and GIP responses, with no differences in postprandial ghrelin and glucagon responses.
In the coupled and delayed coupling interventions, NNS beverages had no postprandial glucose and endocrine effects, with responses similar to water.
The studies generally had low to moderate confidence.
The study was supported by an unrestricted grant from IAFNS. Dr. Khan has received research support from the Canadian Institutes of Health Research, the International Life Sciences Institute, and the National Honey Board. He has received honorariums for lectures from the International Food Information Council and the IAFNS. Dr. Mellor has no disclosures.
A version of this article first appeared on Medscape.com.
Drinking a sugar-sweetened beverage (SSB), however, had a different effect on postprandial levels of glucose and the hormones insulin, glucagonlike peptide–1 (GLP-1), gastric inhibitory polypeptide (GIP), peptide YY (PYY), ghrelin, leptin, and glucagon.
These findings are from a new meta-analysis by Roselyn Zhang and colleagues, supported by the nonprofit organization Institute for the Advancement of Food and Nutrition Sciences. The study was published recently in Nutrients.
“Nonnutritive sweeteners have no acute metabolic or endocrine effects and they are similar to water in that respect, and they show a different response from caloric sweeteners,” study author Tauseef Khan, MBBS, PhD, summarized in an interview following a press briefing from the IAFNS.
“Our study supports that nonnutritive sweeteners are a healthier alternative to sugar-sweetened beverages or caloric beverages,” said Dr. Khan, an epidemiologist in the department of nutritional sciences, University of Toronto.
Most participants in the 36 trials included in the meta-analysis were healthy, he noted. However, for certain types of NNS beverages, “we had enough studies for type 2 diabetes to also assess that separately, and the results were the same: Nonnutritive sweeteners were no different from water; however, they were different from caloric sweeteners.”
Of note, none of the studies included erythritol – a sugar alcohol (polyol) increasingly used as an artificial sweetener in keto and other types of foods – which was associated with a risk for adverse cardiac events in a paper in Nature Medicine.
Are these NNS drinks largely inert?
“This [meta-analysis] implies that sweeteners are largely inert,” in terms of acute postprandial glucose and hormone response, but the review did not include newer reports that differ, Duane Mellor, PhD, RD, RNutr, who was not involved with the research, noted in an email.
“This is possibly,” he said, because the study “only reviewed the literature up until January 2022 and therefore it missed the World Health Organization review ‘Health Effects of the Use of Non-Sugar Sweeteners’ published in April [2022], and a study published in August 2022 in the journal Cell suggesting that some nonnutritive sweeteners may have a minor effect on gut microbiome and glucose response.
“Although there is a place of nonnutritive sweeteners as a way to reduce sugar intake, they are a small part of dietary pattern and lifestyle which can help reduce risk of disease,” said Dr. Mellor, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England.
“So, although we are clear we need to reduce our intake of sugar-sweetened beverages, switching to non-nutritive sweetened beverages (such as diet sodas) is not necessarily the healthiest option, as unlike water, it seems that some nonnutritive sweeteners may influence glucose responses and levels of related hormones in more recent studies.”
NNS beverages ‘are similar to water’
Dr. Khan pointed out that the meta-analysis addressed two major concerns about NNS beverages.
First, the “sweet uncoupling hypothesis” proposes that low-calorie sweeteners affect sweet taste by separating sweet taste from calories. “The body is confused, and then there is hormonal change. Our study shows that actually that’s not true, and [NNS beverages] are similar to water.”
Second, when no-calorie or low-calorie sweeteners are taken with calories (coupling), a concern is that “then you eat more somehow, or your response is different. However, the results [in this meta-analysis] also show that that is not the case for glucose response, insulin response, and other hormonal markers.”
“The strength is not that low-calorie sweeteners have some benefit per se,” he elaborated. “The advantage is that they replace caloric beverages.
“We are not saying that anybody who is not taking low-calorie sweeteners should start taking [them],” he continued. “What we are saying is somebody who is taking sugar-sweetened beverages and has a problem of taking excess calories, if you replace those calories with low-calorie sweetener, replacement of calories itself may be beneficial, and also they should not be concerned of any [acute] issues with a moderate amount of low-calorie sweeteners.”
Postprandial effect of NNS beverages, SSBs, water
Eight NNS are currently approved by the Food and Drug Administration: aspartame, acesulfame potassium (ace-K), luo han guo (monkfruit) extract, neotame, saccharin, stevia, sucralose, and advantame, the researchers noted.
Ms. Zhang and colleagues searched the literature up until Jan. 15, 2022, for studies of NNS beverages and acute postprandial glycemic and endocrine responses.
Trials were excluded if they involved sugar alcohols (eg, erythritol) or rare sugars (eg, allulose), or if they were shorter than 2 hours, lacked a comparator arm, or did not provide suitable endpoint data.
They identified 36 randomized and nonrandomized clinical trials of 472 predominantly healthy participants: 21 trials (15 reports, n = 266) with NNS consumed alone (uncoupled), 3 trials (3 reports, n = 27) with NNS consumed in a solution containing a carbohydrate (coupled), and 12 trials (7 reports, n = 179) with NNS consumed up to 15 minutes before oral glucose carbohydrate load (delayed coupling).
The four types of beverages were single NNS (ace-K, aspartame, cyclamate, saccharin, stevia, and sucralose), NNS blends (ace-K + aspartame; ace-K + sucralose; ace-K + aspartame + cyclamate; and ace-K + aspartame + sucralose), SSBs (glucose, sucrose, and fructose), and water (control).
In the uncoupled interventions, NNS beverages (single or blends) had no effect on postprandial glucose, insulin, GLP-1, GIP, PYY, ghrelin, and glucagon, with responses similar to water.
In the uncoupled interventions, SSBs sweetened with caloric sugars (glucose and sucrose) increased postprandial glucose, insulin, GLP-1, and GIP responses, with no differences in postprandial ghrelin and glucagon responses.
In the coupled and delayed coupling interventions, NNS beverages had no postprandial glucose and endocrine effects, with responses similar to water.
The studies generally had low to moderate confidence.
The study was supported by an unrestricted grant from IAFNS. Dr. Khan has received research support from the Canadian Institutes of Health Research, the International Life Sciences Institute, and the National Honey Board. He has received honorariums for lectures from the International Food Information Council and the IAFNS. Dr. Mellor has no disclosures.
A version of this article first appeared on Medscape.com.
FROM NUTRIENTS
High CV risk factor burden in young adults a ‘smoldering’ crisis
New data show a high and rising burden of most cardiovascular (CV) risk factors among young adults aged 20-44 years in the United States.
In this age group, over the past 10 years, there has been an increase in the prevalence of diabetes and obesity, no improvement in the prevalence of hypertension, and a decrease in the prevalence of hyperlipidemia.
Yet medical treatment rates for CV risk factors are “surprisingly” low among young adults, study investigator Rishi Wadhera, MD, with Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, told this news organization.
The findings are “extremely concerning. We’re witnessing a smoldering public health crisis. The onset of these risk factors earlier in life is associated with a higher lifetime risk of heart disease and potentially life-threatening,” Dr. Wadhera added.
The study was presented March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation and was simultaneously published in JAMA.
The burden of CV risk factors among young adults is “unacceptably high and increasing,” write the co-authors of a JAMA editorial.
“The time is now for aggressive preventive measures in young adults. Without immediate action there will continue to be a rise in heart disease and the burden it places on patients, families, and communities,” say Norrina Allen, PhD, and John Wilkins, MD, with Northwestern University, Chicago.
Preventing a tsunami of heart disease
The findings stem from a cross-sectional study of 12,294 U.S. adults aged 20-44 years (mean age, 32; 51% women) who participated in National Health and Nutrition Examination Survey (NHANES) cycles for 2009-2010 to 2017-2020.
Overall, the prevalence of hypertension was 9.3% in 2009-2010 and increased to 11.5% in 2017-2020. The prevalence of diabetes rose from 3.0% to 4.1%, and the prevalence of obesity rose from 32.7% to 40.9%. The prevalence of hyperlipidemia decreased from 40.5% to 36.1%.
Black adults consistently had high rates of hypertension during the study period – 16.2% in 2009-2010 and 20.1% in 2017-2020 – and significant increases in hypertension occurred among Mexican American adults (from 6.5% to 9.5%) and other Hispanic adults (from 4.4% to 10.5%), while Mexican American adults had a significant uptick in diabetes (from 4.3% to 7.5%).
Equally concerning, said Dr. Wadhera, is the fact that only about 55% of young adults with hypertension were receiving antihypertensive medication, and just 1 in 2 young adults with diabetes were receiving treatment. “These low rates were driven, in part, by many young adults not being aware of their diagnosis,” he noted.
The NHANES data also show that the percentage of young adults who were treated for hypertension and who achieved blood pressure control did not change significantly over the study period (65.0% in 2009-2010 and 74.8% in 2017-2020). Blood sugar control among young adults being treated for diabetes remained suboptimal throughout the study period (45.5% in 2009-2010 and 56.6% in 2017-2020).
“The fact that blood pressure control and glycemic control are so poor is really worrisome,” Jeffrey Berger, MD, director of the Center for the Prevention of Cardiovascular Disease at NYU Langone Heart, who wasn’t involved in the study, told this news organization.
“Even in the lipid control, while it did get a little bit better, it’s still only around 30%-40%. So, I think we have ways to go as a society,” Dr. Berger noted.
Double down on screening
Dr. Wadhera said “we need to double down on efforts to screen for and treat cardiovascular risk factors like high blood pressure and diabetes in young adults. We need to intensify clinical and public health interventions focused on primordial and primary prevention in young adults now so that we can avoid a tsunami of cardiovascular disease in the long term.”
“It’s critically important that young adults speak with their health care provider about whether – and when – they should undergo screening for high blood pressure, diabetes, and high cholesterol,” Dr. Wadhera added.
Dr. Berger said one problem is that younger people often have a “superman or superwoman” view and don’t comprehend that they are at risk for some of these conditions. Studies such as this “reinforce the idea that it’s never too young to be checked out.”
As a cardiologist who specializes in cardiovascular prevention, Dr. Berger said he sometimes hears patients say things like, “I don’t ever want to need a cardiologist,” or “I hope I never need a cardiologist.”
“My response is, ‘There are many different types of cardiologists,’ and I think it would really be helpful for many people to see a prevention-focused cardiologist way before they have problems,” he said in an interview.
“As a system, medicine has become very good at treating patients with different diseases. I think we need to get better in terms of preventing some of these problems,” Dr. Berger added.
In their editorial, Dr. Allen and Dr. Wilkins say the “foundation of cardiovascular health begins early in life. These worsening trends in risk factors highlight the importance of focusing on prevention in adolescence and young adulthood in order to promote cardiovascular health across the lifetime.”
The study was funded by a grant from the National Heart, Lung, and Blood Institute. Dr. Wadhera has served as a consultant for Abbott and CVS Health. Dr. Wilkins has received personal fees from 3M. Dr. Berger has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New data show a high and rising burden of most cardiovascular (CV) risk factors among young adults aged 20-44 years in the United States.
In this age group, over the past 10 years, there has been an increase in the prevalence of diabetes and obesity, no improvement in the prevalence of hypertension, and a decrease in the prevalence of hyperlipidemia.
Yet medical treatment rates for CV risk factors are “surprisingly” low among young adults, study investigator Rishi Wadhera, MD, with Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, told this news organization.
The findings are “extremely concerning. We’re witnessing a smoldering public health crisis. The onset of these risk factors earlier in life is associated with a higher lifetime risk of heart disease and potentially life-threatening,” Dr. Wadhera added.
The study was presented March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation and was simultaneously published in JAMA.
The burden of CV risk factors among young adults is “unacceptably high and increasing,” write the co-authors of a JAMA editorial.
“The time is now for aggressive preventive measures in young adults. Without immediate action there will continue to be a rise in heart disease and the burden it places on patients, families, and communities,” say Norrina Allen, PhD, and John Wilkins, MD, with Northwestern University, Chicago.
Preventing a tsunami of heart disease
The findings stem from a cross-sectional study of 12,294 U.S. adults aged 20-44 years (mean age, 32; 51% women) who participated in National Health and Nutrition Examination Survey (NHANES) cycles for 2009-2010 to 2017-2020.
Overall, the prevalence of hypertension was 9.3% in 2009-2010 and increased to 11.5% in 2017-2020. The prevalence of diabetes rose from 3.0% to 4.1%, and the prevalence of obesity rose from 32.7% to 40.9%. The prevalence of hyperlipidemia decreased from 40.5% to 36.1%.
Black adults consistently had high rates of hypertension during the study period – 16.2% in 2009-2010 and 20.1% in 2017-2020 – and significant increases in hypertension occurred among Mexican American adults (from 6.5% to 9.5%) and other Hispanic adults (from 4.4% to 10.5%), while Mexican American adults had a significant uptick in diabetes (from 4.3% to 7.5%).
Equally concerning, said Dr. Wadhera, is the fact that only about 55% of young adults with hypertension were receiving antihypertensive medication, and just 1 in 2 young adults with diabetes were receiving treatment. “These low rates were driven, in part, by many young adults not being aware of their diagnosis,” he noted.
The NHANES data also show that the percentage of young adults who were treated for hypertension and who achieved blood pressure control did not change significantly over the study period (65.0% in 2009-2010 and 74.8% in 2017-2020). Blood sugar control among young adults being treated for diabetes remained suboptimal throughout the study period (45.5% in 2009-2010 and 56.6% in 2017-2020).
“The fact that blood pressure control and glycemic control are so poor is really worrisome,” Jeffrey Berger, MD, director of the Center for the Prevention of Cardiovascular Disease at NYU Langone Heart, who wasn’t involved in the study, told this news organization.
“Even in the lipid control, while it did get a little bit better, it’s still only around 30%-40%. So, I think we have ways to go as a society,” Dr. Berger noted.
Double down on screening
Dr. Wadhera said “we need to double down on efforts to screen for and treat cardiovascular risk factors like high blood pressure and diabetes in young adults. We need to intensify clinical and public health interventions focused on primordial and primary prevention in young adults now so that we can avoid a tsunami of cardiovascular disease in the long term.”
“It’s critically important that young adults speak with their health care provider about whether – and when – they should undergo screening for high blood pressure, diabetes, and high cholesterol,” Dr. Wadhera added.
Dr. Berger said one problem is that younger people often have a “superman or superwoman” view and don’t comprehend that they are at risk for some of these conditions. Studies such as this “reinforce the idea that it’s never too young to be checked out.”
As a cardiologist who specializes in cardiovascular prevention, Dr. Berger said he sometimes hears patients say things like, “I don’t ever want to need a cardiologist,” or “I hope I never need a cardiologist.”
“My response is, ‘There are many different types of cardiologists,’ and I think it would really be helpful for many people to see a prevention-focused cardiologist way before they have problems,” he said in an interview.
“As a system, medicine has become very good at treating patients with different diseases. I think we need to get better in terms of preventing some of these problems,” Dr. Berger added.
In their editorial, Dr. Allen and Dr. Wilkins say the “foundation of cardiovascular health begins early in life. These worsening trends in risk factors highlight the importance of focusing on prevention in adolescence and young adulthood in order to promote cardiovascular health across the lifetime.”
The study was funded by a grant from the National Heart, Lung, and Blood Institute. Dr. Wadhera has served as a consultant for Abbott and CVS Health. Dr. Wilkins has received personal fees from 3M. Dr. Berger has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New data show a high and rising burden of most cardiovascular (CV) risk factors among young adults aged 20-44 years in the United States.
In this age group, over the past 10 years, there has been an increase in the prevalence of diabetes and obesity, no improvement in the prevalence of hypertension, and a decrease in the prevalence of hyperlipidemia.
Yet medical treatment rates for CV risk factors are “surprisingly” low among young adults, study investigator Rishi Wadhera, MD, with Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, told this news organization.
The findings are “extremely concerning. We’re witnessing a smoldering public health crisis. The onset of these risk factors earlier in life is associated with a higher lifetime risk of heart disease and potentially life-threatening,” Dr. Wadhera added.
The study was presented March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation and was simultaneously published in JAMA.
The burden of CV risk factors among young adults is “unacceptably high and increasing,” write the co-authors of a JAMA editorial.
“The time is now for aggressive preventive measures in young adults. Without immediate action there will continue to be a rise in heart disease and the burden it places on patients, families, and communities,” say Norrina Allen, PhD, and John Wilkins, MD, with Northwestern University, Chicago.
Preventing a tsunami of heart disease
The findings stem from a cross-sectional study of 12,294 U.S. adults aged 20-44 years (mean age, 32; 51% women) who participated in National Health and Nutrition Examination Survey (NHANES) cycles for 2009-2010 to 2017-2020.
Overall, the prevalence of hypertension was 9.3% in 2009-2010 and increased to 11.5% in 2017-2020. The prevalence of diabetes rose from 3.0% to 4.1%, and the prevalence of obesity rose from 32.7% to 40.9%. The prevalence of hyperlipidemia decreased from 40.5% to 36.1%.
Black adults consistently had high rates of hypertension during the study period – 16.2% in 2009-2010 and 20.1% in 2017-2020 – and significant increases in hypertension occurred among Mexican American adults (from 6.5% to 9.5%) and other Hispanic adults (from 4.4% to 10.5%), while Mexican American adults had a significant uptick in diabetes (from 4.3% to 7.5%).
Equally concerning, said Dr. Wadhera, is the fact that only about 55% of young adults with hypertension were receiving antihypertensive medication, and just 1 in 2 young adults with diabetes were receiving treatment. “These low rates were driven, in part, by many young adults not being aware of their diagnosis,” he noted.
The NHANES data also show that the percentage of young adults who were treated for hypertension and who achieved blood pressure control did not change significantly over the study period (65.0% in 2009-2010 and 74.8% in 2017-2020). Blood sugar control among young adults being treated for diabetes remained suboptimal throughout the study period (45.5% in 2009-2010 and 56.6% in 2017-2020).
“The fact that blood pressure control and glycemic control are so poor is really worrisome,” Jeffrey Berger, MD, director of the Center for the Prevention of Cardiovascular Disease at NYU Langone Heart, who wasn’t involved in the study, told this news organization.
“Even in the lipid control, while it did get a little bit better, it’s still only around 30%-40%. So, I think we have ways to go as a society,” Dr. Berger noted.
Double down on screening
Dr. Wadhera said “we need to double down on efforts to screen for and treat cardiovascular risk factors like high blood pressure and diabetes in young adults. We need to intensify clinical and public health interventions focused on primordial and primary prevention in young adults now so that we can avoid a tsunami of cardiovascular disease in the long term.”
“It’s critically important that young adults speak with their health care provider about whether – and when – they should undergo screening for high blood pressure, diabetes, and high cholesterol,” Dr. Wadhera added.
Dr. Berger said one problem is that younger people often have a “superman or superwoman” view and don’t comprehend that they are at risk for some of these conditions. Studies such as this “reinforce the idea that it’s never too young to be checked out.”
As a cardiologist who specializes in cardiovascular prevention, Dr. Berger said he sometimes hears patients say things like, “I don’t ever want to need a cardiologist,” or “I hope I never need a cardiologist.”
“My response is, ‘There are many different types of cardiologists,’ and I think it would really be helpful for many people to see a prevention-focused cardiologist way before they have problems,” he said in an interview.
“As a system, medicine has become very good at treating patients with different diseases. I think we need to get better in terms of preventing some of these problems,” Dr. Berger added.
In their editorial, Dr. Allen and Dr. Wilkins say the “foundation of cardiovascular health begins early in life. These worsening trends in risk factors highlight the importance of focusing on prevention in adolescence and young adulthood in order to promote cardiovascular health across the lifetime.”
The study was funded by a grant from the National Heart, Lung, and Blood Institute. Dr. Wadhera has served as a consultant for Abbott and CVS Health. Dr. Wilkins has received personal fees from 3M. Dr. Berger has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
What impact do carbs have on bone health?
I am often asked about the impact of dietary nutrients on bone health, particularly as many patients with low bone density, many with a history of multiple fractures, are referred to me. Many factors affect bone density, an important predictor of fracture risk, including genetics, body weight and muscle mass, bone loading exercise, menstrual status, other hormonal factors, nutritional status, optimal absorption of dietary nutrients, and medication use.
Dietary nutrients include macronutrients (carbohydrates, proteins, fat, and fiber) and micronutrients (such as dietary minerals and vitamins). The importance of micronutrients such as calcium, phosphorus, magnesium, and vitamins C, D, and K in optimizing bone mineralization and bone formation has been well documented.
The impact of protein intake on bone health is slightly more controversial, with some studies suggesting that increased protein intake may be deleterious to bone by increasing acid load, which in turn, increases calcium loss in urine. Overall data analysis from multiple studies support the finding that a higher protein intake is modestly beneficial for bone at certain sites, such as the spine.
Though data regarding the impact of dietary carbohydrates on bone are not as robust, it’s important to understand these effects given the increasing knowledge of the deleterious impact of processed carbohydrates on weight and cardiometabolic outcomes. This leads to the growing recommendations to limit carbohydrates in diet.
Quality and quantity of carbs affect bone health
Available studies suggest that both the quality and quantity of carbohydrates that are in a diet as well as the glycemic index of food may affect bone outcomes. Glycemic index refers to the extent of blood glucose elevation that occurs after the intake of any specific food. Foods with a higher glycemic index cause a rapid increase in blood glucose, whereas those with a low glycemic index result in a slower and more gradual increase. Examples of high–glycemic index food include processed and baked foods (such as breakfast cereals [unless whole grain], pretzels, cookies, doughnuts, pastries, cake, white bread, bagels, croissants, and corn chips), sugar-sweetened beverages, white rice, fast food (such as pizza and burgers), and potatoes. Examples of low glycemic index foods include vegetables, fruits, legumes, dairy and dairy products (without added sugar), whole-grain foods (such as oat porridge), and nuts.
A high–glycemic index diet has been associated with a greater risk for obesity and cardiovascular disease, and with lower bone density, an increased risk for fracture. This has been attributed to acute increases in glucose and insulin levels after consumption of high–glycemic index food, which causes increased oxidative stress and secretion of inflammatory cytokines, such as interleukin 6 and tumor necrosis factor alpha, that activate cells in bone that increase bone loss.
Higher blood glucose concentrations induced by a higher dietary glycemic index can have deleterious effects on osteoblasts, the cells important for bone formation, and increase bone loss through production of advanced glycation end products that affect the cross linking of collagen in bone (important for bone strength), as well as calcium loss in urine. This was recently reported in a study by Garcia-Gavilan and others, in which the authors showed that high dietary glycemic index and dietary glucose load are associated with a higher risk for osteoporosis-related fractures in an older Mediterranean population who are at high risk for cardiovascular events. Similar data were reported by Nouri and coauthors in a study from Iran.
The quantity and quality of dietary carbohydrates may also have an impact on bone. The quality of carbohydrates has been assessed using the carbohydrate quality index (CQI) and the low carbohydrate diet score (LCDS). The CQI takes into account dietary fiber intake, glycemic index, intake of processed vs. whole grain, and solid vs. total carbohydrates in diet. A higher CQI diet is associated with reduced cardiovascular risk. Higher LCDS reflects lower carbohydrate and higher fat and protein intake.
Diets that are rich in refined or processed carbohydrates with added sugar are proinflammatory and increase oxidative stress, which may lead to increased bone loss, low bone density, and increased fracture risk. These foods also have a high glycemic index.
In contrast, diets that are rich in whole grains, legumes, fruits, vegetables, nuts, and olive oil have a lower glycemic index and are beneficial to bone. These diets have a higher CQI and LCDS (as reported by Nouri and coauthors) and provide a rich source of antioxidants, vitamins, minerals, and other nutrients (such as calcium, magnesium, and vitamins B, C, and K), which are all beneficial to bone. Gao and others have reported that implementing a low glycemic index pulse-based diet (lentils, peas, beans) is superior to a regular hospital diet in preventing the increase in bone loss that typically occurs during hospitalization with enforced bed rest.
Most reports of the impact of carbohydrates on bone health are from observational studies. In an interventional study, Dalskov and coauthors randomly assigned children aged 5-18 years who had parents with overweight to one of five diets (high protein/low glycemic index, high protein/high glycemic index, low protein/low glycemic index, low protein/high glycemic index, or regular) for 6 months.
Contrasting with our understanding that protein intake is overall good for bone, this study found that among patients receiving a high–glycemic index diet, those who were on a high-protein diet had greater reductions in a bone formation marker than did those on a low-protein diet, with no major changes observed with the other diets. This suggests the influence of associated dietary nutrients on bone outcomes and that protein intake may modify the effects of dietary carbohydrates on bone formation. Similarly, the fat content of food can alter the glycemic index and thus may modify the impact of dietary carbohydrates on bone.
In summary, available data suggest that the quantity and quality of carbohydrates, including the glycemic index of food, may affect bone health and that it is important to exercise moderation in the consumption of such foods. However, there are only a few studies that have examined these associations, and more studies are necessary to further clarify the impact of dietary carbohydrates on bone as well as any modifications of these effects by other associated food groups. These studies will allow us to refine our recommendations to our patients as we advance our understanding of the impact of the combined effects of various dietary nutrients on bone.
Madhusmita Misra, MD, MPH, is chief of the division of pediatric endocrinology, Mass General for Children, Boston, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for AbbVie, Sanofi, and Ipsen.
A version of this article first appeared on Medscape.com.
I am often asked about the impact of dietary nutrients on bone health, particularly as many patients with low bone density, many with a history of multiple fractures, are referred to me. Many factors affect bone density, an important predictor of fracture risk, including genetics, body weight and muscle mass, bone loading exercise, menstrual status, other hormonal factors, nutritional status, optimal absorption of dietary nutrients, and medication use.
Dietary nutrients include macronutrients (carbohydrates, proteins, fat, and fiber) and micronutrients (such as dietary minerals and vitamins). The importance of micronutrients such as calcium, phosphorus, magnesium, and vitamins C, D, and K in optimizing bone mineralization and bone formation has been well documented.
The impact of protein intake on bone health is slightly more controversial, with some studies suggesting that increased protein intake may be deleterious to bone by increasing acid load, which in turn, increases calcium loss in urine. Overall data analysis from multiple studies support the finding that a higher protein intake is modestly beneficial for bone at certain sites, such as the spine.
Though data regarding the impact of dietary carbohydrates on bone are not as robust, it’s important to understand these effects given the increasing knowledge of the deleterious impact of processed carbohydrates on weight and cardiometabolic outcomes. This leads to the growing recommendations to limit carbohydrates in diet.
Quality and quantity of carbs affect bone health
Available studies suggest that both the quality and quantity of carbohydrates that are in a diet as well as the glycemic index of food may affect bone outcomes. Glycemic index refers to the extent of blood glucose elevation that occurs after the intake of any specific food. Foods with a higher glycemic index cause a rapid increase in blood glucose, whereas those with a low glycemic index result in a slower and more gradual increase. Examples of high–glycemic index food include processed and baked foods (such as breakfast cereals [unless whole grain], pretzels, cookies, doughnuts, pastries, cake, white bread, bagels, croissants, and corn chips), sugar-sweetened beverages, white rice, fast food (such as pizza and burgers), and potatoes. Examples of low glycemic index foods include vegetables, fruits, legumes, dairy and dairy products (without added sugar), whole-grain foods (such as oat porridge), and nuts.
A high–glycemic index diet has been associated with a greater risk for obesity and cardiovascular disease, and with lower bone density, an increased risk for fracture. This has been attributed to acute increases in glucose and insulin levels after consumption of high–glycemic index food, which causes increased oxidative stress and secretion of inflammatory cytokines, such as interleukin 6 and tumor necrosis factor alpha, that activate cells in bone that increase bone loss.
Higher blood glucose concentrations induced by a higher dietary glycemic index can have deleterious effects on osteoblasts, the cells important for bone formation, and increase bone loss through production of advanced glycation end products that affect the cross linking of collagen in bone (important for bone strength), as well as calcium loss in urine. This was recently reported in a study by Garcia-Gavilan and others, in which the authors showed that high dietary glycemic index and dietary glucose load are associated with a higher risk for osteoporosis-related fractures in an older Mediterranean population who are at high risk for cardiovascular events. Similar data were reported by Nouri and coauthors in a study from Iran.
The quantity and quality of dietary carbohydrates may also have an impact on bone. The quality of carbohydrates has been assessed using the carbohydrate quality index (CQI) and the low carbohydrate diet score (LCDS). The CQI takes into account dietary fiber intake, glycemic index, intake of processed vs. whole grain, and solid vs. total carbohydrates in diet. A higher CQI diet is associated with reduced cardiovascular risk. Higher LCDS reflects lower carbohydrate and higher fat and protein intake.
Diets that are rich in refined or processed carbohydrates with added sugar are proinflammatory and increase oxidative stress, which may lead to increased bone loss, low bone density, and increased fracture risk. These foods also have a high glycemic index.
In contrast, diets that are rich in whole grains, legumes, fruits, vegetables, nuts, and olive oil have a lower glycemic index and are beneficial to bone. These diets have a higher CQI and LCDS (as reported by Nouri and coauthors) and provide a rich source of antioxidants, vitamins, minerals, and other nutrients (such as calcium, magnesium, and vitamins B, C, and K), which are all beneficial to bone. Gao and others have reported that implementing a low glycemic index pulse-based diet (lentils, peas, beans) is superior to a regular hospital diet in preventing the increase in bone loss that typically occurs during hospitalization with enforced bed rest.
Most reports of the impact of carbohydrates on bone health are from observational studies. In an interventional study, Dalskov and coauthors randomly assigned children aged 5-18 years who had parents with overweight to one of five diets (high protein/low glycemic index, high protein/high glycemic index, low protein/low glycemic index, low protein/high glycemic index, or regular) for 6 months.
Contrasting with our understanding that protein intake is overall good for bone, this study found that among patients receiving a high–glycemic index diet, those who were on a high-protein diet had greater reductions in a bone formation marker than did those on a low-protein diet, with no major changes observed with the other diets. This suggests the influence of associated dietary nutrients on bone outcomes and that protein intake may modify the effects of dietary carbohydrates on bone formation. Similarly, the fat content of food can alter the glycemic index and thus may modify the impact of dietary carbohydrates on bone.
In summary, available data suggest that the quantity and quality of carbohydrates, including the glycemic index of food, may affect bone health and that it is important to exercise moderation in the consumption of such foods. However, there are only a few studies that have examined these associations, and more studies are necessary to further clarify the impact of dietary carbohydrates on bone as well as any modifications of these effects by other associated food groups. These studies will allow us to refine our recommendations to our patients as we advance our understanding of the impact of the combined effects of various dietary nutrients on bone.
Madhusmita Misra, MD, MPH, is chief of the division of pediatric endocrinology, Mass General for Children, Boston, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for AbbVie, Sanofi, and Ipsen.
A version of this article first appeared on Medscape.com.
I am often asked about the impact of dietary nutrients on bone health, particularly as many patients with low bone density, many with a history of multiple fractures, are referred to me. Many factors affect bone density, an important predictor of fracture risk, including genetics, body weight and muscle mass, bone loading exercise, menstrual status, other hormonal factors, nutritional status, optimal absorption of dietary nutrients, and medication use.
Dietary nutrients include macronutrients (carbohydrates, proteins, fat, and fiber) and micronutrients (such as dietary minerals and vitamins). The importance of micronutrients such as calcium, phosphorus, magnesium, and vitamins C, D, and K in optimizing bone mineralization and bone formation has been well documented.
The impact of protein intake on bone health is slightly more controversial, with some studies suggesting that increased protein intake may be deleterious to bone by increasing acid load, which in turn, increases calcium loss in urine. Overall data analysis from multiple studies support the finding that a higher protein intake is modestly beneficial for bone at certain sites, such as the spine.
Though data regarding the impact of dietary carbohydrates on bone are not as robust, it’s important to understand these effects given the increasing knowledge of the deleterious impact of processed carbohydrates on weight and cardiometabolic outcomes. This leads to the growing recommendations to limit carbohydrates in diet.
Quality and quantity of carbs affect bone health
Available studies suggest that both the quality and quantity of carbohydrates that are in a diet as well as the glycemic index of food may affect bone outcomes. Glycemic index refers to the extent of blood glucose elevation that occurs after the intake of any specific food. Foods with a higher glycemic index cause a rapid increase in blood glucose, whereas those with a low glycemic index result in a slower and more gradual increase. Examples of high–glycemic index food include processed and baked foods (such as breakfast cereals [unless whole grain], pretzels, cookies, doughnuts, pastries, cake, white bread, bagels, croissants, and corn chips), sugar-sweetened beverages, white rice, fast food (such as pizza and burgers), and potatoes. Examples of low glycemic index foods include vegetables, fruits, legumes, dairy and dairy products (without added sugar), whole-grain foods (such as oat porridge), and nuts.
A high–glycemic index diet has been associated with a greater risk for obesity and cardiovascular disease, and with lower bone density, an increased risk for fracture. This has been attributed to acute increases in glucose and insulin levels after consumption of high–glycemic index food, which causes increased oxidative stress and secretion of inflammatory cytokines, such as interleukin 6 and tumor necrosis factor alpha, that activate cells in bone that increase bone loss.
Higher blood glucose concentrations induced by a higher dietary glycemic index can have deleterious effects on osteoblasts, the cells important for bone formation, and increase bone loss through production of advanced glycation end products that affect the cross linking of collagen in bone (important for bone strength), as well as calcium loss in urine. This was recently reported in a study by Garcia-Gavilan and others, in which the authors showed that high dietary glycemic index and dietary glucose load are associated with a higher risk for osteoporosis-related fractures in an older Mediterranean population who are at high risk for cardiovascular events. Similar data were reported by Nouri and coauthors in a study from Iran.
The quantity and quality of dietary carbohydrates may also have an impact on bone. The quality of carbohydrates has been assessed using the carbohydrate quality index (CQI) and the low carbohydrate diet score (LCDS). The CQI takes into account dietary fiber intake, glycemic index, intake of processed vs. whole grain, and solid vs. total carbohydrates in diet. A higher CQI diet is associated with reduced cardiovascular risk. Higher LCDS reflects lower carbohydrate and higher fat and protein intake.
Diets that are rich in refined or processed carbohydrates with added sugar are proinflammatory and increase oxidative stress, which may lead to increased bone loss, low bone density, and increased fracture risk. These foods also have a high glycemic index.
In contrast, diets that are rich in whole grains, legumes, fruits, vegetables, nuts, and olive oil have a lower glycemic index and are beneficial to bone. These diets have a higher CQI and LCDS (as reported by Nouri and coauthors) and provide a rich source of antioxidants, vitamins, minerals, and other nutrients (such as calcium, magnesium, and vitamins B, C, and K), which are all beneficial to bone. Gao and others have reported that implementing a low glycemic index pulse-based diet (lentils, peas, beans) is superior to a regular hospital diet in preventing the increase in bone loss that typically occurs during hospitalization with enforced bed rest.
Most reports of the impact of carbohydrates on bone health are from observational studies. In an interventional study, Dalskov and coauthors randomly assigned children aged 5-18 years who had parents with overweight to one of five diets (high protein/low glycemic index, high protein/high glycemic index, low protein/low glycemic index, low protein/high glycemic index, or regular) for 6 months.
Contrasting with our understanding that protein intake is overall good for bone, this study found that among patients receiving a high–glycemic index diet, those who were on a high-protein diet had greater reductions in a bone formation marker than did those on a low-protein diet, with no major changes observed with the other diets. This suggests the influence of associated dietary nutrients on bone outcomes and that protein intake may modify the effects of dietary carbohydrates on bone formation. Similarly, the fat content of food can alter the glycemic index and thus may modify the impact of dietary carbohydrates on bone.
In summary, available data suggest that the quantity and quality of carbohydrates, including the glycemic index of food, may affect bone health and that it is important to exercise moderation in the consumption of such foods. However, there are only a few studies that have examined these associations, and more studies are necessary to further clarify the impact of dietary carbohydrates on bone as well as any modifications of these effects by other associated food groups. These studies will allow us to refine our recommendations to our patients as we advance our understanding of the impact of the combined effects of various dietary nutrients on bone.
Madhusmita Misra, MD, MPH, is chief of the division of pediatric endocrinology, Mass General for Children, Boston, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for AbbVie, Sanofi, and Ipsen.
A version of this article first appeared on Medscape.com.
Be vigilant about suspected cases of measles, expert advises
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
HONOLULU – .
“Measles is one of the most contagious of human viruses, and we are seeing a resurgence,” Adelaide A. Hebert, MD, professor of dermatology and pediatrics, and chief of pediatric dermatology at the Universtiy of Texas, Houston, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “This is a re-emerging viral infection that dermatologists must recognize. Measles often starts behind the ears, and the eruption can look a lot like a drug eruption,” she noted. “Many of my pediatric colleagues have never seen a case of measles before because we have had a vaccine since 1963. Measles can almost entirely be prevented with vaccination. You get herd immunity if both doses have been administered to 95% of the population.”
In 2021, the World Health Organization estimated that 25 million children worldwide missed the measles vaccine. This caused 9 million cases of measles and 128,000 deaths in 22 countries, mainly from viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. According to the Centers for Disease Control and Prevention, 1,274 measles cases occurred in 31 states in 2019, mostly in individuals who were not vaccinated against it. Reported cases fell to 13 in 2020 but rose to 49 cases in 2021 and to 121 cases in 2022. As of Feb. 28, 2023, three cases have been reported in the United States.
“Measles spreads through direct contact with an infected person and through airborne transmission,” said Dr. Hebert, who recommended an article published in The Lancet for background on the topic. “Unlike COVID-19, measles has not mutated, so the original measles vaccine will work very well.”
Common clinical signs of measles include a generalized, maculopapular eruption lasting for 3 days or more, a temperature above 101° F plus cough, coryza, or conjunctivitis. Confirmation of measles can be made by PCR for viral RNA. Clinicians can also send a blood draw to the state public health lab for analysis. The serologic standard is a fourfold rise or fall in IgG titer with a paired sample sent 10-14 days after the initial collection.
“You can administer immune globulin up to 6 days after exposure to potentially prevent measles or decrease severity [in] immunocompromised hosts not previously vaccinated,” she said. The recommended intramuscular dose is 0.5 mL/kg, up to a dose of 15 mL/kg. Treatment is supportive and focused on relieving common symptoms and providing nutritional support. Administration of vitamin A is currently recommended for all children with acute measles.
Vitamin A supplements are available either as capsules (50,000 IU; 100,000 IU; 200,000 IU) or in liquid form. Parenteral formulations are also available. “Capsules need to be cut open and the contents squeezed into the mouths of children younger than 2 years,” Dr. Hebert said. “Capsules have the advantage that they can be given to mothers for administration at home.”
The recommended dosage of vitamin A in children is as follows, she said:
- Aged 12 months or older: 200,000 IU daily for 2 days.
- Aged 6 to 11 months: 100,000 IU daily for 2 days.
- Aged 6 months or younger: 50,000 IU daily for 2 days.
The American Academy of Pediatrics recommends a third dose given 2-4 weeks later to children with clinical signs and symptoms of vitamin A deficiency.
In an interview following the meeting, Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas, Austin, emphasized that when clinicians evaluate pediatric patients with viral symptoms such as fever, cough, and skin eruption, “measles should be in the differential diagnosis.” The 2022 uptick in measles cases “would be another reason to engage in regular vaccinations.”
Dr. Hebert disclosed that she is a consultant or advisor for AbbVie, Almirall, Amryt Pharma, Arcutis Biotherapeutics, Beiersdorf, Dermavant Sciences, Galderma Laboratories, L’Oreal, Novan, Ortho Dermatologics, Pfizer, and Verrica.
Dr. Levy disclosed that he is consultant or advisor for Abeona, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi-Genzyme.
MedscapeLIVE! and this news organization are owned by the same parent company.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR









