User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Vitamin D deficiency clearly linked to inflammation
Vitamin D deficiency has a causative role in the systemic inflammation that commonly accompanies it, with inflammation declining, reflected by reductions in elevated C-reactive protein (CRP), as vitamin D levels increase to normal levels, new research shows.
However, there is no reverse effect between the two: Changes in CRP levels did not appear to affect vitamin D levels.
first author Elina Hypponen, PhD, a professor in nutritional and genetic epidemiology and director of the Australian Centre for Precision Health, Adelaide, said in an interview.
“Given that the serum CRP level is a widely used biomarker for chronic inflammation, these results suggest that improving vitamin D status may reduce chronic inflammation, but only for people with vitamin D deficiency,” Dr. Hypponen and coauthors reported in their study, published in the International Journal of Epidemiology.
Vitamin D associated with CRP in ‘L-shaped’ manner
Nutritional factors are known to influence systemic inflammation in a variety of ways. However, there has been debate over the association between vitamin D – specifically, serum 25-hydroxyvitamin D (25[OH]D), an indicator of vitamin D status – and CRP, with some reports of observational associations between the two disputed in more robust randomized trials.
To further evaluate the relationship, the authors performed a bidirectional Mendelian randomization analysis, using a cohort of 294,970 unrelated participants of White/British ancestry in the UK Biobank, the largest cohort to date with measured serum 25(OH)D concentrations, they noted.
Overall, the average 25(OH)D concentration was 50.0 nmol/L (range, 10-340 nmol/L), with 11.7% (n = 34,403) of participants having concentrations of less than 25 nmol/L, considered deficient.
The analysis showed that genetically predicted serum 25(OH)D was associated with serum CRP in an L-shaped manner, with CRP levels, and hence inflammation, sharply decreasing in relation to increasing 25(OH)D concentration to normal levels.
However, the relationship was only significant among participants with 25(OH)D levels in the deficiency range (< 25 nmol/L), with the association leveling off at about 50 nmol/L of 25(OH)D, which is generally considered a normal level.
The association was supported in further stratified Mendelian randomization analyses, which confirmed an inverse association between serum 25(OH)D in the deficiency range and CRP, but not with higher concentrations of serum vitamin D.
Conversely, neither linear nor nonlinear Mendelian randomization analyses showed a causal effect of serum CRP level on 25(OH)D concentrations.
The findings suggest that “improving vitamin D status in the deficiency range could reduce systemic low-grade inflammation and potentially mitigate the risk or severity of chronic illnesses with an inflammatory component,” the authors noted.
Dr. Hypponen added that the greatest reductions in CRP are observed with correction of the most severe vitamin D deficiency.
“The strongest benefits of improving concentrations will be seen for people with severe deficiency,” Dr. Hypponen said in an interview.
“In our study, much of the benefit was achieved when people reached the National Academy of Sciences endorsed cutoff of 50 nmol/L [for vitamin D sufficiency].”
Prohormone effects?
The anti-inflammatory effects observed with serum vitamin D could be related to its role as a prohormone that can impact vitamin D receptor–expressing immune cells, such as monocytes, B cells, T cells, and antigen-presenting cells, the authors noted.
“Indeed, cell experiments have shown that active vitamin D can inhibit the production of proinflammatory cytokines, including [tumor necrosis factor]–alpha, interleukin-1b, IL-6, IL-8, and IL-12, and promote the production of IL-10, an anti-inflammatory cytokine,” they explained.
In that regard, adequate vitamin D concentrations could be important in preventing inflammation-related complications from obesity and reduce the risk or severity of chronic illnesses with an inflammatory component, such as cardiovascular diseases, diabetes, autoimmune diseases, neurodegenerative conditions, and others, the authors noted.
Previous studies unable to assess effect of deficiency
While the current findings contradict other studies that have used Mendelian randomization and showed no causal effect of 25(OH)D on CRP, those previous studies only used a standard linear Mendelian randomization method that could not rule out the possibility of a ‘threshold effect’ restricted to vitamin D deficiency, the authors noted.
“Indeed, it is logical to expect that improving vitamin D status would be relevant only in the presence of vitamin D deficiency, whereas any further additions may be redundant and, in the ... extreme of supplementation, might become toxic,” they wrote.
However, the nonlinear Mendelian randomization approach used in the current study allows for better detection of the association, and the authors point out that the method has also been recently used in research showing an adverse effect of vitamin D deficiency on cardiovascular disease risk and mortality, which would not be visible using the standard linear Mendelian randomization approach.
Meanwhile, the current findings add to broader research showing benefits of increases in vitamin D to be mainly limited to those who are deficient, with limited benefit of supplementation for those who are not, Dr. Hypponen emphasized.
“We have repeatedly seen evidence for health benefits for increasing vitamin D concentrations in individuals with very low levels, while for others, there appears to be little to no benefit,” Dr. Hypponen said in a press statement.
“These findings highlight the importance of avoiding clinical vitamin D deficiency and provide further evidence for the wide-ranging effects of hormonal vitamin D,” she added.
The study was financially supported by the National Health and Medical Research Council, Australia. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vitamin D deficiency has a causative role in the systemic inflammation that commonly accompanies it, with inflammation declining, reflected by reductions in elevated C-reactive protein (CRP), as vitamin D levels increase to normal levels, new research shows.
However, there is no reverse effect between the two: Changes in CRP levels did not appear to affect vitamin D levels.
first author Elina Hypponen, PhD, a professor in nutritional and genetic epidemiology and director of the Australian Centre for Precision Health, Adelaide, said in an interview.
“Given that the serum CRP level is a widely used biomarker for chronic inflammation, these results suggest that improving vitamin D status may reduce chronic inflammation, but only for people with vitamin D deficiency,” Dr. Hypponen and coauthors reported in their study, published in the International Journal of Epidemiology.
Vitamin D associated with CRP in ‘L-shaped’ manner
Nutritional factors are known to influence systemic inflammation in a variety of ways. However, there has been debate over the association between vitamin D – specifically, serum 25-hydroxyvitamin D (25[OH]D), an indicator of vitamin D status – and CRP, with some reports of observational associations between the two disputed in more robust randomized trials.
To further evaluate the relationship, the authors performed a bidirectional Mendelian randomization analysis, using a cohort of 294,970 unrelated participants of White/British ancestry in the UK Biobank, the largest cohort to date with measured serum 25(OH)D concentrations, they noted.
Overall, the average 25(OH)D concentration was 50.0 nmol/L (range, 10-340 nmol/L), with 11.7% (n = 34,403) of participants having concentrations of less than 25 nmol/L, considered deficient.
The analysis showed that genetically predicted serum 25(OH)D was associated with serum CRP in an L-shaped manner, with CRP levels, and hence inflammation, sharply decreasing in relation to increasing 25(OH)D concentration to normal levels.
However, the relationship was only significant among participants with 25(OH)D levels in the deficiency range (< 25 nmol/L), with the association leveling off at about 50 nmol/L of 25(OH)D, which is generally considered a normal level.
The association was supported in further stratified Mendelian randomization analyses, which confirmed an inverse association between serum 25(OH)D in the deficiency range and CRP, but not with higher concentrations of serum vitamin D.
Conversely, neither linear nor nonlinear Mendelian randomization analyses showed a causal effect of serum CRP level on 25(OH)D concentrations.
The findings suggest that “improving vitamin D status in the deficiency range could reduce systemic low-grade inflammation and potentially mitigate the risk or severity of chronic illnesses with an inflammatory component,” the authors noted.
Dr. Hypponen added that the greatest reductions in CRP are observed with correction of the most severe vitamin D deficiency.
“The strongest benefits of improving concentrations will be seen for people with severe deficiency,” Dr. Hypponen said in an interview.
“In our study, much of the benefit was achieved when people reached the National Academy of Sciences endorsed cutoff of 50 nmol/L [for vitamin D sufficiency].”
Prohormone effects?
The anti-inflammatory effects observed with serum vitamin D could be related to its role as a prohormone that can impact vitamin D receptor–expressing immune cells, such as monocytes, B cells, T cells, and antigen-presenting cells, the authors noted.
“Indeed, cell experiments have shown that active vitamin D can inhibit the production of proinflammatory cytokines, including [tumor necrosis factor]–alpha, interleukin-1b, IL-6, IL-8, and IL-12, and promote the production of IL-10, an anti-inflammatory cytokine,” they explained.
In that regard, adequate vitamin D concentrations could be important in preventing inflammation-related complications from obesity and reduce the risk or severity of chronic illnesses with an inflammatory component, such as cardiovascular diseases, diabetes, autoimmune diseases, neurodegenerative conditions, and others, the authors noted.
Previous studies unable to assess effect of deficiency
While the current findings contradict other studies that have used Mendelian randomization and showed no causal effect of 25(OH)D on CRP, those previous studies only used a standard linear Mendelian randomization method that could not rule out the possibility of a ‘threshold effect’ restricted to vitamin D deficiency, the authors noted.
“Indeed, it is logical to expect that improving vitamin D status would be relevant only in the presence of vitamin D deficiency, whereas any further additions may be redundant and, in the ... extreme of supplementation, might become toxic,” they wrote.
However, the nonlinear Mendelian randomization approach used in the current study allows for better detection of the association, and the authors point out that the method has also been recently used in research showing an adverse effect of vitamin D deficiency on cardiovascular disease risk and mortality, which would not be visible using the standard linear Mendelian randomization approach.
Meanwhile, the current findings add to broader research showing benefits of increases in vitamin D to be mainly limited to those who are deficient, with limited benefit of supplementation for those who are not, Dr. Hypponen emphasized.
“We have repeatedly seen evidence for health benefits for increasing vitamin D concentrations in individuals with very low levels, while for others, there appears to be little to no benefit,” Dr. Hypponen said in a press statement.
“These findings highlight the importance of avoiding clinical vitamin D deficiency and provide further evidence for the wide-ranging effects of hormonal vitamin D,” she added.
The study was financially supported by the National Health and Medical Research Council, Australia. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vitamin D deficiency has a causative role in the systemic inflammation that commonly accompanies it, with inflammation declining, reflected by reductions in elevated C-reactive protein (CRP), as vitamin D levels increase to normal levels, new research shows.
However, there is no reverse effect between the two: Changes in CRP levels did not appear to affect vitamin D levels.
first author Elina Hypponen, PhD, a professor in nutritional and genetic epidemiology and director of the Australian Centre for Precision Health, Adelaide, said in an interview.
“Given that the serum CRP level is a widely used biomarker for chronic inflammation, these results suggest that improving vitamin D status may reduce chronic inflammation, but only for people with vitamin D deficiency,” Dr. Hypponen and coauthors reported in their study, published in the International Journal of Epidemiology.
Vitamin D associated with CRP in ‘L-shaped’ manner
Nutritional factors are known to influence systemic inflammation in a variety of ways. However, there has been debate over the association between vitamin D – specifically, serum 25-hydroxyvitamin D (25[OH]D), an indicator of vitamin D status – and CRP, with some reports of observational associations between the two disputed in more robust randomized trials.
To further evaluate the relationship, the authors performed a bidirectional Mendelian randomization analysis, using a cohort of 294,970 unrelated participants of White/British ancestry in the UK Biobank, the largest cohort to date with measured serum 25(OH)D concentrations, they noted.
Overall, the average 25(OH)D concentration was 50.0 nmol/L (range, 10-340 nmol/L), with 11.7% (n = 34,403) of participants having concentrations of less than 25 nmol/L, considered deficient.
The analysis showed that genetically predicted serum 25(OH)D was associated with serum CRP in an L-shaped manner, with CRP levels, and hence inflammation, sharply decreasing in relation to increasing 25(OH)D concentration to normal levels.
However, the relationship was only significant among participants with 25(OH)D levels in the deficiency range (< 25 nmol/L), with the association leveling off at about 50 nmol/L of 25(OH)D, which is generally considered a normal level.
The association was supported in further stratified Mendelian randomization analyses, which confirmed an inverse association between serum 25(OH)D in the deficiency range and CRP, but not with higher concentrations of serum vitamin D.
Conversely, neither linear nor nonlinear Mendelian randomization analyses showed a causal effect of serum CRP level on 25(OH)D concentrations.
The findings suggest that “improving vitamin D status in the deficiency range could reduce systemic low-grade inflammation and potentially mitigate the risk or severity of chronic illnesses with an inflammatory component,” the authors noted.
Dr. Hypponen added that the greatest reductions in CRP are observed with correction of the most severe vitamin D deficiency.
“The strongest benefits of improving concentrations will be seen for people with severe deficiency,” Dr. Hypponen said in an interview.
“In our study, much of the benefit was achieved when people reached the National Academy of Sciences endorsed cutoff of 50 nmol/L [for vitamin D sufficiency].”
Prohormone effects?
The anti-inflammatory effects observed with serum vitamin D could be related to its role as a prohormone that can impact vitamin D receptor–expressing immune cells, such as monocytes, B cells, T cells, and antigen-presenting cells, the authors noted.
“Indeed, cell experiments have shown that active vitamin D can inhibit the production of proinflammatory cytokines, including [tumor necrosis factor]–alpha, interleukin-1b, IL-6, IL-8, and IL-12, and promote the production of IL-10, an anti-inflammatory cytokine,” they explained.
In that regard, adequate vitamin D concentrations could be important in preventing inflammation-related complications from obesity and reduce the risk or severity of chronic illnesses with an inflammatory component, such as cardiovascular diseases, diabetes, autoimmune diseases, neurodegenerative conditions, and others, the authors noted.
Previous studies unable to assess effect of deficiency
While the current findings contradict other studies that have used Mendelian randomization and showed no causal effect of 25(OH)D on CRP, those previous studies only used a standard linear Mendelian randomization method that could not rule out the possibility of a ‘threshold effect’ restricted to vitamin D deficiency, the authors noted.
“Indeed, it is logical to expect that improving vitamin D status would be relevant only in the presence of vitamin D deficiency, whereas any further additions may be redundant and, in the ... extreme of supplementation, might become toxic,” they wrote.
However, the nonlinear Mendelian randomization approach used in the current study allows for better detection of the association, and the authors point out that the method has also been recently used in research showing an adverse effect of vitamin D deficiency on cardiovascular disease risk and mortality, which would not be visible using the standard linear Mendelian randomization approach.
Meanwhile, the current findings add to broader research showing benefits of increases in vitamin D to be mainly limited to those who are deficient, with limited benefit of supplementation for those who are not, Dr. Hypponen emphasized.
“We have repeatedly seen evidence for health benefits for increasing vitamin D concentrations in individuals with very low levels, while for others, there appears to be little to no benefit,” Dr. Hypponen said in a press statement.
“These findings highlight the importance of avoiding clinical vitamin D deficiency and provide further evidence for the wide-ranging effects of hormonal vitamin D,” she added.
The study was financially supported by the National Health and Medical Research Council, Australia. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF EPIDEMIOLOGY
COVID-19 may trigger irritable bowel syndrome
Gastrointestinal symptoms are common with long COVID, also known as post-acute COVID-19 syndrome, according to Walter Chan, MD, MPH, and Madhusudan Grover, MBBS.
Dr. Chan, an assistant professor at Harvard Medical School, Boston, and Dr. Grover, an associate professor of medicine and physiology at Mayo Clinic, Rochester, Minn., conducted a review of the literature on COVID-19’s long-term gastrointestinal effects. Their review was published in Clinical Gastroenterology and Hepatology.
Estimates of the prevalence of gastrointestinal symptoms with COVID-19 have ranged as high as 60%, Dr. Chan and Dr. Grover report, and the symptoms may be present in patients with long COVID, a syndrome that continues 4 weeks or longer.
In one survey of 749 COVID-19 survivors, 29% reported at least one new chronic gastrointestinal symptom. The most common were heartburn, constipation, diarrhea, and abdominal pain. Of those with abdominal pain, 39% had symptoms that met Rome IV criteria for irritable bowel syndrome.
People who have gastrointestinal symptoms after their initial SARS-CoV-2 infection are more likely to have them with long COVID. Psychiatric diagnoses, hospitalization, and the loss of smell and taste are predictors of gastrointestinal symptoms.
Infectious gastroenteritis can increase the risk for disorders of gut-brain interaction, especially postinfection IBS, Dr. Chan and Dr. Grover write.
COVID-19 likely causes gastrointestinal symptoms through multiple mechanisms. It may suppress angiotensin-converting enzyme 2, which protects intestinal cells. It can alter the microbiome. It can cause or worsen weight gain and diabetes. It may disrupt the immune system and trigger an autoimmune reaction. It can cause depression and anxiety, and it can alter dietary habits.
No specific treatments for gastrointestinal symptoms associated with long COVID have emerged, so clinicians should make use of established therapies for disorders of gut-brain interaction, Dr. Chan and Dr. Grover recommend.
Beyond adequate sleep and exercise, these may include high-fiber, low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), gluten-free, low-carbohydrate, or elimination diets.
For diarrhea, they list loperamide, ondansetron, alosetron, eluxadoline, antispasmodics, rifaximin, and bile acid sequestrants.
For constipation, they mention fiber supplements, polyethylene glycol, linaclotide, plecanatide, lubiprostone, tenapanor, tegaserod, and prucalopride.
For modulating intestinal permeability, they recommend glutamine.
Neuromodulation may be achieved with tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, azaperones, and delta ligands, they write.
For psychological therapy, they recommend cognitive-behavioral therapy and gut-directed hypnotherapy.
A handful of studies have suggested benefits from Lactiplantibacillus plantarum and Pediococcus acidilactici as probiotic therapies. Additionally, one study showed positive results with a high-fiber formula, perhaps by nourishing short-chain fatty acid-producing bacteria, Dr. Chan and Dr. Grover write.
Dr. Chan reported financial relationships with Ironwood, Takeda, and Phathom Pharmaceuticals. Dr. Grover reported financial relationships with Takeda, Donga, Alexza Pharmaceuticals, and Alfasigma.
A version of this article first appeared on Medscape.com.
Gastrointestinal symptoms are common with long COVID, also known as post-acute COVID-19 syndrome, according to Walter Chan, MD, MPH, and Madhusudan Grover, MBBS.
Dr. Chan, an assistant professor at Harvard Medical School, Boston, and Dr. Grover, an associate professor of medicine and physiology at Mayo Clinic, Rochester, Minn., conducted a review of the literature on COVID-19’s long-term gastrointestinal effects. Their review was published in Clinical Gastroenterology and Hepatology.
Estimates of the prevalence of gastrointestinal symptoms with COVID-19 have ranged as high as 60%, Dr. Chan and Dr. Grover report, and the symptoms may be present in patients with long COVID, a syndrome that continues 4 weeks or longer.
In one survey of 749 COVID-19 survivors, 29% reported at least one new chronic gastrointestinal symptom. The most common were heartburn, constipation, diarrhea, and abdominal pain. Of those with abdominal pain, 39% had symptoms that met Rome IV criteria for irritable bowel syndrome.
People who have gastrointestinal symptoms after their initial SARS-CoV-2 infection are more likely to have them with long COVID. Psychiatric diagnoses, hospitalization, and the loss of smell and taste are predictors of gastrointestinal symptoms.
Infectious gastroenteritis can increase the risk for disorders of gut-brain interaction, especially postinfection IBS, Dr. Chan and Dr. Grover write.
COVID-19 likely causes gastrointestinal symptoms through multiple mechanisms. It may suppress angiotensin-converting enzyme 2, which protects intestinal cells. It can alter the microbiome. It can cause or worsen weight gain and diabetes. It may disrupt the immune system and trigger an autoimmune reaction. It can cause depression and anxiety, and it can alter dietary habits.
No specific treatments for gastrointestinal symptoms associated with long COVID have emerged, so clinicians should make use of established therapies for disorders of gut-brain interaction, Dr. Chan and Dr. Grover recommend.
Beyond adequate sleep and exercise, these may include high-fiber, low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), gluten-free, low-carbohydrate, or elimination diets.
For diarrhea, they list loperamide, ondansetron, alosetron, eluxadoline, antispasmodics, rifaximin, and bile acid sequestrants.
For constipation, they mention fiber supplements, polyethylene glycol, linaclotide, plecanatide, lubiprostone, tenapanor, tegaserod, and prucalopride.
For modulating intestinal permeability, they recommend glutamine.
Neuromodulation may be achieved with tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, azaperones, and delta ligands, they write.
For psychological therapy, they recommend cognitive-behavioral therapy and gut-directed hypnotherapy.
A handful of studies have suggested benefits from Lactiplantibacillus plantarum and Pediococcus acidilactici as probiotic therapies. Additionally, one study showed positive results with a high-fiber formula, perhaps by nourishing short-chain fatty acid-producing bacteria, Dr. Chan and Dr. Grover write.
Dr. Chan reported financial relationships with Ironwood, Takeda, and Phathom Pharmaceuticals. Dr. Grover reported financial relationships with Takeda, Donga, Alexza Pharmaceuticals, and Alfasigma.
A version of this article first appeared on Medscape.com.
Gastrointestinal symptoms are common with long COVID, also known as post-acute COVID-19 syndrome, according to Walter Chan, MD, MPH, and Madhusudan Grover, MBBS.
Dr. Chan, an assistant professor at Harvard Medical School, Boston, and Dr. Grover, an associate professor of medicine and physiology at Mayo Clinic, Rochester, Minn., conducted a review of the literature on COVID-19’s long-term gastrointestinal effects. Their review was published in Clinical Gastroenterology and Hepatology.
Estimates of the prevalence of gastrointestinal symptoms with COVID-19 have ranged as high as 60%, Dr. Chan and Dr. Grover report, and the symptoms may be present in patients with long COVID, a syndrome that continues 4 weeks or longer.
In one survey of 749 COVID-19 survivors, 29% reported at least one new chronic gastrointestinal symptom. The most common were heartburn, constipation, diarrhea, and abdominal pain. Of those with abdominal pain, 39% had symptoms that met Rome IV criteria for irritable bowel syndrome.
People who have gastrointestinal symptoms after their initial SARS-CoV-2 infection are more likely to have them with long COVID. Psychiatric diagnoses, hospitalization, and the loss of smell and taste are predictors of gastrointestinal symptoms.
Infectious gastroenteritis can increase the risk for disorders of gut-brain interaction, especially postinfection IBS, Dr. Chan and Dr. Grover write.
COVID-19 likely causes gastrointestinal symptoms through multiple mechanisms. It may suppress angiotensin-converting enzyme 2, which protects intestinal cells. It can alter the microbiome. It can cause or worsen weight gain and diabetes. It may disrupt the immune system and trigger an autoimmune reaction. It can cause depression and anxiety, and it can alter dietary habits.
No specific treatments for gastrointestinal symptoms associated with long COVID have emerged, so clinicians should make use of established therapies for disorders of gut-brain interaction, Dr. Chan and Dr. Grover recommend.
Beyond adequate sleep and exercise, these may include high-fiber, low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), gluten-free, low-carbohydrate, or elimination diets.
For diarrhea, they list loperamide, ondansetron, alosetron, eluxadoline, antispasmodics, rifaximin, and bile acid sequestrants.
For constipation, they mention fiber supplements, polyethylene glycol, linaclotide, plecanatide, lubiprostone, tenapanor, tegaserod, and prucalopride.
For modulating intestinal permeability, they recommend glutamine.
Neuromodulation may be achieved with tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, azaperones, and delta ligands, they write.
For psychological therapy, they recommend cognitive-behavioral therapy and gut-directed hypnotherapy.
A handful of studies have suggested benefits from Lactiplantibacillus plantarum and Pediococcus acidilactici as probiotic therapies. Additionally, one study showed positive results with a high-fiber formula, perhaps by nourishing short-chain fatty acid-producing bacteria, Dr. Chan and Dr. Grover write.
Dr. Chan reported financial relationships with Ironwood, Takeda, and Phathom Pharmaceuticals. Dr. Grover reported financial relationships with Takeda, Donga, Alexza Pharmaceuticals, and Alfasigma.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Long COVID case study: persistent hormone deficiencies
A case study of a 65-year-old man in Japan with long COVID describes how he recovered from certain impaired hormone deficiencies that persisted for more than a year.
Days after the patient recovered from respiratory failure and came off a ventilator, he had a sudden drop in blood pressure, which responded to hydrocortisone.
The patient was found to have low levels of growth hormone and adrenocorticotropic hormone (ACTH), hypopituitarism, that persisted for more than a year. He also had low levels of testosterone that remained low at 15 months (the study end).
“An important finding in the present case is the eventual recovery from hypopituitarism over time but not from hypogonadism,” the researchers write in their study published in Endocrine Journal.
, which was confirmed using an insulin tolerance test, Kai Yoshimura, Kakogawa Medical Center, Japan, and colleagues report.
The findings show that “pituitary insufficiency should be considered in patients with prolonged symptoms of COVID-19,” they report, since it can be treated with hormone supplements that markedly improve symptoms and quality of life.
“It might be worthwhile to screen for endocrine dysfunction in patients with such persistent symptoms after their recovery from the acute disease,” the researchers conclude.
Case study timeline
The patient in this study was healthy without obesity, previous endocrine disease, or steroid use. He was admitted to hospital because he had dyspnea and fever for 8 days and a reverse transcription-polymerase chain reaction (RT-PCR) test that was positive for COVID-19.
He received ciclesonide 200 mcg/day for 2 days. Then he was put on a ventilator and the drug was discontinued and “favipiravir, ritonavir, and lopinavir, a standard regimen during the early phase of the COVID-19 pandemic, were initiated;” the researchers explain.
On day 25 of his hospital stay the patient had recovered from respiratory failure and was extubated.
On day 31, he had a negative PCR test for COVID-19.
On day 36, the patient’s blood pressure suddenly dropped from 120/80 mmHg to 80/50 mmHg. His plasma ACTH and serum cortisol levels were low, suggesting secondary adrenal insufficiency. The low blood pressure responded to hydrocortisone 100 mg, which was gradually tapered.
At day 96, the patient was discharged from hospital with a dose of 15 mg/day hydrocortisone.
At 3 months after discharge, an insulin tolerance test revealed that the patient’s ACTH and cortisol responses were blunted, suggestive of adrenal insufficiency. The patient also had moderate growth hormone deficiency and symptoms of hypogonadism.
At 6 months after discharge, the patient started testosterone therapy because his dysspermatism had worsened.
At 12 months after discharge, a repeat insulin tolerance test showed that both ACTH and cortisol responses were low but improved. The patient was no longer deficient in growth hormone.
At 15 months after discharge, early morning levels of ACTH and cortisol were now in the normal range. The patient discontinued testosterone treatment, but the symptoms returned, so he resumed it.
Long COVID symptoms, possible biological mechanism
The present case shows how certain COVID-19–associated conditions develop after the onset of, or the recovery from, respiratory disorders, the authors note.
Symptoms of long COVID-19 include fatigue, weakness, hair loss, diarrhea, arthralgia, and depression, and these symptoms are associated with pituitary insufficiency, especially secondary adrenocortical insufficiency.
In addition, an estimated 25% of sexually active men who recover from COVID have semen disorders such as azoospermia and oligospermia.
The underlying mechanism by which COVID-19 might trigger pituitary insufficiency is unknown, but other viral infections such as influenza-A and herpes simplex are also associated with transient hypopituitarism. An exaggerated immune response triggered by SARS-CoV-2 may explain the dysfunction of multiple endocrine organs, the researchers write.
The researchers have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
A case study of a 65-year-old man in Japan with long COVID describes how he recovered from certain impaired hormone deficiencies that persisted for more than a year.
Days after the patient recovered from respiratory failure and came off a ventilator, he had a sudden drop in blood pressure, which responded to hydrocortisone.
The patient was found to have low levels of growth hormone and adrenocorticotropic hormone (ACTH), hypopituitarism, that persisted for more than a year. He also had low levels of testosterone that remained low at 15 months (the study end).
“An important finding in the present case is the eventual recovery from hypopituitarism over time but not from hypogonadism,” the researchers write in their study published in Endocrine Journal.
, which was confirmed using an insulin tolerance test, Kai Yoshimura, Kakogawa Medical Center, Japan, and colleagues report.
The findings show that “pituitary insufficiency should be considered in patients with prolonged symptoms of COVID-19,” they report, since it can be treated with hormone supplements that markedly improve symptoms and quality of life.
“It might be worthwhile to screen for endocrine dysfunction in patients with such persistent symptoms after their recovery from the acute disease,” the researchers conclude.
Case study timeline
The patient in this study was healthy without obesity, previous endocrine disease, or steroid use. He was admitted to hospital because he had dyspnea and fever for 8 days and a reverse transcription-polymerase chain reaction (RT-PCR) test that was positive for COVID-19.
He received ciclesonide 200 mcg/day for 2 days. Then he was put on a ventilator and the drug was discontinued and “favipiravir, ritonavir, and lopinavir, a standard regimen during the early phase of the COVID-19 pandemic, were initiated;” the researchers explain.
On day 25 of his hospital stay the patient had recovered from respiratory failure and was extubated.
On day 31, he had a negative PCR test for COVID-19.
On day 36, the patient’s blood pressure suddenly dropped from 120/80 mmHg to 80/50 mmHg. His plasma ACTH and serum cortisol levels were low, suggesting secondary adrenal insufficiency. The low blood pressure responded to hydrocortisone 100 mg, which was gradually tapered.
At day 96, the patient was discharged from hospital with a dose of 15 mg/day hydrocortisone.
At 3 months after discharge, an insulin tolerance test revealed that the patient’s ACTH and cortisol responses were blunted, suggestive of adrenal insufficiency. The patient also had moderate growth hormone deficiency and symptoms of hypogonadism.
At 6 months after discharge, the patient started testosterone therapy because his dysspermatism had worsened.
At 12 months after discharge, a repeat insulin tolerance test showed that both ACTH and cortisol responses were low but improved. The patient was no longer deficient in growth hormone.
At 15 months after discharge, early morning levels of ACTH and cortisol were now in the normal range. The patient discontinued testosterone treatment, but the symptoms returned, so he resumed it.
Long COVID symptoms, possible biological mechanism
The present case shows how certain COVID-19–associated conditions develop after the onset of, or the recovery from, respiratory disorders, the authors note.
Symptoms of long COVID-19 include fatigue, weakness, hair loss, diarrhea, arthralgia, and depression, and these symptoms are associated with pituitary insufficiency, especially secondary adrenocortical insufficiency.
In addition, an estimated 25% of sexually active men who recover from COVID have semen disorders such as azoospermia and oligospermia.
The underlying mechanism by which COVID-19 might trigger pituitary insufficiency is unknown, but other viral infections such as influenza-A and herpes simplex are also associated with transient hypopituitarism. An exaggerated immune response triggered by SARS-CoV-2 may explain the dysfunction of multiple endocrine organs, the researchers write.
The researchers have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
A case study of a 65-year-old man in Japan with long COVID describes how he recovered from certain impaired hormone deficiencies that persisted for more than a year.
Days after the patient recovered from respiratory failure and came off a ventilator, he had a sudden drop in blood pressure, which responded to hydrocortisone.
The patient was found to have low levels of growth hormone and adrenocorticotropic hormone (ACTH), hypopituitarism, that persisted for more than a year. He also had low levels of testosterone that remained low at 15 months (the study end).
“An important finding in the present case is the eventual recovery from hypopituitarism over time but not from hypogonadism,” the researchers write in their study published in Endocrine Journal.
, which was confirmed using an insulin tolerance test, Kai Yoshimura, Kakogawa Medical Center, Japan, and colleagues report.
The findings show that “pituitary insufficiency should be considered in patients with prolonged symptoms of COVID-19,” they report, since it can be treated with hormone supplements that markedly improve symptoms and quality of life.
“It might be worthwhile to screen for endocrine dysfunction in patients with such persistent symptoms after their recovery from the acute disease,” the researchers conclude.
Case study timeline
The patient in this study was healthy without obesity, previous endocrine disease, or steroid use. He was admitted to hospital because he had dyspnea and fever for 8 days and a reverse transcription-polymerase chain reaction (RT-PCR) test that was positive for COVID-19.
He received ciclesonide 200 mcg/day for 2 days. Then he was put on a ventilator and the drug was discontinued and “favipiravir, ritonavir, and lopinavir, a standard regimen during the early phase of the COVID-19 pandemic, were initiated;” the researchers explain.
On day 25 of his hospital stay the patient had recovered from respiratory failure and was extubated.
On day 31, he had a negative PCR test for COVID-19.
On day 36, the patient’s blood pressure suddenly dropped from 120/80 mmHg to 80/50 mmHg. His plasma ACTH and serum cortisol levels were low, suggesting secondary adrenal insufficiency. The low blood pressure responded to hydrocortisone 100 mg, which was gradually tapered.
At day 96, the patient was discharged from hospital with a dose of 15 mg/day hydrocortisone.
At 3 months after discharge, an insulin tolerance test revealed that the patient’s ACTH and cortisol responses were blunted, suggestive of adrenal insufficiency. The patient also had moderate growth hormone deficiency and symptoms of hypogonadism.
At 6 months after discharge, the patient started testosterone therapy because his dysspermatism had worsened.
At 12 months after discharge, a repeat insulin tolerance test showed that both ACTH and cortisol responses were low but improved. The patient was no longer deficient in growth hormone.
At 15 months after discharge, early morning levels of ACTH and cortisol were now in the normal range. The patient discontinued testosterone treatment, but the symptoms returned, so he resumed it.
Long COVID symptoms, possible biological mechanism
The present case shows how certain COVID-19–associated conditions develop after the onset of, or the recovery from, respiratory disorders, the authors note.
Symptoms of long COVID-19 include fatigue, weakness, hair loss, diarrhea, arthralgia, and depression, and these symptoms are associated with pituitary insufficiency, especially secondary adrenocortical insufficiency.
In addition, an estimated 25% of sexually active men who recover from COVID have semen disorders such as azoospermia and oligospermia.
The underlying mechanism by which COVID-19 might trigger pituitary insufficiency is unknown, but other viral infections such as influenza-A and herpes simplex are also associated with transient hypopituitarism. An exaggerated immune response triggered by SARS-CoV-2 may explain the dysfunction of multiple endocrine organs, the researchers write.
The researchers have declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
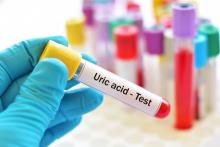
Low urate limits for gout questioned in study
Lower limits on serum urate levels applied in gout management may be based on a misreading of data on mortality risks, researchers say.
Low urate levels may not in themselves pose a risk of death but may be a sign of some other illness, said Joshua F. Baker, MD, MSCE, associate professor of rheumatology and epidemiology at the University of Pennsylvania in Philadelphia.
“It points us towards being more reassured that we can be aggressive in treating gout without a concern about long-term effects for our patients,” he said in an interview. He and colleagues published their findings online in Arthritis & Rheumatology.
Previous research has linked high levels of urate with excessive fat and low levels of urate with loss of skeletal muscle mass. And epidemiologic studies have shown a U-shaped relationship between urate levels and mortality, suggesting that very high and very low levels of urate could be harmful.
Based on this correlation, and the theory that urate could have antioxidant benefits, some professional societies have recommended not lowering urate levels below a defined threshold when treating gout. The European Alliance of Associations for Rheumatology has recommended a lower limit of 3 mg/dL.
But the evidence doesn’t entirely support this caution. For example, in a clinical trial of pegloticase (Krystexxa) in patients with refractory gout, patients whose mean serum urate dropped below 2 mg/dL did not die in higher proportions than patients with higher urate levels.
To better understand the risk of low urate, Dr. Baker and colleagues analyzed data on 13,979 participants in the National Health and Nutrition Examination Survey (NHANES) during 1999-2006. The dataset included whole-body dual-energy x-ray absorptiometry (DXA) body composition measures as well as urate levels.
The researchers argue this measurement reveals more about a person’s overall health than body mass index (BMI), which doesn’t distinguish between mass from fat and mass from muscle.
They defined low lean body mass, or sarcopenia, as an appendicular lean mass index relative to fat mass index z score of –1. And they defined low urate as less than 2.5 mg/dL in women and less than 3.5 mg/dL in men.
They found that 29% of people with low urate had low lean body mass, compared with 16% of people with normal urate levels. The difference was statistically significant (P = .001).
They found an association between low urate and increased mortality (hazard ratio, 1.61; 95% confidence interval, 1.14-2.28; P = .008). But that association lost its statistical significance when the researchers adjusted for body composition and weight loss (HR, 1.30; 95% CI, 0.92-1.85; P = .13).
Dr. Baker thinks the association between elevated mortality and low urate can be explained by conditions such as cancer or lung inflammation that might on one hand increase the risk of death and on the other hand lower urate levels by lowering muscle mass. “Low uric acid levels are observed in people who have lost weight for unhealthy reasons, and that can explain relationships with long-term outcomes,” he said.
Proportions of muscle and fat could not account for the risk of mortality associated with high levels of urate, the researchers found. Those participants with urate levels above 5.7 mg/dL had a higher risk of death with higher levels of urate, and this persisted even after statistical adjustment for body composition.
The study sheds light on an important area of controversy, said Mehdi Fini, MD, of the department of medicine at the University of Colorado at Denver, Aurora, who was not involved in the research.
But body composition does not entirely explain the relationship between urate and mortality, he told this news organization. Medications used to lower urate can cause side effects that might increase mortality, he said.
Also, he said, it’s important to understand the role of comorbidities. He cited evidence that low urate is associated with renal, cardiovascular, and pulmonary conditions. Safe levels of urate might differ depending on these factors. So rather than applying the same target serum level to all patients, perhaps researchers should investigate whether lowering urate by a percentage of the patient’s current level is safer and more effective, he suggested.
He agreed with an editorial that also appeared in Arthritis & Rheumatology saying that there is no evidence for a benefit in lowering urate much below 5 mg/dL. “No matter what, I think we should just be careful,” Dr. Fini said.
Dr. Fini and Dr. Baker report no relevant financial relationships. Dr. Baker acknowledged support from a VA Clinical Science Research & Development Merit Award and a Rehabilitation R&D Merit Award.
A version of this article first appeared on Medscape.com.
Lower limits on serum urate levels applied in gout management may be based on a misreading of data on mortality risks, researchers say.
Low urate levels may not in themselves pose a risk of death but may be a sign of some other illness, said Joshua F. Baker, MD, MSCE, associate professor of rheumatology and epidemiology at the University of Pennsylvania in Philadelphia.
“It points us towards being more reassured that we can be aggressive in treating gout without a concern about long-term effects for our patients,” he said in an interview. He and colleagues published their findings online in Arthritis & Rheumatology.
Previous research has linked high levels of urate with excessive fat and low levels of urate with loss of skeletal muscle mass. And epidemiologic studies have shown a U-shaped relationship between urate levels and mortality, suggesting that very high and very low levels of urate could be harmful.
Based on this correlation, and the theory that urate could have antioxidant benefits, some professional societies have recommended not lowering urate levels below a defined threshold when treating gout. The European Alliance of Associations for Rheumatology has recommended a lower limit of 3 mg/dL.
But the evidence doesn’t entirely support this caution. For example, in a clinical trial of pegloticase (Krystexxa) in patients with refractory gout, patients whose mean serum urate dropped below 2 mg/dL did not die in higher proportions than patients with higher urate levels.
To better understand the risk of low urate, Dr. Baker and colleagues analyzed data on 13,979 participants in the National Health and Nutrition Examination Survey (NHANES) during 1999-2006. The dataset included whole-body dual-energy x-ray absorptiometry (DXA) body composition measures as well as urate levels.
The researchers argue this measurement reveals more about a person’s overall health than body mass index (BMI), which doesn’t distinguish between mass from fat and mass from muscle.
They defined low lean body mass, or sarcopenia, as an appendicular lean mass index relative to fat mass index z score of –1. And they defined low urate as less than 2.5 mg/dL in women and less than 3.5 mg/dL in men.
They found that 29% of people with low urate had low lean body mass, compared with 16% of people with normal urate levels. The difference was statistically significant (P = .001).
They found an association between low urate and increased mortality (hazard ratio, 1.61; 95% confidence interval, 1.14-2.28; P = .008). But that association lost its statistical significance when the researchers adjusted for body composition and weight loss (HR, 1.30; 95% CI, 0.92-1.85; P = .13).
Dr. Baker thinks the association between elevated mortality and low urate can be explained by conditions such as cancer or lung inflammation that might on one hand increase the risk of death and on the other hand lower urate levels by lowering muscle mass. “Low uric acid levels are observed in people who have lost weight for unhealthy reasons, and that can explain relationships with long-term outcomes,” he said.
Proportions of muscle and fat could not account for the risk of mortality associated with high levels of urate, the researchers found. Those participants with urate levels above 5.7 mg/dL had a higher risk of death with higher levels of urate, and this persisted even after statistical adjustment for body composition.
The study sheds light on an important area of controversy, said Mehdi Fini, MD, of the department of medicine at the University of Colorado at Denver, Aurora, who was not involved in the research.
But body composition does not entirely explain the relationship between urate and mortality, he told this news organization. Medications used to lower urate can cause side effects that might increase mortality, he said.
Also, he said, it’s important to understand the role of comorbidities. He cited evidence that low urate is associated with renal, cardiovascular, and pulmonary conditions. Safe levels of urate might differ depending on these factors. So rather than applying the same target serum level to all patients, perhaps researchers should investigate whether lowering urate by a percentage of the patient’s current level is safer and more effective, he suggested.
He agreed with an editorial that also appeared in Arthritis & Rheumatology saying that there is no evidence for a benefit in lowering urate much below 5 mg/dL. “No matter what, I think we should just be careful,” Dr. Fini said.
Dr. Fini and Dr. Baker report no relevant financial relationships. Dr. Baker acknowledged support from a VA Clinical Science Research & Development Merit Award and a Rehabilitation R&D Merit Award.
A version of this article first appeared on Medscape.com.
Lower limits on serum urate levels applied in gout management may be based on a misreading of data on mortality risks, researchers say.
Low urate levels may not in themselves pose a risk of death but may be a sign of some other illness, said Joshua F. Baker, MD, MSCE, associate professor of rheumatology and epidemiology at the University of Pennsylvania in Philadelphia.
“It points us towards being more reassured that we can be aggressive in treating gout without a concern about long-term effects for our patients,” he said in an interview. He and colleagues published their findings online in Arthritis & Rheumatology.
Previous research has linked high levels of urate with excessive fat and low levels of urate with loss of skeletal muscle mass. And epidemiologic studies have shown a U-shaped relationship between urate levels and mortality, suggesting that very high and very low levels of urate could be harmful.
Based on this correlation, and the theory that urate could have antioxidant benefits, some professional societies have recommended not lowering urate levels below a defined threshold when treating gout. The European Alliance of Associations for Rheumatology has recommended a lower limit of 3 mg/dL.
But the evidence doesn’t entirely support this caution. For example, in a clinical trial of pegloticase (Krystexxa) in patients with refractory gout, patients whose mean serum urate dropped below 2 mg/dL did not die in higher proportions than patients with higher urate levels.
To better understand the risk of low urate, Dr. Baker and colleagues analyzed data on 13,979 participants in the National Health and Nutrition Examination Survey (NHANES) during 1999-2006. The dataset included whole-body dual-energy x-ray absorptiometry (DXA) body composition measures as well as urate levels.
The researchers argue this measurement reveals more about a person’s overall health than body mass index (BMI), which doesn’t distinguish between mass from fat and mass from muscle.
They defined low lean body mass, or sarcopenia, as an appendicular lean mass index relative to fat mass index z score of –1. And they defined low urate as less than 2.5 mg/dL in women and less than 3.5 mg/dL in men.
They found that 29% of people with low urate had low lean body mass, compared with 16% of people with normal urate levels. The difference was statistically significant (P = .001).
They found an association between low urate and increased mortality (hazard ratio, 1.61; 95% confidence interval, 1.14-2.28; P = .008). But that association lost its statistical significance when the researchers adjusted for body composition and weight loss (HR, 1.30; 95% CI, 0.92-1.85; P = .13).
Dr. Baker thinks the association between elevated mortality and low urate can be explained by conditions such as cancer or lung inflammation that might on one hand increase the risk of death and on the other hand lower urate levels by lowering muscle mass. “Low uric acid levels are observed in people who have lost weight for unhealthy reasons, and that can explain relationships with long-term outcomes,” he said.
Proportions of muscle and fat could not account for the risk of mortality associated with high levels of urate, the researchers found. Those participants with urate levels above 5.7 mg/dL had a higher risk of death with higher levels of urate, and this persisted even after statistical adjustment for body composition.
The study sheds light on an important area of controversy, said Mehdi Fini, MD, of the department of medicine at the University of Colorado at Denver, Aurora, who was not involved in the research.
But body composition does not entirely explain the relationship between urate and mortality, he told this news organization. Medications used to lower urate can cause side effects that might increase mortality, he said.
Also, he said, it’s important to understand the role of comorbidities. He cited evidence that low urate is associated with renal, cardiovascular, and pulmonary conditions. Safe levels of urate might differ depending on these factors. So rather than applying the same target serum level to all patients, perhaps researchers should investigate whether lowering urate by a percentage of the patient’s current level is safer and more effective, he suggested.
He agreed with an editorial that also appeared in Arthritis & Rheumatology saying that there is no evidence for a benefit in lowering urate much below 5 mg/dL. “No matter what, I think we should just be careful,” Dr. Fini said.
Dr. Fini and Dr. Baker report no relevant financial relationships. Dr. Baker acknowledged support from a VA Clinical Science Research & Development Merit Award and a Rehabilitation R&D Merit Award.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Large study amplifies evidence of COVID vaccine safety in pregnancy
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
FROM BMJ
Exercise limitations in COPD – not everyone needs more inhalers
Chronic obstructive pulmonary disease (COPD) is defined by airway obstruction and alveolar damage caused by exposure to noxious air particles. The physiologic results include varying degrees of gas-exchange abnormality and mechanical respiratory limitation, often in the form of dynamic hyperinflation. There’s a third major contributor, though – skeletal muscle deconditioning. Only one of these abnormalities responds to inhalers.
When your patients with COPD report dyspnea or exercise intolerance, what do you do? Do you attempt to determine its character to pinpoint its origin? Do you quiz them about their baseline activity levels to quantify their conditioning? I bet you get right to the point and order a cardiopulmonary exercise test (CPET). That way you’ll be able to tease out all the contributors. Nah. Most likely you add an inhaler before continuing to rush through your COPD quality metrics: Vaccines? Check. Lung cancer screening? Check. Smoking cessation? Check.
The physiology of dyspnea and exercise limitation in COPD has been extensively studied. Work-of-breathing, dynamic hyperinflation, and gas-exchange inefficiencies interact with each other in complex ways to produce symptoms. The presence of deconditioning simply magnifies the existing abnormalities within the respiratory system by creating more strain at lower work rates. Acute exacerbations (AECOPD) and oral corticosteroids further aggravate skeletal muscle dysfunction.
The Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) Report directs clinicians to use inhalers to manage dyspnea. If they’re already on one inhaler, they get another. This continues until they’re stabilized on a long-acting beta-agonist (LABA), long-acting muscarinic antagonist (LAMA), and an inhaled corticosteroid (ICS). The GOLD report also advises pulmonary rehabilitation for any patient with grade B through D disease. Unfortunately, the pulmonary rehabilitation recommendation is buried in the text and doesn’t appear within the popularized pharmacologic algorithms in the report’s figures.
The data for adding inhalers on top of each other to reduce AECOPD and improve overall quality of life (QOL) are good. However, although GOLD tells us to keep adding inhalers for the dyspneic patient with COPD, the authors acknowledge that this hasn’t been systematically tested. The difference? A statement doesn’t require the same formal, rigorous scientific analysis known as the GRADE approach. Using this kind of analysis, a recent clinical practice guideline by the American Thoracic Society found no benefit in dyspnea or respiratory QOL with step-up from inhaler monotherapy.
Inhalers won’t do anything for gas-exchange inefficiencies and deconditioning, at least not directly. A recent CPET study from the CanCOLD network found ventilatory inefficiency in 23% of GOLD 1 and 26% of GOLD 2-4 COPD patients. The numbers were higher for those who reported dyspnea. Skeletal muscle dysfunction rates are equally high.
Thus, dyspnea and exercise intolerance are major determinants of QOL in COPD, but inhalers will only get you so far. At a minimum, make sure you get an activity/exercise history from your patients with COPD. For those who are sedentary, provide an exercise prescription (really, it’s not that hard to do). If dyspnea persists despite LABA or LAMA monotherapy, clarify the complaint before doubling down. Finally, try to get the patient into a good pulmonary rehabilitation program. They’ll thank you afterwards.
Dr. Holley is Associate Professor, department of medicine, Uniformed Services University of the Health Sciences and Program Director, Pulmonary and Critical Care Medical Fellowship, department of medicine, Walter Reed National Military Medical Center, both in Bethesda, Md. He reported receiving research grants from Fisher-Paykel and receiving income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
Chronic obstructive pulmonary disease (COPD) is defined by airway obstruction and alveolar damage caused by exposure to noxious air particles. The physiologic results include varying degrees of gas-exchange abnormality and mechanical respiratory limitation, often in the form of dynamic hyperinflation. There’s a third major contributor, though – skeletal muscle deconditioning. Only one of these abnormalities responds to inhalers.
When your patients with COPD report dyspnea or exercise intolerance, what do you do? Do you attempt to determine its character to pinpoint its origin? Do you quiz them about their baseline activity levels to quantify their conditioning? I bet you get right to the point and order a cardiopulmonary exercise test (CPET). That way you’ll be able to tease out all the contributors. Nah. Most likely you add an inhaler before continuing to rush through your COPD quality metrics: Vaccines? Check. Lung cancer screening? Check. Smoking cessation? Check.
The physiology of dyspnea and exercise limitation in COPD has been extensively studied. Work-of-breathing, dynamic hyperinflation, and gas-exchange inefficiencies interact with each other in complex ways to produce symptoms. The presence of deconditioning simply magnifies the existing abnormalities within the respiratory system by creating more strain at lower work rates. Acute exacerbations (AECOPD) and oral corticosteroids further aggravate skeletal muscle dysfunction.
The Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) Report directs clinicians to use inhalers to manage dyspnea. If they’re already on one inhaler, they get another. This continues until they’re stabilized on a long-acting beta-agonist (LABA), long-acting muscarinic antagonist (LAMA), and an inhaled corticosteroid (ICS). The GOLD report also advises pulmonary rehabilitation for any patient with grade B through D disease. Unfortunately, the pulmonary rehabilitation recommendation is buried in the text and doesn’t appear within the popularized pharmacologic algorithms in the report’s figures.
The data for adding inhalers on top of each other to reduce AECOPD and improve overall quality of life (QOL) are good. However, although GOLD tells us to keep adding inhalers for the dyspneic patient with COPD, the authors acknowledge that this hasn’t been systematically tested. The difference? A statement doesn’t require the same formal, rigorous scientific analysis known as the GRADE approach. Using this kind of analysis, a recent clinical practice guideline by the American Thoracic Society found no benefit in dyspnea or respiratory QOL with step-up from inhaler monotherapy.
Inhalers won’t do anything for gas-exchange inefficiencies and deconditioning, at least not directly. A recent CPET study from the CanCOLD network found ventilatory inefficiency in 23% of GOLD 1 and 26% of GOLD 2-4 COPD patients. The numbers were higher for those who reported dyspnea. Skeletal muscle dysfunction rates are equally high.
Thus, dyspnea and exercise intolerance are major determinants of QOL in COPD, but inhalers will only get you so far. At a minimum, make sure you get an activity/exercise history from your patients with COPD. For those who are sedentary, provide an exercise prescription (really, it’s not that hard to do). If dyspnea persists despite LABA or LAMA monotherapy, clarify the complaint before doubling down. Finally, try to get the patient into a good pulmonary rehabilitation program. They’ll thank you afterwards.
Dr. Holley is Associate Professor, department of medicine, Uniformed Services University of the Health Sciences and Program Director, Pulmonary and Critical Care Medical Fellowship, department of medicine, Walter Reed National Military Medical Center, both in Bethesda, Md. He reported receiving research grants from Fisher-Paykel and receiving income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
Chronic obstructive pulmonary disease (COPD) is defined by airway obstruction and alveolar damage caused by exposure to noxious air particles. The physiologic results include varying degrees of gas-exchange abnormality and mechanical respiratory limitation, often in the form of dynamic hyperinflation. There’s a third major contributor, though – skeletal muscle deconditioning. Only one of these abnormalities responds to inhalers.
When your patients with COPD report dyspnea or exercise intolerance, what do you do? Do you attempt to determine its character to pinpoint its origin? Do you quiz them about their baseline activity levels to quantify their conditioning? I bet you get right to the point and order a cardiopulmonary exercise test (CPET). That way you’ll be able to tease out all the contributors. Nah. Most likely you add an inhaler before continuing to rush through your COPD quality metrics: Vaccines? Check. Lung cancer screening? Check. Smoking cessation? Check.
The physiology of dyspnea and exercise limitation in COPD has been extensively studied. Work-of-breathing, dynamic hyperinflation, and gas-exchange inefficiencies interact with each other in complex ways to produce symptoms. The presence of deconditioning simply magnifies the existing abnormalities within the respiratory system by creating more strain at lower work rates. Acute exacerbations (AECOPD) and oral corticosteroids further aggravate skeletal muscle dysfunction.
The Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (GOLD) Report directs clinicians to use inhalers to manage dyspnea. If they’re already on one inhaler, they get another. This continues until they’re stabilized on a long-acting beta-agonist (LABA), long-acting muscarinic antagonist (LAMA), and an inhaled corticosteroid (ICS). The GOLD report also advises pulmonary rehabilitation for any patient with grade B through D disease. Unfortunately, the pulmonary rehabilitation recommendation is buried in the text and doesn’t appear within the popularized pharmacologic algorithms in the report’s figures.
The data for adding inhalers on top of each other to reduce AECOPD and improve overall quality of life (QOL) are good. However, although GOLD tells us to keep adding inhalers for the dyspneic patient with COPD, the authors acknowledge that this hasn’t been systematically tested. The difference? A statement doesn’t require the same formal, rigorous scientific analysis known as the GRADE approach. Using this kind of analysis, a recent clinical practice guideline by the American Thoracic Society found no benefit in dyspnea or respiratory QOL with step-up from inhaler monotherapy.
Inhalers won’t do anything for gas-exchange inefficiencies and deconditioning, at least not directly. A recent CPET study from the CanCOLD network found ventilatory inefficiency in 23% of GOLD 1 and 26% of GOLD 2-4 COPD patients. The numbers were higher for those who reported dyspnea. Skeletal muscle dysfunction rates are equally high.
Thus, dyspnea and exercise intolerance are major determinants of QOL in COPD, but inhalers will only get you so far. At a minimum, make sure you get an activity/exercise history from your patients with COPD. For those who are sedentary, provide an exercise prescription (really, it’s not that hard to do). If dyspnea persists despite LABA or LAMA monotherapy, clarify the complaint before doubling down. Finally, try to get the patient into a good pulmonary rehabilitation program. They’ll thank you afterwards.
Dr. Holley is Associate Professor, department of medicine, Uniformed Services University of the Health Sciences and Program Director, Pulmonary and Critical Care Medical Fellowship, department of medicine, Walter Reed National Military Medical Center, both in Bethesda, Md. He reported receiving research grants from Fisher-Paykel and receiving income from the American College of Chest Physicians.
A version of this article first appeared on Medscape.com.
‘Stop pretending’ there’s a magic formula to weight loss
Is there a diet or weight-loss program out there that doesn’t work for those who stick with it during its first 12 weeks?
Truly, the world’s most backwards, upside-down, anti-science, nonsensical diets work over the short haul, fueled by the fact that short-term suffering for weight loss is a skill set that humanity has assiduously cultivated for at least the past 100 years. We’re really good at it!
It’s the keeping the weight off, though, that’s the hitch. Which leads me to the question, why are medical journals, even preeminent nonpredatory ones, publishing 12-week weight-loss program studies as if they have value? And does anyone truly imagine that after over 100 years of trying, there’ll be a short-term diet or program that’ll have the durable, reproducible results that no other short-term diet or program ever has?
Take this study published by Obesity: “Pragmatic implementation of a fully automated online obesity treatment in primary care.” It details a 12-week online, automated, weight-loss program that led completers to lose the roughly 5% of weight that many diets and programs see lost over their first 12 weeks. By its description, aside from its automated provision, the program sounds like pretty much the same boilerplate weight management advice and recommendations that haven’t been shown to lead large numbers of people to sustain long-term weight loss.
Participants were provided with weekly lessons which no doubt in some manner told them that high-calorie foods had high numbers of calories and should be minimized, along with other weight-loss secrets. Users were to upload weekly self-monitored weight, energy intake, and exercise minutes and were told to use a food diary. Their goal was losing 10% of their body weight by consuming 1,200-1,500 calories per day if they weighed less than 250 pounds (113 kg) and 1,500-1,800 calories if they weighed more than 250 pounds, while also telling them to aim for 200 minutes per week of moderate- to vigorous-intensity physical activity.
What was found was wholly unsurprising. Perhaps speaking to the tremendous and wide-ranging degrees of privilege that are required to prioritize intentional behavior change in the name of health, 79% of those who were given a prescription for the program either didn’t start it or stopped it before the end of the first week.
Of those who actually started the program and completed more than 1 week, despite having been selected as appropriate and interested participants by their physicians, only 20% watched all of the automated programs’ video lessons while only 32% actually bothered to submit all 12 weeks of weight data. Of course, the authors found that those who watched the greatest number of videos and submitted the most self-reported weights lost more weight and ascribed that loss to the program. What the authors did not entertain was the possibility that those who weren’t losing weight, or who were gaining, might simply be less inclined to continue with a program that wasn’t leading them to their desired outcomes or to want to submit their lack of loss or gains.
Short-term weight-loss studies help no one and when, as in this case, the outcomes aren’t even mediocre, and the completion and engagement rates are terrible, the study is still presented as significant and important. This bolsters the harmful stereotype that weight management is achievable by way of simple messages and generic goals. It suggests that it’s individuals who fail programs by not trying hard enough and that those who do, or who want it the most, will succeed. It may also lead patients and clinicians to second-guess the use of antiobesity medications, the current generation of which lead to far greater weight loss and reproducibility than any behavioral program or diet ever has.
The good news here at least is that the small percentage of participants who made it through this program’s 12 weeks are being randomly assigned to differing 9-month maintenance programs which at least will then lead to a 1-year analysis on the completers.
Why this study was published now, rather than pushed until the 1-year data were available, speaks to the pervasiveness of the toxic weight-biased notion that simple education will overcome the physiology forged over millions of years of extreme dietary insecurity.
Our food environment is a veritable floodplain of hyperpalatable foods, and social determinants of health make intentional behavior change in the name of health an unattainable luxury for a huge swath of the population.
Dr. Freedhoff is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute. He reported serving as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health and receiving research grants from Novo Nordisk. A version of this article first appeared on Medscape.com.
Is there a diet or weight-loss program out there that doesn’t work for those who stick with it during its first 12 weeks?
Truly, the world’s most backwards, upside-down, anti-science, nonsensical diets work over the short haul, fueled by the fact that short-term suffering for weight loss is a skill set that humanity has assiduously cultivated for at least the past 100 years. We’re really good at it!
It’s the keeping the weight off, though, that’s the hitch. Which leads me to the question, why are medical journals, even preeminent nonpredatory ones, publishing 12-week weight-loss program studies as if they have value? And does anyone truly imagine that after over 100 years of trying, there’ll be a short-term diet or program that’ll have the durable, reproducible results that no other short-term diet or program ever has?
Take this study published by Obesity: “Pragmatic implementation of a fully automated online obesity treatment in primary care.” It details a 12-week online, automated, weight-loss program that led completers to lose the roughly 5% of weight that many diets and programs see lost over their first 12 weeks. By its description, aside from its automated provision, the program sounds like pretty much the same boilerplate weight management advice and recommendations that haven’t been shown to lead large numbers of people to sustain long-term weight loss.
Participants were provided with weekly lessons which no doubt in some manner told them that high-calorie foods had high numbers of calories and should be minimized, along with other weight-loss secrets. Users were to upload weekly self-monitored weight, energy intake, and exercise minutes and were told to use a food diary. Their goal was losing 10% of their body weight by consuming 1,200-1,500 calories per day if they weighed less than 250 pounds (113 kg) and 1,500-1,800 calories if they weighed more than 250 pounds, while also telling them to aim for 200 minutes per week of moderate- to vigorous-intensity physical activity.
What was found was wholly unsurprising. Perhaps speaking to the tremendous and wide-ranging degrees of privilege that are required to prioritize intentional behavior change in the name of health, 79% of those who were given a prescription for the program either didn’t start it or stopped it before the end of the first week.
Of those who actually started the program and completed more than 1 week, despite having been selected as appropriate and interested participants by their physicians, only 20% watched all of the automated programs’ video lessons while only 32% actually bothered to submit all 12 weeks of weight data. Of course, the authors found that those who watched the greatest number of videos and submitted the most self-reported weights lost more weight and ascribed that loss to the program. What the authors did not entertain was the possibility that those who weren’t losing weight, or who were gaining, might simply be less inclined to continue with a program that wasn’t leading them to their desired outcomes or to want to submit their lack of loss or gains.
Short-term weight-loss studies help no one and when, as in this case, the outcomes aren’t even mediocre, and the completion and engagement rates are terrible, the study is still presented as significant and important. This bolsters the harmful stereotype that weight management is achievable by way of simple messages and generic goals. It suggests that it’s individuals who fail programs by not trying hard enough and that those who do, or who want it the most, will succeed. It may also lead patients and clinicians to second-guess the use of antiobesity medications, the current generation of which lead to far greater weight loss and reproducibility than any behavioral program or diet ever has.
The good news here at least is that the small percentage of participants who made it through this program’s 12 weeks are being randomly assigned to differing 9-month maintenance programs which at least will then lead to a 1-year analysis on the completers.
Why this study was published now, rather than pushed until the 1-year data were available, speaks to the pervasiveness of the toxic weight-biased notion that simple education will overcome the physiology forged over millions of years of extreme dietary insecurity.
Our food environment is a veritable floodplain of hyperpalatable foods, and social determinants of health make intentional behavior change in the name of health an unattainable luxury for a huge swath of the population.
Dr. Freedhoff is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute. He reported serving as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health and receiving research grants from Novo Nordisk. A version of this article first appeared on Medscape.com.
Is there a diet or weight-loss program out there that doesn’t work for those who stick with it during its first 12 weeks?
Truly, the world’s most backwards, upside-down, anti-science, nonsensical diets work over the short haul, fueled by the fact that short-term suffering for weight loss is a skill set that humanity has assiduously cultivated for at least the past 100 years. We’re really good at it!
It’s the keeping the weight off, though, that’s the hitch. Which leads me to the question, why are medical journals, even preeminent nonpredatory ones, publishing 12-week weight-loss program studies as if they have value? And does anyone truly imagine that after over 100 years of trying, there’ll be a short-term diet or program that’ll have the durable, reproducible results that no other short-term diet or program ever has?
Take this study published by Obesity: “Pragmatic implementation of a fully automated online obesity treatment in primary care.” It details a 12-week online, automated, weight-loss program that led completers to lose the roughly 5% of weight that many diets and programs see lost over their first 12 weeks. By its description, aside from its automated provision, the program sounds like pretty much the same boilerplate weight management advice and recommendations that haven’t been shown to lead large numbers of people to sustain long-term weight loss.
Participants were provided with weekly lessons which no doubt in some manner told them that high-calorie foods had high numbers of calories and should be minimized, along with other weight-loss secrets. Users were to upload weekly self-monitored weight, energy intake, and exercise minutes and were told to use a food diary. Their goal was losing 10% of their body weight by consuming 1,200-1,500 calories per day if they weighed less than 250 pounds (113 kg) and 1,500-1,800 calories if they weighed more than 250 pounds, while also telling them to aim for 200 minutes per week of moderate- to vigorous-intensity physical activity.
What was found was wholly unsurprising. Perhaps speaking to the tremendous and wide-ranging degrees of privilege that are required to prioritize intentional behavior change in the name of health, 79% of those who were given a prescription for the program either didn’t start it or stopped it before the end of the first week.
Of those who actually started the program and completed more than 1 week, despite having been selected as appropriate and interested participants by their physicians, only 20% watched all of the automated programs’ video lessons while only 32% actually bothered to submit all 12 weeks of weight data. Of course, the authors found that those who watched the greatest number of videos and submitted the most self-reported weights lost more weight and ascribed that loss to the program. What the authors did not entertain was the possibility that those who weren’t losing weight, or who were gaining, might simply be less inclined to continue with a program that wasn’t leading them to their desired outcomes or to want to submit their lack of loss or gains.
Short-term weight-loss studies help no one and when, as in this case, the outcomes aren’t even mediocre, and the completion and engagement rates are terrible, the study is still presented as significant and important. This bolsters the harmful stereotype that weight management is achievable by way of simple messages and generic goals. It suggests that it’s individuals who fail programs by not trying hard enough and that those who do, or who want it the most, will succeed. It may also lead patients and clinicians to second-guess the use of antiobesity medications, the current generation of which lead to far greater weight loss and reproducibility than any behavioral program or diet ever has.
The good news here at least is that the small percentage of participants who made it through this program’s 12 weeks are being randomly assigned to differing 9-month maintenance programs which at least will then lead to a 1-year analysis on the completers.
Why this study was published now, rather than pushed until the 1-year data were available, speaks to the pervasiveness of the toxic weight-biased notion that simple education will overcome the physiology forged over millions of years of extreme dietary insecurity.
Our food environment is a veritable floodplain of hyperpalatable foods, and social determinants of health make intentional behavior change in the name of health an unattainable luxury for a huge swath of the population.
Dr. Freedhoff is an associate professor of family medicine at the University of Ottawa and medical director of the Bariatric Medical Institute. He reported serving as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health and receiving research grants from Novo Nordisk. A version of this article first appeared on Medscape.com.
Ultrasound helps predict gout flares over the next year
Adding ultrasound (US) to the clinical exam helps predict the likelihood of future gout flares, results of a prospective, observational study conducted in Italy suggest.
“Baseline US findings indicative of MSU [monosodium urate] burden and US-detected inflammation are independent predictors of gout flares over 12 months,” lead author Edoardo Cipolletta, MD, of the rheumatology unit, department of clinical and molecular sciences at Marche Polytechnic University in Ancona, Italy, and colleagues wrote in Rheumatology.
“We demonstrated that US findings provided an additional value over clinical data in estimating the risk of flares. Moreover, we reported an association between US findings at a joint and the occurrence of gout flares at the same joint,” they added.
Predicting risk of flares and reducing their occurrence are two main challenges in managing gout, the authors wrote. US can be used to scan multiple joints and is widely used in Europe as a low-cost, radiation-free imaging tool that’s easily integrated into clinical practice.
To investigate whether US can predict gout flares, the researchers enrolled 81 consecutive adult patients with gout in the study between April 2019 and March 2021 at one academic rheumatology treatment site in Italy and followed them for 12 months. The authors compared cases (who developed at least one flare within 12 months of the baseline visit) with controls (who self-reported no gout flares over that period).
Patients diagnosed with other inflammatory arthritis and those with coexisting calcium pyrophosphate deposition disease were excluded from the study.
The 71 participants who completed the study were, on average, in their early 60s, and in both groups, all but one were male. At the baseline visit, all had been on stable urate-lowering therapy for at least 6 months and had not had any gout flares in 4 weeks. The mean gout duration was 7 years in the case group and 8 years in controls.
At baseline, all participants underwent physical examination and US of elbows, wrists, second metacarpophalangeal joints, knees, ankles, and first metatarsophalangeal joints by a member of the research team who was blinded to the clinical and laboratory data.
Clinical assessments were scheduled at baseline and at 6-month intervals, and all participants were evaluated by a second researcher who was blinded to US findings.
During follow-up visits, participants were asked to report any gout flare, considered to meet at least three of four criteria: patient-defined flare, pain at rest score higher than 3 on a 0-10 scale, at least one swollen joint, and at least one warm joint. Patients not reaching their target serum urate goal received escalated urate-lowering therapy dosage and anti-inflammatory prophylaxis.
The US indicators of MSU deposits – aggregates, double contour sign, and tophi – were recorded as present or absent. The power Doppler signal was scored from 0 through 4, and summated scores for each US finding were calculated.
Over 12 months, the researchers found:
- Thirty (42.3%) patients had at least one flare, with a median of 2.0 flares. Patients with flares had higher a US median total MSU score (5.0 vs. 2.0; P = .01) and power Doppler signal (3.0 vs. 0; P < .01) than controls.
- In multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the occurrence of flares. The adjusted odds ratio for total MSU score was 1.75 (95% confidence interval, 1.26-2.43) and for power Doppler score was 1.63 (95% CI, 1.12-2.40).
- Also in a multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the number of flares. The incidence risk ratio for total MSU score adjusted was 1.17 (95% CI, 1.08-1.26) and for power Doppler score was 1.29 (95% CI, 1.19-1.40).
Four rheumatologists welcome findings
Gout remains the most common cause of inflammatory arthritis and a significant reason for hospital visits, noted Narender Annapureddy, MD, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn..
“The study adds to the growing utility of musculoskeletal ultrasound in rheumatology practices to treat various diseases,” he said. “Data that could provide risk prediction for gout flares would be associated with significant benefits in terms of reducing ED visits, hospital admission, and lost work productivity.”
One study limitation, Dr. Annapureddy mentioned, was the single experienced US reader, “which may limit generalizability of results at this time, at least in the United States.”
Yeohan Song, MD, an instructor at Ohio State University Wexner Medical Center, Columbus, integrates US into his practice.
“In gout management, musculoskeletal ultrasound is a useful adjunct to the clinical exam and laboratory markers, particularly [in patients] with recurrent flares despite guideline-directed target serum urate levels,” he said.
Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, pointed out that the US protocol in the study involved imaging knees, ankles, first metatarsophalangeal joints, elbows, wrists, and second metacarpophalangeal joints, and took around 30 minutes to complete.
“That would not be practical in the United States due to time constraints in most rheumatology clinics,” she said.
“The authors report that a ‘reduced scanning protocol’ of the bilateral knees, ankles, and first metatarsophalangeal joints demonstrated similar predictive ability as the full protocol,” she added, “although scanning six joints still might not be feasible during a typical return patient clinic visit in the United States.”
Philip Chu, MD, clinical associate at Duke University, Durham, N.C., uses diagnostic US to help differentiate borderline gout cases from other arthropathies.
“A baseline scan, a follow-up scan before deciding to stop prophylaxis, or a follow-up scan in the setting of recurrent gout flares despite reaching goal serum uric acid, may be cost-effective time points to perform diagnostic US,” he advised.
“Unfortunately,” he added, “reimbursement for diagnostic US has been decreasing over the years, which makes it challenging to increase diagnostic US to the [frequency of its use] in Europe.”
Asked how most gout care being provided by primary care doctors in the United States affects gout management, Dr. Chu said: “Depending on which guidelines one follows for treating gout – from the American College of Rheumatology or the American College of Physicians – one may be more or less likely to start urate-lowering therapy after the first gout flare.”
“Understanding MSU burden in each patient, or even seeing active inflammation at these sites by increased Doppler signal, may change the threshold for physicians to initiate therapy,” he added.
The study received no funding. Three study authors reported financial involvements with pharmaceutical companies. Dr. Cipolletta, Dr. Annapureddy, Dr. Song, Dr. Tedeschi, and Dr. Chu reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Adding ultrasound (US) to the clinical exam helps predict the likelihood of future gout flares, results of a prospective, observational study conducted in Italy suggest.
“Baseline US findings indicative of MSU [monosodium urate] burden and US-detected inflammation are independent predictors of gout flares over 12 months,” lead author Edoardo Cipolletta, MD, of the rheumatology unit, department of clinical and molecular sciences at Marche Polytechnic University in Ancona, Italy, and colleagues wrote in Rheumatology.
“We demonstrated that US findings provided an additional value over clinical data in estimating the risk of flares. Moreover, we reported an association between US findings at a joint and the occurrence of gout flares at the same joint,” they added.
Predicting risk of flares and reducing their occurrence are two main challenges in managing gout, the authors wrote. US can be used to scan multiple joints and is widely used in Europe as a low-cost, radiation-free imaging tool that’s easily integrated into clinical practice.
To investigate whether US can predict gout flares, the researchers enrolled 81 consecutive adult patients with gout in the study between April 2019 and March 2021 at one academic rheumatology treatment site in Italy and followed them for 12 months. The authors compared cases (who developed at least one flare within 12 months of the baseline visit) with controls (who self-reported no gout flares over that period).
Patients diagnosed with other inflammatory arthritis and those with coexisting calcium pyrophosphate deposition disease were excluded from the study.
The 71 participants who completed the study were, on average, in their early 60s, and in both groups, all but one were male. At the baseline visit, all had been on stable urate-lowering therapy for at least 6 months and had not had any gout flares in 4 weeks. The mean gout duration was 7 years in the case group and 8 years in controls.
At baseline, all participants underwent physical examination and US of elbows, wrists, second metacarpophalangeal joints, knees, ankles, and first metatarsophalangeal joints by a member of the research team who was blinded to the clinical and laboratory data.
Clinical assessments were scheduled at baseline and at 6-month intervals, and all participants were evaluated by a second researcher who was blinded to US findings.
During follow-up visits, participants were asked to report any gout flare, considered to meet at least three of four criteria: patient-defined flare, pain at rest score higher than 3 on a 0-10 scale, at least one swollen joint, and at least one warm joint. Patients not reaching their target serum urate goal received escalated urate-lowering therapy dosage and anti-inflammatory prophylaxis.
The US indicators of MSU deposits – aggregates, double contour sign, and tophi – were recorded as present or absent. The power Doppler signal was scored from 0 through 4, and summated scores for each US finding were calculated.
Over 12 months, the researchers found:
- Thirty (42.3%) patients had at least one flare, with a median of 2.0 flares. Patients with flares had higher a US median total MSU score (5.0 vs. 2.0; P = .01) and power Doppler signal (3.0 vs. 0; P < .01) than controls.
- In multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the occurrence of flares. The adjusted odds ratio for total MSU score was 1.75 (95% confidence interval, 1.26-2.43) and for power Doppler score was 1.63 (95% CI, 1.12-2.40).
- Also in a multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the number of flares. The incidence risk ratio for total MSU score adjusted was 1.17 (95% CI, 1.08-1.26) and for power Doppler score was 1.29 (95% CI, 1.19-1.40).
Four rheumatologists welcome findings
Gout remains the most common cause of inflammatory arthritis and a significant reason for hospital visits, noted Narender Annapureddy, MD, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn..
“The study adds to the growing utility of musculoskeletal ultrasound in rheumatology practices to treat various diseases,” he said. “Data that could provide risk prediction for gout flares would be associated with significant benefits in terms of reducing ED visits, hospital admission, and lost work productivity.”
One study limitation, Dr. Annapureddy mentioned, was the single experienced US reader, “which may limit generalizability of results at this time, at least in the United States.”
Yeohan Song, MD, an instructor at Ohio State University Wexner Medical Center, Columbus, integrates US into his practice.
“In gout management, musculoskeletal ultrasound is a useful adjunct to the clinical exam and laboratory markers, particularly [in patients] with recurrent flares despite guideline-directed target serum urate levels,” he said.
Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, pointed out that the US protocol in the study involved imaging knees, ankles, first metatarsophalangeal joints, elbows, wrists, and second metacarpophalangeal joints, and took around 30 minutes to complete.
“That would not be practical in the United States due to time constraints in most rheumatology clinics,” she said.
“The authors report that a ‘reduced scanning protocol’ of the bilateral knees, ankles, and first metatarsophalangeal joints demonstrated similar predictive ability as the full protocol,” she added, “although scanning six joints still might not be feasible during a typical return patient clinic visit in the United States.”
Philip Chu, MD, clinical associate at Duke University, Durham, N.C., uses diagnostic US to help differentiate borderline gout cases from other arthropathies.
“A baseline scan, a follow-up scan before deciding to stop prophylaxis, or a follow-up scan in the setting of recurrent gout flares despite reaching goal serum uric acid, may be cost-effective time points to perform diagnostic US,” he advised.
“Unfortunately,” he added, “reimbursement for diagnostic US has been decreasing over the years, which makes it challenging to increase diagnostic US to the [frequency of its use] in Europe.”
Asked how most gout care being provided by primary care doctors in the United States affects gout management, Dr. Chu said: “Depending on which guidelines one follows for treating gout – from the American College of Rheumatology or the American College of Physicians – one may be more or less likely to start urate-lowering therapy after the first gout flare.”
“Understanding MSU burden in each patient, or even seeing active inflammation at these sites by increased Doppler signal, may change the threshold for physicians to initiate therapy,” he added.
The study received no funding. Three study authors reported financial involvements with pharmaceutical companies. Dr. Cipolletta, Dr. Annapureddy, Dr. Song, Dr. Tedeschi, and Dr. Chu reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Adding ultrasound (US) to the clinical exam helps predict the likelihood of future gout flares, results of a prospective, observational study conducted in Italy suggest.
“Baseline US findings indicative of MSU [monosodium urate] burden and US-detected inflammation are independent predictors of gout flares over 12 months,” lead author Edoardo Cipolletta, MD, of the rheumatology unit, department of clinical and molecular sciences at Marche Polytechnic University in Ancona, Italy, and colleagues wrote in Rheumatology.
“We demonstrated that US findings provided an additional value over clinical data in estimating the risk of flares. Moreover, we reported an association between US findings at a joint and the occurrence of gout flares at the same joint,” they added.
Predicting risk of flares and reducing their occurrence are two main challenges in managing gout, the authors wrote. US can be used to scan multiple joints and is widely used in Europe as a low-cost, radiation-free imaging tool that’s easily integrated into clinical practice.
To investigate whether US can predict gout flares, the researchers enrolled 81 consecutive adult patients with gout in the study between April 2019 and March 2021 at one academic rheumatology treatment site in Italy and followed them for 12 months. The authors compared cases (who developed at least one flare within 12 months of the baseline visit) with controls (who self-reported no gout flares over that period).
Patients diagnosed with other inflammatory arthritis and those with coexisting calcium pyrophosphate deposition disease were excluded from the study.
The 71 participants who completed the study were, on average, in their early 60s, and in both groups, all but one were male. At the baseline visit, all had been on stable urate-lowering therapy for at least 6 months and had not had any gout flares in 4 weeks. The mean gout duration was 7 years in the case group and 8 years in controls.
At baseline, all participants underwent physical examination and US of elbows, wrists, second metacarpophalangeal joints, knees, ankles, and first metatarsophalangeal joints by a member of the research team who was blinded to the clinical and laboratory data.
Clinical assessments were scheduled at baseline and at 6-month intervals, and all participants were evaluated by a second researcher who was blinded to US findings.
During follow-up visits, participants were asked to report any gout flare, considered to meet at least three of four criteria: patient-defined flare, pain at rest score higher than 3 on a 0-10 scale, at least one swollen joint, and at least one warm joint. Patients not reaching their target serum urate goal received escalated urate-lowering therapy dosage and anti-inflammatory prophylaxis.
The US indicators of MSU deposits – aggregates, double contour sign, and tophi – were recorded as present or absent. The power Doppler signal was scored from 0 through 4, and summated scores for each US finding were calculated.
Over 12 months, the researchers found:
- Thirty (42.3%) patients had at least one flare, with a median of 2.0 flares. Patients with flares had higher a US median total MSU score (5.0 vs. 2.0; P = .01) and power Doppler signal (3.0 vs. 0; P < .01) than controls.
- In multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the occurrence of flares. The adjusted odds ratio for total MSU score was 1.75 (95% confidence interval, 1.26-2.43) and for power Doppler score was 1.63 (95% CI, 1.12-2.40).
- Also in a multivariate analysis, baseline US scores indicating MSU deposits and US-detected inflammation were significantly linked with the number of flares. The incidence risk ratio for total MSU score adjusted was 1.17 (95% CI, 1.08-1.26) and for power Doppler score was 1.29 (95% CI, 1.19-1.40).
Four rheumatologists welcome findings
Gout remains the most common cause of inflammatory arthritis and a significant reason for hospital visits, noted Narender Annapureddy, MD, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn..
“The study adds to the growing utility of musculoskeletal ultrasound in rheumatology practices to treat various diseases,” he said. “Data that could provide risk prediction for gout flares would be associated with significant benefits in terms of reducing ED visits, hospital admission, and lost work productivity.”
One study limitation, Dr. Annapureddy mentioned, was the single experienced US reader, “which may limit generalizability of results at this time, at least in the United States.”
Yeohan Song, MD, an instructor at Ohio State University Wexner Medical Center, Columbus, integrates US into his practice.
“In gout management, musculoskeletal ultrasound is a useful adjunct to the clinical exam and laboratory markers, particularly [in patients] with recurrent flares despite guideline-directed target serum urate levels,” he said.
Sara K. Tedeschi, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, pointed out that the US protocol in the study involved imaging knees, ankles, first metatarsophalangeal joints, elbows, wrists, and second metacarpophalangeal joints, and took around 30 minutes to complete.
“That would not be practical in the United States due to time constraints in most rheumatology clinics,” she said.
“The authors report that a ‘reduced scanning protocol’ of the bilateral knees, ankles, and first metatarsophalangeal joints demonstrated similar predictive ability as the full protocol,” she added, “although scanning six joints still might not be feasible during a typical return patient clinic visit in the United States.”
Philip Chu, MD, clinical associate at Duke University, Durham, N.C., uses diagnostic US to help differentiate borderline gout cases from other arthropathies.
“A baseline scan, a follow-up scan before deciding to stop prophylaxis, or a follow-up scan in the setting of recurrent gout flares despite reaching goal serum uric acid, may be cost-effective time points to perform diagnostic US,” he advised.
“Unfortunately,” he added, “reimbursement for diagnostic US has been decreasing over the years, which makes it challenging to increase diagnostic US to the [frequency of its use] in Europe.”
Asked how most gout care being provided by primary care doctors in the United States affects gout management, Dr. Chu said: “Depending on which guidelines one follows for treating gout – from the American College of Rheumatology or the American College of Physicians – one may be more or less likely to start urate-lowering therapy after the first gout flare.”
“Understanding MSU burden in each patient, or even seeing active inflammation at these sites by increased Doppler signal, may change the threshold for physicians to initiate therapy,” he added.
The study received no funding. Three study authors reported financial involvements with pharmaceutical companies. Dr. Cipolletta, Dr. Annapureddy, Dr. Song, Dr. Tedeschi, and Dr. Chu reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM RHEUMATOLOGY
Black Americans’ high gout rate stems from social causes
Gout prevalence is more common in Black Americans than White Americans, and the disparity in prevalence is attributable to social determinants of health, according to a recently published article in JAMA Network Open.
“There has been evidence from recent cohort studies in the U.S. that was suggesting that the prevalence and incidence [of gout] was growing among non-White populations,” said Natalie McCormick, PhD, the study’s lead author and postdoctoral research fellow in medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston. “We wanted to do this at the general population level to see how generalizable [that evidence] is.”
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Medical Group, Franklin, Wisc., noted the findings highlight inequities in care for patients with gout that could be improved with greater emphasis on educating patients about their condition.
“I think that what this shows is that in the U.S. ... there still are some disparities in treating gout,” said Dr. Wells, who was not involved with the study. “And that we have ways to mitigate that, with not only aggressive therapy, but also with other tools like counseling patients. At the end of the day, people all want to be educated about the disease.”
Greater prevalence disappears with adjustment for socioclinical factors
The cross-sectional analysis involved data from U.S. adult participants in the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2016 who self-reported Black or White race.
Investigators considered factors such as excess body mass index (BMI), chronic kidney disease (defined as estimated glomerular filtration rate less than 60 mL/min per 1.73 m2), poverty, poor-quality diet, lower educational level, alcohol consumption, and diuretic use in their analysis.
Dr. McCormick and coinvestigators included a total of 18,693 participants, consisting of 3,304 Black women, 6,195 White women, 3,085 Black men, and 6,109 White men.
They determined that the age-standardized prevalence of gout was 3.5% (95% confidence interval, 2.7%-4.3%) in Black women and 2.0% (95% CI, 1.5% - 2.5%) in White women (age-adjusted odds ratio, 1.81; 95% CI, 1.29-2.53). They calculated that the prevalence was 7.0% (95% CI, 6.2%-7.9%) in Black men and 5.4% (95% CI, 4.7%-6.2%) in White men (age-adjusted OR, 1.26; 95% CI, 1.02-1.55). They found similar differences in the prevalence of hyperuricemia between Black and White Americans.
The increased prevalence of gout in Black Americans, compared with White Americans, does not arise from genetics, according to McCormick. “Our conclusion was that it was due to social determinants of health,” she said. “When we adjusted for all socioclinical risk factors, the racial differences in gout and hyperuricemia prevalence disappeared. Importantly, stepwise regression analysis showed the two biggest drivers of the racial difference in gout prevalence among women were poverty itself, and excess BMI, which can be influenced by poverty.”
Dr. McCormick pointed out that in contrast to the current data, there was no racial difference in the prevalence of gout approximately 2 decades earlier, looking at data from the 1988-1994 NHANES III.
Given the findings, which included the fact that significantly more Black women and men were currently taking diuretics, compared with their White counterparts, Dr. McCormick pointed out clinicians should give more thought to medical therapies prescribed for conditions like high blood pressure to patients with gout or at risk for gout.
“One thing we found was that diuretic use was a driver” of gout, Dr. McCormick said. A prescriber “may want to consider different therapies that present a lower risk of gout if someone has hypertension. There could be greater consideration for prescribing alternatives to diuretics.”
More patient education and rheumatology referrals needed
An impediment to providing that education to patients with gout is unconscious bias on the part of the primary care provider, Dr. Wells said.
“It is about what your perspectives are and what you bring to the table,” he explained. “If you saw [a patient] who looked like someone in your family, that person will be treated differently [than someone who does not look like a family member]. That is where the whole concept [of unconscious bias] comes in.”
Primary care providers need to adopt a holistic approach to gout management that involves counseling about good nutrition, smoking cessation, regular exercise, and limiting alcohol consumption, in addition to medication adherence. Primary care providers may have a bias in treating their Black patients, failing to devote sufficient time and attention to assist them in getting their disease under control, he said.
“Gout should be just like any other chronic disease,” Dr. Wells said. “You need to have a target in mind, and you and your patient need to work together to get to that target. When [patients] end up in rheumatology offices, it is almost too late. I think the take-home message here is that in 2022 ... for any patient who has gout, that patient probably needs to be seen by a rheumatologist because, indeed, with aggressive therapy, preventive therapy, [and] education, and if they are on the right medications, they won’t end up with these crippling joints that we see all the time.”
Dr. McCormick and Dr. Wells disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout prevalence is more common in Black Americans than White Americans, and the disparity in prevalence is attributable to social determinants of health, according to a recently published article in JAMA Network Open.
“There has been evidence from recent cohort studies in the U.S. that was suggesting that the prevalence and incidence [of gout] was growing among non-White populations,” said Natalie McCormick, PhD, the study’s lead author and postdoctoral research fellow in medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston. “We wanted to do this at the general population level to see how generalizable [that evidence] is.”
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Medical Group, Franklin, Wisc., noted the findings highlight inequities in care for patients with gout that could be improved with greater emphasis on educating patients about their condition.
“I think that what this shows is that in the U.S. ... there still are some disparities in treating gout,” said Dr. Wells, who was not involved with the study. “And that we have ways to mitigate that, with not only aggressive therapy, but also with other tools like counseling patients. At the end of the day, people all want to be educated about the disease.”
Greater prevalence disappears with adjustment for socioclinical factors
The cross-sectional analysis involved data from U.S. adult participants in the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2016 who self-reported Black or White race.
Investigators considered factors such as excess body mass index (BMI), chronic kidney disease (defined as estimated glomerular filtration rate less than 60 mL/min per 1.73 m2), poverty, poor-quality diet, lower educational level, alcohol consumption, and diuretic use in their analysis.
Dr. McCormick and coinvestigators included a total of 18,693 participants, consisting of 3,304 Black women, 6,195 White women, 3,085 Black men, and 6,109 White men.
They determined that the age-standardized prevalence of gout was 3.5% (95% confidence interval, 2.7%-4.3%) in Black women and 2.0% (95% CI, 1.5% - 2.5%) in White women (age-adjusted odds ratio, 1.81; 95% CI, 1.29-2.53). They calculated that the prevalence was 7.0% (95% CI, 6.2%-7.9%) in Black men and 5.4% (95% CI, 4.7%-6.2%) in White men (age-adjusted OR, 1.26; 95% CI, 1.02-1.55). They found similar differences in the prevalence of hyperuricemia between Black and White Americans.
The increased prevalence of gout in Black Americans, compared with White Americans, does not arise from genetics, according to McCormick. “Our conclusion was that it was due to social determinants of health,” she said. “When we adjusted for all socioclinical risk factors, the racial differences in gout and hyperuricemia prevalence disappeared. Importantly, stepwise regression analysis showed the two biggest drivers of the racial difference in gout prevalence among women were poverty itself, and excess BMI, which can be influenced by poverty.”
Dr. McCormick pointed out that in contrast to the current data, there was no racial difference in the prevalence of gout approximately 2 decades earlier, looking at data from the 1988-1994 NHANES III.
Given the findings, which included the fact that significantly more Black women and men were currently taking diuretics, compared with their White counterparts, Dr. McCormick pointed out clinicians should give more thought to medical therapies prescribed for conditions like high blood pressure to patients with gout or at risk for gout.
“One thing we found was that diuretic use was a driver” of gout, Dr. McCormick said. A prescriber “may want to consider different therapies that present a lower risk of gout if someone has hypertension. There could be greater consideration for prescribing alternatives to diuretics.”
More patient education and rheumatology referrals needed
An impediment to providing that education to patients with gout is unconscious bias on the part of the primary care provider, Dr. Wells said.
“It is about what your perspectives are and what you bring to the table,” he explained. “If you saw [a patient] who looked like someone in your family, that person will be treated differently [than someone who does not look like a family member]. That is where the whole concept [of unconscious bias] comes in.”
Primary care providers need to adopt a holistic approach to gout management that involves counseling about good nutrition, smoking cessation, regular exercise, and limiting alcohol consumption, in addition to medication adherence. Primary care providers may have a bias in treating their Black patients, failing to devote sufficient time and attention to assist them in getting their disease under control, he said.
“Gout should be just like any other chronic disease,” Dr. Wells said. “You need to have a target in mind, and you and your patient need to work together to get to that target. When [patients] end up in rheumatology offices, it is almost too late. I think the take-home message here is that in 2022 ... for any patient who has gout, that patient probably needs to be seen by a rheumatologist because, indeed, with aggressive therapy, preventive therapy, [and] education, and if they are on the right medications, they won’t end up with these crippling joints that we see all the time.”
Dr. McCormick and Dr. Wells disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout prevalence is more common in Black Americans than White Americans, and the disparity in prevalence is attributable to social determinants of health, according to a recently published article in JAMA Network Open.
“There has been evidence from recent cohort studies in the U.S. that was suggesting that the prevalence and incidence [of gout] was growing among non-White populations,” said Natalie McCormick, PhD, the study’s lead author and postdoctoral research fellow in medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, both in Boston. “We wanted to do this at the general population level to see how generalizable [that evidence] is.”
Alvin Wells, MD, PhD, director of the department of rheumatology at Advocate Aurora Medical Group, Franklin, Wisc., noted the findings highlight inequities in care for patients with gout that could be improved with greater emphasis on educating patients about their condition.
“I think that what this shows is that in the U.S. ... there still are some disparities in treating gout,” said Dr. Wells, who was not involved with the study. “And that we have ways to mitigate that, with not only aggressive therapy, but also with other tools like counseling patients. At the end of the day, people all want to be educated about the disease.”
Greater prevalence disappears with adjustment for socioclinical factors
The cross-sectional analysis involved data from U.S. adult participants in the National Health and Nutrition Examination Survey (NHANES) from 2007 to 2016 who self-reported Black or White race.
Investigators considered factors such as excess body mass index (BMI), chronic kidney disease (defined as estimated glomerular filtration rate less than 60 mL/min per 1.73 m2), poverty, poor-quality diet, lower educational level, alcohol consumption, and diuretic use in their analysis.
Dr. McCormick and coinvestigators included a total of 18,693 participants, consisting of 3,304 Black women, 6,195 White women, 3,085 Black men, and 6,109 White men.
They determined that the age-standardized prevalence of gout was 3.5% (95% confidence interval, 2.7%-4.3%) in Black women and 2.0% (95% CI, 1.5% - 2.5%) in White women (age-adjusted odds ratio, 1.81; 95% CI, 1.29-2.53). They calculated that the prevalence was 7.0% (95% CI, 6.2%-7.9%) in Black men and 5.4% (95% CI, 4.7%-6.2%) in White men (age-adjusted OR, 1.26; 95% CI, 1.02-1.55). They found similar differences in the prevalence of hyperuricemia between Black and White Americans.
The increased prevalence of gout in Black Americans, compared with White Americans, does not arise from genetics, according to McCormick. “Our conclusion was that it was due to social determinants of health,” she said. “When we adjusted for all socioclinical risk factors, the racial differences in gout and hyperuricemia prevalence disappeared. Importantly, stepwise regression analysis showed the two biggest drivers of the racial difference in gout prevalence among women were poverty itself, and excess BMI, which can be influenced by poverty.”
Dr. McCormick pointed out that in contrast to the current data, there was no racial difference in the prevalence of gout approximately 2 decades earlier, looking at data from the 1988-1994 NHANES III.
Given the findings, which included the fact that significantly more Black women and men were currently taking diuretics, compared with their White counterparts, Dr. McCormick pointed out clinicians should give more thought to medical therapies prescribed for conditions like high blood pressure to patients with gout or at risk for gout.
“One thing we found was that diuretic use was a driver” of gout, Dr. McCormick said. A prescriber “may want to consider different therapies that present a lower risk of gout if someone has hypertension. There could be greater consideration for prescribing alternatives to diuretics.”
More patient education and rheumatology referrals needed
An impediment to providing that education to patients with gout is unconscious bias on the part of the primary care provider, Dr. Wells said.
“It is about what your perspectives are and what you bring to the table,” he explained. “If you saw [a patient] who looked like someone in your family, that person will be treated differently [than someone who does not look like a family member]. That is where the whole concept [of unconscious bias] comes in.”
Primary care providers need to adopt a holistic approach to gout management that involves counseling about good nutrition, smoking cessation, regular exercise, and limiting alcohol consumption, in addition to medication adherence. Primary care providers may have a bias in treating their Black patients, failing to devote sufficient time and attention to assist them in getting their disease under control, he said.
“Gout should be just like any other chronic disease,” Dr. Wells said. “You need to have a target in mind, and you and your patient need to work together to get to that target. When [patients] end up in rheumatology offices, it is almost too late. I think the take-home message here is that in 2022 ... for any patient who has gout, that patient probably needs to be seen by a rheumatologist because, indeed, with aggressive therapy, preventive therapy, [and] education, and if they are on the right medications, they won’t end up with these crippling joints that we see all the time.”
Dr. McCormick and Dr. Wells disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Toenail trauma
The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111

The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The patient’s initial injury was probably a subungual hematoma, which can take 12 to 18 months to resolve (the time it takes for a new toenail to grow). However, the precipitating trauma likely created an opportunity for fungal elements to invade the nail plate, resulting in the current complaint of superficial onychomycosis.
Onychomycosis is a frequently seen condition with increasing prevalence in older patients. It has several clinical presentations: Superficial onychomycosis manifests with chalky white changes on the surface of the nail. Distal subungual onychomycosis develops at the distal aspect of the nail with thickening and subungual debris. Proximal subungual onychomycosis occurs in the proximal aspect of the nail.
Although often asymptomatic, onychomycosis can cause thickening of the nails and development of subsequent deformity or pincer nails (which painfully “pinch” the underlying skin). It is especially concerning in patients with diabetes or peripheral neuropathy, in whom the abnormal thickness and shape of the nails can lead to microtrauma at the proximal and lateral attachments of the nail. These patients have an increased risk of secondary infection, possible complications, and even, for some, amputation.
If the patient is asymptomatic, and does not have diabetes, neuropathy, or other risk factors, treatment is not required. For those who would benefit from treatment, it is usually safe and inexpensive with the current generation of oral antifungal medications.
Some recommend confirmatory testing before treatment intiation,1 but the low adverse effect profile of terbinafine and its current cost below $10/month2 make empiric treatment safe and cost effective in most cases.3 If needed, and with access to microscopy, a potassium hydroxide (KOH) prep can be performed on scrapings from the affected portions of the nail. If that is not available, scrapings or clippings can be sent to the lab for KOH and periodic acid-Schiff staining.
The US Food and Drug Administration previously recommended follow-up liver enzyme tests if terbinafine is used for more than 6 weeks. (Fingernails require only 6 weeks of treatment, but toenails grow more slowly and require 12 weeks of treatment.) However, research has demonstrated that hepatotoxicity risk is extremely low and transaminase elevations are rare.4 In the rare cases that liver dysfunction has occurred, patients developed symptoms of jaundice, malaise, dark urine, or pruritis.4
This patient was counseled regarding the fungal nature of onychomycosis and the general safety of a 90-day course of oral terbinafine 250 mg/d—provided he did not have underlying liver or kidney disease or leukopenia. He reported that he had not had any blood work performed in the past year but was due for his annual wellness evaluation, at which he would discuss his overall health with his primary care provider, obtain baseline blood testing, and determine whether to proceed with treatment. He was advised that if, after starting treatment, he developed any symptoms of jaundice, dark urine, or other difficulties, he should report them to his care team.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111
- Frazier WT, Santiago-Delgado ZM, Stupka KC 2nd. Onychomycosis: rapid evidence review. Am Fam Physician. 2021;104:359-367.
- Terbinafine. GoodRx. Accessed August 9, 2022. https://www.goodrx.com/terbinafine
- Mikailov A, Cohen J, Joyce C, et al. Cost-effectiveness of confirmatory testing before treatment of onychomycosis. JAMA Dermatol. 2016;152:276-281. doi: 10.1001/jamadermatol.2015.4190
- Sun CW, Hsu S. Terbinafine: safety profile and monitoring in treatment of dermatophyte infections. Dermatol Ther. 2019;32:e13111. doi: 10.1111/dth.13111