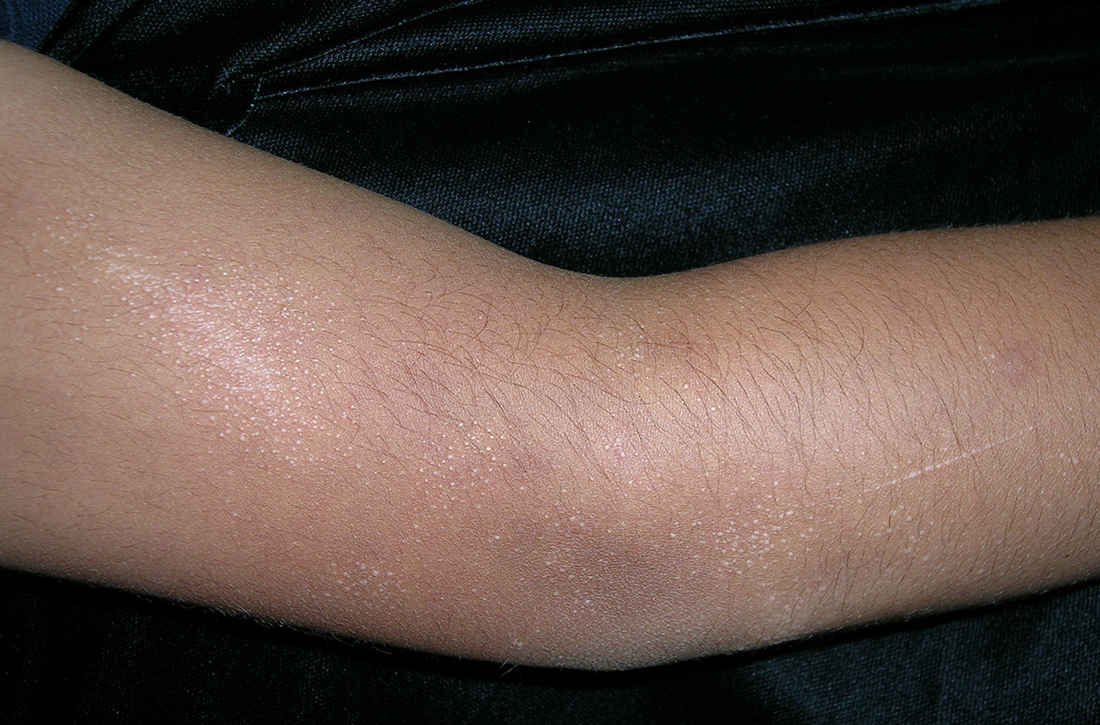
User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Avexitide promising for hypoglycemia after weight-loss surgery
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022
FDA OKs first systemic treatment for alopecia areata
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
Just 20 minutes of vigorous activity daily benefits teens
Vigorous physical activity for 20 minutes a day was enough to maximize cardiorespiratory benefits in adolescents, based on data from more than 300 individuals.
Current recommendations for physical activity in children and adolescents from the World Health Organization call for moderate to vigorous physical activity (MVPA) for an average of 60 minutes a day for physical and mental health; however, guidance on how much physical activity teens need to maximize cardiorespiratory fitness (CRF) has not been determined, Samuel Joseph Burden, BMedSci, of John Radcliffe Hospital Oxford (England), and colleagues wrote.
“Although data in young people are limited, adult studies have shown that regular, brief vigorous physical activity is highly effective at improving health markers, including CRF, which is also an important marker of health in youth,” the researchers wrote.
In a study published in Pediatrics, the researchers examined the associations between physical activity intensity and maximal CRF. The study population included 339 adolescents aged 13-14 years who were evaluated during the 2018-2019 and 2019-2020 school years. Participants wore wrist accelerometers to measure the intensity of physical activity and participated in 20-meter shuttle runs to demonstrate CRF. The researchers used partial multivariable linear regression to assess variables at different intensities including moderate physical activity (MPA), light physical activity (LPA), and sedentary time, as well as vigorous physical activity (VPA).
The wrist monitors measured the intensities of physical activity based on the bandpass-filtered followed by Euclidean norm metric (BFEN), a validated metric. “Previously validated thresholds for BFEN were used to determine the average duration of daily physical activity at each intensity: 0.1 g for LPA, 0.314 g for MPA, and 0.998 g for VPA,” the researchers wrote. Physical activity below the threshold for LPA was categorized as sedentary time.
Participants wore the accelerometers for 1 week; value recording included at least 3 weekdays and 1 weekend day, and each valid day required more than 6 hours of awake time.
Overall, VPA for up to 20 minutes was significantly associated with improved CRF. However, the benefits on CRF plateaued after that time, and longer duration of VPA was not associated with significantly greater improvements in CRF. Neither MPA nor LPA were associated with any improvements in CRF.
Participants who engaged in an average of 14 minutes (range, 12-17 minutes) of VPA per day met the median CRF.
The researchers also conducted independent t tests to assess differences in VPA at different CRF thresholds.
Those in the highest quartile of VPA had CRF z scores 1.03 higher, compared with those in the lower quartiles.
Given that current PA guidelines involve a combination of moderate and vigorous PA that could be met by MPA with no VPA, the current findings have public health implications for improving CRF in adolescents, the researchers wrote.
Even with MPA as an option, most adolescents fail to meet the recommendations of at least 60 minutes of MVPA, they said. “One possible reason is that this duration is quite long, requiring a daily time commitment that some may find difficult to maintain. A shorter target of 20 minutes might be easier to schedule daily and a focus on VPA would simplify messages about the intensity of activity that is likely to improve CRF.”
The study findings were limited by several factors including the use of data from only two schools in the United Kingdom, which may limit generalizability, and future research would ideally include a more direct assessment of VO2 max, the researchers wrote. However, the results were strengthened by the large and diverse study population, including teens with a wide range of body mass index as well as CRF.
Future research is needed to test whether interventions based on a target of 20 minutes of VPA creates significant improvements in adolescent cardiometabolic health, the researchers concluded.
Any activity has value for sedentary teens
The current study suggests that counseling teens about physical activity may be less challenging for clinicians if optimal cardiorespiratory benefits can be reached with shorter bouts of activity, Michele LaBotz, MD, of Intermed Sports Medicine, South Portland, Maine, and Sarah Hoffman, DO, of Tufts University, Boston, wrote in an accompanying editorial.
The results have two key implications for pediatricians, the authors said. First, “optimal CRF can be achieved with much shorter periods of activity than previously recommended.” Second, “current ‘moderate to vigorous’ PA recommendations may not be sufficient to improve CRF in adolescents, if achieved through moderate activity only.”
However, the editorialists emphasized that, although shorter periods of higher-intensity exercise reduce the time burden for teens and families, specific education is needed to explain the extra effort involved in exercising vigorously enough for cardiorespiratory benefits.
Patients can be counseled that activity is vigorous when they start to sweat, their face gets red, and they feel short of breath and unable to talk during activity,” they explained. These sensations may be new and uncomfortable for children and teens who have been quite sedentary or used to low-intensity activity. Dr. LaBotz and Dr. Hoffman advised pediatricians to counsel patients to build intensity gradually, with “exercise snacks,” that involve several minutes of activity that become more challenging over time.
“Exercise snacks can include anything that elevates the heart rate for a minute or more, such as running up and down the stairs a few times; chasing the dog around the backyard; or just putting on some music and dancing hard,” the editorialists wrote.
“Some exercise is better than none, and extrapolating from adult data, the biggest benefit likely occurs when we can help our most sedentary and least fit patients become a bit more active, even if it falls short of currently recommended levels,” they concluded.
The study was supported by grants to various researchers from the British Heart Foundation, the Elizabeth Casson Trust, the U.K. National Institute of Health Research, the Professor Nigel Groome Studentship scheme (Oxford Brookes University), and the U.K. Department of Health. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Vigorous physical activity for 20 minutes a day was enough to maximize cardiorespiratory benefits in adolescents, based on data from more than 300 individuals.
Current recommendations for physical activity in children and adolescents from the World Health Organization call for moderate to vigorous physical activity (MVPA) for an average of 60 minutes a day for physical and mental health; however, guidance on how much physical activity teens need to maximize cardiorespiratory fitness (CRF) has not been determined, Samuel Joseph Burden, BMedSci, of John Radcliffe Hospital Oxford (England), and colleagues wrote.
“Although data in young people are limited, adult studies have shown that regular, brief vigorous physical activity is highly effective at improving health markers, including CRF, which is also an important marker of health in youth,” the researchers wrote.
In a study published in Pediatrics, the researchers examined the associations between physical activity intensity and maximal CRF. The study population included 339 adolescents aged 13-14 years who were evaluated during the 2018-2019 and 2019-2020 school years. Participants wore wrist accelerometers to measure the intensity of physical activity and participated in 20-meter shuttle runs to demonstrate CRF. The researchers used partial multivariable linear regression to assess variables at different intensities including moderate physical activity (MPA), light physical activity (LPA), and sedentary time, as well as vigorous physical activity (VPA).
The wrist monitors measured the intensities of physical activity based on the bandpass-filtered followed by Euclidean norm metric (BFEN), a validated metric. “Previously validated thresholds for BFEN were used to determine the average duration of daily physical activity at each intensity: 0.1 g for LPA, 0.314 g for MPA, and 0.998 g for VPA,” the researchers wrote. Physical activity below the threshold for LPA was categorized as sedentary time.
Participants wore the accelerometers for 1 week; value recording included at least 3 weekdays and 1 weekend day, and each valid day required more than 6 hours of awake time.
Overall, VPA for up to 20 minutes was significantly associated with improved CRF. However, the benefits on CRF plateaued after that time, and longer duration of VPA was not associated with significantly greater improvements in CRF. Neither MPA nor LPA were associated with any improvements in CRF.
Participants who engaged in an average of 14 minutes (range, 12-17 minutes) of VPA per day met the median CRF.
The researchers also conducted independent t tests to assess differences in VPA at different CRF thresholds.
Those in the highest quartile of VPA had CRF z scores 1.03 higher, compared with those in the lower quartiles.
Given that current PA guidelines involve a combination of moderate and vigorous PA that could be met by MPA with no VPA, the current findings have public health implications for improving CRF in adolescents, the researchers wrote.
Even with MPA as an option, most adolescents fail to meet the recommendations of at least 60 minutes of MVPA, they said. “One possible reason is that this duration is quite long, requiring a daily time commitment that some may find difficult to maintain. A shorter target of 20 minutes might be easier to schedule daily and a focus on VPA would simplify messages about the intensity of activity that is likely to improve CRF.”
The study findings were limited by several factors including the use of data from only two schools in the United Kingdom, which may limit generalizability, and future research would ideally include a more direct assessment of VO2 max, the researchers wrote. However, the results were strengthened by the large and diverse study population, including teens with a wide range of body mass index as well as CRF.
Future research is needed to test whether interventions based on a target of 20 minutes of VPA creates significant improvements in adolescent cardiometabolic health, the researchers concluded.
Any activity has value for sedentary teens
The current study suggests that counseling teens about physical activity may be less challenging for clinicians if optimal cardiorespiratory benefits can be reached with shorter bouts of activity, Michele LaBotz, MD, of Intermed Sports Medicine, South Portland, Maine, and Sarah Hoffman, DO, of Tufts University, Boston, wrote in an accompanying editorial.
The results have two key implications for pediatricians, the authors said. First, “optimal CRF can be achieved with much shorter periods of activity than previously recommended.” Second, “current ‘moderate to vigorous’ PA recommendations may not be sufficient to improve CRF in adolescents, if achieved through moderate activity only.”
However, the editorialists emphasized that, although shorter periods of higher-intensity exercise reduce the time burden for teens and families, specific education is needed to explain the extra effort involved in exercising vigorously enough for cardiorespiratory benefits.
Patients can be counseled that activity is vigorous when they start to sweat, their face gets red, and they feel short of breath and unable to talk during activity,” they explained. These sensations may be new and uncomfortable for children and teens who have been quite sedentary or used to low-intensity activity. Dr. LaBotz and Dr. Hoffman advised pediatricians to counsel patients to build intensity gradually, with “exercise snacks,” that involve several minutes of activity that become more challenging over time.
“Exercise snacks can include anything that elevates the heart rate for a minute or more, such as running up and down the stairs a few times; chasing the dog around the backyard; or just putting on some music and dancing hard,” the editorialists wrote.
“Some exercise is better than none, and extrapolating from adult data, the biggest benefit likely occurs when we can help our most sedentary and least fit patients become a bit more active, even if it falls short of currently recommended levels,” they concluded.
The study was supported by grants to various researchers from the British Heart Foundation, the Elizabeth Casson Trust, the U.K. National Institute of Health Research, the Professor Nigel Groome Studentship scheme (Oxford Brookes University), and the U.K. Department of Health. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
Vigorous physical activity for 20 minutes a day was enough to maximize cardiorespiratory benefits in adolescents, based on data from more than 300 individuals.
Current recommendations for physical activity in children and adolescents from the World Health Organization call for moderate to vigorous physical activity (MVPA) for an average of 60 minutes a day for physical and mental health; however, guidance on how much physical activity teens need to maximize cardiorespiratory fitness (CRF) has not been determined, Samuel Joseph Burden, BMedSci, of John Radcliffe Hospital Oxford (England), and colleagues wrote.
“Although data in young people are limited, adult studies have shown that regular, brief vigorous physical activity is highly effective at improving health markers, including CRF, which is also an important marker of health in youth,” the researchers wrote.
In a study published in Pediatrics, the researchers examined the associations between physical activity intensity and maximal CRF. The study population included 339 adolescents aged 13-14 years who were evaluated during the 2018-2019 and 2019-2020 school years. Participants wore wrist accelerometers to measure the intensity of physical activity and participated in 20-meter shuttle runs to demonstrate CRF. The researchers used partial multivariable linear regression to assess variables at different intensities including moderate physical activity (MPA), light physical activity (LPA), and sedentary time, as well as vigorous physical activity (VPA).
The wrist monitors measured the intensities of physical activity based on the bandpass-filtered followed by Euclidean norm metric (BFEN), a validated metric. “Previously validated thresholds for BFEN were used to determine the average duration of daily physical activity at each intensity: 0.1 g for LPA, 0.314 g for MPA, and 0.998 g for VPA,” the researchers wrote. Physical activity below the threshold for LPA was categorized as sedentary time.
Participants wore the accelerometers for 1 week; value recording included at least 3 weekdays and 1 weekend day, and each valid day required more than 6 hours of awake time.
Overall, VPA for up to 20 minutes was significantly associated with improved CRF. However, the benefits on CRF plateaued after that time, and longer duration of VPA was not associated with significantly greater improvements in CRF. Neither MPA nor LPA were associated with any improvements in CRF.
Participants who engaged in an average of 14 minutes (range, 12-17 minutes) of VPA per day met the median CRF.
The researchers also conducted independent t tests to assess differences in VPA at different CRF thresholds.
Those in the highest quartile of VPA had CRF z scores 1.03 higher, compared with those in the lower quartiles.
Given that current PA guidelines involve a combination of moderate and vigorous PA that could be met by MPA with no VPA, the current findings have public health implications for improving CRF in adolescents, the researchers wrote.
Even with MPA as an option, most adolescents fail to meet the recommendations of at least 60 minutes of MVPA, they said. “One possible reason is that this duration is quite long, requiring a daily time commitment that some may find difficult to maintain. A shorter target of 20 minutes might be easier to schedule daily and a focus on VPA would simplify messages about the intensity of activity that is likely to improve CRF.”
The study findings were limited by several factors including the use of data from only two schools in the United Kingdom, which may limit generalizability, and future research would ideally include a more direct assessment of VO2 max, the researchers wrote. However, the results were strengthened by the large and diverse study population, including teens with a wide range of body mass index as well as CRF.
Future research is needed to test whether interventions based on a target of 20 minutes of VPA creates significant improvements in adolescent cardiometabolic health, the researchers concluded.
Any activity has value for sedentary teens
The current study suggests that counseling teens about physical activity may be less challenging for clinicians if optimal cardiorespiratory benefits can be reached with shorter bouts of activity, Michele LaBotz, MD, of Intermed Sports Medicine, South Portland, Maine, and Sarah Hoffman, DO, of Tufts University, Boston, wrote in an accompanying editorial.
The results have two key implications for pediatricians, the authors said. First, “optimal CRF can be achieved with much shorter periods of activity than previously recommended.” Second, “current ‘moderate to vigorous’ PA recommendations may not be sufficient to improve CRF in adolescents, if achieved through moderate activity only.”
However, the editorialists emphasized that, although shorter periods of higher-intensity exercise reduce the time burden for teens and families, specific education is needed to explain the extra effort involved in exercising vigorously enough for cardiorespiratory benefits.
Patients can be counseled that activity is vigorous when they start to sweat, their face gets red, and they feel short of breath and unable to talk during activity,” they explained. These sensations may be new and uncomfortable for children and teens who have been quite sedentary or used to low-intensity activity. Dr. LaBotz and Dr. Hoffman advised pediatricians to counsel patients to build intensity gradually, with “exercise snacks,” that involve several minutes of activity that become more challenging over time.
“Exercise snacks can include anything that elevates the heart rate for a minute or more, such as running up and down the stairs a few times; chasing the dog around the backyard; or just putting on some music and dancing hard,” the editorialists wrote.
“Some exercise is better than none, and extrapolating from adult data, the biggest benefit likely occurs when we can help our most sedentary and least fit patients become a bit more active, even if it falls short of currently recommended levels,” they concluded.
The study was supported by grants to various researchers from the British Heart Foundation, the Elizabeth Casson Trust, the U.K. National Institute of Health Research, the Professor Nigel Groome Studentship scheme (Oxford Brookes University), and the U.K. Department of Health. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose.
FROM PEDIATRICS
SGLT2 inhibitors cut AFib risk in real-word analysis
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
NEW ORLEANS – The case continues to grow for prioritizing a sodium-glucose transporter 2 (SGLT2) inhibitor in patients with type 2 diabetes, as real-world evidence of benefit and safety accumulates on top of the data from randomized trials that first established this class as a management pillar.
Another important effect of these agents gaining increasing currency, on top of their well-established benefits in patients with type 2 diabetes for preventing acute heart failure exacerbations and slowing progression of diabetic kidney disease, is that they cut the incidence of new-onset atrial fibrillation (AFib). That effect was confirmed in an analysis of data from about 300,000 U.S. patients included in recent Medicare records, Elisabetta Patorno, MD, reported at the annual scientific sessions of the American Diabetes Association.
But despite documentation like this, real-world evidence also continues to show limited uptake of SGLT2 inhibitors in U.S. patients with type 2 diabetes. Records from more than 1.3 million patients with type 2 diabetes managed in the Veterans Affairs Healthcare System during 2019 or 2022 documented that just 10% of these patients received an agent from this class, even though all were eligible to receive it, according to findings in a separate report at the meeting.
The AFib analysis analyzed two sets of propensity score–matched Medicare patients during 2013-2018 aged 65 years or older with type 2 diabetes and no history of AFib. One analysis focused on 80,475 matched patients who started on treatment with either an SGLT2 inhibitor or a glucagonlike peptide–1 (GLP-1) receptor agonist, and a second on 74,868 matched patients who began either an SGTL2 inhibitor or a dipeptidyl peptidase–4 (DPP4) inhibitor. In both analyses, matching involved more than 130 variables. In both pair sets, patients at baseline averaged about 72 years old, nearly two-thirds were women, about 8%-9% had heart failure, 77%-80% were on metformin, and 20%-25% were using insulin.
The study’s primary endpoint was the incidence of hospitalization for AFib, which occurred a significant 18% less often in the patients who started on an SGLT2, compared with those who started a DPP4 inhibitor during median follow-up of 6.7 months, and a significant 10% less often, compared with those starting a GLP-1 receptor agonist during a median follow-up of 6.0 months, Elisabetta Patorno, MD, DrPH, reported at the meeting. This worked out to 3.7 fewer hospitalizations for AFib per 1,000 patient-years of follow-up among the people who received an SGLT2 inhibitor, compared with a DPP4 inhibitor, and a decrease of 1.8 hospitalizations/1,000 patient-years when compared against patients in a GLP-1 receptor agonist.
Two secondary outcomes showed significantly fewer episodes of newly diagnosed AFib, and significantly fewer patients initiating AFib treatment among those who received an SGLT2 inhibitor relative to the comparator groups. In addition, these associations were consistent across subgroup analyses that divided patients by their age, sex, history of heart failure, and history of atherosclerotic cardiovascular disease.
AFib effects add to benefits
The findings “suggest that initiation of an SGLT2 inhibitor may be beneficial in older adults with type 2 diabetes who are at risk for AFib,” said Dr. Patorno, a researcher in the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital, Boston. “These new findings on AFib may be helpful when weighing the potential risks and benefits of various glucose-lowering drugs in older patients with type 2 diabetes.”
This new evidence follows several prior reports from other research groups of data supporting an AFib benefit from SGLT2 inhibitors. The earlier reports include a post hoc analysis of more than 17,000 patients enrolled in the DECLARE-TIMI 58 cardiovascular outcome trial of dapagliflozin (Farxiga), which showed a 19% relative decrease in the rate of incident AFib or atrial flutter events during a median 4.2 year follow-up.
Other prior reports that found a reduced incidence of AFib events linked with SGLT2 inhibitor treatment include a 2020 meta-analysis based on data from more than 38,000 patients with type 2 diabetes enrolled in any of 16 randomized, controlled trials, which found a 24% relative risk reduction. And an as-yet unpublished report from researchers at the University of Rochester (N.Y.) and their associates presented in November 2021 at the annual scientific sessions of the American Heart Association that documented a significant 24% relative risk reduction in incident AFib events linked to SGLT2 inhibitor treatment in a prospective study of 13,890 patients at several hospitals in Israel or the United States.
Evidence ‘convincing’ in totality
The accumulated evidence for a reduced incidence of AFib when patients were on treatment with an SGLT2 inhibitor are “convincing because it’s real world data that complements what we know from clinical trials,” commented Silvio E. Inzucchi, MD, professor of medicine at Yale University and director of the Yale Medicine Diabetes Center in New Haven, Conn., who was not involved with the study.
“If these drugs reduce heart failure, they may also reduce AFib. Heart failure patients easily slip into AFib,” he noted in an interview, but added that “I don’t think this explains all cases” of the reduced AFib incidence.
Dr. Patorno offered a few other possible mechanisms for the observed effect. The class may work by reducing blood pressure, weight, inflammation, and oxidative stress, mitochondrial dysfunction, atrial remodeling, and AFib susceptibility. These agents are also known to cause natriuresis and diuresis, which could reduce atrial dilation, a mechanism that again relates the AFib effect to the better documented reduction in acute heart failure exacerbations.
“With the diuretic effect, we’d expect less overload at the atrium and less dilation, and the same mechanism would reduce heart failure,” she said in an interview.
“If you reduce preload and afterload you may reduce stress on the ventricle and reduce atrial stretch, and that might have a significant effect on atrial arrhythmia,” agreed Dr. Inzucchi.
EMPRISE produces more real-world evidence
A pair of additional reports at the meeting that Dr. Patorno coauthored provided real-world evidence supporting the dramatic heart failure benefit of the SGLT2 inhibitor empagliflozin (Jardiance) in U.S. patients with type 2 diabetes, compared with alternative drug classes. The EMPRISE study used data from the Medicare, Optum Clinformatics, and MarketScan databases during the period from August 2014, when empagliflozin became available, to September 2019. The study used more than 140 variables to match patients treated with either empagliflozin or a comparator agent.
The results showed that, in an analysis of more than 130,000 matched pairs, treatment with empagliflozin was linked to a significant 30% reduction in the incidence of hospitalization for heart failure, compared with patients treated with a GLP-1 receptor agonist. Analysis of more than 116,000 matched pairs of patients showed that treatment with empagliflozin linked with a significant 29%-50% reduced rate of hospitalization for heart failure, compared with matched patients treated with a DPP4 inhibitor.
These findings “add to the pool of information” on the efficacy of agents from the SGLT2 inhibitor class, Dr. Patorno said in an interview. “We wanted to look at the full range of patients with type 2 diabetes who we see in practice,” rather than the more selected group of patients enrolled in randomized trials.
SGLT2 inhibitor use lags even when cost isn’t an issue
Despite all the accumulated evidence for efficacy and safety of the class, usage remains low, Julio A. Lamprea-Montealegre, MD, PhD, a cardiologist at the University of California, San Francisco, reported in a separate talk at the meeting. The study he presented examined records for 1,319,500 adults with type 2 diabetes managed in the VA Healthcare System during 2019 and 2020. Despite being in a system that “removes the influence of cost,” just 10% of these patients received treatment with an SGLT2 inhibitor, and 7% received treatment with a GLP-1 receptor agonist.
Notably, his analysis further showed that treatment with an SGLT2 inhibitor was especially depressed among patients with an estimated glomerular filtration rate (eGFR) of 30-44 mL/min per 1.73m2. In this subgroup, usage of a drug from this class was at two-thirds of the rate, compared with patients with an eGFR of at least 90 mL/min per 1.73m2. His findings also documented lower rates of use in patients with higher risk for atherosclerotic cardiovascular disease. Dr. Lamprea-Montealegre called this a “treatment paradox,” in which patients likely to get the most benefit from an SGLT2 inhibitor were also less likely to actually receive it.
While his findings from the VA System suggest that drug cost is not the only factor driving underuse, the high price set for the SGLT2 inhibitor drugs that all currently remain on U.S. patents is widely considered an important factor.
“There is a big problem of affordability,” said Dr. Patorno.
“SGLT2 inhibitors should probably be first-line therapy” for many patients with type 2 diabetes, said Dr. Inzucchi. “The only thing holding it back is cost,” a situation that he hopes will dramatically shift once agents from this class become generic and have substantially lower price tags.
The EMPRISE study received funding from Boehringer Ingelheim, the company that markets empagliflozin (Jardiance). Dr. Patorno had no relevant commercial disclosures. Dr. Inzucchi is an adviser to Abbott Diagnostics, Esperion Therapeutics, and vTv Therapeutics, a consultant to Merck and Pfizer, and has other relationships with AstraZeneca, Boehringer Ingelheim, Lexicon, and Novo Nordisk. Dr. Lamprea-Montealegre had received research funding from Bayer.
AT ADA 2022
Surprising link between herpes zoster and dementia
Herpes zoster does not appear to increase dementia risk – on the contrary, the viral infection may offer some protection, a large population-based study suggests.
“We were surprised by these results [and] the reasons for the decreased risk are unclear,” study author Sigrun Alba Johannesdottir Schmidt, MD, PhD, with Aarhus (Denmark) University Hospital, said in a news release.
The study was published online in Neurology.
Conflicting findings
Herpes zoster (HZ) is an acute, cutaneous viral infection caused by the reactivation of varicella-zoster virus (VZV). Previous population-based studies have reported both decreased and increased risks of dementia after having HZ.
It’s thought that HZ may contribute to the development of dementia through neuroinflammation, cerebral vasculopathy, or direct neural damage, but epidemiologic evidence is limited.
To investigate further, Dr. Schmidt and colleagues used Danish medical registries to identify 247,305 people who had visited a hospital for HZ or were prescribed antiviral medication for HZ over a 20-year period and matched them to 1,235,890 people who did not have HZ. For both cohorts, the median age was 64 years, and 61% were women.
Dementia was diagnosed in 9.7% of zoster patients and 10.3% of matched control persons during up to 21 years of follow-up.
Contrary to the researchers’ expectation, HZ was associated with a small (7%) decreased relative risk of all-cause dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.90-0.95).
There was no increased long-term risk of dementia in subgroup analyses, except possibly among those with HZ that involved the central nervous system (HR, 1.94; 95% CI, 0.78-4.80), which has been shown before.
However, the population attributable fraction of dementia caused by this rare complication is low (< 1%), suggesting that universal vaccination against VZV in the elderly has limited potential to reduce dementia risk, the investigators noted.
Nonetheless, Dr. Schmidt said shingles vaccination should be encouraged in older people because it can prevent complications from the disease.
The research team admitted that the slightly decreased long-term risk of dementia, including Alzheimer’s disease, was “unexpected.” The reasons for this decreased risk are unclear, they say, and could be explained by missed diagnoses of shingles in people with undiagnosed dementia.
They were not able to examine whether antiviral treatment modifies the association between HZ and dementia and said that this topic merits further research.
The study was supported by the Edel and Wilhelm Daubenmerkls Charitable Foundation. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Herpes zoster does not appear to increase dementia risk – on the contrary, the viral infection may offer some protection, a large population-based study suggests.
“We were surprised by these results [and] the reasons for the decreased risk are unclear,” study author Sigrun Alba Johannesdottir Schmidt, MD, PhD, with Aarhus (Denmark) University Hospital, said in a news release.
The study was published online in Neurology.
Conflicting findings
Herpes zoster (HZ) is an acute, cutaneous viral infection caused by the reactivation of varicella-zoster virus (VZV). Previous population-based studies have reported both decreased and increased risks of dementia after having HZ.
It’s thought that HZ may contribute to the development of dementia through neuroinflammation, cerebral vasculopathy, or direct neural damage, but epidemiologic evidence is limited.
To investigate further, Dr. Schmidt and colleagues used Danish medical registries to identify 247,305 people who had visited a hospital for HZ or were prescribed antiviral medication for HZ over a 20-year period and matched them to 1,235,890 people who did not have HZ. For both cohorts, the median age was 64 years, and 61% were women.
Dementia was diagnosed in 9.7% of zoster patients and 10.3% of matched control persons during up to 21 years of follow-up.
Contrary to the researchers’ expectation, HZ was associated with a small (7%) decreased relative risk of all-cause dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.90-0.95).
There was no increased long-term risk of dementia in subgroup analyses, except possibly among those with HZ that involved the central nervous system (HR, 1.94; 95% CI, 0.78-4.80), which has been shown before.
However, the population attributable fraction of dementia caused by this rare complication is low (< 1%), suggesting that universal vaccination against VZV in the elderly has limited potential to reduce dementia risk, the investigators noted.
Nonetheless, Dr. Schmidt said shingles vaccination should be encouraged in older people because it can prevent complications from the disease.
The research team admitted that the slightly decreased long-term risk of dementia, including Alzheimer’s disease, was “unexpected.” The reasons for this decreased risk are unclear, they say, and could be explained by missed diagnoses of shingles in people with undiagnosed dementia.
They were not able to examine whether antiviral treatment modifies the association between HZ and dementia and said that this topic merits further research.
The study was supported by the Edel and Wilhelm Daubenmerkls Charitable Foundation. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Herpes zoster does not appear to increase dementia risk – on the contrary, the viral infection may offer some protection, a large population-based study suggests.
“We were surprised by these results [and] the reasons for the decreased risk are unclear,” study author Sigrun Alba Johannesdottir Schmidt, MD, PhD, with Aarhus (Denmark) University Hospital, said in a news release.
The study was published online in Neurology.
Conflicting findings
Herpes zoster (HZ) is an acute, cutaneous viral infection caused by the reactivation of varicella-zoster virus (VZV). Previous population-based studies have reported both decreased and increased risks of dementia after having HZ.
It’s thought that HZ may contribute to the development of dementia through neuroinflammation, cerebral vasculopathy, or direct neural damage, but epidemiologic evidence is limited.
To investigate further, Dr. Schmidt and colleagues used Danish medical registries to identify 247,305 people who had visited a hospital for HZ or were prescribed antiviral medication for HZ over a 20-year period and matched them to 1,235,890 people who did not have HZ. For both cohorts, the median age was 64 years, and 61% were women.
Dementia was diagnosed in 9.7% of zoster patients and 10.3% of matched control persons during up to 21 years of follow-up.
Contrary to the researchers’ expectation, HZ was associated with a small (7%) decreased relative risk of all-cause dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.90-0.95).
There was no increased long-term risk of dementia in subgroup analyses, except possibly among those with HZ that involved the central nervous system (HR, 1.94; 95% CI, 0.78-4.80), which has been shown before.
However, the population attributable fraction of dementia caused by this rare complication is low (< 1%), suggesting that universal vaccination against VZV in the elderly has limited potential to reduce dementia risk, the investigators noted.
Nonetheless, Dr. Schmidt said shingles vaccination should be encouraged in older people because it can prevent complications from the disease.
The research team admitted that the slightly decreased long-term risk of dementia, including Alzheimer’s disease, was “unexpected.” The reasons for this decreased risk are unclear, they say, and could be explained by missed diagnoses of shingles in people with undiagnosed dementia.
They were not able to examine whether antiviral treatment modifies the association between HZ and dementia and said that this topic merits further research.
The study was supported by the Edel and Wilhelm Daubenmerkls Charitable Foundation. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New guideline for in-hospital care of diabetes says use CGMs
Goal-directed glycemic management – which may include new technologies for glucose monitoring – for non–critically ill hospitalized patients who have diabetes or newly recognized hyperglycemia can improve outcomes, according to a new practice guideline from the Endocrine Society.
Even though roughly 35% of hospitalized patients have diabetes or newly discovered hyperglycemia, there is “wide variability in glycemic management in clinical practice,” writing panel chair Mary Korytkowski, MD, from the University of Pittsburgh, said at the annual meeting of the Endocrine Society. “These patients get admitted to every patient service in the hospital, meaning that every clinical service will encounter this group of patients, and their glycemic management can have a major effect on their outcomes. Both short term and long term.”
This guideline provides strategies “to achieve previously recommended glycemic goals while also reducing the risk for hypoglycemia, and this includes inpatient use of insulin pump therapy or continuous glucose monitoring [CGM] devices, among others,” she said.
It also includes “recommendations for preoperative glycemic goals as well as when the use of correctional insulin – well known as sliding scale insulin – may be appropriate” and when it is not.
The document, which replaces a 2012 guideline, was published online in the Journal of Clinical Endocrinology & Metabolism.
A multidisciplinary panel developed the document over the last 3 years to answer 10 clinical practice questions related to management of non–critically ill hospitalized patients with diabetes or newly discovered hyperglycemia.
Use of CGM devices in hospital
The first recommendation is: “In adults with insulin-treated diabetes hospitalized for noncritical illness who are at high risk of hypoglycemia, we suggest the use of real-time [CGM] with confirmatory bedside point-of-care blood glucose monitoring for adjustments in insulin dosing rather than point-of-care blood glucose rather than testing alone in hospital settings where resources and training are available.” (Conditional recommendation. Low certainty of evidence).
“We were actually very careful in terms of looking at the data” for use of CGMs, Dr. Korytkowski said in an interview.
Although CGMs are approved by the Food and Drug Administration in the outpatient setting, and that’s becoming the standard of care there, they are not yet approved for in-hospital use.
However, the FDA granted an emergency allowance for use of CGMs in hospitals during the COVID-19 pandemic.
That was “when everyone was scrambling for what to do,” Dr. Korytkowski noted. “There was a shortage of personal protective equipment and a real interest in trying to limit the amount of exposure of healthcare personnel in some of these really critically ill patients for whom intravenous insulin therapy was used to control their glucose level.”
On March 1, the FDA granted Breakthrough Devices Designation for Dexcom CGM use in the hospital setting.
The new guideline suggests CGM be used to detect trends in glycemic management, with insulin dosing decisions made with point-of-care glucose measure (the standard of care).
To implement CGM for glycemic management in hospitals, Dr. Korytkowski said, would require “extensive staff and nursing education to have people with expertise available to provide support to nursing personnel who are both placing these devices, changing these devices, looking at trends, and then knowing when to remove them for certain procedures such as MRI or radiologic procedures.”
“We know that not all hospitals may be readily available to use these devices,” she said. “It is an area of active research. But the use of these devices during the pandemic, in both critical care and non–critical care setting has really provided us with a lot of information that was used to formulate this suggestion in the guideline.”
The document addresses the following areas: CGM, continuous subcutaneous insulin infusion pump therapy, inpatient diabetes education, prespecified preoperative glycemic targets, use of neutral protamine Hagedorn insulin for glucocorticoid or enteral nutrition-associated hyperglycemia, noninsulin therapies, preoperative carbohydrate-containing oral fluids, carbohydrate counting for prandial (mealtime) insulin dosing, and correctional and scheduled (basal or basal bolus) insulin therapies.
Nine key recommendations
Dr. Korytkowski identified nine key recommendations:
- CGM systems can help guide glycemic management with reduced risk for hypoglycemia.
- Patients experiencing glucocorticoid- or enteral nutrition–associated hyperglycemia require scheduled insulin therapy to address anticipated glucose excursions.
- Selected patients using insulin pump therapy prior to a hospital admission can continue to use these devices in the hospital if they have the mental and physical capacity to do so with knowledgeable hospital personnel.
- Diabetes self-management education provided to hospitalized patients can promote improved glycemic control following discharge with reductions in the risk for hospital readmission. “We know that is recommended for patients in the outpatient setting but often they do not get this,” she said. “We were able to observe that this can also impact long-term outcomes “
- Patients with diabetes scheduled for elective surgery may have improved postoperative outcomes when preoperative hemoglobin A1c is 8% or less and preoperative blood glucose is less than 180 mg/dL. “This recommendation answers the question: ‘Where should glycemic goals be for people who are undergoing surgery?’ ”
- Providing preoperative carbohydrate-containing beverages to patients with known diabetes is not recommended.
- Patients with newly recognized hyperglycemia or well-managed diabetes on noninsulin therapy may be treated with correctional insulin alone as initial therapy at hospital admission.
- Some noninsulin diabetes therapies can be used in combination with correction insulin for patients with type 2 diabetes who have mild hyperglycemia.
- Correctional insulin – “otherwise known as sliding-scale insulin” – can be used as initial therapy for patients with newly recognized hyperglycemia or type 2 diabetes treated with noninsulin therapy prior to hospital admission.
- Scheduled insulin therapy is preferred for patients experiencing persistent blood glucose values greater than 180 mg/dL and is recommended for patients using insulin therapy prior to admission.
The guideline writers’ hopes
“We hope that this guideline will resolve debates” about appropriate preoperative glycemic management and when sliding-scale insulin can be used and should not be used, said Dr. Korytkowski.
The authors also hope that “it will stimulate research funding for this very important aspect of diabetes care, and that hospitals will recognize the importance of having access to knowledgeable diabetes care and education specialists who can provide staff education regarding inpatient glycemic management, provide oversight for patients using insulin pump therapy or CGM devices, and empower hospital nurses to provide diabetes [self-management] education prior to patient discharge.”
Claire Pegg, the patient representative on the panel, hopes “that this guideline serves as the beginning of a conversation that will allow inpatient caregivers to provide individualized care to patients – some of whom may be self-sufficient with their glycemic management and others who need additional assistance.”
Development of the guideline was funded by the Endocrine Society. Dr. Korytkowski has reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Goal-directed glycemic management – which may include new technologies for glucose monitoring – for non–critically ill hospitalized patients who have diabetes or newly recognized hyperglycemia can improve outcomes, according to a new practice guideline from the Endocrine Society.
Even though roughly 35% of hospitalized patients have diabetes or newly discovered hyperglycemia, there is “wide variability in glycemic management in clinical practice,” writing panel chair Mary Korytkowski, MD, from the University of Pittsburgh, said at the annual meeting of the Endocrine Society. “These patients get admitted to every patient service in the hospital, meaning that every clinical service will encounter this group of patients, and their glycemic management can have a major effect on their outcomes. Both short term and long term.”
This guideline provides strategies “to achieve previously recommended glycemic goals while also reducing the risk for hypoglycemia, and this includes inpatient use of insulin pump therapy or continuous glucose monitoring [CGM] devices, among others,” she said.
It also includes “recommendations for preoperative glycemic goals as well as when the use of correctional insulin – well known as sliding scale insulin – may be appropriate” and when it is not.
The document, which replaces a 2012 guideline, was published online in the Journal of Clinical Endocrinology & Metabolism.
A multidisciplinary panel developed the document over the last 3 years to answer 10 clinical practice questions related to management of non–critically ill hospitalized patients with diabetes or newly discovered hyperglycemia.
Use of CGM devices in hospital
The first recommendation is: “In adults with insulin-treated diabetes hospitalized for noncritical illness who are at high risk of hypoglycemia, we suggest the use of real-time [CGM] with confirmatory bedside point-of-care blood glucose monitoring for adjustments in insulin dosing rather than point-of-care blood glucose rather than testing alone in hospital settings where resources and training are available.” (Conditional recommendation. Low certainty of evidence).
“We were actually very careful in terms of looking at the data” for use of CGMs, Dr. Korytkowski said in an interview.
Although CGMs are approved by the Food and Drug Administration in the outpatient setting, and that’s becoming the standard of care there, they are not yet approved for in-hospital use.
However, the FDA granted an emergency allowance for use of CGMs in hospitals during the COVID-19 pandemic.
That was “when everyone was scrambling for what to do,” Dr. Korytkowski noted. “There was a shortage of personal protective equipment and a real interest in trying to limit the amount of exposure of healthcare personnel in some of these really critically ill patients for whom intravenous insulin therapy was used to control their glucose level.”
On March 1, the FDA granted Breakthrough Devices Designation for Dexcom CGM use in the hospital setting.
The new guideline suggests CGM be used to detect trends in glycemic management, with insulin dosing decisions made with point-of-care glucose measure (the standard of care).
To implement CGM for glycemic management in hospitals, Dr. Korytkowski said, would require “extensive staff and nursing education to have people with expertise available to provide support to nursing personnel who are both placing these devices, changing these devices, looking at trends, and then knowing when to remove them for certain procedures such as MRI or radiologic procedures.”
“We know that not all hospitals may be readily available to use these devices,” she said. “It is an area of active research. But the use of these devices during the pandemic, in both critical care and non–critical care setting has really provided us with a lot of information that was used to formulate this suggestion in the guideline.”
The document addresses the following areas: CGM, continuous subcutaneous insulin infusion pump therapy, inpatient diabetes education, prespecified preoperative glycemic targets, use of neutral protamine Hagedorn insulin for glucocorticoid or enteral nutrition-associated hyperglycemia, noninsulin therapies, preoperative carbohydrate-containing oral fluids, carbohydrate counting for prandial (mealtime) insulin dosing, and correctional and scheduled (basal or basal bolus) insulin therapies.
Nine key recommendations
Dr. Korytkowski identified nine key recommendations:
- CGM systems can help guide glycemic management with reduced risk for hypoglycemia.
- Patients experiencing glucocorticoid- or enteral nutrition–associated hyperglycemia require scheduled insulin therapy to address anticipated glucose excursions.
- Selected patients using insulin pump therapy prior to a hospital admission can continue to use these devices in the hospital if they have the mental and physical capacity to do so with knowledgeable hospital personnel.
- Diabetes self-management education provided to hospitalized patients can promote improved glycemic control following discharge with reductions in the risk for hospital readmission. “We know that is recommended for patients in the outpatient setting but often they do not get this,” she said. “We were able to observe that this can also impact long-term outcomes “
- Patients with diabetes scheduled for elective surgery may have improved postoperative outcomes when preoperative hemoglobin A1c is 8% or less and preoperative blood glucose is less than 180 mg/dL. “This recommendation answers the question: ‘Where should glycemic goals be for people who are undergoing surgery?’ ”
- Providing preoperative carbohydrate-containing beverages to patients with known diabetes is not recommended.
- Patients with newly recognized hyperglycemia or well-managed diabetes on noninsulin therapy may be treated with correctional insulin alone as initial therapy at hospital admission.
- Some noninsulin diabetes therapies can be used in combination with correction insulin for patients with type 2 diabetes who have mild hyperglycemia.
- Correctional insulin – “otherwise known as sliding-scale insulin” – can be used as initial therapy for patients with newly recognized hyperglycemia or type 2 diabetes treated with noninsulin therapy prior to hospital admission.
- Scheduled insulin therapy is preferred for patients experiencing persistent blood glucose values greater than 180 mg/dL and is recommended for patients using insulin therapy prior to admission.
The guideline writers’ hopes
“We hope that this guideline will resolve debates” about appropriate preoperative glycemic management and when sliding-scale insulin can be used and should not be used, said Dr. Korytkowski.
The authors also hope that “it will stimulate research funding for this very important aspect of diabetes care, and that hospitals will recognize the importance of having access to knowledgeable diabetes care and education specialists who can provide staff education regarding inpatient glycemic management, provide oversight for patients using insulin pump therapy or CGM devices, and empower hospital nurses to provide diabetes [self-management] education prior to patient discharge.”
Claire Pegg, the patient representative on the panel, hopes “that this guideline serves as the beginning of a conversation that will allow inpatient caregivers to provide individualized care to patients – some of whom may be self-sufficient with their glycemic management and others who need additional assistance.”
Development of the guideline was funded by the Endocrine Society. Dr. Korytkowski has reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Goal-directed glycemic management – which may include new technologies for glucose monitoring – for non–critically ill hospitalized patients who have diabetes or newly recognized hyperglycemia can improve outcomes, according to a new practice guideline from the Endocrine Society.
Even though roughly 35% of hospitalized patients have diabetes or newly discovered hyperglycemia, there is “wide variability in glycemic management in clinical practice,” writing panel chair Mary Korytkowski, MD, from the University of Pittsburgh, said at the annual meeting of the Endocrine Society. “These patients get admitted to every patient service in the hospital, meaning that every clinical service will encounter this group of patients, and their glycemic management can have a major effect on their outcomes. Both short term and long term.”
This guideline provides strategies “to achieve previously recommended glycemic goals while also reducing the risk for hypoglycemia, and this includes inpatient use of insulin pump therapy or continuous glucose monitoring [CGM] devices, among others,” she said.
It also includes “recommendations for preoperative glycemic goals as well as when the use of correctional insulin – well known as sliding scale insulin – may be appropriate” and when it is not.
The document, which replaces a 2012 guideline, was published online in the Journal of Clinical Endocrinology & Metabolism.
A multidisciplinary panel developed the document over the last 3 years to answer 10 clinical practice questions related to management of non–critically ill hospitalized patients with diabetes or newly discovered hyperglycemia.
Use of CGM devices in hospital
The first recommendation is: “In adults with insulin-treated diabetes hospitalized for noncritical illness who are at high risk of hypoglycemia, we suggest the use of real-time [CGM] with confirmatory bedside point-of-care blood glucose monitoring for adjustments in insulin dosing rather than point-of-care blood glucose rather than testing alone in hospital settings where resources and training are available.” (Conditional recommendation. Low certainty of evidence).
“We were actually very careful in terms of looking at the data” for use of CGMs, Dr. Korytkowski said in an interview.
Although CGMs are approved by the Food and Drug Administration in the outpatient setting, and that’s becoming the standard of care there, they are not yet approved for in-hospital use.
However, the FDA granted an emergency allowance for use of CGMs in hospitals during the COVID-19 pandemic.
That was “when everyone was scrambling for what to do,” Dr. Korytkowski noted. “There was a shortage of personal protective equipment and a real interest in trying to limit the amount of exposure of healthcare personnel in some of these really critically ill patients for whom intravenous insulin therapy was used to control their glucose level.”
On March 1, the FDA granted Breakthrough Devices Designation for Dexcom CGM use in the hospital setting.
The new guideline suggests CGM be used to detect trends in glycemic management, with insulin dosing decisions made with point-of-care glucose measure (the standard of care).
To implement CGM for glycemic management in hospitals, Dr. Korytkowski said, would require “extensive staff and nursing education to have people with expertise available to provide support to nursing personnel who are both placing these devices, changing these devices, looking at trends, and then knowing when to remove them for certain procedures such as MRI or radiologic procedures.”
“We know that not all hospitals may be readily available to use these devices,” she said. “It is an area of active research. But the use of these devices during the pandemic, in both critical care and non–critical care setting has really provided us with a lot of information that was used to formulate this suggestion in the guideline.”
The document addresses the following areas: CGM, continuous subcutaneous insulin infusion pump therapy, inpatient diabetes education, prespecified preoperative glycemic targets, use of neutral protamine Hagedorn insulin for glucocorticoid or enteral nutrition-associated hyperglycemia, noninsulin therapies, preoperative carbohydrate-containing oral fluids, carbohydrate counting for prandial (mealtime) insulin dosing, and correctional and scheduled (basal or basal bolus) insulin therapies.
Nine key recommendations
Dr. Korytkowski identified nine key recommendations:
- CGM systems can help guide glycemic management with reduced risk for hypoglycemia.
- Patients experiencing glucocorticoid- or enteral nutrition–associated hyperglycemia require scheduled insulin therapy to address anticipated glucose excursions.
- Selected patients using insulin pump therapy prior to a hospital admission can continue to use these devices in the hospital if they have the mental and physical capacity to do so with knowledgeable hospital personnel.
- Diabetes self-management education provided to hospitalized patients can promote improved glycemic control following discharge with reductions in the risk for hospital readmission. “We know that is recommended for patients in the outpatient setting but often they do not get this,” she said. “We were able to observe that this can also impact long-term outcomes “
- Patients with diabetes scheduled for elective surgery may have improved postoperative outcomes when preoperative hemoglobin A1c is 8% or less and preoperative blood glucose is less than 180 mg/dL. “This recommendation answers the question: ‘Where should glycemic goals be for people who are undergoing surgery?’ ”
- Providing preoperative carbohydrate-containing beverages to patients with known diabetes is not recommended.
- Patients with newly recognized hyperglycemia or well-managed diabetes on noninsulin therapy may be treated with correctional insulin alone as initial therapy at hospital admission.
- Some noninsulin diabetes therapies can be used in combination with correction insulin for patients with type 2 diabetes who have mild hyperglycemia.
- Correctional insulin – “otherwise known as sliding-scale insulin” – can be used as initial therapy for patients with newly recognized hyperglycemia or type 2 diabetes treated with noninsulin therapy prior to hospital admission.
- Scheduled insulin therapy is preferred for patients experiencing persistent blood glucose values greater than 180 mg/dL and is recommended for patients using insulin therapy prior to admission.
The guideline writers’ hopes
“We hope that this guideline will resolve debates” about appropriate preoperative glycemic management and when sliding-scale insulin can be used and should not be used, said Dr. Korytkowski.
The authors also hope that “it will stimulate research funding for this very important aspect of diabetes care, and that hospitals will recognize the importance of having access to knowledgeable diabetes care and education specialists who can provide staff education regarding inpatient glycemic management, provide oversight for patients using insulin pump therapy or CGM devices, and empower hospital nurses to provide diabetes [self-management] education prior to patient discharge.”
Claire Pegg, the patient representative on the panel, hopes “that this guideline serves as the beginning of a conversation that will allow inpatient caregivers to provide individualized care to patients – some of whom may be self-sufficient with their glycemic management and others who need additional assistance.”
Development of the guideline was funded by the Endocrine Society. Dr. Korytkowski has reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022
Self-injury and suicide ‘all too common’ in type 1 diabetes
Depression, self-harm, and suicide among people with type 1 and type 2 diabetes are “underappreciated” among health care practitioners, according to Katharine Barnard-Kelly, PhD, who founded the Reducing Suicide Rates Among Individuals With Diabetes (RESCUE) advocacy group in 2021.
“We have the most advanced technology to achieve glycemic control, but the mental burden remains underappreciated,” she said at a symposium with other speakers from RESCUE during the annual scientific sessions of the American Diabetes Association.
Notably, suicide and self-harm are “all too common” among young adults with type 1 diabetes who are receiving insulin, said Dr. Barnard-Kelly, a psychologist and visiting professor at Southern Health NHS Foundation Trust, Southampton, United Kingdom. And insulin under- or overdosing is the most common method of self-harm.
However, “with a multipronged approach to awareness, education, and identification, we have the opportunity to intervene on the link between suicide and diabetes,” she said, noting that the aim is to “raise awareness and arm [doctors and others] with messages that can ultimately save a young person’s life if adopted in clinical practice and through mental health screenings.”
The rationale behind the RESCUE initiative is also described in a brief report published in Diabetes Technology & Therapeutics.
Six key messages
RESCUE now has “approximately 30 members across academia, clinical practice, industry, advocacy, government, regulatory bodies [including the U.S. Food and Drug Administration], and people with diabetes from several countries,” Dr. Barnard-Kelly told this news organization.
She identified six key messages from the symposium:
- “Suicide prevalence is considerably higher among people with diabetes than the general population.
- Talking about suicide does not increase an individual’s risk of suicide.
- Current screening tools for depression and suicide are not sufficiently sensitive to be effective among people with diabetes.
- Identification of suicidal acts among people with diabetes is extremely difficult.
- For every suicide, the World Health Organization reports there are 20 suicide attempts.
- Health care providers often underestimate the prevalence of suicidality among their patient population and feel ill-equipped to initiate conversations with their patients about suicide.”
Dr. Barnard-Kelly also presented some sobering statistics that highlight the need for increased awareness.
A study reported that, of 160 cases of insulin overdose, 90% were suicides.
Adolescents and young adults with type 2 diabetes are 61% more likely to report suicidal thoughts than those without diabetes.
The risk of depression is two- to three-times higher in people with diabetes. According to another study, 7% of deaths in individuals with type 1 diabetes are estimated to be from suicide.
Survey about screening for depression, suicide risk in diabetes
During the symposium, Daniel R. Chernavvsky, MD, reported results from a small online survey of health care professionals who treat patients with type 1 or type 2 diabetes, which identified their concerns about screening for depression and assessing suicide risk in patients with diabetes.
Respondents were mainly from the United States (103) but were also from the United Kingdom (18), Slovenia, and the Netherlands (5), said Dr. Chernavvsky, who is senior director of medical affairs at Dexcom, Charlottesville, Va.
They included 59 doctors, 21 nurses,17 diabetes educators, 15 psychologists, seven dieticians, four social workers, and six “other” health care professionals, with a mean age of 46 (range, 25-72 years old) who had been working on average 14 years (range, 0.5-45 years).
Close to three-quarters (72%) reported that at least one of their patients had attempted suicide. The most common self-harm behaviors in their patients were insulin omission or a too large insulin bolus, and less often, binge eating.
Almost all respondents (95%) believed that routine visits to the diabetes clinic were appropriate times to discuss depression, self-injury, and suicidal ideation – at every visit (42% of respondents) or some visits (52%).
Only 30% were comfortable asking patients about self-harm or suicide.
Psychologists and social workers were very comfortable, but others were less comfortable or not comfortable at all.
Many respondents expressed concerns such as, “What do I do?” “Would I make the problem worse?” “Would I give the patient the idea?” Some reported they had “limited resources” or it “feels invasive.”
They identified a need for “a better understanding of what [they could] do to support and care for patients,” and “more knowledge about how to deal with [patients’] answers” to screening questionnaires.
Screening for psychological morbidities in diabetes
Guidelines from the ADA and the International Society for Pediatric and Adolescent Diabetes recommend routine screening of patients with diabetes for psychological morbidities, including depression, said Shideh Majidi, MD.
Depression is associated with higher A1c, noted Dr. Majidi, who is associate director, childhood and adolescent diabetes program at Children’s National Hospital, Washington, D.C.
She identified the following topics that need to be addressed when considering implementing a program for depression screening and suicide risk assessment in a diabetes clinic:
- Conducting screening: Which screening questionnaire will you use? Who will do it? Where? How often?
- Scoring screening questionnaires: Who will do it?
- Depression screening discussion: Who will do it? How will the person be notified of the score?
- Suicide risk assessment: Who will conduct it? What is the process to get someone to the emergency department?
- Resources/referral: Who will initiate and follow-up?
Next steps
The RESCUE advocacy group is preparing educational and support materials for health care professionals who treat patients with diabetes, as well as other materials for patients themselves.
A version of this article first appeared on Medscape.com.
Depression, self-harm, and suicide among people with type 1 and type 2 diabetes are “underappreciated” among health care practitioners, according to Katharine Barnard-Kelly, PhD, who founded the Reducing Suicide Rates Among Individuals With Diabetes (RESCUE) advocacy group in 2021.
“We have the most advanced technology to achieve glycemic control, but the mental burden remains underappreciated,” she said at a symposium with other speakers from RESCUE during the annual scientific sessions of the American Diabetes Association.
Notably, suicide and self-harm are “all too common” among young adults with type 1 diabetes who are receiving insulin, said Dr. Barnard-Kelly, a psychologist and visiting professor at Southern Health NHS Foundation Trust, Southampton, United Kingdom. And insulin under- or overdosing is the most common method of self-harm.
However, “with a multipronged approach to awareness, education, and identification, we have the opportunity to intervene on the link between suicide and diabetes,” she said, noting that the aim is to “raise awareness and arm [doctors and others] with messages that can ultimately save a young person’s life if adopted in clinical practice and through mental health screenings.”
The rationale behind the RESCUE initiative is also described in a brief report published in Diabetes Technology & Therapeutics.
Six key messages
RESCUE now has “approximately 30 members across academia, clinical practice, industry, advocacy, government, regulatory bodies [including the U.S. Food and Drug Administration], and people with diabetes from several countries,” Dr. Barnard-Kelly told this news organization.
She identified six key messages from the symposium:
- “Suicide prevalence is considerably higher among people with diabetes than the general population.
- Talking about suicide does not increase an individual’s risk of suicide.
- Current screening tools for depression and suicide are not sufficiently sensitive to be effective among people with diabetes.
- Identification of suicidal acts among people with diabetes is extremely difficult.
- For every suicide, the World Health Organization reports there are 20 suicide attempts.
- Health care providers often underestimate the prevalence of suicidality among their patient population and feel ill-equipped to initiate conversations with their patients about suicide.”
Dr. Barnard-Kelly also presented some sobering statistics that highlight the need for increased awareness.
A study reported that, of 160 cases of insulin overdose, 90% were suicides.
Adolescents and young adults with type 2 diabetes are 61% more likely to report suicidal thoughts than those without diabetes.
The risk of depression is two- to three-times higher in people with diabetes. According to another study, 7% of deaths in individuals with type 1 diabetes are estimated to be from suicide.
Survey about screening for depression, suicide risk in diabetes
During the symposium, Daniel R. Chernavvsky, MD, reported results from a small online survey of health care professionals who treat patients with type 1 or type 2 diabetes, which identified their concerns about screening for depression and assessing suicide risk in patients with diabetes.
Respondents were mainly from the United States (103) but were also from the United Kingdom (18), Slovenia, and the Netherlands (5), said Dr. Chernavvsky, who is senior director of medical affairs at Dexcom, Charlottesville, Va.
They included 59 doctors, 21 nurses,17 diabetes educators, 15 psychologists, seven dieticians, four social workers, and six “other” health care professionals, with a mean age of 46 (range, 25-72 years old) who had been working on average 14 years (range, 0.5-45 years).
Close to three-quarters (72%) reported that at least one of their patients had attempted suicide. The most common self-harm behaviors in their patients were insulin omission or a too large insulin bolus, and less often, binge eating.
Almost all respondents (95%) believed that routine visits to the diabetes clinic were appropriate times to discuss depression, self-injury, and suicidal ideation – at every visit (42% of respondents) or some visits (52%).
Only 30% were comfortable asking patients about self-harm or suicide.
Psychologists and social workers were very comfortable, but others were less comfortable or not comfortable at all.
Many respondents expressed concerns such as, “What do I do?” “Would I make the problem worse?” “Would I give the patient the idea?” Some reported they had “limited resources” or it “feels invasive.”
They identified a need for “a better understanding of what [they could] do to support and care for patients,” and “more knowledge about how to deal with [patients’] answers” to screening questionnaires.
Screening for psychological morbidities in diabetes
Guidelines from the ADA and the International Society for Pediatric and Adolescent Diabetes recommend routine screening of patients with diabetes for psychological morbidities, including depression, said Shideh Majidi, MD.
Depression is associated with higher A1c, noted Dr. Majidi, who is associate director, childhood and adolescent diabetes program at Children’s National Hospital, Washington, D.C.
She identified the following topics that need to be addressed when considering implementing a program for depression screening and suicide risk assessment in a diabetes clinic:
- Conducting screening: Which screening questionnaire will you use? Who will do it? Where? How often?
- Scoring screening questionnaires: Who will do it?
- Depression screening discussion: Who will do it? How will the person be notified of the score?
- Suicide risk assessment: Who will conduct it? What is the process to get someone to the emergency department?
- Resources/referral: Who will initiate and follow-up?
Next steps
The RESCUE advocacy group is preparing educational and support materials for health care professionals who treat patients with diabetes, as well as other materials for patients themselves.
A version of this article first appeared on Medscape.com.
Depression, self-harm, and suicide among people with type 1 and type 2 diabetes are “underappreciated” among health care practitioners, according to Katharine Barnard-Kelly, PhD, who founded the Reducing Suicide Rates Among Individuals With Diabetes (RESCUE) advocacy group in 2021.
“We have the most advanced technology to achieve glycemic control, but the mental burden remains underappreciated,” she said at a symposium with other speakers from RESCUE during the annual scientific sessions of the American Diabetes Association.
Notably, suicide and self-harm are “all too common” among young adults with type 1 diabetes who are receiving insulin, said Dr. Barnard-Kelly, a psychologist and visiting professor at Southern Health NHS Foundation Trust, Southampton, United Kingdom. And insulin under- or overdosing is the most common method of self-harm.
However, “with a multipronged approach to awareness, education, and identification, we have the opportunity to intervene on the link between suicide and diabetes,” she said, noting that the aim is to “raise awareness and arm [doctors and others] with messages that can ultimately save a young person’s life if adopted in clinical practice and through mental health screenings.”
The rationale behind the RESCUE initiative is also described in a brief report published in Diabetes Technology & Therapeutics.
Six key messages
RESCUE now has “approximately 30 members across academia, clinical practice, industry, advocacy, government, regulatory bodies [including the U.S. Food and Drug Administration], and people with diabetes from several countries,” Dr. Barnard-Kelly told this news organization.
She identified six key messages from the symposium:
- “Suicide prevalence is considerably higher among people with diabetes than the general population.
- Talking about suicide does not increase an individual’s risk of suicide.
- Current screening tools for depression and suicide are not sufficiently sensitive to be effective among people with diabetes.
- Identification of suicidal acts among people with diabetes is extremely difficult.
- For every suicide, the World Health Organization reports there are 20 suicide attempts.
- Health care providers often underestimate the prevalence of suicidality among their patient population and feel ill-equipped to initiate conversations with their patients about suicide.”
Dr. Barnard-Kelly also presented some sobering statistics that highlight the need for increased awareness.
A study reported that, of 160 cases of insulin overdose, 90% were suicides.
Adolescents and young adults with type 2 diabetes are 61% more likely to report suicidal thoughts than those without diabetes.
The risk of depression is two- to three-times higher in people with diabetes. According to another study, 7% of deaths in individuals with type 1 diabetes are estimated to be from suicide.
Survey about screening for depression, suicide risk in diabetes
During the symposium, Daniel R. Chernavvsky, MD, reported results from a small online survey of health care professionals who treat patients with type 1 or type 2 diabetes, which identified their concerns about screening for depression and assessing suicide risk in patients with diabetes.
Respondents were mainly from the United States (103) but were also from the United Kingdom (18), Slovenia, and the Netherlands (5), said Dr. Chernavvsky, who is senior director of medical affairs at Dexcom, Charlottesville, Va.
They included 59 doctors, 21 nurses,17 diabetes educators, 15 psychologists, seven dieticians, four social workers, and six “other” health care professionals, with a mean age of 46 (range, 25-72 years old) who had been working on average 14 years (range, 0.5-45 years).
Close to three-quarters (72%) reported that at least one of their patients had attempted suicide. The most common self-harm behaviors in their patients were insulin omission or a too large insulin bolus, and less often, binge eating.
Almost all respondents (95%) believed that routine visits to the diabetes clinic were appropriate times to discuss depression, self-injury, and suicidal ideation – at every visit (42% of respondents) or some visits (52%).
Only 30% were comfortable asking patients about self-harm or suicide.
Psychologists and social workers were very comfortable, but others were less comfortable or not comfortable at all.
Many respondents expressed concerns such as, “What do I do?” “Would I make the problem worse?” “Would I give the patient the idea?” Some reported they had “limited resources” or it “feels invasive.”
They identified a need for “a better understanding of what [they could] do to support and care for patients,” and “more knowledge about how to deal with [patients’] answers” to screening questionnaires.
Screening for psychological morbidities in diabetes
Guidelines from the ADA and the International Society for Pediatric and Adolescent Diabetes recommend routine screening of patients with diabetes for psychological morbidities, including depression, said Shideh Majidi, MD.
Depression is associated with higher A1c, noted Dr. Majidi, who is associate director, childhood and adolescent diabetes program at Children’s National Hospital, Washington, D.C.
She identified the following topics that need to be addressed when considering implementing a program for depression screening and suicide risk assessment in a diabetes clinic:
- Conducting screening: Which screening questionnaire will you use? Who will do it? Where? How often?
- Scoring screening questionnaires: Who will do it?
- Depression screening discussion: Who will do it? How will the person be notified of the score?
- Suicide risk assessment: Who will conduct it? What is the process to get someone to the emergency department?
- Resources/referral: Who will initiate and follow-up?
Next steps
The RESCUE advocacy group is preparing educational and support materials for health care professionals who treat patients with diabetes, as well as other materials for patients themselves.
A version of this article first appeared on Medscape.com.
FROM ADA 2022
2022 GOLD Report: Tips for diagnosing and evaluating COPD
For many years, COPD has remained one of the top four leading causes of death in the United States according to CDC data. Around the world, it is responsible for about 3 million deaths annually. It is estimated that 16 million Americans are now diagnosed with COPD. However, it is commonly agreed by experts that it is widely underdiagnosed and there may be millions more suffering from this disease.
The direct costs of COPD are around $49 billion a year in direct costs, with billions more in indirect costs. Around the globe, COPD is one of the top three causes of death, with 90% of deaths happening in low- and middle-income countries. The burden of COPD is expected to grow over time because of the aging population and continued exposure to COPD risk factors.
The Global Initiative for Chronic Obstructive Lung Disease report (or GOLD) is revised every year, translated into many languages, and used by health care workers globally. It was started in 1998, and its aim was to produce guidelines based on the best scientific evidence available that was nonbiased to be used for assessment, diagnosis, and treatment of patients with COPD. The first report was issued in 2001. The method of producing the GOLD report was to do a search of PubMed for evidence-based, peer-reviewed studies. Those not captured by this method could be submitted for review. The science committee then meets twice a year and reviews each publication, eventually agreeing on a set of guidelines/updates.
2022 GOLD Report
For the 2022 GOLD report, 160 new references were added. Overall, the GOLD report is five chapters (more than 150 pages) giving in-depth guidance for the diagnosis, prevention, management, and treatment of patients with stable COPD, COPD exacerbations, and hospitalized patients.
The report suggests that COPD is being underdiagnosed.
Family physicians and internists will be seeing more and more cases as the population ages, and we need to do a better job of recognizing patients who have COPD. If possible, we should try to have spirometry available in our practices. Like any other disease, we know prevention works best so primary care physicians also need to be looking for risk factors, such as smoking history, and help patients try to reduce them if possible. Below is more explanation of the latest guidelines.
For most of us, when we learned about COPD as a disease, the terms “chronic bronchitis” and “emphysema” were emphasized. These words are no longer used as synonymous for COPD.
The disease is now described as involving chronic limitation in airflow that results from a combination of small airway disease and parenchymal destruction (emphysema). The rates of each vary from person to person and progress at different rates. Key factors that contribute to COPD disease burden include chronic inflammation, narrowing of small airways, loss of alveolar attachments, loss of elastic recoil, and mucociliary dysfunction, according to the 2022 GOLD report.
Respiratory symptoms may precede the onset of airflow limitation. COPD should be considered in any patient with dyspnea, chronic cough or sputum production, a history of recurrent lower respiratory tract infections, and risk factors for the disease.
The biggest risk factor for COPD is smoking. Other risk factors include occupational exposure, e-cigarette use, pollution, genetic factors, and comorbid conditions. Symptoms of the disease can include chest tightness, wheezing, and fatigue.
To make a diagnosis of COPD, spirometry is required, the latest GOLD report says. A postbronchodilator FEV1/FVC less than 0.70 confirms persistent airflow limitation and hence COPD. This value is used in clinical trials and forms the basis of what most treatment guidelines are derived from. It would be beneficial for any physician treating COPD patients to have easy access to spirometry. It provides the most reproducible and objective measurement of airflow limitation. Also, it was found that assessing the degree of reversibility of airflow limitation to decide therapeutic decisions is no longer recommended and thus, asking the patient to stop inhaled medications beforehand is unnecessary. To access the impact COPD has on a patient’s life beyond dyspnea, the guidelines recommend doing a disease-specific health questionnaire, such as the COPD Assessment Test (CAT).
Along with patient symptoms and history of exacerbations, spirometry is crucial for the diagnosis, prognosis, and therapeutic decisions in COPD patients, according to the GOLD guidance. The best predictor of frequent exacerbations, however, is a history of previous exacerbations. In cases where there is a discrepancy between airflow limitation and symptoms, additional testing should be considered. Alpha-1 antitrypsin deficiency (AATD) screening should be considered in younger patients (under 45 years) with perilobular emphysema, and those in areas of high AATD prevalence. Chest x-rays are not recommended in diagnosing COPD but can be helpful if other comorbidities are present. CT scan is not routinely recommended but should be used only for the detection of bronchiectasis, if the patient meets the criteria for lung cancer screening, if surgery is necessary, or if other diseases may need to be evaluated.
Pulse oximetry can be helpful in accessing degree of severity, respiratory failure, and right heart failure. Walking tests can be helpful for evaluating disability and mortality risk. Other tests that have been used but are not routinely recommended include plethysmography and diffusing capacity of the lungs for carbon monoxide.
Composite scores can identify patients who are at increased risk of mortality. One such score is the BODE (Body mass, Obstruction, Dyspnea, and Exercise) method. Biomarkers are being investigated, but data are still not available to recommend their routine use.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
For many years, COPD has remained one of the top four leading causes of death in the United States according to CDC data. Around the world, it is responsible for about 3 million deaths annually. It is estimated that 16 million Americans are now diagnosed with COPD. However, it is commonly agreed by experts that it is widely underdiagnosed and there may be millions more suffering from this disease.
The direct costs of COPD are around $49 billion a year in direct costs, with billions more in indirect costs. Around the globe, COPD is one of the top three causes of death, with 90% of deaths happening in low- and middle-income countries. The burden of COPD is expected to grow over time because of the aging population and continued exposure to COPD risk factors.
The Global Initiative for Chronic Obstructive Lung Disease report (or GOLD) is revised every year, translated into many languages, and used by health care workers globally. It was started in 1998, and its aim was to produce guidelines based on the best scientific evidence available that was nonbiased to be used for assessment, diagnosis, and treatment of patients with COPD. The first report was issued in 2001. The method of producing the GOLD report was to do a search of PubMed for evidence-based, peer-reviewed studies. Those not captured by this method could be submitted for review. The science committee then meets twice a year and reviews each publication, eventually agreeing on a set of guidelines/updates.
2022 GOLD Report
For the 2022 GOLD report, 160 new references were added. Overall, the GOLD report is five chapters (more than 150 pages) giving in-depth guidance for the diagnosis, prevention, management, and treatment of patients with stable COPD, COPD exacerbations, and hospitalized patients.
The report suggests that COPD is being underdiagnosed.
Family physicians and internists will be seeing more and more cases as the population ages, and we need to do a better job of recognizing patients who have COPD. If possible, we should try to have spirometry available in our practices. Like any other disease, we know prevention works best so primary care physicians also need to be looking for risk factors, such as smoking history, and help patients try to reduce them if possible. Below is more explanation of the latest guidelines.
For most of us, when we learned about COPD as a disease, the terms “chronic bronchitis” and “emphysema” were emphasized. These words are no longer used as synonymous for COPD.
The disease is now described as involving chronic limitation in airflow that results from a combination of small airway disease and parenchymal destruction (emphysema). The rates of each vary from person to person and progress at different rates. Key factors that contribute to COPD disease burden include chronic inflammation, narrowing of small airways, loss of alveolar attachments, loss of elastic recoil, and mucociliary dysfunction, according to the 2022 GOLD report.
Respiratory symptoms may precede the onset of airflow limitation. COPD should be considered in any patient with dyspnea, chronic cough or sputum production, a history of recurrent lower respiratory tract infections, and risk factors for the disease.
The biggest risk factor for COPD is smoking. Other risk factors include occupational exposure, e-cigarette use, pollution, genetic factors, and comorbid conditions. Symptoms of the disease can include chest tightness, wheezing, and fatigue.
To make a diagnosis of COPD, spirometry is required, the latest GOLD report says. A postbronchodilator FEV1/FVC less than 0.70 confirms persistent airflow limitation and hence COPD. This value is used in clinical trials and forms the basis of what most treatment guidelines are derived from. It would be beneficial for any physician treating COPD patients to have easy access to spirometry. It provides the most reproducible and objective measurement of airflow limitation. Also, it was found that assessing the degree of reversibility of airflow limitation to decide therapeutic decisions is no longer recommended and thus, asking the patient to stop inhaled medications beforehand is unnecessary. To access the impact COPD has on a patient’s life beyond dyspnea, the guidelines recommend doing a disease-specific health questionnaire, such as the COPD Assessment Test (CAT).
Along with patient symptoms and history of exacerbations, spirometry is crucial for the diagnosis, prognosis, and therapeutic decisions in COPD patients, according to the GOLD guidance. The best predictor of frequent exacerbations, however, is a history of previous exacerbations. In cases where there is a discrepancy between airflow limitation and symptoms, additional testing should be considered. Alpha-1 antitrypsin deficiency (AATD) screening should be considered in younger patients (under 45 years) with perilobular emphysema, and those in areas of high AATD prevalence. Chest x-rays are not recommended in diagnosing COPD but can be helpful if other comorbidities are present. CT scan is not routinely recommended but should be used only for the detection of bronchiectasis, if the patient meets the criteria for lung cancer screening, if surgery is necessary, or if other diseases may need to be evaluated.
Pulse oximetry can be helpful in accessing degree of severity, respiratory failure, and right heart failure. Walking tests can be helpful for evaluating disability and mortality risk. Other tests that have been used but are not routinely recommended include plethysmography and diffusing capacity of the lungs for carbon monoxide.
Composite scores can identify patients who are at increased risk of mortality. One such score is the BODE (Body mass, Obstruction, Dyspnea, and Exercise) method. Biomarkers are being investigated, but data are still not available to recommend their routine use.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
For many years, COPD has remained one of the top four leading causes of death in the United States according to CDC data. Around the world, it is responsible for about 3 million deaths annually. It is estimated that 16 million Americans are now diagnosed with COPD. However, it is commonly agreed by experts that it is widely underdiagnosed and there may be millions more suffering from this disease.
The direct costs of COPD are around $49 billion a year in direct costs, with billions more in indirect costs. Around the globe, COPD is one of the top three causes of death, with 90% of deaths happening in low- and middle-income countries. The burden of COPD is expected to grow over time because of the aging population and continued exposure to COPD risk factors.
The Global Initiative for Chronic Obstructive Lung Disease report (or GOLD) is revised every year, translated into many languages, and used by health care workers globally. It was started in 1998, and its aim was to produce guidelines based on the best scientific evidence available that was nonbiased to be used for assessment, diagnosis, and treatment of patients with COPD. The first report was issued in 2001. The method of producing the GOLD report was to do a search of PubMed for evidence-based, peer-reviewed studies. Those not captured by this method could be submitted for review. The science committee then meets twice a year and reviews each publication, eventually agreeing on a set of guidelines/updates.
2022 GOLD Report
For the 2022 GOLD report, 160 new references were added. Overall, the GOLD report is five chapters (more than 150 pages) giving in-depth guidance for the diagnosis, prevention, management, and treatment of patients with stable COPD, COPD exacerbations, and hospitalized patients.
The report suggests that COPD is being underdiagnosed.
Family physicians and internists will be seeing more and more cases as the population ages, and we need to do a better job of recognizing patients who have COPD. If possible, we should try to have spirometry available in our practices. Like any other disease, we know prevention works best so primary care physicians also need to be looking for risk factors, such as smoking history, and help patients try to reduce them if possible. Below is more explanation of the latest guidelines.
For most of us, when we learned about COPD as a disease, the terms “chronic bronchitis” and “emphysema” were emphasized. These words are no longer used as synonymous for COPD.
The disease is now described as involving chronic limitation in airflow that results from a combination of small airway disease and parenchymal destruction (emphysema). The rates of each vary from person to person and progress at different rates. Key factors that contribute to COPD disease burden include chronic inflammation, narrowing of small airways, loss of alveolar attachments, loss of elastic recoil, and mucociliary dysfunction, according to the 2022 GOLD report.
Respiratory symptoms may precede the onset of airflow limitation. COPD should be considered in any patient with dyspnea, chronic cough or sputum production, a history of recurrent lower respiratory tract infections, and risk factors for the disease.
The biggest risk factor for COPD is smoking. Other risk factors include occupational exposure, e-cigarette use, pollution, genetic factors, and comorbid conditions. Symptoms of the disease can include chest tightness, wheezing, and fatigue.
To make a diagnosis of COPD, spirometry is required, the latest GOLD report says. A postbronchodilator FEV1/FVC less than 0.70 confirms persistent airflow limitation and hence COPD. This value is used in clinical trials and forms the basis of what most treatment guidelines are derived from. It would be beneficial for any physician treating COPD patients to have easy access to spirometry. It provides the most reproducible and objective measurement of airflow limitation. Also, it was found that assessing the degree of reversibility of airflow limitation to decide therapeutic decisions is no longer recommended and thus, asking the patient to stop inhaled medications beforehand is unnecessary. To access the impact COPD has on a patient’s life beyond dyspnea, the guidelines recommend doing a disease-specific health questionnaire, such as the COPD Assessment Test (CAT).
Along with patient symptoms and history of exacerbations, spirometry is crucial for the diagnosis, prognosis, and therapeutic decisions in COPD patients, according to the GOLD guidance. The best predictor of frequent exacerbations, however, is a history of previous exacerbations. In cases where there is a discrepancy between airflow limitation and symptoms, additional testing should be considered. Alpha-1 antitrypsin deficiency (AATD) screening should be considered in younger patients (under 45 years) with perilobular emphysema, and those in areas of high AATD prevalence. Chest x-rays are not recommended in diagnosing COPD but can be helpful if other comorbidities are present. CT scan is not routinely recommended but should be used only for the detection of bronchiectasis, if the patient meets the criteria for lung cancer screening, if surgery is necessary, or if other diseases may need to be evaluated.
Pulse oximetry can be helpful in accessing degree of severity, respiratory failure, and right heart failure. Walking tests can be helpful for evaluating disability and mortality risk. Other tests that have been used but are not routinely recommended include plethysmography and diffusing capacity of the lungs for carbon monoxide.
Composite scores can identify patients who are at increased risk of mortality. One such score is the BODE (Body mass, Obstruction, Dyspnea, and Exercise) method. Biomarkers are being investigated, but data are still not available to recommend their routine use.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
Coalescing skin-colored papules
AN 8-YEAR-OLD BOY was evaluated by his family physician for a widespread rash that had first appeared on his arms 4 months earlier. Physical examination revealed 1- to 2-mm hypopigmented, smooth, and dome-shaped papules in clusters and linear arrays on the child’s back, shoulders, and extensor surfaces of both arms (FIGURE). There was no tenderness to palpation of the affected areas, but the patient complained of pruritus. Otherwise, he was in good health.
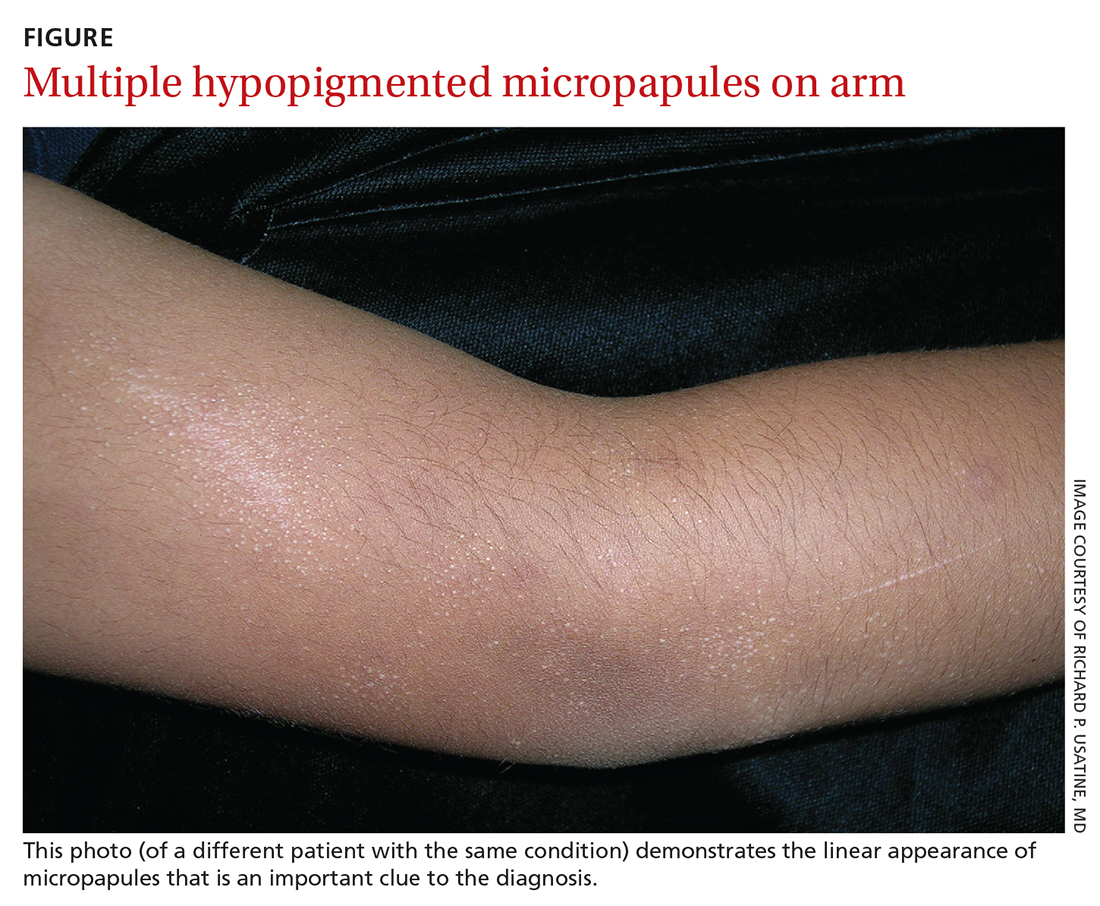
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen nitidus
This clinical manifestation of multiple, hypopigmented, pinhead-sized papules is most consistent with the diagnosis of lichen nitidus.
A rare and chronic inflammatory skin condition, lichen nitidus is characterized by numerous small, skin-colored papules that are often arranged in clusters on the upper extremities, the genitalia, and the anterior trunk.1 The papules are less likely to occur on the face, lower extremities, palms, and soles. Oral mucosal and nail involvement are rare. The condition is usually asymptomatic but can sometimes be associated with pruritus.
Lichen nitidus occurs more frequently in children or young adults and has a female predominance.1 It does not exhibit a predilection of any race.2 The etiology and pathogenesis of lichen nitidus remain unclear. Genetic factors have been proposed as a potential cause; it has also been reported to be associated with Down syndrome.3
Making the Dx with dermoscopy, skin biopsy
Dermoscopy is a useful technique for diagnosing lichen nitidus. Dermoscopic features of lichen nitidus include white, well-demarcated circular areas with a brown shadow.4 Skin biopsy provides a definitive diagnosis. Lichen nitidus has a distinct histopathologic “ball and claw” appearance of rete ridges clutching a lymphohistiocytic infiltrate.1
Consider these common conditions in the differential
The differential diagnosis includes lichen spinulosus, papular eczema, lichen planus, keratosis pilaris, and verruca plana (flat warts).
Continue to: Lichen spinulosus
Lichen spinulosus lesions are similar in appearance to lichen nitidus but are grouped in patches on the neck, arms, abdomen, and buttocks.1 The Koebner phenomenon is not typically present. Lichen spinulosus lesions consist of follicular papules that may exhibit a central keratotic plug.
Papular eczema lesions lack the uniform and discrete appearance observed in lichen nitidus. Pruritus is also more likely to be present in papular eczema.
Lichen planus lesions are typically violaceous, flat, and larger in size than lichen nitidus (measuring 1 mm to 1 cm), and have characteristic Wickham striae. Oral involvement is also more suggestive of lichen planus.
Keratosis pilaris is distinguished by its much more common occurrence and perifollicular erythema.
Verruca plana, in contrast to lichen nitidus, are typically pink, flat-topped lesions. They are also larger in size (2 mm to 5 mm).
Continue to: Topical treatment can help manage the condition
Topical treatment can help manage the condition
Most patients experience spontaneous resolution of lesions within several years; treatment is primarily for symptomatic or cosmetic purposes. When pruritus is present, topical corticosteroids and oral antihistamines may help (eg, hydrocortisone 2.5% cream and oral hydroxyzine). Topical calcineurin inhibitors, such as pimecrolimus cream, have also been reported as an effective therapy in children with lichen nitidus.1 In patients with generalized lichen nitidus who have not responded to topical corticosteroids, phototherapy can be used.5 There are no randomized controlled trials to assess the effectiveness of different types of treatments.
In this case, the patient was advised to start using an over-the-counter topical steroid, such as 1% hydrocortisone cream, to help control pruritus. He was scheduled for a follow-up appointment in 3 months.
1. Shiohara T, Mizukawa Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Elsevier Inc;2008:167-170.
2. Lapins NA, Willoughby C, Helwig EB. Lichen nitidus. A study of forty-three cases. Cutis. 1978;21:634-637.
3. Botelho LFF, de Magalhães JPJ, Ogawa MM, et al. Generalized Lichen nitidus associated with Down’s syndrome: case report. An Bras Dermatol. 2012;87:466-468. doi: 10.1590/s0365-05962012000300018
4. Malakar S, Save S, Mehta P. Brown shadow in lichen nitidus: a dermoscopic marker! Indian Dermatol Online J. 2018;9:479-480. doi: 10.4103/idoj.IDOJ_338_17
5. Synakiewicz J, Polańska A, Bowszyc-Dmochowska M, et al. Generalized lichen nitidus: a case report and review of the literature. Postepy Dermatol Alergol. 2016;33:488-490. doi: 10.5114/ada.2016.63890
AN 8-YEAR-OLD BOY was evaluated by his family physician for a widespread rash that had first appeared on his arms 4 months earlier. Physical examination revealed 1- to 2-mm hypopigmented, smooth, and dome-shaped papules in clusters and linear arrays on the child’s back, shoulders, and extensor surfaces of both arms (FIGURE). There was no tenderness to palpation of the affected areas, but the patient complained of pruritus. Otherwise, he was in good health.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen nitidus
This clinical manifestation of multiple, hypopigmented, pinhead-sized papules is most consistent with the diagnosis of lichen nitidus.
A rare and chronic inflammatory skin condition, lichen nitidus is characterized by numerous small, skin-colored papules that are often arranged in clusters on the upper extremities, the genitalia, and the anterior trunk.1 The papules are less likely to occur on the face, lower extremities, palms, and soles. Oral mucosal and nail involvement are rare. The condition is usually asymptomatic but can sometimes be associated with pruritus.
Lichen nitidus occurs more frequently in children or young adults and has a female predominance.1 It does not exhibit a predilection of any race.2 The etiology and pathogenesis of lichen nitidus remain unclear. Genetic factors have been proposed as a potential cause; it has also been reported to be associated with Down syndrome.3
Making the Dx with dermoscopy, skin biopsy
Dermoscopy is a useful technique for diagnosing lichen nitidus. Dermoscopic features of lichen nitidus include white, well-demarcated circular areas with a brown shadow.4 Skin biopsy provides a definitive diagnosis. Lichen nitidus has a distinct histopathologic “ball and claw” appearance of rete ridges clutching a lymphohistiocytic infiltrate.1
Consider these common conditions in the differential
The differential diagnosis includes lichen spinulosus, papular eczema, lichen planus, keratosis pilaris, and verruca plana (flat warts).
Continue to: Lichen spinulosus
Lichen spinulosus lesions are similar in appearance to lichen nitidus but are grouped in patches on the neck, arms, abdomen, and buttocks.1 The Koebner phenomenon is not typically present. Lichen spinulosus lesions consist of follicular papules that may exhibit a central keratotic plug.
Papular eczema lesions lack the uniform and discrete appearance observed in lichen nitidus. Pruritus is also more likely to be present in papular eczema.
Lichen planus lesions are typically violaceous, flat, and larger in size than lichen nitidus (measuring 1 mm to 1 cm), and have characteristic Wickham striae. Oral involvement is also more suggestive of lichen planus.
Keratosis pilaris is distinguished by its much more common occurrence and perifollicular erythema.
Verruca plana, in contrast to lichen nitidus, are typically pink, flat-topped lesions. They are also larger in size (2 mm to 5 mm).
Continue to: Topical treatment can help manage the condition
Topical treatment can help manage the condition
Most patients experience spontaneous resolution of lesions within several years; treatment is primarily for symptomatic or cosmetic purposes. When pruritus is present, topical corticosteroids and oral antihistamines may help (eg, hydrocortisone 2.5% cream and oral hydroxyzine). Topical calcineurin inhibitors, such as pimecrolimus cream, have also been reported as an effective therapy in children with lichen nitidus.1 In patients with generalized lichen nitidus who have not responded to topical corticosteroids, phototherapy can be used.5 There are no randomized controlled trials to assess the effectiveness of different types of treatments.
In this case, the patient was advised to start using an over-the-counter topical steroid, such as 1% hydrocortisone cream, to help control pruritus. He was scheduled for a follow-up appointment in 3 months.
AN 8-YEAR-OLD BOY was evaluated by his family physician for a widespread rash that had first appeared on his arms 4 months earlier. Physical examination revealed 1- to 2-mm hypopigmented, smooth, and dome-shaped papules in clusters and linear arrays on the child’s back, shoulders, and extensor surfaces of both arms (FIGURE). There was no tenderness to palpation of the affected areas, but the patient complained of pruritus. Otherwise, he was in good health.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lichen nitidus
This clinical manifestation of multiple, hypopigmented, pinhead-sized papules is most consistent with the diagnosis of lichen nitidus.
A rare and chronic inflammatory skin condition, lichen nitidus is characterized by numerous small, skin-colored papules that are often arranged in clusters on the upper extremities, the genitalia, and the anterior trunk.1 The papules are less likely to occur on the face, lower extremities, palms, and soles. Oral mucosal and nail involvement are rare. The condition is usually asymptomatic but can sometimes be associated with pruritus.
Lichen nitidus occurs more frequently in children or young adults and has a female predominance.1 It does not exhibit a predilection of any race.2 The etiology and pathogenesis of lichen nitidus remain unclear. Genetic factors have been proposed as a potential cause; it has also been reported to be associated with Down syndrome.3
Making the Dx with dermoscopy, skin biopsy
Dermoscopy is a useful technique for diagnosing lichen nitidus. Dermoscopic features of lichen nitidus include white, well-demarcated circular areas with a brown shadow.4 Skin biopsy provides a definitive diagnosis. Lichen nitidus has a distinct histopathologic “ball and claw” appearance of rete ridges clutching a lymphohistiocytic infiltrate.1
Consider these common conditions in the differential
The differential diagnosis includes lichen spinulosus, papular eczema, lichen planus, keratosis pilaris, and verruca plana (flat warts).
Continue to: Lichen spinulosus
Lichen spinulosus lesions are similar in appearance to lichen nitidus but are grouped in patches on the neck, arms, abdomen, and buttocks.1 The Koebner phenomenon is not typically present. Lichen spinulosus lesions consist of follicular papules that may exhibit a central keratotic plug.
Papular eczema lesions lack the uniform and discrete appearance observed in lichen nitidus. Pruritus is also more likely to be present in papular eczema.
Lichen planus lesions are typically violaceous, flat, and larger in size than lichen nitidus (measuring 1 mm to 1 cm), and have characteristic Wickham striae. Oral involvement is also more suggestive of lichen planus.
Keratosis pilaris is distinguished by its much more common occurrence and perifollicular erythema.
Verruca plana, in contrast to lichen nitidus, are typically pink, flat-topped lesions. They are also larger in size (2 mm to 5 mm).
Continue to: Topical treatment can help manage the condition
Topical treatment can help manage the condition
Most patients experience spontaneous resolution of lesions within several years; treatment is primarily for symptomatic or cosmetic purposes. When pruritus is present, topical corticosteroids and oral antihistamines may help (eg, hydrocortisone 2.5% cream and oral hydroxyzine). Topical calcineurin inhibitors, such as pimecrolimus cream, have also been reported as an effective therapy in children with lichen nitidus.1 In patients with generalized lichen nitidus who have not responded to topical corticosteroids, phototherapy can be used.5 There are no randomized controlled trials to assess the effectiveness of different types of treatments.
In this case, the patient was advised to start using an over-the-counter topical steroid, such as 1% hydrocortisone cream, to help control pruritus. He was scheduled for a follow-up appointment in 3 months.
1. Shiohara T, Mizukawa Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Elsevier Inc;2008:167-170.
2. Lapins NA, Willoughby C, Helwig EB. Lichen nitidus. A study of forty-three cases. Cutis. 1978;21:634-637.
3. Botelho LFF, de Magalhães JPJ, Ogawa MM, et al. Generalized Lichen nitidus associated with Down’s syndrome: case report. An Bras Dermatol. 2012;87:466-468. doi: 10.1590/s0365-05962012000300018
4. Malakar S, Save S, Mehta P. Brown shadow in lichen nitidus: a dermoscopic marker! Indian Dermatol Online J. 2018;9:479-480. doi: 10.4103/idoj.IDOJ_338_17
5. Synakiewicz J, Polańska A, Bowszyc-Dmochowska M, et al. Generalized lichen nitidus: a case report and review of the literature. Postepy Dermatol Alergol. 2016;33:488-490. doi: 10.5114/ada.2016.63890
1. Shiohara T, Mizukawa Y. Lichen planus and lichenoid dermatoses. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Elsevier Inc;2008:167-170.
2. Lapins NA, Willoughby C, Helwig EB. Lichen nitidus. A study of forty-three cases. Cutis. 1978;21:634-637.
3. Botelho LFF, de Magalhães JPJ, Ogawa MM, et al. Generalized Lichen nitidus associated with Down’s syndrome: case report. An Bras Dermatol. 2012;87:466-468. doi: 10.1590/s0365-05962012000300018
4. Malakar S, Save S, Mehta P. Brown shadow in lichen nitidus: a dermoscopic marker! Indian Dermatol Online J. 2018;9:479-480. doi: 10.4103/idoj.IDOJ_338_17
5. Synakiewicz J, Polańska A, Bowszyc-Dmochowska M, et al. Generalized lichen nitidus: a case report and review of the literature. Postepy Dermatol Alergol. 2016;33:488-490. doi: 10.5114/ada.2016.63890
Current monkeypox outbreak marked by unconventional spread, clinical features
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.