User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Newly defined liver disorder associated with COVID mortality
People with metabolic dysfunction–associated fatty liver disease (MAFLD) – a newly defined condition – may be more likely to die from COVID-19, researchers say.
A cohort of people hospitalized for COVID-19 in Central Military Hospital, Mexico City, who met the criteria for MAFLD died at a higher rate than a control group without fatty liver disease, said Martín Uriel Vázquez-Medina, MSc, a researcher in the National Polytechnic Institute in Mexico City.
Patients who met only the criteria for the traditional classification, nonalcoholic fatty liver disease (NAFLD), also died of COVID-19 at a higher rate than the control group, but the difference was not statistically significant.
“It is important to screen for MAFLD,” Mr. Vázquez-Medina told this news organization. “It’s a new definition, but it has really helped us to identify which patients are going to get worse by COVID-19.”
The study was published in Hepatology Communications.
More evidence for clinical relevance of MAFLD
The finding lends support to an initiative to use MAFLD instead of NAFLD to identify patients whose liver steatosis poses a threat to their health, Mr. Vázquez-Medina said.
NAFLD affects as much as a quarter of the world’s population. No drugs have been approved to treat it. Some researchers have reasoned that the imprecision of the definition of NAFLD could be one reason for the lack of progress in treatment.
“NAFLD is something that doesn’t have positive criteria to be diagnosed,” said Mr. Vázquez-Medina. “You only say NAFLD when you don’t find hepatitis or another disease.”
In an article published in Gastroenterology, an international consensus panel proposed MAFLD as an alternative, arguing that a focus on metabolic dysfunction could more accurately reflect the pathogenesis of the disease and help stratify patients.
Previous research has suggested that patients with MAFLD have a higher risk of atherosclerotic cardiovascular disease and that the prevalence of colorectal adenomas is a higher in these patients, compared with patients with NAFLD.
The high prevalence of MAFLD in Mexico – about 30% – could help explain the country’s high rate of mortality from COVID-19, Mr. Vázquez-Medina said. Almost 6% of people diagnosed with COVID in Mexico have died from it, according to the Johns Hopkins University and Medical Center Coronavirus Resource Center.
Sorting COVID outcomes by liver steatosis
To understand the interaction of MAFLD, NAFLD, liver fibrosis, and COVID-19, Mr. Vázquez-Medina and his colleagues analyzed the records of all patients admitted to the Central Military Hospital with COVID-19 from April 4, 2020, to June 24, 2020.
They excluded patients for whom complete data were lacking or for whom a liver function test was not conducted in the first 24 hours of hospitalization. Also excluded were patients with significant consumption of alcohol (> 30 g/day for men and > 20 g/day for women) and those with a history of autoimmune liver disease, liver cancer, decompensated cirrhosis, platelet disorders, or myopathies.
The remaining patients were divided into three groups – 220 who met the criteria for MAFLD, 79 who met the criteria for NAFLD but not MAFLD, and 60 other patients as a control group.
The researchers defined MAFLD as the presence of liver steatosis detected with a noninvasive method and one of the following: overweight (body mass index, 25-29.9 kg/m2), type 2 diabetes, or the presence of two metabolic abnormalities (blood pressure > 140/90 mm Hg, plasma triglycerides > 150 mg/dL, plasma high-density lipoprotein cholesterol < 40 mg/dL in men and < 50 mg/dL in women, and prediabetes).
They defined NAFLD as the presence of liver steatosis without the other criteria for MAFLD.
The patients with MAFLD were the most likely to be intubated and were the most likely to die (intubation, 44.09%; mortality, 55%), followed by those with NAFLD (intubation, 40.51%; mortality, 51.9%) and those in the control group (intubation, 20%; mortality, 38.33%).
The difference in mortality between the MAFLD group and the control group was statistically significant (P = .02). The mortality difference between the NAFLD and the control group fell just short of statistical significance (P = .07).
For intubation, the difference between the MAFLD and the control group was highly statistically significant (P = .001), and the difference between the NAFLD and the control group was also statistically significant (P = .01)
Patients with advanced fibrosis and either MAFLD or NAFLD were also more likely to die than patients in the control group with advanced fibrosis.
That’s why screening for MAFLD is important, Mr. Vázquez-Medina said.
Next steps and new questions
Future research should examine whether patients with MAFLD have elevated levels of biomarkers for inflammation, such as interleukin 6, Mr. Vázquez-Medina said. A “chronic low proinflammatory state” may be the key to understanding the vulnerability of patients to MAFLD to COVID-19, he speculated.
The metabolic traits associated with MAFLD could explain the higher mortality and intubation rates with COVID, said Rohit Loomba, MD, MHSc, a professor of medicine in the division of gastroenterology at the University of California, San Diego, who was not involved in the study.
“Hypertension, diabetes, and obesity increase the risk of complications from COVID in all patients, whether they have been diagnosed with NAFLD or not,” he told this news organization in an email.
Mr. Vasquez-Medina pointed out that the patients with MAFLD had a higher risk of mortality even after adjusting for age, sex, type 2 diabetes, hypertension, overweight, and obesity (BMI ≥ 30 kg/m2). MAFLD also was more strongly associated with a poor outcome than either hypertension alone or obesity alone. Only age emerged as a significant independent covariate in the study.
Dr. Loomba also questioned whether the regression model used in this study for liver steatosis was “fully reflective of NAFLD.”
The researchers identified liver steatosis with a diagnostic formula that used noninvasive clinical BMI and laboratory tests (alanine aminotransferase), citing a study that found the regression formula was better at diagnosing NAFLD than FibroScan.
Mr. Vázquez-Medina reported no relevant financial relationships. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89bio, Terns Pharmaceuticals, and Viking Therapeutics. He is co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
People with metabolic dysfunction–associated fatty liver disease (MAFLD) – a newly defined condition – may be more likely to die from COVID-19, researchers say.
A cohort of people hospitalized for COVID-19 in Central Military Hospital, Mexico City, who met the criteria for MAFLD died at a higher rate than a control group without fatty liver disease, said Martín Uriel Vázquez-Medina, MSc, a researcher in the National Polytechnic Institute in Mexico City.
Patients who met only the criteria for the traditional classification, nonalcoholic fatty liver disease (NAFLD), also died of COVID-19 at a higher rate than the control group, but the difference was not statistically significant.
“It is important to screen for MAFLD,” Mr. Vázquez-Medina told this news organization. “It’s a new definition, but it has really helped us to identify which patients are going to get worse by COVID-19.”
The study was published in Hepatology Communications.
More evidence for clinical relevance of MAFLD
The finding lends support to an initiative to use MAFLD instead of NAFLD to identify patients whose liver steatosis poses a threat to their health, Mr. Vázquez-Medina said.
NAFLD affects as much as a quarter of the world’s population. No drugs have been approved to treat it. Some researchers have reasoned that the imprecision of the definition of NAFLD could be one reason for the lack of progress in treatment.
“NAFLD is something that doesn’t have positive criteria to be diagnosed,” said Mr. Vázquez-Medina. “You only say NAFLD when you don’t find hepatitis or another disease.”
In an article published in Gastroenterology, an international consensus panel proposed MAFLD as an alternative, arguing that a focus on metabolic dysfunction could more accurately reflect the pathogenesis of the disease and help stratify patients.
Previous research has suggested that patients with MAFLD have a higher risk of atherosclerotic cardiovascular disease and that the prevalence of colorectal adenomas is a higher in these patients, compared with patients with NAFLD.
The high prevalence of MAFLD in Mexico – about 30% – could help explain the country’s high rate of mortality from COVID-19, Mr. Vázquez-Medina said. Almost 6% of people diagnosed with COVID in Mexico have died from it, according to the Johns Hopkins University and Medical Center Coronavirus Resource Center.
Sorting COVID outcomes by liver steatosis
To understand the interaction of MAFLD, NAFLD, liver fibrosis, and COVID-19, Mr. Vázquez-Medina and his colleagues analyzed the records of all patients admitted to the Central Military Hospital with COVID-19 from April 4, 2020, to June 24, 2020.
They excluded patients for whom complete data were lacking or for whom a liver function test was not conducted in the first 24 hours of hospitalization. Also excluded were patients with significant consumption of alcohol (> 30 g/day for men and > 20 g/day for women) and those with a history of autoimmune liver disease, liver cancer, decompensated cirrhosis, platelet disorders, or myopathies.
The remaining patients were divided into three groups – 220 who met the criteria for MAFLD, 79 who met the criteria for NAFLD but not MAFLD, and 60 other patients as a control group.
The researchers defined MAFLD as the presence of liver steatosis detected with a noninvasive method and one of the following: overweight (body mass index, 25-29.9 kg/m2), type 2 diabetes, or the presence of two metabolic abnormalities (blood pressure > 140/90 mm Hg, plasma triglycerides > 150 mg/dL, plasma high-density lipoprotein cholesterol < 40 mg/dL in men and < 50 mg/dL in women, and prediabetes).
They defined NAFLD as the presence of liver steatosis without the other criteria for MAFLD.
The patients with MAFLD were the most likely to be intubated and were the most likely to die (intubation, 44.09%; mortality, 55%), followed by those with NAFLD (intubation, 40.51%; mortality, 51.9%) and those in the control group (intubation, 20%; mortality, 38.33%).
The difference in mortality between the MAFLD group and the control group was statistically significant (P = .02). The mortality difference between the NAFLD and the control group fell just short of statistical significance (P = .07).
For intubation, the difference between the MAFLD and the control group was highly statistically significant (P = .001), and the difference between the NAFLD and the control group was also statistically significant (P = .01)
Patients with advanced fibrosis and either MAFLD or NAFLD were also more likely to die than patients in the control group with advanced fibrosis.
That’s why screening for MAFLD is important, Mr. Vázquez-Medina said.
Next steps and new questions
Future research should examine whether patients with MAFLD have elevated levels of biomarkers for inflammation, such as interleukin 6, Mr. Vázquez-Medina said. A “chronic low proinflammatory state” may be the key to understanding the vulnerability of patients to MAFLD to COVID-19, he speculated.
The metabolic traits associated with MAFLD could explain the higher mortality and intubation rates with COVID, said Rohit Loomba, MD, MHSc, a professor of medicine in the division of gastroenterology at the University of California, San Diego, who was not involved in the study.
“Hypertension, diabetes, and obesity increase the risk of complications from COVID in all patients, whether they have been diagnosed with NAFLD or not,” he told this news organization in an email.
Mr. Vasquez-Medina pointed out that the patients with MAFLD had a higher risk of mortality even after adjusting for age, sex, type 2 diabetes, hypertension, overweight, and obesity (BMI ≥ 30 kg/m2). MAFLD also was more strongly associated with a poor outcome than either hypertension alone or obesity alone. Only age emerged as a significant independent covariate in the study.
Dr. Loomba also questioned whether the regression model used in this study for liver steatosis was “fully reflective of NAFLD.”
The researchers identified liver steatosis with a diagnostic formula that used noninvasive clinical BMI and laboratory tests (alanine aminotransferase), citing a study that found the regression formula was better at diagnosing NAFLD than FibroScan.
Mr. Vázquez-Medina reported no relevant financial relationships. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89bio, Terns Pharmaceuticals, and Viking Therapeutics. He is co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
People with metabolic dysfunction–associated fatty liver disease (MAFLD) – a newly defined condition – may be more likely to die from COVID-19, researchers say.
A cohort of people hospitalized for COVID-19 in Central Military Hospital, Mexico City, who met the criteria for MAFLD died at a higher rate than a control group without fatty liver disease, said Martín Uriel Vázquez-Medina, MSc, a researcher in the National Polytechnic Institute in Mexico City.
Patients who met only the criteria for the traditional classification, nonalcoholic fatty liver disease (NAFLD), also died of COVID-19 at a higher rate than the control group, but the difference was not statistically significant.
“It is important to screen for MAFLD,” Mr. Vázquez-Medina told this news organization. “It’s a new definition, but it has really helped us to identify which patients are going to get worse by COVID-19.”
The study was published in Hepatology Communications.
More evidence for clinical relevance of MAFLD
The finding lends support to an initiative to use MAFLD instead of NAFLD to identify patients whose liver steatosis poses a threat to their health, Mr. Vázquez-Medina said.
NAFLD affects as much as a quarter of the world’s population. No drugs have been approved to treat it. Some researchers have reasoned that the imprecision of the definition of NAFLD could be one reason for the lack of progress in treatment.
“NAFLD is something that doesn’t have positive criteria to be diagnosed,” said Mr. Vázquez-Medina. “You only say NAFLD when you don’t find hepatitis or another disease.”
In an article published in Gastroenterology, an international consensus panel proposed MAFLD as an alternative, arguing that a focus on metabolic dysfunction could more accurately reflect the pathogenesis of the disease and help stratify patients.
Previous research has suggested that patients with MAFLD have a higher risk of atherosclerotic cardiovascular disease and that the prevalence of colorectal adenomas is a higher in these patients, compared with patients with NAFLD.
The high prevalence of MAFLD in Mexico – about 30% – could help explain the country’s high rate of mortality from COVID-19, Mr. Vázquez-Medina said. Almost 6% of people diagnosed with COVID in Mexico have died from it, according to the Johns Hopkins University and Medical Center Coronavirus Resource Center.
Sorting COVID outcomes by liver steatosis
To understand the interaction of MAFLD, NAFLD, liver fibrosis, and COVID-19, Mr. Vázquez-Medina and his colleagues analyzed the records of all patients admitted to the Central Military Hospital with COVID-19 from April 4, 2020, to June 24, 2020.
They excluded patients for whom complete data were lacking or for whom a liver function test was not conducted in the first 24 hours of hospitalization. Also excluded were patients with significant consumption of alcohol (> 30 g/day for men and > 20 g/day for women) and those with a history of autoimmune liver disease, liver cancer, decompensated cirrhosis, platelet disorders, or myopathies.
The remaining patients were divided into three groups – 220 who met the criteria for MAFLD, 79 who met the criteria for NAFLD but not MAFLD, and 60 other patients as a control group.
The researchers defined MAFLD as the presence of liver steatosis detected with a noninvasive method and one of the following: overweight (body mass index, 25-29.9 kg/m2), type 2 diabetes, or the presence of two metabolic abnormalities (blood pressure > 140/90 mm Hg, plasma triglycerides > 150 mg/dL, plasma high-density lipoprotein cholesterol < 40 mg/dL in men and < 50 mg/dL in women, and prediabetes).
They defined NAFLD as the presence of liver steatosis without the other criteria for MAFLD.
The patients with MAFLD were the most likely to be intubated and were the most likely to die (intubation, 44.09%; mortality, 55%), followed by those with NAFLD (intubation, 40.51%; mortality, 51.9%) and those in the control group (intubation, 20%; mortality, 38.33%).
The difference in mortality between the MAFLD group and the control group was statistically significant (P = .02). The mortality difference between the NAFLD and the control group fell just short of statistical significance (P = .07).
For intubation, the difference between the MAFLD and the control group was highly statistically significant (P = .001), and the difference between the NAFLD and the control group was also statistically significant (P = .01)
Patients with advanced fibrosis and either MAFLD or NAFLD were also more likely to die than patients in the control group with advanced fibrosis.
That’s why screening for MAFLD is important, Mr. Vázquez-Medina said.
Next steps and new questions
Future research should examine whether patients with MAFLD have elevated levels of biomarkers for inflammation, such as interleukin 6, Mr. Vázquez-Medina said. A “chronic low proinflammatory state” may be the key to understanding the vulnerability of patients to MAFLD to COVID-19, he speculated.
The metabolic traits associated with MAFLD could explain the higher mortality and intubation rates with COVID, said Rohit Loomba, MD, MHSc, a professor of medicine in the division of gastroenterology at the University of California, San Diego, who was not involved in the study.
“Hypertension, diabetes, and obesity increase the risk of complications from COVID in all patients, whether they have been diagnosed with NAFLD or not,” he told this news organization in an email.
Mr. Vasquez-Medina pointed out that the patients with MAFLD had a higher risk of mortality even after adjusting for age, sex, type 2 diabetes, hypertension, overweight, and obesity (BMI ≥ 30 kg/m2). MAFLD also was more strongly associated with a poor outcome than either hypertension alone or obesity alone. Only age emerged as a significant independent covariate in the study.
Dr. Loomba also questioned whether the regression model used in this study for liver steatosis was “fully reflective of NAFLD.”
The researchers identified liver steatosis with a diagnostic formula that used noninvasive clinical BMI and laboratory tests (alanine aminotransferase), citing a study that found the regression formula was better at diagnosing NAFLD than FibroScan.
Mr. Vázquez-Medina reported no relevant financial relationships. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89bio, Terns Pharmaceuticals, and Viking Therapeutics. He is co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
FROM HEPATOLOGY COMMUNICATIONS
Severe COVID-19 adds 20 years of cognitive aging: Study
adding that the impairment is “equivalent to losing 10 IQ points.”
In their study, published in eClinicalMedicine, a team of scientists from the University of Cambridge and Imperial College London said there is growing evidence that COVID-19 can cause lasting cognitive and mental health problems. Patients report fatigue, “brain fog,” problems recalling words, sleep disturbances, anxiety, and even posttraumatic stress disorder months after infection.
The researchers analyzed data from 46 individuals who received critical care for COVID-19 at Addenbrooke’s Hospital between March and July 2020 (27 females, 19 males, mean age 51 years, 16 of whom had mechanical ventilation) and were recruited to the NIHR COVID-19 BioResource project.
At an average of 6 months after acute COVID-19 illness, the study participants underwent detailed computerized cognitive tests via the Cognitron platform, comprising eight tasks deployed on an iPad measuring mental function such as memory, attention, and reasoning. Also assessed were anxiety, depression, and posttraumatic stress disorder via standard mood, anxiety, and posttraumatic stress scales – specifically the Generalized Anxiety Disorder 7 (GAD-7), the Patient Health Questionnaire 9 (PHQ-9), and the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders 5 (PCL-5). Their data were compared against 460 controls – matched for age, sex, education, and first language – and the pattern of deficits across tasks was qualitatively compared with normal age-related decline and early-stage dementia.
Less accurate and slower response times
The authors highlighted how this was the first time a “rigorous assessment and comparison” had been carried out in relation to the after-effects of severe COVID-19.
“Cognitive impairment is common to a wide range of neurological disorders, including dementia, and even routine aging, but the patterns we saw – the cognitive ‘fingerprint’ of COVID-19 – was distinct from all of these,” said David Menon, MD, division of anesthesia at the University of Cambridge, England, and the study’s senior author.
The scientists found that COVID-19 survivors were less accurate and had slower response times than the control population, and added that survivors scored particularly poorly on verbal analogical reasoning and showed slower processing speeds.
Critically, the scale of the cognitive deficits correlated with acute illness severity, but not fatigue or mental health status at the time of cognitive assessment, said the authors.
Recovery ‘at best gradual’
The effects were strongest for those with more severe acute illness, and who required mechanical ventilation, said the authors, who found that acute illness severity was “better at predicting the cognitive deficits.”
The authors pointed out how these deficits were still detectable when patients were followed up 6 months later, and that, although patients’ scores and reaction times began to improve over time, any recovery was “at best gradual” and likely to be influenced by factors such as illness severity and its neurological or psychological impacts.
“We followed some patients up as late as 10 months after their acute infection, so were able to see a very slow improvement,” Dr. Menon said. He explained how, while this improvement was not statistically significant, it was “at least heading in the right direction.”
However, he warned it is very possible that some of these individuals “will never fully recover.”
The cognitive deficits observed may be due to several factors in combination, said the authors, including inadequate oxygen or blood supply to the brain, blockage of large or small blood vessels due to clotting, and microscopic bleeds. They highlighted how the most important mechanism, however, may be “damage caused by the body’s own inflammatory response and immune system.”
Adam Hampshire, PhD, of the department of brain sciences at Imperial College London, one of the study’s authors, described how around 40,000 people have been through intensive care with COVID-19 in England alone, with many more despite having been very sick not admitted to hospital. This means there is a “large number of people out there still experiencing problems with cognition many months later,” he said. “We urgently need to look at what can be done to help these people.”
A version of this article first appeared on Univadis.
adding that the impairment is “equivalent to losing 10 IQ points.”
In their study, published in eClinicalMedicine, a team of scientists from the University of Cambridge and Imperial College London said there is growing evidence that COVID-19 can cause lasting cognitive and mental health problems. Patients report fatigue, “brain fog,” problems recalling words, sleep disturbances, anxiety, and even posttraumatic stress disorder months after infection.
The researchers analyzed data from 46 individuals who received critical care for COVID-19 at Addenbrooke’s Hospital between March and July 2020 (27 females, 19 males, mean age 51 years, 16 of whom had mechanical ventilation) and were recruited to the NIHR COVID-19 BioResource project.
At an average of 6 months after acute COVID-19 illness, the study participants underwent detailed computerized cognitive tests via the Cognitron platform, comprising eight tasks deployed on an iPad measuring mental function such as memory, attention, and reasoning. Also assessed were anxiety, depression, and posttraumatic stress disorder via standard mood, anxiety, and posttraumatic stress scales – specifically the Generalized Anxiety Disorder 7 (GAD-7), the Patient Health Questionnaire 9 (PHQ-9), and the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders 5 (PCL-5). Their data were compared against 460 controls – matched for age, sex, education, and first language – and the pattern of deficits across tasks was qualitatively compared with normal age-related decline and early-stage dementia.
Less accurate and slower response times
The authors highlighted how this was the first time a “rigorous assessment and comparison” had been carried out in relation to the after-effects of severe COVID-19.
“Cognitive impairment is common to a wide range of neurological disorders, including dementia, and even routine aging, but the patterns we saw – the cognitive ‘fingerprint’ of COVID-19 – was distinct from all of these,” said David Menon, MD, division of anesthesia at the University of Cambridge, England, and the study’s senior author.
The scientists found that COVID-19 survivors were less accurate and had slower response times than the control population, and added that survivors scored particularly poorly on verbal analogical reasoning and showed slower processing speeds.
Critically, the scale of the cognitive deficits correlated with acute illness severity, but not fatigue or mental health status at the time of cognitive assessment, said the authors.
Recovery ‘at best gradual’
The effects were strongest for those with more severe acute illness, and who required mechanical ventilation, said the authors, who found that acute illness severity was “better at predicting the cognitive deficits.”
The authors pointed out how these deficits were still detectable when patients were followed up 6 months later, and that, although patients’ scores and reaction times began to improve over time, any recovery was “at best gradual” and likely to be influenced by factors such as illness severity and its neurological or psychological impacts.
“We followed some patients up as late as 10 months after their acute infection, so were able to see a very slow improvement,” Dr. Menon said. He explained how, while this improvement was not statistically significant, it was “at least heading in the right direction.”
However, he warned it is very possible that some of these individuals “will never fully recover.”
The cognitive deficits observed may be due to several factors in combination, said the authors, including inadequate oxygen or blood supply to the brain, blockage of large or small blood vessels due to clotting, and microscopic bleeds. They highlighted how the most important mechanism, however, may be “damage caused by the body’s own inflammatory response and immune system.”
Adam Hampshire, PhD, of the department of brain sciences at Imperial College London, one of the study’s authors, described how around 40,000 people have been through intensive care with COVID-19 in England alone, with many more despite having been very sick not admitted to hospital. This means there is a “large number of people out there still experiencing problems with cognition many months later,” he said. “We urgently need to look at what can be done to help these people.”
A version of this article first appeared on Univadis.
adding that the impairment is “equivalent to losing 10 IQ points.”
In their study, published in eClinicalMedicine, a team of scientists from the University of Cambridge and Imperial College London said there is growing evidence that COVID-19 can cause lasting cognitive and mental health problems. Patients report fatigue, “brain fog,” problems recalling words, sleep disturbances, anxiety, and even posttraumatic stress disorder months after infection.
The researchers analyzed data from 46 individuals who received critical care for COVID-19 at Addenbrooke’s Hospital between March and July 2020 (27 females, 19 males, mean age 51 years, 16 of whom had mechanical ventilation) and were recruited to the NIHR COVID-19 BioResource project.
At an average of 6 months after acute COVID-19 illness, the study participants underwent detailed computerized cognitive tests via the Cognitron platform, comprising eight tasks deployed on an iPad measuring mental function such as memory, attention, and reasoning. Also assessed were anxiety, depression, and posttraumatic stress disorder via standard mood, anxiety, and posttraumatic stress scales – specifically the Generalized Anxiety Disorder 7 (GAD-7), the Patient Health Questionnaire 9 (PHQ-9), and the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders 5 (PCL-5). Their data were compared against 460 controls – matched for age, sex, education, and first language – and the pattern of deficits across tasks was qualitatively compared with normal age-related decline and early-stage dementia.
Less accurate and slower response times
The authors highlighted how this was the first time a “rigorous assessment and comparison” had been carried out in relation to the after-effects of severe COVID-19.
“Cognitive impairment is common to a wide range of neurological disorders, including dementia, and even routine aging, but the patterns we saw – the cognitive ‘fingerprint’ of COVID-19 – was distinct from all of these,” said David Menon, MD, division of anesthesia at the University of Cambridge, England, and the study’s senior author.
The scientists found that COVID-19 survivors were less accurate and had slower response times than the control population, and added that survivors scored particularly poorly on verbal analogical reasoning and showed slower processing speeds.
Critically, the scale of the cognitive deficits correlated with acute illness severity, but not fatigue or mental health status at the time of cognitive assessment, said the authors.
Recovery ‘at best gradual’
The effects were strongest for those with more severe acute illness, and who required mechanical ventilation, said the authors, who found that acute illness severity was “better at predicting the cognitive deficits.”
The authors pointed out how these deficits were still detectable when patients were followed up 6 months later, and that, although patients’ scores and reaction times began to improve over time, any recovery was “at best gradual” and likely to be influenced by factors such as illness severity and its neurological or psychological impacts.
“We followed some patients up as late as 10 months after their acute infection, so were able to see a very slow improvement,” Dr. Menon said. He explained how, while this improvement was not statistically significant, it was “at least heading in the right direction.”
However, he warned it is very possible that some of these individuals “will never fully recover.”
The cognitive deficits observed may be due to several factors in combination, said the authors, including inadequate oxygen or blood supply to the brain, blockage of large or small blood vessels due to clotting, and microscopic bleeds. They highlighted how the most important mechanism, however, may be “damage caused by the body’s own inflammatory response and immune system.”
Adam Hampshire, PhD, of the department of brain sciences at Imperial College London, one of the study’s authors, described how around 40,000 people have been through intensive care with COVID-19 in England alone, with many more despite having been very sick not admitted to hospital. This means there is a “large number of people out there still experiencing problems with cognition many months later,” he said. “We urgently need to look at what can be done to help these people.”
A version of this article first appeared on Univadis.
FROM ECLINICAL MEDICINE
When it’s not long, but medium COVID?
Symptom timelines surrounding COVID infection tend to center on either the immediate 5-day quarantine protocols for acute infection or the long-COVID symptoms that can last a month or potentially far longer.
People may return to work or daily routines, but something is off: What had been simple exercise regimens become onerous. Everyday tasks take more effort.
Does this ill-defined subset point to a “medium COVID?”
Farha Ikramuddin, MD, MHA, a physiatrist and rehabilitation specialist at the University of Minnesota and M Health Fairview in Minneapolis, points out there is no definition or diagnostic code or shared official understanding of a middle category for COVID.
“But am I seeing that? Absolutely,” she said in an interview.
“I have seen patients who are younger, healthier, [and] with not so many comorbidities have either persistence of symptoms or reappearance after the initial infection is done,” she said.
Some patients report they had very low infection or were nonsymptomatic and returned to their normal health fairly quickly after infection. Then a week later they began experiencing fatigue, lost appetite, loss of smell, and feeling full after a few bites, Dr. Ikramuddin said.
Part of the trouble in categorizing the space between returning to normal after a week and having symptoms for months is that organizations can’t agree on a timeline for when symptoms warrant a “long-COVID” label.
For instance, the Centers for Disease Control and Prevention defines it as 4 or more weeks after infection. The World Health Organization defines it as starting 3 months after COVID-19 symptom onset.
“I’m seeing ‘medium COVID’ – as one would call it – in younger and healthier patients. I’m also noticing that these symptoms are not severe enough to warrant stopping their job or changing their job schedules,” Dr. Ikramuddin said.
They go back to work, she said, but start noticing something is off.
“I am seeing that.”
“I discharge at least two patients a week from my clinic because they have moved on and no longer have symptoms,” Dr. Ikramuddin said.
In a story from Kaiser Health News published last month, WHYY health reporter Nina Feldman writes: “What I’ve come to think of as my ‘medium COVID’ affected my life. I couldn’t socialize much, drink, or stay up past 9:30 p.m. It took me 10 weeks to go for my first run – I’d been too afraid to try.”
She described a dinner with a friend after ending initial isolation protocols: “One glass of wine left me feeling like I’d had a whole bottle. I was bone-achingly exhausted but couldn’t sleep.”
Medical mystery
Dr. Ikramuddin notes the mechanism behind prolonged COVID-19 symptoms is still a medical mystery.
“In one scenario,” she said, “the question is being asked about whether the virus is staying dormant, similar to herpes zoster or HIV.”
“Right now, instead of getting more answers, we’re getting more questions,” Dr. Ikramuddin said.
Mouhib Naddour, MD, a pulmonary specialist with Sharp HealthCare in San Diego, said he’s seeing that it’s taking some patients who have had COVID longer to recover than it would for other viral infections.
Some patients fall between those recovering within 2-3 weeks and patients having long COVID. Those patients in the gap could be lumped into a middle-range COVID, he told this news organization.
“We try to put things into tables and boxes but it is hard with this disease,” Dr. Naddour said.
He agrees there’s no medical definition for “medium” COVID, but he said the idea should bring hope for patients to know that, if their symptoms are persisting they don’t necessarily have long COVID – and their symptoms may still disappear.
“This is an illness that may take longer to completely recover from,” he said. “The majority of patients we’re seeing in this group could be healthy young patients who get COVID, then 2-3 weeks after they test negative, still have lingering symptoms.”
Common symptoms
Some commonly reported symptoms of those with enduring illness, which often overlap with other stages of COVID, are difficulty breathing, chest tightness, dry cough, chest pain, muscle and joint pain, fatigue, difficulty sleeping, and mood swings, Dr. Naddour said.
“We need to do an extensive assessment to make sure there’s no other problem causing these symptoms,” he said.
Still, there is no set timeline for the medium-COVID range, he noted, so checking in with a primary care physician is important for people experiencing symptoms.
It’s a continuum, not a category
Fernando Carnavali, MD, coordinator for Mount Sinai’s Center for Post-COVID Care in New York, said he is not ready to recognize a separate category for a “medium” COVID.
He noted that science can’t even agree on a name for lasting post-COVID symptoms, whether it’s “long COVID” or “long-haul COVID,” “post-COVID syndrome” or “post-acute sequelae of COVID-19 (PASC ).” There’s no agreed-upon pathophysiology or biomarker.
“That creates these gaps of understanding on where we are,” Dr. Carnavali said in an interview.
He said he understands people’s need to categorize symptoms, but rather than a middle ground he sees a continuum.
It doesn’t mean what others may call COVID’s middle ground doesn’t exist, Dr. Carnavali said: “We are in the infancy of defining this. Trying to classify them may create more anxiety.”
The clinicians interviewed for this story report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Symptom timelines surrounding COVID infection tend to center on either the immediate 5-day quarantine protocols for acute infection or the long-COVID symptoms that can last a month or potentially far longer.
People may return to work or daily routines, but something is off: What had been simple exercise regimens become onerous. Everyday tasks take more effort.
Does this ill-defined subset point to a “medium COVID?”
Farha Ikramuddin, MD, MHA, a physiatrist and rehabilitation specialist at the University of Minnesota and M Health Fairview in Minneapolis, points out there is no definition or diagnostic code or shared official understanding of a middle category for COVID.
“But am I seeing that? Absolutely,” she said in an interview.
“I have seen patients who are younger, healthier, [and] with not so many comorbidities have either persistence of symptoms or reappearance after the initial infection is done,” she said.
Some patients report they had very low infection or were nonsymptomatic and returned to their normal health fairly quickly after infection. Then a week later they began experiencing fatigue, lost appetite, loss of smell, and feeling full after a few bites, Dr. Ikramuddin said.
Part of the trouble in categorizing the space between returning to normal after a week and having symptoms for months is that organizations can’t agree on a timeline for when symptoms warrant a “long-COVID” label.
For instance, the Centers for Disease Control and Prevention defines it as 4 or more weeks after infection. The World Health Organization defines it as starting 3 months after COVID-19 symptom onset.
“I’m seeing ‘medium COVID’ – as one would call it – in younger and healthier patients. I’m also noticing that these symptoms are not severe enough to warrant stopping their job or changing their job schedules,” Dr. Ikramuddin said.
They go back to work, she said, but start noticing something is off.
“I am seeing that.”
“I discharge at least two patients a week from my clinic because they have moved on and no longer have symptoms,” Dr. Ikramuddin said.
In a story from Kaiser Health News published last month, WHYY health reporter Nina Feldman writes: “What I’ve come to think of as my ‘medium COVID’ affected my life. I couldn’t socialize much, drink, or stay up past 9:30 p.m. It took me 10 weeks to go for my first run – I’d been too afraid to try.”
She described a dinner with a friend after ending initial isolation protocols: “One glass of wine left me feeling like I’d had a whole bottle. I was bone-achingly exhausted but couldn’t sleep.”
Medical mystery
Dr. Ikramuddin notes the mechanism behind prolonged COVID-19 symptoms is still a medical mystery.
“In one scenario,” she said, “the question is being asked about whether the virus is staying dormant, similar to herpes zoster or HIV.”
“Right now, instead of getting more answers, we’re getting more questions,” Dr. Ikramuddin said.
Mouhib Naddour, MD, a pulmonary specialist with Sharp HealthCare in San Diego, said he’s seeing that it’s taking some patients who have had COVID longer to recover than it would for other viral infections.
Some patients fall between those recovering within 2-3 weeks and patients having long COVID. Those patients in the gap could be lumped into a middle-range COVID, he told this news organization.
“We try to put things into tables and boxes but it is hard with this disease,” Dr. Naddour said.
He agrees there’s no medical definition for “medium” COVID, but he said the idea should bring hope for patients to know that, if their symptoms are persisting they don’t necessarily have long COVID – and their symptoms may still disappear.
“This is an illness that may take longer to completely recover from,” he said. “The majority of patients we’re seeing in this group could be healthy young patients who get COVID, then 2-3 weeks after they test negative, still have lingering symptoms.”
Common symptoms
Some commonly reported symptoms of those with enduring illness, which often overlap with other stages of COVID, are difficulty breathing, chest tightness, dry cough, chest pain, muscle and joint pain, fatigue, difficulty sleeping, and mood swings, Dr. Naddour said.
“We need to do an extensive assessment to make sure there’s no other problem causing these symptoms,” he said.
Still, there is no set timeline for the medium-COVID range, he noted, so checking in with a primary care physician is important for people experiencing symptoms.
It’s a continuum, not a category
Fernando Carnavali, MD, coordinator for Mount Sinai’s Center for Post-COVID Care in New York, said he is not ready to recognize a separate category for a “medium” COVID.
He noted that science can’t even agree on a name for lasting post-COVID symptoms, whether it’s “long COVID” or “long-haul COVID,” “post-COVID syndrome” or “post-acute sequelae of COVID-19 (PASC ).” There’s no agreed-upon pathophysiology or biomarker.
“That creates these gaps of understanding on where we are,” Dr. Carnavali said in an interview.
He said he understands people’s need to categorize symptoms, but rather than a middle ground he sees a continuum.
It doesn’t mean what others may call COVID’s middle ground doesn’t exist, Dr. Carnavali said: “We are in the infancy of defining this. Trying to classify them may create more anxiety.”
The clinicians interviewed for this story report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Symptom timelines surrounding COVID infection tend to center on either the immediate 5-day quarantine protocols for acute infection or the long-COVID symptoms that can last a month or potentially far longer.
People may return to work or daily routines, but something is off: What had been simple exercise regimens become onerous. Everyday tasks take more effort.
Does this ill-defined subset point to a “medium COVID?”
Farha Ikramuddin, MD, MHA, a physiatrist and rehabilitation specialist at the University of Minnesota and M Health Fairview in Minneapolis, points out there is no definition or diagnostic code or shared official understanding of a middle category for COVID.
“But am I seeing that? Absolutely,” she said in an interview.
“I have seen patients who are younger, healthier, [and] with not so many comorbidities have either persistence of symptoms or reappearance after the initial infection is done,” she said.
Some patients report they had very low infection or were nonsymptomatic and returned to their normal health fairly quickly after infection. Then a week later they began experiencing fatigue, lost appetite, loss of smell, and feeling full after a few bites, Dr. Ikramuddin said.
Part of the trouble in categorizing the space between returning to normal after a week and having symptoms for months is that organizations can’t agree on a timeline for when symptoms warrant a “long-COVID” label.
For instance, the Centers for Disease Control and Prevention defines it as 4 or more weeks after infection. The World Health Organization defines it as starting 3 months after COVID-19 symptom onset.
“I’m seeing ‘medium COVID’ – as one would call it – in younger and healthier patients. I’m also noticing that these symptoms are not severe enough to warrant stopping their job or changing their job schedules,” Dr. Ikramuddin said.
They go back to work, she said, but start noticing something is off.
“I am seeing that.”
“I discharge at least two patients a week from my clinic because they have moved on and no longer have symptoms,” Dr. Ikramuddin said.
In a story from Kaiser Health News published last month, WHYY health reporter Nina Feldman writes: “What I’ve come to think of as my ‘medium COVID’ affected my life. I couldn’t socialize much, drink, or stay up past 9:30 p.m. It took me 10 weeks to go for my first run – I’d been too afraid to try.”
She described a dinner with a friend after ending initial isolation protocols: “One glass of wine left me feeling like I’d had a whole bottle. I was bone-achingly exhausted but couldn’t sleep.”
Medical mystery
Dr. Ikramuddin notes the mechanism behind prolonged COVID-19 symptoms is still a medical mystery.
“In one scenario,” she said, “the question is being asked about whether the virus is staying dormant, similar to herpes zoster or HIV.”
“Right now, instead of getting more answers, we’re getting more questions,” Dr. Ikramuddin said.
Mouhib Naddour, MD, a pulmonary specialist with Sharp HealthCare in San Diego, said he’s seeing that it’s taking some patients who have had COVID longer to recover than it would for other viral infections.
Some patients fall between those recovering within 2-3 weeks and patients having long COVID. Those patients in the gap could be lumped into a middle-range COVID, he told this news organization.
“We try to put things into tables and boxes but it is hard with this disease,” Dr. Naddour said.
He agrees there’s no medical definition for “medium” COVID, but he said the idea should bring hope for patients to know that, if their symptoms are persisting they don’t necessarily have long COVID – and their symptoms may still disappear.
“This is an illness that may take longer to completely recover from,” he said. “The majority of patients we’re seeing in this group could be healthy young patients who get COVID, then 2-3 weeks after they test negative, still have lingering symptoms.”
Common symptoms
Some commonly reported symptoms of those with enduring illness, which often overlap with other stages of COVID, are difficulty breathing, chest tightness, dry cough, chest pain, muscle and joint pain, fatigue, difficulty sleeping, and mood swings, Dr. Naddour said.
“We need to do an extensive assessment to make sure there’s no other problem causing these symptoms,” he said.
Still, there is no set timeline for the medium-COVID range, he noted, so checking in with a primary care physician is important for people experiencing symptoms.
It’s a continuum, not a category
Fernando Carnavali, MD, coordinator for Mount Sinai’s Center for Post-COVID Care in New York, said he is not ready to recognize a separate category for a “medium” COVID.
He noted that science can’t even agree on a name for lasting post-COVID symptoms, whether it’s “long COVID” or “long-haul COVID,” “post-COVID syndrome” or “post-acute sequelae of COVID-19 (PASC ).” There’s no agreed-upon pathophysiology or biomarker.
“That creates these gaps of understanding on where we are,” Dr. Carnavali said in an interview.
He said he understands people’s need to categorize symptoms, but rather than a middle ground he sees a continuum.
It doesn’t mean what others may call COVID’s middle ground doesn’t exist, Dr. Carnavali said: “We are in the infancy of defining this. Trying to classify them may create more anxiety.”
The clinicians interviewed for this story report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New data confirm risk of Guillain-Barré with J&J COVID shot
The Janssen vaccine (Ad26.COV2.S) is a replication-incompetent adenoviral vector vaccine.
The data show no increased risk of GBS with the Pfizer (BNT162b2) or Moderna (mRNA-1273) shots – both mRNA vaccines.
“Our findings support the current guidance from U.S. health officials that preferentially recommend use of mRNA COVID-19 vaccines for primary and booster doses,” Nicola Klein, MD, PhD, with Kaiser Permanente Vaccine Study Center, Oakland, Calif., told this news organization.
“Individuals who choose to receive Janssen/J&J COVID-19 vaccine should be informed of the potential safety risks, including GBS,” Dr. Klein said.
The study was published online in JAMA Network Open.
Eleven cases
Between mid-December 2020 and mid-November 2021, roughly 15.1 million doses of COVID-19 vaccine were administered to nearly 7.9 million adults in the United States.
This includes roughly 483,000 doses of the Janssen vaccine, 8.8 million doses of the Pfizer vaccine, and 5.8 million doses of the Moderna vaccine.
The researchers confirmed 11 cases of GBS after the Janssen vaccine.
The unadjusted incidence of GBS (per 100,000 person-years) was 32.4 in the first 21 days after the Janssen vaccine – substantially higher than the expected background rate of 1 to 2 cases per 100,000 person-years.
There were 36 confirmed cases of GBS after mRNA vaccines. The unadjusted incidence in the first 21 days after mRNA vaccination was 1.3 per 100,000 person-years, similar to the overall expected background rate.
In an adjusted head-to-head comparison, GBS incidence during the 21 days after receipt of the Janssen vaccine was 20.6 times higher than the GBS incidence during the 21 days after the Pfizer or Moderna mRNA vaccines, amounting to 15.5 excess cases per million Janssen vaccine recipients.
Most cases of GBS after the Janssen vaccine occurred during the 1- to 21-day risk interval, with the period of greatest risk in the 1-14 days after vaccination.
The findings of this analysis of surveillance data of COVID-19 vaccines are “consistent with an elevated risk of GBS after primary Ad26.COV2.S vaccination,” the authors wrote.
Novel presentation?
The researchers note that nearly all individuals who developed GBS after the Janssen vaccine had facial weakness or paralysis, in addition to weakness and decreased reflexes in the limbs, suggesting that the presentation of GBS after COVID-19 adenoviral vector vaccine may be novel.
“More research is needed to determine if the presentation of GBS after adenoviral vector vaccine differs from GBS after other exposures such as Campylobacter jejuni, and to investigate the mechanism for how adenoviral vector vaccines may cause GBS,” Dr. Klein and colleagues said.
“The Vaccine Safety Datalink continues to conduct safety surveillance for all COVID-19 vaccines, including monitoring for GBS and other serious health outcomes after vaccination,” Dr. Klein said in an interview.
This study was supported by the Centers for Disease Control and Prevention. Dr. Klein reported receiving grants from Pfizer research support for a COVID vaccine clinical trial as well as other unrelated studies, grants from Merck, grants from GlaxoSmithKline, grants from Sanofi Pasteur, and grants from Protein Science (now Sanofi Pasteur) outside the submitted work.
A version of this article first appeared on Medscape.com.
The Janssen vaccine (Ad26.COV2.S) is a replication-incompetent adenoviral vector vaccine.
The data show no increased risk of GBS with the Pfizer (BNT162b2) or Moderna (mRNA-1273) shots – both mRNA vaccines.
“Our findings support the current guidance from U.S. health officials that preferentially recommend use of mRNA COVID-19 vaccines for primary and booster doses,” Nicola Klein, MD, PhD, with Kaiser Permanente Vaccine Study Center, Oakland, Calif., told this news organization.
“Individuals who choose to receive Janssen/J&J COVID-19 vaccine should be informed of the potential safety risks, including GBS,” Dr. Klein said.
The study was published online in JAMA Network Open.
Eleven cases
Between mid-December 2020 and mid-November 2021, roughly 15.1 million doses of COVID-19 vaccine were administered to nearly 7.9 million adults in the United States.
This includes roughly 483,000 doses of the Janssen vaccine, 8.8 million doses of the Pfizer vaccine, and 5.8 million doses of the Moderna vaccine.
The researchers confirmed 11 cases of GBS after the Janssen vaccine.
The unadjusted incidence of GBS (per 100,000 person-years) was 32.4 in the first 21 days after the Janssen vaccine – substantially higher than the expected background rate of 1 to 2 cases per 100,000 person-years.
There were 36 confirmed cases of GBS after mRNA vaccines. The unadjusted incidence in the first 21 days after mRNA vaccination was 1.3 per 100,000 person-years, similar to the overall expected background rate.
In an adjusted head-to-head comparison, GBS incidence during the 21 days after receipt of the Janssen vaccine was 20.6 times higher than the GBS incidence during the 21 days after the Pfizer or Moderna mRNA vaccines, amounting to 15.5 excess cases per million Janssen vaccine recipients.
Most cases of GBS after the Janssen vaccine occurred during the 1- to 21-day risk interval, with the period of greatest risk in the 1-14 days after vaccination.
The findings of this analysis of surveillance data of COVID-19 vaccines are “consistent with an elevated risk of GBS after primary Ad26.COV2.S vaccination,” the authors wrote.
Novel presentation?
The researchers note that nearly all individuals who developed GBS after the Janssen vaccine had facial weakness or paralysis, in addition to weakness and decreased reflexes in the limbs, suggesting that the presentation of GBS after COVID-19 adenoviral vector vaccine may be novel.
“More research is needed to determine if the presentation of GBS after adenoviral vector vaccine differs from GBS after other exposures such as Campylobacter jejuni, and to investigate the mechanism for how adenoviral vector vaccines may cause GBS,” Dr. Klein and colleagues said.
“The Vaccine Safety Datalink continues to conduct safety surveillance for all COVID-19 vaccines, including monitoring for GBS and other serious health outcomes after vaccination,” Dr. Klein said in an interview.
This study was supported by the Centers for Disease Control and Prevention. Dr. Klein reported receiving grants from Pfizer research support for a COVID vaccine clinical trial as well as other unrelated studies, grants from Merck, grants from GlaxoSmithKline, grants from Sanofi Pasteur, and grants from Protein Science (now Sanofi Pasteur) outside the submitted work.
A version of this article first appeared on Medscape.com.
The Janssen vaccine (Ad26.COV2.S) is a replication-incompetent adenoviral vector vaccine.
The data show no increased risk of GBS with the Pfizer (BNT162b2) or Moderna (mRNA-1273) shots – both mRNA vaccines.
“Our findings support the current guidance from U.S. health officials that preferentially recommend use of mRNA COVID-19 vaccines for primary and booster doses,” Nicola Klein, MD, PhD, with Kaiser Permanente Vaccine Study Center, Oakland, Calif., told this news organization.
“Individuals who choose to receive Janssen/J&J COVID-19 vaccine should be informed of the potential safety risks, including GBS,” Dr. Klein said.
The study was published online in JAMA Network Open.
Eleven cases
Between mid-December 2020 and mid-November 2021, roughly 15.1 million doses of COVID-19 vaccine were administered to nearly 7.9 million adults in the United States.
This includes roughly 483,000 doses of the Janssen vaccine, 8.8 million doses of the Pfizer vaccine, and 5.8 million doses of the Moderna vaccine.
The researchers confirmed 11 cases of GBS after the Janssen vaccine.
The unadjusted incidence of GBS (per 100,000 person-years) was 32.4 in the first 21 days after the Janssen vaccine – substantially higher than the expected background rate of 1 to 2 cases per 100,000 person-years.
There were 36 confirmed cases of GBS after mRNA vaccines. The unadjusted incidence in the first 21 days after mRNA vaccination was 1.3 per 100,000 person-years, similar to the overall expected background rate.
In an adjusted head-to-head comparison, GBS incidence during the 21 days after receipt of the Janssen vaccine was 20.6 times higher than the GBS incidence during the 21 days after the Pfizer or Moderna mRNA vaccines, amounting to 15.5 excess cases per million Janssen vaccine recipients.
Most cases of GBS after the Janssen vaccine occurred during the 1- to 21-day risk interval, with the period of greatest risk in the 1-14 days after vaccination.
The findings of this analysis of surveillance data of COVID-19 vaccines are “consistent with an elevated risk of GBS after primary Ad26.COV2.S vaccination,” the authors wrote.
Novel presentation?
The researchers note that nearly all individuals who developed GBS after the Janssen vaccine had facial weakness or paralysis, in addition to weakness and decreased reflexes in the limbs, suggesting that the presentation of GBS after COVID-19 adenoviral vector vaccine may be novel.
“More research is needed to determine if the presentation of GBS after adenoviral vector vaccine differs from GBS after other exposures such as Campylobacter jejuni, and to investigate the mechanism for how adenoviral vector vaccines may cause GBS,” Dr. Klein and colleagues said.
“The Vaccine Safety Datalink continues to conduct safety surveillance for all COVID-19 vaccines, including monitoring for GBS and other serious health outcomes after vaccination,” Dr. Klein said in an interview.
This study was supported by the Centers for Disease Control and Prevention. Dr. Klein reported receiving grants from Pfizer research support for a COVID vaccine clinical trial as well as other unrelated studies, grants from Merck, grants from GlaxoSmithKline, grants from Sanofi Pasteur, and grants from Protein Science (now Sanofi Pasteur) outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Children and COVID: New cases up for third straight week
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.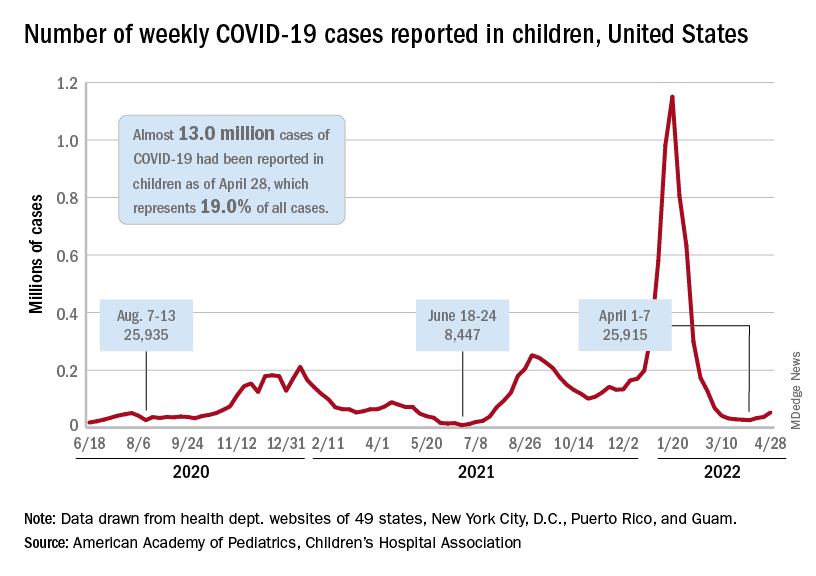
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”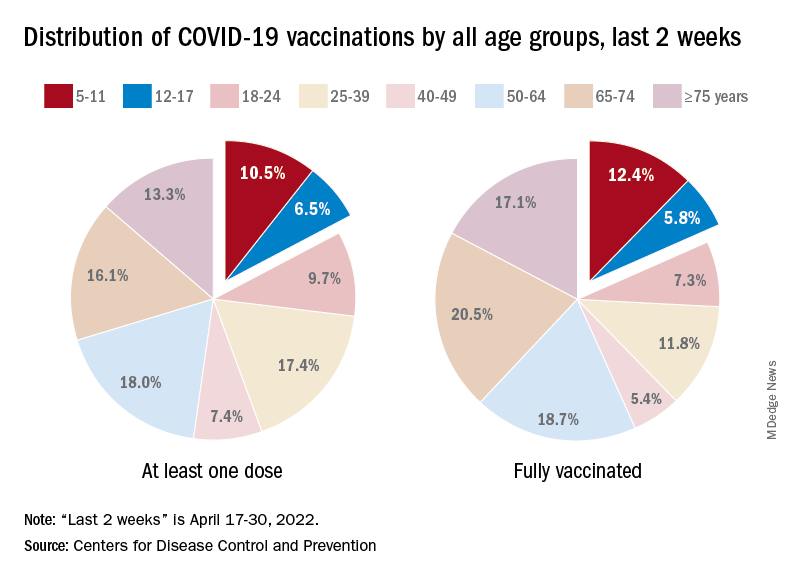
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
New research holds promise for fighting obesity, says expert
Caroline Apovian, MD, codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, described some of the new insights about obesity she has gained during her talk at the annual meeting of the American College of Physicians.
“When I was a medical student a while back, I learned that fat tissue just sat there and stored fat,” she said. “Now we know it’s an endocrine organ.”
This tissue secretes hormones, such as leptin, and other factors that have an array of effects on the brain, pancreas, heart, liver, and muscles. Moreover, it has plasticity, with the ability to change, constantly adjusting our metabolism as nutrient supply and demand changes, she continued.
Obesity leads to a decline in this plasticity, leading to fibrosis and inflammation and other problems. These changes can further impair the function of adipose tissue, leading to metabolic disease. But the central role of adipose tissue, and its dynamic nature, presents an opportunity for treatment, Dr. Apovian said, during her talk.
Hints to why obesity has become more common
More than 42% of the U.S. population – “unbelievably,” Dr. Apovian said – is obese, meaning they have a BMI over 30, according to the Centers for Disease Control and Prevention. That’s up by about 25% since 1960, although calories eaten hasn’t increased, and physical activity has increased somewhat, she said.
The root cause is still a bit of a mystery, but according to “good hints and clues” from animal models that are starting to be translated to the study of human obesity, “it has to do with epigenetics and how our brains and our bodies are perceiving the environment,” she noted, during her presentation.
“Our genes haven’t changed. Our environment has changed,” she said.
The industrialization of the food supply, the use of pesticides and preservatives, the dawn of fast food have all combined, most likely, to do “a number on our bodies,” Dr. Apovian said.
But not all hope is lost thanks to new research, Dr. Apovian suggested.
New treatments show promise for helping patients’ obesity
New research that has increased Dr. Apovian’s understanding of the sophisticated role of adipose tissue may be helpful for treating patients with obesity, offering more targets for intervention, she told the audience.
Some treatment avenues already identified have started producing results, Dr. Apovian noted.
Gastric bypass surgery typically leads to a loss of 25% of body weight, but is often shunned by patients, she said. “With such a great surgical procedure, we still only do 256,000 procedures and we have millions of Americans with a BMI over 30.”
Weight control with obsessive dieting, meal-planning and calorie-counting, “can be done, but it’s really hard,” Dr. Apovian noted.
More appealing therapies targeting hormones and appetite suppression have produced impressive results. Recently approved semaglutide produced 14% weight loss, compared with about 2% for placebo, she said.
Results just released for tirzepatide, a dual agonist of gut hormones GLP-1 and GIP, show a 22% total weight loss, compared with about 2% for placebo, with about 56% of patients losing more than 20% of their body weight, Dr. Apovian said.
Referencing studies finding that several hormones are altered during weight loss, she predicted that targeting multiple hormones with drug treatment will also be necessary for best results.
But, she noted, “we’re treating obesity now with one- or two-drug combos.”
Medication costs are too high for many patients
Isis Smith, MD, an internist at University Medical Center in New Orleans, said in an interview that the cost of the most effective medications – which are not covered by Medicaid – means that many of her patients don’t have access to these treatments.
“We’re talking about $1,000 a month. And so there is no way they can afford [them]. I can prescribe phentermine [but] unless a patient has another indication, Medicaid will not pay for it,” she explained.
“I love hearing about all of the new developments. ... It’s interesting to hear, but we need to get insurance to pay so that I can actually prescribe,” Dr. Smith noted.
Dr. Apovian reports financial relationships with Xeno Biosciences, Cowen, Allergan, Novo Nordisk, Abbott Nutrition, and other companies.
Caroline Apovian, MD, codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, described some of the new insights about obesity she has gained during her talk at the annual meeting of the American College of Physicians.
“When I was a medical student a while back, I learned that fat tissue just sat there and stored fat,” she said. “Now we know it’s an endocrine organ.”
This tissue secretes hormones, such as leptin, and other factors that have an array of effects on the brain, pancreas, heart, liver, and muscles. Moreover, it has plasticity, with the ability to change, constantly adjusting our metabolism as nutrient supply and demand changes, she continued.
Obesity leads to a decline in this plasticity, leading to fibrosis and inflammation and other problems. These changes can further impair the function of adipose tissue, leading to metabolic disease. But the central role of adipose tissue, and its dynamic nature, presents an opportunity for treatment, Dr. Apovian said, during her talk.
Hints to why obesity has become more common
More than 42% of the U.S. population – “unbelievably,” Dr. Apovian said – is obese, meaning they have a BMI over 30, according to the Centers for Disease Control and Prevention. That’s up by about 25% since 1960, although calories eaten hasn’t increased, and physical activity has increased somewhat, she said.
The root cause is still a bit of a mystery, but according to “good hints and clues” from animal models that are starting to be translated to the study of human obesity, “it has to do with epigenetics and how our brains and our bodies are perceiving the environment,” she noted, during her presentation.
“Our genes haven’t changed. Our environment has changed,” she said.
The industrialization of the food supply, the use of pesticides and preservatives, the dawn of fast food have all combined, most likely, to do “a number on our bodies,” Dr. Apovian said.
But not all hope is lost thanks to new research, Dr. Apovian suggested.
New treatments show promise for helping patients’ obesity
New research that has increased Dr. Apovian’s understanding of the sophisticated role of adipose tissue may be helpful for treating patients with obesity, offering more targets for intervention, she told the audience.
Some treatment avenues already identified have started producing results, Dr. Apovian noted.
Gastric bypass surgery typically leads to a loss of 25% of body weight, but is often shunned by patients, she said. “With such a great surgical procedure, we still only do 256,000 procedures and we have millions of Americans with a BMI over 30.”
Weight control with obsessive dieting, meal-planning and calorie-counting, “can be done, but it’s really hard,” Dr. Apovian noted.
More appealing therapies targeting hormones and appetite suppression have produced impressive results. Recently approved semaglutide produced 14% weight loss, compared with about 2% for placebo, she said.
Results just released for tirzepatide, a dual agonist of gut hormones GLP-1 and GIP, show a 22% total weight loss, compared with about 2% for placebo, with about 56% of patients losing more than 20% of their body weight, Dr. Apovian said.
Referencing studies finding that several hormones are altered during weight loss, she predicted that targeting multiple hormones with drug treatment will also be necessary for best results.
But, she noted, “we’re treating obesity now with one- or two-drug combos.”
Medication costs are too high for many patients
Isis Smith, MD, an internist at University Medical Center in New Orleans, said in an interview that the cost of the most effective medications – which are not covered by Medicaid – means that many of her patients don’t have access to these treatments.
“We’re talking about $1,000 a month. And so there is no way they can afford [them]. I can prescribe phentermine [but] unless a patient has another indication, Medicaid will not pay for it,” she explained.
“I love hearing about all of the new developments. ... It’s interesting to hear, but we need to get insurance to pay so that I can actually prescribe,” Dr. Smith noted.
Dr. Apovian reports financial relationships with Xeno Biosciences, Cowen, Allergan, Novo Nordisk, Abbott Nutrition, and other companies.
Caroline Apovian, MD, codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, described some of the new insights about obesity she has gained during her talk at the annual meeting of the American College of Physicians.
“When I was a medical student a while back, I learned that fat tissue just sat there and stored fat,” she said. “Now we know it’s an endocrine organ.”
This tissue secretes hormones, such as leptin, and other factors that have an array of effects on the brain, pancreas, heart, liver, and muscles. Moreover, it has plasticity, with the ability to change, constantly adjusting our metabolism as nutrient supply and demand changes, she continued.
Obesity leads to a decline in this plasticity, leading to fibrosis and inflammation and other problems. These changes can further impair the function of adipose tissue, leading to metabolic disease. But the central role of adipose tissue, and its dynamic nature, presents an opportunity for treatment, Dr. Apovian said, during her talk.
Hints to why obesity has become more common
More than 42% of the U.S. population – “unbelievably,” Dr. Apovian said – is obese, meaning they have a BMI over 30, according to the Centers for Disease Control and Prevention. That’s up by about 25% since 1960, although calories eaten hasn’t increased, and physical activity has increased somewhat, she said.
The root cause is still a bit of a mystery, but according to “good hints and clues” from animal models that are starting to be translated to the study of human obesity, “it has to do with epigenetics and how our brains and our bodies are perceiving the environment,” she noted, during her presentation.
“Our genes haven’t changed. Our environment has changed,” she said.
The industrialization of the food supply, the use of pesticides and preservatives, the dawn of fast food have all combined, most likely, to do “a number on our bodies,” Dr. Apovian said.
But not all hope is lost thanks to new research, Dr. Apovian suggested.
New treatments show promise for helping patients’ obesity
New research that has increased Dr. Apovian’s understanding of the sophisticated role of adipose tissue may be helpful for treating patients with obesity, offering more targets for intervention, she told the audience.
Some treatment avenues already identified have started producing results, Dr. Apovian noted.
Gastric bypass surgery typically leads to a loss of 25% of body weight, but is often shunned by patients, she said. “With such a great surgical procedure, we still only do 256,000 procedures and we have millions of Americans with a BMI over 30.”
Weight control with obsessive dieting, meal-planning and calorie-counting, “can be done, but it’s really hard,” Dr. Apovian noted.
More appealing therapies targeting hormones and appetite suppression have produced impressive results. Recently approved semaglutide produced 14% weight loss, compared with about 2% for placebo, she said.
Results just released for tirzepatide, a dual agonist of gut hormones GLP-1 and GIP, show a 22% total weight loss, compared with about 2% for placebo, with about 56% of patients losing more than 20% of their body weight, Dr. Apovian said.
Referencing studies finding that several hormones are altered during weight loss, she predicted that targeting multiple hormones with drug treatment will also be necessary for best results.
But, she noted, “we’re treating obesity now with one- or two-drug combos.”
Medication costs are too high for many patients
Isis Smith, MD, an internist at University Medical Center in New Orleans, said in an interview that the cost of the most effective medications – which are not covered by Medicaid – means that many of her patients don’t have access to these treatments.
“We’re talking about $1,000 a month. And so there is no way they can afford [them]. I can prescribe phentermine [but] unless a patient has another indication, Medicaid will not pay for it,” she explained.
“I love hearing about all of the new developments. ... It’s interesting to hear, but we need to get insurance to pay so that I can actually prescribe,” Dr. Smith noted.
Dr. Apovian reports financial relationships with Xeno Biosciences, Cowen, Allergan, Novo Nordisk, Abbott Nutrition, and other companies.
AT INTERNAL MEDICINE 2022
Nurses, med staff voice their heartache about California nurse suicide
The Santa Clara Police Department “thoroughly investigated” a report April 27 at Kaiser Permanente Santa Clara Medical Center that a male nurse in the emergency department died from a self-inflicted gunshot wound and ruled it a suicide, according to Wahid Kazem, assistant chief of police.
“This tragic event occurred in a closed room that is not used for patient care, adjacent to the emergency department. No other staff or patients were threatened,” according to Rakesh Chaudhary, MD, physician-in-chief of the medical center.
He added that the emergency department remained open for walk-in patients during the investigation, but ambulances were temporarily diverted to nearby hospitals. “The Santa Clara Police Department and our staff immediately took precautions to isolate the affected area and avoid impact to patient care.”
In terms of the effect on those closer to the victim, Dr. Chaudhary said, “Our hearts go out to the family, friends, and coworkers affected by this terrible loss. Our teams are on site providing emotional support and resources for staff.”
Neither the police nor the hospital released the victim’s name. “Out of respect for the privacy of our colleague and their family, we cannot provide any additional details,” Dr. Chaudhary said.
Among those who tweeted reactions to the news the past few days was someone who worked with the victim, according to the post: “My heart goes out to my coworker who thought he had no one to lean on and to my good friend who had to witness this tragedy. Love my ER fam.”
A male critical care RN tweeted: “My heart hurts for the nurse, his loved ones, and colleagues. Anyone working in ER understands the unique stress that we’ve been under. This is so tragic.”
While others cited the need for more mental health services to care for nurses, a psychiatrist on Twitter added, “Nurses are not OK and pizza and pats on the back aren’t going to fix it. This affects all of us.”
Mental health support was listed as a prime demand of striking workers recently at Stanford (Calif.) Health Care and Lucile Packard Children’s Hospital in Palo Alto, about a half hour away from Santa Clara Medical Center.
The nurses’ strike ended May 2 with an agreement between the health systems and the Committee for Recognition of Nursing Achievement union representing the nurses. The contract includes improvements to existing benefits supporting nurses’ health and well-being, according to a StanfordPackardVoice.com newsletter updating the negotiations.
Earlier this year, an intensive care unit RN from Stanford, Michael Odell, reportedly walked off his shift and was found dead 2 days later in San Francisco by the Alameda County Sheriff’s Office dive team. No foul play was suspected and the incident was believed to be a suicide.
A version of this article first appeared on Medscape.com.
The Santa Clara Police Department “thoroughly investigated” a report April 27 at Kaiser Permanente Santa Clara Medical Center that a male nurse in the emergency department died from a self-inflicted gunshot wound and ruled it a suicide, according to Wahid Kazem, assistant chief of police.
“This tragic event occurred in a closed room that is not used for patient care, adjacent to the emergency department. No other staff or patients were threatened,” according to Rakesh Chaudhary, MD, physician-in-chief of the medical center.
He added that the emergency department remained open for walk-in patients during the investigation, but ambulances were temporarily diverted to nearby hospitals. “The Santa Clara Police Department and our staff immediately took precautions to isolate the affected area and avoid impact to patient care.”
In terms of the effect on those closer to the victim, Dr. Chaudhary said, “Our hearts go out to the family, friends, and coworkers affected by this terrible loss. Our teams are on site providing emotional support and resources for staff.”
Neither the police nor the hospital released the victim’s name. “Out of respect for the privacy of our colleague and their family, we cannot provide any additional details,” Dr. Chaudhary said.
Among those who tweeted reactions to the news the past few days was someone who worked with the victim, according to the post: “My heart goes out to my coworker who thought he had no one to lean on and to my good friend who had to witness this tragedy. Love my ER fam.”
A male critical care RN tweeted: “My heart hurts for the nurse, his loved ones, and colleagues. Anyone working in ER understands the unique stress that we’ve been under. This is so tragic.”
While others cited the need for more mental health services to care for nurses, a psychiatrist on Twitter added, “Nurses are not OK and pizza and pats on the back aren’t going to fix it. This affects all of us.”
Mental health support was listed as a prime demand of striking workers recently at Stanford (Calif.) Health Care and Lucile Packard Children’s Hospital in Palo Alto, about a half hour away from Santa Clara Medical Center.
The nurses’ strike ended May 2 with an agreement between the health systems and the Committee for Recognition of Nursing Achievement union representing the nurses. The contract includes improvements to existing benefits supporting nurses’ health and well-being, according to a StanfordPackardVoice.com newsletter updating the negotiations.
Earlier this year, an intensive care unit RN from Stanford, Michael Odell, reportedly walked off his shift and was found dead 2 days later in San Francisco by the Alameda County Sheriff’s Office dive team. No foul play was suspected and the incident was believed to be a suicide.
A version of this article first appeared on Medscape.com.
The Santa Clara Police Department “thoroughly investigated” a report April 27 at Kaiser Permanente Santa Clara Medical Center that a male nurse in the emergency department died from a self-inflicted gunshot wound and ruled it a suicide, according to Wahid Kazem, assistant chief of police.
“This tragic event occurred in a closed room that is not used for patient care, adjacent to the emergency department. No other staff or patients were threatened,” according to Rakesh Chaudhary, MD, physician-in-chief of the medical center.
He added that the emergency department remained open for walk-in patients during the investigation, but ambulances were temporarily diverted to nearby hospitals. “The Santa Clara Police Department and our staff immediately took precautions to isolate the affected area and avoid impact to patient care.”
In terms of the effect on those closer to the victim, Dr. Chaudhary said, “Our hearts go out to the family, friends, and coworkers affected by this terrible loss. Our teams are on site providing emotional support and resources for staff.”
Neither the police nor the hospital released the victim’s name. “Out of respect for the privacy of our colleague and their family, we cannot provide any additional details,” Dr. Chaudhary said.
Among those who tweeted reactions to the news the past few days was someone who worked with the victim, according to the post: “My heart goes out to my coworker who thought he had no one to lean on and to my good friend who had to witness this tragedy. Love my ER fam.”
A male critical care RN tweeted: “My heart hurts for the nurse, his loved ones, and colleagues. Anyone working in ER understands the unique stress that we’ve been under. This is so tragic.”
While others cited the need for more mental health services to care for nurses, a psychiatrist on Twitter added, “Nurses are not OK and pizza and pats on the back aren’t going to fix it. This affects all of us.”
Mental health support was listed as a prime demand of striking workers recently at Stanford (Calif.) Health Care and Lucile Packard Children’s Hospital in Palo Alto, about a half hour away from Santa Clara Medical Center.
The nurses’ strike ended May 2 with an agreement between the health systems and the Committee for Recognition of Nursing Achievement union representing the nurses. The contract includes improvements to existing benefits supporting nurses’ health and well-being, according to a StanfordPackardVoice.com newsletter updating the negotiations.
Earlier this year, an intensive care unit RN from Stanford, Michael Odell, reportedly walked off his shift and was found dead 2 days later in San Francisco by the Alameda County Sheriff’s Office dive team. No foul play was suspected and the incident was believed to be a suicide.
A version of this article first appeared on Medscape.com.
Paxlovid doesn’t prevent infection in households, Pfizer says
Paxlovid works as a treatment for COVID-19 but not as a preventive measure, particularly if you’ve been exposed to the coronavirus through a household member who is infected, according to a new announcement from Pfizer.
In a clinical trial, the oral antiviral tablets were tested for postexposure prophylactic use, or tested for how well they prevented a coronavirus infection in people exposed to the virus. Paxlovid somewhat reduced the risk of infection, but the results weren’t statistically significant.
“We designed the clinical development program for Paxlovid to be comprehensive and ambitious with the aim of being able to help combat COVID-19 in a very broad population of patients,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the announcement.
“While we are disappointed in the outcome of this particular study, these results do not impact the strong efficacy and safety data we’ve observed in our earlier trial for the treatment of COVID-19 patients at high risk of developing severe illness,” he said.
The trial included nearly 3,000 adults who were living with someone who recently tested positive for COVID-19 and had symptoms. The people in the trial, who tested negative and didn’t have symptoms, were given either Paxlovid twice daily for 5 or 10 days or a placebo. The study recruitment began in September 2021 and was completed during the peak of the Omicron wave.
Those who took the 5-day course of Paxlovid were found to be 32% less likely to become infected than the placebo group. Those who took the 10-day treatment had a 37% risk reduction. But the results weren’t statistically significant and may have been because of chance.
“Traditionally, it’s been difficult to use small-molecule antivirals for true prophylaxis because the biology of treating infection is different from the biology of preventing infection,” Daniel Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, told STAT News.
He also noted that the Omicron variant could have played a role.
“That hyperinfectiousness probably makes it more difficult to prevent infections,” Dr. Barouch said.
The safety data was consistent with that of previous studies, Pfizer said, which found that the treatment was about 90% effective at preventing hospitalization or death in COVID-19 patients with a high risk of severe illness if the pills were taken for 5 days soon after symptoms started.
Paxlovid is approved or authorized for conditional or emergency use in more than 60 countries to treat high-risk COVID-19 patients, Pfizer said. In the United States, the drug is authorized for emergency use for the treatment of mild to moderate COVID-19 in those aged 12 and older who face high risks for severe disease, hospitalization, or death.
The full study data will be released in coming months and submitted to a peer-reviewed publication, the company said. More details are on the ClinicalTrials.gov website (NCT05047601).
A version of this article first appeared on WebMD.com.
Paxlovid works as a treatment for COVID-19 but not as a preventive measure, particularly if you’ve been exposed to the coronavirus through a household member who is infected, according to a new announcement from Pfizer.
In a clinical trial, the oral antiviral tablets were tested for postexposure prophylactic use, or tested for how well they prevented a coronavirus infection in people exposed to the virus. Paxlovid somewhat reduced the risk of infection, but the results weren’t statistically significant.
“We designed the clinical development program for Paxlovid to be comprehensive and ambitious with the aim of being able to help combat COVID-19 in a very broad population of patients,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the announcement.
“While we are disappointed in the outcome of this particular study, these results do not impact the strong efficacy and safety data we’ve observed in our earlier trial for the treatment of COVID-19 patients at high risk of developing severe illness,” he said.
The trial included nearly 3,000 adults who were living with someone who recently tested positive for COVID-19 and had symptoms. The people in the trial, who tested negative and didn’t have symptoms, were given either Paxlovid twice daily for 5 or 10 days or a placebo. The study recruitment began in September 2021 and was completed during the peak of the Omicron wave.
Those who took the 5-day course of Paxlovid were found to be 32% less likely to become infected than the placebo group. Those who took the 10-day treatment had a 37% risk reduction. But the results weren’t statistically significant and may have been because of chance.
“Traditionally, it’s been difficult to use small-molecule antivirals for true prophylaxis because the biology of treating infection is different from the biology of preventing infection,” Daniel Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, told STAT News.
He also noted that the Omicron variant could have played a role.
“That hyperinfectiousness probably makes it more difficult to prevent infections,” Dr. Barouch said.
The safety data was consistent with that of previous studies, Pfizer said, which found that the treatment was about 90% effective at preventing hospitalization or death in COVID-19 patients with a high risk of severe illness if the pills were taken for 5 days soon after symptoms started.
Paxlovid is approved or authorized for conditional or emergency use in more than 60 countries to treat high-risk COVID-19 patients, Pfizer said. In the United States, the drug is authorized for emergency use for the treatment of mild to moderate COVID-19 in those aged 12 and older who face high risks for severe disease, hospitalization, or death.
The full study data will be released in coming months and submitted to a peer-reviewed publication, the company said. More details are on the ClinicalTrials.gov website (NCT05047601).
A version of this article first appeared on WebMD.com.
Paxlovid works as a treatment for COVID-19 but not as a preventive measure, particularly if you’ve been exposed to the coronavirus through a household member who is infected, according to a new announcement from Pfizer.
In a clinical trial, the oral antiviral tablets were tested for postexposure prophylactic use, or tested for how well they prevented a coronavirus infection in people exposed to the virus. Paxlovid somewhat reduced the risk of infection, but the results weren’t statistically significant.
“We designed the clinical development program for Paxlovid to be comprehensive and ambitious with the aim of being able to help combat COVID-19 in a very broad population of patients,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the announcement.
“While we are disappointed in the outcome of this particular study, these results do not impact the strong efficacy and safety data we’ve observed in our earlier trial for the treatment of COVID-19 patients at high risk of developing severe illness,” he said.
The trial included nearly 3,000 adults who were living with someone who recently tested positive for COVID-19 and had symptoms. The people in the trial, who tested negative and didn’t have symptoms, were given either Paxlovid twice daily for 5 or 10 days or a placebo. The study recruitment began in September 2021 and was completed during the peak of the Omicron wave.
Those who took the 5-day course of Paxlovid were found to be 32% less likely to become infected than the placebo group. Those who took the 10-day treatment had a 37% risk reduction. But the results weren’t statistically significant and may have been because of chance.
“Traditionally, it’s been difficult to use small-molecule antivirals for true prophylaxis because the biology of treating infection is different from the biology of preventing infection,” Daniel Barouch, MD, director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center, told STAT News.
He also noted that the Omicron variant could have played a role.
“That hyperinfectiousness probably makes it more difficult to prevent infections,” Dr. Barouch said.
The safety data was consistent with that of previous studies, Pfizer said, which found that the treatment was about 90% effective at preventing hospitalization or death in COVID-19 patients with a high risk of severe illness if the pills were taken for 5 days soon after symptoms started.
Paxlovid is approved or authorized for conditional or emergency use in more than 60 countries to treat high-risk COVID-19 patients, Pfizer said. In the United States, the drug is authorized for emergency use for the treatment of mild to moderate COVID-19 in those aged 12 and older who face high risks for severe disease, hospitalization, or death.
The full study data will be released in coming months and submitted to a peer-reviewed publication, the company said. More details are on the ClinicalTrials.gov website (NCT05047601).
A version of this article first appeared on WebMD.com.
Drug combo holds promise as on-demand contraceptive: Study
A combination of ulipristal acetate (UA) and a cyclo-oxygenase-2 (COX-2) inhibitor holds promise as a pericoital, “on- demand” female oral contraceptive, taken only when needed, according to an exploratory study published in BMJ Sexual & Reproductive Health.
The prospective, open-label, pilot study showed that UA and meloxicam successfully disrupted ovulation at “the peak of luteal surge, when conception risk is highest,” reported lead author Erica P Cahill, MD, of Stanford (Calif.) University, and colleagues.
“There are many people who report being interested in preventing pregnancy who are not using contraception,” Dr. Cahill said in an interview. The ideal is to be able to take a medication to prevent ovulation and know that you wouldn’t ovulate or be able to become pregnant for the next 3-5 days. These would be pericoital contraceptive pills that one could take prior to or immediately after intercourse that would expand the contraceptive options available and meet some of this need, she said.
Dr. Cahill said currently approved emergency contraceptives containing ulipristal acetate or levonorgestrel “work by inhibiting ovulation at the level of the luteal surge, the pituitary signal that starts the ovulation cascade. Because of this mechanism, they are only effective when taken prior to that signal. If they are taken near or after ovulation has occurred, they are not effective.” She said combining meloxicam with UA could address this because meloxicam “has been shown to prevent some of the later steps of ovulation just prior to the egg being released.”
The study included nine healthy women, with a mean age of 31.4 years, and a mean body mass index of 24.5 ± 3.9 kg/m2. All subjects had no exposure to hormonal medication, pregnancy, or lactation in the prior 3 months.
Each participant was followed for two cycles: The first without treatment, to establish normal ovulatory function; and the second during treatment with a one-time dose of UA 30 mg and meloxicam 30 mg during the “fertile window.” This window was defined as when the lead ovarian follicle had a mean diameter of 18 mm, and was determined via thrice-weekly ultrasounds, as well as luteinizing hormone (LH) measurements.
The primary outcome of the study was ovulation disruption, defined as unruptured dominant follicle for 5 days, a blunted LH peak, defined as <15 IU/L, and a nonovulatory luteal phase progesterone level, defined as <3 ng/mL.
Ovulation disruption was achieved in six subjects (67.7%), with eight subjects (88.9%) meeting some criteria.
“When we compare ovulation disruption rates in our study with the previous studies on which our protocol is based, the combination of UA and meloxicam disrupted ovulation at each phase of the fertile window more than any other medication previously studied,” the researchers wrote. “This medication combination is an important candidate to evaluate as oral pericoital contraception.”
When comparing subjects’ baseline cycles with their treatment cycles, the latter were approximately 3 days longer, although there was no difference in endometrial stripe thickness or irregular bleeding.
“Cycle length changes are an important parameter as people interested in oral, on-demand contraception may also be using fertility awareness methods which can be affected by cycle length changes.”
The authors noted that measures of full efficacy and side effects were beyond the scope of the study and would require repeat dosing. Similarly, liver enzymes were not measured, because there was only one dose of study medication, but “given the potential impact of repeat UA on liver enzymes, this measurement is critical for future studies.”
Asked to comment on the study, Eve Espey, MD, said that although it was limited in size and the use of an “intermediate outcome” of ovulation disruption, “the combination does show some promise as a focus of future research.” However, Dr. Espey, distinguished professor and chair in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said it is too early to determine the significance of the findings. “But it does point the way to further research,” she noted. “Compared with existing emergency contraception, this study shows that the UA-meloxicam combination disrupts ovulation over a broader mid-cycle time period – [an] extended duration of action [that] could theoretically translate into increased effectiveness as a contraceptive.”
The study was supported by the Society for Family Planning Research Fund. None of the authors, or Dr. Espey, declared competing interests.
A combination of ulipristal acetate (UA) and a cyclo-oxygenase-2 (COX-2) inhibitor holds promise as a pericoital, “on- demand” female oral contraceptive, taken only when needed, according to an exploratory study published in BMJ Sexual & Reproductive Health.
The prospective, open-label, pilot study showed that UA and meloxicam successfully disrupted ovulation at “the peak of luteal surge, when conception risk is highest,” reported lead author Erica P Cahill, MD, of Stanford (Calif.) University, and colleagues.
“There are many people who report being interested in preventing pregnancy who are not using contraception,” Dr. Cahill said in an interview. The ideal is to be able to take a medication to prevent ovulation and know that you wouldn’t ovulate or be able to become pregnant for the next 3-5 days. These would be pericoital contraceptive pills that one could take prior to or immediately after intercourse that would expand the contraceptive options available and meet some of this need, she said.
Dr. Cahill said currently approved emergency contraceptives containing ulipristal acetate or levonorgestrel “work by inhibiting ovulation at the level of the luteal surge, the pituitary signal that starts the ovulation cascade. Because of this mechanism, they are only effective when taken prior to that signal. If they are taken near or after ovulation has occurred, they are not effective.” She said combining meloxicam with UA could address this because meloxicam “has been shown to prevent some of the later steps of ovulation just prior to the egg being released.”
The study included nine healthy women, with a mean age of 31.4 years, and a mean body mass index of 24.5 ± 3.9 kg/m2. All subjects had no exposure to hormonal medication, pregnancy, or lactation in the prior 3 months.
Each participant was followed for two cycles: The first without treatment, to establish normal ovulatory function; and the second during treatment with a one-time dose of UA 30 mg and meloxicam 30 mg during the “fertile window.” This window was defined as when the lead ovarian follicle had a mean diameter of 18 mm, and was determined via thrice-weekly ultrasounds, as well as luteinizing hormone (LH) measurements.
The primary outcome of the study was ovulation disruption, defined as unruptured dominant follicle for 5 days, a blunted LH peak, defined as <15 IU/L, and a nonovulatory luteal phase progesterone level, defined as <3 ng/mL.
Ovulation disruption was achieved in six subjects (67.7%), with eight subjects (88.9%) meeting some criteria.
“When we compare ovulation disruption rates in our study with the previous studies on which our protocol is based, the combination of UA and meloxicam disrupted ovulation at each phase of the fertile window more than any other medication previously studied,” the researchers wrote. “This medication combination is an important candidate to evaluate as oral pericoital contraception.”
When comparing subjects’ baseline cycles with their treatment cycles, the latter were approximately 3 days longer, although there was no difference in endometrial stripe thickness or irregular bleeding.
“Cycle length changes are an important parameter as people interested in oral, on-demand contraception may also be using fertility awareness methods which can be affected by cycle length changes.”
The authors noted that measures of full efficacy and side effects were beyond the scope of the study and would require repeat dosing. Similarly, liver enzymes were not measured, because there was only one dose of study medication, but “given the potential impact of repeat UA on liver enzymes, this measurement is critical for future studies.”
Asked to comment on the study, Eve Espey, MD, said that although it was limited in size and the use of an “intermediate outcome” of ovulation disruption, “the combination does show some promise as a focus of future research.” However, Dr. Espey, distinguished professor and chair in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said it is too early to determine the significance of the findings. “But it does point the way to further research,” she noted. “Compared with existing emergency contraception, this study shows that the UA-meloxicam combination disrupts ovulation over a broader mid-cycle time period – [an] extended duration of action [that] could theoretically translate into increased effectiveness as a contraceptive.”
The study was supported by the Society for Family Planning Research Fund. None of the authors, or Dr. Espey, declared competing interests.
A combination of ulipristal acetate (UA) and a cyclo-oxygenase-2 (COX-2) inhibitor holds promise as a pericoital, “on- demand” female oral contraceptive, taken only when needed, according to an exploratory study published in BMJ Sexual & Reproductive Health.
The prospective, open-label, pilot study showed that UA and meloxicam successfully disrupted ovulation at “the peak of luteal surge, when conception risk is highest,” reported lead author Erica P Cahill, MD, of Stanford (Calif.) University, and colleagues.
“There are many people who report being interested in preventing pregnancy who are not using contraception,” Dr. Cahill said in an interview. The ideal is to be able to take a medication to prevent ovulation and know that you wouldn’t ovulate or be able to become pregnant for the next 3-5 days. These would be pericoital contraceptive pills that one could take prior to or immediately after intercourse that would expand the contraceptive options available and meet some of this need, she said.
Dr. Cahill said currently approved emergency contraceptives containing ulipristal acetate or levonorgestrel “work by inhibiting ovulation at the level of the luteal surge, the pituitary signal that starts the ovulation cascade. Because of this mechanism, they are only effective when taken prior to that signal. If they are taken near or after ovulation has occurred, they are not effective.” She said combining meloxicam with UA could address this because meloxicam “has been shown to prevent some of the later steps of ovulation just prior to the egg being released.”
The study included nine healthy women, with a mean age of 31.4 years, and a mean body mass index of 24.5 ± 3.9 kg/m2. All subjects had no exposure to hormonal medication, pregnancy, or lactation in the prior 3 months.
Each participant was followed for two cycles: The first without treatment, to establish normal ovulatory function; and the second during treatment with a one-time dose of UA 30 mg and meloxicam 30 mg during the “fertile window.” This window was defined as when the lead ovarian follicle had a mean diameter of 18 mm, and was determined via thrice-weekly ultrasounds, as well as luteinizing hormone (LH) measurements.
The primary outcome of the study was ovulation disruption, defined as unruptured dominant follicle for 5 days, a blunted LH peak, defined as <15 IU/L, and a nonovulatory luteal phase progesterone level, defined as <3 ng/mL.
Ovulation disruption was achieved in six subjects (67.7%), with eight subjects (88.9%) meeting some criteria.
“When we compare ovulation disruption rates in our study with the previous studies on which our protocol is based, the combination of UA and meloxicam disrupted ovulation at each phase of the fertile window more than any other medication previously studied,” the researchers wrote. “This medication combination is an important candidate to evaluate as oral pericoital contraception.”
When comparing subjects’ baseline cycles with their treatment cycles, the latter were approximately 3 days longer, although there was no difference in endometrial stripe thickness or irregular bleeding.
“Cycle length changes are an important parameter as people interested in oral, on-demand contraception may also be using fertility awareness methods which can be affected by cycle length changes.”
The authors noted that measures of full efficacy and side effects were beyond the scope of the study and would require repeat dosing. Similarly, liver enzymes were not measured, because there was only one dose of study medication, but “given the potential impact of repeat UA on liver enzymes, this measurement is critical for future studies.”
Asked to comment on the study, Eve Espey, MD, said that although it was limited in size and the use of an “intermediate outcome” of ovulation disruption, “the combination does show some promise as a focus of future research.” However, Dr. Espey, distinguished professor and chair in the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, said it is too early to determine the significance of the findings. “But it does point the way to further research,” she noted. “Compared with existing emergency contraception, this study shows that the UA-meloxicam combination disrupts ovulation over a broader mid-cycle time period – [an] extended duration of action [that] could theoretically translate into increased effectiveness as a contraceptive.”
The study was supported by the Society for Family Planning Research Fund. None of the authors, or Dr. Espey, declared competing interests.
FROM BMJ SEXUAL & REPRODUCTIVE HEALTH
Vegetarian diet as good for children, with slight risk of underweight
With more families placing their children on vegetarian diets, the results from a Canadian longitudinal cohort study are reassuring: It found no clinically meaningful differences in height, growth, or biochemical measures of nutrition in young children on vegetarian and nonvegetarian diets.
While z scores (the standard deviation above or below the mean) were similar in both dietary groups, there was a weak association between a vegetarian diet and lower mean z height, as well as slightly higher odds of underweight.
No significant associations were identified between vegetarian and nonvegetarian diets for child z body mass index (BMI), serum ferritin, 25(OH)D, and serum lipids, according to Jonathon L. Maguire, MD. MSc, of St. Michael’s Hospital Pediatric Clinic in Toronto.
Moreover, the magnitude of the height and vegetarian diet association was small at just 0.3 cm for a 3-year-old child and unlikely to be clinically meaningful, Dr. Maguire and colleagues wrote online in Pediatrics.
In a secondary study outcome, cow’s milk consumption was associated with higher serum lipid levels for both diets. Serum lipids were similar among those who did or did not consume a vegetarian diet and consumed the recommended 2 cups of cow’s milk per day.
“The vast majority of children with vegetarian diets have similar growth and nutrition as children without vegetarian diets,” said Dr. Maguire, who is also a professor of pediatrics and nutritional sciences at the University of Toronto, in an interview. “But, I think we should be mindful to carefully plan vegetarian diets for children [who are] underweight.”
The study conclusion was based on 8,907 children, 6 months to 8 years of age, including 248 vegetarian and 25 vegan children, at baseline. They were part of theTARGet Kids! practice-based research network in Toronto.
The mean age of children at baseline was 2.2 years (standard deviation, 1.5), and 52.4% were male. Participants were followed for an average of 2.8 years (SD, 1.7).
Those with a vegetarian diet had longer breastfeeding duration: 12.6 months (SD, 9.5) versus 10.0 months (SD, 7.0). They were also more likely to be of Asian ethnicity: 33.8% versus 19.0%. Otherwise, children with and without a vegetarian diet were similar at baseline.
In study outcomes, vegetarian children had higher odds of underweight: body mass index z score less than –2 (odds ratio 1.87; 95% confidence interval, 1.19-2.96, P = .007), while no association with overweight or obesity was found.
In a secondary outcome, cow’s milk consumption was associated with higher levels of non–high-density lipoprotein cholesterol (P = .03), total cholesterol (P = .04), and low-density lipoprotein cholesterol (P = .02) in young children on a vegetarian diet. Levels were similar in children with and without a vegetarian diet who consumed the recommended 2 cups of cow’s milk per day.
Previous studies have found that vegetarian children have normal growth and development but tend to be leaner than their omnivore peers.
As for the potential effect of following a fully vegan diet on these nutritional measures, Dr. Maguire said, “Unfortunately, there were not enough children with vegan diets to make meaningful conclusions.”
Would results likely be similar in older children who have more independence and engage more with their peers?“ I don’t know, but we will be following these children for many years to come through the TARGet Kids! research network, Dr. Maguire said.
Studies such as this are timely as plant-based eating becomes more widespread in the United States. The 2007-2010 National Health and Nutrition Examination Surveys found that 2.1% of American adults followed a vegetarian diet, and that figure appears to have increased, with 5% of American adults self-identifying as vegetarian in a 2019 Gallup poll.
Offering her perspective on the Canadian study but not involved in it, Stephanie Di Figlia-Peck, MS, RDN, agreed the results indicate that “a vegetarian diet is not a negative thing for growth and development.” She is a lead registered dietitian in the division of adolescent medicine at Cohen Children’s Medical Center in New Hyde Park, N.Y. She noted, however, that the study looked only at very young children on average.
She stressed that vegetarian regimens require family commitment and agreed on the need for planning. “For these diets to work, a lot has to go into it. But if they’re carefully planned, there is adequate protein and micro- and macronutrients and there’s a nonnegative effect on growth and development.”
The study results mirror what she sees in clinical practice, with vegetarian children tending to weigh less. “Some obese and overweight children will adopt vegetarian diets to lose weight,” Ms. Di Figlia-Peck said.
And perseverance has rewards. “When people follow a vegetarian diet, they tend to have lower blood pressure and cholesterol. A plant-based diet can favorably impact diseases for an entire lifetime.”
This study was supported by the Canadian Institutes of Health Research and the St. Michael’s Hospital and SickKids Hospital foundations. Dr. Maguire received an unrestricted research grant for a previous investigator-initiated study from Dairy Farmers of Canada, and D drops provided nonfinancial support (vitamin D supplements) for a previous investigator-initiated study on vitamin D and respiratory tract infections. Coauthor David Jenkins, MD, PhD, DSc, reported research support from multiple private-sector and nonprivate organizations; several of his family members are involved in the promotion of vegetarian diets. Ms. Di Figlia-Peck had no competing interests to declare.
With more families placing their children on vegetarian diets, the results from a Canadian longitudinal cohort study are reassuring: It found no clinically meaningful differences in height, growth, or biochemical measures of nutrition in young children on vegetarian and nonvegetarian diets.
While z scores (the standard deviation above or below the mean) were similar in both dietary groups, there was a weak association between a vegetarian diet and lower mean z height, as well as slightly higher odds of underweight.
No significant associations were identified between vegetarian and nonvegetarian diets for child z body mass index (BMI), serum ferritin, 25(OH)D, and serum lipids, according to Jonathon L. Maguire, MD. MSc, of St. Michael’s Hospital Pediatric Clinic in Toronto.
Moreover, the magnitude of the height and vegetarian diet association was small at just 0.3 cm for a 3-year-old child and unlikely to be clinically meaningful, Dr. Maguire and colleagues wrote online in Pediatrics.
In a secondary study outcome, cow’s milk consumption was associated with higher serum lipid levels for both diets. Serum lipids were similar among those who did or did not consume a vegetarian diet and consumed the recommended 2 cups of cow’s milk per day.
“The vast majority of children with vegetarian diets have similar growth and nutrition as children without vegetarian diets,” said Dr. Maguire, who is also a professor of pediatrics and nutritional sciences at the University of Toronto, in an interview. “But, I think we should be mindful to carefully plan vegetarian diets for children [who are] underweight.”
The study conclusion was based on 8,907 children, 6 months to 8 years of age, including 248 vegetarian and 25 vegan children, at baseline. They were part of theTARGet Kids! practice-based research network in Toronto.
The mean age of children at baseline was 2.2 years (standard deviation, 1.5), and 52.4% were male. Participants were followed for an average of 2.8 years (SD, 1.7).
Those with a vegetarian diet had longer breastfeeding duration: 12.6 months (SD, 9.5) versus 10.0 months (SD, 7.0). They were also more likely to be of Asian ethnicity: 33.8% versus 19.0%. Otherwise, children with and without a vegetarian diet were similar at baseline.
In study outcomes, vegetarian children had higher odds of underweight: body mass index z score less than –2 (odds ratio 1.87; 95% confidence interval, 1.19-2.96, P = .007), while no association with overweight or obesity was found.
In a secondary outcome, cow’s milk consumption was associated with higher levels of non–high-density lipoprotein cholesterol (P = .03), total cholesterol (P = .04), and low-density lipoprotein cholesterol (P = .02) in young children on a vegetarian diet. Levels were similar in children with and without a vegetarian diet who consumed the recommended 2 cups of cow’s milk per day.
Previous studies have found that vegetarian children have normal growth and development but tend to be leaner than their omnivore peers.
As for the potential effect of following a fully vegan diet on these nutritional measures, Dr. Maguire said, “Unfortunately, there were not enough children with vegan diets to make meaningful conclusions.”
Would results likely be similar in older children who have more independence and engage more with their peers?“ I don’t know, but we will be following these children for many years to come through the TARGet Kids! research network, Dr. Maguire said.
Studies such as this are timely as plant-based eating becomes more widespread in the United States. The 2007-2010 National Health and Nutrition Examination Surveys found that 2.1% of American adults followed a vegetarian diet, and that figure appears to have increased, with 5% of American adults self-identifying as vegetarian in a 2019 Gallup poll.
Offering her perspective on the Canadian study but not involved in it, Stephanie Di Figlia-Peck, MS, RDN, agreed the results indicate that “a vegetarian diet is not a negative thing for growth and development.” She is a lead registered dietitian in the division of adolescent medicine at Cohen Children’s Medical Center in New Hyde Park, N.Y. She noted, however, that the study looked only at very young children on average.
She stressed that vegetarian regimens require family commitment and agreed on the need for planning. “For these diets to work, a lot has to go into it. But if they’re carefully planned, there is adequate protein and micro- and macronutrients and there’s a nonnegative effect on growth and development.”
The study results mirror what she sees in clinical practice, with vegetarian children tending to weigh less. “Some obese and overweight children will adopt vegetarian diets to lose weight,” Ms. Di Figlia-Peck said.
And perseverance has rewards. “When people follow a vegetarian diet, they tend to have lower blood pressure and cholesterol. A plant-based diet can favorably impact diseases for an entire lifetime.”
This study was supported by the Canadian Institutes of Health Research and the St. Michael’s Hospital and SickKids Hospital foundations. Dr. Maguire received an unrestricted research grant for a previous investigator-initiated study from Dairy Farmers of Canada, and D drops provided nonfinancial support (vitamin D supplements) for a previous investigator-initiated study on vitamin D and respiratory tract infections. Coauthor David Jenkins, MD, PhD, DSc, reported research support from multiple private-sector and nonprivate organizations; several of his family members are involved in the promotion of vegetarian diets. Ms. Di Figlia-Peck had no competing interests to declare.
With more families placing their children on vegetarian diets, the results from a Canadian longitudinal cohort study are reassuring: It found no clinically meaningful differences in height, growth, or biochemical measures of nutrition in young children on vegetarian and nonvegetarian diets.
While z scores (the standard deviation above or below the mean) were similar in both dietary groups, there was a weak association between a vegetarian diet and lower mean z height, as well as slightly higher odds of underweight.
No significant associations were identified between vegetarian and nonvegetarian diets for child z body mass index (BMI), serum ferritin, 25(OH)D, and serum lipids, according to Jonathon L. Maguire, MD. MSc, of St. Michael’s Hospital Pediatric Clinic in Toronto.
Moreover, the magnitude of the height and vegetarian diet association was small at just 0.3 cm for a 3-year-old child and unlikely to be clinically meaningful, Dr. Maguire and colleagues wrote online in Pediatrics.
In a secondary study outcome, cow’s milk consumption was associated with higher serum lipid levels for both diets. Serum lipids were similar among those who did or did not consume a vegetarian diet and consumed the recommended 2 cups of cow’s milk per day.
“The vast majority of children with vegetarian diets have similar growth and nutrition as children without vegetarian diets,” said Dr. Maguire, who is also a professor of pediatrics and nutritional sciences at the University of Toronto, in an interview. “But, I think we should be mindful to carefully plan vegetarian diets for children [who are] underweight.”
The study conclusion was based on 8,907 children, 6 months to 8 years of age, including 248 vegetarian and 25 vegan children, at baseline. They were part of theTARGet Kids! practice-based research network in Toronto.
The mean age of children at baseline was 2.2 years (standard deviation, 1.5), and 52.4% were male. Participants were followed for an average of 2.8 years (SD, 1.7).
Those with a vegetarian diet had longer breastfeeding duration: 12.6 months (SD, 9.5) versus 10.0 months (SD, 7.0). They were also more likely to be of Asian ethnicity: 33.8% versus 19.0%. Otherwise, children with and without a vegetarian diet were similar at baseline.
In study outcomes, vegetarian children had higher odds of underweight: body mass index z score less than –2 (odds ratio 1.87; 95% confidence interval, 1.19-2.96, P = .007), while no association with overweight or obesity was found.
In a secondary outcome, cow’s milk consumption was associated with higher levels of non–high-density lipoprotein cholesterol (P = .03), total cholesterol (P = .04), and low-density lipoprotein cholesterol (P = .02) in young children on a vegetarian diet. Levels were similar in children with and without a vegetarian diet who consumed the recommended 2 cups of cow’s milk per day.
Previous studies have found that vegetarian children have normal growth and development but tend to be leaner than their omnivore peers.
As for the potential effect of following a fully vegan diet on these nutritional measures, Dr. Maguire said, “Unfortunately, there were not enough children with vegan diets to make meaningful conclusions.”
Would results likely be similar in older children who have more independence and engage more with their peers?“ I don’t know, but we will be following these children for many years to come through the TARGet Kids! research network, Dr. Maguire said.
Studies such as this are timely as plant-based eating becomes more widespread in the United States. The 2007-2010 National Health and Nutrition Examination Surveys found that 2.1% of American adults followed a vegetarian diet, and that figure appears to have increased, with 5% of American adults self-identifying as vegetarian in a 2019 Gallup poll.
Offering her perspective on the Canadian study but not involved in it, Stephanie Di Figlia-Peck, MS, RDN, agreed the results indicate that “a vegetarian diet is not a negative thing for growth and development.” She is a lead registered dietitian in the division of adolescent medicine at Cohen Children’s Medical Center in New Hyde Park, N.Y. She noted, however, that the study looked only at very young children on average.
She stressed that vegetarian regimens require family commitment and agreed on the need for planning. “For these diets to work, a lot has to go into it. But if they’re carefully planned, there is adequate protein and micro- and macronutrients and there’s a nonnegative effect on growth and development.”
The study results mirror what she sees in clinical practice, with vegetarian children tending to weigh less. “Some obese and overweight children will adopt vegetarian diets to lose weight,” Ms. Di Figlia-Peck said.
And perseverance has rewards. “When people follow a vegetarian diet, they tend to have lower blood pressure and cholesterol. A plant-based diet can favorably impact diseases for an entire lifetime.”
This study was supported by the Canadian Institutes of Health Research and the St. Michael’s Hospital and SickKids Hospital foundations. Dr. Maguire received an unrestricted research grant for a previous investigator-initiated study from Dairy Farmers of Canada, and D drops provided nonfinancial support (vitamin D supplements) for a previous investigator-initiated study on vitamin D and respiratory tract infections. Coauthor David Jenkins, MD, PhD, DSc, reported research support from multiple private-sector and nonprivate organizations; several of his family members are involved in the promotion of vegetarian diets. Ms. Di Figlia-Peck had no competing interests to declare.
FROM PEDIATRICS



