User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
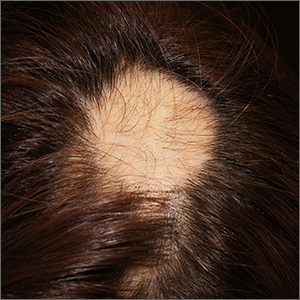
Steroid phobia drives weaker prescribing, nonadherence for AD
, Nanette B. Silverberg, MD, said at the Revolutionizing Atopic Dermatitis meeting.
Up to 40% of parents of children with chronic AD cite anxiety surrounding corticosteroids, according to Dr. Silverberg, chief of pediatric dermatology at the Mount Sinai Health System, New York.
When the potential for adverse events are explained to parents who are anxious about a drug, “they take it in a different way than other individuals,” noted Dr. Silverberg, clinical professor of pediatrics and dermatology at Icahn School of Medicine at Mount Sinai.
In a systematic review of 16 studies examining topical corticosteroid phobia in AD, published between 1946 and 2016, the prevalence of corticosteroid phobia among patients with AD or their caregivers ranged from 21% to 83.7%, with definitions of phobia that ranged from “concern” to “irrational fear.” In two studies where adherence was evaluated, patients with corticosteroid phobia had a higher rate of partial adherence (49.4%) or nonadherence (14.1%) when compared with patients who didn’t have a phobia of corticosteroids (29.3 % and 9.8%, respectively)..
The source of these fears can be information from friends, relatives, media, the Internet, as well as doctors, Dr. Silverberg noted. “We have to be responsible for providing proper data to these individuals,” she said.
Primary care providers also treat young children with AD differently from older children, when compared with other specialties, according to the results of one study that involved a survey and a retrospective chart review, published in 2020. In the survey, 88% of primary care providers in Chicago said they managed AD differently in children under aged 2 years than in older children, with 65% reporting they were more likely to refer a child under 2 years to a specialist, and 64% said they were less likely to prescribe high-potency topical corticosteroids to children in this age group. The retrospective review found that at PCP visits, significantly more children with AD between aged 2 and 5 years were more likely to be prescribed medium-potency topical corticosteroids (0.66% vs. 0.37%, P < .01) and high-potency topical corticosteroids (0.15% vs. 0.05%; P < .01) than children under 2 years old, respectively.
Of the children who had seen a specialist, more dermatologists (57%) prescribed medium-potency and high-potency topical corticosteroids for children under aged 2 years than did allergists (30%) and pediatricians (15%) (P < .01), according to the study.
“These are our colleagues who are often very strong prescribers using systemic agents, and only 15% of pediatricians will do this,” Dr. Silverberg said. “We’re really looking at a big divide between us and other subspecialties and primary care, and [topical corticosteroids] are frequently underutilized because of these fears.”
In another study looking at the use of topical corticosteroids for AD in the pediatric emergency department (mean age of patients, 6.3 years), from 2012 to 2017, patients at 46 of 167 visits were prescribed over-the-counter topical hydrocortisone, while at 63 of 167 visits, patients were not prescribed or recommended any corticosteroid.
The mean class of the topical corticosteroid prescribed was 5.5, and the most commonly recommended corticosteroid was class 7 (the least potent available) in 61 of 104 patients (P < .001). A dermatologist was consulted in 14 of 167 visits (8.6%), and in those cases, topical corticosteroids were often prescribed (P = .018), as was a higher class of corticosteroids (a mean of 3.1 vs. 5.9; P < .001).
Topical corticosteroids also tend to be prescribed less by internal medicine physicians than by family medicine physicians or dermatologists. A 2020 study of ambulatory care data in the United States from 2006 to 2016 found that internists were 22 times less likely to prescribe topical corticosteroids for AD compared with dermatologists (5.1% vs. 52.2%; P = .001). But there was no significant difference in prescribing between family medicine physicians and dermatologists (39.1% vs. 52.2%, P = .27).
“We know they [corticosteroids] work, but so many people are fearful of them ... even with a low, low side effect profile,” Dr. Silverberg said.
For children with AD, corticosteroid use is “suboptimal” across the United States, with evidence that Medicaid-insured pediatric patients with AD are less likely to see a specialist and less likely to be prescribed high-potency topical corticosteroids compared with commercially-insured patients.
Discussing efficacy and safety
Dr. Silverberg said providers who care for children with AD should talk about the fear surrounding these medications and educate parents with anxiety surrounding corticosteroids. “Side effects are usually short term and limited, so we really can assure parents that there is a long safety profile,” she said.
Asked to comment on this topic, Adelaide Hebert, MD, professor of dermatology and director of pediatric dermatology at the University of Texas, Houston, said that she often sees concerns surrounding the use of topical corticosteroids, both in her practice with parents and when teaching residents in other disciplines, such as pediatrics, family medicine, and emergency medicine.
“We don’t do a good job in medical school educating the students about the safety, applicability, and proper use of topical steroids, and I think that leads to some of the confusion when it comes to properly using this class of medications in treating atopic dermatitis,” she said in an interview.
The use of a high-potency topical steroid is important, she noted, as lower doses may not adequately control AD. “If the patient has very mild disease, this may be just fine,” she noted. Those patients often do not see a pediatric dermatologist, “but the ones with moderate or severe atopic dermatitis often do, and I would say [the problem of] undertreatment is all too common.”
Like Dr. Silverberg, Dr. Hebert said that in her clinical experience, side effects from topical corticosteroids have been rare. “I could count on one hand the number of patients in a 38-year pediatric dermatology practice where they had an adverse effect from a topical steroid,” she said.
Dr. Silverberg reports receiving consulting fees from Amryt Pharma, Galderma, Incyte, and Vyne; non-CME related fees from Pfizer and Regeneron; and contracted research fees from Incyte and the Vitiligo Research Foundation. Dr. Hebert reports receiving research funds from GSK, Leo, Ortho Dermatologics, Galderma, Dermavant, Pfizer, and Arcutis Biotherapeutics paid to her institution; honoraria from Pfizer, Arcutis, Incyte; and having served on the data safety monitoring board for Regeneron-Sanofi, GSK, and Ortho Dermatologics.
, Nanette B. Silverberg, MD, said at the Revolutionizing Atopic Dermatitis meeting.
Up to 40% of parents of children with chronic AD cite anxiety surrounding corticosteroids, according to Dr. Silverberg, chief of pediatric dermatology at the Mount Sinai Health System, New York.
When the potential for adverse events are explained to parents who are anxious about a drug, “they take it in a different way than other individuals,” noted Dr. Silverberg, clinical professor of pediatrics and dermatology at Icahn School of Medicine at Mount Sinai.
In a systematic review of 16 studies examining topical corticosteroid phobia in AD, published between 1946 and 2016, the prevalence of corticosteroid phobia among patients with AD or their caregivers ranged from 21% to 83.7%, with definitions of phobia that ranged from “concern” to “irrational fear.” In two studies where adherence was evaluated, patients with corticosteroid phobia had a higher rate of partial adherence (49.4%) or nonadherence (14.1%) when compared with patients who didn’t have a phobia of corticosteroids (29.3 % and 9.8%, respectively)..
The source of these fears can be information from friends, relatives, media, the Internet, as well as doctors, Dr. Silverberg noted. “We have to be responsible for providing proper data to these individuals,” she said.
Primary care providers also treat young children with AD differently from older children, when compared with other specialties, according to the results of one study that involved a survey and a retrospective chart review, published in 2020. In the survey, 88% of primary care providers in Chicago said they managed AD differently in children under aged 2 years than in older children, with 65% reporting they were more likely to refer a child under 2 years to a specialist, and 64% said they were less likely to prescribe high-potency topical corticosteroids to children in this age group. The retrospective review found that at PCP visits, significantly more children with AD between aged 2 and 5 years were more likely to be prescribed medium-potency topical corticosteroids (0.66% vs. 0.37%, P < .01) and high-potency topical corticosteroids (0.15% vs. 0.05%; P < .01) than children under 2 years old, respectively.
Of the children who had seen a specialist, more dermatologists (57%) prescribed medium-potency and high-potency topical corticosteroids for children under aged 2 years than did allergists (30%) and pediatricians (15%) (P < .01), according to the study.
“These are our colleagues who are often very strong prescribers using systemic agents, and only 15% of pediatricians will do this,” Dr. Silverberg said. “We’re really looking at a big divide between us and other subspecialties and primary care, and [topical corticosteroids] are frequently underutilized because of these fears.”
In another study looking at the use of topical corticosteroids for AD in the pediatric emergency department (mean age of patients, 6.3 years), from 2012 to 2017, patients at 46 of 167 visits were prescribed over-the-counter topical hydrocortisone, while at 63 of 167 visits, patients were not prescribed or recommended any corticosteroid.
The mean class of the topical corticosteroid prescribed was 5.5, and the most commonly recommended corticosteroid was class 7 (the least potent available) in 61 of 104 patients (P < .001). A dermatologist was consulted in 14 of 167 visits (8.6%), and in those cases, topical corticosteroids were often prescribed (P = .018), as was a higher class of corticosteroids (a mean of 3.1 vs. 5.9; P < .001).
Topical corticosteroids also tend to be prescribed less by internal medicine physicians than by family medicine physicians or dermatologists. A 2020 study of ambulatory care data in the United States from 2006 to 2016 found that internists were 22 times less likely to prescribe topical corticosteroids for AD compared with dermatologists (5.1% vs. 52.2%; P = .001). But there was no significant difference in prescribing between family medicine physicians and dermatologists (39.1% vs. 52.2%, P = .27).
“We know they [corticosteroids] work, but so many people are fearful of them ... even with a low, low side effect profile,” Dr. Silverberg said.
For children with AD, corticosteroid use is “suboptimal” across the United States, with evidence that Medicaid-insured pediatric patients with AD are less likely to see a specialist and less likely to be prescribed high-potency topical corticosteroids compared with commercially-insured patients.
Discussing efficacy and safety
Dr. Silverberg said providers who care for children with AD should talk about the fear surrounding these medications and educate parents with anxiety surrounding corticosteroids. “Side effects are usually short term and limited, so we really can assure parents that there is a long safety profile,” she said.
Asked to comment on this topic, Adelaide Hebert, MD, professor of dermatology and director of pediatric dermatology at the University of Texas, Houston, said that she often sees concerns surrounding the use of topical corticosteroids, both in her practice with parents and when teaching residents in other disciplines, such as pediatrics, family medicine, and emergency medicine.
“We don’t do a good job in medical school educating the students about the safety, applicability, and proper use of topical steroids, and I think that leads to some of the confusion when it comes to properly using this class of medications in treating atopic dermatitis,” she said in an interview.
The use of a high-potency topical steroid is important, she noted, as lower doses may not adequately control AD. “If the patient has very mild disease, this may be just fine,” she noted. Those patients often do not see a pediatric dermatologist, “but the ones with moderate or severe atopic dermatitis often do, and I would say [the problem of] undertreatment is all too common.”
Like Dr. Silverberg, Dr. Hebert said that in her clinical experience, side effects from topical corticosteroids have been rare. “I could count on one hand the number of patients in a 38-year pediatric dermatology practice where they had an adverse effect from a topical steroid,” she said.
Dr. Silverberg reports receiving consulting fees from Amryt Pharma, Galderma, Incyte, and Vyne; non-CME related fees from Pfizer and Regeneron; and contracted research fees from Incyte and the Vitiligo Research Foundation. Dr. Hebert reports receiving research funds from GSK, Leo, Ortho Dermatologics, Galderma, Dermavant, Pfizer, and Arcutis Biotherapeutics paid to her institution; honoraria from Pfizer, Arcutis, Incyte; and having served on the data safety monitoring board for Regeneron-Sanofi, GSK, and Ortho Dermatologics.
, Nanette B. Silverberg, MD, said at the Revolutionizing Atopic Dermatitis meeting.
Up to 40% of parents of children with chronic AD cite anxiety surrounding corticosteroids, according to Dr. Silverberg, chief of pediatric dermatology at the Mount Sinai Health System, New York.
When the potential for adverse events are explained to parents who are anxious about a drug, “they take it in a different way than other individuals,” noted Dr. Silverberg, clinical professor of pediatrics and dermatology at Icahn School of Medicine at Mount Sinai.
In a systematic review of 16 studies examining topical corticosteroid phobia in AD, published between 1946 and 2016, the prevalence of corticosteroid phobia among patients with AD or their caregivers ranged from 21% to 83.7%, with definitions of phobia that ranged from “concern” to “irrational fear.” In two studies where adherence was evaluated, patients with corticosteroid phobia had a higher rate of partial adherence (49.4%) or nonadherence (14.1%) when compared with patients who didn’t have a phobia of corticosteroids (29.3 % and 9.8%, respectively)..
The source of these fears can be information from friends, relatives, media, the Internet, as well as doctors, Dr. Silverberg noted. “We have to be responsible for providing proper data to these individuals,” she said.
Primary care providers also treat young children with AD differently from older children, when compared with other specialties, according to the results of one study that involved a survey and a retrospective chart review, published in 2020. In the survey, 88% of primary care providers in Chicago said they managed AD differently in children under aged 2 years than in older children, with 65% reporting they were more likely to refer a child under 2 years to a specialist, and 64% said they were less likely to prescribe high-potency topical corticosteroids to children in this age group. The retrospective review found that at PCP visits, significantly more children with AD between aged 2 and 5 years were more likely to be prescribed medium-potency topical corticosteroids (0.66% vs. 0.37%, P < .01) and high-potency topical corticosteroids (0.15% vs. 0.05%; P < .01) than children under 2 years old, respectively.
Of the children who had seen a specialist, more dermatologists (57%) prescribed medium-potency and high-potency topical corticosteroids for children under aged 2 years than did allergists (30%) and pediatricians (15%) (P < .01), according to the study.
“These are our colleagues who are often very strong prescribers using systemic agents, and only 15% of pediatricians will do this,” Dr. Silverberg said. “We’re really looking at a big divide between us and other subspecialties and primary care, and [topical corticosteroids] are frequently underutilized because of these fears.”
In another study looking at the use of topical corticosteroids for AD in the pediatric emergency department (mean age of patients, 6.3 years), from 2012 to 2017, patients at 46 of 167 visits were prescribed over-the-counter topical hydrocortisone, while at 63 of 167 visits, patients were not prescribed or recommended any corticosteroid.
The mean class of the topical corticosteroid prescribed was 5.5, and the most commonly recommended corticosteroid was class 7 (the least potent available) in 61 of 104 patients (P < .001). A dermatologist was consulted in 14 of 167 visits (8.6%), and in those cases, topical corticosteroids were often prescribed (P = .018), as was a higher class of corticosteroids (a mean of 3.1 vs. 5.9; P < .001).
Topical corticosteroids also tend to be prescribed less by internal medicine physicians than by family medicine physicians or dermatologists. A 2020 study of ambulatory care data in the United States from 2006 to 2016 found that internists were 22 times less likely to prescribe topical corticosteroids for AD compared with dermatologists (5.1% vs. 52.2%; P = .001). But there was no significant difference in prescribing between family medicine physicians and dermatologists (39.1% vs. 52.2%, P = .27).
“We know they [corticosteroids] work, but so many people are fearful of them ... even with a low, low side effect profile,” Dr. Silverberg said.
For children with AD, corticosteroid use is “suboptimal” across the United States, with evidence that Medicaid-insured pediatric patients with AD are less likely to see a specialist and less likely to be prescribed high-potency topical corticosteroids compared with commercially-insured patients.
Discussing efficacy and safety
Dr. Silverberg said providers who care for children with AD should talk about the fear surrounding these medications and educate parents with anxiety surrounding corticosteroids. “Side effects are usually short term and limited, so we really can assure parents that there is a long safety profile,” she said.
Asked to comment on this topic, Adelaide Hebert, MD, professor of dermatology and director of pediatric dermatology at the University of Texas, Houston, said that she often sees concerns surrounding the use of topical corticosteroids, both in her practice with parents and when teaching residents in other disciplines, such as pediatrics, family medicine, and emergency medicine.
“We don’t do a good job in medical school educating the students about the safety, applicability, and proper use of topical steroids, and I think that leads to some of the confusion when it comes to properly using this class of medications in treating atopic dermatitis,” she said in an interview.
The use of a high-potency topical steroid is important, she noted, as lower doses may not adequately control AD. “If the patient has very mild disease, this may be just fine,” she noted. Those patients often do not see a pediatric dermatologist, “but the ones with moderate or severe atopic dermatitis often do, and I would say [the problem of] undertreatment is all too common.”
Like Dr. Silverberg, Dr. Hebert said that in her clinical experience, side effects from topical corticosteroids have been rare. “I could count on one hand the number of patients in a 38-year pediatric dermatology practice where they had an adverse effect from a topical steroid,” she said.
Dr. Silverberg reports receiving consulting fees from Amryt Pharma, Galderma, Incyte, and Vyne; non-CME related fees from Pfizer and Regeneron; and contracted research fees from Incyte and the Vitiligo Research Foundation. Dr. Hebert reports receiving research funds from GSK, Leo, Ortho Dermatologics, Galderma, Dermavant, Pfizer, and Arcutis Biotherapeutics paid to her institution; honoraria from Pfizer, Arcutis, Incyte; and having served on the data safety monitoring board for Regeneron-Sanofi, GSK, and Ortho Dermatologics.
FROM RAD 2022
Flu vaccine linked to lower risk for stroke: INTERSTROKE
in a large new case-control study.
“While influenza vaccination is a cost-effective method to prevent influenza, it is also an effective way to reduce the burden of stroke,” said study author Christopher Schwarzbach, MD, of Ludwigshafen (Germany) Hospital.
“Our results therefore encourage the wider use of influenza vaccination,” he concluded.
Dr. Schwarzbach presented these data from the INTERSTROKE study at the 2022 European Stroke Organisation Conference.
He explained that acute inflammatory disease is thought to increase the risk for cerebrovascular events, and the seasonality of influenza-like illness appears to be associated with the seasonality of cardiovascular and cerebrovascular events. Previous observational studies have also shown a link between influenza vaccination and a reduced risk for stroke.
The current INTERSTROKE study was a large international case-control study conducted between 2007 and 2015 that involved 13,447 cases (patients within 5 days of their first stroke) and a similar number of age- and gender-matched people from 32 countries across the world.
All cases and control subjects were systematically asked whether they had acute febrile illness in the previous 4 weeks and whether they had received an influenza vaccination within the previous year.
Conditional logistical regression was used to quantify the results, with adjustment for 13 different possible confounding factors, including hypertension, activity, smoking, cardiovascular risk factors, and socioeconomic factors.
Results showed that having had an acute febrile illness in the previous 4 weeks was more commonly reported in the patients with an acute ischemic stroke (8.7%) than in control patients (5.6%). After adjustment for confounding factors, this gives an adjusted risk ratio of 1.18, which was of borderline statistical significance (95% confidence limits, 1.01-1.39), Dr. Schwarzbach reported.
The association between recent febrile illness and acute ischemic stroke was stronger when compared with community control subjects (adjusted odds ratio, 2.0), but it was absent when compared with hospital control subjects.
The association was also only apparent in Australia, China, North America, and Western Europe; it was not seen in other parts of the world.
There was no association between acute febrile illness and acute cerebral hemorrhage.
Flu vaccine linked to halving of stroke risk
Having received a flu vaccine in the previous year was strongly associated with a lower risk for any type of stroke (aOR, 0.53), ischemic stroke (aOR, 0.57), and hemorrhagic stroke (aOR, 0.34).
Dr. Schwarzbach noted that these results were also consistent in an extended statistical model that included variables that might reflect a willingness to be vaccinated and when compared with both community and hospital-based control subjects.
The strength of the association between influenza vaccination and reduced risk for stroke was similar when compared with either community or hospital control subjects, and was only moderately stronger during than outside the influenza season.
The association was also seen in all regions of the world apart from Africa and South Asia, Dr. Schwarzbach reported, but he noted that vaccination rates in these two regions were extremely low.
The researchers also found that the magnitude of the associations between flu vaccination and lower risk for stroke were stronger in individuals who had multiple annual vaccinations, with an odds ratio of 0.54 in those who had received a vaccine every year for the previous 5 years, and of 0.79 in those who had received one to four vaccinations in the previous 5 years.
Mechanism: Immune stimulation?
Discussing possible mechanisms behind these results, Dr. Schwarzbach noted that the finding that the association with influenza vaccination and reduced stroke risk was independent of seasonality was surprising. “We had expected the protective effect of vaccination to be bigger during the influenza season, but this wasn’t the case.”
He suggested that one explanation might be the inclusion of regions of the world where this seasonality doesn’t exist.
But he pointed out that the finding of a stronger association between flu vaccination and lower stroke risk in those who had received more vaccinations has given rise to another theory: that it is the stimulation of the immune system rather than the protection of infection against influenza that is the key factor.
In an interview with Dr. Schwarzbach, Guillaume Turk, MD, professor of neurology at GHU Paris, pointed out that causal inferences are always difficult in case-control studies and in clinical epidemiology in general.
“What makes you think that this association between influenza vaccination and decreased risk is causal rather than due to unmeasured confounders? For example, patients who received vaccination may have received more medical attention and may have been more aware of the risk factors for stroke,” he asked.
Dr. Schwarzbach replied: “Yes, this is the issue of healthy user bias, which is always a problem in this type of research and is hard to address.”
“What we tried to do here is to adjust for variables that might influence the willingness of people to get vaccinated,” he added. “These were mainly socioeconomic factors. But, of course, this is something that we can’t rule out.”
Dr. Schwarzbach noted that, for more reliable information on this association, prospective studies are needed.
‘A plausible effect’
Discussing the study after the presentation, William Whiteley, BM, PhD, a clinical epidemiologist at the University of Edinburgh and a consultant neurologist in NHS Lothian, said vaccination was a potentially important way to reduce stroke.
“In this study, there was a plausible effect on reducing stroke incidence from vaccination against influenza, and also a plausible increase in the risk of stroke from having a recent febrile illness, which we have seen in other studies,” he commented.
Dr. Whiteley noted that this observation was particularly relevant now because of the COVID-19 pandemic.
“We’ve all been worried about the risk of heart attack and stroke after COVID, where we’ve seen quite early high risks, and we are also optimistic about the effect of vaccination on reducing those incidences. We’ve seen data from the U.K. that there may be around a 20% reduction in risk of stroke from vaccination. So, it’s all quite plausible, but at the moment it’s all based on observational evidence and we really need some randomized evidence,” he said.
“Vaccination and infections have all sorts of odd confounders,” he added. “People who get vaccines tend to be more healthy than those who don’t get vaccines, so you can start to see quite implausible effects of vaccination on overall mortality, which probably aren’t real, and you probably can’t get rid of that totally with statistical methods.”
Alastair Webb, MD, University of Oxford (England), asked how reliable the current findings were, given that the occurrence of febrile illnesses and receipt of vaccines were all self-reported, and although there was an association for ischemic stroke and febrile illness, this seemed to go in the opposite direction for hemorrhagic stroke. He also noted that the 50% reduction in stroke risk with vaccination in this study seemed “quite a large magnitude of effect.”
Dr. Whiteley replied: “Yes, it is large, but it is promising.” He cited a previous meta-analysis of randomized studies that showed a roughly 25%-35% reduction in vascular events after flu vaccination, but noted that there was a lot of heterogeneity between studies.
“I’m not sure we’re going to see much more randomized evidence, but I think we can probably all agree that having a vaccine against flu or COVID is a good thing for all of us,” Dr. Whiteley concluded.
The INTERSTROKE study was funded by the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Stroke Network, Health Research Board Ireland, Swedish Research Council, Swedish Heart and Lung Foundation, The Health & Medical Care Committee of the Regional Executive Board, Region Vastra Gotaland (Sweden), AstraZeneca, Boehringer Ingelheim, Pfizer, MSD, Chest, Heart and Stroke Scotland, and The Stroke Association, with support from The UK Stroke Research Network. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
in a large new case-control study.
“While influenza vaccination is a cost-effective method to prevent influenza, it is also an effective way to reduce the burden of stroke,” said study author Christopher Schwarzbach, MD, of Ludwigshafen (Germany) Hospital.
“Our results therefore encourage the wider use of influenza vaccination,” he concluded.
Dr. Schwarzbach presented these data from the INTERSTROKE study at the 2022 European Stroke Organisation Conference.
He explained that acute inflammatory disease is thought to increase the risk for cerebrovascular events, and the seasonality of influenza-like illness appears to be associated with the seasonality of cardiovascular and cerebrovascular events. Previous observational studies have also shown a link between influenza vaccination and a reduced risk for stroke.
The current INTERSTROKE study was a large international case-control study conducted between 2007 and 2015 that involved 13,447 cases (patients within 5 days of their first stroke) and a similar number of age- and gender-matched people from 32 countries across the world.
All cases and control subjects were systematically asked whether they had acute febrile illness in the previous 4 weeks and whether they had received an influenza vaccination within the previous year.
Conditional logistical regression was used to quantify the results, with adjustment for 13 different possible confounding factors, including hypertension, activity, smoking, cardiovascular risk factors, and socioeconomic factors.
Results showed that having had an acute febrile illness in the previous 4 weeks was more commonly reported in the patients with an acute ischemic stroke (8.7%) than in control patients (5.6%). After adjustment for confounding factors, this gives an adjusted risk ratio of 1.18, which was of borderline statistical significance (95% confidence limits, 1.01-1.39), Dr. Schwarzbach reported.
The association between recent febrile illness and acute ischemic stroke was stronger when compared with community control subjects (adjusted odds ratio, 2.0), but it was absent when compared with hospital control subjects.
The association was also only apparent in Australia, China, North America, and Western Europe; it was not seen in other parts of the world.
There was no association between acute febrile illness and acute cerebral hemorrhage.
Flu vaccine linked to halving of stroke risk
Having received a flu vaccine in the previous year was strongly associated with a lower risk for any type of stroke (aOR, 0.53), ischemic stroke (aOR, 0.57), and hemorrhagic stroke (aOR, 0.34).
Dr. Schwarzbach noted that these results were also consistent in an extended statistical model that included variables that might reflect a willingness to be vaccinated and when compared with both community and hospital-based control subjects.
The strength of the association between influenza vaccination and reduced risk for stroke was similar when compared with either community or hospital control subjects, and was only moderately stronger during than outside the influenza season.
The association was also seen in all regions of the world apart from Africa and South Asia, Dr. Schwarzbach reported, but he noted that vaccination rates in these two regions were extremely low.
The researchers also found that the magnitude of the associations between flu vaccination and lower risk for stroke were stronger in individuals who had multiple annual vaccinations, with an odds ratio of 0.54 in those who had received a vaccine every year for the previous 5 years, and of 0.79 in those who had received one to four vaccinations in the previous 5 years.
Mechanism: Immune stimulation?
Discussing possible mechanisms behind these results, Dr. Schwarzbach noted that the finding that the association with influenza vaccination and reduced stroke risk was independent of seasonality was surprising. “We had expected the protective effect of vaccination to be bigger during the influenza season, but this wasn’t the case.”
He suggested that one explanation might be the inclusion of regions of the world where this seasonality doesn’t exist.
But he pointed out that the finding of a stronger association between flu vaccination and lower stroke risk in those who had received more vaccinations has given rise to another theory: that it is the stimulation of the immune system rather than the protection of infection against influenza that is the key factor.
In an interview with Dr. Schwarzbach, Guillaume Turk, MD, professor of neurology at GHU Paris, pointed out that causal inferences are always difficult in case-control studies and in clinical epidemiology in general.
“What makes you think that this association between influenza vaccination and decreased risk is causal rather than due to unmeasured confounders? For example, patients who received vaccination may have received more medical attention and may have been more aware of the risk factors for stroke,” he asked.
Dr. Schwarzbach replied: “Yes, this is the issue of healthy user bias, which is always a problem in this type of research and is hard to address.”
“What we tried to do here is to adjust for variables that might influence the willingness of people to get vaccinated,” he added. “These were mainly socioeconomic factors. But, of course, this is something that we can’t rule out.”
Dr. Schwarzbach noted that, for more reliable information on this association, prospective studies are needed.
‘A plausible effect’
Discussing the study after the presentation, William Whiteley, BM, PhD, a clinical epidemiologist at the University of Edinburgh and a consultant neurologist in NHS Lothian, said vaccination was a potentially important way to reduce stroke.
“In this study, there was a plausible effect on reducing stroke incidence from vaccination against influenza, and also a plausible increase in the risk of stroke from having a recent febrile illness, which we have seen in other studies,” he commented.
Dr. Whiteley noted that this observation was particularly relevant now because of the COVID-19 pandemic.
“We’ve all been worried about the risk of heart attack and stroke after COVID, where we’ve seen quite early high risks, and we are also optimistic about the effect of vaccination on reducing those incidences. We’ve seen data from the U.K. that there may be around a 20% reduction in risk of stroke from vaccination. So, it’s all quite plausible, but at the moment it’s all based on observational evidence and we really need some randomized evidence,” he said.
“Vaccination and infections have all sorts of odd confounders,” he added. “People who get vaccines tend to be more healthy than those who don’t get vaccines, so you can start to see quite implausible effects of vaccination on overall mortality, which probably aren’t real, and you probably can’t get rid of that totally with statistical methods.”
Alastair Webb, MD, University of Oxford (England), asked how reliable the current findings were, given that the occurrence of febrile illnesses and receipt of vaccines were all self-reported, and although there was an association for ischemic stroke and febrile illness, this seemed to go in the opposite direction for hemorrhagic stroke. He also noted that the 50% reduction in stroke risk with vaccination in this study seemed “quite a large magnitude of effect.”
Dr. Whiteley replied: “Yes, it is large, but it is promising.” He cited a previous meta-analysis of randomized studies that showed a roughly 25%-35% reduction in vascular events after flu vaccination, but noted that there was a lot of heterogeneity between studies.
“I’m not sure we’re going to see much more randomized evidence, but I think we can probably all agree that having a vaccine against flu or COVID is a good thing for all of us,” Dr. Whiteley concluded.
The INTERSTROKE study was funded by the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Stroke Network, Health Research Board Ireland, Swedish Research Council, Swedish Heart and Lung Foundation, The Health & Medical Care Committee of the Regional Executive Board, Region Vastra Gotaland (Sweden), AstraZeneca, Boehringer Ingelheim, Pfizer, MSD, Chest, Heart and Stroke Scotland, and The Stroke Association, with support from The UK Stroke Research Network. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
in a large new case-control study.
“While influenza vaccination is a cost-effective method to prevent influenza, it is also an effective way to reduce the burden of stroke,” said study author Christopher Schwarzbach, MD, of Ludwigshafen (Germany) Hospital.
“Our results therefore encourage the wider use of influenza vaccination,” he concluded.
Dr. Schwarzbach presented these data from the INTERSTROKE study at the 2022 European Stroke Organisation Conference.
He explained that acute inflammatory disease is thought to increase the risk for cerebrovascular events, and the seasonality of influenza-like illness appears to be associated with the seasonality of cardiovascular and cerebrovascular events. Previous observational studies have also shown a link between influenza vaccination and a reduced risk for stroke.
The current INTERSTROKE study was a large international case-control study conducted between 2007 and 2015 that involved 13,447 cases (patients within 5 days of their first stroke) and a similar number of age- and gender-matched people from 32 countries across the world.
All cases and control subjects were systematically asked whether they had acute febrile illness in the previous 4 weeks and whether they had received an influenza vaccination within the previous year.
Conditional logistical regression was used to quantify the results, with adjustment for 13 different possible confounding factors, including hypertension, activity, smoking, cardiovascular risk factors, and socioeconomic factors.
Results showed that having had an acute febrile illness in the previous 4 weeks was more commonly reported in the patients with an acute ischemic stroke (8.7%) than in control patients (5.6%). After adjustment for confounding factors, this gives an adjusted risk ratio of 1.18, which was of borderline statistical significance (95% confidence limits, 1.01-1.39), Dr. Schwarzbach reported.
The association between recent febrile illness and acute ischemic stroke was stronger when compared with community control subjects (adjusted odds ratio, 2.0), but it was absent when compared with hospital control subjects.
The association was also only apparent in Australia, China, North America, and Western Europe; it was not seen in other parts of the world.
There was no association between acute febrile illness and acute cerebral hemorrhage.
Flu vaccine linked to halving of stroke risk
Having received a flu vaccine in the previous year was strongly associated with a lower risk for any type of stroke (aOR, 0.53), ischemic stroke (aOR, 0.57), and hemorrhagic stroke (aOR, 0.34).
Dr. Schwarzbach noted that these results were also consistent in an extended statistical model that included variables that might reflect a willingness to be vaccinated and when compared with both community and hospital-based control subjects.
The strength of the association between influenza vaccination and reduced risk for stroke was similar when compared with either community or hospital control subjects, and was only moderately stronger during than outside the influenza season.
The association was also seen in all regions of the world apart from Africa and South Asia, Dr. Schwarzbach reported, but he noted that vaccination rates in these two regions were extremely low.
The researchers also found that the magnitude of the associations between flu vaccination and lower risk for stroke were stronger in individuals who had multiple annual vaccinations, with an odds ratio of 0.54 in those who had received a vaccine every year for the previous 5 years, and of 0.79 in those who had received one to four vaccinations in the previous 5 years.
Mechanism: Immune stimulation?
Discussing possible mechanisms behind these results, Dr. Schwarzbach noted that the finding that the association with influenza vaccination and reduced stroke risk was independent of seasonality was surprising. “We had expected the protective effect of vaccination to be bigger during the influenza season, but this wasn’t the case.”
He suggested that one explanation might be the inclusion of regions of the world where this seasonality doesn’t exist.
But he pointed out that the finding of a stronger association between flu vaccination and lower stroke risk in those who had received more vaccinations has given rise to another theory: that it is the stimulation of the immune system rather than the protection of infection against influenza that is the key factor.
In an interview with Dr. Schwarzbach, Guillaume Turk, MD, professor of neurology at GHU Paris, pointed out that causal inferences are always difficult in case-control studies and in clinical epidemiology in general.
“What makes you think that this association between influenza vaccination and decreased risk is causal rather than due to unmeasured confounders? For example, patients who received vaccination may have received more medical attention and may have been more aware of the risk factors for stroke,” he asked.
Dr. Schwarzbach replied: “Yes, this is the issue of healthy user bias, which is always a problem in this type of research and is hard to address.”
“What we tried to do here is to adjust for variables that might influence the willingness of people to get vaccinated,” he added. “These were mainly socioeconomic factors. But, of course, this is something that we can’t rule out.”
Dr. Schwarzbach noted that, for more reliable information on this association, prospective studies are needed.
‘A plausible effect’
Discussing the study after the presentation, William Whiteley, BM, PhD, a clinical epidemiologist at the University of Edinburgh and a consultant neurologist in NHS Lothian, said vaccination was a potentially important way to reduce stroke.
“In this study, there was a plausible effect on reducing stroke incidence from vaccination against influenza, and also a plausible increase in the risk of stroke from having a recent febrile illness, which we have seen in other studies,” he commented.
Dr. Whiteley noted that this observation was particularly relevant now because of the COVID-19 pandemic.
“We’ve all been worried about the risk of heart attack and stroke after COVID, where we’ve seen quite early high risks, and we are also optimistic about the effect of vaccination on reducing those incidences. We’ve seen data from the U.K. that there may be around a 20% reduction in risk of stroke from vaccination. So, it’s all quite plausible, but at the moment it’s all based on observational evidence and we really need some randomized evidence,” he said.
“Vaccination and infections have all sorts of odd confounders,” he added. “People who get vaccines tend to be more healthy than those who don’t get vaccines, so you can start to see quite implausible effects of vaccination on overall mortality, which probably aren’t real, and you probably can’t get rid of that totally with statistical methods.”
Alastair Webb, MD, University of Oxford (England), asked how reliable the current findings were, given that the occurrence of febrile illnesses and receipt of vaccines were all self-reported, and although there was an association for ischemic stroke and febrile illness, this seemed to go in the opposite direction for hemorrhagic stroke. He also noted that the 50% reduction in stroke risk with vaccination in this study seemed “quite a large magnitude of effect.”
Dr. Whiteley replied: “Yes, it is large, but it is promising.” He cited a previous meta-analysis of randomized studies that showed a roughly 25%-35% reduction in vascular events after flu vaccination, but noted that there was a lot of heterogeneity between studies.
“I’m not sure we’re going to see much more randomized evidence, but I think we can probably all agree that having a vaccine against flu or COVID is a good thing for all of us,” Dr. Whiteley concluded.
The INTERSTROKE study was funded by the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Stroke Network, Health Research Board Ireland, Swedish Research Council, Swedish Heart and Lung Foundation, The Health & Medical Care Committee of the Regional Executive Board, Region Vastra Gotaland (Sweden), AstraZeneca, Boehringer Ingelheim, Pfizer, MSD, Chest, Heart and Stroke Scotland, and The Stroke Association, with support from The UK Stroke Research Network. The authors reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
Vegan diet helps shed pounds but doesn’t dint diabetes
on average, new research indicates.
No effect was seen on blood pressure, triglycerides, or high-density lipoprotein cholesterol. HbA1c was reduced by a mean of –0.18 percentage points (P = .002), and there was a small reduction in total cholesterol and low-density lipoprotein cholesterol, on average, across all the studies examined in this meta-analysis.
The work, which compared a number of trials looking at vegan diets versus “normal” eating or other kinds of weight loss diets, “indicates with reasonable certainty that adhering to a vegan diet for at least 12 weeks may result in clinically meaningful weight loss [and] can be used in the management of overweight and type 2 diabetes,” said Anne-Ditte Termannsen, PhD, who reported the findings during a press conference at the European Congress on Obesity 2022, where the work was also presented as a poster.
A vegan diet most likely led to weight loss because it is “associated with a reduced calorie intake due to a lower content of fat and higher content of dietary fiber,” added Dr. Termannsen of the Steno Diabetes Center Copenhagen.
Asked to comment, Janet Cade, PhD, who leads the Nutritional Epidemiology Group at the University of Leeds (England) said the results are likely attributable to fewer calories in the vegan diet, compared with the “control” diets. “Of course, a vegan diet can be healthier in a range of ways, such as higher fruit and vegetables, more fiber and antioxidants; however, the same would be true of a vegetarian diet,” she noted.
And she warned that longer-term data are needed on health outcomes associated with vegan diets, noting, “there have been links to poorer bone health and osteoporosis in people consuming a vegan diet.”
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England) told the UK Science Media Centre: “The authors conducted a systematic review of intervention studies and found that, compared with no dietary interventions, vegan diets showed the strongest association with body-weight reduction.”
However, “When comparing vegan diets with other dietary interventions – such as the Mediterranean diet – the association was much weaker,” he noted.
Vegan, habitual, or a range of weight-loss diets
Dr. Termannsen and colleagues set out to look at the effect of a plant-based diet on cardiometabolic risk factors in people with overweight or type 2 diabetes. They searched the literature for randomized controlled trials with adult participants with overweight (body mass index ≥ 25 kg/m2), prediabetes, or type 2 diabetes.
Participants followed a vegan diet that lasted at least 12 weeks; habitual diets without any changes or energy restriction; a Mediterranean diet; a host of different “diabetes” diets; a low-fat diet; or portion-controlled diets.
“The vegan diets were nearly all low-fat vegan diets but vary substantially regarding the protein, fat, carbohydrate content. All but one study was ad libitum fat, and there were no energy restrictions,” Dr. Termannsen said.
Control diets were more varied. “Some continued their habitual diet, and about half were energy restricted and the others were not,” she acknowledged.
Outcomes comprised body weight, BMI, HbA1c, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides, which were assessed across studies.
A total of 11 trials were included in the meta-analysis, and studies were a mean duration of 19 weeks. A total of 796 participants were included.
Compared with control diets, those on vegan diets lost on average –4.1 kg (–9 lb) (P < .001), with a range of –5.9 kg to –2.4 kg.
BMI dropped by –1.38 kg/m2 (P < .001). Total cholesterol dropped by –0.30 mmol/L (–11.6 mg/dL; P = .007) and LDL cholesterol by –0.24 mmol/L (–9.28 mg/dL; P = .005).
Further analyses found even greater reductions in body weight and BMI when vegan diets were compared with continuing a normal diet without dietary changes, on average, at –7.4 kg (–16.3 lb) (P < .001) and –2.78 kg/m2 (P < .001) respectively.
When compared with other intervention diets, however, body weight dropped by –2.7 kg (–6 lb; P < .001) and BMI by –0.87 kg/m2 (P < .001).
Commenting on limitations of studies compared to the real world, Dr. Termannsen said: “Some studies reported high adherence to their diet, usually due to a high level of support, suggesting that providing continued face-to-face contact with participants may partly explain the adherence differences.”
“This also questions the long-term feasibility of the diet and the applicability of this as long-term care,” she added.
Following a vegan diet requires good planning to ensure adequate nutrition and avoid any deficiencies, she urged. “We need to remember that the menu plans in the studies were created by dietitians.”
A version of this article first appeared on Medscape.com.
on average, new research indicates.
No effect was seen on blood pressure, triglycerides, or high-density lipoprotein cholesterol. HbA1c was reduced by a mean of –0.18 percentage points (P = .002), and there was a small reduction in total cholesterol and low-density lipoprotein cholesterol, on average, across all the studies examined in this meta-analysis.
The work, which compared a number of trials looking at vegan diets versus “normal” eating or other kinds of weight loss diets, “indicates with reasonable certainty that adhering to a vegan diet for at least 12 weeks may result in clinically meaningful weight loss [and] can be used in the management of overweight and type 2 diabetes,” said Anne-Ditte Termannsen, PhD, who reported the findings during a press conference at the European Congress on Obesity 2022, where the work was also presented as a poster.
A vegan diet most likely led to weight loss because it is “associated with a reduced calorie intake due to a lower content of fat and higher content of dietary fiber,” added Dr. Termannsen of the Steno Diabetes Center Copenhagen.
Asked to comment, Janet Cade, PhD, who leads the Nutritional Epidemiology Group at the University of Leeds (England) said the results are likely attributable to fewer calories in the vegan diet, compared with the “control” diets. “Of course, a vegan diet can be healthier in a range of ways, such as higher fruit and vegetables, more fiber and antioxidants; however, the same would be true of a vegetarian diet,” she noted.
And she warned that longer-term data are needed on health outcomes associated with vegan diets, noting, “there have been links to poorer bone health and osteoporosis in people consuming a vegan diet.”
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England) told the UK Science Media Centre: “The authors conducted a systematic review of intervention studies and found that, compared with no dietary interventions, vegan diets showed the strongest association with body-weight reduction.”
However, “When comparing vegan diets with other dietary interventions – such as the Mediterranean diet – the association was much weaker,” he noted.
Vegan, habitual, or a range of weight-loss diets
Dr. Termannsen and colleagues set out to look at the effect of a plant-based diet on cardiometabolic risk factors in people with overweight or type 2 diabetes. They searched the literature for randomized controlled trials with adult participants with overweight (body mass index ≥ 25 kg/m2), prediabetes, or type 2 diabetes.
Participants followed a vegan diet that lasted at least 12 weeks; habitual diets without any changes or energy restriction; a Mediterranean diet; a host of different “diabetes” diets; a low-fat diet; or portion-controlled diets.
“The vegan diets were nearly all low-fat vegan diets but vary substantially regarding the protein, fat, carbohydrate content. All but one study was ad libitum fat, and there were no energy restrictions,” Dr. Termannsen said.
Control diets were more varied. “Some continued their habitual diet, and about half were energy restricted and the others were not,” she acknowledged.
Outcomes comprised body weight, BMI, HbA1c, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides, which were assessed across studies.
A total of 11 trials were included in the meta-analysis, and studies were a mean duration of 19 weeks. A total of 796 participants were included.
Compared with control diets, those on vegan diets lost on average –4.1 kg (–9 lb) (P < .001), with a range of –5.9 kg to –2.4 kg.
BMI dropped by –1.38 kg/m2 (P < .001). Total cholesterol dropped by –0.30 mmol/L (–11.6 mg/dL; P = .007) and LDL cholesterol by –0.24 mmol/L (–9.28 mg/dL; P = .005).
Further analyses found even greater reductions in body weight and BMI when vegan diets were compared with continuing a normal diet without dietary changes, on average, at –7.4 kg (–16.3 lb) (P < .001) and –2.78 kg/m2 (P < .001) respectively.
When compared with other intervention diets, however, body weight dropped by –2.7 kg (–6 lb; P < .001) and BMI by –0.87 kg/m2 (P < .001).
Commenting on limitations of studies compared to the real world, Dr. Termannsen said: “Some studies reported high adherence to their diet, usually due to a high level of support, suggesting that providing continued face-to-face contact with participants may partly explain the adherence differences.”
“This also questions the long-term feasibility of the diet and the applicability of this as long-term care,” she added.
Following a vegan diet requires good planning to ensure adequate nutrition and avoid any deficiencies, she urged. “We need to remember that the menu plans in the studies were created by dietitians.”
A version of this article first appeared on Medscape.com.
on average, new research indicates.
No effect was seen on blood pressure, triglycerides, or high-density lipoprotein cholesterol. HbA1c was reduced by a mean of –0.18 percentage points (P = .002), and there was a small reduction in total cholesterol and low-density lipoprotein cholesterol, on average, across all the studies examined in this meta-analysis.
The work, which compared a number of trials looking at vegan diets versus “normal” eating or other kinds of weight loss diets, “indicates with reasonable certainty that adhering to a vegan diet for at least 12 weeks may result in clinically meaningful weight loss [and] can be used in the management of overweight and type 2 diabetes,” said Anne-Ditte Termannsen, PhD, who reported the findings during a press conference at the European Congress on Obesity 2022, where the work was also presented as a poster.
A vegan diet most likely led to weight loss because it is “associated with a reduced calorie intake due to a lower content of fat and higher content of dietary fiber,” added Dr. Termannsen of the Steno Diabetes Center Copenhagen.
Asked to comment, Janet Cade, PhD, who leads the Nutritional Epidemiology Group at the University of Leeds (England) said the results are likely attributable to fewer calories in the vegan diet, compared with the “control” diets. “Of course, a vegan diet can be healthier in a range of ways, such as higher fruit and vegetables, more fiber and antioxidants; however, the same would be true of a vegetarian diet,” she noted.
And she warned that longer-term data are needed on health outcomes associated with vegan diets, noting, “there have been links to poorer bone health and osteoporosis in people consuming a vegan diet.”
Gunter Kuhnle, PhD, professor of nutrition and food science, University of Reading (England) told the UK Science Media Centre: “The authors conducted a systematic review of intervention studies and found that, compared with no dietary interventions, vegan diets showed the strongest association with body-weight reduction.”
However, “When comparing vegan diets with other dietary interventions – such as the Mediterranean diet – the association was much weaker,” he noted.
Vegan, habitual, or a range of weight-loss diets
Dr. Termannsen and colleagues set out to look at the effect of a plant-based diet on cardiometabolic risk factors in people with overweight or type 2 diabetes. They searched the literature for randomized controlled trials with adult participants with overweight (body mass index ≥ 25 kg/m2), prediabetes, or type 2 diabetes.
Participants followed a vegan diet that lasted at least 12 weeks; habitual diets without any changes or energy restriction; a Mediterranean diet; a host of different “diabetes” diets; a low-fat diet; or portion-controlled diets.
“The vegan diets were nearly all low-fat vegan diets but vary substantially regarding the protein, fat, carbohydrate content. All but one study was ad libitum fat, and there were no energy restrictions,” Dr. Termannsen said.
Control diets were more varied. “Some continued their habitual diet, and about half were energy restricted and the others were not,” she acknowledged.
Outcomes comprised body weight, BMI, HbA1c, systolic and diastolic blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides, which were assessed across studies.
A total of 11 trials were included in the meta-analysis, and studies were a mean duration of 19 weeks. A total of 796 participants were included.
Compared with control diets, those on vegan diets lost on average –4.1 kg (–9 lb) (P < .001), with a range of –5.9 kg to –2.4 kg.
BMI dropped by –1.38 kg/m2 (P < .001). Total cholesterol dropped by –0.30 mmol/L (–11.6 mg/dL; P = .007) and LDL cholesterol by –0.24 mmol/L (–9.28 mg/dL; P = .005).
Further analyses found even greater reductions in body weight and BMI when vegan diets were compared with continuing a normal diet without dietary changes, on average, at –7.4 kg (–16.3 lb) (P < .001) and –2.78 kg/m2 (P < .001) respectively.
When compared with other intervention diets, however, body weight dropped by –2.7 kg (–6 lb; P < .001) and BMI by –0.87 kg/m2 (P < .001).
Commenting on limitations of studies compared to the real world, Dr. Termannsen said: “Some studies reported high adherence to their diet, usually due to a high level of support, suggesting that providing continued face-to-face contact with participants may partly explain the adherence differences.”
“This also questions the long-term feasibility of the diet and the applicability of this as long-term care,” she added.
Following a vegan diet requires good planning to ensure adequate nutrition and avoid any deficiencies, she urged. “We need to remember that the menu plans in the studies were created by dietitians.”
A version of this article first appeared on Medscape.com.
FROM ECO 2022
FDA limits use of J&J COVID vaccine over blood clot risk
In a statement issued May 5, the FDA said the J&J vaccine should only be given to people 18 and older who don’t have access to other vaccines or for whom other vaccines are not clinically appropriate. People 18 and older can also get the J&J vaccine if they choose to because they wouldn’t otherwise receive any vaccine, the FDA said.
The FDA statement was similar to the recommendation made in December by a Centers for Disease Control and Prevention committee of experts.
The FDA said the decision was made after more information was shared about the occurrence of a rare blood clotting condition, thrombosis with thrombocytopenia syndrome (TTS), 1 or 2 weeks after people received the J&J vaccine. The finding “warrants limiting the authorized use of the vaccine,” the FDA said.
“We recognize that the Janssen COVID-19 vaccine still has a role in the current pandemic response in the United States and across the global community,” Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said in the statement.
“Our action reflects our updated analysis of the risk of TTS following administration of this vaccine and limits the use of the vaccine to certain individuals.”
The CDC says 16.9 million people are fully vaccinated with the J&J vaccine, compared with 76.5 million with Moderna and 126.3 million with Pfizer.
Through March 18, the CDC and FDA have detected 60 confirmed cases of TTS, including 9 fatal cases, ABC News reported.
The J&J vaccine was granted emergency authorization in February 2021. Health authorities hoped it would help spread vaccines across the nation because it only required one initial dose and didn’t need to be stored at extremely cold temperatures, unlike the two-dose Pfizer and Moderna vaccines.
But 2 months after authorization, the government paused its use for 10 days because of reports of TTS. In December 2021, the CDC’s Advisory Committee on Immunization Practices said the Pfizer and Moderna vaccines were preferred over J&J because J&J carried the rare risk of blood clots and bleeding in the brain.
The FDA said the cause of the blood clotting is not known. But the “known and potential benefits of the vaccine” outweigh the risks for those people now allowed to receive it, the FDA said.
A version of this article first appeared on WebMD.com.
In a statement issued May 5, the FDA said the J&J vaccine should only be given to people 18 and older who don’t have access to other vaccines or for whom other vaccines are not clinically appropriate. People 18 and older can also get the J&J vaccine if they choose to because they wouldn’t otherwise receive any vaccine, the FDA said.
The FDA statement was similar to the recommendation made in December by a Centers for Disease Control and Prevention committee of experts.
The FDA said the decision was made after more information was shared about the occurrence of a rare blood clotting condition, thrombosis with thrombocytopenia syndrome (TTS), 1 or 2 weeks after people received the J&J vaccine. The finding “warrants limiting the authorized use of the vaccine,” the FDA said.
“We recognize that the Janssen COVID-19 vaccine still has a role in the current pandemic response in the United States and across the global community,” Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said in the statement.
“Our action reflects our updated analysis of the risk of TTS following administration of this vaccine and limits the use of the vaccine to certain individuals.”
The CDC says 16.9 million people are fully vaccinated with the J&J vaccine, compared with 76.5 million with Moderna and 126.3 million with Pfizer.
Through March 18, the CDC and FDA have detected 60 confirmed cases of TTS, including 9 fatal cases, ABC News reported.
The J&J vaccine was granted emergency authorization in February 2021. Health authorities hoped it would help spread vaccines across the nation because it only required one initial dose and didn’t need to be stored at extremely cold temperatures, unlike the two-dose Pfizer and Moderna vaccines.
But 2 months after authorization, the government paused its use for 10 days because of reports of TTS. In December 2021, the CDC’s Advisory Committee on Immunization Practices said the Pfizer and Moderna vaccines were preferred over J&J because J&J carried the rare risk of blood clots and bleeding in the brain.
The FDA said the cause of the blood clotting is not known. But the “known and potential benefits of the vaccine” outweigh the risks for those people now allowed to receive it, the FDA said.
A version of this article first appeared on WebMD.com.
In a statement issued May 5, the FDA said the J&J vaccine should only be given to people 18 and older who don’t have access to other vaccines or for whom other vaccines are not clinically appropriate. People 18 and older can also get the J&J vaccine if they choose to because they wouldn’t otherwise receive any vaccine, the FDA said.
The FDA statement was similar to the recommendation made in December by a Centers for Disease Control and Prevention committee of experts.
The FDA said the decision was made after more information was shared about the occurrence of a rare blood clotting condition, thrombosis with thrombocytopenia syndrome (TTS), 1 or 2 weeks after people received the J&J vaccine. The finding “warrants limiting the authorized use of the vaccine,” the FDA said.
“We recognize that the Janssen COVID-19 vaccine still has a role in the current pandemic response in the United States and across the global community,” Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said in the statement.
“Our action reflects our updated analysis of the risk of TTS following administration of this vaccine and limits the use of the vaccine to certain individuals.”
The CDC says 16.9 million people are fully vaccinated with the J&J vaccine, compared with 76.5 million with Moderna and 126.3 million with Pfizer.
Through March 18, the CDC and FDA have detected 60 confirmed cases of TTS, including 9 fatal cases, ABC News reported.
The J&J vaccine was granted emergency authorization in February 2021. Health authorities hoped it would help spread vaccines across the nation because it only required one initial dose and didn’t need to be stored at extremely cold temperatures, unlike the two-dose Pfizer and Moderna vaccines.
But 2 months after authorization, the government paused its use for 10 days because of reports of TTS. In December 2021, the CDC’s Advisory Committee on Immunization Practices said the Pfizer and Moderna vaccines were preferred over J&J because J&J carried the rare risk of blood clots and bleeding in the brain.
The FDA said the cause of the blood clotting is not known. But the “known and potential benefits of the vaccine” outweigh the risks for those people now allowed to receive it, the FDA said.
A version of this article first appeared on WebMD.com.
Calorie counting and exercise ‘of limited value’ for obesity weight loss
Counting calories, joining a gym, and taking part in exercise programs are popular methods used by people in the United Kingdom who want to shed some pounds, but they seem to be fairly ineffective strategies, according to an investigation.
A survey of adults with obesity from six countries in western Europe found that most who set out to reduce a meaningful amount of weight failed in their attempt.
The preliminary results, presented in two posters at the European Congress on Obesity, underlined the need for better support and solutions for weight management, the authors suggested.
Marc Evans, MB, BCh, a consultant physician in diabetes and endocrinology, from University Hospital, Cardiff, Wales, who led the analysis, said that, “while obesity’s impact on health is well known, our finding that a sizable proportion of adults with obesity appear at elevated risk of hospitalization or surgery due to multiple underlying illnesses, undoubtedly adds a sense of urgency to tackling Europe’s growing obesity epidemic.”
The study, which also involved analytics consultancy firm Lane Clark & Peacock, conducted a cross-sectional survey of 1,850 adults. Of those 500 were from the UK, and the remainder from France, Germany, Italy, Spain, and Sweden.
All participants had a body mass index of 30 kg/m2, or higher. More specifically, 56.3%; were classified as obesity class I, 26.8% obesity class II, and 16.9% obesity class III.
Obesity-related conditions
In total, 25.7% of participants reported no obesity-related health conditions, 28.4% had one condition, 19.6% had two, and 26.3% had three or more. The most common comorbidities were hypertension, dyslipidemia, and type 2 diabetes.
Overall, 78.6% of respondents reported having tried to lose weight in the previous year. Asked in a questionnaire about how they had tried to achieve this, the responses indicated that the most common strategies were:
- Calorie-controlled/restricted diet (71.9%)
- Exercise program course (21.9%)
- Pharmaceutical treatment/medication (12.3%)
- Joined a gym (12%)
- Digital health app (9.7%)
Among other participants, 8.1% said they had used alternative treatments, 7.6% a weight loss service, and 2.1% cognitive-behavioral therapy.
Analysis of the survey results showed that 78% of the individuals who attempted to lose weight did not achieve a clinically meaningful loss of 5% or more of their body weight, while some actually weighed more afterward.
Exercise and restricted diet
Notably, while exercise and calorie-controlled or restricted diets were among the most popular weight-loss methods in U.K. participants, they were amongst the least successful strategies. For instance, while 26.5% of adults who controlled their diet said they had lost weight, 17.1% reported their weight had increased. For those who took part in an exercise program, 33.3% said they lost weight, but 15.5% said they gained weight.
Signing up for gym membership also scored poorly, with 27% shedding weight, compared with 32.4% who put weight on.
“Our survey results indicate that, while the majority of adults with obesity are actively trying to reduce their weight, using a variety of strategies, most are unsuccessful,” said Dr. Evans.
Further studies were needed to assess whether people who lose weight succeed in maintaining their weight loss, the authors said.
The conference posters have yet to be published in a journal but were peer reviewed by the ECO selection committee.
The studies were sponsored by Novo Nordisk, a researcher into and manufacturer of diabetes and obesity medications, and employer of several of the coauthors.
A version of this article first appeared on Medscape UK/Univadis.
Counting calories, joining a gym, and taking part in exercise programs are popular methods used by people in the United Kingdom who want to shed some pounds, but they seem to be fairly ineffective strategies, according to an investigation.
A survey of adults with obesity from six countries in western Europe found that most who set out to reduce a meaningful amount of weight failed in their attempt.
The preliminary results, presented in two posters at the European Congress on Obesity, underlined the need for better support and solutions for weight management, the authors suggested.
Marc Evans, MB, BCh, a consultant physician in diabetes and endocrinology, from University Hospital, Cardiff, Wales, who led the analysis, said that, “while obesity’s impact on health is well known, our finding that a sizable proportion of adults with obesity appear at elevated risk of hospitalization or surgery due to multiple underlying illnesses, undoubtedly adds a sense of urgency to tackling Europe’s growing obesity epidemic.”
The study, which also involved analytics consultancy firm Lane Clark & Peacock, conducted a cross-sectional survey of 1,850 adults. Of those 500 were from the UK, and the remainder from France, Germany, Italy, Spain, and Sweden.
All participants had a body mass index of 30 kg/m2, or higher. More specifically, 56.3%; were classified as obesity class I, 26.8% obesity class II, and 16.9% obesity class III.
Obesity-related conditions
In total, 25.7% of participants reported no obesity-related health conditions, 28.4% had one condition, 19.6% had two, and 26.3% had three or more. The most common comorbidities were hypertension, dyslipidemia, and type 2 diabetes.
Overall, 78.6% of respondents reported having tried to lose weight in the previous year. Asked in a questionnaire about how they had tried to achieve this, the responses indicated that the most common strategies were:
- Calorie-controlled/restricted diet (71.9%)
- Exercise program course (21.9%)
- Pharmaceutical treatment/medication (12.3%)
- Joined a gym (12%)
- Digital health app (9.7%)
Among other participants, 8.1% said they had used alternative treatments, 7.6% a weight loss service, and 2.1% cognitive-behavioral therapy.
Analysis of the survey results showed that 78% of the individuals who attempted to lose weight did not achieve a clinically meaningful loss of 5% or more of their body weight, while some actually weighed more afterward.
Exercise and restricted diet
Notably, while exercise and calorie-controlled or restricted diets were among the most popular weight-loss methods in U.K. participants, they were amongst the least successful strategies. For instance, while 26.5% of adults who controlled their diet said they had lost weight, 17.1% reported their weight had increased. For those who took part in an exercise program, 33.3% said they lost weight, but 15.5% said they gained weight.
Signing up for gym membership also scored poorly, with 27% shedding weight, compared with 32.4% who put weight on.
“Our survey results indicate that, while the majority of adults with obesity are actively trying to reduce their weight, using a variety of strategies, most are unsuccessful,” said Dr. Evans.
Further studies were needed to assess whether people who lose weight succeed in maintaining their weight loss, the authors said.
The conference posters have yet to be published in a journal but were peer reviewed by the ECO selection committee.
The studies were sponsored by Novo Nordisk, a researcher into and manufacturer of diabetes and obesity medications, and employer of several of the coauthors.
A version of this article first appeared on Medscape UK/Univadis.
Counting calories, joining a gym, and taking part in exercise programs are popular methods used by people in the United Kingdom who want to shed some pounds, but they seem to be fairly ineffective strategies, according to an investigation.
A survey of adults with obesity from six countries in western Europe found that most who set out to reduce a meaningful amount of weight failed in their attempt.
The preliminary results, presented in two posters at the European Congress on Obesity, underlined the need for better support and solutions for weight management, the authors suggested.
Marc Evans, MB, BCh, a consultant physician in diabetes and endocrinology, from University Hospital, Cardiff, Wales, who led the analysis, said that, “while obesity’s impact on health is well known, our finding that a sizable proportion of adults with obesity appear at elevated risk of hospitalization or surgery due to multiple underlying illnesses, undoubtedly adds a sense of urgency to tackling Europe’s growing obesity epidemic.”
The study, which also involved analytics consultancy firm Lane Clark & Peacock, conducted a cross-sectional survey of 1,850 adults. Of those 500 were from the UK, and the remainder from France, Germany, Italy, Spain, and Sweden.
All participants had a body mass index of 30 kg/m2, or higher. More specifically, 56.3%; were classified as obesity class I, 26.8% obesity class II, and 16.9% obesity class III.
Obesity-related conditions
In total, 25.7% of participants reported no obesity-related health conditions, 28.4% had one condition, 19.6% had two, and 26.3% had three or more. The most common comorbidities were hypertension, dyslipidemia, and type 2 diabetes.
Overall, 78.6% of respondents reported having tried to lose weight in the previous year. Asked in a questionnaire about how they had tried to achieve this, the responses indicated that the most common strategies were:
- Calorie-controlled/restricted diet (71.9%)
- Exercise program course (21.9%)
- Pharmaceutical treatment/medication (12.3%)
- Joined a gym (12%)
- Digital health app (9.7%)
Among other participants, 8.1% said they had used alternative treatments, 7.6% a weight loss service, and 2.1% cognitive-behavioral therapy.
Analysis of the survey results showed that 78% of the individuals who attempted to lose weight did not achieve a clinically meaningful loss of 5% or more of their body weight, while some actually weighed more afterward.
Exercise and restricted diet
Notably, while exercise and calorie-controlled or restricted diets were among the most popular weight-loss methods in U.K. participants, they were amongst the least successful strategies. For instance, while 26.5% of adults who controlled their diet said they had lost weight, 17.1% reported their weight had increased. For those who took part in an exercise program, 33.3% said they lost weight, but 15.5% said they gained weight.
Signing up for gym membership also scored poorly, with 27% shedding weight, compared with 32.4% who put weight on.
“Our survey results indicate that, while the majority of adults with obesity are actively trying to reduce their weight, using a variety of strategies, most are unsuccessful,” said Dr. Evans.
Further studies were needed to assess whether people who lose weight succeed in maintaining their weight loss, the authors said.
The conference posters have yet to be published in a journal but were peer reviewed by the ECO selection committee.
The studies were sponsored by Novo Nordisk, a researcher into and manufacturer of diabetes and obesity medications, and employer of several of the coauthors.
A version of this article first appeared on Medscape UK/Univadis.
FROM ECO 2022
Second COVID booster: Who should receive it and when?
The more boosters the better? Data from Israel show that immune protection in elderly people is strengthened even further after a fourth dose. Karl Lauterbach, MD, German minister of health, recently pleaded for a second booster for those aged 18 years and older, and he pushed for a European Union–wide recommendation. He has not been able to implement this yet.
Just as before, Germany’s Standing Committee on Vaccination (STIKO) is only recommending the second booster for people aged 70 years and older, the European Medicines Agency (EMA) is recommending the fourth vaccination for everyone aged 80 years and older, and the United States has set the general age limit at 50 years.
Specialists remain skeptical about expanding the availability of the second booster. “From an immunologic perspective, people under the age of 70 with a healthy immune system do not need this fourth vaccination,” said Christiane Falk, PhD, head of the Institute for Transplantation Immunology of the Hannover Medical School (Germany) and member of the German Federal Government COVID Expert Panel, at a Science Media Center press briefing.
After the second vaccination, young healthy people are sufficiently protected against a severe course of the disease. Dr. Falk sees the STIKO recommendation as feasible, since it can be worked with. People in nursing facilities or those with additional underlying conditions would be considered for a fourth vaccination, explained Dr. Falk.
Complete protection unrealistic
Achieving complete protection against infection through multiple boosters is not realistic, said Christoph Neumann-Haefelin, MD, head of the Working Group for Translational Virus Immunology at the Clinic for Internal Medicine II, University Hospital Freiburg, Germany. Therefore, this should not be pursued when discussing boosters. “The aim of the booster vaccination should be to protect different groups of people against severe courses of the disease,” said Dr. Neumann-Haefelin.
Neutralizing antibodies that are only present in high concentrations for a few weeks after infection or vaccination are sometimes able to prevent the infection on their own. The immunologic memory of B cells and T cells, which ensures long-lasting protection against severe courses of the disease, is at a high level after two doses, and a third dose increases the protection more.
While people with a weak immune system need significantly more vaccinations in a shorter period to receive the same protection, too many booster vaccinations against SARS-CoV-2 are not sensible for young healthy people.
Immune saturation effect
A recent study in macaques showed that an adjusted Omicron booster did not lead to higher antibody titers, compared with a usual booster. In January 2022, the EMA warned against frequent consecutive boosters that may no longer produce the desired immune response.
If someone receives a booster too early, a saturation effect can occur, warned Andreas Radbruch, PhD, scientific director of the German Rheumatism Research Center Berlin. “We know this from lots of experimental studies but also from lots of other vaccinations. For example, you cannot be vaccinated against tetanus twice at 3- or 4-week intervals. Nothing at all will happen the second time,” explained Dr. Radbruch.
If the same antigen is applied again and again at the same dose, the immune system is made so active that the antigen is directly intercepted and cannot have any new effect on the immune system. This mechanism has been known for a long time, said Dr. Radbruch.
‘Original antigenic sin’
Premature boosting could even be a handicap in the competition between immune response and virus, said Dr. Radbruch. This is due to the principle of “original antigenic sin.” If the immune system has already come into contact with a virus, contact with a new virus variant will cause it to form antibodies predominantly against those epitopes that were already present in the original virus. As a result of this, too many boosters can weaken protection against different variants.
“We have not actually observed this with SARS-CoV-2, however,” said Dr. Radbruch. “Immunity is always extremely broad. With a double or triple vaccination, all previously existing variants are covered by an affinity-matured immune system.”
Dr. Neumann-Haefelin confirmed this and added that all virus mutations, including Omicron, have different epitopes that affect the antibody response, but the T-cell response does not differ.
Dr. Radbruch said that the vaccine protection probably lasts for decades. Following an infection or vaccination, the antibody concentration in the bone marrow is similar to that achieved after a measles or tetanus vaccination. “The vaccination is already extremely efficient. You have protection at the same magnitude as for other infectious diseases or vaccinations, which is expected to last decades,” said Dr. Radbruch.
He clarified that the decrease in antibodies after vaccination and infection is normal and does not indicate a drop in protection. “Quantity and quality must not be confused here. There is simply less mass, but the grade of remaining antibody increases.”
In the competition around the virus antigens (referred to as affinity maturation), antibodies develop that bind 10 to 100 times better and are particularly protective against the virus. The immune system is thereby sustainably effective.
For whom and when?
Since the immune response is age dependent, it makes more sense to administer an additional booster to elderly people than to young people. Also included in this group, however, are people whose immune system still does not provide the same level of protection after the second or even third vaccination as that of younger, healthy people.
Dr. Radbruch noted that 4% of people older than 70 years exhibited autoantibodies against interferons. The effects are huge. “That is 20% of patients in an intensive care unit – and they all have a very poor prognosis,” said Dr. Radbruch. These people are extremely threatened by the virus. Multiple vaccinations are sensible for them.
Even people with a weak immune response benefit from multiple vaccinations, confirmed Dr. Neumann-Haefelin. “We are not seeing the antibody responses here that we see in young people with healthy immune systems until the third or fourth vaccination sometimes.”
Although for young healthy people, it is particularly important to ensure a sufficient period between vaccinations so that the affinity maturation is not impaired, those with a weak immune response can be vaccinated again as soon as after 3 months.
The “optimum minimum period of time” for people with healthy immune systems is 6 months, according to Dr. Neumann-Haefelin. “This is true for everyone in whom a proper response is expected.” The vaccine protection probably lasts significantly longer, and therefore, frequent boosting may not be necessary in the future, he said. The time separation also applies for medical personnel, for whom the Robert Koch Institute also recommends a second booster.
A version of this article first appeared on Medscape.com.
The more boosters the better? Data from Israel show that immune protection in elderly people is strengthened even further after a fourth dose. Karl Lauterbach, MD, German minister of health, recently pleaded for a second booster for those aged 18 years and older, and he pushed for a European Union–wide recommendation. He has not been able to implement this yet.
Just as before, Germany’s Standing Committee on Vaccination (STIKO) is only recommending the second booster for people aged 70 years and older, the European Medicines Agency (EMA) is recommending the fourth vaccination for everyone aged 80 years and older, and the United States has set the general age limit at 50 years.
Specialists remain skeptical about expanding the availability of the second booster. “From an immunologic perspective, people under the age of 70 with a healthy immune system do not need this fourth vaccination,” said Christiane Falk, PhD, head of the Institute for Transplantation Immunology of the Hannover Medical School (Germany) and member of the German Federal Government COVID Expert Panel, at a Science Media Center press briefing.
After the second vaccination, young healthy people are sufficiently protected against a severe course of the disease. Dr. Falk sees the STIKO recommendation as feasible, since it can be worked with. People in nursing facilities or those with additional underlying conditions would be considered for a fourth vaccination, explained Dr. Falk.
Complete protection unrealistic
Achieving complete protection against infection through multiple boosters is not realistic, said Christoph Neumann-Haefelin, MD, head of the Working Group for Translational Virus Immunology at the Clinic for Internal Medicine II, University Hospital Freiburg, Germany. Therefore, this should not be pursued when discussing boosters. “The aim of the booster vaccination should be to protect different groups of people against severe courses of the disease,” said Dr. Neumann-Haefelin.
Neutralizing antibodies that are only present in high concentrations for a few weeks after infection or vaccination are sometimes able to prevent the infection on their own. The immunologic memory of B cells and T cells, which ensures long-lasting protection against severe courses of the disease, is at a high level after two doses, and a third dose increases the protection more.
While people with a weak immune system need significantly more vaccinations in a shorter period to receive the same protection, too many booster vaccinations against SARS-CoV-2 are not sensible for young healthy people.
Immune saturation effect
A recent study in macaques showed that an adjusted Omicron booster did not lead to higher antibody titers, compared with a usual booster. In January 2022, the EMA warned against frequent consecutive boosters that may no longer produce the desired immune response.
If someone receives a booster too early, a saturation effect can occur, warned Andreas Radbruch, PhD, scientific director of the German Rheumatism Research Center Berlin. “We know this from lots of experimental studies but also from lots of other vaccinations. For example, you cannot be vaccinated against tetanus twice at 3- or 4-week intervals. Nothing at all will happen the second time,” explained Dr. Radbruch.
If the same antigen is applied again and again at the same dose, the immune system is made so active that the antigen is directly intercepted and cannot have any new effect on the immune system. This mechanism has been known for a long time, said Dr. Radbruch.
‘Original antigenic sin’
Premature boosting could even be a handicap in the competition between immune response and virus, said Dr. Radbruch. This is due to the principle of “original antigenic sin.” If the immune system has already come into contact with a virus, contact with a new virus variant will cause it to form antibodies predominantly against those epitopes that were already present in the original virus. As a result of this, too many boosters can weaken protection against different variants.
“We have not actually observed this with SARS-CoV-2, however,” said Dr. Radbruch. “Immunity is always extremely broad. With a double or triple vaccination, all previously existing variants are covered by an affinity-matured immune system.”
Dr. Neumann-Haefelin confirmed this and added that all virus mutations, including Omicron, have different epitopes that affect the antibody response, but the T-cell response does not differ.
Dr. Radbruch said that the vaccine protection probably lasts for decades. Following an infection or vaccination, the antibody concentration in the bone marrow is similar to that achieved after a measles or tetanus vaccination. “The vaccination is already extremely efficient. You have protection at the same magnitude as for other infectious diseases or vaccinations, which is expected to last decades,” said Dr. Radbruch.
He clarified that the decrease in antibodies after vaccination and infection is normal and does not indicate a drop in protection. “Quantity and quality must not be confused here. There is simply less mass, but the grade of remaining antibody increases.”
In the competition around the virus antigens (referred to as affinity maturation), antibodies develop that bind 10 to 100 times better and are particularly protective against the virus. The immune system is thereby sustainably effective.
For whom and when?
Since the immune response is age dependent, it makes more sense to administer an additional booster to elderly people than to young people. Also included in this group, however, are people whose immune system still does not provide the same level of protection after the second or even third vaccination as that of younger, healthy people.
Dr. Radbruch noted that 4% of people older than 70 years exhibited autoantibodies against interferons. The effects are huge. “That is 20% of patients in an intensive care unit – and they all have a very poor prognosis,” said Dr. Radbruch. These people are extremely threatened by the virus. Multiple vaccinations are sensible for them.
Even people with a weak immune response benefit from multiple vaccinations, confirmed Dr. Neumann-Haefelin. “We are not seeing the antibody responses here that we see in young people with healthy immune systems until the third or fourth vaccination sometimes.”
Although for young healthy people, it is particularly important to ensure a sufficient period between vaccinations so that the affinity maturation is not impaired, those with a weak immune response can be vaccinated again as soon as after 3 months.
The “optimum minimum period of time” for people with healthy immune systems is 6 months, according to Dr. Neumann-Haefelin. “This is true for everyone in whom a proper response is expected.” The vaccine protection probably lasts significantly longer, and therefore, frequent boosting may not be necessary in the future, he said. The time separation also applies for medical personnel, for whom the Robert Koch Institute also recommends a second booster.
A version of this article first appeared on Medscape.com.
The more boosters the better? Data from Israel show that immune protection in elderly people is strengthened even further after a fourth dose. Karl Lauterbach, MD, German minister of health, recently pleaded for a second booster for those aged 18 years and older, and he pushed for a European Union–wide recommendation. He has not been able to implement this yet.
Just as before, Germany’s Standing Committee on Vaccination (STIKO) is only recommending the second booster for people aged 70 years and older, the European Medicines Agency (EMA) is recommending the fourth vaccination for everyone aged 80 years and older, and the United States has set the general age limit at 50 years.
Specialists remain skeptical about expanding the availability of the second booster. “From an immunologic perspective, people under the age of 70 with a healthy immune system do not need this fourth vaccination,” said Christiane Falk, PhD, head of the Institute for Transplantation Immunology of the Hannover Medical School (Germany) and member of the German Federal Government COVID Expert Panel, at a Science Media Center press briefing.
After the second vaccination, young healthy people are sufficiently protected against a severe course of the disease. Dr. Falk sees the STIKO recommendation as feasible, since it can be worked with. People in nursing facilities or those with additional underlying conditions would be considered for a fourth vaccination, explained Dr. Falk.
Complete protection unrealistic
Achieving complete protection against infection through multiple boosters is not realistic, said Christoph Neumann-Haefelin, MD, head of the Working Group for Translational Virus Immunology at the Clinic for Internal Medicine II, University Hospital Freiburg, Germany. Therefore, this should not be pursued when discussing boosters. “The aim of the booster vaccination should be to protect different groups of people against severe courses of the disease,” said Dr. Neumann-Haefelin.
Neutralizing antibodies that are only present in high concentrations for a few weeks after infection or vaccination are sometimes able to prevent the infection on their own. The immunologic memory of B cells and T cells, which ensures long-lasting protection against severe courses of the disease, is at a high level after two doses, and a third dose increases the protection more.
While people with a weak immune system need significantly more vaccinations in a shorter period to receive the same protection, too many booster vaccinations against SARS-CoV-2 are not sensible for young healthy people.
Immune saturation effect
A recent study in macaques showed that an adjusted Omicron booster did not lead to higher antibody titers, compared with a usual booster. In January 2022, the EMA warned against frequent consecutive boosters that may no longer produce the desired immune response.
If someone receives a booster too early, a saturation effect can occur, warned Andreas Radbruch, PhD, scientific director of the German Rheumatism Research Center Berlin. “We know this from lots of experimental studies but also from lots of other vaccinations. For example, you cannot be vaccinated against tetanus twice at 3- or 4-week intervals. Nothing at all will happen the second time,” explained Dr. Radbruch.
If the same antigen is applied again and again at the same dose, the immune system is made so active that the antigen is directly intercepted and cannot have any new effect on the immune system. This mechanism has been known for a long time, said Dr. Radbruch.
‘Original antigenic sin’
Premature boosting could even be a handicap in the competition between immune response and virus, said Dr. Radbruch. This is due to the principle of “original antigenic sin.” If the immune system has already come into contact with a virus, contact with a new virus variant will cause it to form antibodies predominantly against those epitopes that were already present in the original virus. As a result of this, too many boosters can weaken protection against different variants.
“We have not actually observed this with SARS-CoV-2, however,” said Dr. Radbruch. “Immunity is always extremely broad. With a double or triple vaccination, all previously existing variants are covered by an affinity-matured immune system.”
Dr. Neumann-Haefelin confirmed this and added that all virus mutations, including Omicron, have different epitopes that affect the antibody response, but the T-cell response does not differ.
Dr. Radbruch said that the vaccine protection probably lasts for decades. Following an infection or vaccination, the antibody concentration in the bone marrow is similar to that achieved after a measles or tetanus vaccination. “The vaccination is already extremely efficient. You have protection at the same magnitude as for other infectious diseases or vaccinations, which is expected to last decades,” said Dr. Radbruch.
He clarified that the decrease in antibodies after vaccination and infection is normal and does not indicate a drop in protection. “Quantity and quality must not be confused here. There is simply less mass, but the grade of remaining antibody increases.”
In the competition around the virus antigens (referred to as affinity maturation), antibodies develop that bind 10 to 100 times better and are particularly protective against the virus. The immune system is thereby sustainably effective.
For whom and when?
Since the immune response is age dependent, it makes more sense to administer an additional booster to elderly people than to young people. Also included in this group, however, are people whose immune system still does not provide the same level of protection after the second or even third vaccination as that of younger, healthy people.
Dr. Radbruch noted that 4% of people older than 70 years exhibited autoantibodies against interferons. The effects are huge. “That is 20% of patients in an intensive care unit – and they all have a very poor prognosis,” said Dr. Radbruch. These people are extremely threatened by the virus. Multiple vaccinations are sensible for them.
Even people with a weak immune response benefit from multiple vaccinations, confirmed Dr. Neumann-Haefelin. “We are not seeing the antibody responses here that we see in young people with healthy immune systems until the third or fourth vaccination sometimes.”
Although for young healthy people, it is particularly important to ensure a sufficient period between vaccinations so that the affinity maturation is not impaired, those with a weak immune response can be vaccinated again as soon as after 3 months.
The “optimum minimum period of time” for people with healthy immune systems is 6 months, according to Dr. Neumann-Haefelin. “This is true for everyone in whom a proper response is expected.” The vaccine protection probably lasts significantly longer, and therefore, frequent boosting may not be necessary in the future, he said. The time separation also applies for medical personnel, for whom the Robert Koch Institute also recommends a second booster.
A version of this article first appeared on Medscape.com.
Topline results for dapagliflozin in HFpEF: DELIVER
Topline results from the phase 3 DELIVER trial show dapagliflozin (Farxiga) significantly reduced the primary endpoint of cardiovascular death or worsening heart failure in patients with mildly reduced or preserved ejection fraction, AstraZeneca announced today.
The sodium-glucose cotransporter 2 (SGLT2) inhibitor is not approved in this setting but is already approved for treatment of type 2 diabetes, chronic kidney disease, and heart failure with reduced ejection fraction.
“The results of DELIVER extend the benefit of dapagliflozin to the full spectrum of patients with heart failure,” principal investigator of the trial, Scott Solomon, MD, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in the news release.
The safety and tolerability of dapagliflozin in the trial were consistent with its established safety profile, the company says.
The full trial results will be submitted for presentation at a forthcoming medical meeting, and regulatory submissions will be made in the coming months, it notes.
A version of this article first appeared on Medscape.com.
Topline results from the phase 3 DELIVER trial show dapagliflozin (Farxiga) significantly reduced the primary endpoint of cardiovascular death or worsening heart failure in patients with mildly reduced or preserved ejection fraction, AstraZeneca announced today.
The sodium-glucose cotransporter 2 (SGLT2) inhibitor is not approved in this setting but is already approved for treatment of type 2 diabetes, chronic kidney disease, and heart failure with reduced ejection fraction.
“The results of DELIVER extend the benefit of dapagliflozin to the full spectrum of patients with heart failure,” principal investigator of the trial, Scott Solomon, MD, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in the news release.
The safety and tolerability of dapagliflozin in the trial were consistent with its established safety profile, the company says.
The full trial results will be submitted for presentation at a forthcoming medical meeting, and regulatory submissions will be made in the coming months, it notes.
A version of this article first appeared on Medscape.com.
Topline results from the phase 3 DELIVER trial show dapagliflozin (Farxiga) significantly reduced the primary endpoint of cardiovascular death or worsening heart failure in patients with mildly reduced or preserved ejection fraction, AstraZeneca announced today.
The sodium-glucose cotransporter 2 (SGLT2) inhibitor is not approved in this setting but is already approved for treatment of type 2 diabetes, chronic kidney disease, and heart failure with reduced ejection fraction.
“The results of DELIVER extend the benefit of dapagliflozin to the full spectrum of patients with heart failure,” principal investigator of the trial, Scott Solomon, MD, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in the news release.
The safety and tolerability of dapagliflozin in the trial were consistent with its established safety profile, the company says.
The full trial results will be submitted for presentation at a forthcoming medical meeting, and regulatory submissions will be made in the coming months, it notes.
A version of this article first appeared on Medscape.com.
Bone, breath, heart, guts: Eight essential papers in primary care
1. Adding a New Medication Versus Maximizing Dose to Intensify Hypertension Treatment in Older Adults: A Retrospective Observational Study
Roughly one in three adults with hypertension have inadequate blood pressure control, and clinicians have two options for intensifying treatment: “The dose of the current drug regimen can be maximized, or a new drug can be added,” said deputy editor Christina C. Wee, MD, MPH, at the annual meeting of the American College of Physicians.
Data from randomized controlled trials suggest treatment with lower doses of combination therapy may be more effective, with fewer side effects – although the best strategy in older adults remains unclear.
To answer that question, researchers conducted a large-scale, population-based, retrospective cohort study, and observational data were used to emulate a target trial with two groups: new medication and maximizing dose.
The cohort comprised people aged 65 years or older with hypertension and was limited to those with a systolic blood pressure of 130 mm Hg or higher. Two intensification approaches were used: adding a new medication, defined as a total dose increase with a new medication; and maximizing dose, defined as a total dose increase without new medication.
A total of 178,562 patients were included in the study, and 45,575 (25.5%) had intensification by adding a new medication and 132,987 (74.5%) by maximizing dose.
“Both produced systolic blood pressure reduction with a slight advantage in the ‘add a new medication’ group,” Dr. Wee said. “That group reduced their systolic blood pressure by over 4.5 points as compared to 3.8 points in the maximized [dose] group.”
At 12 months the results were similar, but only 50% of patients in the new medication group were able to sustain that strategy, compared with two-thirds of patients who had their dose increased.
“This suggests that, in older adults, adding a new antihypertensive medication versus maximizing dosing of existing regimen is less common, only minimally more effective, and less sustainable,” Dr. Wee said. “Maximizing dose of antihypertensive medication is a reasonable approach [and] may be easier to sustain.”
2. Cost-Effectiveness of Screening Mammography Beyond Age 75 Years: A Cost-Effectiveness Analysis
The U.S. Preventive Services Task Force recommends biennial screening mammograms through the age of 74 years, and a meta-analysis of randomized controlled trials suggests mortality is reduced among women with at least a 10-year life expectancy, Dr. Wee said.
However, whether screening beyond age 75 years is cost effective, especially among women with comorbidities, is unclear.
To address that question, researchers estimated benefits, harms, and cost-effectiveness of extending mammography to age 80, 85, or 90 years according to comorbidity burden, using data from the Surveillance, Epidemiology, and End Results program and the Breast Cancer Surveillance Consortium.
The results showed that extending annual mammography beyond age 75 years was not cost effective, but biennial mammography was. “It was cost effective to age 80 regardless of baseline comorbidity score, but it averted only small, absolute numbers of breast cancer deaths – especially for women with comorbidities,” Dr. Wee said. “It was not cost effective beyond age 80.”
3. Prediction of End-Stage Kidney Disease Using Estimated Glomerular Filtration Rate With and Without Race: A Prospective Cohort Study
Estimated glomerular filtration rate (eGFR) is associated with end-stage kidney disease (ESKD) and is used to make dialysis and transplant decisions. “However, the accuracy of using eGFR alone has been questioned and, previously, some eGFR equations included a correction for race and this has been quite controversial,” Dr. Wee said. “And just last year, the Chronic Kidney Disease Epidemiology Collaboration released their new equations, removing the adjustment for race.”
The study authors posed two questions:
- How well does eGFR alone predict risk of ESKD, compared with Kidney Function Risk Equation (KFRE)?
- Does using different eGFR equations affect performance of either eGFR alone or KFRE in predicting the risk of ESKD?
During a maximum 16 years of follow-up, 856 participants (n = 3,873) developed ESKD. Across all eGFR equations, the KFRE score was superior for predicting 2-year incidence of end-stage kidney disease, compared with eGFR alone.
“KFRE score better predicted 2-year risk of ESKD than eGFR alone regardless of eGFR equations used,” Dr. Wee said. “Correcting eGFR equations for race did not improve performance and validates recent guidelines.”
4. Comparative Fracture Risk During Osteoporosis Drug Holidays After Long-Term Risedronate Versus Alendronate Therapy: A Propensity Score-Matched Cohort Study
The study looked at the comparative risks of drug holidays after long-term (≥ 3 years) risedronate versus alendronate therapy in a cohort of individuals aged 66 years or older. The primary outcome was hip fracture within 3 years after a 120-day ascertainment period.
The cohort included 25,077 propensity score–matched pairs (81% female) with a mean age of 81 years. Hip fracture rates were higher among risedronate than alendronate drug holidays, although this association was attenuated when any fracture was included as the outcome.
Overall, risedronate treatment before a drug holiday was associated with an 18% greater risk of hip fractures than alendronate, and this relative increase translated to a small absolute increase of 0.6%.
“These differences primarily manifested after 24 months, but given these small differences, I’m not sure if we need to change our current management strategy,” Dr. Wee said. “But further study is warranted.”
5. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults: A Response-Adaptive, Randomized Clinical Trial
This study assessed the effects of four doses of vitamin D3 supplements on the risk of falls.
The cohort included 688 participants, aged 70 years and older, with an elevated fall risk and a serum 25-hydroxyvitamin D level of 25-72.5 nmol/L. The intervention was 200 (control), 1,000, 2,000, or 4,000 IU of vitamin D3 per day.
“Their results showed that supplementation at doses of 1,000 IU/day or higher did not prevent falls compared with 200 IU/day,” said deputy editor Stephanie Chang, MD, MPH. “Several analyses raised safety concerns about vitamin D3 doses of 1,000 IU/day or higher.”
6. Postdiagnosis Smoking Cessation and Reduced Risk for Lung Cancer Progression and Mortality: A Prospective Cohort Study
This study sought to determine if quitting smoking after a diagnosis of lung cancer reduced the risk for disease progression and mortality. Researchers prospectively analyzed patients with non–small cell lung cancer (NSCLC) who were recruited between 2007 and 2016 and followed annually through 2020. The cohort comprised 517 current smokers who were diagnosed with early-stage (IA-IIIA) NSCLC.
The adjusted median overall survival time was 21.6 months higher among patients who quit smoking versus those who continued smoking, and a higher 5-year overall and progression-free survival were observed among patients who quit than those who continued smoking. After adjusting for confounders, smoking cessation remained associated with a lower risk for all-cause mortality, cancer-specific mortality, and disease progression.
7. Acute Consumption of Alcohol and Discrete Atrial Fibrillation Events
This study sought to determine if alcohol consumption heightened the risk for an episode of atrial fibrillation (AFib). The cohort included 100 individuals with paroxysmal AFib who were fitted with a continuous electrocardiogram monitor and an ankle-worn transdermal ethanol sensor for 4 weeks. Real-time documentation of each alcoholic drink consumed was self-recorded and finger-stick blood tests for phosphatidylethanol were used to corroborate ascertainments of drinking events.
Phosphatidylethanol testing correlated with the number of real-time recorded drinks and with the transdermal alcohol sensor. Consuming one alcoholic drink was associated with a twofold increased risk of AFib over the next 4 hours. The risk rose threefold with the consumption of two drinks.
“There is evidence of dose-response relationship with higher risk with more drinks,” Dr. Chang said. “Even one drink may predispose to an acute episode of AF[ib] in those so predisposed.”
8. Evaluation and Management After Acute Left-Sided Colonic Diverticulitis: A Systematic Review
Management of uncomplicated diverticulitis is usually conservative and includes bowel rest and fluids. However, uncertainty remains about the role of hospitalization and antibiotics, Dr. Chang said. The new review included 51 studies looking at colonoscopy, nonsurgical treatments, and elective surgery for patients with diverticulitis.
It was unclear if patients with recent acute diverticulitis are at increased risk for colorectal cancer, although those with complicated diverticulitis do appear to be at a higher risk of the disease. Treatment with mesalamine was shown to be ineffective in preventing recurrence, and other nonsurgical treatments lacked adequate evidence.
As for surgery, elective procedures reduce recurrence in patients with prior complicated or smoldering or frequently recurrent diverticulitis, but it is unclear which of these patients may benefit most.
“The ACP recommends initial management without antibiotics,” said Dr. Chang, adding that other questions need to be addressed, such as inpatient versus outpatient management and elective surgery after an acute episode.
Dr. Wee and Dr. Chang disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
1. Adding a New Medication Versus Maximizing Dose to Intensify Hypertension Treatment in Older Adults: A Retrospective Observational Study
Roughly one in three adults with hypertension have inadequate blood pressure control, and clinicians have two options for intensifying treatment: “The dose of the current drug regimen can be maximized, or a new drug can be added,” said deputy editor Christina C. Wee, MD, MPH, at the annual meeting of the American College of Physicians.
Data from randomized controlled trials suggest treatment with lower doses of combination therapy may be more effective, with fewer side effects – although the best strategy in older adults remains unclear.
To answer that question, researchers conducted a large-scale, population-based, retrospective cohort study, and observational data were used to emulate a target trial with two groups: new medication and maximizing dose.
The cohort comprised people aged 65 years or older with hypertension and was limited to those with a systolic blood pressure of 130 mm Hg or higher. Two intensification approaches were used: adding a new medication, defined as a total dose increase with a new medication; and maximizing dose, defined as a total dose increase without new medication.
A total of 178,562 patients were included in the study, and 45,575 (25.5%) had intensification by adding a new medication and 132,987 (74.5%) by maximizing dose.
“Both produced systolic blood pressure reduction with a slight advantage in the ‘add a new medication’ group,” Dr. Wee said. “That group reduced their systolic blood pressure by over 4.5 points as compared to 3.8 points in the maximized [dose] group.”
At 12 months the results were similar, but only 50% of patients in the new medication group were able to sustain that strategy, compared with two-thirds of patients who had their dose increased.
“This suggests that, in older adults, adding a new antihypertensive medication versus maximizing dosing of existing regimen is less common, only minimally more effective, and less sustainable,” Dr. Wee said. “Maximizing dose of antihypertensive medication is a reasonable approach [and] may be easier to sustain.”
2. Cost-Effectiveness of Screening Mammography Beyond Age 75 Years: A Cost-Effectiveness Analysis
The U.S. Preventive Services Task Force recommends biennial screening mammograms through the age of 74 years, and a meta-analysis of randomized controlled trials suggests mortality is reduced among women with at least a 10-year life expectancy, Dr. Wee said.
However, whether screening beyond age 75 years is cost effective, especially among women with comorbidities, is unclear.
To address that question, researchers estimated benefits, harms, and cost-effectiveness of extending mammography to age 80, 85, or 90 years according to comorbidity burden, using data from the Surveillance, Epidemiology, and End Results program and the Breast Cancer Surveillance Consortium.
The results showed that extending annual mammography beyond age 75 years was not cost effective, but biennial mammography was. “It was cost effective to age 80 regardless of baseline comorbidity score, but it averted only small, absolute numbers of breast cancer deaths – especially for women with comorbidities,” Dr. Wee said. “It was not cost effective beyond age 80.”
3. Prediction of End-Stage Kidney Disease Using Estimated Glomerular Filtration Rate With and Without Race: A Prospective Cohort Study
Estimated glomerular filtration rate (eGFR) is associated with end-stage kidney disease (ESKD) and is used to make dialysis and transplant decisions. “However, the accuracy of using eGFR alone has been questioned and, previously, some eGFR equations included a correction for race and this has been quite controversial,” Dr. Wee said. “And just last year, the Chronic Kidney Disease Epidemiology Collaboration released their new equations, removing the adjustment for race.”
The study authors posed two questions:
- How well does eGFR alone predict risk of ESKD, compared with Kidney Function Risk Equation (KFRE)?
- Does using different eGFR equations affect performance of either eGFR alone or KFRE in predicting the risk of ESKD?
During a maximum 16 years of follow-up, 856 participants (n = 3,873) developed ESKD. Across all eGFR equations, the KFRE score was superior for predicting 2-year incidence of end-stage kidney disease, compared with eGFR alone.
“KFRE score better predicted 2-year risk of ESKD than eGFR alone regardless of eGFR equations used,” Dr. Wee said. “Correcting eGFR equations for race did not improve performance and validates recent guidelines.”
4. Comparative Fracture Risk During Osteoporosis Drug Holidays After Long-Term Risedronate Versus Alendronate Therapy: A Propensity Score-Matched Cohort Study
The study looked at the comparative risks of drug holidays after long-term (≥ 3 years) risedronate versus alendronate therapy in a cohort of individuals aged 66 years or older. The primary outcome was hip fracture within 3 years after a 120-day ascertainment period.
The cohort included 25,077 propensity score–matched pairs (81% female) with a mean age of 81 years. Hip fracture rates were higher among risedronate than alendronate drug holidays, although this association was attenuated when any fracture was included as the outcome.
Overall, risedronate treatment before a drug holiday was associated with an 18% greater risk of hip fractures than alendronate, and this relative increase translated to a small absolute increase of 0.6%.
“These differences primarily manifested after 24 months, but given these small differences, I’m not sure if we need to change our current management strategy,” Dr. Wee said. “But further study is warranted.”
5. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults: A Response-Adaptive, Randomized Clinical Trial
This study assessed the effects of four doses of vitamin D3 supplements on the risk of falls.
The cohort included 688 participants, aged 70 years and older, with an elevated fall risk and a serum 25-hydroxyvitamin D level of 25-72.5 nmol/L. The intervention was 200 (control), 1,000, 2,000, or 4,000 IU of vitamin D3 per day.
“Their results showed that supplementation at doses of 1,000 IU/day or higher did not prevent falls compared with 200 IU/day,” said deputy editor Stephanie Chang, MD, MPH. “Several analyses raised safety concerns about vitamin D3 doses of 1,000 IU/day or higher.”
6. Postdiagnosis Smoking Cessation and Reduced Risk for Lung Cancer Progression and Mortality: A Prospective Cohort Study
This study sought to determine if quitting smoking after a diagnosis of lung cancer reduced the risk for disease progression and mortality. Researchers prospectively analyzed patients with non–small cell lung cancer (NSCLC) who were recruited between 2007 and 2016 and followed annually through 2020. The cohort comprised 517 current smokers who were diagnosed with early-stage (IA-IIIA) NSCLC.
The adjusted median overall survival time was 21.6 months higher among patients who quit smoking versus those who continued smoking, and a higher 5-year overall and progression-free survival were observed among patients who quit than those who continued smoking. After adjusting for confounders, smoking cessation remained associated with a lower risk for all-cause mortality, cancer-specific mortality, and disease progression.
7. Acute Consumption of Alcohol and Discrete Atrial Fibrillation Events
This study sought to determine if alcohol consumption heightened the risk for an episode of atrial fibrillation (AFib). The cohort included 100 individuals with paroxysmal AFib who were fitted with a continuous electrocardiogram monitor and an ankle-worn transdermal ethanol sensor for 4 weeks. Real-time documentation of each alcoholic drink consumed was self-recorded and finger-stick blood tests for phosphatidylethanol were used to corroborate ascertainments of drinking events.
Phosphatidylethanol testing correlated with the number of real-time recorded drinks and with the transdermal alcohol sensor. Consuming one alcoholic drink was associated with a twofold increased risk of AFib over the next 4 hours. The risk rose threefold with the consumption of two drinks.
“There is evidence of dose-response relationship with higher risk with more drinks,” Dr. Chang said. “Even one drink may predispose to an acute episode of AF[ib] in those so predisposed.”
8. Evaluation and Management After Acute Left-Sided Colonic Diverticulitis: A Systematic Review
Management of uncomplicated diverticulitis is usually conservative and includes bowel rest and fluids. However, uncertainty remains about the role of hospitalization and antibiotics, Dr. Chang said. The new review included 51 studies looking at colonoscopy, nonsurgical treatments, and elective surgery for patients with diverticulitis.
It was unclear if patients with recent acute diverticulitis are at increased risk for colorectal cancer, although those with complicated diverticulitis do appear to be at a higher risk of the disease. Treatment with mesalamine was shown to be ineffective in preventing recurrence, and other nonsurgical treatments lacked adequate evidence.
As for surgery, elective procedures reduce recurrence in patients with prior complicated or smoldering or frequently recurrent diverticulitis, but it is unclear which of these patients may benefit most.
“The ACP recommends initial management without antibiotics,” said Dr. Chang, adding that other questions need to be addressed, such as inpatient versus outpatient management and elective surgery after an acute episode.
Dr. Wee and Dr. Chang disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
1. Adding a New Medication Versus Maximizing Dose to Intensify Hypertension Treatment in Older Adults: A Retrospective Observational Study
Roughly one in three adults with hypertension have inadequate blood pressure control, and clinicians have two options for intensifying treatment: “The dose of the current drug regimen can be maximized, or a new drug can be added,” said deputy editor Christina C. Wee, MD, MPH, at the annual meeting of the American College of Physicians.
Data from randomized controlled trials suggest treatment with lower doses of combination therapy may be more effective, with fewer side effects – although the best strategy in older adults remains unclear.
To answer that question, researchers conducted a large-scale, population-based, retrospective cohort study, and observational data were used to emulate a target trial with two groups: new medication and maximizing dose.
The cohort comprised people aged 65 years or older with hypertension and was limited to those with a systolic blood pressure of 130 mm Hg or higher. Two intensification approaches were used: adding a new medication, defined as a total dose increase with a new medication; and maximizing dose, defined as a total dose increase without new medication.
A total of 178,562 patients were included in the study, and 45,575 (25.5%) had intensification by adding a new medication and 132,987 (74.5%) by maximizing dose.
“Both produced systolic blood pressure reduction with a slight advantage in the ‘add a new medication’ group,” Dr. Wee said. “That group reduced their systolic blood pressure by over 4.5 points as compared to 3.8 points in the maximized [dose] group.”
At 12 months the results were similar, but only 50% of patients in the new medication group were able to sustain that strategy, compared with two-thirds of patients who had their dose increased.
“This suggests that, in older adults, adding a new antihypertensive medication versus maximizing dosing of existing regimen is less common, only minimally more effective, and less sustainable,” Dr. Wee said. “Maximizing dose of antihypertensive medication is a reasonable approach [and] may be easier to sustain.”
2. Cost-Effectiveness of Screening Mammography Beyond Age 75 Years: A Cost-Effectiveness Analysis
The U.S. Preventive Services Task Force recommends biennial screening mammograms through the age of 74 years, and a meta-analysis of randomized controlled trials suggests mortality is reduced among women with at least a 10-year life expectancy, Dr. Wee said.
However, whether screening beyond age 75 years is cost effective, especially among women with comorbidities, is unclear.
To address that question, researchers estimated benefits, harms, and cost-effectiveness of extending mammography to age 80, 85, or 90 years according to comorbidity burden, using data from the Surveillance, Epidemiology, and End Results program and the Breast Cancer Surveillance Consortium.
The results showed that extending annual mammography beyond age 75 years was not cost effective, but biennial mammography was. “It was cost effective to age 80 regardless of baseline comorbidity score, but it averted only small, absolute numbers of breast cancer deaths – especially for women with comorbidities,” Dr. Wee said. “It was not cost effective beyond age 80.”
3. Prediction of End-Stage Kidney Disease Using Estimated Glomerular Filtration Rate With and Without Race: A Prospective Cohort Study
Estimated glomerular filtration rate (eGFR) is associated with end-stage kidney disease (ESKD) and is used to make dialysis and transplant decisions. “However, the accuracy of using eGFR alone has been questioned and, previously, some eGFR equations included a correction for race and this has been quite controversial,” Dr. Wee said. “And just last year, the Chronic Kidney Disease Epidemiology Collaboration released their new equations, removing the adjustment for race.”
The study authors posed two questions:
- How well does eGFR alone predict risk of ESKD, compared with Kidney Function Risk Equation (KFRE)?
- Does using different eGFR equations affect performance of either eGFR alone or KFRE in predicting the risk of ESKD?
During a maximum 16 years of follow-up, 856 participants (n = 3,873) developed ESKD. Across all eGFR equations, the KFRE score was superior for predicting 2-year incidence of end-stage kidney disease, compared with eGFR alone.
“KFRE score better predicted 2-year risk of ESKD than eGFR alone regardless of eGFR equations used,” Dr. Wee said. “Correcting eGFR equations for race did not improve performance and validates recent guidelines.”
4. Comparative Fracture Risk During Osteoporosis Drug Holidays After Long-Term Risedronate Versus Alendronate Therapy: A Propensity Score-Matched Cohort Study
The study looked at the comparative risks of drug holidays after long-term (≥ 3 years) risedronate versus alendronate therapy in a cohort of individuals aged 66 years or older. The primary outcome was hip fracture within 3 years after a 120-day ascertainment period.
The cohort included 25,077 propensity score–matched pairs (81% female) with a mean age of 81 years. Hip fracture rates were higher among risedronate than alendronate drug holidays, although this association was attenuated when any fracture was included as the outcome.
Overall, risedronate treatment before a drug holiday was associated with an 18% greater risk of hip fractures than alendronate, and this relative increase translated to a small absolute increase of 0.6%.
“These differences primarily manifested after 24 months, but given these small differences, I’m not sure if we need to change our current management strategy,” Dr. Wee said. “But further study is warranted.”
5. The Effects of Four Doses of Vitamin D Supplements on Falls in Older Adults: A Response-Adaptive, Randomized Clinical Trial
This study assessed the effects of four doses of vitamin D3 supplements on the risk of falls.
The cohort included 688 participants, aged 70 years and older, with an elevated fall risk and a serum 25-hydroxyvitamin D level of 25-72.5 nmol/L. The intervention was 200 (control), 1,000, 2,000, or 4,000 IU of vitamin D3 per day.
“Their results showed that supplementation at doses of 1,000 IU/day or higher did not prevent falls compared with 200 IU/day,” said deputy editor Stephanie Chang, MD, MPH. “Several analyses raised safety concerns about vitamin D3 doses of 1,000 IU/day or higher.”
6. Postdiagnosis Smoking Cessation and Reduced Risk for Lung Cancer Progression and Mortality: A Prospective Cohort Study
This study sought to determine if quitting smoking after a diagnosis of lung cancer reduced the risk for disease progression and mortality. Researchers prospectively analyzed patients with non–small cell lung cancer (NSCLC) who were recruited between 2007 and 2016 and followed annually through 2020. The cohort comprised 517 current smokers who were diagnosed with early-stage (IA-IIIA) NSCLC.
The adjusted median overall survival time was 21.6 months higher among patients who quit smoking versus those who continued smoking, and a higher 5-year overall and progression-free survival were observed among patients who quit than those who continued smoking. After adjusting for confounders, smoking cessation remained associated with a lower risk for all-cause mortality, cancer-specific mortality, and disease progression.
7. Acute Consumption of Alcohol and Discrete Atrial Fibrillation Events
This study sought to determine if alcohol consumption heightened the risk for an episode of atrial fibrillation (AFib). The cohort included 100 individuals with paroxysmal AFib who were fitted with a continuous electrocardiogram monitor and an ankle-worn transdermal ethanol sensor for 4 weeks. Real-time documentation of each alcoholic drink consumed was self-recorded and finger-stick blood tests for phosphatidylethanol were used to corroborate ascertainments of drinking events.
Phosphatidylethanol testing correlated with the number of real-time recorded drinks and with the transdermal alcohol sensor. Consuming one alcoholic drink was associated with a twofold increased risk of AFib over the next 4 hours. The risk rose threefold with the consumption of two drinks.
“There is evidence of dose-response relationship with higher risk with more drinks,” Dr. Chang said. “Even one drink may predispose to an acute episode of AF[ib] in those so predisposed.”
8. Evaluation and Management After Acute Left-Sided Colonic Diverticulitis: A Systematic Review
Management of uncomplicated diverticulitis is usually conservative and includes bowel rest and fluids. However, uncertainty remains about the role of hospitalization and antibiotics, Dr. Chang said. The new review included 51 studies looking at colonoscopy, nonsurgical treatments, and elective surgery for patients with diverticulitis.
It was unclear if patients with recent acute diverticulitis are at increased risk for colorectal cancer, although those with complicated diverticulitis do appear to be at a higher risk of the disease. Treatment with mesalamine was shown to be ineffective in preventing recurrence, and other nonsurgical treatments lacked adequate evidence.
As for surgery, elective procedures reduce recurrence in patients with prior complicated or smoldering or frequently recurrent diverticulitis, but it is unclear which of these patients may benefit most.
“The ACP recommends initial management without antibiotics,” said Dr. Chang, adding that other questions need to be addressed, such as inpatient versus outpatient management and elective surgery after an acute episode.
Dr. Wee and Dr. Chang disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2022
Focal hair loss
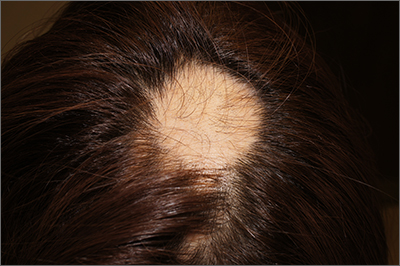
The findings of smooth, round alopecia occurring rapidly without associated scarring, pain, or itching, is consistent with the diagnosis of alopecia areata.
Alopecia areata is a common autoimmune disease caused by T lymphocytes targeting hair follicles and resulting in rapid and nonscarring hair loss. It is usually self-resolving and about 2% of all individuals are affected at some point during their lifetime, with an average age of onset of 33 years.1 Some patients may progress to loss of all scalp hair (alopecia totalis) or all hair on the scalp and body (alopecia universalis).1
It is important to inspect a patient’s scalp, face, and body for more subtle areas of loss that could signal other disorders, such as lichen planopilaris, discoid lupus, or telogen effluvium. It is worth noting that alopecia areata is not associated with scalp lesions, crusting, or scars without follicles. Such findings should be further investigated with a 4-mm punch biopsy of affected and adjacent follicular units. Carefully labeling biopsy specimens as scalp specimens for hair loss will aid in a correct histopathologic diagnosis.
Systematic data comparing treatments for alopecia areata are lacking. For localized disease, topical or intradermal triamcinolone injections at a concentration of 5 to 10 mg/mL, with about 0.1 mL to 0.05 mL injected every square centimeter of affected area (up to 40 mg per visit), can provide rapid regrowth.1 Within 4 months of the monthly injections, 63% of patients experience complete regrowth.1 Despite this favorable outcome, there is also a high rate of recurrence.
For more widespread disease, contact immunotherapy with squaric acid dibutyl ester or diphencyprone can provoke a low-grade contact allergy and induce antigenic completion. This therapy is painless but can be itchy; medications must be compounded and titrated to activity.
The patient in this case opted to receive monthly triamcinolone injections in an undiluted concentration of 10 mg/mL for 3 months, at which point she experienced excellent hair regrowth. A small patch of recurrence was noted a year later and treated twice with monthly triamcinolone injections.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options. Int J Trichology. 2018;10:51-60. doi: 10.4103/ijt.ijt_99_17

The findings of smooth, round alopecia occurring rapidly without associated scarring, pain, or itching, is consistent with the diagnosis of alopecia areata.
Alopecia areata is a common autoimmune disease caused by T lymphocytes targeting hair follicles and resulting in rapid and nonscarring hair loss. It is usually self-resolving and about 2% of all individuals are affected at some point during their lifetime, with an average age of onset of 33 years.1 Some patients may progress to loss of all scalp hair (alopecia totalis) or all hair on the scalp and body (alopecia universalis).1
It is important to inspect a patient’s scalp, face, and body for more subtle areas of loss that could signal other disorders, such as lichen planopilaris, discoid lupus, or telogen effluvium. It is worth noting that alopecia areata is not associated with scalp lesions, crusting, or scars without follicles. Such findings should be further investigated with a 4-mm punch biopsy of affected and adjacent follicular units. Carefully labeling biopsy specimens as scalp specimens for hair loss will aid in a correct histopathologic diagnosis.
Systematic data comparing treatments for alopecia areata are lacking. For localized disease, topical or intradermal triamcinolone injections at a concentration of 5 to 10 mg/mL, with about 0.1 mL to 0.05 mL injected every square centimeter of affected area (up to 40 mg per visit), can provide rapid regrowth.1 Within 4 months of the monthly injections, 63% of patients experience complete regrowth.1 Despite this favorable outcome, there is also a high rate of recurrence.
For more widespread disease, contact immunotherapy with squaric acid dibutyl ester or diphencyprone can provoke a low-grade contact allergy and induce antigenic completion. This therapy is painless but can be itchy; medications must be compounded and titrated to activity.
The patient in this case opted to receive monthly triamcinolone injections in an undiluted concentration of 10 mg/mL for 3 months, at which point she experienced excellent hair regrowth. A small patch of recurrence was noted a year later and treated twice with monthly triamcinolone injections.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

The findings of smooth, round alopecia occurring rapidly without associated scarring, pain, or itching, is consistent with the diagnosis of alopecia areata.
Alopecia areata is a common autoimmune disease caused by T lymphocytes targeting hair follicles and resulting in rapid and nonscarring hair loss. It is usually self-resolving and about 2% of all individuals are affected at some point during their lifetime, with an average age of onset of 33 years.1 Some patients may progress to loss of all scalp hair (alopecia totalis) or all hair on the scalp and body (alopecia universalis).1
It is important to inspect a patient’s scalp, face, and body for more subtle areas of loss that could signal other disorders, such as lichen planopilaris, discoid lupus, or telogen effluvium. It is worth noting that alopecia areata is not associated with scalp lesions, crusting, or scars without follicles. Such findings should be further investigated with a 4-mm punch biopsy of affected and adjacent follicular units. Carefully labeling biopsy specimens as scalp specimens for hair loss will aid in a correct histopathologic diagnosis.
Systematic data comparing treatments for alopecia areata are lacking. For localized disease, topical or intradermal triamcinolone injections at a concentration of 5 to 10 mg/mL, with about 0.1 mL to 0.05 mL injected every square centimeter of affected area (up to 40 mg per visit), can provide rapid regrowth.1 Within 4 months of the monthly injections, 63% of patients experience complete regrowth.1 Despite this favorable outcome, there is also a high rate of recurrence.
For more widespread disease, contact immunotherapy with squaric acid dibutyl ester or diphencyprone can provoke a low-grade contact allergy and induce antigenic completion. This therapy is painless but can be itchy; medications must be compounded and titrated to activity.
The patient in this case opted to receive monthly triamcinolone injections in an undiluted concentration of 10 mg/mL for 3 months, at which point she experienced excellent hair regrowth. A small patch of recurrence was noted a year later and treated twice with monthly triamcinolone injections.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options. Int J Trichology. 2018;10:51-60. doi: 10.4103/ijt.ijt_99_17
1. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options. Int J Trichology. 2018;10:51-60. doi: 10.4103/ijt.ijt_99_17
Omicron sublineages evade immunity from past infection
A South African study based on blood samples found that the BA.4 and BA.5 sublineages of Omicron were more likely to evade antibodies produced by previous Omicron infections than the immunity provided by vaccinations.
Scientists took blood samples from 39 people infected with Omicron, with 24 people not vaccinated and 15 vaccinated with the Pfizer or the Johnson & Johnson vaccines, Reuters reported.
“The vaccinated group showed about a fivefold higher neutralization capacity ... and should be better protected,” the investigators found, according to Reuters.
There was an eightfold decrease in antibody protection in unvaccinated blood samples when exposed to the subvariants compared to a threefold decrease in the blood samples from vaccinated people.
“Based on neutralization escape, BA.4 and BA.5 have potential to result in a new infection wave,” the investigators found.
The finding is important because health authorities say cases caused by the sublineages are increasing in South Africa to a degree that the nation may be entering a fifth wave of COVID, Reuters said.
Health Minister Joe Phaahla said recently that hospitalizations were increasing but that ICU admissions had not greatly gone up yet.
A version of this article first appeared on WebMD.com.
A South African study based on blood samples found that the BA.4 and BA.5 sublineages of Omicron were more likely to evade antibodies produced by previous Omicron infections than the immunity provided by vaccinations.
Scientists took blood samples from 39 people infected with Omicron, with 24 people not vaccinated and 15 vaccinated with the Pfizer or the Johnson & Johnson vaccines, Reuters reported.
“The vaccinated group showed about a fivefold higher neutralization capacity ... and should be better protected,” the investigators found, according to Reuters.
There was an eightfold decrease in antibody protection in unvaccinated blood samples when exposed to the subvariants compared to a threefold decrease in the blood samples from vaccinated people.
“Based on neutralization escape, BA.4 and BA.5 have potential to result in a new infection wave,” the investigators found.
The finding is important because health authorities say cases caused by the sublineages are increasing in South Africa to a degree that the nation may be entering a fifth wave of COVID, Reuters said.
Health Minister Joe Phaahla said recently that hospitalizations were increasing but that ICU admissions had not greatly gone up yet.
A version of this article first appeared on WebMD.com.
A South African study based on blood samples found that the BA.4 and BA.5 sublineages of Omicron were more likely to evade antibodies produced by previous Omicron infections than the immunity provided by vaccinations.
Scientists took blood samples from 39 people infected with Omicron, with 24 people not vaccinated and 15 vaccinated with the Pfizer or the Johnson & Johnson vaccines, Reuters reported.
“The vaccinated group showed about a fivefold higher neutralization capacity ... and should be better protected,” the investigators found, according to Reuters.
There was an eightfold decrease in antibody protection in unvaccinated blood samples when exposed to the subvariants compared to a threefold decrease in the blood samples from vaccinated people.
“Based on neutralization escape, BA.4 and BA.5 have potential to result in a new infection wave,” the investigators found.
The finding is important because health authorities say cases caused by the sublineages are increasing in South Africa to a degree that the nation may be entering a fifth wave of COVID, Reuters said.
Health Minister Joe Phaahla said recently that hospitalizations were increasing but that ICU admissions had not greatly gone up yet.
A version of this article first appeared on WebMD.com.