User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
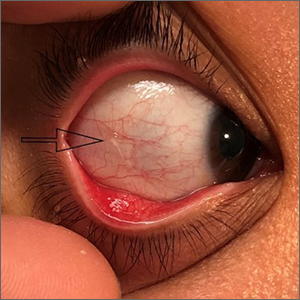
Use of bone densitometry to grade hip OA could be boon to diagnosis, prognosis
Bone densitometry scans provide useful information that can be used to classify radiographic hip osteoarthritis more objectively than does currently used methods, UK researchers believe.
Based on detecting osteophytes using high-resolution dual energy x-ray absorptiometry (DEXA), the novel grading system they have developed showed an exponential relationship with worsening clinical outcomes such as hip pain, hospital-diagnosed OA, and total hip replacement (THR).
“Given the low radiation doses involved in DEXA, this could open up opportunities for ascertaining OA in larger population-based cohorts than those available for x-rays,” Ben G. Faber, MBBS, BSc, reported at the annual meeting of the British Society for Rheumatology during the best oral abstracts session.
This not only supports further research into OA but also means that it might be possible to use DEXA scans to help screen for hip OA and assess the risk for hip replacement in the future, added Dr. Faber, a Medical Research Council Clinical Research Fellow at the University of Bristol and rheumatology registrar for the North Bristol NHS Trust in England.
Session chair Tonia Vincent, MBBS, PhD, FRCP, a consultant rheumatologist and director of the Centre for Osteoarthritis Pathogenesis at the Kennedy Institute of Rheumatology at the University of Oxford (England), found the relationship between the DEXA findings and Kellgren and Lawrence (KL) grade and clinical outcomes to be “really striking.”
It highlights “a very important structure-symptom relationship, which people in the textbooks say doesn’t exist for osteoarthritis,” Dr. Vincent observed.
New scanners, new score
DEXA scans are a mainstay of assessing fracture risk in osteoporosis. Although originally developed for assessing bone mineral density, the newer scanners have such high resolution that they can now show radiographic features such as joint space narrowing (JSN) and the presence of osteophytes.
Both are given equal weighting in existing x-ray grading or scoring systems, which are fairly subjective, Dr. Faber said, but recent research conducted by him and his collaborators has suggested that the presence of osteophytes may be a better indicator of hip pain than JSN.
Using more than 40,000 DEXA scans obtained from the UK Biobank, Dr. Faber and associates developed a semi-automated tool that measured both JSN and osteophytes, giving greater weight to the latter. These patients with DEXA scans in the Biobank had a mean age of 63.7 years. Hip pain was present in 8.1%, hospital-diagnosed OA in 1.3%, and total hip replacement occurred in 0.6%.
The tool the researchers developed automatically calculated the minimum joint space width using a machine-learning-based approach, whereas they manually identified osteophytes at three key locations – the lateral acetabulum, the superior lateral femoral head, and the inferior medial femoral head. However, Dr. Faber said, “we’re now very close to fully automating that part of the process.”
Minimum JSN and osteophyte presence at each location was quantified using a scale of 0 (none) to 3 (greatest) to give a total score out of a possible 12; they then used this score to create five ‘grades’ from 0 (least) to 4 (most).
Applying these new radiographic hip OA grades to the Biobank DEXA scans revealed a strong and increasing association between the presences of osteophytes and the clinical outcomes considered.
For instance, when any osteophytes were detected, the odds ratios (ORs) for having hip pain for more than 3 months, a hospital diagnosis of OA, or THR were a respective 2.05, 4.98, and 6.17.
The presence of inferior or superior femoral osteophytes carried higher ORs for the three outcomes than did acetabular osteophytes, with the greatest ORs seen in patients with osteophytes at all three locations (6.95, 20.53, and 21.79, respectively). By comparison, ORs for JSN were 1.37, 3.48, and 3.91.
There were “strong progressive relationships between each grade of OA and the clinical outcomes,” Dr. Faber said, noting that “the headline figure” was that comparing people with grade 4 with grade 0, the risk for needing THR was 58 times higher. This tallies with what would be expected, Dr. Faber said, since “one would expect to see OA on imaging findings before someone had a total hip replacement.”
What might the future hold?
“One of the strengths of this study is that by using a semi-automated approach, we feel that this is a more objective measure of radiographic hip OA, which hopefully will mean that it’s more reproducible in the future when repeating in other cohorts,” Dr. Faber said.
Asked what he thought the future held, Dr. Faber responded: “A grand vision might be that you’re already doing DEXA scans to look at bone health in individuals, and from those same DEXAs you could get information on radiographic hip OA,” he hypothesized.
“We do this with BMD and we feed that into FRAX [Fracture Risk Assessment Tool] to give someone a fracture risk. Could we do the same for total hip replacement to really identify people are high risk of OA in the future?” he wondered. “Then could we intervene to potentially prevent that ... or increase the duration that they’re healthy before they require the operation? There’s still plenty of work needed to get there.”
Dr. Faber and colleagues work was recently published in Rheumatology.
Dr. Faber had no conflicts of interest to disclose. Dr. Vincent had nothing to declare; her research is funded by Versus Arthritis, the Medical Research Council, the European Research Council, FOREUM (Foundation for Research in Rheumatology), the Dunhill Trust, and the Kennedy Trust for Rheumatology Research.
Bone densitometry scans provide useful information that can be used to classify radiographic hip osteoarthritis more objectively than does currently used methods, UK researchers believe.
Based on detecting osteophytes using high-resolution dual energy x-ray absorptiometry (DEXA), the novel grading system they have developed showed an exponential relationship with worsening clinical outcomes such as hip pain, hospital-diagnosed OA, and total hip replacement (THR).
“Given the low radiation doses involved in DEXA, this could open up opportunities for ascertaining OA in larger population-based cohorts than those available for x-rays,” Ben G. Faber, MBBS, BSc, reported at the annual meeting of the British Society for Rheumatology during the best oral abstracts session.
This not only supports further research into OA but also means that it might be possible to use DEXA scans to help screen for hip OA and assess the risk for hip replacement in the future, added Dr. Faber, a Medical Research Council Clinical Research Fellow at the University of Bristol and rheumatology registrar for the North Bristol NHS Trust in England.
Session chair Tonia Vincent, MBBS, PhD, FRCP, a consultant rheumatologist and director of the Centre for Osteoarthritis Pathogenesis at the Kennedy Institute of Rheumatology at the University of Oxford (England), found the relationship between the DEXA findings and Kellgren and Lawrence (KL) grade and clinical outcomes to be “really striking.”
It highlights “a very important structure-symptom relationship, which people in the textbooks say doesn’t exist for osteoarthritis,” Dr. Vincent observed.
New scanners, new score
DEXA scans are a mainstay of assessing fracture risk in osteoporosis. Although originally developed for assessing bone mineral density, the newer scanners have such high resolution that they can now show radiographic features such as joint space narrowing (JSN) and the presence of osteophytes.
Both are given equal weighting in existing x-ray grading or scoring systems, which are fairly subjective, Dr. Faber said, but recent research conducted by him and his collaborators has suggested that the presence of osteophytes may be a better indicator of hip pain than JSN.
Using more than 40,000 DEXA scans obtained from the UK Biobank, Dr. Faber and associates developed a semi-automated tool that measured both JSN and osteophytes, giving greater weight to the latter. These patients with DEXA scans in the Biobank had a mean age of 63.7 years. Hip pain was present in 8.1%, hospital-diagnosed OA in 1.3%, and total hip replacement occurred in 0.6%.
The tool the researchers developed automatically calculated the minimum joint space width using a machine-learning-based approach, whereas they manually identified osteophytes at three key locations – the lateral acetabulum, the superior lateral femoral head, and the inferior medial femoral head. However, Dr. Faber said, “we’re now very close to fully automating that part of the process.”
Minimum JSN and osteophyte presence at each location was quantified using a scale of 0 (none) to 3 (greatest) to give a total score out of a possible 12; they then used this score to create five ‘grades’ from 0 (least) to 4 (most).
Applying these new radiographic hip OA grades to the Biobank DEXA scans revealed a strong and increasing association between the presences of osteophytes and the clinical outcomes considered.
For instance, when any osteophytes were detected, the odds ratios (ORs) for having hip pain for more than 3 months, a hospital diagnosis of OA, or THR were a respective 2.05, 4.98, and 6.17.
The presence of inferior or superior femoral osteophytes carried higher ORs for the three outcomes than did acetabular osteophytes, with the greatest ORs seen in patients with osteophytes at all three locations (6.95, 20.53, and 21.79, respectively). By comparison, ORs for JSN were 1.37, 3.48, and 3.91.
There were “strong progressive relationships between each grade of OA and the clinical outcomes,” Dr. Faber said, noting that “the headline figure” was that comparing people with grade 4 with grade 0, the risk for needing THR was 58 times higher. This tallies with what would be expected, Dr. Faber said, since “one would expect to see OA on imaging findings before someone had a total hip replacement.”
What might the future hold?
“One of the strengths of this study is that by using a semi-automated approach, we feel that this is a more objective measure of radiographic hip OA, which hopefully will mean that it’s more reproducible in the future when repeating in other cohorts,” Dr. Faber said.
Asked what he thought the future held, Dr. Faber responded: “A grand vision might be that you’re already doing DEXA scans to look at bone health in individuals, and from those same DEXAs you could get information on radiographic hip OA,” he hypothesized.
“We do this with BMD and we feed that into FRAX [Fracture Risk Assessment Tool] to give someone a fracture risk. Could we do the same for total hip replacement to really identify people are high risk of OA in the future?” he wondered. “Then could we intervene to potentially prevent that ... or increase the duration that they’re healthy before they require the operation? There’s still plenty of work needed to get there.”
Dr. Faber and colleagues work was recently published in Rheumatology.
Dr. Faber had no conflicts of interest to disclose. Dr. Vincent had nothing to declare; her research is funded by Versus Arthritis, the Medical Research Council, the European Research Council, FOREUM (Foundation for Research in Rheumatology), the Dunhill Trust, and the Kennedy Trust for Rheumatology Research.
Bone densitometry scans provide useful information that can be used to classify radiographic hip osteoarthritis more objectively than does currently used methods, UK researchers believe.
Based on detecting osteophytes using high-resolution dual energy x-ray absorptiometry (DEXA), the novel grading system they have developed showed an exponential relationship with worsening clinical outcomes such as hip pain, hospital-diagnosed OA, and total hip replacement (THR).
“Given the low radiation doses involved in DEXA, this could open up opportunities for ascertaining OA in larger population-based cohorts than those available for x-rays,” Ben G. Faber, MBBS, BSc, reported at the annual meeting of the British Society for Rheumatology during the best oral abstracts session.
This not only supports further research into OA but also means that it might be possible to use DEXA scans to help screen for hip OA and assess the risk for hip replacement in the future, added Dr. Faber, a Medical Research Council Clinical Research Fellow at the University of Bristol and rheumatology registrar for the North Bristol NHS Trust in England.
Session chair Tonia Vincent, MBBS, PhD, FRCP, a consultant rheumatologist and director of the Centre for Osteoarthritis Pathogenesis at the Kennedy Institute of Rheumatology at the University of Oxford (England), found the relationship between the DEXA findings and Kellgren and Lawrence (KL) grade and clinical outcomes to be “really striking.”
It highlights “a very important structure-symptom relationship, which people in the textbooks say doesn’t exist for osteoarthritis,” Dr. Vincent observed.
New scanners, new score
DEXA scans are a mainstay of assessing fracture risk in osteoporosis. Although originally developed for assessing bone mineral density, the newer scanners have such high resolution that they can now show radiographic features such as joint space narrowing (JSN) and the presence of osteophytes.
Both are given equal weighting in existing x-ray grading or scoring systems, which are fairly subjective, Dr. Faber said, but recent research conducted by him and his collaborators has suggested that the presence of osteophytes may be a better indicator of hip pain than JSN.
Using more than 40,000 DEXA scans obtained from the UK Biobank, Dr. Faber and associates developed a semi-automated tool that measured both JSN and osteophytes, giving greater weight to the latter. These patients with DEXA scans in the Biobank had a mean age of 63.7 years. Hip pain was present in 8.1%, hospital-diagnosed OA in 1.3%, and total hip replacement occurred in 0.6%.
The tool the researchers developed automatically calculated the minimum joint space width using a machine-learning-based approach, whereas they manually identified osteophytes at three key locations – the lateral acetabulum, the superior lateral femoral head, and the inferior medial femoral head. However, Dr. Faber said, “we’re now very close to fully automating that part of the process.”
Minimum JSN and osteophyte presence at each location was quantified using a scale of 0 (none) to 3 (greatest) to give a total score out of a possible 12; they then used this score to create five ‘grades’ from 0 (least) to 4 (most).
Applying these new radiographic hip OA grades to the Biobank DEXA scans revealed a strong and increasing association between the presences of osteophytes and the clinical outcomes considered.
For instance, when any osteophytes were detected, the odds ratios (ORs) for having hip pain for more than 3 months, a hospital diagnosis of OA, or THR were a respective 2.05, 4.98, and 6.17.
The presence of inferior or superior femoral osteophytes carried higher ORs for the three outcomes than did acetabular osteophytes, with the greatest ORs seen in patients with osteophytes at all three locations (6.95, 20.53, and 21.79, respectively). By comparison, ORs for JSN were 1.37, 3.48, and 3.91.
There were “strong progressive relationships between each grade of OA and the clinical outcomes,” Dr. Faber said, noting that “the headline figure” was that comparing people with grade 4 with grade 0, the risk for needing THR was 58 times higher. This tallies with what would be expected, Dr. Faber said, since “one would expect to see OA on imaging findings before someone had a total hip replacement.”
What might the future hold?
“One of the strengths of this study is that by using a semi-automated approach, we feel that this is a more objective measure of radiographic hip OA, which hopefully will mean that it’s more reproducible in the future when repeating in other cohorts,” Dr. Faber said.
Asked what he thought the future held, Dr. Faber responded: “A grand vision might be that you’re already doing DEXA scans to look at bone health in individuals, and from those same DEXAs you could get information on radiographic hip OA,” he hypothesized.
“We do this with BMD and we feed that into FRAX [Fracture Risk Assessment Tool] to give someone a fracture risk. Could we do the same for total hip replacement to really identify people are high risk of OA in the future?” he wondered. “Then could we intervene to potentially prevent that ... or increase the duration that they’re healthy before they require the operation? There’s still plenty of work needed to get there.”
Dr. Faber and colleagues work was recently published in Rheumatology.
Dr. Faber had no conflicts of interest to disclose. Dr. Vincent had nothing to declare; her research is funded by Versus Arthritis, the Medical Research Council, the European Research Council, FOREUM (Foundation for Research in Rheumatology), the Dunhill Trust, and the Kennedy Trust for Rheumatology Research.
FROM BSR 2022
Tirzepatide succeeds in obesity in SURMOUNT-1, says Lilly
More than half of patients taking the two highest doses of tirzepatide as a once-weekly injection lost at least 20% of their body weight in the first phase 3 trial to examine this agent in patients with obesity, but without diabetes, according to preliminary top-line results from the SURMOUNT-1 trial announced by Lilly.
The full results will be reported at an upcoming medical conference and published at a later date, Lilly added.
There was much excitement in response to the news, but others have urged caution and noted that, even if tirzepatide is eventually approved for obesity, one of the major barriers to use in the United States will be insurance coverage.
“Wow (and a double Wow!) 52lb weight loss (22.5%) at highest dose of tirzepatide,” tweeted Sek Kathiresan, MD, a cardiologist who is cofounder of Verve Therapeutics and on leave from Harvard (@skathire).
“Thus far the challenge with GLP-1s [agonists] for management of obesity is that insurance usually isn’t covering them. This makes them unaffordable for most people,” replied James Marroquin, MD, of the University of Texas at Austin. (@Jamesmarroquin).
Yoni Freedhoff, MD, of the University of Ottawa (Ont.) who writes a column for this news organization on obesity, said if tirzepatide pans out, along with other similar agents already on the market for this indication, “the next few decades should see the pharmaceutical management of obesity rival its surgical management.”
Would compete with ‘game-changer’ semaglutide?
Tirzepatide has been dubbed a “twincretin” because it works not only as an agonist of the glucagonlike peptide-1 (GLP-1) receptor, but also of the glucose-dependent insulinotropic polypeptide (GIP) receptor. It has been much hyped based on the results of the series of SURPASS clinical trials, which have formed the basis of the application for type 2 diabetes approval, about which the U.S. Food and Drug Administration is expected to make a decision soon.
Several GLP-1 agonists are on the market for both type 2 diabetes and for obesity indications separately, including semaglutide (marketed as Wegovy for obesity, also a once-weekly injection) and liraglutide (Saxenda for obesity, a daily injection), both Novo Nordisk agents.
Wegovy was approved for weight loss in the United States last year, with doctors telling this news organization then that a third of patients who take the drug are likely to lose 20% or more of their starting weight, an outcome that approaches reductions seen with bariatric surgery.
Dr. Freedhoff said he’d like to see “reimbursement by insurers who will see these drugs serving as important ancillary treatments for the myriad of weight-responsive conditions they’re already covering.”
SURMOUNT-1 data: ‘Impressive body weight’ reductions
The new tirzepatide data come from the multicenter, randomized, double-blind, placebo-controlled SURMOUNT-1 trial, which included 2539 participants from the United States, Argentina, Brazil, China, India, Japan Mexico, Russia, and Taiwan. They had obesity or overweight plus at least one comorbidity but not diabetes. They were randomized to 5-mg, 10-mg, or 15-mg once-weekly tirzepatide or placebo injections for 18 months (72 weeks).
Efficacy was analyzed in two ways. Prior to factoring in drug discontinuation, participants taking tirzepatide experienced weight loss of 16.0% (35 lb/16 kg) with 5 mg, 21.4% (49 lb/22 kg) with 10 mg, and 22.5% (52 lb/24 kg) on 15 mg. In contrast, the placebo group lost just 2.4% of body weight (5 lb/2 kg).
But treatment discontinuation rates because of adverse events were 4.3%, 7.1%, 6.2%, and 2.6%, for tirzepatide 5 mg, 10 mg, 15 mg, and placebo, respectively. Overall treatment discontinuation rates were 14.3%, 16.4%, 15.1%, and 26.4%, respectively.
When efficacy was assessed regardless of treatment discontinuation, average body weight reductions were 15.0%, 19.5%, 20.9%, and 3.1% for tirzepatide 5 mg, 10 mg, 15 mg, and placebo, respectively.
More than half of patients taking tirzepatide 10 mg and 15 mg (55% and 63%, respectively) lost at least 20% of their body weight, compared with just 1.3% taking placebo.
Overall safety and tolerability were similar to those of other GLP-1 agonists, with adverse events being gastrointestinal in nature and increasing with higher doses. Nausea affected 24.6%, 33.3%, and 31.0% of the tirzepatide 5-mg, 10-mg, and 15-mg dose groups, respectively, and vomiting was experienced by 8.3%, 10.7%, and 12.2% of patients, respectively. Diarrhea and constipation were also reported more often with the drug than placebo.
“Tirzepatide delivered impressive body weight reductions in SURMOUNT-1, which could represent an important step forward for helping the patient and physician partnership treat this complex disease,” said study investigator Louis J. Aronne, MD, director of the Comprehensive Weight Control Center and the Sanford I. Weill Professor of Metabolic Research at Weill Cornell Medicine, New York, in a press release.
Further studies are ongoing for tirzepatide as a potential treatment for obesity or overweight, according to the Lilly statement. SURMOUNT is a phase 3 global clinical development program for tirzepatide that began in late 2019 with over 5,000 people with obesity or overweight across six clinical trials. Results from SURMOUNT-2, SURMOUNT-3, and SURMOUNT-4 are expected in 2023.
Tirzepatide is also being studied as a potential treatment for nonalcoholic fatty liver disease and heart failure with preserved ejection fraction. Studies of tirzepatide in obstructive sleep apnea and of morbidity/mortality in obesity are also planned.
Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly. Dr. Freedhoff has served or is serving as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health and has received a research grant from Novo Nordisk.
A version of this article first appeared on Medscape.com.
More than half of patients taking the two highest doses of tirzepatide as a once-weekly injection lost at least 20% of their body weight in the first phase 3 trial to examine this agent in patients with obesity, but without diabetes, according to preliminary top-line results from the SURMOUNT-1 trial announced by Lilly.
The full results will be reported at an upcoming medical conference and published at a later date, Lilly added.
There was much excitement in response to the news, but others have urged caution and noted that, even if tirzepatide is eventually approved for obesity, one of the major barriers to use in the United States will be insurance coverage.
“Wow (and a double Wow!) 52lb weight loss (22.5%) at highest dose of tirzepatide,” tweeted Sek Kathiresan, MD, a cardiologist who is cofounder of Verve Therapeutics and on leave from Harvard (@skathire).
“Thus far the challenge with GLP-1s [agonists] for management of obesity is that insurance usually isn’t covering them. This makes them unaffordable for most people,” replied James Marroquin, MD, of the University of Texas at Austin. (@Jamesmarroquin).
Yoni Freedhoff, MD, of the University of Ottawa (Ont.) who writes a column for this news organization on obesity, said if tirzepatide pans out, along with other similar agents already on the market for this indication, “the next few decades should see the pharmaceutical management of obesity rival its surgical management.”
Would compete with ‘game-changer’ semaglutide?
Tirzepatide has been dubbed a “twincretin” because it works not only as an agonist of the glucagonlike peptide-1 (GLP-1) receptor, but also of the glucose-dependent insulinotropic polypeptide (GIP) receptor. It has been much hyped based on the results of the series of SURPASS clinical trials, which have formed the basis of the application for type 2 diabetes approval, about which the U.S. Food and Drug Administration is expected to make a decision soon.
Several GLP-1 agonists are on the market for both type 2 diabetes and for obesity indications separately, including semaglutide (marketed as Wegovy for obesity, also a once-weekly injection) and liraglutide (Saxenda for obesity, a daily injection), both Novo Nordisk agents.
Wegovy was approved for weight loss in the United States last year, with doctors telling this news organization then that a third of patients who take the drug are likely to lose 20% or more of their starting weight, an outcome that approaches reductions seen with bariatric surgery.
Dr. Freedhoff said he’d like to see “reimbursement by insurers who will see these drugs serving as important ancillary treatments for the myriad of weight-responsive conditions they’re already covering.”
SURMOUNT-1 data: ‘Impressive body weight’ reductions
The new tirzepatide data come from the multicenter, randomized, double-blind, placebo-controlled SURMOUNT-1 trial, which included 2539 participants from the United States, Argentina, Brazil, China, India, Japan Mexico, Russia, and Taiwan. They had obesity or overweight plus at least one comorbidity but not diabetes. They were randomized to 5-mg, 10-mg, or 15-mg once-weekly tirzepatide or placebo injections for 18 months (72 weeks).
Efficacy was analyzed in two ways. Prior to factoring in drug discontinuation, participants taking tirzepatide experienced weight loss of 16.0% (35 lb/16 kg) with 5 mg, 21.4% (49 lb/22 kg) with 10 mg, and 22.5% (52 lb/24 kg) on 15 mg. In contrast, the placebo group lost just 2.4% of body weight (5 lb/2 kg).
But treatment discontinuation rates because of adverse events were 4.3%, 7.1%, 6.2%, and 2.6%, for tirzepatide 5 mg, 10 mg, 15 mg, and placebo, respectively. Overall treatment discontinuation rates were 14.3%, 16.4%, 15.1%, and 26.4%, respectively.
When efficacy was assessed regardless of treatment discontinuation, average body weight reductions were 15.0%, 19.5%, 20.9%, and 3.1% for tirzepatide 5 mg, 10 mg, 15 mg, and placebo, respectively.
More than half of patients taking tirzepatide 10 mg and 15 mg (55% and 63%, respectively) lost at least 20% of their body weight, compared with just 1.3% taking placebo.
Overall safety and tolerability were similar to those of other GLP-1 agonists, with adverse events being gastrointestinal in nature and increasing with higher doses. Nausea affected 24.6%, 33.3%, and 31.0% of the tirzepatide 5-mg, 10-mg, and 15-mg dose groups, respectively, and vomiting was experienced by 8.3%, 10.7%, and 12.2% of patients, respectively. Diarrhea and constipation were also reported more often with the drug than placebo.
“Tirzepatide delivered impressive body weight reductions in SURMOUNT-1, which could represent an important step forward for helping the patient and physician partnership treat this complex disease,” said study investigator Louis J. Aronne, MD, director of the Comprehensive Weight Control Center and the Sanford I. Weill Professor of Metabolic Research at Weill Cornell Medicine, New York, in a press release.
Further studies are ongoing for tirzepatide as a potential treatment for obesity or overweight, according to the Lilly statement. SURMOUNT is a phase 3 global clinical development program for tirzepatide that began in late 2019 with over 5,000 people with obesity or overweight across six clinical trials. Results from SURMOUNT-2, SURMOUNT-3, and SURMOUNT-4 are expected in 2023.
Tirzepatide is also being studied as a potential treatment for nonalcoholic fatty liver disease and heart failure with preserved ejection fraction. Studies of tirzepatide in obstructive sleep apnea and of morbidity/mortality in obesity are also planned.
Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly. Dr. Freedhoff has served or is serving as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health and has received a research grant from Novo Nordisk.
A version of this article first appeared on Medscape.com.
More than half of patients taking the two highest doses of tirzepatide as a once-weekly injection lost at least 20% of their body weight in the first phase 3 trial to examine this agent in patients with obesity, but without diabetes, according to preliminary top-line results from the SURMOUNT-1 trial announced by Lilly.
The full results will be reported at an upcoming medical conference and published at a later date, Lilly added.
There was much excitement in response to the news, but others have urged caution and noted that, even if tirzepatide is eventually approved for obesity, one of the major barriers to use in the United States will be insurance coverage.
“Wow (and a double Wow!) 52lb weight loss (22.5%) at highest dose of tirzepatide,” tweeted Sek Kathiresan, MD, a cardiologist who is cofounder of Verve Therapeutics and on leave from Harvard (@skathire).
“Thus far the challenge with GLP-1s [agonists] for management of obesity is that insurance usually isn’t covering them. This makes them unaffordable for most people,” replied James Marroquin, MD, of the University of Texas at Austin. (@Jamesmarroquin).
Yoni Freedhoff, MD, of the University of Ottawa (Ont.) who writes a column for this news organization on obesity, said if tirzepatide pans out, along with other similar agents already on the market for this indication, “the next few decades should see the pharmaceutical management of obesity rival its surgical management.”
Would compete with ‘game-changer’ semaglutide?
Tirzepatide has been dubbed a “twincretin” because it works not only as an agonist of the glucagonlike peptide-1 (GLP-1) receptor, but also of the glucose-dependent insulinotropic polypeptide (GIP) receptor. It has been much hyped based on the results of the series of SURPASS clinical trials, which have formed the basis of the application for type 2 diabetes approval, about which the U.S. Food and Drug Administration is expected to make a decision soon.
Several GLP-1 agonists are on the market for both type 2 diabetes and for obesity indications separately, including semaglutide (marketed as Wegovy for obesity, also a once-weekly injection) and liraglutide (Saxenda for obesity, a daily injection), both Novo Nordisk agents.
Wegovy was approved for weight loss in the United States last year, with doctors telling this news organization then that a third of patients who take the drug are likely to lose 20% or more of their starting weight, an outcome that approaches reductions seen with bariatric surgery.
Dr. Freedhoff said he’d like to see “reimbursement by insurers who will see these drugs serving as important ancillary treatments for the myriad of weight-responsive conditions they’re already covering.”
SURMOUNT-1 data: ‘Impressive body weight’ reductions
The new tirzepatide data come from the multicenter, randomized, double-blind, placebo-controlled SURMOUNT-1 trial, which included 2539 participants from the United States, Argentina, Brazil, China, India, Japan Mexico, Russia, and Taiwan. They had obesity or overweight plus at least one comorbidity but not diabetes. They were randomized to 5-mg, 10-mg, or 15-mg once-weekly tirzepatide or placebo injections for 18 months (72 weeks).
Efficacy was analyzed in two ways. Prior to factoring in drug discontinuation, participants taking tirzepatide experienced weight loss of 16.0% (35 lb/16 kg) with 5 mg, 21.4% (49 lb/22 kg) with 10 mg, and 22.5% (52 lb/24 kg) on 15 mg. In contrast, the placebo group lost just 2.4% of body weight (5 lb/2 kg).
But treatment discontinuation rates because of adverse events were 4.3%, 7.1%, 6.2%, and 2.6%, for tirzepatide 5 mg, 10 mg, 15 mg, and placebo, respectively. Overall treatment discontinuation rates were 14.3%, 16.4%, 15.1%, and 26.4%, respectively.
When efficacy was assessed regardless of treatment discontinuation, average body weight reductions were 15.0%, 19.5%, 20.9%, and 3.1% for tirzepatide 5 mg, 10 mg, 15 mg, and placebo, respectively.
More than half of patients taking tirzepatide 10 mg and 15 mg (55% and 63%, respectively) lost at least 20% of their body weight, compared with just 1.3% taking placebo.
Overall safety and tolerability were similar to those of other GLP-1 agonists, with adverse events being gastrointestinal in nature and increasing with higher doses. Nausea affected 24.6%, 33.3%, and 31.0% of the tirzepatide 5-mg, 10-mg, and 15-mg dose groups, respectively, and vomiting was experienced by 8.3%, 10.7%, and 12.2% of patients, respectively. Diarrhea and constipation were also reported more often with the drug than placebo.
“Tirzepatide delivered impressive body weight reductions in SURMOUNT-1, which could represent an important step forward for helping the patient and physician partnership treat this complex disease,” said study investigator Louis J. Aronne, MD, director of the Comprehensive Weight Control Center and the Sanford I. Weill Professor of Metabolic Research at Weill Cornell Medicine, New York, in a press release.
Further studies are ongoing for tirzepatide as a potential treatment for obesity or overweight, according to the Lilly statement. SURMOUNT is a phase 3 global clinical development program for tirzepatide that began in late 2019 with over 5,000 people with obesity or overweight across six clinical trials. Results from SURMOUNT-2, SURMOUNT-3, and SURMOUNT-4 are expected in 2023.
Tirzepatide is also being studied as a potential treatment for nonalcoholic fatty liver disease and heart failure with preserved ejection fraction. Studies of tirzepatide in obstructive sleep apnea and of morbidity/mortality in obesity are also planned.
Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly. Dr. Freedhoff has served or is serving as a director, officer, partner, employee, adviser, consultant, or trustee for the Bariatric Medical Institute and Constant Health and has received a research grant from Novo Nordisk.
A version of this article first appeared on Medscape.com.
Inappropriate antibiotic use in U.S. hospitals increased during pandemic
LISBON – During the pandemic, critical and acute care hospitals with medium and high rates of antimicrobial resistance (AMR) showed significant increases in antibiotic prescriptions and longer durations of antibiotic treatment among all hospital admissions, and also in those patients who were bacterial culture negative, according to a large U.S.-based study.
The analysis across 271 U.S. hospitals also showed that AMR rates were significantly higher for pathogens during the pandemic period, compared with the prepandemic period in patients who were tested for SARS-CoV-2, and highest in SARS-CoV-2–positive patients.
More than a third of SARS-CoV-2–positive patients who were prescribed antibiotics were bacterial culture negative.
Findings of the study were presented by Vikas Gupta, PharmD, director of medical affairs at medical technology firm Becton Dickinson, at this year’s European Congress of Clinical Microbiology & Infectious Diseases. He conducted the study jointly with Karri Bauer, PharmD, from Merck Sharp & Dohme, Kenilworth, N.J., and colleagues.
“There are differences in AMR that go beyond COVID-positive admissions,” Dr. Gupta told this news organization. “There is opportunity for improvement especially with those hospitalized patients who had a negative culture result, or no culture collected.”
“We found a higher percentage of COVID-positive admissions that were prescribed antibacterial therapy even in those having [tested negative for bacteria] or no culture result,” said Dr. Gupta. “Our data also shows that the percentage of admissions with duration of antibacterial therapy over 3 days was significantly higher in COVID-positive but culture-negative/no culture patients, compared to other groups evaluated.”
Of all admissions prescribed antibiotics during the pandemic, 57.8% of SARS-CoV-2–positive patients were prescribed antibiotics whereas 88.1% of SARS-CoV-2–positive admissions were bacterial culture negative/no culture. Overall, prepandemic, 35% of admissions were prescribed antibiotics.
Duration of antibiotic therapy in the prepandemic era was an average of 3.5 days, compared with an average of 3.8 days overall in the pandemic and 5.7 days in patients who tested positive for SARS-CoV-2. Similarly, the percentage of patients who were bacterial culture negative or had no culture and received antibiotic therapy for more than 72 hours was 17.6% in the prepandemic era, compared with 19.2% overall in the pandemic era, and 41.1% in patients who tested positive for COVID-19.
Dr. Gupta and Dr. Bauer wanted to look at all patients admitted to hospitals segmented by SARS-CoV-2 positive, negative, and not tested, to get a sense of how much antibiotic use there was and how long patients were on antibiotics. “We ultimately want to optimize and not overuse antibiotics and prescribe them for right period of time,” said Dr. Gupta.
“To date, there has been no conclusive evidence about the suggestion that the pandemic has led to increased AMR rates, so we aimed to evaluate the pandemic’s impact on AMR and antibiotic use across U.S. hospitals,” he explained.
The multicenter, retrospective cohort analysis made use of BD’s infection surveillance platform (BD HealthSight Infection Advisor with MedMined Insights) and was conducted across 271 U.S. critical access/acute care facilities, representing approximately 10%-13% of U.S. hospital admissions. It included all hospitalized patients with more than 1 day of in-patient admission. Patients were considered SARS-CoV-2 positive by polymerase chain reaction test or antigen test either 7 days or less prior to or within 14 days of admission.
Patients were categorized as hospitalized during the “prepandemic” period (July 1, 2019 through February 29, 2020) and the “pandemic” period (March 1, 2020 through Oct. 30, 2021) and were stratified based on their SARS-CoV-2 result.
Investigators included all hospital admissions with an AMR event (first positive culture for select gram-negative or gram-positive pathogens that were reported as nonsusceptible across blood, urine, respiratory, intra-abdominal, skin/wound, and other sources).
The investigators calculated AMR rates at the patient-admission level and defined per 100 admissions. Also, they further evaluated AMR rates based on community onset (defined as culture collected ≤2 days from admission) or hospital onset (>2 days from admission). Finally, AMR rates were determined according to whether they related to prepandemic or pandemic periods.
Hospitals were also categorized according to their AMR rates as low (<25%), medium (25%-75%), and high (>75%).
Overall AMR rates were lower in the pandemic period, compared with the prepandemic period. However, reported Dr.Gupta, for hospital-onset pathogens specifically, AMR rates were significantly higher overall in the pandemic period and mostly driven by admissions tested for SARS-CoV-2 (whether positive or negative).
Hospitals with high AMR rates also tended to have more SARS-CoV-2 positive admissions (6.1% in high-AMR hospitals vs. 3% in low-AMR hospitals). The highest antibiotic-prescribing rates and highest duration of antibiotic use was also seen in those hospitals with highest AMR rates.
Of the SARS-CoV-2 patients who were bacterial culture negative/no culture and were prescribed antibiotics, 36.5% were in hospitals with a high AMR rate. “Roughly one-third of patients without culture evidence of a bacterial infection were prescribed antibiotics in hospitals with a high AMR rate,” said Dr. Gupta.
The researchers wanted to tease out whether hospitals with high, moderate, or low AMR rates look different with respect to antibiotic-prescribing patterns. During the pandemic period, they found that hospitals with high and medium AMR rates experienced significant increases in antibiotic prescriptions and longer durations. Prepandemic, the overall hospital-onset AMR rate was 0.8 per 100 admissions, whereas during the pandemic this rose to 1.4 per 100 admissions in high-AMR hospitals and dropped to 0.4 in low-AMR hospitals.
SARS-CoV-2–positive admission rates were higher in facilities with medium (5.6%) and high AMR (6.1%) rates than those with low (3%) AMR rates. “We found that those with medium and high AMR rates were more likely to have COVID-positive admissions than facilities with low AMR rates,” Dr. Gupta said. “It appears as if COVID is contributing to AMR in the facilities.”
Asked for independent comment, Jason C. Gallagher, PharmD, BCPS, clinical professor at Temple University School of Pharmacy in Philadelphia, said in an interview, “It is not surprising that there was more antimicrobial resistance in patients with COVID than those without. Even though antibiotics do not work for COVID, they are often prescribed, and antibiotic use is a major risk factor for antimicrobial resistance. This is likely because clinicians are sometimes concerned about coinfections with bacteria (which are rare) and because hospitalized patients with severe COVID can acquire other infections as they are treated.”
Antibiotic stewardship programs
Antibiotic stewardship programs have been highly stressed during the pandemic, so the researchers hope their data support the need for better antibiotic stewardship practices during pandemic surges when control is more challenging.
Dr. Gupta explained that they were seeing interesting associations that can inform antimicrobial stewardship programs and teams. “We are not trying to imply causality,” he stressed.
It is a common practice for stewardship teams to evaluate the need for continuation of antibiotic therapy at 3 days, especially in patients who are culture negative or did not have a culture collected.
“Antibiotic time-out at 3 days is a recommended practice to evaluate for continuing antibiotic therapy based on the patient’s condition and culture results,” he said. “This is what made our study unique because we wanted to look at what percentage of admissions were prescribed antibiotics beyond 3 days and compare to the prepandemic period.”
Session moderator Evangelos J. Giamarellos-Bourboulis, MD, PhD, an assistant professor of internal medicine and infectious diseases, University of Athens, Greece, thanked Dr. Gupta for his “eloquent presentation” and sought to clarify whether the data “refer to antimicrobial use that was empirical or whether use was in hospitals with high AMR rates, or whether the approach was driven through microbiology?”
Dr. Gupta replied that this was why they evaluated the negative-culture and no-culture patients. “We wanted to get a measure of antibacterial use in this population too,” he said. “Definitely, there is empirical therapy as well as definitive therapy, but I think the negative and no-culture group provide a reference point where we see similar signals and trends to that of the overall population.”
An audience member also addressed a question to Dr. Gupta: “Did you look at the patient population, because in many cases, during COVID, these patients may have been more severe than in the prepandemic period?”
Dr. Gupta replied: “In our manuscript we’ve done an analysis where we adjusted for patient-level facility and regional-level factors. There are definitely differences in the patient populations but overall, these are pretty sick patients when we look at the level of severity overall.”
Dr. Gupta is an employee of and a shareholder in Becton Dickinson. Dr. Bauer is an employee of and a shareholder in Merck. Dr. Gallagher consults for many pharmaceutical companies including Merck.
Dr. Giamarellos-Bourboulis disclosed honoraria (paid to the University of Athens) from Abbott CH, Brahms Thermo Fisher GMBH Germany, GlaxoSmithKline, and Sobi; serving as a consultant for Abbott CH, Fab’nTech, InflaRx GmbH, UCB, Sobi, and Xbiotech; research grants (paid to the Hellenic Institute for the Study of Sepsis) from Abbott CH, BioMerieux France, Johnson & Johnson, MSD, Sobi, Thermo Fisher Brahms GmbH; and EU research funding: Horizon 2020 ITN European Sepsis Academy (granted to the University of Athens); Horizon 2020 ImmunoSep and RISinCOVID (granted to the Hellenic Institute for the Study of Sepsis); Horizon Health EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis).
A version of this article first appeared on Medscape.com.
LISBON – During the pandemic, critical and acute care hospitals with medium and high rates of antimicrobial resistance (AMR) showed significant increases in antibiotic prescriptions and longer durations of antibiotic treatment among all hospital admissions, and also in those patients who were bacterial culture negative, according to a large U.S.-based study.
The analysis across 271 U.S. hospitals also showed that AMR rates were significantly higher for pathogens during the pandemic period, compared with the prepandemic period in patients who were tested for SARS-CoV-2, and highest in SARS-CoV-2–positive patients.
More than a third of SARS-CoV-2–positive patients who were prescribed antibiotics were bacterial culture negative.
Findings of the study were presented by Vikas Gupta, PharmD, director of medical affairs at medical technology firm Becton Dickinson, at this year’s European Congress of Clinical Microbiology & Infectious Diseases. He conducted the study jointly with Karri Bauer, PharmD, from Merck Sharp & Dohme, Kenilworth, N.J., and colleagues.
“There are differences in AMR that go beyond COVID-positive admissions,” Dr. Gupta told this news organization. “There is opportunity for improvement especially with those hospitalized patients who had a negative culture result, or no culture collected.”
“We found a higher percentage of COVID-positive admissions that were prescribed antibacterial therapy even in those having [tested negative for bacteria] or no culture result,” said Dr. Gupta. “Our data also shows that the percentage of admissions with duration of antibacterial therapy over 3 days was significantly higher in COVID-positive but culture-negative/no culture patients, compared to other groups evaluated.”
Of all admissions prescribed antibiotics during the pandemic, 57.8% of SARS-CoV-2–positive patients were prescribed antibiotics whereas 88.1% of SARS-CoV-2–positive admissions were bacterial culture negative/no culture. Overall, prepandemic, 35% of admissions were prescribed antibiotics.
Duration of antibiotic therapy in the prepandemic era was an average of 3.5 days, compared with an average of 3.8 days overall in the pandemic and 5.7 days in patients who tested positive for SARS-CoV-2. Similarly, the percentage of patients who were bacterial culture negative or had no culture and received antibiotic therapy for more than 72 hours was 17.6% in the prepandemic era, compared with 19.2% overall in the pandemic era, and 41.1% in patients who tested positive for COVID-19.
Dr. Gupta and Dr. Bauer wanted to look at all patients admitted to hospitals segmented by SARS-CoV-2 positive, negative, and not tested, to get a sense of how much antibiotic use there was and how long patients were on antibiotics. “We ultimately want to optimize and not overuse antibiotics and prescribe them for right period of time,” said Dr. Gupta.
“To date, there has been no conclusive evidence about the suggestion that the pandemic has led to increased AMR rates, so we aimed to evaluate the pandemic’s impact on AMR and antibiotic use across U.S. hospitals,” he explained.
The multicenter, retrospective cohort analysis made use of BD’s infection surveillance platform (BD HealthSight Infection Advisor with MedMined Insights) and was conducted across 271 U.S. critical access/acute care facilities, representing approximately 10%-13% of U.S. hospital admissions. It included all hospitalized patients with more than 1 day of in-patient admission. Patients were considered SARS-CoV-2 positive by polymerase chain reaction test or antigen test either 7 days or less prior to or within 14 days of admission.
Patients were categorized as hospitalized during the “prepandemic” period (July 1, 2019 through February 29, 2020) and the “pandemic” period (March 1, 2020 through Oct. 30, 2021) and were stratified based on their SARS-CoV-2 result.
Investigators included all hospital admissions with an AMR event (first positive culture for select gram-negative or gram-positive pathogens that were reported as nonsusceptible across blood, urine, respiratory, intra-abdominal, skin/wound, and other sources).
The investigators calculated AMR rates at the patient-admission level and defined per 100 admissions. Also, they further evaluated AMR rates based on community onset (defined as culture collected ≤2 days from admission) or hospital onset (>2 days from admission). Finally, AMR rates were determined according to whether they related to prepandemic or pandemic periods.
Hospitals were also categorized according to their AMR rates as low (<25%), medium (25%-75%), and high (>75%).
Overall AMR rates were lower in the pandemic period, compared with the prepandemic period. However, reported Dr.Gupta, for hospital-onset pathogens specifically, AMR rates were significantly higher overall in the pandemic period and mostly driven by admissions tested for SARS-CoV-2 (whether positive or negative).
Hospitals with high AMR rates also tended to have more SARS-CoV-2 positive admissions (6.1% in high-AMR hospitals vs. 3% in low-AMR hospitals). The highest antibiotic-prescribing rates and highest duration of antibiotic use was also seen in those hospitals with highest AMR rates.
Of the SARS-CoV-2 patients who were bacterial culture negative/no culture and were prescribed antibiotics, 36.5% were in hospitals with a high AMR rate. “Roughly one-third of patients without culture evidence of a bacterial infection were prescribed antibiotics in hospitals with a high AMR rate,” said Dr. Gupta.
The researchers wanted to tease out whether hospitals with high, moderate, or low AMR rates look different with respect to antibiotic-prescribing patterns. During the pandemic period, they found that hospitals with high and medium AMR rates experienced significant increases in antibiotic prescriptions and longer durations. Prepandemic, the overall hospital-onset AMR rate was 0.8 per 100 admissions, whereas during the pandemic this rose to 1.4 per 100 admissions in high-AMR hospitals and dropped to 0.4 in low-AMR hospitals.
SARS-CoV-2–positive admission rates were higher in facilities with medium (5.6%) and high AMR (6.1%) rates than those with low (3%) AMR rates. “We found that those with medium and high AMR rates were more likely to have COVID-positive admissions than facilities with low AMR rates,” Dr. Gupta said. “It appears as if COVID is contributing to AMR in the facilities.”
Asked for independent comment, Jason C. Gallagher, PharmD, BCPS, clinical professor at Temple University School of Pharmacy in Philadelphia, said in an interview, “It is not surprising that there was more antimicrobial resistance in patients with COVID than those without. Even though antibiotics do not work for COVID, they are often prescribed, and antibiotic use is a major risk factor for antimicrobial resistance. This is likely because clinicians are sometimes concerned about coinfections with bacteria (which are rare) and because hospitalized patients with severe COVID can acquire other infections as they are treated.”
Antibiotic stewardship programs
Antibiotic stewardship programs have been highly stressed during the pandemic, so the researchers hope their data support the need for better antibiotic stewardship practices during pandemic surges when control is more challenging.
Dr. Gupta explained that they were seeing interesting associations that can inform antimicrobial stewardship programs and teams. “We are not trying to imply causality,” he stressed.
It is a common practice for stewardship teams to evaluate the need for continuation of antibiotic therapy at 3 days, especially in patients who are culture negative or did not have a culture collected.
“Antibiotic time-out at 3 days is a recommended practice to evaluate for continuing antibiotic therapy based on the patient’s condition and culture results,” he said. “This is what made our study unique because we wanted to look at what percentage of admissions were prescribed antibiotics beyond 3 days and compare to the prepandemic period.”
Session moderator Evangelos J. Giamarellos-Bourboulis, MD, PhD, an assistant professor of internal medicine and infectious diseases, University of Athens, Greece, thanked Dr. Gupta for his “eloquent presentation” and sought to clarify whether the data “refer to antimicrobial use that was empirical or whether use was in hospitals with high AMR rates, or whether the approach was driven through microbiology?”
Dr. Gupta replied that this was why they evaluated the negative-culture and no-culture patients. “We wanted to get a measure of antibacterial use in this population too,” he said. “Definitely, there is empirical therapy as well as definitive therapy, but I think the negative and no-culture group provide a reference point where we see similar signals and trends to that of the overall population.”
An audience member also addressed a question to Dr. Gupta: “Did you look at the patient population, because in many cases, during COVID, these patients may have been more severe than in the prepandemic period?”
Dr. Gupta replied: “In our manuscript we’ve done an analysis where we adjusted for patient-level facility and regional-level factors. There are definitely differences in the patient populations but overall, these are pretty sick patients when we look at the level of severity overall.”
Dr. Gupta is an employee of and a shareholder in Becton Dickinson. Dr. Bauer is an employee of and a shareholder in Merck. Dr. Gallagher consults for many pharmaceutical companies including Merck.
Dr. Giamarellos-Bourboulis disclosed honoraria (paid to the University of Athens) from Abbott CH, Brahms Thermo Fisher GMBH Germany, GlaxoSmithKline, and Sobi; serving as a consultant for Abbott CH, Fab’nTech, InflaRx GmbH, UCB, Sobi, and Xbiotech; research grants (paid to the Hellenic Institute for the Study of Sepsis) from Abbott CH, BioMerieux France, Johnson & Johnson, MSD, Sobi, Thermo Fisher Brahms GmbH; and EU research funding: Horizon 2020 ITN European Sepsis Academy (granted to the University of Athens); Horizon 2020 ImmunoSep and RISinCOVID (granted to the Hellenic Institute for the Study of Sepsis); Horizon Health EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis).
A version of this article first appeared on Medscape.com.
LISBON – During the pandemic, critical and acute care hospitals with medium and high rates of antimicrobial resistance (AMR) showed significant increases in antibiotic prescriptions and longer durations of antibiotic treatment among all hospital admissions, and also in those patients who were bacterial culture negative, according to a large U.S.-based study.
The analysis across 271 U.S. hospitals also showed that AMR rates were significantly higher for pathogens during the pandemic period, compared with the prepandemic period in patients who were tested for SARS-CoV-2, and highest in SARS-CoV-2–positive patients.
More than a third of SARS-CoV-2–positive patients who were prescribed antibiotics were bacterial culture negative.
Findings of the study were presented by Vikas Gupta, PharmD, director of medical affairs at medical technology firm Becton Dickinson, at this year’s European Congress of Clinical Microbiology & Infectious Diseases. He conducted the study jointly with Karri Bauer, PharmD, from Merck Sharp & Dohme, Kenilworth, N.J., and colleagues.
“There are differences in AMR that go beyond COVID-positive admissions,” Dr. Gupta told this news organization. “There is opportunity for improvement especially with those hospitalized patients who had a negative culture result, or no culture collected.”
“We found a higher percentage of COVID-positive admissions that were prescribed antibacterial therapy even in those having [tested negative for bacteria] or no culture result,” said Dr. Gupta. “Our data also shows that the percentage of admissions with duration of antibacterial therapy over 3 days was significantly higher in COVID-positive but culture-negative/no culture patients, compared to other groups evaluated.”
Of all admissions prescribed antibiotics during the pandemic, 57.8% of SARS-CoV-2–positive patients were prescribed antibiotics whereas 88.1% of SARS-CoV-2–positive admissions were bacterial culture negative/no culture. Overall, prepandemic, 35% of admissions were prescribed antibiotics.
Duration of antibiotic therapy in the prepandemic era was an average of 3.5 days, compared with an average of 3.8 days overall in the pandemic and 5.7 days in patients who tested positive for SARS-CoV-2. Similarly, the percentage of patients who were bacterial culture negative or had no culture and received antibiotic therapy for more than 72 hours was 17.6% in the prepandemic era, compared with 19.2% overall in the pandemic era, and 41.1% in patients who tested positive for COVID-19.
Dr. Gupta and Dr. Bauer wanted to look at all patients admitted to hospitals segmented by SARS-CoV-2 positive, negative, and not tested, to get a sense of how much antibiotic use there was and how long patients were on antibiotics. “We ultimately want to optimize and not overuse antibiotics and prescribe them for right period of time,” said Dr. Gupta.
“To date, there has been no conclusive evidence about the suggestion that the pandemic has led to increased AMR rates, so we aimed to evaluate the pandemic’s impact on AMR and antibiotic use across U.S. hospitals,” he explained.
The multicenter, retrospective cohort analysis made use of BD’s infection surveillance platform (BD HealthSight Infection Advisor with MedMined Insights) and was conducted across 271 U.S. critical access/acute care facilities, representing approximately 10%-13% of U.S. hospital admissions. It included all hospitalized patients with more than 1 day of in-patient admission. Patients were considered SARS-CoV-2 positive by polymerase chain reaction test or antigen test either 7 days or less prior to or within 14 days of admission.
Patients were categorized as hospitalized during the “prepandemic” period (July 1, 2019 through February 29, 2020) and the “pandemic” period (March 1, 2020 through Oct. 30, 2021) and were stratified based on their SARS-CoV-2 result.
Investigators included all hospital admissions with an AMR event (first positive culture for select gram-negative or gram-positive pathogens that were reported as nonsusceptible across blood, urine, respiratory, intra-abdominal, skin/wound, and other sources).
The investigators calculated AMR rates at the patient-admission level and defined per 100 admissions. Also, they further evaluated AMR rates based on community onset (defined as culture collected ≤2 days from admission) or hospital onset (>2 days from admission). Finally, AMR rates were determined according to whether they related to prepandemic or pandemic periods.
Hospitals were also categorized according to their AMR rates as low (<25%), medium (25%-75%), and high (>75%).
Overall AMR rates were lower in the pandemic period, compared with the prepandemic period. However, reported Dr.Gupta, for hospital-onset pathogens specifically, AMR rates were significantly higher overall in the pandemic period and mostly driven by admissions tested for SARS-CoV-2 (whether positive or negative).
Hospitals with high AMR rates also tended to have more SARS-CoV-2 positive admissions (6.1% in high-AMR hospitals vs. 3% in low-AMR hospitals). The highest antibiotic-prescribing rates and highest duration of antibiotic use was also seen in those hospitals with highest AMR rates.
Of the SARS-CoV-2 patients who were bacterial culture negative/no culture and were prescribed antibiotics, 36.5% were in hospitals with a high AMR rate. “Roughly one-third of patients without culture evidence of a bacterial infection were prescribed antibiotics in hospitals with a high AMR rate,” said Dr. Gupta.
The researchers wanted to tease out whether hospitals with high, moderate, or low AMR rates look different with respect to antibiotic-prescribing patterns. During the pandemic period, they found that hospitals with high and medium AMR rates experienced significant increases in antibiotic prescriptions and longer durations. Prepandemic, the overall hospital-onset AMR rate was 0.8 per 100 admissions, whereas during the pandemic this rose to 1.4 per 100 admissions in high-AMR hospitals and dropped to 0.4 in low-AMR hospitals.
SARS-CoV-2–positive admission rates were higher in facilities with medium (5.6%) and high AMR (6.1%) rates than those with low (3%) AMR rates. “We found that those with medium and high AMR rates were more likely to have COVID-positive admissions than facilities with low AMR rates,” Dr. Gupta said. “It appears as if COVID is contributing to AMR in the facilities.”
Asked for independent comment, Jason C. Gallagher, PharmD, BCPS, clinical professor at Temple University School of Pharmacy in Philadelphia, said in an interview, “It is not surprising that there was more antimicrobial resistance in patients with COVID than those without. Even though antibiotics do not work for COVID, they are often prescribed, and antibiotic use is a major risk factor for antimicrobial resistance. This is likely because clinicians are sometimes concerned about coinfections with bacteria (which are rare) and because hospitalized patients with severe COVID can acquire other infections as they are treated.”
Antibiotic stewardship programs
Antibiotic stewardship programs have been highly stressed during the pandemic, so the researchers hope their data support the need for better antibiotic stewardship practices during pandemic surges when control is more challenging.
Dr. Gupta explained that they were seeing interesting associations that can inform antimicrobial stewardship programs and teams. “We are not trying to imply causality,” he stressed.
It is a common practice for stewardship teams to evaluate the need for continuation of antibiotic therapy at 3 days, especially in patients who are culture negative or did not have a culture collected.
“Antibiotic time-out at 3 days is a recommended practice to evaluate for continuing antibiotic therapy based on the patient’s condition and culture results,” he said. “This is what made our study unique because we wanted to look at what percentage of admissions were prescribed antibiotics beyond 3 days and compare to the prepandemic period.”
Session moderator Evangelos J. Giamarellos-Bourboulis, MD, PhD, an assistant professor of internal medicine and infectious diseases, University of Athens, Greece, thanked Dr. Gupta for his “eloquent presentation” and sought to clarify whether the data “refer to antimicrobial use that was empirical or whether use was in hospitals with high AMR rates, or whether the approach was driven through microbiology?”
Dr. Gupta replied that this was why they evaluated the negative-culture and no-culture patients. “We wanted to get a measure of antibacterial use in this population too,” he said. “Definitely, there is empirical therapy as well as definitive therapy, but I think the negative and no-culture group provide a reference point where we see similar signals and trends to that of the overall population.”
An audience member also addressed a question to Dr. Gupta: “Did you look at the patient population, because in many cases, during COVID, these patients may have been more severe than in the prepandemic period?”
Dr. Gupta replied: “In our manuscript we’ve done an analysis where we adjusted for patient-level facility and regional-level factors. There are definitely differences in the patient populations but overall, these are pretty sick patients when we look at the level of severity overall.”
Dr. Gupta is an employee of and a shareholder in Becton Dickinson. Dr. Bauer is an employee of and a shareholder in Merck. Dr. Gallagher consults for many pharmaceutical companies including Merck.
Dr. Giamarellos-Bourboulis disclosed honoraria (paid to the University of Athens) from Abbott CH, Brahms Thermo Fisher GMBH Germany, GlaxoSmithKline, and Sobi; serving as a consultant for Abbott CH, Fab’nTech, InflaRx GmbH, UCB, Sobi, and Xbiotech; research grants (paid to the Hellenic Institute for the Study of Sepsis) from Abbott CH, BioMerieux France, Johnson & Johnson, MSD, Sobi, Thermo Fisher Brahms GmbH; and EU research funding: Horizon 2020 ITN European Sepsis Academy (granted to the University of Athens); Horizon 2020 ImmunoSep and RISinCOVID (granted to the Hellenic Institute for the Study of Sepsis); Horizon Health EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis).
A version of this article first appeared on Medscape.com.
Long-COVID symptoms a serious challenge for older patients, physicians
Even mundane tasks such as making a meal can be exhausting for Louise Salant.
“I’m totally wiped out,” said the 71-year-old former private music instructor with asthma who lives in New York City and has been coping with debilitating symptoms of fatigue, shortness of breath, and gastrointestinal symptoms since recovering from a severe bout of COVID-19 2 years ago. “I just don’t have the energy.”
Ms. Salant is not alone. Many older people who contract COVID-19 experience prolonged symptoms of the disease. An analysis of Medicare Advantage claims data published in the BMJ found that about one-third of roughly 87,000 adults aged 65 in the database with a COVID-19 diagnosis sought care for persistent or new symptoms 21 or more days later.
That figure is about twice the rate of persistent COVID-19 related symptoms seen in a cohort of adults younger than age 65 with commercial insurance analyzed by the same group of researchers in a separate BMJ study. Compared with a 2020 comparator group of patients in this age cohort, these patients had a greater likelihood of respiratory failure, fatigue, hypertension, memory problems, kidney injury, mental health conditions, hypercoagulability, and cardiac rhythm disorders. When they compared post–COVID-19 symptoms to lasting symptoms of another serious viral disease – influenza – the researchers found that only respiratory failure, dementia, and post-viral fatigue were more common in the COVID-19 group.
“It became clear early in the pandemic that there is going to be a second pandemic related to all of the complications that we’ve seen related to COVID-19 infections,” said Ken Cohen, MD, executive director of translational research and national senior medical director for Optum Labs in Minnetonka, Minn., who coauthored the BMJ studies.
The results are among a growing body of evidence suggesting that older adults are at high risk of persistent post-COVID-19 symptoms.
Researchers in Rome, for example, found that 83% of 165 patients aged 65 or older who had been hospitalized for COVID-19 reported at least one lasting symptom – problems like fatigue, shortness of breath, joint pain, and coughing – in the months after hospitalization. One-third of those had two symptoms, and 46% had three or more.
A similar study in Norway found that two-thirds of patients aged 60 or older reported reduced health-related quality of life during follow-up visits 6 months after hospitalization for COVID-19. The most-reported impairments among those patients were the inability to perform the tasks of daily life, reduced mobility, and increased pain and discomfort.
Cognitive concerns
Mounting evidence indicates that COVID-19 may contribute to chronic cognitive impairment in older adults. A multisite U.S. study found that 28% of 817 adults presenting to emergency departments with COVID-19 had delirium and poorer outcomes. A Chinese case-control study that enrolled 1,438 individuals hospitalized in Wuhan for COVID-19, along with 438 of their uninfected spouses, found that 12% of COVID-19 survivors experienced cognitive impairment a year after discharge. Matteo Tosato, MD, PhD, head of the outpatient clinic for patients with long COVID symptoms at Gemelli Hospital in Rome, called those findings “very concerning.”
Jin Ho Han, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tenn., said cognitive impairment is common after an acute illness, particularly in frail or vulnerable patients.
“Hospitalization and the acute illness itself accelerate cognitive decline,” said Dr. Han, and previous evidence links delirium with worsening cognition. He and his colleagues are studying the potential role of delirium in longer-term cognitive decline in older patients after COVID-19.
Dr. Han emphasized the importance of preventing COVID-19-related delirium through vaccines and other strategies to reduce exposure of older patients to the virus. “Once you have cognitive decline, there are no interventions to reverse it,” he said.
Alarm bells for long-term care
Experts expressed concern that the situation might be even worse for people living in long-term care facilities. Many already need assistance with tasks of daily living and could be particularly vulnerable to lasting effects of COVID-19, said Karl Steinberg, MD, president of the Society for Post-Acute and Long-Term Care Medicine. He estimated that roughly half of his patients who have had COVID-19, regardless of the severity of their symptoms, have endured some degree of functional decline.
“It’s common for long-term care facility residents to experience functional and cognitive decline, even after seemingly minor things, like a cold or a trip to the hospital,” Dr. Steinberg, who has been a medical director of long-term care facilities in San Diego County for more than 2 decades, told this news organization. “It makes it a little harder to determine whether the declines we’ve been seeing post COVID in these residents are attributable to post COVID versus just an accelerated step in their overall expected decline.”
The pandemic may have contributed to worse outcomes for people in long-term care facilities in several ways: the disease itself, its effects on health care delivery, and necessary preventive measures to protect long-term care residents from exposure to the virus.
“During the many months where family visits were prohibited, we saw people – whether they had COVID-19 or not – suffer major clinical, functional, cognitive declines or severe psychological symptoms,” Dr. Steinberg said.
He emphasized the importance of preventive measures such as vaccines and boosters in patients in long-term care facilities. He said the benefit of preventing lasting symptoms is often a strong motivator for family caregivers of people with dementia to get them vaccinated or boosted.
“It’s clear that vaccination and booster reduce the incidence of post-COVID symptoms,” he said. Almost all studies have been in younger cohorts, but he expects the benefits would also apply to older patients.
Easing symptoms and offering support
As with long COVID generally, many questions remain about the causes of lasting symptoms of COVID-19 in older patients, and how best to treat them. Dr. Tosato, who led the study of long-COVID patients in Rome, is focusing on inflammation as a critical factor in the condition. He and colleagues across Europe hope to answer some of them by launching a multicenter study of lasting COVID-19 symptoms.
In the meantime, Dr. Steinberg and Dr. Tosato said they are doing their best to evaluate and treat patients empirically.
“We pull from our armamentarium to treat system-specific symptoms,” Dr. Steinberg said. “We want to improve the quality of life and help each day be the best it can.”
Physicians in long-term care facilities might use medications such as antidepressants or nonpharmacologic approaches for patients experiencing depression symptoms. Families are also crucial in helping patients by bringing in home-cooked meals and encouraging loved ones who may be experiencing loss of taste or smell to eat, Dr. Steinberg said.
“We’ve seen with the return of families and loved ones visiting to some extent has alleviated some people’s symptoms, especially psychological ones,” he said.
Dr. Tosato said he and his colleagues start with an individualized, multidisciplinary assessment to determine what types of care may help. He noted that physicians might recommend medications or rehabilitative therapies depending on the patient’s needs.
“A personalized approach is key,” Dr. Tosato said. His study also found that the proportion of older patients experiencing symptoms declined over time – a glimmer of hope that many will recover.
Dr. Cohen emphasized the need for a multimodal rehabilitation, an evidence-based approach used to care for patients who survived hospitalization with severe COVID-19 – a group that has substantially higher rates of persistent symptoms. This approach includes cognitive rehabilitation, physical therapy, occupational therapy, and a graded exercise program.
Dr. Han and colleagues are studying potential therapies such as cognitive rehabilitation in adults who’ve experienced delirium. But until evidence-based treatments are available, they stress the role of support for patients with cognitive decline and their families.
“A lot of the work we do is teach patients and their families to compensate for newly acquired cognitive deficits from any illness, including COVID-19,” Dr. Han said.
Ms. Salant said she has experienced some improvement in her energy since her pulmonologist recommended a new inhaler based on her symptoms. Her sense of smell and taste, lost to the infection, returned after she received her first dose of a vaccine against COVID-19. She takes comfort in participating in Survivor Corps, a group of more than 170,000 COVID-19 survivors and their families who advocate for more scientific research on the disease.
She also expressed gratitude for the support she receives from her primary care physician, who she said has taken the time to learn more about the symptoms of long COVID, listens to her, and respects what she has to say.
“I have hope that I will keep getting better by baby steps,” Ms. Salant said.
Dr. Tosato, Dr. Steinberg, and Dr. Han have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even mundane tasks such as making a meal can be exhausting for Louise Salant.
“I’m totally wiped out,” said the 71-year-old former private music instructor with asthma who lives in New York City and has been coping with debilitating symptoms of fatigue, shortness of breath, and gastrointestinal symptoms since recovering from a severe bout of COVID-19 2 years ago. “I just don’t have the energy.”
Ms. Salant is not alone. Many older people who contract COVID-19 experience prolonged symptoms of the disease. An analysis of Medicare Advantage claims data published in the BMJ found that about one-third of roughly 87,000 adults aged 65 in the database with a COVID-19 diagnosis sought care for persistent or new symptoms 21 or more days later.
That figure is about twice the rate of persistent COVID-19 related symptoms seen in a cohort of adults younger than age 65 with commercial insurance analyzed by the same group of researchers in a separate BMJ study. Compared with a 2020 comparator group of patients in this age cohort, these patients had a greater likelihood of respiratory failure, fatigue, hypertension, memory problems, kidney injury, mental health conditions, hypercoagulability, and cardiac rhythm disorders. When they compared post–COVID-19 symptoms to lasting symptoms of another serious viral disease – influenza – the researchers found that only respiratory failure, dementia, and post-viral fatigue were more common in the COVID-19 group.
“It became clear early in the pandemic that there is going to be a second pandemic related to all of the complications that we’ve seen related to COVID-19 infections,” said Ken Cohen, MD, executive director of translational research and national senior medical director for Optum Labs in Minnetonka, Minn., who coauthored the BMJ studies.
The results are among a growing body of evidence suggesting that older adults are at high risk of persistent post-COVID-19 symptoms.
Researchers in Rome, for example, found that 83% of 165 patients aged 65 or older who had been hospitalized for COVID-19 reported at least one lasting symptom – problems like fatigue, shortness of breath, joint pain, and coughing – in the months after hospitalization. One-third of those had two symptoms, and 46% had three or more.
A similar study in Norway found that two-thirds of patients aged 60 or older reported reduced health-related quality of life during follow-up visits 6 months after hospitalization for COVID-19. The most-reported impairments among those patients were the inability to perform the tasks of daily life, reduced mobility, and increased pain and discomfort.
Cognitive concerns
Mounting evidence indicates that COVID-19 may contribute to chronic cognitive impairment in older adults. A multisite U.S. study found that 28% of 817 adults presenting to emergency departments with COVID-19 had delirium and poorer outcomes. A Chinese case-control study that enrolled 1,438 individuals hospitalized in Wuhan for COVID-19, along with 438 of their uninfected spouses, found that 12% of COVID-19 survivors experienced cognitive impairment a year after discharge. Matteo Tosato, MD, PhD, head of the outpatient clinic for patients with long COVID symptoms at Gemelli Hospital in Rome, called those findings “very concerning.”
Jin Ho Han, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tenn., said cognitive impairment is common after an acute illness, particularly in frail or vulnerable patients.
“Hospitalization and the acute illness itself accelerate cognitive decline,” said Dr. Han, and previous evidence links delirium with worsening cognition. He and his colleagues are studying the potential role of delirium in longer-term cognitive decline in older patients after COVID-19.
Dr. Han emphasized the importance of preventing COVID-19-related delirium through vaccines and other strategies to reduce exposure of older patients to the virus. “Once you have cognitive decline, there are no interventions to reverse it,” he said.
Alarm bells for long-term care
Experts expressed concern that the situation might be even worse for people living in long-term care facilities. Many already need assistance with tasks of daily living and could be particularly vulnerable to lasting effects of COVID-19, said Karl Steinberg, MD, president of the Society for Post-Acute and Long-Term Care Medicine. He estimated that roughly half of his patients who have had COVID-19, regardless of the severity of their symptoms, have endured some degree of functional decline.
“It’s common for long-term care facility residents to experience functional and cognitive decline, even after seemingly minor things, like a cold or a trip to the hospital,” Dr. Steinberg, who has been a medical director of long-term care facilities in San Diego County for more than 2 decades, told this news organization. “It makes it a little harder to determine whether the declines we’ve been seeing post COVID in these residents are attributable to post COVID versus just an accelerated step in their overall expected decline.”
The pandemic may have contributed to worse outcomes for people in long-term care facilities in several ways: the disease itself, its effects on health care delivery, and necessary preventive measures to protect long-term care residents from exposure to the virus.
“During the many months where family visits were prohibited, we saw people – whether they had COVID-19 or not – suffer major clinical, functional, cognitive declines or severe psychological symptoms,” Dr. Steinberg said.
He emphasized the importance of preventive measures such as vaccines and boosters in patients in long-term care facilities. He said the benefit of preventing lasting symptoms is often a strong motivator for family caregivers of people with dementia to get them vaccinated or boosted.
“It’s clear that vaccination and booster reduce the incidence of post-COVID symptoms,” he said. Almost all studies have been in younger cohorts, but he expects the benefits would also apply to older patients.
Easing symptoms and offering support
As with long COVID generally, many questions remain about the causes of lasting symptoms of COVID-19 in older patients, and how best to treat them. Dr. Tosato, who led the study of long-COVID patients in Rome, is focusing on inflammation as a critical factor in the condition. He and colleagues across Europe hope to answer some of them by launching a multicenter study of lasting COVID-19 symptoms.
In the meantime, Dr. Steinberg and Dr. Tosato said they are doing their best to evaluate and treat patients empirically.
“We pull from our armamentarium to treat system-specific symptoms,” Dr. Steinberg said. “We want to improve the quality of life and help each day be the best it can.”
Physicians in long-term care facilities might use medications such as antidepressants or nonpharmacologic approaches for patients experiencing depression symptoms. Families are also crucial in helping patients by bringing in home-cooked meals and encouraging loved ones who may be experiencing loss of taste or smell to eat, Dr. Steinberg said.
“We’ve seen with the return of families and loved ones visiting to some extent has alleviated some people’s symptoms, especially psychological ones,” he said.
Dr. Tosato said he and his colleagues start with an individualized, multidisciplinary assessment to determine what types of care may help. He noted that physicians might recommend medications or rehabilitative therapies depending on the patient’s needs.
“A personalized approach is key,” Dr. Tosato said. His study also found that the proportion of older patients experiencing symptoms declined over time – a glimmer of hope that many will recover.
Dr. Cohen emphasized the need for a multimodal rehabilitation, an evidence-based approach used to care for patients who survived hospitalization with severe COVID-19 – a group that has substantially higher rates of persistent symptoms. This approach includes cognitive rehabilitation, physical therapy, occupational therapy, and a graded exercise program.
Dr. Han and colleagues are studying potential therapies such as cognitive rehabilitation in adults who’ve experienced delirium. But until evidence-based treatments are available, they stress the role of support for patients with cognitive decline and their families.
“A lot of the work we do is teach patients and their families to compensate for newly acquired cognitive deficits from any illness, including COVID-19,” Dr. Han said.
Ms. Salant said she has experienced some improvement in her energy since her pulmonologist recommended a new inhaler based on her symptoms. Her sense of smell and taste, lost to the infection, returned after she received her first dose of a vaccine against COVID-19. She takes comfort in participating in Survivor Corps, a group of more than 170,000 COVID-19 survivors and their families who advocate for more scientific research on the disease.
She also expressed gratitude for the support she receives from her primary care physician, who she said has taken the time to learn more about the symptoms of long COVID, listens to her, and respects what she has to say.
“I have hope that I will keep getting better by baby steps,” Ms. Salant said.
Dr. Tosato, Dr. Steinberg, and Dr. Han have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Even mundane tasks such as making a meal can be exhausting for Louise Salant.
“I’m totally wiped out,” said the 71-year-old former private music instructor with asthma who lives in New York City and has been coping with debilitating symptoms of fatigue, shortness of breath, and gastrointestinal symptoms since recovering from a severe bout of COVID-19 2 years ago. “I just don’t have the energy.”
Ms. Salant is not alone. Many older people who contract COVID-19 experience prolonged symptoms of the disease. An analysis of Medicare Advantage claims data published in the BMJ found that about one-third of roughly 87,000 adults aged 65 in the database with a COVID-19 diagnosis sought care for persistent or new symptoms 21 or more days later.
That figure is about twice the rate of persistent COVID-19 related symptoms seen in a cohort of adults younger than age 65 with commercial insurance analyzed by the same group of researchers in a separate BMJ study. Compared with a 2020 comparator group of patients in this age cohort, these patients had a greater likelihood of respiratory failure, fatigue, hypertension, memory problems, kidney injury, mental health conditions, hypercoagulability, and cardiac rhythm disorders. When they compared post–COVID-19 symptoms to lasting symptoms of another serious viral disease – influenza – the researchers found that only respiratory failure, dementia, and post-viral fatigue were more common in the COVID-19 group.
“It became clear early in the pandemic that there is going to be a second pandemic related to all of the complications that we’ve seen related to COVID-19 infections,” said Ken Cohen, MD, executive director of translational research and national senior medical director for Optum Labs in Minnetonka, Minn., who coauthored the BMJ studies.
The results are among a growing body of evidence suggesting that older adults are at high risk of persistent post-COVID-19 symptoms.
Researchers in Rome, for example, found that 83% of 165 patients aged 65 or older who had been hospitalized for COVID-19 reported at least one lasting symptom – problems like fatigue, shortness of breath, joint pain, and coughing – in the months after hospitalization. One-third of those had two symptoms, and 46% had three or more.
A similar study in Norway found that two-thirds of patients aged 60 or older reported reduced health-related quality of life during follow-up visits 6 months after hospitalization for COVID-19. The most-reported impairments among those patients were the inability to perform the tasks of daily life, reduced mobility, and increased pain and discomfort.
Cognitive concerns
Mounting evidence indicates that COVID-19 may contribute to chronic cognitive impairment in older adults. A multisite U.S. study found that 28% of 817 adults presenting to emergency departments with COVID-19 had delirium and poorer outcomes. A Chinese case-control study that enrolled 1,438 individuals hospitalized in Wuhan for COVID-19, along with 438 of their uninfected spouses, found that 12% of COVID-19 survivors experienced cognitive impairment a year after discharge. Matteo Tosato, MD, PhD, head of the outpatient clinic for patients with long COVID symptoms at Gemelli Hospital in Rome, called those findings “very concerning.”
Jin Ho Han, MD, associate professor of emergency medicine at Vanderbilt University, Nashville, Tenn., said cognitive impairment is common after an acute illness, particularly in frail or vulnerable patients.
“Hospitalization and the acute illness itself accelerate cognitive decline,” said Dr. Han, and previous evidence links delirium with worsening cognition. He and his colleagues are studying the potential role of delirium in longer-term cognitive decline in older patients after COVID-19.
Dr. Han emphasized the importance of preventing COVID-19-related delirium through vaccines and other strategies to reduce exposure of older patients to the virus. “Once you have cognitive decline, there are no interventions to reverse it,” he said.
Alarm bells for long-term care
Experts expressed concern that the situation might be even worse for people living in long-term care facilities. Many already need assistance with tasks of daily living and could be particularly vulnerable to lasting effects of COVID-19, said Karl Steinberg, MD, president of the Society for Post-Acute and Long-Term Care Medicine. He estimated that roughly half of his patients who have had COVID-19, regardless of the severity of their symptoms, have endured some degree of functional decline.
“It’s common for long-term care facility residents to experience functional and cognitive decline, even after seemingly minor things, like a cold or a trip to the hospital,” Dr. Steinberg, who has been a medical director of long-term care facilities in San Diego County for more than 2 decades, told this news organization. “It makes it a little harder to determine whether the declines we’ve been seeing post COVID in these residents are attributable to post COVID versus just an accelerated step in their overall expected decline.”
The pandemic may have contributed to worse outcomes for people in long-term care facilities in several ways: the disease itself, its effects on health care delivery, and necessary preventive measures to protect long-term care residents from exposure to the virus.
“During the many months where family visits were prohibited, we saw people – whether they had COVID-19 or not – suffer major clinical, functional, cognitive declines or severe psychological symptoms,” Dr. Steinberg said.
He emphasized the importance of preventive measures such as vaccines and boosters in patients in long-term care facilities. He said the benefit of preventing lasting symptoms is often a strong motivator for family caregivers of people with dementia to get them vaccinated or boosted.
“It’s clear that vaccination and booster reduce the incidence of post-COVID symptoms,” he said. Almost all studies have been in younger cohorts, but he expects the benefits would also apply to older patients.
Easing symptoms and offering support
As with long COVID generally, many questions remain about the causes of lasting symptoms of COVID-19 in older patients, and how best to treat them. Dr. Tosato, who led the study of long-COVID patients in Rome, is focusing on inflammation as a critical factor in the condition. He and colleagues across Europe hope to answer some of them by launching a multicenter study of lasting COVID-19 symptoms.
In the meantime, Dr. Steinberg and Dr. Tosato said they are doing their best to evaluate and treat patients empirically.
“We pull from our armamentarium to treat system-specific symptoms,” Dr. Steinberg said. “We want to improve the quality of life and help each day be the best it can.”
Physicians in long-term care facilities might use medications such as antidepressants or nonpharmacologic approaches for patients experiencing depression symptoms. Families are also crucial in helping patients by bringing in home-cooked meals and encouraging loved ones who may be experiencing loss of taste or smell to eat, Dr. Steinberg said.
“We’ve seen with the return of families and loved ones visiting to some extent has alleviated some people’s symptoms, especially psychological ones,” he said.
Dr. Tosato said he and his colleagues start with an individualized, multidisciplinary assessment to determine what types of care may help. He noted that physicians might recommend medications or rehabilitative therapies depending on the patient’s needs.
“A personalized approach is key,” Dr. Tosato said. His study also found that the proportion of older patients experiencing symptoms declined over time – a glimmer of hope that many will recover.
Dr. Cohen emphasized the need for a multimodal rehabilitation, an evidence-based approach used to care for patients who survived hospitalization with severe COVID-19 – a group that has substantially higher rates of persistent symptoms. This approach includes cognitive rehabilitation, physical therapy, occupational therapy, and a graded exercise program.
Dr. Han and colleagues are studying potential therapies such as cognitive rehabilitation in adults who’ve experienced delirium. But until evidence-based treatments are available, they stress the role of support for patients with cognitive decline and their families.
“A lot of the work we do is teach patients and their families to compensate for newly acquired cognitive deficits from any illness, including COVID-19,” Dr. Han said.
Ms. Salant said she has experienced some improvement in her energy since her pulmonologist recommended a new inhaler based on her symptoms. Her sense of smell and taste, lost to the infection, returned after she received her first dose of a vaccine against COVID-19. She takes comfort in participating in Survivor Corps, a group of more than 170,000 COVID-19 survivors and their families who advocate for more scientific research on the disease.
She also expressed gratitude for the support she receives from her primary care physician, who she said has taken the time to learn more about the symptoms of long COVID, listens to her, and respects what she has to say.
“I have hope that I will keep getting better by baby steps,” Ms. Salant said.
Dr. Tosato, Dr. Steinberg, and Dr. Han have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fifth COVID shot recommended for patients with cancer
The National Comprehensive Cancer Network (NCCN) has recommended a fifth COVID-19 mRNA shot for people who are immunocompromised, including many with cancer or a history of cancer.
A fifth shot of an mRNA vaccine represents a second booster, the group explained, because the primary mRNA immunization series for immunocompromised individuals involves three doses of either the Pfizer or Moderna vaccine.
The update, issued today, comes from the NCCN’s Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis, which released its first vaccine guidelines for patients with cancer in January 2021. The NCCN has issued numerous updates since then as information about the virus and vaccines has evolved.
“We know a lot more about COVID-19 and the vaccines now, and we can use that knowledge to minimize the confusion and enhance the protection we can offer to our immunocompromised patients,” said advisory committee co-leader Lindsey Baden, MD, an infectious diseases specialist at the Dana-Farber Cancer Institute, Boston.
The latest iteration of the NCCN’s COVID guidelines includes an update for patients who initially received Johnson & Johnson’s single-shot vaccine, including a recommendation that patients receive an mRNA vaccine for both the first and second booster.
The group also updated dosing recommendations for pre-exposure prevention with tixagevimab plus cilgavimab (Evusheld, AstraZeneca), suggesting 300 mg of each monoclonal antibody instead of 150 mg, based on in vitro activity against Omicron variants.
The group noted that the Moderna and Pfizer shots can be used interchangeably for boosters.
“The NCCN Committee considers both homologous and heterologous boosters to be appropriate options,” the experts wrote.
A version of this article first appeared on Medscape.com.
The National Comprehensive Cancer Network (NCCN) has recommended a fifth COVID-19 mRNA shot for people who are immunocompromised, including many with cancer or a history of cancer.
A fifth shot of an mRNA vaccine represents a second booster, the group explained, because the primary mRNA immunization series for immunocompromised individuals involves three doses of either the Pfizer or Moderna vaccine.
The update, issued today, comes from the NCCN’s Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis, which released its first vaccine guidelines for patients with cancer in January 2021. The NCCN has issued numerous updates since then as information about the virus and vaccines has evolved.
“We know a lot more about COVID-19 and the vaccines now, and we can use that knowledge to minimize the confusion and enhance the protection we can offer to our immunocompromised patients,” said advisory committee co-leader Lindsey Baden, MD, an infectious diseases specialist at the Dana-Farber Cancer Institute, Boston.
The latest iteration of the NCCN’s COVID guidelines includes an update for patients who initially received Johnson & Johnson’s single-shot vaccine, including a recommendation that patients receive an mRNA vaccine for both the first and second booster.
The group also updated dosing recommendations for pre-exposure prevention with tixagevimab plus cilgavimab (Evusheld, AstraZeneca), suggesting 300 mg of each monoclonal antibody instead of 150 mg, based on in vitro activity against Omicron variants.
The group noted that the Moderna and Pfizer shots can be used interchangeably for boosters.
“The NCCN Committee considers both homologous and heterologous boosters to be appropriate options,” the experts wrote.
A version of this article first appeared on Medscape.com.
The National Comprehensive Cancer Network (NCCN) has recommended a fifth COVID-19 mRNA shot for people who are immunocompromised, including many with cancer or a history of cancer.
A fifth shot of an mRNA vaccine represents a second booster, the group explained, because the primary mRNA immunization series for immunocompromised individuals involves three doses of either the Pfizer or Moderna vaccine.
The update, issued today, comes from the NCCN’s Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis, which released its first vaccine guidelines for patients with cancer in January 2021. The NCCN has issued numerous updates since then as information about the virus and vaccines has evolved.
“We know a lot more about COVID-19 and the vaccines now, and we can use that knowledge to minimize the confusion and enhance the protection we can offer to our immunocompromised patients,” said advisory committee co-leader Lindsey Baden, MD, an infectious diseases specialist at the Dana-Farber Cancer Institute, Boston.
The latest iteration of the NCCN’s COVID guidelines includes an update for patients who initially received Johnson & Johnson’s single-shot vaccine, including a recommendation that patients receive an mRNA vaccine for both the first and second booster.
The group also updated dosing recommendations for pre-exposure prevention with tixagevimab plus cilgavimab (Evusheld, AstraZeneca), suggesting 300 mg of each monoclonal antibody instead of 150 mg, based on in vitro activity against Omicron variants.
The group noted that the Moderna and Pfizer shots can be used interchangeably for boosters.
“The NCCN Committee considers both homologous and heterologous boosters to be appropriate options,” the experts wrote.
A version of this article first appeared on Medscape.com.
30 years of fake nursing ends with 7-year prison sentence
A Canadian woman who officials allege faked being a registered nurse for some 30 years in Canada and the United States is scheduled to appear in court next month after being sentenced to 7 years in prison.
She was previously sentenced April 22 in an Ontario court after she pled guilty in January to seven offenses, including impersonation, assault with a weapon, and assault, according to CBC Radio-Canada.
Ms. Cleroux, who uses several aliases, had a long history of deception in three provinces in Canada, as well as in Colorado and Florida. The sentencing in Ontario stemmed from incidents at a medical and dental clinic in Ottawa last year, which included administration of medications to patients through needle injections, Ottawa Police reported in a press statement obtained by this news organization.
Authorities charged Ms. Cleroux in September with assault with a weapon and criminal negligence causing bodily harm, along with “personation to gain advantage,” obtaining by false pretense, and using a forged document, this news organization reported.
Ms. Cleroux has been in custody since her arrest by Ottawa Police in August.
The Vancouver Police Department (VPD) charged Ms. Cleroux last year with fraud of over $5,000 and personation with intent. VPD investigated claims that an employee at BC Women’s Hospital fraudulently identified herself as a nurse while working there between June 2020 and June 2021, according to a VPD press release.
Nursing colleges in British Columbia and Ontario issued warnings that she had used aliases and purported to be a registered nurse to gain employment. The aliases included Melanie Thompson, Melanie Smith, and Melanie Cleroux.
Ms. Cleroux was believed to be a student in a nursing school in Colorado, but she only completed 2 years of a 4-year nursing course and was never certified as a nurse, according to CBC. Her criminal record dates back 30 years and includes 67 adult convictions and other convictions in her youth, CBC reported.
A version of this article first appeared on Medscape.com.
A Canadian woman who officials allege faked being a registered nurse for some 30 years in Canada and the United States is scheduled to appear in court next month after being sentenced to 7 years in prison.
She was previously sentenced April 22 in an Ontario court after she pled guilty in January to seven offenses, including impersonation, assault with a weapon, and assault, according to CBC Radio-Canada.
Ms. Cleroux, who uses several aliases, had a long history of deception in three provinces in Canada, as well as in Colorado and Florida. The sentencing in Ontario stemmed from incidents at a medical and dental clinic in Ottawa last year, which included administration of medications to patients through needle injections, Ottawa Police reported in a press statement obtained by this news organization.
Authorities charged Ms. Cleroux in September with assault with a weapon and criminal negligence causing bodily harm, along with “personation to gain advantage,” obtaining by false pretense, and using a forged document, this news organization reported.
Ms. Cleroux has been in custody since her arrest by Ottawa Police in August.
The Vancouver Police Department (VPD) charged Ms. Cleroux last year with fraud of over $5,000 and personation with intent. VPD investigated claims that an employee at BC Women’s Hospital fraudulently identified herself as a nurse while working there between June 2020 and June 2021, according to a VPD press release.
Nursing colleges in British Columbia and Ontario issued warnings that she had used aliases and purported to be a registered nurse to gain employment. The aliases included Melanie Thompson, Melanie Smith, and Melanie Cleroux.
Ms. Cleroux was believed to be a student in a nursing school in Colorado, but she only completed 2 years of a 4-year nursing course and was never certified as a nurse, according to CBC. Her criminal record dates back 30 years and includes 67 adult convictions and other convictions in her youth, CBC reported.
A version of this article first appeared on Medscape.com.
A Canadian woman who officials allege faked being a registered nurse for some 30 years in Canada and the United States is scheduled to appear in court next month after being sentenced to 7 years in prison.
She was previously sentenced April 22 in an Ontario court after she pled guilty in January to seven offenses, including impersonation, assault with a weapon, and assault, according to CBC Radio-Canada.
Ms. Cleroux, who uses several aliases, had a long history of deception in three provinces in Canada, as well as in Colorado and Florida. The sentencing in Ontario stemmed from incidents at a medical and dental clinic in Ottawa last year, which included administration of medications to patients through needle injections, Ottawa Police reported in a press statement obtained by this news organization.
Authorities charged Ms. Cleroux in September with assault with a weapon and criminal negligence causing bodily harm, along with “personation to gain advantage,” obtaining by false pretense, and using a forged document, this news organization reported.
Ms. Cleroux has been in custody since her arrest by Ottawa Police in August.
The Vancouver Police Department (VPD) charged Ms. Cleroux last year with fraud of over $5,000 and personation with intent. VPD investigated claims that an employee at BC Women’s Hospital fraudulently identified herself as a nurse while working there between June 2020 and June 2021, according to a VPD press release.
Nursing colleges in British Columbia and Ontario issued warnings that she had used aliases and purported to be a registered nurse to gain employment. The aliases included Melanie Thompson, Melanie Smith, and Melanie Cleroux.
Ms. Cleroux was believed to be a student in a nursing school in Colorado, but she only completed 2 years of a 4-year nursing course and was never certified as a nurse, according to CBC. Her criminal record dates back 30 years and includes 67 adult convictions and other convictions in her youth, CBC reported.
A version of this article first appeared on Medscape.com.
NB-UVB phototherapy plays a key role in psoriasis treatment, expert says
BOSTON – In 2012, about 50% of patients receiving phototherapy at Brigham and Women’s Hospital in Boston were being treated for psoriasis. A decade later, that proportion has dropped to 20%.
Several factors have contributed to this trend, namely, the development of biologics, the COVID-19 pandemic, “and the rise of home phototherapy options,” Elizabeth A. Buzney, MD, codirector of the phototherapy center at Brigham and Women’s department of dermatology, said at the annual meeting of the American Academy of Dermatology. In her clinical opinion, phototherapy plays an essential role in the treatment of psoriasis.
“It is medically and financially responsible to review the option of phototherapy with every psoriasis patient,” Dr. Buzney said. “Many patients are not medical or financial candidates for biologic/apremilast therapy, or just would prefer nonsystemic therapy.”
In one meta-analysis, the proportion of patients achieving Psoriasis Area and Severity Index (PASI) 75 with NB-UVB therapy was 70% after 20-40 sessions, just below the efficacy of newer biologics – but better than ustekinumab and adalimumab.
“Phototherapy is not so far out of range as you might think it is,” she said, noting that other studies of NB-UVB therapy show PASI 75 responses of 62% and PASI 90 responses of 40%.
Phototherapy can also be an appealing option because biologics aren’t the best option for all patients with psoriasis. They are expensive for the health care system and potentially for patients, require initial and potentially continued lab testing and monitoring, and require injections, “which some patients don’t like,” said Dr. Buzney, who is also vice-chair of clinical affairs at the Brigham and Women’s Hospital department of dermatology. “There’s an infrequent risk of serious infection and there is risk in patients with HIV, TB, and hepatitis that you have to address. There is also concern for the impact of biologics on patients with a recent cancer.”
On the other hand, few contraindications to NB-UVB exist. According to joint American Academy of Dermatology-National Psoriasis Foundation guidelines on the management and treatment of psoriasis with phototherapy, published in 2019, NB-UVB therapy is only contraindicated in patients with xeroderma pigmentosa and other photosensitive disorders. Concurrent use of cyclosporine and NB-UVB treatment is also contraindicated because of the calculated increase in risk of skin cancer, extrapolated from data on risk with cyclosporine and PUVA (psoralen and ultraviolet A therapy).
The guidelines state that NB-UVB can be used with caution in lupus patients with no history of photosensitivity and who are SS-A negative, as well as patients with a history of melanoma or multiple nonmelanoma skin cancers, a history of recurrent oral herpes simplex virus infection, a history of arsenic intake, prior exposure to ionizing radiation, and those taking photosensitizing medications (since NB-UVB lamps emit “negligible” UVA).
It’s also safe to use during pregnancy and in children. “It’s safe and effective for the right patient,” Dr. Buzney said, discussing how phototherapy can be modified to accommodate children. “You can consider a slower dose-increased regimen. Will children keep the eye protection on? That’s a tricky one. How are you going to manage their anxiety during treatment and involve their family?”
Subgroups of patients who demonstrate a better response to NB-UVB treatment include those with guttate psoriasis, compared with plaque psoriasis, nonsmokers, those with a lower BMI, those with a higher baseline PASI, and those who demonstrate a faster trajectory of clinical response over the first 2-3 weeks of treatment.
Why would one not use phototherapy for psoriasis? “Cost and convenience,” Dr. Buzney said. “There is lost time/revenue to commute to treatment, which may involve multiple times per week. Coming to a public space when COVID-19 is still lingering is another concern, as are the out-of-pocket costs for copays and parking.”
For these reasons, she considers home phototherapy as a transformative option for many patients. Home phototherapy booths provide a safe and effective way to use NB-UVB phototherapy while minimizing copays and commuting costs. The one-time price tag of home NB-UVB booths runs between $5,000 and $7,000, but that is “much less expensive than the biologics,” which can cost $40,000-$50,000 per year, she said.
A small cross-sectional study of office- versus home-based NB-UVB in patients with vitiligo found a cost savings for home-based NB-UVB after 3 months.
One of the challenges with home phototherapy is the lack of long-term studies on patient use. In a small study Dr. Buzney conducted of 30 patients who were prescribed home phototherapy in the last 5 years, 65% practiced (or had practiced) conservative dosing, 83% had continued care with a dermatologist, 19% reported sunburns (5 mild and 1 severe), and 50% had discontinued the therapy at the time of survey because of a perceived lack of efficacy and inconvenience. But 30% of those who had stopped had done so within one month of getting their home booth.
“This tells me that we have to educate our patients better about what expectations should be and make sure they understand how to use their booths,” she said. “Home phototherapy has changed my practice, but not everyone is a candidate for it. Some patients are not dependable. Others are unable to understand instructions.”
Cost to purchase a NB-UVB booth is also an issue, she noted. “Typically, a percentage of cost is covered by insurance, but it’s problematic to purchase a booth if patients don’t know it’s going to work for them or not. Then you have college students who don’t have the space in their apartment or dorm room for a booth.”
Dr. Buzney reported having no relevant financial conflicts.
BOSTON – In 2012, about 50% of patients receiving phototherapy at Brigham and Women’s Hospital in Boston were being treated for psoriasis. A decade later, that proportion has dropped to 20%.
Several factors have contributed to this trend, namely, the development of biologics, the COVID-19 pandemic, “and the rise of home phototherapy options,” Elizabeth A. Buzney, MD, codirector of the phototherapy center at Brigham and Women’s department of dermatology, said at the annual meeting of the American Academy of Dermatology. In her clinical opinion, phototherapy plays an essential role in the treatment of psoriasis.
“It is medically and financially responsible to review the option of phototherapy with every psoriasis patient,” Dr. Buzney said. “Many patients are not medical or financial candidates for biologic/apremilast therapy, or just would prefer nonsystemic therapy.”
In one meta-analysis, the proportion of patients achieving Psoriasis Area and Severity Index (PASI) 75 with NB-UVB therapy was 70% after 20-40 sessions, just below the efficacy of newer biologics – but better than ustekinumab and adalimumab.
“Phototherapy is not so far out of range as you might think it is,” she said, noting that other studies of NB-UVB therapy show PASI 75 responses of 62% and PASI 90 responses of 40%.
Phototherapy can also be an appealing option because biologics aren’t the best option for all patients with psoriasis. They are expensive for the health care system and potentially for patients, require initial and potentially continued lab testing and monitoring, and require injections, “which some patients don’t like,” said Dr. Buzney, who is also vice-chair of clinical affairs at the Brigham and Women’s Hospital department of dermatology. “There’s an infrequent risk of serious infection and there is risk in patients with HIV, TB, and hepatitis that you have to address. There is also concern for the impact of biologics on patients with a recent cancer.”
On the other hand, few contraindications to NB-UVB exist. According to joint American Academy of Dermatology-National Psoriasis Foundation guidelines on the management and treatment of psoriasis with phototherapy, published in 2019, NB-UVB therapy is only contraindicated in patients with xeroderma pigmentosa and other photosensitive disorders. Concurrent use of cyclosporine and NB-UVB treatment is also contraindicated because of the calculated increase in risk of skin cancer, extrapolated from data on risk with cyclosporine and PUVA (psoralen and ultraviolet A therapy).
The guidelines state that NB-UVB can be used with caution in lupus patients with no history of photosensitivity and who are SS-A negative, as well as patients with a history of melanoma or multiple nonmelanoma skin cancers, a history of recurrent oral herpes simplex virus infection, a history of arsenic intake, prior exposure to ionizing radiation, and those taking photosensitizing medications (since NB-UVB lamps emit “negligible” UVA).
It’s also safe to use during pregnancy and in children. “It’s safe and effective for the right patient,” Dr. Buzney said, discussing how phototherapy can be modified to accommodate children. “You can consider a slower dose-increased regimen. Will children keep the eye protection on? That’s a tricky one. How are you going to manage their anxiety during treatment and involve their family?”
Subgroups of patients who demonstrate a better response to NB-UVB treatment include those with guttate psoriasis, compared with plaque psoriasis, nonsmokers, those with a lower BMI, those with a higher baseline PASI, and those who demonstrate a faster trajectory of clinical response over the first 2-3 weeks of treatment.
Why would one not use phototherapy for psoriasis? “Cost and convenience,” Dr. Buzney said. “There is lost time/revenue to commute to treatment, which may involve multiple times per week. Coming to a public space when COVID-19 is still lingering is another concern, as are the out-of-pocket costs for copays and parking.”
For these reasons, she considers home phototherapy as a transformative option for many patients. Home phototherapy booths provide a safe and effective way to use NB-UVB phototherapy while minimizing copays and commuting costs. The one-time price tag of home NB-UVB booths runs between $5,000 and $7,000, but that is “much less expensive than the biologics,” which can cost $40,000-$50,000 per year, she said.
A small cross-sectional study of office- versus home-based NB-UVB in patients with vitiligo found a cost savings for home-based NB-UVB after 3 months.
One of the challenges with home phototherapy is the lack of long-term studies on patient use. In a small study Dr. Buzney conducted of 30 patients who were prescribed home phototherapy in the last 5 years, 65% practiced (or had practiced) conservative dosing, 83% had continued care with a dermatologist, 19% reported sunburns (5 mild and 1 severe), and 50% had discontinued the therapy at the time of survey because of a perceived lack of efficacy and inconvenience. But 30% of those who had stopped had done so within one month of getting their home booth.
“This tells me that we have to educate our patients better about what expectations should be and make sure they understand how to use their booths,” she said. “Home phototherapy has changed my practice, but not everyone is a candidate for it. Some patients are not dependable. Others are unable to understand instructions.”
Cost to purchase a NB-UVB booth is also an issue, she noted. “Typically, a percentage of cost is covered by insurance, but it’s problematic to purchase a booth if patients don’t know it’s going to work for them or not. Then you have college students who don’t have the space in their apartment or dorm room for a booth.”
Dr. Buzney reported having no relevant financial conflicts.
BOSTON – In 2012, about 50% of patients receiving phototherapy at Brigham and Women’s Hospital in Boston were being treated for psoriasis. A decade later, that proportion has dropped to 20%.
Several factors have contributed to this trend, namely, the development of biologics, the COVID-19 pandemic, “and the rise of home phototherapy options,” Elizabeth A. Buzney, MD, codirector of the phototherapy center at Brigham and Women’s department of dermatology, said at the annual meeting of the American Academy of Dermatology. In her clinical opinion, phototherapy plays an essential role in the treatment of psoriasis.
“It is medically and financially responsible to review the option of phototherapy with every psoriasis patient,” Dr. Buzney said. “Many patients are not medical or financial candidates for biologic/apremilast therapy, or just would prefer nonsystemic therapy.”
In one meta-analysis, the proportion of patients achieving Psoriasis Area and Severity Index (PASI) 75 with NB-UVB therapy was 70% after 20-40 sessions, just below the efficacy of newer biologics – but better than ustekinumab and adalimumab.
“Phototherapy is not so far out of range as you might think it is,” she said, noting that other studies of NB-UVB therapy show PASI 75 responses of 62% and PASI 90 responses of 40%.
Phototherapy can also be an appealing option because biologics aren’t the best option for all patients with psoriasis. They are expensive for the health care system and potentially for patients, require initial and potentially continued lab testing and monitoring, and require injections, “which some patients don’t like,” said Dr. Buzney, who is also vice-chair of clinical affairs at the Brigham and Women’s Hospital department of dermatology. “There’s an infrequent risk of serious infection and there is risk in patients with HIV, TB, and hepatitis that you have to address. There is also concern for the impact of biologics on patients with a recent cancer.”
On the other hand, few contraindications to NB-UVB exist. According to joint American Academy of Dermatology-National Psoriasis Foundation guidelines on the management and treatment of psoriasis with phototherapy, published in 2019, NB-UVB therapy is only contraindicated in patients with xeroderma pigmentosa and other photosensitive disorders. Concurrent use of cyclosporine and NB-UVB treatment is also contraindicated because of the calculated increase in risk of skin cancer, extrapolated from data on risk with cyclosporine and PUVA (psoralen and ultraviolet A therapy).
The guidelines state that NB-UVB can be used with caution in lupus patients with no history of photosensitivity and who are SS-A negative, as well as patients with a history of melanoma or multiple nonmelanoma skin cancers, a history of recurrent oral herpes simplex virus infection, a history of arsenic intake, prior exposure to ionizing radiation, and those taking photosensitizing medications (since NB-UVB lamps emit “negligible” UVA).
It’s also safe to use during pregnancy and in children. “It’s safe and effective for the right patient,” Dr. Buzney said, discussing how phototherapy can be modified to accommodate children. “You can consider a slower dose-increased regimen. Will children keep the eye protection on? That’s a tricky one. How are you going to manage their anxiety during treatment and involve their family?”
Subgroups of patients who demonstrate a better response to NB-UVB treatment include those with guttate psoriasis, compared with plaque psoriasis, nonsmokers, those with a lower BMI, those with a higher baseline PASI, and those who demonstrate a faster trajectory of clinical response over the first 2-3 weeks of treatment.
Why would one not use phototherapy for psoriasis? “Cost and convenience,” Dr. Buzney said. “There is lost time/revenue to commute to treatment, which may involve multiple times per week. Coming to a public space when COVID-19 is still lingering is another concern, as are the out-of-pocket costs for copays and parking.”
For these reasons, she considers home phototherapy as a transformative option for many patients. Home phototherapy booths provide a safe and effective way to use NB-UVB phototherapy while minimizing copays and commuting costs. The one-time price tag of home NB-UVB booths runs between $5,000 and $7,000, but that is “much less expensive than the biologics,” which can cost $40,000-$50,000 per year, she said.
A small cross-sectional study of office- versus home-based NB-UVB in patients with vitiligo found a cost savings for home-based NB-UVB after 3 months.
One of the challenges with home phototherapy is the lack of long-term studies on patient use. In a small study Dr. Buzney conducted of 30 patients who were prescribed home phototherapy in the last 5 years, 65% practiced (or had practiced) conservative dosing, 83% had continued care with a dermatologist, 19% reported sunburns (5 mild and 1 severe), and 50% had discontinued the therapy at the time of survey because of a perceived lack of efficacy and inconvenience. But 30% of those who had stopped had done so within one month of getting their home booth.
“This tells me that we have to educate our patients better about what expectations should be and make sure they understand how to use their booths,” she said. “Home phototherapy has changed my practice, but not everyone is a candidate for it. Some patients are not dependable. Others are unable to understand instructions.”
Cost to purchase a NB-UVB booth is also an issue, she noted. “Typically, a percentage of cost is covered by insurance, but it’s problematic to purchase a booth if patients don’t know it’s going to work for them or not. Then you have college students who don’t have the space in their apartment or dorm room for a booth.”
Dr. Buzney reported having no relevant financial conflicts.
AT AAD 22
Unilateral eye irritation
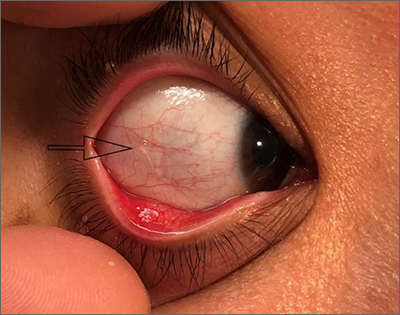
Physical examination revealed an irregularly shaped conjunctival cyst on the lateral (temporal) field of the right eye. (This diagnosis is usually made based on the clinical examination alone.)
Primary care physicians encounter patients with a variety of eye conditions; pruritis and foreign body sensation are among the most common complaints.1 While viral or allergic conjunctivitis is often to blame for “itchy eyes,” the cause can also be a conjunctival mass.
Conjunctival masses can be divided into 2 groups: solid tumors or cysts.2 Conjunctival cysts form due to trauma, infection, or inflammation that disrupts the conjunctival epithelium. They can be congenital or acquired (more common) and are rarely caused by over-the-counter eye drops.2,3 The differential diagnosis for a conjunctival cyst includes conjunctival bleb, pinguecula, pterygium, pyogenic granuloma, and tumors of the conjunctiva. An external eye exam plus a slit-lamp examination can help confirm the diagnosis.
Small, asymptomatic conjunctival cysts will mostly resolve on their own and can be managed conservatively with lubricating eye drops.3 When inflammation surrounds the cyst, short-term use of a mild topical corticosteroid is reasonable.2 Simple needle aspiration can be performed but may lead to recurrence of the cyst. Lesions larger than 15 mm, or those that have grown or changed, should be evaluated by an ophthalmologist for biopsy and further management.2,3
After a discussion of the benefits and risks of different approaches, this patient decided on conservative management. Supportive care with lubricating eye drops was started. At her 1-month follow-up, all symptoms had resolved.
Photos courtesy of Morteza Khodaee, MD, MPH. Text courtesy of Amy S. Li, MD, Department of Internal Medicine, Jennifer Cogburn, MD, and Morteza Khodaee, MD, MPH, Department of Family Medicine, University of Colorado School of Medicine, Denver
1. Pflipsen M, Massaquoi M, Wolf S. Evaluation of the painful eye. Am Fam Physician. 2016 Jun 15;93:991-998.
2. Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Indian J Ophthalmol. 2019;67:1930-1948. doi: 10.4103/ijo.IJO_2040_19
3. Olivier JF. Common conjunctival lesions. S Afr J CPD. 2013;31:134-137.

Physical examination revealed an irregularly shaped conjunctival cyst on the lateral (temporal) field of the right eye. (This diagnosis is usually made based on the clinical examination alone.)
Primary care physicians encounter patients with a variety of eye conditions; pruritis and foreign body sensation are among the most common complaints.1 While viral or allergic conjunctivitis is often to blame for “itchy eyes,” the cause can also be a conjunctival mass.
Conjunctival masses can be divided into 2 groups: solid tumors or cysts.2 Conjunctival cysts form due to trauma, infection, or inflammation that disrupts the conjunctival epithelium. They can be congenital or acquired (more common) and are rarely caused by over-the-counter eye drops.2,3 The differential diagnosis for a conjunctival cyst includes conjunctival bleb, pinguecula, pterygium, pyogenic granuloma, and tumors of the conjunctiva. An external eye exam plus a slit-lamp examination can help confirm the diagnosis.
Small, asymptomatic conjunctival cysts will mostly resolve on their own and can be managed conservatively with lubricating eye drops.3 When inflammation surrounds the cyst, short-term use of a mild topical corticosteroid is reasonable.2 Simple needle aspiration can be performed but may lead to recurrence of the cyst. Lesions larger than 15 mm, or those that have grown or changed, should be evaluated by an ophthalmologist for biopsy and further management.2,3
After a discussion of the benefits and risks of different approaches, this patient decided on conservative management. Supportive care with lubricating eye drops was started. At her 1-month follow-up, all symptoms had resolved.
Photos courtesy of Morteza Khodaee, MD, MPH. Text courtesy of Amy S. Li, MD, Department of Internal Medicine, Jennifer Cogburn, MD, and Morteza Khodaee, MD, MPH, Department of Family Medicine, University of Colorado School of Medicine, Denver

Physical examination revealed an irregularly shaped conjunctival cyst on the lateral (temporal) field of the right eye. (This diagnosis is usually made based on the clinical examination alone.)
Primary care physicians encounter patients with a variety of eye conditions; pruritis and foreign body sensation are among the most common complaints.1 While viral or allergic conjunctivitis is often to blame for “itchy eyes,” the cause can also be a conjunctival mass.
Conjunctival masses can be divided into 2 groups: solid tumors or cysts.2 Conjunctival cysts form due to trauma, infection, or inflammation that disrupts the conjunctival epithelium. They can be congenital or acquired (more common) and are rarely caused by over-the-counter eye drops.2,3 The differential diagnosis for a conjunctival cyst includes conjunctival bleb, pinguecula, pterygium, pyogenic granuloma, and tumors of the conjunctiva. An external eye exam plus a slit-lamp examination can help confirm the diagnosis.
Small, asymptomatic conjunctival cysts will mostly resolve on their own and can be managed conservatively with lubricating eye drops.3 When inflammation surrounds the cyst, short-term use of a mild topical corticosteroid is reasonable.2 Simple needle aspiration can be performed but may lead to recurrence of the cyst. Lesions larger than 15 mm, or those that have grown or changed, should be evaluated by an ophthalmologist for biopsy and further management.2,3
After a discussion of the benefits and risks of different approaches, this patient decided on conservative management. Supportive care with lubricating eye drops was started. At her 1-month follow-up, all symptoms had resolved.
Photos courtesy of Morteza Khodaee, MD, MPH. Text courtesy of Amy S. Li, MD, Department of Internal Medicine, Jennifer Cogburn, MD, and Morteza Khodaee, MD, MPH, Department of Family Medicine, University of Colorado School of Medicine, Denver
1. Pflipsen M, Massaquoi M, Wolf S. Evaluation of the painful eye. Am Fam Physician. 2016 Jun 15;93:991-998.
2. Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Indian J Ophthalmol. 2019;67:1930-1948. doi: 10.4103/ijo.IJO_2040_19
3. Olivier JF. Common conjunctival lesions. S Afr J CPD. 2013;31:134-137.
1. Pflipsen M, Massaquoi M, Wolf S. Evaluation of the painful eye. Am Fam Physician. 2016 Jun 15;93:991-998.
2. Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Indian J Ophthalmol. 2019;67:1930-1948. doi: 10.4103/ijo.IJO_2040_19
3. Olivier JF. Common conjunctival lesions. S Afr J CPD. 2013;31:134-137.
More evidence links asthma severity to age of onset
A recently published multinational cohort study may be the largest to date that’s found the age of asthma onset is an integral factor in defining the severity of disease and the frequency of comorbidities.
“It’s very simple to ask your patient: ‘Did you have asthma as a child? When did your asthma start?’ ” coauthor Guy Brusselle, MD, a professor at the University of Ghent (Belgium), said in an interview. “You do not need expensive investigations, CT scans or proteomics or genomics; just two simple questions.”
The retrospective cohort study, published in the Journal of Allergy and Clinical Immunology: In Practice, combined national electronic health records databases from five different countries – the United Kingdom, Spain, Italy, the Netherlands, and Denmark – that included 586,436 adult asthma patients. The study divided the patients into three subtypes: childhood-onset asthma, meaning a diagnosis before age 18 (n = 81,691); adult-onset disease, defined as a diagnosis between ages 18 and 40 (n = 218,184); and late onset, defined as a diagnosis made after age 40 (n = 286,561).
Dr. Brusselle said the study found stark differences in characteristics between the three subtypes, including an increasing risk for women with later age of onset. Across the five databases, females comprised approximately 45% of those with childhood-onset asthma, but about 60% of those with later-onset disease, Dr. Brusselle said.
As for characteristics of asthma, 7.2% of the cohort (n = 42,611) had severe asthma, but the proportion was highest in late-onset asthma, 10% versus 5% in adult onset and 3% in childhood onset. The percentage of uncontrolled asthma followed a similar trend: 8%, 6%, and 0.4% in the respective treatment groups.
The most common comorbidities were atopic disorders (31%) and overweight/obesity (50%). The prevalence of atopic disorders was highest in the childhood-onset group, 45% versus 35%, and 25% in the adult-onset and late-onset patients. However, the trend for overweight/obesity was reversed: 30%, 43%, and 61%, respectively.
“The larger differences were when late-onset asthma was compared to adult-onset asthma with respect to comorbidities,” Dr. Brusselle said. “The late-onset asthma patients more frequently had nasal polyposis.” These patients typically lose their sense of smell, as in COVID-19. However, in nasal polyposis the loss is chronic rather than transient.
Pulmonologists should be attuned to the prevalence of overweight/obesity in the late-onset group, Dr. Brusselle said. “We know that obesity is an important risk factor for diabetes, and then obesity is also associated with gastroesophageal reflux – and we know that gastroesophageal reflux is a risk factor for asthma exacerbations.”
Smaller studies have arrived at the same conclusions regarding the relationships between asthma severity and age of onset, Dr. Brusselle said. What’s notable about this study is its size and the consistency of findings across different national databases.
“In childhood onset you need to watch for different allergies – atopic dermatitis and allergic rhinitis – but in late-onset asthma look for obesity, diabetes and reflux disease, and nasal polyposis,” he said.
Sally E. Wenzel, MD, professor at the University of Pittsburgh and director of the Asthma and Environmental Lung Health Institute at the University of Pittsburgh Medical Center, concurred that the size of this study makes it noteworthy.
“It’s certainly far and away the largest study of its kind that’s ever been done, and it’s multinational,” she said in an interview. “Just doing a study like this with thousands and thousands of patients is a step in the right direction. That’s probably what’s very unique about it, to bring all of these clinical cohorts as it were together and to look at what is the relationship of the age of onset.”
She also said the study is unique in how it delineates the groups by age of onset.
“In addition to this concept that there’s a difference in asthma by the age that you got diagnosed with it, I think it’s also important to just remember that when any physician, be they a specialist or nonspecialist, sees a patient with asthma, they should ask them when did their symptoms develop,” she said. “These are really simple questions that don’t take any sophisticated training and don’t take any sophisticated instruments to measure, but they can be really helpful.”
GlaxoSmithKline supplied a grant for the study. Dr. Brusselle disclosed relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Sanofi, and Teva. A study coauthor is an employee of GSK. Dr. Wenzel reported no disclosures.
A version of this article first appeared on Medscape.com.
A recently published multinational cohort study may be the largest to date that’s found the age of asthma onset is an integral factor in defining the severity of disease and the frequency of comorbidities.
“It’s very simple to ask your patient: ‘Did you have asthma as a child? When did your asthma start?’ ” coauthor Guy Brusselle, MD, a professor at the University of Ghent (Belgium), said in an interview. “You do not need expensive investigations, CT scans or proteomics or genomics; just two simple questions.”
The retrospective cohort study, published in the Journal of Allergy and Clinical Immunology: In Practice, combined national electronic health records databases from five different countries – the United Kingdom, Spain, Italy, the Netherlands, and Denmark – that included 586,436 adult asthma patients. The study divided the patients into three subtypes: childhood-onset asthma, meaning a diagnosis before age 18 (n = 81,691); adult-onset disease, defined as a diagnosis between ages 18 and 40 (n = 218,184); and late onset, defined as a diagnosis made after age 40 (n = 286,561).
Dr. Brusselle said the study found stark differences in characteristics between the three subtypes, including an increasing risk for women with later age of onset. Across the five databases, females comprised approximately 45% of those with childhood-onset asthma, but about 60% of those with later-onset disease, Dr. Brusselle said.
As for characteristics of asthma, 7.2% of the cohort (n = 42,611) had severe asthma, but the proportion was highest in late-onset asthma, 10% versus 5% in adult onset and 3% in childhood onset. The percentage of uncontrolled asthma followed a similar trend: 8%, 6%, and 0.4% in the respective treatment groups.
The most common comorbidities were atopic disorders (31%) and overweight/obesity (50%). The prevalence of atopic disorders was highest in the childhood-onset group, 45% versus 35%, and 25% in the adult-onset and late-onset patients. However, the trend for overweight/obesity was reversed: 30%, 43%, and 61%, respectively.
“The larger differences were when late-onset asthma was compared to adult-onset asthma with respect to comorbidities,” Dr. Brusselle said. “The late-onset asthma patients more frequently had nasal polyposis.” These patients typically lose their sense of smell, as in COVID-19. However, in nasal polyposis the loss is chronic rather than transient.
Pulmonologists should be attuned to the prevalence of overweight/obesity in the late-onset group, Dr. Brusselle said. “We know that obesity is an important risk factor for diabetes, and then obesity is also associated with gastroesophageal reflux – and we know that gastroesophageal reflux is a risk factor for asthma exacerbations.”
Smaller studies have arrived at the same conclusions regarding the relationships between asthma severity and age of onset, Dr. Brusselle said. What’s notable about this study is its size and the consistency of findings across different national databases.
“In childhood onset you need to watch for different allergies – atopic dermatitis and allergic rhinitis – but in late-onset asthma look for obesity, diabetes and reflux disease, and nasal polyposis,” he said.
Sally E. Wenzel, MD, professor at the University of Pittsburgh and director of the Asthma and Environmental Lung Health Institute at the University of Pittsburgh Medical Center, concurred that the size of this study makes it noteworthy.
“It’s certainly far and away the largest study of its kind that’s ever been done, and it’s multinational,” she said in an interview. “Just doing a study like this with thousands and thousands of patients is a step in the right direction. That’s probably what’s very unique about it, to bring all of these clinical cohorts as it were together and to look at what is the relationship of the age of onset.”
She also said the study is unique in how it delineates the groups by age of onset.
“In addition to this concept that there’s a difference in asthma by the age that you got diagnosed with it, I think it’s also important to just remember that when any physician, be they a specialist or nonspecialist, sees a patient with asthma, they should ask them when did their symptoms develop,” she said. “These are really simple questions that don’t take any sophisticated training and don’t take any sophisticated instruments to measure, but they can be really helpful.”
GlaxoSmithKline supplied a grant for the study. Dr. Brusselle disclosed relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Sanofi, and Teva. A study coauthor is an employee of GSK. Dr. Wenzel reported no disclosures.
A version of this article first appeared on Medscape.com.
A recently published multinational cohort study may be the largest to date that’s found the age of asthma onset is an integral factor in defining the severity of disease and the frequency of comorbidities.
“It’s very simple to ask your patient: ‘Did you have asthma as a child? When did your asthma start?’ ” coauthor Guy Brusselle, MD, a professor at the University of Ghent (Belgium), said in an interview. “You do not need expensive investigations, CT scans or proteomics or genomics; just two simple questions.”
The retrospective cohort study, published in the Journal of Allergy and Clinical Immunology: In Practice, combined national electronic health records databases from five different countries – the United Kingdom, Spain, Italy, the Netherlands, and Denmark – that included 586,436 adult asthma patients. The study divided the patients into three subtypes: childhood-onset asthma, meaning a diagnosis before age 18 (n = 81,691); adult-onset disease, defined as a diagnosis between ages 18 and 40 (n = 218,184); and late onset, defined as a diagnosis made after age 40 (n = 286,561).
Dr. Brusselle said the study found stark differences in characteristics between the three subtypes, including an increasing risk for women with later age of onset. Across the five databases, females comprised approximately 45% of those with childhood-onset asthma, but about 60% of those with later-onset disease, Dr. Brusselle said.
As for characteristics of asthma, 7.2% of the cohort (n = 42,611) had severe asthma, but the proportion was highest in late-onset asthma, 10% versus 5% in adult onset and 3% in childhood onset. The percentage of uncontrolled asthma followed a similar trend: 8%, 6%, and 0.4% in the respective treatment groups.
The most common comorbidities were atopic disorders (31%) and overweight/obesity (50%). The prevalence of atopic disorders was highest in the childhood-onset group, 45% versus 35%, and 25% in the adult-onset and late-onset patients. However, the trend for overweight/obesity was reversed: 30%, 43%, and 61%, respectively.
“The larger differences were when late-onset asthma was compared to adult-onset asthma with respect to comorbidities,” Dr. Brusselle said. “The late-onset asthma patients more frequently had nasal polyposis.” These patients typically lose their sense of smell, as in COVID-19. However, in nasal polyposis the loss is chronic rather than transient.
Pulmonologists should be attuned to the prevalence of overweight/obesity in the late-onset group, Dr. Brusselle said. “We know that obesity is an important risk factor for diabetes, and then obesity is also associated with gastroesophageal reflux – and we know that gastroesophageal reflux is a risk factor for asthma exacerbations.”
Smaller studies have arrived at the same conclusions regarding the relationships between asthma severity and age of onset, Dr. Brusselle said. What’s notable about this study is its size and the consistency of findings across different national databases.
“In childhood onset you need to watch for different allergies – atopic dermatitis and allergic rhinitis – but in late-onset asthma look for obesity, diabetes and reflux disease, and nasal polyposis,” he said.
Sally E. Wenzel, MD, professor at the University of Pittsburgh and director of the Asthma and Environmental Lung Health Institute at the University of Pittsburgh Medical Center, concurred that the size of this study makes it noteworthy.
“It’s certainly far and away the largest study of its kind that’s ever been done, and it’s multinational,” she said in an interview. “Just doing a study like this with thousands and thousands of patients is a step in the right direction. That’s probably what’s very unique about it, to bring all of these clinical cohorts as it were together and to look at what is the relationship of the age of onset.”
She also said the study is unique in how it delineates the groups by age of onset.
“In addition to this concept that there’s a difference in asthma by the age that you got diagnosed with it, I think it’s also important to just remember that when any physician, be they a specialist or nonspecialist, sees a patient with asthma, they should ask them when did their symptoms develop,” she said. “These are really simple questions that don’t take any sophisticated training and don’t take any sophisticated instruments to measure, but they can be really helpful.”
GlaxoSmithKline supplied a grant for the study. Dr. Brusselle disclosed relationships with AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Sanofi, and Teva. A study coauthor is an employee of GSK. Dr. Wenzel reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Almost 60% of U.S. population has been infected by COVID-19: CDC
The percentage of Americans who have been infected with COVID-19 jumped from 34% in December 2021 to 58% in February 2022, a new study from the Centers for Disease Control and Prevention reveals.
This is the first time the seroprevalence of prior infection is more than 50% in the American population.
“I definitely expected that we were going to see an increase continue ... but I didn’t expect it to increase quite this much. But we follow the data ... and this is what the evidence is showing us,” lead study researcher Kristie E. N. Clarke, MD, said during a CDC media briefing April 26.
Researchers found that presence of antinucleocapsid (anti-N) antibodies from prior infection varied by age. The rate varied from as high as 75% in children and teenagers 17 years and younger to 33% in those 65 and older, for example.
The study showed that the anti-N antibodies were more common in age groups with the lowest vaccination numbers.
Combined with up-to-date CDC data on deaths, hospitalizations, and cases, the study provides a clearer picture of where we are now and where we might be headed in terms of the pandemic.
Vaccination still valuable
The fact that nearly 60% of Americans have antibodies from prior infection is not a reason to think people with a history of COVID-19 should skip vaccination, said CDC director Rochelle P. Walensky, MD.
“I can’t underscore enough that those with detectable antibodies from previous infection, we encourage them to still get vaccinated,” Dr. Walensky said.
“We do know that reinfections happen,” she said, “so that’s important in terms of thinking forward.”
The CDC continues to encourage all Americans to stay up to date with their COVID-19 vaccinations, said Dr. Clarke, colead for the CDC’s COVID-19 Epidemiology and Surveillance Taskforce Seroprevalence Team. “Having infection-induced antibodies does not necessarily mean you are protected against future infections.”
The study, published in the CDC’s Morbidity and Mortality Weekly Report (MMWR), did not evaluate antibody protection from COVID-19 vaccination.
It should also be noted that the study looked at presence or absence of anti-N antibodies, and not whether certain levels were linked to less or more protection.
Where are we now?
Dr. Walensky used the media briefing as an opportunity to share current COVID-19 numbers.
“Overall, we can continue to have some mixed trends. Deaths, fortunately, are continuing to trend downward with a 7-day average of about 300 per day, which represents an estimated 18% decline from the prior week,” she said.
Hospital admissions also remain low, at about 1,500 per day. “But we should note that for the second week in a row, they are slowly trending upwards,” Dr. Walensky said. There was an increase of about 9% at press time compared with the prior week.
Cases remain “comparatively low” to even where we were a month ago, at 44,000 per day,” Dr. Walensky said. “Although this too represents an increase of about 25% in the past week.”
Dr. Walensky noted that positive test numbers are not as reliable a metric as they were before the growth in use of rapid home tests. But it’s not the only measure. “We continue to believe that our PCR testing data, especially when we corroborate it with information from our other surveillance systems – like wastewater surveillance and emergency department surveillance – provide us a reliable picture of the trajectory of COVID-19 across our country.”
She recommended that people continue to consult the CDC’s COVID-19 county tracker to monitor local levels of COVID-19.
Dr. Walensky also shared recent findings from genomic sequencing that continue to show the predominance of the Omicron variant. “Essentially a hundred percent of what we’re finding now is Omicron,” she said. In terms of individual variants, the Omicron BA.1 variant is about 3% of circulating virus, the BA.2 variant is about 68%, and BA.2.12.1 makes up about 35%.
“We’re just starting to learn about the impact of BA2.121,” Dr. Walensky said. “It appears it might have a transmission advantage of about 25% over the BA2 subvariant.”
A version of this article first appeared on Medscape.com.
The percentage of Americans who have been infected with COVID-19 jumped from 34% in December 2021 to 58% in February 2022, a new study from the Centers for Disease Control and Prevention reveals.
This is the first time the seroprevalence of prior infection is more than 50% in the American population.
“I definitely expected that we were going to see an increase continue ... but I didn’t expect it to increase quite this much. But we follow the data ... and this is what the evidence is showing us,” lead study researcher Kristie E. N. Clarke, MD, said during a CDC media briefing April 26.
Researchers found that presence of antinucleocapsid (anti-N) antibodies from prior infection varied by age. The rate varied from as high as 75% in children and teenagers 17 years and younger to 33% in those 65 and older, for example.
The study showed that the anti-N antibodies were more common in age groups with the lowest vaccination numbers.
Combined with up-to-date CDC data on deaths, hospitalizations, and cases, the study provides a clearer picture of where we are now and where we might be headed in terms of the pandemic.
Vaccination still valuable
The fact that nearly 60% of Americans have antibodies from prior infection is not a reason to think people with a history of COVID-19 should skip vaccination, said CDC director Rochelle P. Walensky, MD.
“I can’t underscore enough that those with detectable antibodies from previous infection, we encourage them to still get vaccinated,” Dr. Walensky said.
“We do know that reinfections happen,” she said, “so that’s important in terms of thinking forward.”
The CDC continues to encourage all Americans to stay up to date with their COVID-19 vaccinations, said Dr. Clarke, colead for the CDC’s COVID-19 Epidemiology and Surveillance Taskforce Seroprevalence Team. “Having infection-induced antibodies does not necessarily mean you are protected against future infections.”
The study, published in the CDC’s Morbidity and Mortality Weekly Report (MMWR), did not evaluate antibody protection from COVID-19 vaccination.
It should also be noted that the study looked at presence or absence of anti-N antibodies, and not whether certain levels were linked to less or more protection.
Where are we now?
Dr. Walensky used the media briefing as an opportunity to share current COVID-19 numbers.
“Overall, we can continue to have some mixed trends. Deaths, fortunately, are continuing to trend downward with a 7-day average of about 300 per day, which represents an estimated 18% decline from the prior week,” she said.
Hospital admissions also remain low, at about 1,500 per day. “But we should note that for the second week in a row, they are slowly trending upwards,” Dr. Walensky said. There was an increase of about 9% at press time compared with the prior week.
Cases remain “comparatively low” to even where we were a month ago, at 44,000 per day,” Dr. Walensky said. “Although this too represents an increase of about 25% in the past week.”
Dr. Walensky noted that positive test numbers are not as reliable a metric as they were before the growth in use of rapid home tests. But it’s not the only measure. “We continue to believe that our PCR testing data, especially when we corroborate it with information from our other surveillance systems – like wastewater surveillance and emergency department surveillance – provide us a reliable picture of the trajectory of COVID-19 across our country.”
She recommended that people continue to consult the CDC’s COVID-19 county tracker to monitor local levels of COVID-19.
Dr. Walensky also shared recent findings from genomic sequencing that continue to show the predominance of the Omicron variant. “Essentially a hundred percent of what we’re finding now is Omicron,” she said. In terms of individual variants, the Omicron BA.1 variant is about 3% of circulating virus, the BA.2 variant is about 68%, and BA.2.12.1 makes up about 35%.
“We’re just starting to learn about the impact of BA2.121,” Dr. Walensky said. “It appears it might have a transmission advantage of about 25% over the BA2 subvariant.”
A version of this article first appeared on Medscape.com.
The percentage of Americans who have been infected with COVID-19 jumped from 34% in December 2021 to 58% in February 2022, a new study from the Centers for Disease Control and Prevention reveals.
This is the first time the seroprevalence of prior infection is more than 50% in the American population.
“I definitely expected that we were going to see an increase continue ... but I didn’t expect it to increase quite this much. But we follow the data ... and this is what the evidence is showing us,” lead study researcher Kristie E. N. Clarke, MD, said during a CDC media briefing April 26.
Researchers found that presence of antinucleocapsid (anti-N) antibodies from prior infection varied by age. The rate varied from as high as 75% in children and teenagers 17 years and younger to 33% in those 65 and older, for example.
The study showed that the anti-N antibodies were more common in age groups with the lowest vaccination numbers.
Combined with up-to-date CDC data on deaths, hospitalizations, and cases, the study provides a clearer picture of where we are now and where we might be headed in terms of the pandemic.
Vaccination still valuable
The fact that nearly 60% of Americans have antibodies from prior infection is not a reason to think people with a history of COVID-19 should skip vaccination, said CDC director Rochelle P. Walensky, MD.
“I can’t underscore enough that those with detectable antibodies from previous infection, we encourage them to still get vaccinated,” Dr. Walensky said.
“We do know that reinfections happen,” she said, “so that’s important in terms of thinking forward.”
The CDC continues to encourage all Americans to stay up to date with their COVID-19 vaccinations, said Dr. Clarke, colead for the CDC’s COVID-19 Epidemiology and Surveillance Taskforce Seroprevalence Team. “Having infection-induced antibodies does not necessarily mean you are protected against future infections.”
The study, published in the CDC’s Morbidity and Mortality Weekly Report (MMWR), did not evaluate antibody protection from COVID-19 vaccination.
It should also be noted that the study looked at presence or absence of anti-N antibodies, and not whether certain levels were linked to less or more protection.
Where are we now?
Dr. Walensky used the media briefing as an opportunity to share current COVID-19 numbers.
“Overall, we can continue to have some mixed trends. Deaths, fortunately, are continuing to trend downward with a 7-day average of about 300 per day, which represents an estimated 18% decline from the prior week,” she said.
Hospital admissions also remain low, at about 1,500 per day. “But we should note that for the second week in a row, they are slowly trending upwards,” Dr. Walensky said. There was an increase of about 9% at press time compared with the prior week.
Cases remain “comparatively low” to even where we were a month ago, at 44,000 per day,” Dr. Walensky said. “Although this too represents an increase of about 25% in the past week.”
Dr. Walensky noted that positive test numbers are not as reliable a metric as they were before the growth in use of rapid home tests. But it’s not the only measure. “We continue to believe that our PCR testing data, especially when we corroborate it with information from our other surveillance systems – like wastewater surveillance and emergency department surveillance – provide us a reliable picture of the trajectory of COVID-19 across our country.”
She recommended that people continue to consult the CDC’s COVID-19 county tracker to monitor local levels of COVID-19.
Dr. Walensky also shared recent findings from genomic sequencing that continue to show the predominance of the Omicron variant. “Essentially a hundred percent of what we’re finding now is Omicron,” she said. In terms of individual variants, the Omicron BA.1 variant is about 3% of circulating virus, the BA.2 variant is about 68%, and BA.2.12.1 makes up about 35%.
“We’re just starting to learn about the impact of BA2.121,” Dr. Walensky said. “It appears it might have a transmission advantage of about 25% over the BA2 subvariant.”
A version of this article first appeared on Medscape.com.
FROM MMWR