User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Obesity linked to combined OSA syndrome and severe asthma
Almost all patients with both obstructive sleep apnea syndrome and severe asthma fell into the obesity phenotype, not the allergy phenotype, based on data from nearly 1,500 adults.
Both asthma and sleep-disordered breathing are common conditions worldwide, and previous research suggests that obstructive sleep apnea syndrome (OSAS) and severe asthma in particular could be associated, wrote Laurent Portel, MD, of Centre Hospitalier de Libourne, France, and colleagues.
“Even if the underlying mechanisms are not well established, it is clear that both OSAS and obesity act to aggravate existing asthma, making it more difficult to control,” they said. However, the pathology of this relationship is not well-understood, and data on severe asthma phenotypes and OSAS are limited, they said.
In a study published in Respiratory Medicine and Research, the investigators reviewed data from 1,465 patients older than 18 years with severe asthma who were part of a larger, prospective multicenter study of the management of asthma patients. The larger study, developed by the Collège des Pneumologues des Hôpitaux Généraux (CPHG) is known as the FASE-CPHG (France Asthme SEvère-CPHG) and includes 104 nonacademic hospitals in France.
Diagnosis of OSAS was reported by physicians; diagnosis of severe asthma was based on the Global Initiative for Asthma criteria. The average age of the patients was 54.4 years, 63% were women, and 60% were nonsmokers.
A total of 161 patients were diagnosed with OSAS. The researchers conducted a cluster analysis on 1,424 patients, including 156 of the OSAS patients. They identified five clusters: early-onset atopic asthma (690 patients), obese asthma (153 patients), late-onset asthma (299 patients), eosinophilic asthma (143 patients), and aspirin sensitivity asthma (139 patients).
All 153 patients in the obese asthma cluster had OSAS, by contrast, none of the patients in the early atopic asthma cluster had OSAS.
Overall, obesity, male sex, high blood pressure, depression, late-onset asthma, and early-onset atopic asthma were independently associated with OSAS, with odds ratios of 5.782, 3.047, 2.875, 2.552, 1.789, and 0.622, respectively.
Notably, OSAS patients were more frequently treated with long-term oral corticosteroids than those without OSAS (30% vs. 15%, P < .0001), the researchers said. “It is possible that this treatment may be responsible for obesity, and it represents a well-known risk factor for developing OSAS,” they wrote.
Uncontrolled asthma was significantly more common in OSAS patients than in those without OSAS (77.7% vs. 69%, P = .03), and significantly more OSAS patients reported no or occasional physical activity (79.8% vs. 68.2%, P ≤ .001).
The study findings were limited by several factors including the lack of patients from primary care or university hospitals, which may limit the generalizability of the results, the reliance on physician statements for diagnosis of OSAS, and the lack of data on OSAS severity or treatment, the researchers noted.
However, the results fill a needed gap in the literature because of the limited data on severe asthma patients in real life, and identifying severe asthma patients by phenotype may help identify those at greatest risk for OSAS, they said.
“Identified patients could more easily benefit from specific examinations such as poly(somno)graphy and, consequently, could benefit from a better management of both asthma and OSAS,” they emphasized.
The larger FASE-CPHG study was supported in part by ALK, AstraZeneca, Boehringer Ingelheim, GSK, and Le Nouveau Souffle. The researchers had no financial conflicts to disclose.
Almost all patients with both obstructive sleep apnea syndrome and severe asthma fell into the obesity phenotype, not the allergy phenotype, based on data from nearly 1,500 adults.
Both asthma and sleep-disordered breathing are common conditions worldwide, and previous research suggests that obstructive sleep apnea syndrome (OSAS) and severe asthma in particular could be associated, wrote Laurent Portel, MD, of Centre Hospitalier de Libourne, France, and colleagues.
“Even if the underlying mechanisms are not well established, it is clear that both OSAS and obesity act to aggravate existing asthma, making it more difficult to control,” they said. However, the pathology of this relationship is not well-understood, and data on severe asthma phenotypes and OSAS are limited, they said.
In a study published in Respiratory Medicine and Research, the investigators reviewed data from 1,465 patients older than 18 years with severe asthma who were part of a larger, prospective multicenter study of the management of asthma patients. The larger study, developed by the Collège des Pneumologues des Hôpitaux Généraux (CPHG) is known as the FASE-CPHG (France Asthme SEvère-CPHG) and includes 104 nonacademic hospitals in France.
Diagnosis of OSAS was reported by physicians; diagnosis of severe asthma was based on the Global Initiative for Asthma criteria. The average age of the patients was 54.4 years, 63% were women, and 60% were nonsmokers.
A total of 161 patients were diagnosed with OSAS. The researchers conducted a cluster analysis on 1,424 patients, including 156 of the OSAS patients. They identified five clusters: early-onset atopic asthma (690 patients), obese asthma (153 patients), late-onset asthma (299 patients), eosinophilic asthma (143 patients), and aspirin sensitivity asthma (139 patients).
All 153 patients in the obese asthma cluster had OSAS, by contrast, none of the patients in the early atopic asthma cluster had OSAS.
Overall, obesity, male sex, high blood pressure, depression, late-onset asthma, and early-onset atopic asthma were independently associated with OSAS, with odds ratios of 5.782, 3.047, 2.875, 2.552, 1.789, and 0.622, respectively.
Notably, OSAS patients were more frequently treated with long-term oral corticosteroids than those without OSAS (30% vs. 15%, P < .0001), the researchers said. “It is possible that this treatment may be responsible for obesity, and it represents a well-known risk factor for developing OSAS,” they wrote.
Uncontrolled asthma was significantly more common in OSAS patients than in those without OSAS (77.7% vs. 69%, P = .03), and significantly more OSAS patients reported no or occasional physical activity (79.8% vs. 68.2%, P ≤ .001).
The study findings were limited by several factors including the lack of patients from primary care or university hospitals, which may limit the generalizability of the results, the reliance on physician statements for diagnosis of OSAS, and the lack of data on OSAS severity or treatment, the researchers noted.
However, the results fill a needed gap in the literature because of the limited data on severe asthma patients in real life, and identifying severe asthma patients by phenotype may help identify those at greatest risk for OSAS, they said.
“Identified patients could more easily benefit from specific examinations such as poly(somno)graphy and, consequently, could benefit from a better management of both asthma and OSAS,” they emphasized.
The larger FASE-CPHG study was supported in part by ALK, AstraZeneca, Boehringer Ingelheim, GSK, and Le Nouveau Souffle. The researchers had no financial conflicts to disclose.
Almost all patients with both obstructive sleep apnea syndrome and severe asthma fell into the obesity phenotype, not the allergy phenotype, based on data from nearly 1,500 adults.
Both asthma and sleep-disordered breathing are common conditions worldwide, and previous research suggests that obstructive sleep apnea syndrome (OSAS) and severe asthma in particular could be associated, wrote Laurent Portel, MD, of Centre Hospitalier de Libourne, France, and colleagues.
“Even if the underlying mechanisms are not well established, it is clear that both OSAS and obesity act to aggravate existing asthma, making it more difficult to control,” they said. However, the pathology of this relationship is not well-understood, and data on severe asthma phenotypes and OSAS are limited, they said.
In a study published in Respiratory Medicine and Research, the investigators reviewed data from 1,465 patients older than 18 years with severe asthma who were part of a larger, prospective multicenter study of the management of asthma patients. The larger study, developed by the Collège des Pneumologues des Hôpitaux Généraux (CPHG) is known as the FASE-CPHG (France Asthme SEvère-CPHG) and includes 104 nonacademic hospitals in France.
Diagnosis of OSAS was reported by physicians; diagnosis of severe asthma was based on the Global Initiative for Asthma criteria. The average age of the patients was 54.4 years, 63% were women, and 60% were nonsmokers.
A total of 161 patients were diagnosed with OSAS. The researchers conducted a cluster analysis on 1,424 patients, including 156 of the OSAS patients. They identified five clusters: early-onset atopic asthma (690 patients), obese asthma (153 patients), late-onset asthma (299 patients), eosinophilic asthma (143 patients), and aspirin sensitivity asthma (139 patients).
All 153 patients in the obese asthma cluster had OSAS, by contrast, none of the patients in the early atopic asthma cluster had OSAS.
Overall, obesity, male sex, high blood pressure, depression, late-onset asthma, and early-onset atopic asthma were independently associated with OSAS, with odds ratios of 5.782, 3.047, 2.875, 2.552, 1.789, and 0.622, respectively.
Notably, OSAS patients were more frequently treated with long-term oral corticosteroids than those without OSAS (30% vs. 15%, P < .0001), the researchers said. “It is possible that this treatment may be responsible for obesity, and it represents a well-known risk factor for developing OSAS,” they wrote.
Uncontrolled asthma was significantly more common in OSAS patients than in those without OSAS (77.7% vs. 69%, P = .03), and significantly more OSAS patients reported no or occasional physical activity (79.8% vs. 68.2%, P ≤ .001).
The study findings were limited by several factors including the lack of patients from primary care or university hospitals, which may limit the generalizability of the results, the reliance on physician statements for diagnosis of OSAS, and the lack of data on OSAS severity or treatment, the researchers noted.
However, the results fill a needed gap in the literature because of the limited data on severe asthma patients in real life, and identifying severe asthma patients by phenotype may help identify those at greatest risk for OSAS, they said.
“Identified patients could more easily benefit from specific examinations such as poly(somno)graphy and, consequently, could benefit from a better management of both asthma and OSAS,” they emphasized.
The larger FASE-CPHG study was supported in part by ALK, AstraZeneca, Boehringer Ingelheim, GSK, and Le Nouveau Souffle. The researchers had no financial conflicts to disclose.
FROM RESPIRATORY MEDICINE AND RESEARCH
New ACC guidance on cardiovascular consequences of COVID-19
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
The American College of Cardiology has issued an expert consensus clinical guidance document for the evaluation and management of adults with key cardiovascular consequences of COVID-19.
The document makes recommendations on how to evaluate and manage COVID-associated myocarditis and long COVID and gives advice on resumption of exercise following COVID-19 infection.
The clinical guidance was published online March 16 in the Journal of the American College of Cardiology.
“The best means to diagnose and treat myocarditis and long COVID following SARS-CoV-2 infection continues to evolve,” said Ty Gluckman, MD, MHA, cochair of the expert consensus decision pathway. “This document attempts to provide key recommendations for how to evaluate and manage adults with these conditions, including guidance for safe return to play for both competitive and noncompetitive athletes.”
The authors of the guidance note that COVID-19 can be associated with various abnormalities in cardiac testing and a wide range of cardiovascular complications. For some patients, cardiac symptoms such as chest pain, shortness of breath, fatigue, and palpitations persist, lasting months after the initial illness, and evidence of myocardial injury has also been observed in both symptomatic and asymptomatic individuals, as well as after receipt of the COVID-19 mRNA vaccine.
“For clinicians treating these individuals, a growing number of questions exist related to evaluation and management of these conditions, as well as safe resumption of physical activity,” they say. This report is intended to provide practical guidance on these issues.
Myocarditis
The report states that myocarditis has been recognized as a rare but serious complication of SARS-CoV-2 infection as well as COVID-19 mRNA vaccination.
It defines myocarditis as: 1.cardiac symptoms such as chest pain, dyspnea, palpitations, or syncope; 2. elevated cardiac troponin; and 3. abnormal electrocardiographic, echocardiographic, cardiac MRI, and/or histopathologic findings on biopsy.
The document makes the following recommendations in regard to COVID-related myocarditis:
When there is increased suspicion for cardiac involvement with COVID-19, initial testing should consist of an ECG, measurement of cardiac troponin, and an echocardiogram. Cardiology consultation is recommended for those with a rising cardiac troponin and/or echocardiographic abnormalities. Cardiac MRI is recommended in hemodynamically stable patients with suspected myocarditis.
Hospitalization is recommended for patients with definite myocarditis, ideally at an advanced heart failure center. Patients with fulminant myocarditis should be managed at centers with an expertise in advanced heart failure, mechanical circulatory support, and other advanced therapies.
Patients with myocarditis and COVID-19 pneumonia (with an ongoing need for supplemental oxygen) should be treated with corticosteroids. For patients with suspected pericardial involvement, treatment with NSAIDs, colchicine, and/or prednisone is reasonable. Intravenous corticosteroids may be considered in those with suspected or confirmed COVID-19 myocarditis with hemodynamic compromise or MIS-A (multisystem inflammatory syndrome in adults). Empiric use of corticosteroids may also be considered in those with biopsy evidence of severe myocardial infiltrates or fulminant myocarditis, balanced against infection risk.
As appropriate, guideline-directed medical therapy for heart failure should be initiated and continued after discharge.
The document notes that myocarditis following COVID-19 mRNA vaccination is rare, with highest rates seen in young males after the second vaccine dose. As of May 22, 2021, the U.S. Vaccine Adverse Event Reporting System noted rates of 40.6 cases per million after the second vaccine dose among male individuals aged 12-29 years and 2.4 cases per million among male individuals aged 30 and older. Corresponding rates in female individuals were 4.2 and 1 cases per million, respectively.
But the report says that COVID-19 vaccination is associated with “a very favorable benefit-to-risk ratio” for all age and sex groups evaluated thus far.
In general, vaccine-associated myocarditis should be diagnosed, categorized, and treated in a manner analogous to myocarditis following SARS-CoV-2 infection, the guidance advises.
Long COVID
The document refers to long COVID as postacute sequelae of SARS-CoV-2 infection (PASC), and reports that this condition is experienced by up to 10%-30% of infected individuals. It is defined by a constellation of new, returning, or persistent health problems experienced by individuals 4 or more weeks after COVID-19 infection.
Although individuals with this condition may experience wide-ranging symptoms, the symptoms that draw increased attention to the cardiovascular system include tachycardia, exercise intolerance, chest pain, and shortness of breath.
Nicole Bhave, MD, cochair of the expert consensus decision pathway, says: “There appears to be a ‘downward spiral’ for long-COVID patients. Fatigue and decreased exercise capacity lead to diminished activity and bed rest, in turn leading to worsening symptoms and decreased quality of life.” She adds that “the writing committee recommends a basic cardiopulmonary evaluation performed up front to determine if further specialty care and formalized medical therapy is needed for these patients.”
The authors propose two terms to better understand potential etiologies for those with cardiovascular symptoms:
PASC-CVD, or PASC-cardiovascular disease, refers to a broad group of cardiovascular conditions (including myocarditis) that manifest at least 4 weeks after COVID-19 infection.
PASC-CVS, or PASC-cardiovascular syndrome, includes a wide range of cardiovascular symptoms without objective evidence of cardiovascular disease following standard diagnostic testing.
The document makes the following recommendations for the management of PASC-CVD and PASC-CVS.
For patients with cardiovascular symptoms and suspected PASC, the authors suggest that a reasonable initial testing approach includes basic laboratory testing, including cardiac troponin, an ECG, an echocardiogram, an ambulatory rhythm monitor, chest imaging, and/or pulmonary function tests.
Cardiology consultation is recommended for patients with PASC who have abnormal cardiac test results, known cardiovascular disease with new or worsening symptoms, documented cardiac complications during SARS-CoV-2 infection, and/or persistent cardiopulmonary symptoms that are not otherwise explained.
Recumbent or semirecumbent exercise (for example, rowing, swimming, or cycling) is recommended initially for PASC-CVS patients with tachycardia, exercise/orthostatic intolerance, and/or deconditioning, with transition to upright exercise as orthostatic intolerance improves. Exercise duration should also be short (5-10 minutes/day) initially, with gradual increases as functional capacity improves.
Salt and fluid loading represent nonpharmacologic interventions that may provide symptomatic relief for patients with tachycardia, palpitations, and/or orthostatic hypotension.
Beta-blockers, nondihydropyridine calcium-channel blockers, ivabradine, fludrocortisone, and midodrine may be used empirically as well.
Return to play for athletes
The authors note that concerns about possible cardiac injury after COVID-19 fueled early apprehension regarding the safety of competitive sports for athletes recovering from the infection.
But they say that subsequent data from large registries have demonstrated an overall low prevalence of clinical myocarditis, without a rise in the rate of adverse cardiac events. Based on this, updated guidance is provided with a practical, evidence-based framework to guide resumption of athletics and intense exercise training.
They make the following recommendations:
- For athletes recovering from COVID-19 with ongoing cardiopulmonary symptoms (chest pain, shortness of breath, palpitations, lightheadedness) or those requiring hospitalization with increased suspicion for cardiac involvement, further evaluation with triad testing – an ECG, measurement of cardiac troponin, and an echocardiogram – should be performed.
- For those with abnormal test results, further evaluation with cardiac MRI should be considered. Individuals diagnosed with clinical myocarditis should abstain from exercise for 3-6 months.
- Cardiac testing is not recommended for asymptomatic individuals following COVID-19 infection. Individuals should abstain from training for 3 days to ensure that symptoms do not develop.
- For those with mild or moderate noncardiopulmonary symptoms (fever, lethargy, muscle aches), training may resume after symptom resolution.
- For those with remote infection (≥3 months) without ongoing cardiopulmonary symptoms, a gradual increase in exercise is recommended without the need for cardiac testing.
Based on the low prevalence of myocarditis observed in competitive athletes with COVID-19, the authors note that these recommendations can be reasonably applied to high-school athletes (aged 14 and older) along with adult recreational exercise enthusiasts.
Future study is needed, however, to better understand how long cardiac abnormalities persist following COVID-19 infection and the role of exercise training in long COVID.
The authors conclude that the current guidance is intended to help clinicians understand not only when testing may be warranted, but also when it is not.
“Given that it reflects the current state of knowledge through early 2022, it is anticipated that recommendations will change over time as our understanding evolves,” they say.
The 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19: Myocarditis, Post-Acute Sequelae of SARS-CoV-2 Infection (PASC), and Return to Play will be discussed in a session at the American College of Cardiology’s annual scientific session meeting in Washington in April.
A version of this article first appeared on Medscape.com.
French fries vs. almonds every day for a month: What changes?
Eat french fries every day for a month? Sure, as long as it’s for science.
That’s exactly what 107 people did in a scientific study, while 58 others ate a daily serving of almonds with the same number of calories.
At the end of the study, the researchers found no significant differences between the groups in people’s total amount of fat or their fasting glucose measures, according to the study, published Feb. 18 in the American Journal of Clinical Nutrition.
The french fry eaters gained a little more weight, but it was not statistically significant. The people who ate french fries gained 0.49 kilograms (just over a pound), vs. about a tenth of a kilogram (about one-fifth of a pound) in the group of people who ate almonds.
“The take-home is if you like almonds, eat some almonds. If you like potatoes, eat some potatoes, but don’t overeat either,” said study leader David B. Allison, PhD, a professor at Indiana University’s School of Public Health in Bloomington. ‘It’s probably good to have a little bit of each – each has some unique advantages in terms of nutrition.”
“This study confirms what registered dietitian nutritionists already know – all foods can fit. We can eat almonds, french fries, kale, and cookies,” said Melissa Majumdar, a registered dietitian and certified specialist in obesity and weight management at Emory University Hospital Midtown in Atlanta. ‘The consumption of one food or the avoidance of another does not make a healthy diet.”
At the same time, people should not interpret the results to mean it’s OK to eat french fries all day, every day. “We know that while potatoes are nutrient dense, the frying process reduces the nutritional value,” Ms. Majumdar said.
“Because french fries are often consumed alongside other nutrient-poor or high-fat foods, they should not be consumed daily but can fit into an overall balanced diet,” she added.
Would you like fries with that?
The researchers compared french fries to almonds because almonds are known for positive effects on energy balance, body composition, and low glycemic index. The research was partly funded by the Alliance for Potato Research and Education.
French fries are an incredibly popular food in the United States. According to an August 2021 post on the food website Mashed, Americans eat an average of 30 pounds of french fries each year.
Although consumption of almonds is increasing, Americans eat far less in volume each year than they do fries – an estimated 2.4 pounds of almonds per person, according to August 2021 figures from the Almond Board of California.
Dr. Allison and colleagues recruited 180 healthy adults for the study. Their average age was 30, and about two-thirds were women.
They randomly assigned 60 people to add about a medium serving of plain french fries (Tater Pals Ovenable Crinkle Cut Fries, Simplot Foods) to their diet. Another 60 people were assigned to the same amount of Tater Pals fries with herbs (oregano, basil, garlic, onion, and rosemary), and another 60 people ate Wonderful brand roasted and salted almonds.
Investigators told people to add either the potatoes or nuts to their diet every day for a month and gave no further instructions.
After some people dropped out of the study, results were based on 55 who ate regular french fries, 52 who ate french fries with herbs and spices, and 58 who ate the nuts.
The researchers scanned people to detect any changes in fat mass. They also measured changes in body weight, carbohydrate metabolism, and fasting blood glucose and insulin.
Key findings
Changes in total body fat mass and fat mass were not significantly different between the french fry groups and the almond group.
In terms of glycemic control, eating french fries for a month “is no better or worse than consuming a caloric equivalent of nuts,” the researchers noted.
Similarly, the change in total fat mass did not differ significantly among the three treatment groups.
Adding the herb and spice mix to the french fries did not make a significant difference on glycemic control, contrary to what the researchers thought might happen.
And fasting glucose, insulin, and HbA1c levels did not differ significantly between the combined french fry and almond groups. When comparisons were made among the three groups, the almond group had a lower insulin response, compared to the plain french fry group.
Many different things could be explored in future research, said study coauthor Rebecca Hanson, a registered dietitian nutritionist and research study coordinator at the University of Alabama at Birmingham. “People were not told to change their exercise or diet, so there are so many different variables,” she said. Repeating the research in people with diabetes is another possibility going forward.
The researchers acknowledged that 30 days may not have been long enough to show a significant difference. But they also noted that many previous studies were observational while they used a randomized controlled trial, considered a more robust study design.
Dr. Allison, the senior author, emphasized that this is just one study. “No one study has all the answers.
“I don’t want to tell you our results are the be all and end all or that we’ve now learned everything there is to learn about potatoes and almonds,” he said.
“Our study shows for the variables we looked at ... we did not see important, discernible differences,” he said. “That doesn’t mean if you ate 500 potatoes a day or 500 kilograms of almonds it would be the same. But at these modest levels, it doesn’t seem to make much difference.”
The study was funded by grants from the National Institutes of Health and from the Alliance for Potato Research and Education.
Asked if the industry support should be a concern, Ms. Majumdar said, “Funding from a specific food board does not necessarily dilute the results of a well-designed study. It’s not uncommon for a funding source to come from a food board that may benefit from the findings. Research money has to come from somewhere.
“This study has reputable researchers, some of the best in the field,” she said.
The U.S. produces the most almonds in the world, and California is the only state where almonds are grown commercially. Asked for the almond industry’s take on the findings, “We don’t have a comment,” said Rick Kushman, a spokesman for the Almond Board of California.
A version of this article first appeared on WebMD.com.
Eat french fries every day for a month? Sure, as long as it’s for science.
That’s exactly what 107 people did in a scientific study, while 58 others ate a daily serving of almonds with the same number of calories.
At the end of the study, the researchers found no significant differences between the groups in people’s total amount of fat or their fasting glucose measures, according to the study, published Feb. 18 in the American Journal of Clinical Nutrition.
The french fry eaters gained a little more weight, but it was not statistically significant. The people who ate french fries gained 0.49 kilograms (just over a pound), vs. about a tenth of a kilogram (about one-fifth of a pound) in the group of people who ate almonds.
“The take-home is if you like almonds, eat some almonds. If you like potatoes, eat some potatoes, but don’t overeat either,” said study leader David B. Allison, PhD, a professor at Indiana University’s School of Public Health in Bloomington. ‘It’s probably good to have a little bit of each – each has some unique advantages in terms of nutrition.”
“This study confirms what registered dietitian nutritionists already know – all foods can fit. We can eat almonds, french fries, kale, and cookies,” said Melissa Majumdar, a registered dietitian and certified specialist in obesity and weight management at Emory University Hospital Midtown in Atlanta. ‘The consumption of one food or the avoidance of another does not make a healthy diet.”
At the same time, people should not interpret the results to mean it’s OK to eat french fries all day, every day. “We know that while potatoes are nutrient dense, the frying process reduces the nutritional value,” Ms. Majumdar said.
“Because french fries are often consumed alongside other nutrient-poor or high-fat foods, they should not be consumed daily but can fit into an overall balanced diet,” she added.
Would you like fries with that?
The researchers compared french fries to almonds because almonds are known for positive effects on energy balance, body composition, and low glycemic index. The research was partly funded by the Alliance for Potato Research and Education.
French fries are an incredibly popular food in the United States. According to an August 2021 post on the food website Mashed, Americans eat an average of 30 pounds of french fries each year.
Although consumption of almonds is increasing, Americans eat far less in volume each year than they do fries – an estimated 2.4 pounds of almonds per person, according to August 2021 figures from the Almond Board of California.
Dr. Allison and colleagues recruited 180 healthy adults for the study. Their average age was 30, and about two-thirds were women.
They randomly assigned 60 people to add about a medium serving of plain french fries (Tater Pals Ovenable Crinkle Cut Fries, Simplot Foods) to their diet. Another 60 people were assigned to the same amount of Tater Pals fries with herbs (oregano, basil, garlic, onion, and rosemary), and another 60 people ate Wonderful brand roasted and salted almonds.
Investigators told people to add either the potatoes or nuts to their diet every day for a month and gave no further instructions.
After some people dropped out of the study, results were based on 55 who ate regular french fries, 52 who ate french fries with herbs and spices, and 58 who ate the nuts.
The researchers scanned people to detect any changes in fat mass. They also measured changes in body weight, carbohydrate metabolism, and fasting blood glucose and insulin.
Key findings
Changes in total body fat mass and fat mass were not significantly different between the french fry groups and the almond group.
In terms of glycemic control, eating french fries for a month “is no better or worse than consuming a caloric equivalent of nuts,” the researchers noted.
Similarly, the change in total fat mass did not differ significantly among the three treatment groups.
Adding the herb and spice mix to the french fries did not make a significant difference on glycemic control, contrary to what the researchers thought might happen.
And fasting glucose, insulin, and HbA1c levels did not differ significantly between the combined french fry and almond groups. When comparisons were made among the three groups, the almond group had a lower insulin response, compared to the plain french fry group.
Many different things could be explored in future research, said study coauthor Rebecca Hanson, a registered dietitian nutritionist and research study coordinator at the University of Alabama at Birmingham. “People were not told to change their exercise or diet, so there are so many different variables,” she said. Repeating the research in people with diabetes is another possibility going forward.
The researchers acknowledged that 30 days may not have been long enough to show a significant difference. But they also noted that many previous studies were observational while they used a randomized controlled trial, considered a more robust study design.
Dr. Allison, the senior author, emphasized that this is just one study. “No one study has all the answers.
“I don’t want to tell you our results are the be all and end all or that we’ve now learned everything there is to learn about potatoes and almonds,” he said.
“Our study shows for the variables we looked at ... we did not see important, discernible differences,” he said. “That doesn’t mean if you ate 500 potatoes a day or 500 kilograms of almonds it would be the same. But at these modest levels, it doesn’t seem to make much difference.”
The study was funded by grants from the National Institutes of Health and from the Alliance for Potato Research and Education.
Asked if the industry support should be a concern, Ms. Majumdar said, “Funding from a specific food board does not necessarily dilute the results of a well-designed study. It’s not uncommon for a funding source to come from a food board that may benefit from the findings. Research money has to come from somewhere.
“This study has reputable researchers, some of the best in the field,” she said.
The U.S. produces the most almonds in the world, and California is the only state where almonds are grown commercially. Asked for the almond industry’s take on the findings, “We don’t have a comment,” said Rick Kushman, a spokesman for the Almond Board of California.
A version of this article first appeared on WebMD.com.
Eat french fries every day for a month? Sure, as long as it’s for science.
That’s exactly what 107 people did in a scientific study, while 58 others ate a daily serving of almonds with the same number of calories.
At the end of the study, the researchers found no significant differences between the groups in people’s total amount of fat or their fasting glucose measures, according to the study, published Feb. 18 in the American Journal of Clinical Nutrition.
The french fry eaters gained a little more weight, but it was not statistically significant. The people who ate french fries gained 0.49 kilograms (just over a pound), vs. about a tenth of a kilogram (about one-fifth of a pound) in the group of people who ate almonds.
“The take-home is if you like almonds, eat some almonds. If you like potatoes, eat some potatoes, but don’t overeat either,” said study leader David B. Allison, PhD, a professor at Indiana University’s School of Public Health in Bloomington. ‘It’s probably good to have a little bit of each – each has some unique advantages in terms of nutrition.”
“This study confirms what registered dietitian nutritionists already know – all foods can fit. We can eat almonds, french fries, kale, and cookies,” said Melissa Majumdar, a registered dietitian and certified specialist in obesity and weight management at Emory University Hospital Midtown in Atlanta. ‘The consumption of one food or the avoidance of another does not make a healthy diet.”
At the same time, people should not interpret the results to mean it’s OK to eat french fries all day, every day. “We know that while potatoes are nutrient dense, the frying process reduces the nutritional value,” Ms. Majumdar said.
“Because french fries are often consumed alongside other nutrient-poor or high-fat foods, they should not be consumed daily but can fit into an overall balanced diet,” she added.
Would you like fries with that?
The researchers compared french fries to almonds because almonds are known for positive effects on energy balance, body composition, and low glycemic index. The research was partly funded by the Alliance for Potato Research and Education.
French fries are an incredibly popular food in the United States. According to an August 2021 post on the food website Mashed, Americans eat an average of 30 pounds of french fries each year.
Although consumption of almonds is increasing, Americans eat far less in volume each year than they do fries – an estimated 2.4 pounds of almonds per person, according to August 2021 figures from the Almond Board of California.
Dr. Allison and colleagues recruited 180 healthy adults for the study. Their average age was 30, and about two-thirds were women.
They randomly assigned 60 people to add about a medium serving of plain french fries (Tater Pals Ovenable Crinkle Cut Fries, Simplot Foods) to their diet. Another 60 people were assigned to the same amount of Tater Pals fries with herbs (oregano, basil, garlic, onion, and rosemary), and another 60 people ate Wonderful brand roasted and salted almonds.
Investigators told people to add either the potatoes or nuts to their diet every day for a month and gave no further instructions.
After some people dropped out of the study, results were based on 55 who ate regular french fries, 52 who ate french fries with herbs and spices, and 58 who ate the nuts.
The researchers scanned people to detect any changes in fat mass. They also measured changes in body weight, carbohydrate metabolism, and fasting blood glucose and insulin.
Key findings
Changes in total body fat mass and fat mass were not significantly different between the french fry groups and the almond group.
In terms of glycemic control, eating french fries for a month “is no better or worse than consuming a caloric equivalent of nuts,” the researchers noted.
Similarly, the change in total fat mass did not differ significantly among the three treatment groups.
Adding the herb and spice mix to the french fries did not make a significant difference on glycemic control, contrary to what the researchers thought might happen.
And fasting glucose, insulin, and HbA1c levels did not differ significantly between the combined french fry and almond groups. When comparisons were made among the three groups, the almond group had a lower insulin response, compared to the plain french fry group.
Many different things could be explored in future research, said study coauthor Rebecca Hanson, a registered dietitian nutritionist and research study coordinator at the University of Alabama at Birmingham. “People were not told to change their exercise or diet, so there are so many different variables,” she said. Repeating the research in people with diabetes is another possibility going forward.
The researchers acknowledged that 30 days may not have been long enough to show a significant difference. But they also noted that many previous studies were observational while they used a randomized controlled trial, considered a more robust study design.
Dr. Allison, the senior author, emphasized that this is just one study. “No one study has all the answers.
“I don’t want to tell you our results are the be all and end all or that we’ve now learned everything there is to learn about potatoes and almonds,” he said.
“Our study shows for the variables we looked at ... we did not see important, discernible differences,” he said. “That doesn’t mean if you ate 500 potatoes a day or 500 kilograms of almonds it would be the same. But at these modest levels, it doesn’t seem to make much difference.”
The study was funded by grants from the National Institutes of Health and from the Alliance for Potato Research and Education.
Asked if the industry support should be a concern, Ms. Majumdar said, “Funding from a specific food board does not necessarily dilute the results of a well-designed study. It’s not uncommon for a funding source to come from a food board that may benefit from the findings. Research money has to come from somewhere.
“This study has reputable researchers, some of the best in the field,” she said.
The U.S. produces the most almonds in the world, and California is the only state where almonds are grown commercially. Asked for the almond industry’s take on the findings, “We don’t have a comment,” said Rick Kushman, a spokesman for the Almond Board of California.
A version of this article first appeared on WebMD.com.
FROM AMERICAN JOURNAL OF CLINICAL NUTRITION
High-intensity exercise vs. omega-3s for heart failure risk reduction
A year of high-intensity interval training seemed to benefit obese middle-aged adults at a high risk of heart failure, but omega-3 fatty acid supplementation didn’t have any effect on cardiac biomarkers measured in a small, single-center, prospective study.
“One year of HIIT training reduces adiposity but had no consistent effect on myocardial triglyceride content or visceral adiposity,” wrote lead author Christopher M. Hearon Jr., PhD, and colleagues in JACC: Heart Failure. “However, long-duration HIIT improves fitness and induces favorable cardiac remodeling.” Omega-3 supplementation, however, had “no independent or additive effect.” Dr. Hearon is an instructor of applied clinical research at University of Texas Southwestern Medical Center in Dallas.
Investigators there and at the Institute for Exercise and Environmental Medicine at Texas Health Presbyterian Hospital Dallas studied 80 patients aged 40-55 years classified as high risk for HF and obese, randomizing them to a year of high-intensity interval training (HIIT) with supplementation of either 1.6 g omega-3 FA or placebo daily; or to a control group split between supplementation or placebo. Fifty-six patients completed the 1-year study, with a compliance rate of 90% in the HIIT group and 92% in those assigned omega-3 FA supplementation.
Carl J. “Chip” Lavie, MD, of the John Ochsner Heart and Vascular Institute in New Orleans, commented that, although the study was “extremely well done from an excellent research group,” it was limited by its small population and relatively short follow-up. Future research should evaluate HIIT and moderate exercise on clinical events over a longer term as well as different doses of omega-3 “There is tremendous potential for omega-3 in heart failure prevention and treatment.”
HIIT boosts exercise capacity, more
In the study, the HIIT group showed improvement in a number of cardiac markers: around a 22% improvement in exercise capacity as measured by absolute peak and relative peak oxygen uptake (VO2), even without significant weight loss. They improved an average of 0.43 L/min (0.32-0.53; P < .0001) and 4.46 mL/kg per minute (3.18-5.56; P < .0001), respectively.
The researchers attributed the increase in peak VO2 to an increase in peak cardiac output averaging 2.15 L/min (95% confidence interval, 0.90-3.39; P = .001) and stroke volume averaging 9.46 mL (95% CI, 0.65-18.27; P = .04). A year of exercise training also resulted in changes in cardiac remodeling, including increases in left ventricle mass and LV end diastolic volume, averaging 9.4 g (95% CI, 4.36-14.44; P < .001) and 12.33 mL (95% CI, 5.61-19.05; P < .001), respectively.
The study also found that neither intervention had any appreciable impact on body weight, body mass index, body surface area or lean mass, or markers of arterial or local carotid stiffness. The exercise group had a modest decrease in fat mass, averaging 2.63 kg (95% CI,–4.81 to –0.46; P = .02), but without any effect from omega-3 supplementation.
The study acknowledged that high-dose omega-3 supplements have been found to lower triglyceride levels in people with severe hypertriglyceridemia, and hypothesized that HIIT alone or with omega-3 supplementation would improve fitness and biomarkers in people with stage A HF. “Contrary to our hypothesis, we found that one year of n-3FA [omega-3 FA] supplementation had no detectable effect on any parameter related to cardiopulmonary fitness, cardiovascular remodeling/stiffness, visceral adiposity, or myocardial triglyceride content,” Dr. Hearon and colleagues wrote.
The study “shows that obese middle-aged patients with heart failure with preserved ejection fraction [HFpEF] can markedly improve their fitness with HIIT and, generally, fitness is one of the strongest if not the strongest predictor of prognosis and survival,” said Dr. Lavie.
“Studies are needed on exercise that improves fitness in both HF with reduced ejection fraction and HFpEF, but especially HFpEF,” he said.
The study received funding from the American Heart Association Strategically Focused Research Network. Dr. Hearon and coauthors have no relevant disclosures. Dr. Lavie is a speaker and consultant for PAI Health, the Global Organization for EPA and DHA Omega-3s and DSM Nutritional Products.
A year of high-intensity interval training seemed to benefit obese middle-aged adults at a high risk of heart failure, but omega-3 fatty acid supplementation didn’t have any effect on cardiac biomarkers measured in a small, single-center, prospective study.
“One year of HIIT training reduces adiposity but had no consistent effect on myocardial triglyceride content or visceral adiposity,” wrote lead author Christopher M. Hearon Jr., PhD, and colleagues in JACC: Heart Failure. “However, long-duration HIIT improves fitness and induces favorable cardiac remodeling.” Omega-3 supplementation, however, had “no independent or additive effect.” Dr. Hearon is an instructor of applied clinical research at University of Texas Southwestern Medical Center in Dallas.
Investigators there and at the Institute for Exercise and Environmental Medicine at Texas Health Presbyterian Hospital Dallas studied 80 patients aged 40-55 years classified as high risk for HF and obese, randomizing them to a year of high-intensity interval training (HIIT) with supplementation of either 1.6 g omega-3 FA or placebo daily; or to a control group split between supplementation or placebo. Fifty-six patients completed the 1-year study, with a compliance rate of 90% in the HIIT group and 92% in those assigned omega-3 FA supplementation.
Carl J. “Chip” Lavie, MD, of the John Ochsner Heart and Vascular Institute in New Orleans, commented that, although the study was “extremely well done from an excellent research group,” it was limited by its small population and relatively short follow-up. Future research should evaluate HIIT and moderate exercise on clinical events over a longer term as well as different doses of omega-3 “There is tremendous potential for omega-3 in heart failure prevention and treatment.”
HIIT boosts exercise capacity, more
In the study, the HIIT group showed improvement in a number of cardiac markers: around a 22% improvement in exercise capacity as measured by absolute peak and relative peak oxygen uptake (VO2), even without significant weight loss. They improved an average of 0.43 L/min (0.32-0.53; P < .0001) and 4.46 mL/kg per minute (3.18-5.56; P < .0001), respectively.
The researchers attributed the increase in peak VO2 to an increase in peak cardiac output averaging 2.15 L/min (95% confidence interval, 0.90-3.39; P = .001) and stroke volume averaging 9.46 mL (95% CI, 0.65-18.27; P = .04). A year of exercise training also resulted in changes in cardiac remodeling, including increases in left ventricle mass and LV end diastolic volume, averaging 9.4 g (95% CI, 4.36-14.44; P < .001) and 12.33 mL (95% CI, 5.61-19.05; P < .001), respectively.
The study also found that neither intervention had any appreciable impact on body weight, body mass index, body surface area or lean mass, or markers of arterial or local carotid stiffness. The exercise group had a modest decrease in fat mass, averaging 2.63 kg (95% CI,–4.81 to –0.46; P = .02), but without any effect from omega-3 supplementation.
The study acknowledged that high-dose omega-3 supplements have been found to lower triglyceride levels in people with severe hypertriglyceridemia, and hypothesized that HIIT alone or with omega-3 supplementation would improve fitness and biomarkers in people with stage A HF. “Contrary to our hypothesis, we found that one year of n-3FA [omega-3 FA] supplementation had no detectable effect on any parameter related to cardiopulmonary fitness, cardiovascular remodeling/stiffness, visceral adiposity, or myocardial triglyceride content,” Dr. Hearon and colleagues wrote.
The study “shows that obese middle-aged patients with heart failure with preserved ejection fraction [HFpEF] can markedly improve their fitness with HIIT and, generally, fitness is one of the strongest if not the strongest predictor of prognosis and survival,” said Dr. Lavie.
“Studies are needed on exercise that improves fitness in both HF with reduced ejection fraction and HFpEF, but especially HFpEF,” he said.
The study received funding from the American Heart Association Strategically Focused Research Network. Dr. Hearon and coauthors have no relevant disclosures. Dr. Lavie is a speaker and consultant for PAI Health, the Global Organization for EPA and DHA Omega-3s and DSM Nutritional Products.
A year of high-intensity interval training seemed to benefit obese middle-aged adults at a high risk of heart failure, but omega-3 fatty acid supplementation didn’t have any effect on cardiac biomarkers measured in a small, single-center, prospective study.
“One year of HIIT training reduces adiposity but had no consistent effect on myocardial triglyceride content or visceral adiposity,” wrote lead author Christopher M. Hearon Jr., PhD, and colleagues in JACC: Heart Failure. “However, long-duration HIIT improves fitness and induces favorable cardiac remodeling.” Omega-3 supplementation, however, had “no independent or additive effect.” Dr. Hearon is an instructor of applied clinical research at University of Texas Southwestern Medical Center in Dallas.
Investigators there and at the Institute for Exercise and Environmental Medicine at Texas Health Presbyterian Hospital Dallas studied 80 patients aged 40-55 years classified as high risk for HF and obese, randomizing them to a year of high-intensity interval training (HIIT) with supplementation of either 1.6 g omega-3 FA or placebo daily; or to a control group split between supplementation or placebo. Fifty-six patients completed the 1-year study, with a compliance rate of 90% in the HIIT group and 92% in those assigned omega-3 FA supplementation.
Carl J. “Chip” Lavie, MD, of the John Ochsner Heart and Vascular Institute in New Orleans, commented that, although the study was “extremely well done from an excellent research group,” it was limited by its small population and relatively short follow-up. Future research should evaluate HIIT and moderate exercise on clinical events over a longer term as well as different doses of omega-3 “There is tremendous potential for omega-3 in heart failure prevention and treatment.”
HIIT boosts exercise capacity, more
In the study, the HIIT group showed improvement in a number of cardiac markers: around a 22% improvement in exercise capacity as measured by absolute peak and relative peak oxygen uptake (VO2), even without significant weight loss. They improved an average of 0.43 L/min (0.32-0.53; P < .0001) and 4.46 mL/kg per minute (3.18-5.56; P < .0001), respectively.
The researchers attributed the increase in peak VO2 to an increase in peak cardiac output averaging 2.15 L/min (95% confidence interval, 0.90-3.39; P = .001) and stroke volume averaging 9.46 mL (95% CI, 0.65-18.27; P = .04). A year of exercise training also resulted in changes in cardiac remodeling, including increases in left ventricle mass and LV end diastolic volume, averaging 9.4 g (95% CI, 4.36-14.44; P < .001) and 12.33 mL (95% CI, 5.61-19.05; P < .001), respectively.
The study also found that neither intervention had any appreciable impact on body weight, body mass index, body surface area or lean mass, or markers of arterial or local carotid stiffness. The exercise group had a modest decrease in fat mass, averaging 2.63 kg (95% CI,–4.81 to –0.46; P = .02), but without any effect from omega-3 supplementation.
The study acknowledged that high-dose omega-3 supplements have been found to lower triglyceride levels in people with severe hypertriglyceridemia, and hypothesized that HIIT alone or with omega-3 supplementation would improve fitness and biomarkers in people with stage A HF. “Contrary to our hypothesis, we found that one year of n-3FA [omega-3 FA] supplementation had no detectable effect on any parameter related to cardiopulmonary fitness, cardiovascular remodeling/stiffness, visceral adiposity, or myocardial triglyceride content,” Dr. Hearon and colleagues wrote.
The study “shows that obese middle-aged patients with heart failure with preserved ejection fraction [HFpEF] can markedly improve their fitness with HIIT and, generally, fitness is one of the strongest if not the strongest predictor of prognosis and survival,” said Dr. Lavie.
“Studies are needed on exercise that improves fitness in both HF with reduced ejection fraction and HFpEF, but especially HFpEF,” he said.
The study received funding from the American Heart Association Strategically Focused Research Network. Dr. Hearon and coauthors have no relevant disclosures. Dr. Lavie is a speaker and consultant for PAI Health, the Global Organization for EPA and DHA Omega-3s and DSM Nutritional Products.
FROM JACC: HEART FAILURE
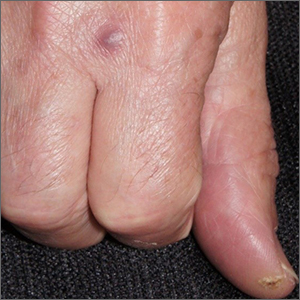
“Fishy” papule
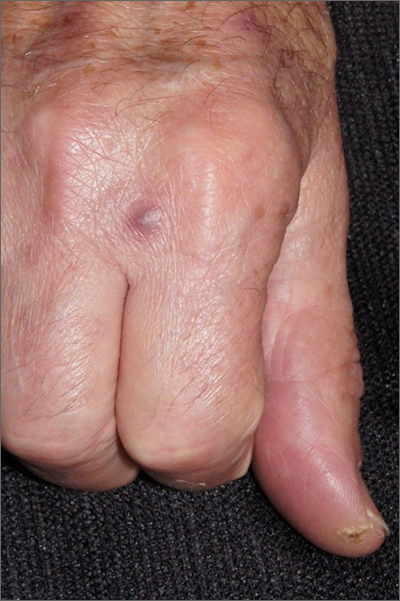
A biopsy was performed to exclude squamous cell carcinoma and an additional biopsy was sent for tissue culture for aerobic and acid-fast bacteria. The culture revealed a surprising diagnosis: cutaneous mycobacterium marinum.
Mycobacterium marinum is one of many nontuberculosis mycobacteria that may rarely cause infections in immunocompetent patients. M marinum is found worldwide in saltwater and freshwater. Infections may occur in individuals working in fisheries or fish markets, natural marine environments, or with aquariums. The infection may gain access through small, even unnoticed breaks in the skin. Papules, pustules, or abscesses caused by M marinum develop a few weeks after exposure and share many features with other common skin infections, including Staphylococcus aureus. Lymphatic involvement and sporotrichoid spread may occur. Immunocompromised patients can experience deeper involvement into tendons. Patients with significant soft tissue pain should undergo computed tomography, or preferably magnetic resonance imaging, to determine the extent of disease.
For immunocompetent patients and those with limited disease, as in this case, spontaneous resolution can occur after a year or more. However, because of the potential risk of more severe disease, treatment is recommended. M marinum is resistant to multiple antibiotics and there are no standardized treatment guidelines. Minocycline 100 mg bid for 3 weeks to 3 months is 1 accepted regimen for limited disease; treatment should be continued for 3 to 4 weeks following clinical resolution.1 Patients with more widespread disease benefit from evaluation by Infectious Diseases. Patients exposed to atypical mycobacteria may have false positive QuantiFERON-TB Gold tests that are commonly performed prior to biologic therapies.2
This patient achieved complete resolution of his signs and symptoms after receiving minocycline 100 mg bid for 6 weeks. He continues to fish recreationally.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978. doi: 10.1517/14656566.8.17.2965
2. Gajurel K, Subramanian AK. False-positive QuantiFERON TB-Gold test due to Mycobacterium gordonae. Diagn Microbiol Infect Dis. 2016;84:315-317. doi: 10.1016/j.diagmicrobio.2015.10.020

A biopsy was performed to exclude squamous cell carcinoma and an additional biopsy was sent for tissue culture for aerobic and acid-fast bacteria. The culture revealed a surprising diagnosis: cutaneous mycobacterium marinum.
Mycobacterium marinum is one of many nontuberculosis mycobacteria that may rarely cause infections in immunocompetent patients. M marinum is found worldwide in saltwater and freshwater. Infections may occur in individuals working in fisheries or fish markets, natural marine environments, or with aquariums. The infection may gain access through small, even unnoticed breaks in the skin. Papules, pustules, or abscesses caused by M marinum develop a few weeks after exposure and share many features with other common skin infections, including Staphylococcus aureus. Lymphatic involvement and sporotrichoid spread may occur. Immunocompromised patients can experience deeper involvement into tendons. Patients with significant soft tissue pain should undergo computed tomography, or preferably magnetic resonance imaging, to determine the extent of disease.
For immunocompetent patients and those with limited disease, as in this case, spontaneous resolution can occur after a year or more. However, because of the potential risk of more severe disease, treatment is recommended. M marinum is resistant to multiple antibiotics and there are no standardized treatment guidelines. Minocycline 100 mg bid for 3 weeks to 3 months is 1 accepted regimen for limited disease; treatment should be continued for 3 to 4 weeks following clinical resolution.1 Patients with more widespread disease benefit from evaluation by Infectious Diseases. Patients exposed to atypical mycobacteria may have false positive QuantiFERON-TB Gold tests that are commonly performed prior to biologic therapies.2
This patient achieved complete resolution of his signs and symptoms after receiving minocycline 100 mg bid for 6 weeks. He continues to fish recreationally.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

A biopsy was performed to exclude squamous cell carcinoma and an additional biopsy was sent for tissue culture for aerobic and acid-fast bacteria. The culture revealed a surprising diagnosis: cutaneous mycobacterium marinum.
Mycobacterium marinum is one of many nontuberculosis mycobacteria that may rarely cause infections in immunocompetent patients. M marinum is found worldwide in saltwater and freshwater. Infections may occur in individuals working in fisheries or fish markets, natural marine environments, or with aquariums. The infection may gain access through small, even unnoticed breaks in the skin. Papules, pustules, or abscesses caused by M marinum develop a few weeks after exposure and share many features with other common skin infections, including Staphylococcus aureus. Lymphatic involvement and sporotrichoid spread may occur. Immunocompromised patients can experience deeper involvement into tendons. Patients with significant soft tissue pain should undergo computed tomography, or preferably magnetic resonance imaging, to determine the extent of disease.
For immunocompetent patients and those with limited disease, as in this case, spontaneous resolution can occur after a year or more. However, because of the potential risk of more severe disease, treatment is recommended. M marinum is resistant to multiple antibiotics and there are no standardized treatment guidelines. Minocycline 100 mg bid for 3 weeks to 3 months is 1 accepted regimen for limited disease; treatment should be continued for 3 to 4 weeks following clinical resolution.1 Patients with more widespread disease benefit from evaluation by Infectious Diseases. Patients exposed to atypical mycobacteria may have false positive QuantiFERON-TB Gold tests that are commonly performed prior to biologic therapies.2
This patient achieved complete resolution of his signs and symptoms after receiving minocycline 100 mg bid for 6 weeks. He continues to fish recreationally.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978. doi: 10.1517/14656566.8.17.2965
2. Gajurel K, Subramanian AK. False-positive QuantiFERON TB-Gold test due to Mycobacterium gordonae. Diagn Microbiol Infect Dis. 2016;84:315-317. doi: 10.1016/j.diagmicrobio.2015.10.020
1. Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978. doi: 10.1517/14656566.8.17.2965
2. Gajurel K, Subramanian AK. False-positive QuantiFERON TB-Gold test due to Mycobacterium gordonae. Diagn Microbiol Infect Dis. 2016;84:315-317. doi: 10.1016/j.diagmicrobio.2015.10.020
Just one extra drink a day may change the brain
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
It’s no secret that heavy drinking is linked to potential health problems, from liver damage to a higher risk of cancer. But most people probably wouldn’t think a nightcap every evening is much of a health threat.
Now, new evidence published in Nature Communications suggests
Previous research has found that people with alcohol use disorder have structural changes in their brains, compared with healthy people’s brains, such as reduced gray-matter and white-matter volume.
But those findings were in people with a history of heavy drinking, defined by the National Institute on Alcohol Abuse and Alcoholism as more than four drinks a day for men and more than three drinks a day for women.
The national dietary guidelines from the U.S. Department of Health & Human Services advise drinking no more than two standard drinks for men and one drink for women each day. A standard drink in the United States is 12 ounces of beer, 5 ounces of wine, or 1½ ounce of liquor.
But could even this modest amount of alcohol make a difference to our brains?
Researchers examined functional MRI brain scans from 36,678 healthy adults, aged 40-69 years, in the United Kingdom and compared those findings with their weekly alcohol consumption, adjusting for differences in age, sex, height, social and economic status, and country of residence, among other things.
In line with past studies, the researchers found that, as a person drank more alcohol, their gray-matter and white-matter volume decreased, getting worse the more drinks they had in a week.
But the researchers also noted that they could tell the difference between brain images of people who never drank alcohol and those who had just one or two drinks a day.
Going from 1 unit of alcohol to 2 – which in the United Kingdom means a full pint of beer or standard glass of wine – was linked to changes similar to 2 years of aging in the brain.
Other than comparing the changes with aging, it’s not yet clear what the findings mean until the scientists do more research, including looking at the genes of the people who took part in the study.
The study also has several drawbacks. The people who were studied are all middle-aged Europeans, so findings might be different in younger people or those with different ancestries. People also self-reported how much alcohol they drank for the past year, which they might not remember correctly or which might be different from previous years, including past years of heavy drinking.
And since the researchers compared drinking habits with brain imaging at one point in time, it’s not possible to say whether alcohol is actually causing the brain differences they saw.
Still, the findings raise the question of whether national guidelines should be revisited, and whether it’s better to cut that evening drink to a half-glass of wine instead.
A version of this article first appeared on WebMD.com.
FROM NATURE COMMUNICATIONS
FDA approves generic Symbicort for asthma, COPD
The U.S. Food and Drug Administration approved the first generic of Symbicort (budesonide and formoterol fumarate dihydrate) inhalation aerosol for the treatment of asthma in patients 6 years of age and older and for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
The approval was given for a complex generic drug-device combination product – a metered-dose inhaler that contains both budesonide (a corticosteroid that reduces inflammation) and formoterol (a long-acting bronchodilator that relaxes muscles in the airways to improve breathing). It is intended to be used as two inhalations, two times a day (usually morning and night, about 12 hours apart), to treat both diseases by preventing symptoms, such as wheezing for those with asthma and for improved breathing for patients with COPD.
The inhaler is approved at two strengths (160/4.5 mcg/actuation and 80/4.5 mcg/actuation), according to the March 15 FDA announcement. The device is not intended for the treatment of acute asthma.
“Today’s approval of the first generic for one of the most commonly prescribed complex drug-device combination products to treat asthma and COPD is another step forward in our commitment to bring generic copies of complex drugs to the market, which can improve quality of life and help reduce the cost of treatment,” said Sally Choe, PhD, director of the Office of Generic Drugs in the FDA’s Center for Drug Evaluation and Research.
The most common side effects associated with budesonide and formoterol fumarate dihydrate oral inhalation aerosol for those with asthma are nasopharyngitis pain, sinusitis, influenza, back pain, nasal congestion, stomach discomfort, vomiting, and oral candidiasis (thrush). For those with COPD, the most common side effects are nasopharyngitis, oral candidiasis, bronchitis, sinusitis, and upper respiratory tract infection, the FDA reported.
The approval of this generic drug-device combination was granted to Mylan Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration approved the first generic of Symbicort (budesonide and formoterol fumarate dihydrate) inhalation aerosol for the treatment of asthma in patients 6 years of age and older and for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
The approval was given for a complex generic drug-device combination product – a metered-dose inhaler that contains both budesonide (a corticosteroid that reduces inflammation) and formoterol (a long-acting bronchodilator that relaxes muscles in the airways to improve breathing). It is intended to be used as two inhalations, two times a day (usually morning and night, about 12 hours apart), to treat both diseases by preventing symptoms, such as wheezing for those with asthma and for improved breathing for patients with COPD.
The inhaler is approved at two strengths (160/4.5 mcg/actuation and 80/4.5 mcg/actuation), according to the March 15 FDA announcement. The device is not intended for the treatment of acute asthma.
“Today’s approval of the first generic for one of the most commonly prescribed complex drug-device combination products to treat asthma and COPD is another step forward in our commitment to bring generic copies of complex drugs to the market, which can improve quality of life and help reduce the cost of treatment,” said Sally Choe, PhD, director of the Office of Generic Drugs in the FDA’s Center for Drug Evaluation and Research.
The most common side effects associated with budesonide and formoterol fumarate dihydrate oral inhalation aerosol for those with asthma are nasopharyngitis pain, sinusitis, influenza, back pain, nasal congestion, stomach discomfort, vomiting, and oral candidiasis (thrush). For those with COPD, the most common side effects are nasopharyngitis, oral candidiasis, bronchitis, sinusitis, and upper respiratory tract infection, the FDA reported.
The approval of this generic drug-device combination was granted to Mylan Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration approved the first generic of Symbicort (budesonide and formoterol fumarate dihydrate) inhalation aerosol for the treatment of asthma in patients 6 years of age and older and for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
The approval was given for a complex generic drug-device combination product – a metered-dose inhaler that contains both budesonide (a corticosteroid that reduces inflammation) and formoterol (a long-acting bronchodilator that relaxes muscles in the airways to improve breathing). It is intended to be used as two inhalations, two times a day (usually morning and night, about 12 hours apart), to treat both diseases by preventing symptoms, such as wheezing for those with asthma and for improved breathing for patients with COPD.
The inhaler is approved at two strengths (160/4.5 mcg/actuation and 80/4.5 mcg/actuation), according to the March 15 FDA announcement. The device is not intended for the treatment of acute asthma.
“Today’s approval of the first generic for one of the most commonly prescribed complex drug-device combination products to treat asthma and COPD is another step forward in our commitment to bring generic copies of complex drugs to the market, which can improve quality of life and help reduce the cost of treatment,” said Sally Choe, PhD, director of the Office of Generic Drugs in the FDA’s Center for Drug Evaluation and Research.
The most common side effects associated with budesonide and formoterol fumarate dihydrate oral inhalation aerosol for those with asthma are nasopharyngitis pain, sinusitis, influenza, back pain, nasal congestion, stomach discomfort, vomiting, and oral candidiasis (thrush). For those with COPD, the most common side effects are nasopharyngitis, oral candidiasis, bronchitis, sinusitis, and upper respiratory tract infection, the FDA reported.
The approval of this generic drug-device combination was granted to Mylan Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Food insecurity linked to metabolic syndrome in Hispanic/Latino youth
Severe food insecurity was associated with metabolic syndrome and unfavorable cardiometabolic markers in Hispanic/Latino youth, researchers report.
The findings, published March 16 in Pediatrics, highlight the need to investigate interventions that address food insecurity among Hispanic/Latino youth, a segment of the U.S. population at high risk of cardiometabolic complications.
“Among Hispanic/Latino youth, no study, to our knowledge has evaluated food insecurity’s role in metabolic syndrome and metabolic syndrome–relevant cardiometabolic markers in this population,” lead author Luis E. Maldonado, PhD, of the University of North Carolina at Chapel Hill, and colleagues explained.
The researchers conducted a cross-sectional study to evaluate the associations between lower household and child food security and metabolic syndrome, as well as clinically measured cardiometabolic markers, including fasting plasma glucose, waist circumference, triglycerides, systolic and diastolic blood pressure, and high-density lipoprotein cholesterol (HDL-C).
Household food security (high, marginal, low, very low) and child food security (high, marginal, low/very low) measures were evaluated separately, and were adjusted for participant age, sex, site, parental education, and poverty-income ratio.
Data were obtained from the Hispanic Community Children’s Health Study/Study of Latino Youth, a study of offspring of adults enrolled in the Hispanic Community Health Survey/Study of Latinos.
Results
The study cohort included 1,325 Hispanic/Latino youth aged 8-16 years. For both household food security and child food security, youth in the lowest food security category had significantly lower HDL-C compared with youth with high food security (household food security, –3.17; 95% confidence interval, –5.65 to –0.70; child food security, –1.81; 95% CI, –3.54 to –0.09).
In addition, low/very low compared with high child food security was associated with higher triglycerides (beta, 8.68; 95% CI, 1.75-15.61), higher fasting plasma glucose (beta, 1.37; 95% CI, 0.08-2.65), and metabolic syndrome composite variable expected log counts (beta, 2.12; 95% CI, 0.02-0.45).
Furthermore, the researchers found statistically significant interactions between each of the two food security measures and receipt of any food assistance in the previous year in models of triglycerides (P for interactions: household food security, .03 and child food security, .005) and HDL-C (P for interactions: household food security, .01 and child food security, .04).
After evaluating the effect of parental place of birth, they found a statistically significant association for triglycerides only (P for interactions: household food security, .05 and child food security, .008).
“Our study is among the first to document adverse associations between household and child food security measures with a metabolic syndrome score variable and several metabolic syndrome–relevant cardiometabolic markers among US Hispanic/Latino youth,” the researchers wrote.
The researchers acknowledged that the cross-sectional nature of the study was a key limitation; thus, causality could not be inferred.
“In the future, we plan to conduct more qualitative work to better understand how Hispanic/Latino families respond to food insecurity, which may identify the factors that shape their response,” study author Sandra S. Albrecht, PhD, of Columbia University, New York, NY, said in an interview.
Recommendations for pediatricians
Food insecurity researcher Yankun Wang, PhD candidate at Indiana University, Bloomington, commented: “I would recommend pediatricians pay more attention to children from low-income households since they are more likely to have mental and physical health issues due to food insecurity.
“It can be very helpful if pediatricians could help families obtain SNAP benefits, enroll youth in the school breakfast and lunch programs, and promote nutrition education in schools,” Mr. Wang added.
This study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported no relevant disclosures.
Severe food insecurity was associated with metabolic syndrome and unfavorable cardiometabolic markers in Hispanic/Latino youth, researchers report.
The findings, published March 16 in Pediatrics, highlight the need to investigate interventions that address food insecurity among Hispanic/Latino youth, a segment of the U.S. population at high risk of cardiometabolic complications.
“Among Hispanic/Latino youth, no study, to our knowledge has evaluated food insecurity’s role in metabolic syndrome and metabolic syndrome–relevant cardiometabolic markers in this population,” lead author Luis E. Maldonado, PhD, of the University of North Carolina at Chapel Hill, and colleagues explained.
The researchers conducted a cross-sectional study to evaluate the associations between lower household and child food security and metabolic syndrome, as well as clinically measured cardiometabolic markers, including fasting plasma glucose, waist circumference, triglycerides, systolic and diastolic blood pressure, and high-density lipoprotein cholesterol (HDL-C).
Household food security (high, marginal, low, very low) and child food security (high, marginal, low/very low) measures were evaluated separately, and were adjusted for participant age, sex, site, parental education, and poverty-income ratio.
Data were obtained from the Hispanic Community Children’s Health Study/Study of Latino Youth, a study of offspring of adults enrolled in the Hispanic Community Health Survey/Study of Latinos.
Results
The study cohort included 1,325 Hispanic/Latino youth aged 8-16 years. For both household food security and child food security, youth in the lowest food security category had significantly lower HDL-C compared with youth with high food security (household food security, –3.17; 95% confidence interval, –5.65 to –0.70; child food security, –1.81; 95% CI, –3.54 to –0.09).
In addition, low/very low compared with high child food security was associated with higher triglycerides (beta, 8.68; 95% CI, 1.75-15.61), higher fasting plasma glucose (beta, 1.37; 95% CI, 0.08-2.65), and metabolic syndrome composite variable expected log counts (beta, 2.12; 95% CI, 0.02-0.45).
Furthermore, the researchers found statistically significant interactions between each of the two food security measures and receipt of any food assistance in the previous year in models of triglycerides (P for interactions: household food security, .03 and child food security, .005) and HDL-C (P for interactions: household food security, .01 and child food security, .04).
After evaluating the effect of parental place of birth, they found a statistically significant association for triglycerides only (P for interactions: household food security, .05 and child food security, .008).
“Our study is among the first to document adverse associations between household and child food security measures with a metabolic syndrome score variable and several metabolic syndrome–relevant cardiometabolic markers among US Hispanic/Latino youth,” the researchers wrote.
The researchers acknowledged that the cross-sectional nature of the study was a key limitation; thus, causality could not be inferred.
“In the future, we plan to conduct more qualitative work to better understand how Hispanic/Latino families respond to food insecurity, which may identify the factors that shape their response,” study author Sandra S. Albrecht, PhD, of Columbia University, New York, NY, said in an interview.
Recommendations for pediatricians
Food insecurity researcher Yankun Wang, PhD candidate at Indiana University, Bloomington, commented: “I would recommend pediatricians pay more attention to children from low-income households since they are more likely to have mental and physical health issues due to food insecurity.
“It can be very helpful if pediatricians could help families obtain SNAP benefits, enroll youth in the school breakfast and lunch programs, and promote nutrition education in schools,” Mr. Wang added.
This study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported no relevant disclosures.
Severe food insecurity was associated with metabolic syndrome and unfavorable cardiometabolic markers in Hispanic/Latino youth, researchers report.
The findings, published March 16 in Pediatrics, highlight the need to investigate interventions that address food insecurity among Hispanic/Latino youth, a segment of the U.S. population at high risk of cardiometabolic complications.
“Among Hispanic/Latino youth, no study, to our knowledge has evaluated food insecurity’s role in metabolic syndrome and metabolic syndrome–relevant cardiometabolic markers in this population,” lead author Luis E. Maldonado, PhD, of the University of North Carolina at Chapel Hill, and colleagues explained.
The researchers conducted a cross-sectional study to evaluate the associations between lower household and child food security and metabolic syndrome, as well as clinically measured cardiometabolic markers, including fasting plasma glucose, waist circumference, triglycerides, systolic and diastolic blood pressure, and high-density lipoprotein cholesterol (HDL-C).
Household food security (high, marginal, low, very low) and child food security (high, marginal, low/very low) measures were evaluated separately, and were adjusted for participant age, sex, site, parental education, and poverty-income ratio.
Data were obtained from the Hispanic Community Children’s Health Study/Study of Latino Youth, a study of offspring of adults enrolled in the Hispanic Community Health Survey/Study of Latinos.
Results
The study cohort included 1,325 Hispanic/Latino youth aged 8-16 years. For both household food security and child food security, youth in the lowest food security category had significantly lower HDL-C compared with youth with high food security (household food security, –3.17; 95% confidence interval, –5.65 to –0.70; child food security, –1.81; 95% CI, –3.54 to –0.09).
In addition, low/very low compared with high child food security was associated with higher triglycerides (beta, 8.68; 95% CI, 1.75-15.61), higher fasting plasma glucose (beta, 1.37; 95% CI, 0.08-2.65), and metabolic syndrome composite variable expected log counts (beta, 2.12; 95% CI, 0.02-0.45).
Furthermore, the researchers found statistically significant interactions between each of the two food security measures and receipt of any food assistance in the previous year in models of triglycerides (P for interactions: household food security, .03 and child food security, .005) and HDL-C (P for interactions: household food security, .01 and child food security, .04).
After evaluating the effect of parental place of birth, they found a statistically significant association for triglycerides only (P for interactions: household food security, .05 and child food security, .008).
“Our study is among the first to document adverse associations between household and child food security measures with a metabolic syndrome score variable and several metabolic syndrome–relevant cardiometabolic markers among US Hispanic/Latino youth,” the researchers wrote.
The researchers acknowledged that the cross-sectional nature of the study was a key limitation; thus, causality could not be inferred.
“In the future, we plan to conduct more qualitative work to better understand how Hispanic/Latino families respond to food insecurity, which may identify the factors that shape their response,” study author Sandra S. Albrecht, PhD, of Columbia University, New York, NY, said in an interview.
Recommendations for pediatricians
Food insecurity researcher Yankun Wang, PhD candidate at Indiana University, Bloomington, commented: “I would recommend pediatricians pay more attention to children from low-income households since they are more likely to have mental and physical health issues due to food insecurity.
“It can be very helpful if pediatricians could help families obtain SNAP benefits, enroll youth in the school breakfast and lunch programs, and promote nutrition education in schools,” Mr. Wang added.
This study was supported by grant funding from the National Heart, Lung, and Blood Institute. The authors reported no relevant disclosures.
FROM PEDIATRICS
Children and COVID: Decline in new cases reaches 7th week
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.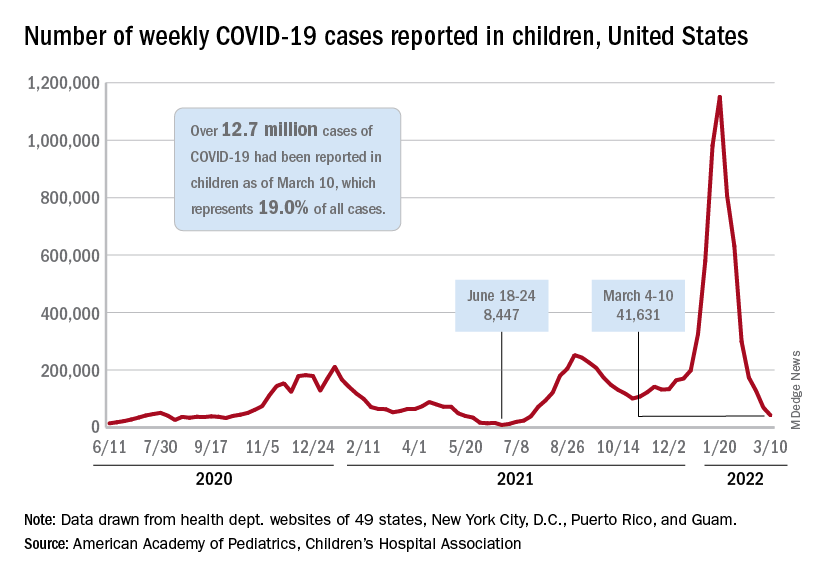
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
Lights on during sleep can play havoc with metabolism
“The most important finding” is that, compared with one night in a dim light environment, “one night of exposure to a moderate level of room light while sleeping with eyes closed increased heart rate and sympathetic [nervous system] activity during the entire sleep period,” said senior author Phyllis C. Zee, MD, PhD.
And on the morning following the moderate room light condition, a higher amount of insulin secretion was required to normalize glucose levels following ingestion of a bolus of glucose in an oral glucose tolerance test, consistent with higher insulin resistance, Dr. Zee, director of the center for circadian and sleep medicine at Northwestern University, Chicago, told this news organization in an email.
The study by Ivy C. Mason, PhD, also of Northwestern University, and colleagues was published March 14 in the Proceedings of the National Academy of Sciences.
Melatonin levels were similar under the two light conditions, Dr. Zee added, which “suggests that the effect of light during sleep on these cardiometabolic measures were more likely due to activation of the sympathetic [nervous] system and less likely due to changes in sleep or suppression of melatonin by light.”
“Attention to avoiding exposure to light at night during sleep may be beneficial for cardiometabolic health,” the researchers conclude.
That means “turn lights off before sleeping,” Dr. Zee elaborated. If a light is needed for safety reasons, keep it as dim as possible, she advises, and avoid exposure to blue or green light, but instead try red-amber colors.
How light during sleep may affect insulin, melatonin, heart rate
Several studies have investigated the effect of light on sleep and metabolic outcomes, the researchers explain.
In one study, light in the bedroom was associated with obesity in women, and in another study, it was associated with risk of type 2 diabetes in an elderly population.
Research has suggested that nighttime light exposure may alter glucose metabolism by increasing insulin resistance; lowering melatonin levels, which alters insulin secretion; and having an arousing effect on the sympathetic autonomic nervous system (increasing the stress hormone cortisol or heart rate, and decreasing heart rate variability).
However, the effect of a single night of moderate room light exposure across the entire nighttime sleep period has not been fully investigated.
The researchers enrolled and randomized 20 healthy young adults who were 18-40 years old and regularly went to sleep between 9 p.m. and 1 a.m. and slept 6.5-8.5 hours, to sleep 2 nights in the sleep laboratory under two conditions.
Ten participants (eight women, two men) slept in a dim light condition on night 1 and in a moderate light condition on night 2. The other 10 participants (six women, four men) slept 2 nights in the dim light condition.
The moderate light condition consisted of four 60-watt incandescent overhead ceiling light bulbs (a total of 100 lux), which “is bright enough to see, but not to read comfortably,” Dr. Zee explained. “It’s like hallway light in an apartment. But the people were sleeping, so about 90% of the light would be blocked by the eyelids.”
The dim light condition was less than 3 lux, which is dimmer than a night light.
When participants were awake, the room lighting was 240 lux.
Participants in each group were a mean age of 27 years and had a mean body mass index of 23 and 24 kg/m2.
The week before the study, participants went to bed at 11 p.m. and slept for 7 hours (based on actigraphy measures). During the laboratory stay, the participants were allowed to sleep 8 hours, during which polysomnography was performed.
They received standard meals at 2.5, 5, and 11 hours after waking and had 30 minutes to eat them. Snacking and caffeine were not permitted.
Participants were instructed to remain seated or standing in their room, but not exercise, when they were not sleeping. Blood samples to determine melatonin levels were collected hourly during wake and sleep via an intravenous line.
Participants slept for a similar time, around 7 hours, in both conditions.
Although melatonin levels were similar in both conditions, this was a relatively small sample, the researchers caution.
In the room light condition, participants spent proportionately more time in stage N2 sleep and less in slow-wave and rapid eye movement sleep. There was no increase in sleep fragmentation or arousals.
The research was partly supported by the Center for Circadian and Sleep Medicine at Northwestern University, the National Center for Advancing Translational Sciences, the National Institutes of Health, and the American Heart Association. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The most important finding” is that, compared with one night in a dim light environment, “one night of exposure to a moderate level of room light while sleeping with eyes closed increased heart rate and sympathetic [nervous system] activity during the entire sleep period,” said senior author Phyllis C. Zee, MD, PhD.
And on the morning following the moderate room light condition, a higher amount of insulin secretion was required to normalize glucose levels following ingestion of a bolus of glucose in an oral glucose tolerance test, consistent with higher insulin resistance, Dr. Zee, director of the center for circadian and sleep medicine at Northwestern University, Chicago, told this news organization in an email.
The study by Ivy C. Mason, PhD, also of Northwestern University, and colleagues was published March 14 in the Proceedings of the National Academy of Sciences.
Melatonin levels were similar under the two light conditions, Dr. Zee added, which “suggests that the effect of light during sleep on these cardiometabolic measures were more likely due to activation of the sympathetic [nervous] system and less likely due to changes in sleep or suppression of melatonin by light.”
“Attention to avoiding exposure to light at night during sleep may be beneficial for cardiometabolic health,” the researchers conclude.
That means “turn lights off before sleeping,” Dr. Zee elaborated. If a light is needed for safety reasons, keep it as dim as possible, she advises, and avoid exposure to blue or green light, but instead try red-amber colors.
How light during sleep may affect insulin, melatonin, heart rate
Several studies have investigated the effect of light on sleep and metabolic outcomes, the researchers explain.
In one study, light in the bedroom was associated with obesity in women, and in another study, it was associated with risk of type 2 diabetes in an elderly population.
Research has suggested that nighttime light exposure may alter glucose metabolism by increasing insulin resistance; lowering melatonin levels, which alters insulin secretion; and having an arousing effect on the sympathetic autonomic nervous system (increasing the stress hormone cortisol or heart rate, and decreasing heart rate variability).
However, the effect of a single night of moderate room light exposure across the entire nighttime sleep period has not been fully investigated.
The researchers enrolled and randomized 20 healthy young adults who were 18-40 years old and regularly went to sleep between 9 p.m. and 1 a.m. and slept 6.5-8.5 hours, to sleep 2 nights in the sleep laboratory under two conditions.
Ten participants (eight women, two men) slept in a dim light condition on night 1 and in a moderate light condition on night 2. The other 10 participants (six women, four men) slept 2 nights in the dim light condition.
The moderate light condition consisted of four 60-watt incandescent overhead ceiling light bulbs (a total of 100 lux), which “is bright enough to see, but not to read comfortably,” Dr. Zee explained. “It’s like hallway light in an apartment. But the people were sleeping, so about 90% of the light would be blocked by the eyelids.”
The dim light condition was less than 3 lux, which is dimmer than a night light.
When participants were awake, the room lighting was 240 lux.
Participants in each group were a mean age of 27 years and had a mean body mass index of 23 and 24 kg/m2.
The week before the study, participants went to bed at 11 p.m. and slept for 7 hours (based on actigraphy measures). During the laboratory stay, the participants were allowed to sleep 8 hours, during which polysomnography was performed.
They received standard meals at 2.5, 5, and 11 hours after waking and had 30 minutes to eat them. Snacking and caffeine were not permitted.
Participants were instructed to remain seated or standing in their room, but not exercise, when they were not sleeping. Blood samples to determine melatonin levels were collected hourly during wake and sleep via an intravenous line.
Participants slept for a similar time, around 7 hours, in both conditions.
Although melatonin levels were similar in both conditions, this was a relatively small sample, the researchers caution.
In the room light condition, participants spent proportionately more time in stage N2 sleep and less in slow-wave and rapid eye movement sleep. There was no increase in sleep fragmentation or arousals.
The research was partly supported by the Center for Circadian and Sleep Medicine at Northwestern University, the National Center for Advancing Translational Sciences, the National Institutes of Health, and the American Heart Association. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“The most important finding” is that, compared with one night in a dim light environment, “one night of exposure to a moderate level of room light while sleeping with eyes closed increased heart rate and sympathetic [nervous system] activity during the entire sleep period,” said senior author Phyllis C. Zee, MD, PhD.
And on the morning following the moderate room light condition, a higher amount of insulin secretion was required to normalize glucose levels following ingestion of a bolus of glucose in an oral glucose tolerance test, consistent with higher insulin resistance, Dr. Zee, director of the center for circadian and sleep medicine at Northwestern University, Chicago, told this news organization in an email.
The study by Ivy C. Mason, PhD, also of Northwestern University, and colleagues was published March 14 in the Proceedings of the National Academy of Sciences.
Melatonin levels were similar under the two light conditions, Dr. Zee added, which “suggests that the effect of light during sleep on these cardiometabolic measures were more likely due to activation of the sympathetic [nervous] system and less likely due to changes in sleep or suppression of melatonin by light.”
“Attention to avoiding exposure to light at night during sleep may be beneficial for cardiometabolic health,” the researchers conclude.
That means “turn lights off before sleeping,” Dr. Zee elaborated. If a light is needed for safety reasons, keep it as dim as possible, she advises, and avoid exposure to blue or green light, but instead try red-amber colors.
How light during sleep may affect insulin, melatonin, heart rate
Several studies have investigated the effect of light on sleep and metabolic outcomes, the researchers explain.
In one study, light in the bedroom was associated with obesity in women, and in another study, it was associated with risk of type 2 diabetes in an elderly population.
Research has suggested that nighttime light exposure may alter glucose metabolism by increasing insulin resistance; lowering melatonin levels, which alters insulin secretion; and having an arousing effect on the sympathetic autonomic nervous system (increasing the stress hormone cortisol or heart rate, and decreasing heart rate variability).
However, the effect of a single night of moderate room light exposure across the entire nighttime sleep period has not been fully investigated.
The researchers enrolled and randomized 20 healthy young adults who were 18-40 years old and regularly went to sleep between 9 p.m. and 1 a.m. and slept 6.5-8.5 hours, to sleep 2 nights in the sleep laboratory under two conditions.
Ten participants (eight women, two men) slept in a dim light condition on night 1 and in a moderate light condition on night 2. The other 10 participants (six women, four men) slept 2 nights in the dim light condition.
The moderate light condition consisted of four 60-watt incandescent overhead ceiling light bulbs (a total of 100 lux), which “is bright enough to see, but not to read comfortably,” Dr. Zee explained. “It’s like hallway light in an apartment. But the people were sleeping, so about 90% of the light would be blocked by the eyelids.”
The dim light condition was less than 3 lux, which is dimmer than a night light.
When participants were awake, the room lighting was 240 lux.
Participants in each group were a mean age of 27 years and had a mean body mass index of 23 and 24 kg/m2.
The week before the study, participants went to bed at 11 p.m. and slept for 7 hours (based on actigraphy measures). During the laboratory stay, the participants were allowed to sleep 8 hours, during which polysomnography was performed.
They received standard meals at 2.5, 5, and 11 hours after waking and had 30 minutes to eat them. Snacking and caffeine were not permitted.
Participants were instructed to remain seated or standing in their room, but not exercise, when they were not sleeping. Blood samples to determine melatonin levels were collected hourly during wake and sleep via an intravenous line.
Participants slept for a similar time, around 7 hours, in both conditions.
Although melatonin levels were similar in both conditions, this was a relatively small sample, the researchers caution.
In the room light condition, participants spent proportionately more time in stage N2 sleep and less in slow-wave and rapid eye movement sleep. There was no increase in sleep fragmentation or arousals.
The research was partly supported by the Center for Circadian and Sleep Medicine at Northwestern University, the National Center for Advancing Translational Sciences, the National Institutes of Health, and the American Heart Association. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES