User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
FDA committee recommends 2022-2023 influenza vaccine strains
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee has chosen the influenza vaccine strains for the 2022-2023 season in the northern hemisphere, which begins in the fall of 2022.
On March 3, the committee unanimously voted to endorse the World Health Organization’s recommendations as to which influenza strains to include for coverage by vaccines for the upcoming flu season. Two of the four recommended strains are different from last season.
The committee also heard updates on flu activity this season. So far, data from the U.S. Flu Vaccine Effectiveness (VE) network, which consists of seven study sites, have not shown that the vaccine is protective against influenza A. “We can say that it is not highly effective,” Brendan Flannery, PhD, who leads the U.S. Flu VE network for the Centers for Disease Control and Prevention, said in an interview. He was not involved with the advisory committee meeting. Flu activity this season has been low, he explained, so there are fewer cases his team can use to estimate vaccine efficacy. “If there’s some benefit, it’s hard for us to show that now,” he said.
Vaccine strains
The panel voted to include a A/Darwin/9/2021-like strain for the H3N2 component of the vaccine; this is changed from A/Cambodia/e0826360/2020. For the influenza B Victoria lineage component, the committee voted to include a B/Austria/1359417/2021-like virus, a swap from this year’s B/Washington/02/2019-like virus. These changes apply to the egg-based, cell-culture, and recombinant vaccines. Both new strains were included in WHO’s 2022 influenza vaccine strain recommendations for the southern hemisphere.
For the influenza A H1N1 component, the group also agreed to include a A/Victoria/2570/2019 (H1N1) pdm09-like virus for the egg-based vaccine and the A/Wisconsin/588/2019 (H1N1) pdm09-like virus for cell culture or recombinant vaccines. These strains were included for the 2021-2022 season. The panel also voted for the inclusion of a B/Phuket/3073/2013-like virus (B/Yamagata lineage) as the second influenza B strain for the quadrivalent egg-based, cell culture, or recombinant vaccines, which is unchanged from this flu season.
‘Sporadic’ flu activity
While there was an uptick in influenza activity this year compared to the 2020-2021 season, hospitalization rates are lower than in the four seasons preceding the pandemic (from 2016-2017 to 2019-2020). As of Feb. 26, the cumulative hospitalization rate for this flu season was 5.2 hospitalizations per 100,000 individuals. There have been eight pediatric deaths due to influenza so far this season, compared to one pediatric death reported to the CDC during the 2020-2021 flu season.
About 4.1% of specimens tested at clinical laboratories were positive for flu. Since Oct. 30, 2.7% of specimens have been positive for influenza this season. Nearly all viruses detected (97.7%) have been influenza A.
Lisa Grohskopf, MD, MPH, a medical officer in the influenza division at the CDC who presented the data at the meeting, described flu activity this season as “sporadic” and noted that activity is increasing in some areas of the country. According to CDC’s weekly influenza surveillance report, most states had minimal influenza-like illness (ILI) activity, although Arkansas, Idaho, Iowa, Kansas, Minnesota, and Utah had slightly higher ILI activity as of Feb. 26. Champaign-Urbana, Illinois; St. Cloud, Minnesota; and Brownwood, Texas, had the highest levels of flu activity in the country.
Low vaccine effectiveness
As of Jan. 22, results from the U.S. Flu VE network do not show statistically significant evidence that the flu vaccine is effective. Currently, the vaccine is estimated to be 8% effective against preventing influenza A infection (95% confidence interval, –31% to 36%) and 14% effective against preventing A/H3N2 infection (95% CI, –28% to 43%) for people aged 6 months and older.
The network did not have enough data to provide age-specific VE estimates or estimates of effectiveness against influenza B. This could be due to low flu activity relative to prepandemic years, Dr. Flannery said. Of the 2,758 individuals enrolled in the VE flu network this season, just 147 (5%) tested positive for the flu this season. This is the lowest positivity rate observed in the Flu VE network participants with respiratory illness over the past 10 flu seasons, Dr. Grohskopf noted. In comparison, estimates from the 2019 to 2020 season included 4,112 individuals, and 1,060 tested positive for flu.
“We are really at the bare minimum of what we can use for a flu vaccine effectiveness estimate,” Dr. Flannery said about the more recent data. The network was not able to produce any estimates about flu vaccine effectiveness for the 2020-2021 season because of historically low flu activity.
The Department of Defense also presented vaccine efficacy estimates for the 2021–2022 season. The vaccine has been 36% effective (95% CI, 28%-44%) against all strains of the virus, 33% effective against influenza A (95% CI, 24%-41%), 32% effective against A/H3N2 (95% CI, 3%-53%), and 59% effective against influenza B (95% CI, 42%-71%). These results are from a young, healthy adult population, Lieutenant Commander Courtney Gustin, DrPH, MSN, told the panel, and they may not be reflective of efficacy rates across all age groups.
Though these findings suggest there is low to no measurable benefit against influenza A, Dr. Flannery said the CDC still recommends getting the flu vaccine, as it can be protective against other circulating flu strains. “We have been able to demonstrate protection against other H3 [viruses], B viruses, and H1 viruses in the past,” he said. And as these results only show protection against mild disease, “there is still possibility that there’s benefit against more severe disease,” he added. Studies measuring effectiveness against more severe outcomes are not yet available.
A version of this article first appeared on Medscape.com.
Extensive scarring alopecia and widespread rash
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
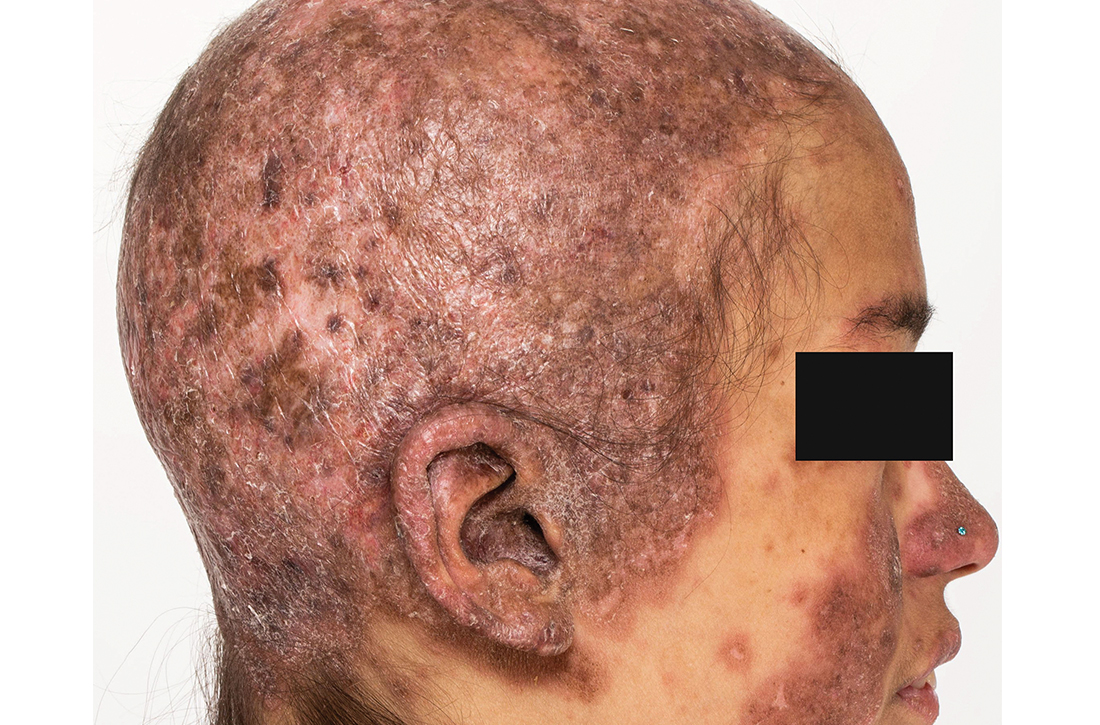
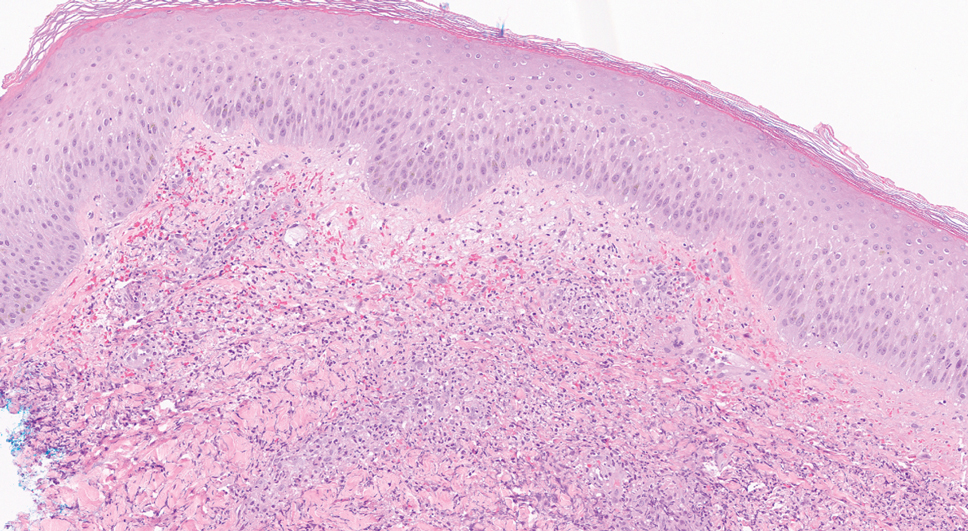
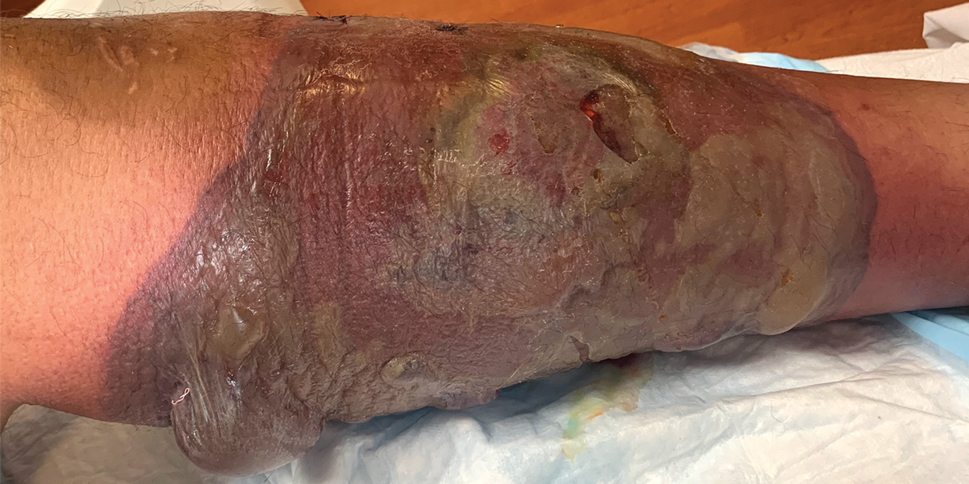
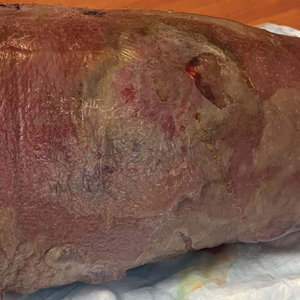
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
A 23-year-old woman with systemic lupus erythematosus (SLE) and a history of poor adherence to recommended treatment presented with a widespread pruritic rash and diffuse hair loss. The rash had rapidly progressed following sun exposure during the summer. The patient cited her mental health status (anxiety, depression), socioeconomic factors, and challenges with prescription insurance coverage as reasons for nonadherence to treatment.
Clinical examination revealed diffuse scarring alopecia and abnormal pigmentation of the scalp (FIGURE 1A), as well as large, red-brown, scaly, atrophic plaques on the face, ears, extremities, back, and buttocks (FIGURES 1B and 1C).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Generalized chronic cutaneouslupus erythematosus
The clinical features of our patient were most consistent with generalized chronic cutaneous lupus erythematosus (CCLE), which is 1 of 3 subtypes of cutaneous lupus erythematosus (CLE). The other 2 are acute and subacute cutaneous lupus erythematosus (ACLE and SCLE, respectively). CCLE is further divided into 3 distinct entities: discoid lupus erythematosus (DLE), chilblain lupus erythematosus, and lupus erythematosus panniculitis.
Distinguishing between the different forms of cutaneous lupus can be challenging; diagnosis is based on differences in clinical features and duration of skin changes, as well as biopsy and lab results.1 The clinical features of our patient were most consistent with DLE, based on the scarring alopecia with scaly atrophic plaques, dyspigmentation, and exacerbation following sun exposure.
DLE is the most common form of CCLE and frequently manifests in a localized, photosensitive distribution involving the scalp, ears, and/or face.2 Less commonly, it can demonstrate a more generalized distribution involving the trunk and/or extremities (reported incidence of 1.04 per 100,000 people).3 Longstanding DLE lesions commonly exhibit scarring and dyspigmentation. DLE occurs in approximately 15% to 30% of SLE patients,4 whereas about 10% of patients with DLE will progress to SLE.3
Positive antinuclear antibodies (ANA) are found in 54% of patients with CCLE, compared to 74% and 81% of patients with SCLE and ACLE, respectively.5 Thus, a negative ANA should not rule out the possibility of CLE.
Comprehensive lab work and biopsy could expose a systemic origin
While our patient already had a diagnosis of SLE, many patients will present with no prior history of autoimmune connective tissue disease, and, in that case, the objective should be to confirm the diagnosis and evaluate for systemic involvement. This includes a thorough review of systems; skin biopsy; complete blood count; liver function tests; urinalysis; and measurement of creatinine, inflammatory markers, ANA, extractable nuclear antigens, double-stranded DNA, complement levels (C3, C4, total), and antiphospholipid antibodies.6
Continue to: Biopsy
Biopsy features of DLE include vacuolar interface dermatitis, basement membrane zone thickening, follicular plugging, superficial and deep perivascular and periadnexal lymphohistiocytic inflammation with plasma cells, and increased mucin deposition. Direct immunofluorescence biopsy may show a continuous granular immunoglobulin (Ig) G/IgA/IgM and C3 band at the basement membrane zone.
Abnormal serologic tests may support the diagnosis of SLE based on American College of Rheumatology criteria and could suggest additional organ involvement or associated conditions, such as lupus nephritis or antiphospholipid syndrome (respectively). Currently, no clear consensus exists on monitoring patients with cutaneous lupus for systemic disease.
A gamut of skin-changing conditions should be considered
The differential diagnosis in this case includes SCLE, dermatitis, tinea corporis, cutaneous drug eruptions, and graft-versus-host disease (GVHD).
SCLE classically manifests with annular or psoriasiform lesions on the sun-exposed areas of the upper trunk (eg, the chest, neck, and upper extremities), while the central face and scalp are typically spared. Differentiating between generalized DLE and SCLE may be the most difficult, given similarities in the associated skin changes.
Dermatitis (atopic or contact) manifests as pruritic erythematous eczematous plaques, most commonly involving the flexural areas in atopic dermatitis and an exposure-dependent distribution pattern in contact dermatitis. The patient may have a history of atopy.
Continue to: Tinea corporis
Tinea corporis will manifest with annular scaly patches or plaques and may demonstrate erythematous papules around hair follicles in Majocchi granuloma. A positive potassium hydroxide exam demonstrating fungal hyphae confirms the diagnosis.
Cutaneous drug eruptions can have various morphologies and timing of onset. Certain photosensitive drug reactions can be triggered or exacerbated with sun exposure. Therefore, it is necessary to obtain a thorough medication history, including any new medications that were started within the past 4 to 6 weeks, although onset can be delayed beyond this timeframe.
GVHD is a complication that more commonly follows allogeneic hematopoietic stem cell transplants, although it may be seen following solid-organ transplantation or transfusion of nonirradiated blood. Chronic GVHD has an onset ≥ 100 days after transplant and is divided into nonsclerotic (lichenoid, atopic dermatitis-like, psoriasiform, poikilodermatous) and sclerotic morphologies.
Successful Tx requires adherence but may not prevent flare-ups
First-line treatment options for severe and widespread skin manifestations of CLE include photoprotection, smoking cessation, topical corticosteroids, hydroxychloroquine, and systemic corticosteroids. Second-line treatments include chloroquine, methotrexate, or mycophenolate mofetil; thalidomide or lenalidomide may be considered for patients with refractory disease.7,8
With successful treatment and photoprotection, patients may achieve significant skin clearing. Occasional flares, especially during warmer months, may occur if they are not diligent about photoprotection. Systemic treatments will also improve the patient’s systemic symptoms if the patient has concomitant SLE.
Our patient was advised to use topical steroids and to restart hydroxychloroquine 300 mg/d and mycophenolate mofetil 1000 mg/d (a regimen with which she had previously been nonadherent). The patient followed up with her family physician for assessment of her other medical issues. No new interventions for her mental health were initiated during this visit, as the severity of her depression was considered mild. She was referred to a case manager to navigate multiple medical appointments and prescription insurance coverage issues. The patient’s dose of mycophenolate mofetil was increased gradually to 3 g/d, and the patient experienced improvement in both her cutaneous lesions and systemic symptoms.
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226
1. Petty AJ, Floyd L, Henderson C, et al. Cutaneous lupus erythematosus: progress and challenges. Curr Allergy Asthma Rep. 2020;20:12. doi: 10.1007/s11882-020-00906-8
2. Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48-49:14-19. doi: 10.1016/j.jaut.2014.01.021
3. Durosaro O, Davis MDP, Reed KB, et al. Incidence of cutaneous lupus erythematosus, 1965-2005: a population-based study. Arch Dermatol. 2009;145:249-253. doi: 10.1001/archdermatol.2009.21
4. Merola JF. Overview of cutaneous lupus erythematosus. UpToDate. Updated September 19, 2021. Accessed February 17, 2022. www.uptodate.com/contents/overview-of-cutaneous-lupus-erythematosus
5. Biazar C, Sigges J, Patsinakidis N, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12:444-454. doi: 10.1016/j.autrev.2012.08.019
6. O’Brien JC, Chong BF. not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Investig Dermatol Symp Proc. 2017;18:S69-S74. doi: 10.1016/j.jisp.2016.09.001
7. Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part I. J Am Acad Dermatol. 2011;65:e179-e193. doi: 10.1016/j.jaad.2010.06.018
8. Kindle SA, Wetter DA, Davis MDP, et al. Lenalidomide treatment of cutaneous lupus erythematosus: the Mayo Clinic experience. Int J Dermatol. 2016;55:e431-e439. doi: 10.1111/ijd.13226
COVID-19 found in 29 types of animals, scientists say
according to researchers’ latest tally.
In most cases, humans infect animals, and animals don’t transmit the virus back to humans. But scientists have expressed concerns about recent research that shows some animals – such as mink and deer – appear to be able to spread the virus to humans.
In addition, the virus will likely continue to circulate in wild animals, which could lead to new mutations, some of which may make the virus less susceptible to people’s immunity from current vaccines. Researchers are calling for better surveillance of animals, especially in the wild, to track any new variants.
“It could be evolving in hosts we are not aware of,” Eman Anis, PhD, an assistant professor of microbiology at the University of Pennsylvania, Philadelphia, told the Philadelphia Inquirer.
Scientists have identified the virus in a growing list of animals, according to the Centers for Disease Control and Prevention, including cats, dogs, ferrets, gorillas, hamsters, hippos, hyenas, mice, otters, pigs, rabbits, and tigers. In many cases, humans spread the coronavirus to pets at home or to wildlife in zoos and sanctuaries.
In the study, published in bioRxiv, researchers identified a person who tested positive after close contact with infected white-tailed deer. The coronavirus had evolved dozens of mutations not found in other strains.
Even with the changes, the virus they found doesn’t appear different enough to evade current vaccines, the researchers reported. The vaccines target the spike protein on the outside of coronavirus cells, and the mutations that happened in deer occurred elsewhere in the virus.
At the same time, scientists have noted that this points to the need to step up monitoring in wild animals before mutations become a problem.
“This is no need to panic, but this is not something we can ignore,” Suresh Kuchipudi, PhD, a professor of veterinary and biomedical sciences at Pennsylvania State University in University Park, told the Inquirer.
Dr. Kuchipudi, who wasn’t involved with the Canadian study, has done other studies that found COVID-19 in deer. As the coronavirus continues to circulate in deer, more mutations will arise, he noted.
“It’s hard to predict what evolution’s going to come up with,” Frederic Bushman, a microbiology professor at the University of Pennsylvania, told the Inquirer.
“The virus will probably change different ways in different animals. Some of them probably won’t infect humans as well,” he said. “But the fear is that maybe some new one will come along that does infect humans well.”
A version of this article first appeared on WebMD.com.
according to researchers’ latest tally.
In most cases, humans infect animals, and animals don’t transmit the virus back to humans. But scientists have expressed concerns about recent research that shows some animals – such as mink and deer – appear to be able to spread the virus to humans.
In addition, the virus will likely continue to circulate in wild animals, which could lead to new mutations, some of which may make the virus less susceptible to people’s immunity from current vaccines. Researchers are calling for better surveillance of animals, especially in the wild, to track any new variants.
“It could be evolving in hosts we are not aware of,” Eman Anis, PhD, an assistant professor of microbiology at the University of Pennsylvania, Philadelphia, told the Philadelphia Inquirer.
Scientists have identified the virus in a growing list of animals, according to the Centers for Disease Control and Prevention, including cats, dogs, ferrets, gorillas, hamsters, hippos, hyenas, mice, otters, pigs, rabbits, and tigers. In many cases, humans spread the coronavirus to pets at home or to wildlife in zoos and sanctuaries.
In the study, published in bioRxiv, researchers identified a person who tested positive after close contact with infected white-tailed deer. The coronavirus had evolved dozens of mutations not found in other strains.
Even with the changes, the virus they found doesn’t appear different enough to evade current vaccines, the researchers reported. The vaccines target the spike protein on the outside of coronavirus cells, and the mutations that happened in deer occurred elsewhere in the virus.
At the same time, scientists have noted that this points to the need to step up monitoring in wild animals before mutations become a problem.
“This is no need to panic, but this is not something we can ignore,” Suresh Kuchipudi, PhD, a professor of veterinary and biomedical sciences at Pennsylvania State University in University Park, told the Inquirer.
Dr. Kuchipudi, who wasn’t involved with the Canadian study, has done other studies that found COVID-19 in deer. As the coronavirus continues to circulate in deer, more mutations will arise, he noted.
“It’s hard to predict what evolution’s going to come up with,” Frederic Bushman, a microbiology professor at the University of Pennsylvania, told the Inquirer.
“The virus will probably change different ways in different animals. Some of them probably won’t infect humans as well,” he said. “But the fear is that maybe some new one will come along that does infect humans well.”
A version of this article first appeared on WebMD.com.
according to researchers’ latest tally.
In most cases, humans infect animals, and animals don’t transmit the virus back to humans. But scientists have expressed concerns about recent research that shows some animals – such as mink and deer – appear to be able to spread the virus to humans.
In addition, the virus will likely continue to circulate in wild animals, which could lead to new mutations, some of which may make the virus less susceptible to people’s immunity from current vaccines. Researchers are calling for better surveillance of animals, especially in the wild, to track any new variants.
“It could be evolving in hosts we are not aware of,” Eman Anis, PhD, an assistant professor of microbiology at the University of Pennsylvania, Philadelphia, told the Philadelphia Inquirer.
Scientists have identified the virus in a growing list of animals, according to the Centers for Disease Control and Prevention, including cats, dogs, ferrets, gorillas, hamsters, hippos, hyenas, mice, otters, pigs, rabbits, and tigers. In many cases, humans spread the coronavirus to pets at home or to wildlife in zoos and sanctuaries.
In the study, published in bioRxiv, researchers identified a person who tested positive after close contact with infected white-tailed deer. The coronavirus had evolved dozens of mutations not found in other strains.
Even with the changes, the virus they found doesn’t appear different enough to evade current vaccines, the researchers reported. The vaccines target the spike protein on the outside of coronavirus cells, and the mutations that happened in deer occurred elsewhere in the virus.
At the same time, scientists have noted that this points to the need to step up monitoring in wild animals before mutations become a problem.
“This is no need to panic, but this is not something we can ignore,” Suresh Kuchipudi, PhD, a professor of veterinary and biomedical sciences at Pennsylvania State University in University Park, told the Inquirer.
Dr. Kuchipudi, who wasn’t involved with the Canadian study, has done other studies that found COVID-19 in deer. As the coronavirus continues to circulate in deer, more mutations will arise, he noted.
“It’s hard to predict what evolution’s going to come up with,” Frederic Bushman, a microbiology professor at the University of Pennsylvania, told the Inquirer.
“The virus will probably change different ways in different animals. Some of them probably won’t infect humans as well,” he said. “But the fear is that maybe some new one will come along that does infect humans well.”
A version of this article first appeared on WebMD.com.
Side effects of COVID mRNA vaccines are mild and short, large study confirms
Data from the first 6 months after the rollout of mRNA COVID-19 vaccines in the United States released today show that adverse effects from shots are typically mild and short-lived.
Findings of the large study, compiled after nearly 300 million doses were administered, were published online March 7 in The Lancet Infectious Diseases.
Researchers, led by Hannah G. Rosenblum, MD, with the Centers for Disease Control and Prevention COVID Response Team, used passive U.S. surveillance data collected through the Vaccine Adverse Event Reporting System (VAERS), and the active system, v-safe, starting in December 2020 through the first 6 months of the U.S. COVID-19 vaccination program. V-safe is a voluntary, smartphone-based system set up in 2020 specifically for monitoring reactions to COVID-19 and health effects after vaccination. The health effects information from v-safe is presented in this study for the first time.
Of the 298.7 million doses of mRNA vaccines administered in the U.S. during the study period, VAERS processed 340,522 reports. Of those, 313,499 (92.1%) were nonserious; 22,527 (6.6%) were serious (nondeath); and 4,496 (1.3%) were deaths.
From v-safe reporting, researchers learned that about 71% of the 7.9 million participants reported local or systemic reactions, more frequently after dose 2 than after dose 1. Of those reporting reactions after dose 1, about two-thirds (68.6%) reported a local reaction and 52.7% reported a systemic reaction.
Among other findings:
- Injection-site pain occurred after dose 1 in 66.2% of participants and 68.6% after dose 2.
- One-third of participants (33.9%) reported fatigue after dose 1 and 55.7% after dose 2.
- Headache was reported among 27% of participants after dose 1 and 46.2% after dose 2.
- When injection site pain, fatigue, or headaches were reported, the reports were usually in the first week after vaccination.
- Reports of being unable to work or do normal daily activities, or instances of seeking medical care, occurred more commonly after dose 2 (32.1%) than after dose 1 (11.9%). Fewer than 1% of participants reported seeking medical care after dose 1 or 2 of the vaccine.
- Reactions and health effects were reported more often in female than in male recipients, and in people younger than 65 years, compared with older people.
- Serious adverse events, including myocarditis, have been identified following mRNA vaccinations, but the events are rare.
The authors wrote that these results are consistent with preauthorization clinical trials and early postauthorization reports.
“On the basis of our findings, mild to moderate transient reactogenicity should be anticipated,” they said, “particularly among younger and female vaccine recipients.”
‘Robust and reassuring data’
“The safety monitoring of the mRNA COVID-19 vaccines stands out as the most comprehensive of any vaccine in U.S. history. The use of these complementary monitoring systems has provided robust and reassuring data,” Matthew S. Krantz, MD, with the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., and Elizabeth J. Phillips, MD, with the department of pathology, microbiology, and immunology at Vanderbilt, wrote in a related commentary in The Lancet Infectious Diseases.
They point out that the v-safe reports of reactions are consistent with those reported from clinical trials and a large population study in the United Kingdom.
Dr. Phillips said in a press release, “[A]lthough approximately one in 1,000 individuals vaccinated may have an adverse effect, most of these are nonserious. No unusual patterns emerged in the cause of death or serious adverse effects among VAERS reports. For adverse events of special interest, it is reassuring that there were no unexpected signals other than myopericarditis and anaphylaxis, already known to be associated with mRNA vaccines.”
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from the first 6 months after the rollout of mRNA COVID-19 vaccines in the United States released today show that adverse effects from shots are typically mild and short-lived.
Findings of the large study, compiled after nearly 300 million doses were administered, were published online March 7 in The Lancet Infectious Diseases.
Researchers, led by Hannah G. Rosenblum, MD, with the Centers for Disease Control and Prevention COVID Response Team, used passive U.S. surveillance data collected through the Vaccine Adverse Event Reporting System (VAERS), and the active system, v-safe, starting in December 2020 through the first 6 months of the U.S. COVID-19 vaccination program. V-safe is a voluntary, smartphone-based system set up in 2020 specifically for monitoring reactions to COVID-19 and health effects after vaccination. The health effects information from v-safe is presented in this study for the first time.
Of the 298.7 million doses of mRNA vaccines administered in the U.S. during the study period, VAERS processed 340,522 reports. Of those, 313,499 (92.1%) were nonserious; 22,527 (6.6%) were serious (nondeath); and 4,496 (1.3%) were deaths.
From v-safe reporting, researchers learned that about 71% of the 7.9 million participants reported local or systemic reactions, more frequently after dose 2 than after dose 1. Of those reporting reactions after dose 1, about two-thirds (68.6%) reported a local reaction and 52.7% reported a systemic reaction.
Among other findings:
- Injection-site pain occurred after dose 1 in 66.2% of participants and 68.6% after dose 2.
- One-third of participants (33.9%) reported fatigue after dose 1 and 55.7% after dose 2.
- Headache was reported among 27% of participants after dose 1 and 46.2% after dose 2.
- When injection site pain, fatigue, or headaches were reported, the reports were usually in the first week after vaccination.
- Reports of being unable to work or do normal daily activities, or instances of seeking medical care, occurred more commonly after dose 2 (32.1%) than after dose 1 (11.9%). Fewer than 1% of participants reported seeking medical care after dose 1 or 2 of the vaccine.
- Reactions and health effects were reported more often in female than in male recipients, and in people younger than 65 years, compared with older people.
- Serious adverse events, including myocarditis, have been identified following mRNA vaccinations, but the events are rare.
The authors wrote that these results are consistent with preauthorization clinical trials and early postauthorization reports.
“On the basis of our findings, mild to moderate transient reactogenicity should be anticipated,” they said, “particularly among younger and female vaccine recipients.”
‘Robust and reassuring data’
“The safety monitoring of the mRNA COVID-19 vaccines stands out as the most comprehensive of any vaccine in U.S. history. The use of these complementary monitoring systems has provided robust and reassuring data,” Matthew S. Krantz, MD, with the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., and Elizabeth J. Phillips, MD, with the department of pathology, microbiology, and immunology at Vanderbilt, wrote in a related commentary in The Lancet Infectious Diseases.
They point out that the v-safe reports of reactions are consistent with those reported from clinical trials and a large population study in the United Kingdom.
Dr. Phillips said in a press release, “[A]lthough approximately one in 1,000 individuals vaccinated may have an adverse effect, most of these are nonserious. No unusual patterns emerged in the cause of death or serious adverse effects among VAERS reports. For adverse events of special interest, it is reassuring that there were no unexpected signals other than myopericarditis and anaphylaxis, already known to be associated with mRNA vaccines.”
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Data from the first 6 months after the rollout of mRNA COVID-19 vaccines in the United States released today show that adverse effects from shots are typically mild and short-lived.
Findings of the large study, compiled after nearly 300 million doses were administered, were published online March 7 in The Lancet Infectious Diseases.
Researchers, led by Hannah G. Rosenblum, MD, with the Centers for Disease Control and Prevention COVID Response Team, used passive U.S. surveillance data collected through the Vaccine Adverse Event Reporting System (VAERS), and the active system, v-safe, starting in December 2020 through the first 6 months of the U.S. COVID-19 vaccination program. V-safe is a voluntary, smartphone-based system set up in 2020 specifically for monitoring reactions to COVID-19 and health effects after vaccination. The health effects information from v-safe is presented in this study for the first time.
Of the 298.7 million doses of mRNA vaccines administered in the U.S. during the study period, VAERS processed 340,522 reports. Of those, 313,499 (92.1%) were nonserious; 22,527 (6.6%) were serious (nondeath); and 4,496 (1.3%) were deaths.
From v-safe reporting, researchers learned that about 71% of the 7.9 million participants reported local or systemic reactions, more frequently after dose 2 than after dose 1. Of those reporting reactions after dose 1, about two-thirds (68.6%) reported a local reaction and 52.7% reported a systemic reaction.
Among other findings:
- Injection-site pain occurred after dose 1 in 66.2% of participants and 68.6% after dose 2.
- One-third of participants (33.9%) reported fatigue after dose 1 and 55.7% after dose 2.
- Headache was reported among 27% of participants after dose 1 and 46.2% after dose 2.
- When injection site pain, fatigue, or headaches were reported, the reports were usually in the first week after vaccination.
- Reports of being unable to work or do normal daily activities, or instances of seeking medical care, occurred more commonly after dose 2 (32.1%) than after dose 1 (11.9%). Fewer than 1% of participants reported seeking medical care after dose 1 or 2 of the vaccine.
- Reactions and health effects were reported more often in female than in male recipients, and in people younger than 65 years, compared with older people.
- Serious adverse events, including myocarditis, have been identified following mRNA vaccinations, but the events are rare.
The authors wrote that these results are consistent with preauthorization clinical trials and early postauthorization reports.
“On the basis of our findings, mild to moderate transient reactogenicity should be anticipated,” they said, “particularly among younger and female vaccine recipients.”
‘Robust and reassuring data’
“The safety monitoring of the mRNA COVID-19 vaccines stands out as the most comprehensive of any vaccine in U.S. history. The use of these complementary monitoring systems has provided robust and reassuring data,” Matthew S. Krantz, MD, with the division of allergy, pulmonary, and critical care medicine at Vanderbilt University, Nashville, Tenn., and Elizabeth J. Phillips, MD, with the department of pathology, microbiology, and immunology at Vanderbilt, wrote in a related commentary in The Lancet Infectious Diseases.
They point out that the v-safe reports of reactions are consistent with those reported from clinical trials and a large population study in the United Kingdom.
Dr. Phillips said in a press release, “[A]lthough approximately one in 1,000 individuals vaccinated may have an adverse effect, most of these are nonserious. No unusual patterns emerged in the cause of death or serious adverse effects among VAERS reports. For adverse events of special interest, it is reassuring that there were no unexpected signals other than myopericarditis and anaphylaxis, already known to be associated with mRNA vaccines.”
The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antivaccine physician pleads guilty to role in Capitol riot
California-based emergency physician Simone Melissa Gold, MD, JD, founder of the antivaccine group America’s Frontline Doctors (AFD) and leading voice in the antivaccine movement, has pleaded guilty to one of five charges related to the Jan. 6 Capitol riot.
According to the plea deal, Dr. Gold pleaded guilty to charges that she “did unlawfully and knowingly enter and remain in a restricted building and grounds, that is, any posted, cordoned-off, or otherwise restricted area within the United States Capitol and its grounds, during a time when the vice president was in the building without lawful authority to do so.” As part of the agreement, additional charges against her – obstructing an official proceeding and intent to disrupt the orderly conduct of government business – will be dismissed. She also agreed to cooperate with investigators, including allowing them to review social media postings made during the time surrounding the event.
Shortly after she was indicted, Dr. Gold told The Washington Post that she did not see any violence and that the event was “peaceful.” However, according to news reports, Dr. Gold acknowledged in her plea deal that she and her codefendant, John Herbert Strand, witnessed the assault of a police officer while they were outside the building.
Dr. Gold, 56, based in Beverly Hills, Calif., founded AFD in 2019. The group notes its goal is to “amplify the voices of concerned physicians and patients nationwide to combat those who push political and economic agendas at the expense of science and quality health care solutions.” Mr. Strand is the organization’s communication’s director.
The group has been a leading proponent of the use of ivermectin as a “safe and effective treatment” for COVID-19, according to its website.
In 2021, Dr. Gold spoke at an event called The Stand, representing AFD, where she promised to tell “the truth” about COVID vaccines, including that it was actually giving people the virus, that COVID was renamed from the “Wuhan Virus” as part of a cover-up, and touted treatments, including hydroxycholoroquine and ivermectin.
Dr. Gold has been one of the leading voices in the anti-vaccine movement. She has more than 400,000 Twitter followers; her Twitter profile includes a pinned tweet saying: “We are living in Orwellian times.” In addition to spreading vaccine misinformation, Dr. Gold has promoted the use of unproven treatments such as hydroxychloroquine and ivermectin.
Calls and emails to AFD regarding a statement on Gold’s plea made by this news organization were not returned by press time.
In October, Representative James E. Clyburn (D-S.C.), chairman of the Select Subcommittee on the Coronavirus Crisis, launched an investigation into organizations, including AFD, that spread misinformation and facilitate access to disproven and potentially hazardous treatments for COVID-19. According to news reports, Rep. Clyburn called the AFD and other such groups “predatory actors.”
Hospitals where Dr. Gold previously worked, including Providence St. Joseph Medical Center in Santa Monica, Calif., and Cedars-Sinai in Los Angeles, have disassociated themselves from her. On July 29, 2020, Cedars-Sinai Medical Center, where Gold previously worked, issued a statement that said, in part, “Simone Gold, MD, has not worked with Cedars-Sinai Medical Center or any of its offices or affiliates since 2015. For 3 weeks in late 2015, Dr. Gold was employed on a per diem basis by Cedars-Sinai Medical Network, a component of Cedars-Sinai. She worked during this brief time in a network urgent care clinic. Dr. Gold is not authorized to represent or speak about any information on behalf of Cedars-Sinai.”
Dr. Gold’s medical license in the state of California is current and she has no pending hearings before the state medical board, according to its website. On her own website, Dr. Gold says she “voluntarily refused” to renew her board certification last year, “due to the unethical behavior of the medical boards.”
Dr. Gold is also a licensed attorney, having earned her law degree in health policy analysis at Stanford (Calif.) Law School.
Dr. Gold faces 6 months in prison. Sentencing is scheduled for June 16.
A version of this article first appeared on Medscape.com.
California-based emergency physician Simone Melissa Gold, MD, JD, founder of the antivaccine group America’s Frontline Doctors (AFD) and leading voice in the antivaccine movement, has pleaded guilty to one of five charges related to the Jan. 6 Capitol riot.
According to the plea deal, Dr. Gold pleaded guilty to charges that she “did unlawfully and knowingly enter and remain in a restricted building and grounds, that is, any posted, cordoned-off, or otherwise restricted area within the United States Capitol and its grounds, during a time when the vice president was in the building without lawful authority to do so.” As part of the agreement, additional charges against her – obstructing an official proceeding and intent to disrupt the orderly conduct of government business – will be dismissed. She also agreed to cooperate with investigators, including allowing them to review social media postings made during the time surrounding the event.
Shortly after she was indicted, Dr. Gold told The Washington Post that she did not see any violence and that the event was “peaceful.” However, according to news reports, Dr. Gold acknowledged in her plea deal that she and her codefendant, John Herbert Strand, witnessed the assault of a police officer while they were outside the building.
Dr. Gold, 56, based in Beverly Hills, Calif., founded AFD in 2019. The group notes its goal is to “amplify the voices of concerned physicians and patients nationwide to combat those who push political and economic agendas at the expense of science and quality health care solutions.” Mr. Strand is the organization’s communication’s director.
The group has been a leading proponent of the use of ivermectin as a “safe and effective treatment” for COVID-19, according to its website.
In 2021, Dr. Gold spoke at an event called The Stand, representing AFD, where she promised to tell “the truth” about COVID vaccines, including that it was actually giving people the virus, that COVID was renamed from the “Wuhan Virus” as part of a cover-up, and touted treatments, including hydroxycholoroquine and ivermectin.
Dr. Gold has been one of the leading voices in the anti-vaccine movement. She has more than 400,000 Twitter followers; her Twitter profile includes a pinned tweet saying: “We are living in Orwellian times.” In addition to spreading vaccine misinformation, Dr. Gold has promoted the use of unproven treatments such as hydroxychloroquine and ivermectin.
Calls and emails to AFD regarding a statement on Gold’s plea made by this news organization were not returned by press time.
In October, Representative James E. Clyburn (D-S.C.), chairman of the Select Subcommittee on the Coronavirus Crisis, launched an investigation into organizations, including AFD, that spread misinformation and facilitate access to disproven and potentially hazardous treatments for COVID-19. According to news reports, Rep. Clyburn called the AFD and other such groups “predatory actors.”
Hospitals where Dr. Gold previously worked, including Providence St. Joseph Medical Center in Santa Monica, Calif., and Cedars-Sinai in Los Angeles, have disassociated themselves from her. On July 29, 2020, Cedars-Sinai Medical Center, where Gold previously worked, issued a statement that said, in part, “Simone Gold, MD, has not worked with Cedars-Sinai Medical Center or any of its offices or affiliates since 2015. For 3 weeks in late 2015, Dr. Gold was employed on a per diem basis by Cedars-Sinai Medical Network, a component of Cedars-Sinai. She worked during this brief time in a network urgent care clinic. Dr. Gold is not authorized to represent or speak about any information on behalf of Cedars-Sinai.”
Dr. Gold’s medical license in the state of California is current and she has no pending hearings before the state medical board, according to its website. On her own website, Dr. Gold says she “voluntarily refused” to renew her board certification last year, “due to the unethical behavior of the medical boards.”
Dr. Gold is also a licensed attorney, having earned her law degree in health policy analysis at Stanford (Calif.) Law School.
Dr. Gold faces 6 months in prison. Sentencing is scheduled for June 16.
A version of this article first appeared on Medscape.com.
California-based emergency physician Simone Melissa Gold, MD, JD, founder of the antivaccine group America’s Frontline Doctors (AFD) and leading voice in the antivaccine movement, has pleaded guilty to one of five charges related to the Jan. 6 Capitol riot.
According to the plea deal, Dr. Gold pleaded guilty to charges that she “did unlawfully and knowingly enter and remain in a restricted building and grounds, that is, any posted, cordoned-off, or otherwise restricted area within the United States Capitol and its grounds, during a time when the vice president was in the building without lawful authority to do so.” As part of the agreement, additional charges against her – obstructing an official proceeding and intent to disrupt the orderly conduct of government business – will be dismissed. She also agreed to cooperate with investigators, including allowing them to review social media postings made during the time surrounding the event.
Shortly after she was indicted, Dr. Gold told The Washington Post that she did not see any violence and that the event was “peaceful.” However, according to news reports, Dr. Gold acknowledged in her plea deal that she and her codefendant, John Herbert Strand, witnessed the assault of a police officer while they were outside the building.
Dr. Gold, 56, based in Beverly Hills, Calif., founded AFD in 2019. The group notes its goal is to “amplify the voices of concerned physicians and patients nationwide to combat those who push political and economic agendas at the expense of science and quality health care solutions.” Mr. Strand is the organization’s communication’s director.
The group has been a leading proponent of the use of ivermectin as a “safe and effective treatment” for COVID-19, according to its website.
In 2021, Dr. Gold spoke at an event called The Stand, representing AFD, where she promised to tell “the truth” about COVID vaccines, including that it was actually giving people the virus, that COVID was renamed from the “Wuhan Virus” as part of a cover-up, and touted treatments, including hydroxycholoroquine and ivermectin.
Dr. Gold has been one of the leading voices in the anti-vaccine movement. She has more than 400,000 Twitter followers; her Twitter profile includes a pinned tweet saying: “We are living in Orwellian times.” In addition to spreading vaccine misinformation, Dr. Gold has promoted the use of unproven treatments such as hydroxychloroquine and ivermectin.
Calls and emails to AFD regarding a statement on Gold’s plea made by this news organization were not returned by press time.
In October, Representative James E. Clyburn (D-S.C.), chairman of the Select Subcommittee on the Coronavirus Crisis, launched an investigation into organizations, including AFD, that spread misinformation and facilitate access to disproven and potentially hazardous treatments for COVID-19. According to news reports, Rep. Clyburn called the AFD and other such groups “predatory actors.”
Hospitals where Dr. Gold previously worked, including Providence St. Joseph Medical Center in Santa Monica, Calif., and Cedars-Sinai in Los Angeles, have disassociated themselves from her. On July 29, 2020, Cedars-Sinai Medical Center, where Gold previously worked, issued a statement that said, in part, “Simone Gold, MD, has not worked with Cedars-Sinai Medical Center or any of its offices or affiliates since 2015. For 3 weeks in late 2015, Dr. Gold was employed on a per diem basis by Cedars-Sinai Medical Network, a component of Cedars-Sinai. She worked during this brief time in a network urgent care clinic. Dr. Gold is not authorized to represent or speak about any information on behalf of Cedars-Sinai.”
Dr. Gold’s medical license in the state of California is current and she has no pending hearings before the state medical board, according to its website. On her own website, Dr. Gold says she “voluntarily refused” to renew her board certification last year, “due to the unethical behavior of the medical boards.”
Dr. Gold is also a licensed attorney, having earned her law degree in health policy analysis at Stanford (Calif.) Law School.
Dr. Gold faces 6 months in prison. Sentencing is scheduled for June 16.
A version of this article first appeared on Medscape.com.
Brown fat, white fat. Is one better than the other?
“Adipose tissue is an underappreciated and misunderstood organ.” It’s with these words that Aaron M. Cypess, MD, PhD, begins his recent review published in the New England Journal of Medicine.
As obesity rates steadily rise, “the riskiest approach to human adipose tissue is to dismiss its importance,” he adds, especially because there has been “an explosive growth” in our understanding of white and brown adipose tissue over the past 5 to 10 years.
This news organization asked Dr. Cypess, a National Institutes of Health (NIH) scientist whose research focuses on brown fat, to discuss some of the main points in his review, titled, “Reassessing Human Adipose Tissue,” and clear up some misconceptions about fat.
You write that, for people who struggle to lose weight, “fat is often a source of misery, not marvel.” Why is fat a marvel?
When I started medical school in 1992, fat was just a thing that stored calories. You had to get it out of the way when you operated on the stomach or intestines. Now we know it’s not just one cell, it’s multiple types of cells, including immune cells and some blood cells. There’s cell turnover, and cells can get bigger or smaller, so it’s a dynamic tissue. It impacts the immune system and affects insulin sensitivity.
Why use the term “adipose tissue” and not just “fat”?
People think of fat cells and that’s it. However, adipose tissue (fat) has multiple cell types, and they each matter. There are adipocytes (fat cells) – which can be white, brown, beige, or pink – as well as immune cells, fibroblasts, blood vessels, and parts of nerve cells.
The main function of white adipose tissue is to store energy in the form of triglycerides. Brown adipose tissue consumes glucose and triglycerides, generating heat. Brown fat cells within depots of white fat are termed brite cells (a portmanteau of brown and white) or beige cells. Pink fat cells have been found in breast tissue in mice.
What do we now know about white fat and brown fat? Can brown fat change to white fat or vice versa?
White adipose tissue is commonly separated into visceral fat and subcutaneous fat, which have negative and neutral or positive metabolic effects, respectively. It is capable of more than doubling in mass and then returning to baseline.
White adipocyte-derived hormones include leptin, which is low in starvation, and adiponectin, which regulates glucose and lipid metabolism. White adipose tissue is essential for the proper function of the reproductive system, including secretion of hormones and lactation.
Brown adipose tissue protects newborns from cold as they develop the ability to shiver, and in adults it is found in depots in the neck, shoulders, posterior thorax, and abdomen. The amount of brown adipose tissue varies according to sex and lowers with increasing age and increasing body mass index.
There is much more white fat in the body than brown fat. It appears that activating brown fat leads to beneficial effects on metabolism, though we don’t know yet all the steps for how that happens.
In mice, you’ve got white fat depots and brown fat depots, and some brown fat can be found in the white fat.
With humans it’s much more complicated, and I’ve seen this in the operating room myself, and on slides. Where you find brown fat cells you also find a certain proportion of white fat cells, not an exclusive brown fat depot like you see in a mouse.
It is hotly debated right now whether brown fat can change to white fat and vice versa (transdifferentiation). The beige fat cells are supposed to be the kind that can shuttle between more white-like or brown-like. They can sometimes be white or sometimes brown. It can be very contentious in [scientific] papers and meetings.
Are humans born with all the fat cells they will ever have?
No. New fat cells are made throughout our lives. When the white adipocytes store too much triglyceride, they get really big and they get “sick” and die faster. It’s the rate at which the white cells take up the fat to store it and then get rid of it that can impact whether someone gains a lot of weight and whether they can successfully lose it after reasonable effort.
The average lifespan of a white fat cell is 15 years. We have no idea yet of the lifespan of a brown fat cell.
Is there a single “fat gene”? What role do fat genes play in the likelihood of developing metabolic diseases and type 2 diabetes?
Genes are very important for influencing the development of obesity and probably influence 50%-70% of obesity, based on studies in populations of predominantly European origin. But that high percentage reflects the impact of hundreds of genes. For most people, there is no one gene that exerts all of the effects. There are extremely rare diseases where one gene is responsible. Currently, only 20% of the entire phenotypic variation in obesity can be explained by the thousands of loci identified so far.
Why is it “correct but too simplistic” to attribute the increasing rates of obesity to excessive triglyceride storage in white adipose tissue?
Saying obesity is caused by too much triglyceride storage ignores the reasons how and why the triglycerides got there. There are likely to be multiple contributing factors to drive obesity, and those have billions of dollars of policy implications. Is obesity resulting from portion sizes? Then we should work on educating the public on how to estimate their caloric intake. Is it the types of foods, such as ultra-processed foods? Then we can discourage eating certain food groups while promoting others. Is it about physical activity? Then we should prioritize exercise programs.
Why is obesity “not simply a failure of will power”?
Genetic factors in adipose tissue impact how easy it is to store triglycerides, how easy it is to get fat out of the tissue and burn it up, and what kinds of hormones are released by the tissue to regulate appetite, insulin resistance, and inflammation. Ten different people can all overeat the same amount of the same foods, yet there will be differences in the amount of weight gain and metabolic complications experienced. And at the brain level, some people will feel “full” sooner than others.
How can excess adipose tissue lead to disease? Do some people have “metabolically healthy obesity”?
Excess adipose tissue leads to chronic inflammation that can then cause insulin resistance, hypertension, fatty liver disease, and other complications. It appears that there are metabolically healthy obese people, but it is not clear if that is only a temporary state.
Could long-term brown adipose tissue activation help treat obesity or related metabolic disease?
Our research group at the NIH and others have shown that long-term brown adipose tissue activation produces metabolic benefit such as improved insulin resistance, lower plasma glucose, and higher HDL [good] cholesterol. However, there is no evidence yet that it will lead to actual weight loss.
We are trying to use brown adipose tissue activation to treat obesity-related metabolic disease to see if it could lead to reduction in inflammation, improvement in the cholesterol profile, and decrease in blood pressure.
A large observational study published Jan. 4, 2021, in Nature Medicine by Paul Cohen’s group at Rockefeller University, in tens of thousands of people at Memorial Sloan Kettering Cancer Center, showed that people who had brown fat were generally healthier and had less high blood pressure and less cardiovascular disease. This study could not show causation, but at every BMI, people were healthier if they had more brown fat than if they had less. So, there’s something going on. We’re still trying to figure that out.
Dr. Cypess has no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Adipose tissue is an underappreciated and misunderstood organ.” It’s with these words that Aaron M. Cypess, MD, PhD, begins his recent review published in the New England Journal of Medicine.
As obesity rates steadily rise, “the riskiest approach to human adipose tissue is to dismiss its importance,” he adds, especially because there has been “an explosive growth” in our understanding of white and brown adipose tissue over the past 5 to 10 years.
This news organization asked Dr. Cypess, a National Institutes of Health (NIH) scientist whose research focuses on brown fat, to discuss some of the main points in his review, titled, “Reassessing Human Adipose Tissue,” and clear up some misconceptions about fat.
You write that, for people who struggle to lose weight, “fat is often a source of misery, not marvel.” Why is fat a marvel?
When I started medical school in 1992, fat was just a thing that stored calories. You had to get it out of the way when you operated on the stomach or intestines. Now we know it’s not just one cell, it’s multiple types of cells, including immune cells and some blood cells. There’s cell turnover, and cells can get bigger or smaller, so it’s a dynamic tissue. It impacts the immune system and affects insulin sensitivity.
Why use the term “adipose tissue” and not just “fat”?
People think of fat cells and that’s it. However, adipose tissue (fat) has multiple cell types, and they each matter. There are adipocytes (fat cells) – which can be white, brown, beige, or pink – as well as immune cells, fibroblasts, blood vessels, and parts of nerve cells.
The main function of white adipose tissue is to store energy in the form of triglycerides. Brown adipose tissue consumes glucose and triglycerides, generating heat. Brown fat cells within depots of white fat are termed brite cells (a portmanteau of brown and white) or beige cells. Pink fat cells have been found in breast tissue in mice.
What do we now know about white fat and brown fat? Can brown fat change to white fat or vice versa?
White adipose tissue is commonly separated into visceral fat and subcutaneous fat, which have negative and neutral or positive metabolic effects, respectively. It is capable of more than doubling in mass and then returning to baseline.
White adipocyte-derived hormones include leptin, which is low in starvation, and adiponectin, which regulates glucose and lipid metabolism. White adipose tissue is essential for the proper function of the reproductive system, including secretion of hormones and lactation.
Brown adipose tissue protects newborns from cold as they develop the ability to shiver, and in adults it is found in depots in the neck, shoulders, posterior thorax, and abdomen. The amount of brown adipose tissue varies according to sex and lowers with increasing age and increasing body mass index.
There is much more white fat in the body than brown fat. It appears that activating brown fat leads to beneficial effects on metabolism, though we don’t know yet all the steps for how that happens.
In mice, you’ve got white fat depots and brown fat depots, and some brown fat can be found in the white fat.
With humans it’s much more complicated, and I’ve seen this in the operating room myself, and on slides. Where you find brown fat cells you also find a certain proportion of white fat cells, not an exclusive brown fat depot like you see in a mouse.
It is hotly debated right now whether brown fat can change to white fat and vice versa (transdifferentiation). The beige fat cells are supposed to be the kind that can shuttle between more white-like or brown-like. They can sometimes be white or sometimes brown. It can be very contentious in [scientific] papers and meetings.
Are humans born with all the fat cells they will ever have?
No. New fat cells are made throughout our lives. When the white adipocytes store too much triglyceride, they get really big and they get “sick” and die faster. It’s the rate at which the white cells take up the fat to store it and then get rid of it that can impact whether someone gains a lot of weight and whether they can successfully lose it after reasonable effort.
The average lifespan of a white fat cell is 15 years. We have no idea yet of the lifespan of a brown fat cell.
Is there a single “fat gene”? What role do fat genes play in the likelihood of developing metabolic diseases and type 2 diabetes?
Genes are very important for influencing the development of obesity and probably influence 50%-70% of obesity, based on studies in populations of predominantly European origin. But that high percentage reflects the impact of hundreds of genes. For most people, there is no one gene that exerts all of the effects. There are extremely rare diseases where one gene is responsible. Currently, only 20% of the entire phenotypic variation in obesity can be explained by the thousands of loci identified so far.
Why is it “correct but too simplistic” to attribute the increasing rates of obesity to excessive triglyceride storage in white adipose tissue?
Saying obesity is caused by too much triglyceride storage ignores the reasons how and why the triglycerides got there. There are likely to be multiple contributing factors to drive obesity, and those have billions of dollars of policy implications. Is obesity resulting from portion sizes? Then we should work on educating the public on how to estimate their caloric intake. Is it the types of foods, such as ultra-processed foods? Then we can discourage eating certain food groups while promoting others. Is it about physical activity? Then we should prioritize exercise programs.
Why is obesity “not simply a failure of will power”?
Genetic factors in adipose tissue impact how easy it is to store triglycerides, how easy it is to get fat out of the tissue and burn it up, and what kinds of hormones are released by the tissue to regulate appetite, insulin resistance, and inflammation. Ten different people can all overeat the same amount of the same foods, yet there will be differences in the amount of weight gain and metabolic complications experienced. And at the brain level, some people will feel “full” sooner than others.
How can excess adipose tissue lead to disease? Do some people have “metabolically healthy obesity”?
Excess adipose tissue leads to chronic inflammation that can then cause insulin resistance, hypertension, fatty liver disease, and other complications. It appears that there are metabolically healthy obese people, but it is not clear if that is only a temporary state.
Could long-term brown adipose tissue activation help treat obesity or related metabolic disease?
Our research group at the NIH and others have shown that long-term brown adipose tissue activation produces metabolic benefit such as improved insulin resistance, lower plasma glucose, and higher HDL [good] cholesterol. However, there is no evidence yet that it will lead to actual weight loss.
We are trying to use brown adipose tissue activation to treat obesity-related metabolic disease to see if it could lead to reduction in inflammation, improvement in the cholesterol profile, and decrease in blood pressure.
A large observational study published Jan. 4, 2021, in Nature Medicine by Paul Cohen’s group at Rockefeller University, in tens of thousands of people at Memorial Sloan Kettering Cancer Center, showed that people who had brown fat were generally healthier and had less high blood pressure and less cardiovascular disease. This study could not show causation, but at every BMI, people were healthier if they had more brown fat than if they had less. So, there’s something going on. We’re still trying to figure that out.
Dr. Cypess has no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Adipose tissue is an underappreciated and misunderstood organ.” It’s with these words that Aaron M. Cypess, MD, PhD, begins his recent review published in the New England Journal of Medicine.
As obesity rates steadily rise, “the riskiest approach to human adipose tissue is to dismiss its importance,” he adds, especially because there has been “an explosive growth” in our understanding of white and brown adipose tissue over the past 5 to 10 years.
This news organization asked Dr. Cypess, a National Institutes of Health (NIH) scientist whose research focuses on brown fat, to discuss some of the main points in his review, titled, “Reassessing Human Adipose Tissue,” and clear up some misconceptions about fat.
You write that, for people who struggle to lose weight, “fat is often a source of misery, not marvel.” Why is fat a marvel?
When I started medical school in 1992, fat was just a thing that stored calories. You had to get it out of the way when you operated on the stomach or intestines. Now we know it’s not just one cell, it’s multiple types of cells, including immune cells and some blood cells. There’s cell turnover, and cells can get bigger or smaller, so it’s a dynamic tissue. It impacts the immune system and affects insulin sensitivity.
Why use the term “adipose tissue” and not just “fat”?
People think of fat cells and that’s it. However, adipose tissue (fat) has multiple cell types, and they each matter. There are adipocytes (fat cells) – which can be white, brown, beige, or pink – as well as immune cells, fibroblasts, blood vessels, and parts of nerve cells.
The main function of white adipose tissue is to store energy in the form of triglycerides. Brown adipose tissue consumes glucose and triglycerides, generating heat. Brown fat cells within depots of white fat are termed brite cells (a portmanteau of brown and white) or beige cells. Pink fat cells have been found in breast tissue in mice.
What do we now know about white fat and brown fat? Can brown fat change to white fat or vice versa?
White adipose tissue is commonly separated into visceral fat and subcutaneous fat, which have negative and neutral or positive metabolic effects, respectively. It is capable of more than doubling in mass and then returning to baseline.
White adipocyte-derived hormones include leptin, which is low in starvation, and adiponectin, which regulates glucose and lipid metabolism. White adipose tissue is essential for the proper function of the reproductive system, including secretion of hormones and lactation.
Brown adipose tissue protects newborns from cold as they develop the ability to shiver, and in adults it is found in depots in the neck, shoulders, posterior thorax, and abdomen. The amount of brown adipose tissue varies according to sex and lowers with increasing age and increasing body mass index.
There is much more white fat in the body than brown fat. It appears that activating brown fat leads to beneficial effects on metabolism, though we don’t know yet all the steps for how that happens.
In mice, you’ve got white fat depots and brown fat depots, and some brown fat can be found in the white fat.
With humans it’s much more complicated, and I’ve seen this in the operating room myself, and on slides. Where you find brown fat cells you also find a certain proportion of white fat cells, not an exclusive brown fat depot like you see in a mouse.
It is hotly debated right now whether brown fat can change to white fat and vice versa (transdifferentiation). The beige fat cells are supposed to be the kind that can shuttle between more white-like or brown-like. They can sometimes be white or sometimes brown. It can be very contentious in [scientific] papers and meetings.
Are humans born with all the fat cells they will ever have?
No. New fat cells are made throughout our lives. When the white adipocytes store too much triglyceride, they get really big and they get “sick” and die faster. It’s the rate at which the white cells take up the fat to store it and then get rid of it that can impact whether someone gains a lot of weight and whether they can successfully lose it after reasonable effort.
The average lifespan of a white fat cell is 15 years. We have no idea yet of the lifespan of a brown fat cell.
Is there a single “fat gene”? What role do fat genes play in the likelihood of developing metabolic diseases and type 2 diabetes?
Genes are very important for influencing the development of obesity and probably influence 50%-70% of obesity, based on studies in populations of predominantly European origin. But that high percentage reflects the impact of hundreds of genes. For most people, there is no one gene that exerts all of the effects. There are extremely rare diseases where one gene is responsible. Currently, only 20% of the entire phenotypic variation in obesity can be explained by the thousands of loci identified so far.
Why is it “correct but too simplistic” to attribute the increasing rates of obesity to excessive triglyceride storage in white adipose tissue?
Saying obesity is caused by too much triglyceride storage ignores the reasons how and why the triglycerides got there. There are likely to be multiple contributing factors to drive obesity, and those have billions of dollars of policy implications. Is obesity resulting from portion sizes? Then we should work on educating the public on how to estimate their caloric intake. Is it the types of foods, such as ultra-processed foods? Then we can discourage eating certain food groups while promoting others. Is it about physical activity? Then we should prioritize exercise programs.
Why is obesity “not simply a failure of will power”?
Genetic factors in adipose tissue impact how easy it is to store triglycerides, how easy it is to get fat out of the tissue and burn it up, and what kinds of hormones are released by the tissue to regulate appetite, insulin resistance, and inflammation. Ten different people can all overeat the same amount of the same foods, yet there will be differences in the amount of weight gain and metabolic complications experienced. And at the brain level, some people will feel “full” sooner than others.
How can excess adipose tissue lead to disease? Do some people have “metabolically healthy obesity”?
Excess adipose tissue leads to chronic inflammation that can then cause insulin resistance, hypertension, fatty liver disease, and other complications. It appears that there are metabolically healthy obese people, but it is not clear if that is only a temporary state.
Could long-term brown adipose tissue activation help treat obesity or related metabolic disease?
Our research group at the NIH and others have shown that long-term brown adipose tissue activation produces metabolic benefit such as improved insulin resistance, lower plasma glucose, and higher HDL [good] cholesterol. However, there is no evidence yet that it will lead to actual weight loss.
We are trying to use brown adipose tissue activation to treat obesity-related metabolic disease to see if it could lead to reduction in inflammation, improvement in the cholesterol profile, and decrease in blood pressure.
A large observational study published Jan. 4, 2021, in Nature Medicine by Paul Cohen’s group at Rockefeller University, in tens of thousands of people at Memorial Sloan Kettering Cancer Center, showed that people who had brown fat were generally healthier and had less high blood pressure and less cardiovascular disease. This study could not show causation, but at every BMI, people were healthier if they had more brown fat than if they had less. So, there’s something going on. We’re still trying to figure that out.
Dr. Cypess has no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Innumerable pulmonary nodules
A 56-year-old woman with a history of a thyroid goiter following a partial thyroidectomy presented to the emergency department with shortness of breath, progressive weakness, and fatigue. She also reported a poor appetite and unintentional weight loss of approximately 40 lbs over the past 2 months but had not sought medical care. She denied having a cough, chest pain, hemoptysis, hematemesis, fevers, chills, or night sweats.
Physical examination revealed a cachectic woman with tachycardia and tachypnea, along with diffuse coarse rales throughout both lungs. The patient’s initial heart rate was 101 beats/min; respiratory rate, 25 breaths/min; and oxygen saturation, 74% on room air. The results of a complete blood count and comprehensive metabolic panel were within normal limits. A chest radiograph was performed, showing innumerable subcentimeter- and pericentimeter-sized soft-tissue densities in both lungs (FIGURE 1). Computed tomography (CT) of the chest was subsequently obtained to better characterize the nodules (FIGURES 2A and 2B). The CT revealed innumerable noncalcified pulmonary nodules as well as multiple hilar masses suggestive of malignancy; however, infectious etiologies could not be ruled out.
As the patient was visiting from a tuberculosis-endemic area, she was empirically started on rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) therapy for treatment of Mycobacterium tuberculosis infection. Subsequently, polymerase chain reaction (PCR) testing and acid-fast bacillus sputum smear came back negative for M tuberculosis and RIPE therapy was stopped. Bacterial culture and fungal screens for Pneumocystis jirovecii, Coccidioides, Cryptococcus, and Histoplasma capsulatum were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Metastatic follicular thyroid carcinoma
A biopsy of one of the lung nodules was performed and showed strong positive staining for thyroglobulin and thyroid transcription factor-1, establishing a diagnosis of metastatic follicular thyroid carcinoma. Further imaging revealed additional metastases to the brain, liver, adrenal glands, and spine.
Follicular thyroid carcinoma is the second most common histologic type of thyroid cancer, comprising 10% to 15% of thyroid cancer cases.1 Distant metastases are not uncommon in this histologic type; about 11% of patients have distant metastases at presentation.2 Compared with papillary thyroid cancer, which spreads via the lymphatics, the follicular type can metastasize via vascular invasion, thus leading to its “aggressiveness” and frequent occurrence of metastasis outside the neck (most commonly to the lung).1 For this reason, an early diagnosis is important.3 In this case, the patient revealed that she hadn’t sought medical care at the onset of her symptoms due to financial limitations and limited access to medical providers; this likely contributed to the patient’s diagnosis at a late stage of disease.
Infectious and septic diseases are part of the differential Dx
The differential diagnosis for diffuse pulmonary nodules includes various infections, silicosis, coal worker’s pneumoconiosis, septic pulmonary emboli, and pulmonary sarcoidosis. All of these diagnoses can cause constitutional, as well as pulmonary, symptoms.
Infections, such as pulmonary tuberculosis, pulmonary coccidioidomycosis, or other cavitating infections, can be ruled out with specific testing for the organism of concern and/or culture.
Silicosis and coal worker’s pneumoconiosis generally cause lesions in the upper lobes and require a significant occupational exposure to silica or coal, respectively.
Continue to: Septic pulmonary emboli
Septic pulmonary emboli are usually seen in the context of sepsis, and there are positive blood cultures at the time of imaging.
Pulmonary sarcoidosis typically manifests with hilar and mediastinal lymphadenopathy and is often accompanied by other organ involvement.
There are options for treatment if diagnosis is made early
Thyroidectomy, radioiodine treatment, and thyroid-stimulating hormone suppressive therapy are the most commonly used therapies for treatment of follicular thyroid carcinoma.4 If follicular thyroid carcinoma is caught early (with only local involvement), 5-year relative survival rates are near 100%.5 However, outcomes are generally poor in patients who are of older age and those who have macronodular lung metastases or multiple bone metastases.6
Our patient was started on levothyroxine. Radioiodine and targeted drug therapy with a tyrosine kinase inhibitor were attempted, but the patient was unable to tolerate them due to her poor functional status. She was thus transitioned to hospice care.
1. Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid. 2013;23:1015-1021. doi: 10.1089/thy.2012.0356
2. Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474-476. doi: 10.1016/s0002-9610(97)00158-x
3. Lim IIP, Hochman T, Blumberg SN, et al. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid. 2012;22:269-274. doi: 10.1089/thy.2010.0385
4. Grani G, Lamartina L, Durante C, et al. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6:500-514. doi: 10.1016/S2213-8587(17)30325-X
5. Thyroid cancer survival rates, by type and stage. American Cancer Society. Updated January 25, 2021. Accessed February 1, 2022. www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html
6. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. doi: 10.1210/jc.2005-2838
A 56-year-old woman with a history of a thyroid goiter following a partial thyroidectomy presented to the emergency department with shortness of breath, progressive weakness, and fatigue. She also reported a poor appetite and unintentional weight loss of approximately 40 lbs over the past 2 months but had not sought medical care. She denied having a cough, chest pain, hemoptysis, hematemesis, fevers, chills, or night sweats.
Physical examination revealed a cachectic woman with tachycardia and tachypnea, along with diffuse coarse rales throughout both lungs. The patient’s initial heart rate was 101 beats/min; respiratory rate, 25 breaths/min; and oxygen saturation, 74% on room air. The results of a complete blood count and comprehensive metabolic panel were within normal limits. A chest radiograph was performed, showing innumerable subcentimeter- and pericentimeter-sized soft-tissue densities in both lungs (FIGURE 1). Computed tomography (CT) of the chest was subsequently obtained to better characterize the nodules (FIGURES 2A and 2B). The CT revealed innumerable noncalcified pulmonary nodules as well as multiple hilar masses suggestive of malignancy; however, infectious etiologies could not be ruled out.
As the patient was visiting from a tuberculosis-endemic area, she was empirically started on rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) therapy for treatment of Mycobacterium tuberculosis infection. Subsequently, polymerase chain reaction (PCR) testing and acid-fast bacillus sputum smear came back negative for M tuberculosis and RIPE therapy was stopped. Bacterial culture and fungal screens for Pneumocystis jirovecii, Coccidioides, Cryptococcus, and Histoplasma capsulatum were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Metastatic follicular thyroid carcinoma
A biopsy of one of the lung nodules was performed and showed strong positive staining for thyroglobulin and thyroid transcription factor-1, establishing a diagnosis of metastatic follicular thyroid carcinoma. Further imaging revealed additional metastases to the brain, liver, adrenal glands, and spine.
Follicular thyroid carcinoma is the second most common histologic type of thyroid cancer, comprising 10% to 15% of thyroid cancer cases.1 Distant metastases are not uncommon in this histologic type; about 11% of patients have distant metastases at presentation.2 Compared with papillary thyroid cancer, which spreads via the lymphatics, the follicular type can metastasize via vascular invasion, thus leading to its “aggressiveness” and frequent occurrence of metastasis outside the neck (most commonly to the lung).1 For this reason, an early diagnosis is important.3 In this case, the patient revealed that she hadn’t sought medical care at the onset of her symptoms due to financial limitations and limited access to medical providers; this likely contributed to the patient’s diagnosis at a late stage of disease.
Infectious and septic diseases are part of the differential Dx
The differential diagnosis for diffuse pulmonary nodules includes various infections, silicosis, coal worker’s pneumoconiosis, septic pulmonary emboli, and pulmonary sarcoidosis. All of these diagnoses can cause constitutional, as well as pulmonary, symptoms.
Infections, such as pulmonary tuberculosis, pulmonary coccidioidomycosis, or other cavitating infections, can be ruled out with specific testing for the organism of concern and/or culture.
Silicosis and coal worker’s pneumoconiosis generally cause lesions in the upper lobes and require a significant occupational exposure to silica or coal, respectively.
Continue to: Septic pulmonary emboli
Septic pulmonary emboli are usually seen in the context of sepsis, and there are positive blood cultures at the time of imaging.
Pulmonary sarcoidosis typically manifests with hilar and mediastinal lymphadenopathy and is often accompanied by other organ involvement.
There are options for treatment if diagnosis is made early
Thyroidectomy, radioiodine treatment, and thyroid-stimulating hormone suppressive therapy are the most commonly used therapies for treatment of follicular thyroid carcinoma.4 If follicular thyroid carcinoma is caught early (with only local involvement), 5-year relative survival rates are near 100%.5 However, outcomes are generally poor in patients who are of older age and those who have macronodular lung metastases or multiple bone metastases.6
Our patient was started on levothyroxine. Radioiodine and targeted drug therapy with a tyrosine kinase inhibitor were attempted, but the patient was unable to tolerate them due to her poor functional status. She was thus transitioned to hospice care.
A 56-year-old woman with a history of a thyroid goiter following a partial thyroidectomy presented to the emergency department with shortness of breath, progressive weakness, and fatigue. She also reported a poor appetite and unintentional weight loss of approximately 40 lbs over the past 2 months but had not sought medical care. She denied having a cough, chest pain, hemoptysis, hematemesis, fevers, chills, or night sweats.
Physical examination revealed a cachectic woman with tachycardia and tachypnea, along with diffuse coarse rales throughout both lungs. The patient’s initial heart rate was 101 beats/min; respiratory rate, 25 breaths/min; and oxygen saturation, 74% on room air. The results of a complete blood count and comprehensive metabolic panel were within normal limits. A chest radiograph was performed, showing innumerable subcentimeter- and pericentimeter-sized soft-tissue densities in both lungs (FIGURE 1). Computed tomography (CT) of the chest was subsequently obtained to better characterize the nodules (FIGURES 2A and 2B). The CT revealed innumerable noncalcified pulmonary nodules as well as multiple hilar masses suggestive of malignancy; however, infectious etiologies could not be ruled out.
As the patient was visiting from a tuberculosis-endemic area, she was empirically started on rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) therapy for treatment of Mycobacterium tuberculosis infection. Subsequently, polymerase chain reaction (PCR) testing and acid-fast bacillus sputum smear came back negative for M tuberculosis and RIPE therapy was stopped. Bacterial culture and fungal screens for Pneumocystis jirovecii, Coccidioides, Cryptococcus, and Histoplasma capsulatum were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Metastatic follicular thyroid carcinoma
A biopsy of one of the lung nodules was performed and showed strong positive staining for thyroglobulin and thyroid transcription factor-1, establishing a diagnosis of metastatic follicular thyroid carcinoma. Further imaging revealed additional metastases to the brain, liver, adrenal glands, and spine.
Follicular thyroid carcinoma is the second most common histologic type of thyroid cancer, comprising 10% to 15% of thyroid cancer cases.1 Distant metastases are not uncommon in this histologic type; about 11% of patients have distant metastases at presentation.2 Compared with papillary thyroid cancer, which spreads via the lymphatics, the follicular type can metastasize via vascular invasion, thus leading to its “aggressiveness” and frequent occurrence of metastasis outside the neck (most commonly to the lung).1 For this reason, an early diagnosis is important.3 In this case, the patient revealed that she hadn’t sought medical care at the onset of her symptoms due to financial limitations and limited access to medical providers; this likely contributed to the patient’s diagnosis at a late stage of disease.
Infectious and septic diseases are part of the differential Dx
The differential diagnosis for diffuse pulmonary nodules includes various infections, silicosis, coal worker’s pneumoconiosis, septic pulmonary emboli, and pulmonary sarcoidosis. All of these diagnoses can cause constitutional, as well as pulmonary, symptoms.
Infections, such as pulmonary tuberculosis, pulmonary coccidioidomycosis, or other cavitating infections, can be ruled out with specific testing for the organism of concern and/or culture.
Silicosis and coal worker’s pneumoconiosis generally cause lesions in the upper lobes and require a significant occupational exposure to silica or coal, respectively.
Continue to: Septic pulmonary emboli
Septic pulmonary emboli are usually seen in the context of sepsis, and there are positive blood cultures at the time of imaging.
Pulmonary sarcoidosis typically manifests with hilar and mediastinal lymphadenopathy and is often accompanied by other organ involvement.
There are options for treatment if diagnosis is made early
Thyroidectomy, radioiodine treatment, and thyroid-stimulating hormone suppressive therapy are the most commonly used therapies for treatment of follicular thyroid carcinoma.4 If follicular thyroid carcinoma is caught early (with only local involvement), 5-year relative survival rates are near 100%.5 However, outcomes are generally poor in patients who are of older age and those who have macronodular lung metastases or multiple bone metastases.6
Our patient was started on levothyroxine. Radioiodine and targeted drug therapy with a tyrosine kinase inhibitor were attempted, but the patient was unable to tolerate them due to her poor functional status. She was thus transitioned to hospice care.
1. Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid. 2013;23:1015-1021. doi: 10.1089/thy.2012.0356
2. Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474-476. doi: 10.1016/s0002-9610(97)00158-x
3. Lim IIP, Hochman T, Blumberg SN, et al. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid. 2012;22:269-274. doi: 10.1089/thy.2010.0385
4. Grani G, Lamartina L, Durante C, et al. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6:500-514. doi: 10.1016/S2213-8587(17)30325-X
5. Thyroid cancer survival rates, by type and stage. American Cancer Society. Updated January 25, 2021. Accessed February 1, 2022. www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html
6. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. doi: 10.1210/jc.2005-2838
1. Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid. 2013;23:1015-1021. doi: 10.1089/thy.2012.0356
2. Shaha AR, Shah JP, Loree TR. Differentiated thyroid cancer presenting initially with distant metastasis. Am J Surg. 1997;174:474-476. doi: 10.1016/s0002-9610(97)00158-x
3. Lim IIP, Hochman T, Blumberg SN, et al. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid. 2012;22:269-274. doi: 10.1089/thy.2010.0385
4. Grani G, Lamartina L, Durante C, et al. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6:500-514. doi: 10.1016/S2213-8587(17)30325-X
5. Thyroid cancer survival rates, by type and stage. American Cancer Society. Updated January 25, 2021. Accessed February 1, 2022. www.cancer.org/cancer/thyroid-cancer/detection-diagnosis-staging/survival-rates.html
6. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892-2899. doi: 10.1210/jc.2005-2838
Rapidly Enlarging Bullous Plaque
The Diagnosis: Bullous Pyoderma Gangrenosum
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.
First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
The Diagnosis: Bullous Pyoderma Gangrenosum
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.
First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
The Diagnosis: Bullous Pyoderma Gangrenosum
A bone marrow biopsy revealed 60% myeloblasts, leading to a diagnosis of acute myeloid leukemia (AML). A biopsy obtained from the edge of the bullous plaque demonstrated a dense dermal neutrophilic infiltrate with extravasated erythrocytes (Figure). Fite, Gram, and Grocott-Gomori methenamine-silver staining failed to reveal infectious organisms. Tissue and blood cultures were negative. Given the pathologic findings, clinical presentation including recent diagnosis of AML, and exclusion of other underlying disease processes including infection, the diagnosis of bullous pyoderma gangrenosum (PG) was made. The lesion improved with systemic steroids and treatment of the underlying AML with fludarabine and venetoclax chemotherapy.
First recognized in 1916 by French dermatologist Louis Brocq, MD, PG is a sterile neutrophilic dermatosis that predominantly affects women older than 50 years.1,2 This disorder can develop idiopathically; secondary to trauma; or in association with systemic diseases such as inflammatory bowel disease, rheumatoid arthritis, and hematologic malignancies. The pathogenesis of PG remains unclear; however, overexpression of inflammatory cytokines may mediate its development by stimulating T cells and promoting neutrophilic chemotaxis.3
Pyoderma gangrenosum classically presents as a rapidly enlarging ulcer with cribriform scarring but manifests variably. Four variants of the disorder exist: classic ulcerative, pustular, bullous, and vegetative PG. Ulcerative PG is the most common variant. Bullous PG is associated with hematologic malignancies such as primary myelofibrosis, myelodysplastic disease, and AML. In these patients, hematologic malignancy often exists prior to the development of PG and portends a poorer prognosis. This association underscores the importance of timely diagnosis and thorough hematologic evaluation by obtaining a complete blood cell count with differential, peripheral smear, serum protein electrophoresis with immunofixation, and quantitative immunoglobulins (IgA, IgG, IgM). If any of the results are positive, prompt referral to a hematologist and bone marrow biopsy are paramount.3
The diagnosis of PG remains elusive, as no validated clinical or pathological criteria exist. Histopathologic evaluation may be nonspecific and variable depending on the subtype. Biopsy results for classic ulcerative PG may reveal a neutrophilic infiltrate with leukocytoclasia. Bullous PG may include subepidermal hemorrhagic bullae. Notably, bullous PG appears histologically similar to the superficial bullous variant of Sweet syndrome.
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a type of neutrophilic dermatosis characterized by fever, neutrophilia, and the sudden onset of tender erythematous lesions. Variations include idiopathic, subcutaneous, and bullous Sweet syndrome, which present as plaques, nodules, or bullae, respectively.4 Similar to PG, Sweet syndrome can manifest in patients with hematologic malignancies. Both PG and Sweet syndrome are thought to exist along a continuum and can be considered intersecting diagnoses in the setting of leukemia or other hematologic malignancies.5 There have been reports of the coexistence of distinct PG and Sweet syndrome lesions on a single patient, further supporting the belief that these entities share a common pathologic mechanism.6 Sweet syndrome also commonly can be associated with upper respiratory infections; pregnancy; and medications, with culprits including granulocyte colony-stimulating factor, azathioprine, vemurafenib, and isotretinoin.7
Other differential diagnoses include brown recluse spider bite, bullous fixed drug eruption (FDE), and necrotizing fasciitis (NF). Venom from the brown recluse spider (Loxosceles reclusa) can trigger toxin-mediated hemolysis, complement-mediated erythrocyte destruction, and basement membrane zone degradation due to the synergistic effects of the toxin’s sphingomyelinase D and protease content.8 The inciting bite is painless. After 8 hours, the site becomes painful and pruritic and presents with peripheral erythema and central pallor. After 24 hours, the lesion blisters. The blister ruptures within 3 to 4 days, resulting in eschar formation with the subsequent development of an indurated blue ulcer with a stellate center. Ulcers can take months to heal.9 Based on the clinical findings in our patient, this diagnosis was less likely.
Fixed drug eruption is a localized cutaneous reaction that manifests in fixed locations minutes to days after exposure to medications such as trimethoprimsulfamethoxazole, nonsteroidal anti-inflammatory drugs, salicylates, and oral contraceptives. Commonly affected areas include the hands, legs, genitals, and trunk. Lesions initially present as well-demarcated, erythematous to violaceous, round plaques. A rarer variant manifesting as bullae also has been described. Careful consideration of the patient’s history and physical examination findings is sufficient for establishing this diagnosis; however, a punch biopsy can provide clarity. Histopathology reveals a lichenoid tissue reaction with dyskeratosis, broad epidermal necrosis, and damage to the stratum basalis. A lymphocytic perivascular infiltrate also may appear in the dermis.10 Both the clinical findings and histopathology of our case were not characteristic of FDE.
Necrotizing fasciitis is a fulminant, life-threatening, soft-tissue infection precipitated by polymicrobial flora. Early recognition of NF is difficult, as in its early stages it can mimic cellulitis. As the infection takes its course, necrosis can extend from the skin and into the subcutaneous tissue. Patients also develop fever, leukocytosis, and signs of sepsis. Histopathology demonstrates neutrophilic infiltration with bacterial invasion as well as necrosis of the superficial fascia and subepidermal edema.11 Pyoderma gangrenosum previously has been reported to mimic NF; however, lack of responsiveness to antibiotic therapy would favor a diagnosis of PG over NF.12
Treatment of PG is driven by the extent of cutaneous involvement. In mild cases, wound care and topical therapy with corticosteroids and tacrolimus may suffice. Severe cases necessitate systemic therapy with oral corticosteroids or cyclosporine; biologic therapy also may play a role in treatment.4 In patients with hematologic malignancy, chemotherapy alone may partially or completely resolve the lesion; however, systemic corticosteroids commonly are included in management.3
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
- Brocq L. A new contribution to the study of geometric phagedenism. Ann Dermatol Syphiligr. 1916;9:1-39.
- Xu A, Balgobind A, Strunk A, et al. Prevalence estimates for pyoderma gangrenosum in the United States: an age- and sexadjusted population analysis. J Am Acad Dermatol. 2020;83:425-429. doi:10.1016/j.jaad.2019.08.001
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359. doi:10.1016/j.jaad.2019.09.032
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224‐228. doi:10.7861/clinmedicine.19-3-224
- Caughman W, Stern R, Haynes H. Neutrophilic dermatosis of myeloproliferative disorders. atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9:751-758. doi:10.1016/s0190-9622(83)70191-x
- Wallach D, Vignon-Pennamen M. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Manzoni-de-Almeida D, Squaiella-Baptistão CC, Lopes PH, et al. Loxosceles venom sphingomyelinase D activates human blood leukocytes: role of the complement system. Mol Immunol. 2018;94:45-53.
- Wilson JR, Hagood CO Jr, Prather ID. Brown recluse spider bites: a complex problem wound. a brief review and case study. Ostomy Wound Manage. 2005;51:59-66.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Bakleh M, Wold LE, Mandrekar JN, et al. Correlation of histopathologic findings with clinical outcome in necrotizing fasciitis. Clin Infect Dis. 2005;40:410-414. doi:10.1086/427286
- de Souza EF, da Silva GA, Dos Santos GR, et al. Pyoderma gangrenosum simulating necrotizing fasciitis. Case Rep Med. 2015;2015:504970. doi:10.1155/2015/504970
A 26-year-old previously healthy man presented to the emergency department with a new asymptomatic enlarging lesion on the lower leg that had appeared 4 days prior as a self-described “pimple” and rapidly evolved. The patient also reported chills, fatigue, and decreased appetite during that time. Physical examination revealed a red to violaceous, well-demarcated, bullous plaque involving much of the left lower leg. Laboratory studies demonstrated a hemoglobin level of 8.1 g/dL (reference range, 14.0–17.5 g/dL), hematocrit level of 23.7% (reference range, 41%–50%), platelet count of 26×103 /μL (reference range, 150–350×103 /μL), and a population of circulating blast cells and metamyelocytes.
Lawsuit: 18-inch sponge left in stomach for 5 years; migrates internally
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
Carolyn Boerste underwent aortobifemoral bypass surgery at the University of Louisville (Ky.) Hospital in March 2011 to improve circulation in her lower extremities. She had a history of peripheral vascular disease, hypertension, and diabetes, which caused a wound on her toe to become infected and gangrenous, according to court records.
During the surgery, performed by Marvin Morris, MD, the surgical team left a laparotomy sponge in Ms. Boerste’s abdomen. Because of its size, Ms. Boerste’s attorneys characterized the 18-by-18-inch object as “more like a towel,” according to court documents.
During the years that the sponge went undetected, the object eroded via transmural migration from Ms. Boerste’s abdomen into her intestine, causing diarrhea, vomiting, and nausea. In March 2015, Ms. Boerste was transferred by ambulance to an emergency department because of abdominal pain. An emergency physician ordered an abdominal CT scan, which showed the x-ray detectable sponge marker inside Ms. Boerste’s intestine, according to her complaint.
Although the radiologist called the emergency physician to advise him of the sponge marker, the information was not shared with Ms. Boerste and she was discharged from the hospital with a urinary tract infection diagnosis. The emergency physician later testified he had no memory of the call with the radiologist.
The CT scan was faxed to Ms. Boerste’s family physician. She testified that she read the report but did not mention the sponge marker to Ms. Boerste because she believed the issue had been handled by the emergency physician. Thus the sponge remained inside Ms. Boerste for another 20 months.
In November 2016, Ms. Boerste returned to the same emergency department with more intense gastrointestinal issues. Another CT scan was ordered, which revealed the sponge. The object was removed by exploratory laparotomy later that month. In her complaint, Ms. Boerste claimed that the removal surgery resulted in amputation of her leg because of wounds developed on her lower extremities while she was bedridden during recovery.
In 2017, she filed a negligence lawsuit against Dr. Morris, the hospital, and several others involved in her care. On the first day of trial in December 2019, the hospital conceded liability. The trial continued against Dr. Morris and the other defendants as to liability and damages and proceeded against the hospital as to damages.
At trial, evidence showed there was significant confusion among nurses on how to document sponge counts, according to the appellate decision. In general, nurses used a perioperative nursing record to document the surgical procedure, and that record had a place to document some but not all sponge counts required by hospital policy. The nursing record did not have a place to document sponge counts required to be recorded at every break, lunch, and shift change. Nurses also used a worksheet to track sponge counts, but that worksheet was not part of the medical record.
Dr. Morris testified that he relies on nurses regarding sponge counts, but that he also performs a visual and tactile inspection of the abdominal cavity. He acknowledged during trial that the standard of care required him to keep track of the sponges before closing. Dr. Morris also testified that the surgeon and nurses are a team, and “the entire team did not count the sponges correctly when finishing the bypass surgery,” according to the appellate decision.
After a 10-day trial, jurors found Dr. Morris and several other defendants liable. They apportioned 60% liability to the hospital, 10% to Morris, 15% to the family physician, 0% to the emergency physician, and 15% to the rehabilitation center. Ms. Boerste was awarded $9.5 million in damages and an additional $1 million in punitive damages, for a total of $10.5 million.
Dr. Morris and the hospital appealed to the Commonwealth of Kentucky Court of Appeals. As the appeal was pending, Ms. Boerste died, and her son took over the plaintiff’s role.
In their appeal, Dr. Morris and the hospital said they should be granted a new trial for a number of reasons, including that the pain and suffering award was grossly excessive and reflected improper jury sympathy, that the punitive damages award should be vacated because jurors were not properly instructed on the issue, and that the judgment against Dr. Morris should be overturned because there was no evidence he deviated from the standard of care.
The defendants also argued that they were entitled to instructions on “apportionment of fault and mitigation of damages against Boerste.” The mitigation of damages doctrine prevents an injured plaintiff from recovering unreasonable expenses associated with the injury if they could have been avoided through reasonable efforts. Specifically, attorneys for Dr. Morris emphasized that Ms. Boerste failed to follow medical advice for follow-up care, to obtain recommended podiatrist care, and to make necessary efforts to control her diabetes. Had Ms. Boerste taken more proactive steps to manage her health, leg amputation may not have been needed because the sponge may have been found during other treatment, they contended.
In its Jan. 7, 2022, opinion, the appeals court upheld the majority of the jury award. Judges wrote that Dr. Morris’ testimony alone was sufficient for the jury to determine whether he breached the standard of care, and that the defendants are not entitled to a new trial on pain and suffering damages. In addition, judges rejected mitigation of damages.
“The fact that Boerste was a poor patient who failed to properly treat her diabetes is irrelevant,” the panel wrote in their decision. “She was a poor patient prior to the bypass surgery, and Appellants knew Boerste might ultimately need to have her lower leg amputated at the time of the bypass surgery. Therefore, we hold Appellants were not entitled to instructions on apportionment of fault or mitigation of damages.”
The appeals court, however, vacated the $1 million punitive damages award, ruling that the lower court did not give a proper instruction to the jury on punitive damages. The appeals court sent the case back to the lower court for a retrial as it pertains to punitive damages.
Attorneys for Dr. Morris and the hospital did not return messages seeking comment.
Bo Bolus, an attorney for Ms. Boerste’s family, said there will be no retrial on punitive damages, and that the plaintiff is satisfied with the outcome of the case.
“While we are pleased that Carolyn’s family and, equally importantly, her memory, now finally have closure on this extremely trying matter, our pleasure is severely tempered by the loss of Carolyn in November of last year,” Mr. Bolus said. “After having endured all she did, it is, frankly, painful for all concerned that she will not reap the reward of the jury’s verdict.”
A version of this article first appeared on Medscape.com.
COVID-19 vaccine does not affect in vitro fertilization outcomes
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
Getting a COVID-19 mRNA vaccine did not affect pregnancy rates for women trying to conceive with in vitro fertilization or ovarian response to treatment, findings of a new study indicate.
The study was led by Sarit Avraham, MD, with the IVF unit, department of obstetrics and gynecology, Shamir Medical Center in Tzrifi, Israel. The findings were published online in Fertility and Sterility in a preproof version.
“Women should be vaccinated for COVID-19 prior to attempting to conceive via IVF treatments, given the higher risk of severe illness in pregnant women,” the authors wrote.
Doubts arose from “the theoretical concept of the supposed similarity between the SARS-CoV-2 spike protein and the syncytin protein that is speculated to take part in the fertilization process and the formation of the placenta,” the authors wrote.
Some then assumed that the COVID vaccine might kick off an immune response that could affect implantation and pregnancy. But this study and others before it found otherwise.
Researchers included 200 vaccinated women trying to conceive with IVF treatments in the retrospective study, and compared them with 200 unvaccinated patients of similar age (average age in both groups, 36 years) who were not previously infected with COVID-19. All the women were undergoing IVF from January to April 2021 and all the vaccinated women completed two doses of the BNT162b2 (Pfizer/BioNTech) vaccine at least 2 weeks before ovarian stimulation.
Researchers compared the average number of oocytes retrieved and clinical pregnancy rates between the two groups.
No difference between groups
Two hundred patients underwent oocyte retrieval 14-68 days after receiving a COVID shot; there was no significant difference by vaccination status in the number retrieved per cycle (10.63 in the vaccinated group vs. 10.72 in the unvaccinated group; P = .93).
There was also no difference in the clinical pregnancy rates after fresh embryo transfers. The rate among 128 vaccinated patients was 32.8% versus 33.1% in the 133 unvaccinated patients (P = .96), with 42 and 44 clinical pregnancies, respectively.
A total of 113 patients (66 in the study group and 47 in the controls) underwent freeze-all cycles to preserve fertility and fertilization rates were similar between vaccinated and unvaccinated (55.43% vaccinated vs. 54.29% unvaccinated; P = .73). The average number of cryopreserved embryos was 3.59 (vaccinated) versus 3.28 (unvaccinated) (P = .80).
In a subanalysis of outcomes by age, researchers found vaccination status had no effect on number of oocytes or pregnancy rates in the 39-and-older group. That’s important because it shows the vaccine did not affect outcomes even in a population with reduced ovarian reserves, the authors wrote.
The authors noted one of the study’s limitations is that it didn’t include information about vaccination or past infection status of the male partners.
Question should be put to rest
Sarah Cross, MD, a maternal-fetal medicine specialist at the University of Minnesota, Minneapolis, said the study is the biggest she’s seen that concludes COVID vaccinations are safe and highly encouraged for women before trying to conceive, but other smaller studies have come to the same conclusion.
She pointed to research including a study from 2021 with similar findings that concluded: “Physicians and public health personnel can counsel women of reproductive age that neither previous illness with COVID-19 nor antibodies produced from vaccination to COVID-19 will cause sterility.”
She said she thinks the question of whether COVID shots are safe with IVF has been answered and the results of the latest study add proof to counter misinformation around the issue.
“The COVID-19 vaccine does not affect fertility,” she said. “I don’t know how many more [studies] we need.”
The harm is in not getting vaccinated, she said. Pregnancy significantly increases a woman’s chance of getting severe COVID, the need for hospitalization, mechanical ventilation, and risk of death.
“I personally have never had a hospitalized patient who’s been vaccinated,” Dr. Cross said. “The worst thing for the fetus is to have a critically ill mother.”
Dr. Cross, whose high-risk patients include those seeking counseling before IVF, added: “I would counsel all of them that they should absolutely get vaccinated prior to pregnancy, when they’re pregnant, whenever it is, as soon as they possibly can.”
The study authors and Dr. Cross report no relevant financial relationships.
FROM FERTILITY AND STERILITY