User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
The battle of egos behind the life-saving discovery of insulin
Leonard Thompson’s father was so desperate to save his 14-year-old child from certain death due to diabetes that, on Jan. 11, 1922, he took him to Toronto General Hospital to receive what is arguably the first dose of insulin given to a human. From an anticipated life expectancy of weeks – months at best – Thompson lived for an astonishing further 13 years, eventually dying from pneumonia unrelated to diabetes.
By all accounts, the story is a centenary celebration of a remarkable discovery. Insulin has changed what was once a death sentence to a near-normal life expectancy for the millions of people with type 1 diabetes over the past 100 years.
But behind the life-changing success of the discovery – and the Nobel Prize that went with it – lies a tale blighted by disputed claims, twisted truths, and likely injustices between the scientists involved, as they each vied for an honored place in medical history.
Kersten Hall, PhD, honorary fellow, religion and history of science, at the University of Leeds, England, has scoured archives and personal records held at the University of Toronto to uncover the personal stories behind insulin’s discovery.
Despite the wranglings, Dr. Hall asserts: “There’s a distinction between the science and the scientists. Scientists are wonderfully flawed and complex human beings with all their glorious virtues and vices, as we all are. It’s no surprise that they get greedy, jealous, and insecure.”
At death’s door: Diabetes before the 1920s
Prior to insulin’s discovery in 1921, a diagnosis of type 1 diabetes placed someone at death’s door, with nothing but starvation – albeit a slightly slower death – to mitigate a fast-approaching departure from this world. At that time, most diabetes cases would have been type 1 diabetes because, with less obesogenic diets and shorter lifespans, people were much less likely to develop type 2 diabetes.
Nowadays, it is widely recognized that the prevalence of type 2 diabetes is on a steep upward curve, but so too is type 1 diabetes. In the United States alone, there are 1.5 million people diagnosed with type 1 diabetes, a number expected to rise to around 5 million by 2050, according to JDRF, the type 1 diabetes advocacy organization.
Interestingly, 100 years since the first treated patient, life-long insulin remains the only real effective therapy for patients with type 1 diabetes. Once pancreatic beta cells have ceased to function and insulin production has stopped, insulin replacement is the only way to keep blood glucose levels within the recommended range (A1c ≤ 48 mmol/mol [6.5%]), according to the UK National Institute for Health and Care Excellence (NICE), as well as numerous diabetes organizations, including the American Diabetes Association (ADA).
Preliminary clinical trials have looked at stem cell transplantation, prematurely dubbed as a “cure” for type 1 diabetes, as an alternative to insulin therapy. The procedure involves transplanting stem cell–derived cells, which become functional beta cells when infused into humans, but requires immunosuppression, as reported by this news organization.
Today, the life expectancy of people with type 1 diabetes treated with insulin is close to those without the disease, although this is dependent on how tightly blood glucose is controlled. Some studies show life expectancy of those with type 1 diabetes is around 8-12 years lower than the general population but varies depending on where a person lives.
In some lower-income countries, many with type 1 diabetes still die prematurely either because they are undiagnosed or cannot access insulin. The high cost of insulin in the United States is well publicized, as featured in numerous articles by this news organization, and numerous patients in the United States have died because they cannot afford insulin.
Without insulin, young Leonard Thompson would have been lucky to have reached his 15th birthday.
“Such patients were cachectic and thin and would have weighed around 40-50 pounds (18-23 kg), which is very low for an older child. Survival was short and lasted weeks or months usually,” said Elizabeth Stephens, MD, an endocrinologist in Portland, Ore.
“The discovery of insulin was really a miracle because without it diabetes patients were facing certain death. Even nowadays, if people don’t get their insulin because they can’t afford it or for whatever reason, they can still die,” Dr. Stephens stressed.
Antidiabetic effects of pancreatic extract limited
Back in 1869, Paul Langerhans, MD, discovered pancreatic islet cells, or islets of Langerhans, as a medical student. Researchers tried to produce extracts that lowered blood glucose but they were too toxic for patient use.
In 1908, as detailed in his recent book, Insulin – the Crooked Timber, Dr. Hall also refers to the fact that a German researcher, Georg Zuelzer, MD, demonstrated in six patients that pancreatic extracts could reduce urinary levels of glucose and ketones, and that in one case, the treatment woke the patient from a coma. Dr. Zuelzer had purified the extract with alcohol but patients still experienced convulsions and coma; in fact, they were experiencing hypoglycemic shock, but Dr. Zuelzer had not identified it as such.
“He thought his preparation was full of impurities – and that’s the irony. He had in his hands an insulin prep that was so clean and so potent that it sent the test animals into hypoglycemic shock,” Dr. Hall pointed out.
By 1921, two young researchers, Frederick G. Banting, MD, a practicing medical doctor in Toronto, together with a final year physiology student at the University of Toronto, Charles H. Best, MD, DSc, collaborated on the instruction of Dr. Best’s superior, John James Rickard Macleod, MBChB, professor of physiology at the University of Toronto, to make pancreatic extracts, first from dogs and then from cattle.
Over the months prior to treating Thompson, working together in the laboratory, Dr. Banting and Dr. Best prepared the pancreatic extract from cattle and tested it on dogs with diabetes.
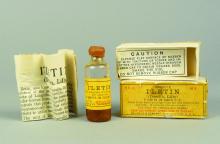
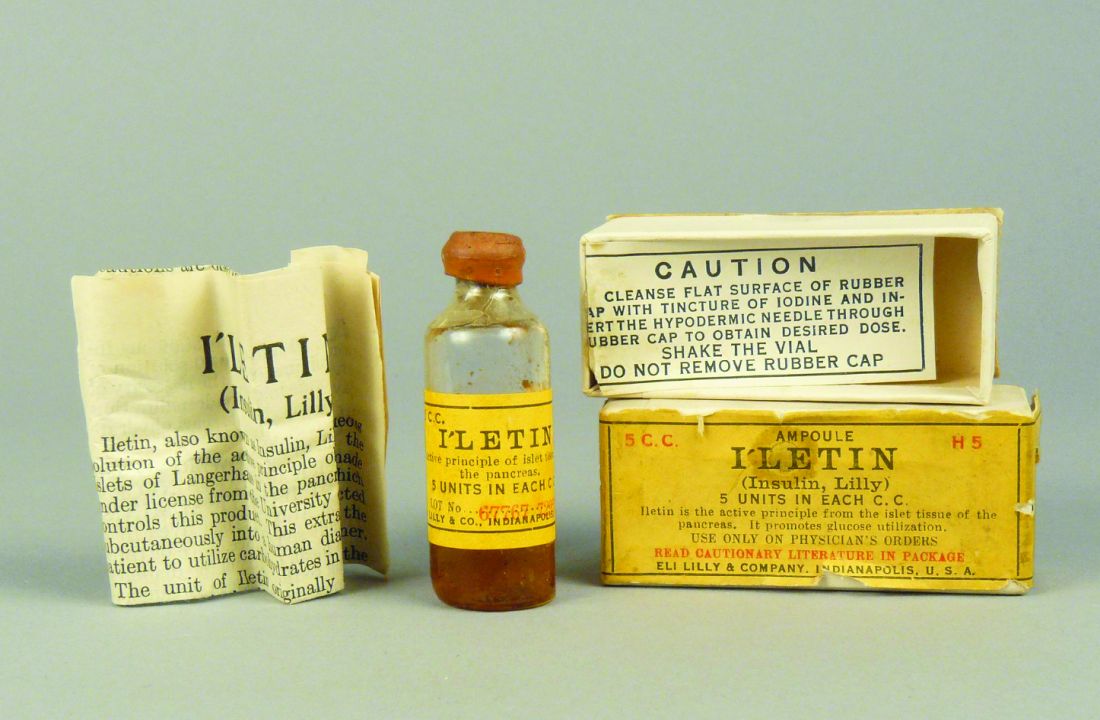
Then, in what amounted to a phase 1 trial of its day, with an “n of one,” a frail and close-to-death Thompson was given 15 cc of pancreatic extract at Toronto General Hospital in January 1922. His blood glucose level dropped by 25%, but unfortunately, his body still produced ketones, indicating the antidiabetic effect was limited. He also experienced an adverse reaction at the injection site with an accumulation of abscesses.
So despite success with isolating the extract and administering it to Thompson, the product remained tainted with impurities.
At this point, colleague James Collip, MD, PhD, came to the rescue. He used his skills as a biochemist to purify the pancreatic extract enough to eliminate impurities.
When Thompson was treated 2 weeks later with the purified extract, he experienced a more positive outcome. Gone was the injection site reaction, gone were the high blood glucose levels, and Thompson “became brighter, more active, looked better, and said he felt stronger,” according to a publication describing the treatment.
Dr. Collip also determined that by over-purifying the product, the animals he experimented on could overreact and experience convulsions, coma, and death due to hypoglycemia from too much insulin.
Fighting talk
Recalling an excerpt from Dr. Banting’s diary, Dr. Hall said that Dr. Banting had a mercurial temper and testified to his loss of patience with Dr. Collip when the chemist refused to share his formula of purification. His diary reads: “I grabbed him in one hand by the overcoat ... and almost lifting him I sat him down hard on the chair ... I remember telling him that it was a good job he was so much smaller – otherwise I would ‘knock hell out of him.’ ”
According to Dr. Hall, in 1923, when Dr. Banting and Dr. Macleod were jointly awarded the Nobel Prize for Medicine, Dr. Best resented being excluded, and despite Dr. Banting’s sharing half his prize money with Dr. Best, animosity prevailed.
At one point, before leaving on a plane for a wartime mission to the United Kingdom, Dr. Banting noted that if he didn’t make it back alive, “and they give my [professorial] chair to that son-of-a-bitch Best, I’ll never rest in my grave.” In a cruel twist of fate, Dr. Banting’s plane crashed and all aboard died.
The Nobel Prize had also been a source of rivalry between Dr. Banting and his boss, Dr. Macleod. In late 1921, while presenting the findings from animal models at the American Physiological Society conference, Dr. Banting’s nerves got the better of him and Dr. Macleod took over at the podium to finish the talk. Dr. Banting perceived this as his boss stealing the limelight.
Only a few months later, at the Association of American Physicians annual conference, Dr. Macleod played to an audience for a second time by making the first formal announcement of the discovery to the scientific community. Notably, Dr. Banting was absent.
The Nobel Prize or a poisoned chalice?
Awarded annually for physics, chemistry, medicine/physiology, literature, peace, and economics, Nobel Prizes are usually considered the holy grail of achievement. In 1895, funds for the prizes were bequeathed by Alfred Nobel in his last will and testament, with each prize worth around $40,000 at the time (approximately $1,000,000 in today’s value).
Writing in 2001 in the journal Diabetes Voice, Professor Sir George Alberti, DPhil, BM BCh, former president of the UK Royal College of Physicians, summarized the burden that accompanies the Nobel Prize: “I personally believe that such prizes and awards do more harm than good and should be abolished. Many a scientist has gone to their grave feeling deeply aggrieved because they were not awarded a Nobel Prize.”
Such high stakes surround the prize that, in the case of insulin, the course of its discovery meant courtesies and truth were swept aside in hot pursuit of fame. After Dr. Macleod died in 1935 and Dr. Banting died in 1941, Dr. Best took the opportunity to try to revise history. There was the small obstacle of Dr. Collip, but Dr. Best managed to play down Dr. Collip’s contribution by focusing on the eureka moment as being the first insulin dose administered, despite the fact that a more complete recovery without side effects was later achieved only with Dr. Collip’s help.
Despite exclusion from the Nobel Prize, Dr. Best nevertheless became recognized as the “go-to-guy” for the discovery of insulin, said Dr. Hall. When Dr. Best spoke about the discovery of insulin at the New York Diabetes Association meeting in 1946, he was introduced as a speaker whose reputation was already so great that he did “not require much of an introduction.”
“And when a new research institute was opened in Toronto in 1953, it was named in his honor. The opening address, by Sir Henry Dale of the UK Medical Research Council, sang Best’s praises to the rafters, much to the disgruntlement of Best’s former colleague, James Collip, who was sitting in the audience,” Dr. Hall pointed out.
Both Dr. Hall and Dr. Stephens live with type 1 diabetes and have benefited from the efforts of Dr. Banting, Dr. Best, Dr. Collip, Dr. Zuelzer, and Dr. Macleod.
“The discovery of insulin was a miracle, it has allowed people to survive,” said Dr. Stephens. “Few medicines can reverse a death sentence like insulin can. It’s easy to forget how it was when insulin wasn’t there – and it wasn’t that long ago.”
Dr. Hall reflects that scientific progress and discovery are often portrayed as being the result of towering geniuses standing on each other’s shoulders.
“But I think that when German philosopher Immanuel Kant remarked that ‘Out of the crooked timber of humanity, no straight thing can ever be made,’ he offered us a much more accurate picture of how science works. And I think that there’s perhaps no more powerful example of this than the story of insulin,” he said.
A version of this article first appeared on Medscape.com.
Leonard Thompson’s father was so desperate to save his 14-year-old child from certain death due to diabetes that, on Jan. 11, 1922, he took him to Toronto General Hospital to receive what is arguably the first dose of insulin given to a human. From an anticipated life expectancy of weeks – months at best – Thompson lived for an astonishing further 13 years, eventually dying from pneumonia unrelated to diabetes.
By all accounts, the story is a centenary celebration of a remarkable discovery. Insulin has changed what was once a death sentence to a near-normal life expectancy for the millions of people with type 1 diabetes over the past 100 years.
But behind the life-changing success of the discovery – and the Nobel Prize that went with it – lies a tale blighted by disputed claims, twisted truths, and likely injustices between the scientists involved, as they each vied for an honored place in medical history.
Kersten Hall, PhD, honorary fellow, religion and history of science, at the University of Leeds, England, has scoured archives and personal records held at the University of Toronto to uncover the personal stories behind insulin’s discovery.
Despite the wranglings, Dr. Hall asserts: “There’s a distinction between the science and the scientists. Scientists are wonderfully flawed and complex human beings with all their glorious virtues and vices, as we all are. It’s no surprise that they get greedy, jealous, and insecure.”
At death’s door: Diabetes before the 1920s
Prior to insulin’s discovery in 1921, a diagnosis of type 1 diabetes placed someone at death’s door, with nothing but starvation – albeit a slightly slower death – to mitigate a fast-approaching departure from this world. At that time, most diabetes cases would have been type 1 diabetes because, with less obesogenic diets and shorter lifespans, people were much less likely to develop type 2 diabetes.
Nowadays, it is widely recognized that the prevalence of type 2 diabetes is on a steep upward curve, but so too is type 1 diabetes. In the United States alone, there are 1.5 million people diagnosed with type 1 diabetes, a number expected to rise to around 5 million by 2050, according to JDRF, the type 1 diabetes advocacy organization.
Interestingly, 100 years since the first treated patient, life-long insulin remains the only real effective therapy for patients with type 1 diabetes. Once pancreatic beta cells have ceased to function and insulin production has stopped, insulin replacement is the only way to keep blood glucose levels within the recommended range (A1c ≤ 48 mmol/mol [6.5%]), according to the UK National Institute for Health and Care Excellence (NICE), as well as numerous diabetes organizations, including the American Diabetes Association (ADA).
Preliminary clinical trials have looked at stem cell transplantation, prematurely dubbed as a “cure” for type 1 diabetes, as an alternative to insulin therapy. The procedure involves transplanting stem cell–derived cells, which become functional beta cells when infused into humans, but requires immunosuppression, as reported by this news organization.
Today, the life expectancy of people with type 1 diabetes treated with insulin is close to those without the disease, although this is dependent on how tightly blood glucose is controlled. Some studies show life expectancy of those with type 1 diabetes is around 8-12 years lower than the general population but varies depending on where a person lives.
In some lower-income countries, many with type 1 diabetes still die prematurely either because they are undiagnosed or cannot access insulin. The high cost of insulin in the United States is well publicized, as featured in numerous articles by this news organization, and numerous patients in the United States have died because they cannot afford insulin.
Without insulin, young Leonard Thompson would have been lucky to have reached his 15th birthday.
“Such patients were cachectic and thin and would have weighed around 40-50 pounds (18-23 kg), which is very low for an older child. Survival was short and lasted weeks or months usually,” said Elizabeth Stephens, MD, an endocrinologist in Portland, Ore.
“The discovery of insulin was really a miracle because without it diabetes patients were facing certain death. Even nowadays, if people don’t get their insulin because they can’t afford it or for whatever reason, they can still die,” Dr. Stephens stressed.
Antidiabetic effects of pancreatic extract limited
Back in 1869, Paul Langerhans, MD, discovered pancreatic islet cells, or islets of Langerhans, as a medical student. Researchers tried to produce extracts that lowered blood glucose but they were too toxic for patient use.
In 1908, as detailed in his recent book, Insulin – the Crooked Timber, Dr. Hall also refers to the fact that a German researcher, Georg Zuelzer, MD, demonstrated in six patients that pancreatic extracts could reduce urinary levels of glucose and ketones, and that in one case, the treatment woke the patient from a coma. Dr. Zuelzer had purified the extract with alcohol but patients still experienced convulsions and coma; in fact, they were experiencing hypoglycemic shock, but Dr. Zuelzer had not identified it as such.
“He thought his preparation was full of impurities – and that’s the irony. He had in his hands an insulin prep that was so clean and so potent that it sent the test animals into hypoglycemic shock,” Dr. Hall pointed out.
By 1921, two young researchers, Frederick G. Banting, MD, a practicing medical doctor in Toronto, together with a final year physiology student at the University of Toronto, Charles H. Best, MD, DSc, collaborated on the instruction of Dr. Best’s superior, John James Rickard Macleod, MBChB, professor of physiology at the University of Toronto, to make pancreatic extracts, first from dogs and then from cattle.
Over the months prior to treating Thompson, working together in the laboratory, Dr. Banting and Dr. Best prepared the pancreatic extract from cattle and tested it on dogs with diabetes.
Then, in what amounted to a phase 1 trial of its day, with an “n of one,” a frail and close-to-death Thompson was given 15 cc of pancreatic extract at Toronto General Hospital in January 1922. His blood glucose level dropped by 25%, but unfortunately, his body still produced ketones, indicating the antidiabetic effect was limited. He also experienced an adverse reaction at the injection site with an accumulation of abscesses.
So despite success with isolating the extract and administering it to Thompson, the product remained tainted with impurities.
At this point, colleague James Collip, MD, PhD, came to the rescue. He used his skills as a biochemist to purify the pancreatic extract enough to eliminate impurities.
When Thompson was treated 2 weeks later with the purified extract, he experienced a more positive outcome. Gone was the injection site reaction, gone were the high blood glucose levels, and Thompson “became brighter, more active, looked better, and said he felt stronger,” according to a publication describing the treatment.
Dr. Collip also determined that by over-purifying the product, the animals he experimented on could overreact and experience convulsions, coma, and death due to hypoglycemia from too much insulin.
Fighting talk
Recalling an excerpt from Dr. Banting’s diary, Dr. Hall said that Dr. Banting had a mercurial temper and testified to his loss of patience with Dr. Collip when the chemist refused to share his formula of purification. His diary reads: “I grabbed him in one hand by the overcoat ... and almost lifting him I sat him down hard on the chair ... I remember telling him that it was a good job he was so much smaller – otherwise I would ‘knock hell out of him.’ ”
According to Dr. Hall, in 1923, when Dr. Banting and Dr. Macleod were jointly awarded the Nobel Prize for Medicine, Dr. Best resented being excluded, and despite Dr. Banting’s sharing half his prize money with Dr. Best, animosity prevailed.
At one point, before leaving on a plane for a wartime mission to the United Kingdom, Dr. Banting noted that if he didn’t make it back alive, “and they give my [professorial] chair to that son-of-a-bitch Best, I’ll never rest in my grave.” In a cruel twist of fate, Dr. Banting’s plane crashed and all aboard died.
The Nobel Prize had also been a source of rivalry between Dr. Banting and his boss, Dr. Macleod. In late 1921, while presenting the findings from animal models at the American Physiological Society conference, Dr. Banting’s nerves got the better of him and Dr. Macleod took over at the podium to finish the talk. Dr. Banting perceived this as his boss stealing the limelight.
Only a few months later, at the Association of American Physicians annual conference, Dr. Macleod played to an audience for a second time by making the first formal announcement of the discovery to the scientific community. Notably, Dr. Banting was absent.
The Nobel Prize or a poisoned chalice?
Awarded annually for physics, chemistry, medicine/physiology, literature, peace, and economics, Nobel Prizes are usually considered the holy grail of achievement. In 1895, funds for the prizes were bequeathed by Alfred Nobel in his last will and testament, with each prize worth around $40,000 at the time (approximately $1,000,000 in today’s value).
Writing in 2001 in the journal Diabetes Voice, Professor Sir George Alberti, DPhil, BM BCh, former president of the UK Royal College of Physicians, summarized the burden that accompanies the Nobel Prize: “I personally believe that such prizes and awards do more harm than good and should be abolished. Many a scientist has gone to their grave feeling deeply aggrieved because they were not awarded a Nobel Prize.”
Such high stakes surround the prize that, in the case of insulin, the course of its discovery meant courtesies and truth were swept aside in hot pursuit of fame. After Dr. Macleod died in 1935 and Dr. Banting died in 1941, Dr. Best took the opportunity to try to revise history. There was the small obstacle of Dr. Collip, but Dr. Best managed to play down Dr. Collip’s contribution by focusing on the eureka moment as being the first insulin dose administered, despite the fact that a more complete recovery without side effects was later achieved only with Dr. Collip’s help.
Despite exclusion from the Nobel Prize, Dr. Best nevertheless became recognized as the “go-to-guy” for the discovery of insulin, said Dr. Hall. When Dr. Best spoke about the discovery of insulin at the New York Diabetes Association meeting in 1946, he was introduced as a speaker whose reputation was already so great that he did “not require much of an introduction.”
“And when a new research institute was opened in Toronto in 1953, it was named in his honor. The opening address, by Sir Henry Dale of the UK Medical Research Council, sang Best’s praises to the rafters, much to the disgruntlement of Best’s former colleague, James Collip, who was sitting in the audience,” Dr. Hall pointed out.
Both Dr. Hall and Dr. Stephens live with type 1 diabetes and have benefited from the efforts of Dr. Banting, Dr. Best, Dr. Collip, Dr. Zuelzer, and Dr. Macleod.
“The discovery of insulin was a miracle, it has allowed people to survive,” said Dr. Stephens. “Few medicines can reverse a death sentence like insulin can. It’s easy to forget how it was when insulin wasn’t there – and it wasn’t that long ago.”
Dr. Hall reflects that scientific progress and discovery are often portrayed as being the result of towering geniuses standing on each other’s shoulders.
“But I think that when German philosopher Immanuel Kant remarked that ‘Out of the crooked timber of humanity, no straight thing can ever be made,’ he offered us a much more accurate picture of how science works. And I think that there’s perhaps no more powerful example of this than the story of insulin,” he said.
A version of this article first appeared on Medscape.com.
Leonard Thompson’s father was so desperate to save his 14-year-old child from certain death due to diabetes that, on Jan. 11, 1922, he took him to Toronto General Hospital to receive what is arguably the first dose of insulin given to a human. From an anticipated life expectancy of weeks – months at best – Thompson lived for an astonishing further 13 years, eventually dying from pneumonia unrelated to diabetes.
By all accounts, the story is a centenary celebration of a remarkable discovery. Insulin has changed what was once a death sentence to a near-normal life expectancy for the millions of people with type 1 diabetes over the past 100 years.
But behind the life-changing success of the discovery – and the Nobel Prize that went with it – lies a tale blighted by disputed claims, twisted truths, and likely injustices between the scientists involved, as they each vied for an honored place in medical history.
Kersten Hall, PhD, honorary fellow, religion and history of science, at the University of Leeds, England, has scoured archives and personal records held at the University of Toronto to uncover the personal stories behind insulin’s discovery.
Despite the wranglings, Dr. Hall asserts: “There’s a distinction between the science and the scientists. Scientists are wonderfully flawed and complex human beings with all their glorious virtues and vices, as we all are. It’s no surprise that they get greedy, jealous, and insecure.”
At death’s door: Diabetes before the 1920s
Prior to insulin’s discovery in 1921, a diagnosis of type 1 diabetes placed someone at death’s door, with nothing but starvation – albeit a slightly slower death – to mitigate a fast-approaching departure from this world. At that time, most diabetes cases would have been type 1 diabetes because, with less obesogenic diets and shorter lifespans, people were much less likely to develop type 2 diabetes.
Nowadays, it is widely recognized that the prevalence of type 2 diabetes is on a steep upward curve, but so too is type 1 diabetes. In the United States alone, there are 1.5 million people diagnosed with type 1 diabetes, a number expected to rise to around 5 million by 2050, according to JDRF, the type 1 diabetes advocacy organization.
Interestingly, 100 years since the first treated patient, life-long insulin remains the only real effective therapy for patients with type 1 diabetes. Once pancreatic beta cells have ceased to function and insulin production has stopped, insulin replacement is the only way to keep blood glucose levels within the recommended range (A1c ≤ 48 mmol/mol [6.5%]), according to the UK National Institute for Health and Care Excellence (NICE), as well as numerous diabetes organizations, including the American Diabetes Association (ADA).
Preliminary clinical trials have looked at stem cell transplantation, prematurely dubbed as a “cure” for type 1 diabetes, as an alternative to insulin therapy. The procedure involves transplanting stem cell–derived cells, which become functional beta cells when infused into humans, but requires immunosuppression, as reported by this news organization.
Today, the life expectancy of people with type 1 diabetes treated with insulin is close to those without the disease, although this is dependent on how tightly blood glucose is controlled. Some studies show life expectancy of those with type 1 diabetes is around 8-12 years lower than the general population but varies depending on where a person lives.
In some lower-income countries, many with type 1 diabetes still die prematurely either because they are undiagnosed or cannot access insulin. The high cost of insulin in the United States is well publicized, as featured in numerous articles by this news organization, and numerous patients in the United States have died because they cannot afford insulin.
Without insulin, young Leonard Thompson would have been lucky to have reached his 15th birthday.
“Such patients were cachectic and thin and would have weighed around 40-50 pounds (18-23 kg), which is very low for an older child. Survival was short and lasted weeks or months usually,” said Elizabeth Stephens, MD, an endocrinologist in Portland, Ore.
“The discovery of insulin was really a miracle because without it diabetes patients were facing certain death. Even nowadays, if people don’t get their insulin because they can’t afford it or for whatever reason, they can still die,” Dr. Stephens stressed.
Antidiabetic effects of pancreatic extract limited
Back in 1869, Paul Langerhans, MD, discovered pancreatic islet cells, or islets of Langerhans, as a medical student. Researchers tried to produce extracts that lowered blood glucose but they were too toxic for patient use.
In 1908, as detailed in his recent book, Insulin – the Crooked Timber, Dr. Hall also refers to the fact that a German researcher, Georg Zuelzer, MD, demonstrated in six patients that pancreatic extracts could reduce urinary levels of glucose and ketones, and that in one case, the treatment woke the patient from a coma. Dr. Zuelzer had purified the extract with alcohol but patients still experienced convulsions and coma; in fact, they were experiencing hypoglycemic shock, but Dr. Zuelzer had not identified it as such.
“He thought his preparation was full of impurities – and that’s the irony. He had in his hands an insulin prep that was so clean and so potent that it sent the test animals into hypoglycemic shock,” Dr. Hall pointed out.
By 1921, two young researchers, Frederick G. Banting, MD, a practicing medical doctor in Toronto, together with a final year physiology student at the University of Toronto, Charles H. Best, MD, DSc, collaborated on the instruction of Dr. Best’s superior, John James Rickard Macleod, MBChB, professor of physiology at the University of Toronto, to make pancreatic extracts, first from dogs and then from cattle.
Over the months prior to treating Thompson, working together in the laboratory, Dr. Banting and Dr. Best prepared the pancreatic extract from cattle and tested it on dogs with diabetes.
Then, in what amounted to a phase 1 trial of its day, with an “n of one,” a frail and close-to-death Thompson was given 15 cc of pancreatic extract at Toronto General Hospital in January 1922. His blood glucose level dropped by 25%, but unfortunately, his body still produced ketones, indicating the antidiabetic effect was limited. He also experienced an adverse reaction at the injection site with an accumulation of abscesses.
So despite success with isolating the extract and administering it to Thompson, the product remained tainted with impurities.
At this point, colleague James Collip, MD, PhD, came to the rescue. He used his skills as a biochemist to purify the pancreatic extract enough to eliminate impurities.
When Thompson was treated 2 weeks later with the purified extract, he experienced a more positive outcome. Gone was the injection site reaction, gone were the high blood glucose levels, and Thompson “became brighter, more active, looked better, and said he felt stronger,” according to a publication describing the treatment.
Dr. Collip also determined that by over-purifying the product, the animals he experimented on could overreact and experience convulsions, coma, and death due to hypoglycemia from too much insulin.
Fighting talk
Recalling an excerpt from Dr. Banting’s diary, Dr. Hall said that Dr. Banting had a mercurial temper and testified to his loss of patience with Dr. Collip when the chemist refused to share his formula of purification. His diary reads: “I grabbed him in one hand by the overcoat ... and almost lifting him I sat him down hard on the chair ... I remember telling him that it was a good job he was so much smaller – otherwise I would ‘knock hell out of him.’ ”
According to Dr. Hall, in 1923, when Dr. Banting and Dr. Macleod were jointly awarded the Nobel Prize for Medicine, Dr. Best resented being excluded, and despite Dr. Banting’s sharing half his prize money with Dr. Best, animosity prevailed.
At one point, before leaving on a plane for a wartime mission to the United Kingdom, Dr. Banting noted that if he didn’t make it back alive, “and they give my [professorial] chair to that son-of-a-bitch Best, I’ll never rest in my grave.” In a cruel twist of fate, Dr. Banting’s plane crashed and all aboard died.
The Nobel Prize had also been a source of rivalry between Dr. Banting and his boss, Dr. Macleod. In late 1921, while presenting the findings from animal models at the American Physiological Society conference, Dr. Banting’s nerves got the better of him and Dr. Macleod took over at the podium to finish the talk. Dr. Banting perceived this as his boss stealing the limelight.
Only a few months later, at the Association of American Physicians annual conference, Dr. Macleod played to an audience for a second time by making the first formal announcement of the discovery to the scientific community. Notably, Dr. Banting was absent.
The Nobel Prize or a poisoned chalice?
Awarded annually for physics, chemistry, medicine/physiology, literature, peace, and economics, Nobel Prizes are usually considered the holy grail of achievement. In 1895, funds for the prizes were bequeathed by Alfred Nobel in his last will and testament, with each prize worth around $40,000 at the time (approximately $1,000,000 in today’s value).
Writing in 2001 in the journal Diabetes Voice, Professor Sir George Alberti, DPhil, BM BCh, former president of the UK Royal College of Physicians, summarized the burden that accompanies the Nobel Prize: “I personally believe that such prizes and awards do more harm than good and should be abolished. Many a scientist has gone to their grave feeling deeply aggrieved because they were not awarded a Nobel Prize.”
Such high stakes surround the prize that, in the case of insulin, the course of its discovery meant courtesies and truth were swept aside in hot pursuit of fame. After Dr. Macleod died in 1935 and Dr. Banting died in 1941, Dr. Best took the opportunity to try to revise history. There was the small obstacle of Dr. Collip, but Dr. Best managed to play down Dr. Collip’s contribution by focusing on the eureka moment as being the first insulin dose administered, despite the fact that a more complete recovery without side effects was later achieved only with Dr. Collip’s help.
Despite exclusion from the Nobel Prize, Dr. Best nevertheless became recognized as the “go-to-guy” for the discovery of insulin, said Dr. Hall. When Dr. Best spoke about the discovery of insulin at the New York Diabetes Association meeting in 1946, he was introduced as a speaker whose reputation was already so great that he did “not require much of an introduction.”
“And when a new research institute was opened in Toronto in 1953, it was named in his honor. The opening address, by Sir Henry Dale of the UK Medical Research Council, sang Best’s praises to the rafters, much to the disgruntlement of Best’s former colleague, James Collip, who was sitting in the audience,” Dr. Hall pointed out.
Both Dr. Hall and Dr. Stephens live with type 1 diabetes and have benefited from the efforts of Dr. Banting, Dr. Best, Dr. Collip, Dr. Zuelzer, and Dr. Macleod.
“The discovery of insulin was a miracle, it has allowed people to survive,” said Dr. Stephens. “Few medicines can reverse a death sentence like insulin can. It’s easy to forget how it was when insulin wasn’t there – and it wasn’t that long ago.”
Dr. Hall reflects that scientific progress and discovery are often portrayed as being the result of towering geniuses standing on each other’s shoulders.
“But I think that when German philosopher Immanuel Kant remarked that ‘Out of the crooked timber of humanity, no straight thing can ever be made,’ he offered us a much more accurate picture of how science works. And I think that there’s perhaps no more powerful example of this than the story of insulin,” he said.
A version of this article first appeared on Medscape.com.
Medical boards pressured to let it slide when doctors spread COVID misinformation
Tennessee’s Board of Medical Examiners unanimously adopted in September 2021 a statement that said doctors spreading COVID misinformation – such as suggesting that vaccines contain microchips – could jeopardize their license to practice.
“I’m very glad that we’re taking this step,” Dr. Stephen Loyd, MD, the panel’s vice president, said at the time. “If you’re spreading this willful misinformation, for me it’s going to be really hard to do anything other than put you on probation or take your license for a year. There has to be a message sent for this. It’s not okay.”
The board’s statement was posted on a government website.
The growing tension in Tennessee between conservative lawmakers and the state’s medical board may be the most prominent example in the country. But the Federation of State Medical Boards, which created the language adopted by at least 15 state boards, is tracking legislation introduced by Republicans in at least 14 states that would restrict a medical board’s authority to discipline doctors for their advice on COVID.
Humayun Chaudhry, DO, the federation’s CEO, called it “an unwelcome trend.” The nonprofit association, based in Euless, Tex., said the statement is merely a COVID-specific restatement of an existing rule: that doctors who engage in behavior that puts patients at risk could face disciplinary action.
Although doctors have leeway to decide which treatments to provide, the medical boards that oversee them have broad authority over licensing. Often, doctors are investigated for violating guidelines on prescribing high-powered drugs. But physicians are sometimes punished for other “unprofessional conduct.” In 2013, Tennessee’s board fined U.S. Rep. Scott DesJarlais for separately having sexual relations with two female patients more than a decade earlier.
Still, stopping doctors from sharing unsound medical advice has proved challenging. Even defining misinformation has been difficult. And during the pandemic, resistance from some state legislatures is complicating the effort.
A relatively small group of physicians peddle COVID misinformation, but many of them associate with America’s Frontline Doctors. Its founder, Simone Gold, MD, has claimed patients are dying from COVID treatments, not the virus itself. Sherri Tenpenny, DO, said in a legislative hearing in Ohio that the COVID vaccine could magnetize patients. Stella Immanuel, MD, has pushed hydroxychloroquine as a COVID cure in Texas, although clinical trials showed that it had no benefit. None of them agreed to requests for comment.
The Texas Medical Board fined Dr. Immanuel $500 for not informing a patient of the risks associated with using hydroxychloroquine as an off-label COVID treatment.
In Tennessee, state lawmakers called a special legislative session in October to address COVID restrictions, and Republican Gov. Bill Lee signed a sweeping package of bills that push back against pandemic rules. One included language directed at the medical board’s recent COVID policy statement, making it more difficult for the panel to investigate complaints about physicians’ advice on COVID vaccines or treatments.
In November, Republican state Rep. John Ragan sent the medical board a letter demanding that the statement be deleted from the state’s website. Rep. Ragan leads a legislative panel that had raised the prospect of defunding the state’s health department over its promotion of COVID vaccines to teens.
Among his demands, Rep. Ragan listed 20 questions he wanted the medical board to answer in writing, including why the misinformation “policy” was proposed nearly two years into the pandemic, which scholars would determine what constitutes misinformation, and how was the “policy” not an infringement on the doctor-patient relationship.
“If you fail to act promptly, your organization will be required to appear before the Joint Government Operations Committee to explain your inaction,” Rep. Ragan wrote in the letter, obtained by Kaiser Health News and Nashville Public Radio.
In response to a request for comment, Rep. Ragan said that “any executive agency, including Board of Medical Examiners, that refuses to follow the law is subject to dissolution.”
He set a deadline of Dec. 7.
In Florida, a Republican-sponsored bill making its way through the state legislature proposes to ban medical boards from revoking or threatening to revoke doctors’ licenses for what they say unless “direct physical harm” of a patient occurred. If the publicized complaint can’t be proved, the board could owe a doctor up to $1.5 million in damages.
Although Florida’s medical board has not adopted the Federation of State Medical Boards’ COVID misinformation statement, the panel has considered misinformation complaints against physicians, including the state’s surgeon general, Joseph Ladapo, MD, PhD.
Dr. Chaudhry said he’s surprised just how many COVID-related complaints are being filed across the country. Often, boards do not publicize investigations before a violation of ethics or standards is confirmed. But in response to a survey by the federation in late 2021, two-thirds of state boards reported an increase in misinformation complaints. And the federation said 12 boards had taken action against a licensed physician.
“At the end of the day, if a physician who is licensed engages in activity that causes harm, the state medical boards are the ones that historically have been set up to look into the situation and make a judgment about what happened or didn’t happen,” Dr. Chaudhry said. “And if you start to chip away at that, it becomes a slippery slope.”
The Georgia Composite Medical Board adopted a version of the federation’s misinformation guidance in early November and has been receiving 10-20 complaints each month, said Debi Dalton, MD, the chairperson. Two months in, no one had been sanctioned.
Dr. Dalton said that even putting out a misinformation policy leaves some “gray” area. Generally, physicians are expected to follow the “consensus,” rather than “the newest information that pops up on social media,” she said.
“We expect physicians to think ethically, professionally, and with the safety of patients in mind,” Dr. Dalton said.
A few physician groups are resisting attempts to root out misinformation, including the Association of American Physicians and Surgeons, known for its stands against government regulation.
Some medical boards have opted against taking a public stand against misinformation.
The Alabama Board of Medical Examiners discussed signing on to the federation’s statement, according to the minutes from an October meeting. But after debating the potential legal ramifications in a private executive session, the board opted not to act.
In Tennessee, the Board of Medical Examiners met on the day Rep. Ragan had set as the deadline and voted to remove the misinformation statement from its website to avoid being called into a legislative hearing. But then, in late January, the board decided to stick with the policy – although it did not republish the statement online immediately – and more specifically defined misinformation, calling it “content that is false, inaccurate or misleading, even if spread unintentionally.”
Board members acknowledged they would likely get more pushback from lawmakers but said they wanted to protect their profession from interference.
“Doctors who are putting forth good evidence-based medicine deserve the protection of this board so they can actually say: ‘Hey, I’m in line with this guideline, and this is a source of truth,’” said Melanie Blake, MD, the board’s president. “We should be a source of truth.”
The medical board was looking into nearly 30 open complaints related to COVID when its misinformation statement came down from its website. As of early February, no Tennessee physician had faced disciplinary action.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN.
Tennessee’s Board of Medical Examiners unanimously adopted in September 2021 a statement that said doctors spreading COVID misinformation – such as suggesting that vaccines contain microchips – could jeopardize their license to practice.
“I’m very glad that we’re taking this step,” Dr. Stephen Loyd, MD, the panel’s vice president, said at the time. “If you’re spreading this willful misinformation, for me it’s going to be really hard to do anything other than put you on probation or take your license for a year. There has to be a message sent for this. It’s not okay.”
The board’s statement was posted on a government website.
The growing tension in Tennessee between conservative lawmakers and the state’s medical board may be the most prominent example in the country. But the Federation of State Medical Boards, which created the language adopted by at least 15 state boards, is tracking legislation introduced by Republicans in at least 14 states that would restrict a medical board’s authority to discipline doctors for their advice on COVID.
Humayun Chaudhry, DO, the federation’s CEO, called it “an unwelcome trend.” The nonprofit association, based in Euless, Tex., said the statement is merely a COVID-specific restatement of an existing rule: that doctors who engage in behavior that puts patients at risk could face disciplinary action.
Although doctors have leeway to decide which treatments to provide, the medical boards that oversee them have broad authority over licensing. Often, doctors are investigated for violating guidelines on prescribing high-powered drugs. But physicians are sometimes punished for other “unprofessional conduct.” In 2013, Tennessee’s board fined U.S. Rep. Scott DesJarlais for separately having sexual relations with two female patients more than a decade earlier.
Still, stopping doctors from sharing unsound medical advice has proved challenging. Even defining misinformation has been difficult. And during the pandemic, resistance from some state legislatures is complicating the effort.
A relatively small group of physicians peddle COVID misinformation, but many of them associate with America’s Frontline Doctors. Its founder, Simone Gold, MD, has claimed patients are dying from COVID treatments, not the virus itself. Sherri Tenpenny, DO, said in a legislative hearing in Ohio that the COVID vaccine could magnetize patients. Stella Immanuel, MD, has pushed hydroxychloroquine as a COVID cure in Texas, although clinical trials showed that it had no benefit. None of them agreed to requests for comment.
The Texas Medical Board fined Dr. Immanuel $500 for not informing a patient of the risks associated with using hydroxychloroquine as an off-label COVID treatment.
In Tennessee, state lawmakers called a special legislative session in October to address COVID restrictions, and Republican Gov. Bill Lee signed a sweeping package of bills that push back against pandemic rules. One included language directed at the medical board’s recent COVID policy statement, making it more difficult for the panel to investigate complaints about physicians’ advice on COVID vaccines or treatments.
In November, Republican state Rep. John Ragan sent the medical board a letter demanding that the statement be deleted from the state’s website. Rep. Ragan leads a legislative panel that had raised the prospect of defunding the state’s health department over its promotion of COVID vaccines to teens.
Among his demands, Rep. Ragan listed 20 questions he wanted the medical board to answer in writing, including why the misinformation “policy” was proposed nearly two years into the pandemic, which scholars would determine what constitutes misinformation, and how was the “policy” not an infringement on the doctor-patient relationship.
“If you fail to act promptly, your organization will be required to appear before the Joint Government Operations Committee to explain your inaction,” Rep. Ragan wrote in the letter, obtained by Kaiser Health News and Nashville Public Radio.
In response to a request for comment, Rep. Ragan said that “any executive agency, including Board of Medical Examiners, that refuses to follow the law is subject to dissolution.”
He set a deadline of Dec. 7.
In Florida, a Republican-sponsored bill making its way through the state legislature proposes to ban medical boards from revoking or threatening to revoke doctors’ licenses for what they say unless “direct physical harm” of a patient occurred. If the publicized complaint can’t be proved, the board could owe a doctor up to $1.5 million in damages.
Although Florida’s medical board has not adopted the Federation of State Medical Boards’ COVID misinformation statement, the panel has considered misinformation complaints against physicians, including the state’s surgeon general, Joseph Ladapo, MD, PhD.
Dr. Chaudhry said he’s surprised just how many COVID-related complaints are being filed across the country. Often, boards do not publicize investigations before a violation of ethics or standards is confirmed. But in response to a survey by the federation in late 2021, two-thirds of state boards reported an increase in misinformation complaints. And the federation said 12 boards had taken action against a licensed physician.
“At the end of the day, if a physician who is licensed engages in activity that causes harm, the state medical boards are the ones that historically have been set up to look into the situation and make a judgment about what happened or didn’t happen,” Dr. Chaudhry said. “And if you start to chip away at that, it becomes a slippery slope.”
The Georgia Composite Medical Board adopted a version of the federation’s misinformation guidance in early November and has been receiving 10-20 complaints each month, said Debi Dalton, MD, the chairperson. Two months in, no one had been sanctioned.
Dr. Dalton said that even putting out a misinformation policy leaves some “gray” area. Generally, physicians are expected to follow the “consensus,” rather than “the newest information that pops up on social media,” she said.
“We expect physicians to think ethically, professionally, and with the safety of patients in mind,” Dr. Dalton said.
A few physician groups are resisting attempts to root out misinformation, including the Association of American Physicians and Surgeons, known for its stands against government regulation.
Some medical boards have opted against taking a public stand against misinformation.
The Alabama Board of Medical Examiners discussed signing on to the federation’s statement, according to the minutes from an October meeting. But after debating the potential legal ramifications in a private executive session, the board opted not to act.
In Tennessee, the Board of Medical Examiners met on the day Rep. Ragan had set as the deadline and voted to remove the misinformation statement from its website to avoid being called into a legislative hearing. But then, in late January, the board decided to stick with the policy – although it did not republish the statement online immediately – and more specifically defined misinformation, calling it “content that is false, inaccurate or misleading, even if spread unintentionally.”
Board members acknowledged they would likely get more pushback from lawmakers but said they wanted to protect their profession from interference.
“Doctors who are putting forth good evidence-based medicine deserve the protection of this board so they can actually say: ‘Hey, I’m in line with this guideline, and this is a source of truth,’” said Melanie Blake, MD, the board’s president. “We should be a source of truth.”
The medical board was looking into nearly 30 open complaints related to COVID when its misinformation statement came down from its website. As of early February, no Tennessee physician had faced disciplinary action.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN.
Tennessee’s Board of Medical Examiners unanimously adopted in September 2021 a statement that said doctors spreading COVID misinformation – such as suggesting that vaccines contain microchips – could jeopardize their license to practice.
“I’m very glad that we’re taking this step,” Dr. Stephen Loyd, MD, the panel’s vice president, said at the time. “If you’re spreading this willful misinformation, for me it’s going to be really hard to do anything other than put you on probation or take your license for a year. There has to be a message sent for this. It’s not okay.”
The board’s statement was posted on a government website.
The growing tension in Tennessee between conservative lawmakers and the state’s medical board may be the most prominent example in the country. But the Federation of State Medical Boards, which created the language adopted by at least 15 state boards, is tracking legislation introduced by Republicans in at least 14 states that would restrict a medical board’s authority to discipline doctors for their advice on COVID.
Humayun Chaudhry, DO, the federation’s CEO, called it “an unwelcome trend.” The nonprofit association, based in Euless, Tex., said the statement is merely a COVID-specific restatement of an existing rule: that doctors who engage in behavior that puts patients at risk could face disciplinary action.
Although doctors have leeway to decide which treatments to provide, the medical boards that oversee them have broad authority over licensing. Often, doctors are investigated for violating guidelines on prescribing high-powered drugs. But physicians are sometimes punished for other “unprofessional conduct.” In 2013, Tennessee’s board fined U.S. Rep. Scott DesJarlais for separately having sexual relations with two female patients more than a decade earlier.
Still, stopping doctors from sharing unsound medical advice has proved challenging. Even defining misinformation has been difficult. And during the pandemic, resistance from some state legislatures is complicating the effort.
A relatively small group of physicians peddle COVID misinformation, but many of them associate with America’s Frontline Doctors. Its founder, Simone Gold, MD, has claimed patients are dying from COVID treatments, not the virus itself. Sherri Tenpenny, DO, said in a legislative hearing in Ohio that the COVID vaccine could magnetize patients. Stella Immanuel, MD, has pushed hydroxychloroquine as a COVID cure in Texas, although clinical trials showed that it had no benefit. None of them agreed to requests for comment.
The Texas Medical Board fined Dr. Immanuel $500 for not informing a patient of the risks associated with using hydroxychloroquine as an off-label COVID treatment.
In Tennessee, state lawmakers called a special legislative session in October to address COVID restrictions, and Republican Gov. Bill Lee signed a sweeping package of bills that push back against pandemic rules. One included language directed at the medical board’s recent COVID policy statement, making it more difficult for the panel to investigate complaints about physicians’ advice on COVID vaccines or treatments.
In November, Republican state Rep. John Ragan sent the medical board a letter demanding that the statement be deleted from the state’s website. Rep. Ragan leads a legislative panel that had raised the prospect of defunding the state’s health department over its promotion of COVID vaccines to teens.
Among his demands, Rep. Ragan listed 20 questions he wanted the medical board to answer in writing, including why the misinformation “policy” was proposed nearly two years into the pandemic, which scholars would determine what constitutes misinformation, and how was the “policy” not an infringement on the doctor-patient relationship.
“If you fail to act promptly, your organization will be required to appear before the Joint Government Operations Committee to explain your inaction,” Rep. Ragan wrote in the letter, obtained by Kaiser Health News and Nashville Public Radio.
In response to a request for comment, Rep. Ragan said that “any executive agency, including Board of Medical Examiners, that refuses to follow the law is subject to dissolution.”
He set a deadline of Dec. 7.
In Florida, a Republican-sponsored bill making its way through the state legislature proposes to ban medical boards from revoking or threatening to revoke doctors’ licenses for what they say unless “direct physical harm” of a patient occurred. If the publicized complaint can’t be proved, the board could owe a doctor up to $1.5 million in damages.
Although Florida’s medical board has not adopted the Federation of State Medical Boards’ COVID misinformation statement, the panel has considered misinformation complaints against physicians, including the state’s surgeon general, Joseph Ladapo, MD, PhD.
Dr. Chaudhry said he’s surprised just how many COVID-related complaints are being filed across the country. Often, boards do not publicize investigations before a violation of ethics or standards is confirmed. But in response to a survey by the federation in late 2021, two-thirds of state boards reported an increase in misinformation complaints. And the federation said 12 boards had taken action against a licensed physician.
“At the end of the day, if a physician who is licensed engages in activity that causes harm, the state medical boards are the ones that historically have been set up to look into the situation and make a judgment about what happened or didn’t happen,” Dr. Chaudhry said. “And if you start to chip away at that, it becomes a slippery slope.”
The Georgia Composite Medical Board adopted a version of the federation’s misinformation guidance in early November and has been receiving 10-20 complaints each month, said Debi Dalton, MD, the chairperson. Two months in, no one had been sanctioned.
Dr. Dalton said that even putting out a misinformation policy leaves some “gray” area. Generally, physicians are expected to follow the “consensus,” rather than “the newest information that pops up on social media,” she said.
“We expect physicians to think ethically, professionally, and with the safety of patients in mind,” Dr. Dalton said.
A few physician groups are resisting attempts to root out misinformation, including the Association of American Physicians and Surgeons, known for its stands against government regulation.
Some medical boards have opted against taking a public stand against misinformation.
The Alabama Board of Medical Examiners discussed signing on to the federation’s statement, according to the minutes from an October meeting. But after debating the potential legal ramifications in a private executive session, the board opted not to act.
In Tennessee, the Board of Medical Examiners met on the day Rep. Ragan had set as the deadline and voted to remove the misinformation statement from its website to avoid being called into a legislative hearing. But then, in late January, the board decided to stick with the policy – although it did not republish the statement online immediately – and more specifically defined misinformation, calling it “content that is false, inaccurate or misleading, even if spread unintentionally.”
Board members acknowledged they would likely get more pushback from lawmakers but said they wanted to protect their profession from interference.
“Doctors who are putting forth good evidence-based medicine deserve the protection of this board so they can actually say: ‘Hey, I’m in line with this guideline, and this is a source of truth,’” said Melanie Blake, MD, the board’s president. “We should be a source of truth.”
The medical board was looking into nearly 30 open complaints related to COVID when its misinformation statement came down from its website. As of early February, no Tennessee physician had faced disciplinary action.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation. This story is part of a partnership that includes Nashville Public Radio, NPR, and KHN.
16 toddlers with HIV at birth had no detectable virus 2 years later
Hours after their births, 34 infants began a three-drug combination HIV treatment. Now, 2 years later, a third of those toddlers have tested negative for HIV antibodies and have no detectable HIV DNA in their blood. The children aren’t cured of HIV, but as many as 16 of them may be candidates to stop treatment and see if they are in fact in HIV remission.
If one or more are,
At the Conference on Retroviruses and Opportunistic Infections, Deborah Persaud, MD, interim director of pediatric infectious diseases and professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, Md., told this news organization that the evidence suggests that more U.S. clinicians should start infants at high risk for HIV on presumptive treatment – not only to potentially prevent transmission but also to set the child up for the lowest possible viral reservoir, the first step to HIV remission.
The three-drug preemptive treatment is “not uniformly practiced,” Dr. Persaud said in an interview. “We’re at a point now where we don’t have to wait to see if we have remission” to act on these findings, she said. “The question is, should this now become standard of care for in-utero infected infants?”
Every year, about 150 infants are born with HIV in the United States, according to the Elizabeth Glaser Pediatric AIDS Foundation. Current U.S. perinatal treatment guidelines already suggest either treatment with one or more HIV drugs at birth to attempt preventing transmission or initiating three-drug regimens for infants at high risk for perinatally acquired HIV. In this case “high risk” is defined as infants born to:
- people who haven’t received any HIV treatment before delivery or during delivery,
- people who did receive treatment but failed to achieve undetectable viral loads, or
- people who acquire HIV during pregnancy, or who otherwise weren’t diagnosed until after birth.
Trying to replicate the Mississippi baby
The Mississippi baby did eventually relapse. But ever since Dr. Persaud reported the case of that 2-year-old who went into treatment-free remission in 2013, she has been trying to figure out how to duplicate that initial success. There were several factors in that remission, but one piece researchers could control was starting treatment very early – before HIV blood tests even come back positive. So, in this trial, researchers enrolled 440 infants in Africa and Asia at high risk for in utero HIV transmission.
All 440 of those infants received their first doses of the three-drug preemptive treatment within 24 hours of birth. Of those 440 infants, 34 tested positive for HIV and remained in the trial.*
Meanwhile, in North America, South America, and African countries, another 20 infants enrolled in the trial – not as part of the protocol but because their clinicians had been influenced by the news of the Mississippi baby, Dr. Persaud said, and decided on their own to start high-risk infants on three-drug regimens preemptively.
“We wanted to take advantage of those real-world situations of infants being treated outside the clinical trials,” Dr. Persaud said.
Now there were 54 infants trying this very early treatment. In Cohort One, they started their first drug cocktail 7 hours after delivery. In Cohort Two, their first antiretroviral combination treatment was at 32.8 hours of life, and they enrolled in the trial at 8 days. Then researchers followed the infants closely, adding on lopinavir and ritonavir when age-appropriate.
Meeting milestones
To continue in the trial and be considered for treatment interruption, infants had to meet certain milestones. At 24 weeks, HIV RNA needed to be below 200 copies per milliliter. Then their HIV RNA needed to stay below 200 copies consistently until week 48. At week 48, they had to have an HIV RNA that was even lower – below 20 or 40 copies – with “target not detected” in the test in HIV RNA. That’s a sign that there weren’t even any trace levels of viral nucleic acid RNA in the blood to indicate HIV. Then, from week 48 on, they had to maintain that level of viral suppression until age 2.
At that point, not only did they need to maintain that level of viral suppression, they also needed to have a negative HIV antibody test and a PCR test for total HIV DNA, which had to be undetectable down to the limit of 4 copies per 106 – that is, there were fewer than 4 copies of the virus out of 1 million cells tested. Only then would they be considered for treatment interruption.
“After week 28 there was no leeway,” Dr. Persaud said. Then “they had to have nothing detectable from the first year of age. We thought the best shot at remission were cases that achieved very good and strict virologic control.”
Criteria for consideration
Of the 34 infants in Cohort One, 24 infants made it past the first hurdle at 24 weeks and 6 had PCR tests that found no cell-associated HIV DNA. In Cohort Two, 15 made it past the week-24 hurdle and 4 had no detectable HIV DNA via PCR test.
Now, more than 2 years out from study initiation, Dr. Persaud and colleagues are evaluating each child to see if any still meet the requirements for treatment interruption. The COVID-19 pandemic has delayed their evaluations, and it’s possible that fewer children now meet the requirements. But Dr. Persaud said there are still candidates left. An analysis suggests that up to 30% of the children, or 16, were candidates at 2 years.
“We have kids who are eligible for [antiretroviral therapy] cessation years out from this, which I think is really important,” she said in an interview. “It’s not game over.”
And although 30% is not an overwhelming victory, Dr. Persaud said the team’s goal was “to identify an N of 1 to replicate the Mississippi baby.” The study team, led by Ellen Chadwick, MD, of Northwestern University’s Feinberg School of Medicine, Chicago, and a member of the board that creates HIV perinatal treatment guidelines, is starting a new trial, using more modern, integrase inhibitor-based, three-drug regimens for infants and pairing them with broadly neutralizing antibodies. The combination used in this trial included zidovudine, or AZT.
If one of the children is able to go off treatment, it would be the first step toward creating a functional cure for HIV, starting with the youngest people affected by the virus.
“This trial convinces me that very early treatment was the key strategy that led to remission in the Mississippi baby,” Dr. Persaud said in an interview. “We’re confirming here that the first step toward remission and cure is reducing reservoirs. We’ve got that here. Whether we need more on top of that – therapeutic vaccines, immunotherapies, or a better regimen to start out with – needs to be determined.”
The presentation was met with excitement and questions. For instance, if very early treatment works, why does it work for just 30% of the children?
Were some of the children able to control HIV on their own because they were rare post-treatment controllers? And was 30% really a victory? Others were convinced of it.
“Amazing outcome to have 30% so well suppressed after 2 years with CA-DNA not detected,” commented Hermione Lyall, MBChB, a pediatric infectious disease doctor at Imperial College Healthcare NHS Trust in the United Kingdom, in the virtual chat.
As for whether the study should change practice, Elaine Abrams, MD, professor of epidemiology and pediatrics at Columbia University Medical Center, New York, and CROI cochair, said that this study proves that the three-drug regimen is at the very least safe to start immediately.
Whether it should become standard of care everywhere is still up for discussion, she told this news organization.
“It very much depends on what you’re trying to achieve,” she said. “Postnatal prophylaxis is provided to reduce the risk of acquiring infection. That’s a different objective than early treatment. If you have 1,000 high-risk babies, how many are likely to turn out to have HIV infection? And how many of those will you be treating with three drugs and actually making this impact by doing so? And how many babies are going to be getting possibly extra treatment that they don’t need?”
Regardless, what’s clear is that treatment is essential – for mother and infant, said Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases. It needs to start, he said, by making sure all mothers know their HIV status and have access early in pregnancy to the treatment that can prevent transmission.
“So much of what’s wrong in the world is about implementation of health care,” he said in an interview. Still, “if you could demonstrate that early treatment to the mother plus early treatment to the babies [is efficacious], we could really talk about an HIV-free generation of kids.”
The study was funded by the National Institutes of Health. Dr. Persaud, Dr. Dieffenbach, Dr. Abrams, and Dr. Lyall all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 2/16/22: An earlier version of this article misstated the number that tested positive for HIV and remained in the trial.
This article was updated 2/16/22.
Hours after their births, 34 infants began a three-drug combination HIV treatment. Now, 2 years later, a third of those toddlers have tested negative for HIV antibodies and have no detectable HIV DNA in their blood. The children aren’t cured of HIV, but as many as 16 of them may be candidates to stop treatment and see if they are in fact in HIV remission.
If one or more are,
At the Conference on Retroviruses and Opportunistic Infections, Deborah Persaud, MD, interim director of pediatric infectious diseases and professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, Md., told this news organization that the evidence suggests that more U.S. clinicians should start infants at high risk for HIV on presumptive treatment – not only to potentially prevent transmission but also to set the child up for the lowest possible viral reservoir, the first step to HIV remission.
The three-drug preemptive treatment is “not uniformly practiced,” Dr. Persaud said in an interview. “We’re at a point now where we don’t have to wait to see if we have remission” to act on these findings, she said. “The question is, should this now become standard of care for in-utero infected infants?”
Every year, about 150 infants are born with HIV in the United States, according to the Elizabeth Glaser Pediatric AIDS Foundation. Current U.S. perinatal treatment guidelines already suggest either treatment with one or more HIV drugs at birth to attempt preventing transmission or initiating three-drug regimens for infants at high risk for perinatally acquired HIV. In this case “high risk” is defined as infants born to:
- people who haven’t received any HIV treatment before delivery or during delivery,
- people who did receive treatment but failed to achieve undetectable viral loads, or
- people who acquire HIV during pregnancy, or who otherwise weren’t diagnosed until after birth.
Trying to replicate the Mississippi baby
The Mississippi baby did eventually relapse. But ever since Dr. Persaud reported the case of that 2-year-old who went into treatment-free remission in 2013, she has been trying to figure out how to duplicate that initial success. There were several factors in that remission, but one piece researchers could control was starting treatment very early – before HIV blood tests even come back positive. So, in this trial, researchers enrolled 440 infants in Africa and Asia at high risk for in utero HIV transmission.
All 440 of those infants received their first doses of the three-drug preemptive treatment within 24 hours of birth. Of those 440 infants, 34 tested positive for HIV and remained in the trial.*
Meanwhile, in North America, South America, and African countries, another 20 infants enrolled in the trial – not as part of the protocol but because their clinicians had been influenced by the news of the Mississippi baby, Dr. Persaud said, and decided on their own to start high-risk infants on three-drug regimens preemptively.
“We wanted to take advantage of those real-world situations of infants being treated outside the clinical trials,” Dr. Persaud said.
Now there were 54 infants trying this very early treatment. In Cohort One, they started their first drug cocktail 7 hours after delivery. In Cohort Two, their first antiretroviral combination treatment was at 32.8 hours of life, and they enrolled in the trial at 8 days. Then researchers followed the infants closely, adding on lopinavir and ritonavir when age-appropriate.
Meeting milestones
To continue in the trial and be considered for treatment interruption, infants had to meet certain milestones. At 24 weeks, HIV RNA needed to be below 200 copies per milliliter. Then their HIV RNA needed to stay below 200 copies consistently until week 48. At week 48, they had to have an HIV RNA that was even lower – below 20 or 40 copies – with “target not detected” in the test in HIV RNA. That’s a sign that there weren’t even any trace levels of viral nucleic acid RNA in the blood to indicate HIV. Then, from week 48 on, they had to maintain that level of viral suppression until age 2.
At that point, not only did they need to maintain that level of viral suppression, they also needed to have a negative HIV antibody test and a PCR test for total HIV DNA, which had to be undetectable down to the limit of 4 copies per 106 – that is, there were fewer than 4 copies of the virus out of 1 million cells tested. Only then would they be considered for treatment interruption.
“After week 28 there was no leeway,” Dr. Persaud said. Then “they had to have nothing detectable from the first year of age. We thought the best shot at remission were cases that achieved very good and strict virologic control.”
Criteria for consideration
Of the 34 infants in Cohort One, 24 infants made it past the first hurdle at 24 weeks and 6 had PCR tests that found no cell-associated HIV DNA. In Cohort Two, 15 made it past the week-24 hurdle and 4 had no detectable HIV DNA via PCR test.
Now, more than 2 years out from study initiation, Dr. Persaud and colleagues are evaluating each child to see if any still meet the requirements for treatment interruption. The COVID-19 pandemic has delayed their evaluations, and it’s possible that fewer children now meet the requirements. But Dr. Persaud said there are still candidates left. An analysis suggests that up to 30% of the children, or 16, were candidates at 2 years.
“We have kids who are eligible for [antiretroviral therapy] cessation years out from this, which I think is really important,” she said in an interview. “It’s not game over.”
And although 30% is not an overwhelming victory, Dr. Persaud said the team’s goal was “to identify an N of 1 to replicate the Mississippi baby.” The study team, led by Ellen Chadwick, MD, of Northwestern University’s Feinberg School of Medicine, Chicago, and a member of the board that creates HIV perinatal treatment guidelines, is starting a new trial, using more modern, integrase inhibitor-based, three-drug regimens for infants and pairing them with broadly neutralizing antibodies. The combination used in this trial included zidovudine, or AZT.
If one of the children is able to go off treatment, it would be the first step toward creating a functional cure for HIV, starting with the youngest people affected by the virus.
“This trial convinces me that very early treatment was the key strategy that led to remission in the Mississippi baby,” Dr. Persaud said in an interview. “We’re confirming here that the first step toward remission and cure is reducing reservoirs. We’ve got that here. Whether we need more on top of that – therapeutic vaccines, immunotherapies, or a better regimen to start out with – needs to be determined.”
The presentation was met with excitement and questions. For instance, if very early treatment works, why does it work for just 30% of the children?
Were some of the children able to control HIV on their own because they were rare post-treatment controllers? And was 30% really a victory? Others were convinced of it.
“Amazing outcome to have 30% so well suppressed after 2 years with CA-DNA not detected,” commented Hermione Lyall, MBChB, a pediatric infectious disease doctor at Imperial College Healthcare NHS Trust in the United Kingdom, in the virtual chat.
As for whether the study should change practice, Elaine Abrams, MD, professor of epidemiology and pediatrics at Columbia University Medical Center, New York, and CROI cochair, said that this study proves that the three-drug regimen is at the very least safe to start immediately.
Whether it should become standard of care everywhere is still up for discussion, she told this news organization.
“It very much depends on what you’re trying to achieve,” she said. “Postnatal prophylaxis is provided to reduce the risk of acquiring infection. That’s a different objective than early treatment. If you have 1,000 high-risk babies, how many are likely to turn out to have HIV infection? And how many of those will you be treating with three drugs and actually making this impact by doing so? And how many babies are going to be getting possibly extra treatment that they don’t need?”
Regardless, what’s clear is that treatment is essential – for mother and infant, said Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases. It needs to start, he said, by making sure all mothers know their HIV status and have access early in pregnancy to the treatment that can prevent transmission.
“So much of what’s wrong in the world is about implementation of health care,” he said in an interview. Still, “if you could demonstrate that early treatment to the mother plus early treatment to the babies [is efficacious], we could really talk about an HIV-free generation of kids.”
The study was funded by the National Institutes of Health. Dr. Persaud, Dr. Dieffenbach, Dr. Abrams, and Dr. Lyall all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 2/16/22: An earlier version of this article misstated the number that tested positive for HIV and remained in the trial.
This article was updated 2/16/22.
Hours after their births, 34 infants began a three-drug combination HIV treatment. Now, 2 years later, a third of those toddlers have tested negative for HIV antibodies and have no detectable HIV DNA in their blood. The children aren’t cured of HIV, but as many as 16 of them may be candidates to stop treatment and see if they are in fact in HIV remission.
If one or more are,
At the Conference on Retroviruses and Opportunistic Infections, Deborah Persaud, MD, interim director of pediatric infectious diseases and professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, Md., told this news organization that the evidence suggests that more U.S. clinicians should start infants at high risk for HIV on presumptive treatment – not only to potentially prevent transmission but also to set the child up for the lowest possible viral reservoir, the first step to HIV remission.
The three-drug preemptive treatment is “not uniformly practiced,” Dr. Persaud said in an interview. “We’re at a point now where we don’t have to wait to see if we have remission” to act on these findings, she said. “The question is, should this now become standard of care for in-utero infected infants?”
Every year, about 150 infants are born with HIV in the United States, according to the Elizabeth Glaser Pediatric AIDS Foundation. Current U.S. perinatal treatment guidelines already suggest either treatment with one or more HIV drugs at birth to attempt preventing transmission or initiating three-drug regimens for infants at high risk for perinatally acquired HIV. In this case “high risk” is defined as infants born to:
- people who haven’t received any HIV treatment before delivery or during delivery,
- people who did receive treatment but failed to achieve undetectable viral loads, or
- people who acquire HIV during pregnancy, or who otherwise weren’t diagnosed until after birth.
Trying to replicate the Mississippi baby
The Mississippi baby did eventually relapse. But ever since Dr. Persaud reported the case of that 2-year-old who went into treatment-free remission in 2013, she has been trying to figure out how to duplicate that initial success. There were several factors in that remission, but one piece researchers could control was starting treatment very early – before HIV blood tests even come back positive. So, in this trial, researchers enrolled 440 infants in Africa and Asia at high risk for in utero HIV transmission.
All 440 of those infants received their first doses of the three-drug preemptive treatment within 24 hours of birth. Of those 440 infants, 34 tested positive for HIV and remained in the trial.*
Meanwhile, in North America, South America, and African countries, another 20 infants enrolled in the trial – not as part of the protocol but because their clinicians had been influenced by the news of the Mississippi baby, Dr. Persaud said, and decided on their own to start high-risk infants on three-drug regimens preemptively.
“We wanted to take advantage of those real-world situations of infants being treated outside the clinical trials,” Dr. Persaud said.
Now there were 54 infants trying this very early treatment. In Cohort One, they started their first drug cocktail 7 hours after delivery. In Cohort Two, their first antiretroviral combination treatment was at 32.8 hours of life, and they enrolled in the trial at 8 days. Then researchers followed the infants closely, adding on lopinavir and ritonavir when age-appropriate.
Meeting milestones
To continue in the trial and be considered for treatment interruption, infants had to meet certain milestones. At 24 weeks, HIV RNA needed to be below 200 copies per milliliter. Then their HIV RNA needed to stay below 200 copies consistently until week 48. At week 48, they had to have an HIV RNA that was even lower – below 20 or 40 copies – with “target not detected” in the test in HIV RNA. That’s a sign that there weren’t even any trace levels of viral nucleic acid RNA in the blood to indicate HIV. Then, from week 48 on, they had to maintain that level of viral suppression until age 2.
At that point, not only did they need to maintain that level of viral suppression, they also needed to have a negative HIV antibody test and a PCR test for total HIV DNA, which had to be undetectable down to the limit of 4 copies per 106 – that is, there were fewer than 4 copies of the virus out of 1 million cells tested. Only then would they be considered for treatment interruption.
“After week 28 there was no leeway,” Dr. Persaud said. Then “they had to have nothing detectable from the first year of age. We thought the best shot at remission were cases that achieved very good and strict virologic control.”
Criteria for consideration
Of the 34 infants in Cohort One, 24 infants made it past the first hurdle at 24 weeks and 6 had PCR tests that found no cell-associated HIV DNA. In Cohort Two, 15 made it past the week-24 hurdle and 4 had no detectable HIV DNA via PCR test.
Now, more than 2 years out from study initiation, Dr. Persaud and colleagues are evaluating each child to see if any still meet the requirements for treatment interruption. The COVID-19 pandemic has delayed their evaluations, and it’s possible that fewer children now meet the requirements. But Dr. Persaud said there are still candidates left. An analysis suggests that up to 30% of the children, or 16, were candidates at 2 years.
“We have kids who are eligible for [antiretroviral therapy] cessation years out from this, which I think is really important,” she said in an interview. “It’s not game over.”
And although 30% is not an overwhelming victory, Dr. Persaud said the team’s goal was “to identify an N of 1 to replicate the Mississippi baby.” The study team, led by Ellen Chadwick, MD, of Northwestern University’s Feinberg School of Medicine, Chicago, and a member of the board that creates HIV perinatal treatment guidelines, is starting a new trial, using more modern, integrase inhibitor-based, three-drug regimens for infants and pairing them with broadly neutralizing antibodies. The combination used in this trial included zidovudine, or AZT.
If one of the children is able to go off treatment, it would be the first step toward creating a functional cure for HIV, starting with the youngest people affected by the virus.
“This trial convinces me that very early treatment was the key strategy that led to remission in the Mississippi baby,” Dr. Persaud said in an interview. “We’re confirming here that the first step toward remission and cure is reducing reservoirs. We’ve got that here. Whether we need more on top of that – therapeutic vaccines, immunotherapies, or a better regimen to start out with – needs to be determined.”
The presentation was met with excitement and questions. For instance, if very early treatment works, why does it work for just 30% of the children?
Were some of the children able to control HIV on their own because they were rare post-treatment controllers? And was 30% really a victory? Others were convinced of it.
“Amazing outcome to have 30% so well suppressed after 2 years with CA-DNA not detected,” commented Hermione Lyall, MBChB, a pediatric infectious disease doctor at Imperial College Healthcare NHS Trust in the United Kingdom, in the virtual chat.
As for whether the study should change practice, Elaine Abrams, MD, professor of epidemiology and pediatrics at Columbia University Medical Center, New York, and CROI cochair, said that this study proves that the three-drug regimen is at the very least safe to start immediately.
Whether it should become standard of care everywhere is still up for discussion, she told this news organization.
“It very much depends on what you’re trying to achieve,” she said. “Postnatal prophylaxis is provided to reduce the risk of acquiring infection. That’s a different objective than early treatment. If you have 1,000 high-risk babies, how many are likely to turn out to have HIV infection? And how many of those will you be treating with three drugs and actually making this impact by doing so? And how many babies are going to be getting possibly extra treatment that they don’t need?”
Regardless, what’s clear is that treatment is essential – for mother and infant, said Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases. It needs to start, he said, by making sure all mothers know their HIV status and have access early in pregnancy to the treatment that can prevent transmission.
“So much of what’s wrong in the world is about implementation of health care,” he said in an interview. Still, “if you could demonstrate that early treatment to the mother plus early treatment to the babies [is efficacious], we could really talk about an HIV-free generation of kids.”
The study was funded by the National Institutes of Health. Dr. Persaud, Dr. Dieffenbach, Dr. Abrams, and Dr. Lyall all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 2/16/22: An earlier version of this article misstated the number that tested positive for HIV and remained in the trial.
This article was updated 2/16/22.
FROM CROI 22
New study shows natural immunity to COVID has enduring strength
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
It’s a matter of quality, not quantity. That’s the gist of a new Israeli study that shows that unvaccinated people with a prior SARS-CoV-2 infection create antibodies that are more effective in the long run compared with others who were vaccinated but never infected.
“While the quantity of antibodies decreases with time in both COVID-19 recovered patients and vaccinated individuals, ” lead author Carmit Cohen, PhD, said in an interview.
This difference could explain why previously infected patients appear to be better protected against a new infection than those who have only been vaccinated, according to a news release attached to the research.
One key caveat: This research does not include people from the later part of the pandemic.
This means there is a catch in terms of timing, William Schaffner, MD, Vanderbilt University School of Medicine, Nashville, Tenn., said when asked to comment on the study: “The study involved only the early COVID strains – it has no information on either the Delta or Omicron variants. Thus, the results primarily are of scientific or historical interest but are not immediately relevant to the current situation.”
The findings come from an early release of a study to be presented at the European Congress of Clinical Microbiology & Infectious Diseases in April.
An unexpected finding of the study showed that obese people had better protection – a higher and more sustained immune response – compared with overweight and normal-weight individuals.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” Dr. Schaffner said. “Obesity does predispose to more severe disease.”
A focus on earlier strains
Dr. Cohen – a senior research assistant in infectious disease prevention at the Sheba Medical Center in Ramat Gan, Israel – and her colleagues recruited participants between March 25, 2020 and Nov. 25, 2020 and completed analysis in April 2021. This means they assessed people with a history of infection from the original, the Alpha, and some Beta strains of SARS-CoV-2.
Dr. Cohen indicated that the next phase of their research will examine innate and acquired immune responses to the more recent Delta and Omicron variants.
The investigators analyzed the antibody-induced immune response up to 1 year in 130 COVID-19 recovered but unvaccinated individuals versus up to 8 months among 402 others matched by age and body mass index (BMI) and without previous infection who received two doses of the Pfizer vaccine.
The numbers of antibodies a month after vaccination were higher than those in the COVID-19 recovered patients. However, these numbers also declined more steeply in the vaccinated group, they note.
To assess the antibody performance, the investigators used the avidity index. This assay measures antibody function based on the strength of the interactions between the antibody and the viral antigen.
They found that the avidity index was higher in vaccinated individuals than in recovered patients initially but changes over time. At up to 6 months, the index did not significantly change in vaccinated individuals, whereas it gradually increased in recovered patients. This increase would potentially protect them from reinfection, the authors note.
These findings stand in stark contrast to an Oct. 29, 2021, Centers for Disease Control and Prevention study that found that COVID-19 vaccines provided five times the protection of natural immunity.
Those results, published in the organization’s Morbidity and Mortality Weekly Report, suggest that vaccination helps people mount a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least 6 months.
Protection linked to obesity
Another finding that ran against the scientific grain was the data about obesity.
There was a higher and more persistent antibody performance among people with a BMI of 30 kg/m2.
This could relate to greater disease severity and/or a more pronounced initial response to infection among the obese group.
“Our hypothesis is that patients with obesity begin with a more pronounced response – reflected also by the disease manifestation – and the trend of decline is similar, therefore the kinetics of immune response remain higher throughout the study,” Dr. Cohen said.
“The results in the obese group were indeed unexpected and need further research to confirm or dispute,” said Dr. Schaffner, who is also the current medical director of the National Foundation for Infectious Diseases. “Obesity does predispose to more severe disease.”
Before the boosters
Along with using participants from only the earlier part of the pandemic, another limitation of the study was that the vaccinated group had only two doses of vaccine; boosters were not given during the time of the study, Dr. Schaffner said.
“Again, not the current situation.”
“That said, the strength and duration of natural immunity provided by the early variants was solid for up to a year, confirming previous reports,” he said.
A version of this article first appeared on Medscape.com.
A third person living with HIV has been cured by transplant
In a first, If she remains off treatment without any hint of HIV, she would be only the third person in the world – after the Berlin Patient and the London Patient – to be cured through a transplant.
“Her own virus could not infect her cells,” said Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, who presented the study at the Conference on Retroviruses and Opportunistic Infections, which both presenters and the audience attended remotely.
The middle-aged New York woman of mixed race, who has asked that her specific race and age not be shared to protect her privacy, was diagnosed with HIV in 2013 when she was still in the very early stages of infection. She started treatment immediately and quickly achieved an undetectable viral load. An undetectable viral load not only prevents someone from transmitting HIV to others but also reduces or eliminates HIV replication, which means fewer variants and less time for the virus to infiltrate cells where it can hide.
But in 2017, she was diagnosed with leukemia. As a last resort to cure her of the cancer, she received a combination of adult stem cells from a relative’s blood that closely matched her own and umbilical cord blood obtained from a cord blood bank. That particular sample of cord blood was selected for its genetic mutation against the CCR5 receptor on immune cells, CD4 T cells. That mutation makes the immune system resistant to HIV.
The two previous HIV cures, of Berlin Patient Timothy Ray Brown and London Patient Adam Castillejo, also used stem cell transplantation with a CCR5 mutation, but theirs were bone marrow transplants. Bone marrow transplants are more arduous than cord blood transplants, which are commonly used in pediatric cancer treatment.
In this case, the physicians treating her used both.
“This allows the adult cells to accelerate and grow up until the cord blood takes over,” said Dr. Bryson. During her presentation, Dr. Bryson pointed to two types of data: First, she presented data showing the level of HIV in the patient’s blood. Soon after HIV diagnosis and treatment, her viral load dropped to undetectable levels. She had a spike of virus when she received the transplant, but then it went back to undetectable and has stayed that way ever since.
Meanwhile, following the transplant, her immune system started rebuilding itself using the new, HIV-resistant cells provided in the transplant. As her care team watched, no graft-versus-host (GVH) disease, a common side effect of stem cell transplants, emerged. In fact, the transplant went so well that she was discharged early from the hospital.
One hundred days after the transplant, the immune system contained within the cord blood had taken over. Her CD4 immune cells returned to normal levels a little more than a year after the transplant. By 27 months, she decided to stop all HIV treatment to see if the transplant had worked.
This was the real test. But as Dr. Bryson and colleagues continued to watch her HIV viral load and her CD4 counts and search for infectious virus, they didn’t find any. She tested negative for HIV by antibody test. Dr. Bryson grew 75 million of her cells in a lab to look for any HIV. None. Aside from one blip in detectable HIV DNA at 14 weeks, researchers never found HIV in the patient again.
“Her cells are resistant to HIV now – both her own strains and laboratory strains,” Dr. Bryson told this news organization. “It’s been 14 months since then. She has no rebound and no detectable virus.”
The presentation drew as raucous as praise gets in a virtual environment. The comments began pouring in.
“Impressive results,” wrote Jim Hoxie, MD, professor emeritus at the University of Pennsylvania, Philadelphia.
“Exciting case,” wrote Allison Agwu, MD, a professor of pediatrics at Johns Hopkins University, Baltimore.
And Dennis Copertino, a research specialist at Weill Cornell Medicine, New York, wrote: “Thank you so much for translating this important cure strategy to people of color.”
Most donors with CCR5 mutations are White, Dr. Bryson said, suggesting that this approach, in a mixed-race woman, could expand the pool of people living with HIV and cancer who are good candidates for the approach.
But other observers had questions, ones that may require more research to answer. Some asked why this woman’s virus, after transplantation, wasn’t just immune to viruses with CCR5 but also another variant, called CXCR4, that one wouldn’t expect. Luis Montaner, DVM, director of the Immunopathogenesis Laboratory at the Wistar Institute in Philadelphia, wondered whether it was more than the blood that had cleared HIV. Did it get into the tissue, too? That question has not yet been answered.
For Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases, the lack of GVH disease was a powerful and hopeful finding.
“There’s been this ongoing hypothesis that maybe graft-versus-host disease was needed at some level to help clear out every last single CD4+ T cell that may or may not have been harboring replication-competent virus,” Dr. Dieffenbach said in an interview. “But there was no GVH disease. That’s incredible. It’s a wonderful thing.”
Now the challenge is to move from a single case to making cure available to other people living with HIV.
The case also got cure researchers thinking.
Dr. Montaner called the case “an encouraging roadmap supporting anti-CCR5 strategies by CRISPR Cas9,” studies that are now underway.
Steven Deeks, MD, called the case “perhaps a model for how we might do this using a person’s own cells. Because we were never really going to be transplanting cells from another person as a scalable cure.”
For people living with HIV, particularly women of color, the results raise hopes and questions. Nina Martinez knows something about being a “first.” In 2019, she was the first American woman of color living with HIV to donate a kidney to another person living with the virus. To her, the excitement over the first woman of color being cured of HIV just shines a light on how very White and male HIV cure studies have been until now.
“For me, I’m not looking for a cure in which the successful step forward is me getting cancer,” she said in an interview. “I’m looking at, what’s going to be sustainable? I want to know what’s going to work for a group of people.”
Gina Marie Brown, a social worker living with HIV in New Orleans, is also thinking of groups of people.
“Every time we get a breakthrough, it’s like the sun is taken from behind the clouds a little more,” said Ms. Brown. “I think about people in the South, who bear a huge burden of HIV. I think about trans women. I think about Black women, and gay, bisexual, and same-gender-loving men. This could really impact HIV – in the same way that PrEP [pre-exposure prophylaxis] has, the same way that one pill once a day has.”
When Ms. Brown was diagnosed with HIV 22 years ago, she started to plan her funeral.
“That’s how much I thought HIV was a death sentence,” she told this news organization. “Oh my goodness! Glad you stuck around, Gina.”
The study was funded by the National Institutes of Health. Dr. Bryson, Dr. Dieffenbach, Dr. Deeks, and Dr. Montaner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a first, If she remains off treatment without any hint of HIV, she would be only the third person in the world – after the Berlin Patient and the London Patient – to be cured through a transplant.
“Her own virus could not infect her cells,” said Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, who presented the study at the Conference on Retroviruses and Opportunistic Infections, which both presenters and the audience attended remotely.
The middle-aged New York woman of mixed race, who has asked that her specific race and age not be shared to protect her privacy, was diagnosed with HIV in 2013 when she was still in the very early stages of infection. She started treatment immediately and quickly achieved an undetectable viral load. An undetectable viral load not only prevents someone from transmitting HIV to others but also reduces or eliminates HIV replication, which means fewer variants and less time for the virus to infiltrate cells where it can hide.
But in 2017, she was diagnosed with leukemia. As a last resort to cure her of the cancer, she received a combination of adult stem cells from a relative’s blood that closely matched her own and umbilical cord blood obtained from a cord blood bank. That particular sample of cord blood was selected for its genetic mutation against the CCR5 receptor on immune cells, CD4 T cells. That mutation makes the immune system resistant to HIV.
The two previous HIV cures, of Berlin Patient Timothy Ray Brown and London Patient Adam Castillejo, also used stem cell transplantation with a CCR5 mutation, but theirs were bone marrow transplants. Bone marrow transplants are more arduous than cord blood transplants, which are commonly used in pediatric cancer treatment.
In this case, the physicians treating her used both.
“This allows the adult cells to accelerate and grow up until the cord blood takes over,” said Dr. Bryson. During her presentation, Dr. Bryson pointed to two types of data: First, she presented data showing the level of HIV in the patient’s blood. Soon after HIV diagnosis and treatment, her viral load dropped to undetectable levels. She had a spike of virus when she received the transplant, but then it went back to undetectable and has stayed that way ever since.
Meanwhile, following the transplant, her immune system started rebuilding itself using the new, HIV-resistant cells provided in the transplant. As her care team watched, no graft-versus-host (GVH) disease, a common side effect of stem cell transplants, emerged. In fact, the transplant went so well that she was discharged early from the hospital.
One hundred days after the transplant, the immune system contained within the cord blood had taken over. Her CD4 immune cells returned to normal levels a little more than a year after the transplant. By 27 months, she decided to stop all HIV treatment to see if the transplant had worked.
This was the real test. But as Dr. Bryson and colleagues continued to watch her HIV viral load and her CD4 counts and search for infectious virus, they didn’t find any. She tested negative for HIV by antibody test. Dr. Bryson grew 75 million of her cells in a lab to look for any HIV. None. Aside from one blip in detectable HIV DNA at 14 weeks, researchers never found HIV in the patient again.
“Her cells are resistant to HIV now – both her own strains and laboratory strains,” Dr. Bryson told this news organization. “It’s been 14 months since then. She has no rebound and no detectable virus.”
The presentation drew as raucous as praise gets in a virtual environment. The comments began pouring in.
“Impressive results,” wrote Jim Hoxie, MD, professor emeritus at the University of Pennsylvania, Philadelphia.
“Exciting case,” wrote Allison Agwu, MD, a professor of pediatrics at Johns Hopkins University, Baltimore.
And Dennis Copertino, a research specialist at Weill Cornell Medicine, New York, wrote: “Thank you so much for translating this important cure strategy to people of color.”
Most donors with CCR5 mutations are White, Dr. Bryson said, suggesting that this approach, in a mixed-race woman, could expand the pool of people living with HIV and cancer who are good candidates for the approach.
But other observers had questions, ones that may require more research to answer. Some asked why this woman’s virus, after transplantation, wasn’t just immune to viruses with CCR5 but also another variant, called CXCR4, that one wouldn’t expect. Luis Montaner, DVM, director of the Immunopathogenesis Laboratory at the Wistar Institute in Philadelphia, wondered whether it was more than the blood that had cleared HIV. Did it get into the tissue, too? That question has not yet been answered.
For Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases, the lack of GVH disease was a powerful and hopeful finding.
“There’s been this ongoing hypothesis that maybe graft-versus-host disease was needed at some level to help clear out every last single CD4+ T cell that may or may not have been harboring replication-competent virus,” Dr. Dieffenbach said in an interview. “But there was no GVH disease. That’s incredible. It’s a wonderful thing.”
Now the challenge is to move from a single case to making cure available to other people living with HIV.
The case also got cure researchers thinking.
Dr. Montaner called the case “an encouraging roadmap supporting anti-CCR5 strategies by CRISPR Cas9,” studies that are now underway.
Steven Deeks, MD, called the case “perhaps a model for how we might do this using a person’s own cells. Because we were never really going to be transplanting cells from another person as a scalable cure.”
For people living with HIV, particularly women of color, the results raise hopes and questions. Nina Martinez knows something about being a “first.” In 2019, she was the first American woman of color living with HIV to donate a kidney to another person living with the virus. To her, the excitement over the first woman of color being cured of HIV just shines a light on how very White and male HIV cure studies have been until now.
“For me, I’m not looking for a cure in which the successful step forward is me getting cancer,” she said in an interview. “I’m looking at, what’s going to be sustainable? I want to know what’s going to work for a group of people.”
Gina Marie Brown, a social worker living with HIV in New Orleans, is also thinking of groups of people.
“Every time we get a breakthrough, it’s like the sun is taken from behind the clouds a little more,” said Ms. Brown. “I think about people in the South, who bear a huge burden of HIV. I think about trans women. I think about Black women, and gay, bisexual, and same-gender-loving men. This could really impact HIV – in the same way that PrEP [pre-exposure prophylaxis] has, the same way that one pill once a day has.”
When Ms. Brown was diagnosed with HIV 22 years ago, she started to plan her funeral.
“That’s how much I thought HIV was a death sentence,” she told this news organization. “Oh my goodness! Glad you stuck around, Gina.”
The study was funded by the National Institutes of Health. Dr. Bryson, Dr. Dieffenbach, Dr. Deeks, and Dr. Montaner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a first, If she remains off treatment without any hint of HIV, she would be only the third person in the world – after the Berlin Patient and the London Patient – to be cured through a transplant.
“Her own virus could not infect her cells,” said Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, who presented the study at the Conference on Retroviruses and Opportunistic Infections, which both presenters and the audience attended remotely.
The middle-aged New York woman of mixed race, who has asked that her specific race and age not be shared to protect her privacy, was diagnosed with HIV in 2013 when she was still in the very early stages of infection. She started treatment immediately and quickly achieved an undetectable viral load. An undetectable viral load not only prevents someone from transmitting HIV to others but also reduces or eliminates HIV replication, which means fewer variants and less time for the virus to infiltrate cells where it can hide.
But in 2017, she was diagnosed with leukemia. As a last resort to cure her of the cancer, she received a combination of adult stem cells from a relative’s blood that closely matched her own and umbilical cord blood obtained from a cord blood bank. That particular sample of cord blood was selected for its genetic mutation against the CCR5 receptor on immune cells, CD4 T cells. That mutation makes the immune system resistant to HIV.
The two previous HIV cures, of Berlin Patient Timothy Ray Brown and London Patient Adam Castillejo, also used stem cell transplantation with a CCR5 mutation, but theirs were bone marrow transplants. Bone marrow transplants are more arduous than cord blood transplants, which are commonly used in pediatric cancer treatment.
In this case, the physicians treating her used both.
“This allows the adult cells to accelerate and grow up until the cord blood takes over,” said Dr. Bryson. During her presentation, Dr. Bryson pointed to two types of data: First, she presented data showing the level of HIV in the patient’s blood. Soon after HIV diagnosis and treatment, her viral load dropped to undetectable levels. She had a spike of virus when she received the transplant, but then it went back to undetectable and has stayed that way ever since.
Meanwhile, following the transplant, her immune system started rebuilding itself using the new, HIV-resistant cells provided in the transplant. As her care team watched, no graft-versus-host (GVH) disease, a common side effect of stem cell transplants, emerged. In fact, the transplant went so well that she was discharged early from the hospital.
One hundred days after the transplant, the immune system contained within the cord blood had taken over. Her CD4 immune cells returned to normal levels a little more than a year after the transplant. By 27 months, she decided to stop all HIV treatment to see if the transplant had worked.
This was the real test. But as Dr. Bryson and colleagues continued to watch her HIV viral load and her CD4 counts and search for infectious virus, they didn’t find any. She tested negative for HIV by antibody test. Dr. Bryson grew 75 million of her cells in a lab to look for any HIV. None. Aside from one blip in detectable HIV DNA at 14 weeks, researchers never found HIV in the patient again.
“Her cells are resistant to HIV now – both her own strains and laboratory strains,” Dr. Bryson told this news organization. “It’s been 14 months since then. She has no rebound and no detectable virus.”
The presentation drew as raucous as praise gets in a virtual environment. The comments began pouring in.
“Impressive results,” wrote Jim Hoxie, MD, professor emeritus at the University of Pennsylvania, Philadelphia.
“Exciting case,” wrote Allison Agwu, MD, a professor of pediatrics at Johns Hopkins University, Baltimore.
And Dennis Copertino, a research specialist at Weill Cornell Medicine, New York, wrote: “Thank you so much for translating this important cure strategy to people of color.”
Most donors with CCR5 mutations are White, Dr. Bryson said, suggesting that this approach, in a mixed-race woman, could expand the pool of people living with HIV and cancer who are good candidates for the approach.
But other observers had questions, ones that may require more research to answer. Some asked why this woman’s virus, after transplantation, wasn’t just immune to viruses with CCR5 but also another variant, called CXCR4, that one wouldn’t expect. Luis Montaner, DVM, director of the Immunopathogenesis Laboratory at the Wistar Institute in Philadelphia, wondered whether it was more than the blood that had cleared HIV. Did it get into the tissue, too? That question has not yet been answered.
For Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases, the lack of GVH disease was a powerful and hopeful finding.
“There’s been this ongoing hypothesis that maybe graft-versus-host disease was needed at some level to help clear out every last single CD4+ T cell that may or may not have been harboring replication-competent virus,” Dr. Dieffenbach said in an interview. “But there was no GVH disease. That’s incredible. It’s a wonderful thing.”
Now the challenge is to move from a single case to making cure available to other people living with HIV.
The case also got cure researchers thinking.
Dr. Montaner called the case “an encouraging roadmap supporting anti-CCR5 strategies by CRISPR Cas9,” studies that are now underway.
Steven Deeks, MD, called the case “perhaps a model for how we might do this using a person’s own cells. Because we were never really going to be transplanting cells from another person as a scalable cure.”
For people living with HIV, particularly women of color, the results raise hopes and questions. Nina Martinez knows something about being a “first.” In 2019, she was the first American woman of color living with HIV to donate a kidney to another person living with the virus. To her, the excitement over the first woman of color being cured of HIV just shines a light on how very White and male HIV cure studies have been until now.
“For me, I’m not looking for a cure in which the successful step forward is me getting cancer,” she said in an interview. “I’m looking at, what’s going to be sustainable? I want to know what’s going to work for a group of people.”
Gina Marie Brown, a social worker living with HIV in New Orleans, is also thinking of groups of people.
“Every time we get a breakthrough, it’s like the sun is taken from behind the clouds a little more,” said Ms. Brown. “I think about people in the South, who bear a huge burden of HIV. I think about trans women. I think about Black women, and gay, bisexual, and same-gender-loving men. This could really impact HIV – in the same way that PrEP [pre-exposure prophylaxis] has, the same way that one pill once a day has.”
When Ms. Brown was diagnosed with HIV 22 years ago, she started to plan her funeral.
“That’s how much I thought HIV was a death sentence,” she told this news organization. “Oh my goodness! Glad you stuck around, Gina.”
The study was funded by the National Institutes of Health. Dr. Bryson, Dr. Dieffenbach, Dr. Deeks, and Dr. Montaner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CROI 2022
Tips for connecting with your patients
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
It is a tough time to be a doctor. With the stresses of the pandemic, the continued unfettered rise of insurance company BS, and so many medical groups being bought up that we often don’t even know who makes the decisions, the patient can sometimes be hidden in the equation.
Be curious
When physicians are curious about why patients have symptoms, how those symptoms will affect their lives, and how worried the patient is about them, patients feel cared about.
Ascertaining how concerned patients are about their symptoms will help you make decisions on whether symptoms you are not concerned about actually need to be treated.
Limit use of EHRs when possible
Use of the electronic health record during visits is essential, but focusing on it too much can put a barrier between the physician and the patient.
Marmor and colleagues found there is an inverse relationship between time spent on the EHR by a patient’s physician and the patient’s satisfaction.1
Eye contact with the patient is important, especially when patients are sharing concerns they are scared about and upsetting experiences. There can be awkward pauses when looking things up on the EHR. Fill those pauses by explaining to the patient what you are doing, or chatting with the patient.
Consider teaching medical students
When a medical student works with you, it doubles the time the patient gets with a concerned listener. Students also can do a great job with timely follow-up and checking in with worried patients.
By having the student present in the clinic room, with the patient present, the patient can really feel heard. The student shares all the details the patient shared, and now their physician is hearing an organized, thoughtful report of the patients concerns.
In fact, I was involved in a study that showed that patients preferred in room presentations, and that they were more satisfied when students presented in the room.2
Use healing words
Some words carry loaded emotions. The word chronic, for example, has negative connotations, whereas the term persisting does not.
I will often ask patients how long they have been suffering from a symptom to imply my concern for what they are going through. The term “chief complaint” is outdated, and upsets patients when they see it in their medical record.
As a patient of mine once said to me: “I never complained about that problem, I just brought it to your attention.” No one wants to be seen as a complainer. Substituting the word concern for complaint works well.
Explain as you examine
People love to hear the term normal. When you are examining a patient, let them know when findings are normal.
I also find it helpful to explain to patients why I am doing certain physical exam maneuvers. This helps them assess how thorough we are in our thought process.
When patients feel their physicians are thorough, they have more confidence in them.
In summary
- Be curious.
- Do not overly focus on the EHR.
- Consider teaching a medical student.
- Be careful of word choice.
- “Overexplain” the physical exam.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Marmor RA et al. Appl Clin Inform. 2018 Jan;9(1):11-4.
2. Rogers HD et al. Acad Med. 2003 Sep;78(9):945-9.
Children and COVID: Weekly cases down by more than half
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
Long COVID symptoms linked to effects on vagus nerve
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Several long COVID symptoms could be linked to the effects of the coronavirus on a vital central nerve, according to new research being released in the spring.
The vagus nerve, which runs from the brain into the body, connects to the heart, lungs, intestines, and several muscles involved with swallowing. It plays a role in several body functions that control heart rate, speech, the gag reflex, sweating, and digestion.
Those with long COVID and vagus nerve problems could face long-term issues with their voice, a hard time swallowing, dizziness, a high heart rate, low blood pressure, and diarrhea, the study authors found.
Their findings will be presented at the 2022 European Congress of Clinical Microbiology and Infectious Diseases in late April.
“Most long COVID subjects with vagus nerve dysfunction symptoms had a range of significant, clinically relevant, structural and/or functional alterations in their vagus nerve, including nerve thickening, trouble swallowing, and symptoms of impaired breathing,” the study authors wrote. “Our findings so far thus point at vagus nerve dysfunction as a central pathophysiological feature of long COVID.”
Researchers from the University Hospital Germans Trias i Pujol in Barcelona performed a study to look at vagus nerve functioning in long COVID patients. Among 348 patients, about 66% had at least one symptom that suggested vagus nerve dysfunction. The researchers did a broad evaluation with imaging and functional tests for 22 patients in the university’s Long COVID Clinic from March to June 2021.
Of the 22 patients, 20 were women, and the median age was 44. The most frequent symptoms related to vagus nerve dysfunction were diarrhea (73%), high heart rates (59%), dizziness (45%), swallowing problems (45%), voice problems (45%), and low blood pressure (14%).
Almost all (19 of 22 patients) had three or more symptoms related to vagus nerve dysfunction. The average length of symptoms was 14 months.
Of 22 patients, 6 had a change in the vagus nerve in the neck, which the researchers observed by ultrasound. They had a thickening of the vagus nerve and increased “echogenicity,” which suggests inflammation.
What’s more, 10 of 22 patients had flattened “diaphragmatic curves” during a thoracic ultrasound, which means the diaphragm doesn’t move as well as it should during breathing, and abnormal breathing. In another assessment, 10 of 16 patients had lower maximum inspiration pressures, suggesting a weakness in breathing muscles.
Eating and digestion were also impaired in some patients, with 13 reporting trouble with swallowing. During a gastric and bowel function assessment, eight patients couldn’t move food from the esophagus to the stomach as well as they should, while nine patients had acid reflux. Three patients had a hiatal hernia, which happens when the upper part of the stomach bulges through the diaphragm into the chest cavity.
The voices of some patients changed as well. Eight patients had an abnormal voice handicap index 30 test, which is a standard way to measure voice function. Among those, seven patients had dysphonia, or persistent voice problems.
The study is ongoing, and the research team is continuing to recruit patients to study the links between long COVID and the vagus nerve. The full paper isn’t yet available, and the research hasn’t yet been peer reviewed.
“The study appears to add to a growing collection of data suggesting at least some of the symptoms of long COVID is mediated through a direct impact on the nervous system,” David Strain, MD, a clinical senior lecturer at the University of Exeter (England), told the Science Media Centre.
“Establishing vagal nerve damage is useful information, as there are recognized, albeit not perfect, treatments for other causes of vagal nerve dysfunction that may be extrapolated to be beneficial for people with this type of long COVID,” he said.
A version of this article first appeared on WebMD.com.
Estrogen supplementation may reduce COVID-19 death risk
Estrogen supplementation is associated with a reduced risk of death from COVID-19 among postmenopausal women, new research suggests.
The findings, from a nationwide study using data from Sweden, were published online Feb. 14 in BMJ Open by Malin Sund, MD, PhD, of Umeå (Sweden) University Faculty of Medicine and colleagues.
Among postmenopausal women aged 50-80 years with verified COVID-19, those receiving estrogen as part of hormone replacement therapy for menopausal symptoms were less than half as likely to die from it as those not receiving estrogen, even after adjustment for confounders.
“This study shows an association between estrogen levels and COVID-19 death. Consequently, drugs increasing estrogen levels may have a role in therapeutic efforts to alleviate COVID-19 severity in postmenopausal women and could be studied in randomized control trials,” the investigators write.
However, coauthor Anne-Marie Fors Connolly, MD, PhD, a resident in clinical microbiology at Umeå University, cautioned: “This is an observational study. Further clinical studies are needed to verify these results before recommending clinicians to consider estrogen supplementation.”
Stephen Evans, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, agrees.
He told the U.K. Science and Media Centre: “This is an observational study comparing three groups of women based on whether they used hormonal therapy to boost estrogen levels or who had, as a result of treatment for breast cancer ... reduced estrogen levels or neither. The findings are apparently dramatic.”
“At the very least, great caution should be exercised in thinking that menopausal hormone therapy will have substantial, or even any, benefits in dealing with COVID-19,” he warned.
Do women die less frequently from COVID-19 than men?
Studies conducted early in the pandemic suggest women may be protected from poor outcomes of SARS-CoV-2 infection, compared with men, even after adjustment for confounders.
According to more recent data from the Swedish Public Health Agency, of the 16,501 people who have died from COVID-19 since the start of the pandemic, about 45% are women and 55% are men. About 70% who have received intensive care because of COVID-19 are men, although cumulative data suggest that women are nearly as likely to die from COVID-19 as men, Dr. Connolly told this news organization.
For the current study, a total of 14,685 women aged 50-80 years were included, of whom 17.3% (2,535) had received estrogen supplementation, 81.2% (11,923) had native estrogen levels with no breast cancer or estrogen supplementation (controls), and 1.5% (227) had decreased estrogen levels because of breast cancer and antiestrogen treatment.
The group with decreased estrogen levels had a more than twofold risk of dying from COVID-19 compared with controls (odds ratio, 2.35), but this difference was no longer significant after adjustments for potential confounders including age, income, and educational level, and weighted Charlson Comorbidity Index (wCCI).
However, the group with augmented estrogen levels had a decreased risk of dying from COVID-19 before (odds ratio, 0.45) and after (OR, 0.47) adjustment.
The percentages of patients who died of COVID-19 were 4.6% of controls, 10.1% of those with decreased estrogen, and 2.1% with increased estrogen.
Not surprisingly, the risk of dying from COVID-19 also increased with age (OR of 1.15 for every year increase in age) and comorbidities (OR, 1.13 per increase in wCCI). Low income and having only a primary level education also increased the odds of dying from COVID-19.
Data on obesity, a known risk factor for COVID-19 death, weren’t reported.
“Obesity would have been a very relevant variable to include. Unfortunately, this information is not present in the nationwide registry data that we used for our study,” Dr. Connolly told this news organization.
While the data are observational and can’t be used to inform treatment, Dr. Connolly pointed to a U.S. randomized clinical trial currently recruiting patients that will investigate the effect of estradiol and progesterone therapy in 120 adults hospitalized with COVID-19.
In the meantime, she warned doctors and patients: “Please do not consider ending antiestrogen treatment following breast cancer – this is a necessary treatment for the cancer.”
Dr. Evans noted, “There are short-term benefits of menopausal hormone therapy but women should not, based on this or other observational studies, be advised to take HRT [hormone replacement therapy] for a supposed benefit on death from COVID-19.”
The study had several nonpharmaceutical industry funders, including Umeå University and the Knut and Alice Wallenberg Foundation. The authors and Dr. Evans have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Estrogen supplementation is associated with a reduced risk of death from COVID-19 among postmenopausal women, new research suggests.
The findings, from a nationwide study using data from Sweden, were published online Feb. 14 in BMJ Open by Malin Sund, MD, PhD, of Umeå (Sweden) University Faculty of Medicine and colleagues.
Among postmenopausal women aged 50-80 years with verified COVID-19, those receiving estrogen as part of hormone replacement therapy for menopausal symptoms were less than half as likely to die from it as those not receiving estrogen, even after adjustment for confounders.
“This study shows an association between estrogen levels and COVID-19 death. Consequently, drugs increasing estrogen levels may have a role in therapeutic efforts to alleviate COVID-19 severity in postmenopausal women and could be studied in randomized control trials,” the investigators write.
However, coauthor Anne-Marie Fors Connolly, MD, PhD, a resident in clinical microbiology at Umeå University, cautioned: “This is an observational study. Further clinical studies are needed to verify these results before recommending clinicians to consider estrogen supplementation.”
Stephen Evans, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, agrees.
He told the U.K. Science and Media Centre: “This is an observational study comparing three groups of women based on whether they used hormonal therapy to boost estrogen levels or who had, as a result of treatment for breast cancer ... reduced estrogen levels or neither. The findings are apparently dramatic.”
“At the very least, great caution should be exercised in thinking that menopausal hormone therapy will have substantial, or even any, benefits in dealing with COVID-19,” he warned.
Do women die less frequently from COVID-19 than men?
Studies conducted early in the pandemic suggest women may be protected from poor outcomes of SARS-CoV-2 infection, compared with men, even after adjustment for confounders.
According to more recent data from the Swedish Public Health Agency, of the 16,501 people who have died from COVID-19 since the start of the pandemic, about 45% are women and 55% are men. About 70% who have received intensive care because of COVID-19 are men, although cumulative data suggest that women are nearly as likely to die from COVID-19 as men, Dr. Connolly told this news organization.
For the current study, a total of 14,685 women aged 50-80 years were included, of whom 17.3% (2,535) had received estrogen supplementation, 81.2% (11,923) had native estrogen levels with no breast cancer or estrogen supplementation (controls), and 1.5% (227) had decreased estrogen levels because of breast cancer and antiestrogen treatment.
The group with decreased estrogen levels had a more than twofold risk of dying from COVID-19 compared with controls (odds ratio, 2.35), but this difference was no longer significant after adjustments for potential confounders including age, income, and educational level, and weighted Charlson Comorbidity Index (wCCI).
However, the group with augmented estrogen levels had a decreased risk of dying from COVID-19 before (odds ratio, 0.45) and after (OR, 0.47) adjustment.
The percentages of patients who died of COVID-19 were 4.6% of controls, 10.1% of those with decreased estrogen, and 2.1% with increased estrogen.
Not surprisingly, the risk of dying from COVID-19 also increased with age (OR of 1.15 for every year increase in age) and comorbidities (OR, 1.13 per increase in wCCI). Low income and having only a primary level education also increased the odds of dying from COVID-19.
Data on obesity, a known risk factor for COVID-19 death, weren’t reported.
“Obesity would have been a very relevant variable to include. Unfortunately, this information is not present in the nationwide registry data that we used for our study,” Dr. Connolly told this news organization.
While the data are observational and can’t be used to inform treatment, Dr. Connolly pointed to a U.S. randomized clinical trial currently recruiting patients that will investigate the effect of estradiol and progesterone therapy in 120 adults hospitalized with COVID-19.
In the meantime, she warned doctors and patients: “Please do not consider ending antiestrogen treatment following breast cancer – this is a necessary treatment for the cancer.”
Dr. Evans noted, “There are short-term benefits of menopausal hormone therapy but women should not, based on this or other observational studies, be advised to take HRT [hormone replacement therapy] for a supposed benefit on death from COVID-19.”
The study had several nonpharmaceutical industry funders, including Umeå University and the Knut and Alice Wallenberg Foundation. The authors and Dr. Evans have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Estrogen supplementation is associated with a reduced risk of death from COVID-19 among postmenopausal women, new research suggests.
The findings, from a nationwide study using data from Sweden, were published online Feb. 14 in BMJ Open by Malin Sund, MD, PhD, of Umeå (Sweden) University Faculty of Medicine and colleagues.
Among postmenopausal women aged 50-80 years with verified COVID-19, those receiving estrogen as part of hormone replacement therapy for menopausal symptoms were less than half as likely to die from it as those not receiving estrogen, even after adjustment for confounders.
“This study shows an association between estrogen levels and COVID-19 death. Consequently, drugs increasing estrogen levels may have a role in therapeutic efforts to alleviate COVID-19 severity in postmenopausal women and could be studied in randomized control trials,” the investigators write.
However, coauthor Anne-Marie Fors Connolly, MD, PhD, a resident in clinical microbiology at Umeå University, cautioned: “This is an observational study. Further clinical studies are needed to verify these results before recommending clinicians to consider estrogen supplementation.”
Stephen Evans, professor of pharmacoepidemiology, London School of Hygiene & Tropical Medicine, agrees.
He told the U.K. Science and Media Centre: “This is an observational study comparing three groups of women based on whether they used hormonal therapy to boost estrogen levels or who had, as a result of treatment for breast cancer ... reduced estrogen levels or neither. The findings are apparently dramatic.”
“At the very least, great caution should be exercised in thinking that menopausal hormone therapy will have substantial, or even any, benefits in dealing with COVID-19,” he warned.
Do women die less frequently from COVID-19 than men?
Studies conducted early in the pandemic suggest women may be protected from poor outcomes of SARS-CoV-2 infection, compared with men, even after adjustment for confounders.
According to more recent data from the Swedish Public Health Agency, of the 16,501 people who have died from COVID-19 since the start of the pandemic, about 45% are women and 55% are men. About 70% who have received intensive care because of COVID-19 are men, although cumulative data suggest that women are nearly as likely to die from COVID-19 as men, Dr. Connolly told this news organization.
For the current study, a total of 14,685 women aged 50-80 years were included, of whom 17.3% (2,535) had received estrogen supplementation, 81.2% (11,923) had native estrogen levels with no breast cancer or estrogen supplementation (controls), and 1.5% (227) had decreased estrogen levels because of breast cancer and antiestrogen treatment.
The group with decreased estrogen levels had a more than twofold risk of dying from COVID-19 compared with controls (odds ratio, 2.35), but this difference was no longer significant after adjustments for potential confounders including age, income, and educational level, and weighted Charlson Comorbidity Index (wCCI).
However, the group with augmented estrogen levels had a decreased risk of dying from COVID-19 before (odds ratio, 0.45) and after (OR, 0.47) adjustment.
The percentages of patients who died of COVID-19 were 4.6% of controls, 10.1% of those with decreased estrogen, and 2.1% with increased estrogen.
Not surprisingly, the risk of dying from COVID-19 also increased with age (OR of 1.15 for every year increase in age) and comorbidities (OR, 1.13 per increase in wCCI). Low income and having only a primary level education also increased the odds of dying from COVID-19.
Data on obesity, a known risk factor for COVID-19 death, weren’t reported.
“Obesity would have been a very relevant variable to include. Unfortunately, this information is not present in the nationwide registry data that we used for our study,” Dr. Connolly told this news organization.
While the data are observational and can’t be used to inform treatment, Dr. Connolly pointed to a U.S. randomized clinical trial currently recruiting patients that will investigate the effect of estradiol and progesterone therapy in 120 adults hospitalized with COVID-19.
In the meantime, she warned doctors and patients: “Please do not consider ending antiestrogen treatment following breast cancer – this is a necessary treatment for the cancer.”
Dr. Evans noted, “There are short-term benefits of menopausal hormone therapy but women should not, based on this or other observational studies, be advised to take HRT [hormone replacement therapy] for a supposed benefit on death from COVID-19.”
The study had several nonpharmaceutical industry funders, including Umeå University and the Knut and Alice Wallenberg Foundation. The authors and Dr. Evans have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
Can cancer patients get approved COVID therapies?
In mid-November, Kevin Billingsley, MD, MBA, chief medical officer at Yale Cancer Center, New Haven, Conn., was keeping a close eye on the new COVID variant sweeping across South Africa. Six weeks later, the Omicron variant had become the dominant strain in the U.S. – and the Yale health system was no exception.
“As we entered January, we had a breathtaking rate of infection in our hospital,” said Dr. Billingsley, who also leads clinical care at the Smilow Cancer Hospital. “Some of the newly authorized COVID agents were available but not widely enough to make a clinically meaningful impact to protect all high-risk individuals during this surge.”
That left the team at Yale with difficult decisions about who would receive these treatments and who wouldn’t.
The health system convened a COVID-19 immunocompromised working group to identify which patients should get priority access to one of the promising drugs authorized to treat the infection – the monoclonal antibody sotrovimab and antiviral pills Paxlovid and molnupiravir – or the sole available option to prevent it, Evusheld.
“Although clinically sound, none of these decisions have been easy,” Dr. Billingsley told this news organization. “We have done a lot of case-by-case reviewing and a lot of handwringing. Omicron has been a wild ride for us all, and we have been doing the best we can with limited resources.”
‘We’re seeing incredible variability’
The team at Yale is not alone. The restricted supply of COVID-19 treatments has led many oncologists and other experts across the U.S. to create carefully curated lists of their most vulnerable patients.
In late December, the National Institutes of Health published broad criteria to help clinicians prioritize patients most likely to benefit from these therapies. A handful of state health departments, including those in Michigan and Minnesota, established their own standards. Patients with cancer – specifically those with hematologic malignancies and receiving oncology therapies that compromise the immune system – appeared at the top of everyone’s list.
But ultimately individual decisions about who receives these drugs and how they’re allocated fell to institutions.
“Overall, what we’re seeing is incredible variability across the country, because there’s no uniform agreement on what comprises best practices on allocating scarce resources,” said Matthew Wynia, MD, MPH, professor of medicine and director of the Center for Bioethics and Humanities at the University of Colorado, Aurora. “There are so many people at the top of most lists, and the drugs are in such short supply, that there’s no guarantee even those in the top tier will get it.”
This news organization spoke to experts across the country about their experiences accessing these treatments during the Omicron surge and their strategies prioritizing patients with cancer.
Dealing with limited supply
Overall, the limited supply of COVID-19 drugs means not every patient who’s eligible to receive a treatment will get one.
A snapshot of the past 2 weeks, for instance, shows that the count of new infections hit almost 4.3 million, while distribution of the two antiviral pills Paxlovid and molnupiravir and the monoclonal antibody sotrovimab reached just over 600,000 courses.
Since receiving emergency use authorization in early December, almost 500,000 courses of the pre-exposure prophylactic agent Evusheld – which offers about 6 months of protection for immunocompromised individuals – have been distributed; however, about 7 million adults in the U.S. could potentially benefit from it.
In addition, the distribution of drugs is uneven. The federal government manages the overall distribution to states, but states then decide how to divvy up these allocations to hospitals, pharmacies, and medical centers. In Ohio, for instance, the antivirals go to providers who already receive monoclonal antibodies, while in Tennessee, the supply of antiviral agents only goes to Walmart pharmacies.
This strategy, Dr. Wynia explained, can leave clinicians at the mercy of where and how much states decide to allocate to each location. “I’ve heard of some hospitals and health systems in Colorado that aren’t using all they’ve got, but most don’t have nearly enough,” Dr. Wynia said. However, he noted, “some of that is inevitable. We will never get a perfect distribution of these drugs when there is such variable need and demand.”
And, according to Nicolette Louissaint, PhD, MBA, senior vice president of policy and strategic planning at the Healthcare Distribution Alliance in Arlington, Virginia, “we can take some comfort that the federal government is actively looking at cases from week to week and working with state and local health departments to see who needs these products, which means the process is constantly being reviewed and adjusted.”
Plus, not every positive COVID-19 case, even among immunocompromised individuals, necessarily warrants treatment. “If, for instance, an individual with cancer has a mild case of COVID-19, their provider may not deem it necessary for them to receive treatment,” Dr. Louissaint noted.
Still, given the limited and unpredictable supply, “we have had to be thoughtful about who gets these drugs,” said Derek Raghavan, MD, PhD, president of the Levine Cancer Institute, part of the 40-hospital Atrium Health system in Charlotte, North Carolina.
Dr. Raghavan said the highest priority goes to patients with hematologic malignancies, those receiving or coming off chemotherapy or experiencing myelosuppression and immune paresis, as well as those who have undergone organ transplants. Age and other comorbidities, such as diabetes or obesity, play into the lineup as well.
To further hone their priority list, the Levine Cancer Institute has implemented a cancer-centered Hospital at Home initiative. The program includes 40 oncology nurse navigators who routinely screen and score all cancer patients who test positive for COVID-19 by their symptoms and risk factors. For a time-sensitive treatment like Paxlovid, this close monitoring allows patients with COVID to access the pills within 5 days of symptom onset.
Ultimately, “the decision regarding who gets these drugs is [made] by a team to overcome any risk of personal bias, and some of it just comes down to the interface between clinical judgment and available data,” Dr. Raghavan told this news organization. “Although we’d like to have more COVID drugs available and fewer patients with COVID, we have been able to get adequate supplies for our most at-risk patients.”
Like Dr. Raghavan, Karen Bloch, MD, MPH, the medical director for the COVID Infusion Clinic at Vanderbilt University Medical Center (VUMC), said the clinic has had to be highly selective about which patients would benefit most from the COVID monoclonal antibodies. For patients with cancer, her team prioritizes individuals who would be least able to develop antibodies through vaccination or natural infection – which includes patients with B cell malignancies, acute myeloid leukemia, or multiple myeloma receiving active treatment, as well as those who recently received an allogeneic or autologous stem cell transplant.
“Since our criteria for treatment with therapies such as sotrovimab and Evusheld are pretty stringent, we have had sufficient supply to treat those who meet our internal ‘category 1’ predetermined criteria,” said Dr. Bloch, professor of medicine and associate division director for clinical affairs at VUMC, Nashville. “More recently, as the supply chain has begun to open up, we’ve been able to loosen our criteria for sotrovimab, though not for Evusheld yet.”
The Yale team described a similar evolution. “Initially, only a small subset of oncology patients could get these drugs,” said Osama (Sam) Abdelghany, PharmD, MHA, associate director of Oncology Pharmacy Services at Smilow Cancer Hospital. But as the caseload has diminished, Dr. Abdelghany noted, “we have been able to reach many more patients with COVID-19.”
An equitable system?
Dr. Wynia, who has written many reports on crisis standards of care, has spent thousands of hours delving into the ethics of allocating scarce resources during a disaster.
A core problem arises when there are too many people who need a scarce resource and no way of differentiating among them.
In response to the limited supply of COVID-19 treatments, some institutions, such as the University of Pittsburgh Medical Center and Massachusetts General Hospital, have created a lottery system. Others, such as Johns Hopkins Medicine, have opted for first come, first served. Each strategy comes with caveats.
“First come, first served prioritization may be quicker, but it gives more well-resourced people an advantage and lends itself to people abusing the system or exacerbating existing disparities,” Dr. Wynia said.
While a lottery system may be more equitable, this strategy often comes at the price of efficiency. “The practicality of doing a lottery when you have to make a decision about whether or not to treat the patient sitting in front of you comes with its own challenges,” Dr. Wynia said.
At the University of Colorado, he explained, the health center constantly scans medical records for patients who have been diagnosed with COVID and fall into a high-risk group. That way clinicians can call or email those most likely to benefit from these drugs.
“It ends up being a bit of a first come, first served strategy,” Dr. Wynia said. “But we also do not have a huge supply coming in each week, so reaching out to the most eligible people when we have the drugs in hand means more privileged patients are less likely to game the system.”
To manage the supply of Evusheld, Timothy Kubal, MD, MBA, and colleagues also reach out to patients most likely to benefit – specifically, those who can’t mount an adequate antibody response after vaccination.
“We screen all of our patients who have been receiving anti-CD20 agents and other chemotherapy agents known to suppress antibody response,” Dr. Kubal, a medical oncologist/hematologist at the Moffitt Institute in Tampa, Florida, said in an interview. “We then test those patients for antibodies and deliver Evusheld if they have no evidence of antibodies.”
Fortunately, in the coming months, distribution of these drugs should improve significantly. Pfizer says it expects to deliver 10 million courses of Paxlovid by the end of June, and another 10 million by the end of September. More than 1 million courses of sotrovimab should be distributed by GlaxoSmithKline through the end of March. And, recently, the Biden administration announced it purchased 1.2 million courses of Evusheld from AstraZeneca.
“Every few weeks, because the COVID picture changes, the demand changes,” said Dr. Louissaint. “With vaccination rates going up and cases going down, fewer patients will need these products.”
Still, the constant barrage of supply shortages over the past 2 years – from COVID tests, ventilators, and personal protective equipment early on to COVID vaccines a year later and more recently health care staff and COVID tests once again – has taken its toll.
“We have faced supply challenge after challenge and have had to be creative in each situation,” said Lisa Barbarotta, MSN, APRN, program director of Oncology Education and Clinical Practice at Smilow Cancer Hospital. “Nothing has been easy about this.”
And, Dr. Bloch cautioned, even with broader access to COVID-19 drugs on the horizon, there is still no substitute for vaccination. “Getting vaccinated is the best and first line of defense for most people,” she said.
A version of this article first appeared on Medscape.com.
In mid-November, Kevin Billingsley, MD, MBA, chief medical officer at Yale Cancer Center, New Haven, Conn., was keeping a close eye on the new COVID variant sweeping across South Africa. Six weeks later, the Omicron variant had become the dominant strain in the U.S. – and the Yale health system was no exception.
“As we entered January, we had a breathtaking rate of infection in our hospital,” said Dr. Billingsley, who also leads clinical care at the Smilow Cancer Hospital. “Some of the newly authorized COVID agents were available but not widely enough to make a clinically meaningful impact to protect all high-risk individuals during this surge.”
That left the team at Yale with difficult decisions about who would receive these treatments and who wouldn’t.
The health system convened a COVID-19 immunocompromised working group to identify which patients should get priority access to one of the promising drugs authorized to treat the infection – the monoclonal antibody sotrovimab and antiviral pills Paxlovid and molnupiravir – or the sole available option to prevent it, Evusheld.
“Although clinically sound, none of these decisions have been easy,” Dr. Billingsley told this news organization. “We have done a lot of case-by-case reviewing and a lot of handwringing. Omicron has been a wild ride for us all, and we have been doing the best we can with limited resources.”
‘We’re seeing incredible variability’
The team at Yale is not alone. The restricted supply of COVID-19 treatments has led many oncologists and other experts across the U.S. to create carefully curated lists of their most vulnerable patients.
In late December, the National Institutes of Health published broad criteria to help clinicians prioritize patients most likely to benefit from these therapies. A handful of state health departments, including those in Michigan and Minnesota, established their own standards. Patients with cancer – specifically those with hematologic malignancies and receiving oncology therapies that compromise the immune system – appeared at the top of everyone’s list.
But ultimately individual decisions about who receives these drugs and how they’re allocated fell to institutions.
“Overall, what we’re seeing is incredible variability across the country, because there’s no uniform agreement on what comprises best practices on allocating scarce resources,” said Matthew Wynia, MD, MPH, professor of medicine and director of the Center for Bioethics and Humanities at the University of Colorado, Aurora. “There are so many people at the top of most lists, and the drugs are in such short supply, that there’s no guarantee even those in the top tier will get it.”
This news organization spoke to experts across the country about their experiences accessing these treatments during the Omicron surge and their strategies prioritizing patients with cancer.
Dealing with limited supply
Overall, the limited supply of COVID-19 drugs means not every patient who’s eligible to receive a treatment will get one.
A snapshot of the past 2 weeks, for instance, shows that the count of new infections hit almost 4.3 million, while distribution of the two antiviral pills Paxlovid and molnupiravir and the monoclonal antibody sotrovimab reached just over 600,000 courses.
Since receiving emergency use authorization in early December, almost 500,000 courses of the pre-exposure prophylactic agent Evusheld – which offers about 6 months of protection for immunocompromised individuals – have been distributed; however, about 7 million adults in the U.S. could potentially benefit from it.
In addition, the distribution of drugs is uneven. The federal government manages the overall distribution to states, but states then decide how to divvy up these allocations to hospitals, pharmacies, and medical centers. In Ohio, for instance, the antivirals go to providers who already receive monoclonal antibodies, while in Tennessee, the supply of antiviral agents only goes to Walmart pharmacies.
This strategy, Dr. Wynia explained, can leave clinicians at the mercy of where and how much states decide to allocate to each location. “I’ve heard of some hospitals and health systems in Colorado that aren’t using all they’ve got, but most don’t have nearly enough,” Dr. Wynia said. However, he noted, “some of that is inevitable. We will never get a perfect distribution of these drugs when there is such variable need and demand.”
And, according to Nicolette Louissaint, PhD, MBA, senior vice president of policy and strategic planning at the Healthcare Distribution Alliance in Arlington, Virginia, “we can take some comfort that the federal government is actively looking at cases from week to week and working with state and local health departments to see who needs these products, which means the process is constantly being reviewed and adjusted.”
Plus, not every positive COVID-19 case, even among immunocompromised individuals, necessarily warrants treatment. “If, for instance, an individual with cancer has a mild case of COVID-19, their provider may not deem it necessary for them to receive treatment,” Dr. Louissaint noted.
Still, given the limited and unpredictable supply, “we have had to be thoughtful about who gets these drugs,” said Derek Raghavan, MD, PhD, president of the Levine Cancer Institute, part of the 40-hospital Atrium Health system in Charlotte, North Carolina.
Dr. Raghavan said the highest priority goes to patients with hematologic malignancies, those receiving or coming off chemotherapy or experiencing myelosuppression and immune paresis, as well as those who have undergone organ transplants. Age and other comorbidities, such as diabetes or obesity, play into the lineup as well.
To further hone their priority list, the Levine Cancer Institute has implemented a cancer-centered Hospital at Home initiative. The program includes 40 oncology nurse navigators who routinely screen and score all cancer patients who test positive for COVID-19 by their symptoms and risk factors. For a time-sensitive treatment like Paxlovid, this close monitoring allows patients with COVID to access the pills within 5 days of symptom onset.
Ultimately, “the decision regarding who gets these drugs is [made] by a team to overcome any risk of personal bias, and some of it just comes down to the interface between clinical judgment and available data,” Dr. Raghavan told this news organization. “Although we’d like to have more COVID drugs available and fewer patients with COVID, we have been able to get adequate supplies for our most at-risk patients.”
Like Dr. Raghavan, Karen Bloch, MD, MPH, the medical director for the COVID Infusion Clinic at Vanderbilt University Medical Center (VUMC), said the clinic has had to be highly selective about which patients would benefit most from the COVID monoclonal antibodies. For patients with cancer, her team prioritizes individuals who would be least able to develop antibodies through vaccination or natural infection – which includes patients with B cell malignancies, acute myeloid leukemia, or multiple myeloma receiving active treatment, as well as those who recently received an allogeneic or autologous stem cell transplant.
“Since our criteria for treatment with therapies such as sotrovimab and Evusheld are pretty stringent, we have had sufficient supply to treat those who meet our internal ‘category 1’ predetermined criteria,” said Dr. Bloch, professor of medicine and associate division director for clinical affairs at VUMC, Nashville. “More recently, as the supply chain has begun to open up, we’ve been able to loosen our criteria for sotrovimab, though not for Evusheld yet.”
The Yale team described a similar evolution. “Initially, only a small subset of oncology patients could get these drugs,” said Osama (Sam) Abdelghany, PharmD, MHA, associate director of Oncology Pharmacy Services at Smilow Cancer Hospital. But as the caseload has diminished, Dr. Abdelghany noted, “we have been able to reach many more patients with COVID-19.”
An equitable system?
Dr. Wynia, who has written many reports on crisis standards of care, has spent thousands of hours delving into the ethics of allocating scarce resources during a disaster.
A core problem arises when there are too many people who need a scarce resource and no way of differentiating among them.
In response to the limited supply of COVID-19 treatments, some institutions, such as the University of Pittsburgh Medical Center and Massachusetts General Hospital, have created a lottery system. Others, such as Johns Hopkins Medicine, have opted for first come, first served. Each strategy comes with caveats.
“First come, first served prioritization may be quicker, but it gives more well-resourced people an advantage and lends itself to people abusing the system or exacerbating existing disparities,” Dr. Wynia said.
While a lottery system may be more equitable, this strategy often comes at the price of efficiency. “The practicality of doing a lottery when you have to make a decision about whether or not to treat the patient sitting in front of you comes with its own challenges,” Dr. Wynia said.
At the University of Colorado, he explained, the health center constantly scans medical records for patients who have been diagnosed with COVID and fall into a high-risk group. That way clinicians can call or email those most likely to benefit from these drugs.
“It ends up being a bit of a first come, first served strategy,” Dr. Wynia said. “But we also do not have a huge supply coming in each week, so reaching out to the most eligible people when we have the drugs in hand means more privileged patients are less likely to game the system.”
To manage the supply of Evusheld, Timothy Kubal, MD, MBA, and colleagues also reach out to patients most likely to benefit – specifically, those who can’t mount an adequate antibody response after vaccination.
“We screen all of our patients who have been receiving anti-CD20 agents and other chemotherapy agents known to suppress antibody response,” Dr. Kubal, a medical oncologist/hematologist at the Moffitt Institute in Tampa, Florida, said in an interview. “We then test those patients for antibodies and deliver Evusheld if they have no evidence of antibodies.”
Fortunately, in the coming months, distribution of these drugs should improve significantly. Pfizer says it expects to deliver 10 million courses of Paxlovid by the end of June, and another 10 million by the end of September. More than 1 million courses of sotrovimab should be distributed by GlaxoSmithKline through the end of March. And, recently, the Biden administration announced it purchased 1.2 million courses of Evusheld from AstraZeneca.
“Every few weeks, because the COVID picture changes, the demand changes,” said Dr. Louissaint. “With vaccination rates going up and cases going down, fewer patients will need these products.”
Still, the constant barrage of supply shortages over the past 2 years – from COVID tests, ventilators, and personal protective equipment early on to COVID vaccines a year later and more recently health care staff and COVID tests once again – has taken its toll.
“We have faced supply challenge after challenge and have had to be creative in each situation,” said Lisa Barbarotta, MSN, APRN, program director of Oncology Education and Clinical Practice at Smilow Cancer Hospital. “Nothing has been easy about this.”
And, Dr. Bloch cautioned, even with broader access to COVID-19 drugs on the horizon, there is still no substitute for vaccination. “Getting vaccinated is the best and first line of defense for most people,” she said.
A version of this article first appeared on Medscape.com.
In mid-November, Kevin Billingsley, MD, MBA, chief medical officer at Yale Cancer Center, New Haven, Conn., was keeping a close eye on the new COVID variant sweeping across South Africa. Six weeks later, the Omicron variant had become the dominant strain in the U.S. – and the Yale health system was no exception.
“As we entered January, we had a breathtaking rate of infection in our hospital,” said Dr. Billingsley, who also leads clinical care at the Smilow Cancer Hospital. “Some of the newly authorized COVID agents were available but not widely enough to make a clinically meaningful impact to protect all high-risk individuals during this surge.”
That left the team at Yale with difficult decisions about who would receive these treatments and who wouldn’t.
The health system convened a COVID-19 immunocompromised working group to identify which patients should get priority access to one of the promising drugs authorized to treat the infection – the monoclonal antibody sotrovimab and antiviral pills Paxlovid and molnupiravir – or the sole available option to prevent it, Evusheld.
“Although clinically sound, none of these decisions have been easy,” Dr. Billingsley told this news organization. “We have done a lot of case-by-case reviewing and a lot of handwringing. Omicron has been a wild ride for us all, and we have been doing the best we can with limited resources.”
‘We’re seeing incredible variability’
The team at Yale is not alone. The restricted supply of COVID-19 treatments has led many oncologists and other experts across the U.S. to create carefully curated lists of their most vulnerable patients.
In late December, the National Institutes of Health published broad criteria to help clinicians prioritize patients most likely to benefit from these therapies. A handful of state health departments, including those in Michigan and Minnesota, established their own standards. Patients with cancer – specifically those with hematologic malignancies and receiving oncology therapies that compromise the immune system – appeared at the top of everyone’s list.
But ultimately individual decisions about who receives these drugs and how they’re allocated fell to institutions.
“Overall, what we’re seeing is incredible variability across the country, because there’s no uniform agreement on what comprises best practices on allocating scarce resources,” said Matthew Wynia, MD, MPH, professor of medicine and director of the Center for Bioethics and Humanities at the University of Colorado, Aurora. “There are so many people at the top of most lists, and the drugs are in such short supply, that there’s no guarantee even those in the top tier will get it.”
This news organization spoke to experts across the country about their experiences accessing these treatments during the Omicron surge and their strategies prioritizing patients with cancer.
Dealing with limited supply
Overall, the limited supply of COVID-19 drugs means not every patient who’s eligible to receive a treatment will get one.
A snapshot of the past 2 weeks, for instance, shows that the count of new infections hit almost 4.3 million, while distribution of the two antiviral pills Paxlovid and molnupiravir and the monoclonal antibody sotrovimab reached just over 600,000 courses.
Since receiving emergency use authorization in early December, almost 500,000 courses of the pre-exposure prophylactic agent Evusheld – which offers about 6 months of protection for immunocompromised individuals – have been distributed; however, about 7 million adults in the U.S. could potentially benefit from it.
In addition, the distribution of drugs is uneven. The federal government manages the overall distribution to states, but states then decide how to divvy up these allocations to hospitals, pharmacies, and medical centers. In Ohio, for instance, the antivirals go to providers who already receive monoclonal antibodies, while in Tennessee, the supply of antiviral agents only goes to Walmart pharmacies.
This strategy, Dr. Wynia explained, can leave clinicians at the mercy of where and how much states decide to allocate to each location. “I’ve heard of some hospitals and health systems in Colorado that aren’t using all they’ve got, but most don’t have nearly enough,” Dr. Wynia said. However, he noted, “some of that is inevitable. We will never get a perfect distribution of these drugs when there is such variable need and demand.”
And, according to Nicolette Louissaint, PhD, MBA, senior vice president of policy and strategic planning at the Healthcare Distribution Alliance in Arlington, Virginia, “we can take some comfort that the federal government is actively looking at cases from week to week and working with state and local health departments to see who needs these products, which means the process is constantly being reviewed and adjusted.”
Plus, not every positive COVID-19 case, even among immunocompromised individuals, necessarily warrants treatment. “If, for instance, an individual with cancer has a mild case of COVID-19, their provider may not deem it necessary for them to receive treatment,” Dr. Louissaint noted.
Still, given the limited and unpredictable supply, “we have had to be thoughtful about who gets these drugs,” said Derek Raghavan, MD, PhD, president of the Levine Cancer Institute, part of the 40-hospital Atrium Health system in Charlotte, North Carolina.
Dr. Raghavan said the highest priority goes to patients with hematologic malignancies, those receiving or coming off chemotherapy or experiencing myelosuppression and immune paresis, as well as those who have undergone organ transplants. Age and other comorbidities, such as diabetes or obesity, play into the lineup as well.
To further hone their priority list, the Levine Cancer Institute has implemented a cancer-centered Hospital at Home initiative. The program includes 40 oncology nurse navigators who routinely screen and score all cancer patients who test positive for COVID-19 by their symptoms and risk factors. For a time-sensitive treatment like Paxlovid, this close monitoring allows patients with COVID to access the pills within 5 days of symptom onset.
Ultimately, “the decision regarding who gets these drugs is [made] by a team to overcome any risk of personal bias, and some of it just comes down to the interface between clinical judgment and available data,” Dr. Raghavan told this news organization. “Although we’d like to have more COVID drugs available and fewer patients with COVID, we have been able to get adequate supplies for our most at-risk patients.”
Like Dr. Raghavan, Karen Bloch, MD, MPH, the medical director for the COVID Infusion Clinic at Vanderbilt University Medical Center (VUMC), said the clinic has had to be highly selective about which patients would benefit most from the COVID monoclonal antibodies. For patients with cancer, her team prioritizes individuals who would be least able to develop antibodies through vaccination or natural infection – which includes patients with B cell malignancies, acute myeloid leukemia, or multiple myeloma receiving active treatment, as well as those who recently received an allogeneic or autologous stem cell transplant.
“Since our criteria for treatment with therapies such as sotrovimab and Evusheld are pretty stringent, we have had sufficient supply to treat those who meet our internal ‘category 1’ predetermined criteria,” said Dr. Bloch, professor of medicine and associate division director for clinical affairs at VUMC, Nashville. “More recently, as the supply chain has begun to open up, we’ve been able to loosen our criteria for sotrovimab, though not for Evusheld yet.”
The Yale team described a similar evolution. “Initially, only a small subset of oncology patients could get these drugs,” said Osama (Sam) Abdelghany, PharmD, MHA, associate director of Oncology Pharmacy Services at Smilow Cancer Hospital. But as the caseload has diminished, Dr. Abdelghany noted, “we have been able to reach many more patients with COVID-19.”
An equitable system?
Dr. Wynia, who has written many reports on crisis standards of care, has spent thousands of hours delving into the ethics of allocating scarce resources during a disaster.
A core problem arises when there are too many people who need a scarce resource and no way of differentiating among them.
In response to the limited supply of COVID-19 treatments, some institutions, such as the University of Pittsburgh Medical Center and Massachusetts General Hospital, have created a lottery system. Others, such as Johns Hopkins Medicine, have opted for first come, first served. Each strategy comes with caveats.
“First come, first served prioritization may be quicker, but it gives more well-resourced people an advantage and lends itself to people abusing the system or exacerbating existing disparities,” Dr. Wynia said.
While a lottery system may be more equitable, this strategy often comes at the price of efficiency. “The practicality of doing a lottery when you have to make a decision about whether or not to treat the patient sitting in front of you comes with its own challenges,” Dr. Wynia said.
At the University of Colorado, he explained, the health center constantly scans medical records for patients who have been diagnosed with COVID and fall into a high-risk group. That way clinicians can call or email those most likely to benefit from these drugs.
“It ends up being a bit of a first come, first served strategy,” Dr. Wynia said. “But we also do not have a huge supply coming in each week, so reaching out to the most eligible people when we have the drugs in hand means more privileged patients are less likely to game the system.”
To manage the supply of Evusheld, Timothy Kubal, MD, MBA, and colleagues also reach out to patients most likely to benefit – specifically, those who can’t mount an adequate antibody response after vaccination.
“We screen all of our patients who have been receiving anti-CD20 agents and other chemotherapy agents known to suppress antibody response,” Dr. Kubal, a medical oncologist/hematologist at the Moffitt Institute in Tampa, Florida, said in an interview. “We then test those patients for antibodies and deliver Evusheld if they have no evidence of antibodies.”
Fortunately, in the coming months, distribution of these drugs should improve significantly. Pfizer says it expects to deliver 10 million courses of Paxlovid by the end of June, and another 10 million by the end of September. More than 1 million courses of sotrovimab should be distributed by GlaxoSmithKline through the end of March. And, recently, the Biden administration announced it purchased 1.2 million courses of Evusheld from AstraZeneca.
“Every few weeks, because the COVID picture changes, the demand changes,” said Dr. Louissaint. “With vaccination rates going up and cases going down, fewer patients will need these products.”
Still, the constant barrage of supply shortages over the past 2 years – from COVID tests, ventilators, and personal protective equipment early on to COVID vaccines a year later and more recently health care staff and COVID tests once again – has taken its toll.
“We have faced supply challenge after challenge and have had to be creative in each situation,” said Lisa Barbarotta, MSN, APRN, program director of Oncology Education and Clinical Practice at Smilow Cancer Hospital. “Nothing has been easy about this.”
And, Dr. Bloch cautioned, even with broader access to COVID-19 drugs on the horizon, there is still no substitute for vaccination. “Getting vaccinated is the best and first line of defense for most people,” she said.
A version of this article first appeared on Medscape.com.